Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02860118 2014-08-21
METHOD FOR IMPROVING GOLD RECOVERY
FIELD OF THE INVENTION
[0001] The disclosed methods are useful in leach processes, particularly
those that use
activated carbon for extracting precious metals e.g., gold, from leachate
using carbon-in-leach (CIL),
carbon-in-pulp (CIP) and/or carbon-in-column (CIC) configurations.
[0002] The present invention relates to the treatment of aqueous cyanide-
containing
compositions that comprise at least one anionic surfactant and/or an anionic
polymer, and to a process
for extracting metals, in particular gold and/or silver, from materials or
minerals which comprise the
corresponding metal with the aid of this aqueous cyanide-containing
composition. In particular, the
present invention can be used to reduce the impact of additives utilized in
producing a concentrated
metal solution including, for example, surfactants, anti-scalants and other
additives that are used to
improve upstream processes such as leach extraction and flotation. Certain of
these additives, while
useful in the upstream process, tend to reduce the effectiveness of the
activated carbon downstream,
thereby suppressing the overall process yield. The disclosed methods involve
treating these
concentrated metal solutions using one or more cationic compounds to improve
the performance of
the activated carbon processes.
[0003] Embodiments of this invention are useful in combination with
various gold extraction
processes. Gold is among the rarest elements in our world comprising perhaps
only about 4 mg/t (4
ppb) of the Earth's crust. Gold is not uniformly distributed, however, and is
typically found in higher
concentration as lodes, veins or other deposits in quartz rock, often in
combination with pyrites,
arsenopyrite, copper ores and silver ores. Most gold occurs in elemental form,
although
most gold particles are relatively small. Gold is commonly alloyed with silver
but may also include
copper, platinum and other metals as impurities. Some gold minerals are also
found in naturally
occurring tellurides including, for example, ealayerite (AuTe,), sylvanite
(AgAuTe4) and nagyagite
(Pb5Au(Te,Sb)4S54)-
[0004] Most modern gold extraction processes treat the gold ore using
amalgamation or
cyanide leaching. Amalgamation, in which the gold-containing ore is crushed
and mixed with water
and mercury to form an alloy (amalgam) from which the mercury can be
subsequently removed by
distilling at elevated temperatures, e.g., 600 C or above.
[0005] In cyanide leaching a sodium or potassium cyanide solution is added
to a heap of gold
containing ore, or to a vessel containing finely milled rock while supplying
atmospheric oxygen and,
if appropriate, in the presence of lime to form a complex gold cyanide
compound according to the
following reaction:
1
Au + 8NaCN + Q,+ 2R20 --* 4Na[Au(CN)7] + 4NaOH [1].
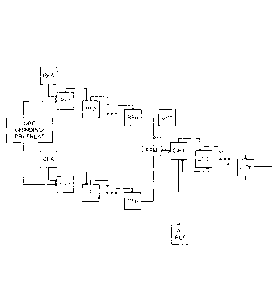
[00061 The gold can be recovered from the cyanide compound using a number
of techniques
including, for example, by reduction (for example using zinc turnings),
selective adsorption onto active carbon
(e.g., CIP, CIL and C1C processes) or ion exchangers (e.g., resin-in--pulp
process (RIP)) with subsequent
desorption using concentrated cyanide solution or zinc cyanate in the case of
ion exchangers. The gold ions can
then be reduced from the cyanide complexes electrolytically (electrowinning)
or by adding zinc powder.
[0007] In the heap leaching process ore is mined, crushed and formed into
large heaps and then
sprayed from above with the cyanide liquor so that the cyanide liquor can
percolate or trickle through the rock.
The mother liquor enriched with gold, i.e., the pregnant solution, collects at
the bottom of the heap.
[0008] As noted above, other metals are frequently found with the gold
including, in particular, silver
and copper. As with the gold, silver may be extracted by cyanide leaching in
which ore material comminuted to
a fine sludge is leached with 0.1 to 0.2% strength sodium cyanide solution
with thorough ventilation, both
metallic silver and silver sulfide and silver chloride going into solution as
dicyanoargentate (I):
2Ag + H20 + 1/202 + 4NaCN 2Na[Ag(CN)21 + 2NaOH [2]
Ag2S + 4NaCN2 Na[Ag(CN)2] + Na2S [3]
2AgC1 + 4NaCN 2Na[Ag(CN)2] + 2NaC1 [4]
[0009] Because reaction [3] leads to an equilibrium, the sodium sulfide
Na2S formed during the
leaching of sulfidie silver ores must be oxidized by blowing in air:
2S2- + 202 + H20¨. 82032- + 20H- 151
or precipitated by adding lead salt:
+ PbS [6]
to shift the equilibrium in favor of the forward reaction. The silver can then
be precipitated from the resulting
clear liquors by introducing zinc or aluminum dust
2Na[Ag(CN)2] + Zn Na2[Zn(CN)4] + 2Ag [7]
after which the slurry is subjected to further processing to recover the
silver.
