Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
USE OF MAGNESIUM COMPOUNDS IN THE TREATMENT
OF POTABLE WATER
TECHNICAL FIELD
[001] The present disclosure generally relates to potable water treatment, and
more
particularly relates to the use of magnesium compounds in the treatment of
potable water.
BACKGROUND
[002] Potable water treatment systems may typically be designed to take a raw
water
supply, whether from surface sources, such as rivers, lakes, reservoirs, or
from groundwater
sources, such as wells and aquifers, and process the raw water for
distribution and
consumption.
[0003] The processing may involve removing constituents from the water that
may
be harmful, and may also include removing other constituents that may impart
undesirable
color, taste, turbidity or odor. The removed constituents may be in the form
of dissolved
solids or gases, suspended solids or gases, miscible or immiscible liquids,
and may be
organic and/or inorganic in nature. These constituents are often measured as
Total Organic
Carbon ("TOC"), Total Dissolved Solids ("TDS"), Total Suspended Solids
("TSS"), and
Turbidity ("NTU"). In some situations, specific constituents such as minerals
may be
identified in more detail.
SUMMARY
[004] In an implementation, a method may include adding an effective amount of
a
magnesium compound to supply water to be treated. The method may also include
adding an
effective amount of a flocculation aiding metal salt to the supply water. The
method
1
Date Recue/Date Received 2021-05-31
CA 02867472 2014-10-17
may further include removing one or more contaminants from the supply water to
provide
treated water.
[005] One or more of the following features may be included. The magnesium
compound includes one or more of magnesium hydroxide and magnesium oxide. The
magnesium hydroxide may exhibit an alkaline magnesium hydroxide purity of
between
about 85% to about 100%. The magnesium hydroxide may exhibit a caustic
magnesia
activity of between about 50 seconds to about 1440 minutes. The magnesium
hydroxide
may exhibit a particle size of between about 0.1 micron to about 50 microns.
The
magnesium hydroxide may exhibit a specific surface area of between about 9
m2/g to about
200 m2/g. The magnesium hydroxide may exhibit a stabilized residuals test
value of
between about 1 milligram to about 50 grams. The magnesium oxide may exhibit
an
alkaline magnesium oxide purity of between about 85% to about 100%. The
magnesium
oxide may exhibit a caustic magnesia activity of between about 50 seconds to
about 1000
seconds. The magnesium oxide may exhibit a particle size of between about 0.1
micron to
about 30 microns. The magnesium oxide may exhibit a specific surface area of
between
about 9 m2/g to about 300 m2/g.
[006] The flocculation aiding metal salt may include one or more of alum,
ferrous
sulfate, ferric sulfate, ferrous chloride, and ferric chloride. Adding the
effective amount of
the magnesium compound may include adding the magnesium compound to the supply
water prior to adding the flocculation aiding metal salt. Adding the effective
amount of
the magnesium compound may include adding the magnesium compound generally
along
with adding the flocculation aiding metal salt.
[007] Adding the effective amount of the magnesium compound includes measuring
a quality of the treated water. The method may further include adjusting the
effective
amount of the magnesium compound based upon, at least in part, the measured
quality of
the treated water. The quality of the treated water may include one or more of
an alkalinity
of the treated water and a pH of the treated water. The quality of the treated
water may
include a corrosivity of the treated water as indicated by a Langelier Index
of the treated
water. The method may further include determining an anticipated alkalinity
depletion
associated with adding the flocculation aiding metal salt. The effective
amount of the
2
CA 02867472 2014-10-17
magnesium compound may be based upon, at least in part, amount offsetting at
least a
portion of the anticipated alkalinity depletion.
[008] The method may further include adding an effective amount of a polymer
contaminant removal aid including one or more of polyepichlorohydrin-
dimethylamine,
polyamine, poly-diallyl-dimethylammonium chloride (polyDADMAC), polyacrylate,
polyamide, a Mannich polymer, and polyacrylamide. Adding the magnesium
compound
may include adding an admixture of the magnesium compound and a alkaline earth
metal
compound. Removing one or more contaminants from the supply water may include
one
or more of flocculation, coagulation, sedimentation, and filtration. Removing
one or more
contaminants from the supply water may include filtration. The method may
further
include recycling a residual magnesium compound from a filter element and
recycling the
residual magnesium compound into the supply water.
[009] According to another implementation, a method may include treating
supply
water. Treating the supply water may include adding an effective amount of a
flocculation
aiding metal salt to the supply water and removing one or more contaminants
from the
supply water. Treating the supply water may provide treated potable water. The
method
may also include determining a corrosivity associated with the treated potable
water. The
method may further include adding an effective amount of a magnesium compound
to the
supply water based upon, at least in part, the determined corrosivity
associated with the
treated potable water to achieve a desired reduced corrosivity associated with
the treated
potable water.
[0010] One or more of the following features may be included. Determining the
corrosivity associated with the treated potable water may include determining
a Langelier's
Index associated with the treated potable water. Adding the effective amount
of the
magnesium compound may include adding the magnesium compound to the supply
water
prior to adding the flocculation aiding metal salt. Adding the effective
amount of the
magnesium compound may include adding the magnesium compound generally along
with
adding the flocculation aiding metal salt.
[0011] The magnesium compound may include magnesium hydroxide exhibiting an
alkaline magnesium hydroxide purity of between about 85% to about 100%. The
magnesium hydroxide may exhibit a caustic magnesia activity of between about
50 seconds
3
CA 02867472 2014-10-17
to about 1440 minutes. The magnesium hydroxide may exhibit a particle size of
between
about 0.1 micron to about 50 microns. The magnesium hydroxide may exhibit a
specific
surface area of between about 9 m2/g to about 200 m2/g. The magnesium
hydroxide may
exhibit a stabilized residuals test value of between about 1 milligram to
about 50 grams.
The magnesium compound may include magnesium oxide exhibiting an alkaline
magnesium oxide purity of between about 85% to about 100%. The magnesium oxide
may
exhibit a caustic magnesia activity of between about 50 seconds to about 1000
seconds.
The magnesium oxide may exhibit a particle size of between about 0.1 micron to
about 30
microns. The magnesium oxide may exhibit a specific surface area of between
about 9
m2/g to about 300 m2/g.
[0012] According to yet another implementation, a method may include adding an
effective amount of a flocculation aiding metal salt to supply water. The
method may also
include removing one or more contaminants from the supply water using a bio-
filtration
process. The method may further include adding an effective amount of a
magnesium
compound to the supply water to improve performance of the bio-filtration
process.
[0013] One or more of the following features may be included. The magnesium
compound may include magnesium hydroxide exhibiting an alkaline magnesium
hydroxide purity of between about 85% to about 100%. The magnesium hydroxide
may
exhibit a caustic magnesia activity of between about 50 seconds to about 1440
minutes.
