Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02863698 2014-08-06
1
IMIDAZOLIUM SALTS AS ADDITIVES FOR FUELS
Description
The present invention relates to the use of imidazolium salts as additives for
fuels, especially
as detergent additives for diesel fuels, in particular for those diesel fuels
which are
combusted in direct injection diesel engines, especially in common rail
injection systems. The
present invention further relates to an additive concentrate and to a fuel
composition
comprising such imidazolium salts. The present invention further relates to
novel imidazolium
salts and to the use thereof in industrial fluids.
In direct injection diesel engines, the fuel is injected and distributed
ultrafinely (nebulized) by
a multihole injection nozzle which reaches directly into the combustion
chamber of the
engine, instead of being introduced into a prechamber or swirl chamber as in
the case of the
conventional (chamber) diesel engine. The advantage of the direct injection
diesel engines
lies in their high performance for diesel engines and nevertheless low fuel
consumption.
Moreover, these engines achieve a very high torque even at low speeds.
At present, essentially three methods are being used for injection of the fuel
directly into the
combustion chamber of the diesel engine: the conventional distributor
injection pump, the
pump-nozzle system (unit-injector system or unit-pump system), and the common
rail
system.
In the common rail system, the diesel fuel is conveyed by a pump with
pressures up to 2000
bar into a high-pressure line, the common rail. Proceeding from the common
rail, branch
lines run to the different injectors which inject the fuel directly into the
combustion chamber.
The full pressure is always applied to the common rail, which enables multiple
injection or a
specific injection form. In the other injection systems, in contrast, only a
smaller variation in
the injection is possible. The injection in the common rail is divided
essentially into three
groups: (1.) pre-injection, by which essentially softer combustion is
achieved, such that harsh
combustion noises ("nailing") are reduced and the engine seems to run quietly;
(2.) main
injection, which is responsible especially for a good torque profile; and (3.)
post-injection,
which especially ensures a low NO value. In this post-injection, the fuel is
generally not
combusted, but instead vaporized by residual heat in the cylinder. The exhaust
gas/fuel
mixture formed is transported to the exhaust gas system, where the fuel, in
the presence of
suitable catalysts, acts as a reducing agent for the nitrogen oxides NOR.
CA 02863698 2014-08-06
2
The variable, cylinder-individual injection in the common rail injection
system can positively
influence the pollutant emission of the engine, for example the emission of
nitrogen oxides
(N0x), carbon monoxide (CO) and especially of particulates (soot). This makes
it possible, for
example, for engines equipped with common rail injection systems to meet the
Euro 4
standard theoretically even without additional particulate filters.
In modern common rail diesel engines, under particular conditions, for example
when
biodiesel-containing fuels or fuels with metal impurities such as zinc
compounds, copper
compounds, lead compounds and other metal compounds are used, deposits can
form on
the injector orifices, which adversely affect the injection performance of the
fuel and hence
impair the performance of the engine, i.e. especially reduce the power, but in
some cases
also worsen the combustion. The formation of deposits is enhanced further by
further
developments in the injector construction, especially by the change in the
geometry of the
nozzles (narrower, conical orifices with rounded outlet). For lasting optimal
functioning of
engine and injectors, such deposits in the nozzle orifices must be prevented
or reduced by
suitable fuel additives.
International application WO 2012/004300 (1) describes acid-free quaternized
nitrogen
compounds as fuel additives, which are obtainable by addition of a compound
comprising at
least one oxygen- or nitrogen-containing group reactive with an anhydride and
additionally at
least one quaternizable amino group onto a polycarboxylic anhydride compound
and
subsequent quaternization with an epoxide in the absence of free acid.
Suitable compounds
having an oxygen- or nitrogen-containing group reactive with an anhydride and
additionally a
quaternizable amino group are especially polyamines having at least one
primary or
secondary amino group and at least one tertiary amino group. Useful
polycarboxylic
anhydrides include especially dicarboxylic acids such as succinic acid with a
relatively long-
chain hydrocarbyl substituent. Such a quaternized nitrogen compound is, for
example, the
reaction product, obtained at 40 C, of polyisobutenylsuccinic anhydride with 3-
(dimethylamino)propylamine, which is a polyisobutenylsuccinic monoamide and
which is
subsequently quaternized with styrene oxide in the absence of free acid at 70
C. Such acid-
free quaternized nitrogen compounds are especially suitable as a fuel additive
for reducing or
preventing deposits in the injection systems of direct injection diesel
engines, especially in
common rail injection systems, for reducing the fuel consumption of direct
injection diesel
engines, especially of diesel engines with common rail injection systems,
and/or for
minimizing power loss in direct injection diesel engines, especially in diesel
engines with
common rail injection systems.
CA 02863698 2014-08-06
3
International application PCT/EP2011/071683 (2) describes
polytetrahydrobenzoxazines and
bistetrahydrobenzoxazines as fuel additives, which are obtainable by, in a
first reaction step,
gradually reacting a C1- to C20-alkylenediamine having two primary amino
functions, e.g. 1,2-
ethylenediamine, with a Cl- to C12-aldehyde, e.g. formaldehyde, and a C1- to
C8-alkanol at a
temperature of 20 to 80 C with elimination and removal of water, both the
aldehyde and the
alcohol being used in more than twice the molar amount relative to the
diamine, reacting the
condensation product thus obtained in a second reaction step with a phenol
which bears at
least one long-chain substituent, for example a tert-octyl, n-nonyl, n-dodecyl
or polyisobutyl
radical, in a stoichiometric ratio of the alkylenediamine originally used of
1.2: 1 to 3: 1 at a
temperature of 30 to 120 C and optionally heating the bistetrahydrobenzoxazine
thus
obtained in a third reaction step to a temperature of 125 to 280 C for at
least 10 minutes.
Such polytetrahydrobenzoxazines and bistetrahydrobenzoxazines are especially
suitable as
a fuel additive for reducing or preventing deposits in the injection systems
of direct injection
diesel engines, especially in common rail injection systems, for reducing the
fuel
consumption of direct injection diesel engines, especially of diesel engines
with common rail
injection systems, and/or for minimizing power loss in direct injection diesel
engines,
especially in diesel engines with common rail injection systems.
However, the acid-free quaternized nitrogen compounds and
polytetrahydrobenzoxazines or
bistetrahydrobenzoxazines mentioned are still in need of improvement in terms
of their
properties as detergent additives for fuels. In addition, they should also
have improved
anticorrosive action, improved motor oil compatibility and improved low-
temperature
properties.
It was therefore an object of the present invention to provide improved fuel
additives which
no longer have the disadvantages detailed from the prior art.
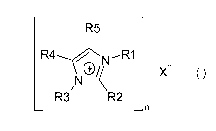
Accordingly, the use of imidazolium salts of the general formula (I)
R5
Xn-
N /K
R3 R2
n
(I)
in which
CA 02863698 2014-08-06
4
the variables R1 and R3 are each independently an organic radical having 1 to
3000 carbon
atoms,
the variables R2, R4 and R5 are each independently hydrogen or an organic
radical having 1
to 3000 carbon atoms,
X is an anion and
n is the number 1, 2 or 3
as additives for fuels has been found.
Imidazolium salts of the (I) type ¨ as well as, for example, open-chain
quaternary ammonium
salts, pyridinium salts, pyridazinium salts, pyrimidinium salts, pyrazinium
salts, pyrazoliunn
salts, pyrazolinium salts, imidazolinium salts, thiazolium salts, triazolium
salts, pyrrolidinium
salts and imidazolidinium salts ¨ are among what are known as ionic liquids,
which are
understood to mean salts (i.e. compounds composed of cations and anions)
which, at
standard pressure, have a melting point of less than 200 C, usually even of
less than 80 C.
Ionic liquids often comprise an organic compound as a cation (organic cation).
According to
the valency of the anion, the ionic liquid may, as well as the organic cation,
comprise further
cations such as metal cations.
lmidazolium salts of the (I) type are known in their application as detergents
or dispersants in
lubricant formulations. For instance, WO 2010/101801 Al (3) describes oil-
soluble ionic
detergents as additive components in lubricant oils for internal combustion
engines;
examples cited are, as well as open-chain ionic systems and quaternized
pyridinium
detergents, quaternized imidazolium phenoxides, imidazolium chlorides and
imidazolium
salicylates.
WO 2010/096168 Al (4) describes ionic liquids such as pyridinium salts as
additives for
control of deposit formation on the internal surfaces of internal combustion
engines. In
contrast to the present invention, however, such additives are added to the
lubricant oil and
not to the fuel used to operate these engines. Moreover, WO 2010/096168 Al
explicitly does
not disclose imidazolium salts as such additives.
US 4 108 858 (5) discloses high molecular weight N-hydrocarbyl-substituted
quaternized
ammonium salts with a molecular weight of 350 to 3000 carbon atoms for the
hydrocarbyl
group as detergents and dispersants for fuels such as gasoline fuels and
diesel fuels and for
CA 02863698 2014-08-06
lubricant oils. Specified as such high molecular weight N-hydrocarbyl-
substituted quaternized
ammonium salts are, as well as open-chain systems, salts of piperidines,
piperazines,
morpholines and pyridines. Useful relatively long-chain hydrocarbyl radicals
include, for
example, polybutene or polypropylene radicals.
5
In a preferred embodiment of the present invention, the imidazolium salts (I)
are used as
detergent additives for diesel fuels. In this embodiment, particular
preference is given to the
individual uses of the imidazolium salts (I) as an additive for reducing or
preventing deposits
in the injection systems of direct injection diesel engines, especially in
common rail injection
systems, for reducing the fuel consumption of direct injection diesel engines,
especially of
diesel engines with common rail injection systems, and/or for minimizing power
loss in direct
injection diesel engines, especially in diesel engines with common rail
injection systems.
In a further preferred embodiment embodiment, the imidazolium salts (I) are
used as a wax
antisettling additive (WASA) for middle distillate fuels, especially diesel
fuels.
In a further preferred embodiment, the imidazolium salts (I) are used as a
lubricity improver
for fuels, especially as friction modifiers for gasoline fuels and as
lubricity additives for middle
distillate fuels or diesel fuels.
The organic radicals for the variables R1 to R5 in the imidazolium salts of
the general
formula (I) comprise preferably 1 to 1000, especially 1 to 500 and in
particular 1 to 250
carbon atoms. In general, these organic radicals are low molecular weight
radicals, for
example alkyl, cycloalkyl, alkenyl, cycloalkenyl, aryl or heteroaryl radicals,
or polymeric
radicals, for example polypropyl radicals or especially polyisobutyl radicals.