[00101 Although the cyanide processes avoid the problems associated with
mercury, it does require
large quantities of cyanide salts for separating the metal from the metal-
containing ore and other materials.
Thus, when extracting gold or silver, up to 300 g of cyanide per metric ton of
material can be required,
depending on the characteristics of the gold- and/or silver-containing
z
Date Recue/Date Received 2021-01-25
material. Even with large quantities of cyanide salts, it can still be
difficult to achieve a high recovery
percentage of the desired metals from the starting material(s).
[0011] A number of prior art references have addressed the addition of a
variety of surfactants to the
cyanide liquor for improving the effectiveness of the cyanide leaching by
which the amount of cyanide required
can be decreased and/or the duration of the extraction process can he reduced
while maintaining or increasing
the process yield.
[0012] For example, U.S. Pat. No. 5,827,348 describes the use of
fluoroaliphatic surfactants for
cyanide leaching to produce a cyanide-containing liquor that exhibits a
surface tension of less than 40 Dyn/cm.
U.S. Pat. No. 8,287,616 describes compositions for cyanide leaching which
include at least one nonionic
surfactant and a process for extracting metals utilizing those
compositions.U.S. Pat. No. 4,929,274 describes a
process for heap leaching that utilizes surfactant compositions including
ethoxylated fatty esters,
alkylsulfosuccinates or fatty alcohols in the cyanide-containing liquor. U.S.
Pat. App!. 2003/0192403 Al also
describes the use of surtactants including ethoxylated aliphatic alcohols
during gold extraction.
[0013] Froth flotation of gold-bearing ore utilizes comminution (i.e.,
crushing and grinding the ore to
fine particulates), thereby increasing the surface area of the ore for
subsequent processing. The resulting fine
powder is then mixed with water to form a slurry in which the desired mineral
is rendered hydrophobic through
the addition of one or more surfactants (sometimes referred to as "collector
chemicals"). The particular additives
selected will depend on both which mineral or metal is being refined and the
chemical nature of the ore from
which the desired material is being extracted. The resulting slurry (often
referred to as "pulp") comprising a
mixture of hydrophobic particles and hydrophilic particles is then introduced
to a water bath and aerated,
creating bubbles. The hydrophobic particles containing the desired mineral
then attach themselves to the air
bubbles, which carry the particles to the surface to become a part of a froth
forming on the surface of the
aeration vessel. This froth, with its increased concentration of the desired
mineral can then be removed for
additional processing.
[0014] To be effective on a given ore slurry, the surfactants are chosen
based upon their selective
wetting of the types of particles being separated. A well-chosen surfactant
will selectively adsorb, physically or
chemically, with one of the types of particles. This combination of the
particle and the surfactant allows the
particles to bind effectively to the surface of a bubble. Flotation can be
performed using a number of techniques
and apparatus including, for example, mechanically agitated cells or tanks,
flotation columns or Jameson cells.
[0015] Mechanical cells typically include a large mixer and diffuser
mechanism at the bottom of the
mixing tank to introduce air and provide mixing action. Flotation columns
typically
3
Date Recue/Date Received 2021-01-25
CA 02860118 2014-08-21
incorporate one or more air spargers for introducing air at or near the bottom
of a tall column while
introducing slurry near the top of the column. The countercurrent motion of
the slurry flowing down
the column and the air flowing up the column provides a mixing action. Jameson
cells do not
incorporate impellers or spargers, but instead combine the slurry with air in
a downcomer where high
shear creates the turbulent conditions required for bubble particle
contacting.
[0016] Regardless of the equipment selected, the process conditions
including, for example,
the flow rate(s), tank dimensions and/or agitation conditions are typically
selected to activate the
desired minerals and achieve the desired degree of bubble attachment. In some
configurations, a
conditioner pulp is fed to a bank of rougher cells which remove most of the
desired minerals as a
concentrate. The rougher pulp then passes to a bank of scavenger cells where
additional reagents may
be added. The scavenger cell froth can then be returned to the rougher cells
for additional treatment
or sent a separate set of cleaner cells with the residual scavenger pulp
usually being discarded as tails.
More complex flotation circuits may include several sets of cleaner and re-
cleaner cells, and
intermediate re-grinding of pulp or concentrate.
[0017] Surfactants either chemically bond (chemisorption) on a hydrophobic
mineral
surface, or adsorb onto the surface physisorption (or physical adsorption).
Surfactants are typically
selected to increase the natural hydrophobicity of the surface, thereby
increasing the separability of
the hydrophobic and hydrophilic particles. A number of xanthates are used in
this manner including,
for example, potassium amyl xanthate (PAX), potassium isobutyl xanthate
(P1BX), potassium ethyl
xanthate (KEX), sodium isobutyl xanthate (SIBX), sodium isopropyl xanthate
(SIPX) and sodium
ethyl xanthate (SEX). Other surfactants include, for example,
dithiophosphates, thiocarbamates,
xanthogen formates, thionocarbamates, thiocarbanilide, palmitic acid and
various amines.
