Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02868594 2014-09-24
1
ELECTROCHEMICAL REDUCTION DEVICE, AND METHOD FOR PRODUCING
HYDROGENATED PRODUCT OF AROMATIC HYDROCARBON COMPOUND OR
NITROGEN-CONTAINING HETEROCYCLIC AROMATIC COMPOUND
[TECHNICAL FIELD]
[0001] The present invention relates to a device and a
method for electrochemically hydrogenating an aromatic
hydrocarbon compound or an N-containing heterocyclic aromatic
compound.
[BACKGROUND ART]
[0002] It is known that cyclic organic compounds such as
cyclohexane and decalin are able to be obtained efficiently
by hydrogenating a benzene ring of corresponding aromatic
hydrocarbon compounds (benzene and naphthalene) using a
hydrogen gas. Since high temperature and high pressure are
required for this reaction, this reaction is not suitable for
small and medium-scale manufacturing. On the other hand, in
the case of an electrochemical reaction where an electrolysis
cell is used, it is not necessary to treat gaseous hydrogen
since water can be used as a source of hydrogen, and the
reaction is known to progress under relatively mild reaction
conditions (at about room temperature to 200 C and under
normal pressure).
CA 068594 9-24
2
[prior art document]
[patent document]
[0003] [Patent document No. 1] JP 2003-045449
[Patent document No. 2] JP 2005-126288
[Patent Document No. 3] JP 2005-239479
[non-patent document]
[0004] [non-patent document No. 1] Masaru Ichikawa, J.
Jpn. Inst. Energy, vol. 85, 517 (2006)
[DISCLOSURE OF THE INVENTION]
[PROBLEM TO BE SOLVED BY THE INVENTION]
[0005] As an example for electrochemically hydrogenating
a benzene ring of an aromatic hydrocarbon compound such as
toluene or the like, a method has been reported for obtaining
methylcyclohexane, which is a hydride in which a benzene ring
is hydrogenated, without going through a state of a hydrogen
gas by sending toluene that is vaporized into a gaseous state
to the side of a reduction electrode in a configuration
similar to that of water electrolysis (see non-patent
document No. 1). However, the amount of substance (current
density) that can be transformed per electrode area and time
is not large, and it has been difficult to industrially
hydrogenate a benzene ring of aromatic hydrocarbon compounds.
[0006] In this background, a purpose of the present
invention is to provide a technology capable of
CA 02868594 2014-09-24
3
electrochemically hydrogenating at least one benzene ring of
an aromatic hydrocarbon compound or an N-containing
heterocyclic aromatic compound with high efficiency.
[0007] One embodiment of the present invention relates to
an electrochemical reduction device. The electrochemical
reduction device includes: an electrode unit including an
electrolyte membrane having ionic conductivity, a reduction
electrode that is provided on one side of the electrolyte
membrane and that contains a reduction catalyst for
hydrogenating at least one benzene ring of an aromatic
hydrocarbon compound, and an oxygen evolving electrode that
is provided on the other side of the electrolyte membrane; a
power control unit that applies a voltage Va between the
reduction electrode and the oxygen evolving electrode; and a
control unit that controls the power control unit such that a
relationship, VRER - arbitrarily-defined acceptable potential
< VcA < VTR13., can be satisfied when the potential at a
reversible hydrogen electrode, the standard redox potential
of the aromatic hydrocarbon compound, and the potential of
the reduction electrode are expressed as VHERr VTRR, and VCAr
respectively. A potential in the present invention means a
true electrode potential with respect to a reference
potential. Therefore, when there exist, for example, an
electrolyte membrane resistance, an electrode catalyst layer
CA 02868594 2014-09-24
4
resistance, an ohmic loss derived from various electrical
connections, and the like, a true electrode potential needs
to be calculated and/or corrected in consideration of these
as described later.
[0008] In the electrochemical reduction device according
to the above embodiment, the arbitrarily-defined acceptable
potential may be 20 mV. The electrochemical reduction device
may further include: a reference electrode that is arranged
to be in contact with the electrolyte membrane and to be
electrically isolated from the reduction electrode and the
oxygen evolving electrode and that is held at a reference
electrode potential VRef; and a voltage detection unit that
detects a potential difference n,Volk between the reference
electrode and the reduction electrode, and the control unit
may acquire the potential VcA of the reduction electrode based
on the potential difference AVcA and the reference electrode
potential VRef. The control unit may control the potential VCA
of the reduction electrode to be in a predetermined range by
changing the voltage Va. When an oxygen evolution equilibrium
potential in the electrolysis of water is expressed as VoER,
the control unit controls the power control unit such that an
expression, Va (V0ER - VoA), is satisfied. The reference
electrode may be arranged on the side of the electrolyte
membrane on which the reduction electrode is provided.
CA 02868594 2014-09-24
[0009] Another embodiment of the present invention
relates to an electrochemical reduction device. The
electrochemical reduction device includes: an electrode unit
assembly in which a plurality of electrode units are
5 electrically connected to one another in series, the
electrode units each including an electrolyte membrane having
ionic conductivity, a reduction electrode that is provided on
one side of the electrolyte membrane and that contains a
reduction catalyst for hydrogenating at least one benzene
ring of an aromatic hydrocarbon compound, and an oxygen
evolving electrode that is provided on the other side of the
electrolyte membrane; a power control unit that applies a
voltage VA between a positive electrode terminal and a
negative electrode terminal of the electrode unit assembly;
and a control unit that controls the power control unit such
that a relationship, VHER - arbitrarily-defined acceptable
potential VCA VTRRi can be satisfied when the potential at
a reversible hydrogen electrode, the standard redox potential
of the aromatic hydrocarbon compound, and the potential of
the reduction electrode of each electrode unit are expressed
as VHER, VTR12/ and VcA, respectively.
