Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
ENERGY RECOVERY WHEN PROCESSING MATERIALS WITH REACTIVE FLUIDS
FIELD OF THE INVENTION
[0001] The invention generally relates to recovering and reusing energy
when
processing materials with reactive fluids. More particularly, the invention
relates to methods
and apparatuses for recovering and reusing energy from processes in which
materials
comprising polymers and/or oligomers are treated with a reactive fluid.
BACKGROUND OF THE INVENTION
[0002] Reactive fluids, such as sub-critical, near-critical, and/or
supercritical fluids, are
highly energetic fluids having a high temperature and high pressure. These
reactive fluids
can be used to treat materials comprising polymers and/or oligomers to
decrease the degree of
polymerization or oligomerization of the polymers or oligomers in the
material. For
example, biomass, which typically is composed of natural polymers or
oligomers, such as
cellulose, hemicellulose, and lignin, or waste feedstocks, which may contain
natural or
synthetic polymers or oligomers, can be hydrolyzed by treatment with sub-
critical, near-
critical, and/or supercritical water to produce monomers and/or shorter chain
polymers and
oligomers.
[0003] Generating reactive fluids having a high temperature and high
pressure is an
energy intensive process. Without effective management of the system, the heat
and pressure
associated with the reactive fluid will dissipate into the ambient environment
during or after
treatment of a material, thereby losing the energy associated therewith. To
economically
operate a system that employs reactive fluids, an energy recovery system may
be employed.
Although some energy recovery methods and systems are known, not all recovery
methods or
systems are applicable to systems that employ reactive fluids. Moreover, it is
not obvious
which methods or systems should be employed to recover energy from processes
that treat
biomass or waste feedstocks, or how this recovered energy should be redeployed
in the
energy-originating method or system, or in other co-located or nearby methods
or systems.
For example, if heat that is recovered from a reactive fluid is not properly
reintegrated into
the process, undesirable reactions may occur or desirable reactions may not
occur at all.
Thus, there remains a need in the art for improved methods and systems for
recovering and
reusing energy when processing materials with reactive fluids.
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
BRIEF SUMMARY OF THE INVENTION
[0004] The invention provides a method comprising, consisting of, or
consisting
essentially of:
providing a composition at a first temperature and a first pressure, wherein
the
composition comprises:
at least one material selected from the group consisting of a polymer, an
oligomer, and combinations thereof; and
a liquid;
optionally, preheating the composition in a first preheating stage to form a
preheated
composition, wherein the preheated composition is characterized by a second
temperature
and a second pressure;
heating the composition or the preheated composition with a reactive fluid in
a first
heating stage to form a heated composition, wherein the reactive fluid is
characterized by a
third temperature and a third pressure, and the heated composition is
characterized by a
fourth temperature and a fourth pressure;
wherein the reactive fluid is produced by a process comprising:
providing a fluid;
optionally, preheating the fluid in a second preheating stage to produce
a preheated fluid having a fifth temperature and a fifth pressure; and
heating the fluid or the preheated fluid in a second heating stage to
produce the reactive fluid; and
cooling the heated composition in a cooling stage to form a cooled
composition,
wherein the cooled composition is characterized by a sixth temperature and a
sixth pressure;
wherein:
the cooled composition comprises one or more reaction products derived from
the
material;
the cooling stage comprises a first flash evaporation, thereby producing a
first flashed
vapor and a first flashed composition;
the first flashed composition is the same as or different from the cooled
composition;
and
at least a portion of the first flashed vapor is used to perform a first
useful
function.
2
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
[0005] The invention further provides an apparatus, wherein the apparatus
comprises,
consists of, or consists essentially of:
optionally, a module configured for preheating a composition to form a
preheated
composition having a second temperature and a second pressure;
wherein the composition comprises:
at least one material comprising a polymer, an oligomer, or a
combination thereof; and
a liquid;
and wherein the composition has a first temperature and a first pressure;
a reactor configured for reacting the composition or the preheated composition
with a
reactive fluid to form a heated composition having a fourth temperature and a
fourth pressure;
wherein the reactive fluid has a third temperature and a third pressure;
a reactive fluid generator comprising:
optionally, a fluid preheater configured for preheating a fluid to produce a
preheated fluid having a fifth temperature and a fifth pressure; and
a heater configured for heating the fluid or the preheated fluid to form the
reactive fluid;
a first flash unit configured for a first flash evaporation to form a first
flashed vapor
and a first flashed composition, wherein the first flashed composition has a
seventh
temperature and a seventh pressure, the seventh temperature is less than the
fourth
temperature, and at least a portion of the first flashed vapor is used for a
first useful function;
optionally, a second flash unit configured for a second flash evaporation to
form a
second flashed vapor and a second flashed composition, wherein the second
flashed
composition has an eighth temperature and an eighth pressure, the eighth
temperature is less
than the fourth temperature, and optionally at least a portion of the second
flashed vapor is
used for a fourth useful function, wherein the fourth useful function is the
same as or
different from the first useful function;
optionally, at least one clean vapor exchanger, wherein at least one of the
first flashed
vapor and the second flashed vapor indirectly provides heat to a clean fluid
in the clean vapor
exchanger thereby producing a first clean vapor and/or a second clean vapor,
respectively,
and optionally at least a portion of the first clean vapor and/or the second
clean vapor is used
for a second useful function and or fifth useful function, respectively,
wherein the second
useful function is the same as or different from the first, fourth, and/or
fifth useful functions;
and
3
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
optionally, at least one indirect heat exchanger comprising a heat transfer
fluid,
wherein the at least one heat exchanger is configured for indirectly cooling a
process stream
processed by the apparatus, thereby producing an energized heat transfer
fluid, and the
energized heat transfer fluid optionally is used for a third useful function,
wherein the third
useful function is the same as or different from the first, second, fourth,
and fifth useful
functions.
BRIEF DESCRIPTION OF THE DRAWINGS
[0006] The accompanying drawings, which are included to provide a further
understanding of the invention and are incorporated in and constitute a part
of this
specification, illustrate aspects of the invention, and, together with the
description, serve to
explain the principles of the invention. Dashed lines generally indicate the
flow path of
energy recovered in the process. In the drawings:
[0007] FIGURE lA illustrates an embodiment of a method of the invention, in
which
at least a portion of the first flashed vapor is used to perform a first
useful function.
[0008] FIGURE 1B illustrates an embodiment of a method of the invention, in
which
the first useful function is using at least a portion of the first flashed
vapor to indirectly
preheat the composition.
[0009] FIGURE 1C illustrates an embodiment of a method of the invention, in
which
the first useful function is using at least a portion of the first flashed
vapor to preheat both the
composition and the fluid.
[0010] FIGURE 1D illustrates an embodiment of a method of the invention, in
which
the first useful function is using at least a portion of the first flashed
vapor to preheat the
composition. At least a portion of the lower energy vapor issuing from the
first preheating
stage is used to preheat the fluid.
[0011] FIGURE 2A illustrates an embodiment of a method of the invention, in
which
the first useful function is using at least a portion of the first flashed
vapor to indirectly
generate a clean vapor, and optionally at least a portion of the clean vapor
is used to perform
a second useful function.
[0012] FIGURE 2B illustrates an embodiment of a method of the invention, in
which
the first useful function is using at least a portion of the first flashed
vapor to indirectly
generate a clean vapor. The second useful function is using at least a portion
of the clean
vapor to directly or indirectly preheat the composition.
4
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
[0013] FIGURE 2C illustrates an embodiment of a method of the invention, in
which
the first useful function is using at least a portion of the first flashed
vapor to indirectly
generate a clean vapor. The second useful function is using at least a portion
of the clean
vapor to directly or indirectly preheat the fluid.
[0014] FIGURE 2D illustrates an embodiment of a method of the invention, in
which
the first useful function is using at least a portion of the first flashed
vapor to indirectly
generate a clean vapor. The second useful function is using at least a portion
of the clean
vapor to directly or indirectly preheat the composition and the fluid.
[0015] FIGURE 3A illustrates an embodiment of a method of the invention, in
which
the cooling stage comprises a first flash evaporation followed by an indirect
heat exchange
using a heat transfer fluid. At least a portion of the first flashed vapor is
used to perform a
first useful function, and optionally at least a portion of the energized heat
transfer fluid is
used to perform a third useful function.
[0016] FIGURE 3B illustrates an embodiment of a method of the invention, in
which
the cooling stage comprises a first flash evaporation preceded by an indirect
heat exchange
using a heat transfer fluid. At least a portion of the first flashed vapor is
used to perform a
first useful function, and optionally at least a portion of the energized heat
transfer fluid is
used to perform a third useful function.
[0017] FIGURE 3C illustrates an embodiment of a method of the invention, in
which
the cooling stage comprises a first flash evaporation preceded by an indirect
heat exchange
using a heat transfer fluid. At least a portion of the first flashed vapor is
used to perform a
first useful function. The energized heat transfer fluid is cycled in a
continuous loop between
the cooling stage, the first preheating stage, the second preheating stage,
and back to the
cooling stage.
[0018] FIGURE 4A illustrates an embodiment of a method of the invention, in
which
the cooling stage comprises a first flash evaporation followed by a second
flash evaporation.
The first flashed vapor is used to perform a first useful function, and
optionally the second
flashed vapor is used to perform a second useful function.
[0019] FIGURE 4B illustrates an embodiment of a method of the invention, in
which
the cooling stage comprises a first flash evaporation preceded by a second
flash evaporation.
The first flashed vapor is used to perform a first useful function, and
optionally the second
flashed vapor is used to perform a second useful function.
[0020] FIGURE 4C illustrates an embodiment of a method of the invention, in
which
the cooling stage comprises a first flash evaporation followed by a second
flash evaporation.
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
The first useful function is using at least a portion of the first flashed
vapor to preheat the
fluid. The second useful function is using at least a portion of the second
flashed vapor to
indirect generate a second clean vapor, and the second clean vapor optionally
is used to
perform a fifth useful function.
[0021] FIGURE 5 illustrates an embodiment of an apparatus of the invention.
The
apparatus comprises an optional preheater, a reactor, a first flash unit, and
a second flash unit.
The apparatus also comprises an optional fluid preheater, and a fluid heater.
The second
flashed vapor issuing from the second flash unit is fed to an indirect clean
vapor exchanger,
thereby producing clean vapor, which optionally can be used for a fifth useful
function.
DETAILED DESCRIPTION OF THE INVENTION
[0022] As employed above and throughout the disclosure, the following
terms, unless
otherwise indicated, shall be understood to have the following meanings. Other
term
definitions are explicitly provided throughout the disclosure, or may be
implicitly understood
by the context of the disclosure.
[0023] As used herein, the phrase "substantially free" means have no more
than about
1%, preferably less than about 0.5%, or more preferably less than about 0.1%,
by weight of a
component, based on the total weight of any composition containing the
component.
[0024] As used herein, the singular forms "a," "an," and "the" include the
plural
reference unless the context clearly indicates otherwise.
[0025] The use of numerical values in the various quantitative values
specified in this
application, unless expressly indicated otherwise, are stated as
approximations as though the
minimum and maximum values within the stated ranges were both preceded by the
word
"about." In this manner, slight variations from a stated value can be used to
achieve
substantially the same results as the stated value. Also, the disclosure of
ranges is intended as
a continuous range including every value between the minimum and maximum
values recited
as well as any ranges that can be formed by such values. Also disclosed herein
are any and
all ratios (and ranges of any such ratios) that can be formed by dividing a
recited numeric
value into any other recited numeric value. Accordingly, the skilled person
will appreciate
that many such ratios, ranges, and ranges of ratios can be unambiguously
derived from the
numerical values presented herein and in all instances such ratios, ranges,
and ranges of ratios
represent various embodiments of the present invention.
6
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
[0026] As used herein, a "reactive fluid" includes sub-critical, near-
critical, and
supercritical fluids, as defined herein.
[0027] As used herein, "recovered energy" is energy that is recaptured or
reclaimed
from a system, method, and/or apparatus. Energy (e.g., recovered energy)
typically is in the
form of heat and/or pressure. For example, recovered heat or thermal energy
can be reused
for a variety of useful functions as described elsewhere herein, such as to
heat other process
streams. Moreover, recovered energy in the form of pressure can also be used
for a variety of
useful functions as described elsewhere herein, such as actuating turbine
blades to generate
electricity.
[0028] A supercritical fluid is a fluid at a temperature above its critical
temperature and
at a pressure above its critical pressure. A supercritical fluid exists at or
above its "critical
point," the point of highest temperature and pressure at which the liquid and
vapor (gas)
phases can exist in equilibrium with one another. Above critical pressure and
critical
temperature, the distinction between liquid and gas phases disappears. A
supercritical fluid
possesses approximately the penetration properties of a gas simultaneously
with the solvent
properties of a liquid. Accordingly, supercritical fluid extraction has the
benefit of high
penetrability and good solvation.
[0029] Reported critical temperatures and pressures include: for pure
water, a critical
temperature of about 374.2 C, and a critical pressure of about 221 bar; for
carbon dioxide, a
critical temperature of about 31 C and a critical pressure of about 72.9
atmospheres (about
1072 psig). Near critical water has a temperature at or above about 300 C and
below the
critical temperature of water (374.2 C), and a pressure high enough to ensure
that all fluid is
in the liquid phase. Sub-critical water has a temperature of less than about
300 C and a
pressure high enough to ensure that all fluid is in the liquid phase. Sub-
critical water
temperature may be greater than about 250 C and less than about 300 C, and
in many
instances sub-critical water has a temperature between about 250 C and about
280 C. The
term "hot compressed water" is used interchangeably herein for water that is
at or above its
critical state, or defined herein as near-critical or sub-critical, or any
other temperature above
about 50 C (e.g., at least about 100 C, at least about 150 C, at least
about 200 C, at least
about 250 C) but typically less than subcritical and at pressures such that
water is in a liquid
state.
[0030] As used herein, a fluid which is "supercritical" (e.g. supercritical
water,
supercritical ethanol, supercritical CO2, etc.) indicates a fluid which would
be supercritical if
present in pure form under a given set of temperature and pressure conditions.
