Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
NOVEL PYRROLIDINE DERIVATIVES AS INHIBITORS OF CATHEPSIN
The present invention relates to organic compounds useful for therapy and/or
prophylaxis in a mammal, and in particular to compounds that are preferential
inhibitors of
the cysteine protease cathepsin, in particular of the cysteine protease
cathepsin S or L.
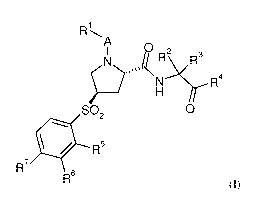
The invention relates in particular to a compound of formula (I)
A
0 2
P,;:R4
SO2 0
=R5
R7
R6 (I)
wherein
A is carbonyl or absent;
R1 is alkoxy, nitrophenyl, 1H-pyrazoly1 substituted with alkyl and cycloalkyl,
alkylcycloalkyl, haloalkylcycloalkyl, phenylcycloalkyl, halophenylcycloalkyl,
pyridinylcycloalkyl or halopyridinylcycloalkyl;
R2 and R3 are independently selected from hydrogen, alkyl and cycloalkylalkyl;
or R2 and R3 together with the carbon atom to which they are attached form
cycloalkyl;
R4 is -C(0)NR8R9 or benzooxazolyl;
DP/12.09.12
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 2 -
R5, R6 and R7 are independently selected from hydrogen, alkyl, halogen,
haloalkyl,
alkoxy, haloalkoxy and morpholinyl; and
one of R8 and R9 is hydrogen or alkyl and the other one is alkyl, alkoxyalkyl,
cycloalkyl, haloalkyl, phenylalkyl, naphtylalkyl or tetrahydropyranyl;
or a pharmaceutically acceptable salt or ester thereof.
The compounds of the invention are preferential inhibitors of the cysteine
protease
Cathepsin (Cat), in particular Cathepsin S or Cathepsin L and are therefore
useful to treat
metabolic diseases like diabetes, atherosclerosis, abdominal aortic aneurysm,
peripheral
arterial disease, cancer, reduction of cardiovascular events in chronic kidney
disease,
glomerulonephritis, age related macular degeneration, diabetic nephropathy and
diabetic
retinopathy. In addition, immune mediated diseases like rheumatoid arthritis,
crohn's
disease, multiple sclerosis, sjorgen syndrome, lupus erythematosus,
neuropathic pain,
diabetes type I, asthma and allergy and skin related immune disease are
suitable diseases
to be treated with a cathepsin S inhibitor. Objects of the present invention
are the
compounds of formula (I) and their aforementioned salts per se and their use
as
therapeutically active substances, a process for the manufacture of the said
compounds,
intermediates, pharmaceutical compositions, medicaments containing the said
compounds,
their pharmaceutically acceptable salts, the use of the said compounds and
salts for the
prophylaxis and/or therapy of illnesses, especially in the treatment or
prophylaxis of
diabetes, atherosclerosis, abdominal aortic aneurysm, peripheral arterial
disease, cancer,
reduction of cardiovascular events in chronic kidney disease and diabetic
nephropathy, and
the use of the said compounds and salts for the production of medicaments for
the
treatment or prophylaxis of diabetes, atherosclerosis, abdominal aortic
aneurysm,
peripheral arterial disease, cancer, reduction of cardiovascular events in
chronic kidney
disease and diabetic nephropathy.
Mammalian cathepsins are cysteine-type proteases involved in key steps of
biological and pathological events. Cathepsins are considered tractable drug
targets as it is
feasible to inhibit enzymatic activity with small molecules and are therefore
of interest to
the pharmaceutical industry (Bromme, D. (2001), 'Papain-like cysteine
proteases', Curr
Protoc Protein Sci, Chapter 21, Unit 21 2; Roberts, R. (2005), 'Lysosomal
cysteine
proteases: structure, function and inhibition of cathepsins', Drug News
Perspect, 18 (10),
605-14).
Cathepsin S is prominently expressed in antigen presenting cells like
macrophages
and dendritic cells and smooth muscle cells. (Hsing, L. C. and Rudensky, A. Y.
(2005),
'The lysosomal cysteine proteases in MHC class II antigen presentation',
Immunol Rev,
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
-3-
207, 229-41; Rudensky, A. and Beers, C. (2006), 'Lysosomal cysteine proteases
and
antigen presentation', Ernst Schering Res Found Workshop, (56), 81-95). While
Cathepsin
S is only weakly expressed in normal arterial tissue, strong upregulation is
seen in
atherosclerotic arteries (Liu, J., et al. (2006), 'Increased serum cathepsin S
in patients with
atherosclerosis and diabetes', Atherosclerosis, 186 (2), 411-9; Sukhova, G.
K., et al.
(1998), 'Expression of the elastolytic cathepsins S and K in human atheroma
and regulation
of their production in smooth muscle cells', J Clin Invest, 102 (3), 576-83).
Preclinical data suggest that the function of Cathepsin S is critical for
atherosclerosis
as Cathepsin S deficient mice have a reduced atherosclerosis-phenotype when
tested in
appropriate mouse models. In LDL-Rec deficient mice reduced lipid
accumulation, elastin-
fibre breakdown and chronic arterial inflammation is reported. In APO E
deficient mice a
significant reduction of acute plaque rupture events was reported. When
chronic renal
disease is introduced into CatS/In APO-E deficient mice a strong reduction of
accelerated
calcification is seen on top of the anti atherosclerotic activity in arteries
and heart valves
Aikawa, E., et al. (2009), 'Arterial and aortic valve calcification abolished
by elastolytic
cathepsin S deficiency in chronic renal disease', Circulation, 119 (13), 1785-
94; de
Nooijer, R., et al. (2009), 'Leukocyte cathepsin S is a potent regulator of
both cell and
matrix turnover in advanced atherosclerosis', Arterioscler Thromb Vasc Biol,
29 (2), 188-
94; Rodgers, K. J., et al. (2006), 'Destabilizing role of cathepsin S in
murine
atherosclerotic plaques', Arterioscler Thromb Vasc Biol, 26 (4), 851-6;
Sukhova et al.
(2003), 'Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-
deficient mice',
J Clin Invest, 111(6), 897-906). This suggests a potential inhibitor of
Cathepsin S would
stabilise atherosclerotic plaque by reducing extracellular matrix breakdown,
by reducing
the proinflammatory state and by reducing accelerated calcification and
subsequently its
clinical manifestations.
These phenotypes described in atherosclerosis models are in agreement with
known
cellular functions of Cathepsin S. Firstly, Cathepsin S is involved in the
degradation of
extracellular matrix that stabilises the plaque. In particular, Cathepsin S
has potent
elastinolytic activity and can exert this at neutral pH, a feature that
distinguishes Cathepsin
S from all other Cathepsins. Secondly, Cathepsin S is the major protease
involved in
antigen processing, in particular cleavage of the invariant chain in antigen
presenting cells,
resulting in reduced contribution of Tcells to the chronic inflammation of the
atherosclerotic tissue. Elevated inflammation results in further oxidative and
proteolytic
tissue damage and subsequently plaque destabilisation (Cheng, X. W., et al.
(2004),
'Increased expression of elastolytic cysteine proteases, cathepsins S and K,
in the neointima
of balloon-injured rat carotid arteries', Am J Pathol, 164 (1), 243-51;
Driessen, C., et al.
(1999), 'Cathepsin S controls the trafficking and maturation of MHC class II
molecules in
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 4 -
dendritic cells', J Cell Biol, 147 (4), 775-90; Rudensky, A. and Beers, C.
(2006),
'Lysosomal cysteine proteases and antigen presentation', Ernst Schering Res
Found
Workshop, (56), 81-95).
The anti-inflammatory and anti-elastinolytic properties of a Cat S inhibitor
make it
also a prominent target for chronic obstructive pulmonary disease (Williams,
A. S., et al.
(2009), 'Role of cathepsin S in ozone-induced airway hyperresponsiveness and
inflammation', Pulm Pharmacol Ther, 22 (1), 27-32). Furthermore due to its
extracellular
functions in matrix degradation, inhibition of cathepsin S will impact
neointima formation
and angiogenesis (Burns-Kurtis, C. L., et al. (2004), 'Cathepsin S expression
is up-
regulated following balloon angioplasty in the hypercholesterolemic rabbit',
Cardiovasc
Res, 62 (3), 610-20; Cheng, X. W., et al. (2004), 'Increased expression of
elastolytic
cysteine proteases, cathepsins S and K, in the neointima of balloon-injured
rat carotid
arteries', Am J Pathol, 164 (1), 243-51; Shi, G. P., et al. (2003),
'Deficiency of the cysteine
protease cathepsin S impairs microvessel growth', Circ Res, 92 (5), 493-500;
Wang, B., et
al. (2006), 'Cathepsin S controls angiogenesis and tumor growth via matrix-
derived
angiogenic factors', J Biol Chem, 281 (9), 6020-9). An inhibitor of Cathepsin
S might
therefore be useful in several different disease settings.
Cathepsin S plays also a role in the reduction of tumor growth and tumor cell
invasion as described by Roberta E. Burden in Clin Cancer Res 2009;15(19). In
addition,
nephrectomized Cathepsin S knock out mice showed a significant reduction of
arterial
calcification when compared to nephrectomized wild type mice. This indicates
that
inhibition of Cathepsin S may have a beneficial effect on the reduction of
cardiovascular
events in chronic kidney disease patients (Elena Aikawa, Circulation, 2009,
1785-1794).
Cathepsin L shows a broader expression profile than cathepsin S and there are
also
data which suggest a role of cathepsin L in atherosclerosis, e.g. LDLrec & Cat
L deficient
mice show a reduced atherosclerotic phenotype (Kitamoto, S., et al. (2007),
'Cathepsin L
deficiency reduces diet-induced atherosclerosis in low-density lipoprotein
receptor-
knockout mice', Circulation, 115 (15), 2065-75). In addition, Cat L was
suggested to be
involved in metabolic syndrome as it controls adipogenesis and peripheral
glucose
tolerance. In renal disease Cathepsin L is described to regulate podocyte
function by
proteolytically processing dynamin and thereby proteinuria (Sever, S., et al.
(2007),
'Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism
for
proteinuric kidney disease', J Clin Invest, 117 (8), 2095-104).
Tissue remodelling, extracellular matrix degradation, the generation of active
neuropeptides and roles in antigen presentation in thymic epithelial cells are
cellular
activities described for Cathepsin L (Funkelstein et al. 2008; Rudensky and
Beers 2006).
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 5 -
In the present description the term "alkyl", alone or in combination,
signifies a
straight-chain or branched-chain alkyl group with 1 to 8 carbon atoms,
preferably a straight
or branched-chain alkyl group with 1 to 6 carbon atoms and particularly
preferred a straight
or branched-chain alkyl group with 1 to 4 carbon atoms. Examples of straight-
chain and
branched C1-C8 alkyl groups are methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, tert.-
butyl, the isomeric pentyls, the isomeric hexyls, the isomeric heptyls and the
isomeric
octyls. Particular alkyls are methyl, ethyl, propyl, isopropyl and butyl.
The term "cycloalkyl", alone or in combination, signifies a cycloalkyl ring
with 3 to
8 carbon atoms and preferably a cycloalkyl ring with 3 to 6 carbon atoms.
Examples of C3-
C8 cycloalkyl are cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl and
cyclooctyl. Particular cycloalkyl are cyclopropyl and cyclobutyl.
The term "alkoxy", alone or in combination, signifies a group of the formula
alkyl-0- in which the term "alkyl" has the previously given significance, such
as methoxy,
ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy, sec. butoxy and tert.
butoxy.
Particular alkoxy are ethoxy, isopropoxy and tert. butoxy.
The term "oxy", alone or in combination, signifies the -0- group.
The term "halogen" or "halo", alone or in combination, signifies fluorine,
chlorine,
bromine or iodine.
The terms "haloalkyl", "halocycloalkyl", "haloalkoxy", "halophenyl",
"halpyridinyl",
alone or in combination, denote an alkyl group, a cycloalkyl group, an alkoxy
group, a
phenyl group and a pyridinyl group substituted with at least one halogen,
preferably
substituted with one to five halogens, preferably one to three halogens. A
particular
haloalkyl is trifluoromethyl. Particular haloalkoxy are trifluoroethyl and
trifluoroisopropoxy.
The terms "hydroxyl" and "hydroxy", alone or in combination, signify the -OH
group.
The term "pharmaceutically acceptable salts" refers to those salts which
retain the
biological effectiveness and properties of the free bases or free acids, which
are not
biologically or otherwise undesirable. The salts are formed with inorganic
acids such as
hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric
acid, preferably
hydrochloric acid, and organic acids such as acetic acid, propionic acid,
glycolic acid,
pyruvic acid, oxylic acid, maleic acid, malonic acid, succinic acid, fumaric
acid, tartaric
acid, citric acid, benzoic acid, cinnamic acid, mandelic acid, methanesulfonic
acid,
ethanesulfonic acid, p-toluenesulfonic acid, salicylic acid, N-acetylcystein.
In addition
CA 02854569 2014-05-05
WO 2013/076063
PCT/EP2012/073073
- 6 -
these salts may be prepared form addition of an inorganic base or an organic
base to the
free acid. Salts derived from an inorganic base include, but are not limited
to, the sodium,
potassium, lithium, ammonium, calcium, magnesium salts. Salts derived from
organic
bases include, but are not limited to salts of primary, secondary, and
tertiary amines,
"Pharmaceutically acceptable esters" means that the compound of general
formula (I)
may be derivatised at functional groups to provide derivatives which are
capable of
conversion back to the parent compounds in vivo. Examples of such compounds
include
20 If one of
the starting materials or compounds of formula (I) contain one or more
functional groups which are not stable or are reactive under the reaction
conditions of one
or more reaction steps, appropriate protecting groups (as described e.g. in
"Protective
Groups in Organic Chemistry" by T. W. Greene and P. G. M. Wutts, 3rd Ed.,
1999, Wiley,
New York) can be introduced before the critical step applying methods well
known in the
carbobenzyloxy (Cbz) and p-methoxybenzyloxycarbonyl (Moz).
