Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
1
METHODS AND DEVICES FOR SOFT TISSUE DISSECTION
PRIORITY APPLICATIONS
[0001] The present application is a continuation-in-part application of,
and claims priority to,
U.S. Patent Application Serial No. 13/872,766, entitled "Instruments, Devices,
and Related
Methods for Soft Tissue Dissection," filed April 29, 2013, which claims
priority to: U.S.
Provisional Patent Application No. 61/687,587, entitled "Instrument for Soft
Tissue Dissection,"
filed on April 28, 2012; U.S. Provisional Patent Application No. 61/744,936,
entitled
"Instrument for Soft Tissue Dissection," filed on October 6, 2012; and U.S.
Provisional Patent
Application No. 61/783,834, entitled "Instruments, Devices, and Related
Methods for Soft
Tissue Dissection," filed on March 14, 2013, all of which are incorporated
herein by reference in
their entireties.
BACKGROUND
Kehl of the Dirclosure
[0002] The field of the disclosure relates to methods or devices used to
dissect tissue during
surgery or other medical procedures.
TechnicaLeackground
[0003] Surgeons frequently are required to sever or separate tissues during
a surgical
procedure. Two techniques are commonly used: (1) "sharp dissection" in which
the surgeon
uses a cutting instrument to slice a tissue, cutting with either scissors, a
scalpel, electrosurgery,
or other slicing instrument and (2) blunt dissection.
[0004] The advantage of sharp dissection is that the cutting instrument
easily cuts through
any tissue. The cut itself is indiscriminate, slicing through any and all
tissues to which the
instrument is applied. This is also the disadvantage of sharp dissection,
especially when trying to
isolate a first tissue without damaging it, when the first tissue is embedded
in and obscured by a
second tissue or, more commonly, in many tissues. Accidental cutting of a
blood vessel, a nerve,
or of the bowel, for example, is not an uncommon occurrence for even the most
experienced
surgeons and can lead to serious, even life-threatening, intra-operative
complications and can
have prolonged consequences for the patient.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
2
[0005] Isolation of a first tissue that is embedded in other tissues is
thus frequently
performed by blunt dissection. In blunt dissection, a blunt instrument is used
to force through a
tissue, to force apart two tissues, or to otherwise separate tissues by
tearing rather than cutting.
Almost all surgeries require blunt dissection of tissues to expose target
structures, such as blood
vessels to be ligated or nerve bundles to be avoided. Examples in thoracic
surgery include
isolation of blood vessels during hilar dissection for lobectomy and exposure
of lymph nodes.
[0006] Blunt dissection includes a range of maneuvers, including various
ways to tear soft
tissues, such as the insertion of blunt probes or instruments, inverted action
(i.e., spreading) of
forceps, and pulling of tissues with forceps or by rubbing with a "swab
dissector" (e.g. surgical
gauze held in a forceps). When needed, sharp dissection is used judiciously to
cut tissues that
resist tearing during blunt dissection.
[0007] The general goal is to tear or otherwise disrupt tissue, such as
membranes and
mesenteries, away from the target structure without tearing or disrupting
either the target
structure or critical structures such as nearby vessels or nerves. The surgeon
capitalizes on the
different mechanical behaviors of tissues, such as the different stiffness of
adjacent tissues or the
existence of planes of softer tissue between firmer tissues. Frequently, the
goal is to isolate a
target tissue that is mechanically firm, being composed of more tightly packed
fibrous
components, and is embedded in a tissue that is mechanically soft, being
composed of more
loosely packed fibrous components (for example, loose networks of collagen,
reticulin, and
elastin). More tightly packed fibrous tissues include tissues composed of
tightly packed
collagen and other fibrous connective tissues, usually having highly organized
anisotropic
distributions of fibrous components, often with hierarchical composition.
Examples include
blood vessels, nerve sheaths, muscles, fascia, bladders, and tendons. More
loosely packed
fibrous tissues have a much lower number of fibers per unit volume or are
composed of less well
organized materials such as fat and mesenteries. Fibrous components include
fibers, fibrils,
filaments, and other filamentous components. When a tissue is referred to as
"fibrous", the
reference is typically to extracellular filamentous components, such as
collagen and elastin ¨
proteins that polymerize into linear structures of varying and diverse
complexity to form the
extracellular matrix. As mentioned in the previous paragraph, the density,
orientation, and
organization of fibrous components greatly determine the tissue's mechanical
behavior.
Sometimes, tissues are referred to as "tough, fibrous tissues" indicating that
the fibrous or
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
3
filamentous components are densely packed and comprise a significant fraction
of the bulk of the
tissue. However, all tissues are fibrous, to one extent or another, with
fibers and other
filamentous extracellular components being present in virtually every tissue.
[0008] What is important to the present discussion is that softer tissues
tear more easily than
firmer tissues, so blunt dissection attempts to proceed by exerting sufficient
force to tear softer
tissue but not firmer tissue.
[0009] Blunt dissection can be difficult and is often time-consuming.
Judging the force to
tear a soft tissue, but not a closely apposed firm tissue is not easy. Thus,
blood vessels can be
torn. Nerves can be stretched or torn. In response, surgeons attempt judicious
sharp dissection,
but blood vessels and nerves can be cut, especially a smaller side branch.
This all leads to long,
tedious dissections and increased risk of complications, like bleeding, air
leaks from the lungs,
and nerve damage.
[0010] Surgeons frequently use forceps for blunt dissection. FIGS. 1A and
1B show a
typical forceps 10 of the prior art. FIG. 1A shows the forceps 10 in the
closed position for
clamping a tissue 34 between the opposing first clamp element 30 and second
clamp element 31.
FIG. 1B shows the forceps 10 in the open position, forcing tissue 34 apart. A
first finger
engager 20 and an opposing second finger engager 21 are used to actuate the
mechanism. First
finger engager 20 drives first clamp element 30, and second finger engager 21
drives second
clamp element 31. A pivot 40 attaches the first clamp element 30 and the
second clamp element
31, permitting a scissor-like action to force the first clamp element 30 and
the second clamp
element 31 together or apart, thereby clamping tissue 34 between the two clamp
surfaces 35 and
36 or rending tissue 34 by the spreading of the first clamp element 30 and the
second clamp
element 31. Frequently, a ratcheting clasp 50 is used to lock the first clamp
element 30 and the
second clamp element 31 together.
[0011] Laparoscopic and thoracoscopic (collectively referred here as
"endoscopic")
instruments use a similar action. FIG. 2 shows an example of an endoscopic
forceps 110 of the
prior art. A first finger engager 120 and an opposing second finger engager
121 are used to
actuate the mechanism. First finger engager 120 is rigidly mounted to the
instrument body 150.
Second finger engager 121 drives opposing clamp elements 130 and 131. A pivot
140 attaches
the two clamp elements 130 and 131, such that actuation of second finger
engager 121 forces
clamp elements 130 and 131 together, thereby clamping a tissue between two
clamp surfaces 135
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
4
and 136. As in FIG. 1, endoscopic forceps 110 can be used to force a tissue
apart. Clamp
elements 130 and 131 are closed, inserted into a tissue, and then opened to
tear the tissue.
[0012] For either instrument, forceps 10 or endoscopic forceps 110, a
surgeon performs blunt
dissection by closing the forceps, pushing the closed forceps into a tissue
and then, optionally,
opening the forceps inside the tissue, using the force applied by opening of
the jaws of the
forceps to tear the tissue apart. A surgeon thus proceeds to dissect a tissue
by a combination of
pushing into the tissue and opening the jaws of the forceps.
[0013] Blunt dissection is commonly used for wet and slick tissues, and the
smooth, passive
surfaces of most surgical instruments slide easily along the tissue, impairing
the instrument's
ability to gain purchase and separate the tissue. Furthermore, the surgeon has
only limited
control, being able only to jab, move sideways, or separate. An improved
instrument for blunt
dissection that could differentially separate soft tissues while not
disrupting firm tissues would
greatly facilitate many surgeries.
SUMMARY OF THE DETAILED DESCRIPTION
[0014] Embodiments disclosed include methods and devices for blunt dissection,
which
differentially disrupt a patient's soft tissues while not disrupting that
patient's firm tissues. In
one embodiment, a differential dissecting instrument for differentially
dissecting complex tissue
is disclosed. The differential dissecting instrument comprises a handle, a
central longitudinal
axis, and an elongate member having a proximal end and a distal end. The
differential dissecting
instrument also comprises a differential dissecting member configured to be
rotatably attached to
the distal end, the differential dissecting member comprising at least one
tissue engaging surface,
a first torque-point, the first torque-point disposed to a first side of the
axis of rotation of the
differential dissecting member, and a mechanism, configured to mechanically
rotate the
differential dissecting member around the axis of rotation thereby causing the
at least one tissue
engaging surface to move in at least one direction against the complex tissue.
The mechanism
comprises at least one force-transmitting member possessing a distal end and a
proximal end, the
distal end being attached to the first torque-point member. The proximal end
of the at least one
force-transmitting member is attached to a motive source configured to
oscillate the differential
dissecting member. Further, the at least one tissue engaging surface is
configured to selectively
engage the complex tissue such that when the differential dissecting member is
pressed by the
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
surgeon into the patient's complex tissue, the at least one tissue engaging
surface moves across
the complex tissue and the at least one tissue engaging surface disrupts at
least one soft tissue in
the complex tissue, but does not disrupt firm tissue in the complex tissue.
BRIEF DESCRIPTION OF THE FIGURES
[0015] FIGS. 1A and 1B show examples of the prior art. FIG. 1A shows
forceps used to
grasp tissue;
[0016] FIG. 1B shows exemplary forceps used in blunt dissection to divide
tissue;
[0017] FIG. 2 shows laparoscopic forceps of the prior art;
[0018] FIG. 3A through 3F show an exemplary differential dissecting
instrument. FIGS.
3A through 3C show a differential dissecting instrument having a rotating
differential dissecting
member within a shroud. FIG. 3D-1 through 3D-3 show front and side views of a
differential
dissecting member[[.]]; FIG. 3D-1 is a side view of a differential dissecting
member, while FIG.
3D-2 depicts a close-up of the surface of the differential dissecting member,
and FIG. 3D-3
shows a front view of that same differential dissecting member. FIG. 3E-1
through FIG. 3E-4
show four different types of differential dissecting members [[.11,
differential dissecting member
type I, type II, type III, and type IV, respectively. FIG. 3F-1 and FIG. 3F-2
show a differential
dissecting member in front and side view, respectively, including a tissue to
be dissected;
[0019] FIGS. 4A through 4F show how an exemplary differential dissecting
instrument
disrupts soft tissue, but not firm tissue, in a complex tissue, exposing the
firm tissue. FIGS. 4D
through 4F illustrate how a differential dissecting member engages and
disrupts tissues having
dispersed fibrous components but is unable to engage, and thus disrupt,
fibrous components;
[0020] FIGS. 5A through 5C show the tissue engaging end of different
exemplary
differential dissecting instruments comprising a dissecting wheel mounted in a
shroud. FIGS.
5A through 5B show an instrument with one configuration of a dissecting wheel
and FIG. 5C-1
and FIG. 5C-2 show another instrument with a different configuration of a
dissecting wheel;
FIG. 5C-1 depict the dissecting wheel in exploded view away from the
instrument, while FIG.
5C-2 shows the dissecting wheel in place;
[0021] FIGS. 6A through 6D show different configurations of an exemplary
differential
dissecting member in a differential dissecting instrument showing how the axis
of rotation of the
differential dissecting member can have many different orientations with
respect to the
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
6
differential dissecting instrument, including differential dissecting
instruments having flexible or
articulating elongate members;
[0022] FIGS. 7A and 7B show an exemplary differential dissecting instrument
that uses a
dissecting wire instead of a dissecting wheel or other differential dissecting
member;
[0023] FIGS. 8A through 8C show an exemplary differential dissecting
instrument that uses
a flexible belt as a differential dissecting member;
[0024] FIGS. 9A through 9C show how a varying the exposure of the tissue
engaging
surface of a differential dissecting member changes the behavior of a
differential dissecting
instrument, especially the range of angles of exposure of the tissue engaging
surface;
[0025] FIGS. 10A through 10C show how a varying the exposure of the tissue
engaging
surface of a differential dissecting member changes the directions of the
friction forces on a
tissue and thus the angles of strain on that tissue;
[0026] FIGS. 11A and 11B show an exemplary differential dissecting
instrument with water
outlets that emit beside the differential dissecting member;
[0027] FIG. 12 shows an exemplary differential dissecting instrument having
two opposing
flexible belts that generate opposing frictional forces and thus reducing
torque on the differential
dissecting instrument;
[0028] FIG. 13 shows an exemplary differential dissecting instrument can
have multiple
components placed into the shroud, including suction lines, water tubes, and
light emitting
diodes;
[0029] FIG. 14-1 through FIG. 14-3 show how the elongate member of an
exemplary
differential dissecting instrument can be articulated with a bendable region
to facilitate
placement of the differential dissecting member; FIG. 14-1 depicts the
elongate member of the
differential dissecting instrument in Position 1, straight, FIG. 14-2 shows
the elongate member
of the differential dissecting instrument bent at 45 degrees, and FIG. 14-3
illustrates the elongate
member of the differential dissecting instrument bent at 90 degrees;
[0030] FIGS. 15A through 15E show different exemplary differential
dissecting members
illustrating several important dimensions and features of differential
dissecting members; FIG.
15A shows a top view of an exemplary differential dissecting member that
rotates about a
rotational joint; further, FIG. 15B-1 through 15B-3 depict a differential
dissecting member as in
FIG. 15A; FIG. 15B-1 shows the differential dissecting member in side view
cross-section,
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
7
FIG. 15B-2 depicts a close-up view of the tip of the differential dissecting
member shown in
FIG. 15B-1, and FIG. 15B-3 shows a close-up view of the surface of the
differential dissecting
member shown in FIG. 15B-2; FIG. 15C illustrates another embodiment of a
differential
dissecting member having a scalloped tissue engaging surface; FIG. 15D shows
an oblique view
of the differential dissecting member depicted in FIG. 15C; FIG. 15E-1
illustrates an end-on
view of the differential dissecting member depicted in FIG. 15C, FIG. 15E-2
depicts a close-up
view of the tissue-engaging surface of the differential dissecting member
shown in FIG. 15E-1,
and FIG. 15E-3 details a very close-up view of the surface features of the
differential dissecting
member shown in FIG. 15E-1 and FIG. 15E-2;
[0031] FIG. 16-1 through FIG. 16-3 show one exemplary means for changing
the level of
aggressiveness of a differential dissecting member; FIG. 16-1 shows a
differential dissecting
member with some pointed, but still-not-sharp features, FIG. 16-2 shows a
differential dissecting
member with more rounded features than shown in FIG. 16-1, and FIG. 16-3 shows
a
differential dissecting member with even more blunt features than those
differential dissecting
members shown in FIG. 16-1 or FIG. 16-2;
[0032] FIGS. 17A, FIG. 17B-1, and 17B-2 show how features, such as
scalloping, of the
tissue engaging surface result in the tissue engaging surface having varying
angles of attack as it
moves over a tissue; FIG. 17A depicts a differential dissecting member with a
lobate form, FIG.
17B-1 shows that same differential dissecting member impinging on a tissue,
and FIG. 17B-2 is
a close-up view of the lobes of the lobate differential dissecting member
detailing the angles of
attack of the tissue engaging surface with respect to the tissue;
[0033] FIG. 18 shows how relative placements of the center of rotation and
the center of
gravity of an oscillating differential dissecting member can cause a
differential dissecting
instrument to vibrate;
[0034] FIGS. 19A through 19D show how an exemplary differential dissecting
member, or
a shroud surrounding it, strain a tissue in the direction perpendicular to the
direction of motion of
the tissue engaging surface. FIG. 19D illustrates how this strain can align
fibrous components
inside the tissue, thereby facilitating their disruption by the tissue
engaging surface;
[0035] FIG. 20 further illustrates how an exemplary differential dissecting
member disrupts
tissue, including how the differential dissecting member strains the tissue
and disrupts fibrous
components, such as interstitial fibers;
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
8
[0036] FIGS. 21A through 21C-4 show how relative movement of the shroud and
the
differential dissecting member of a differential dissecting instrument vary
the wedge angle and
thus can produce more or less strain in a tissue; FIG. 21A shows a side view
of a differential
dissecting member that has a thin dissecting wheel and is wrapped in a shroud;
FIG. 21B-1 and
FIG. 21B-2 further illustrate a front view of the shrouded differential
dissecting member in FIG.
21A and a close-up view of same, respectively; FIG. 21C-1 through FIG. 21C-4
show four
different positions of a shroud covering the differential dissecting member of
the differential
dissecting instrument;
[0037] FIG. 22 shows one example of an exemplary reciprocating mechanism
for a
differential dissecting member that uses a scotch yoke mechanism to convert
rotation of a shaft
to reciprocal oscillation of a differential dissecting member;
[0038] FIGS. 23A through 23C further illustrate the scotch yoke mechanism
shown in FIG.
22;
[0039] FIGS. 24A and 24B further illustrate the scotch yoke mechanism shown
in FIG. 22;
[0040] FIGS. 25A through 24D further illustrate the scotch yoke mechanism
shown in FIG.
22, including how more of the differential dissecting member can be shrouded
to reduce trauma
to a patient's tissues;
[0041] FIGS. 26A-1, FIG. 26A-2, FIG. 26B-1, and 26B-2 show how an exemplary
differential dissecting member can be fitted with retractable blade to permit
a differential
dissecting instrument to also perform sharp dissection of tissues; FIG. 26A-1
and FIG. 26B-1
show side views while FIG. 26A-2 and FIG. 26B-2 show top views; FIG. 26A-1 and
FIG. 26A-
2 show the differential dissecting member with a retractable scalpel
withdrawn, while FIG. 26B-
1 and FIG. 26B-2 show the same differential dissecting member with the
retractable scalpel
extended;
[0042] FIGS. 27A and 27B show how an exemplary differential dissecting
member can be
fitted with a clasping member to permit a differential dissecting instrument
to act as forceps;
[0043] FIG. 28 shows an exemplary differential dissecting member having a
tissue engaging
surface and a lateral surface;
[0044] FIGS. 29A through 29E-2 show magnified views of the tissue engaging
surface and
lateral surfaces of the differential dissecting member in FIG. 28 with the
tissue engaging surface
being comprised of an alternating series of valleys and projections; FIG. 29C-
2 depicts a close-
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
9
up of the corner of a projection shown in FIG. 29C-1; FIG. 29E-1 and FIG. 29E-
2 show two
alternative versions of arrangements of valleys and projections forming the
surface of a
differential dissecting member;
[0045] FIGS. 30A through 30D show how the lateral surface of the
differential dissecting
member in FIGS. 28 and 29A through 29C align and strain tissues, including
interstitial fibrous
components and how straining of the interstitial fibrous components
facilitates their alignment
and entering a valley and then being torn by a projection;
[0046] FIG. 31 further illustrates from a different view how fibrous
components of a tissue
enter a valley and are then strained and torn by a projection;
[0047] FIG. 32 shows an exploded view of a complete exemplary differential
dissecting
instrument;
[0048] FIGS. 33A through 33C show an enlarged view of the differential
dissecting member
of the differential dissecting instrument in FIG. 32, with emphasis on how a
scotch yoke
mechanism permits a rotating shaft to drive the reciprocal oscillations of the
differential
dissecting member;
[0049] FIG. 34 shows an exploded view of another exemplary differential
dissecting
instrument having a retractable blade;
[0050] FIGS. 35A through 35C-2 show an enlarged view of the differential
dissecting
member of the differential dissecting instrument in FIG. 34, including how
this mechanism can
also be used to vary the amplitude of oscillation of the differential
dissecting member; FIG. 35A
shows an exploded view of an exemplary Differential Dissecting Instrument;
FIG. 35B depicts
the details of assembly of an exemplary differential dissecting member; FIG.
35C-1 and FIG.
35C-2 depict how the angular amplitude of a differential dissecting member can
be controlled
via the longitudinal position of the cam receiver body;
[0051] FIGS. 36A-1, 36A-2, 36B-1, 36B-2, 36B-3, and 36B-4 show an exemplary
retractable blade that is a retractable hook having a more aggressive tissue
engaging surface plus
a hook with a sharpened elbow permitting selective slicing of tissue for sharp
dissection; FIG.
36A-1 depicts the hook extended from the differential dissecting member, FIG.
36A-2 shows it
retracted into the differential dissecting member; FIG. 36B-1 and 36B-2 show
the hook
extended, and FIG. 36B-3 and 36B-4 show the hook retracted; FIG. 36B-1 and
FIG. 36B-3
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
depict the differential dissecting member in static position, while FIG. 36B-2
and FIG. 36B-4
show the differential dissecting member actively oscillating;
[0052] FIGS. 37-1, 37-2, 37-3, and 37-4 illustrate how the retractable hook
shown in FIGS.
36A and 36B can be used to quickly and safely divide a membranous structure,
like the
peritoneum; FIG. 37-1 shows the hook extended from the tip of a static
differential dissecting
member while the differential dissecting instrument is suspended by the
surgeon above a
patient's tissue, FIG. 37-2 depicts the hook extended from the oscillating
differential dissecting
member and so oscillating against the surface of the tissue, FIG. 37-3 shows
the static
differential dissecting member with extended hook engaging the edge of a
tissue capsule, and
FIG. 37-4 depicts the differential dissecting member oscillating with extended
hook, so cutting
the tissue capsule layer;
[0053] FIG. 38 shows a complete exemplary differential dissecting
instrument having a
pistol grip and the ability to rotate the instrument insertion tube and, thus,
turn the plane of
oscillation of the differential dissecting member;
[0054] FIG. 39 shows how an exemplary differential dissecting instrument
can be fitted to
the arm of a surgical robot and can, optionally, be fitted with an
electrically conducting patch for
electrocautery;
[0055] FIGS. 40-1 and 40-2 show an exemplary laparoscopic version of a
differential
dissecting instrument having electromechanical actuators distal to an
articulation[[;]], and in the
straight and bent positions, respectively;
[0056] FIG. 41 shows one exemplary version of a differential dissecting
instrument driven
by a flexible drive shaft;
[0057] FIGS. 42A through 42[[B]]E show an oblique view and expanded views
of one
embodiment of a differential dissecting instrument in slender pencil grip form
designed
especially for open surgery;
[0058] FIGS. 43A through 43C show different embodiments of some mechanisms
that can
drive the oscillation of a differential dissecting member;
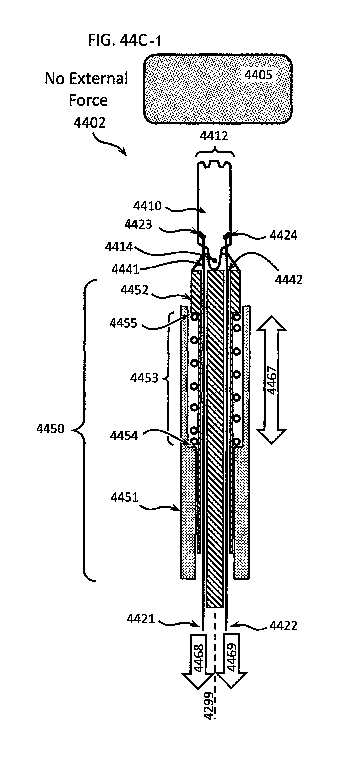
[0059] FIGS. 44A through 44C show different embodiments of mechanisms that
protect a
both a differential dissecting instrument and a tissue being dissected from
excessive loading;
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
11
[0060]
FIGS. 45A through 45G show a method for using a differential dissecting
instrument for separating a tissue plane without damaging blood vessels and
other anatomical
structures in the tissue plane;
[0061]
FIGS. 46A-1, 46A-2, 46B-1, 46B-2, 46C-1, and 46C-2 show an instrument for
tunneling with a differential dissecting instrument coupled with an endoscope;
and
[0062]
FIGS. through 47D show another instrument for tunneling with a differential
dissecting instrument coupled with an endoscope and including accessory
components to
enhance dissection and to improve the field of view for the endoscope.
