Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02930349 2016-05-11
WO 2015/075317 PCT/F12014/050891
METHOD FOR RECOVERING RARE EARTH METALS FROM WASTE
SULPHATES
Field of the Invention
The present invention relates to recovery of rare earth metals from waste
sulphate
materials. In particular, the invention relates to reductive fractionation of
waste gypsum,
comprising sulphate salts of calcium and other metals to their dispersed
sulphides, in which
form the metal components with high magnetic susceptibility can be recovered
by using
magnetic separation. Sulphate reducing bacteria are preferably used for the
reductive
fractionation.
Description of Related Art
Igneous apatite minerals, industrially utilized for manufacturing phosphate
fertilizers, are a
known secondary source of rare earth metals. The rare earth (RE) content of
apatites varies
between 0,5 to 1 % as oxides. Several pilot processes to recover the valuable
rare earth
metals in connection with the adjacent fertiliser production have been
developed, so far
without economic success (Jorjani et al., 2011; Al-Shawi et al., 2002).
The for long time leading fertilizer manufacturing process includes the
leaching of the ore
with sulphuric acid, which includes formation of phosphogypsum as Ca504=2H20
(dihydrate) as the side product. This process, used for many decades for
example in the
Finnish Siilinjarvi fertiliser plant has already produced ca. 45 million
tonnes of
phosphopgypsum, concurrently gathered in the storage pile in the fertiliser
plant area. In
the former studies performed by Kemira Oy it was concluded that even 80 % of
the rare
earth content of the phosphate raw material will be carried into the waste
gypsum
(Lounamaa et al., 1980). A similar experience is reported from several other
sites in the
world.
The rare earth metals are present in the gypsum as their respective sulphates,
even though
particulates of monazite, non-dissolved in the sulphuric acid process may also
appear.
Typical techniques for the chemical fractionation of rare earths from
phosphogypsum
usually include leaching with dilute sulphuric acid solution, separation of
rare earth
concentrates from leaching sulphuric acid by pre-concentration via
evaporation, liquid-
liquid extraction or precipitation method and anhydrite production from
purified
CA 02930349 2016-05-11
WO 2015/075317 PCT/F12014/050891
2
phosphogypsum by recrystallization of concentrated sulphuric acid solution.
All such
methods have so far rendered complex and uneconomical (Preston et al., 1996;
WO
2011/008137 A3), due to ineffectiveness of the multi-stage procedures and low
initial
concentration of rare earths in the phosphogypsum.
On the other hand, a combined mechanical-magnetic separation method (FI 101787
B) has
been proposed to purify phosphogypsum waste from its heavy metal impurities.
WO
2009/125064 Al discloses a respective method for purification of flue gas
desulfurization
(FGD) gypsum. In such techniques gypsum is subjected to grinding in various
degrees of
fineness, then slurried by water addition and finally led to high gradient
magnetic
separation (HGMS) to collect the magnetized fraction. The main goal of the
method has
been to purify waste gypsum for its possible future use as contaminant free
filler in various
components such as boards in construction industry or as pigment in paper-
making, but the
method has shown potential in using the magnetic separation to recover metals
from waste
gypsum. Yet the recovery of such metals is highly dependent on the consistency
of the
slurry as well as of the fineness of the gypsum stock and in average but 35 %
of the rare
earth metals such as La, Nd, Ce and Y could be recovered. It is obvious that
despite the
rare earth metal sulphates generally have high magnetic susceptibilities, the
crystallisation
of the gypsum encapsulates the REs to a large amount within the Ca-sulphate
granules,
which has a low magnetisation and thus leads to high losses in the separation
stage.
The use of sulphate reducing bacteria (SRB) for removing contaminants such as
heavy
metals from aqueous solutions is disclosed by Kaksonen and Puhakka (2007). The
SRB
can be used for treating ground and surface waters contaminated with acid mine
drainage
(AMD), and for recovering metals from wastewater and process streams. The
biologically
produced H2S precipitates metals as metal sulfides, while biogenic bicarbonate
alkalinity
neutralizes acidic waters. In such method, the aqueous sulphate solution,
provided with
appropriate electron donor is inoculated with micro-organisms, which promote
the
reduction of the sulphate ion to hydrogen sulphide:
8H2 + 2S042- ¨> 0 H2S + HS- + 5H20 + 30H
Instead of hydrogen, organic compounds descending from e.g. fermentation
processes or
waste streams with anaerobic degradation stages and including e.g. organic
acids or
CA 02930349 2016-05-11
WO 2015/075317 PCT/F12014/050891
3
alcohols can be used as electron donors. Thus, for example waste water from
dairy
industries or from farming have been used (Kaksonen and Puhakka, 2007;
Rzeczycka et
al., 2010) for the treatment of phosphogypsum by SRB.
