Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
POLYSILOCARB MATERIALS, METHODS AND USES
[0001] This application: (i) claims under 35 U.S.C. 119(e)(1) the
benefit of the filing date of February 28, 2014 of US provisional application
serial
number 61/946,598; (ii) claims under 35 U.S.C. 119(e)(1) the benefit of the
filing
date of January 21, 2015 of US provisional application serial number
62/106,094;
(iii) is a continuation-in-part of US patent application serial number
14/268,150
filed May 2, 2014; and (iv) is a continuation-in-part of US patent application
serial
number 14/212,896 filed March 14, 2014, the entire disclosures of each of
which
are incorporated herein by reference.
BACKGROUND OF THE INVENTION
Field of the Invention
[0002] The present inventions relate to polyorganic compositions,
structures and materials; polymer derived preceramic and ceramic materials;
and
in particular polysilocarb compositions, structures and materials. The present
inventions further relate to methods for making these compositions, structures
and materials. Polymeric derived ceramic materials are disclosed and taught in
US Patent Applications Serial Number 61/818,981, 61/818,906, 61/788,632,
61/843,014, and 61/890808, the entire disclosures of each of which are
incorporated herein by reference.
[0003] Materials made of, or derived from, carbosilane or
polycarbosilane (Si-C), silane or polysilane (Si-Si), silazane or polysilazane
(Si-
N-Si), silicon carbide (SiC), carbosilazane or polycarbosilazane (Si-N-Si-C-
Si),
siloxane or polysiloxanes (Si-0) are known. These general types of materials
have great, but unrealized promise; and have failed to find large-scale
applications or market acceptance. Instead, their use has been relegated to
very
narrow, limited, low volume, high priced and highly specific applications,
such as
a ceramic component in a rocket nozzle, or a patch for the space shuttle.
Thus,
they have failed to obtain wide spread use ceramics, and it is believed they
have
obtained even less acceptance and use, if any, as a plastic material, e.g.,
cured
but not pyrolized.
1
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[0004] To a greater or lesser extent all of these materials and the
process used to make them suffer from one or more failings, including for
example: they are exceptionally expensive and difficult to make, having costs
in
the thousands and tens-of-thousands of dollars per pound; they require high
and
very high purity starting materials; the process requires hazardous organic
solvents such as toluene, tetrahydrofuran (THF), and hexane; the materials are
incapable of making non-reinforced structures having any usable strength; the
process produces undesirable and hazardous byproducts, such as hydrochloric
acid and sludge, which may contain magnesium; the process requires multiple
solvent and reagent based reaction steps coupled with curing and pyrolizing
steps; the materials are incapable of forming a useful prepreg; and their
overall
physical properties are mixed, e.g., good temperature properties but highly
brittle.
[0005] As a result, although believed to have great promise, these
types of materials have failed to find large-scale applications or market
acceptance and have remained essentially scientific curiosities.
SUMMARY
[0006] Accordingly, there has been a long-standing and unfulfilled
need
for new materials that have the performance characteristic and features of
high
priced ceramics but with lower costs and greater flexibility in manufacturing
and
using the material. The present inventions, among other things, solve these
needs by providing the articles of manufacture, devices and processes taught,
disclosed and claimed herein.
[0007] There is provided a polysilocarb derived reinforced composite
grinding or cutting member; having: a bulk phase and a cutting material;
wherein
the bulk phase is derived from a polysilocarb formulation.
[0008] There is provided the materials, methods, articles that have
one
or more of the following features: wherein the polysilocarb formulation is a
reaction type formulation; wherein the polysilocarb formulation is a reaction
type
formulation, wherein the formulation has at least one precursor selected from
the
group consisting of Phenyltriethoxysilane, Phenylmethyldiethoxysilane,
2
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
Methyldiethoxysilane, Vinylmethyldiethoxysilane, Trimethyethoxysilane
Triethoxysilane, and TES 40; wherein the polysilocarb formulation is a
reaction
type formulation, whereby the formulation has at least two precursors selected
from the group consisting of Phenyltriethoxysilane,
Phenylmethyldiethoxysilane,
Methyldiethoxysilane, Vinylmethyldiethoxysilane, Trimethyethoxysilane
Triethoxysilane, and TES 40; wherein the cutting material is selected from the
group consisting of polycrystalline diamond compact, SiC, Aluminum oxide and
diamond; wherein the cutting material is evening distributed through the
entirety
of the member; wherein the cutting material is evening distributed through a
majority of a outer volume of the member, wherein the outer volume defines at
least about 50% of the total volume of the member; wherein the polysilocarb
formulation is a mixing type formulation; wherein the polysilocarb formulation
is a
mixing type formulation, wherein the formulation has at least one precursor
selected from the group consisting of methyl terminated vinyl polysiloxane,
vinyl
terminated vinyl polysiloxane, hydride terminated vinyl polysiloxane, vinyl
terminated dimethyl polysiloxane, hydroxy terminated dimethyl polysiloxane,
phenyl terminated dimethyl polysiloxane, methyl terminated phenylethyl
polysiloxane, and tetravinyl cyclosiloxane; wherein the polysilocarb
formulation is
substantially solvent free.
[0009] Yet
further there is provided a structural building member having
a cured polysilocarb formulation.
[0010] There
is provided the materials, methods, articles that have one
or more of the following features: having a structural component and a cured
polysiloxane formulation; wherein the structural component is selected from
the
group consisting of dry wall, sheet rock, gypsum board, MDF board, plywood,
plastics and particleboard; having a second component selected from the group
consisting of paints, glues and plastics; and wherein the second component has
a cured polysiloxane formulation; wherein the cured polysiloxane formulation
is a
volumetric shape; wherein the polysilocarb formulation is a reaction type
formulation;, wherein the polysilocarb formulation is a mixing type
formulation;
wherein the polysilocarb formulation is a mixing type formulation, wherein the
3
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
formulation has at least one precursor selected from the group consisting of
methyl terminated vinyl polysiloxane, vinyl terminated vinyl polysiloxane,
hydride
terminated vinyl polysiloxane, vinyl terminated dimethyl polysiloxane, hydroxy
terminated dimethyl polysiloxane, phenyl terminated dimethyl polysiloxane,
methyl terminated phenylethyl polysiloxane, and tetravinyl cyclosiloxane;
wherein
the polysilocarb formulation is substantially solvent free.
[0011] Yet further there is provided a structural building member
having
a pyrolized polysilocarb ceramic, the ceramic having from about 30 weight
(:)/0 to
about 60 weight (:)/0 silicon, from about 5 weight (:)/0 to about 40 weight
(:)/0 oxygen,
and from about 3 weight (:)/0 to about 35 weight (:)/0 carbon, and wherein 20
weight
(:)/0 to 80 weight (:)/0 of the carbon is silicon-bound-carbon and 80 weight
(:)/0 to about
20 weight (:)/0 of the carbon is free carbon.
[0012] There is provided the materials, methods, articles that have
one
or more of the following features: wherein the ceramic material has about 40
weight (:)/0 to about 50 weight (:)/0 silicon, and wherein about 25 weight
(:)/0 to about
40 weight (:)/0 of the carbon is silicon-bound-carbon; wherein the ceramic
material
has about 40 weight (:)/0 to about 50 weight (:)/0 silicon, and wherein about
55
weight (:)/0 to about 75 weight (:)/0 of the carbon is free carbon; wherein
the ceramic
material has about 20 weight (:)/0 to about 30 weight (:)/0 oxygen, and
wherein about
25 weight (:)/0 to about 40 weight (:)/0 of the carbon is silicon-bound-
carbon; wherein
the ceramic material has about about 20 weight (:)/0 to about 30 weight (:)/0
oxygen,
and wherein about 55 weight (:)/0 to about 75 weight (:)/0 of the carbon is
free
carbon; wherein the ceramic material has about 20 weight (:)/0 to about 30
weight
(:)/0 carbon, and wherein about 25 weight (:)/0 to about 40 weight (:)/0 of
the carbon is
silicon-bound-carbon; wherein the ceramic material has about about 20 weight
(:)/0
to about 30 weight (:)/0 carbon, and wherein about 55 weight (:)/0 to about 75
weight
(:)/0 of the carbon is free carbon.
[0013] Still further there is provided a method of providing flame
protection to a structural assembly, the method having selecting a structural
assembly for treating with a polysiloxane formulation; applying the
polysiloxane
4
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
formulation to the structural assembly; the polysiloxane formulation capable
of
reacting to a hard cure in less than two days at 75 degrees F; curing the
polysiloxane formulation; whereby the structural assembly with the hard cured
polysiloxane application has a standard fire test of at least about 1/2-h
under
ASTM E-119.
[0014] There is provided the materials, methods, articles that have
one
or more of the following features: whereby the structural assembly with the
hard
cured polysiloxane application has a standard fire test of at least about 2-h
under
ASTM E-119; whereby the structural assembly with the hard cured polysiloxane
application has a standard fire test of at least about 4-h under ASTM E-119;
wherein the structural assembly is selected from the group consisting of
bearing
walls, masonry units, composite assemblies of structural materials for
buildings,
non-bearing walls, partitions, columns, girders, beams, slabs, and composite
slab
and beam assemblies for floors and roofs.
