Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
SYSTEM AND METHOD FOR LUNG VISUALIZATION
USING ULTRASOUND
BACKGROUND
Technical Field
[0001] The present disclosure relates to systems and methods for
visualizing a
lung using ultrasound imaging techniques. More particularly, the present
disclosure
relates to systems and methods that augment images of a luminal networks
obtained by
other imaging modality with ultrasound images.
Discussion of Related Art
[0002] Standard of care for lung diseases, such as asthma, chronic
obstructive
pulmonary disease (COPD), and chronic obstructive lung disease (COLD), or for
lung-
related diseases has been focused largely on medical and/or drug management
which are
highly invasive to patients in general. For example, it has been reported for
decades that
lung denervation via localized and invasive means (e.g., surgery) may provide
therapeutic benefit for asthma or emphysema.
[0003] Electromagnetic navigation (EMN) has helped expand the
possibilities of
treatment of luminal networks such as the lungs. EMN relies on non-invasive
imaging
technologies, such as computed tomography (CT) scanning, magnetic resonance
imaging
(MRI), or fluoroscopic technologies. EMN in combination with these non-
invasive
imaging technologies has been also used to identify a location of a target and
to help
clinicians navigate a luminal network of the lung to the target. However,
images
generated by these non-invasive imaging technologies have been unable to
provide a
resolution sufficient to identify features such locations of nerves that run
parallel to the
luminal network. Further, when a treatment is performed, additional images
using these
non-invasive imaging technologies must have been performed to determine
whether the
1
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
treatment has been complete. That increases the number of exposures of harmful
X-rays
or substances to the patient and costs of treatments. Still further, every
clinician is
desirous of a greater resolution of the area being treated. Accordingly there
is a need for
an imaging modality, which provides the desired resolution and is clinically
efficient in
operation.
SUMMARY
[0004] In an aspect, the present disclosure features a system for US
based
interrogation of a lung. The system includes a memory, an electromagnetic (EM)
board,
an extended working channel (EWC), an EM sensor, a US transducer, and a
processor.
The memory stores a three dimensional (3D) model of a luminal network and a
pathway
plan for navigating a luminal network and the EM board is configured to
generate an EM
field. The EWC is configured to navigate the luminal network of a patient
toward a
target in accordance with the pathway plan and the EM sensor extends distally
from a
distal end of the EWC and is configured to sense the EM field. The US
transducer is
configured to generate US waves and receive US waves reflected from the
luminal
network and the processor is configured to process the sensed EM field to
synchronize a
location of the EM sensor in the 3D model, to process the reflected US waves
to generate
US images, or to integrate the generated images with the 3D model.
[0005] In another aspect, the system further includes a display device
configured
to display the integrated 3D model and US images. The display is further
configured to
display a status based on the location of the EM sensor. The status indicates
whether the
EM sensor is located at a not-in-target location, the target, or a location
adjacent to
healthy tissue. The status further indicates whether treatment of the target
is complete.
[0006] In another aspect, a resolution of the generated images is finer
than a
resolution of the 3D model.
2
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
[0007] In another aspect, the EM sensor is located at or around a distal
end of the
EWC.
[0008] In another aspect, the system further includes a plurality of
reference
sensors located on a patient and configured to create a breathing pattern of
the patient.
The system still further includes a tracking device, which is coupled to the
plurality of
reference sensors and the EM sensor, and is configured to identify the
location of the EM
sensor by compensating for patient's breathing based on the breathing pattern.
[0009] In another aspect, a location of integration of the generated
images is
based on the location of the EM sensor in the 3D model.
[0010] In another aspect, the processor is further configured to
identify tissue
density based on the reflected US waves. The processor is still further
configured to
determine whether a treatment device is at a center of the target.
[0011] In yet another aspect, the processor is further configured to
determine a
sufficiency of treatment based on a density of the target according to the
reflected US
waves.
[0012] In yet another aspect, the processor is further configured to
detect a size
of the target.
[0013] In yet another aspect, the processor is further configured to
determine
shrinkage of the target real-time during and after a treatment of the target.
[0014] In another aspect, the generated images show outside of the
luminal
network.
[0015] In another aspect, the processor is further configured to
determine an
offset between the EM sensor and the US transducer. Integration of the
generated
images with the 3D model is based on the offset.
[0016] In yet another aspect, the US transducer is positioned in a
forward looking
manner before the EM sensor.
3
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
[0017] Any of the above aspects and embodiments of the present
disclosure may
be combined without departing from the scope of the present disclosure.
BRIEF DESCRIPTION OF THE DRAWINGS
[0018] Objects and features of the presently disclosed systems and
methods will
become apparent to those of ordinary skill in the art when descriptions of
various
embodiments are read with reference to the accompanying drawings, of which:
[0019] FIG. 1 is a perspective view of a system for visualizing a lung
of a patient
in accordance with an embodiment of the present disclosure;
[0020] FIG. 2A is a profile view of a catheter guide assembly in
accordance with
an embodiment of the present disclosure;
[0021] FIG. 2B is
[0022] an expanded view of the indicated area of detail, which shows a
distal tip
of an extended working channel of FIG. 2A in accordance with an embodiment of
the
present disclosure;
[0023] FIG. 3 is an anatomical illustration of a three dimensional model
for a
lung in accordance with an embodiment of the present disclosure;
[0024] FIG. 4A is an illustration of a pathway from the entry point to
the target in
accordance with an embodiment of the present disclosure;
[0025] FIG. 4B is a transverse cross-sectional view of the section of
the lung of
FIG. 4A taken along section line B-B;
[0026] FIG. 4C is an illustration of a catheter guide assembly inserted
into a lung
following the pathway plan of FIG. 4A;
[0027] FIG. 4D is an enlarged detail view of the circled area of FIG.
