Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
1
:FLUORESCENT MEASUREMENT IN A DISPOSABLE MICRO:FLUIDIC DEVICE,
AND minuoD THEREOF
FIELD OF THE INVENTION
The present invention relates to the quantitative optical detection of target
biological.
analytes of the type in a biological specimen, such as a patient body fluid.
The present invention
is more specifically related to a device and method for achieving a true and
specific optical.
signal emitted from fluorescently labeled target analytes. The true and
specific optical signal is
achieved by attenuating the interfering fluorescence emitted from fluorescent
detector molecules
that are non-specifically bound to the lumina' surface of an assay chamber.
The optical signal
accurately reflects the concentration of the target analyte in the biological
specimen when
assayed in an assay chamber of a microfluidic device according to the
invention described
herein.
BACKGROUND OF THE INVENTION
Fluorescent measurement of a target analyte in biomedical assays may be
conducted in an
assay chamber in which one portion of the chamber has an optically clear
surface that is coated.
with binding partners specific for a target analyte of interest in a
biological sample. In a cell-
based assay, cells are grown on the optically clear lumina' surface of a cell
assay vessel. In cell
based assays the vessel must be sufficiently large, he, capable of holding
sufficient fluid (often
greater than 100 microliters), to maintain the cells with appropriate needs
such as nutrition,
oxygen, and waste removal. The optically clear luminal surface of such cell-
based assay vessels
is specifically treated to allow the cells to attach to its lumina' surface.
Other luminal surfaces of
the cell assay vessel are treated with blocking agents to minimize non-
specific binding to these
other luminal surfaces. Cell membrane potential changes, for example, may be
assayed based on
fluorescence changes of membrane potential-sensitive dyes which interact with
the cells to emit
fluorescent signals. Fluorescence is optically measured by an optical detector
through the
optically clear lumina] surface, typically the bottom surface, of the vessel.
With respect to fluorescent measurement of a target biological analyte not
bound to a cell
in a biological sample, the biological sample suspected of having the target
analyte of interest
typically is mixed in a solution with fluorescent detector molecules having a
binding partner that
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
2
is specific for and binds to the target analyte, The biological sample with
the target analyte of
interest bound to the fluorescent detector molecule flows as a solution into
the lumen of an assay
chamber having a portion that is optically clear. The lumina' surface of the
optically clear
portion is coated with binding partners of the target analyte. The target
analyte in the biological
in another typical assay format for detecting target analytes, the biological
specimen is
introduced (with or without first mixing with an appropriate assay reagent)
into the assay
chamber such that any an.alytes will be specifically bound to the binding
partners on the optically
15 A typical problem encountered in biomedical assays of the above types is
non-specific
binding of fluorescent detector molecules to lumina' surfaces of the chamber.
Such non-specific
surface binding may occur directly or indirectly by fluorescent detector
molecules complexing
with a biological moiety found in the sample, for example, a protein. The
complex binds to
luminal surfaces of the assay chamber other than to the binding partner-coated
luminal surface of
In a cell-based assay, similar assay steps are taken. However, washing in a
cell-based
assay may be undesirable because washing may disrupt cells attached to the
optically clear
surface of the assay vesselõAdditionally, the lumina' surfaces of the cell-
based assay vessel,
other than the optically clear lumina' surface, may be treated with blocking
agents such as
30 As mentioned above, typical problems encountered in diagnostic. assay
designs in which
the assay detects the presence of a target analyte and is performed in an
assay chamber, include
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
3
non-specific binding of fluorescent detector molecules to surfaces other than
the coated optically
clear surface. This could potentially give rise to detectable fluorescence
even in the absence of
the target analyte, loading to a falsely positive or elevated diagnostic
result. This effect is
particularly problematic in a closed assay chamber where the depth of the
chamber is extremely
shallow, i.e., the optically clear surface is fractions of a millimeter away
from the opposite
chamber surface. In this chamber type, the opposite chamber surface remains
accessible to the
optical system that provides excitation light and collects the emitted
fluorescence. Accordingly,
non-specific binding and background fluorescence adulterates the actual
fluorescent signal
emitted from the target analyte obscuring the optical signal that would
otherwise accurately
reflect the quantity of target analyte in a biological sample, such as a
patient body fluid.
SUMMARY OF THE INVENTION
The present invention is directed to automated, cost-effective, high
throughput solutions
that minimize background fluorescence of detector molecules bound non-
specifically to lumina]
surfaces of an assay chamber, while avoiding the problems and cost associated
with blocking
non-functionalized chamber luminal surfaces. In particular, background
fluorescence arising
from the lumina' surface opposite an actively treated optically clear surface
is substantially
reduced, without attenuating the optical signal originating from the target
analyte bound to the
optically clear activated surface. The assay for measuring a specific target
analyte as defined by
the invention is conducted in a inicrofundic device which permits extremely
rapid test results
while simultaneously improving assay sensitivity, and accuracy and minimizing
the expenditure
of costly reagents,
l.n one aspect, the invention relates to a device, kit, or a composition of
matter for
achieving a true and specific optical signal emitted from fiuorescently
labeled target biological
analytes in an assay chamber, in one embodiment, the invention includes a
microfiuidic device
having an assay chamber for detecting a target analyte. The assay chamber
includes a first wall
with at least a portion of the first wall being optically clear, an opposite
wall, and a lumen.
Optionally, the entire first wall is optically clear. The first wall is coated
on the luminal surface
with binding partners specific for a target analyte in the biological sample.
The luminal surface
of the opposite wall may be coated or, optionally, uncoated with binding or
blocking agents,
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
4
The device, kit, or composition of matter includes a fluorescent detector
molecule
comprising a binding partner fbr the target analyte, a solution in the assay
chamber comprising
dye which is capable of absorbing the light of a wavelength range selected
from the group
consisting of emission wavelength range, excitation wavelength range, or their
combination, of
any fluorescent detector molecule that is non-specifically bound .to the
luminal surface of the
chamber. The dye may be a single standard dye selected from the group
amaranth, brilliant
green, erioglaucine, for example, or a combination of standard dyes.
