Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
1
TOPICAL COMPOSITIONS COMPRISING BIMATOPROST AND METHODS
FOR STIMULATING HAIR GROWTH THEREWITH
RELATED APPLICATION
[0001] This present application claims priority to U.S. Provisional
Application
Serial No. 61/783,962, filed on March 14, 2013, and this present application
is
also a continuation-in-part of U.S. Application Serial No. 14/163,954, filed
January 24, 2014, which is a continuation of U.S. Application Serial No.
12/940,711, filed November 5, 2010, which claims priority to U.S. Provisional
Application Serial No. 61/259,368, filed on November 9, 2009, the disclosures
of which are hereby incorporated herein by reference.
FIELD OF THE INVENTION
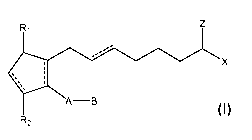
[0002] Disclosed herein are topical compositions and methods for stimulating
the growth of hair and treating disorders resulting in hair loss wherein said
compositions include a cyclopentane heptanoic acid, 2-cycloalkyl or arylalkyl
compound represented by the formula l:
z
R1
.-----
x
ss, :
\
A-B
R2
wherein the dashed bonds represent the presence or absence of a double
bond which can be in the cis or trans configuration and A, B, Z, X, R1 and R2
are as defined in the specification and a penetration enhancer. Such
compositions are used in stimulating hair growth of human or non-human
animals.
BACKGROUND OF THE INVENTION
[0003] Dermatologists recognize many different types of hair loss, the most
common being "alopecia" or "baldness" wherein humans (mostly males) begin
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
2
losing scalp hair at the temples and on the crown of their head. However, hair
loss may be due to many other disorders.
[0004] Hair loss is often accompanied by a change in the hair growth cycle.
All
mammalian hair passes through a life cycle that includes the anagen phase,
the catagen phase and the telogen phase. The anagen phase is the period of
active hair growth. In the scalp, this phase lasts from 3-5 years. The catagen
phase is a short 1-2 week transitional phase between the anagen phase and
the telogen phase. The final telogen phase is considered a "resting phase"
where all growth ceases. This phase is also relatively short-lived lasting
about 3
- 4 months before the hair is shed and a new one begins to grow. With the
onset of baldness, a successively greater proportion of hairs are in the
telogen
phase with correspondingly fewer in the active growth anagen phase.
[0005]Additionally, different types of hair exist including terminal hairs,
vellus
hairs and modified terminal hairs. Terminal hairs are coarse, pigmented, long
hairs in which the bulb of the hair follicle is seated deep in the dermis.
Vellus
hairs, on the other hand, are fine, thin, non-pigmented short hairs in which
the
hair bulb is located superficially in the dermis. Modified terminal hairs are
seen
in eye lashes and eye brows. As alopecia progresses, a transition takes place
wherein the hairs themselves change from the terminal to the vellus type.
Accordingly, alopecia (baldness) also includes a deficiency in terminal hairs.
[0006]One non-drug treatment for alopecia is hair transplantation. Plugs of
skin
containing hair are transplanted from areas of the scalp where hair is growing
to bald areas. This approach can be reasonably successful, however it is
costly, time-consuming and painful. Other non-drug related approaches to
treating alopecia include ultra-violet radiation, massage, psychiatric
treatment
and exercise therapy. None of these approaches, however, have been
generally accepted as effective. Even such things as revascularization surgery
or acupuncture have shown little, if any, effect.
SUMMARY OF THE INVENTION
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
3
[0007] Compositions and methods are disclosed herein for topical application
of
an effective amount of at least one penetration enhancer and cyclopentane
heptanoic acid, 2-cycloalkyl or arylalkyl compound represented by the formula
I:
R1 z
.--
x
\ :
\ .
A¨B
R2
wherein the dashed bonds represent the presence or absence of a double
bond which can be in the cis or trans configuration, A is an alkylene or
alkenylene radical having from two to six carbon atoms, which radical can be
interrupted by one or more oxo radicals and substituted with one or more
hydroxy, oxo, alkyloxy or akylcarboxy groups wherein the alkyl radical
comprises from one to six carbon atoms; B is a cycloalkyl radical having from
three to seven carbon atoms, or an aryl radical, selected from the group
consisting of hydrogen, a lower alkyl radical having from four to ten carbon
atoms wherein the heteroatom is selected from the group consisting of
nitrogen, oxygen and sulfur atoms; X is --N(R4)2 wherein R4 is selected from
the
group consisting of hydrogen, a lower alkyl radical having from one to six
carbon atoms,
0 0
11 11
R5¨C¨ and R5-0¨C¨
wherein R5 is a lower alkyl radical having from one to six carbon atoms; Z is
=0; one of R1 and R2 is =0, --OH or a --0(CO)R6 group, and the other one is --
OH or --0(CO)R6, or R1 is =0 and R2 is H, wherein R6 is a saturated or
unsaturated acyclic hydrocarbon group having from 1 to about 20 carbon
atoms, or
--(CH2)mR7 wherein m is 0 or an integer of from 1 to 10, and R7 is cycloalkyl
radical, having from three to seven carbon atoms, or a hydrocarbyl aryl or
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
4
heteroaryl radical, as defined above in free form or a pharmaceutically
acceptable salt thereof, in association with a penetration enhancer in
particular
formulations adapted for topical application to mammalian skin.
[0008] In one embodiment, the cyclopentane heptanoic acid, 2-cycloalkyl or
arylalkyl compound represented by the formula I is the compound bimatoprost.
[0009] Another embodiment includes a composition comprising bimatoprost at
a concentration of about 0.001 - 1.5% w/w, from 0.01 -1.0% w/w, from 0.02 -
1.0% w/w, 0.03 to about 1.0 % w/w, 0.03 to 0.9% w/w, 0.04 to 0.8% w/w, 0.05-
0.7% w/w, 0.06% - 0.6% w/w, 0.07% - 0.5% w/w, 0.08 - 0.4% w/w, 0.09 - 0.3%
w/w, 0.03% - 5% w/w, 0.3% - 3% w/w, 1% - 3% w/w, 0.1% w/w, 0.15%w/w,
0.2% w/w, 0.3% w/w, 0.4%w/w, 0.5% w/w, 0.6% w/w, 0.7% w/w, 0.8% w/w,
0.9%w/w, 1.0 %w/w, 1.5%w/w, 2%w/w, How/w, 3.5%w/w, 4%w/w, 5%w/w,
5.5%w/w, 6%w/w, 6.5%w/w, 7%w/w, 8%w/w, 9%w/w, and 10%w/w. The
preferred bimatoprost concentration range is about 2-4% w/w, more preferably
about 2.5-3.5% w/w. These preferred bimatoprost concentration ranges allow a
surprisingly good balance to be achieved between the wanted pharmacologic
effects of the composition and any unwanted side-effects. It had previously
been
thought that bimatoprost compositions for stimulating growth of hair should
have
a much lower bimatoprost concentration; this has now surprisingly been found
not to be the case.
[0010]The following excipients may also be included: Carbomer at a
concentration of about 0.05 - 1.0 % w/w; base at a concentration of about 0.01
to about 2.0 % w/w; ethanol at a concentration of about 10 to about 90 % w/w;
glycerin at a concentration of about 1.0 to about 20 % w/w; diethylene glycol
monoethyl ether at a concentration of about 1.0 to about 50 % w/w; polysorbate
20 at a concentration of about 0.1 to about 5.0 % w/w; polysorbate 40 at a
concentration of about 0.1 to about 5.0 % w/w; polysorbate 60 at a
concentration of about 0.1 to about 5.0 % w/w; polysorbate 80 at a
concentration of about 0.1 to about 5.0 % w/w; PPG-5 ceteth-20 at a
concentration of about 0.1 to about 5.0 % w/w; oleic acid at a concentration
of
about 0.1 to about 5.0 % w/w; isostearyl isostearate at a concentration of
about
0.1 to about 10 % w/w; isopropyl myristate at a concentration of about 0.1 to
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
about 10 % w/w; dipropylene glycol dimethyl ether at a concentration of about
1
to about 50 % w/w; diethylene glycol at a concentration of about 1 to about 50
%
w/w; dipropylene glycol at a concentration of about 1 to about 50 % w/w;
caprylic/capric at a concentration of about 0.1 to about 10 % w/w; benzyl
alcohol
at a concentration of about 0.1 to about 2.0 % w/w; silicone at a
concentration
of about 0.1 to about 10 % w/w; PEG 40 castor oil at a concentration of about
0.1 to 20%w/w; PEG 35 castor oil at a concentration of about 0.1 to 20%w/w;
()leyl alcohol at a concentration of about 0.1 to 10%w/w; glyceryl monooleate
at a
concentration of about 0.1 to 10%w/w; and/or water at a concentration of about
0 to about 90 % w/w.
[0011]Another embodiment includes a composition comprising bimatoprost at
about 0.1 % w/w; carbomer at about 0.10 % w/w; NaOH at about 0.035 % w/w;
ethanol at about 15.0 % w/w; diethylene glycol monoethyl ether at about 10.0 %
w/w; and water at about 74.8 % w/w.
[0012]Another embodiment includes a composition comprising bimatoprost at
about 0.1 % w/w; carbomer at about 0.15 % w/w; triethylamine (TEA) at about
0.22 % w/w; ethanol at about 15.0 % w/w; diethylene glycol monoethyl ether at
about 10.0 % w/w; polysorbate 20 at about 4.0 % w/w; and water at about 70.5 %
w/w.
[0013]Another embodiment includes a composition comprising bimatoprost at
about 0.1 % w/w; carbomer at about 0.125 % w/w; TEA at about 0.18 % w/w;
ethanol at about 30.0 % w/w; diethylene glycol monoethyl ether at about 20.0 %
w/w; and water at about 49.59 % w/w.
[0014]Another embodiment includes a composition comprising bimatoprost at
about 0.1 % w/w; carbomer at about 0.10 % w/w; TEA at about 0.15 % w/w;
ethanol at about 30.0 % w/w; propylene glycol at about 20 % w/w; and water at
about 49.7 % w/w.
[0015]Another embodiment includes a composition comprising bimatoprost at
about 0.1 % w/w; carbomer at about 0.20 % w/w; TEA at about 0.22 % w/w;
ethanol at about 60.0 % w/w; glycerin at about 5.0 % w/w; and water at about
34.48 % w/w.
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
6
[0016]Another embodiment includes a composition comprising bimatoprost at
about 0.1 % w/w; carbomer at about 0.25 % w/w; TEA at about 0.38 % w/w;
ethanol at about 60.0 % w/w; polysorbate 20 at about 4.0 % w/w; and water at
about 35.27 % w/w.
[0017]Another embodiment includes a composition comprising bimatoprost at
about 0.1 % w/w; carbomer at about 0.25 % w/w; TEA at about 0.38 % w/w;
ethanol at about 50.0 % w/w; diethylene glycol monoethyl ether at about 10 %
w/w; polysorbate 20 at about 4.0 % w/w; and water at about 35.27 % w/w.