For example,
7
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
"supercritical water" indicates water present at a temperature of at least
about 374.2 C and a
pressure of at least about 221 bar, whether the water is pure water, or
present as a mixture
(e.g. water and ethanol, water and CO2, etc). Thus, for example, "a mixture of
sub-critical
water and supercritical carbon dioxide" indicates a mixture of water and
carbon dioxide at a
temperature and pressure above that of the critical point for carbon dioxide
but below the
critical point for water, regardless of whether the supercritical phase
contains water and
regardless of whether the water phase contains any carbon dioxide. For
example, a mixture
of sub-critical water and supercritical CO2 may have a temperature of about
250 C to about
280 C and a pressure of at least about 225 bar.
[0031] As used herein, the term "biomass" means a renewable energy source
generally
comprising carbon-based biological material derived from living or recently-
living
organisms. The organisms are or may have been plants, animals, fungi, etc.
Examples of
biomass include, without limitation, wood, lignocellulosic biomass, waste
feedstocks,
manufacturing waste (wood residues such as sawmill and paper mill discards),
agricultural
residues (including corn stover, sugarcane bagasse, rice hulls, oat hulls,
etc.), food waste,
plastic, black liquor (a byproduct of wood pulping processes), etc. Wood can
be, for
example, hardwood, softwood, annual fibers, and combinations thereof Biomass
typically
comprises cellulose, hemicellulose, and lignin. Any suitable type of biomass
can be used as a
feedstock for the invention described herein. Fossil fuels are generally not
considered
biomass even though ultimately derived from carbon-based biological material.
The term
"biomass" as used herein does not include fossil fuel sources.
[0032] As used herein, "degree of polymerization" refers to the number of
monomeric
units in a macromolecule or polymer or oligomer molecule, including those
monomeric units
that are not identical (such as in an oligomer with different monomeric
residues). The degree
of polymerization (DP) of the various saccharides in the compositions of the
invention may
be measured using gel permeation chromatography (GPC), high pressure liquid
chromatography (HPLC), such as DIONEX with an electrochemical detector, matrix-
assisted
laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, or
other
conventional molecular weight determination methods.
[0033] As used herein, "flash evaporation" means a reduction in pressure by
means of a
valve or other pressure control device, whereby a vapor portion and a residual
liquid portion
are produced. The vapor portion (i.e., the "flashed vapor") typically exits
the system through
the valve or other pressure control device, whereas the residual liquid
portion (i.e., the
"flashed composition") remains fore of the valve or other pressure control
device and does
8
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
not exit therethrough. The vapor portion typically contains at least a portion
of a liquid in
gaseous form.
[0034] As used herein, a "useful function" includes any function having
industrial
utility. Examples of useful functions include, but are not limited to, active
heating, electricity
generation (e.g., turbine-generated electricity), feeding to a co-located or
nearby plant (e.g.,
in which the co-located or nearby plant benefits in some manner from the
recovered energy),
evaporation (e.g., solutions or suspension containing desirable compounds or
products can be
concentrated by evaporating a solvent or liquid using the recovered energy),
clean vapor
generation (e.g., using a clean vapor exchanger to produce clean vapor, as
described
elsewhere herein), vacuum generation (e.g., a vacuum jet), or combinations
thereof.
Examples of active heating include, e.g., active heating of: compositions
(e.g., preheating or
heating a composition), liquid media (e.g,. preheating or heating a liquid for
process streams
or refining streams), and/or product streams (e.g., evaporating a liquid for
product
concentration). Clean vapor production is a useful function as defined herein,
because clean
vapor has many industrially useful functions. For example, clean vapor can be
used to
directly heat process streams without introducing any impurities into the
system. Passive heat
dissipation is an example of a function that is not included in the definition
of a "useful
function," as used herein.
[0035] As used herein, a "co-located plant" is a factory, mill, or other
type of
manufactory located on the same site, or within about 10 miles of the site,
where the energy
is produced and recovered. Typically, the flashed vapors or energized heat
transfer fluids are
fed to a co-located plant by way of piping or other means of conveyance.
[0036] As used herein, a "nearby plant" is a factory, mill, or other type
of manufactory
located about 10 miles to about 100 miles from the location where the energy
is produced and
recovered. Typically, the flashed vapors or energized heat transfer fluids are
fed to a nearby
plant by way of piping or other means of conveyance.
[0037] As used herein, "preheating" means a lower temperature heating stage
that
occurs prior to (e.g., immediately prior to) a subsequent higher temperature
heating stage.
[0038] As used herein, "clean vapor" means a vapor that is substantially
free of
impurities that are typically present in a flashed vapor (i.e., "dirty vapor")
obtained from flash
evaporation of a high temperature and high pressure process stream. For
example, when
processing biomass using reactive fluids, flashed vapors from the high
temperature and high
pressure process streams may contain volatile impurities, such as acetic acid,
formic acid, and
furfural, i.e., impurities that are produced in the process. In this
situation, the flashed "dirty
9
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
vapor" can be passed through an indirect contact heat exchanger containing a
clean fluid (i.e.,
a clean vapor exchanger), thereby generating a "clean vapor." The "clean
vapor" does not
contain the volatile impurities of the "dirty vapor," but still contains a
substantial amount of
recovered energy (in the form of heat and pressure). The clean fluid that
becomes the clean
vapor can be any suitable clean fluid that does not contain any components
that may be
undesirable to have in the process stream. Suitable clean fluids can comprise,
consist of, or
consist essentially of water, methanol, ethanol, propanol, butanol, pentanol,
or combinations
thereof Clean vapor is desirable in industrial processes, because the clean
vapor can be used
to directly admix with or inject into process streams without adding
undesirable impurities.
Moreover, directly admixing/injecting a clean vapor into process streams is
beneficial from
the standpoint of providing latent heat that is transferred upon condensation.
As used herein,
indirectly generating a clean vapor using recovered energy (e.g., in the form
of a flashed
vapor) is not considered to involve a heat transfer fluid.
[0039] As used herein, a "slurry" means a mixture comprising solids in a
liquid carrier.
In some embodiments, a "slurry" may be a suspension of fine particulates
(e.g., less than
1000 microns, less than about 750 microns, less than about 500 microns, less
than about 400
microns, less than about 300 microns, less than about 200 microns, or less
than about 100
microns) in a liquid carrier. In other embodiments, a slurry may be a mixture
comprising
larger particles (e.g., wood chips, about 0.25 inches to about 1 inch in
diameter) in a liquid
carrier. In some embodiments, a slurry is a suspension of fine particles in a
liquid carrier, in
which the slurry is a viscous paste having the consistency of, e.g.,
toothpaste, honey,
molasses, etc., at ambient conditions. As used herein, a slurry that is a
viscous paste is a
slurry having a viscosity of about 500 cP or more, e.g., about 1,000 cP or
more, 2,000 cP or
more, about 3,000 cP or more, about 4,000 cP or more, about 5,000 cP or more,
about 6,000
cP or more, about 7,000 cP or more, about 8,000 cP or more, about 9,000 cP or
more, about
10,000 cP or more, about 15,000 cP or more, about 20,000 cP or more, about
25,000 cP or
more, about 30,000 cP or more, about 50,000 cP or more, about 75,000 cP or
more, or about
100,000 cP or more. Alternatively, or in addition, a slurry that is a viscous
paste as defined
herein has a viscosity of about 100,000 cP or less, e.g., about 75,000 cP or
less, about 50,000
cP or less, about 30,000 cP or less, about 25,000 cP or less, about 20,000 cP
or less, about
15,000 cP or less, about 10,000 cP or less, about 9,000 cP or less, about
8,000 cP or less,
about 7,000 cP or less, about 6,000 cP or less, about 5,000 cP or less, about
4,000 cP or less,
about 3,000 cP or less, about 2,000 cP or less, or about 1,000 cP or less.
Thus, a viscous
paste as defined herein may have a viscosity defined by any or the foregoing
endpoints. For
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
example, the viscosity can be about 1,000 cP to about 50,000 cP, about 500 cP
to about 7,000
cP, or about 4,000 cP to about 25,000 cP.
[0040] As used herein, "continuous" means a process which is uninterrupted
for its
duration, or interrupted, paused or suspended only momentarily, relative to
the duration of the
process. A method is "continuous" when, for example, a slurry comprising a
feedstock
material is fed into an apparatus without interruption or without a
substantial interruption, or
when the method is not performed in a batch process.
[0041] The interaction of a reactive fluid and a material comprising
polymers and/or
oligomers typically produces reaction products, and these reaction products
can be
transformed into other products by any suitable method, such as enzymatically,
catalytically,
non-catalytically, biocatalytically, or by a combination thereof
[0042] As used herein, "transformed enzymatically" or "enzymatic
transformation"
means that the transformation (e.g., reaction) is effected by one or more
enzymes, or by
proteins or polypeptides having enzymatic activity (i.e., activity similar to
that of bona fide
enzymes).
[0043] As used herein, "transformed catalytically" or "catalytic
transformation" means
that the transformation (e.g., reaction) is effected by a catalyst or other
agent having catalytic
activity (e.g., acid, base, metal, and the like).
[0044] As used herein, "transformed non-catalytically" or "non-catalytic
transformation" means that the transformation (e.g., reaction) is effected by
a reactant or
reagent that is consumed in the reaction.
[0045] As used herein, "transformed biocatalytically" or "biocatalytic
transformation"
means that the transformation is effected by one or more organisms (e.g.,
bacteria, yeast,
algae, and the like).
[0046] As used herein, the terms "soluble" or "insoluble" refer to the
solubility of a
component or material in a liquid at ambient conditions, unless otherwise
specified or clearly
contradicted by context.
[0047] While the present invention is capable of being embodied in various
forms, the
description below of several embodiments is made with the understanding that
the present
disclosure is to be considered as an exemplification of the invention, and is
not intended to
limit the invention to the specific embodiments illustrated. Headings or
sections may be
provided for convenience only and are not to be construed to limit the
invention in any
manner. Embodiments illustrated under any heading or section may be combined
with
embodiments illustrated under any other heading or section.
11
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
[0048] The invention provides a method comprising, consisting of, or
consisting
essentially of:
providing a composition at a first temperature and a first pressure, wherein
the
composition comprises:
at least one material selected from the group consisting of a polymer, an
oligomer, and combinations thereof; and
a liquid;
optionally, preheating the composition in a first preheating stage to form a
preheated
composition, wherein the preheated composition is characterized by a second
temperature
and a second pressure;
heating the composition or the preheated composition with a reactive fluid in
a first
heating stage to form a heated composition, wherein the reactive fluid is
characterized by a
third temperature and a third pressure, and the heated composition is
characterized by a
fourth temperature and a fourth pressure;
wherein the reactive fluid is produced by a process comprising:
providing a fluid;
optionally, preheating the fluid in a second preheating stage to produce
a preheated fluid having a fifth temperature and a fifth pressure; and
heating the fluid or the preheated fluid in a second heating stage to
produce the reactive fluid; and
cooling the heated composition in a cooling stage to form a cooled
composition,
wherein the cooled composition is characterized by a sixth temperature and a
sixth pressure;
wherein:
the cooled composition comprises one or more reaction products derived from
the
material;
the cooling stage comprises a first flash evaporation, thereby producing a
first flashed
vapor and a first flashed composition;
the first flashed composition is the same as or different from the cooled
composition;
and
at least a portion of the first flashed vapor is used to perform a first
useful
function.
[0049] The steps in the method can be performed in any suitable order, as
would be
apparent to those of ordinary skill in the art.
12
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
[0050] Heating the composition or the preheated composition with a reactive
fluid
typically takes place by way of direct contact. For example, the reactive
fluid is directly
contacted with the composition or the preheated composition, in which energy
from the
reactive fluid is transferred to the composition or the preheated composition.
In some
embodiments, an indirect contact heat exchanger may be employed, in which the
reactive
fluid is not brought into direct contact with the composition or preheated
composition (e.g.,
through the use of a tube-in-tube indirect heat exchanger, as described
elsewhere herein).
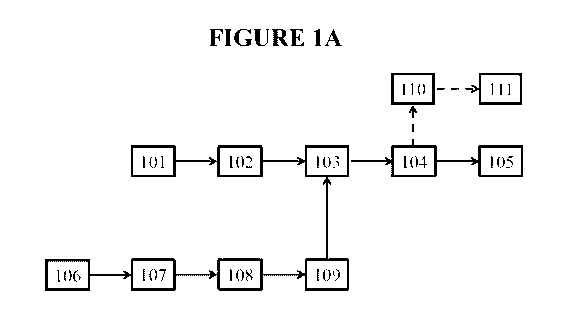
[0051] FIGURE lA depicts an embodiment of the invention. Dashed lines
generally
indicate the flow path of energy recovered in the process. A composition (101)
is provided
and optionally is preheated in a first preheating stage (102) to form a
preheated composition
(not shown). The composition (101) or preheated composition is heated with a
reactive fluid
(109) in a first heating stage (103) to form a heated composition (not shown).
The heated
composition is cooled in a cooling stage (104) to form a cooled composition
(105), in which
the cooling stage (104) comprises a first flash evaporation, thereby producing
a first flashed
vapor (110) and a first flashed composition (not shown). At least a portion of
the first flashed
vapor (110) is used to perform a first useful function (111). The reactive
fluid (109) is
produced by a process comprising providing a fluid (106), optionally
preheating the fluid
(106) in a second preheating stage (107) to form a preheated fluid (not
shown), and then
heating the fluid (106) or the preheated fluid in a second heating stage (108)
to form the
reactive fluid (109). In some embodiments, the cooled composition (105) may be
the same as
or different from the first flashed composition.
[0052] In some embodiments of the invention, in the cooling stage, the
heated
composition is contacted with a cool fluid having a temperature less than the
fourth
temperature (e.g., the heated composition is quenched with a cooler fluid)
prior to the first
flash evaporation. In some embodiments, in the cooling stage, after the first
flash
evaporation, the first flashed composition is contacted with a cool fluid
having a temperature
that is lower than a temperature of the first flashed composition (e.g., the
first flashed
composition is quenched with a cooler fluid). In some embodiments, the cooling
stage may
have any number of flash evaporations (e.g., one, two, three, four, five, six,
seven, and so on).