[0010] In the electrochemical reduction device according
to the above embodiment, the arbitrarily-defined acceptable
potential may be 20 mV. The electrochemical reduction device
CA 02868594 2014-09-24
6
may further include: a reference electrode that is arranged
to be in contact with an electrolyte membrane of any one of
electrolytic layers contained in the electrode unit assembly
and to be electrically isolated from the reduction electrode
and the oxygen evolving electrode; and a voltage detection
unit that detects a potential difference AVcA between the
reference electrode and the reduction electrode, and the
control unit may acquire the potential VcA of the reduction
electrode based on the potential difference LVcA and the
reference electrode potential VRef= The control unit may
control the potential VcA of the reduction electrode of each
electrode unit to be in a predetermined range by changing the
voltage VA. When an oxygen evolution equilibrium potential in
the electrolysis of water is expressed as VOER, the control
unit may control the power control unit such that an
expression, VA (V0ER - VcA) x N, is satisfied where N (two or
greater) is the number of serially-concatenated electrode
units. The reference electrode may be arranged on the side of
the electrolyte membrane on which the reduction electrode is
provided. The reference electrode may be arranged on the side
of the electrolyte membrane on which the reduction electrode
is provided.
[0011] Another embodiment of the present invention
relates to a method for manufacturing a hydride of an
CA 02868594 2014-09-24
7
aromatic hydrocarbon compound or an N-containing heterocyclic
aromatic compound. The method for manufacturing a hydride of
an aromatic hydrocarbon compound or an N-containing
heterocyclic aromatic compound includes introducing an
aromatic hydrocarbon compound or an N-containing heterocyclic
aromatic compound to the reduction electrode side of the
electrode unit, circulating water or a humidified gas to the
oxygen evolving electrode side, and hydrogenating at least
one benzene ring of the aromatic hydrocarbon compound or the
N-containing heterocyclic aromatic compound introduced to the
reduction electrode side, by using the electrochemical
reduction device according to any one of above-stated
embodiments. In the method for manufacturing a hydride of an
aromatic hydrocarbon compound or an N-containing heterocyclic
aromatic compound according to the embodiment, the aromatic
hydrocarbon compound or the N-containing heterocyclic
aromatic compound to be introduced to the reduction electrode
side may be introduced to the reduction electrode side in a
liquid state at a reaction temperature.
[0012] Combinations of the above-stated elements will
also be within the scope of the present invention sought to
be patented by the present patent application.
CA 068594 2014 -09-24
8
[ADVANTAGE OF THE INVENTION]
[00]3] According to the present invention, at least one
benzene ring of an aromatic hydrocarbon compound or an
N-containing heterocyclic aromatic compound can be
electrochemically hydrogenated with high efficiency.
[BRIEF DESCRIPTION OF THE DRAWINGS]
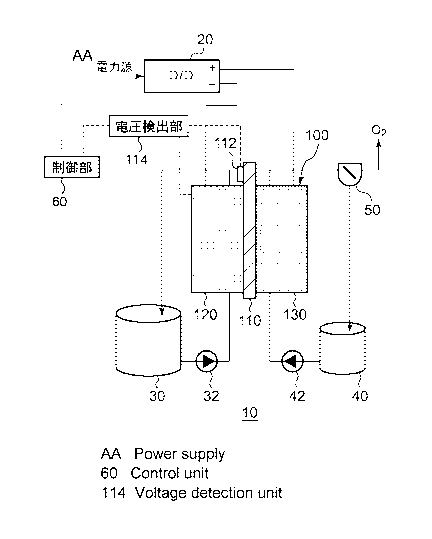
[0014] Fig. 1 is a schematic diagram illustrating the
configuration of an electrochemical reduction device
according to a first embodiment;
Fig. 2 is a diagram illustrating the configuration of an
electrode unit of the electrochemical reduction device
according to the first embodiment;
Fig. 3 is a flowchart illustrating an example of
potential control of a reduction electrode by a control unit;
Fig. 4 is a graph illustrating a relationship between
the potential of the reduction electrode and various types of
current density;
Fig. 5 is a schematic diagram illustrating the
configuration of an electrochemical reduction device
according to a second embodiment; and
Fig. 6 is a schematic diagram illustrating the
configuration of an electrochemical reduction device
according to a third embodiment.
CA 02868594 2014-09-24
9
[BEST MODE FOR CARRYING OUT THE INVENTION]
[0015] Described below is an explanation of the
embodiments of the present invention with reference to
figures. In the figures, like numerals represent like
constituting elements, and the description thereof is omitted
appropriately.
[0016] (First Embodiment)
Fig. 1 is a schematic diagram illustrating the
configuration of an electrochemical reduction device 10
according to an embodiment. Fig. 2 is a diagram illustrating
the configuration of an electrode unit of the electrochemical
reduction device 10 according to the embodiment. As shown in
Fig. 1, the electrochemical reduction device 10 has an
electrode unit 100, a power control unit 20, an organic
material storage tank 30, a water storage tank 40, a
gas-liquid separator 50, and a control unit 60.
[0017] The power control unit 20 is, for example, a DC/DC -
converter for converting the output voltage of a power source
into a predetermined voltage. The positive electrode output
terminal of the power control unit 20 is connected to the
positive electrode of the electrode unit 100. The negative
electrode output terminal of the power control unit 20 is
connected to the negative electrode of the electrode unit 100.
With this, a predetermined voltage is applied between an
CA 02868594 2014-09-24
oxygen evolving electrode (positive electrode) 130 of the
electrode unit 100 and a reduction electrode (negative
electrode) 120. A reference electrode input terminal of the
power control unit 20 is connected to a reference electrode
5 112 provided on an electrolyte membrane 110, which will be
described later, and the potential of the positive electrode
output terminal and the potential of the negative electrode
output terminal are determined based on the potential of the
reference electrode 112 in accordance with an instruction
10 from the control unit 60. As the power source, electrical
power derived from natural energy such as sunlight, wind
power, and the like can be used. The mode of the potential
control of the positive electrode output terminal and the
negative electrode output terminal by the cont.rol unit 60
will be described later.
