Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
CHEMICALLY PREPARED TONER FORMULATION INCLUDING A BORAX
COUPLING AGENT
CROSS REFERENCES TO RELATED APPLICATIONS
This patent application is related to United States Patent Application Serial
No. 13/339,565 (Attorney Docket No. P7-US1), filed December 29, 2011, entitled
"Process
for Preparing Toner including a Borax Coupling Agent", and assigned to the
assignee of the
present application.
BACKGROUND
[0002] 1. Field of the Disclosure
[0003] The present invention relates generally to chemically prepared toner
for use in
electrophotography and more particularly to chemically prepared toner
including a borax
coupling agent.
[0004] 2. Description of the Related Art
[0005] Toners for use in electrophotographic printers include two
primary types,
mechanically milled toners and chemically prepared toners (CPT). Chemically
prepared
toners have significant advantages over mechanically milled toners including
better print
quality, higher toner transfer efficiency and lower torque properties for
various components
of the electrophotographic printer such as a developer roller, a fuser belt
and a charge roller.
The particle size distribution of CPTs is typically narrower than the particle
size distribution
of mechanically milled toners. The size and shape of CPTs are also easier to
control than
mechanically milled toners.
[0006] There are several known types of CPT including suspension
polymerization
toner (SPT), emulsion aggregation toner (EAT)/latex aggregation toner (LAT),
toner made
from a dispersion of pre-formed polymer in solvent (DPPT) and "chemically
milled" toner.
While emulsion aggregation toner requires a more complex process than other
CPTs, the
resulting toner has a relatively narrower size distribution. Emulsion
aggregation toners can
also be manufactured with a smaller particle size allowing improved print
resolution. The
emulsion aggregation process also permits better control of the shape and
structure of the
1
CA 02858396 2014-06-05
WO 2013/101995
PCMJS2012/071932
toner particles which allows them to be tailored to fit the desired cleaning,
doctoring and
transfer properties. The shape of the toner particles may be optimized to
ensure proper and
efficient cleaning of the toner from various electrophotographic printer
components, such as
the developer roller, charge roller and doctoring blades, in order to prevent
filming or
unwanted deposition of toner on these components.
[0007] In a typical process for preparing EAT, emulsion aggregation is
carried out in
an aqueous system resulting in good control of both the size and shape of the
toner particles.
The toner components typically include a polymer binder, one or more colorants
and a
release agent. A styrene-acrylic copolymer polymer binder is often used as the
latex binder
to in the emulsion aggregation process. However, the use of a styrene-
acrylic copolymer latex
binder requires a tradeoff between the toner's fusing properties and its
shipping and storage
properties. A toner's fusing properties include its fuse window. The fuse
window is the
range of temperatures at which fusing is satisfactorily conducted without
incomplete fusion
and without transfer of toner to the heating element, which may be a roller,
belt or other
member contacting the toner during fusing. Thus, below the low end of the fuse
window the
toner is incompletely melted and above the high end of the fuse window the
toner flows onto
the fixing member where it mars subsequent sheets being fixed. It is preferred
that the low
end of the fuse window be as low as possible to reduce the required
temperature of the fuser
in the electrophotographic printer to improve the printer's safety and to
conserve energy.
However, the toner must also be able to survive the temperature and humidity
extremes
associated with storage and shipping without caking or blocking which may
result in print
flaws. As a result, the low end of the fuse window cannot be so low that the
toner could melt
during the storing or shipping of a toner cartridge containing the toner.
[0008] Toners formed from polyester binder resins typically possess
better
mechanical properties than toners formed from a styrene-acrylic copolymer
binder of similar
melt viscosity characteristics. This makes them more durable and resistant to
filming of
printer components. Polyester toners also have better compatibility with color
pigments
resulting in a wider color gamut. Until recently, polyester binder resins were
frequently used
in preparing mechanically milled toners but rarely in chemically prepared
toners. Polyester
.. hinder resins are manufactured using condensation polymerization. This
method is time
consuming due to the involvement of long polymerization cycles and therefore
limits the use
of polyester binder resins to polyester polymers having low to moderate
molecular weights,
2
which limits the fusing properties of the toner. Further, polyester binder
resins are more
difficult to disperse in an aqueous system due to their polar nature, pH
sensitivity and gel
content thereby limiting their applicability in the emulsion aggregation
process.
[0009] However, with advancement in toner manufacturing technology, it
has become
possible to obtain stable emulsions formed using polyester binder resins by
first dissolving
them in an organic solvent, such as methyl ethyl ketone (MEK), methylene
chloride, ethyl
acetate, or tetrahydrofuran (THF), and then performing a phase-inversion
process where
water is added slowly to the organic solvent. The organic solvent is then
evaporated to allow
the polyester binder resins to form stable emulsions. U.S. Patent No.
7,939,236 entitled
"Chemically Prepared Toner and Process Therefor," which is assigned to the
assignee of the
present application, teaches a similar process for obtaining a stable emulsion
using an organic
solvent. These advances have permitted the use of polyester binder resins to
form emulsion
aggregation toner. For example, U.S. Patent No. 7,923,191 entitled "Polyester
Resin
Produced by Emulsion Aggregation" and U.S. Patent Application Serial No.
12/206,402
entitled "Emulsion Aggregation Toner Formulation," which are assigned to the
assignee of
the present application, disclose processes for preparing emulsion aggregation
toner using
polyester binder resins.
[0010] These techniques provide the ability to produce emulsion
aggregation toner
that possesses excellent fusibility; however, issues related to surface
migration of lower
molecular weight resins, waxes and colorants persist. The migration of these
ingredients to
the surface of the toner particle weakens the toner's fusing and ship/store
properties and
increases the occurrence of filming on printer components. Accordingly, it
will be
appreciated that an emulsion aggregation toner formulation and process that
reduces the
migration of lower molecular weight resins, waxes and colorants to the toner
particle surface
is desired. It is also desired to minimize the overall number of fine toner
particles, which
contribute to filming on the printer components.
SUMMARY
[0011] A chemically prepared toner composition according to one
example
embodiment includes a core including a first polymer binder, a colorant and a
release agent; a
shell that is formed around the core and includes a second polymer binder; and
a borax
coupling agent between the core and the shell.
3
Date Recue/Date Received 2020-06-04
Various embodiments relate to a chemically prepared toner composition,
comprising: a core having an outer surface, the core having components
including a first
polymer binder having functional groups, a colorant and a release agent; a
borax coupling
agent located on the outer surface of the core; and a shell formed around the
outer surface of
the core and the borax coupling agent, the shell including a second polymer
binder having
functional groups, wherein the borax coupling agent is located between the
core and the shell
and bonds the shell to the outer surface of the core by forming hydrogen
bonding between its
hydroxyl groups and the functional groups present in the first and second
polymers and
collects the components of the toner core on the core before the shell is
added thereby
reducing the residual fine particles in the toner.
BRIEF DESCRIPTION OF THE DRAWINGS
[0012] The above-mentioned and other features and advantages of the
various
embodiments, and the manner of attaining them, will become more apparent and
will be
better understood by reference to the accompanying drawings.
[0013] Figure 1 is an image of a conventional emulsion aggregation toner
particle
taken using a scanning electron microscope.
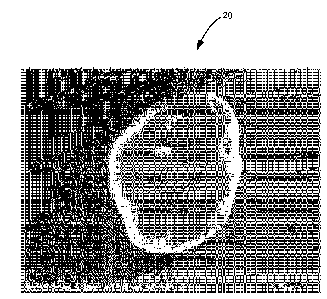
[0014] Figure 2 is an image of an emulsion aggregation toner particle
that includes a
borax coupling agent between core and shell layers of the toner according to
one example
embodiment.