In some embodiments, in the cooling stage, the at least one flash evaporation
can be
combined, in any suitable order, with one or more indirect cooling steps with
a heat transfer
fluid (e.g., one, two three, four, five, six, seven, and so on). In some
embodiments, the
cooling stage comprises passive cooling. For example, the cooling stage may
comprise any
number and order of active cooling steps (e.g., flash evaporation, indirect
cooling with a heat
13
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
transfer fluid, and so on), and passive cooling may be performed at any point
before or after
the active cooling steps. As an example, a heated composition may be cooled
via flash
evaporation (to form a flashed vapor and a flashed composition), and then the
flashed
composition, still above ambient temperature, may be passively cooled (e.g.,
to ambient
temperature), if desired. In some embodiments, the cooled composition is at
ambient
conditions. In other embodiments, the cooled composition is at an elevated
temperature
and/or pressure, as compared to ambient conditions. In yet other embodiments,
the cooled
composition is cooled below ambient conditions, if desired.
[0053] In some embodiments of the invention, at least one of the optional
first and
second preheating stages is performed. In other embodiments, the first
preheating stage is
performed and the second preheating stage is not performed. In yet other
embodiments, the
first preheating stage is not performed and the second preheating stage is
performed. In some
embodiments, both the first preheating stage and the second preheating stage
are performed.
In other embodiments, neither the first preheating stage nor the second
preheating stage is
performed.
[0054] In some embodiments of the invention, the first useful function is
selected from
the group consisting of indirectly preheating the composition, indirectly
preheating the fluid,
and combinations thereof. In some embodiments, the first useful function is
selected from
the group consisting of active heating, electricity generation, feeding to a
co-located or
nearby plant, evaporation, clean vapor generation, and combinations thereof
[0055] The indirect preheating typically occurs by way of a tube-in-tube
heat
exchanger, in which the flashed vapor may be fed through an outer tube that
surrounds an
inner tube containing the composition. Alternate arrangements are
contemplated, for
example, in which the inner tube contains the first flashed vapor and the
outer tube contains
the composition. The flashed vapor and the composition may flow co-currently
or counter-
currently. Alternative apparatuses that are not tube-in-tube heat exchangers
are also
contemplated and may also be used for heat exchange.
[0056] In the course of the indirect preheating using flashed vapors, at
least a portion of
the flashed vapors may be condensed as heat is transferred from the relatively
hotter flashed
vapors to the relatively cooler composition, thereby forming a condensate
derived from the
flashed vapors. In some embodiments, at least a portion of the first flashed
vapor is
condensed to form a condensate, and at least a portion of the condensate can
be used for a
function selected from the group consisting of forming the composition used in
the process of
the invention, forming another composition that is different from the
composition used in the
14
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
process of the invention, directly cooling the heated composition prior to or
during the
cooling step (e.g., quenching the heated composition by direct contact of the
condensate with
the heated composition), directly cooling the first flashed composition, and
combinations
thereof Reusing/recycling at least a portion of the condensate may be
beneficial for
conserving water and lowering the overall cost of the process.
[0057] At least a portion of any vapor used in an indirect preheating stage
and exiting
the indirect preheating stage may be used for a useful function as described
herein. Any
exiting vapor typically will have a lower total energy (e.g., lower
temperature and/or lower
pressure) than the original flashed vapor, as a consequence of heat transfer
from the original
flashed vapor in the preheating stage. This lower energy vapor may be
desirable for a
number of reasons, for example, less sophisticated and/or less expensive
equipment may be
needed to handle the lower energy vapor, and/or the lower energy vapor may
have a proper
temperature and/or pressure for reusing in other parts of the process (or a co-
located or
nearby process) where a higher temperature vapor would be detrimental to a
process,
processing equipment, and/or the material being processed. For example,
certain equipment
may fail or the material being processed may decompose if exposed to a vapor
having too
high of a temperature and/or pressure.
[0058] In some embodiments of the invention, the method is continuous, semi-
continuous, batch, semi-batch, or combinations thereof.
[0059] In some embodiments of the invention, at least one of the optional
first and
second preheating stages is performed, and at least one of the first and
second preheating
stages does not employ energy recovered from the inventive process, and/or
does not reuse
energy recovered from a process occurring at a co-located or nearby plant. For
example, at
least one of the optional first and second preheating stages may instead
employ electrical
heating, combustion heating, induction heating, steam heating, saturated steam
heating, and
the like, and combinations thereof, as the source(s) for providing heat. In
some
embodiments, the optional first preheating stage is performed, and energy
other than that
recovered in the inventive method is used in the first preheating stage. In
some
embodiments, the optional second preheating stage is performed, and energy
other than that
recovered in the inventive method is used in the second preheating stage. In
some
embodiments, both the optional first and second preheating stages are
performed, and energy
other than that recovered in the inventive method is used in both the first
and second
preheating stages. In some embodiments, at least one of the first and second
preheating does
not use energy other than that recovered in the inventive method. In some
embodiments,
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
neither the first nor second preheating uses energy other than that recovered
in the inventive
method.
[0060] FIGURES 1B-1D depict some embodiments of the invention, in which at
least
one of the first and second preheating stages is performed, and in which the
first useful
function is selected from the group consisting of indirectly preheating the
composition,
indirectly preheating the fluid, and combinations thereof. Features 101-110 in
FIGURES
1B-1D are the same as the features having the same numbers in FIGURE 1A.
[0061] FIGURE 1B depicts an embodiment of the invention, in which at least
a first
preheating stage (102) is performed, and the first useful function is
indirectly preheating the
composition (101) with at least a portion of the first flashed vapor (110) in
the first preheating
stage (102) to form a preheated composition (not shown). After at least a
portion of the first
flashed vapor (110) is used in the first preheating stage (102), lower energy
vapor (112) and
condensate (113) may exit the first preheating stage (102) and at least a
portion of one or both
of the lower energy vapor (112) and condensate (113) may be used for other
useful functions,
as described elsewhere herein.
[0062] FIGURE 1C depicts an embodiment of the invention, in which both the
first
preheating stage (102) and the second preheating stage (107) are performed,
and in which the
first useful function is indirectly preheating the composition (101) and the
fluid (106) with at
least a portion of the first flashed vapor (110) in the first (102) and second
(107) preheating
stages, respectively.
[0063] FIGURE 1D depicts an embodiment of the invention, in which both the
first
preheating stage (102) and the second preheating stage (107) are performed,
and in which the
first useful function is indirectly preheating the composition (101) with at
least a portion of
the first flashed vapor (110) in the first preheating stage (102). Lower
energy vapor (112)
and condensate (113) issue from first preheating stage (102), and at least a
portion of the
lower energy vapor (112) is used to indirectly preheat fluid (106) in the
second preheating
stage (107). Even lower energy vapor (112) and condensate (113) may similarly
exit from
the second preheating stage (107) and at least a portion of one or both of the
lower energy
vapor (112) and condensate (113) may be used for other functions, as described
elsewhere
herein. The vapor exiting from the second preheating stage (107) typically has
an even lower
amount of total energy than the vapor exiting from the first preheating stage
(102), and thus
the vapor exiting from the second preheating stage (107) may be suitably
employed in
processes and equipment amenable to this even lower energy vapor. Although
FIGURE 1D
shows recovered energy flowing from the cooling stage (104) to the first
preheating stage
16
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
(102) to the second preheating stage (107), it is also contemplated that the
recovered energy
may instead flow from the cooling stage (104) to the second preheating stage
(107) to the first
preheating stage (102).
[0064] In some embodiments, the first useful function is indirectly
generating a first
clean vapor, and at least a portion of the first clean vapor is used to
perform a second useful
function. In some embodiments, the second useful function is not performed. In
some
embodiments, the second useful function is selected from the group consisting
of directly or
indirectly preheating the composition, directly or indirectly preheating the
fluid, and
combinations thereof In some embodiments, the second useful function is
selected from the
group consisting of active heating, electricity generation, feeding to a co-
located or nearby
plant, evaporation, clean vapor generation, and combinations thereof (as
described more fully
elsewhere herein).
[0065] Direct preheating of the composition or fluid with clean vapor
typically takes
place by way of a direct contact heat exchanger. In a direct contact heat
exchanger, the clean
vapor is directly contacted with the composition or the preheated composition,
in which
energy from the clean vapor is transferred to the composition or the preheated
composition.
In some embodiments, the clean vapor may provide energy to preheat the
composition and/or
fluid by way of an indirect contact heat exchanger, in which the clean vapor
is not brought
into direct contact with the composition or preheated composition, but instead
preheating is
carried out through the use of a tube-in-tube indirect heat exchanger, or some
other indirect
means, as described elsewhere herein.
[0066] It is notable that the use of an indirect contact heat exchanger to
cool a process
stream with a heat transfer fluid typically results in fouling on the surface
of the heat
exchanger, which is expensive and time consuming to remove. Removal of the
fouling
typically is necessary to ensure optimal performance of the process or
apparatus (e.g.,
optimum flow, pressure, etc.). In fact, if the fouling is not periodically
removed, the system
may fail. However, cooling a process stream by flash evaporation, and then
using a clean
vapor exchanger to indirectly generate clean vapors from the flashed vapors,
does not result
in any fouling (or at least results in significantly less fouling than the
fouling that occurs
when cooling a process stream in an indirect contact heat exchanger using a
heat transfer
fluid). Moreover, flash evaporation of a process stream also cools the process
stream much
faster than when cooling solely using an indirect contact heat exchanger with
a heat transfer
fluid. Therefore, the generation and use of clean vapors from the flashed
vapors of a process
17
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
stream has advantages over cooling a process stream with an indirect contact
heat exchanger
with a heat transfer fluid.
[0067] FIGURE 2A depicts an embodiment of the invention. Features 101-110
in
FIGURE 2A are the same as the features having the same numbers in FIGURE 1A.
Dashed
lines generally indicate the flow path of energy recovered in the process. In
FIGURE 2A,
the first useful function is indirectly generating a first clean vapor (115)
in clean vapor
exchanger (114) using at least a portion of the first flashed vapor (110), and
at least a portion
of the first clean vapor (115) is used to perform a second useful function
(116).
[0068] FIGURES 2B-2D depict some embodiments of the invention, in which at
least
one of the first and second preheating stages is performed, and in which the
second useful
function is selected from the group consisting of directly or indirectly
preheating the
composition, directly or indirectly preheating the fluid, and combinations
thereof Features
101-110 in FIGURES 2B-2D are the same as the features having the same numbers
in
FIGURE 1A. Dashed lines generally indicate the flow path of energy recovered
in the
process.
[0069] FIGURE 2B depicts an embodiment of the invention, in which at least
a first
preheating stage (102) is performed, and the second useful function is
directly or indirectly
preheating the composition (101) with at least a portion of the clean vapor
(115) in the first
preheating stage (102) to form a preheated composition (not shown). In
embodiments where
the second useful function is indirect preheating of the composition (101)
with at least a
portion of the clean vapor (115) in the first preheating stage (102), lower
energy vapor (not
shown) and condensate (not shown) may exit the first preheating stage (102)
and at least a
portion of one or both of the lower energy vapor and condensate may be used
for other
functions, as described elsewhere herein. For example, the lower energy vapor
exiting the
first preheating stage (102) may be used in the second preheating stage (107)
to preheat the
fluid (106).
[0070] FIGURE 2C depicts an embodiment of the invention, in which at least
a second
preheating stage (107) is performed, and the second useful function is
directly or indirectly
preheating the fluid (106) with at least a portion of the clean vapor (115) in
the second
preheating stage (107) to form a preheated fluid (not shown). In embodiments
where the
second useful function is indirect preheating of the fluid (106) with at least
a portion of the
clean vapor (115) in the second preheating stage (107), lower energy vapor
(not shown) and
condensate (not shown) may exit the second preheating stage (107) and at least
a portion of
one or both of the lower energy vapor and condensate may be used for other
functions, as
18
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
described elsewhere herein. For example, the lower energy vapor exiting the
second
preheating stage (107) may be used in the first preheating stage (102) to
preheat the
composition (101).
[0071] FIGURE 2D depicts an embodiment of the invention, in which both the
first
preheating stage (102) and the second preheating stage (107) are performed,
and in which the
second useful function is directly or indirectly preheating the composition
(101) and the fluid
(106) with at least a portion of the clean vapor (115) in the first (102) and
second (107)
preheating stages, respectively. In embodiments where the second useful
function is indirect
preheating of the composition (101) or the fluid (106) with at least a portion
of the clean
vapor (115) in the first preheating stage (102) or the second preheating stage
(107), lower
energy vapor (not shown) and condensate (not shown) may exit the first (102)
or second
(107) preheating stages, and at least a portion of one or both of the lower
energy vapor and
condensate may be used for other functions, as described elsewhere herein.
[0072] In some embodiments of the invention, in the cooling stage, the
first flash
evaporation is preceded by or followed by indirect cooling using a heat
transfer fluid, thereby
producing an energized heat transfer fluid and a heat-exchanged composition.
In other
words, the composition that is at an elevated temperature is cooled by
indirect cooling using a
heat transfer fluid, and the composition that now has a lower temperature is
considered the
heat-exchanged composition. In some embodiments, the energized heat transfer
fluid is used
to perform a third useful function. In some embodiments, the third useful
function is not
performed. In some embodiments, the third useful function is the same as or
different from
the first and/or second useful functions. In some embodiments, a heat transfer
fluid is not
employed, e.g., the method does not employ indirect cooling using a heat
transfer fluid.
[0073] The use of heat transfer fluids to cool process streams typically
takes place by
way of an indirect contact heat exchanger, in which the higher temperature
process stream
may be fed through an outer tube than surrounds an inner tube containing the
heat transfer
fluid. Alternate arrangements are contemplated, for example, in which the
inner tube
contains the higher temperature process stream and the outer tube contains the
heat transfer
fluid. The higher temperature process stream and the heat transfer fluid may
flow co-
currently or counter-currently.
