Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02927381 2016-12-02
TRAJECTORY ALIGNMENT SYSTEM AND METHODS
TECHNICAL FIELD
[0001] The subject matter of the present disclosure generally relates to the
field of image guided
medical procedures.
[0002] More particularly, the subject matter of the present disclosure
technically relates to the
field of patient reference toots for rapid registration in relation to image
guided medical
procedures. Even more particularly, the subject matter of the present
disclosure technically
relates to the field of assisting patient reference tools for rapid
registration in relation to image
guided medical procedures.
BACKGROUND
[0003] In the related art, surgery, such as neurosurgery, for example, brain
tumors are typically
excised through an open craniotomy approach guided by imaging. The data
collected in these
solutions typically consists of CT scans with an associated contrast agent,
such as iodinated
contrast agent, as well as MRI scans with an associated contrast agent, such
as gadolinium
contrast agent. Also, optical imaging is often used in the form of a
microscope to differentiate
the boundaries of the tumor from healthy tissue, known as the peripheral zone.
Tracking of
instruments relative to the patient and the associated imaging data is also
often achieved by way
of external hardware systems such as mechanical arms, radiofrequeney, or
optical tracking
devices. As a set, these devices are commonly referred to as surgical
navigation systems and are
often cumbersome and provide inaccurate tracking.
[0004] Port-based surgery is a minimally invasive surgical technique where a
port is introduced
to access a surgical region of interest using surgical tools. Unlike other
minimally invasive
techniques, such as laparoscopic techniques, a port diameter is larger than a
tool diameter.
Hence, the tissue region of interest is visible through the port, wherein
exposed tissue in a region
of interest, at a depth few centimetres below the skin surface, is accessible
through a narrow
corridor in the port.
CA 02927381 2016-12-02
[0005] Several related art problems generally preclude or impair the ability
to perform port-
based navigation in an intra-operative setting. For example, the position of
the port axis relative
to a typical tracking device (TD) is a free and uncontrolled parameter that
prohibits the
determination of access port orientation. Further, the limited access which is
available, due to
the required equipment for the procedure, causes indirect access port tracking
to be impractical
and unfeasible. Also, the requirement for angulation of the access port to
access many areas
within the brain during a procedure makes navigation of the access port a
difficult and
challenging problem that has not yet been addressed.
[0006] Further, a recent paper by Stieglitz et al., "The Silent Loss of
Neuronavigation Accuracy:
A Systematic Retrospective Analysis of Factors Influencing the Mismatch of
Frameless
Stereotactic Systems in Cranial Neurosurgery," highlights the need for
accurate navigation,
wherein after patient registration, an ongoing loss of neuro-navigation
accuracy remains due to
other mitigating factors related to the surgical procedure, i.e., draping,
attachment of skin
retractors, and duration of surgery. Surgeons should be aware of this "silent"
loss of accuracy
when using related art navigation systems.
[0007] Accordingly, challenges experienced in the related art include an
inability to perform a
real-time registration of a surgical trajectory in relation to the unique
characteristics of a
particular tissue types or sub-types, such as in relation to cerebral tissue.
Therefore, a need exists
for a system and method that integrates and updates pre-operative and intra-
operative plans into
navigation systems for minimally invasive surgical procedures, such as an
improved system and
method for mapping navigation space to patient space in a medical procedure,
e.g., as a real-time
registration of a surgical trajectory in relation to the unique
characteristics of a particular tissue
types or sub-types, for example, cerebral tissue.
BRIEF SUMMARY
[0008] The present disclosure addresses at least many of the foregoing
challenges experienced
by related art registration devices and methods, by way of a system and
methods for aligning a
trajectory, such as a therapeutic trajectory, a medical trajectory, and/or a
surgical trajectory, in
real-time, whereby axonal connections, neural fibers, and neural pathways are
preserved, and
2
CA 02927381 2016-12-02
whereby damage to brain circuitry is prevented. The presently disclosed system
and methods for
aligning a surgical trajectory in real-time involve registration by way of
multi-modal imaging for
providing transformed real-time data to a user interface, the transformed data
comprising real-
time registration data in relation to real-time neural network data, such real-
time registration data
renderable by way of user interface, e.g., a display device. The present
disclosure applies
equally well to catheters, DBS needles, a biopsy procedure, and also to
biopsies and/or catheters
in other medical procedures performed on other parts of the body. To date,
such capabilities
have been hitherto unknown in the related art.
[0009] In accordance with an embodiment of the present disclosure, a medical
navigation system
and methods are used to execute a surgical plan during brain medical
procedures. These
procedures may include port-based surgery using a port with an introducer,
deep brain
stimulation, or brain biopsy using needles. The navigation system, comprising
navigation
software module, is configured to utilize a medical plan or a surgical plan
("plan") based on a
multi-segment path trajectory, previously prepared or predetermined using pre-
operative
anatomical information of a given patient's brain. This plan is imported into
the navigation
software module.
[0010] Prior to commencing the procedure, the brain is registered using the
corresponding pre-
operative anatomical information, in accordance with an embodiment of the
present disclosure.
Once the craniotomy has been performed, the navigation system and methods
utilize a user
interface for displaying an overlay image of the brain and the multipoint path
trajectory. In
addition, the user interface provides a guidance mechanism to assist the
surgeon in aligning the
surgical tool, such as a port, a biopsy needle, a catheter, and the like,
e.g., coaxially along a first
path trajectory segment. Using port-based surgery as an example, once the port
is aligned with
the first path trajectory segment, the surgeon begins a cannulation procedure
and moves the port
introducer along the first path trajectory segment while the system and method
assist the surgeon
in remaining consistently coaxial in relation to the first path trajectory
segment, the user interface
displaying, to the surgeon, the distance of the introducer along the first
path trajectory segment
until the end of the first path trajectory segment is reached. The surgeon
then changes direction
to follow a second path trajectory segment. The process is repeated until the
target location is
reached.
3
CA 02927381 2016-12-02
[00111 The system and methods of the present disclosure provide the surgeon
with positional
information of the patient's anatomy of interest throughout the course of the
medical procedure
using video overlay, e.g., allowing the surgeon to see the brain through the
drapes and, therefore,
know his/her orientation relative to the patient. By so doing, the surgeon
more accurately
identifies potential locations of anatomical structures of the brain intra-
operatively, as opposed to
performing the procedure without a rendered overlay of the anatomical part as
otherwise
practiced in the related art. The system and methods allow facilitates
confirmation that the
correct anatomical data of the patient more effectively than presently used
systems for at least
that the imaged anatomy is rendered onto the real-time imaging of the patient
anatomy, thereby
allowing the surgeon to compare the rendered image of the anatomical part with
the real
anatomical part, for example, comparing the sulci locations during a port
procedure.
[0012] The system and methods of the present disclosure provide tracking of
multiple tools
relative to the brain during surgery so that the surgeon is not "flying
blind." For example the
system can track the port as well as any tool being used in conjunction with
the port, such as a
resection tool in the case of tumor resection, whereas related art systems
track only a pointer
tool. The navigation system and methods provide the surgical team with a setup
for the surgery
based on a predetermined plan, e.g., a setup of the head clamp, position of
patient, tracking
device, etc., to prevent readjustments of such elements during surgery. The
navigation system
and methods adaptively update a section of a larger pre-operative MRI image by
using a
localized intra-operative MRI image (given that the brain is internally
accessible from within the
skull). The navigation system and methods may provide positionally accurate
maps (images)
correlating intra-operative information acquired during surgery, such as
hyperspectral and
Raman signatures, to locations at which the information is acquired. For
example, these Raman
signatures may be represented by spatially correlated color maps.
[0013] The system and methods of the present disclosure, while primarily
described for port-
based brain surgery, is not limited to port based brain surgery, but is also
applicable to any
surgical or medical procedure that utilizes a navigation system. Thus, a port
may not be
necessary; and the anatomical part may be any part of the anatomy. This system
can be utilized
with any animal, including humans.
4
CA 02927381 2016-12-02
[0014] In accordance with an embodiment of the present disclosure, a an
alignment system for
aligning a tool in relation to a trajectory in real-time comprises: a
processor configurable by a
set of executable instructions storable in relation to a non-transitory memory
device to: receive
input data from at least one source of at least one pre-operative plan image,
at least one multi-
modal image, and at least one real-time multi-modal image; interactively track
at least one neural
fiber, whereby interactively tracked neural fiber data is obtained;
automatically generate output
data by way of data transformation using the input data and the interactively
tracked neural fiber
data; and transmit the output data to at least one of: at least one display
device for rendering at
least one real-time interactive navigation display for facilitating neural
navigation, and at least
one drive device for positioning at least one tracking device in relation to
the tool in real-time,
whereby real-time alignment data is achieved, and whereby at least one
neurological structure is
preserved.
[0015] In accordance with another embodiment of the present disclosure, a
method of fabricating
a an alignment system for aligning a tool in relation to a trajectory in real-
time comprises:
providing a processor configurable by a set of executable instructions
storable in relation to a
non-transitory memory device to: receive input data from at least one source
of at least one pre-
operative plan image, at least one multi-modal image, and at least one real-
time multi-modal
image; interactively track at least one neural fiber, whereby interactively
tracked neural fiber
data is obtained; automatically generate output data by way of data
transformation using the
input data and the interactively tracked neural fiber data; and transmit the
output data to at least
one of: at least one display device for rendering at least one real-time
interactive navigation
display for facilitating neural navigation, and at least one drive device for
positioning at least one
tracking device in relation to the tool in real-time, whereby real-time
alignment data is achieved,
and whereby at least one neurological structure is preserved.
CA 02927381 2016-12-02
[0016] In accordance with another embodiment of the present disclosure, a
method of using an
alignment system for aligning a tool in relation to a trajectory in real-time
comprises: providing
the alignment system, the alignment system providing comprising providing a
processor
configurable by a set of executable instructions storable in relation to a non-
transitory memory
device to: receive input data from at least one source of at least one pre-
operative plan image, at
least one multi-modal image, and at least one real-time multi-modal image;
interactively track at
least one neural fiber, whereby interactively tracked neural fiber data is
obtained; automatically
generate output data by way of data transformation using the input data and
the interactively
tracked neural fiber data; and transmit the output data to at least one of: at
least one display
device for rendering at least one real-time interactive navigation display for
facilitating neural
navigation, and at least one drive device for positioning at least one
tracking device in relation to
the tool in real-time, whereby real-time alignment data is achieved, and
whereby at least one
neurological structure is preserved; calibrating the tool by using a
calibration block; if
performing a port procedure, verifying a port; evaluating an approach by
determining whether a
planned engagement point is appropriate using the at least one real-time
interactive navigation
display of the alignment system; if the planned engagement point is
appropriate, performing the
approach; if the planned engagement point is inappropriate, interactively
setting a new
engagement point by way of at least one interactive feature of the alignment
system; and
optionally returning to the evaluating step.
[0017] Benefits of the system and methods of the present disclosure include,
but are not limited
to, eliminating the necessity of a tracked sheath for aligning a port,
facilitating alignment of
compatible miniframes, such as Monteris miniframes, facilitating an approach
by way of a
pointer, facilitating locating an entry point by using real-time registration
data renderable, such
as by real-time graphics, via a user interface, e.g., on a display device, and
displaying a trajectory
length (or pathway) in at least one of the stage of craniotomy, approach, and
resection.
[0018] Some of the features in the present disclosure are broadly outlined in
order that the
section entitled Detailed Description is better understood and that the
present contribution to the
art may be better appreciated. Additional features of the present disclosure
are described
hereinafter. In this respect, understood is that the present disclosure is not
limited in its
application to the details of the components or steps set forth herein or as
illustrated in the
6
CA 02927381 2016-12-02
several figures of the being carried out in various ways. Also, understood is
that the phraseology
and terminology employed herein are for the purpose of the description and
should not be
regarded as limiting.
BRIEF DESCRIPTION OF THE DRAWINGS
[0019] The above, and other, aspects, features, and advantages of several
embodiments of
the present disclosure will be more apparent from the following Detailed
Description as
presented in conjunction with the following several figures of the Drawing.