DETAILED DESCRIPTION
[0063] Embodiments disclosed include methods and devices for blunt dissection,
which
differentially disrupt a patient's soft tissues while not disrupting that
patient's firm tissues. In
one embodiment, a differential dissecting instrument for differentially
dissecting complex tissue
is disclosed. The differential dissecting instrument comprises a handle, a
central longitudinal
axis, and an elongate member having a proximal end and a distal end. The
differential dissecting
instrument also comprises a differential dissecting member configured to be
rotatably attached to
the distal end, the differential dissecting member comprising at least one
tissue engaging surface,
a first torque-point, the first torque-point disposed to a first side of the
axis of rotation of the
differential dissecting member, and a mechanism, configured to mechanically
rotate the
differential dissecting member around the axis of rotation thereby causing the
at least one tissue
engaging surface to move in at least one direction against the complex tissue.
The mechanism
comprises at least one force-transmitting member possessing a distal end and a
proximal end, the
distal end being attached to the first torque-point member. The proximal end
of the at least one
force-transmitting member is attached to a motive source configured to
oscillate the differential
dissecting member. Further, the at least one tissue engaging surface is
configured to selectively
engage the complex tissue such that when the differential dissecting member is
pressed by the
surgeon into the patient's complex tissue, the at least one tissue engaging
surface moves across
the complex tissue and the at least one tissue engaging surface disrupts at
least one soft tissue in
the complex tissue, but does not disrupt firm tissue in the complex tissue.
[0064] Specifically, "Differential Dissecting Instruments" are disclosed.
The term
"differential" is used because a Differential Dissecting Instrument can
disrupt Soft Tissue while
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
12
avoiding disruption of Firm Tissue. The effector end of a Differential
Dissecting Instrument can
be pressed against a tissue comprised of both Firm Tissue and Soft Tissue, and
the Soft Tissue is
disrupted far more readily than the Firm Tissue. Thus, when a Differential
Dissecting Instrument
is pressed into a Complex Tissue, the Differential Dissecting Instrument
disrupts Soft Tissue,
thereby exposing Firm Tissues. This differential action is automatic ¨ a
function of the device's
design. Far less attention is required of an operator than traditional methods
for blunt dissection,
and risk of accidental damage to tissues is greatly reduced.
[0065] For the purposes of this application, "Soft Tissue" is defined as
the various softer
tissues separated, torn, removed, or otherwise typically disrupted during
blunt dissection.
"Target Tissue" is defined as the tissue to be isolated and its integrity
preserved during blunt
dissection, such as a blood vessel, gall bladder, urethra, or nerve bundle.
"Firm Tissue" is
defined as tissue that is mechanically stronger, usually including one or more
layers of tightly
packed collagen or other extracellular fibrous matrices. Examples of Firm
Tissues include the
walls of blood vessels, the sheaths of nerve fibers, fascia, tendons,
ligaments, bladders,
pericardium, and many others. A "Complex Tissue" is a tissue composed of both
Soft Tissue
and Firm Tissue and can contain a Target Tissue.
[0066] FIGS. 3A, 3B, and 3C show the effector end of a Differential
Dissecting Instrument
300 that can differentially disrupt Soft Tissue while not disrupting Firm
Tissues. In this
embodiment, a dissecting member comprises a dissecting wheel 310 that rotates
around shaft 320
that is held inside cavity 331 inside shroud 330. FIG. 3A shows the separate
parts. FIGS. 3B
and 3C show two different views of the assembly. The dissecting wheel 310 is
turned by any of
several mechanisms, such as a motor or a manually driven drive with
appropriate means of
transmission. Dissecting wheel 310 has tissue engaging surface 340 that can
grab and disrupt
Soft Tissue but not Firm Tissue. Examples of tissue engaging surface 340 and
dissecting wheel
310 include a diamond grinding wheel or an abrasive stone or a surface
otherwise covered by
small obtrusions or projections (further defined below) from the surface.
Shroud 330 obscures
portions of dissecting wheel 310 such that only one portion of dissecting
wheel 310 is exposed.
In use, dissecting wheel 310 rotates at a speed ranging from approximately
sixty (60) to
approximately twenty-five thousand (25,000) rpm or from approximately sixty
(60) to
approximately one hundred thousand (100,000) rpm, with speed being operator
selectable.
Additionally, the direction of rotation of dissecting wheel 310 can be
reversed by the operator.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
13
Alternately, dissecting wheel 310 can oscillate (reciprocal oscillation) with
a frequency ranging
from about sixty 60 to approximately twenty thousand (20,000) cycles per
minute in one
embodiment. In another embodiment, the dissecting wheel 310 can oscillate
(reciprocal
oscillation) with a frequency ranging from about 2,000 to 1,000,000 cycles per
minute.
[0067] Dissecting wheel 310 is one example of a "Differential Dissecting
Member"
(hereinafter "DDM") that can differentially disrupt Soft Tissue but not Firm
Tissue. FIG. 3D
shows side, front, and oblique views of one embodiment of a DDM 350 that has
been separated
from the rest of the Differential Dissecting Instrument 300 for clarity. DDM
350 is comprised of
a body 360 having an axis of rotation 365 about which body 360 rotates.
Rotation can be
oscillatory (i.e. back-and-forth) or continuous. Body 360 has an outer surface
361 with a tissue
engaging surface 370 distributed over at least a portion of the outer surface
361 of body 360.
Non-tissue engaging surface 371 is the portion of outer surface 361 not
covered by tissue
engaging surface 370. In this embodiment, no portion of outer surface 361 that
contacts a tissue,
and especially tissue engaging surface 370, should have features that are
sufficiently sharp to
slice tissue, so there should be no knife edges (like a scalpel or scissors),
no sharply pointed teeth
(like a saw), no sharp corners, and no sharp-edged fluting (like a drill bit
or an arthroscopic
shaver), where sharp means possessing a radius of curvature less than 25 pm.
Typical maximum
dimensions of a DDM are between approximately three (3) and approximately
twenty (20)
millimeters (mm). Alternatively, a small version for microsurgery can measure
between
approximately two (2) and approximately five (5) mm.
[0068] The tissue engaging surface 370 is further comprised of a plurality
of projections 375
(shown in expanded detail view of FIGS. 3D-1 through 3D-3) from the outer
surface 361 of
body 360, each projection 375 having a projection length 380 measured from
trough to peak in a
direction substantially perpendicular to that local region of outer surface
361 of body 360.
Different projections 375 on tissue engaging surface 370 can all have the same
projection length
380, or they can have different projection lengths 380. Projections 375
preferably have a
projection length 380 less than approximately one (1) mm. Alternatively, for
some embodiments
the projection length can be greater than approximately one (1) mm but less
than approximately
five (5) mm. Collectively, all projections 375 on a tissue engaging surface
370 have an average
projection length (Pavg). Projections 375 are separated by gaps 385,
preferably spanning a
distance of approximately 0.1 mm to approximately ten (10) mm.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
14
[0069] Referring now to FIGS. 3D-1 through 3D-3, FIG. 3D-1 through 3D-3
show front and
side views of a differential dissecting member. FIG. 3D-1 is a side view of a
differential
dissecting member, while FIG. 3D-2 depicts a close-up of the surface of the
differential
dissecting member, and FIG. 3D-3 shows a front view of that same differential
dissecting
member. Body 360 of FIGS. 3D-1 through 3D-3 can optionally be shaped such that
tissue
engaging surface 370 is located at varying distances from the axis of rotation
365. Thus, a
placement radius R can be measured in a plane perpendicular to the axis of
rotation 365 from the
axis of rotation 365 to any point on tissue engaging surface 370. There will
thus be a minimum
placement radius Rm., having the shortest length and a maximum placement
radius Rmax having
the longest length, and as shown in FIGS. 3D-1 through 3D-3 and 3E-1 through
3E-4, Rm., is
greater than zero whenever the tissue engaging surface 370 does not completely
cover the
surface 361 of the DDM 350. Thus, if body 360 is shaped such that tissue
engaging surface 370
is located at varying distances from the axis of rotation 365, then (Rmax ¨
Rmin) will be greater
than zero. In some embodiments of a DDM, this relationship (Rmax ¨ Rmin) is
greater than
approximately one (1) mm. In other embodiments this relationship (Rmax ¨ Rmm)
is greater than
Pavg. Alternatively, as shown in the examples in FIG. 3D-1 through 3D-3 and
FIG. 3E-1
through 3E-4, Rm., is typically at least 5% shorter than Rmax. Typical sizes
for a DDM are Rm., >
approximately one (1) mm and Rmax < approximately fifty (50) mm; however,
smaller versions
for microscopic dissections can have smaller dimensions of Rm., >
approximately 0.5 mm and
Rmax < approximately five (5) mm.
[0070] Referring now to FIGS. 3E-1 through 3E-4, four different embodiments
of a DDM
are shown in side view, with the axis of rotation 365 being perpendicular to
the plane of the
page. The cross-sectional profile of a DDM in a plane perpendicular to the
axis of rotation 365
is important, as will be discussed in subsequent paragraphs. Below are four
scenarios for a
cross-sectional profile of a DDM.
DDM Type I: The cross-sectional profile can be any shape, except circular or a
wedge of a circle. The axis of rotation 365 is located at any point within the
cross-
section as shown in FIG. 3D-1 through 3D-3 that yields the result that Pavg <
(Rmax -
Rmin). As shown in FIG. 3D-1 through 3D-3, a DDM Type I can include regular
cross-sectional profiles and irregular cross-sectional profiles, including
various
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
asymmetries, wavy/undulating/scalloped borders, cut-outs, involute borders,
etc. In
this example, the DDM Type I reciprocally oscillates between two end positions
(dotted outlines). Alternatively, motion can be rotational.
DDM Type II: The cross-sectional profile is circular or the wedge of a circle.
The
axis of rotation 365 is located at any point within the cross-section such
that it yields
the result that Pavg < (Rmax - Rmm) (i.e. the axis of rotation 365 is not
close to the
center of the circle).
DDM Type III: The cross-sectional shape is circular or the wedge of a circle.
The
axis of rotation 365 is located at any point within the cross-section
sufficiently close
to the center of the circle such that it yields the result that Pavg (Rmax -
Rmm) (i.e. the
axis of rotation 365 is approximately at the center of the circle).
DDM Type IV: The cross-sectional shape has a regularly repeating feature on
the
perimeter, such as scalloping, that yields the result that Pavg < (Rmax - Rmm)
no matter
where the axis of rotation 365 is located, including at the centroid of the
cross-
sectional shape. A Type I DDM and a Type IV DDM are closely related in that
the
axis of rotation 365 can be anywhere within the cross-sectional shape and
still yield
the result that Pavg < (Rmax - Rmm).
[0071] The scallops, undulations, or any regularly repeating feature of a
DDM do not include
perforations or holes in the tissue engaging surface 370 for which the walls
of the perforations do
not significantly contact tissue. For example, the aspirating passages
disclosed in U.S. Patent
Number 6,423,078 comprise holes in the abrasive surface, which act as the
tissue engaging
surface, of an abrading member. These holes do not comprise the features
disclosed for DDMs
because the holes act only as fluidic ports in the tissue engaging surface,
and the walls of the
aspirating passages are not brought to bear on tissue. Nevertheless, DDMs
disclosed herein can
include aspirating passages such as these.
[0072] DDMs of Type I through IV can also include any variety of shape out
of the plane of
the page. As stated earlier, "The cross-sectional profile of a DDM in a plane
perpendicular to the
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
16
axis of rotation 365 is important". Thus, dissecting wheel 310 in FIG. 3A
through FIG. 3C is
an example of a DDM Type III.
[0073] FIGS. 3F-1 and 3F-2 illustrates a DDM 390 that is similar to the DDM
350 shown in
FIGS. 3D-1 through 3D-3. DDM 390 has a first end and a second end 392 wherein
the first end
391 is directed away from the Complex Tissue 399 and is rotatably engaged with
a mechanism
(not shown) such that DDM 390 is rotated about an axis of rotation 365 by the
mechanism. The
mechanism can include motorized and manual drives. The second end 392 is
directed toward the
Complex Tissue 399 and comprises a semi-ellipsoid shape defined by three
orthogonal semi-
axes: the major semi-axis A, the first minor semi-axis B, and the second minor
semi-axis C,
wherein major semi-axis A lies in the direction of a line connecting the first
end 391 and the
second end 392; minor semi-axis C is parallel to the axis of rotation 365
(i.e. A is perpendicular
to the axis of rotation 365); and minor semi-axis B is perpendicular to both
major semi-axis A
and minor semi-axis C . The semi-ellipsoid can have a range of shapes (e.g.,
there may be
different relationships between the lengths of the three semi-axes, including
A = B = C, A # B #
C, A > B and A > C). In one embodiment, A > B > C has been found to be very
effective for a
DDM.
[0074] FIGS. 4A through 4C show how the effector end of Differential
Dissecting
Instrument 300 can be used for dissection of a Complex Tissue, comprised of
both Soft Tissue
and Firm Tissue, wherein the DDM is a dissecting wheel 310. In FIG. 4A, an
operator initiates
rotation of dissecting wheel 310, as indicated by arrow 410, before or upon
contact with a Soft
Tissue 400. In FIG. 4B, the operator then presses the exposed tissue engaging
surface 340 of
dissecting wheel 310 into the volume of the Soft Tissue 400 for blunt
dissection to reach the
Target Tissue 420 within. The arrows 430 and 440 in FIG. 4B show two possible
operator-
executed motions of the Differential Dissecting Instrument 300. Only the
portion of tissue
engaging surface 340 of dissecting wheel 310 exposed outside of shroud 330
contacts the Soft
Tissue 400 and thereby disrupts that portion of Soft Tissue 400 in contact
with tissue engaging
surface 340. Because the exposed, moving portion of tissue engaging surface
340 can disrupt
tissue without further action by the surgeon (e.g. without the surgeon's
forcefully scrubbing a
Differential Dissecting Instrument 300 against Soft Tissue 400), tissue can be
disrupted simply
by application of the rotating dissecting surface 340 of dissecting wheel 310
to any part of Soft
Tissue 400; however, when dissecting wheel 310 contacts the Firm Tissue of
Target Tissue 420,
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
17
it does not disrupt the Target Tissue 420. Note that pushing dissecting wheel
310 into Soft
Tissue 400 as indicated by the arrowhead on arrow 430 is a "plunge" ¨ the
dissecting wheel 310
can be pushed blindly into Soft Tissue 400 because it will not disrupt Firm
Tissue and will,
therefore, not disrupt Target Tissue 420. Other motions of Differential
Dissecting Instrument
300 can be used to dissect Soft Tissue 400, including motion orthogonal to
arrows 430 and 440,
curvaceous motions, and other 3D motions. Once Target Tissue 420 has been
exposed,
Differential Dissecting Instrument 300 can be withdrawn, exposing the Target
Tissue 420, as
shown in FIG. 4C.
[0075] FIG. 4D through FIG. 4F show how one embodiment of a DDM disrupts
Soft Tissue
but won't disrupt Firm Tissue. FIG. 4D depicts a sectional view of a DDM as
dissecting wheel
310 with tissue engaging surface 340 having projections 375. Dissecting wheel
310 moves in
and out of the plane of the page, with shaft 320 (not shown) substantially
parallel to the plane of
the page. The projections 375 thus move through the plane of the page. FIG. 4D
further shows
a volume of Soft Tissue 400 that remains substantially in place as dissecting
wheel 310, tissue
engaging surface 340, and projections 375 travel through the plane of the
page. Given the
motion of the projections 375 relative to the roughly stationary Soft Tissue
400, dissecting wheel
310 disrupts Soft Tissue 400. In detail, the Soft Tissue 400 is comprised of
both fibrous
components 401 and gel-like material 402. (Soft Tissues are frequently
composed of
extracellular material with fibrous components 401, e.g. collagen fibers and
small bundles of
fibers, and with thin sheet components, e.g. thinner membranes, dispersed in
water-swollen gel-
like materials.) Projections 375 are capable of sweeping through gel-like
material 402 such that
they encounter and then snag individual fibrous components 401 (e.g. at points
450 and 451);
fibrous components 401 are then torn by the relative motion of projections 375
on the dissecting
wheel 310 through the plane of the page and Soft Tissue 400. As dissecting
wheel 310 is pushed
deeper into tissue 400, projections 375 will snag deeper and deeper fibrous
components, also
tearing them. Thus, Soft Tissues 400 with dispersed components can be
dissected with a DDM.
[0076] FIG. 4E shows, in contrast to FIG. 4D, how a tightly packed fibrous
tissue can resist
dissection by a dissecting wheel 310. Firm Tissues 403 are frequently
comprised of fibrous
components 401 that are tightly packed either into parallel, crossed, or other
organized arrays
(e.g. fascia and blood vessel walls), or into tightly packed 2D and 3D meshes,
and a gel-like
material 402 covers the arrays of fibrous components 401. In FIG. 4E, a Firm
Tissue 403 is
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
18
composed of a gel-like material 402 (stippled region) thinly coating a layer
of tightly packed
fibrous components 401, the filaments of which are depicted with their long
axes perpendicular
to the plane of the page, thus the cross-section of the fibrous components 401
is depicted as
circular. In this image the dissecting wheel 310 reciprocally oscillates left-
right on the page, as
indicated by arrow 405, sweeping projections 375 over the surface of Firm
Tissue 403. Due to
the tight packing of fibrous components 401 in this Firm Tissue 403,
projections 375 are unable
to separately engage and snag fibrous components 401, and are thus unable to
apply sufficient
stress to tear fibrous components 401. Furthermore, gel-like material 402
serves as a lubricant,
causing projections 375 to tend to slip off of the tightly packed fibrous
components 401 of Firm
Tissue 403. Finally, any compliance of the surface of Firm Tissue 403 exposed
to dissecting
wheel 310 will prevent developing tension in the Firm Tissue 403 or fibrous
components 401,
resulting in the Firm Tissue 403 deflecting away from any pressure exerted by
dissecting wheel
310. Firm Tissues 403 thus resist disruption by DDMs by a combination of tight
packing of
fibrous and sheet components 401, lubrication of these components by gel-like
materials 402,
and compliance of the Firm Tissue 403.
[0077] Motion of a DDM, as stated above, can be either rotational or
oscillatory. The
velocity of a point on a DDM past a specific region of tissue strongly
influences the ability of a
DDM to disrupt that tissue. FIG. 4F depicts a dissecting wheel 310 that sweeps
left-right within
the plane of the page (as shown by double headed arrow 460) over a Soft Tissue
400 with a point
of contact 470. The translational velocity of point of contact 470 is
determined by the rotational
velocity of the DDM and the distance 480 separating point of contact 470 from
the center of
rotation (not shown). For rotational motion, the translational velocity equals
2700), where D is
the distance 480 and w is the rotational frequency in rotations per second.
For oscillatory
motion, the translational velocity equals DY2X, where D is the distance 480,
'I' is the oscillatory
frequency in cycles per second, and X is the angle swept in radians. For a
differential dissector,
distance 480 ranges from about one (1) mm to about forty (40) mm; rotational
velocity ranges
from approximately two (2) rotations per second to approximately three hundred
fifty (350
rotations per second; oscillatory frequency ranges from about two (2) hertz
(Hz) to about three
hundred fifty (350) Hz; and angle swept ranges from 2 to 270 . Thus, the
translational velocity
of point of contact 470 on a differential dissector can range from about one
(1) mm per second to
about sixty thousand (60,000) mm per second. In one embodiment, a distance 480
of
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
19
approximately fifteen (15) mm and an oscillatory motion with frequency of
approximately one
hundred (100) Hz sweeping through about forty-five degrees (45 ), yielding
about twenty-four
hundred (2400) mm per second, is very effective for a number of Soft Tissues.
Note that this
means that the velocities of operator-executed motions (as shown in FIG. 4)
are always smaller
than the velocity of a point of contact on a DDM during dissection because
surgeons are careful
during dissections, moving their instruments only slowly (usually much less
than one hundred
(100) mm per second). Additionally, motion of the DDM is described throughout
this document
as arising from a rotational motion (continuous rotation or reciprocal, i.e.,
back-and-forth,
oscillation). However, any motion of a DDM, including rectilinear motion,
relative to a tissue
such that the tissue engaging surface of the DDM appropriately engages the
tissue, as described
above, can be used.
[0078] A DDM can be forced against a blood vessel wall, the pleura, the
pericardium, the
esophagus, the gall bladder, and almost any other organ or tissue comprised of
or covered by a
tightly packed fibrous tissue, and the DDM will not significantly disrupt such
a Firm Tissue
under light hand pressure. Conversely, a DDM can be forced against a mesentery
or other Soft
Tissue, and the Soft Tissue will rapidly disrupt under light hand pressure.
Differential dissectors
fitted with any one of a variety of DDMs as disclosed herein have been found
by the inventors to
rapidly dissect between the planes of lobes in the lung, to dissect an
interior mammary artery
away from the inner wall of the chest, to separate the blood vessels and
bronchiole in the hilum
of a lung lobe, to dissect the esophagus from surrounding tissues, to
penetrate through bulk
muscle between, rather than through, the fiber bundles, to dissect fascia and
tendons away from
muscle fibers, to clean dissected fascia, to expose branched vascular and
lymphatic structures, to
dissect pockets into tissues and to separate tissue planes in many different
tissues. The utility of
a differential dissector is broad and, thus, has many potential uses.
Importantly, due to the
composition of skin and of surgical gloves, the skin or surgical gloves are
not cut or otherwise
disrupted by a DDM, even when significant pressure is applied. The inventors
have shown that
an oscillating DDM of the type disclosed herein can be held against a cheek of
the face without
any harm. Thus, a differential dissector is inherently safe to use, which
simplifies use during
surgery, especially when the surgeon's fingers must be near the point of
dissection.
[0079] DDMs are preferably formed from a rigid material, such as a metal or
a rigid polymer
(e.g., Shore A equal to or greater than 70), rather than from softer polymers
and elastomers (e.g.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
Shore A less than 70). Use of a rigid material keeps the projections from the
tissue engaging
surface from deflecting away from the tissue, as might occur if a softer
material was used.
DDMs or their component portions can be machined from bulk material,
constructed via
stereolithography, molded by any of the means well known in the art (e.g.
injection molding), or
by any such method known in the art.
[0080]
The projections of a tissue engaging surface of a DDM can be fabricated by any
of
several means. Projections can be formed by coating the tissue engaging
surface with grit
similar to sandpaper using grit coarser than 1000 but finer than 10 on the
Coated Abrasive
Manufacturers Institute standard.
Grit can include particles composed of diamond,
carborundum, metal, glass, sand or other materials known in the art.
Projections can be formed
into the surface of the material composing a DDM by sanding, sandblasting,
machining,
chemical treatment, electrical discharge machining, or other methods known in
the art.
Projections can be molded directly into the surface of a DDM. Projections can
be formed onto
the surface by stereolithography. Projections can be irregularly shaped, like
particles of grit, or
they can be regularly shaped having defined faceted, curved, or sloped
surfaces. The projections
may be elongate, and the long axis of these projections may have an angle with
respect to the
tissue engaging surface. Projections possess a cross-sectional shape when
viewing the tissue
engaging surface from above, and this shape may be round, faceted, or complex.
The cross-
sectional shapes of projections may be oriented with respect to the direction
of travel of the
DDM.
[0081]
Keeping the tissue wet helps differential dissection. A well-wetted Firm
Tissue is
better lubricated, greatly reducing disruption by a DDM. Conversely, a well-
wetted Soft Tissue
remains water-swollen and soft, separating the spacing of individual fibers,
facilitating their
being engaged and torn by the projections from the tissue engaging surface of
a DDM. Wetting
of the tissue can be accomplished by any of several means, including simply
irrigating the tissue
with physiological saline during dissection. Irrigation can be performed with
procedures already
used in surgery, such as an irrigation line, or by one of the devices
disclosed below.
Additionally, wetting of the tissue, and thus also the tissue engaging surface
of the DDM,
reduces clogging of the tissue engaging surface with disrupted tissue.
[0082]
FIG. 5A and FIG. 5B show another embodiment of the effector end of a
Differential
Dissecting Instrument 500 which has a DDM Type III configured as a circular
cylinder 510.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
21
FIG. 5A shows circular cylinder 510, with shaft 520 separate from the shroud
530. The tissue
engaging surface 540 covers the side of circular cylinder 510. The two-headed
arrow indicates
rotation about the axis of rotation 575. FIG. 5B shows both parts configured
for use with only a
limited portion of tissue engaging surface 540 exposed.