[0074] FIGURES 3A and 3B depict two embodiments of the invention. Features
101-
110 in FIGURES 3A and 3B are the same as the features having the same numbers
in
FIGURE 1A. Dashed lines generally indicate the flow path of energy recovered
in the
process.
19
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
[0075] In FIGURE 3A, the cooling stage (104) comprises both a first flash
evaporation
(121) and indirect cooling (118) with a heat transfer fluid. More
particularly, in the cooling
stage (104) the first flash evaporation (121) is followed by indirectly
cooling (118) the first
flashed composition with a heat transfer fluid (117), thereby producing a heat
exchanged
composition (not shown) and an energized heat transfer fluid (119). The first
flashed vapor
(110) is used to perform a first useful function (111). The energized heat
transfer fluid (119)
is used to perform a third useful function (120), if desired. The energized
heat transfer fluid
(119) has a lower energy than it otherwise would have, had the indirect
cooling using the heat
transfer fluid not been preceded by the first flash evaporation. This lower
energy energized
heat transfer fluid may be desirable for a number of applications, such as
where a higher
energy energized heat transfer fluid would be detrimental to the equipment or
processed
material, as described elsewhere herein.
[0076] In FIGURE 3B, the cooling stage (104) comprises both indirect
cooling (118)
with a heat transfer fluid, as well as a first flash evaporation (121).
Specifically, the first
flash evaporation (121) is preceded by indirectly cooling (118) the first
flashed composition
with a heat transfer fluid (117), thereby producing a heat exchanged
composition (not shown)
and an energized heat transfer fluid (119). The energized heat transfer fluid
(119) is used to
perform a third useful function (120), if desired. The first flashed vapor
(110) is used to
perform a first useful function (111), and the first flashed vapor has a lower
energy than it
otherwise would have, had the first flash evaporation not been preceded by the
indirect
cooling using the heat transfer fluid. This lower energy first flashed vapor
may be desirable
for a number of applications, such as where a higher energy first flashed
vapor would be
detrimental to the equipment or processed material, as described elsewhere
herein.
[0077] In some embodiments, at least one of the first and second preheating
stages is
performed, and the third useful function is selected from the group consisting
of indirectly
preheating the composition, indirectly preheating the fluid, and combinations
thereof In
some embodiments, the third useful function is selected from the group
consisting of active
heating, electricity generation, feeding to a co-located or nearby plant,
evaporation, clean
vapor generation, and combinations thereof (as described more fully elsewhere
herein). In
some embodiments, the third useful function comprises indirectly preheating at
least one of
the composition and the fluid. In some embodiments, the third useful function
comprises
indirectly preheating both the composition and the fluid. In some embodiments,
the heat
transfer fluid is circulated in a continuous loop between the cooling stage
and at least one of
the first preheating stage and the second preheating stage. In some
embodiments, the heat
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
transfer fluid is circulated in a continuous loop between the cooling stage
and both the first
preheating stage and the second preheating stage, in any suitable order. As
used herein,
"circulating the heat transfer fluid in a continuous loop" means that the heat
transfer fluid
may first flow to the cooling stage where it obtains energy (e.g., acquires
thermal energy
from the heated composition), then the energized heat transfer fluid flows to
at least one of
the first and second preheating stages where it releases at least some of the
obtained energy
(e.g., thermal energy), and then the heat transfer fluid flows back to the
cooling stage where
the cycle is repeated. The heat transfer fluid need not (although may) cool to
ambient
temperature prior to restarting the cycle.
[0078] FIGURE 3C depicts an embodiment of the invention. Features 101-110
in
FIGURE 3C are the same as the features having the same numbers in FIGURE 1A.
Dashed
lines generally indicate the flow path of energy recovered in the process. In
FIGURE 3C,
the cooling stage (104) comprises both indirect cooling (118) with a heat
transfer fluid, as
well as a first flash evaporation (121). The first preheating stage (102) and
the second
preheating stage (107) are performed, and the third useful function is
indirectly preheating the
composition (101) and the fluid (106) in the first (102) and second (107)
preheating stages,
respectively. In this embodiment, the heat transfer fluid is circulated in a
continuous loop
from the indirect cooling (118) in the cooling stage (104), to the first
preheating stage (102),
to the second preheating stage (107), and then back to the indirect cooling
(118) in the
cooling stage (104) where the cycle repeats in a continuous loop. After
indirect cooling (118)
with the heat transfer fluid (117), the process stream is then subjected to a
first flash
evaporation (121), thereby producing a first flashed vapor (110) and a first
flashed
composition (not shown). The first flashed vapor (110) is used to perform a
first useful
function (111). The first flashed vapor (110) has a lower energy than it
otherwise would
have, had the first flash evaporation not been preceded by the indirect
cooling using the heat
transfer fluid. This lower energy first flashed vapor may be desirable for a
number of
applications, such as where a higher energy first flashed vapor would be
detrimental to the
equipment or processed material, as described elsewhere herein. Although
FIGURE 3C
shows recovered energy flowing from the cooling stage (104) to the first
preheating stage
(102) to the second preheating stage (107), it is also contemplated that the
recovered energy
may instead flow from the cooling stage (104) to the second preheating stage
(107) to the first
preheating stage (102).
[0079] Cooling higher temperature process streams with lower temperature
process
streams (e.g., within the same process, or from related or compatible
processes) can be
21
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
beneficial from the standpoint of directly and efficiently cooling higher
temperature process
streams, without substantially diluting the process streams. Cooling in this
manner prevents
the need to evaporate additional liquid from a process stream that may have
been added when
directly cooling with a cooler liquid (typically a pure liquid not containing
desirable solute or
solids) that is not a process stream. For example, the cooler process stream
may contain the
same or similar compounds as in the higher temperature process stream, and
thus directly
cooling the higher temperature process stream with the cooler process stream
would not
result in much (or at least would not result in a substantial amount of)
dilution. As used
herein, a "substantial" amount of dilution includes a change (decrease) in
concentration of at
least about 1% (e.g., at least about 5%, at least about 10%, at least about
15%, at least about
20%, or at least about 25%). In some embodiments, the heat-exchanged
composition can be
used to cool the first flashed composition. For example, when the heat-
exchanged
composition has a lower temperature than the first flashed composition, the
heat-exchanged
composition can be used to cool the first flashed composition. FIGURE 3A shows
an
embodiment of the invention, in which the heat-exchanged composition would
have a lower
temperature than the first flashed composition, as a consequence of the order
in the cooling
stage of the first flash evaporation and the indirect cooling with a heat
transfer fluid. In other
embodiments, the first flashed composition can be used to cool the heat-
exchanged
composition. For example, when the first flashed composition has a lower
temperature than
the heat-exchanged composition, the first flashed composition can be used to
cool the heat-
exchanged composition. FIGURES 3B and 3C show embodiments of the invention, in
which the first flashed composition would have a lower temperature than the
heat-exchanged
composition, as a consequence of the order in the cooling stage of the first
flash evaporation
and the indirect cooling with a heat transfer fluid.
[0080] In some embodiments of the invention, in the cooling stage, the
first flash
evaporation is preceded by or followed by a second flash evaporation, thereby
producing a
second flashed vapor and a second flashed composition. In some embodiments,
the second
flashed vapor is used to perform a fourth useful function. In some
embodiments, the fourth
useful function is not performed. In some embodiments, the fourth useful
function is the
same as or different from the first, second, and/or third useful functions. In
some
embodiments, a second flash evaporation is not performed. In some embodiments,
the
second flashed composition is the same as or different from the cooled
composition.
[0081] FIGURES 4A and 4B depict two embodiments of the invention. Features
101-
110 in FIGURES 4A and 4B are the same as the features having the same numbers
in
22
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
FIGURE 1A. Dashed lines generally indicate the flow path of energy recovered
in the
process.
[0082] In FIGURE 4A, the cooling stage (104) comprises both a first flash
evaporation
(121) and a second flash evaporation (122). More particularly, in the cooling
stage (104) the
first flash evaporation (121) is followed by a second flash evaporation (122),
thereby
producing a first flashed vapor (110) that is used to perform a first useful
function (111), and
a second flashed vapor (123) that is optionally used to perform a fourth
useful function (124).
The second flashed vapor (123) has a lower energy than it otherwise would
have, had the
second flash evaporation (122) not been preceded by the first flash
evaporation (121). This
lower energy second flashed vapor (123) may be desirable for a number of
applications, such
as where a higher energy flashed vapor would be detrimental to the equipment
or material
that is processed, as described elsewhere herein.
[0083] In FIGURE 4B, the cooling stage (104) comprises both a first flash
evaporation
(121) and a second flash evaporation (122). More particularly, in the cooling
stage (104) the
first flash evaporation (121) is preceded by a second flash evaporation (122),
thereby
producing a first flashed vapor (110) that is used to perform a first useful
function (111), and
a second flashed vapor (123) that is optionally used to perform a fourth
useful function (124).
The first flashed vapor (110) has a lower energy than it otherwise would have,
had the first
flash evaporation (121) not been preceded by the second flash evaporation
(122). This lower
energy first flashed vapor (110) may be desirable for a number of
applications, such as where
a higher energy flashed vapor would be detrimental to the equipment or
processed material,
as described elsewhere herein.
[0084] In some embodiments, at least one of the first and second preheating
stages is
performed, and the fourth useful function is selected from the group
consisting of indirectly
preheating the composition, indirectly preheating the fluid, and combinations
thereof In
some embodiments, the fourth useful function is selected from the group
consisting of active
heating, electricity generation, feeding to a co-located or nearby plant,
evaporation, clean
vapor generation, and combinations thereof (as described more fully elsewhere
herein). In
some embodiments, the fourth useful function is not performed. In some
embodiments, the
fourth useful function is indirectly generating a second clean vapor, and at
least a portion of
the second clean vapor is used to perform a fifth useful function. In some
embodiments, the
fifth useful function is the same as or different from the first, second,
third, and/or fourth
useful functions. In some embodiments, the fifth useful function is not
performed. In some
embodiments, at least one of the first and second preheating stages is
performed, and the fifth
23
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
useful function is selected from the group consisting of directly or
indirectly preheating the
composition, directly or indirectly preheating the fluid, and combinations
thereof. In some
embodiments, (i) at least one of the first and second preheating stages is
performed, (ii) the
first flash evaporation is followed by the second flash evaporation, and (iii)
the first useful
function is at least one of indirectly preheating the composition and
indirectly preheating the
fluid. In some embodiments, (i) the first flash evaporation is preceded by the
second flash
evaporation, (ii) the first useful function comprises indirectly generating a
first clean vapor,
and (iii) at least a portion of the first clean vapor is used to perform a
second useful function
(as described elsewhere herein). Many suitable arrangements of flash
evaporations and
recovered energy utilization are contemplated.
[0085] FIGURE 4C depicts an embodiment of the invention. Features 101-110
in
FIGURE 4C is the same as the features having the same numbers in FIGURE 1A.
Dashed
lines generally indicate the flow path of energy recovered in the process.
[0086] In FIGURE 4C, the cooling stage (104) comprises both a first flash
evaporation
(121) and a second flash evaporation (122). More particularly, in the cooling
stage (104) the
first flash evaporation (121) produces a first flashed vapor (110) and a first
flashed
composition (not shown). At least the second preheating stage (107) is
performed, and at
least a portion of the first flashed vapor (110) is used in the second
preheating stage (107) to
indirectly preheat the fluid (106). After at least a portion of the first
flashed vapor (110) is
used in the second preheating stage (107), lower energy vapor (112) and
condensate (113)
may exit the second preheating stage (107) and at least a portion of one or
both of the lower
energy vapor (112) and condensate (113) may be used for other useful
functions, as described
elsewhere herein. The second flash evaporation (122) produces a second flashed
vapor (123)
and a second flashed composition (not shown), and at least a portion of the
second flashed
vapor (123) is used to indirectly generate a first clean vapor (115) in clean
vapor exchanger
(114). Optionally, at least a portion of the first clean vapor (115) is used
for a second useful
function. The second flashed vapor (123) has a lower energy than it otherwise
would have,
had the second flash evaporation (122) not been preceded by the first flash
evaporation (121)
in the cooling stage (104). For this same reason, the first clean vapor (115)
also has a lower
energy.
[0087] In some embodiments of the invention, at least a portion of the
second flashed
vapor is condensed to form a condensate, and at least a portion of the
condensate can be used
for a function selected from the group consisting of forming the composition
used in the
process of the invention, forming another composition that is different from
the composition
24
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
used in the process of the invention, directly cooling the heated composition
prior to or
during the cooling step (e.g., quenching the heated composition by direct
contact of the
condensate with the heated composition), directly cooling the first or second
flashed
composition, and combinations thereof Reusing/recycling at least a portion of
the
condensate may be beneficial for conserving water and lowering the overall
cost of the
process. In FIGURE 4C, for example, when the second flashed vapor (123)
generates the
first clean vapor (115) in the clean vapor exchanger (114), at least a portion
of the second
flashed vapor (123) will condense to form a condensate (not shown) in clean
vapor exchanger
(114). At least one of lower energy vapor (not shown) and condensate (not
shown) likely
will issue from clean vapor exchanger (114) in this process.
[0088] In some embodiments of the invention, the second flashed composition
can be
used to cool the first flashed composition. For example, when the second
flashed
composition has a lower temperature than the first flashed composition, the
second flashed
composition can be used to cool the first flashed composition. FIGURES 4A and
4C show
embodiments of the invention, in which the second flashed composition would
have a lower
temperature than the first flashed composition, as a consequence of the order
in the cooling
stage of the first flash evaporation and the second flash evaporation. In
other embodiments,
the first flashed composition can be used to cool the second flashed
composition. For
example, when the first flashed composition has a lower temperature than the
second flashed
composition, the first flashed composition can be used to cool the second
flashed
composition. FIGURE 4B shows an embodiment of the invention, in which the
first flashed
composition would have a lower temperature than the second flashed
composition, as a
consequence of the order in the cooling stage of the first flash evaporation
and the second
flash evaporation. As described elsewhere herein, there are benefits to
recycling cooler
process streams to directly cool higher temperature process streams.