[0020] FIG. 1 is a diagram illustrating a perspective view of a navigation
system, such as a
medical navigation system, comprising a patent reference device, in an
environmental context,
such as an operation room, of in accordance with an embodiment of the present
disclosure.
[0021] FIG. 2 is a schematic diagram illustrating a navigation system, such as
a medical
navigation system, comprising a patent reference device, in accordance with an
embodiment of
the present disclosure.
[0022] FIG. 3A is a flow diagram illustrating a method of performing a medical
procedure,
such as a surgical procedure, e.g., a brain biopsy using an access port, by
way of a navigation
system, in accordance with an embodiment of the present disclosure.
[0023] FIG. 3B is a flow diagram illustrating a partial view of a portion of
the method of
performing a medical procedure, such as a surgical procedure, e.g., a brain
biopsy using an
access port, by way of a navigation system, as shown in FIG. 3A, in accordance
with an
embodiment of the present disclosure.
[0024] FIG. 4A is a screenshot illustrating at least one image of a brain
renderable on a display
device during a step of a positioning and fixing step in the method, as shown
in FIGS. 3A and
3B, by way of a navigation system, in accordance with an embodiment of the
present
disclosure.
[0025] FIG. 4B is a screenshot illustrating at least one image of a brain
renderable on a display
device during a step of initiating registration, e.g., by using fiducial touch-
points, in the
7
CA 02927381 2016-12-02
method, as shown in FIGS. 3A and 3B, by way of a navigation system, in
accordance with an
embodiment of the present disclosure.
[0026] FIG. 4C is a screenshot illustrating at least one image of a brain
renderable on a display
device during a step of preparing and planning a craniotomy, in the method, as
shown in FIGS.
3A and 3B, by way of a navigation system, in accordance with an embodiment of
the present
disclosure.
[0027] FIG. 4D is a screenshot illustrating at least one image of a brain
renderable on a display
device during steps of confirming engagement and motion range within a cranial
space and
cutting a dura at the engagement point and identifying a sulcus, in the
method, as shown in
FIGS. 3A and 3B, by way of a navigation system, in accordance with an
embodiment of the
present disclosure.
[0028] FIG. 4E is a screenshot illustrating at least one image of a brain
renderable on a display
device during iterative cannulating steps, in the method, as shown in FIGS. 3A
and 3B, by way
of a navigation system, in accordance with an embodiment of the present
disclosure.
[0029] FIG. 5 is a diagram illustrating an access port based surgical
procedure being conducted
by way of the navigation system and methods, in accordance with some
embodiments of the
present disclosure.
[0030] FIG. 6A is a diagram illustrating a perspective view of a patient
reference device
comprising a tracking tool, in accordance with an embodiment of the present
disclosure.
= [0031] FIG. 6B is a diagram illustrating a perspective view of a patient
reference device
comprising a tracking tool, in accordance with an embodiment of the present
disclosure.
[0032] FIG. 6C is a diagram illustrating a perspective view of a patient
reference device
comprising a tracking tool, in accordance with an embodiment of the present
disclosure.
[0033] FIG. 6D is a diagram illustrating a perspective view of a patient
reference device
= comprising a tracking tool, in accordance with an embodiment of the
present disclosure.
8
CA 02927381 2016-12-02
[0034] FIG. 6E is a diagram illustrating a perspective view of a patient
reference device
comprising an access port, in accordance with an embodiment of the present
disclosure.
[0035] FIG. 6F is a diagram illustrating a front view of a patient reference
device comprising an
access port, as shown in FIG. 6E, in accordance with an embodiment of the
present disclosure.
[0036] FIG. 6G is a diagram illustrating a side view of a patient reference
device comprising an
access port, as shown in FIG. 6E, in accordance with an embodiment of the
present disclosure.
[0037] FIG. 6H is a diagram illustrating a top view of a patient reference
device comprising an
access port, as shown in FIG. 6E, in accordance with an embodiment of the
present disclosure.
[0038] FIG. 7 is a diagram illustrating a perspective view of a patient
reference device
comprising a tracking tool, as shown in FIG. 6C, engaged with a patient
reference device
comprising an access port, in accordance with an embodiment of the present
disclosure.
[0039] FIG. 8 is a schematic diagram illustrating a relationship between
components of the
navigation system, such as a control and processing unit, a tracking system, a
data storage
device for the tracking system, and system devices, and medical instruments,
in accordance
with an embodiment of the present disclosure.
[0040] FIG. 9 is a schematic diagram illustrating a pre-operative surgical
planning system for
use with a medical navigation system, in accordance with an embodiment of the
present
disclosure.
[0041] FIG. 10 is a schematic diagram illustrating an intra-operative surgical
management
system for use with a medical navigation system, in accordance with an
embodiment of the
present disclosure.
[0042] FIG. 11A is a flow diagram illustrating a method of performing a
medical procedure,
=
such as a port-based procedure, by way of a navigation system, in accordance
with an
alternative embodiment of the present disclosure.
[0043] FIG. 11B is a flow diagram illustrating a method of performing a
medical procedure,
9
CA 02927381 2016-12-02
such as a frameless brain biopsy, by way of a navigation system, in accordance
with an
alternative embodiment of the present disclosure.
[0044] FIG. 11C is a flow diagram illustrating a method of performing a
medical procedure,
such as a frameless deep brain stimulation (DBS), by way of a navigation
system, in
accordance with an alternative embodiment of the present disclosure.
[0045] FIG. 11D is a flow diagram illustrating a method of performing a
medical procedure,
such as a catheter/shunt placement, by way of a navigation system, in
accordance with an
alternative embodiment of the present disclosure.
[0046] FIG. 12 is a screenshot illustrating various elements of a display
renderable on at least
one display device by way of using a navigation system, in accordance with an
embodiment of
the present disclosure.
[0047] FIG. 13A is a screenshot illustrating a display, renderable on at least
one display device,
by way of a navigation system, as an trajectory alignment system, in
accordance with an
embodiment of the present disclosure.
[0048] FIG. 13B is a flow diagram illustrating a method of fabricating a
navigation system, as an
trajectory alignment system, for aligning a tool in relation to a trajectory
during an approach
phase of a surgical procedure, in accordance with an embodiment of the present
disclosure.
[0050] FIG. 13C is flow diagram illustrating a method of using a navigation
system, as an
trajectory alignment system, for aligning a tool in relation to a trajectory
during an approach
phase of a surgical procedure, in accordance with an embodiment of the present
disclosure.
[0049] FIG. 14 is a screenshot illustrating a display, renderable on at least
one display device, by
way of a navigation system, as a trajectory alignment system, in accordance
with an embodiment
of the present disclosure.
[0050] Corresponding reference numerals or characters indicate corresponding
components
throughout the several figures of the Drawing. Elements in the several figures
are illustrated
for simplicity and clarity and have not necessarily been drawn to scale. For
example, the
CA 02927381 2016-12-02
dimensions of some of the elements in the figures may be emphasized relative
to other elements
for facilitating understanding of the various presently disclosed embodiments.
Also, common,
but well-understood, elements that are useful or necessary in commercially
feasible
embodiment are often not depicted in order to facilitate a less obstructed
view of these various
embodiments of the present disclosure.
DETAILED DESCRIPTION
[0051] The systems and methods described herein are useful in the field
neurosurgery,
including oncological care, neurodegenerative disease, stroke, brain trauma,
and orthopedic
surgery. However, the subject matter of the present disclosure may extend or
apply to other
conditions or fields of medicine; and such extensions or applications are
encompassed by the
present disclosure. The systems and methods described herein encompass
surgical processes
that are applicable to surgical procedures for brain, spine, knee, and any
other region of the
body that will benefit from the use of an access port or small orifice to
access the interior of an
animal body, such as a human body.
[0052] Various systems, apparatuses, devices, or processes are below-described
and provide
examples of the navigation systems and methods embodiments, in accordance with
embodiments of the present disclosure. None of the below-described embodiments
limits any
claimed embodiment; and any claimed embodiment may also encompass systems,
apparatuses,
devices, or processes which may differ from below-described examples. The
claimed
embodiments are not limited to systems, apparatuses, devices, or processes
having all of the
features of any one of the below-described systems, apparatuses, devices, or
processes or to
features common to some or all of the below-described systems, apparatuses,
devices, or
processes.
I-00531 Furthermore, this Detailed Description sets forth numerous specific
details in order to
provide a thorough understanding of the various embodiments described
throughout the present
disclosure. However, it will be understood by those of ordinary skill in the
art that the
embodiments described herein may be practiced without these specific details.
In other
I
CA 02927381 2016-12-02
instances, well-known methods, procedures and components have not been
described in detail
so as not to obscure the embodiments described herein.
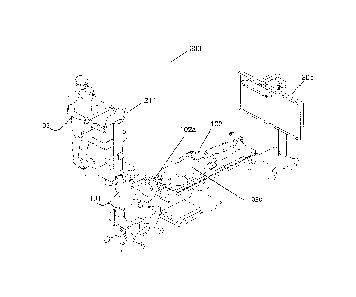
[0054] Referring to FIG. 1, this diagram illustrates, in a perspective view, a
navigation system
200, such as a medical navigation system, comprising a patent reference
device, in an
environmental context, such as an operation room (OR), of in accordance with
an embodiment
of the present disclosure. The system 200 supports, facilitates, and enhances
minimally
invasive access port based surgery using a minimally invasive access port
based surgical
procedure. By example only, a surgeon 101 conducts a minimally invasive access
port based
surgery on a subject, such as a patient 102, in an OR environment. The
navigation system 200,
comprising an equipment tower 201, a tracking system 213, at least one display
device, e.g., a
primary display device 211 and a secondary display device 205, the system 200
configured to
track at least one instrument, such as a surgical instrument, e.g., an access
port 206 and an
introducer 210, and/or a tool, such as a tracking tool, e.g., a pointer, for
assisting the surgeon
101 during the minimally invasive access port based surgical procedure (FIG.
2). By example
only, an operator 103 is also present to operate, control, and provide
assistance for the system
200.
100551 Referring to FIG. 2, this schematic diagram illustrates a medical
navigation system,
comprising an equipment tower 201, a tracking system 213, at least one display
device. e.g., a
primary display device 211 and a secondary display device 205, the system 200
configured to
track at least one instrument, such as a surgical instrument, e.g., an access
port 206 and an
introducer 210, and/or a tool, such as a tracking tool, e.g., a pointer, for
assisting the surgeon
101 during the minimally invasive access port based surgical procedure, in
accordance with an
embodiment of the present disclosure. By example only, the navigation system
200 comprises
a display device 211, such as a monitor, for displaying a video image, an
equipment tower 201
for accommodating at least one piece of equipment, a robotic arm 202, an
optical scope 204
coupled with at least one piece of equipment and supportable by the robotic
arm 202.
[0056] Still referring to FIG. 2, the equipment tower 201 is mountable on a
frame, e.g., a rack
or a cart, and is configured to accommodate at least one of a computer
operable by at least one
a set of instructions, storable in relation to at least one non-transitory
memory device,
12
CA 02927381 2016-12-02
corresponding to at least one of planning software, navigation software, and
robotic software
for managing at least one of the robotic arm 202 and the at least one
instrument, such as a
surgical instrument, e.g., an access port 206 and an introducer 210, and/or a
tool, such as a
tracking tool, e.g., a pointer, and a power supply, e.g., an AC adapter power
supply. The
computer comprises at least one of a control unit and a processing unit, e.g.,
a control and
processing unit 400 (FIG. 8), for example. The equipment tower 201 comprises a
single tower
configured to facilitate coupling of the at least one display device. e.g., a
primary display
device 211 and a secondary display device 205, with the at least one piece of
equipment.
However, other configurations are also encompassed by the present disclosure,
such as the
equipment tower 201 comprising dual towers configured to facilitate coupling
of a single
display, etc. Also, the equipment tower 201 is also configurable to
accommodate an
uninterruptible power supply (UPS) for providing emergency power.
