Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
1
BEVERAGE CARTRIDGE CONTAINING PHARMACEUTICAL ACTIVES
FIELD OF THE INVENTION
The present invention is related to a beverage cartridge and more
particularly, a cartridge
that is adapted to make beverages containing pharmaceutical actives.
BACKGROUND OF THE INVENTION
Currently, there are a number of cartridges for use in automatic brewing
machines that
make a single serving of a beverage product, such as coffee or tea. However,
current cartridges
are not suitable for use with pharmaceutical actives because the actives are
sensitive to
environmental conditions, such as humidity and oxygen, and the actives can
degrade in current
cartridges. Furthermore, the Consumer Product Safety Commission (CPSC)
requires the primary
package is child-resistant if the cartridge contains certain active
ingredients, such as
acetaminophen and diphenhydramine.
As such, there remains a need for a cartridge which is adapted for use in an
automatic
brewing machine that is both child-resistant and provides a barrier from
environmental
conditions to provide long term stability for pharmaceutical actives.
SUMMARY OF THE INVENTION
A child-resistant cartridge adapted to fit an automatic brewing machine
comprising: (a) a
base wherein the base contains a pharmaceutical active; and (b) a lidding
wherein the lidding
comprises a multilayer laminate comprising an outer layer and an inner layer;
wherein the
cartridge is made of tear resistant materials sufficient to prevent 70% of
children from accessing
the contents of five or more cartridges during a Child-Resistant Screening
Test.
A child-resistant multi-layer laminate lidding adapted for a cartridge
comprising: (a) an
outer layer; and (b) an inner layer; wherein the lidding is made of tear
resistant materials
sufficient to prevent 70% of children from accessing the contents of five or
more cartridges by
puncturing the lidding during a Child-Resistant Screening Test and wherein the
lidding is
penetrable by a needle or other penetrator of an automatic brewing machine.
A child-resistant cartridge adapted to fit an automatic brewing machine
comprising: (a) a
base wherein the base contains a pharmaceutical active; and (b) a lidding;
wherein the cartridge
is made of tear resistant materials sufficient to prevent 70% of children from
accessing the
contents of five or more cartridges during a Child-Resistant Screening Test.
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
2
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a three-dimensional exploded view of a beverage cartridge;
FIG. 2 is a side sectional view of a beverage cartridge of FIG. 1;
FIG. 3 is a side sectional view of a lidding for a beverage cartridge;
FIG. 4A is a lidding for a beverage cartridge;
FIG. 4B is a lidding for a beverage cartridge;
FIG. 5 is a side sectional view of a beverage cartridge with two compartments;
FIG. 6 is an example of a side sectional view of a beverage cartridge with two
chambers;
and
FIG. 7 is an example three-dimensional exploded view of a beverage cartridge
with a
filter.
DETAILED DESCRIPTION OF THE INVENTION
Many consumers enjoy drinking hot or cold beverages with pharmaceutical
actives. A
convenient and easy way to make a single serving of a hot or cold beverage is
to use an
automatic brewing machine with a cartridge. However, the current cartridges
are not adapted to
be used with pharmaceutical actives for two reasons. First, the current
cartridge does not provide
a sufficient barrier to environmental conditions to prevent degradation.
Second, the current
cartridges are not child-resistant. It has been observed that children are
able to open the current
cartridges with their teeth and then using their fingers to peel off the
lidding to access the
contents or by applying pressure to the lidding with their thumb to puncture
the lidding.
Furthermore, once a child figured out how to open one cartridge, he was able
to quickly open
additional cartridges.
The cartridges of the present invention can provide a sufficient barrier from
environmental conditions including humidity and gases, including oxygen, and
can be child-
resistant. The structure and/or material(s) of the base and/or the lidding can
provide long term
stability for the pharmaceutical actives and/or child-resistance. The
cartridge can have a base and
a lidding structure. In one example, the base can be a multi-layer laminate,
which can include
polychlorotrifluoroethylene (PCTFE), an adhesive, and polyethylene
terephthalate with a glycol
modifier (PETG). In another example, the lidding can be a multi-layer laminate
structure that can
comprise aluminum foil, adhesive, and a plastic. The base and/or the lidding
can be tear resistant.
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
3
Furthermore, while being child-resistant, the bottom of the base and/or the
lidding can still be
penetrable by a needle or penetrator of the automatic brewing machine.
The cartridge can meet the definition of child-resistant under the U.S. Poison
Prevention
Packaging Act of 1970 (16 C.F.R. 1700.14). Specifically, the cartridges can
meet the definition
of F=3 according to the Child-Resistant Testing as described herein. In
another example, the
cartridge can meet the definition of F=1 according to the Child-Resistant
Testing, in another
example F=2, in another example F=4, in another example F=5, and in another
example F=6. In
one example, the cartridge alone meets the definition of child-resistant
without the secondary
packaging.
The cartridge can be adapted for use in an automatic machine such as a single
serving
automatic brewing machine. Some of these machines can have a penetrator or
needle that can
penetrate the lidding and then provide a flow of water, frequently hot water,
through the hole in
the lidding, while a second penetrator or needle pushes through the bottom of
the base to receive
the outflow of the beverage and dispense it into a cup or container. Even
though the base and the
lidding layer are child resistant, in some examples, they can be penetrated by
a penetrator or
needle of a brewing machine. In another example, the child-resistant and/or
barrier features do
not interfere with normal operation of the automatic brewing machine.
As used herein, "active" or "pharmaceutical active" includes all compounds and
compositions that can be used to treat and/or prevent illness and/or provide
overall health and
wellness benefits in mammals, particularly humans. Non-limiting examples of
particularly useful
actives include over-the-counter (OTC) actives, behind the counter actives,
and prescription
actives, vitamins, minerals, plant-derived materials, energy boosting
materials, probiotics, fiber,
prebiotics, and combinations thereof.
