Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
1
COMBINATION TREATMENT
The present invention relates to the use of combinations comprising 8-[(1R)-1-
(3,5-
difluorophenylamino)ethyll-N,N-dimethy1-2-morpholino-4-oxo-4H-chromene-6-
carboxamide (hereafter "Compound [IF) or a pharmaceutically acceptable salt
thereof and
a taxane in the treatment or prophylaxis of cancer. Taxanes include
established cancer
drugs such as docetaxel (TaxotereTr''') and paclitaxel (TaxolTm). Other
taxanes include
cabazitaxel, larotaxel, ortataxel, and tesetaxel. The invention also relates
to pharmaceutical
compositions comprising Compound [I] or a pharmaceutically acceptable salt
thereof and a
taxane; and to kits comprising Compound [I] or a pharmaceutically acceptable
salt thereof
and a taxane, optionally with instructions for use. The invention further
relates to methods
of treatment comprising the simultaneous, sequential or separate
administration of
Compound [I] or a pharmaceutically acceptable salt thereof and a taxane to
warm-blooded
animal, such as man.
It has been discovered that a cell may become cancerous by virtue of the
transformation of a portion of its DNA into an oncogene, a gene which, on
activation, leads
to the formation of malignant tumour cells (Bradshaw, Mutagenesis, 1986, 1,
91). Several
such oncogenes give rise to the production of peptides, which are receptors
for growth
factors. Activation of the growth factor receptor complex subsequently leads
to an
increase in cell proliferation. It is known, for example, that several
oncogenes encode
tyrosine kinase enzymes and that certain growth factor receptors are also
tyrosine kinase
enzymes (Yarden etal., Ann. Rev. Biochem., 1988, 57, 443; Larsen et al., Ann.
Reports in
Med. Chem., 1989, Chpt. 13). The first group of tyrosine kinases to be
identified arose
from viral oncogenes, for example pp60v-sit tyrosine kinase (otherwise known
as v-Src),
and the corresponding tyrosine kinases in normal cells, for example pp60's'
tyrosine
kinase (otherwise known as c-Src).
Receptor tyrosine kinases are important in the transmission of biochemical
signals
which initiate cell replication. They are large enzymes which span the cell
membrane and
possess an extracellular binding domain for growth factors such as epidermal
growth factor
(EGF) and an intracellular portion which functions as a kinase to
phosphorylate tyrosine
amino acids in proteins and hence to influence cell proliferation. Various
classes of
receptor tyrosine kinases are known (Wilks, Advances in Cancer Research, 1993,
60,
CA 02900136 2015-08-04
WO 2014/135851 PCT/GB2014/050618
2
43-73) including lipid kinases, which are located intracellularly and are
involved in the
transmission of biochemical signals such as those that influence tumour cell
growth and
invasiveness. Various classes of lipid kinases are known including the PI 3-
kinase family,
which is alternatively known as the phosphatidylinosito1-3-kinase family.
It is now well understood that deregulation of oncogenes and tumour-suppressor
genes contributes to the formation of malignant tumours, for example by way of
increased
cell proliferation or increased cell survival. It is also now known that
signalling pathways
mediated by the PI 3-kinase family have a central role in a number of cell
processes
including proliferation and survival, and deregulation of these pathways is a
causative
factor a wide spectrum of human cancers and other diseases (Katso et al.,
Annual Rev. Cell
Dev. Biol., 2001, 17: 615-617 and Foster et al., J. Cell Science, 2003, 116:
3037-3040).
The PI 3-kinase family of lipid kinases is a group of enzymes that
phosphorylate
the 3-position of the inositol ring of phosphatidylinositol (PI). Three major
groups of PI 3-
kinase enzymes are known which are classified according to their physiological
substrate
specificity (Vanhaesebroeck et al., Trends in Biol. Sci., 1997, 22, 267).
Class III PI 3-
kinase enzymes phosphorylate PI alone. In contrast, Class II PI 3-kinase
enzymes
phosphorylate both PI and PI 4-phosphate [abbreviated hereinafter to PI(4)13].
Class I PI 3-
kinase enzymes phosphorylate PI, PI(4)P and PI 4,5-bisphosphate [abbreviated
hereinafter
to PI(4,5)P2], although only PI(4,5)P2 is believed to be the physiological
cellular substrate.
Phosphorylation of PI(4,5)P2 produces the lipid second messenger PI 3,4,5-
triphosphate
[abbreviated hereinafter to PI(3,4,5)P3]. More distantly related members of
this
superfamily are Class IV kinases such as mTOR and DNA-dependent kinase that
phosphorylate serine/threonine residues within protein substrates. The most
studied and
understood of these lipid kinases are the Class I PI 3-kinase enzymes.
Class I PI 3-kinase is a heterodimer consisting of a p110 catalytic subunit
and a
regulatory subunit, and the family is further divided into Class la and Class
lb enzymes on
the basis of regulatory partners and mechanism of regulation. Class Ia
enzymes, include PI
3-kinase (3, and consist of three distinct catalytic subunits (p 1 lOcc,
p11013 and p1106) that
dimerise with five distinct regulatory subunits (p85cc, p55cc, p50c,c, p851:3
and p55y), with
all catalytic subunits being able to interact with all regulatory subunits to
form a variety of
heterodimers. Class Ia PI 3-kinase enzymes are generally activated in response
to growth
factor-stimulation of receptor tyrosine kinases, via interaction of the
regulatory subunit
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
3
SH2 domains with specific phospho-tyrosine residues of the activated receptor
or adaptor
proteins such as IRS-1. Both p1 10a, and p11013 are constitutively expressed
in all cell
types, whereas p1106 expression is more restricted to leukocyte populations
and some
epithelial cells. In contrast, the single Class lb enzyme consists of a pllOy
catalytic
subunit that interacts with a p101 regulatory subunit. Furthermore, the Class
lb enzymes
are activated in response to G-protein coupled receptor (GPCR) systems as well
as by the
mechanisms described above.