Kaufman et al. (1997) present a combined chemical and biological process for
the
recycling of flue gas desulfurization (FGD) gypsum into calcium carbonate and
elemental
sulphur. In this process, a mixed culture of sulfate-reducing bacteria (SRB)
utilizes
inexpensive carbon sources, such as sewage digestor synthesis gas, to reduce
FGD gypsum
to hydrogen sulfide. In the process concept, the sulphide is further oxidized
to elemental
sulfur via reaction with ferric sulfate, and accumulating calcium ions are
precipitated as
calcium carbonate using carbon dioxide. Employing anaerobically digested
municipal
sewage sludge (AD-MSS) medium as a carbon source, SRBs in serum bottles
demonstrated an FGD gypsum reduction rate of 8 mg/L/h (109 cells) -1. A
chemostat with
continuous addition of both AD-MSS media and gypsum exhibited sulfate
reduction rates
as high as 1,3 kg FGD gypsum/m3=d, with 100 % conversion of sulphate.
The sulphide ions generated by the SRB however further react with the metal
cations in the
solution producing low solubility metal sulphides:
H25 + M2'¨>MS(s) + 2H'
EP 0844981 B1 proposes a biomagnetic separation method for the recovery of
metals from
an influent liquid containing e.g. radioactive heavy metal contaminants from
waste water
of a nuclear plant. The technique involves adding specific adsorbent material
to the
contaminated solution to attach the contaminants by chemical or electrostatic
adsorption.
As the ferromagnetic adsorbent, bacterially generated ferrous sulphide from
the respective
sulphate is preferably used (Watson et al., 1996). The method is targeted to
remove toxic
heavy metals from the influent solution, and proved successful in dropping the
concentrations of e.g. mercury, cadmium, chromium and lead contents of the
solution by
several orders of magnitude.
WO 2013/044376 Al relates to magnetic separation of different rare earth
compounds,
wherein a quantitative fractionation of various rare earth metal compounds is
described in
terms of their magnetic susceptibilities by using a separation channel rigged
with magnets
CA 02930349 2016-05-11
WO 2015/075317 PCT/F12014/050891
4
arranged progressively from weakest to strongest along the length axis and
respective
output channels to fractionate compounds with various susceptibilities and
specific
gravities. This publication shows the feasibility of separating and refining
rare individual
rare earth compounds by HGMS techniques, yet chemical formulation of the rare
earth
compounds as a necessary pre-treatment before magnetic fractionation is not
disclosed.
Thus, it has been estimated that even 60 to 80 % of rare earth metals, which
are used as
ingredients in phosphate production industry, end up to waste gypsum. Another
recent
analysis of the Finnish phosphogypsum gives the contents of La, Ce and Y in
the 390,
1100 and 23 ppm, respectively. It would therefore be beneficial to develop an
economic
process to recover the valuable metal contents from waste gypsum.
Summary of the Invention
The present invention is based on reductive and enriching treatment of
sulphate materials
combined with magnetic separation to recover rare earth metals.
Particularly, the present invention relates to a method for recovering rare
earth metal
enrichment from waste sulphates by first reducing rare earth metal sulphates
to a metal
sulphide precipitate and then separating a highly magnetized fraction of the
metal sulphide
precipitate with a magnetic separator.
In the present method sulphate reduction may be carried out for example by
utilizing
sulphate reducing bacteria, by applying thermal treatment or by using
hydrometallurgical
reduction with H2S.
More precisely, the method according to the present invention is characterized
by what is
stated in the characterizing part of claim 1. In addition, the use of said
method is
characterized in claim 16.
