Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
.` CA 02910375 2015-10-23
DESCRIPTION
CATALYST AND ELECTRODE CATALYST LAYER FOR FUEL CELL HAVING THE
CATALYST
Technical Field
[0001]
The present invention relates to a catalyst and an electrode
catalyst layer for fuel cell having the catalyst.
Background Art
[0002]
A polymer electrolyte fuel cell using a proton conductive solid
polymer membrane operates at a low temperature in comparison to other
types of fuel cells, for example, a solid oxide fuel cell or a molten
carbonate fuel cell. For this reason, the polymer electrolyte fuel
cell has been expected to be used as a power source for energy storage
system or a driving power source for a vehicle such as a car, and
practical uses thereof have been started.
[0003]
In general, such a polymer electrolyte fuel cell uses expensive
noble metal catalyst represented by Pt (platinum) or a Pt alloy, which
leads to high cost of the fuel cell. Therefore, development of
techniques capable of lowering the cost of the fuel cell by reducing
a used amount of noble metal catalyst has been required.
[0004]
For example, Patent Literature 1 discloses the invention
relating to a catalyst for an air electrode of a solid polymer
electrolyte fuel cell where catalyst particles (alloy particles)
obtained by alloy formation of platinum and one supplementary metal
are supported on a carbon powder support. In this case, the catalyst
has features in that the supplementary metal is iron or cobalt and
¨ 1 ¨
CA 02910375 2015-10-23
a compounding ratio of the platinum and the supplementary metal is
in a range of 6 : 1 to 3 : 2 (molar ratio). According to the catalyst
disclosed in Patent Literature 1, iron or cobalt is selected as the
supplementary metal, and the platinum and the supplementary metal
are compounded with a predetermined compounding ratio, and a catalyst
activity is improved and a deterioration in characteristics caused
by infiltration of the supplementary metal into a polymer membrane
can be prevented.
[0005]
The catalyst disclosed in Patent Literature 1 has a
configuration where particles of a platinum/iron alloy or a
platinum/cobalt alloy are supported on the surface of fine carbon
powder. In this case, since the catalyst has a high catalyst activity,
an appropriate catalyst activity is exhibited in a three-phase
interface of a fuel cell. As a result, an amount of expensive
platinum used in the catalyst can be reduced, which contributes to
lowering the cost.
[0006]
In addition, the catalyst disclosed in Patent Literature 1 is
manufactured by immersing a fine carbon powder into a platinum
solution, performing reduction, then, immersing the resulting
product into an iron solution or a cobalt solution, and after that,
performing drying in 100% of hydrogen gas in Example.
Citation List
Patent Literature
[0007]
Patent Literature 1: Japanese Patent Application Laid-Open No.
2003-142112
Summary of Invention
[0008]
¨ 2 ¨
CA 02910375 2015-10-23
However, in the catalyst disclosed in Patent Literature 1,
there are problems in that it is difficult to obtain the catalyst
where the alloy particles with a desired composition are supported
inside the support, and the catalyst activity is not sufficient.
[0009]
The present invention is to provide a catalyst where alloy
particles with a desired composition are supported inside a support.
[0010]
The present inventors had studied hard. As a result, they
found out that the problems were able to be solved by controlling
a size of the pores in which the catalyst metals were supported, so
that the present invention was completed.
Brief Description of Drawings
[0011]
Fig. 1 is a schematic cross-sectional diagram illustrating a
basic configuration of a solid polymer electrolyte fuel cell
according to an embodiment of the present invention.
Fig. 2 is a schematic cross-sectional explanation diagram
illustrating a shape and a structure of a catalyst according to an
embodiment of the present invention.
Fig. 3 is a schematic diagram illustrating a relationship
between a catalyst and an electrolyte in a catalyst layer according
to an embodiment of the present invention.
Description of Embodiments
.. [0012]
According to the present invention, by controlling a size of
pores in which catalyst metals are supported, metals constituting
alloy particles appropriately enter into a support, so that the alloy
particles with a desired composition can be supported inside the
support. Therefore, a catalyst having a high catalyst activity can
¨ 3 ¨
CA 02910375 2015-10-23
be obtained.
[0013]
A catalyst (in this specification, sometimes referred to as
an "electrode catalyst") according to the present invention is
configured to include a support and alloy particles supported on the
support. In this case, the catalyst satisfies the following
configurations (a) to (d) :
(a) the alloy particle is an alloy of platinum and a metal other
than the platinum;
(b) the catalyst contains mesopores having a radius of 1 to
10 nm originated from the support;
(c) a mode radius of the mesopores is in a range of 2.5 to 10
nm; and
(d) the alloy particles have a catalyst function, and at least
a portion of the alloy particles is supported inside the mesopores.
[0014]
Herein, the "mesopore" denotes a pore of which radius is within
a range of 1 to 10 nm among the pores contained in the catalyst. In
addition, the catalyst may also has the pores that are not classified
as the mesopores, that is, the pores having a radius of less than
1 nm, and the pores having a radius of more than 10 nm.
[0015]
The present inventors recognize that, in the case where the
alloy particles are supported on the surface of the support like the
related art as described above-in Patent Literature 1, the alloy
particles are abraded or dislocated. On the other hand, the
inventors recognize that, when the alloy particles are intended to
be supported inside the support, it is difficult to support the alloy
particles with a desired composition inside the support.
[0016]
¨ 4 ¨
CA 02910375 2015-10-23
On the contrary, the present inventors recognize that, by using
alloy particles satisfying the configuration (a) and controlling so
that the catalyst satisfies the configurations (b) and (c), the alloy
particles with a desired composition are supported inside the
mesopores (namely, the configuration (d) is satisfied). Therefore,
the catalyst where the alloy particles with a desired composition
are supported inside the support can be obtained.
[0017]
For example, in the case of supporting the platinum on a support
and, after that, supporting metals other than the platinum like
Patent Literature 1, there is a tendency in that the metals other
than the platinum are difficult to enter into the support. In this
case, a small amount of metals other than the platinum exists in the
alloy formation, and thus, a desired alloy-formation ratio cannot
be obtained, so that a desired composition of the alloy particles
cannot be obtained. As a result, an excellent catalyst activity
cannot be obtained, and a used amount of the platinum is also increased.
However, it is recognized that, if the support capable of satisfying
the configurations (b) and (c) is used, the metals other than the
platinum can appropriately enter into the support which supports the
platinum. As a result, a desired alloy-formation ratio is achieved,
so that the alloy particles with a desired composition can be
obtained.
[0018]
Since the alloy particles satisfying the configuration (a)
which is obtained in this manner exhibit an excellent catalytic
activity as disclosed in Patent Literature 1, the used amount of
platinum can be reduced.
[0019]
In addition, according to the study of the present inventors,
¨ 5 ¨
CA 02910375 2015-10-23
it is recognized that, if the configuration (d) is satisfied, the
alloy particles exhibit an excellent catalytic activity in
comparison with the case where the alloy particles are supported on
the surface of the support. Specifically, in the case where the alloy
particles are supported on the surface of the support, an electrolyte
(electrolyte polymer) is easily adsorbed to the surfaces of the alloy
particles in comparison with a gas such as oxygen. In addition, if
the alloy particles are in contact with the electrolyte (electrolyte
polymer) , a reaction active area of the surface is decreased, so that
the catalyst activity is relatively decreased. On the contrary,
since the electrolyte cannot enter into the mesopores, the decrease
in reaction active area due to the adsorption of the electrolyte can
be prevented by supporting the alloy particles inside the support.
In addition, as for a three-phase interface, water existing inside
the fuel cell or being likely to be generated from the fuel cell plays
the role, so that the alloy particles existing inside the support
can be effectively used.
[0020]
Heretofore, the catalyst according to the present invention
can support the alloy particles with a desired composition inside
the support. By doing so, (1) it is possible to prevent the alloy
particles from being abraded or dislocated, (2) it is possible to
improve a reaction activity of the alloy particles, and (3) it is
possible to reduce a used amount of the platinum. As a result, the
catalyst according to the present invention can realize lowering the
cost of a fuel cell. In addition, the catalyst has an excellent
durability.
[0021]
Hereinafter, embodiments of the present invention will be
described with reference to the drawings. However, the scope of the
¨ 6 ¨
CA 02910375 2015-10-23
present invention should be defined based on the Claims, and not
limited to only the embodiment described below. In addition, in the
figures, scaling factors are exaggerated for the convenience of
description, and thus, the scaling factors may be different from
actual factors.
[0022]
[Fuel Cell]
A fuel cell comprises a membrane electrode assembly (MEA) and
a pair of separators including an anode-side separator having a fuel
gas passage through which a fuel gas flows and a cathode-side
separator having an oxidant gas passage through which an oxidant gas
flows. The fuel cell according to the present embodiment has
excellent durability and can exhibit a high power generation
performance.
[0023]
Fig. 1 is a schematic diagram illustrating a basic
configuration of a polymer electrolyte fuel cell (PEFC) 1 according
to an embodiment of the present invention. First, a PEFC 1 is
configured to include a solid polymer electrolyte membrane 2 and a
pair of catalyst layers (anode catalyst layer 3a and cathode catalyst
layer 3c) interposing the solid polymer electrolyte membrane 2. A
stacked body of the solid polymer electrolyte membrane 2 and the
catalyst layers (3a, 3c) is sandwiched by a pair of gas diffusion
layers (GDLs) (anode gas diffusion layer 4a and cathode gas diffusion
layer 4c) . In this manner, the solid polymer electrolyte membrane
2, a pair of the catalyst layers (3a, 3c), and a pair of gas diffusion
layers (4a, 4c) in the stacked state constitute a membrane electrode
assembly (MEA) 10.