Low molecular
weight radicals comprise preferably 1 to 20 carbon atoms.
Useful organic radicals having 1 to 3000 carbon atoms for the variables R1 to
R5 in the
imidazolium salts of the general formula (I) preferably include Ci- to C20-
alkyl radicals,
especially Ci- to C12-alkyl radicals, in particular C1- to Cs-alkyl radicals,
and the aryl-,
heteroaryl-, cycloalkyl-, halogen-, hydroxy-, amino-, carboxy-, formyl-, -0-, -
CO-, -00-0- or ¨
CO-N<-substituted components thereof, for example methyl, ethyl, 1-propyl, 2-
propyl, 1-
butyl, 2-butyl, 2-methyl-1-propyl (isobutyl), 2-methyl-2-propyl (tert-butyl),
1-pentyl, 2-pentyl, 3-
pentyl, 2-methyl-1-butyl, 3-methyl-1-butyl, 2-methyl-2-butyl, 3-methyl-2-
butyl, 2,2-dimethy1-1-
propyl, 1-hexyl, 2-hexyl, 3-hexyl, 2-methyl-1-pentyl, 3-methyl-1-pentyl, 4-
methyl-1-pentyl, 2-
methy1-2-pentyl, 3-methyl-2-pentyl, 4-methyl-2-pentyl, 2-methyl-3-pentyl, 3-
methyl-3-pentyl,
2,2-dimethy1-1-butyl, 2,3-dimethy1-1-butyl, 3,3-dimethy1-1-butyl, 2-ethyl-1-
butyl, 2,3-dimethyl-
2-butyl, 3,3-dimethy1-2-butyl, n-heptyl, n-octyl, 2-ethylhexyl, n-nonyl, n-
decyl, 2-propylheptyl,
CA 02863698 2014-08-06
6
n-undecyl, n-dodecyl, n-tridecyl, isotridecyl, n-tetradecyl, n-pentadecyl, n-
hexadecyl, n-
heptadecyl, n-octadecyl, n-nonadecyl, n-eicosyl, phenylmethyl (benzyl),
diphenylmethyl,
triphenylmethyl, 2-phenylethyl, 3-phenylpropyl, cyclopentylmethyl, 2-
cyclopentylethyl, 3-
cyclopentylpropyl, cyclohexylmethyl, 2-cyclohexylethyl, 3-cyclohexylpropyl,
methoxy, ethoxy,
formyl, acetyl, and also fluoroalkyl radicals such as monofluoromethyl,
difluoromethyl,
trifluoromethyl, pentafluoroethyl, 3,3,3-trifluoropropyl, perfluorohexyl,
perfluorooctyl,
perfluorodecyl or perfluorododecyl.
Further suitable organic radicals having 1 to 20 carbon atoms for variables R1
to R5 in the
imidazolium salts of the general formula (I) are also 03- to 012-cycloalkyl
radicals, especially
05- to 07-cycloalkyl radicals, and the aryl-, heteroaryl-, cycloalkyl-,
halogen-, hydroxy-,
amino-, carboxy-, formyl-, -0-, -CO- or -00-0-substituted components thereof,
for example
cyclopentyl, 2-methyl-1-cyclopentyl, 3-methyl-1-cyclopentyl, cyclohexyl, 2-
methyl-1-
cyclohexyl, 3-methyl-1-cyclohexyl, 4-methyl-1-cyclohexyl, and also
fluorocyclohexyl radicals
such as perfluorocyclohexyl.
Further suitable organic radicals having 1 to 20 carbon atoms for variables R1
to R5 in the
imidazolium salts of the general formula (I) are also 02- to 020-alkenyl
radicals, especially 03-
to C8-alkenyl radicals, and the aryl-, heteroaryl-, cycloalkyl-, halogen-,
hydroxy-, amino-,
carboxy-, formyl-, -0-, -CO- or -00-0-substituted components thereof, for
example vinyl, 2-
propenyl (allyl), 3-butenyl, cis-2-butenyl, trans-2-butenyl, and also
fluoroalkenyl radicals such
as perfluoro-2-propenyl, perfluoro-3-butenyl or perfluoro-2-butenyls.
Further suitable organic radicals having 1 to 20 carbon atoms for variables R1
to R5 in the
imidazolium salts of the general formula (I) are also 03- to 012-cycloalkenyl
radicals,
especially 05- to C7-cycloalkenyl radicals, and the aryl-, heteroaryl-,
cycloalkyl-, halogen-,
hydroxy-, amino-, carboxy-, formyl-, -0-, -CO- or -00-0-substituted components
thereof, for
example 3-cyclopentenyl, 2-cyclohexenyl, 3-cyclohexenyl, 2,5-cyclohexadienyl,
and also
fluorocycloalkenyl radicals such as fluorocyclohexenyl radicals.
Further suitable organic radicals having 1 to 20 carbon atoms for variables R1
to R5 in the
imidazolium salts of the general formula (I) are also aryl or heteroaryl
radicals having 3 to 20
and especially 5 to 10 carbon atoms and the alkyl-, aryl-, heteroaryl-,
cycloalkyl-, halogen-,
hydroxy-, amino-, carboxy-, formyl-, -0-, -00- or -00-0-substituted components
thereof, for
example phenyl, 2-methylphenyl (2-toly1), 3-methylphenyl (3-toly1), 4-
methylphenyl (4-toly1),
2-ethylphenyl, 3-ethylphenyl, 4-ethylphenyl, 2,3-dimethylphenyl, 2,4-
dimethylphenyl, 2,5-
dimethylphenyl, 2,6-dimethylphenyl, 3,4-dimethylphenyl, 3,5-dimethylphenyl, 4-
phenylphenyl,
1-naphthyl, 2-naphthyl, 1-pyrrolyl, 2-pyrrolyl, 3-pyrrolyl, 2-pyridinyl, 3-
pyridinyl, 4-pyridinyl,
CA 02863698 2014-08-06
7
and also fluoroaryl or fluoroheteroaryl radicals such as mono-, di-, tri-,
tetra- or
pentafluorophenyl.
It is also possible for two adjacent radicals of the variables R1 to R5 to
form an unsaturated,
saturated or aromatic ring which may optionally be substituted by functional
groups, aryl,
alkyl, aryloxy, alkyloxy, halogen, heteroatoms and/or heterocycles and may
optionally be
interrupted by one or more oxygen and/or sulfur atoms and/or one or more
substituted or
unsubstituted imino groups.
The organic radicals having 1 to 3000 carbon atoms for the variables R1 to R5
may be
synthetic radicals or ¨ especially in the case of alkyl and alkenyl radicals ¨
radicals based on
naturally occurring compounds. The latter derive particularly from naturally
occurring
glycerides or fatty acids, for example from stearic acid, palmitic acid, oleic
acid, linoleic acid,
linolenic acid or tallow fatty acid. Such radicals based on naturally
occurring compounds are
often mixtures of different, usually homologous alkyl or alkenyl radicals.
Further preferred organic radicals having 1 to 3000 carbon atoms for the
variables R1 to R5
in the imidazolium salts of the general formula (I) also include polyisobutyl
radicals having 16
to 3000, especially having 20 to 1000, in particular having 25 to 500 and most
preferably
having 30 to 250 carbon atoms. Such polyisobutyl radicals have number-average
molecular
weights Mrõ determined by gel permeation chromatography, of 200 to 40 000,
preferably of
500 to 15 000, especially of 700 to 7000, in particular of 900 to 3000 and
most preferably of
900 to 1100. The polyisobutyl radicals may be joined to the imidazolium ring
directly or by a
methylene group (-CH2-).
The organic radicals having 1 to 3000 carbon atoms for the variables R1 to R5,
especially
the alkyl, cycloalkyl, alkenyl, cycloalkenyl, aryl and heteroaryl radicals
mentioned, and also
the polymeric radicals mentioned, may comprise one or more heteroatoms in
their skeletons,
such as oxygen atoms, sulfur atoms, or nitrogen atoms optionally substituted
by further,
usually low molecular weight organic radicals, or bear one or more
substituents or one or
more functional groups, for example hydroxyl groups, halogen atoms such as
fluorine,
chlorine or bromine, pseudohalide groups such as thiocyanato or dicyanamido,
cyano
groups, nitro groups, sulfo groups, sulfonic acid groups, sulfonic ester
groups, sulfonamide
groups, amino groups, carboxylic acid groups, carboxylic ester groups or
carboxamide
groups.
CA 02863698 2014-08-06
8
In general, imidazolium salts of the general formula (I) in which the
variables R1 and R3 each
have the above definitions of an organic radical having 1 to 3000 carbon atoms
and the
variables R2, R4 and R5 are each hydrogen are used.
In a preferred embodiment of the present invention, imidazolium salts of the
general formula
(I) in which the variables R1 and R3 are each independently C1- to C20-alkyl
groups, C2- to
C20-alkenyl groups and/or polyisobutyl radicals having a number-average
molecular weight
(Me) of 200 to 40 000 and the variables R2, R4 and R5 are each hydrogen are
used. These
Ci- to C20-alkyl groups are preferably pure hydrocarbyl radicals. Typical
examples of such
pure C1- to C20-hydrocarbyl radicals are the 2-ethylhexyl and tallow fatty
alkyl radicals.
Useful anions X in the imidazolium salts of the general formula (I) include,
for example:
chloride; bromide, iodide; thiocyanate; hexafluorophosphate;
trifluoromethanesulfonate;
methanesulfonate; carboxylates, especially formate, acetate, propionate,
butyrate or
benzoate; mandelate; nitrate; nitrite; trifluoroacetate; sulfate;
hydrogensulfate; methylsulfate;
ethylsulfate; 1-propylsulfate; 1-butylsulfate; 1-hexylsulfate; 1-octylsulfate;
phosphate;
dihydrogenphosphate; hydrogenphosphate; C1-C4-dialkylphosphates; propionate;
tetrachloroaluminate; Al2C17-; chlorozincate; chloroferrate;
bis(trifluoromethylsulfonyl)imide;
bis(pentafluoroethylsulfonyl)imide; bis(methylsulfonyl)imide; bis(p-
tolylsulfonyl)imide;
tris(trifluoromethylsulfonyl)methide; bis(pentafluoroethylsulfonyl)methide; p-
tolylsulfonate;
tetracarbonylcobaltate; dimethyleneglycolmonomethylethersulfate; oleate;
stearate; acrylate;
methacrylate; maleate; hydrogencitrate; vinylphosphonate;
bis(pentafluoroethyl)phosphinate;
borates such as bis[salicylato(2-)]borate, bis[oxalato(2-)]borate, bis[1,2-
benzenediolato(2-)-
0,01]borate, tetracyanoborate or tetrafluoroborate; dicyanamide;
tris(pentafluoroethyl)trifluorophosphate;
tris(heptafluoropropyl)trifluorophosphate, cyclic
arylphosphates such as pyrocatecholphosphate of the formula (C61-1402)P(0)0-;
chlorocobaltate.