[0089] In some embodiments of the invention, at least one condition is
satisfied,
wherein the condition is selected from the group consisting of:
(a) the at least one material comprises, consists of, or consists essentially
of biomass,
cellulosic material, paper, cardboard, lignocellulosic material, municipal
waste, municipal
solid waste, manufacturing waste, food waste, agricultural residue, corn
stover, sugarcane
bagasse, grass, bark, dedicated energy crops, wood residue, sawmill and paper
mill discards,
hardwood, softwood, plastic, waste plastic, synthetic polymers or oligomers,
natural
polymers or oligomers, or combinations thereof;
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
(b) the composition comprises, consists of, or consists essentially of a first
solid
fraction and a first liquid fraction, wherein the first solid fraction
comprises, consists of, or
consists essentially of cellulose, insoluble lignin, and optionally insoluble
C5
oligosaccharides, and the first liquid fraction comprises, consists of, or
consists essentially of
at least one first soluble component selected from the group consisting of C5
monosaccharides, C5 oligosaccharides, xylose, arabinose, lyxose, ribose,
soluble lignin, and
combinations thereof;
(c) the cooled composition comprises, consists of, or consists essentially of
a second
solid fraction and a second liquid fraction, wherein the second solid fraction
comprises,
consists of, or consists essentially of insoluble lignin, and the second
liquid fraction
comprises, consists of, or consists essentially of at least one second soluble
component
selected from the group consisting of C6 monosaccharides, C6 oligosaccharides,
glucose,
galactose, mannose, fructose, soluble lignin, and combinations thereof;
(d) no more than about 10 wt.% of the reaction products, based on the total
weight of
the reaction products, is dihydrogen, methane, carbon dioxide, carbon
monoxide, tar, or
combinations thereof; and
(e) the reaction products comprise, consist of, or consist essentially of C6
monosaccharides, C5 monosaccharides, C6 oligosaccharides having a degree of
polymerization of 2 to 15, C5 oligosaccharides having a degree of
polymerization of 2 to 15,
depolymerization products of a plastic, or combinations thereof
[0090] In some embodiments of the invention, the material present in the
composition
can be any suitable material that comprises, consists of, or consists
essentially of a polymer,
an oligomer, or a combination thereof. Suitable materials that may be employed
are selected
from the group consisting of biomass, cellulosic material, paper, cardboard,
lignocellulosic
material, municipal waste, municipal solid waste, manufacturing waste, food
waste,
agricultural residue, corn stover, sugarcane bagasse, grass, bark, dedicated
energy crops,
wood residue, sawmill and paper mill discards, hardwood, softwood, plastic,
waste plastic,
synthetic polymers or oligomers, natural polymers or oligomers, and
combinations thereof
The polymer can be any polymer, such as a homopolymer, a co-polymer, a block
co-polymer,
a triblock co-polymer, a random copolymer, or combinations thereof
[0091] In some embodiments of the invention, the composition can comprise a
first
solid fraction and a first liquid fraction. In some embodiments, the first
solid fraction can
comprise cellulose, insoluble lignin, and optionally insoluble C5
oligosaccharides. In some
embodiments, the first liquid fraction may comprise at least one first soluble
component
26
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
selected from the group consisting of C5 monosaccharides, C5 oligosaccharides,
xylose,
arabinose, lyxose, ribose, soluble lignin, and combinations thereof. In some
embodiments,
the at least one material present in the composition can be part of the first
solid fraction, part
of the first liquid fraction, or a combination thereof.
[0092] In some embodiments of the invention, the cooled composition can
comprise a
second solid fraction and a second liquid fraction. In some embodiments, the
second solid
fraction can comprise insoluble lignin. In some embodiments, the second liquid
fraction can
comprise at least one second soluble component selected from the group
consisting of C6
monosaccharides, C6 oligosaccharides, glucose, galactose, mannose, fructose,
soluble lignin,
and combinations thereof
[0093] In some embodiments of the invention, no more than about 10 wt.% of
the
reaction products (e.g., no more than about 9 wt.%, no more than about about 8
wt.%, no
more than about about 7 wt.%, no more than about about 6 wt.%, no more than
about about 5
wt.%, no more than about about 4 wt.%, no more than about about 3 wt.%, no
more than
about about 2 wt.%, or no more than about about 1 wt.% of the reaction
products), based on
the total weight of the reaction products, is dihydrogen, methane, carbon
dioxide, carbon
monoxide, tar, or combinations thereof The amount of reaction products can
refer to the
reaction products individually, or in combination (e.g., in some embodiments
the reaction
products may contain no more than about 4 wt.% of dihydrogen, or the reaction
products may
contain no more than 4 wt.% of dihydrogen and carbon dioxide, for example). As
used
herein, "tar" is a mixture of hydrocarbons and free carbon. Dihydrogen,
methane, carbon
dioxide, carbon monoxide, and tar typically are reaction products of biomass
gasification
processes or biomass pyrolysis processes, or both. In some embodiments, the
method is not a
gasification process (e.g., a biomass gasification process) or a pyrolysis
process (e.g.,
biomass pyrolysis process), both of which are well known in the art.
[0094] In some embodiments of the invention, the reaction products are
selected from
the group consisting of C6 monosaccharides, C5 monosaccharides, C6
oligosaccharides having
a degree of polymerization of 2 to 15, C5 oligosaccharides having a degree of
polymerization
of 2 to 15, depolymerization products of a plastic, and combinations thereof.
C6
monosaccharides include, for example, glucose, galactose, mannose, fructose,
or
combinations thereof C6 oligosaccharides include, for example, oligomers of
glucose,
galactose, mannose, fructose, or combinations thereof, having a degree of
polymerization of 2
to 15. C5 monosaccharides include, for example, xylose, arabinose, lyxose,
ribose, or
combinations thereof C5 oligosaccharides include, for example, oligomers of
xylose,
27
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
arabinose, lyxose, ribose, or combinations thereof, having a degree of
polymerization of 2 to
15.
[0095] In some embodiments of the invention, the composition can be a
slurry, a
mixture, a suspension, a dispersion, a solution, a sludge, a syrup, a paste,
or a combination
thereof
[0096] In some embodiments of the invention, the composition comprises a
liquid, and
the liquid comprises, consists of, or consists essentially of water, methanol,
ethanol, propanol,
butanol, pentanol, carbon dioxide, sulfur dioxide, or combinations thereof In
a preferred
embodiment, the liquid comprises water. In some embodiments, the liquid does
not
comprise, consist of, or consist essentially of an organic solvent (e.g., an
exogenous organic
solvent).
[0097] In some embodiments of the invention, the solids content of the
composition,
based on the total weight of the composition, is about 1 wt.% or more, e.g.,
about 5 wt.% or
more, about 10 wt.% or more, about 11 wt.% or more, about 12 wt.% or more,
about 13 wt.%
or more, about 14 wt.% or more, about 15 wt.% or more, about 16 wt.% or more,
about 17
wt.% or more, about 18 wt.% or more, about 19 wt.% or more, about 20 wt.% or
more, about
21 wt.% or more, about 22 wt.% or more, about 23 wt.% or more, about 24 wt.%
or more,
about 25 wt.% or more, about 26 wt.% or more, about 27 wt.% or more, about 28
wt.% or
more, about 29 wt.% or more, about 30 wt.% or more, about 32 wt.% or more, or
about 34
wt.% or more. Alternatively, or in addition, the solids content of the
composition, based on
the total weight of the composition, is about 35 wt.% or less, e.g., about 34
wt.% or less,
about 32 wt.% or less, about 30 wt.% or less, about 29 wt.% or less, about 28
wt.% or less,
about 27 wt.% or less, about 26 wt.% or less, about 25 wt.% or less, about 24
wt.% or less,
about 23 wt.% or less, about 22 wt.% or less, about 21 wt.% or less, about 20
wt.% or less,
about 19 wt.% or less, about 18 wt.% or less, about 17 wt.% or less, about 16
wt.% or less,
about 15 wt.% or less, about 14 wt.% or less, about 13 wt.% or less, about 12
wt.% or less,
about 11 wt.% or less, about 10 wt.% or less, about 5 wt.% or less. Thus, the
solids content
of the composition can be bounded by any two of the foregoing endpoints. For
example, the
solids content of the composition can be about 15 wt.% to about 29 wt.%, about
10 wt.% to
about 18 wt.%, or about 24 wt.% to about 27 wt.%.
[0098] In some embodiments of the invention, the method is carried out
substantially
free of exogenous acid. In some embodiments of the invention, an exogenous
acid is not
employed. In other embodiments, an exogenous acid is employed. In some
embodiments,
the exogenous acid comprises, consists of, or consists essentially of an
organic acid, an
28
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
inorganic acid, or combinations thereof. In some embodiments, the exogenous
acid
comprises, consists of, or consists essentially of sulfuric acid, sulfonic
acid, phosphoric acid,
phosphonic acid, nitric acid, nitrous acid, hydrochloric acid, hydrofluoric
acid, hydrobromic
acid, hydroiodic acid, aliphatic carboxylic acids (such as acetic acid and
formic acid),
aromatic carboxylic acids (such as benzoic acid and salicylic acid),
dicarboxylic acids (such
as oxalic acid, phthalic acid, sebacic acid, and adipic acid), aliphatic fatty
acids (such as oleic
acid, palmitic acid, and stearic acid), aromatic fatty acids (such as
phenylstearic acid), amino
acids, carbonic acid (e.g., formed in situ by the addition of carbon dioxide),
sulfurous acid
(e.g., formed in situ by the addition of sulfur dioxide), or combinations
thereof
[0099] In some embodiments of the invention, at least one of the reactions
products are
transformed into a substance selected from the group consisting of gasoline,
jet fuel, butanol,
acetic acid, acetic anhydride, acetone, acrylic acid, adipic acid, benzene,
ethanol, ethylene,
ethylene glycol, ethylene oxide, methanol, polypropylene, terephthalic acid,
toluene, xylene,
1,3-propanediol, 1,4-butanediol, acetoin, alanine, arabitol, ascorbic acid,
aspartic acid, citric
acid, coumaric acid, fumaric acid, glycerol, glycine, kojic acid, lactic acid,
lysine, malonic
acid, proline, propionic acid, serine, sorbitol, succinic acid, threonine,
xylitol, sugar acids,
glucaric acid, gluconic acid, xylonic acids, acontic acid, glutamic acid,
malic acid, oxalic
acid, formic acid, acetaldehyde, 3-hydroxypropionic acid, 2,5-furan
dicarboxylic acid,
furfural, glutaric acid, itaconic acid, levulinic acid, and combinations
thereof. In some
embodiments, the transformation occurs enzymatically, catalytically, non-
catalytically,
biocatalytically, or by a combination thereof
[0100] In some embodiments of the invention, at least one condition is
satisfied,
wherein the condition is selected from the group consisting of:
(a) the first temperature is about 1 C to about 100 C, and/or the first
pressure is
about 14 psia to about 50 psia;
(b) the second temperature is about 100 C to about 250 C, and/or the second
pressure is about 14 psia to about 575 psia
(c) the third temperature is about 250 C to about 600 C, and/or the third
pressure is
about 575 psia to about 5500 psia;
(d) the fourth temperature is about 250 C to about 550 C, and/or the fourth
pressure
is about 575 psia to about 5500 psia;
(e) the fifth temperature is about 100 C to about 300 C, and/or the fifth
pressure is
about 14 psia to about 5500 psia; and
29
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
(f) the sixth temperature is about 1 C to about 500 C, the sixth pressure is
about 14
psia to less than about 5500 psia, the sixth temperature is lower than the
fourth temperature,
and/or the fifth temperature and the fifth pressure are the same as or
different from the first
temperature and the first pressure, respectively.
[0101] The first temperature can be any suitable temperature. For example,
the first
temperature can be about 1 C or more, e.g., about 5 C or more, about 10 C
or more, about
15 C or more, about 20 C or more, about 25 C or more, about 30 C or more,
about 35 C
or more, about 40 C or more, about 45 C or more, about 50 C or more, about
55 C or
more, about 60 C or more, about 65 C or more, about 70 C or more, about 75
C or more,
about 80 C or more, about 85 C or more, about 90 C or more, or about 95 C
or more.
Alternatively, or in addition, the first temperature can be about 100 C or
less, e.g., about 95
C or less, about 90 C or less, about 85 C or less, about 80 C or less,
about 75 C or less,
about 70 C or less, about 65 C or less, about 60 C or less, about 55 C or
less, about 50 C
or less, about 45 C or less, about 40 C or less, about 35 C or less, about
30 C or less,
about 25 C or less, about 20 C or less, about 15 C or less, about 10 C or
less, or about 5
C or less. Thus, the first temperature can be bounded by any two of the
foregoing endpoints.
For example, the first temperature can be about 35 C to about 75 C, about 65
C to about 95
C, or about 20 C to about 30 C.
[0102] The first pressure can be any suitable pressure. For example, the
first pressure
can be about 14 psia or more, e.g., about 14.7 psia or more, about 15 psia or
more, about 20
psia or more, about 25 psia or more, about 30 psia or more, about 35 psia or
more, about 40
psia or more, or about 45 psia or more. Alternatively, or in addition, the
first pressure can be
about 50 psia or less, e.g., about 45 psia or less, about 40 psia or less,
about 35 psia or less,
about 30 psia or less, about 25 psia or less, about 20 psia or less, about 15
psia or less, or
about 14.7 psia or less. Thus, the first pressure can be bounded by any two of
the foregoing
endpoints. For example, the first pressure can be about 14.7 psia to about 15
psia, about 25
psia to about 45 psia, or about 14 psia to about 30 psia.