There is now considerable evidence indicating that Class Ia PI 3-kinase
enzymes,
which include PI 3-kinase 13, contribute to tumourigenesis in a wide variety
of human
cancers, either directly or indirectly (Vivanco and Sawyers, Nature Reviews
Cancer, 2002,
2, 489-501). For example, the pllOct subunit is amplified in some tumours such
as those
of the ovary (Shayesteh etal., Nature Genetics, 1999, 21: 99-102) and cervix
(Ma etal.,
Oncogene, 2000, 19: 2739-2744). Activating mutations within the catalytic site
of pllOcc
have been associated with various other tumours such as those of the
colorectal region and
of the breast and lung (Samuels etal., Science, 2004, 304, 554). Tumour-
related mutations
in p85a have also been identified in cancers such as those of the ovary and
colon (Philp et
al., Cancer Research, 2001, 61, 7426-7429). PI 3 kinase-13 plays a critical
role in B-cell
function and has been shown to be a mediator of survival signalling in a range
of B-cell
malignancies. This includes, but may not be limited to, chronic lymphocytic
leukaemia
(CLL), acute lymphoblastic leukaemia (ALL), follicular lymphoma, diffuse large
B-cell
lymphoma (DLBCL) and mantle cell lymphoma (Ikeda et al., Blood, 2010, 116,
1460-
1468; Herman etal., Blood, 2010, 116, 2078-2088; Lannutti etal., Blood, 2011,
117, 591-
594; Hoellenriegel etal., Blood, 2011, 118, 3603-3612). In addition to direct
effects, it is
believed that activation of Class Ia PI 3-kinase contributes to tumourigenic
events that
occur upstream in signalling pathways, for example by way of ligand-dependent
or ligand-
independent activation of receptor tyrosine kinases, GPCR systems or integrins
(Vara et
al., Cancer Treatment Reviews, 2004, 30, 193-204). Examples of such upstream
signalling
pathways include over-expression of the receptor tyrosine kinase Erb2 in a
variety of
tumours leading to activation of PI 3-kinase-mediated pathways (Harari etal.,
Oncogene,
2000, 19, 6102-6114) and over-expression of the oncogene Ras (Kauffmann-Zeh
etal.,
Nature, 1997, 385, 544-548). In addition, Class Ia PI 3-kinases may contribute
indirectly
to tumourigenesis caused by various downstream signalling events. For example,
loss of
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
4
the effect of the PTEN tumour-suppressor phosphatase that catalyses conversion
of
P1(3,4,5)P3 back to PI(4,5)P2 is associated with a very broad range of tumours
via
deregulation of PI 3-kinase-mediated production of P1(3,4, 5)P3 (Simpson and
Parsons,
Exp. Cell Res., 2001, 264, 29-41). Furthermore, augmentation of the effects of
other P13-
kinase-mediated signalling events is believed to contribute to a variety of
cancers, for
example by activation of AKT (Nicholson and Anderson, Cellular Signalling,
2002, 14,
381-395)
In addition to a role in mediating proliferative and survival signalling in
tumour
cells, there is also good evidence that Class Ia PI 3-kinase enzymes will also
contribute to
tumourigenesis via its function in tumour-associated stromal cells. For
example, PI 3-
kinase signalling is known to play an important role in mediating angiogenic
events in
endothelial cells in response to pro-angiogenic factors such as VEGF (Abid et
al.,
Arterioscler. Thromb. Vasc. Biol., 2004, 24, 294-300). As Class I PI 3-kinase
enzymes are
also involved in motility and migration (Sawyer, Expert Opinion Investig.
Drugs, 2004, 13,
1-19), PI 3-kinase inhibitors should provide therapeutic benefit via
inhibition of tumour
cell invasion and metastasis.
In addition, Class I PI 3-kinase enzymes play an important role in the
regulation of
immune cells with PI 3-kinase activity contributing to pro-tumourigenic
effects of
inflammatory cells (Coussens and Werb, Nature, 2002, 420, 860-867).
These findings suggest that pharmacological inhibitors of Class I PI 3-kinase
enzymes should be of therapeutic value for treatment of the various forms of
the disease of
cancer comprising solid tumours such as carcinomas and sarcomas and the
leukaemias and
lymphoid malignancies. In particular, inhibitors of Class I PI 3-kinase
enzymes should be
of therapeutic value for treatment of, for example, cancer of the breast,
colorectum, lung
(including small cell lung cancer, non-small cell lung cancer and
bronchioalveolar cancer)
and prostate, and of cancer of the bile duct, bone, bladder, brain, head and
neck, kidney,
liver, gastrointestinal tissue, oesophagus, ovary, pancreas, skin, testes,
thyroid, uterus,
cervix and vulva, and of leukaemias (including acute lymphoblastic leukaemia,
Chronic
Lymphocytic Leukaemia and chronic myelogenous leukaemia), multiple myeloma and
lymphomas (including non-Hodgkin's lymphomas such as diffuse large B-cell
lymphoma,
follicular lymphoma, and mantle cell lymphoma).
CA 02900136 2015-08-04
WO 2014/135851 PCT/GB2014/050618
Generally, investigators have explored the physiological and pathological
roles of
the PI 3-kinase enzyme family using the aforementioned PI 3-kinase inhibitors
LY294002
and wortmannin. Although use of those compounds may suggest a role for PI 3-
kinase in a
cellular event, they are not sufficiently selective within the PI 3-kinase
family to allow
5 dissection of the individual roles of the family members. For this
reason, more potent and
selective pharmaceutical PI 3-kinase inhibitors would be useful to allow a
more complete
understanding of PI 3-kinase function and to provide useful therapeutic
agents.
In addition to tumourigenesis, there is evidence that Class I PI 3-kinase
enzymes
play a role in other diseases (Wymann et al., Trends in Pharmacological
Science, 2003, 24,
366-376). Both Class Ia PI 3-kinase enzymes and the single Class lb enzyme
have
important roles in cells of the immune system (Koyasu, Nature Immunology,
2003, 4, 313-
319) and thus they are therapeutic targets for inflammatory and allergic
indications.
Inhibition of PI 3-kinase is also, as described earlier, useful to treat
cardiovascular disease
via anti-inflammatory effects or directly by affecting cardiac myocytes
(Prasad et al.,
Trends in Cardiovascular Medicine, 2003, 13, 206-212). Inhibition of PI 3-
kinase is also
useful to treat thrombosis. W02004016607 provides a method of disrupting
platelet
aggregation and adhesion occurring under high shear conditions, and a method
for
inhibiting platelet activation induced by shear, where both methods comprise
the
administration of a selective PI 3-kinasef3 inhibitor. W02004016607 also
provides an
antithrombotic method comprising administering an effective amount of a
selective PI 3-
kinase 13 inhibitor. According to the method, specific inhibition of
thrombosis can be
obtained without affecting normal haemostasis by targeting PI 3-kinase 1 that
is important
for shear-induced platelet activation. Said antithrombotic method therefore
does not
involve side effects caused by disruption of normal haemostasis, such as
extending of
bleeding time.
Compound [I] is a selective inhibitor of phosphoinositide (PI) 3-kinase 13
which is
disclosed amongst many other Examples in international patent application
publication
number W02011/051704 Compound [I] has the following structure:
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
6
0 0
0 N-
,==
FF
Compound [I]
In W02011/051704 it is stated that the compounds disclosed therein "may be
applied as a sole therapy or may involve, in addition to the compound of the
invention,
conventional surgery or radiotherapy or chemotherapy". W02011/051704 then
lists many
potential anti-tumour agents for use in such chemotherapy.
It has now been found that the use of Compound [I] in combination with a
taxane
surprisingly provides a synergistic effect, and may therefore provide an
improved method
of treating cancer compared to the use of either Compound [I] or a taxane
alone.
A combination treatment may be considered to provide a synergistic effect if
the
effect is therapeutically superior, as measured by, for example, the extent of
the response,
the response rate, the time to disease progression or the survival period, to
that achievable
on dosing one or other of the components of the combination treatment at their
conventional dose. For example, the effect of the combination treatment is
synergistic if
the use of the combination is superior to the effect achievable with Compound
[I] or one of
the specified combination partners, when used alone. In particular, the effect
of the
combination treatment is synergistic if efficacy can be maintained during
combination
treatment at a lower dose of one or more of the combination partners than is
required for
the corresponding monotherapy treatment. Further, the effect of the
combination treatment
is synergistic if a beneficial effect is obtained in a group of patients that
does not respond
(or responds poorly) to Compound [I] or one of the specified combination
partners, when
used alone.
