Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 1 -
Flash Coating Treatments For Proppant Solids
Cross-Reference to Related Applications
[0001] The present application claims priority to U.S. provisional patent
application serial
number 61/904,833, filed on November 15, 2013 and U.S. provisional patent
application serial
number 61/898,328 filed on October 31, 2013, the disclosures of each of which
are hereby
incorporated by reference.
Field
[0002] Embodiments disclosed herein relate to, for example, treatments for
coated or uncoated
proppants that can, among other things, control fugitive dust during typical
handling procedures
with typical transport equipment and/or add functional features to the
proppant solid.
Background
[0003] Dust generated by the handling of proppant (both coated and uncoated)
has been an area
of concern for a number of years. The dust can be a nuisance, a health hazard
and also disrupt
production of oil and gas products produced during the fracturing process.
Prior methods of
controlling dust have not been sufficient.
[0004] Defects in the manufacturing process for proppants have been attempted
to be cured by
the use of additives or increased washing. However, they have not been
effective while
maintaining the necessary properties of the proppant (e.g. flow and strength).
Examples can be
found, for example, in U.S. Patent No. 7,270,879, which shows dust being
generated that would
likely be a nuisance but should be avoided. In addition, a variety of methods
can be used to
decrease the effects of the dust, which include, for example, mechanical
isolation (e.g., masks),
atmospheric venting and other containment strategies to reduce exposure to the
dusts of the
operations. These methods, however, do not reduce dust, but rather reduce the
effect of the dust.
[0005] Prior methods for reducing dust include converting the potential dust
source into a solid,
paste or liquid. However, none of these would be acceptable for frac proppants
or sands which
must remain dry and free-flowing for use with existing pneumatic and dry
solids material
transfer handling equipment. This same requirement effectively eliminates the
use of
conventional wet treatments that make the particulates perceptibly wet to the
eye and touch.
Such wetness in finely divided solids causes clumping, aggregation and
enhanced difficulties
with gravity-fed discharge system or pneumatic conveyance equipment. Other
chemical methods
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 2 -
are described, for example, in U.S. Patent No. 5,480,584 and U.S. Patent No
7,270,879, but the
process is not suitable for frac proppants or sands because the proppants
would clump,
aggregate, or would otherwise materially change their the free-flowing
characteristics so that
conventional pneumatic conveyance equipment exhibits a diminished or
compromised
effectiveness. The processes can also not be very cost effective.
[0006] Accordingly, fugitive proppant dust presents unique treatment and
control issues
relative to other forms of dust. For example, road surfaces are generally
fixed in position so that
treatments can be applied and allowed a period of time to penetrate and set.
Coal mines similarly
see a fixed treatment surface. Proppants are often moved, usually by gravity
discharge or
pneumatic conveyance, and rely heavily on a free-flowing form to be loaded and
discharged with
conventional handling equipment. Proppants should also be chemically
compatible with, and
wettable by, frac fluids that have relatively complex physical and chemical
properties to be
effective. Thus, traditional forms of dust control have not been sufficiently
effective when used
with proppants. Therefore, there is a need for improved products and processes
for controlling
dust. Additionally, there is a need to functionalize proppants by including
functional molecules
in any coating that is applied to the proppant to control the dust. The
embodiments disclosed
herein satisfy these needs as well as others.
Summary
[0007] Embodiments disclosed herein provide methods for treating proppant
solids (e.g.,
coated or uncoated frac sands, bauxite, ceramic and the like) with a liquid
treatment agent that
can suppress or reduce the formation and release of dust. In some embodiments,
the methods do
not adversely affect the wettability, free-flowing character, or proppant
performance of the
treated solids when the treated solids are used as proppants in a well.
[0008] Embodiments disclosed herein provide methods that can apply a liquid
treatment agent
quickly, on-the-fly during transport and/or discharge and without necessarily
using an installed
manufacturing facility. Therefore, in some embodiments, the method can be
performed in a
transfer vessel, at a manufacturing site, or at any point in the transfer or
discharge process and/or
before use at a well site.
[0009] In some embodiments, the method is performed with proppants after the
proppants have
been collected from a bulk storage site and during the process of loading,
transport to, or
unloading for delivery at a wellsite. In some embodiments, the method allows
the treated
proppant to retain its free-flowing characteristics for continued use of
conventional pneumatic
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 3 -
handling equipment. In some embodiments, the method is performed without
adversely
affecting its use in conventional fracturing fluids. In some embodiments, the
method comprises
contacting one or more proppant solids with a coating composition during one
or more of such
steps where the coating composition forms a protective coating within seconds
of its application
that controls fugitive dust from the coated proppants. In some embodiments,
the coating
provides coated proppants with a functional benefit, other than reducing dust
production or
release. In some embodiments, the coating includes or is a hydrophobic
coating. Examples of
additional functional features that can be added to proppants using
embodiments described
herein include, but are not limited to, scale inhibition, friction reduction,
tracer-containing
coatings, impact modifiers, controlled delivery of chemical additives, sulfide
control, composite
coatings, staged and time release coatings, coatings that are acid and base-
resistant, corrosion
inhibition agents, additives that improve crush resistance, agents that
inhibit paraffin or
asphaltene deposits, coatings that improve conductivity, and coatings for the
removal of targeted
anions and halogens from produced fluids.
[0010] In some embodiments, the method comprises contacting finely divided
proppant solids
(e.g. sized sand or ceramic particulates) with a liquid treatment agent
comprising one or more of:
(a) monosaccharide and/or polysaccharide solutions, (b) low molecular weight
mineral oils, (c)
vegetable oils, (d) mixtures of polyethylene and oil, (e) C6-C16 alkoxylated
alcohols, (f) polymer
and copolymer mixtures containing one or more ionic and nonionic acrylic
polymers, acrylate
polymers, acrylamide polymers, vinyl acetate polymers, styrene/acrylic
acid/acrylonitrile
copolymers, (f) crosslinked guar gum, (g) carboxymethylcellulose, (h) starch,
(i) psyllium
powder, (j) potassium-based superabsorbent polymers, (k) copolymers, and (1)
mixtures of the
preceding.
[0011] Embodiments described herein can be used to quickly coat frac sands,
ceramics, bauxite
and other, finely divided, solids that are used in hydraulic fracturing or
similar industries that
make use of gravity feeder systems, belt conveyors, and pneumatic conveyance
devices. The
methods described herein can be used to suppress the formation of fugitive
dust, prevent existing
dust that might cling to the surfaces of the solids or which is intermingled
in the bulk solids from
becoming airborne, preserve the sphericity and integrity of the treated
solids, substantially
reduce or avoid the generation of fugitive dust emissions from the conveyed
solids while
maintaining free-flowing characteristics by the treated particle, and/or add
functional chemical
effects to the treated proppants.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-4-
100121 Embodiments described herein provide processes for treating free-
flowing, finely
divided proppant solids. In some embodiments, the processes comprises
contacting the solids
less than five seconds with a liquid treatment agent with an amount of the
liquid treatment agent
that substantially retains free-flowing characteristics of the treated solids.
In some embodiments,
the solids are contacted with the liquid treatment agent more than once and
each contacting step
is for less than five seconds.
[0013] In some embodiments, the contacting comprises spraying solids
substantially
simultaneously from more than one direction. In some embodiments, the solids
are contacted for
less than two seconds with the liquid treatment agent. In some embodiments,
the solids are
contacted for less than one second with the liquid treatment agent. In some
embodiments, the
solids are contacted with the liquid treatment agent for the time it takes the
solids to fall a
distance of four feet.
[0014] In some embodiments, the liquid treatment agent comprises a
polysaccharide solution.
In some embodiments, the liquid treatment agent comprises a C6-C16 alkoxylated
alcohol. In
some embodiments, the liquid treatment agent comprises at least one acrylic
polymer. In some
embodiments, the liquid treatment agent comprises an acrylic copolymer. In
some embodiments,
the liquid treatment agent comprises a mixture of at least one C6-C16
alkoxylated alcohol and at
least one acrylic polymer.
[0015] In some embodiments, the liquid treatment agent is applied to the
solids in an amount
of less than 1 wt% per weight based on the weight of the proppant solids. In
some embodiments,
the liquid treatment agent is contacted with the solids in an amount of less
than 0.5 wt%. In some
embodiments, the liquid treatment agent is contacted with the solids in an
amount of less than
0.35 wt%. In some embodiments, the liquid treatment agent is contacted with
the solids in an
amount of less than 0.25 wt%.
[0016] In some embodiments, the liquid treatment agent is contacted with the
solids
immediately before, concurrently with, or immediately after passing the solids
through a static
mixer.
[0017] In some embodiments, the contacting step comprises: applying a first
liquid treatment
agent with a first spray assembly onto the solids for less than five seconds;
passing the treated
solids through a static mixer; and applying a second liquid treatment agent
with a second spray
assembly onto the solids for less than five seconds. In some embodiments, the
first liquid
treatment and the second liquid treatment are different solutions. In some
embodiments, the
second liquid treatment is applied to the solids immediately after the solids
are passed through
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 5 -
the static mixer. In some embodiments, at least one of the first and second
liquid treatment
agents is effective to coat the solids with a dust reduction coating.
[0018] In some embodiments, at least one of the first and second liquid
treatments is effective
to coat the solids with an additional coating. In some embodiments, the
additional coating is one
or more of: a hydrophobic coating, a coating that reduces friction, a coating
that comprises a
tracer, an impact modifier coating, a coating for timed or staged release of
an additive, a coating
that controls sulfides, a different polymeric coating, an acid or base
resistant coating, a coating
that inhibits corrosion, a coating that increases proppant crush resistance, a
coating that inhibits
paraffin precipitation or aggregation, a coating that inhibits asphaltene
precipitation, or a coating
comprising an ion exchange resin that removes anions and/or halogens, or any
combination
thereof
[0019] The present disclosure also provides coated, free-flowing proppants
comprising a dried
and/or cured coating that comprises less than about 3 wt% of a treating agent.
In some
embodiments, the coated, free-flowing, proppant exhibits reduced fugitive dust
generation as
compared to the uncoated proppant. In some embodiments, the coated, free-
flowing proppants
comprise 0.0009-0.5 wt% of the coating. In some embodiments, the coated, free-
flowing
proppants comprise 0.001-0.35 wt% of the coating. In some embodiments, the
coating comprises
one or more of: monosaccharides or polysaccharides, surfactants, alkoxylated
alcohols, acrylic
polymers, methacrylic polymers, copolymers of acrylic acid and/or methacrylic
acid,
methacrylates and copolymers thereof, polyvinyl acetates, vinyl acrylic
copolymers,
polybutadiene, low molecular weight mineral oils, acrylamide polymers,
lignosulfonates, water-
dispersible natural gums, water-dispersible pectins, water-dispersible starch
derivatives, water-
dispersible cellulose derivatives, or any mixture thereof In some embodiments,
the coating
comprises one or more monosaccharides or polysaccharides. In some embodiments,
the coating
comprises one or more alkoxylated alcohols. In some embodiments, the coating
comprises at
least one C6-C12 alkoxylated alcohol and at least one Cio-C16 alkoxylated
alcohol. In some
embodiments, the coating comprises one or more acrylic polymers. In some
embodiments, the
coating comprises at least one C6-C12 alkoxylated alcohol, at least one Cio-
C16 alkoxylated
alcohols, and at least one acrylic polymer. In some embodiments, the coating
comprises one or
more methacrylic polymers, one or more copolymers of acrylic acid and/or
methacrylic acid, and
one or more of methacrylates. In some embodiments, the coating comprises any
one or more of:
a hydrophobic coating, a coating that reduces friction, a coating that
comprises a tracer, an
impact modifier coating, a coating for timed or staged release of an additive,
a coating that
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 6 -
controls sulfides, a different polymeric coating, an acid or base resistant
coating, a coating that
inhibits corrosion, a coating that increases proppant crush resistance, a
coating that inhibits
paraffin precipitation or aggregation, a coating that inhibits asphaltene
precipitation, and/or a
coating comprising an ion exchange resin that removes anions and/or halogens,
or any
combination thereof In some embodiments, the coating further comprises a
sulfide scavenger or
scale inhibitor.
Brief Description of the Drawings
[0020] Figure 1 is a diagram showing the types of equipment and process flow
sequence
described herein.
[0021] Figure 2 shows a representative spray point in an optional static mixer
that can be used
as described herein.
[0022] Figure 3 shows the outside of a static mixer and the representative
locations of a series
of static mixing bars helically arranged within the static mixer.
[0023] Figure 4 is a view downwardly through a static mixer that shows the
helical disposition
of static mixing bars disposed within the mixer.
[0024] Figure 5 shows the use of a series of spray nozzles located around the
perimeter of a
ring disposed around a discharge spout in a proppant handling facility.
[0025] Figure 6 is a side view of the ring sprayer shown in Figure 5.
[0026] Figure 7 shows a configuration that combines the sprayer assembly of
Figures 5 and 6
with the drum-shaped static mixer of Figures 3 and 4.
[0027] Figure 8 shows an alternative configuration in which spray nozzles
precede and follow a
static mixer.
[0028] Figure 9 illustrates non-limiting embodiments of a vertical treatment
mixer that
combines a partially enclosed, upper spray section above a static mixing
section followed by a
lower, inwardly tapered discharge section.
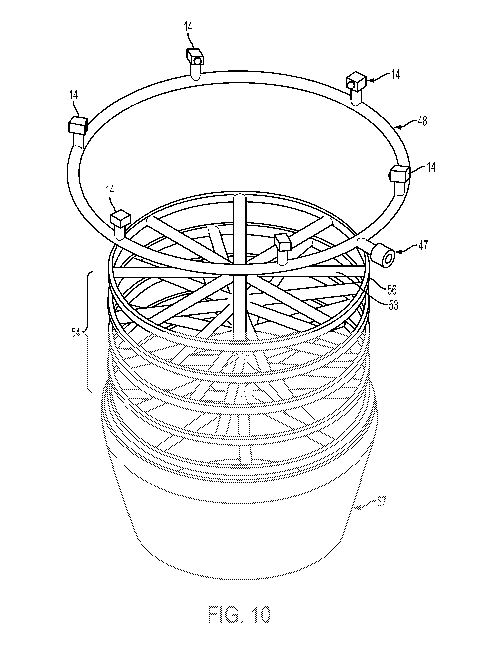
[0029] Figure 10 illustrates non-limiting embodiments of a vertical treatment
mixer that
combines a partially enclosed, upper spray section above a static mixing
section followed by a
lower, inwardly tapered discharge section.
[0030] Figure 11 illustrates non-limiting embodiments of a vertical treatment
mixer that
combines a partially enclosed, upper spray section above a static mixing
section followed by a
lower, inwardly tapered discharge section.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-7-
100311 Figurel2 illustrates non-limiting embodiments of a vertical treatment
mixer that
combines a partially enclosed, upper spray section above a static mixing
section followed by a
lower, inwardly tapered discharge section.
Description
[0032] Embodiments disclosed herein provide methods and compositions for
treating frac
sands, whether or not provided with a cured coating, as well as other finely
divided proppant
solids (e.g., resin-coated sand, bauxite or ceramics), that are effective for
reducing the amount of
fugitive dust associated with processing, handling, transporting and using,
for example, such
finely divided proppant materials in hydraulic fracturing.
[0033] Embodiments disclosed herein also provide methods that reduce fugitive
dust associated
with the proppant material itself and do not require users, transporters and
well sites to purchase
or use additional equipment to handle the thus-treated solids.
[0034] Embodiments disclosed herein provide compositions and methods for
maintaining or
improving performance of the proppant solids pack by reducing loss of
sphericity and/or
minimizing the inclusion of fine particles that could affect the performance
of the proppant
solids.
[0035] Embodiments disclosed herein provide methods for treating a proppant
quickly and with
minimal effect on the conventional handling techniques and equipment currently
in use for
loading, moving, and unloading coated or uncoated proppant sands or ceramics.
[0036] Embodiments disclosed herein include, but are not limited to, free-
flowing proppant
solids being treated with a liquid treatment agent quickly and at a
sufficiently low application
rate in order to maintain the free-flowing properties of the treated solids.
Without wishing to be
bound by any particular theory, such low levels of treatment with the agents
allow the treated
solids to be handled with conventional handling equipment without adversely
affecting the
handling and conveying process. The treatment agent can also help to avoid the
degradation or
deterioration of the proppant solids. Some of the unexpected advantages of the
processes and
compositions described herein include, but are not limited to, preserving
sphericity and the
crush resistance benefits associated with the proppants while avoiding the
formation of fines
(e.g. dust) that can become an airborne health hazard or in a high enough
concentration to affect
the properties of the fracturing fluid. Embodiments described herein can also
be used to provide
the proppant with additional functions and/or benefits of value for oil and
gas well operation by
incorporating functional molecules into the coating.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-8-
100371 Advantages of the embodiments described throughout and others would be
readily
apparent to one of skill in the art. In addition, certain advantages, the
embodiments described
herein include, but are not limited to, that the method that protects the
proppant grains from the
abrasion during handling or pneumatic transfer can also help to reduce wear on
the pneumatic
trucks that transport the sand for the transload to the wellsite. Thus,
embodiments described
herein not only help to control fugitive dust but also limit the wear on pipes
and fittings used in
moving and handling the solids. The embodiments described herein can also be
effective in
reducing the wear on the high pressure pipes and fittings that connect the
discharge end of the
high pressure pumps to a wellhead. For example, because a large amount of
proppant is
pumped, the high pressure pipes and fittings must be tested frequently to
determine the effect of
proppant abrasion on that strength. The embodiments described herein can help
to reduce the
wear on the equipment and thereby increase its useful life.