[0015] Figure 3 is a graph depicting the pH adjusting windows for an
emulsion
aggregation toner that includes a borax coupling agent between core and shell
layers of the
toner according to one example embodiment compared to a conventional emulsion
aggregation toner, a toner that includes a zinc sulfate coupling agent and a
toner that includes
an aluminum sulfate coupling agent.
DETAILED DESCRIPTION
[0016] The following description and drawings illustrate embodiments
sufficiently to
enable those skilled in the art to practice the present invention. It is to be
understood that the
disclosure is not limited to the details of construction and the arrangement
of components set
forth in the following description or illustrated in the drawings. The
invention is capable of
4
Date Recue/Date Received 2021-02-12
other embodiments and of being practiced or of being carried out in various
ways. For
example, other embodiments may incorporate structural, chronological, process,
and other
changes. Examples merely typify possible variations. Individual components and
functions
are optional unless explicitly required, and the sequence of operations may
vary. Portions
and features of some embodiments may be included in or substituted for those
of others. The
scope of the application encompasses the appended claims and all available
equivalents. The
following description is, therefore, not to be taken in a limited sense and
the scope of the
present invention is defined by the appended claims. Also, it is to be
understood that the
4a
Date Recue/Date Received 2020-06-04
phraseology and terminology used herein is for the purpose of description and
should not be
regarded as limiting. The use of "including," "comprising," or "having" and
variations
thereof herein is meant to encompass the items listed thereafter and
equivalents thereof as
well as additional items.
[0017] The present disclosure relates to a chemically prepared toner
containing a
borax coupling agent between core and shell layers of the toner and an
associated emulsion
aggregation method of preparation. The toner may be utilized in an
electrophotographic
printer such as a printer, copier, multi-function device or an all-in-one
device. The toner may
be provided in a cartridge that supplies toner to the electrophotographic
printer. Example
methods of forming toner using conventional emulsion aggregation techniques
may be found
in U.S. Patent Nos. 6,531,254 and 6,531,256.
[0018] In the present emulsion aggregation process, the toner
particles are provided
by chemical methods as opposed to physical methods such as pulverization.
Generally, the
toner includes one or more polymer binders, a release agent, a colorant, a
borax coupling
agent and one or more optional additives such as a charge control agent (CCA).
An emulsion
of a polymer binder is formed in water, optionally with organic solvent, with
an inorganic
base such as sodium hydroxide, potassium hydroxide, ammonium hydroxide, or an
organic
amine compound. A stabilizing agent having an anionic functional group (A-),
e.g., an
anionic surfactant or an anionic polymeric dispersant may also be included. It
will be
appreciated that a cationic (C+) functional group, e.g., a cationic surfactant
or a cationic
polymeric dispersant, may be substituted as desired. The polymer latex is used
at two points
during the toner formation process. A first portion of the polymer latex is
used to form the
core of the resulting toner particle and a second portion of the polymer latex
is used to form a
shell around the toner core. The first and second portions of the polymer
latex may be
formed separately or together. Where the portions of the polymer latex forming
the toner
core and the toner shell are formed separately, either the same or different
polymer binders
may be used. The ratio of the amount of polymer binder in the toner core to
the amount of
toner in the shell is between about 20:80 (wt.) and about 80:20 (wt.)
including all values and
increments therebetween, such as between about 50:50 (wt.) and about 80:20
(wt.),
depending on the particular resin(s) used.
5
Date Recue/Date Received 2020-06-04
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
[0019] The colorant, release agent, and the optional CCA are dispersed
separately in
their own aqueous environments or in one aqueous mixture, as desired, in the
presence of a
stabilizing agent having similar functionality (and ionic charge) as the
stabilizing agent
employed in the polymer latex. The polymer latex forming the toner core, the
release agent
dispersion, the colorant dispersion and the optional CCA dispersion are then
mixed and
stirred to ensure a homogenous composition. As used herein, the term
dispersion refers to a
system in which particles are dispersed in a continuous phase of a different
composition (or
state) and may include an emulsion. Acid is then added to reduce the pH and
cause
flocculation. Flocculation refers to the process by which destabilized
particles conglomerate
(due to e.g., the presence of available counterions) into relatively larger
aggregates. In this
case, flocculation includes the formation of a gel where resin, colorant,
release agent and
CCA form an aggregate mixture, typically from particles 1-2 microns (J.tm) in
size. Unless
stated otherwise, reference to particle size herein refers to the largest
cross-sectional
dimension of the particle. The aggregated toner particles may then be heated
to a temperature
that is less than or around (e.g., 5 C) the glass transition temperature (Tg)
of the polymer
latex to induce the growth of clusters of the aggregate particles. Once the
aggregate particles
reach the desired size of the toner core, the borax coupling agent is added so
that it fotins on
the surface of the toner core. Following addition of the borax coupling agent,
the polymer
latex forming the toner shell is added. This polymer latex aggregates around
the toner core to
form the toner shell. Once the aggregate particles reach the desired toner
size, base may be
added to increase the pH and reionize the anionic stabilizing agent to prevent
further particle
growth or one can add additional anionic stabilizing agents. The temperature
is then raised
above the glass transition temperature of the polymer latex(es) to fuse the
particles together
within each cluster. This temperature is maintained until the particles reach
the desired
circularity. The toner particles are then washed and dried.
[0020] The toner particles produced may have an average particle size
of between
about 3i_tm and about 20[tm (volume average particle size) including all
values and
increments therebetvveen, such as between about 41am and about 14tm or, more
particularly,
between about 51.1.m and about 7[1.m. The toner particles produced may have an
average
degree of circularity between about 0.90 and about 1.00, including all values
and increments
therebetween, such as about 0.93 to about 0.98. The average degree of
circularity and
6
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
average particle size may be deteimined by a Sysmex Flow Particle Image
Analyzer (e.g.,
FPIA-3000) available from Malvern Instruments.
[0021] The various components for the emulsion aggregation method to
prepare the
above referenced toner will be described below. It should be noted that the
various features
of the indicated components may all be adjusted to facilitate the step of
aggregation and
foimation of toner particles of desired size and geometry. It may therefore be
appreciated
that by controlling the indicated characteristics, one may first form
relatively stable
dispersions, wherein aggregation may proceed along with relatively easy
control of final
toner particle size for use in an electrophotographic printer or printer
cartridge.
[0022] Polymer Binder
[0023] As mentioned above, the toners herein include one or more
polymer binders.
The terms resin and polymer are used interchangeably herein as there is no
technical
difference between the two. In one embodiment, the polymer binder(s) include
polyesters.
The polyester binder(s) may include a semi-crystalline polyester binder, a
crystalline
polyester binder or an amorphous polyester binder. Alternatively, the
polyester binder(s)
may include a polyester copolymer binder resin. For example, the polyester
binder(s) may
include a styrene/acrylic-polyester graft copolymer. The polyester binder(s)
may be formed
using acid monomers such as terephthalic acid, trimellitic anhydride,
dodecenyl succinic
anhydride and fumaric acid. Further, the polyester binder(s) may be formed
using alcohol
monomers such as ethoxylated and propoxylated bisphenol A. Example polyester
resins
include, but are not limited to, T100, TF-104, NE-1582, NE-701, NE-2141, NE-
1569, Binder
C, FPESL-2, W-85N, TL-17, TPESL-10, TPESL-11 polyester resins from Kao
Corporation,
Bunka Sumida-ku, Tokyo, Japan, or mixtures thereof.
[0024] In other embodiments, the polymer binder(s) include a
thermoplastic type
polymer such as a styrene and/or substituted styrene polymer, such as a
homopolymer (e.g.,
polystyrene) and/or copolymer (e.g., styrene-butadiene copolymer and/or
styrene-acrylic
copolymer, a styrene-butyl methacrylate copolymer and/or polymers made from
styrene-butyl
acrylate and other acrylic monomers such as hydroxy acrylates or hydroxyl
methacrylates);
polyvinyl acetate, polyalkenes, poly(vinyl chloride), polyurethanes,
polyamides, silicones,
epoxy resins, or phenolic resins.
