Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
1
BIOMEDICAL PATCHES WITH SPATIALLY ARRANGED FIBERS
BACKGROUND
[0001] Numerous pathological conditions and surgical procedures result in
substantial
defects in a variety of organs, tissues, and anatomical structures. In the
majority of such cases,
surgeons and physicians are required to repair such defects utilizing
specialized types of surgical
meshes, materials, and/or scaffolds. Unfortunately, the in vivo performance of
known surgical
materials is negatively impacted by a number of limiting factors. For
instance, existing synthetic
surgical meshes typically result in excessive fibrosis or scarification
leading to poor tissue
integration and increased risk, of post-operative pain. Simultaneously. known
biologic materials
may induce strong immune reactions and aberrant tissue ingrowth which
negatively impact
patient outcomes. Additionally, existing synthetic surgical meshes can create
scarification, post-
operative pain, limited mobility, limited range of motion, adhesions,
infections, erosion, poor
biomechanical properties, and/or poor intraoperative handling.
[0002] Nanofabricated or nanofiber meshes or materials composed of
reabsorbabie
polymer fibers tens to thousands of times smaller than individual human cells
have recently been
proposed as a unique substrate for implantable surgical meshes and materials.
Generally,
existing nanofiber materials tend to possess suboptimal mechanical performance
compared to
known surgical meshes. Existing nanofiber materials do not possess the tensile
strength, tear
resistance, and burst strength needed for numerous surgical applications or
for basic
intraoperative handling prior to in vivo placement. To combat this deficiency,
known meshes are
formed using higher fiber densities as a means of improving mechanical
strength. Yet,
utilization of such high-density meshes can decrease effective cellular
ingrowth into the mesh,
decrease mesh integration with native tissue, and reduce the biocompatibility
of the polymeric
implant. As a result, nanofiber materials with increased thickness and/or
strength and favorable
cellular and/or tissue integration and biocompatibility is needed as well as a
method for
producing nanofiber materials.
SUMMARY
[0003] A system for producing a structure including a plurality of fibers is
provided. The
system includes a polymer collector having a predefined pattern, wherein the
collector is charged
at a first polarity, and a spinneret configured to dispense a polymer, wherein
the spinneret is
charged at a second polarity substantially opposite the first polarity such
that polymer dispensed
from the spinneret forms a plurality of fibers on the predefined pattern of
the fiber collector.
81786882
2
[0004] A method for producing a structure including a plurality of fibers is
provided.
The method includes providing a collector with a predefined pattern, charging
the collector
with a first polarity, providing a spinneret, the spinneret configured to
dispense a polymer on
the provided collector, charging the spinneret to a second polarity
substantially opposite the
first, and dispensing a polymer on the collector, such that the polymer forms
a plurality of
fibers defining the structure, wherein the structure has at least two
densities formed by the
plurality of fibers.
[0005] A method for repairing a defect of a substrate. The method includes
providing a
substrate with a defect, providing a structure formed from a plurality of
polymeric fibers, the
structure having a plurality of densities, and applying the structure to the
substrate.
[0006] A method for producing a structure for use in repairing a defect in a
substrate is
provided. The method includes providing a first layer formed by a plurality of
polymeric
fibers, providing a second layer formed by a plurality of polymeric fibers,
the second layer
having a plurality of densities, and coupling the first layer and the second
layer together using
a first coupling process such that the first and second layers are configured
to separate after at
least one of a predetermined time and an environmental condition.
[0007] A structure for use in repairing a defect in a substrate is provided.
The structure
includes a first layer formed by a plurality of polymeric fibers and a second
layer coupled to
the first layer using a first coupling process, the second layer having a
plurality of densities
formed by a plurality of polymeric fibers, wherein the first and second layers
are configured
to separate after at least one of a predetermined time and an environmental
condition.
[0008] A method for repairing a defect of a substrate is provided. The method
includes
providing a substrate with a defect, providing a structure formed from a
plurality of polymeric
fibers, the structure comprising a first layer formed by a plurality of
polymeric fibers, and a
second layer coupled to the first layer, the second layer having a plurality
of densities formed
by a plurality of polymeric fibers, wherein the first and second layers are
configured to
separate after at least one of a predetermined time and an environmental
condition, and
applying the structure to the substrate.
CA 2885682 2018-12-04
81786882
2a
[0008a] There is further provided a system for producing a structure including
a
plurality of fibers, the system comprising: a collector having a predefined
pattern, wherein the
collector is charged at a first polarity; a spinneret configured to dispense a
polymer, wherein
the spinneret is charged at a second polarity substantially opposite the first
polarity such that
the polymer dispensed from the spinneret forms the plurality of fibers on the
predefined
pattern of the collector; and a focusing device, wherein the focusing device
is charged with a
polarity similar to the spinneret.
[0008b] There is further provided a method for producing a structure including
a
plurality of fibers, the method comprising: providing a collector with a
predefined pattern;
charging the collector with a first polarity; providing a spinneret, the
spinneret configured to
dispense a polymer on the provided collector; charging at least a portion of
the spinneret to a
second polarity substantially opposite at least a portion of the first
polarity; and dispensing the
polymer on the collector via a focusing device, such that at least a portion
of the polymer
forms the plurality of fibers thereby defining the structure, wherein the
structure has at least
two densities formed by the plurality of fibers.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] The embodiments described herein may be better understood by referring
to the
following description in conjunction with the accompanying drawings.
CA 2885682 2019-03-22
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
3
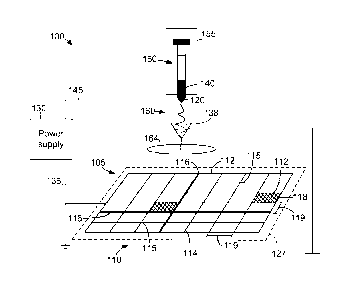
[00101 FIG. 1 is a diagram illustrating an electrospinning system for
producing a structure
of spatially arranged fibers.