The compound of formula (I) can contain several asymmetric centers and can be
The term "asymmetric carbon atom" means a carbon atom with four different
substituents. According to the Cahn-Ingold-Prelog Convention an asymmetric
carbon atom
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 7 -
The invention relates in particular to the following:
A compound of formula (I) wherein
A is carbonyl or absent;
R1 is alkoxy, nitrophenyl, 1H-pyrazoly1 substituted with alkyl and cycloalkyl,
alkylcycloalkyl, haloalkylcycloalkyl, phenylcycloalkyl, halophenylcycloalkyl,
pyridinylcycloalkyl or halopyridinylcycloalkyl;
R2 and R3 are independently selected from hydrogen, alkyl and cycloalkylalkyl;
or R2 and R3 together with the carbon atom to which they are attached form
cycloalkyl;
R4 is -C(0)NR8R9 or benzooxazolyl;
R5, R6 and R7 are independently selected from hydrogen, alkyl, halogen,
haloalkyl,
alkoxy and haloalkoxy; and
one of R8 and R9 is hydrogen or alkyl and the other one is alkyl, cycloalkyl,
haloalkyl,
phenylalkyl or naphtylalkyl;
or a pharmaceutically acceptable salt or ester thereof;
A compound of formula (I), wherein R1 is halophenylcycloalkyl or
halopyridinylcycloalkyl;
A compound of formula (I), wherein R1 is tert-butoxy, nitrophenyl, 1H-
pyrazoly1
substituted with methyl and cyclobutyl, trifluoromehtycyclopropyl,
phenylcycloalkyl,
chlorophenylcyclopropyl, bromophenylcyclopropyl, iodophenylcyclopropyl,
chlorofluorophenylcyclopropyl, bromofluorophenylcyclopropyl,
pyridinylcycloalkyl or
chlorofluoropyridinylcypropyl or bromofluoropyridinylcyclopropyl;
A compound of formula (I), wherein R1 is chlorophenylcyclopropyl,
chlorofluorophenylcyclopropyl, bromophenylcyclopropyl,
bromofluorophenylcyclopropyl
or chlorofluoropyridinylcyclopropyl;
A compound of formula (I), wherein R2 and R3 are independently selected from
hydrogen and alkyl;
A compound of formula (I), wherein R2 and R3 are independently selected from
hydrogen, methyl, ethyl, propyl and butyl;
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 8 -
A compound of formula (I), wherein R5, R6 and R7 are independently selected
from
halogen, haloalkyl and haloalkoxy;
A compound of formula (I), wherein R5, R6 and R7 are independently selected
from
hydrogen, chloro, trifluoromethyl and trifluoroethoxy;
A compound of formula (I), wherein R5 is methyl, trifluoromethyl or chloro;
A compound of formula (I), wherein R5 is chloro or trifluoromethyl;
A compound of formula (I), wherein R6 is hydrogen;
A compound of formula (I), wherein R7 is hydrogen, methyl, chloro, fluoro,
bromo,
fluoro, trifluoroethoxy or trifluoropropoxy;
A compound of formula (I), wherein R7 is hydrogen, chloro or trifluoroethoxy;
A compound of formula (I), wherein one of R8 and R9 is hydrogen and the other
one
is alkyl or cycloalkyl;
A compound of formula (I), wherein one of R8 and R9 is hydrogen and the other
one
is ethyl, propyl, butyl or cyclopropyl;
A compound of formula (I) selected from
(2S,4R)-tert-butyl 2-((S)-1-(cyclopropylamino)-1,2-dioxopentan-3-ylcarbamoy1)-
4-
(2,4-dimethylphenylsulfonyl)pyrrolidine-1-carboxylate;
(2S,4R)-tert-butyl 4-(4-chloro-2-methylphenylsulfony1)-2-((S)-1-
(cyclopropylamino)-1,2-dioxopentan-3-ylcarbamoyl)pyrrolidine-1-carboxylate;
(2S,4R)-N-((S)-1-(cyclopropylamino)-1,2-dioxopentan-3-y1)-1-(2-nitropheny1)-4-
(2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxamide;
(2S,4R)-1-(1-cyclobuty1-3-methy1-1H-pyrazol-5-y1)-N-((S)-1-(cyclopropylamino)-
1,2-dioxopentan-3-y1)-4-(4-fluoro-2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S,4R)-1-(2-Nitro-pheny1)-4-(2-trifluoromethyl-benzenesulfony1)-pyrrolidine-2-
carboxylic acid [(S)-1-(benzooxazole-2-carbony1)-propyll-amide;
(2S,4R)-4-(4-bromo-2-(trifluoromethyl)phenylsulfony1)-1-(1-cyclobuty1-3-methyl-
1H-pyrazol-5-y1)-N-((S)-1-(cyclopropylamino)-1,2-dioxopentan-3-y1)pyrrolidine-
2-
carboxamide;
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 9 -
(2S ,4R)-N-((S)- 1- (cyclopropylamino)- 1,2-dioxopentan-3-y1)- 1-( 1-
(trifluoromethyl)cyclopropanecarbony1)-4- (2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (cyclopropylamino)- 1,2-dioxopentan-3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (cyclopropylamino)- 1,2-dioxohexan-3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-(1-(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chloro-4-
(2,2,2-trifluoroethoxy)phenylsulfony1)-N-((S)- 1-(cyclopropylamino)- 1,2-
dioxopentan-3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-N-((S)- 1-
(cyclopropylamino)-
1,2-dioxopentan-3-y1)-4- (4- (2,2,2-trifluoroethoxy)-2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxamide;
(2S,4R)-N-((S)- 1- (benzo [d] oxazol-2-y1)- 1-oxobutan-2-y1)- 1-( 1-(4-
chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1-( 1-(4-chloro-2-fluorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)- 1- (cyclopropylamino)- 1,2-dioxopentan-3-
yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-bromo-2-fluorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)- 1- (cyclopropylamino)- 1,2-dioxopentan-3-
yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-bromophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (cyclopropylamino)- 1,2-dioxopentan-3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-bromophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (cyclopropylamino)- 1,2-dioxohexan-3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)-4-(2-chlorophenylsulfony1)-N-((S)- 1- (cyclopropylamino)- 1,2-
dioxopentan-
3-y1)- 1- (1- (4-iodophenyl)cyclopropanecarbonyl)pyrrolidine-2-carboxamide;
(2S ,4R)-4-(2-chlorophenylsulfony1)-N-((S)- 1- (cyclopropylamino)- 1,2-
dioxohexan-3-
y1)- 1-(1-(4-iodophenyl)cyclopropanecarbonyl)pyrrolidine-2-carboxamide;
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 10 -
(2S ,4R)- 1 -( 1 -(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-N-((S)-
1 -
(cyclopropylamino)- 1,2-dioxopentan-3-y1)-4- (2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1 -( 1 -(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-N-((S)-
1-
(cyclopropylamino)-1,2-dioxohexan-3-y1)-4-(2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1 -( 1 -(4-chlorophenyl)cyclopropanecarbony1)-N-((S)- 1-
(cyclopropylamino)-
1,2-dioxopentan-3-y1)-4- (2- (trifluoromethyl)phenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1 -( 1 -(4-chlorophenyl)cyclopropanecarbony1)-N-((S)- 1-
(cyclopropylamino)-
1,2-dioxohexan-3-y1)-4-(2-(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1 -( 1 -(5-bromo-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)- 1- (cyclopropylamino)- 1,2-dioxopentan-3-
yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1 -( 1 -(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)- 1- (cyclopropylamino)- 1,2-dioxohexan-3-
yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1 -( 1 -(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)- 1- (cyclopropylamino)- 1,2-dioxopentan-3-
yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1 -( 1 -(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
(4-(cyclopropylamino)-2-methy1-3 ,4-dioxobutan-2-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1 -( 1 -(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
(1 -(2-(cyclopropylamino)-2-oxoacetyl)cyclopropyl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1 -( 1 -(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (cyclopropylamino)-4-methyl- 1,2-dioxopentan-3-yl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1 -( 1 -(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (cyclopropylamino)-5-methyl- 1,2-dioxohexan-3-yl)pyrrolidine-2-
carboxamide;
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 1 1 -
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1-cyclohexy1-4-(cyclopropylamino)-3,4-dioxobutan-2-yl)pyrrolidine-2-
carboxamide;
(2S ,4R)-N-((S)- 1- (butylamino)- 1,2-dioxopentan-3-y1)- 1-( 1-(4-
chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S ,4R)-N-((S)- 1- (butylamino)- 1,2-dioxohexan-3-y1)- 1-( 1- (4-
chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S ,4R)-N-((S)- 1- (benzylamino)- 1,2-dioxopentan-3-y1)- 1- (1- (4-
chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)-4- (cyclopropylamino)-3,4-dioxobutan-2-yl)pyrrolidine-2-carboxamide;
(2S,4R)-N-((S)- 1- (benzylamino)- 1,2-dioxohexan-3-y1)- 1-( 1-(4-
chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((3S)- 1,2-dioxo- 1- (pentan-2-ylamino)pentan-3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((3S)- 1,2-dioxo- 1- (pentan-2-ylamino)hexan-3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-N-((S)- 1-
(cyclopropylamino)-
1,2-dioxopentan-3-y1)-4- (2,4-dichlorophenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S ,4R)-4-(2-chloro-4-fluorophenylsulfony1)- 1-( 1-(4-
chlorophenyl)cyclopropanecarbony1)-N-((S)- 1- (cyclopropylamino)- 1,2-
dioxopentan-
3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1,2-dioxo- 1- (2,2,2-trifluoroethylamino)hexan-3 -yl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (is opropylamino)- 1,2-dioxohexan-3-yl)pyrrolidine-2-carboxamide;
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 12 -
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-N-((S)- 1-
(cyclopropylamino)-
1,2-dioxohexan-3-y1)-4-(2,4-dichlorophenylsulfonyl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (ethylamino)- 1,2-dioxopentan-3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (ethylamino)- 1,2-dioxohexan-3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)-4-(2-chloro-4- ((S)- 1,1, 1-trifluoropropan-2-yloxy)phenylsulfony1)-
1- (1- (4-
chlorophenyl)cyclopropanecarbony1)-N-((S)- 1- (cyclopropylamino)- 1,2-
dioxopentan-
3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)-4-(2-chloro-4- ((S)- 1,1, 1-trifluoropropan-2-yloxy)phenylsulfony1)-
1- (1- (4-
chlorophenyl)cyclopropanecarbony1)-N-((S)- 1- (cyclopropylamino)- 1,2-
dioxohexan-
3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (cyclopropyl(methyl)amino)- 1,2-dioxohexan-3-yl)pyrrolidine-2-
carboxamide;
(2S ,4R)-4-(2-chloro-4-fluorophenylsulfony1)- 1-( 1-(4-
chlorophenyl)cyclopropanecarbony1)-N-((S)- 1- (cyclopropylamino)- 1,2-
dioxohexan-
3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1,2-dioxo- 1- (phenethylamino)pentan-3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (2- (naphthalen- 1-yl)ethylamino)- 1,2-dioxopentan-3-yl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (2- (naphthalen-2-yl)ethylamino)- 1,2-dioxopentan-3-yl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)- 1- (naphthalen- 1-ylmethylamino)-1,2-dioxopentan-3-yl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1-( 1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-
((S)-1,2-dioxo- 1- (tetrahydro-2H-pyran-4-ylamino)pentan-3-yl)pyrrolidine-2-
carboxamide;
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 1 3 -
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(2-methoxyethylamino)-1,2-dioxopentan-3-yl)pyrrolidine-2-carboxamide;
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(isobutylamino)-1,2-dioxopentan-3-yl)pyrrolidine-2-carboxamide;
(2S,4R)-4-(2-chloro-4-morpholinophenylsulfony1)-1-(1-(4-
chlorophenyl)cyclopropanecarbony1)-N-((S)-1-(cyclopropylamino)-1,2-dioxopentan-
3-yl)pyrrolidine-2-carboxamide;
(2S,4R)-4-(2-chloro-4-morpholinophenylsulfony1)-1-(1-(4-
chlorophenyl)cyclopropanecarbony1)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-
1 0 3-yl)pyrrolidine-2-carboxamide;
(2S,4R)-1-(1-(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)-1-(cyclopropylamino)-4-methy1-1,2-dioxopentan-3-
yl)pyrrolidine-2-carboxamide;
(2S,4R)-1-(1-(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)-1-(cyclopropylamino)-5-methy1-1,2-dioxohexan-3-
yl)pyrrolidine-2-carboxamide;
(2S,4R)-N-((S)-1-(butylamino)-1,2-dioxopentan-3-y1)-1-(1-(5-chloro-3-
fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-
2-
carboxamide;
(2S,4R)-N-((S)-1-(butylamino)-1,2-dioxohexan-3-y1)-1-(1-(5-chloro-3-
fluoropyridin-
2-yl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-
carboxamide;
and
(2S,4R)-N-((S)-1-(benzylamino)-1,2-dioxohexan-3-y1)-1-(1-(5-chloro-3-
fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-
2-
carboxamide;
A compound of formula (I) selected from
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(cyclopropylamino)-1,2-dioxopentan-3-yl)pyrrolidine-2-carboxamide;
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)pyrrolidine-2-carboxamide;
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 1 4 -
(2S ,4R)- 1 -( 1 -(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chloro-4-
(2,2,2-trifluoroethoxy)phenylsulfony1)-N- ((S)- 1 -(cyclopropylamino)- 1,2-
dioxopentan-3-yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1 -( 1 -(4-chlorophenyl)cyclopropanecarbony1)-N-((S)- 1-
(cyclopropylamino)-
1,2-dioxopentan-3-y1)-4- (4- (2,2,2-trifluoroethoxy)-2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxamide;
(2S,4R)-N-((S)- 1- (benzo [d] oxazol-2-y1)- 1-oxobutan-2-y1)- 1-(1-(4-
chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1 -( 1 -(4-chloro-2-fluorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)- 1- (cyclopropylamino)- 1,2-dioxopentan-3-
yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1 -( 1 -(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-N-((S)-
1 -
(cyclopropylamino)- 1,2-dioxopentan-3-y1)-4- (2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1 -( 1 -(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-N-((S)-
1 -
(cyclopropylamino)- 1,2-dioxohexan-3-y1)-4-(2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1 -( 1 -(4-chlorophenyl)cyclopropanecarbony1)-N-((S)- 1-
(cyclopropylamino)-
1,2-dioxopentan-3-y1)-4- (2- (trifluoromethyl)phenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1 -( 1 -(4-chlorophenyl)cyclopropanecarbony1)-N-((S)- 1-
(cyclopropylamino)-
1,2-dioxohexan-3-y1)-4-(2-(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S ,4R)- 1 -( 1 -(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)- 1- (cyclopropylamino)- 1,2-dioxohexan-3-
yl)pyrrolidine-2-carboxamide;
(2S ,4R)- 1 -( 1 -(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)- 1- (cyclopropylamino)- 1,2-dioxopentan-3-
yl)pyrrolidine-2-carboxamide;
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 1 5 -
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(cyclopropylamino)-4-methy1-1,2-dioxopentan-3-yl)pyrrolidine-2-
carboxamide;
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(cyclopropylamino)-5-methy1-1,2-dioxohexan-3-yl)pyrrolidine-2-
carboxamide;
(2S,4R)-N-((S)-1-(butylamino)-1,2-dioxopentan-3-y1)-1-(1-(4-
chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-
carboxamide;
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-4-(cyclopropylamino)-3,4-dioxobutan-2-yl)pyrrolidine-2-carboxamide;
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-N-((S)-1-(cyclopropylamino)-
1,2-dioxopentan-3-y1)-4-(2,4-dichlorophenylsulfonyl)pyrrolidine-2-carboxamide;
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(isopropylamino)-1,2-dioxohexan-3-yl)pyrrolidine-2-carboxamide;
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(ethylamino)-1,2-dioxopentan-3-yl)pyrrolidine-2-carboxamide; and
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(ethylamino)-1,2-dioxohexan-3-yl)pyrrolidine-2-carboxamide.
The compounds of the present invention can be prepared, for example, by the
general
synthetic procedures described below.
In the following schemes and description, R1 to R7 and A have, unless
otherwise
indicated, the meaning of R1 to R7 and A as defined above.
Abbreviations:
BOP: Benzotriazolyl-N-oxy-tris(dimethylamino)-phosphonium hexafluorophosphate;
BOP-CI: Bis-(2-oxo-3-oxazolidiny1)-phosphinic acid chloride;
CDI: 1,1'-Carbonyldiimidazole;
DCC : N,N'-Dicyclohexylcarbodiimide ;
DIPEA: Diisopropyl ethyl amine;
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 16 -
DMA: N,N-Dimethylacetamide;
DMF: N,N-Dimethylformamide;
EDCI: N-(3-Dimetylaminopropy1)-N'-ethyl-carbodiimide hydrochloride;
HATU: 0-(7-azabenzotriazol-1-y1)-1,1,3,3-tetramethyluronium
hexafluorophosphate;
HOBT: 1-Hydroxybenzotriazole;
LiHMDS: Lithium bis(trimethylsilyeamide;
MCPBA: 3-Chloroperbenzoic acid;
NMP = N-Methylpyrrolidinone;
PyBOP: Benzotriazol-1-yl-oxytripyrrolidinephosphonium hexafluorophosphate;
TEA: Triethylamine;
TBTU: 0-(Benzotriazol-1-y1)-N,N,N',N'-tetramethyluronium terafluoroborate;
THF: Tetrahydrofurane;
The hydroxy function of an orthogonally protected cis-4-hydroxy-proline
derivative
A, such as (2S,4S)-4-hydroxy-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl
ester 2-methyl
ester, is transformed into an appropriate leaving group, e.g., by reaction
with a sulfonyl
chloride in the presence of a base such as triethylamine, to yield compound B
(scheme 1).
Reaction of B with thiophenols in the presence of an appropriate base such as
NaH,
LiHMDS, DIPEA, TEA, etc yields compounds of type C. Oxidation of the obtained
thioether is accomplished by an appropriate oxidizing agent such as H202,
Oxone,
MCPBA, etc. to yield sulfones D. Deprotection under appropriate conditions,
depending
on the nature of the protection group PG, gives E. E can be transformed into F
by a large
variety of conditions, which depend on the nature of the substituent R1-A-,
and which will
be known to a person skilled in the art. For instance, if R1-A- is an
arylalkylcarbonyl
substituent, the transformation can be accomplished by a reaction of E with
the appropriate
carboxylic acid, activated by one of the various coupling reagents such as BOP-
C1, TBTU,
BOP, PyBop, HATU, EDCl/HOBT, DIC/HOBT; DCC/HOBT, etc. Saponification with a
base such as Li0H, NaOH, KOH or K2CO3, Na2CO3, Cs2CO3, yields compounds G.
