Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02859128 2014-06-12
1
Process for preparing formic acid
Description
The present application incorporates the provisional US application No.
61/577,701, filed
on December 20, 2011, by reference.
The present invention relates to a process for obtaining formic acid by
thermal
separation of a stream comprising formic acid and a tertiary amine (I), in
which a liquid
stream comprising formic acid and tertiary amine (I) in a molar ratio of from
0.5 to 5 is
produced by combining tertiary amine (I) and a formic acid source, and formic
acid is
removed by distillation at a temperature at the bottom of from 100 to 300 C
and a
pressure of from 30 to 3000 hPa abs from the resulting liquid stream in a
distillation
apparatus, where the bottom output from the distillation apparatus is
separated into two
liquid phases and the upper liquid phase is recirculated to the formic acid
source and the
lower liquid phase is recirculated to the removal of the secondary components
and/or to
the distillation apparatus.
Formic acid is an important and versatile product. It is used, for example,
for acidification
in the production of animal feeds, as preservative, as disinfectant, as
assistant in the
textile and leather industry, as a mixture with its salts for deicing aircraft
and runways
and also as synthetic building block in the chemical industry.
The most widespread process at present for preparing formic acid is the
hydrolysis of
methyl formate which can be obtained, for example, from methanol and carbon
monoxide. The aqueous formic acid obtained by hydrolysis is subsequently
concentrated, for example using an extraction auxiliary such as a
dialkylformamide
(DE 25 45 658 Al).
In addition, obtaining formic acid by thermal dissociation of compounds of
formic acid
and a tertiary nitrogen base is also known. These compounds are generally
acidic
ammonium formates of tertiary nitrogen bases, in which the formic acid has
reacted
beyond the stage of classical salt formation with the tertiary nitrogen bases
to form
CA 02859128 2014-06-12
2
stable addition compounds bridged by hydrogen bonds. The addition compounds of
formic acid and tertiary nitrogen bases can be formed by combining the
tertiary nitrogen
base and a formic acid source. Thus, for example, WO 2006/021,411 discloses
the
preparation of such addition compounds in general by (i) direct reaction of
the tertiary
nitrogen base with formic acid, (ii) by transition metal-catalyzed
hydrogenation of carbon
dioxide to formic acid in the presence of the tertiary nitrogen base, (iii) by
reaction of
methyl formate with water and subsequent extraction of the resulting formic
acid by
means of the tertiary nitrogen base and (iv) by reaction of methyl formate
with water in
the presence of the tertiary nitrogen base.
The general advantages of using addition compounds of formic acid and tertiary
nitrogen
bases for obtaining formic acid are that the addition compounds firstly bind
the formic
acid strongly enough to withdraw the formic acid as free formic acid from the
medium,
for example the reaction medium, in which the formic acid has been formed by
chemical
synthesis or, for example, from a dilute formic acid solution and thereby
allow the formic
acid to be separated off more readily in the form of its addition compounds,
but are weak
enough for the formic acid subsequently to be able to be released again from
the
addition compounds by thermal dissociation in order to obtain it in
concentrated and
purified free form.
EP 0 001 432 A discloses a process for obtaining formic acid by hydrolysis of
methyl
formate in the presence of a tertiary amine, in particular an alkylimidazole,
to form
addition compounds of formic acid and the tertiary amine. The hydrolysis
mixture
obtained, which comprises unreacted methyl formate, water, methanol, addition
compounds and tertiary amine, is freed of the low boilers methyl formate and
methanol
in a first distillation column. In a second column, the remaining bottom
product is
dewatered. The dewatered bottom product from the second column, which still
comprises addition compounds and tertiary amine, is then fed to a third column
and in
this the addition compounds are thermally dissociated into formic acid and
tertiary
amine. The formic acid liberated is removed as overhead product. The tertiary
amine
collects in the liquid phase and is recirculated to the hydrolysis.
CA 02859128 2014-06-12
3
DE 34 28 319 A discloses a process for obtaining formic acid by hydrolysis of
methyl
formate. The hydrolysis mixture obtained, which comprises unreacted methyl
formate,
water, methanol and formic acid, is freed of the low boilers methyl formate
and methanol
in a first distillation column. The aqueous formic acid obtained at the bottom
is
subsequently extracted with a relatively high-boiling amine, in particular a
relatively long-
chain, hydrophobic C6-C14-trialkylamine, in the presence of an additional
hydrophobic
solvent, in particular an aliphatic, cycloaliphatic or aromatic hydrocarbon,
and thereby
converted into an aqueous addition compound of formic acid and the amine. This
is
dewatered in a second distillation column. The dewatered addition compound
obtained
at the bottom is then, according to the teaching of DE 34 28 319 A, fed to the
uppermost
plate of a distillation column (in fig. 1 denoted as "K4") and thermally
dissociated. The
hydrophobic solvent is present both in the overhead stream and the bottoms
from the
column. The gaseous overhead stream comprises mainly the formic acid liberated
together with the hydrophobic solvent. This stream is liquefied again in the
condenser.
This results in formation of two phases, namely a polar formic acid phase and
a
hydrophobic solvent phase. The formic acid phase is discharged as product and
the
solvent phase is returned as runback to the column. Due to the presence of the
hydrophobic solvent, complete dissociation of the adduct, which according to
the
teaching of the DE first publication occurs without decomposition of formic
acid, can be
achieved. The (virtually) formic acid-free bottoms comprise the hydrophobic
amine and
the hydrophobic solvent. This is recirculated to the extraction stage.
EP 0 181 078 A and EP 0 126 524 A describe processes for obtaining formic acid
by
hydrogenation of carbon dioxide in the presence of a transition metal catalyst
and a
tertiary amine such as a C1-C10-trialkylamine to form an addition compound of
formic
acid and the tertiary amine, work-up of the hydrogenation output to separate
off the
catalyst and the low boilers, replacement of the amine base by a weaker,
higher-boiling
tertiary amine, in particular by an alkylimidazole, with splitting-off of the
first tertiary
amine and subsequent thermal dissociation of the newly formed addition
compound in a
distillation column. According to EP 0 181 078 A, fig. 1, the stream
comprising formic
acid and amine is for this purpose fed into the middle region of the column
"30". The
formic acid liberated in the thermal dissociation is removed as overhead
product. The
CA 02859128 2014-06-12
4
weaker, higher-boiling tertiary amine collects at the bottom and is
recirculated to the
stage of base exchange.
WO 2008/116,799 discloses a process for obtaining formic acid by hydrogenation
of
carbon dioxide in the presence of a transition metal catalyst, a high-boiling
polar solvent
such as an alcohol, ether, sulfolane, dimethyl sulfoxide or amide and a polar
amine
bearing at least one hydroxyl group to form an addition compound of formic
acid and the
amine. According to the teaching of WO 2008/116,799, the hydrogenation output
can be
fed directly to a distillation apparatus for thermal dissociation of the
addition compound.
This can comprise a distillation column and, if short residence times are
desired, also a
thin film evaporator or falling film evaporator. The formic acid liberated is
removed as
overhead product. The polar amine and the polar solvent and any catalyst which
has not
been separated off collect at the bottom and can be recirculated to the
hydrogenation
stage.
WO 2006/021,411 describes a process for obtaining formic acid by thermal
dissociation
of an addition compound of formic acid and a tertiary amine (quaternary
ammonium
formate), in which the tertiary amine has a boiling point of from 105 to 175
C.
Alkylpyridines are mentioned as preferred tertiary amines. The specific
boiling range of
the tertiary amines increases the color stability of the formic acid obtained.
The addition
compound to be used can in general be obtained from the tertiary amine and a
formic
acid source. The output from the adduct synthesis is advantageously firstly
freed of
volatile constituents and then fed to the thermal dissociation. The thermal
dissociation is
carried out as usual in a distillation column, with the stream comprising
formic acid and
amine being fed as per fig. 1 of WO 2006/021,411 into the middle region of the
column
(C). The formic acid liberated is removed as overhead product. The tertiary
amine which
may still comprise residues of formic acid collects in the liquid phase and
can be
recirculated to the formic acid source.
EP 0 563 831 A reports an improved process for the thermal dissociation of an
addition
compound of formic acid and a tertiary amine (quaternary ammonium formate) to
give
formic acid. The addition compound to be used can in general be obtained from
the
tertiary amine and a formic acid source. The output from the synthesis is
advantageously
CA 02859128 2014-06-12
firstly freed of volatile constituents and then fed into the middle of a
distillation column for
thermal dissociation. The improvement comprises essentially carrying out the
thermal
dissociation of the addition compound in the presence of a secondary formamide
which
increases the color stability of the formic acid obtained. The formic acid
liberated is
5 removed as overhead product. The tertiary amine and the secondary
formamide collect
in the liquid phase and can be recirculated to the formic acid source.
PCT/EP2011/060770 teaches a process for obtaining formic acid by thermal
separation
of a stream comprising formic acid and a tertiary amine (I), in which
combining tertiary
amine (I) and a formic acid source produces a liquid stream comprising formic
acid and
a tertiary amine (I) in a molar ratio of from 0.5 to 5, from 10 to 100% by
weight of the
secondary components comprised therein are separated off and formic acid is
removed
by distillation from the resulting liquid stream in a distillation apparatus
at a temperature
at the bottom of from 100 to 300 C and a pressure of from 30 to 3000 hPa, and
the
bottom output from the distillation apparatus is separated into two liquid
phases of which
the upper liquid phase is enriched in tertiary amine (I) and is recirculated
to the formic
acid source and the lower liquid phase is enriched in formic acid and is
recirculated to
removal of the secondary components and/or to the distillation apparatus.
It is an object of the present invention to discover an improved process for
obtaining
formic acid by thermal separation of a stream comprising formic acid and a
tertiary
amine, which process has advantages over the prior art and is able to give
formic acid in
high yield and high concentration. In particular, the improved process should
also
function stably over long operating times and produce formic acid in constant
high purity.
The process should naturally be able to be carried out very simply and with a
very low
energy consumption.
We have surprisingly found a process for obtaining formic acid by thermal
separation of
a stream comprising formic acid and a tertiary amine (I) which at a pressure
of 1013 hPa
abs has a boiling point which is at least 5 C higher than that of formic acid,
in which
CA 02859128 2014-06-12
6
(a) a liquid stream comprising formic acid and tertiary amine (I) and
having a molar
ratio of formic acid to tertiary amine (I) of from 0.5 to 5 is produced by
combining
tertiary amine (I) and a formic acid source;
(b) from 10 to 100% by weight of the secondary components comprised therein
are
separated off from the liquid stream obtained from step (a);
(c) formic acid is removed by distillation from the liquid stream
comprising formic acid
and tertiary amine (I) obtained from step (b) in a distillation apparatus at a
temperature at the bottom of from 100 to 300 C and a pressure of from 30 to
3000 hPa abs, where the tertiary amine (I) to be used in step (a) and the
degree of
separation in the abovementioned distillation apparatus are selected so that
two
liquid phases are formed in the bottom output;
(d) the bottom output from the distillation apparatus mentioned in step (c) is
separated
into two liquid phases, where the upper liquid phase has a molar ratio of
formic
acid to tertiary amine (I) of from 0 to 0.5 and the lower liquid phase has a
molar
ratio of formic acid to tertiary amine (I) of from 0.5 to 4;
(e) the upper liquid phase from the phase separation in step (d) is
recirculated to step
(a); and
(f) the lower liquid phase from the phase separation in step (d) is
recirculated to step
(b) and/or (c),
wherein
(g) low boilers which at a pressure of 1013 hPa abs have a boiling point
which is at
least 5 C lower than that of the tertiary amine (I) are separated off by
distillation
from the upper liquid phase from the phase separation in step (d) in a
distillation
apparatus at a temperature at the bottom of from 100 to 300 C and a pressure
of
from 1 to 1000 hPa abs and the stream depleted in low boilers is recirculated
to
one of the abovementioned steps (a) to (f).