[0024]
In the PEFC 1, the MEA 10 is sandwiched by a pair of separators
¨ 7 ¨
CA 02910375 2015-10-23
(anode separator 5a and cathode separator 5c). In Fig. 1, the
separators (5a, 5c) are illustrated to be positioned at two ends of
the MEA 10 illustrated. In general, in a fuel cell stack where a
plurality of MEAs are stacked, the separator is also used as a
separator for adjacent PEFC (not shown). In other words, MEAs in
a fuel cell stack are sequentially stacked through the separator to
constitute the stack. In an actual fuel cell stack, a gas sealing
member is disposed between the separators (3a, 5c) and the solid
polymer electrolyte membrane 2 and between the PEFC 1 and a different
PEFC adjacent thereto. However, it is omitted in Fig. 1.
[0025]
The separators (5a, 5c) are obtained by applying a pressing
process to a thin board having a thickness of, for example, 0.5 mm
or less to form a corrugating shape illustrated in Fig. 1. Convex
portions of the separators 5a and 5c seen from the MEA side are in
contact with the MEA 10. This secures an electrical connection with
the MEA 10. Concave portions (spaces between the separator and the
MEA formed by the corrugating shapes of the separators) of the
separators (5a and 5c) seen from the MEA side function as a gas passage
for passing a gas during the operation of the PEFC 1. Specifically,
a fuel gas (for example, hydrogen) flows through a gas passage 6a
of the anode separator 5a, and an oxidant gas (for example, air) flows
through a gas passage 6c of the cathode separator 5c.
[0026]
On the other hand, concave portions of the separators (5a, 5c)
seen from the side opposite to the MEA side function as a coolant
passage 7 for passing a coolant (e.g. water) for cooling the PEFC
during the operation of the PEFC 1. In addition, manifolds (not
shown) are typically installed in the separators. The manifold
functions as a connecting means for connecting cells when the stack
¨ 8 ¨
CA 02910375 2015-10-23
is configured. According to the configuration, a mechanical
strength of the fuel cell stack can be secured.
[0027]
In the embodiment illustrated in Fig. 1, each of the separators
(5a, 5c) is formed in a corrugating shape. However, the separator
is not limited to such a corrugating shape. If it can serve as a
gas passage and a coolant passage, arbitrary shape such as a flat
shape and a partially corrugating shape may be employed.
[0028]
The fuel cell including the MEA according to the present
invention as described above has excellent performance of power
generation. Herein, the type of the fuel cell is not particularly
limited. In the above description, the polymer electrolyte fuel cell
is exemplified, but besides, an alkali fuel cell, a direct methanol
fuel cell, a micro fuel cell, and the like maybe exemplified. Among
the fuel cells, due to a small size and capability of obtaining high
density and high power, a polymer electrolyte fuel cell (2EFC) is
preferred. In addition, the fuel cell is useful as a power source
for energy storage system besides a power source for a vehicle such
as a car where amounting space is limited. Among the power sources,
the fuel cell is particularly preferably used as a power source for
a vehicle such as a car where a high output voltage is required after
the stopping of operation for a relatively long time.
[0029]
A fuel used for operating the fuel cell is not particularly
limited. For example, hydrogen, methanol, ethanol, 1-propanol,
2-propanol, 1-butanol, secondary butanol, tertiary butanol,
dimethyl ether, diethyl ether, ethylene glycol, diethylene glycol,
or the like can be used. Among them, in view of capability of high
output, hydrogen or methanol is preferably used.
¨ 9 ¨
CA 02910375 2015-10-23
[0030]
In addition, although application use of the fuel cell is not
particularly limited, the fuel cell is preferably applied to vehicles.
The electrolyte membrane-electrode assembly according to the present
invention has excellent power generation performance and durability,
and can be downsized. Therefore, in terms of mountability on a
vehicle, the fuel cell according to the present invention is
particularly advantageous in the case where the fuel cell is applied
to a vehicle.
[0031]
Hereinafter, members constituting the fuel cell according to
the present embodiment will be described in brief, but the scope of
the present invention is not limited only to the following forms.
[0032]
[Catalyst (Electrode Catalyst)]
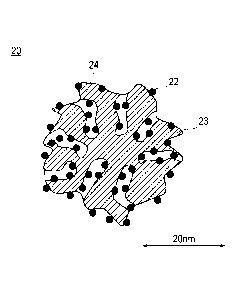
Fig. 2 is a schematic cross-sectional diagram illustrating a
shape and a structure of a catalyst according to an embodiment of
the present invention. As illustrated in Fig. 2, a catalyst 20
according to the embodiment consists of alloy particles 22 and
supports 23. In addition, the catalyst 20 has pores (mesopores) 24
having a radius of 1 to 10 nm originated from the supports. In this
case, a mode radius of the mesopores is in a range of 2.5 to 10 nm.
In addition, the alloy particles 22 include an alloy containing
platinum and metal components other than the platinum. Herein, the
alloy particles 22 are supported inside the mesopores 24. In
addition, at least a portion of the alloy particles 22 is supported
inside the mesopores 24, and a portion thereof may be supported on
the surfaces of the supports 23. However, in terms of preventing
the electrolyte and the alloy particles in the catalyst layer from
being in contact with each other, it is preferable that substantially
¨ 10 ¨
CA 02910375 2015-10-23
all the alloy particles 22 are supported inside the mesopores 24.
Herein, the amount of "substantially all the alloy particles" is not
particularly limited if the amount can improve a sufficient catalyst
activity. The amount of "substantially all the alloy particles" is
preferably 50 wt% or more (upper limit: 100 wt%) with respect to all
the alloy particles, more preferably 80 wt% or more (upper limit:
100 wt%) .
[0033]
In this specification, "the alloy particles are supported
inside the mesopores" can be recognized by a decrease in pore volume
of the mesopores before and after the supporting of the alloy
particles on the support. Specifically, a support has mesopores at
a certain pore volume, and if the alloy particles are supported inside
the mesopores, the pore volume of the mesopores is decreased.
Therefore, the case where a difference [= (pore volume of the
mesopores before supporting) - (pore volume of the mesopores after
supporting) ] between the pore volume of the mesopores of the support
before the supporting of the alloy particles and the pore volume of
the mesopores of the support after the supporting of the alloy
particles exceeds 0 corresponds to "the alloy particles are supported
inside the mesopores" . In terms of the reduction in the gas transport
resistance and the securing of the path for gas transportation, the
decreased value of the pore volume of the mesopores before and after
the supporting of the alloy particles is preferably 0.02 cc/g or more,
more preferably in a range of 0.02 to 0.21 cc/g.
[0034]
In an embodiment of the present invention, the radius of the
mesopores originated from the support of the catalyst (after the
supporting of the alloy particles) is in a range of 1 to 10 nm,
preferably in a range of 2.5 to 10 nm, more preferably in a range
¨ 11 ¨
CA 02910375 2015-10-23
of 5 to 10 nm. By controlling so that the mesopores has the radius
as described above, the alloy particles or the metals constituting
the alloy particles appropriately enter into the support during the
manufacturing, and the alloy particles with a desired composition
can be obtained.
[0035]
In addition, the maximum frequent diameter (in the present
invention, simply referred to as a "mode radius of the mesopores")
of the pore distribution of the mesopores originated from the
supports of the catalyst (after the supporting of the alloy
particles) is in a range of 2.5 to 10 nm, preferably in a range of
3 to 10 nm, more preferably in a range of 5 to 10 nm. If the mode
radius of the mesopores is controlled so as to be within such a range,
a sufficient number of the alloy particles can be stored (supported),
and thus the electrolyte and the alloy particles in the catalyst layer
are physically separated from each other (contact of the alloy
particles and the electrolyte can be more effectively suppressed and
prevented). Therefore, the activity of the alloy particles can be
more effectively used. In addition, due to existence of a large
volume of the mesopores, the function and effect of the present
invention are further remarkably exhibited, so that a catalyst
reaction can be more effectively facilitated.
[0036]
A pore volume of the mesopores of the support of the catalyst
(after the supporting of the alloy particles) (in the present
invention, simply referred to as a "pore volume of the mesopores")
is not particularly limited, but it is preferably 0.6 cc/g support
or more, more preferably in a range of 0.6 to 3 cc/g support, even
more preferably in a range of 0.6 to 1.5 cc/g support. If the pore
volume is 0.6 cc/g support or more, a large number of the alloy
¨ 12 ¨
CA 02910375 2015-10-23
particles can be stored in (supported by) the mesopores, and thus,
the electrolyte and the alloy particles in the catalyst layer are
physically separated from each other (contact of the alloy particles
and the electrolyte can be more effectively suppressed and prevented) .
Therefore, the activity of the alloy particles can be more
effectively used. In addition, due to existence of a large number
of the mesopores, the function and effect of the present invention
are further remarkably exhibited, so that a catalyst reaction can
be more effectively facilitated.
[0037]
The BET specific surface area (BET specific surface area (m2/g
support) of the catalyst per 1 g of support) of the catalyst (after
supporting of the alloy particles) is not particularly limited, but
it is preferably 1000 m2/g support or more, more preferably in a range
of 1000 to 3000 m2/g support, even more preferably in a range of 1100
to 1800 m2/g support. If the specific surface area is within such
a range, a large number of the alloy particles can be stored in
(supported by) the mesopores. In addition, the electrolyte and the
alloy particles in the catalyst layer are physically separated from
each other (contact of the alloy particles and the electrolyte can
be more effectively suppressed and prevented). Therefore, the
activity of the alloy particles can be more effectively used. In
addition, due to existence of a large number of the mesopores or a
large number of other pores, the function and effect of the present
invention are further remarkably exhibited, so that a catalyst
reaction can be more effectively facilitated.