Amide coupling of G with "warhead predecessors" 0 or Y is accomplished by one
of the
various coupling reagents such as BOP-C1, TBTU, BOP, PyBop, HATU, EDCl/HOBT,
DIC/HOBT; DCC/HOBT, etc. to give H. Oxidation of H by an oxidizing agent such
as
Dess-Martin Periodinane gives the final product I. It will be appreciated by
the person
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 17 -
skilled in the art that, at different stages of the reaction sequence, groups
R5, R6, and R7
can be transformed. For instance, a halogen can be transformed into an alkoxy
substituent
by the reaction with the appropriate alkcohol in the presence of a base, such
as Cs2CO3.
Scheme 1
SH 5
a R6 PGµ sCOORI
PGµ sCOORI PG R
R µ sCOORI 7
_).... 10 S
R'
OH LG
411 R6
A B R7
C
PGµ sCOORI¨Aµ s
li?COOR' R1 COORI
N7
0=S=0 5 _No. 0=S=0 _ 5 _No. 0=S=0 R5 -NMI"
am R6 a R6
W R ''I R 4111 R6
R7 R7 R7
D E F
R2:13 4 2 R3 n 2 R3
R1¨A\ ,COOH
H2N R R1....A, ___ .K.T.,R4 R1
RN
OH
H OH
H 0
0
R5 0= =0 0= =0
R5 R5
=R6
R7 lei Rs
lei Rs
R7 R7
G
H I
PG = protecting group, e.g., Boc, Cbz, Fmoc
LG = leaving group, e.g. -0S02R
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 18 -
The "warhead predecessors" 0 can be prepared by a variety of conditions, which
may be exemplified by the general synthetic procedures below, named here the
"Boc-
Passerini-Route" (scheme 2), and the "Bn-amide-coupling route" (scheme 3). The
person
skilled in the art will appreciate that compounds 0 can also be prepared by
variations of
these procedures, in particular by procedures involving alternative protection
groups.
A 2-aminoalkanol J is transformed into the corresponding tert-butyl carbamate,
e.g.
by reaction with di-tert-butyl dicarbonate in the presence of a base.
Oxidation under
suitable conditions, such as Swern's conditions, gives aldehyde L. Reaction
with an
isocyanide and acetic acid gives, in a transformation known as Passerini
reaction,
compound M. Saponification by a base sich as NaOH gives compound N, which is
finally
transformed by Boc-deprotection under acidic conditions (e.g., trifluoroacetic
acid), into
the "warhead predecessor" 01.
Scheme 2
2 3 2 3
2 3 R R \ ,R
R OH õ ,?0
OH HN - rir, IN -)1111-
H2N
J K L
2 3 0
R \zR ii 2 3 0
R=??L R2=l3)Z
11 1111, R8 _)110.1 H N N ,R8
-)illw H2N N-R8
0 0 I- A OH H OH H
''0 0
0
M N 01
Alternatively, "warhead predecessor" 0 may be prepared as outlined in scheme 3
("Bn-amide-coupling route").
The reaction of an amino acid P with an excess of benzyl halide in the
presence of a
base gives compound Q, which can be saponified by a base, such as Li0H, NaOH,
KOH
or K2CO3, Na2CO3, Cs2CO3, to compound R. The carboxylic acid function of R is
then
reduced to an aldehyde, as in compound T. This can be accomplished by several
ways, for
instance, by transformation of R into a "Weinreb Amide" S and subsequent
reaction with a
suitable reducing agent, such as LiA1H4 to give T. Reaction with a bisulfite
salt, such as
sodium bisulfite, and a cyanide salt, such as potassium cyanide, gives rise to
a 2-
hydroxynitrile U. Compound U can be saponified under acidic conditions to
carboxylic
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 19 -
acid V. Acid V is then coupled with a primary or secondary amine to give amide
W, using
one of the various coupling reagents such as BOP-C1, TBTU, BOP, PyBop, HATU,
EDCl/HOBT, DIC/HOBT; DCC/HOBT, etc. In a final step, the compound is
deprotected
by hydrogenolysis under appropriate conditions to give "warhead predecessor"
02, e.g. by
reaction with hydrogen under elevated pressure in the presence of a catalyst
such as
Pearlman's catalyst.
Scheme 3
2 3 R2R3
0
RR
R2 \/0 * N COOH
(1=13 * Nr
_21,,. .
H2N 000H 0
* 1110
P Q R
2 3
11110 1110 110
S T U
R2 R3
2 30
R R
= Ncr COO H . NxN.R8
2 3 0
19
OH -311P- OH R -211" H2N R
N
* 110 OH R9
/ W 02
The preparation of benzoxazole-containing "warhead predecessors" can be
accomplished as outlined in scheme 4. A suitably N-protected amino-aldehyde M
is
reacted with a carbanion equivalent of benzoxazole, which may in turn be
prepared by
reaction of benzoxazole with a metallating agent, such as isopropylmagnesium
chloride.
The resulting N-protected aminoalcohol X is then deprotected under the
appopriate
conditions to give "warhead predecessor" 03.
Scheme 4
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 20 -
N 0 2 3
HN)0 02 3 N *
R=1).L. 2 3 N *
R=Iruss
>
0 0 HN 0 icyc, OH H2N 0
OH
M X 03
The invention also relates to a process for the preparation of a compound of
formula
(I) as define above, comprising the reaction of a compound of formula (II)
R
A
I 02
N ii IR._
N R4
SO2 HO
=R5
R7
R6 (1)
in the presence of an oxidant, wherein A and R1 to R7 are as defined above.
Suitable oxidants are known to those skilled in the ert. In particular, the
process of
the invention can be carried out in the presence of Dess-Martin Periodinane,
Cr03, PDC
(pyridinium dichromate), 02/V205, NaI04, Na0C1/2,2,6,6-tetramethylpiperidine-1-
oxyl or
MX (2-iodoxybenzoic acid).
The above process can be performed at a temperature of -20 C to 150 C.
Suitable solvents for the process of the invention are dichloromethane,
pyridine,
toluene, DMSO, acetone, water or acetic acid.
A compound of formula (I), when manufactured according to the above process is
also an object of the invention.
The compounds of formula (I) and their pharmaceutically acceptable salts can
be
used as medicaments (e.g. in the form of pharmaceutical preparations). The
pharmaceutical
preparations can be administered internally, such as orally (e.g. in the form
of tablets,
coated tablets, dragees, hard and soft gelatin capsules, solutions, emulsions
or
suspensions), nasally (e.g. in the form of nasal sprays) or rectally (e.g. in
the form of
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 21 -
suppositories). However, the administration can also be effected parentally,
such as
intramuscularly or intravenously (e.g. in the form of injection solutions).
The compounds of formula (I) and their pharmaceutically acceptable salts can
be
processed with pharmaceutically inert, inorganic or organic adjuvants for the
production of
tablets, coated tablets, dragees and hard gelatin capsules. Lactose, corn
starch or
derivatives thereof, talc, stearic acid or its salts etc. can be used, for
example, as such
adjuvants for tablets, dragees and hard gelatin capsules.
Suitable adjuvants for soft gelatin capsules are, for example, vegetable oils,
waxes,
fats, semi-solid substances and liquid polyols, etc.
Suitable adjuvants for the production of solutions and syrups are, for
example, water,
polyols, saccharose, invert sugar, glucose, etc.
Suitable adjuvants for injection solutions are, for example, water, alcohols,
polyols,
glycerol, vegetable oils, etc.
Suitable adjuvants for suppositories are, for example, natural or hardened
oils,
waxes, fats, semi-solid or liquid polyols, etc.
Moreover, the pharmaceutical preparations can contain preservatives,
solubilizers,
viscosity-increasing substances, stabilizers, wetting agents, emulsifiers,
sweeteners,
colorants, flavorants, salts for varying the osmotic pressure, buffers,
masking agents or
antioxidants. They can also contain still other therapeutically valuable
substances.
The invention thus also relates in particular to the following:
A compound of formula (I) for use as therapeutically active substance;
A pharmaceutical composition comprising a compound of formula (I) and a
therapeutically inert carrier;
The use of a compound of formula (I) for the preparation of medicaments for
the
treatment or prophylaxis of diabetes, atherosclerosis, abdominal aortic
aneurysm,
peripheral arterial disease, cancer, reduction of cardiovascular events in
chronic kidney
disease, diabetic nephropathy, diabetic rethinopathy or age related macular
degeneration;
A compound of formula (I) for the treatment or prophylaxis of diabetes,
atherosclerosis, abdominal aortic aneurysm, peripheral arterial disease,
cancer, reduction of
cardiovascular events in chronic kidney disease, diabetic nephropathy,
diabetic
rethinopathy or age related macular degeneration; and
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 22 -
A method for the treatment or prophylaxis of diabetes, atherosclerosis,
abdominal
aortic aneurysm, peripheral arterial disease, cancer, reduction of
cardiovascular events in
chronic kidney disease, diabetic nephropathy, diabetic rethinopathy or age
related macular
degeneration, which method comprises administering an effective amount of a
compound
of formula (I).
The invention will now be illustrated by the following examples which have no
limiting character.
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 23 -
Examples
Abbreviations
DIPEA: Diisopropyl ethyl amine;
DMF: N,N-Dimethylformamide;
EDC: N-(3-Dimetylaminopropy1)-N'-ethyl-carbodiimide hydrochloride;
Eq: equivalent(s)
HATU: 0-(7-azabenzotriazol-1-y1)-1,1,3,3-tetramethyluronium
hexafluorophosphate;
HOBt: 1-Hydroxybenzotriazole;
LiHMDS: Lithium bis(trimethylsilyl)amide;
MCPBA: 3-Chloroperbenzoic acid;
Satd. : saturated
RT : room temperature;
TBTU: 0-(Benzotriazol-1-y1)-N,N,N',N'-tetramethyluronium terafluoroborate;
THF: Tetrahydrofurane;
TFA: Trifluoroacetic acid
Synthesis of Intermediates
Representative Procedure A ("Boc-Passerini-Route"): Synthesis of intermediate
(25,35)-3-amino-N-cyclopropy1-2-hydroxypentanamide dihydrochloride:
Step Al: (5)-tert-butyl-1-hydroxybutan-2-ylcarbamate
Under an atmosphere of argon, (S)-2-aminobutan-l-ol (5.12 g, 56.3 mmol, Eq:
1.00) was
combined with water (20 ml) and dioxane (20 ml) to give a colorless solution.
At 0 C,
NaOH (2.7 g, 67.5 mmol, Eq: 1.2) and di-tert-butyl dicarbonate (14.7 g, 67.5
mmol, Eq:
1.2) were added, and the reaction was stirred for 2 h at RT. The reaction
mixture was
poured into 50 ml H20 and extracted with Et0Ac (2 x 75 ml) .The organic layers
were
dried over Na2504 and concentrated in vacuo. The title compound was obtained
as a
colorless liquid (11.62 g, quant., MS (m/e) = 190.3 [M+H ]).
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 24 -
Step A2: (S)-tert-butyl 1-oxobutan-2-ylcarbamate
Under an atmosphere of argon, oxalyl chloride (7.01 g, 4.75 ml, 55.3 mmol, Eq:
1.00) was
combined with CH2C12 (100 ml) to give a colorless solution. The reaction was
cooled to -
60 C. Then dimethyl sulfoxide (10.8 g, 9.8 ml, 138 mmol, Eq: 2.5) diluted in
CH2C12
(20m1) was added dropwise at - 60 C. The reaction was stirred for 10 min at -
60 C. Then
(5)-tert-butyl -1-hydroxybutan-2-ylcarbamate (11.62 g, 55.3 mmol, Eq: 1.00)
dissolved in
20m1 CH2C12 was added dropwise at -70 C. The reaction was allowed to warm to -
40 C
for 10 min and then cooled to -70 C again. A solution of triethylamine (15.7
g, 21.6 ml,
155 mmol, Eq: 2.8) in 20 ml CH2C12 was added dropwise. The reaction mixture
was
allowed to warm to room temperature over 2 hours. The reaction mixture was
poured into
100 ml satd. sodium dihydrogenphosphate solution and extracted with ethyl
acetate (2 x
150 mL).The organic layer was back-extracted with brine (1 x 50 mL). The
organic layers
were dried over Na2SO4 and concentrated in vacuo. The title compound was
obtained as a
light yellow oil (11.12 g, quant., MS (m/e) = 188.2 [M+H ]).
Step A3: (2S,35)-3-(tert-butoxycarbonylamino)-1-(cyclopropylamino)-1-oxopentan-
2-y1
acetate
Under an atmosphere of argon, (S)-tert-butyl-1-oxobutan-2-ylcarbamate (650 mg,
3.47
mmol, Eq: 1.00), isocyanocyclopropane (256 mg, 3.82 mmol, Eq: 1.1) and acetic
acid (417
mg, 397 pi, 6.94 mmol, Eq: 2) were combined with CH2C12 (24.0 ml) to give a
light
yellow solution. The reaction was stirred overnight at RT. The solvents were
removed
under reduced pressure. The residue was purified by flash chromatography
(silica gel, 20 g,
20% to 60% Et0Ac in heptane). The title compound was obtained as a brown oil
(1.09 g,
quant., MS (m/e) = 315.4 [M+H 1).
Step A4: tert-butyl (2S,3S)-1-(cyclopropylamino)-2-hydroxy-1-oxopentan-3-
ylcarbamate
Under an atmosphere of argon, (2S,3S)-3-(tert-butoxycarbonylamino)-1-
(cyclopropylamino)-1-oxopentan-2-y1 acetate (1.09 g, 3.47 mmol, Eq: 1.00) and
NaOH 1N
in H20 (5.2 ml, 5.2 mmol, Eq: 1.5) were combined with methanol (10 ml) to give
a
colorless solution. The reaction was stirred for 2 h at RT. The reaction
mixture was poured
into 25 mL H20 and extracted with ethyl acetate (2 x 50 mL). The organic
layers were
dried over Na2504 and concentrated in vacuo. The title compound was obtained
as a light
brown solid (540mg, 57.2 %, MS (m/e) = 273.4 [M+H 1).
Step AS: (2S,35)-3-amino-N-cyclopropy1-2-hydroxypentanamide dihydrochloride
Under an atmosphere of argon, tert-butyl (2S,3S)-1-(cyclopropylamino)-2-
hydroxy-l-
oxopentan-3-ylcarbamate (540mg, 1.98 mmol) and HC1 4N in dioxane (10.4 ml)
were
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 25 -
combined to give a light brown solution. The reaction was stirred for 4 h at
RT. The
solvent was evaporated. The residual solvent was removed under vacuum. The
title
compound was obtained as a brown solid (480 mg, quant., MS (m/e) = 173.2 [M+H
] .
Representative procedure B ("Bn-amide-coupling route"): Synthesis of
intermediate
(25,35)-3-amino-N-cyclopropy1-2-hydroxypentanamide
Step Bl: (S)-benzyl 2-(dibenzylamino)butanoate
Under an atmosphere of argon, sodium hydroxide (15.5 g, 388 mmol, Eq: 2) and
potassium
carbonate (53.6 g, 388 mmol, Eq: 2) were combined with water (300 ml) at 0 C
to give a
colorless solution. (S)-2-Aminobutanoic acid (20 g, 194 mmol, Eq: 1.00) was
added slowly
between 0 - 5 C. The suspension was then heated to 90 C. Then benzyl bromide
(133 g,
92.1 ml, 776 mmol, Eq: 4) was added dropwise. The reaction was stirred
overnight at
90 C. The reaction mixture was poured into water (300 ml) and extracted with
ethyl
acetate. The organic layers were dried over Na2504 and concentrated in vacuo.
The title
compound was obtained as a light yellow liquid (84 g, 98.6 %, MS (m/e) = 374.3
[M+H D .