In one embodiment, the binding partner that is coated on the lumina' surface
of the first
wall or just the optically clear portion of the lumina' surface of the first
wall comprises an
antibody specific for the target analyte. The binding partner of the
fluorescent detector molecule
comprises another antibody specific for the target analyte. Optionally, the
binding partners that
are coated on the lumina.' surface of .the first wall may comprise an
intermediate binding partner.
In one ernbodiment, the distance between the first wall and the opposite wall
is in the
range of about 10 microns to 5.0 millimeters, about 75 microns, about 50
microns to 200
microns, or about 70 microns to 100 microns.
in another embodiment, the composition, kit, or device includes fluorescently
labeled
target analyte molecules. Fluorescently labeled target analyte molecules may
be useful in a
competitor binding assay.
In another aspect, the invention relates to a method for attenuating non-
specific
fluorescence in a microfluidic device used to measure fluoreseently labeled
target analytes in a
biological specimen.. According to one embodiment of the method of the
invention, a sample is
introduced into the chamber lumen of the microlluidie device described above.
A fluorescent
detector molecule comprising a binding partner for the target analyte is
introduced into the
chamber lumen. Optionally, the chamber lumen may be washed. The volume of wash
solution
may be less than, the same as, or greater than the volume of the chamber
lumen.
After introduction of the fluorescent detector molecule, a solution comprising
an
attenuating dye, for example, amaranth, erioglaucine, brilliant green, or
combinations of standard
dyes, is introduced into the chamber, The dye is capable of absorbing light of
a wavelength
range selected from the group consisting of emission wavelength range,
excitation wavelength.
range, or their combination of any fluorescent detector molecule that is non-
specifically bound to
the lumina' surface of the chamberõAn optical measurement is made and is
related to the target
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
analyte concentration in the sample. Optically measuring comprises measuring
an optical signal
arising from the lumina" surface of the first wall,.
In one embodiment, the method of the invention is a competitive binding assay
including
the step of introducing fluorescently labeled target analyte molecules into
the chamber lumen.
5 in a particular embodiment of the method of the invention, the sample
and fluorescent
detector molecule comprising a binding partner for said target analyte are
mixed together before
introducing the sample and the fluorescent detector molecule into the chamber.
Alternatively,
the lumen of the chamber is washed after introducing the sample into the
chamber lumen and
prior to introducing the fluorescent detector molecule into the chamber lumen.
The lumen of the
chamber may be washed with a wash reagent before introducing the dye.
Alternatively, the
lumen of the chamber is washed with a wash reagent containing the attenuating
dye. The
volume of the wash reagent is the same as or exceeds the volume of the
chamber, In one
embodiment, the washing step introduces a wash reagent through an inlet port
of the chamber
and removes the wash reagent through an outlet port of the chamber.
In one embodiment, the non-specifically bound fluorescent detector molecule
according
to the method of the invention is coupled to another molecule, e.g., a non-
target analyte.
The foregoing and other features and advantages of the invention will be more
apparent
from the description drawings, and claims which follow.
BRIEF DESCRIPTION OF THE DRAWINGS
These embodiments and other aspects of this invention will be readily apparent
from the
detailed description below and the appended drawings, which are meant to
illustrate and not to
limit the invention, and in which:
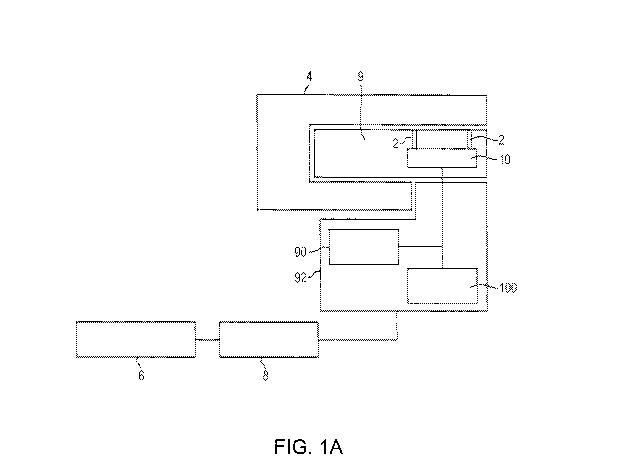
FIG. IA is a plan view of an exemplary instrument system including a
microfluidic
device according to one embodiment of the invention.
IB illustrates a top cutaway view of an exemplary assay chamber according to
one
embodiment of the invention.
FIG. IC illustrates a bottom cut away view of the exemplary assay chamber
illustrated in
FIG. I B.
10 FIG. 1.D illustrates a top cut away view of another exemplary
cylindrical assay chamber
according to one embodiment of the invention.
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
6
FIG, lE illustrates a bottom cut away view of the exemplary cylindrical assay
chamber
illustrated in FIG, ID.
HQ. 1.F illustrates a top view of an exemplary assay chamber and method of
making
according to one embodiment of the invention.
FIG-. 2 is a diagrammatic cross-sectional view of an assay chamber without
attenuating
dye.
FIG. 3 is a diagrammatic cross-sectional view of an exemplary assay chamber
including
an attenuating dye according to one embodiment of the invention.
FIG. 4 is a perspective view of an exemplary assay chamber including an
optical signal
portion of a wall according to one embodiment of the invention,
DESCRIPTION
The present invention wi II be more completely understood through the
following
description, which should be read in conjunction with the attached drawings.
In this description,
like numbers refer to similar elements within various embodiments of the
present invention.