[0018] In some embodiments, the composition comprises water, bimatoprost at
a concentration of about 1% w/w to about 4% w/w, preferably about 2-4% w/w
and most preferably 2.5-3.5% w/w, and one or more selected from the group
consisting of: cetostearyl alcohol at a concentration of about 0.5% w/w to
about
1% w/w, glyceryl mono-oleate at a concentration of about 1% w/w to about 3%
w/w, preferably about 2% w/w, ()leyl alcohol at a concentration of about 1%
w/w
to about 3% w/w, preferably about 2% w/w, ethanol at a concentration of about
30% w/w to about 75% w/w, propylene glycol at a concentration of about 10%
w/w to about 25% w/w, benzyl alcohol at a concentration of about 0.5% w/w to
about 2% w/w, preferably about 1% w/w, ultrez at a concentration of about
0.15% w/w, trolamine at a concentration of about 0.16% w/w, and glycerol at a
concentration of about 0.5% w/w to about 10% w/w, preferably 2% w/w.
[0019] In some embodiments, the composition comprises water, bimatoprost at
a concentration of about about 1-5% w/w, preferably about 2-4% w/w, more
preferably about 2.5-3.5% w/w, the most preferred value being 3% w/w, and
one or more selected from the group consisting of: transcutol at a
concentration
of about 1% w/w to about 25% w/w, preferably about 10% w/w, propylene
glycol at a concentration of about 1% w/w to about 25% w/w, glycerol
monooleate at a concentration of about 1% w/w to about 3% w/w, preferably
about 2% w/w, ()leyl alcohol at a concentration of about 1% w/w to about 3%
w/w, preferably about 2% w/w, ethanol at a concentration of about 30% w/w to
about 75% w/w, propylene glycol at a concentration of about 10% w/w to about
25% w/w, benzyl alcohol at a concentration of about 0.5% w/w to about 2%
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
7
w/w, preferably about 1% w/w, carbomer ultrez at a concentration of about
0.15% w/w to about 0.2% w/w, triethanolamine at a concentration of about
0.16% w/w, and glycerin at a concentration of about 0.5% w/w to about 10%
w/w, preferably 2% w/w.
[0020]Some embodiments may also comprise one or more additional
ingredients in addition to those specified in the paragraph above, wherein the
one or more ingredients are selected from the group consisting of linoleic
acid
at a concentration of about 1% w/w to about 5% w/w, preferably 2% w/w,
sodium lauryl sulfate at a concentration between 0.1% w/w to about 0.5% w/w,
preferably 0.2% w/w, and docusate sodium at a concentration between 0.1%
w/w to about 0.5% w/w, preferably 0.2% w/w.
[0021] In some embodiments, the composition comprises water, bimatoprost at
a concentration of about 1-5% w/w, preferably about 2-4% w/w, more
preferably about 2.5-3.5% w/w the most preferred value being 3% w/w, and
one or more selected from the group consisting of: transcutol at a
concentration
of about 1% w/w to about 25% w/w, preferably about 10% w/w, propylene
glycol at a concentration of about 1% w/w to about 25% w/w, glycerol
monooleate at a concentration of about 1% w/w to about 3% w/w, preferably
about 2% w/w, oleic acid at a concentration of about 1% w/w to about 3% w/w,
preferably about 2% w/w, linoleic acid at a concentration of about 1% w/w to
about 3% w/w, preferably about 2% w/w, ethanol at a concentration of about
30% w/w to about 75% w/w, propylene glycol at a concentration of about 10%
w/w to about 25% w/w, benzyl alcohol at a concentration of about 0.5% w/w to
about 2% w/w, preferably about 1% w/w, carbomer ultrez at a concentration of
about 0.15% w/w to about 0.2% w/w, triethanolamine at a concentration of
about 0.16% w/w, glycerin at a concentration of about 0.5% w/w to about 10%
w/w, preferably about 2% w/w, terpinolene at a concentration of about 0.5%
w/w to about 5% w/w, preferably about 2% w/w, limonene at a concentration of
about 0.5% w/w to about 5% w/w, preferably about 2% w/w, nerol at a
concentration of about 0.5% w/w to about 5% w/w, preferably about 2% w/w,
cineol at a concentration of about 0.5% w/w to about 5% w/w, preferably about
2% w/w, octyl salicylate at a concentration of about 0.5% w/w to about 5% w/w,
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
8
preferably about 2% w/w, DMSO at a concentration of about 0.5% w/w to about
5% w/w, preferably about 2% w/w, DDAB at a concentration of about 0.01%
w/w to about 1% w/w, preferably about 0.2% w/w, sodium taurodeoxycholate at
a concentration of about 0.01% w/w to about 5% w/w, preferably about 2%
w/w, docusate sodium at a concentration of about 0.01% w/w to about 1% w/w,
preferably about 0.2% w/w, Crodamol MM at a concentration of about 1% w/w
to about 30% w/w, preferably about 25% w/w, polysorbate 80 at a
concentration of about 1% w/w to about 5% w/w, preferably about 2% w/w.,
Dow ST-Elastomer 10 at a concentration of about 40% w/w to about 80% w/w,
preferably about 73.5% w/w, Dow Silky Wax 10 at a concentration of about 1%
w/w to about 20% w/w, preferably about 8% w/w, Isopropyl Myristate at a
concentration of about 1% w/w to about 20% w/w, preferably about 8% w/w.
[0022] In some embodiments, the composition comprises water; bimatoprost,
for example at a concentration from about 0.3% w/w to about 5% w/w,
preferably about 1-5% w/w or about 2-4% w/w, more preferably about 2.5-3.5%
w/w the most preferred value being 3% w/w; and one or more selected from the
following: ethanol, for example at a concentration between 0% w/w to about
89% w/w; propylene glycol, for example at a concentration between 0% w/w to
about 89% w/w; diethylene glycol monoethyl ether, for example at a
concentration between 0% w/w to about 89% w/w; benzyl alcohol, for example
at a concentration between 0% w/w to about 89% w/w; and one or more fatty
acids and/or fatty ester excipients, for example at a concentration between 0%
w/w to about 10% w/w. In some embodiments, the fatty acids may include one
or more C8-C28 fatty acids, and which may be saturated, monounsaturated, or
polyunsaturated. In some embodiments, a saturated fatty acid may be stearic
acid. In some embodiments, a monounsaturated fatty acid may be oleic acid.
In some embodiments, a polyunsaturated fatty acid may be linoleic acid. In
some embodiments, the fatty ester may one or more include C8-C28 fatty acids,
and which may be saturated, monounsaturated, or polyunsaturated. In some
embodiments, a saturated fatty ester may be glyceryl monostearate. In some
embodiments, a monounsaturated fatty ester may be glyceryl monooleate. In
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
9
some embodiments, a polyunsaturated fatty ester may be ethyl ester of linoleic
acid.
[0023]A preferred composition comprises bimatoprost, ()leyl alcohol, ethanol
and propylene glycol. Bimatoprost is comprised in an amount of about 1-5%
w/w, preferably about 2-4% w/w, more preferably about 2.5-3.5% w/w the most
preferred value being 3% w/w. ()leyl alcohol is comprised in an amount of
about
1-10% w/w. Ethanol is comprised in an amount of about 50-80% w/w.
Propylene glycol is comprised in an amount of 15-15% w/w.
[0024] Examples of particularly preferred compositions for growing hair by
topical application comprise bimatoprost in free form or a pharmaceutically
acceptable salt thereof, wherein the bimatoprost is contained in an amount of
about 0.3% w/w to about 4% w/w; at least one first compound selected from a
fatty acid, fatty acid alcohol and fatty ester, wherein said composition is
formulated for topical administration to the skin.
[0025] In some embodiments, the first compound is a fatty acid. The fatty acid
may be saturated or unsaturated. In some embodiments, the fatty acid is
selected from the group consisting of stearic acid, oleic acid, linoleic acid,
and
mixtures thereof. In some embodiments, the first compound is a fatty ester.
The fatty ester may be saturated or unsaturated. The fatty ester may be
selected from the group consisting of glyceryl monostearate, glyceryl
monooleate, and ethyl ester of linoleic acid. In some embodiments, the
composition comprises at least two first compounds. The composition may
comprise a mixture of at last one fatty acid and at least one fatty ester. The
first compound may have 12-24 carbon atoms. The composition may further
comprise at least one second compound selected from the group consisting of
ethanol, propylene glycol, diethylene glycol monoethyl ether, and benzyl
alcohol. The composition may further comprise at least one third compound
selected from the group consisting of terpenes, occlusive agents, surface
active
agents, sulfoxides, cyclic ethers, amides, amines, and dimethylaminopropionic
acid derivatives. In some embodiments, the terpene is selected from the group
consisting of terpinolene, limonene, nerol, and cineol. In some embodiments,
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
the occlusive agent is selected from the group consisting of silicones,
mineral
oils, and water insoluble polymers. In some embodiments, the surface active
agent is selected from the group consisting of polysorbate 20, polysorbate 40,
polysorbate 60, polysorbate 80, sodium dodecyl sulfate, sodium lauryl sulfate,
DMSO, and docusate sodium. In some embodiments, the
dimethylaminopropionic acid derivative is 2-dimethylaminopropionic acid
dodecyl ester. The composition may comprise bimatoprost in an amount of
about 1% w/w to about 4% w/w. More preferably, the composition may
comprise bimatoprost in an amount of about 2.5% w/w to about 3.5% w/w.
Most preferably, the composition may comprise bimatoprost in an amount of
about 3% w/w. In some embodiments, the composition is in the form of one
selected from the group consisting of solutions, gels, ointments, foams,
films,
liniments, creams, shampoos, lotions, pastes, jellies, sprays and aerosols. In
some embodiments, the composition is packaged in a kit with an applicator for
application to the skin.
[0026] In some preferred embodiments, a method for stimulating hair growth
comprises administering to the skin of a patient an effective amount of a
bimatoprost composition according to any embodiment previously described,
wherein the administration causes increased hair growth. The composition
may be applied to the scalp. The composition may be applied at least once
daily. In some embodiments, the composition is applied to the scalp for
treatment of a condition selected from the group consisting of alopecia
areata,
telogen effluvium, anagen effluvium, cicatricial alopecia, scarring alopecia;
hair
shaft abnormalities, trichorrexis nodosa, loose anagen syndrome,
trichotillomania, traction alopecia; infectious hair disorders, tiniea
capitis,
sebohorreic dermatitis, follicullitus of the scalp, and androgenetic alopecia.
In
some embodiments, wherein the composition is applied to one or both of the
scalp and the eyebrows for patients experiencing hair loss due to
chemotherapy, hormonal imbalance, fungal infection of the scalp, anti-
coagulants, medicine for gout, depression, high blood pressure and heart
disease.
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
11
[0027] In one embodiment, a composition for promoting the growth of hair
comprises: at least one penetration enhancer; and bimatoprost in free form or
a
pharmaceutically acceptable salt thereof, wherein the bimatoprost is contained
in an amount of about 1-4% w/w; wherein said composition is formulated for
topical administration to the skin. In some embodiments, the penetration
enhancer is selected from one or more of the group consisting of alcohols,
glycols, fatty acids, fatty esters, fatty ethers, occlusive agents, surface
active
agents, dimethylaminoproprionic acid derivatives, terpenes, sulfoxides, cyclic
ethers, amides, and amines.
