Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049
TITLE
PROCESS FOR PRODUCING A REFINERY STREAM-COMPATIBLE BIO-OIL
FROM A LIGNOCELLULOSIC FEEDSTOCK
FIELD
[001] The invention relates generally to compositions and methods for
preparing
biofuels, including lignocellulose-derived bio-oils compatible with refinery
process streams.
BACKGROUND
[002] A bio-oil produced from a lignocellulosic feedstock, typically has a
high oxygen
content, in the range of 10-25% 0, or higher. In order for a bio-oil to be
processed with a
conventional refinery stream, the bio-oil needs to be miscible, or soluble,
with the refinery
stream. Incompatible liquids frequently will have flow or phase separation
problems in flow
lines, vessels or reactors.
[003] Biomass liquefaction processes, such as wood pyrolysis or wood
liquefaction with
a donor solvent, are coarse transformations with minimal control on the
chemical makeup of the
product bio-oil. Specifically, the oxygen content and average molecular weight
of the bio-oil has
not been controlled sufficiently to make the oil compatible (miscible) with
the refinery stream for
further hydroprocessing. In addition, these coarse bio-oils may be highly
reactive during
catalytic hydrogenation with the result that they are prone to easy
degradation and unwanted side
reactions, such as charring.
[004] There is a need in the art for systems enabling the use of
lignocellulosic
feedstock-derived bio-oils compatible with refinery process streams. The
present disclosure
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049
addresses this need and more and describes: 1) compositions of processed bio-
oil compatible
with refinery streams; and, 2) processes and systems for converting a bio-oil
precursor to a bio-
oil compatible with refinery process streams.
SUMMARY OF THE INVENTION
[005] In one aspect, compatibility of a bio-oil with a refinery process stream
is achieved
by using a previously hydrotreated bio-oil blend as a recycle oil in a
process, whereby a coarse
bio-oil is mixed with the recycled bio-oil in a controlled ratio to form a
mixture from which
oxygen and water are removed. The resultant bio-oil product in from this
process has reduced
oxygen content, lower molecular weight, and is suitably miscible for use in a
refinery stream for
further hydroprocessing, or it can be used as recycle oil for processing
additional coarse bio-oil.
[006] In one embodiment, a method for rendering biomass-derived pyrolysis oil
miscible with refinery hydrocarbons comprises mixing a high oxygen content bio-
oil having an
oxygen content of at least about 10 wt. % with a low oxygen content bio-oil
having an oxygen
content of less than about 8 wt. % to produce a blended oil. The blended oil
has an oxygen
content for suitable miscibility.
[007] In a further step, the blended oil is hydrotreated to yield a
hydrotreated mixture
comprising (i) low oxygen-content hydrotreated bio-oil and (ii) water, wherein
the low oxygen-
content hydrotreated bio-oil has an oxygen content of 10 wt. % or less.
[008] In a further step, water is removed from the hydrotreated mixture via a
phase
separation between the low oxygen content hydrotreated bio-oil and the water
to yield a low
oxygen content hybrid bio-oil intermediate. Addition of a solvent may be
unnecessary. The
water may readily phase separate from the hydrotreated bio-oil, with the bio-
oil floating on top
2
CA 02850510 2014-03-28
WO 2013/049665 PCT/US2012/058049
of the water, so a typical water separator process may be used. Azeotropic
distillation may be
useful for determining the amount of water in a mixed bio-oil/water sample
where good phase
separation does not occur. One aspect of the claimed process is that it
produces a bio-oil which
readily phase separates from any associated water produced or carried into the
process. A
portion of the low oxygen content hybrid bio-oil intermediate may be recycled
with the high
oxygen content bio-oil according to the above described embodiments.
Alternatively, a portion
of the low oxygen content hybrid bio-oil intermediate may be removed for use
in a refinery
stream for further hydroprocessing.
[009] In another aspect, a system for producing a bio-oil comprises a mixing
unit, a
hydrotreater, and a separator. The system may further comprise biomass
conversion unit and a
second mixing unit. The biomass conversion unit facilitates production of a
high oxygen content
bio-oil from a biomass feedstock. The mixing unit comprises a first inlet
receiving the high
oxygen content bio-oil from the conversion unit and a second inlet receiving a
low oxygen
content bio-oil from a bio-oil feedstock to form a blended oil. The
hydrotreater comprises an
inlet receiving the blended oil from the mixing unit to produce a hydrotreated
bio-oil mixture.
The separator separates and removes water from the hydrotreated bio-oil
mixture to produce a
low oxygen content hybrid bio-oil intermediate and includes an inlet receiving
the hydrotreated
bio-oil mixture from the hydrotreater, an outlet supplying at least at least a
portion the low
oxygen content hybrid bio-oil intermediate to the mixing unit, and optionally
an outlet supplying
at least another portion of the low oxygen content hybrid bio-oil intermediate
to a source of
refinery hydrocarbons.
[0010] In some embodiments, the system may further comprise a second mixing
unit
comprising a first inlet receiving the low oxygen content hybrid bio-oil
intermediate from the
3
CA 02850510 2014-03-28
WO 2013/049665 PCT/US2012/058049
separator, a second inlet receiving a low oxygen content bio-oil from a bio-
oil feedstock to
produce a low oxygen blended oil, and an outlet supplying the low oxygen
blended oil formed in
the second mixing unit to the first mixing unit. The separator further
comprises an outlet
supplying the low oxygen content hybrid bio-oil intermediate to the second
mixing unit.