In general, the anions X are selected from the following group:
= alkylsulfates of the formula Ra0S03- where Ra is a Cr to C12-alkyl group,
preferably a Ci-
to Cs-alkyl group;
= the alkylsulfonates of the formula RaS03- where Ra is a C1- to C12-alkyl
group, preferably a
Ci- to Cs-alkyl group;
= halides, especially chloride and bromide;
CA 02863698 2014-08-06
9
= pseudohalides, especially thiocyanate and dicyanamide;
= carboxylates of the
formula Ra000- where Ra is a to C60-alkyl group, a 02- to 060-
alkenyl group, a 06- to C60-aryl group or a 07- to C60-alkylaryl or -arylalkyl
group,
preferably a to C20-alkyl group, a 02- to 020-alkenyl group, a 06- to C20-
aryl group or a
C7- to Caralkylaryl or arylalkyl group, in particular a 02- to Ca-alkenyl
group, a 06- to 012-
aryl group or a 07- to C14-alkylaryl or -arylalkyl group, especially acetate,
but also
formate, propionate, butyrate, acrylate, methacrylate, benzoate, phenylacetate
or o-, m-
or p-methylbenzoate;
= polycarboxylates of the formula Rb(COO-) where n is the number 1, 2 or 3
and Rn is an
n-valent hydrocarbyl radical having 1 to 60, especially 1 to 20 and in
particular 1 to 14
carbon atoms; typical radicals of this kind are malonate, succinate,
glutarate, adipate,
phthalate or terephthalate; a further suitable polycarboxylate anion is also
the oxalate
anion -000-000-;
= phosphates, especially dialkylphosphates of the formula RaRbPO4- where Ra
and Rb are
each independently a C1- to C6-alkyl group; more particularly, Ra and Rb are
each the
same alkyl group as in dimethylphosphate and diethylphosphate;
= phosphonates, especially monoalkyl phosphonates of the formula R8RbP03-
where Ra
and Rb are each independently a to C6-alkyl group;
= the TFSI anion of the formula N(SO2CF3)2-;
= tricyanomethanide of the formula (ON)3 C.
Frequently selected anions X are chloride, bromide, hydrogensulfate,
tetrachloroaluminate,
thiocyanate, dicyanamide, methylsulfate, ethylsulfate, methanesulfonate,
formate, acetate,
dimethylphosphate, diethylphosphate, p-tolylsulfonate, tetrafluoroborate,
hexafluorophosphate, methylmethylphosphonate, methylphosphonate, the TFSI
anion,
tricyanomethanide and trifluoromethanesulfonate.
In a preferred embodiment of the present invention, imidazolium salts of the
general formula
(I) in which the anion X denotes sulfate, an alkylsulfate, an alkylsulfonate,
an alkylcarbonate,
a halide, a pseudohalide, a carboxylate, a phosphate, a phosphonate, nitrate,
nitrite, the
TFSI anion of the formula N(SO2CF3)2- or the tricyanomethanide anion are used.
The anion X
is most preferably an alkylcarbonate, a pseudohalide, a carboxylate or the
tricyanomethanide
CA 02863698 2014-08-06
anion. It is frequently also advantageous when the anion X does not comprise
any
phosphorus atom, any sulfur atom, any halogen atom and/or any boron atom.
The charge n of the anion X depends on its nature and may assume the value of
1, 2 or 3. n
5 is most frequently 1 or 2, especially 1.
Typical individual examples of imidazolium salts (1) are 1,3-
dimethylimidazolium acetate, 1,3-
diethylimidazolium acetate, 1-ethy1-3-methylimidazolium acetate, 1-propy1-3-
methylimidazolium acetate, 1-buty1-3-methylimidazolium acetate, 1-penty1-3-
10 methylimidazolium acetate, 1-hexy1-3-methylimidazolium acetate, 1-octy1-
3-
methylimidazolium acetate, 1-(2-ethylhexyl)-3-methylimidazolium acetate, 1,3-
di(2-
ethylhexyl)imidazolium acetate, 1-decy1-3-methylimidazolium acetate, 1-(2-
propylheptyI)-3-
methylimidazolium acetate, 1,3,4,5-tetramethylimidazolium acetate, 1,3-
dimethy1-4,5-
diphenylimidazolium acetate, 1,4,5-trimethy1-3-ethylimidazolium acetate, 1-
methy1-3-ethyl-
4,5-diphenylimidazolium acetate, 1,3-dimethylimidazolium methylcarbonate, 1,3-
diethylimidazolium methylcarbonate, 1-ethy1-3-methylimidazolium
methylcarbonate, 1-propy1-
3-methylimidazolium methylcarbonate, 1-buty1-3-methylimidazolium
methylcarbonate, 1-
penty1-3-methylimidazolium methylcarbonate, 1-hexy1-3-methylimidazolium
methylcarbonate,
1-octy1-3-methylimidazolium methylcarbonate, 1-(2-ethylhexyl)-3-
methylimidazolium
methylcarbonate, 1,3-di(2-ethylhexyl)imidazolium methylcarbonate, 1-decy1-3-
methylimidazolium methylcarbonate, 1-(2-propylheptyI)-3-methylimidazolium
methylcarbonate, 1,3,4,5-tetramethylimidazolium methylcarbonate, 1,3-dimethy1-
4,5-
diphenylimidazolium methylcarbonate, 1,4,5-trimethy1-3-ethylimidazolium
methylcarbonate,
1-methy1-3-ethy1-4,5-diphenylimidazolium methylcarbonate, 1,3-
dimethylimidazolium
methylsulfate, 1,3-diethylimidazolium methylsulfate, 1-ethy1-3-
methylimidazolium
methylsulfate, 1-propy1-3-methylimidazolium methylsulfate, 1-buty1-3-
methylimidazolium
methylsulfate, 1-penty1-3-methylimidazolium methylsulfate, 1-hexy1-3-
methylimidazolium
methylsulfate, 1-octy1-3-methylimidazolium methylsulfate, 1-(2-ethylhexyl)-3-
methylimidazolium methylsulfate, 1,3-di(2-ethylhexyl)imidazolium
methylsulfate, 1-decy1-3-
methylimidazolium methylsulfate, 1-(2-propylhepty1)-3-methylimidazolium
methylsulfate,
1,3,4,5-tetramethylimidazolium methylsulfate, 1,3-dimethy1-4,5-
diphenylimidazolium
methylsulfate, 1,4,5-trimethy1-3-ethylimidazolium methylsulfate, 1-methy1-3-
ethy1-4,5-
diphenylimidazolium methylsulfate, 1,3-dimethylimidazolium methylsulfonate,
1,3-
diethylimidazolium methylsulfonate, 1-ethyl-3-methylimidazolium
methylsulfonate, 1-propy1-3-
methylimidazolium methylsulfonate, 1-butyl-3-methylimidazolium
methylsulfonate, 1-penty1-3-
methylimidazolium methylsulfonate, 1-hexy1-3-methylimidazolium
methylsulfonate, 1-octy1-3-
methylimidazolium methylsulfonate, 1-(2-ethylhexyl)-3-methylimidazolium
methylsulfonate,
1,3-di(2-ethylhexyl)imidazolium methylsulfonate, 1-decy1-3-methylimidazolium
CA 02863698 2014-08-06
11
methylsulfonate, 1-(2-propylheptyI)-3-methylimidazolium methylsulfonate,
1,3,4,5-
tetramethylimidazolium methylsulfonate, 1,3-dimethy1-4,5-diphenylimidazolium
methylsulfonate, 1,4,5-trimethy1-3-ethylimidazolium methylsulfonate, 1-methy1-
3-ethy1-4,5-
diphenylimidazolium methylsulfonate, 1,3-dimethylimidazolium diethylphosphate,
1,3-
diethylimidazolium diethylphosphate, 1-ethyl-3-methylimidazolium
diethylphosphate, 1-
propy1-3-methylimidazolium diethylphosphate, 1-buty1-3-methylimidazolium
diethylphosphate,
1-penty1-3-methylimidazolium diethylphosphate, 1-hexy1-3-methylimidazolium
diethylphosphate, 1-octy1-3-methylimidazolium diethylphosphate, 1-(2-
ethylhexyl)-3-
methylimidazolium diethylphosphate, 1,3-di(2-ethylhexyl)imidazolium
diethylphosphate, 1-
decy1-3-methylimidazolium diethylphosphate, 1-(2-propylheptyI)-3-
methylimidazolium
diethylphosphate, 1,3,4,5-tetramethylimidazolium diethylphosphate, 1,3-
dimethy1-4,5-
diphenylimidazolium diethylphosphate, 1,4,5-trimethy1-3-ethylimidazolium
diethylphosphate
and 1-methyl-3-ethyl-4,5-diphenylimidazolium diethylphosphate.
