Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02862300 2014-07-22
WO 2013/119230
PCT/US2012/024382
Description
Method of producing alcohols
Technical Field
[0001] The disclosure relates to a method of making alcohols, and more
specifically alkanols, from alkanes, and more specifically from alkane
halides.
Background
[0002] Alcohols are industrially produced from direct hydration of alkenes,
such as ethylene, or from cracking of appropriate fractions of distilled (or
fractionated) crude oil. While demands for alcohols, and especially for
ethanol,
continue to increase, crude oil reserves continue to he depleted. Moreover,
the
processes of alkene hydration and fractionation and cracking of crude oil are
themselves energy intensive processes.
[0003] There remains a need therefore, for a method of producing alcohols
from more readily available starting materials and for a process which does
not
require the energy input necessary for current industrial alcohol production.
Summary of the Disclosure
[0004] The disclosure provides a method of making alcohols. More
specifically, an illustrative disclosed method comprises reacting an alkane
gas
with a halogen gas in a halogenation reactor to form a halogenation reaction
product mixture comprising alkane halide and hydrogen halide mixture;
contacting the halogenation reaction product mixture with a metal organic salt
thereby forming an extractor product mixture of a metal halide, organic ester,
and
organic acid; separating the organic ester and organic acid mixture from the
metal
halide; oxygenating the metal halide to form a metal oxide and halide
containing
gasses; separating the metal oxide from the halide containing gasses; mixing
the
CA 02862300 2014-07-22
WO 2013/119231) PCT/US2012/024382
metal oxide with water to form a metal oxide slurry; mixing the metal oxide
slurry with a countercurrent flow of the organic ester and organic acid
mixture to
form a raw product comprising alkanol and a metal organic salt.
[0005] In an improvement method of making alcohols, the disclosed method
involves liquid phase forming of alcohol esters from liquid alkane halides and
a
solution of metal salts of organic acids whose alkanes esters are less soluble
in
water than that of the alkane halide and treating of the alcohol ester formed
with
magnesium or metal hydroxides to form the alcohol and the metal salt of the
organic acids.
Brief Description of the Drawings
[0006] The drawings illustrate an exemplary form of the disclosure; it being
understood, however, that this disclosure is not limited to the precise
arrangements and instrumentalities shown in the drawing.
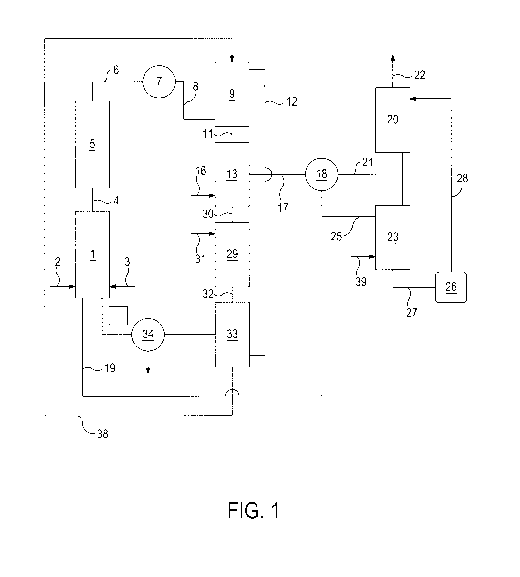
[0007] FIG. 1 is a schematic diagram of one embodiment of the disclosed
process.
[0008] FIG. 2 is a schematic diagram of an alternative illustrative method of
the disclosed process.
[0009] FIG. 3 is a schematic diagram of another aspect of the method of the
disclosed process.
Detailed Description
[0010] The disclosure is a method for making alcohols. The disclosure is
a method for producing organic alcohols, including for example, methanol,
ethanol, propanol, and combinations thereof.
[0011] In one illustrative embodiment, the instant disclosure provides a
method comprising: reacting an alkane with a halogen gas in a halogenations
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-3-
reactor to form a halogenation reaction product mixture comprising alkane
halide
and hydrogen halide mixture; contacting the halogenation reaction product
mixture with a metal organic salt thereby forming an extractor product mixture
of
a metal halide, organic salt, organic ester, and organic acid; separating the
organic ester and organic acid mixture from the metal halide; oxygenating the
metal halide to form a metal oxide and halide containing gasses; separating
the
metal oxide from the halide containing gasses; mixing the metal oxide with
water
to form a metal oxide slurry; mixing the metal oxide slun-y with a
countercurrent
flow of the organic ester and organic acid mixture to form a raw product
comprising alkanol and a metal organic salt.