Considerable advantages are obtained with the method of the invention, which
provides a
cost-effective and environmentally friendly technical solution for recovering
valuable rare
earth metals for example from the wastes of phosphate production industry. In
addition the
method can be used for recycling of waste gypsum back to calcium carbonate and
sulphuric acid.
CA 02930349 2016-05-11
WO 2015/075317 PCT/F12014/050891
Next, the invention will be described more closely with references to the
attached drawing
and detailed description.
Brief Description of the Drawings
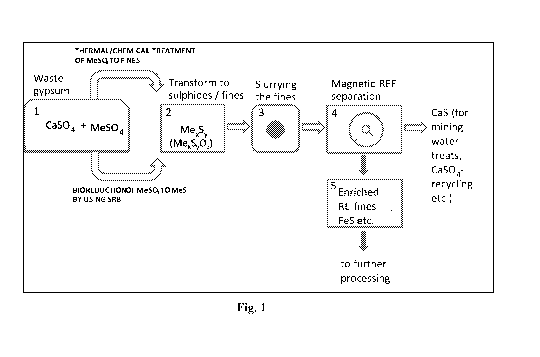
5 Figure 1 is a schematic description of a process according to the present
invention.
Numbers 1-5 are process steps, which are explained in the detailed description
below.
Detailed Description of Embodiments
Herein below the following short terms are used:
"SRB" as in sulphate reducing bacteria
"RE" as in rare earth or rare earth metal
"HGMS" as in high grade/gradient magnetic separation/separator
Characterizing to the method of the present invention is to combine a
reductive treatment
of a waste sulphate material and a following magnetic separation to recover
valuable rare
earth metals. In one embodiment the waste sulphate material is waste gypsum,
for example
waste phosphogypsum.
In the method waste sulphates containing rare earth metal compounds are
reduced in a
liquid phase e.g. by sulphate reducing bacteria (SRB) to form a finely divided
rare earth
metal precipitate, followed by separation of the magnetized fraction of the
precipitate by a
magnetic separator, such as high grade magnetic separator (HGMS). Thus, the
present
invention is based on an enrichment of the rare earth metal content of e.g.
waste gypsum
into a metal sulphide precipitate, and to a higher magnetic susceptibility of
the RE
compounds in the precipitate compared to other substances present in said
precipitate (such
as calcium sulphate/sulphide/phosphate). Preferably, the method relates to a
recovery of a
rare earth metal enrichment, which comprises rare earth metals as their
corresponding
sulphides, oxides or phosphates, or as a combination thereof Said enrichment
may also
comprise small amounts of other compounds than rear earth metals compounds,
for
example K, Fe, Ca, Mg and Al sulphides.
Thus, the magnetized fraction of the metal sulphide precipitate comprises rare
earth metals
and has a higher magnetic susceptibility compared to other substances, such as
calcium
compounds, present in said precipitate. It has been discovered that he
magnetic
CA 02930349 2016-05-11
WO 2015/075317 PCT/F12014/050891
6
susceptibility of e.g. rare earth metal sulphides is often exceptionally high,
whereas that of
calcium sulphide is low. The same applies to corresponding oxides and
sulphates.
In a preferred embodiment the process comprises the following steps (numbers 1
to 5 are
also correspondingly marked into Figure 1):
1. Dissolving a waste gypsum to a dilute sulphuric acid or water,
2. Inoculating SRB with appropriate nutrient solution (and pH),
3. SRB reducing process (for example with rates exceeding 10 kg/m3 of
gypsum),
4. Recovery of the finely dispersed sulphide slurry by precipitation or
filtration,
5. Use of HGMS for the recovery of the highly magnetised fraction of the fine
sulphides.
One suitable sulphate reducing bacteria for use in the present method
originate from genus
Desulfovibrio. As an example bacterium such as Desulfovibrio desulfuricans can
be used.
In addition, SRB belonging to the genera Desulfobulbus and Desulfotomaculum
have
shown to be promising. In order to carry out the reduction mechanism, SRB need
some
organic nutrients for their metabolism. Thereby SRB may use carbon sources,
such as
sewage digests, alcohol or synthesis gas, as microbial nutrients, and, also as
electron
donors. This biological reduction, i.e. bioreduction is preferably carried out
in anaerobic
reaction conditions and at temperatures between 20 C and 50 C, more
preferably
between 30 and 40 C and particularly about 37 C.