Within this description, the claimed invention will be explained with respect
to embodiments.
The skilled artisan will readily appreciate that the methods and systems
described herein are
merely exemplary and that variations can be made without departing from the
spirit and. scope of
the invention,
As used herein, microfluidic device shall mean devices for biological assays
that utilize
fluid volumes on the order of picoliters to microliters, The devices have
channels and/or
chambers with dimensions ranging from millimeters to micrometers.
As used herein, target biological analyte shall mean an analyte or a group of
analytes of
interest in a biological specimen such as but not limited to pathogens,
proteins, nucleic acids,
lipids, antibodies, antigens, and enzymes. For example, a group of anal:yles
may be a plurality of
proteins, for example, myoglobin, proBNP, and myosin, proteins that are useful
in detecting
heart failure.
As used herein, a fluorescent detector molecule shall mean any molecule,
binding
partner, or entity that can complex directly or indirectly with another
molecule or substance and
can be detected using a suitable fluorescence optic system, wherein the
molecule, binding partner
or entity is excited by light of an appropriate wavelength and the emitted
light at a different
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
7
wavelength) is measured. The molecule, binding partner or entity may be
intrinsically
fluorescent or rendered fluorescent by attachment of an appropriate
fluorophore.
As used herein, an attenuating dye shall mean a dye that absorbs light of a
wavelength
range including emission wavelength range, excitation wavelength range, or the
combination of
emission wavelength range and excitation wavelength range of any fluorescent
detector
molecule.
As used herein, a binding partner shall mean a molecule, for example, an
antibody which
binds specifically to a target biological analyte, or an intermediate in a
binding cascade, for
example, where strepavidin is coated onto a surface as an intermediate binding
partner, and the
strepavidin then binds to biotin which has been conjugated to an antibody that
is a specific
binding partner for a target biological analyte,
As used herein, background fluorescence shall mean fluorescence that has not
originated
from a fluorescent detector molecule bound to a target analyte of interest,
In one aspect, the invention relates to a disposable microfluidic device for
optical
measurement of a target biological analyte in a biological specimen such as,
but not limited to,
body tissues, or a patient body fluid, libr example, blood, serum, plasma,
urine, sputum,
cerebrospinal fluid, joint fluid, digestive fluid, .tissue aspirates,
exudates, and transudates.
Embodiments of the invention relate to an apparatus, kit, composition of
matter, or
method, for example, an immunoassay method, for detecting target analytes in
an assay chamber
of a microfluidic device.
Figures 1 A-F are exemplary embodiments of a disposable microfluidic device
and
instrument system according to the invention that has been developed for
sensitive, accurate,
cost-effective, and automated diagnostic testing of a target analyte of
interest and generates rapid
test results. In one embodiment, referring to FIG. IA, the instrument system
includes a
microfluidic device 9 having an assay chamber 10 and fluid conduits 2, a
microfluidic device
holder 4, microprocessor 6, electronics 8, and an optical system 92 comprising
an optical source
90 and an optical detector 100 for measuring optical signals such as optical
signals generated by
a fluorescent detector molecule bound to a target analyte in an assay chamber.
Referring to FIG. 1.B, in one embodiment, the microfluidic device includes a
rectangular
assay chamber 10 which has 6 walls 12,, specifically, 12a, 12b, 12c, 12d, 12e,
and 12f,
surrounding a chamber lumen 16. The assay chamber 10 is capable of holding a
fluid when any
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
8
wall could be the wall closest to the source of gravitational pull. In other
words, following
assembly, the chamber 10 is completely enclosed on all sides with the
exception of optional
ports, for example, inlet or outlet ports. In one embodiment, the chamber 10
may be a channel
with optional inlet and/or outlet ports at the channel ends. The shape of the
chamber 10 of the
micro-fluidic device is not limited by the shapes illustrated in the appended
figures.
Each wall 12a-12f of the chamber 10 has a luminal surface 14 adjacent the
lumen 16, in
one embodiment according to the invention, the chamber 10 has an inlet port 20
and an outlet
port 22.
An active, optically clear wall portion is positioned within wall 12f, or
optionally, as
illustrated in FIG, 1B, the entire wall 1.2f is optically clear. The luminal
surface 14f of the wall
12f, or optionally only the optically clear portion of wall 12f is activated
by coating the surface
with binding partners specific for a target analyte of interest. The walls 12d
and 12f may be
planar or may have one or more radii, in one embodiment, the chamber wall 12d
that is opposite
to the optically clear wall 12f is substantially parallel to, 0 to 45 degrees,
0 to 10 degrees, or 10
to 45 degrees, for example, relative to the plane of the optically clear wall
12f, Alternatively, the
luminal surface of chamber wall 12d is substantially parallel to, 0 to 45
degrees, 0 to 10 degrees,
or 10 to 45 degrees, for example, relative to the plane of the luminal surface
of optically clear
wall 12f. In one embodiment, the luminal surface 14 of the chamber walls 12a-
12e other than
the lumina" surface 14f of the optically clear wall 12f are uncoated with
binding partners or with
blocking agents or any other agents prior to initiation of an assay that would
otherwise block
non-specific binding to the lumina' surfaces of these walls.
The assay chamber 10 may be made from a polymer, for example, but not limited
to,
polystyrene.