[0038] Controlling fugitive dust from frac sands and other proppants can be
accomplished by
methods and processes described herein. In some embodiments, the processes
comprise
contacting finely divided proppant solids with a liquid treatment agent at an
amount that is
sufficient to suppress fugitive dust emissions from the treated solids and/or
impart additional
functional chemical benefits while still maintaining the freely flowing
character of the treated
solids, like those of the proppants before treatment, that continues to allow
the effective use of
gravity feed, pneumatic and belt conveyor handling systems. In some
embodiments, the
treatment occurs in 10 seconds or less and while the solids are in free fall,
guided free fall (as in
falling through a static mixer), or during pneumatic conveyance. During these
periods, the free-
flowing properties of the solids make them particularly amenable to contact
with one or more
dispersive liquid sprays and turbulent mixing.
[0039] Even when treated at an amount less than that required to make the
solids perceptibly
wet, i.e., in an amount of less than 0.7 wt% moisture to preserve free-flowing
characteristics, or
in some embodiments from 0.05-0.4 wt%, dust emissions are substantially
reduced and what
particulates are ejected due to discharge impact quickly settle. Such
performance allows treated
proppants to continue to be handled effectively with existing handling
equipment like gravity-
based discharge systems, moving belts, pneumatic conveyance systems, etc.
[0040] The solids that can be treated are, and remain, finely divided, free-
flowing, solids that
generally have a size of about 0.2 mm to about 1 mm. Such solid sizes are used
in hydraulic
fracturing to prop open cracks formed downhole within the fractured strata.
Such crack props, or
"proppants" as they are known, must resist the crushing forces of crack
closure to help maintain
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 9 -
the flow of liquids and gases that have been trapped in the strata. Materials
often used as
proppant include coated and uncoated sand, bauxite, and ceramic proppant
materials. All such
materials are suitable for use in the methods and processes described herein.
[0041] In some embodiments described herein, embodiments use a liquid
treatment agent that
is applied at extremely low levels, e.g., at levels that avoid making the
particulates perceptibly
wet such as observed by, e.g., drips, puddles, a visible wet sheen or a wet
"feel" upon handling
the treated solids. In some embodiments, some treatments might require mild
drying after contact
with the sprayed treating agent in order to avoid "perceptibly wet" particles,
especially those
prepared using non-aqueous based solvent carriers.
[0042] In some embodiments, the treatment agent level is also fast and
sufficiently low in
applied volumes to avoid the formation of firmly agglomerated masses of
treated solids that are
not readily transported by conventional dry proppant solids handling
equipment, e.g., gravity-fed
conveying systems, pneumatic transport, and the like. In other words, the
proppant solids that are
treated according to the presently disclosed methods continue to act and be
subject to handling
by conventional proppant solids handling equipment and systems. In some
embodiments, the
liquid treatment agent is applied or contacted with the solids for less than
or equal to 20, 19, 18,
17, 16, 15, 14, 13, 12, 11, 10, 9, 8, 7, 6, 5, 4, 3, 2, or 1 seconds. As used
herein, the phrase "less
than" when used in reference to a certain of period of time does not include
zero unless explicitly
stated. In some embodiments, the liquid treatment agent is contacted with the
solids for about
0.1 to about 5 seconds, about 0.1 to about 10 seconds, about 0.1 to about 15
seconds, or about
0.1 to about 20 seconds. In some embodiments, the liquid treatment agent is
contacted with the
solids for about 1 to about 10, about 1 to about 9, about 1 to about 8, about
1 to about 7, about 1
to about 6, about 1 to about 5, about 1 to about 4, about 1 to about 3, or
about 1 to about 2
seconds. In some embodiments, the liquid treatment agent is contacted with the
solids for about
0.5 to about 10, about 0.5 to about 9, about 0.5 to about 8, about 0.5 to
about 7, about 0.5 to
about 6, about 0.5 to about 5, about 0.5 to about 4, about 0.5 to about 3,
about 0.5 to about 2, or
about 0.5 to about 1 seconds. In some embodiments, the liquid treatment agent
is contacted with
the solids for about 2 to about 10, about 2 to about 9, about 2 to about 8,
about 2 to about 7,
about 2 to about 6, about 2 to about 5, about 2 to about 4, or about 2 to
about 3 seconds. In some
embodiments, the liquid treatment agent is contacted with the solids for about
3 to about 10,
about 3 to about 9, about 3 to about 8, about 3 to about 7, about 3 to about
6, about 3 to about 5,
or about 3 to about 4 seconds. In some embodiments, the liquid treatment agent
is contacted
with the solids for about 4 to about 10, about 4 to about 9, about 4 to about
8, about 4 to about 7,
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 10 -
about 4 to about 6, or about 4 to about 5 seconds. The time periods described
herein can be used
in conjunction with any embodiment of the processes described herein involving
the contacting
of a solid with a liquid treatment agent. The phrase "time period as described
herein" refers to
these time periods in addition to any time periods described specifically with
any particular
embodiment.
[0043] In some embodiments, the liquid treatment agent is presented as an
aqueous solution,
dispersion, or emulsion. In some embodiments, suitable levels of the liquid
treatment agent can
be characterized as a weight of applied solids per unit weight of treated
solids. In some
embodiments, with such a reference frame, suitable application rates of liquid
treatment agent
are less than 5 wt% treating agent solids per unit weight of treated solid
(e.g. sand). In some
embodiments, the liquid treatment agent is applied at a rate of less than
about 3 wt% and without
adversely affecting free-flowing characteristics by the treated proppants
after the applied
materials have dried. In some embodiments, the treatment agent is applied at
an amount from
about 0.0002 to about 1.5 wt%, about 0.0002 to about 1 wt%, about 0.0005 to
about 0.85 wt%,
about 0.0007 to about 0.75 wt%, about 0.0008 to about 0.65 wt%, about 0.0009
to about 0.5
wt%, about 0.001 to about 0.35 wt% and about 0.0013 to about 0.25 wt%. In some
embodiments,
the amount of the liquid treatment agent is from about 3 to about 8 lb of the
liquid treatment
agent per ton of proppant solid. In some embodiments, the solids can be
contacted with the
liquid treatment agent at a rate of about 400 tons/hour at commercial
application rates depending
on the equipment used. In some embodiments, the about 3 to about 8 lb of
treatment agent is
based upon a dispersion that has about 40% solids.
[0044] As described herein, the solids are contacted with the liquid treatment
agent very
quickly thereby making the process amenable to treatment rapidly, "on-the-
fly", at loading,
handling in transport or at unloading events. As described herein, the solids
can be contacted
with the treatment for short periods of time, which include, but are not
limited to for a period of
time that is less than five seconds, but greater than zero. In some
embodiments, the time period
is about 1 to about 3 seconds. In some embodiments, the solids are contacted
with the liquid
treatment agent in the time it takes the solids to fall 3-4 feet (1-1.3 m). In
some embodiments, the
liquid treatment agent is contacted with the solids using a spray dispersion
nozzle. In some
embodiments, the liquid treatment agent is contacted with the solids via a
plurality of spray
dispersion nozzles that impinge on a falling or guided falling stream of
proppants, or which
introduce the liquid treatment agent onto the proppant solids as the solids
are pneumatically
conveyed for loading or unloading.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-11-
100451 The liquid treatment agent can be contacted with the solids in any way
that is effective
to provide the solids with a substantially uniform dispersion of liquid
treatment agent over as
much of the solids within the treatment zone as is reasonably possible. The
methods can be
dependent, for example, on the existing equipment, budget and space. In some
embodiments, the
contacting equipment is a spraying system of at least one nozzle that
distributes the liquid
treatment agent over, under, around and within the treated solids as they move
past and through
the treatment zone. In some embodiments there are a plurality of nozzles.
[0046] In some embodiments, a typical treatment zone might be located along a
conveyor belt
as proppants are unloaded from a transport vehicle and conveyed by a belt to
discharge
equipment. In some embodiments, a treatment zone includes 1 to 8 nozzles
and/or atomizing
spray nozzles, to create a fine spray, mist or fog that contacts the moving
proppants from both
above and below the conveyor belt or as the solids fall from the conveyor belt
to effect a
substantially uniform treatment.
[0047] In some embodiments, the treatment zone could be within an enclosure
located around
the conveying system/belt to better contain the treatment additive as it is
applied, to better
control the environment around the application point, or to make the
contacting process more
efficient.
[0048] The proppant solids can also be heated or allowed to become heated to
an elevated
temperature, i.e., at a temperature above 25 C or from about 300 to about 85
C, immediately
before or after the contacting step so that higher concentrations of the
liquid treatment agent can
be applied to increase performance or allow a less expensive additive to be
utilized.
[0049] In some embodiments, another treatment zone might be located in or in
conjunction
with a pneumatic conveyor. One or more spray nozzles (e.g. fine spray nozzles)
can be aligned
and directed to discharge the liquid treatment agent into the pneumatic air
stream at one or more
locations at the appropriate injection rate so as to contact the conveyed
solids as they are mixed
and moving in the conveyance stream.
[0050] In some embodiments, treatment zones are located at one or more
transfer points within
the handling process where the solids are in motion and sufficient mixing can
be performed
readily. In some embodiments, they are mixed with a static mixer to enhance
mixing of the
treated solids and encourage a substantially even distribution of the liquid
treatment agent over
the solids. In some embodiments, the locations include loading ports where
stored proppant
solids are delivered for transport to a delivery truck, discharge ports used
for loading pneumatic
transport trucks, and discharge belts when a truck unloads proppants at a well
site. In some
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 12 -
embodiments, the process comprises applying a first liquid treatment agent
with a first spray
assembly onto the solids for a period of time as described herein; passing the
treated solids
through a static mixer; and applying a second liquid treatment agent with a
second spray
assembly onto said solids for a period of time as described herein. In some
embodiments, the
first liquid treatment and the second liquid treatment are different
solutions. In some
embodiments, the second liquid treatment is applied to the solids immediately
after the solids are
passed through the static mixer. In some embodiments, at least one of the
first and second liquid
treatment agents is effective to coat the solids with a dust reduction
coating. In some
embodiments, at least one of the first and second liquid treatments is
effective to coat the solids
with an additional coating. In some embodiments, the additional coating is a
hydrophobic
coating, a coating that reduces friction, a coating that comprises a tracer,
an impact modifier
coating, a coating for timed or staged release of an additive, a coating that
controls sulfides, a
different polymeric coating, an acid or base resistant coating, a coating that
inhibits corrosion, a
coating that increases proppant crush resistance, a coating that inhibits
paraffin precipitation or
aggregation, a coating that inhibits asphaltene precipitation, or a coating
comprising an ion
exchange resin that removes anions and/or halogens. Such coatings are
described herein, but
other coatings can also be applied.
[0051] In some embodiments, the liquid treatment agent is contacted and mixed
with the
proppant solids at a transfer point location where the proppant solids are
discharged and
experience some period of free fall to a vertically lower point. Such
locations permit the use of
one or more spray nozzles. For example, 1 to 12 nozzles in 1 to 3 stages can
be disposed around
the falling solids such as around a discharge port in a substantially circular
pattern. In some
embodiments, multiple nozzles are used. In some embodiments, multiple nozzles
are used each
with a fan-shaped or conical spray pattern that are aligned and aimed to spray
the falling solids
with the liquid treatment agent and coat the solids. In some embodiments, the
contacting occurs
immediately before, during, and/or after passage through a static mixer that
uses the momentum
of the falling solids to encourage better mixing and distribution of the
liquid treatment agent over
the solids. In some embodiments, a diagram of such a process is shown is
illustrated in Figure 1.
[0052] As shown, an insulated and/or heated enclosure (1) protects the water
storage tank (2)
and liquid treatment agent concentrate storage units (3), (4), (5), (6) from
substantial variations
in ambient temperature. A pump (7) is used to move water from a storage tank
(2) through a
strainer (8) into a liquid treatment agent mixer (9). A pump (10) delivers the
liquid treatment
agent from the storage units (3-5) to the mixer (9), or to a point immediately
above and
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 13 -
preceding the mixer (9), at a controlled rate sufficient to meet the desired
concentration rate for
use in the presently disclosed methods. A pump (11) is used to transfer the
diluted liquid
treatment agent (12) to a mixer (13) and dispersed with one or more spray
nozzles (14) at, e.g., a
rate within the range of 1.7-5 gallons per minute at 40-60 psi when treating
sand moved at
typical commercial volumes of, e.g., 100-400 tons per hour. The proppant sand
(15) is delivered
to the top of the mixer (13) which is suitably a static mixer sized to handle
commercial volumes
of sand, where the proppant sand (15) is mixed with the liquid treatment agent
issuing from the
first spray assembly of spray nozzles (14).
[0053] A recirculation circuit (16) can be used to keep the liquid treatment
agent in motion
within the conduits if a valve (17) is closed.
[0054] An optional air compressor (18) can be used to provide a source of
pressurized air to the
enclosure (1) and/or the mixer (13). An optional power generator (19) serves
as a source of
backup power for the enclosure (1), including the pumps (7), (10), (11) and
the mixer (9).
[0055] A mixer (13), such as a static sand mixer, is shown in somewhat more
detail in Figure 2.
In this view, liquid treatment agent (12) is passed through nozzles (14)
surrounding a sand inlet
(20) of the mixer (13) where the liquid treatment agent (12) contacts the sand
(21) as it passes
through a spraying zone (22). The sand (21) then contacts a series of mounted,
impingement-
type, rods or mixing members (23) that are located throughout the vertical
height of the mixing
zone (24). In some embodiments, the mixing members (23) are round, ovoid,
curved, ramp-
shaped, triangular, square (suitably disposed with an edge pointed upwardly)
or diamond-shaped,
or otherwise chosen to exhibit a cross-sectional shape that serves to re-
direct or direct individual
grains of sand (21) as they fall through the mixing zone (24) and thereby
effect a mixing action.
By impingement and deflection off of the lateral surfaces of rounded mixing
members (23), the
liquid treatment agent (12) on the sand (21) is re-distributed to more evenly
distribute the liquid
treatment agent across the bulk of the sand (21) in a manner that is
substantially uniform. The
use of pipes or rods with a sufficient material hardness to resist the
abrasive effects of falling
sand are shown to facilitate construction and maintenance as members (23)
become worn.
[0056] In some embodiments, the mixing members (23) are releasably connected,
secured or
retained within the mixer (13) by a suitable fastener or bracket to retain the
members (23) within
the mixer (13) despite the friction and forces of sand falling there through.
Suitable fasteners can
include, but are not limited to, bolts into the members (23) in a horizontal
direction, transverse
bolts that secure the members (23) to the mixer (13) with one or more flanges
or brackets that are
themselves secured, welded or connected to the lateral walls of the mixer
(13), or retention
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 14 -
brackets (not shown) having a U- or L-shape into which the member (23) is
secured from
vertical movement.
[0057] In some embodiments of the mixer (13), there is a transition zone (25)
that allows the
treated sand to settle before discharge through an outlet (26). Such a
transition also serves to
reduce the momentum of the discharged sand and thereby limit the forces that
might serve to
eject fugitive dust as the falling, treated sand is deposited.
[0058] An acceptable, alternative type of static mixer (13) is shown in
Figures 3 and 4. The
static mixer shown is substantially cylindrical in shape (like a 55 gallon
drum where the top inlet
(27) is substantially the same diameter as the bottom outlet (28)) and
dimensioned to receive,
mix, and discharge high volumes of proppant sand. In this embodiment, the
static, impingement-
type, mixing members (23) are formed by a series of rods or pipes (29) that
horizontally traverse
a drum (30) and are vertically distributed in a helical pattern (31) at an
inter-rod distance (32)
over the height of the drum (30). Three eyelets (33) attached to the top of
the drum (30) provide
supports for hanging the mixer below a free-fall discharge port of
conventional proppant sand
handling equipment.
[0059] A spray assembly (34) is shown in Figures 5 and 6 that can be used in
combination with
the static mixer (13) of Figures 3 and 4 in a configuration like that of
Figure 7. More specifically,
a spray assembly (34) is attached around the perimeter of a sand discharge
port with a series of
one or more, suitably 3-7, spray nozzles (14) that are substantially evenly
distributed around the
spray assembly (34). Each nozzle (14) is oriented radially inwardly and
downwardly with
overlapping spray pattern areas (36) so that sand introduced into the top
inlet (27) is contacted
with one or more spray streams of liquid treatment agent issuing through
nozzles (14) at the top
end of, or immediately before, the static mixer (13) located immediately below
the spray
assembly (34) to discharge a treated sand (35). Connectors or straps (37) on
the spray assembly
(34) are distributed to cooperate with eyehooks (33) on the static mixer for
suspending the static
mixer below the spray assembly.
[0060] Figure 8 illustrates an alternative version of the mixer that is shown
in Figure 7 but with
the addition of a second spray assembly (38) connected to a second liquid
treatment agent (39)
that can be the same or different than liquid treatment agent (12). Exemplary
second liquid
treatment agents can include: the dust control agents introduced as the first
liquid treatment agent
(12) as well as the functional treatments that are described above. The second
spray region can
be used to add a second functionality to the coating or simply to help insure
that more of the
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 15 -
proppant's surface area is covered by the coating process. Second nozzles (40)
are oriented to
spray the second liquid treatment agent (39) downwardly as treated sand (41)
is discharged.