[0011] FIG. 2 is a diagram of a collector removed from the electrospinning
system of FIG.
1 and having a plurality of fibers deposited thereon forming a patch.
[0012] FIG. 3 is an illustration of a biomedical patch including a plurality
of spatially
arranged electrospun fibers deposited on a collector shown in FIG. 1.
[0013] FIG. 4 is another illustration of a biomedical patch including a
plurality of spatially
arranged electrospun fibers deposited on a collector shown in FIG. 1.
[0014] FIG. 5 is an illustration of a solid fiber spinneret shown in FIG. .1.
[0015] HG. 6 is an illustration of a co-axial fiber spinneret shown in FIG. 1.
[0016] FIG. 7 is an illustration of a multi-layer biomedical patch.
[0017] FIG. 8 is an illustration of a delamination of patches, such as the
patch shown in
FIG. 7, using various fusion strengths over time.
[0018] FIGS. 9 and 10 are histological cross-sections of regenerated dura
repaired with
multi-laminar nanofiber material such as a patch shown in Fig. 8.
[0019] Figs. 11 and 12 are histological cross-sections of regenerated dura
repaired with
multi-laminar nanofiber material such as a patch shown in Fig. 8.
[0020] Fig. 13 is an illustration of a delamination of patches, such as the
patch shown in
FIG. 7, using various fusion methods and strengths over time.
[0021] FIG. 14 is a flowchart of an exemplary method 700 for producing a
structure of
spatially arranged fibers using system 100 shown in FIG. 1.
[0022] FIG. 15 is a flowchart of an exemplary method 750 for fusing or
coupling together
structures or patch layers produced by method 700 shown in FIG. 14.
[0023] FIG. 16 is a flowchart of an exemplary method 800 for repairing a
defect in a
biological tissue using the structures produced by methods 700 and 750 shown
in FIGS. 14 and
ic.
DETAILED DESCRIPTION
[0024] Embodiments provided herein facilitate repairing biological tissue or
reinforcing
biomedical material with the use of a biomedical patch including a plurality
of fibers. Such
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
4
fibers may have a very small cross-sectional diameter (e.g., from 1-3000
nanometers) and,
accordingly, may be referred to as nanofibers and/or microfibers. While
biomedical patches are
described herein with reference to dura mater and use as a surgical mesh,
embodiments described
may be applied to any biological tissue. Moreover, although described as
biomedical patches,
structures with aligned fibers may be used for other purposes. Accordingly,
embodiments
described are not limited to biomedical patches.
[0025] In operation, biomedical patches provided herein facilitate cell
growth, provide
reinforcement, and may be referred to as "membranes," "scaffolds," "matrices,"
"meshes",
"implants", or "substrates." Biomedical patches with varying densities, as
described herein, may
promote significantly faster healing and/or regeneration of tissue such as the
dura niater than
existing patches constructed using conventional designs.
[0026] Dum mater is a membranous connective tissue comprising the outermost
layer of
the meninges surrounding the brain and spinal cord, which covers and supports
the dural sinuses.
Surgical meshs are often needed during neurosurgical, orthopedic, or
reconstructive surgical
procedures to repair, expand, reinforce, or replace the incised, damaged, or
resected dura mater.
[0027] Although many efforts have been made, the challenge to develop a
suitable surgical
mesh for dural repair has been met with limited success. A.utografis (e.g.,
fascia lata, temporalis
fascia, and pericranium) are preferable because they do not provoke severe
inflammatory or
immunologic reactions. Potential drawbacks of autografts include the
difficulty in achieving a
watertight closure, formation of scar tissue, insufficient availability of
graft materials to close
large dural defects, increased risk of infection, donor site morbidity, and
the need for an
additional operative site. Allografts and xenograft materials are often
associated with adverse
effects such as graft dissolution, encapsulation, foreign body reaction.
immunological reaction,
contracture, scarring, adhesion formation, and toxicity-induced side effects
from
immunosuppressive regimens. Lyophilized human dura mater as a dural substitute
has also been
reported as a source of transmittable diseases, specifically involving prions,
such as Creutzfeldt-
Jakob disease.
[0028] In terms of synthetic surgical mesh materials, non-absorbable synthetic
polymers,
such as silicone and expanded polytetrafluoroethylene (ePTFE), often cause
serious
complications that may include induction of granulation tissue formation due
to their chronic
stimulation of the foreign body response, Natural absorbable polymers,
including collagen,
fibrin, and cellulose, may present a risk of infection and disease
transmission. As a result,
synthetic absorbable polymers such as poly (3-hydroxybutymte-co-3-
hydroxyvalerate) (PHBV),
CA 02885682 2015-03-20
WO 2014/046669
PCT/1JS2012/056548
poly (lactic acid) (PLA), polyglycolic acid (PGA), poly (lactic-co-glycolic
acid) (PLGA), PLA.-
PCL-PGA ternary copolymers, and hydroxyethylmetharrylate hydrogels have
recently attracted
attention as biodegradable implant materials for dural repair. Methods and
systems described
herein may be practiced with these materials and/or any biomedical polymer
whether the
polymer is non-absorbable or absorbable, or synthetic in origin.
[0029] In order to facilitate successful regeneration and/or repair of the
dura mater
following surgery, a synthetic surgical mesh or biomedical patch should
promote: 1) adhesion of
dural fibroblasts (the primary cell type present in the dura) to the surface
of the biomedical patch;
ii) migration of dural fibroblasts from the periphery of the biomedical patch
into the center of the
patch; iii) reinforcement or replacement of existing tissues; iv) minimal
immune response; v)
water tight closure of the dural membrane dura mater: vi) mechanical support
of the native
dural post-operatively and during tissue regeneration ./ neoduralization; vii)
rapid closure of the
dural defect; and viii) increased ease of use,
[0030] Electrospinning is an enabling technique which can produce nanoscale
fibers from a
large number of polymers. The electrospun nanofibers are typically collected
as a randomly-
oriented, nonwoven mat. Uniaxially or radially aliened arrays of nanofibers
can also be obtained
under certain conditions. However, traditional nanofiber scaffolds may lack
the optimal
mechanical and biological properties necessary for some biomedical or surgical
applications
post-operatively.