4C;
[0028] FIG. 5A is a flowchart of a method for visualizing a lung using
US waves
in accordance with an embodiment of the present disclosure;
4
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
[0029] FIG. 5B is a flowchart of a method for navigation to the target
in
accordance with an embodiment of the present disclosure; and
[0030] FIG. 5C is a flowchart of a method for checking the level of
treatment in
accordance with an embodiment of the present disclosure.
DETAILED DESCRIPTION
[0031] The present disclosure is related to systems and methods for
visualizing
the luminal network of a lung using ultrasound (US) imaging technologies which
provide
a sufficient resolution to identify and locate a target for diagnostic,
navigation, and
treatment purposes. US imaging, particularly in conjunction with non-invasive
imaging
can provide a greater resolution and enable luminal network mapping and target
identification. Further, additional clarity is provided with respect to tissue
adjacent
identified targets which can result in different treatment options being
considered to
avoid adversely affecting the adjacent tissue. Still further, the use of US
imaging in
conjunction with treatment can provide detailed imaging for post treatment
analysis and
identification of sufficiency of treatment. Although the present disclosure
will be
described in terms of specific illustrative embodiments, it will be readily
apparent to
those skilled in this art that various modifications, rearrangements, and
substitutions may
be made without departing from the spirit of the present disclosure. The scope
of the
present disclosure is defined by the claims appended to this disclosure.
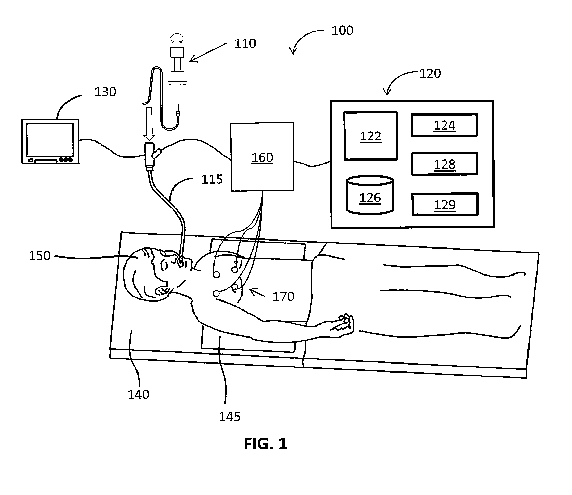
[0032] FIG. 1 illustrates an electromagnetic navigation (ENM) system
100,
which is configured to augment CT, MRI, or fluoroscopic images, with US image
data
assisting in navigation through a luminal network of a patient's lung to a
target. One
such ENM system may be the ELECTROMAGNETIC NAVIGATION
BRONCHOSCOPY system currently sold by Covidien LP. The system 100 includes a
catheter guide assembly 110, a bronchoscope 115, a computing device 120, a
monitoring
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
device 130, an EM board 140, a tracking device 160, and reference sensors 170.
The
bronchoscope 115 is operatively coupled to the computing device 120 and the
monitoring device 130 via wired connection (as shown in FIG. 1) or wireless
connection
(not shown).
[0033] The bronchoscope 115 is inserted into the mouth of the patient
150 and
captures images of the luminal network of the lung. In the EMN system 100,
inserted
into the bronchoscope 115 is a catheter guide assembly 110 for achieving
access to the
periphery of the luminal network of the patient 150. The catheter guide
assembly 110
may include an extended working channel (EWC) 230 into which a locatable guide
catheter (LG) 220 with EM sensor 265 (FIG. 2B) at the distal tip is inserted.
EWC 230,
the LG 220, and an EM sensor 265 are used to navigate through the luminal
network of
the lung as described in greater detail below.
[0034] The computing device 120, such as, a laptop, desktop, tablet, or
other
similar computing device, includes a display 122, one or more processors 124,
memory
126, a network card 128, and an input device 129. The system 100 may also
include
multiple computing devices, wherein the multiple computing devices 120 are
employed
for planning, treatment, visualization, or helping clinicians in a manner
suitable for
medical operations. The display 122 may be touch-sensitive and/or voice-
activated,
enabling the display 122 to serve as both an input and output device. The
display 122
may display a two dimensional (2D) images or three dimensional (3D) model of a
lung
to locate and identify a portion of the lung that displays symptoms of lung
diseases. The
generation of such images and models is described in greater detail below. The
display
122 may further display options to select, add, and remove a target to be
treated and
settable items for the visualization of the lung. In an aspect, the display
122 may also
display the location of the catheter guide assembly 110 in the luminal network
of the
lung based on the 2D images or 3D model of the lung. For ease of description
not
6
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
intended to be limiting on the scope of this disclosure, a 3D model is
described in detail
below but one of skill in the art will recognize that similar features and
tasks can be
accomplished with 2D models and images.
[0035] The one or more processors 124 execute computer-executable
instructions.