Typical individual examples of imidazolium salts (1) with polyisobutenyl
radicals are 1-
polyisobuty1-3-methylimidazolium acetate, 1-polyisobuty1-3-ethylimidazolium
acetate, 1-
polyisobuty1-3-propylimidazolium acetate, 1-polyisobuty1-3-butylimidazolium
acetate, 1-
polyisobuty1-3-(2-ethylhexyl)imidazolium acetate, 1,3-
di(polyisobutyl)imidazolium acetate, 1-
polyisobuty1-3-methylimidazolium methylcarbonate, 1-polyisobuty1-3-
ethylimidazolium
methylcarbonate, 1-polyisobuty1-3-propylimidazolium methylcarbonate, 1-
polyisobuty1-3-
butylimidazolium methylcarbonate, 1-polyisobuty1-3-(2-ethylhexyl)imidazolium
methylcarbonate, 1,3-di(polyisobutyl)imidazolium methylcarbonate, 1-
polyisobuty1-3-
methylimidazolium thiocyanate, 1-polyisobuty1-3-ethylimidazolium thiocyanate,
1-
polyisobuty1-3-propylimidazolium thiocyanate, 1-polyisobuty1-3-
butylimidazolium thiocyanate,
1-polyisobuty1-3-(2-ethylhexyl)imidazolium thiocyanate, 1,3-
di(polyisobutyl)imidazolium
thiocyanate, 1-polyisobuty1-3-methylimidazolium tricyanomethanide, 1-
polyisobuty1-3-
ethylimidazolium tricyanomethanide, 1-polyisobuty1-3-propylimidazolium
tricyanomethanide,
1-polyisobuty1-3-butylimidazolium tricyanomethanide, 1-polyisobuty1-3-(2-
ethylhexyl)imidazolium tricyanomethanide and 1,3-di(polyisobutyl)imidazolium
tricyanomethanide.
lmidazolium salts of the (I) type with low molecular weight radicals are sold
commercially
under the BasionicsTM name by BASF SE.
The preparation of the imidazolium salts of the (1) type is familiar to the
person skilled in the
art. A typical synthesis route proceeds from imidazole formation from 1 mol of
a 1,2-
dicarbonyl compound, 1 mol of an appropriately substituted primary amine, 1
mol of
ammonia and 1 mol of an aldehyde, conducts an N-alkylation with a suitable
alkylating agent
CA 02863698 2014-08-06
12
and then, if desired, exchanges the anion. For example, glyoxal or benzil, a
low molecular
weight primary alkylamine or alkenylamine, for example a Ci- to C13-
alkylamine, or a
polyisobutylamine, ammonia and formaldehyde are used to prepare an N-alky1-4,5-
diphenylimidazole or an N-alkylimidazole or an N-polyisobuty1-4,5-
diphenylimidazole or an N-
polyisobutylimidazole, and the unsubstituted second nitrogen atom is alkylated
with an
epoxide such as ethylene oxide, propylene oxide, butylene oxide or styrene
oxide in the
presence of acetic acid, or with a dialkyl carbonate, in which case the
imidazolium salt has
an acetate anion or an alkylcarbonate anion. To introduce a polyisobutyl
radical on the
unsubstituted second nitrogen atom, can with a polyisobutene epoxide as the
alkylating
agent be used.
In the preparation of imidazolium salts of the (1) type with the same
variables R1 and R3, 1
mol of a 1,2-dicarbonyl compound is advantageously used together with 2 mol of
an
appropriately substituted primary amine and 1 mol of an aldehyde, optionally
in the presence
of a suitable solvent (for example of acetic acid and water when an
imidazolium acetate is to
be obtained) in a one-stage synthesis, usually at 20 to 120 C, especially at
25 to 80 C.
The fuel additized with one or more imidazolium salts (I) is a gasoline fuel
or especially a
middle distillate fuel, in particular a diesel fuel. The fuel may comprise
further customary
additives ("coadditives") to improve efficacy and/or suppress wear.
In the case of diesel fuels, these are primarily customary detergent
additives, carrier oils,
cold flow improvers, lubricity improvers, corrosion inhibitors, demulsifiers,
dehazers,
antifoams, cetane number improvers, combustion improvers, antioxidants or
stabilizers,
antistats, metallocenes, metal deactivators, dyes and/or solvents.
In the case of gasoline fuels, these are in particular lubricity improvers
(friction modifiers),
corrosion inhibitors, demulsifiers, dehazers, antifoams, combustion improvers,
antioxidants
or stabilizers, antistats, metallocenes, metal deactivators, dyes and/or
solvents.
Typical examples of suitable coadditives are listed in the following sections:
The customary detergent additives are preferably amphiphilic substances which
possess at
least one hydrophobic hydrocarbyl radical with a number-average molecular
weight (Me) of
85 to 20 000 and at least one polar moiety selected from:
(Da) mono- or polyamino groups having up to 6 nitrogen atoms, at least one
nitrogen atom
having basic properties;
CA 02863698 2014-08-06
13
(Db) nitro groups, optionally in combination with hydroxyl groups;
(Dc) hydroxyl groups in combination with mono- or polyamino groups, at least
one nitrogen
atom having basic properties;
(Dd) carboxyl groups or the alkali metal or alkaline earth metal salts
thereof;
(De) sulfonic acid groups or the alkali metal or alkaline earth metal salts
thereof;
(Df) polyoxy-C2- to C4-alkylene moieties terminated by hydroxyl groups, mono-
or
polyamino groups, at least one nitrogen atom having basic properties, or by
carbamate
groups;
(Dg) carboxylic ester groups;
(Dh) moieties derived from succinic anhydride and having hydroxyl and/or amino
and/or
amido and/or imido groups; and/or
(Di) moieties obtained by Mannich reaction of substituted phenols with
aldehydes and
mono- or polyamines.
The hydrophobic hydrocarbyl radical in the above detergent additives, which
ensures the
adequate solubility in the fuel, has a number-average molecular weight (Me) of
85 to 20 000,
preferably of 113 to 10 000, more preferably of 300 to 5000, even more
preferably of 300 to
3000, even more especially preferably of 500 to 2500 and especially of 700 to
2500, in
particular of 800 to 1500. Typical hydrophobic hydrocarbyl radicals especially
include
polypropenyl, polybutenyl and polyisobutenyl radicals with a number-average
molecular
weight Mr, of preferably in each case 300 to 5000, more preferably 300 to
3000, even more
preferably 500 to 2500, even more especially preferably 700 to 2500 and
especially 800 to
1500.
Examples of the above groups of detergent additives include the following:
Additives comprising mono- or polyamino groups (Da) are preferably
polyalkenemono- or
polyalkenepolyamines based on polypropene or on high-reactivity (i.e. having
predominantly
terminal double bonds) or conventional (i.e. having predominantly internal
double bonds)
polybutene or polyisobutene having Me = 300 to 5000, more preferably 500 to
2500 and
CA 02863698 2014-08-06
14
especially 700 to 2500. Such additives based on high-reactivity polyisobutene,
which can be -
prepared from the polyisobutene which may comprise up to 20% by weight of n-
butene units
by hydroformylation and reductive amination with ammonia, monoamines or
polyamines
such as dimethylaminopropylamine, ethylenediamine, diethylenetriamine,
triethylenetetramine or tetraethylenepentamine, are known especially from EP-A
244 616.
When polybutene or polyisobutene having predominantly internal double bonds
(usually in
the 13 and y positions) are used as starting materials in the preparation of
the additives, a
possible preparative route is by chlorination and subsequent amination or by
oxidation of the
double bond with air or ozone to give the carbonyl or carboxyl compound and
subsequent
amination under reductive (hydrogenating) conditions. For the amination, it is
possible here
to use amines such as ammonia, monoamines or the abovementioned polyamines.
Corresponding additives based on polypropene are described more particularly
in WO-A
94/24231.
1=5 Further particular additives comprising monoamino groups (Da) are the
hydrogenation
products of the reaction products of polyisobutenes having an average degree
of
polymerization P = 5 to 100 with nitrogen oxides or mixtures of nitrogen
oxides and oxygen,
as described more particularly in WO-A 97/03946.
Further particular additives comprising monoamino groups (Da) are the
compounds
obtainable from polyisobutene epoxides by reaction with amines and subsequent
dehydration and reduction of the amino alcohols, as described more
particularly in DE-A 196
20 262.
Additives comprising nitro groups (Db), optionally in combination with
hydroxyl groups, are
preferably reaction products of polyisobutenes having an average degree of
polymerization P
= 5 to 100 or 10 to 100 with nitrogen oxides or mixtures of nitrogen oxides
and oxygen, as
described more particularly in WO-A 96/03367 and in WO-A 96/03479. These
reaction
products are generally mixtures of pure nitropolyisobutenes (e.g. a,I3-
dinitropolyisobutene)
and mixed hydroxynitropolyisobutenes (e.g. a-nitro-P-hydroxypolyisobutene).
Additives comprising hydroxyl groups in combination with mono- or polyamino
groups (Dc)
are especially reaction products of polyisobutene epoxides obtainable from
polyisobutene
having preferably predominantly terminal double bonds and Mr, = 300 to 5000,
with ammonia
or mono- or polyamines, as described more particularly in EP-A 476 485.
Additives comprising carboxyl groups or their alkali metal or alkaline earth
metal salts (Dd)
are preferably copolymers of C2- to Cao-olefins with maleic anhydride which
have a total
CA 02863698 2014-08-06
molar mass of 500 to 20 000 and some or all of whose carboxyl groups have been
converted
to the alkali metal or alkaline earth metal salts and any remainder of the
carboxyl groups has
been reacted with alcohols or amines. Such additives are disclosed more
particularly by EP-
A 307 815. Such additives serve mainly to prevent valve seat wear and can, as
described in
5 WO-A 87/01126, advantageously be used in combination with customary fuel
detergents
such as poly(iso)buteneamines or polyetheramines.
Additives comprising sulfonic acid groups or their alkali metal or alkaline
earth metal salts
(De) are preferably alkali metal or alkaline earth metal salts of an alkyl
sulfosuccinate, as
10 described more particularly in EP-A 639 632. Such additives serve mainly
to prevent valve
seat wear and can be used advantageously in combination with customary fuel
detergents
such as poly(iso)buteneamines or polyetheramines.
Additives comprising polyoxy-C2-C4-alkylene moieties (Df) are preferably
polyethers or
15 polyetheramines which are obtainable by reaction of C2- to C60-alkanols,
06- to 030'
alkanediols, mono- or di-C2- to C30-alkylamines, to C30-alkylcyclohexanols
or Ci- to C30"
alkylphenols with 1 to 30 mol of ethylene oxide and/or propylene oxide and/or
butylene oxide
per hydroxyl group or amino group and, in the case of the polyetheramines, by
subsequent -
reductive amination with ammonia, monoamines or polyamines. Such products are
described
more particularly in EP-A 310 875, EP-A 356 725, EP-A 700 985 and US-A 4 877
416. In the
case of polyethers, such products also have carrier oil properties. Typical
examples thereof
are tridecanol butoxylates or isotridecanol butoxylates, isononylphenol
butoxylates and also
polyisobutenol butoxylates and propoxylates, and also the corresponding
reaction products
with ammonia.