CA 02859128 2014-06-12
7
The tertiary amine (I) used in step (a) of the process of the invention has,
at a pressure
of 1013 hPa abs, a boiling point which is at least 5 C higher than that of
formic acid. The
tertiary amine (I) to be used preferably has a boiling point which is at least
10 C higher,
particularly preferably at least 50 C higher and very particularly preferably
at least 100 C
higher, than that of formic acid. A restriction in respect of an upper limit
value for the
boiling point is not necessary since a very low vapor pressure of the tertiary
amine (I) is
basically advantageous for the process of the invention. In general, the
boiling point of
the tertiary amine (I) is below 500 C at a pressure optionally extrapolated by
known
methods from vacuum to 1013 hPa abs.
The formic acid source mentioned in step (a) is a stream which comprises
formic acid in
dilute, contaminated and/or chemically bound form or comprises a precursor
from which
formic acid is produced by chemical reaction. The formic acid source in step
(a)
ultimately ensures the direct or indirect introduction of formic acid.
Addition in chemically
bound form can, for example, be effected in the form of a complex, a salt or
an addition
compound of formic acid and an amine other than the tertiary amine (I).
Possible
chemical reactions are in principle all chemical reactions in which formic
acid is
produced. However, the production of formic acid by hydrolysis of methyl
formate and
production of formic acid by transition metal-catalyzed hydrogenation of
carbon dioxide
are of particular industrial importance at the time of the present patent
application. Both
the possible syntheses mentioned are well known in the art and have been
described in
a variety of variants and embodiments. A further industrially relevant
possibility for
producing formic acid by chemical reaction is, for example, direct reaction of
carbon
monoxide with water.
In the case of the hydrolysis of methyl formate, it is usual to introduce
methyl formate,
water and tertiary amine (I) either together or in succession into the
hydrolysis reactor in
order to trap the formic acid formed by hydrolysis in the form of an addition
compound by
means of the tertiary amine (I) and thus withdraw it from the hydrolysis
equilibrium. This
makes it possible to achieve a higher conversion of methyl formate and allows
particularly advantageous removal of the unreacted water by means of a
subsequent
distillation.
CA 02859128 2014-06-12
8
In the case of the transition metal-catalyzed hydrogenation of carbon dioxide,
the tertiary
amine (I) is generally introduced into the hydrogenation reactor in order to
form a stream
comprising formic acid and a tertiary amine (I) in the hydrogenation itself.
The stream comprising formic acid and tertiary amine (I) is preferably
produced by
hydrolysis of methyl formate in the presence of water and tertiary amine (I)
in step (a).
Production of the stream comprising formic acid and tertiary amine (I) by
concentration
of dilute formic acid in the presence of tertiary amine (I) in step (a) is
also preferred.
However, the stream comprising formic acid and tertiary amine (I) is
particularly
preferably produced by hydrolysis of methyl formate in the presence of water
and tertiary
amine (I) in step (a).
The tertiary amine (I) and the formic acid source can be combined in the
presence of
water in step (a). In the preferred hydrolysis of methyl formate, water is
actually needed
as reactant for the conversion of methyl formate. If the tertiary amine (I)
and the formic
acid source are combined in the presence of water in step (a), the content of
water is
generally set, taking account of the amount of chemically consumed water, so
that the
liquid stream produced in step (a) comprises not only formic acid and tertiary
amine (I)
but also water.
The combining of tertiary amine (I) and the formic acid source can be carried
out in a
variety of ways. If the formic acid source is a stream comprising formic acid
in dilute,
contaminated and/or chemically bound form, simple contacting, preferably with
mixing,
with the tertiary amine (I) is often sufficient. This can, for example, be
carried out in
tubes which preferably comprise suitable mixing internals. Contacting can
likewise be
carried out in other apparatuses, for example stirred vessels. Stepwise
combining in
which the tertiary amine (I) is added stepwise to the formic acid source or,
conversely,
the formic acid source is added stepwise to the tertiary amine (I) is also
possible and
may even be advantageous. If the formic acid source is a stream from which the
formic
acid is to be produced from a number of materials by chemical reaction, it is
generally
advantageous to produce the formic acid source by combining the individual
components in the reactor. Possible reactors are, in particular, the reactors
known to
CA 02859128 2014-06-12
9
those skilled in the art for this type of reaction. The tertiary amine (I)
can, for example, be
initially charged, introduced in parallel to the individual components of the
formic acid
source, introduced during the course of the chemical reaction or introduced
only at the
end of the chemical reaction. It is also possible to distribute these
individual steps over a
plurality of reactors. Depending on the heat involved on combining tertiary
amine (I) and
the formic acid source, it may be advantageous to cool the apparatus itself or
the stream
obtained therefrom.
Suitable ways of combining tertiary amine (I) and the formic acid source can
be
determined without great difficulty on the basis of routine knowledge in the
art.
The liquid stream produced on combining tertiary amine (I) and a formic acid
source in
step (a) has a molar ratio of formic acid to tertiary amine (I) of from 0.5 to
5. The molar
ratio is preferably 1 and preferably 3. The molar ratio mentioned is based on
the total
liquid stream, regardless of whether it is present as a single phase or a
plurality of
phases.
The liquid stream comprising formic acid and tertiary amine (I) which is
produced in step
(a) generally has a concentration of formic acid plus tertiary amine (I) of
from 1 to 99%
by weight, based on the total amount of the stream. The stream mentioned
preferably
has a concentration of formic acid plus tertiary amine (I) of 5% by weight and
particularly preferably 15% by weight and also preferably 95% by weight and
particularly preferably 90% by weight.
From 10 to 100% by weight of the secondary components present in the liquid
stream
obtained from step (a) are separated off from this liquid stream. The range
mentioned is
based on the concentration of secondary components in the liquid stream
produced in
step (a). This concentration will hereinafter be referred to as
"Csecondarycomponents (stream
from step (a))". The liquid stream depleted in secondary components
corresponds to the
stream which is fed to the distillation apparatus as per step (c). This
concentration will
hereinafter be referred to as "Csecondarycomponents (feed stream to step
(c))". The
abovementioned removal of secondary components is thus based on the quotient
CA 02859128 2014-06-12
C second a ryc amp n en rs eed stream to step (0)[g 11]
.100% by weight
C second a ry arnp an sr (strewn from step (a))[911]
Preference is given to 20% by weight and particularly preferably 30% by weight
and
5 also preferably 99.99% by weight and particularly preferably 99.9% by
weight of the
secondary components being separated off in step (b).
Here, the term secondary components refers to all components which are
comprised in
the liquid stream obtained in step (a) and are neither formic acid nor
tertiary amine (I).
10 Examples which may be mentioned are water, methanol (in particular in
the case of the
hydrolysis of methyl formate), dissolved unhydrolyzed methyl formate (in
particular in the
case of the hydrolysis of methyl formate), possible degradation products of
the tertiary
amine (I), dissolved inert gases, homogeneous catalyst (in particular in the
case of the
hydrogenation of carbon dioxide), dissolved carbon dioxide or dissolved
hydrogen (in
particular in the case of the hydrogenation of carbon dioxide), solvents,
other
components.
The way in which the secondary components may be separated off is
inconsequential
for the process of the invention. Thus, for example, it is possible to use the
customary
and known methods for the separation of liquid mixtures. Particular mention
may be
made of separation by distillation. In this case, the liquid mixture is
separated in a
distillation apparatus. Thus, for example, low-boiling secondary components
such as
methanol, methyl formate or water can be separated off at the top or as a side
offtake
stream. However, it is also conceivable to separate off high-boiling secondary
components at the bottom and the mixture comprising formic acid and tertiary
amine (I)
as side stream or overhead product. Apart from separation by distillation,
membrane,
absorption, adsorption, crystallization, filtration, sedimentation or
extraction processes
are, however, also possible. Preference is given to extraction processes in
the
concentration of dilute aqueous formic acid and the use of tertiary amines (I)
which are
immiscible or only miscible to a small extent with water.
CA 02859128 2014-06-12
11
It is naturally also possible to combine a plurality of separation steps which
may also be
based on different methods. The design of the separation step or separation
steps can
be undertaken using conventional technical knowledge.
Of course, further process steps apart from step (b) can be carried out
between steps (a)
and (c) in the process of the invention.
Finally, formic acid is removed by distillation in a distillation apparatus at
a temperature
at the bottom of from 100 to 300 C and a pressure of from 30 to 3000 hPa abs
from the
liquid stream obtained from step (b). As distillation apparatuses for this
purpose, it is in
principle possible to use the apparatuses known to those skilled in the art
for such
separation tasks or can be designed by a person skilled in the art using
general
technical knowledge.
The distillation apparatus usually comprises not only the actual column body
with
internals but also, inter alia, an overhead condenser and a bottom vaporizer.
In addition,
these can naturally also comprise further peripheral apparatuses or internals,
for
example a flash vessel in the feed line (for example to separate gas and
liquid in the
feed to the column body), an intermediate vaporizer (for example for improved
heat
integration of the process) or internals for avoiding or reducing aerosol
formation (for
example heatable trays, demisters, coalescers or deep-bed diffusion filters).
The column
body can be equipped, for example, with ordered packing, random packing
elements or
trays. The number of theoretical plates required is dependent, in particular,
on the type
of tertiary amine (I), the concentration of formic acid and tertiary amine (I)
in the feed to
the distillation apparatus in step (c) and the desired concentration or the
desired purity of
the formic acid, and can be determined in a conventional way by a person
skilled in the
art. The number of theoretical plates required is generally 3, preferably ?..
6 and
particularly preferably 7. There are in principle no upper limits. However,
for practical
reasons it will be usual to use generally 70, optionally 50, theoretical
plates or even
30 theoretical plates.
CA 02859128 2014-06-12
12
The stream comprising formic acid and tertiary amine (I) from step (b) can be
fed, for
example, as side stream to the column body in the distillation apparatus.
A flash evaporator, for example, can optionally also precede the addition. To
keep the
thermal stress on the stream fed into the distillation apparatus as small as
possible, it is
generally advantageous to feed this in in a relatively low region of the
distillation
apparatus. Thus, in step (c), the stream comprising formic acid and tertiary
amine (I) is
preferably fed in in the region of the lower quarter, preferably in the region
of the lower
fifth and particularly preferably in the region of the lower sixth, of the
theoretical plates
present, with direct introduction into the bottom naturally also being
comprised here.
As an alternative, preference is also given, in step (c), to feed said stream
comprising
formic acid and a tertiary amine (I) from step (b) into the bottom vaporizer
of the
distillation apparatus.
The distillation apparatus is operated at a temperature at the bottom of from
100 to
300 C and a pressure of from 30 to 3000 hPa abs. The distillation apparatus is
preferably operated at a temperature at the bottom of 120 C, particularly
preferably
140 C, and preferably 220 C and particularly preferably 200 C. The pressure is
preferably 30 hPa abs, particularly preferably 60 hPa abs, and preferably 1500
hPa
abs and particularly preferably 500 hPa abs.
Depending on the composition and origin of the feed comprising formic acid and
a
tertiary amine (I) to the distillation apparatus, formic acid can be obtained
as overhead
product and/or side product from the distillation apparatus. If the feed
comprises
constituents having boiling points lower than that of formic acid, it may be
advantageous
to separate these off as overhead product and separate off the formic acid at
a side
offtake in the distillation. In the case of possible dissolved gases (for
example carbon
monoxide or carbon dioxide) in the feed, it is generally also possible to
separate off the
formic acid together with these as overhead product. If the feed comprises
constituents
having boiling points higher than that of formic acid, formic acid is
preferably separated
off by distillation as overhead product, but optionally instead or
additionally in the form of
CA 02859128 2014-06-12
13
a second stream at the side offtake. The constituents which have boiling
points higher
than that of formic acid are in this case preferably taken off in an
additional side stream.
