Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
HOST CELLS WITH ARTIFICIAL ENDOSYMBIONTS
CROSS-REFERENCES TO RELATED APPLICATION(S)
[1] The present invention claims the benefit of priority from U.S. Patent
Application Serial
No 13/374,799, entitled "HOST CELLS WITH ARTIFICIAL ENDOSYMBIONTS," filed on
January 13, 2012, the entire disclosure of which is hereby incorporated by
reference in its entirety
for all purposes.
FIELD OF THE INVENTION
[2] The present invention relates generally to the field of endosymbiosis,
artificial
endosymbionts, and magnetotactic bacteria. In particular, the invention
provides single-cell
organisms such as artificial endosymbionts including magnetotactic bacteria,
eukaryotic cells to
host those single-celled organisms, and methods of introducing the single-
celled organisms into
the eukaryotic cells.
BACKGROUND OF THE INVENTION
[31 Mitochondria, chloroplast and other membrane bound organelles add
heritable
functionalities, such as photosynthesis, to eukaryotic cells. Such organelles
(identified by their
vestigial circular DNA) are believed to be endosymbiotically derived.
[4] Bacteria exist with a wide range of functionalities not present in
various eukaryotic cells.
For example, in 1975 Blakemore identified magnetotactic bacteria (MTB) that
orient and swim
along a geomagnetic field. (Blakemore, R. Magnetotactic bacteria. Science 24:
377-379 (1975)
(which is incorporated by reference in its entirety for all purposes)). These
magnetotactic bacteria
produce magnetic structures called magnetosomes that are composed of magnetite
(Fe304) or
greigite (Fe3S4) enclosed by a lipid membrane. (Id). A large number of MTB
species have been
identified since their initial discovery. (Id.).
[5] Magnetotactic bacteria have been used to selectively bind to and
separate substances.
(U.S. Patent No. 4,677,067 (which is incorporated by reference in its entirety
for all purposes)).
Additionally, attempts have been made to add magnetic functionality to cells
through external
tags. (Swiston, A.J., Cheng, C., Soong, H.U., Irvine, D.J., Cohen, R.J.,
Rubner, M.F. Surface
Functionalization of Living Cells with Multilayer Patches. Nano Lett. 8(12):
4446-53 (2008)
(which is incorporated by reference in its entirety for all purposes)).
Bacterial magnetite has also
1
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
been introduced into red blood cells by cell fusion (Matsunaga, T., Kamiya,
S., (1988), In:
Atsumi, K., Kotani, M., Ueno, S., Katila T., Williamsen, S.J. (eds) 6th
International Conference
on Biomagnetisms (1987). Tokyo Denki University Press, Tokyo, pp. 50-51 (which
is
incorporated by reference in its entirety for all purposes)), and MTB have
been introduced into
granulocytes and monocytes by phagocytosis. (Matsunaga, T., Hashimoto, K.,
Nakamura, N.,
Nakamura, K., Hashimoto, S. Phagocytosis of bacterial magnetite by leucocytes.
Applied
Microbiology and Biotechnology 31(4): 401-405 (1989) (which is incorporated by
reference in its
entirety for all purposes)). However, none of these alterations are heritable
to daughter cells.
[6]
It is an object of the present invention to provide eukaryotic cells
containing a single-
celled organism that is introduced into the eukaryotic cell through human
intervention which
transfers to daughter cells of the eukaryotic cell through at least five cell
divisions, and which
maintains sufficient copy number in the daughter cells so that a desired
functionality introduced
by the single-celled organism is maintained in the daughter cells. It is
further an object of the
present invention to provide eukaryotic host cells containing artificial
endosymbionts that are
heritable to daughter cells. It is also an object of the present invention to
provide methods of
introducing artificial endosymbionts into the cytosol of eukaryotic host
cells. It is another object
of the present invention to provide eukaryotic cells with a heritable magnetic
phenotype.
SUMMARY OF THE INVENTION
ri
The present invention relates to eukaryotic cells comprising single-celled
organisms, such
as artificial endosymbionts, and methods of introducing such single-celled
organisms into
eukaryotic cells. In one embodiment, the single-celled organism provides the
eukaryotic cell with
a desired functionality.
In one embodiment, the single-celled organisms are artificial
endosymbionts heritable to daughter cells. In another embodiment, the
artificial endosymbiont is
a magnetotactic bacterium. In one embodiment, the magnetotactic bacterium
provides the
eukaryotic cell with a magnetic functionality. The artificial endosymbiont of
the invention may
be modified by deleting, adding, and/or mutating at least one gene whereby the
artificial
endosymbiont acquires a trait useful for endosymbiosis or biotrophy. The genes
to be mutated,
added, and/or deleted in the artificial endosymbiont may be genes encoding
components of the
flagellar assembly and genes encoding enzymes for synthesizing essential
macromolecules, such
as amino acids, nucleotides, vitamins, and co-factors. In certain embodiments,
the MTB may
further be modified to express an antibiotic resistance gene or other
selectable marker.
2
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
[8] In some embodiments the eukaryotic cells of the invention are
mammalian, such as
mouse, rat, rabbit, hamster, human, porcine, bovine, or canine. In another
embodiment the
artificial endosymbiont is transmitted from the host cell to daughter progeny
host cells. In another
embodiment, the method further comprises deleting, inserting, and/or mutating
at least one gene
from the eukaryotic cell.
[9] The single-celled organisms of the invention can be introduced into
eukaryotic cells by a
number of methods known to those of skill in the art including, but not
limited to, microinjection,
natural phagocytosis, induced phagocytosis, macropinocytosis, other cellular
internalization
processes, liposome fusion, erythrocyte ghost fusion, or electroporation.
BRIEF DESCRIPTION OF THE FIGURES
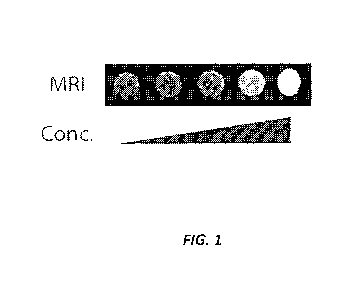
[10] FIG. 1 shows positive contrast generated with a Ti pulse sequence over
a log scale
concentration up to ¨108 MTB/mL for gfp+AMB suspended in agar plugs using a
1.5T instrument
to optimize and characterize the imaging properties.
[11] FIG. 2 shows a blastula stage mouse embryo that has had one of its two
cells at the 2-cell
embryo stage microinjected with gfp+AMB. The embryo is imaged with Leica SP2
AOBS
spectral confocal inverted microscope surrounded by an environmental control
chamber for live-
cell imaging with 20X, 0.7 NA objective, and optical zoom of 3X. Panel A shows
differential
interference contrast (DIC) image and Panel B shows a gray scale fluorescence
capture of the
same image.
[12] FIG. 3 shows the change of total embryo OFF fluorescence of four mouse
embryos over
time as measured by confocal microscopy. One of the two cells from the 2-cell
stage of each
embryo had been microinjected with gfp+AMB, and the total GFP fluorescence of
each embryo
was measured beginning at the 8-cell stage, 24 hours after microinjection.
DETAILED DESCRIPTION OF THE INVENTION
[13] The invention is illustrated by way of example and not by way of
limitation. It should be
noted that references to "an" or "one" or "some" embodiment(s) in this
disclosure are not
necessarily to the same embodiment, and all such references mean at least one.