As used herein, "child-resistant" means a cartridge or other packaging that is
designed or
constructed to be significantly difficult for young children to open or obtain
a toxic or harmful
amount of the substance contained therein within a reasonable time and not
difficult for normal
adults to use properly, but does not mean packaging which all such children
cannot open or
obtain a toxic or harmful amount within a reasonable time.
As used herein, "dissolve" refers to passing into solution.
As used herein, "disintegrate" refers to breaking up into small parts.
As used herein, "permanently joined" refers to configurations in which a first
element is
secured to a second element such that the elements generally cannot be
separated from one
another without at least partially destroying one or both of the elements.
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
4
As used herein, "primary packaging" refers a packaging component that is or
may be in
direct contact with the dosage form.
As used herein, "releasably joined" refers to configurations in which a first
element is
secured to a second element, such that the first element and the second
element can be separated
with no or minimal damage to the first and second elements.
As used herein, "tear resistant" means capable of experiencing a reasonable
level of stress
and/or deformation without experiencing a significant loss of integrity.
Stress and/or deformation
can be applied by a number of movements including, but not limited to, biting,
pulling, pealing,
poking, pushing and/or jabbing at the cartridge. In one example, any of these
movements can be
performed by a child, and tear resistant refers to a material that is capable
of experiencing a
reasonable level of stress and/or deformation without experiencing a
significant loss of integrity
when undergoing forces that can be applied by a child. In one example, the
child is six years or
younger, in another example five years or younger, in another example four
years or younger, in
another example three or younger, in another example two or younger, and in
another example
18 months or younger. In one example, even though the cartridge is tear
resistant, it can still be
punctured with the needle or penetrator of an automatic brewing machine.
As used herein, the term "treat" or "treating" includes preventing,
alleviating,
ameliorating, inhibiting, or mitigating one or more health conditions in a
mammal, in particular a
human and in one example an adult human. Non-limiting examples of health
conditions can
include respiratory conditions.
As used herein, the articles "a" and "an" are understood to mean one or more
of the
material that is claimed or described, for example, "an active" or "a
compartment".
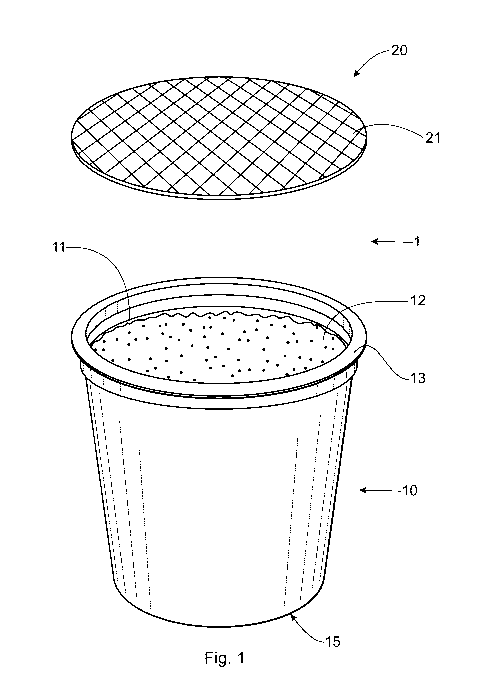
FIG. 1 shows an exploded view of cartridge 1 and FIG. 2 shows a side sectional
view of
cartridge 1. Cartridge 1 includes base 10 and lidding 20. Base 10 includes
opening 11 and
outwardly facing rim 13. Base 10 can have the shape of a cup, cylinder, bowl,
or an inverted
truncated cone. Base 10 can include component 12, which can include an active
and/or an
excipient. In one example, the component can be a powder. Bottom 15 of base 10
can be
penetrable by a penetrator or needle during use.
With reference to FIG. 2, inner surface 26 of lidding 20 can be permanently
joined to rim
13 of base 10 at seal 25. The lidding can be joined to the base by any
suitable method. In one
example, the lidding has a coating that is heat activated, causing it to
attach to the rim of the base
when exposed to heat. In another example, the lidding can be attached using
ultrasonic frequency
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
and in another example the lidding can be attached using induction heating.
Alternatively, the
lidding can be attached with a contact adhesive.
Lidding 20 can be a child-resistant laminate with outer layer 21 and inner
layer 22. Outer
layer 21 can provide the child-resistance and can be tear resistant while
still be penetrable by a
5
needle or other penetrator of an automatic brewing machine. In one example,
outer layer 21 can
contain reinforced strands 24, which can provide additional child resistance.
Reinforced strands
24 can form a mesh across lidding 20 and in one example, the mesh can be open
at the location
where the lidding is intended to be punctured by a needle or penetrator. In
one example, the
reinforced strands can be embedded in the child-resistant laminate. In another
example, the
reinforced strands are underneath the outer layer. In another example, the
reinforced strands can
be underneath the inner layer. In another example, the reinforced strands can
be between the
outer layer and the inner layer. In another example, the reinforced strands
can be embedded in the
outer layer and/or the inner layer. The reinforced strands can be made of any
suitable material
including nylon, polypropylene, fiberglass, and combinations thereof.
Inner layer 22 can reduce the amount of gasses, including oxygen, from
entering cartridge
1. Inner layer 22 can be made out of any suitable material and in one example,
inner layer 22 can
be made out of aluminum foil. Inner layer 22 can be opaque which can provide
additional child-
resistance. In another example, inner layer 22 can also include ink. In one
example, outer layer
21 and inner layer 22 are permanently joined by an adhesive.
The lidding can be both child-resistant and provide an adequate barrier from
gasses and
humidity, while still being penetrable by a needle or other penetrator of an
automatic brewing
machine. In one example, the lidding is a laminate. The laminate can have any
number of layers.