[0012] In an alternative illustrative improvement embodiment, the instant
disclosure provides a method comprising: reacting an alkane with a halogen gas
in a halogenations reactor to form a halogenation reaction product mixture
comprising alkane halide and hydrogen halide mixture; contacting the
halogenation reaction product mixture with a metal organic salt under aqueous
conditions thereby forming an aqueous extractor product mixture of a soluble
metal halide, and an insoluble organic ester; separating the insoluble organic
ester
from the aqueous metal halide; oxygenating the metal halide to form a metal
oxide and halide containing gasses: separating the metal oxide from the halide
containing gasses; mixing the metal oxide with water to form a metal oxide
slurry; mixing the metal oxide slurry with a countercurrent flow of the
insoluble
organic ester and insoluble organic salt mixture to fonn a raw product
comprising
alkanol.
[0013] In yet another alternative illustrative improvement method of
making alcohols, the instant disclosure provides a method for the production
of
alcohols comprising: contacting an alkane gas with an aqueous halide saturated
solution to strip the halide from the solution to form a product mixture of an
alkane and a halide; reacting a halogen gas with the alkane halide mixture to
form
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-4-
an alkyl halide, an alkyl di halide, an alkyl tri halide, and a hydrogen
halide
gasses; neutralizing the alkyl di halide, the alkyl tri halide, and the
hydrogen
halide gasses with a suspension of magnesium hydroxide; cooling the gasses
from the neutralizing step to a temperature to liquefy the gasses to form
liquefied
gasses; mixing the liquefied gasses with a magnesium benzoate or butyrate
solution to form an aqueous solution of benzoate or butyrate ethyl esters;
separating the benzoate or butyrate ethyl esters in the aqueous solution which
are
water insoluble from water in the aqueous solution to form an ester insoluble
layer comprising benzoate or butyrate ethyl esters and a water layer
containing
magnesium halide; contacting the ester insoluble layer with a suspension of
magnesium hydroxide to form an alcohol.
[0014] Alkanes useful in various embodiments of the disclosed methods may
be selected from the group consisting of Cl-C20 alkanes, including most
preferably, methane, ethane, propane, butane and mixtures thereof. All
combinations and subcombinations of such alkanes are included and disclosed
herein. For example, the alkanes may comprise a mixture of methane and ethane;
or in the alternative, a mixture of methane and propane; or in the
alternative, a
mixture of ethane and butane. In the alternative, the alkane may comprise only
a
single alkane. For example, the alkane may comprise methane with no other
alkane component; or in the alternative, the alkane may comprise ethane with
no
other alkane component; or in the alternative the alkane may comprise propane
with no other alkane component.
[0015] Ilalogen gasses useful in various embodiments of the disclosed
methods may be selected from the group consisting of chlorine gas, bromine
gas,
iodine gas, and combinations thereof. All combinations and subcombinations of
such halogen gasses are included and disclosed herein. For example, the
halogen
gasses may comprise a mixture of chlorine and bromine gasses; or in the
alternative the halogen gasses may comprise a mixture of chlorine and iodine
CA 02862300 2014-07-22
WO 2013/119230
PCT/US2012/024382
-5-
gasses. In the alternative, the halogen gas useful in the halogenations step
of the
disclosed method may comprise only a single halogen gas. For example the
halogen gas may be bromine gas; or in the alternative, the halogen gas may be
chlorine gas. The halogen gas or gasses used in the halogenation reactor may
he
supplied directly into the halogenations reactor, as for example, by injection
through a dedicated supply line. Alternatively, the halogen gas or gasses used
in
the halogenation reactor may be formed in situ in the halogenation reactor.
[0016] Metal organic salts useful in the disclosed method of FIG. I may be
selected from the group consisting of metal formate, metal acetate, metal
benzoate, and combinations thereof. The metal of the metal organic salt in
various embodiments of the disclosed method of FIG. 1 may be selected from
Magnesium, Zinc, and combinations thereof, for example. All combinations and
subcombinations of the metal organic salts are disclosed and included herein.