Additives comprising carboxylic ester groups (Dg) are preferably esters of
mono-, di- or
tricarboxylic acids with long-chain alkanols or polyols, especially those
having a minimum
viscosity of 2 mm2/s at 100 C, as described more particularly in DE-A 38 38
918. The mono-,
di- or tricarboxylic acids used may be aliphatic or aromatic acids, and
particularly suitable
ester alcohols or ester polyols are long-chain representatives having, for
example, 6 to 24
carbon atoms. Typical representatives of the esters are adipates, phthalates,
isophthalates,
terephthalates and trimellitates of isooctanol, of isononanol, of isodecanol
and of
isotridecanol. Such products also satisfy carrier oil properties.
Additives comprising moieties derived from succinic anhydride and having
hydroxyl and/or
amino and/or amido and/or especially imido groups (Dh) are preferably
corresponding
derivatives of alkyl- or alkenyl-substituted succinic anhydride and especially
the
corresponding derivatives of polyisobutenylsuccinic anhydride which are
obtainable by
CA 02863698 2014-08-06
16
reacting conventional or high-reactivity polyisobutene having M,, = preferably
300 to 5000,
more preferably 300 to 3000, even more preferably 500 to 2500, even more
especially
preferably 700 to 2500 and especially 800 to 1500, with maleic anhydride by a
thermal route
in an ene reaction or via the chlorinated polyisobutene. The moieties having
hydroxyl and/or
amino and/or amido and/or imido groups are, for example, carboxylic acid
groups, acid
amides of monoamines, acid amides of di- or polyamines which, in addition to
the amide
function, also have free amine groups, succinic acid derivatives having an
acid and an amide
function, carboximides with monoamines, carboximides with di- or polyamines
which, in
addition to the imide function, also have free amine groups, or diimides which
are formed by
the reaction of di- or polyamines with two succinic acid derivatives. Such
fuel additives are
described more particularly in US-A 4 849 572. They are preferably the
reaction products of
alkyl- or alkenyl-substituted succinic acids or derivatives thereof with
amines and more
preferably the reaction products of polyisobutenyl-substituted succinic acids
or derivatives
thereof with amines. Of particular interest in this context are reaction
products with aliphatic
polyamines (polyalkyleneimines) such as especially ethylenediamine,
diethylenetriamine,
triethylenetetramine, tetraethylenepentamine, pentaethylenehexamine and
hexaethyleneheptamine, which have an imide structure.
Additives comprising moieties (Di) obtained by Mannich reaction of substituted
phenols with
aldehydes and mono- or polyamines are preferably reaction products of
polyisobutene-
substituted phenols with formaldehyde and mono- or polyamines such as
ethylenediamine,
diethylenetriamine, triethylenetetramine, tetraethylenepentamine or
dimethylaminopropylamine. The polyisobutenyl-substituted phenols may stem from
conventional or high-reactivity polyisobutene having Mn = 300 to 5000. Such
"polyisobutene
Mannich bases" are described more particularly in EP-A 831 141.
One or more of the detergent additives from groups (Da) to (Di) mentioned can
be added to
the fuel in such an amount that the dosage of these detergent additives is
preferably 25 to
2500 ppm by weight, especially 75 to 1500 ppm by weight, in particular 150 to
1000 ppm by
weight.
Carrier oils additionally used as a coadditive may be of mineral or synthetic
nature. Suitable
mineral carrier oils are fractions obtained in crude oil processing, such as
brightstock or base
oils having viscosities, for example, from the SN 500 - 2000 class; but also
aromatic
hydrocarbons, paraffinic hydrocarbons and alkoxyalkanols. Likewise useful is a
fraction
which is obtained in the refining of mineral oil and is known as "hydrocrack
oil" (vacuum
distillate cut having a boiling range of from about 360 to 500 C, obtainable
from natural
mineral oil which has been catalytically hydrogenated under high pressure and
isomerized
CA 02863698 2014-08-06
17
and also deparaffinized). Likewise suitable are mixtures of the abovementioned
mineral
carrier oils.
Examples of suitable synthetic carrier oils are polyolefins (polyalphaolefins
or
polyinternalolefins), (poly)esters, (poly)alkoxylates, polyethers, aliphatic
polyether-amines,
alkylphenol-started polyethers, alkylphenol-started polyetheramines and
carboxylic esters of
long-chain alkanols.
Examples of suitable polyolefins are olefin polymers having Mn = 400 to 1800,
in particular
based on polybutene or polyisobutene (hydrogenated or unhydrogenated).
Examples of suitable polyethers or polyetheramines are preferably compounds
comprising
polyoxy-C2- to C4-alkylene moieties which are obtainable by reacting C2- to
C60-alkanols, C6-
to C30-alkanediols, mono- or di-C2- to C30-alkylamines, Cl- to C30-
alkylcyclohexanols or Ci- to
C30-alkylphenols with 1 to 30 mol of ethylene oxide and/or propylene oxide
and/or butylene
oxide per hydroxyl group or amino group, and, in the case of the
polyetheramines, by
subsequent reductive amination with ammonia, monoamines or polyamines. Such
products
are described more particularly in EP-A 310 875, EP-A 356 725, EP-A 700 985
and US-A
4,877,416. For example, the polyetheramines used may be poly-C2- to C6-
alkylene oxide
amines or functional derivatives thereof. Typical examples thereof are
tridecanol butoxylates
or isotridecanol butoxylates, isononylphenol butoxylates and also
polyisobutenol butoxylates
and propoxylates, and also the corresponding reaction products with ammonia.
Examples of carboxylic esters of long-chain alkanols are more particularly
esters of mono-,
di- or tricarboxylic acids with long-chain alkanols or polyols, as described
more particularly in
DE-A 38 38 918. The mono-, di- or tricarboxylic acids used may be aliphatic or
aromatic
acids; particularly suitable ester alcohols or ester polyols are long-chain
representatives
having, for example, 6 to 24 carbon atoms. Typical representatives of the
esters are
adipates, phthalates, isophthalates, terephthalates and trimellitates of
isooctanol, isononanol,
isodecanol and isotridecanol, for example di(n- or isotridecyl) phthalate.
Further suitable carrier oil systems are described, for example, in DE-A 38 26
608, DE-A 41
42 241, DE-A 43 09 074, EP-A 452 328 and EP-A 548 617.
Examples of particularly suitable synthetic carrier oils are alcohol-started
polyethers having
about 5 to 35, preferably about 5 to 30, more preferably 10 to 30 and
especially 15 to 30 C3-
to C6-alkylene oxide units, for example propylene oxide, n-butylene oxide and
isobutylene
oxide units, or mixtures thereof, per alcohol molecule. Nonlimiting examples
of suitable
CA 02863698 2014-08-06
18
starter alcohols are long-chain alkanols or phenols substituted by long-chain
alkyl in which
the long-chain alkyl radical is especially a straight-chain or branched 06- to
Cis-alkyl radical.
Particular examples include tridecanol and nonylphenol. Particularly preferred
alcohol-started
polyethers are the reaction products (polyetherification products) of
monohydric aliphatic 06-
to GIs-alcohols with 03- to C6-alkylene oxides. Examples of monohydric
aliphatic C6-C18-
alcohols are hexanol, heptanol, octanol, 2-ethylhexanol, nonyl alcohol,
decanol, 2-
propylheptanol, undecanol, dodecanol, tridecanol, tetradecanol, pentadecanol,
hexadecanol,
octadecanol and the constitutional and positional isomers thereof. The
alcohols can be used
either in the form of the pure isomers or in the form of technical grade
mixtures. A particularly
preferred alcohol is tridecanol. Examples of 03- to C6-alkylene oxides are
propylene oxide,
such as 1,2-propylene oxide, butylene oxide, such as 1,2-butylene oxide, 2,3-
butylene oxide,
isobutylene oxide or tetrahydrofuran, pentylene oxide and hexylene oxide.
Particular
preference among these is given to 03- to C4-alkylene oxides, i.e. propylene
oxide such as
1,2-propylene oxide and butylene oxide such as 1,2-butylene oxide, 2,3-
butylene oxide and
isobutylene oxide. Especially butylene oxide is used.
Further suitable synthetic carrier oils are alkoxylated alkylphenols, as
described in DE-A 10
102 913.
Particular carrier oils are synthetic carrier oils, particular preference
being given to the above-
described alcohol-started polyethers.
The carrier oil or the mixture of different carrier oils is added to the fuel
in an amount of
preferably 1 to 1000 ppm by weight, more preferably of 10 to 500 ppm by weight
and
especially of 20 to 100 ppm by weight.
Cold flow improvers suitable as coadditives are in principle all organic
compounds which are
capable of improving the flow performance of middle distillate fuels or diesel
fuels under cold
conditions. For the intended purpose, they must have sufficient oil
solubility. More
particularly, useful cold flow improvers for this purpose are the cold flow
improvers (middle
distillate flow improvers, MDFIs) typically used in the case of middle
distillates of fossil origin,
i.e. in the case of customary mineral diesel fuels. However, it is also
possible to use organic
compounds which partly or predominantly have the properties of a wax
antisettling additive
(WASA) when used in customary diesel fuels. The imidazolium salts (I) used in
accordance
with the invention, in middle distillate fuels, especially in diesel fuels,
themselves have
properties as WASAs, which is of course also subject matter of the present
invention.
Coadditives used as cold flow improvers can also act partly or predominantly
as nucleators.
CA 02863698 2014-08-06
19
It is also possible to use mixtures of organic compounds effective as MDFIs
and/or effective
as WASAs and/or effective as nucleators.
The cold flow improver is typically selected from
(K1) copolymers of a C2- to C40-olefin with at least one further ethylenically
unsaturated
monomer;
(K2) comb polymers;
(K3) polyoxyalkylenes;
(K4) polar nitrogen compounds;
(K5) sulfocarboxylic acids or sulfonic acids or derivatives thereof; and
(K6) poly(meth)acrylic esters.
It is possible to use either mixtures of different representatives from one of
the particular
classes (K1) to (K6) or mixtures of representatives from different classes
(K1) to (K6).
Suitable C2- to Cacrolefin monomers for the copolymers of class (K1) are, for
example, those
having 2 to 20 and especially 2 to 10 carbon atoms, and 1 to 3 and preferably
1 or 2 carbon-
carbon double bonds, especially having one carbon-carbon double bond. In the
latter case,
the carbon-carbon double bond may be arranged either terminally (a-olefins) or
internally.
However, preference is given to a-olefins, particular preference to a-olefins
having 2 to 6
carbon atoms, for example propene, 1-butene, 1-pentene, 1-hexene and in
particular
ethylene.
