Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
81788428
ENDOBRONCHIAL TUBE APPARATUS
Background
[01] Endobronchial tubes (also known as dual-lumen endotracheal tubes)
provide an
open airway for patient ventilation during surgery. In particular,
endobronchial tubes are used
during surgical procedures to provide ventilation to individual lungs
separately. Current
endobronchial tubes include a first, tracheal lumen and a second, bronchial
lumen. Each
lumen includes an associated inflatable cuff, the cuff associated with the
tracheal lumen being
positioned within the trachea and the cuff associated with the bronchial lumen
being
positioned within one of the bronchus.
Summary
[02] According to an aspect of the present invention, there is provided an
apparatus for
monitoring electromyographic signals of a patient's laryngeal muscles,
comprising: an
endobronchial tube defining first and second lumens, the endobronchial tube
having a
proximal portion and a distal portion; a first cuff disposed at said distal
portion and coupled to
the endobronchial tube and positioned proximate a first opening fluidly
coupled with the first
lumen, the first cuff sized to be positioned within a bronchus of the patient;
a second cuff
disposed at said distal portion and coupled to the endobronchial tube and
positioned proximate
a second opening fluidly coupled with the second lumen, the second cuff
configured to be
positioned within a trachea of the patient; an electrode cuff coupled to the
endobronchial tube
and positioned nearer to said proximal portion of said endobronchial tube than
the first cuff
and the second cuff are to said proximal portion of said endobronchial tube;
and electrodes
positioned on an exterior surface of the electrode cuff.
[02a] According to another aspect of the present invention, there is
provided a method for
monitoring electromyographic signals of a patient's laryngeal muscles,
comprising: providing
an endobronchial tube defining first and second lumens, the endobronchial tube
having a
proximal portion and a distal portion, the endobronchial tube including
electrodes positioned
on an exterior surface of an electrode cuff of the endobronchial tube;
positioning a bronchial
cuff within a bronchus of the patient, the bronchial cuff being disposed at
said distal portion
- 1 -
Date Recue/Date Received 2020-11-02
81788428
and coupled to the endobronchial tube; positioning a tracheal cuff within a
trachea of the
patient, the tracheal cuff being disposed at said distal portion and coupled
to the
endobronchial tube, wherein the electrode cuff is positioned nearer to said
proximal portion of
said endobronchial tube than the bronchial cuff and the tracheal cuff are to
said proximal
portion of said endobronchial tube; and measuring signals of the patient using
the electrodes.
102b] Concepts presented herein include an apparatus for monitoring
electromyographic
(EMG) signals of a patient's laryngeal muscles. The apparatus includes an
endobronchial tube
having an exterior surface and two lumens for providing ventilation.
Conductive ink
electrodes are formed on the exterior surface of the endobronchial tube. The
conductive ink
electrodes are configured to receive the EMG signals from the laryngeal
muscles when the
endotracheal tube is placed in a trachea of the patient. At least one
conductor is coupled to the
conductive ink electrodes and is configured to carry the EMG signals received
by the
conductive ink electrodes to a processing apparatus.
Brief Description of the Drawings
[03] Fig. 1 is a schematic view of an EMG endobronchial tube.
[04] Figs. 2A and 2B are different side views of an endobronchial tube.
[05] Fig. 2C is a sectional view of the endobronchial tube illustrated in
Fig. 2A
la
Date Recue/Date Received 2020-11-02
CA 02893048 2015-05-28
PCT
M190.524.111/C00003563.WOU2
[06] Fig. 3 is a partial side view of an endobronchial tube having an
electrode
cuff.
Detailed Description
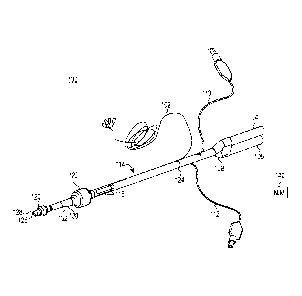
[07] Figure 1 shows an EMG endobronchial tube 100 made from extruded
polymer. Endobronchial tube 100 includes solid wires 102, a bronchial fitting
104, a tracheal fitting 106, a y-connector 108, a bronchial cuff inflating
conduit
110, a tracheal cuff inflating conduit 112, extruded polymer tube 114,
electrodes
116, bronchial cuff 120 and tracheal cuff 122. Solid wires 102 are connected
to
electrodes 116 at an interconnection 124. Tube 114 transports gases to and
from
the lungs. In particular, tube 114 defines a first, bronchial lumen 126
extending
from bronchial fitting 104 to an opening 128 distal the bronchial cuff 120 and
a
second, tracheal lumen 130 extending from tracheal fitting 106 to an opening
132
distal the tracheal cuff 122. The Y-connector 108 fluidly couples the
bronchial
fitting 104 and tracheal fitting 106 to bronchial lumen 126 and tracheal lumen
130, respectively.