The side stream comprising secondary components can optionally be recirculated
to
step (b) in order to separate off the secondary components.
Formic acid having a content of up to 100% by weight can be obtained in this
way. In
general, formic acid contents of from 75 to 99.995% by weight can be achieved
without
problems. The balance to 100% by weight is mainly water, with other components
such
as solvents or possible decomposition products naturally also being
conceivable as
materials apart from formic acid and the tertiary amine (I) introduced into
the distillation
apparatus. Thus, water can, for example, be comprised in the feed to the
distillation
apparatus or else may also be formed in small amounts only in the thermal
separation
by decomposition of formic acid.
In the isolation of concentrated formic acid having a content of from 95 to
100% by
weight as overhead or side product, water is discharged in a side stream
together with
part of the formic acid split off. The formic acid content of the side stream
is typically
from 75 to 95% by weight. The aqueous formic acid in the side stream can
optionally be
recirculated to step (b) in order to separate off the water.
However, it is also possible to discharge the water and the formic acid split
off in a joint
overhead or side stream. The formic acid content of the product obtained in
this way is
then generally from 85 to 95% by weight.
To largely suppress, in particular, the formation of organic decomposition
products of the
tertiary amine (I) which are formed by oxidation, it is particularly
advantageous,
especially when the distillation apparatus is operated at pressures below 0.1
MPa abs,
for the intrusion of oxygen through a large number of connections, ports and
flanges to
be avoided or at least kept extremely low by special care during installation,
by use of
particularly well-sealed flange connections (for instance those having comb
profile seals
or weld lip seals) or by means of nitrogen-blanketed flange connections. A
suitable
flange connection is disclosed, for example, in DE 10 2009 046 310 Al.
CA 02859128 2014-06-12
14
The formic acid which can be obtained by the process of the invention has a
low color
number and also a high color number stability. In general, a color number of
5_ 20 APHA,
in particular even 5_ 10 APHA and possibly even 5 APHA, can be achieved
without
problems. Even on storage for a number of weeks, the color number remains
virtually
constant or increases only insignificantly.
Owing to the removal of the organic decomposition products of the tertiary
amine (I)
according to the invention in step (b), a particularly pure formic acid in
which said
decomposition products are generally present in a concentration of 70 ppm by
weight,
preferably 30 ppm by weight and very particularly preferably 20 ppm by weight,
can
be obtained without a further outlay.
The content of secondary components is extremely low and is generally 100 ppm
by
weight, preferably 50 ppm by weight and very particularly preferably 25 ppm by
weight.
It may also be advantageous to use a plurality of distillation apparatuses in
step (c),
particularly when further fractions, for example accompanying materials
comprised,
reaction by-products, impurities and/or formic acid fractions of various
purities and
concentrations, are to be obtained in addition to the free formic acid and the
amine (1)-
comprising bottom product.
The distillation apparatus for separating off the formic acid can naturally
also be
configured as thermally coupled distillation columns or as a dividing wall
column.
In the process of the invention, the tertiary amine (I) to be used in step (a)
and the
degree of separation in the distillation apparatus mentioned in step (c) are
selected so
that two liquid phases are formed in the bottom output from the distillation
apparatus
mentioned in step (c).
CA 02859128 2014-06-12
The formation of two liquid phases is determined mainly by the chemical and
physical
properties of the two phases. These can in turn be influenced by the choice of
the
tertiary amine (I) to be used, by the degree of separation in the distillation
apparatus and
also by the presence of any additional components such as solvents and the
5 concentrations thereof.
For the present purposes, the degree of separation is the quotient
Mprmic acid (feed stream to step (c))[g I h] m forme. acid (bottom
output)[g I h]
= 100%
M formic acid (feed stream to step (c))[g I h]
wherem
"¨formic acid(feed stream to step (c))" is the amount of formic acid fed per
unit time
to the distillation apparatus andm
"¨formic acid(bottom output)" corresponds to the amount of
formic acid discharged per unit time in the bottom output. In this preferred
embodiment
of the process of the invention, the degree of separation selected is
generally 10%,
preferably 25% and particularly preferably 40%, and generally 99.9%,
preferably
99.5% and particularly preferably 99.0%. The degree of separation can, for
example,
be easily influenced by the temperature and pressure conditions in the
distillation
apparatus and by the residence time in the distillation apparatus. It can be
determined
by means of simple tests, optionally also during operation of the process of
the
invention.
The suitability of a tertiary amine (I) or a solvent which is optionally
additionally desired
can be determined, for example, in simple tests in which the number of phases
is
determined under the conditions envisaged.
The phase separation can, for example, be carried out in a separate phase
separator
located downstream of the distillation apparatus. However, it is also possible
to integrate
the phase separator into the bottom region of the distillation apparatus, in
the region of
the bottom vaporizer or else in the region of the bottom vaporizer circuit.
Here, it is also
possible or may even be advantageous to use, for example, a centrifugal
separator.
CA 02859128 2014-06-12
16
Since the formation of two liquid phases is also influenced by the temperature
in addition
to the chemical and physical properties of the two phases and the miscibility
generally
increases with temperature, it may be advantageous to operate the phase
separation at
a lower temperature than the temperature at the bottom previously selected in
order to
improve the phase separation. For this purpose, the bottom output is usually
cooled to a
temperature in the range from 30 to 180 C in an intermediate heat exchanger.
The
phase separation is preferably carried out at a temperature of 50 C and at a
temperature of 160 C and particularly preferably at a temperature of 130 C.
The upper liquid phase in step (d) has a molar ratio of formic acid to
tertiary amine (I) of
in general from 0 to 0.5, preferably 0.005 and particularly preferably 0.015
and also
preferably 0.25 and particularly preferably 0.125. The lower liquid phase in
step (d)
has a molar ratio of formic acid to tertiary amine (I) of in general from 0.5
to 4, preferably
0.75 and particularly preferably 1 and also preferably 3.5 and particularly
preferably 3. However, depending on the choice of the amine, it can of course
also be
possible for the phase comprising formic acid to form the upper phase and the
amine
phase having a molar formic acid/amine ratio of from 0 to 0.5 to form the
lower phase. It
is merely important that there is a phase separation with one phase having a
molar ratio
of formic acid to tertiary amine of in general from 0 to 0.5 and a second
phase having a
molar ratio of formic acid to tertiary amine of in general from 0.5 to 4. The
upper phase is
preferably that having a molar ratio of formic acid to tertiary amine of in
general from 0 to
0.5 and the lower phase is preferably that having a molar ratio of formic acid
to tertiary
amine of in general from 0.5 to 4.
Furthermore, it is advantageous in the process of the invention to select the
degree of
separation of the distillation apparatus mentioned in step (c) in such a way
that the molar
ratio of formic acid to tertiary amine (I) in the bottom output is from 0.1 to
2Ø For the
purposes of the present invention, the bottom output is the totality of the
liquid bottom
condensates which leave the distillation apparatus and are separated into two
liquid
phases in step (d). It is inconsequential whether the bottom condensates
originate, for
example, directly from the bottom of the distillation apparatus, the bottom of
the bottom
vaporizer or from both. The degree of separation of the distillation apparatus
mentioned
CA 02859128 2014-06-12
17
in step (c) is preferably selected so that the molar ratio of formic acid to
tertiary amine (I)
in the bottom output is preferably 1.5.
As a result of the recirculation of the upper liquid phase from the phase
separation in
step (d) to step (a) as per step (e), the tertiary amine (I) comprised in the
upper liquid
phase can be used, by combination with the formic acid source, for further
generation of
a stream comprising formic acid and tertiary amine (I). In general, from 10 to
100%,
preferably from 50 to 100%, particularly preferably from 80 to 100%, very
particularly
preferably from 90 to 100% and in particular from 95 to 100%, of the upper
liquid phase
is recirculated to step (a).
In the context of the present invention, it has surprisingly been found that
the upper
liquid phase from the phase separation in step (d) is particularly enriched
with low-
boiling, organic degradation products of the tertiary amine (I), compared to
other low-
formic acid streams.
For the purposes of the present invention, the term organic decomposition
products of
the tertiary amine (I) refers to compounds which are formed by chemical
transformation
of the tertiary amine (I) with parting of bonds originally present, new
formation of
nitrogen-carbon bonds or chemical transformation of the radicals bound to the
nitrogen.
Thus, it has been recognized in the context of the invention that tertiary
amines (I) tend,
for example, to decompose in the presence of formic acid at elevated
temperature and
elevated pressure, as prevail in individual steps of the process of the
invention, to form
the corresponding formamide which is N,N-substituted by the radicals of the
tertiary
amine (I) and the corresponding formate comprising the other radical of the
tertiary
amine (I). In the case of a tertiary amine (I) having three identical radicals
R, for example
C5-C8-alkyl, the abovementioned decomposition reaction would, for example, be
as
follows:
/0 0
NR3 + 2 H H _________________________________ + H
-H20
OHNR2 OR
, (A)
CA 02859128 2014-06-12
18
forming the corresponding dialkylformamide and the corresponding alkyl formate
as
organic decomposition products of the tertiary amine (I).
Furthermore, it has been recognized in the context of the invention that
tertiary amines
(I) also tend, for example, to decompose in the presence of formic acid and
traces of
oxygen at elevated temperature, as can prevail in individual steps of the
process of the
invention, to form the corresponding formamide which is N,N-substituted by the
radicals
of the tertiary amine (I) and the aldehyde formed from the other radical. In
the case of a
tertiary amine (I) having three identical radicals CH2-R, for example C5-C8-
alkyl, the
abovementioned decomposition reaction would, for example, be as follows:
0 + 1/ 0 ,0 0
N(CH2R)3 + H ____________________ 2 2 - H 2 0 H + R
OH N(CH2R)2 H , (B)
forming the corresponding dialkylformamide and the corresponding alkanal as
organic
decomposition products of the tertiary amine (I).
Furthermore, it was recognized in the context of the invention that tertiary
amines (I)
tend in the presence of methyl formate which is used in obtaining formic acid
by
hydrolysis of methyl formate to be methylated to the corresponding methyl
ammonium
cation. In the case of a tertiary amine (I) having three identical radicals R,
for example
C5-C8-alkyl, the abovementioned methylation reaction would, for example, be as
follows,
where Me is methyl:
NR3 H __ /< [MeNR3]. [H000]-
\OMe
(C)
This can redissociate, also forming a tertiary amine having a methyl group. In
the case of
the abovementioned system, this reaction equation would be as follows:
CA 02859128 2014-06-12
19
1/0
[MeNR3]- [H000]- ________________ MeNR2 + H
OR (D)
According to reaction equation (A), the tertiary amine comprising a methyl
group then
likewise leads to formation of dialkylformamide:
0 0 0
MeNR2 + 2 -HO 2 H __ 1( + H
OH 'NR2 OMe
(E)
Organic decomposition products of the tertiary amine (I) can lead to
contamination of the
formic acid to be obtained as per step (c). In addition, organic decomposition
products of
the tertiary amine (I) having a boiling point between that of formic acid and
the tertiary
amine (I) tend to accumulate in the distillation apparatus used in step (c)
and thereby
increase the energy consumption in the distillation apparatus.
In the context of the invention, it was recognized that any interfering
components can be
separated off particularly well and in a simple manner by distillation from
the
abovementioned upper liquid phase from the phase separation in step (d). In
the
process of the invention, low boilers which at a pressure of 1013 hPa abs have
a boiling
point which is at least 5 C lower than that of the tertiary amine (I) are, in
step (g),
separated off by distillation from the upper liquid phase from the phase
separation in
step (d) in a distillation apparatus at a temperature at the bottom of from
100 to 300 C
and a pressure of from 1 to 1000 hPa abs and the stream depleted in low
boilers is
recirculated to one of the abovementioned steps (a) to (f).