[0011] In another aspect, the present invention provides a blended oil
composition that
can be used in the above described system. In one embodiment, the blended oil
composition
comprises a high oxygen content bio-oil with an oxygen content of at least
about 10 wt. % bio-
oil blended with a low oxygen content bio-oil with an oxygen content of less
than about 8 wt. %,
wherein the ratio of the high oxygen content bio-oil to the low oxygen content
bio-oil in the
blended oil is at least about 5%, 10%, or 20% up to about 30%, 40%, 50%, and
up to about 90%.
An average molecular weight of the low oxygen content bio-oil may be about 100
g/mol, 125
g/mol, or 150 g/mol up to about 300 g/mol, 400 g/mol, or 500 g/mol. An average
molecular
weight of the high oxygen content bio-oil may be about 150 g/mol, 200 g/mol,
or 250 g/mol up
to about 800 g/mol, 900 g/mol, or 1000 g/mol.
BRIEF DESCRIPTION OF THE DRAWINGS
[0012] The accompanying drawings illustrate one or more embodiments of the
invention
and, together with the written description, serve to explain the principles of
the invention.
Wherever possible, the same reference numbers are used throughout the drawings
to refer to the
same or like elements of an embodiment.
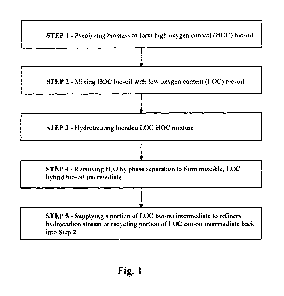
[0013] FIG. 1 depicts, in stepwise fashion, a method for rendering biomass-
derived bio-
oil miscible with refinery hydrocarbons in accordance with some embodiments of
the present
invention.
4
CA 02850510 2014-03-28
WO 2013/049665 PCT/US2012/058049
[0014] FIG. 2 depicts a system for rendering biomass-derived bio-oil miscible
with
refinery hydrocarbons in accordance with some embodiments of the present
invention.
DETAILED DESCRIPTION OF THE INVENTION
[0015] Certain terms and phrases are defined throughout this description as
they are first
used, while certain other terms used in this description are defined below:
[0016] As used herein, the term "biomass" refers to any organic matter
collected for use
as a source of energy as further described herein.
[0017] The term "biofuel" refers to a fuel product at least partly derived
from "biomass,"
the latter comprising a renewable resource of organic materials.
[0018] The term "bio-oil" refers to a liquid biofuel product comprising oxygen-
containing organic compounds produced by thermochemical treatment of a solid
biomass
feedstock, such as by pyrolyis, or a natural oil, already present in the
feedstock, and typically
produced by mechanical and/or solvent extraction methods.
[0019] The terms "feed' or "feedstock" refer to a hydrocarbonaceous material
fed into
one or more of the systems or processes of the present invention in order to
make a fuel,
lubricant, or other commercial product. A biomass feedstock useful for the
methods described
herein can be a solid fuel, bio-oil, fluid fuel (e.g., a fuel that includes a
liquid or a gas).
[0020] The term "pyrolysis" or "pyrolyzing" refer to the thermal processing
and/or
thermal decomposition of hydrocarbonaceous material, typically carried out in
a non-oxidative
environment.
[0021] The term "pyrolysis oil" refers to a liquid hydrocarbon product
resulting from the
pyrolyzing treatment of hydrocarbonaceous material.
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049
[0022] The terms "hydroprocessing" or "hydrotreating" are used interchangeably
herein
with reference to processes or treatments that react a hydrocarbon-based
material with hydrogen,
typically under pressure and with a catalyst (hydroprocessing can be non-
catalytic). Such
processes include, but are not limited to, hydrodeoxygenation (of oxygenated
species),
hydrotreating, hydrocracking, hydroisomerization, and hydrodewaxing. For
examples of such
processes, see Cash et al., U.S. Pat. No. 6,630,066; and Elomari, U.S. Pat.
No. 6,841,063.
[0023] The present invention provides a processed bio-oil composition
compatible with
refinery streams and a process for converting a bio-oil precursor to a bio-oil
compatible with
refinery streams. In one aspect, compatibility of a bio-oil with a refinery
process stream is
achieved by using a previously hydrotreated bio-oil blend as a recycle oil in
a process, whereby a
coarse bio-oil is mixed with the recycled bio-oil in a controlled ratio to
form a mixture from
which oxygen and water are removed. The resultant bio-oil product formed from
this process
has reduced oxygen content, lower molecular weight, and is suitably miscible
for use in a
refinery stream for further hydroprocessing, or it can be used as recycle oil
for processing
additional coarse bio-oil.
[0024] In one embodiment, exemplified in FIG. 1, a method for rendering
biomass-
derived pyrolysis oil miscible with refinery hydrocarbons, comprises: (Step 1)
pyrolyzing
biomass to form a high oxygen content (HOC) bio-oil; (Step 2) mixing HOC bio-
oil with low
oxygen content (LOC) bio-oil; (Step 3) hydrotreating the blended LOC/HOC
mixture; (Step 4)
removing water by phase separation to form a miscible, LOC hybrid bio-oil
intermediate; and
(Step 5) supplying a portion of LOC hybrid bio-oil intermediate to refinery
hydrocarbon stream
or recycling a portion of the LOC hybrid bio-oil intermediate back into Step
2.
6
CA 02850510 2014-03-28
WO 2013/049665 PCT/US2012/058049
[0025] In one embodiment, a method for rendering biomass-derived pyrolysis oil
miscible with refinery hydrocarbons comprises mixing a high oxygen content bio-
oil having an
oxygen content of at least about 10 wt. % with a low oxygen content bio-oil
having an oxygen
content of less than about 8 wt. % to produce a blended oil.
[0026] In a further embodiment, the blended oil is hydrotreated to yield a
hydrotreated
mixture comprising (i) low oxygen-content hydrotreated bio-oil and (ii) water,
wherein the low
oxygen-content hydrotreated bio-oil has an oxygen content of 10 wt. % or less.