[0083] FIGS. 5C-1 and 5C-2 show another embodiment of the effector end of a
Differential
Dissecting Instrument with a different configuration for the shroud and DDM,
here another
DDM Type III. FIGS. 5C-1 and 5C-2 show a Differential Dissecting Instrument
550 with a
dissecting wheel 560, with shaft 570 separate from the shroud 580. Tissue
engaging surface 590
covers the periphery of dissecting wheel 560. The two-headed arrow indicates
the axis of
rotation 575. FIG. 5C-2 shows both parts configured for use with only a
limited portion of
tissue engaging surface 590 exposed. This configuration is problematic because
shroud 580
makes it difficult to position the tissue engaging surface 590 against a
tissue, and shroud 580
blocks the operator's view.
[0084] FIG. 6A shows one embodiment of a Differential Dissecting Instrument
600 that
includes a handle 610 for an operator. Handle 610 connects to elongate member
620 comprising
a first end 621 connected to handle 610 and a second end 622 connected to a
DDM 630.
Elongate member 620 can be shorter, allowing better manual control of the DDM
630 on an
instrument for open surgery, or it can be longer, allowing Differential
Dissecting Instrument 600
to be a laparoscopic instrument. The drive mechanisms for rotating DDM 630,
such as a rotating
drive shaft for a Scotch yoke or a crank/slider, are readily adapted to any
elongate member 620,
long or short, or to any device capable of driving DDM 630. DDM 630 is a Type
III DDM
rotatably mounted to elongate member 620 at second end 622 such that DDM 630
reciprocally
oscillates about its axis of rotation 640, as indicated by the double-headed
arrow (Axis of
rotation 640 is perpendicular to the plane of the page in FIG. 6A). First end
621 and second end
622 define a centerline 650 of elongate member 620. The tangent 651 of
centerline 650, as
centerline 650 approaches second point 622, and axis of rotation 640 thus
define a presentation
angle 670 (not shown ¨ perpendicular to page). In this example, the
presentation angle 670 is
90 (i.e., axis of rotation 640 is aligned perpendicular to tangent 651).
Rather than a handle 610,
first end 621 of elongate member 620 can attach to the arm of a robot for
robotic surgery. A
DDM can easily be adapted to any other device capable of moving or rotating
the DDM.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
22
[0085] FIG. 6B shows another embodiment of a similar Differential
Dissecting Instrument
601 but with the axis of rotation parallel to the centerline. Handle 610
connects to elongate
member 620 comprising a first end 621 connected to the handle 610 and a second
end 622
connected to a Type III DDM 631. DDM 631 is rotatably mounted to elongate
member 620 at
second end 622 such that DDM 631 reciprocally oscillates about its axis of
rotation 640. The
axis of rotation 640 is parallel to the plane of the page in FIG. 6B. First
end 621 and second end
622 define a centerline 650 of elongate member 620 with tangent 651 as
centerline 650
approaches second end 622. Axis of rotation 640 is thus aligned parallel to
tangent 651 (i.e., the
presentation angle 670 is 0 ). (Again, presentation angle 670 is not presented
in FIG. 6B
because presentation angle is 0 .) Differential Dissecting Instrument 601 is
thus similar to
Differential Dissecting Instrument 550 in FIG. 5C and thus has similar
limitations, including
that it is difficult to position the tissue engaging surface of DDM 631
against a tissue without
blocking the operator's view.
[0086] FIG. 6C shows another embodiment of a Differential Dissecting
Instrument 603
having a curved elongate member 620 with curved centerline 650 and tangent 651
to centerline
650 as centerline 650 approaches second point 622. The axis of rotation 640 is
perpendicular to
tangent 651 forming presentation angle 670, which is 90 in this example.
Elongate member 620
may similarly be bent, jointed, articulated, or otherwise made of a plurality
of parts. In all cases,
the presentation angle 670 is formed by the axis of rotation of a DDM and the
tangent of the
centerline as it approaches second point 622.
[0087] FIG. 6D shows another embodiment of a Differential Dissecting
Instrument 604
similar to Differential Dissecting Instrument 602 in FIG. 6B. Handle 610
connects to elongate
member 620 comprising a first end 621 connected to the handle 610 and a second
end 622
connected to a Type III DDM 631. DDM 631 is rotatably mounted to elongate
member 620 at
second end 622 such that DDM 631 reciprocally oscillates about its axis of
rotation 640. The
axis of rotation 640 is parallel to the plane of the page in FIG. 6D. First
end 621 and second end
622 define a centerline 650 of elongate member 620 with tangent 651 as
centerline 650
approaches second point 622. Axis of rotation 640 is thus aligned at a non-
zero angle to tangent
651 (i.e., the presentation angle 670 is between 0 and 90 ). In preferred
embodiments,
presentation angle 670 does not equal 0 , for the reasons described for
Differential Dissecting
Instrument 603 in FIG. 5C and FIG. 6B.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
23
[0088] FIG. 7A and FIG. 7B show another embodiment of the effector end of a
Differential
Dissecting Instrument 700 that uses a dissecting wire 710 as the DDM. FIG. 7A
shows the
assembled device. Dissecting wire 710 stands out a distance 725 from the
backing surface 726
of a shroud 730, the dissecting wire 710 emitting from a first post 720,
spanning gap 722, and
entering a second post 721 on the end of shroud 730. Dissecting wire 710 is a
continuous loop of
wire driven such that the exposed section of dissecting wire 710 travels in
the direction indicated
by arrow 723 across gap 722 in FIG. 7A.
[0089] FIG. 7B shows a schematic side view of this embodiment of a
Differential Dissecting
Instrument 700 that depicts the loop of dissecting wire 710 and drive
mechanism. Dissecting
wire 710 is a continuous loop that passes over a first idler bearing 750
housed in first post 720
and then emits from first post 720. Dissecting wire 710 travels across gap
722, moving in the
direction of arrow 723, and enters second post 721 where it passes over second
idler bearing 751.
The loop of dissecting wire 710 travels further back in shroud 730 where it
passes over a drive
wheel 760 which is turned by, for example, a motor in the direction of curved
arrow 724. Thus,
rotation of drive wheel 760 drives dissecting wire 710. Note that dissecting
wire 710 can be a
flexible linear element with any cross-sectional shape, so instead of being a
wire of circular
cross-sectional shape, dissecting wire 710 could be a flexible flat belt with
the outward-facing
side possessing a tissue engaging surface. Similarly, dissecting wire 710 can
be a flexible cord
having greater diameter than a wire would permit turning over idler bearings
750 and 751; the
flexible cord having a tissue engaging surface. Further, the distance 725
between the dissecting
wire 710 and the backing surface 726 can be arbitrarily large or small, for
example the distance
725 can be large enough to create a substantial area encircled by the
dissecting wire 710, the
backing surface 726 and the first post 720 and the second post 721, thus able
to surround a
Target Tissue to be removed. In contrast, distance 725 can be zero, where the
dissecting wire
710 runs along the surface of the shroud 730, or even in a slight
accommodating groove that
supports the dissecting wire 710 from behind. Such an accommodating groove can
have a semi-
circular cross-sectional shape thus exposing just a portion of the cross-
sectional shape of the
dissecting wire 710 to the tissue to be dissected. Further, the shape of the
backing surface 726
can be flat, or it can be curved, subtly or pronounced, and the curved surface
can possess convex
areas, concave areas, or a combination.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
24
[0090] FIG. 8A-8C show the effector end of a Differential Dissecting
Instrument 800 that
uses a flexible belt as the DDM. FIG. 8A shows the separate parts. Flexible
belt 840 has an
outer tissue engaging surface 850. Flexible belt 840 travels over idler wheel
810, which rotates
around shaft 820, all of which are housed in shroud 830.
[0091] FIG. 8B shows the assembled effector end of Differential Dissecting
Instrument 800
with only a limited portion of tissue engaging surface 850 of flexible belt
840 exposed.
[0092] FIG. 8C shows a top view of a schematic of one example of how a
flexible belt, such
as flexible belt 840, can be driven. Idler wheel 810 and drive wheel 860 are
mounted inside
shroud 830. Flexible belt 840 wraps around idler wheel 810 and drive wheel
860. Drive wheel
860 is powered to rotate such that flexible belt 840 is driven in the
direction indicated by curved
arrow 870. The tissue engaging surface 850 exposed outside the shroud 830 is
then used to
disrupt tissue. The drive wheel 860 can be driven by any of several
mechanisms, such as a
motor, hand crank, etc. The drive wheel 860 and the idler wheel 810 need not
be right circular
cylinders, nor must their rotational axes be parallel.
[0093] The extent of exposure of tissue engaging surfaces outside of the
shrouding can be
greater or less than those shown in the prior examples. In fact, varying the
exposure changes
several aspects of the behavior of the Differential Dissecting Instruments.
[0094] First, a larger exposure, increases the exposed area of the tissue
engaging surface,
which increases the amount of tissue disrupted per unit time and increases the
surface area of
tissue removed. Thus, decreasing the exposure allows more precise removal of
tissue, but it
reduces the total amount of material removed. Second, increasing the exposure
changes the
angle of exposed tissue engaging surface. Consider FIGS. 9A through 9C, which
show top view
schematics of the effector end of Differential Dissecting Instrument 800 with
successively
restricted exposure of tissue engaging surface 850 as controlled by the
aperture 900 in the
shroud. Aperture 900 is largest in FIG. 9A and smallest in FIG. 9C. As the
exposure is
restricted, the range of angles of the arrows normal to tissue engaging
surface 850 decreases. In
FIG. 9A, the tissue engaging surface 850 disrupts both forward and on the
sides. In FIG. 9C,
the tissue engaging surface 850 disrupts only forward. Thus, when the tissue
engaging surface
850 is applied to a tissue, different directions of contact are applied,
depending on the angle of
the exposed tissue engaging surface.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
[0095] Second, this increasing angle of exposure of the tissue engaging
surface 850 also
changes both the angles at which the contacted surface of a tissue is strained
and the torque on
the instrument. Consider FIGS. 10A-10C which show the friction on a tissue 400
created by
application of a tissue engaging surface 1010.
[0096] In FIG. 10A, tissue engaging surface 1010 is moving in the direction
of arrow 1020.
This produces a friction force in the direction of arrow 1030. The larger the
area of contact, the
larger the friction force. The friction force both pulls the tissue 400
sideways (in the direction of
arrow 1030), shearing the tissue 400, and forces the tissue engaging surface
1010 in the direction
opposite arrow 1020. If tissue engaging surface 1010 is mounted on an
instrument 1060 at a
distance from the point 1040 held by an operator, then the friction force
places a torque 1050
about point 1040. This torque can cause the end 1070 opposite point 1040 of
instrument 1060 to
be pulled away from the desired point of application, making control of
dissection more difficult.
Thus, limiting the extent of exposure of a tissue engaging surface reduces the
friction force and
improves control by reducing torque on the handle.
[0097] FIG. 10B shows how a circular tissue engaging surface 850 produces
friction forces
normal to the tissue engaging surface 850 and thus, in different directions
depending on the
range of contact of the tissue 400 on the circular tissue engaging surface
850. The resulting
multidirectional shearing forces on the tissue 400 produce more complex strain
patterns in the
tissue 400. As in FIG. 10A, the friction force still produces a net upward
force 1080 on the tip
of shroud 830; however, it does not produce a net left/right (into and out of
the tissue 400) force
on the tip of shroud 830. FIG. 10C shows that reducing the exposure of tissue
engaging surface
850 by narrowing aperture 900 makes the friction force on the tissue more 1-
dimensional,
simplifying strain patterns in the tissue.
[0098] Despite this discussion of friction against a tissue, as discussed
above with respect to
wetted tissues, a DDM as described herein has the unusual quality of being
effective when it has
low friction with respect to a Complex Tissue. The non-tissue engaging surface
and the tissue
engaging surface are effective even when the entire DDM is fully bathed with a
lubricant, such
as a surgical lubricant or a hydrogel lubricant.
[0099] In surgery, it is preferable to minimize unintended transport of
tissues to other parts
of the body. Disrupted pieces of tissue can adhere to the tissue engaging
surfaces of the
Differential Dissecting Instruments disclosed here. Unintended transport can
be minimized in
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
26
two ways. First, narrowing and controlling the shape of the aperture 900 as
shown in FIG. 10B
and FIG. 10C means that fragments of disrupted tissue adhering to the tissue
engaging surface
850 will only be transported a short distance before being deposited on or
entering the shroud.
Similarly, if they attach to but are then thrown tangentially away from the
tissue engaging
surface 850 by inertia, then narrowing the aperture 900 will reduce the
surface area available for
adhesion, the time available for adhesion and the distance that material can
be accelerated.
Second, the tissue engaging surface 850 can be made resistant to tissue
adhesion. Surface
treatment of a tissue engaging surface 850 can be achieved by any of several
techniques known
in the art, such as chemical treatment, vapor deposition, sputtering, and
others. For example,
fluorinating the tissue engaging surface 850 by any of several known methods
(e.g. dip coating,
chemical deposition, chemical cross-linking such as with silanes, etc.), can
make the tissue
engaging surface 850 resist tissue adhesion by both hydrophilic materials and
carbon-based
hydrophobic tissue components. In one embodiment, diamond/carbide coated
tissue engaging
surfaces may be used, which we have discovered to be much less likely to have
tissue adhere to
these surfaces.
[00100] Transport of materials can also be reduced by the use of an
oscillating (reciprocating)
motion of the DDM, rather than a continuous unidirectional or continuous
rotational motion.
Oscillation prevents transport over distances exceeding the distance of
oscillation, which can be
over only a few degrees of rotation (e.g. 5 degrees to 90 degrees). Any of a
number of
mechanisms can be used to drive reciprocating oscillating motion with a
rotating motor, such as
a Scotch yoke or crank/slider.
[00101] Tissue adherence is also a problem for decreasing the effectiveness of
the tissue
engaging surface 850. Clogging of the tissue engaging surface 850 creates a
thick coat of
material over the tissue engaging surface 850, making it much less effective
at ablating Soft
Tissue. As above, making the surface resistant to adhesion by tissues
decreases this problem.
Fluorinated tissue engaging surfaces and diamond/carbide tissue engaging
surfaces don't clog as
readily, especially when disrupting fatty tissues.
[00102] Clogging is also reduced if the tissue is wet and further if the
tissue engaging surface
850 is flushed with water, as discussed earlier. FIG. 11A and FIG. 11B show a
Differential
Dissecting Instrument 1100 in which a first array of 3 water outlets 1111
emits beside tissue
engaging surface 850 from shroud 830. A second array of 3 water outlets 1112
emits on the
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
27
opposite side of tissue engaging surface 850. Other arrangements of water
outlets are possible.
FIG. 11A shows a solid model in oblique view. FIG. 11B shows the top view for
a schematic of
Differential Dissecting Instrument 1100 in which water tube 1121 carries
water, or other fluid
such as physiological saline, inside and to one side of shroud 830 to water
outlets 1111, and a
second water tube 1122 carries fluid inside and to the other side of shroud
830 to water outlets
1112. Water outlets 1111 and 1112 emit from opposite sides of aperture 900,
providing fluid to
both sides of tissue engaging surface 850. The liquid emitting from water
outlets can, optionally,
carry physiologically active materials, either dissolved or suspended in the
liquid.
Physiologically active materials can include various pharmaceutical compounds
(antibiotics,
anti-inflammatories, etc.) and active biomolecules (e.g. cytokines,
collagenases, etc.)
[00103] Appropriate arrangement of tissue engaging surfaces 850 creates
friction forces on
tissues that can be used to advantage during blunt dissection. FIG. 12 shows a
Differential
Dissecting Instrument 1200 having two, opposing flexible belts 1201 and 1202
exposed in
aperture 1230. Each belt is configured as in FIG. 10B with flexible belt 1201
running over idle
1211 and flexible belt 1202 running over idle 1212, but the flexible belts
1201 and 1202 circulate
in opposite sense with respect to each other. Thus, the flexible belt 1201 and
the flexible belt
1202 run side by side in the same direction as shown by arrows 1203 and 1204
but in opposite
directions when exposed to the tissue 1205, as shown by arrows 1271 and 1272.
Thus, the
flexible belt 1201 creates a net force 1251 downward and the flexible belt
1202 creates a net
force 1252 upward on shroud 1220, whereby these forces 1251 and 1252 cancel,
leaving little or
no net force on the shroud 1220. This eliminates any torqueing of Differential
Dissecting
Instrument 1200 (as described in FIG. 10A), making it easier for an operator
to control.
Additionally, the opposing directions of motion 1271 and 1272 of flexible
belts 1201 and 1202
create opposing frictional forces on tissue 1205 during dissection, thereby
pulling the tissue 1205
apart in the region identified by double headed arrow 1260. This pulling
action can facilitate
blunt dissection by tearing the tissue in the region of double headed arrow
1260. Note that the
gap 1280 between flexible belts 1201 and 1202 inside shroud 1220 can be varied
and can be
reduced to zero such that flexible belts 1201 and 1202 are in contact. Contact
between flexible
belts 1201 and 1202 can help a drive mechanism match the rates of travel of
flexible belts 1201
and 1202. In fact, friction between flexible belts 1201 and 1202 can allow one
belt, for example
1201, to drive the other belt, in this example 1202. Thus a motor, for
example, can actively drive
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
28
flexible belt 1201, and flexible belt 1202 is then driven by flexible belt
1201. This can simplify
the drive mechanism for two belts.
[00104] FIG. 13 shows how the shroud 1330 of a Differential Dissecting
Instrument 1300 can
house other items, permitting greater functionality. Dissecting wheel 810 is
exposed at aperture
900. Suction lines 1301 and 1302 can connect to the front of the shroud
1330 near tissue
engaging surface 850, helping to remove any debris from disruption or excess
fluid, such as fluid
from water tubes 1121 and 1122 which emit through water outlets 1111 and 1112.
Light
emitting diodes (LEDs) can be placed on shroud 1330 to better illuminate an
area for blunt
dissection; for example, LEDs 1311 and 1312 are supplied with power by cables
1313 and 1314,
respectively, and light from LEDs 1311 and 1312 directly illuminates the
tissue in the region of
disruption.
[00105] FIGS. 14-1 through 14-3 show how the elongate member 1410 of a
Differential
Dissecting Instrument 1400 can be articulated with a bendable region 1430 such
that a user can
achieve variable bending of the elongate member 1410 to facilitate placement
of the DDM 1420.
FIG. 14-1 depicts the elongate member of the differential dissecting
instrument in Position 1,
straight, FIG. 14-2 shows the elongate member of the differential dissecting
instrument bent at
45 degrees, and FIG. 14-3 illustrates the elongate member of the differential
dissecting
instrument bent at 90 degrees. In Position 1 (Fig 14-1), the elongate member
1410 is straight.
In Position 2 (FIG. 14-2) and then in Position 3 (FIG. 14-3), elongate member
1410 is
successively bent at bendable region 1430 such that the DDM 1420 moves from
forward-facing
in Position 1 to side-facing in Position 3. Bendable region 1430 can be an
articulated joint or
any other mechanism to permit bending.
[00106] FIGS. 15A-15E show different DDMs, illustrating several important
dimensions and
features of DDMs. FIG. 15A shows a top view of an exemplary differential
dissecting member
that rotates about a rotational joint. FIG. 15A shows a top view of a DDM 1500
that rotates
about a rotational joint 1510. Actuation of DDM 1500 causes it to reciprocally
oscillate up and
down, as shown by the double headed arrow 1506 such that tissue engaging
surface 1520
(pebbled section) swings through an arc with radius RA. Oscillation of DDM
1500 can swing
through a range of 90 degrees. The tissue engaging surface has a minimum
radius Rs in the
plane of rotation (the plane perpendicular to the plane of rotation ¨ the
plane of the page here).
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
29
[00107] FIG. 15B shows a side view in cross-section with two successively
enlarged views.
(DDM 1500 thus oscillates in and out of the page in this view.) FIG. 15B-1
through 15B-3
depict a differential dissecting member as in FIG. 15A; FIG. 15B-1 shows the
differential
dissecting member in side view cross-section, FIG. 15B-2 depicts a close-up
view of the tip of
the differential dissecting member shown in FIG. 15B-1, and FIG. 15B-3 shows a
close-up view
of the surface of the differential dissecting member shown in FIG. 15B-2.
First side 1530 and
tissue engaging surface 1520 join at first margin 1540, having a radius of
curvature RE, and
second side 1531 and tissue engaging surface 1520 join at second margin 1541,
having radius of
curvature RE, where the radii of curvature of first margin 1540 and second
margin 1541 can be
different, but should be large enough such that the first margin 1540 and the
second margin 1541
are not sharp. Tissue engaging surface 1520 is then created by projections
1550 with a
maximum length Lma,,, defined as the maximum length of a feature from the
innermost trough to
the outermost peak.
[00108] FIG. 15C illustrates a different DDM 1501 having a scalloped tissue
engaging
surface formed by surface features 1560. Here, the surface feature 1560 is a
convex lobe, but a
surface feature 1560 can be any regular or repeating feature on the tissue
engaging surface 1520
having a minimum radius of curvature R. Furthermore, surface features can have
a profile that
is not in the plane of rotation, as shown in FIG. 15D and FIG. 15E. FIG. 15D
shows an oblique
view and FIG. 15E shows an end-on view. FIG. 15E-1 illustrates an end-on view
of the
differential dissecting member depicted in FIG. 15C, FIG. 15E-2 depicts a
close-up view of the
tissue-engaging surface of the differential dissecting member shown in FIG.
15E-1, and FIG.
15E-3 details a very close-up view of the surface features of the differential
dissecting member
shown in FIG. 15E-1 and FIG. 15E-2. The inserts in FIG. 15E-1 through 15E-3
show
successively magnified sections of the DDM 1502 taken along the 45 angle. DDM
1502 has
surface features 1570 with a profile in a plane at 45 to the plane of
rotation. As with DDM 1501
in FIG. 15C, the tissue engaging surface 1520 of DDM 1502 has projections 1550
with a
maximum length Lma, . In one embodiment, RA can be between approximately one
(1) mm and
approximately one hundred (100) mm. In one embodiment, Rs can be between
approximately
0.1 mm and approximately ten (10) mm. In one embodiment, RE can be between
approximately
0.05 mm and approximately ten (10) mm, such that no slicing edge is presented
to a tissue.
Alternatively, for some embodiments of a DDM, Rs and Re can be as small as
about 0.025 mm.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
[00109] DDMs can have tissue engaging surfaces that are scalloped, or notched,
or have
undulating profiles such that the angle of attack of the tissue engaging
surface with respect to the
surface of the tissue varies as the tissue engaging surface passes over a
given point in the tissue.
In fact, the angle of attack varies for any DDM for which Pavg < (Rim, -
Rmiii), e.g. for a DDM
Type I, Type II, or Type IV. A varying angle of attack makes the dissecting
action more
aggressive, in which a more aggressive DDM is better able to disrupt a firmer
tissue and a less
aggressive DDM is less able to disrupt that same tissue.
[00110] FIGS. 16-1 through 16-3 show an alternate means by which DDMs can be
made with
different levels of aggressiveness, i.e. the aggressiveness of a DDM can be
designed. DDM
1600 rotates about an axis of rotation 1610 and has a tissue engaging surface
1620 bearing
projections 1622. These projections (FIG. 16-1) have more pointed tips (but
still not sharp
enough to slice). DDM 1640 has a tissue engaging surface 1650 bearing
projections having
more rounded tips 1652 (FIG. 16-2). DDM 1680 has a tissue engaging surface
1690 bearing
projections with even more rounded tips 1692 (FIG. 16-3). DDM 1600 is more
aggressive than
DDM 1640 which is more aggressive than DDM 1680.
[00111] FIG. 17A shows one embodiment of a DDM 1700 having a scalloped tissue
engaging
surface 1710 and a center of rotation 1720. DDM 1700 is thus an example of a
DDM Type IV.
Oscillation of DDM 1700 back and forth as shown by double headed arrow 1730
causes tissue
engaging surface 1710 to move over a tissue such that the edges of the scallop
bring the tissue
engaging surface 1710 to bear at different angles of attack as each scallop
passes over the tissue.
