Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02908497 2015-09-29
WO 2014/131037
PCT/US2014/018410
THIN FILM VASCULAR STENT FOR ARTERIAL DISEASE
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional Application No.
61/769,042, filed on February 25, 2013. In addition, this application is
related to
International Application No. PCT/U52010/026430, filed on March 5, 2010, which
claims the benefit of U.S. Provisional Application No 61/158,200, filed March
6, 2009
and U.S. Provisional Application No. 61/158,221, filed March 6,2009. All the
foregoing applications are hereby incorporated by reference in their entirety.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR
DEVELOPMENT
[0002] This invention was made with Government support under Grant No.
HL099445, awarded by the National Institutes of Health. The Government has
certain
rights in the invention.
TECHNICAL FIELD
[0003] This invention pertains generally to implantable devices, and more
particularly to an implantable medical device, and surface treatments for the
same, for
treating peripheral artery disease (PAD).
- 1 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
BACKGROUND
[0004] Lower extremity peripheral arterial disease (PAD) is characterized by
the
accumulation of atherosclerotic plaque in the arteries of the legs. PAD
commonly
presents with intermittent claudication, which can be lifestyle-limiting, but
may also
present as chronic or acute limb ischemia and ultimately require amputation.
The
prevalence of symptomatic PAD increases with age and is as high as 8% of the
general
population in persons over 70. In 2008, -1 million endovascular procedures for
PAD
were performed in the United States, representing a 5-fold increase from a
decade
earlier and -70% of the total PAD interventions. Due to the aging of our
population,
endovascular procedures to treat PAD are increasing, with an estimated 2
million
procedures performed annually by 2020. Unfortunately, current endovascular
treatments
are often associated with poor outcomes and new endovascular devices need to
be
considered for the aging population
[mos] One endovascular device for treating PAD is the Viabahn endoprosthesis
from Gore. This device uses a self-expandable Nitinol stent backbone lined
circumferentially with an expandable polytetrafluoroethylene (ePTFE) liner
that is
approximately 150 gm in thickness. Data on this device indicate that 1 year
primary
patency rates for superficial femoral artery disease are between 65-85%. In
order to
understand the relatively high failure rate of these devices, investigators
have identified
three key problems. These problems are as follows: First, stenosis tends to
occur at the
proximal and distal ends of the device. Second, patency rates are independent
of treated
lesion length. Third a significant percentage of patients (-40% after 5 years)
experience
late-term thrombosis.
[0006] Many of the vessels subject to PAD are relatively small such as having
a
diameter of just a few millimeters. The relatively thick (-150 gm) ePTFE
lining is thus
- 2 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
appreciable with regard to such vessel lumens. The resulting restriction in
vessel lumen
diameter by 300 microns causes or exacerbates proximal and distal restenosis.
The
impermeability of the ePTFE lining is another issue. Because ePTFE is a thick
polymer, it is an impermeable barrier to cell growth and migration. FIG. 1
shows
images taken of a stented sheep iliac artery three months after stenting. Due
to the
image magnification, only two struts 10 of a stent 5 are shown adjacent the
iliac artery
lining 12. An ePTFE stent liner 12 is on the luminal side of struts 10. Struts
10 are thus
abluminal with regard to ePTFE stent liner 12. Given the impermeability of
ePTFE
stent liner 12, intercellular communication between any luminal endothelial
layer on a
luminal surface 14 of ePTFE stent liner 12 and an artery wall 15 is
impossible. Luminal
surface 14 is thus chronically exposed and acellular. This endothelialization
failure on
luminal surface 14 greatly increases the risk of thrombosis. In contrast, a
neointima 11
has proliferated around struts 10. A large body of evidence has shown that
intercellular
communication between a luminal endothelial monolayer and the underlying
vessel wall
is critical for preventing excessive smooth muscle cell proliferation leading
to
neointimal hyperplasia (NIH) and eventually stenosis. Although the
impermeability of
ePTFE stent liner 12 prevents neointima 11 from invading the vessel lumen, the
proliferation of neointima 11 on struts 10 is undesirable and increases the
probability of
restenosis. The relatively thick and impermeable ePTFE barrier, while
preventing
smooth muscle cell proliferation (i.e. a beneficial attribute), also prevents
nutrient
exchange and paracrine communication between intima and media that are key
features
of normal vessel physiology. Thus, a thick impermeable ePTFE covering is a
poor
choice for an endovascular device where the goal is to establish non-
pathologic vascular
homeostasis as quickly and efficiently as possible.
- 3 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
[0007] Accordingly, there is a need in the art for improved techniques and
devices with regard to preventing restenosis and thrombosis in stented
vessels.