Hydrotreating
processes in accordance with one or more embodiments disclosed herein are
expected to reduce
oxygen content to less than about 10%, reduce average molecular weight, and
generally to
"stabilize" the bio-oil so aging or phase stability may no longer be an issue
or concern. Oxygen
is removed by hydrotreatment via reaction with hydrogen over a suitable
catalyst.
[0027] In a further step, water is removed from the hydrotreated mixture via a
phase
separation between the low oxygen content hydrotreated bio-oil and the water
to yield a low
oxygen content hybrid bio-oil intermediate. A portion of the low oxygen
content hybrid bio-oil
intermediate may be removed for use in a refinery stream or it may be combined
and recycled
with the high oxygen content bio-oil according to the above described
embodiments.
[0028] In one embodiment, the high oxygen content bio-oil is produced in a
biomass
conversion unit by a conversion process comprising fast pyrolysis, slow
pyrolysis, liquefaction,
gasification, enzymatic conversion, cellulolysis, Fischer-Tropsch processing
or combinations
thereof. In a preferred embodiment, the high oxygen content bio-oil comprises
a liquid
hydrocarbon product resulting from pyrolysis of a lignocellulosic feedstock.
[0029] Bio-oils are a complex mixture of biomass compounds, including
oxygenates, that
are obtained from an organic matter collected for use as a source of energy.
Any biomass source
7
CA 02850510 2014-03-28
WO 2013/049665 PCT/US2012/058049
may be used as starting materials for the bio-oils of the present invention.
Non-fossilized
biomass includes plant biomass (defined below), animal biomass (any animal by-
product, animal
waste, etc.), and municipal waste biomass (residential and light commercial
refuse with
recyclables such as metal and glass removed). Biomass may also include any
type of
carbonaceous material from a fossilized source. Fossilized biomass, therefore,
can further
encompass various petroleum products, including, but not limited to petroleum
and coal.
[0030] Plant biomass or lignocellulosic biomass includes virtually any plant-
derived
organic matter (woody or non-woody) available for energy on a sustainable
basis. Exemplary
plants include grasses, trees, and other sources of lignocellulosic material,
including those
derived from municipal waste, food processing wastes, forestry wastes and pulp
and paper
byproducts. "Plant-derived" necessarily includes both sexually reproductive
plant parts involved
in the production of seed (e.g., flower buds, flowers, fruit and seeds) and
vegetative parts (e.g.,
leaves, roots, leaf buds and stems). Examples of such plants include, but are
not limited to, corn,
soybeans, cotton, wheat, rice, and algae. Plant biomass can include, but is
not limited to,
agricultural crop wastes and residues such as corn stover, wheat straw, rice
straw, and sugar cane
bagasse. Plant biomass can further include, but is not limited to, woody
energy crops, wood
wastes, and residues such as trees, softwood forest thinnings, barky wastes,
sawdust, paper and
pulp industry waste streams, and wood fiber. Examples of such trees, include,
but are not limited
to, hybrid poplar trees (e.g., Aspen). Additionally, any type of grasses, such
as switch grass, for
example, can be used as a plant biomass source. Typically, the plant biomass
for use in the
present invention includes starch, cellulose, hemicellulose, lignin, and
combinations thereof.
[0031] Biomass starting materials may also include waste products from
industry,
agriculture, forestry, and households. Examples of such waste products that
can be used as
8
CA 02850510 2014-03-28
WO 2013/049665 PCT/US2012/058049
biomass include fermentation waste, straw, lumber, sewage, garbage, vegetable
processing
waste, yard waste, including grass clippings, tree clippings, leaves, and
brush, and food leftovers.
[0032] Plant-derived starting materials typically include at least 5 wt. %
water, in some
embodiments at least 10% wt. % water, 20 wt. % water, or more. In some
embodiments there
may be 5 to 50 wt. % water, 10 to 40 wt. % water or up to about 35 wt. %
water. The water may
be present in a single phase with the oil, or primarily in a second phase (for
an example an
emulsion with the aqueous phase as either the major or minor component), or in
mixture of
phases. In some preferred embodiments, a second (primarily) water phase is
formed during a
hydrogenation reaction and is removed during or after the hydrogenation
treatment.
[0033] The biomass starting materials may be thermally processed using any
conventional process for preparing bio-oils therefrom, including fast
pyrolysis, slow pyrolysis,
liquefaction, gasification, enzymatic conversion, cellulolysis, Fischer-
Tropsch processing, and
combinations thereof. Bio-oils resulting therefrom represent a complex mixture
of compounds,
often derived from the thermal breakdown of solid biomass components,
including cellulose,
hemicellulose and lignin present in lignocellulosic biomass.
[0034] In a preferred embodiment, the biomass starting materials are thermally
processed
via fast pyrolysis. Fast pyrolysis is a high temperature process (350 to 800
C.) in which a
biologically based feedstock, such as lignocellulosic biomass, is rapidly
heated in the absence of
air and vaporizes into a product gas stream. Fast pyrolysis of solid biomass
causes the major part
of its solid organic material to be instantaneously transformed into a vapor
phase. This vapor
phase contains both non-condensable gases (including methane, hydrogen, carbon
monoxide,
carbon dioxide and olefins) and condensable vapors. It is the condensable
vapors that constitute
the final liquid bio-oil.
9
CA 02850510 2014-03-28
WO 2013/049665 PCT/US2012/058049
[0035] The feedstocks of the present invention may be processed using a fast
pyrolysis
reactor, such as that disclosed in U.S. Pat. Nos. 5,961,780 and 5,792,340.