[0018] The organic material storage tank 30 stores an
aromatic compound. An aromatic compound used in the present
embodiment is an aromatic hydrocarbon compound or an
N-containing heterocyclic aromatic compound containing at
least one aromatic ring and includes benzene, naphthalene,
anthracene, diphenylethane, pyridine, pyrimidine, pyrazine,
quinoline, isoquinoline, N-alkylpyrrole, N-alkylindole,
N-alkyldibenzopyrrole and the like. One through four hydrogen
atoms of the aromatic ring of the aromatic hydrocarbon
CA 02868594 2014-09-24
11
compound or the N-containing heterocyclic aromatic compound
may be substituted by alkyl groups. An "alkyl" of the above
aromatic compounds is a linear or branched alkyl group with
one through six carbons. For example, alkylbenzene includes
toluene, ethyl benzene, and the like, dialkylbenzene includes
xylene, diethylbenzene, and the like, and trialkylbenzene
includes mesitylene and the like. An example of
alkylnaphthalene includes methylnaphthalene. The above-stated
aromatic ring of the aromatic hydrocarbon compound or the
N-containing heterocyclic aromatic compound may have one
through three substituents. In the following explanation, an
aromatic hydrocarbon compound and an N-containing
heterocyclic aromatic compound used in the present invention
are often referred to as "aromatic compounds". Preferably,
the aromatic compound is a liquid at room temperature. When a
mixture of a plurality of aromatic compounds among the
above-described aromatic compounds is used, the aromatic
compounds need to be a liquid as the mixture. With this, the
aromatic compound can be supplied to the electrode unit 100
in a liquid state without performing processes such as
heating, pressurizing, and the like. Thus, the simplification
of the electrochemical reduction device 10 can be achieved.
The concentration of the aromatic hydrocarbon compound in a
liquid state is 0.1 percent or greater, preferably 0.3
CA 02868594 2014-09-24
12
percent or greater, and more preferably 0.5 percent or
greater.
[0019] The aromatic compound stored in the organic
material storage tank 30 is supplied to the reduction
electrode 120 of the electrode unit 100 by a first liquid
supply device 32. For the first liquid supply device 32, for
example, various types of pumps such as a gear pump, a
cylinder pump, or the like or a gravity flow device or the
like can be used. Instead of the aromatic compound, an
N-substitution product of the above-stated aromatic compound
may be used. A circulation pathway is provided between the
organic material storage tank 30 and the reduction electrode
of the electrode unit 100. An aromatic compound in which at
least one benzene ring is hydrogenated by the electrode unit
100 and an unreacted aromatic compound are stored in the
organic material storage tank 30 via the circulation pathway.
No gas is generated by a major reaction that progresses at
the reduction electrode 120 of the electrode unit 100. In the
case where hydrogen is evolved as a byproduct, a gas-liquid
separation device may be provided in the middle of the
circulation pathway.
[0020] The water storage tank 40 stores ion-exchanged
water, purified water, and the like (hereinafter, simply
referred to as "water"). Water stored in the water storage
CA 02868594 2014-09-24
13
tank 40 is supplied to the oxygen evolving electrode 130 of
the electrode unit 100 by a second liquid supply device 42.
As in the case of the first liquid supply device 32, for
example, various types of pumps such as a gear pump, a
cylinder pump, or the like or a gravity flow device or the
like can be used for the second liquid supply device 42. A
circulation pathway is provided between the water storage
tank 40 and the oxygen evolving electrode of the electrode
unit 100. Water that is unreacted in the electrode unit 100
is stored in the water storage tank 40 via the circulation
pathway. The gas-liquid separator 50 is provided in the
middle of a pathway where unreacted water is sent back to the
water storage tank 40 from the electrode unit 100. The
gas-liquid separator 50 separates oxygen evolved by the
electrolysis of water in the electrode unit 100 from water
and discharges the oxygen outside the system.
[0021]
As shown in Fig. 2, the electrode unit 100 has an
electrolyte membrane 110, a reduction electrode 120, an
oxygen evolving electrode 130, liquid diffusion layers 140a
and 140b, and separators 150a and 150b. In Fig. 1, the
electrode unit 100 is simplified for illustration, and the
liquid diffusion layers 140a and 140b and the separators 150a
and 150 are omitted.
CA 02868594 2014-09-24
14
[0022] The electrolyte membrane 110 is formed of a
material (ionomer) having protonic conductivity. While
selectively conducting protons, the electrolyte membrane 110
is required to prevent substances from getting mixed or being
diffused between the reduction electrode 120 and the oxygen
evolving electrode 130. The thickness of the electrolyte
membrane 110 is preferably from 5 to 300 pm, more preferably
from 10 to 150 pm, and most preferably from 20 to 100 pm. If
the thickness of the electrolyte membrane 110 is less than 5
pm, the barrier property of the electrolyte membrane 110 is
lowered, and the amount of cross-leaking substances is more
likely to increase. If the thickness of the electrolyte
membrane 110 is more than 300 pm, ion transfer resistance
becomes too large. Thus, the thickness of more than 300 pm is
not preferred.
[0023] The area specific resistance, i.e., ion transfer
resistance per geometric area, of the electrolyte membrane
110 is preferably 2000 mQ.cm2 or less, more preferably 1000
mQ-cm2 or less, and most preferably 500 mQ.cm2 or less. If
the area specific resistance of the electrolyte membrane 110
is 2000 mQ.cm2 or greater, protonic conductivity becomes
insufficient. An example of a material having protonic
conductivity (which is a cation-exchanging ionomer) = includes
a perfluorosulfonic acid polymer such as Nafion (registered
CA 02868594 2014-09-24
trademark), Flemion (registered trademark), etc. The ion
exchange capacity (IEC) of the cation-exchanging ionomer is
preferably from 0.7 to 2 meq/g and more preferably from 1 to
1.2 meq/g. If the ion exchange capacity of the
5 cation-exchanging ionomer is less than 0.7 meq/g, ionic
conductivity becomes insufficient. On the other hand, if the
ion exchange capacity of the cation-exchanging ionomer is
greater than 2 meq/g, the solubility of the ionomer in water
becomes increased, and the strength of the electrolyte
10 membrane 110 thus becomes insufficient.