[08] Fittings 104 and 106 are configured to be connected to a respirating
machine (not shown) for injecting air into the lungs and withdrawing air from
the
lungs. Cuff inflating conduits 110 and 112 are configured to be connected to a
source of compressed air (not shown) for inflating cuffs 120 and 122. Cuff
inflating conduit 110 communicates with a lumen located in the wall of tube
114,
and the lumen communicates with bronchial cuff 120. Likewise, tracheal cuff
inflating conduit 112 communicates within a lumen located in the wall of tube
114, and the lumen communicates with tracheal cuff 122. During use, one of the
fittings (e.g., bronchial fitting 104) is configured to inject air into one
lung while
the other fitting (e.g., tracheal fitting 106) is configured to injected air
into the
other lung. For example, cuff 120 can be positioned into the left bronchus and
cuff 122 positioned into the trachea. In this case, opening 126 is positioned
to
direct air into the left lung from bronchial fitting 104 while opening 132 is
positioned to direct air into the right lung from tracheal fitting 106.
Selectively,
air can be provided to only one of the fittings 104, 106 so as to provide air
to only
- 2 -
CA 02893048 2015-05-28
?CT
M190.524.111/C00003563.WOU2
a single lung and collapsing the other lung. In such a case, a surgeon can
operate
proximate the collapsed lung or on the collapsed lung. After endobronchial
tube
100 is inserted into the trachea of a patient, electrodes 116 sense EMG
signals,
which are output to an EMG processing machine, such as nerve integrity monitor
(NIM) device 140, via solid wires 102. Die cut tape may be used to tape tube
114
to a patient's mouth to secure the tube and keep it appropriately positioned.
[09] In one embodiment, the NIM 140 is configured to determine when the
electrodes 116 are in contact with the vocal folds, and is configured to
provide an
alert to the surgeon when such contact is lost. In one embodiment, the NIM 140
is also configured to determine whether the electrodes 116 are in contact with
muscle or tissue based on the received signals
110] In one embodiment, tube 114 is a braided tube that is more flexible
than
conventional solid polymer tubes, and that reduces kinking. Tube 114 according
to one embodiment is formed from a braided polymer or nitinol within a thin-
walled tube, and reduces or eliminates rotation of the tube at the vocal
folds,
while allowing a proximal portion of the tube to rotate.
[11] Figure 2A shows a first side view (posterior side) of endobronchial
tube
114 with four electrodes 116. Figure 2B shows a second side view (rotated 90
degrees from the view shown in Figure 2A) of the endobronchial tube 114 shown
in Figure 2A. Figure 2C is a diagram illustrating a cross-sectional view of
the
endobronchial tube 114 shown in Figures 2A and 2B.
[12] Electrodes 116 include four electrodes 116A-116D, which arc formed
around a circumference of the tube 114 and extend in a longitudinal direction
of
the tube 114. Electrodes 116A and 116B are positioned entirely on the
posterior
side of the tube 114 and are also referred to herein as posterior electrodes
116A
and 116B. Electrodes 116C and 116D are positioned entirely on the anterior
side
of the tube 114 and are also referred to as anterior electrodes 116C and 116D.
The anterior side of the tube 114 is the bottom half of the tube 114 shown in
Figure 2C, and the posterior side of the tube 114 is the top half of the tube
114
- 3 -
CA 02893048 2015-05-28
PCT
M190.524.111/C00003563.WOU2
shown in Figure 2C. Each of the electrodes 116A-116D is coupled to a
respective trace 150A-150D (trace 150D is not visible in the Figures). Traces
150A-150D are positioned in a protected (masked) region 152 of tube 114.
Posterior electrodes 116A and 116B are positioned in an exposed (unmasked)
region 154 of tube 114. Anterior electrodes 116C and 116D are positioned in an
exposed (unmasked) region 156 of tube 114.
[13] In one embodiment, each of the electrodes 116A-116D has a length of
about one inch, and extends laterally around a circumference of the tube for a
distance corresponding to an angle 160 of about 60 degrees (i.e., each of the
electrodes 116A-116D has a width of about 16.67 percent of the total
circumference of the tube). The electrodes are laterally spaced apart around
the
circumference of the tube by a distance corresponding to an angle 160 of about
30 degrees (i.e., the lateral spacing between each of the electrodes 116A-116D
is
about 8.333 percent of the total circumference of the tube). The posterior
electrodes 116A and 116B are longitudinally offset or displaced from the
anterior
electrodes 116C and 116D. The posterior electrodes 116A and 116B are
positioned closer to the distal end (right side in Figures 2A and 2B) of the
tube
114 than the anterior electrodes 116C and 116D. and the anterior electrodes
116C
and 116D are positioned closer to the proximal end (left side in Figures 2A
and
2B) of the tube 114 than the posterior electrodes 116A and 116B.