In one example the laminate has at least two layers, in another example at
least three layers, in
another example at least four layers, and in another example at least five
layers. In another
example, the lidding can be only one layer. In one example, one or more layers
of the lidding can
be opaque and in another example one or more layers of the lidding can be
transparent.
FIG. 3 is an example of a side sectional view of lidding 30 for a beverage
cartridge.
Lidding 30 can comprise outer layer 32, second layer 33, third layer 34, and
inner layer 35. In
one example, outer layer 32 can comprise polyethylene terephthalate (PET),
second layer 33 can
comprise orientated polyamide (OPA), third layer 34 can comprise aluminum, and
inner layer 35
can comprise polyethylene vinyl acetate (PE-EVA). In another example, inner
layer 35 can
comprise linear low-density polyethylene (LLDPE). In one example, each layer
is attached to an
adjacent layer with an adhesive. In another example, ink is between the outer
layer and the
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
6
second layer. In another example, ink is printed on the outer layer. In
another example, a heat
seal coating partially or entirely covers the inner layer.
FIGS. 4A and 4B are examples of liddings with weakened areas. The weakened
area can
be in the region that is intended to be punctured by a needle or other
penetrator of an automatic
brewing machine. This weakened area can allow the lidding to be child
resistant while still
allowing the lid to be penetrated by a needle or penetrator. The weakened area
can extend
partially or completely through one or more layers of the laminate lidding. In
one example, the
weakened area is on the outer layer of the lidding. In another example, the
weakened area is not
on the outer layer of the lidding because a child could see the weakened area
and gain access or
pick and peel at an edge of the weakened area. In one example, the weakened
area does not
penetrate the inner layer. In another example, the weakened area only
penetrates or partially
penetrates the outer layer. In one example, the weakened can be small enough
so it is difficult for
a child to use it to access the cartridge contents. In one example, the
weakened area is from about
1 mm to about 12 mm at its widest point, in another example from about 2 mm to
about 10 mm,
and in another example from about 3 mm to about 8 mm.
FIG. 4A is an example of lidding 40 with a weakened area, hole 42. The hole
can be any
suitable shape. Non-limiting examples of shapes can include a circle, square,
diamond, rectangle,
triangle, star, cross, oval, and combinations thereof.
FIG. 4B is an example of lidding 45 with a weakened area, scored portion 47.
Scored
portion 47 can be in any of the shapes listed above, as well as an "x", cross-
hatching, multiple
dots, and combinations thereof.
FIG. 5 is an example of a side sectional view of beverage cartridge 5 with
more than one
compartment. For some components it could be advantageous to separate some of
the ingredients
until immediately before they are consumed. In one example, the actives and/or
excipients are
incompatible and separating them prolongs the stability of the components. In
another example,
the components are in different states, for example one component is a solid
and the other is a
liquid. In another example, one compartment contains a component with an
active and another
compartment contains a component with a flavor, which could allow for more
robust flavor
delivery, particularly if the flavor was a liquid.
In FIG. 5, beverage cartridge 5 has first compartment 51 containing first
component 53
and second compartment 52 containing second component 54. First compartment 51
and second
compartment 52 are separated by divider 55.
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
7
The divider can be any suitable material. In one example the divider partially
or
completely dissolves or partially or completely disintegrates, when exposed to
hot or cold water.
In another example, the divider can be a mesh or woven material that separates
the components
when they are solid but when the components dissolve in water, the solution
can travel through
the mesh or woven material. In another example, the divider can be punctured
with the needle or
penetrator from the automatic brewing machine. When the divider is punctured,
water and the
second component can flow from the second compartment to the first
compartment.
The beverage cartridge can have multiple compartments. In one example, the
beverage
cartridge can have at least two compartments, in another example at least
three compartments,
and in another example at least four compartments. The compartments can extend
in any
direction including horizontally across the cartridge, vertically from the
bottom of the base to the
lidding, diagonally, or combinations thereof.
FIG. 6 is an example of side sectional view of beverage cartridge 6 with
multiple
chambers. Cartridge 6 has first chamber 61 containing first component 63 and
second chamber
62 containing second component 64. The chambers not only can keep the
components separate
until just before use but they can also allow the user to select which actives
she wants in her
beverage.
In one example, the automatic brewing machine can have an upper needle 66,
which
punctures lidding 65, approximately in the center, and does not puncture first
chamber 61 or
second chamber 62. Simultaneously, lower needle 67 punctures the bottom 68 of
base 69 and
chamber 63. Lower needle 67 does not puncture the middle of base 67, instead
it punctures off
center. Thus by arranging cartridge 6 with a particular orientation in the
brewing machine, one
chamber could be broken, while the other chamber remains intact. In one
example, the chambers
can be made of a frangible material and/or can be pre-scored so when the lower
needle punctures
the chamber it cracks or breaks completely in two or more parts. Non-limiting
examples of
frangible materials can include starch based films, water soluble modified
cellulose films such as
hydroxyethyl cellylose, ethyl vinyl acetate films, and combinations thereof.
When the brewing
begins, water will flow out the upper needle and flow around the intact
chamber, while
combining with the components of the broken chamber and making a beverage with
the desired
components.
For instance, the first chamber may contain an active to treat aches and
pains, such as
acetaminophen or ibuprofen, and the second chamber may contain an active to
treat cough, such
as dextromethorphan. If the consumer does not have a cough she can position
the cartridge in the
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
8
automatic beverage brewer such that only the chamber that has the actives for
aches and pains is
penetrated by the bottom needle. However, if the consumer wants both actives
she can brew
twice, puncturing both chambers.
In another example, the chamber can contain different flavors allowing the
user to select a
flavor of her beverage.
In another example, the upper needle can puncture both chambers. The lower
needle
punctures only one chamber and liquid from the upper needle can flow into both
chambers but
the beverage will only be able to flow out of the chamber punctured by lower
needle. Thus, the
consumer can make a beverage with the desired components.
