Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
Method for Synthesizing 3-Ethoxy-4-Ethoxycarbonyl Phenylacetic Acid
Field of the Invention
The present invention belongs to the pharmaceutical field, and relates to a
method for synthesizing a
Repaglinide intermediate, particularly a method for synthesizing 3-ethoxy-4-
ethoxycarbonyl
phenylacetic acid.
Background of the Invention
Repaglinide, with chemical name as S(+)-2-ethoxy-4-1243-methyl-1-(2-(1-
piperidyl) phenyl) butyl)
amino]-2-oxyethyll benzoic acid, belongs to megliginides, with the following
structural formula:
7 0 0 11
110 cols
Repaglinide is jointly developed by Boehringer IngelheimTM (a German company)
and Novo
NordiskTM (a Danish company), is a non-sulfonylurea insulinotropic agent, can
stimulate the pancreas
in the body of a Type-II diabetic patient and thereby simulate physiological
insulin secretion, has a
high protein binding ratio, can be absorbed and digested quickly, and has high
safety. It can be
administrated separately, or administrated in combination with other
hypoglycemic drugs to enhance
the therapeutic effect, and can adapt to patients with different life styles,
and thereby improves the
life quality of the patients.
Repaglinide is mainly synthesized from two intermediates: (S)-3-methyl-1-(2-(1-
piperidyl) phenyl)
butyl amine and 3-ethoxy-4-ethoxycarbonyl phenylacetic acid. In the prior
arts, the methods for
synthesizing 3-ethoxy-4-ethoxycarbonyl phenylacetic acid include:
A synthetic route of 3-ethoxy-4-ethoxycarbonyl phenylacetic acid reported by
M. Salman, et al. in
the literature "Synthesis of 3-Ethoxy-4-Ethoxycarbonyl Phenylacetic Acid, A
Key Acid Synthon of
Repaglinide" as follows:
0 0 0
CO2(g)
H CH3CH2Br 0"2' L DA /DM Pli 0Xi'. 'OC 2H 5
finc- K2C 0 3 I-1 3C - OC 2H 5 HO :C.A.'
002H5
In the reaction, 4-methyl salicylic acid is used as the raw material, and
reacts with bromoethane to
generate an ester compound under an alkaline condition; then, the ester
compound reacts with LDA
in a mixture of anhydrous THF and DMPU at -75 C, and then CO2 is added to
participate in the
carbonyl synthesis reaction, so that 3-ethoxy-4-ethoxycarbonyl phenylacetic
acid is obtained. The
method employs highly toxic reaction reagents including LDA and DMPU and
involves ultralow
temperature reaction (-75 C); therefore it is not suitable for industrial mass
production.
A synthetic route of 3-ethoxy-4-ethoxycarbonyl phenylacetic acid reported by
M. S. Reddy, et al. in
the document "Process for the Preparation of 3-Ethoxy-4-Alkoxy Carbonyl-Phenyl
Acetic Acid" as
follows:
CA 2918096 2017-06-30
CA 02918096 2016-01-12
2
follows:
0
1110 OH NAN%."2"6 NB% AIDA
fCTAVO2H3
H30 OH K2coa N3C :-4"."003843
rt _.
cciT '
002116
NCI(0)
NoON `002113
C2H9ON....
(&0022116
=
0C2H3 cow 00A5
L 00gH3
IUk
003N3
In the method, 4- methyl salicylic acid is used as the raw material, and
reacts with bromoethane under
an alkaline condition to generate an ester compound; then, the ester compound
is bromized, cyanided,
esterified, and hydrolyzed to obtain 3-ethoxy-4-ethoxycarbonyl phenylacetic
acid. The method employs
extremely toxic sodium cyanide and reagents including NBS, AIBN and carbon
tetrachloride, etc.,
involves dangerous operation and may result in severe environmental pollution;
therefore, it is also not
suitable for industrial mass production.
Thus, it is of great significance to develop a method for synthesizing 3-
ethoxy-4-ethoxycarbonyl
phenylacetic acid, which is safe to operate, has mild reaction conditions, and
.is suitable for industrial
mass production.
Contents of the Invention
The technical problem to be solved in the present invention is to provide a
method for synthesizing
3-ethoxy-4-ethoxycarbonyl phenylacetic acid, which employs three routes to
synthesize
3-ethoxy-4-ethoxycarbonyl phenylacetic acid, involves mild reaction reagents
and reaction conditions,
and the operation difficulties and the reaction risk are lowered .
To solve the technical problem described above, the present invention employs
the following technical
scheme:
A method for synthesizing 3-ethoxy-4-ethoxycarbonyl phenylacetic acid, which
employs
m-hydroxyphenylacetic acid (i.e., compound 11) as the raw material, and
utilizes one of the following
three routes to synthesize 3 -ethoxy-4-ethoxycarbonyl phenylacetic acid (i.e.,
compound 4):
(1) Route
The compound 11 is used as the raw, material, first, 4-formy173-
hydroxyphenylethyl acetate (i.e.,
compound 1) is synthesized, then, 4Lacetoacetate-2-hydroxybenzoic acid (i.e.,
compound 2) is
synthesized through an oxidation reaction, next, 3 -ethoxy-4-ethoxycarbonyl-
phenylethyl acetate (i.e.,
compound 3) is synthesized through esterification and etherification
reactions, and finally, the
compound 4 is obtained through a hydrolysis reaction;
The reaction equation is as equation I:
CA 02918096 2016-01-12
3
coom = rzooEt ...7,
OH (14114/0 ti
OEt 0E1
1/0 Oita ikv ''s.
metal' v5AsEirar. i
iiydrolysli w I
__________________________ lb,
142COOH H2COOEI (?-11:000E1 11143000EI óH2COOH
' (II) (1) (2) . ,(3) (4)
Equation I;
(2) Route II
The compound 11 is used as the raw material, first, 4-formy1-3-
ethoxyphenylethyl acetate (i.e.,
compound 5) is synthesized, next, 4-acetylcarbethoxy-2-ethoxybenzoic acid
(i.e,, compound 6) is
synthesized through an oxidation reaction, next, the compound 3 is synthesized
through an
esterification reaction, and finally, the compound 4 is obtained through a
hydrolysis reaction;
The reaction equation is as equation II:
i
,
Iireoa
zent) t Om t croet
OH........1 Et rLiiOrd (..,,,,()Et
Ederlamllaa Hydrobth
6. n
y
9:00011 POOR CHACOOEt CH2C008 H2CODII
(II) ($) (4) (5) (4)
Equation II;
(3) Route III
The compound 11 is used as the raw material, first, 4-formy1-3-
hydroxyphenylacetic acid (i.e.,
compound 7) is synthesized through a formylation reaction, then, 4-
carboxymethy1-2-hydroxybenzoic
acid (i.e., compound 8) is synthesized through an oxidation reaction, next,
the compound 3 is
synthesized through esterilication and etherification reactions, and finally,
the compound 4 is obtained
through a hydrolysis reaction;
The reaction equation is equation III:
HO Olki. ,.,õ
00gIpay..o. 00E1 1
OH L...t. OR
1
9., ___________________________ Chtlfttlon ri
ii...
01420001-1 ft:(NC-00H Cll*C 0 011 1-12000Et
àHCOO4
( I 1 ) ( 7? ( 8) ( 3) ( i)
=.