[14] Tube 114 includes an overlap region 166 where a proximal portion of
the
posterior electrodes 116A and 116B longitudinally overlap with a distal
portion
of the anterior electrodes 116C and 116D. The electrodes 116 do not physically
overlap each other since they are laterally offset from each other. In one
embodiment, the overlap region 166 is about 0.1 inches long, and the overall
length from a proximal end of the anterior electrodes 116C and 116D to a
distal
end of the posterior electrodes 116A and 116B is about 1.9 inches. In another
embodiment, the overlap region 166 is about 0.2 inches long, and the overall
length from a proximal end of the anterior electrodes 116C and 116D to a
distal
end of the posterior electrodes 116A and 116B is about 1.8 inches. Tube 114 is
configured to be positioned such that the vocal folds of a patient are
positioned in
- 4 -
CA 02893048 2015-05-28
PCT
M190.524.111/C00003563.WOU2
the overlap region 166. Thus, the configuration of the electrodes 116 above
the
vocal folds is different than the configuration below the vocal folds. The
posterior electrodes 116A and 116B are configured to be positioned primarily
below the vocal folds, and the anterior electrodes 116C and 116D are
configured
to be positioned primarily above the vocal folds. In one embodiment,
electrodes
116A and 116C are used for a first EMG channel, and electrodes 116B and 116D
are used for a second EMG channel.
[15] In an alternate embodiment, all four surface printed electrodes, 112A,
112B, 112C and 112D, are equal in length. This will allow the finish product
to
be placed with little concerns of rotational alignment.
[16] As illustrated in Fig. 2C, conduits 110 and 112 are formed in a
thickness
of the tube 114 to carry compressed air to bronchial cuff 120 and tracheal
cuff
122, respectively. Additionally, inside tube 114 are formed bronchial lumen
126
and tracheal lumen 130. During use, one of the lumens 126 and 130 can be used
to inject gases into a particular lung while the other lumen is sealed from
injecting gases into the opposite lung.
[17] With reference to Fig. 3, another embodiment includes an electrode
cuff
170 positioned proximal the tracheal cuff 122. In the embodiment of Fig. 3,
cuff
122 is of a different shape than that illustrated in Figs. 1-2C. Other shapes
for the
cuffs 122 and 170 can be utilized. Electrodes 116 are applied directly to the
electrode cuff 170 and are similar to that discussed above. Cuffs 122 and 170
are
sized so as to both provide suitable sealing between the trachea and cuff 122
yet
provide suitable compliance of electrode cuff 170 in contact with the vocal
folds
of a patient when inflated by pressurized fluid provided within inflating
conduit
110. Upon inflation, the tracheal cuff 122 has a larger diameter DI than a
diameter D2 of electrode cuff 170. In some embodiments, the diameter D2 is
selected to be approximately half the diameter DI. In one example, D1 is about
20 millimeters, whereas D2 is about 9 millimeters. In yet a further
embodiment,
D1 is approximately 27 millimeters, whereas D2 is approximately 14
millimeters.
Moreover, a length Li of the cuff 170 is selected to be greater than a length
L2
- 5 -
CA 02893048 2015-05-28
PCT
M190.524.111/C00003563.WOU2
for cuff 122. In one embodiment, the L 1 is approximately 1.875 inches. In
another embodiment, L 1 is in a range from approximately 1.5 inches to 2.5
inches. In a further embodiment, a ratio of DI :L1 is selected to be in a
range
from approximately 15:100 to 30:100.
[18] Furthermore, a compliance for cuff 170 is selected so as to prevent
trauma
due to cuff 170 contacting the vocal folds of the patient. In one embodiment,
the
cuff 170 is formed of a semi-compliant balloon. The semi-compliant balloon
will
increase in diameter about 10 to 20 percent from a nominal pressure to a rated
burst pressure for the balloon. In a further embodiment, cuff 170 is formed of
a
compliant balloon such that the balloon will increase in diameter from 20 to
200
percent from a nominal pressure to a rated burst pressure of the balloon. In a
further embodiment, the cuff 170 is formed of a compliant material that has a
greater compliance than a material selected for cuff 122. In one embodiment,
cuff 122 has a compliance defined as increasing in diameter about 20 to 200
percent from a nominal pressure to a rated burst pressure for the cuff 122.
[19] Inflating conduit 110 extends along the length of tube 114 to
electrode
cuff 170 and continues in extension to the tracheal cuff 122. Due to relative
compliance of the cuffs 122 and 170, cuff 122 is configured to fluidly seal
the
trachea of a patient when positioned, whereas electrode cuff 170 inflates to
contact the vocal folds of the patient so as to prevent trauma from occurring
due
to contact between the cuff 170 and the vocal folds. Furthermore, by selecting
diameters D1 and D2 of cuffs 122 and 170, tension exerted on an exterior
surface
of each cuff is adjusted. In one embodiment, thickness and diameter for cuffs
122 and 170 are selected such that cuff 122 will absorb pressure and reduce
pressure on cuff 170. In this configuration, cuff 170 can conform to a shape
of
vocal folds and ensure sufficient electrical contact between the electrodes
112
and the vocal folds without causing irritation by exerting too much pressure
on
the vocal folds.
[20] Although the present disclosure has been described with reference to
preferred embodiments, workers skilled in the art will recognize that changes
can
- 6 -
CA 02893048 2015-05-28
PCT
M190.524.111/C00003563.WOU2
be made in form and detail without departing from the spirit and scope of the
present disclosure.
- 7 -