The magnesium hydroxide may exhibit a particle size of between about 0.1
micron to about
50 microns. The magnesium hydroxide may exhibit a specific surface area of
between
about 9 m2/g to about 200 m2/g. The magnesium hydroxide may exhibit a
stabilized
residuals test value of between about 1 milligram to about 50 grams. The
magnesium
compound may include magnesium oxide exhibiting an alkaline magnesium oxide
purity
of between about 85% to about 100%. The magnesium oxide may exhibit a caustic
magnesia activity of between about 50 seconds to about 1000 seconds. The
magnesium
oxide may exhibit a particle size of between about 0.1 micron to about 30
microns. The
magnesium oxide may exhibit a specific surface area of between about 9 m2/g to
about 300
m2/g.
[0014] Adding the effective amount of the magnesium compound may include
adding
the magnesium compound to the supply water prior to adding the flocculation
aiding metal
4
salt. Adding the effective amount of the magnesium compound may include adding
the
magnesium compound generally along with adding the flocculation aiding metal
salt.
According to an aspect of the present invention, there is provided a method
comprising:
adding a magnesium compound to supply water to be treated, wherein the
magnesium compound includes magnesium hydroxide exhibiting an alkaline
magnesium
hydroxide purity of between about 85% to 100%; a caustic magnesia activity of
between
about 50 seconds to about 1440 minutes; a particle size of between about 0.1
micron to about
50 microns; a specific surface area of between about 9 m2/g to about 200 m2/g;
and a
stabilized residuals test value of between about 1 milligram to about 50
grams;
adding a flocculation aiding metal salt to the supply water; and
removing one or more contaminants from the supply water to provide treated
water.
According to another aspect of the present invention, there is provided a
method
comprising:
treating supply water, including adding a flocculation aiding metal salt to
the supply
water and removing one or more contaminants from the supply water, to provide
treated
potable water;
determining a corrosivity associated with the treated potable water;
adding a magnesium compound to the supply water based upon the determined
corrosivity associated with the treated potable water to achieve a desired
reduced corrosivity
associated with the treated potable water.
According to another aspect of the present invention, there is provided a
method
comprising:
adding an a magnesium compound to supply water to be treated, wherein the
magnesium compound includes magnesium oxide exhibiting an alkaline magnesium
oxide
purity of between about 85% to 100%; a caustic magnesia activity of between
about 30
Date Recue/Date Received 2021-05-31
seconds to about 3600 seconds; a particle size of between about 0.1 micron to
about 30
microns; and a specific surface area of between about 9 m2/g to about 300
m2/g;
adding a flocculation aiding metal salt to the supply water; and
removing one or more contaminants from the supply water to provide treated
water.
[0015] The details of one or more implementations are set forth in the
accompanying
drawings and the description below. Other features and advantages will become
apparent
from the description, the drawings, and the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
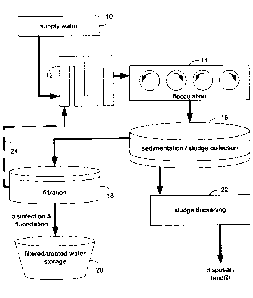
[0016] FIG. 1 schematically depicts an example water treatment system, wherein
the
identified coagulant may include the use of metal salts or a polymer.
DESCRIPTION OF EXAMPLE EMBODIMENTS
[0017] Supply water may typically be treated in a variety of manners to make
it suitable for
potable use. For example, water that may ultimately be intended for potable
use may often
be supplied from surface sources such as rivers, lakes, reservoirs, etc., or
from ground water
sources, such as wells, aquifers, and the like. The water supply may typically
include various
contaminants that may make the supply water unsuitable, or undesirable for
potable use.
Consistent with embodiments of the present disclosure, supply water may be
processed to
provide treated water that may be suitable for potable use. In an embodiment,
treatment of
supply water may generally include adding a magnesium compound to the supply
water and
adding a flocculation aiding metal salt to the supply water. One or more
contaminants may
be removed from the supply water to provide treated water that may, in some
embodiments,
be suitable for potable use.
[0018] Consistent with some embodiments, magnesium compounds, such as
magnesium hydroxide and/or magnesium oxide, may be utilized in combination
with
flocculation aiding metal salts to improve the efficiency of treatment of
water for potable use
5a
Date Recue/Date Received 2021-05-31
(e.g., which may also be referred to as "treating potable water," or similar
phrasing). For
example, flocculation aiding metal salts that may be utilized in connection
with the treatment
of potable water may include, but are not limited to, alum, ferrous sulfate,
ferric sulfate,
ferrous chloride, and ferric chloride. Such metal salts may serve as
flocculation aids to
facilitate the removal of contaminants from supply water. In some situations,
the flocculation
aiding metal salts may deplete the alkalinity of the supply water, which may
5b
Date Recue/Date Received 2021-05-31
CA 02867472 2014-10-17
lead to treated water having an alkalinity, and/or pH, below a desired level.
In some
embodiments, the magnesium compounds may, at least in part, offset the
alkalinity
reduction caused by the flocculation aiding metal salts. In some such
embodiments, the
treated water may have an alkalinity within a desired alkalinity range.
Further, in some
such embodiments, the treated water may have a pH that may be within a desired
pH range
for the treated water. In some embodiments, the magnesium compounds may
provide
treated water having an alkalinity and/or a pH within a desired range, which
may reduce
and/or eliminate the need for alkalinity and/or pH modifying processes and/or
substances,
such as caustic soda and/or lime, which may be expensive and/or potentially
dangerous to
handle.
[0019] In various embodiments, a magnesium compound may be uniquely utilized
in
connection with treatment of water for potable use. For example, the
relatively low
solubility of magnesium compounds, such as magnesium hydroxide and/or
magnesium
oxide, may have generally dissuaded the use of magnesium compounds for the
treatment
of potable water. For example, the relatively low solubility of magnesium
compounds may
have given rise to concerns about increased turbidity, increased total
suspended solids in
the water and/or deleterious effects on filtration processes. Consistent with
some
embodiments, magnesium compounds may be utilized in connection with the
treatment of
potable water without undesirably contributing to the turbidity of the water,
undesirable
levels of total suspended solids in the water, and/or may not excessively
negatively impact
filtration, or may even improve filtration efficiency. In some embodiments,
magnesium
compounds having a relatively high reactivity may be utilized in connection
with the
treatment of potable water. In some embodiments, magnesium compounds, such as
Brucite
and/or other relatively lower reactivity magnesium compounds may be suitably
utilized for
achieving certain results. Further, in some embodiments, relatively high
reactivity
magnesium compounds and relatively lower reactivity magnesium compounds may be
used together to achieve a particular effect in treating the water. For
example, a relatively
high reactivity magnesia and a relatively low reactivity magnesia may be
combined when
it is desirable to have both a high dissolution effect (e.g., which may
supplement soluble
Mg+2, OH- and increase pH facilitating flocculation), but also have sufficient
particulate
magnesia of either high and/or low reactivity to facilitate adsorption of
certain
6
CA 02867472 2014-10-17
contaminants. The relatively low reactivity magnesia may come from the group
of
compounds including Brucite (naturally occurring Mg(OH)2) and/or Magnesite
(naturally
occurring MgCO3), both of which may be in the family of magnesia compounds.