[00112] FIGS. 17B-1 and 17B-2 illustrate the action of DDM 1700 against a
tissue 1750.
FIG. 17B-1 shows that same differential dissecting member impinging on a
tissue, and FIG.
17B-2 is a close-up view of the lobes of the lobate differential dissecting
member detailing the
angles of attack of the tissue engaging surface with respect to the tissue.
The angle of attack (the
angle 0 between the direction of motion and the tangent to the tissue engaging
surface 1710 at a
point of contact) is shown at two points P1 and P2 on the tissue engaging
surface 1710. 01 is
smaller than 02. Similar action can be achieved with a DDM 1800, as shown in
FIG. 18, by
using a circular tissue engaging component 1805 with tissue engaging surface
1810 and a center
of rotation 1820 that is not the center of circular tissue engaging component
1805 (e.g., a DDM
Type II). Oscillation of tissue engaging component 1805 back and forth as
shown by double
headed arrow 1830 causes tissue engaging surface 1810 to move over a tissue
such that the tissue
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
31
engaging surface 1810 moves such that the angle of attack varies at each point
on the tissue
engaging surface 1810 on the perimeter of the circular tissue engaging
component 1805.
[00113] FIG. 18 illustrates another important point, especially for
accelerating motions of a
DDM against a tissue 1850, and accelerations occur whenever a DDM is loaded or
unloaded and
whenever an oscillating DDM decelerates after sweeping one direction and
accelerates to sweep
in the opposite direction. DDM 1800 is mounted with its center of gravity 1870
displaced from
the center of rotation 1820. The solid double-headed arrow 1830 shows the
rotation about the
center of rotation 1820, and the dashed double-headed arrow 1840 shows the
motion of center of
gravity 1870. The force of accelerating the mass of DDM 1800 and the distance
between the
center of gravity 1870 and the center of rotation 1820 create a moment about
the center of
rotation 1820 which causes a differential dissector to vibrate. This moment
will cause the handle
of a differential dissector, to which the DDM 1800 is attached, to shake. DDMs
composed of
denser materials will make the shaking more extreme. It can, thus, be
advantageous to make
DDMs from less dense materials, like rigid polymers rather than metals, to
decrease shaking of
the handle. Conversely, one might arrange a countering moment through
appropriate distribution
of mass within a DDM to place the center of gravity at the axis of rotation.
[00114] The entirety of the surface of a DDM can be tissue engaging.
Alternatively, selected
portions of the surface can be tissue engaging. This can be advantageous to
restrict dissection
effects to one region of the surface of the DDM, the forward-looking surface,
for example. FIG.
19A through FIG. 19D show a Differential Dissecting Instrument 1900 that has a
DDM that is a
dissecting wheel 1910 that is similar to that shown in FIG. 3A through FIG.
3C; however, the
tissue engaging surface is restricted to a thin tissue engaging strip 1920
around the outer
perimeter of dissecting wheel 1910 which rotates around axis of rotation 365.
The remainder
comprises the non-tissue engaging surface 1930, disposed laterally to either
side of tissue
engaging strip 1920, of the exposed surface of the dissecting wheel 1910 and
has a much
smoother surface, optionally being glass smooth, free of projections, or
otherwise unable to
engage fibers in the tissues. FIG. 19B illustrates how a dissecting wheel 1910
fits into shroud
1940 and is pressed by an operator in the direction 367. As FIG. 19C
illustrates, non-tissue
engaging surface 1930, which is smoother than tissue engaging strip 1920,
reduces disruption of
tissue 1950 after it has been separated by tissue engaging strip 1920. Shroud
1940 further
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
32
protects tissue 1950 from disruption by dissecting wheel 1910 as the dissector
penetrates further
into tissue 1950 in the direction of pressing 367.
[00115] FIG. 19D illustrates an additional, important action of non-tissue
engaging surface
1930 and of shroud 1940. When there is a component of motion 1901 in the
direction of
pressing 367 (not shown here) of Differential Dissecting Instrument 1900 into
tissue 1950, these
wider portions (non-tissue engaging surface 1930 and of shroud 1940) of
Differential Dissecting
Instrument 1900 force apart, or wedge, recently separated portions of tissue
1950, aligning and
straining the fibrous components 1980 of tissue 1950, putting them in tension
and aligning them
perpendicular to the motion of tissue engaging strip 1920. This strain in
fibrous components
1980 facilitates the ability of the projections of the tissue engaging
materials in tissue engaging
strip 1920 to grab and tear individual fibers.
[00116] As tissue engaging strip 1920 moves past tissue 1950, moving in a
direction
perpendicular to (and so through) the plane of the page, the projections on
tissue engaging strip
1920 therein disrupt tissue 1950, including tearing individual fibrous
components 1980 of tissue
1950 (e.g. collagen or elastin fibers). Such fibrous components 1980
frequently have irregular
alignments (i.e., irregular orientations) in Soft Tissues. However, as tissue
1950 is disrupted,
Differential Dissecting Instrument 1900 pushes into tissue 1950 in the
direction of component of
motion 1901 such that as remaining tissue engaging surface 1930 and shroud
1940 push into the
separated tissue 1950, they push tissue 1950, including severed fibrous
components 1990, aside
in the direction of arrows 1960 and 1961, aligning previously irregularly
oriented fibers and
straining material at the point of contact of tissue engaging strip 1920. This
local region of strain
aligns and strains (and so pre-stresses) unsevered fibrous components 1980 in
a direction
perpendicular to the direction of motion of tissue engaging strip 1920, as
shown by double-ended
arrow 1970, facilitating their being grabbed and increasing the likelihood of
their being severed
by projections from tissue engaging strip 1920. Non-tissue engaging surface
1930 and of shroud
1940 will act as a wedge if they are angled with respect to one another, as
shown in FIG. 19C
and FIG. 19D or even if they have a width that is wider than the tissue
engaging surface 1910.
In one embodiment, a semi-ellipsoid shape, as described in FIG. 3F, in which
the second minor
semi-axis C is a significant fraction of the first minor semi-axis B (e.g., in
one embodiment,
where 0.2B <C < 0.8B), is an effective shape for wedging.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
33
[00117] Alignment of fibers, as described in the preceding paragraph, can
greatly alter how a
DDM performs. Alignment can be achieved by the surgeon straining a tissue in
the appropriate
directions with their hands or with a separate instrument. Alignment can be
achieved by the
DDM, as described in the preceding paragraph, by a smooth portion on a tissue
engaging wheel,
such as non-tissue engaging surface 1930 in FIG. 19C through FIG. 19D, by a
smooth shroud,
such as shroud 1940 in FIG. 19A through FIG. 19D, or by a separate mechanism
on a DDM.
[00118] FIG. 20 shows details of one version of disruption of tissue segments
in a human
patient. The region of interest 2000 of the patient is depicted within a
circular window, showing
a section view through two apposed volumes, namely a tissue segment A apposed
to a tissue
segment B; the apposition occurs in a region 2010 bridged by both interstitial
fibers 2012 and
taut interstitial fibers 2015 and further associated with broken interstitial
fibers 2020. Also
depicted in the circular window is a DDM 2030 possessing a tissue engaging
surface 2034 that
further possesses projections 2032 and a smooth non-tissue engaging surface
2033. In this view,
the DDM 2030 reciprocates about an axis 2036, so that the motion of the fiber-
engaging
projections 2032 is in and out of the plane of the page (i.e., reciprocally
toward and away from
the viewer).
[00119] Each of the tissue segment A and tissue segment B further has a tissue
segment
surface 2005 and a tissue segment surface 2006, respectively, composed of
relatively tightly
packed fibers aligned parallel to tissue segment surface 2005 and tissue
segment surface 2006,
forming a membranous covering over tissue segment A and tissue segment B
(e.g., tissue
segments A and B comprise Firm Tissues). Tissue segment A's surface 2005 and
tissue segment
B's surface 2006 are also three-dimensionally curvaceous. While these tissue
segment surfaces
2005 and 2006 may not be in contact with one another at every point, tissue
surface 2005 and
tissue segment surface 2006 do meet in a region 2010 where tissue segment
surface 2005 and
tissue segment surface 2006 are apposed in a locally, roughly parallel manner,
and are frequently
substantially in contact with one another.
[00120] In that region 2010, the tissue segment surface 2005 and the tissue
segment surface
2006 are secured to one another by a population of relatively loose
interstitial fibers 2012 that
run substantially perpendicularly to the two apposed tissue segment surfaces
2005 and 2006.
This sparse population of interstitial fibers 2012 may or may not also be
derived from (or be
members of) the populations of fibers comprising the more tightly packed woven
surfaces that
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
34
form the tissue segment surfaces 2005 and 2006. For example, a given fiber
comprising part of a
tissue segment surface 2005 may run along that surface for some distance
before turning away
and continuing across the region 2010, thereby becoming a member of the
population of
interstitial fibers 2012, and further, may continue across the region 2010 to
tissue segment
surface 2006, where it can turn and interweave therein, thereby becoming a
member of the
population of fibers comprising tissue segment surface 2006. Thus, the
definition of interstitial
fibers 2012 includes any fibers crossing, bridging, traversing or otherwise
connecting (or
intimately associated with) the region 2010 where tissue segment surface 2005
and tissue
segment surface 2006 are in apposition. The interstitial fibers 2012 may be
the same type of
fibers as those comprising the tissue segment surface 2005 and tissue segment
surface 2006 of
tissue segment A and tissue segment B in one embodiment. In another
embodiment, the
interstitial fibers 2012 may be a distinct type, and the interstitial fibers
2012 may be strongly or
weakly bound, directly or indirectly, to the tissue segment surface 2005 and
the tissue segment
surface 2006.
[00121] In each case, all fibers involved are mechanically capable of
transmitting force (via
tension) either along the surface of each individual tissue segment, or
interstitially, between the
two tissue segments, or both. For example, the state of tension of the
interstitial fibers 2010 and
the fibers comprising the tissue segment surface 2005 and the tissue segment
surface 2006
depends on the forces that act upon tissue segment A and tissue segment B, for
example when
smooth non-tissue engaging surface 2033 wedges into and forces apart these
tissue segments in
the directions 2040 and 2041. For example, the fibers 2010 resist tensile
strains that arise from
the motion of tissue segment surface 2005 in the direction 2040 and the motion
of tissue segment
surface 2006 in the direction 2041 relative to one another, and further, this
resistance varies
according to the mechanical properties of the fibers. For example, if the
unstrained interstitial
fibers 2012 are aligned perpendicularly to the two apposed tissue segment
surfaces 2005 and
2006, then the distance between tissue segment A and tissue segment B may be
increased (as
shown by arrow 2030) until the interstitial fibers 2010 first become
straightened like the taut
interstitial fibers 2015, and then finally the fibers may fail, as is shown by
the broken interstitial
fibers 2020. The most common fiber type in humans is collagen, which possesses
a breaking
strain of about 5% beyond unstressed normal length. If tissue segment A and
tissue segment B
are moved apart as shown by arrow 2030, the collagen fibers (here, unstrained
interstitial fibers
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
2012) will first become taut (as are taut fibers 2015). If the two tissue
segments A and B are
moved even further apart, collagen fibers will stretch about 5%. Crucially, at
this point, if tissue
segment A is moved further than 5% beyond taut from tissue segment B, either
the taut
interstitial fibers 2015 will break, or, if the taut fibers 2012 do not break,
the tissue segments
themselves may rupture, with deleterious consequences for the patient.
[00122] Since surgeons very often must separate, dissever, or otherwise move
tissue segments
with respect to one another to gain access to various areas inside patients,
surgeons are
constantly straining fiber populations equivalent to interstitial fibers 2010
throughout patients'
bodies. Current practice requires either slicing interstitial fibers to free
one tissue segment from
another, or tearing interstitial fibers wholesale by applying blunt force with
forceps (by opening
the jaws, forcing the tissue segments apart, and so tearing the interstitial
fibers). Common
complications are either slicing into the tissue segments while attempting to
cut only the
interstitial fibers via sharp dissection or tearing off smaller or larger
portions of the tissue
segments while attempting blunt dissection of the interstitial fibers. Either
approach first strains
to tautness the interstitial fibers 2010, then stretches them, and then tears
them. The
consequences (for example, air leaks and bleeding of segments of the lung) of
the
aforementioned intimate connection of the interstitial fibers 2010 with the
tissue segment
surfaces 2005 and 2006 now becomes clear: one must segregate the forces
required to cause the
interstitial fibers to fail without also subjecting the integrated tissue
segments themselves to the
same forces.
[00123] The embodiments of the Differential Dissecting Instruments disclosed
herein are
specifically designed to segregate forces on fiber populations by generating
an initial separating
motion of apposed tissue segments A and B via impingement of the smooth
surfaces 2033, thus
exposing and tensioning (pre-stressing) individual interstitial fibers 2010,
making these fibers
much more likely to break, exploiting the opportunity provided by these now
taut interstitial
fibers 2015, and further allowing those to be discreetly encountered, engaged
and converted into
broken interstitial fibers 2020 by the local impingement of projections 2032
of the tissue
engaging surface 2034 of the Differential Dissecting Member 2030. In this way,
a DDM having
a smooth-sided non-tissue engaging surface and/or shroud can greatly increase
both the speed
and effectiveness of dissection of tissues while limiting the extent of that
dissection effect to just
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
36
those fibers within Soft Tissues that connect adjacent regions of Firm Tissues
and still preserving
those Firm Tissues.
[00124] FIG. 21A through FIG. 21C-4 illustrate another Differential Dissecting
Instrument
2100 that uses a very thin dissecting wheel 2110 as the DDM. Dissecting wheel
2110 is nearly
entirely wrapped in a shroud 2120 to achieve a very thin tissue engaging
surface 2009 with
shroud 2120 acting to protect, separate and pre-stress the tissue to be
dissected, as shown in FIG.
19D.
[00125] FIG. 21A shows a side view, and FIG. 21B shows a front view. FIG. 21A
shows a
side view of a differential dissecting member that has a thin dissecting wheel
and is wrapped in a
shroud; FIG. 21B-1 and FIG. 21B-2 further illustrate a front view of the
shrouded differential
dissecting member in FIG. 21A and a close-up view of same, respectively.
Dissecting wheel
2110 is mounted on two posts, first post 2130 and second post 2131 (seen in
side view of FIG.
21B-1) via rotational axle 2135. Rotational axle 2135 is free to rotate within
first post 2130 and
second post 2131, but is firmly affixed to dissecting wheel 2110. Sprocket
2140 is also firmly
affixed to axle 2135. Sprocket 2140 is turned by drive belt 2150. Thus, a
drive mechanism 2160
is created by first post 2130 and second post 2131, axle 2135, sprocket 2140,
and drive belt 2150
to turn dissecting wheel 2110 inside shroud 2120 in the direction of arrow
2161. Alternate drive
mechanisms can be used, and motion can either be rotational or oscillatory.
The first margin
2111 and second margin 2112 of dissecting wheel 2110 preferably are not sharp,
as shown in the
enlarged portion of FIG. 21B-2. (First and second margins 2111 and 2112 are
like first and
second margins 1540 and 1541 in FIGS. 15B-1 through 15B-3.) Sharp margins can
disrupt
more aggressively than a rounded margin; nevertheless, a sharper margin can be
used if more
aggressive disruption or even disrupting is desired. Furthermore, one margin
can be sharper than
the other if a differential disruption or disrupting is desired. For example,
first margin 2111 can
be square or even sharp, while second margin 2112 can be rounded to achieve
more aggressive
disruption or disrupting on the side of first margin 2111.
[00126] Shroud 2120 nearly encloses dissecting wheel 2110, leaving only a fine
portion of
dissecting wheel 2110 exposed as the tissue engaging surface 2111, and forming
a wedge angle
w that determines the strain on tissue at the point of disruption of
dissecting wheel 2110. Larger
wedge angles w strain tissue more as DDM 2100 is pushed into a tissue. FIGS.
21C-1 through
21C-4 depict DDM 2100 with shroud 2120 in four different positions. Shroud
2120 can be
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
37
moved independently of drive mechanism 2160 and dissecting wheel 2110, shroud
2120 being
able to move in the direction of double headed arrow 2190. Thus, in Position 1
(FIG. 21C-1)
only a thin portion of dissecting wheel 2110 is exposed. In Position 2 (FIG.
21C-2), shroud
2120 has been moved in the direction of arrow 2191, leaving a thinner portion
of dissecting
wheel 2110 exposed and also creating a larger wedge angle (0. In Position 3
(FIG. 21C-3),
shroud 2120 has been moved in the direction of arrow 2192 such that shroud
2120 completely
encloses dissecting wheel 2110. Thus, dissecting wheel 2110 can no longer
disrupt tissue. In
this position, the dissecting wheel 2110 effectively acts as a smooth, flat,
blunt probe. In
Position 4 (FIG. 21C-4), shroud 2120 has moved in the direction of arrow 2193,
increasing the
exposure seen in Position 1 or Position 2 of dissecting wheel 2110 and
decreasing wedge angle
(0.
[00127] FIG. 22 shows the distal end of a differential dissector 2210,
including one
embodiment of a reciprocating mechanism, here a scotch yoke. The distal end of
differential
dissector 2210 includes a housing 2212, which further contains a pivot bearing
2214, a motor
shaft bearing 2216, and a shaft drum bearing 2218. FIG. 22 also shows a motor
shaft 2220, a
shaft drum 2222 coaxial with and affixed to the motor shaft 2220, and a driver
pin 2224 which
may be parallel but not coaxial to motor shaft 2220, and is itself affixed to
the shaft drum 2222.
Further, there is a Differential Dissecting Member, DDM 2230, which is
associated with the
differential dissector housing 2212, and further comprises an outer surface
2231 defining the
body of the DDM 2230, a tissue engaging surface 2232 forming at least a
portion of the outer
surface 2231, a DDM pivot shaft 2234 that fits into the pivot bearing 2214,
and further comprises
a hollow DDM pin follower 2236 that effectively captures the driver pin 2224.
The internal
three-dimensional shape of the hollow DDM pin follower 2236 is here shown as a
prism, so that
in the view shown in FIG. 22 the cross-sectional shape resembles an hourglass,
while
perpendicular to that view, the cross-sectional shape is rectilinear.
[00128] FIG. 23A, FIG. 23B, and FIG. 23C show a sectional view of a portion of
the DDM
2230 of FIG. 22 through the narrowest portion of the waist of the hourglass-
shaped hollow
DDM pin follower 2236 and perpendicular to the rotational axis of the shaft
drum 2222. The
shape of the DDM pin follower 2236 is in this view rectangular; further, in
this view showing the
dimensions through the waist of 2236 the height of the rectangle is equal or
larger than a
diameter described by the outer diameter of the driver pin 2224 along its
circular path 2237. The
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
38
width of the rectangle in this view corresponds to the outer diameter of the
driver pin 2224. The
DDM 2230 containing the hollow DDM pin follower 2236 rotates about the axis
2233 of the
shaft 2234. Thus, the position of the hollow DDM pin follower 2236 and so the
rotational
position of the DDM 2230 is determined by the rotational position of the
driver pin 2224.
[00129] In operation, referring to FIG. 22, along with FIGS. 23A-23C, the
motor (not shown)
turns the motor shaft 2220, which turns the drum 2222 about its axis of
rotation, which causes
the driver pin 2224 to travel about a circular path 2237, the plane of which
is here perpendicular
to the rotational axis of the drum 2222. As in a scotch yoke, the
rectangularly hollow DDM pin
follower 2236 converts the circular path 2237 of the driver pin 2224 into
linear travel 2238 of the
hollow DDM pin follower 2236; given that the pin follower 2236 is located some
distance away
from the axis 2233, the DDM 2230 is leveraged about the axis 2233, so
converting the rotational
path 2237 into linear travel 2238 and so reciprocating motion of the DDM 2230
rotating about
the DDM pivot shaft 2234 held by the pivot bearing 2214. The pattern of the
reciprocal motion
of the DDM 2230 can be controlled by varying the shape of the hollow DDM pin
follower 2236,
the driver pin 2224, the 3D angle of the axis 2233 about which the shaft 2234
rotates, the
distance from the driver pin 2224 to the axis 2233, and also by varying the
rotational speed of the
motor.
[00130] The DDM 2230 of FIG. 22 may have reciprocating motion 2250 and 2251,
as shown
in side view in FIG. 24A and FIG. 24B. The oscillation sequence shown depicts
the extreme
positions of the DDM 2230 as the driver pin 2224 travels about circular path
2237 when
provided with rotational motion 2299 from the motor (not shown). The action of
the tissue
engaging surface 2232 of the DDM 2230 on the surface of the tissues to be
dissected is best
shown in an edge-on view in FIG. 20.
[00131] A surgeon operating inside a patient desires to create the least
trauma possible to
tissues which are not the focus of the procedure, or are simply in the way of
the Target Tissue.
To this end, FIGS. 25A through 25C depict the profile view of an embodiment of
a largely
shrouded DDM assembly 2500, further comprising a shrouded pivot shaft 2510
that projects
perpendicularly to the page (i.e., at the viewer), an internal motor shaft
2550, an internal driver
drum 2522, a driver pin 2524, a DDI housing 2512, a DDM 2520 that reciprocates
about the
shrouded pivot shaft 2510 (and so within the plane of the page), a tissue
engaging DDM surface
2534, a smooth DDM surface 2518, a substantially circular DDM region 2516, a
shroud margin
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
39
2517, and a shroud-DDM gap 2514. Considered as a whole, with all exterior
surfaces of the
DDM assembly 2500 included as one, a shrouded DDM assembly 2500 presents a
nearly
continuous smooth surface to a patient's tissues. In this regard, other than
the limited extent of
the tissue engaging DDM surface 2534, the entire Differential Dissecting
Instrument fitted with
the DDM assembly 2500 acts like nothing more than a polished probe.
[00132] Once activated, the DDM 2520 reciprocates within and relative to the
housing 2512.
At the edge of the housing 2512 closest to the DDM 2520 is the shroud margin
2517. Between
the shroud margin 2517 and the DDM 2520 is found the shroud-DDM gap 2514. In
one
embodiment, a Differential Dissecting Instrument fitted with a DDM assembly
2500 includes
provisions for preserving the outwardly smooth character of the Differential
Dissecting
Instrument. The shroud-DDM gap 2514 thus presents a challenge, in that any
relative motion of
the DDM 2520 with respect to the housing 2512 could enlarge the shroud-DDM gap
2514,
presenting sharp edges to the tissues. Alternatively, a portion of the DDM
2520 could impact the
housing 2512. Also, in one embodiment, the shroud-DDM gap 2514 is kept as
small as possible
at all times. To facilitate this, the DDM 2520 has a circular DDM region 2516,
defined in this
perspective as a portion of the mass of the DDM 2520 having the cross-section
of a circle with
its center coincident with the axis of the shrouded pivot shaft 2510. This
circular DDM region
2516 defines and occupies that portion of the outer surface of the DDM 2520
that passes the
shroud margin 2517 during reciprocating motion of the DDM 2520, and at a
distance that defines
the shroud-DDM gap 2514. Because the circular DDM region 2516 preserves over
the angle of
rotation the same radius of DDM 2520, this preserves the shroud-DDM gap 2514
at a constant
value (i.e., shroud-DDM gap 2514 does not change despite motion of the DDM
2520). Thus, the
Differential Dissecting Instrument that is fitted with this DDM assembly
presents to the tissues a
continuously smooth surface everywhere through time.
[00133] FIG. 25D depicts an oblique view of the largely shrouded DDM assembly
2500,
showing a housing 2512, a DDM 2520 that reciprocates about the shrouded pivot
shaft 2510 (see
FIGS. 25A through 25C), a tissue engaging DDM surface 2534, a smooth DDM
surface 2518, a
substantially circular DDM region 2516, a shroud margin 2517, and a shroud-DDM
gap 2514.