[0018] Other additives may be incorporated including, for example, wetting
agents, frothing
agents that can include, for example, pine oil, alcohols, for example, methyl
isobutyl carbinol
(MIBC), polyglycols, polyoxyparafins, cresylic acid (xylenol), pII modifiers
including, for example,
lime (CaO), soda ash (Na2C01), caustic soda (NaOH) and mineral and/or organic
acids.
Cationic modifiers may include, for example, Ba24, Ca2*, Cu', Pb2', Zn2, AgH-,
anionic modifiers may
include, for example, Si032-, P043-, CN-, C032-, S2- and organic modifiers may
include, for example,
dextrin, starch, gum(s) and/or carboxymethyl cellulose (CMC).
[0019] However, while these surfactants and other additives may tend to
improve the
flotation yield, some of these same materials tend to reduce the effectiveness
of the activated carbon
downstream, thereby suppressing the overall process yield because of carbon
fouling or other effects,
and/or may interfere with subsequent extraction processes including, for
example, electrowinning.
[0020] It is, therefore, an object of the invention to provide a
combination of compositions
and processes that increase the overall yields of the desired metal(s), in
particular of gold and/or
4
silver, based on the starting material used.
BRIEF DESCRIPTION
[0021] The disclosed methods incorporate a range of cationic chemical
additives and associated dosing
methods for treating post-flotation process streams to increase the activated
carbon capacity and/or improving
electrowinning performance to increase the overall extraction of precious
metals from a particular ore_
[0022] The methods disclosed herein utilize at least one cationically charged
compound selected from, for
example, a group consisting of amines, particularly quaternary amines such as
alkyldimethylbenzylamrnonium
chloride (ADBAC) compositions, diallyldirnethylarnmonium chloride (DADMAC)
compositions (e.g., CAS
7398-69-8), polyamines and other suitable cationic materials and mixtures
thereof, for neutralizing and
coagulating excess flotation reagents and flotation reagent by-products to
reduce the fouling of the activated
carbon by these compounds downstream of the flotation circuit.
[0023] Lab studies have indicated that an appropriate dosage of a composition
containing one or more
cationic compounds, such as 40 mg/1 of ML2005, available from ChemTreat, Inc.
of Glen Allen, VA, that
comprises a solution of quaternary ammonium compounds, water and ethanol,
provides significant improvement
of the downstream activated carbon capacity and increases the gold content in
ounces per ton of carbon which
can be extracted from a given ore.
Lab studies also indicated no reduction in the activated carbon's gold
capacity at IVIL2005 treatment dosages
up to 200 nig/I (200 ppm). The copper content in the carbon was also
unaffected by the addition of the cationic
reagents at all dosages tested.
[0024] Process monitoring can be implemented by utilizing the particle charge
density ("PCD") technology
developed by BTG industries. This technology is widely used in the pulp and
paper industry for improving
control of the amount of cationic or anionic charge on paper machines and may
be implemented using any
appropriate analyzers including, for example, BTG/MiltekTm analyzers. It is
anticipated that this PCD
technology may be useful for monitoring the floatation circuit concentrate
and/or tails chemistry and/or
activated carbon tails chemistry. Field trials using a portable lab analyzer
have indicated that this type of
analyzer may be used for monitoring the demand, sufficiency and residual
concentrations of the cationic
additive(s) and performance benefits during plant operation. Turbidimetrie,
colorimetric and/or other analytical
test methods to measure low levels (less than 10 pprn) of residual cationic
additives may also be used during
plant operations to prevent overfeed of the additive. The testing can be
conducted on a continuous or periodic
basis and can be automated, serniautomated or manual and/or may include, for
example, "grab" sampling of any
of the relevant process streams at various points of interest for controlling
and/or monitoring the
Date Recue/Date Received 2021-01-25
CA 02860118 2014-08-21
=
process(es).
[00251 Methods for recovering a metal in a leaching process according to
this disclosure
include the steps of obtaining a metal concentrate stream that contains a
concentration of a free
anionic organic compound, combining a quantity of a cationic amine compound
with the metal
concentrate stream to form an adjusted metal concentrate stream, the quantity
of the cationic amine
compound being sufficient to neutralize at least a majority of the free
anionic organic compound; and
mixing the adjusted metal concentrate stream with activated carbon under
conditions whereby the
metal is selectively adsorbed on the activated carbon.