In the copolymers of class (K1), the at least one further ethylenically
unsaturated monomer is
preferably selected from alkenyl carboxylates, (meth)acrylic esters and
further olefins.
When further olefins are also copolymerized, they are preferably higher in
molecular weight
than the abovementioned C2- to C40-olefin base monomer. When, for example, the
olefin
base monomer used is ethylene or propene, suitable further olefins are
especially C10- to C40-
a-olefins. Further olefins are in most cases only additionally copolymerized
when monomers
with carboxylic ester functions are also used.
Suitable (meth)acrylic esters are, for example, esters of (meth)acrylic acid
with C1- to 020-
alkanols, especially to Curalkanols, in particular with methanol, ethanol,
propanol,
isopropanol, n-butanol, sec-butanol, isobutanol, tert-butanol, pentanol,
hexanol, heptanol,
octanol, 2-ethylhexanol, nonanol and decanol, and structural isomers thereof.
CA 02863698 2014-08-06
Suitable alkenyl carboxylates are, for example, 02- to 014-alkenyl esters, for
example the
vinyl and propenyl esters, of carboxylic acids having 2 to 21 carbon atoms,
whose
hydrocarbyl radical may be linear or branched. Among these, preference is
given to the vinyl
esters. Among the carboxylic acids with a branched hydrocarbyl radical,
preference is given
5 to those whose branch is in the a position to the carboxyl group, and the
a-carbon atom is
more preferably tertiary, i.e. the carboxylic acid is what is called a
neocarboxylic acid.
However, the hydrocarbyl radical of the carboxylic acid is preferably linear.
Examples of suitable alkenyl carboxylates are vinyl acetate, vinyl propionate,
vinyl butyrate,
10 vinyl 2-ethylhexanoate, vinyl neopentanoate, vinyl hexanoate, vinyl
neononanoate, vinyl
neodecanoate and the corresponding propenyl esters, preference being given to
the vinyl
esters. A particularly preferred alkenyl carboxylate is vinyl acetate; typical
copolymers of
group (K1) resulting therefrom are ethylene-vinyl acetate copolymers ("EVAs"),
which are
some of the most frequently used.
15 Ethylene-vinyl acetate copolymers usable particularly advantageously and
the preparation
thereof are described in WO 99/29748.
Suitable copolymers of class (K1) are also those which comprise two or more
different
alkenyl carboxylates in copolymerized form, which differ in the alkenyl
function and/or in the
20 carboxylic acid group. Likewise suitable are copolymers which, as well
as the alkenyl
carboxylate(s), comprise at least one olefin and/or at least one (meth)acrylic
ester in
copolymerized form.
Terpolymers of a 02- to 040-a-olefin, a 01- to 020-alkyl ester of an
ethylenically unsaturated
monocarboxylic acid having 3 to 15 carbon atoms and a 02- to 014-alkenyl ester
of a
saturated monocarboxylic acid having 2 to 21 carbon atoms are also suitable as
copolymers
of class (K1). Terpolymers of this kind are described in WO 2005/054314. A
typical
terpolymer of this kind is formed from ethylene, 2-ethylhexyl acrylate and
vinyl acetate.
The at least one or the further ethylenically unsaturated monomer(s) are
copolymerized in
the copolymers of class (K1) in an amount of preferably 1 to 50% by weight,
especially 10 to
45% by weight and in particular 20 to 40% by weight, based on the overall
copolymer. The
main proportion in terms of weight of the monomer units in the copolymers of
class (K1)
therefore originates generally from the 02- to 040 base olefins.
The copolymers of class (K1) preferably have a number-average molecular weight
Mr, of
1000 to 20 000, more preferably of 1000 to 10 000 and especially of 1000 to
8000.
CA 02863698 2014-08-06
21
Typical comb polymers of component (K2) are, for example, obtainable by the
copolymerization of maleic anhydride or fumaric acid with another
ethylenically unsaturated
monomer, for example with an a-olefin or an unsaturated ester, such as vinyl
acetate, and
subsequent esterification of the anhydride or acid function with an alcohol
having at least 10
carbon atoms. Further suitable comb polymers are copolymers of a-olefins and
esterified
comonomers, for example esterified copolymers of styrene and maleic anhydride
or
esterified copolymers of styrene and fumaric acid. Suitable comb polymers may
also be
polyfumarates or polymaleates. Homo- and copolymers of vinyl ethers are also
suitable
comb polymers. Comb polymers suitable as components of class (K2) are, for
example, also
those described in WO 2004/035715 and in "Comb-Like Polymers. Structure and
Properties",
N. A. Plate and V. P. Shibaev, J. Poly. Sci. Macromolecular Revs. 8, pages 117
to 253
(1974). Mixtures of comb polymers are also suitable.
Polyoxyalkylenes suitable as components of class (K3) are, for example,
polyoxyalkylene
esters, polyoxyalkylene ethers, mixed polyoxyalkylene ester/ethers and
mixtures thereof.
These polyoxyalkylene compounds preferably comprise at least one linear alkyl
group,
preferably at least two linear alkyl groups, each having 10 to 30 carbon atoms
and a
polyoxyalkylene group having a number-average molecular weight of up to 5000.
Such
polyoxyalkylene compounds are described, for example, in EP A 061 895 and also
in US 4
491 455. Particular polyoxyalkylene compounds are based on polyethylene
glycols and
polypropylene glycols having a number-average molecular weight of 100 to 5000.
Additionally suitable are polyoxyalkylene mono- and diesters of fatty acids
having 10 to 30
carbon atoms, such as stearic acid or behenic acid.
Polar nitrogen compounds suitable as components of class (K4) may be either
ionic or
nonionic and preferably have at least one substituent, especially at least two
substituents, in
the form of a tertiary nitrogen atom of the general formula >NR7 in which R7
is a C8- to C40-
hydrocarbyl radical. The nitrogen substituents may also be quaternized, i.e.
be in cationic
form. An example of such nitrogen compounds is that of ammonium salts and/or
amides
which are obtainable by the reaction of at least one amine substituted by at
least one
hydrocarbyl radical with a carboxylic acid having 1 to 4 carboxyl groups or
with a suitable
derivative thereof. The amines preferably comprise at least one linear 08- to
C40-alkyl radical.
Primary amines suitable for preparing the polar nitrogen compounds mentioned
are, for
example, octylamine, nonylamine, decylamine, undecylamine, dodecylamine,
tetradecylamine and the higher linear homologs; secondary amines suitable for
this purpose
are, for example, dioctadecylamine and methylbehenylamine. Also suitable for
this purpose
are amine mixtures, especially amine mixtures obtainable on the industrial
scale, such as
fatty amines or hydrogenated tallamines, as described, for example, in
Ullmann's
CA 02863698 2014-08-06
22
Encyclopedia of Industrial Chemistry, 6th Edition, "Amines, aliphatic"
chapter. Acids suitable
for the reaction are, for example, cyclohexane-1,2-dicarboxylic acid,
cyclohexene-1,2-
dicarboxylic acid, cyclopentane-1,2-dicarboxylic acid, naphthalenedicarboxylic
acid, phthalic
acid, isophthalic acid, terephthalic acid, and succinic acids substituted by
long-chain
hydrocarbyl radicals.
More particularly, the component of class (K4) is an oil-soluble reaction
product of poly(C2- to
C20-carboxylic acids) having at least one tertiary amino group with primary or
secondary
amines. The poly(C2- to C20-carboxylic acids) which have at least one tertiary
amino group
and form the basis of this reaction product comprise preferably at least 3
carboxyl groups,
especially 3 to 12 and in particular 3 to 5 carboxyl groups. The carboxylic
acid units in the
polycarboxylic acids have preferably 2 to 10 carbon atoms, and are especially
acetic acid.
units. The carboxylic acid units are suitably bonded to the polycarboxylic
acids, usually via
one or more carbon and/or nitrogen atoms. They are preferably attached to
tertiary nitrogen
atoms which, in the case of a plurality of nitrogen atoms, are bonded via
hydrocarbon chains.
The component of class (K4) is preferably an oil-soluble reaction product
based on poly(C2-
to C20-carboxylic acids) which have at least one tertiary amino group and are
of the general
formula Ha or Ilb
HOOC,B B,COOH
HOOC,B,N,A,N, COOH
B,
(11a)
HOOCB N BCOON
e'COOH (11b)
in which the variable A is a straight-chain or branched C2- to C6-alkylene
group or the moiety
of the formula III
HOOCB ,CH2-C H2-
õN
CH2-C H2-
(111)
and the variable B is a Cl- to Ci9-alkylene group. The compounds of the
general formulae Ila
and 1lb especially have the properties of a WASA.
CA 02863698 2014-08-06
23
Moreover, the preferred oil-soluble reaction product of component (K4),
especially that of the
general formula ha or Ilb, is an amide, an amide-ammonium salt or an ammonium
salt in
which no, one or more carboxylic acid groups have been converted to amide
groups.
Straight-chain or branched 02- to C6-alkylene groups of the variable A are,
for example, 1,1-
ethylene, 1,2-propylene, 1,3-propylene, 1,2-butylene, 1,3-butylene, 1,4-
butylene, 2-methyl-
1,3-propylene, 1,5-pentylene, 2-methyl-1,4-butylene, 2,2-dimethy1-1,3-
propylene, 1,6-
hexylene (hexamethylene) and especially 1,2-ethylene. The variable A comprises
preferably
2 to 4 and especially 2 or 3 carbon atoms.
to Ci9-alkylene groups of the variable B are, for example, 1,2-ethylene, 1,3-
propylene,
1,4-butylene, hexamethylene, octamethylene, decamethylene, dodecamethylene,
tetradecamethylene, hexadecamethylene, octadecamethylene, nonadecamethylene
and
especially methylene. The variable B comprises preferably 1 to 10 and
especially 1 to 4
carbon atoms.
The primary and secondary amines as a reaction partner for the polycarboxylic
acids to form
component (K4) are typically monoamines, especially aliphatic monoamines.
These primary
and secondary amines may be selected from a multitude of amines which bear
hydrocarbyl
radicals which may optionally be bonded to one another.