[0020] In some embodiments of the present disclosure, the use of magnesium
compound in connection with the treatment of potable water (i.e., the
treatment of water
for potable use) may provide for improvements in flocculation performance and
may also
provide pH control and/or alkalinity control for the treated potable water. In
some
embodiments, the use of magnesium compound in connection with the treatment of
potable
water may allow similar and/or improved turbidity removal from gravity
sedimentation
while utilizing less flocculation aiding metal salts. In some embodiments, the
use of
magnesium compound in connection with the treatment of potable water may allow
similar
and/or improved removal of dissolved organic compounds, or total organic
compounds
while utilizing less flocculation aiding metal salts. In some embodiments, the
use of
magnesium compounds in connection with potable water treatment may provide a
permanent hardness addition to the water to achieve a desired level of
hardness in the
treated potable water. In some embodiments, the use of magnesium compounds in
connection with the treatment of potable water may allow a desired pH and/or
alkalinity to
be achieved, which may, for example, reduce or eliminate the need for chemical
additions,
such as caustic soda or lime, for pH and/or alkalinity control. Accordingly,
in various
embodiments, magnesium compounds may be used in connection with potable water
treatment to achieve various results and/or synergistic performance
improvements.
[0021] As generally discussed above, in an implementation, an effective amount
of a
magnesium compound may be added to supply water that is to be treated.
Treating the
supply water may be for the purpose of achieving potable water standards,
e.g., which may
allow the treated water to be distributed for residential or domestic use, or
otherwise
suitable for use and/or consumption by individuals. The method may also
include adding
an effective amount of a flocculation aiding metal salt to the supply water.
One or more
contaminants may be removed from the supply water to provide the treated
water.
[0022] For example, and referring also to FIG. 1, as example embodiment of a
water
treatment system for treating potable water (e.g., for treating supply water
to provide
potable treated water) is generally shown. Supply water 10, which is to be
treated, may
7
CA 02867472 2014-10-17
include any suitable source of water. For example, supply water 10 may include
surface
water from lakes, rivers, reservoirs, or the like. Similarly, supply water 10
may include
ground water, such as from wells, aquifers, or the like. Various other
suitable sources of
supply water 10 may also be utilized. One or more coagulants may be added to
supply
water 10 at coagulation addition 12. In general, and as will be described in
greater detail
below, the one or more coagulants may aggregate at least a portion of the
suspended and/or
dispersed contaminants within supply water 10, such that the aggregated
contaminants may
form larger particles that may be separated from supply water 10 via various
contaminant
removal mechanisms. In an example embodiment, supply water 10 including the
added
coagulants may be mixed in flocculation process 14. In flocculation process
14, the
coagulants may be effectively mixed with supply water 10 and contaminants
within supply
water 10 may be allowed to at least partially coalesce or aggregate to form
larger particles.
[0023] With continued reference to FIG. 1, consistent with the illustrative
water
treatment process, following flocculation 14, the water being treated may
undergo
sedimentation / sludge removal 16. During sedimentation / sludge removal 16
the heavier
and/or larger aggregated and/or flocculated contaminants may settle out from
the water
being treated as sediment or sludge. Clear water (e.g., water from which the
aggregated
and/or flocculated contaminants have settled out) from the sedimentation /
sludge removal
process 16 may be provided to filtration process 18. Filtration process 18 may
utilize one
or more physical and/or biological filtration processes to remove at least a
portion of
remaining contaminants from the water received from the sedimentation / sludge
removal
process 16. The filtered water may be provided to filtered / treated water
storage 20, which
may include one or more storage tanks or reservoirs and/or a distribution
system for
providing the treated potable water to individuals for potable use. In some
embodiments,
the filtered water may undergo disinfection and/or fluoridation, e.g., to
ensure the water is
free from any potentially harmful residual contaminants and/or to add any
desired additions
to the water. Further, in some embodiments, sludge and/or sediment collected
during the
sedimentation / sludge removal process 16 may be collected and at least
partially dewatered
or dried during sludge thickening process 22. The at least partially dewatered
sludge and/or
sediment 16 may be removed for disposal, e.g., in a landfill or via
horticultural use.
8
CA 02867472 2014-10-17
[0024] The magnesium compound and the flocculation aiding metal salts may be
added in the coagulation addition process 12. For example, the effective
amount of the
magnesium compound may be added to supply water 10 at coagulation addition 12.
Further, in an example embodiment, the effective amount of the flocculation
aiding metal
salt may also be added to supply water 10 at coagulation addition 12. In an
illustrative
embodiment, the magnesium compound may include one or more of magnesium
hydroxide
and magnesium oxide, as well as some mixture of magnesium hydroxide and
magnesium
oxide. In some embodiments, mixtures of relatively high reactivity magnesium
compounds
(e.g., as may be discussed in greater detail below) may be using in
combination with one
or more relatively lower reactivity magnesium compounds, such as Brucite or
magnesite.
Further, the flocculation aiding metal salts may include one or more of alum,
ferrous
sulfate, ferric sulfate, ferrous chloride, and ferric chloride. Various
additional and/or
alternative coagulants and/or flocculation aids may be utilized.
[0025] In general, flocculation aiding metal salts may be utilized as a
primary agent for
the removal of contaminants from supply water that is to be treated for
potable use. For
example, often turbidity in supply water, which may be desirably removed to
provide
potable water, may include silts, sand or silica, clays or alumino-silicates,
and natural
organic matter ("NOM"). These materials may be at least partially negatively
charged in
water at near neutral pH. The flocculation aiding metal salts, as well as some
other
flocculation aiding agents, may often be at least partially positively charged
at near-neutral
pH. In some embodiments, the flocculation aiding agents (including the
flocculation aiding
metal salts) may form mixed particulates of the at least partially positively
charged
flocculation aiding agents and the at least partially negatively charged
contaminants,
thereby forming enmeshed floc. Various additional and/or alternative
mechanisms may
also be involved in the flocculation of contaminants within the supply water
through the
use of the flocculation aiding metal salts.
[0026] In some embodiments, the magnesium compounds may be used to improve the
removal of unwanted or undesirable raw water constituents, while reducing or
eliminating
the consequences of using the flocculation aiding metal salts, polymer
flocculation aiding
agents, as well as other flocculation aiding agents, on the quality of the
resultant treated
water, as well as on the potable water infrastructure, through which the
treated potable
9
CA 02867472 2014-10-17
water may be distributed to end users for consumption. For example,
flocculation aiding
metal salts may reduce the pH of the water being treated, and may reduce the
alkalinity of
the water being treated. As such, the flocculation aiding metal salts may
result in a needed
increase in the use of pH-dependent disinfection chemicals. Further, the
flocculation
aiding metal salts may increase the corrosivity of the water being treated,
which may result
in an increase in the need for corrosion control chemicals. Further, in some
situations the
use of flocculation aiding metal salts may increase filtration requirements
and loading, etc.
associated with removal of contaminants from the water being treated.