7
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
[0025] As discussed above, in some embodiments, the toner core may be
formed from
one polymer binder (or mixture) and the toner shell formed from another.
Further, the ratio
of the amount of polymer binder in the toner core to the amount of toner in
the toner shell
may be between about 20:80 (wt.) and about 80:20 (wt.) or more specifically
between about
50:50 (wt.) and about 80:20 (wt.) including all values and increments
therebetween. The
total polymer binder may be provided in the range of about 70% to about 95% by
weight of
the final toner formulation including all values and increments therebetween.
[0026] Borax Coupling Agent
[(027] The coupling agent used herein is borax (also known as sodium
borate,
sodium tetraborate, or disodium tetraborate). As used herein the term coupling
agent refers to
a chemical compound having the cross-linking ability to bond two or more
components
together. Typically, coupling agents have multivalent bonding ability. Borax
differs from
commonly used permanent coupling agents, such as multivalent metal ions (e.g.,
aluminum
and zinc), in that its bonding is reversible. In the electrophotographic
process, toner is
preferred to have a low fusing temperature to save energy and a low melt
viscosity ("soft") to
permit high speed printing at low fusing temperatures. However, in order to
maintain the
stability of the toner during shipping and storage and to prevent filming of
the printer
components, toner is preferred to be "harder" at temperatures below the fusing
temperature.
Borax provides cross-linking through hydrogen bonding between its hydroxy
groups and the
functional groups of the molecules it is bonded to. The hydrogen bonding is
sensitive to
temperature and pressure and is not a stable and permanent bond. For example,
when the
temperature is increased to a certain degree or stress is applied to the
polymer, the bond will
partially or completely break causing the polymer to "flow- or tear off. The
reversibility of
the bonds formed by the borax coupling agent is particularly useful in toner
because it
permits a "soft" toner at the fusing temperature but a "hard" toner at the
storage temperature.
[0028] It has also been observed that borax surprisingly causes fine
particles to collect
on larger particles. As a result, borax is particularly suitable as a coupling
agent between the
core and shell layers of the toner because it collects the components of the
toner core to the
core particle before the shell is added thereby reducing the residual fine
particles in the toner.
This, in turn, reduces the amount of acid needed in the agglomeration stage
and narrows the
particle size distribution of the toner.
8
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
[0029] Borax also serves as a good buffer in the toner formation
reaction as a result of
the equilibrium formed by its boric acid and conjugate base. The presence of
borax makes
the reaction more resistant to pH changes and broadens the pH adjusting window
of the
reaction in comparison with a conventional emulsion aggregation process. The
pH adjusting
window is crucial in the industrial scale up of the process to control the
particle size. With a
broader window, the process is easier to control at an industrial scale.
[0030] The quantity of the borax coupling agent used herein can be
varied. The borax
coupling agent may be provided at between about 0.1% and about 5.0% by weight
of the total
polymer binder in the toner including all values and increments therebetween,
such as
between about 0.1% and about 1.0% or between about 0.1% and about 0.5%. If too
much
coupling agent is used, its bonding may not be completely broken at high
temperature fusing.
On the other hand, if too little coupling agent is used, it may fail to
provide the desired
bonding and buffering effects.
[0031] Colorant
[0032] Colorants are compositions that impart color or other visual effects
to the toner
and may include carbon black, dyes (which may be soluble in a given medium and
capable of
precipitation), pigments (which may be insoluble in a given medium) or a
combination of the
two. A colorant dispersion may be prepared by mixing the pigment in water with
a
dispersant. Alternatively, a self-dispersing colorant may be used thereby
permitting omission
of the dispersant. The colorant may be present in the dispersion at a level of
about 5% to
about 20% by weight including all values and increments therebetween. For
example, the
colorant may be present in the dispersion at a level of about 10% to about 15%
by weight.
The dispersion of colorant may contain particles at a size of about 50
nanometers (nm) to
about 500nm including all values and increments therebetween. Further, the
colorant
dispersion may have a pigment weight percent divided by dispersant weight
percent (P/D
ratio) of about 1:1 to about 8:1 including all values and increments
therebetween, such as
about 2:1 to about 5:1. The colorant may be present at less than or equal to
about 15% by
weight of the final toner formulation including all values and increments
therehetween.
[0033] Release Agent
9
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
[0034] The release agent may include any compound that facilitates the
release of
toner from a component in an electrophotographic printer (e.g., release from a
roller surface).
For example, the release agent may include polyolefin wax, ester wax,
polyester wax,
polyethylene wax, metal salts of fatty acids, fatty acid esters, partially
saponified fatty acid
esters, higher fatty acid esters, higher alcohols, paraffin wax, carnauba wax,
amide waxes and
polyhydric alcohol esters.
[(035] The release agent may therefore include a low molecular weight
hydrocarbon
based polymer (e.g., Mn < 10,000) having a melting point of less than about
140 C including
all values and increments between about 50 C and about 140 C. For example, the
release
agent may have a melting point of about 60 C to about 135 C, or from about 65
C to about
100 C, etc. The release agent may be present in the dispersion at an amount of
about 5% to
about 35% by weight including all values and increments therebetween. For
example, the
release agent may be present in the dispersion at an amount of about 10% to
about 18% by
weight. The dispersion of release agent may also contain particles at a size
of about 50nm to
about 1im including all values and increments therebetween. In addition, the
release agent
dispersion may be further characterized as having a release agent weight
percent divided by
dispersant weight percent (RA/D ratio) of about 1:1 to about 30:1. For
example, the RA/D
ratio may he about 3:1 to about 8:1. The release agent may be provided in the
range of about
2% to about 20% by weight of the final toner formulation including all values
and increments
therebetween.
[0036] Surfactant/Dispersant
[(037] A surfactant, a polymeric dispersant or a combination thereof
may be used.
The polymeric dispersant may generally include three components, namely, a
hydrophilic
component, a hydrophobic component and a protective colloid component.
Reference to
hydrophobic refers to a relatively non-polar type chemical structure that
tends to self-
associate in the presence of water. The hydrophobic component of the polymeric
dispersant
may include electron-rich functional groups or long chain hydrocarbons. Such
functional
groups are known to exhibit strong interaction and/or adsorption properties
with respect to
particle surfaces such as the colorant and the polyester binder resin of the
polyester resin
emulsion. llydrophilic functionality refers to relatively polar functionality
(e.g., an anionic
group) which may then tend to associate with water molecules. The protective
colloid
component includes a water soluble group with no ionic function. The
protective colloid
component of the polymeric dispersant provides extra stability in addition to
the hydrophilic
component in an aqueous system. Use of the protective colloid component
substantially
reduces the amount of the ionic monomer segment or the hydrophilic component
in the
polymeric dispersant. Further, the protective colloid component stabilizes the
polymeric
dispersant in lower acidic media. The protective colloid component generally
includes
polyethylene glycol (PEG) groups. The dispersant employed herein may include
the
dispersants disclosed in U.S. Patent No. 6,991,884 and U.S. Patent No.
5,714,538.
[0038] The surfactant, as used herein, may be a conventional
surfactant known in the
art for dispersing non self-dispersing colorants and release agents employed
for preparing
toner formulations for electrophotography. Commercial surfactants such as the
AKYPO
series of carboxylic acids from AKYPO from Kao Corporation, Bunko Sumida-ku,
Tokyo,
Japan may be used. For example, alkyl ether carboxylates and alkyl ether
sulfates, preferably
lauryl ether carboxylates and lauryl ether sulfates, respectively, may be
used. One particular
suitable anionic surfactant is AKYPO RLM-100 available from Kao Corporation,
Bunka
Sumida-ku, Tokyo, Japan, which is laureth-11 carboxylic acid thereby providing
anionic
carboxylate functionality. Other anionic surfactants contemplated herein
include alkyl
phosphates, alkyl sulfonates and alkyl benzene sulfonates. Sulfonic acid
containing polymers
or surfactants may also be employed.
