Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02853991 2014-06-11
Methods And Compositions For Deterring Feeding/Repelling
The Brown Marmorated Stink Bug (BMSB), Halyomorpha halys
Background Of The Invention
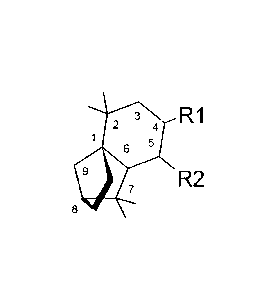
100011 Compositions (feeding deterrent/repellent for Halyomorpha halys)
containing at
least two compounds selected from tridecane, E-2-decenal, isolongifolanone,
isolongifolenone, and
at least one isolongifolenone analog having the following formula:
3 R1
4
1 5
Ili 7 R2
8
wherein RI is hydrogen, an oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen; and optionally a carrier or carrier material. Also methods
for deterring
feeding/repelling Halyomorpha halys involving treating an object or area with
a Halyomorpha
halys deterring feeding/repelling effective amount of at least one compound
selected from
tridecane, E-2-decenal, isolongifolanone, isolongifolenone, and at least one
isolongifolenone
analog having the following formula:
3 2 R1
4
1 5
R2
8
wherein R1 is hydrogen, an oxygen, a Ci_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C110 saturated or unsaturated, straight or branched acid and R2
is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen; and optionally a carrier or carrier material.
1
CA 02853991 2014-06-11
100021 The brown marmorated stink bug, Halyornorpha halys (Stal) (Order
Hemiptera:
Family Pentatomidae), is an extremely polyphagous insect pest originating from
Asia (Funayama, K.,
Japanese Journal of Applied Entomology and Zoology, 49(4): 265-268 (2005);
Funayama. K.,
Japanese Journal of Applied Entomology and Zoology, 51(3): 238-240 (2007);
Son, J,Kõ et al., Acta
Horticulturae, pages 325-330 (2009)). This exotic insect invaded the United
States in 2001 (Hoebeke,
E.R., and M.E. Carter, Proc. Entomol. Soc. Wash., 105: 225-237 (2003)) and has
been detected in 35
states and the District of Columbia, and has been found to feed on over 300
host plants including
forest trees, ornamentals, tree fruits, soybean, cotton, and garden vegetables
(Hoebeke and Carter
2003; Nielsen, A.L., and G.C. Hamilton, Journal of Economic Entomology, 102:
1133-1140 (2009);
Nielsen, A.L., and G.C. Hamilton, Annals of the Entomological Society of
America 102: 608-616
(2009); Jacobs, S., Brown marmorated stink bug Halyomorpha halys,
http://ento.psu.edu/extension/factsheets/brown-marmorated-stink-bug, February
6, 2012). Damage to
fruit from H. halys in mid-Atlantic States has reached critical levels,
causing serious impairment to
peach and apple crops, with some growers losing 60 to 100 percent of their
yield (Marder, J., Stink
Bug Invasion: Is a Wasp the Solution to Save Valued Crops? (2011),
http://www.pbs.org/newshour/rundown/2011/05/fighting-the-stink-bug.html,
February 6, 2012; Sun-
Gazette W., Brown marmorated stink bug update (2011),
http://www.sungazette.com/page/content.detail/id/561129/Brown-marmorated-stink-
bug-
update.html?nav=5014, February 6, 2012; Leskey, T.C., and G. Hamilton, Brown
marmorated D.N.
0136.12 stink bug working group meeting summary report (2010),
http://projects.ipmeenters.org/Northeastem/FundedProjects/ReportFiles/Pship2010
/Pship2010-
Leskey-ProgressReport-237195-Meeting-2010_11_17.pdf, February 6, 2012). In
addition, H. halys is
a considerable homeowner nuisance when insects are seeking ovcrwintering sites
in the late summer
and early fall (Hoebeke and Carter 2003; Nielsen and Hamilton, Annals of the
Entomological Society
of America, 102: 608-616 (2009)).
[0003] Isolongifolenone is a naturally-occurring sesquiterpene isolated from
the
Tauroniro tree (Humiria balsamifera) of South America (Da Silva, T.B.C.,
etal., Pharm. Biol.,
42: 94-97 (2004)). It is an important and well-known compound in the chemical
industry.
Recently, isolongifolenone and isolongifolenone have been found as novel
sesquiterpene
repellents of ticks and mosquitoes (Zhang, A., et al., U.S. Patent 7,378,557
BI; Zhang, A.. et al.,
2
CA 02853991 2014-06-11
J. Med. Entomol., 46: 100-106 (2009); Zhang, A., et al.. U.S. Patent
7,579,016; Carroll, J.F., et
al., (2011) Using lone star ticks, Amblyomma arnericanum (Aeari: Ixodidae), in
in vitro
laboratory bioassays of repellents: dimensions, duration, and variability. In:
Paluch, G., and J.
Coats, eds, Recent Developments in Invertebrate Repellents, ACS Symposium
Series,
Washington, DC: American Chemical Society, pp. 97-120) and can be easily
synthesized from a
precursor widely distributed in pine oil (Wang, S., and A. Zhang, Organic
Preparations and
Procedures International, 40: 405-410 (2008)).
[0004] Currently, there are no useful tools for successful management of H
halys. Thus a
feeding deterrent/repellent would be useful for crop protection and management
of this invasive
species.
Summary Of The Invention
[0005] Compositions (feeding deterrent/repellent for Halyomorpha halys)
containing at
least two compounds selected from tridecane, E-2-decenal, isolongifolanone,
isolongifolenone, and
at least one isolongifolenone analog having the following formula:
2
3 R1
4
1 5
Ili 7 R2
8
wherein R1 is hydrogen, an oxygen, a Clio alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a Ci_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen; and optionally a carrier or carrier material. Also methods
for deterring
feeding/repelling Halyomorpha halys involving treating an object or area with
a Halyomorpha
halys deterring feeding/repelling effective amount of at least one compound
selected from
tridecane, E-2-decenal, isolongifolanone. isolongifolenone, and at least one
isolongifolenone
analog having the following formula:
3
CA 02853991 2014-06-11
3 R1
4
1 5
*7 R2
V
8
wherein R1 is hydrogen, an oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a Ci_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen; and optionally a carrier or carrier material.
100061 This summary is provided to introduce a selection of concepts in a
simplified form
that are further described below in the detailed description. This summary is
not intended to identify
key features or essential features of the claimed subject matter, nor is it
intended as an aid in
determining the scope of the claimed subject matter.
Brief Description Of The Drawings
100071 Figure 1 shows GC/MS total ion traces of aeration extracts from group
H. halys
(top, 17 males) versus individual (bottom, 1 male) on a DB-5MS column as
described below. The
aerations were conducted using 30 day-old males on May 2, 2011 for 24 hours.
Six compounds are
indicated: (1) 2-nonanone, (2) C-12 (dodecane), (3) E-2-decenal, (4) C-13
(tridecane), (5) E-2-
deeenyl acetate, and (6) 2-tridecanone. Figure 2 shows GC/MS total ion traces
of aeration extracts
from individual female H. halys (top) versus male (bottom) on a DB-5MS column
as described
below. The aerations were conducted using 13 day-old H. halys on October 25,
2010 for 24 hours.