Step B2: (S)-2-(dibenzylamino)butanoic acid
Under an atmosphere of argon, (S)-benzyl 2-(dibenzylamino)butanoate (84 g, 191
mmol,
Eq: 1.00) was combined with methanol (136 ml) to give a light yellow solution.
Then cold
(0 C) NaOH solution (15.3 g in 150 ml water, 382 mmol, Eq: 2) was added at
once to the
reaction mixture. The reaction was stirred at 90 C for 4 h. Upon cooling to
RT, the
reaction mixture was poured into water (75 ml) and extracted with tert-butyl
methyl ether:
heptane = 1:1 (2x150 ml), to remove the benzylalcohol formed in the
hydrolysis. The basic
aqueous layer was acidified to pH 2 with HC1 (conc.). The aqueous layer was
then
extracted with ethyl acetate (2x250 ml). The organic layers were dried over
Na2504,
filtered and concentrated under reduced pressure. The residual solvent was
removed under
vacuum. The title compound was obtained as a white solid (68.13 g, quant., MS
(m/e) =
284.1 [M+H ], MS (m/e) = 282.3 [M-H1).
Step B3: (S)-2-(dibenzylamino)-N-methoxy-N-methylbutanamide
Under an atmosphere of argon, (S)-2-(dibenzylamino)butanoic acid (27 g, 80.0
mmol, Eq:
1.00) was combined with dichloromethane (250 ml) to give a white suspension.
At 0 C,
TBTU (36.0 g, 112 mmol, Eq: 1.4) was added. The reaction was stirred for 2 h
at 0 C.
Then N-methylmorpholine (24.3 g, 26.4 ml, 240 mmol, Eq: 3) and N,0-
dimethylhydroxylamine hydrochloride (19.5 g, 200 mmol, Eq: 2.5) were added.
The
reaction was stirred overnight at RT. The reaction mixture was extracted with
water (2 x
75 ml) and brine (50 ml). The organic layers were dried over Na2504 and
concentrated in
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 26 -
vacuo.The residual solvent was removed under vacuum. The title compound was
obtained
as a light yellow oil (35.5 g, 95.1 %, MS (m/e) = 327.3 [M+H ]).
Step B4: (S)-2-(dibenzylamino)butanal
Under an atmosphere of argon, at -20 to -30 C, LiA1H4 (2.34 g, 61.8 mmol, Eq:
1.2) was
combined with THF (150 ml) to give a light grey suspension. Then (S)-2-
(dibenzylamino)-
N-methoxy-N-methylbutanamide (24 g, 51.5 mmol, Eq: 1.00), diluted in THF (50
ml) was
added at -30 C. The reaction was stirred for 2.5 h at -30 C. The excess
reagent was
quenched by the dropwise additon of ethyl acetate and water (1/1, -30m1). The
reaction
was vigorously stirred for 15 mm at RT. After filtration through dicalite, the
mixture was
diluted with water (100 ml) and extracted with ethyl acetate. The organic
layers were dried
over Na2504 and concentrated in vacuo. The title compound was obtained as a
light brown
liquid (13.41 g, 97.5 %, MS (m/e) = 268.3 [M+H 1).
Step B5: (2S,35)-3-(dibenzylamino)-2-hydroxypentanenitrile
Under an atmosphere of argon, (S)-2-(dibenzylamino)butanal (8.55 g, 32.0 mmol,
Eq:
1.00) was combined with dioxane (70 ml) to give a light yellow solution. The
reaction was
stirred for 10 mm at 0 C. Then sodium bisulfite in water (40%, 26.5 ml) was
added. The
reaction was stirred for another 10 mm at 0 C. Then potassium cyanide (8.33 g,
128 mmol,
Eq: 4), diluted in water (35 ml) was added. The reaction was stirred overnight
at RT. The
reaction mixture was poured into water (75 ml) and extracted with ethyl
acetate (2 x 75
m1). The organic layers were dried over Na2504 and concentrated in vacuo. The
crude
material was purified by flash chromatography (silica gel, 50 g, 0% to 40%
ethyl acetate in
heptane). The title compound was obtained as a light yellow oil (6.49 g, 68.9
%, MS (m/e)
= 295.3 [M+H 1).
Step B6: (2S,35)-3-(dibenzylamino)-2-hydroxypentanoic acid
Under an atmosphere of argon, (25,35)-3-(dibenzylamino)-2-
hydroxypentanenitrile (7.05
g, 23.9 mmol, Eq: 1.00) and HC1 (37%, 47.2 g, 39.3 ml, 479 mmol, Eq: 20) were
combined to give a light brown solution. The reaction was stirred for 2.5h at
100 C. The
reaction was cooled with an ice bath and adjusted to pH 6 by addition of NaOH
solution.
The reaction was then extracted with dichloromethane and back-extracted with
brine. The
organic layers were dried over Na2504 and concentrated in vacuo. The residual
solvent
was removed under vacuum. The title compound was obtained as a brown foam
(6.85 g,
91.3 %, MS (m/e) = 312.3 [M-H+1, MS (m/e) = 314.0 [M+H 1).
Step B7: (2S,35)-N-cyclopropy1-3-(dibenzylamino)-2-hydroxypentanamide
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 27 -
Under an atmosphere of argon, (2S,3S)-3-(dibenzylamino)-2-hydroxypentanoic
acid (5.85
g, 18.7 mmol, Eq: 1.00), cyclopropanamine (2.13 g, 2.62 ml, 37.3 mmol, Eq: 2)
were
combined with dichloromethane (100 ml) to give a light yellow solution. At 0 C
HOBt
(4.29 g, 28.0 mmol, Eq: 1.5), EDC (5.37 g, 28.0 mmol, Eq: 1.5) and N-
methylmorpholine
(3.78 g, 4.1 ml, 37.3 mmol, Eq: 2) were added to the reaction. The reaction
was stirred
overnight at RT. The reaction mixture was poured into water (150 ml) and
extracted with
dichloromethane. The organic layer was back-extracted with water. The organic
layers
were dried over Na2SO4 and concentrated in vacuo. The crude material was
purified by
flash chromatography (silica gel, 70 g, 0% to 60% ethyl acetate in heptane).
The title
compound was obtained as a light brown crystalline (4.42 g, 67.2 %, MS (m/e) =
353.3
[M+H 1).
Step B8: (2S,35)-3-amino-N-cyclopropy1-2-hydroxypentanamide
(2S,35)-N-Cyclopropy1-3-(dibenzylamino)-2-hydroxypentanamide (4.42 g, 12.5
mmol, Eq:
1.00) was hydrogenated with Pd(OH)2 (20% on carbon, 890 mg, 1.27 mmol, Eq:
0.101) in
methanol (50 ml) under a hydrogen pressure of 3.5 bar for 4 h. The catalyst
was removed
by filtration, and residual solvent was removed under vacuum. The title
compound was
obtained as a light yellow solid (2.09 g, 96.8 %, MS (m/e) = 173.2 [M+H 1).
(2S,3S)-3-Amino-N-cyclopropy1-2-hydroxyhexanamide:
The title compound was prepared in analogy to (25,35)-3-amino-N-cyclopropy1-2-
hydroxypentanamide, Representative Procedure B, using (S)-2-aminopentanoic
acid in the
first step (step B1).
3-Amino-N-cyclopropy1-2-hydroxy-3-methylbutanamide:
The title compound was prepared in analogy to (25,35)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, starting with
tert-butyl
1-hydroxy-2-methylpropan-2-ylcarbamate in the second step (A2).
2-(1-Aminocyclopropy1)-N-cyclopropy1-2-hydroxyacetamide:
The title compound was prepared in analogy to (25,35)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, starting with
tert-butyl
1-formylcyclopropylcarbamate in the third step (A3).
(2S,3S)-3-Amino-N-cyclopropy1-2-hydroxy-4-methylpentanamide dihydrochloride:
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 28 -
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, starting with
(S)-2-
amino-3-methylbutan-l-ol in the first step (Al).
(2S,3S)-3-Amino-N-cyclopropy1-2-hydroxy-5-methylhexanamide dihydrochloride:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, starting with
(S)-2-
amino-4-methylpentan-l-ol in the first step (Al).
(2S,3S)-3-Amino-4-cyclohexyl-N-cyclopropy1-2-hydroxybutanamide
dihydrochloride:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, starting with
(S)-tert-
butyl 1-cyclohexy1-3-hydroxypropan-2-ylcarbamate in the second step (A2).
(2S,3S)-3-Amino-N-butyl-2-hydroxypentanamide dihydrochloride:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, using 1-
isocyanobutane in the third step (A3).
(2S,3S)-3-Amino-N-butyl-2-hydroxyhexanamide dihydrochloride:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, using (S)-2-
aminopentan-l-ol in the first step (Al), and 1-isocyanobutane in the third
step (A3).
(2S,3S)-3-Amino-N-benzy1-2-hydroxypentanamide dihydrochloride:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, using
(isocyanomethyl)benzene in the third step (A3).
(2S,3S)-3-Amino-N-cyclopropy1-2-hydroxybutanamide dihydrochloride:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, starting with
(S)-tert-
butyl 1-hydroxypropan-2-ylcarbamate in the second step (A2).
(2S,3S)-3-Amino-N-benzy1-2-hydroxyhexanamide dihydrochloride:
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 29 -
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, starting with
(S)-2-
aminopentan-l-ol in the first step (Al), and using (isocyanomethyl)benzene in
the third
step (A3).
(2S,3S)-3-Amino-2-hydroxy-N-(pentan-2-yl)pentanamide dihydrochloride:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, using 2-
isocyanopentane in the third step (A3).
(2S,3S)-3-Amino-2-hydroxy-N-(pentan-2-yl)hexanamide dihydrochloride:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, starting with
(S)-2-
aminopentan-l-ol in the first step (Al), and using 2-isocyanopentane in the
third step (A3).
(2S,3S)-3-Amino-2-hydroxy-N-(2,2,2-trifluoroethyl)hexanamide:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide, Representative Procedure B, using (S)-2-aminopentanoic
acid in the
first step (step B1), and 2,2,2-trifluoro-ethylamine in the seventh step (step
B7).
(2S,3S)-3-Amino-2-hydroxy-N-isopropylhexanamide:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide, Representative Procedure B, using (S)-2-aminopentanoic
acid in the
first step (step B1), and isopropylamine in the seventh step (step B7).
(2S,3S)-3-Amino-N-ethyl-2-hydroxypentanamide dihydrochloride:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, using
isocyanoethane
in the third step (A3).
(2S,3S)-3-Amino-N-ethyl-2-hydroxyhexanamide dihydrochloride:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide dihydrochloride, Representative Procedure A, starting with
(S)-2-
aminopentan-l-ol in the first step (Al), and using isocyanoethane in the third
step (A3).
(2S,3S)-3-Amino-N-cyclopropy1-2-hydroxy-N-methylhexanamide:
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 30 -
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide, Representative Procedure B, using (S)-2-aminopentanoic
acid in the
first step (step B1), and N-methylcyclopropanamine in the seventh step (step
B7).
(2S,3S)-3-Amino-2-hydroxy-N-phenethylpentanamide:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide, Representative Procedure B, using 2-phenylethanamine in
the
seventh step (step B7).
(2S,3S)-3-Amino-2-hydroxy-N-(2-(naphthalen-l-yl)ethyl)pentanamide:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide, Representative Procedure B, using 2-(naphthalen-1-
yl)ethanamine in
the seventh step (step B7).
(2S,3S)-3-Amino-2-hydroxy-N-(2-(naphthalen-2-yl)ethyl)pentanamide:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide, Representative Procedure B, using 2-(naphthalen-2-
yl)ethanamine
hydrochloride in the seventh step (step B7).
(2S,3S)-3-Amino-2-hydroxy-N-(naphthalen-1-ylmethyl)pentanamide:
The title compound was prepared in analogy to (2S,3S)-3-amino-N-cyclopropy1-2-
hydroxypentanamide, Representative Procedure B, using naphthalen-l-
ylmethanamine in
the seventh step (step B7).
Preparation of (2S)-2-amino-1-(benzo[d]oxazol-2-yl)butan-1-ol dihydrochloride,
the
"alpha-ketobenzoxazole predecessor" building block
Step 1: tert-butyl (2S)-1-(benzo[d]oxazol-2-y1)-1-hydroxybutan-2-ylcarbamate
Under an atmosphere of argon, benzo[d]oxazole (5 g, 42.0 mmol, Eq: 1.00) was
combined
with THF (100 ml) to give a light yellow solution. At -5 C isopropylmagnesium
chloride
2M in THF (21.0 ml, 42.0 mmol, Eq: 1.00) was added dropwise. The reaction was
stirred
for 1.5 h at -5 C. Then (S)-tert-butyl 1-oxobutan-2-ylcarbamate (4.72 g, 25.2
mmol, Eq:
0.6) diluted in 20m1 THF was added. The reaction was stirred overnight at RT.
Satd.
ammoniumchloride solution (6 ml) was added to the reaction. The THF was
evaporated
under vacuum.The reaction mixture was poured into 50 ml H20 and extracted with
ethyl
acetate (2x100 m1). The crude material was purified by flash chromatography
(silica gel,
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 31 -
20g, 0% to 40% ethyl acetate in heptane). The title compound was obtained as a
brown
gum (5.08 g, 39.5 %, MS (m/e) = 307.2 [M+H 1).
Step 2: (2S)-2-amino-1-(benzo[d]oxazol-2-yl)butan-1-ol dihydrochloride
Under an atmosphere of argon, tert-butyl (25)-1-(benzo[d]oxazol-2-y1)-1-
hydroxybutan-2-
ylcarbamate (800 mg, 2.61 mmol, Eq: 1.00) and HC1 4M in dioxane (17.3 ml) were
combined to give a dark brown solution. The reaction was stirred for 2 h at
RT. The crude
reaction mixture was concentrated in vacuo, and the residual solvent was
removed under
vacuum. The title compound was obtained as a dark brown gum (910 mg, quant.,
MS
(m/e) = 207.1 [M+H ]).
1-(5-Chloro-3-fluoropyridin-2-yl)cyclopropanecarboxylic acid
Step 1: 1-(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbonitrile
Under an atmosphere of argon, 5-chloro-2,3-difluoropyridine (6 g, 4.17 ml,
40.1 mmol,
Eq: 1.00) was combined with toluene (60.0 ml). Then cyclopropanecarbonitrile
(2.69 g,
3.02 ml, 40.1 mmol, Eq: 1.00) was added to the solution. At -5 C, potassium
bis(trimethylsilyl)amide (80.3 ml, 40.1 mmol, Eq: 1.00) was added dropwise
over 30min.
The dark brown reaction was stirred for 1 hour at -5 C, and then for 2h at
room
temperature. The reaction mixture was poured into satd. NH4C1 solution (50 ml)
and
extracted with 2x100 ml ethyl acetate. The organic layer was dried over
Na2SO4, filtered
and concentrated in vacuo. The crude material was purified by flash
chromatography (70 g
column, from 100% to 50% heptane in ethyl acetate). The title compound was
obtained as
a orange oil (1.55 g, 13.8 %, MS (m/e) = 197.2 [M+H].
Step 2: 1-(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarboxylic acid
Under an atmosphere of argon, 1-(5-chloro-3-fluoropyridin-2-
yl)cyclopropanecarbonitrile
(1.55 g, 7.88 mmol, Eq: 1.00) was dissolved in KOH aq. (0.2 M, 43.4 ml, 8.67
mmol, Eq:
1.10). The reaction was stirred overnight at 100 C. The pH of the reaction
mixture was
adjusted to pH 6 bei addition of HC1 (1M). Then the reaction mixture was
extracted with
dichloromethane. The organic layer was dried over Na2SO4, filtered and
concentrated in
vacuo. The crude product was purified by flash chromatography (50 g column,
from 50%
to 100% ethyl acetate in heptane). The title compound was obtained as a orange
solid (800
mg, 47.1 %, MS (m/e) = 216.3 [M+H 1).
1-(5-Bromo-3-fluoropyridin-2-yl)cyclopropanecarboxylic acid
Step 1: 1-(5-bromo-3-fluoropyridin-2-yl)cyclopropanecarbonitrile
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 32 -
Under an atmosphere of argon, 5-bromo-2,3-difluoropyridine (5 g, 25.8 mmol,
Eq: 1.00)
and cyclopropanecarbonitrile (1.78 g, 2.00 ml, 25.8 mmol, Eq: 1.00) were
combined with
toluene (50.0 ml) to give a colorless solution. The reaction mixture was
cooled to -5 C.