(0024] On the electrolyte membrane 110, a reference
electrode 112 is provided in an area spacedapart from the
reduction electrode 120 and the oxygen evolving electrode 130
in such a manner that the reference electrode 112 is in
15 contact with the electrolyte membrane 110. In other words,
the reference electrode 112 is electrically isolated from the
reduction electrode 120 and the oxygen evolving electrode 130.
The reference electrode 112 is held at a reference electrode
potential VRef. Examples of the reference electrode 112
include a standard hydrogen reduction electrode (reference
electrode potential VRef = 0 V) and an Ag/AgC1 electrode
(reference electrode potential VRef = 0.199 V). However, the
reference electrode 112 is not limited to these. The
reference electrode 112 is preferably provided on the surface
CA 068594 2014-0
16
of the electrolyte membrane 110 on the side of the reduction
electrode 120.
[0025] A potential difference LVcA between the reference
electrode 112 and the reduction electrode 120 is detected by
a voltage detection unit 114. The value of the potential
difference AVcA detected by the voltage detection unit 114 is
input to the control unit 60.
[0026] The reduction electrode 120 is provided on one
side of the electrolyte membrane 110. The reduction electrode
120 is a reduction electrode catalyst layer containing a
reduction catalyst for hydrogenating at least one benzene
ring of an aromatic compound. A reduction catalyst used for
the reduction electrode 120 is not particularly limited but
is composed of, for example, a composition containing a first
catalyst metal (noble metal) that contains at least one of Pt
and Pd and containing one or more kinds of second catalyst
metals selected from among Cr, Mn, Fe, Co, Ni, Cu, Zn, Mo, Ru,
Sn, W, Re, Pb, and Bi. The form of the composition is an
alloy of the first catalyst metal and the second catalyst
metal or an intermetallic compound composed of the first
catalyst metal and the second catalyst metal. The ratio of
the first catalyst metal to the total mass of the first
catalyst metal and the second catalyst metal is preferably
from 10 to 95 wt%, and more preferably from 20 to 90 wt%, and
CA 02868594 2014-09-24
17
most preferably from 25 to 80 wt%. The ratio of the first
catalyst metal of less than 10 wt% may result in
deterioration in durability from the perspective of
resistance to dissolving or the like. On the other hand, if
the ratio of the first catalyst metal is greater than 95 wt%,
the properties of the reduction catalyst become similar to
the properties of a noble metal alone, and the electrode
activity thus becomes insufficient. In the following
explanation, a first catalyst metal and a second catalyst
metal are often collectively referred to as "catalyst metals".
[0027] The above-described catalyst metals may be
supported by a conductive material (support). The electrical
conductivity of the conductive material is preferably 1.0 x
10-2 S/cm or greater, more preferably 3.0 x 10-2 S/cm or
greater, and most preferably 1.0 x 10-1 S/cm or greater. If
the electrical conductivity of the conductive material is
less than 1.0 x 10-2 S/cm, sufficient conductivity cannot be
provided. Examples of the conductive material include a
conductive material containing any one of a porous carbon, a
porous metal, and a porous metal oxide as a major component.
An example of the porous carbon includes carbon black such as
Ketjenblack (registered trademark), acetylene black, Vulcan
(registered trademark), or the like. The BET specific surface
area of the porous carbon measured by a nitrogen adsorption
CA 02868594 2014-09-24
18
method is preferably 100 m2/g or greater, more preferably 150
m2/g or greater, and most preferably 200 m2/g or greater. If
the BET specific surface area of the porous carbon is less
than 100 m2/g, it is difficult to uniformly support the
catalyst metals. Therefore, the rate of utilization of a
catalyst metal surface is lowered, causing the catalyst
performance to be lowered. Examples of the porous metal
include, for example, Pt black, Pd black, a Pt metal
deposited in a fractal shape, and the like. Examples of a
porous metal oxide include an oxide of Ti, an oxide of Zr, an
oxide of Nb, an oxide of Mo, an oxide of Hf, an oxide of Ta,
and an oxide of W. Furthermore, examples of a porous
conductive material for supporting a catalyst metal include a
nitride, a carbide, an oxynitride, a carbonitride, a
partially-oxidized carbonitride of a metal such as Ti, Zr, Nb,
Mo, Hf, Ta, W, or the like (hereinafter, these are
collectively referred to as porous metal carbonitrides and
the like). The respective BET specific surface areas of the
porous metal, the porous metal oxide, the porous metal
carbonitrides, and the like measured by a nitrogen adsorption
method are preferably 1 m2/g or greater, more preferably 3
m2/g or greater, and most preferably 10 m2/g or greater. If
the respective BET specific surface areas of the porous metal,
the porous metal oxide, the porous metal carbonitrides, and
CA 02868594 2014-09-24
19
the like are less than 1 m2/g, it is difficult to uniformly
support the catalyst metals. Therefore, the rate of
utilization of a catalyst metal surface is lowered, causing
the catalyst performance to be lowered.
[0028] Depending on the type and composition of the first
catalyst metal and the second catalyst metal, a simultaneous
impregnation method or a sequential impregnation method can
be employed as a method for supporting the catalyst metals on
the support. The first catalyst metal and the second catalyst
metal are simultaneously impregnated into the support in the
simultaneous impregnation method, and the second catalyst
metal is impregnated into the support after the first
catalyst metal is impregnated into the support in the
sequential impregnation method. In the case of the sequential
impregnation method, after the first catalyst metal is loaded
onto the support, a heat treatment or the like may be
performed once, and the second catalyst metal may be then
loaded onto the support. After the impregnation of both the
first catalyst metal and the second catalyst metal is
completed, the alloying of the first catalyst metal and the
second catalyst metal or the formation of an intermetallic
compound composed of the first catalyst metal and the second
catalyst metal is performed by a heat treatment process.