Four compounds are indicated: (2) C-12, (3) E-2-decenal, (4) C-13, and (5) E-2-
decenyl acetate.
100081 Figure 3 shows the average male-specific compounds (pheromone), E-2-
decenal,
and C-13 per chamber by month as described below. Data was collected in 24-
hour increments
throughout 2011 and 2012 using 13-90 day-old adult male H. halys, and was
analyzed per individual
male per day in a month using ANOVA and Tukey HSD comparisons of log-
transformed data with
a=0.05 (JM13 TO). ANOVA model of square root-transformed data: Amount of
Emission =
Compound, Month Started (Year), Year. R2-0.21, ANOVA: F 1 9 389- 5 . 1 92 ,
p<0.0001; Compound: F-
7.757, p=0.001; Month Started (year): F-5.169, p<0.0001; Year: F-3.118,
p=0.078.
4
CA 02853991 2014-06-11
100091 Figure 4 shows the average male-specific compounds (pheromone), C-13,
and E-2-
decenal per day for single and multiple adult males as described below.
Average and standard error
of the male specific compounds, C-13, and aldehyde (E-2-decenal) were
calculated for single-and
multiple-male chambers using data collected in 24-hour increments throughout
2011 on 13-90 day-
old adult male H halys. Treatments were compared using ANOVA of log-
transformed data with
repeated measures. Figure 4A shows data for average male specific compounds
and C-13 per day for
single v. multiple males. C-13: F71,1 = 4.256; p =0.003; Male specific
compounds: F71,1= 6.634; p <
0.0001. Figure 4B shows data for average E-2-decenal per day for single v.
multiple males.
Aldehyde: F71,I =3.070; p =0.003.
100101 Figure 5 shows the amount of pheromone, C-13, and E-2-decenal present
by
number of H. halys adults in chamber as described below. This was analyzed
with data collected in
24-hour increments throughout 2011 on 13-90 day-old adult male H. halys, with
log-transformed
data using repeated measures ANOVA for Compound = subject, # males (subject).
Pheromone F71,1-
6.634; p <0.0001; C-13 F71,1 = 5.701; p=0.001; Aldehyde F71,1 = 1.617;
p=0.003. Regression
equations: Pheromone = -0.17x + 2.52; C-13 = 0.22x + 1.15; Aldehyde = -0.773x
+ 0.290. The
relationship between C-13 and the male-produced male-specific compounds was
analyzed via
ANOVA using pairwise correlations, p < 0.0001. The C-13 and male specific
compounds interaction
overlaps at 2.50 males.
[0011] Figures 6A-E show the effectiveness of different repellent compounds on
deterring
H halys feeding on green beans in closed-air petri dish tests as described
below. Young adult males
showed a slight preference for pentane-treated green beans over the untreated
control bean. Based on
these results, we chose pentane as the solvent for subsequent repellent assays
in petri dishes to
emphasize repellency effects. Repellents were dissolved in pentane and applied
to filter paper under
green bean in 75u1 applications which yielded a 1-inch diameter circle of
repellent under the bean.
Figure 6A shows that starved nymphs (31d stage) were significantly less likely
to feed on beans
treated with 100ps islongifolanone as well as I ug or 10Oug concentrations of
islongifolenone and C-
13. Figure 6B shows that young adult males (ages 3-7 days into adult stage)
were significantly less
likely to feed on 100ug concentrations of isolongifolanone, isolongifolenone,
and C-13, as well as
1 ug concentrations of E-2-decenal. Figure 6C shows that young adult females
were significantly less
likely to feed on 100 g concentrations of isolongifolanone and
isolongifolenone. Figure 6D shows
that old adult males (ages 13-23 days) were significantly less likely to feed
on beans treated with
CA 02853991 2014-06-11
10Oug isolongifolanone or lug C-13, and Figure 6E shows that old adult females
were significantly
less likely to feed on 100tig concentrations of isolongifolanone and
isolongifolenone. Asterisks
above groups of bars indicate the significance of the three behavioral
observations (blank bean, odor
bean, off bean) relative to the pentane control shown as the first set of bars
in the figure, with p
<0.05 (*), p <0.01(**), and p <0.001 (***), n=30-40 for adults, 40-80 for
nymphs.
[0012] Figures 7A-D show the effectiveness of both individual and combined
compounds
at deterring H. halys feeding in open-air small cage bioassays as described
below. Letters above bars
indicate significance of treatment relative to other treatments; shared
letters are not significantly
different from each other. In Figure 7A, the average number of nymphs (3rd
stage) feeding on
peaches over 8 hours of observations was lower for all compounds and
combinations of compounds
relative to the Tween and H20 controls, except for the aldehyde/C-13
combination. The three-
component blend of equal concentrations isolongifolanone + isolongifolenone +
C-13 yielded the
highest repellency. Statistical analysis of ANOVA (Model: Insects on fruit =
Treatment,
Rep(Treatment) were as follows: for nymphs: F10,830-41.82, p<0.000I. There
were 15 nymphs per
cage, and 4-28 reps tested.
[0013] Figures 7B-D show male and female behavior in small cages for
repellents tested
on peaches (Figure 7C) and apples (7D) as described below. Results did not
significantly differ by
fruit or age group tested, so data was combined in Figure 7B. The 3- and 4-
component blends of
isolongifolanone + isolongifolenone + C-13, and isolongifolanone +
isolongifolenone + C-I3 +
Aldehyde were significantly more repellent to starved male and female adults
than the Tween and
H20 control. C-13 and Anone/C-13 were not tested for adults in apples.
Repeated-measures
ANOVA showed a significant effect for repellent treatments in peaches
(p<0.0001), apples
(p<0.0001), and combined apples and peaches data (p=0.007). There were 10 male
or female adults
per cage and 2-6 reps conducted per sex (4-12 reps total).
Detailed Description Of The Invention
10014] Disclosed are compositions (feeding deterrent/repellent for Halyomorpha
halys)
containing at least two compounds selected from tridecane, E-2-decenal,
isolongifolanone,
isolongifolenone, and at least one isolongifolenone analog having the
following formula:
6
CA 02853991 2014-06-11
3 R1
2 4
1 6 5
lb 7 R2
8
wherein R1 is hydrogen, an oxygen, a Ci_io alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a Ci_io saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a Ci_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a Ci_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen; and optionally a carrier or carrier material. Also
disclosed are methods for
deterring feeding/repelling Halyomorpha halys involving treating (or exposing)
an object or area
(e.g., field, orchard) with a Halyomorpha halys deterring feeding/repelling
effective amount of at
least one compound selected from tridecane. E-2-decenal, isolongifolanone,
isolongifolenone, and
at least one isolongifolenone analog having the following formula:
3 2 R1
4
1 5
);16
1117 R2
8
wherein It] is hydrogen, an oxygen, a Ci_10 alcohol, aldehyde, alkyl, ether,
or esters of said
alcohol with a Ci_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a Ci_io alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a Ci_io saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen; and optionally a carrier or carrier material.