[0039] Optional Additives
[0040] The toner formulation of the present disclosure may also
include one or more
conventional charge control agents, which may optionally be used for preparing
the toner
formulation. A charge control agent may be understood as a compound that
assists in the
production and stability of a tribocharge in the toner. The charge control
agent(s) also help in
preventing deterioration of charge properties of the toner formulation. The
charge control
agent(s) may be prepared in the form of a dispersion in a manner similar to
that of the
colorant and release agent dispersions discussed above.
[0041] The toner formulation may include one or more additional
additives, such as
acids and/or bases, emulsifiers, UV absorbers, fluorescent additives,
pearlescent additives,
plasticizers and combinations thereof These additives may be desired to
enhance the
11
Date Recue/Date Received 2020-06-04
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
properties of an image printed using the present toner formulation. For
example, UV
absorbers may be included to increase UV light fade resistance by preventing
gradual fading
of the image upon subsequent exposures to ultraviolet radiations. Suitable
examples of the
UV absorbers include, but are not limited to, benzophenone, benzotriazole,
acetanilide,
triazine and derivatives thereof. Commercial plasticizers that are known in
the art may also
be used to adjust the coalescening temperature of the toner formulation.
[(042] The following examples are provided to further illustrate the
teachings of the
present disclosure, not to limit the scope of the present disclosure.
[0043] EXAMPLES
[0044] Example Magenta Pigment Dispersion
[0045] About lOg of AKYPO REM-100 polyoxyethylene(10) lauryl ether
carboxylic
acid from Kao Corporation, Bunka Sumida-ku, Tokyo, Japan was combined with
about 350g
of de-ionized water and the pH was adjusted to ¨7-9 using sodium hydroxide.
About lOg of
Solsperse 27000 from Lubrizol Advanced Materials, Cleveland, Ohio, USA was
added and
.. the dispersant and water mixture was blended with an electrical stirrer
followed by the
relatively slow addition of 100g of red 122 pigment. Once the pigment was
completely
wetted and dispersed, the mixture was added to a horizontal media mill to
reduce the particle
size. The solution was processed in the media mill until the particle size was
about 200nm.
The final pigment dispersion was set to contain about 20% to about 25% solids
by weight.
[(046] Example Cyan Pigment Dispersion
[(047] About lOg of AKYPO REM-100 polyoxyethylene(10) lauryl ether
carboxylic
acid from Kao Corporation, Bunka Sumida-ku, Tokyo, Japan was combined with
about 350g
of de-ionized water and the pH was adjusted to ¨7-9 using sodium hydroxide.
About lOg of
Solsperse 27000 from Lubrizol Advanced Materials, Cleveland, Ohio, USA was
added and
the dispersant and water mixture was blended with an electrical stirrer
followed by the
relatively slow addition of 100g of pigment blue 15:3. Once the pigment was
completely
wetted and dispersed, the mixture was added to a horizontal media mill to
reduce the particle
size. The solution was processed in the media mill until the particle size was
about 200nm.
The final pigment dispersion was set to contain about 20% to about 25% solids
by weight.
12
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
[0048] Example Wax Emulsion
[0049] About 12g of AKYPO RLM-100 polyoxyethylene(10) lauryl ether
carboxylic
acid from Kao Corporation, Bunka Sumida-ku, Tokyo, Japan was combined with
about 325g
of de-ionized water and the pII was adjusted to -7-9 using sodium hydroxide.
The mixture
was then processed through a microfluidizer and heated to about 90 C. About
60g of
polyethylene wax from Petrolite, Corp., Westlake, Ohio, USA was slowly added
while the
temperature was maintained at about 90 C for about 15 minutes. The emulsion
was then
removed from the microfluidizer when the particle size was below about 300nm.
The
solution was then stirred at room temperature. The wax emulsion was set to
contain about
10% to about 18% solids by weight.
[0050] Example Polyester Resin Emulsion A
[(051] A mixed polyester resin having a peak molecular weight of about
9,000, a
glass transition temperature (Tg) of about 53 C to about 58 C, a melt
temperature (Tm) of
about 110 C, and an acid value of about 15 to about 20 was used. The glass
transition
temperature is measured by differential scanning calorimetry (DSC), wherein,
in this case,
the onset of the shift in baseline (heat capacity) thereby indicates that the
Tg may occur at
about 53 C to about 58 C at a heating rate of about 5 per minute. The acid
value may be due
to the presence of one or more free carboxylic acid functionalities (¨COOH) in
the
polyester. Acid value refers to the mass of potassium hydroxide (KOH) in
milligrams that is
required to neutralize one gram of the polyester. The acid value is therefore
a measure of the
amount of carboxylic acid groups in the polyester.
[0052] 150g of the mixed polyester resin was dissolved in 450g of
methyl ethyl
ketone (MEK) in a round bottom flask with stirring. The dissolved resin was
then poured
into a beaker. The beaker was placed in an ice bath directly under a
homogenizer. The
homogenizer was turned on at high shear and lOg of 10% potassium hydroxide
(KOH)
solution and 500g of de-ionized water were immediately added to the beaker.
The
homogenizer was run at high shear for about 2-4 minutes then the homogenized
resin solution
was placed in a vacuum distillation reactor. The reactor temperature was
maintained at about
43 C and the pressure was maintained between about 22inHg and about 23inHg.
About
500mL of additional de-ionized water was added to the reactor and the
temperature was
gradually increased to about 70 C to ensure that substantially all of the MEK
was distilled
13
CA 02858396 2014-06-05
WO 2013/101995 PCT/US2012/071932
out. The heat to the reactor was then turned off and the mixture was stirred
until it reached
room temperature. Once the reactor reached room temperature, the vacuum was
turned off
and the resin solution was removed and placed in storage bottles.
[0053] The particle size of the resin emulsion was between about 185nm
and about
235nm (volume average) as measured by a NANOTRAC Particle Size Analyzer. The
pH of
the resin solution was between about 6.5 and about 7Ø
14
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
[0054] Example Polyester Resin Emulsion B
[(055] A polyester resin having a peak molecular weight of about
11,000, a glass
transition temperature of about 55 C to about 60 C, a melt temperature of
about 110 C, and
an acid value of about 15 to about 20 was used to foun an emulsion using the
procedure
described in Example Polyester Resin A, except using 8g of the 10% potassium
hydroxide
(KOH) solution.
[0056] The particle size of the resin emulsion was between about 195nm
and about
235nm (volume average) as measured by a NANOTRAC Particle Size Analyzer. The
pH of
the resin solution was between about 6.7 and about 7.2.
[0057] Example Polyester Resin Emulsion C
[0058] A polyester resin having a peak molecular weight of about
11,000, a glass
transition temperature of about 55 C to about 58 C, a melt temperature of
about 115 C, and
an acid value of about 8 to about 13 was used to form an emulsion using the
procedure
described in Example Polyester Resin A, except using 7g of the 10% potassium
hydroxide
(KOH) solution.
[0059] The particle size of the resin emulsion was between about 190nm
and about
240nm (volume average) as measured by a NANOTRAC Particle Size Analyzer. The
pH of
the resin solution was between about 7.5 and about 8.2.