The cartridge can have any suitable number of chambers. In one example, the
cartridge
can have at least two chambers, in another example at least three chambers,
and in another
example at least four chambers. In one example, the cartridge could have a
single chamber that
could allow the consumer to make a beverage with just the component outside
the chamber or
with both components.
In another example, the chambers can be used to separate incompatible
components.
FIG. 7 is an example of a cartridge 7 with a base 70, lidding 71, and a filter
72. Filter 72
contains component 73. The filter can be used when brewing a drink, such as
coffee or tea. The
water will seep through the filter and extract the essence of the components
and the solid
particles will be retained in the filter. In one example, the actives and/or
other excipients can
dissolve into the water as it passes through the filter. In one example, the
cartridge with a filter
can be used to brew coffee or tea containing actives.
In one example, the cartridge does not contain a filter.
In another example, at least one of the ingredients are packaged in a separate
container
and added by the consumer, or person preparing the beverage for the consumer,
before or after
their beverage is brewed by the automatic brewing machine. In one example the
ingredient can
be a flavor and in another example the ingredient can contain one or more
actives. In one
example, the separate container can be a sachet. In one example, the separate
container can
contain a liquid. In another example the separate container can contain a
solid, where the solid
can be a powder, nonpareils, capsule, tablet, or combinations thereof. In
another example, the
consumer uses the automatic brewing system to puncture the top of the
cartridge but before
brewing the beverage the consumer reopens the machine and adds the separately
packaged
ingredient to the cartridge through the hole made by the automatic brewing
machine and then
recloses the machine and brews their beverage.
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
9
The base can be made of any suitable material(s) that provide the desired
barrier
properties and child-resistance and can still be penetrated by the needle or
penetrator of the
automatic brewing machine. Non-limiting examples of materials that can be
incorporated into the
laminate material of the base can include high-density polyethylene (HDPE),
cyclic olefin
copolymers (COC), biaxally orientated polyamide (OPA), polypropylene (PP),
polyester (PET),
amorphous polyethylene terephthalate (APET), polyvinyl chloride (PVC),
polyethylene (PE),
aluminum foil, and combinations thereof. In another example the laminate can
include a
polyvinylidene chloride coating and in another example the laminate can
include ethylvinyl
alcohol (EVOH). In one example, the base can be transparent and in another
example the base
can be opaque.
In one example, the base can be a laminate made from polychlorotrifluoroethene
(PCTFE), an optional adhesive, and polyethylene terepthalate glycol (PETG). In
one example,
the PETG can be from about 25 lam to about 1.5 mm thick, in another example
from about 50 lam
to about 1 mm, in another example from about 100 lam to about 500 lam, and in
another example
from about 200 lam to about 300 lam. In another example, the PCTFE can be from
about 10 lam
to about 250 lam, in another example from about 25 lam to about 150 lam, in
another example
from about 30 lam to about 100 lam, and in another example from about 40 lam
to about 75 lam.
In one example, the base can be a laminate with an inner layer and an outer
layer. In one
example, the inner layer can be EVOH, or another material that is difficult
for a child to puncture
or tear. The inner layer and the outer layer of the base can be releasably
joined. When the child
tries to open the container, for instance by puncturing, tearing, or biting,
the inner layer can
separate from the outer layer. This separation can slow the child down, which
can give a parent
or caregiver more time to intervene and prevent the child from accessing
cartridges. This
separation may also make the child feel like they are making progress getting
the package open,
when in reality delaminating these two layers is not contributing to gaining
access to the
component, since the child cannot break through the inner and/or outer layer.
In one example, the maximum thickness of the base material can be greater than
the
maximum thickness of current pharmaceutical product barrier materials, such as
cavities on a
blister card, because the cartridge has dimensions larger than a typical
pharmaceutical product
enclosure. Using base material with a greater starting thickness provides for
sufficient thickness
when the base of the cartridge is formed because the material is stretched by
a greater degree
towards the bottom of the cartridge base.
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
Current lidding can be removed by children with a combination of biting and
scratching.
Thus, the lidding can be tear resistant. In one example, the lidding is a
multi-layer laminate. Non-
limiting examples of materials that can be incorporated into the laminate
material of the lidding
can include aluminum foil, PCTFE, EVOH, HDPE, OPA, COC, PP, PET, APET, PVC,
PE, and
5 combinations thereof.
For both the laminate in the lidding layer and the base, the layers of the
laminate can be
affixed by an adhesive layer, which can comprise a polyolefin material like
low density
polyethylene (LDPE).
In one example, the laminate can have an outer layer, a second layer, and an
inner layer.
10 The outer layer can be PET, the second layer can be foil, and the inner
layer can be LLDPE.
In one example the outer layer or PET layer can be from about 5 lam to about
40 lam
thick, in another example from about 10 lam to about 35 lam thick, in another
example from about
lam to about 30 lam thick, and in another example from about 20 lam to about
25 lam thick. In
one example the outer layer or PET layer can be greater than about 8 lam, in
another example
15 greater than about 11 lam, in another example greater than about 16 lam,
in another example
greater than about 20 lam, and in another example greater than about 22 lam.
In another example, the second layer or foil layer can be from about 3 lam to
about 30
lam, in another example from about 8 lam to about 25 lam thick, and in another
example from
about 15 lam to about 22 lam thick. In another example the second layer or
foil layer can be
greater than about 5 lam thick, in another example greater than about 8 lam
thick, in another
example greater than about 12 lam thick, in another example greater than about
15 lam thick, and
in another example greater than about 17 lam thick.