' Equation III;
As a definition in the present invention, the method for preparing the
compound 1 in the route I is
represented by the following equation IV or V:
CA 02918096 2016-01-12
4
OH OH OH
&Navaho% 13...71416..
1.12C0014 H2000Et ' (
''
.52:001E1
(I 1 ) (9) (1) :
. Equation IV;
cHO
OH OH jiilOccoi
1110 PtT.14110,. ellalinall kill I
EM.Ed....i/d1M1444/4,400 1110 = ======.1.¶04,11. I
H2C0 OH 0113C 00 it H2000E1
(11) (7) (1) = Equation V.
As another definition in the present invention, the method for preparing the
compound 5 in the route II
is represented by the following equation VI or VII:
r 0
OH 0E1 E. 0 t
7H2CEmanation snd ati
1 Elhortribn ib. = ""Ui'tk" a. i'I
00 H &IICOOlit Hacooet
(14 (to) (5) , Equation VI;
CHO CHO
# OR
p.¨,Ipii... . "Elle rifinnllen nrul Iti
.......,,..4.. 1 ,2111.11.._.4...
H2C00H CHO 0 ll CH21CICISI
=
(I I) (7) (5) : Equation VII.
As a third definition in the present invention, the process of the oxidation
reaction in the routes I, II or
III is:
The compound 1, compound 5, or compound 7 is used as the raw material, and is
mixed with an
oxidizer at 1:2-10 mole ratio, the mixture is kept to react for 1-5 hours at
room temperature, and the
reaction product is acidified and extracted, and the solvent is removed; thus,
the compound 2,
compound 6, or compound 8 is obtained respectively, as indicated by the
equation I, II, or III,
. As a further definition of the above-mentioned definitions, the oxidizer
is one of silver oxide, silver
nitrate, tert-butyl hydroperoxide, hydrogen peroxide, chromium trioxide,
chromic acid, sodium
dichromate, potassium dichromate, pyridinium dichromate, pyridinium
chlorochromate, sodium
hypohalitc, potassium hypohalite, manganese scsquioxide, and active manganese
dioxide.
As a fourth definition in the present invention, the process of the
formylation reaction in the route I, II
CA 02918096 2016-01-12
or III is:
The compound 9, compound 10, or compound 11 is used as the raw material, and
is mixed with a
formylation reagent at 1:1-8 mole ratio, the mixture is kept to react for 1-6
hours at 25-60r
temperature, water is added into the mixture, and the reaction product is
extracted, and the solvent is
removed; thus, the compound 1, compound 5, or compound 7 is obtained
respectively, as indicated by
the equation I, II, or III.
As a further definition of the above-mentioned definitions, the formylation
reagent is one of dimethyl
formamide, diethyl formamide, diisopropyl formamide, dibutyl formamide,
hexamethylenetetramine,
N-methyl-N-phenyl formamide, N-ethyl-N-phenyl forrnamide, paraforma1dehyde,
chloroform,
dichloromethyl methyl ether, dichloromethyl ethyl ether, and dichloromethyl
butyl ether.
As a fifth confinement in the present invention,
(1) The process of the esterification and etherification reactions in the
route I or route II is:
The compound 2, compound 7, or compound 11 is used as the raw material, and is
mixed with
bromoethane and an alkaline reagent at 1:2-9:1-6 mole ratio; the mixture is
refluxed for 2-7
hours, water is added into the mixture, and then the reaction product is
extracted and the solvent is
removed, so that the compound 3, compound 5, or compound 10 is obtained
respectively, as indicated
by the equation I or II;
(2) The process of the esterification and etherification reactions in the
route III is:
The compound 8 is mixed with bromoethane and an alkaline reagent at 1:3-9:3-6
mole ratio, the
mixture is refluxed for 2-7 hours, water is added into the mixture, and the
reaction product is extracted
and the solvent is removed, so that the compound 3 is obtained, as indicated
by the equation
HI, wherein, the alkaline reagent is one of potassium acetate, sodium acetate,
sodium methoxide,
sodium carbonate, potassium carbonate, sodium ethylate, and potassium ethyl
ate.
As a sixth definition in the present invention, the process of the
esterification reaction in the route I or
His:
The compound 6, compound 7, or compound 11 is used as the raw material, and is
mixed with absolute
ethyl alcohol and concentrated sulfuric acid; then, the mixture is refluxed
for 1-4 hours, the ethyl
alcohol is removed, the reaction product is extracted, and the solvent is
removed, so that the compound
I, compound 3, or compound 9 is obtained, as indicated by the equation I or
II, wherein, the mole ratio
of the raw material to the concentrated sulfuric acid is 1:0.05-1.
As another definition in the present invention, the process of the hydrolysis
reaction in the route I, II or
HI is:
Absolute ethyl alcohol, the compound 3, and an alkaline reagent are mixed, the
mixture is kept to react
for 0.5-3 hours at room temperature, water is added into the mixture, the
reaction product is extracted,
and the solvent is removed, so that 3-ethoxy-4-ethoxycarbonyl phenylacetic
acid (4) is obtained, as
indicated by equation I, II, or III, wherein,
The mole ratio of the compound 3 to the alkaline reagent is 1:1-3;
The alkaline reagent is sodium hydroxide or potassium hydroxide.
With the technical scheme described above, the present invention achieves the
following technical
CA 02918096 2016-01-12
6
progresses, when compared with the prior art:
In the present invention, 3-ethoxy-4-ethoxycalbonyl phenylacetic acid is
synthesized through three
routes respectively, all of the reaction reagents and reaction conditions
invOlved in the reactions are
mild; thus, the operation difficulties and the reaction risk are lowered.
In the present invention, the key factors that have influences on the yield in
the synthesis process of
3-ethoxy-4-ethoxycarbonyl phenylacetic acid mainly include:
(1) Oxidation reaction
The mole ratio of the raw material to the oxidizer is determined as 1:2-10, If
the mole ratio is smaller
than 1:2, the reaction of the raw material will be incomplete; if the mole
ratio is greater than 1:10, a
part of the oxidizer will be wasted, and the byproducts will increase.
In addition, the time of reaction between the raw material and the oxidizer is
1-5 hours, If the reaction
time is shorter than 1 hour, the reaction will be incomplete; if the reaction
time is longer than 5 hour,
the byproducts will increase significantly, and the product yield will be
decreased.
(2) Esterification and etherification reactions
=
The mole ratio of the raw material to bromoethane to the alkaline reagent is
determined as 1:2-9;1-6.
If the mole ratio is smaller than 1:2:1, the reaction will be incomplete, and
the impurities in the
product will increase; if the mole ratio is greater than 1:9:6, a part of the
reaction reagent will be wasted.
If the reaction temperature is lower than the reflux temperature, the reaction
time will increase
significantly, and the reaction will be incomplete.
(3) Formylation reaction
The mole ratio of the raw material to the formylation reagent is determined as
1:1-8. If the mole ratio
is smaller than 1:1, the reaction of the raw material will be incomplete, and
the product yield will be
low; if the mole ratio is greater than 1:8, a part of the formylation reagent
will be wasted, the
byproducts will increase, and the product yield will be decreased.
In addition, the temperature of the formylation reaction is 25-60 C. If the
reaction temperature is lower
than 25 , the raw material and the formylation reagent will hardly have a
reaction; if the reaction
temperature is higher than 60 r, the byproducts will be significantly
increased, and thc product yield
and purity will be decreased.
The present invention is applicable to preparation of a Repaglinide
intermediate -
3 -eth oxy-4-ethoxy carbonyl phenylacetic acid.