[0026] Embodiments of these methods may include other features including
steps for
determining a free anionic organic content of the metal concentrate stream and
adjusting the quantity
of the cationic amine compound in response to the free anionic organic
content, The quantity of the
cationic amine compound added to the metal concentrate stream may be
sufficient to establish an
excess concentration of the cationic amine compound and, in such instances,
the methods may include
determining the excess cationic compound content of the adjusted metal
concentrate stream and
adjusting the quantity of the cationic compound in response to the excess
cationic compound content.
[0027] The source of the metal concentrate stream may be, for example, a
concentrate stream
from a flotation process, a pregnant liquor stream from a heap leach operation
or any other source
which includes an excess of one or more anionic organic compounds.
[0028] Certain of the cationic amine compounds useful for practicing the
disclosed methods
may be represented by the fonnula:
cr
R\CH3
CH3
wherein R may be expressed as CõII,õ+i with n satisfying the relationship 8 <
n < 18 and, preferably,
satisfying the relationship 12 < n < 16. Other cationic amine compounds
selected from the range of
amines, polyamines and other cationic organic species capable of interacting
with the excess anionic
organic compounds including, for example, anionic surfactants and/or polymers,
present in the
untreated metal concentrate stream may also be utilized. The cationic amine
compounds may be used
alone or in combinations and mixtures of such compounds and may include, for
example, one or more
alkyldimethylbenzyl ammonium chloride compounds.
[0029] The disclosed methods may be monitored and/or controlled using a
variety of
techniques including, for example, adding sufficient quantities of the
cationic amine compounds so
that the adjusted metal concentrate stream exhibits a particle charge density
of no more than
6
CA 02860118 2014-08-21
+100 mV, preferably +25 mV and more preferably 5 mV.
[0030] The disclosed methods may also be configured for recycling a
portion of an activated
carbon tails stream into a feed stream for the flotation process and/or into
the adjusted metal
concentrate stream. The content of the activated carbon tails stream may be
monitored or controlled
by analyzing an ionic content of the activated carbon tails stream and
adjusting the activated carbon
tails stream to form an adjusted carbon tails stream exhibiting a
substantially neutral particle charge
density of +100 mV and/or including an excess quantity of one or more cationic
amine compounds
within a certain target range of, for example, 10-200 ppm, 25-55 ppm or 35-45
ppm.
[0031] The disclosed methods may be configured to provide an excess
concentration of the
cationic amine compound in the adjusted metal concentrate stream of no more
than 200 ppm or such
other concentration whereby the performance of the activated carbon is
unchanged or degraded by
less than 5%, preferably less than 2% and more preferably by less than 1%. The
disclosed methods
may also be configured to provide a concentration of the cationic amine
compound in the adjusted
metal concentrate stream of from 10-200 ppm, from 25-55 ppm or 35-45 ppm.
[0032] Certain other of the cationic amine compounds useful for practicing
the disclosed
methods may be represented by the formula:
RIR2(CH3)71\PX0
wherein RI is a saturated or olefinically-unsaturated acyclic aliphatic
hydrocarbyl group, R2 is a
saturated or olefinically unsaturated acyclic aliphatic hydrocarbyl group, a
benzyl group or an alkyl-
substituted benzyl group, and X is an anion, wherein if R2 is a benzyl or
alkyl-substituted benzyl
group, le includes a first carbon chain of 12 to 16 carbon atoms, and further
wherein, if both RI and
R2 are saturated or olefinically-unsaturated acyclic aliphatic hydrocarbyl
groups including first and
second carbon chains, a sum of the first and second carbon chains includes 16
to 20 carbon atoms.
BRIEF DESCRIPTION OF THE DRAWINGS
[0033] In order to provide a more complete explanation of the treatment
compositions,
methods and associated systems as disclosed and claimed herein, and the
various embodiments
thereof, attention is directed to the accompanying figures, wherein:
[0034] FIG. IA illustrates a first conventional prior art process,
specifically a simplified
process diagram for portion of a mining operation including a pair of
flotation arrays and a carbon-in-
pulp array used for recovering target metals including, for example, gold,
silver and/or copper;
[0035] FIG. 1B illustrates a second conventional prior art process,
specifically a simplified
process diagram for portion of a mining operation including a heap leach and a
carbon-in-pulp array
used for recovering target metals including, for example, gold, silver and/or
copper from the pregnant
7
CA 02860118 2014-08-21
liquor extracted from the heap;
[0036] FIG. 2 illustrates a process diagram for a process modified in
accord with an
embodiment of the invention in which the metal concentrate composition
entering the activated
carbon process is modified with the addition of a cationically charged
compound;
[0037] FIG. 3 illustrates a process diagram for a process modified in
accord with another
embodiment of the invention in which the metal concentrate composition
entering the activated
carbon process is modified with the addition of a cationically charged
compound and at least a portion
of the carbon process tail solution is recycled into at least part of the
flotation process;
[0038] FIG. 4 illustrates a process diagram for a process modified in
accord with another
embodiment of the invention in which the metal concentrate composition
entering the activated
carbon process is modified with the addition of a cationically charged
compound and at least a portion
of the carbon process tail solution is recycled into at least part of the
flotation process or the pre-
carbon adjustment solution;
[0039] FIG. 5 illustrates a process diagram for a process modified in
accord with another
embodiment of the invention in which the metal concentrate composition
entering the activated
carbon process is modified with the addition of a cationically charged
compound and composition
entering a least one of the flotation processes is modified with the addition
of a cationically charged
compound; and
[0040] FIGS. 6-9 illustrate test results for the recovery of gold (in
ounces per ton (OPT) of
carbon by fire assay), FIG. 6, silver, FIG. 7, and copper, FIG. 8, as well as
the carbon activity, FIG. 9,
achieved at various loadings of a solution containing components selected from
a group consisting of
amines, particularly quaternary amines such as ADBACs, and polyamines.