These parent amines of the oil-soluble reaction products of component (K4) are
usually
secondary amines and have the general formula HN(R8)2 in which the two
variables R8 are
each independently straight-chain or branched Cm- to C30-alkyl radicals,
especially 014- to
C24-alkyl radicals. These relatively long-chain alkyl radicals are preferably
straight-chain or
only slightly branched. In general, the secondary amines mentioned, with
regard to their
relatively long-chain alkyl radicals, derive from naturally occurring fatty
acids and from
derivatives thereof. The two R8 radicals are preferably the same.
The secondary amines mentioned may be bonded to the polycarboxylic acids by
means of
amide structures or in the form of the ammonium salts; it is also possible for
only a portion to
be present as amide structures and another portion as ammonium salts.
Preferably only few,
if any, free acid groups are present. The oil-soluble reaction products of
component (K4) are
preferably present completely in the form of the amide structures.
CA 02863698 2014-08-06
24
Typical examples of such components (K4) are reaction products of
nitrilotriacetic acid, of
ethylenediaminetetraacetic acid or of propylene-1,2-diaminetetraacetic acid
with in each case
0.5 to 1.5 mol per carboxyl group, especially 0.8 to 1.2 mol per carboxyl
group, of
dioleylamine, dipalmitamine, dicocoamine, distearylamine, dibehenylamine or
especially
ditallamine. A particularly preferred component (K4) is the reaction product
of 1 mol of
ethylenediaminetetraacetic acid and 4 mol of hydrogenated ditallamine.
Further typical examples of component (K4) include the N,N-dialkylammonium
salts of 2-
N',N'-dialkylamidobenzoates, for example the reaction product of 1 mol of
phthalic anhydride
and 2 mol of ditallamine, the latter being hydrogenated or unhydrogenated, and
the reaction
product of 1 mol of an alkenylspirobislactone with 2 mol of a dialkylamine,
for example
ditallamine and/or tallamine, the latter two being hydrogenated or
unhydrogenated.
Further typical structure types for the component of class (K4) are cyclic
compounds with
tertiary amino groups or condensates of long-chain primary or secondary amines
with
carboxylic acid-containing polymers, as described in WO 93/18115.
Sulfocarboxylic acids, sulfonic acids or derivatives thereof which are
suitable as cold flow
improvers of the component of class (K5) are, for example, the oil-soluble
carboxamides and
carboxylic esters of ortho-sulfobenzoic acid, in which the sulfonic acid
function is present as
a sulfonate with alkyl-substituted ammonium cations, as described in EP-A 261
957.
Poly(meth)acrylic esters suitable as cold flow improvers of the component of
class (K6) are
either homo- or copolymers of acrylic and methacrylic esters. Preference is
given to
copolymers of at least two different (meth)acrylic esters which differ with
regard to the
esterified alcohol. The copolymer optionally comprises another different
olefinically
unsaturated monomer in copolymerized form. The weight-average molecular weight
of the
polymer is preferably 50 000 to 500 000. A particularly preferred polymer is a
copolymer of
methacrylic acid and methacrylic esters of saturated C14- and C15-alcohols,
the acid groups
having been neutralized with hydrogenated tallamine. Suitable
poly(meth)acrylic esters are
described, for example, in WO 00/44857.
The cold flow improver or the mixture of different cold flow improvers is
added to the middle
distillate fuel or diesel fuel in a total amount of preferably 10 to 5000 ppm
by weight, more
preferably of 20 to 2000 ppm by weight, even more preferably of 50 to 1000 ppm
by weight
and especially of 100 to 700 ppm by weight, for example of 200 to 500 ppm by
weight.
CA 02863698 2014-08-06
Lubricity improvers or friction modifiers suitable as coadditives are based
typically on fatty
acids or fatty acid esters. Typical examples are tall oil fatty acid, as
described, for example,
in WO 98/004656, and glyceryl monooleate. The reaction products, described in
US 6 743
266 B2, of natural or synthetic oils, for example triglycerides, and
alkanolamines are also
5 suitable as such lubricity improvers.
Corrosion inhibitors suitable as coadditives are, for example, succinic
esters, in particular
with polyols, fatty acid derivatives, for example oleic esters, oligomerized
fatty acids,
substituted ethanolamines, N-acylated sarcosine, imidazoline derivatives, for
example those
10 which bear an alkyl group in the 2 position and a functional organic
radical on the trivalent
nitrogen atom (a typical imidazoline derivative of this kind is the reaction
product of excess
oleic acid with diethylenetriamine), and products which are sold under the
trade names RC
4801 (Rhein Chemie Mannheim, Germany) or HiTEC 536 (Ethyl Corporation). The
imidazoline derivatives mentioned are particularly effective as corrosion
inhibitors when they
15 are combined in this application with one or more carboxamides having
one or more
= carboxamide functions in the molecule and having relatively long-chain
radicals on the amide
nitrogens, for example with the reaction product of maleic anhydride with a
long-chain amine
in an equimolar ratio.
20 Demulsifiers suitable as coadditives are, for example, the alkali metal
or alkaline earth metal
salts of alkyl-substituted phenol- and naphthalenesulfonates and the alkali
metal or alkaline
earth metal salts of fatty acids, and also neutral compounds such as alcohol
alkoxylates, e.g.
alcohol ethoxylates, phenol alkoxylates, e.g. tert-butylphenol ethoxylate or
tert-pentylphenol
ethoxylate, fatty acids, alkylphenols, condensation products of ethylene oxide
(E0) and
25 propylene oxide (PO), for example including in the form of E0/P0 block
copolymers,
polyethyleneimines or else polysiloxanes.
Dehazers suitable as coadditives are, for example, alkoxylated phenol-
formaldehyde
condensates, for example the products available under the trade names NALCO
7007
(Nalco) and TOLAD 2683 (Petrolite).
Antifoams suitable as coadditives are, for example, polyether-modified
polysiloxanes, for
example the products available under the trade names TEGOPREN 5851
(Goldschmidt), Q
25907 (Dow Corning) and RHODOSIL (Rhone Poulenc).
Cetane number improvers suitable as coadditives are, for example, aliphatic
nitrates such as
2-ethylhexyl nitrate and cyclohexyl nitrate and peroxides such as di-tert-
butyl peroxide.
CA 02863698 2014-08-06
=
26
Antioxidants suitable as coadditives are, for example, substituted, i.e.
sterically hindered
phenols, such as 2,6-di-tert-butylphenol, 2,6-di-tert-butyl-3-methylphenol or
products sold
under the IRGANOXO (BASF SE) trade name, for example 2,6-di-tert-buty1-4-
alkoxycarbonylethylphenol (IRGANOX L135), and also phenylenediamines such as
N,N'-di-
sec-butyl-p-phenylenediamine.
Metal deactivators suitable as coadditives are, for example, salicylic acid
derivatives such as
N,N'-disalicylidene-1,2-propanediamine or products sold under the IRGAMETO
(BASF SE)
trade name, based on N-substituted triazoles and tolutriazoles.
Suitable solvents to be used in addition are, for example, nonpolar organic
solvents such as
aromatic and aliphatic hydrocarbons, for example toluene, xylenes, white
spirit and products
which are sold under the SHELLSOL (Royal Dutch/Shell Group) and EXXSOL
(ExxonMobil)
trade names, and also polar organic solvents, for example alcohols such as 2-
ethylhexanol,
decanol and isotridecanol, and carboxylic esters with relatively long-chain
alkyl groups, such
as C12- to C20-fatty acid methyl ester. Such solvents are usually added to the
fuel, especially
the diesel fuel, together with the imidazolium salts (I) and the
aforementioned coadditives,
which they are intended to dissolve or dilute for better handling.
The imidazolium salts (I) for use in accordance with the invention are
outstandingly suitable
as a fuel additive and can in principle be used in any fuels. They bring about
a whole series
of advantageous effects in the operation of internal combustion engines with
fuels. The
imidazolium salts (I) for use in accordance with the invention are preferably
used in middle
distillate fuels, especially diesel fuels.
The present invention therefore also provides a fuel composition, especially a
middle
distillate fuel composition, with a content of the imidazolium salts (I) to be
used in accordance
with the invention which is effective as an additive for achieving
advantageous effects in the
operation of internal combustion engines, for example of diesel engines,
especially of direct
injection diesel engines, in particular of diesel engines with common rail
injection systems,
alongside the majority of a customary base fuel. This effective content
(dosage) is generally
10 to 5000 ppm by weight, preferably 20 to 1500 ppm by weight, especially 25
to 1000 ppm
by weight, in particular 30 to 750 ppm by weight, based in each case on the
total amount of
fuel.
Middle distillate fuels such as diesel fuels or heating oils are preferably
mineral oil raffinates
which typically have a boiling range from 100 to 400 C. These are usually
distillates having a
95% point up to 360 C or even higher. These may also be what is called "ultra
low sulfur
CA 02863698 2014-08-06
27
diesel" or "city diesel", characterized by a 95% point of, for example, not
more than 345 C
and a sulfur content of not more than 0.005% by weight or by a 95% point of,
for example,
285 C and a sulfur content of not more than 0.001% by weight. In addition to
the mineral
middle distillate fuels or diesel fuels obtainable by refining, those
obtainable by coal
gasification or gas liquefaction ["gas to liquid" (GTL) fuels] or by biomass
liquefaction
["biomass to liquid" (BTL) fuels] are also suitable. Also suitable are
mixtures of the
aforementioned middle distillate fuels or diesel fuels with renewable fuels,
such as biodiesel
or bioethanol.
The qualities of the heating oils and diesel fuels are laid down in detail,
for example, in DIN
51603 and EN 590 (cf. also Ullmann's Encyclopedia of Industrial Chemistry, 5th
edition,
Volume Al2, p. 617 ff.).
In addition to the use thereof in the abovementioned middle distillate fuels
of fossil, vegetable
or animal origin, which are essentially hydrocarbon mixtures, the imidazolium
salts (I) for use
in accordance with the invention can also be used in mixtures of such middle
distillates with
biofuel oils (biodiesel). Such mixtures are also encompassed by the term
"middle distillate
fuel" in the context of the present invention. They are commercially available
and usually
comprise the biofuel oils in minor amounts, typically in amounts of 1 to 30%
by weight,
especially of 3 to 10% by weight, based on the total amount of middle
distillate of fossil,
vegetable or animal origin and biofuel oil.