Low boilers are generally secondary components as defined in the present
description
which at a pressure of 1013 hPa abs have a boiling point which is at least 5 C
lower
than that of the tertiary amine (I). This preferably has a boiling point which
is at least 7 C
lower and particularly preferably at least 10 C lower than that of the
tertiary amine (I). A
CA 02859128 2014-06-12
restriction in terms of a lower limit for the boiling point is not necessary
since particularly
low-boiling low boilers can generally also be separated off particularly
easily by
distillation. However, the boiling point of the low boilers at the
abovementioned pressure
of 1013 hPa abs is generally above 100 C.
5
The low boilers to be separated off in the process of the invention are either
present in
the tertiary amine (I) fed to step (a) and/or are formed only during the
course of the
process up to the present step (g). Thus, it is, for example, possible for the
tertiary amine
(I) fed to step (a) to comprise various Organic decomposition products of the
tertiary
10 amine (I) as a result of its production or pretreatment. However, it is
also possible and
usually also the case that the low boilers to be separated off are formed,
either
exclusively or in addition to those introduced in the tertiary amine (I), in
steps (a) to (c)
under appropriate conditions.
15 The separation in step (g) of the low boilers is carried out by
distillation. Possible
distillation apparatuses for this purpose are in principle apparatuses which
are known to
those skilled in the art for such separation tasks or can be designed by a
person skilled
in the art using general technical knowledge. The distillation apparatus is
operated at a
temperature at the bottom of from 100 to 300 C and a pressure of from 1 to
1000 hPa
20 abs. The distillation apparatus is preferably operated at a temperature
at the bottom of ?_
120 C, particularly preferably 140 C, and also preferably 220 C and
particularly
preferably 200 C. The pressure is preferably 5 hPa abs, particularly
preferably 10
hPa abs, and also preferably 500 hPa abs and particularly preferably 250 hPa
abs.
The stream depleted in low boilers is generally obtained as bottom product.
However, it
is also possible to obtain it as side stream, especially when high boilers
possibly present,
i.e. generally components having boiling points higher than that of the
tertiary amine (I),
are to be removed at the same time in the removal of the low boilers by
distillation.
In the process of the invention, it is usual to feed from 0.01 to 50% of the
upper liquid
phase from the phase separation in step (d) to step (g). This amount is
sufficient firstly to
keep the low boilers present at a sufficiently low level, and secondly to keep
the outlay,
CA 02859128 2014-06-12
21
for example, the size of the distillation apparatus or the ongoing energy
consumption,
within limits. Preference is given to feeding 0.1% and particularly preferably
0.5%
and also preferably 20%, particularly preferably 10% and very particularly
preferably
5%, of the upper liquid phase from the phase separation in step (d) to step
(g).
The removal according to the invention of the low boilers in step (g) enables
the amount
thereof in the process to be kept at a low level. In particular, an
accumulation which
increases as time goes on is also effectively and cleverly countered in this
way.
The concentration of low boilers, based on a stream which comprises the bottom
product
from step (g) and the remaining upper liquid phase from step (e) which has not
been fed
to step (g) combined with one another, can easily be kept at a value of 25% by
weight,
preferably 15% by weight and particularly preferably 10% by weight. In
general, the
abovementioned concentration is 0.001% by weight, usually 0.1% by weight. The
degree of depletion of low boilers
in 'ow b oilerstdepieted strearn)IstAl
=
(1 rn!owµboaersCfeed stream to step (g))Lg 1.00%
is generally from 1 to 100%, preferably 10%, particularly preferably 50%.
The low boilers which have been separated off can, for example, be disposed
of.
The stream depleted in low boilers obtained in step (g) is recirculated to one
of the
abovementioned steps (a) to (f) in the process of the invention. In general, a
total of from
10 to 100%, preferably from 50 to 100%, particularly preferably from 80 to
100%, very
particularly preferably from 90 to 100% and in particular from 95 to 100%, of
the stream
depleted in low boilers is recirculated to steps (a) to (f). It is naturally
also possible, for
example, to recirculate the stream depleted in low boilers to a selected
point, i.e., for
example, also to split it and recirculate it to various points. Preference is
given to
recirculating the stream which has been depleted in low boilers to one of the
abovementioned steps (a) to (e). In a particularly preferred embodiment, the
stream
CA 02859128 2014-06-12
22
which has been depleted in low boilers is recirculated to step (a). In
another, particularly
preferred embodiment, the stream which has been depleted in low boilers is
recirculated
to step (b).
It is of course also possible in general for further process steps to be
integrated into the
recirculation of the upper liquid phase from the phase separation from step
(d) to step
(a), in addition to step (g). The type of intermediate process steps are in
principle not
subject to any limits. It is also possible to remove part of the upper liquid
phase in a
targeted manner as "purge stream". Missing amounts of tertiary amine (I) or
amounts of
this which have been lost can naturally be replaced again by fresh tertiary
amine (I)
which, for example, can be introduced via the recycle stream or directly into
step (a) or
at any point in the process, for example in step (b) or step (c).
In the process of the invention, as per step (f), the lower liquid phase from
the phase
separation is recirculated in step (d) to step (b) and/or (c). This enables
the formic acid
comprised in the lower liquid phase likewise to be utilized for isolating
formic acid by
removal by distillation. Depending on the desired embodiment, the lower liquid
phase
can thus be recirculated (i) to step (b), (ii) partly to step (b) and partly
to (c) or (iii) to step
(c). However, preference is generally given to recirculation to step (c) since
the stressing
of the lower liquid phase comprising formic acid and tertiary amine (I) is
usually the
lowest in this case and the quantity of the stream in step (b) is not
increased, which
would otherwise have the consequence of correspondingly larger dimensions. In
general, from 10 to 100%, preferably from 50 to 100%, particularly preferably
from 80 to
100%, very particularly preferably from 90 to 100% and in particular from 80
to 100%, of
the lower liquid phase is recirculated to step (b) and/or (c).
However, it is also possible to recirculate a further part of the lower liquid
phase to step
(a) in addition to the abovementioned recirculation to step (b) and/or (c).
This is, for
example, advantageous when the formic acid is produced by transition metal-
catalyzed
hydrogenation of carbon dioxide, since this is generally carried out in the
presence of a
polar solvent which likewise accumulates in the lower liquid phase and can
thus be
recirculated to step (a).
CA 02859128 2014-06-12
23
It is of course also possible for further process steps to be integrated into
the
recirculation of the lower liquid phase. As a nonlimiting example, mention
may, here too,
be made of a purification of the lower liquid phase to be recirculated or of
the tertiary
amine (I) comprised therein and/or the formic acid comprised therein in order
to remove
undesirable accompanying materials, reaction by-products or further
impurities. The type
of intermediate process steps is also in principle not subject to any limits.
It is also
possible to discharge part of the lower liquid phase in a targeted manner as
"purge
stream" in order to remove, for example, undesirable accompanying materials,
reaction
by-products or further impurities.
The tertiary amine (I) which is preferably to be used in the process of the
invention has
the general formula (la)
NR1R2R3 (la),
where the radicals R1 to R3 are identical or different and are each,
independently of one
another, an unbranched or branched, acyclic or cyclic, aliphatic, araliphatic
or aromatic
radical having in each case from 1 to 16 carbon atoms, preferably from 1 to 12
carbon
atoms, where individual carbon atoms can also be, independently of one
another,
replaced by a heterogroup selected from the group consisting of -0- and >N-
and two or
all three radicals can also be joined to one another to form a chain
comprising at least
four atoms.
Examples of suitable amines are:
= Tri-n-propylamine (bp1013 hPa = 156 C), tri-n-butylamine, tri-n-
pentylamine, tri(3-
methylbutyl)amine, tri-n-hexylamine, tri-n-heptylamine, tri-n-octylamine, tri-
n-
nonylamine, tri-n-decylamine, tri-n-undecylamine, tri-n-dodecylamine, tri-n-
tridecyl-
amine, tri-n-tetradecylamine, tri-n-pentadecylamine, tri-n-hexadecylamine,
tri(2-
ethylhexyl)amine, tri(2-propylheptyl)amine.
= Dimethyldecylamine, dimethyldodecylamine, dimethyltetradecylamine, ethyl-
di(2-
propyl)amine (bp1013 hpa = 127 c), di-n-octylmethylamine, di-n-
hexylmethylamine, di-n-
CA 02859128 2014-06-12
24
hexyl(2-methylpropyl)amine, di-n-hexyl(3-methylbutyl)amine, methyl-di(2-
ethylhexyl)amine, di-n-hexyl(1-methyl-n-hexyl)amine, di-2-propyldecylamine.
= Tricyclopentylamine, tricyclohexylamine, tricycloheptylamine,
tricyclooctylamine and
derivatives thereof substituted by one or more methyl, ethyl, 1-propyl, 2-
propyl, 1-butyl,
2-butyl or 2-methyl-2-propyl groups.
= Dimethylcyclohexylamine, methyldicyclohexylamine, diethylcyclohexylamine,
ethyl-
dicyclohexylamine, dimethylcyclopentylamine, methyldicyclopentylamine, methyl-
dicyclohexylamine.
= Triphenylamine, methyldiphenylamine, ethyldiphenylamine,
propyldiphenylamine,
butyldiphenylamine, 2-ethylhexyldiphenylamine, dimethylphenylamine, diethyl-
phenylamine, dipropylphenylamine, dibutylphenylamine, bis(2-ethylhexyl)phenyl-
amine, tribenzylamine, methyldibenzylamine, ethyldibenzylamine and derivatives
thereof substituted by one or more methyl, ethyl, 1-propyl, 2-propyl, 1-butyl,
2-butyl
or 2-methyl-2-propyl groups.
= 1,5-Di(1-piperidyl)pentane, N-C1-C12-alkylpiperidines, N,N-di-C1-C12-
alkylpiperazines, N-
C1-C12-alkylpyrrolidines, N-C1-C12-alkylimidazoles and derivatives thereof
substituted by
one or more methyl, ethyl, 1-propyl, 2-propyl, 1-butyl, 2-butyl or 2-methyl-2-
propyl
groups.
= 1,8-Diazabicyclo[5.4.0]undec-7-ene ("DBU"), 1,4-
diazabicyclo[2.2.2]octane, N-
methyl-8-azabicyclo[3.2.1]octane ("tropane"), N-methyl-9-
azabicyclo[3.3.1]nonane
("granatane"), 1-azabicyclo[2.2.2]octane ("quinuclidine"), 7,15-
diazatetracyclo-
[7.7.1.027.010.15]heptadecane ("sparteine").
It is naturally also possible to use mixtures of various tertiary amines (1)
in the process of
the invention. Naturally, all tertiary amines (1) used then preferably have,
at a pressure of
1013 hPa abs, a boiling point which is at least 5 C higher than that of formic
acid.
CA 02859128 2014-06-12
Among the above-described tertiary amines of the general formula (la),
preference is in
turn given to those in which the radicals R1 to R3 are identical or different
and are each,
independently of one another, an unbranched or branched, acyclic or cyclic,
aliphatic,
araliphatic or aromatic radical having in each case from 1 to 16 carbon atoms,
preferably
5 from 1 to 12 carbon atoms, where individual carbon atoms may also be,
independently
of one another, replaced by a heterogroup selected from the group consisting
of -0- and
>N- and two or all three radicals can also be joined to one another to form a
saturated
chain comprising at least four atoms.
10 Preference is given to at least one of the radicals on the alpha-carbon
atom, i.e. on the
carbon atom bound directly to the amine nitrogen atom, having two hydrogen
atoms.
In the process of the invention, particular preference is given to using an
amine of the
general formula (la) in which the radicals R1 to R3 are selected independently
from the
15 group consisting of C1-C12-alkyl, C5-C8-cycloalkyl, benzyl and phenyl as
tertiary amine
(I).