The processors 124 may perform image-processing functions so that the 3D model
of the
lung can be displayed on the display 122. In embodiments, the computing device
120
may further include a separate graphic accelerator (not shown) that performs
only the
image-processing functions so that the one or more processors 124 may be
available for
other programs.
[0036] The memory 126 stores data and programs. For example, data may be
image data for the 3D model or any other related data such as patients'
medical records,
prescriptions and/or history of the patient's diseases. One type of programs
stored in the
memory 126 is a 3D model and pathway planning software module (planning
software).
An example of the 3D model generation and pathway planning software may be the
ILOGIC planning suite currently sold by Covidien LP. When image data of a
patient,
which is typically in digital imaging and communications in medicine (DICOM)
format,
from for example a CT image data set (or image data set by other imaging
modality) is
imported into the planning software, a 3D model of the bronchial tree is
generated. In an
aspect, imaging may be done by CT imaging, magnetic resonance imaging (MRI),
functional MRI, X-ray, and/or any other imaging modalities. To generate the 3D
model,
the planning software employs segmentation, surface rendering, and/or volume
rendering.
The planning software then allows for the 3D model to be sliced or manipulated
into a
number of different views including axial, coronal, and sagittal views that
are commonly
used to review the original image data. These different views allow the user
to review all
of the image data and identify potential targets in the images.
[0037] Once a target is identified, the software enters into a pathway
planning
7
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
module. The pathway planning module develops a pathway plan to achieve access
to the
targets and the pathway plan pin-points the location and identifies the
coordinates of the
target such that they can be arrived at using the EMN system 100, and
particularly the
catheter guide assembly 110 together with the EWC 230 and the LG 220. The
pathway
planning module guides a clinician through a series of steps to develop a
pathway plan
for export and later use in during navigation to the target in the patient
150. The term,
clinician, may include doctor, surgeon, nurse, medical assistant, or any user
of the
pathway planning module involved in planning, performing, monitoring and/or
supervising a medical procedure.
[0038] Details of these processes and the pathway planning module can be
found
in concurrently filed with this disclosure and commonly assigned U.S. Patent
Application number 62/035,863 filed August 11, 2014 entitled "Treatment
procedure
planning system and method" and U.S. Patent Application number 13/838,805
filed by
Covidien LP on Jun 21, 2013, and entitled "Pathway planning system and
method," the
entire contents of each of which are incorporated in this disclosure by
reference. Such
pathway planning modules permit clinicians to view individual slices of the CT
image
data set and to identify one or more targets. These targets may be, for
example, lesions
or the location of a nerve which affects the actions of tissue where lung
disease has
rendered the lung function compromised.
[0039] The memory 126 may store navigation and procedure software which
interfaces with the EMN system 100 to provide guidance to the clinician and
provide a
representation of the planned pathway on the 3D model and 2D images derived
from the
3D model. An example of such navigation software may be the ILOGIC navigation
and procedure suite sold by Covidien LP. In practice, the location of the
patient 150 in
the EM field generated by the EM field generating device 145 must be
registered to the
3D model and the 2D images derived from the model.
8
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
[0040] Such registration may be manual or automatic and is described in
detail in
concurrently filed with this disclosure and commonly assigned U.S. Patent
Application
62/020,240 filed by Covidien LP on July 2, 2014, and entitled "System and
method for
navigating within the lung."
[0041] As shown in FIG. 1, the EM board 140 is configured to provide a
flat
surface for the patient to lie down and includes an EM field generating device
145.
When the patient 150 lies down on the EM board 140, the EM field generating
device
145 generates an EM field sufficient to surround a portion of the patient 150.
The EM
sensor 265 at the distal tip 260 of the LG 220 is used to determine the
location of the EM
sensor 265 in the EM field generated by the EM field generating device 145.
[0042] In embodiment, the EM board 140 may be configured to be
operatively
coupled with the reference sensors 170 which are located on the chest of the
patient 170.
The reference sensors 170 move up and down following the chest while the
patient 150
is inhaling and move down following the chest while the patient 150 is
exhaling. The
movement of the reference sensors 170 in the EM field is captured by the
reference
sensors 170 and transmitted to the tracking device 160 so that the breathing
pattern of the
patient 150 may be recognized. The tracking device 160 also receives outputs
of the EM
sensor 265, combines both outputs, and compensates the breathing pattern for
the
location of the EM sensor 265. In this way, the location identified by the EM
sensor 265
may be compensated for so that the compensated location of the EM sensor 265
is
synchronized with the 3D model of the lung. Once the patient 150 is registered
to the 3D
model, the position of the EWC 230 and particularly the LG 220 can be tracked
within
the EM field generated by the EM field generator 145, and the position of the
LG 220
can be depicted in the 3D model or 2D images of the navigation and procedure
software.
[0043] FIG. 2A illustrates an embodiment of the catheter guide assembly
110 of
FIG. 1. The catheter guide assembly 110 includes a control handle 210. The
control
9
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
handle 210 has an actuator and a selector mechanism for selectively
mechanically
steering, rotating, and advancing an extended working channel (EWC) 230 or
locatable
guide catheter (LG) 220 inserted in the EWC 230, meaning that the distal tip
260 of the
LG 220 is turning to a direction in accordance with the movement of the
control handle
210. A locking mechanism 225 secures the EWC 230 and the LG 220 to one
another.