[14]
The present invention is directed to eukaryotic cells containing single-celled
organisms,
such as host cells containing artificial endosymbionts in the cytosol of the
host cell, and methods
3
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
of introducing the single-celled organism into the eukaryotic cell. In one
embodiment the single-
celled organism is an artificial endosymbiont that is genetically altered. In
some embodiments the
single-celled organisms are magnetotactic bacteria (MTB).
Definitions
[15] As used herein, the term "AMB" refers to Magnetospirillum magneticum
strain AMB-1.
[16] As used herein, the term "artificial endosymbiont" refers to a to a
single-celled organism
that is or has been introduced into the cytosol of a eukaryotic cell through
human intervention,
which has been or can be transferred to daughter cells of the eukaryotic cell
through at least five
cell divisions, and which maintains sufficient copy number in the daughter
cells so that a
phenotype introduced by the artificial endosymbiont is maintained in the
daughter cells.
[17] As used herein, the term "cellular life cycle" refers to series of
events involving the
growth, replication, and division of a eukaryotic cell. It is divided into
five stages, known as Go,
in which the cell is quiescent, G1 and G2, in which the cell increases in
size, S, in which the cell
duplicates its DNA, and M, in which the cell undergoes mitosis and divides.
[18] As used herein, the term "cytosol" refers to the portion of the
cytoplasm not within
membrane-bound sub-structures of the cell.
[19] As used herein, the term "daughter cell" refers to cells that are
formed by the division of a
cell.
[20] As used herein, the term "essential molecule" refers to a molecule
needed by a host cell
for growth or survival.
[21] As used herein, the term "genetically modified" refers to altering the
DNA of a cell so
that a desired property or characteristic of the cell is changed.
[22] As used herein, the term "host cell" refers to a eukaryotic cell in
which an artificial
endosymbiont can reside.
[23] As used herein, the term "liposome mediated" refers to artificial
microscopic vesicles
consisting of an aqueous core enclosed in one or more lipid layers, used to
convey artificial
endosymbionts to host cells.
4
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
[24] As used herein, the term "magnetosome" refers to particles of
magnetite (i.e., Fe3 04) or
greigite (Fe3S4) enclosed by a sheath or membrane, either as individual
particles or in chains of
particles.
[25] As used herein, the term "magnetotactic bacteria" or "MTB" refers to
bacteria with genes
encoding magnetosomes.
[26] As used herein, the term "mammal" refers to warm-blooded vertebrate
animals all of
which possess hair and suckle their young.
[27] As used herein, the term "microinjection" refers to the injection of
artificial
endosymbionts into host cells.
[28] As used herein, the term "tagged artificial endosymbiont" refers to
artificial
endosymbionts that have a ligand on the surface of the endosymbiont.
[29] As used herein, the term "parent cell" refers to a cell that divides to
form two or more
daughter cells.
[30] As used herein, the term "receptor mediated" refers to a molecular
structure or site on the
surface of a host cell that binds with an artificial endosymbiont or a tagged
artificial
endosymbiont followed by internalization of the artificial endosymbiont.
Artificial Endosymbionts
[31] Single-celled organisms of the invention include bacteria that are
capable of surviving in
a eukaryotic cell and maintain copy number such that the phenotype introduced
by the single-
celled organism is maintained in daughter cells. In some embodiments, the
single-celled
organism does not kill the eukaryotic host cell without further human
intervention. In some
embodiments, the single-cell organism has a functionality that is acquired by
the eukaryotic cell
following the introduction of the single-celled organism. In some embodiments,
the functionality
of the single-cell organism is magnetism, production of a nutrient,
desalinization, photosynthesis,
or tolerance to harsh environmental challenges. Magnetism includes
diamagnetism and
paramagnetism. In some embodiments, the eukaryotic cell maintains the
functionality for at least
48 hours. In some embodiments, the single-celled organism can stably maintain
phenotype in the
eukaryotic daughter cells through at least 3 cell divisions, or at least 4
division, or at least 5
divisions, or at least 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or
20 cell divisions. In
5
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
another embodiment, the single-celled organism can stably maintain phenotype
in the eukaryotic
daughter cells through 3-5 divisions, or 5-10 divisions, or 10-15 divisions,
or 15-20 divisions.
[32] In an embodiment of the invention, the single-celled organisms of
the invention are
genetically modified. Methods for genetically modifying bacteria are well
known in the art.
Typically, the bacteria will be genetically modified to improve their survival
in eukaryotic host
cells, and/or to reduce the toxicity of the single-celled organism to the
eukaryotic cell, and/or to
provide the eukaryotic cell with a useful phenotype. In one embodiment, the
flagellar proteins of
a single-celled organism are modified so that the single-celled organism no
longer expresses
flagellar proteins in the eukaryotic host cell. In another embodiment, the
single-celled organism
is modified so that it can no longer synthesize an essential molecule that is
preferably provided by
the eukaryotic host cell. In an embodiment, the single-celled organism is
genetically modified so
that its cell cycle is coordinated with the cell cycle of the eukaryotic host
cell so that copy number
of the single-celled organism can be maintained at a sufficient level to
impart the phenotype to
daughter cells.
[33] Embodiments of the invention include singled-celled organisms that are
Proteobacteria.
Embodiments of the invention include single-celled organisms that are a-
Proteobacteria. In the
current taxonomic scheme based on 16S rRNA, a-proteobacteria are recognized as
a Class within
the phylum Proteobacteria, and are subdivided into 7 main subgroups or orders
(Caulobacterales,
Rh izobiales, Rhodobacterales, Rhodospirillales, Rickettsiales, Sph
ingomonadales and
Parvularculales). (Gupta, R.S. Phylogenomics and signature proteins for the
alpha Proteobacteria
and its main groups. BMC Microbiology, 7:106 (2007) (which is incorporated by
reference in its
entirety for all purposes)).
[34] A large number of a-proteobacterial genomes that cover all of the main
groups within a-
proteobacteria have been sequenced, providing information that can be used to
identify unique
sets of genes or proteins that are distinctive characteristics of various
higher taxonomic groups
(e.g. families, orders, etc.) within a-proteobacteria. (Id. (which is
incorporated by reference in its
entirety for all purposes)).
[35] Embodiments of the invention include single-celled organisms that are
magnetotactic
bacteria ("MTB"). A large number of MTB species are known to those of ordinary
skill in the art
since their initial discovery in 1975 by Blakemore (Blakemore, R.
Magnetotactic bacteria. Science
24: 377-379 (1975) (which is incorporated by reference in its entirety for all
purposes)) and
6
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
represent a group of microbes (Faivre, D. & Schiller, D. Magnetotactic
bacteria and
magnetosomes. Chemistry Reviews 108: 4875-4898 (2008) (which is incorporated
by reference in
its entirety for all purposes)). MTB have been identified in different
subgroups of the
Proteobacteria and the Nitrospira phylum with most of the phylotypes grouping
in a-
Proteobacteria. Currently, culturable MTB strains assigned as a-Proteobacteria
by 16S rRNA
sequence similarity include the strain originally isolated by Blakemore in
1975, Magnetospirillum
magnetotactium (formerly Aquasprillium magnetotactium), M gryphiswaldense, M
magneticum
strain AMB-1 ("AMB"), M polymorp hum, Magnetosprillum sp. MSM-4 and MSM-6,
Magnetococcus marinus, marine vibrio strains MV-1 and MV-2, a marine spirillum
strain MMS-1
and Magnetococcus sp. strain MC-1, as well as others. A number of MTB are
available in pure
culture, including AMB. The doubling time of AMB in pure culture is
approximately eight hours
and is close to that of a typical mammalian cell.