[0057] Still referring to FIG. 2, a patient's head is retained by a head
holder 217, a craniotomy
is performed, a dura flap is formed and retracted, and the access port 206 and
the introducer
210 are inserted into the patient's brain 102a. The introducer 210 further
comprises a pointing
tool. The introducer 210 is trackable by way of the tracking system 213,
whereby position
information is used in the navigation system 200. The tracking system 213 is
configured to
track and determine, e.g., in real-time by way of a set of instructions
corresponding to tracking
software and storable in relation to at least one non-transitory memory
device, location data of
at least one OR item, such as the robotic arm 202 and the at least one
instrument, such as a
surgical instrument, e.g., an access port 206 and an introducer 210, and/or a
tool, such as a
tracking tool, e.g., a pointer,. The tracking system 213 comprises at least
one sensor (not
shown) for detecting at least one fiducial marker 212 disposable in relation
to the at least one
OR item, e.g., the robotic arm 202 and the at least one instrument, such as a
surgical
instrument, e.g., an access port 206 and an introducer 210, and/or a tool,
such as a tracking tool,
e.g., a pointer,. The tracking system 213 comprises a three-dimensional (3D)
optical tracking
stereo camera, such as a Northern Digital Imaging (NDI) optical tracking
stereo camera, by
example only. The secondary display device 205 is configured to display real-
time output 205a
from the tracking system 213. The output 205a comprises a display of at least
one of an axial
view, a sagittal view, at least one coronal view, and a view oriented relative
to the at least one
13
CA 02927381 2016-12-02
= instrument, such as perpendicular to a tool tip, in-plane of a tool
shaft, etc. The output 205a
further comprises a display of multiple views.
[0058] Still referring to FIG. 2, minimally invasive brain surgery using
access ports is a recent
method of performing surgery on brain tumors. In order to introduce an access
port 206 into a
brain, such as the patient brain 102b, of a patient head 102a, an introducer,
e.g., the introducer
210, comprises an atraumatic tip disposable within the access port 206 for
facilitating
positioning the access port 206 within the patient brain 102a. The introducer
210 further
comprises at least one fiducial marker 212 for facilitating tracking by the
tracking system 213.
The at least one fiducial marker 212 comprises at least one of at least one
reflective sphere (not
shown) for use with a tracking system 213 comprising an optical tracking
stereo camera (not
shown) and at least one pick-up coil (not shown) for use with a tracking
system 213 comprising
an electromagnetic tracking device (not shown). The at least one fiducial
marker 212 is
detectable by the at least one sensor (not shown) of the tracking system 213;
and the position of
the at least one fiducial marker 212 is determined by the tracking system 213
operating by way
of the tracking software. In a preferred embodiment of the present disclosure,
the at least one
fiducial marker 212 comprises a plurality of fiducial markers 212.
[0059] Still referring to FIG. 2, after the introducer 210 and the access port
206 are inserted into
the brain 102b, the introducer 210 is removed to facilitate access to tissue
of the brain 102b
through a central opening of the access port 206. However, after the
introducer 210 is
removed, the access port 206 is no longer being trackable by the tracking
system 213.
Accordingly, the access port 206 is indirectly trackable by way of additional
pointing tools (not
shown) configured for identification by the navigation system 200.
[0060] Still referring to FIG. 2, the navigation system 200 further comprises
a guide clamp 218
for retaining the access port 206. The guide clamp 218 is configured to
optionally engage and
disengage the access port 206, eliminating the need to remove the access port
206 from the
patient 102. In some embodiments, the access port 206 is configured to slide
up and down
within the guide clamp 218 in a closed position. The guide clamp 218 further
comprises a
locking mechanism (not shown), the locking mechanism being attachable or
integrable in
14
CA 02927381 2016-12-02
relation to the guide clamp 218, and the locking mechanism being optionally
manually
actuable, e.g., using one hand as further below described.
[00611 Still referring to FIG. 2, the navigation system 200 further comprises
an articulating arm
219, such as a small articulating arm, configured to couple with the guide
clamp 218. The
articulating arm 219 comprises up to six (6) degrees of freedom for
facilitating positioning of
the guide clamp 218. The articulating arm 219 is attachable at a location in
relation to the head
holder 217, or in relation to any other suitable patient support structure, to
ensure, when locked
in place, that the guide clamp 218 is fixed in relation to the patient's head
102a. The
articulating arm 219 comprises an interface 219a disposable in relation to the
guide clamp 218,
wherein the interface 219a is at least one of flexible and lockable into
place. Flexibility of the
interface 219a facilitates movability of the access port 206 into various
positions within the
brain 102b, yet still maintains rotability about a fixed point.
[0062] Still referring to FIG. 2, by example only, the interface 219a
comprises a linkage, such
as a slender bar or a slender rod. When the access port 206 is moved to
various positions, the
interface 219a is configured to oppose a bending force, whereby the access
port 206 is
returnable to a centered position. The interface 219a further comprises an
optional collar
engageable with the linkage between the articulating arm 219, and the guide
clamp 218, such
that, when engaged, the linkage becomes rigid. Currently, no such mechanisms
are known to
exist in the related art to enable positioning an access port 206 in such
manner.
1_0063J Still referring to FIG. 2, the navigation system 200, comprising
preset equipment and
components, further facilitates setup of a surgical procedure which may be
otherwise complex
and lengthy in the related art for at least the reason that many pieces of
equipment associated
with a surgical procedure must be coordinated. In an alternative embodiment of
the present
disclosure, navigation system 200 provides a solution to the related art
problems, and
comprises a plurality of wide-field cameras, e.g., two additional wide-field
cameras (not
shown) being implemented with video overlay information, wherein one camera,
e.g., a first
additional camera, of the two additional wide-field cameras is mountable in
relation to the
optical scope 204; and the other camera, e.g., a second additional camera, of
the two additional
wide-field cameras is mountable in relation to the navigation system 213.
Alternatively, in the
CA 02927381 2016-12-02
case of the navigation system 213 comprising an optical tracking device, a
video image is
directly extractable from the second additional camera of the tracking system
213. Video
overlay information is then insertable into the images, wherein the video
overlay provides at
least one of type of information, such an image displaying a physical space
and confirm
tracking system registration alignment and optional corresponding text and/or
indicia, an image
displaying a motion range of the robotic arm 202 holding the optical scope 204
and optional
corresponding text and/or indicia, and an image displaying a guide head
positioning and a
patient positioning and optional corresponding text and/or indicia.
[0064] Referring to FIG. 3A, this flow diagram illustrates a method Ml of
performing a
medical procedure, such as a surgical procedure, e.g., a brain biopsy using an
access port 206,
by way of a navigation system 200, in accordance with an embodiment of the
present
disclosure. The method Ml comprises: importing a surgical plan, e.g., a port-
based surgical
plan, as indicated by block 302; positioning and fixing a patient, as
indicated by block 304;
initiating a registration, as indicated by block 306; confirming registration,
as indicated by
block 308; draping the patient, as indicated by block 310; confirming a
patient engagement
point, as indicated by block 312; preparing and planning a craniotomy, as
indicated by block
314; cutting (e.g., incising) a cranium, as indicated by block 316; updating
registration, as
indicated by block 322, and confirming engagement and motion range within a
cranial space, as
indicated by block 318; cutting a dura at the engagement point and identifying
a sulcus, as
indicated by block 320; updating registration, as indicated by block 322, and
determining
whether a planned trajectory plan is complete, as indicated by block 324; if
the planned
trajectory plan is complete, performing a resection, as indicated by block
326, decannulating, as
indicated by block 327, and closing the dura and closing the cranium, as
indicated by block
330; or, if the planned trajectory plan is incomplete, aligning the access
port 206 at the
engagement point and setting the planned trajectory, as indicated by block
332, cannulating to a
target depth, as indicated by block 334, and determining whether a planned
trajectory is
complete, as indicated by block 324.
[0065] Still referring to FIG. 3A, by example only, the method M1 further
comprises: using
pre-operative 3D imaging data, such as MRI data, CT scan data, ultrasound data
etc.;
overlaying imaging data (real-time), from received input data (interactively
measured data),
16
CA 02927381 2016-12-02
such as data relating to sulci entry points, target locations, surgical
outcome criteria, and
additional 3D image data information, on the pre-operative 3D imaging data;
and displaying at
least one trajectory path based on a calculated score corresponding to a
projected surgical path,
as described by the present disclosure and by the disclosurc(s) of the
priority document(s). At
least one of the pre-operative 3D imaging data and the interactively measured
data comprise
three (3) spatial dimensions of the data set. In another embodiment of the
present disclosure,
the relevant parameters comprise two (2) spatial dimensions, e.g., as in the
case of MR "slice"
images as acquired by conventional MRI equipment) and time t being a third
dimension of the
data set. In another embodiment of the present disclosure, the relevant
parameters comprise
three (3) spatial dimensions and time t being a fourth dimension of the data
set. Some imaging
modalities and estimation methods, such as diffusion tensor imaging (DTI)
data, may contain
more than four dimensions of information at each spatial location. The method
M1 may
comprise executing a variety of surgical plans by way of the navigation system
200.
[0066] Still referring to FIG. 3A, the method M1 includes further detailed sub-
steps. After
importing a surgical plan into the navigation system 200, as indicated by
block 302, positioning
and fixing the patient comprises affixing the patient's head 102a into
position using a head
holder 217 and/or the patient's body 102c using a body holding mechanism (not
shown), and
confirming the head position with the patient plan using the navigation
software, as indicated
by block 304. In the step of initiating registration of the patient, as
indicated by block 306,
initiating registration , the word "registration," or the phrase "image
registration" comprises
transforming different sets of data into one coordinate system, whereby
transformed data is
provided.
[00671 Still referring to FIG. 3A, the method M1 includes yet further detailed
sub-steps. For
instance, registration of the patient, as indicated by block 306, can be
performed in relation to a
base reference frame is performable by various sub-steps, such as (a)
identifying features
(natural or engineered) on the MR and CT images and point to those same
features in the live
scene using a pointer tool that is tracked by the tracking system; (b) tracing
a line on the curved
profile of the patient's face or forehead with a pointer tool that is tracked
by the tracking system
and matching this curved profile to the 3D MR or CT volume; (c) applying a
tool of known
geometry to the patient's face or forehead, wherein the tool comprises at
least one of an active
17
CA 02927381 2016-12-02
target and a passive target, trackable by the tracking system 213; and (d)
using a surface
acquisition tool based on structured light and matching an extracted surface
to the 3D MR or
CT volume.
[0068] Still referring to FIG. 3A, those skilled in the art will appreciate
that there are numerous
registration techniques available and one or more of them may be used in the
present
application. Non-limiting examples include intensity-based methods which
compare intensity
patterns in images via correlation metrics, while feature-based methods find
correspondence
between image features such as points, lines, and contours. Image registration
algorithms may
also be classified according to the transformation models they use to relate
the target image
space to the reference image space. Another classification can be made between
single-
modality and multi-modality methods. Single-modality methods typically
register images in
the same modality acquired by the same scanner/sensor type, for example, a
series of MR
images can be co-registered, while multi-modality registration methods are
used to register
images acquired by different scanner/sensor types, for example in MRI and PET.
[0069] Still referring to FIG. 3A, in the present disclosure, the method M1
further comprises
using multi-modality registration techniques from medical imaging of the
head/brain obtained
from different scanners e.g., from registration of brain CT/MRI images or
PET/CT images for
tumor localization, registration of contrast-enhanced CT images in relation to
non-contrast-
enhanced CT images, and registration of ultrasound and CT, and transforming
such data for
better interactively refining alignment of a surgical trajectory.
[0070] Referring to FIG. 3B, this flow diagram illustrates, in a partial view,
a portion of the
method M1 of performing a medical procedure, such as a surgical procedure,
e.g., a brain
biopsy using an access port 206, by way of a navigation system 200, as shown
in FIG. 3A, in
accordance with an embodiment of the present disclosure. The method M1 further
comprises:
completing registration by using fiducial touch-points (FIG. 4B) captured by a
pointing tool as
indicated by block 340 (FIGS. 6A-6D), wherein completing registration by using
fiducial
touch-points comprises first identifying fiducial touch-points on images, as
indicated by block
342, touching the fiducial touch-points with a tracked instrument, as
indicated by block 344,
and determining registration data in relation to reference markers, as
indicated by block 346.