Referring to FIGS. 1B-1C, in a particular embodiment according to the
invention, the
assay chamber 10 is substantially rectangular with an optically clear wall 12f
(or portion thereof)
and a wall 12d opposite the optically clear wall 12f. The distance 80 between
the luminal
surface 14f of the optically clear wall 12f and the lumina' surface 14d of the
wall 12d opposite
the optically clear wall 12f is in the range of about 10 microns to 5
millimeters, 10 microns to 2
millimeters, 10 microns to 1 millimeter, 50 microns to 200 microns, 50 microns
to 125 microns,
70 microns to 100 microns, 75 microns to 150 microns, preferably 50 to 100
microns, more
preferably 75 microns, The chamber lumen 16 is bounded and enclosed by the
walls 12a-12f
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
9
including the optically clear wall 12f and the wall 12d opposite the optically
clear wall of the
chamber 10. The walls other than the optically clear wall may be made from a
light blocking
material, for example, a black plastic. Alternatively, the walls may be
optically clear,
Referring to FIGS, 1D-1E, in another embodiment according to the invention,
assay
chamber 10 is substantially cylindrical with wall 12f and wall 12d at opposite
ends of the
cylindrical chamber 10, and wall 12b joining wall 12f and 12d. Wall 12f of the
chamber 10 is
optically clear or, optionally, a portion of wall 12f is optically clear. The
chamber wall 12d that
is opposite to the optically clear wall 12f is substantially parallel, 0 to
45', 0 to 10', or 10 to 45'
relative to the plane of the optically clear wall 12f. Alternatively, the
lumina" surface of chamber
wall 12d is substantially parallel, 0 to 45 degrees, 0 to 10 degrees, or 10 to
45 degrees, for
example, relative to the plane of the lumina' surface of optically clear wall
12f.
Referring still to FIGS. 1D-1E, the lumina' surface 14f of the optically clear
wall 12f or a
portion of the lumina.' surface wall 121 of the cylindrical chamber 10 is
activated by coating the
surface with binding partners specific for a target anal yte of interest by
standard methods known
to the skilled artisan. In one embodiment, the :lumina" surface of the walls
12b and 12d are
uncoated with binding partners or with blocking agents or any other agents
prior to initiation of
an assay that would otherwise block non-specific binding to the lumina"
surfaces of these walls,
The distance 80 between the ltuninal surface 14f of the optically clear wall
12f and the lumina'
surface 14d of the wall 12d is in the range of about 10 microns to 5
millimeters, 10 microns to 2
millimeters, 10 microns to 1 millimeter, 50 microns to 200 microns, 50 microns
to 125 microns,
70 microns to 100 microns, 75 microns to 150 microns, preferably 50 to 100
microns, more
preferably 75 microns.
The chamber may assume other shapes (e.g. shapes with curved side portions as
opposed
to orthogonal edges may facilitate optimal fluidic properties when introducing
and removing
solutions from the chamber), a channel for example, and is not limited to the
illustrated
rectangular or cylindrical shapes. The walls other than the optically clear
wall may be made
from a light blocking material, for example, a black plastic. Alternatively,
the walls may be
optically clear.
Referring to FIG. 1.f, in one embodiment of the inicrofluidic device for
detecting target
analytes in a biological specimen according to the invention, the chamber 10
is assembled from
parts into a single integrated chamber 10. For example, in one embodiment, a
first chamber part
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
is a shallow well 40 made from a polymeric material and having a wall 12d at
the bottom of the
shallow well 40, an open face 42 at the top of the shallow well, and well side
walls 12a, 1.2b, 12c
and 12e. The shape of the well 40 is not limited to rectangular but may be
oval, circular, or other
shapes, for example.
The depth of the shallow well 40 is in the range of about 10 microns to 5
millimeters, 10
microns to 2 millimeters, 10 microns to 1 millimeter, 50 microns to 200
microns, 50 microns to
12.5 microns, 70 microns to 100 microns, 75 microns to 150 microns, preferably
50 to 100
microns, more preferably 75 microns. An optically clear, planar wall 12f or a
wall with an
optically clear portion, with dimensions that correspond substantially to the
open face 42. of the
10 shallow well 40 forms a second chamber part to be joined to the shallow
well 40 to form the
assay chamber 10. The optically clear wall 12f, or optionally, a portion of
wall 12f of the assay
chamber 10 is activated by coating the surface on one side of the wall with
binding partners,
defined above, for the target analyte of interest (see, e,g,, .FIG. 2), The
binding partner coated on
the surface may be, but is not limited to, for example, polyclonal or
monoclonal antibodies and
fragments thereof specific for a target analyte, other proteins, lectins,
antibodies,
oligonucleotides, protein biomarkers, aptamers, receptors, protein A, protein
G, biotin, or
strepavidin. The coated surface of the optically clear wall 12f is placed face
down on the open
face 42 of the shallow polymeric well 40 such that the coated surface is on
the luminal side of
the newly formed chamber 10.
The optically clear wall 12f is affixed to the top of the walls of the shallow
polymeric
well 40 by adhesives, heat bonding, ultrasonic welding, or other methods of
permanent
attachment. Optionally, the luminal surfaces 14 of the shallow well portion 40
of the chamber
10, including the luminal surface 14d of the wall 12d at the base of the
shallow well 40, are not
treated with any agents prior to initiation of an assay, such as blocking
agents, for example, but
not limited to the blocking agents casein, bovine serum albumin, and newborn
calf serum.
Referring to FIG, 2, chamber 10, as described above, is readied for an assay.