[0027] An effective amount of the magnesium compound added to supply water 10
may facilitate the removal of total organic carbon ("TOC"), as well as the
removal of other
contaminants. For example, in some embodiments, the effective amount of the
magnesium
compound may reduce and/or minimize the pfl depression caused by the
flocculation
aiding metal salts. Further, in some embodiments, the effective amount of the
magnesium
compound may facilitate the formation of metal hydroxides Al0Hx, Fe0Hx, and/or
Mg(OH)2, which may enhance flocculation and sedimentation of settleable
solids,
containing total suspended solids ("TSS") or TOC, in the water. For example,
at least
partially positively charged metal hydroxides and the at least partially
negatively charged
contaminant particles may form an enmeshed floc that may facilitate separation
from the
water being treated. Furthermore, in some embodiments, the adsorption of
positive cations,
such as Mg', onto negatively charged particles of contaminants may result in a
charge
reduction, and thereby provide less resistance to flocculation of the
negatively charged
contaminants. Additionally, in some embodiments, an increased salt
concentration in the
water being treated may result in a reduction in the electrical double layer,
which may
facilitate removal of contaminant particles from the water being treated. As
generally
discussed above, the effective amount of the magnesium compound may further
enhance
the quality of treated water in that the treated water may require relatively
lower amounts
of chemicals and polymers used for disinfection, scale prevention, and
reducing water
corrosivity. The reduction in the use of the chemicals and polymers may
thereby reduce
any potentially harmful by-products that such chemicals may cause.
Accordingly, in an
embodiment, adding the effective amount of the magnesium compound and the
CA 02867472 2014-10-17
flocculation aiding metal salt may be based upon, at least in part, a desired
removal of TSS,
TOC, dissolved organic carbon ("DOC"), and/or NOM.
[0028] In addition improving TOC, DOC and NOM removal in conjunction with
flocculation aiding metal salts, in some embodiments the effective amount of
the
magnesium compound may facilitate and/or aid in the removal of inorganic
constituents,
such as arsenic, selenium, or the like. For example, the ability to remove,
and/or aid in the
removal of, inorganic constituents may be a function of residual magnesium
hydroxide
and/or magnesium oxide solids that may be on a physical filter (e.g. during
filtration 18).
For example, in some situations the residual magnesium compound may complex
aluminum from alum (which may be utilized as a flocculation aiding metal
salt). Such a
magnesium - aluminum complex may absorb and/or adsorb the inorganics such as
arsenic,
selenium and other potentially toxic metals. Other processes may be involved
and/or
responsible for the removal of inorganic materials from the water being
treated. Removal
of inorganic materials resulting from the use of magnesium compounds for the
treatment
of potable water may provide a significant benefit to potable water treatment
plants, e.g.,
as stricter EPA guidelines may be established to minimize such contaminants
for drinking
water.
[0029] As generally discussed above, magnesium compounds may be utilized in
combination with one or more flocculation aiding metal salts to facilitate
contaminant
removal from water that may be treated for potable use. For example. an
effective amount
of the magnesium compound may be added to the supply water to facilitate,
promote,
and/or improve flocculation efficiency and effectiveness. In some such
embodiments, the
flocculation efficiency and/or effectiveness may be improved to allow the
amount of
flocculation aiding metal salts to be reduced, e.g., as compared to the amount
that may be
required for treating potable water without the use of the magnesium
compounds. Further,
in some embodiments, the effective amount of the magnesium compound may
additionally
and/or alternatively compensate for at least a portion of the pH reduction in
the water being
treated as a result of the addition of the flocculation aiding metal salts
and/or compensate
for at least a portion of the alkalinity depression in the water being treated
as a result of the
addition of the flocculation aiding metal salts. In some such embodiments, the
need for
additional and/or separate chemical additions to the water to compensate for
the reduced
11
CA 02867472 2014-10-17
pH and/or the depressed alkalinity may be reduced and/or eliminated. Further,
in some
embodiments, the effective amount of the magnesium compound may facilitate
and/or
promote the removal of inorganic materials from the water being treated. For
example, the
effective amount of the magnesium compound may facilitate and/or promote the
removal
of arsenic and/or selenium, and/or various other potentially toxic metals from
the water
being treated.
[0030] In an embodiment, the effective amount of the magnesium compound and
the
one or more flocculation aiding metal salts may facilitate and/or promote
removing organic
materials, which may affect color, odors and taste, from the supply water.
Example of such
organic materials may include, but are not limited to tannic compounds and/or
humic
compounds. Organic acids, such as tannic acids and/or humic acids, may be a
breakdown
product of, or be the result of, decomposed plant matter. Such contaminants
have been
known to result in unwanted taste, coloration and turbidity in potable water,
especially in
U.S. geographic regions such as South Florida, even after final filtration
from the potable
water plant into drinking reservoirs. An effective amount of the magnesium
compound
may be added to the water being treated, along with an effective amount of the
one or more
flocculation aiding metal salts, and/or polymer treatment agents, to reduce
and/or eliminate
organic materials in the treated potable water including tannins, tannic acid,
humus, humic
acid, and other similar organics that may impart undesirable taste or color.
In some
implementations the removal of organics, and/or the removal of inorganics, may
be
promoted and/or improved by the further addition of an oxidizer, such as
hypochlorite salts,
percarbonate salts, peroxides, nitrates and associated salts, or similar, to
the supply water.
[0031] Adding the effective amount of the magnesium compound may include
adding
the magnesium compound to the supply water prior to adding the flocculation
aiding metal
salt. Adding the magnesium compound prior to adding the flocculation aiding
metal salt
may include adding the magnesium compound at a location that is upstream in
the water
treatment process relative to a location at which the flocculation aiding
metal salt is added.
For example, the magnesium compound may be added upstream of, or prior to, the
addition
of acidic, or low pH, products such as, but not limited to, flocculation
aiding metal salts.