[0031] in order to increase the strength of nanofiber scaffolds, custom
fabrication of
scaffolds into particular patterns would be highly advantageous. Additionally,
multiple layers of
nanofiber materials fused/coupled together in a manner that allows for a
purposeful degradation
of the layers can also provide strength while allowing for cellular
penetration and/or tissue
integration.
[0032] Many polymers are available for use in electrospinning. In some
embodiments
described herein, nanofibers for dura substitutes are produced as the
electrospun polymer from
poly (e-caprolactone) (PCL), an FDA approved, semiciystalline polyester that
can degrade via
hydrolysis of its ester linkages under physiological conditions with nontoxic
degradation
products. This polymer has been extensively utilized and studied in the human
body as a
material for fabrication of drug delivery carriers, sutures, or adhesion
barriers. As described
herein, electrospun PCL nanofibers may be used to generate scaffolds that are
useful as surgical
meshes.
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
6
[0033] Embodiments provided herein facilitate producing a novel type of
artificial tissue
substitute including a polymeric nanofiber material, which is formed through a
novel method of
electrospinning. This polymeric material includes non-woven nanofibers (e.g.,
fibers having a
diameter of 1-3000 nanometers) which are arranged or organized and aligned
into patterns both
within and across a material sheet.
[0034] FIG. I is a diagram illustrating a perspective view of an exemplary
electrospinnina
system 100 for producing a structure of spatially arranged or organized
fibers. System 100
includes a collector 105 with a predetermined pattern 110 including a
plurality of reinforcement
features 112. System .100 also includes a spinneret 120.
[0035] System 100 is configured to create an electric potential between one or
more
collectors 105 and one or more spinnerets 120. In one embodiment, collector
105 and features
.112 are configured to be electrically charged at a first amplitude and/or
polarity. For example,
collector 105 and features 112 may be electrically coupled to one or more
power supplies 130 via
one or more conductors 135. Power supply 130 is configured to charge collector
105 and
features 112 at the first amplitude and/or polarity via conductor 135.
[0036] In the embodiment illustrated in Fig. 1, collector 105 includes pattern
110 that is a
grid pattern formed by features 112 such that collector 105 is substantially
rectangular. In other
embodiments, collector 105 may have any shape including, but not limited to,
circular, elliptical,
ovular, square, and/or triangular. In one embodiment, features 112 include
ribs 114, seams 116,
and surfaces 118 configured to receive and/or collect polymer fibers. In one
embodiment, rib
114 is substantially cylindrical and has a circumference between Sum - 100cm,
seam 116 is
substantially rectangular having a thickness between 5um- 100cm, and surface
118 is a filling of
a void or feature space 119 formed between ribs 114 and/or seams 116. In one
embodiment,
surface has a thickness between 5um ¨10cm. In the exemplary embodiment,
features 112 are
made fabricated from at least a portion of metallic substance, including, but
not limited to steel,
aluminum, tin, copper, silver, gold, platinum, and any alloy or mixture
thereof In one
embodiment, features 112 include a coating applied to collector 105. coatings
can include, but
are not limited to anorlization, chemical coatings, material coatings
(conductive or non-
conductive), and gradient coatings that facilitate the creation of continuous
gradients of fibers.
However, it should be noted that features 112 (e.g., ribs 114, seams 116, and
surface 118) can
have any shape and be fabricated from any material that facilitates producing
patches as
disclosed herein.
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
7
[0037] In the exemplary embodiment, pattern 110 is formed by spatially
organizing
features 112. In one embodiment, features 112 (e.g., ribs 114 and seams 116)
are interconnected
at nodes 115 such that a feature space 119 is formed between features 112 in
the range of 10 um
and 10 cm. In one embodiment, pattern 110 includes a plurality of spaces 119
such that multiple
varying distances are formed between features 112. It should be noted that
pattern can be
formed to be symmetrical, repeating, and asymmetrical. In the exemplary
embodiment, the
shape of collector 105 enables the biomedical patch formed on collector to
include additional
support and/or reinforcement properties. Such additional support and/or
reinforcement
properties are achieved by creating high density fiber deposition areas on
charged features 112
and having low density fiber deposition areas over feature spaces 119.
[0038] For example, a diamond shaped collector 105 including a diamond shaped
array
pattern 110 enables a diamond-shaped patch to be produced on the diamond
shaped collector 105
to have different mechanical properties from a rectangular-shaped or a
circular-shaped patch
such as, but not limited to, tensile strength, tear resistance, terminal
strain, failure mechanisms or
rates, and/or controlled artiotropic properties, such as greater strength in
one axis relative to
another.
[0039] In one embodiment, pattern 110 defines a collector plane 127 and
spinneret 120 is
orthogonally offset from. the collector plane 127 at a variable distance. For
example. spinneret
120 may be orthogonally offset from the collector plane 127 at a distance of
50 micrometers to
100 centimeters. Alternatively, spinneret 120 can be offset from collector 105
in any manner
that facilitates creating patches as described herein, including but not
limited to, horizontal and
diagonal or skew.
[0040] Spinneret 120 is configured to dispense a polymer 140 while
electrically charged at
a second amplitude and/or polarity opposite the first amplitude and/or
polarity. As shown in
FIG. 1, spinneret 120 is electrically coupled to one or more power supplies
130 by one or more
conductors 145. Power supply 130 is configured to charge one or more
spinnerets 120 at the
second amplitude and/or polarity via conductor 145. In some embodiments, power
supplies 130
provides a direct current and/or static or time variant voltage (e.g., between
1 - 50 kilovolts). In
one embodiment, conductor 145 is charged positively, and collector 105 is also
charged
positively. In all embodiments, power supply 130 is configured to allow
adjustment of a current,
a voltage, and/or a power.