[0038]
In addition, in this specification, the "BET specific surface
area (m2/g support)" of the catalyst is measured by a nitrogen
adsorption method. More specifically, about 0.04 to 0.07 g of the
¨ 13 ¨
CA 02910375 2015-10-23
catalyst powder is accurately weighed and enclosed in a sample tube.
The sample tube is preliminarily dried by a vacuum drier at 90 C for
several hours, and a sample for measurement is obtained. For the
weighing, an electronic balance (A1220) produced by Shimadzu Co.,
Ltd. is used. In addition, in case of a coated sheet, about 0.03
to 0.04 g of a net weight of a coat layer obtained by subtracting
a weight of Teflon (registered trademark) (substrate) having the same
area from a total weight of the coated sheet is used as the sample
weight. Next, in the following measurement condition, the BET
specific surface area is measured. In an adsorption side of
adsorption and desorption isotherms, a BET plot is produced from a
relative pressure (P/PO) range of about 0.00 to 0.45, and the surface
area and the BET specific surface area are calculated from the slope
and the intercept.
[0039]
[Chem. 1]
< Measurement Condition >
Measurement Apparatus : BELSOROP 36, High - Precession Automatic Gas
Adsorption Apparatus produced by
BEL Japan, Inc.
Adsorption Gas : N2
Dead Volume Measurement Gas : He
Adsorption Temperature : 77 K (Liquid Nitrogen Temperature)
Measurement Preparation : 90 C, Several hours in Vacuum Drier (After He
Purging, Setting on Measurement Stage)
Measurement Mode : Adsorption Process and Desorption Process in Isotherm
Measurement Relative Pressure P/Po : about 0 to 0.99
Equilibrium Setting Time :180 sec for 1 relative pressure
[0040]
The "pore radius (nm) of the mesopores" denotes a radius of
the pores measured by a nitrogen adsorption method (DH method). In
addition, the "mode radius (rim) of the pore distribution of the
mesopores" denotes a pore radius taking a peak value (maximum
frequency) in a differential pore distribution curve obtained by the
nitrogen adsorption method (DH method).
[0041]
¨ 14 ¨
CA 02910375 2015-10-23
The "pore volume of the mesopores" denotes a total volume of
the mesopores having a radius of 1 to 10 nm of the support existing
in the catalyst and is expressed by volume (cc/g support) per 1 g
of support. The "pore volume of the mesopores (cc/g support)" is
calculated as an area (integration value) under a differential pore
distribution curve obtained according to a nitrogen adsorption
method (DH method).
[0042]
The "differential pore distribution" is a distribution curve
where a pore diameter is plotted in the horizontal axis and a pore
volume corresponding to the pore diameter in the catalyst is plotted
in the vertical axis. Namely, when the pore volume of the catalyst
obtained by the nitrogen adsorption method (DH method) is denoted
by V and the pore diameter is denoted by D, a value (dV/d(logD)) is
obtained by dividing the differential pore volume dV by a
differential logarithm of the pore diameter. Next, the differential
pore distribution curve is obtained by plotting the dV/d(logD) for
the average pore diameter of each section. The differential pore
volume dV denotes an increment of pore volume between measurement
points.
[0043]
In this specification, the measurement methods of the radius
and pore volume of the mesopores in accordance with the nitrogen
adsorption method (DH method) are not particularly limited. For
example, methods disclosed in well-known literatures such as
"Science of Adsorption" (second edition written by Kondo Seiichi,
Ishikawa Tatsuo, and Abe Ikuo, Maruzen Co., Ltd.), "Fuel Cell
Analysis Method" (compiled by Takasu Yoshio, Yoshitake Yu, and
Ishihara Tatsumi of KAGAKU DCJIN), and an article by D. Dollion and
G. R. Heal in J. Appl. Chem. 14, 109 (1964) maybe employed. In this
¨ 15 ¨
CA 02910375 2015-10-23
specification, the radius and pore volume of the mesopores in
accordance with the nitrogen adsorption method (DH method) are values
measured according to the method disclosed in the article written
by D. DoIlion and G. R. Heal in J. Appl. Chem. 14, 109 (1964) .
[0044]
The method of manufacturing the catalyst having a specific pore
distribution described above is not particularly limited, but in
general it is important to set the pore distribution of the mesopores
of the support to the above-described pore distribution (that is,
the same pore distribution as that of the catalyst) . Specifically,
as the method of manufacturing the support having the mesopores
having a radius of 1 to 10 nm wherein the mode radius of the mesopores
is in a range of 2.5 to 10 nm, the method disclosed in Japanese Patent
Application Publication No. 2010-208887 (US Patent Application
Publication No. 2011/0318254) or the like is preferably used. In
addition, as the method of manufacturing the support where the pore
volume of the mesopores is controlled to be 0.6 cc/g support or more,
the method disclosed in Japanese Patent Application Publication No.
2010-208887 (US Patent Application Publication No. 2011/0318254) or
the like is preferably used.
[0045]
A material of the support is not particularly limited if pores
(primary pores) having mesopores of a radius of 1 to 10 nm and a mode
radius of the mesopores in a range of 2.5 to 10 nm can be formed inside
the support and if the support has enough specific surface area and
enough electron conductivity to support a catalyst component inside
the mesopores in a dispersed state. Preferably, a main component
is carbon. Specifically, carbon particles made of carbon black
(Ketjen Black, oil furnace black, channel black, lamp black, thermal
black, acetylene black, or the like) , activated charcoal, or the like
¨ 16 ¨
CA 02910375 2015-10-23
may be exemplified. The expression "main component is carbon"
denotes that the support contains carbon atoms as a main component,
and includes both of the configurations that the support consists
only of carbon atoms and that the support substantially consists of
carbon atoms. An element (s) other than carbon atom may be contained.
The expression "substantially consists of carbon atoms" denotes that
impurities of about 2 to 3 wt% or less can be contaminated.
[0046]
More preferably, since it is easy to form a desired pore space
inside the support, carbon black is used, and more preferably, carbon
manufactured according to the method disclosed in Japanese Patent
Application Publication No. 2010-208887 (US Patent Application
Publication No. 2011/0318254) or the like is used. For example, the
radius or mode radius of the mesopores of the support and the pore
volume of the mesopores can be controlled by changing a diameter of
template particles such as magnesium oxide used for manufacturing
the support or a type of resin.
[0047]
Besides the aforementioned carbon materials, a porous metal
such as Sn (tin) or Ti (titanium) or a conductive metal oxide can
also be used as the support.
[0048]
The BET specific surface area of the support may be a specific
surface area enough to highly dispersedly support the catalyst
component. The BET specific surface area of the support is
substantially equivalent to the BET specific surface area of the
catalyst. The BET specific surface area of the support is preferably
in a range of 1000 to 3000 m2/g, more preferably in a range of 1100
to 1800 m2/g. If the specific surface area is within such a range,
a sufficient number of the mesopores can be secured, and thus, a large
¨ 17 ¨
CA 02910375 2015-10-23
number of the alloy particles can be stored in (supported by) the
mesopores. In addition, the electrolyte and the alloy particles in
the catalyst layer are physically separated from each other (contact
of the alloy particles and the electrolyte can be more effectively
suppressed and prevented). Therefore, the activity of the alloy
particles can be more effectively used. In addition, the balance
between dispersibility of the catalyst component and an effective
utilization rate of the catalyst component on the catalyst support
can be appropriately controlled.
[0049]
An average particle diameter of the support is preferably in
a range of 20 to 100 nm. If the average primary particle diameter
is within such a range, even in the case where the above-described
pore structure is formed in the support, mechanical strength can be
maintained, and a catalyst layer can be controlled within an
appropriate range. As a value of the "average particle diameter of
a support", unless otherwise noted, a value calculated as an average
value of particle diameters of particles observed within several or
several tens of fields by using observation means such as a scanning
electron microscope (SEM) or a transmission electron microscope
(TEM) is employed. In addition, the "particle diameter" denotes a
maximum distance among distances between arbitrary two points on an
outline of a particle.
[0050]
In the present invention, there is no need to use the
above-described granular porous support, so long as the support has
the above-described pore distributions of mesopores in the catalyst.
[0051]
Namely, as the support, a non-porous conductive support,
nonwoven fabric, carbon paper, carbon cloth, or the like made of
¨ 18 ¨
CA 02910375 2015-10-23
carbon fiber constituting a gas diffusion layer, or the like may be
exemplified. In this case, the catalyst can be supported on the
non-porous conductive support or can be directly attached to the
nonwoven fabric, the carbon paper, the carbon cloth, or the like made
of the carbon fiber constituting the gas diffusion layer of the
membrane electrode assembly.
[0052]
An alloy particle which can be used in the present invention
performs catalysis of electrochemical reaction. As an alloy
particle used for an anode catalyst layer, a well-known catalyst can
be used in a similar manner without particular limitation if the
catalyst has catalytic effects on oxidation reaction of hydrogen.
In addition, as an alloy particle used for a cathode catalyst layer,
a well-known catalyst can be used in a similar manner without
particular limitation if the catalyst has catalytic effects on
reduction reaction of oxygen. In general, an alloy is obtained by
mixing a metal element with at least one metal element or non-metal
element, and is a general term for substances having metallic
properties. The structure of the alloy includes an eutectic alloy
which is a mixture where component elements form separate crystals,
an alloy where component elements are completely fused to form a solid
solution, an alloy where component elements form a intermetallic
compound or a compound between a metal and a non-metal, and the like,
and any one thereof may be employed in the present application.