In some embodiments, the magnesium compound may be added to the supply water
and
may be dosed to reduce and/or to minimize the potential contribution to
finished water
12
CA 02867472 2014-10-17
turbidity or suspended solids resulting from the magnesium compound. In some
embodiments, adding the magnesium compound to reduce and/or minimize the
turbidity
or suspended solids in the finished, or treated water, may include adding the
magnesium in
sufficient quantities such that the turbidity and/or suspended solids in the
finished water
may be minimized and/or reduced. In some embodiments, adding the magnesium
compound to reduce and/or minimize the turbidity or suspended solids in the
finished water
may include adding the magnesium compound sufficiently upstream in the water
treatment
process to allow sufficient dissolution or collection (e.g., via sedimentation
and/or
filtration) of the magnesium compound to reduce and/or minimize turbidity or
suspended
solids in the finished water. In an embodiment, adding the magnesium compound
prior to
adding the flocculation aiding metal salts may include adding the magnesium
compound
at an upstream location within coagulant addition process 12 relative to the
location at
which the flocculation aiding metal salt is added. In some embodiments, adding
the
effective amount of the magnesium compound may include adding the magnesium
compound generally along with adding the flocculation aiding metal salt. For
example,
the magnesium compound may be added at generally the same location within the
coagulant addition process 12 as the flocculation aiding metal salts. Still
further, in some
embodiments, the magnesium compound may be added to the supply water after the
addition of the flocculation aiding metal salt.
[0032] In some embodiments, the quality and reactivity of magnesium compound
may
be selected to provide desirable performance. For example, in some situations
it may be
possible that any un-dissolved magnesium compound that accumulates in a filter
utilized
in connection with potable water treatment may continue to dissolve and may
improve
capture in the filter. In some situations, a relatively less reactive grade of
magnesia, such
as Brucite, may not provide a comparable rate of dissolution, and thus may
exhibit more
characteristics of fouling than might be achieved with relatively more
reactive grades of
magnesia. Illustrative examples of relatively more reactive magnesium compound
may
include Thioguard and Magoxt brands of magnesium hydroxide and magnesium
oxide
available from Premier Magnesia, LLC. The mechanisms for improved performance
associated with relatively higher reactivity magnesium compounds may, in some
implementations, be based upon, at least in part, the characteristics of the
matrix formed
13
CA 02867472 2014-10-17
by the media, the filter, the magnesia and the metal salt, and may account for
the better
overall performance. However, in some implementations less reactive grades of
magnesia
may be acceptably utilized to varying degrees of efficacy. In some
implementations,
relatively less reactive grades of magnesium compounds may be used in
conjunction with
relatively higher reactivity grades of magnesium compounds to achieve a
specific result.
[0033] In an example embodiment, magnesium compounds (e.g., magnesium oxide
and/or magnesium hydroxide) may be utilized having a relatively high degree of
purity. In
an example embodiment, magnesium compounds may be provided having an alkaline
magnesium oxide and/or alkaline magnesium hydroxide purity of between about
85% to
about 100% pure alkaline magnesium oxide and/or magnesium hydroxide. In an
illustrative embodiment, a magnesium compound may be provided having an
alkaline
magnesium oxide and/or alkaline magnesium hydroxide purity of between about
91% to
about 98% pure alkaline magnesium oxide and/or magnesium hydroxide.
[0034] In some embodiments, the stability of the magnesium hydroxide may be
generally related to the ability of a magnesium hydroxide slurry to maintain
pumpability
while minimizing solids residue that may accumulate in a storage and/or
transportation
tank (e.g., rail tank, tanker truck, etc.), which may become difficult to re-
suspend.
Accordingly, the stability of the magnesium hydroxide may be indicative of the
ability of
a magnesium hydroxide slurry to withstand transportation and storage, while
remaining
susceptible to dispensing, as through pumping. In an example embodiment, the
stability
of a magnesium hydroxide slurry may be quantified using the stabilized
residuals test
("SRT-Tap Test"). In general, the SRT-Tap test may assess the solids settling
stability of
magnesium hydroxide suspensions. According to an embodiment, an eight fluid
ounce test
bottle may be filled with a magnesium hydroxide slurry. The magnesium
hydroxide slurry
may be retained in the test bottle for a fourteen hour period, with the test
bottle maintained
in an upright position. After fourteen hours, sediment collecting in the
bottom of the test
bottle (e.g., as a result of magnesium hydroxide falling out of suspension)
may be
evaluated. The test bottle containing the slurry and/or any collected sediment
may be
vigorously shaken in a horizontal orientation of the test bottle for fifteen
seconds, and the
slurry may then be poured out of the test bottle. The test bottle may
subsequently be filled
with approximately an inch and a half of water, which may be swirled within
the bottle to
14
CA 02867472 2014-10-17
remove slurry film from the side of the bottled. The water may be poured from
the test
bottle and the test bottle may be inverted to drain for fifteen minutes. A
difference between
the post draining weight of the test bottle and an initial weight (e.g., prior
to initially filling
the test bottle with magnesium hydroxide slurry) may be determined. According
to various
embodiments, a suitable magnesium hydroxide may provide an SRT-Tap test value
of
between about 1 milligram and about 50 grams. In some embodiments, a suitable
magnesium hydroxide may provide an SRI-Tap test value of between about 0.1
gram to
about 50 grams. In an embodiment, a suitable magnesium hydroxide may provide
an SRT-
Tap test value of between about 1 gram to about 50 grams. In an example
embodiment, a
suitable magnesium hydroxide may provide an SRI-Tap Test value of between
about 1
gram and about 20 grams. In a particular embodiment, a suitable magnesium
hydroxide
may provide an SRT-TAP Test value of between about 1 gram and about 20 grams,
with
an average value of about 10 grams.
[0035] In some embodiments, a magnesium compound may include magnesium
hydroxide exhibiting a caustic magnesia activity ("CMA") neutralization time
of between
about 50 seconds to about 1440 minutes using 1.0N acetic acid and a magnesium
hydroxide
content of between about 10% to about 100%. In some embodiments, a magnesium
compound may include magnesium oxide exhibiting a caustic magnesia activity
neutralization time of between about 30 seconds to about 3600 seconds using
1.0N acetic
acid and a magnesium oxide content of between about 10% to about 100%. In some
embodiments, a magnesium compound may include magnesium oxide exhibiting a
caustic
magnesia activity neutralization time of between about 50 seconds to about
1000 seconds
using 1.0N acetic acid and a magnesium oxide content of between about 10% to
about
100%. In an embodiment, the magnesium compound may be provided exhibiting a
caustic
magnesia activity neutralization time of between about 50 seconds to about 200
seconds
using a 1.0N acetic acid and a magnesium oxide and/or magnesium hydroxide
content of
between about 10% to about 100%. In a particular example embodiment, the
magnesium
compound may be provided exhibiting a caustic magnesia activity neutralization
time of
about 125 seconds using a 1.0N acetic acid and a magnesium oxide and/or
magnesium
hydroxide content of between about 10% to about 100%.