However, it is also possible to replace the steps 1 to 3 in the above process
example by a
thermal treatment of the gypsum by using e.g. syngas produced by gasification
of biomass
or by using hydrometallurgical reduction with hydrogen sulphide H25. In one
possible
aspect the sulphate reduction is thus performed with calcium sulphide received
from
thermal roasting or sulphidisation of waste gypsum.
According to one embodiment, the waste sulphate material is reduced to a
finely divided
precipitate having a maximum particle size of below 0,50 gm, such as between
0,10 and
0,50 gm. The precipitate is typically formed as an ultimately fine sludge,
with low or
negligible degree of co-precipitated granules. In addition, the sulphides have
higher
magnetic susceptibility than the corresponding sulphates. Thus, the enriched
sludge of such
CA 02930349 2016-05-11
WO 2015/075317 PCT/F12014/050891
7
rare earth metal sulphides, which have potentially high magnetic
susceptibility, can be
subjected to an effective fractionation process by applying high magnetic
fields.
According to another embodiment of the present invention, the metal
precipitate obtained
by the bioreduction or such reductive treatment of waste gypsum, consists of
elements,
which are selected from the group of La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy,
Ho, Er, Tm,
Yb, Lu, Ga, Ge, Ho, Nb, Sc, Ta, Th, U, Y, In, Al, Ca, Fe, K, Mg, Mn, Na, P and
S, as their
corresponding sulphides, phosphates or oxides, or as any combination thereof.
In a further embodiment, the formed non-rear earth metal comprising substances
(i.e. non-
magnetic e.g. calcium sulphate fraction from step 5) can be used for the
treatment of acid
mine waters to precipitate heavy metal sulphates or it may be recycled in a
thermal process
to recycle sulphur as sulphuric acid and calcium as quicklime.
As mentioned earlier, the sulphate reduction may also be performed by a
chemical reaction
or reactions in aqueous sulphate slurry by using hydrogen sulphide. Sulphate
waste
material can also be treated thermally to produce metal precipitates. Thereby,
according to
one embodiment of the invention, it is possible to combine the chemical
reaction and the
thermal treatment or carry out each presented reduction scheme solely.
HGMS equipment is preferred for an efficient separation of the finely
dispersed magnetic
REs. The equipment itself is usually rather simple and provides an easy
flushing of
magnetics. In addition the maintenance cost is low as well as the power
consumption.
Herein it is preferred to choose HGMS equipment capable of recovering rare
earth metals
having a magnetic susceptibility x of at least 1 000, more preferably at least
5 000.
However, also lower (below 1 000) and higher (up to even 150 000)
susceptibilities may
exist after sulphate reduction, so the separator should preferably be
adjustable or able to
perform within a wide magnetic susceptibility scale.
Thus, the present invention wherein sulphate reduction is combined with
magnetic
separation provides an environmentally friendly and an efficient method for
recovering
valuable rare earth metals from waste sulphate materials. The said method is
targeted to
metal companies and is usable around the world, especially in areas where
industrial
phosphate production takes place.
CA 02930349 2016-05-11
WO 2015/075317 PCT/F12014/050891
8
Herein below the present invention is illustrated by a non-limiting working
example. It
should be understood, however, that the embodiments given in the description
above and
in the example are for illustrative purposes only, and that various changes
and
modifications are possible within the scope of the claims.
Example 1
Phosphogypsum samples were first dried in oven (105 C, 20 h). Gypsum leachate
was
then prepared by adding dry phosphogypsum powder to water (50 g/L), followed
by 24 h
mixing in Erlenmeyer glass. Obtained solution was filtered (0,45 gm) to remove
solid
phosphogypsum particles. Clear solution was used for sulphate reducing
bacteria (SRB)
studies. The phosphogypsum filtrate was rendered anaerobic by flushing with N2
gas
through a 0,22 gm pore size filter for 1 hour, after which the flask
containing the gypsum
leachate was sealed with a gas tight butyl rubber stopper and open top screw
cap. The
phosphogypsum leachate was amended with 0,2 g yeast extract and 3,75 ml
lactate L-1.
Pre-grown Desulfovibrio desulfuricans bacteria were added to the 2,5 L volume
of
phosphogypsum leachate.