[0041] In one embodiment, spinneret 120 is coupled to a dispensing mechanism
150
containing polymer 140 in a liquid solution form. In such an embodiment,
dispensing
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
8
mechanism 150 is operated manually by a dispensing pump 155. Alternatively,
dispensing
mechanism 150 can be operated automatically with any mechanism configured to
dispense
nanofibers as described herein. In the exemplary embodiment, spinneret 120
includes a metallic
needle having an aperture between 10 micrometers and 3 millimeters in diameter
for dispensing
nanafibers.
[0042] As dispensing mechanism 150 pressurizes polymer 140, spinneret 120
dispenses
polymer 140 as a jet or stream 160. In one embodiment, stream 160 is dispensed
in a horizontal
or sideways stream from spinneret 120. Stream 160 has a diameter approximately
equal to the
aperture diameter of spinneret 120. Stream 160 descends toward collector 105
forming a Taylor
cone. For example, stream 160 may fall downward under the influence of gravity
and/or may be
attracted downward by charge distributed on the fibers and on features 112. As
stream 160
descends, polymer 140 forms one or more solid polymeric fibers 165. In the
exemplary
embodiment, fibers 165 are solid, however it should be noted that fibers 165
can have any
structure including by not limited to, core or shell, porous, co-axial, and co-
axial. Alternatively,
polymer 140 deposition can be accomplished by any other fiber deposition
method including but
not limited to, solvent electrospinning, force electrospinning, melt
electrospinning, extrusion,
and melt blowing.
[0043] In some embodiments, a mask .164 composed of a conducting or non-
conducting
material is applied to collector 105 to manipulate deposition of fibers 165.
For example, mask
164 may be positioned between spinneret 120 and collector 105 such that no
fibers 165 are
deposited on collector 105 beneath mask 164. Moreover, mask 164 may be used as
a time-
variant mask by adjusting its position between the spinneret and the collector
while spinneret
.120 dispenses polymer 140, facilitating spatial variation of fiber density
on. collector 105. While
mask 164 is shown as circular, mask 164 may have any shape (e.g., rectangular
or semi-circular)
and size suitable for use with system 100. Alternatively, or in addition,
deposition of fibers 165
on collector 105 may be manipulated by adjusting the position a collector 105
with respect to
spinneret .120 or by spatially varying the electrical potential applied
between the spinneret 120
and/or the electrodes making up the collector 105. For example, positioning
one side of collector
105 directly beneath spinneret 120 may cause more fibers 165 to be deposited
on that side than
are deposited on the opposite side of collector 105 in a Gaussian
distribution. To modulate the
spatial distribution of fibers forming on collector 105, in some embodiments,
a focusing device
138 is utilized to focus fiber deposition in a particular special region. In
such an. embodiment,
focusing device 138 is charged with a polarity similar to spinneret 120 and
includes an aperture
allowing fiber deposition to occur substantially in the space under the
aperture. Focusing device
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
9
138 may have any geometry that allows for receipt of nanofibers from spinneret
120 and
deposition of the received nanofibers onto collector 105 as described herein.
[0044] FIG. 2 is a diagram of collector 105 removed from electrospinning
system 100
(shown in FIG. .1) and having a plurality of fibers 165 deposited thereon
forming a patch 170.
Fibers 165 are oriented such that they correspond to the position of features
112 (shown in FIG.
1).
[0045] Patch 170 is illustrated with a small quantity of fibers 165 in FIG. 2
for clarity. in
some embodiments, patch 170 includes thousands, tens of thousands, hundreds of
thousands, or
more fibers 165, distributed on collector 105. Even with millions of fibers
165, patch 170 retains
predictable properties such as being flexible and/or pliable. As such, the
predictable properties
facilitate the application of patch 170 to uneven biological tissue surfaces,
such as the surface of
the dura mater.
[0046] The alignment of fibers 165 illustrates a patch 170 with varying
densities. Patch
170 enables reinforcement or structural integrity to be provided in
predetermined locations. For
example, a larger deposition of fibers occurs in various locations, such as
portion 167, which
provide structural reinforcement. Accordingly, system 100 enables the creation
of customized
materials 170 for individual biomedical or clinical and non-clinical
applications.
[0047] In the exemplary embodiment, fibers 165 have a diameter of 1-30(X)
nanometers. In
one embodiment, fibers have a diameter of approximately 220 nanotneters (e.g.,
215 nm to 225
am). It should be noted that the diameter of the fibers 165, thickness of the
patch 170, and/or
fiber density within the patch 170 may affect the durability (e.g., tensile
strength, suture pullout
strength, conformability, etc.) of patch 170. As such., the diameter of the
fibers lAi . LS-31M Of
the patch 170, and/or fiber density within the patch 170 can be selected
according to the
requirements of the end application of the material. Patch 170 may be produced
with various
mechanical properties by varying the thickness andlor the fiber density,
spatial patterning,
polymer composition, and/or number of layers of the patch 170 by operating
electrospinning
system. 100 for relatively longer or shorter durations, changing the polymeric
solution, changing
the chemical composition, changing collector 105, changing collector design,
and/or changing
the manner of fiber deposition.
[0048] Fig. 3 is an illustration 305 of a patch 170 including a plurality of
electrospun fibers
deposited on collector 105 and Fig. 4 is an illustration 405 of a patch 170
including a plurality of
eleetrospun fibers deposited on collector 105. In the exemplary embodiment,
collectors 105
respectively provide an increased deposition of fibers on and substantial near
features 112. Such
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
additional support and/or reinforcement properties are achieved by creating
high. density fiber
deposition areas on charged features 112 and having low density fiber
deposition areas over
feature spaces 119. It should be noted that collector 105 can include any
pattern or combination
of patterns such as the grid pattern shown in Fig. 3 and the hexagonal or
honeycomb pattern
shown in Fig. 4.