Catheter guide assemblies usable with the instant disclosure are currently
marketed and
sold by Covidien LP under the name SUPERDIMENSION Procedure Kits and
EDGETM Procedure Kits. For a more detailed description of the catheter guide
assemblies is made to commonly-owned U.S. Patent Application Serial No.
13/836,203
filed on March 15, 2013 by Ladtkow et al. and U.S. Patent No. 7,233,820, the
entire
contents of which are hereby incorporated by reference.
[0044] FIG. 2B is an expanded view of the distal end 250 of the EWC 230
of FIG.
2A. A US transducer 265 located at the distal end 250 of the EWC 230. The EM
sensor
265 is located at the distal tip 260 of the LG 220, which is depicted
extending beyond the
distal end 250 of the EWC 230. As described briefly above, the EM sensor 265
senses
the EM field generated by the EM field generating device 145. The sensed EM
field is
used to identify the location of the EM sensor 265 in accordance with the
coordinate
system of the EM field. When the location of the EM sensor 265 is determined
by the
tracking device 160, the computing device 120 compares the location of the EM
sensor
265 with the 3D model of the lung and registers the location of the EM sensor
265 into
the coordinate system of the 3D model.
[0045] For example, when the EM sensor 265 is near at the entrance to
the
trachea, the EM sensor 265 senses the EM field and the location of the EM
sensor is then
compared with the trachea portion of the 3D model so that the location of the
EM sensor
265 is depicted in the corresponding location of the 3D model and 2D images of
the
navigation and procedure software. And when the EM sensor 265 is further
inserted
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
through the trachea to a location where separate bronchial trees are branched,
the
distance the EM sensor 265 travels from the entrance of the trachea to the
branching
location is scaled to match to the corresponding distance in the 3D model and
2D images
of the navigation and procedure software. Specifically, when the EM sensor 265
travels
along the trachea, the distance is measured in accordance with the coordinate
system of
the EM field. Since the coordinate system of the EM field is different from
the
coordinate system of the 3D model, there is a scaling factor to match the
coordinate
system of the EM field to the coordinate system of the 3D model. Thus, by
multiplying a
scale factor to the distance the EM sensor 265 travels, the coordinate system
of the EM
field is synchronized with the coordinate system of the 3D model. In this way,
the EM
field may be synchronized with the 3D model and 2D images of the navigation
and
procedure software. Or other suitable method may be employed to synchronize
the
coordinate system of the EM field with the coordinate system of the 3D model.
[0046] As noted above, the 3D model may not provide a resolution
sufficient for
guiding the EWC 230 of the catheter guide assembly 110 to a target, meaning
that the 3D
model becomes blurred or ceases to recognize the luminal network as the EWC
230
approaches a certain point. For example, when CT scan images are taken by 1 mm
thick
and 1 cm apart by a CT scan device, corresponding 3D model and/or pathway
plans may
not be able to show full perspective of a target whose size is less than 1 cm
or a portion
of a luminal network whose diameter is less than 1 cm. Thus, another imaging
modality
is necessary to find and/or identify a target and/or a terminal bronchial
branch, whose
size is less than a certain size which CT scan images are unable to show with
sufficient
details. For this purpose, the memory 126 also stores another program that can
process
and convert image data captured by an imaging modality associated with the
catheter
guide assembly 110, as will be described in detail below. This image data may
be
converted into visual images having sufficient resolutions to identify such
targets and
11
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
terminal bronchial branches or be incorporated into and used to update the
data from the
CT scans in an effort to provide a greater resolution and fill-in data that
was missing in
the CT scan.
[0047] One such imaging modality is depicted in FIG. 2B where the US
transducer 255 is depicted on the EWC 230 proximal the distal end. One of
skill in the
art will recognize that the location of the US transducer 255 and the EM
sensor 265 may
be alternated between the LG 220 and the EWC 230, or that more than one of
each
sensor and transducer may be employed without departing from the scope of the
present
disclosure. The US transducer 255 transmits ultrasound waves and receives
reflected
ultrasound waves. Generally, ultrasound waves penetrate tissue based on the
frequency
of the ultrasound waves. For example, 1 megahertz (MHz) ultrasound waves
penetrate
to a depth of 2 cm to 5 cm and 3 MHz ultrasound waves penetrate to a depth of
1.5 cm.
Thus, US waves are suitable for imaging bronchial trees. In an aspect, the US
transducer
255 may be a radial US transducer.
[0048] Generally, US waves are reflected at a boundary where density
changes or
at the interface between tissues. While the US transducer 255 is navigating
the luminal
network of the lung, the US waves are reflected from the inside wall of a
bronchial tree,
from the outside wall of the bronchial tree, and from a diseased portion or
cancerous
portion located at the outside wall of the bronchial tree and provide finite
details of the
lung structure and the tissue patency that could not otherwise be revealed
using non-
invasive imaging means.
[0049] The reflected US waves have information such as amplitude and a
delayed time between transmission of the US waves and reception of the
reflected US
waves. Since the US waves travels differently and attenuates amplitudes
differently in
accordance with the density of tissue, the amplitude and the delayed time may
be used to
identify a type of tissue, a density of the tissue, and/or a size of the
tissue. Since the
12
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
density of abnormal tissues (e.g., diseased or cancerous cells) are different
from the
normal lung tissue, the reflected US waves may be used to identify the
diseased or
cancerous cells from normal cells and the size and/or thickness of the
diseased or
cancerous cells.