[0028] It will of course be understood that the ranges described above, and
throughout this document, are also intended to encompass single values
contained within these ranges. For example, for a formulation comprising a
particular ingredient in a range between 1-50%, a percentage of 5% or 49% is
also intended to be disclosed.
[0029]The compositions were manufactured using the following general
procedure. Non-aqueous components (e.g. bimatoprost, ethanol, glycols) were
combined in a beaker and stirred using a propeller type overhead mixer until
the solution was clear. Water was added to the non-aqueous mixture followed
by the addition of the thickening agent. Upon dispersion of the thickening
agent, a base was added to neutralize the polymer and thicken the solution
into
a gel other desired composition. For example, ethanol and bimatoprost were
combined in a beaker and stirred using a propeller type overhead mixer until
the solution was clear. This mixture was then added to the non-aqueous
ingredients to form a non-aqueous mixture. In a separate vessel the thickening
agent was dispersed in water to form an aqueous mixture, which was then
added to the non-aqueous mixture. Upon mixing of the non-aqueous and
aqueous mixtures, a base was added to neutralize the polymer and to thicken
the solution into a gel.
[0030] In yet another embodiment, a composition for growing hair by topical
application comprises
at least one penetration enhancer comprising ()leyl alcohol; and
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
12
bimatoprost in free form or a pharmaceutically acceptable salt thereof;
wherein said composition is formulated for topical administration to the skin.
[0031] In some embodiments, the composition comprises from between about
0.3% to about 10% by weight of bimatoprost, preferably about 1-5% w/w or
about 2-4% w/w, more preferably about 2.5-3.5% w/w the most preferred value
being 3% w/w. In some embodiments, the composition comprises about 1% by
weight of bimatoprost. In some embodiments, the composition comprises
about 3% by weight of bimatoprost. The composition may comprise about 3%
by weight of bimatoprost, about 5% by weight of ()leyl alcohol, about 66% by
weight of ethanol, and about 22% by weight of propylene glycol. The
composition may be in the form of one selected from the group consisting of
solutions, gels, ointments, foams, films, liniments, creams, shampoos,
lotions,
pastes, jellies, sprays and aerosols. The composition may be packaged in a kit
with an applicator for application to the skin.
[0032] In another embodiment, a method for stimulating hair growth comprises
administering to the skin of a patient an effective amount of a bimatoprost
composition as described herein, wherein the administration causes increased
hair growth.
[0033] In some embodiments, the composition is applied to the scalp. In some
embodiments, the composition is applied at least once daily. The composition
may be applied to the scalp for treatment of conditions selected from the
group
consisting of alopecia areata, telogen effluvium, anagen effluvium,
cicatricial
alopecia, scarring alopecia; hair shaft abnormalities, trichorrexis nodosa,
loose
anagen syndrome, trichotillomania, traction alopecia; infectious hair
disorders,
tiniea capitis, sebohorreic dermatitis,
follicullitus of the scalp, and
androgenetic alopecia. The composition may be applied to one or both of the
scalp and the eyebrows for patients experiencing hair loss due to
chemotherapy, hormonal imbalance, fungal infection of the scalp, anti-
coagulants, medicine for gout, depression, high blood pressure and heart
disease.
CA 02900285 2015-08-04
WO 2014/158373 PCT/US2014/015430
13
[0034] In some embodiments, a suitable formulation for topical administration
of
bimatoprost may comprise one or more of the following ingredients listed in
the
table below:
Ingredient (%
Function Bimatoprost Bimatoprost
w/w) solution 1 solution 2
Bimatoprost Active 0.3-6% 0.3-6%
Oleyl Alcohol 0 0.1-10%
Ethanol Penetration 40-80% 40-80%
enhancer
Propylene
10-30% 10-30%
glycol
Thickener
Carbopol or
other thickeners Thickener 0-6% 0-6%
(Sepineo,
cellulose, etc)
Triethanolamine
or other Neutralizing
QSAD QSAD
neutralizer as Agent
appropriate
Purified water Vehicle QS 100% QS 100%
DETAILED DESCRIPTION
[0035] Bimatoprost is a moderately soluble compound intended for topical
delivery to the skin to stimulate hair growth. Hair growth includes, without
limitation, stimulating the conversion of vellus hair to growth as terminal
hair as
well as increasing the rate of growth of terminal hair. Embodiments disclosed
herein provide formulations of bimatoprost and similar compounds with
penetration enhancers. These penetration enhancers facilitate active
component penetration and/or maintenance at their site of action in the skin.
Formulations disclosed herein can be self-preserved or contain an
antimicrobial
agent such as benzyl alcohol.
[0036] In accordance with embodiments disclosed herein, active components
are represented by
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
14
R1 Z
---
---
X
1
1
\ 1
\ 1
A-B
R2
[0037]The active components are provided in particular formulations that
include penetration enhancers. Some examples of representative compounds
useful in the practice of embodiments disclosed herein include the compounds
shown in Table 1:
TABLE 1. Representative Compounds
cyclopentane heptenamide-5-cis-2-(3a-hydroxy-5-pheny1-1-trans-penteny1)-3,5-
dihydroxy, [1,2 ,3,S] cyclopentane N,N-dimethylheptenamide-5-cis-2-(3a-
hydroxy-5-pheny1-1-trans-penten- yI)-3,5-dihydroxy, [1 a,2R,3a,5a]
cyclopentane heptenylamide-5-cis-2-(3a-hydroxy-4-meta-chlorophenoxy-1-
trans-pent-eny1)-3,5-dihydroxy, [1a,2R,3a,5a1
cyclopentane heptenylamide-5-cis-2-(3a-hydroxy-4-trifluoromethylphenoxy-1-
trans-- pentenyI)-3,5-dihydroxy, [1a,2R,3a,5a1
cyclopentane N-isopropyl heptenamide-5-cis-2-(3a-hydroxy-5-pheny1-1-trans-
pentenyI)-3,5-dihy droxy, [1 ci,20a,5a]
cyclopentane N-ethyl heptenamide-5-cis-2-(3a-hydroxy-5-pheny1-1-trans-
penteny1)-3,5-dihydroxy, [1a,20a,5a]
cyclopentane N-methyl heptenamide-5-cis-2-(3a-hydroxy-5-pheny1-1-trans-
penteny1)-3,5-dihy droxy, [1a,20a,5ai
cyclopentane heptenamide-5-cis-2-(3a-hydroxy-4-meta-chlorophenoxy-1-trans-
buteny- 1)-3,5-dihydroxy, [1 a,2R,3a,5ai
CA 02900285 2015-08-04
WO 2014/158373 PCT/US2014/015430
[0038] In one embodiment, the compound is a cyclopentane heptanoic acid, 2-
(phenyl alkyl or phenyloxyalkyl) represented by the formula II:
z
R1
oõ
.--
x
\ :
\
,
..='...'. _.....)007
R2 R3 \ __
(C1-12)y(0)x<
/
wherein y is 0 or 1, x is 0 or 1 and x and y are not both 1, Y is selected
from the
group consisting of alkyl, halo, e.g. fluoro, chloro, etc., nitro, amino,
thiol,
hydroxy, alkyloxy, alkylcarboxy, halo substituted alkyl wherein said alkyl
radical
comprises from one to six carbon atoms, etc. and n is 0 or an integer of from
1
to 3 and R3 is =0, --OH or --0(CO)R6 wherein R6 is as defined above or a
pharmaceutically acceptable salt thereof.
[0039] In another embodiment, the compound is a compound of formula 111:
R1 z
x
\ :
= . Mil
\
..%-... _,... .........7
R2 R3 \ __
(C1-12)y(0)x<
/
wherein hatched lines indicate a configuration, solid triangles are used to
indicate 13 configuration. In another embodiment, y is 1 and x is 0 and R1, R2
and R3 are hydroxy.
[0040] One exemplary active compound is cyclopentane N-ethyl heptanamide-
5-cis-2-(3a-hydroxy-5-phenyl-1-trans-pentenyI)-3,5-aihy- droxy, [1 a,20a,5a1,
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
16
also known as bimatoprost and sold under the name of LUMIGAN by Allergan,
Inc., California, USA. This compound has the following structure:
OH
0
E
E
E
(5H =
(5H
[0041] The synthesis of the above compounds has been disclosed in U.S. Pat.
No. 5,607,978 which is incorporated by reference in its entirety.
[0042]The compound will generally range from about 1 x 10-7 to about 50%
w/w of the composition, in one embodiment from about 0.001 to about 50% w/w
of the composition and in another embodiment from about 0.1 to about 30%
w/w of the composition. In some embodiments, a preferred range of the active
compound may be about 0.03% w/w to about 5%, more preferably about 0.3%
w/w to about 3% w/w, and even more preferably, about 1% w/w to about 3%
w/w. Ranges and percentages within about 0.3% w/w, 0.5% w/w, 1% w/w,
1.5% w/w, 2% w/w, 3% w/w, 3.5% w/w, 4% w/w, 5% w/w, 5.5% w/w, 6% w/w,
6.5% w/w, 7% w/w, 8% w/w, 9% w/w, 10% w/w; 10-50% w/w; about 20-50%
w/w; about 30-40% w/w and about 35% are also included.
[0043]The pharmaceutical formulations disclosed herein can include one or
more penetration enhancers. The phrase "penetration enhancers" includes any
agent that facilitates the transfer or delivery of active components to their
site of
action and/or maintains the active component at their site of action. Non-
limiting
examples of classes of appropriate penetration enhancers include alcohols,
glycols, fatty acids, ethers, esters, occlusive agents and surface active
agents.
Representative and non-limiting examples of these classes are provided below.
It will of course be understood that one or more penetration enhancers or
classes thereof may be combined in the various embodiments disclosed herein.
[0044]Alcohols include, without limitation, aliphatic and aromatic alcohols,
including ethanol, propanol, n-propanol, isopropanol, butyl alcohol, octanol,
benzyl alcohol and acetyl alcohol, in one embodiment, as described in U.S.
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
17
Pat. No. 5,789,244, the entire contents of which are incorporated by reference
herein. Fatty alcohols include, for example, saturated and unsaturated fatty
alcohols, including for example those with C8-C28 chain length, stearyl
alcohol,
()leyl alcohol, palmityl alcohol, and lauryl alcohol, and combinations
thereof. In
some embodiments, ()leyl alcohol may be used in a range between about 0.5%
w/w to about 50% w/w, preferably between about 1% w/w and about 10% w/w,
and even more preferably between about 3% w/w and about 6% w/w.
Percentages of 1% w/w, 1.5% w/w, 2% w/w, 3% w/w, 3.5% w/w, 4% w/w, 5%
w/w, 5.5% w/w, 6% w/w, 7% w/w, 8% w/w, 9% w/w, and 10% w/w are also
contemplated. Most preferably, ()leyl alcohol may be present at about 5% w/w.
[0045]Glycols include, without limitation, propylene glycol, polyethylene
glycols
(including for example polyethylene glycols with a molecular weight from about
300-8000 Da!tons), glycol derivatives, and other low molecular weight glycols
such as glycerol and thioglycerol.