[0015] Generally the isolongifolenone analogs have the following formula:
263 R1
1 5
R2
8
wherein R1 is hydrogen, an oxygen, a C110 alcohol (straight or branched),
aldehyde, alkyl, ether
(e.g. methanol, ethanol, ethanal, 4-methylhexane, heptyloxymethane), or esters
of said alcohol
7
CA 02853991 2014-06-11
with a Ci_io saturated or unsaturated, straight or branched acid (e.g., formic
acid, acetic acid, 2-
methylbutyric acid, 3-methy1-2-butenoic acid) and R2 is hydrogen, an oxygen, a
C1_10 alcohol
(straight or branched), aldehyde, alkyl, ether (e.g. methanol, ethanol,
ethanal, 4-methylhexane.
heptyloxymethane), or esters of said alcohol with a C1_10 saturated or
unsaturated, straight or
branched acid (e.g., formic acid, acetic acid, 2-methylbutyric acid, 3-methyl-
2-butenoic acid);
optionally there is a double bond between carbons 5 and 6 and R2 is hydrogen.
Preferably R1 or
R2 are hydrogen (in other words, R1 can be hydrogen or R2 can be hydrogen or
both RI and R2
can be hydrogen). Preferably R1 and R2 are not both hydrogen (in other words
if R1 is hydrogen
then R2 is not hydrogen or if R2 is hydrogen then R1 is not hydrogen).
[0016] The isolongifolenone analogs can be a (1R,83)-2,2,7,7-
tetramethyltricyclo[6.2.1.01 undec-5-ene having the formula:
3 R1
- 4
1 0
W77 R2
By
wherein R1 is hydrogen, an oxygen, a C1_10 alcohol (straight or branched),
aldehyde, alkyl, ether
(e.g. methanol, ethanol, ethanal, 4-methylhexane, heptyloxymethane), or esters
of said alcohol
with a C1_10 saturated or unsaturated, straight or branched acid (e.g., formic
acid, acetic acid, 2-
methylbutyric acid, 3-methy1-2-butenoic acid) and R2 is hydrogen.
[0017] The isolongifolenone analogs can be a (1S,8S)-2,2,7,7-
tetramethyltricyclo[6.2.1.01'6]undecane having the formula:
2
3 R1
4
1 5
R2
8
8
CA 02853991 2014-06-11
wherein R1 is hydrogen, an oxygen. a C1_10 alcohol (straight or branched),
aldehyde, alkyl, ether
(e.g. methanol, ethanol, ethanal, 4-methylhexane, heptyloxymethane), or esters
of said alcohol
with a Ci_io saturated or unsaturated, straight or branched acid (e.g., formic
acid, acetic acid, 2-
methylbutyric acid, 3-methyl-2-butenoic acid) and R2 is hydrogen; and
optionally a carrier or
carrier material.
[0018] The isolongifolenone analogs can be a tricyclo(1S,85)-2,2,7,7-
tetramethyltricyclo[6.2.1.01'61undecane having the formula:
, 3 R1
4
1 5
Ili 7 R2
a
wherein R2 is an oxygen, a C1_10 alcohol (straight or branched), aldehyde,
alkyl, ether (e.g.
methanol, ethanol, ethanal, 4-methylhexane, heptyloxymethane), or esters of
said alcohol with a
Ci_io saturated or unsaturated, straight or branched acid (e.g., formic acid,
acetic acid, 2-
methylbutyric acid, 3-methyl-2-butenoic acid) and R1 is hydrogen; and
optionally a carrier or
carrier material.
[0019] The isolongifolenone analogs can be a (1S,85)-2,2,7,7-
tetramethyltricyclo[6.2.1.01'6]undecane having the formula:
, 4
3 R1
1 5
107
R2
8
9
CA 02853991 2014-06-11
wherein RI is an oxygen, a C1_10 alcohol (straight or branched), aldehyde,
alkyl, ether (e.g.
methanol, ethanal, 4-methylhexane, heptyloxymethane), or esters of said with a
C1_10 saturated or
unsaturated, straight or branched acid (e.g., formic acid, acetic acid, 2-
methylbutyric acid, 3-
methy1-2-butenoic acid) and R2 is an oxygen, a Ci_10 alcohol (straight or
branched). aldehyde,
alkyl, ether (e.g. methanol, ethanal, 4-methylhexane, heptyloxymethane), or
esters of said
alcohol with a C1_10 saturated or unsaturated, straight or branched acid
(e.g., formic acid, acetic
acid, 2-methylbutyric acid, 3-methyl-2-butenoic acid); and optionally a
carrier or carrier
material.
[0020] Preferably the isolongifolenone analogs are one of the compounds in
Figure 5
in U.S. Patent 7,579,016 (excluding isolongifolenone).
[0021] The optical isomers of isolongifolenone analogs could be (+)- or (-)-;
depending
on the starting material isolongifolene or isolongifolene.
[0022] The isolongifolenone analogs were synthesized according to known
methods:
Synthesis of dihydroisolongifolenone (J4-120A) (Prahlad, J. R., et al.,
Tetrahedron Lett., 5: 417-
427 (1964); Ranganathan, R., et al., Tetrahedron, 26: 621-630 (1970)).
Synthesis of
isolongifolenyl alcohol (J4-120B) (Curtis, A. J., et al., GB Patent No.
1,256,535 (1971);
Banthorpe, D. V., Tetrahedron Lett., 36: 3865-3868 (1972); Piekenhagen, W.,
and D.
Schatkowski, U.S. Patent No. 6,734,159 B2 (2004)). Synthesis of
dihydroisolongifolenyl
alcohol (J4-120E), same procedure as J4-120B. Synthesis of
dihydroisolongifolenyl alcohol
acetate (J4-120C) (Raucher, S., et al., J. Am. Chem. Soc., 103: 1853-1855
(1981); Pinheiro, S.,
et al., Tetrahedron Asymmetry, 11:3495-3502 (2000)). Synthesis of
isolongifolenyl alcohol
acetate (J4-120D), same procedure as J4-120C. Synthesis of isolongifolanone
(J4-120F) (U.S.
Patent No. 5,426,095); it was obtained from Bedoukian Research Inc. as a gift.
[0023] The carrier or carrier material may be, for example, agronomically,
physiologically, or pharmaceutically acceptable carriers or carrier materials.
An insect repellent is
any compound or composition which deters insects from an object or area. Thus,
the term "repelling"
is defined as eliciting insects (e.g., male Halyomorpha halys) to make
oriented movements away
from a source of a chemical repellent (Dethier, V. L., et al., J. Econ. Ent.,
53: 134-136 (1960)), and
also includes inhibiting feeding by insects when a chemical is present in a
place where insects would,
in the absence of the chemical, feed.
[0024] The feeding deterrent/repellent of the present invention may be
applied with a
CA 02853991 2014-06-11
carrier component. The carrier component can be a liquid or a solid material.
As is known in the art,
the vehicle or carrier to be used refers to a substrate such as water,
membranes, hollow fiber,
microcapsule, cigarette filters, gel, polymers, or the like. All of these
substrates have been used to
release insect feeding deterrent/repellents in general and are well known in
the art. The carrier or
carrier material as used herein is defined as not including the body of an
insect (e.2.,
Halyomorpha halys).