Potassium bis(trimethylsilyl)amide (0.5 M, 51.6 ml, 25.8 mmol, Eq: 1.00) was
added
dropwise at -5 C. The reaction mixture was stirred 1 hr at -5 C, and over the
weekend at
RT. The reaction mixture was then poured into NH4C1 solution (satd., 50 ml).
The aqueous
layer was extracted with ethyl acetate (3 x 60 ml). The organic layers were
combined and
dried over Na2SO4 and concentrated in vacuo. The crude material was purified
by flash
chromatography (silica gel, 70 g, 0% to 50% ethyl acetate in heptane as
eluent) to yield the
title compound as a light brown waxy solid (0.49 g, 7.89 %, MS (m/e) =
248.1[M+H D.
Step 2: 1-(5-bromo-3-fluoropyridin-2-yl)cyclopropanecarboxylic acid
Under an atmosphere of argon, 1-(5-bromo-3-fluoropyridin-2-
yl)cyclopropanecarbonitrile
(0.49 g, 2.03 mmol, Eq: 1.00) was combined with KOH solution (1%, 12.5 ml) to
give a
light brown suspension. The reaction mixture was heated to 100 C and stirred
over night.
Upon cooling to RT, HC1 (1N, 3 ml) was added.The mixture was extracted with
dichloromethane (3 x 15 ml). The organic layers were dried over Na2SO4 and
concentrated
in vacuo to yield the title compound as a light brown solid (0.420 g, 61.4 %,
MS (m/e) =
260.1 [M+H 1). The compound was used in the next steps with no further
purification.
Representative Procedure C: (2S,4R)-1-(1-(4-chlorop henypcyclopropanecarbony1)-
4-
(2-chlorophenylsulfonyl)pyrrolidine-2-carboxylic acid
Step Cl: (2S,4S)-1-tert-butyl 2-methyl 4-(3-nitrophenylsulfonyloxy)pyrrolidine-
1,2-
dicarboxylate
Under an atmosphere of argon, (25,45)-1-tert-butyl 2-methyl 4-
hydroxypyrrolidine-1,2-
dicarboxylate (10 g, 39.5 mmol, Eq: 1.00) was combined with dichloromethane
(75 ml) to
give a colorless solution. Then at 0 C, 3-nitrobenzene-1-sulfonyl chloride
(9.58 g, 41.9
mmol, Eq: 1.06) was added. Also at 0 C, triethylamine (12.0 g, 16.5 ml, 119
mmol, Eq:
3.00) was added dropwise to the reaction mixture. The reaction was stirred for
1.5 h at 0 C
and then for 2 days at room temperature. The reaction mixture was diluted in
dichloromethane (200 ml) and then extracted with 2x50 ml HC1 0.5N, 70 ml satd.
NaHCO3 solution and 50 ml satd. NaC1 solution. The organic layer was stirred
over
Na2504, filtered and concentrated under reduced pressure. The title compound
was
obtained as a brown gum (17.25 g, quant., MS (m/e) = 431.3 [M+H ]. The product
was
used in the next step without further purification.
Step C2: (2S,4R)-1-tert-butyl 2-methyl 4-(2-chlorophenylthio)pyrrolidine-1,2-
dicarboxylate
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 33 -
Under an atmosphere of argon, (2S,4S)-1-tert-butyl 2-methyl 4-(3-
nitrophenylsulfonyloxy)pyrrolidine-1,2-dicarboxylate (8.5 g, 19.7 mmol, Eq:
1.00) was
combined with propionitrile (82.0 ml) to give a light brown solution. Then 2-
chlorobenzenethiol (4.28 g, 3.38 ml, 29.6 mmol, Eq: 1.50) and triethylamine
(4.00 g, 5.5
ml, 39.5 mmol, Eq: 2.00) were added dropwise. The reaction was stirred
overnight at
reflux (100 C). The reaction was cooled to room temperature, and poured in 50
ml
Na2CO3 solution (10%), and extracted with 2x100 ml ethyl acetate. The organic
layers
were washed with 50 ml HC1 0.1M and 50 ml satd. NaC1 solution. The organic
layer was
stirred over Na2SO4, filtered and concentrated in vacuo. The crude material
was purified by
flash chromatography (70g column, from 100% to 80% heptane in ethyl acetate).
The title
compound was obtained as a yellow oil (5.7 g, 77.6 %, MS (m/e) = 372.1 [M+H
1).
Step C3: (2S,4R)-1-tert-butyl 2-methyl 4-(2-chlorophenylsulfonyl)pyrrolidine-
1,2-
dicarboxylate
Under an atmosphere of argon, (25,4R)-1-tert-butyl 2-methyl 4-(2-
chlorophenylthio)pyrrolidine-1,2-dicarboxylate (5.7 g, 15.3 mmol, Eq: 1.00)
was combined
with dichloromethane (80.0 ml) to give a light yellow solution. At 0 C, 3-
chlorobenzoperoxoic acid (7.21 g, 32.2 mmol, Eq: 2.10) was added slowly. The
reaction
was stirred overnight at room temperature. The reaction mixture was extracted
with
dichloromethane and washed with 80 ml satd. Na2CO3 solution and 50 ml brine.
The
organic layers were dried over Na2504, filtered and concentrated in vacuo. The
crude
material was purified by flash chromatography (70 g column, from 100% to 60%
heptane
in ethyl acetate). The title compound was obtained as a light yellow gum (5.76
g, 93.0 %,
MS (m/e) = 404.3 [M+H 1).
Step C4: (2S ,4R)-methyl 4-(2-chlorophenylsulfonyl)pyrrolidine-2-carboxylate
Under an atmosphere of argon, (25,4R)-1-tert-butyl 2-methyl 4-(2-
chlorophenylsulfonyl)pyrrolidine-1,2-dicarboxylate (5.76 g, 14.3 mmol, Eq:
1.00) was
combined with dichloromethane (35.0 ml) to give a light yellow solution. At 0
C, 2,2,2-
trifluoroacetic acid (24.4 g, 16.5 ml, 214 mmol, Eq: 15.0) was added dropwise.
The
reaction was warmed up to room temperature and stirred for 3 h. The reaction
mixture was
evaporated to dryness. The crude material was dissolved in 100 ml
dichloromethane, then
extracted with 50 ml satd. Na2CO3 solution and 50 ml H20. The aqueous layers
were
washed with 40 ml dichloromethane. The organic layer was dried over Na2504,
filtered,
and concentrated in vacuo. The title compound was obtained as a orange viscous
oil (4.02
g, 92.8 %, MS (m/e) = 304.2 [M+H 1).
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 34 -
Step C5: (2S ,4R)-methyl 1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfonyl)pyrrolidine-2-carboxylate
Under an atmosphere of argon, 1-(4-chlorophenyl)cyclopropanecarboxylic acid
(1.57 g, 7.9
mmol, Eq: 1.20) was combined with tetrahydrofuran (70.0 ml) to give a
colorless solution.
Then EDC (3.09 g, 15.8 mmol, Eq: 2.40), HOBt (2.47 g, 15.8 mmol, Eq: 2.40) and
triethylamine (6.66 g, 9.18 ml, 65.8 mmol, Eq: 10.0) was added. (A white
suspesion was
obtained.) The reaction mixture was stirred for 15 min at room temperature. A
solution of
(25,4R)-methyl 4-(2-chlorophenylsulfonyl)pyrrolidine-2-carboxylate (2 g, 6.58
mmol, Eq:
1.00) in tetrahydrofuran (14.0 ml) was added dropwise. The reaction was
stirred for 3 days
at room temperature. The reaction mixture was poured into 75 ml HC1 1M and
extracted
with 2x100 ml ethyl acetate. The organic layers were washed with 75 ml satd.
Na2CO3
soluion and 75 ml brine. The organic layer was dried over Na2SO4, filtered and
concentrated in vacuo. The crude material was purified by flash chromatography
(50 g
column, from 0% to 100% ethyl acetate in heptane). The title compound was
obtained as a
light yellow solid (1.5 g, 47.2%, MS (m/e) = 482.3 [M+H 1).
Step C6: (2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfonyl)pyrrolidine-2-carboxylic acid
Under an atmosphere of argon, (25,4R)-methyl 1-(1-(4-
chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-
carboxylate
(1.5 g, 3.11 mmol, Eq: 1.00) was combined with THF (20 ml) to give alight
yellow
solution. Then lithium hydroxide monohydrate (196 mg, 4.66 mmol, Eq: 1.5) in 4
ml water
and 4 ml methanol was added. The reaction was stirred for 2 h at RT. The
solvents were
evaporated. The reaction was extracted with ethyl acetate, H20 and brine. The
organic
layers were dried over Na2SO4 and concentrated in vacuo. The title compound
was
obtained as a white foam (1.52 g, 97.1 %, MS (m/e) = 468.2 [M+H ] ; MS (m/e) =
466.1
[M-H1).
Preparation of (2S,4R)-1-(1-(trifluoromethypcyclopropanecarbony1)-4-(2-
(trifluoromethyl)phenylsulfonyppyrrolidine-2-carboxylic acid
The title compound was prepared in analogy to Representative Procedure C,
using 2-
trifluoromethyl-benzenethiol in step C2 and 1-trifluoromethyl-
cyclopropanecarboxylic acid
/ HATU in step C5.
Preparation of (2S,4R)-1-(1-(5-chloro-3-fluoropyridin-2-
ypcyclopropanecarbony1)-4-
(2-chloro-4-(2,2,2-trifluoroethoxy)phenylsulfonyppyrrolidine-2-carboxylic acid
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 35 -
Step 1: (2S,4R)-1-tert-butyl 2-methyl 4-(2-chloro-4-(2,2,2-
trifluoroethoxy)phenylsulfonyl)
pyrrolidine-1,2-dicarboxylate:
Under an atmosphere of argon, (2S,4R)-1-tert-butyl 2-methyl 4-(2-chloro-4-
fluorophenylsulfonyl)pyrrolidine-1,2-dicarboxylate ([prepared in analogy to
General
Procedure C, steps C1-C3, using 2-chloro-4-fluorothiophenol in step C2] 1.5 g,
1.0 eq.)
was combined with N,N-dimethylacetamide (15 ml) to give a light yellow
solution. 2,2,2-
Trifluoroethanol (854 mg, 617 pi, 8.53 mmol, Eq: 2.4) and cesium carbonate
(2.09 g, 6.4
mmol, Eq: 1.8) were added. The reaction mixture was stirred over night at RT.
The crude
reaction mixture was concentrated in vacuo. The crude material was purified by
flash
chromatography (silica gel, 50 g, 0% to 50% ethyl acetate in heptane) to yield
the title
compound as a white foam (0.95 g, 53.2 %, MS (m/e) = 502.09 [M+H 1).
Step 2: (2S,4R)-1-(1-(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chloro-4-
(2,2,2-trifluoroethoxy)phenylsulfonyl)pyrrolidine-2-carboxylic acid:
The title compound was prepared in analogy to General Procedure C, steps C4-
C6, using
(25,4R)-1-tert-butyl 2-methyl 4-(2-chloro-4-(2,2,2-
trifluoroethoxy)phenylsulfonyl)pyrrolidine-1,2-dicarboxylate in step C4, and 1-
(5-chloro-
3-fluoro-pyridin-2-y1)-cyclopropanecarboxylic acid in step C5. MS (m/e) =
584.9 [M+H ] .
Preparation of (2S,4R)-141-(4-chloro-pheny1)-cyclopropanecarbonyl]-444-(2,2,2-
trifluoro-ethoxy)-2-trifluoromethyl-benzenesulfonyl]-pyrrolidine-2-carboxylic
acid
Step 1: (25,4R)-1-tert-Butyl 2-methyl 4-(4-(2,2,2-trifluoroethoxy)-2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-1,2-dicarboxylate:
Under an atmosphere of argon, (25,4R)-1-tert-butyl 2-methyl 4-(4-fluoro-2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-1,2-dicarboxylate ([prepared in
analogy to
General Procedure C, steps C1-C3, using 2-trifluoromethy1-4-fluorothiophenol
in step C2]
0.79 g, 1.73 mmol, Eq: 1.00) was combined with N,N-dimethylacetamide (10 ml)
to give a
light yellow solution. 2,2,2-Trifluoroethanol (416 mg, 301 pi, 4.16 mmol, Eq:
2.4) and
cesium carbonate (1.02 g, 3.12 mmol, Eq: 1.8) were added. The reaction mixture
was
stirred over night at RT. The crude reaction mixture was concentrated in
vacuo. The
residue was poured into HC1 (0.5 M, 20 ml) and extracted with ethyl acetate.
The organic
layers were dried over Na2504 and concentrated in vacuo. The crude material
was purified
by flash chromatography (silica gel, 50 g, 0% to 40% ethyl acetate in heptane)
to yield the
title compound as a white foam (610 mg, 65.7 %, MS (m/e) = 536.1 [M+H 1).
Step 2: (2S,4R)-1-(1-(4-Chlorophenyl)cyclopropanecarbony1)-4-(4-(2,2,2-
trifluoroethoxy)-
2-(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxylic acid:
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 36 -
The title compound was prepared in analogy to General Procedure C, steps C4-
C6, using
(2S,4R)-1-tert-butyl 2-methyl 4-(4-(2,2,2-trifluoroethoxy)-2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-1,2-dicarboxylate in step C4. MS
(m/e) =
600.0 [M+H 1.
Preparation of (2S,4R)-4-(2-chloro-benzenesulfony1)-141-(4-chloro-2-fluoro-
pheny1)-
cyclopropanecarbonyl]-pyrrolidine-2-carboxylic acid
The title compound was prepared in analogy to Representative Procedure C,
using 1-(4-
chloro-2-fluoro-pheny1)-cyclopropanecarboxylic acid in step C5.
Preparation of (2S,4R)-141-(4-bromo-2-fluoro-pheny1)-cyclopropanecarbony1]-4-
(2-
chloro-benzenesulfony1)-pyrrolidine-2-carboxylic acid
The title compound was prepared in analogy to Representative Procedure C,
using 1-(4-
bromo-2-fluoro-pheny1)-cyclopropanecarboxylic acid in step C5.
Preparation of (2S,4R)-141-(4-Bromo-pheny1)-cyclopropanecarbony1]-4-(2-chloro-
benzenesulfony1)-pyrrolidine-2-carboxylic acid
The title compound was prepared in analogy to Representative Procedure C,
using 1-(4-
bromo-pheny1)-cyclopropanecarboxylic acid in step C5.
Preparation of (2S,4R)-4-(2-chloro-benzenesulfony1)-141-(4-iodo-pheny1)-
cyclopropanecarbonyl]-pyrrolidine-2-carboxylic acid
The title compound was prepared in analogy to Representative Procedure C,
using 1-(4-
iodo-phenyl)-cyclopropanecarboxylic acid in step C5.
Preparation of (2S,4R)-141-(5-chloro-3-fluoro-pyridin-2-y1)-
cyclopropanecarbony1]-
4-(2-trifluoromethyl-benzenesulfony1)-pyrrolidine-2-carboxylic acid
The title compound was prepared in analogy to Representative Procedure C,
using 145-
chloro-3-fluoropyridin-2-yl)cyclopropanecarboxylic acid in step C5 and 2-
(trifluoromethyl)thiophenol in step C2.
Preparation of (2S,4R)-141-(4-chloro-pheny1)-cyclopropanecarbony1]-4-(2-
trifluoromethyl-benzenesulfony1)-pyrrolidine-2-carboxylic acid
The title compound was prepared in analogy to Representative Procedure C,
using 2-
(trifluoromethyl)thiophenol in step C2.
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 37 -
Preparation of (2S,4R)-4-(2-chloro-benzenesulfony1)-1-[1-(5-chloro-3-fluoro-
pyridin-
2-y1)-cyclopropanecarbony1]-pyrrolidine-2-carboxylic acid
The title compound was prepared in analogy to Representative Procedure C,
using 145-
chloro-3-fluoropyridin-2-yl)cyclopropanecarboxylic acid in step C5.