In another example, the inner layer or LLDPE layer can be from about 10 lam to
about 40
lam thick, in another example from about 15 lam to about 35 lam thick, in
another example from
about 20 lam to about 30 lam thick, and in another example from about 22 lam
to about 27 lam
thick. In another example, the inner layer or LLDPE layer can be greater than
about 8 lam thick,
in another example greater than about 13 lam thick, in another example greater
than about 18 lam
thick, in another example greater than about 20 lam thick, in another example
greater than about
22 lam thick, and in another example greater than 24 lam thick.
The base, lidding, and/or cartridge can provide an effective barrier. In one
example, the
base, lidding, and/or cartridge can have a final moisture vapor transmission
rate of about 2 x 10-5
to about 2 x 10-3 g/cartridge/day, in another example from about 1 x 104 to
about 6 x 104
g/cartridge/day, and in another example from about 2 x 104 to about 3 x 104
g/cartridge/day, as
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
11
determined by the United States Pharmacopeial (USP) <671> (August 1, 2013).
Follow Method
1 of the procedure for Single-Unit Containers and Unit-Dose Containers for
Capsules and
Tablets.
The cartridge can provide long term stability to the pharmaceutical actives.
In one
example, the pharmaceutical active in the cartridge have a shelf life of at
least about 1 year, in
another example at least about 18 months, in another example at least about 2
years, and in
another example at least about 2.5 years. As used herein, "shelf life" refers
to the time period
during which a drug product is expected to remain within the approved shelf
life specification,
provided that it is stored under the conditions defined on the container
label. The conditions
under which shelf life testing is conducted, the specific measurements and the
minimum
requirements for usable, fit for consumption or saleable can be set by
governmental or regulatory
bodies and can be published in guidance documents such as the United States
Pharmacopeial
(USP) or ICH Harmonised Tripartite Guideline Stability Testing of New Drug
Substances and
Products Q1A(R2), Step 4, of the version published February 6th, 2003.
In one example, the pharmaceutical active is at least about 90% of label for
at least 1
year, in another example for at least about 1.5 years, in another example for
at least about 2
years, and in another example for at least about 3 years. In another example
the pharmaceutical
active is at least about 95% of label for at least 1 year, alternatively at
least about 1.5 years,
alternatively at least about 2 years. The cartridge may provide long term
stability by maintaining
the pharmaceutical active above about 80% or label for at least about 1 year,
alternatively at least
about 1.5 years, alternatively at least 2 years.
The cartridges can be in secondary packaging. In one example, the secondary
package
can be a standard paperboard box that contains from about 1 to about 12
cartridges, and in
another example from about 3 to about 6 cartridges. In another example, the
secondary package
can be child-resistant. In one example, the secondary package can contribute
to the overall child-
resistance of the cartridge. In another example the secondary package can be
an F=1 child-
resistant package, in another example an F=2 child-resistant package, and in
another example an
F=3 child-resistant package. In another example, each cartridge is
individually packaged in a
secondary package. In another example, the secondary packaging can provide
additional barrier
properties to help prolong stability of the components.
In one example, the cartridge can be used with an automatic brewing machine
and can be
adapted to fit in an automatic brewing machine. In another example, the
cartridge can be used
without an automatic brewing machine.
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
12
In one example, the cartridges are disposable which means that the cartridge
can be
disposed of or discarded after a limited number of uses. In one example, the
cartridge can be used
five or fewer times, in another example three or fewer times, in another
example two or fewer
times, and in another example the cartridge is used only one time. In another
example, the
cartridge can be reused and can be refilled with a component containing an
active and/or an
excipient.
The component can include both one or more actives and/or one or more
excipients. The
component can be in any suitable form including, but not limited to, a powder,
a liquid, a gel, or
a tablet. Non-limiting example of excipients can include flavors, sweeteners,
disintegrants, fillers,
colors, lubricants, glidants, sorbents, preservatives, sensates, and
combinations thereof. In one
example, the component is a powder that dissolves as the water passes through
the cartridge. In
another example, the component is a concentrated liquid that can be diluted
when the water
passes through the cartridge. In yet another example, the component is only
partially dissolved or
extracted to make the beverage.
The cartridge can contain one or more actives. In one example, the beverage
containing
actives can be consumed by adults and children 12 years and over. In another
example, the
beverage containing actives can be intended to be consumed by children, often
under the
supervision of an adult.
The actives can treat a variety of symptoms including, but not limited to,
symptoms from
cold, flu, or allergies. Non-limiting examples of actives can include
decongestants, expectorants,
antihistamines, antitussives, pain relievers, and combinations thereof.
In one example, the cartridge can provide multi-symptom relief of cold and/or
flu
symptoms and can be intended for use at night. The cartridge can deliver about
650 mg
acetaminophen, about 30 mg dextromethorphan HBr, about 10 mg of phenylephrine,
about 30 to
about 60 mg of pseudoephedrine, and/or about 12.5 mg doxylamine succinate
during a single
brew cycle.
In some examples, how much active is delivered, does not necessarily mean that
the
cartridge contains this amount of active. The cartridge will likely contain
more active than what
is delivered to the user since in some examples, not all of the active will be
transferred from the
cartridge to the beverage. Furthermore, according to government regulations,
such as the USP,
the delivered amount of active can be within 10% of the monographed amount.
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
13
In another example, the cartridge can help relieve occasional sleeplessness
and/or can
help reduce the time to fall asleep if you have difficulty falling asleep. The
cartridge can provide
about 50 mg diphenhydramine HC1 or about 25 mg of doxylamine succinate.
In another example, the cartridge can help the user treat allergy symptoms.
The cartridge
can deliver about 25 mg to about 50 mg diphenhydramine HC1, about 10mg
loratadine, about 650
mg to about 1000 mg acetaminophen, about 7.5 mg to about 12.5 mg doxylamine,
and/or about
mg phenylephrine.