A combination treatment may also be considered to provide a synergistic effect
if
one or both of the components may be dosed less frequently than the dosing
schedule used
for conventional dosing of each component when used alone, while not adversely
impacting the beneficial effect otherwise achieved by the use of conventional
amounts of
81519558
7
an agent used alone. In particular, synergy is deemed to be present if the
frequency of
dosing of Compound [I] and/or a specified combination partner may be reduced
relative to
what would otherwise be conventional/required when using one of the
combination
partners alone, without detriment to one or more factors such as: extent of
the response, the
response rate, the time to disease progression and survival data and in
particular without
detriment to the duration of the response. Decreasing the dosing amount and
frequency for
a particular compound can lead to fewer and/or less troublesome side-effects
than those
that occur when conventional scheduling/doses are used.
In a first aspect of the invention there is provided a combination comprising
Compound [I] or a pharmaceutically acceptable salt thereof and a taxane, for
use in the
treatment of cancer.
A pharmaceutically acceptable salt is, for example, an acid-addition salt with
an
inorganic or organic acid, for example hydrochloric acid, hydrobromic acid,
methanesulphonic acid, sulphuric acid or trifluoroacetic acid.
Herein where the term "taxane" is used it is to be understood that this may
refer to
any chemical analogue which exerts its anticancer effect by stabilization of
the tubulin
microtubules involved in cell division.
Examples of taxanes that may be combined with Compound [I] include:
(2aR,3aR,4aR,6R,9S,11S,12S,12aR,12b S)-6,12b-diacetoxy-943(S)-(tert-
butoxycarbonylamino)-2(R)-hydroxy-3-phenylpropionyloxy]-12-b enzoyloxy-11-
hydroxy-
8,13,13-trimethy1-2a,3,3a,4,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-
methanocyclodeca[3,4]-cyclopropa[4,5]benz[1,2-b]oxet-5-one dihydrate;
paclitaxel
(Taxol), BMS-184476 (7-methylthiomethylpaclitaxel); BMS-188797; BMS-275183;
BMS-
. TM
188797; BMS-109881; CYC-3204 (a penetratin-paclitaxel conjugate); Taxoprexin;
DJ-
927; Docetaxel (Taxoterem4); Larotexel (XRP9881; RPR-109881A); XRP6258
(RPR112658); Milataxel (MAC-321); MST 997; MBT-206; NBT-287; Ortataxel; Protax-
3; PG-TXL; PNU-166945; PNU-106258; Orataxel (BAY 59-8862; IDN 5109;
semisynthetic taxane);TPI-287; Protaxel and MAC-321 (Taxalog).
Examples of formulations for taxanes include:
- Conventional formulations of paclitaxel or docetaxel, for example the
currently
approved Taxollm and TaxotereTm formulations;
Date Recue/Date Received 2020-07-21
CA 02900136 2015-08-04
WO 2014/135851 PCT/GB2014/050618
8
- Formulations with biocompatible polymers, particularly proteins such as
albumin,
more particularly nano-particle or micro-particle formulations of paclitaxel
or
docetaxel with albumin, for example AbraxaneTm (described in US 5,439,686 and
US 6,749,868) or NAB-docetaxel (described in, for example US 20080161382,
US20070117744 and US 20070082838);
- Polymer conjugates, particularly polymer conjugates of paclitaxel or
docetaxel,
more particularly conjugates of docetaxel or paclitaxel with poly-L-glutamate,
for
example Opaxio (also known as Xyotax, paclitaxel poliglumex, CT-2103 and
described in for example Li C.; Poly ( L -glutamic acid) ¨ anticancer drug
conjugates; Adv. Drug Deliv. Rev. 2002; 54: 695 ¨713);
- Conjugates of docetaxel or paclitaxel with a fatty acid, particularly
conjugates of
paclitaxel or docetaxel with docosahexaenoic acid (DHA), for example,
Taxoprexin
(DHA-paclitaxel, described in for example Bradley MO et al. Tumor targeting by
covalent conjugation of a natural fatty acid to paclitaxel; Clin. Cancer Res.
2001; 7:
3229 ¨38);
- Microparticle compositions such as the porous microparticle formulations
described in US 6,645,528, for example the microparticle formulation of
paclitaxel
AI-850, comprising paclitaxel nanoparticles in a porous, hydrophilic matrix,
composed primarily of a sugar; and
- Emulsions of paclitaxel or docetaxel in vitamin E, for example Tocosol.
In one embodiment the taxane is selected from paclitaxel, docetaxel and
Abraxane.
In one embodiment the taxane is selected from docetaxel and paclitaxel.
In one embodiment the taxane is paclitaxel.
In one embodiment the taxane is docetaxel.
In one embodiment the taxane is abraxane.
In one embodiment the taxane is cabazitaxel.
In one embodiment the taxane is selected from docetaxel, paclitaxel,
cabazitaxel,
larotaxel, ortataxel and tesetaxel.
Herein, where the term "combination" is used it is to be understood that this
may
refer to simultaneous, separate or sequential administration of the components
of the
combination.
81519558
9
In one embodiment "combination" refers to simultaneous administration of the
components of the combination.
In one embodiment "combination" refers to separate administration of the
components
of the combination.
In one embodiment "combination" refers to sequential administration of the
components of the combination.
As shown hereinafter, there are benefits in combining Compound [I] with a
taxane
(such as docetaxel) in the PC3 and HCC70 xenograft models. Furthermore, as
also shown
herein intermittent dosage of Compound [I] in combination with a taxane (such
as doectaxel)
has similar effectiveness at regulating tumour size as continuous dosage of
Compound [I] in
combination with a taxane (such as docetaxel). In particular, intermittent
dosage of
Compound [I] following only a single dose of a taxane (such as docetaxel) has
similar
effectiveness at regulating tumour size as continuous dosage of Compound [I]
in combination
with a taxane (such as docetaxel).
Therefore, in one embodiment there is provided a combination comprising
Compound
[I] or a pharmaceutically acceptable salt thereof and a taxane for use in the
treatment of
cancer, wherein the Compound [I] or a pharmaceutically acceptable salt thereof
is dosed
intermittently.
In one embodiment the dosage cycle comprises the intermittent dosage of
Compound
[I] or a pharmaceutically acceptable salt thereof.
For the avoidance of doubt, intermittent dosage of Compound [I] or a
pharmaceutically acceptable salt thereof means that in a given dosage cycle
there will be one
or more days (for example 1, 2, 3, 4, 5, 6 or 7 days) where no Compound [I] or
a
pharmaceutically acceptable salt thereof is administered.
In one embodiment, there is provided a combination comprising Compound [I]:
Date Recue/Date Received 2020-07-21
81519558
9a
o
II
N
1
0 N
0
''.. NH
F F
Compound II]
or a pharmaceutically acceptable salt thereof and docetaxel, for use in the
treatment of cancer,
wherein the Compound [I], or a pharmaceutically acceptable salt thereof is for
intermittent
administration, wherein within a given dosage cycle, 1, 2, 3, 4, 5, 6 or 7
days of the given
dosage cycle are free of administration of the Compound [I] or a
pharmaceutically acceptable
salt thereof.