Hereunder the present invention will be further detailed in embodiments, with
reference to the
accompanying drawings.
Description of the Drawings
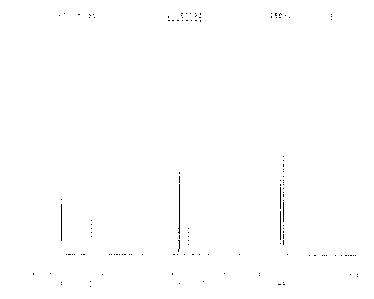
Fig.. 1 is a NMR spectrogram of the compound I (i.e, 4-formy1-3-
hydroxyphenylethyl acetate) in the
route 1(a) in example 1 of the present invention;
Fig. 2 is a NMR spectrogram of the compound 3 (i.e.) 3-ethoxy-4-ethoxycarbonyl-
phenylethyl acetate)
in the route 1(a) in example 1 of the present invention;
Fig. 3 is a NMR spectrogram of the compound 4 (i.e., 3-ethoxy-4-ethoxycarbonyl
phenylacetic acid) in
CA 02918096 2016-01-12
7
the route 1(a) in example 1 of the present invention.
Detailed Description of the Embodiments
Example 1: Method for synthesizing 3-ethoxy-4-ethoxycarbonyl phenylacetic acid
A method for synthesizing 3-ethoxy-4-ethoxycarbonyl phenylacetic acid,'
wherein m-hydroxy phenyl
acetic acid (i.e., compound 11) is used as the raw material, and finally 3-
ethoxy-4-ethoxycarbonyl
phenylacetic acid (i.e., compound 4) is synthesized.
Route I (a);
= i; CHO Or1.111. get
' OM
Emerlflulibi rdb., Hydrolysi;
t r
THICOOH HAMM HACoott &1ccOEt H2com
(1) (0) (1) (2) (3) (4)
The compound 11 is used as the raw material, first, m-hydroxyphenyl ethyl
acetate (i.e., compound 9)
is synthesized through an esterification reaction, then, 4-formy1-3-
hydroxyphenylethyl acetate (i.e.,
compound 1) is synthesized via a formylation reaction, next, 4-
acetylcarbethoxy-2-hydroxybenzoic
acid (i.e., compound 2) is synthesized through an oxidation reaction, then, 3-
.ethoxy-4- ethoxy
carbonyl-phenyl ethyl acetate (i,e,, compound 3) is synthesized through
esterification and etherific,ation
reactions, and finally, the compound 4 is obtained through a hydrolysis
reaction.
Specifically, the preparation process is:
(I) Esterification reaction
149.4m1 of absolute ethyl alcohol, 15.0g of compound 11, and 9.67g of
concentrated sulfuric acid are
mixed, the mixture is refluxed for 2 hours, ethyl alcohol is removed by rotary
evaporation, the product
is extracted with methylene chloride, and then the liquid extract is merged
and concentrated; thus,
17,0g of compound 9 is obtained. The yield ratio is 95.7%. Wherein, the
reaction mole ratio of the
compound 11 to the concentrated sulfuric acid is 1:1.
(II) Formylation reaction
5.0g of compound 9 and 4.06g of dimethyl formamide are mixed, the mixture is
kept to react for 2
hours at 30 C, water is added after the reaction, the product is extracted
with ethyl acetate, and then the
liquid extract is merged and concentrated; thus, 4.51g of compound 1 is
obtained. The yield ratio is
78.0%., wherein, the reaction mole ratio of the compound 9 to the dirnethyl
formamide is 1:2.
The NMR data of the compound 1 (i.e., 4-formy1-3-hydroxyphenyl ethyl acetate)
is as follows:
1H NIV1R (CDC13, 500 Hz, 5: ppm), 11.040 (s, 1H), 9.869 (s, 1H), 7,525 (s,
1H), 6.960 (s, 1H), 6,948(s,
1H), 4,177 (q, 2H), 3.631 (s, 2H), 1.264 (t, 3H). The NMR.spectrogram is shown
in Fig, 1,
(III) Oxidation reaction
2.5g of compound 1 and 13.93g of silver oxide are mixed, the mixture is kept
to react for 3 hours at
room temperature, the product is acidified with hydrochloric acid after the
reaction and is
CA 02918096 2016-01-12
,
,
8
extracted with ethyl acetate, and then the liquid extract is merged and
concentrated; thus, 2.35g of
, compound 2 is obtained. The yield ratio is 87.3%, wherein, the
reaction mole ratio of the compound 1
:
to the silver oxide is 1:5,
(IV) Esterification and etherifieation reactions
1.3g of compound 2, 2.53g of bromoethane, and 1.18g of sodium ethylate are
mixed, the mixture is
refiuxed for 4 hours, water is added after the reaction, the product is
extracted with ethyl ether, and the
liquid extract is merged and concentrated; thus, 1.46g of compound 3 is
obtained. The yield ratio is
90%. Wherein, the reaction mole ratio of the compound 2 to bromoethane to
sodium ethylate is 1:4:3.
The NMR data of the compound 3 (i.e., 3-ethoxy-4-ethoxycarbony1-phenylethyl
acetate) is as follows:
111 NMR (CDC13, 500 Hz, 8:ppm), 7.742 (s, 1H), 6.898 (s, 1H), 6.883 (s, 1H),
4.350 (q, 21-1), 4.155(q,
2H), 4.119 (q, 2H), 3.604 (s, 2H), 1.452 (t, 3H), 1,368 (t, 311), 1.243 (t,
3H). The NMR spectrogram is
shown in Fig. 2,
(V) Hydrolysis reaction
31.2m11 of absolute ethyl alcohol, 3.0g of compound 3, and 0.86g of sodium
hydroxide are mixed, the
mixture is kept to react for 1 hour at room temperature, water is added after
the reaction, the product is
extracted with methylene chloride, and the liquid extract is merged and
concentrated; thus, 2.40g of
compound 4 is obtained. The yield ratio is 89.0%. Wherein, the reaction mole
ratio of the compound 3
to the sodium hydroxide is 1:2.
.
.
The NMR data of the compound 4 (i.e., 3-ethoxy-4-ethoxyoarbonyl phenylacetic
acid) is as follows:
1H NMR (CDC13, 500 Hz, 8:ppm), 7.751 (s, 111), 6.890 (s, 111), 6.879 (s, 1H),
4.352 (q, 2H), 4.112 (q,
2H), 3.650 (s, 2H), 1.449 (t, 311), 1.367 (t, 31-1). The NMR spectrogram is
shown in Fig. 3.
Route 1(b):
. = qa COOEt
H =
aid.. (0-6,
Rm.
tilcoori :::::=i, 't.-lei a 11
yr
142COOEt 1-52000E
mi:rmonion
Hydrolyrls 1101
,.......,
HICOOEtOEt
H2C0(511
(11) (7) (1) (2) (3) (4)
The compound 11 is used as the raw material, first, 4-formy1-3-
hydroxyphenylacetic acid (i.e.,
compound 7) is synthesized through a formylation reaction, then, the compound
1 is synthesized
through an esterification reaction, next, the compound 2 is synthesized
through an oxidation reaction,
then, the compound 3 is synthesized through esterification and etherification
reactions, and finally, the
compound 4 is obtained through a hydrolysis reaction.
. .
.