[00134] Sharp dissection is frequently performed alternately with blunt
dissection when
exposing a Target Tissue. This occurs whenever a membrane or a large fibrous
component,
which resists blunt dissection, is encountered and must be severed for the
surgeon to penetrate
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
further into a tissue. Current practice requires that a surgeon either use a
suboptimal instrument
for blunt dissection (e.g., an inactive electrosurgery scalpel) or to swap
instruments while
exposing a Target Tissue. Use of a suboptimal instrument decreases the ease of
blunt dissection
and increases potential risk to a Target Tissue. Swapping consumes time and is
distracting,
especially for many minimally invasive procedures in which the instrument must
pass through a
narrow orifice in the body wall and then be gently guided to the site, such as
during laparoscopy
and thoracoscopy. A Differential Dissecting Instrument can be equipped with a
sharp dissecting
component that can be selectively activated by a surgeon, eliminating the need
for instrument
swapping while still providing the surgeon with an optimal instrument.
[00135] FIGS. 26A-1 and 26A-2 show a top and side view, respectively, of one
embodiment
of a Differential Dissecting Instrument 2600, similar to Differential
Dissecting Instrument 2000,
as shown in FIG. 20, but now also comprising a retractable scalpel blade that
is covered during
blunt dissection. FIG. 26A-1 and FIG. 26B-1 show side views while FIG. 26A-2
and FIG.
26B-2 show top views; FIG. 26A-1 and FIG. 26A-2 show the differential
dissecting member
with a retractable scalpel withdrawn. The retractable scalpel blade can be
projected outward by a
surgeon for sharp dissection and then retracted before proceeding with further
blunt dissection.
Differential Dissecting Instrument 2600 has an elongate member comprised of
shroud 2620 to
which DDM 2610 is rotatably mounted via rotational axle 2635. To one side of
DDM 2610 is a
slot 2612 under which lies retractable scalpel blade 2622 such that
retractable scalpel blade 2622
is completely covered by shroud 2620. Retractable scalpel blade 2622 is
actuated by a retraction
mechanism (not illustrated) controlled by a surgeon. Actuation of the
retractable scalpel blade
2622 can be controlled manually via a slider, by electrical actuation (such as
a solenoid), or by
any suitable mechanism controllable by an operator.
[00136] FIGS. 26B-1 and 26B-2 show Differential Dissecting Instrument 2600
with
retractable scalpel blade 2622 extended for sharp dissection. FIG. 26B-1 and
FIG. 26B-2 show
the same differential dissecting member with the retractable scalpel extended.
Retractable scalpel
blade 2622 is one example of a sharp dissecting tool. In other embodiments,
the Differential
Dissecting Instrument 2600 could include other sharp dissection tools, such as
an electrosurgery
blade, ultrasonic cutter, or a disrupting hook. In other embodiments, the
Differential Dissecting
Instrument 2600 could include a tool for energetic disruption, for example an
electrocautery
blade or electrosurgery head. Additionally, instead of retraction, retractable
scalpel blade 2622,
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
41
or other suitable tool, could be selectively be exposed for use by one of
several mechanisms,
such as by pop-out, by unfolding, or other mechanism known in the art.
[00137] FIGS. 27A and 27B show a top and side view, respectively, of another
embodiment
of a Differential Dissecting Instrument 2700, similar to Differential
Dissecting Instrument 2600
shown in FIG. 26A and FIG. 26B, but now possessing a grasping member to allow
the
Differential Dissecting Instrument 2700 to also function as forceps.
Differential Dissecting
Instrument 2700 has a DDM 2710 rotatably attached to an instrument shaft 2720
and is rotated
by a motorized mechanism (not shown). A push rod 2730 is inside instrument
shaft 2720 and is
activated by a mechanism residing in a handle (not shown) and activated
manually by an
operator. When DDM 2710 is active, it oscillates back-and-forth as indicated
by arrow 2740.
When the operator switches off the action of DDM 2710, the operator can then
push with push
rod 2730 on forceps jaw 2750 which has a control horn 2760 that causes forceps
jaw 2750 to
rotate around pivot point 2770 and thus to open. The opposing jaw for the
forceps is the DDM
2710. The operator can then grasp and release objects between forceps jaw 2750
and DDM 2710
by pushing or pulling on push rod 2730.
[00138] FIG. 28, and FIGS. 29A through 29D depict another embodiment of a DDM.
In
practice, this embodiment has provided great differential action and rapid
dissection through
complex tissues. For this embodiment of a DDM, the projections of the tissue
engaging surface
are formed by valleys cut into the surface of the DDM. Referring to FIG. 28,
DDM 2800 has a
first end 2810 and a second end 2820, with a central axis 2825 connecting the
first end 2810 and
second end 2820. First end 2810 is directed away from the complex tissue to be
dissected (not
shown) and is engaged with a drive mechanism (not shown) that moves DDM 2800
such that
second end 2820 sweeps along a direction of motion. Here, the mechanism
oscillates DDM
2800 about an axis of rotation 2830 that is perpendicular to the central axis
2825 such that the
direction of motion 2840 is an arc of motion lying in a plane perpendicular to
the axis of rotation
2830. The second end 2820 has a tissue-facing surface 2850 that is directed
toward the complex
tissue comprising at least one tissue engaging surface 2860 and at least one
lateral surface 2870.
[00139] The motion of DDM 2800 in this example is a reciprocal (back-and-
forth) oscillation,
but other DDMs can have a continuous rotation or a rectilinear motion. The
rotation is
preferably between 2,000 and 25,000 cycles per minute, but can range from 60
cycles per minute
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
42
up to 900,000 cycles per minute, all of which are well below ultrasonic. In
certain embodiments,
speeds of 300 to 25,000 cycles per minute have been found to be very
effective.
[00140] FIGS. 29A through 29E-2 show magnified views of the tissue-facing
surface 2850 of
DDM 2800 from FIG. 28. FIG. 29A shows an oblique view of tissue-facing surface
2850 with
components identified. FIGS. 29B-D show different views of tissue-facing
surface 2850 with
the geometry of the shape better described, especially with respect to
components of tissue-
facing surface 2850. FIG. 29C-2 depicts a close-up of the corner of a
projection shown in FIG.
29C-1; FIG. 29E-1 and FIG. 29E-2 show two alternative versions of arrangements
of valleys
and projections forming the surface of a differential dissecting member.
Finally, FIGS. 29E-1
and 29E-2 show different embodiments of some of these components. The tissue-
facing surface
2850 has a tissue engaging surface 2860 and two lateral surfaces, a first
lateral surface 2871
disposed lateral to and to one side of the tissue engaging surface 2860 and a
second lateral
surface 2872 disposed lateral to and to the opposing side of the tissue
engaging surface.
Referring to FIGS. 29A, 29C-1, and 29C-2, the tissue engaging surface 2860 is
comprised of an
alternating series of at least one valley 2910 and one projection 2920 arrayed
along the direction
of motion 2840 which is an arc of motion on the tissue-facing surface 2850
such that the
intersection of the at least one valley 2910 and at least one projection 2920
define at least one
valley edge 2930 oriented such that it has a component of direction
perpendicular to the direction
of motion 2840.
[00141] No valley edge 2930 should be sharp, e.g. it should not be capable of
slicing into
Complex Tissue, especially into Firm Tissue. For example, no point on a valley
edge 2930
should have a radius of curvature Re smaller than approximately 0.025 mm (see
FIG. 29C-1,
expanded view). This radius of curvature Re is similar to the radius of
curvature of the surface
Rs and of the edge Re as depicted in FIG. 15. We have shown through testing
that edges with
radius of curvature Re no smaller than approximately 0.050 mm can be
effective, too.
Additionally, the radius of curvature Re can vary along the length of valley
edge 2930. In the
embodiment shown in FIGS. 29A through 29D, the radius of curvature Re is
smallest where the
valley edge 2930 is furthest from the axis of rotation 2830 and increases
closer to the first lateral
surface 2871 and the second lateral surface 2872. Furthermore, the minimum
radius of curvature
Re for a valley edge 2930 can be different for different valley edges in the
same DDM and even
for the valley edges on opposing sides of the same valley.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
43
[00142] Projections 2920 in DDM 2800 may be formed by subtractive manufacture
in one
embodiment. In effect, the valleys 2910 are cut out of the surface of a semi-
ellipsoid, as shown
in FIGS. 29B, 29C-1, and 29C-2, having a major semi-axis A aligned
perpendicular to the
rotational velocity 2830 and parallel to the central axis 2825 (see FIG. 28)
(i.e. pointing toward
the Complex Tissue), a first minor semi-axis B, and a second minor semi-axis C
that is parallel
to the rotational velocity 2830. The projections 2920 thus have projection
tops 2940 that are the
remaining semi-ellipsoidal surface and are continuous with the lateral
surfaces 2971 and 2972.
Tissue engaging surfaces 2860 are thus created by the lateral limits of the
valleys 2910 in this
embodiment and span the tissue-facing surface between the valleys 2910 that
form the projection
2920. In other embodiments, projections can be formed by other means and can
thus have more
differently shaped projection tops, including projection tops that are not
formed as the remainder
of a surface. For example, in one embodiment, the projections can effectively
be built up from a
surface, enabling more complex projection tops.
[00143] Referring to FIG. 29A, FIG. 29C, and 29C-2, each valley 2910 may have
a first
valley side 2911, a second valley side 2912, and a valley bottom 2913, whereby
the first valley
side 2911 and the second valley side 2912 lie on opposing sides of the valley
2910. The valley
bottom 2913 is linear or curvilinear and can be two-dimensional or 3-
dimensional. For example,
the valley bottoms in DDM 2800 are straight lines aligned parallel to the
rotational velocity
2830. The first valley side 2911 and the second valley side 2912 rise from the
valley bottom
2913 to a valley edge 2930. The transition from valley bottom can be gradual
and indeterminate,
as in the valleys 2910 in DDM 2800, or the transition can be faceted. A valley
2910 may be
curved in two dimensions, being straight in the direction parallel to the
valley bottom 2913 (and
thus also parallel to the axis of rotation 2830). Valley sides, however, can
be any shape,
including surfaces curved in three dimensions.
[00144] A valley edge is formed by the intersection of a valley wall with a
projection top.
Valley edges can thus have different shapes, depending on the shapes of the
projection top and
the valley edge. The valley edges 2930 on DDM 2800 trace three dimensional
curves and thus
have both curvature and torsion (as defined mathematically in geometry) that
are non-zero and
varying along the valley edge. Valley edges can have smoothly varying
curvature and torsion (as
do valley edges 2930), or a valley edge can be bent.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
44
[00145] FIG. 29C-1 presents an expanded view of a valley edge, in the plane
perpendicular to
the valley edge. The projection top 2920 and the valley side (2911 or 2912
here) form a face
angle F in this plane that is rounded at the intersection (i.e. it is
"radiused" as a machinist would
describe it) having the radius of curvature IZ, described above. The face
angle F can form an
angle less than 90 , which appears sharp on first inspection, but sharpness is
determined by the
radius of curvature IZ, of the edge. The face angle F can vary along the
length of the valley edge,
as it does for DDM 2800 where the face angle F is smallest at the points on
the valley edge
furthest from the axis of rotation 2830. In one embodiment, face angles of
about thirty degrees
(30 ) to about one hundred fifty degrees (150 ) may be effective.
[00146] Valleys have a length, width, and depth where the valley length is the
length of the
valley bottom, the valley width is the distance separating the valley edges of
one valley measured
at their longest distance of separation, and the valley depth is the maximum
vertical distance
from a valley edge to the valley bottom (e.g. peak-to-trough height). Typical
dimensions for a
valley include valley lengths of 0.25 mm to 10 mm, valley widths of 0.1 mm to
10 mm, and
valley depths of 0.1 mm to 10 mm. In one embodiment, a valley length of
approximately three
(3) mm, a valley depth of approximately three (3) mm, and a valley width of
approximately two
(2) mm has been found to be very effective.
[00147] When a DDM has multiple valleys, like DDM 2800, the valleys can be
parallel, like
valleys 2910 of DDM 2800, having valley bottoms 2913 that are all parallel, or
they can be non-
parallel with valley bottoms lying at non-zero angles with respect to each
other or at variable
angles with respect to each other.
[00148] The valleys 2910 of DDM 2800 have a single channel (the space
bounded by the
valley sides and valley bottom); however, valleys can have multiple,
intersecting channels such
that valley bottoms can fork or multiply branch or form networks on the tissue
engaging surface.
FIG. 29E-1 and 29E-2 show top views of two DDMs, the left DDM 2980 having
parallel valleys
2981 with valley bottoms that are not parallel to the rotational velocity
while the right DDM
2990 has network 2991 of multiple intersecting valleys all at different angles
with respect to the
rotational velocity and to each other.
[00149] As described above, the tissue-facing surface 2850 of DDM 2800 has the
surface of a
semi-ellipsoid having a major semi-axis A aligned perpendicular to the axis of
rotation 2830 and
parallel to the central axis 2825, a first minor semi-axis B, and a second
minor semi-axis C that is
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
parallel to the axis of rotation 2830. Tissue-facing surface 2850 may have an
ellipsoid shape in
one embodiment, in which A > B > C. However, any relationship is possible
between the
lengths of the semi-axes. For example, in other embodiments, DDMs may be
fabricated for
which A = B = C (e.g., the tissue-facing surface is hemi-spherical).
[00150] The first lateral surface 2871 and the second lateral surface 2872 of
DDM 2800 are
continuations of the hemi-ellipsoidal shape. As such, they lie at an angle to
one another, forming
a wedge, as earlier depicted in FIG. 19D and FIG. 20, that aligns and strains
fibrous components
of a Complex Tissue allowing the projections to snag and break the fibrous
components.
[00151] FIG. 30A presents the situation of a first tissue region 3011 encased
in first
membrane 3016 and second tissue region 3012 encased in second membrane 3017.
First
membrane 3016 and second membrane 3017 abut at tissue plane 3020. First
membrane 3016
and second membrane 3017 are formed of densely packed fibrous components and
thus comprise
a Firm Tissue. The interstitial materials spanning the tissue plane from first
membrane 3016 to
second membrane 3017 include fibrous components 3030. These fibrous components
3030 are
less densely packed, so the interstitial materials comprise a Soft Tissue. As
tissue-facing surface
2850 is pressed in the direction of arrow 3050 into the tissue plane 3020 to
separate the two
tissue regions 3011 and 3012, the first lateral surface 2871 and the second
lateral surface 2872
exert a first spreading force 3041 and a second spreading force 3042 on tissue
regions 3011 and
3012, respectively, that align and strain fibrous components 3030 at the
projection tops 2940 (see
FIG. 29C-1 and 29C-2). This enables the fibrous components 3030 to enter the
valleys 2910
and thus be snagged and then torn by a projection 2920 as the tissue-facing
surface 2850 rotates
about axis of rotation 2830 and so moves out of the plane of the page (toward
the viewer).
Additionally, as the projection tops 2940 are continuous with the lateral
sides, the more lateral
areas of the projection tops 2940 also exert additional spreading forces 3043
and 3044 that also
wedge tissue regions 3011 and 3012 apart, further increasing the strain on
fibrous components
3030.
[00152] FIGS. 30B through 30D show how the curvature of first lateral surface
2871 and
second lateral surface 2872 can be changed to make a DDM more or less
aggressive. Consider
first DDM 3060 in FIG. 30B. As explained in FIG. 30A, the more lateral areas
of the projection
tops 2940 exert spreading forces 3043 and 3044 that wedge adjacent tissue
regions apart.
Furthermore, the first lateral surface 2871 and the second lateral surface
2872 exert a first
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
46
spreading force 3041 and a second spreading force 3042. As first angle 3065
formed by
spreading forces 3043 and 3044 approaches 1800 (as shown for DDM 3061 in FIG.
30C), the
wedging action of the lateral areas of the projection tops 2940 decreases.
(Note that angle 3065
is similar to the wedge angle (0 described in FIGS. 21A through 21C.) If
projections 2920
become also laterally (left-right in this figure) thinner, then the
projections will more rapidly
disrupt Soft Tissue but will also be more proned to abrade or disrupt Firm
Tissue. As in FIG.
30A, first and second lateral surfaces 2871 and 2872 create spreading forces
3041 and 3042,
respectively, forming a second angle 3066 (effectively, a second wedge angle
(0). If second
angle 3066 is similar to first angle 3065 (as shown for DDM 3060 in FIG. 30B),
then these
surfaces combine to create a single wedging surface. If, as in FIG. 30C,
second angle 3066' is
larger than first angle 3065 (i.e. lateral surfaces 2871 and 2872 are more
nearly parallel), then
second angle 3066' exerts little or no wedging action. Conversely, if, as in
FIG. 30D, second
angle 3066" is smaller than first angle 3065 (i.e. lateral surfaces 2871 and
2872 become more
nearly perpendicular, then second angle 3066" exerts a greater wedging action.
DDMs like 3061
have proven more effective in dissecting tissue planes possessing prominent
collagen fibrils that
span the tissue plane, crossing from one surface to the other.
[00153] Referring back to FIG. 30A, FIG. 30A also illustrates an important
aspect of a DDM.
A DDM will automatically follow a tissue plane. Because tissue planes tend to
be bounded by
Firm Tissues (e.g. membranes, ducts, etc.) and are spanned by Soft Tissues, a
DDM will, by
virtue of its differential action, not move into the Firm Tissue and will move
into the Soft Tissue,
thus following and separating a tissue plane will little or no guidance from
an operator. This
means that the operator need not have as detailed an understanding of the
anatomy as is required
by current practice or, conversely, a DDM allows a skilled surgeon to more
confidently dissect
an uncertain anatomy, e.g. when tissue planes are distorted by a tumor or when
tissues are
swollen or inflamed.
[00154] FIG. 31 shows an end-on view of the tissue-facing surface 2850 as it
snags and then
stretches to breaking the fibrous components 3030 shown in FIG. 30A. Three
fibrous
components (first fibrous component 3031, second fibrous component 3032, and
third fibrous
component 3033) have been snagged by three projections (first projection 2921,
second
projection 2922, and third projection 2923, respectively). Tissue facing
surface 2850 rotates,
generating a direction of motion 2840 which is an arc of motion as depicted by
arrows 3100.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
47
First fibrous component 3031 has just entered the first valley 2911 and has
not yet been snagged
by first projection 2921. Second fibrous component 3032 entered the second
valley 2912 at an
earlier point in time and has been snagged and strained by second projection
2922. Third fibrous
component 3033 entered the third valley 2913 at an even earlier point in time
and has been
snagged and strained even further by third projection 2923. Ultimately, all
three fibrous
components 3031, 3032, and 3033 will be strained to breaking.
[00155] FIG. 31 illustrates an important aspect of DDM 2800's design. Because
the valleys
span from one lateral surface 2871 to the opposing lateral surface 2872, each
valley creates an
open space spanning across the end of DDM 2800 into which strained fibrous
components can
enter, thus facilitating their being snagged by the projections.
[00156] It is important to note that DDM 2800 does not have arrays of small
projections that
give any part of its surface texture, as described earlier. Rather, all
surfaces of DDM 2800 are
smooth and, preferably, possess low friction surfaces. The shapes and
configurations of the
surface features of DDM 2800 are responsible for its ability to differentially
dissect Complex
Tissues. In fact, DDM 2800 works best when all of its surfaces that are in
contact with tissue are
well lubricated with, for example, a surgical lube.
[00157] FIG. 32 shows an exploded view of one embodiment of a complete
differential
dissecting instrument. The differential dissecting instrument 3200 is grossly
comprised of an
instrument handle 3212 from which projects an instrument insertion tube 3290
which has a first
end 3291 attached to instrument handle 3212 and a second end 3293, to which is
rotatably
mounted a DDM 3292. The instrument handle 3212 is assembled from an upper
housing 3220,
which includes upper battery cover 3222, and a lower housing 3230, which are
held together by
instrument housing bolts 3236. Included in the upper housing 3220 and lower
housing 3230 are
a motor 3260 and a battery pack 3270. In the upper housing 3220 is a switch
port 3224, through
which can be accessed a switch 3282 (which may be a momentary switch or an on-
off switch)
for providing power to the motor 3260 from the battery pack 3270. A printed
circuit board 3280
further containing a power level adjustment 3281 (which can be any convenient
component, but
is here shown as a linear potentiometer) is provided and can be accessed
through a flexible
switch cover 3284 mounted in surface of the upper housing 3220. Also included
are forward
spring battery connectors 3272 and aft spring battery connectors 3274, which
route electric
power from the battery pack 3270. The upper housing 3220 further contains an
instrument
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
48
insertion tube support 3226 to secure and orient the instrument insertion tube
3290 near and
coaxial with the motor 3260.
[00158] The lower housing 3230 further provides access to and secures the
battery pack 3270
with an integral lower battery cover 3232 and motor housing section 3234,
further held to the
upper housing 3220 using the three instrument housing bolts 3236. The motor
housing section
3234 holds and secures the motor 3260 coaxial with the instrument insertion
tube 3290, which
passes through the instrument insertion tube support 3226. The motor 3260 is
pressed forward
by the motor housing section 3234 against the motor collar 3264, the inside
diameter of which
leaves room for the motor shaft coupler 3262. The motor shaft coupler 3262,
with the help of the
motor shaft coupler bolts 3266, mounts securely onto the end of the shaft of
the motor 3260 and
further grips a first end 3295 of a drive shaft 3294. The drive shaft 3294 is
rotated by the motor
3260 inside of and concentrically with the instrument insertion tube 3290. The
drive shaft 3294
also has a second end 3297 of drive shaft 3294, which is concentrically
supported by a shaft
bearing 3296 that is mounted onto a second end 3293 of instrument insertion
tube 3290. The
DDM 3292 is rotatably mounted onto shaft bearing 3296 such that drive shaft
3294 causes DDM
3292 to rotate. DDM 3292, shaft bearing 3296, drive shaft 3294, and instrument
insertion tube
3290 collectively form the DDM assembly 3299, which is described next.
[00159] FIG. 33A, FIG. 33B, and FIG. 33C depict the details of the DDM
assembly 3299,
including how the DDM 3292 is assembled with other components such that the
motor 3260
drives oscillation of DDM 3292.
[00160] Referring now to FIG. 33A, DDM 3292 in this embodiment comprises a
tissue facing
surface 3322 on a first end 3321 and a shaft bearing grip 3324 on a second end
3323. The shaft
bearing grip 3324 is further fitted with two pivot pins 3325. The DDM 3292 may
be partially
hollow, possessing a shaft bearing cavity 3326 that permits the shaft bearing
3296 to fit inside.
The shaft bearing cavity 3326 further sports a cam following cavity 3328. The
shape of the cam
following cavity 3328 may be oblong in that it is much narrower in one
direction, forming a slot.
Shaft bearing 3296 has a bore 3336, a shaft bearing tip 3332, a threaded
bearing end 3338, and
two pivot pin holes 3334. Threaded shaft bearing end 3338 screws into threaded
shaft bearing
mount 3342 on the second end 3293 of instrument insertion tube 3290. Bore 3336
can have a
diameter greater than the diameter 3385 of drive shaft 3294 everywhere along
its length, except
at shaft bearing tip 3332, thereby decreasing the contact surface between
shaft bearing 3296 and
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
49
drive shaft 3294. The second end 3297 of drive shaft 3294 is modified to
include a main shaft
section 3352 and a cam shaft section 3354. The various sub-components of these
components
allow for their assembly and operation, as can be seen in FIG. 33B and FIG.
33C.
[00161] Referring now to FIG. 33B, drive shaft 3294 is shown as fitting
coaxially within shaft
bearing 3296 and instrument insertion tube 3290 of the DDM assembly 3299. This
aligns
threaded bearing end 3338 of shaft bearing 3296 for screwing into the threaded
shaft bearing
mount 3342 located at the second end 3293 of instrument insertion tube 3290. A
shaft bearing
tip 3332 accommodates drive shaft 3294, preventing misalignment with respect
to the DDM
3292. The second end 3293 of drive shaft 3294 emits from shaft bearing tip
3332 such that cam
shaft section 3354 is fully exposed. Once the instrument insertion tube 3290,
shaft bearing 3296,
and drive shaft 3294 are assembled, the DDM 3292 mounts onto shaft bearing
3296 such that (a)
the pivot point pins 3325 insert into pivot pin holes 3334 and (b) cam shaft
section 3354 inserts
into cam following cavity 3328, as shown in FIG. 33C.