Other known riser
reactors with short residence times may also be employed, for example, but not
limited to U.S.
Pat. Nos. 4,427,539, 4,569,753, 4,818,373, 4,243,514 (which are incorporated
by reference).
The reactor is preferably run at a temperature of from about 450 C. to about
600 C, more
preferably from about 480 C. to about 550 C. The contact times between the
heat carrier and
feedstock is preferably from about 0.01 to about 20 sec, more preferably from
about 0.1 to about
sec., most preferably, from about 0.5 to about 2 sec.
[0036] Preferably, the heat carrier used within the pyrolysis reactor is
catalytically inert
or exhibits low catalytic activity. Such a heat carrier may be a particulate
solid, preferably sand,
for example, silica sand. By silica sand it is meant any sand comprising
greater than about 80%
silica, preferably greater than about 95% silica, and more preferably greater
than about 99%
silica. It is to be understood that the above composition is an example of a
silica sand that can be
used as a heat carrier as described herein, however, variations within the
proportions of these
ingredients within other silica sands may exist and still be suitable for use
as a heat carrier.
Other known inert particulate heat carriers or contact materials, for example
kaolin clays, rutile,
low surface area alumina, oxides of magnesium and calcium are described in
U.S. Pat. No.
4,818,373 or U.S. Pat. No. 4,243,514.
[0037] As used herein, the phrase "high oxygen content bio-oil" (HOC bio-oil)
comprises
an oxygen content of at least 15 wt % oxygen. In some embodiments, the high
oxygen content
bio-oil comprises at least 20 wt % oxygen, at least 25 wt % oxygen, at least
30 wt % oxygen, at
least 35 wt % oxygen, 40 wt % oxygen, between 25 wt. % and 50 wt. %, and
combinations or
ranges therefrom.
CA 02850510 2014-03-28
WO 2013/049665 PCT/US2012/058049
[0038] The low oxygen content bio-oil comprises an oxygen content of about 8
wt. % or
less. In some embodiments, the low oxygen bio-oil comprises an oxygen content
between about
wt. % and about 8 wt % or less than 5 wt. %. In other embodiments, the low
oxygen content
bio-oil has a total acid number less than about 10 mg KOH/g.
[0039] The low oxygen content bio-oil may comprise a biomass feedstock
produced by
any convenient method, including, but not limited to, pyrolysis or catalytic
pyrolysis,
hydroliquefaction via catalytic hydrogenation or by hydrogen donor solvent
liquefaction.
Reduction may be by conventional hydrotreating using hydrogen, or with
synthesis gas (CO/H2),
or with aqueous reduction using water plus CO with suitable catalysts. In some
embodiments,
the low oxygen content bio-oil is produced from a hydrotreated lignocellulosic
feedstock.
[0040] In one embodiment, the high oxygen content bio-oil is mixed with the
low oxygen
content bio-oil, wherein the ratio of the high oxygen content bio-oil to the
low oxygen content
bio-oil in the blended oil is less than or equal to 0.3. In some embodiments,
the high oxygen
content bio-oil and the low oxygen content bio-oil are mixed so that the low
oxygen content bio-
oil serves as a solvent for the high oxygen content bio-oil. In other
embodiments, the high
oxygen content bio-oil and the low oxygen content bio-oil are mixed so that
the blended oil has a
total acid number between about 50-100 mg KOH/g. In yet other embodiments, a
bio-oil is
pretreated by hydrotreating the bio-oil prior to admixture in the blended oil.
[0041] In the present invention, the oxygenates present in the feed are
removed by
hydrotreating. "Hydrotreating" may be defined as a catalytic process, usually
carried out in the
presence of free hydrogen, in which the primary purpose when used to process
conventional
petroleum derived feed stocks is the removal of various contaminants, such as
arsenic;
11
CA 02850510 2014-03-28
WO 2013/049665 PCT/US2012/058049
heteroatoms, such as sulfur, oxygen, and nitrogen; and aromatics from the feed
stock. In the
present process, the primary purpose is to remove the oxygenates in the feed.
[0042] The method comprises hydrotreating the blended oil by passing the oil
to a
hydrotreating unit where the oil is contacted with a hydrotreating catalyst
under a hydrogen
atmosphere. The hydrotreating unit can also be a hydrocracking unit with a
hydrocracking
catalyst for breaking additional oxygenates out of the lignin compounds or any
other unit known
to remove oxygenates from a feed.
[0043] In one embodiment, the blended oil is passed to a hydrotreater where
the blended
oil is contacted with a hydrotreating catalyst under a hydrogen atmosphere.
Alternatively, the
hydrotreater can comprise a hydrocracking unit with a hydrocracking catalyst
for breaking
additional oxygenates out of e.g, the lignin compounds in the blended oil
composition. When
hydrotreating the blended oil, hydrogen is added separately or together with
the blended oil in a
reactor, thereby resulting in the production of low oxygen-content
hydrotreated bio-oil and
water. In a continuous process, hydrogen is added along the length of a
reactor. The hydrogen is
preferably added in excess of stoichiometry to maximize reaction rate by
minimizing mass
transfer limitations. Exemplary hydrogen flow rates may range between about 50
to about 5,000
standard cubic feet (SCF) of hydrogen per barrel (bbl) of oil feed. When
milder hydrotreatment
conditions are desired, the hydrogen between about 200 to about 2,000 SCF/bbl
of oil feed, from
about 400 to about 1,600 SCF/bbl, from about 600 to about 1,200 SCF/bbl, or
combinations
thereof.