[0025] A repellent is any compound or composition which deters insects from
biting
and feeding on a host. Thus, the term "repelling" is defined as inhibiting
feeding by insects
when a chemical is present in a place where insects (e.g., Halyomorpha halys)
would, in the
absence of the chemical. feed, and it also includes causing insects (e.g.,
Halyomorpha halys) to
make oriented movements away from a source of a chemical repellent (Dethier,
V. L., et al., J.
Econ. Ent., 53: 134-136 (1960)). Thus, the term "repelling" also includes
reducing the number
of insect (e.g., Halyomorpha halys) bites on a treated area or object (e.g.,
fruit skin which has
been treated topically with the compositions or compounds of the present
invention) when
compared to the same area or object which is untreated, and the term
"repelling" also includes
causing insects (e.g., Halyomorpha halys) to make oriented movements away from
a treated area
or object (e.g., fruit skin which has been treated topically with the
compositions or compounds of
the present invention) when compared to the same area or object which is
untreated. The amount
of the feeding deterrent/repellent used will be at least an effective amount.
The term "effective
amount," as used herein, means the minimum amount of the compound needed to
cause
Halyomorpha halys to make oriented movements away from a treated area or
object when
compared to the same area or object which is untreated. Effective
concentrations of the feeding
deterrent/repellent in the compositions may vary between about 0.00001% to
about 99.99%
(preferably about 0.00001% to about 50%, more preferably about 0.00001% to
about 10%, more
preferably about 0.00001% to about 1%, more preferably about 0.00001% to about
0.1%, more
preferably about 0.00001% to about 0.01%). Of course, the precise amount
needed will vary in
accordance with the particular feeding deterrent/repellent composition used;
the type of area or
object to be treated; the number of hours or days of feeding
deterring/repelling needed; and the
environment in which the area or object is located. The precise amount of
feeding
deterrent/repellent can easily be determined by one skilled in the art given
the teaching of this
application. For example, one skilled in the art could follow the procedure
utilized below.
11
CA 02853991 2014-06-11
[0026] The method for repelling Halyomorpha halys from an object (e.g.,
structures)
or area (e.g., a surface such as fruit skin) involves treating (or exposing)
the object or area with
the compounds, and optionally including a carrier material or carrier. The
terms "object" or
"area" as used herein include any place where the presence of target pests
(e.g., Halyomorpha
halys) is not desirable, including any type of premises, which can be out-of-
doors, such as in
gardens, lawns, tents, camping bed nets, camping areas, and so forth, or
indoors, such as in
barns, garages, commercial buildings, homes, and so forth, or any area where
pests are a
problem, such as in shipping or storage containers (e.g., bags, boxes, crates,
etc.), packing
materials, bedding, and so forth; also includes the outer covering of fruits
and vegetables.
[0027] Unless defined otherwise, all technical and scientific terms used
herein have the
same meaning as commonly understood by one of ordinary skill in the art to
which the invention
belongs. The term "about" is defined as plus or minus ten percent; for
example, about 100 0C means
90 C to 110 C. Although any methods and materials similar or equivalent to
those described herein
can be used in the practice or testing of the present invention, the preferred
methods and materials are
now described.
[0028] The following examples are intended only to further illustrate the
invention and are
not intended to limit the scope of the invention as defined by the claims.
Examples
[0029] Materials and Methods. Insects Rearing: The H. halys colony was
established in
2007 from adults collected in Allentown, PA, and reared on a diet of organic
green beans, water
(supplied by two 7 cm x 2-cm OD test tube)s, and seeds (2:1 sunflower:
buckwheat seeds) in plastic
containers (21 cm x 21 cm OD). Eggs were collected twice weekly throughout the
study and put into
separate containers containing the same diet. After emerging, the five stages
of nymphal instars were
reared in the same container until they developed into adult. Within two days
after final imaginal
ecdysis, a subset of males were separated and reared in different containers
for aeration analyses
while other adults were held together. Insects were maintained in Thermo Forma
chambers (Thermo
Fisher Scientific ) at 25 C and 72% relative humidity with 16L : SD
photoperiod. The colony was
replenished with ¨20 field-collected bugs annually, collected in Beltsville.
MD.
[0030] Volatile Secretion Compounds Collection and Isolation: Volatile
aeration
collections were initiated using two-day-old adult virgin males and females
held under different
densities in a collection device (e.g.. 1, 2, 3, 5, 7, 9. and 20 virgin males
per group). The males and
12
CA 02853991 2014-06-11
females were separately placed into two 1-liter, 4-necked glass containers
(Zhang, A., etal., Journal
of Chemical Ecology, 20: 2415-2427 (1994)). Humidified air was drawn into the
container through
6-14 mesh activated charcoal (Fisher Scientific , Pittsburgh, PA) and out
through two traps (15 cm x
1.5-cm OD) containing Super Q (200 mg each; Alltech Associates, Inc. ,
Deerfield, IL) by vacuum
(-1 liter/min) (Zhang, A., Zeitschrift fur Naturforschung, Section C
Biosciences, 57: 553558 (2002)).
Insects were fed with organic green beans (replaced every 2-3 days), provided
water on cotton balls,
and aerated continuously for 20 to 90 days depending on insect living
conditions at room temperature
(23-25 C) and 16L : 8D photoperiod. The adsorbent traps were changed every day
(some of them in
3 days for weekend) and eluted with methylene chloride (0.5 ml/each sample).
The elutants were
stored in ¨30 C freezer until analyses.
[0031] Analytical Methods: An Agilent 6890 gas chromatography (GC) equipped
with
an auto sampler and a 30-m x 0.25-mm ID, 0.25-nm film-thickness HP-5MS (J&W
Scientific Inc.,
Folsom, CA) capillary column in the splitless mode with hydrogen (1.4 ml/min)
as carrier was
used for quantitative analysis. A hydrocarbon, 1-tetradecene (10 ng/til and 1
ng/n1 in CH2C12), was
used as external standard for quantitative analysis of C-13. while E-2-decenal
was analyzed using
ng/n1 and 10 ng/ 1 of E-2-decenal. The oven temperature was programmed at 40 C
for 2 min,
then heated to 280 C at 15 C /min and held for 10 min. Electron impact mass
spectrometry (El
MS) was conducted on an Agilent 6890 GC coupled to an Agilent 5973 Mass
Selective Detector
using a 60-m x 0.25-mm ID, 0.25-nm film-thickness DB-WAXETR (J&W Scientific
Inc. ,
Folsom, CA) capillary column at 50 C for 2 min, then programmed to 250 C at 15
C /min and
held for 15 min or a 60-m x 0.25-mm ID, 0.25-nm film-thickness DB-5M5
capillary column
(50 C for 2 min, then programmed to 280 C at 20 C/min and held for 15 min)
with helium as
carrier gas, unless other temperature programs indicated. A 70 eV electron
beam was employed for
sample ionization. Chemicals tridecane, E-2-decenal, and Tween-80 were
purchased from Sigma-
Aldrich (St. Louis, MO) and Bedoukian Research, Inc.CRADanbury, CT).