[0041] The foregoing figures, being merely exemplary, have been simplified
to represent
various basic operational components that may be utilized in practicing one or
more embodiments of
the disclosed methods depending upon the particular operational parameters. It
is believed that these
figures are sufficient to illustrate the basic design elements to a degree
that one of ordinary skill in the
art can readily develop an understanding of the various embodiments and
aspects of the invention as
disclosed and claimed herein.
DETAILED DESCRIPTION
[0042] As illustrated in FIG. IA, a conventional gold extraction process
may include an ore
preparation process during which the ore is reduced to particles within a
target size range. These ore
particles are then introduced to one or more multi-stage flotation processes.
As illustrated in FIG. IA,
the flotation process includes a rough flotation sequence, RF I RFn into
which corresponding
8
CA 02860118 2014-08-21
additive compositions RFA are introduced to provide the desired combination of
surfactants, frothing
agents and other modifiers for promoting the separation of the desired
minerals (metal(s)) during the
flotation process.
[0043] As illustrated in FIG. 1B, a conventional heap leach gold
extraction process may
include an ore preparation process during which the ore is reduced to
particles within a target size
range. These ore particles are then formed into large piles (heaps) and
irrigated with an extraction
solution that removes the desired metal from the ore and forms a metal
concentrate solution (also
referred to as pregnant liquor) that is collected at the bottom of the heap.
[0044] The separated froth or metal concentrate stream (or pregnant
liquor) is then fed into
an activated carbon process for collecting the desired metal. As noted above,
the activated carbon can
be arranged in a variety of configurations including, for example, CIP, CIC
(not shown) and CIL (not
shown), each of which also has an associated range of equipment and techniques
that can be
incorporated as desired to control the collection process. In the illustrated
CIP arrangement, the
pregnant solution from the flotation processes is passed through a series of
CIP vessels, CIP I ...
CIPn, while a counter current flow of activated carbon is passed through the
same series of CIP
vessels, CIPn CIP I . In addition to the pregnant solution, one or more
additives, e.g., a cyanide
solution, can be introduced before the solution enters the activated carbon
process. The loaded carbon
retrieved from the activated carbon process is then subjected to additional
processing, AU REC (not
illustrated), to recover the loading mineral, typically gold and/or other
precious or rare metal(s).
[0045] A number of fundamental physical and chemical parameters affect
adsorption by the
activated carbon. The equilibrium capacity of activated carbon for the
adsorption of gold is
influenced by a number of factors. These include, for example, temperature,
the nature of the raw
material used to manufacture the carbon, the activation conditions used during
carbon manufacture,
pH, the concentration of free cyanide ions and spectator ions such as Ca2+,
Na+ and K+, the presence
of organic solvents such as acetone, ethanol and acetonitrile, the presence of
organic and inorganic
foulants including, for example, xanthates and calcium carbonates.
[0046] Researchers have quantified some of the effects on the equilibrium
behavior of the
carbon-aurocyanide system under typical industrial adsorption conditions
indicating that the
aurocyanide only transforms into AuCN or metallic gold at high temperatures,
in a strong acid
solution or a combination of both acid and elevated temperature. At ambient
temperature and in an
alkaline solution, conditions typically found in a CIP/CIL adsorption circuit,
the aurocyanide complex
is generally adsorbed in a fully reversible manner.
[0047] A number of parameters relating to the carbon particles and the
operating conditions
can affect the kinetics of this adsorption. For example, smaller carbon
particles tend to exhibit higher
adsorption rates. The surface roughness of the carbon can affect the film mass
transfer coefficient for
9
CA 02860118 2014-08-21
adsorption while the internal surface and pore diffusion properties of the
carbon affect the physio-
chemical characteristics of the carbon. Process parameters may include, for
example, the temperature,
the energy and mechanism used in agitating the pulp and the solids content of
the pulp. Under
conventional industrial conditions it is suspected that adsorption rates are
limited by film-transfer
effects whereby increased agitation should improve adsorption rates. Other
parameters include
oxygen concentration, pH with lower pH values tending to increase adsorption.