18
CA 02927381 2016-12-02
The method Ml alternatively further comprises: completing registration by
conducting a
surface scan procedure, as indicated by block 350, wherein conducting a
surface scan procedure
comprises scanning the face using a 3D scanner, as indicated by block 352,
extracting the face
surface data from MR/CT data, as indicated by block 354, and determining
registration data
points by matching the face surface data from the 3D scanner with the face
surface data from
.
MR/CT data, as indicated by block 356. Upon completing registration by using
fiducial touch-
points procedure, as indicated by block 340, or surface scan completing
registration by
conducting a surface scan procedure, as indicated by block 350, and
transforming and
confirming the determined registration data, as indicated by block 308.
[0071] Still referring to FIG. 3B, during a navigation procedure, such via the
method Ml, a
handheld instrument is trackable by using a tracking system 213, and a
representation of the
= instrument's position and orientation may be provided and displayed as an
overlay on a
previously acquired or current image (such as a three-dimensional scan) of a
patient's anatomy
obtained with an imaging device or system (such as ultrasound, CT or MRI). To
achieve this, a
registration is needed between the coordinate frame of a tracking system 213,
the physical
location of the patient 102 in space, and the coordinate frame of the
corresponding image of the
patient 102. This registration is typically obtained relative to a tracked
reference marker, which
is placed in a fixed position relative to the patient anatomy of interest and
thus can be used as a
fixed reference for the anatomy. Generally, this can be accomplished by
attaching the
reference to a patient immobilization frame (such as a clamp for skull
fixation in neurosurgery),
which itself is rigidly attached to the patient 102. However, the reference
may be held to the
frame, for example, through an arm, which can be bumped and accidentally
moved, which
creates a loss of registration.
[0072] Still referring to FIG. 3B, additionally, since the reference marker
must be positioned so
that it is visible by the navigation hardware (typically requiring line-of-
sight for optical
tracking, or otherwise within the observation or communication field of the
tracking system
213), this tends to position the reference such that it is in the open thus
more susceptible to
accidental interaction and loss of registration. In situations of lost
registration, a surgical
procedure tends to be stopped while a new registration is computed, although
this may not
always be possible if, for example, the registration fiducial-points or
patient skin surface are no
19
CA 02927381 2016-12-02
longer accessible due to the progression of the surgical procedure, and thus
creating a need for
a full re-registration or, in some cases even disabling navigation for the
remainder of the
procedure.
[0073] Still referring to FIG. 3B and referring back to FIG. 3A, in the method
Ml, after
confirming registration, as indicated by block 308, draping the patient 102,
as indicated by
block 310, comprises covering the patient 102 and surrounding areas with a
sterile barrier (not
shown) to create and maintain a sterile field during the surgical procedure.
The purpose of the
draping step is to eliminate the passage of microorganisms, e.g., bacteria,
between non-sterile
and sterile areas. After performing the draping step, as indicated by block
310, the method M1
comprises confirming patient engagement points, as indicated by block 312, and
preparing and
planning the craniotomy, as indicated by block 314 (FIG 4C).
[0074] Still referring to FIG. 3B and referring back to FIG. 3A, in the method
Ml, after
preparing and planning the craniotomy, as indicated by block 314, the method
M1 comprises
cutting the cranium e.g., by way of a craniotomy, wherein a bone flap is
temporarily removed
from the skull to access the brain 102b, as indicated by block 316, updating
registration data, as
indicated by block 322, such as by adding additional registration
correspondence points within
the craniotomy, e.g. the location of a visible blood vessel, confirming the
engagement within
the craniotomy location and the motion range, as indicated by block 318, and
cutting the dura at
the engagement points and identifying the sulcus, as indicated by block 320
(FIG 4D).
[0075] Still referring to FIG. 3B and referring back to FIG. 3A, the method M1
also comprises
updating the registration data, as indicated by block 322, wherein updating
comprises adding
further registration correspondence points near the engagement point, e.g. a
bifurcation of the
entry sulcus. In an embodiment of the present disclosure, by focusing the wide
field camera's
gaze on the surgical area of interest, updating the registration data
comprises manipulating or
transforming the registration data to ensure the best match for the surgical
area of interest,
while ignoring any non-uniform tissue deformation affecting areas outside of
the surgical area
of interest. Additionally, by matching overlay representations of tissue with
an actual view of
the tissue of interest, the particular tissue representation can be matched to
the video image,
thereby tending to ensure registration of the tissue of interest.
CA 02927381 2016-12-02
[00761Still referring to FIG. 3B and referring back to FIG. 3A, in the method
MI, for example,
matching overlay representations of tissue with an actual view of the tissue
of interest is
automatically performable by at least one of: (a) matching a video of a post
craniotomy brain,
e.g., an exposed brain, with an imaged sulcal map; (b) matching a video
position of exposed
vessels with image segmentation of vessels; (c) matching a video position of a
lesion or a
tumour with a image segmentation of a tumour; and (d) matching a video image
from an
endoscopy up-nasal cavity with a bone rendering of a bone surface on a nasal
cavity for an
endonasal alignment. The method M1 further comprises using multiple cameras
and
overlaying images with tracked instrument(s) views, thereby allowing multiple
views of the
data and overlayed images to be simultaneously presented, e.g., in real-time,
thereby improving
registration or correction.
[0077] Still referring to FIG. 3B and referring back to FIG. 3A, in the method
MI, completing
the planned trajectory, as indicated by block 324, comprises initiating
cannulation, wherein
cannulation comprises inserting a port (not shown) into the brain 102b,
typically along a sulci
path after identifying sulci, as indicated by block 320, along a planned
trajectory. Cannulation
is an iterative process that involves repeating the steps of aligning the port
on engagement and
setting the planned trajectory, as indicated by block 332, and then
cannulating to the target
depth, as indicated by block 334, until the planned trajectory is completed,
as indicated by
block 324 (FIG 4E).
[0078]Still referring to FIG. 3B and referring back to FIG. 3A, in the method
Ml, the iterative
cannulation process, as indicated by blocks 324, 332, 334, together, may also
support multi-
point trajectories where a target, e.g., a tumour, is accessible by pushing to
intermediate points,
then adjusting the angle to get to the next point in planned trajectory. This
process allows
trajectories to be redefined around tissue that one may want to preserve, or
ensure that the
trajectory stays within a sulcus to avoid damaging neighbouring tissue, e.g.,
healthy tissue.
Navigating multi-point trajectories may be accomplished by physically
reorienting a straight
port at different points along a (planned) path, or by having a flexible port
that has a number of
manipulable bends that can be set along the path.
21
CA 02927381 2016-12-02
[0079] Still referring to FIG. 3B and referring back to FIG. 3A, in the method
Ml,
decannulating, as indicated by block 326, comprises: removing the access port
206 and any
tracking instruments from the brain 102b; resecting by removing at least one
of a part of the
brain 102b and a tumour of interest, as indicated by block 328; and closing
the dura and closing
the cranium, thereby completing the craniotomy, as indicated by block 330. In
a further
embodiment of the present disclosure, the method MI, using the navigation
system 200, further
comprises at least one of imaging, re-imaging, and registering, by using
different modalities,
fiber structures of the brain, such as nerves, ligaments, etc., for intra-
operatively addressing
(avoiding) such fiber structures.
[0080] Referring to FIG. 4A, this screenshot illustrates at least one image I
of a brain 102b
renderable on a display device 205 during a step of positioning and fixing a
patient 102, as
indicated by block 304, in the method MI, as shown in FIGS. 3A and 3B, by way
of a
navigation system 200, in accordance with an embodiment of the present
disclosure. In FIG.
4A at least one image I are renderable during the step of positioning and
fixing the patient 102,
as indicated by block 304, wherein positioning is performed in response to
instructions from
the navigation software, and wherein positioning comprises at least one of
reviewing the
imported surgical plan, confirming whether a patient positioning is consistent
with craniotomy
needs, and selecting a planned trajectory from a list of planned trajectories
corresponding to the
imported surgical plan.
[0081] Referring to FIG. 4B, this screenshot illustrates at least one image I
of a brain 102b
renderable on a display device 205 during execution of an initiating
registration400 of a brain
102b, e.g., by using fiducial touch-points, in the method Ml, as shown in
FIGS. 3A and 3B, by
way of a navigation system 200, operable in response to instructions from
navigation software,
in accordance with an embodiment of the present disclosure.
[0082] Referring to FIG. 4C, this screenshot illustrates at least one image I
of a brain 102b
renderable on a display device 205 during a step of preparing and planning a
craniotomy, as
indicated by block 306, in the method Ml, as shown in FIGS. 3A and 3B, by way
of a
navigation system 200, operable in response to instructions from navigation
software, in
accordance with an embodiment of the present disclosure.
22
CA 02927381 2016-12-02
[0083] Referring to FIG. 4D, this screenshot illustrates at least one image I
of a brain 102b
renderable on a display device 205 during steps of confirming engagement and
motion range
within a cranial space, as indicated by block 318, and cutting a dura at the
engagement point
and identifying a sulcus, as indicated by block 320, in the method Ml, as
shown in FIGS. 3A
and 3B, by way of a navigation system 200, operable in response to
instructions from
navigation software, in accordance with an embodiment of the present
disclosure.
[0084] Referring to FIG. 4E, this screenshot illustrates at least one image I
of a brain 102b
renderable on a display device 205 during iterative cannulating steps, as
indicated by blocks
324, 332, 334, together, in the method Ml, as shown in FIGS. 3A and 3B, by way
of a
navigation system 200, operable in response to instructions from navigation
software, in
accordance with an embodiment of the present disclosure.
[0085] Referring to FIG. 5, this diagram illustrates a access port based
surgical procedure being
conducted by way of the navigation system 200 and methods, such as the method
Ml, in
accordance with some embodiments of the present disclosure. In this example, a
surgeon 501
is resecting a tumor from the brain 102b of a patient 502 through an access
port 506. An
external scope 505 is coupled with a robotic arm 504, and is used to view down
port 506 at a
sufficient magnification to allow for enhanced visibility down port 506. The
output of external
scope 505 is rendered on a visual display, such as the display device 205.
[0086] Still referring to FIG. 5, the method Ml, as shown in FIGS 3A and 3B
may further
comprise a step of quantitatively registering that least one image I by way of
the system 200,
wherein quantitatively registering comprises measuring at least one absolute
quantitative metric
and using that absolute quantitative metric to register images among a
plurality of imaging
modalities, thereby providing transformed imaging data. The at least one
absolute quantitative
metric comprises at least one of Ti, T2, cell density, tissue density, tissue
anisotropy, tissue
= stiffness, fluid flow per volume or area, electrical conductivity, pH,
and pressure. The method
M1 further comprises disposing active or passive fiduciary markers,
respectively, 507, 508,
e.g., spherical markers, in relation to at least one of the access port 506
and the external scope
505 for facilitating their tracking (location of these tools) by the tracking
system 213. The
active or passive fiduciary markers, 507, 508, are sensed by sensors of the
tracking system 213,
23
CA 02927381 2016-12-02
whereby identifiable points are provided. A tracked instrument is typically
indicated by a
sensing a grouping of active or passive fiduciary markers, 507, 508, whereby a
rigid body, such
as a tool, such as a tracking tool, is identified by the tracking system 213,
and whereby the
position and pose in 3D of a tracked instrument, such as a tool, is
determinable. Typically, a
minimum of 3 active or passive fiduciary markers, 507, 508, are placed on a
tracked tool to
define the instrument. In the several figures of the Drawing, four active or
passive fiduciary
markers, 507, 508, are used to track each tool, by example only.
[0087] Still referring to FIG. 5, in a preferred embodiment, the navigation
system 200 may
comprise fiduciary markers, the fiduciary markers comprising reflectosphere
markers in
combination with an optical tracking system to determine spatial positioning
of the surgical
instruments within the operating field. The spatial position of automated
mechanical arm(s) or
robotic arms used during surgery may be also tracked in a similar manner.