Isolongifolenone was
synthesized at Beltsville (Wang, S., and A. Zhang, Organic Preparations and
Procedures
International, 40: 405-410 (2008)) and isolongifolanone was provided by
International Flavors
& Fragrances Inc. (New York, NY) as a gift.
100321 Optimized petri dish bioassays: Insects tested were moved in groups
from
Thermo Forma cages to plastic petri dishes for food and water deprivation 16-
20 hours before
bioassays. Adults were separated by age and sex and held in groups of five,
nymphs were held
13
CA 02853991 2014-06-11
in groups of 20. Bioassays were conducted from April-June of 2012 between 8
a.m. and 4 p.m.,
in a fume hood lined with black paper and covered with black curtain to
prevent external visual
stimuli. The fume hood was illuminated by a single 60 watt light bulb with a
light intensity of
800 LUX, placed 50 cm above bio assay arenas, with 26 -28 C temperature and
50% relative
humidity. Glass petri dishes (10cm diameter) were used for the bioassay arena
and were lined
with Whatman's #1 filter paper (9cm) that was changed before each assay.
Adults were
bioassayed individually. Nymphs were tested in groups of four because in
preliminary assays
they were observed continually walking for up to four hours unless
conspecifics were present.
[0033] We tested the effects of solvent on H halys survival and feeding
behavior to
optimize the solvent used in petri dish assays. Mortality of 3rd stage nymphs,
and adult males
and females (ages 3-7 days or 13-23 days into adult stage). was quantified as
the percentage of
individuals who died after exposure to methylene chloride (DCM) after 2.25
hours. Since young
adult males were the most susceptible to DCM, they were tested in bioassays
for feeding
aversion to four additional solvents (acetone, pentane, hexane, and ethanol).
Insects were
starved for 16-20 hours prior to being exposed to 1 inch cut sections of
organic green beans in a
10cm petri covered glass petri dish. One bean was coated with 35 1 solvent
while the other was
untreated. Behaviors of young adult male individuals were recorded at three
time points (45, 90,
and 135 minutes) as feeding on the blank (untreated) bean, feeding on the odor
(solvent-treated)
bean, or sitting on the wall of the petri dish. Percent response for each
choice was averaged over
the three time periods.
[0034] We then used the optimized solvent (i.e., pentane) to test the effects
of different
repellents on deterring H halys feeding on organic green beans in petri
dishes. Insects were
tested in a glass Petri dish for their attraction/aversion to 1 g and 100 g
concentrations of
isolongifolanone and isolongifolenone (International Flavors and Fragrances,
NY), C-13, and E-
2-decenal, diluted in pentane. Pure pentane was tested as a control. Whatman's
#1 filter paper
(9cm) was placed in petri dish and 2 x one-inch diameter impressions were made
with a metal
circle. One circle was treated with 75u1 of pentane (control) and the other
with pentane +
repellant (treatment) and dried for five minutes. One-inch sections of organic
green beans were
placed over the treated or untreated circle. Starved insects were introduced
to the petri dish
assay arena and observed at three time points in 45 minute increments for 2.25
hours. Their
14
CA 02853991 2014-06-11
location was recorded as feeding with stylet inserted on the treatment (i.e.,
odor) bean, blank
bean, or off bean (i.e., walking off of food source or no movement in petri
dish and not on bean).
Individuals were only used for one bioassay at the adult or nymphal stage in
their lifetime. Petri
dishes were rinsed with water daily and baked overnight at 100 C, and were
rinsed with acetone
after every six uses.
[0035] Small Cage Bioassays: Nymphs and adults were tested for effects of
feeding
deterrents/repellents from islongifolanone, islongifolenone, C-13, and E-2-
decenal applied to
organic peaches and gala apples in a small cage. Insects were starved for 16
hours prior to
experiments before being transferred in groups of 15 (nymphs) or 10 (adults
male or female) to
two gallon containers containing one treated fruit, and observed hourly for
the number of
nymphs on the fruit. Experiments were conducted in a fume hood under the same
light and heat
conditions as above. Different concentrations of isolongifolanone,
isolongifolenone, C-13, and
E-2-decenal were tested both individually and combined (equal amount of each
component),
with a final concentration of 70-80 mg/fruit chosen as an effective treatment.
Compounds (400
mg) were combined with the emulsifier Tween-80 (2 mg), mixed with water (1120
10 ml) in
glass vial (20 ml), and sonicated for 20 min. The solution containing
emulsifier Tween-80 (2
mg) in 10 ml water was used as control. The emulsified solutions were diluted
in 30 ml water
(H20) and then evenly sprayed on the fruit surfaces with 6 oz. spray bottles
(The Bottle Crew,
West Bloomfield, MI) and treated fruits were dried in a fume hood for 5 mm.
The treatments
tested were Isolongifolanone, C-13, E-2-decenal, Isolongifolanone + C-13,
Isolongifolanone +
Isolongifolenone, E-2-decenal + C-13, Isolongifolenone + C-13,
Isolongifolanone +
Isolongifolenone + C-13, Isolongifolanone + Isolongifolenone + C-13 + E-2-
decenal, Tween-80,
and distilled water (H20). Isolongifolenone was not tested individually
because it is solid at
room temperature and could not form an emulsion.
[0036] Statistical Analyses: For aeration analyses, we measured the average
pheromone, C-13, and E-2-decenal collected per individual male per day in a
month, and tested
whether this amount varied by month collected using repeated measures ANOVA
and Tukey
HSD comparisons of log-transformed data with ct=.05 (JMP10). Average and
standard error of
the male-produced pheromone, C-13, and aldehyde (E-2-decenal) were calculated
for single-and
multiple-male chambers. The effect of male H. halys density on the amount of
the compounds in
CA 02853991 2014-06-11
aeration chambers was analyzed for log-transformed data using repeated
measures ANOVA. The
relationship between C-13 and the male specific compounds was analyzed via
repeated measures
ANOVA pairwise correlations of log-transformed data, and the relationship
between C-13 and
E-2-decenal were analyzed using repeated measures ANOVA of log-transformed
data.
100371 Optimized petri dish bioassays were analyzed for each age group, sex,
and
treatment using Likelihood Ratio tests (JMP*). Percent response for each
choice per compound
was averaged over the three time periods observed. The likelihood of the three
choices was set
to the level determined by the pentane control for each age/sex group, and the
confidence
interval set at 95%. P-values are indicated as follows: * = 0.05; ** = 0.01;
*** = 0.001.
100381 Small cage bioassays were analyzed by replicate, treatment, and time
using
repeated measures ANOVA and multiple comparisons for treatments were tested
using Tukeys
HSD, Model: Nymphs on Fruit = Treatment, Rep(Treatment), Time, Treatment*Time.
Letters
above bars indicate significance of treatment relative to other treatments at
p=0.05; shared letters are
not significantly different from each other.
100391 Results: The GC/MS total ion traces of airborne extracts from group and
single male H. halys are shown in Figure 1. Tridecane (4) and E-2-decenal (3)
were surprisingly
the major components emitted by the group (top) compared with individual male
(bottom).