The culture developed a precipitation, which was collected on a 0,22 gm pore
size filter
funnel by vacuum suction. The precipitate was rinsed from the filter with
sterile double
distilled water, collected in 50 ml cone tubes and dried prior to analysis.
The formed
precipitate was analysed by using standard ICM-MS and ICP-OES methods. The
contents
of La, Ce and Y in the SRB precipitate were observed as 30 400, 66 200 and 8
800 ppm
(mg/kg), respectively. The Nd-content of the SRB precipitate was 45 000 ppm.
The result
indicates substantial enrichment of the said metals and also of other rare
earth metals in the
formed SRB precipitate.
The highly magnetized fraction of the precipitate was then recovered with high
grade
magnetic separation (HGMS), providing an enrichment, wherein the content of
above
mentioned rare earth metals was high, as disclosed in Table 1:
CA 02930349 2016-05-11
WO 2015/075317 PCT/F12014/050891
9
Table 1. Rare earth metal content of the enrichment after recovery.
Component ppm w-%
La 137290 13,7
Ce 263940 26,4
Y 25600 2,6
Nd 396610 39,7(*)
(*) from separate experiment (FI 101787 B)
Example 2
The experiment as described in Example 1 was identically repeated to test the
reproducibility of the procedure. The contents of La, Ce and Y in the test 2
SRB precipitate
were observed as 33 900, 77 300 and 5 200 ppm (mg/kg), respectively. The Nd-
content of
the SRB precipitate was 38 900 ppm.
By using the similar magnetic separation as in test 1, the final enrichment is
as disclosed in
Table 2:
Table 2. Rare earth metal content of the enrichment after recovery
Component ppm w-%
La 153097 15,3
Ce 308196 30,8
Y 15127 1,5
Nd 342848 34,3(*)
(*) from separate experiment (FI 101787 B)
Example 3
Phosphogypsum samples of the same origin as used in the aforementioned patent
FI
101787 B were dried in oven (105 C, 20 h). Gypsum leachate was prepared by
adding dry
phosphogypsum powder to water (50 g/L), followed by 24 h mixing in Erlenmeyer
glass.
Obtained solution was filtered (0,45 gm) to remove solid phosphogypsum
particles. Clear
solution was used for sulphate reducing bacteria (SRB) studies.
CA 02930349 2016-05-11
WO 2015/075317 PCT/F12014/050891
The continuously operated sulphate reduction and REE precipitation experiment
was done
in 0,7-liter UASB (upflow anaerobic sludge blanket) column, equipped also with
solution
recycling line with a powerful pump to adjust the sludge fluidization and, if
needed, to mix
and homogenize the sludge in column. The column was inoculated with 500 ml of
5 -- anaerobic granular sludge from an operating waste water treatment plant,
and filled up to a
total volume of 700 ml with sulphate rich water. Microbial activity was
ensured by
continuing the sulphate rich water, ethanol and substrates pumping. When
sulphate
reduction was performing reliably, sludge inside the column was agitated with
recycle line
pumping (300 ml/h for 1 minute) and homogenized sludge sample was taken from
the
10 -- column for elemental analysis, corresponding an initial situation of the
sludge. Then
phosphogypsum filtrate was pumped to the column.
The phosphogypsum filtrate used in the experiment was rendered anaerobic by
flushing
with N2 gas for 1 hour and pumped then to 0,7-liter column with the speed of
27 ml/h for
-- 20 days. Simultaneously, substrate-nutrition solution was pumped to the
column with the
speed of 1,75 ml/h for providing following concentrations to the total feed:
ethanol (0,16
v-%), KH2PO4 (13,8 mg/1), (NH4)2SO4 (33,7 mg/1), ascorbic acid (2,7 mg/1),
thioglycolic
acid (2,7 mg/1) and yeast extract (2,7 mg/1). With these parameters, the
hydraulic retention
time (HRT) was maintained at 24 hours. During 20 days of running, the pH, ORP
and
-- sulphate reduction rates were observed. The pH remained in the area of 5,5
¨ 5,8, while
ORP remained in values less than -200 mV (Ag/AgC1/ 3M KC1 electrode). Sulphate
reduction rates were fluctuating from 38 to 80 %. After the 20 day experiment,
sludge in
column was again agitated with recycle line pumping (300 ml/h for 1 minute)
and
homogenized sludge sample was taken from the column for elemental analysis.