BRIEF SUMMARY OF THE INVENTION
[mos] A stent cover is provided that inhibits smooth muscle cell migration and
resulting neointimal hyperplasia while promoting a healthy luminal endothelial
lining.
The stent cover comprises micro-patterned-thin-film nitinol (MTFN) forming a
cylinder
for enclosing and covering stent struts or truss members. The micro-pattern
comprises a
plurality of fenestrations in the thin-film nitinol that are large enough to
allow sufficient
intercellular communication yet are small enough to inhibit neointimal
hyperplasia. The
stent cover extends in a longitudinal dimension from a proximal end to a
distal end.
There is a corresponding longitudinal dimension or extent across each
fenestration. In
that regard, the blood flow within the stented vessel flows generally in the
longitudinal
dimension. Similarly, there is a transverse dimension or extent across each
fenestration
that is orthogonal to the longitudinal dimension. These dimensions exist
whether each
fenestration comprises a similar polygon or are instead irregular. Regardless
of the
fenestration geometry, the transverse and longitudinal dimensions for each
fenestration
do not exceed a critical dimension so as to inhibit neointimal hyperplasia.
This
maximum or critical dimension is comparable to the dimensions of a smooth
muscle
cell. In one embodiment, the maximum dimension is 10 microns. More generally,
the
maximum dimension is that which prevents or at least substantially inhibits
migration of
smooth muscle cells through the fenestrations such as 25 microns or less.
[0009] The micro-patterned thin film stent cover is quite advantageous as
compared to conventional ePTFE barriers. For example, the fenestrations
promote
endothelialization on the luminal surface of the stent cover. In contrast to
the
- 4 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
conventional ePTFE barrier, which lacks such endothelialization, the micro-
patterned-
thin-film stent cover thus inhibits thrombosis. Although the fenestrations
enable
endothelialization of the luminal surface and thus inhibit thrombosis, the
fenestrations
also prevent neointimal hyperplasia on the stent cover luminal surface because
the
fenestration dimensions are too small to permit smooth muscle cell migration
through
the fenestrations. In addition, the resulting cellular communication between
the
endothelial lining on the stent cover luminal surface and the vessel wall
adjacent to the
stent cover abluminal surface is believed to inhibit hyperplasia on the
abluminal surface
of the stent cover. In contrast, the neointimal proliferation (neointimal 11
of Figure 1)
on the abluminal surface of conventional ePTFE barriers is plainly
undesirable.
Furthermore, the micro-patterned thin-film stent cover is markedly thinner
than
conventional ePTFE barriers and thus resists restenosis resulting from flow
constrictions. These and other advantages of the advantageous micro-patterned-
thin-
film stent cover disclosed herein may be better appreciated from the following
detailed
description without placing limitations thereon.
BRIEF DESCRIPTION OF THE DRAWINGS
[oolo] The invention will be more fully understood by reference to the
following drawings which are for illustrative purposes only:
[ow 1] FIG. 1 shows an image taken from an ePTFE covered stent after 3
months in a sheep iliac artery.
[0012] FIG. 2A illustrates an assembled view of a PAD stent in accordance with
the present invention.
[0013] FIG. 2B illustrates an exploded view of the PAD stent of FIG. 1.
- 5 -
CA 02908497 2015-09-29
WO 2014/131037
PCT/US2014/018410
[0014] FIG. 3A shows a schematic diagram of an implanted stent with MTFN
covering of the present invention and its effect on "edge effect" stenosis.
[0015] FIG. 3B shows a schematic diagram of an implanted stent with ePTFE
covering and its effect on "edge effect" stenosis.
[0016] FIG. 4 shows a schematic diagram of an implanted stent with MTFN
covering of the present invention and effect on SMC (smooth muscle cell)
migration.
[0017] FIG. 5 shows a schematic diagram of an implanted stent with MTFN
covering of the present invention and resulting endothelial monolayer.
[0018] FIG. 6 is a graph showing the influence of treatment time on the
wetting
angle of the MTFN film of the present invention.
[0019] FIG. 7 shows SEM images of four MTFN sheets, each with different
micro patterns of fenestrations in accordance with the present invention.
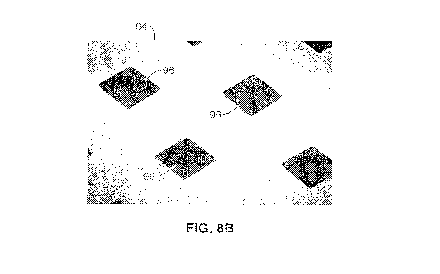
[0020] FIG. 8A and FIG. 8B show optical microscopy images of two films
having diamond pattern fenestrations with dimensions of 7.5 m x 10 gm and 45
gm x
60 gm, respectively.