Competing ions that
are adsorbed with gold including, for example, silver, copper and nickel that,
if present in sufficiently
high concentrations, will tend to retard gold adsorption and finally, physical
blocking or occlusion of
the carbon pores by fine solids or precipitated material.
[0048] As illustrated in FIG. 2, a first embodiment of the disclosed
processes includes a
second (optional) flotation process comprising a cleaner and/or scavenger
flotation sequence, CFI ...
CFn, into which corresponding additive compositions CFA, are introduced to
provide the desired
combination of surfactants, frothing agents and other modifiers for promoting
the separation of the
desired minerals (metal(s)) during the flotation process. This first
embodiment further involves the
addition of at least one cationically charged compound selected from, for
example, a group consisting
of amines, particularly quaternary amines, polyamines, diallyldimethylammonium
chloride
(DADMAC) compositions and other suitable cationic materials and mixtures
thereof. Also of
particular interest are those quaternary amines classified as
alkyldimethylbenzylammonium chlorides
(ADBAC) that incorporate a positively charged nitrogen (cation) that is
covalently bonded to three
alkyl group substituents and a benzyl substituent that can be characterized by
the structure:
ci
//CH3 [8]
\ +
CH3
where R may be characterized by Cõ1-12õ, with n satisfying the relationship 8
< n < 18. The ADBAC
composition will typically include compounds exhibiting a mixture of carbon
chain lengths, the
majority of the carbon chains typically satisfying the relationship 12 5_ n 5.
16.
[0049] The cationically charged compound(s) is introduced, CPA, in an
amount sufficient to
suppress the carbon fouling effects of excess surfactant, typically one or
more anionic surfactant(s),
exiting the flotation process. The dosing of the cationically charged
compound(s) can be controlled in
response to the solution monitor, FPM, that may, for example, be configured to
provide particle
charge density (PCD) readings obtained by analyzing the flotation circuit
concentrate chemistry.
[0050] The addition of the amine cationic compounds will tend to
neutralize the anionic
organic compounds including, for example, surfactants and polymers, and tend
to make the particle
CA 02860118 2014-08-21
charge density of the metal concentrate stream more positive (less negative).
It is believed that when
the dosing of the amine cationic compounds approaches or slightly surpasses
that of the anionic
organic compounds, a substantially neutral charge density will be obtained.
This substantially neutral
charge density may reflect a charge density measurement within +100 mV,
preferably within +25 mV,
more preferably within +5 mV and most preferably within +1 mV.
[0051] Confirmation of the PCD sensor readings may be made using
potentiometric titration
technique as reflected, for example, in ASTM D5806-95 for determining active
matter in quaternary
ammonium salts (in disinfectants), particularly n-alkyldimethylbenzylammonium
chloride,
eetyltrimethylammonium chloride, blends of n-oetyldecyl dimethylammonium
chloride, di-n-octyl
dimethylammoniu in chloride, and di-n-decyldirnethyl ammonium chloride or ASTM
D5070-90 for
determining active matter in quaternary ammonium salts (in fabric softeners),
particularly dialkyl
dimethyl quaternary ammonium compound type and the diamidoamine based
quaternary ammonium
compound type.
[0052] As testing has indicated that an excess of the amine cationic on
the order of at least up
to 200 ppm does not degrade the performance of the activated carbon, it may be
preferable to run the
process under conditions that result in an excess of the cationic compound in
the adjusted metal
concentrate stream. Operating under these conditions will also allow the
monitoring and/or analysis
to focus not on particle charge density, but rather utilize one or more
technique for ascertaining the
presence of an excess of the cationic compound(s) including, for example,
quaternary ammonium
salts, in the adjusted metal concentrate stream.
[0053] Lab testing on flotation metal concentrate samples has suggested
that a feed of the
cationically charged compound(s) necessary to achieve concentrations on the
order of 10-200 ppm
may reasonably be expected to achieve a neutral charge density depending on
the quantity and nature
of the excess anionic organic compounds (and convert the initially negative
PCD readings to a
substantially neutral or positive value). Initial testing has also suggested
that a feed rate sufficient to
maintain about 30-60 ppm of the cationic compound provides acceptable results,
with a feed rate
sufficient to maintain about 40 ppm showing the best results.
[0054] Compounds useful for practicing the disclosed methods include a
range of water-
soluble quaternary ammonium salts that can be represented by the formula:
R1R2(CH3)2NeXe 191
wherein RI is a saturated or olefinically-unsaturated acyclic aliphatic
hydrocarbyl group, R2 is a
saturated or olefinically unsaturated acyclic aliphatic hydrocarbyl group, a
benzyl group or an alkyl-
substituted benzyl group, and X is an anion, wherein (a) if R2 is a benzyl or
alkyl-substituted benzyl
group, R1 has in the range of (or an average in the range of) about 12 to
about 16 carbon atoms, and
11
(b) if W and R2 arc saturated or olefinically-unsaturated acyclic aliphatic
hydrocarbyl groups, they need not
be identical and each can have in the range of 4 to about 16 carbon atoms as
long as the total number of carbon
atoms in these two groups is in the range of (or comprise an average in the
range of) about 16 to about 20 carbon
atoms.
