Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02883147 2015-02-19
1
METHOD FOR THE BIOLOGICAL REMOVAL OF SULFATE AND METALS
APPLICATION FIELD FOR THE INVENTION
This invention relates to a process for the biological removal of sulfate and
metals from mining
effluents, mine acid drainages and different industrial liquid residues.
DESCRIPTION OF WHAT IS KNOWN IN THE ART
During the wastewater anaerobic treatment the sulfate contained is reduced
producing sulfhydric
acid. The sulfhydric acid is a toxic gas that corrodes metallic structures and
produces an
unpleasant odor in the water in which it is contained. The water from mines
and effluents
produced in different industries may contain high concentrations of sulfate
and require a
treatment to avoid the issues mentioned (Tait et al., 2009. "Removal of
sulfate from high-strength
wastewater by crystallization". Water Res. 43: 762-772; US 5.587.079). There
are currently some
alternatives to remove sulfate from water, anyhow these alternatives pose
important issues such
as not reducing the concentration of sulfate to the levels required or being
cost-ineffective.
Considering the above issues the need for developing an alternative that is
profitable and efficient
for the removal of sulfate is required. One of the alternatives proposed for
the treatment of water
with high levels of sulfate is the use of sulfate-reducing microorganisms.
Anyhow, this alternative
is limited by two reasons: the first one is that a profitable substrate for
the sulfate-reducing
microorganisms is not available; and the second one is that in the case of
mining effluents that, on
top of having high concentrations of sulfate, they contain metals that have a
toxic effect on the
sulfate-reducing microorganisms (Utgikar et al., 2002. "Quantification of
toxic and inhibitory
impact of copper and zinc on mixed cultures of sulfate-reducing bacteria".
Biotechnol. Bioeng. 82:
306 ¨ 312).
The fact that the typical substrates for sulfate-reducing microorganisms are
not profitable is
because they are low weight molecules such as ethanol, lactate or pyruvate
that have high costs.
The use of complex substrates for the reduction of sulfate represents
advantages since they are
CA 02883147 2015-02-19
2
more profitable than the low molecular weight substrates (Boshoff et al.,
2004. "The use of micro-
algal biomass as a carbon source for biological sulphate reducing systems".
Water. Res. 38: 2659-
2666).Therefore it is necessary a set of microorganisms capable of hydrolizing
and fermenting high
molecular weight substrates producing low molecular weight metabolites that
are substrates
useful for the sulfate-reducing microorganisms (US 5.587.079).
Different patents presenting systems for the removal of sulfate in waters can
be found. Some of
these patents propose the use of sulfate-reducing microorganisms to achieve
the removal of
sulfate in waters. This is the case of patent application EP 0436254 Al and
patent US 6228.263.
One important aspect that differentiates these patents is the substrate they
consider to maintain
the sulfate-reducing microorganism. Thus, as a substrate for the reduction of
sulfate the patent
application EP 0436254 Al proposes the use of ethanol or alcohol blends and US
6.228.263
proposes the use effluents with organic load such as wastewaters, tannery
waters, brewery
effluents, industries working with starch and remains of pulp and paper. A
common place among
the two patents is that they propose precipitating metals that may be present
in the waters with
the sulfhydric acid producing the sulfate reduction. Another alternative in
relation with those
mentioned is the patent application US 2004/0168975 consisting of a system for
the reduction of
sulfate present in waters based on the use of a set of microorganisms. This
set of microorganisms
would be comprised by sulfate-reducing microorganisms and others with the
capability of being
used as complex substrates, which are more profitable than the typical
substrates of the sulfate-
reducing bacteria.
All the patents mentioned so far correspond to biological systems, anyhow
there are treatments
that are in part or completely physicochemical. Thus the patent US 7.914.676
presents an
alternative for the treatment of water with high contents of metals and
sulfate. This system
considers the removal of metals by precipitating them as metallic sulfurs. For
the removal of
sulfate in water the precipitating of sulfate using lime is considered. Then,
the precipitated sulfate
is reduced using sulfate-reducing microorganisms producing the necessary
sulfur for the
precipitation of the metals in the first stage of the treatment. On the other
hand the patent
application US 2010/0108603 corresponds to a chemical alternative for the
treatment of waters
with high levels of sulfate and metals. It is based on the use of basic
substances allowing the
precipitation of metals present in the waters. Then by means of a filtration
system two effluents
CA 02883147 2015-02-19
3
are obtained: one with low concentration of sulfate and metals and another one
with high
concentration of sulfate and metals that is introduced again into the
treatment.
It is important to note that part of the patents mentioned aim to resolve the
issue of the presence
of metals in the waters. On the other hand, patent US 7.326.344 depicts a
system for the removal
of heavy metals based on the biosorption process without considering the issue
of removing the
sulfate. This technology results in concentration of metals below 10 mg/L.
This alternative may be
used to treat mine acid drainages and remove the metals, although it does not
solve the problem
of the sulfate high concentrations.
The alternatives currently available have different drawbacks so none of them
represent a
complete alternative for the treatment of waters with high concentration of
sulfate. Thus, the use
of lime to precipitate the sulfate is not much of an efficient alternative
since it does not allow
decreasing the concentration of sulfate below 1000 mg/L. Consequently, with
this treatment the
compliance with the environmental rules for discharging effluents in different
countries is not
achieved. On the other hand, in the case of effluents with a high
concentration of metals, the
precipitation of metals in hydroxide form has been suggested. This generates
muds that are
difficult to treat. Another disadvantage regarding the generation of these
muds is that they
prevent metals from being removed in waters that in some cases, have a
considerable economic
value. In the more complex case, the effluent with a high concentration of
metals and sulfate, the
use of sulfate-reducing microorganisms is difficult because the toxic or
inhibitory effect of the
metals affect the capacity of reducing the sulfate. Besides, these bacteria
use only organic
molecules of low molecular weight as electrons donors, such as pyruvate,
lactate, ethanol or
blends of alcohols. This represents a disadvantage from an economic standpoint
because the
current high cost of these substrates makes the use of this process at an
industrial scale expensive
and almost non-viable.
This invention solves the issues of the state of the technique using a
synergic combination of a
treatment system for the removal of metals by means of biosorption with a
bacterial biomass to
reduce the inhibiting concentration of metals in waters, followed by a process
of sulfate removal
that uses a halotolerant sulfate-reducing microbial consortium capable of
using complex organic
substrates such as agroindustrial products or residues thereby decreasing
operations costs of the
system. The halotolerant characteristic of the consortium provides the process
with a higher
CA 02883147 2015-02-19
4
flexibility to treat mining effluents contaminated with metals that frequently
show high levels of
salts concentration.