[36] Standard MTB growth media uses succinic acid as the main carbon source,
but MTB can
be grown with fumarate, tartrate, malate, lactate, pyruvate, oxaloacetate,
malonate, P-
hydroxybutyrate and maleate as the sole carbon source. These metabolites are
present inside
eukaryotic cells. Microaerophillic, facultative anaerobic, and obligate
anaerobic MTB strains
have been identified. Oxygen concentrations in the cytosol of eukaryotic cells
are low due to
sequestration by proteins such as myoglobin and concentration in specific
cellular locations, e.g.,
mitochondria, thus the microaerophilic or facultative anaerobic environment
necessary for MTB
growth is already present in a eukaryotic cell.
[37] MTBs can also be classified by the magnetic particles they synthesize,
either magnetite
(Fe304) or greigite (Fe3S4). Magnetite producers are microaerophilic or
facultative anaerobic,
need some oxygen source for magnetosome synthesis, and have optimal growth
temperatures near
physiological temperature.
[38] In some embodiments, the single-celled organisms of the invention are
genetically
modified. Molecular biology tools have been developed for genetic
manipulations of MTB most
extensively in AMB and M gtyphis-waldense strain MSR-1 (reviewed in Jogler, C.
and Schtiler,
D. in Magnetoreception and Magnetosomes in Bacteria, New York, Springer, 2007
p 134-138
(which is incorporated by reference in its entirety for all purposes)). Since
the genome of AMB
was the first sequenced of any MTB, all MTB gene references herein refer to
this genome unless
otherwise specified. The genomes of two other Magnetospirillum strains and
Magnetococcus sp.
strain MC-1 have also been recently sequenced. Genes from these strains or
other MTB strains,
7
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
presently culturable or unculturable, sequenced or unsequenced, know or
unknown, can be used in
the present invention.
[39] The genes responsible for magnetosome formation in MTB cluster in
genomic islands,
known as the magnetosome island (MAI). In M pyphiswaldense, the 130 kb MAI is
generally
structured into four polycistronic operons: the mamAB operon has 17 identified
ORFs extending
over 16.4 kb; the mamGFDC operon has 4 identified ORFs is 2.1 kb and 15 kb
upstream
ofinamAB; the mms6 operon has 6 identified ORFs is 3.6 kb and 368 bp upstream
of the
mamGFDC; the mamXY operon has 4 identified ORFs is located about 30 kb
downstream of
mamAB; and the monocistronic mamW gene. In the MAI the proteins: Mam W,
Mg1457, Mg1458,
Mg1459, Mms6, Mg1462, MamG, MamF, MamD, MamC, MamH, Maml, MamE, MamJ, MamK,
MamL, MamM, MamN, MamO, MamP, MamA, MamQ, MamR, MamB, MamS, MamT, MamU,
and Mg1505 have been identified, many of which have been given specific
functions in
magnetosome formation. Four genes outside the MAI have been liked to
magnetosome
formation, mamY, mtx44, mmsF and mamX. Conserved MAI's have been found in
other MTB
with some differences in genomic organization and size.
[40] In some embodiments, genetic modifications are made to the single-
celled organism.
Such modifications can be directed modifications, random mutagenesis, or a
combination thereof.
Natural endosymbionts are donors of novel metabolic capabilities and derive
nutritional
requirements from the host.
[41] Natural colonization of a host by the symbionts occurs in seven stages:
I) transmission, 2)
entry, 3) countering of host defense, 4) positioning, 5) providing advantage
to the host, 6)
surviving in host environment, and 7) regulation.
[42] In some embodiments, mutual nutritional dependence (biotrophy) may
be established
between the single-celled organism and the eukaryotic cell. In one embodiment,
the single celled
organism comprises at least one deletion of a gene encoding an enzyme for
synthesizing an
essential molecule, wherein said essential molecule is produced by the
eukaryotic host cell. An
essential molecule can include, but is not limited to, an amino acid, a
vitamin, a cofactor, and a
nucleotide. For instance, biotrophy can be accomplished by knocking-out the
ability of the
single-celled organism to make an amino acid, which will then be derived from
the host. Glycine
is a reasonable choice as it is highly abundant in mammalian cells and a
terminal product in
bacterial amino acid biogenesis; at least 22 other possibilities exist. The
enzyme serine
8
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
hydroxymethyltransferase converts serine into glycine at the terminus of the 3-
phosphoglycerate
biosynthetic pathway for amino acid production. In one embodiment, the single-
celled organism
is an AMB in which the gene amb2339 (which encodes the enzyme serine
hydroxymethyltransferase) is genetically modified. There are numerous methods
for mutating or
knocking-out genes known to those of ordinary skill in the art, including in
vitro mutagenesis,
targeted insertion of DNA into the gene of interest by homologous
recombination or deletion of
the gene (or operon, as most of the genes in the bacteria cluster in operons),
or using
endonucleases provided appropriate sites only around the target are present in
the genome.
[43] In another embodiment, nutritional dependence for a single-celled
organism on the host
cell could also be established by eliminating the ability of the single-celled
organism to synthesize
various metabolites, cofactors, vitamins, nucleotides, or other essential
molecules.
[44] In some embodiments of the invention, an MTB has mutations and/or
deletions in genes
associated with mobility and/or secretion. MTB are flagellated, and in some
embodiments of the
invention the MTB has a deletion and/or mutation in at least one gene encoding
molecular
machinery associated with the flagella, such that the magnetic bacterium does
not produce a
functional flagellum. Additionally, many MTB secrete various compounds, such
as hydroxamate
and catechol siderophores, which may be detrimental to or elicit an immune
response from the
host. In the sequenced genome of AMB, of the 4559 ORF's, 83 genes have been
related to cell
mobility and secretion. The flagellar assembly is known to be composed of the
gene products of
amb0498, amb0500, amb0501, amb0502, amb0503, amb0504, amb0505, amb0610,
amb0614,
amb0615, amb0616, amb0617, amb0618, amb0619, amb0628, amb1289, amb1389,
amb2558,
amb2559, amb2578, amb2579, amb2856, amb3493, amb3494 amb3495, amb3496,
amb3498,
amb3824, and amb3827. The flagella is controlled by the chemotaxis machinery
which is
composed of at least the gene products of amb0322, amb0323, amb0324, amb0325,
amb0326,
amb1806, amb1963, amb1966, amb2333, amb2635, amb2640, amb2648, amb2652,
amb2826,
amb2932, amb3002, amb3003, amb3004, amb3007, amb3102, amb3329, amb3501,
amb3502,
amb3654, amb3879, and amh3880.
[45] In one embodiment, genes encoding antibiotic resistance are inserted
into the genome of
the single-celled organism. Eukaryotic cells cultured in media containing the
antibiotic will
require the single-celled organism for survival. Neomycin resistance is
conferred by either one of
two aminoglycoside phosphotransferase genes, which also provide resistance
against geneticin
(G418), a commonly used antibiotic for eukaryotes. Hygromycin B resistance is
conferred by a
9
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
kinase that inactivates hygromcin B by phosphorylation. Puromycin is a
commonly used
antibiotic for mammalian cell culture and resistance is conferred by the pac
gene encoding
puromycin N-acetyl-transferase. External control of the antibiotic
concentration allows
intracellular regulation of the copy number of the single-celled organism. Any
other system
where resistance or tolerance to an external factor is achieved by chemical
modification of this
factor can also be employed. An indirect nutritive advantage on eukaryotic
cells may also be
established by using MTB and a magnetic culture method. In this embodiment,
magnetic fields
are established to confer an advantage to eukaryotic cells containing MTB.