[0046] Fatty acids, esters and ethers include, without limitation, saturated,
monounsaturated, and polyunsaturated C8-C28 fatty acids and fatty esters, such
as C4-C20 saturated monocarboxylic and dicarboxylic acids, straight chain
fatty
acids, stearic acid, oleic acid, linoleic acid, palmitoleic acid, octanoic and
decanoic acids, methyl laurate, ethyl oleate, polyethylene glycol monolaurate,
propylene glycol monolaurate, propylene glycerol dilaurate, glycerol
monolaurate, glyceryl monooleate, glyceryl monostearate, ethyl esters of
linoleic acid, isopropyl n-decanoate, octyldodecyl myristate, diethylene
glycol
monoethyl ether, diethylene glycol monomethyl ether, Crodamol MM, Isopropyl
myristate, and compounds wherein a C2_C4 alkane diol or triol is substituted
with one or two fatty ether substituents.
[0047] Occlusive agents include, without limitation, silicones (including Dow
ST-
Elastomer 10, Dow Silky Wax 10), mineral oils and greases, long chain acids,
animal fats and greases, vegetable fats and greases, water insoluble polymers,
paraffin, paraffin oil, liquid paraffin, petrolatum, liquid petrolatum, white
petrolatum, yellow petrolatum, microcrystalline wax and ceresin.
CA 02900285 2015-08-04
WO 2014/158373 PCT/US2014/015430
18
[0048]Surface active agents include without limitation nonionic, anionic, and
cationic agents, and combinations thereof, such as polysorbate 20, 40, 60 and
80,
TWEEN (20, 40, 60, 80) and optionally corresponding SPAN Series (20, 40,
60, 80), POLOXAMER (231, 182, 184), sodium dodecyl sulfate (SDS),
macrogol 15 hydroxystearate, polyvinyl caprolactam - polyvinyl acetate -
polyethylene glycol graft co-polymer. lecithin,
lysolecith in,
nonylphenoxypolyoxyethylene, lysophosphatidylcholine, polyethylenglycol 400,
polyoxyethylene ethers, polyglycol ether surfactants, sodium laurate, sodium
lauryl sulfate, cetyltrimethylammonium bromide, docusate sodium, and
benzalkonium chloride.
[0049] Other penetration enhancers that may be useful include
dimethylaminoproprionic acid derivatives, such as 2-dimethylaminopropionic
acid dodecyl ester (DDAIP); terpenes, including terpinolene, limonene, nerol,
cineol; sulfoxides such as DMSO; cyclic ethers; amides and amines, such as
Didecyldimethylammonium bromide (DDAB), sodium taurodeoxycholate,
triethylamine; octyl salicylate, and combinations thereof.
[0050]Additional penetration enhancers will be known to those of ordinary
skill
in the art of topical drug delivery, and/or are described in the pertinent
texts and
literature.
[0051]Embodiments disclosed herein can also include viscosity increasing
agents. Appropriate agents include, without limitation, methylcellulose, ethyl
cellulose, hydroxyethyl cellulose, acrylamide/sodium acryloyldimethyltaurate
copolymer, polyacrylic acid, polyvinyl alcohol, polyvinyl pyrrolidone,
hyaluronic
acid and chondroitin sulfate.
[0052]Certain embodiments disclosed herein can include preservatives
including, without limitation, phenoxyethanol, benzyl alcohol, benzalkonium
chloride, chlorhexidine, chlorobutanol, methyl-, propyl-, or butyl-
parahydroxybenzoic acids, phenylmercuric salts including, without limitation,
nitrate, chloride, acetate, and borate and betain.
[0053]Various other additives may be included in the compositions of the
present invention in addition to those identified above. These include, but
are
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
19
not limited to, antioxidants, astringents, perfumes, emollients, pigments,
dyes,
humectants, propellants, and sunscreen agents, as well as other classes of
materials whose presence may be cosmetically, medicinally or otherwise
desirable. The compositions and formulations may also be taken in conjunction
with minoxidil and propecia.
[0054]Compositions can also be formulated as "slow-releasing" formulations so
that the activity of active components is sustained for a longer period of
time
between treatments.
[0055]While particular embodiments disclosed herein can include each of the
components discussed above, other particular embodiments can be required to
be "substantially free" of one or more of these components in various
combinations. "Substantially free", as used herein, means that the component
is not added to a formulation and cannot be present in any amount greater than
about 1% w/w.
[0056]While not limiting the scope of express exclusion of the preceding
paragraph, particular embodiments disclosed herein can be substantially free
of
one or more of bimatoprost, carbomer, NaOH, TEA, ethanol, glycerin,
diethylene glycol, monoethyl ether, propylene glycol, polysorbate 20,
polysorbate 40, polysorbate 60, polysorbate 80, PPG-5 ceteth-20, oleic acid,
isostearyl isostearate, isopropyl myristate, dipropylene glycol dimethyl
ether,
diethylene glycol, dipropylene glycol, triglycerides, caprylic/capric, benzyl
alcohol,
silicone and water.
[0057]All components of formulations described herein will be included in
amounts that are dermatologically-acceptable. As used herein,
"dermatologically-acceptable" means that the compositions or components
thereof are suitable for use in contact with human skin without undue
toxicity,
incompatibility, instability, allergic response, and the like. As used in
herein as
applied to active agents and excipients, the term "about" refers to variations
in
concentrations which are considered to be bioequivalent.
[0058] Embodiments disclosed herein find application in mammalian species,
including both humans and animals. In humans, the compounds of
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
embodiments disclosed herein can be applied without limitation, to the scalp,
face, beard, head, pubic area, upper lip, eyebrows, and eyelids. The
compositions of the present inventions may be used for treating various hair
loss disorders including but not limited to alopecia areata, telogen
effluvium,
anagen effluvium, cicatricial alopecia and scarring alopecia; hair shaft
abnormalities such as trichorrexis nodosa, loose anagen syndrome,
trichotillomania and traction alopecia; infectious hair disorders such as
tiniea
capitis, sebohorreic dermatitis, and follicullitus of the scalp; genetic
disorders
such as androgenetic alopecia and patients undergoing hair loss due to
chemotherapy, hormonal imbalance (e.g., thyroid conditions such as
hypothyroidism and hyperthyroidism, pregnancy, child birth, discontinuation of
birth control pills and changes in menstrual cycle), fungal infection of the
scalp
such as ringworm, medicines which cause hair loss such as anti-coagulants,
medicine for gout, depression, high blood pressure and certain heart
medications. The formulations of the present invention may be used to treat
hair loss related to other disease such as diabetes, lupus, and poor
nutrition,
mental and physical stress such as due to surgery, illness and high fever.
Environmental factors and chemicals used in hair treatment (dying, tinting and
bleaching).
[0059] In animals raised for their pelts, e.g., mink, the formulations can be
applied over the entire surface of the body to improve the overall pelt for
commercial reasons. The process can also be used for cosmetic reasons in
animals, e.g., applied to the skin of dogs and cats having bald patches due to
mange or other diseases causing a degree of alopecia.
[0060]The compositions and methods of the present invention may be applied
to patients suffering from hair loss or in healthy patients simply wanting to
increase hair growth in any part of the body.
[0061]The compositions disclosed herein are formulated for topical
administration. The term "topical administration" as used herein includes
applying a formulation as described herein to the outer skin or hair. The
application will generally occur at or near the area of desired hair growth.
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
21
[0062]Accordingly, appropriate formulation or composition types include,
without limitation, solutions, gels, ointments, foams, films, liniments,
creams,
shampoos, lotions, pastes, jellies, sprays and aerosols. Such formulation
types
can be applied in swaths, patches, applicators or through the use of
impregnated dressings depending on the situation and part of the body to be
treated.
[0063]Typically, the formulations described herein will be applied repeatedly
for
a sustained period of time to the part of the body to be treated. In
particular
embodiments, formulations disclosed herein can include one or more
applications daily, one or more applications weekly, one or more applications
monthly or one or more applications yearly for a period of treatment of at
least
one day, at least one week, at least one month, at least one year or until the
treatment has achieved or achieved and maintained a desired result.
[0064] Formulations described herein will be administered in safe and
effective
amounts. As used herein, "safe and effective amounts" include an amount
sufficient so that the composition provides the desired hair growth
stimulation
effect at a reasonable benefit/risk ratio attendant with any medical
treatment.
Within the scope of sound medical judgment, the amount of active components
used can vary with the particular condition being treated, the severity of the
condition, the cause of the condition, the duration of the treatment, the
specific
active component employed, its concentration, the specific vehicle utilized,
the
general health of the patient, the tolerance of the patient to various effects
of
the administration, other drugs being administered to the patient, and like
factors within the specific knowledge and expertise of the patient or
attending
physician.
[0065] For daily administration, an appropriate dose can include, without
limitation, about 0.1 ng to about 100 mg, about 1 ng to about 10 mg per day or
in another embodiment about 10 ng to about 1 mg per day.
[0066] Non-limiting examples of some components with their appropriate
concentration ranges and function are provided in Tables 1A and B below.
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
22
Particular examples of non-limiting formulations or compositions are provided
in
Table 2.
Table 1A: Example Components with Function and Concentration Ranges
Ingredient Function Composition (% w/w)
bimatoprost Active 0.03 - 1.0
carbomer Thickener 0.05 - 1.0
base Neutralizing Agent 0.01 - 2.0
ethanol 10 - 90
glycerin 1.0 - 20
diethylene glycol monoethyl 1.0 - 50
ether
propylene glycol 1- 50
polysorbate 20 0.1 - 5.0
polysorbate 40 0.1 - 5.0
polysorbate 60 0.1- 5.0
polysorbate 80
Penetration enhancers a
PPG-5 ceteth-20 0.1- 5.0
oleic acid 0.1 - 5.0
isostearyl isostearate 0.1 - 10
isopropyl myristate 0.1 - 10
dipropylene glycol dimethyl 1-50
ether
diethylene glycol 1-50
dipropylene glycol 1-50
caprylic/capric triglycerides 0.1-10
benzyl alcohol Preservative 0.1 - 2.0
silicone Occlusive Agent 0.1 - 10
water Vehicle 0 - 90
Table 1B: Example Components with Function and Concentration Ranges
Ingredient Function Composition (% w/w)
bimatoprost Active 0.03 - 1.0
carbomer Thickener 0.05 - 1.0
base Neutralizing Agent 0.01 - 2.0
ethanol 10 - 90
glycerin 1.0 - 20
diethylene glycol monoethyl 1.0 - 50
ether
propylene glycol Penetration enhancers 1- 50
polysorbate 20 0.1 - 5.0
polysorbate 40 0.1 - 5.0
polysorbate 60 0.1- 5.0
polysorbate 80 0.1 - 5.0
CA 02900285 2015-08-04
WO 2014/158373 PCT/US2014/015430
23
PPG-5 ceteth-20 0.1- 5.0
oleic acid 0.1 - 5.0
isostearyl isostearate 0.1 - 10
isopropyl myristate 0.1 - 10
dipropylene glycol dimethyl 1-50
ether
diethylene glycol 1-50
dipropylene glycol 1-50
caprylic/capric triglycerides 0.1-10
()ley! alcohol 0.1-10
benzyl alcohol Preservative 0.1 - 2.0
silicone Occlusive Agent 0.1 - 10
water Vehicle 0 - 90
Table 2: Example Compositions
Ingredient Function Composition (% w/w)
bimatoprost Active 0.1
0.1 0.1 0.1 0.1 0.1 0.1
carbomer Thickener 0.10 0.15 0.125 0.10 0.20 0.25 0.25
NaOH (s) Neutralizing 0.035
Agent
TEA Neutralizing 0.22 0.18 0.15 0.22 0.38 0.38
Agent
ethanol 15.0 15.0 30.0 30.0 60.0 60.0 50.0
glycerin 5.0
diethylene glycol . 10.0 10.0 20.0 10
monoethyl ether Penetration
enhancers
propylene glycol 20
polysorbate 20 4.0 4.0 4.0
water Vehicle 74.8 70.5 49.595 49.7 34.48 35.27 35.27
EXAMPLE 1: Preparations of Bimatoprost Scalp Hair Growth Gel
Compositions
[0067] Ethyl alcohol is weighed into a suitable media jar equipped for mixing,
bimatoprost is then added to the ethyl alcohol and stirred at moderate speed
until bimatoprost is dissolved. Into separate mixing tank, glycerin,
diethylene
glycol monoethyl ether, and propylene glycol are added and mixed until the
solvents are dispersed. Ethyl alcohol/bimatoprost solution is then added into
the non-aqueous solution and mixed until the components are homogenously
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
24
mixed (about 5 minutes of mixing). To the above mixture the carbomer
thickener previously dispersed in water is added and mixed until well
dispersed,
once dispersed a base is added to thicken the solution into a gel.