In another example, the cartridges can be sold in combination with other
products, in
particular products containing over-the-counter actives or vitamins and/or
minerals. In one
10 example, the cartridge can contain actives intended for nighttime relief
of cold and/or flu
symptoms and the cartridge can be packaged or otherwise sold in combination
with actives
intended for daytime relief of cold and/or flu symptoms such as acetaminophen,
dextromethorphan, and phenylephrine. In another example, the cartridge can
contain vitamins,
for instance a tea containing vitamins, and it can be packaged or otherwise
sold in combination
with over-the-counter actives, for instance actives containing daytime and/or
nighttime relief of
cold and/or flu symptoms. The over-the-counter actives can be any dosage form
including, but
not limited to, a liquid, solid dosage forms such as liquicaps or tablets, or
a cartridge.
The automatic brewing machine can dispense an entire dose of active as
determined by
the monograph. Thus, the active can quickly dissolve or can be extracted into
water as it is being
brewed. In one example, the cartridge can contain more than the monographed
dosage of the
active in order to ensure that the correct dosage is in the consumer's cup.
The beverage can be
any volume. In one example the beverage is from about 100 mL to about 500 mL,
in another
example from about 125 mL to about 250 mL, in another example from about 130
mL to about
250 mL, and in another example about 150 mL to about 200 mL. In one example,
the beverage is
about 6 fluid ounces (177.41 mL) and in another example the beverage is about
8 fluid ounces
(236.59 mL).
In one example, following each use the automatic brewing machine can be rinsed
with
water or otherwise cleaned to remove residual actives and/or excipients. For
example, a brewing
cycle can be conducted by the consumer without a cartridge in the machine. In
one example, the
automatic brewing machine can be cleaned with water. In another example, the
automatic
brewing machine can be cleaned with a cleaning agent, for instance a cleaning
agent comprising
vinegar. Some consumers may also want to clean the brewing machine before
brewing the
beverage.
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
14
In one example, the cartridge can be child-resistant and can be given a rating
that is
referred to as the F value. The F Value refers to the number of unit doses to
which access is
considered a test failure. The number following the "F" refers to the number
of unit doses that
may produce serious personal injury or serious illness based on a 25-pound
(11.4 kg) child.
The Child-Resistant Test is a standardized test and can be found in 16 C.F.R.
1700
Poison Prevention Packaging. In one example, the child-resistant package is an
F=1 package, in
another example an F=2 package, in yet another example an F=3 package, in
another example an
F=4 package, and in yet another example an F=5 package.
In one example about 80% or more of the children in the child resistance test
cannot
access the contents of 5 cartridges or fewer, in another example about 70% or
more of the
children cannot the contents of 5 cartridges or fewer, in another example
about 60% or more of
the children cannot access the contents of 5 cartridges or fewer. In another
example about 80% or
more of the children in the child resistance test cannot access the contents
of 4 cartridges or
fewer, in another example about 70% or more of the children cannot access the
contents of 4
cartridges or fewer, in another example about 60% or more of the children
cannot access the
contents of 4 cartridges or fewer. In another example, about 80% or more of
the children in the
child resistance test cannot access the contents of 3 cartridges or fewer, in
another example about
70% or more of the children cannot access the contents of 3 cartridges or
fewer, in another
example about 60% or more of the children cannot access the contents of 3
cartridges or fewer.
In another example, about 80% or more of the children in the child resistance
test cannot access
the contents of 2 cartridges or fewer, in another example about 70% or more of
the children
cannot access the contents of 2 cartridges or fewer, in another example about
60% or more of the
children cannot access the contents of 2 cartridges or fewer.
Test Methods
Child-Resistant Testing
The child-resistant testing can be conducted according to the Code of Federal
Regulations
Title 16: Part 1700.
Child-Resistant Screening Test
The Child-Resistant Screening Test can be conducted as follows:
For the Child-Resistant Screening Test the children are between 42-51 months
of age.
Both boys and girls are selected in approximately even numbers.
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
A test failure is defined as any child who gains access to the cartridge
contents. For the
following experiment, the test was a failure if any child accessed the
contents of three or more
cartridges during the full 10 minutes of testing. As used herein, "gained
access to" means that the
actives have been removed or can be removed in whole or in part. In the case
of a cartridge, the
5 active can be removed if any amount of active can spill out of the
cartridge if it is inverted. If a
cartridge or package is breached and the contents are not removed but could be
removed, this is
still considered access.
The children are tested two at a time. The two children are escorted to the
test area and
are seated so there is no physical barrier between the children and the
tester. The tester will talk
10 to the children to make them at ease. The children are not given the
impression that they are in a
race or a contest, they are not offered a reward, and they are not told that
the test is a game or that
it is fun. To begin the test the tester shall hand the children identical
cartridges and say "Please
try and open this for me." If the child refuses to participate after the test
has started, the tester
shall reassure the child and gently encourage the child to try. If the child
continues to refuse, the
15 tester shall ask the child to hold the cartridge in his/her lap until
the other child is finished. This
pair of children shall not be eliminated from the results unless the refusing
child disrupts the
participation of the other child.
Each child will be given 5 minutes to open his/her cartridge. The tester shall
minimize
conversations with the children as long as they continue to attempt to open
their packages. The
tester shall not discourage the children verbally or with facial expressions.
If a child gets
frustrated or bored and stops trying to open his/her cartridge, the tester
shall reassure the child
and gently encourage the child to keep trying. The children should be allowed
freedom of
movement to work on their cartridges as long as the tester can watch both
children (e.g. they can
stand up, get down on the floor, or bang or pry the package). The children
shall be allowed to talk
to each other about opening the cartridges and shall be allowed to watch each
other try to open
packages. If the child opens his/her cartridge, the tester shall say, "Thank
you," and take the
opened cartridge from the child and give the child another unopened cartridge.