This could be either
by providing the means for attachment to culture matrix or the access to
necessary growth or
media factors.
[46] In another embodiment, genetic modifications are made the MTB genome to
enhance
intracellular stability against the host defense mechanisms for a particular
host cell type. Many
eukaryote endosymbionts and endoparasites, such as the proteobacterial
endosymbionts of insects
such as Buchnera, Wigglesworthia, and Wolhachia; the methanogenic
endosymbionts of
anaerobic ciliates; the nitrogen-fixing symbionts in the diatom Rhopalodia;
the chemosynthetic
endosymbiont consortia of gutless tubeworms (Olavius or Jnanidrillus), the
cyanobacterial
endosymbionts of sponges, the endosymbionts of all five extant classes of
Echinodermata, the
Rhizobia endosymbionts of plants, various endosymbiotic algae, the Legionella-
like X bacteria
endosymbionts of Ameoba proteus, numerous Salmonella sp., Mycobacterium
tuberculosis,
Legionella pneumophila, etc. reside in membrane-bound vacuoles often termed
symbiosomes,
while some species, such as Blochmannia, the rickettsia, Shigella,
enteroinvasive Escherichia
coli, and Listeria, have the ability to inhabit the cytosol. The Dot-Icm Type
IV secretory system
is employed by many intracellular bacteria acquired by phagocytosis to evade
the endocytic
pathway and persist in the host cell. This system has been well-studied in L.
pneumophila and
consists of the proteins: DotA through DotP, DotU, DotV, IcmF, IcmQ through
IcmT, IcmV,
IcmW and IcmX. In Photorhabdus luminescens, the luminescent endosymbiont of
nematodes, the
genes encoding RTX-like toxins, proteases, type III secretion system and iron
uptake systems
were shown to support intracellular stability and replication. The gene bacA
and the regulatory
system ByrRS are essential for maintenance of symbiosis between Rhizobia and
plants as well as
the survival of Brucella ahortus in mammalian cells. The PrfA regulon enables
some Listeria
species to escape the phagesome and inhabit the cytosol. The desired cellular
location (e.g.,
symbiosome or cytosol) of the intracellular MTB will dictate which genes are
required to be
expressed in the MTB (either directly from the genome or through a stable
vector) for survival
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
and proliferation in the host environment. The endogenous plasmid pMGT is
highly stable in
MTB and a number of other broad range vectors (those ofincQ, IncP, pBBR1,
etc.) are capable of
stable replication in MTh.
[47] In another embodiment, the single-celled organism is genetically
modified by knocking in
genes, such as bacteriostatic gene(s), siderophore gene(s), metabolic
requirement gene(s), suicide
gene(s), life cycle regulation gene(s), transporter gene(s), and escape from
the phagosome
gene(s). In another embodiment, the single-celled organisms are randomly
mutated and
subsequently screened for enhanced integration within the host cell. Random
mutation can be
accomplished by treatment with mutagenic compounds, exposure to UV -light or
other methods
know to those skilled in the art.
[48] In another embodiment, transgenetic modification(s) are made to
counter eukaryotic cell
defenses using genes from various parasites or endosymbionts. In another
embodiment, the
population of the single-celled organisms in the eukaryotic host cell is
regulated though a balance
of intrinsic use of host mechanisms (nutrient availability, control of
reproduction, etc.) and
antibiotic concentration.
[49] In another embodiment, a natural endosymbiont or an intracellular
parasite is genetically
modified to produce magnetosomes. Endosymbionts of insects such as
Buchnera,
Wigglesworthia, and Wolbachia; the methanogenic endosymbionts of anaerobic
ciliates; the
nitrogen-fixing symbionts in the diatom Rhopalodia; the chemosynthetic
endosymbiont consortia
of gutless tubeworms (Olavius or lnanidrillus), the cyanobacterial
endosymbionts of sponges, the
endosymbionts of all five extant classes of Echinodermata, the Rhizobia
endosymbionts of plants,
various endosymbiotic algae, the Legionella-like X bacteria endosymbionts of
Ameoba proteus,
numerous Salmonella sp., Mycobacterium tuberculosis, Legionella pneumophila
belong to a-
proteobacteria and could be genetically engineered to produce magnetosomes. In
another
embodiment, a pre-existing organelle can be genetically modified to express
one or more
magnetosome genes to produce an artificial endosymbiont. For instance,
mitochondria, plastids,
hydrogenosomes, apicoplasts or other organelles, which harbor their own
genetic material, can be
genetically altered.
[50] In a preferred embodiment, the single-celled organism is an MTB, which
may or may not
be genetically altered, that produces magnetic particles upon culturing of the
eukaryotic cells.
11
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
Eukaryotic Cells
[51] The invention provides eukaryotic cells comprising single-celled
organisms in the
eukaryotic cells that are heritable and methods of introducing the single-
celled organisms into
host cells.
[52] In some embodiments the eukaryotic cells are plant cells. In some
embodiments the
eukaryotic cells are cells of monocotyledonous or dicotyledonous plants
including, but not limited
to, maize, wheat, barely, rye, oat, rice, soybean, peanut, pea, lentil and
alfalfa, cotton, rapeseed,
canola, pepper, sunflower, potato, tobacco, tomato, eggplant, eucalyptus, a
tree, an ornamental
plant, a perennial grass, or a forage crop. In other embodiments the
eukaryotic cells are algal,
including but not limited to algae of the genera Chlorella, Chlamydomonas,
Scenedesmus,
Isochrysis, Dunaliella, Tetraselmis, Nannochloropsis, or Prototheca, In some
embodiments the
eukaryotic cells are fungi cells, including but not limited to fungi of the
genera Saccharomyces,
Klyuveromyces, Candida, Pichia, Debaromyces, Hansenula, Yarrowia,
Zygosaccharomyces, or
Schizosaccharomyces.
[53] In some embodiments the eukaryotic cells of the invention are animal
cells. In some
embodiments the eukaryotic cells are mammalian, such as mouse, rat, rabbit,
hamster, human,
porcine, bovine, or canine. Mice routinely function as a model for other
mammals, most
particularly for humans. (See, e.g., Hanna, J., Wernig, M., Markoulaki, S.,
Sun, C., Meissner, A.,
Cassady, J.P., Beard, C., Brambrink, T., Wu, L., Townes, T.M., Jaenisch, R.
Treatment of sickle
cell anemia mouse model with iPS cells generated from autologous skin. Science
318: 1920-1923
(2007); Holtzman, D.M., Bales, K.R., Wu, S., Bhat, P., Parsadanian, M., Fagan,
A., Chang, L.K.,
Sun, Y., Paul, S.M. Expression of human apolipoprotein E reduces amyloid-p
deposition in a
mouse model of Alzheimer's disease. .1 Clin. Invest. 103(6): R15¨R21 (1999);
Warren, R.S.,
Yuan, H., Math, M.R., Gillett, N.A., Ferrara, N. Regulation by vascular
endothelial growth factor
of human colon cancer tumorigenesis in a mouse model of experimental liver
metastasis. J. Clin.