[0061] Figures 9-12 depict further alternatives for a contact device for a
sprayed dust control
liquid treatment agent that contacts the proppant solids on-the-fly while the
solids are in a guided
free fall under the effects of gravity. It is contemplated that the use of
inline spray dispersion
systems can be used with minor modifications of conventional pneumatic
conveyance systems to
provide dust control treatment as the proppant solids are transported to or
from storage.
[0062] As shown in Figures 9-12, a contact mixer (42) is vertically oriented
to allow proppant
solids to fall therethrough. The top section (43) has a reinforcing vertical
lip (44) about the intake
opening (45) of a cover (55). The diameter of the top section (43) is greater
than that of the
diameter of the opening (45) to allow the nozzles (14) to disperse the dust
control liquid
treatment agent inwardly into a falling stream of proppants to be treated from
a relatively safe
perimeter position that is not impacted by the stream of falling solids and
the abrasion associated
therewith.
[0063] As shown, a supply connector (47) connects to a circular manifold (48)
that is in fluid
communication with nozzles (14) oriented inwardly toward the center of the
device for the
supply, under pressure, of liquid treatment agent to proppants as they fall
through the opening
(45). A horizontal upper surface (49) of the cover (55) extends inwardly
toward the lip (44) to
provide a partial upper enclosure of the contact zone that also reduce
upwelling fugitive dust
during the treatment process. An inward taper of the sidewalls below the
nozzles (14) helps to
guide solids from the sidewalls toward the middle mixing section.
[0064] Handles (50), such as 2-4 handles, and/or lifting lugs (51), such as 2-
4 lugs, can be
secured to the outside of the sidewall of the uppermost end (43) for handling
and positioning the
device.
[0065] The middle section (52) of the contact mixer (42) can be cylindrical in
external shape
and include plurality of static mixing deflector members (53). As shown, the
static mixing
deflector members (53) can be disposed as a plurality of spoke members within
an outer ring
(56) as a modular, substantially planar, spoke-containing hoop unit (54).
Figure 10 shows the use
of five such spoked hoop units (54), each having six deflector spoke members
(55) that are
evenly distributed around the interior of a ring (56) and that meet at
substantially the geometric
center of their respective hoop unit (54). The mixing deflector members (53)
can be secured to
the outer ring (56) by any method including welding, soldering, brazing and/or
fasters. Each
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 16 -
deflection hoop member (54) can be secured to the ring (56) by welding,
brazing, soldering or
similarly permanent and durable connection.
[0066] Each successive hoop unit (54) is then stacked vertically within middle
portion (52)
above the bottom section (57) and offset an appropriate angular amount
relative to the preceding
hoop unit (54) to provide a helical progression of deflector members (53) down
the length of the
middle portion (52) in the mixer (46). The lowest hoop unit (54) can rest on
the top of the bottom
section (57) but can be supported by a support flange or bracket (not shown)
that is secured to
the interior sidewall at the bottom (61) of the middle section (52).
[0067] The modular nature of this form of mixing device permits the degree and
duration of
mixing to be adjusted based on the number of mixing spokes found in each unit
and the number
of mixing modules that are used in the device.
[0068] The bottom section (57) of the mixer (46) can be in the form of a
straight cylinder (i.e.,
about 180 degrees relative to the outer sides of the middle section (52)) but
can exhibit an
inwardly tapered frustoconical cross section (60) that is at an angle (58)
that is within the range
from about 150-175 degrees, or at an angle within the range of about 160-170
degrees. This
tapering section helps to channel and settle the particulates at the outer
perimeter of the treated
proppant stream for discharge from the bottom opening (59). Similarly, the
bottom of the top
section (43) can exhibit an inward taper at an angle (62) that is within the
range from about 15-
45 degrees, or 25-35 degrees from vertical.
[0069] Accordingly, in some embodiments, a process for treating free-flowing,
finely divided
proppant solids is provided. In some embodiments, the process comprises
contacting the solids
less than five seconds with a liquid treatment agent with an amount of the
liquid treatment agent
that substantially retains free-flowing characteristics of the treated solids.
The liquid treatment
agent can be any agent described herein and contain one or more of the
compositions described
herein. In some embodiments, the solids are contacted with the liquid
treatment agent more than
once and each contacting step is for less than five seconds. The time period
for contact can also
be any time period as described herein.
[0070] The processes described herein are suitable for applying coatings or
agents to various
finely divided proppant solids. Examples include, but are not limited to,
uncoated sand, sand
with a cured or partially cured coating, bauxite, ceramic, coated bauxite, or
ceramic. In some
embodiments, the finely divided proppant solids are uncoated sand or resin-
coated sand.
[0071] In some embodiments, the process comprises spraying the liquid
treatment agent onto
the proppant solids while the solids are in free fall, guided free fall, or
during pneumatic
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 17 -
transport. Other embodiments are described herein can also be part of the
process. The solids
can also be sprayed substantially simultaneously from more than one direction.
[0072] As described herein, the processes described herein can be used to
apply a dust
reduction coating. The liquid treatment agent can also be effective or used to
coat the solids with
any one or more of: a hydrophobic coating, a coating that reduces friction, a
coating that
comprises a tracer, an impact modifier coating, a coating for timed or staged
release of an
additive, a coating that controls sulfides, a different polymeric coating, an
acid or base resistant
coating, a coating that inhibits corrosion, a coating that increases proppant
crush resistance, a
coating that inhibits paraffin precipitation or aggregation, a coating that
inhibits asphaltene
precipitation, and/or a coating comprising an ion exchange resin that removes
anions and/or
halogens, or any combination thereof Examples of such coatings are described
herein.
[0073] In some embodiments, a process for producing free-flowing, finely
divided proppant
solids with reduced dust properties is provided. In some embodiments, the
process comprise
contacting the solids for a period of time as described herein with a dust
reducing liquid
treatment agent with an amount of the dust reducing liquid treatment agent
that substantially
retains free-flowing characteristics of the treated solids and reduces the
dust produced by the
solids. In some embodiments, the dust produced by free-flowing, finely divided
proppant solids
with reduced dust properties is less than dust produced by solids not
contacted with the dust
reducing liquid treatment agent. In some embodiments, the dust reducing liquid
treatment agent
is effective to coat the solids with a hydrophobic coating, a coating that
reduces friction, a
coating that comprises a tracer, an impact modifier coating, a coating for
timed or staged release
of an additive, a coating that controls sulfides, a different polymeric
coating, an acid or base
resistant coating, a coating that inhibits corrosion, a coating that increases
proppant crush
resistance, a coating that inhibits paraffin precipitation or aggregation, a
coating that inhibits
asphaltene precipitation, and/or a coating comprising an ion exchange resin
that removes anions
and/or halogens. That is, in some embodiments, the coating can have more than
one function. In
some embodiments, the dust reducing treatment agent comprises a polysaccharide
solution. In
some embodiments, the dust reducing treatment agent comprises a C6-C16
alkoxylated alcohol.
In some embodiments, the dust reducing treatment agent comprises at least one
acrylic polymer.
In some embodiments, the dust reducing treatment agent comprises an acrylic
copolymer. In
some embodiments, the dust reducing treatment agent comprises a mixture of at
least one C6-C16
alkoxylated alcohol and at least one acrylic polymer. In some embodiments, the
amount of the
dust reducing treatment agent that is applied to the solids is an amount of
less than 1 wt% per
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 18 -
weight based on the weight of said proppant solids. In some embodiments, the
amount is an
amount of less than 0.5 wt%. In some embodiments, the amount is an amount of
less than 0.35
wt%. In some embodiments, In some embodiments, the amount is an amount of less
than 0.25
wt%.
[0074] In some embodiments, the dust reducing treatment agent comprises an
emulsion of
ethoxylated, propoxylated C6-C12 alcohols, ethoxylated, propoxylated Cio-C16
alcohols, acrylic
polymers, and water. In some embodiments, the dust reducing treatment agent
comprises a
surfactant. In some embodiments, the dust reducing treatment agent comprises
less than 0.1%
aqueous ammonia. In some embodiments, the dust reducing treatment agent
comprises less than
0.05% free (e.g. residual) monomers. In some embodiments, the dust treatment
agent comprises
about 15% to about 30%, about 17 to about 28%, or about 20% to about 25% of
ethoxylated,
propoxylated C6-C12 alcohols. In some embodiments, the dust treatment agent
comprises about
5% to about 20%, about 8 to about 18%, or about 10% to about 15% of
ethoxylated,
propoxylated C10-C16 alcohols. In some embodiments, the dust reducing reagent
comprises
about 20% to about 25% of ethoxylated, propoxylated C6-C12 alcohols, about 10%
to about 15%
of ethoxylated, propoxylated C10-C16 alcohols, about 5% to about 10% acrylic
polymers, less
than 0.1% ammonia, less than 0.05% free monomers. In some embodiments, the
dust reducing
reagent comprises about 20% to about 25% of ethoxylated, propoxylated C6-C12
alcohols, about
10% to about 15% of ethoxylated, propoxylated C10-C16 alcohols, about 5% to
about 10% acrylic
polymers, less than 0.1% ammonia, less than 0.05% free monomers with the
remaining being
water.
[0075] In some embodiments, a process for coating a free-flowing proppant is
provided. In
some embodiments, the process comprises contacting the proppant for a period
of time as
described herein with a liquid treatment agent with an amount of the liquid
treatment agent that
substantially retains free-flowing characteristics of the proppant to produce
coated free-flowing
proppant, wherein the coating is a dust reducing coating, a hydrophobic
coating, a coating that
reduces friction, a coating that comprises a tracer, an impact modifier
coating, a coating for
timed or staged release of an additive, a coating that controls sulfides, a
different polymeric
coating, an acid or base resistant coating, a coating that inhibits corrosion,
a coating that
increases proppant crush resistance, a coating that inhibits paraffin
precipitation or aggregation, a
coating that inhibits asphaltene precipitation, and/or a coating comprising an
ion exchange resin
that removes anions and/or halogens, or any combination thereof In some
embodiments, the
coating is a dust reducing coating. In some embodiments, the coating is a
hydrophobic coating, a
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 19 -
coating that reduces friction, a coating that comprises a tracer, an impact
modifier coating, a
coating for timed or staged release of an additive, a coating that controls
sulfides, a different
polymeric coating, an acid or base resistant coating, a coating that inhibits
corrosion, a coating
that increases proppant crush resistance, a coating that inhibits paraffin
precipitation or
aggregation, a coating that inhibits asphaltene precipitation, or a coating
comprising an ion
exchange resin that removes anions and/or halogens, or any combination thereof
[0076] Coated free-flowing proppants comprising a dried and/or cured coating
that comprises
less than about 3 wt% of a liquid treatment agent are also provided. In some
embodiments, the
coated, free-flowing proppant exhibits reduced fugitive dust generation as
compared to the
uncoated proppant. In some embodiments, the coated, free-flowing proppant
comprises 0.0009-
0.5 wt% of the coating. In some embodiments, the coated, free-flowing proppant
comprises
0.001-0.35 wt% of the coating. In some embodiments, the coating comprises one
or more of:
monosaccharides or polysaccharides, surfactants, alkoxylated alcohols, acrylic
polymers,
methacrylic polymers, copolymers of acrylic acid and/or methacrylic acid,
methacrylates and
copolymers thereof, polyvinyl acetates, vinyl acrylic copolymers,
polybutadiene, low molecular
weight mineral oils, acrylamide polymers, lignosulfonates, water-dispersible
natural gums,
water-dispersible pectins, water-dispersible starch derivatives, water-
dispersible cellulose
derivatives, or any mixture thereof
[0077] In some embodiments, the coating comprises one or more monosaccharides
or
polysaccharides. In some embodiments, the coating comprises one or more
alkoxylated
alcohols. In some embodiments, the coating comprises at least one C6-C12
alkoxylated alcohol
and at least one C10-C16 alkoxylated alcohol. In some embodiments, the coating
comprises one
or more acrylic polymers. In some embodiments, the coating comprises at least
one C6-C12
alkoxylated alcohol, at least one C10-C16 alkoxylated alcohols, and at least
one acrylic polymer.
In some embodiments, the coating comprises one or more methacrylic polymers,
one or more
copolymers of acrylic acid and/or methacrylic acid, and one or more of
methacrylates. In some
embodiments, the coating is a hydrophobic coating, a coating that reduces
friction, a coating that
comprises a tracer, an impact modifier coating, a coating for timed or staged
release of an
additive, a coating that controls sulfides, a different polymeric coating, an
acid or base resistant
coating, a coating that inhibits corrosion, a coating that increases proppant
crush resistance, a
coating that inhibits paraffin precipitation or aggregation, a coating that
inhibits asphaltene
precipitation, or a coating comprising an ion exchange resin that removes
anions and/or
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 20 -
halogens. In some embodiments, the coating further comprises a sulfide
scavenger or scale
inhibitor.
[0078] Various liquid treatment agents are described herein. The liquid
treatment agents can be
applied to the solids according to any of the various embodiments described
herein. The liquid
treatment agents can be applied simultaneously or consecutively. Additionally,
the processes
described herein can be used to add multiple layers or coatings to the solids.
The liquid
treatment agents can also be applied singularly or in any combination with one
another. The
process is not limited to applying any one coating, unless explicitly stated
to the contrary.
[0079] The liquid treatment agent that can be used in the methods described
herein can be an
aqueous solution or emulsion. In some embodiments, the liquid treatment agent
can be used to
reduce dust produced by the solids. This can be referred to as "fugitive dust
control." In some
embodiments, a liquid treatment agent for controlling dust can be, for
example, an aqueous
solution or emulsion comprising one or more polysaccharides, surfactants and
alkoxylated
alcohols, acrylic polymers, methacrylic polymers and copolymers of acrylic
acid and/or
methacrylic acid, polyvinyl acetates, vinyl acrylic copolymers, methacrylates
(see U.S. Patent
No. 4,594,268) and copolymers with methacrylates, polybutadiene, low molecular
weight
mineral oils, and mixtures thereof The use of aqueous solutions permit the
liquid treatment
agent to be purchased as a concentrate and then diluted to a working
concentration when needed
or when there is access to a supply of dilution water. The use of water-based
dispersions also
avoids the need to handle another hydrocarbon material at the wellsite.
[0080] Suitable monosaccharides and polysaccharides include starches, sugars
and sugar-based
materials. Examples of such materials include, but are not limited to,
molasses, glycerol, hydrol,
black-strap, residual syrups, mother liquors, bagasse, sorgo molasses, wood
molasses, or corn
molasses and/or beet or cane sugar juices formed during the raw preparation or
refining of sugar.
For example, see the fertilizer treatment described in WO 2013/029140 made
with (a) raffinate
and (b) sugar-containing solution. The raffinate (a) is an aqueous solution
effluent (for instance
syrup or liquor) from fermentation processes (residuary or not). Raffinate (a)
is an aqueous
solution comprising at least citric acid, inorganic matter (such as minerals),
proteic matter and
sugar matter. The sugar includes carbohydrate selected from fructose,
dextrose, maltose and/or
polyol selected from arabitol, erythritol, or mixtures thereof See also US
Patent Nos. 6,790,245
and 7,157,021.
[0081] Non-limiting examples of surfactants and alkoxylated alcohols that can
be used include,
but are not limited to, C10-C14 alpha-olefin sulfonates, Cio-C16 alcohol
sulfates, C2-C16 alcohol
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 21 -
ether sulfates, C2-C16 alpha sulfo esters, highly branched anionic
surfactants, nonionic
surfactants that are block copolymers of molecular weight less than 600 and
derived from
ethylene oxide/propylene oxide or other epoxide, nonionic surfactants that are
C8-C16 branched
alcohols that have been ethoxylated with four to ten moles of ethylene oxide
per mole alcohol,
and mixtures thereof For example, see the coal dust treatment described in CA
Patent No.
2,163,972 and US Patent No. 4,592,931. See also U.S. Patent Nos. 6,372,842;
5,194,174;
4,417,992 and 4,801,635. Other examples include those described in
EP01234106A2; U.S. Pat.
No. 3,900,611; U.S. Pat. No. 3,763,072; WO 2005/121272 and U.S. Patent
Application
Publication No. 2007/073590. Any overlap in molecular length in the above
ranges is due to the
realities of commercial production and separation and would be so recognized
by those in this
technology.
[0082] A variety of water soluble or water-dispersed polymers or polymer
emulsions can also
be a part of the liquid treatment agent. Examples include, but are not limited
to, acrylic
polymers and copolymers, methacrylic polymers and copolymers of acrylic acid
and/or
methacrylic acid. Examples of alkoxylated alcohols that can be used include,
but are not limited
to, acrylic acid copolymers of acrylic acid and one or more of unsaturated
aliphatic carboxylic
acids such as 2-chloroacrylic acid, 2-bromoacrylic acid, maleic acid, fumaric
acid, itaconic acid,
methacrylic acid, mesaconic acid or the like or unsaturated compounds
copolymerizable
with acrylic acid, for example, acrylonitrile, methyl acrylate, methyl
methacrylate, vinyl acetate,
vinyl propionate, methyl itaconate, styrene, 2-hydroxylethyl methacrylate, and
the like.
[0083] In some embodiments, the polyacrylic acid or acrylic acid copolymer has
a weight
average molecular weight of from about 5,000 to about 30 million or from about
1 million to
about 5 million. In some embodiments, the amount of acrylic polymer present in
the mixture
with the polybasic acid is about 2 to about 50, about 3 to about 10, or about
4, parts by weight
per weight part of polybasic acid. See, U.S. Patent No. 4,592,931 the
disclosure of which is
hereby incorporated by reference.