For
example, the metal organic salt may be magnesium formate, zinc acetate,
magnesium benzoate, zinc dichlorobenzoate, zinc dichloroacetate, or any
combination of two or more of the tbregoing. Metal organic salts useful in the
improvement methods disclosed below are selected based on the water solubility
of the alkane halide with which the metal organic salt is reacted and the
alkane
ester product resulting from that reaction in water. Specifically, if the
alkane
ester product has a solubility that is less than the solubility of the
reactant alkane
halide, then the alkane ester product will precipitate out of the water into
an
insoluble layer which can be easily separated from the metallic halide which
remains in the water. This separation of insoluble alkane ester from the
metallic
halide permits the metallic halide to be processed downstream independent from
the alkane ester stream to form the metallic hydroxide required to be
contacted
with the alkane ester to form the alkane alcohol. If, however, the alkane
ester
product has a solubility that is greater than the solubility of the reactant
alkane
halide, the alkane ester product will not precipitate out of the water hut
remain
CA 02862300 2014-07-22
WO 2013/119230
PCT/US2012/024382
-6-
with the metallic halide as a mixture in the water; making the reaction of the
metallic halide into metallic hydroxide not possible. The metal organic salt
may
be a magnesium benzoate or a magnesium butyrate or a magnesium salicylate, for
example, which are the magnesium salts of benzoic acid, butyric acid, and
salicyclic acid, respectivily. When magnesium butyrate is used, for example,
the
solubility of the ethyl bromide in water is 0.91 grams per 100 ml of water, or
0.0835 moles per 1000 ml of water and the solubility of ethyl butyrate is 0.68
grams per 100 ml of water or 0.059 moles per 1000 ml. Because the solubility
of
the ethyl butyrate is less than the solubility of the magnesium butyrate in
water,
the ethyl butyrate will precipitate out of the water as an insoluble layer
according
the teachings of this disclosure. When methyl formate is used, however, the
solubility of the methyl formate is greater than the solubility of the ethyl
bromide
and hence the methyl formate will remain with the ethyl bromide in the aqueous
solution.
[0017] In one embodiment of the disclosed method of FIG. 1, the
alkane
is methane, the metal organic salt is magnesium tbniiate, the halide gas is
bromine gas, and the alkanol is methanol.
[0018] In an alternative embodiment with all of the disclosed
methods,
the disclosure provides a method of making alkanols except that the halogen
gas
is chlorine gas.
[0019] In an alternative embodiment with all the disclosed methods,
the
disclosure provides a method of making alkanols except that the halogen gas is
a
mixture of bromine and chlorine gasses.
[0020] In an alternative embodiment with all the disclosed methods,
the
disclosure provides a method of making alkanols except that the alkane is
ethane.
[0021] In an alternative embodiment with all the disclosed methods,
the
disclosure provides a method of making alkanols except that the alkane is
propane.
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-7-
[0022] In an alternative embodiment with all the disclosed methods, the
disclosure provides a method of making alkanols except that the alkane is
butane.
[0023] In an alternative embodiment with all the disclosed methods, the
disclosure provides a method of making alkanols except that the alkane is a
mixture of methane and ethane.
[0024] In an alternative embodiment of FIG. 1,the invention provides a
method of making alkanols except that the metal organic salt is magnesium
acetate.
[0025] In an alternative embodiment of all the disclosed methods, the
invention provides a method of making alkanols except that the metal organic
salt
is magnesium benzoate.
[0026] In an alternative embodiment of all the disclosed methods, the
invention provides a method of making alkanols except that the metal organic
salt
is zinc benzoate.
[0027] In an alternative embodiment of FIG. 1, the invention provides a
method of making alkanols except that the metal organic salt is magnesium
acetate. In an alternative embodiment of FIG. I, the invention provides a
method
of making alkanols except that the metal organic salt is zinc formate.
[0028] The various steps of the disclosed method may be conducted in
any appropriate reactor. For example, in the disclosure of both methods, the
step
of oxygenating the metal halide may occur in a fluidized bed reactor,
otherwise
known as. a fluo-solids reactor. In some embodiments of the disclosed method
the step of contacting the halogenation reaction product mixture with a metal
organic salt occurs in a column packed with an inert packing material. Any one
or
more inert materials as are known in the art may he used in the step of
contacting
the halogenations reaction product mixture with a metal organic salt may he
used,
including for example Berl saddles. In some embodiments of the disclosed
methods, the step of contacting the halogenation reaction product mixture with
a
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-8-
metal organic salt solution occurs by flowing the halogenation reaction
product
mixture against a countercurrent flow of the metal organic salt.
[0029] In some embodiments, the disclosed methods further comprises
stripping the bromine from the bromine containing gasses and recycling the
bromine into the halogenations reactor in the case of FIG. 1 and a brominator
reactor in the case of FIG. 2.
[0030] The reactors, condensers, mixers, distillation reactor, decanter,
and
other equipment used in the illustrative embodiments shown in the FIGS. are
well
known in function and operation.
[0031] In certain embodiments of the disclosed method, the alkane to
halogen gas molar ratio may be greater than 2:1. All individual values and
subranges greater than a 2:1 ratio are included herein and disclosed herein;
for
example, the alkane to halogen gas molar ration can be from a lower limit of
2:1,
2.2:1, 2.4:1, 2.6:1, 2.8:1, 3:1, 3.5:1, 3.8:1, 4:1; 4.2:1. In at least one
aspect of the
present disclosure the alkane to halogen gas molar ratio is greater than or
equal to
4: I .The halogenations step in all the methods wherein the alkane and halogen
gas
are reacted to form an alkane halide and the hydrogen halide is, in some
embodiments of the disclosed methods, autocatalytic following initiation. In
such
embodiments, the halogenations reaction may be initiated by application of
heat
to a temperature between 350 and 450° C. All individual values and
subranges from 350 and 450° C. are included herein and disclosed
herein;
for example, the halogenation reaction initiation temperature can be from a
lower
limit of 350, 360, 370, 380, 390, 400, 410, 420, 430, or 440° C. to an
upper limit of 360, 370, 380, 390, 400, 410, 420, 430, 440 or 450° C.