In one embodiment, there is provided use of a combination comprising Compound
[I]:
0
I)
N
1
0 N
1(:)
F F
Compound II]
or a pharmaceutically acceptable salt thereof and docetaxel, for the treatment
of cancer,
wherein the use comprises intermittent administration of the Compound [I], or
a
pharmaceutically acceptable salt thereof, and wherein within a given dosage
cycle, 1, 2, 3, 4,
Date Recue/Date Received 2020-07-21
81519558
9b
5, 6 or 7 days of the given dosage cycle are free of administration of the
Compound [I] or a
pharmaceutically acceptable salt thereof.
It may be advantageous, within a given dosage cycle, to administer one
specific
component of the combination before the other, i.e. to dose sequentially.
Therefore, in one embodiment the dosage cycle comprises the sequential
administration of the Compound [I] or a pharmaceutically acceptable salt
thereof prior to the
administration of the taxane (such as docetaxel).
In another embodiment the dosage cycle comprises the sequential administration
of the
taxane (such as docetaxel) prior to the administration of Compound [I] or a
pharmaceutically
acceptable salt thereof.
Date Recue/Date Received 2020-07-21
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
In one embodiment the dosage cycle involves only a single dose of the taxane
(such
as docetaxel).
In one embodiment the dosage cycle comprises the sequential administration of
the
taxane (such as docetaxel) only within the 2 days prior to the first
administration of
5 .. Compound [I] or a pharmaceutically acceptable salt thereof within a
dosage cycle.
In one embodiment the dosage cycle comprises the sequential administration of
the
taxane (such as docetaxel) only within the 24 hours prior to the first
administration of
Compound [I] or a pharmaceutically acceptable salt thereof within a dosage
cycle
In one embodiment the dosage cycle comprises the sequential administration of
the
10 taxane (such as docetaxel) only within the 12 hours prior to the first
administration of
Compound [I] or a pharmaceutically acceptable salt thereof within a dosage
cycle.
In one embodiment the dosage cycle comprises the sequential administration of
the
taxane (such as docetaxel) only within the 6 hours prior to the first
administration of
Compound [I] or a pharmaceutically acceptable salt thereof within a dosage
cycle.
In one embodiment the dosage cycle comprises the sequential administration of
the
taxane (such as docetaxel) only within the 3 hours prior to the first
administration of
Compound [I] or a pharmaceutically acceptable salt thereof within a dosage
cycle.
In one embodiment the dosage cycle comprises the sequential administration of
the
taxane (such as docetaxel) only within the 1.5 hours prior to the first
administration of
.. Compound [I] or a pharmaceutically acceptable salt thereof within a dosage
cycle.
For the avoidance of doubt "within the x hours prior to the first
administration of
Compound" means any time up to x hours before the first dosing of Compound [I]
or a
pharmaceutically acceptable salt thereof within a given dosage cycle, and
includes
substantially simultaneous dosing of the taxane (such as docetaxel) with the
first dosing of
Compound [I] or a pharmaceutically acceptable salt thereof of a given dosage
cycle.
In one embodiment the dosage cycle is from 8 to 29 days in length.
In one embodiment the dosage cycle is from 15 to 29 days in length
In one embodiment the dosage cycle is from 15 to 22 days in length
In one embodiment the dosage cycle is from 22 to 29 days in length
In one embodiment the dosage cycle is from 8 to 22 days in length
In one embodiment the dosage cycle is from 8 to 15 days in length.
In one embodiment the dosage cycle is 29 days in length.
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
11
In one embodiment the dosage cycle is 22 days in length.
In one embodiment the dosage cycle is 15 days in length.
In one embodiment the dosage cycle is 8 days in length.
In one embodiment the dosage cycle comprises Compound [I] or a
pharmaceutically acceptable salt thereof being dosed for at least one period
(for example 1,
2, or 3 periods) of 3-5 consecutive days (for example 3, 4 or 5 days), which
period(s) are
immediately followed by a further period of least one day (for example 2, 3, 4
or 5
consecutive days) where no Compound [I] or a pharmaceutically acceptable salt
thereof is
dosed
In one embodiment the dosage cycle comprises at least one 7 day period, in
which
period Compound [I] or a pharmaceutically acceptable salt thereof is dosed
only for 3-5
consecutive days
In one embodiment the dosage cycle comprises one 7 day period in which
Compound [I] or a pharmaceutically acceptable salt thereof is only dosed for 3-
5
consecutive days
In one embodiment the dosage cycle comprises two 7 day periods in which
Compound [I] or a pharmaceutically acceptable salt thereof is only dosed for 3-
5
consecutive days
In one embodiment the dosage cycle comprises three7 day periods in which
Compound [I] or a pharmaceutically acceptable salt thereof is only dosed for 3-
5
consecutive days
In one embodiment the dosage cycle comprises Compound [I] or a
pharmaceutically acceptable salt thereof being dosed for at least one period
of 5
consecutive days immediately followed by a further period of 2 consecutive
days where no
Compound [I] or a pharmaceutically acceptable salt thereof is dosed.
In one embodiment the dosage cycle comprises Compound [I] or a
pharmaceutically acceptable salt thereof being dosed for at least one period
of 4
consecutive days immediately followed by a further period of 3 consecutive
days where no
Compound [I] or a pharmaceutically acceptable salt thereof is dosed
In one embodiment the dosage cycle comprises Compound [I] or a
pharmaceutically acceptable salt thereof being dosed for at least one period
of 3
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
12
consecutive days immediately followed by a further period of 4 consecutive
days where no
Compound [I] or a pharmaceutically acceptable salt thereof is dosed.
In one embodiment the dosage cycle is 29 days long and comprises four 7 day
periods, each such period consisting of 5 days where Compound [I] or a
pharmaceutically
__ acceptable salt thereof is dosed followed by 2 days where no Compound [I]
or a
pharmaceutically acceptable salt thereof is dosed.
In one embodiment the dosage cycle is 29 days long and comprises four 7 day
periods, each such period consisting of 4 days where Compound [I] or a
pharmaceutically
acceptable salt thereof is dosed followed by 3 days where no Compound [I] or a
__ pharmaceutically acceptable salt thereof is dosed.
In one embodiment the dosage cycle is 29 days long and comprises four 7 day
periods, each such period consisting of 3 days where Compound [I] or a
pharmaceutically
acceptable salt thereof is dosed followed by 4 days where no Compound [I] or a
pharmaceutically acceptable salt thereof is dosed.
In one embodiment the dosage cycle is 22 days long and comprises three 7 day
periods, each such period consisting of 5 days where Compound [I] or a
pharmaceutically
acceptable salt thereof is dosed followed by 2 days where no Compound [I] or a
pharmaceutically acceptable salt thereof is dosed.
In one embodiment the dosage cycle is 22 days long and comprises three 7 day
__ periods, each such period consisting of 4 days where Compound [I] or a
pharmaceutically
acceptable salt thereof is dosed followed by 3 days where no Compound [I] or a
pharmaceutically acceptable salt thereof is dosed.