[0103] The second temperature can be any suitable temperature. For example,
the
second temperature can be about 100 C or more, e.g., about 110 C or more,
about 120 C or
more, about 130 C or more, about 140 C or more, about 150 C or more, about
160 C or
more, about 170 C or more, about 180 C or more, about 190 C or more, about
200 C or
more, about 210 C or more, about 220 C or more, about 230 C or more, or
about 240 C or
more. Alternatively, or in addition, the second temperature can be about 250
C or less, e.g.,
about 240 C or less, about 230 C or less, about 220 C or less, about 210 C
or less, about
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
200 C or less, about 190 C or less, about 180 C or less, about 170 C or
less, about 160 C
or less, about 150 C or less, about 140 C or less, about 130 C or less,
about120 C or less,
or about 110 C or less. Thus, the second temperature can be bounded by any
two of the
foregoing endpoints. For example, the second temperature can be about 130 C
to about 230
C, about 110 C to about 140 C, or about 220 C to about 240 C.
[0104] The second pressure can be any suitable pressure. For example, the
second
pressure can be about 14 psia or more, e.g., about 14.7 psia or more, about 15
psia or more,
about 25 psia or more, about 50 psia or more, about 75 psia or more, about 100
psia or more,
about 125 psia or more, about 150 psia or more, about 175 psia or more, about
200 psia or
more, about 225 psia or more, about 250 psia or more, about 275 psia or more,
about 300 psia
or more, about 325 psia or more, about 350 psia or more, about 375 psia or
more, about 400
psia or more, about 425 psia or more, about 450 psia or more, about 475 psia
or more, about
500 psia or more, about 525 psia or more, or about 550 psia or more.
Alternatively, or in
addition, the second pressure can be about 575 psia or less, e.g., about 550
psia or less, about
525 psia or less, about 500 psia or less, about 475 psia or less, about 450
psia or less, about
425 psia or less, about 400 psia or less, about 375 psia or less, about 350
psia or less, about
325 psia or less, about 300 psia or less, about 275 psia or less, about 250
psia or less, about
225 psia or less, about 200 psia or less, about 175 psia or less, about 150
psia or less, about
125 psia or less, about 100 psia or less, about 75 psia or less, about 50 psia
or less, about 25
psia or less, about 15 psia or less, or about 14.7 psia or less. Thus, the
second pressure can be
bound by any two of the foregoing endpoints. For example, the second pressure
can be about
50 psia to about 275 psia, about 125 psia to about 500 psia, or about 400 psia
to about 450
psia.
[0105] The third temperature can be any suitable temperature. For example,
the third
temperature can be about 250 C or more, e.g., about 275 C or more, about 300
C or more,
about 325 C or more, about 350 C or more, about 375 C or more, about 400 C
or more,
about 425 C or more, about 450 C or more, about 475 C or more, about 500 C
or more,
about 525 C or more, about 550 C or more, or about 575 C or more.
Alternatively, or in
addition, the third temperature can be about 600 C or less, e.g., about 575
C or less, about
550 C or less, about 525 C or less, about 500 C or less, about 475 C or
less, about 450 C
or less, about 425 C or less, about 400 C or less, about 375 C or less,
about 350 C or less,
about 325 C or less, about 300 C or less, or about 275 C or less. Thus, the
third
temperature can be bound by any two of the foregoing endpoints. For example,
the third
31
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
temperature can be about 375 C to about 500 C, about 325 C to about 425 C,
or about
525 C to about 600 C.
[0106] The third pressure can be any suitable third pressure. For example,
the third
pressure can be about 575 psia or more, e.g., about 600 psia or more, about
700 psia or more,
about 800 psia or more, about 900 psia or more, about 1000 psia or more, about
1100 psia or
more, about 1200 psia or more, about 1300 psia or more, about 1400 psia or
more, about
1500 psia or more, about 1600 psia or more, about 1700 psia or more, about
1800 psia or
more, about 1900 psia or more, about 2000 psia or more, about 2100 psia or
more, about
2200 psia or more, about 2300 psia or more, about 2400 psia or more, about
2500 psia or
more, about 2600 psia or more, about 2700 psia or more, about 2800 psia or
more, about
2900 psia or more, about 3000 psia or more, about 3100 psia or more, about
3200 psia or
more, about 3300 psia or more, about 3400 psia or more, about 3500 psia or
more, about
3600 psia or more, about 3700 psia or more, about 3800 psia or more, about
3900 psia or
more, about 4000 psia or more, about 4100 psia or more, about 4200 psia or
more, about
4300 psia or more, about 4400 psia or more, about 4500 psia or more, about
4600 psia or
more, about 4700 psia or more, about 4800 psia or more, about 4900 psia or
more, about
5000 psia or more, about 5100 psia or more, about 5200 psia or more, about
5300 psia or
more, or about 5400 psia or more. Alternatively, or in addition, the third
pressure can be
about 5500 psia or less, e.g., about 5400 psia or less, about 5300 psia or
less, about5200 psia
or less, about 5100 psia or less, about 5000 psia or less, about 4900 psia or
less, about 4800
psia or less, about 4700 psia or less, about 4600 psia or less, about 4500
psia or less, about
4400 psia or less, about 4300 psia or less, about 4200 psia or less, about
4100 psia or less,
about 4000 psia or less, about 3900 psia or less, about 3800 psia or less,
about 3700 psia or
less, about 3600 psia or less, about 3500 psia or less, about 3400 psia or
less, about 3300 psia
or less, about 3200 psia or less, about 3100 psia or less, about 3000 psia or
less, about 2900
psia or less, about 2800 psia or less, about 2700 psia or less, about 2600
psia or less, about
2500 psia or less, about 2400 psia or less, about 2300 psia or less, about
2200 psia or less,
about 2100 psia or less, about 2000 psia or less, about 1900 psia or less,
about 1800 psia or
less, about 1700 psia or less, about 1600 psia or less, about 1500 psia or
less, about 1400 psia
or less, about 1300 psia or less, about 1200 psia or less, about 1100 psia or
less, about 1000
psia or less, about 900 psia or less, about 800 psia or less, about 700 psia
or less, or about 600
psia or less. Thus, the third pressure can be bounded by any two of the
foregoing endpoints.
For example, the third pressure can be about 1200 psia to about 3800 psia,
about 2000 psia to
about 2800 psia, or about 3700 psia to about 5400 psia.
32
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
[0107] The fourth temperature can be any suitable temperature. For example,
the
fourth temperature can be about 250 C or more, e.g., about 275 C or more,
about 300 C or
more, about 325 C or more, about 350 C or more, about 375 C or more, about
400 C or
more, about 425 C or more, about 450 C or more, about 475 C or more, about
500 C or
more, or about 525 C or more. Alternatively, or in addition, the fourth
temperature can be
about 550 C or less, e.g., about 525 C or less, about 500 C or less, about
475 C or less,
about 450 C or less, about 425 C or less, about 400 C or less, about 375 C
or less, about
350 C or less, about 325 C or less, about 300 C or less, or about 275 C or
less. Thus, the
fourth temperature can be bounded by any two of the foregoing endpoints. For
example, the
fourth temperature can be about 425 C to about 550 C, about 325 C to about
375 C, or
about 350 C to about 500 C.
[0108] The fourth pressure can be any suitable pressure. For example, the
fourth
pressure can be about 575 psia or more, e.g., about 600 psia or more, about
700 psia or more,
about 800 psia or more, about 900 psia or more, about 1000 psia or more, about
1100 psia or
more, about 1200 psia or more, about 1300 psia or more, about 1400 psia or
more, about
1500 psia or more, about 1600 psia or more, about 1700 psia or more, about
1800 psia or
more, about 1900 psia or more, about 2000 psia or more, about 2100 psia or
more, about
2200 psia or more, about 2300 psia or more, about 2400 psia or more, about
2500 psia or
more, about 2600 psia or more, about 2700 psia or more, about 2800 psia or
more, about
2900 psia or more, about 3000 psia or more, about 3100 psia or more, about
3200 psia or
more, about 3300 psia or more, about 3400 psia or more, about 3500 psia or
more, about
3600 psia or more, about 3700 psia or more, about 3800 psia or more, about
3900 psia or
more, about 4000 psia or more, about 4100 psia or more, about 4200 psia or
more, about
4300 psia or more, about 4400 psia or more, about 4500 psia or more, about
4600 psia or
more, about 4700 psia or more, about 4800 psia or more, about 4900 psia or
more, about
5000 psia or more, about 5100 psia or more, about 5200 psia or more, about
5300 psia or
more, or about 5400 psia or more. Alternatively, or in addition, the fourth
pressure can be
about 5500 psia or less, e.g., about 5400 psia or less, about 5300 psia or
less, about5200 psia
or less, about 5100 psia or less, about 5000 psia or less, about 4900 psia or
less, about 4800
psia or less, about 4700 psia or less, about 4600 psia or less, about 4500
psia or less, about
4400 psia or less, about 4300 psia or less, about 4200 psia or less, about
4100 psia or less,
about 4000 psia or less, about 3900 psia or less, about 3800 psia or less,
about 3700 psia or
less, about 3600 psia or less, about 3500 psia or less, about 3400 psia or
less, about 3300 psia
or less, about 3200 psia or less, about 3100 psia or less, about 3000 psia or
less, about 2900
33
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
psia or less, about 2800 psia or less, about 2700 psia or less, about 2600
psia or less, about
2500 psia or less, about 2400 psia or less, about 2300 psia or less, about
2200 psia or less,
about 2100 psia or less, about 2000 psia or less, about 1900 psia or less,
about 1800 psia or
less, about 1700 psia or less, about 1600 psia or less, about 1500 psia or
less, about 1400 psia
or less, about 1300 psia or less, about 1200 psia or less, about 1100 psia or
less, about 1000
psia or less, about 900 psia or less, about 800 psia or less, about 700 psia
or less, or about 600
psia or less. Thus, the fourth pressure can be bounded by any two of the
foregoing endpoints.
For example, the fourth pressure can be about 675 psia to about 1000 psia,
about 3000 psia to
about 3400 psia, or about 2800 psia to about 3300 psia.
[0109] The fifth temperature can be any suitable temperature. For example,
the fifth
temperature can be about 100 C or more, e.g., about 110 C or more, about 120
C or more,
about 130 C or more, about 140 C or more, about 150 C or more, about 160 C
or more,
about 170 C or more, about 180 C or more, about 190 C or more, about 200 C
or more,
about 210 C or more, about 220 C or more, about 230 C or more, about 240 C
or more,
about 250 C or more, about 260 C or more, about 270 C or more, about 280 C
or more, or
about 290 C or more. Alternatively, or in addition, the fifth temperature can
be about 300
C or less, e.g., about 290 C or less, about 280 C or less, about 270 C or
less, about 260 C
or less, about 250 C or less, about 240 C or less, about 230 C or less,
about 220 C or less,
about 210 C or less, about 200 C or less, about 190 C or less, about 180 C
or less, about
170 C or less, about 160 C or less, about 150 C or less, about 140 C or
less, about 130 C
or less, about 120 C or less, or about 110 C or less. Thus, the fifth
temperature can be
bounded by any two of the foregoing endpoints. For example, the fifth
temperature can be
about 120 C to about 180 C, about 140 C to about 290 C, or about 200 C to
about 250
C.
[0110] The fifth pressure can be any suitable pressure. For example, the
fifth pressure
can be about 14 psia or more, e.g., 14.7 psia or more, about 15 psia or more,
about 25 psia or
more, about 50 psia or more, about 75 psia or more, about 100 psia or more,
about 125 psia
or more, about 150 psia or more, about 175 psia or more, about 200 psia or
more, about 225
psia or more, about 250 psia or more, about 275 psia or more, about 300 psia
or more, about
325 psia or more, about 350 psia or more, about 375 psia or more, about 400
psia or more,
about 425 psia or more, about 450 psia or more, about 475 psia or more, about
500 psia or
more, about 525 psia or more, about 550 psia or more, about 575 psia or more,
about 600 psia
or more, about 700 psia or more, about 800 psia or more, about 900 psia or
more, about 1000
psia or more, about 1100 psia or more, about 1200 psia or more, about 1300
psia or more,
34
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
about 1400 psia or more, about 1500 psia or more, about 1600 psia or more,
about 1700 psia
or more, about 1800 psia or more, about 1900 psia or more, about 2000 psia or
more, about
2100 psia or more, about 2200 psia or more, about 2300 psia or more, about
2400 psia or
more, about 2500 psia or more, about 2600 psia or more, about 2700 psia or
more, about
2800 psia or more, about 2900 psia or more, about 3000 psia or more, about
3100 psia or
more, about 3200 psia or more, about 3300 psia or more, about 3400 psia or
more, about
3500 psia or more, about 3600 psia or more, about 3700 psia or more, about
3800 psia or
more, about 3900 psia or more, about 4000 psia or more, about 4100 psia or
more, about
4200 psia or more, about 4300 psia or more, about 4400 psia or more, about
4500 psia or
more, about 4600 psia or more, about 4700 psia or more, about 4800 psia or
more, about
4900 psia or more, about 5000 psia or more, about 5100 psia or more, about
5200 psia or
more, about 5300 psia or more, or about 5400 psia or more. Alternatively, or
in addition, the
fifth pressure can be about 5500 psia or less, e.g., about 5400 psia or less,
about 5300 psia or
less, about5200 psia or less, about 5100 psia or less, about 5000 psia or
less, about 4900 psia
or less, about 4800 psia or less, about 4700 psia or less, about 4600 psia or
less, about 4500
psia or less, about 4400 psia or less, about 4300 psia or less, about 4200
psia or less, about
4100 psia or less, about 4000 psia or less, about 3900 psia or less, about
3800 psia or less,
about 3700 psia or less, about 3600 psia or less, about 3500 psia or less,
about 3400 psia or
less, about 3300 psia or less, about 3200 psia or less, about 3100 psia or
less, about 3000 psia
or less, about 2900 psia or less, about 2800 psia or less, about 2700 psia or
less, about 2600
psia or less, about 2500 psia or less, about 2400 psia or less, about 2300
psia or less, about
2200 psia or less, about 2100 psia or less, about 2000 psia or less, about
1900 psia or less,
about 1800 psia or less, about 1700 psia or less, about 1600 psia or less,
about 1500 psia or
less, about 1400 psia or less, about 1300 psia or less, about 1200 psia or
less, about 1100 psia
or less, about 1000 psia or less, about 900 psia or less, about 800 psia or
less, about 700 psia
or less, about 600 psia or less, about 575 psia or less, about 550 psia or
less, about 525 psia or
less, about 500 psia or less, about 475 psia or less, about 450 psia or less,
about 425 psia or
less, about 400 psia or less, about 375 psia or less, about 350 psia or less,
about 325 psia or
less, about 300 psia or less, about 275 psia or less, about 250 psia or less,
about 225 psia or
less, about 200 psia or less, about 175 psia or less, about 150 psia or less,
about 125 psia or
less, about 100 psia or less, about 75 psia or less, about 50 psia or less,
about 25 psia or less,
about 15 psia or less, or about 14.7 psia or less. Thus, the fifth pressure
can be bounded by
any two of the foregoing endpoints. For example, the fifth pressure can be
about 300 psia to
about 4500 psia, about 2400 psia to about 3200 psia, or about 1300 psia to
about 2800 psia.