[0084] Polyvinyl acetate and vinyl acrylic solutions and emulsions can also be
used in the
liquid treatment agent. For example, water-dispersible acrylic and vinyl
polymers are suitable,
include but are not limited to the homo-, co-, and ter- polymers of acrylic
acid, vinyl alcohol,
vinyl acetate, dimethyl diacrylyl ammonium chloride (DMDAAC), acrylaminyl
propyl sulfonate
(AMPS) and the like, and combinations thereof
[0085] Acrylamide polymers can also be used in the liquid treatment agent.
Examples of
acrylamide polymers include, but are not limited to, a polyacrylamide
copolymer in an amount
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 22 -
within the range from about 0.5 to about 20 wt% of the resulting mixture. In
some embodiments,
the acrylamide is added in an amount from about 1 to about 2 wt%. Examples of
suitable
acrylamides include, but are not limited to, anionic charged polyacrylamides
or polyacrylamide
polyacrylate copolymers with an average molecular weight from 3 million to 25
million g/mol
and a charge density from 10% to 60%. Non-limiting examples of commercial
acrylamide
products include: AN934XD from SNF, Inc., AF306 from Hychem, Inc., and
Magnafloc 336
from CIBA.
[0086] The polyacrylamide can be used alone or in combination with a starch
that has been
modified for enhanced solubility in cold water. See U.S. Patent No. 5,242,248
(polyacrylamide
treatment for horse arenas) and Published U.S. Patent Application Publication
No. 20130184381,
the disclosures of which are hereby incorporated by reference.
[0087] Lignosulfonates can also be used as the liquid treatment agent or as a
component of the
liquid treatment agent. Examples include, but are not limited to, lignin
sulfonate salts such as
ammonium lignin sulfonate, and alkali metal and alkaline earth metal salts of
lignosulfonic acid,
such as sodium lignin sulfonate, calcium lignin sulfonate and the like, and
combinations thereof
In some embodiments, ammonium lignin sulfonate can be used. Without wishing to
be bound by
any particular theory, ammonium lignin sulfonate can be used in order to avoid
the addition of
inorganic materials such as calcium and sodium, particularly sodium.
[0088] The liquid treatment agent can also include one or more water-
dispersible natural gums,
water-dispersible pectins, water-dispersible starch derivatives, or water-
dispersible cellulose
derivatives. Examples of natural gums include: terrestrial plant exudates
including, but not
limited to, gum arabic (acacia), gum tragacanth, gum karaya, and the like;
terrestrial plant seed
mucilages, including but not limited, to psyllium seed gum, flax seed gum,
guar gum, locust
bean gum, tamarind kernel powder, okra, and the like; derived marine plant
mucilages, including
but not limited to, algin, alginates, carrageenan, agar, furcellaran, and the
like; other terrestrial
plant extracts including but not limited to arabinogalactan, pectin, and the
like; microbial
fermentation products including but not limited to xanthan, dextran,
scleroglucan, and the like.
Cellulose derivatives include chemical derivatives of cellulose, including but
not limited to,
alkyl, carboxyalkyl, hydroxyalkyl and combination ethers, and the sulfonate
and phosphate
esters.
[0089] In some embodiments, the guar gum is a solution whose viscosity can be
adjusted to
accommodate variations in the treated solids. For example, the viscosity of a
guar gum solution
can be adjusted by treatment with gamma radiation to achieve a viscosity of
about 40 to about
CA 02927111 2016-04-11
WO 2015/066283
PCT/US2014/063086
-23-
140 cps at 1% concentration at application temperature. Guar gum (such as that
sold by Rantec,
Inc. under the trade names Super Tack, C7000, J3000, and HVX); carboxymethyl
guar gum
(such as CM Guar sold by Maharashtra Traders); carboxymethyl cassia seed
powder (such as
CM Cassia sold by Maharashtra Traders); carboxymethyl cellulose (such as
FinnFix300 sold by
Noviant); starch (corn, maize, potato, tapioca, and wet milled/spray dried
starch such as
GW8900 sold by KTM Industries); starches pre-treated with crosslinking agents
such as
epiclorohydrin and phosphorus oxychloride; Carboxymethyl starch (0.2 to 0.3
degree of
substitution (DS), such as AquaBloc, KogumHS, RT3063 and RT3064 sold by
Process Products
N.W.); hydroxypropyl guar gum; hydroxyethyl guar gum; carboxymethyl-
hydroxypropyl guar
gum; ethyl starch; oxidized starch; and hydroxyethyl cellulose. Other examples
of polymers
include Cassia seed powder, psyllium husk powder, xanthan gum, any cereal
grain, annual or
perennial dicot seed derived polysaccharide (sesbania, locust, bean gum, flax
seed, and gum
karaya).
[0090] In some embodiments, prior to the addition of guar gum, the water for
the treatment
agent formulation can be treated with a crosslinking agent made with a blend
of one part glyoxal
and two parts zirconium lactate (e.g., the DuPont product sold under the brand
name TYZOR
217) at a rate of 30 to 50 parts crosslinking agent per 100 parts of polymer.
For example, to 15
gallons of water (125.1-1b) a dose of 1.75-lb of guar gum is to be added;
prior to the polymer
addition a dose of 0.70-lb of crosslinking agent (40% of 1.75-lb of polymer)
is added. The guar
gum polymer can, in some embodiments, be added to the water at a rate of 0.70%
to 1.4% by
weight. A plasticizer, glycerin, can also be added at a rate of 0.5 to 5% by
weight of the guar
gum solution.
[0091] Water-dispersible starch derivatives include, but are not limited to,
alkyl, carboxyalkyl,
hydroxyalkyl and combination ethers of starch, phosphate or sulfonate esters
of starch and the
like which are prepared by various chemical or enzymatic reaction processes.
[0092] Tables 1 and 2 are non-limiting exemplary lists of liquid, dust
suppressing, chemical
liquid treatment agents by category and commercial product name that can be
used to treat
proppant solids for fugitive dust control according to the processes and
methods described
herein.
Table 1
SUPPRESSANT PRODUCT NAME MANUFACTURER OR PRIMARY
CATEGORY DISTRIBUTOR
Molassas/Sugar Beet Dust Down Amalgamated Sugar Co.
CA 02927111 2016-04-11
WO 2015/066283
PCT/US2014/063086
- 24 -
Table 1
SUPPRESSANT PRODUCT NAME MANUFACTURER
OR PRIMARY
CATEGORY DISTRIBUTOR
Tall Oil Emulsion Dust Control E Pacific Chemicals, Inc./
Lyman Dust Control
Dustrol EX Pacific Chemicals, Inc / Lyman Dust
Control
Road Oyl Soil Stabilization Products Co., Inc.
Vegetable Oils Soapstock Kansas Soybean Association
Indiana Soybean Association
Dust Control Agent SS Greenland Corp.
Enzymes Bio Cat 300-1 Soil Stabilization Products Co., Inc.
EMCSQUARED Soil Stabilization Products Co., Inc.
Perma-Zyme 11X The Charbon Group, Inc.
UBIX No. 0010 Enzymes Plus,
Div of Anderson
Affiliates
Ionic Road Bond EN-1 C.S.S. Technology, Inc.
Terrastone Moorhead Group
Sulfonated Oils CBR Plus CBR Plus, Inc. (Canada)
Condor SS Earth Sciences Products Corp.
SA-44 System Dallas Roadway Products, Inc.
Settler Mantex
Ten-aBond Clay Fluid Sciences, LLC
Stabilizer
Polyvinyl Acetate Aerospray 70A Cytec Industries
Soil Master WR Enviromental Soil Systems, Inc.
Vinyl Acrylic Earthbound L Earth Chem Inc.
ECO-110 Chem-crete
PolyPavement PolyPavement Company
Liquid Dust Control Enviroseal Corp.
Marloc Reclamare Co.
Soiloc-D Hercules Soiloc
CA 02927111 2016-04-11
WO 2015/066283
PCT/US2014/063086
- 25 -
Table 1
SUPPRESSANT PRODUCT NAME MANUFACTURER OR PRIMARY
CATEGORY DISTRIBUTOR
Soil Seal Soil Stabilization Products Co., Inc.
Soil Sement Midwestern Industrial Supply, Inc.
Ten-aBond PolySeal Fluid Sciences, LLC
Combination of Top Shield Base Seal International, Inc.
Polymers
Table 2
Polymers
= Ten-aLOC ¨ polyvinyl alcohol from MonoSol, LLC, Portage IN 46368
= Tracer Tackifier ¨ copolymer of sodium acrylate and acrylamide with
pre-gelatinized starch from Reinco Inc., Plainfield, NJ 07061
= DirtGlue ¨ acrylate ester polymer emulsion and organosilicon
waterproofing agent (US 2012020755) from TerraFirmer Corporation,
Amesbury, Massachusetts 01913
= Soil Sement ¨ emulsion of acrylic and vinyl acetate polymer plus a
resin-modified emulsion made with a mixture of pitch and rosin (US
2013019128) from Midwest Industrial Supply, Akron, Ohio
= Enviroseal LDC ¨ inorganic acrylic copolymers from Enviroseal
Corporation, Port St. Lucie, Florida 34952
= Envirotac II ¨ acrylic copolymers from Environmental Products &
Applications, La Quinta, California 92253
= DustShield -- acrylic styrene emulsion polymer from Soil-Loc, Inc.,
Scottsdale, Arizona 85255
= SoilShield-LS ¨ Poly vinyl acrylic copolymer from Soil-Loc, Inc.,
Scottsdale, Arizona 85255
CA 02927111 2016-04-11
WO 2015/066283
PCT/US2014/063086
- 26 -
= Marloc ¨ copolymer emulsion from Rantec Corp., Ranchester, WY
82839
= SOILOC-MQ ¨ liquid blend of acrylic resins from Hercules
Environmental, Inc., Doraville, GA 30340
= Polytac ¨ acrylic co-polymer from DustPro, Inc., Phoenix, AZ 85034
= Soiltac0 ¨ synthetic copolymer emulsion from Soilworks, LLC.,
Chandler, AZ 85286
Lignin Sulfonates
= Lignosite 458 -- from Georgia-Pacific Chemicals LLC, Atlanta, GA
= LS-50 from Prince Minerals, New York, NY 10036
Other Chemical Suppressants
= EK-35 -- high viscosity synthetic iso-alkane from Midwest Industrial
Supply, Inc., Canton, OH
= EnviroKleen ¨ sodium salt of a secondary alkane sulphonate and D-
limonene from Milestone Chemicals Australia Pty Ltd., West Heidelberg, Vic.
3081, Australia
= Earthzyme -- multi-enzyme product from Cypher International Ltd.,
Winnepeg, MB Canada RG3 0J8
= Diamond Doctor ¨ severely hydrotreated, hydorcracked,
hydroisomerized, high viscosity synthetic iso-alkane (CAS 178603-64-0) from
Midwest Industrial Supply, Inc., Canton, OH
= DUSTRACT ¨ mixture of diethylene glycol, ethyl alcohol and sodium
dioctyl succinate from Midwest Industrial Supply, Inc., Canton, OH
= DustFloc -- blend of natural and organic polysaccharides from Apex
Resources, Inc., Louisville, KY 40228
= Roadbond EN1 ¨ sulphonates and surfactants from C.S.S. Technology,
Inc., Tolar, TX 76476
= TERGITOLTm NP- or NP-9 -- nonionic surfactants from Dow
Chemical
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-27 -
= PAVECRYLTM SUPPRESS -- vinyl/acrylic emulsion from Dow
Chemical
Other Emulsions
= ArenaPro -- natural soy-lecithin blend from Dustkill LLC, Columbus, IN
47203
= Road Oyl Resin Modified Emulsion ¨ a pine rosin and pitch emulsion
alleged to be made in accordance with US Patent No. 4,822,425; from Midwest
Industrial Supply, Inc., Canton, OH
[0093] The products described herein can be contacted with the solids as
described herein.
The processes are not limited to the specific examples. Other liquid, dust
suppression, liquid
treatment agents that are typically commercially available and described as
useful for controlling
unpaved road dust, dust from storage piles, and similar structures can also be
used. Such agents
can be aqueous or solvent-based, but are not just water or a volatile solvent.
That is, in some
embodiments, a liquid treatment agent is not water or a volatile solvent not
containing any other
components. A listing of such materials has been published by the City of
Albuquerque and can
be found at goo"dot"gl/wlehmI.
[0094] In some embodiments, the liquid treatment agent can be in the form of
thin coatings that
can cure by contact with ambient water or moisture, e.g., an alkyd that can
cure on exposure to
moisture.
[0095] In some embodiments, the liquid treatment agent comprises a light
mineral oil which
can be contacted with the proppant solids in the form of a light oil or in an
aqueous form with a
surfactant. Mineral oils that can be used as/in the liquid treatment agent
include, but are not
limited to, mineral oils characterized by a pour point of from about 30 F to
about 120 F, a
viscosity from about 50 SSU to about 350 SSU at 100 F, a distillation
temperature above about
500 F, a distillation end point below about 1000 F, a distillation residue
of not more than about
15%, and an aromatic content of not more than about 60%.
[0096] In some embodiments, mineral oils are characterized by a pour point of
from about 35
F to about 100 F, a viscosity from about 100 SSU to about 310 SSU at 100 F,
a 10%
distillation temperature from about 500 F to about 700 F, a distillation end
point below about
900 F, a distillation residue of not more than about 15%, and an aromatic
content of not more
than about 50%.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-28-
100971 The mode or modes by which the liquid treatment agent according to the
methods
disclosed herein reduces fugitive dust is not, as yet, fully understood. While
not wishing to be
bound by any particular theory, it may be that the applied liquid treatment
agent provides a
sufficiently adhesive surface that generated fugitive dust merely sticks to
the outer surface of a
treated solid. It may also be that the treated surface acts as a wetted
surface of reduced friction
that allows impacts to slide off rather than impart a structural shock impact
to the proppant. A
further possibility is that the small amount of applied dust control liquid
treatment agent acts as
an adhesive and that fugitive dust captured on the surface of the treated
proppant acts as an
impact modifier to cushion impacts and friction that might otherwise generate
fugitive dust from
the proppant surface. It may also be that when the chosen polymer is applied
to some substantial
part of the exposed surface area that the polymer acts as an impact modifier
to cushion the
impact of the grain-to-metal or grain-to-grain contacts. It may also be that,
if the treatment
process does not fully cover the exposed surface area, that the collision of
an uncoated grain with
a partially-coated grain still can minimize the generation of dust/broken
particles. The exact
reason that the processes described herein can be used to reduce dust is not
necessarily
significant, but rather the result that is achieved is.
[0098] The processes described herein can also be used to apply other coatings
to proppants.
Such other coatings can provide the proppants with additional, functional
properties at the same
time as the dust control treatment or an independent treatment step. Such
other coatings can
include the following. The processes can also be used to provide a coating
that does not result in
fugitive dust control.
[0099] Hydrophobic coatings. Water barriers are useful to prevent reaction or
dissolution of
proppant under acidic or basic conditions downhole. Chemical reactions of
proppant are known
to cause reductions in crush resistance, and potential scale formation through
diagenesis, i.e.,
dissolution of the proppant and re-precipitation with dissolved minerals in
the formation water.
[0100] A water resistant coating can be formed by contacting the proppant
solids with one or
more organofunctional alkoxy silanes to develop a hydrophobic surface.
Examples of
organofunctional alkoxy silanes include, but are not limited to, waterborne or
anhydrous alkyl or
aryl silanes. Triethoxy RCH3CH20)3Sin or trimethoxy [(CH30)3SiR] where R
represents a
substituted or unsubstituted alkyl or substituted or unsubstituted aryl
moiety, silanes and
chlorosilanes could be used as well if a lower reaction temperature and higher
speed of reaction
are necessary. It should be noted that HC1 can be generated as a byproduct of
the treatment
process, which can cause issues with corrosion. Therefore, in some
embodiments, corrosion-
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 29 -
resistant treatment heads and handling equipment immediately after the
chlorosilane treatment
can be used.
[0101] In some embodiments, if a hydrophobic and oleophobic surface is
required, treatment of
the proppant with a fluoroalkyl silane is performed.
[0102] If a thicker crosslinked, polymeric coating is needed for enhanced
durability and
hydrophobicity, a polymer can be applied after the silane treatment. In such a
treatment, the
silanes can include, but are not limited to, a triethoxy RCH3CH20)3Sin or
trimethoxy
[(CH30)3SiR] silane, where the R can include a functional group that could
either react with
crosslinkable polymers after they are applied on the surface of the proppant,
or can be
chemically compatible with the polymer for van der Waals force of adhesion of
the polymer. In
some embodiments, the R Groups for the silanes include, but are not limited
to:
amines (for preparation or polyurethanes, polyureas, polyamides, polyimides or
epoxies.
Amines can also be used for polysulfones);
isocyanates (for polyurethane, polyurea coatings);
vinyl (for reaction with polybutadiene, polystyrenebutadiene, other addition
type
olefinic polymers, or reaction with residual vinyl groups in any copolymer
blends used as
coatings);
epoxides (for reaction with epoxies);
methacrylate or ureido groups (for polyacrylates); and
phenyl groups (for use with aromatic-containing polymers such as the
polyaryletherketones (PAEKs) and their composites such as
polyetherketoneketone (PEKK)/
50:50 terephthallic:isothallic/ amorphous polyetherketoneetherketoneketone
(PEKEKK),
polyethersulfone (PES), polyphenylsulfone (PPSU), polyetherimine (PEI), or
poly(p-phenylene
oxide) (PPO)).