DEFINITION OF THE INVENTION
The main objective of this invention is a method for the biological removal of
sulfate and metals
from mining effluents, mine acid drainages and different industrial liquid
residues comprising at
least the steps of:
a) subjecting the effluent to at least a first step of removing the metals in
solution by
biosorption with a bacterial biomass added on itself forming floccules of easy
sedimentation and separation or a bacterial biomass attached to inert support
materials
forming a biofilm, and
b) subjecting the liquid previously treated in the biosorption step to a
second halotolerant
sulfate continuous removal step in an anaerobic bioreactor by a halotolerant
sulfate-
reducing microbial consortium capable of using complex carbonaceous organic
compounds as electrons donors.
In an embodiment of the invention, and as additional step before the treatment
by biosorption,
the invention comprises subjecting the effluent to a pre-treatment with lime
to reduce the
concentrations of sulfate and metals.
In another embodiment of the invention, the bacterial biomass is comprised by
natural bacteria
consortium that form a biofilm isolated from the environment.
In another additional embodiment of the invention, the bacterial biomass is
comprised by a
population of bacteria selected from the Bacillus, Pseudomonas, Klebisella,
Enterobacter genders.
In another preferred embodiment of the invention, the bacterial biomass is
comprised by the
Bacillus sp. VCHB-10 strain deposited as NRRL-B-30881.
CA 02883147 2015-02-19
In an embodiment of the invention, the first removal step for the metals in
solution by means of
biosorption with a bacterial biomass added on itself, comprises at least the
steps of:
a) growing the bacteria forming aggregates in a bioreactor,
b) sedimenting the aggregates and removing the culture medium from the
bioreactor,
c) contacting the water containing metal ions with the aggregates in the
bioreactor to
remove them by biosorption, sedimenting the aggregates, removing the treated
water,
contacting again the water containing the metal ions with the aggregates as
many times as
necessary until its biosorption capacity decreases due to the saturation,
d) leaving the aggregates to sediment and remove the remaining water from the
bioreactor,
e) adding a diluted acid to elute the metal ions captured by the aggregates
and
f) repeating the process from step c.
In another embodiment of the invention the first step of removing the metals
in solution by
biosorption with a bacterial biomass attached to inert support materials
forming a biofilm
comprises at least the steps of:
a) growing bacteria forming a biofilm in a fixed-bed bioreactor,
b) removing the culture medium from the bioreactor,
c) contacting in a continuous or semi-continuous way the water containing
metal ions with
the biofilm from the fixed-bed bioreactor to remove them by biosorption until
its
biosorption capacity starts to decrease due to the saturation,
d) adding a diluted acid to elute the metal ions captured by the biofilm, and
e) repeating the process from step c.
In an embodiment of the invention, the second step of continuous removal of
sulfate in a fixed-
bed anaerobic bioreactor by halotolerant sulfate-reducing microbial consortium
comprises at least
the steps of:
CA 02883147 2015-02-19
6
a) growing the sulfate-reducing microbial consortium in a fixed-bed bioreactor
containing the
support material of the bioreactor and a culture medium comprised by at least
one or
more complex carbonaceous organic compounds as electrons donors and sulfate,
b) re-circulating the culture medium of the bioreactor until a sulfate-
reducing consortium
biofilm is formed on the support material of the bioreactor,
c) contacting in a continuous or semi-continuous way the water previously
treated by means
of the step of metals biosorption with the biofilm of the sulfate-reducing
consortium in
the anaerobic bioreactor and simultaneously adding a suspension of one or more
complex
carbonaceous organic compounds as electrons donors, and
d) removing the treated water from the anaerobic bioreactor.
In another embodiment of the invention, the second step of the continuous
removal of the sulfate
in an anaerobic bioreactor by a halotolerant sulfate-reducing microbial
consortium comprises at
least the steps of:
a) growing the sulfate-reducing microbial consortium in a bioreactor
containing a culture
medium comprised by, at least one or more complex carbonaceous organic
compounds in
particulate form as electrons donor and sulfate,
b) re-circulating the culture medium of the bioreactor until the sulfate-
reducing consortium
biofilm is formed on the complex carbonaceous organic compound(s) in
particulate form,
c) contacting in a continuous or semi-continuous way the water previously
treated by the
step of metals biosorption with the sulfate-reducing consortium biofilm in the
anaerobic
bioreactor and simultaneously adding a suspension of one or more particulate
complex
carbonaceous organic compounds as electrons donors, and
d) removing the treated water from the anaerobic bioreactor.
In an embodiment of the invention, the halotolerant sulfate-reducing microbial
consortium is
enriched from an environment sample.
CA 02883147 2015-02-19
7
In another embodiment of the invention the environment sample is anaerobic mud
from a saline
pond or a salt flat.
In an embodiment of the invention the halotolerant sulfur-reducing microbial
consortium is
comprised of at least hydrolytic, fermentative, acetogenic and sulfur-reducing
microorganisms.
In an embodiment of the invention the halotolerant sulfate-reducing microbial
consortium is
comprised by bacteria and arqueas.
In another embodiment of the invention the bacteria belong to at least the
phylogenetic groups of
proteobacteria a, 13, y and 6 and bacteria of the Cytophaga-Flavobacterium
group.
In an additional embodiment of the invention the halotolerant sulfate-reducing
microbial
consortium presents the capacity of growing at sodium chloride concentrations
between 0 and
100 g/L.
In an embodiment of the invention the complex organic compound(s) are products
of natural
origin rich in polymeric organic compounds.
In another embodiment of the invention the products of natural origin rich in
polymeric organic
compounds are selected from the cellulose group, the products or residues from
lignocellulosic
vegetables, the starch, the vegetable products or residues that are rich in
starch, the sea algae, the
microalgae and cyanobacteria.
In an embodiment of the invention the support materials used in the
biosorption step or sulfate
reduction are selected from the group of ceramic, siliceous rock, glass and
plastic.