[0049] Referring to FIGS. 1-4, fibers 165 may be solid, core/shell, co-axial,
or porous. In
some embodiments, the size and/or structure of fibers 165 is determined by the
design and/or
size of spinneret 120, and/or polymer solution which includes viscosity,
solvent or method of
preparation of the solution, voltage or electric charge, distance between
spinneret 120 and
collector 105, and rate of deposition. FIG. 5 is an illustration of a solid
fiber spinneret 120A.
Solid fiber spinneret 120A includes a truncated conical body 180 defining a
center line 182. At a
dispensing end 184, body 180 includes an annulus 186. Annulus 186 defines a
circular aperture
190A, through which polymer 140 may be dispensed. Fibers 165 produced with
solid fiber
spinneret 120A have a solid composition.
[0050] FIG. 6 is an illustration of a co-axial fiber spinneret 120B. Like
solid fiber
spinneret 120A, co-axial fiber spinneret 120B includes a truncated conical
body 180 with an
annulus 186 at a dispensing end 184. Co-axial fiber spinneret 12013 also
includes a central body
188B positioned within annulus 186. Annulus 186 and central body 18813 define
an annular
aperture 19013. Accordingly, when polymer 140 is dispensed by co-axial fiber
spinneret 120B,
fibers 165 have a co-axial composition, with an exterior wail surrounding a
cavity. The exterior
wall of a fiber 165 dispensed by co-axial fiber spinneret 12013 defines an
outer diameter
corresponding to the inner diameter of annulus 186 and an inner diameter
corresponding to the
diameter of central body .188B. Accordingly, the outer diameter and inner
diameter of co-axial
fibers 165 may be adjusted by adjusting the diameters of annulus 186 and
central body 188B.
[0051] Fiber spinnerets 120A and 120B facilitate incorporating a substance,
such as a
biological agent, growth factor, and/or a drug (e.g., a chemotherapeutic
substance), into patch
170. For example, the substance may be deposited within a cavity defined by co-
axial fibers 165
of patch 170. In one embodiment, polymer 140 is selected to create porous
and/or semi-soluble
fibers 165, and the substance is dispensed from the cavity through fibers 165.
In another
embodiment, polymer 140 is degradable, and the substance is dispensed as
fibers 165 degrade in
vivo. For example, fibers 165 may be configured to degrade within a second to
1 second to 12
months. In one embodiment, a burst release of the substance occurs upon entry
into a system
and an elution occurs over a predetermined period of time. The degradation
rate of polymer 140
CA 02885682 2015-03-20
WO 2014/046669
PCT/1JS2012/056548
i
may be manipulated by any loading and/or release method such as adjusting a
ratio of constituent
polymers within polymer 140, loading the agent into the bulk of the material,
functionalizing the
agent to the surface of the fibers, and/or releasing the agent by degradation
of the polymer or by
diffusion of the agent from the polymer. In another embodiment, a substance is
delivered by
solid fibers 165. For example, a solid fiber 165 may be created from a polymer
140 including
the substance in solution. As solid fiber 165 degrades, the substance is
released into the
surrounding tissue.
[0052] As shown in FIGS. 5 and 6, annulus 186 is perpendicular to center line
182. In an
alternative embodiment, annulus 186 is oblique (e.g., oriented at an. acute or
obtuse angle) with
respect to center line 182. The outside diameter of fibers 165 may be
determined by the inside
diameter of annulus 186.
[0053] FIG. 7 is an illustration of a multi-layer biomedical patch 435. Patch
435 includes a
biomedical patch layer with a plurality of sydrrmietrical spatially organized
fibers 420 and a
biomedical patch layer with a plurality of spatially organized fibers having
varying densities 425
such as increased density portions 430. As shown in FIG. 7, biomedical patch
layers 420 and
425 are combined (e.g., fused, joined, adhered, overlaid) to produce multi-
layer biomedical patch
435 with reinforcement fiber layers. It should be noted that any combination,
number, or type of
fiber layers may be combined to create biomedical patch 435. Combining the
patches, especially
layers 420 and 425, facilitates providing a biomedical patch that promotes
cell migration to a
center of the biomedical patch while exhibiting potentially greater durability
(e.g., tensile
strength) than a biomedical patch having only standard, randomly-organized
fibers. It should be
noted that patch 435 can be formed of layers having various densities and/or
thicknesses (both
individually and collectively), fiber organizations, polymer compositions,
surface coatings, and
types of concentrations of agents and/or drugs.
[0054] In some embodiments, multiple biomedical patch layers 410-425 may be
combined
to create a multi-layer biomedical patch. For example, referring to FIGS. 1-4,
after depositing a
first set of fibers on collector 105, one may wait for the first set of fibers
165 to solidify
completely or cure and then deposit a second set of fibers 165 on collector
105. The second set
of fibers 165 may be deposited directly over the first set of fibers 165 on
collector 105.
Alternatively, the first set of fibers 165 may be removed from collector 105,
and the second set
of fibers 165 may be deposited on conductive surface 162 and/or collector 105
and then removed
and adhered/overlaid on the first set of fibers 165. Such embodiments
facilitate increased
structural or mechanical reinforcement of the patch in predetermined
locations, and added spatial
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
17
control of cell migationlactivity imparted by the layers 2-dimensionally and
stacked layers 3-
dimensionally. In some embodiments, a non nanofiber intermediate layer (e.g.,
hydrogel or
polymeric scaffold) may be disposed between biomedical patch layers 400 and/or
biomedical
patch layers 410.