For
example, the halogenation reaction initiation temperature may be in the ran2e
of
from 350 to 380° C., or in the alternative, halogenation reaction
initiation
temperature may be in the range of from 380 to 400° C., or in the
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-9-
alternative, the halogenation reaction initiation temperature may be in the
range
of from 400 to 450° C.
[0032] In alternative embodiments, the halogenations reaction may he
initiated at lower temperatures in the presence of ultraviolet radiation. In
such
embodiments, the halogenations reaction initiation temperature may be in the
range from 250 to 350° C. All individual values and subranges from 250
and 350° C. are included herein and disclosed herein; for example, the
halogenation reaction initiation temperature can be from a lower limit of 250,
260, 270, 280, 290, 300, 310, 320, 330, or 340° C. to an upper limit of
260, 270,280, 290, 300, 310, 320, 330, 340 or 350° C. for example, the
halogenation reaction initiation temperature may be in the range of from 250
to
280° C., or in the alternative, halogenation reaction initiation
temperature
may be in the range of from 280 to 300° C., or in the alternative, the
halogenation reaction initiation temperature may be in the range of from 300
to
350° C.
[0033] Following initiation, in some embodiments of the disclosed
method, the heat generated by the halogenation reaction is sufficient to
maintain
the halogenation reaction.
[0034] The following examples and description of the drawings is an
example of one or more embodiments of the disclosed methods and is not
intended to limit the scope of the disclosure.
EXAMPLES
ORIGINAL EXAMPLE 1
[0035] Referring to Ha 1, natural gas comprising methane enters a
mixing chamber 1 through line 2 in which it is mixed with bromine vapor
entering mixing chamber 1 through line 3. The natural gas/bromine vapor
CA 02862300 2014-07-22
WO 2013/119230
PCT/US2012/024382
-10-
mixture passes into the halogenations reactor 5 wherein methyl bromide and
hydrobromic acid are formed. The halogenations reaction product mixture which
may further comprise unreacted gasses passes through line 6 to condenser 7
wherein the mixture is cooled. Following cooling the halogenation reaction
product mixture is passed through line 8 and flowed upward into extractor 9
through an inert packing material 10 (not shown) against a counterflow of
magnesium formate solution which enters extractor 9 through line 38.
Magnesium bromide is formed and magnesium bromide solution exits extractor 9
through line 11. Also formed in extractor 9 is methyl formate and formic acid
gasses which exit extractor 9 through line 12. Magnesium bromide solution
enters reactor 13 wherein it is heated and reacted with oxygen entering
through
line 16. Bromine containing gasses are led from reactor 13 through line 17 to
cooler 18 where most of the bromine is recovered and exits cooler 18 through
line 19. Gasses containing traces of bromine are led from cooler 18 to
absorber
20 through line 21 wherein the gasses are contacted with a counterflow of
solvent, thereby recovering the remainder of the bromine. Bromine tree gasses
may be vented or otherwise routed through line 22. "[he bromine containing
solvent exits the bottom of absorber 20 and enters the top of stripper 23
through
line 24. In the stripper 23, the bromine containing solvent is contacted with
methane entering the stripper 23 through line 39, thereby stripping the
bromine
from the bromine containing solvent. Stripped solvent may he recovered from
the
bottom of stripper 23 and pumped using pump 26 through line 28 into the top of
absorber 20. Magnesium oxide from oxidation reactor 13 enter reactor 29
through
line 30. Water is added to reactor 29 through line 31. A slurry of magnesium
oxide is formed in reactor 29 and is passed through line 32 into the top of
stripper
33 wherein the magnesium oxide slurry is contacted with a counterllow of
methyl
formate and formic acid gasses. In stripper 33, methanol is formed and exits
stripper 33 to condenser 34. Condenser 34 cools the methanol which is
collected
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-11-
through line 35. Gas products of stripper 33 which are substantially free of
methanol are passed into mixing chamber 1 through line 36. Magnesium formate
solution leaves stripper 33 through line 38 through which it is passed into
extractor 9.