In one embodiment the dosage cycle is 22 days long and comprises three 7 day
periods, each such period consisting of 3 days where Compound [I] or a
pharmaceutically
__ acceptable salt thereof is dosed followed by 4 days where no Compound [I]
or a
pharmaceutically acceptable salt thereof is dosed.
In one embodiment the dosage cycle is 15 days long and comprises two 7 day
periods, each such period consisting of 5 days where Compound [I] or a
pharmaceutically
acceptable salt thereof is dosed followed by 2 days where no Compound [I] or a
__ pharmaceutically acceptable salt thereof is dosed.
In one embodiment the dosage cycle is 15 days long and comprises two 7 day
periods, each such period consisting of 4 days where Compound [I] or a
pharmaceutically
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
13
acceptable salt thereof is dosed followed by 3 days where no Compound [I] or a
pharmaceutically acceptable salt thereof is dosed.
In one embodiment the dosage cycle is 15 days long and comprises two 7 day
periods, each such period consisting of 3 days where Compound [I] or a
pharmaceutically
acceptable salt thereof is dosed followed by 4 days where no Compound [I] or a
pharmaceutically acceptable salt thereof is dosed.
In one embodiment the dosage cycle is 8 days long and comprises one 7 day
period
consisting of 5 days where Compound [I] or a pharmaceutically acceptable salt
thereof is
dosed followed by 2 days where no Compound [I] or a pharmaceutically
acceptable salt
thereof is dosed.
In one embodiment the dosage cycle is 8 days long and comprises one 7 day
period
consisting of 4 days where Compound [I] or a pharmaceutically acceptable salt
thereof is
dosed followed by 3 days where no Compound [I] or a pharmaceutically
acceptable salt
thereof is dosed.
In one embodiment the dosage cycle is 8 days long and comprises one 7 day
period
consisting of 3 days where Compound [I] or a pharmaceutically acceptable salt
thereof is
dosed followed by 4 days where no Compound [I] or a pharmaceutically
acceptable salt
thereof is dosed.
In one embodiment the dosage cycle is 8-29 days long and comprises a single
administration of the taxane (such as docetaxel) within the 24 hours prior to
the first
administration of Compound [I] or a pharmaceutically acceptable salt thereof,
followed by
intermittent dosing of Compound [I] or a phatmaceutically acceptable salt
thereof.
In one embodiment the dosage cycle is 15-29 days long and comprises the
administration of the taxane (such as docetaxel) within the 24 hours prior to
the first
administration of Compound [I] or a pharmaceutically acceptable salt thereof,
followed by
intermittent dosing of Compound [I] or a pharmaceutically acceptable salt
thereof in 7 day
periods consisting of 5 days where Compound [I] or a pharmaceutically
acceptable salt
thereof is dosed followed by 2 days where no Compound [I] or a
pharmaceutically
acceptable salt thereof is dosed.
In one embodiment the dosage cycle is 15-29 days long and comprises the
administration of the taxane (such as docetaxel) within the 24 hours prior to
the first
administration of Compound [I] or a pharmaceutically acceptable salt thereof,
followed by
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
14
intermittent dosing of Compound [I] or a phaimaceutically acceptable salt
thereof in 7 day
periods consisting of 4 days where Compound [I] or a pharmaceutically
acceptable salt
thereof is dosed followed by 3 days where no Compound [I] or a
pharmaceutically
acceptable salt thereof is dosed.
In one embodiment the dosage cycle is 15-29 days long and comprises the
administration of the taxane (such as docetaxel) within the 24 hours prior to
the first
administration of Compound [I] or a pharmaceutically acceptable salt thereof,
followed by
intermittent dosing of Compound [I] or a pharmaceutically acceptable salt
thereof in 7 day
periods consisting of 3 days where Compound [I] or a pharmaceutically
acceptable salt
thereof is dosed followed by 4 days where no Compound [I] or a
phaimaceutically
acceptable salt thereof is dosed.
In one embodiment the dosage cycle comprises the following steps:
a) Day 1: Administration of taxane (for example docetaxel);
b) Days 2-6: Administration of Compound [I] or a pharmaceutically acceptable
salt thereof;
c) Days 7-8: No dosage of either Compound [I] or a pharmaceutically acceptable
salt thereof or taxane;
d) Days 9-13: Administration of Compound [I] or a pharmaceutically acceptable
salt thereof;
e) Days 14-15: No dosage of either Compound [I] or a pharmaceutically
acceptable salt thereof or taxane;
f) Days 16-20: Administration of Compound [I] or a pharmaceutically
acceptable
salt thereof;
g) Days 21-22: No dosage of either Compound [I] or a pharmaceutically
acceptable salt thereof or taxane.
In one embodiment the dosage cycle comprises the following steps:
a) Day 1: Administration of taxane (for example docetaxel);
b) Days 2-5: Administration of Compound [I] or a pharmaceutically acceptable
salt thereof;
c) Days 6-8: No dosage of either Compound [I] or a pharmaceutically acceptable
salt thereof or taxane;
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
d) Days 9-12: Administration of Compound [I] or a pharmaceutically acceptable
salt thereof;
e) Days 13-15: No dosage of either Compound [I] or a pharmaceutically
acceptable salt thereof or taxane;
5 f) Days 16-19: Administration of Compound [I] or a pharmaceutically
acceptable
salt thereof.
g) Days 20-22: No dosage of either Compound [I] or a pharmaceutically
acceptable salt thereof or taxane.
In one embodiment the dosage cycle comprises the following steps.
10 a) Day 1: Administration of taxane (for example docetaxel);
b) Days 2-4: Administration of Compound [I] or a phaimaceutically acceptable
salt thereof;
c) Days 5-8: No dosage of either Compound [I] or a pharmaceutically acceptable
salt thereof or taxane;
15 d) Days 9-11: Administration of Compound [I] or a pharmaceutically
acceptable
salt thereof;
e) Days 12-15: No dosage of either Compound [I] or a pharmaceutically
acceptable salt thereof or taxane;
f) Days 16-18: Administration of Compound [I] or a pharmaceutically
acceptable
salt thereof.
g) Days 19-22: No dosage of either Compound [I] or a pharmaceutically
acceptable salt thereof or taxane.
In one embodiment of the invention, Compound [I] or a phaimaceutically
acceptable salt thereof is administered once daily on the days of a dosage
cycle when it is
dosed.
In one embodiment of the invention, Compound [I] or a phaimaceutically
acceptable salt thereof is administered twice daily on the days of a dosage
cycle when it is
dosed.
For the avoidance of doubt, dosage cycles may be separated by a number of days
(for example 1, 2, 3, 4, 5, 6 or 7 days) where none of the active combination
components
are administered.
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
16
In one aspect where Compound [I] is mentioned, the Compound [I] is 8-[(1R)-1-
(3,5-difluorophenylamino)ethy1]-N,N-dimethyl-2-morpholino-4-oxo-4H-chromene-6-
carboxamide.
In another aspect where Compound [I] is mentioned, the Compound [I] is a
pharmaceutically acceptable salt of 841R)-1-(3,5-difluorophenylamino)ethyli-
N,N-
dimethy1-2-m orphol ino-4-oxo-4H-chromene-6-carboxami de.