Representative formulations made according to the method above are shown in
Table 3 below.
Table 3. Bimatoprost Scalp Hair Growth Topical Gel Formulations
Bimatopros Bimatoprost Bimatoprost Bimatoprost
t 0.03% 0.1% 0.3% 0.2%
Ingredient (% (Propylene (Propylene (Propylene (Propylene
w/w) Glycol) Glycol) Glycol) Glycol)
Solution Solution Solution Solution
Bimatoprost 0.03 0.1 0.3 0.2
Propylene glycol 10.0 10.0 10.0 10.0
Diethylene
glycol 10.0 10.0 10.0 10.0
monoethyl ether
Ethyl alcohol 30.0 30.0 30.0 30.0
Glycerin 2.0 2.0 2.0 2.0
Carbomer
0.15 0.15 0.15 0.15
(Ultrez 10)
Triethanolamine 0.16 0.16 0.16 0.16
Purified water 47.66 47.59 47.39 47.49
EXAMPLE 2: In Vivo Treatment
[0068]A study is initiated to systematically evaluate the appearance of hair
on
the scalp and eyebrows who are administered bimatoprost gel formulations as
in Table 3. The study involves 10 subjects, 5 male, 5 female, average age 70
years, (ranging from 50-94 years). Each subject is treated daily by the
topical
application of bimatoprost by the 0.3% w/w bimatoprost formulation of Table 3.
[0069]The study is limited to subjects who have administered bimatoprost for
more than 3 months. The mean duration of exposure to the 0.3% w/w
bimatoprost gel formulation prior to assessing the parameter of hair or
eyebrow
growth between the control and study eye is 129 days (range 90-254 days).
Observations are made under high magnification at a slit lamp biomicroscope.
Documentation of differences between the control and treatment areas is
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
accomplished using a camera specially adapted for use with a slit lamp
biomicroscope.
[0070]The Results of the Observations Will Be as Follows:
[0071] Length of hair and eyebrows: Increased length of hair in both groups is
regularly observed. The difference in length varies from approximately 10% to
as much as 30%.
[0072] Number of hairs and eyebrows: Increased numbers of hairs are
observed on the scalp and eyebrows of each patient. The difference in number
of hair and eyebrows varies from approximately 5% to as much as 30%.
Whether statistically significant or not, bimatoprost with a penetration
enhancer
will provide better and/or faster results than bimatoprost without a
penetration
enhancer.
[0073]The foregoing observations will establish that 0.03% w/w bimatoprost
composition penetrates skin and grows hair.
EXAMPLE 3: Topical Cream.
[0074]A topical 0.2% w/w bimatoprost cream is prepared as follows: Tegacid
and spermaceti are melted together at a temperature of 70-80 C.
Methylparaben is dissolved in about 500 gm of water and propylene glycol,
polysorbate 80, bimatoprost and a penetration enhancer are added in turn,
maintaining a temperature of 75-80 C. The methylparaben mixture is added
slowly to the Tegacid and spermaceti melt, with constant stirring. The
addition
is continued for at least 30 minutes with additional stirring until the
temperature
has dropped to 40-45 C. Finally, sufficient water is added to bring the final
weight to 1000 gm and the preparation stirred to maintain homogeneity until
cooled and congealed.
EXAMPLE 4: Topical Cream.
[0075]A 0.1% w/w bimatoprost topical cream is prepared as follows: Tegacid
and spermaceti are melted together at a temperature of 70-80 C.
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
26
Methylparaben is dissolved in water and propylene glycol, polysorbate 80,
bimatoprost and a penetration enhancer are added in turn, maintaining a
temperature of 75-80 C. The methylparaben mixture is added slowly to the
Tegacid and spermaceti melt, with constant stirring. The addition is continued
for at least 30 minutes with additional stirring until the temperature has
dropped
to 40-45 C. Finally, sufficient water is added to bring the final weight to
1000
gm and the preparation stirred to maintain homogeneity until cooled and
congealed.
EXAMPLE 5: Topical Ointment.
[0076]An Ointment Containing 2.0% w/w Bimatoprost is Prepared as Follows:
[0077]White petrolatum and wool fat are melted, strained and liquid petrolatum
is added thereto. Bimatoprost, a penetration enhancer, zinc oxide, and
calamine are added to the remaining liquid petrolatum and the mixture milled
until the powders are finely divided and uniformly dispersed. The mixture is
stirred into the white petrolatum, melted and cooled with stirring until the
ointment congeals. In other variants, the zinc oxide and/or calamine can be
omitted such that the formulation is substantially free of the zinc oxide or
calamine.
EXAMPLE 6: Ointment.
[0078]An ointment containing 5% w/w bimatoprost and a penetration enhancer
is prepared by adding the active compound to light liquid petrolatum. White
petrolatum is melted together with wool fat, strained, and the temperature
adjusted to 45-50 C. The liquid petrolatum slurry is added and the ointment
stirred until congealed. The ointment can be packaged in 30 gm tubes.
EXAMPLE 7: Spray Formulation.
[0079]An aqueous spray formulation containing 0.03%, w/w bimatoprost and a
penetration enhancer are prepared as follows. Bimatoprost and a penetration
enhancer are dissolved in water and the resulting solution is sterilized by
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
27
filtration. The solution is aseptically filled into sterile containers with a
spray
nozzle for application on top of the head. The formulation is presented in
Table
4A below. An alternative formulation is also listed in Table 4B.
Table 4A. Bimatoprost Spray Formulation of Example 7
Ingredient (% w/w) Spray formulation (w/w%)
Bimatoprost 0.03
Propylene glycol 5
Diethylene glycol monoethyl ether 5
Ethyl alcohol 15
Light mineral oil
Ceteareth 12
Glycerin 1
Carbomer (Ultrez 10)
Triethanolamine
Purified water 24
Hydrofluoro carbon, hydrocarbon
49.97
propellant, CO2, or, Nitrogen
Table 4B. Alternative bimatoprost Spray Formulation
Ingredient (% w/w) Spray formulation (w/w%)
Bimatoprost 0.03
Propylene glycol 5
Diethylene glycol monoethyl ether 5
Ethyl alcohol 15
Glycerin 1
PVP or Cellulose 0.1-1%
Purified water 24
Hydrofluoro carbon, hydrocarbon
49.97
propellant, CO2, or, Nitrogen
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
28
EXAMPLE 8: Lotion.
[0080]A sample of bimatoprost and a penetration enhancer is dissolved in the
vehicle of N-methyl pyrrolidone and propylene glycol to make a 0.5% w/w
bimatoprost lotion for application to the scalp or other parts of the body for
growing hair.
EXAMPLE 9: Aerosol
[0081]An aerosol containing approximately 0.1% w/w bimatoprost and a
penetration enhancer is prepared by dissolving the bimatoprost and a
penetration enhancer in absolute alcohol. The resulting solution is filtered
to
remove particles and lint. This solution is chilled to about -30 C. A chilled
mixture of dichlorodifluoromethane and dichlorotetrafluoroethane is then added
to the solution. Thirteen ml plastic-coated amber bottles can be cold filled
with
11.5 gm each of the resulting solution and capped. The aerosol may be
sprayed onto the scalp or other parts of the body to grow hair.
EXAMPLE 10: Topical Foam Formulation
[0082]A 0.1% w/w bimatoprost topical foam formulation is prepared as follows:
Methylparaben is dissolved in about 500 gm of water and propylene glycol,
polysorbate 80, bimatoprost and a penetration enhancer are added in turn,
maintaining a temperature of 75-80 C. The methylparaben mixture is added
slowly to Tegacid and spermaceti, with constant stirring. The addition is
continued for at least 30 minutes with additional stirring until the
temperature
has dropped to 40-45 C. Finally, sufficient water is added to bring the final
weight to 1000 gm and the preparation stirred to maintain homogeneity until
cooled and congealed.
[0083] Alternative foam formulations prepared in a similar manner as taught in
Example 10 are described below in Tables 5A-B.
Table 5A:
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
29
Ingredient (% w/w) Foam formulation (w/w%)
Bimatoprost 0.03
Propylene glycol
Diethylene glycol monoethyl ether 5
Ethyl alcohol 10
Light mineral oil 6
Ceteareth 12 5
Glycerin
Carbomer (Ultrez 10)
Table 5B:
Ingredient (% w/w) Foam formulation (w/w%)
Bimatoprost 0.03
Propylene glycol
Diethylene glycol monoethyl ether 5
Ethyl alcohol 10
Light mineral oil 6
Myrj 45 5
Glycerin
EXAMPLE 11: Dusting Powder
[0084]A powder of the compound bimatoprost and a penetration enhancer is
prepared by mixing in dry form with talcum powder at a weight/weight ratio of
1:1:10.
EXAMPLE 12: Related Compounds
[0085] Following the procedures of the preceding Examples, compositions are
similarly prepared substituting an equimolar amount of a compound of Table 1A
for the bimatoprost disclosed in the preceding Examples.
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
[0086] Unless otherwise indicated, all numbers expressing quantities of
ingredients, properties such as molecular weight, reaction conditions, etc.
used
in the specification and claims are to be understood as being modified in all
instances by the term "about." "About" refers to variations in concentrations
of
excipients and types of excipients which are considered to be bioequivalent
according to the FDA and other regulatory authorities.
Example 13
[0087]A 44 year old Caucasian male undergoing hair loss due to alopecia
areata applies once daily before sleeping the 0.1% w/w bimatoprost
composition of Table 3 for a period of 6 months. After 3 months of
application,
the subject will notice new hair growth where there previously had been none
and darkening of the follicles of old hair. Observations of new hair growth
are
made under high magnification at the slit lamp biomicroscope and by computer
assisted image analysis. Documentation of differences between the control and
treatment areas is accomplished using a camera specially adapted for use with
the slit lamp biomicroscope.