[0050] The computing device 120 analyzes the reflected US waves and
generates
visual images which has a higher resolution than that of the 3D model or the
CT scan
images. The generated visual images may be augmented to and integrated with
the 3D
model of the lung or 2D images such as the CT scan images.
[0051] In embodiments, when a treatment is performed to treat an
abnormal
tissue located at the outside wall of a bronchial tree, generally, the size of
the abnormal
tissue shrinks and density of the abnormal tissue changes to the density of
the normal
lung tissue. Traditionally, when a treatment is performed, another CT scan is
performed
to obtain another set of CT images to check the size of the diseased or
cancerous cells so
that clinicians may determine whether the treatment is complete or another one
is to be
made. Since the US transducer 255 is able to check the size and the density of
the
abnormal tissue, the level of treatment may also be checked at the spot
without
performing another CT scan.
[0052] As shown in FIG. 2B, the US transducer 255 and the EM sensor 265
are
separated by a distance, DOFF. This distance, DOFF, may be sensed, coded into
the
navigation and procedure software, measured and sent by the clinician, or
sensed by the
US transducer 255 and the EM sensor 265. The computing device 120 uses the
distance,
DOFF, to adjust the incorporation of the US images into the 3D model or 2D
images
derived therefrom. For example, when the EM sensor 265 is located at the
distal tip 260
of the LG 220, the US transducer 255 is located at or circumscribing the
distal end 250
of the EWC 230, and both sensors are 1 cm distance apart from each other, this
distance
is recognized by the software and the US data or images is offset and
integrated into the
13
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
3D model or 2D images derived therefrom by a distance in the coordinate system
of the
3D model, which corresponds to 1 cm in the coordinate system of the EM field.
[0053] When the EWC 230 and the LG 220 reaches a target by manipulation
of
the catheter guide assembly 110 following the pathway plan, the EM sensor 265
confirms its location at the target and a clinician may visually confirm the
location at the
target by looking at visual images generated from the US images. The LG
catheter 220
may be removed from the catheter guide assembly 110 and a biopsy tool may be
inserted
into the EWC 230 to the target to retrieve sample of the target for
confirmation of the
disease. An anchoring tool may be employed to anchor the EWC 230 at the
target.
Further, treatment tools such as an ablation catheter may be inserted through
the EWC
230 and into the target. The US transducer 255 may then be used to transmit
and receive
US waves and the computing device 120 determines whether the treatment tool is
at the
epicenter of the target by comparing the densities of the tissue surrounding
the treatment
tool or by generating US images of the target for clinical comparison. By
being located
at the epicenter of the target, the treatment tool may perform treatment with
high
efficiency. In an aspect, the EM sensor 265 and the US transducer 255 may be
located at
or around the EWC 230 with a distance apart from each other or at or around
the LG 220
with a distance apart from each other.
[0054] In embodiments, the US transducer 255 and the computing device
120
may check the size of the target either before or after treatment. When the
size of the
target is greater than a threshold size, another treatment may be necessary to
complete
the treatment. Thus, the treatment continues until the size of the target is
decreased
under the threshold size. In this way, visualization using US waves may be
utilized for
checking the level of treatment.
[0055] In embodiments, the US transducer 255 may be a sacrificial US
transducer 255 which may be positioned in a forward looking manner to identify
the
14
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
target. The US transducer 255 is sacrificial because it may be rendered
ineffective
following treatments of the target by the application of microwave energy of
the
treatment device.
[0056] In embodiments, in a pre-treatment step, one or more markers can
be
placed through the EWC 230 to identify the location of the target. The marker
may
assist in navigating to a desired location and confirming placement of the EWC
230,
particularly after removal of the LG 220 and the EM sensor 265 when the EM
navigation
features of the present disclosure may not be effective. The marker may give a
clinician
an ability to re-visit the target after the target has been treated and to
collect further
samples. The marker may be a fiducial marker, fluorescent dye, or FLUOROGOLD .
In the case of fluorescent dye markers, the US imaging capabilities may
further increase
the determination of sufficiency of treatment, or provide greater clarity as
to the exact
location of the target. Other markers for marking the location of a target may
be
employed by those of ordinary skill in the art without departing from the
scope of the
present disclosure.
[0057] FIG. 3 illustrates a 3D model 300 for a patent's bronchial trees
and the
trachea together with the lung. The 3D model 300 may include information of
most of
the organs so that a clinician may selectively see particular organs or
portions of organs
of interest as shown in FIG. 3. In this case, these selected organs are the
lungs including
right lobe 310, the left lobe 320, the trachea 330 and bronchial trees 340.
The right lobe
310 has three sub-lobes, i.e., superior lobe 312, middle lobe 314, and
inferior lobe 316,
and the left lobe 320 has two sub-lobes, i.e., superior lobe 322 and inferior
lobe 324.
[0058] The trachea 330 is a tube that connects the pharynx and larynx to
the lung
310 and 320. At the lower end of the trachea 330, left or right primary
bronchus 342 is
divided. Secondary bronchus 344 also divides at the lower end of the primary
bronchus
342. The circumference of the primary bronchus 342 is greater than that of the
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
secondary bronchus 344. In the same manner, tertiary bronchus 346 divides at
the lower
end of the secondary bronchus 344 and terminal bronchiole 348 divides at the
lower end
of the tertiary bronchus 346. The primary bronchus 342, the secondary bronchus
344,
and the tertiary bronchus 346 are supported by cartilaginous plates. However,
when the
size of the tertiary bronchus 346 becomes smaller and smaller, the
cartilaginous plates
disappear and outer wall is dominated by smooth muscle. The outer wall of the
terminal
bronchiole 348 is also dominated by smooth muscle.