[0015] Moreover, there is provided a flame resistant structural
assembly, having: a structural assembly and a hard cured polysiloxane
formulation; the polysiloxane formulation being at least substantially free
from
halogens; wherein the flame resistant structural assembly exceeds a standard
fire test of at least about 1/2-h under ASTM E-119.
[0016] Furthermore there is provided a flame resistant outer wrap for
structural assembly, having: a flame resistant outer wrap for structural
assembly
and a hard cured polysiloxane formulation impregnated into the outer wrap; the
polysiloxane formulation being at least substantially free from halogens.
[0017] Still further there is provided a fire resistant plastic having
a first
plastic and a halogen free fire retardant, the fire retardant having a hard
cured
polysiloxane formulation; the plastic capable of meeting at least VO rating
under
UL-94.
[0018] Yet further there is provided an end product having a
polysilocarb formulation, the end product selected from the group consisting
of
fibers, proppants, silane coated proppants, silane and antistatic coated
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
proppants, blast shield, a ballistic composite, structural member, trailer,
mobile
building, shipping container, friction member, grinding device, armored
vehicle,
body armor, insulation, paint, fire resistant coatings, counter tops, exhaust
systems, tubular structures, wiring insulation, pipe insulation, pipe linings,
concrete, and vapor barrier.
[0019] Additionally there is provided end products wherein the
polysilocarb formulation has a pyrolized ceramic, the ceramic having from
about
30 weight % to about 60 weight % silicon, from about 5 weight % to about 40
weight % oxygen, and from about 3 weight % to about 35 weight % carbon, and
wherein 20 weight % to 80 weight % of the carbon is silicon-bound-carbon and
80 weight % to about 20 weight % of the carbon is free carbon.
[0020] Yet further there is provided a method of making an article,
wherein in the article is selected from the group consisting of fibers,
proppants,
silane coated proppants, silane and antistatic coated proppants, blast shield,
a
ballistic composite, structural member, trailer, mobile building, shipping
container,
friction member, grinding device, armored vehicle, body armor, insulation,
paint,
fire resistant coatings, counter tops, exhaust systems, tubular structures,
wiring
insulation, pipe insulation, pipe linings, concrete, and vapor barrier; the
method
having selecting a polysilocarb formulation, making the polysilocarb
formulation,
making the article by processing the polysilocarb formulation as at least from
0.05% to 100% of the article.
[0021] Still additionally, there is provided an article product having
a
pyrolized polysilocarb ceramic, the ceramic having from about 30 weight % to
about 60 weight % silicon, from about 5 weight % to about 40 weight % oxygen,
and from about 3 weight % to about 35 weight % carbon, and wherein 20 weight
% to 80 weight % of the carbon is silicon-bound-carbon and 80 weight % to
about
20 weight % of the carbon is free carbon.
6
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
BRIEF DESCRIPTION OF THE DRAWINGS
[0022] FIG. 1A is a perspective view of an embodiment of polysilocarb
structural members in accordance with the present inventions.
[0023] FIG. 1B is a perspective view of an embodiment of polysilocarb
structural members in accordance with the present inventions.
[0024] FIG. 1C is a perspective view of an embodiment of polysilocarb
structural members in accordance with the present inventions.
[0025] FIG. 1D is a perspective view of an embodiment of polysilocarb
structural members in accordance with the present inventions.
[0026] FIG. 2 is an exploded perspective view of an embodiment of
polysilocarb building support members in accordance with the present
inventions.
[0027] FIG. 3A is a side view of an an embodiment of armored vehicle
having polysilocarb based armor panels in accordance with the present
inventions.
[0028] FIG. 3B is a front view of the vehicle of FIG. 3A.
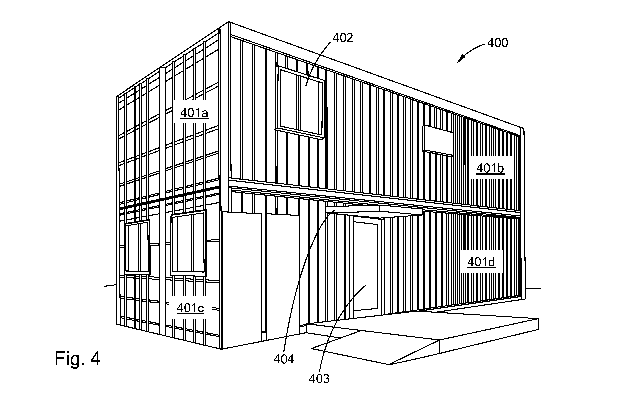
[0029] FIG. 4 is a perspective view of an embodiment of a polysilocarb
modular housing unit in accordance with the present inventions.
[0030] FIG. 5 is a perspective view of an embodiment of a polysilocarb
shipping container in accordance with the present inventions.
[0031] FIG. 6 is perspective view of an embodiment of a polysilocarb
based panel in accordance with the present inventions.
[0032] FIG. 7 is a perspective view of an embodiment of a polysilocarb
body arm in accordance with the present inventions.
[0033] FIG. 8 is a perspective view of an embodiment of a polysilocarb
rope in accordance with the present inventions.
[0034] FIG. 9 is a perspective view of an embodiment of a polysilocarb
trailer in accordance with the present inventions.
[0035] FIG. 10 is perspective view of an embodiment of a polysilocarb
grinding wheel in accordance with the present inventions.
7
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[0036] FIG. 11 is a perspective view of a belt grinding machine, with
embodiment of a polysilocarb abrasive belt in accordance with the present
inventions.
[0037] FIG. 12 is a perspective view of a polysilocarb tubular in
accordance with the present inventions.
[0038] FIG. 13 is a cross sectional view of a polysilocarb break
assembly in accordance with the present inventions.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0039] In general, the present inventions relate to unique and novel
silicon (Si) based materials that are easy to manufacture, handle and have
surprising and unexpected properties and applications. These silicon based
materials have applications and utilizations as a liquid material, a cured
material,
e.g., a plastic, a preceramic, and a pyrolized material, e.g., a ceramic.
[0040] The silicon based materials of the present inventions go
against
the general trends of the art of silicon chemistry and uses. Generally, the
art of
silicon chemistry, and in particular organosilicon chemistry, has moved toward
greater and greater complexity in the functional groups that are appended to,
and
a part of, a silicon based polymeric backbone. Similarly, in general, the
processes that are utilized to make these polymers have moved toward greater
and greater complexity. The present inventions move away from this trend, by
preferably functionalizing a silicon based polymeric backbone with simpler
structures, such as phenyl, phenylethyl and smaller groups, and do so with
processes that are simplified, e.g., solvent free, reduced solvent, lower cost
starting materials, fewer steps, and reduction of reaction intermediates.
[0041] Further, and generally, the art views silicones as tacky, soft
or
liquid materials that are used with, on, or in conjunction with, other
materials to
enhance or provide a performance feature to those other materials. Silicon
based materials generally are not viewed as stand alone products, primary
products, or structural elements. The silicon based materials of the present
inventions, however, move away from this trend and understanding in the art.
8
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
The silicon based materials of the present inventions provide materials that,
among other things, can function as stand alone products, primary products and
structural elements. The silicon based materials of the present invention can
also function as composites, coatings, components, additives, material
performance enhancers, and other applications and utilizations.
[0042] Thus, the present inventions provide a new material systems
and platform having many varied formulations, applications and uses, which
could not generally have been obtained with prior silicon based products, and
in
particular, could not generally have been obtained with prior silicon based
products at acceptable costs, volumes, manufacturing conditions, handling
requirements, or processing conditions among other things.
[0043] Generally, the present inventions are directed toward
"polysilocarb" materials, e.g., material containing silicon (Si), oxygen (0)
and
carbon (C), and materials that have been pyrolized from such materials.
Polysilocarb materials may also contain other elements. Polysilocarb materials
are made from one or more polysilocarb precursor formulation or precursor
formulation. The polysilocarb precursor formulation contains one or more
functionalized silicon polymers, or monomers, as well as, potentially other
ingredients, such as for example, inhibitors, catalysts, pore formers,
fillers,
reinforcers, fibers, particles, colorants, pigments, dies, polymer derived
ceramics
("PDC"), ceramics, metals, metal complexes, and combinations and variations of
these and other materials and additives.
[0044] The polysilocarb precursor formulation is then cured to form a
solid or semi-sold material, e.g., a plastic. The polysilocarb precursor
formulation
may be processed through an initial cure, to provide a partially cured
material,
which may also be referred to, for example, as a preform, green material, or
green cure (not implying anything about the material's color). The green
material
may then be further cured. Thus, one or more curing steps may be used. The
material may be "end cured," i.e., being cured to that point at which the
material
has the necessary physical strength and other properties for its intended
purpose. The amount of curing may be to a final cure (or "hard cure"), i.e.,
that
9
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
point at which all, or essentially all, of the chemical reaction has stopped
(as
measured, for example, by the absence of reactive groups in the material, or
the
leveling off of the decrease in reactive groups over time). Thus, the material
may
be cured to varying degrees, depending upon it's intended use and purpose. For
example, in some situations the end cure and the hard cure may be the same.