Very particular preference is given to using a saturated amine of the general
formula (la)
as tertiary amine (I) in the process of the invention.
In particular, an amine of the general formula (la) in which the radicals R1
to R3 are
selected independently from the group consisting of C5-C8-alkyl, in particular
tri-n-
pentylamine, tri-n-hexylamine, tri-n-heptylamine, tri-n-octylamine,
dimethylcyclohexyl-
amine, methyldicyclohexylamine, dioctylmethylamine and dimethyldecylamine, is
used
as tertiary amine (I) in the process of the invention.
In a further embodiment, amines which have a branch on the alpha-carbon atom
(the
carbon atom bound directly to the amine nitrogen atom), on the beta-carbon
atom (the
second carbon atom from the amine nitrogen atom) or the gamma-carbon atom (the
third carbon atom from the amine nitrogen atom) are used. Here, alkyl, aryl
and other
substituents are conceivable in principle, with preference being given to
alkyl groups
such as methyl, ethyl or propyl groups or piperidinyl groups. In this
embodiment,
particular preference is given to N-ethylpiperidine, tri(3-methylbutyl)amine,
di-n-hexyl(2-
CA 02859128 2014-06-12
26
methylpropyl)amine, di-n-hexyl(3-methylbutyl)amine, methyldi(2-
ethylhexyl)amine, di-n-
hexyl(1-methyl-n-hexyl)amine, di-2-propyldecylamine, methyldicyclohexylamine,
1,5-
di(1-piperidyl)pentane.
The streams comprising formic acid and tertiary amine (I) which are formed in
the
process of the invention can comprise not only free formic acid and the free
tertiary
amine (I) but also, in admixture with these, formic acid and the tertiary
amine (I) in
various other forms. The type and amount of the individual forms can differ as
a function
of the prevailing conditions, for instance the relative ratio of formic acid
to tertiary amine
(I), the presence of further components (for example water, solvents, by-
products,
impurities) and thus ultimately also the concentration of formic acid and
tertiary amine
(I), the temperature and the pressure. Thus, for example, the following
conceivable
forms may be mentioned:
- Ammonium formate (molar ratio of formic acid to tertiary amine (I) of 1)
or formic
acid-rich adduct with the tertiary amine (I) (molar ratio of formic acid to
tertiary
amine (I) of > 1).
Ionic liquid.
The type and amount of the individual forms is inconsequential for carrying
out the
process of the invention.
The liquid stream from step (b) to be fed to step (c) can optionally also
comprise
solvents.
If a solvent is to be used, it is advantageous, particularly in the preferred
variant in which
two liquid phases are formed in the bottom output from the distillation
apparatus
mentioned in step (c), for this to be immiscible or only insignificantly
miscible with the
tertiary amine (I) but readily miscible with the formic acid-comprising amine
phase and
therefore tending to be present in the lower liquid phase in step (d). A
critical parameter
here has been found to be an electrostatic factor, also referred to as EF for
short, of
CA 02859128 2014-06-12
27
preferably 200 x 10-3 Cm, at 25 C. The electrostatic factor EF is defined as
the
product of the relative dielectric constant Er and the dipole moment p of the
solvent (see,
for example, C. Reichardt, "Solvents and Solvent Effects in Organic
Chemistry", 3rd
edition, Wiley-VCH Verlag GmbH & Co KGaA, Weinheim 2003, Chapter 3.2, page 67
bottom to page 68 top). This preferred value ensures that the optional solvent
has a
certain minimum polarity and is miscible with the lower liquid phase in step
(d).
The use of solvents can, depending on the respective system (for example type
of
tertiary amine (I), concentrations, temperature, pressure and the like)
improve, for
example, the separation of the two liquid phases.
As classes of substances which are particularly suitable as optional solvent,
possibilities
are, in particular, formic esters, diols and formic esters thereof, polyols
and formic esters
thereof, sulfones, sulfoxides, open-chain or cyclic amides and also mixtures
of the
classes of substances mentioned.
Suitable diols and polyols are, for example, ethylene glycol (EF = 290.3 x 10-
3 Cm),
diethylene glycol (EF = 244.0 x 10-3 Cm), triethylene glycol, polyethylene
glycol, 1,3-
propanediol (EF = 285.6 x 10-3 Cm), 2-methyl-1,3-propanediol, 1,4-butanediol
(EF =
262.7 x 10-3 Cm), dipropylene glycol, 1,5-pentanediol (EF = 212.5 x 10-3
Cm), 1,6-
hexanediol and glycerol. Due to their OH groups, diols and polyols can be
esterified in
the presence of formic acid. In the process of the invention, this occurs
mainly in step (c)
during the thermal separation of the stream comprising formic acid and
tertiary amine (I)
in the distillation apparatus mentioned. Since the formic esters formed
display very
similar phase behavior, they are generally likewise well suited as solvents.
The water
formed in the esterification also does no harm in the thermal separation. An
accumulation of water in continuous operation of the process of the invention
does not
occur since water in these small amounts can be separated off via a side
offtake on the
distillation apparatus.
Suitable sulfoxides are, for example, dialkyl sulfoxides, preferably C1-C6-
dialkyl
sulfoxides, in particular dimethyl sulfoxide (EF = 627.1 x 10-3 Cm).
CA 02859128 2014-06-12
28
Suitable open-chain or cyclic amides are, for example, formamide (EF =
1243.2 x 10-3 Cm), N-methylformamide (EF = 2352.9 x 10-3 Cm), N,N-dimethyl-
formamide (EF = 396.5 x 10-3 Cm), N-methylpyrrolidone (EF = 437.9 x 10-3
Cm),
acetamide and N-methylcaprolactam.
However, it may also be advantageous to use a rather nonpolar solvent having
an EF of
<200 x 10-3 Cm, at 25 C. Nonpolar solvents may be able to reduce the
concentration of
formic acid in the upper liquid phase.
However, the process of the invention is preferably carried out without
addition of a
solvent.
In a preferred variant of the process of the invention, a formic acid source
which
comprises methyl formate and from which a liquid stream comprising formic
acid, tertiary
amine (I) and methanol is obtained by hydrolysis of methyl formate is used in
the
presence of water in step (a). In this variant, the methanol formed from the
hydrolysis of
methyl formate is then generally also separated off via step (b), in addition
to excess
water. The methanol which has been separated off can then, for example, be
reused in
the synthesis of methyl formate. Since methanol has a significantly lower
boiling point
than water and can thus be separated off relatively easily by distillation
from a
corresponding mixture comprising methanol, water, formic acid and tertiary
amine (I), it
is advantageous in this variant to separate off methanol straight away as
separate
stream from the stream obtained from step (a).
If methanol is separated off in the variant described in the previous
paragraph, it is
particularly advantageous in step (b) to separate off, likewise straight away,
a further
stream comprising unreacted methyl formate and to recirculate the latter to
step (a). In
this way, the yield of formic acid based on the methyl formate used can be
increased
significantly. Since methyl formate has a significantly lower boiling point
than methanol
and can thus be separated off even more easily by distillation from a
corresponding
mixture comprising methyl formate, methanol, water, formic acid and tertiary
amine (I), it
CA 02859128 2014-06-12
29
is advantageous in this variant to separate off methyl formate and methanol
straight
away as separate streams from the stream obtained from step (a). This can, for
example, be carried out in two separate distillation apparatuses in which
methyl formate
is separated off in the first column and methanol is separated off in the
second column.
However, it is also possible, for example, to separate off the two components
in separate
streams in a single distillation apparatus. For example, methyl formate can be
obtained
as overhead product and methanol can be obtained as side stream product.
The hydrolysis of methyl formate in step (a) usually takes place in a
temperature range
from 80 to 150 C and a pressure range from 0.4 to 25 MPa abs. It is in
principle possible
to use all apparatuses in which an exothermic reaction of fluid streams is
possible as
apparatus for carrying out the hydrolysis in step (a). Examples which may be
mentioned
are stirred vessels, tube reactors or shell-and-tube reactors, in each case
without
internals or with internals (for example beds, packing elements, perforated
plates and
the like). The hydrolysis is preferably carried out with removal of heat or
adibatically.
In another preferred variant of the process of the invention, a formic acid
source which
comprises carbon dioxide, hydrogen and a homogeneous catalyst and from which a
liquid stream comprising formic acid and tertiary amine (I) is obtained by
homogeneously
catalyzed hydrogenation of carbon dioxide is used in step (a). If step (a) was
additionally
carried out in the presence of water and/or methanol, which is a particularly
preferred
embodiment of this variant, then in general, in step (b), water and/or
methanol are
separated off again, where, in the case of the removal of methanol, this is
preferably
recirculated back to step (a). In this variant, methanol and water serve first
and foremost
as polar solvents.
The specific steps and process features of the homogeneously catalyzed
hydrogenation
of carbon dioxide to formic acid in the presence of water and methanol are
described in
PCT/EP 2011/060012.
As homogeneous catalyst, preference is given to using a metal-organic complex
comprising an element of group 8, 9 or 10 of the Periodic Table. The complex
preferably
further comprises at least one phosphine group having at least one unbranched
or
CA 02859128 2014-06-12
branched, acyclic or cyclic aliphatic radical having from 1 to 12 carbon
atoms, where
individual carbon atoms can also be replaced by >P-. The hydrogenation is
preferably
carried out at from 20 to 200 C and from 0.2 to 30 MPa abs. The output from
the
hydrogenation stage (a) is preferably a two-phase mixture. The upper phase
comprises
5 tertiary amine (I) and homogeneous catalyst, while the lower phase
comprises formic
acid, tertiary amine (I), water, methanol and likewise homogeneous catalyst.
The two
phases are separated and the upper phase comprising tertiary amine (I) and
homogeneous catalyst is recirculated to the hydrogenation stage (a). The lower
phase
comprising formic acid, tertiary amine (I), water, methanol and homogeneous
catalyst is
10 preferably extracted with tertiary amine (I) in order to extract the
major part of the
homogeneous catalyst present therein and recirculate it together with the
tertiary amine
(I) likewise to the hydrogenation stage (a). The remainder of the lower phase,
which
comprises formic acid, tertiary amine (I), water and methanol, is then
recirculated to step
(b) in order then to separate off, as described above, methanol and according
to the
15 invention water and organic decomposition products of the tertiary amine
(I).
As regards the further work-up, mention may also be made, for the purpose of
supplementary information, of the specific steps and process features
mentioned in
PCT/EP 2011/060012.
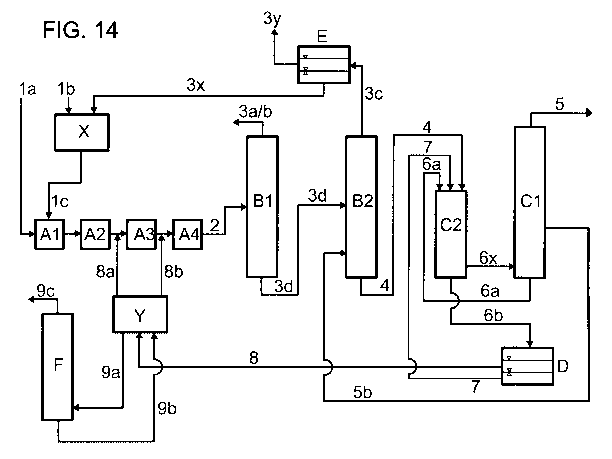
Fig. 1 shows a simplified block diagram of a general embodiment of the process
of the
invention. In the figure, the individual letters have the following meanings:
A = apparatus for producing a stream comprising formic acid and tertiary amine
(I)
B = apparatus for separating off secondary components
C = distillation apparatus
D = phase separation vessel
F = distillation apparatus
A formic acid source is fed via stream (1) and tertiary amine (I) is fed via
stream (8c) to
the apparatus A for producing a stream comprising formic acid and tertiary
amine (I). As
indicated above, the formic acid source to be fed in can comprise, for
example, formic
acid in chemically bound form or a precursor by means of which formic acid is
produced
CA 02859128 2014-06-12
31
by chemical reaction in apparatus A. The stream (2) comprising formic acid and
tertiary
amine (I) is taken off from apparatus A and fed to apparatus B for the removal
of
secondary components. This apparatus can be, for example, a distillation
apparatus in
which low-boiling secondary components are removed by distillation. Secondary
components which have been separated off are taken off via stream (3). The
stream
concentrated in formic acid and tertiary amine (I) is fed via stream (4) to
the distillation
apparatus C. Here, formic acid is separated off by distillation as stream (5).