[0055] In the exemplary embodiment, individual layers are fused or coupled
together such
that the layers delaminate or separate under specific environmental or
temporal conditions. Such
controlled delamination results in maximization of mechanical stability of the
nanofiber material
and the biological interaction (e.g. cellular ingrowth, tissue integration,
cellular exposure, etc.)
between adjacent layers of nanofibers. In the exemplary embodiment, the
process of fusing or
coupling layers includes, but is not limited to, heating, applying mechanical
stress/pressure,
applying an adhesive, chemical processing, cross-linking, and
functionalization.
[0056] In one embodiment, adjacent layers are similarly or variably fused,
adhered, or
joined such that each layer delaminates or separates at a substantially
similar rate within patch
435. Alternatively, layers can be fused together with variable methods such
that each layer
delaminates at different rates. FIG. 8 illustrates delamination of patches
440, 445, and 450 with
various fusion strengths over time. In the exemplary embodiment, a low
strength adhesion 455,
such as but not limited to mild-chemical treatment or crosslinking, low-
pressure physical
lamination, or low-temperature thermal processing, is used to fuse layers of
patch 440 together.
Similarly, a moderate strength adhesion 460 such as but not limited to
moderate chemical
crosslinking, prolonged thermal processing, moderate mechanical entanglement,
application of
moderate adhesives, or high-pressure physical lamination is used to fuse the
layers of patch 445
and a high strength adhesion 465, such as but not limited to extensive
chemical crosslinking,
extensive high-temperature thermal processing, extensive mechanical
entanglement, fiber
interweaving or knitting, or application of aggressive adhesives is used to
fuse layers of patch
450 together. In the exemplary embodiment, a separation 470 of patches 440,
445, and 450 is
shown after a short increment of time, such as, but not limited to I day -- 30
days and a
separation 475 of patches 440, 445, and 450 is shown after a long increment of
time, such as, but
not limited to 30 days --- 3 years. As is shown, patch 440 is substantially
separated 470 after a
short period of time acting as an accelerated separation, patch 445 is
substantially separated 475
after the long period of time acting as a delayed separation, and patch 450
provided substantially
no separation.
[0057] FIGS. 9 and I 0 are histological cross-sections 500 and 502 of dura
mater repaired
with multi-laminar nanofiber material such as patch 440 shown in FIG. 8.
Referring to FIG. 9,
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
13
patch 440 is shown as being inserted into dura 504 two weeks post-operatively.
Regenerative
dural tissue ("neodura") 504 is demonstrated extending on and around the
implanted nanofiber
material 440. Regenerative dural fibroblasts are also shown to have penetrated
the bulk of the
nanofiber material 440, demonstrating progressive cellularization of the
implanted nanofiber
construct. Two weeks following implantation of the multi-layer nanofiber
material 440 no
delamination is noted upon histological examination of the explained tissue.
The nanofiber
material 440 is observed as a homogeneous block of material with low to
moderate cellular
ingowth, yet no singular nanofter layer or separation of nanofiber layers is
observed. FIG. 10
illustrates controlled delamination of patch 440 six weeks post-operatively
and integration of the
patch within the native and/or regenerated dural tissue 504. Regenerative dual
tissue
("neodura") 504 is demonstrated extending on and around the implanted
nanofiber material 440.
Additionally, regenerative dural tissue ("neodura) is demonstrated extending
inbetween
delaminated layers of the nanofiber material. Regenerative dural fibroblasts
are shown to have
significantly penetrated the bulk of the nanofiber material 440, demonstrating
robust
cellularization and integration of the implanted nanofiber construct.
Delamination of individual
layers of nanofibers within the implant construct is noted upon histological
examination of the
explained tissue. The nanofiber material 440 is observed as two heterogenous
layers of material
separated by a thin layer of regenerated dural tissue extending along the
adjoining surface of the
nanofiber monolayers. Evidence of controlled delamination of the implanted
material post-
operatively is specifically demonstrated by observation that multiple layers
of the material
remain fused in proximity of sutures utilized to secure the material to the
native tissue.
[0058] FIGS. 11 and 12 are histological cross-sections 506 and 508 of
regenerated dura
repaired with multi-laminar nanofiber material such as patch 450 shown in FIG.
8. Referring to
FIG. 11, patch 450 is shown as being inserted into dura 504 two weeks post-
operatively.
Regenerative dural tissue ("neodura") 504 is demonstrated extending on and
around the
implanted nanofiber material 450. Regenerative dural fibroblasts are also
shown to have
penetrated the bulk of the nanofiber material 450, demonstrating
cellularization of the implanted
nanofiber construct. No delamination is noted upon histological examination of
the explanted
tissue. The nanofiber material 450 is observed as a homogeneous block of
material with low to
moderate cellular ingowth, yet no singular nanofiber layer or separation of
nanofiber layers is
observed. FIG. 12 illustrates that the high strength adhesion has enabled
layers of patch 450 to
remain substantially fused together six week post-operatively as dural tissue
504 regenerated
around patch 450. Regenerative dural tissue ("neodura") 504 is again
demonstrated extending on
and around the implanted nanofiber material 450. Dural fibroblasts
substantially penetrate the
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
14
bulk of the nanofiber material 450, demonstrating robust cellulariz.ation of
the implanted
nanofiber construct. Unlike nanofiber patch 440, no delamination of nanofiber
patch 450 is noted
upon histological examination of the explained tissue following chronic
implantation. The
nanofiber material 450 is observed as a secure composite material
demonstrating cellular
ingowth yet no separation or observation of singular nanofiber layers.
[0059] FIG. 13 illustrates separation of layers within patches 600, 602, and
604 at varying
rates. Each patch 600, 602, and 604 includes a first layer 606, a second
layer, 608, a third layer
610, and a fourth layer 612. It should be noted that while patches 600, 602,
and 604 are shown
with four layers, patches can be fabricated to have any number of layers.