CA 02868594 2014-09-24
[0029] A material having conductivity such as the
previously-stated conductive oxide, carbon black, or the like
may be added to the reduction electrode 120 in addition to a
conductive compound on which a catalyst metal is supported.
5 With this, the number of electron-conducting paths among
reduction catalyst particles can be increased. Thus,
resistance per geometric area of a reduction catalyst layer
can be lowered in some cases.
[0030] As an additive agent, a fluorine-based resin such
10 as polytetrafluoroethylene (PTFE) may be contained in the
reduction electrode 120.
[0031] The reduction electrode 120 may contain an ionomer
having protonic conductivity. Preferably, the reduction
electrode 120 contains ionically conducting materials
15 (ionomers) having a structure that is identical or similar to
that of the above-stated electrolyte membrane 110 in a
predetermined mass ratio. This allows the ionic conductivity
of the reduction electrode 120 to be improved. In particular,
in the case where a catalyst support is porous, the reduction
20 electrode 120 makes a significant contribution to the
improvement of the ionic conductivity by containing an
ionomer that has protonic conductivity. An example of an
ionomer having protonic conductivity (a cation-exchanging
ionomer) includes a perfluorosulfonic acid polymer such as
CA 02868594 2014-09-24
21
Nafion (registered trademark), Flemion (registered trademark),
etc. The ion exchange capacity (IEC) of the cation-exchanging
ionomer is preferably from 0.7 to 3 meq/g, more preferably
from 1 to 2.5 meq/g, and most preferably from 1.2 to 2 meq/g.
When a catalyst metal is supported on porous carbon (carbon
support), a mass ratio I/C of the cation-exchanging ionomer
(I) to the carbon support (C) is preferably from 0.1 to 2,
more preferably from 0.2 to 1.5, and most preferably from 0.3
to 1.1. It is difficult to obtain sufficient ionic
conductivity if the mass ratio I/C is less than 0.1. On the
other hand, if the mass ratio I/C is 2 or greater, an
increase in the thickness of an ionomer coating for the
catalyst metal inhibits an aromatic compound, which is a
reactant, from touching a catalytic site, or a decrease in
the electron conductivity lowers the electrode activity.
[0032] Preferably, the ionomers contained in the
reduction electrode 120 partially coat a reduction catalyst.
This allows three elements (an aromatic compound, a proton,
and an electron) that are necessary for an electrochemical
reaction at the reduction electrode 120 to be efficiently
supplied to a reaction field.
[0033] The liquid diffusion layer 140a is laminated on
the surface of the reduction electrode 120 on the opposite
side of the electrolyte membrane 110. The liquid diffusion
CA 068594 9-24
22
layer 140a plays a function of uniformly diffusing, to the
reduction electrode 120, a liquid aromatic compound supplied
from the separator 150a that is described later. As the
liquid diffusion layer 140a, for example, carbon paper and
carbon cloth are used.
[0034] The separator 150a is laminated on the surface of
the liquid diffusion layer 140a on the side opposite to the
electrolyte membrane 110. The separator 150a is formed of a
carbon resin, an anticorrosion alloy of Cr-Ni-Fe, Cr-Ni-Mo-Fe,
Cr-Mo-Nb-Ni, Cr-Mo-Fe-W-Ni, or the like. A single or a
plurality of groove-like flow channels 152a is/are provided
on the surface of the separator 150a on the side of the
liquid diffusion layer 140a. The liquid aromatic compound
supplied from the organic material storage tank 30 circulates
through the flow channel 152a. The liquid aromatic compound
soaks into the liquid diffusion layer 140a from the flow
channel 152a. The form of the flow channel 152a is not
particularly limited. For example, a straight flow channel or
a serpentine flow channel can be used. when a metal material
is used for the separator 150a, the separator 150a may be a
structure in which ball-like or pellet-like metal fine powder
is sintered.
[0035] The oxygen evolving electrode 130 is provided on
the other side of the electrolyte membrane 110. The oxygen
CA 02868594 2014-09-24
23
evolving electrode 130 that contains catalysts of noble metal
oxides such as Ru02, Ir02, and the like is preferably used.
These catalysts may be supported in a dispersed manner or
coated by a metal substrate such as a metal wire or mesh of
metals such as Cr, Mn, Fe, Co, Ni, Cu, Zn, Nb, Mo, Ta, W, and
the like or of alloys composed primarily of these metals. In
particular, since Ir02 is high-priced, manufacturing costs can
be lowered by performing thin film coating on a metal
substrate when Ir02 is used as a catalyst.
[0036] The liquid diffusion layer 140b is laminated on
the surface of the oxygen evolving electrode 130 on the side
opposite to the electrolyte membrane 110. The liquid
diffusion layer 140b plays a function of uniformly diffusing,
to the oxygen evolving electrode 130, water supplied from the
separator 150b that is described later. As the liquid
diffusion layer 140b, for example, carbon paper and carbon
cloth are used.
[0037] The separator 150b is laminated on the surface of
the liquid diffusion layer 140b on the side opposite to the
electrolyte membrane 110. The separator 150b is formed of an
anticorrosion alloy of Cr-Ni-Fe, Cr-Ni-Mo-Fe, Cr-Mo-Nb-Ni,
Cr-Mo-Fe-W-Ni, or the like or of a material in which the
surfaces of these metals are coated by an oxide layer. A
single or a plurality of groove-like flow channels 152b
CA 02868594 2014-09-24
24
is/are provided on the surface of the separator 150b on the
side of the liquid diffusion layer 140b. The water supplied
from the water storage tank 40 circulates through the flow
channel 152b. The water soaks into the liquid diffusion layer
140b from the flow channel 152b. The form of the flow channel
152b is not particularly limited. For example, a straight
flow channel or a serpentine flow channel can be used. When a
metal material is used for the separator 150b, the separator
150b may be a structure in which ball-like or pellet-like
metal fine powder is sintered.
[0038] In the present embodiment, liquid water is '
supplied to the oxygen evolving electrode 130. Alternatively,
a humidified gas (e.g., air) may be used in place of liquid
water. In this case, the dew-point temperature of the
humidified gas is preferably from room temperature to 100 C
and more preferably from 50 to 100 C.