[0053]
The alloy particle which can be used in the present invention
is an alloy including platinum and a metal other than the platinum.
In this case, the metal other than the platinum is not particularly
limited, but ruthenium, iridium, rhodium, palladium, osmium,
tungsten, lead, iron, copper, silver, chromium, cobalt, nickel,
¨ 19 ¨
CA 02910375 2015-10-23
manganese, vanadium, molybdenum, gallium, aluminum, or the like may
be exemplified.
[0054]
As disclosed in Patent Literature 1, such alloy particles can
exhibit a high activity. The composition of the alloy is preferably
in a range of 4 : 1 to 1 : 1 (molar ratio), more preferably in a range
of 3 : 1 to 1 : 1 (molar ratio). If the compounding ratio is within
such a range, a high catalytic activity can be exhibited while
reducing the platinum content, so that it is possible to lower the
cost of a fuel cell.
[0055]
Together with the alloy particles, a different catalyst of
alloy particles such as platinum, ruthenium, iridium, rhodium,
palladium, osmium, tungsten, lead, iron, copper, silver, chromium,
cobalt, nickel, manganese, vanadium, molybdenum, gallium, or
aluminum and an alloy thereof (excluding the aforementioned alloy
particles) can be simultaneously used.
[0056]
The shape and size of the alloy particles or the different
catalyst (catalyst component) are not particularly limited, but the
shapes and sizes of well-known catalyst components may be employed.
As the shape, for example, a granular shape, a squamous shape, a
laminar shape, or the like may be used, but the granular shape is
preferred.
[0057]
The average particle diameter of the alloy particles is not
particularly limited, but it is preferably 3 nm or more, more
preferably more than 3 nm and 30 nm or less, even more preferably
more than 3 nm and 10 nm or less. If the average particle diameter
of the alloy particles is 3 nm or more, the alloy particles are
¨ 20 -
CA 02910375 2015-10-23
relatively strongly supported inside the mesopores, so that contact
with the electrolyte in the catalyst layer is more effectively
suppressed and prevented. In addition, elution according to a change
in voltage can be prevented, and degradation in performance overtime
can be also suppressed. Therefore, the catalyst activity can be
further improved, and namely, the catalyst reaction can be more
efficiently facilitated. On the other hand, if the average particle
diameter of the alloy particles is 30 nm or less, the alloy particles
can be supported inside the mesopores of the supports by a simple
method, so that a covering ratio of the electrolyte on the alloy
particles can be reduced. In addition, in the present invention,
the "average particle diameter of the alloy particles" can be
measured as an average value of a crystallite diameter obtained from
a half-value width of a diffraction peak of the alloy particle
component in the X-ray diffraction spectroscopy or as an average
value of a particle diameter of the alloy particles examined from
a transmission electron microscope (TEM) image.
[0058]
In this embodiment, the catalyst content (mg/cm2) per unit
catalyst-coated area is not particularly limited if a sufficient
degree of dispersion of the catalyst on the support and a power
generation performance are obtained. For example, the catalyst
content is in a range of 0.01 to 1 mg/cm. In addition, the platinum
content per unit catalyst-coated area is preferably 0.5 mg/cm2 or
less. The usage of expensive noble-metal catalyst represented by
platinum constituting the alloy particles results in a high price
of the fuel cell. Therefore, it is preferable that the cost be
reduced by decreasing the used amount (platinum content) of the
expensive platinum down to the above-described range. The lower
limit value is not particularly limited if the power generation
¨ 21 ¨
CA 02910375 2015-10-23
performance is obtained, and for example, the lower limit value is
0.01 mg/cm2 or more. The content of the platinum is more preferably
in a range of 0.02 to 0.4 mg/cm2. In this embodiment, since the alloy
particles having a high activity can be used. and an activity per
catalyst weight can be improved by controlling the pore structure
of the support, the used amount of the expensive catalyst can be
reduced.
[0059]
In addition, in this specification, an inductively coupled
plasma emission spectroscopy (ICP) is used for measurement
(determination) of a "content (mg/cm2) of catalyst (platinum) per
unit catalyst-coated area". A method of obtaining a desired "content
(mg/cm2) of catalyst (platinum) per unit catalyst-coated area" is
also easily performed by the skilled in the art, and the amount can
be adjusted by controlling slurry composition (catalyst
concentration) and the coated amount.
[0060]
In addition, the supported amount (in some cases, referred to
as a support ratio) of the alloy particles on the support is preferably
40 wt% or less with respect to a total amount of the supported catalyst
body (that is, the support and the alloy particles) , more preferably
in a range of 20 to 30 wt%. In the art, if the supported concentration
of the alloy particles is decreased, there is a tendency for the alloy
formation to be difficult to proceed. However, in the catalyst
according to the embodiment, even in the case where the supported
amount of the alloy particles is such a small as 40 wt% or less, the
alloy formation can be appropriately promoted. Therefore, it is
possible to provide a catalyst having a small supported amount.
[0061]
[Catalyst Layer]
¨ 22 ¨
CA 02910375 2015-10-23
The catalyst according to the present invention can be
appropriately used for an electrode catalyst layer for fuel cell.
Namely, the present invention also provides an electrode catalyst
layer for fuel cell including the catalyst according to the present
invention and an electrolyte.
[0062]
Fig. 3 is a schematic diagram illustrating a relationship
between a catalyst and an electrolyte in a catalyst layer according
to an embodiment of the present invention. As illustrated in Fig.
3, in the catalyst layer according to the present invention, the
catalyst is covered with the electrolyte 25, but the electrolyte 25
does not enter into the mesopores 24 of the catalyst (support 23).
Therefore, although the alloy particles 22 on the surfaces of the
supports 23 are in contact with the electrolyte 25, the alloy
particles 22 supported in the mesopores 24 are not in contact with
the electrolyte 25. The alloy particles in the mesopores form a
three-phase interface with respect to an oxygen gas and water in the
state that the alloy particles are not in contact with the electrolyte,
so that a reaction active area of the alloy particles can be secured.
[0063]
Although the catalyst according to the present invention may
exist either in a cathode catalyst layer or an anode catalyst layer,
the catalyst is preferably used in a cathode catalyst layer. As
described above, although the catalyst according to the present
invention is not in contact with the electrolyte, the catalyst can
be effectively used by forming three-phase interface of the catalyst
and water. This is because water is formed in the cathode catalyst
layer.
[0064]
An electrolyte is not particularly limited, but it is
¨ 23 ¨
CA 02910375 2015-10-23
preferably an ion-conductive polymer electrolyte. Since the
polymer electrolyte serves to transfer protons generated in the
vicinity of the catalyst active material on a fuel electrode side,
the polymer electrolyte is also referred to as a proton conductive
polymer.
[0065]
The polymer electrolyte is not particularly limited, but
well-known knowledge in the art can be appropriately referred to.
The polymer electrolytes are mainly classified into fluorine-based
polymer electrolytes and hydrocarbon-based polymer electrolytes
depending on a type of an ion-exchange resin as a constituent
material.
[0066]
As an ion-exchange resin constituting the fluorine-based
polymer electrolyte, for example, perfluorocarbon sulfonic acid
based polymers such as Nafion (registered trademark, produced by
DuPont), Aciplex (registered trademark, produced by Asahi Kasei Co.,
Ltd.), and Flemion (registered trademark, produced by Asahi Glass
Co., Ltd.), perfluorocarbon phosphoric acid based polymers,
trifluorostyrene sulfonic acid based polymers, ethylene
tetrafluoroethylene-g-styrene sulfonic acid based polymers,
ethylene-tetrafluoroethylene copolymers,
polyvinylidene
fluoride-perfluorocarbon sulfonic acid based polymers, and the like
may be exemplified. In terms excellent heat resistance, chemical
stability, durability, and mechanical strength, the fluorine-based
polymer electrolyte is preferably used, and a fluorine-based polymer
electrolyte formed of a perfluorocarbon sulfonic acid based polymer
is particularly preferably used.
[0067]
As a hydrocarbon-based electrolyte, sulfonated polyether
¨ 24 ¨
CA 02910375 2015-10-23
sulfones (S-PES), sulfonated polyaryl ether ketones, sulfonated
polybenzimidazole alkyls, phosphonated polybenzimidazole alkyls,
asulfonated polystyrenes, sulfonated polyether ether ketones
(S-PEEK), sulfonated polyphenylenes (S-PPP), and the like may be
exemplified. In terms
of manufacturing advantages such as
inexpensive raw materials, simple manufacturing processes, and high
selectivity of materials, a hydrocarbon-based polymer electrolyte
is preferably used.
[0068]
These ion-exchange resins may be singly used, or two or more
resins may be used together. In addition, the material is not limited
to the above-described material, but another material may be used.
[0069]
With respect to the polymer electrolyte which serves to
transfer protons, proton conductivity is important. In the case
where EW of a polymer electrolyte is too large, ion conductivity with
in the entire catalyst layer would be decreased. Therefore, the
catalyst layer according to the embodiment preferably includes a
polymer electrolyte having a small EW. Specifically, catalyst layer
according to the embodiment preferably includes a polymer
electrolyte having an EW of 1500 g/eq. or less, more preferably
includes a polymer electrolyte having an EW of 1200 g/eq. or less,
and particularly preferably includes a polymer electrolyte having
an EW of 1000 g/eq. or less.