The bottoms
from the distillation apparatus C are fed as stream (6) to the phase
separation vessel D
for phase separation. The lower liquid phase is recirculated as stream (7) to
the
distillation apparatus C. The upper liquid phase is taken off as stream (8a)
and fed to the
distillation apparatus F. In this, low boilers are removed by distillation as
stream (8z) and
the stream depleted in low boilers is recirculated as stream (8c) to the
apparatus A.
Fig. 2 shows a simplified block diagram of a modified, preferred embodiment in
which
only part of the upper liquid phase from the phase separation vessel D is fed
to the
distillation apparatus F for the removal of low boilers. The other part is
recirculated via
stream (8b) and subsequently (8c) directly to the apparatus A.
The recirculation of the stream depleted in low boilers from the distillation
apparatus F
can be carried out to other points in the process. Thus, fig. 3 shows, by
means of the
streams (8y(i)) to (8y(iii)) shown as broken lines, illustrative
recirculations to apparatus
A, to apparatus B and to the phase separation vessel D. The streams shown as
broken
lines are alternatives which can in each case be present either alone or in
combination.
However, the recirculation can, for example, also be effected at other points
in the
process, for example to the distillation apparatus C.
Fig. 4 shows a preferred embodiment in which the removal according to the
invention of
the low boilers as per the variant shown in fig. 2 is combined with a
particular variant for
separating off the secondary components. This particular variant is especially
advantageous in the presence of water as secondary component and also allows
organic decomposition products of the tertiary amine (I) which are formed in
the process
of the invention to be separated off in one step without being passed on to an
CA 02859128 2014-06-12
32
appreciable extent to the distillation apparatus C. In fig. 4, the additional
letter E has the
following meaning:
E = phase separation vessel
The stream (2) comprising formic acid, tertiary amine (I) and water is taken
off from
apparatus A and fed to apparatus B in order to separate off water and organic
decomposition products of the tertiary amine (I). This apparatus can be, for
example, a
distillation apparatus. Water and organic decomposition products of the
tertiary amine (I)
which have been separated off are taken off via stream (3) and fed to the
phase
separation vessel E. In this, two liquid phases are formed. The lower, water-
comprising
liquid phase is recirculated as stream (3x) to the apparatus A. The upper
liquid phase
enriched in organic decomposition products of the tertiary amine (I) is taken
off as
stream (3y) and discharged from the process. The stream enriched in formic
acid and
tertiary amine (I) is fed via stream (4) to the distillation apparatus C.
In the region of the distillation apparatus C and the phase separation D,
various
embodiments are possible. They differ not only in whether the phase separation
is
carried out in a separate vessel or integrated into the bottom of the
distillation column,
but also in the location of the introduction of the stream comprising formic
acid and
tertiary amine (I) into the distillation apparatus and in the flow between the
column
vessel and the bottom vaporizer and also the place at which the bottom output
is taken
off. The embodiments shown in fig. 2 to 7 of PCT/EP2011/060,770 and described
in the
text can also be employed for the purposes of the preferred process according
to the
invention.
Two preferred embodiments for preferred fields of use of the process of the
invention are
described below.
Preparation of formic acid by hydrolysis of methyl formate
A preferred embodiment for obtaining formic acid by hydrolysis of methyl
formate is
shown in fig. 5 by means of a simplified block diagram.
CA 02859128 2014-06-12
33
In the figure, the individual letters have the following meanings:
A =apparatus for the hydrolysis of methyl formate and production of a stream
comprising formic acid, tertiary amine (I) and water
B =distillation apparatus for separating off methyl formate, methanol and
water
C =distillation apparatus for obtaining formic acid
D =phase separation vessel
F =distillation apparatus
Methyl formate (streams (la) and (3b)), water (streams (1 b) and (3c)) and
tertiary amine
(I) (stream (8c)) are fed to the apparatus A. A stream comprising formic acid,
tertiary
amine (I), methanol, water and methyl formate is formed by hydrolysis of
methyl formate
and is taken off as stream (2) from the apparatus A and fed to the apparatus
B. The
methyl formate conversion and thus the composition of the stream (2) depends
first and
foremost on the relative amounts of the three feed streams methyl formate,
water and
tertiary amine (I) fed to the apparatus A, the type of tertiary amine (I)
used, the residence
time and the reaction temperature. The conditions appropriate for the
respective reaction
system can easily be determined by a person skilled in the art, for example by
means of
preliminary tests. The molar ratio of formic acid to tertiary amine (I) in
stream (2) is
usually from 0.5 to 5, preferably from 0.5 to 3, with deviations from this
range naturally
also being possible.
In the distillation apparatus B, unreacted methyl formate (stream (3b)),
methanol formed
in the hydrolysis (stream (3a)) and water and organic decomposition products
of the
tertiary amine (I) (stream (3c)) are separated off from stream (2). Stream
(3b) comprising
the unreacted starting material methyl formate is recirculated to the
apparatus A. The
methanol separated off via stream (3a) can, for example, be reused for
preparing methyl
formate. Stream (3c) is likewise recirculated to the apparatus A. Formic acid
and tertiary
amine (I) are taken off via stream (4). This additionally comprises residual
amounts of
water. Depending on the way in which the process is carried out, these can
amount to a
few percent by weight or even some tens of percent by weight of the stream
(4). The
water content of stream (4) is preferably 20% by weight, particularly
preferably 10%
by weight and very particularly preferably 5% by weight. The molar ratio of
formic acid
CA 02859128 2014-06-12
34
to tertiary amine (I) is not changed or only insignificantly changed by the
distillation
apparatus B, so that this ratio is usually also from 0.5 to 5, preferably from
0.5 to 3 in
stream (4), with deviations from this range naturally also being possible.
Stream (4) is fed to the distillation apparatus C. In this, the formic acid is
removed by
distillation via stream (5) as overhead product, via stream (5a) as side
product and/or via
stream (5b) as side product. Depending on the boundary conditions, i.e.
especially the
composition of the feed stream (4) to the distillation apparatus C and the
desired purity
of the formic acid, formic acid can be obtained as stream (5) at the top or as
stream (5a)
as side product in the present embodiment. Water-comprising formic acid is
then taken
off as side product via stream (5a) or (5b). In some cases, it may even be
sufficient to
remove formic acid or water-comprising formic acid purely via stream (5) as
overhead
product. Depending on the specific embodiment, the side stream (5b) or even
both side
streams (5a) and (5b) can thus be dispensed with. The distillation apparatus C
can
naturally also have the embodiments disclosed in fig. 2 to 7 of
PCT/EP2011/060,770.
The bottom product from the distillation apparatus C is fed as stream (6) to
the phase
separation vessel D. As an alternative, the phase separator vessel D can also
be
integrated into the distillation apparatus C. The bottom product is separated
into two
liquid phases in the phase separation vessel D. A heat exchanger, for example,
can also
optionally be installed between the distillation apparatus C and the phase
separation
vessel D in order to cool the bottom stream taken off. Although a lower phase
separation
temperature generally leads to somewhat better separation in respect of the
formic acid
content, it results in an additional outlay and energy consumption because of
the use of
a heat exchanger. Advantages and disadvantages therefore have to be weighed
against
one another in each case. The lower liquid phase from the phase separation
vessel D is
recirculated via stream (7) to the distillation apparatus C. The lower liquid
phase can
also be preheated. This can be effected by means of a heat exchanger which is
separate in energy terms or by heat integration with the heat exchanger used
for cooling
the bottom output from the distillation apparatus C or a combination of the
two.
The upper liquid phase from the phase separation vessel D is taken off via
stream (8a).
A substream (8x) is fed to the distillation apparatus F. In this, low boilers
are removed by
CA 02859128 2014-06-12
distillation as stream (8z) and the stream depleted in low boilers is
recirculated as
stream (8y) and subsequently (8c) to the apparatus A. The remaining, other
substream
(8b) is recirculated directly via stream (8c) to the apparatus A.
5 In another, preferred embodiment for obtaining formic acid by hydrolysis
of methyl
formate, the methyl formate stream (la) is introduced into the distillation
apparatus B as
shown in fig. 6. This embodiment is generally advantageous when the methyl
formate
available as stream (1a) is still contaminated with residual amounts of
methanol, for
example due to a preceding methyl formate synthesis stage with partial
conversion of
10 methanol and incomplete work-up of the methyl formate. As a result of
the direct
introduction of stream (la) into the distillation apparatus B, the methanol
comprised can
be separated off as stream (3a) and, for example, recirculated to the methyl
formate
synthesis stage. This variant makes it possible to omit a methyl
formate/methanol
separation entirely in the methyl formate synthesis stage and thus to save an
entire
15 distillation column and thus also energy in ongoing operation.
In a further, preferred embodiment for obtaining formic acid by hydrolysis of
methyl
formate, both the methyl formate stream (la) and the water stream (1 b) are
introduced
into the distillation apparatus B as shown in fig. 7. As regards the water
stream (1b), this
20 embodiment is generally advantageous when hot condensate or steam is
available as
water source, since in this way the thermal energy stored therein can be
utilized in the
distillation apparatus B.
For the sake of completeness, it may be mentioned that, in a further
embodiment, it is
25 naturally also possible to introduce the methyl formate stream (la) into
the apparatus A
but the water stream (1b) into the distillation apparatus B. This is
advantageous when,
for example, low-pressure excess steam is available.
In the preparation of formic acid by hydrolysis of methyl formate, too, it is
naturally also
30 possible and even advantageous to combine the variants shown in fig. 5
to 7 with the
specific removal of secondary components shown in fig. 4. This is shown by way
of
example as a combination of the variants from fig. 4 and 5 in fig. 8.
CA 02859128 2014-06-12
36
It is likewise possible, in the variants shown in fig. 5 to 8, in a manner
similar to that
mentioned above in connection with fig. 3, to recirculate the stream depleted
in low
boilers from the distillation apparatus F not only to the apparatus A but also
or
exclusively to other points in the process. Thus, fig. 9 shows, by means of
the streams
(8y(i)) to (8y(iv)) shown as broken lines, illustrative recirculations to
apparatus A, to
apparatus B (two different positions of introduction) and to the phase
separation vessel
D. The streams shown as broken lines are alternatives which can in each case
be
present individually or in combination. Of course, recirculations not shown,
e.g. to the
distillation apparatus C are likewise conceivable.
In the variants of fig. 5 to 9, specific variants in respect of the embodiment
of the
distillation apparatus B having one, two or even three distillation columns
are possible.
Fig. 10a shows an embodiment having one distillation column. Fig. 10b to 10e
show
different embodiments having two distillation columns. Fig. 11a to 11 c show
different
embodiments having three distillation columns. The variants having one or two
distillation columns are preferred for the design of the distillation
apparatus B. For the
sake of completeness, it may be mentioned that, particularly in the
embodiments having
one or two distillation columns, these can also be configured as thermally
coupled
columns or a dividing wall column.
Preparation of formic acid by hydrogenation of carbon dioxide
A preferred embodiment for obtaining formic acid by hydrogenation of carbon
dioxide is
shown in fig. 12 by means of a simplified block diagram.