[0045] The curing may be done at standard ambient temperature and
pressure ("SATP", 1 atmosphere, 25 C), at temperatures above or below that
temperature, at pressures above or below that pressure, and over varying time
periods (both continuous and cycled, e.g., heating followed by cooling and
reheating), from less than a minute, to minutes, to hours, to days (or
potentially
longer), and in air, in liquid, or in a preselected atmosphere, e.g., Argon
(Ar) or
nitrogen (N2).
[0046] The polysilocarb precursor formulations can be made into non-
reinforced, non-filled, composite, reinforced, and filled structures,
intermediates
and end products, and combinations and variations of these and other types of
materials. Further, these structures, intermediates and end products can be
cured (e.g., green cured, end cured, or hard cured), uncured, pyrolized to a
ceramic, and combinations and variations of these (e.g., a cured material may
be
filled with pyrolized beads derived from the same polysilocarb as the cured
material).
[0047] The precursor formulations may be used to form a "neat"
materials, (by "neat" material it is meant that all, and essentially all of
the
structure is made from the precursor material or unfilled formulation; and
thus,
there are no fillers or reinforcements). They may be used to form composite
materials, e.g., reinforced products. They may be used to form non-reinforced
materials, which are materials that are made of primarily, essentially, and
preferably only from the precursor materials, for example a pigmented
polysiloxane structure having only precursor material and a colorant would be
considered non-reinforced material.
[0048] In making the polysilocarb precursor formulation into a
structure,
part, intermediate, or end product, the polysilocarb formulation can be, for
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
example, sprayed, flowed, thermal sprayed, painted, molded, formed, extruded,
spun, dropped, injected or otherwise manipulated into essentially any
volumetric
shape, including planer shape (which still has a volume, but is more akin to a
coating, skin, film, or even a counter top, where the thickness is
significantly
smaller, if not orders of magnitude smaller, than the other dimensions), and
combinations and variations of these. These volumetric shapes would include,
for example, spheres, pellets, rings, lenses, disks, panels, cones,
frustoconical
shapes, squares, rectangles, trusses, angles, channels, hollow sealed
chambers,
hollow spheres, blocks, sheets, coatings, films, skins, particulates, beams,
rods,
angles, columns, fibers, staple fibers, tubes, cups, pipes, and combinations
and
various of these and other more complex shapes, both engineering and
architectural. Additionally, they may be shaped into preforms, or preliminary
shapes that correspond to, or with, a final product, such as for example use
in or
with, a break pad, a clutch plate, a break shoe, a motor, high temperature
parts
of a motor, a diesel motor, rocket components, turbine components, air plane
components, space vehicle components, building materials, shipping container
components, and other structures or components.
[0049] The polysilocarb precursor formulations may be used with
reinforcing materials to form a composite material. Thus, for example, the
formulation may be flowed into, impregnated into, absorbed by or otherwise
combined with a reinforcing material, such as carbon fibers, glass fiber,
woven
fabric, non-woven fabric, copped fibers, fibers, rope, braided structures,
ceramic
powders, glass powders, carbon powders, graphite powders, ceramic fibers,
metal powders, carbide pellets or components, staple fibers, tow,
nanostructures
of the above, PDCs, any other material that meets the temperature requirements
of the process and end product, and combinations and variations of these.
Thus,
for example, the reinforcing materials may be any of the high temperature
resistant reinforcing materials currently used, or capable of being used with,
existing plastics and ceramic composite materials. Additionally, because the
polysilocarb precursor formulation may be formulated for a lower temperature
cure (e.g., SATP) or a cure temperature of for example about 100 F to about
11
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
400 F, the reinforcing material may be polymers, organic polymers, such as
nylons, polypropylene, and polyethylene, as well as aramid fibers, such as
NOMEX or KEVLAR.
[0050] The reinforcing material may also be made from, or derived
from the same material as the formulation that has been formed into a fiber
and
pyrolized into a ceramic, or it may be made from a different precursor
formulation
material, which has been formed into a fiber and pyrolized into a ceramic. In
addition to ceramic fibers derived from the precursor formulation materials
that
may be used as reinforcing material, other porous, substantially porous, and
non-
porous ceramic structures derived from a precursor formulation material may be
used.
[0051] The polysilocarb precursor formulation may be used to form a
filled material. A filled material would be any material having other solid,
or semi-
solid, materials added to the polysilocarb precursor formulation. The filler
material may be selected to provide certain features to the cured product, the
ceramic product or both. These features may relate to or be aesthetic,
tactile,
thermal, density, radiation, chemical, magnetic, electric, and combinations
and
variations of these and other features. These features may be in addition to
strength. Thus, the filler material may not affect the strength of the cured
or
ceramic material, it may add strength, or could even reduce strength in some
situations. The filler material could impart color, magnetic capabilities,
fire
resistances, flame retardance, heat resistance, electrical conductivity, anti-
static,
optical properties (e.g., reflectivity, refractivity and iridescence),
aesthetic
properties (such as stone like appearance in building products), chemical
resistivity, corrosion resistance, wear resistance, abrasions resistance,
thermal
insulation, UV stability, UV protective, and other features that may be
desirable,
necessary, and both, in the end product or material. Thus, filler materials
could
include copper lead wires, thermal conductive fillers, electrically conductive
fillers, lead, optical fibers, ceramic colorants, pigments, oxides, dyes,
powders,
ceramic fines, PDC particles, pore-formers, carbosilanes, silanes, silazanes,
silicon carbide, carbosilazanes, siloxane, powders, ceramic powders, metals,
12
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
metal complexes, carbon, tow, fibers, staple fibers, boron containing
materials,
milled fibers, glass, glass fiber, fiber glass, and nanostructures (including
nanostructures of the forgoing) to name a few. For example, crushed, PDC
particles, e.g., fines or beads, can be added to a polysilocarb formulation
and
then cured to form a filled cured plastic material, which has significant fire
resistant properties as a coating or structural material.
[0052] As used herein, unless specifically provided otherwise, the
terms flame retardant, fire retardant, flame resistant, fire resistant, flame
protection, fire protection, flame suppression, fire suppression, and similar
such
terms are to be given their broadest possible meanings, and would include all
burning, fire, combustion or flame related meanings that are found, described
or
set forth in standards, codes, certifications, regulations, and guidelines,
and
would include the lessening, reduction, and avoidance of fire, combustion or
smoke.
[0053] The fill material may also be made from, or derived from the
same material as the formulation that has been formed into a cured or
pyrolized
solid, or it may be made from a different precursor formulation material,
which
has been formed into a cured solid or semi-solid, or pyrolized solid.
[0054] The polysilocarb formulation and products derived or made from
that formulation may have metals and metal complexes. Thus, metals as oxides,
carbides or silicides can be introduced into precursor formulations, and thus
into
a silica matrix in a controlled fashion. Thus, using organometallic, metal
halide
(chloride, bromide, iodide), metal alkoxide and metal amide compounds of
transition metals and then copolymerizing in the silica matrix, through
incorporation into a precursor formulation is contemplated.
[0055] For example, Cyclopentadienyl compounds of the transition
metals can be utilized. Cyclopentadienyl compounds of the transition metals
can
be organized into two classes: Bis-cyclopentadienyl complexes; and Mono-
cyclopentadienyl complexes. Cyclopentadienyl complexes can include C5H5,
C5Me5, C5H4Me, CH5R5 (where R = Me, Et, Propyl, i-Propyl, butyl, Isobutyl, Sec-
butyl). In either of these cases Si can be directly bonded to the
Cyclopentadienyl
13
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
ligand or the Si center can be attached to an alkyl chain, which in turn is
attached
to the Cyclopentadienyl ligand.
[0056] Cyclopentadienyl complexes, that can be utilized with precursor
formulations and in products, can include: bis-cyclopentadienyl metal
complexes
of first row transition metals (Titanium, Vanadium, Chromium, Iron, Cobalt,
Nickel); second row transition metals (Zirconium, Molybdenum, Ruthenium,
Rhodium, Palladium); third row transition metals (Hafnium, Tantalum, Tungsten,
Iridium, Osmium, Platinum); Lanthanide series (La, Ce, Pr, Nd, Pm, Sm, Eu, Gd,
Tb, Dy, Ho); Actinide series (Ac, Th, Pa, U, Np).
[0057] Monocyclopentadienyl complexes may also be utilized to
provide metal functionality to precursor formulations and would include
monocyclopentadienyl complexes of: first row transition metals (Titanium,
Vanadium, Chromium, Iron, Cobalt, Nickel); second row transition metals
(Zirconium, Molybdenum, Ruthenium, Rhodium, Palladium); third row transition
metals (Hafnium, Tantalum, Tungsten, Iridium, Osmium, Platinum) when
preferably stabilized with proper ligands, (for instance Chloride or
Carbonyl).
[0058] Alky complexes of metals may also be used to provide metal
functionality to precursor formulations and products. In these alkyl complexes
the Si center has an alkyl group (ethyl, propyl, butyl, vinyl, propenyl,
butenyl)
which can bond to transition metal direct through a sigma bond. Further, this
would be more common with later transition metals such as Pd, Rh, Pt, Ir.