[0070]
On the other hand, in the case where the EW is too small, since
hydrophilicity is too high, water is hard to smoothly move. Due to
such a point of view, the EW of polymer electrolyte is preferably
600 or more. The EW (Equivalent Weight) represents an equivalent
weight of an exchange group having proton conductivity. The
¨ 25 ¨
CA 02910375 2015-10-23
equivalent weight is a dry weight of an ion exchange membrane per
1 eq. of ion exchange group, and is represented in units of "g/eq.".
[0071]
It is preferable that the catalyst layer includes two types
or more of polymer electrolytes having different EWs in a power
generation surface, and in this case, among the polymer electrolytes,
the polymer electrolyte having the lowest EW is used in an area where
relative humidity of a gas in a passage is 90% or less. By employing
such material arrangement, resistance is decreased irrespective of
a current density area, so that cell performance can be improved.
The EW of polymer electrolyte used in the area where relative humidity
of the gas in a passage is 90% or less, that is, EW of polymer
electrolyte having the lowest EW is preferably 900 g/eq. or less.
By this, the above-described effects can be further more certainly
and more remarkably attained.
[0072]
The polymer electrolyte having the lowest EW is preferably used
in an area of which temperature is higher than an average temperature
of inlet and outlet for cooling water. By this, resistance is
decreased irrespective of a current density area, so that cell
performance can be further improved.
[0073]
In terms decreased resistance value of a fuel cell system, the
polymer electrolyte having the lowest EW is preferably provided in
an area within the range of 3/5 or less of the passage length from
a gas supply inlet of at least one of a fuel gas and an oxidant gas.
[0074]
The catalyst layer according to the embodiment may include,
between the catalyst and the polymer electrolyte, a liquid proton
conducting material capable of connecting the catalyst and the
¨ 26 ¨
CA 02910375 2015-10-23
polymer electrolyte in a proton conductible state. By introducing
the liquid proton conducting material, a proton transport path
through the liquid proton conducting material is provided between
the catalyst and the polymer electrolyte, so that protons necessary
for the power generation can be efficiently transported on the
surface of the catalyst. By this, availability of the catalyst is
improved, and thus an amount of used catalyst can be reduced while
maintaining power generation performance. The liquid proton
conducting material may be interposed between the catalyst and the
polymer electrolyte. The liquid proton conducting material may be
disposed in pores (secondary pores) between porous supports in a
catalyst layer or may be disposed in pores (mesopores and the like:
primary pores) in porous supports.
[0075]
The liquid proton conducting material is not particularly
limited if the material has ion conductivity and has a function of
forming a proton transport path between the catalyst and the polymer
electrolyte. Specifically, water, a protic ionic liquid, an aqueous
solution of perchloric acid, an aqueous solution of nitric acid, an
aqueous solution of formic acid, an aqueous solution of acetic acid,
and the like may be exemplified.
[0076]
In the case of using water as the liquid proton conducting
material, the water can be introduced as the liquid proton conducting
material into the catalyst layer by wetting the catalyst layer with
a small amount of liquid water or a humidified gas before the start
of power generation. In addition, water generated through
electrochemical reaction during the operation of a fuel cell may be
used as the liquid proton conducting material. Therefore, in a state
where a fuel cell starts to be operated, the liquid proton conducting
¨ 27 ¨
CA 02910375 2015-10-23
material is not necessarily retained. For example, a surface
distance between the catalyst and the electrolyte is preferably set
to be a diameter of an oxygen ion constituting a water molecule, that
is, 0.28 nm or more. By maintaining such a distance, water (liquid
proton conducting material) can be interposed between the catalyst
and the polymer electrolyte (in the liquid conducting material
retaining portion) while maintaining the non-contact state between
the catalyst and the polymer electrolyte, so that a proton transport
path can be secured by water therebetween.
[0077]
In the case of using a material such as an ionic liquid other
than water as the liquid proton conducting material, the ionic liquid,
the polymer electrolyte, and the catalyst are preferably allowed to
be dispersed in a solution in the preparation of a catalyst ink.
However, the ionic liquid may be added at the time of coating a
catalyst layer substrate with a catalyst.
[0078]
In the catalyst according to the present invention, a total
area of the catalyst which is in contact with the polymer electrolyte
is set to be smaller than a total area of the catalyst exposed to
the liquid conducting material retaining portion.
[0079]
Comparison of these areas can be performed, for example, by
obtaining a magnitude relationship between capacitance of an
electrical double layer formed in a catalyst-polymer electrolyte
interface and capacitance of an electrical double layer formed in
a catalyst-liquid proton conducting material interface in a state
where the liquid conducting material retaining portion is filled with
the liquid proton conducting material. Namely, since capacitance
of an electrical double layer is proportional to an area of an
¨ 28 ¨
CA 02910375 2015-10-23
electrochemically effective interface, if the capacitance of the
electrical double layer formed in the catalyst-electrolyte interface
is smaller than the capacitance of the electrical double layer formed
in the catalyst-liquid proton conducting material interface, a
contact area of the catalyst with the electrolyte is smaller than
an area thereof exposed to the liquid conducting material retaining
portion.
[0080]
Herein, a measuring method for capacitance of an electrical
double layer formed in a catalyst-electrolyte interface and
capacitance of an electrical double layer formed in a catalyst-liquid
proton conducting material interface, that is, a magnitude
relationship between a contact area of the catalyst with the
electrolyte and a contact area of the catalyst and the liquid proton
conducting material (determination method for a magnitude
relationship between a contact area of the catalyst and the
electrolyte and an area of the catalyst exposed to the liquid
conducting material retaining portion) will be described.
[0081]
Namely, in the catalyst layer according to the embodiment, the
following four types of interfaces can contribute as capacitance of
electrical double layer (Cdl):
(1) catalyst-polymer electrolyte (C-S)
(2) catalyst-liquid proton conducting material (C-L)
(3) porous support-polymer electrolyte (Cr-S)
(4) porous support-liquid proton conducting material (Cr-L)
[0082]
As described above, since capacitance of an electrical double
layer is proportional to an area of an electrochemically effective
interface, Cdlc-s (capacitance of an electrical double layer in a
¨ 29 ¨
CA 02910375 2015-10-23
catalyst-polymer electrolyte interface) and Cd1c-L (capacitance of
an electrical double layer in a catalyst-liquid proton conducting
material interface) may be obtained. Therefore, the contribution
of the four types of interfaces to capacitance of an electrical double
layer (Cdl) can be identified as follows.
[0083]
First, for example, under a high humidity condition such as
100% RH and under a lower humidity condition such as 10% RH or less,
each capacitance of electrical double layers is measured. As a
measurement method for the capacitance of electrical double layer,
cyclic voltammetry, electrochemical impedance spectroscopy, or the
like may be exemplified. From the comparison, the contribution of
the liquid proton conducting material (in this case, "water"), that
is, the above-described contributions (2) and (4) can be identified.
[0084]
In addition, the contributions to capacitance of an electrical
double layer can be identified by deactivating a catalyst, for
example, in the case of using Pt as the catalyst, by deactivating
the catalyst by supplying CO gas to an electrode to be measured to
allow CO to be adsorbed on the surface of Pt. In this state, as
described above, under the high humidity condition and under the low
humidity condition, each capacitance of electrical double layers is
measured by the same method, and from the comparison, the
contributions of the catalyst, that is, the above-described
contributions (1) and (2) can be identified.
[0085]
By using the above-described method, all the contributions (1)
to (4) described above can be identified, the capacitance of the
electrical double layer in the interface between the catalyst and
the polymer electrolyte and the capacitance of the electrical double
¨ 30 ¨
CA 02910375 2015-10-23
layer in the interface between the catalyst and the liquid proton
conducting material can be obtained.
[0086]
Namely, a measurement value (A) in a highly-humidified state
can be regarded as capacitance of electrical double layer formed in
all the interfaces (1) to (4), and a measurement value (B) in a
lowly-humidified state can be regarded as capacitance of the
electrical double layer formed in the interfaces (1) and (3). In
addition, a measurement value (C) in a catalyst-deactivated and
highly-humidified state can be regarded as capacitance of the
electrical double layer formed in the interfaces (3) and (4), and
a measurement value (D) in a catalyst-deactivated and
lowly-humidified state can be regarded as capacitance of the
electrical double layer formed in the interface (3).
[0087]
Therefore, the difference between A and C can be regarded as
the capacitance of the electrical double layer formed in the
interfaces (1) and (2), and the difference between B and D can be
regarded as the capacitance of the electrical double layer formed
in the interface (1). Next, by calculating the difference between
these values, i.e., (A-C)-(B-D), the capacitance of the electrical
double layer formed in the interface (2) can be obtained. In addition,
a contact area of the catalyst with the polymer electrolyte or an
exposed area thereof to the conducting material retaining portion
can be obtained by, for example, TEN (transmission electron
microscope) tomography besides the above-described method.
[0088]
If necessary, the catalyst layer may contain additives of a
water repellent such as
polytetrafluoroethylene,
polyhexafluoropropylene, and
¨ 31 ¨
CA 02910375 2015-10-23
tetrafluoroethylene-hexafluoropropylene copolymer, a dispersant
such as a surfactant, a thickener such as glycerin, ethylene glycol
(EG), polyvinyl alcohol (PVA), and propylene glycol (PG), a
pore-forming agent, or the like.
[0089]
A layer thickness (as a dried thickness) of the catalyst layer
is preferably in the range of 0.05 to 30 gm, more preferably in the
range of 1 to 20 gm, even more preferably in the range of 2 to 15
gm. These can be applied to both of the cathode catalyst layer and
the anode catalyst layer. However, the layer thickness of the
cathode catalyst layer and the thickness of the anode catalyst layer
may be equal to or different from each other.