[0055] Examples of the benzyl type compounds that may be used in
practicing the disclosed methods
include dodecyldimethyl benzyl ammonium chloride, tridecyldimethyl benzyl
ammonium chloride,
tetradecyldimetbyl benzyl ammonium chloride, pentadecyldimethyl benzyl
ammonium chloride,
hexadecyldirriethyl benzyl ammonium chloride, dodecenyldirriethyl benzyl
ammonium chloride,
tridecenyldimethyl bc..,rizyl ammonium chloride, tc..,tradecenyldimethyl
benzyl ammonium chloride,
pentadecenyldimethyl benzyl ammonium chloride, hexadecenyldimethyl benzyl
ammonium chloride, a mixture
of dodecyl- and tetradecyldimethyl benzyl ammonium chlorides, a mixture of
dodecyl-, tetrad.ecyl-, and
hexadecyldimethyl benzyl ammonium chlorides, a mixture of dodecyl-, tetradecyl-
, hexadecyl-, and
octadecyldirnethyl benzyl ammonium chlorides having an average of about 14
carbon atoms in the molecule, a
mixture of decyl-, dodecyl-, and tetradecyldimethyl benzyl ammonium chlorides
having an average of about 12
carbon atoms in the molecule, a mixture of d.odecyl- and d.odecenyldimethyl
benzyl ammonium chlorides, the
bromide analogs of these compounds, and other analogous quaternary ammonium
compounds.
[0056] Examples of the dihydrocarbyl-type of quaternary ammonium
compounds that may be utilized
in practicing the disclosed methods include (butyl)(dodecyl)dirnethylammonium
chloride,
(hexyl)(decyl)dimethylammonium chloride, (hexyl)(undecyl)dimethylammoniu.m
chloride,
dioctyldimethylammonium chloride, dinonyldimethylammonium chloride,
didecyldimethylammonium chloride,
(octyl)(decyl)dimethyl-arrimonium chloride, (oetyl)(undecyl)dimethylammonium
chloride,
(octyl)(dodecyl)dimethyl-ammonium chloride,
(hexyl)(tridecyl)dirnethyla,mmonium chloride,
(hexyl)(tetrad.ecyl)dimethyl-ammonium chloride, (octyl)(7-
methylnonypdimethylarnmonium chloride, di(3,4-
dimethyloctyl)dimethylammonium chloride, di(4-octenyl)dirnethylammonium
chloride, di(8-
nonenyl)dirnethylarmnonium chloride, di(5-decenyl)dimethylammonium chloride,
(butyl)(2-
hexadecenyl)dimethylarnmonium chloride, (octenyl)(octyl)dimethylammonium
chloride, the bromide analogs of
these compounds, and other analogous quaternary ammonium compounds.
[0057] Methods for the preparation of quaternary ammonium compounds are
well known and
published in the literature and, indeed, many such compounds are readily
available on the commercial chemical
market. Such compounds are discussed in, for example, U.S. Pat. No. 6,010,996.
[0058] Utilizing the system configuration illustrated in FIG. 2, the
cationically charged compound(s) can be
introduced into the flotation concentrates stream after exiting the flotation
circuit
12
Date Recue/Date Received 2021-01-25
CA 02860118 2014-08-21
or before entering the activated carbon circuit, CPA. The monitoring, FPM, if
utilized, can be
conducted before injection of the cationic compound(s) to provide an
opportunity to adjust to dose to
match the anionic nature of the concentrated metal stream. Alternatively,
monitoring, FPM, if
utilized, can be conducted after injection and reaction of the cationic
compound(s) to ensure that the
dose matched the anionic nature of the concentrated metal stream. Additional
equipment (not shown)
may be incorporated to ensure that the cationic compound(s) being introduced
experience sufficient
contact time and mixing energy to neutralize and/or complex the excess anionic
species, e.g., one or
more xanthate surfactants, and reduce the fouling of the activated carbon.
Reducing the fouling of the
activated carbon by the flotation surfactants will tend to increase the rate
and quantity of gold and
consequently improve the percent gold recovery.
[0059] Depending on the particular surfactant species involved, the dosing
point and the
equipment configuration downstream of the dosing point may affect the
effectiveness of the method.