[00162] FIG. 33C depicts the assembled DDM assembly 3299. The DDM 3292 fits
over the
shaft bearing 3296, which is screwed into the threaded shaft bearing mount
3342 of the
instrument insertion tube 3290, all of which coaxially encompass the drive
shaft 3294. It is
notable that the pivot pins 3325 on the shaft bearing grip 3324 fit into the
pivot pin holes 3334 of
the shaft bearing 3296. This arrangement, combined with the shaft bearing
cavity 3326, allows
the hollow DDM 3292 to rotate freely on the pivot pins 3325. Rotation of drive
shaft 3294
causes cam shaft section 3354 to rotate inside cam following cavity 3328,
driving DDM 3292 to
oscillate about the pivot pin holes 3334 and sweeping tissue facing surface
3322 side-to-side as
indicated by double sided arrow 3377.
[00163] In operation, referring to FIG. 32 and FIGS. 33A through 33C, a
surgeon holds the
differential dissecting instrument 3210 by the instrument handle 3212 and
orients the distal tip
sporting the DDM 3292 toward the complex tissue to be dissected. The surgeon
selects the
power level by sliding the power level adjustment 3281 to the desired position
and then places
his or her thumb upon the switch 3282 and presses it to close the switch. When
switch 3282
closes, motor 3260 is turned on and rotates the motor shaft coupler 3262 and,
in turn, the drive
shaft 3294. The drive shaft 3294 is held coaxially and quite precisely in
place by the shaft
bearing 3296 and especially the shaft bearing tip 3332, so that the cam shaft
section 3354 of the
drive shaft 3294 oscillates rotationally inside the cam following cavity 3328
of the shaft bearing
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
cavity 3326 of the DDM 3292. The cam following cavity 3328 is oblong, and in
the
embodiment shown in FIGS. 33A through 33C has its narrowest dimension
occurring in the
direction perpendicular to the axis of the rotational joint formed by the
pivot pins 3325 and the
pivot pin holes 3334. In this embodiment, the narrowest dimension of the cam
following cavity
3328 just barely permits the passage of the cam shaft section 3354 of the now
rotating drive shaft
3294. Accordingly, the rotational oscillation of the cam shaft section 3354
impinges on the long
walls of the cam following cavity 3328, forcing the entire DDM 3292 to rotate
through an
oscillation arc 3377 lying in a plane perpendicular to the axis of the
rotational joint formed by
the pivot pins 3325 and the pivot pin holes 3334. In this embodiment, the
amplitude of the
oscillation arc 3377 through which the tissue facing surface 3322 of the
differential dissecting
member 3292 swings is a function of the diameter 3385 of the drive shaft 3294
out of which the
cam shaft section 3354 is cut and the distance 3379 separating tissue facing
surface 3322 and
pivot pin holes 3334. The frequency of the oscillation matches the frequency
of the oscillation
of rotation of the motor 3260. The operator may control the oscillation
frequency of the tissue
facing surface 3322 by varying the position of the power level adjustment
3281. Note that this
mechanism for converting rotation of motor 3260 and thus rotation of drive
shaft 3294 into
oscillation of the DDM 3292 is similar to the scotch yoke depicted in FIGS. 22
through 25C.
[00164] Differential Dissecting Instrument 3200 is one example of
implementation of a DDM,
and many variants are possible. For example, oscillation of a DDM can be
driven by a crank and
slider mechanism with the slider moving back-and-forth longitudinally inside
an instrument
insertion tube. Alternatively, a motor could be placed adjacent to the DDM,
with the motor shaft
directly driving the DDM and only electrical wires to power the motor running
down the
instrument insertion tube. Additionally, because a DDM adapts well to the end
of a tube, greatly
lengthening the instrument insertion tube allows differential dissecting
instruments, such as
Differential Dissecting Instrument 3200, for example, to be laparoscopic
instruments.
Differential Dissecting Instruments with instrument insertion tubes as long as
thirty-six (36) cm
may be used, although longer or shorter tubes are easily accommodated in the
design. DDMs as
disclosed herein can easily be adapted to the arm of a surgical robot, such as
the Da Vinci
Surgical Robot from Intuitive Surgical (Sunnyvale, CA). A DDM can be made very
small; for
example, effective Differential Dissecting Instruments in which the DDM and
instrument
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
51
insertion tube fit through a five (5) mm hole, such as a surgical port, can be
built, enabling
minimally invasive surgery. These smaller devices are easily built.
[00165] Further, Differential Dissecting Instruments can be used in which the
drive shaft is
replaced by a flexible drive shaft, and the instrument insertion tube is
curved. This creates
Differential Dissecting Instruments with curved instrument insertion tubes,
like that shown in
FIG. 6C. Articulation of the instrument insertion tube is also possible, using
for example a drive
shaft having a universal joint or other bendable coupler at the articulation.
[00166] As previously disclosed, additional functionality can be added to the
end of a
Differential Dissecting Instrument. For example,
= FIG. 11B and FIG. 13 show how the design of the DDM permits fluids to be
delivered to
a DDM for irrigation, or how suction can be applied to clear the surgical
field, or how a
light source can be placed on or near a DDM to illuminate the surgical field.
= FIG. 26A through FIG. 26D disclose a Differential Dissecting Instrument
having an
retractable cutting blade that can be made sharp for cutting or can be
energized by a
electrosurgical generator (unipolar or bipolar) for electrosurgery,
= FIGS. 27A and 27B show how the design of the DDM permits a DDM to be
adapted to
function as forceps.
[00167] Additional functionality can readily be added to a Differential
Dissecting Instrument.
For example, a patch of any size on the side of a DDM or a shroud holding a
DDM can be
energized such that the patch can be used for electrocautery. To simplify
fabrication, the drive
shaft can be used to conduct the electricity from the handle to the DDM. The
design of the DDM
permits the forceps shown in FIGS. 27A and 27B to instead be used as scissors.
Additional
functionalities can include a video camera for imaging or ultrasonic surgery
for sharp dissection.
The improved design of the DDM permits many of these additional
functionalities to be
combined together in one Differential Dissecting Instrument. Advantages
realized from
combining functionalities with a DDM at the working end of a Differential
Dissecting
Instrument include: reducing the number of instruments a surgeon needs for a
procedure;
simplifying inventory for the hospital and logistics for support staff; and,
most importantly,
reducing instrument changes during surgery, which slow surgery and are a major
source of
surgical complications. This is especially true in laparoscopic and robotic
surgeries, which
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
52
require positioning instruments into the body through small incisions,
frequently with airtight
ports.
[00168] FIG. 34 shows an oblique view of one embodiment of an assembled
Differential
Dissecting Instrument. The Differential Dissecting Instrument 3400 is grossly
comprised of an
instrument handle 3412 from which projects an instrument insertion tube 3490
which has a first
end 3491 attached to instrument handle 3412 and a second end 3493, to which is
rotatably
mounted a DDM 3492. The instrument handle 3412 is assembled from an upper
housing 3420,
which includes upper battery cover 3422, and a lower housing 3430, which
includes lower
battery cover 3432. Enclosed in the upper housing 3420 and lower housing 3430
are a motor
3460 and batteries 3470, which can, optionally, be assembled into a battery
pack. In the upper
housing 3420 is a switch 3482 (which may be a momentary switch or an on-off
switch) for
providing power to the motor 3460 from the battery pack 3470. A flexible
switch cover 3484
mounted in the surface of the upper housing 3420 allows access to the power
level adjustment
3581 (FIG. 35A) inside. The upper housing 3420 further comprises a retractable
blade hook
control button 3499 (secured by a control button bolt 3498), as well as an
instrument insertion
tube support 3426 to orient the instrument insertion tube 3490 near and
coaxial with the motor
3460.
[00169] FIG. 35A shows an exploded view of Differential Dissecting Instrument
3400. The
Differential Dissecting Instrument 3400 is grossly comprised of an instrument
handle 3412 from
which projects an instrument insertion tube 3490 which has a first end 3491
attached to
instrument handle 3412 and a second end 3493, to which is rotatably mounted a
DDM 3492.
The instrument handle 3412 is assembled from an upper housing 3420, which
includes upper
battery cover 3422, and a lower housing 3430, which includes a lower battery
cover 3432,
which are held together by instrument housing bolts 3536. Included within the
upper housing
3420 and lower housing 3430 are a motor 3460 and batteries 3470, here shown as
battery type
CR123A (3V each, 18V for all 6 batteries 3470) but other battery types and
voltages can be used.
We've used batteries totaling as low as 3V in some embodiments. In the upper
housing 3420 is a
switch port 3524, through which can be accessed switch 3482 (which may be a
momentary
switch or an on-off switch) for providing power to the motor 3460 from the
battery pack 3470.
A printed circuit board 3580 further containing a power level adjustment 3581
(which can be any
convenient component, but is here shown as a linear potentiometer) is provided
and can be
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
53
accessed through a flexible switch cover 3484 mounted to the surface of the
upper housing 3420.
Also included are forward spring battery connectors 3572 and aft spring
battery connectors 3574,
which route electric power from the batteries 3470. The upper housing 3420
further contains an
instrument insertion tube support 3426 to secure and orient the instrument
insertion tube 3490
near and coaxial with the motor 3460. An instrument insertion tube retaining
bolt 3527 holds the
instrument insertion tube 3490 securely in the instrument insertion tube
support 3426.
[00170] The lower housing 3430 further provides access to and secures the
batteries 3470
with an integral lower battery cover 3432 and motor housing section 3534,
further held to the
upper housing 3420 using the three instrument housing bolts 3536. The motor
housing section
3534 holds and secures the motor 3460 coaxial with the instrument insertion
tube 3490, which
passes through the instrument insertion tube support 3426. The motor 3460 is
pressed forward
by the motor housing section 3534 against the motor spring 3562, the inside
diameter of which
leaves room for the motor shaft coupler 3562. The motor shaft coupler 3562,
with the help of the
motor shaft coupler bolts 3566, mounts securely onto the end of the shaft of
the motor 3460 and
further grips a first end 3595 of a drive shaft 3494. The motor 3460 can slide
longitudinally fore
and aft within the motor housing section 3534 under the control of the
retractable blade hook
control button 3499. The motor 3460 further comprises a power contact plate
3569 which
operably slides against sprung motor power contacts 3563 mounted on circuit
board 3580. Also
mounted on circuit board 3580 is an adjustable power contact pressure control
bolt 3561.
Normally, spring 3567 keeps motor 3460 aft. In that position, the sprung motor
power contacts
3563 mounted on the printed circuit board 3580 are aligned with and press
against the power
contact plate 3569 on motor 3460, and so electric power from battery pack 3470
can drive motor
rotation. Pressing the retractable blade hook control button 3499 forward
causes motor 3460 to
slide forward. The power contact plate 3569 is shorter than the full extent of
travel of motor 3460
under the influence of retractable blade hook control button 3499, such that
electric power from
battery pack 3470 is automatically cut off when the motor 3460 is slid
sufficiently far forward
toward insertion tube second end 3493 to break contact with sprung motor power
contacts 3563.
[00171] The drive shaft 3494 also has a second end 3597, which passes through
and is
concentrically supported by a shaft bearing 3496 that is mounted onto the
second end 3493 of
instrument insertion tube 3490. Referring also to FIG. 35 B, the second end
3597 of drive shaft
3494 further comprises (from the tip of second end of 3597 and working inward)
a cam receiver
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
54
retainer 3555, a cam receiver driver 3554, and a shaft bearing clearance
section 3552. DDM
3492 is rotatably mounted onto shaft bearing 3496 such that drive shaft 3494
causes DDM 3492
to rotate with a reciprocal oscillation. DDM 3492, shaft bearing 3496, a cam
receiver 3596, a
cam receiver retainer 3555, drive shaft 3494, and instrument insertion tube
3490 collectively
form the DDM assembly 3598, which is described next.
[00172] FIG. 35B depicts the details of DDM assembly 3598, including how DDM
3492 is
assembled with other components such that the motor 3460 drives reciprocal
oscillation of DDM
3492. DDM 3492 in this embodiment comprises a tissue facing surface 3522 on a
first end 3521
and a shaft bearing grip 3524 on a second end 3543. The shaft bearing grip
3524 is further fitted
with two pivot pin holes 3525. The DDM 3492 may be partially hollow,
possessing a shaft
bearing cavity 3526 that permits the shaft bearing 3496 to fit inside. The
shaft bearing cavity
3526 further sports a cam receiver cavity 3548 shaped to permit cam receiver
3596 to easily slide
therein. In this embodiment, tissue facing surface 3522 of DDM 3492 further
comprises a
retractable blade slot 3506. Shaft bearing 3496 has a bore 3536, a shaft
bearing tip 3532, a
threaded bearing end 3538, and two insertable pivot pins 3535 that fit into
threaded holes 3534.
The threaded shaft bearing end 3538 screws into threaded shaft bearing mount
3542 on the
second end 3493 of instrument insertion tube 3490. Bore 3536 can have a
diameter greater than
the diameter 3585 of drive shaft 3494 everywhere along its length, except at
shaft bearing tip
3532, thereby decreasing the contact surface between shaft bearing 3496 and
drive shaft 3494.
Second end 3497 of drive shaft 3494 is modified to include main shaft section
3552, cam shaft
section 3554, and cam receiver retainer 3555. Cam receiver 3596 further
comprises a cam
receiver body 3502, a cam receiver chamber 3505, and a retractable blade 3501.
Retractable
blade 3501 can further comprise a hook 3504 and a tissue engaging surface
3503. Tissue
engaging surface 3503 of retractable blade 3501 can be more or less aggressive
than tissue
engaging surface of DDM 3492. The various sub-components of these components
allow for
their assembly and operation, as is disclosed elsewhere in this document.
[00173] DDM 3492 fits over shaft bearing 3496, which is screwed into threaded
shaft bearing
mount 3542 of the instrument insertion tube 3490, all of which coaxially
encompass drive shaft
3494. It is notable that pivot pin holes 3525 on shaft bearing grip 3524 fit
onto pivot pins 3535
of shaft bearing 3496. This arrangement, combined with shaft bearing cavity
3526, allows DDM
3492 to rotate freely on the pivot pins 3535. Rotation of drive shaft 3494
causes cam shaft
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
section 3554 to rotate inside cam receiver 3596, driving DDM 3492 to
reciprocally oscillate
about the pivot pin holes 3525 and sweeping tissue facing surface 3522 side-to-
side. Tissue
facing surface 3522 may possess a tissue-engaging surface (not depicted here)
such that it
performs as a DDM.
[00174] In operation, a surgeon holds the differential dissecting instrument
3400 by the
instrument handle 3412 and orients the distal tip sporting the DDM 3492 toward
the complex
tissue to be dissected. The surgeon selects the power level by sliding the
power level adjustment
3581 to the desired setting and then places his or her thumb upon the switch
3482 and presses it
to close the switch. When switch 3482 closes, motor 3460 is turned on and
rotates the motor
shaft coupler 3562 and, in turn, the drive shaft 3494. The drive shaft 3494 is
held coaxially and
quite precisely in place by the shaft bearing 3496 and especially the shaft
bearing tip 3532, so
that the cam shaft section 3554 of the drive shaft 3494 oscillates
rotationally inside the cam
receiver chamber 3505 of cam receiver 3502 captured within the DDM 3492. The
rotational
oscillation of the cam shaft section 3554 impinges on the walls of the cam
receiver chamber
3505 of cam receiver 3502 which is configured as a scotch yoke as described
earlier, forcing the
entire DDM 3492 to rotate through an oscillation arc lying in a plane
perpendicular to the axis of
the rotational joint formed by the pivot pins 3535 and the pivot pin holes
3525. The surgeon can
extend retractable blade 3501 by pushing retractable blade hook control button
3499 forward.
Forward motion of retractable blade hook control button 3499 causes motor 3460
and power
contact plate 3569 to move forward, separating power contact plate 3569 from
sprung motor
power contacts 3563 and cutting power to the motor, as described earlier, and
preventing
oscillation of DDM 3492. Simultaneously, forward motion of motor 3460 pushes
drive shaft
3494 forward, toward second end 3493 of instrument insertion tube 3490.
Forward motion of
drive shaft 3494 in turn pushes cam receiver retainer 3555 against the top of
cam receiver
chamber 3505 inside cam receiver body 3502, thereby pushing cam receiver body
3502 further
up cam receiver cavity 3548 and extending retractable blade 3501 out of
retractable blade slot
3506. Thus, forward motion of retractable blade hook control button 3499
causes the motor
3460 to stop and retractable blade 3501 to extend out of DDM 3492. When
retractable blade
hook control button 3499 is released, motor spring 3562 pushes motor 3460 aft,
retracting
retractable blade 3501 and restoring electrical contacts for the motor.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
56
[00175] In this embodiment the amplitude of the oscillation through which the
tissue facing
surface 3522 of the differential dissecting member 3492 swings is a function
of the diameter
3585 of the drive shaft 3494 out of which the cam shaft section 3554 is cut
and the distance 3579
separating tissue facing surface 3522 and pivot pin holes 3525. The frequency
of the reciprocal
oscillation (cycles per minute) of the DDM 3492 against the complex tissue
matches the
frequency of rotation (rotations per minute) of the motor 3460. The operator
may control the
oscillation frequency of the tissue facing surface 3342 by varying the
position of the power level
adjustment 3581. Note that this mechanism for converting rotation of motor
3460 and thus
rotation of drive shaft 3494 into oscillation of the DDM 3492 is similar to
the scotch yoke
depicted in FIGS. 22 through 25C.
[00176] FIG. 35C-1 and 35C-2 illustrate that fore/aft motion of drive shaft
3494 and, thus of
cam receiver body 3502, also alters the amplitude of reciprocal oscillation of
DDM 3492. Drive
shaft 3494 is depicted in the aft position (having moved in the direction of
arrow 3595) in FIG.
35C-1 and in the fore position (having moved in the direction of arrow 3597)
in FIG. 35C-2.
Thus, as cam receiver body 3502 moves forward inside cam receiver cavity 3548,
the distance D
from cam receiver body 3548 and pivot pin holes 3525 increases to D' while the
lateral
displacement of the receiver 3599 remains constant (because it is determined
by the diameter
3585 of drive shaft 3494, as described above). As D' increases, the larger
angular amplitude of
DDM 3596 in the left frame decreases to the smaller angular amplitude of DDM
3492 in the
right frame. This effect can be used to decrease the amplitude of oscillation
when a retractable
blade is extended. It can also be used to alter the amplitude of oscillation
during blunt dissection
by the DDM, for example when a surgeon wants a narrower oscillation for more
precise
dissection.
[00177] FIGS. 36A-1, 36A-2, 36B-1, 36B-2, 36B-3, and 36B-4 show the end of a
Differential
Dissecting Instrument 3600 having a DDM 3610 rotatably mounted to instrument
insertion tube
3620 via rotational joint 3630. Differential Dissecting Instrument 3600 also
has a retractable
hook 3640 that can be extended or retracted by motion in the direction
indicated by double
headed arrow 3650. Retractable hook 3640 can be retracted or extended using,
for example, the
mechanism described in FIGS. 34, 35A, and 35B. FIG 36A demonstrates how
retractable hook
3640 can be placed into two configurations. CONFIGURATION 1 (FIG. 36A-1) shows
retractable hook 3640 in the extended position, and CONFIGURATION 2 1 (FIG.
36A-2) shows
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
57
retractable hook 3640 in the retracted position. Retractable hook 3640 can
have a tip 3670 that
can be pointed or rounded and a tissue engaging surface 3660 that can be more
aggressive than
tissue engaging surface 3690 of DDM 3610, or it can be less aggressive.
Retractable hook 3640
possesses an elbow 3680 that can be sharpened to slice, as shown here, or it
can be dull;
furthermore, it can be serrated, and the sharpened region can be located
anywhere within the
elbow. In CONFIGURATION 2, retractable hook is hidden inside DDM 3610, and DDM
3610
alone interacts with the tissue. In CONFIGURATION 1, retractable hook 3640 is
exposed and
can be used to interact with the tissue such that tissue engaging surface 3690
interacts with the
tissue (e.g. to disrupt softer tissues), or such that tip 3670 interacts with
tissue (e.g. to pierce a
tissue), or elbow 3680 interacts with tissue (e.g. to slice a tissue),
depending on how an operator
positions retractable hook 3640 with respect to the tissue. Additionally,
retractable hook 3640
can be held at any intermediate position between CONFIGURATION 1 and
CONFIGURATION
2, including being able to be variably extended by an operator.
[00178] FIGS. 36B-1 through 36B-4 show the end of a Differential Dissecting
Instrument
3600 and illustrates that DDM 3610 can oscillate with retractable hook in the
extended
configuration (CONFIGURATION 1) 1 (FIG. 36B-1) or the retracted configuration
(CONFIGURATION 2) 1 (FIG. 36B-2) and that retractable hook 3640 can be
retracted or
extended before activation of oscillation of DDM 3610 or during oscillation of
DDM 3610.
Arrow 3601 shows retractable hook moving between the retracted configuration
(lower left
frame) to the extended configuration (upper left frame ¨ FIG. 36B-3) while DDM
3610 is not
oscillating. Arrow 3602 shows that DDM 3610 can be switched from stationary
(upper left
frame) to oscillating (upper right frame ¨ FIG. 36B-4) while retractable hook
3640 is in the
extended configuration. Arrow 3603 shows that retractable hook 3640 can be
moved from the
extended configuration (upper right frame) to the retracted configuration
(lower right frame)
while DDM 3610 is oscillating. Arrow 3604 shows that DDM 3610 can change from
stationary
(lower left frame) to oscillating (lower right frame) while retractable hook
3640 is in the
retracted configuration. Retractable hook 3640 can optionally be made of an
electrically
conductive material, like stainless steel, and electrically connected to an
external surgical
electrosurgical generator to allow retractable hook 3640 to act as an
electrosurgical hook.
[00179] Many tissues to be dissected are wrapped in a membrane or capsule that
a surgeon
must divide to gain access to that tissue. Once that membrane or capsule has
been divided, the
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
58
surgeon proceeds with dissection through that tissue. FIGS. 37-1 through 37-4
illustrate in four
panels a method by which a Differential Dissecting Instrument 3600 can be used
to safely and
quickly divide a membrane 3710 overlying a tissue 3700, such as the peritoneum
overlying the
gall bladder or the capsule surrounding a liver. In the upper left panel (FIG.
37-1), the
Differential Dissecting Instrument is seen approaching membrane 3710 with the
retractable hook
3640 in the extended configuration. In the upper right panel (FIG. 37-2), the
tissue engaging
surface 3660 of retractable hook 3640 is pressed by the surgeon against
membrane 3710, and the
DDM 3610 is oscillated such that tissue engaging surface 3660 abrades membrane
3710.
(Alternatively, the retractable hook 3640 can be held in the retracted
configuration, and the tissue
engaging surface 3690 of DDM 3610 can be used to abrade membrane 3710. If the
two tissue
engaging surfaces 3660 and 3690 have different levels of aggressiveness, the
surgeon then has
the flexibility of choosing either the more aggressive or the less aggressive
tissue engaging
surface to abrade the membrane 3710.) The tissue is abraded until a small
opening 3720 is made
in membrane 3710. Next, as shown in the lower left panel (FIG. 37-3), the
surgeon then pries
the tip 3670 of retractable hook 3640 through opening 3720 and under membrane
3710, lifting or
"tenting" a flap 3730 of membrane 3710 away from tissue 3700. The surgeon then
moves DDM
3600 in the direction of arrow 3740, thereby forcing flap 3730 into the elbow
3680 of retractable
hook 3640, the elbow 3680 being sharpened to slice tissue. Finally, as shown
in the lower right
panel (FIG. 37-4), the surgeon makes DDM 3610 oscillate, causing retractable
hook 3640 to
oscillate and, thus, the sharp edge of the elbow 3680 of retractable hook 3640
to quickly move
into membrane 3710 as the surgeon continues moving DDM 3600 in the direction
of arrow 3740.