[0090]
(Method of Manufacturing Catalyst Layer)
Hereinafter, a method for manufacturing the catalyst layer
will be described as an exemplary embodiment, but the scope of the
present invention is not limited to the following embodiment. In
addition, all the conditions for the components and the materials
of the catalyst layer are as described above, and thus, the
description thereof is omitted.
[0091]
First, a support (in this specification, sometimes referred
to as a "porous support" or a "conductive porous support") is prepared.
Specifically, the support may be manufactured as described above in
the method of manufacturing the support. By doing so, pores having
a specific pore distribution (pores including mesopores having a
radius of 1 to 10 nm and a mode radius of the mesopores being in a
range of 2.5 to 10 nm) can be formed in the support.
[0092]
Next, the alloy particles are supported on the porous support,
¨ 32 ¨
CA 02910375 2015-10-23
so that a catalyst powder is formed. The supporting of the alloy
particles on the porous support can be performed by a well-known
method. For example, a well-known method such as an impregnation
method, a liquid phase reduction supporting method, an evaporation
drying method, a colloid adsorption method, a spray pyrolysis method,
or reverse micelle (micro-emulsion method) may be used. Among the
methods, the impregnation method is preferably used.
[0093]
In an embodiment, the aforementioned impregnation method
includes a process (1) of manufacturing a primary support by
immersing the support into a solution containing platinum and
reducing the resulting product, a process (2) of manufacturing a
secondary support by immersing the primary support into a solution
containing metal other than the platinum, and a process (3) of forming
an alloy of the platinum of the secondary support and the alloy other
than the platinum.
[0094]
Process (1)
The process (1) is a process of manufacturing the primary
support by immersing the support into the solution containing
platinum and reducing the resulting product.
[0095]
The solution containing platinum includes a
platinum-containing compound and a solvent.
[0096]
The platinum-containing compound is not particularly limited,
but platinum powder, platinum chloride (II), platinum chloride (IV),
platinum (IV) chloride acid, platinum oxide (IV), diammine dinitro
platinum (II), dichloro tetraammine platinum (II), hexahydroxo
platinum acid (IV), tetrachloroplatinate (II) potassium,
¨ 33 ¨
CA 02910375 2015-10-23
tetrachloroplatinate (IV) potassium, and the like may be exemplified.
The platinum-containing compounds may be used alone or may be used
in a combination of two or more types.
[0097]
As the aforementioned solvent, water or the like may be
exemplified.
[0098]
In addition, if necessary, the solution containing platinum
may include an acid. As the acid, hydrochloric acid, nitric acid,
sulfuric acid, and a mixed acid (for example, aqua regia) thereof
may be exemplified.
[0099]
The concentration of platinum in the solution containing
platinum is preferably in a range of 0.1 to 50 mass%, more preferably
in a range of 0.5 to 20 mass%.
[0100]
The reducing agent which can be used is not particularly limited,
but hydrogen, hydrazine, sodium hydroborate, sodium thiosulfate,
citric acid, sodium citrate, L-ascorbic acid, sodium borohydride,
formaldehyde, methanol, ethanol, ethylene, carbon monoxide, or the
like may be exemplified.
[0101]
After the reduction, if necessary, the solvent or the like
remaining in the primary support is preferably removed by heating
or the like.
[0102]
In process (1), by appropriately setting the concentration of
the platinum in the solution containing platinum, the immersion time,
the reduction condition, and the like, the supported amount of the
platinum on the support can be controlled.
¨ 34 ¨
CA 02910375 2015-10-23
[0103]
Process (2)
The process (2) is a process of manufacturing a secondary
support by immersing the primary support manufactured in the process
(1) into a solution containing the metal other than the platinum.
[0104]
The solution containing the metal other than the platinum
includes a metal-containing compound containing the metal other than
the platinum and a solvent.
[0105]
The metal-containing compound other than the above-described
platinum is appropriately selected according to to-be-manufactured
alloy particles. The metal-containing compound is not particularly
limited, but ruthenium chloride, ruthenium nitrate, sodium ruthenium
acid, potassium ruthenium acid, iridium chloride, iridium nitrate,
hexaammine iridium hydroxide, iridium chloride, ammonium iridium
chloride acid, potassium iridium chloride acid, rhodium chloride,
rhodium nitrate, palladium chloride, palladium nitrate,
dinitrodiammine palladium, iron chloride, cobalt chloride, cobalt
hydroxide, and the like may be exemplified.
[0106]
As the above-described solvent, water and the like may be
exemplified.
[0107]
The concentration of metal other than the platinum in a solution
containing the metal other the platinum is preferably in a range of
0.1 to 50 mass%, more preferably in a range of 0.5 to 20 mass%.
[0108]
By immersing a primary support into the solution containing
the metal other than the platinum, a secondary support supporting
¨ 35 ¨
CA 02910375 2015-10-23
the platinum and the metal other than the platinum can be
manufactured.
[0109]
After the immersion, if necessary, the solvent or the like
remaining in the secondary support is preferably removed by heating
or the like.
[0110]
In the process (2), by appropriately setting the concentration
of the metal other than the platinum in the solution containing the
metal other than the platinum and the immersion time, the supported
amount of the metal other than the platinum on the primary support
can be controlled.
[0111]
Process (3)
The process (3) is a process of forming an alloy of the platinum
and the alloy other than the platinum on the secondary support
manufactured in the process (2).
[0112]
A specific method of forming an alloy is not particularly
limited, but a well-known method can be appropriately employed. For
example, a method of heating in a 100% hydrogen gas or the like can
be exemplified.
[0113]
In addition, when the alloy particles are intended to be
supported inside the support by using the support of the related art
by the method according to the embodiment, since the metals other
than the platinum are difficult to enter into the support in the
above-described process (2), the alloy particles with a desired
alloy-formation ratio (particularly, alloy particles with a low
ratio of the platinum) cannot be obtained. As a result, the ratio
¨ 36 ¨
CA 02910375 2015-10-23
of the platinum as the catalyst is increased, and it is difficult
to lower the cost.
[0114]
However, in the case of using the support used for the catalyst
according to the present invention, specifically, the support of
which the radius and mode radius of the mesopores are controlled,
the metals other than the platinum can appropriately enter into the
support in comparison with the support of the related art. As a
result, a desired alloy-formation ratio can be achieved, so that the
catalyst where the alloy particles with a desired composition are
supported can be obtained.
[0115]
In addition, it can be determined based on the alloy-formation
ratio expressed by the following Formula whether or not the alloy
particles are obtained with a desired composition.
[0116]
[Formula 1]
Alloy - Formation Ratio = (Platinum Content Ratio of Manufactured Alloy
Particles) (Desired Platinum Content Ratio of
Alloy Particles). (Platinum Ratio/Ratio of Metals Other Than Platinum)
(Platinum Ratio with Desired Composition/Ratio of
Metals Other Than Platinum)
[0117]
In the above Formula, it can be stated that, as the
alloy-formation ratio is closer to 1, the alloy particles with a
desired composition can be further obtained.
[0118]
In addition, in another embodiment, in the above-described
impregnation method, alloy metals may be supported on the support
according to an immersion method including a process of manufacturing
alloy particles by performing alloy formation on platinum and metals
other than the platinum and a process of immersing the support into
a solution containing the alloy particles.
¨ 37 ¨
CA 02910375 2015-10-23
[0119]
Subsequently, a catalyst ink containing the catalyst powder,
polymer electrolyte, and a solvent is prepared. As the solvent,
there is no particular limitation. A typical solvent used for
forming a catalyst layer may be similarly used. Specifically, water
such as tap water, pure water, ion-exchanged water, distilled water,
cyclohexanol, a lower alcohol having 1 to 4 carbons such as methanol,
ethanol, n-propanol, isopropanol, n-butanol, sec-butanol,
isobutanol, and tert-butanol, propylene glycol, benzene, toluene,
xylene, or the like may be used. Besides, acetic acid butyl alcohol,
dimethyl ether, ethylene glycol, or the like may be used as a solvent.
These solvents may be used alone or may be used in a state of a mixture
of two or more solvents.
[0120]
An amount of solvent for preparing the catalyst ink is not
particularly limited so long as the electrolyte can be completely
dissolved. Specifically, a concentration (a solid content) of the
catalyst powder and the polymer electrolyte is preferably in the
range of 1 to 50 wt% in the electrode catalyst ink, more preferably
in the range of about 5 to 30 wt%.
[0121]
In the case of using an additive such as a water repellent,
a dispersant, a thickener, and a pore-forming agent, the additive
may be added to the catalyst ink. In this case, an added amount of
the additive is not particularly limited so long as it does not
interfere with the above-described effects by the present invention.
For example, the added amount of the additive is preferably in the
range of 5 to 20 wt%, with respect to the total weight of the electrode
catalyst ink.
[0122]
¨ 38 ¨
CA 02910375 2015-10-23
Next, a surface of a substrate is coated with the catalyst ink.
A method of coating the substrate is not particularly limited, but
a well-known method may be used. Specifically, a well-known method
such as a spray (spray coat) method, a Gulliver printing method, a
die coater method, a screen printing method, or a doctor blade method
can be used.
[0123]
As the substrate coated with the catalyst ink, a solid polymer
electrolyte membrane (electrolyte layer) or a gas diffusion
substrate (gas diffusion layer) may be used. In this case, after
the catalyst layer is formed on a surface of a solid polymer
electrolyte membrane (electrolyte layer) or a gas diffusion
substrate (gas diffusion layer) , the resultant laminate may be used
as it is for manufacturing a membrane electrode assembly.