[0039] When toluene is used as the aromatic compound,
reactions that occur in the electrode unit 100 are as
follows:
<Electrode Reaction at Oxygen Evolving Electrode>
3H20 , 1.502 + 6H++ 6e : E0 = 1.23 V
CA 02868594 2014-09-24
<Electrode Reaction at Reduction Electrode>
toluene + 61-1++ 6e- --, methylcyclohexane : E0 = 0.153 V
(vs RHE)
In other words, the electrode reaction at the oxygen
5 evolving electrode and the electrode reaction at the
reduction electrode progress in parallel, and protons evolved
by electrolysis of water are supplied to the reduction
electrode via the electrolyte membrane 110 by the electrode
reaction at the oxygen evolving electrode and used for
10 hydrogenation of at least one benzene ring of the aromatic
compound in the electrode reaction at the reduction electrode.
[0040] Referring back to Fig. 1, the control unit 60
controls the power control unit 20 such that a relationship,
VHER - 20 mV VcA VTRRt can be satisfied when the potential
15 at a reversible hydrogen electrode, the standard redox
potential of the aromatic compound, and the potential of the
reduction electrode 120 are expressed as VHER, VTRIlt and VcA
respectively. If the potential VcA is below VHER - 20 mV,
competition with a hydrogen generation reaction will occur,
20 and the reduction selectivity of the aromatic compound will
become insufficient. Thus, the potential VcA of below VHER -
20 mV is not preferred. On the other hand, the potential VCA
of higher than the standard redox potential VTRR is not
preferred since the hydrogenation of at least one benzene
CA 02868594 2014-09-24
26
ring of the aromatic compound will not progress at a
practically sufficient reaction speed. In other words, by
setting the potential VcA to be in a range that satisfies the
above-stated relational expression, an electrochemical
reaction can be progressed at the both electrodes, and the
hydrogenation of at least one benzene ring of the aromatic
compound can thus be industrially practiced.
[0041]
Furthermore, the following reaction conditions are
used favorably for the hydrogenation of at least one benzene
ring of an aromatic compound using the electrochemical
reduction device 10. The temperature of the electrode unit
100 is preferably from room temperature to 100 C and more
preferably from 40 to 80 C. The temperature of the electrode
unit 100 of below the room temperature is not preferred since
there is a possibility that the progress of an electrolytic
reaction is slowed down or an enormous amount of energy is
required to remove heat generated as the reaction progresses.
On the other hand, the temperature of the electrode unit 100
of above 100 C is not preferred for the electrochemical
reduction device 10 in which reactions of the both electrodes
are performed in a liquid phase since water will be boiled at
the oxygen evolving electrode 130 and the vapor pressure of
an organic substance will be increased at the reduction
CA 02868594 2014-09-24
27
electrode 120. Since the reduction electrode potential VcA is
a true electrode potential, the reduction electrode potential
VcA may be different from a potential VcA_actual that is actually
measured. If there are resistance components, among various
resistance components that exist in an electrolytic cell used
in the present invention, that result in ohmic resistance, a
resistance value per electrode area of the entirety of these
components is set to be the entire ohmic resistance Rohmic, and
the true electrode potential VcA is calculated using the
following expression.
VcA = VCA_actual Rohmic x J (current density)
Examples of the resistance components that result in ohmic
resistance are proton transfer resistance of the electrolyte
membrane, electron transfer resistance of the electrode
catalyst layer, and, furthermore, contact resistance on an
electric circuit. Rohmic can be obtained as an actual
resistance component on an equivalent circuit by using an
alternating-current impedance method or an
alternating-current resistance measurement at a fixed
frequency. Alternatively, once the configuration of an
electrolytic tank and a material system to be used are
determined, a method is preferably employed where Rohnuc is
used in the following control while considering Rohrnic as an
almost stationary value.
CA 02868594 2014-09-24
28
[0042] Fig. 3 is a flowchart illustrating an example of
potential control of the reduction electrode 120 by the
control unit 60. An explanation is given in the following
regarding the mode of the potential control of the reduction
electrode 120 by using, as an example, a case where an
Ag/AgC1 electrode (reference electrode potential VRef = 0.199
V) is used.
[0043] First, a potential VCA (target value) that
satisfies the expression, VHER - 20 mV VCA VTRR, is set
(S10). In one embodiment, the potential VCA (target value) is
a value that is stored in advance in memory such as ROM. In
another embodiment, the potential VCA (target value) is set by
a user.
[0044] The potential difference AVcA between the
reference electrode 112 and the reduction electrode 120 is
then detected by the voltage detection unit 114 (S20).
[0045] The control unit 60 then calculates a potential
VCA (actual measurement value) of the reduction electrode 120
by using an expression, VCA = AVCA
VRef = L\VcA - 0.199 v (S30).
[0046] Then, the control unit 60 determined whether the
potential VCA (actual measurement value) satisfies the
following expressions (1) and (2) (S40).
'potential VCA (actual measurement value) - potential VCA
(target value)! acceptable value (1)
CA 02868594 2014-09-24
29
VHER - 20 mV VcA (actual measurement value) VTRR
(2)
In the above expression, the acceptable value is, for
example, 1 mV.
[0047] If the potential VcA (actual measurement value)
satisfies the expressions (1) and (2), the step proceeds to
"yes" in S40, and the process performed at this point is
ended. On the other hand, if the potential VcA (actual
measurement value) does not satisfy the expressions (1) and
(2), the step proceeds to "no" in S40, and the control unit
60 adjusts a voltage Va that is applied between the reduction
electrode 120 and the oxygen evolving electrode 130 (S50).
After the adjustment of the voltage Va, the process goes back
to the above-stated process in S10.