This has been demonstrated with fresh tissues to be an easy, quick, and safe
way to divide a
membrane, such as the peritoneum overlying the gall bladder and bile duct,
without damaging
underlying structures (e.g. the gall bladder, bile duct, or liver). The tip
3680 of retractable hook
3640 can be made sufficiently blunt that it does not easily penetrate the
membrane 3710 or
underlying structures; furthermore, the placement of the sharp edge only at
elbow 3680 prevents
critical structures from being exposed to the sharp edge 3680 and thus
reducing the likelihood of
such critical structures being cut. Examples of membranes or capsules
overlying critical
structures include the peritoneum overlying the liver, gall bladder, cystic
duct, and cystic artery;
and the pleura overlying the lung, pulmonary artery, pulmonary vein, and
bronchus.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
59
[00180] A retractable hook can be used in a method similar to that shown in
FIGS. 37-1
through 37-4 to dissect tougher fibrous structures, like adhesions, fibrous
tissues surrounding the
renal artery or vein, and scar tissue. For example, a surgeon can use the tip
of a retractable hook
to grab all or a portion of a fibrous structure and then can push the tissue
into the sharpened
elbow of the hook. The surgeon can then oscillate the DDM and hook to use the
sharp edge
inside the hook to cut the tissue. An advantage of this approach is that it
applies the stresses in
the immediate location of the tissue to be divided. In current practice,
surgeons divide such
tissues by a variety of techniques, including simply grabbing the sides or
ends of such tissues and
pulling them until they break. This can at times put large stresses on the
tissues being pulled,
such as the wall of the intestine, leading to accidental tearing of critical
tissues, such as the wall
of the intestine (and thereby perforating the bowel). By applying the stresses
more locally and
directly to the tissue to be divided (specifically at the sharpened elbow of
the hook), and not over
larger expanses of tissues (e.g. between two pairs of forceps), a surgeon can
have greater
certainty that a more distant tissue, like the wall of the intestine, is
unharmed.
[00181] It is important to note that these methods of dividing tissues by
using a hook that is
oscillated does not heat the tissues, in stark contrast to the extreme heat
that arises from current
practice using electrosurgery. The heat from electrosurgery is widely
acknowledged as a major
risk leading to accidental thermal damage of surrounding tissues. Competing
technologies for
sharp dissection, such as ultrasonic ablation (e.g. the "harmonic shears" from
Ethicon
Endosurgery), have been developed to reduce the heat and thereby decrease the
risk of thermal
damage to tissues. Nevertheless, local heating remains significant and the
risk of thermal
damage is still present. On the contrary, dividing a membrane or dissecting a
fibrous structure as
described here with an oscillating hook causes no heating of tissues,
eliminating this major
source of iatrogenic trauma.
[00182] FIG. 38 shows one embodiment of a Differential Dissecting Instrument
3800 for
laparoscopic surgery. It uses the mechanism for oscillation of the DDM 3810
shown in FIGS.
34, 35A and 35B, including a retractable blade (not visible in this picture
because it is in the
retracted configuration). Differential Dissecting Instrument 3800 uses a
pistol-style handle 3820
having a trigger 3830 to start/stop oscillation of the DDM 3810 and a speed
control 3840 for
controlling the speed of oscillation. A thumb-activated push-button 3850 is
used to extend the
retractable blade which is held in a normally retracted configuration by a
spring mechanism
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
inside handle 3820. A rotational wheel 3860 can be reached and turned with an
index finger, and
rotation of rotational wheel 3860 rotates instrument insertion tube 3870 and
attached DDM 3810
such that the plane of oscillation 3880 of DDM 3810 can be easily turned
through 360 degrees,
thereby allowing a surgeon to orient the plane of oscillation 3880 with a
tissue plane inside the
body while maintaining good ergonomics for the handle 3820. An indicator 3862
on rotational
wheel 3860 provides the surgeon with a visual cue outside the body as to the
orientation of the
plane of oscillation 3880, and, similarly, visual cues, such as embossed
stripes, can be placed on
the instrument insertion tube 3870 or on DDM 3810 thereby providing a visual
cue on camera
during laparoscopic viewing. An electrical plug 3890 allows optional
attachment via cable to an
external electrosurgical generator for electrosurgery and electrocautery
(controlled by external
foot pedals attached to the electrosurgical generator for control of the
electrosurgical generator
or, alternatively, push buttons (not shown) can be placed onto handle 3820 and
used for control
of the electrosurgical generator). Differential Dissecting Instrument 3800,
therefore, allows a
surgeon to perform blunt dissection (via differential dissection), sharp
dissection (via retractable
hook or electrosurgery), and coagulation (via electrocautery) with a single
instrument, thereby
reducing instrument changes which is complicated for laparoscopic surgery.
[00183] FIG. 39 shows a Differential Dissecting Instrument 3900 configured as
a tool to be
attached to the arm of a surgical robot, such as the da Vinci Robot from
Intuitive Surgical, Inc.
DDM 3610 is rotatably attached to instrument insertion tube 3910 via
rotational joint 3630.
Retractable hook 3640 can move between retracted and extended configurations,
as indicated by
double headed arrow 3650. Retractable hook 3640 has a tissue engaging surface
3660, tip 3670,
and elbow 3680 with a sharpened edge for sharp dissection. Retractable hook
3640 can,
optionally, be electrically conductive and electrically connected to an
external electrosurgical
generator. Similarly, DDM 3610 or a small electrically conductive patch 3625
on DDM 3610
can be used for electrocautery. (Note that an electrically conductive patch
can be placed
anywhere on DDM 3610, including the tissue engaging surface 3690.) Instrument
insertion tube
3910 attaches to housing 3920 which contains a motor to drive oscillation of
DDM 3610 and
retractable hook 3640 as described earlier. Housing 3920 is configured with
socket 3930 having
electrical and mechanical connections for connecting to the surgical robot's
arm. Instrument
insertion tube 3910 can be made long, such that housing 3920 is located
outside the patient's
body. Conversely, instrument insertion tube 3910 can be made short, such that
housing 3920 is
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
61
located inside the body, with articulations located in the robot arm and
inside the patient's body
to permit articulated motion of Differential Dissecting Instrument 3900 inside
the patient's body.
[00184] Placement of a small motor in a housing closer to a DDM and inside the
patient's
body facilitates articulation of the instrument insertion tube of a
Differential Dissecting
Instrument because all connections from the housing to the handle or housing,
and thus through
the articulation, can be electrical, which can be much simpler than designs
requiring the
transmission of mechanical drives through an articulation. This is true for
Differential
Dissecting Instruments designed both for surgical robots and for laparoscopy.
[00185] FIGS. 40-1 and 40-2 show one embodiment of such a device as the end of
a
laparoscopic Differential Dissecting Instrument 4000. FIGS. 40-1 and 40-2 show
an exemplary
laparoscopic version of a differential dissecting instrument having
electromechanical actuators
distal to an articulation, and in the straight and bent positions,
respectively. A DDM 3610 is
fitted with a retractable hook 3640 and electrically conducting patch 3625.
DDM 3610 is
rotatably attached to distal instrument insertion tube 4010 which is
articulated at rotational joint
4030 to proximal instrument insertion tube 4020. Mounted inside distal
instrument insertion
tube 4010 are a motor 4040 with motor shaft 4050 and a solenoid 4060 with
solenoid plunger
4070. Rotation of motor shaft 4050 by motor 4040 drives oscillation of DDM
4010 and, thus,
retractable hook 3640, as described earlier. Solenoid 4060 is rigidly attached
to distal instrument
insertion tube 4010, and solenoid plunger 4070 is attached to motor 4040,
which is free to slide
inside distal insertion tube 4010. Thus, when solenoid 4060 is activated,
solenoid plunger moves
up/down (in the direction indicated by arrow 4080) thereby driving motor 4040,
motor shaft
4050, and retractable hook 3640 up/down (as indicated by arrows 4080).
Flexible conductor
ribbon 4090 supplies the necessary electrical power and signals to drive motor
4040 and solenoid
4060. Articulation of laparoscopic Differential Dissecting Instrument 4000 at
rotational joint
4030 allows distal instrument insertion tube 4010 to bend with respect to
proximal instrument
insertion tube 4020, as shown in the right hand panel. Motion of distal
instrument insertion tube
4010 with respect to proximal instrument insertion tube 4020 can be driven by
any of several
mechanisms, such as a control horn driven by a push-pull rod actuated by a
hand-powered
mechanism in the handle of the laparoscopic Differential Dissecting Instrument
4000. This
configuration of actuators (i.e. motor 4040 and solenoid 4060) and flexible
conductor ribbon
4090 facilitates the transmission of complex actions past articulation at
rotational joint 4030,
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
62
transmission that would otherwise require complex mechanical parts that are
expensive, add
bulk, and are prone to failure.
[00186] FIG. 41 shows a Differential Dissecting Instrument 4100 possessing a
thin, flexible
instrument insertion tube 4110 for use in surgical procedures like single-
incision laparoscopic
surgery (SILS) or natural orifice translumenal endoscopic surgery (NOTES). The
actuation
mechanisms are similar to the DDM 3492 and retractable hook 3596 in FIGS. 35A
and 35B are
identical to that shown in FIGS. 35A and 35B; however, the rigid instrument
insertion tube 3490
and rigid drive shaft 3494 are replaced by flexible instrument insertion tube
4110 and flexible
drive shaft 4120, and the retractable hook 3596 is replaced with an
electrosurgical hook 4130.
Flexible drive shaft 4120 can rotate (as shown by double-headed arrow 4160) to
drive the
oscillation of the DDM 3492 or it can push-pull (as shown by double-headed
arrow 4162) to
retract and extend the electrosurgical hook 4130. A multi-lumen flexible
instrument insertion
tube 4110 can be used to reduce wander of flexible drive shaft 4120 inside the
flexible
instrument insertion tube, thereby providing greater authority to the push-
pull mechanism of the
flexible drive shaft 4120 for extending and retracting a electrosurgical hook
4130. A flexible
wire 4140 can also travel inside flexible insertion tube 4110 to allow
conduction of electricity to
electrosurgical hook 4130, with flexible wire 4140 and electrosurgical hook
4130 being
connected via a solder weld 4150 or other appropriate mechanism to cam
receiver body 3502.
Thus, the Differential Dissecting Instrument 4100 is capable of blunt
dissection, electrosurgical
sharp dissection, and electrocautery with controls located on a handset
outside the body or, for
electro surgery or electrocautery, via foot pedals.
[00187] FIGS. 42A through 42E show oblique and expanded views of one
embodiment of a
Differential Dissecting Instrument 4200 that has a slender, pencil grip handle
that can be easily
rotated in the hand, enabling 360 rotation of the plane of rotation of the
DDM 4250 about the
central, longitudinal axis 4299 of Differential Dissecting Instrument 4200.
[00188] FIGS. 42A and 42B show Differential Dissecting Instrument 4200 in
oblique view,
assembled in FIG. 42A and expanded in FIG. 42B. Differential Dissecting
Instrument 4200 has
an approximately cylindrical handle 4210 possessing a longitudinal, central
axis 4299. In use, a
distal end 4201 of the handle 4210 is directed toward a tissue to be
dissected, and a proximal end
4202 is pointed away from the complex tissue and toward the user. Attached to
the distal end
4201 is an elongate member 4220 parallel to the longitudinal axis 4299, having
a proximal end
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
63
4222 attached to the distal end 4201 of the handle 4210 and a distal end 4221
pointing toward the
tissue to be dissected. DDM 4250 attaches to the distal end 4221 of the
elongate member 4220.
In this embodiment, cylindrical handle 4210 is hollow, with a clamshell
construction, such that it
houses a mechanism 4260 configured to mechanically rotate the DDM 4250, as
described in the
next paragraph.
[00189] Referring now to FIGS. 42B, 42D, and 42E, FIG. 42B presents an
expanded view of
the Differential Dissecting Instrument 4200; FIG. 42D shows a closer view of
the drive
mechanism of the DDM 4250; and FIG 42E shows a simplified view of the drive
mechanism
with emphasis on how the DDM 4250 is driven. DDM 4250 is rotatably attached to
the distal
end 4221 of the elongate member 4220 such that DDM 4250 rotates about an axis
of rotation
4252. DDM 4250 possesses a first tissue engaging surface 4251 at the distal
end 4221 of DDM
4250, such that it is directed toward the tissue to be dissected, and a first
torque-point 4253
disposed to a first side 4255 of the axis of rotation 4252 of the DDM 4250
(see FIG. 42E).
Application of a first force 4270 to first torque-point 4253 creates a moment
on DDM 4250
about axis of rotation 4252 and thus drives clockwise rotation of DDM 4250
about axis of
rotation 4252. In the embodiment presented here, there is a second torque-
point 4254 disposed
to a second side 4256 of the axis of rotation 4252, whereby application of a
second force 4271
drives counterclockwise rotation of DDM 4250. Thus the moment created by
application of first
force 4270 at first torque-point 4253 creates a counter-torque to second force
4271 at second
torque point 4254. Alternating application of first force 4270 and then second
force 4271
thereby drives oscillation (clockwise then counterclockwise) of DDM 4250 about
axis of rotation
4252. Note that first force transmitting member 4261 and second force
transmitting member
4262 can be a flexible tension member, such as a cable, wire, string, rope,
tape, belt, or chain, or
a rigid member, such as a push rod or connecting rod. In the embodiment
presented here, the
first force transmitting member 4261 and the second for transmitting member
4264 are flexible
tension members, such as cables.
[00190] Oscillation is driven by a motive source 4290 that is powered by a
motor 4291. Thus,
motive source 4290 drives at least one force-transmitting member 4261 axially,
with respect to
longitudinal axis 4299, proximally and distally, thereby driving first torque-
point 4253 of DDM
4250 around axis of rotation 4252 and thereby making DDM 4250 oscillate around
its axis of
rotation 4252 such that the at least one tissue engaging surface 4251 is
configured to selectively
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
64
engage the tissue to be dissected and such that the at least one tissue
engaging surface 4251
moves across the tissue to be dissected whereby the at least one tissue
engaging surface 4251
disrupts at least one soft tissue in the tissue to be dissected, but does not
disrupt firm tissue in the
tissue to be dissected.
[00191] Referring now to FIGS. 42B, 42D, and 42E, mechanism 4260 drives the
rotation of
DDM 4250. Mechanism 4260 (see especially FIG. 42D) comprises at least one
force-
transmitting member 4261 that drives oscillation of DDM 4250, as described in
the preceding
paragraph. As seen in FIGS. 42B, 42D, and 42E first force-transmitting member
4261 and
second force-transmitting member 4262 extend approximately parallel to
longitudinal axis 4299
inside elongate member 4220, and distal end 4264 of first force-transmitting
member 4261
attaches to first torque-point 4253 of DDM 4250, and distal end 4266 of second
force-
transmitting member 4262 attaches to second torque-point 4254 of DDM 4250. As
seen in
FIGS. 42B and 42D, proximal end 4263 of first force-transmitting member 4261
attaches to a
first follower 4231 of a cam shaft 4230, and proximal end 4265 of second force-
transmitting
member 4262 attaches to a second follower 4232 of cam shaft 4230. First
follower 4231 rides on
a first eccentric cam 4233 (see inset) on cam shaft 4230, and second follower
4232 rides on a
second eccentric cam 4234 (see inset) on cam shaft 4230. Cam shaft 4230
rotates about an axis
of rotation 4235 that is perpendicular to longitudinal axis 4299, and first
eccentric cam 4233 and
second eccentric cam 4234 are positioned on opposite sides of axis of rotation
4235 such that
first follower 4231 moves 180 out of phase with respect to second follower
4232 (see FIGS.
42D and 42E for more detail). Thus, rotation of cam shaft 4230 pulls
alternately on first force-
transmitting member 4261 and second force-transmitting member 4262, creating
the alternating
first and second forces 4270 and 4271, respectively, that drives oscillation
of DDM 4250, as
described above.
[00192] Rotation of cam shaft 4230 is driven by motor 4291 via a gear train.
In the
embodiment presented here, motor 4291 is a DC electric motor forming part of
an electric circuit
4292 (see FIG. 42B) powered by at least one battery 4294, whereupon the device
further
includes at least one switch 4296 operatively associated with the motor 4291
and the at least one
battery 4294 to at least start and stop the motor 4291. Further controls can
be added to permit
proportional speed control of motor 4291 or speed control via incremental
steps. Motor 4291
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
can be one of several types of motors, including brushless DC motors, coreless
DC motors,
stepper motors, etc.
[00193] Spring mechanism 4280, compression spring 4281, compression nut 4282,
lock nut
4283, inner sleeve 4284, outer sleeve 4286, and spring stop 4285 are described
more completely
after FIGS. 44A through 44C below.
[00194] In the embodiment shown in FIGS. 42A through 42C, switch 4296 is part
of an
omnidirectional control switch 4298 that makes the on-off switch for motor
4291 accessible from
substantially any direction about the longitudinal axis 4299 of handle 4210 of
Differential
Dissecting Instrument 4200. In this embodiment, omnidirectional control switch
4298 is
comprised of five (5) switches 4296 in a radial array distributed about the
handle 4210, proximal
to distal end 4201 of handle 4210, such that a switch 4296 can be easily
activated with any finger
regardless of the rotational orientation (about longitudinal axis 4299) of
Differential Dissecting
Instrument 4200 while in the user's hand. In this embodiment, the radial array
of five switches
4296 is covered by a flexible boot 4297 made of a soft elastomer (e.g.
silicone rubber) that
prevents intrusion of fluids into switches 4296 but still permits easy
actuation of switches 4296.
The switches can be either momentary or latching. There may be any number of
switches that
form the radial array; they may lie in a single plane oriented transverse to
the longitudinal axis
4299, or they may not, in which case the array of switches may further be
distributed both
around and along the longitudinal axis 4299 of the Differential Dissecting
Instrument 4200. The
array of switches may or may not be the same; each of the switches 4296 may
vary in size,
shape, type, function (momentary, latching on-off, normally on, normally off.
single-pole single
throw, single-pole double throw, double-pole double throw, double-pole single
throw, or digital
or analog proportional control), or distance from the longitudinal axis 4299,
or any combination.
Further, instead of an array of switches, the omnidirectional control switch
4298 may be
substantially monolithic or of toroidal construction, and may be comprised of,
for example, a
first ring conductor held in abeyance from contact with second ring conductor,
whereupon the
surgeon may apply pressure to this version of the omnidirectional control
switch 4298 from any
direction, causing the first ring conductor to come into electric contact with
the second ring
conductor. Either the first ring conductor or the second ring conductor or
both can be either rigid
or flexible. For example, if the second ring conductor forms a small diameter
rigid ring around
the longitudinal axis 4299 of the Differential Dissecting Instrument 4200, the
second ring
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
66
conductor might be a large diameter ring elastically suspended out of contact
and substantially
coaxially with the first ring conductor. As one example, the surgeon's fingers
might displace a
rigid second ring conductor off-center until it contacts the first ring
conductor, or, alternatively,
the second ring conductor might flexibly deform until contact is established
with the first ring
conductor. The omnidirectional control switch 4298 might take the form of a
power switch
directly controlling the flow of electricity to a motor 4291, or, the
omnidirectional control switch
4298 might transduce surgeon finger inputs into changes in resistance,
capacitance, or other
parameters in order to drive a logic circuit that then controls the motor
4291.
[00195] FIGS. 43A through 43C show different embodiments for imparting a
torque and
counter-torque on a DDM 4300. In FIG. 43A, first tension element 4261 and
second tension
element 4262 are two halves of a single cable 4302 wrapped around a drive
cylinder 4310
attached to DDM 4300. Single cable 4302 drives rotation of drive cylinder 4310
either by
friction or by being physically attached to drive cylinder 4310. Single cable
4302 has a first end
4311 and a second end 4312 with first end 4311 acting as the proximal end of
the first force-
transmitting member and the second end 4312 acting as the proximal end of the
second force-
transmitting member. First and second forces 4270 and 4271, respectively, are
created by the
motion of rocker arm 4320 that rocks about rocker pin 4323 when driven by
linkage 4340 which
is acentrically or eccentrically attached to drive pulley 4343 which rotates
(as indicated by arrow
4344) due to motor 4342.
[00196] Another embodiment is shown in FIG. 43B where a linear spring 4350
attaches to
second torque-point 4254 and a stationary anchor point 4351 such that
application of first force
4270 rotates DDM 4300 and thereby stretches linear spring 4350 and the return
force 4271 of
linear spring 4350 generates the counter-torque when first force 4270
decreases. Similarly the
counter-torque can be generated by a torsion spring 4360, as depicted in FIG.
43C.
[00197] FIGS. 44A-1 through 44C-1 show different embodiments of mechanisms
that
protect a both a differential dissecting instrument and a tissue being
dissected from excessive
loading. FIGS 44A-1 and 44A-2 illustrate two difficulties for the construct
and use of a
Differential Dissecting Instrument 4400 that uses tension members as a force-
transmitting
member. DDM 4410 has a tissue engaging surface 4412 on its distal end and a
rotational joint
4414 on its proximal end to rotatably connect to the distal end of an elongate
member 4430. A
first tension member 4421 connects to a first torque-point 4423, and a second
tension member
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
67
4422 connects to a second torque-point 4424 such that first tension member
4421 and second
tension member 4422 create a counter-torque around rotational joint 4414 to
drive oscillation of
DDM 4410. Difficulty #1: For first tension member 4421 and second tension
member 4422 to
effectively provide a counter-torque about rotational joint 4414, they must
remain taut.
However, poor fit of components, stretching of first tension member 4421 or
second tension
member 4422, wear, or other "play" in the Differential Dissecting Instrument
4400 will cause
Differential Dissecting Instrument 4400 to perform poorly or to fail.
Difficulty #2: During
dissection of tissue 4405, application of an external force FA to the DDM 4410
can force the
Differential Disecting Instrument 4400 into an extreme position, creating
excessive bending or
wear of first tension member 4421 at point 4441 inside elongate member 4430 or
of second
tension member 4422 at point 4442 inside of elongate member 4430 (or at other
points of contact
between either first or second tension member 4421 or 4422 and another
component). More
broadly, excessive forces applied to the DDM of a Differential Dissecting
Instrument can
damage the Differential Dissecting Instrument or the tissue under dissection.
Thus a means of
preventing damage to either the instrument or the tissue would be helpful.
[00198] FIG. 44B illustrates an embodiment of a Differential Dissecting
Instrument 4401 that
addresses these difficulties including means for preventing damage due to an
overload condition.
Differential Dissecting Instrument 4401 possesses two overload mechanisms, a
first overload
mechanism 4477 that is responsive to a first threshold force Fri applied to
DDM 4410 and a
second overload mechanism 4470 that is responsive to a second threshold force
Fr2 applied to
DDM 4410. During dissection of tissue 4405, if force FA is applied to the DDM
4410 that
exceeds a first threshold force FTi, first overload mechanism 4477 stops
rotation of DDM 4410
to reduce the risk of damage to either Differential Dissecting Instrument 4401
or to the tissue
4405 being dissected. For example, first overload mechanism 4477 can include a
force sensor
4461 that measures the force FA applied to DDM 4410. Examples of force sensor
4461 include
load cells, strain gauges, and spring-loaded electrical contacts. In this
example, overload
mechanism 4450 resides in the handle 4411 where elongate member 4413 attaches
to handle
4411 but could be placed elsewhere, for example inside elongate member 4413.
Force sensor
4461 is in communication via wire 4462 with electric circuit 4292, and when
Fri exceeds FA a
signal is sent via wire 4462 to electric circuit 4292 which responds by
cutting power to motor
4290 thereby stopping oscillation of DDM 4410. Alternate means exist for
stopping rotation of
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
68
DDM 4410. For example, a clutch on motor 4290 could limit torque being applied
by motor
4290 such that when external force FA is too large, the torque becomes too
great and the clutch
slips, or the motor 4290 could simply be sufficiently small that it stalls,
etc.
[00199] If force FA is applied to DDM 4410 that exceeds a second threshold
force Fr2, second
overload mechanism 4470 withdraws DDM 4410 proximally away (in the direction
of arrow
4471) from the tissue 4405 thereby reducing external force FA. Note that first
overload
mechanism 4477 and second overload mechanism 4470 can be activated in response
to any
force, not just an axial force, as shown in FIG. 44B. Furthermore, first
threshold force Fri can
be equal to, greater than, or less than second threshold force Fr2, depending
on the desired
response. Also note that a Differential Dissecting Instrument can be fitted
with only one of the
two overload mechanisms 4470 and 4477.
[00200] FIGS 44C-1 AND 44C-2 illustrate an embodiment of a Differential
Dissecting
Instrument 4402 with a single overload mechanism as described above for FIG.
44B.