[0103] The thicker, crosslinked, polymeric coatings can be prepared by a first
step of
application of silanes, followed by a second step of flash coating with the
polymer, prepolymers,
or monomers. As used herein, the phrase "flash coating" refers to the process
of applying the
agent according to a process described herein. In some embodiments, catalysts
can be used for
inducing reactions at typical operating temperatures of the flash coating
process, i.e. room
temperature to 85 C. In some embodiments, methoxysilanes tend to react faster
than ethoxy
silanes, so methoxysilanes can be used for fast, flash-type coatings. If speed
of reaction of the
silane treatment is a limiting factor for proper coating, chlorosilanes can be
used as substitutes
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 30 -
for methoxy or ethoxysilanes. In some embodiments, corrosion resistant
materials are used in
the application process.
[0104] In some embodiments, methods for forming flash coatings of high
temperature aromatic
polymers use a solvent-based slurry or fully dissolved solution. Suitable
solvents include, but are
not limited to, N-methylpyrrolidone (NMP), dimethylformamide (DMF), and
dimethylsulfoxide
(DMSO). If excess solvents remain after application, they can be removed via a
drying step prior
to transfer into containers for shipment.
[0105] Scale inhibition. Several polymeric substances can be used on proppants
to inhibit
scale formation, including phosphino-polycarboxylates, polyacrylates, poly
vinyl sulphonic
acids, and sulphonated polyacrylate co-polymers, or any combination thereof In
the past, these
polymers had to be injected into the formation where they would then disperse
to be effective.
See US Patent No. 5,092,404. Such injections often lead to a substantial
volume of the inhibitor
being produced back out of the well early in the production cycle. By applying
them directly to
the proppant as described herein, the coated proppants can provide a targeted,
positionable, anti-
scale treatment on the relatively large surface area of the proppants in
fractured strata. With a
large portion of the active surface area treated, the effective surface area
where scale can form is
reduced as well as prevent scale formation in the spaces between proppant
particles (i.e., pores)
where scale deposits can have a large negative impact on proppant
conductivity.
[0106] Suitable scale inhibitors include, but are not limited to, carboxylates
and acrylates.
These inhibitors can be applied to the surface of a proppant in a similar
manner to those other
functional coatings described above. Also suitable are fumaric acid (CAS 110-
17-8), Diethylene
Glycol (CAS 111-46-6), phosphorous acid (CAS 13598-36-2), trisodium 2,2'4{2-
[(carboxylatomethyl)amino]ethyll imino)diacetate (CAS 19019-43-3), sodium
glycolate (CAS
2836-32-0), glycine (CAS 38011-25-5), trisodium nitrilotriacetate (CAS 5064-31-
3), 1,2-
propylene glycol (CAS 57-55-6), methoxyacetic acid (CAS 625-45-6),
methylphosphonic acid
(CAS 6419-19-8), polyphosphoric acids (CAS 68131-71-5), alkylbenzene (CAS
68648-87-3),
phosphino-carboxylic acid (CAS 71050-62-9), trisodium ortho phosphate CAS 7601-
54-9), and
sodium polyacrylate (CAS 9003-04-7), or any combination thereof
[0107] If additional adhesion to the proppant surface is needed due to too
high of a solubility of
the scale-inhibiting polymer in the production fluid, amines or ureidosilanes
can be used as
tethering agents for the acrylates and carboxylates. Full chemical bonding can
also be achieved
by adding a vinyl silane, and also retaining some vinyl functionality in the
carboxylates,
acrylates, and polyvinylphosphonic or polyvinylsulfonic acids. Peroxides can
be used to initiate
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 31 -
coupling of the vinyl silane with the vinyl polymer treatment, via addition of
the peroxide in a
subsequent treatment, and applying it to a heated substrate. In some
embodiments, additives can
be mixed with inert polymers to be sprayed to impart scale reduction
functionality to the
coatings. They could also be imbedded in water soluble polymers to allow timed
release of the
scale additives. The release time of the additives from the polymeric coating
can be adjusted by
modifying the swell rates of the polymer via adjustments to the crosslink
density or density of
concentrations of hydrophilic moieties on the polymer backbones.
[0108] Friction reduction. Currently, when those in the industry refer to
"friction reduction"
they are talking about the friction pressure generated when moving the frac
fluid down the well,
typically through tubular conduits to the formation to be treated. Of the
mechanisms for friction
reduction, the most accepted is thought to involve a reduction in turbulent
flow due to the
presence of stretched oligomers or high molecular weight polymers that extend
into the fluid and
disrupt the formation of turbulent eddies in the flowing fluid, often along
the walls of a conduit.
[0109] Proppant treatment for reduced friction can take the form of a
released, high molecular
weight polymer that can help with fugitive dust control aboveground but which
releases from the
proppant into the frac fluid where it serves a second function as a turbulence
reducer. Therefore,
one can create a proppant that has fugitive dust control and reduced friction
properties. In some
embodiments, these properties can be imparted onto the solids with the same
treatment agent.
[0110] In some embodiments, a direct coating of the proppant with one or more
releasable or
dissolvable polymers can deliver the turbulence-reducing agents for the well
via a surface on the
proppant. The coating can be designed to release the turbulence-reducing
agents immediately or
after some time delay. If delayed, such a coating can help reduce the volume
of turbulence-
reducing polymers in the frac fluid and avoid the associated deposits and loss
of conductivity
that can accompany such excess quantities. Once the proppant is placed in the
fracture, the
delayed dissolution or release of the polymeric turbulence-reducing coating on
the proppant
occurs in-situ for enhanced control and reduced opportunities for unintended
deposits and
accumulations of polymeric agents.
[0111] The turbulence-reducing coatings can be designed by those in this art
for immediate
release via use of water soluble polymers, or for timed release via tailoring
of the water soluble
polymer for delayed swelling. Materials that can be used for friction-reducing
coatings include
caprylic alcohol caprylic alcohol (CAS 111-87-5), polyacrylamide (CAS 25085-02-
3),
copolymer of acrylamide and sodium acrylate (CAS 25987-30-8),
acrylamide/ammonium
acrylate copolymer (CAS 26100-47-0), ethoxylated oleylamine (CAS 26635-93-8),
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-32 -
acrylamide/sodium acryloyldimethyltaurate copolymer (CAS 38193-60-1), 2-
propenamide,
polymer with 2-propenoic acid and sodium 2-propenoate (CAS 62649-23-4),
alcohols, c6-c12,
ethoxylated (CAS 68002-97-1), alcohols,c12-14, ethoxylated (CAS 68439-50-9),
alcohols,c12-
16, ethoxylated (CAS 68551-12-2), ammonium sulfate (CAS 7783-20-2), acrylamid
(CAS 79-
06-1), ptfe (teflon) (CAS 9002-84-0), polyacrylamide (CAS 9003-05-8),
poly(acrylamide-co-
acrylic acid) (CAS 9003-06-9), or any combination thereof
[0112] In the so-called "water fracs" where there is no frac fluid system and
only a friction
reducer in water, the concentration of the friction reducer is very low (< 5
lb/1000 gallons). In
such a case, the turbulence-reducing polymer is less likely to cause
significant damage but
surface friction along the proppant pack pores can retard flow and thereby
reduce conductivity.
Such a situation can benefit from the second type of coating having
hydrophobic and/or
oleophobic properties to allow flowing fluids to slide off the proppant
surfaces and through the
pore spaces. A coating that is either hydrophobic and/or oleophobic can permit
both materials to
move by with reduced friction.
[0113] In the so-called "water fracs" where there is no frac fluid system and
only a friction
reducer in water, the concentration of the friction reducer is very low (< 5
lb/1000 gallons). In
such a case, the turbulence-reducing polymer is less likely to cause
significant damage but
surface friction along the proppant pack pores can retard flow and thereby
reduce conductivity.
Such a situation can benefit from the second type of coating having
hydrophobic and/or
oleophobic properties to allow flowing fluids to slide off the proppant
surfaces and through the
pore spaces. A coating that is neither hydrophobic nor oleophobic can permit
both materials to
move by with reduced friction.
[0114] Treatment in this manner can also result in improvement in removal of
static water
trapped in the interstices of the proppant particle surface and between the
particles. This can help
minimize water lock, and thus improve overall hydrocarbon production from a
well by reducing
the surface tension and the amount of force needed to remove the water from
the pores and allow
hydrocarbons to flow through the proppant pack.
[0115] Suitable materials for flash coating the proppant with such hydrophobic
and/or
oleophobic agents include, but are not limited to, superhydrophobic coatings
such as those found
in US Patent Nos. 8,431,220 (hydrophobic core-shell nano-fillers dispersed in
an elastomeric
polymer matrix); 8,338,351 (hydrophobic nanoparticles of silsesquioxanes
containing adhesion
promoter groups and low surface energy groups); 8,258,206 (hydrophobic
nanoparticles of
fumed silica and/or titania in a solvent); and 3.931,428 (hydrophobic fumed
silicon dioxide
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-33 -
particles in resin) and the durable hydrophobic coatings of US Patent Nos.
8,513,342 (acrylic
polymer resin, polysiloxane oil, and hydrophobic particles); 7,999,013 (a
fluorinated monomer
with at least one terminal trifluoromethyl group and a urethane resin); and
7,334,783 (solid
silsesquioxane silicone resins), or any combination thereof Additional
materials that can be
used include, but are not limited to, aliphatic or aromatic polymers that
exhibit water contact
angles of greater than about 90 , such as polybutadiene-containing polymers,
polyurethanes with
high proportions of soft segments (e.g., aliphatic segments),
polymethylmethacrylate, and
siloxane resins, including polydimethylsiloxane, or any combination thereof
[0116] The use of a hydrophobic coating on the proppant can also have the
effect of preventing
water from reaching the surface of the sand grain. It has long been documented
that uncoated
sand's conductivity decreases with an increasing test temperature. This
implies that the
combination of elevated temperature and water contact may be damaging to the
integrity of the
sand particle and the corresponding proppant pack. Therefore, a hydrophobic
coating can be used
to slow down or minimize the detrimental effects that are observed with
increased temperature in
water-rich environments like those found downhole.
[0117] If some embodiments, the proppant is coated with multiple coatings. In
some
embodiments, the proppant is coated with a first layer of
hydrophobic/oleophobic coating
followed by a turbulence-reducing coating. Such a layered structure can permit
the treated
proppant to both reduce turbulence from separation of the top layer and then
reduce surface drag
by the flowing fluids by the underlying layer.
[0118] Friction reducing coatings can also take the form of materials with a
low external,
interparticle friction that function as a slip aid. A suitable material for
use as such an slip aid is a
product sold under the tradename POLYOX from Dow Chemical. This material is a
non-ionic
water-soluble poly(ethylene) oxide polymer with a high-molecular weight.
[0119] Tracer coatings. Tracers are radioactive isotopes or non-radioactive
chemicals that are
injected in a well at specific sites with the intent that they will come out
in detectable levels at
some point in the effluent. Thus, they allow flow tracking of injected fluids
from the source of
introduction to the effluent stream. In addition, tracers that are location-
specific can be used to
track production of fluids from specific areas/zones in a well. Often, the
tracers are introduced as
an additive into the fracturing fluid during completion of a particular zone
of interest.
[0120] Common radio-isotope chemistries used as tracers include tritiated
water (3H20);
tritiated methane (3CH4); 36C1_1311_; 355042- ; 5
1
4cN-; H14c -0 3-;
and 22 Na.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 34 -
[0121] Common non-radioactive tracer chemicals include halohydrocarbons,
halocarbons, SF6,
and cobalt hexacyanide, where the cobalt is present as an anionic complex
because cationic
cobalt can react and precipitate downhole. Various organic compounds of
usefulness include
sulfonic acids and salts of those acids, mapthalenediol, aniline, substituted
analine, and pyridine.
[0122] Tracers can be embedded in proppants but usually require actual
movement of the
proppant particle out of the well (i.e., flowback). The tagged proppant
particle itself is then
collected as a sample and analyzed for the presence/absence of the tracer. See
US Patent Nos.
7,921,910 and 8,354,279. Others have sought to incorporate non-radioactive
tagging chemicals
into the proppant resin coating, but such an introduction method has required
custom proppant
formulations that must be manufactured well in advance of planned usage in a
particular well.
This can cause issues as the reactive phenolic coated proppants can sometimes
have short useful
shelf life as the taggants must be released before the phenolic resin becomes
fully cured.
[0123] One feature in common among the tagged proppant techniques to date is
that all of them
require substantial pre¨planning for production of multiple, different, tagged
proppants for
different well zones in advance of injection. For example, if five different
zones need to be
mapped, five different tagged proppant formulations might be needed. This
means that five
different types of proppants must be prepared at the resin coating plant and
stored in inventory
by either the proppant manufacturer or by the well completion group.
[0124] The present methods and processes occur so quickly and with such small
amounts of
applied polymers, resins, or organic compounds that the same tracers, metals,
salts and organic
compounds could be used as have been used previously in resin coating
facilities. Additionally,
new polymers or oligomers can be used that contain specific functional groups
that have not
been previously used, such as fluorescent dyes or phosphorescent pigments that
can be detected
in even small quantities in produced effluent, whether water or hydrocarbon.
Suitable
fluorescents include coumarins, napthalimides, perylenes, rhodamines,
benzanthrones,
benzoxanthrones, and benzothioxanthrones. Phosphorescent pigments include zinc
sulfide and
strontium aluminate. The coating used in the present process can be tailored
to allow for
selective or timed release leaching of the tracer salts from the coating into
the downhole
environment. This would allow the effluent to be used for analysis rather than
requiring an
analysis of recovered proppants in the flowback. In addition, very short lead
times can be gained
through use of this process, to allow greater flexibility for the customer to
specify numbers of
different tagging sections needed in a particular well. In some embodiments,
the coatings applied
by the processes described herein are applied immediately before moving the
sand from
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-35 -
terminals into containers for shipment to the well pad. This means that the
inventory is reduced
to the containers of tracer agent.
[0125] Some metal agents, e.g., tin and copper, that were previously used as
biocides can also
serve the function of a tracer in a proppant coating.
[0126] Suitable polymers to prepare tracer coatings include acrylate
copolymers with
hydrolysable silylacrylate functional groups, such as those described by US
Patent No.
6,767,978. Briefly described, such polymers are made from at least three
distinct monomers units
selected from the group consisting of fluorinated acrylic monomers, (e.g.
2,2,2-
Trifluoroethylmethacrylate (matrife)), triorganosilylacrylic monomers, (e.g.,
trimethylsilyl
methacrylate) and acrylic monomers not containing an organosilyl moiety, (e.g.
methyl
methacrylate). The three component polymer (i.e. terpolymer) can optionally
contain from 0-5
weight percent of a crosslinking agent. Such polymers are a copolymers
comprising the reaction
product of:
a) a monomer of the formula:
C-0¨RF
H,C=C
wherein:
R is CH3 or H, and
RF is (C).(CH)v(CH2)w(CF)õ(CF2)y(CF3)z where u is from 0 to 1, v is from 0 to
1, w is
from 0 to 20, x is from 0 to 1, y is from 0 to 20, z is from 1 to 3, and the
sum of w and y is from
0 to 20,
b) a monomer of the formula:
0
C ¨RI
H2C =C
wherein: R is CH3 or H, and R1 alkyl or aryl, and
c) a monomer of the formula:
0,µ
II2C=C\
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 36 -
wherein:
R is CH3 or H, and
R1, R2, and R3 can be the same or different and are non-hydrolysable alkyl
groups
containing from 1 to 20 carbon atoms and/or non-hydrolysable aryl groups
containing from 6 to
20 carbon atoms.
[0127] In addition, depending on the chemistry used, metal-containing tracer
moieties can also
be used as biocides, similar to marine antifouling coatings. For example, tin
and copper are
commonly used as biocides in marine paints. These metals or their salts could
also be
incorporated into the acrylate latexes for flash coating onto the proppant or
added to insoluble
polymers for permanent attachment to the exterior of the proppant surface.
[0128] Suitable water soluble and dissolvable polymers are described in US
Patent No.
7,678,872. Such polymers can be applied to proppants according to the present
flash coating
process to allow for introduction timed release functionality of the tracers
into the produced fluid
as the polymer swells or dissolves while also serving to control fugitive dust
from the proppant.
[0129] Impact Modifiers. Fines in a well can severely affect the conductivity
of a proppant
pack. Production of 5% fines can reduce conductivity by as much as 60%.
Particle size analysis
on pneumatically transferred 20/40 sand with a starting fines distribution of
0.03% showed an
increase in fines to 0.6% after one handling step, and 0.9% after two handling
steps prior to
shipment to a well pad. Transport and further handling at the well site will
likely also produce
significantly more impact-related fines.
[0130] The processes described herein can be used to coat proppants with
polymers specifically
designed to be more deformable, which will greatly aid in the reduction of
impact induced fines
production. These polymers reduce the number of grain failures when closure
stress is applied,
effectively increasing the K value of the proppant, and can reduce fines
migration by keeping
failed grains encapsulated.
[0131] There are at least three ways that a thin, deformable coating on a
proppant can improve
fracture conductivity. The first is a benefit addressing the handling process.