Definitions:
Microbial consortium: in this invention the concept of microbial consortium is
understood as a
group of different microorganisms acting together. In a microbial consortium
microorganisms with
different metabolic capacities can be found. In the particular case of the
sulfur-reducing microbial
consortium it is comprised by, for example, hydrolytic, fermentative,
acetogenic and sulfur-
reducing microorganisms. Among the hydrolytic microorganisms, proteolytic
microorganisms
CA 02883147 2015-02-19
8
(capable of degrading proteins), saccharolytic microorganisms (capable of
degrading several
sugars), lipolytic microorganisms (capable of digesting the lipids or fats),
or celullitic
microorganisms (capable of degrading the cellulose or the vegetable material)
could be found.
These different metabolic capacities allow the consortium to be capable of
degrading a variety of
complex organic residues.
Description of the Figures
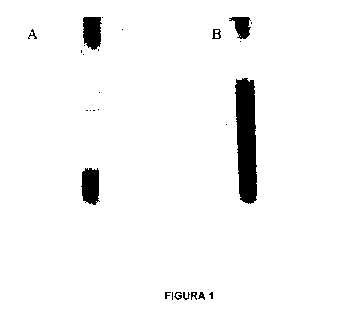
FIGURE 1:
This figure shows the change occurred in the aspect of the culture medium with
starch as
substrate before and after culturing the sulfate-reducing microbial
consortium. The black color is
due to the precipitation of the iron sulfide due to the reaction between the
suifhydric acid
produced by the microorganisms and the ferrous ion present in the culture
medium.
FIGURE 2:
This figure shows the hybridization in situ of the microbial consortium
cultured in a culture
medium with spirulina. The percentage of each group is shown marked with the
specific probes
with regards to the total microorganisms marked with DAPI. The error bars
correspond to the
standard deviation between the percentages of microorganisms marked with the
probe obtained
from at least 3 different images.
FIGURE 3:
This figure shows the hybridization in situ of the microbial consortium
cultures in a medium with
starch. The percentage of each group is shown marked with the specific probes
with regards to the
total microorganisms marked with DAPI. The error bars correspond to the
standard deviation
between the percentages of microorganisms marked with the probe obtained from
at least 3
different images.
FIGURE 4:
This figure shows the hybridization in situ of the microbial consortium
cultures in a medium with
cellulose. The percentage of each group is shown marked with the specific
probes with regards to
the total microorganisms marked with DAR The error bars correspond to the
standard deviation
CA 02883147 2015-02-19
9
between the percentages of microorganisms marked with the probe obtained from
at least 3
different images.
FIGURE 5:
This figure shows the concentration of sulfate in media with spirulina and pH
4.0 (-4,-); 5.0 (-4-);
6.0 (-.-) and 7.5 (-4-) at different times. The error bars correspond to the
standard deviation of
three independent cultures. * Shows a significant difference between the
concentration of sulfate
in media at pH 7.5 and media at pH 4 and 5 as per Duncan trial p<0.05.
FIGURE 6:
This figure shows the concentration of sulfate in media with starch and pH 4.0
(-*-); 5.0 (-0-); 6.0
(.4-) and 7.5 (-4-) at different culture times. The error bars correspond to
the standard deviation
of three independent cultures. * Shows a significant difference between the
concentration of
sulfate in media with pH 7.5 and 6 and the media at pH 4 and 5 as per Duncan
trial p<0.05.
FIGURE 7:
This figure shows the concentration of sulfate in media with cellulose and pH
4.0 (-0-); 5.0 (-0-);
6.0 (.4-) and 7.5 (-4-) at different culture times. The error bars correspond
to the standard
deviation of three independent cultures.. * Shows a significant difference
between the
concentration of sulfate in media with pH 7.5 and media with pH 4.0; 5.0 and
6.0 as per Duncan
trial p<0.05.
FIGURE 8:
This figure shows the concentration of sulfate in culture media with spirulina
with the following
concentration of copper 0 (.), 100 120 (-A-
), 140 (->(-), 160 (-A-) and 180 (-0-) mg/L
at different culture times. The error bars correspond to the standard
deviation of three
independent cultures. * Shows a significant difference between the
concentration of sulfate in
media without copper and in media with copper as per Duncan trial p<0.05.
FIGURE 9:
CA 02883147 2015-02-19
This figure shows the concentration of sulfate in culture media with starch
with the following
concentration of copper 0 (-=-), 40 (-&-), 60 (-0.-),
80 and 100 (-44---) mg/L at different
culture times. The error bars correspond to the standard deviation of three
independent cultures.
* Shows a significant difference between the concentration of sulfate in media
without copper
and in media with copper as per Duncan trial p<0.05.
FIGURE 10:
This figure shows the concentration of sulfate in culture media with cellulose
with the following
concentration of copper 0 20 (-4-), 40 60 and
80 (-)4-) mg/L at different
culture times. The error bars correspond to the standard deviation of three
independent cultures.
* Shows a significant difference between the concentration of sulfate in media
without copper
and in media with copper as per Duncan trial p<0.05.
FIGURE 11:
This figure shows the concentration of sulfate in culture media with starch
with the following zinc
concentrations 0 (--=-), 100 (-i-), 120 (-*-.), 140 (-X--), 160 and 180 (-
4-) mg/L at
different culture times. The error bars correspond to the standard deviation
of three independent
cultures. * Shows a significant difference between the concentration of
sulfate in media without
zinc and in media with zinc as per Duncan trial p<0.05.
FIGURE 12:
This figure shows the concentration of sulfate in culture media with cellulose
with the following
zinc concentrations 0 40 {-00-), 60 (-*-), 80 (-.-) and 100 mg/L at
different culture
times. The error bars correspond to the standard deviation of three
independent cultures. *
Shows a significant difference between the concentration of sulfate in media
without zinc and in
media with zinc as per Duncan trial p<0.05.
FIGURE 13:
This figure shows the concentration of sulfate and sulfhydric acid in the
effluent of the bioreactor
without support during the semi-continuous operation period. Day 66
corresponds to the day the
feeding of the bioreactor is started in a semi-continuous way. The
concentration of sulfate (-fr-)
CA 02883147 2015-02-19
11
and sulfhydric acid (-4-) in the effluent of the bioreactor, concentration of
initial sulfate of the
culture medium (¨), maximum limit of sulfate established for superficial
waters (rule 182637,
Decree Supreme 90, Chile) (---). The error bars correspond to the standard
deviation of three
measurements done to the same sample.