[0160] Toner Formulation Examples
[0061] Comparative Example Toner I
[0162] Comparative Example Toner I was prepared using a conventional
emulsion
aggregation process and did not include a borax coupling agent. The emulsion
aggregation
CPT used in this example was an acid agglomeration with a pH reversal used to
stop the
growth of the toner particles. Components were added to a 2.5 liter reactor in
the following
relative proportions: 88.2 parts (polyester by weight) of the Example
Polyester Resin
Emulsion A, 6.8 parts (pigment by weight) of the Example Magenta Pigment
Dispersion, and
5 parts (release agent by weight) of the Example Wax Emulsion. Deionized water
was then
added so that the mixture contained about 12.5% solids by weight.
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
[0063] The mixture was heated in the reactor to 30 C and a circulation
loop was
started consisting of a high shear mixer and an acid addition pump. The
mixture was sent
through the loop and the high shear mixer was set at 10,000 revolutions per
minute (rpm).
Acid was slowly added to the high shear mixer to evenly disperse the acid in
the toner
.. mixture so that there were no pockets of low pH. Acid addition took about 4
minutes using
306g of 1% sulfuric acid solution. The flow of the loop was then reversed to
return the toner
mixture to the reactor. The reactor temperature was increased to about 50 C to
grow the
particles. The temperature was held around 50 C until the particles reached
the desired size
(number average size of about 51.1m to about Qum and volume average size of
about 61.1.m to
about 71_1m). Once the particles reached their desired size, 4% NaOH was added
to raise the
pH to 6.00 to stop the particle growth. The reaction was held at about 50 C
for about an hour
and then the temperature was increased to 91 C to cause the particles to
coalesce. The
particles were held at 91 C until the particles reached the desired
circularity (about 0.97).
The toner was then washed and dried.
[0064] The dried toner had a volume average particle size of 6.0 m,
measured by a
COULTER COUNTER Multisizer 3 analyzer. Fines (< 24.tin) were present at 4.16%
(by
number) and the toner possessed a circularity of 0.970, both measured by the
SYSMEX
FPIA-3000 particle characterization analyzer, manufactured by Malvern
Instruments, Ltd.,
Malvern, Worcestershire UK. The amount of fines in Comparative Example Toner I
was
consistent with other emulsion aggregation polyester toners that did not
include a borax
coupling agent, which possessed fines between 1% and 7% (by number).
[0065] Additional toners were made using the formulation and procedure
from the
Comparative Example Toner I, except the neutralization pH was altered to test
the pH
adjusting window. The results of these toners are shown in Table 2 below.
[0066] Example Toner A
[0067] The Example Polyester Resin Emulsion A was divided into two
batches, split
70:30 by weight to form the core and the shell of the toner, respectively. The
total polyester
content represented about 87.7% of the total toner solids. Accordingly, the
first batch
contained 61.4% of the total toner solids and the second batch contained 26.3%
of the total
toner solids. Components were added to a 2.5 liter reactor in the following
percentages: the
16
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
first batch of the Example Polyester Resin Emulsion A having 61.4 parts
(polyester by
weight), 6.8 parts (pigment by weight) of the Example Magenta Pigment
Dispersion, and 5
parts (release agent by weight) of the Example Wax Emulsion. Deionized water
was then
added so that the mixture contained about 12% to about 15% solids by weight.
[0068] The mixture was heated in the reactor to 30 C and a circulation loop
was
started consisting of a high shear mixer and an acid addition pump. The
mixture was sent
through the loop and the high shear mixer was set at 10,000 rpm. Acid was
slowly added to
the high shear mixer to evenly disperse the acid in the toner mixture so that
there were no
pockets of low pH. Acid addition took about 4 minutes with 200g of 1% sulfuric
acid
solution. The flow of the loop was then reversed to return the toner mixture
to the reactor
and the temperature of the reactor was increased to about 40-45 C. Once the
particle size
reached 4.01.tm (number average), 5% (wt.) borax solution (30g of solution
having 1.5g of
borax) was added. The borax content represented about 0.5% by weight of the
total toner
solids. After the addition of borax, the second batch of the Example Polyester
Resin
Emulsion A was added, which contained 26.3 parts (polyester by weight). The
mixture was
stirred for about 5 minutes and the pH was monitored. Once the particle size
reached 5.4tm
(number average), 4% NaOH was added to raise the pH to about 5.95 to stop the
particle
growth. The reaction temperature was held for one hour. The particle size was
monitored
during this time period. Once particle growth stopped, the temperature was
increased to 88 C
to cause the particles to coalesce. This temperature was maintained until the
particles
reached their desired circularity (about 0.97). The toner was then washed and
dried.
[0069] The dried toner had a volume average particle size of 6.651.tm
and a number
average particle size of 5.49p.m. Fines (< 21.tm) were present at 0.11% (by
number) and the
toner possessed a circularity of 0.978.
[0070] Additional toners were made using the formulation and procedure from
the
Example Toner A, except the neutralization pH was altered to test the pH
adjusting window.
The results of these toners are shown in Table 2 below.
[0071] Example Toner B
[0072] The Example Polyester Resin Emulsion A was divided into two
batches, split
60:40 by weight to form the core and the shell of the toner, respectively. The
total polyester
17
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
content represented about 87.9% of the total toner solids. Accordingly, the
first batch
contained 52.7% of the total toner solids and the second batch contained 35.2%
of the total
toner solids. Components were added to a 2.5 liter reactor in the following
percentages: the
first batch of the Example Polyester Resin Emulsion A having 52.7 parts
(polyester by
weight), 6.8 parts (pigment by weight) of the Example Magenta Pigment
Dispersion and 5
parts (release agent by weight) of the Example Wax Emulsion. Deionized water
was then
added so that the mixture contained about 12% to about 15% solids by weight.
[0073] The mixture was heated in the reactor to 30 C and a circulation
loop was
started consisting of a high shear mixer and an acid addition pump. The
mixture was sent
l() through the loop and the high shear mixer was set at 10,000 rpm. Acid
was slowly added to
the high shear mixer to evenly disperse the acid in the toner mixture so that
there were no
pockets of low pH. Acid addition took about 4 minutes with 150g of 1% sulfuric
acid
solution. The flow of the loop was then reversed to return the toner mixture
to the reactor
and the temperature of the reactor was increased to about 40-45 C. Once the
particle size
reached 4.0[rm (number average), 5% borax solution (15g of solution having
0.75g borax)
was added. The borax content represented about 0.3% by weight of the total
toner solids.
After the addition of borax, the second batch of the Example Polyester Resin
Emulsion A was
added, which contained 35.2 parts (polyester by weight). The mixture was
stirred for about 5
minutes and the pH was monitored. Once the particle size reached 5.51.rm
(number average),
4% NaOH was added to raise the pH to about 5.95 to stop the particle growth.
The reaction
temperature was held for one hour. The particle size was monitored during this
time period.
Once particle growth stopped, the temperature was increased to 88 C to cause
the particles to
coalesce. This temperature was maintained until the particles reached their
desired circularity
(about 0.97). The toner was then washed and dried.
[0074] The dried toner had a volume average particle size of 6.241.rm and a
number
average particle size of 5.48j.rm. Fines (< 21.rm) were present at 0.09% (by
number) and the
toner possessed a circularity of 0.983.
[0075] Example Toner C
[0076] A combination of Example Polyester Resin Emulsion A and Example
Polyester Resin Emulsion C was used in a 70:30 ratio by weight to form the
core and the
18
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
shell of the toner, respectively. The total polyester content represented
about 87.9% of the
total toner solids. Accordingly, Example Polyester Resin Emulsion A contained
61.5% of the
total toner solids and Example Polyester Resin Emulsion C contained 26.4% of
the total toner
solids. Components were added to a 2.5 liter reactor in the following
percentages: Example
Polyester Resin Emulsion A having 61.5 parts (polyester by weight), 6.8 parts
(pigment by
weight) of the Example Magenta Pigment Dispersion and 5 parts (release agent
by weight) of
the Example Wax Emulsion. Deionized water was then added so that the mixture
contained
about 12% to about 15% solids by weight.