[0059] Diseased or cancerous cells or simply a target may exist on any
bronchial
trees, the primary bronchus 342, the secondary bronchus 344, the tertiary
bronchus 346,
and the terminal bronchioles 348. No matter where a target is located, when a
target is
too small to be detected by a CT imaging modality, the target may still be
detected by the
US imaging modality while the EWC 230 with US transducer 255 is navigating
toward
another target through the luminal network of the lung. The US transducer 255
provides
greater specificity and greater accuracy in detecting and identifying a
target's location in
the patient. In accordance with at least one embodiment, the US transducer 255
may be
a radial ultrasound transducer employed to further refine the image data of
the lungs by
following the pathway plan described above and capturing US image data along
the
pathway. This US image data may be registered to the CT scan images and/or the
3D
model 300 to provide greater clarity with respect to the detection, location,
and size of a
target. For example, this data may also be used diagnostically to help the
clinician
confirm that all likely targets have been identified or treated completely
after treatments.
[0060] In addition, when the US transducer 255 captures image data the
captured
image data is transferred to the computing device 120 wirelessly or via a
wired
connection. Image data captured by an ultrasound imaging modality, is not yet
readily
apprehended by a clinician. The computing device 120 processes and converts it
to an
image with which a clinician can identify a type of tissue, diagnose a
disease, identify a
16
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
location of the catheter guide assembly 110, which is the place of image
taking, or
determine a level of treatment.
[0061] FIG. 4A shows a planar view of bronchial trees of the 3D model or
of the
slices of images of the lung such as the bronchial trees of FIG. 3 and a
pathway plan to a
target. When a target is located at the tip of the bottom left end of the
terminal
bronchiole of FIG. 3, a pathway plan shows how to get to the target via the
luminal
network of the lung.
[0062] FIG. 4B shows an expanded transverse cross-sectional view of the
terminal bronchiole of FIG. 4A taken along section line B-B. The terminal
bronchiole is
surrounded by smooth muscle 405. Nerves 410 and veins 415 are located on the
outer
wall of the smooth muscle 405. The US imaging modality, as described above,
provides
a local view of the airways even out to the terminal bronchiole so that even
the thin
nerves 410 and the veins 415 on the smooth muscle 405 can be detected and
identified.
Thus, by using US imaging in addition to the CT imaging, navigation to and
direction of
therapies such as denervation can be accomplished even at the lung periphery
enabling
greater granularity of treatment options and with greater precision.
[0063] FIG. 4C illustrates a bronchoscope 420 with a catheter guide
assembly
inserted into the lungs via a natural orifice (e.g., the mouth) of a patient
toward the target
following a pathway plan. When the bronchoscope 420 reaches a certain location
of the
lung, the bronchoscope 420 becomes wedged and cannot go further into bronchial
tree
due to the size constraints. Then, the EWC 430 of the catheter guide assembly
may be
used to navigate the luminal network to a target 450 following the pathway
plan, as
described above. The EWC 430 is small and thin enough to reach the target 450.
FIG.
4D illustrates an enlarged detail view of the circled area of FIG. 4C, where a
locatable
guide (LG) may stick out of the distal tip of the EWC 430 which navigates the
luminal
network to the target 450 located at the terminal bronchiole of the lung.
17
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
[0064] FIG. 5A is a flowchart of a method 500 for visualizing a lung
using US
imaging technology. The method 500 starts at step 505 by importing a 3D model
of a
lung and a pathway plan to a target into the navigation and procedure software
stored on
a computer such as the computing device 120 of FIG. 1.
[0065] In step 510, an EM field is generated by an EM board, such as the
EM
field generating device 145 of the EM board 140 as shown in FIG. 1. In step
515, an EM
sensor 265 and a US transducer 255 are inserted into the lung via a natural
orifice or an
incision. The EM sensor 265 and the US transducer 255 may be located on the
EWC
230 with a distance apart or may be located at different places. For example,
the EM
sensor 265 may be located at or around the distal tip 260 of the LG 220 and
the US
transducer 255 may be located at or around the distal end 250 of the EWC 230,
or vice
versa.
[0066] In step 520, the EM sensor 265 senses the EM field and the sensed
results
are transmitted to the computing device 120. The sensed results are used to
calculate a
location of the EM sensor 265 in the coordinate system of the EM field. When
the
location is calculated, the computing device compares the location of the EM
sensor 265
with the 3D model, the 2D images derived therefrom, and the pathway plan. In
an aspect,
the location of the EM sensor 265 may be compensated according to the
breathing
pattern of the patient by the tracking device 160 and the reference sensors
170 before
transmitted to the computing device. Thus, the location of the ME sensor 255
may not
vary in the coordinate system of the 3D model while the patient inhales or
exhales.
[0067] In step 525, the location of the EM sensor 265 is synchronized to
the 3D
model and the 2D images derived therefrom. This location may be the starting
location
of the 3D model, or the entrance of the trachea of the 3D model. Even though
the
location is synchronized, the actual movement of the EM sensor 265 is not
synchronized
to the 3D model yet, here.