FIGURE 14:
This figure shows the concentration of sulfate and generation of sulfhydric
acid in the effluent of
the bioreactor with silica gravel as support during the semi-continuous
operation period. Day 98
corresponds to the day when the feeding of the bioreactor in a semi-continuous
way starts. The
concentration of sulfate (-*-) and sulfhydric acid (-el-) in the effluent of
the bioreactor, sulfate
initial concentration of the culture medium (¨}, maximum limit of sulfate
established for
superficial waters (rule 182637, Decree Supreme 90, Chile) (---). The error
bars correspond to
the standard deviation of three measurements done to the same sample.
FIGURE 15:
This figure shows the concentration of sulfate and generation of sulfhydric
acid in the effluent of
the bioreactor with Celite R-635 as support during the semi-continuous
operation period. Day 98 =
corresponds to the day when the feeding of the bioreactor in a semi-continuous
way starts. The
concentration of sulfate (-h-) and sulfhydric acid (-4,-) in the effluent of
the bioreactor, sulfate
initial concentration of the culture medium (¨), maximum limit of sulfate
established for
superficial waters (rule 182637, Decree Supreme 90, Chile) (---). The error
bars correspond to
the standard deviation of three measurements done to the same sample.
FIGURE 16:
This figure shows the concentration of sulfate and sulfhydric acid in the
effluent of the bioreactor
with Celite R-635 fed with MAD in a semi-continuous way. Day 1 the feeding
with culture medium
started and day 9 with MAD. Sulfate and sulfhydric acid (-4,-)
concentration in the effluent
of the bioreactor, concentration of sulfate of the culture medium or the MAD
(¨), maximum
limit of sulfate established for superficial waters (rule 182637, Decree
Supreme 90, Chile) (---).
The error bars correspond to the standard deviation of three measurements done
to the same
sample.
CA 02883147 2015-02-19
12
FIGURE 17:
This figure depicts the flow diagram of a particular application of the
process for the biological
removal of sulfate and metals from mining effluents, mine acid drainages or
different industrial
liquid residues.
The following examples describe some actual applications of the invention
although they do not
intend to limit the framework or scope of this invention.
EXAMPLES
Example 1
Sulfate-reducing microbial consortium culture using complex carbonaceous
substrates.
The microbial consortium is enriched from the anaerobic sediment of a saline
pond. The culture of
the microbial consortium with complex substrates (microcrystalline cellulose,
starch, spirulina and
industrial starch) is done in 15 cm high by 1.5 cm wide test tubes with 10 mL
culture medium. The
modified Postage "C" culture medium is used (Barton and Tomei, 1995.
"Characteristics and
activities of sulfate-reducing bacteria". In: Sulfate-Reducing Bacteria. Ed:
Baron I. L. 1-32.). Table 1
shows the composition of each of the culture media with their respective
substrates. The starch
corresponds to insoluble corn starch from Merck, Germany. The cellulose
corresponds to
Microcrystalline Cellulose Sigmacell provided by Sigma USA. The spirulina
corresponds to Spirulina
GNC supplied by General Nutrition Centers, U.S.A. The industrial corn starch
corresponds to
normal corn starch 034010, Buffalo supplied by Inducorn S.A., Chile. Once the
culture media are
prepared the pH is set at 7.5 and are autoclaved for 15 minutes at 121 C.
Once sterilized the
media are covered with paraffin oil (Biomerieux, France) still hot in order to
keep the anaerobiosis
in the culture medium. The sterile media are inoculated with approximately 500
iL of the former
culture and is kept at 28 C.
Table 1: Composition of the modified Postgate "C" culture media with different
nutrients.
Compound [g/L] Culture media
Starch Cellulose Spirulina
Industrial starch
CA 02883147 2015-02-19
,
13
K2HPO4 0,5 0,5 0,5 0,5
NH4CI 1,0 1,0 1,0 1,0
Na2SO4 1,0 1,0 1,0 1,0
CaC12=61-120 0,1 0,1 0,1 0,1
MgSO4=7H20 2,0 2,0 2,0 2,0
NaCI 60 60 60 60
FeSO4=7H20 0,5 0,5 0,5 0,5
Yeast extract 0,5 0,5 0,5 0,5
Starch 40
Cellulose 40
Spirulina 20
Industrial starch 20
The growth of the microbial consortium is determined by means of the
appearance of black
precipitate which corresponds to FeS. The former is produced by the reaction
of H2S produced by
the reduction of sulfate with iron present in the culture medium. Figure 1 is
an exemplary
illustration of the change produced when culturing the microbial consortium in
a medium
containing starch as substrate. Figure 1A shows the sterile culture medium and
Figure 1B shows
the culture medium after having inoculated and cultured for 20 days. The same
change in color
can be seen in all culture media with the different substrates (cellulose,
spirulina and industrial
starch).
The characterization of the microbial populations enriched in the different
complex organic
compounds was done using the in situ hybridization technique. Table 2 shows
the probes and their
characteristics used to perform the in situ hybridization with fluorescent
probe.
Table 2: Sequences, position in the target rRNA and specificity of the probes
used in the
hybridization in situ (Amann et al. 1995. "Phylogenetic identification and in
situ detection of
individual microbial cells without cultivation". Microbiol. Rev. 59: 143-169).
CA 02883147 2015-02-19
14
Probe Sequence rRNA position Specificity
EUB 338 GCTGCCTCCCGTAGGAGT 16S, 338-355 Bacteria
Archaea GTGCTCCCCCGCCAATTCCT 16S, 915-934 Arqueas
Sub-class a of the
ALF lb CGTTCGYTCTGAGCCAG 16S, 19-35
Proteobacteria
Sub-class f3 of the
BET 42a GCCTTCCCACTTCGTIT 23S, 1027-1043 Proteobacteria.
Sub-classy of the
GAM 42a GCCTTCCCACATCGTTT 23S, 1027-1043 Proteobacteria.
Most of the members of sub-
BRS 385 CGGCGTCGCTGCGTCAGG 16S, 385-402
class 6 of the Proteobacteria.
CF319a TGGTCCGTGTCTCAGTAC 16S, 319-336
Cytophaga-Flavobacterium
In order to perform the microbial consortium hybridization in situ 100 IA of
culture is taken and
placed in 900 l.IL PBS and centrifuged for 5 minutes at 4724 x g. Once the
centrifuging is done the
supernatant is discarded and the pellet is re-suspended in 900 1_ PBS, then
centrifuged for 3
minutes at 111.8 x g. From the supernatant of the second centrifuging 50 pi
are taken and placed
on a microscope slide where the sample is fixed with heat. Once fixed, 20 pl
37% formaldehyde is
added on each sample for 20 minutes. Then 50 L of the hybridization solution
is added to each
sample (see Table 3) containing 20 mg of probe marked on each of the samples.