[0077] The mixture was heated in the reactor to 30 C and a circulation
loop was
started consisting of a high shear mixer and an acid addition pump. The
mixture was sent
through the loop and the high shear mixer was set at 10,000 rpm. Acid was
slowly added to
the high shear mixer to evenly disperse the acid in the toner mixture so that
there were no
pockets of low pH. Acid addition took about 4 minutes with 200g of 1% sulfuric
acid
solution. The flow of the loop was then reversed to return the toner mixture
to the reactor
and the temperature of the reactor was increased to about 37-42 C. Once the
particle size
reached 4.0pm (number average), 5% (wt.) borax solution (15g of solution
having 0.75g of
borax) was added. The borax content represented about 0.25% by weight of the
total toner
solids. After the addition of borax, the Example Polyester Resin Emulsion C
was added,
which contained 26.4 parts (polyester by weight). The mixture was stirred for
about 5
minutes and the pH was monitored. Once the particle size reached 5.51.tm
(number average),
4% NaOH was added to raise the pH to about 6.60 to stop the particle growth.
The reaction
temperature was held for one hour. The particle size was monitored during this
time period.
Once particle growth stopped, the temperature was increased to 88 C to cause
the particles to
coalesce. This temperature was maintained until the particles reached their
desired circularity
(about 0.97). The toner was then washed and dried.
[0078] The dried toner had a volume average particle size of 6.40 m and
a number
average particle size of 5.181.tm. Fines (< 2pm) were present at 0.92% (by
number) and the
toner possessed a circularity of 0.970.
[0079] Example Toner D
19
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
[0080] A combination of Example Polyester Resin Emulsion A and an
emulsion of
ACT-004 polyester resin available from Toyobo Co., Ltd., Kita-ku, Osaka, Japan
was used in
a 70:30 ratio by weight to foim the core and the shell of the toner,
respectively. The ACT-
004 polyester resin had a peak molecular weight of about 11,000, a glass
transition
temperature of about 57 C to about 61 C, a melt temperature of about 104 C,
and an acid
value of about 16. The emulsion particle size was about 200nm (volume
average). The total
polyester content represented about 87.9% of the total toner solids.
Accordingly, Example
Polyester Resin Emulsion A contained 61.5% of the total toner solids and the
ACT-004
polyester emulsion contained 26.4% of the total toner solids. Components were
added to a
2.5 liter reactor in the following percentages: Example Polyester Resin
Emulsion A having
61.5 parts (polyester by weight), 6.8 parts (pigment by weight) of the Example
Magenta
Pigment Dispersion and 5 parts (release agent by weight) of the Example Wax
Emulsion.
Deionized water was then added so that the mixture contained about 12% to
about 15% solids
by weight.
[0081] The mixture was heated in the reactor to 30 C and a circulation loop
was
started consisting of a high shear mixer and an acid addition pump. The
mixture was sent
through the loop and the high shear mixer was set at 10,000 rpm. Acid was
slowly added to
the high shear mixer to evenly disperse the acid in the toner mixture so that
there were no
pockets of low pH. Acid addition took about 4 minutes with 200g of 1% sulfuric
acid
solution. The flow of the loop was then reversed to return the toner mixture
to the reactor
and the temperature of the reactor was increased to about 35-40 C. Once the
particle size
reached 4.0mm (number average), 5% (wt.) borax solution (15g of solution
having 0.75g of
borax) was added. The borax content represented about 0.25% by weight of the
total toner
solids. After the addition of borax, the ACT-004 polyester resin emulsion was
added, which
contained 26.4 parts (polyester by weight). The mixture was stirred for about
5 minutes and
the pH was monitored. Once the particle size reached 5.51,1m (number average),
4% NaOH
was added to raise the pH to about 6.20 to stop the particle growth. The
reaction temperature
was held for one hour. The particle size was monitored during this time
period. Once
particle growth stopped, the temperature was increased to 88 C to cause the
particles to
coalesce. This temperature was maintained until the particles reached their
desired circularity
(about 0.97). The toner was then washed and dried.
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
[0082] The dried toner had a volume average particle size of 6.18pm and
a number
average particle size of 5.28pm. Fines (< 2p,m) were present at 0.42% (by
number) and the
toner possessed a circularity of 0.973.
[0083] Example Toner E
[0084] The Example Polyester Resin Emulsion B was divided into two batches,
split
70:30 by weight to form the core and the shell of the toner, respectively. The
total polyester
content represented about 87.9% of the total toner solids. Accordingly, the
first batch
contained 61.5% of the total toner solids and the second batch contained 26.4%
of the total
toner solids. Components were added to a 2.5 liter reactor in the following
percentages: the
first batch of Example Polyester Resin Emulsion B having 61.5 parts (polyester
by weight),
6.8 parts (pigment by weight) of the Example Magenta Pigment Dispersion, and 5
parts
(release agent by weight) of the Example Wax Emulsion. Deionized water was
then added so
that the mixture contained about 12% to about 15% solids by weight.
[0085] The mixture was heated in the reactor to 30 C and a circulation
loop was
started consisting of a high shear mixer and an acid addition pump. The
mixture was sent
through the loop and the high shear mixer was set at 10,000 rpm. Acid was
slowly added to
the high shear mixer to evenly disperse the acid in the toner mixture so that
there were no
pockets of low pH. Acid addition took about 4 minutes with 200g of 1% sulfuric
acid
solution. The flow of the loop was then reversed to return the toner mixture
to the reactor
and the temperature of the reactor was increased to about 40-45 C. Once the
particle size
reached 5.0 m (number average), 5% (wt.) borax solution (15g of solution
having 0.75g of
borax) was added. The borax content represented about 0.25% by weight of the
total toner
solids. After the addition of borax, the second batch of Example Polyester
Resin Emulsion B
was added, which contained 26.4 parts (polyester by weight). The mixture was
stiffed for
about 5 minutes and the pH was monitored. Once the particle size reached
5.51.tm (number
average), 4% NaOH was added to raise the pH to about 7.10 to stop the particle
growth. The
reaction temperature was held for one hour. The particle size was monitored
during this time
period. Once particle growth stopped, the temperature was increased to 88 C to
cause the
particles to coalesce. This temperature was maintained until the particles
reached their
desired circularity (about 0.97). The toner was then washed and dried.
21
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
[0086] The dried toner had a volume average particle size of 7.2411m
and a number
average particle size of 5.86 m. Fines (< 24im) were present at 1.76% (by
number) and the
toner possessed a circularity of 0.974.
[0087] Accordingly, it can be seen that the emulsion aggregation
process used to
prepare Example Toners A thru E, which included a borax coupling agent between
core and
shell layers of the toner particles, significantly reduced the percentage of
fine particles in
comparison with the conventional emulsion aggregation process used to prepare
Comparative
Example Toner I. Further, Example Toners A thru E each exhibit a comparable
average
particle size and circularity relative to Comparative Example Toner I as
desired.
[0088] TEST RESULTS
[0089] Surface Migration
[0090] Figure 1 shows an image of a conventional emulsion aggregation
toner
particle 10 prepared according to Comparative Example I taken using a scanning
electron
microscope (SEM). Figure 2 shows an image of an emulsion aggregation toner
particle 20
prepared according to Example A that includes a borax coupling agent between
the core and
shell layers of the toner. As illustrated, toner particle 20 has a smoother,
more uniform
surface than conventional emulsion aggregation toner particle 10. The smooth,
uniform
surface of toner particle 20 reduces the occurrence of filming on the
developer roller and
improves the toner's fusing performance at higher temperatures. In contrast,
toner particle 10
has significantly more colorant, release agent and low molecular weight resin
particles 12 that
have migrated to its surface. As discussed above, borax surprisingly causes
these particles to
collect on the toner core before the shell layer is added, which prevents them
from migrating
to the toner surface.