Preparation of (2S,4R)-1-[1-(4-chloro-pheny1)-cyclopropanecarbony1]-4-(2,4-
dichloro-benzenesulfony1)-pyrrolidine-2-carboxylic acid:
The title compound was prepared in analogy to Representative Procedure C,
using 2,4-
dichlorothiophenol in step C2.
Preparation of (2S,4R)-4-(2-chloro-4-fluoro-benzenesulfony1)-1-[1-(4-chloro-
pheny1)-
cyclopropanecarbony1]-pyrrolidine-2-carboxylic acid:
The title compound was prepared in analogy to Representative Procedure C,
using 2-
chloro-4-fluorothiophenol in step C2.
Preparation of (2S,4R)-141-(4-chloro-pheny1)-cyclopropanecarbonyl]-442-chloro-
4-
((S)-2,2,2-trifluoro-1-methyl-ethoxy)-benzenesulfonyl]-pyrrolidine-2-
carboxylic acid:
Step 1: (2S ,4R)-Methyl 4-(2-chloro-4-((S)-1,1,1-trifluoropropan-2-
yloxy)phenylsulfony1)-
1-(1-(4-chlorophenyl)cyclopropanecarbonyl)pyrrolidine-2-carboxylate:
Under an atmosphere of argon, (25,4R)-methyl 4-(2-chloro-4-
fluorophenylsulfony1)-1-(1-
(4-chlorophenyl)cyclopropanecarbonyl)pyrrolidine-2-carboxylate (200 mg, 331
[tmol, Eq:
1.00, [prepared in analogy to General Procedure C, steps C1-05, using 2-chloro-
4-
fluorothiophenol in step C2]) and cesium carbonate (194 mg, 595 [tmol, Eq:
1.8) were
combined with N,N-dimethylacetamide (2 ml) to give a colorless solution. (R)-
1,1,1-
trifluoropropan-2-ol (90.5 mg, 793 [tmol, Eq: 2.4) was added. The reaction
mixture was
stirred over night at rt. The crude reaction mixture was concentrated in
vacuo. The residue
was poured into HC1 (0.5 M, 5 ml) and extracted with ethyl acetate (5 x 20
m1). The
organic layers were dried over Na2504 and concentrated in vacuo. The crude
material was
purified by flash chromatography (silica gel, 50 g, 0% to 40% Et0Ac in
heptane) to yield
the title compound as a white foam (178 mg, 67.1 %, MS (m/e) = 594.07 [M+H 1)
Step 2: (2S,4R)-1-[1-(4-Chloro-pheny1)-cyclopropanecarbony1]-4-[2-chloro-4-
((S)-2,2,2-
trifluoro-1-methyl-ethoxy)-benzenesulfonyll-pyrrolidine-2-carboxylic acid:
The title compound was prepared in analogy to General Procedure C, step C6,
using
(2S ,4R)-methyl 4-(2-chloro-4-((5)-1,1,1-trifluoropropan-2-
yloxy)phenylsulfony1)-1-(1-(4-
chlorophenyl)cyclopropanecarbonyl)pyrrolidine-2-carboxylate.
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 38 -
Example 1
(2S,4R)-tert-butyl 24(S)-1-(cyclopropylamino)-1,2-dioxopentan-3-ylcarbamoy1)-4-
(2,4-dimethylphenylsulfonyl)pyrrolidine-1-carboxylate
0
y 0 0
N õ1(1-IN)y171.
N
Ozszo 0
5 Step 1 ("coupling"): (2S,4R)-tert-butyl 2-((25,35)-1-(cyclopropylamino)-2-
hydroxy-1-
oxopentan-3-ylcarbamoy1)-4-(2,4-dimethylphenylsulfonyl)pyrrolidine-1-
carboxylate
(2S,4R)-1-(tert-butoxycarbony1)-4-(2,4-dimethylphenylsulfonyl)pyrrolidine-2-
carboxylic
acid (120 mg, 313 lamol, Eq: 1.00, prepared as described in U.S. Pat. Appl.
US20100267722), (2S,35)-3-amino-N-cyclopropy1-2-hydroxypentanamide
10 dihydrochloride (99.7 mg, 407 lamol, Eq: 1.3), N,N-diisopropylethylamine
(121 mg, 164
pi, 939 [tmol, Eq: 3) and HATU (202 mg, 532 lamol, Eq: 1.7) were combined with
DMF
(2 ml) to give a light brown solution. The reaction was stirred overnight at
RT. The crude
material was purified by preparative HPLC (column: ymc C18 (120A), 75x30mm,
acetonitrile / water (+0.1% formic acid) = 95%-5% to 5%-95% in 8 min, flow: 25
15 ml/min). After evaporation of solvents, the title compound was obtained
as a light brown
foam (64.3mg, 38.2 %, MS (m/e) = 538.3 [M+H 1).
Step 2 ("oxidation"): (2S,4R)-tert-butyl 2-((S)-1-(cyclopropylamino)-1,2-
dioxopentan-3-
ylcarbamoy1)-4-(2,4-dimethylphenylsulfonyl)pyrrolidine-1-carboxylate
(2S,4R)-tert-butyl 2-((25,35)-1-(cyclopropylamino)-2-hydroxy-1-oxopentan-3-
20 ylcarbamoy1)-4-(2,4-dimethylphenylsulfonyl)pyrrolidine-1-carboxylate
(64mg, 119 lamol,
Eq: 1.00) and Dess-Martin periodinane (572 mg, 202 lamol, Eq: 1.7) were
combined with
CH2C12 (1 m1). The reaction was stirred overnight at RT. Dess-Martin
periodinane (280
mg) was added again and stirred for 5h, until near complete conversion. The
reaction was
extracted with 15 ml CH2C12 and 2x5 ml satd. Na25203. The organic layers were
dried over
25 Na2504 and concentrated in vacuo. The crude material was purified by
preparative HPLC.
The title compound was obtained as a light yellow gum (52.5 mg, 82.3 %, MS
(m/e) =
536.24 [M+H ]).
Example 2
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 39 -
(2S,4R)-tert-butyl 4-(4-chloro-2-methylphenylsulfony1)-24(S)-1-
(cyclopropylamino)-
1,2-dioxopentan-3-ylcarbamoyl)pyrrolidine-1-carboxylate
0
+
Y 0 0
N sokHr1-1
p== N : N---.c
Ozsz0 :\ 0
140
CI
The title compound was prepared in analogy to Example 1, using (2S,4R)-1-(tert-
butoxycarbony1)-4-(4-chloro-2-methylphenylsulfonyl)pyrrolidine-2-carboxylic
acid
(prepared as described in U.S. Pat. Appl. US20100267722) in step 1. MS (m/e) =
556.19
[M+H ].
Example 3
(2S,4R)-N-((S)-1-(cyclopropylamino)-1,2-dioxopentan-3-y1)-1-(2-nitrophenyl)-4-
(2-
1 0 (trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxamide
- 10
lit H 0 H
0, =
S 0
F
SO
F
F 4
The title compound was prepared in analogy to Example 1, using (25,4R)-1-(2-
nitropheny1)-4-(2-(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxylic
acid (prepared
as described in U.S. Pat. Appl. U520100267722) in step 1. MS (m/e) = 597.16
[M+H ] .
Example 4
(2S,4R)-1-(1-cyclobuty1-3-methy1-1H-pyrazol-5-y1)-N-((S)-1-(cyclopropylamino)-
1,2-
dioxopentan-3-y1)-4-(4-fluoro-2-(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-
carboxamide
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 40
I 0
N okhl 0 H
---f
0
0
F,C
The title compound was prepared in analogy to Example 1, using (2S,4R)-1-(1-
cyclobuty1-
3-methy1-1H-pyrazol-5-y1)-N-((2S,3S)-1-(cyclopropylamino)-2-hydroxy-1-
oxopentan-3-
y1)-4-(4-fluoro-2-(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxamide
(prepared as
described in U.S. Pat. Appl. US20100267722) in step 1. MS (m/e) = 628.22 [M+H
1.
Example 5
(2S,4R)-1-(2-Nitro-pheny1)-4-(2-trifluoromethyl-benzenesulfonyl)-pyrrolidine-2-
carboxylic acid [(S)-1-(benzooxazole-2-carbonyl)-propyl]-amide
0= o
H 0
F S740
F
The title compound was prepared in analogy to Example 1, using (25,4R)-1-(2-
nitropheny1)-4-(2-(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxylic
acid (prepared
as described in U.S. Pat. Appl. US20100267722) and (25)-2-amino-1-
(benzo[d]oxazol-2-
yl)butan-1-ol dihydrochloride in step 1. MS (m/e) = 631.15 [M+H 1.
Example 6
(2S,4R)-4-(4-bromo-2-(trifluoromethyl)phenylsulfony1)-1-(1-cyclobutyl-3-methyl-
1H-
pyrazol-5-y1)-N-OS)-1-(cyclopropylamino)-1,2-dioxopentan-3-yppyrrolidine-2-
carboxamide
Cr4r>1.-
ZH
0
0
F,C H
Br
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
-41 -
The title compound was prepared in analogy to Example 1, using (2S,4R)-1-(2-
nitropheny1)-4-(2-(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-carboxylic
acid (prepared
as described in U.S. Pat. Appl. US20100267722) in step 1. MS (m/e) = 690.14
[M+H 1.
Example 7
(2S,4R)-N-((S)-1-(cyclopropylamino)-1,2-dioxopentan-3-y1)-1-(1-
(trifluoromethyl)
cyclopropanecarbony1)-4-(2-(trifluoromethypphenylsulfonyppyrrolidine-2-
carboxamide
FFF 0 cLIRI 0
>,T_4
IN kHN,v
0=S=0 F
F
0 F
The title compound was prepared in analogy to Example 1, using (25,4R)-1-(1-
(trifluoromethyl)cyclopropanecarbony1)-4-(2-
(trifluoromethyl)phenylsulfonyl)pyrrolidine-
2-carboxylic acid in step 1. MS (m/e) = 612.16 [M+H 1.
Example 8
(2S,4R)-1-(1-(4-chlorophenypcyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)-N-
((S)-1-(cyclopropylamino)-1,2-dioxopentan-3-yl)pyrrolidine-2-carboxamide
01 40
0 0, H 0
'-N
A r\17riCro
\ HN,__
0=s=0 V
0 CI
The title compound was prepared in analogy to Example 1, using (25,4R)-1-(1-(4-
chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-
carboxylic
acid in step 1. MS (m/e) = 620.13 [M+H 1.
Example 9
(2S,4R)-1-(1-(4-chlorophenypcyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)-N-
OS)-1-(cyclopropylamino)-1,2-dioxohexan-3-yppyrrolidine-2-carboxamide
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 42 -
CI 00 0 H 0
\,-N 11
A IN nr
o=s=0
0 CI
The title compound was prepared in analogy to Example 1, using (2S,3S)-3-amino-
N-
cyclopropy1-2-hydroxyhexanamide and (2S,4R)-1-(1-(4-
chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-
carboxylic
acid in step 1. MS (m/e) = 634.15 [M+1-1].
Example 10
(2S,4R)-1-(1-(5-chloro-3-fluoropyridin-2-ypcyclopropanecarbony1)-4-(2-chloro-4-
(2,2,2-trifluoroethoxy)phenylsulfony1)-N-OS)-1-(cyclopropylamino)-1,2-
dioxopentan-
3-yppyrrolidine-2-carboxamide
e_
F 0 CLNVNA
\ IN
CI 0=S=0
'CI
0
F-,L
F F
The title compound was prepared in analogy to Example 1, using (25,4R)-1-(1-(5-
chloro-
3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-chloro-4-(2,2,2-
trifluoroethoxy)phenylsulfonyl)pyrrolidine-2-carboxylic acid step 1. MS (m/e)
= 737.12
[M+H ].
Example 11
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-N-((S)-1-(cyclopropylamino)-
1,2-dioxopentan-3-y1)-4-(4-(2,2,2-trifluoroethoxy)-2-
trifluoromethyl)phenylsulfonyl)
pyrrolidine-2-carboxamide
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 43
0
0 0
111P H 0
CI 0=S=0 F
1101 F F
r0
F>L
F F
The title compound was prepared in analogy to Example 1, using (2S,4R)-141-(4-
chloro-
pheny1)-cyclopropanecarbony1]-4-[4-(2,2,2-trifluoro-ethoxy)-2-trifluoromethyl-
benzenesulfonyThpyrrolidine-2-carboxylic acid in step 1. MS (m/e) = 752.16
[M+H 1.
Example 12
(2S,4R)-N-((S)-1-(benzo[d]oxazol-2-y1)-1-oxobutan-2-y1)-1-(1-(4-chlorophenyl)
cyclopropanecarbony1)-4-(2-chlorophenylsulfonyppyrrolidine-2-carboxamide
CI
*
A IQ 0
0=s=0
=01
The title compound was prepared in analogy to Example 1, using 2-amino-1-
benzooxazol-
2-yl-butan-1-ol in step 1. MS (m/e) = 654.12 [M+H 1.
Example 13
(2S,4R)-1-(1-(4-chloro-2-fluorophenypcyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)-1-(cyclopropylamino)-1,2-dioxopentan-3-
yppyrrolidine-
2-carboxamide
CI 4o)
0 0 H
N
A
0=S=0 V
CI
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 44 -
The title compound was prepared in analogy to Example 1, using (2S,4R)-4-(2-
chloro-
benzenesulfony1)-1-[1-(4-chloro-2-fluoro-phenyl)-cyclopropanecarbonyll-
pyrrolidine-2-
carboxylic acid in step 1. MS (m/e) = 638.13 [M+H ].
Example 14
(2S,4R)-1-(1-(4-bromo-2-fluorophenypcyclopropanecarbony1)-4-(2-
chlorophenylsulfonyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxopentan-3-
yppyrrolidine-
2-carboxamide
Br 0000 H 0
y¨N\Ar
A
0=S=0= V
CI
The title compound was prepared in analogy to Example 1, using (25,4R)-141-(4-
bromo-
2-fluoro-pheny1)-cyclopropanecarbony11-4-(2-chloro-benzenesulfony1)-
pyrrolidine-2-
carboxylic acid in step 1. MS (m/e) = 684.08 [M+H 1.
Example 15
(2S,4R)-1-(1-(4-bromophenypcyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)-N-
((S)-1-(cyclopropylamino)-1,2-dioxopentan-3-yl)pyrrolidine-2-carboxamide
Br
0 oJ 0
A o
0=S=0 V
CI
The title compound was prepared in analogy to Example 1, using (25,4R)-141-(4-
bromo-
pheny1)-cyclopropanecarbony11-4-(2-chloro-benzenesulfony1)-pyrrolidine-2-
carboxylic
acid in step 1. MS (m/e) = 666.08 [M+H 1.
Example 16
(2S,4R)-1-(1-(4-bromophenypcyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)-N-
OS)-1-(cyclopropylamino)-1,2-dioxohexan-3-yppyrrolidine-2-carboxamide
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 45 -
Br 0000 H
y¨N\Ar
A 0
0=s=0 )hiN=v
CI
The title compound was prepared in analogy to Example 1, using (2S,4R)-141-(4-
bromo-
pheny1)-cyclopropanecarbony11-4-(2-chloro-benzenesulfony1)-pyrrolidine-2-
carboxylic
acid and (2S,3S)-3-amino-N-cyclopropy1-2-hydroxyhexanamide in step 1. MS (m/e)
=
680.1 [M+H ].
Example 17
(28,4R)-4-(2-chlorophenylsulfony1)-N-08)-1-(cyclopropylamino)-1,2-dioxopentan-
3-
y1)-1-(1-(4-iodophenypcyclopropanecarbonyppyrrolidine-2-carboxamide
I 0 0 H
A 0
irir
0=S=0 V
CI
1 0
The title compound was prepared in analogy to Example 1, using (25,4R)-4-(2-
chloro-
benzenesulfony1)-1-[1-(4-iodo-phenyl)-cyclopropanecarbonyll-pyrrolidine-2-
carboxylic
acid in step 1. MS (m/e) = 712.07 [M+H 1.