[0044] Preferably, hydrogen is reacted with the blended oil feedstock at a
level of at least
50 liter/liter, more preferably at least 100 liter/liter, and still more
preferably at least 200
12
CA 02850510 2014-03-28
WO 2013/049665 PCT/US2012/058049
liter/liter, in some embodiments in the range of 100 to 300 liter/liter, and
in some embodiments
in the range of 100 to 175 liter/liter. Excess hydrogen may be recycled into a
reactor.
[0045] The hydrotreater may comprise a metal-containing catalyst comprising Co-
Mo,
Ni-Mo, a transition metal, a noble metal, a metal oxide therefrom, a metal
sulfide therefrom, or a
combination thereof. Metal-containing catalysts useful for the methods
described herein include,
for example, a transition metal, a noble metal, or a combination thereof. The
term "metal-
containing catalyst" refers to a catalyst that includes a metal, a metal-
containing compound, or a
metal-containing composite. Exemplary metals include Ni, Co, Pd, Pt, Mo, W,
Ru, Cu, Cr, Zn,
and combinations thereof. In some embodiments, the metal-containing catalyst
can optionally
include a second metal, a second metal-containing compound, or a second metal-
containing
composite. The term "mixed-metal catalyst" refers to a catalyst that contains
more than one
metal, metal-containing compound, or metal-containing composite. Preferred
catalysts include
those comprising Ni, Co, Mo, W or combinations thereof, for example, one or
more Group VIII
metals and one or more Group VIB metals, for example comprising Ni and/or Co
and W and/or
Mo, preferably comprising a combination of Ni and Mo, or Co and Mo, or a
ternary combination
such as Ni, Co, and Mo or Ni, Mo, and W. Particular catalysts include ICR 181
and ICR511
(commercially available from Chevron Lummus Global), and Molyvan A (R.T.
Vanderbilt Co.,
Norwalk, CT)
[0046] The hydrotreatment catalyst may be further supported on a suitable
support
material. In some embodiments, the support comprises alumina, especially gamma
or eta
alumina. Chromia and rare earth oxides may make take up at least part of the
support. Other
useful support oxides include titania, zirconia, hafnia, thoria, vanadia,
urania, oxides of
manganese, molybdenum and tungsten, and combined oxides and supports thereof.
The support
13
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049
material typically has a pore volume over 0.2 cm3/g and a surface area of at
least 1.0, preferably
over 15, especially in the range 50-200 m2/g.
[0047] The catalyst can be present as a wall coating, fluidized bed, fixed bed
of particles
or pellets, etc. A fixed bed of catalyst particles has the advantage of ease
of design and operation
(clean-up and catalyst replacement). In some embodiments, fluidized bed
reactors may be
preferred, especially if the bio-oil is contaminated with inorganic material.
In some
embodiments, wall coated reactors, which have certain advantages for heat and
mass transfer,
may be preferred.
[0048] The hydrotreater may include any conventional hydrotreatment device or
hydrotreatment process, including but not limited to hydrodeoxygenation (of
oxygenated
species), hydrotreating, hydrocracking, hydroisomerization, hydrodewaxing, and
the like. The
hydrotreater may include down flow reactor, autoclave batch reactor, fixed bed
reactor, moving
bed reactor, dynamic bed reactor, fluid bed reactor, slurry reactor,
countercurrent free fall
reactor, concurrent riser reactor, ebullated bed reactor, and reactors with
continuous replacement
or replenishment of the catalyst bed.
[0049] Preferably, the hydrotreatment is carried out relatively mild
hydrotreating
conditions. In one embodiment, hydrotreatment of the blended oil is carried
out under a
hydrogen atmosphere under a hydrogen partial pressure of about 15 pounds-force
per square inch
gauge (psig) to about 3,000 psig, from about 200 psig to about 2,000 psig,
from about 400 psig to
about 1,500 psig, from about 200 to about 1,000 psig, or combinations thereof.
[0050] In addition, the hydrotreatment may be carried out in a range of
different
temperature conditions. In one embodiment, the hydrotreatment is carried out
at a temperatures
between about 100 C to about 500 C, between about 150 C to about 350 C,
below 300 C, or
14
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049
combinations thereof. In another embodiment, the hydrotreatment process
employs a
temperature gradient across a catalyst bed. In a specific embodiment, the
temperature gradient
comprises temperatures between about 100 C to 200 C at the lower temperature
range up to
about 300 C or less at the upper temperature range.
[0051] In a further embodiment, water is removed from the hydrotreated mixture
in a
separator to form a low oxygen content hybrid bio-oil intermediate. In one
embodiment, a
portion of the low oxygen content hybrid bio-oil intermediate may be recycled
with the high
oxygen content bio-oil according to the above described embodiments.
Alternatively, a portion
of the low oxygen content hybrid bio-oil intermediate may be removed for use
in a refinery
stream for further hydroprocessing.
[0052] Generally, the separator will remove water by a phase separation
process based on
differences in volatility. Exemplary separators or separation methods include
phase separation
by decanting, distillation, or separation using membranes. Exemplary separator
units include a
phase separators, extractors, purifiers, distillation columns and the like.
[0053] In a preferred embodiment, water is separated by azeotropic
distillation.
Azeotrope selection is driven by the amount and cost of the azeotrope-forming
liquids, the
desired boiling temperature, and the compatibility of the azeotrope-forming
liquid with the
hydrotreated mixture. "Compatibility" as used herein means that the azeotrope-
forming liquid is
co-soluble with the hydrotreated mixture, i.e., there is no phase separation
upon mixing of the
hydrotreated mixture and the azeotrope-forming liquid(s). While certain
azeotrope-forming
liquids and azeotropes have been identified, the present invention is not so
limited. Other
azeotrope-forming liquids and azeotropes may be used if they form an azeotrope
with water
alone or with water in combination with other azeotrope-forming liquids.