[0048] An explanation is now given regarding an example
for the adjustment of the voltage Va. For example, when
potential VcA (actual measurement value) - potential VCA
(target value) > acceptable value, the control unit 60
transmits to the power control unit 20 an instruction to
increase the voltage Va by only 1 mV. Even when the
expression, !potential VcA (actual measurement value) -
potential VcA (target value)!
acceptable value, is satisfied
as a result of increasing the voltage Va, if VcA (actual
measurement value) is less than (VHER - Vanow), the expression
CA 02868594 2014-09-24
(2) is not satisfied. Thus, the control unit 60 instructs to
lower the voltage Va by 1 mV in the following process.
[0049] On the other hand, when potential VcA (actual
measurement value) - potential VcA (target value) < acceptable
5 value, the control unit 60 transmits to the power control
unit 20 an instruction to lower the voltage Va by only 1 mV.
Even when the expression, !potential VcA (actual measurement
value) - potential VcA (target value)! acceptable value, is
satisfied as a result of lowering the voltage Va, if VCA
10 (actual measurement value) is greater than VTRR, the
expression (2) is not satisfied. Thus, the control unit 60
instructs to increase the voltage Va by 1 mV in the following
process. In this way, the control unit 60 adjusts the voltage
Va until the expressions (1) and (2) are finally satisfied.
15 [0050] A value (adjustment range) for increasing or
decreasing the voltage Va is not limited to 1 mV. For example,
the adjustment range of the voltage Va may be set to be equal
to the above-stated acceptable value in a first adjustment of
the voltage Va, and the adjustment range of the voltage Va
20 may be set to be, e.g., one-fourth of the above-stated
acceptable value in a second or subsequent adjustment of the
voltage Va. With this, the control unit 60 can more promptly
adjust the potential VcA (actual measurement value) to be in a
range where the expressions (1) and (2) are satisfied.
CA 02868594 2014-09-24
31
[0051] When an oxygen evolution equilibrium potential in
the electrolysis of water is expressed as VOER, the control
unit 60 controls the power control unit 20 in such a manner
that an expression, Va (V0ER - VcA) , is satisfied. This
allows a potential VAN of the oxygen evolving electrode 130 to
be maintained to be the oxygen evolution equilibrium
potential VoER or greater.
[0052] (Relationship between Toluene Reduction Property
and Reduction Electrode Potential)
Using an electrode cell having composition shown in
Table 1, a hydrogenation reaction of a benzene ring of
toluene was performed at different reduction electrode
potentials. Fig. 4 is a graph illustrating a relationship
between the potential of the reduction electrode and various
current density. The mass of reduction catalyst metals is 0.5
mg/cm2.
[TABLE 1] =
REDUCTION ELECTRODE ELECTROLYTE
MEMBRANE 'OXYGEN EVOLV LNG
ELECTRODE
REDUCTION CATALYST CONDUCTIVE MATERIAL IONOMER DEPTH(gm)
ION CONDUCTOR DEPTHiunD METAL CATALYST
Pt(50wt%), Ru(50wth) Ketjen black EC600JD Nafion DE2020(EW-1100) .11C-0.8 30
Nafion NRE212C5(EW-1100) 50 Ir02/Mo
A current density A, a current density B, and a current
density C that are shown in Fig. 4 are as shown in the
following.
CA 068594 2314-13
32
Current density A: the entire current density flowing
through the electrode cell
Current density B: current density used for the
reduction of toluene that is back-calculated from the
evolution amount of methylcyclohexane determined
quantitatively by gas chromatography or the like
Current density C: Current density A - Current density B
(current density that was not used for the reduction of
toluene but was mainly used for hydrogen generation)
Faraday efficiency shown in Fig. 4 is calculated by
current density B / current density A x 100 (%).
[0053] As shown in Fig. 4, if the potential of the
reduction electrode is below (potential VHER - 20 mV), in
other words, - 20 mV, the amount of hydrogen generation is
increased, and the faraday efficiency becomes below 50%. Thus,
it has been verified that the potential of the reduction
electrode of below - 20 my is not preferred.
[0054] (Second Embodiment)
Fig. 5 is a schematic diagram illustrating the
configuration of an electrochemical reduction device
according to a second embodiment. As shown in Fig. 5, an
electrochemical reduction device 10 comprises an electrode
unit assembly 200, a power control unit 20, an organic
material storage tank 30, a water storage tank 40, a
CA 068594 9-24
33
gas-liquid separator 50, and a control unit 60. The electrode
unit assembly 200 has a laminated structure where a plurality
of electrode units 100 are connected in series. In the
present embodiment, the number N of the electrode units 100
is five. The configuration of each electrode unit 100 is
similar to the configuration according to the first
embodiment. In Fig. 5, the electrode units 100 are simplified
for illustration, and liquid diffusion layers 140a and 140b
and separators 150a and 150 are omitted.
[0055] The positive electrode output terminal of the
power control unit 20 is connected to the positive electrode
terminal of the electrode unit assembly 200. On the other
hand, the negative electrode output terminal of the power
control unit 20 is connected to the negative electrode
terminal of the electrode unit assembly 200. With this, a
predetermined voltage VA is applied between the positive
electrode terminal and the negative electrode terminal of the
electrode unit assembly 200. A reference electrode input
terminal of the power control unit 20 is connected to a
reference electrode 112 provided on an electrolyte membrane
110 of a specific electrode unit 100, which will be described
later, and the potential of the positive electrode output
terminal and the potential of the negative electrode output
CA 068594 2014-0
34
terminal are determined based on the potential of the
reference electrode 112.
[0056] A first circulation pathway is provided between
the organic material storage tank 30 and reduction electrodes
120 of the respective electrode units 100. Aromatic compounds
stored in the organic material storage tank 30 is supplied to
the reduction electrodes 120 of the respective electrode
units 100 by a first liquid supply device 32. More
specifically, a pipeline that forms the first circulation
pathway is branched on the downstream side of the first
liquid supply device 32, and the aromatic compounds are
supplied to the reduction electrodes 120 of the respective
electrode units 100 in a distributed manner. Aromatic
compounds in which at least one benzene ring are hydrogenated
by the electrode units 100 and unreacted aromatic compounds
merge into a pipeline that communicates with the organic
material storage tank 30 and are then stored in the organic
material storage tank 30 via the pipeline.