Differentiation of
the types of tools and targets and their corresponding virtual geometrically
accurate volumes
could be determined by the specific orientation of the reflectospheres
relative to one another
giving each virtual object an individual identity within the navigation
system. The individual
identifiers would relay information to the system as to the size and virtual
shape of the tool
within the system. The identifier could also provide information such as the
tools central point,
the tools central axis, etc. The virtual tool may also be determinable from a
database of tools
provided to the navigation system 200. The marker positions could be tracked
relative to an
object in the operating room such as the patient. Other types of markers that
could be used
would be RF, EM, LED (pulsed and un-pulsed), glass spheres, reflective
stickers, unique
structures and patterns, wherein the RF and EM would have specific signatures
for the specific
tools to which they would be attached. The reflective stickers, structures,
and patterns, glass
spheres, LEDs could all be detected using optical detectors, while RF and EM
could be
detected by using antennas. Advantages to using EM and RF tags would include
removal of
the line of sight condition during the operation, where using optical system
removes the
additional noise from electrical emission and detection systems.
[0088] Still referring to FIG. 5, in a further embodiment, printed or 3-D
design markers could
be used for detection by an auxiliary camera and / or external scope. The
printed markers could
also be used as a calibration pattern to provide distance information (3D) to
the optical detector.
24
CA 02927381 2016-12-02
These identification markers may include designs such as concentric circles
with different ring
spacing, and / or different types of bar codes. Furthermore, in addition to
using markers, the
contours of known objects (e.g., side of the port, top ring of the port, shaft
of pointer tool, etc.)
could be made recognizable by the optical imaging devices through the tracking
system 213.
[0089] Referring to FIG. 6A, this diagram illustrates, in a perspective view,
a patient reference
device D1 comprising a tracking tool, such as a pointing tool 600, in
accordance with an
embodiment of the present disclosure. The patient reference device D1 further
comprises a
tracking marker 610 disposed on a connector beam 615 attached to an arm 620 of
a pointing
=
tool 600. A minimum of three (3) tracking markers 610, and preferably four (4)
tracking
markers 610, facilitate tracking the device D by the tracking system 213.
[0090] Referring to FIG. 6B, this diagram illustrates, in a perspective view,
a patient reference
device D1 comprising a tracking tool 640, in accordance with an embodiment of
the present
disclosure. The tracking tool 640 is coupled with a supporting arm structure
642 to which four
= tracking markers 610 arc rigidly attached.
[0091] Referring to FIG. 6C, this diagram illustrates, in a perspective view,
a patient reference
device Dl comprising a tracking tool 650, in accordance with an embodiment of
the present
disclosure. The tracking tool 650 is coupled with a supporting arm structure
652 to which four
tracking markers 610 are rigidly attached.
= [0092] Referring to FIG. 6D, this diagram illustrates, in a perspective
view, a patient reference
device D1 comprising a tracking tool 660, in accordance with an embodiment of
the present
disclosure. The tracking tool 660 is coupled with a supporting arm structure
662 to which four
tracking markers 610 are rigidly attached.
[0093] Referring to FIG. 6E, this a diagram illustrates, in a perspective
view, a patient reference
device D2 comprising an access port 680, in accordance with an embodiment of
the present
disclosure. The patient reference device D2 further comprises "fiducial,"
fiducial marker, or
tracking markers 610 placed on an extended arm 682 that is firmly attached to
the access port
680. This arrangement enables clear visibility of the "fiducial," fiducial
marker, or tracking
markers 610 to the tracking system 213. Further, the extended arm 682 ensures
that the
CA 02927381 2016-12-02
"fiducial," fiducial marker, or tracking markers 610 do not interfere with
surgical tools that may
be inserted through the access port 680. The non-uniform structure of the
extended arm 682 for
the tracking markers 610 enables the tracking system 213 to discern both the
position and
orientation of the access port 680 in response to instructions corresponding
to the tracking
software.
[0094] Referring to FIG. 6F, this diagram illustrates, in a front view, a
patient reference device
D2 comprising an access port 680, as shown in FIG. 6E, in accordance with an
embodiment of
the present disclosure.
[0095] Referring to FIG. 6G, this diagram illustrates, in a side view, a
patient reference device
D2 comprising an access port 680, as shown in FIG. 6E, in accordance with an
embodiment of
the present disclosure.
[0096] Referring to FIG. 6H, this diagram illustrates, in a top view, a
patient reference device
D2 comprising an access port 680, as shown in FIG. 6E, in accordance with an
embodiment of
the present disclosure.
[0097] Referring to FIG. 7, this diagram illustrates, in a perspective view, a
patient reference
device DI_ comprising a tracking tool 650, such as a pointing tool, as shown
in FIG. 6C,
engaged with a patient reference device D2 comprising an access port 690, in
accordance with
an embodiment of the present disclosure. The patient reference device DI
comprises the
tracking tool 650, an associated support arm structure 652 (FIG. 6C) with
associated "fiducial,"
fiducial marker, or fiducial markers 610, inserted into a port 690 of the
patient tracking device
D2 further comprising "fiducials" or fiducial markers 692 on associate arm
support structure
694. Both the tracking tool 650 and the access port 690 are equipped with
respective arms 652,
694 configured with respective tracking markers 610, 692. These patient
reference devices D1,
1)2 with respective tracking markers 610, 692 are separately trackable by the
tracking system
213 of the navigation system 200 and are differentiable as unique objects in
images rendered on
the display device 205.
[0098] Referring to FIG. 8, this schematic diagram illustrates a relationship
between
components of the navigation system 200, such as a control and processing unit
400, a tracking
26
CA 02927381 2016-12-02
system 213, a data storage device 442 for the tracking system 213, and system
devices 420, and
medical instruments 460, in accordance with an embodiment of the present
disclosure. The
control and processing unit 400 comprises at least one processor 402, a memory
404, such as a
non-transitory memory device, a system bus 406, at least one input/output
interface 408, a
communications interface 410, and storage device 412. The control and
processing unit 400 is
interfaced with other external devices, such as the tracking system 213, data
storage 442 for the
tracking system 213, and external user input and output devices 444,
optionally comprising, for
example, at least one of a display device, such as display devices 211, 205, a
keyboard, a
mouse, a foot pedal, a microphone, and a speaker.
[0099] Still referring to FIG. 8, the data storage 442 comprises any suitable
data storage device,
such as a local or remote computing device, e.g. a computer, hard drive,
digital media device,
or server, having a database stored thereon. The data storage device 442
includes identification
data 450 for identifying at least one medical instrument 460 and configuration
data 452 for
associating customized configuration parameters with at least one medical
instrument 460. The
data storage device 442 further comprises at least one of preoperative image
data 454 and
medical procedure planning data 456. Although data storage device 442 is shown
as a single
device, understood is that, in other embodiments, the data storage device 442
comprises
multiple storage devices. The data storage device 442 is also configured to
store data in a
custom data structure corresponding to various 3D volumes at different
resolutions, each be
captured with a unique time-stamp and/or quality metric. This custom data
structure provides
the system 200 with an ability to move through contrast, scale, and time
during the surgical
procedure.
[0100] Still referring to FIG. 8, medical instruments 460 are identifiable by
the control and
processing unit 400, wherein the medical instruments 460 are coupled with, and
controlled by,
the control and processing unit 400. Alternatively, the medical instruments
460 are operable or
otherwise independently employable without the control and processing unit
400. The tracking
system 213 may be employed to track at least one of medical instruments 460
and spatially
register the at least one of medical instrument 460 in relation to an intra-
operative reference
frame. The control and processing unit 400 is also interfaceable with a number
of configurable
devices, and may intra-operatively reconfigure at least one such device based
on configuration
27
CA 02927381 2016-12-02
parameters obtained from configuration data 452. Examples of devices 420
include, but are not
limited to, at least one external imaging device 422, at least one
illumination device 424,
robotic arm 202, at least one projection devices 428, and at least one display
device, such as
display devices 211, 205.
[0101] Still referring to FIG. 8, the control and processing unit 400 is
operable by the at least
one processor 402 and the at least one memory 404. For example, the
functionalities described
herein are at least partially implemented via hardware logic in processor 402
by way of the
instructions stored in memory 404 though at least one processing engine 470.
Examples of
processing engines 470 include, but are not limited to, user interface engine
472, tracking
engine 474, motor controller 476, image processing engine 478, image
registration engine 480,
procedure planning engine 482, navigation engine 484, and context analysis
module 486.
Understood is that the system 200 is not intended to be limited to the
components shown in the
several figures of the Drawing. One or more components of the control and
processing 400
may be provided as an external component or device. In one alternative
embodiment,
navigation module 484 may be provided as an external navigation system that is
integrated with
control and processing unit 400.
[0102] Still referring to FIG. 8, embodiments of the system 200 may be
implemented using
processor 402 without additional instructions stored in memory 404.
Embodiments may also
be implemented using the instructions stored in the memory 404 for execution
by one or more
general purpose microprocessors. Thus, the disclosure is not limited to a
specific configuration
of hardware, firmware, and/or software. While some embodiments can be
implemented in fully
functioning computers and computer systems, various embodiments are capable of
being
distributed as a computing product in a variety of forms and are capable of
being applied
regardless of the particular type of machine or computer readable media used
to actually effect
the distribution. At least some aspects disclosed can be embodied, at least in
part, in software.
That is, the techniques may be carried out in a computer system or other data
processing system
in response to its processor, such as a microprocessor, executing sequences of
instructions
contained in a memory, such as ROM, volatile RAM, non-volatile memory, cache
or a remote
storage device. A computer readable storage medium can be used to store
software and data
which when executed by a data processing system causes the system to perform
various
28
CA 02927381 2016-12-02
methods. The executable software and data may be stored in various places
including for
example ROM, volatile RAM, nonvolatile memory and/or cache. Portions of this
software
and/or data may be stored in any one of these storage devices.
[0103] Still referring to FIG. 8, the preceding example embodiments involve
systems and
methods in which a device is intra-operatively configured based on the
identification of a
medical instrument. In other example embodiments, one or more devices may be
automatically
controlled and/or configured by determining one or more context measures
associated with a
medical procedure. A "context measure", as used herein, refers to an
identifier, data element,
parameter or other form of information that pertains to the current state of a
medical procedure.
In one example, a context measure may describe, identify, or be associated
with, the current
phase or step of the medical procedure. In another example, a context measure
may identity the
medical procedure, or the type of medical procedure, that is being performed.
In another
example, a context measure may identify the presence of a tissue type during a
medical
procedure. In another example, a context measure may identify the presence of
one or more
fluids, such as biological fluids or non-biological fluids (e.g. wash fluids)
during the medical
procedure, and may further identify the type of fluid. Each of these examples
relate to the
image-based identification of information pertaining to the context of the
medical procedure.
[0104] Still referring to FIG. 8, examples of computer-readable storage media
include, but are
not limited to, recordable and non-recordable type media such as volatile and
non-volatile
memory devices, read only memory (ROM), random access memory (RAM), flash
memory
devices, floppy and other removable disks, magnetic disk storage media,
optical storage media
(e.g., compact discs (CDs), digital versatile disks (DVDs), etc.), among
others. The instructions
can be embodied in digital and analog communication links for electrical,
optical, acoustical or
other forms of propagated signals, such as carrier waves, infrared signals,
digital signals, and
the like. The storage medium may be the internet cloud, or a computer readable
storage
medium such as a disc.
[0105] Still referring to FIG. 8, at least some of the methods described
herein are capable of
being distributed in a computer program product comprising a computer readable
medium that
bears computer usable instructions for execution by one or more processors, to
perform aspects
29
CA 02927381 2016-12-02
of the methods described. The medium may be provided in various forms such as,
but not
limited to, one or more diskettes, compact disks, tapes, chips, USB keys,
external hard drives,
wire-line transmissions, satellite transmissions, internet transmissions or
downloads, magnetic
and electronic storage media, digital and analog signals, and the like. The
computer useable
instructions may also be in various forms, including compiled and non-compiled
code.