They are
incubated for one and a half hours at 37 C. Then a rinse with the rinsing
solution (see Table 4) is
performed for half hour at 37 C.
Table 3: Hybridization solution composition for the hybridization in situ. The
composition of this
solution depends on the probe used. The hybridization solution 1 was used for
probes ALF lb and
EUB 338, while the hybridization solution 2 was used for probes BET42a,
GAM42a, CF319a and
BRS385.
Compound Hybridization 1 Hybridization 2
CA 02883147 2015-02-19
Formamide 20% 35%
NaCI 0,9 M 0,9 M
Tris/HCI pH 7,2 20 mM 20 mM
SDS 0,01 % 0,01 %
Once rinsed the microscope slides are left to dry and dyed later with DAPI
(4',6-Diamidino-2-
phenylindole). The stain with DAPI is done adding 20 L of a solution
containing this fluorochrome
at a 50 concentration. After 10-15 minutes it is rinsed with distilled
water to eliminate the
excess of DAPI. Once the hybridization is done, it is watched in an
epifluorescence microscope
with Zeiss N 20 filter for probe marked with CY3 and Zeiss N 09 filter to
see the bacteria marked
with DAPI. The samples were photographed using a Canon PowerShot sx110 IS
camera and the
Remote Capture v.3Ø1.8 software. The images were processed using the ImageJ
software to
decrease the background in those that needed it. With the images obtained a
counting of the
microorganisms marked is done with each of the specific probes and the total
microorganisms
marked with DAPI.
The hybridization in situ is applied to the microbial consortiums cultures
between 5 and 7 days in
test tubes with cellulose, starch and spirulina media as nutrients.
Table 4: Composition of the rinse solution for hybridization in situ. The
composition of this solution
depends on the probe to be used. The rinse solution 1 was used for probes ALF
lb and EUB 338,
while rinse solution 2 was used for probes BET42a, GAM42a, CF319a and BRS385.
Compound Rinse 1 Rinse 2
Tris/HCI pH 7,2 20 mM 20 mM
SDS 0,01% 0,021%
NaCI 180 mM 40 mM
EDTA 5 mM 5 mM
When spirulina is used as complex organic compound the results show that the
microbial
consortium is comprised by microorganisms of all the groups studied (Figure
2). So, approximately
56% of microorganisms correspond to bacteria while 7% are arqueas. On the
other hand, the
CA 02883147 2015-02-19
16
proteobacteria 5 correspond to a considerable percentage within the
microorganisms present in
the sample reaching 21% of the total. This percentage is higher to the one
found using the specific
probes for proteobacteria a, p and y and bacteria of the group Cytophaga-
Flavobacterium since
the percentages found with these are below 11%.
As can be seen in Figure 3, the microbial consortium kept in a medium with
starch is comprised by
microorganisms of all the groups studied as well as the consortium cultured in
media with
spirulina. This way near 47% of the microorganisms correspond to bacteria,
while 13% of
microorganisms present are Arqueas. On the other hand, the presence of
proteobacteria 5 can be
observed in the sample and reach 14% of the total microorganisms. Unlike what
is observed in the
culture medium with spirulina, the proteobacteria 6 do not represent a
majority and can be found
in a proportion similar to the microorganisms marked with the probes specific
for proteobacteria a
and bacteria of the Cytophaga-Flavobacterium group that correspond to 15 and
12% respectively
of the total microorganisms. Instead in a similar way to what was found in the
medium with
spirulina, the microorganisms marked with the specific probe for
proteobacteria (3 and y reach 5
and 4% respectively.
When cellulose is used as complex organic compound, the results also show that
the sulfate-
reducing consortium is formed by all the groups studied (Figure 4). In the
sample of the microbial
consortium, 34% of the microorganisms correspond to bacteria and near 2% of
microorganisms
present are Arqueas, both percentages i..re lower as compared to what was
found with the culture
media with spirulina and starch. The percentage of proteobacteria 5 within the
sulfate-reducing
consortium reaches 13% of the total microorganisms. Also, the presence of
proteobacteria a, 13
and y and bacteria of the Cytophaga-Flavobacterium group is detected in
percentages below 9%.
Therefore, the halotolerant sulfate-reducing microbial consortium is comprised
by bacteria and
arqueas. Its proportion depends on the complex carbonaceous organic compound
used for its
culture. Regarding bacteria, these belong to, at least, the phylogenetic
groups of proteobacteria a,
13, y and 5 and bacteria of the Cytophaga-Flavobacterium group. Its ratio also
depends on the type
of electrons donor with which it is cultured.
Example 2
CA 02883147 2015-02-19
17
Effect of the pH on the microbial consortium's capacity of reducing sulfate.
The culture media composition is the olie used for Example 1, although the pH
is set at 4.0, 5.0,
6.0 and 7.5; using potassium hydroxide (KOH) to alkalify and phosphoric acid
(H3PO4) to acidify.
The effect of the pH on the microbial consortium's capacity of reducing
sulfate is determined by
the concentrations of sulfate in media with spirulina, cellulose and starch at
different culture
times. To determine the concentration of sulfate a turbidimetric technique is
used. Between 600-
1000 pL sample from the culture media are taken and centrifuged for 15 minutes
at 4724 x g.
From the supernatant obtained 500 pL was taken and placed in 39.5 mL distilled
water. From the
resulting solution 10 mL is taken and the initial turbidity is measured, then
3g barium chloride is
added (BaCl2) in the 10 mL sample and shaken for 1 minute. When adding the
BaCl2a precipitate is
produced and this corresponds to BaSO4. The turbidity measured is proportional
to the amount of
precipitate and therefore to the amount of sulfate present in the sample since
BaCL2 is added in
excess (American Public Health Association, American Water Works Association y
Water
Environment Federation. 1998. "4500-S042-E". En: "Standard Methods for the
Examination of
Water and Wastewater". Ed. 20.). Once shaken, the sample is left to decant
those larger particles
for 5 minutes and the turbidity of the sample is measured at 890 nm. By means
of a calibration
curve prepared from a sulfate standard solution, the correlation between
turbidity and
concentration of sulfate is obtained. So, by the difference between the
initial turbidity and the
turbidity obtained after adding barium chloride, the concentration of sulfate
in the samples is
determined.