18
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
[0068] The EM sensor 265 travels a certain distance (e.g., from the
entrance of
the trachea to the branching point at the bottom of the trachea). This
distance may be
measured in the coordinate system of the EM field after the EM sensor 265
starts to
sense the EM field. In step 530, the travelling distance by the EM sensor 265
according
to the coordinate system of the EM field may be scaled so that the scaled
distance is
matched to the coordinate system of the 3D model. After this step, the
location and the
movement of the EM sensor 265 are substantially mapped into the 3D model. This
is the
synchronization or registration of the patient to the 3D model and the 2D
images derived
therefrom.
[0069] In step 535, the EM sensor 265, the LG 220, and the EWC 230
navigate
the luminal network of the lung to the target following the pathway plan. In
step 540, it
is determined whether the sensor 265 has reached the target. If it is
determined that the
EM sensor 265 has not reach the target, step 535, i.e., the navigation step,
is continued
until the target is reached following the pathway plan.
[0070] In embodiments, when it is determined that the target is reached
in step
540, step 545 may be performed to image the target with the US transducer 255
to
confirm its location. This may involve confirming tissue densities or
confirming position
relative to markers and other location confirmatory steps. In addition,
imaging of the
target may be employed after treatment to ensure sufficiency of treatment.
Step 545 is
described in further detail in FIG. 5C below.
[0071] FIG. 5B shows detail steps of navigation to the target, step 535
of the
method 500 of FIG. 5A. In step 550 US waves are transmitted by the US
transducer 255
while the distal end of the EWC 230 navigates to the target following the
pathway plan.
In step 555, the US transducer 255 receives and sends US waves reflected from
the lung
tissue to the computing device 120, which in turn processes the reflected US
waves in
step 560. The reflected US waves have information such as amplitude and
delayed time
19
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
from the transmission to the reception. The computing device 120 process the
information to determine the density or size of the lung tissue and/or
determine whether
there are new targets (i.e., diseased or cancerous cells to be treated) not
found in the CT
scan images.
[0072] In step 565, it is determined whether there is a new target along
the
pathway plan to the target. When it is determined that there is a new target,
in step 570,
the new target is identified and registered to the 3D model for later
treatment. In step
575, the route to the new target, which is a part of the pathway plan to the
target, is also
saved as a pathway plan to the new target. Then, the method 535 goes back to
step 565
to continue checking whether there are any further new targets.
[0073] When it is determined that there is no new target in step 565,
the
computing device may generate images based on the processed reflected US
waves.
Since the US waves are reflected from an interface between tissues where
density
changes, the generated images show details both inside and outside of the
bronchial tree.
The generated images may depict a diseased or cancerous cells residing on the
outside of
the bronchial tree. In an aspect, when a treatment device penetrates the
target for
treatment purposes, the generated images can also be used to show whether the
treatment
device is in the center of the target.
[0074] In step 585, the generated images are integrated into the 3D
model based
on the location of the EM sensor 265 and the offset distance DOFF between the
EM
sensor 265 and the US transducer 255. In embodiments, the generated images may
be
overlaid on CT scan images so that a lower resolution portion of the CT scan
images
may be replaced with a higher resolution images (i.e., the generated US
images), the
image data may be selectively fused to create a composite image data set, or
the data can
be incorporated into the CT image data. In step 590, the computing device
displays the
generated images with the 3D model or simply the integrated 3D model. These
steps
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
550-590 of navigation are repeated until the target is reached as shown in the
method 500
of FIG. 5A.
[0075] In an embodiment, visualization using the US waves may also be
used to
determine the sufficiency of treatment. When one treatment is performed on a
target, the
attributes of the target including size, density, and water content of the
target is generally
altered. Thus, in order to check whether the treatment is complete, the
attributes of the
target must be checked and compared to similar measurements taken before
treatment.
FIG. 5C illustrates a flowchart of a method for checking the sufficiency of
treatment
after it is determined that the EM sensor 265 reaches the target in step 540
of FIG. 5A.
In step 605, a treatment device, such as an ablation catheter, is inserted
into the EWC
230 after removal of the LG 220 and its EM sensor 265. In step 610, it is
determined
whether the treatment device is at the epicenter of the target. This is done
by use of the
US transducer 255. US images show where the density of imaged tissue changes
and the
target has a different density from normal lung tissue.
[0076] When it is determined that the treatment device is not at the
epicenter of
the target, the treatment device is inserted or retreated more or less to
adjust its location
in step 615. Then, in step 610, the location of the treatment device is again
checked.
When it is determined that the treatment device is located at the epicenter of
the target in
step 610, the treatment device treats the target.
[0077] In embodiments, similar steps as steps 605-615 of FIG. 5C may be
applied for biopsy. When a biopsy tool is inserted to take samples of the
target, the US
transducer 255 is used to check whether the biopsy tool is at the correct
location of the
target. When it is determined that the biopsy tool is at the right place, then
the biopsy
tool takes samples. Or when it is determined that the biopsy tools is not at
the target, the
biopsy tool may be adjusted to reach correctly at the target.
[0078] In step 620, the treatment device treats the target. Following
treatment
21
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
application, the US transducer 255 may be employed to image the target,
determine the
attributes of the target in step 625 (e.g., the size), and compares the
attributes of the
target with threshold values in step 630. Here, the threshold size may be
predetermined
based on a type of disease and may indicate that the disease is treated
completely.