[0036] Advantageously, the magnesium bromide, the liquid metallic
halide in line 11 is separated from the liquid methyl formate, the alkane
ester,
under gaseous conditions in extractor 9 in Example 1 as follows:
(1) Metallic Fonnate (1) + Methyl Bromide (I)
Methyl Formate (g) + Magnesium Bromide (1)
In other words, the following is the condition for the separation of the
alkane
ester from the alkane halide in a reaction:
(2) Metallic Organic Salt (1) + Alkane Halide (1)
Alkane Ester (g) + Metallic Halide (1)
which allows for the processing of the liquid magnesium bromide by oxidation
reactor 13 and reactor 29 for the formation of the slurry of magnesium oxide
found in line 6 required to form the methyl alkanol. More specifically, the
alcohol is formed by reaction of the liquid magnesium hydroxide, the liquid
metallic hydroxide in line 6, and methyl Ibrmate gas, the alkane ester gas in
line
12 to form the liquid metallic salt, the metal ester in line 38, and the
methanol
eas, the alkane alcohol that enter condenser 34 Ibr condensation and recover
as
follows:
(3) Methyl Forrnate (2) + Magnesium 11yrdoxide (1) ¨0.
Methanol (2) + Magnesium Formate (I)
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-1/-
IMPROVEMENT EXAMPLES
[0037] In the Improvement Examples, at the point where the alkane esters
are formed from the reaction of the liquid alkane halide and the metallic
organic
salt in accordance with chemical equation 3 above, the reaction advantageously
occurs under conditions such that the alkane esters formed are insoluble in
the
aqueous solution containing the liquid alkane halides and the liquid metal
organic
salt as the reactants. This allows for the separation of the liquid alkane
esters
from the liquid metallic halide so that the liquid metallic halide can be
further
processed into the slurry of metallic oxides that may be reacted with the
liquid
ester to form the alkane alcohol. Advantageously, the alkane halides that are
contacted with the metallic organic salt in the Improvement Examples are
selected such that the alkane esters formed from the reaction of the alkane
halide
and the metallic organic salt have a solubility in water that is less than the
solubility of the alkane halide in water. If the alkane ester product has a
solubility that is less than the solubility of the reactant alkane halide,
then the
alkane ester product will precipitate out of the water into an insoluble layer
which
can he easily separated from the metallic halide which remains in the water.
This
separation of insoluble alkane ester from the metallic halide permits the
metallic
halide to be processed downstream independent from the alkane ester stream to
form the metallic hydroxide required to be contacted with the alkane ester to
form
the alkane ethanol. If, however, the alkane ester product has a solubility
that is
greater than the solubility of the reactant alkane halide, the alkane ester
product
will not precipitate out of the water but remain with the metallic halide as a
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-13-
mixture in the water; making the reaction of the metallic halide into metallic
hydroxide not possible.
IMPROVEMENT EXAMPLE 1
[0038] Improvement Example 1 uses the same process and equipment as
shown in FIG. 1 except that equipment 9 which is an extractor in the Example 1
is replaced with a separator in Example 2 for the purpose of separating alkane
esters from metal halides in the mix of products that is formed in the
separator.
As previously discussed, the alkane esters in Improvement Example 1 are formed
from the reaction of the liquid alkane halide and the metallic organic salt,
such
that the reaction advantageously occurs under conditions such that the alkane
esters formed are insoluble in the aqueous solution containing the liquid
alkane
halides and the liquid metal organic salt as the reactants. More specifically,
the
alkane esters formed in separator 9 in Improvement Example 1 are chosen to
have a solubility in water that is less than the solubility of the alkane
halide in
water.
[0039] In other words, the following is the condition for the separation of
the alkane ester from the alkane halide in a reaction in Improvement Example
1:
(4) Metallic Organic Salt (1) + Alkane Halide (1)
Alkane Ester (1) + Metallic Halide (1)
[0040] As seen in chemical equation 4, the alkane ester remains as a
liquid in the product mix in this reaction. In Improvement Example I. an ethyl
butyrate is used as the alkane ester. From chemical reaction 4, in Improvement
Ixample I, ethyl bromide is the alkane halide of choice as the alkane halide
14
use as a reactant to the metallic organic salt according to the following
reaction
occurring in separator 9:
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-14-
(5) Metallic Butyrate (1) + Ethyl Bromide (1)
Ethyl Butyrate (1) + Magnesium Bromide (1)
[0041] This is because the solubility of the ethyl bromide (i.e., which
reacts with the metallic organic salt to form the alkane ester according to
chemical equation 5 above) in water is 0.91 grams per 100 ml of water, or
0.0835
moles per 1000 ml of water which is greater than the solubility of ethyl
butyrate
in water which is 0.68 grams per 100 ml of water or 0.059 moles per 1000 ml.