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
[0 1 1 1] The sixth temperature can be any suitable temperature. For
example, the sixth
temperature can be about 1 C or more, e.g., about 5 C or more, about 10 C
or more, about
25 C or more, about 50 C or more, about 75 C or more, about 100 C or more,
about 125
C or more, about 150 C or more, about 175 C or more, about 200 C or more,
about 225
C or more, about 250 C or more, about 275 C or more, about 300 C or more,
about 325
C or more, about 350 C or more, about 375 C or more, about 400 C or more,
about 425
C or more, about 450 C or more, or about 475 C or more. Alternatively, or in
addition, the
sixth temperature can be about 500 C or less, e.g., about 475 C or less,
about 450 C or less,
about 425 C or less, about 400 C or less, about 375 C or less, about 350 C
or less, about
325 C or less, about 300 C or less, about 275 C or less, about 250 C or
less, about 225 C
or less, about 200 C or less, about 175 C or less, about 150 C or less,
about 125 C or less,
about 100 C or less, about 75 C or less, about 50 C or less, about 25 C or
less, about 10
C or less, or about 5 C or less. Thus, the sixth temperature can be bounded
by any two of
the foregoing endpoints. For example, the sixth temperature can be about 275
C to about
425 C, about 10 C to about 150 C, or about 200 C to about 300 C.
[0112] The sixth pressure can be any suitable pressure. For example, the
sixth pressure
can be about 14 psia or more, e.g., 14.7 psia or more, about 15 psia or more,
about 25 psia or
more, about 50 psia or more, about 75 psia or more, about 100 psia or more,
about 125 psia
or more, about 150 psia or more, about 175 psia or more, about 200 psia or
more, about 225
psia or more, about 250 psia or more, about 275 psia or more, about 300 psia
or more, about
325 psia or more, about 350 psia or more, about 375 psia or more, about 400
psia or more,
about 425 psia or more, about 450 psia or more, about 475 psia or more, about
500 psia or
more, about 525 psia or more, about 550 psia or more, about 575 psia or more,
about 600 psia
or more, about 700 psia or more, about 800 psia or more, about 900 psia or
more, about 1000
psia or more, about 1100 psia or more, about 1200 psia or more, about 1300
psia or more,
about 1400 psia or more, about 1500 psia or more, about 1600 psia or more,
about 1700 psia
or more, about 1800 psia or more, about 1900 psia or more, about 2000 psia or
more, about
2100 psia or more, about 2200 psia or more, about 2300 psia or more, about
2400 psia or
more, about 2500 psia or more, about 2600 psia or more, about 2700 psia or
more, about
2800 psia or more, about 2900 psia or more, about 3000 psia or more, about
3100 psia or
more, about 3200 psia or more, about 3300 psia or more, about 3400 psia or
more, about
3500 psia or more, about 3600 psia or more, about 3700 psia or more, about
3800 psia or
more, about 3900 psia or more, about 4000 psia or more, about 4100 psia or
more, about
4200 psia or more, about 4300 psia or more, about 4400 psia or more, about
4500 psia or
36
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
more, about 4600 psia or more, about 4700 psia or more, about 4800 psia or
more, about
4900 psia or more, about 5000 psia or more, about 5100 psia or more, about
5200 psia or
more, about 5300 psia or more, or about 5400 psia or more. Alternatively, or
in addition, the
sixth pressure can be less than about 5500 psia, e.g., about 5400 psia or
less, about 5300 psia
or less, about5200 psia or less, about 5100 psia or less, about 5000 psia or
less, about 4900
psia or less, about 4800 psia or less, about 4700 psia or less, about 4600
psia or less, about
4500 psia or less, about 4400 psia or less, about 4300 psia or less, about
4200 psia or less,
about 4100 psia or less, about 4000 psia or less, about 3900 psia or less,
about 3800 psia or
less, about 3700 psia or less, about 3600 psia or less, about 3500 psia or
less, about 3400 psia
or less, about 3300 psia or less, about 3200 psia or less, about 3100 psia or
less, about 3000
psia or less, about 2900 psia or less, about 2800 psia or less, about 2700
psia or less, about
2600 psia or less, about 2500 psia or less, about 2400 psia or less, about
2300 psia or less,
about 2200 psia or less, about 2100 psia or less, about 2000 psia or less,
about 1900 psia or
less, about 1800 psia or less, about 1700 psia or less, about 1600 psia or
less, about 1500 psia
or less, about 1400 psia or less, about 1300 psia or less, about 1200 psia or
less, about 1100
psia or less, about 1000 psia or less, about 900 psia or less, about 800 psia
or less, about 700
psia or less, about 600 psia or less, about 575 psia or less, about 550 psia
or less, about 525
psia or less, about 500 psia or less, about 475 psia or less, about 450 psia
or less, about 425
psia or less, about 400 psia or less, about 375 psia or less, about 350 psia
or less, about 325
psia or less, about 300 psia or less, about 275 psia or less, about 250 psia
or less, about 225
psia or less, about 200 psia or less, about 175 psia or less, about 150 psia
or less, about 125
psia or less, about 100 psia or less, about 75 psia or less, about 50 psia or
less, about 25 psia
or less, about 15 psia or less, or about 14.7 psia or less. Thus, the sixth
pressure can be
bounded by any two of the foregoing endpoints. For example, the sixth pressure
can be about
3000 psia to about 3700 psia, about 300 psia to about 700 psia, or about 1500
psia to about
3200 psia.
[0113] The first flashed composition has a seventh temperature and a
seventh pressure,
and the seventh temperature and seventh pressure can be any suitable
temperature and
pressure. For example, the seventh temperature can be about 1 C or more,
e.g., about 5 C
or more, about 10 C or more, about 25 C or more, about 50 C or more, about
75 C or
more, about 100 C or more, about 125 C or more, about 150 C or more, about
175 C or
more, about 200 C or more, about 225 C or more, about 250 C or more, about
275 C or
more, about 300 C or more, about 325 C or more, about 350 C or more, about
375 C or
more, about 400 C or more, about 425 C or more, about 450 C or more, about
475 C or
37
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
more, about 500 C or more, about 525 C or more, or about 535 C or more.
Alternatively,
or in addition, the seventh temperature can be about 545 C or less, e.g.,
about 535 C or less,
about 525 C or less, about 500 C or less, about 475 C or less, about 450 C
or less, about
425 C or less, about 400 C or less, about 375 C or less, about 350 C or
less, about 325 C
or less, about 300 C or less, about 275 C or less, about 250 C or less,
about 225 C or less,
about200 C or less, about 175 C or less, about 150 C or less, about 125 C
or less, about
100 C or less, about 75 C or less, about 50 C or less, about 25 C or less,
about 10 C or
less, or about 5 C or less. Thus, the seventh temperature can be bounded by
any two of the
foregoing endpoints. For example, the seventh temperature can be about 75 C
to about 450
C, about 25 C to about 125 C, or about 350 C to about 545 C. The seventh
temperature
typically is lower than the fourth temperature.
[0114] The first flashed composition can have any suitable pressure
(seventh pressure).
For example, the seventh pressure can be about 14 psia or more, e.g., about
14.7 psia or more,
about 15 psia or more, about 25 psia or more, about 50 psia or more, about 75
psia or more,
about 100 psia or more, about 125 psia or more, about 150 psia or more, about
175 psia or
more, about 200 psia or more, about 225 psia or more, about 250 psia or more,
about 275 psia
or more, about 300 psia or more, about 325 psia or more, about 350 psia or
more, about 375
psia or more, about 400 psia or more, about 425 psia or more, about 450 psia
or more, about
475 psia or more, about 500 psia or more, about 525 psia or more, or about 550
psia or more.
Alternatively, or in addition, the seventh pressure can be about 575 psia or
less, e.g., about
550 psia or less, about 525 psia or less, about 500 psia or less, about 475
psia or less, about
450 psia or less, about 425 psia or less, about 400 psia or less, about 375
psia or less, about
350 psia or less, about 325 psia or less, about 300 psia or less, about 275
psia or less, about
250 psia or less, about 225 psia or less, about 200 psia or less, about 175
psia or less, about
150 psia or less, about 125 psia or less, about 100 psia or less, about 75
psia or less, about 50
psia or less, about 25 psia or less, about 15 psia or less, or about 14.7 psia
or less. Thus, the
seventh pressure can be bound by any two of the foregoing endpoints. For
example, the
seventh pressure can be about 50 psia to about 275 psia, about 125 psia to
about 500 psia, or
about 400 psia to about 450 psia.
[0115] The first flashed vapor has a ninth temperature and a ninth
pressure, and the
ninth temperature and ninth pressure can be any suitable temperature and
pressure. For
example, the ninth temperature can be about 1 C or more, e.g., about 5 C or
more, about 10
C or more, about 25 C or more, about 50 C or more, about 75 C or more,
about 100 C or
more, about 125 C or more, about 150 C or more, about 175 C or more, about
200 C or
38
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
more, about 225 C or more, about 250 C or more, about 275 C or more, about
300 C or
more, about 325 C or more, about 350 C or more, about 375 C or more, about
400 C or
more, about 425 C or more, about 450 C or more, about 475 C or more, about
500 C or
more, about 525 C or more, about 535 C or more, or about 545 C or more.
Alternatively,
or in addition, the ninth temperature can be about 550 C or less, e.g., about
545 C or less,
about 535 C or less, about 525 C or less, about 500 C or less, about 475 C
or less, about
450 C or less, about 425 C or less, about 400 C or less, about 375 C or
less, about 350 C
or less, about 325 C or less, about 300 C or less, about 275 C or less,
about 250 C or less,
about 225 C or less, about200 C or less, about 175 C or less, about 150 C
or less, about
125 C or less, about 100 C or less, about 75 C or less, about 50 C or
less, about 25 C or
less, about 10 C or less, or about 5 C or less. Thus, the ninth temperature
can be bounded
by any two of the foregoing endpoints. For example, the ninth temperature can
be about 375
C to about 425 C, about 125 C to about 250 C, or about 350 C to about 400
C. The
ninth temperature typically is lower than the fourth temperature.
[0116] The first flashed vapor can have any suitable pressure (ninth
pressure). For
example, the ninth pressure can be about 14 psia or more, e.g., 14.7 psia or
more, about 15
psia or more, about 25 psia or more, about 50 psia or more, about 75 psia or
more, about 100
psia or more, about 125 psia or more, about 150 psia or more, about 175 psia
or more, about
200 psia or more, about 225 psia or more, about 250 psia or more, about 275
psia or more,
about 300 psia or more, about 325 psia or more, about 350 psia or more, about
375 psia or
more, about 400 psia or more, about 425 psia or more, about 450 psia or more,
about 475 psia
or more, about 500 psia or more, about 525 psia or more, about 550 psia or
more, about 575
psia or more, about 600 psia or more, about 700 psia or more, about 800 psia
or more, about
900 psia or more, about 1000 psia or more, about 1100 psia or more, about 1200
psia or
more, about 1300 psia or more, about 1400 psia or more, about 1500 psia or
more, about
1600 psia or more, about 1700 psia or more, about 1800 psia or more, about
1900 psia or
more, about 2000 psia or more, about 2100 psia or more, about 2200 psia or
more, about
2300 psia or more, about 2400 psia or more, about 2500 psia or more, about
2600 psia or
more, about 2700 psia or more, about 2800 psia or more, about 2900 psia or
more, about
3000 psia or more, about 3100 psia or more, about 3200 psia or more, about
3300 psia or
more, about 3400 psia or more, about 3500 psia or more, about 3600 psia or
more, about
3700 psia or more, about 3800 psia or more, about 3900 psia or more, about
4000 psia or
more, about 4100 psia or more, about 4200 psia or more, about 4300 psia or
more, about
4400 psia or more, about 4500 psia or more, about 4600 psia or more, about
4700 psia or
39
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
more, about 4800 psia or more, about 4900 psia or more, about 5000 psia or
more, about
5100 psia or more, about 5200 psia or more, about 5300 psia or more, or about
5400 psia or
more. Alternatively, or in addition, the ninth pressure can be less than about
5500 psia, e.g.,
about 5400 psia or less, about 5300 psia or less, about5200 psia or less,
about 5100 psia or
less, about 5000 psia or less, about 4900 psia or less, about 4800 psia or
less, about 4700 psia
or less, about 4600 psia or less, about 4500 psia or less, about 4400 psia or
less, about 4300
psia or less, about 4200 psia or less, about 4100 psia or less, about 4000
psia or less, about
3900 psia or less, about 3800 psia or less, about 3700 psia or less, about
3600 psia or less,
about 3500 psia or less, about 3400 psia or less, about 3300 psia or less,
about 3200 psia or
less, about 3100 psia or less, about 3000 psia or less, about 2900 psia or
less, about 2800 psia
or less, about 2700 psia or less, about 2600 psia or less, about 2500 psia or
less, about 2400
psia or less, about 2300 psia or less, about 2200 psia or less, about 2100
psia or less, about
2000 psia or less, about 1900 psia or less, about 1800 psia or less, about
1700 psia or less,
about 1600 psia or less, about 1500 psia or less, about 1400 psia or less,
about 1300 psia or
less, about 1200 psia or less, about 1100 psia or less, about 1000 psia or
less, about 900 psia
or less, about 800 psia or less, about 700 psia or less, about 600 psia or
less, about 575 psia or
less, about 550 psia or less, about 525 psia or less, about 500 psia or less,
about 475 psia or
less, about 450 psia or less, about 425 psia or less, about 400 psia or less,
about 375 psia or
less, about 350 psia or less, about 325 psia or less, about 300 psia or less,
about 275 psia or
less, about 250 psia or less, about 225 psia or less, about 200 psia or less,
about 175 psia or
less, about 150 psia or less, about 125 psia or less, about 100 psia or less,
about 75 psia or
less, about 50 psia or less, about 25 psia or less, about 15 psia or less, or
about 14.7 psia or
less. Thus, the ninth pressure can be bounded by any two of the foregoing
endpoints. For
example, the ninth pressure can be about 3000 psia to about 3700 psia, about
300 psia to
about 700 psia, or about 1500 psia to about 3200 psia.