Chamber
10 is filled with the biological sample suspected of having the target analyte
of interest. After an
appropriate incubation period to allow binding of target analytes to the
binding partners on the
optically clear wall, the chamber lumen 16 is washed by introducing a volume
of wash solution
through the inlet port 20 that exceeds or is equal to the volume of the
chamber lumen. The wash
solution may be removed through outlet port 22. The fluorescent detector
molecules with
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
11
binding affinity for the target analytes are added to the chamber lumen and
incubated for
sufficient time to allow binding to occur. The chamber is again washed prior
to optical detection
to remove unbound fluorescent detector molecules. Optionally, the fluorescent
detector
molecules with binding affinity for the target of interest may be pre-mixed
with sample. The
mixture is then introduced into the chamber, followed by washing the chamber
lumen, which is
followed by optical detection.
in one embodiment according to the invention, the binding partners of the
fluorescent
detector molecules that are mixed with the biological sample are different
than the binding
partners fOr the target analyte coated on the lumina' surface of the optically
clear wall,
Alternatively, the binding partners integral to the fluorescent detector
molecules and the binding
partners coated on the lumina' surface may be the same, for example, when the
target analyte is
multivalent. in some cases, the binding partners may be purposefully designed
to bind to a group
of closely related target analytes, for example to detect all members of the
distinct, but closely
related subtypes of HIV viruses, Furthermore, the binding partners may be
intermediates in a
binding cascade, for example where streptaNidin is coated onto the surface as
an intermediate
binding partner. Streptavidin then binds to biotin which has been conjugated
to an antibody that
is specific. for the analyte of interest. The target analyte in the sample
binds to the binding
partner of the fluorescent detector molecules when the target analyte and
binding partner are
contacted in solution, thereby forming a fluorescendy labeled target analyte,
For optical detection, excitation light from an optical source 90 of the
instrument system
is directed through the optically clear wall 12f or a portion of the optically
clear wall 12f of the
assay chamber 10 to excite fluorescence 56 of the fluorescent detector
molecules 52. bound to the
target analytes 55 which in turn are bound to the binding partners 57 on the
lumina" surface 14f
of the optically clear wall 1211 Fluorescence 50 detected by an optical
detector 100 from
fluorescent detector molecules 52 non-specifically bound to the untreated
lurninal surfaces of
portions of the assay chamber, the opposite wall luminal surface 14d in
particular, is unwanted
background fluorescence. The background fluorescence 50 overlaps the
fluorescence 56 emitted
from the target analyte bound 55 to the binding partners 57 on the lumina'
surface 14f of the
optically clear wall 121 of the assay chamber 10. Thus, without a modification
of the above
chamber and method discussed below, the optical signal received by the optical
detector includes
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
12
a background contribution that is not related to the concentration of the
target analyte,
Accordingly, sensitivity and accuracy of the assay are compromised.
Referring to Fla 3, in the assay chamber of a microfluidic device according to
the
invention discussed above, the efficiency of fluorescence excitation and
collection from the non-
treated lumina' surface 14d of the opposite wall 12d of the assay chamber 10
and the activated
lumina' surface 14f of the optically clear wall 12f is essentially identical
given the narrow
distance 80 (in one embodiment described above, as little as 10 microns)
between the luminal
surface 14f of the optically clear wall 12f and the lumina' surface 14d of the
wall 12d opposite to
the optically clear wall, In contrast, the efficiency of fluorescence
excitation and collection from
the surfaces other than the active surface of an open-top vessel lacking a
vessel surface opposite
to the active surface, or in assay chambers where the distance between the
treated optically clear
luminal surface and the surface of the opposite wall is greater than the depth
of field, of the
optical system, for example, greater than about 5 millimeters, is much less
than the efficiency of
fluorescence from the active (treated optically clear lumina]) surface of the
chamber. In the
instant chamber, the active surface is merely about 10 microns to 5
millimeters, 10 microns to 2
millimeters, 10 microns to I millimeter, 50 microns to 200 microns, 50 microns
to 125 microns,
70 microns to100 microns, 75 microns to 150 microns, preferably 50 to 100
microns, more
preferably 75 microns from the opposite surface. Therefore, the depth 80 in
the instant
application is less than the depth of field of the optical system. Only the
fluorescence emitted
from the target analyte bound to the binding partners on the treated optically
clear luminal
surface and not the fluorescence of detector label non-specifically bound to
other portions of the
lutnin.al surface of the chamber is relevant to accurately detecting the
target analyte. When
background fluorescence is also detected, accuracy and sensitivity of the
assay directed to
detection of the specific target analyte is severely compromised.
The introduction of a dye 60 that has particular characteristics into the
assay chamber of
the microfluidic device is yet an additional modification of the invention
that is illustrated in
FIG. 3 and described below. The introduced dye 60 attenuates the effect of
background
fluorescence 50 emitted by non-specific binding of the fluorescent detector
molecules 52 to the
lumina' surface 14d of the wall 12d opposite to the optically clear wall 12f,
but not the specific
fluorescence 56 emitted by the fluorescent-labeled target analyte 54 that is
specifically bound to
the activated luminal surface 14f of the optically clear wall 12f.
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
13
In this embodiment of the invention, the sample and any unbound material
including
unbound fluorescent detector molecules are removed from the chamber and the
Chamber lumen
is washed with a volume of wash solution exceeding or equal to the volume of
the chamber
lumen as described above. Next, an attenuating dye 60, as defined above, is
introduced into the
lumen of the chamber. The. optimal concentration of the dye is the highest
concentration of the
dye that meets the following criteria: the dye must remain in solution under
all conditions of
transportation, storage and use and must not cause chemical or biochemical
effects that alter the
results of the assay, The dye solution volume is approximately equal to the
volume of the
chamber. In one embodiment, the attenuating dye may be included with the
fluorescent detector
molecules or, optionally, in the wash solution that is used to remove unbound
fluorescent
detector molecules and the sample from the chamber. The attenuating dye 60
includes such
standard dyes as amaranth, erioglaucine, brilliant green or combinations of
various standard
dyes. Fluorescent labels include fluorescent molecules from common dye
families derived from
xantherie (e,g. Fluorescein, Texas Red), cyanine, naphthalene, coumarin,
oxadiazole, pyrene,
oxazine, acridine, arylmethine, tetrapyrrrite and commercial dyes including
TOTO-1, YOY0-1,
Alexa. Fluors, Cy family (e,g. Cy2, Cy5, Cy7) and many others, as well as
fluorescent molecules
useful in time-resolved fluorescence such as chelates of the lanthanides,
europium., samarium,
and terbium, Fluorescence 50 from the fluorescent detector molecules 52 that
are non-
specifically bound to the lumina' surface of the chamber, for example, surface
14d, is "masked"
by the one or more attenuating dyes 60 that are introduced into the chamber
lumen. The specific
fluorescence 56 of the fluorescent labeled target analyte 54 bound to the
lumina]. surface 14f of
the optically clear wall 121 is not masked. By masking non-specific
fluorescence, that is the
fluorescence arising from fluorescent detector molecules non-specifically
bound to the wall
opposite the optically clear wall in particular, the sensitivity and accuracy
of the chamber 10 for
detecting the target analyte is increased. Thus, measuring the concentration
of the target analyte
of interest is accomplished without the obscuring effect caused by the
fluorescence 50 of non-
specifically bound fluorescence detector molecules 52 on the measurement of
the concentration
of the target analyte reflected by the optical signal.