[0106] Still referring to FIG. 8, the navigation system 200 provides tools to
the neurosurgeon
that will lead to the most informed, least damaging neurosurgical operations.
In addition to
port-based removal of brain tumours and intracranial hemorrhages (ICH), the
navigation
system 200 can also be applied to at least one of: (a) a brain biopsy, (b) a
functional/deep-brain
stimulation, (c) a catheter/shunt placement, (c) an open craniotomies, (d) an
endonasal/skull-
based/ENT, and (e) spine procedures.
[0107] Referring to FIG. 9, this schematic diagram illustrates a pre-operative
surgical planning
system 900 for use with a navigation system 200, in accordance with an
embodiment of the
present disclosure. The pre-operative surgical planning system 900 comprises
components and
inputs for planning and scoring surgical paths as disclosed herein and as
disclosed in at least
one priority document.
[0108] Referring to FIG. 10, this schematic diagram illustrates an intra-
operative surgical
management system 1000 for use with a navigation system 200, in accordance
with an
embodiment of the present disclosure. The intra-operative surgical management
system 1000
comprises components and inputs for navigation along the surgical paths
produced by the pre-
operative surgical planning system 900, as shown in FIG. 9. The intra-
operative surgical
management system 1000 can be used as a surgical planning and navigation tool
in the pre-
operative and intra-operative stages. Data input(s) of the surgical planning
steps and surgical
procedures, as shown in FIG. 9, can be used as input(s) to the intra-operative
navigation stage
performable by the intra-operative surgical management system 1000.
[0109] Still referring to FIG. 10, the intra-operative surgical management
system 1000 of the
navigation system 200 provides a user, such as a surgeon, with a unified
technique for
navigating through a surgical region by utilizing pre-operative data input(s)
and updated intra-
CA 02927381 2016-12-02
operative data input(s). The processor(s), such as the at least one processor
402, is operable by
way of a set of instructions 11 and/or algorithms storable in relation to a
non-transitory memory
device, such as the at least one memory 404, wherein the at least one
processor 402 is
configured to: analyze pre-operative data input(s) and intra-operative data
input(s) and update
surgical plans during the course of surgery accordingly.
[0110] Still referring to FIG. 10, for example, if intra-operative input(s) in
the form of newly
acquired images identified a previously unknown or unidentified nerve bundle
or a previously
unknown or unidentified fiber track, the at least one processor 402 can use
these intra-operative
input(s), if desired, for updating the surgical plan during surgery to avoid
contacting the nerve
bundle. The intra-operative input(s) may include a variety input(s), including
local data
gathered using a variety of sensor(s), such as at least one intra-operative
imaging sensor (not
shown). In some embodiments, the intra-operative surgical management system
1000 of the
navigation system 200 may provide continuously updated, e.g., in real-time,
intra-operative
input(s) in the context of a specific surgical procedure by way of the at
least one intra-operative
imaging sensor to: validate tissue position, update tissue imaging after tumor
resection, and
update surgical device position during surgery.
[0111] Still referring to FIG. 10, the intra-operative surgical management
system 1000 of the
navigation system 200 may provide for re-formatting of the image, for example,
to warn of
possible puncture of, or collision with, critical tissue structures with a
surgical tool during
surgery. In addition, the intra-operative surgical management system 1000 may
provide
imaging and input updates for any shifts or surgical errors that might occur
from a needle
deflection, tissue deflection, or patient movement as well as provide analysis
and
transformation of data to correct for imaging distortions, e.g., in real-time.
The magnitude of
these combined shifts or surgical errors is clinically significant and may
regularly exceed 2 cm.
Some the most significant are MRI based distortions such gradient non-
linearity, susceptibility
shifts, eddy current artifacts which may exceed lcm on standard MRI scanners
(1.5 T and 3.0 T
systems). The intra-operative surgical management system 1000 mitigates, and
may eliminate,
these combined shifts or surgical errors.
31
CA 02927381 2016-12-02
[0112] Still referring to FIG. 10, in accordance with embodiments of the
present disclosure, by
using the a intra-operative surgical management system 1000, a variety of
intra-operative
imaging techniques can be implemented to generate intra-operative input(s) by
way of a variety
of imaging devices, including anatomy specific MRI devices, surface array MRI
scans, endo-
nasal MRI devices, anatomy specific US scans, endo-nasal US scans, anatomy
specific CT or
PET scans, port-based or probe based photo-acoustic imaging, as well as
optical imaging done
with remote scanning, or probe based scanning, whereby multi-modal imaging and
data are
providable and transformable into useful images and data in real-time.
[0113] Referring to FIG. 11A, this flow diagram illustrates a method M2 of
performing a port-
based procedure by way of a navigation system 200, in accordance with an
alternative
embodiment of the present disclosure. The method M2 comprises initiating setup
in relation to
an OR and a patient 102, as indicated by block 1102, wherein initiating setup
comprises setting
each piece of relevant equipment, such as lights and surgical tools, in
relation to the navigation
system 200, and "preparing" and pinning, e.g., by a head clamp, the patient
102 in relation to
the headrest; registering a portion of a the patient's anatomy, such as a
patient's head 102a, as
indicated by block 1104, wherein registering comprises determining a pose of
the portion of a
the patient's anatomy, e.g., the patient's head 102a, in relation to a base
reference frame, and
correlating the location of the base reference frame in relation to the
imaging frame of
reference.
[0114] Still referring to FIG. 11A, the method M2 further comprises:
confirming the trajectory,
as indicated by block 1106, wherein confirming comprises positioning a port,
such as the
access port 206, is at an engagement point and displaying the trajectory on
the at least one
display device, such as the display devices 205, 211; determining whether a
surgical plan
requires adjustment, as indicated by block 1107, wherein determining comprises
confirming
that each piece of relevant equipment has a sufficient line of sight and reach
for the port-based
procedure; if an adjustment is required, adjusting the surgical plan based on
data comprising at
least one observable constraint in the OR, as indicated by block 1108, wherein
at least one of a
new engagement point and a new target point is defined; if an adjustment is
not required,
setting pre-incision, as indicated by block 1110, wherein setting pre-incision
comprises draping
the patient 102 and the relevant equipment and shaving and sterilizing a
surgical site of the
32
CA 02927381 2016-12-02
patient 102; and checking the registration and the trajectory for accuracy, as
indicated by block
1112.
101151 Still referring to FIG. 11A, the method M2 further comprises:
approaching the surgical
site, as indicated by block 1114, wherein approaching comprises commencing a
craniotomy by
forming a hole in a cranium of the patient head 102a, thereby forming a
cranial hole such as by
forming a burr-hole, and a bone portion, such as a cranial flap, testing a
range of motion of the
port, and intra-operatively adjusting the trajectory if required, forming an
opening in a dura,
thereby forming a dural flap, stitching-back the dural flap, inserting the
port, along the
trajectory via navigation guidance, such as provided on the at least one
display device, and
coaxially positioning a surgical camera, such as the optical camera 204, in
relation to the port.
[0116] Still referring to FIG. 11A, the method M2 further comprises: resecting
a target tissue,
e.g., immediately after the approaching step, as indicated by block 1116,
wherein resecting
comprises removing the target tissue, such as a tumour, using a surgical tool,
e.g., a NICO
Myriad tool, moving the port within constraints of the cranium hole, e.g., by
the surgeon
and/or robotics, for facilitating removal of all the target tissue, e.g., by
detecting all the target
tissue by using immunohistochemistry (ICH) techniques, re-positioning the
surgical camera as
required for viewing through the port, and cauterizing any tissue having
bleeding as required.
[0117] Still referring to FIG. 11A, the method M2 further comprises:
reconstructing the
surgical site, as indicated by block 1118, wherein reconstructing comprises
irrigating the
= surgical site through the port, slowly retracting the port while viewing
surgical site via the
surgical camera, coupling a graft to at least one portion of the surgical
site, e.g., using an
adhesive, such as a physiologically compatible glue, unstitching the dural
flap, stitching the
dural flap into its original position, and redisposing the bone flap into the
cranial hole, e.g., by
stapling the bone flap; and removing the head clamp; and recovering the
patient 102, as
indicated by block 1120, wherein recovering the patient 102 comprises sending
the patient 102
to a recovery area of a hospital, by example only, and, shortly thereafter,
sending the patient
102 home in the absence of any hemorrhage.
33
CA 02927381 2016-12-02
[0118] Still referring to FIG. 11A and ahead to FIG 11B, for a brain biopsy,
instead of
resecting, the method M2 comprises inserting a thin needle into a patient's
brain 102b,
removing a sample of brain tissue using the thin needle, assessing the sample
of brain tissue,
e.g., by a pathologist (human and/or robotic characterizing equipment) to
determine whether
the sample of brain tissue is cancerous (malignant). For a brain biopsy, the
method M2
optionally comprises using a stereotactic frame. While both types of
procedures, e.g., resection
and brain biopsy, are performed in the method M2 using image-guidance, the
navigation
system 200 is well-suited for handling frameless biopsies.
[0119] Referring to FIG 11B, this flow diagram illustrates a method M3 of
performing a
medical procedure, such as a frameless brain biopsy, by way of a navigation
system 200, in
accordance with an alternative embodiment of the present disclosure. The brain
biopsy surgical
procedure is very similar to a port-based surgical procedure (FIG. 11A) with
the exception that
the method M3 comprises: performing a biopsy, as indicated by block 1122,
reconstructing the
surgical site, as indicated by block 1124, and recovering the patient 102, as
indicated by block
1126, wherein such steps having different aspects. In the biopsy step (step
1122), a small hole
is drilled into the skull at the engagement point.
[0120] Still referring to FIG 11B, in the method M3, performing the biopsy, as
indicated by
block 1122, comprises guiding the biopsy needle through a hole, such as a
cranial hole, into the
brain 102b, and to the planned or relevant target tissue, tracking the biopsy
needle in real-time,
obtaining a biopsy sample, and disposing the biopsy sample in a container for
transportation to
a pathology laboratory. In the method M3, reconstructing the surgical site, as
indicated by
block 1124, and recovering the patient 102, as indicated by block 1126, have
shorter durations
than the corresponding steps in a resection for at least the reason that the
cranial hole is much
smaller. As noted above, the biopsy needle is also tracked continuously by the
navigation
system 200. In a further embodiment, the surgeon holds the biopsy needle, free-
hand, during
the procedure. In other embodiments, in the method M3, performing the biopsy,
as indicated
by block 1122, further comprises adhering a needle guide, e.g., to the skull
of the patient 102,
positioning and orienting the needle guide using the navigation system 200. If
the needle guide
comprises a depth-stop, continuous navigation for the biopsy needle may be
minimized or
eliminated.
34
CA 02927381 2016-12-02
[0121] Referring to FIG. 11C, this flow diagram illustrates a method M4 of
performing a
medical procedure, such as a frameless DBS, by way of a navigation system 200,
in accordance
with an alternative embodiment of the present disclosure. In a DBS procedure,
the method M4
comprises implanting an electrode, such as a small electrode, into a specific
area of the brain
102b for reduction of tremors from Parkinson's disease and dystonia, wherein
the electrode is
connected to a control device implantable elsewhere in the body of the patient
102, typically
near the clavicle. In the method M4. DBS is performable via a stereotactic
frame or frameless
technique; and the steps of the method M4 are similar to those of the method
M3, as shown in
FIG. 11C, and to the method M2, as shown in FIG. 11B, with the exception that
the method
M4 comprises: implanting an electrode, as indicated by block 1128, confirming
placement, as
indicated by block 1130, and implanting a control device, as indicated by
block 1132.
[0122] Still referring to FIG. 11C, in the method M4, implanting an electrode,
as indicated by
=
block 1128, comprises forming a small hole, e.g., by drilling, in the skull at
the engagement
point, positioning and orienting a guidance device on the skull using the
navigation system 200,
guiding the electrode through the guidance device into the brain 102b to the
planned target,
e.g., the target tissue, and tracking the electrode in real-time using the
navigation system 200.