In
other words, the solubility of the ethyl butyrate (i.e., the alkane ester) in
water is
less than the solubility of its reactant ethyl bromide (i.e., its alkane
halide
reactant) in water. This advantageously keeps the ethyl butyrate in a liquid
phase
in separator 9. Since liquid ethyl butyrate is insoluble in water, the liquid
ethyl
butyrate advantageously forms an insoluble layer with the aqueous magnesium
bromide solution layer. This allows the liquid ethyl butyrate to be
advantageously separated in separator 9 from the magnesium bromide solution
which allows the magnesium bromide solution to be processed into the
magnesium oxide slurry in line 32 required for reaction with the liquid ethyl
butyrate in line 12 to form the ethanol in stripper 33.
IMPROVEMENT EXAMPLE 2
[0042] In Improvement Example 2, an methyl benzoate is used as the
alkane ester. As in Improvement Example 1, ethyl methyl benzoate is chosen as
the alkane halide for use as a reactant to the metallic organic salt according
to the
following reaction occurring in separator 9:
(6) Metallic Benzoate (1) + Methyl Bromide (1)
Methyl I3enzoate (1) + Magnesium Bromide (1)
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-15-
[0043] This is because the solubility of methyl bromide (i.e., which reacts
with the metallic manic salt to form the alkane ester according to chemical
equation 6 above) in water is 0.09 grams per 100 ml of water, or .0094 moles
per
1000 ml of water which is greater than the solubility of methyl benzoate in
water
which is 0.157 grams per 100 ml of water or .0011532 moles per 1000 ml. In
other words, the solubility of the methyl benzoate (i.e., the alkane ester) in
water
is less than the solubility of its reactant methyl bromide (i.e., its alkane
halide
reactant) in water. This advantageously keeps the methyl benzoate in a liquid
phase in separator 9. Since liquid methyl benzoate is insoluble in water, the
liquid methyl benzoate advantageously forms an insoluble layer with the
aqueous
magnesium bromide solution. This allows the liquid methyl benzoate to be
advantageously separated in separator 9 from the magnesium bromide solution
which allows the magnesium bromide solution to be processed into the
magnesium oxide slurry in line 32 required for reaction with the liquid methyl
benzoate in line 12 to tOrni the methanol in stripper 33.
IMPROVEMENT EXAMPI.1 3
[0044] In Improvement Example 3, a methyl formate is used as the alkane
ester. In this Improvement Example 3, the methyl formate is quickly seen to be
an unworkable choice since the methyl halide for use as a reactant to the
metallic
organic salt does not follow the following reaction required to keep the
alkane
ester as a liquid in separator 9 according to the following teachings of my
improvement disclosure:
(7) Metallic organic salt (1) + Alkane Halide (1)
Alkane Ester (1) + Metallic Halide (I)
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-16-
[0045] This is because the solubility of methyl bromide (i.e., which reacts
with the metallic organic salt to form the alkane ester according) in water is
0.09_
grams per 100 ml of water, or .0094 moles per 1000 ml of water which is less
than the solubility of methyl formate in water which is 30.4 grams per 100 ml
of
water or 5.0624479 moles per 1000 ml. In other words, the solubility of the
methyl formate (i.e., the alkane ester) in water is greater than the
solubility of its
reactant methyl halide (i.e., its alkane halide reactant) in water. As a
result, the
methyl formate remains in the aqueous metal hydroxide solution in liquid
separator 9 as a solvent which prevents the magnesium bromide solution to be
processed into the magnesium oxide slurry in line 32 required for reaction to
form the methanol. In other words, because the solubility of the methyl
formate
was not less than the solubility of its reactant methyl bromide, there is no
methyl
formate product in line 12 for use in forming the methanol in stripper 33. In
contradistinction, in Example 1, there was a gaseous methyl formate product
formed in line 12 for use in the methanol production reaction in stripper 33
because the extractor 9 evaporates the methyl formate from the magnesium
bromide solution to create the required separation between the magnesium
bromide and the methyl formate in extractor 9.
IMPROVEMENT EXAMPLE 4
[00461 Referring to FIG 2, bromine is produced in fluo-solids reactor 1.
The bromine vapor passes through line 3 to condenser 2 where most of the
bromine, typically up to about 95%, for example, may be is condensed. The
uncondensed bromine vapor passes through line 5 to absorber 4 and is flowed
against a counterflow of water. Clean gasses from the cross-flow are vented to
the atmosphere through line 6. Water saturated with bromine forming an aqueous
bromine saturated solution passes through line 8 to extractor 7. In extractor
7. the
aqueous bromine saturated solution flows against a counterflow of ethane gas
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-17-
which strips all, or substantially all, of the bromine from the water. The
ethane
containing the stripped bromine passes through line 9 to brominator reactor
10.