CA 02867472 2014-10-17
[0036] In an embodiment, the magnesium compound may be provided having a
particle size that may provide an enhanced specific surface area ("SSA"). For
example,
generally, a magnesium compound having a smaller particle size may enhance the
overall
specific surface area of the magnesium compound (e.g., which may include
magnesium
oxide and/or magnesium hydroxide). In an embodiment, a magnesium compound may
include a magnesium hydroxide exhibiting a particle size of between about 0.1
micron to
about 50 micron. In some embodiments, a magnesium compound may include
magnesium
oxide exhibiting a particle size of between about 0.1 micron to about 30
micron. For
example, in an embodiment, the magnesium compound may include a magnesium
oxide
and/or magnesium hydroxide having a particle size of between about 1 micron to
about 20
microns. In one illustrative embodiment, the magnesium compound may include a
magnesium oxide and/or magnesium hydroxide having an average particle size of
about 10
micron.
[0037] In addition to the average particle size, the magnesium compound may be
provided having a particle size distribution that may improve the stability of
a slurry
produced using the magnesium compound. As generally discussed above, a higher
degree
of stability of a slurry produced using the magnesium compound may generally
relate to
the ability to maintain pumpability of the slurry while minimizing solids
residue that may
accumulate in a storage ancUor transportation tank. In some situations, a
relatively more
narrow particle size distribution of the magnesium compound may increase the
stability of
a slurry produced using the magnesium compound. In an embodiment, particle
size and
particle size distribution may be measured and/or controlled using screen
analysis and a
particle size distribution analyzer.
[0038] In an embodiment, a magnesium compound may be provided having a desired
reactivity. A magnesium compound having a relatively higher reactivity may
provide more
complete and efficient use within a desired application, and may, in some
instances, at least
partially offset a relatively low solubility that may be associated with
magnesium
compounds such as magnesium oxide and/or magnesium hydroxide. In an
embodiment,
specific surface area ("SSA") of the magnesium compound may be correlated to
reactivity,
e.g., in which a relatively higher specific surface area may be correlated to
a relatively
higher reactivity. In some embodiments, a magnesium compound may include
magnesium
16
CA 02867472 2014-10-17
hydroxide exhibiting a specific surface area of between about 9 m2/g to about
200 m2/g.
For example, in an example embodiment, a magnesium compound may include
magnesium
hydroxide having a specific surface area in the range of between about 9
m2/gram to about
50 m2/gram. In one particular embodiment, a magnesium hydroxide may include a
specific
surface area of about 12 m2/gram. In some embodiments, a magnesium compound
may
include magnesium oxide exhibiting a specific surface area of between about 9
m2/g to
about 300 m2/g, or greater. For example, in an example embodiment, a magnesium
compound may include magnesium oxide having a specific surface area in the
range of
between about 9 m2/gram to about 150 m2/gram, or greater.
[0039] While magnesium compounds having various different characteristics
(such as
purity, SRT-Tap Test values, CMA, particle size, and SSA) have been described,
it will be
understood that such characteristics, and representative values, are provided
for the purpose
of illustration and example. Consistent with the present disclosure, magnesium
oxide
and/or magnesium hydroxide compounds having different characteristic values
may be
utilized in connection with treating potable water to varying degrees of
efficacy, either
individually or in combination.
[0040] In some embodiments, combinations of magnesium compounds having
different reactivities may be utilized in connection with the treatment of
potable water. For
example, in an embodiment relatively high reactivity magnesium compounds and
relatively lower reactivity magnesium compounds may be used together to
achieve a
particular effect in treating the water. In some embodiments, the combination
of
magnesium compounds having different reactivities may achieve synergistic
benefits. For
example, combinations of magnesium compounds having differing reactivities may
be
added to water being treated to aid in removing a particular contaminant from
the water.
In some such embodiments, the different magnesium compounds having different
reactivities may provide a synergistic result, for example, in terms of
removing particular
contaminants, controlling pH or alkalinity of the potable water, etc.
[0041] The method may further include adding an effective amount of a polymer
contaminant removal aid including one or more of polyepichlorohydrin-
dimethylamine,
polyamine, polydiallyl-dimethylammonium chloride (polyDADMAC), polyacrylate,
polyamide, a Mannich polymer, and polyacrylamide. For example, and as
generally
17
CA 02867472 2014-10-17
described above, organic solids, which may often be measured and/or reported
as TOC,
may be removed, at least in part, through the addition of flocculation aiding
metal salt such
as alum (e.g., aluminum sulfate), ferrous chloride, ferric chloride, ferrous
sulfate, ferric
sulfate, or other metal chlorides or salts to promote flocculation and
coagulation of solids.
The flocculated and/or coagulated solids may then be removed through the
process of
sedimentation 16 or filtration process 18. An effective amount of the
magnesium
compound may be added to the water being treated (e.g., supply water 10). For
example,
the magnesium compound may be added to the water being treated upstream
relative to the
addition of the flocculation aiding metal salt. The addition of the effective
amount of the
magnesium compound may reduce, and/or prevent, the depletion of alkalinity
from water
and/or may reduce, and/or prevent, the depression of pH. In such an
implementation, the
addition of the magnesium compound may increase processing efficiency and/or
reduce
corrosiveness of the water, which may be, at least in part, attributed to the
use of the
flocculation aiding metal salts for treating the water. In some
implementations, magnesia
may similarly be used in connection with polymers (such as, but not limited
to,
polyepichlorohydrin-dimethylamines,
polyamines, polydiallyl-dimethy lammoni um
chloride (polyDADMAC), polyacrylates, polyamides, Mannichs, polyacrylamides,
etc.)
which may also be used in connection with water treatment. Such polymers, or
polymeric
compounds, may aid the coagulation/flocculation process, for example, by
adding
positively or negatively charged substrates to facilitate the agglomeration of
oppositely
charged contaminants and flocculation aids. Accordingly, in some embodiments,
an
effective amount of a polymer flocculating or solids conditioning aid may be
added to the
water supply. In some such embodiments, the further combination of magnesium
compounds and polymer coagulation/flocculation aids may provide improved
contaminant
removal. Further, in some embodiments, the use of magnesium compounds in
combination
with polymer coagulation/flocculation aids may reduce the quantity of polymer
coagulation/flocculation aids that may be required to achieve a similar
contaminant
removal (e.g., as compared to a quantity of polymer coagulation/flocculation
aid that may
be utilized in the absence of the magnesium compound).
[0042] Adding the magnesium compound may include adding an admixture of the
magnesium compound and an alkaline earth metal compound. For example, in an
18
CA 02867472 2014-10-17
embodiment, the effective amount of the magnesium compound may increase
divalent
cation concentration in the water, which may improve wastewater treatment,
after the
potable water has been used by individuals and/or businesses. For example,
increasing the
divalent cation concentration may improve wastewater treatment in terms of
clarification,
sedimentation, dewatering etc., by counteracting the imbalances created by
sodium and
other monovalent compounds that may be discharged to the sewer system. In an
embodiment, adding the magnesium compound in admixture with one or more
alkaline
earth metal compounds, such as beryllium compounds, other magnesium compounds,
calcium compounds, strontium compounds, barium compounds, and/or radium
compounds, may facilitate increasing the divalent cation concentrations in the
treated
potable water.