Referring to patch 600,
low strength adhesion 455 is used to fuse layers 606, 608. and 610 together
and high strength
adhesion 465 is used to fuse layers 610 and 612 together. After a short time
period, a separation
614 of layers 606, 608, and 610 has occurred and layers remain substantially
fused together. As
shown in patch 602. moderate strength adhesion 460 is used to fuse together
layers 606 and 608,
while high strength adhesion 465 is used to fuse together layers 608, 610, and
612. A separation
616 of layers 606 and 608 occurs after a long period of time while
substantially no separation
occurs between layers 608, 610, and 612. Referring to patch 604, a high
strength adhesion 465 is
used between layers 606, 608, 610, and 612 such that substantially no
separation occurs between
the layers.
[0060] A multi-layered biomedical patch may be useful for dural grafts as well
as other
tissue engineering applications. Sequential layers of fibers can be created
with varying orders
(e.g., radially aligned, reinforced, or randomly oriented), densities (e.g.,
low, high, or mixture of
fiber density), patterns or reinforcement, and compositions (polymer). which
may allow specific
types of cells to infiltrate and populate select layers of the artificial
biomedical patch. For
example, biomedical patches containing a high fiber density generally prohibit
cellular migration
and infiltration, while biomedical patches containing a low fiber density
generally enhance
cellular migration and infiltration. Such additional support and/or
reinforcement properties are
achieved by creating high density fiber deposition that discourages cellular
penetration and
having low density fiber deposition areas that promote cellular penetration
and/or ingrowth.
[0061] Overall, the ability to form multi-layered fiber materials, as
described herein, may
be extremely beneficial in the construction of biomedical patches designed to
recapitulate the
natural multi-laminar structure of not only dura mater, but also other
biological tissues such as
skin, heart valve leaflets, pericardium, and/or any other biological tissue.
Furthermore, one or
more layers of a biomedical patch may be fabricated from bioresorbable
polymers such that the
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
resulting nanofiber materials fully resorb following implantation.
Manipulation of the chemical
composition of the polymers utilized to fabricate these scaffolds may further
allow for specific
control of the rate of degradation and/or resorption of a biomedical patch
following implantation.
[0062] FIG. 14 is a flowchart of an exempla*, method 700 for producing a
structure of
spatially organized fibers using system 100 shown in FIG. 1. While one
embodiment of method
700 is shown in FIG, 14, it is contemplated that any of the operations
illustrated may be omitted
and that the operations may be performed in a different order than is shown.
In the exemplary
embodiment, method 700 includes electrically charging 705 collector 105 at a
first amplitude
and/or polarity (e.g., negatively charging or grounding). Spinneret 120 is
electrically charged
710 at a second amplitude andlor polarity opposite the first amplitude and/or
polarity (e.g.,
positively charged). A polymer (e.g., a liquid polymer) is dispensed 715 from
spinneret 120. In
the exemplary embodiment, dispensed 715 polymers are collected 720 on
collector 105 to form a
plurality of polymeric fibers on or substantially near features 112 that
creates a structure or
patch. After the dispensed 615 polymers are collected 720 and a structure is
created, the
structure may undergo post-processing 725. Such post-processing 725 can
include, but is not
limited to, lamination, layer stacking, coupling and/or fusing, chemically
treating, and applying a
biological agent, growth factor, and/or drug.
[0063] FIG. 15 is a flowchart of an exemplary method 750 for fusing or
coupling together
structures or patch layers produced by method 700 shown in FIG. 14. Method 750
includes
providing 755 a first, second, and third patch layer. First patch layer is
coupled 760 to second
patch layer using a first coupling technique. The coupled 760 first and second
layers are then
coupled 765 to the third patch layer using a second coupling teclmique
different than the first
coupling. technique. In the exemplary embodiment, coupling techniques, include
but are not
limited to, heating, applying mechanical stress/pressure, chemical processing,
cross-linking, and
functionalization. While method 750 illustrates a first patch layer coupled to
a second patch
layer, it should be noted that multiple layers (e.g., 3, 5, 6,) can be coupled
together
simultaneously. Additionally, the process may be repeated to add layers to
structures produced
by method 750.
[0064] FIG. 16 is a flowchart of an exemplary method 800 for repairing a
defect of a
substrate using a structure produced by methods 700 and 750 shown in FIGS. 14
and 15. In one
embodiment, method 800 includes providing 805 a substrate substance with a
defect. The defect
may include a void, tissue defect, injury, insult, and/or any other condition
resulting in
diminished function of biological tissue. In the exemplary embodiment, the
substrate is
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
16
biological tissue. Alternatively, the substrate can be any substrate including
but not limited to,
filtration media, textiles, membrane media, and coatings. In one embodiment,
the defect
provided 805 includes a void created by surgical incision to provide access to
an underlying
tissue (e.g., a tumor). In another embodiment, a void is created 805 by
excising necrotic tissue
(e.g., skin cells). In the exemplary embodiment, one or more patches capable
of covering the
defect are selected 810. For example, a plurality of biomedical patches may be
selected 810 for
a large and/or complex (e.g., irregularly shaped) defect. In the exemplary
embodiment, a
biomedical patch having a diameter greater than the diameter of an
approximately circular defect
is selected 810. Alternatively, a patch is selected 810 and customized, pre-
operation or intra-
operation, to fit the defect. It should be noted that any size, shape, and/or
geometry of structure
may be used in the selection 810 of the patch.
[0065] In one embodiment, a substance such as a growth factor and/or a drug
(e.g., a
chemotherapeutic drug) is applied 815 to the biomedical patch. In the
exemplary embodiment
growth factor and/or a drug is applied 815 pre-operatively. However, it should
be noted that
growth factor and/or a drug may be applied 815 at any time including, but not
limited to, intra-
operatively and post-operatively. In one embodiment, the biomedical patch may
be immersed in
the substance to allow the substance to occupy a cavity within co-axial fibers
of the biomedical
patch, dope the polymer comprising the fibers in the biomedical patch, or coat
the surface of the
fibers within the biomedical patch.