[0057] A second circulation pathway is provided between
the water storage tank 40 and oxygen evolving electrodes 130
of the respective electrode units 100. Water stored in the
water storage tank 40 is supplied to the oxygen evolving
electrodes 130 of the respective electrode units 100 by a
second liquid supply device 42. More specifically, a pipeline
CA 02868594 2014-09-24
that forms the second circulation pathway is branched on the
downstream side of the second liquid supply device 42, and
the water is supplied to the oxygen evolving electrodes 130
of the respective electrode units 100 in a distributed manner.
5 Unreacted water merges into a pipeline that communicates with
the water storage tank 40 and is then stored in the water
storage tank 30 via the pipeline.
[0058] On an electrolyte membrane 110 of a specific
electrode unit 100, a reference electrode 112 is provided in
10 an area spaced apart from the reduction electrode 120 and the
oxygen evolving electrode 130 in such a manner that the
reference electrode 112 is in contact with the electrolyte
membrane 110 in the same way as in the first embodiment. The
specific electrode unit 100 needs to be any one of the
15 plurality of electrode units 100.
[0059] A potential difference AVcA between the reference
electrode 112 and the reduction electrodes 120 is detected by
a voltage detection unit 114. The value of the potential
difference Z\VcA detected by the voltage detection unit 114 is
20 input to the control unit 60.
[0060] The control unit 60 controls the power control
unit 20 such that a relationship, VHER - 20 mV VcA
VTRI2r can
be satisfied when the potential at a reversible hydrogen
electrode, the standard redox potential of an aromatic
CA 02868594 2014-09-24
36
compound, and the potential of the reduction electrodes 120
of the respective electrode units 100 are expressed as VHER/
VTRR and VcA, respectively.
[0061] The mode of the potential control of the reduction
electrode 120 by the control unit 60 is similar to the mode
according to the first embodiment. Note that while an applied
voltage Va is adjusted by the control unit 60 in the first
embodiment, a voltage VA applied between the positive
electrode terminal and the negative electrode terminal of the
electrode unit assembly 200 is adjusted by the control unit
60 in the present embodiment.
[0062] The control unit 60 controls the power control
unit 20 in such a manner that an expression, VA >_ (V0ER - VcA)
x N, is satisfied where N (two or greater) is the number of
electrode units and is five in the present embodiment. This
allows the potential VAN to be maintained to be the oxygen
evolution equilibrium potential VoER or greater.
[0063] According to the present embodiment, the
hydrogenation of an aromatic compound can be progressed in
parallel in a plurality of electrode units. Thus, the amount
of hydrogenation of at least one benzene ring of aromatic
compounds per unit time can be dramatically increased.
Therefore, the hydrogenation of at least one benzene ring of
aromatic compounds can be industrially practiced.
CA 02868594 2014-09-24
37
[0064] (Third Embodiment)
Fig. 6 is a schematic diagram illustrating the
configuration of an electrochemical reduction device
according to a third embodiment. The basic configuration of
an electrochemical reduction device 10 according to the
present embodiment is similar to the basic configuration
according to the second embodiment. In the present embodiment,
an electrode unit assembly 200 is held in an electrolytic
tank 300. A second circulation pathway is provided between
the electrolytic tank 300 and a water storage tank 40, and
the electrolytic tank 300 is filled with water supplied from
the water storage tank 40. Water that fills the electrolytic
tank 300 can circulate in oxygen evolving electrodes 130 of
the respective electrode units 100.
[0065] In addition to effects that can be obtained in the
second embodiment, the electrochemical reduction device 10
according to the present embodiment has an advantage of
decreasing an in-plane temperature difference of the oxygen
evolving electrodes 130, a temperature difference among
electrode units, and an interelectrode temperature difference
from reduction electrodes 120 by increasing the heat capacity
of a water tank in the electrolytic tank.
[0066] The invention is not limited to the
above-mentioned embodiments, and various modifications, such
CA 02868594 2014-09-24
38
as a design change, may be added thereto on the basis of
knowledge of those skilled in the art. It should be
understood that any embodiment to which one or more of the
modifications are added is also included in the scope of the
invention. For example, in the above-described embodiments, a
configuration is employed where an aromatic compound and
water circulate inside a circulation pathway, and a reservoir
connected to the inlet side of an electrolysis unit and a
reservoir connected to the outlet side of the electrolysis
unit may be provided separately.
[0067] In the above-stated embodiments, a reduction
electrode 120 contains an ionomer having protonic
conductivity. Alternatively, a reduction electrode 120 may
contain an ionomer having hydroxy ion conductivity.
[0068] In the second and third embodiments, a reference
electrode 112 is provided on an electrolyte membrane 110 of a
single electrode unit. Alternatively, a reference electrode
112 may be provided on respective electrolyte membranes 110
of a plurality of electrode units 100. In this case, by the
voltage detection unit 114, a potential difference LVcA
between each reference electrode 112 and a corresponding
reduction electrode 120 is detected, and a potential VcA is
calculated by using an average value of a plurality of
potential differences AVCA that are detected. With this, a
CA 068594 2014-0
39
voltage VA can be adjusted to be in a more appropriate range
when variation in potential is caused among the electrode
units 100.
[DESCRIPTION OF THE REFERENCE NUMERALS]
[0069] 10 electrochemical reduction device,
20 power supply unit,
30 organic material storage tank,
40 water storage tank,
50 gas-liquid separator,
100 electrode unit,
112 reference electrode,
114 voltage detection unit,
110 electrolyte membrane,
120 reduction electrode,
130 oxygen evolving electrode,
140a, 140b liquid diffusion layer,
150a, 150b separator,
200 electrode unit assembly,
300 electrolytic tank.
[INDUSTRIAL APPLICABILITY]
[0070] The present invention can be applied to
technologies for electrochemically hydrogenating an aromatic
hydrocarbon compound or an N-containing heterocyclic aromatic
compound.
=