[0043] Adding the effective amount of the magnesium compound includes
measuring
a quality of the treated water. For example, as generally discussed,
flocculation aiding
metal salts, as well as various other agents used in the treatment of potable
water may
impact various water quality attributes, e.g., which may render such quality
attributes less
desirable. Additionally, the supply water may have various quality attributes
that may be
less desirable, e.g., even prior to treatment with flocculation aiding metal
salts and/or other
agents. As such, the effective amount of the magnesium compound may at least
partially
compensate for less desirable water quality attributes. For example, the
quality of the
treated water may include one or more of an alkalinity of the treated water
and a pH of the
treated water. Adding the effective amount of the magnesium compound may
include
measuring the pH and/or alkalinity of the treated water to determine the
effective amount
of the magnesium compound, e.g., which may at least partially provide a
desirable pH
and/or alkalinity in the treated water.
[0044] As generally discussed above, the flocculation aiding metal salt, as
well as other
agents used to treat the potable water, may deplete the alkalinity of the
treated water. In
an embodiment, an anticipated alkalinity depletion associated with adding the
flocculation
aiding metal salt may be determined. Additionally / alternatively an
anticipated pH
decrease associated with adding the flocculation aiding metal salt may be
determined. One
or more of the determined anticipated alkalinity depletion and/or pH decrease
associated
with adding the flocculation aiding metal salt may allow an effective amount,
and/or an
19
CA 02867472 2014-10-17
initial anticipated effective amount, of the magnesium compound to be
determined. For
example, the effective amount, and/or the initial anticipated effective amount
of the
magnesium compound may be based upon, at least in part, an amount of the
magnesium
compound offsetting at least a portion of the anticipated alkalinity
depletion.
[0045] The effective amount of the magnesium compound may be adjusted based
upon, at least in part, the measured quality of the treated water. For
example, the initial
anticipated effective amount of the magnesium compound may be added to the
supply
water based upon, at least in part, an anticipated alkalinity depletion and/or
pH decrease.
Subsequently, the actual alkalinity, pH, and/or another water quality
attribute of the treated
water may be determined. The effective amount of the magnesium compound added
to the
supply water may be adjusted from the initial anticipated effective amount,
e.g., to achieve
and/or more closely approach a desired water quality attribute.
[0046] In an embodiment, the magnesium compound may be added in sufficient
quantities to the finished potable water to increase pH and/or alkalinity
levels in the
wastewater collection system that may receive the potable water after it has
been used by
homes or businesses. In an example, the pH and/or alkalinity levels present in
the waste
water may be sufficient to prevent and/or reduce such issues as odors,
corrosion, FOG, etc
once the water passes to the wastewater collection system. In addition / as an
alternative
to compensating for any depletion in the alkalinity of the treated water
resulting from the
use of the flocculation aiding metal salt, in an embodiment the alkaline value
of the potable
water to be distributed may be increased to be greater than the alkaline value
of the supply
water. In some such embodiments, the treated potable water may have a greater
value in
terms of wastewater treatment. That is, once the treated potable water has
been used by
consumers and passed into the wastewater collection system, the resultant
wastewater may
have a higher alkaline value than the initial supply water, and thereby may
render the
wastewater more resistant to problems in wastewater treatment such as odors,
corrosion,
and acid production from biological treatment.
[0047] In an embodiment, the magnesium value of the potable water to be
distributed
may be greater than before treatment, and therefore may have a greater value
in tei ins of
health benefits to the consumers of the potable water. For example, many
individuals may
be at least partially deficient in nutritional magnesium. In a similar manner
as other
CA 02867472 2014-10-17
beneficial additions, such a fluoride, the effective amount of magnesium
compound may
provide beneficial nutritional magnesium to individuals who drink the treated
potable
water. In this regard, there is suggestion and/or evidence that appropriate
nutritional
magnesium intake may prevent Type 2 diabetes, prevent osteoporosis, reduce
migraine
headaches, reduce cardiovascular disease, and/or lower blood pressure. In some
embodiments, the effective amount of the magnesium compound may provide the
potential
for one or more such health benefits to individuals consuming the treated
potable water.
In an embodiment, such an effective amount of the magnesium compound may be
based
upon, at least in part, a measured effective nutritional magnesium quality of
the treated
potable water. Selection of the quality of magnesia may be based in part on
the quantity
of magnesium desired in the treated water. In some situations, lower
reactivity forms of
magnesia may be captured during sedimentation or filtration, which may prevent
the
desired magnesium fortification from being achieved through magnesia
introduced to the
treatment process. Magnesium may still be added to the finished water in the
forms of
more highly soluble salts, including but not limited to magnesium chloride,
magnesium
sulfate, magnesium citrate, etc.
[0048] In an embodiment, the quality of the treated water may include a
corrosivity of
the treated water as indicated by a Langelier Index of the treated water. For
example, in
an implementation, the magnesium compound may be utilized for treating supply
water, at
least in part to control and/or reduce the corrosivity of the treated water.
Accordingly, in
an implementation, supply water may be treated including adding an effective
amount of a
flocculation aiding metal salt to the supply water and removing one or more
contaminants
from the supply water. Treating the supply water may provide treated potable
water.
Further, a corrosivity associated with the treated potable water may be
determined. An
effective amount of a magnesium compound may be added to the supply water
based upon,
at least in part, the determined corrosivity associated with the treated
potable water to
achieve a desired reduced corrosivity associated with the treated potable
water. For
example, the determined corrosivity associated with the treated potable water
may be based
upon, at least in part, one or more of a quality of the supply water, a
corrosivity resulting
from the flocculation aiding metal salts, and/or one or more other agents
utilized in treating
the potable water. As generally described above, corrosivity of the water may
be
21
CA 02867472 2014-10-17
quantified, or represented, using the Langelier's Index. Accordingly, in an
embodiment,
determining the corrosivity associated with the treated potable water may
include
determining a Langelier's Index associated with the treated potable water.
Further, the
effective amount of the magnesium compound added to the supply water and/or to
the
treated potable water may include an amount sufficient to provide an
Langelier's Index
value associated with the treated potable water that may indicate a desired
degree of
corrosivity, for example, a relatively low level of corrosivity associated
with the treated
potable water. In some embodiments, the reduction in corrosivity associated
with the
treated potable water may eliminate, or reduce, the need for corrosion
inhibiting agents,
such as phosphates, which may conventionally be utilized in connection with
treated water.
Accordingly, in some embodiment, the use of corrosion control agents, such as
phosphates,
polyphosphates, phosphonates, may be optimized and/or reduced based upon, at
least in
part, the corrosivity of the water and the effective amount of magnesium
compound added
to the supply water.