Specifically, the preparation process is;
(I) Formylation reaction
2.8g of compound 11 and 9.32g of diethyl fotmamide are mixed, the mixture is
kept to react for 3 hours
at 40 C, water is added after the reaction, the product is extracted with
ethyl acetate, and then the liquid
CA 02918096 2016-01-12
9
extract is merged and concentrated; thus, 2.58g of compound 7 is obtained. The
yield ratio is
77.8%, wherein, the reaction mole ratio of the compound 11 to the diethyl
formamide is 1:5.
(II) Esterification reaction
6.5m1 of absolute ethyl alcohol, 2.5g of compound 7, and 0.07g concentrated
sulfuric acid are mixed,
the mixture is refluxed for 1 hour, ethyl alcohol is removed by rotary
evaporation, the product is
extracted with methylene chloride, and then the liquid extract is merged and
concentrated; thus, 2,75g
of compound 1 is obtained. The yield ratio is 95.3%. Wherein, the reaction
mole ratio of the compound
7 to the concentrated sulfuric acid is 1:0.05.
The NMR data of the compound 1 (i.e., 4-formy1-3-hydroxyphenyl ethyl acetate)
is as follows:
1H NMR_ (CDC13, 500 Hz, 8:ppm), 11.042 (s, 1H), 9.870 (s, 1H), 7.527 (s, 1H),
6.963 (s, 1H), 6.945 (s,
11-1), 4.179 (q, 211), 3.630 (s, 211), 1.266 (t, 311).
(III) Oxidation reaction
3.2g of compound 1 and 1.57g hydrogen peroxide are mixed, the mixture is kept
to react for 3 hour at
room temperature, the product is acidified with dilute sulfuric acid after the
reaction and is
extracted with ethyl ether, and then the liquid extract is merged and
concentrated; thus, 3.0g of
compound 2 is obtained. The yield ratio is 87%, wherein, the reaction mole
ratio of the compound 1 to
the hydrogen peroxide is 1:3,
= (IV) Esterification and etherification reactions
2.5g of compound 2, 2.43g of bromoethane, and 0.76g of sodium ethylate are
mixed, the mixture is
refiuxed for 2 hours, water is added after the reaction, the product is
extracted with ethyl ether, and the
liquid extract is merged and concentrated; thus, /Sg of compound 3 is
obtained. The yield ratio is
89.6%, wherein, the reaction mole ratio of the compound 2 to bromoethane to
sodium ethylate is 1:2:1,
The NMR data of the compound 3 (i.e., 3-ethoxy-4-ethoxyoarbonyl-phenylethyl
acetate) is as follows:
11-1 NMR (CDC13, 500 Hz, 8:ppm), 7.744 (s, 111), 6.895 (s, 1H), 6.882 (s, IH),
4.336 (q, 211), 4.152 (q,
211), 4.123 (q, 211), 1601 (s, 2H), 1.453 (t, 3111), 1.365 (t, 3H), 1.246 (t,
3H),
(V) Hydrolysis reaction
3.7m1 of absolute ethyl alcohol, 1.8g of compound 3, and 0,36g of potassium
hydroxide are mixed, the
mixture is kept to react for 2 hours at room temperature, water is added after
the reaction, the product is
= extracted with methylene chloride, and the liquid extract is merged and
concentrated; thus, 1.45g of
3-ethoxy-4-ethoxycarbonyl pheny1acetic acid (compound 4) is obtained. The
yield ratio is 89,2%.
Wherein, the reaction mole ratio of the compound 3 to the potassium hydroxide
is 1;1,
The NMR data of the compound 4 (i.e., 3-ethoxy-4-ethoxycarbonyl phenylacetic
acid) is as follows:
1H NMR (CDC13, 500 Hz, 8:ppm), 7.749 (s, 1H), 6.891 (s, 111), 6.877 (s, 111),
4,350 (q, 2H), 4.110 (q,
211), 3.651 (s, 2H), 1.448 (t, 3H), 1.366 (t, 3H).
Route 11(a):
CA 02918096 2016-01-12
1.6nsa....1
HO H 0E
Et
Et
adOzion
O
Et
0t.
Et
Hyd
11:1" (1
.C1 13 111
HIC150/4 H2C001Et HICOOEt HAO0E1 Ha000Et 00Et
=et
H2cooti
0.1) (10) = 0) (3) (4)
The compound 11 is used as the raw material, first, 3-ethoxyphenylethyl
acetate (i.e., compound 10) is
synthesized through esterification and etherification reactions, then, 4-
formy1-3-ethoxyphenylethyl
acetate (i.e., compound 5) is synthesized through a formylation reaction,
next,
4-acetylcarbethoxy-2-ethoxybenzoic acid (i.e., compound 6) is synthesized
through an oxidation
reaction, then, the compound 3 is synthesized through an esterification
reaction, and finally, the
compound 4 is obtained through a hydrolysis reaction.
Specifically, the preparation process is:
. (I) Esterification and etherification reactions
3.2g of compound 11, 6.88g of bromoethane, and 10.33g of potassium acetate are
mixed, the mixture is
refluxed for 6 hours, water is added after the reaction, the product is
extracted with methylene chloride,
and the liquid extract is merged and concentrated; thus, 3.97g of compound 10
is obtained. The yield
ratio is 90,6%, Wherein, the reaction mole ratio of the compound 11 to
bromoethane to potassium
acetate is 1:3:5,
(II) Forniylation reaction
4.0g of compound 10 and 11.46g of N-ethyl-N-phenyl formamide are mixed, the
mixture is kept to
react for 6 hours at 25V , water is added after the reaction, the product is
extracted with ethyl acetate,
and then the liquid extract is merged and concentrated; thus, 3.55g of
compound 5 is obtained. The
yield ratio is 78.3%, wherein, the reaction mole ratio of the compound 10 to
the N-ethyl-N-phenyl
formarnide is 1:4.
OM Oxidation reaction
4.2g of compound 5 and 14.24g of chromium trioxide are mixed, the mixture is
kept to react for 4
hours at room temperature, the product is acidified with dilute hydrochloric
acid after the reaction and
' is extracted with methylene chloride, and then the liquid extract is merged
and concentrated; thus,
3.90g of compound 6 is obtained. The yield ratio is 86.9%, wherein, the
reaction mole ratio of the
compound 5 to the chromium trioxide is 1:8.
(IV) Esterification reaction
8.1m1 of absolute ethyl alcohol, 3.5g of compound 6, and 0.109g of
concentrated sulfuric acid are
mixed, the mixture is refluxed for 4 hours, ethyl alcohol is removed by rotary
evaporation, the, product
is extracted with ethyl acetate, and then the liquid extract is merged and
concentrated; thus, 3.73g of
compound 3 is obtained. The yield ratio is 96.0%, wherein, the reaction mole
ratio of the compound 6
to the concentrated sulfuric acid is 1:0.08.
. The NMR data of the compound 3 (i.e., 3-ethoxy-4-ethoxycarbonyl-
phenylethyl acetate) is as follows:
CA 02918096 2016-01-12
=
=
11
1H NMR (CDC13, 500 Hz, 8:ppm), 7,740 (s, 111), 6.897 (s, 1H), 6.884 (s, IH),
4.337 (q, 2H), 4.154 (q,
2H), 4.125 (q, 2H), 3.602 (s, 2H), 1.450 (t, 3H), 1.367 (t, 3H), 1.244 (t,
3H).