[0059] Coordination complexes of metals may also be used to provide
metal functionality to precursor formulations and products. In these
coordination
complexes the Si center has an unsaturated alkyl group (vinyl, propenyl,
butenyl,
acetylene, butadienyl) which can bond to carbonyl complexes or ene complexes
of Cr, Mo, W, Mn, Re, Fe, Ru, Os, Co, Rh, Ir, Ni. The Si center may also be
attached to a phenyl, substituted phenyl or other aryl compound (pyridine,
pyrimidine) and the phenyl or aryl group can displace carbonyls on the metal
centers.
[0060] Metal alkoxides may also be used to provide metal functionality
to precursor formulations and products. Metal alkoxide compounds can be
14
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
mixed with the Silicon precursor compounds and then treated with water to form
the oxides at the same time as the polymer, copolymerize. This can also be
done with metal halides and metal amides. Preferably, this may be done using
early transition metals along with Aluminum, Gallium and Indium, later
transition
metals: Fe, Mn, Cu, and alkaline earth metals: Ca, Sr, Ba, Mg.
[0061] Compounds where Si is directly bonded to a metal center which
is stabilized by halide or organic groups may also be utilized to provide
metal
functionality to precursor formulations and products.
[0062] Additionally, it should be understood that the metal and metal
complexes may be the continuous phase after pyrolysis, or subsequent heat
treatment. Formulations can be specifically designed to react with selected
metals to in situ form metal carbides, oxides and other metal compounds,
generally known as cermets (e.g., ceramic metallic compounds). The
formulations can be reacted with selected metals to form in situ compounds
such
as mullite, alumino silicate, and others. The amount of metal relative to the
amount of silica in the formulation or end product can be from about 0.1 mole
(:)/0
to 99.9 mole %, about 1 mole (:)/0 or greater, about 10 mole (:)/0 or greater,
about 20
mole percent or greater (:)/0 and greater. The forgoing use of metals with the
present precursor formulas can be used to control and provide predetermined
stoichiometries.
[0063] Filled materials would include reinforced materials. In many
cases, cured, as well as pyrolized polysilocarb filled materials can be viewed
as
composite materials. Generally, under this view, the polysilocarb would
constitute the bulk or matrix phase, (e.g., a continuous, or substantially
continuous phase), and the filler would constitute the dispersed (e.g., non-
continuous), phase.
[0064] It should be noted, however, that by referring to a material as
"filled" or "reinforced" it does not imply that the majority (either by
weight, volume,
or both) of that material is the polysilocarb. Thus, generally, the ratio
(either
weight or volume) of polysilocarb to filler material could be from about
0.1:99.9 to
99.9:0.1. Smaller amounts of filler material or polysilocarb could also be
present
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
or utilized, but would more typically be viewed as an additive or referred to
in
other manners. Thus, the terms composite, filled material, polysilocarb filled
materials, reinforced materials, polysilocarb reinforced materials,
polysilocarb
filled materials, polysilocarb reinforced materials and similar such terms
should
be viewed as non-limiting as to amounts and ratios of the material's
constitutes,
and thus in this context, be given their broadest possible meaning.
[0065] The polysilocarb precursor formulation may be specifically
formulated to cure under conditions (e.g., temperature, and perhaps time) that
match, e.g., are predetermined to match, the properties of the reinforcing
material, filler material or substrate. These materials may also be made from,
or
derived from, the same material as the polysilocarb precursor formulation that
is
used as the matrix, or it may be made from a different polysilocarb precursor
formulation. In addition to ceramic fibers derived from the polysilocarb
precursor
formulation materials, porous, substantially porous, and non-porous ceramic
structures derived from a polysilocarb precursor formulation material may be
used as filler or reinforcing material.
[0066] The polysilocarb precursor formulations may be used to coat or
impregnate a woven or non-woven fabric, made from for example carbon fiber,
glass fibers or fibers made from a polysilocarb precursor formulation (the
same
or different formulation), to from a prepreg material. Further, a polysilocarb
precursor formulation may be used as an interface coating on the reinforcing
material, for use either with a polysilocarb precursor formulation as the
matrix
material. Further, carbon fiber may be heat treated to about 1,400 to about
1,800 or higher, which creates a surface feature that eliminates the need for
a
separate interface coating, for use with polysilocarb precursor formulations.
[0067] Fillers can reduce the amount of shrinkage that occurs during
the processing of the formulation into a ceramic, they can be used to provide
a
predetermined density of the product, either reducing or increasing density,
and
can be used to provide other customized and predetermined product and
processing features. Fillers, at larger amounts, e.g., greater than 10%, can
have
the effect of reducing shrinkage during cure.
16
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[0068] Depending upon the particular application, product or end use,
the filler can be evenly distributed in the precursor formulation, unevenly
distributed, a predetermined rate of settling, and can have different amounts
in
different formulations, which can then be formed into a product having a
predetermined amounts of filler in predetermined areas, e.g., striated layers
having different filler concentration.
[0069] Preferably, for a typical filled product, the filler is
substantially
evenly distributed and more preferably evenly distributed within the end
product.
In this manner localize stresses or weak points can be avoided. Generally, for
a
non-reinforced material each filler particle may have a volume that is less
than
about 0.3%, less than about 0.2%, less than about 0.1`)/0, and less than about
0.05% of the volume of a product, intermediate or part. For example if the
product is spherical in shape and the filler is spherical in shape the
diameter of
the filler should preferable be about 1/10 to about 1/20 of the diameter of
the
proppant particle, and more preferably the filler diameter should be less than
about 1/20 of the diameter of the proppant particle. Generally, the relative
amount of filler used in a material should preferable be about 30% to about
65%
of the volume of the sphere, e.g., volume %.
[0070] Generally, when a small particulate filler, e.g., fines, beads,
pellets, is used for the purposes of increasing strength, without the presence
of
fibers, fabric, etc., generally at least about 2% to at least about 5 volume
%, can
show an increase in the strength, although this may be greater or smaller
depending upon other factors, such as the shape and volume of the product,
later processing conditions, e.g., cure time, temperature, number of pyrolysis
reinfiltrations. Generally, as the filler level increases from about above 5
volume
% no further strength benefits may be realized. Such small particulate filled
products, in which appreciable strength benefits are obtained from the filler,
and
in particular an increase in strength of at least about 5%, at last about 10%
and
preferably at least about 20% would be considered to be reinforced products
and
materials.
17
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[0071] As used herein, unless specified otherwise the terms %, weight
% and mass % are used interchangeably and refer to the weight of a first
component as a percentage of the weight of the total, e.g., formulation,
mixture,
material or product. As used herein, unless specified otherwise "volume `)/0"
and
"`"/0 volume" and similar such terms refer to the volume of a first component
as a
percentage of the volume of the total, e.g., formulation, material or product.
[0072] At various points during the manufacturing process, the
polysilocarb structures, intermediates and end products, and combinations and
variations of these, may be machined, milled, molded, shaped, drilled or
otherwise mechanically processed and shaped.
[0073] Generally, the term "about" is meant to encompass a variance
or range of 10%, the experimental or instrument error associated with
obtaining
the stated value, and preferably the larger of these.
[0074] The precursor formulations are preferably clear or are
essentially colorless and generally transmissive to light in the visible
wavelengths. They may, depending upon the formulation have a turbid, milky or
clouding appearance. They may also have color bodies, pigments or colorants,
as well as color filler (which can survive pyrolysis, for ceramic end
products, such
as those used in ceramic pottery glazes). The precursor may also have a yellow
or amber color or tint, without the need of the addition of a colorant.
[0075] The precursor formulations may be packaged, shipped and
stored for later use in forming products, e.g., structures or parts, or they
may be
used directly in these processes, e.g., continuous process to make a product.
Thus, a precursor formulation may be stored in 55 gallon drums, tank trucks,
rail
tack cars, onsite storage tanks having the capable of holding hundreds of
gals,
and shipping totes holding 1,000 liters, by way of example. Additionally, in
manufacturing process the formulations may be made and used in a continuous,
and semi-continuous processes.
[0076] The present inventions, among other things, provide substantial
flexibility in designing processes, systems, ceramics, having processing
properties and end product performance features to meet predetermined and
18
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
specific performance criteria. Thus, for example the viscosity of the
precursor
formulation may me predetermined by the formulation to match a particular
morphology of the reinforcing material, the cure temperature of the precursor
formulation may be predetermined by the formulation to enable a prepreg to
have
an extended shelf life. The viscosity of the of the precursor formulation may
be
established so that the precursor readily flows into the reinforcing material
of the
prepreg while at the same time being thick enough to prevent the precursor
formulation from draining or running off of the reinforcing material. The
formulation of the precursor formulation may also, for example, be such that
the
strength of a cured preform is sufficient to allow rough or initial machining
of the
preform, prior to pyrolysis.