[0049] As generally described above, the treatment of the supply water to
provide
treated potable water may include removing one or more contaminants from the
supply
water to provide treated water. Removing one or more contaminants from the
supply water
may include one or more of flocculation, coagulation, sedimentation and
filtration. For
example, one or more contaminants may be removed from the water via the
flocculation
process 14 and/or the sedimentation process 16. Further, and as also described
with respect
to FIG. 1, removing one or more contaminants may include filtration (e.g., via
filtration
process 18). In some embodiments utilizing filtration to remove one or more
contaminants,
the performance of the filtration process may be evaluated in terms or
contaminant removal
performance and maintenance. Conventionally, because magnesium hydroxide may
have
a relatively low solubility, magnesium hydroxide has generally been dismissed
as an
alkaline modifier in potable water treatment, due to potential increases in
TSS and
turbidity, or clogging and scaling of filters. Uniquely, consistent with an
aspect of the
present disclosure, magnesium compounds may be used to improve the performance
of the
filters, both in terms of reduced final TSS and turbidity, but also in terms
of backwash
requirements for filtration. In some embodiments, at least a portion of any
residual
magnesium compound captured in a filter may be recycled from a filter element
back into
22
CA 02867472 2014-10-17
the supply water (e.g., as generally indicated by arrow 24 in FIG. 1). For
example, when
a filter element is backwashed, e.g., to remove any build-up of material that
may reduce
the performance of the filter, at least a portion of the backwash, which may
include residual
magnesium compound, may be returned to specific parts of the treatment
process.
Returning at least a portion of the backwash (e.g., recycling residual
magnesium
compound) to specific parts of the treatment process may enhance the
flocculation and
sedimentation processes preceding filtration, and may minimize and/or reduce
the
requirements of the magnesium compounds and/or the flocculation aiding metal
salts to
the raw supply water.
[0050] In some embodiments, removing one or more contaminants from the supply
water may include using a bio-filtration process. Bio-filtration processes may
include
removing organic material from the water using a biological process. In such
an
embodiment, adding the effective amount of the magnesium compound may include
adding an effective amount of the magnesium compound to the supply water to
improve
the performance of the bio-filtration process. As generally mentioned bio-
filtration may
include biological removal of contaminants through a bio-filter or bio-media.
In such bio-
filtration processes, organic material may be removed biologically, and the
magnesium
compound may serve as a nutrient and cationic enhancement to the
microorganisms and/or
to the structure of the biological matrix, which may thereby improve
biological
performance. In addition / as an alternative to improving biological
performance, the
magnesium compound may reduce and/or minimize problems associated with
sloughing
and clogging of biomass. Further, in some embodiments, the magnesium compound
may
serve to make the organics more digestible.
[0051] Consistent with the foregoing, in some implementations, the magnesium
compound may be optimized not only for flocculation aiding metal salt use but
may also
be optimized for filtration performance. In some implementations, the
magnesium
compound may be added in sufficient quantities to the water being treated to
improve the
performance of the filters, whether stratified media or membrane. The
magnesium
compound may be dosed sufficiently to actively reduce the required quantities
of
flocculation aiding metal salts, to reduce inorganic and organic loading to
the filtration
process, to reduce the impacts of scaling/fouling, and/or to reduce or
minimize backwash
23
CA 02867472 2014-10-17
or replacement rates of media and filters. In implementations utilizing bio-
filtration, the
magnesium compound may be added in sufficient quantities to improve
performance of
the bio-filters in terms of organics removal from the water stream and overall
health of the
biomass.
[0052] Consistent with the foregoing, in some implementations, processing the
supply
water to provide potable water may include removing constituents (such as
contaminants)
from the water that may be harmful. Processing the supply water to provide
potable water
may also include removing other constituents that may impart undesirable
color, taste,
turbidity or odor. The removed constituents may be in the form of dissolved
solids or
gases, suspended solids or gases, miscible or immiscible liquids, and may be
organic and/or
inorganic in nature. These constituents may be measured as Total Organic
Carbon
("TOC"), Total Dissolved Solids ("TDS"), and Total Suspended Solids ("TSS").
In some
situations, specific constituents such as minerals may be identified in more
detail.
[0053] According to various embodiments and implementations, the present
disclosure
may utilize magnesium compounds in combination with flocculation aiding metal
salts in
the treatment of water to make the water suitable for potable use. In some
embodiments,
the utilization of the magnesium compounds may improve the effectiveness of
flocculation
aiding metal salt use in the treatment of potable water. Consistent with some
embodiments,
the magnesium compound and the flocculation aiding metal salt may be dosed to
achieve
optimal and/or improved TSS and/or TOC removal, and may achieve a reduction in
turbidity. In some embodiments, the magnesium compound may be added in
sufficient
quantities to reduce or eliminate aluminum and/or iron in the finished potable
water and/or
in the sludge or sediment recovered during the treatment process. Similarly,
the
magnesium compound may also reduce or eliminate the need for polymer
additives.
[0054] Often in other areas of water treatment, such as wastewater treatment,
there has
been suggestion that flocculation performance may deteriorate as pH rises.
However,
consistent with the present disclosure, the use of magnesium compounds and the
resultant
increase and/or stabilization of pH has been found to improve flocculation
performance. It
may be possible that according to prior processes, which realized a decrease
in flocculation
performance as pH rises, pH may have been modified using caustic soda or lime.
In the
case of caustic soda use, for example, the sodium added to the system when
using sodium
24
CA 02867472 2014-10-17
hydroxide may be deleterious to flocculation and sedimentation. Consistent
with the
present disclosure, it may be the case that because of the specific
particulate and cationic
properties, magnesium compounds used for pH elevation in conjunction with the
flocculation aiding metal salts may lead to improved performance of the system
for
flocculation and sedimentation.
[0055] In some embodiments, the magnesium compounds may be added in sufficient
quantities to increase and/or optimize metal hydroxide formation. Further, in
some
implementations, magnesium compounds may be added in sufficient quantities to
reduce
the required amount of disinfection chemicals necessary to achieve potable
water
standards. As generally described above, the magnesium compounds may be added
to the
water being treated in sufficient quantities, and along with one or more
flocculation aiding
metal salts, polymers, and/or one or more oxidizers, in order to remove and/or
reduce
organics or undesirable inorganics such as arsenic or selenium. Further, in
some
implementations, the magnesium compounds may be added to the water being
treated in
sufficient quantities to alter the I.angelier index to indicate the potable
water produced may
be less corrosive.
[0056] In addition, as an alternative to possible potable water treatment
performance
increases, in some implementations magnesium compounds utilized in connection
with the
treatment of potable water may provide various health benefits. For example,
increasing
the residual magnesium content in potable water, which is valued by the WHO
(World
Health Organization) for providing specific health benefits, may provide some
reduction
in heart disease and diabetes, and/or may provide other health benefits.
[0057] A number of implementations have been described. Nevertheless, it will
be
understood that various modifications may be made. Accordingly, other
implementations
are within the scope of the following claims.