[0021] FIG. 9A through FIG. 9C show molecular analysis of the
hemocompatibility of TFN as compared to ePTFE, with FIG. 9A showing total
thrombus and FIGS. 9B and 9C showing fibrin and platelet deposition,
respectively.
[0022] FIG. 10A through FIG. 10C show images of the effects of surface
wettability and the endothelial monolayer in vitro after 1 week for contact
angles of 00,
40', and 65', respectively.
[0023] FIG. 11 shows a representative image of a vessel wall treated with the
stent of FIG. 8A having 7.5 gm x10 gm perforations.
[0024] FIG. 12 shows the contralateral iliac artery treated with the stent
covering of FIG. 8B having 45 gm x 60 gm perforations.
- 6 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
[0025] FIG. 13 shows a graph of neointimal area for various fenestration
sizes.
[0026] FIG. 14A through FIG. 14C show images of the wall of an artery treated
with a 45 gm x 60 gm stent covering at low magnification, medium
magnification, and
high magnification respectively.
[0027] FIG. 15A shows images of HAECs grown on un-patterned TFN.
[0028] FIG. 15B (scale bar 100 gm) and FIG. 15C (scale bar 50 gm) show
HAECs grown on MTFN with a lattice pattern.
DETAILED DESCRIPTION
[0029] Referring more specifically to the drawings, for illustrative purposes
the
present invention is embodied in the apparatus generally shown in FIG. 2
through FIG.
15C. It will be appreciated that the apparatus may vary as to configuration
and as to
details of the parts, and that the method may vary as to the specific steps
and sequence,
without departing from the basic concepts as disclosed herein.
[0030] FIG. 2A and FIG. 2B illustrate assembled and exploded views
(respectively) of PAD stent 20 in accordance with the present invention. Stent
20
generally comprises a micro-patterned-thin-film nitinol (MTFN) cover 22 that
is
disposed around a collapsible truss 24. As shown in FIG. 2B, truss 24 may
comprise a
plurality of undulating wire segments or stent struts 28 that are coupled to
anchor points
26 that allow the truss 24 to be compressed in a collapsed configuration (not
shown) to a
delivery location. In one embodiment, struts 28 may comprise nitinol. The MTFN
sheet 22 generally forms an extremely low profile (e.g. 5 gm thick) tubular
structure
around the truss 24 and has a plurality of perforations shown in greater
detail in FIGS. 7
through 8B.
- 7 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
[0031] Referring to FIG. 3A through FIG. 5, it will be appreciated that MTFN
stent cover 22 has a number of advantages. For example, FIG. 3A shows a
portion of
an MTFN stent cover 22 contacting a vessel wall 21. For illustration clarity,
the stent
struts on the luminal side of MTFN stent cover 22 are not illustrated in FIG.
3A.
Because stent cover 22 comprises thin-film nitinol, it presents an
insignificant barrier to
the blood flow direction as indicated by arrow F. This lack of flow
restriction inhibits
restenosis of the stented vessel. In contrast, the much greater thickness of
conventional
ePTFE stent liner 12 as shown in FIG. 3B presents a much greater obstruction
to blood
flow as indicated by flow separation zone F. In addition, MTFN stent cover 22
promotes endothelialization 30 on its luminal surface 23 to inhibit thrombosis
whereas
luminal surface 14 of conventional ePTFE stent liner 12 is acellular and thus
promotes
thrombosis.
[0032] An example fenestration 40 in MTFN stent cover 22 is shown in FIG. 4.
The longitudinal and transverse dimensions across fenestration 40 are small
enough to
prevent migration of smooth muscle cells 32 in the stented vessel wall through
fenestration 40 (i.e., from an abluminal surface 42 of MTFN stent cover 22 to
the
luminal surface 44). For example, these dimensions in one embodiment do not
exceed
microns with 1 micron precision. Thus, endothelial cells 34 on luminal surface
44
do not get invaded by smooth muscle cells 32 such that neointimal hyperplasia
is
prevented while still allowing for intercellular communication through the
graft's
thickness.
[0033] In addition, the MTFN stent covers disclosed herein may include a
surface treatment to increase hydrophilicity. This increased hydrophilicity is
illustrated
symbolically in FIG. 5 by dotted line 51. The resulting chemical modification
of stent
- 8 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
surfaces such as luminal surface 44 facilitates growth of a robust endothelial
monolayer
34.