[0077] Custom and predetermined control of when chemical reactions
occur in the various stages of the process from raw material to final end
product
can provide for reduced costs, increased process control, increased
reliability,
increased efficiency, enhanced product features, and combinations and
variation
of these and other benefits. The sequencing of when chemical reactions take
place can be based primarily upon the processing or making of precursors, and
the processing or making of precursor formulations; and may also be based upon
cure and pyrolysis conditions. Further, the custom and predetermined selection
of these steps, formulations and conditions, can provide enhanced product and
processing features through chemical reactions, molecular arrangements and
rearrangements, and microstructure arrangements and rearrangements, that
preferably have been predetermined and controlled.
[0078] Generally, the process form making the present polysilocarb
materials involves one or more steps. The starting materials are obtained,
made
or derived. Precursors are obtained or can be made from starting materials.
The
precursors are combined to form a precursor formulation. The precursor
formulation is then shaped, formed, molded, etc. into a desired form, which
form
is then cured, which among other things transforms the precursor formulation
into
a plastic like material. This cured plastic like material can then be
pyrolyzed into
a ceramic. It being understood, that these steps may not all be used, that
some
19
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
of these steps may be repeated, once, twice or several times, and that
combinations and variations of these general steps may be utilized to obtain a
desired product or result.
[0079] Depending upon the specific process and desired features of
the product the precursors and starting materials, the process type and
conditions and the precursors can be predetermined and preselected. This
regime of precursors and processes provides great flexibility to create custom
features for intermediate, end and final products, and thus, typically,
combinations and variations of them, can provide a specific predetermined
product. Factors such as cost, controllability, shelf life, scale up,
manufacturing
ease, etc., can also be considered.
[0080] In embodiments, precursor materials may be methyl hydrogen,
and substituted and modified methyl hydrogens, siloxane backbone additives,
reactive monomers, hydrocarbons, reaction products of a siloxane backbone
additive with a silane modifier or an organic modifier, and other similar
types of
materials, such as silane based materials, silazane based materials,
carbosilane
based materials, phenol/formaldehyde based materials, and combinations and
variations of these and others.
[0081] Additionally, inhibitors such as cyclohexane, 1-Ethyny1-1-
cyclohexanol (which may be obtained from ALDRICH),
Octamethylcyclotetrasiloxane, tetramethyltetravinylcyclotetrasiloxane (which
may
act, depending upon amount and temperature as a reactant or a reactant
retardant (i.e., slows down a reaction to increase pot life), e.g., at room
temperature it is a retardant and at elevated temperatures it is a reactant),
may
be added to the polysilocarb precursor formulation, e.g., an inhibited
polysilocarb
precursor formulation. Other materials, as well, may be added to the
polysilocarb
precursor formulation, e.g., a filled polysilocarb precursor formulation, at
this
point in processing, including fillers such as SiC powder, carbon black, PDC
particles, pigments, particles, nano-tubes, whiskers, or other materials,
discussed
in this specification or otherwise known to the arts. Further, a formulation
with
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
both inhibitors and fillers would be considered an inhibited, filled
polysilocarb
precursor formulation.
[0082] Precursors and precursor formulations are preferably non-
hazardous materials. Generally they have flash points that are preferably
above
room and typical storage temperatures, are preferably noncorrosive, preferably
have low vapor pressure, low or no odor, and may be non- or mildly irritating
to
the skin. A catalyst may be used, and can be added at the time of, prior to,
shortly before, or at an earlier time before the precursor formulation is
formed or
made into a structure, prior to curing. Precursor formulations can have pot
lives,
that meet the needs of the manufacturing process. Generally, catalysts can be
used and can be balance with, or used in conjunction with the inhibitor to
have a
predetermined and predictable shelf life, cure time, cure temperature
profiles.
These profiles can be tailored to the specific manufacturing processes, e.g.,
ship
and hold for a month before curing, or ship hold and process directly from
liquid
to ceramic, or make and cure with minimal shipping or hold times.
[0083] In this mixing type process for making a precursor formulation,
preferably chemical reactions or molecular rearrangements only take place
during the making of the precursors, the curing process of the preform, and in
the
pyrolizing process. Thus, chemical reactions, e.g., polymerizations,
reductions,
condensations, substitutions, take place or are utilized in the making of a
precursor. In making a polysilocarb precursor formulation preferably no and
essentially no, chemical reactions and molecular rearrangements take place.
These embodiments of the present mixing type process, which avoid the need to,
and do not, utilize a polymerization or other reaction during the making of a
precursor formulation, provides significant advantages over prior methods of
making polymer derived ceramics. Preferably, in the embodiments of these
mixing type of formulations and processes, polymerization, crosslinking or
other
chemical reactions take place primarily, preferably essentially, and more
preferably solely in the preform during the curing process.
[0084] The precursors may also be selected from the following:
SiSiB0 TRIMETHYLSILYL TERMINATED METHYL 63148-
21
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
HF2020 HYDROGEN SILICONE FLUID 57-2
This is a type of material commonly called methylhydrogen fluid, and has the
formula below:
CH;)
H3C 0 __ Si-0 CH3
CH3 611 H3
-4 1
[0085]
TRIMETHYLSILYL TERMINATED
SiSiB0 68037-
METHYLHYDROSILOXANE DIMETHYLSILOXANE
HF2050 59-2
COPOLYMER
This may be called methyl terminated with dimethyl groups and has the formula
below.
" CH=a H =
11,4C¨ 0¨ Si 0 ___________ Si 0 ____ S¨CH3
'Ha (I C1Ha
m -
In some embodiments this precursor can decrease the exotherm and decrease
shrinkage
[0086]
SiSiB0 HYDRIDE TERMINATED METHYLHYDROSILOXANE 69013-
HF2060 DIMETHYLSILOXANE COPOLYMER 23-6
This may be called hydride terminated with dimethyl groups and has the formula
below.
22
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
CH3 CH,3 - H -
r
H ..... 0 0 .......... 0 .... H
1
CH3 CH,
CH a
m In some
embodiments this
precursor can decrease the exotherm and decrease shrinkage and provide
branch points
[0087]
SiSiB0 HF2038 HYDROGEN TERMINATED POLYDIPHENYL SILOXANE
H ...... 0 .......... o---:H
CH.3 CH3
\:4µ'
In some embodiments this precursor
can improve as-cured toughness and decrease shrinkage and improve thermal
stability of as-cured material
[0088]
SiSiB0 HYDRIDE TERMINATED METHYLHYDROSILOXANE 115487-
HF2068 DIMETHYLSILOXANE COPOLYMER 49-5
23
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
=õ,
-
CH3 H = e,
.4413
1
H ..... 0 ........ 0 ...... :Si .. 0 ... H
CH3
CH3 H
s=-= 3
m n
In some embodiments this precursor can improve as-cured toughness and
decrease shrinkage and improve thermal stability of as-cured material; but,
may
allow for higher cross-link density
[0089]
iSiB0 HYDRIDE TERMINATED POLY(PHENYL- 68952-
HF2078 DIMETHYLSILOXY) SILOXANE 30-7
PHENYL SILSESQUIOXANE, HYDROGEN-
TERMINATED
CH,
H 0 _____________ 0 ____
CH3O
=,s
................... . .. CH3
In some embodiments this
precursor's tri-functionality can be used for controlled branching, as well as
in
some embodiments to reduced shrinkage.
24
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[0090]
SiSiB0 VINYLDIMETHYL TERMINATED VINYLMETHYL- 68083-
VF6060 DIMETHYL POLYSILOXANE COPOLYMERS 18-1
C -
iõrs3
11:2C _ CH ¨ 0 ____ ¨ 0 --c=¨&¨ CH ¨
CH3 CH..;
CH3
m
In some embodiments this precursor's tri-functionality can be used for
controlled
branching, as well as in some embodiments to reduced shrinkage.
[0091]
SiSiB0 VINYLDIMETHYL TERMINATED
DIMETHYL- 68951-
VF6862 DIPHENYL POLYSILOXANE COPOLYMER 96-2
Cft; CH3
H2C .. CH- __ - -0¨ __ -0 ________________________
..........................................................
CH3 C H
- ¨
In some embodiments this precursor can be used to improve as cured toughness
and decreased shrinkage
[0092]
SiSiB0 VINYLDIMETHYL TERMINATED DIMETHYL-
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
VF6872 METHYLVINYL-DIPHENYL POLYSILOXANE COPOLYMER
CH2
CH3 CH 111-1 ' CH3
H2C=CH .... i:=;=ii ..... 0 ............ 0 ........... 0- + ........... 0
CH=3: i.,
CH3 CH3 CH3 CH3
In some embodiments this precursor can be used to improve as cured toughness
and decreased shrinkage; as well as providing the ability to improve crosslink
density through branching if needed.
[0093]
SiSiB P09401 1,1,3,3-TETRAMETHYL-1,3-DIVINYLDISILOXANE 2627-95-4
cii, 013
I -
H.:L._ i¨CH¨CH2
cH3
In some embodiments this precursor may provided for less shrinkage than the
tetravinyl; but still can provide for high crosslink density due to high vinyl
percentage, but primarily through 2-dimensional crosslinking, without any
branching
[0094]
SiSiB0 SILANOL TERMINATED 70131-
26
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
PF1070 POLYDIMETHYLSILOXANE (0F1070) 67-8
Cit-s
HO¨
In some embodiments this precursor may assist in decreasing the density by in-
situ nano/micro pore formation.