Example 18
(28,4R)-4-(2-chlorophenylsulfony1)-N-08)-1-(cyclopropylamino)-1,2-dioxohexan-3-
y1)-1-(1-(4-iodophenypcyclopropanecarbonyppyrrolidine-2-carboxamide
I A
0 H 0
irir
)HN,
0=S=0= v
CI
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 46 -
The title compound was prepared in analogy to Example 1, using (2S,4R)-4-(2-
chloro-
benzenesulfony1)-1-[1-(4-iodo-phenyl)-cyclopropanecarbonyll-pyrrolidine-2-
carboxylic
acid and (2S,3S)-3-amino-N-cyclopropy1-2-hydroxyhexanamide in step 1. MS (m/e)
=
726.09 [M+H 1.
Example 19
(2S,4R)-1-(1-(5-chloro-3-fluoropyridin-2-ypcyclopropanecarbony1)-N-OS)-1-
(cyclopropylamino)-1,2-dioxopentan-3-y1)-4-(2-(trifluoromethyl)
phenylsulfonyl)pyrrolidine-2-carboxamide
ci
I i-N)Lr0
E
0=S=0 F F
F
The title compound was prepared in analogy to Example 1, using (25,4R)-141-(5-
chloro-
3-fluoro-pyridin-2-y1)-cyclopropanecarbony11-4-(2-trifluoromethyl-
benzenesulfony1)-
pyrrolidine-2-carboxylic acid in step 1. MS (m/e) = 673.15 [M+1-11.
Example 20
(2S,4R)-1-(1-(5-chloro-3-fluoropyridin-2-ypcyclopropanecarbony1)-N-OS)-1-
1 5 (cyclopropylamino)-1,2-dioxohexan-3-y1)-4-(2-
(trifluoromethyl)phenylsulfonyl)
pyrrolidine-2-carboxamide
ci
o ohi
0=S=0 F F
F
The title compound was prepared in analogy to Example 1, using (25,4R)-141-(5-
chloro-
3-fluoro-pyridin-2-y1)-cyclopropanecarbony11-4-(2-trifluoromethyl-
benzenesulfony1)-
pyrrolidine-2-carboxylic acid and (2S,35)-3-amino-N-cyclopropy1-2-
hydroxyhexanamide
in step 1. MS (m/e) = 687.17 [M+H ].
Example 21
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 47 -
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-N-((S)-1-(cyclopropylamino)-
1,2-dioxopentan-3-y1)-4-(2-(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-
carboxamide
CI 0 0 H 0
N NyLr0
A (i)
0=S=0 F
F
The title compound was prepared in analogy to Example 1, using (2S,4R)-141-(4-
chloro-
pheny1)-cyclopropanecarbonyll -4- (2-trifluoromethyl-benzenesulfony1)-
pyrrolidine-2-
carboxylic acid in step 1. MS (m/e) = 654.16 [M+H ].
Example 22
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-N-((S)-1-(cyclopropylamino)-
1 0 1,2-dioxohexan-3-y1)-4-(2-(trifluoromethyl)phenylsulfonyl)pyrrolidine-2-
carboxamide
CI 0 0_ H 0
A 1\1,1 ritci
0=S=0 F
SF
The title compound was prepared in analogy to Example 1, using (2S,4R)-141-(4-
chloro-
pheny1)-cyclopropanecarbonyll -4- (2-trifluoromethyl-benzenesulfony1)-
pyrrolidine-2-
carboxylic acid and (25,35)-3-amino-N-cyclopropy1-2-hydroxyhexanamide in step
1. MS
(m/e) = 668.18 [M+H 1.
Example 23
(2S,4R)-1-(1-(5-bromo-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)-1-(cyclopropylamino)-1,2-dioxopentan-3-
yl)pyrrolidine-
2-carboxamide
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
F 0 0___INIVI NA
\ IN
e_
H 0 H
Br 0=S=0
'CI
The title compound was prepared in analogy to Example 1, using 1-(5-bromo-3-
fluoropyridin-2-yl)cyclopropanecarboxylic acid in step 1. MS (m/e) = 685.07
[M+H 1.
Example 24
(2S,4R)-1-(1-(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-
yl)pyrrolidine-
2-carboxamide
ci . F 0 0. FI :1) 0
N IQ ENrci
0=s=0
0 ci
The title compound was prepared in analogy to Example 1, using (25,4R)-4-(2-
chloro-
benzenesulfony1)-1- [1-(5-chloro-3-fluoro-pyridin-2-y1)-cyclopropanecarbonyl] -
pyrrolidine-
2-carboxylic acid and (25,35)-3-amino-N-cyclopropy1-2-hydroxyhexanamide in
step 1.
MS (m/e) = 653.14 [M+H ].
Example 25
(2S,4R)-1-(1-(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)-1-(cyclopropylamino)-1,2-dioxopentan-3-
yl)pyrrolidine-
2-carboxamide
ci F 0 Ra L0
N
0=S=0
'CI
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 49 -
The title compound was prepared in analogy to Example 1, using (2S,4R)-4-(2-
chloro-
benzenesulfony1)-1-[1-(5-chloro-3-fluoro-pyridin-2-y1)-cyclopropanecarbonyll-
pyrrolidine-
2-carboxylic acid in step 1. MS (m/e) = 639.12 [M+1-11.
Example 26
(2S,4R)-1-(1-(4-chlorophenypcyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)-N-
(4-(cyclopropylamino)-2-methyl-3,4-dioxobutan-2-yppyrrolidine-2-carboxamide
CI 0 0
A
0=S=0
=CI
The title compound was prepared in analogy to Example 1, using 3-amino-N-
cyclopropy1-
2-hydroxy-3-methylbutanamide in step 1. MS (m/e) = 620.13 [M+1-11.
Example 27
(2S,4R)-1-(1-(4-chlorophenypcyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)-N-
(1-(2-(cyclopropylamino)-2-oxoacetyl)cyclopropyl)pyrrolidine-2-carboxamide
CI
o 0 7 it
1\ H
A
o=s=o
=01
The title compound was prepared in analogy to Example 1, using 2-(1-
aminocyclopropy1)-
1 5 N-cyclopropy1-2-hydroxyacetamide in step 1. MS (m/e) = 618.12 [M+H 1.
Example 28
(2S,4R)-1-(1-(4-chlorophenypcyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)-N-
((S)-1-(cyclopropylamino)-4-methyl-1,2-dioxopentan-3-yl)pyrrolidine-2-
carboxamide
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 50 -
= ci
O
A i µ,
N
0=s=0
=01
The title compound was prepared in analogy to Example 1, using (2S,3S)-3-amino-
N-
cyclopropy1-2-hydroxy-4-methylpentanamide dihydrochloride in step 1. MS (m/e)
=
632.14 [M-Hl.
Example 29
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(cyclopropylamino)-5-methy1-1,2-dioxohexan-3-yl)pyrrolidine-2-
carboxamide
CI
VI A T
y0
0=s=0
di
The title compound was prepared in analogy to Example 1, (25,35)-3-amino-N-
cyclopropy1-2-hydroxy-5-methylhexanamide dihydrochloride in step 1. MS (m/e) =
646.15
Example 30
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-cyclohexy1-4-(cyclopropylamino)-3,4-dioxobutan-2-yl)pyrrolidine-2-
carboxamide
CI ai
o 0õ
A i\Q
0=S=0
CI
The title compound was prepared in analogy to Example 1, using (25,35)-3-amino-
4-
cyclohexyl-N-cyclopropy1-2-hydroxybutanamide dihydrochloride in step 1. MS
(m/e) =
686.19 [M-Hl.
CA 02854569 2014-05-05
WO 2013/076063
PCT/EP2012/073073
- 51 -
Example 31
(2S,4R)-N-((S)-1-(butylamino)-1,2-dioxopentan-3-y1)-1-(1-(4-chlorophenyl)
cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-carboxamide
CI opO03 H
=)-N
A IQ 0
0=s=0
CI
The title compound was prepared in analogy to Example 1, using (2S,3S)-3-amino-
N-
buty1-2-hydroxypentanamide dihydrochloride in step 1. MS (m/e) = 634.15 [M-F-
11.
Example 32
(2S,4R)-N-((S)-1-(butylamino)-1,2-dioxohexan-3-y1)-1-(1-(4-chlorophenyl)
cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-carboxamide
CI
O03
p-N
A T
0
0=s=0
01
The title compound was prepared in analogy to Example 1, using (25,35)-3-amino-
N-
buty1-2-hydroxyhexanamide dihydrochloride in step 1. MS (m/e) = 648.17 [M-F11.
Example 33
(2S,4R)-N-((S)-1-(benzylamino)-1,2-dioxopentan-3-y1)-1-(1-(4-chlorophenyl)
cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-carboxamide
Cl 0 El,)0rEl
N
A 1\ L
0=s=0
=a
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 52 -
The title compound was prepared in analogy to Example 1, using (2S,3S)-3-amino-
N-
benzy1-2-hydroxypentanamide dihydrochloride in step 1. MS (m/e) = 668.14 EM-H
]
Example 34
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-4-(cyclopropylamino)-3,4-dioxobutan-2-yl)pyrrolidine-2-carboxamide
CI
0=S=0
CI
The title compound was prepared in analogy to Example 1, using (25,35)-3-amino-
N-
cyclopropy1-2-hydroxybutanamide dihydrochloride in step 1. MS (m/e) = 604.11
[M-H ]
Example 35
(2S,4R)-N-((S)-1-(benzylamino)-1,2-dioxohexan-3-y1)-1-(1-(4-chlorophenyl)
cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-carboxamide
CI 0 0 HArH
)¨N N
A r\Q 0
0=S=0
=CI
The title compound was prepared in analogy to Example 1, using (25,35)-3-amino-
N-
benzy1-2-hydroxyhexanamide dihydrochloride in step 1. MS (m/e) = 682.15 EM-H ]
Example 36
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((3S)-1,2-dioxo-1-(pentan-2-ylamino)pentan-3-yl)pyrrolidine-2-carboxamide
CI =
A IQ 0
0=S=0
CI
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 53 -
The title compound was prepared in analogy to Example 1, using (2S,3S)-3-amino-
2-
hydroxy-N-(pentan-2-yl)pentanamide dihydrochloride in step 1. MS (m/e) =
648.17 [M-
H].
Example 37
(28,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
038)-1,2-dioxo-1-(pentan-2-ylamino)hexan-3-yl)pyrrolidine-2-carboxamide
CI140
A 0
0=s=0
CI
The title compound was prepared in analogy to Example 1, using (25,35)-3-amino-
2-
hydroxy-N-(pentan-2-yl)hexanamide dihydrochloride in step 1. MS (m/e) = 664.2
[M-Hl.
Example 38
(28,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-N-08)-1-(cyclopropylamino)-
1,2-dioxopentan-3-y1)-4-(2,4-dichlorophenylsulfonyl)pyrrolidine-2-carboxamide
CI 0 NV A
A H
0=S=0
a
a
The title compound was prepared in analogy to Example 1, using (2S,4R)-1-[1-(4-
chloro-
1 5 phenyl)-cyclopropanecarbony11-4-(2,4-dichloro-benzenesulfony1)-
pyrrolidine-2-carboxylic
acid in step 1. MS (m/e) = 656.09 [M+H ].
Example 39
(28,4R)-4-(2-chloro-4-fluorophenylsulfony1)-1-(1-(4-chlorophenyl)
cyclopropanecarbony1)-N-08)-1-(cyclopropylamino)-1,2-dioxopentan-3-
20 yl)pyrrolidine-2-carboxamide
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 54 -
CI o
19--NreA
H
A
o=s=0
=01
The title compound was prepared in analogy to Example 1, using (2S,4R)-4-(2-
chloro-4-
fluoro-benzenesulfony1)-1-[1-(4-chloro-phenyl)-cyclopropanecarbonyll-
pyrrolidine-2-
carboxylic acid in step 1. MS (m/e) = 638.12 [M+H ].
Example 40
(2S,4R)-1-(1-(4-chlorophenypcyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)-N-
((S)-1,2-dioxo-1-(2,2,2-trifluoroethylamino)hexan-3-yl)pyrrolidine-2-
carboxamide
CI
)-N,Lf0 F
A p
0=S=0
'CI
The title compound was prepared in analogy to Example 1, using (25,35)-3-amino-
2-
hydroxy-N-(2,2,2-trifluoroethyl)hexanamide in step 1. MS (m/e) = 676.12 [M+1-
11.
Example 41
(2S,4R)-1-(1-(4-chlorophenypcyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)-N-
((S)-1-(isopropylamino)-1,2-dioxohexan-3-yl)pyrrolidine-2-carboxamide
= 01 o
A
0=S=0
CI
The title compound was prepared in analogy to Example 1, using (25,35)-3-amino-
2-
hydroxy-N-isopropylhexanamide in step 1. MS (m/e) = 636.17 [M+1-11.
Example 42
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 55 -
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-N-((S)-1-(cyclopropylamino)-
1,2-dioxohexan-3-y1)-4-(2,4-dichlorophenylsulfonyl)pyrrolidine-2-carboxamide
CI. 0(:)A
}---N N
A IQ H 0 H
0=S=0
'CI
CI
The title compound was prepared in analogy to Example 1, using (2S,4R)-1-(1-(4-
chlorophenyl)cyclopropanecarbony1)-4-(2,4-dichlorophenylsulfonyl)pyrrolidine-2-
carboxylic acid and (2S,3S)-3-amino-N-cyclopropy1-2-hydroxyhexanamide in step
1. MS
(m/e) = 670.11 [M+H 1.
Example 43
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
1 0 ((S)-1-(ethylamino)-1,2-dioxopentan-3-yl)pyrrolidine-2-carboxamide
Cl 0o 0 H 0 H
)¨NIN)cNN
A 1\ L 8
0=s=0
'CI
The title compound was prepared in analogy to Example 1, using (25,35)-3-amino-
N-
ethy1-2-hydroxypentanamide dihydrochloride in step 1. MS (m/e) = 606.12 [M-H ]
.
Example 44
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(ethylamino)-1,2-dioxohexan-3-yl)pyrrolidine-2-carboxamide
CI 0o 0 H 0 H
)¨NIN)cNN
A y
o=s=o
'CI
CA 02854569 2014-05-05
WO 2013/076063
PCT/EP2012/073073
- 56 -
The title compound was prepared in analogy to Example 1, using (2S,3S)-3-amino-
N-
ethy1-2-hydroxyhexanamide dihydrochloride in step 1. MS (m/e) = 622.15 [M+H 1.
Example 45
(2S,4R)-4-(2-chloro-44(S)-1,1,1-trifluoropropan-2-yloxy)phenylsulfony1)-1-(1-
(4-
chlorophenyl)cyclopropanecarbony1)-N-((S)-1-(cyclopropylamino)-1,2-dioxopentan-
3-yl)pyrrolidine-2-carboxamide
CI, 0 A
P---N N
1\7
A H H
0
0=s=0
0 ci
FIF
F
The title compound was prepared in analogy to Example 1, using (25,4R)-141-(4-
chloro-
pheny1)-cyclopropanecarbonyll -4- [2-chloro-4- ((S)-2,2,2-trifluoro-1-methyl-
ethoxy)-
benzenesulfonyll-pyrrolidine-2-carboxylic acid in step 1. MS (m/e) = 732.15
[M+H ].
Example 46
(2S,4R)-4-(2-chloro-44(S)-1,1,1-trifluoropropan-2-yloxy)phenylsulfony1)-1-(1-
(4-
chlorophenyl)cyclopropanecarbony1)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-
3-
yppyrrolidine-2-carboxamide
CI, 0 (:),\ dc31( A
IQ
A H H
0
0=s=0
0 a
0
FIss F
F
The title compound was prepared in analogy to Example 1, using (25,35)-3-amino-
N-
cyclopropy1-2-hydroxyhexanamide and (2S,4R)-1-[1-(4-chloro-pheny1)-
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 57 -
cyclopropanecarbony11-4-[2-chloro-44(S)-2,2,2-trifluoro-l-methyl-ethoxy)-
benzenesulfonyll-pyrrolidine-2-carboxylic acid in step 1. MS (m/e) = 746.17
[M+H 1.
Example 47
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(cyclopropyl(methyl)amino)-1,2-dioxohexan-3-yl)pyrrolidine-2-
carboxamide
o 1 0 ON
yN,c0
....r
Al LcHzi
0= S= 0
0 CI
The title compound was prepared in analogy to Example 1, using (25,35)-3-amino-
N-
cyclopropy1-2-hydroxy-N-methylhexanamide in step 1. MS (mile) = 648.17 [M+H ].