Invest. 95: 1789-1797 (1995) (each of these three publications is incorporated
by reference in its
entirety for all purposes)).
[54] In some embodiments, the eukaryotic cell is a human cancer cell.
There are many human
cancer cell lines that are well known to those of ordinary skill in the art,
including common
epithelial tumor cell lines such as Coco-2, MDA-MB231 and MCF7, non-epithelial
tumor cell
lines, such as HT-1080 and HL60, the NCI60-cell line panel (see, e.g.,
Shoemaker, R., The NCI60
12
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
human tumor cell line anticancer drug screen. Nature Reviews Cancer 6, 813-823
(2006) (which
is incorporated by reference in its entirety for all purposes)). Additionally,
those of ordinary skill
in the art are familiar with obtaining cancer cells from primary human tumors.
[55] In other embodiments, the eukaryotic cells are stem cells. Those of
ordinary skill in the
art are familiar with a variety of stem cell types, including Embryonic Stem
Cells, Inducible
Pluripotent Stem Cells, Hematopoietic Stem Cells, Neural Stem Cells, Epidermal
Neural Crest
Stem Cells, Mammary Stem Cells, Intestinal Stem Cells, Mesenchymal stem cells,
Olfactory adult
stem cells, and Testicular cells.
[56] In an embodiment, the eukaryotic cell is a cell found in the
circulatory system of a human
host. For example, red blood cells, platelets, plasma cells, T-cells, natural
killer cells, or the like,
and precursor cells of the same. As a group, these cells are defined to be
circulating host cells of
the invention. The present invention may be used with any of these circulating
cells. In an
embodiment, the eukaryotic host cell is a T-cell. In another embodiment, the
eukaryotic cell is a
B-cell. In an embodiment the eukaryotic cell is a neutrophil. In an
embodiment, the eukaryotic
cell is a megakaryocyte.
[57] In another embodiment, at least one gene from the eukaryotic cell is
genetically altered.
In some embodiments, mutual nutritional dependence (biotrophy) may be
established between the
artificial endosymbiont and the eukaryotic cell by genetic modification of the
eukaryotic cell,
using the appropriate molecular biology techniques specific to the target host
cell type known to
those of ordinary skill in the art, creating eukaryotic cell dependence on the
single-celled
organism for some essential macromolecule thus establishing the environmental
pressures for
biotrophy. In another embodiment, nutritional dependence for a single-celled
organism on the
eukaryotic cell may be established by genetically altering the eukaryotic cell
to eliminate the
ability of the single-celled organism to synthesize various metabolites,
cofactors, vitamins,
nucleotides, or other essential molecules. In such embodiments, the essential
molecule may be
provided by the single-celled organism. In another embodiment, the eukaryotic
cell gene
encoding the enzyme serine hydroxymethyltransferase, which converts serine
into glycine at the
terminus of the 3-phosphoglycerate biosynthetic pathway for amino acid
production, may be
modified.
13
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
Methods of Introducing Single-Celled Organisms into Eukaryotic Cells
[58] The single-celled organisms of the invention can be introduced into
eukaryotic cells by a
number of methods known to those of skill in the art including, but not
limited to, microinjection,
natural phagocytosis, induced phagocytosis, macropinocytosis, other cellular
uptake processes,
liposome fusion, erythrocyte ghost fusion, electroporation, receptor mediated
methods, and the
like. (See Microinjection and Organelle Transplantation Techniques, Celis et
al. Eds.; Academic
Press: New York, 1986 and references therein, (incorporated by reference in
its entirety for all
purposes)).
[59] In one embodiment, a single-celled organism is introduced to the host
cell by
microinjection into the cytoplasm of the host cell. A variety of
microinjection techniques are
known to those skilled in the art. Microinjection is the most efficient of
transfer techniques
available (essentially 100%) and has no cell type restrictions (Id.; Xi, Z. &
Dobson, S.
Characterization of Wolbachia transfection efficiency by using microinjection
of embryonic
cytoplasm and embryo homogenate. App!. Environ. MicrobioL 71(6): 3199-3204
(2005); Goetz,
M., Bubert, A., Wang, G., Chico-Calero, I., Vazquez-Boland, J.A., Beck, M.,
Slaghuis, J., Szalay,
A. A., Goebel, W. Microinjection and growth of bacteria in the cytosol of
mammalian host cells.
Proc. Natl. Acad. ScL USA 98:12221-12226 (2001) (each of these three
publications is
incorporated by reference in its entirety for all purposes)).
[60] Naturally phagocytotic cells have been show to take up bacteria,
including MTB
(Burdette, D.L., Seemann, J., Orth, K. Vibrio VopQ induces P13-kinase
independent autophagy
and antagonizes phagocytosis. Molecular microbiology 73: 639 (2009);
Wiedemann, A., Linder,
S., Grassi, G., Albert, M., Autenrieth, I., Aepfelbacher, M. Yersinia
enterocolitica invasin
triggers phagocytosis via 01 integrins, CDC42Hs and WASp in macrophages.
Cellular
Microbiology 3: 693 (2001); Hackam, D.J., Rotstein, 0.D., Schreiber, A.,
Zhang, W., Grinstein,
S. Rho is required for the initiation of calcium signaling and phagocytosis by
Fey receptors in
macrophages../. of Exp. Med. 186(6): 955-966 (1997); Matsunaga, T., Hashimoto,
K., Nakamura,
N., Nakamura, K., Hashimoto, S. Phagocytosis of bacterial magnetite by
leucocytes. Applied
Microbiology and Biotechnology 31(4): 401-405 (1989) (each of these four
publications is
incorporated by reference in its entirety for all purposes)).
[61] This method is scalable, but may be limited to specific cell types
(e.g., macrophage).
However, recent studies have shown that non-phagocytotic cell types can be
induced to
14
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
endocytose bacteria when co-cultured with various factors: media and chemical
factors, biologic
factors (e.g., baculovirus, protein factors, genetic knock-ins, etc.). (See,
e.g., Salminen, M.,
Airenne, K.J., Rinnankoski, R., Reimari, J., Valilehto, 0., Rinne, J.,
Suikkanen, S., Kuldconen, S.,
Yla-Herttuala, S., Kulomaa, M.S., Vihinen-Ranta, M. Improvement in nuclear
entry and transgene
expression of baculoviruses by disintegration of microtubules in human
hepatocytes. J. Viral
79(5): 2720-2728 (2005); Modalsli, K.R., Mikalsen, S., Bukholm, G., Degre, M.
Microinjection
of HEp-2 cells with coxsackie B1 virus RNA enhances invasiveness of Shigella
flexneri only after
prestimulation with UV-inactivated virus. APMIS 101: 602-606 (1993); Hayward,
R.D. &
Koronakis, V. Direct nucleation and bundling of actin by the SipC protein of
invasive Salmonella.
The EMBO Journal 18: 4926-4934 (1999); Yoshida, S., Katayama, E., Kuwae, A.,
Mimuro, H.,
Suzuki, T., Sasakawa, C. Shigella deliver an effector protein to trigger host
microtubule
destabilization, which promotes Rac 1 activity and efficient bacterial
internalization. The EMBO
Journal 21: 2923-2935 (2002); Bigildeev et al. J. Exp HematoL, 39: 187 (2011);
Finlay, B.B. &
Falkow, S. Common themes in microbial pathogenicity revisited. MicrobioL and
MoL Biol. Rev.