An additive that
controls/prevent the generation of dust (through handling and pneumatic
transfer) is helping to
minimize the generation and inclusion of fine particles that are created
through movement of
such an abrasive that material as uncoated sand. Without wishing to be bound
by any theory, the
process that causes the creation of fines is simultaneously creating weakened
points everywhere
the grain was abraded. Conductivity tests have documented that uncoated sand
samples that were
moved pneumatically had measurably lower conductivity than the same sand not
so handled. The
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-37 -
impact-modifying polymer coating can further reduce grain failure by spreading
out point-to-
point stresses that occur when one grain is pushed against another during the
closure of the
fracture and subsequent increase of closure stress that occurs as the well is
produced. The
deformable coating effectively increases the area of contact between two
grains. This increase in
contact area reduces the point loading that is trying to make the grains fail.
Minimizing the
generation of fines that occur either during handling or from the pressure
applied in the fracture,
will mean there are less fines that can be mobilized to create conductivity
damage. If the flash
coating results in a uniformly distributed film around the sand grain, the
coating can be an
effective means of preventing fines movement through the encapsulation of any
failed grains.
Preventing or minimizing the movement of fines can result in controlling a
condition that has
been proven to be capable of reducing fracture conductivity by as much as 75%.
[0132] In some embodiments, for an impact modified layer, the layer comprises
lower Tg
polyurethanes or lightly crosslinked polyurethanes. The polyurethane formula
could be tailored
for lower Tg and better resilience by using a very soft polyols (e.g.,
polybutadiene-based polyols
with very light crosslinking). Another embodiment uses the application of a
thin coating of
polybutadiene polymer as the impact layer. Such a flash coating is applied
with either a latex-
based or solvent-based formulation, and a peroxide for lightly
curing/crosslinking the
polybutadiene coating. Other embodiments include, but are not limited to,
other rubbery
polymers including polyisoprene, polychloroprene, polyisobutylene, crosslinked
polyethylene,
styrene-butadiene, nitrile rubbers, silicones, polyacrylate rubbers, or
fluorocarbon rubbers. The
rubber or gum should be in a water-based latex or dispersion or dissolved in a
solvent for spray
application.
[0133] Polybutadiene coatings with unreacted vinyl or alkene groups can also
be crosslinked
through use of catalysts or curative agents. When catalysts, fast curatives,
or curatives with
accelerants are introduced during processes described herein, the result will
be a very hard, tough
coating. Alternately, curative agents can be added that will activate
thermally after the materials
are introduced downhole at elevated temperatures. This may have the effect of
having a soft
rubbery coating to protect against handling damage, but that soft rubbery
coating could then
convert to a hard coating after placement downhole at and cured elevated
temperatures.
[0134] Curative agents that can be used are those that are typically used for
rubbers, including
sulfur systems, sulfur systems activated with metal soaps, and peroxides.
Accelerators such as
sulfonamide thiurams or guanadines might also be used, depending on cure
conditions and
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 38 -
desired properties. Other curing catalysts could also be employed to perform
similarly include
ionic catalysts, metal oxides, and platinum catalysts.
[0135] Additive Delivery. "Self-suspending proppants" can have an external
coating that
contains a water swellable polymer that changes the proppant density upon
contact with water.
See, for example, U.S. 2013/0233545. Such coatings are taught to have about
0.1-10 wt%
hydrogel based on the weight of the proppant and can contain one or more
chemical additives,
such as scale inhibitors, biocides, breakers, wax control agents, asphaltene
control agents and
tracers.
[0136] In some embodiments, the water swellable polymer can be applied by
processes
described herein and present at a much lower concentration, e.g., less than
about 0.1 wt%, or
from about 0.001 to about 0.07 wt%. At such low levels, the swellable coating
is unlikely to
produce a self-suspending proppant but, rather, imparts enhanced mobility
relative into the
fracture to untreated sand while also providing dust control as well as a
delivery system upon
contact with water for biocides and tracers. For example the swellable polymer
coating could act
as a dust control when first applied, could swell to enhance mobility for
placement, and could
also contain tracers, biocides, or other active ingredients that could be
released over time through
diffusion out of the swollen polymer.
[0137] Soluble and semi-soluble polymers that can be used as delivery coatings
include, but are
not limited to, 2,4,6-tribromophenyl acrylate, cellulose-based polymers,
chitosan-based
polymers, polysaccharide polymers, guar gum, poly(1-glycerol methacrylate),
poly(2-
dimethylaminoethyl methacrylate), poly(2-ethyl-2-oxazoline) , poly(2-ethyl-2-
oxazoline),
poly(2-hydroxyethyl methacrylate/methacrylic acid), poly(2-hydroxypropyl
methacrylate) ,
poly(2-methacryloxyethyltrimethylammonium bromide), poly(2-vinyl-1-
methylpyridinium
bromide), poly(2-vinylpyridine n-oxide), polyvinylpyridines, polyacrylamides,
polyacrylic acids
and their salts (crosslinked and partially crosslinked), poly(butadiene/maleic
acid),
polyethylenglycol, polyethyleneoxides, poly(methacrylic acids,
polyvynylpyrrolidones,
polyvinyl alcohols, polyvinylacetates, sulfonates of polystyrene, sulfonates
of polyolefins,
polyaniline, and polyethylenimines, or any combination thereof
[0138] Biocidal Coatings. A number of nonpolymeric biocides have been used in
fracturing
fluids. Any of these can be used in solid forms or adsorbed into solid or
dissolvable solid carriers
for use as additives in an applied coating according to the present disclosure
to impart biocidal
activity to the proppant coatings. Exemplary biocidal agents include, but are
not limited to: 2,2-
dibromo-3-nitrilopropionamide (CAS 10222-01-2); magnesium nitrate (CAS 10377-
60-3);
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 39 -
glutaraldehyde (CAS 111-30-8); 2-bromo-2-cyanoacetamide (CAS 1113-55-9);
caprylic alcohol
(CAS 111-87-5); triethylene glycol (CAS 112-27-6); sodium dodecyl diphenyl
ether disulfonate
(CAS 119345-04-9); 2-amino-2-methyl-1-propanol (CAS 124-68-5);
ethelenediaminetetraacetate
(CAS 150-38-9); 5-chloro-2-methyl-4-isothiazolin-3-one (CAS 26172-55-4);
benzisothiazolinone and other isothiazolinones (CAS 2634-33-5); ethoxylated
oleylamine (CAS
26635-93-8); 2-methyl-4-isothiazolin-3-one (CAS 2682-20-4); formaldehyde (CAS
30846-35-
6); dibromoacetonitrile (CAS 3252-43-5); dimethyl oxazolidine (CAS 51200-87-
4); 2-bromo-2-
nitro-1,3-propanediol (CAS 52-51-7); tetrahydro-3, 5-dimethyl-2h- 1,3,5- thia
(CAS 533-73-2);
3,5-dimethyltetrahydro-1,3,5-thiadiazine-2-thione (CAS 533-74-4); tetrakis
hydroxymethyl-
phosphonium sulfate (CAS 55566-30-8); formaldehyde amine (CAS 56652-26-7);
quaternary
ammonium chloride (CAS 61789-71-1); C6-C12 ethoxylated alcohols (CAS 68002-97-
1);
benzalkonium chloride (CAS 68424-85-1); C12-C14 ethoxylated alcohols (CAS
68439-50-9);
C12-C16 ethoxylated alcohols (CAS 68551-12-2); oxydiethylene bis(alkyldimethyl
ammonium
chloride) (CAS 68607-28-3); didecyl dimethyl ammonium chloride (CAS 7173-51-
5); 3,4,4-
trimethyl oxazolidine (CAS 75673-43-7); cetylethylmorpholinium ethyl sulfate
(CAS 78-21-7);
and tributyltetradecylphosphonium chloride (CAS 81741-28-8), or any
combination thereof
[0139] Alternatively, an erodible outer coating with a timed release or staged
release can be
used that will dissolve and/or release included additives into the groundwater
or hydrocarbons
downhole. Such coatings can be based on polymers that were substantially
insoluble in cool
water but soluble in water at downhole temperatures where the active is
intended to begin
functioning shortly after introduction. Alternatively, the outer layer coating
can be prepared in
such a way as to render it insoluble in the well fluids and subject to release
when crack closure
stresses are applied.
[0140] The time frame for release of an encapsulated ingredient (biocide,
scale inhibitor, etc.)
via diffusion can be tailored based on the crosslink density of the coating. A
polymer with little
to no crosslinking can result a fast dissolving coating. Highly crosslinked
materials can have a
much slower release of soluble ingredients in the coating. If mobility of the
chemicals of interest
is too low in a crosslinked membrane, dissolvable fillers like salts, organic
crystalline solids, etc.
can be incorporated in the coating mixture. Once the coated proppant is
introduced downhole,
the particles can dissolve to leave larger pores as done for filtration
membranes. See U.S. Patent
No. 4,177,228. Insoluble polymers like the thermosets (e.g., alkyds, partially
cured acrylics,
phenolics, and epoxies) and thermoplastics (e.g., polysulfones, polyethers,
and most
polyurethanes) can also be used as insoluble outer coatings applied as
described herein. Alkyds,
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 40 -
which are polyesters, are likely to hydrolyze over time under the hot, wet
downhole conditions
and can thereby use this property to impart a delayed release through
combination of
environmental hydrolysis and situational erosion. Polyamides, which can
hydrolyze and degrade
over time, can be used as well for this type of coating.
[0141] Coatings can be prepared by mixing thermoset polymers with the soluble
fillers and
applying them to the proppant particles according to the various embodiments
described herein.
Thermoplastic membrane coatings can be applied via dissolving in solvent,
mixing with the
soluble fillers, and coating the resulting mixture onto the proppant particles
with subsequent
removal of the solvent by drying with pneumatic conveyance air or air forced
through the coated
materials. Timings for release can be tailored by proper selection of filler
size, shape, and filler
concentration.
[0142] Biocidal polymer coatings. Biocides are often used at low
concentrations in the
hydraulic fracturing fluid mixtures, on the order of 0.001% in the fracturing
fluid, which
corresponds to approximately 0.01% of the total proppant weight.
Microorganisms have a
significant economic impact on the health and productivity of a well. For
example, unchecked
bacteria growth can result in "souring" of wells, where the bacteria produces
hydrogen sulfide as
a waste product of their metabolic function. Such sour gases in the produced
fluids are highly
undesirable and can be a source for corrosion in the production equipment as
well as a cost for
sulfur removal from the produced hydrocarbons.
[0143] Therefore, in some embodiments, a biocidal polymer can be applied to
the proppants as
an aid to both fugitive dust control as well as inhibition of bacterial growth
downhole. Suitable
polymers that can be used as biocides include: acrylate copolymer, sodium salt
(CAS 397256-
50-7), and formaldehyde, polymer with methyloxirane, 4-nonylphenol and oxirane
(CA563428-
92-2), or any combination thereof
[0144] In addition, depending on the chemistry used, metals used as marine
antifouling
coatings can also serve as biocides on a proppant. For example tin and copper
are commonly
used as biocides in marine paint. These same agents could be incorporated into
the acrylate
latexes for flash coating onto the proppant as a biocidal coating.
[0145] Sulfide Control. Hydrogen sulfide is a toxic chemical that is also
corrosive to metals.
The presence of hydrogen sulfide in hydrocarbon reservoirs raises the cost of
production,
transportation and refining due to increased safety and corrosion prevention
requirements.
Sulfide scavengers are often used to remove sulfides while drilling as
additives in muds or as
ingredients in flush treatments.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-41 -
[0146] Depending on the concentration of hydrogen sulfide in the fractured
reservoir, the
concentrations of the scavengers included on the surface of the proppant can
be varied to remove
more or less hydrogen sulfide. In sufficient volume, proppants with sulfide
scavenging
capabilities can reduce the concentration from levels that pose safety hazards
(in the range of
500-1000 ppm) to levels where the sulfides are only a nuisance (1-20ppm). If
the surface area of
the proppants is high and dispersion of the scavengers is good, high
efficiencies in hydrogen
sulfide reaction and removal are possible.
[0147] A timed release dosage can be delivered according to the present
disclosure by
including copper salts, such as copper carbonate (CuCO3), in the proppant that
can be delivered
very slowly into the fracture to treat hydrogen sulfide before it can reach
steel components in the
wellbore.
[0148] Zinc oxide (ZnO) and ferric oxide (Fe203) are used directly as solid
particulates to
address hydrogen sulfide. These can be incorporated onto the surface of coated
proppants or be
formed as functional fillers within the proppant coating that is applied. The
use of high surface
area fillers, even nanometer-sized particulates, can be used to maximize the
interaction area
between the hydrogen sulfide and the metal oxide.
[0149] Also useful are oxidizing agents, such as solid forms of oxidizing
agents. Exemplary
materials include solid permanganates, quinones, benzoquinone, napthoquinones,
and agents
containing quinone functional groups, such as chloranil, 2,3-dichloro-5,6-
dicyanobenzoquinone,
anthroquinone, and the like, or any combination thereof
[0150] Polymers with pendant aldehyde groups can also be used introduce an
aldehyde
functionality in a proppant coating for control of hydrogen sulfides.
Polyurethanes can be made
with such functionalities. See US Patent No. 3,392,148. Similarly, other
polymers can be formed
with pendant aldehyde groups, such as polyethers, polyesters, polycarbonates,
polybutadiene,
hydrogenated polybutadiene, epoxies, and phenolics, or any combination thereof
[0151] In addition, dendrimers can be prepared with multiple terminal aldehyde
groups that are
available for reaction. These aldehyde-rich dendrimers can be used as fillers,
copolymers, or
alloys and applied to the proppants as a coating, or a layered coating.
[0152] Dioxole monomers and polymers allow introduction of this functionality
as pendant
groups in polymers. Such dioxane functional groups can serve as oxidative
agents to control the
production of hydrogen sulfides. Homopolymers of dioxole can be used as well
as copolymers of
dioxoles with fluorinated alkenes, acrylates, methacrylates, acrylic acids and
the like.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 42 -
[0153] Amines and triazines also used as scavengers for hydrogen sulfide.
Amine-terminated
polymers or dendrimers can be used and have the advantage of being tethered to
a polymer so
they can stay in place in a proppant coating. High functionality can be
achieved by the use of
dendrimers, i.e., using multiple functional groups per single polymer
molecule.
[0154] Triazines can be incorporated into polyurethane crosslink bridges via
attachment of
isocyanates to the R groups of the triazines. See US Patent No. 5,138,055
"Urethane-functional
s-triazine crosslinking agents". Through variations of the ratio of ¨OH groups
and the use of triol
functionality and monofunctional triazine isocyanate, pendant triazines can
also be prepared.
These functionalized polymers can be added as fillers or prepared as the
coating itself to both
impart fugitive dust control as well as hydrogen sulfide control downhole.
[0155] Metal carboxylates and chelates, some of which are based on or contain
zinc or iron,
can be used on proppants to remove hydrogen sulfide. See US Patent No.
4,252,655 (organic
zinc chelates in drilling fluid). These carboxylates or chelates are provided
in the proppant
coating as water soluble complexes which, upon interaction with hydrogen
sulfide in-situ
downhole, will form insoluble metal sulfates.
[0156] Hydrogen sulfide can also be controlled with polymers having functional
groups that
can act as ligands. Polycarboxylates that have been pretreated with metals to
create metal
carboxylate complexes can be mixed with other polymers, such as those
described elsewhere
herein, and applied as a coating to proppant particles. This is also
applicable to other polymers
with pendant functional groups that act as complexing ligands for sulfide,
such as amines and
ethers.
[0157] In some embodiments, the metals used for sulfide control are not
present as a complex
in the polymeric backbone so that removal of the metal would not have to
involve polymer
decomposition. Polymers with metal side chain complexes can be used.
Polyvinylferrocenes,
polyferrocenylacrylates are two such examples of this class of material. In
some embodiments,
the main chain metal containing polymer can also be used, but the polymer will
degrade upon
reaction with hydrogen sulfide.
[0158] If the production fluid which contains hydrogen sulfide at a basic pH
(i.e., pH of greater
than 7 or greater than 8-9), most of the hydrogen sulfide will be present as
HS-anion. In this
case, anion exchange resins or zeolites can be used to extract the HS-anions
from the fluid. The
zeolites or anionic exchange resins can be used as active fillers in a resin
coated proppant
composition include aluminosilicates such as clinoptilolite, modified
clinoptilolite, vermiculite,
montmorillonite, bentonite, chabazite, heulandite, stilbite, natrolite,
analcime, phillipsite,
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 43 -
permatite, hydrotalcite, zeolites A, X, and Y; antimonysilicates;
silicotitanates; and sodium
titanates, and those listed in US 8,763,700, the disclosure of which is hereby
incorporated by
reference. Suitable ion exchange resins are generally categorized as strong
acid cation exchange
resins, weak acid cation exchange resins, strong base anion exchange resins,
and weak base
anion exchange resins, as described in US 8,763,700. Hydrogen sulfide that is
produced through
biological activity is controlled through use of biocides and biocidal
coatings (as discussed
above), and removal of sulfate anions (H504- or 504-2). Anion exchange resins
can be used for
removal of sulfate. Nitrates can also be used to disrupt the sulfate
conversion by bacterial.
Nitrate salts can also be added in a coating layer and then protected from
premature release with
an erodible or semipermeable coating to allow an extended release of the
nitrates.
[0159] Composite Coatings. In some embodiments, the processes described can be
carried out
effectively in series, and such a process provides a cost-effective process to
apply multiple layers
of coatings with different compositions and different functional attributes. A
variety of
combinations are possible. For example, in some embodiments, multiple spray
heads could be
used, each of which can apply a different formulation. If the successive
coating formulation is
chemically incompatible in that the applied layer does not wet the undercoated
layer, one or
more primer agents, e.g., block or graft copolymers with similar surface
energies and or
solubility parameters as the two incompatible layers, can be used for better
interfacial bonding.