Referring to FIG. 4, in one embodiment according to the invention, the optics
of the
instrument are arranged to detect fluorescence only from the optically clear
wall 12f or a portion
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
14
of wall 12f and from the wall 12d opposite to the optically clear wall while
not detecting
fluorescence that may be emitted from the side walls or any other wall of the
chamber 10.
For example, referring still to FIG, 4, in a rectangular assay chamber 10
according to the
invention having a chamber depth of 0.1 rum and outside dimensions of 6 mm x 2
mm, in one
embodiment, the optically clear wall 12f of the chamber is 6 ram x 2 mm.
Referring still to FIG.
4, in this embodiment, only a 1 mm. x 1 min optical signal portion 120 of the
6 mm x 2 mm
optically clear wall 12f, the center, for example, is utilized for the optical
signal. Accordingly,
the signal due to non-specific binding of the fluorescent detector molecules
on wall surfaces such
as the sides of the chamber other than the opposite wall surface 12d is
substantially eliminated.
The outside dimensions of the chamber may be larger than the optically clear
area which
in turn may be larger than the portion used to make optical measurements.
Exemplification
Nlyoglobin is an exemplary target analyte found in a biological specimen that
may be
detected in the microfluidic device according to the invention described
above. Referring again
to FIG. 2, the exemplary chamber is shallow having a. depth 80, for example,
of about 75
microns. A binding partner, a monoclonal antibody, for example, directed to a
specific epitope
of myoglobin may be used as the binding partner that is applied to the
turninal. surface 14f of the
optically clear wail 12f. Another monoclonal antibody directed to a different
epitope of
myoglobin is labeled with a fluorescent detector molecule such as fluorescent
chelates of
europium, The fluorescently labeled monoclonal antibody is mixed with the
biological specimen
that may contain the myoglobin target analyteõAfter sufficient. incubation
time, the fluorescently
labeled monoclonal antibody binds the myoglobin analyte to form a
fluorescently labeled
myoglobin target analyte. Without the addition of an attenuating dye,
amaranth, for example, to
the system, non-specific fluorescence from the luminal surface 14d of the
opposite wall 12d
caused by non-specific binding of the fluorescent detector molecule, and
specific fluorescence
from the binding of the fluorescently labeled myoglobin target analyte to the
specific monoclonal
antibody-binding partner on the lumina' surface 14f of the optically clear
wall 12f, is measured
by an optical detector 100. The measured optical signal from the assay chamber
10 includes
fluorescence 50 from non-specific binding of fluorescent detector molecules 52
to the untreated.
luminal surface 14d of the wall I 2d and fluorescence 56 emitted by the
fluorescent chelates of
europium labeled myoglobin target analyte 55 specifically bound to the
monoclonal antibody
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
binding partner 57 on the luminal surface 14f of the optically clear wall 12f,
leading to an
artificially elevated fluorescence value that does not accurately reflect the
concentration of
myoglobin in the biological specimen. Following removal of unbound fluorescent
detector
molecules by a wash reagent without dye, the remaining non-specifically bound
fluorescent
5 detector molecules on the lumina.' surfaces of the chamber, particularly
on the lumina" surface
14d of wall 12d opposite the optically clear wall 12f of the shallow chamber,
interfere with the
true and specific optical signal emitted from the fluorescently labeled
myo,c,,lobin target analyte
bound to the specific monoclonal antibody binding partners on the luminat
surface 14f of the
optically clear wall 12f.
10 The method to detect the target analyte myoglobin, for example, as
described above,
preferably also incorporates the addition of an attenuating dye such as but
not limited to
amaranth, or combinations of dyes as described above. Referring to FIG, 3, in
the preferred
embodiment of the invention, the exemplary chamber is a shallow chamber having
a depth 80,
for example, of about 75 microns, .After sufficient incubation to allow
binding to occur, unbound.
15 fluorescent labeled monoclonal antibodies directed to the myoglobin
target analyte are removed
and the chamber lumen 16 is washed with a volume of wash reagent exceeding or
equal to the
volume of the chamber lumen. The wash reagent may contain or may be free of an
attenuating
dye, amaranth in this example, as described above with respect to FIG. 3. if
the wash reagent
does not contain the attenuating dye, the dye is added to the chamber lumen
after the wash. In
this exemplary embodiment, non-specific binding of fluorescent detector
molecule to the
untreated luminal surface 14d of the assay chamber 10 occurs, as discussed
above with respect to
FIG. 2. However, the amaranth dye 60 molecules positioned between the non-
specifically bound
fluorescent detector molecules 52 on the luminal surfaces 14d of the wall 12d
opposite to the
optically clear wall 12f, in particular, and the optical system 92 effectively
attenuate the non-
specific fluorescence. 'File application of amaranth in this example leads to
an accurate
determination of the specific fluorescence of the myoglobin target analyte 55
bound to the
rn.onoclonal antibody-binding partner 57 that is coated on the lumina' surface
14f of the optically
clear wail 12f.