In the method M4, confirming placement, as indicated by block 1130, comprising
at least one
of: listening to activity on the electrode; and performing a test stimulation
of an area of the
brain 102b via the electrode and observing a patient response.
[0123] Still referring to FIG. 11C, in the method M4, implanting a control
device, as indicated
by block 1132, comprises:
forming an incision at a location proximate a clavicle;
subcutaneously inserting a control device; attaching the control device to the
clavicle;
subcutaneously routing at least one lead from the electrode leads to the
control device. As in
the method M2 (FIG. 11A), the method M4 comprises: reconstructing the surgical
site, as
indicated by block 1118; and recovering the patient 102, as indicated by block
1120.
[0124] Referring to FIG. 11D, this flow diagram illustrates a method M5 of
performing a
medical procedure, such as a catheter/shunt placement, by way of a navigation
system 200, in
accordance with an alternative embodiment of the present disclosure. In
general, catheter or
shunt placement is assisted by the navigation system 200. Shunts or catheters
are inserted into
CA 02927381 2016-12-02
the brain cavity to treat patients with hydrocephalus. Cranial pressure is too
great in these
patients as a result of excessive cerebral spinal fluid (CS F). A shunt or
catheter is introduced
under image guidance of the navigation system 200; and the excess CSF is
drained into another
part of the body for reabsorption.
[0125] Still referring to FIG. 11D, a method M5 comprises steps that are
similar to the method
M3 (FIG. 11B) with the replacement of performing the biopsy, as indicated by
block 1122,
placing a shunt or catheter, as indicated by block 1134, wherein placing a
shunt, as indicated by
block 1134, comprises forming a small hole, e.g., by drilling, in the skull at
the engagement
point, positioning and orienting a guidance device on the skull using the
navigation system 200,
guiding the shunt or catheter through the guidance device into the brain 102h
to the planned
target, e.g., the target tissue, and tracking the shunt or catheter in real-
time using the navigation
system 200.
[0126] Referring back to FIGS. 1-11D, in an embodiment, during a surgical
procedure, such as
a port-base procedure, brain displacement or deformation can be predicted
(modeled) with
accurate simulation, using information, such as a priori tissue stiffness
information, geometric
information relating to the introducer and port, a biomechanical model of
tissue deformation,
(using the skull as a boundary condition) and using pre-operative imaging
data. This model is
updateable by using real-time imaging information as the introducer is
positioned inside of the
head, and more accurately, by real-time imaging being performed using data
obtained via the
in-situ port for obtaining and updating intra-operative data. For instance,
real-time ultrasound
imaging, being performed on the tip of the port, can detect tissue stiffness
inside the brain.
This information is useable instead of the a priori predicted stiffness and
can provide a better
estimate of tissue movement. In addition, ultrasound can be used to identify
sulci patterns as
the port is being introduced. These sulci patterns can be matched to the pre-
operative sulcus
patterns; and a deformed pre-operative model can be generated based on this
information.
[0127] Referring back to FIGS. 1-11D, in this iterative manner, the model will
be updated by
the system according to information obtained during the procedure to provide
for accurate
representations of the tumor location, e.g., modeling of tumor roll within the
brain and
measurement of the total stress and strain on nerve fibers as the port is
inserted into the brain.
36
CA 02927381 2016-12-02
This information may be represented by the system as a global value; and, as
with the
weighting of the hierarchy of the fibers, the actual strain of the fibers may
be used to calculate a
value associated with the invasiveness of a surgical approach.
[01281 Referring back to FIGS. 1-11D, a discrepancy may exist among the pre-
operative
imaging data and the real-time port information (US, OCT, photo acoustic,
optical). This
discrepancy can be measured by matching sulci patterns, blood vessel
positions, or by
quantifiable common contrast mechanisms such as elastic modulus, tissue
anisotropy, blood-
flow, etc. The real-time port information is expected to represent accurate
information; and,
when a significant discrepancy is found, a scan is performed for updating the
volumetric MRI
and/or CT scans to update the pre-operative, or intra-operative, scanning
volume. In the optimal
configuration, an MRI port coil would be used in conjunction with an external
MRI system to
acquire a 3D volume demonstrating sulci path, tumor, nerve fascicles by way of
diffusion tensor
imaging (DTI) acquisition, and blood vessels. As the acquisition time is
typically much longer
than US, OCT or photo-acoustic imaging, a real-time modality is not expected
to be used;
however, it can be effectively utilized as a single modality to position the
access port with
pseudo-real time capability (typically not faster than lfps). Future
availability of faster
acquisition technologies may provide improved real-time DTI information using
a port coil and
is encompassed by the present disclosure.
[0129] Referring to FIG. 12, this diagram illustrates various elements of a
display D renderable
on at least one display device, such as the display devices 205, 211, by way
of a navigation
system 200 using an trajectory alignment system, in accordance with an
embodiment of the
present disclosure. The display D comprises at least one of an interactive
navigation window
W, at least one real-time navigation image I', a dashboard DB, and a sidebar
SB (FIGS. 13 and
14). The interactive navigation window W displays information corresponding to
a current
stage, e.g., an "approach" stage, of a therapeutic procedure, such as a
medical procedure and a
surgical procedure, and comprises at least one feature for interactively
confirming, revising,
and updating trajectory information. The real-time navigation image I
comprises a real-time
neural image NI and at least one indicia, such as textual navigation
information 120, a
navigation symbol S, e.g., a generally circular symbol, and an alignment
symbol A, the
alignment symbol A comprising a crosshair symbol CH and a generally circular
boundary or a
37
CA 02927381 2016-12-02
broken generally circular boundary CB, the crosshair symbol CH in movable
relation to the
generally circular boundary or a broken generally circular boundary CB.
[0130] Still referring to FIG. 12, the navigation symbol S is rendered at a
location relative to
the real-time neural image NI, the location of the navigation symbol S
corresponding to at least
one of a planned trajectory and an updated trajectory. The alignment symbol A
is rendered at a
location relative to the real-time neural image NI, the location of the
alignment symbol A
corresponding to real-time data corresponding to movement of a tracked or
tracking tool (not
shown), such as an access port, a pointer tool, a surgical tool, a stimulation
tool, and the like.
The navigation symbol S and the alignment symbol A are renderable as elements
overlaying
the real-time neural image NI, together, provide real-time feedback regarding
alignment of the
tracked tool in relation to a planned trajectory or an updated trajectory for
facilitating neural
navigation, whereby real-time alignment data is achievable, and whereby at
least one
neurological structure is preservable.
[0131] Still referring to FIG. 12, the navigation symbol S and the alignment
symbol A, each
comprise a color-coding feature for enhancing neural navigation. By example
only, the
navigation symbol S comprises a red color for indicating that the tracked tool
is not aligned and
a green color for indicating that the tracked tool is aligned. The generally
circular boundary or
a broken generally circular boundary CB of the alignment symbol A comprises a
white "color"
(or an absence of color) for indicating the tracked tool is outside a
predetermined, or
interactively set, proximity threshold in relation to the planned, or updated,
trajectory and the
crosshair symbol CH of the alignment symbol A comprises a yellow color for
indicating that
the tracked tool is near, or inside, a predetermined, or interactively set,
proximity threshold in
relation to the planned, or updated, trajectory. For example, when the system
200, using an
trajectory alignment system, determines that the tracked tool is aligned
within a predetermined,
or interactively set, proximity threshold in relation to the planned, or
updated, trajectory both
the crosshair symbol CH and the generally circular boundary or a broken
generally circular
boundary CB (turns from white to yellow) of the alignment symbol A comprise a
yellow color;
and the navigation symbol S comprises a green color, wherein the alignment
symbol A is
disposed within the navigation symbol S.
38
CA 02927381 2016-12-02
[0132] Still referring to FIG. 12, by example only, the color green indicates
that the tracked or
tracking tool is on the planned trajectory; and the color yellow indicates
that the tracked or
tracking tool has reached the target, such the relevant target tissue, e.g., a
tumour. If a surgeon
is using a port tool or an access port, the sheath is advanced by the virtual
tip distance for
positioning an opening of the sheath at the location of the target. When the
sheath has reached
the target, the sheath is secured, such as by a "Shepherd's Hook;" and the
obturator is removed.
By example only, a red color indicates that the tracked tool is off the
planned trajectory or is
past the target.
[0133] Still referring to FIG. 12, by example only, the textual navigation
information 120
comprises at least one of planned trajectory information, updated trajectory
information,
tracked tool identification information, and tracked tool location
information, e.g., interactive
data relating to a distance between a distal end of the tracked or tracking
tool and a target, such
as a target tissue. Further, such textual information is also renderable by
the system 200, using
a trajectory alignment system, on the at least one display device, such as the
display devices
205, 211 via the interactive navigation window W. The window W also comprises
interactive
features, such as buttons for moving forward and backward in the therapeutic
procedure,
buttons for loading images and/or information from at least one database, and
a dropdown
menu for selecting a tool for tracking, by example only.
[0134] Still referring to FIG. 12, by using a pointer for aligning a tool to a
trajectory, the
alignment system facilitates alignment of an access port without the need for
a tracked sheath,
sup[ports alignment of compatible miniframes, is available for use with a
laptop computer, is
configured to reconcile patient identification and name in a "merge" series,
and facilitates
changing a port length and to eliminate the need for port verification, in
accordance with an
alternative embodiment of the present disclosure. The alignment system
facilitates performing
an "approach" by using a pointer, provides a graphic feature whereby alignment
becomes
intuitive, and displays a trajectory length in the approach.
[0135] Referring to FIG. 13A, this screenshot illustrates a display D
renderable on at least one
display device, such as the display devices 205, 211, by way of a navigation
system 200 using
an trajectory alignment system, in accordance with an embodiment of the
present disclosure.
39
CA 02927381 2016-12-02
The display D comprises at least one of an interactive navigation window W, at
least one real-
time navigation image I', a dashboard DB, and a sidebar SB. The interactive
navigation
window W displays information corresponding to a current stage, e.g., an
"approach" stage, of
a therapeutic procedure, such as a medical procedure and a surgical procedure,
and comprises
at least one feature for interactively confirming, revising, and updating
trajectory information.
The real-time navigation image I' comprises a real-time neural image NI and at
least one
indicia, such as textual navigation information 120, a navigation symbol S,
e.g., a generally
circular symbol, and an alignment symbol A, the alignment symbol A comprising
a crosshair
symbol CH and a generally circular boundary or a broken generally circular
boundary CB, the
crosshair symbol CH in movable relation to the generally circular boundary or
a broken
generally circular boundary CB.
L01361 Still referring to FIG. 13A, a system, such as the navigation system
200, e.g., as an
alignment system, for aligning a tool, such as the access port 206, in
relation to a trajectory in
real-time comprises: a processor, such as the processor 402, configurable by a
set of executable
instructions storable in relation to a non-transitory memory device. such as
the memory 404, to:
receive input data from at least one source, such as the devices 420, the
tracking system 213, and
the external devices 444, of at least one pre-operative plan image, at least
one multi-modal
image, and at least one real-time multi-modal image; interactively track at
least one neural fiber,
whereby interactively tracked neural fiber data is obtained; automatically
generate output data by
way of data transformation using the input data and the interactively tracked
neural fiber data;
and transmit the output data to at least one of: at least one display device
205, 211, for rendering
at least one real-time interactive navigation display D for facilitating
neural navigation, and at
least one drive device for positioning at least one tracking device in
relation to the tool in real-
time, whereby real-time alignment data is achieved, and whereby at least one
neurological
structure is preserved, in accordance with an embodiment of the present
disclosure.
[0137] Still referring to FIG. 13A, the at least one indicia further comprises
a tracked tool
indicia 130, wherein the tracked tool indicia 130 comprise a color-coding
feature for enhancing
neural navigation. The color-coding feature comprises a blue color, by example
only. Each
real-time neural image NI comprises a distinct color coding feature, such as
in the
representation of bone or boney structures, wherein a distinct color is
assignable for
CA 02927381 2016-12-02
representing a particular cross-section of a patient's anatomy. In an
"approach" stage of phase,
the display D shows information about the trajectory created for this
procedure, such as a
planned trajectory, and the location of at least one tracked tool rendered as
the tracked tool
indicia 130. The window W, corresponding to an "approach" phasc, is available
if a planned
trajectory exists for the given procedure. For example, a planned trajectory
is creatable by
using the BrightMatter Plan or during the "targeting" phase.