[0091] Developer Roller and Doctor Blade Filming
[0092] The developer roller and doctor blade filming of Example Toners A
and B and
Comparative Example Toner I were also tested. The toners were each placed in a
toner
cartridge. Each cartridge was then inserted into a testing robot and run at 50
ppm.
Periodically, each cartridge's developer roller and doctor blade were visually
examined to
assess the amount of toner filming on the components. The level of toner
filming was graded
22
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
on a scale of 1 to 4, where a higher grade (e.g., 4) indicates more filming
and poorer
performance. The testing results are shown in Table 1 below.
TABLE 1
Developer Roll Filming Doctor Blade
Filming
No. of Comparative Toner A Toner B Comparative Toner A Toner B
Pages Ex. Toner I Ex. Toner I
0 0 0 0 0 0 0
500 1 0 1 0 0 0
1,000 2 1 1 0 0 0
1,500 2 1 1 0 0 0
2,000 3 2 1 0 1 1
3,000 3 3 3 1 1 3
4,000 3 4 4 2 2 3
5,000 4 - - 2 - -
[0093] As shown in
Table 1, Example Toners A and B, which included a borax
coupling agent, exhibited improved resistance to developer roll filming and
comparable
resistance to doctor blade filming in comparison with Comparative Example
Toner I.
[0094] In order to further evaluate the performance of the borax
coupling agent,
additional comparative example toners were prepared using a zinc sulfate and
an aluminum
sulfate coupling agent, respectively, between core and shell layers of the
toner.
[0095] Comparative Example Toner II
[0096] Comparative Example Toner II was prepared using a zinc sulfate
coupling
agent instead of a borax coupling agent. The Example Polyester Resin Emulsion
A was
divided into two batches, split 70:30 by weight to form the core and the shell
of the toner,
respectively. The total polyester content represented about 90.3% of the total
toner solids.
Accordingly, the first batch contained 63.2% of the total toner solids and the
second batch
contained 27.1% of the total toner solids. Components were added to a 2.5
liter reactor in the
following percentages: the first batch of the Example Polyester Resin Emulsion
A having
63.2 parts (polyester by weight), 4.4 parts (pigment by weight) of the Example
Cyan Pigment
Dispersion, and 5 parts (release agent by weight) of the Example Wax Emulsion.
Deionized
23
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
water was then added so that the mixture contained about 12% to about 15%
solids by
weight.
[0097] The mixture was heated in the reactor to 30 C and a circulation
loop was
started consisting of a high shear mixer and an acid addition pump. The
mixture was sent
through the loop and the high shear mixer was set at 10,000 rpm. Acid was
slowly added to
the high shear mixer to evenly disperse the acid in the toner mixture so that
there were no
pockets of low pH. Acid addition took about 4 minutes with 175g of 1% sulfuric
acid
solution. The flow of the loop was then reversed to return the toner mixture
to the reactor
and the temperature of the reactor was increased to about 40-45 C. Once the
particle size
reached 4.0ittm (number average), 5% (wt.) zinc sulfate solution (18g of
solution having 0.9g
of zinc sulfate) was added. The zinc sulfate content represented about 0.3% by
weight of the
total toner solids. After the addition of zinc sulfate, the second hatch of
the Example
Polyester Resin Emulsion A was added, which contained 27.1 parts (polyester by
weight).
The mixture was stirred for about 5 minutes and the pH was monitored. Once the
particle
size reached 5.5[tm (number average), 4% NaOH was added to raise the pH to
about 6.82 to
stop the particle growth. The reaction temperature was held for one hour. The
particle size
was monitored during this time period. Once particle growth stopped, the
temperature was
increased to 88 C to cause the particles to coalesce. This temperature was
maintained until
the particles reached their desired circularity (about 0.97). The toner was
then washed and
dried.
[0098] The dried toner had a volume average particle size of 5.871.tm
and a number
average particle size of 4.98p.m. Fines (< 21.tm) were present at 1.12% (by
number) and the
toner possessed a circularity of 0.972.
[0099] Additional toners were made using the formulation and procedure
from the
Comparative Example Toner II, except the neutralization pII was altered to
test the pII
adjusting window. The results of these toners are shown in Table 2 below.
[00100] Comparative Example Toner III
[00101] Comparative Example Toner III was prepared using an aluminum
sulfate
coupling agent instead of a borax coupling agent. The Example Polyester Resin
Emulsion A
was divided into two batches, split 70:30 by weight to foim the core and the
shell of the
24
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
toner, respectively. The total polyester content represented about 90.3% of
the total toner
solids. Accordingly, the first batch contained 63.2% of the total toner solids
and the second
batch contained 27.1% of the total toner solids. Components were added to a
2.5 liter reactor
in the following percentages: the first batch of the Example Polyester Resin
Emulsion A
having 63.2 parts (polyester by weight), 4.4 parts (pigment by weight) of the
Example Cyan
Pigment Dispersion, and 5 parts (release agent by weight) of the Example Wax
Emulsion.
Deionized water was then added so that the mixture contained about 12% to
about 15% solids
by weight.
[00102] The mixture was heated in the reactor to 30 C and a circulation
loop was
started consisting of a high shear mixer and an acid addition pump. The
mixture was sent
through the loop and the high shear mixer was set at 10,000 rpm. Acid was
slowly added to
the high shear mixer to evenly disperse the acid in the toner mixture so that
there were no
pockets of low pH. Acid addition took about 4 minutes with 175g of 1% sulfuric
acid
solution. The flow of the loop was then reversed to return the toner mixture
to the reactor
and the temperature of the reactor was increased to about 40-45 C. Once the
particle size
reached 4.0pm (number average), 5% (wt.) aluminum sulfate solution (18g of
solution having
0.9g of aluminum sulfate) was added. The aluminum sulfate content represented
about 0.3%
by weight of the total toner solids. After the addition of aluminum sulfate,
the second batch
of the Example Polyester Resin Emulsion A was added, which contained 27.1
parts
(polyester by weight). The mixture was stirred for about 5 minutes and the pH
was
monitored. Once the particle size reached 5.5pm (number average), 4% NaOH was
added to
raise the pH to about 6.47 to stop the particle growth. The reaction
temperature was held for
one hour. The particle size was monitored during this time period. Once
particle growth
stopped, the temperature was increased to 88 C to cause the particles to
coalesce. This
temperature was maintained until the particles reached their desired
circularity (about 0.97).
The toner was then washed and dried.
[00103] The dried toner had a volume average particle size of 6.10 tn
and a number
average particle size of 5.20 m. Fines (< 2 m) were present at 0.24% (by
number) and the
toner possessed a circularity of 0.970.
CA 02858396 2014-06-05
WO 2013/101995 PCT/US2012/071932
[00104] Additional toners were made using the formulation and procedure
from the
Comparative Example Toner III, except the neutralization pH was altered to
test the pH
adjusting window. The results of these toners are shown in Table 2 below.
[00105] pII Adjusting Window
[00106] The results of the pH adjusting window testing referred to above
for
Comparative Example Toners 1-Ill and Example Toner A are shown in Figure 3 and
Table 2
below. Specifically, Figure 3 shows a graph summarizing the data presented in
Table 2.
26
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
TABLE 2
Toner Coupling Agent pH Volume Avg. Fines
Acceptable?