[0034] MTFN stent cover 22 is processed to specific dimensions and
composition to promote adaptation within the patient's body. Nitinol, or
Nickel
Titanium, is an equiatomic (1 atom Ni, 1 atom Ti) shape memory alloy, and is
commonly used in endovascular devices in the form of bulk Nitinol (>100
microns
thick). MTFN stent cover 22 of the present invention comprises thin film
nitinol (TFN)
that is fabricated in sheets approximately 5 m thick via sputter deposition
that has only
recently become available for practical uses.
[0035] In one embodiment, MTFN stent cover 22 may be generated using a
"hot-target" sputter deposition process, detailed in International Application
No.
PCT/US2010/026430 (the '430 application) that consistently generates thin-film
nitinol
(TFN) with a <0.5% atomic compositional variation. In addition to its high
purity, the
TFN produced by this method is both extremely smooth (surface roughness of
5nm) and
strong (tensile strength of 500MPa).
[0036] As discussed in the '430 application, a semiconductor substrate may be
patterned using a deep reactive ion etching (DRIE) process to create a
patterned
substrate. Nitinol is then sputtered onto the patterned substrate. Although
the bottoms
of the etched trenches in the substrate also receive a sputtered layer of
nitinol, those
areas are separated from the nitinol deposited on the non-trenched portions of
the
patterned substrate by the vertical trench walls produced by the DRIE process.
When
the nitinol film is then released from the patterned substrate, the nitinol
film will then
have fenestrations corresponding to where the trenches were produced on the
patterned
substrate. As detailed further in the '430 application, the use of the DRIE-
patterned
substrate is quite advantageous because of its relatively tight tolerance ¨
for example,
- 9 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
the trench shapes (and thus the resulting fenestrations in the patterned thin-
film nitinol)
may have a tolerance of a 1 micron or less. In contrast, wet etching
techniques
typically have much coarser tolerances. Since the fenestrations disclosed
herein are
relatively small (e.g., having longitudinal and transverse dimensions of 10
microns or
less), it is advantageous to employ the DRIE process discussed in the '430
application.
However, it will be appreciated that nitinol may be sputtered onto an un-
patterned
substrate such that the fenestrations are subsequently formed using
conventional wet-
etching techniques in alternative embodiments.
[0037] Because the patterned substrate is typically planar, the DRIE process
discussed in the '430 application typically produces a planar thin-film
nitinol sheet. In
contrast, stent cover 22 is cylindrical. To form such a three-dimensional
structure from
the nitinol sheet, the longitudinal edges of the sheet are sealed together
along a seam 23
as shown in FIG. 2B. It will thus be appreciated the stent cover length (the
longitudinal
extent of a stent cover) depends upon the longitudinal length of the initial
thin-film
nitinol sheet that is then sealed along its longitudinal edges to form seam
23.
Conversely, the initial thin-film nitinol sheet will have some transverse
length along its
proximal and distal edges. It is this transverse length for the initial thin-
film nitinol
sheet that determines the stent cover lumen diameter. Alternatively, nitinol
could be
sputtered onto a patterned tubular mandrel and then released from the mandrel
to
produce stent cover 22. In such embodiments, there would be no intermediate
stage of
forming a planar sheet and then sealing the sheet into a tubular structure. To
distinguish the sealed stent cover from the initial thin-film sheet, the term
"MTFN sheet
22" refers to the initial planar thin-film sheet whereas "MTFN stent cover 22"
refers to
the stent cover that results from sealing the thin-film sheet along seam 23.
In yet
additional alternative embodiments, nitinol may be sputtered onto a patterned
planar
- 10 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
substrate. A sacrificial layer such as a chromium layer may then be deposited
along a
swath of the sputtered nitinol and then additional nitinol sputtered onto this
sacrificial
layer and the initially-deposited nitinol outside of the area covered by the
sacrificial
layer. A "layer cake" nitinol sheet results that has sealed edges (nitinol
deposited on
nitinol) and also nitinol layers that are separated by the sacrificial layer.
This sacrificial
layer is exposed in the fenestrations of the nitinol layers and may thus be
etched away.
A three-dimensional (i.e., tubular) structure results that needs no sealing
along any
longitudinal edges despite the use of planar substrates. Additional details
regarding the
manufacture of such a tubular thin-film nitinol structure may be found in U.S.
Patent
No. 6,790,298, the contents of which are incorporated by reference in their
entirety.
[0038] The MTFN stent cover 22 of the present disclosure generally has a
thickness of less than 50 microns, and preferably has a thicknesses ranging
from about
0.1 microns to about 30 microns. Preferably, the thin films may have a
thickness
ranging from about 0.1, 1, 2, 4, 5, 10, 15, 20, 25, 30 or 50 microns to about
4, 5, 10, 15,
20, 25, or 30 microns. More preferably, the thin films may have a thickness of
from
about 4 microns to about 12 microns.