Figure 5 shows the concentration of sulfate in time when cultivating the
sulfate-reducing microbial
consortium in media with different pH and spirulina as substrate. In the
culture media with
spirulina at pH 6.0 and 7.5after nine days culture of the microbial consortium
there is a decrease in
the concentration of sulfate, reaching values near to 11 and 10 mM
respectively. On the other
hand, in the culture media with pH 5.0 and 4.0 until day 17 of culture, no
decrease in the levels of
sulfate is observed. The statistical analysis, by means of the Duncan trial
with p<0.05 indicates
there is a significant difference between the concentration of sulfate in the
culture medium with
pH 7.5 with regards to the media with pH 4.0 and 5.0 on days 14 and 17 of
culture.
Figure 6 shows the concentration of sulfate in time when culturing the sulfate-
reducing microbial
consortium in media with different pH and starch as substrate. In the media
culture with pH 6.0
CA 02883147 2015-02-19
18
and 7.5 there is a decrease in the concentration of sulfate unlike the media
with pH 4.0 and 5Ø
The difference between initial and final concentration of sulfate in the media
with pH 6.0 and 7.5
is statistically significant as per Duncan's trial. The levels of sulfate
decrease up to approximate
values of 5.5 mM sulfur in media with pH 7.5. On the other hand, in the
culture medium at pH 6.0
the concentration of sulfate is reduced at levels below 7.0 mM. Duncan's
statistical trial shows
that there are significant differences among the concentrations of sulfate
measured in the media
at pH 4.0 and 5.0 as compared to the media with pH 7.5 and 6.0 on days 19 and
26 of culture.
Moreover, there is not a significant statistical difference between the
concentration of initial and
final sulfate in the culture media with pH 4.0 and 5.0; unlike the media with
pH 6.0 and 7.5 where
there actually is difference.
Figure 7 shows the concentration of sulfate in time when culturing the sulfate-
reducing microbial
consortium in media with different pH and cellulose as substrate. The higher
decrease in the
sulfate levels is produced at pH 7.5 being the initial and final
concentrations of sulfates
significantly different as per Duncan's trial. In the culture medium with pH
6.0 also a decrease in
sulfate levels between the initial and final concentration of sulfate is also
observed. Although this
decrease is smaller than that with the medium at pH 7.5, likewise it is
statistically significant. The
culture media with pH 4.0 and 5.0 do not show a considerable sulfate decrease
and this is
confirmed by the statistical analysis indicating that there is no significant
difference. Moreover,
there is a statistically significant difference between the concentration of
sulfate in the medium
with pH 7.5 as compared to the media with pH 4.0, 5.0 and 6.0 on day 26 of
culture.
Example 3
Effect of the presence of metals in the microbial consortium's capacity of
reducing sulfate.
Preparing culture media with spirulina, starch and cellulose as substrate the
same way as was
done in Example 1 although with different metal concentrations. Metals used Ze
and Cu2+ are
added as salts (ZnCl2 y CuCl2 respectively). The culture media with spirulina
have the following
concentrations 0, 100, 120, 140, 160 and 180 mg/L copper. The culture media
with starch have the
following concentrations 40, 60, 80 and 100 mg/L copper and 100, 120, 140, 160
and 180 mg/L
zinc. For the media with cellulose culture the following concentrations are
used 20, 40, 60 and 80
mg/L copper and 40, 60, 80 and 100 mg/L zinc.
CA 02883147 2015-02-19
19
Figure 8 shows the concentration of sulfates in media with spirulina and
different concentration of
coppers where the microbial consortium was cultured. The control shows a
tendency to decrease
the levels of sulfate on culture days 2, 4 and 6 that is not seen in culture
media with copper.
Duncan's statistical trial indicates that there is a significant difference
between the control without
copper and the medium with 200 mg/L copper on day 2 and between the control
and the medium
with 140 mg/L on culture day 4. This means that the removal of sulfate in a
culture medium
without copper would happen before than in the media with copper.
Figure 9 shows the concentration of sulfate in cultures with starch at
different concentration of
copper. The microbial consortium cultured in a medium with starch is capable
of reducing the
sulfate levels in the presence of copper but in less amount with respect to
the control without
copper. Using the Duncan trial a significant difference in the concentration
of sulfate on culture
day 11 between the control and the media with copper can be found. Moreover,
there is a
significant difference between the control and the media with 60, 80 and 100
m/L copper on days
22 and 27.
Figure 10 shows the concentration of sulfate in cultures with cellulose and
different concentration
of copper. Duncan's statistical trial indicates that there is only significant
difference between the
initial and final concentration of sulfate of the control cultures without
copper. Moreover, there is
a significant statistical difference in the concentration of sulfate on
culture days 29 and 38
between the control without copper and the culture media with copper. With all
concentrations of
copper used there was an inhibition in the capacity of the microbial
consortium of reducing
sulfate.
Figure 11 shows the concentration of sulfate in cultures with starch and
different concentration of
zinc. The control condition without zinc is the only one where a decrease in
the levels of sulfate
occurs. Duncan's statistical analysis indicates that there is a significant
difference in the sulfate
levels from culture day 7 on between the control condition without zinc and
the culture media
with zinc.
Figure 12 shows the concentration of sulfate in cultures with cellulose and
different
concentrations of zinc. In the control condition without zinc, such as in
cultures with 40 and 60
CA 02883147 2015-02-19
mg/L zinc there is a decrease in the concentration of sulfate. Anyhow, this
decrease is only
statistically significant in control cultures and with 40 mg/L zinc. Duncan's
trial indicates that on
culture day 29 there are significant differences between the concentration of
sulfate of the control
without zinc and the culture media with zinc. On the other hand on culture day
39 there is a
significant difference between the control and culture media with 60, 80 and
100 mg/L zinc.
,Examole 4
Removal of sulfate using a sulfate-reducing microbial consortium kept in a
bioreactor without
support material.
Use a glass bioreactor with a useful volume of 496 cm3, (dimensions: 49 cm
high x 3.6 cm wide).
The bioreactor is filled with culture medium with the composition shown in
Table 1 and industrial
starch as substrate at a concentration of 2 g/L. In order to keep the
anaerobiosis thioglycolic acid
in the culture medium at a 0.1 g/L concentration is used. The bioreactor is
inoculated with an
already grown culture from the sulfate-reducing microbial consortium kept
without support
material. The bioreactor is kept at 28 C. The bioreactor operates for 65 days
as batch until the
sulfate-reducing consortium biofilm is formed on the starch. In this case, and
because it is partially
in the form of solid particles in the culture medium, the starch itself acts
simultaneously as
substrate and solid material for the attachment of the microorganisms. As of
day 66 it starts to be
fed daily in a semi-continuous way. For the feeding and re-circulation of the
bioreactors Cole-
Parmer Instrument Co., U:S.A. peristaltic pumps model 7554-30,1 ¨ 100 rpm are
used.