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049
[0054] Azeotropic distillation can be conducted using a Dean Stark trap or
equivalent
apparatus and the temperature is set to an elevated temperature in the range
of about 130 C. to
about 150 C., such as about 145 C., and it should be appreciated that
distillation may start after
a period of time to allow the reaction mixture to reach about 95 C. to 105
C. Once the
distillation commences, the gas flow for the inert atmosphere (such as a
blanket under N2) can be
increased to about 0.1 SCFH to about 1.0 SCFH, such as 0.5 SCFH as an example.
The
temperature is maintained at the selected elevated temperature for sufficient
time, which may be
about an additional 2 hours to about 2.5 hours.
[0055] Effective azeotrope-forming liquids for preparing low oxygen content
hybrid bio-
oil intermediate compositions include toluene, ethanol, acetone, 2-propanol,
cyclohexane, 2-
butanone, octane, benzene, ethyl acetate, and combinations thereof. Exemplary
suitable
azeotropes formed during process include binary azeotropes such as
ethanol/water,
toluene/water, acetone/water, 2-propanol/water, cyclohexane/water, 2-
butanone/water, and
octane/water and ternary azeotropes such as ethanol/toluene/water, 1-
butanolloctane/water,
benzene/2-propanol/water, ethano1/2-butanone/water, and ethanol/ethyl
acetate/water
[0056] The low oxygen content hybrid bio-oil intermediate is preferably formed
with a
moderately low oxygen content, generally <10%, and has a total acid number
(TAN) of <20 mg
KOH/g. In one embodiment, the low oxygen content hybrid bio-oil intermediate
has a total acid
number less than or equal to 20 mg KOH/g. In other embodiments, the low oxygen
content
hybrid bio-oil intermediate has an average molecular weight between about 200-
300 g/mol, with
the highest molecular weight components not greater than 500-600 g/mol. The
boiling point
range low oxygen content hybrid bio-oil intermediate is generally no more than
500 C with a
typical midpoint of about 300 C, and may be as low as about 180 C, 200 C,
or 220 C.
16
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049
[0057] Preferably, the low oxygen content hybrid bio-oil intermediate is
formed to be
substantially miscible in a non-polar solvent. For example, suitable
miscibility, as used herein,
may refer to a low oxygen content hybrid bio-oil intermediate that may be
greater than about
95% soluble when mixed with the non-polar solvent in a 10:90 ratio of bio-oil
to solvent.
[0058] Where a portion of the low oxygen content hybrid bio-oil intermediate
is
combined and recycled with the high oxygen content bio-oil according to the
above described
embodiments, the low oxygen content hybrid bio-oil intermediate may be added
to a second low
oxygen content bio-oil in a second mixing unit to yield a second blended oil,
wherein the second
blended oil is added to the high oxygen content bio-oil in a first mixing
unit.
[0059] In other embodiments, at least a portion of the low oxygen content
hybrid bio-oil
intermediate is directly applied for use in a refinery process stream. A
suitable refinery process
stream will have sufficiently high aromatic content, and will be compatible
with the bio-oil and
able to completely dissolve or be miscible with the bio-oil, without causing
phase separation of
the highest boiling or the highest oxygen content components.
[0060] In another aspect, the present invention provides a blended oil
composition,
comprising a high oxygen content bio-oil with an oxygen content of at least
about 15 wt. % bio-
oil blended with a low oxygen content bio-oil with an oxygen content of less
than about 8 wt. %,
wherein the blended oil has an oxygen content for suitable miscibility. The
blended oil
composition may be modified in accordance with the above method teachings.
[0061] In another aspect, a system for producing a bio-oil comprises a mixing
unit, a
hydrotreater, and a separator. The system may further comprise biomass
conversion unit and a
second mixing unit. With reference to FIG. 2, in one embodiment of the present
invention, an
17
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049
exemplary system 100 for producing a refinery stream compatible bio-oil
comprises a mixing
unit 112, a hydrotreater 136, and a separator 150.
[0062] In one embodiment, the system 100 further comprises a biomass
conversion unit
104 (depicted as a pyrolysis unit) producing a high oxygen content (HOC) bio-
oil 108 or
pyrolysis oil (Py-Oil) from a biomass feedstock 102. In FIG. 2, the biomass
conversion unit 104
comprises an inlet 106 receiving the biomass feedstock 102 and an outlet 110
supplying the
HOC bio-oil 108 or Py-Oil to a first mixing unit 112.
[0063] The first mixing unit 112 comprises a first inlet 116 receiving the HOC
bio-oil
108 from the conversion unit 104 and a second inlet 120 receiving a low oxygen
content (LOC)
bio-oil 124 from a second biomass feedstock 128 to form a blended LOC/HOC bio-
oil 132. The
blended LOC/HOC bio-oil 132 exits from an outlet 134 in the first mixing unit
112 and is
received in a hydrotreater 136.
[0064] The hydrotreater 136 comprises a first inlet 140 receiving the blended
LOC/HOC
oil 132 from the first mixing unit 112 and a second inlet 142 receiving
hydrogen gas 146. The
hydrotreater 136 produces a hydrotreated LOC/water mixture 148, which exits
from an outlet
144 and passes on to a separator 150.
[0065] The separator 150 separates water 164 from the hydrotreated bio-oil
mixture 148
to produce a miscible, LOC hybrid bio-oil intermediate 156 and water 164. The
separator 150
comprises an inlet 152 receiving the hydrotreated bio-oil mixture 148 from the
hydrotreater 136
and may additionally include an outlet 158 supplying at least a portion of the
LOC hybrid bio-oil
intermediate 156 to the first mixing unit 112, an outlet 160 supplying at
least a portion of the
LOC hybrid bio-oil intermediate 156 to a source of refinery hydrocarbons 162,
an outlet 166
18
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049
supplying at least a portion of the LOC hybrid bio-oil intermediate 156 to a
second mixing unit
170, and an outlet 168 for water 164 to exit.