In the figure, the individual letters have the following meanings:
A =apparatus for the hydrogenation of carbon dioxide and production of a
stream
comprising formic acid, tertiary amine (I) and water
Al = hydrogenation reactor
A2 = phase separation vessel
A3 = extraction unit
CA 02859128 2014-06-12
37
B =distillation apparatus for separating off methanol, water and organic
decomposition
products of the tertiary amine (I)
C =distillation apparatus for obtaining formic acid
D =phase separation vessel
F =distillation apparatus
Carbon dioxide (stream (la)), hydrogen (stream (1 b)) and tertiary amine (I)
(stream (8c))
are fed to the hydrogenation reactor Al in the apparatus A. In the
hydrogenation reactor
Al, the hydrogenation proceeds in the presence of a homogeneous catalyst and
of
water and methanol as solvent to form a stream (2a) comprising formic acid,
tertiary
amine (I), methanol, water and homogeneous catalyst. This is fed to the phase
separation vessel A2 in which two liquid phases are formed. The upper liquid
phase
comprising tertiary amine (I) and homogeneous catalyst is recirculated via
stream (2b) to
the hydrogenation reactor Al. The lower liquid phase comprising formic acid,
tertiary
amine (I), water, methanol and likewise homogeneous catalyst is conveyed via
stream
(2c) to the extraction unit A3. In this, the residues of the homogeneous
catalyst still
present are largely extracted by means of the tertiary amine (I) fed in as
stream (8) and
are recirculated together with the tertiary amine (I) as stream (2d) to the
hydrogenation
reactor Al. A stream comprising formic acid, tertiary amine (I) and water is
thus obtained
as stream (2) and fed to the distillation apparatus B.
Methanol (stream (3b)) and water and organic decomposition products of the
tertiary
amine (I) (stream (3c)) are separated off from stream (2) in the distillation
apparatus B.
Stream (3b) comprising methanol is recirculated to the hydrogenation reactor
Al in
apparatus A. Stream (3c) is likewise recirculated to the hydrogenation reactor
Al in the
apparatus A. Formic acid and tertiary amine (I) are taken off via stream (4)
and
conveyed to the distillation apparatus C. With regard to the process steps in
respect of
the distillation apparatus C, the phase separation vessel D and the
distillation apparatus
F, reference may be made to the above description of the preparation of formic
acid by
hydrolysis of methyl formate.
Naturally, in the preparation of formic acid by hydrogenation of carbon
dioxide, it is also
possible, in a manner similar to that mentioned above in connection with fig.
3, to
CA 02859128 2014-06-12
38
recirculate the stream depleted in low boilers from the distillation apparatus
F not only to
the apparatus A but also or exclusively to other points in the process.
The process of the invention makes it possible to obtain formic acid in high
yield and
high concentration by thermal separation of a stream comprising formic acid
and a
tertiary amine.
The removal according to the invention of low boilers from the upper liquid
phase from
the phase separation of the bottom output from the thermal separation of the
stream
comprising formic acid and tertiary amine enables the concentration of the low
boilers in
the system to be kept at a low level. In this way, the gradual accumulation of
low boilers
is avoided and a slow increase in the energy consumption in the distillation
apparatus for
the thermal separation of the stream comprising formic acid and tertiary amine
and also
a slow deterioration in the formic acid quality as a result of increasing
contamination with
low boilers are thus effectively countered. The process of the invention can
thus be
operated very stably with, at the same time, constant high purity of the
formic acid
produced over long operating times. The formic acid obtained has a low color
number
and a high color number stability. The process can be carried out simply,
reliably and
with a low energy consumption.
The process of the invention can, in particular, also be used particularly
advantageously
in conjunction with the hydrolysis of methyl formate as formic acid source and
has
technical and economic advantages over the production process of methyl
formate
hydrolysis with subsequent dewatering by means of an extractant or a two-
pressure
distillation which is at present performed in the industry.
CA 02859128 2014-06-12
39
Examples
Laboratory plant 1 (for comparative example 1)
Laboratory plant 1 was employed for examining the continuous process without
use of
the present invention. The simplified block diagram of laboratory plant 1 is
shown in fig.
13. In the figure, the individual letters have the following meaning:
Al = stirred vessel (volume 0.3 I, electrically heated)
A2,3,4 = in each case (internal diameter 80 mm, length 1200 mm,
filled with 2 mm glass spheres, electrically heated)
X = Mixing vessel (volume 5 I)
= Vessel (volume 5 I)
Bl= Distillation apparatus comprising column body (internal diameter
55 mm,
equipped with two mesh packings each having a packing height of 1.3 m and
a specific surface area of 750 m2/m3, with the inlet for stream (2) being
located between the two mesh packings), oil-heated falling film evaporator
and condenser and also regulable runback distributor at the top of the
column
B2= Distillation apparatus comprising column body (internal diameter
55 mm,
equipped with 12 bubble cap trays in the stripping section and 10 bubble cap
trays in the enrichment section, with the inlet for stream (3d) being located
between the two sections and the inlet for stream (5b) being located in the
stripping section), oil-heated falling film evaporator and condenser and
alsoregulable runback distributor at the top of the column
Cl= column body (internal diameter 43 mm, equipped with a mesh packing
above
the bottom having a packing height of 0.66 m and a specific surface area of
500 m2/m3 and also a further mesh packing having a packing height of
1.82 m and a specific surface area of 750 m2/m3, with the side offtake for
stream (5b) being located between the two mesh packings) and condenser
and also regulable runback distributorat the top of the column
C2= oil-heated falling film evaporator
D= separate phase separation vessel (volume 0.3 I, oil-heated)
CA 02859128 2014-06-12
The apparatus and lines are composed of a nickel-based alloy having the
material
number 2.4610. The mass flows were measured by means of a coriolis flow meter.
Laboratory plant 1 was operated continuously.
5 In all experiments in the laboratory plant 1, the content of formic acid
was in each case
determined by potentiometric titration with 0.5 N NaOH in water and the
content of water
was determined by the Karl Fischer method. All other organic components were
in each
case determined by gas chromatography.
10 Laboratory plant 2 (for example 2 according to the invention)
Laboratory plant 2 is laboratory plant 1 expanded by a separate phase
separation vessel
for stream (3c) and was employed for examining the continuous process using
the
present invention. The simplified block diagram of laboratory plant 2 is shown
in figure
15 14. In the figure, the additional letters have the following meanings:
E= Phase separation vessel
F= Distillation apparatus comprising column body (internal diameter
30 mm,
equipped with 1 m of Sulzer CY packing (750 m2/m3), with the inlet for
20 stream (9a) being located below the packing), oil-heated bottom
vessel
and also regulable runback distributor at the top of the column
otherwise, reference is made to the description of laboratory plant 1.
Example 1
25 (Comparative example)
Example 1 was carried out in laboratory plant 1. By means of metering pumps,
1760 g/h
of methyl formate as stream (1a) and 849 g/h of water as stream (1c) were
metered into
the stirred vessel Al. Stream (1c) was taken off from the mixing vessel X and
was
30 composed of fresh water via stream (1b) and recycle water from the
distillation
apparatus B2 via stream (3c). Stream (1b) was selected so that the sum of
stream (1b)
and stream (3c) gave the desired stream (lc). The stirred vessel Al was
operated at
110 C and 1.3 MPa abs. The output was introduced into tube reactor A2 which
was
CA 02859128 2014-06-12
41
likewise operated at 110 C and 1.3 MPa abs. The output from tube reactor A2
was
introduced into tube reactor A3. 1964 g/h of tri-n-hexylamine were fed via
stream (8a)
into the latter. The output from tube reactor A3 was introduced into tube
reactor A4. A
further 1661 g/h of tri-n-hexylamine were fed via stream (8b) into the latter.
Streams (8a)
Stream (2) was depressurized and introduced into the column body of the
distillation
apparatus B1. At a pressure at the top of 0.18 MPa abs and a reflux ratio of
2.5, a
mixture comprising methanol formed and unreacted methyl formate was taken off
as
addition, 277 g/h of the side offtake stream from the column body of the
distillation
apparatus Cl, comprising 79.3% by weight of formic acid and 16.6% by weight of
water,
were fed in via stream (5b). As overhead product from the distillation
apparatus B2,
450 g/h of stream (3c) were taken off at a pressure at the top of 0.10 MPa abs
and a
As bottom product, 4821 g/h of a mixture comprising 75.3% by weight of tri-n-
CA 02859128 2014-06-12
42
161 C. The gaseous output from the evaporator was fed as stream (6x) to the
column
body Cl. The latter was operated at a pressure at the top of 0.015 MPa abs and
a reflux
ratio of runback to distillate of 4. As overhead product from Cl 907 g/h of
99.6% by
weight strength formic acid were obtained as stream (5). As side offtake
stream, 277 g/h
were taken off as stream (5b) and recirculated to the column body B2. The
liquid output
from the column body Cl was fed as stream (6a) to the top of the evaporator
C2.
The liquid output from the evaporator C2 was introduced as stream (6b) into
the phase
separation vessel D. This was operated at atmospheric pressure and a
temperature of
80 C. Two liquid phases were formed. The upper liquid phase was continuously
taken
off at 3587 g/h as stream (8) and conveyed to the vessel Y. Stream (8)
comprised 95.7%
by weight of tri-n-hexylamine and 1.2% by weight of formic acid. The lower
liquid phase
was conveyed continuously as stream (7) to the evaporator C2. The remaining
stream
was fed into the top of the evaporator C2.
To ensure the abovementioned operating state, the plant was firstly run-in for
seven
days. During this time, the methyldi-n-hexylamine concentration in stream (8)
rose to
0.31% by weight and continued to rise steadily in the following days. 9 days
after start-
up, the concentration was 0.77% by weight. An end to the rise could not be
discerned.
The methyldi-n-hexylamine concentration is shown in tabular form in table 1
and in
graphical form in figure 15.
Example 1 shows that without the use of the measure according to the invention
for the
targeted isolation and discharge of low boilers, in the present example
especially
methyldi-n-hexylamine, the concentration of these in stream (8) increases
continuously.
Example 1 also demonstrates that methyldi-n-hexylamine is also formed under
real
operating conditions. Disadvantages in the long-term operation of such a
process would
be preprogrammed.
Example 2 (example according to the invention)
Example 2 was carried out in laboratory plant 2. By means of metering pumps,
2280 g/h
of methyl formate as stream (1a) and 950 g/h of water as stream (1c) were
metered into
CA 02859128 2014-06-12
43
the stirred vessel Al. Stream (1c) was taken off from the mixing vessel X and
was
composed of fresh water via stream (1b) and recycle water from the
distillation
apparatus B2 via stream (3c). Stream (1b) was selected so that the sum of
stream (1b)
and stream (3c) gave the desired stream (1c). The stirred vessel Al was
operated at
110 C and 1.3 MPa abs. The output was introduced into tube reactor A2 which
was
operated at 108 C and 1.3 MPa abs. The output from tube reactor A2 was
introduced
into tube reactor A3. 1603 g/h of tri-n-hexylamine were fed via stream (8a)
into the latter.
The output from tube reactor A3 was introduced into tube reactor A4. A further
1603 g/h
of tri-n-hexylamine were fed via stream (8b) into the latter. Streams (8a) and
(8b) were
taken off from the vessel Y which served to distribute tri-n-hexylamine
recirculated via
stream (8) over the two tube reactors A3 and A4. Tube reactor A3 was operated
at
105 C and 1.3 MPa abs, and tube reactor A4 was operated at 106 C and 1.3 MPa
abs.
A product mixture comprising 49.8% by weight of tri-n-hexylamine, 16.9% by
weight of
formic acid, 12.3% by weight of methanol, 7.9% by weight of water and 11.5% by
weight
of methyl formate was obtained as stream (2).