In one embodiment of the invention there is provided a combination comprising
Compound [I] or a pharmaceutically acceptable salt thereof and docetaxel, for
use in the
treatment of cancer.
In one embodiment of the invention there is provided a combination comprising
Compound [I] or a pharmaceutically acceptable salt thereof and docetaxel, for
use in the
treatment of cancer.
In one embodiment of the invention there is provided a combination comprising
a
pharmaceutically acceptable salt of Compound [I] and docetaxel, for use in the
treatment
of cancer.
According to a further aspect of the invention there is provided a
pharmaceutical
composition which comprises Compound [I] or a pharmaceutically acceptable salt
thereof
and a taxane in association with a pharmaceutically acceptable diluent or
carrier.
According to a further aspect of the invention there is provided a
pharmaceutical
composition which comprises Compound [I] or a pharmaceutically acceptable salt
thereof
and docetaxel in association with a pharmaceutically acceptable diluent or
carrier.
In one embodiment there is provided a pharmaceutical product comprising:
(i) a pharmaceutical composition which comprises Compound [I], or a
pharmaceutically acceptable salt thereof, in association with a
pharmaceutically
acceptable diluent or carrier; and
(ii) a pharmaceutical composition which comprises a taxane, in association
with a
pharmaceutically acceptable diluent or carrier.
In one embodiment there is provided a pharmaceutical product comprising:
(i) a pharmaceutical composition which comprises Compound [I], or a
pharmaceutically acceptable salt thereof, in association with a
pharmaceutically
acceptable diluent or carrier; and
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
17
(ii) a pharmaceutical composition which comprises docetaxel, in
association with a
pharmaceutically acceptable diluent or carrier.
In one aspect there is provided a method of treating cancer, in a warm-blooded
animal, such as a human, which comprises administering to said animal an
effective
amount of Compound [I], or a pharmaceutically acceptable salt thereof, in
combination
with an effective amount of a taxane.
In one aspect there is provided a method of treating cancer, in a warm-blooded
animal, such as a human, which comprises administering to said animal an
effective
amount of Compound [I], or a pharmaceutically acceptable salt thereof, in
combination
with an effective amount of docetaxel.
Where the treatment of cancer is indicated, it is to be understood that this
may refer
to the prevention of metastases and the treatment of metastases, i.e. cancer
spread.
Therefore the combination of the present invention might be used to treat a
patient who has
no metastases to stop them occurring, or to lengthen the time period before
they occur, and
to a patient who already has metastases to treat the metastases themselves.
Furthermore the
treatment of cancer may refer to treatment of an established primary tumour or
tumours
and developing primary tumour or tumours.
Therefore, in one aspect the treatment of cancer relates to the prevention of
metastases.
In another aspect of the invention the treatment of cancer relates to the
treatment of
metastases.
In another aspect of the invention the treatment of cancer relates to
treatment of an
established primary tumour or tumours or developing primary tumour or tumours.
Herein, the treatment of cancer may refer to the prevention of cancerper se.
According to a further aspect of the invention, there is provided a kit
comprising
Compound [I], or a pharmaceutically acceptable salt thereof and a taxane,
optionally with
instructions for use.
According to a further aspect of the invention, there is provided a kit
comprising
Compound [I], or a pharmaceutically acceptable salt thereof and docetaxel,
optionally with
instructions for use.
According to a further aspect of the invention, there is provided a kit
comprising:
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
18
a) Compound [I], or a pharmaceutically acceptable salt thereof, in a first
unit dosage
form;
b) a taxane, in a second unit dosage form;
c) container means for containing said first and second dosage forms; and
optionally
d) instructions for use.
According to a further aspect of the invention, there is provided a kit
comprising
a) Compound [I], or a pharmaceutically acceptable salt thereof, in a first
unit dosage
form;
b) docetaxel, in a second unit dosage form;
c) container means for containing said first and second dosage forms, and
optionally
d) instructions for use.
An example of a unit dosage form is a tablet for oral administration.
According to a further aspect of the invention there is provided a
pharmaceutical
composition which comprises Compound [I], or a pharmaceutically acceptable
salt thereof,
and a taxane in association with a pharmaceutically acceptable diluent or
carrier, for use in
the treatment of cancer.
According to a further aspect of the invention there is provided a
pharmaceutical
composition which comprises Compound [I], or a pharmaceutically acceptable
salt thereof,
and docetaxel in association with a pharmaceutically acceptable diluent or
carrier, for use
in the treatment of cancer.
According to a further aspect of the invention there is provided a
pharmaceutical
composition which comprises Compound [I], or a pharmaceutically acceptable
salt thereof,
in association with a pharmaceutically acceptable diluent or carrier; in
combination with a
pharmaceutical composition which comprises a taxane, in association with a
pharmaceutically acceptable diluent or carrier, for use in the treatment of
cancer.
According to a further aspect of the invention there is provided a
pharmaceutical
composition which comprises Compound [I], or a pharmaceutically acceptable
salt thereof,
in association with a pharmaceutically acceptable diluent or carrier; in
combination with a
pharmaceutical composition which comprises docetaxel, in association with a
pharmaceutically acceptable diluent or carrier, for use in the treatment of
cancer.
The pharmaceutical compositions may be in a form suitable for oral
administration,
for example as a tablet or capsule, for parenteral injection (including
intravenous,
CA 02900136 2015-08-04
WO 2014/135851 PCT/GB2014/050618
19
subcutaneous, intramuscular, intravascular or infusion) as a sterile solution,
suspension or
emulsion, for topical administration as an ointment or cream or for rectal
administration as
a suppository. In general the above compositions may be prepared in a
conventional
manner using conventional excipients.
According to a further aspect of the present invention there is provided a kit
comprising Compound [I], or a pharmaceutically acceptable salt thereof and a
taxane;
optionally with instructions for use; for use in the treatment of cancer.
According to a further aspect of the present invention there is provided a kit
comprising Compound [I], or a pharmaceutically acceptable salt thereof and
docetaxel;
optionally with instructions for use, for use in the treatment of cancer.
According to a further aspect of the present invention there is provided a kit
comprising:
a) Compound [I], or a pharmaceutically acceptable salt thereof, in a first
unit dosage form,
b) a taxane;
c) container means for containing said first and second dosage forms; and
optionally
d) instructions for use;
for use in the treatment of cancer.
According to a further aspect of the present invention there is provided a kit
comprising:
a) Compound [I], or a pharmaceutically acceptable salt thereof, in a first
unit dosage form;
b) docetaxel;
c) container means for containing said first and second dosage forms; and
optionally
d) instructions for use;
for use in the treatment of cancer.
According to the present invention, there is provided a combination which
comprises Compound [I], or a pharmaceutically acceptable salt thereof and a
taxane for use
as a medicament.
According to the present invention, there is provided a combination which
comprises Compound [I], or a pharmaceutically acceptable salt thereof and
docetaxel for
use as a medicament.
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
According to another feature of the invention there is provided the use of
Compound [I], or a pharmaceutically acceptable salt thereof, in combination
with a taxane,
in the manufacture of a medicament for the treatment of cancer.
According to another feature of the invention there is provided the use of
5 Compound [I], or a pharmaceutically acceptable salt thereof, in
combination with
docetaxel, in the manufacture of a medicament for the treatment of cancer.