[0039] As a result of the relative thinness, covering a stent truss 24 (FIG.
2B)
with the thin memory metal film of the present disclosure results in a minimal
and
inconsequential increase in the size of the overall device. For example, MTFN
can be
manufactured in films of from about 5 to about 8 gm thickness, so that
covering a stent
with MTFN adds very little bulk to the devices. The stent struts can have a
thickness in
the range of, for example, about 2 gm, 4 gm, 6 gm, 7 gm, 10 gm, 17 gm, 18 gm,
or 20
gm.
[0040] Both truss 24 and MTFN stent cover 22 can be produced in a range of
shapes and sizes. For example, thin memory metal alloy films can be made
square or
- 11 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
rectangular e.g. when laid flat, the sheet can have the appearance of a
rectangle with a
longer longitudinal dimension and a shorter transverse dimension. Each
dimension of
such a square or rectangle can be selected from a wide range.
[0041] In some embodiments, the width (the transverse dimension) of such a
square or rectangle may be in the range of, for example, about 0.5 mm, 1 mm, 3
mm, 5
mm, 10 mm, 16 mm, 20 mm, 25 mm, 30 mm, or 40 mm. The width is generally a
function of the internal diameter of the lumen to be treated.
[0042] Correspondingly, the length (longitudinal dimension) of such a square
or
rectangle may be in the range of, for example, about 0.5 mm, 2 mm, 5 mm, 15
mm, 20
mm, mm, 50 mm, or 100 mm. Generally, the length is a function of the size of
the
region to be treated.
[0043] Adjacent sides of sheet 22 need not be perpendicular. The sheet 22 can
have a form that is not an endless loop; for example, the sheet can have two
distal edges
as ends of the sheet, bounding the length dimension.
[0044] Thin memory metal alloy films may be made in a wide variety of shapes
other than square or rectangular. For example, thin memory metal alloy films
may be
made to resemble other polygons, circles, ovals, crescents, or an arbitrary
shape.
[0045] In one embodiment, the sheet 22 comprises a generally rectangular thin
film sheet wrapped into a generally tubular shape having a longitudinal and
radial
direction. The two distal edges of the sheet define two ends of the tubular
shape and
meet or overlap.
[0046] In another embodiment, the sheet has a compacted form with a first
internal diameter and a deployed form with a second internal diameter larger
than the
first internal diameter such that the sheet contacts the lumen wall at a
radius equal to or
slightly larger than the radius of the lumen.
- 12 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
[0047] Another advantage of MTFN sheet 22 of the present invention is the
ability to control its surface characteristics by chemical treatment. In a
preferred
embodiment, the MTFN sheet 22 is treated in accordance with the methods
disclosed in
the '430 application, which includes removal of the film's native surface
oxide layer
with a buffered oxide etchant, followed by passivation in nitric acid (HNO3)
and
submersion in hydrogen peroxide (H202). This process produces a TiO layer
(e.g.,
100nm thick) and allows charged hydroxyl groups to attach to the surface as
confirmed
with high resolution transmission electron microscopy (HRTEM). The negative
charge
mimics the negative charge of the vascular endothelium and can be manipulated
to
facilitate rapid endothelialization (see FIGS. 10A-10C described in further
detail
below).
[0048] One tool to characterize the hydrophilicity of MTFN sheet 22 surface 44
is wetting angle. FIG. 6 shows the influence of treatment time on the wetting
angle of
the sheet 22. At the extremes, untreated TFN has a wetting contact angle of
65', whereas
the sheet 22 treated for 15 hours in H202 has a contact angle of 0 (i.e. a
super-
hydrophilic surface). This treatment modifies the surface characteristics
(i.e. negative
charge and TiO layer) to achieve contact angles ranging from 0 to 65', which
can be
used to vary the characteristics of the stent 20.
[0049] Another significant advantage of MTFN stent cover 22 is the ability to
precisely control permeability (i.e. porosity) and geometry. As discussed
earlier, the
deep reactive ion etching (DRIE) method disclosed in the '430 application may
be used
to produce relatively small fenestrations with high precision (tolerance of 1
micron or
less). Thus, fenestrations having the maximum dimensions of, for example, 25
microns
or less, or even 10 microns or less, to inhibit smooth muscle migration
through the
fenestrations is achievable.
- 13 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
[0050] Examples of four different fabricated MTFN sheets are shown in FIG. 7
to illustrate the wide variety of fenestration shapes that may be sized so as
to inhibit
smooth muscle cell migration yet enable fluid exchange to promote
endothelialization.