The different spray heads can also be used to apply the same formulation if
multiple layers are
desired. Some examples of composite coatings include the following.
[0160] Two layers for improved proppant physical performance. Different,
successive layers
are applied with different performance characteristics, such as a hard
urethane layer (urethane,
crosslinker (such as polyaziridine), and isocyanate) followed by an outer,
softer urethane layer.
This coating structure can allow some compaction for proppant particle bonding
due to the soft
outer layer but inhibit further compaction/crushing due to the hard inner
layer. The relatively
softer outer layer can also tend to reduce interparticle impact damage as well
as wear damage on
the associated handling and conveying equipment used to handle the proppants
after the flash
coating was applied.
[0161] Successive layers for a timed release functionality. Successive layers
can be used to add
a first layer with an additive having a first functionality followed by a
second layer having
properties that control when and how ambient liquids get access to the first
layer additive
materials. For example, a soft, lightly crosslinked urethane layer with
biocide additives is
covered with a hard urethane layer that contains dissolvable particles. When
the dissolvable
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 44 -
particles are removed, the outer coating forms a semipermeable coating that
allows slow
diffusion of the underlying biocidal additive.
[0162] Layers of strongly-bonded polymer followed by weakly-bonded polymer. A
silane
treatment for silica compatabilization can be applied to the sand proppant
outer surface. This
treatment is followed by coating with an inner polymer layer containing
functional additives,
such as Fe203 particulates to provide sulfide scavenging. The outer layer
coating contains
polyacrylamides that are loosely bonded to the first coating. Once downhole,
the polyacrylamide
is released and collects on the internal surfaces of metal pipes in the well.
This formulation can
deliver friction reduction in the short term and offer a level of sulfide
control over the lifetime of
the well until the iron oxide particles were fully exhausted.
[0163] Staged Release Coatings. For example, oxygen related corrosion and
asphaltene often
are more problematic at the beginning of a well life cycle, while bacterial
growth occurs later in
the well life cycle. A composite coating of three layers can address such
delayed developments.
The first, innermost, layer can comprise, for example, a biocidal
functionality. The second
coating layer can comprise, for example, an asphaltene inhibitor, and the
third layer can
comprise, for example, a loosely bound polyhydroxyl compound as an oxygen
scavenger. The
outer layer of this proppant can reduce oxygen levels immediately, especially
in dead
zones/zones of limited flow from the entrance of the well, which can't be
flushed with fluids
containing oxygen scavengers. As the well begins production, the outer layer
can be consumed
and erode from the surface to expose the asphaltene-inhibiting layer of a
sulfonated alkylphenol
polymer that can also erode or dissolve over time. As the well continues to
produce, asphaltene
issues can lessen, and the remaining innermost coating can slowly release its
biocides to ensure
continued health of the well. A single, composite provides these extended
benefits with less cost
and easier logistics than the use of multiple proppants with different
functions introduced into
the well as a mixture.
[0164] Timed Release Coatings. The use of an outer layer made with dissolvable
particles
and/or dissolvable or erodible polymers can be used to provide a controlled,
timed release of
functional additives much like an enteric coating of a medicament. Unlike a
staged release
structure, a timed release coating does not have a second stage of release.
Importantly, the coated
proppants according to the present disclosure provide for release over time,
in situ, and
throughout the fractured strata. Exemplary functional additives can include
biocides, scale
inhibitors, tracers, and sulfide control agents. Suitable water soluble and
dissolvable polymers
are described in US Patent No. 7,678,872. Erodible matrix materials include
one or more
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 45 -
cellulose derivatives, crystalline or noncrystalline forms that are either
soluble or insoluble in
water.
[0165] The time frame for release of an encapsulated ingredient via diffusion
can be adjusted
and tailored to the need by adjusting the crosslink density of the
encapsulating coating. A
polymer with little to no crosslinking exhibits a fast-dissolving coating for
a short interval before
release. Highly crosslinked materials can have a much slower rate of release
of soluble
ingredients in the coating. If mobility of the chemicals of interest is too
low in a crosslinked
membrane, dissolvable fillers like salts, organic crystalline solids, etc. can
be incorporated in the
coating mixture. Once the coated proppant is introduced downhole, the
particles can dissolve to
leave larger pores, as has been done with filtration membranes as in US Patent
No. 4,177,228
entitled "Method of Production of a Micro-Porous Membrane for Filtration
Plants." If lightly
crosslinked or a hydrogel, the polymer swells and will allow a controlled
diffusion of the
encapsulated additives.
[0166] Insoluble polymers, such as the thermosets (e.g., alkyds, partially-
cured acrylics,
phenolics, and epoxies) and the thermoplastics (e.g., polysulfones,
polyethers, and
polyurethanes) can be used as thin coatings with dissolvable additives. Such
coatings are
prepared by mixing, e.g., a thermoset polymer with finely divided, dissolvable
solids and
applying the resulting mixture to the proppant particles. Thermoplastics can
be applied by
dissolving the thermoplastic polymer in a solvent, mixing in the finely
divided, dissolvable
solids, and coating the proppants with the mixture. The solvent is then
removed with a drying
stage, which may be no more than a cross-flowing air stream. The time before
release can be
adjusted based on the size, shape, and solids concentration.
[0167] In some embodiments, the processes described herein provide for the
formation of a
self-polishing coating that dissolves over time or is eroded as fluid passes
over the surface of the
coating. Suitable materials for such coatings include acrylate copolymers with
hydrolysable
silylacrylate functional groups. (See US Patent No. 6,767,978.) Alkyds, which
are polyesters,
can also be used as they tend to hydrolyze over time under downhole conditions
and thereby
impart a delayed-release mechanism through combination of hydrolysis and
erosion.
[0168] Cellulosic coatings can also provide a timed release coating. Suitable
and include, but
are not limited to, the hydroxyalkyl cellulose family such as hydroxyethyl
methylcellulose and
hydroxypropyl methylcellulose (also known as hypromellose). A suitable
material is
commercially available under the tradename METHOCEL from Dow Chemical. This
material is
a cellulose ether made from water-soluble methylcellulose and hydroxypropyl
methylcellulose
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 46 -
polymers. Rheological modification can also be provided from the use of a
hydroxyethyl
cellulose agent, such as those commercially available under the tradename
CELLOSIZE, from
Dow Chemical.
[0169] Polyamides, which can be hydrolyzed under downhole conditions, can be
used as well.
[0170] Acid/Base-Resistant Coatings. Chemical attack of a proppant is a
concern in hydraulic
fracturing. For silica sand, the acid number of a proppant is often used to
designate the sand's
quality. The test in ISO 13503-2, section 8 describes the specific testing of
proppant sand under
acid exposure as a way to determine its suitability for specific well
conditions. If components or
impurities in the sand dissolve or are unstable in acidic environments, the
proppant grains will
gain porosity and exhibit a lower overall crush resistance. It can, therefore,
be desirable to have a
coating that could minimize the attack on the silica sand by acids found in
downhole
groundwaters.
[0171] Basic solutions can also dissolve or partially degrade silica proppants
and the resin
coating on such proppants, especially at a pH of nine or higher. This can
cause issues in
conductivities of proppant packs placed in fractures, due to weakening of the
grains and possible
reduction in particle size due to dissolving of outer layer of the particles.
[0172] Ceramic proppants can also suffer under highly basic or acidic waters
as a result of
diagenesis, a phenomenon in which the ceramic dissolves in aqueous solutions
under pressure
followed by a re-precipitation with other elements present in the fluid. The
re-formed solid is
unlikely to be as strong or the same size as the original ceramic proppant and
can be a significant
concern for its effects on conductivity of a ceramic proppant pack.
[0173] In some embodiments, the coatings that are applied are acid resistant,
base resistant, or
both, and can offer new protections for proppants of all types, including, but
not limited to, sand
and ceramic proppants. Some of the acid-resistant polymers that can be applied
include:
polypropylene, acrylic polymers, and most fluoropolymers. For increased
coverage of the total
exterior surface of the proppants, multiple coating applications of the same
base polymer might
be needed, depending on the equipment and number of dispersion nozzles that
are used. The
processes described herein can be repeated until the appropriate number of
coatings are applied.
[0174] Suitable base-resistant polymers include the polyolefins, some
fluoropolymers (except
that PVDF and FKM are not particularly resistant to strong bases) and many
polyurethanes.
[0175] Corrosion inhibitors. Corrosion of metals in downhole applications is a
significant
problem in the oil and gas industry. Corrosion can occur via either an acid-
induced process or via
oxidation. Acidic conditions can be caused by acid treatment of the formation,
acid or H25
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-47 -
producing bacteria, or CO2 that can dissolve in water under pressure to form
carbonic acid.
Oxidation/oxidative corrosion of the metal can occur in the presence of water
and oxygen.
[0176] Corrosion in downhole applications is often addressed by addition of
corrosion
inhibitors and/or acid scavengers during drilling, completion, or hydraulic
fracturing. The
corrosion inhibitor provides a coating to passivate the metal surfaces exposed
to the fluids.
Passivating layers of small molecules are also applied via addition of these
molecules in a
treating fluid, followed by use of complexation chemistry to attach the
molecules to the metal,
e.g., the use of active ligand sites on small organic molecules or polymers to
bind to the metal.
Acid scavengers are acid-accepting and basic compounds. Periodic washing or
flushing with
fluids containing such materials after the initial treatment is also a common
method to keep
corrosion under control.
[0177] Oxygen scavengers are used to remove dissolved oxygen from downhole
fluids. Once a
well is completed, oxygen is not usually a significant problem as it is not
normally present in
producing formations, but it can be a problem in drilling muds and fracture
fluids. Oxygen
scavengers are used in these fluids during drilling, fracturing or completion.
[0178] Polymeric coatings for the metallic surfaces to prevent corrosion are
often used, and
applied to the metals prior to their use. Baked resins, or epoxy coatings, are
two examples, but
other polymers can be used on the metals. Cathodic protection is also used
where possible, by
placing a more reactive metal near the metal to be protected, and using the
more reactive metal
to react or oxidize with the chemistries in the fluid, rather than the metals
which are desired to be
protected. Zinc, aluminum and other metals which are more reactive than iron
(Fe) are used for
cathodic protection.
[0179] Chemicals that can be applied to the solids for corrosion protection
include 1-
benzylquinolinium chloride (CAS 15619-48-4), acetaldehyde (CAS 57-07-0),
ammonium
bisulfite (CAS 10192-30-0), benzylideneacetaldehyde (CAS 104-55-2), castor oil
(CAS 8001-
79-4), copper chloride anhydrous (CAS 7447-39-4), fatty acid esters (CAS 67701-
32-0),
formamide (CAS 75-12-7), octoxynol 9 (CAS 68412-54-4), potassium acetate (CAS
127-08-2),
propargyl alcohol (CAS 107-19-7), propylene glycol butyl ether (CAS 15821-83-
7),
pyridinium, 1-(phenylmethyl)- (CAS 68909-18-2), tall oil fatty acids (CAS
61790-12-3), tar
bases, quinoline derivatives, benzyl chloride-quaternized (CAS 72480-70-7),
and
triethylphosphate (CAS 78-40-0), or any combination thereof
[0180] Corrosion inhibitors that are solids can be mixed into resin
formulations as a filler, then
applied to proppants to form a coating that can deliver the corrosion
protection directly
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 48 -
downhole. The coatings can be designed to deliver corrosion protection
immediately, as might be
desired for oxygen scavengers during drilling or completion. The coatings can
also be tailored
for timed release of corrosion, as discussed above. Cathodic protection can be
provided by also
including one or more metal particles (Zn, Al, and the like) in highly
conductive produced
waters/brines.
[0181] Corrosion inhibitors that are liquids can be introduced into these
systems via selection
of a polymer proppant coating in which the liquids/organic chemicals are
miscible or semi-
soluble. Some examples include digycolamines mixed with polyacrylamides, or
lightly
crosslinked or thermoplastic polyurethanes.
[0182] Other polymers, such as 2-vinyl-2-oxyzoline can be used as water
soluble polymer
fillers that can be encapsulated in a resin coating on proppant particles, and
dissolved over time
from the coating. The soluble molecules can then passivate metal surfaces, and
inhibit acidic
corrosion.
[0183] Acid scavenging activity can be provided with a flash coating of
polymers having acid
scavenging attributes. For example, polymers with nitrogen containing
heteroatoms such as
polyvinylpyridine and polyvinylpyrrolidone, carboxylates, or pendant amines
can provide such
acid scavenging activity, i.e., nitrogen can interact with acids to form a
salt. The scavenging
power of these polymers can be related to the concentration of functional
groups on the polymer
as well as the mobility and accessibility of these groups to react with the
produced fluids and
remove acidic impurities.
[0184] Improvement in Crush resistance. Water-based dispersions of precured
polyurethanes
can be mixed with a polyurethane crosslinking agent such as polyaziridine,
isocyanate or
carbodiimides to generate a hard, crosslinked, coating in low concentration.
Variations of the
nature and amount of the crosslinking agent, as exists for one of no more than
an ordinary level
of skill in this art, allow the cure levels of the coating to be adjusted and
tailored for more or less
hardness, crosslink density, glass transition temperature, and permeation
rate. In some
embodiments, coating levels per treatment of up to 0.5% or 01-0.3 wt% based on
the weight of
the proppant can be applied. In some embodiments, multiple coatings are
applied to generate
thicker coatings, if desired. In some embodiments, the proppant has, or at
least, 1, 2, 3, 4, 5, 6, 7,
8, 9, or 10 coatings.
[0185] Increased crush resistance ("K values") can be obtained with
polyurethane-treated
proppant sand relative to its untreated version at even low coating levels.
See Table 3 below.
Other types of thermoplastic and thermoset polymeric coatings should exhibit
similar results.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 49 -
Table 3
K values From Crush Tests, per ISO 13503-2
PU Coating Weight Crush test, K value Improvement over Raw
Sand
0% 6 0%
(untreated 20/40 sand) (control)
0.25% 7 17%
0.25% 7 17%
0.31% 7 17%
0.50% 10 67%
0.53% 10 67%
[0186] Paraffin Inhibitors. Paraffins are long chain hydrocarbons, typically
C18 to Clop or
more (18-100 carbons) that often precipitate out of a hydrocarbon solution due
to changes in
temperature or composition that decrease the solubility of the paraffin in the
hydrocarbon fluids.
Once precipitated, those paraffins can crystallize to form a waxy buildup.
[0187] In some embodiments, paraffin inhibitors can be coated into or onto
proppants. Such a
coating places the treatment in the fractured strata and at the elevated
temperatures found
downhole before the paraffins have begun to precipitate or crystallize. By
introducing the
inhibitors in the fractured strata while the paraffins are still soluble, the
treatment can affect the
crystallization rate of paraffin as the produced hydrocarbon stream cools
and/or mixes with water
as it moves towards the surface and consolidates with other frac streams for
recovery. Such
conditions often result in reduced paraffin solubility and create conditions
where paraffin
precipitation and crystallization become problematic.
[0188] The paraffin inhibitors of the present disclosure can be added as a
polymeric coating on
the proppants or as released additives. The coated polymers can stay
associated with the
proppant particles until the proppant was exposed to hydrocarbons whereupon
the polymers can
dissolve in the hydrocarbon or mixed hydrocarbon/water effluent. Releasable
additives contained
in timed release or staged release coatings of the types discussed above allow
the paraffin
inhibitor additives to be released over time via diffusion out of the swelled
or dissolving coating
or by migration out of a coating whose soluble particulates had left openings
for egress of the
paraffin additives.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 50 -
[0189] Polymers that can serve as paraffin inhibitors include, e.g., styrene
ester copolymers and
terpolymers, esters, novalacs, polyalkylated phenol, and fumerate-vinyl
acetate copolymers.
Tailoring the molecular weight of the inhibitor as well as the lengths of the
pendant chains can
be used to modify the nature of the inhibition effects. These characteristics
affect both the
crystallization rate and size distribution of paraffin crystals and thus the
pour point of the
resulting solutions.
[0190] Paraffin pour point can be decreased by adding solvents to a
hydrocarbon mixture to
increase solubility of paraffin, and thus reduce the crystallization rate and
overall crystallite size
distribution of the paraffin crystals. These are often copolymers of acrylic
esters with ally'
ethers, urea and its derivatives, ethylene-vinlyacetate backbone with
unsaturated dicarboxylic
acid imides, dicarboxylic acid amides, and dicarboxylic acid half amides.
[0191] Polymers that are useful for paraffin crystal modification include
ethylene-vinyl acetate
copolymers, acrylate polymers/copolymers, and maleic anhydride copolymers and
esters.
[0192] Paraffin dispersants work via changing the paraffin crystal surface,
causing repulsion of
the paraffin particles and thus inhibit formation of larger paraffin
agglomerates that could
precipitate from suspension in the reservoir fluids. Typical chemistries
include olefin
sulphonates, polyalkoxylates and amine ethoxylates.
[0193] Asphaltene Inhibitors. Asphaltenes are complex polycyclic aromatic
compounds,
often with heteroatoms and with aliphatic side chains. They are present in
many hydrocarbon
reserves at concentrations that vary from <1 to 20%. They are soluble in
benzene or aromatic
solvents but insoluble in in low molecular weight alkanes.