[0066] In the exemplary embodiment, the patch is applied 820 to (e.g.,
overlaid on) the
biological tissue to cover, repair, reinforce, and/or fill at least a portion
of the defect. For
example, the biomedical patch may be applied 820 to dura mater tissue, cardiac
tissue, and/or
any biological tissue including a defect. In one embodiment, the perimeter of
the biomedical
patch extends past the perimeter of the defect, such that the entire defect is
covered by the
biomedical patch. In some embodiments, the biomedical patch is coupled 825 to
the biological
tissue with a plurality of sutures, adhesive, and/or any other means of
attaching the biomedical
patch to the biological tissue (inlay). In an alternative embodiment, the
biomedical patch is
simply allowed to fuse to the biological tissue, such as by adhesion of
biological cells to the
biomedical patch (onlay). In another embodiment, biomedical patch may be
directly coupled to
the edge of the tissue with no overlap. In one embodiment, biomedical patch
may be overlaid on
Lop of a wound/defect or injury covering the entirety of the defect or injury
without filling the
defect.
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
17
[00671 In one embodiment, after the biomedical patch is applied 820 and
optionally
coupled 825 to the biological tissue, the biological tissue is covered 830.
Alternatively, the patch
may be the terminal covering. In such an embodiment, a backing that is either
non-permeable or
permeable may be coupled to the patch. In one embodiment, other tissue
overlaying the defect
(e.g., dermis and/or epidermis) is repaired (e.g., sutured closed). In another
embodiment, one or
more protective layers are applied over the biological tissue. For example, a
bandage may be
applied to a skin graft, with or without a protective substance, such as a
gel, an ointment, and/or
an antibacterial agent. In one embodiment, the protective layer includes, but
is not limited to, a
covering, film tissue, dressing, mesh, and nanofiber structure, such as an
additional biomedical
patch, as described herein..
100681 Embodiments described herein are operable with any surgical procedure
involving
the repair, replacement, or expansion of the dura mater, including, but not
limited to, a
transphenoidal procedure (e.g., surgical removal of pituitary adenomas),
various types of skull
base surgeries, and/or surgical removal of cranial or spinal tumors (e.g.,
meningiomas and/or
astrocytomas). In one embodiment, a biomedical patch may be applied to a bone
fracture (e.g., a
complex fracture). In another embodiment, a biomedical patch may be applied to
a defect in the
skin (e.g. a burn).
(0069] Moreover, such embodiments provide a dura mater substitute, a
biomedical patch
for a skin graft (e.g., dermal or epidermal), a biomedical patch for tracheal
repair, a scaffold for
an artificial heart valve leaflet, an artificial mesh for surgical repair of a
gastrointestinal tract
(e.g., an abdominal hernia or an ulcer), an artificial mesh for surgical
repair of cardiac defects.
Embodiments described herein facilitate providing a cardiac patch of
sufficient flexibility to
enable movement of the biomedical patch by a biological tissue (e.g.,
cardionayocytes or cardiac
tissue, muscle, skin, connective tissue, intestinal tissue, stomach tissue,
bone, gastrointestinal
tract, and mucosa).
[00701 In some embodiments, a biomedical patch has a thickness greater or less
than a
thickness of the biological tissue being repaired. Biomedical patches with
spatially organized
polymeric fibers facilitate reducing the expense of tissue repair, improving
tissue healing time,
and reducing or eliminating the risk of zoonotic infection. Moreover, such
biomedical patches
are relatively simple to manufacture, enabling, customization of shape, size,
and chemical
composition and improved availability and non-immunogenicity. In addition,
biomedical
patches with spatially organized polymeric fibers exhibit excellent handling
properties due to
their cloth-like composition, eliminate the need for a second surgery to
harvest autologous graft
tissue, and reduce the risk of contracture and adhesion when compared with
known products.
CA 02885682 2015-03-20
WO 2014/046669
PCT/US2012/056548
18
Additionally, the patches described herein facilitate reinforcement,
buttressing, lamination,
and/or sealing in a variety of applications such as but not limited to
clinical and non-clinical
applications.
[0071 [ Although the foregoing description contains many specifics, these
should not be
construed as limiting the scope of the present disclosure, but merely as
providing illustrations of
some of the presently preferred embodiments. Similarly, other embodiments of
the invention
may be devised which do not depart from the spirit or scope of the present
invention. For
example, while the illustrative examples have been used in with clinical
applications, the above
described nanofiber structures can have non-clinical application such as
filtration, textiles,
membrane technology, and coatings. Features from different embodiments may be
employed in
combination. The scope of the invention is, therefore, indicated and limited
only by the
appended claims and their legal equivalents, rather than by the foregoing
description. All
additions, deletions, and modifications to the invention as disclosed herein
which fall within the
meaning and scope of the claims are to be embraced thereby.
[0072] As used herein, an element or step recited in the singular and
proceeded with the
word "a" or "an" should be understood as not excluding plural elements or
steps, unless such
exclusion is explicitly recited. Furthermore, references to "one embodiment"
of the present
invention are not intended to be interpreted as excluding: the existence of
additional embodiments
that also incorporate the recited features.
[0073] This written description uses examples to disclose various
enibodiments, which
include the best mode, to enable any person skilled in the art to practice
those embodiments,
including making and using any devices or systems and performing any
incorporated methods.
The patentable scope is defined by the claims, and may include other examples
that occur to
those skilled in the art. Such other examples are intended to be within the
scope of the claims if
they have structural elements that do not differ from the literal language of
the claims, or if they
include equivalent structural elements with insubstantial differences from the
literal languages of
the claims.