61: 136-169 (1997) (each of these six publications is incorporated by
reference in its entirety for
all purposes).
[62] The related process, macropinocytosis or "cell drinking," is a method
numerous bacteria
and viruses employ for intracellular entry (Zhang (2004) In: Molecular Imaging
and Contrast
Agent Database (MICAD) [database online]; Bethesda (MD): National Library of
Medicine (US),
NCBI; 2004-2011 (each of these two publications is incorporated by reference
in its entirety for
all purposes)). Various protocols exist which can be employed to induce cells
to take up bacteria.
Several agents, such as nucleic acids, proteins, drugs and organelles have
been encapsulated in
Liposomes and delivered to cells (Ben-Haim, N., Broz, P., Marsch, S., Meier,
W., Hunziker, P.
Cell-specific integration of artificial organelles based on functionalized
polymer vesicles. Nano
Lea. 8(5): 1368-1373 (2008); Lian, W., Chang, C., Chen, Y., Dao, R., Luo, Y.,
Chien, J., Hsieh,
S., Lin, C. Intracellular delivery can be achieved by bombarding cells or
tissues with accelerated
molecules or bacteria without the need for carrier particles. Experimental
Cell Research 313(1):
53-64 (2007); Heng, B.C. & Cao, T. Immunoliposome-mediated delivery of
neomycin
phosphotransferase for the lineage-specific selection of
differentiated/committed stem cell
progenies: Potential advantages over transfection with marker genes,
fluorescence-activated and
magnetic affinity cell-sorting. Med Hypotheses 65(2): 334-336 (2005); Potrykus
(1990) Ciba
Found Symp, Vol. 1 54: 198 (each of these four publications is incorporated by
reference in its
entirety for all purposes)). This method is inexpensive, relatively simple and
scalable.
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
Additionally, liposome uptake can be enhanced by manipulation of incubation
conditions,
variation of liposome charge, receptor mediation, and magnetic enhancement.
(See, e.g., Pan et
al. Int. J Pharm, 358: 263 (2008); Sarbolouki, M.N. & Toliat, T. Storage
stability of stabilized
MLV and REV liposomes containing sodium methotrexate (acqueous & lyophilized).
J. Pharm.
ScL Techno., 52(10): 23-27 (1998); Elorza, B., Elorza, M.A., Sainz, M.C.,
Chantres, J.R.
Comparison of particle size and encapsulation parameters of three liposomal
preparations.
Microencapsul. 10(2): 237-248 (1993); Mykhaylyk, 0., Sanchez-Antequera, Y.,
Vlaskou, D.,
Hammerschmid, E., Anton, M., Zelphati, 0. and Plank, C. Liposomal
Magnetofection. Methods
Mol. Bio., 605: 487-525 (2010) (each of these four publications is
incorporated by reference in its
entirety for all purposes)).
[63] Erythrocyte-mediated transfer is similar to liposome fusion and has
been shown to have
high efficiency and efficacy across all cell types tested (Microinjection and
Organelle
Transplantation Techniques; Celis et al. Eds.; Academic Press: New York, 1986
(which is
incorporated by reference in its entirety for all purposes)). Typically
erythrocytes are loaded by
osmotic shock methods or electroporation methods (Schoen, P., Chonn, A.,
Cullis, P.R., Wilschut,
J., and Schuerrer, P. Gene transfer mediated by fusion protein hemagglutinin
reconstituted in
cationic lipid vesicles. Gene Therapy 6: 823-832 (1999); Li, L.H., Hensen,
M.L., Zhao, Y.L., Hui,
S.W. Electrofusion between heterogeneous-sized mammalian cells in a pellet:
potential
applications in drug delivery and hybridoma formation. Biophysical Journal
71:479-486 (1996);
Carruthers, A., & Melchior, D.L. A rapid method of reconstituting human
erythrocyte sugar
transport proteins. Biochem. 23: 2712-2718 (1984) (each of these three
publications is
incorporated by reference in its entirety for all purposes). Alternatively,
erythrocytes may be
loaded indirectly by loading hematopoietic progenitors with single-celled
organisms and inducing
them to differentiate and expand into erythrocytes containing single-celled
organisms.
[64] Electroporation is a commonly used, inexpensive method to deliver
factors to cells.
(Potrykus, I. Gene transfer methods for plants and cell cultures. Ciba Found
Symp 154, 198-208;
discussion 208-112 (1990); Wolbank, S. et al. Labeling of human adipose-
derived stem cells for
non-invasive in vivo cell tracking. Cell Tissue Bank 8, 163-177 (2007) (each
of these two
publications is incorporated by reference in its entirety for all purposes)).
[65] In another embodiment, a eukaryotic cell that naturally endocytoses
bacteria (e.g.,
Chinese hamster ovary (CHO)) is used. In one embodiment, the modified single-
celled bacteria
are added to the CHO culture directly. CHO cells are cultured by standard
procedures in Ham's
16
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
F-12 media with 10% fetal calf serum media prior to infection with the MTB.
Post infection, the
media is augmented with additional iron (40 to 80 [tM) as either ferric malate
or FeCl3.
Numerous other cell types internalize bacteria by endocytosis or more
specifically phagocytosis;
endosymbionts or parasites have their own methods for cellular entry and these
natural processes
can be exploited for internalization of the artificial endosymbionts resulting
in the generation of
so-called symbiosomes. In another embodiment, symbiosomes from one cell can be
transplanted
to another cell type (i.e., one incapable of endocytosis of artificial
endosymbionts) using
microinjection, organelle transplantation, and chimera techniques. These host
cells are cultured in
typical media and techniques for the specific cell type with the.
[66] In one embodiment, a single-celled organism is introduced to the host
cell by a liposome
mediated process. Mitochondria and chloroplasts, which are larger than MTB,
have been
efficiently introduced into eukaryotic cells when encapsulated into liposomes.
(Bonnett, H. T.
Planta 131, 229 (1976); Giles, K.; Vaughan, V.; Ranch, J.; Emery, J. Liposome-
mediated uptake
of chloroplasts by plant protoplasts. In Vitro Cellular & Developmental
Biology - Plant 16(7)
581-584 (each of these two publications is incorporated by reference in its
entirety for all
purposes)). Numerous liposome fusion protocols and agents are available and
can be used by the
skilled artisan without undue experimentation. (See, e.g., Ben-Haim, N., Broz,
P., Marsch, S.,
Meier, W., Hunziker, P. Cell-specific integration of artificial organelles
based on functionalized
polymer vesicles. Nano Lett. 8(5): 1368-1373 (2008); Lian, W., Chang, C.,
Chen, Y., Dao, R.,
Luo, Y., Chien, J., Hsieh, S., Lin, C. Intracellular delivery can be achieved
by bombarding cells
or tissues with accelerated molecules or bacteria without the need for carrier
particles.
Experimental Cell Research 313(1): 53-64 (2007); Heng, B.C. & Cao, T.
Immunoliposome-
mediated delivery of neomycin phosphotransferase for the lineage-specific
selection of
differentiated/committed stem cell progenies: Potential advantages over
transfection with marker
genes, fluorescence-activated and magnetic affinity cell-sorting. Med.