For example, a sheet 50 may comprise a plurality of oval slots 52, a sheet 60
may
comprise a pattern of circular holes 62, a sheet 70 may comprise thin diamond-
shaped
borders 72 separating the fenestrations in a "chain-link fence" fashion, and a
sheet 80
may comprise a plurality of diamond-shaped fenestrations 82.
[0051] The fenestrations may be aligned in precise regular arrays (i.e. 2
micron
resolution or less). This presents a unique advantage of MTFN sheet 22 over
ePTFE
and other biomaterials. For example, FIG. 8A is an optical microscopy image of
a sheet
90 having diamond-shaped fenestrations 92 with a longitudinal dimension of 10
microns across each fenestration 92 and a transverse dimension across each
fenestration
92 of 7.5 microns (7.5 gm x10 gm). Similarly, FIG. 8B is an optical microscopy
image
of a sheet 94 having diamond-shaped fenestrations 96 having dimensions of 45
gm x 60
gm. These patterns were used in the stent cover pilot studies discussed below.
Each
fenestration 92 is smaller than a smooth muscle cell (SMC) 32 (see FIG. 4),
allowing
the sheet 90 to act as a filter that prevents SMC migration onto the stent
luminal surface
and the resulting NIH, but still permits exchange of nutrients and cell-to-
cell signaling
molecules through fenestrations 92. Such aperture dimensions allow biologic
interactions to achieve improved outcomes.
[0052] The following discussion detail tests performed on the MTFN 22 of the
present invention experimentally correlate TFN's wetting contact angle to its
hemocompatibility and ability to support endothelial cell growth in vitro as
well as
neointimal growth in vivo. To demonstrate hemo-compatibility of the MTFN-based
stents of the present invention, a series of experiments were conducted.
Prototype stents
- 14 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
were fabricated using non-micropatterned TFN with the extreme contact angles
of 65
or 00. The resulting devices, along with an ePTFE control, were deployed in a
custom in
vitro model that circulates fresh whole blood simulating moderate arterial
stenosis.
Following testing, the three materials were analyzed qualitatively with
scanning
electron microscopy (SEM) and quantitatively via a series of molecular assays.
[0053] FIG. 9A through FIG. 9C are graphs of the results from molecular
analysis of the hemocompatibility of TFN as compared to ePTFE. In particular,
FIG.
9A shows the total thrombus deposition for the ePTFE control and the two TFN
examples whereas FIGS. 9B and 9C show fibrin and platelet deposition,
respectively,
for those devices. In the test, the devices were placed in a whole blood
circulation
model at a wall shear rate simulating a moderate arterial stenosis for 3
hours.
[0054] Both the 00 and the 65 degree TFN devices demonstrated markedly less
blood product as compared to the deposition on the ePTFE control device. In
the case
of platelets (FIG. 9C), the prototype ePTFE stent had more than two orders of
magnitude greater deposition than the prototype TFN devices. Scanning electron
microscopy (SEM) and mass spectrometry data confirmed all of these findings.
On
SEM, the ePTFE device was almost completely obscured by thrombus, while both
types
of TFN were clearly visible beneath a sparse covering of fibrin, platelets,
and RBCs.
Likewise, mass spectrometry confirmed the trends measured in FIGS. 9A through
FIG.
9C. For example, mass spectrometry analysis demonstrated approximately ten
times
more deposition of hemoglobin a and 0 chains on the ePTFE device than on
either TFN
device. This data strongly suggests that TFN has markedly improved
hemocompatibility
as compared to ePTFE, and that contact angle exerts significant effects on the
interaction with blood. Based on these results, it is believed that MTFN stent
20 of the
- 15 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
present disclosure will have a reduced incidence of both acute and late-term
thrombosis
as compared to ePTFE counterparts.
[0055] In addition, the MTFN surface wettability was studied for its effects
on
endothelial growth and neointimal architecture. A primary measure of an
indwelling
intravascular device's success is its ability to rapidly and completely
endothelialize with
a minimal amount of neointimal growth. As discussed with regard to FIG. 1,
this is a
significant limitation of ePTFE covered stents. To evaluate the correlation
between
contact angle and endothelialization, TFN with 3 different contact angles (00,
40 , and
65 ) was tested. In particular, Human Aortic Endothelial Cells (HAECs, Lonza,
Switzerland) were cultured on the TFN samples for 1, 3, and 7 days. After each
test
time, samples were stained with AlexaFluor 488 phalloidin (f-actin specific)
and DAPI
(nucleus).
[0056] Representative images of the effects of surface wettability and the
endothelial monolayer in vitro after 1 week are shown in FIG. 10A through FIG.