[0117] Other flashed evaporations may be employed, e.g., a third, fourth,
fifth, sixth,
etc. flashed evaporations, having third, fourth, fifth, sixth, etc. flashed
compositions and
flashed vapors, respectively, associated therewith. The temperatures and
pressures for a
second flashed composition and second flashed vapor, third flashed composition
and third
flashed vapor, fourth flashed composition and fourth flashed vapor, etc., can
be the same as
the temperatures and pressure disclosed herein for the first flashed
composition and first
flashed vapor. For example, the temperatures for the third, fourth, fifth,
etc., flashed
compositions can be about 1 C to about 545 C, or can be within any of the
temperature
ranges disclosed for the first flashed composition. Moreover, the pressures
for the third,
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
fourth, fifth, etc., flashed compositions can be about 14 psia to about 575
psia, or can be
within any of the pressure ranges disclosed for the first flashed composition.
Additionally,
the temperatures for the third, fourth, fifth, etc., flashed vapors can be
about 100 C to about
545 C, or can be within any of the temperature ranges disclosed for the first
flashed vapor.
Furthermore, the pressures for the third, fourth, fifth, etc., flashed vapors
can be about 14 psia
to about 570 psia, or can be within any of the pressure ranges disclosed for
the first flashed
vapor. The second flashed composition has an eighth temperature and pressure
associated
therewith, and the second flashed vapor has a tenth temperature and pressure
associated
therewith. Similar numbering schemes may be employed to differentiate the
temperature and
pressure of the third, fourth, fifth, sixth, etc., flashed compositions and
flashed vapors.
Typically, when multiple flash evaporations are employed in the inventive
method, the actual
temperatures and pressures of each flashed composition and vapor likely would
be different,
but the temperatures and pressures disclosed herein for the first flashed
composition are
applicable to these other flashed compositions.
[0118] The numbered temperatures and/or pressures (e.g., "first
temperature," "fifth
temperature," etc.) can have any suitable relationship to any other numbered
temperature
and/or pressure. For example, the relationship can be the same, the different,
higher than, or
lower than. In some embodiments, the second temperature is higher than the
first
temperature. In some embodiments, the second pressure is higher than the first
pressure. In
some embodiments, the fourth temperature is higher than the second
temperature. In some
embodiments, the fourth pressure is higher than the second pressure. In some
embodiments,
the fourth temperature is higher than the first temperature. In some
embodiments, the fourth
pressure is higher than the first pressure. In some embodiments, the third
temperature is
higher than at least one of the first, second, fourth, fifth, sixth, seventh,
eighth, and ninth
temperatures. In some embodiments, the third pressure is higher than at least
one of the first,
second, fourth, fifth, sixth, seventh, eighth, and ninth pressures. In some
embodiments, the
sixth temperature is lower than or higher than the second temperature. In some
embodiments,
the sixth temperature is the same as or different from the first temperature.
In some
embodiments, the sixth temperature is lower than or higher than the first
temperature. In
some embodiments, the sixth pressure is lower than or higher than the second
pressure. In
some embodiments, the sixth pressure is the same as or different from the
first pressure. In
some embodiments, the sixth pressure is lower than or higher than the first
pressure. In some
embodiments, the sixth temperature is lower than the fourth temperature. In
some
embodiments, the sixth pressure is lower than the fourth pressure. Other
suitable
41
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
comparisons can be made between numbered temperatures or pressures, and/or
numbered
flash compositions and flashed vapors, but are not necessarily explicitly
disclosed herein,
simply for brevity.
[0119] The heated composition typically is maintained at the fourth
temperature and
fourth pressure for about 0.02 s to about 600 s, preferably about 0.1 s to
about 60 s. This time
period is typically termed the "residence time." The residence time of the
heated
composition at the fourth temperature and fourth pressure typically is about
0.02 s or more,
e.g., about 0.05 s or more, about 0.1 s or more, about 0.15 s or more, about
0.2 s or more,
about 0.25 s or more, about 0.3 s or more, about 0.35 s or more, about 0.4 s
or more, about
0.45 s or more, about 0.5 s or more, about 0.55 s or more, about 0.6 s or
more, about 0.65 s or
more, about 0.7 s or more, about 0.75 s or more, about 0.8 s or more, about
0.85 s or more,
about 0.9 s or more, about 0.95 s or more, about 1 s or more, about 1.1 s or
more, about 1.2 s
or more, about 1.3 s or more, about 1.4 s or more, about 1.5 s or more, about
1.6 s or more,
about 1.7 s or more, about 1.8 s or more, about 1.9 s or more, about 2 s or
more, about 2.5 s
or more, about 3 s or more, about 3.5 s or more, about 4 s or more, about 4.5
s or more, about
s or more, about 5.5 s or more, about 6 s or more, about 6.5 s or more, about
7 s or more,
about 7.5 s or more, about 8 s or more, about 8.5 s or more, about 9 s or
more, about 9.5 s or
more, about 10 s or more, about 15 s or more, about 20 s or more, about 25 s
or more, about
30 s or more, about 35 s or more, about 40 s or more, about 45 s or more,
about 50 s or more,
about 55 s or more, about 60 s or more, about 70 s or more, about 80 s or
more, about 90 s or
more, about 100 s or more, about 110 s or more, about 120 s or more, about 130
s or more,
about 140 s or more, about 150 s or more, about 200 s or more, about 250 s or
more, about
300 s or more, about 350 s or more, about 400 s or more, about 450 s or more,
about 500 s or
more, or about 550 s or more. Alternatively, or in addition, the residence
time is about 600 s
or less, e.g., about 550 s or less, about 500 s or less, about 450 s or less,
about 400 s or less,
about 350 s or less, about 300 s or less, about 250 s or less, about 200 s or
less, about 150 s or
less, about 140 s or less, about 130 s or less, about 120 s or less, about 110
s or less, about
100 s or less, about 90 s or less, about 80 s or less, about 70 s or less,
about 60 s or less, about
55 s or less, about 50 s or less, about 45 s or less, about 40 s or less,
about 35 s or less, about
30 s or less, about 25 s or less, about 20 s or less, about 15 s or less,
about 10 s or less, about
9.5 s or less, about 9 s or less, about 8.5 s or less, about 8 s or less,
about 7.5 s or less, about 7
s or less, about 6.5 s or less, about 6 s or less, about 5.5 s or less, about
5 s or less, about 4.5 s
or less, about 4 s or less, about 3.5 s or less, about 3 s or less, about 2.5
s or less, about 2 s or
less, about 1.9 s or less, about 1.8 s or less, about 1.7 s or less, about 1.6
s or less, about 1.5 s
42
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
or less, about 1.4 s or less, about 1.3 s or less, about 1.2 s or less, about
1.1 s or less, about 1 s
or less, about 0.95 s or less, about 0.9 s or less, about 0.85 s or less,
about 0.8 s or less, about
0.75 s or less, about 0.7 s or less, about 0.65 s or less, about 0.6 s or
less, about 0.55 s or less,
about 0.5 s or less, about 0.45 s or less, about 0.4 s or less, about 0.35 s
or less, about 0.3 s or
less, about 0.25 s or less, about 0.2 s or less, about 0.15 s or less, about
0.1 s or less, or about
0.05 s or less. Thus, the residence time of the heated composition at the
fourth temperature
and fourth pressure can be bounded by any two of the foregoing endpoints. For
example, the
residence time can be about 0.01 s to about 0.9 s, about 60 s to about 550 s,
or about 1.5 s to
about 9.5 s.
[0120] The invention also provides an apparatus comprising, consisting of,
or
consisting essentially of:
optionally, a module configured for preheating a composition to form a
preheated
composition having a second temperature and a second pressure;
wherein the composition comprises:
at least one material comprising a polymer, an oligomer, or a
combination thereof; and
a liquid;
and wherein the composition has a first temperature and a first pressure;
a reactor configured for reacting the composition or the preheated composition
with a
reactive fluid to form a heated composition having a fourth temperature and a
fourth pressure;
wherein the reactive fluid has a third temperature and a third pressure;
a reactive fluid generator comprising:
optionally, a fluid preheater configured for preheating a fluid to produce a
preheated fluid having a fifth temperature and a fifth pressure; and
a heater configured for heating the fluid or the preheated fluid to form the
reactive fluid;
a first flash unit configured for a first flash evaporation to form a first
flashed vapor
and a first flashed composition, wherein the first flashed composition has a
seventh
temperature and a seventh pressure, the seventh temperature is less than the
fourth
temperature, and at least a portion of the first flashed vapor is used for a
first useful function;
optionally, a second flash unit configured for a second flash evaporation to
form a
second flashed vapor and a second flashed composition, wherein the second
flashed
composition has an eighth temperature and an eighth pressure, the eighth
temperature is less
than the fourth temperature, and optionally at least a portion of the second
flashed vapor is
43
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
used for a fourth useful function, wherein the fourth useful function is the
same as or
different from the first useful function;
optionally, at least one clean vapor exchanger, wherein at least one of the
first flashed
vapor and the second flashed vapor indirectly provides heat to a clean fluid
in the clean vapor
exchanger thereby producing a first clean vapor and/or a second clean vapor,
respectively,
and optionally at least a portion of the first clean vapor and/or the second
clean vapor is used
for a second useful function and or fifth useful function, respectively,
wherein the second
useful function is the same as or different from the first, fourth, and/or
fifth useful functions;
and
optionally, at least one indirect heat exchanger comprising a heat transfer
fluid,
wherein the at least one heat exchanger is configured for indirectly cooling a
process stream
processed by the apparatus, thereby producing an energized heat transfer
fluid, and the
energized heat transfer fluid optionally is used for a third useful function,
wherein the third
useful function is the same as or different from the first, second, fourth,
and fifth useful
functions.
[0121] The temperatures, pressures, and useful functions described
elsewhere herein
with respect to the inventive method are applicable to the temperatures,
pressures, and useful
functions, respectively, described for the inventive apparatus (e.g., the
ranges disclosed
herein for the first temperature of the method are applicable to the first
temperature for the
apparatus, the fourth useful function disclosed herein for the method are
applicable to the
fourth useful function for the apparatus, and so on).
[0122] The components of the inventive apparatus can be arranged in any
suitable
manner, relative to the flow direction of the process stream(s). In some
embodiments, the
first flash unit can be located before or after the second flash unit. In some
embodiments, the
heat exchanger can be located before or after the first flash unit. In some
embodiments, the
heat exchanger can be located before or after the second flash unit. In some
embodiments, a
heat exchanger is not employed. In some embodiments, a second flash unit is
not employed.
In some embodiments, three, four, five, six, seven, or eight flash units can
be employed. In
some embodiments, two, three, four, five, six, seven, or eight heat exchangers
can be
employed.
[0123] The first flash unit, second flash unit, and indirect heat
exchanger, if employed,
are typically used in a cooling stage to cool the heated composition.
[0124] FIGURE 5 depicts an embodiment of the invention. FIGURE 5 is
representative of an apparatus of the invention, although alternate
embodiments are
44
CA 02917125 2015-12-30
WO 2015/005918 PCT/US2013/049921
contemplated in accordance with the disclosures herein. Dashed lines generally
indicate the
flow path of energy recovered in the process. A composition (101) is provided
and optionally
is preheated in an optional first preheater (123) to form a preheated
composition (not shown).
The composition (101) or preheated composition is heated with a reactive fluid
(109) in
reactor (124) to form a heated composition (not shown). The heated composition
is cooled in
a cooling stage (104) to form a cooled composition (not shown). Typically,
energy is
recovered during the cooling stage. The cooled composition optionally is
contained in
optional receiving vessel (130). The cooling stage (104) comprises a first
flash unit (127) and
a second flash unit (128). The first flash unit (127) controls a first flash
evaporation and
produces a first flashed vapor (110) and a first flashed composition (not
shown). At least a
portion of the first flashed vapor (110) is used to perform a first useful
function (not shown).
The second flash unit (128) controls a second flash evaporation and produces a
second
flashed vapor (121) and a second flashed composition (not shown). At least a
portion of the
second flashed vapor (121) is fed to a clean vapor exchanger (129) to
indirectly generate
clean vapor (115). Optionally, at least a portion of clean vapor (115) can be
used for a useful
function (not shown). The reactive fluid (109) is produced in a reactive fluid
generator by a
process comprising providing a fluid (106), optionally preheating the fluid
(106) in an
optional second preheater (125) to form a preheated fluid (not shown), and
then heating the
fluid (106) or the preheated fluid in a heater (126) to form the reactive
fluid (109).
[0125] When ranges are used herein for conditions, such as temperature or
pressure, all
combinations and sub-combinations of the ranges therein are intended to be,
and are,
included.
[0126] The disclosures of each patent, patent application, and publication
cited or
described in this document are hereby incorporated herein by reference, in
their entireties.
[0127] Those skilled in the art will appreciate that numerous changes and
modifications
can be made to the preferred embodiments of the invention and that such
changes and
modifications can be made without departing from the spirit of the invention.