[0079] When it is determined that the size of the treated target is
greater than the
threshold size, the computing device 120 notifies a clinician of incomplete
treatment by
displaying on the display screen such notice in step 635. The method 545 then
goes back
to step 620 for another treatment. These steps 620-635 repeat until the
treatment is
complete. In an aspect, these treatments may be performed at the spot or for a
period. In
a case when the treatments are performed during a period, a marker may be
placed at or
near the target so that a treating device can be inserted to the target with
certainty during
a later treatment.
[0080] When it is determined that the size of the target is less than or
equal to the
threshold size in step 630, the computing device 120 notifies a clinician of
complete
treatment by displaying that the treatment is complete in step 640, and the
method 545 of
checking the level of treatment is ended. Thus, the US transducer 255 and US
imaging
features of the present disclosure may be employed to confirm the sufficiency
of
treatment of a target.
[0081] In another embodiment, the monitoring device 130 and/or the
computer
120 may display a color code on the display, notifying a clinician of a
status. The status
may be based on a location of the EWC 230 of the catheter guide assembly 110.
The
status may indicate whether the distal end of the EWC 230 is located at a not-
in-target
location, at the target, or at a location adjacent to healthy tissue, and
whether treatment of
the target is complete. For example, the color code may be used in a way that
a red color
indicates that the EWC 230 is at a not-in-target location, a green color
indicates that the
EWC 230 is at a target, a yellow color indicates that the EWC 230 is adjacent
to healthy
22
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
tissue, and an orange color indicates that the treatment is complete. However,
this is an
example and is not meant to limit the scope of this disclosure. Other status
indication
systems may be employed as people in the ordinary skill in the art would
apprehend.
[0082] Though not described in detail above, with respect to FIG. 1, the
network
interface 128 enables other computing devices 120, the bronchoscope 115, and
the
catheter guide assembly 110 to communicate through a wired and/or wireless
network
connection. In FIG. 1, the bronchoscope 115 and catheter guide assembly 110
may
transmit or receive medical images, medical data, and control data to and from
the
computing device 120 via a wired connection. In a case where the network
interface 128
connects to other computing devices or the bronchoscope 115 and catheter guide
assembly 110 wirelessly, the network interface 128 uses a frequency for
communication,
which may be different from the frequency the bronchoscope 115 or the catheter
guide
assembly 110 uses for transmitting the captured images.
[0083] The memory 126 of computing device 120 may include one or more
among solid-state storage devices, flash memory chips, mass storage, tape
drive, or any
computer-readable storage medium which is connected to a processor through a
storage
controller and a communications bus. Computer readable storage media include
non-
transitory, volatile, non-volatile, removable, and non-removable media
implemented in
any method or technology for storage of information such as computer-readable
instructions, data structures, program modules or other data. For example,
computer-
readable storage media includes random access memory (RAM), read-only memory
(ROM), erasable programmable read only memory (EPROM), electrically erasable
programmable read only memory (EEPROM), flash memory or other solid state
memory
technology, CD-ROM, DVD or other optical storage, magnetic cassettes, magnetic
tape,
magnetic disk storage or other magnetic storage devices, or any other medium
which can
be used to store desired information and which can be accessed by the
computing device
23
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
120.
[0084] In embodiments, the display 122 may work as an input device such
that
the display 122 may receive multiple finger actions, such as pinching or
spreading
fingers. For example, when fingers are pinched, the portion of the displayed
image,
where the fingers are located on the display 122 before pinching, may be
zoomed out and,
when fingers are spread, the portion of the lung, where the fingers are
located on the
display 122 before spreading, is zoomed in. Or when multiple fingers swipe the
display
122 together in one direction, the displayed image may be rotated in the same
direction
as the swiping direction and the amount of rotation is proportional to a
distance and/or a
speed of the swiping motion. These features may be also implemented using the
input
device 129.
[0085] The input device 129 is used for inputting data or control
information,
such as setting values, or text information. The input device 129 includes a
keyboard,
mouse, scanning devices, or other data input devices. The input device 129 may
be
further used to manipulate displayed images or the 3D model to zoom in and
out, and
rotate in any direction.
[0086] The monitoring device 130 is operatively connected with the
bronchoscope 115 and the computing device 120. The monitoring device 130
includes
buttons and switches for setting settable items of the monitoring device 130.
The
monitoring device 130 may be touch-sensitive and/or voice-activated, enabling
the
monitoring device 130 to serve as both an input and output device. Thus,
settable items
of the monitoring device 130 may be set, changed, or adjusted by using the
buttons,
touches to the screen of the monitoring device 130, or voices.
[0087] When the bronchoscope 115 captures images of the luminal network
of
the lung and the captured images do not need to be processed for visualization
for human
eyes, the monitoring device 130 may receive and display the captured images on
the
24
CA 02923457 2016-03-04
WO 2015/034906
PCT/US2014/053878
monitoring device 130 so that a clinician may confirm that the location of the
catheter
guide assembly 110 is in an intended place, particularly for use in
confirmation of
registration.
[0088] Although embodiments have been described in detail with reference
to the
accompanying drawings for the purpose of illustration and description, it is
to be
understood that the inventive processes and apparatus are not to be construed
as limited.
It will be apparent to those of ordinary skill in the art that various
modifications to the
foregoing embodiments may be made without departing from the scope of the
disclosure.