At the end of the 5-minute period, the tester shall ask the children to put
their cartridges
down and then shall demonstrate how to open the cartridge. This demonstration
is done by
performing a demonstration of how to use the cartridge in the machine, a
Keurig K75 Platinum
Edition brewer. The children shall not be allowed to continue to try to open
their cartridges
during the demonstration period. The tester shall say, "watch me use this
package." Once the
tester gets the children's full attention, the tester shall hold the demo
package approximately two
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
16
feet from the children and open the package at a normal speed as if the tester
were going to use
the contents. There shall be no exaggerated opening movements. The tester
shall not discuss or
describe how to open the package. After opening the package, the tester shall
show the children
the package.
Then, the children are given a second five-minute period to try and open their
cartridges.
The tester begins the five minute period by saying, "now you try to open your
packages." If both
children have not used their teeth to try to open their packages during the
first 5 minutes, the
tester shall say immediately before or soon after beginning the second 5-
minute period, "You can
use your teeth if you want to." This is the only statement that the tester
shall make about using
teeth. The test shall continue for another five minutes or until both children
have opened their
packages, whichever comes first.
Example 1
Example 1 shows the results from the Child-Resistant Screening Test. The
procedures for
the Child-Resistant Screening Test were followed as described above. The trial
used Swiss Miss
K-Cups (purchased at Keurig.com, on October 25th, 2013, lot number 6696
PL120, expiration
date January 17, 2015). Table 1, below, shows how many cartridges were opened
and how long it
took to open each one. Table 2, describes how each of the cartridges that were
opened was
accessed.
Table 1
First 5 min test period
Second 5 min test period
Time to open cartridge (min) Time to open
cartridge (min)
Age 1
2 3 4 5 6 1 2 3 4 5
6
Gender (mo)
1 Male 42
2 Female 43
3 Female 43
4 Male 45
5 Female 45
6 Male 46
7* Female 45 1:42 2:54 3:22 3:42 4:20 4:31 6:10
8 Female 49 4:30 6:29 6:43 7:04 7:14 7:24
9 Male 51
6:00 6:25 6:31 6:50 6:58 7:05
10 Female 49
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
17
11 Male 51
12* Male 50 5:58 6:57 7:38 9:12
13 Male 46
14 Female 42
15 Female 43
16 Female 43
17 Male 48 5:32 6:01 6:31 6:39 6:59
7:21
18 Male 50 5:21 5:46 6:01 6:13 6:32
7:16
19 Female 48 7:49 8:06 8:32 8:57 9:59
10:12
20 Male 43 7:00 7:36 8:07 8:32 9:19
10:00
21 Male 50
*Child was given a cartridge that had a reinforced top for second five minute
test period.
The top was reinforced with packing tape that was reinforced with fiber. The
tape was adhered
over the lidding of the Swiss Miss K-Cup and a hotplate was used to further
bond the tape to
the lidding.
Table 2
Description of how cartridges were Description of how
cartridges were
Age opened during the first 5 min test opened during
the second 5 min test
Gender (mo) period period
Child was given a cartridge with
reinforced tape for the second five
7* Female 45 Child used teeth.
minutes. Child peeled re-enforced tape
and then used teeth.
Child used teeth to puncture foil top and
8 Female 49 Child used teeth.
then used fingers to tear the foil.
Child punctured with finger to open the
9 Male 51 n/a first cartridge and then
used his teeth to
open the five others.
Child was given a cartridge with
reinforced tape for the second five
12* Male 50 n/a
minutes. Child peeled the the reinforced
tape and then used teeth.
Child used teeth to puncture foil top and
17 Male 48 n/a
then used fingers to tear the foil.
Child used teeth to puncture foil top and
18 Male 50 n/a
then used fingers to tear the foil.
19 Female 48 n/a Child punctured foil top
with thumb.
Child used teeth to puncture foil top and
20 Male 43 n/a
then used fingers to tear the foil.
The Child-Resistance Screening Test with the Swiss Miss K-Cups had a 65% pass
rate.
The container passed if the child opened two or fewer cartridges. This is not
an acceptable level
of child-resistance for beverage products that contain pharmaceutical
products, especially
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
18
products containing acetaminophen or diphenhydramine. Almost all of the
children who accessed
the contents, used their teeth to puncture the lidding. Thus, in order to make
a child resistant
cartridge it can be important to have a lidding that cannot be punctured or
torn by biting. One
child opened the package by applying steady pressure with his thumb to
puncture the foil lidding.
Thus, the lidding can also be made child resistant if it is not susceptible to
being opened by
pressing.
It is also interesting to note that after the children saw the demonstration
and were told
they could use their teeth, many more children gained access to the contents
of the cartridge.
Also, if a child was able to open one cartridge, he or she was generally able
to open the
remaining cartridges very quickly. Therefore, in order to make the cartridge
child-resistant, it
may be necessary to make the cartridge tear resistant.
Although the cartridges which were reinforced with packing tape were not child
resistant,
it did take the children longer to access the contents of these cartridges.
Thus, if the heat seal was
stronger, the child-resistant properties of the package may be improved.
Although the two children who received cartridges with reinforced tops were
able to
access the contents, they were not able to access the contents by biting or
otherwise puncturing
the reinforced tape. Instead, they were able to separate the reinforced tape
from the K-Cup
lidding by pealing. Therefore, if the packing tape was more securely adhered
to the lidding, for
instance in a laminate structure, a sufficient level of child-resistance could
be achieved.
Example 2
Example 2 shows the results from the second Child-Resistant Screening Test
utilizing
laminate lidding structure believed to be superior to those tested in Example
1. The procedures
for the Child-Resistant Screening Test were followed as described above. This
example used the
same base as Example 1; however the lidding had a different laminate
structure. The laminate
had an outer layer, a second layer, and an inner layer. The outer layer was 12
lam PET, the
second layer was 9 lam foil, and the inner layer was 25 lam LLDPE. The outer
layer was
connected to the second layer with adhesive and the second layer was connected
to the inner
layer with adhesive. The cartridges were filled with Ovaltine (batch number
#401158801G).