10C,
(N=3, p<0.05). These results indicate that TFN with a 40 contact angle
supports a
more confluent, and faster-growing endothelial monolayer than the 65 or 0
films.
[0057] In vivo data was also acquired showing the effects of contact angle on
the
healing response that follows endovascular device placement. For this study,
two non-
micropatterned TFN covered stents with a contact angle of either 0 or 65
were
fabricated and deployed in the iliac arteries of swine. After 30 days, devices
were
harvested, and sectioned for pathology. The 0 specimen demonstrated a
thinner, more
organized neointima with less inflammatory infiltrate as compared to the 65
device.
This data correlates well with the in vitro results, showing increased
endothelial growth
on the 0 thin films as compared to the 65 films.
- 16 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
[0058] Accordingly, an MTFN sheet 22 may be fabricated according to an
optimal contact angle (e.g. below at or 400) by controlling the processing
time (e.g.
treatment time within a hydrogen peroxide (H202) bath (see FIG. 6)) to support
rapid
growth of a functional endothelial monolayer on the MTFN device 20, while also
producing a surface that minimizes thrombus deposition (see FIG. 9A).
[0059] Micropattern pore size was also studied with respect to neointimal
thickness and abluminal SMC migration. Pilot studies were performed to examine
the
effects of MTFN perforation size (i.e. permeability) on SMC migration and
neointimal
growth in vivo. Three types of MTFN sheets were used for this study. Each
sheet had
diamond-shaped apertures with dimensions of 7.5 x 10 gm (sheet 90 of FIG. 8A),
10 x
20gm, and 45 x 60 gm (sheet 94 of FIG. 8B). MTFN covered stents were then
placed in
the iliac arteries of swine and harvested after 30 days.
[0060] FIG. 11 shows a representative image of a vessel wall 100 treated with
an MTFN stent cover 90 having 7.5 gm x10 gm perforations (FIG. 8A). The
neointima
102 is thin, well-organized, and does not extend into the vessel lumen 108
beyond the
level of the stent struts 28. FIG. 12 shows the contralateral iliac artery 104
of the same
animal treated with the stent cover 94 of FIG. 8B having 45 gm x 60 gm
perforations
(at the same magnification. The neointima 106 is thick, disorganized, and has
increased
numbers of inflammatory cells.
[0061] For quantitative comparison, FIG. 13 shows a graph of neointimal area
(NIA ¨ defined as the area between the MTFN stent cover and open vessel lumen
108)
for various fenestration sizes, ranging from 7.5 X 10 micron fenestrations to
45 X 60
microns fenestrations. FIG. 13 shows an increase in NIA with increasing MTFN
fenestration size. Note that the NIA (6.0 0.7mm2) for the 7.5 gm x10 gm device
is
substantially smaller than the NIA for an ePTFE covered stent (not
illustrated), which
- 17 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
showed an NIA of 11.9 4.3mm2 after 3 months of implantation in a sheep. On the
far
right of FIG. 13, NIA measurements are included for two non-micropatterned
films with
00 and 65 contact angles (N = 1 for the un-patterned devices). These results
demonstrate
that an absence of fenestrations leads to increased NIA, regardless of contact
angle (i.e.
similar to the relatively impermeable ePTFE devices), likely from a lack of
intercellular
communication between the layers of the vessel wall for a rapid, well-
organized healing
response.
[0062] This study demonstrated that SMCs 32 clearly migrate across the MTFN
barrier in devices with large fenestrations (e.g. 45 gm x 60 gm) but not in
devices with
small fenestrations (e.g. 7.5 gm x 10 gm). FIG. 14A through 14C show the wall
of an
artery treated with a 45 gm x 60 gm device at low magnification, medium
magnification, and high magnification, respectively. The fenestrations can be
seen as
small breaks in the MTFN stent cover. At the site of the fenestrations, robust
smooth
muscle cell migration is observed (marked by the black arrows, most notably in
FIG.
14B and FIG. 14C). These areas of cell migration appear as "mini-volcanoes,"
and
significant SMC migration from the abluminal side of the MTFN stent covers is
observed.
[0063] These images provide evidence of a "critical dimension" whereby SMC
migration across the MTFN is inhibited while a path for intercellular
communication is
still in place. Accordingly, the longitudinal and transverse dimensions for
the
fenestrations are ideally less than 10 gm, and preferably between 5 gm and 10
gm.
More generally, these dimensions should be less than 25 microns, and even more
generally should be less than or equal to a dimension that inhibits smooth
muscle
migration.