in a preferred embodiment, the assay chamber 10 is a microfluidic element
within a
microfluidic assay device, in order to achieve the short incubation times and
small sample and
reagent volumes that are well-known characteristics of microfluidie assay
devices. These
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
16
characteristics can only be achieved if the assay chamber is kept shallow as
disclosed above,
preferably with depth 10 ¨ 200 microns. If the assay chamber is excessively
deep, mass
transport by diffusion will require long incubation times, and filling and
washing of the assay
chamber will require larger volumes of costly reagents. However, referring
again to FIG. 3, in
order to achieve highly sensitive assays, it is also desirable to detect only
the fluorescence from
detector molecules specifically bound to the active treated luminal surface
14f of the optically
clear 12f of the assay chamber 10, and not to detect the fluorescence from
detector molecules
non-specifically bound to the non-treated luminal surface 14d of the opposite
wall 12d of the
assay chamber 10. If the assay chamber is kept shallow as disclosed above,
surface 14d and
surface 14f will both be within the depth of field of practical optical
systems that deliver the
excitation light and collect the fluorescence. (While specialized optical
designs to address this
problem may be possible, they add cost, complexity, and risk of malfunction,)
According to the
invention, the use of attenuating dye resolves this fundamental conflict
between microfluidic
design and optical design.
In another embodiment according to the invention, a competitive binding assay
may be
performed. According to this embodiment, a binding partner for the target
analyte is coated on
the luminal surface of the optically clear wall, as described previously.
Fluorescently labeled
target analyte molecules are prepared that compete with the target analyte for
binding
specifically to the binding partner coated on the luminal surface of the
optically clear wall, The
fluoreseently labeled target analyte molecules and the unlabeled analyte
molecules in the sample
compete to bind with the binding partner coated on the lumina' surface of the
optically clear
wall Thus, as the concentration of unlabeled analyte molecules in the sample
increases, there is
a corresponding decrease in the number of labeled molecules specifically bound
to the binding
partners coated on the lumina' surface. Nevertheless, as is true for other
embodiments discussed
above, quantitation of unlabeled analyte in the sample is based on measurement
of fluorescence
from specifically bound fluorescent molecules on the luminal surface of the
optically clear wall.
Fluorescence from non-specifically bound fluorescent molecules on other
surfaces within the
depth of field of the optical system degrades the analytical perfOrmance of
the assay, and the use
of an attenuating dye according to the present invention resolves this
problem.
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
17
Specific Examples of Surface Fluorescence Attenuation:
For proof of principle, studies were conducted to determine the effect of
various dyes in.
solution on attenuating non-specifically bound fluorescently labeled particles
to the lumina'
surfaces, the surface opposite the optically clear surface in particular, of
the assay chamber
described above. For this study, latex nanoparticles labeled with fluorescent
ehelates of
europium were directly added to the non-treated luminal surface 14d of the
wall 12d opposite to
the optically clear wall 12f of the chamber described above to simulate non-
specifically bound
fluorescent label that could occur during an actual diagnostic assay.
Latex nanoparticies labeled with fluorescent chelates of europium were
dispensed
directly onto the luminal surface of th.e wall opposite the optically clear
wall of the polystyrene
chambers (6 mm x 2.5 ann. x .075mm) described above. 1 x 105, 1 x 106 or 1 x
107 nanoparticles
were added to the surface in 1 ut aqueous buffer and allowed to air dryõAn
optically clear wall
that was not treated was then ultrasonically welded onto the chamber to form
the assay chamber
10 described above.
The lumen of each of the assay chambers described above was then washed three
times
with 100 WI., of an aqueous solution without attenuating &ye in order to
remove loosely bound
material on the lumina' surface of the chamber. Fluorescence was measured
after each wash
using 340 um excitation and collecting the emitted light using a 615 rim band
pass filter. The
amount of fluorescence from the lumina' surface 14d of the wall 12d opposite
the optically clear
wall 12f was measured through the optically clear wall 12f As shown in Table I
below,
although subsequent washes continued to remove additional fluorescence from
the surface, the
first three washes removed the majority of the loosely bound nanoparticles, as
the fluorescence
decreased 92% after the first wash. About 41% of the remaining counts were
removed after the
second wash, and only about 10% of the remaining counts were removed after the
third wash
(excluding the 1 x 105 case in which the fluorescence actually increased
slightly after th.e third
wash, the decline was about 22% after the third wash).
Following the third wash, the assay chambers were then washed with 100 uI. of
wash
reagent plus 7 mg/mL amaranth dye (CAS No: [915-67-3]), which strongly absorbs
at 360 nrn,
near the wavelength used for excitation of fluorescent &elates of europium. In
the presence of
amaranth dye, the measured fluorescence was on average about 67% less than the
measurements
without dye, a decline that was too great to be explained solely by removal of
additional
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
18
fluorescent nanoparticles from the surface (although undoubtedly, a minor
amount of additional
loosely bound material was likely removed, see below). Rather, these results
were interpreted as
attenuation by the dye of the fluorescence arising from the nanoparticles
hound to the surface
14d of the wall opposite (which is not an activated surface) the optically
clear wall 12f.
This conclusion was supported by washing the chamber lumens a fifth time, this
time
again using a wash reagent without dye. After removal of the dye by a fifth
wash, the
fluorescence increased --- an average of 2 fold ¨ showing that the large
decrease in fluorescence
after the fourth wash with dye could not have been due solely to the removal
of loosely bound
nanoparticles from the surface 14d.
After the fifth wash the fluorescence did not return to the levels achieved
after the third
wash, indicating that additional loosely bound material was removed during the
fourth and fifth
washes. Assuming equal loses by removal of loosely bound material from the
surface in each of
these final two washes, the loss was estimated at about 19% per wash, similar
to that seen from
the third wash as shown below in Table 1.