It may be convenient or medically appropriate for a physician to determine the
exact dosage and scheduling for use of a combination product, such that the
active
components of the combination product may necessarily not be present together
within a
10 single dosage form at a fixed dose Therefore a physician or pharmacist
may prepare a
combination medicament comprising the active combination products in readiness
for
simultaneous, separate or sequential combination use in medicine, for example
to treat
cancer in a warm-blooded animal, such as human.
According to another feature of the invention there is provided the use of
15 Compound [I], or a pharmaceutically acceptable salt thereof, in
combination with a taxane
(such as docetaxel), in the preparation of a combination medicament for use in
medicine.
According to another feature of the invention there is provided the use of
Compound [I], or a pharmaceutically acceptable salt thereof, in combination
with a taxane
(such as docetaxel), in the preparation of a combination medicament for
simultaneous,
20 separate or sequential combination use in medicine.
According to another feature of the invention there is provided the use of
Compound [I], or a pharmaceutically acceptable salt thereof, in combination
with a taxane
(such as docetaxel), in the preparation of a combination medicament for
simultaneous,
separate or sequential combination use for the treatment of cancer.
According to another feature of the invention there is provided the use of
Compound [I], or a pharmaceutically acceptable salt thereof, in combination
with a taxane
(such as docetaxel), in the preparation of a combination medicament for
simultaneous,
separate or sequential combination use for the treatment of cancer in a warm-
blooded
animal such as a human
According to another feature of the invention there is provided the use of
Compound [I], or a pharmaceutically acceptable salt thereof, in combination
with a taxane
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
21
(such as docetaxel), in the preparation of a combination medicament for
separate
combination use for the treatment of cancer in a warm-blooded animal such as a
human.
According to another feature of the invention there is provided the use of
Compound [I], or a pharmaceutically acceptable salt thereof, in combination
with a taxane
.. (such as docetaxel), in the preparation of a combination medicament for
sequential
combination use for the treatment of cancer in a warm-blooded animal such as a
human.
According to another feature of the invention there is provided the use of
Compound [I], or a pharmaceutically acceptable salt thereof, in combination
with a taxane
(such as docetaxel), in the preparation of a combination medicament for the
treatment of
cancer.
According to a further aspect of the present invention there is provided a
combination comprising Compound [I], or a pharmaceutically acceptable salt
thereof, and
a taxane (such as docetaxel), for use in the treatment of cancer.
In one embodiment there is provided Compound [I], or a pharmaceutically
acceptable salt thereof, and a taxane (such as docetaxel) for use in the
treatment of cancer
in a warm-blooded animal such as a human.
In one embodiment there is provided Compound [I], or a pharmaceutically
acceptable salt thereof, and a taxane (such as docetaxel), for use in the
treatment of cancer
in a warm-blooded animal such as a human; wherein the Compound [I], or a
.. pharmaceutically acceptable salt thereof, and the taxane are administered
simultaneously,
separately or sequentially to the wattn-blooded animal.
In one embodiment there is provided a method for the production of an anti-
cancer
effect in a warm-blooded animal such as a human, which comprises administering
to said
animal an effective amount of Compound [I] or a pharmaceutically acceptable
salt thereof,
optionally together with a pharmaceutically acceptable diluent or carrier;
before, after or
simultaneously with an effective amount of a taxane (such as docetaxel),
optionally
together with a phal maceutically acceptable diluent or carrier.
Where cancer is referred to, it may refer to oesophageal cancer, myeloma,
hepatocellular cancer, pancreatic cancer, cervical cancer, ewings tumour,
neuroblastoma,
kaposis sarcoma, ovarian cancer, breast cancer, colorectal cancer, prostate
cancer, bladder
cancer, melanoma, lung cancer - non small cell lung cancer, and small cell
lung cancer,
gastric cancer, head and neck cancer, brain cancer, renal cancer, lymphoma and
leukaemia.
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
22
In one embodiment the cancer is prostate cancer.
In one embodiment the cancer is pancreatic cancer.
In one embodiment the cancer is castrate-resistant prostate cancer.
In one embodiment the cancer is small cell lung cancer.
In one embodiment the cancer is non-small cell lung cancer.
In one embodiment the cancer is squamous non-small cell lung cancer.
In one embodiment the cancer is colorectal cancer.
In one embodiment the cancer is ovarian cancer.
In one embodiment the cancer is breast cancer.
In one embodiment the cancer is triple negative breast cancer.
In one embodiment the cancer is prostate cancer, non-small cell lung cancer or
breast cancer.
In one embodiment the cancer is castrate-resistant prostate cancer, squamous
non-
small cell lung cancer, colorectal cancer, pancreatic cancer or triple
negative breast cancer.
In one embodiment the cancer is castrate-resistant prostate cancer, squamous
non-
small cell lung cancer or triple negative breast cancer.
In one embodiment the cancer is bladder cancer, oesophageal cancer, gastric
cancer, melanoma, cervical cancer or renal cancer.
In one embodiment the cancer is endometrial, liver, stomach, thyroid, rectal
or
.. brain cancer.
In one embodiment the cancer is cancer which is deficient in the gene P
IEN.
In another embodiment the cancer is in a metastatic state, and more
particularly the
cancer produces metastases to the bone.
In one embodiment of the invention, particularly the cancer is in a metastatic
state,
.. and more particularly the cancer produces skin metastases.
In one embodiment of the invention, particularly the cancer is in a metastatic
state,
and more particularly the cancer produces lymphatic metastases.
In one embodiment of the invention, the cancer is in a non-metastatic state
The compositions of the invention may be obtained by conventional procedures
using conventional pharmaceutical excipients, well known in the art. Thus,
compositions
intended for oral use may contain, for example, one or more colouring,
sweetening,
flavouring and/or preservative agents.
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
23
A compound such as Compound I may normally be administered to a
warm-blooded animal at a unit dose within the range 5-5000 mg/m2 body area of
the
animal, i.e. approximately 0.1-200 mg/kg, and this normally provides a
therapeutically-effective dose. A unit dose form such as a tablet or capsule
will usually
contain, for example 1-250 mg of active ingredient. Preferably a unit dose
form such as a
tablet or capsule will usually contain, for example 10-70 mg of active
ingredient
Preferably a daily dose in the range of 10-150 mg/kg is employed, for example
10-50
mg/kg administered twice daily. However the daily dose will necessarily be
varied
depending upon the host treated, the particular route of administration, and
the severity of
the illness being treated. Accordingly the practitioner who is treating any
particular patient
may determine the optimum dosage. For example, a pharmaceutical composition of
the
present invention suitable for oral administration could comprise 1-200 mg/mL
of
Compound Tin 0.5% hydroxypropylmethylcellulose (HPMC).
A taxane (such as docetaxel) will normally be administered to a warm-blooded
animal at a unit dose known to the skilled practitioner as a therapeutically
effective dose.
For a single dosage form, the active ingredients may be compounded with an
appropriate
and convenient amount of excipients which may vary from about 5 to about 98
percent by
weight of the total composition. Dosage unit forms will generally contain
about 20 mg to
about 500 mg of active ingredient. However the daily dose will necessarily be
varied
depending upon the host treated, the particular route of administration, and
the severity of
the illness being treated. Accordingly the optimum dosage may be determined by
the
practitioner who is treating any particular patient.