(V) Hydrolysis reaction
15ml of absolute ethyl alcohol, 3.6g of compound 3, and 1,54g of sodium
hydroxide are mixed, the
mixture is kept to react for 3 hours at room temperature, water is added after
the reaction, the product is
extracted with methylene chloride, and the liquid extract is merged and
concentrated; thus, 2.87g of
3-ethoxy-4-ethoxycarbonyl phenylacetic acid (i.e. compound 4) is obtained. The
yield ratio is
88.5%, wherein, the reaction mole ratio of the compound 3 to the sodium
hydroxide is 1:3.
The NMR data of the compound 4 (i.e,, 3-ethoxy-4-ethoxycarbonyl phenylacetic
acid) is as follows:
I H NMR (CDC13, 500 Hz, 8:pprn), 7.750 (s, 1H), 6,891 (s, 1H), 6.877 (s, 111),
4.351 (q, 2H), 4.110 (q,
2H), 3.651 (s, 2H), 1,447 (t, 3H), 1.366 (t, 311).
Route 11(b):
HO= HO 00H ir =Et Et
1'1 dik.u....d -,.. = I
9P4 el õ,õõ. ,. 4 .t am
."..... 1 ., 0 , MIME 1 , , ,..,... So iva.o.
H2COOH H2000H KoCOOEt H2000Et I4OO H2cOH
(11) (7) OD (0) , (3) ' (4)
M-hydroxyphenyl acetic acid (i.e., compound 11) is used as the raw material,
first,
4-formy1-3-hydroxyphenylacetic acid (i.e., compound 7) is synthesized through
an formylation reaction,
then, 4-formy1-3-ethoxyphenylethyl acetate (i.e., compound 5) is synthesized
through esterification and
etherification reactions, next, 4-acetylcarbethoxy-2-ethoxybenzoic acid (i.e.,
compound 6) is
synthesized through an oxidation reaction, then, 3-ethoxy-4-ethoxycarbonyl-
phenylethyl acetate (i.e.,
compound 3) is synthesized through an esterification reaction, and finally, 3-
ethoxy-4-ethoxycarbonyl
phenylacetic acid (i.e., compound 4) is obtained through a hydrolysis
reaction.
Specifically, the preparation method is:
(1) Formylation reaction
2,9g of compound 11 and 1.72g of paraformaldehyde are mixed, the mixture is
kept to react for 1 hour
at 60 C, water is added after the reaction, the product is extracted with
ethyl ether, and then the liquid
extract is merged and concentrated; thus, 2.7g of compound 7 is obtained. The
yield ratio is
78.5%, wherein, the reaction mole ratio of the compound 11 to the
paraformaldehyde is 1:3.
(II) Esterification and etherification reactions
5.0g of compound 7, 27.24g of bromoethane, and 23.04g of potassium carbonate
are mixed, the
' mixture is refluxed for 6 hours, water is added after the reaction, the
product is extracted with
methylene chloride, and the liquid extract is merged and concentrated; thus,
5,95g of compound 5 is
obtained. The yield ratio is 90.8%. Wherein, the reaction mole ratio of the
compound 7 to bromoethane
to potassium carbonate is 1:9:6.
(III) Oxidation reaction
CA 02918096 2016-01-12
12
2.6g of compound 5 and 6.96g of manganese sesquioxide are mixed, the mixture
is kept to react for 5
hours at room temperature, the product is acidified with dilute sulfuric acid
after the reaction and is
extracted with ethyl acetate, and then the liquid extract is merged and
concentrated; thus, 2.4g of
compound 6 is obtained. The yield ratio is 86.9%, wherein, the reaction mole
ratio of the compound 5
to the manganese sesquioxide is 1:4.
(IV) Esterification reaction
14.3m1 of absolute ethyl alcohol, 5.68 of compound 6, and 0.131g of
concentrated sulfuric acid are
mixed, the mixture is refluxed for 4 hours, ethyl alcohol is removed by rotary
evaporation, the product
is extracted with ethyl acetate, and then the liquid extract is merged and
concentrated; thus, 5.97g of
compound 3 is obtained. The yield ratio is 96.0%,wherein, the reaction mole
ratio of the compound 6 to
the concentrated sulfuric acid is 1:0.06.
The NMR data of the compound 3 is as follows:
11-1 NMR (CDC13, 500 Hz, 8:ppm), 7.741 (s, 1H), 6.899 (s, 111), 6.8831 (s,
1H), 4.333 (q, 2H), 4.153
(q, 21-I), 4.125 (q, 2H), 3.603 (s, 211), 1.451 (t, 311), 1.366 (t, 3H), 1.242
(t, 31.1).
(V) Hydrolysis reaction ,
12m1 of absolute ethyl alcohol, 4.8g of compound 3, and 1.03g of sodium
hydroxide are mixed, the
mixture is kept to react for 2,5 hour at room temperature, water is added
after the reaction, the product
is extracted with methylene chloride, and the liquid extract is merged and
concentrated; thus, 3.85g of
3-ethoxy-4-ethoxycarbonyl phenylacetic acid (i.e. compound 4) is obtained. The
yield ratio is
89.2%, wherein, the reaction mole ratio of the compound 3 to the sodium
hydroxide is 1:1.5.
The NMR data of the compound 4 is as follows:
11-1 NMR (CDC13,500 Hz, 8:ppm), 7.749 (s, 111), 6.892 (s, 1H), 6.878 (s, 1H),
4.351 (q, 2H), 4.110 (q,
21-1), 3.651 (s, 2H), 1.446 (t, 3H), 1.366 (t, 3H).
Route III:
X..** soH 00E1 *see
OH 014 = Et Ott
Pormylallon Ilmenflenlo.ud
Oxidollon Hydrobrdi
õco. '1:41COCH 2COOH RACCIOEt H2COOH
(11) (7) (4) (3) (4)
The m-hydroxyphenyl acetic acid (i.e., compound 11) is used as the raw
material, first,
4-formy1-3-hydroxyphenyl acetic acid (i.e., compound 7) is synthesized through
a formylation reaction,
then, 4-carboxymethy1-2-hydroxybenzoic acid (i.e., compound 8) is synthesized
through an oxidation
reaction, next, 3-ethoxy-4-ethoxycarbony1-pheny1ethy1 acetate (i.e., compound
3) is synthesized
through esterifieation and etherification reactions, and finally, 3-ethoxy-4-
ethoxycarbonyl phenylacetic
acid (i.e., compound 4) is obtained through a hydrolysis reaction.
Specifically, the preparation method is:
CA 02918096 2016-01-12
13
(I) Formylation reaction
4,6g compound 11 and 12.73g hexamethyleneteiramine are mixed, the mixture is
kept to react for 3
hours at 30'C, water is added after the reaction, the product is extracted
with ethyl ether, and then the
liquid extract is merged and concentrated; thus, 4.25g of compound 7 is
obtained. The yield ratio is
78.1%, wherein, the reaction mole ratio of the compound 11 to the
hexamethylenetetramine is 1:3.
(II) Oxidation reaction
3.5g of compound 7 and 7.04g of potassium hypochlorite are mixed, the mixture
is kept to react for 5
hours at room temperature, the product is acidified with dilute hydrochloric
acid after the reaction and
is extracted with methylene chloride, and then the liquid extract is merged
and concentrated; thus, 3.3g
of compound 8 is obtained. The yield ratio is 86.6%, wherein, the reaction
mole ratio of the compound
7 to the potassium hypochlorite acid is 1:4.