Other minor components, including 2-nonanone, dodecane, E-2-decenyl acetate,
and 2-
tridecanone, varied by amount in different volatile collections; surprisingly,
these compounds
were virtually undetectable in the airborne extracts obtained from single
males, with male-
specific compounds appearing as the major components (bottom). In addition,
surprisingly,
tridecane was the only major volatile component emitted by individual female
H. halys
compared to individual males (Figure 2).
100401 Average amount of C-13, E-2-decenal, and male-specific compound
(pheromone)
emission per individual per day was determined for each month of collections
(Figure 3). Adult
males emitted more pheromone per day in March than December and January, more
C-13 per day in
August than in January and February, and more E-2-decenal per day in June than
in March, May, and
November. Whole model ANOVA: Square root(Amount of Emission) = Compound. Month
Started
(Year), Year. R2-0.21, ANOVA: F9389-5.192. p<0.0001; Compound: F-7.757,
p=0.001; Month
Started (year): F-5.169, p<0.0001; Year: F-3.118, p=0.078. ANOVA by compound:
Square
Root(Compound) = Month Started (Year), Year as follows: For Pheromone, R2-
0.354, F17,141-3.997,
16
CA 02853991 2014-06-11
p<0.0001; Month Started (year): F-4.070, p<0.0001; Year: F-0.344, p=0.559. For
C-13, R2-0.337,
F15,123-3.653, p<0.0001; Month Started (year): F-3.752, p<0.0001; Year: F-
2.701, p=0.103. For E-2-
decenal, R2-0.497, F16,67-3.150, p=0.001; Month Started (year): F-1.211,
p=0.295; Year: F-4.397,
p=0.041. .
[0041] H. halys males surprisingly produced significantly more C-13 when
held in
groups of two or more than when held individually (Figure 4A); F71.1 = 4.256;
p =0.003.
Conversely, male-specific compound (pheromone) emission was surprisingly lower
for groups of
males than those held individually F71,1= 6.634; p <0.0001. Surprisingly, H
halys males produced
significantly more of the defensive aldehyde when held in groups of two or
more than when held
individually (Figure 4B); F71,1=3.070 ; p =0.003.
[0042] The concentration of C-13 and aldehyde surprisingly increased
proportionately as
the number of males increased, while the amount of male-specific compounds
emitted surprisingly
decreased (Figure 5); Pheromone= subject, #males (subject): F71,1-6.634; p
<0.0001; C-13 = subject,
# males (subject): F71,1 = 5.701; p=0.001; Aldehyde = subject, # males
(subject): F71,1 = 1.617;
p=0.003. Regression equations: Pheromone = -0.17x + 2.52; C-13 = 0.22x + 1.15;
Aldehyde = -
0.773x + 0.290. Multivariate pairwise correlations p < 0.0001. The C-13 and
male-specific
compounds interaction overlapped at 2.5 males. Groups of males began releasing
C-13 nine days
post-imaginal ecdysis and release in cyclic bursts throughout their adulthood.
[0043] Surprisingly, different solvents had different effects on II.
halys behavior. When
35111 of solvent was applied to a fresh 1-inch section of green bean, starved
adult males ages (3-7
days) were significantly less likely to feed on beans treated with methylene
chloride (DCM), hexane,
or acetone. Less feeding aversion was seen for beans treated with 35u1
ethanol. Young adult males
showed a slight preference for pentane-treated green beans over the untreated
control bean. Based on
these results, we chose pentane as the solvent for our subsequent repellent
assays in petri dishes.
[0044] In closed-air optimized bioassays, starved nymphs (3rd stage)
were significantly less
likely to feed on beans treated with 1001.tg isolongifolanone, as well as lug
or 1001g concentrations
of isolongifolenone and C-13 (Figure 6A). Young adult males (ages 3-7 days
into adult stage) were
significantly less likely to feed on 100u.g concentrations of
isolongifolanone, isolongifolenone, and
C-13, as well as 1 g E-2-decenal (Figure 6B), and young adult females were
deterred by 100ug
concentrations of isolongifolanone and isolongifolenone (Figure 6C). Old adult
males (ages 13-23
days) were significantly less likely to feed on beans treated with 100ug
isolongifolanone or l[tg C-13
(Figure 6D), and old adult females were deterred by 1001ag concentrations of
isolongifolanone and
17
CA 02853991 2014-06-11
isolongifolenone (Figure 6E). For nominal logistic regression statistical
analysis of all compounds
by age group (Model: age group response = Compound), the likelihood ratio
statistics were as
follows: Nymph x2-72.047,p<0.0001; Young Adult Male -Z-33.697, p=0.006; Young
Adult Female
;(2_31446, p=0.012; Old Adult Male x2-18.947, p=0.167; Old Adult Female x2-
20.021, p=0.130.
Asterisks above groups of bars indicate the significance of the three
behavioral observations (blank
bean, odor bean, off bean) relative to the pentane control shown as the first
set of bars in the figure,
with p <0.05 (*), p <0.01(**), and p <0.001 (***), n=30-40 for adults, 40-80
for nymphs.
[0045] Table 1 summarizes the results for compounds and concentrations tested
in
optimized petri dish assays from Figures 6A-E. Isolongifolanone at 100p.g
concentration was
significantly repellent to all age groups and sexes. lsolongifolenone at
100i.tg concentration was the
next most effective, deterring all groups except for old adult males. C-13 was
the next most effective
and was deterrent to young males and old adult males and nymphs. E-2-decenal
11,tg was effective at
deterring Nymphs, young adult males, and old adult females, while E-2-decenal
1001.tg was effective
at deterring old adult males. Boxes indicated with n.s. mean that compound
tested was not
significant in repelling insect from treated bean.
[0046] Figures 7A-D show the effectiveness of both individual and combined
compounds
at deterring H halys feeding in small cage bioassays. Letters above bars
indicate significance of
treatment relative to other treatments; shared letters are not significantly
different from each other.
The average number of nymphs (3rd stage) feeding on peaches over 8 hours of
observations was
lower for all compounds and combinations of compounds relative to the Tween
and H20 controls,
except for the aldehyde/C-13 combination (Figure 7A). The three-component
blend of equal
concentrations isolongifolanone + isolongifolenone + C-13 yielded the highest
repellency. Statistical
analysis of ANOVA (Model: Insects on fruit ¨ Treatment, Rep(Treatment) were as
follows: For
nymphs: F10,830-41.82, p<0.0001. Nymphs were tested in groups of 15
individuals, with 4-28 reps per
treatment.
[0047] Male and female behavior in small cages was observed for
repellents on peaches
(Figure 7C) and apples (7D), and did not significantly differ by fruit or age
group tested (see detailed
statistical model below), so data was combined in Figure 7B. The 3- and 4-
component blends of
isolongifolanone + isolongifolenone + C-I3, and isolongifolanone +
isolongifolenone + C-13 +
Aldehyde were significantly more repellent to starved male and female adults
than the Tween and
1I20 control. C-13 and Anone/C-13 were not tested for adults in apples. We
used repeated-
measures ANOVA to test the following model: Number of H. hulys on fruit =
Treatment, Sex,
18
CA 02853991 2014-06-11
Time(Treatment), Rep(Treatment), Age, Time*Treatment. There was a significant
effect for
treatment in peaches (p<0.0001) and apples (p<0.0001), and a significant
effect for time in apples
(p<0.0001). For the same test run on combined apples and peaches data, there
was a significant
effect for treatment (p=0.007) and time (p<0.0001), but not for fruit. The
significant effect of time
indicated that the deterrent properties of the tested repellents wore off in
open cage systems several
hours after their application to the food source. There were 10 male or female
adults per cage and 2-
6 reps conducted per sex (4-12 reps total).