[0095]
SILANOL TERMINATED
70131-67-
POLYDIMETHYSILOXANE
SiSiB0 8
OH-ENDCAPPED POLYDIMETHYLSILOXANE
0F1070 73138-87-
HYDROXY TERMINATED
1
POLYDIMETHYLSILOXANE
CH3 CH3 CHa,,
CH3
In some embodiments this precursor may assist in decreasing the density by in-
situ nano/micro pore formation.
[0096]
SiSiB0 VINYL TERMINATED POLYDIMETHYL 68083-19-
VF6030 SILOXANE 2
27
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
CR2 CH3
CH=3 CH3
In some embodiments this precursor can increase cure speed, decrease
shrinkage slightly, and improves thermal /structural stability of cured and
pyrolyzed material
[0097]
SiSiB0 HYDROGEN TERMINATED 70900-
HF2030 POLYDIMETHYLSILOXANE FLUID 21-9
CH,
= ..s
.... i .. 0 ... 0 H
CHn CH3 613
n
[0098] In general, embodiments of formulations for polysilocarb
formulations may for example have from about 20% to about 99% MH, about 0%
to about 30% siloxane backbone additives, about 1`)/0 to about 60% reactive
monomers, and, about 0% to about 90% reaction products of a siloxane
backbone additives with a silane modifier or an organic modifier reaction
products.
[0099] In mixing the formulations a sufficient time to permit the
precursors to become effectively mixed and dispersed. Typically, the precursor
formulations are relatively, and essentially, shear insensitive, and thus the
type of
pumps or mixing are not critical. It is further noted that in higher viscosity
formulations additional mixing time may be required. Embodiments of processes
can provide the ability to build custom precursor formulations that when cured
can provide plastics having unique and desirable features such as high
28
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
temperature, flame resistance and retardation, strength and other features.
The
cured materials can also be pyrolized to form ceramics having unique features.
This allows for the predetermined balancing of different types of
functionality in
the end product by selecting function groups for incorporation into the
polymer
that makes up the precursor formulation.
[00100] Embodiments of a preform can be cured in a controlled
atmosphere, such as an inert gas, or it can be cured in the atmosphere. The
cure conditions, e.g., temperature, time, rate, etc., can be predetermined by
the
formulation and other processing conditions. For example, such conditions can
be selected to match, the size of the preform, the shape of the preform, or
the
mold holding the preform to prevent stress cracking, off gassing, or other
problems associated with the curing process. Further, the curing conditions
may
be such as to take advantage of, in a controlled manner, what may have been
previously perceived as problems associated with the curing process. Thus, for
example, off gassing may be used to create a foam material having either open
or closed structure. Further, the porosity of the material may be
predetermined
such that, for example, a particular pore size may be obtained, and in this
manner a filter or ceramic screen having predetermined pore sizes, flow
characteristic may be made.
[00101] The preforms, either unreinforced, neat, or reinforced, may be
used as a stand alone product, an end product, a final product, or a
preliminary
product for which later machining or processing may be performed on. The
preforms may also be subject to pyrolysis, which converts the preform material
into a ceramic.
[00102] In pyrolizing the preform, or cured structure or cured material, in
some embodiments it is heated to above about 650 C to about 1,200 C. At
these temperatures typically all organic structures are either removed or
combined with the inorganic constituents to form a ceramic. Typically at
temperatures in the 650 C to 1,200 C range the material is an amorphous
glassy ceramic. When heated above 1,200 C the material may from nano
29
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
crystalline structures, or micro crystalline structures, such as SiC, Si3N4,
SiCN, 13
SiC, and above 1,900 C an a SiC structure may form.
[00103] During pyrolysis material is loss through off gassing. The
amount of material remaining at the end of a pyrolysis set is referred to as
char
yield (or pyrolysis yield). Embodiments of formulations are capable of being
air
pyrolized to form a ceramic and to preferably do so at char yield in excess of
at
least about 80% and above 88%. The initial or first pyrolysis step generally
yields
a structure that is not very dense. However, in some examples, such as the use
of light weight spheres, the first pyrolysis may be sufficient. Thus, there
can be
in some embodiments a reinfiltration process that may be performed on the
pyrolized material, to add in additional polysilocarb precursor formulation
material, to fill in, or fill the voids and spaces in the structure. This
reinfiltrated
material is they repyrolized. This process of pyrolization, reinfiltration may
be
repeated, through one, two, three, and up to 10 or more times to obtain the
desired density of the final product. Additionally, with formulations of
embodiments of the present inventions, the viscosity of the formulation may be
tailored to provide more efficient reinfiltrations, and thus, a different
formulation
may be used at later reinfiltration steps, as the voids or pores become
smaller
and more difficult to get the formulation material into it. The high char
yields, and
other features of embodiments of the present invention, enable the manufacture
of completely closed structures, e.g., "helium tight" materials, with less
than
twelve reinfiltration steps, less than about 10 reinfiltrations steps and less
than
five reinfiltrations steps. Thus, by way of example, an initial inert gas
pyrolysis
may be performed with a high char yield formulation followed by four
reinfiltration
air pyrolysis steps.
[00104] Embodiments of the present inventions have the ability to utilize
precursors that have impurities, high-level impurities and significant
impurities.
Thus, the precursors may have more than about 0.1% impurities, more than
about 0.5%, more than about 1`)/0 impurities, more than about 5% impurities,
more than about 10% impurities, and more than about 50% impurities. In using
materials with impurities, the amounts of these impurities, or at least the
relative
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
amounts, so that the amount of actual precursor is known, should preferably be
determined by for example GPC (Gel Permeation Chromatography) or other
methods of analysis. In this manner the formulation of the polysilocarb
precursor
formulation may be adjusted for the amount of impurities present. The ability
of
embodiments of the present invention to utilize lower level impurity
materials, and
essentially impure materials, and highly impure materials, provides
significant
advantages over other method of making polymer derived ceramics. This
provides two significant advantages, among other things. First, the ability to
use
impure, lower purity, materials in embodiments of the present inventions,
provides the ability to greatly reduce the cost of the formulations and end
products, e.g., cured preforms, cured parts, and ceramic parts or structures.
Second, the ability to use impure, lower purity, materials in embodiments of
the
present inventions, provides the ability to have end products, e.g., cured
preforms, cured parts, and ceramic parts or structures, that have a
substantially
greater consistence from part to part, because variations in starting
materials can
be adjusted for during the formulation of each polysilocarb precursor
formulation.
[00105] The equipment, processes and techniques to make the present
inventions can be any of the systems, processes and techniques disclosed and
taught in this specification, know to the art for molding, forming, extruding,
coating, and assembling components, as well as, those disclosed and taught in
US Patent Applications Serial Numbers 14/212,986, 14/268,150, 14/324,056,
14/514,257, 61/946,598 and 62/055,397 and 62/106,094, the entire disclosure of
each of which are incorporated herein by reference.
[00106] It should be understood that the use of headings in this
specification is for the purpose of clarity, reference, and is not limiting in
any way.
Thus, the processes compositions, and disclosures described under a heading
should be read in context with the entirely of this specification, including
the
various examples. The use of headings in this specification should not limit
the
scope of protection afford the present inventions.
31
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
General Processes for Obtaining a Polysilocarb Precursor
[00107] Typically polymer derived ceramic precursor formulations, and
in particular polysilocarb precursor formulations can generally be made by
three
types of processes, although other processes, and variations and combinations
of these processes may be utilized. These processes generally involve
combining precursors to form a precursor formulation. One type of process
generally involves the mixing together of precursor materials in preferably a
solvent free process with essentially no chemical reactions taking place,
e.g.,
"the mixing process." The other type of process generally involves chemical
reactions, e.g., "the reaction type process," to form specific, e.g., custom,
precursor formulations, which could be monomers, dimers, trimers and polymers.
A third type of process has a chemical reaction of two or more components in a
solvent free environment, e.g., "the reaction blending type process."
Generally,
in the mixing process essentially all, and preferably all, of the chemical
reactions
take place during subsequent processing, such as during curing, pyrolysis and
both.
[00108] It should be understood that these terms - reaction type
process, reaction blending type process, and the mixing type process - are
used
for convenience and as a short hand reference. These terms are not, and should
not be viewed as, limiting. For example, the reaction process can be used to
create a precursor material that is then used in the mixing process with
another
precursor material.
[00109] These process types are described in this specification, among
other places, under their respective headings. It should be understood that
the
teachings for one process, under one heading, and the teachings for the other
processes, under the other headings, can be applicable to each other, as well
as,
being applicable to other sections, embodiments and teachings in this
specification, and vice versa. The starting or precursor materials for one
type of
process may be used in the other type of processes. Further, it should be
understood that the processes described under these headings should be read in
32
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
context with the entirely of this specification, including the various
examples and
embodiments.