Hypotheses 65(2): 334-336
(2005); Potrykus (1990) Ciba Found Symp, Vol. 1 54: 198 (each of these four
publications is
incorporated by reference in its entirety for all purposes)).
[67] The inventions disclosed herein will be better understood from the
experimental details
which follow. However, one skilled in the art will readily appreciate that the
specific methods
and results discussed are merely illustrative of the inventions as described
more fully in the claims
which follow thereafter.
17
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
EXAMPLES
Example 1. Microinjection of Op+AMB into murine cells
A. Construction of gfp+AMB.
[68] Expression vectors for eGFP, one including a Shine-Dalgamo sequence
upstream of the
gfi, gene and one without a Shine Dalgamo, sequence were cloned into cryptic
broad host range
vector pBBR1MCS-2 (Kovach, M.E., et al. Four new derivatives of the broad-host-
range cloning
vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166,
175-176, (1995)
(which is incorporated by reference in its entirety for all purposes)). AMB
(ATCC 700264) was
transformed with this construct. (Matsunaga, T. et al. Complete genome
sequence of the
facultative anaerobic magnetotactic bacterium Magnetospirillum sp. strain AMB-
1. DNA Res. 12,
157-166 (2005); Burgess J.G., et al. Evolutionary relationships among
Magnetospirillum strains
inferred from phylogenetic analysis of 16S rDNA sequences. J Bacteriol. 175:
6689-6694 (1993);
Matsunaga T, et al. Gene transfer in magnetic bacteria: transposon mutagenesis
and cloning of
genomic DNA fragments required for magnetosome synthesis. J. BacterioL 174:
2748-2753
(1992); Kawaguchi R, et al. Phylogeny and 16s rRNA sequence of
Magnetospirillum sp. AMB-1,
an aerobic magnetic bacterium. Nucleic Acids Res. 20: 1140, (1992) (each of
these four
publications is incorporated by reference in its entirety for all purposes)).
[69] Transformation was achieved by conjugation using a donor Escherichia coli
strain as
described by Goulian, M. van der Woude, M.A. A simple system for converting
lacZ to gfp
reporter fusions in diverse bacteria. Gene 372, 219-226 (2006); Scheffel, A.
Schtiler, D. The
Acidic Repetitive Domain of the Magnetospirillum gryphiswaldense MamJ Protein
Displays
Hypervariability but Is Not Required for Magnetosome Chain Assembly. J
BacterioL September;
189(17): 6437-6446 (2007) (each of these two publications is incorporated by
reference in its
entirety for all purposes). The mating reactions were cultured for 10 days
under defined
microaerophilic conditions in the absence of DAP to select for positive
transformants.
[70] Following conjugation, gfp AMB transformants with and without the Shine-
Dalgamo
sequence successfully displayed GFP fluorescence. The tranformants containing
the Shine-
Dalgarno sequence displayed higher levels of GFP fluorescence than the
transformants without
this sequence. The resulting fluorescence did not leave the gfp+AMB cells when
viewed at 100x
magnification at 488 nm excitation.
18
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
[71] The magnetic properties of the gfp AMB were analyzed by MRI. The gVAMB
was
suspended in agar plugs using a 1.5T instrument to optimize and characterize
the imaging
properties. FIG. 1 shows the positive contrast generated with a Ti pulse
sequence over a log scale
concentration up to ¨108 MTB/mL. Signal intensity was related to
concentration.
B. Microinjection into murine embryonic cells.
[72] The ,g)V-AMB was mircoinjected into one cell of each of 170 mouse
embryos at the 2-cell
stage. Six concentrations over a log scale up to ¨105 gfp+AMB were injected
per cell, estimated
by the optical density at 565nm. Death rate of cells following microinjection
was constant across
the different injected concentrations. Images overlaying fluorescent and
differential interference
contrast (DIC) images of cells injected with the highest concentration (105
MTB/cell) were
compared. An uninjected control exhibited low levels of autofluorescence.
Slices at different
horizontal planes in 8-cell embryos at a given time point were compared. In
each embryo, all four
cells derived from the injected cell showed significant fluorescence while
none of the four cells
derived from the uninjected internal controls displayed significant
fluorescence.
[73] The embryos were allowed to develop for three days after the
injection. In each
concentration level, embryos survived for up to the full three days developing
to the 256 cell
blastula stage and appeared healthy enough for implantation. Numerous cells
within each blastula
displayed significant fluorescence, demonstrating that the artificial
endosymbionts were
transferred to daughter cells across multiple cell divisions as the embryos
comprising the
eukaryotic host cells developed to the blastula stage. One such blastula is
shown in FIG. 2, where
Panel A shows a differential interference contrast (DIC) image of the blastula
and Panel B) shows
a gray scale fluorescence capture of the same image, showing fluorescence in
numerous cells
throughout the blastula.
[74]
Confocal microscopy was used to quantify total expression of GFP throughout
four
individual embryos by measuring total GFP fluorescence in the entire embryo
over time at various
points beginning at the eight cell stage of the embryo. FIG. 3 shows the
change of embryo
fluorescence over time. This indicates that the copy number of artificial
endosymbionts was
maintained in daughter cells for at least seven generations, such that the
fluorescent phenotype of
the host cells was maintained as the embryo progressed from the 2-cell stage
to the 256-cell
blastula stage.
19
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
[75] These results demonstrate that, when delivered by microinjection,
gfp+AMB were not
immediately cleared or degraded and were not toxic to the developing embryo
over the course of
the three day experiment. Microinjected embryos divided normally, suggesting
that gVAMB do
not display pathogenic markers or secret toxic compounds. They were
transferred to daughter
cells across many cell divisions, were contained in the cytoplasm, were
punctate and well
distributed, and maintained copy number within the daughter host cells, such
that the fluorescent
phenotype of the eukaryote host cells was maintained in daughter cells through
at least seven
generations. These results demonstrate that AMB can be stably maintained
intracellularly and are
transferred to daughter cells over at least seven cell divisions.
Example 2. Phagocytic entry of AMB
[76] Receptor mediated: The inIAB gene is amplified from L. monocytogenes
genomic DNA
(ATCC 19114) and is inserted into pBBRIMCS-5,107 the gentamicin cognate of
pBBR1MCS-2
(Kovach, M.E., et al. Four new derivatives of the broad-host-range cloning
vector pBBRIMCS,
carrying different antibiotic-resistance cassettes. Gene 166, 175-176,
(1995)), and gfp+inIAB+
AMB is generated. The gfp+inIAB+ AMB is co-cultured with eukaryotic host
cells, including
common epithelial tumor cell lines Coco-2, MDA-MB231 and MCF7, non-epithelial
tumor cell
lines, such as HT-1080 and HL60, and murine stem cells. Fluorescent microscopy
and FACS are
used to monitor and quantify internalization and intracellular location.
[77] Expression of pore-forming haemolysin (hlyA) in AMB is achieved
through amplification
of hlyA from L. monocytogenes genomic DNA (ATCC 19114). The amplified hlyA is
inserted
into pBBR1MCS-3 (the tetracycline cognate of pBBR1MCS-2) which is then used to
transform
geAMB. The resulting AMB strain is exposed to murine macrophage cell line
J774, capable of
spontaneous phagocytosis. Gentomycin treatment is used to eliminate bacteria
not internalized
and hlyA-AMB is used as negative control. Fluorescent microscopy is used to
monitor the
intracellular fate and localization of AMB.