Alternatively, as the substrate, a peelable substrate such as a
polytetrafluoroethylene (PTFE) [Teflon (registered trademark) ]
sheet can be used, and after a catalyst layer is formed on the
substrate, the catalyst layer portion can be peeled off from the
substrate, so that the catalyst layer may be obtained.
[0124]
Finally, the coat layer (film) of the catalyst ink is dried
under an air ambience or under an inert gas ambience at a temperature
ranging from room temperature to 150 C for a time ranging from 1 to
60 minutes. By this, the catalyst layer can be formed.
[0125]
(Membrane Electrode Assembly)
According to another embodiment of the present invention,
provided is a membrane electrode assembly for a fuel cell including
the above-described electrode catalyst layer for fuel cell. Namely,
provided is a membrane electrode assembly for fuel cell which
¨ 39 ¨
CA 02910375 2015-10-23
comprises a solid polymer electrolyte membrane 2, a cathode catalyst
layer disposed on one side of the electrolyte membrane, an anode
catalyst layer disposed on the other side of the electrolyte membrane,
and a pair of gas diffusion layers (4a, 4c) interposing the
electrolyte membrane 2, the anode catalyst layer 3a, and the cathode
catalyst layer 3c. In the membrane electrode assembly, at least one
of the cathode catalyst layer and the anode catalyst layer is the
catalyst layer according to the embodiment described above.
[0126]
However, by taking into consideration necessity of improved
proton conductivity and improved transport characteristic (gas
diffusibility) of a reaction gas (particularly, 02), at least the
cathode catalyst layer is preferably the catalyst layer according
to the embodiment described above. However, the catalyst layer
according to the embodiment is not particularly limited. The
catalyst layer may be used as the anode catalyst layer or may be used
as the cathode catalyst layer and the anode catalyst layer.
[0127]
According to further embodiment of the present invention,
provided is a fuel cell including the membrane electrode assembly
according to the embodiment. Namely, according to one aspect, the
present invention provides a fuel cell comprising a pair of anode
separator and cathode separator interposing the membrane electrode
assembly according to the embodiment.
[0128]
Hereinafter, members of a PEFC 1 using the catalyst layer
according to the embodiment will be described with reference to Fig.
1. However, the present invention has features with respect to the
catalyst layer. Therefore, among members constituting the fuel cell,
specific forms of members other than the catalyst layer may be
¨ 40 ¨
CA 02910375 2015-10-23
appropriately modified with reference to well-known knowledge in the
art.
[0129]
(Electrolyte Membrane)
An electrolyte membrane is configured with a solid polymer
electrolyte membrane 2 in the same form illustrated in, for example,
Fig. 1. The solid polymer electrolyte membrane 2 serves to
selectively transmit protons generated in an anode catalyst layer
3a to a cathode catalyst layer 3c in the thickness direction during
the operation of the PEFC 1. In
addition, the solid polymer
electrolyte membrane 2 also serves as a partition wall for preventing
a fuel gas supplied to an anode side from being mixed with an oxidant
gas supplied to a cathode side.
[0130]
An electrolyte material constituting the solid polymer
electrolyte membrane 2 is not particularly limited, but well-known
knowledge in the art may be appropriately referred to. For example,
the fluorine-based polymer electrolyte or the hydrocarbon-based
polymer electrolyte described above as the polymer electrolyte can
be used. There is no need to use the polymer electrolyte which is
necessarily the same as the polymer electrolyte used for the catalyst
layer.
[0131]
A thickness of the electrolyte layer is not particularly
limited, but it may be determined by taking into consideration
characteristics of the obtained fuel cell. The thickness of the
electrolyte layer is typically in the range of about 5 to 300 pin.
If the thickness of the electrolyte layer is within such a range,
balance between strength during the film formation or durability
during the use and output characteristics during the use can be
¨ 41 ¨
CA 02910375 2015-10-23
appropriately controlled.
[0132]
(Gas Diffusion Layer)
A gas diffusion layer (anode gas diffusion layer 4a, cathode
gas diffusion layer 4c) serves to facilitate diffusion of a gas (fuel
gas or oxidant gas) supplied through a gas passage (6a, 6c) of a
separator to a catalyst layer (3a, 3c) and also serves as an electron
conducting path.
[0133]
A material constituting a substrate of the gas diffusion layers
(4a, 4c) is not particularly limited, but well-known knowledge in
the related art may be appropriately referred to. For example, a
sheet-shaped material having conductivity and porous property such
as a fabric made of carbon, a sheet-shaped paper, felt, and a nonwoven
fabric may be exemplified.
[0134]
A thickness of the substrate may be appropriately determined
by considering characteristics of the obtained gas diffusion layer.
The thickness of the substrate may be in the range of about 30 to
500 m. If the thickness of the substrate is within such a range,
balance between mechanical strength and diffusibility of gas, water,
and the like can be appropriately controlled.
[0135]
The gas diffusion layer preferably includes a water repellent
for the purpose of preventing a flooding phenomenon or the like by
improving water repellent property. The water repellent is not
particularly limited, but fluorine-based polymer materials such as
polytetrafluoroethylene (PTFE), polyvinylidene fluoride (PVdF),
polyhexafluoropropylene, and
tetrafluoroethylene-hexafluoropropylene copolymer (FEP),
42 ¨
CA 02910375 2015-10-23
polypropylene, polyethylene, and the like may be exemplified.
[0136]
In order to further improve water repellent property, the gas
diffusion layer may include a carbon particle layer (microporous
layer (MPL), not shown) configured with an assembly of carbon
particles including a water repellent provided at the catalyst-layer
side of the substrate.
[0137]
Carbon particles included in the carbon particle layer are not
particularly limited, but well-known materials in the art such as
carbon black, graphite, and expandable graphite maybe appropriately
employed. Among the materials, due to excellent electron
conductivity and a large specific surface area, carbon black such
as oil furnace black, channel black, lamp black, thermal black, and
acetylene black can be preferably used.
[0138]
An average particle diameter of the carbon particles may be
set to be in the range of about 10 to 100 nm. By this, high
water-repellent property by a capillary force can be obtained, and
contacting property with the catalyst layer can be improved.
[0139]
As the water repellent used for the carbon particle layer, the
above-described water repellent may be exemplified. Among the
materials, due to excellent water repellent property and excellent
corrosion resistance during the electrode reaction, the
fluorine-based polymer material can be preferably used.
[0140]
A mixing ratio of the carbon particles and the water repellent
in the carbon particle layer may be set to be in the range of weight
ratio of about 90:10 to 40:60 (carbon particle: water repellent) by
¨ 43 ¨
CA 02910375 2015-10-23
taking into consideration balance between water repellent property
and electron conductivity. Meanwhile, a thickness of the carbon
particle layer is not particularly limited, but it may be
appropriately determined by taking into consideration water
repellent property of the obtained gas diffusion layer.
[0141]
(Method of Manufacturing Membrane Electrode Assembly)
A method of manufacturing a membrane electrode assembly is not
particularly limited, and a well-known method in the art may be used.
For example, a method which comprises transferring a catalyst layer
to a solid polymer electrolyte membrane by using a hot press, or
coating a solid polymer electrolyte membrane with a catalyst layer
and drying the coating, and joining the resulting laminate with gas
diffusion layers, or a method which comprises coating a microporous
layer (in the case of not including a microporous layer, one surface
of a substrate layer) of a gas diffusion layer with a catalyst layer
in advance and drying the resulting product to produce two gas
diffusion electrodes (GDEs), and joining both surfaces of the solid
polymer electrolyte membrane with the two gas diffusion electrodes
by using a hot press can be used. The coating and joining conditions
by hot press and the like may be appropriately adjusted according
to a type of the polymer electrolyte (perfluorosulfonic acid-based
or hydrocarbon-based) in the solid polymer electrolyte membrane or
the catalyst layer.
[0142]
(Separator)
In the case of configuring a fuel cell stack by connecting a
plurality of unit fuel cells of polymer electrolyte fuel cells in
series, a separator serves to electrically connect the cells in
series. The separator also serves as a partition wall for separating
¨ 44 ¨
CA 02910375 2015-10-23
a fuel gas, an oxidant gas, and a coolant from each other. In order
to secure a passage thereof, as described above, gas passages and
coolant passages are preferably installed in each of the separators.
As a material constituting the separator, well-known materials in
the art of carbon such as dense carbon graphite and a carbon plate,
a metal such as a stainless steel, or the like can be employed without
limitation. A thickness or size of the separator, a shape or size
of the installed passages, and the like are not particularly limited,
but they can be appropriately determined by taking into consideration
desired output characteristics and the like of the obtained fuel
cell.
[0143]
A manufacturing method for the fuel cell is not particularly
limited, and well-known knowledge in the art in the field of fuel
cell may be appropriately referred to.
[ 0144]
Furthermore, in order that the fuel cell can generate a desired
voltage, a fuel cell stack may be formed by connecting a plurality
of membrane electrode assemblies in series through a separator. A
shape and the like of the fuel cell are not particularly limited,
and they may be appropriately determined so as to obtain desired cell
characteristics such as a voltage.
[0145]
Since the above-described PEFC or membrane electrode assembly
uses the catalyst layer having an excellent durability, the PEFC or
membrane electrode assembly has an excellent durability. In
addition, by using the above-described catalyst layer, it is possible
to lower the cost of the PEFC.