(III) Esterification and etherification reactions
4.2g of compound 8, 9.34g of bromoethane, and 4.63g of sodium methoxide are
mixed, the mixture is
refluxed for 3 hours, water is added after the reaction, the product is
extracted with ethyl acetate, and
the liquid extract is merged and concentrated; thus, 5.46g of compound 3 is
obtained. The yield ratio is
91%, wherein, the reaction mole ratio of the compound 8 to bromoethane to
sodium methoxide is 1:4:4.
The NMR data of the compound 3 is as follows:
11-1 NMR (CDC13, 500 Hz, 8:ppm), 7.742 (s, 1H), 6.898 (s, 11-1), 6.883 (s,
1H), 4,335 (q, 2H), 4.155 (q,
2H), 4.126 (q, 2H), 3.604 (s, 2H), 1.452 (t, 3H), 1.368 (t, 3H), 1.243 (t,
3H).
(IV) Hydrolysis reaction
13.8m1 of absolute ethyl alcohol, 5.1g of compound 3, and 1.09g of sodium
hydroxide are mixed, the
mixture is kept to react for 2.5 hours at room temperature, water is added
after the reaction, the product
is extracted with methylene chloride, and the liquid extract is merged and
concentrated; thus, 4.22g of
compound 4 is obtained. The yield ratio is 92%, wherein, the reaction mole
ratio of the compound 3 to
the sodium hydroxide is 1:1.5.
The NMR data of the compound 4 is as follows:
1H NMR (CDC13,500 Hz, 8:ppm),7.753 (s, 1H), 6.889 (s, 1H), 6.878 (s, 1H),
4.352 (q, 2H), 4.110 (q,
2H), 3,651 (s, 2H), 1.447 (t, 3H), 1.366 (t, 31-I),
Examples 2-26: method for synthesizing 3-ethoxy-4-ethoxycarbonyl phenylacetic
acid
The examples 2-26 are methods for synthesizing 3-ethoxy-4-ethoxycarbonyl
phenylacetic acid
respectively, which zre similar to the method in the example 1, but involves
different technical
parameters, as shown in the following tables.
Technical parameters involved in the Route 1(a)
Example
Technical Parameter . .
2 3 4 5 - 6
Compound 11(g) 15 15 15 15 15¨
Esterifi- Concentrated
If c acid (g) 0.484 4.84 9.68 7.74 0.774
cation suri
=
reaction M [compound 11
10.05 10.5 1Ø8 1:0.08
concentrated gulfuric - ¨
CA 02918096 2016-01-12
14
--
.
- -- -
acid] -
Absolute ethyl
46 35 30 29 *3
alcohol (m1) _ __
Reflux reaction time 1 1.5 4 3 2
(h)
-
=
Obtained compound
17.03 1638 17.09 15.49 16.02
9(g) .
-
Yield ratio (%) 95.9 92.2 __ 96.2 87.2 90.2
Compound 9 (g) 5 5 5 5 5 . ....
Type of formylation Diethyl N-mthyl-N-phonyl Dichloromethyl
Dii
Chloroform
sopropylforalamide
reagent formamide formamidc
26.53 butyl ether
25.12
Amount (g) 2,81 18.75 11.09
, M [compound 9 ;
1:1 1:5 1:8 113 1:7
P rmY' formylation reagent] .
ation
Reaction
reaction 60 30 25 50 40
teFiperature (C)
Reaction time (h) 6 4 , 2 5 1 .
Obtained compound
4.54 4,55 4.5 4.48 4.45
1(i)
Yield ratio (%) 78.5 78.8 , 77.8 77-5 77-0
= Compound 1(g) 2.5 2.5 2_5 2.52.5
- ,
" Tett-butyl Sodium Potassium
Active manganese
Type of oxidizer
hydroperoxide Chromium trioxide
dichromate hypochloritc . dioxide
Amount OD 2.40
6.50 28 65 10.88 3.13 .
. .
Oxidati-
M [compound I :
on 1:6 1:2 1:8 1:10 1:3
oxidizer] .
reaction
=
Reaction time (h) 3 2.5 1 4 5
Obtained compound
2.34 2.37 2.36 2.33 2.35
2(g) .
Yield ratio (%). 87. 88 87.8 86.5 0.5
Compound 2(g) 1.3 1.3 1.3 L3 1.3
Bromoethane (g). 1.90 1,26 3.79 _ 5.69
4.43
. _.. -
Type of alkaline Potassium . Sodium
Sodium methoxide Sodium aeetate Potassium ethylate
reagent acetate carbonate
Esterifi- 0 2.86 1.47
Amount (g) 2.85 , 314 , 2.46
cation
M [compound 2:
and
bromoethane : 1:3:5 1:2;1 1:6:4 1:9:6 1:7:3
etherific
alkaline reagent)
- -
-ation
Reflux reaction time
reaction 3 7 2 5 6
(11) .õ.., _.
Obtained compound
1,48 1,43 1.39 1.50 1_45
3(g)
Yield ratio (%) 90_5 88 85 92 89
-
Compound 3 (g) 3.0 3.0 3.0 3,0 3.0
_
Type of alkaline Sodium Potassium Potassium Sodium
Sodium hydroxide
=
. reagent hydroxide . hydroxide hydroxide hydroxide
0.64
Amount (g) . . 0.43 1.50 1.80 0.86
Hydroiy M [compound 3 :
1:1 1:2.5 1:3 1:2 1:1.5
alkaline reagent]
reaction Absolute ethyl
20 25 . 32 15 10
alcohol (m1) ._ .
,. Reaction time (ti) 1 2.5 0.5 2 3
Compound 4(g) 2.43 2,48 2.38 2.46 2.32
Yield ratio (%) 90 . . 92 88 91 86
_ .
In this example, the reaction material feed in the reactions I-.V are
increased to 0.5-1.0kg, and the yield
ratios are similar to those in the examples 2.6, be,
The yield ratio of the reaction I is 87.2-962%; the yield ratio of the
reaction II is 77.-78.8%; the yield .
CA 02918096 2016-01-12
ratio of the reaction III is 86.5-88%; the yield ratio of the reaction IV is
85-92%; the yield ratio of the
reaction V is 86-92%.
Technical parameters involved in the Route 1(b)
. Technical Parameter Example -
-
7 8 9 10
ii
Compound 11(g) 2.8 2.8 2.8 2.8 .
2.8 .
, Type of formylation,c.orn4thocno.h....6, N-
othy1-N-phenyl Dichloromelhyl
Dibutylfermamide
Peteforrnaldchyde
reagent 8.69 20,66 for:timid methyl ether
3,32
Amount (g) 13.72 2.12
M [compound 11.
1:3 1:8 1:5 1:1
1:6
Pormyiation formylatlon reagent] ,
reaction Reaction
30 45 25 60
50
temperature ('C) _ ..
Reaction time (h) 3 1 6 4-
2
= -
Obtained compound
2.60 2.32 2.62 2.59
2.55
7(g) _
Yield ratio (%) 78.5 76 78.9 78
77
_ - _
Compound 7 (g) 2.5 2,5 2.5 2.5
2.5
Concentrated 0,12 0.48 0.07 1.36
1.09
sulfuric acid (g) -__; .
M [compound 7:
concentrated sulfuric 1Ø09 1:0.35 1:0.05 1:1
1:0.8
acid]
Esterification
Absolute cthyl
reaction 8.5 15 6 25
22
alcohol (ml)
.