[78] If bacteria remain confined to the phagosomes, two genes, plcA and
plcB, implicated in
escape of L. monocytogenes into the cytosol, are introduced. (Smith, G. A.,
Marquis, H., Jones,
S., Johnston, N. C., Portnoy, D. A., Goldfine, H. Infection and immunity 63:
4231 (1995); Camilli,
A.; Goldfine, H.; Portnoy, D. A. The Journal of Experimental Medicine 173: 751
(1991) (each of
these two publications is incorporated by reference in its entirety for all
purposes)). If bacteria
escape successfully, but fail to propagate, hpt is introduced. (Goetz, M.,
Bubert, A., Wang, G.,
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
Chico-Calero, I., Vazquez-Boland, J. A., Beck, M.; Slaghuis, J., Szalay, A.
A., Goebel, W. Proc
Nall Acad Sci USA 98: 12221 (2001); Chico-Calero, I., Suarez, M., Gonzalez-
Zorn, B., Scortti,
M., Slaghuis, J., Goebel, W., Vazquez-Boland, J. A. Proc Nail Acad Sci U S A
99: 431(2002)
(each of these two publications is incorporated by reference in its entirety
for all purposes)). In L.
monocytogenes, hpt encodes the transporter responsible for uptake of glucose-6-
phosphate from
the cytosol. Other genes from L. monocytogenes have been implicated in
sustaining growth
within host (glnA and gltAB and argD) and these are systematically introduced
as needed.
(Joseph, B., Przybilla, K., Stuhler, C., Schauer, K., Slaghuis, J., Fuchs, T.
M., Goebel, W. Journal
of Bacteria 188: 556 (2006) (which is incorporated by reference in its
entirety for all purposes)).
Example 3. Regulation of AMB Growth
[79] Regulation of AMB growth in embryonic stem cells can be regulated as
follows.
Coleoptericin-A (ColA) is amplified from total Sitophilus wyzae cDNA.
Expression of ColA in
beetles of genus Sitophilus regulates titers of y-Protobacterium, which has
naturally developed
close symbiotic relationship the beetles, and resides in specific cells called
bacteriocytes. (Login,
F.H., Balmain, S., Vanier, A., Vincent-Monegat, C., Vigneron, A., Weiss-Gayet,
M., Rochat, D.,
Heddi, A. Antimicrobial peptides keep insect endosymbionts under control.
Science 334(6054):
362-365 (2011) (which is incorporated by reference in its entirety for all
purposes)).
[80] Murine embryonic stem cells comprising gfp+AMB are treated using a neural
differentiation protocol. MTB expression levels are quantified using qPCR and
fluorescent
microscopy. Amplified colA is then expressed in the gfp+AMB embryonic stem
cells. A
promoter is selected to provide optimal ColA expression levels.
Example 4. Magnetic pheonotype of murine cells containing gfp+AMB
[81] Cells from macrophage cell line J774.2 derived from murine ascites and
solid tumor with
introduced geAMB were applied to a magnetic column and were retained by the
column. These
results demonstrate that, following introduction of geAMB, J774.2 murine cells
were
magnetically detected and magnetically manipulated, as they were magnetically
concentrated and
magnetically collected.
Example 5. Gfp+AMB in human breast cancer cell line MDA-MB-231
[82] Gfp+AMB, Gfp+InlA/B+AMB, and Gfp+Plal+AMB were each introduced to human
breast cancer cells from cell line MDA-MB-231. GFP fluorescence was detected
in more than
21
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
90% of the MDA-MB-231 cells 48 hours after the introduction of each of Gfp
AMB,
Gfp+In1A/B+AMB, and Gfp+Plal +AMB. GFP fluorescence in Gfp+AMB was observed in
these
MDA-MB-231 cells at least 13 days after introduction of gfp+AMB, in the fourth
passage of the
MDA-MB-231 cells following the introduction, where MDA-MB-231 cell population
doubled
three to four times between each passage. GFP fluorescence was observed in
both forming
daughter cells of an MDA-MB-231 cell with introduced geAMB in the process of
cell division.
[83] Following the introduction of gfp+AMB, MDA-MB-231 cells were stained with
Lysotrackere Red DND-99 dye and Hoechst 33342 stain purchased from Life
Technologies.
Green GFP fluorescence was observed as localized within individual MDA-MB-231
cells in
[84] At 24 hours and 72 hours after introduction, plated MDA-MB-231 cells and
plated control
MDA-MB-231 cells were fixed in formalin and glutaraldehyde following a wash
with PBS. Cells
were then stained with Prussian Blue, and observed by microscopy at 40x
magnification. Iron
staining was observed in some of the MDA-MB-231 cells with introduced gfp+AMB
but not in
the control MDA-MB-231 cells without introduced gfp+AMB. The proportion of
cells displaying
iron staining was similar between the MDA-MB-231 cells 72 hours after gfp AMB
introduction
and the MDA-MB-231 cells 24 hours after gfp+AMB introduction.
[85] 48 hours after the introduction of gfp+AMB, MDA-MB231 cells were
trypsinized and
resuspended in PBS. One sample of these cells was placed into a glass slide
chamber. A magnet
was aligned to the side of chamber and cell movement was observed under
microscope at 20X
magnification. MDA-MB231 cells with introduced gfp+AMB, but not control MDA-
MB231 cells
which had not had gfp+AMB introduced, exhibited movement toward the magnet.
Another
sample of these cells was placed into small tubes, which were taped to a
magnet for one hour.
Control MDA-MB231 cells which had not had gfp+AMB introduced settled down at
the bottom of
the tube. However, MDA-MB231 cells with introduced gfp+AMB were aligned to the
magnet
side of the tubes.
[86] These results indicate that gfp+AMB were not immediately cleared from
human breast
cancer MDA-MB-231 cells. They were transferred to daughter cells across at
least 12 cell
divisions and were located within the MDA-MB-231 cells outside of both the
lysosomes and
nuclei. These results also demonstrate that at least 48 hours following
introduction of gfp+AMB,
the MDA-MB-231 cells containing gfp+AMB displayed were magnetically detected
and
magnetically manipulated, as they were magnetically moved, magnetically
targeted to a location,
22
CA 02863331 2014-07-09
WO 2013/106814
PCT/US2013/021414
magnetically concentrated, and magnetically collected. Additionally, at least
72 hours following
introduction of geAMB, the MDA-MB-231 cells contained observable quantities of
iron.
Example 6. Gfp+AMB in human Induced Pluripotent Stem Cells
[87] GFP fluorescence was observed in Human Induced Pluripotent Stem ("IPS")
cells at least
eight days following introduction of gfil+AMB to the IPS cells, in the second
passage of the IPS
cells. These results indicate that gfp+AMB were not immediately cleared from
human IPS cells,
and were transferred to daughter cells.
[88] All publications, patents and patent applications discussed and cited
herein are
incorporated herein by reference in their entireties. It is understood that
the disclosed invention is
not limited to the particular methodology, protocols and materials described
as these can vary. It
is also understood that the terminology used herein is for the purposes of
describing particular
embodiments only and is not intended to limit the scope of the present
invention which will be
limited only by the appended claims.
[89] Those skilled in the art will recognize, or be able to ascertain using
no more than routine
experimentation, many equivalents to the specific embodiments of the invention
described herein.
Such equivalents are intended to be encompassed by the following claims.
23