Table I: Attenuation of Surface-Bound Fluorescence by Amaranth Dye
Surface-Bound Fluorescence*
Nanoparticles 1-x10 lxle lx10I
.==
.=%.==
Starting fluorescence 99,346 1,162,483 8,000,834
.==
:.:
After 1st wash, no dye 9,035 74,630 749,524
...
..
.==
..
After 2nd wash, no dye 5,086 47,429 421,524 .
...
=
After 3rd wash, no dye 6,118 30,042 315,188
After 4th wash + dye 2,360 10,645 99,854
=
:
After 5th wash, no dye 3,739 23,497 214,367
...
..
:
:
Attenuation 61.4% 72.7% 68.3% :
:.:
-
.....::
Maximum fluorescence measurements within the chambers.
As a further exemplification of the invention (See Table II), the above
experiment was
repeated with one of the following dyes added to the wash reagent: 1) 7
mg/mi... amaranth
(control); 2) 14 mg/mL amaranth; 3) 22,5 mg/mL erioglaucine (CAS No: 13844-45-
9Th and 4)
ril &IL brilliant green (CAS No: [633-03-41). Unlike amaranth which absorbs
near the
wavelength of the excitation light, erioglauchie and brilliant green absorb
strongly pear 615 urn,
the fluorescence emission wavelength of chelates of europium. In this
experiment, 2 x 106
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
19
fluorescent nanoparticles were dispensed directly onto the luminal surface 14d
of the wall
opposite the optically clear wall 12f of polystyrene chambers as described
above.
As illustrated in Table II below, with fluorescent particles attached to the
luminal surface
14d of the wall 12d opposite the optically clear wall 12f, 63% attenuation of
surface-bound
fluorescence was observed using 7 ing/mL amaranth, similar to the level of
attenuation observed
above. Increasing the amaranth dye concentration from 7 mg/mL to 14 trigtml,
resulted in even
greater attenuation of -fluorescence from the surface 14d, now at 77%
reduction. When
erioglaucine or brilliant green dyes were used, only about 2% of the surface-
bound fluorescence
was measured, indicating about 98% attenuation of the fluorescence bound to
the luminal surface
14d of the wall 12d opposite to the optically clear wall 12f of the chamber in
the presence of the
these dyes at the concentration used.
As in the first exemplification described, after the fifth wash to remove the
attenuating
dye, a substantial increase of fluorescence was measured, again demonstrating
that the
attenuating dye blocked the fluorescence of the nanoparticles bound to the
luminal surface 14d of
the wall 12d opposite the optically clear wall 12f rather than removing them.
Table 11: Attenuation of Surface-Bound Fluorescence with Additional Dyes
'Percentage of Fluorescence Remaining After 3rd Wash
Dye 4th Wash + Dye 5th Wash, No Dye.:
Amaranth (7 mgirril_) 37% 80%
Amaranth (14 mg/mL) 23% 66%
Erioglaucine (22.5 rngimL) 1.5% 70%
Brilliant Greeft(60.ingimi.,) 2.1% 42%
First principles dictate that the magnitude of attenuation caused by dye
molecule
absorption should increase with increasing concentration of the dye. Indeed,
this was observed
with a doubling of the amaranth concentration (See Table II). Furthermore,
high concentrations
of erioglaucine and brilliant green blocked about 98% of the surface-bound
fluorescence (See
Table ID. Subsequent removal of the dyes led to recovery of fluorescence,
proving that the
effect of tb.e dyes was to block fluorescence from . the surface-bound
nanoparticles rather than
removing the nanoparticles. This proof of principle experiment shows that it
is possible to
almost completely block the non-specifically bound fluorescence from the
surface of the luminal
wall opposite the optically clear wall by the application of an attenuating
dye. As the results
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
indicate, the choice of dye and concentration are important parameters
affecting the magnitude of
attenuation, The optimal concentration of the dye is the highest concentration
of the dye that
meets the following criteria: the dye must remain in solution under all
conditions of
transportation, storage and use and must not cause chemical or biochemical
effects that alter the
According to one embodiment of a method of the invention for reducing the
unwanted
background fluorescence in an assay for measuring a target anal yte in a
biological sample, a
microfluidic device having an assay chamber is provided. The assay chamber has
a lumen
enclosed by walls and an optional inlet and an outlet port. One chamber wall
or alternatively, a
The luminal surface or a portion of the optically clear wall of the chamber is
coated
(activated) with specific binding partners, as defined above, for a target
analyte of interest in the
The biological sample is mixed with a fluorescent detector molecule that
includes another
binding partner specific for the target analyte. This binding partner may be
the same as or,
optional ly, different than the binding partner coated on the optically clear
surface. The sample
After incubation to allow binding, the solution including the biological
sample and the
fluorescent detector molecules are removed from the chamber, In one
embodiment, the chamber
CA 02859019 2014-06-11
WO 2013/090107
PCT/US2012/068110
21
The above described device and method can be used to reduce interfering signal
arising
from fluorescent detector molecules that non-specifically bind to non-treated
luminal surfaces of
diagnostic test devices of wide and varied designs, excluding the primary,
optically clear,
funetionalized active reaction surface. Accordingly, the described device and
method of the
invention improves the accuracy sensitivity, manat'acturing costs and
minimizes use of costly
reagents in fluorescence-based in vitro medical diagnostic tests thereby
leading to improved
patient care.
The invention may be embodied in other specific forms without departing from
the spirit
or essential characteristics thereof. The foregoing embodiments are therefore
to be considered in
all respects illustrative rather than limiting on the invention. Scope of the
invention is indicated
by the claims, and all changes that come within the meaning and range of
equivalency of the
claims are intended to be embraced.
We claim: