Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
1
SMOKING COMPOSITION COMPRISING FLAVOUR PRECURSOR
This disclosure relates to smoking compositions having enhanced sensory
components, and articles and methods related to such smoking compositions.
Smoking articles that heat tobacco or other sources of nicotine, without
combustion
of the tobacco or nicotine source, have been proposed. Such articles generate
aerosols that
can deliver nicotine and other tobacco constituents to a user. However, a user
may perceive
significantly different flavour notes from those experienced with conventional
smoking
articles such as cigarettes.
Accordingly, it may be desirable to incorporate flavour compounds into tobacco
or
other sources of nicotine that are used in non-combustion smoking articles to
better
approximate an experience associated with smoking a conventional cigarette. A
variety of
flavour compounds are known for addition to tobacco products. However, the
volatility of
such compounds presents challenges for incorporating the compounds into
tobacco or
smoking articles. For example, losses may occur during production or storage
due to the
volatility of the compounds.
As described herein, smoking articles, in which the tobacco or other source of
nicotine is heated but not combusted, deliver to a user an aerosol containing
a flavour to
provide the user with an experience similar to that of smoking a conventional
cigarette. The
articles contain a flavour composition that includes a flavour precursor,
which does not have
a significant odour. The flavour precursor converts into one or more flavour
compounds or
intermediates for flavour compounds upon heating to produce flavour notes that
provide the
composition with a profile more closely resembling that of conventional
cigarettes.
Smoking articles described herein may provide one or more advantages. For
example, the smoking articles described herein provide an enhanced flavour
experience
relative to smoking articles that do not include a flavour precursor. Upon
heating, the flavour
precursors described herein release flavour compounds or intermediates of
flavour
compounds comprising a thiol group. Thiol-containing flavour compounds or
intermediates,
such as hydrogen sulfide, methanethiol, and furfurylthiol, are more volatile
than the flavour
precursor compounds. Because the flavour precursors are not volatile or are
less volatile
than the flavour compounds, losses during production may be mitigated and the
articles may
have a longer shelf life. Further, because the flavour precursor compounds are
less
odourous than the flavour compounds, the article does not impart substantial
sulfur notes
when it is not in use.
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
2
As used herein, "thiol" means a compound of the formula RSH, where R is H or
an
organic group.
As used herein, "thiol-containing flavour compound" and "flavour compound"
mean a
thiol-containing flavour compound that is released from the flavour precursor
compound or
that is generated from the reaction of a thiol-containing intermediate with an
aerosol forming
substrate.
As used herein, "thiol-containing intermediate" and "intermediate" mean any
thiol-
containing compound that is released from the flavour precursor compound and
that can
react with an aerosol forming substrate, such as tobacco or other nicotine
source to
generate a thiol-containing flavour compound.
The thiol-containing flavour compound or intermediate is a compound that is
more
volatile than the flavour precursor compound. Preferably, the flavour compound
has flavour
properties that enhance the experience of non-combustible smoking articles to,
for example,
provide an experience more similar to that resulting from smoking a
combustible smoking
article. For instance, the flavour compound can enhance flavour properties
such as
complexity and mouth fullness. Complexity is generally known as the overall
balance of the
flavour being richer without dominating single sensory attributes. Mouth
fullness is
described as perception of richness and volume in the mouth and throat of the
smoke. The
released or generated thiol-containing flavour compounds may provide one or
more of these
or other flavour properties. In embodiments, the released or generated
thiol-containing
flavour compound is selected from the group consisting of hydrogen sulfide,
methanethiol, 3-
methy1-2-butene-1-thiol and furfurylthiol. Hydrogen sulphide and methanethiol
are
particularly preferred for their generation of flavour notes particularly
associated with tobacco
smoke.
Any suitable test may be employed to determine whether the thiol-containing
flavour
compound produces a flavour component that is typically perceptible with
smoking of
cigarettes. For example, a compound that invokes a subjective perception of a
flavour note,
associated with smoking of cigarettes would be considered to have or to
produce a flavour
for purposes of the present disclosure. In embodiments, trained flavourists
may determine
whether a flavour compound is released or generated on heating a flavour
precursor
compound, such as in the presence of an aerosol forming compound during
smoking of a
non-combustible smoking article. In embodiments, randomly or non-randomly
selected
people may be chosen to determine whether they perceive the compound to
produce a
flavour. By way of example, if 25% or more, e.g., 50% or more, 60% or more,
70% or more,
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
3
or 80% or more of the selected people perceive a flavour, the compound will be
determined
to be flavour producing.
Any suitable sulfur-containing flavour precursor compound may be employed to
release a thiol-containing flavour compound or intermediate. In embodiments,
the flavour
precursor compound has a structure of Formula I, as follows:
R2 9
HN
R1
1 CH2 I
,S
R4 R3 (I),
where:
R1 is OH or an amino acid residue,
R2 is H, C(0)CH3, or an amino acid residue,
R3 is H or C1-C3 unsubstituted alkyl,
R4 is optional and, if present, is C1-C3 unsubstituted alkyl, and
x is an integer from 1 to 3.
R1 is preferably OH or a glycine residue. R2 is preferably H, C(0)CH3, or a
glutamate
residue. R3 is preferably H or CH3. R4 is preferably CH3, if present, to
produce a positively
charged sulfur species. Preferably, R4 is not present. X is preferably 1 or 2.
In embodiments, the flavour precursor compound has a structure of Formula II,
as
follows:
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
4
R2 0
HN R1
( CH2
\ R3 (II)
where:
R1 is OH or an amino acid residue,
R2 is H, C(0)CH3, or an amino acid residue,
R3 is H, C1-C3 unsubstituted alkyl or an amino acid residue, and
x is an integer from 1 to 3.
R1 is preferably OH or a glycine residue. R2 is preferably H, C(0)CH3, or a
glutamate
residue. R3 is preferably H, CH3 or a cysteine residue. X is preferably 1 or
2.
In embodiments, the flavour precursor compound has a structure of Formula III,
as
follows:
R2 0
HN
I CH2 lx
SH (III)
where:
R1 is OH or an amino acid residue,
R2 is H, C(0)CH3, or an amino acid residue, and
x is an integer from 1 to 3.
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
R1 is preferably OH or a glycine residue. R2 is preferably H, C(0)CH3 or a
glutamate
residue. X is preferably 1 or 2.
In embodiments, the flavour precursor compound has a structure of Formula IV,
as
follows:
R2 0
HN
R1
\SH
5 (IV)
where:
R1 is OH or an amino acid residue, and
R2 is H, C(0)CH3, or an amino acid residue.
R1 is preferably OH or a glycine residue. R2 is preferably H, C(0)CH3, or a
glutamate
residue.
It will be understood that, with regard to Formulas I-IV presented above, that
an
amino acid residue may be bound to the core structure via a peptide bond or
via a suitable
side chain, such as a carboxylic acid group.
In embodiments, the flavour precursor compound is a compound selected from the
group consisting of cysteine (CAS No. 3374-33-9), cysteine (CAS No. 56-89-3),
glutathione
(CAS No. 70-18-8), methionine (CAS No. 59-51-8), DL methionine methylsulfonium
(CAS
No. 582174), N-acetyl cysteine (CAS No. 616-91-1), S-methyl cysteine (CAS No.
1187-84-
4), DL-homocyceteine (CAS No. 454-29-5), N-acetyl methionine (CAS No. 65-82-
7),
Farnesyl-Met-Ome (CAS No. 218962-72-2), albumin (CAS No. 9006-59-1) and 2-
hydroxy-4-
(methylthio)butyric acid (CAS No. 922-50-9).
Disclosure of a compound herein, whether by name, structure, reference number
or
the like, refers to the particular disclosed compound, as well as suitable
salts, polymorphs,
isomers, etc. thereof.
In embodiments, the flavour precursor compound is a compound comprising a
cysteine residue. Examples of such compounds include cysteine, glutathione, N-
acetyl
cysteine, S-methyl cysteine, and cysteine.
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
6
Preferably, the flavour precursor compound is cysteine or glutathione. More
preferably, the flavour precursor compound is cysteine.
As used herein, a flavour composition is a composition comprising a flavour
precursor compound and a further compound typically used in flavour
compositions, for
example. In particular, the composition may comprise one or more flavour
compounds
known in the art, which include but are not limited to, menthol, spearmint,
peppermint,
eucalyptus, vanilla, cocoa, chocolate, coffee, tea, spices (such as cinnamon,
clove and
ginger), fruit extracts, and combinations thereof. In embodiments, the flavour
composition
further comprises menthol or eugenol. Such flavourants are commonly used to
provide a
refreshing flavour to the smoke of a smoking article.The composition may also
comprise
adjuvants that allow the composition to meet technical requirements, such as
stability or
tonality persistence.
A delivery system may be provided, which encapsulates a flavour composition or
flavour precursor compound. The
delivery system protects the flavour composition or
flavour precursor compound during manufacture and upon storage.
In embodiments, a delivery system may take any suitable form which is capable
of
retaining the flavour composition or flavour precursor compound within the
structure of the
system until the release is desired.
Preferably, the delivery system comprises a closed matrix or network
structure, which
traps the flavour precursor compound within the closed structure. For example,
the flavour
composition or precursor may be trapped in domains within a matrix structure.
Upon
compression or deformation of the material, the flavour composition or
precursor is forced
out from the matrix structure, for example, through the breakage of the
surrounding
structure. In embodiments, the delivery system comprises a polymer matrix
comprising one
or more matrix-forming polymers. For example, the gradual breakdown of the
polymer
matrix with increasing compressive force, temperature, or both provides the
controlled
release of the flavour precursor compound from the delivery system. The
release of the
flavour precursor compound can thus vary as a function of temperature or the
deformation
resulting from the compressive or shear force that is applied to the delivery
system.
The delivery system may advantageously be provided within smoking articles in
a
variety of different forms so that there is flexibility in the way in which
the composition or
precursor can be incorporated into the smoking article. In embodiments the
delivery system
is provided in the form of particles, beads or capsules. The particles, beads
or capsules may
be formed into any suitable shape, but are preferably substantially
cylindrical or spherical.
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
7
In various embodiments, the flavour precursor is synthesized or purified prior
to its
addition to the flavour composition or flavour delivery composition.
A flavour precursor compound, as described above, may be included in a smoking
composition in any suitable manner and in any suitable amount. The term
"smoking
composition" is used to describe a composition that produces an aerosol when
it is heated,
and is used to make a smoking article. The term "smoking article" includes
articles that heat
the smoking composition directly or indirectly, or smoking articles that
neither combust nor
heat the smoking composition, but rather use air flow or a chemical reaction
to deliver
nicotine, a flavour compound or other materials from the tobacco or other
nicotine source.
As used herein, the term "smoke" is used to describe an aerosol produced by a
smoking
article. An aerosol produced by a smoking article may be, for example,
produced by non-
combustible smoking articles, such as heated smoking articles or non-heated
smoking
articles.
A flavour delivery system can controllably release a flavour precursor
compound to
its surrounding environment by any suitable methods, for example by
deformation of the
flavour delivery system or by changing the temperature. The amount of flavour
precursor
compound released over a time interval, as well as the start or end of the
interval(s) can be
controlled. The amount of flavour precursor compound released during each
interval and the
length of time intervals need not be equal.
A smoking composition of the invention comprises a flavor precursor compound
or a
flavor composition, the composition or precursor optionally being provided as
part of a
delivery system. A smoking composition may comprise, for example, one or more
of:
powder, granules, pellets, shreds, strips or sheets comprising one or more of:
herb leaf,
tobacco leaf, tobacco stems, fragments of tobacco ribs, homogenized tobacco,
reconstituted
tobacco, processed tobacco, extruded tobacco and expanded tobacco. The smoking
composition may be in loose form, or may be provided in a suitable container
or cartridge.
For example, the smoking composition may be contained within a paper or wrap
and have
the form of a plug.
Smoking articles that include aerosol-generating devices typically comprise an
aerosol-forming substrate that is assembled, often with other components, in
the form of a
rod. Typically, such a rod is configured in shape and size to be inserted into
an aerosol-
generating device that comprises a heating element for heating the aerosol-
forming
substrate.
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
8
"Aerosol-forming substrate" as used herein is a type of smoking composition
that can
be used in an aerosol-generating device to produce an aerosol. The aerosol-
forming
substrate can release a flavor compound upon heating. The aerosol-forming
substrate can
be in solid form or liquid form. The substrate can comprise both liquid and
solid components.
The aerosol-forming substrate may comprise tobacco and a flavour composition
wherein a
flavor precursor compound or a disassociated flavor compound is released from
the
substrate upon heating. In embodiments, the aerosol-forming substrate does not
include
tobacco, but does include a flavor composition wherein a flavor precursor
compound as
described herein or a disassociated flavor compound is released from the
substrate upon
heating, and optionally nicotine. The aerosol-forming substrate may further
comprise an
aerosol former. Examples of suitable aerosol formers are glycerine and
propylene glycol.
Optionally, the aerosol-forming substrate may be provided on or embedded in a
carrier
which may take the form of powder, granules, pellets, shreds, spaghetti
strands, strips or
sheets. The aerosol-forming substrate may be deposited on the surface of the
carrier in the
form of, for example, a sheet, foam, gel or slurry. The aerosol-forming
substrate may be
deposited on the entire surface of the carrier, or alternatively, may be
deposited in a pattern
in order to provide a non-uniform flavor delivery during use.
Smoking articles may comprise a filter which may be a single segment filter or
a
multi-component filter comprising two or more connected or unconnected filter
segments.
Any suitable filter or filter segment may be included in a smoking article
described herein.
One or more of the filter segments may each comprise a flavor precursor
compound or a
flavor composition, the precursor or composition optionally being provided in
a delivery
system. Smoking articles may be packaged in containers for sale, for example
in soft packs
or hinge-lid packs, with an inner liner coated or impregnated with a flavor
compound, a flavor
composition or a delivery system.
A smoking composition comprising a flavor precursor compound or a flavor
composition, the precursor or composition optionally being provided in a
delivery system,
can be manufactured using any suitable technique. For example, a flavour
composition
comprising a flavour precursor compound, may be sprayed on tobacco. To
facilitate
spraying, the flavor precursor compound can be added to a liquid carrier to
form a mixture,
such as solution, suspension or slurry, and the slurry can be applied onto the
tobacco.
Slurries comprising the flavor precursor compound can include any liquid or
liquid mixtures
suitable for dispersing and dispensing (e.g., spraying) particles comprising
the flavor
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
9
precursor compound. A preferred liquid is water (e.g., deionized water),
though other liquids,
such as alcohols, can be used.
In some embodiments, tobacco may be dipped in a solution, suspension or
dispersion comprising the flavour precursor. A solid such as a powder or
crystals comprising
the flavour precursor may be added to tobacco. The smoking composition can
then be
processed for use in a smoking article.
In some embodiments, the flavor precursor compound may be added to cut filler
tobacco stock supplied to a cigarette-making machine or applied to a pre-
formed tobacco
column prior to wrapping a cigarette wrapper around the tobacco column.
The concentration of a flavor precursor compound in the slurry can be any
amount
suitable for dispensing the slurry onto tobacco. Slurries comprising a
dispersion of a flavor
precursor compound in a liquid can comprise from about 0.01 to about 2% by
weight of a
flavor precursor compound. The flavor precursor compound can be provided in
the form of a
dried powder and applied to tobacco as such. If dried powder is used, it can
be dusted onto
tobacco. Another technique for incorporating the flavor precursor compound in
a tobacco
smoking composition involves adding the flavor precursor compound to a slurry
of
ingredients used to make reconstituted tobacco. The slurry, including the
flavor precursor
compound, can be formed into a reconstituted tobacco sheet and the sheet can
be cut to
shreds for incorporation as cut filler of a rod of smoking composition or
other forms of
smoking article.
Homogenized tobacco can also be used to make aerosol-forming substrate for use
in
smoking articles that are being heated in an aerosol-generating device. As
used herein, the
term "homogenized tobacco" denotes a material formed by agglomerating
particulate
tobacco. Tobacco dust created by tobacco breakage during shipping and
manufacturing,
leaf lamina, stems and other tobacco by-products that are finely ground may be
mixed with a
binder to agglomerate the particulate tobacco. Homogenized tobacco may
comprise other
additives in addition to a flavor precursor or flavour composition, including
but not limited to,
aerosol-formers, plasticisers, humectants, and non-tobacco fibers, fillers,
aqueous and non-
aqueous solvents and combinations thereof. Homogenized tobacco can be cast,
extruded,
or rolled. A number of reconstitution processes for producing homogenized
tobacco
materials are known in the art. These include, but are not limited to: paper-
making
processes of the type described in, for example, US5,724,998; casting
processes of the type
described in, for example, US5,724,998; dough reconstitution processes of the
type
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
described in, for example, US3,894,544; and extrusion processes of the type
described in,
for example, in GB983,928.
It will be appreciated that aerosol-forming substrates may have different
shapes and
sizes depending upon, for example, the type of smoking article in which they
are intended to
5 be used. Aerosol-forming substrates may be substantially three-
dimensional. For example,
aerosol-forming substrates may be bricks or plugs comprising a plurality of
strands of
homogenized tobacco material. Alternatively, aerosol-generating substrates may
be
substantially two-dimensional. For example, aerosol-generating substrates may
be mats or
sheets comprising a plurality of strands of homogenized tobacco.
1.0 The amount of flavour precursor compound desired in a smoking
composition may
vary depending on the final product in which the smoking composition is to be
included. For
example, the flavour precursor compound may be added to tobacco to enhance the
flavour
or aroma of conventional smoking articles, such as cigarettes, cigars, and the
like. As such
articles may already produce a similar flavour or aroma when smoked, flavour
precursor
compounds that release flavour compounds having similar flavour qualities may
be added in
relatively low concentrations or amounts. In contrast, it may be desirable to
add higher
concentrations or amounts of flavour precursor compounds to smoking
compositions, such
as tobacco-containing aerosol-forming substrates, that will be used in smoking
articles in
which constituents are aerosolized without combustion. Smoking articles having
tobacco-
containing aerosol-forming substrates for use with an aerosol-generating
device typically
provide flavour characteristics that differ from those of combustible smoking
articles. It may
also be desirable to include higher concentrations or amounts of flavour
compounds to
smoking compositions that do not include tobacco, such as non-tobacco
containing aerosol-
forming substrates. Preferably, the flavour precursor compounds are included
in the smoking
composition for use in smoking articles in amounts that provide a user with a
perceived note
similar to a combustible smoking article, such as a cigarette. For example,
the flavour
precursor may be included in a non-combustion smoking article or a smoking
article that
does not include tobacco in an amount that results in the release or delivery
of the flavour
compound in a concentration or amount similar to that delivered to a user of a
conventional
smoking article such as a cigarette.
In embodiments, the amount of flavour compound delivered to a user may be
determined on a per puff basis. Any suitable method may be used to determine
the amount
of flavour compound delivered per puff. For example, ISO, Health Canada
Intense, or other
standard or automated procedures may be employed or modified to determine
content per
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
11
puff. Examples of ISO methods that may be employed or modified include: (i)
ISO
4387:1991 Cigarettes ¨ Determination of total and nicotine free dry matter
using a routine
analytical smoking machine, 1991-10-15; (ii) ISO 8454:1995 Cigarettes ¨
Determination of
carbon monoxide in the vapour phase of cigarette smoke ¨ NDIR method, 1991-11-
15; (iii)
ISO 10315:1991 Cigarettes ¨ Determination of nicotine in smoke condensates ¨
Gas-
chromatographic method, 1991-08-01; and (iv) ISO 10362-1:1991 Cigarettes ¨
Determination of water in smoke condensates ¨ Part 1: Gas-chromatographic
method, 1991-
09-15. The Health Canada Intensive method refers to: Health Canada ¨ Official
Method T-
115, Determination of "Tar", Nicotine and Carbon Monoxide in Mainstream
Tobacco Smoke,
December 1999. Regardless of the method employed, it may be desirable to
determine the
amount of a flavour compound that is delivered per puff of a conventional
cigarette and
formulate a non-conventional smoking article that aerosolizes tobacco or other
constituents
without combustion so that it delivers similar amounts of the flavour compound
per puff. The
amount of flavour precursor compound added to a smoking composition for such
articles
may be varied and tested to determine how much flavour precursor is needed to
deliver a
similar amount of the flavour compound.
Of course, the desired amount of flavour compound delivered per puff will
depend on
the flavour compound itself. In embodiments, a smoking article is configured
to deliver about
5 nanograms or more of the flavour compound per puff. In embodiments, a
smoking article
is configured to deliver from about 10 nanograms of the flavour compound per
puff to 1
milligram of the flavour compound per puff, such as from about 50 nanograms
per puff to
about 750 nanograms per puff or from about 75 nanograms per puff to about 500
nanograms per puff.
The amount of flavour precursor compound added to a smoking composition to
deliver a desired amount of disassociated flavour compound to a user will
depend on the
components and configuration of the article and the flavour precursor
compound.
Of course, a flavour precursor may be added to a smoking composition in any
suitable amount or concentration. In embodiments, a smoking composition
comprises 0.001
weight % or greater, based on the total weight of tobacco, of the flavour
precursor
compound. In embodiments, a smoking composition includes 0.005 weight % or
greater of
the flavour precursor compound. In embodiments, a smoking composition includes
0.01
weight % or less of the flavour precursor compound. In embodiments, a smoking
composition includes from about 0.001 weight % to about 2 weight % of the
flavour
precursor compound, such as from about 0.005 weight % to about 1 weight % of
the flavour
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
12
precursor compound or from about 0.01 weight % to about 0.25 weight % of the
flavour
precursor compound.
The flavour precursor compound may be added to tobacco at any suitable time,
such
as before or during processing of the tobacco for incorporation into a smoking
article or the
like. Preferably the flavour precursor compound is stable (that is, the
flavour compound is
not released or generated) under such processing.
In embodiments, one or more flavour precursor compounds is dissolved in a food
grade solvent and then applied to a tobacco substrate or other suitable
substrate in
concentrations up to 1000ppm. The substrate may then be equilibrated at room
temperature
to a humidity of approximately 60 % for at least 48 hours. For application
during the
substrate generation (e.g. cast leaf process, extrusion) the compounds can be
added directly
as solid material to any available food grade solvent system required in the
process. In case
the application as a solid is not feasible, the compound can be applied as a
solution,
suspension, etc. created as described above. The concentration to the
substrate during
generation may reach 1 % of the total dry weight.
In embodiments, the reconstitution of tobacco is performed by drying and
casting
homogeneous slurry of tobacco powder, water, glycerin, binder and cellulose
fibres. This
type of process is known as cast leaf process and is widely used by the
tobacco industry for
the manufacturing of reconstituted or homogenized tobacco for use in
conventional cigarette.
A cast leaf process may involve applying temperatures of up to about 140 C,
such as
between about 90 C and about 140 C. Accordingly, if the smoking composition
that
includes the flavour precursor compound is to be manufactured in a cast leaf
process, the
flavour precursor compound is preferably stable at such temperatures.
Preferably, the flavour precursor compound releases the flavour compound or
the
intermediate under general use conditions of the article in which the flavour
precursor
compound is incorporated. By way of example, smoking articles that heat, but
do not
combust, tobacco to aerosolize tobacco constituents typically heat the tobacco
at about
200 C to about 450 C. Accordingly, the flavour precursor compound preferably
releases the
flavour compound at a temperature from about 200 C to about 450 C when a
smoking
composition having the flavour precursor compound is included in such
articles. By way of
another example, combustion of tobacco typically occurs at a temperature of
about 450 C.
Accordingly, the flavour precursor compound preferably releases the flavour
compound at a
temperature below about 450 C when a tobacco composition having the flavour
precursor
compound is included in combustible smoking articles such as cigarette or
cigars.
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
13
In embodiments, the flavour precursor compound releases the flavour compound
at a
temperature of about 150 C or greater. In embodiments, the flavour precursor
compound
releases the flavour compound at a temperature of about 450 C or less. In
embodiments,
the flavour precursor compound releases the flavour compound at a temperature
of from
about 100 C to about 450 C, such as from about 150 C to about 450 C or from
about 200 C
to about 450 C.
Tobacco compositions, as used herein refer to smoking compositions that
include
tobacco and a flavour precursor. For example, the tobacco composition may be
used in
combustible smoking articles or non-combustible smoking articles in which an
aerosol-
forming substrate is used. The tobacco composition can be reconstituted
tobacco containing
various tobacco types of different origins, as well as binders and humectants.
The
humectants facilitate the production of an aerosol. When heated, the
humectants evaporate
and re-condense into small droplets to generate a visible aerosol. Preferably,
the flavour
precursor composition releases the flavour compound under use conditions, but
not under
storage, processing, or manufacturing conditions.
In the case of combustible smoking articles such as cigarettes, the smoking
composition may be used in any portion of the smoking article having a tobacco
substrate,
for example in the tobacco rod of a conventional cigarette, or in one or more
segments of the
filter of a conventional cigarette. In the case of smoking articles in which
the smoking
composition, or a component thereof, is not combusted, the smoking composition
may be
used in any portion of the smoking article having an aerosol-forming
substrate.
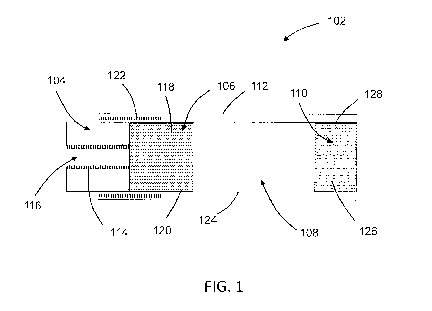
By way of example and with reference to FIG. 1, a schematic drawing of a
smoking
article 102 in which the smoking composition is not combusted is shown. As
shown in FIG.
1, the smoking article 102 includes a combustible heat source 104, an aerosol-
forming
substrate 106, an elongate expansion chamber 108 and a mouthpiece 110 in
abutting
coaxial alignment, which are overwrapped in an outer wrapper of cigarette
paper 112 of low
air permeability.
The combustible heat-source 104 is a pyrolised porous carbon-based heat
source.
The combustible heat source 104 is cylindrical and comprises a central airflow
channel 116
that extends longitudinally through the combustible heat source 104. A
substantially air
impermeable, heat resistant coating 114 of iron oxide is provided on the inner
surface of the
central airflow channel 116. The aerosol-forming substrate 106 is located
immediately
downstream of the combustible heat source 104 and comprises a cylindrical plug
of
homogenised tobacco material 118 comprising a flavor precursor compound and
glycerine
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
14
as aerosol former and circumscribed by filter plug wrap 120. The homogenized
tobacco
material 118 consists of longitudinally aligned filaments of extruded tobacco
material.
A heat-conducting element 122 consisting of a tube of aluminum foil surrounds
and is
in contact with a rear portion of the combustible heat source 104 and an
abutting front
portion of the aerosol-generating substrate 106. As shown in FIG. 1, a rear
portion of the
aerosol-generating substrate 106 is not surrounded by the heat-conducting
element 122.
The elongate expansion chamber 108 is located downstream of the aerosol-
generating substrate 106 and comprises a cylindrical open-ended tube of
cardboard 124.
The mouthpiece 110 of the smoking article 102 is located downstream of the
expansion
chamber 108 and comprises a cylindrical plug of cellulose acetate tow 126 of
very low
filtration efficiency circumscribed by filter plug wrap 128. The mouthpiece
110 may be
circumscribed by tipping paper (not shown). The dimensions of the smoking
article 102 may
be similar to a conventional cigarette.
In use, a user ignites the combustible carbon-based heat source 4 and then
draws air
through the central airflow channel 116 downstream towards the mouthpiece 110.
The front
portion of the aerosol-generating substrate 106 is heated primarily by
conduction through the
abutting non-combusting rear portion of the combustible heat source 104 and
the heat-
conducting element 122. The drawn air is heated as it passes through the
central airflow
channel 116 and then heats the aerosol-forming substrate 106 by convection.
The heating of
the aerosol-forming substrate 106 releases volatile and semi-volatile
compounds, including
the disassociated flavor compound, and glycerine from the aerosol forming
substrate 118,
which are entrained in the heated drawn air as it flows through the aerosol-
forming
substrate.
The heated air and entrained compounds pass downstream through the expansion
chamber 108, cool and condense to form an aerosol that passes through the
mouthpiece
into the mouth of the user (at about ambient temperature).
To make the smoking article 102, a rectangular piece of the heat-conducting
element
122 is glued to cigarette paper 112. The heat source 104, the plug of the
aerosol-forming
substrate 106 and the expansion chamber 108 are suitably aligned and
positioned on the
cigarette paper 112 with the attached heat-conducting element 122. The
cigarette paper 112
with the attached heat-conducting element 122 is wrapped around the rear
portion of the
heat source 104, the aerosol-generating substrate 106 and the expansion
chamber 108 and
glued. The mouthpiece 110 is attached to the open end of the expansion chamber
using
known filter combining technology.
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
In another example of a heated smoking article, the smoking composition
comprising
the flavor precursor compound is brought into direct contact with a heat
source that is not
combusted, such as an electrical heat source. FIG. 2 shows a smoking article
that is heated
by an electrical heat source when engaged with a device for consumption. The
smoking
5 article 101 comprises a front-plug 103, an aerosol-forming substrate 111,
a hollow cellulose
acetate tube 109, a transfer section 107, and a mouthpiece filter 105. These
five elements
are arranged sequentially and in coaxial alignment and are assembled by a
cigarette paper
115 to form a rod. The rod has a mouth-end, which a user inserts into his or
her mouth
during use, and a distal end located at the opposite end of the rod to the
mouth end. When
10 assembled, the rod is 52 mm long and has a diameter of 7.2 mm. The front-
plug 103 is a
cylindrical portion of cellulose acetate tow. The aerosol-forming substrate
111 is located
downstream of the front-plug 103 and comprises a bundle of crimped cast-leaf
tobacco
wrapped in a filter paper. The cast-leaf tobacco includes additives, including
glycerine as an
aerosol former. The tube 109 is located immediately downstream of the aerosol-
forming
15 substrate 111 and is formed from cellulose acetate. The transfer section
107 allows volatile
substances including the flavor compound released from the aerosol-forming
substrate 111
to pass along the rod towards the mouth end. The volatile substances may cool
within the
transfer section 107 to form an aerosol. The mouthpiece filter 105 is a
conventional
mouthpiece filter formed from cellulose acetate tow. The elements identified
above are
assembled by being tightly wrapped within a cigarette paper 115.
The aerosol-generating device 119 comprises a sheath 121 for receiving the
smoking
article 101 for consumption. A heating element 113 is located within the
sheath 121 and
positioned to engage with the distal end of the smoking article 101. The
heating element 113
is shaped in the form of a blade terminating in a point. As the smoking
article 101 is pushed
into the sheath the point of the heating element 113 engages first with the
front-plug 103 and
then penetrates into the aerosol-forming substrate 111. When the smoking
article 101 is
properly engaged with the aerosol-generating device 119, the heating element
113 is
located within the aerosol-forming substrate 111. Heat generated by the
heating element
113 is transferred by conduction and convection to the aerosol-forming
substrate 111 which
comprises the flavor precursor compound. An insulating collar 117 may surround
a portion of
the heating element 113 that is in contact with and protect the front-plug 103
from burning or
melting. Of course, it will be understood that the smoking articles described
with regard to
FIG. 1 and FIG. 2 are just two examples of a smoking article that may employ a
smoking
composition comprising a flavor precursor compound as described herein. It
will be further
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
16
understood that methods for making a smoking article having a smoking
composition that
includes a flavor precursor compound other than those described in connection
with FIG. 1
and FIG. 2 may be employed.
Any suitable method for making a smoking article having a smoking composition
that
includes a flavor precursor compound may be employed. In general, a method for
making a
smoking article having a smoking composition that includes a flavor precursor
compound
includes adding or incorporating the flavor precursor compound or a flavor
delivery
composition into a smoking composition, such as tobacco or other substrate;
and
incorporating the smoking composition into a smoking article.
In general and as described herein, a method for enhancing flavor of a smoking
composition comprises adding a flavor precursor compound, optionally
encapsulated in a
delivery system, to the smoking composition. The flavor precursor compound
releases or
dissociates a flavor compound or intermediate upon heating. As used herein
"release" and
"dissociate" are used interchangeably. The heating of the flavor precursor
compound occurs
when a smoking article is used (combusted or heated) by a smoker.
All scientific and technical terms used herein have meanings commonly used in
the
art unless otherwise specified. The definitions provided herein are to
facilitate understanding
of certain terms used frequently herein.
As used in this specification and the appended claims, the singular forms "a",
"an",
and "the" encompass embodiments having plural referents, unless the content
clearly
dictates otherwise.
As used in this specification and the appended claims, the term "or" is
generally
employed in its sense including "and/or" unless the content clearly dictates
otherwise.
As used herein, "have", "having", "include", "including", "comprise",
"comprising" or
the like are used in their open ended sense, and generally mean "including,
but not limited
to". It will be understood that "consisting essentially of', "consisting of",
and the like are
subsumed in "comprising," and the like.
Non-limiting examples illustrating certain aspects of the compounds,
compositions,
processes and articles described in this disclosure are provided below.
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
17
Examples
Example 1
A variety of non-volatile sulfur compounds, as shown in table 1, are screened
by
(ATD) GC-MS on a cellulose filter to determine whether and how much hydrogen
sulfide and
methanethiol they release upon heating.
The compounds are also added to non-combusted smoking articles to assess the
change in sensory attributes of such compounds during aerosolisation of such
articles in the
presence and absence of the compounds.
Certain of the non-volatile sulfur compounds are also applied to a blended
tobacco
by syringe injection and used for non-combusted smoking substrates. The
tobacco with the
non-volatile sulfur compounds is formed into sticks and equilibrated for at
least 12 hours
prior to assessment.
The ortho-nasal impact of the selected compounds is evaluated in different
concentrations on global aroma by usage of the smoke simulator in pushing
mode. Selected
compounds are then selected for sensory testing.
Table 1: Non-volatile sulfur compounds
non-volatile thiol-containing compounds CAS No. category
Cysteine 3374-33-9 amino acid
Cystin 56-89-3 amino acid derivate
Glutathione 70-18-8 natural antioxidant in
cells
Methionine 59-51-8 amino acid
DL-Methionine methylsulfonium chloride 582174 amino acid derivate
N-Acetyl L-cysteine 616-91-1 amino acid derivate
S-methyl L-cysteine 1187-84-4 amino acid derivate
DL-Homocysteine 454-29-5 amino acid derivate
N-Acetyl L-methionine 65-82-7 amino acid derivate
Farnesyl-Met-Ome 218962-72-2
amino acid derivate
Egg albumin 9006-59-1 sulfur rich protein
2-Hydroxy-4-(methylthio)butyric acid Ca salt 922-50-9 non-amino acid
sulfur source
Aqueous solutions are prepared according to table 2. Additionally 1:10 and
1:100
dilutions are prepared for validation.
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
18
Table 2: Stock solution preparation of tested compounds
non-volatile sulfur compounds weight (g) mL of H20
Cysteine 1.00 10
Cystin n/a n/a
Glutathione 1.00 10
Methionine 0.50 20
DL-Methionine methylsulfonium chloride 1.08 10
N-Acetyl L-cysteine 1.50 10
S-methyl L-cysteine 0.98 20
DL-Homocysteine 0.50 10
N-Acetyl L-methionine 1.91 10
Farnesyl-Met-Ome 1 pL 20 pL_
Egg albumin 0.50 10
2-Hydroxy-4-(methylthio)butyric acid Ca salt 1.00 20
ATD-GC-MS analyses are performed using following instrumental set-up:
=
Table 3: ATD-GC-MS instrumentation
Instrument Details
Injector Perkin Elmer Thermal Desorber Turbo Matrix 350
Gas chromatograph Agilent 7890A GC
Column 60m x 0.25 pm, FFAP
Mass chromatograph Agilent 5975C
Table 4: ATD-GC-MS parameters
ATD parameters Value
tube temperature 250 C
trap temperature -40 C
temperature gradient 99 C per second to 275 C
desorption time 5 min
N2 pressure 170 kPa; constant
Of each compound solution (stock, 1:10, 1:100) 20pL are spiked on a pre-
equilibrated Cambridge pad piece which is then inserted into glass tube. Tubes
are ortho-
nasally assessed after thermal desorption and detected aroma notes determined.
The
results are given in Table 5.
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
19
Table 5: Flavour Evaluation
non-volatile sulfur
comments and ortho-nasal evaluation
compounds
fresh tubes (250 C heated) gave burnt phenolic roasty impression
Cysteine
with weak sulfury meaty note
Glutathione fresh tubes (350 C heated) gave only weak roasty
impression
Methionine fresh tube (350 C heated) smelled cooked potato and
meaty-like
N-Acetyl L-methionine Methanethiol note stronger than hydrogen sulfide note
Farnesyl-Met-Ome cooked potato-like
Egg albumin Methanethiol and hydrogen sulphide balanced out
2-Hydroxy-4-
fresh tubes (250 C heated) smelled cooked potato-like with a sulfury
(methylthio)butyric acid
roasty aspect
Ca salt
DL-Methionine
methylsulfonium chloride green, cabbage, fishy, sulfury green
N-Acetyl-Cysteine popcorn, meaty, roasty
DL-Homocysteine roasty, very weak
S-Methyl-L-Cysteine cabbage, sulfury
The results indicate that there are mainly two families of compounds, the
first
generating predominantly hydrogen sulphide (H2S) and the second generating
predominantly methanethiol (MSH). The exception is egg albumin for which the
generation
seems to be similar.
The tubes of those candidates generating mostly H2S were ortho-nasally
evaluated
after heating as being sulfury, roasty or burnt. H2S could not be directly
detected. In contrast
the methanethiol generating compounds all were judged having a potato-like
aroma,
indicating the presence of methional as side product.
Example 2
Orthonasal & smoking evaluation of selected non-volatile sulfur compounds is
performed
using a smoke simulator in pushing mode. The following prototypes are tested:
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
Table 6: Prototypes to be tested
concentration concentration
non-volatile sulfur
spiked in mg spiked in mg
compounds
(concept A) (concept B)
Cysteine 1.82 0.182
Glutathione 1.75 0.175
Methionine 0.47 0.047
2-Hydroxy-4-
(methylthio)butyric acid 0.94 0.094
Ca salt
DL-Methionine
1.00 0.10
methylsulfoniunn chloride
S-Methyl-L-Cysteine 2.07 0.20
Testing is performed as follows. The smoking regime is Health Canada. The
power
setting of smoke simulator is 54W. Smoking evaluation is performed for
concepts showing
5 preference in ortho-nasal evaluation. Smoking evaluation is performed by
comparison of
untreated blend versus treated concept
For submission to sensory testing in Expert Panel only Cysteine and
Glutathione
were selected. For Glutathione Concept B and Cysteine Concept B was chosen.
10 Table 7: Observations recorded during the experimental run
Thiol-containing
compound Concept Panel description
Cysteine A sulfury, cold smoke, less off-notes
sulfury, cold smoke, less off-notes
Glutathione A sulfury, cold smoke, less off-notes
sulfury, cold smoke, less off-notes
Methionine A cooked potato and sulfury vegetable are dominating
cooked potato, sulfury vegetable, less off-notes
2-Hydroxy-4- A increased sulfury notes; direction sulfury green &
smoked ham
(methylthio) butyric
increased sulfury smoked perception with less off-notes
acid Ca salt
DL-Methionine A cooked potato, cabbage notes, untypical aroma
- -
methylsulfonium
no significant difference
chloride
CA 02928020 2016-04-19
WO 2015/075650
PCT/1B2014/066172
21
S-Methyl-L-Cysteine A increase of sulfury notes; green, bell pepper-like
& meaty notes
close to reference with increased hay-like, greenish notes
The addition of sulfur containing compounds, especially with free thiol
groups,
improves the flavor perception of non-combustible products and addresses
issues, such as
off-notes, complexity and mouth fullness. In particular, addition of the thiol-
containing
precursors Cysteine and Glutathione contributes positively to the global
flavor of aerosols
formed upon heating by adding smokiness, mouth fullness, harshness, bitterness
and
complexity.
In summary the addition of sulfur containing precursors was shown to have a
positive
effect on the overall sensory perception of prototypes by increasing smoky
dark attributes,
reducing non-combustible product off-notes and increasing mouth and throat
sensations, like
bitterness and mouth fullness.
=