[0066] In some embodiments the system 100 comprises a second mixing unit 170
comprising an inlet 172 receiving the LOC hybrid bio-oil intermediate 156 from
the separator
150 and an inlet 174 receiving a LOC bio-oil 124 from a bio-oil feedstock 128
to form a blended
LOC oil 176 exiting from an outlet 178, which supplies the blended LOC oil 176
formed in the
second mixing unit 170 to the first mixing unit 112. The first mixing unit 112
may further
include an inlet 180 receiving the LOC hybrid oil intermediate 156 from the
separator 150 and an
inlet 182 receiving the blended LOC oil 176 from the second mixing unit 170.
[0067] Each reactor vessel of the invention preferably includes an inlet and
an outlet
adapted to remove the product stream from the vessel or reactor. The vessels
and reactors may
include additional outlets to allow for the removal of portions of the
reactant stream to help
maximize the desired product formation, and allow for collection and recycling
of byproducts for
use in other portions of the system. Further, the apparatuses for conducting
the inventive
processes can be conducted batchwise or continuously.
[0068] In another aspect, the present invention provides a blended oil
composition that
can be used in the above described system. The blended oil composition may
comprise any of
the above described LOC/HOC bio-oil compositions. In one embodiment, the
blended oil
composition comprises a high oxygen content bio-oil with an oxygen content of
at least about 15
wt. % bio-oil blended with a low oxygen content bio-oil with an oxygen content
of less than
about 8 wt. %.
[0069] The present invention is further illustrated by the following examples
which
should not be construed as limiting. The contents of all references, patents
and published patent
19
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049
applications cited throughout this application, as well as the Figures and
Tables are incorporated
herein by reference to the extent that they are not inconsistent.
Example 1: Pyrolysis Oil
[0070] A pyrolysis oil was produced from pine sawdust by a fast pyrolysis
method.
Chemical analysis of the pyrolysis oil showed 21% water content, and elemental
analyses of
48.72 % carbon, 5.97 % hydrogen, <0.05 % nitrogen, and 44.64 % oxygen (by
difference) on a
moisture and ash free basis (MAF). The total acid number (TAN) of the
Pyrolysis Oil was 331
mg KOH/g.
[0071] This resulting Py-Oil was immiscible with n-dodecane. The low
solubility of the
Py-Oil in an aromatic solvent was demonstrated using toluene as a solvent. The
Py-Oil was
mixed with five-to-ten times the volume of toluene. The mixture was heated to
boiling and the
water removed by azeotropic distillation (Dean Stark method). The resulting Py-
Oil/toluene
mixture was allowed to cool and two phases resulted: a thin, light colored
toluene rich phase and
a thick, viscous Py-Oil phase immiscible in toluene. The toluene-insoluble Py-
Oil accounted for
38% of the original Py-Oil.
[0072] The elemental composition of the toluene-insoluble Py-Oil phase was
59.3 % C,
6.56 % H, 0.1 % N, and 34.5 % 0 (by difference). As determined by vapor
pressure osmometry,
the number average molecular weight (Mn) was 730g/mol. The high oxygen content
and
molecular weight of the toluene-insoluble Py-Oil is consistent with its low
solubility in an
aromatic solvent, such as toluene.
Example 2: Lignin bio-oil
[0073] A lignin bio-oil was produced by hydrotreating a purified pine Kraft
lignin with
hydrogen at 2000 psig and 420 C and a suspended iron based catalyst. The
chemical analysis of
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049
the resultant lignin oil showed <0.34% water content, and an elemental
analyses of 83.47 %
carbon, 9.23 % hydrogen, 1.19 % nitrogen, 0.40 % sulfur, and 5.71 % oxygen (by
difference).
The TAN of lignin bio-oil was 7 mg KOH/g. The number average molecular weight
of the
lignin bio-oil was 229 g/mol.
[0074] The solubility of the lignin bio-oil in toluene was determined. The bio-
oil was
mixed with nine times the volume of toluene. The mixture was heated to reflux,
and then
allowed to cool to room temperature. A single organic phase resulted, with the
lignin bio-oil
being miscible with the toluene. The lignin bio-oil was miscible with the
toluene due to its low
oxygen content, molecular weight, and TAN value, as compared to the toluene-
insoluble Py-Oil
in Example 1.
Example 3: Mild Hydrotreating of Py-Oil + Lignin Oil
[0075] A Py-Oil, as described in Example 1, was blended in line with a lignin
bio-oil,
described in Example 2, to yield a 1:3 mixture and directly fed into a
hydrotreating, down flow
reactor containing a sulfided NiO/Mo03 supported catalyst (ICR181). The
process pressure was
800 psig of hydrogen. A temperature gradient was applied across the catalyst
bed, with the inlet
temperature at 140 C, and an outlet temperature of 245 C. The product bio-
oil was
homogeneous and the water phase could be separated. Chemical analysis of the
bio-oil product
was 80.93 % carbon, 9.90 % hydrogen, 1.11 % nitrogen, and 8.07 % oxygen (by
difference).
The TAN of the product bio-oil was 17 mg KOH/g. Simple dilution of the Py-Oil
by the lignin
oil produced a TAN value of 88 mg KOH/g.
[0076] The product bio-oil was mixed with about ten times the volume of
toluene. The
mixture was heated to boiling and residual water entrained in the oil was
removed by azeotropic
distillation (Dean Stark method). The resulting bio-oil/toluene mixture was
then allowed to cool.