Reflux reaction time
2 3 4 1
2.5
_
Obtained compound
2.77 2.72 2.60 2.57
2.80
. 1 (g)
97
Yield ratio (%) 96 94 90 89
. ..
Compound 1 (g) 3,2 3.2 3.2 3.2
3.2
Sodium Manganese Pyridinium
Type of oxidizer Silver nitrate Chromic acid
Amount (g) 3.63
hypochlorite
sesquioxide dichromate
13,06
9.16 24.29 . 17.36
Oxidation M [compound 1 :
1:5 1:2 1:8 1:10
1:3
reaction oxidizer]
Reaction time (h) 2,5 1 3.5 5
4
Obtained compound
3.03 3.02 2,96 2.93
3.05
2(g) _
Yield ratio (110 . 88 87.5 86
8588.5
. .
- Compound 2(g) 2.5 2,5 2.5 2.5
2.5
Bromoethane (g) 2.43 . .6.08 8,51 10.94
3.65
TYPe of alkaline Potassium .
Sodium acetate Sodium
Potassium Sodium
reagent carbonate 2.75 rriethoxide ethylale carbonate
Amount (g) 1.54 8.51 5.64
3.55
...
. EsteriticRtian m. [compound 2:
and
' bromoethane : 1:21 1:5:3 1:7:2 1:9:6 1:3:3
etheriticinion
ratetiona alkaline reagent'
Reflux reaction time .
5 2 3.5 7
4
(h)
-- - =
Obtained compound
2.84 2.75 2.78 2.75
2.81
. 3(g) .
.
' .
Yield ratio (%). . 91 88 89 88,1_
90
Compound 3(g) 1.8 1.8 1.8 1.8
1,8
Type of alkaline Sodium Sodium
Potassium Potassium
Potassium hydroxide
Hydrolysis reagent hydroxide 0 36 hydroxide
hydroxide hydroxide
reaction Amount (g) 0.46 0,77 0.90
0.72
= M
[compound 3: =
1:1.8 1:1 1:3 1:2.5
1:2
alkaline reagent]
CA 02918096 2016-01-12
16
=
Absolute ethyl
12 10 20 25. 18
alcohol (m1) .
-
Reaction time (h) 1 3 0.5 2.5
1.5
Compound 4(g) 1.44 1.51 1,43 1.47 .
1.39
Yield ratio (%) 89. 93 88 91
86
In this example, the reaction material feed in the reactions I-V are increased
to 0.5-1.0kg, and the yield
ratios are similar to those in the examples 7-11, i.e.: '
The yield ratio of the reaction I is 76-78.9%; the yield ratio of the reaction
H is 89-97%; the yield ratio
of the reaction III is 85-88.5%; the yield ratio of the reaction IV is 88-91%;
the yield ratio of the
reaction V is 86-93%.
Technical parameters involved in the Route H (a)
Example =
Technical Parameter
12 13 14 . 15 16
Compound 11 .
3.2 3.2 3.2 31 3.2
(8) - _
Bromoetbane (g) 8.03 4,59 11.47 20.65 4.59
Type of alkaline Potassium Sodium Potassium
Sodium methoxide Sodium
carbonate
= reagent carbonate ethylato
acetate
6,82 /23
Amount (8) 8,73 . 8,60 8.26
. 13aterlfication M [compound -
I and 11 :
113,53 1:2:6 1:5:4 1:9:6 . 1:2:1
ethorilicAlion broinoethane :
,
reactiona alkaline reagent]
Reflux reaction '
2.5 4 2 7 ' 5
time (h)
Obtained
compound 10 3.99 3.85 4.01 3,94 3.81
(13) - -
Yield ratio (%) 91 88 91.5 90 .. 87
Compound 10
4.0 4.0 4.0 4.0 4.0
60 .
Type of
=
formylation NInethyl-N=phonyl Dichloromethyl
Ditnethyl
Chloroform D i is opropylforrnemide
town mid c ethyl ethor formamidc
reagent 7.79 10,91 1.41 = 18.37
6.21
Amount (8) _
M [compound
Formylation 10 : formylation 1:3 1:6 1:1 1:8
1:2.5
reaction reagent]
Reaction
45 25 60 30 50
temperatu_re,(O. ) .,
Reaction time 25 1 3.5 6
, (h) . .
Obtained
3.63 3.54 3.59 3.50 3,52
compound 5 (a) _
Yield ratio (%) 80 78 79 77 77.-C--- -
- .
Compound 5 (g) 4.2 4.2 4.24.2 4.2
. . = _y .
Hydrogen Potassium Active
manganese
Typo of oxidizer Silver oxide Prldln iu ma
loroch romp te
peroxide dichromate
dioxide
Amount (g) 8.25 3.03 5236 23,02
. .
6.19
'
M [compound
1:2 . 1:5 1:10 . 1:6 . 1:4
Oxidation = 5: oxidizer] _
reaction Reaction time
2.5 5 3,5 2 1
(h) - -
.
Obtained
3.92 3.81 1.94 3.85 3.76
compound 6 (g) -
Yield ratio (%) 87.5 85 88 86 84
Compound 6 (g) 3.5 3.5 r- - 3,5 3.5
. .
CA 02918096 2016-01-12
17
. ..__
Concentrated
0.14 0.068 0.82 0.41
1.36
sulfuric acid (g)
M [compound
6 : concentrated 1:0.1 1:0,05 1:0.6 1:0,3
1 !I
sulfuric acid] ,
_
Absolute ethyl
EsterlficatIon /5 8 30 20
35
alcohol (nil)
reaction -
RCIlux reaction
2 4 3 2.5
1
time (h) -
Obtained
3.77 3.70 3.50 3.38
3.75
compound 3(g)
Yield ratio (%) 97 _ 95 90 87
96.5
Compound 3 _) 3.6 3.6 3.6 3.6
_ 3.6
Type of alkaline Potassium Sodium Sodium
Potassium hydroxide
Sodium hydroxide
reagent hydroxide hydroxide hydroxide
2,16 1,03
Amount (g) 1.80 0.51 0.77 ., .
M [compound
3 :alkaline I:2.5 1:1 1:1.5 1:3
12
Hydrolysis reagent'
reaction Absolute ethyl
21 7 12 Si
15
alcohol (ml)
Reaction time
2 1 0.5 1.5 3 "
(h)
Compound 4(g) 2.92 2.88 2.95 2.79
2.75
Yield ratio cio 90 89 91 86
85
, In this example, the reaction material feed in the reactions I-V are
increased to 0.5-1.0 kg, and the
actual yield ratios are similar to that in the examples 12-16, i.e.:
-
The yield ratio of the reaction I is 87-91.5%; the yield ratio of the reaction
II is 77-80%; the yield ratio
of the reaction III is 84-88%; the yield ratio of the reaction IV is 87-97%;
the yield ratio of the
reaction V is 85-91%.
= Technical parameters involved in the Route 11(b)
F .
Example ,
Technical Parameter -
17 18 19 20
21
Compound 11
2.9 2.9 2.9 2.9
2.9
(8) __
Type of
Diethyl Dichleromethyl bl-eUtyl-N-phenyl
Ilexamethyleneletraminc
nigopropyllormanude
fmnYladm formamide butyl ether fartnamIde
r 13.37
19.72
reagent
11.58 3.0 8.53
Amy u_nt(g) ., , _ .