Table 3, below, shows how many cartridges were opened and how long it took to
open each one.
Table 4, describes the method used for each child who opened three or more
cartridges during the
Child-Resistant Screening Test. Age groups are as follows: A=42-44 months,
B=45-48 months,
and C=49-51 months old.
CA 02929863 2016-05-05
WO 2015/081145 PCT/US2014/067518
19
Table 3
First 5 min test period Second 5 min test
period
A Time to open cartridge (min)
Time to open cartridge (min)
ge
1 2 3 4 5 6 1 2 3 4 5 6
Gender (group)
1 Female A
2 Male B
3 Male A
4 Male B 4:40
Female B
6 Female B
7 Female C 6:00 8:02
8 Male C
9 Female B 6:01 7:12 7:16
Female C 7:16 7:45 8:22
11 Male C 1:40 1:54 2:31
12** Male C 7:34 8:01 9:05
13 Female C
14 Male C
Female C
16 Female C
17 Female B
18 Female A
19 Male A
Female A 7:20 8:43 9:21
**Child found a way to access the product using an artifact of the method used
to make
the prototype samples for testing. It is believed, that normal manufacturing
would not allow this
5 access method to be as successful.
CA 02929863 2016-05-05
WO 2015/081145 PCT/US2014/067518
Table 4
Age
Description of how cartridges were opened
Gender (group)
9 Female B Peeled lid, poked with thumbs/finger, struck
against floor
10 Female C Peeled lid, struck against floor
11 Male C Poked hard with thumb/knuckle
Male Peeled lid, crushed until plastic formed small hole, banged on
12** C package, poking at thin spot in plastic (artifact
of
prototyping)
20 Female A Peeled lid, used teeth after demo
This Child-Resistance Screening Test with the laminate lidding of Example 2
had a 75%
pass rate counting child #12 and a 80% pass rate if he was excluded. The
container passed if the
5 child opened three or fewer cartridges. 75% is not an acceptable level of
child-resistance (80% is
the minimum level of acceptance) for beverage products that contain
pharmaceutical products,
especially products containing acetaminophen or diphenhydramine. Almost all of
the children
who accessed the contents, used their teeth or fingers to puncture the
lidding. Thus, in order to
make a child resistant cartridge it can be important to have a lidding that
cannot be punctured or
10 torn by biting.
Example 3
Example 3 shows the results from the third Child-Resistant Screening Test
utilizing new
packaging component materials believed to be superior to those tested in
Example 1 and
15 Example 2. The procedures for the Child-Resistant Screening Test were
followed as described
above. The trial used the same base as Example 1; however the lidding had a
different laminate
structure. The laminate had an outer layer, a second layer, and an inner
layer. The outer layer was
23 lam PET, the second layer was 18 lam foil, and the inner layer was 25 lam
LLDPE. The outer
layer was connected to the second layer with adhesive and the second layer was
connected to the
20 inner layer with adhesive. The cartridges were filled with Ovaltine (batch
number
#401158801G). Table 5, below, shows how many cartridges were opened and how
long it took to
open each one. Table 6, describes the method used for each child who opened
three or more
cartridges during the Child-Resistant Screening Test. Age groups are as
follows: A=42-44
months, B=45-48 months, and C=49-51 months old.
CA 02929863 2016-05-05
WO 2015/081145 PCT/US2014/067518
21
Table 5
First 5 min test period Second 5 min test
period
A Time to open cartridge (min)
Time to open cartridge (min)
ge
1 2 3 4 5 6 1 2 3 4 5 6
Gender (group)
1 Male B
2 Male B
3 Female A
4 Male A
Male B
6 Female A
7 Female B
8 Male B
9 Male B 6:27 6:53 7:02
10 Female B 9:58
11 Female C
12 Male B
13 Male C
14 Female C 7:58
Male C
16 Female C 2:17 2:40 3:09
17 Female C
18 Male A
19 Male B
Female B
Table 6
Age
Description of how cartridges were opened
Gender (mo)
Peeled lid, used teeth after demo to access all three
9 Male B
containers
16 Female C No detail given except required very little
effort
5 This Child-Resistance Screening Test with the laminate lidding of
Example 3 had a 90%
pass rate. The container passed if the child opened three or fewer cartridges.
90% is an acceptable
level of child-resistance (80% is the minimum level of acceptance) for
beverage products which
CA 02929863 2016-05-05
WO 2015/081145
PCT/US2014/067518
22
contain pharmaceutical products, especially products containing acetaminophen
or
diphenhydramine.
The dimensions and values disclosed herein are not to be understood as being
strictly
limited to the exact numerical values recited. Instead, unless otherwise
specified, each such
dimension is intended to mean both the recited value and a functionally
equivalent range
surrounding that value. For example, a dimension disclosed as "40 mm" is
intended to mean
"about 40 mm."
Every document cited herein, including any cross referenced or related patent
or
application and any patent application or patent to which this application
claims priority or
benefit thereof, is hereby incorporated herein by reference in its entirety
unless expressly
excluded or otherwise limited. The citation of any document is not an
admission that it is prior art
with respect to any invention disclosed or claimed herein or that it alone, or
in any combination
with any other reference or references, teaches, suggests or discloses any
such invention. Further,
to the extent that any meaning or definition of a term in this document
conflicts with any
meaning or definition of the same term in a document incorporated by
reference, the meaning or
definition assigned to that term in this document shall govern.
While particular embodiments of the present invention have been illustrated
and
described, it would be obvious to those skilled in the art that various other
changes and
modifications can be made without departing from the spirit and scope of the
invention. It is
therefore intended to cover in the appended claims all such changes and
modifications that are
within the scope of this invention.