[0048] Discussion: From H halys airborne collection extracts, we found that
groups of
males surprisingly produced significantly more C-13 and E-2-decenal than
single individuals
(Figures 1 and 4), indicating that communication between males could influence
the emission
rate of the semiochemicals. Bug density having an effect on emission rate of
defensive
compounds has not been previously reported in the Pentatomid family of
insects. Our results clearly
show that the amounts of C-13 and E-2-decenal were positively proportional to
the number of the
males (Figure 5). Thus, higher densities of males yielded higher amounts of C-
13 and E-2-decenal.
Conversely, male-specific semiochemicals (pheromone) were severely reduced, or
absent, when C-
13 and the defensive aldehyde were present in aeration analyses (Figure 5).
These results suggest that
high densities are not profitable environment for mate-seeking males, so that
males produce more
tridecane and E-2-decenal to repel conspecific males away in order to maintain
an optimal population
density in their habitat. We confirmed the repellency of C-13 by showing that
it deterred H halys
feeding when diluted in pentane, a solvent that was slightly attractive to H.
halys feeding (Figures 6a-
6e). The decreased repellency of C-13 in small cage tests was likely due to
the high evaporation rate
of this compound in open-air systems and we are currently optimizing the
longevity of C-13.
[0049] Based on the above information, we conclude that tridecane (i.e.,
C-13), a
hydrocarbon from the II. halys secretion, not only acts as a defensive odor
carrier, but also is a
semiochemical component that has significant biological function to influence
H halys behavior.
To our knowledge, no applications of using tridecane and E-2-decenal in insect
pest management
have been previously reported. Furthermore, based on the information above, we
found that
isolongifoloanone and isolongifolenone were highly effective repellents for
deterring all age groups
and sexes of a halys, especially when combined with their natural
semiochemical secretions C-13
and E-2-decenal.
19
[0050] The effectiveness of various insecticide classes to control of H. halys
have been
tested in laboratory conditions (Nielsen, A.L., et al., Journal of Economic
Entomology, 101: 1439-
1442 (2008); however, some pesticide application must be conducted by a pest
control applicator and
causes additional cost/time to growers. An egg parasitoid, Trissolcus
halymorphae Yang
(Hymenoptera: Scelionidae), is a biological control agent of H. halys
currently used in northern
China (Yang, Z.Q., et al., Annals of the Entomological Society of America,
102: 39-47 (2009).
Although it has a potential as a biocontrol agent to be used in the U.S., it
still is under risk assessment
and evaluation. We have identified isolongifolanone, isolongifolenone,
tridecane, and E-2-decenal as
feeding deterrents/repellents of H. halys male and nymph, and they can be
immediately used for
management of H. halys populations in orchards or other commodity-based
fields. Because they are
natural products, there are no environmental pollution concerns related to
using synthetic
insecticides. These identified feeding deterrents/repellents are commercially
available and can be
easily commercialized as different formulations and used for protecting
agricultural crops from H.
halys damage in support of ongoing H. halys management programs.
[0051] References: Aligiannis, N., et al., Flavour and Fragrance Journal, 19:
320-324
(2004); Ashour, M.L., et al., Journal of Pharmacy and Pharmacology, 61: 1079-
1087 (2009); Baser,
K.H.C., et al., Journal of Essential Oil Research, 18: 515-517 (2006); Borges,
M., et al.,
Entomologia experimentalis et applicata, 44: 205-212 (1987); Brahmi, F., et
al., International
Journal of Food Science and Technology, 46: 1316-1322 (2011); Calam, D.H., and
A. Youdeowei, Journal of Insect Physiology, 14: 1147-1158 (1968); Favaro,
C.F., et al., Journal of
the Brazilian Chemical Society, 22: 58-64 (2011); Fons, F., et al., Natural
Product Communications,
5: 1655-1658 (2010); Fucarino, A., et al., Journal of Chemical Ecology, 30:
1257-1269 (2004);
Gough, A.J.E., et al., Journal of Chemical Ecology, 11: 343-352 (1985); Ho,
H.Y., and J.G. Millar,
Zoological Studies, 40: 193-198 (2001); Ho, H.Y., et al., Journal of Chemical
Ecology, 29: 2101-
2114 (2003); Ho, H.Y., et al., Journal of Chemical Ecology, 31: 29-37 (2005);
Kou, R., et al.,
Journal of Chemical Ecology, 15: 2695-2702 (1989); Kraft, B.S., et al.,
Journal of Chemical
Ecology, 25: 2477-2494 (1999); Lockwood, J.A., and R.N. Story, Ann. Entomol.
Soc. Am., 78:
474-479 (1985); Marques, F.A., et al., Journal of the Brazilian Chemical
Society, 18: 1242-1246
(2007); Moronkola, D.O., et al., Journal of Essential Oil Research, 21: 264-
266 (2009); Ortiz
Moreno, A., et al., Journal of
Date Recue/Date Received 2020-11-20
CA 02853991 2014-06-11
Agricultural and Food Chemistry, 51: 2216-2221(2003); Nagalakshmi, M.A.H.. et
al., Flavour and
Fragrance Journal, 16: 241-244 (2001); Nagnan, P., et al., International
Journal of Insect
Morphology and Embryology, 23: 355-370 (1994); Pareja, M., et al., Journal of
Insect Physiology,
53: 639-648 (2007); Rapior, S., et al., Journal of Essential Oil Research, 8:
199-201 (1996);
Raspotnig, G., et al., Experimental and Applied Acarology, 25: 933-946 (2001);
Sosa-Gomez, D.R.,
et al., Journal of Invertebrate Pathology, 69: 31-39 (1997); Sturaro, A., et
al., Chromatographia, 39:
103-106 (1994); Telci, 1., and Y. Hisil, European Journal of Horticultural
Science, 73: 267-272
(2008); Tu, N.T.M., et al., Flavour and Fragrance Journal, 17: 169-174 (2002);
Wang, J., et al.,
Natural Product Communications, 6: 1749-1753 (2011); Williams, L., III, et
al., Journal of
Chemical Ecology, 27: 203-216 (2001);Witte, V., et al., Chemoecology, 17: 63-
69 (2007); Zahn,
D.K., et al., Journal of Chemical Ecology, 34: 238251 (2008); U.S. Patent No.
7,378,557; U.S.
Patent No. 7,579,016.
[0052] Thus, in view of the above, the present invention concerns (in part)
the following:
100531 A composition comprising (or consisting essentially of or consisting
of) at least two
compounds selected from the group consisting of tridecane, E-2-decenal,
isolongifolanone,
isolongifolenone, and at least one isolongifolenone analog having the
following formula:
0 3 R1
4
1 5
Ili 7 R2
8
wherein R1 is hydrogen, an oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen; and optionally a carrier or carrier material. The
composition may contain any
combination of the compounds so long as at least two of the compounds are
present.