[00110] It should be understood that combinations and variations of
these processes may be used in reaching a precursor formulation, and in
reaching intermediate, end and final products. Depending upon the specific
process and desired features of the product the precursors and starting
materials
for one process type can be used in the other. A formulation from the mixing
type process may be used as a precursor, or component in the reaction type
process, or the reaction blending type process. Similarly, a formulation from
the
reaction type process may be used in the mixing type process and the reaction
blending process. Similarly, a formulation from the reaction blending type
process may be used in the mixing type process and the reaction type process.
Thus, and preferably, the optimum performance and features from the other
processes can be combined and utilized to provide a cost effective and
efficient
process and end product. These processes provide great flexibility to create
custom features for intermediate, end, and final products, and thus, any of
these
processes, and combinations of them, can provide a specific predetermined
product. In selecting which type of process is preferable, factors such as
cost,
controllability, shelf life, scale up, manufacturing ease, etc., can be
considered.
[00111] In addition to being commercially available the precursors may
be made by way of an alkoxylation type process, e.g., an ethoxylation process.
In this process chlorosilanes are reacted with ethanol in the presences of a
catalysis, e.g., HCI, to provide the precursor materials, which materials may
further be reacted to provide longer chain precursors. Other alcohols, e.g.,
methanol may also be used. Thus, for example SiCI4, SiCI3H, SiCl2(C1-13)2,
SiCl2(CH3)H, Si(CH3)3CI, Si(CH3)CIH, are reacted with ethanol CH3CH2OH to
form precursors. In some of these reactions phenols may be the source of the
phenoxy group, which is substituted for a hydride group that has been placed
on
the silicon. One, two or more step reactions may need to take place.
[00112] Precursor materials may also be obtained by way of an
acetylene reaction route. In general there are several known paths for adding
33
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
acetylene to Si-H. Thus, for example, tetramethylcyclotetrasiloxane can be
reacted with acetylene in the presence of a catalyst to produce
tetramethyltetravinylcyclotetrasiloxane. This product can then be ring opened
and polymerized in order to form linear vinyl,methylsiloxanes. Alternatively,
typical vinyl silanes can be produced by reacting methyl,dichlorosilane
(obtained
from the direct process or Rochow process) with acetylene. These monomers
can then be purified (because there may be some scrambling) to form vinyl,
methyl, dichlorosilane. Then the vinyl monomer can be polymerized via
hydrolysis to form many cyclic, and linear siloxanes, having various chain
lengths, including for example various cyclotetrasiloxanes (e.g., D4') and
various
cyclopentasiloxanes (e.g., D5'). These paths, however, are costly, and there
has
been a long standing and increasing need for a lower cost raw material source
to
produce vinyl silanes. Prior to the present inventions, it was not believed
that
MHF could be used in an acetylene addition process to obtain vinyl silanes.
MHF is less expensive than vinyl,methyl (either linear or cyclic), and adding
acetylene to MHF to make vinyl meets, among other things, the long standing
need to provide a more cost effective material and at relatively inexpensive
costs.
In making this addition the following variables, among others, should be
considered and controlled: feed (D4', linear methyl, hydrogen siloxane
fluids);
temperature; ratio of acetylene to Si-H; homogeneous catalysts (Karstedt's,
DBT
Laureate, no catalyst, Karstedt's with inhibitor); supported catalysts (Pt on
carbon, Pt on alumina, Pd on alumina); flow rates (liquid feed, acetylene
feed);
pressure; and, catalyst concentration. Examples of embodiments of reactions
providing for the addition of acetylene to MHF (cyclic and linear) are
provided in
Tables A and B. Table A are batch acetylene reactions. Table B are continuous
acetylene reactions. It should be understood that batch, continuous, counter
current flow of MHF and acetylene feeds, continuous recycle of single pass
material to achieve higher conversions, and combinations and variations of
these
and other processes can be utilized.
34
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00113] TABLE A: Batch Acetylene Reactions
Pt
'F'
rt'.
'e-;' t,
=11
t g
(,)i:,4 41 6- ,, co ,, _. .., .......
¨ ¨ .
-, :
m s m if,
t
1 MHF 400 0.48% 0.00% -- -- 80 - -- 0.20 --
100
2 MHF 1000 0.27% 0.00% -- -- 65 - 276 - 0.75
3.4%
75 328
3 MHF 1000 0.00% 0.00% -- -- 80 378 - 6.33
49.4%
100 729
120
4 MHF 117 0.20% 0.00% Hexane 1000 60- 155- 4.50 188.0%
66 242
MHF 1000 0.40% 0.40% -- -- 55 - 102 7.5 15.7%
6 MHF 360 1.00% 0.00% Hexane 392 65 102 6.4 40.3%
7a MHF 360 0.40% 0.00% Hexane 400 65 -- 2.0 23.4%
7b MHF 280 0.40% 0.00% Hexane 454 68 -- 137.0 23.4%
8 D4' 1000 0.27% 0.00% -- -- 79 327 - 6.5
61.3%
745
9 MHF 370 0.40% 0.00% Hexane 402 65 155 -
8.0 140.3%
412
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
[00114] TABLE B: Continuous Acetylene Reactions
t-D
4 g rD
1
4
F., c
D4' 5% Pt on 0.00% 100.0% -- 60 - 100 50 40.0%
Carbon
11 D4' 5% Pt on 0.00% 100.0% 50 - 90 100 20.0%
Carbon
12 D4' 1% Pt on 0.00% 100.0% 40 - 50 50 23.8%
Alumina
13 MHF 5% Pt on 0.00% 100.0% 55 -60 55 - 60
13.6%
Carbon
14 MHF 0.01% Pt on 0.00% 20.0% Hexane 20 - 25 50
108.5%
Alumina
MHF 0.01% Pt on 0.00% 20.0% Hexane 60 50 - 55 117.1%
Alumina
16 MHF 0.01% Pt on 0.00% 20.0% Hexane 70 50 125.1%
Alumina
17 MHF 0.12% Pt on 0.00% 20.0% Hexane 60 50 133.8%
Alumina
18 MHF 0.12% Pt on 0.00% 4.0% Hexane 60 50 456.0%
Alumina
(D4' is tetramethyl tetrahydride cyclotetrasiloxane)
[00115] Continuous High Pressure Reactor ("CHPR") embodiments
may be advantageous for, among other reasons: reaction conversion saving
more acetylene needed in liquid phase; tube reactors providing pressures which
in turn increases solubility of acetylene; reaction with hexyne saving
concentration and time (e.g., 100 hours,); can eliminate homogeneous catalyst
and thus eliminate hydrosilylation reaction with resultant vinyls once
complete;
and, using a heterogeneous (Solid) catalyst to maintain product integrity,
increased shelf-life, increase pot-life and combinations and variations of
these.
[00116] In addressing the various conditions in the acetylene
addition reactions, some factors may be: crosslinking retardation by dilution,
acetylene and lower catalyst concentration; and conversion (using
heterogeneous catalyst) may be lower for larger linear molecules compared to
smaller molecules.
36
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
[00117] The presence and quality of vinyl and vinyl conversions can
be determined by, among other things,: FT-IR for presence of vinyl
absorptions,
decrease in SiH absorption ; 1H NMR for presence of vinyls and decrease in
SiH; 130 NMR for presence of vinyls.
[00118] As used herein, unless specified otherwise the terms %, weight
(:)/0 and mass (:)/0 are used interchangeably and refer to the weight of a
first
component as a percentage of the weight of the total, e.g., formulation,
mixture,
material or product. As used herein, unless specified otherwise "volume (:)/0"
and
"(:)/0 volume" and similar such terms refer to the volume of a first component
as a
percentage of the volume of the total, e.g., formulation, material or product.
The Mixing Type Process
[00119] Precursor materials may be methyl hydrogen, and substituted
and modified methyl hydrogens, siloxane backbone additives, reactive
monomers, reaction products of a siloxane backbone additive with a silane
modifier or an organic modifier, and other similar types of materials, such as
silane based materials, silazane based materials, carbosilane based materials,
phenol/formaldehyde based materials, and combinations and variations of these.
The precursors are preferably liquids at room temperature, although they may
be
solids that are melted, or that are soluble in one of the other precursors.
(In this
situation, however, it should be understood that when one precursor dissolves
another, it is nevertheless not considered to be a "solvent" as that term is
used
with respect to the prior art processes that employ non-constituent solvents,
e.g.,
solvents that do not form a part or component of the end product, are treated
as
waste products, and both.)
[00120] The precursors are mixed together in a vessel, preferably at
room temperature. Preferably, little, and more preferably no solvents, e.g.,
water, organic solvents, polar solvents, non-polar solvents, hexane, THF,
toluene, are added to this mixture of precursor materials. Preferably, each
precursor material is miscible with the others, e.g., they can be mixed at any
relative amounts, or in any proportions, and will not separate or precipitate.
At
this point the "precursor mixture" or "polysilocarb precursor formulation" is
37
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
compete (noting that if only a single precursor is used the material would
simply
be a "polysilocarb precursor" or a "polysilocarb precursor formulation" or a
"formulation"). Although complete, fillers and reinforcers may be added to the
formulation. In preferred embodiments of the formulation, essentially no, and
more preferably no chemical reactions, e.g., crosslinking or polymerization,
takes
place within the formulation, when the formulation is mixed, or when the
formulation is being held in a vessel, on a prepreg, or over a time period,
prior to
being cured.