Particle Size (%<21.tm)
(Pm)
Comp. Ex. Toner I None 5.97 7.43 0.9 Marginal
Comp. Ex. Toner I 6.01 6.02 2.04 Yes
Comp. Ex. Toner I 6.13 6.32 0.67 Yes
Comp. Ex. Toner I 6.25 6.07 1.1 Yes
Comp. Ex. Toner I 6.28 6.49 10.26 No
Comp. Ex. Toner I 6.33 5.51 35.5 No
Example Toner A Borax 5.77 7.51 0.28 Marginal
Example Toner A 5.86 6.9 0.53 Yes
Example Toner A 5.97 6.54 0.45 Yes
Example Toner A 6.24 6.17 3.04 Yes
Example Toner A 6.44 5.83 4.2 Yes
Example Toner A 6.53 5.33 7.46 Marginal
Comp. Ex. Toner II Zinc Sulfate 6.5 7.67 0.22 Marginal
Comp. Ex. Toner II 6.59 7.38 0.29 Marginal
Comp. Ex. Toner II 6.82 5.87 1.1 Yes
Comp. Ex. Toner II 7.24 5.77 4.38 Yes
Comp. Ex. Toner IT 7.29 5.82 5.21 Marginal
Comp. Ex. Toner III Aluminum 6.26 8.23 0.26 No
Comp. Ex. Toner III Sulfate 6.31 7.16 0.54 Marginal
Comp. Ex. Toner HI 6.47 6.10 0.24 Yes
Comp. Ex. Toner III 6.61 5.52 4.16 Yes
Comp. Ex. Toner III 7.03 5.40 3.05 Yes
Comp. Ex. Toner III 7.23 4.83 36.47 No
[00107] As
illustrated in Table 2 and Figure 3, the pH adjusting window for the toners
having a coupling agent (borax, zinc sulfate or aluminum sulfate) are
significantly broader
than the pH adjusting window for the conventional emulsion aggregation toner
of
Comparative Example Toner I. As discussed above, when the pH adjusting window
is
broader, the process is easier to control at an industrial scale.
27
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
[00108] Fusing Window
[00109] Each toner composition was used to print 24# Hammeimill laser
paper
(HMLP) using a fusing robot at 50 pages per minute (ppm) with a toner coverage
of 1.1
mg/cm2 employing various fusing temperatures as shown in Tables 3 and 4 below.
The
temperatures indicated in Tables 3 and 4 are the temperatures of the fusing
robot's heating
element/heater. For each toner composition, various fuse grade measurements
were
performed. These fuse grade measurements include a scratch resistance test
shown in Table
3 and a conventional 60 degree gloss test shown in Table 4. For the scratch
resistance test,
the printed samples were evaluated using a TABER ABRADER device from TABER
Industries, North Tonawanda, New York, USA. The printed samples were evaluated
on the
TABER ABRADER scale from 0 to 10 (where a rating of 10 indicates the most
scratch
resistance). The TABER ABRADER device scratches the printed samples multiple
times
with different forces until the toner is scratched off the sample. The point
at which the toner
is scratched off corresponds with a number rating between 0 and 10 on the
TABER
ABRADER scale. As is known in the art, the conventional 60 degree gloss test
includes
shining a known amount of light at the surface of the printed sheet at a 60
degree angle and
measuring its reflectance. A higher gloss test value indicates that more
energy was
transferred to the substrate when it moved through the fuser. The gloss of the
print also
relates to the resin and release agent used in the toner.
28
CA 02858396 2014-06-05
WO 2013/101995 PCT/US2012/071932
TABLE 3
Scratch Test
Fusing Comp. Toner Toner Toner Toner Toner Comp. Comp.
Temp. Toner I A B C D E Toner II Toner III
( C)
190 CO CO CO CO -
195 4 6.7 9 3.7 -
200 CO 5.5 7.7 10 10 CO -
205 CO 8.7 9.7 10 10 4 CO CO
210 6 10 10 10 10 9.7 2.3 5.3
215 8 10 10 10 10 10 4 9.7
770 8.3 10 10 10 10 10 10 9.7
225 9 10 10 10 10 10 7.3 9.7
230 10 10 10 10 10 10 10 10
TABLE 4
Gloss Test
Fusing Comp. Toner Toner Toner Toner Toner Comp. Comp.
Temp. Toner I A B C D E Toner II Toner III
( C)
190
195 5.9 6.5 9.3 -
200 6.4 6.8 9.1 10.4 -
205 7.9 7.8 9.1 10.7 -
210 8.8 8.3 8.5 10.4 11.5 10.1 - 4.3
215 9.2 9 10.5 13 13.1 12 - 4.9
770 11.2 10.2 11.5 14.1 14.9 13.4 7.7 5
225 10.7 10.9 12.6 14.3 16 14 9.2 6.1
230 12.8 12.1 12.1 16.9 16.8 16.7 10.4 6.3
[00110] As shown in Table 3, Example Toners A and B, which included a borax
coupling agent and were founed using the same resin as Comparative Example
Toners 1-III,
29
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
exhibited superior fusing performance compared to the conventional emulsion
aggregation
toner (Comparative Example Toner I) and the toners having a zinc sulfate or an
aluminum
sulfate coupling agent (Comparative Example Toners II and III). The low ends
of the fusing
windows for Example Toners A and B were lower than the low ends of the fusing
windows
for Comparative Examples I-III. Specifically, Example Toners A and B provided
acceptable
scratch resistance at temperatures as low as 200 C and 195 C, respectively.
Comparative
Example Toners I-III were unable to provide acceptable scratch resistance at
these
temperatures and instead showed cold offset ("CO"), which means the toner
failed to fuse to
the paper. Accordingly, less energy was required to accomplish an acceptable
fusing
operation for Example Toners A and B than for Comparative Example Toners 1-
III. Example
Toners A and B also provided improved scratch resistance at elevated
temperatures from
210 C - 230 C in comparison with Comparative Example Toners I-III.
[00111] The cores of Example Toners C and D were foimed using the same
resin as
Example Toners A and B and Comparative Example Toners I-III but different
resins were
used to form the shells of Example Toners C and D. Nonetheless, as shown in
Table 3, the
low ends of the fusing windows for Example Toners C and D, which included a
borax
coupling agent, were lower than the low ends of the fusing windows for
Comparative
Examples Example Toners C and D also exhibited improved scratch
resistance at
elevated temperatures from 210 C - 230 C in comparison with Comparative
Example Toners
I-III.
[00112] Example Toner E was formed using a higher molecular weight resin
having a
higher glass transition temperature than the resin used to form Example Toners
A and B and
Comparative Example Toners I-III. It will be appreciated by one skilled in the
art that the
higher molecular weight and the higher glass transition temperature of this
resin were
expected to compromise the low end of the fusing window. Table 3 shows that
Example
Toners A and B outperformed Example Toner E due to the lower molecular weight
and lower
glass transition temperature resin used in Example Toners A and B. However,
the fusing
perfoimance of Example Toner E, which included a borax coupling agent and a
higher
molecular weight and higher glass transition temperature resin, was comparable
to the fusing
performance of Comparative Example Toners I-III even though Comparative
Example
Toners included a lower molecular weight and lower glass transition
temperature resin.
CA 02858396 2014-06-05
WO 2013/101995
PCT/US2012/071932
[00113] As shown in Table 4, Example Toners A thru E exhibited
comparable gloss
test perfoimance in comparison with the Comparative Example Toner I.
Comparative
Example Toners II and III showed poorer gloss values in comparison with
Example Toners A
thru E and Comparative Example Toner I.
[00114] The foregoing description of several embodiments has been presented
for
purposes of illustration. It is not intended to be exhaustive or to limit the
application to the
precise forms disclosed, and obviously many modifications and variations are
possible in
light of the above teaching. It is understood that the invention may be
practiced in ways other
than as specifically set forth herein without departing from the scope of the
invention. It is
to intended that the scope of the application be defined by the claims
appended hereto.
[00115] What is claimed is:
31