100541 The above composition, wherein said composition comprises at least
three
compounds selected from the group consisting of E-2-decenal, tridecane,
isolongifolanone,
isolongifolenone, and at least one isolongifolenone analog having the
following formula:
21
CA 02853991 2014-06-11
3 R
2 4 1
1 6 5
R
*72
8
wherein RI is hydrogen, an oxygen, a C1.10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a Ci_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen. The composition may contain any combination of the
compounds so long as at
least three of the compounds are present.
100551 The above composition, wherein said composition comprises at least four
compounds selected from the group consisting of E-2-decenal, tridecane,
isolongifolanone,
isolongifolenone, and at least one isolongifolenone analog having the
following formula:
0 3 54 R1
1
R2
11/ 7
8
wherein R1 is hydrogen, an oxygen. a Ci_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen. The composition may contain any combination of the
compounds so long as at
least four of the compounds are present.
100561 The above composition, wherein said composition comprises E-2-decenal,
tridecane, isolongifolanone, isolongifolenone, and at least one
isolongifolenone analog having the
following formula:
22
CA 02853991 2014-06-11
3 R
2 4 1
1 5
14, 7 R2
8
wherein R1 is hydrogen, an oxygen, a Ci_io alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a Ci_io saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1.10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen.
[0057] The above composition, wherein said composition contains E-2-decenal.
The
above composition, wherein said composition does not contain E-2-decenal.
[0058] The above composition, wherein said composition contains tridecane. The
above
composition, wherein said composition does not contain tridecane.
[0059] The above composition, wherein said composition contains
isolongifolanone.
The above composition, wherein said composition does not contain
isolongifolanone.
[0060] The above composition, wherein said composition contains
isolongifolenone.
The above composition, wherein said composition does not contain
isolongifolenone.
[0061] The above composition, wherein said composition contains at least one
isolongifolenone analog having the following formula:
3 R
2 4 1
1
lAr 7 R2
8
wherein R1 is hydrogen, an oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen. The above composition, wherein said composition does not
contain at least
one isolongifolenone analog having the following formula:
23
CA 02853991 2014-06-11
3 R
2 4 1
1 6 5
111) 7 R2
8
wherein R1 is hydrogen, an oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1.10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen.
[0062] The above composition, wherein said composition contains tridecane and
E-2-
decenal.
[0063] A method for deterring feeding/repelling Halyomorpha halys, said method
comprising (or consisting essentially of or consisting of) treating an object
or area with a composition
comprising (or consisting essentially of or consisting of) a Halyomorpha halys
deterring
feeding/repelling effective amount of at least one compound selected from the
group consisting of
tridecane, E-2-decenal, isolongifolanone, isolongifolenone, and at least one
isolongifolenone
analog having the following formula:
0 3 R1
< 4
1 5
IA
R2
8
wherein R1 is hydrogen, an oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen; and optionally a carrier or carrier material. The
composition may contain any
combination of the compounds so long as at least one of the compounds are
present.
24
CA 02853991 2014-06-11
[0064] The above method, wherein said composition comprises at least two
compounds
selected from the group consisting of E-2-decenal, tridecane,
isolongifolanone, isolongifolenone,
and at least one isolongifolenone analog having the following formula:
3 R
2 4 1
1 5
R2
11/7
8
wherein R1 is hydrogen, an oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen. The composition may contain any combination of the
compounds so long as at
least two of the compounds are present.
[0065] The above method, wherein said composition comprises at least three
compounds
selected from the group consisting of E-2-decenal, tridecane,
isolongifolanone, isolongifolenone,
and at least one isolongifolenone analog having the following formula:
3 R1
2 4
1 5
R2
Ili 7
8
wherein Ri is hydrogen, an oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen. The composition may contain any combination of the
compounds so long as at
least three of the compounds are present.
[0066] The above method, wherein said composition comprises at least four
compounds
selected from the group consisting of E-2-decenal, tridecane,
isolongifolanone, isolongifolenone,
and at least one isolongifolenone analog having the following formula:
CA 02853991 2014-06-11
2 3 4 R1
1 5
Ili 7 R2
8
wherein R1 is hydrogen, an oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1.10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen. The composition may contain any combination of the
compounds so long as at
least four of the compounds are present.
[0067] The above method, wherein said composition comprises E-2-decenal,
tridecane,
isolongifolanone, isolongifolenone, and at least one isolongifolenone analog
having the
following formula:
3 R
2 4 1
1 5
67
R2
a
wherein R1 is hydrogen, an oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen.
[0068] The above method, wherein said composition contains E-2-decenal. The
above
method, wherein said composition does not contain E-2-decenal.
[0069] The above method, wherein said composition contains tridecane. The
above
method, wherein said composition does not contain tridecane.
[0070] The above method, wherein said composition contains isolongifolanone.
The
above method, wherein said composition does not contain isolongifolanone.
[0071] The above method, wherein said composition contains isolongifolenone.
The
26
CA 02853991 2014-06-11
above method, wherein said composition does not contain isolongifolenone.
[0072] The above method, wherein said composition contains at least one
isolongifolenone analog having the following formula:
3 2 R1
4
1 5
III 7 R2
8
wherein R1 is hydrogen, an oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1.40 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1_10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen. The above method, wherein said composition does not
contain at least one
isolongifolenone analog having the following formula:
3 R1
2 4
1 5
R2
8171
wherein RI is hydrogen, an oxygen, a Ci_10 alcohol, aldehyde, alkyl, ether, or
esters of said
alcohol with a C1_10 saturated or unsaturated, straight or branched acid and
R2 is hydrogen, an
oxygen, a C1.10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol
with a C1_10 saturated or
unsaturated, straight or branched acid; optionally there is a double bond
between carbons 5 and 6
and R2 is hydrogen.
[0073] The above method, wherein said composition contains tridecane and E-2-
decenal.
[0074] The above method, wherein said composition contains tridecane and E-2-
decenal
in a 10:1 molar ratio.
[0075] The above method, wherein said Halyomorpha halys is selected from the
group
consisting of males, females, and mixtures thereof.
[0076] The above method, wherein said Halyomorpha halys are males.
[0077] The above method, wherein said Halyomorpha halys are females.
27
CA 02853991 2014-06-11
[00781 The above method, wherein said Halyomorpha halys arc in an immature
stage
(e.g., nymphs).
[0079] Other embodiments of the invention will be apparent to those
skilled in the art
from a consideration of this specification or practice of the invention
disclosed herein. It is intended
that the specification and examples be considered as exemplary only, with the
true scope and spirit
of the invention being indicated by the following claims.
28
CA 02853991 2014-06-11
,
Anone C13 Enone E-2-decenal
1 jig 100ttg 1 ttg 100pg 1 jig 100ttg 11.tg
100ttg
Nymph *** *** ** *** ** ** n.s.
Young Adult Male *** n.s. * n.s. *** ** n.s.
Young Adult Female *** n.s. n.s. n.s. *** n.s. n.s.
Old Adult Male *** /Ls. * n.s. n.s. n.s. *
Old Adult Female *** n.s. n.s. n.s. * n.s. *
TABLE 1
29