Example 14
[0088]A 37 year old Hispanic male suffering from male pattern baldness due to
androgenetic alopecia applies the 0.2% w/w bimatoprost composition of Table
3 twice daily in areas where hair is noticeably thinning. After 63 days of
application, increased growth of hair will be noticed as will be new hair
growth
as measured by high magnification at the slit lamp biomicroscope and by
computer assisted image analysis. After satisfactory levels of hair growth are
observed, the patient applies the 0.2% w/w bimatoprost composition only twice
a week.
Example 15
[0089]A 29 year old Caucasian healthy female wishes to have fuller hair and
more hair growth even though no disease or hair loss condition has been
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
31
diagnosed by doctors. The patient will apply the 0.3% w/w bimatoprost
composition of Table 3 once daily until more hair growth is observed after
approximately three months of use. The patient continues to apply the
composition once a week to maintain the increased hair growth.
Example 16
[0090]A 35 year old African American male diagnosed with follicular
degeneration syndrome and associated hair loss will apply the 0.03% w/w
bimatoprost composition of Table 3. The composition will be applied twice
daily,
once in the morning after showering and once in the evening. After 46 days of
application, increased hair growth will be noticed and easing of the symptoms
of follicular degeneration syndrome. The patient continues application for
another 6 months.
[0091]Further examples of formulations containing bimatoprost are also
possible, and may be used to further stimulate hair growth. In some
embodiments, some formulations may be particularly beneficial to stimulate
hair growth in the scalp.
EXAMPLE 17: bimatoprost solution formulation with ley! alcohol
[0092] A formulation of bimatoprost solution containing 0.3% w/w bimatoprost
and a penetration enhancer comprising ()leyl alcohol may be prepared as
follows. The ingredients of Table 7 below are weighed and dispensed into a
suitable media jar equipped for mixing. Preferably, bimatoprost is dissolved
into the ethanol, and then combined with ()leyl alcohol, propylene glycol and
water. The various components are mixed together until homogenously mixed.
It will be understood that the formulation provided in Table 7 is non limiting
and
that other formulations are of course possible and envisioned. The bimatoprost
solution below had a ratio of bimatoprost to ()leyl alcohol of 0.06, a ratio
of
bimatoprost to ethanol of 0.005, and a ratio of ()leyl alcohol to ethanol of
0.083.
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
32
Table 7. bimatoprost solution formulation with oleyl alcohol
Ingredient (% Bimatoprost
w/w) solution
Bimatoprost 0.3
Oleyl Alcohol 5
Ethanol 60
Propylene glycol 20
Purified water 14.97
EXAMPLE 18: bimatoprost solution formulation without oleyl alcohol
[0093]Of course, while the example above comprises oleyl alcohol, which is
believed to act as a penetration enhancer, some compositions may
nevertheless not comprise this compound. Table 8 below illustrates additional
non-limiting examples of such formulations. The bimatoprost solutions 8A and
8B below had a ratio of bimatoprost to ethanol of 0.005.
Table 8. bimatoprost solution formulation without oleyl alcohol
Bimatopros
Ingredient (% Bimatoprost
t solution
w/w) solution 8A
8B
Bimatoprost 0.3 0.3
Ethanol 60 60
Propylene glycol 20 0
Transcuto10 0 20
Benzyl Alcohol 0 2.5
Purified water 19.97 17.47
In the preceding and throughout, Transcuto10 refers to a commercial product
sold by Gattefosse, and which comprises diethylene glycol monoethyl ether.
Example 19: bimatoprost gel formulation without oleyl alcohol
CA 02900285 2015-08-04
WO 2014/158373 PCT/US2014/015430
33
[0094] In addition to the gel formulation provided in Example 1 above, a gel
formulation comprising 0.3% w/w bimatoprost may also be manufactured using
the ingredients listed in Table 9 below.
[0095]The ingredients in Table 9 are formulated into a gel according to the
following procedure. First, ascorbic acid and EDTA are dissolved in a portion
of the total water. Then, carbopol 974P is added to this solution to disperse
and wet the carbopol. Next, poloxamer 407 is added to another portion of the
total water in a separate container and mixed to disperse. The carbopol
portion
is then added to this part and mixed. Polysorbate 80, hexylene glycol and PEG
400 are next combined in another container and mixed until homogeneous.
BHA, BHT and bimatoprost are weighed into another container, followed by the
addition of benzyl alcohol. The ingredients are mixed together until
homogeneous. Subsequently, this part is added to the Polysorbate 80 part and
mixed. All parts are mixed together, followed by mixing in the remaining water
and tromethamine (which have been previously mixed together) so as to
neutralize the gel
Table 9: bimatoprost gel formulation
Bimatoprost
Ingredient (%
Gel
w/w)
Bimatoprost 0.3
Benzyl alcohol 1
Tromethamine 0.8
Hexylene Glycol 2
PEG 400 45
Carbomer (Ultrez
1.25
10)
Poloxamer 407 0.2
Polysorbate 40 0.2
Ascorbic Acid 0.5
BHT 0.5
BHA 0.5
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
34
EDTA 0.5
Purified water 47.66
Example 20: In Vitro testing
[0096]The formulations described in Tables 7, 8, and 9 above were tested with
an in vitro system comprising a 1.0 cm2 Franz Cell diffusion chamber. During
this testing, the Franz Cell comprises a sample of dermatomized ex-vivo
human cadaver posterior trunk skin overlaying a diffusion cell filled with
receptor solution fluid configured to simulate body fluid.
[0097]The tested formulations were applied to the skin samples overlaying the
diffusion cell. Two donor cadavers were used, the first from a 43-year old
black
male, and the second from a 59-year old white male. Testing was performed in
triplicate for each donor and formulation tested. Ten pl of tested solution
was
applied per square centimeter of skin. At 2, 4, 7, 24, and 48 hour intervals,
receptor solution fluid was collected at each time point, and the amount of
bimatoprost was quantified. To obtain the cumulative receptor solution
concentration, the amount of bimatoprost calculated at each time interval
above
were added together. Analysis and bimatoprost quantification for the skin
stratum corneum and dermis was performed at the 48 hour endpoint.
Table 10. Summary of in vitro skin penetration results
Stratum Corneum Cumulative Receptor
and Epidermis Conc. Dermis Conc. at 48 Solution Fluid
Formulation
at 48 hrs. (ng hrs. (ng bimatoprost) Concentration at
48
bimatoprost) hrs. (ng. bimatoprost)
Bimatoprost 0.3% 700 18.8 6.95
(Propylene Glycol)
Solution, Table 3
Bimatoprost solution, 1600 164 2390
Table 7
Bimatoprost solution 410 41.3 28.9
8A, Table 8
Bimatoprost solution 200 51.4 30.2
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
8B, Table 8
Bimatoprost gel, Table 64 14.2 BLQ
9
[0098]As shown in Table 10 above, the 0.3% bimatoprost formulation
described in Table 3 was compared to the new formulations of Tables 7, 8, and
9. The new formulations (all containing 0.3% bimatoprost) all exhibited
greater
cumulative amounts of bimatoprost permeating through the skin sample and
into the receptor solution, with the exception of the gel formulation which
exhibited minimal skin penetration. Unexpectedly, the formulation from table 7
that contained ()leyl alcohol as a skin penetrant showed a surprisingly higher
skin penetration (stratum corneum/epidermis and dermis concentration), and
greater cumulative receptor solution fluid concentration relative to the other
tested formulations.
Example 21: Additional 1% and 3% bimatoprost formulations
[0099]Additional formulations were prepared in a similar manner to what has
already been described. Table 11 illustrates examples of such formulations,
illustrating that the formulations do not necessarily need to comprise ()ley!
alcohol. The bimatoprost solution A below had a ratio of bimatoprost to
ethanol of 0.03. The bimatoprost solution B had a ratio of bimatoprost to
ethanol of 0.01. The bimatoprost solution C had a ratio of bimatoprost to
ethanol of 0.005. The bimatoprost solution D had a ratio of bimatoprost to
ethanol of 0.0167. The bimatoprost solution E had a ratio of bimatoprost to
ethanol of 0.05.
Table 11. 1% and 3% bimatoprost formulations
Bimatopros Bimatopros Bimatopros Bimatopros Bimatopros
Ingredient
t solution A t solution B t solution C t solution D t solution E
(% w/w)
1% 3% 0.3% 1% 3%
Bimatoprost 1 3 0.3 1 3
Transcutol 10 10 0 0 0
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
36
Ethanol 30 30 60 60 60
Propylene
10 20 20 20
glycol
Glycerin 2 2 0 0 0
Carbomer
0.15 0.15 0 0 0
(Ultrez 10)
Triethanola
0.16 0.16 0 0 0
mine
Purified
46.69 44.69 19.7 19 17
water
Example 22: In vitro testing
[00100] The formulations of Table 11 were compared to the Bimatoprost
0.3% Solution formulation of Table 3. Testing was done using the same
apparatus and general setup as Example 20 above. Here, however, the
cadaver skin samples came from a 44 year old white female, and a 60 year old
white female.
Table 12. Summary of in vitro skin penetration results
Formulation Dermis Conc. at 48 Cumulative Receptor
hrs. (ng bimatoprost) Solution Fluid
Concentration at 48
hrs. (ng. bimatoprost)
Bimatoprost 0.3% 69.1 28.7
(Propylene Glycol)
Solution, Table 3
Bimatoprost solution A 35.3 58.3
1%, Table 11
Bimatoprost solution B 787 133
3%, Table 11
Bimatoprost solution C 139 83.1
0.3%, Table 11
Bimatoprost solution D 1060 175
1%, Table 11
Bimatoprost solution E 1410 230
3%, Table 11
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
37
[00101] The results above indicate that the bimatoprost formulations with
a
higher relative ethanol and propylene glycol content, i.e., the solutions C,
D,
and E from Table 10, showed higher receptor solution and dermis
concentration relative to the other formulations.
Example 23: Additional bimatoprost formulations
[00102] Further formulations were also prepared using similar
manufacturing techniques as have been previously described. Here, the
formulations described below in Table 13 were manufactured comprising oleyl
alcohol as a skin penetrating agent. In table 13, bimatoprost solution A had a
ratio of bimatoprost to oleyl alcohol of 0.6, a ratio of bimatoprost to
ethanol of
0.045, and a ratio of oleyl alcohol to ethanol of 0.076. The bimatoprost
solution
B had a ratio of bimatoprost to oleyl alcohol of 0.6, a ratio of bimatoprost
to
ethanol of 0.05, and a ratio of oleyl alcohol to ethanol of 0.083. The
bimatoprost solution C had a ratio of bimatoprost to oleyl alcohol of 0.6, a
ratio
of bimatoprost to ethanol of 0.05, and a ratio of oleyl alcohol to ethanol of
0.083.