21
CA 02850510 2014-03-28
WO 2013/049665 PCT/US2012/058049
A single organic phase resulted, with the product bio-oil being completely
miscible with the
toluene.
Example 4: Effect of catalyst and Py-Oil Ratio on the Hydrotreated Py-Oil
Product
[0077] Similar to Example 3, but the percent of Py-Oil to lignin bio-oil was
varied at 25
%, 37.5 %, and 50 % Py-Oil for three separate runs. A different sulfided
NiO/Mo03 supported
catalyst (ICR511) was used for these runs. A similar temperature gradient for
the catalyst bed
was used as in Example 3, with the outlet temperature being about 265 C.
[0078] The product bio-oil from each condition was separated from the water
phase. As
shown in Table 1 below, determination of the percent toluene solubility showed
that these
products exhibited increasing toluene insolubility, consistent with higher
molecular weight
components.
Table 1
Py-Oil / Lignin Oil Ratio % toluene-insoluble %C %H %N %0 (by
cliff.)
25:75 1.4% 79.15 9.7 1.02 9.76
37.5:62.5 7.0%
50:50 10.3%
[0079] As in Example 3, the hydrotreated product bio-oil from the 25:75 ratio
appeared
to be homogeneous. The Total Acid Number (TAN) of this product bio-oil was 15
mg KOH/g.
[0080] The hydrotreated product bio-oils from the 37.5% and 50% ratio mixtures
were
non-uniform and each had two distinct organic phases. This is reflected in
their higher toluene
insoluble values.
Example 5: Effect of Hydrotreating Temperature
[0081] Similar to Example 3, but for these runs the reaction temperature was
increased in
the lower third of the catalyst bed to produce six separate run conditions
with increasing reaction
22
CA 02850510 2014-03-28
WO 2013/049665 PCT/US2012/058049
temperatures. Table 2 shows the effect of hydrotreating temperatures on
product oil density, %
0 content, number average molecular weight, and toluene solubility.
Table 2
Reactor Outlet Temp C density % 0 (by cliff.) Mol
Wt % toluene-insoluble
250 1.0291 9.28% 225 0.00%
260 1.0318 10.01% 0.02%
270 1.0216 8.38% 234 0.24%
280 1.0101 7.76% 0.15%
300 0.9967 6.04% 241 0.00%
320 0.9794 4.92% 231 0.00%
Example 6: Lignin Liquefaction in Tetralin using a catalyst
[0082] Approximately 2.97 g of Kraft lignin was slurried with 0.0029 g of Moly
Van A
and 29 mL of tetralin in a 300 mL Autoclave batch reactor. The reactor was
purged and filled
with H2 to 800 psig. After 60 minutes at 400 C, the external heat was removed
and the reactor
was allowed to cool. The resulting product solution was removed from the
reactor, collected,
and filtered. The tetralin soluble phase was 21.6 g. The resulting solid was
washed with
acetone, and the acetone washings were combined and stripped to yield a polar
phase (tetralin
insoluble product, 2.27 g). The third phase was the residual solid (0.321 g)
after the tetralin
filtration and acetone washing steps. The analyses of the products are shown
in Table 3 below.
Table 3
Relative Amounts %C %H %N %S %0 (by difference)
Tetralin Soluble Phase 21.6 (89.3%) 89.07 9.41 0.0 0.10
1.52
Tetralin Insoluble Product 2.27 (9.4%) 89.82 9.75 0.0 0.43
Residual Solids 0.321 (1.3%) 61.00 3.11 0.3 0 20.1
[0083] Based on 'craft lignin, the yield of Tetralin insoluble product was
76%.
Example 7: Lignin Liquefaction in Lignin Bio-Oil Using a Catalyst
[0084] Approximately 10.02 g of Kraft lignin was slurried with 0.1071 g of
Moly Van A
and 51.65 g of lignin bio-oil (from Example 2) in a 300 mL Autoclave batch
reactor. The reactor
23
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049
was purged and filled with 112 to 800 psig. After 60 minutes at 400 C, the
external heat was
removed and the reactor was allowed to cool. The resulting material was
removed from the
reactor and collected as described by the following. The lignin bio-oil
soluble phase was
collected by filtering the entire solution (z 39.47 g). The resulting solid
was washed with
acetone and stripped to collect the polar phase (6.53 g). The third phase was
the residual solid
after the lignin bio-oil filtration and acetone washing steps (1.518 g). The
analyses of the
products are shown in Table 4 below.
Table 4
Relative Amounts %C %II %N S % 0
(by difference)
Lignin Bio-Oil Soluble Phase 39.47 (83.1%) 84.60 8.79 0.15 0.105
6.35
Lignin Bio-Oil Insoluble Product 6.53 (13.7%) 82.52 9.08 0 na
7.33
Residual Solids 1.52 ( 3.2%)
[0085] Based on kraft lignin, the yield of lignin bio-oil insoluble product
was 65%.
[0086] In both Examples 6 and 7, the solvent insoluble products are similar,
as neither is
soluble in the reaction solvent. The data indicates tetralin is a slightly
poorer solvent than the lignin
oil, because the amount of Tetralin insoluble product was higher than the
amount of lignin bio-oil
insoluble product.
[0087] The above description is for the purpose of teaching the person of
ordinary skill in
the art how to practice the present invention, and it is not intended to
detail all those obvious
modifications and variations of it which will become apparent to the skilled
worker upon reading
the description. It is intended, however, that all such obvious modifications
and variations be
included within the scope of the present invention, which is defined by the
following claims.
The claims are intended to cover the claimed components and steps in any
sequence which is
effective to meet the objectives there intended, unless the context
specifically indicates the
contrary.
24
CA 02850510 2014-03-28
WO 2013/049665
PCT/US2012/058049