Liquid bromine from condenser 2 passes through line 11 to vaporizer 12 where
the liquid bromine is vaporized and preheated before passing through line 13
to
brominator reactor 10. Bromine and ethane in brominator reactor 10 react to
form ethyl bromide, ethyl dibromide, ethyl tri bromide, and hydrogen bromide
gasses. These gasses exiting reactor 10 pass through line 15 to neutralizing
reactor 14 where ethyl di-bromide, ethyl tri-bromide, and hydrogen bromide are
neutralized with a suspension of magnesium hydroxide flowing through line 19
from reactor 18. Liquids from neutralizing reactor 14 pass through line 32 to
fluo
solids reactor 1. Gasses from neutralizing reactor 14 pass through line 20 to
condenser 12 where the gasses are cooled.
[0047] These gases are cooled to temperatures below and preferably well
below the boiling point of ethyl bromide. Liquid products from condenser 21
pass through line 22 to intensive mixer 23, which also receives magnesium
benzoate or butyrate solution from reactor 29 through line 40. The liquid
product
from intensive mixer 23 passes through line 26 to decanter 27 where benzoate
or
butyrate ethyl esters separate as a substantially insoluble ester layer (phase
2
liquid) from the water layer (phase 1 liquid). The water layer containing
magnesium bromide, or phase I liquid, flows out of decanter 27 along line 36
to
reactor 1. The insoluble ester layer containing benzoate or butyrate ethyl
esters,
or phase 2 liquid, flows out of decanter 27 through line 28 to distillation
reactor
29. At distillation reactor 29, the phase 2 insoluble ester layer contacts a
suspension of magnesium hydroxide which enters distillation reactor 29 through
line 41 from reactor 18. Alcohol (ethanol) from distillation reactor 29 is
recovered as product through line 31. Solid product from fluo-solid reactor 1,
containing magnesium oxide, flows through line 42 to reactor 18 where it
contacts water entering reactor 18 from line 34 and reacts to form magnesium
CA 02862300 2014-07-22
WO 2013/119230 PCT/US2012/024382
-18-
hydroxide. Magnesium hydroxide flows from reactor 18 to brominator reactor 10
and distillation reactor 29.
[0048] Advantageously, the alkane halides that are contacted with the
metallic organic salt in intensive mixer 23 are selected such that the alkane
esters
formed in intensive mixer 23 have a solubility in water that is less than.the
solubility of the alkane halide in water. If the alkane ester product has a
solubility that is less than the solubility of the reactant alkane halide,
then the
alkane ester product will precipitate out of the water into an insoluble layer
which
can be easily separated from the metallic halide which remains in the water.
This
separation of insoluble alkane ester from the metallic halide permits the
metallic
halide to be processed downstream independent from the alkane ester stream to
form the metallic hydroxide required to be contacted with the alkane ester to
form
the alkane alcohol. If, however, the alkane ester product has a solubility
that is
greater than the solubility of the reactant alkane halide, the alkane ester
product
will not precipitate out of the water but remain with the metallic halide as a
mixture in the water; making the reaction of the metallic halide into metallic
hydroxide not possible. hence, unlike Example I where the organic ester going
to stripper 33 on line 12 is a gas, the organic ester flowing on line 28 in
Example
3 from decanter 27 to distillation reactor 29 is a liquid; just as the organic
ester
flowing into stripper 33 on line 12 in Example 2 is a liquid. The liquid
organic
ester may enable the use of smaller equipment in the process, eliminate the
need
for a as pump, have lower energy requirements, be less expensive and produce
more alcohol output per unit volume than is possible using the method of
Example 1.
IMPROVEMENT EXAMP1,E 5
[0049] An illustrative alternative method for the production of alcohols is
shown in FIG. 3 and includes the steps of reacting,:
CA 02862300 2014-07-22
WO 2013/119230
PCT/US2012/024382
-19-
1. producing a halogen gas in a fluo -solids reactor 101, solids comprising
a metal and a halogen forms a halogen gas and a metal oxide;
2. condensing the halogen gas in a condenser 102, forming a liquid
halogen and a gas comprising trace halogen, the liquid halogen may be
vaporized
in vaporizer 112 and fed to a brominator 110; .