Biofuel oils are generally based on fatty acid esters, usually essentially on
alkyl esters of
fatty acids which derive from vegetable and/or animal oils and/or fats. Alkyl
esters are
typically understood to mean lower alkyl esters, especially C1-C4-alkyl
esters, which are
obtainable by transesterifying the glycerides which occur in vegetable and/or
animal oils
and/or fats, especially triglycerides, by means of lower alcohols, for example
ethanol or in
particular methanol ("FAME"). Typical lower alkyl esters based on vegetable
and/or animal
oils and/or fats, which find use as a biofuel oil or components thereof, are,
for example,
sunflower methyl ester, palm oil methyl ester ("PME"), soya oil methyl ester
("SME") and
especially rapeseed oil methyl ester ("RME").
The middle distillate fuels or diesel fuels are more preferably those having a
low sulfur
content, i.e. having a sulfur content of less than 0.05% by weight, preferably
of less than
0.02% by weight, more particularly of less than 0.005% by weight and
especially of less than
0.001% by weight of sulfur.
CA 02863698 2014-08-06
28
Useful gasoline fuels include all commercial gasoline fuel compositions. One
typical
representative which shall be mentioned here is the Eurosuper base fuel to EN
228, which is
customary on the market. In addition, gasoline fuel compositions of the
specification
according to WO 00/47698 are also possible fields of use for the present
invention.
The present invention also provides an additive concentrate which, in
combination with at
least one further fuel additive, especially with at least one further diesel
fuel additive,
comprises at least one imidazolium salt (I) for use in accordance with the
invention. Typically,
such an additive concentrate comprises 10 to 60% by weight of at least one
solvent or
diluent, which may be an abovementioned solvent or the fuel itself. The
inventive additive
concentrate preferably comprises, as well as the at least one imidazolium salt
(I) for use in
accordance with the invention, at least one detergent additive from the
abovementioned
group (Da) to (Di), especially at least one detergent additive of the (Dh)
type, and generally
additionally also at least one lubricity improver and/or a corrosion inhibitor
and/or a
demulsifier and/or a dehazer and/or an antifoam and/or a cetane number
improver and/or an
antioxidant and/or a metal deactivator, in the relative amounts customary
therefor in each
case.
The imidazolium salts (I) for use in accordance with the invention are
especially suitable as
an additive in fuel compositions, especially in diesel fuels, for overcoming
the problems
outlined at the outset in direct injection diesel engines, in particular in
those with common rail
injection systems.
Since some of the imidazolium salts described are novel substances, the
present invention
likewise provides imidazolium salts of the general formula (la)
R5
R4
Xn-
N2-1(
R3 R2
n
(la)
in which
one of the variables R1 and R3 is or both variables R1 and R3 are
independently a linear
alkyl or alkenyl radical having 14 to 3000 carbon atoms or a branched alkyl or
alkenyl radical
having 4 to 3000 carbon atoms,
CA 02863698 2014-08-06
29
the variable R1 or R3 which is not a linear alkyl or alkenyl radical having 14
to 3000 carbon
atoms or a branched alkyl or alkenyl radical having 4 to 3000 carbon atoms is
an alkyl radical
having 1 to 13 carbon atoms or an alkenyl radical having 2 to 13 carbon atoms,
the variables R2, R4 and R5 are each independently hydrogen, an alkyl radical
having 1 to
20 carbon atoms or an alkenyl radical having 2 to 20 carbon atoms,
X is an anion and
n is the number 1, 2 or 3,
where the said variables R1 to R5, X and n each have the abovementioned
relevant
individual definitions and preferred ranges.
Particularly preferred imidazolium salts of the general formula (la) are those
in which one of
the variables R1 and R3 is or both variables R1 and R3 are independently a
linear alkyl or
alkenyl radical having 14 to 20 carbon atoms or a branched alkyl or alkenyl
radical having 4
to 13 carbon atoms, and the variables R2, R4 and R5 are each independently
hydrogen, an
alkyl radical having 1 to 20 carbon atoms or an alkenyl radical having 2 to 20
carbon atoms.
Particularly preferred imidazolium salts of the general formula (la) are
additionally those in
which one of the variables R1 and R3 is or both variables R1 and R3 are
independently a
polyisobutyl radical having a number-average molecular weight of 200 to 40
000, and the
variables R2, R4 and R5 are each independently hydrogen, an alkyl radical
having 1 to 20
carbon atoms or an alkenyl radical having 2 to 20 carbon atoms.
The novel imidazolium salts of the general formula (la) are suitable, as well
as their possible
use as additives for fuels, especially as detergent additives for diesel
fuels, also for
improvement of the use properties of mineral and synthetic nonaqueous
industrial fluids.
Nonaqueous industrial fluids, which in individual cases may comprise water
components, but
the essential effect of which is based on nonaqueous components, shall be
understood here
to mean lubricants, lubricant compositions and lubricant oils in the widest
sense, especially
motor oils, transmission oils, axle oils, hydraulic fluids, hydraulic oils,
compressor fluids,
compressor oils, circulation oils, turbine oils, transformer oils, gas motor
oils, wind turbine
oils, slideway oils, lubricant greases, cooling lubricants, antiwear oils for
chains and conveyor
systems, metalworking fluids, food-compatible lubricants for the industrial
processing of
foods, and boiler oils for industrial cookers, sterilizers and steam peelers.
Use properties
CA 02863698 2014-08-06
which are improved by the imidazolium salts (la) are especially lubricity,
frictional wear,
lifetime, corrosion protection, antimicrobial protection, demulsification
capacity with regard to
easier removal of water and impurities, and filterability.
5 The invention is now illustrated in detail by the working examples which
follow:
Examples
Preparation of 1,3-di(2-ethylhexyl)imidazolium acetate
300.3 g (3.0 mol) of a 30% by weight aqueous formaldehyde solution, 435.3 g
(3.0 mol) of
glyoxal and 180.2 g (3.0 mol) of anhydrous acetic acid were initially charged
in a flask, and
791.3 g (6.0 mol) of 98% by weight 2-ethylhexylamine were gradually added
thereto at room
temperature, while stirring. In the course of this, the temperature of the
reaction mixture rose
rapidly to 38 C and was kept there by ice bath cooling until the addition of
the amine had
ended. This was followed by stirring at 80 C for 5 hours. After removing the
upper aqueous
phase, 1038.4 g of 1,3-(2-ethylhexyl)imidazolium acetate were obtained.
Preparation of 1,3-di(polyisobutyl)imidazolium acetate
Analogously to the above-described preparation of 1,3-di(2-
ethylhexyl)imidazolium acetate,
3.0 mol of 30% by weight aqueous formaldehyde solution, 3.0 mol of glyoxal,
3.0 mol of
anhydrous acetic acid and 6.0 mol of polyisobutylamine C4H9-(C4H8)x-CH2NH2
where x = 17-
18 (commercial product, Kerocom PIBA from BASF SE) were used to obtain 1,3-
di(polyisobutyl)imidazolium acetate.
Use examples
To study the influence of the additives on the performance of direct injection
diesel engines,
the test method used was the DW10 engine test, in which the power loss was
determined by
injector deposits in the common rail diesel engine, based on the official test
method CEO F-
098-08.
The power loss is a direct measure of formation of deposits in the injectors.
A direct injection diesel engine with common rail system according to test
method CEO F-
098-08 was used. The fuel used was a commercial diesel fuel from Haltermann
(DF-79-
07/5). To artificially induce the formation of deposits at the injectors, 1
ppm by weight of zinc
in the form of a zinc didodecanoate solution was added thereto. The results
illustrate the
CA 02863698 2014-08-06
31
relative power loss at 4000 rpm, measured during sustained operation over 12
hours. The
value "t0" indicates the power in kW at the start of the test, and the value
112" the power in
kW at the end of the test.
The following imidazolium salts were used as additives for use in
accordance with invention:
(1.1) 1-ethyl-3-methylimidazolium acetate
(1.2) 1-butyl-3-methylimidazolium acetate
(1.3) 1-octy1-3-methylimidazolium methylcarbonate
(1.4) 1,3-di(2-ethylhexyl)imidazolium acetate
Compounds (1.1) and (1.2) are commercial products; compound (1.3) was prepared
from N-
octylimidazole by quaternization with dimethyl carbonate as a 30% by weight
solution in
methanol by a customary synthesis method; compound (1.4) was prepared by the
synthesis
method specified above.
In the test runs performed, additives (1.1) and (1.2) were used as pure
substances and
additives (1.3) and (1.4) as solutions. The dosages specified are based on the
active
ingredient.
The results of the power or power loss determinations of the DW10 engine test
runs are
compiled in the following table:
Additive Dosage [ppm by tO [kW] t12 [kW] power loss [%]
wt.]
none 0 93.9 88.8 -5.4
(1.1) 100 _98.9 98.0 -0.9
(1.2) 100 97.1 97.0 -0.1
(1.2) 30 95.2 94.4 -0.8
(1.3) ..33 96.9 97.2 +0.3
(1.4) 50 95.8 95.1 -0.7
With additives (1.2) and (1.4), a soiling and cleaning run according to the
DW10 test was
additionally performed. For this purpose, the direct injection diesel engine
with a common rail
System used was first operated with the same commercial diesel fuel (with a
content of 1
ppm by weight of zinc in the form of a zinc didodecanoate solution) without
detergent additive
CA 02863698 2014-08-06
32
for 12 hours, in the course of which the value t for the power in the
experiment with (1.2) at
first fell gradually from 96.2 kW to 89.8 kW. After addition of 30 ppm by
weight of additive
(1.2) and further operation for 5 hours, the value t for the power rose again
to 95.7 kW, with
the greatest jump for t within the first two hours after addition of (1.2)
(after 1 hour t = 91.4
kW, after 2 hours t = 94.5 kW).
In experiment with additive (1.4), the power fell from 98.4 kW to 93.9 kW in
the first 13 hours
of operation without additive. After addition of 50 ppm by weight of additive
(1.4) and further
operation for 12 hours, the value t for the power rose again to 96.3 kW, with
the greatest
jump in power within the first two hours after addition of (1.4) (after
further lowering of the
value after the change of fuel to 92.8 kW, power rose after one hour back to t
= 94.5 kW,
then after 2 hours to t = 95.5 kW).
Additive (1.4) was also used to run a "keep clean" engine test according to
test method CEO
F-23-01 with the PSA XUD-9 A engine. The additive was used with a dosage of 50
ppm in a
commercial diesel fuel from Haltermann (DF-79-07/5). For comparison, the
engine was
operated in a separate test run with the same diesel fuel without additive.
The flow restriction
at 0.1 mm needle elevation in the fuel was 63% without additive, and -32% with
50 ppm by
weight of additive (1.4).