Table 5: Modified parameters during the operation of the bioreactor without
support.
Batch Semi-continuous
Days 1-34 35 ¨ 65 66 ¨ 78 79 ¨ 81 82 - 85 86 ¨ 90
Recirculation* 50 % 100 % 100 % 100% 100 %
Feeding* 10% 20% 40% 30%
pH 7.5 7.5 9 9
* Percentage of the total volume of the bioreactor re-circulated and fed
daily.
CA 02883147 2015-02-19
21
Table 5 shows the modifications in re-circulation, feeding and pH of the
medium used to feed the
bioreactor at different times.
Figure 13 shows the concentration of sulfate and sulfhydric acid in the
effluent of the bioreactor
during the semi-continuous period. The concentration of sulfate at the moment
of starting the
feeding is over the regulation (maximum sulfate level established for
superficial waters in rule
182637 Decree Supreme 90, Chile) and is kept until day 72 when it decreases to
approximately 9
mM. By day 74 a concentration of sulfate in the vicinity of 7.3 mM is
registered which is kept
stable between 7.0 and 7.5 mM until day 81. The concentration of sulfate is
kept below 7.5 mM
despite that on day 79 of operation the daily feeding volume is increased from
10 to 20%.
Anyhow, when the daily feeding volume is increased to 40% of the volume of the
bioreactor, an
increase in the concentration of sulfate in the bioreactor's effluent is
produced thereby causing an
increase in the concentration of sulfate in the bioreactor's effluent hence
taking its value on day
85 above the regulation. Finally it can be seen that the concentration of
sulfate at the end of the
experiment reaches values below the regulation because the feeding volume
decreases by 30%.
The sulfhydric acid concentration in the bioreactor's effluent at the
beginning of the operation as
semi-continued produces an exponential increase reaching an approximate value
of 1.3 mM on
day 70. As of this day an important variability in the concentration of
sulfhydric acid can be
observed reaching values between 1.0 and 2.5 mM.
Example 5
Removal of sulfate using a sulfate-reducing microbial consortium kept in a
bioreactor with silica
gravel as support material.
Use a Teflon bioreactor with 4120 cm3 useful volume (dimensions: 49 cm high x
3.3 cm wide). The
bioreactor is filled with culture medium as described in Example 4 although it
is further added 313
g silica gravel as support material. The bioreactor is inoculated with a
culture that grew in contact
with the silica gravel.
Table 6: Modified parameters during the bioreactor operation with silica
gravel as support
material.
CA 02883147 2015-02-19
22
=
Batch Semi-continuous
Days 1-66 67 - 97 98 - 105 106 - 112 113 - 116
117 - 121
Recirculation* 50 % 100 % 100 % 100 % 100 %
Feeding* 10% 10% 20% 30%
pH 7.5 8 9 9
*Percentage of the total volume of the bioreactor re-circulated and fed daily.
Table 6 shows the re-circulation, feeding and pH modifications of the medium
used to feed the
bioreactor at different times.
Figure 14 shows the sulfate and sulfhydric concentrations in the bioreactor's
effluent with silica
gravel once the feeding is started as semi-continuous system. Regarding the
concentration of
sulfate, it can be seen that when starting the feeding of the bioreactor the
rule of sulfate is
exceeded (rule 182637 Decree Supreme 90, Chile). By keeping the feeding volume
at 10% no
decrease in the concentration of sulfate is observed. As of day 106 a culture
medium with pH 8
was used to feed the bioreactor. On day 107 a decrease in the sulfate levels
is observed so the
concentration decreases below the rule. A sustained decrease in the
concentration of sulfate is
observed with a feeding corresponding to 10% of the volume with pH8 until
reaching on day 112
an approximate value of 5.5 mM. An increase in the feeding volume on day 113
from 10 to 20% of
the volume generates a slight increase in the concentration of sulfate. On day
117 of operation the
lower concentration of sulfate is reached which corresponds to approximately
4.8 mM. The
increase in the feeding volume on day 117 from 20 to 30% of the volume
produces a considerable
increase in the concentration of sulfate, notwithstanding this is kept below
the rule. Regarding the
concentration of sulfhydric acid a gradual increase until day 113 is observed
reaching an
approximate concentration of 3.0 mM. From day 116 on the concentration varies
between 2.8 and
mM sulfhydric acid.
Example 6
CA 02883147 2015-02-19
23
Removal of sulfate using a sulfate-reducing microbial consortium kept in a
bioreactor with Celite
R-635 as support material.
A bioreactor as the one described in Example 4 is used and further added 200 g
Celite R-635 as
support material. The bioreactor is inoculated with a culture grown in contact
with Celite R-635.
Table 7 shows the re-circulation, feeding and pH modifications of the medium
used to feed the
bioreactor at different times.
Table 7: Modified parameters during the operation of the bioreactor with
Celite R-635 as support.
Batch Semi-continuous
Days 1-66 67 - 97 98 - 109 110 - 112 113 - 116
117 ¨ 121
Recirculation* 50 % 100 % 100 % 100 % 100 %
Feeding* 10% 20% 40% 30%
pH 7,5 7,5 9 9
*Percentage of the total volume of the bioreactor re-circulated and fed daily.
Figure 15 shows the concentrations of sulfate and sulfhydric acid in the
bioreactor's effluent with
silica gravel once the feeding is started as semi-continuous system. At the
moment the feeding
starts the concentration of sulfate is below the rule (rule 182637 Decree
Supreme 90, Chile). This
condition is kept until day 109 in which period it is fed with 10% of the
volume. It can be observed
that there is no increase in the concentration of sulfate as of day 110 when
the feeding volume is
increased from 10 to 20%. The volume feeding increase from 20 to 40% of the
bioreactor's volume
as of day 113 produces an increase in the concentration of sulfate so it
reaches a 10 mM
concentration on day 116. By decreasing the daily feeding volume up to 30% the
concentration of
sulfate decreases.
The sulfhydric acid concentration increases until day 112 when a value of 4.8
mM is reached. On
day 119 of operation a maximum value of 7 mM is reached.