Stream (2) was depressurized and introduced into the column body of the
distillation
apparatus Bl. At a pressure at the top of 0.18 MPa abs and a reflux ratio of
1.4, a
mixture comprising methanol formed and unreacted methyl formate was taken off
as
overhead product stream (3ab). As bottom product, 5007 g/h of a mixture
comprising
59.5% by weight of tri-n-hexylamine, 9.9% by weight of water, 26.3% by weight
of formic
acid and 0.1% by weight of methanol was obtained as stream (3d). The
temperature at
the bottom of B1 was 117 C.
Stream (3d) was introduced into the column body of the distillation apparatus
B2. In
addition, 265 g/h of the side offtake stream from the column body of the
distillation
apparatus Cl, comprising 83.2% by weight of formic acid and 16.6% by weight of
water,
were fed in via stream (5b). As overhead product from the distillation
apparatus B2,
600 g/h of stream (3c) were taken off at a pressure at the top of 0.18 MPa abs
and a
reflux ratio of 0.25. Stream (3c) which comprised 97.9% by weight of water and
2.0% by
weight of formic acid, was fed to the mixing vessel X for recirculation to the
stirred vessel
Al.
CA 02859128 2014-06-12
44
As bottom product, 4512 g/h of a mixture comprising 63.9% by weight of tri-n-
hexylamine, 27.9% by weight of formic acid and 1.0% by weight of water were
obtained
as stream (4) at a temperature at the bottom of B2 of 177 C and were fed to
the top of
the evaporator C2. The evaporator C2 and the column body Cl were operated
under
reduced pressure. The temperature at the lower outlet from the evaporator C2
was
161 C. The gaseous output from the evaporator was fed as stream (6x) to the
column
body Cl. The latter was operated at a pressure at the top of 0.015 MPa abs and
a reflux
ratio of runback to distillate of 2.6. As overhead product from Cl 930 g/h of
99.6% by
weight strength formic acid were obtained as stream (5). As side offtake
stream, 265 g/h
were taken off as stream (5b) and recirculated to the column body B2. The
liquid output
from the column body Cl was fed as stream (6a) to the top of the evaporator
C2.
The liquid output from the evaporator C2 was introduced as stream (6b) into
the phase
separation vessel D. This was operated at atmospheric pressure and a
temperature of
80 C. Two liquid phases were formed. The upper liquid phase was continuously
taken
off at 3250 g/h as stream (8) and conveyed to the vessel Y. Stream (8)
comprised 95.1%
by weight of tri-n-hexylamine and 1.2% by weight of formic acid. The lower
liquid phase
was taken off continuously as stream (7) and fed into the top of the
evaporator C2.
Every weekday (Monday to Friday), 790 g were taken off from vessel Y and
distilled in
the distillation apparatus F at a pressure at the top of 15 hPa abs and a
temperature at
the bottom of 162 C. Each time, about 35 g of overhead product were obtained
and
discarded. The overhead product in each case comprised about 67.1% by weight
of
methyldi-n-hexylamine, about 0.2% by weight of tri-n-hexylamine and about
28.5% by
weight of formic acid. The bottom output remaining in each case was fed back
to the
vessel Y. At weekends (Saturday and Sunday) no work-up by distillation using
the
distillation apparatus F was carried out.
The methyldi-n-hexylamine concentration in stream (8) was monitored
analytically during
the experiment. It is shown in tabular form in table 2 and in graphical form
in figure 16.
The broken, vertical lines in fig. 16 in each case symbolize the weekend
(Saturday and
Sunday) during which no work-up by distillation in the distillation apparatus
F was carried
out.
CA 02859128 2014-06-12
Example 2 shows that without the use of the measure according to the invention
for the
targeted isolation and discharge of low boilers, in the present example
especially
methyldi-n-hexylamine, the concentration of these increases continually. Thus,
for
5 example, a rise in stream (8) from 2.99% by weight to 3.08% by weight of
methyldi-n-
hexylamine was found on the first weekend of the measurements. On the other
hand,
the concentration of methyldi-n-hexylamine could be reduced again by means of
the
measure according to the invention during the following five weekdays (Monday
to
Friday).
The ultimate result is therefore that the concentration of methyldi-n-
hexylamine could be
kept to a value of about 3% by weight under the process conditions indicated.
Examples 3 to 4
(Decomposition of tri-n-hexylamine in comparison with methyldi-n-hexylamine)
Example 3
(Decomposition of tri-n-hexylamine in the presence of formic acid and water)
95.3 g (0.35 mol) of tri-n-hexylamine, 16.3 g (0.35 mol) of formic acid (98-
100% by
weight) and 6.3 g (0.35 mol) of water were mixed in an ice bath in the
laboratory. The
solution obtained was subsequently warmed to room temperature (about 20 C) and
degassed by evacuation (2 hPa abs) and admission of pure nitrogen, carried out
a total
of three times. A two-phase solution was obtained. This was then transferred
under an
N2 atmosphere in a glove box into a 270 ml autoclave (material: HC) and the
autoclave
was closed. The autoclave was subsequently pressurized with nitrogen to 1.0
MPa abs
and heated to 160 C while stirring vigorously. After the temperature had been
reached, a
total pressure of 2.5 MPa abs was set by injection of further N2. The reaction
mixture
was then stirred at 160 C for 72 hours. The autoclave was subsequently cooled
to room
temperature, depressurized to atmospheric pressure and the contents were
transferred
to a glass vessel. The output separated into two phases. 48.1 g of upper phase
and
57.9 g of lower phase were obtained. Both phases were analyzed by gas
chromatography to determine their di-n-hexylformamide content. The upper phase
CA 02859128 2014-06-12
46
comprised 0.16% by weight (0.077 g) of di-n-hexylformamide, and the lower
phase
comprised 0.69% by weight (0.4 g) of di-n-hexylformamide.
Example 4
(Decomposition of methyldi-n-hexylamine in the presence of formic acid and
water)
69.8 g (0.35 mol) of methyldi-n-hexylamine, 16.3 g (0.35 mol) of formic acid
(98-100% by
weight) and 6.3 g (0.35 mol) of water were mixed in an ice bath in the
laboratory. The
solution obtained was subsequently warmed to room temperature (about 20 C) and
degassed by evacuation (2 hPa abs) and admission of pure nitrogen, carried out
a total
of three times. A two-phase solution was obtained. This was then transferred
under an
N2 atmosphere in a glove box into a 270 ml autoclave (material: HC) and the
autoclave
was closed. The autoclave was subsequently pressurized with nitrogen to 1.0
MPa abs
and heated to 160 C while stirring vigorously. After the temperature had been
reached, a
total pressure of 2.5 MPa abs was set by injection of further N2. The reaction
mixture
was then stirred at 160 C for 72 hours. The autoclave was subsequently cooled
to room
temperature, depressurized to atmospheric pressure and the contents were
transferred
to a glass vessel. The output separated into two phases. 25.0 g of upper phase
and
54.3 g of lower phase were obtained. Both phases were analyzed by gas
chromatography to determine their di-n-hexylformamide content. The upper phase
comprised 0.52% by weight (0.13 g) of di-n-hexylformamide, and the lower phase
comprised 1.1% by weight (0.597 g) of di-n-hexylformamide.
Examples 3 and 4 show that the acidolytic formation of di-n-hexylformamide
from
methyldi-n-hexylamine occurs significantly more quickly than that from tri-n-
hexylamine.
Since the formatin of di-n-hexylformamide equates to a direct loss of tertiary
amine (I), it
is advantageous in the preparation of formic acid by thermal separation of a
stream
comprising formic acid and a tertiary amine (I) to keep the amount of methyldi-
n-
hexylamine as low as possible.
Examples 5 to 7
(Influence of methyldi-n-hexylamine on the energy consumption of the pure
distillation of
formic acid)
CA 02859128 2014-06-12
47
Example 5
670 g/h of a mixture from stream (4) from operation of the laboratory plant
were fed into
a distillation column (internal diameter 30 mm) having 25 bubble trays and an
oil-heated
Sambay evaporator (thin film evaporator) at an oil temperature of 200 C and a
pressure
at the top of 150 hPa abs. The mixture used comprises 20% by weight of formic
acid,
74% by weight of tri-n-hexylamine, 2% by weight of water and 1% by weight of
di-n-
hexylformamide. The reflux ratio at the top of the column was 5:1. Under these
conditions, 105 g/h of 99.8% strength formic acid were obtained as overhead
product,
10 g/h of 78% strength aqueous formic acid were taken off via a side offtake
on the 13th
tray and 555 g/h of bottom output were obtained. All streams were combined
again in a
mixing vessel and fed back to the column. During the experiment, the entire
energy input
was regulated via the oil temperature in the Sambay evaporator.
Example 6
Example 6 was carried out like example 5 but a mixture derived from stream (4)
from the
operation of the laboratory plant and enriched with 4% by weight of methyldi-n-
hexylamine was fed in. The mixture used comprised 20% by weight of formic
acid, 70%
by weight of tri-n-hexylamine, 4% by weight of methyldi-n-hexylamine, 2% by
weight of
water and 1% by weight of di-n-hexylformamide. In contrast to example 5, only
90 g/h of
99.8% strength formic acid were able to be obtained as overhead product when
using
the methyl di-n-hexylamine-comprising feed stream. The amount of the side
offtake
stream was 14 g/h, with 80% strength by weight formic acid being obtained
here. The
remainder was discharged as bottom stream.
Example 7
In example 7, an attempt was made in the apparatus described in example 5 to
obtain a
similarly large amount of 99.8% strength formic acid as overhead product using
the
methyldi-n-hexylamine-comprising feed stream mentioned in example 6 by
increasing
the oil temperature. For this purpose, 666 g/h of the feed stream mentioned in
example 6
were fed in. At an oil temperature of 205 C, 103 g/h of 99.8% strength formic
acid were
able to be obtained as overhead product. 20 g/h of 79% strength by weight
formic acid
were taken off as side stream. The remainder was discharged as bottom stream.
CA 02859128 2014-06-12
48
Examples 5, 6 and 7 demonstrate a significantly adverse effect of the presence
of
methyldi-n-hexylamine in the pure distillation of formic acid. Under otherwise
constant
conditions, the amount of pure formic acid which can be achieved decreases
significantly. In the present case, only 90 g/h instead of 105 g/h of 99.8%
strength formic
acid were obtained as overhead product in the presence of 4% by weight of
methyldi-n-
hexylamine. To compensate for this decrease, an increase in the temperature at
the
bottom and thus in the energy input is required. In the present case, an
increase from
200 C to 205 C enabled 103 g/h of 99.8% strength formic acid to be obtained
again as
overhead product.
Example 8
In the distillation column described in example 5, 650 g/h of the mixture from
experiment
7 comprising 20% by weight of formic acid, 2% by weight of water, 4% by weight
of
methyldi-n-hexylamine and 70% by weight of tri-n-hexylamine were fed in at the
bottom
at an oil temperature of 194 C and a pressure at the top of 150 hPa abs. The
reflux ratio
at the top of the distillation column was 3:1. Under these conditions, an
overhead stream
of 50 g/h of 99.8% strength formic acid was taken off at the top of the
distillation column,
a side stream of 75 g/h of 75% strength aqueous formic acid was taken off from
the 6th
tray of the distillation column and a bottom output of 515 g/h was taken off.
The side
stream obtained was analyzed to determine its content of tri-n-hexylamine and
methyldi-
n-hexylamine. It comprises 3000 ppm by weight of tri-n-hexylamine and 35 000
ppm by
weight of methyldi-n-hexylamine.
Example 8 shows that a selective increase in the concentration of methyldi-n-
hexylamine
compared to tri-n-hexylamine by a factor of 10 can be achieved in the side
offtake
stream of the pure formic acid column.