M [compound
11:
1:6 1:1 1;3 ,1:5
1:8
formylalion
.
Formylation reagent]
-
reaction Reaction
temperature 30 50 25 45
60
CC) .
Reaction time
2.5 1 4 6
3.5
(h) ,
- -
Obtained
compound 7 2.71 2.61 2.68 2.59
2.57
(8)
Yield ratio -
79 76 78 75.5
75
e/o)
. ... ,
Eaterificntion Compound 7
5.0 5.0 5.0 5.0
5.0
and _ (g)
ethenficarion Bromoethane
reactions OZ) 15.13 27.24 6.05 21.19
6,05
. .
CA 02918096 2016-01-12
I
i
I
is
- _____________________________________________________________
Type of
Sodium Potassium
alkaline Sodium ethylate
Potassium acetate Sodium carbonate
acetate carbonate
reagent 1.89 8.18
14.72
9.11 23.03
Amountlizi
M [compound
7:
bromoethane : 1:5:4 1:9:6 1:2:1 1:7:31:2.5
alkaline
reagentt
Reflux
reaction time 4 2 5 7 3.5
(ii)
Obtained
compound 5 5.84 5 9 6.04 5.64
5.71
(8)
Yield ratio
89 90 92 86 87
(%)
Compound 5
2,6 2,6 2.6 2.6 2.6
(8)
Type of Tart-butyl Silver oxide Chromium
Potassium dichromate
Potaasiurn
oxidizer hydroperoxide trioxide
hypochlorite
5,11 12.96
Arnountlg) 9'93 5.51
7.98
M [compound
. 1:10 1:2 . 1:5 1:4 1:8
Oxidation 5 : oxidizer] .
reaction Reaction time 5 3.5 1 2.5 5
(h) _
Obtained
compound 6 2.42 2.45 . 2.31 222
2.28 .
,
(8)
Yield ratio
87 88 83 80 82
(%) .._
Compound -6--
5.6 5.6 5.6 5.6 5.6
(8) _
____________
Concentrated
sulfuric acid 0.87 0.22 0.11 1.52
2.18
(8)
M [compound
6:
1:0.4 1:0.1 1:0,05 1:0.7 1;1
concentrated
sultbric acid]
Esserification
-.
Absolute ethyl reaction Ab hl 20 12 8
26 35
alcohol (ml)
_____________________________________________________________________
Rell ux .
reaction time 4 2.5 I 3 2
(h)
Obtained
, compound 3 6.03 5.6 5.47 6.0
5.72
(8)
--Yield ratio.. --
97 90 88 96.5 92
CYO
Compound 3
4.8 4.8 4.8 4.8 4,8
(8) -- ._
Type of
Sodium Potassium Sodium
alkaline Sodium hydroxide
Potassium hydroxide
hydroxide hydroxide hydroxide
. 1.37
2.40 .
reagent
' 0.69 1.44 ' 2.06
Hydrolysis Amount (A) _
reaction M [compound
3 : alkaline 1:1 1:1.5 1:3 1:2
1:2.5
reagent]
Absolute ethyl
15 20 40 25 30
alcohol (m1) -
Reaction time . 3 1 0.5 2.5 1.5
CA 02918096 2016-01-12
19
(h)
_
Compound 4
3.89 3.80 4.02 3.93 3.87
(11)
Yield ratio
90 88 93 91 89.5
(%)
In this example, the reaction material inputs in the reactions I-V are
increased to 0.5-1.0 kg, and the
actual yield ratios are similar to that in the examples 17-21, i.e.:
The yield ratio of the reaction I is 75-79%; the yield ratio of the reaction
II is 86-92%; the yield ratio
of the reaction III is 80-88%; the yield ratio of the reaction IV is 88-97%;
the yield ratio of the
reaction V is 88-93%.
Technical parameters involved in the Route III
= =
Example
Technical Parameter
22 - 23 24 25 26
_
_
Compound 11(g) 4.6 4.6 4.6 4.6 4.6
- --
Type of formylation reagent Chloroform methyl
DichloromcthylDibutylfonnamide Punfoimaldehyde
formamide
methyl ether
Amount (g) 25.29 23.79 7.26
221 10.44
Formylation = M [compound 11 : =
1:7 1:1 1:5 1:8 1:3
reaction formylation reagent]
. .
Reaction temperature (r) 25 60 45 3550
-= -
Reaction time_fh)1 5 6 3.5 4.5
. ,
Obtained compound 7(g) 4.14 4.31 4.36 3.92
4.03
Yield ratio (%) 76 79 80 72 74
Compound 7 (g) 3.5 3.5 3.5 3.5 3.5 ,
Silver Sodium Active manganese
Hydrogen Sodium
Type of oxidizer
nitrate dichromate dioxide peroxide
hypochlorite
Amount (g)
Oxidation 11.56 11.59 10.14 6.61
10,13
- = ,
reaction M [compound 7 : oxidizer] 1:15 1:2 1:6 -
1:10 1;7
Reaction time (h) 2 5 1 4,5 3 .
= Obtained compound 8 (g) 3.24
343 3.05 3.35 3.09
Yield ratio (%) 85 90 80 88 81
Compound 8 (g) 4.2 4.2 4,2 -
4,2
4,2
Bromoethane (g) 21.01 11.67 9.34- 7.0 16.34
-
_
Potassium Sodium Sodium
Type of alkaline reagent carbonate methoxide Potassium acetate
Sodium acetate
carbonate
EsterIfication Amount (g) .
631 5.27
17.77 4.63
11.36
and
ethcrification M [compound 8:
bromoetbane : alkaline 1:9:6 1:5:4 1:4:3 1:3:3 1:7:5
reactions
reagent]
Reflux reaction time (h) 5 3.5 7 2 4
_
Obtained compound 3 (g) 5.28 5.52 4.92 5.4
5.04
' Yield ratio (%). 88 92 '
82' 90 84
' Compound 3 (g) 5.1 5.1 5.1 - .
5,1 5.1
Potassium Sodium Potassium Sodium
Sodium hydroxide hydroxide hydroxide
Type of alkaline reagent
hydroxide hydroxide
Amount (g) 1.82
..., 1.02 2,19 2,04
1.09
Hydrolysis M [compound 3 : alkaline
1:1 1.3 1:2.5 1;2 1;1,5
reaction reagent]
Absolute ethyl alcohol (m1) 10 40 26 18
,12
_ Reaction time (h) 2.5 0.5 3 1 2
Compound 4(g) 422 4.09 4.13 4.18 4.27
Yield ratio (%) 92 _ 89 90 91
93
In this example, the reaction material feed in the reactions 1--TV are
increased to 0.5-4 kg, and the yield
ratios are similar to that in the examples 22-26, i.e.: '
CA 02918096 2016-01-12
The yield ratio of the reaction I is 72-80%; the yield ratio of the reaction
11 is 80-90%; the yield ratio
of the reaction III is 82-92%; the yield ratio of thc reaction IV is 89-93%.
While the present invention is described via some preferred embodiments, the
present invention is not
limited to those specific embodiments. Based on the teaching from the above
description, those skilled
in the art can make modifications or alternations to those embodiments to
obtain equivalent
embodiments. However, any simple modification, equivalent variation or
derivation made to the above
embodiments on the basis of the technical nature of the present invention
without departing from the
scope of the technical scheme of the present invention shall be deemed as
falling into the protection
scope of the present invention as defined by the claims.
=