Example 7
1
CA 02883147 2015-02-19
24
Removal of sulfate present in a mine acid drainage (MAD) using a sulfate-
reducing microbial
consortium kept in a bioreactor with Celite R-635 as support material.
A bioreactor like the one described in Example 6 is used to eliminate the
sulfate present in a MAD
previously treated. The pre-treatment consists of adding lime to increase the
pH and precipitate
the copper that is present. The amount of lime used is the necessary one to
reach a pH equal to
6.3. The biosorption allows the decrease in the concentrations of metals
present in the MAD. The
treatment consists of putting the MAD in contact with a biomass obtained from
a culture of the
strain Bacillus sp VCHB-10 deposited as NRRL-B-30881 (US 7.951.578; US
7.479.220). Thereby, the
metals present in the MAD are adsorbed by the biomass thus obtaining a MAD
with a lower metal
concentration. The obtaining of the biomass used in the biosorption is done as
per the following
protocol. Bacillus sp. VCHB-10 is cultured in solid TSA medium for 24 hours at
28 'C. From this
culture a stripe is taken and inoculated in a fermenter (Multigen F-1000
Fermenter, 2 liters
capacity, with aeration, temperature and agitation control, New Brunswick
Scientific, U.S.A.) filled
with sterile medium, the composition of the culture medium used can be seen in
Table 8.
The culture of Bacillus sp. VCHB-10 in a fermenter is done at 28 C during 16
hours with 200 rpm
agitation and 0,75 vvm aeration. Once the culture time elapsed the biomass is
left to decant and
the supernatant is discarded. The biomass obtained is used for the biosorption
of the metals
present in the MAD. For this purpose 2 L of mine acid drainage are put in
contact with the biomass
in the bioreactor for 1 hour with agitation at 75 rpm. Once the biosorption is
done the biomass is
left to decant and the supernatant corresponding to the treated MAD with a low
metal
concentration is taken.
Table 8: Composition of the culture medium for Bacillus sp. VCHB-10.
Compound Concentration g/L
Na2HPO4.2H20 1,3
KH2PO4 0,3
K2SO4 0,1
NaCI 0,1
M gSO4=7 H20 0,02
CaC12=2H20 0,013
I
CA 02883147 2015-02-19
FeSO4=7H20 0,0018
Yeast extract 1,0
Triptone 1,0
Glucose 10,0
With a biosorption treatment the concentration of copper present in the MAD
already treated
with lime is decreased. Both treatments allow decreasing the concentration of
copper from 1400
mg/L to 1.8 mg/L. After the MAD's pre-treatment K2HPO4, NH4CI is added and
yeast extract at the
same concentration of the culture medium.
Table 9: Modified parameters during the bioreactor operation with Celite R-635
as support fed
with culture medium or MAD
Semi-continuous
Days 1 - 9 10 - 13 14 - 18 19 - 23
Feeding Culture medium MAD MAD MAD
Recirculation* 100 % 100 % 100 % 100%
Feeding* 30% 30% 20% 20%
pH 9 9 10 11
*Percentage of the total volume of the bioreactor re-circulated and fed daily
The treatment with lime allows decreasing the concentration of sulfate from
37.5 mM up to 18.75
mM and the concentration of copper from 1.4 g/L up to 20 mg/L. The treatment
by biosorption
decreased the copper concentration from 20 mg/I up to 1.8 mg/L.
Table 9 shows the re-circulation, feeding and pH modification of the medium
used to feed the
sulfate-reducing anaerobic bioreactor at different times.
Figure 16 shows the concentrations of sulfate and sulfhydric acid in the
bioreactor's effluent with
Celite R-635 used to remove the sulfate present in a MAD. At the beginning the
bioreactor is fed
daily with culture medium corresponding to a 30% of the bioreactor's volume.
As of day 10 the
operation is fed with MAD corresponding to 30% of the bioreactor's volume. As
of day 10 an
CA 02883147 2015-02-19
26
increase in the concentration of sulfate is produced. On day 13 of the
operation the feeding
volume is decreased to 20% of the bioreactor's volume. The concentration of
sulfate continues to
increase until it reaches a stable concentration. Thereby the concentration of
sulfate is kept as of
day 18 of the operation at approximately 11.5 mM. The concentration of
sulfhydric acid is kept
stable in time in the vicinity of 6 mM.
Example 8
Process for the biological removal of sulfate and metals
The process consists of treating waters contaminated with sulfate or with
sulfate and metals
coming from industries of different sectors, mining among them. The process is
comprised by a
pre-treatment of the physicochemical type and biological and later with a
biological treatment to
decrease the concentration of sulfate using a sulfate-reducing microbial
consortium capable of
using complex substrates.
As shown in Figure 17, the process begins with a pre-treatment divided in two
stages. The waters
with a high concentration of sulfate and metals enter through conduit 1 to the
reactor 3, to which,
through conduit 2 quicklime is added, which allows to decrease the
concentration of metal and
sulfate.
Through conduit 4 the precipitate produced in the reactor 3 is removed.
Through conduit 5 the
effluent of the reactor 3 is taken to the reactor 7 where the removal of the
metals is done by
biosorption with a bacterial biomass. For the biosorption process biomass of
Bacillus sp. VCHI3-10
is used. Through conduit 6 an acid solution to do the metal desorption process
is added from the
bacterial biomass into the reactor. Later through conduit 8 an effluent with
high concentration of
metals is obtained, The biomass present inside the reactor 7 is active again
and can be used in a
new biosorption/desorption cycle. Alternatively two or more bioreactors may be
used to do the
biosorption process in an alternate way and make the process continuous,
keeping one of the
bioreactors in biosorption stage and the other one in desorption stage.
Through conduit 9 the
effluent from reactor 7 is taken to the anaerobic bioreactor 11, where the
sulfate-reducing
microbial consortium removes the sulfate present in the effluent of the
reactor 2. The nutrients
for the sulfate-reducing microbial consortium are entered directly into the
anaerobic bioreactor 11
through conduit 10. The anaerobic bioreactor can maintain the biomass of the
sulfate-reducing
microbial consortium with and without the support material. In case of using
support material this
may correspond to Celite R-635, silica gravel, polyurethane, charcoal or
polyethylene. A re-
1
CA 02883147 2015-02-19
27
circulating system inside the anaerobic bioreactor 11 allows the optimization
of the sulfate-
reducing process. Thereby through conduit 12 an effluent with low
concentration of metals and
sulfate is obtained.