Table 13. Additional bimatoprost formulations
Bimatopros Bimatopros Bimatopros
Ingredient
t solution A t solution B t solution C
(% w/w)
3% 3% 3%
Bimatoprost 3 3 3
Oleyl alcohol 5 5 5
Ethanol 66 60 60
Propylene
22 20 20
glycol
Cremophor
RH40 (PEG o 5 o
40 castor oil)
Cremophor
ELP o o 8
(PEG 35
castor oil)
Purified
4 7 4
water
CA 02900285 2015-08-04
WO 2014/158373 PCT/US2014/015430
38
Example 24: Additional bimatoprost gel formulation
[00103] An additional gel formulation containing 10% w/w bimatoprost was
also prepared using similar manufacturing techniques as have been previously
described. The bimatoprost gel formulation below had a ratio of bimatoprost to
ethanol of 0.33. Table 14 below describes the specific ingredients comprising
this formulation.
Table 14. Additional bimatoprost gel formulation
Bimatoprost
Ingredient (%
Gel
w/w)
Bimatoprost 10
Ethanol 30
Transcuto10 10
Propylene Glycol 10
Glycerin 2
Carbomer Ultrez
0.15
Triethanolamine 0.16
Purified water 37.69
Example 25: in vitro testing
[00104] The formulations of Tables 13 and 14 were compared to the
bimatoprost 0.03% Solution formulation of Table 3. Testing was done using the
same apparatus and general setup as used in Examples 20 and 22 above.
Here, the cadaver skin samples were from three donor cadavers¨a 54 year
old white male, a 42 year old black male, and a 26 year old white male.
Table 15. Summary of in vitro skin penetration results
Formulation Dermis Conc. at 48 Cumulative Receptor
hrs. (ng bimatoprost) Solution Fluid
Concentration at 48
hrs. (ng. bimatoprost)
Bimatoprost 0.3% 79.1 250
(Propylene Glycol)
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
39
Solution, Table 3
Bimatoprost solution A 1970 17200
3%, Table 13
Bimatoprost solution B 1240 8100
3%, Table 13
Bimatoprost solution C 2900 5640
3%, Table 13
Bimatoprost 10% gel 2820 646
formulation, Table 14
[00105] Relative to the control formulation of Table 3, the 3% bimatoprost
solutions containing ()leyl alcohol demonstrated high skin permeation and
permeation into the receptor solution. Solution A demonstrated a particularly
high receptor fluid concentration relative to the other ()leyl alcohol
formulations.
[00106] Additionally, the 10% w/w bimatoprost gel formulation from Table
14, which did not include any ()leyl alcohol, showed a high dermal
concentration, but did not exhibit as much penetration into the receptor
solution
as the 3% w/w bimatoprost solutions. Nevertheless, the tested characteristics
of all four new formulations were superior compared to the original control
formulation.
Example 26: Additional bimatoprost formulations
[00107] Examples of formulations containing bimatoprost and one or more
penetration enhancers may be found in Table 16 below, and were
manufactured in accordance with the techniques described previously.
Table 16: Additional 3% bimatoprost formulations
Formulation
1 2 3 4 5 6 7
#
Ingredient
(% w/w)
Bimatoprost 3 3 3 3 3 3 3
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
QS QS
Water 2 2 2 6 4
100 100
cetostearyl-- --
0.5 1 -- --
alcohol
glyceryl
2 2 2 --
mono-oleate
Oleyl alcohol -- -- 2 --
Ethanol 73.5 73 72 68 68.25 68.25 30
Propylene
10 10 10 10 22.75 22.75 10
Glycol
Transcutol P 10 10 10 10 10
Benzyl -- --
1 1 1 1
alcohol
Ultrez 0.15
Trolamine 0.16
-- --
Glycerol 2
Example 27: Additional in vitro testing
[00108] The formulations from Table 16 above were tested in an in vitro
skin
penetration testing method. Testing was done using the same apparatus and
general setup as used in Examples 20 and 22 above.
Table 17: Summary of in vitro skin penetration results
Cumulative
Formulation # (fromDermis
amount in
Table 16) Concentration at
receptor
48 hours. (ng)
solution (ng)
F-1 567 722 278 73
F-2 907 554 861 395
F-3 2050 660 685 108
F-4 2740 260 894 615
F-5 3110 230 819 356
F-6 4080 1640 1480 980
CA 02900285 2015-08-04
WO 2014/158373 PCT/US2014/015430
41
F-7 365 317 757 123
[00109] Compared to the formulation F-7, formulations F-2 through F-6 were
found to have demonstrated comparable or higher bimatoprost permeation into
the receptor fluid. Also formulations F-2 through F-6 were shown to have a
higher amount of bimatoprost in the dermis. Formulation F-6, which contains
oleyl alcohol, had the highest bimatoprost concentration in the dermis and
receptor solution, respectively.
Example 28: Additional bimatoprost formulations
[00110] Examples of formulations containing bimatoprost and one or more
penetration enhancers may be found in Table 18 below, and were
manufactured in accordance with the techniques described previously. It will
be noted that the formulations F-1 and F-2 are the same as those shown in
Table 11.
Table 18: Additional bimatoprost formulations
Formulation # -1 -2 -3 -4 -5 -6 -7 -8 -9 -10
Ingredient
(% w/w)
Bimatoprost 3 1 1 1 1 1 1 1 1 1
Glycerin 2 2 2 2
Ethanol 30 30 69.4 69.4 70 46 45 69.4 69.4 69.4
Transcutol 10 10 21.1 10 10 4 21.1
Propylene
Glycol 10 10 23.1 10 10 4 23.1 23.1
Benzyl Alcohol 1 1 1
Carbomer
Ultrez 10 0.15 0.15 0.2
Triethanolamine 0.16 0.16 0.3
Oleyl Alcohol 0.5 0.5
Oleic Acid 0.5 2 2
Glycerol
monooleate
(GMO) 2 2 2
Water 44.69 46.69 6 6 6 30 42.5 6 4.5 4.5
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
42
Example 29: Additional in vitro testing
[00111] The formulations from Table 18 above were tested in an in vitro
skin
penetration testing method. Testing was done using the same apparatus and
general setup as used in Examples 20 and 22 above.
Table 19: Additional in vitro testing
Cumulative
Dermis
amount in
Concentration at
receptor
48 hours. (ng)
solution (ng)
Description
F-1 45.7 6.5 449 231
F-2 36.6 36.3 132 70
F-3 209 41 618 457
F-4 104 32 145 126
F-5 328 183 476 56
F-6 178 41 830 25
F-7 66.7 29.7 452 6
F-8 66.0 28.1 391 227
F-9 193 134 935 680
F-10 206 155 229 72
[00112] Compared to the formulation F-1 (which has a 3% bimatoprost
concentration), formulations F-3 through F-10 have comparable or higher
bimatoprost permeation into the receptor fluid while containing bimatoprost at
a
1% concentration. Additionally, formulations F-3, and F-5 through F-10
demonstrate a higher amount of bimatoprost in the dermis versus formulation
F-1. This study shows that glycerol monooleate and oleic acid, respectively,
may be useful in enhancing the penetration of bimatoprost into and through the
skin in an in vitro study compared to formulations F-1 and F-2.
Example 30: Additional bimatoprost formulations
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
43
[00113] Examples of formulations containing bimatoprost and one or more
penetration enhancers may be found in Table 20 below, and were
manufactured in accordance with the techniques described previously. It will
be noted that the formulations F-1 and F-2 are the same as those shown in
Table 11.
Table 20: Additional bimatoprost formulations
Formulation 1 2 3 4 6 7 8 9
Ingredient
(% w/w)
Bimatoprost 3 1 1 1 1 1 1 1
Glycerin 2 2
Ethanol 30 30 69.4 68.25 68.25 68.25 68.25 68.25
Transcutol 10 10
Propylene
Glycol 10 10 23.1 22.75 22.75 22.75 22.75
22.75
Benzyl Alcohol
Carbomer
Ultrez 10 0.15 0.15
Triethanolamine 0.16 0.16
Oleyl Alcohol 0.5
Oleic Acid 2
GMO 2 2
Linoleic Acid 2
Sodium Lauryl
Sulfate 0.2 0.2
Docusate
Sodium 0.2
Water 44.69 46.69 6 4 7.8 5.8 7.8 6
Example 31: Additional in vitro testing
[00114] The formulations from Table 20 above were tested in an in vitro
skin
penetration testing method. Testing was done using the same apparatus and
general setup as used in Examples 20 and 22 above.
Table 21: Additional in vitro testing
Cumulative
Dermis
Formulation ID amount in
Concentration at
receptor
48 hours (ng)
solution (ng)
F-1 42.7 3.1 348 57
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
44
F-2 55.3 31.7 135 54
F-3 594 301 202 35
F-4 1070 530 514 168
F-6 101 58 387 435
F-7 624 394 331 156
F-8 171 93 377 204
F-9 936 419 378 62
[00115] Compared
to the formulation F-1, formulations F-3 through F-9 have
comparable or higher bimatoprost permeation into the receptor fluid while
using
a comparatively lower percentage of bimatoprost (1% versus 3%). A
combination of GMO and oleic acid (Formulation F-4) and linoleic acid (
Formulation F-9), showed the highest permeation of bimatoprost into the
receptor solution.
Example 32: Additional bimatoprost formulations
[00116] Examples
of formulations containing bimatoprost and one or more
penetration enhancers may be found in Table 22 below, and were
manufactured in accordance with the techniques described previously. It will
be noted that the formulation F-1 is the same as those shown in Table 11.
Table 22: Additional bimatoprost formulations
Formulation 1 2 3 4 5 6 7 8 9
Ingredient
(% w/w)
Bimatoprost 3 1 1 1 1 1 1 1 1
Glycerin 2
Ethanol 30 68.25 68.25 68.25 68.25 68.25 68.25 68.25 67
Transcutol 10 10
Propylene
Glycol 10 22.75 22.75 22.75 22.75 22.75 22.75 22.75 10
Benzyl Alcohol 1
Oleic Acid 2 2 2 2 2 2 2 2
CA 02900285 2015-08-04
WO 2014/158373 PCT/US2014/015430
glycerol
monooleate 2 2 2 2 2 2 2 2
Linoleic Acid 2
Terpinolene 2
Limonene 2
Nerol 2
Cineol 2
Octyl Sal icylate 2
DMSO 2
Carbomer
Ultrez 10 0.15
triethylamine 0.16
Water 44.69 2 2 2 2 2 2 2 7
Example 33: Additional in vitro testing
[00117] The formulations from Table 22 above were tested in an in vitro
skin
penetration testing method. Testing was done using the same apparatus and
general setup as used in Examples 20 and 22 above.
Table 23: Additional in vitro testing
Cumulative Dermis
amount in Concentration
Formulation ID
receptor at 48 hours
solution (ng) (ng)
F-1 10.5 2.1 72 27
F-2 1360 360 581 360
F-3 2030 2170 183 107
F-4 5200 2720 251 172
F-5 3570 1100 538 583
F-6 4650 1010 362 163
F-7 5890 4150 678 644
F-8 6810 1020 632 314
F-9 3640 2020 279 48
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
46
[00118] Compared to the formulation F-1, formulations F-2 through F-9 have
demonstrated comparable or higher bimatoprost permeation into receptor
solution and into the dermis, even with a lower overall concentration of
bimatoprost.