[0194] Asphaltenes pose similar issues to the paraffins in that they are
typically soluble in the
pressurized, heated hydrocarbon mixture in a reservoir field, but changes in
temperature and
pressure during production from that reservoir can cause precipitation or
flocculation. Either of
these can have the effect of reducing fluid flow or, in the worst case,
stopping fluid flow
completely. Once the asphaltenes precipitate, the well must be remediated by
mechanically
scraping or dislodging the deposits through the application of differential
pressures or by
cleaning with toluene, xylene, or other suitable aromatic solvent. Cleaning is
expensive and stops
well production during the process so the asphaltene additives carried by
treated proppants
represent a substantial economic benefit for well owners and operators.
[0195] Asphaltene is controlled via use of dispersing additives or inhibitors.
Dispersants reduce
the particle size of the precipitated asphaltenes and keep them in suspension.
Dispersants are
often used as frac fluid additives at a point after asphaltene precipitation
is likely to occur, i.e.,
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-51 -
after a pressure drop or temperature drop as the oil moves from the reservoir
into the production
channels. Dispersants are usually nonpolymeric surfactants. Some asphaltene
dispersants that
have been used in frac fluids include: very low polarity alkylaromatics;
alklarylsulfonic acids;
phosphoric esters and phosphonocarboxylic acids; sarcosinates; amphoteric
surfactants;
ethercarboxlic acids; aminoalkylene carboxylic acids; alkylphenols and their
ethoxylates;
imidazolines and alkylamine imidazolines; alkylsuccinimides;
alkylpyrrolidones; fatty acid
amides and their ethoxylates; fatty esters of polyhydric alcohols; ion-pair
salts of imines and
organic acids; and ionic liquids.
[0196] Inhibitors actually prevent the aggregation of asphaltene molecules and
prevent
precipitation. Asphaltene inhibitors are typically polymers. Common asphaltene
inhibitors that
have typically been used in frac fluids include: alkylphenol/aldehyde resins
and sulfonated
variants of these resins; polyolefin esters, amides, or imides with alkyl,
alkylene phenyl, or
alkylene pyridyl functional groups; alkenyl/vinylpyrolidone copolymers; graft
polymers of
polyolefins with maleic anhydride or vinylimidazole; hyperbranched
polyesterimides;
lignosulfonates; and polyalkoxylated asphaltenes.
[0197] Polymeric asphaltene inhibitors can be introduced directly as coatings
on the proppant
particles. They can be applied as coatings that can be released in a
controlled fashion either
immediately or slowly over time by the timed release and staged release
coatings discussed
above.
[0198] The asphaltene inhibitors can also be used as an additive in a
polymeric coating.
[0199] Asphaltene dispersants can be used mainly as ingredients/fillers in a
coating to be
released over time. Their release over time can be controlled with the
coatings discussed herein
depending on whether an immediate release or timed release dosing is desired.
Branched
polymers with arms that contain the dispersant functionality can also be used
where the branches
are connected to the polymer backbone by reactive groups that might degrade
over time, such as
esters, hydrolysable groups, and the like to release the dispersants over
time.
[0200] An advantage of using asphaltene control agents directly on proppant
particles is that
these agents can be released within the formation prior to asphaltene
precipitation. Such an in-
situ delivery allows effective treatment before development of the problem and
in controlled
concentrations.
[0201] Fines Migration Control. In addition to higher crush resistance and
decreased
equipment wear from handling, flash coatings of the present disclosure can
help control fines
migration downhole and thereby help to maintain conductivity.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 52 -
[0202] Fines produced through crushing of the proppant pack can fill a portion
of the
interparticle porosity, which is directly linked to conductivity. More
importantly fines can be
mobilized under pressure in downhole conditions during fluid production to
cause a great
amount of damage, sometimes more than a 75% reduction in conductivity.
[0203] The effect of fines migration is not obvious in a standard conductivity
test, as the test is
performed at too low of a flow rate to mobilize fines. Some control over fines
migration
downhole can be added to proppants by applying to the treated proppants an
external tackifier
that will capture fines encountered downhole. The coated proppants are then
placed in the well
during fracturing. This ensures the fines control treatment is accurately
placed on the surface of
the particles and ensures that the coating penetrates the fracture as deeply
as the proppant
particles.
[0204] Common tackifier resins or resin dispersions that can be used for fines
control on a
proppant include: a) rosin resins from aged tree stumps (wood rosin), sap (gum
rosin), or by-
products of the paper making process (tall oil rosin); b) hydrocarbon resins
from petroleum
based feedstocks either aliphatic (C5), aromatic (C9), dicyclopentadiene, or
mixtures of these;
and c) terpene resins from wood sources or from citrus fruit.
[0205] Removal of anions/halogens from produced water. Halogens, particularly
bromines,
can cause issues in produced water due to the reaction with disinfectants to
make disinfection by-
product compounds. For bromide, a concentration value of 0.1 mg/L poses a risk
for unintended
by-product production. These by-products can also be potential carcinogens.
For example, some
by-product compounds have toxicologic characteristics of human carcinogens,
four which are
already regulated, e.g., bromodichloromethane, dichloroacetic acid,
dibromoacetic acid, and
bromate.
[0206] The removal of bromines can occur in the context of the present
disclosure by adding
anion exchange resins into or onto a resin coating on a proppant. Such
exchange resins can be
added during application of a flash coating as described herein or at the end
thereof as the
coating dries for adhesive-type incorporation into the coated surface.
[0207] The processes and compositions described herein are well-suited to the
treatment of a
variety of proppant solids in a context other than a formal resin-coating
operation or facility. As
such, the process can be used to apply, for example, a dust suppressing,
liquid treatment agent as
an uncured coating over at least a portion, such as a large portion, of the
proppant solids within
the bulk mixture. Such a treatment process affords the possibility that the
process can be used to
provide the proppant solids with additional properties without the need for a
formal,
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
-53 -
manufacturing facility-based coating process. Such types of additional
functionalities are
described in our co-pending US patent application serial number 10/872,532
entitled "Dual
Function Proppants", now US Patent No. 8,763,700, the disclosure of which is
hereby
incorporated by reference. Such additional materials can include, e.g.,
pigments, tints, dyes, and
fillers in an amount to provide visible coloration in the coatings. Other
materials can include, but
are not limited to, reaction enhancers or catalysts, crosslinking agents,
optical brighteners,
propylene carbonates, coloring agents, fluorescent agents, whitening agents,
UV absorbers,
hindered amine light stabilizers, defoaming agents, processing aids, mica,
talc, nano-fillers,
impact modifiers, and lubricants. Other additives can also include, for
example, solvents,
softeners, surface-active agents, molecular sieves for removing the reaction
water, thinners
and/or adhesion agents can be used. The additives can be present in an amount
of about 15
weight percent or less. In one embodiment, the additive is present in an
amount of about 0.005-5
percent by weight of the coating composition. The processes described herein
can also be used
to add other functionalities as described herein.
[0208] The proppants described herein can be used in a gas or oil well. For
example, the
proppants can be used in a fractured subterranean stratum to prop open the
fractures as well as
use the properties of the proppant in the process of producing the oil and/or
gas from the well. In
some embodiments, the proppants are contacted with the fractured subterranean
stratum. The
proppants can be contacted with the fractured subterranean stratum using any
traditional methods
for introducing proppants and/or sand into a gas/oil well. In some
embodiments, a method of
introducing a proppant into a gas and/or oil well is provided. In some
embodiments, the method
comprises placing the proppants into the well.
Examples
Example 1
[0209] An ineffective initial test was performed using a poorly designed spray
pattern at an
existing sand plant. The spray was applied at several sites along the conveyor
belt. It was learned
that if the sand was not heated, that one would be unlikely to be able to
exceed 0.5% addition of
the treatment agent in water. The tested coating efficiency was so poor as to
not be able to
properly evaluate the effectiveness of the treatment agents being tested.
Example 2
[0210] In a second example, the treatment agent solution was applied by hand
to sand as it was
agitated by a mixer that was used to coat the sand like the equipment used in
a conventional
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 54 -
coating process. This approach was taken to focus on whether the technology
would be effective
if uniformly applied. Application levels were tested at levels ranging from a
high of 0.5% (by
weight) of a mineral oil or a diluted polymer solution having a concentration
as low as 0.12%
(by weight).
[0211] The results showed that, even if perfectly applied, a concentration of
0.5% (by weight)
was likely to create a particle surface that was too wet and would likely
create issues with
moving the treated proppant using conventional handling equipment. A
concentration of 0.25%
was found to be effective, but a further reduction to 0.12% was somewhat
ineffective.
Example 3
[0212] Uncoated, unheated, proppant sand was treated at the rate of 0.25 wt%
with a mixture
containing acrylic polymers, and alkoxylated alcohols (commercially available
under the name
ROHMIN DC-5500 Emulsion from Rohm and Haas Chemicals, LLC, 100 Independence
mall
West, Philadelphia, PA 19106). This treatment agent was applied by a plurality
of nozzles
located on either side of a curtain of sands falling from a conveyor belt. The
nozzles formed a
cone-shaped fog of fine spray that impinged on the falling sand solids as they
fell into a
receptacle that fed a pneumatic test pipe through a series of turns to
discharge into an open
container. Some nozzles were positioned to treat the top portion of the stream
coming off the belt
while others sprayed from beneath to coat the underside of the particle
stream.
[0213] All of the treated sand remained dry to the touch and free-flowing.
There was no
discernible clumping, aggregation or pooling of excess treatment agent.
[0214] Compared to the untreated standard, the treated sand showed markedly
reduced levels,
i.e., subjectively 50-80% reduction, of fugitive dust rising from the open
discharge chamber.
What solids did rise with ambient air currents produced by the discharge were
seen to settle
quickly back into the open container.
Example 4
[0215] The same treatment agent as described in Example 3 was used to treat
uncoated frac
sand at a commercial sand handling facility. The treatment nozzles were
disposed on a ring
sprayer (as in Figs. 5 and 6) and whose conical spray patterns were directed
to apply treating
agent to the falling sand at substantially the same rate as an Example 1. The
treated sand then
passed through a static mixer of the type shown in Figs. 3 and 4 in a
configuration as shown in
Fig. 7. All treatments were done at ambient temperature. Visual observation of
showed that the
treated sand exhibited substantially the same, marked reduction in fugitive
dust from the open
CA 02927111 2016-04-11
WO 2015/066283
PCT/US2014/063086
- 55 -
discharge and dust carried upwardly from ambient air currents quickly settled
down and did not
escape the discharge area.
Example 5
[0216] Measurement tests were done on the proppant described in Examples 3 and
4 to
compare the effects of the treatment against untreated 30/50 sized sand. The
results are shown in
Table 4 below.
Table 4
Uncoated Formulation
Bulk Density (1b/ft3) 94.65 99.11
#20 0.00 0.01
#30 0.33 2.75
#35 10.96 22.22
#40 46.21 45.00
#45 30.10 18.98
#50 9.86 7.67
#70 2.46 3.20
PAN 0.08 0.16
Mean Diameter (mm) 0.432 0.458
MPD (mm) 0.475 0.497
8.47% at
Crush 9.47 at 8K
8K
LOT 0.10% 0.12%
Turbidity 198 NTU 10 NTU
Acid Solubility 0.91% 0.13%
CA 02927111 2016-04-11
WO 2015/066283
PCT/US2014/063086
- 56 -
Uncoated Formulation
Roundness 0.7 0.7
Sphericity 0.7 0.7
[0217] Even though the formulation was applied so quickly, the treatment
provided an
improvement in crush resistance with a significant decrease in turbidity.
Turbidity relates to the
proportion of small solids suspended in solution. The decrease in turbidity
shows that fines are
not dispersed in solution in a treated sample but are entrapped or
agglomerated in the proppant.
This shows the use of the additive treatment is effective for minimization of
fines mobility in
solution and translates into reduced mobility in air (reduced introduction of
dust into the
atmosphere after handling of the treated sand vs. the untreated sand).
[0218] It was also found that the applied coating also increased the
resistance of the treated
proppant to the effects of acids ( a mixture of 12% hydrochloric and 3%
hydrofluoric acids) and
increased the K Value (crush resistance) of the treated proppant.
Examples 6 and 7
[0219] Additional tests were performed to measure the compatibility of the
dust control
treatment on sand with certain properties of a test frac fluid. The frac fluid
used guar gum, a
natural water soluble polymer.
[0220] Example 6 was a crosslink test using a borate crosslinker in 200 F
deionized water
compared to water containing the dust control treatment components. The base
gel was 20 parts
per thousand (ppt) polymer loading and 2.2 grams per thousand (gpt) of the
borate crosslinker.
The system was buffered to a pH above 8.5 and then crosslinked with the borate
solution. The
dust additive was the ROHMIN DC-5500 at a concentration of 0.25% by weight.
The sand was
coated.
[0221] The test starts with a slurry of water and 4 pounds/gallon of sand that
has been treated
with an emulsion containing acrylic acid polymers and a mixture of ethoxylated
alcohols. The
slurry is heated for an hour at 200 F while being stirred. In that time,
anything that can be
extracted from the coating will be moved into the water. The sand is then
separated from the
water, and the water is then used to make the fracturing fluid system.
[0222] At the polymer loading identified above and with a pH in deionized
water control of
6.67, the viscosity of the frac fluid was initially 15.4 cp. When crosslinked
with the borate, the
pH was 11.05.
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 57 -
[0223] At 15.4 cp, the pH in deionized water control was 6.67. When
crosslinked with the
borate, the pH was 11.05. The treatment pH at 15.2 cp was 8.06 initially and
was 10.70 when
crosslinked.
Table 5
Time Crosslinked Gel Viscosity at 100 sec-1, cp
(min) Deionized Water Water with Dust Control
30 355 cP 348 cP
60. 380 cP 350 cP
90 367 cP 328 cP
120 min. 360 cP 325 cP
[0224] The viscosity data presented in Table 5 shows that the control sample
(made in
deionized water) had a very similar rheology profile to the fracturing fluid
made with the water
that had been exposed to the chemicals used in the present dust control
treatment process. These
tests show that the viscosity increases to >300cP for both the deionized water
and the treated
water once the cross-linker is added. Thus, the crosslinking reaction is
equivalent in deionized
water either with or without the dust control additive. This test result
confirms that the chemistry
used in the treatment of the present process will not alter or interfere with
the rheological
properties to the frac fluid.
[0225] Example 7 is a breaker test with 200 F water containing a dissolved
sample of the dust
control treatment. One purpose of the test is to determine whether a dissolved
sample of the dust
control treatment adversely affects the efficiency of the frac fluid gel
breaker. Stated another
way, the test sought to find out whether the chemistry used in the dust
additive would require
more breaker to decrease the viscosity of the fracturing fluid or change the
rate at which the
viscosity is decreased with respect to time. The water used in this test was
prepared following the
same procedure that was explained in connection with Example 6.
Table 6
Crosslinked Gel Viscosity at 100 sec-1, cP
Time (min)
Deionized water Water with coating material
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 58 -
829 655
7 464 446
64 93
6 3
0 0
[0226] The results from the side by side tests revealed a similar viscosity
profile with very
similar values from the 7 minute mark until the last reading at the 20 minute
mark. Therefore,
this industry value test confirms that the coating treatment chemistry has no
effect on the
efficiency of the breaker system that was tested.
Example 8
[0227] In Example 8, a 40/70 ceramic proppant was treated with 0.003 wt%
coating of a water-
based emulsion that included a combination of materials including acrylic
polymers, C6-C12
ethoxylated alcohols and Cio-C16 ethoxylated alcohols. The coating had three
positive effects: (1)
it decreased the ceramic's turbidity from 524 NTU's to 110; (2) it decreased
the solubility of the
ceramic in 12% HCL and 3% HF acid from 3.4% to 2.6%, and (3) it increased the
K Value from
13 to 15. These results could not have been predicted based upon the process
that the proppant
was coated with.
[0228] This description is not limited to the particular processes,
compositions, or
methodologies described, as these may vary. The terminology used in the
description is for the
purpose of describing the particular versions or embodiments only, and it is
not intended to limit
the scope of the embodiments described herein. Unless defined otherwise, all
technical and
scientific terms used herein have the same meanings as commonly understood by
one of ordinary
skill in the art. In some cases, terms with commonly understood meanings are
defined herein for
clarity and/or for ready reference, and the inclusion of such definitions
herein should not
necessarily be construed to represent a substantial difference over what is
generally understood
in the art. However, in case of conflict, the patent specification, including
definitions, will
prevail.
[0229] It must also be noted that as used herein and in the appended claims,
the singular forms
"a", "an", and "the" include plural reference unless the context clearly
dictates otherwise.
[0230] As used in this document, terms "comprise," "have," and "include" and
their
conjugates, as used herein, mean "including but not limited to." While various
compositions,
methods, and devices are described in terms of "comprising" various components
or steps
CA 02927111 2016-04-11
WO 2015/066283 PCT/US2014/063086
- 59 -
(interpreted as meaning "including, but not limited to"), the compositions,
methods, and devices
can also "consist essentially of" or "consist of' the various components and
steps, and such
terminology should be interpreted as defining essentially closed-member
groups.
[0231] Various references and patents are disclosed herein, each of which are
hereby
incorporated by reference for the purpose that they are cited.
[0232] From the foregoing, it will be appreciated that various embodiments of
the present
disclosure have been described herein for purposes of illustration, and that
various modifications
can be made without departing from the scope and spirit of the present
disclosure. Accordingly,
the various embodiments disclosed herein are not intended to be limiting.