[0138] Referring to FIG. 13B, this flow diagram illustrates a method M6 of
fabricating a
navigation system, such as the system 200, e.g., as an alignment system, for
aligning a tool, such
as an access port 206, in relation to a trajectory in real-time comprises:
providing a processor,
such as the processor 402, configurable by a set of executable instructions
storable in relation to
a non-transitory memory device, such as the memory 404, as indicated by block
1310, to:
receive input data from at least one source, such as the devices 420, the
tracking system 213, and
the external devices 444, of at least one pre-operative plan image, at least
one multi-modal
image, and at least one real-time multi-modal image, as indicated by block
1311; interactively
track at least one neural fiber, whereby interactively tracked fiber data is
obtained, as indicated
by block 1312; automatically generate output data by way of data
transformation using the input
data and the interactively tracked neural fiber data, as indicated by block
1313; and transmit the
output data to at least one display device, such as the display devices 205,
211, for rendering at
least one real-time interactive navigation display D for facilitating neural
navigation, whereby
real-time alignment data is achieved, and whereby at least one neurological
structure is
preserved, as indicated by block 1314, in accordance with an embodiment of the
present
disclosure.
[0139] Referring to FIG. 13C, this flow diagram illustrates a method M7 of
aligning a tool in
relation to a trajectory during an approach phase of a surgical procedure by
way of an alignment
system, in accordance with an embodiment of the present disclosure. The method
M7 comprises
evaluating an approach by determining whether a planned engagement point is
appropriate, as
indicated by block 1301; if the planned engagement point is appropriate,
proceeding to engage
the tool at the planned engagement point, as indicated by block 1302; if the
planned engagement
point is not, or no longer, appropriate, e.g., by changed circumstances,
interactively specifying a
new engagement point, such as by at least one interactive feature of the
window W, as indicated
41
CA 02927381 2016-12-02
by block 1303; and returning to evaluating an approach by determining whether
a planned
engagement point is appropriate, as indicated by block 1301.
[0140] Still referring to FIG. 13C, a method of aligning a tool in relation to
a trajectory in real-
time by way of an alignment system comprises: providing the alignment system,
as indicated by
block 1300, the alignment system providing comprising providing a processor,
such as the
processor 402, configurable by a set of executable instructions storable in
relation to a non-
transitory memory device, such as the memory 404, to: receive input data from
at least one
source, such as the devices 420, the tracking system 213, and the external
devices 444, of at least
one pre-operative plan image, at least one multi-modal image, and at least one
real-time multi-
modal image; interactively track at least one neural fiber, whereby
interactively tracked fiber
data is obtainable; automatically generate output data by way of data
transformation using the
input data and the interactively tracked neural fiber data; and transmit the
output data to at least
one of: at least one display device 205, 211, for rendering at least one real-
time interactive
navigation display D for facilitating neural navigation, and at least one
drive device for
positioning at least one tracking device in relation to the tool in real-time,
whereby real-time
alignment data is achievable, and whereby at least one neurological structure
is preservable;
calibrating the tool by using a calibration block; if performing a port
procedure, verifying a port;
= evaluating an approach by determining whether a planned engagement point
is appropriate using
the at least one real-time interactive navigation display of the alignment
system; if the planned
engagement point is appropriate, performing the approach; if the planned
engagement point is
inappropriate, interactively setting a new engagement point by way of at least
one interactive
feature of the alignment system; and optionally returning to the evaluating
step, in accordance
with another embodiment of the present disclosure.
= [0141] Still referring to FIG. 13C, the method M7 further comprises
calibrating a tool, such as a
sterile pointer tool, as indicated by block 1304, wherein calibrating the
pointer tool comprises:
clicking a pointer tool icon 131 on the left side, such as on the sidebar SB,
of the screen, such as
the display D; positioning the pointer tool in a sterile calibration block by
following a set of on-
screen instructions; and orienting the pointer tool and the calibration block,
wherein all marker,
such as all eight reflective markers, are visible to a tracking camera, such
as the optical camera
204.
42
CA 02927381 2016-12-02
[0142] Still referring to FIG. 13C, the method M7 further comprises verifying
a port for
performing a port procedure, as indicated by block 1305. Before using the
system 200 to guide
the approach for a port procedure, by example only, a Synaptive tracking
array is assembled
in relation to the NICO BrainPath device and verified by using the
calibration block. In the
method M6, verifying the port comprises: (a) if a tool other than the planned
tool is needed,
selecting the tool from the drop-down list, such as via the window W; (b)
positioning the
assembled BrainPath' device and the Synaptive tracking array in a sterile
calibration block by
following a set of on-screen instructions; and (c) orienting the tool and the
calibration block so
that all eight reflective markers are visible to the tracking camera. If a
port tool verification
attempt fails, the system 200, using a trajectory alignment system discards
any previous port
tool verifications. For example, if a 50 mm tool is verified, and a 60 mm tool
has an attempted
and failed verification, only the 50 mm tool is useable another tool is
properly verified.
[0143] Still referring to FIG. 13C, the method M7 further comprises working in
the approach
phase, as indicated by block 1306, wherein working in the approach phase
comprises: (a)
positioning the tool tip at the engagement point, wherein a tool tip is
indicated by the crosshair
symbol CH of the alignment symbol A, and wherein a target point is indicated
by the generally
circular boundary or the broken generally circular boundary CB in a yellow
color of the
alignment symbol A; and (b) when the crosshair symbol CH is positioned in the
generally
circular boundary or the broken generally circular boundary CB and the
navigation symbol S or
"tool graphic" is green, positioning the tool and aligning the tool to
correctly to reach the
target; and (c) orienting the tool and calibration block so that all eight
reflective markers are
visible to the tracking camera. For example, if a 50 mm tool is verified and a
60 mm tool has
an attempted and failed verification, only the 50 mm tool is useable another
tool is properly
verified at this stage as well. The viewport shows a target-centric view of
the tool's distance
from the target. By keeping the tool graphic concentric with the yellow broken
circle, the tool
stays on the planned trajectory, wherein a "distance to target" notification
text in the viewport.
This text changes color to indicate the status of the approach, wherein green
indicates that the
tool is on the planned trajectory, yellow indicates that the tool tip has
reached the target, and
red indicates that the Tool is off the planned trajectory or is past the
target. If performing a port
procedure, switching between tracking the BrainPath device and the pointer
tool is possible by
43
CA 02927381 2016-12-02
selecting another tool from the drop-down list in the phase panel of the
window W. If an open
craniotomy procedure is being performed, only the pointer tool is available.
[0144] Still referring to FIG. 13C, in executing the method M7, the system 200
further uses a
virtual tip feature in the approach phase, as indicated by block 1307, wherein
the length of the
sheath of the BrainPath device is used as the length of the BrainPath tool.
The distance the
obturator extends past the opening of the sheath is indicated by the virtual
tip. In the approach
phase, the "distance to target" indicated in the viewports is the distance
between the target and
the virtual tip of the BrainPatV tool. To position the opening of the sheath
at the target, the
virtual tip length must be set to the distance that the obturator extends past
the opening of the
sheath. The system 200, using a trajectory alignment system, automatically
sets the distance
that the obturator extends past the opening of the sheath based on the
selected tool for the
procedure. If the opening of the sheath at the target is repositioned, for
example to view the
tractography beyond the tip of the tool, resetting the "distance to target" to
the default is
required before completing the approach phase. When using the BrainPath''
tool, once the
sheath is advanced to the target and secured with the Shepherd's Hook, the
virtual tip length for
the BrainPath tool is settable to 0 mm so that the viewports display the
slices (anatomical
cross-section images) at the opening of the sheath rather than those ahead of
the tool by the
virtual tip length.
[0145] Still referring to FIG. 13C, in the method M7, setting a new engagement
point
comprises: (a) moving the tip of a tracked tool to a new location
corresponding to a new
engagement point; (b) clicking an interactive feature, comprising a relocating
engagement
feature in the phase panel, such as the window W; (c) reviewing at least one
of the original or
planned craniotomy location, the original or planned craniotomy size, and the
original or
planned tool, in light of the newly set engagement point; and, (d) if
necessary, adjusting at
least one of the original or planned craniotomy location, the original or
planned craniotomy
size, and the original or planned tool.
[0146] Referring to FIG. 14, this screenshot illustrates a display D
renderable on at least one
display device, such as the display devices 205, 211, by way of a navigation
system 200 using
an trajectory alignment system, in accordance with an embodiment of the
present disclosure.
44
CA 02927381 2016-12-02
The display D comprises at least one of an interactive navigation window W, at
least one real-
time navigation image I', a dashboard DB, and a sidebar SB. The interactive
navigation
window W displays information corresponding to a current stage, e.g., a
"resection" stage, of a
therapeutic procedure, such as a medical procedure and a surgical procedure,
and comprises at
least one feature for interactively confirming, revising, and updating
trajectory information.
The real-time navigation image I' comprises a real-time neural image NI and at
least one
indicia, such as the tracked tool indicia 130. The viewports, such as the at
least one real-time
navigation image I', in the resection phase or stage facilitate orienting the
anatomy to the at
least one plan image. One viewport shows the plan images from the perspective
of the
BrainPath sheath at its current position. The other viewports show orthogonal
views of the
plan images. When the pointer tool is in the tracking camera's field of view,
its image appears
in the viewports. The virtual tip length in the resection phase is set to 0 mm
by default in this
phase. To show the tractography ahead of the tool in the viewports, clicking
the up arrow
increases the virtual tip length by a desired amount.
[0147] While the present disclosure describes various embodiments for
illustrative purposes,
such description is not intended to be limited to such embodiments. On the
contrary, the
applicant's teachings described and illustrated herein encompass various
alternatives,
modifications, and equivalents, without departing from the embodiments, the
general scope of
which is defined in the appended claims. Except to the extent necessary or
inherent in the
processes themselves, no particular order to steps or stages of methods or
processes described
in this disclosure is intended or implied. In many cases the order of process
steps may be
varied without changing the purpose, effect, or import of the methods
described.
[0148] Information as herein shown and described in detail is fully capable of
attaining the
above-described object of the present disclosure, the presently preferred
embodiment of the
present disclosure, and is, thus, representative of the subject matter which
is broadly
contemplated by the present disclosure. The scope of the present disclosure
fully encompasses
other embodiments which may become obvious to those skilled in the art, and is
to be limited,
accordingly, by nothing other than the appended claims, wherein any reference
to an element
being made in the singular is not intended to mean one and only one unless
explicitly so
stated, but rather "one or more." All structural and functional equivalents to
the elements of
CA 02927381 2016-12-02
the above-described preferred embodiment and additional embodiments as
regarded by those of
ordinary skill in the art are hereby expressly incorporated by reference and
are intended to be
encompassed by the present claims. Moreover, no requirement exists for a
system or method to
address each and every problem sought to be resolved by the present
disclosure, for such to be
encompassed by the present claims. Furthermore, no element, component, or
method step in
the present disclosure is intended to be dedicated to the public regardless of
whether the
element, component, or method step is explicitly recited in the claims.
However, that various
changes and modifications in form, material, work-piece, and fabrication
material detail may be
made, without departing from the spirit and scope of the present disclosure,
as set forth in the
appended claims, as may be apparent to those of ordinary skill in the art, are
also encompassed by
the present disclosure.
INDUSTRIAL APPLICABILITY
[0149] The subject matter of the present disclosure industrially applies to
the field of image
guided medical procedures. More
particularly, subject matter of the present disclosure
industrially applies to the field of patient reference tools for rapid
registration in relation to
image guided medical procedures. Even more particularly, subject matter of the
present
disclosure industrially applies to the field of assisting patient reference
tools for rapid registration
in relation to image guided medical procedures.
46