Example 34: Additional bimatoprost formulations
[00119] Examples of formulations containing bimatoprost and one or more
penetration enhancers may be found in Table 24 below, and were
manufactured in accordance with the techniques described previously. It will
be noted that the formulation F-1 is the same as those shown in Table 11.
Table 24: Additional bimatoprost formulations
Formulation 1 2 3 4 5 6 7 8 9
Ingredient
(% w/w)
Bimatoprost 3 1 1 1 1 1 1 1 0.5
Glycerin 2
Ethanol 30 46 68.25 68.25 68.25 68.25 68.25 10
Transcutol 10 10
Propylene Glycol 10 10 22.75 22.75 22.75 22.75 22.75
Benzyl Alcohol 1
Oleic Acid 2 0.8 3.2 2 2
GMO 2 3.2 0.8 2 2
Sodium Lauryl
Sulfate 0.2 0.2
DDAB 0.2
Sodium
Taurodeoxycholat
2
Docusate Sodium 0.2
Polysorbate 80 2
Crodamol MM 25
Dow ST-
Elastomer 10 73.5
Dow Silky Wax
8
Isopropyl
Myristate 8
Carbomer Ultrez 0.15
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
47
Trolamine 0.16
Water QS QS QS QS QS QS QS QS
Example 35: Additional bimatoprost formulations
[00120] In some embodiments, several possible formulations capable of
superior skin penetration may be manufactured as set forth in the two
following
tables. Using a base formulation set forth in Table 25, a mixture of one or
more
fatty acids or fatty esters (as set forth in Table 26) may be added thereto.
Preferably, a base formulation from Table 25 will be combined with at least
two
ingredients from Table 26. Even more preferably, the at least two ingredients
from Table 26 are one fatty acid and one fatty ester. Of course, it will be
understood that these formulations do not presume any particular order of
manufacture, and are only presented thusly for ease of understanding. While
the base formulations may be prepared with any appropriate concentration of
bimatoprost, this concentration is preferably between about 0.3% w/w and
about 5% w/w, more preferably about 1% w/w to about 3% w/w, and even more
preferably about 3% w/w.
Table 25: Base formulations
Function Ingredient Composition (% w/w)
Active Ingredient Bimatoprost 0.3-5%
Excipients Ethanol 0-89
Propylene glycol 0-89
Diethylene glycol
monoethyl ether 0-89
Benzyl alcohol 0-89
Water 0-89
Table 26: Example fatty acid and fatty ester excipients
Ingredient Example Composition (% w/w)
Fatty acids (C8-C28)
Saturated stearic acid 0-10
Monounsaturated oleic acid 0-10
Polyunsaturated linoleic acid 0-10
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
48
Fatty esters (C8-C28)
Saturated glyceryl monostearate 0-10
Monounsaturated glyceryl monooleate 0-10
Polyunsaturated ethyl ester of linoleic acid 0-10
Example 35: Clinical testing to evaluate the efficacy and safety of once-
daily topical bimatoprost solution for increasing scalp hair growth in men
with androgenic alopecia
[00121] In a phase 2 multicenter trial, men 18-49 years of age with mild to
moderate androgenic alopecia (AGA) were randomized in a 1:1:1:1:1 ratio to
receive in a double-blind manner bimatoprost (BIM) 0.3%, 0.1%, 0.03%, or
vehicle applied once daily, or open label over-the-counter minoxidil 5%
solution
(MIN) applied twice daily to the vertex area of the scalp for 6 months.
Subjects
were evaluated every 2 months during treatment and at 2 months after
completing treatment. The BIM formulations used in this study were as
described in Table 3 above.
[00122] The following assessments were made with digital image analysis
(DIA; macrophotographs of a prespecified 1-cm2 circular area at the anterior
leading edge of the vertex thinning area of the scalp identified by microdot
tattoo) using customized validated software: Target Area Hair Count (TAHC)
measured in terminal hairs/cm2; Target Area Hair Width (TAHW) measured in
mm/cm2; and, Target Area Hair Darkness (TAHD) measured in intensity units;
[00123] Other assessments included Subject Self Assessment (SSA),
Investigator Global Assessment (IGA), and Global Panel Review (GPR) by an
independent panel of 3 dermatologists while viewing standardized global
photographs of the scalp at baseline (day 1) and at the current visit (or live
assessment at the current visit in the case of IGA) Change in scalp hair
growth
was measured on 7-point ordinal scale from ¨3 (greatly decreased) to +3
(greatly increased).
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
49
[00124] Co-primary efficacy endpoints measured included the change from
baseline in TAHC and the SSA of change in scalp hair growth. Secondary
efficacy endpoints included changes from baseline in TAHW and TAHD, and
IGA/GPR of changes in scalp hair growth.
[00125] Safety measures included adverse event monitoring, local
tolerability assessment, clinical laboratory testing, vital signs, 12-lead
electrocardiograms (ECGs), and physical examinations.
[00126] Statistical efficacy analyses were performed on the modified intent-
to-treat population, which included all randomized subjects who received study
treatment and had baseline measurements The co-primary efficacy analyses
were conducted at month 6. Percent changes from baseline in TAHC, TAHW,
and TAHD were analyzed using the Wilcoxon rank-sum test. Frequency
distributions of SSA, IGA, and GPR scores were analyzed using a Cochran-
Mantel-Haenszel test stratified for age group (18-34 vs 35-49 years), and
missing data were imputed to month 6 using a last-observation-carried-forward
approach. Safety measures were evaluated in all subjects who received study
medication; among-group and pairwise comparisons of the incidence of
adverse events were performed using a chi-square or Fisher's exact test
Results
[00127] A total of 307 men with AGA received study treatment and were
included in the efficacy and safety analyses. Treatment arms were generally
well balanced with respect to demographic and baseline characteristics
[00128] Digital image analysis (DIA) showed that bimatoprost 0.3%
produced a significantly greater mean percentage change from baseline to
month 6 in TAHC compared with vehicle (11.1% vs 3.3%; P=0.008). DIA also
revealed a significant improvement in TAHW, but not in TAHD, with
bimatoprost 0.3% in comparison to the vehicle at month 6; significant
improvements were also seen at month 4 for TAHW. Two months after
stopping treatment, the changes in TAHC and TAHW with bimatoprost were no
longer significantly different from vehicle.
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
[00129] The
distribution of SSA scores at month 6 did not differ between
bimatoprost and vehicle. GPR assessments at month 6, but not IGA
assessments, showed that the percentage of subjects with 1-grade
improvement (i.e., scores of +3, +2, or +1) was significantly greater with
bimatoprost 0.3% compared with vehicle (21.1% vs 8.9%; P=0.030). The
improvement in GPR assessment with bimatoprost 0.3% was maintained at 2
months post-treatment (16.4% vs 0%; P=0.017)
[00130] In the
open-label minoxidil 5% treatment arm with twice-daily
application, treatment resulted in an increase in TAHC of 18.4% from baseline
at month 6. This was higher than published reports of an .---13% increase in
TAHC after 4 and 12 months of treatment. A majority of subjects (67.3%) on
minoxidil 5% indicated 1-grade improvement in hair growth. At the doses
tested, the efficacy of any bimatoprost group was less than that of the MIN
group
[00131] With
reference to Table 27 below, the incidence of treatment-related
adverse events was slightly higher with bimatoprost compared with vehicle, but
lower than the rate with minoxidil.
Table 27: Summary of Treatment-Related Adverse Events (AEs)
Incidence, n Vehicle
Bimatoprost Bimatoprost Bimatoprost Minoxidil 5%
(%) (n=62) 0.03% 0.1% 0.3% (n=61)
(n=62) (n=61) (n=61)
Subjects with
treatment- 3(4.8) 3(48) 7(11.5) 5(8.2) 13 (21.3)
related AEs
Most common treatment-related AEs (reported by n% of subjects in any group)
Application-
site dryness 1 (1.6) 0 0 2 (3.3) 2 (3.3)
Application-
site pruritus 0 0 4 (6.6) 1 (1.6) 7 (11.5)
Application--
site pain 0 0 3 (4.9) 1 (1.6) 2 (3,3)
[00132] With reference to Table 28 below, the most common dermal
tolerability symptoms were itching based on subject report and dryness/scaling
based on dermatologist report; both were less frequent with bimatoprost 0.3%
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
51
compared with minoxidil. However, folliculitis was more common with
bimatoprost 0.3% than with minoxidil on the dermatologist report.
Table 28: Dermal Tolerability Based on Subject and Dermatologist Reports
Subjects Vehicle Bimatoprost Bimatoprost Bimatoprost Minoxidil 5%
With ?1 (n=62) 0.03% 0.1% 0.3% (n=61)
Severity (n=62) (n=61) (n=61)
Grade
Increase
From
Baseline at
Any Visit, n
(%)
Subject assessment
Burning 0 1 (1.6) 3 (4.9) 5 (8.2)* 3 (4.9)
Itching 2 (3.2) 2 (3.2) 3 (4.9) 7 (11.5) 14 (23.0)t
Stinging 0 1 (1.6) 2 (3.3) 5 (8.2)* 2 (3.3)
Dermatologist assessment
Dryness/scalin
8 (12.9) 9 (14.5) 7 (11.5) 9 (14.8) 20 (32.8)t
Erythema 4(6.5) 5(8.1) 7(11.5) 9(14.8) 7(11.5)
Edema 0 0 1 (1.6) 2 (3.3) 3 (4.9)
Pigmentation 0 1 (1.6) 2 (3.3) 2 (3.3) 3 (4.9)
Folliculitis 0 3 (4.8) 2 (3.3) 8 (13.1) 2 (3.3)
* P=0.028; t P=0.001; $ P=0.01; P=0.003 versus vehicle.
[00133] There were no clinically meaningful or dose-dependent changes in
clinical laboratory parameters, vital signs, ECGs, or physical examinations
with
bimatoprost
Conclusions
[00134] The study demonstrated that topical bimatoprost 0.3% applied once
daily significantly increased scalp hair growth compared with vehicle in
subjects
with mild-to-moderate AGA. Topical Minoxidil 5% applied twice daily in an
open-label manner showed efficacy higher than any of the bimatoprost doses
tested. Topical bimatoprost exhibited a safety and tolerability profile that
was
consistent with that of an established topical treatment for scalp hair growth
CA 02900285 2015-08-04
WO 2014/158373
PCT/US2014/015430
52
[00135] While the above detailed description has shown, described, and
pointed out novel features as applied to various embodiments, it will be
understood that various omissions, substitutions, and changes in the form and
details of the device or process illustrated may be made without departing
from
the spirit of the disclosure. Additionally, the various features and processes
described above may be used independently of one another, or may be
combined in various ways. All possible combinations and subcombinations are
intended to fall within the scope of this disclosure. Many of the embodiments
described above include similar components, and as such, these similar
components can be interchanged in different embodiments. Although the
invention has been disclosed in the context of certain embodiments and
examples, it will be understood by those skilled in the art that the invention
extends beyond the specifically disclosed embodiments to other alternative
embodiments and/or uses and obvious modifications and equivalents thereof.
Accordingly, the invention is not intended to be limited by the specific
disclosures of preferred embodiments herein.