- 18 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
[0064] Based on these in vivo an MTFN covered stent designed with a "critical
dimension" for its fenestrations (e.g. less than 10 gm and ideally between
about 5 gm
and about 10 gm) will reduce NIH, while still providing a channel for
communication
between an endothelial layer on the device's luminal side with the underlying
vessel
wall in a manner not possible with ePTFE-based devices.
[0065] The effects of micropattern geometry on endothelial growth have also
been examined. For these studies, Human Aortic Endothelial Cells (HAECs) were
grown on MTFN with different geometries and allowed to proliferate for 3 days.
Samples were stained with DAPI and Phalloidin and imaged with a fluorescent
microscope. FIG. 15A shows HAECs grown on unpatterned TFN. Their rounded
"cobblestone" morphology is typical of ECs grown under normal culture
conditions.
FIGS. 15B (scale bar 100 gm) and 15C (scale bar 50 gm) show HAECs grown on
MTFN with a lattice pattern (similar to MTFN sheet 70 of FIG. 7). The lower-
left inset
shows the pore geometry used. The cells adopt an elongated morphology that
follows
the MTFN geometric designs, indicating that micropattern geometry regulates
endothelial morphology (e.g. elongate and/or faceted, straight-edged geometry
being
preferred). Of note, these results were obtained using TFN with a 650 contact
angle,
which explains why HAEC growth was not confluent given the data presented in
the
previous section, i.e. a wetting angle of 40 appears to be optimal.
Regardless, these
findings are significant when one considers the relationship between
endothelial
morphology and function. It is well established that arterial regions (such as
bifurcations) prone to atherosclerosis are exposed to low-oscillating shear
stress. ECs in
these regions adopt a round, "cobblestone" morphology and have increased
expression
of immune-regulating surface receptors. Conversely, "atheroprotected" regions
are
typically straight and exposed to high unidirectional shear stress. ECs found
here adopt
- 19 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
an elongated "spindle" morphology and have decreased expression of pro-
inflammatory
surface receptors. Based on these findings, an appropriately patterned MTFN
stent 20
of the present invention may serve as a scaffold to encourage an
atheroprotective EC
morphology in ways not possible with ePTFE-based stents.
[0066] The MTFN-based stents of the present invention address two main
problems associated with ePTFE covered stents: 1. Patency independent of
treated
lesion length, and 2. late-term graft thrombosis. Based on the above findings,
it is
believed that ePTFE's thickness causes a size mismatch with the vessel wall
that leads to
restenosis, and that the relatively impermeable ePTFE barrier prevents
communication
between the luminal neointima and abluminal vessel wall. This causes a failure
of
ePTFE grafts to endothelialize and creates a chronically exposed thrombogenic
surface
that predisposes patients to late-term thrombosis.
[0067] The MTFN-based stent of the present invention overcomes these
limitations in at least three ways. First, the ultra-low profile of the MTFN
stent 20 of the
present invention eliminates edge-effect stenosis and persistent flow
separation zones by
allowing for proximal and distal cell migration. Second, the MTFN stent 20 of
the
present invention has a porosity which can be controlled such that abluminal
SMC
migration is prevented, but still allows for intercellular communication
between
neointima and vessel wall throughout the length of the stent. Third, the MTFN
stent 20
of the present invention has surface characteristics and fenestration geometry
that can be
optimized to encourage growth of a non-thrombogenic, non-immunogenic
endothelial
layer on the stent's luminal surface that is in direct communication with the
underlying
vessel wall to maintain long-term patency.
[0068] Although the description above contains many details, these should not
be construed as limiting the scope of the invention but as merely providing
illustrations
- 20 -
CA 02908497 2015-09-29
WO 2014/131037 PCT/US2014/018410
of some of the presently preferred embodiments of this invention. Therefore,
it will be
appreciated that the scope of the present invention fully encompasses other
embodiments which may become obvious to those skilled in the art, and that the
scope
of the present invention is accordingly to be limited by nothing other than
the appended
claims, in which reference to an element in the singular is not intended to
mean "one
and only one" unless explicitly so stated, but rather "one or more." All
structural,
chemical, and functional equivalents to the elements of the above-described
preferred
embodiment that are known to those of ordinary skill in the art are expressly
incorporated herein by reference and are intended to be encompassed by the
present
claims. Moreover, it is not necessary for a device or method to address each
and every
problem sought to be solved by the present invention, for it to be encompassed
by the
present claims. Furthermore, no element, component, or method step in the
present
disclosure is intended to be dedicated to the public regardless of whether the
element,
component, or method step is explicitly recited in the claims. No claim
element herein is
to be construed under the provisions of 35 U.S.C. 112, sixth paragraph, unless
the
element is expressly recited using the phrase "means for."
-21 -