While the inoculated waste suspension dilutes the observed REE contents, the
experiment
yet indicated significant enrichment of those into the sludge. The following
enrichment
factors were found for La, Ce, Y and Nd during the treatment:
La initial 7,3 mg/kg; final 202,0 mg/kg (enrichment ratio of 28);
-- Ce initial 13 mg/kg; final 477 mg/kg (enrichment ratio of 37);
Y initial 3,6 mg/kg, final 48,8 mg/kg (enrichment ratio of 14) and
Nd initial 7,2 mg/kg, final 295 mg/kg (enrichment ratio of 41).
Elemental contribution studies of phosphogypsum filtrate, fed to the column,
revealed that
precipitation rate was 100 % for La, Ce, Y and Nd.
CA 02930349 2016-05-11
WO 2015/075317 PCT/F12014/050891
11
While the above description and example show and describe and point out
fundamental
novel features of the invention as applied to a preferred embodiment thereof,
it will be
understood that various omissions and substitutions and changes in the details
of the
method may be made by those skilled in the art without departing from the
spirit of the
invention. For example, it is expressly intended that all combinations of
those elements
and/or method steps which perform substantially the same operations or give
substantially
the same results as those achieved above are within the scope of the
invention.
Substitutions of the elements from one described embodiment to another are
also fully
intended and contemplated. It is the intention, therefore, to be limited only
as indicated by
the scope of the claims appended hereto.
Citation list - patent literature
1. WO 2011/008137 A3
2. FI 101787 B
3. WO 2009/125064 Al
4. EP 0844981 B1
5. WO 2013/044376 Al
Citation list - non-patent literature
Al-Shawi, A. W., Engdal, S. E., Jenssen, O. B., Jorgenssen, T. R., Rosaeg, M.,
The
integrated recovery of rare earths from apatite in the Odda process of
fertilizer production
by solvent extraction. A plant experience, Proc. Int. Solvent Extraction
Conf., ISEC 2002,
Johannesburg.
Jorjani, E., Bagherieh, A. H., Chelgani, S. C., Rare earth elements leaching
from
Chadormalu apatite concentrate: Laboratory studies and regression predictions,
Korean
Journal of Chemical Engineering, Vol. 28, pp. 557-562, 2011.
Kaksonen, A. H., Puhakka, J. A., Sulfate Reduction Based Bioprocesses for the
Treatment
of Acid Mine Drainage and the Recovery of Metals, Engineering in Life
Sciences, Vol. 7,
pp. 541-564, 2007.
CA 02930349 2016-05-11
WO 2015/075317 PCT/F12014/050891
12
Kaufman, E. N., Little, M. H., Selvaraj, P., A biological process for the
reclamation of flue
gas desulfurization gypsum using mixed sulfate-reducing bacteria with
inexpensive carbon
sources, Applied Biochemistry and Biotechnology, Vol. 63-65, pp. 677-693,
1997.
Lounamaa, N., Mattila, T., Judin, V. P., Sund, H. E., Recovery of rare earths
from
phosphorus rock by solvent extraction, Proc. 2nd. Int. Congr. Phosphorus
Compounds,
Institut Mondial du Phosphate, Paris, 1980, pp. 759-768.
Preston, J. S., Cole, P. M., Craig, W. M., Feather, A. M., The recovery of
rare earth oxides
from a phosphoric acid by-product. Part 1. Leaching of rare earth values and
recovery of
a mixed rare earth oxide by solvent extraction, Hydrometallurgy, Vol. 41, pp.
1-19, 1996.
Rzeczycka, M., Miernik, A., Markiewicz, Z., Simultaneous Degradation of Waste
Phosphogypsum and Liquid Manure from Industrial Pig Farm by a Mixed Community
of
Sulfate-Reducing Bacteria, Polish Journal of Microbiology, Vol. 59, pp. 241-
247, 2010.
Watson, J. H. P., Ellwood, D. C., Duggleby, C. J., A chemostat with magnetic
feedback for
the growth of sulphate reducing bacteria and its application to the removal
and recovery
of heavy metals from solution, Minerals Engineering, Vol. 9, pp. 973-983,
1996.