Differential Dissecting Instrument 4402 is similar to Differential Dissecting
Instrument 4401,
however, now elongate member 4430 is replaced by another exemplary overload
mechanism
4450 which acts in the same way as second overload mechanism 4470 described
above, by
withdrawing DDM 4410 proximally away from tissue 4405. Overload mechanism 4450
comprises an outer sleeve 4451 with a first spring stop 4454, an inner sleeve
4452 with a second
spring stop 4455, and a compression spring 4453. Outer sleeve 4451 and inner
sleeve 4452 are
aligned parallel to the longitudinal axis 4299 of the handle. DDM 4410 is
attached to inner
sleeve 4452 at rotational joint 4414. Inner sleeve 4452 is free to slide
proximally inside outer
sleeve 4451. As shown on the left-hand side (with no external force applied)
compression spring
4453 causes inner sleeve 4452 to slide distally inside outer sleeve 4451 due
to compression force
4460 being applied to first spring stop 4454 and second spring stop 4455.
Sliding is limited by
forces 4462 and 4463 exerted by first and second tension members 4421 and
4422, respectively,
such that the combined force of force 4462 and force 4463 equals compression
force 4460.
Thus, overload mechanism 4450 fixes Difficulty #1 described above in that
compression spring
will adjust the position of inner sleeve 4452 relative to outer sleeve 4451
whenever any play
accumulates, such as stretching of first and second tension members 4421 and
4422,
respectively. The right-hand side of FIG. 44B shows how overload mechanism
4450 also
alleviates the problems of Difficulty #2. When an external force FA is applied
to DDM 4410, a
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
69
moment is created about rotational joint 4414 forcing DDM 4410 into an extreme
position and
also applies a moment at torque-point 4424 that stretches second tension
member 4422, creating
a larger force 4463 on second tension member 4422. The increase in force 4463
thereby
increases the compression force 4460 on compression spring 4453. In response,
compression
spring 4453 compresses allowing inner sleeve 4452 to slide proximally (arrow
4456) inside outer
sleeve 4451 and thus shorten overload mechanism 4450. This withdraws DDM 4410
proximally
away from the tissue being dissected thereby decreasing the magnitude of force
4463 on second
tension member 4422 and reducing the risk of damage to Differential Dissecting
Instrument
4402, and especially to second tension member 4422. Note that other
embodiments for
withdrawing a DDM in response to an overload are possible. Different
configurations of springs,
flexible or bendable elongate members, friction pads that slip on overload,
etc. are all possible.
[00201] Returning now to FIG. 42D, spring mechanism 4280 comprises compression
spring
4281, compression nut 4282, lock nut 4283, inner sleeve 4284, and spring stop
4285.
Compression spring 4281 surrounds inner sleeve 4284 and is compressed between
compression
nut 4282 (which serves as the first spring stop 482) and spring stop 4285
(which serves as the
second spring stop) such that it pulls on first tension element 4261 and
second tension element
4262. The strength with which compression spring 4282 pulls is set by
compression nut 4282
which is threaded onto inner sleeve 4284 ¨ advancing compression nut 4282
downward (with
respect to the page) compresses compression spring 4281, increasing the
strength with which
compression spring 4281 pulls on first tension element 4261 and second tension
element 4262.
After an appropriate pull is established, compression nut 4283 can be locked
with lock nut 4283.
This means for varying the strength with which compression spring pulls on
first tension element
4261 and second tension element 4262 effectively sets the threshold force at
which the
compression spring 4281 is overcome by an external force, as discussed in FIG.
44B.
Furthermore, the distance of advance of compression nut 4283 along inner
sleeve 4284 defines
the distance over which compression spring can remove slack from the mechanism
arising from,
for example, stretch of first tension element 4261 and second tension element
4262. The travel
of inner sleeve
[00202] FIGS. 45A through 45G show a method for using a differential
dissecting instrument
for separating a tissue plane without damaging blood vessels and other
anatomical structures in
the tissue plane. FIGS. 45A through 46G depict a method for using a
Differential Dissecting
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
Instrument 4530 to dissect apart two tissues adjoining at a tissue plane. In
FIG. 45A, first tissue
4501 and second tissue 4502 adhere at a common border 4504, with Soft Tissue
4505 acting as
an adhesive between the first capsule 4506 of first tissue 4501 and a second
capsule 4507 of
second tissue 4502. In this example, one blood vessel 4520 (depicted in cross-
section) lies in the
plane of the common border 4504, in between the first capsule 4506 and the
second capsule
4507; a second blood vessel is a "perforator" 4510 that crosses the common
border 4504 going
from tissue 4501 to tissue 4502; and one collagenous bundle 4515 also crosses
the common
border 4504 going from first tissue 4501 to second tissue 4502. Thus, if first
tissue 4501 is to be
separated from second tissue 4502 by blunt dissection, then soft tissue 4505
must be disrupted,
preferably without disrupting the perforator 4510, collagenous bundle 4515, or
blood vessel
4520. (Disruption of the blood vessels can lead to unnecessary bleeding.)
Again, 4505 is a Soft
Tissue, typically comprised of gelatinous materials, mesenteries, reticular
fibers, and loosely
organized collagen fibrils. Firm Tissues include first and second capsules
4506 and 4507,
respectively, the walls of blood vessels 4510 and 4520, and collagenous bundle
4515.
[00203] Blunt dissection is performed by first grasping first tissue 4501 with
forceps 4540 and
pulling in the direction of arrow 4550 to apply tension at the edge of common
border 4504, as
indicated by double-headed arrow 4536. Application of tension across common
border 4504 is
important throughout this dissection as such tension assists the differential
action of the
Differential Dissecting Instrument 4530, as discussed above. Differential
Dissecting Instrument
4530 comprises a DDM 4532 with tissue engaging surface 4533, with DDM being
rotatably
mounted on instrument insertion tube 4531 such that it oscillates into and out
of the plane of the
page (as indicated by rotational axis 4535), causing tissue engaging surface
4533 to swipe
against the edge of common border 4504. Force 4551 is applied by the operator
to push tissue
engaging surface 4533 into the edge of common border 4504, thereby causing
ablation of Soft
Tissue 4505 and ensuing separation of the first and second capsules 4506 and
4507 of the first
and second tissues 4501 and 4502, respectively, as shown in FIG. 45B. If the
tissue engaging
surface 4533 wanders up or down due to inaccuracy of placement or misdirection
of force 4551
by the operator, the tissue engaging surface 4533 will not disrupt and,
therefore, will not cross
either capsule 4506 or 4507. Thus, Differential Dissecting Instrument
automatically follows the
plane between the tissues, defined by common border 4504.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
71
[00204] In FIG. 45C, tissue engaging surface 4533 continues along common
border 4504
until it impinges on blood vessel 4520. Again, tissue engaging surface 4533
will not disrupt the
Firm Tissue comprising the wall of blood vessel 4520. Instead, tissue engaging
surface 4533
moves to one side or the other of blood vessel 4520 (here seen moving below
blood vessel 4520),
depending on whether Soft Tissue 4505 is more easily disrupted above or below
blood vessel
4520 or if the operator pushes Differential Dissecting Instrument 4530 above
or below the blood
vessel 4520. The operator knows to push Differential Dissecting Instrument
4530 in a different
direction because the operator can sense tissue engaging surface 4533
impinging on blood vessel
4520 as an increase in resistance to pushing Differential Dissecting
Instrument 4530 into the
common border 4504 ¨ progress of the Differential Dissecting Instrument 4530
practically stops
because the tissue engaging surface 4533 will not disrupt and thus cross the
Firm Tissue
composing the wall of blood vessel 4520.
[00205] Blunt dissection continues in FIG. 45D along common border 4504 as the
operator
continues to apply tension 4536 across common border 4504 with forceps 4540
and to push
Differential Dissecting Instrument 4530 into the common border 4504. Capsules
4506 and 4507
continue channeling the Differential Dissecting Instrument 4530 along common
border 4504 by
preventing the tissue engaging surface 4533 from crossing either first capsule
4506 or second
capsule 4507 until tissue engaging surface 4503 impinges onto collagenous
bundle 4515. Again,
tissue engaging surface 4533 cannot disrupt collagenous bundle 4515, and,
again, the operator
senses that further progress of Differential Dissecting Instrument 4530 into
the common border
4504 is blocked. The operator then works the Differential Dissecting
Instrument to one side or
the other, which as seen in FIG. 45E is to the rear of collagenous bundle 4515
for this example,
and then continues dissecting along common border 4504 until tissue engaging
surface 4533
impinges now on perforator 4510. Again, the operator senses an obstruction and
moves the
Differential Dissecting Instrument 4530 to one side or the other, which as
seen in FIG. 45F is to
the rear of perforator 4510 in this example.
[00206] FIG. 45G shows the resulting dissection after Differential Dissecting
Instrument
4530 has been removed. The common border 4504 has now been dissected such that
the
capsules 4506 and 4507 of tissues 4501 and 4502, respectively, are separated,
providing a critical
view for the surgeon. Importantly, blood vessel 4520 is unharmed; collagenous
bundle 4515 is
stretched in the gap between first capsule 4506 and second capsule 4507, and
perforator 4510 is
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
72
stretched across the gap between first capsule 4506 and second capsule 4507.
Collagenous
bundle 4515 and perforator 4520 are, thus, "skeletonized", they are seen now
in open space
where they can be cauterized and cut without touching either the capsules 4506
or 4507 or
tissues 4501 or 4502. This is especially important if bleeding from perforator
4510 is to be
controlled when tissues 4501 and 4502 are separated and, also, if contact with
or thermal spread
from the electrocautery surface might cause thermal damage to either tissue
4501 or 4502.
[00207] The dissection technique, such as that shown in FIGS. 45A through 45F,
has been
used by the inventors to perform several surgical dissections (in ex vivo
animal tissues, live
animal tissues (pig), and in human cadavers) such as, for example, to separate
the gall bladder
from the bed of the liver, to separate adjacent muscles, to separate a blood
vessel from a bladder
or from another blood vessel, to separate adjacent lobes of the lung, to
isolate the pulmonary
artery and the cystic duct and cystic artery, and many others. Strikingly,
each of these
dissections has been remarkably blood-free, owing to the Differential
Dissector's ability to
dissect without disrupting either blood vessels, even blood vessels as small
as 0.5 mm outer
diameter, or tissue capsules. Furthermore, the dissection has been remarkably
safe. During these
surgeries the surgeon deliberately attempted maneuvers that would have been
catastrophic with
another instrument. For example, the surgeon stabbed the liver repeatedly with
the Differential
Dissecting Instrument set on high speed, and bounced the Differential
Dissecting Instrument on
the pulmonary artery, and stabbed into the large bowel, urinary bladder, and
lung ¨ there was no
damage to any organ. As described earlier, the absence of sharp edges in a DDM
allows it to
perform blunt dissection safely, unlike any other surgical instrument.
[00208] A dissection with a Differential Dissecting Instrument, such as that
shown in FIGS.
45A through 45F can be used, for example, to dissect fascial planes during a
tummy tuck
procedure. In fact, it is possible to dissect these tissue planes without
cauterizing or cutting
perforators. Rather, by working around perforators to skeletonize them during
dissection, using
the Differential Dissecting Instruments shown herein, sufficient separation of
tissue planes can
be achieved to permit dissection to be advanced without having to cut
perforators, which is
usually done to avoid accidental tearing or to permit sufficient separation of
the tissues to permit
viewing the dissection as it advances. Preserving perforators, rather than
cutting them, maintains
normal blood flow to the superior layers which is otherwise compromised by
disruption of
perforators. This result is truly remarkable and of great clinical importance.
Maintenance of
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
73
normal blood flow lessens the chance of tissue necrosis (due to insufficient
blood flow) and
increases the chance for a rapid and complete recovery (due to sufficient
blood flow). This is
extremely important whenever skin has been lifted from underlying tissues
(e.g. for cosmetic or
reconstructive procedures) or whenever a flap of tissue is to be isolated but
preserved.
[00209] A Differential Dissecting Instrument, such as any of those disclosed
herein, can be
used to dissect through fatty tissues; however, in such a dissection through
fat there are no organ
capsules or other Firm Tissues to guide the Differential Dissecting
Instrument, and the dissection
proceeds solely under guidance of the operator, rather than being guided by
the bordering Firm
Tissues. Such a dissection has been used to separate the skin from underlying
tissues for a face
lift in a human cadaver. Importantly, as described above, a sufficient gap was
generated, without
accidentally or intentionally disrupting perforating blood vessels, to advance
the dissection
through to completion. In a living patient, such a procedure would maintain
normal blood flow
to the tissues throughout the surgical procedure and, thus, into recovery.
This is in stark contrast
to the prior art which cauterizes perforation blood vessels, cutting off this
circulation and badly
comprising normal blood flow. As discussed above, preserving perforators,
rather than cutting
them, maintains normal blood flow to the skin which is otherwise compromised
by disruption of
perforators. Maintenance of normal blood flow lessens the chance of tissue
necrosis (due to
insufficient blood flow) and increases the chance for a rapid and complete
recovery (due to
sufficient blood flow). Both are strongly desired outcomes of all surgical
procedures, but
especially cosmetic surgical procedures.
[00210] A Differential Dissecting Instrument, such as any of those disclosed
herein, can be
used, in similar fashion, to tunnel into and through a portion of the body,
allowing tissue
capsules, blood vessel walls, nerve bundles, and other Firm Tissues to guide
the tissue engaging
surface along existing tissue planes. For tunneling, however, the operator
does not move the
Differential Dissecting Instrument from side to side to separate broad
sections of tissue planes;
rather, the operator pushes the Differential Dissecting Instrument into the
tissue plane, with only
limited motion to the side, to create a narrow tunnel. Such tunnels are used
in many surgical
procedures, such as tunneling to position pacing leads for pacemakers and
other heart rhythm
management devices, and are increasingly being used in minimally invasive
surgical procedures,
such as robotic, thoracoscopic, and laparoscopic surgery, to reduce the
disruption of tissues, and
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
74
thus trauma to tissues, during surgery. One problem that arises in tunneling
is lack of visibility
at the terminal end of the tunnel ¨ surgeons do not like to work blind.
[00211] FIGS. 46A-1, 46A-2, 46B-1, 46B-2, 46C-1, and 46C-2 show an instrument
for
tunneling with a differential dissecting instrument coupled with an endoscope.
FIGS. 46A
through 46C depict a dissection system 4600 for tunneling with a Differential
Dissecting
Instrument and with visibility being provided by a television camera or other
viewing device. As
shown in FIGS. 46A-1 and 46A-2, dissection system 4600 is comprised of an
instrument tube
4610 having two lumens, endoscope lumen 4620 and instrument lumen 4630.
Additional lumens
can be used to simultaneously introduce multiple instruments.
[00212] As seen in FIGS. 46B-1 and 46B-2, endoscope lumen 4620 houses an
endoscope
4640 that is fitted with a television camera or other viewing device at the
opposite end (not
shown), thereby providing a view of the dissection to the operator. Endoscope
4640 can also
include fiberoptics, separate from those used for the camera, to deliver light
into the field of
dissection. Instrument lumen 4630 is used to insert one of several different
instruments into the
field of view of the camera whereby they are used to dissect or otherwise
manipulate tissue under
view of the endoscope 4640. FIGS. 46B-1 and 46B-2 show instrument tube 4610
equipped with
an endoscope 4640 inside endoscope lumen 4620 and a Differential Dissecting
Instrument 4650
having a DDM 4655 inside instrument lumen 4630. Endoscope 4640 has a field of
view 4645
that permits viewing of the DDM 4655 of Differential Dissecting Instrument
4650 and its
interaction with tissue. Differential Dissecting Instrument 4650 can be
rotated inside instrument
lumen 4630 to permit the plane of oscillation of the DDM 4655 to be rotated to
align with
different tissue planes. (The plane of oscillation should be parallel to the
tissue plane.)
[00213] Multiple instruments can be inserted, one at a time, into instrument
lumen 4630, as
needed. FIGS. 46C-1 and 46C-2 show an electrosurgical instrument (e.g. a hook)
4660 inserted
into instrument lumen 4630. Electrosurgical hook 4660 can also be inserted
into and rotated
inside instrument lumen 4630 to allow the hook to point in any direction. In
use, instrument tube
4610 is loaded with endoscope 4640 inside endoscope lumen 4620 and with
Differential
Dissecting Instrument 4650 loaded into instrument lumen 4630. The instrument
tube is
positioned by an operator at the correct point on a patient, as determined by
viewing the display
of endoscope 4640, who activates Differential Dissecting Instrument 4650 to
initiate blunt
dissection. Differential Dissecting Instrument 4650 can be rotated inside
instrument lumen 4630,
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
as indicated by curved double arrow 4657, to align the plane of oscillation
with a tissue plane;
furthermore, Differential Dissecting Instrument 4650 can be advanced into and
out of instrument
lumen 4630, as indicated by straight double arrow 4656, such that DDM 4655
projects further or
less from the face 4605 of instrument tube 4610, as needed for dissection. As
the tunnel is
opened, dissection system 4600 is advanced into the tunnel, with endoscope
4640 providing a
view for the operator as the tunnel is opened up. If sharp dissection or
electrocautery is needed,
then Differential Dissecting Instrument 4650 can be removed and
electrosurgical hook 4660 can
be introduced into instrument lumen 4630 to cut or to cauterize.
[00214] Conversely, a Differential Dissecting Instrument having an extendable
electrosurgical
hook, such as the Differential Dissecting Instrument shown in FIG. 41, can be
used to avoid
having to switch back and forth between Differential Dissecting Instrument
4650 and
electrosurgical hook 4660. Other instruments, such as scissors, forceps,
bipolar forceps, or
ultrasonic cutters, can also be introduced via instrument lumen 4630 as needed
for the dissection,
or they can be part of a multi-function Differential Dissecting Instrument, as
described earlier.
[00215] A dissection system such as dissection system 4600 can be used for
many types of
endoscopic tunneling, such as endoscopic saphenous vein harvesting, endoscopic
tunneling for
anterior access to the vertebral column, for tunneling into the neck, for
tunneling into the lung for
lobectomy, or for tunneling to the heart for minimally invasive valve
replacement. A major
advantage of dissection system 4600 over existing endoscopic saphenous vein
harvesting
systems is that addition of differential dissection decreases the chance of
side branch evulsion or
damage to the vessel wall. Normally, such trauma to the vessel requires
surgical repair, such as
suturing evulsions, and is thought to greatly impair the quality of the graft
during coronary artery
bypass grafting, degrading the long-term durability of the graft.
[00216] In one demonstration of the effectiveness of a Differential Dissecting
Instrument, as
disclosed herein, for safely dissecting a major vessel with side grafts, a
surgeon inserted a
Differential Dissecting Instrument into an incision over a vessel in a live
pig (approximately 120
lbs) and then blindly advanced the Differential Dissecting Instrument along
the path of least
resistance, assuming this was the tissue plane overlying the vessel. At the
conclusion of
dissection along a 20 cm path, the surgeon dissected down, to the shaft of the
Differential
Dissecting Instrument, discovering that, yes, the Differential Dissecting
Instrument had followed
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
76
the vessel and that the vessel had been freed from surrounding tissue with no
evulsions of side
branches or bruising of the main vessel wall.
[00217] FIGS. 47A through 47D show another instrument for tunneling with a
differential
dissecting instrument coupled with an endoscope and including accessory
components to
enhance dissection and to improve the field of view for the endoscope. FIG.
47A through 47D
depict a dissecting system 4700 like the dissecting system 4600 for tunneling
into a tissue, such
as along a blood vessel. However, the dissecting system 4700 includes:
= an inflatable annular balloon 4710 located at the distal end of the
instrument tube 4610
that, on inflation, both expands the diameter of the tunnel into the tissue
4701 and forms
an airtight seal between the instrument tube 4610 and the surrounding tissue
4701 and
= an insufflation system 4720 (a system that injects air to expand a cavity
inside the body)
that permits both inflation/deflation of the balloon 4710 and injection of
pressurized air
into the end of the tunnel and thus into the tissue 4701 to expand the end of
the tunnel,
assisting blunt dissection, and providing a cavity 4702 distal to the face
4605 allowing
the camera 4640 to view the tissue 4701 and the action of instruments inserted
into the
second instrument lumen 4630.
[00218] FIGS. 47A-1 and 47A-2 show front and side views, respectively, of the
distal end of
the dissecting system 4700. As with the dissecting system 4600, there is a
multi-lumen
instrument tube 4610 with an endoscope 4640 inserted into the first instrument
lumen 4620 and a
Differential Dissecting Instrument 4650 (or other instrument) inserted into
the second instrument
lumen 4630. The balloon 4710 wraps the end of the instrument tube 4610 and can
be inflated by
air flow 4714 through an inflation tube 4712. The balloon 4710 is shown
deflated in FIG. 47A
whereby it lies closely apposed to the instrument tube 4610 to facilitate
insertion of the
instrument tube 4610 into the tissue 4701.
[00219] FIGS. 47B-1 and 47B-2 show front and side views, respectively,
inflation of the
balloon 4710 by an air flow 4714 which is provided by the balloon inflation
tube 4712 and
driven by air pumping device 4718 (shown in FIG. 47D). Air pumping device 4718
can be on of
any number of devices for providing regulated air flows including syringes,
air pumps, and such.
Note that the airflow 4714 can be in the opposite direction as drawn,
permitting deflation of the
balloon 4710 when needed.
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
77
[00220] As shown in FIG. 47C, inflation of the balloon 4710 pushes the tissue
4701 radially
away from the distal end of the instrument tube 4610, as indicated by the
arrows 4716. Thus the
instrument tube 4610 can be inserted into a tissue 4701 with the balloon 4710
deflated. After
insertion, the balloon 4710 can be inflated to help create a cavity 4702 and
thereby improve the
view for the camera 4740 attached to the endoscope 4640.
[00221] An insufflation system 4720 can also be attached to the proximal end
of the
instrument tube 4610 (see FIG. 47D). The insufflation system 4720 comprises an
insufflation
tube 4726 that connects the second instrument lumen 4630 to an air pump 4728
that provides a
regulated air flow. The regulated air flow is controllable by the operator
such that air can be
injected into or withdrawn from the second instrument lumen 4630 via
insufflation tube 4726.
Air pump 4728 can be one of any number of devices for providing regulated air
flows, including
syringes, air pumps, and such. Pressurized air flows into the insufflation
tube 4726 (as shown by
the arrow 4724), into and along the second instrument lumen 4630, and exits
into the cavity 4702
at the distal end of the instrument tube 4610 as shown by arrow 4724 in FIG.
47C. Air is
blocked from exiting the second instrument lumen 4630 by a seal 4722 between
the Differential
Dissecting Instrument 4650 (or any other instrument inserted into the second
instrument lumen
4630) and second instrument lumen 4630. Air inside the cavity 4702 can thus be
pressurized
which further expands the cavity 4702 to improve visibility for the camera
4740 attached to the
endoscope 4640 and maneuverability for the Differential Dissecting Instrument
4650.
Pressurized air inside the cavity 4702 also tensions the tissues along the
periphery of the cavity
4702 including the region of dissection 4704 for the DDM 4655. (As described
earlier,
tensioning of the tissue facilitates differential dissection; this can also be
done by placing the
differential dissecting member inside the balloon, working on and dissecting
the tissues through
the balloon membrane, and letting the balloon expansion apply the tension
normally supplied by
other instruments.) The seal 3022 can operate to block air flow both when an
instrument, such as
the Differential Dissecting Instrument 4650 or the electrosurgical hook 4660,
is inserted into the
second instrument lumen 4630. A second seal 4723 can optionally be placed
between the
endoscope 4640 and the first instrument lumen 4620 to stop airflow out any
gaps.
[00222] The embodiments set forth herein are examples and are not intended to
encompass
the entirety of the invention. Many modifications and embodiments of the
inventions set forth
herein will come to mind to one skilled in the art to which these inventions
pertain having the
CA 02928627 2016-04-22
WO 2015/065898 PCT/US2014/062382
78
benefit of the teaching presented in the foregoing descriptions and the
associated drawings.
Therefore, it is to be understood that the inventions are not to be limited to
the specific
embodiments disclosed and that modifications and other embodiments are
intended to be
included within the scope of the appended claims. Although specific terms are
used herein, they
are used in a generic and descriptive sense only and not for the purposes of
limitation.