The dosing point and configuration should be selected whereby sufficient
contact time is allowed and
mixing provided in order for the relatively low concentrations (generally less
than 200 ppin and more
typically less than 100 ppm) of cationic and anionic species to react/complex
without losing a
substantial quantity of the cationic species to reaction/complexing with other
solids present in the
stream. It is believed that in most instances, a contact time on the order of
1-10 minutes will be
sufficient to achieve the desired result. For example, it is believed that
contact times of 4-6 minutes at
moderate mixing energy levels (for example, G factors in the 100-200 range)
will achieve sufficient
reaction/complexing of the cationic and anionic species without undue demand
for the cationic
species attributable to the additional solids in the flotation stream.
[0060] As illustrated in FIG. 3, a second embodiment of the disclosed
processes expands
upon the embodiment illustrated in FIG. 2 to provide for recycling at least a
portion of the activated
carbon circuit tails into the feed stream for at least one of the flotation
circuits, whereby a
predetermined concentration of one or more cationically charged compound(s)
used in the activated
carbon circuit can be used for modifying the performance of at least one of
the flotation circuits. As
noted above, these cationic species will tend to bind with anionic species,
e.g, to form one or more
compounds with different physiochemical properties than either of the source
compounds. The new
compound(s) may be hydrophobic and may partition into sludge or other
particles present in the
activated carbon circuit.
[0061] Despite the conventional wisdom regarding the segregation of the
cationic surfactants
and any resulting cationic/anionic complexes, however, in practice it appears
that some portion of the
cationic species or their byproducts may survive the activated carbon circuit
and, in the process
illustrated in FIG. 3, may comprise a portion of the recycle stream that is
subsequently introduced into
the rough flotation circuit. Further, it appears that this residual cationic
species or complex may
constitute a surprisingly beneficial additive to the flotation circuit.
13
[0062] As illustrated in FIG. 4, a third embodiment of the disclosed
processes expands upon the
embodiment illustrated in FIG. 3 to provide for recycling at least a portion
of the activated carbon circuit tails
into the feed stream for at least one of the flotation circuits and/or the
additive feed stream for the activated
carbon circuit. This third embodiment also provides for independent monitoring
of the tails from the rough,
RFM, and cleaner, CFM, flotation circuits.
[0063] As illustrated in FIG. 5, a fourth embodiment of the disclosed
processes expands upon the
embodiment illustrated in FIG. 4 to provide for recycling at least a portion
of the activated carbon circuit tails
into the feed stream for at least one of the flotation circuits and/or the
additive feed stream for the activated
carbon circuit. This fourth embodiment also provides a control circuit for
monitoring one or more characteristic
properties of the recycle stream, RCM, and adjusting the composition of the
recycle stream, RC ADJ, through
introduction of additional surfactant species or other additive(s), QA, before
it is introduced into one or more
flotation circuits and may provide for additional adjustment, RFX, for
controlling the composition and/or
particle charge density of the solution entering at least one of the flotation
circuits.
[0064] Although, as discussed above, it is anticipated that the
concentration of the excess anionic
species will typically be less than 200 ppm, and more frequently, less than
100 ppm after completing a
conventional flotation circuit, periodic excursions from these typical values
are expected to result from, for
example, overfeeding of flotation reagent(s) and/or other factors including,
for example, ore composition, grind
size, and environmental factors such as temperature and pH from lime addition.
[0065] Depending on the configuration utilized, the disclosed methods and
systems will detect these
excursions in the flotation circuit effluent using the streaming
current/particle charge analyzers or other suitable
detectors FPM. RFM and/or CFM, before entering the activated carbon circuit.
The cationic surfactant dosage
can then be adjusted in response to the magnitude of the excursion detected to
ensure that the cationic demand
(a function of excess reagent and the inherent anionic charge nature of ore
slurries in general) can be satisfied in
a timely and cost-effective manner.
[0066] Although several exemplary embodiments of this invention have been
described in detail, it
will be readily apparent to those skilled in the art that the disclosed mining
processes, and the apparatus for
implementing these processes, may easily be modified from the exact
embodiments provided herein without
matenally departing from the essential characteristics thereof: In particular,
the disclosed methods need not be
practiced on systems that include two flotation circuits and, if multiple
flotation circuits are present and recycle
streams are utilized, they can be directed to one or more of the flotation
and/or activated carbon circuits without
departing from the disclosed invention.
[0067] Accordingly, therefore, these disclosures are to be considered in
all respects as illustrative and
not restrictive. As will be appreciated by those skilled in the art, a number
of other
14
Date Recue/Date Received 2021-01-25
CA 02860118 2014-08-21
embodiments of the methods according to the disclosure are both feasible and
would be expected to
provide similar advantages. The scope of the invention, therefore, should be
understood as
encompassing those variations of the example embodiments detailed herein that
would be readily
apparent to one of ordinary skill in the art.
[0068] Further, while certain process steps are described for the purpose
of enabling the
reader to make and use certain mining treatment processes shown, such
suggestions shall not serve in
any way to limit the claims to the exact variation disclosed, and it is to be
understood that other
variations, including various treatment additives may be utilized in
practicing the disclosed methods.
* * * * *