3. recovering the trace halogen from the gas by absorbing the trace
halogen in water, in absorber 104;
4. contacting an alkane to the absorbed trace halogen and water and
forming a gas comprising the alkane and trace halogen (e.g. gas with an
aqueous
halide saturated solution), in extractor 107;
5. stripping the trace halogen from the solution to form a product mixture
of an alkane and a halide;
6. feeding the product mixture of an alkane and a halide to brominator
110;
7. reacting the halogen and the alkane in brominator 110 to form
halogenated alkanes;
S. reacting the metal oxide, fonned in fluo solids reactor 101, with water
forming a metal hydroxide in reactor 118;
9. feeding a portion of the metal hydroxide to a neutralizer 114;
10. neutralizing at least a portion of the halogenated alkanes with the
metal hydroxide, forming neutralized gasses and liquids in neutralizer 114;
11. feeding the neutralized liquids to fluo solids reactor 101;
12. condensing a portion of the neutralized gasses in condenser 121
forming a condensate and a gas;
13. feeding the gas from condenser 121 to brominator reactor 110;
14. feeding the condensate from condenser 121 to intensive mixer 1 23 ;
15. mixing, in intensive mixer 123, the condensate from condenser 121
with metallic organic salt, fed from distiller/reactor 129, forming a reacted
liquid;
CA 02862300 2014-07-22
WO 2013/119230
PCT/US2012/024382
-20-
16. decanting the reacted liquid into a first liquid phase and a second
liquid phase in decanter 127 wherein the first liquid phase comprises a metal
halogen and water and is fed to fluo solids reactor 101;
17. feeding the second liquid phase to distiller/reactor 129 wherein the
second liquid may comprise metal hydroxide;
18. distilling and reacting of the second liquid phase in distiller/reactor
129, removing alcohol therefrom; and
19. removing the metal benzoate or butyrate from distiller/reactor 129 and
feeding to the intensive mixer 123.
[0050] In at least one aspect of the process shown in FIG. 3, the metals
comprised in the solids fed to fluo solids reactor 101 comprises magnesium. In
this aspect the metal oxide fed to reactor 118 from fluo solids reactor 101
comprises MgO. The metal hydroxide fed to neutralizer 114 and
distiller/reactor
129, from reactor 118, comprises Mg0H. Additionally, the metal benzoate or
butyrate fed from distiller/reactor 129 comprises magnesium benzoate or
butyrate.
[0051] In at least one additional aspect of the process shown in FIG. 3, the
halogens comprised in the solids fed to fluo solids reactor 101 comprises
bromine. In this aspect of the process, the halogens fed to broininator 110
= comprise bromine. In at least further aspect of the process shown in FIG.
3, the
alkane fed to extractor 107 comprises ethane. In this aspect, the halogen and
alkane fed to brominator 110, from extractor 107, comprises ethane. In yet
another aspect of the process shown in HG. 3, the halogens comprised in the
solids fed to fluo solids reactor 101 comprises bromine and the metals
comprised
in the solids fed to fluo solids reactor 101 comprises magnesium. In this
aspect,
the phase 1 liquid fed to fluo solids reactor 101, from decanter 127,
comprises
Mglir-7.
CA 02862300 2014-07-22
WO 2013/119230
PCT/US2012/024382
-21-
[0052] As with the other Improvement Examples, the method disclosed in
connection with Improvement Example 5 involves liquid phase forming of
alcohol esters from liquid alkane halides and a solution of metal salts of
organic
acids whose alkanes esters are less soluble in water than that of the alkane
halide
and treating of the alcohol ester formed with magnesium or metal hydroxides to
form the alcohol and the metal salt of the organic acids.
[0053] From all of the examples above and the entirety of this disclosure, the
following can be seen. A method of making alcohols may involve forming of
alcohol esters from liquid alkane halides and a solution of metallic salts of
organic acids to produce gaseous alcohol esters for reaction with magnesium or
metal hydroxides to form the alcohol and the metal salt of the organic acids.
In
an improvement method, liquid phase alcohol esters instead of gaseous alcohol
esters are produced from liquid alkane halides and a solution of metal salts
of
organic acids whose alkane esters are less soluble in water than that of the
alkane
halide and treating of the alcohol ester formed with magnesium or metal
hydroxides to form the alcohol and the metal salt of the organic acids.
Industrial Applicability
[0054] The disclosed methods have wide use for producing alcohols from more
readily available starting materials and may provide efficiencies in energy
input
requirements and costs over current industrial alcohol production from direct
hydration of alkenes, or from cracking of appropriate fractions of distilled
(or
fractionated) crude oil.
[0055] More specifically, the disclosed improvement methods illustrated by the
Improvement Examples in connection with FIG. 1 and the methods disclosed in
connection with FIGS. 2 and 3 involve liquid phase forming of alcohol esters
from liquid alkane halides and a solution of metal salts of organic acids
whose
alkanes esters are less soluble in water than that of the alkane halide and
treating
CA 02862300 2014-07-22
WO 2013/119230
PCT/US2012/024382
of the alcohol ester formed with magnesium or metal hydroxides to form the
alcohol and the metal salt of the organic acids. In these disclosed
improvement
methods, the liquid organic ester may enable the use of smaller equipment in
the
process, eliminate the need for a gas pump, have lower energy requirements, he
less expensive and produce more alcohol output.per unit volume than is
possible
using the method of Original Example 1.
[0056] The present disclosure may be embodied in other forms without
departing from the spirit and the essential attributes thereof, and,
accordingly,
reference should be made to the appended claims, rather than to the foregoing
specification, as indicating the scope of the disclosure.