In any embodiment described herein, the taxane (for example docetaxel) may be
dosed at 50-140 mg/m2 body area of the animal on the day(s) when it is dosed,
for example
60-120 mg/m2 body area of the animal, or for example 65-110 mg/m2 body area of
the
animal. In any embodiment described herein, the Compound [I] or a
pharmaceutically
acceptable salt thereof may be dosed at 10-50 mg/kg twice daily on days when
it is dosed.
The skilled person understands that if a pharmaceutically acceptable salt of
Compound [I]
is used, the Compound [I] content of the dose is less than 100% and the actual
mass of the
salt being dosed will be higher than the aforementioned 10-50 mg/kg twice
daily,
depending on the mass of the counterion used to make the particular salt, and
the
81519558
24
stoichiometry of the salt. The actual doses to be used for a given patient
should be
determined by an appropriately qualified physician.
The dosage of each of the drugs and their proportions have to be composed so
that
the best possible treatment effects, as defined by national and international
guidelines
(which are periodically reviewed and re-defined), will be met.
Experimental Details
Compound [I] may be prepared according to the procedures described in
W02011/051704.
Female Swiss athymic nu/nu mice were implanted subcutaneously in the left
flank
with 0.1m1 PC3 cells (1x106 cells in Iscove's serum free medium mixed 50:50
with
matrigel) or HCC70 cells (1x106 cells in RPMI serum free medium mixed 50:50
with
matrigel). Once tumours reached ¨200-500mm3 in size animals were randomised
into
control and treatment groups.
Compound [I] in the free base form was dosed either in the presence or absence
of
ABT (aminobenzotriazole). For groups where ABT was administered, Compound [I]
was
formulated once weekly either alone in 10% DMSO/ 60% TEG/30% WFI or in the
presence of ABT at 10 mg/ml. For twice daily dosing Compound [I] was co-dosed
with
ABT at hour 0, and then administered alone as the single formulation at hour 6-
8. For
groups where ABT was not administered, Compound [I] was formulated once weekly
as a
suspension in HPMC/Tween and dosed once or twice daily (0 and 6-8 hours)
Docetaxel (Taxoterel) was formulated fresh in physiological saline at 1.5mg/m1
and
dosed as a single i.v. bolus at a rate of 0.1m1/10g on Day 0,24 hours prior to
the
administration of Compound [I].
Tumour volume was calculated twice weekly from bilateral calliper measurements
using the formula (length x width x width) x /1/6. Growth inhibition from the
start of
treatment was assessed by comparison of the geometric mean change in tumour
volume for
the control and treated groups. Statistical significance was evaluated using a
one-tailed "t"-
test.
Date Recue/Date Received 2020-07-21
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
List of Figures
FIG. 1 - Long Term Continuous Dosing of Compound [I] formulated with ABT in
Combination With Docetaxel in a mouse PC3 Xenograft Model.
5
This figure shows the change in tumour volume in a mouse PC3 (a prostate
cancer cell
line) xenograft model over a 42-day period when treated with:
i. Compound [I] alone at a dose of 10 mg/Kg bid;
10 ii. Compound [I] alone at a dose of 30 mg/Kg bid;
iii. A taxane (docetaxel) alone;
iv. A combination of Compound [I] at a dose of 10 mg/Kg bid and a taxane
(docetaxel) together; and
v. A combination of Compound [I] at a dose of 30 mg/Kg bid and a taxane
15 (docetaxel) together.
Fig. 1 shows that use of the combination of Compound [I] and docetaxel appears
to
achieve additional tumour shrinkage when compared to the use of either
Compound [I] or
docetaxel alone.
FIG. 2 - Anti-tumour Activity of Compound [I] formulated in the Absence of ABT
When
Dosed Continually in Combination With Docetaxel in a mouse HCC70 Xenograft
Model.
This figure shows the change in tumour volume in a mouse HCC70 (a breast
cancer cell
line) xenograft model over a 21-day period when treated with:
i. Compound [I] alone at a dose of 25 mg/Kg bid;
ii. Compound [I] alone at a dose of 50 mg/Kg bid;
iii. A taxane (docetaxel) alone;
iv. A combination of Compound [I] at a dose of 25 mg/Kg bid and a taxane
(docetaxel) together; and
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
26
v. A combination of Compound [I] at a dose of 50 mg/Kg bid and a taxane
(docetaxel) together.
Fig. 2 again shows that use of the combination treatment appears to achieve
additional
tumour shrinkage over the use of either Compound [I] or docetaxel alone.
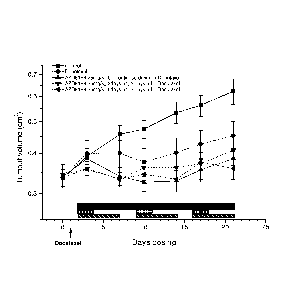
FIG. 3 - Anti-tumour Activity of Intermittent High Dose Compound [I]
formulated in the
absence of ABT when dosed in combination with Docetaxel in an HCC70 Xenograft
Model.
Fig. 3 shows the change in tumour volume in a mouse HCC70 (a breast cancer
cell line)
xenograft model over a 23-day period when treated with.
i. A taxane (docetaxel) alone;
ii. Compound [I] alone at a dose of 100 mg/Kg bid, wherein the Compound [I]
was
administered on 5 consecutive days of a 7 day period only, with no agent
administered on the remaining 2 days; and
iii. A combination of Compound [I] at a dose of 100 mg/Kg bid and docetaxel
together, wherein the Compound [I] was administered on 5 consecutive days of a
7
day period only, with no agent administered on the remaining 2 days.
Fig. 3 shows that even intermittent dosage of Compound [I] in combination with
a single
dose of docetaxel can result in sustained additional anti-tumour activity over
either
docetaxel or Compound [I] alone.
FIG. 4 - Anti-tumour Activity of Intermittent Low Dose Compound [I] formulated
in the
absence of ABT when dosed in combination with Docetaxel in an HCC70 Xenograft
Model.
This figure shows the change in tumour volume in a mouse HCC70 (a breast
cancer cell
line) xenograft model over a 21-day period when treated with:
CA 02900136 2015-08-04
WO 2014/135851
PCT/GB2014/050618
27
i. A taxane (docetaxel) alone;
ii. A combination of Compound [I] at a dose of 25 mg/Kg bid and a taxane
(docetaxel) together, continuously dosed;
iii. A combination of Compound [I] at a dose of 25 mg/Kg bid and docetaxel
together,
wherein the Compound [I] was administered on 5 consecutive days of a 7 day
period only, with no agent administered on the remaining 2 days.
iv. A combination of Compound [I] at a dose of 25 mg/Kg bid and docetaxel
together,
wherein the Compound [I] was administered on 2 consecutive days of a 7 day
period only, with no agent administered on the remaining 5 days.
Fig. 4 shows that even intermittent dosage of Compound [I] at a relatively low
dose in
combination with a single dose of docetaxel can result in sustained additional
anti-tumour
activity when compared to either docetaxel or Compound [I] alone.