[0146]
The PEFC according to the embodiment or a fuel cell stack using
¨ 45 ¨
CA 02910375 2015-10-23
the PEFC can be installed, for example, as a driving power supply
for a vehicle.
Example
[0147]
The effects of the present invention will be described with
reference to the following Examples and Comparative Examples.
However, the scope of the present invention is not limited to the
following Examples.
[0148]
<Manufacturing of Support>
[Synthesis Example 1]
A support A having a mode radius of mesopores of 6.1 nm and
a pore volume of mesopores of 0.95 cc/g support was manufactured.
Specifically, heat treatment was performed on a composite obtained
by mixing a magnesium oxide having an average crystallite size of
10 nm and a thermoplastic resin with 3 : 7 of mass ratio under a
nitrogen ambience at 900 C, and after that, the resulting product
was washed with dilute sulfuric acid and drying is performed, so that
the support A was manufactured.
[0149]
[Synthesis Example 2]
A support B having a mode radius of mesopores of 2.4 nm and
a pore volume of mesopores of 1.53 cc/g support was manufactured.
Specifically, heat treatment was performed on a composite obtained
by mixing a magnesium oxide having an average crystallite size of
5 nm and a thermoplastic resin with 2 : 8 of mass ratio under a nitrogen
ambience at 900 C, and after that, the resulting product was washed
with dilute sulfuric acid and drying is performed, so that the support
B was manufactured.
[0150]
¨ 46 ¨
CA 02910375 2015-10-23
[Synthesis Example 3]
Black pearls (registered trademark) 2000 (pore volume of the
mesopores: 0.49 cc/g support, having no clear mode diameter of the
mesopores, produced by Cabot) was prepared as a support C.
[0151]
[Synthesis Example 4]
Ketjen Black EC300J (pore volume of the mesopores: 0.39 cc/g
support, having no clear mode diameter of the mesopores mode diameter,
produced by Ketjen Black International) was prepared as a support
D.
[0152]
<Manufacturing of Catalyst>
[Example 1]
In the case of supporting the alloy particles on the surface
of the support disclosed in Patent Literature 1, a catalyst was
manufactured in a condition that a compounding ratio of platinum and
cobalt was 3 : 1. Namely, the catalyst was manufactured by setting
a desired composition ratio of platinum and cobalt to 3 : 1.
[0153]
More specifically, 12 g of the support A manufactured in
Synthesis Example I was immersed into a solution containing platinum,
and stirring was performed. Next, the solution was stirred and mixed
at a boiling point (about 95 C) for 7 hours, and after that, filtering
and drying were performed, so that a primary support was manufactured.
At this time, the solution containing platinum used above was 1000
g (platinum content: 8 g) of a dinitrodiammine platinum nitric acid
solution having a platinum concentration of 0.8 mass%.
[0154]
Next, 10 g of the primary support obtained above was immersed
into a solution containing cobalt, and stirring was performed for
¨ 47 ¨
CA 02910375 2015-10-23
1 hour. Next, the resulting solution was dried at 60 C, so that a
secondary support was manufactured. At this time, the solution
containing cobalt used above was 60 g (cobalt content: 0.4 g) of an
aqueous cobalt chloride solution having a cobalt concentration of
0.66 mass%.
[0155]
Finally, the alloy formation process was performed in 100% of
hydrogen gas at 1000 C for 2 hours, so that a catalyst was
manufactured.
[0156]
In addition, with respect to the obtained catalyst, the mode
radius of the mesopores and the pore volume of the mesopores were
measured by a nitrogen adsorption method (DH method) , and the values
were 6.1 nm and 0.81 cc/g support, respectively.
[0157]
In addition, the supported amount of the alloy particles was
measured by an ICP-MS (inductively coupled plasma mass spectrometer),
and the value was 30 wt%.
[0158]
[Comparative Example I]
Except for using the support B manufactured in Synthesis
Example 2, the same method as that of Example 1 was performed, so
that a catalyst was manufactured.
[0159]
In addition, the mode radius of the mesopores, the pore volume
of the mesopores and the supported amount of the alloy particles were
measured by the same method as that of Example 1, and the values were
2.1 nm, 1.35 cc/g support, and 30 wt%, respectively.
[0160]
[Comparative Example 21
¨ 48 ¨
CA 02910375 2015-10-23
Except for using the support C manufactured in Synthesis
Example 3, the same method as that of Example 1 was performed, so
that a catalyst was manufactured.
[0161]
In addition, the pore volume of the mesopores and the supported
amount of the alloy particles were measured by the same method as
that of Example 1, and the values were 0.49 cc/g support and 30 wt%,
respectively. In addition, with respect to the catalyst, clear mode
diameter of the mesopores was not observed.
[0162]
[Comparative Example 3]
Except for using the support C of Synthesis Example 3 and
changing the dinitrodiammine platinum nitric acid solution to 600
g (platinum content: 4.8 g) and changing the aqueous cobalt chloride
solution to 36 g (cobalt content: 0.24 g) , the same method as that
of Example I was performed, so that a catalyst was manufactured.
[0163]
In addition, the pore volume of the mesopores and the supported
amount of the alloy particles were measured by the same method as
that of Example 1, and the values were 0.36 cc/g support and 50 wt%,
respectively. In addition, with respect to the catalyst, clear mode
diameter of the mesopores is not observed.
[0164]
[Comparative Example 4]
Except for using the support D of Synthesis Example 4, the same
method as that of Example 1 was performed, so that a catalyst was
manufactured.
[0165]
In addition, the pore volume of the mesopores and the supported
amount of the alloy particles were measured by the same method as
¨ 49 ¨
CA 02910375 2015-10-23
that of Example 1, and the values were 0.36 cc/g support and 50 wt%,
respectively. In addition, with respect to the catalyst, clear mode
diameter of the mesopores was not observed.
[0166]
The catalysts manufactured in Example 1 and Comparative
Examples 1 to 4 are listed in Table 1.
[0167]
[Table 1]
Support (Before Supporting of Alloy Catalyst (After Supporting of Alloy,
Particles) Particles)
Pore
Type of Mode Radius Pore Volume of Mode Radius Volume of Supported ,
Amount of Alloy'
of Mesopore Mesopore cpfM.esopore
Mesopore
Support , Particles
(nm) (cc/g support)' (nm) (cc/g
(wt%)
support)
, Example I Support A 6.1 0.95 6.1 ' 0.81 30
;
'Comparative'
'Support BI 2.4 1.53 2.1 , 1.35 30
Example 11
Comparative
Example 2 'Support CI None 0.49 None -- 0.49 -- 30
'Comparative
Support C None 0.39 None i 0.36 SO
, Example 3
,Comparative
Support DI None 0.39 None ' 0.36 50
Example 4
[0168]
<Performance Evaluation>
Performance evaluation was performed on the catalysts
manufactured in Example 1 and Comparative Examples 1 to 4.
[0169]
[Measurement of Alloy-Formation Ratio]
The composition of the alloy particles of the manufactured
catalyst was measured by an ICP-MS (inductively coupled plasma mass
spectrometer).
[0170]
In Examples and Comparative Examples, since the condition is
set so that the compounding ratio of platinum and cobalt is 3 : 1,
it was evaluated based on the compounding amount of the platinum in
the alloy particles by using the following Formula whether or not
an alloy with a desired composition was able to be obtained.
¨ 50 ¨
CA 02910375 2015-10-23
[ 0 1 7 1
[Formula 2]
Alloy - Formation Ratio = (Platinum Content Ratio of Manufactured Alloy
Particles) (Desired Platinum Content Ratio of
Alloy Particles) = (Platinum Ratio/Cobalt Ratio) (3/1)
[0172]
The obtained results are listed in the following Table 2.
[0173]
[Table 2]
Catalyst (After Supporting of Alloy Particles)
Mode Radius of Pore Volume of Supported Amount of Alloy-
Formation
Mesopore Mesopore Alloy Particles Ratio
1
(nm) (cc/g support) (wt%)
Example 1 6.1 0.81 30 1.004
Comparative
2.1 1.35 30 1.521
Example 1
Comparative
None 0.49 30 1.367
Example 2
Comparative
None 0.36 50 1.127
Example 3
Comparative
None 0.36 50 0.917
Example 4
[0174]
In Table 2, in Example 1 where the catalyst is manufactured
so that the catalyst including the mesopores having a radius of 1
to 10 nm originated from the support and the mode radius of the
mesopores is in a range of 2.5 to 10 nm, the alloy-formation ratio
is very close to 1. Namely, it is found out that, in the catalyst
of Example 1, the alloy particles with a desired composition
(platinum : cobalt = 3 : 1) are supported.
[0175]
On the contrary, for example, in the catalyst of Comparative
Example 1, in the case where the mode diameter of the mesopores is
as small as 2.1 nm, the alloy-formation ratio has a high value (1.5) .
Namely, it is found out that the alloy particles are supported in
the obtained catalyst with a compounding ratio of platinum and cobalt
of 4.5 : 1, so that the alloy particles with a desired composition
(platinum : cobalt = 3 : 1) cannot be obtained.
¨ 51 ¨
[0176]
Reference Signs List
1 Solid polymer electrolyte fuel cell (PEFC)
2 Solid polymer electrolyte membrane
3a Anode catalyst layer
3c Cathode catalyst layer
4a Anode gas diffusion layer
4c Cathode gas diffusion layer
5a Anode separator
5c Cathode separator
6a Anode gas passage
6c Cathode gas passage
7 Coolant passage
10 Membrane electrode assembly (MEA)
20 Catalyst
22 Alloy particle
23 Support
24 Mesopore
Electrolyte
- 52 -
Date Recue/Date Received 2020-07-31