Example 48
(2S,4R)-4-(2-chloro-4-fluorophenylsulfony1)-1-(1-(4-chlorophenyl)
cyclopropanecarbony1)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-
y1)pyrrolidine-
2-carboxamide
CI.1---N N
A IQ H 0 H
0=S=0
'CI
F
The title compound was prepared in analogy to Example 1, using (25,35)-3-amino-
N-
cyclopropy1-2-hydroxyhexanamide and (25,4R)-4-(2-Chloro-4-fluoro-
benzenesulfony1)-1-
[1-(4-chloro-pheny1)-cyclopropanecarbonyl]-pyrrolidine-2-carboxylic acid in
step 1. MS
(mile) = 652.14 [M+H ].
Example 49
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1,2-dioxo-1-(phenethylamino)pentan-3-yl)pyrrolidine-2-carboxamide
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 58 -
CI 0 o 0 H
)-N,Lr0
A
IN H N
0
0=S=0
=01
The title compound was prepared in analogy to Example 1, using (2S,3S)-3-amino-
2-
hydroxy-N-phenethylpentanamide in step 1. MS (m/e) = 684.16 [M+H 1.
Example 50
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
OS)-1-(2-(naphthalen-1-ypethylamino)-1,2-dioxopentan-3-yppyrrolidine-2-
carboxamide
CI, o 0 H
)-Nr0
A
IN7 HN fp
0=s=0 ir
0 ci
The title compound was prepared in analogy to Example 1, using (25,35)-3-amino-
2-
hydroxy-N-(2-(naphthalen-1-yl)ethyl)pentanamide in step 1. MS (m/e) = 734.18
[M+H 1.
Example 51
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(2-(naphthalen-2-yl)ethylamino)-1,2-dioxopentan-3-yl)pyrrolidine-2-
carboxamide
ci 0 0
m
A 9 LHN
0=S=0 O.
0 a
The title compound was prepared in analogy to Example 1, using (2S,3S)-3-amino-
2-
hydroxy-N-(2-(naphthalen-2-yl)ethyl)pentanamide in step 1. MS (m/e) = 734.18
[M+H 1.
Example 52
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 59 -
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(naphthalen-l-ylmethylamino)-1,2-dioxopentan-3-yl)pyrrolidine-2-
carboxamide
CI 0O0)
H
YNr0
A IN =HN Ws
0=S=0
'CI
The title compound was prepared in analogy to Example 1, using (2S,3S)-3-amino-
2-
hydroxy-N-(naphthalen-l-ylmethyl)pentanamide in step 1. MS (m/e) = 720.17
[M+Hl.
Example 53
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1,2-dioxo-1-(tetrahydro-2H-pyran-4-ylamino)pentan-3-yl)pyrrolidine-2-
carboxamide
CI 0o H
-N)Lr0
A
0=S=0 (!)
=01
The title compound was prepared in analogy to Example 1, using (25,4R)-4-(2-
Chloro-
benzenesulfony1)-1-[1-(4-chloro-phenyl)-cyclopropanecarbonyll-pyrrolidine-2-
carboxylic
acid and (S)-3-Amino-2-oxo-pentanoic acid (tetrahydro-pyran-4-y1)-am in step
1. MS
(m/e) = 664.16 [M+1-11.
Example 54
(2S,4R)-1-(1-(4-chlorophenyl)cyclopropanecarbony1)-4-(2-chlorophenylsulfony1)-
N-
((S)-1-(2-methoxyethylamino)-1,2-dioxopentan-3-yl)pyrrolidine-2-carboxamide
CI =
o 0 H
,-N,Lr0
A IQ =:HN,.(:)
0=S=0
0 CI
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 60 -
The title compound was prepared in analogy to Example 1, using (2S,4R)-4-(2-
Chloro-
benzenesulfony1)-1-[1-(4-chloro-phenyl)-cyclopropanecarbonyll-pyrrolidine-2-
carboxylic
acid and (S)-3-Amino-2-oxo-pentanoic acid (2-methoxy-ethyl)-a in step 1. MS
(m/e) =
638.15 [M+H ].
Example 55
(2S,4R)-1-(1-(4-chlorophenypcyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)-N-
((S)-1-(isobutylamino)-1,2-dioxopentan-3-yl)pyrrolidine-2-carboxamide
CI. o 0,L
H
-Nr0
A IQ .:HNL
0=S=0
'CI
The title compound was prepared in analogy to Example 1, using (25,4R)-4-(2-
Chloro-
benzenesulfony1)-1-[1-(4-chloro-phenyl)-cyclopropanecarbonyll-pyrrolidine-2-
carboxylic
acid and (S)-3-Amino-2-oxo-pentanoic acid isobutyl-amide in step 1. MS (m/e) =
636.17
[M+1-1].
Example 56
(2S,4R)-4-(2-chloro-4-morpholinophenylsulfony1)-1-(1-(4-
chlorophenypcyclopropanecarbony1)-N-((S)-1-(cyclopropylamino)-1,2-dioxopentan-
3-yppyrrolidine-2-carboxamide
CI, 0
cLNNA
1\ H H
A 0
0=s=0
=01
N
Co)
The title compound was prepared in analogy to Example 1, using (25,4R)-4-(2-
Chloro-4-
morpholin-4-yl-benzenesulfony1)-1-[1-(4-chloro-phenyl)-cyclopropanecarbonyll -
pyrrolidine-2-carboxylic acid and (S)-3-Amino-2-oxo-pentanoic acid
cyclopropylamide in
step 1. MS (m/e) = 705.19 [M+H ].
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 61 -
Example 57
(2S,4R)-4-(2-chloro-4-morpholinophenylsulfony1)-1-(1-(4-
chlorophenyl)cyclopropanecarbony1)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-
3-
yl)pyrrolidine-2-carboxamide
CI 401 0
ON 194--1,(reA
A
H H
0
0=s=0
'CI
N
Co)
The title compound was prepared in analogy to Example 1, using (2S,4R)-4-(2-
Chloro-4-
morpholin-4-yl-benzenesulfony1)-1-[1-(4-chloro-phenyl)-cyclopropanecarbonyll-
pyrrolidine-2-carboxylic acid and (S)-3-Amino-2-oxo-hexanoic acid
cyclopropylamide in
step 1. MS (m/e) = 719.20 [M+H 1.
Example 58
(2S,4R)-1-(1-(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)-1-(cyclopropylamino)-4-methyl-1,2-dioxopentan-3-
yl)pyrrolidine-2-carboxamide
choõ..ri, 0 HArH
, I u
0= S=0
'CI
The title compound was prepared in analogy to Example 1, using (25,4R)-4-(2-
Chloro-
benzenesulfony1)-1-[1-(5-chloro-3-fluoro-pyridin-2-y1)-cyclopropanecarbonyll-
pyrrolidine-
2-carboxylic acid and (S)-3-Amino-4-methyl-2-oxo-pentanoic acid
cyclopropylamide in
step 1. MS (m/e) = 653.14 [M+H 1.
Example 59
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 62 -
(2S,4R)-1-(1-(5-chloro-3-fluoropyridin-2-yl)cyclopropanecarbony1)-4-(2-
chlorophenylsulfony1)-N-((S)-1-(cyclopropylamino)-5-methyl-1,2-dioxohexan-3-
yl)pyrrolidine-2-carboxamide
ch....r.vi... 0 H JiyH
I
N 1\Q 0 V
0=S=0
=01
The title compound was prepared in analogy to Example 1, using (2S,4R)-4-(2-
Chloro-
benzenesulfony1)-1-[1-(5-chloro-3-fluoro-pyridin-2-y1)-cyclopropanecarbonyll-
pyrrolidine-
2-carboxylic acid and (S)-3-Amino-5-methyl-2-oxo-hexanoic acid
cyclopropylamide in
step 1. MS (m/e) = 667.15 [M+H ] .
Example 60
(2S,4R)-N-OS)-1-(butylamino)-1,2-dioxopentan-3-y1)-1-(1-(5-chloro-3-
fluoropyridin-
2-y1)cyclopropanecarbonyl)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-carboxamide
a i Fo cµ4 Cli) H
N
N IQ U
0=S=0
=01
The title compound was prepared in analogy to Example 1, using (25,4R)-4-(2-
Chloro-
benzenesulfonyl )-1-[1-(5-chloro-3-fluoro-pyridin-2-y1)-cyclopropanecarbonyll-
pyrrolidine-2-carboxylic acid and (S)-3-Amino-2-oxo-pentanoic acid butylamide
in step 1.
MS (m/e) = 655.15 [M+H ] .
Example 61
(2S,4R)-N-((S)-1-(butylamino)-1,2-dioxohexan-3-y1)-1-(1-(5-chloro-3-
fluoropyridin-2-
y1)cyclopropanecarbony1)-4-(2-chlorophenylsulfonyl)pyrrolidine-2-carboxamide
a F 0 cLEI 9
N 1\ nr
.)
0=S=0
=01
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 63 -
The title compound was prepared in analogy to Example 1, using (2S,4R)-4-(2-
Chloro-
benzenesulfony1)-1-[1-(5-chloro-3-fluoro-pyridin-2-y1)-cyclopropanecarbonyll-
pyrrolidine-
2-carboxylic acid and (S)-3-Amino-2-oxo-hexanoic acid butylamide in step 1. MS
(m/e) =
669.17 [M+Hl.
Example 62
(2S,4R)-N-OS)-1-(benzylamino)-1,2-dioxohexan-3-y1)-1-(1-(5-chloro-3-
fluoropyridin-
2-ypcyclopropanecarbonyl)-4-(2-chlorophenylsulfonyppyrrolidine-2-carboxamide
a F 0 cL ICI FRI Op
N IQ 7
0=s=0
0 CI
The title compound was prepared in analogy to Example 1, using (25,4R)-4-(2-
Chloro-
benzenesulfony1)-1-[1-(5-chloro-3-fluoro-pyridin-2-y1)-cyclopropanecarbonyll-
pyrrolidine-
2-carboxylic acid and (S)-3-Amino-2-oxo-hexanoic acid benzylamide in step 1.
MS (m/e)
= 703.15 [M+H 1.
Example 63
Cathepsin enzyme inhibition assay
Enzyme activity is measured by observing the increase in fluorescence
intensity caused by
cleavage of a peptide substrate containing a fluorophore whose emission is
quenched in the
intact peptide.
Assay buffer: 100 mM potassium phosphate pH 6.5, EDTA-Na 5 mM, Triton X-100
0.001%, DTT 5 mM.
Enzymes (all at 1 nM): human and mouse Cathepsin S, Cat K, Cat B, Cat L.
Substrate (20 [1.M): Z-Val-Val-Arg-AMC, except for Cat K which uses Z-Leu-Arg-
AMC
(both from Bachem).
Z = Benzyloxycarbonyl.
AMC = 7-Amino-4-Methyl-Coumarin.
DTT = dithiothreitol.
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 64 -
Final volume: 100 p.L.
Excitation 360 nm, Emission 465 nm.
Enzyme is added to the substance dilutions in 96-well microtitre plates and
the reaction is
started with substrate. Fluorescence emission is measured over 20 minutes,
during which
time a linear increase is observed in the absence of inhibitor. IC50 are
calculated by
standard methods.
Inhibition of human Cat S, mouse Cat S, human Cat K, mouse Cat K, human Cat B,
mouse Cat B, human Cat L and mouse Cat L have been measured separately. The
results
obtained for human Cat S, L, K and B for representative compounds of the
invention are
expressed in the following table in 1AM.
1c50 1c50 1c50 1c50
Example cathepsin cathepsin cathepsin cathepsin
1 0.000829 1.9145 7.9045 11.0695
2 0.001122 2.5925 9.4285 22.6235
3 0.001002 1.5505 7.4255 3.181
4 0.002979 0.5508 5.892 2.918
5 0.00183 25 25 105.5
6 0.000976 0.2022 0.1678 1.2985
7 0.003783 3.04 11.252 1.8525
8 0.000296 0.0043 0.0276 0.0627
9 0.000367 0.0047 0.0196 0.0433
10 0.002876 0.0554 0.1183 0.8288
11 0.001379 0.0632 0.3898 0.2911
12 0.000737 0.0212 0.3603 0.1826
CA 02854569 2014-05-05
WO 2013/076063
PCT/EP2012/073073
- 65 -
13 0.000704 0.0113 0.0679 0.1422
14 0.000668 0.0104 0.0373 0.1503
15 0.00052 0.0082 0.0448 0.0985
16 0.000442 0.0059 0.0202 0.0454
17 0.000652 0.0101 0.0301 0.1068
18 0.0006 0.0084 0.021 0.0701
19 0.000593 0.0324 0.7583 0.4243
20 0.00056 0.0185 0.3474 0.2452
21 0.000668 0.0287 0.406 0.165
22 0.000983 0.0266 0.4054 0.1436
23 0.002978 0.0421 0.2988 1.229
24 0.00055 0.0108 0.0717 0.1896
25 0.000403 0.0098 0.0977 0.2514
26 0.010474 3.1045 25 25
27 0.017875 6.795 25 25
28 0.000328 0.0052 0.0382 0.0973
29 0.001265 0.0184 0.069 0.1782
30 0.001417 0.0234 0.1372 0.21
31 0.000827 0.0112 0.0356 0.0478
32 0.000734 0.0077 0.024 0.0294
33 0.001114 0.0136 0.0529 0.077
CA 02854569 2014-05-05
WO 2013/076063
PCT/EP2012/073073
- 66 -
34 0.000787 0.028 0.4437 0.3994
35 0.001213 0.0098 0.0215 0.031
36 0.000776 0.0165 0.1554 0.3495
37 0.001024 0.0151 0.1074 0.2459
38 0.00034 0.0106 0.0533 0.1542
39 0.000558 0.0114 0.0636 0.1397
40 0.00106 0.0299 0.4062 0.3662
41 0.000568 0.0117 0.1522 0.1871
42 0.000634 0.0117 0.0287 0.1154
43 0.000508 0.0136 0.1614 0.1123
44 0.000779 0.0136 0.0918 0.1076
45 0.005426 0.0749 0.212 0.3352
46 0.005982 0.0671 0.251 0.2238
47 0.02226 0.2827 0.9976 1.537
48 0.000527 0.0094 0.035 0.0832
49 0.000901 0.002 0.0344 0.0302
50 0.002758 0.0086 0.1192 0.0688
51 0.003076 0.013 0.1594 0.1724
52 0.001959 0.0094 0.3166 0.3855
53 0.000676 0.0017 0.0393 0.1884
54 0.00063 0.0026 0.082 0.0854
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 67 -
55 0.000876 0.0026 0.0565 0.0616
56 0.002568 0.0206 0.0654 0.1011
57 0.003328 0.0249 0.075 0.0932
58 0.000478 0.0009 0.0491 0.3234
59 0.002608 0.0064 0.1414 0.5185
60 0.002866 0.0061 0.1436 0.3541
61 0.002934 0.0062 0.1062 0.1722
62 0.002348 0.006 0.0412 0.12
The compounds according to the invention have, an 1050 at Cathepsin S or at
Cathepsin L,
or at both Cathepsin S and Cathepsin L, which is 0.00001 and 100 [tM,
preferably between
0.00001 and 50 p,M, more preferably between 0.00001 and 20 M. The particular
compounds of the invention have an 1050 at Cathepsin S or at Cathepsin L, or
at both
Cathepsin S and Cathepsin L below 0.03 1AM.
Example A
A compound of formula (I) can be used in a manner known per se as the active
ingredient
for the production of tablets of the following composition:
Per tablet
Active ingredient 200 mg
Microcrystalline cellulose 155 mg
Corn starch 25 mg
Talc 25 mg
Hydroxypropylmethylcellulose 20 mg
425 mg
CA 02854569 2014-05-05
WO 2013/076063 PCT/EP2012/073073
- 68 -
Example B
A compound of formula (I) can be used in a manner known per se as the active
ingredient
for the production of capsules of the following composition:
Per capsule
Active ingredient 100.0 mg
Corn starch 20.0 mg
Lactose 95.0 mg
Talc 4.5 mg
Magnesium stearate 0.5 mg
220.0 mg