[00121] The precursors can be mixed under numerous types of
atmospheres and conditions, e.g., air, inert, N2, Argon, flowing gas, static
gas,
reduced pressure, elevated pressure, ambient pressure, and combinations and
variations of these.
[00122] Additionally, inhibitors such as cyclohexane, 1-Ethyny1-1-
cyclohexanol (which may be obtained from ALDRICH),
Octamethylcyclotetrasiloxane, and tetramethyltetravinylcyclotetrasiloxane, may
be added to the polysilocarb precursor formulation, e.g., an inhibited
polysilocarb
precursor formulation. It should be noted that
tetramethyltetravinylcyclotetrasiloxane may act as both a reactant and a
reaction
retardant (e.g., an inhibitor), depending upon the amount present and
temperature, e.g., at room temperature it is a retardant and at elevated
temperatures it is a reactant. Other materials, as well, may be added to the
polysilocarb precursor formulation, e.g., a filled polysilocarb precursor
formulation, at this point in processing, including fillers such as SiC
powder,
carbon black, sand, polymer derived ceramic particles, pigments, particles,
nano-
tubes, whiskers, or other materials, discussed in this specification or
otherwise
known to the arts. Further, a formulation with both inhibitors and fillers
would be
considered an inhibited, filled polysilocarb precursor formulation.
[00123] Depending upon the particular precursors and their relative
amounts in the polysilocarb precursor formulation, polysilocarb precursor
formulations may have shelf lives at room temperature of greater than 12
hours,
greater than 1 day, greater than 1 week, greater than 1 month, and for years
or
38
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
more. These precursor formulations may have shelf lives at high temperatures,
for example, at about 90 F, of greater than 12 hours, greater than 1 day,
greater
than 1 week, greater than 1 month, and for years or more. The use of
inhibitors
may further extend the shelf life in time, for higher temperatures, and
combinations and variations of these. The use of inhibitors, may also have
benefits in the development of manufacturing and commercial processes, by
controlling the rate of reaction, so that it takes place in the desired and
intended
parts of the process or manufacturing system.
[00124] As used herein the term "shelf life" should be given its broadest
possible meaning, unless specified otherwise, and would include, for example,
the formulation being capable of being used for its intended purpose, or
performing, e.g., functioning, for its intended use, at 100% percent as well
as a
freshly made formulation, at least about 90% as well as a freshly made
formulation, at least about 80% as well as a freshly made formulation, and at
at
least about 70% as well as a freshly made formulation.
[00125] Precursors and precursor formulations are preferably non-
hazardous materials. They have flash points that are preferably above about 70
C, above about 80 C, above about 100 C and above about 300 C, and above.
Preferably, they may be noncorrosive. Preferably, they may have a low vapor
pressure, may have low or no odor, and may be non- or mildly irritating to the
skin.
[00126] A catalyst or initiator may be used, and can be added at the
time of, prior to, shortly before, or at an earlier time before the precursor
formulation is formed or made into a structure, prior to curing. The catalysis
assists in, advances, and promotes the curing of the precursor formulation to
form a preform.
[00127] The time period where the precursor formulation remains useful
for curing after the catalysis is added is referred to as "pot life", e.g.,
how long
can the catalyzed formulation remain in its holding vessel before it should be
used. Depending upon the particular formulation, whether an inhibitor is being
used, and if so the amount being used, storage conditions, e.g., temperature,
low
39
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
02 atmosphere, and potentially other factors, precursor formulations can have
pot lives, for example, of from about 5 minutes to about 10 days, about 1 day
to
about 6 days, about 4 to 5 days, about 30 minutes, about 15 minutes, about 1
hour to about 24 hours, and about 12 hours to about 24 hours.
[00128] The catalyst can be any platinum (Pt) based catalyst, which can,
for example, be diluted to a ranges of: about 0.01 parts per million (ppm) Pt
to
about 250 ppm Pt, about 0.03 ppm Pt, about 0.1 ppm Pt, about 0.2 ppm Pt,
about 0.5 ppm Pt, about 0.02 to 0.5 ppm Pt, about 1 ppm to 200 ppm Pt and
preferably, for some applications and embodiments, about 5 ppm to 50 ppm Pt.
The catalyst can be a peroxide based catalyst with, for example, a 10 hour
half
life above 90 C at a concentration of between 0.1`)/0 to 3% peroxide, and
about
0.5% and 2% peroxide. It can be an organic based peroxide. It can be any
organometallic catalyst capable of reacting with Si-H bonds, Si-OH bonds, or
unsaturated carbon bonds, these catalysts may include: dibutyltin dilaurate,
zinc
octoate, peroxides, organometallic compounds of for example titanium,
zirconium, rhodium, iridium, palladium, cobalt or nickel. Catalysts may also
be
any other rhodium, rhenium, iridium, palladium, nickel, and ruthenium type or
based catalysts. Combinations and variations of these and other catalysts may
be used. Catalysts may be obtained from ARKEMA under the trade name
LUPEROX, e.g., LUPEROX 231; and from Johnson Matthey under the trade
names: Karstedt's catalyst, Ashby's catalyst, Speier's catalyst.
[00129] Further, custom and specific combinations of these and other
catalysts may be used, such that they are matched to specific formulations,
and
in this way selectively and specifically catalyze the reaction of specific
constituents. Moreover, the use of these types of matched
catalyst¨formulations
systems may be used to provide predetermined product features, such as for
example, pore structures, porosity, densities, density profiles, high purity,
ultra
high purity, and other morphologies or features of cured structures and
ceramics.
[00130] In this mixing type process for making a precursor formulation,
preferably chemical reactions or molecular rearrangements only take place
during the making of the starting materials, the curing process, and in the
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
pyrolizing process. Chemical reactions, e.g., polymerizations, reductions,
condensations, substitutions, take place or are utilized in the making of a
starting
material or precursor. In making a polysilocarb precursor formulation by the
mixing type process, preferably no and essentially no, chemical reactions and
molecular rearrangements take place. These embodiments of the present mixing
type process, which avoid the need to, and do not, utilize a polymerization or
other reaction during the making of a precursor formulation, provides
significant
advantages over prior methods of making polymer derived ceramics. Preferably,
in the embodiments of these mixing type of formulations and processes,
polymerization, crosslinking or other chemical reactions take place primarily,
preferably essentially, and more preferably solely during the curing process.
[00131] The precursor may be a siloxane backbone additive, such as,
methyl hydrogen (MH), which formula is shown below.
CH3 CH& CH3 C11,3
CHa Si 0- S 0
CH3 H. CH3 CHla
The
[00132] The MH may have a molecular weight ("mw" which can be
measured as weight averaged molecular weight in amu or as g/mol) from about
400 mw to about 10,000 mw, from about 600 mw to about 3,000 mw, and may
have a viscosity preferably from about 20 cps to about 60 cps. The percentage
of methylsiloxane units "X" may be from 1`)/0 to 100%. The percentage of the
dimethylsiloxane units "Y" may be from 0% to 99%. This precursor may be used
to provide the backbone of the cross-linked structures, as well as, other
features
and characteristics to the cured preform and ceramic material. This precursor
may also, among other things, be modified by reacting with unsaturated carbon
compounds to produce new, or additional, precursors. Typically, methyl
hydrogen fluid (MHF) has minimal amounts of "Y", and more preferably "Y" is
for
all practical purposes zero.
41
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
[00133] The precursor may be a siloxane backbone additive, such as
vinyl substituted polydimethyl siloxane, which formula is shown below.
C1-13 CHa CH3 CH3
CH3 0 St 0 __ Sii¨ 0 I Sti ¨C1-13
11
C1-fa C: CH3 CH
C
[00134] This precursor may have a molecular weight (mw) from about
400 mw to about 10,000 mw, and may have a viscosity preferably from about 50
cps to about 2,000 cps. The percentage of methylvinylsiloxane units "X" may be
from 1% to 100%. The percentage of the dimethylsiloxane units "Y" may be from
0% to 99%. Preferably, X is about 100%. This precursor may be used to
decrease cross-link density and improve toughness, as well as, other features
and characteristics to the cured preform and ceramic material.
[00135] The precursor may be a siloxane backbone additive, such as
vinyl substituted and vinyl terminated polydimethyl siloxane, which formula is
shown below.
_
CH3 CH3 CH CHI
C
C- Si- CI --Si -0 = Si 0 - C
e5, II
CH3 C =I CFI CH.
2_ =ks-
C
[00136] This precursor may have a molecular weight (mw) from about
500 mw to about 15,000 mw, and may preferably have a molecular weight from
about 500 mw to 1,000 mw, and may have a viscosity preferably from about 10
cps to about 200 cps. The percentage of methylvinylsiloxane units "X" may be
from 1% to 100%. The percentage of the dimethylsiloxane units "Y" may be from
0% to 99%. This precursor may be used to provide branching and decrease the
cure temperature, as well as, other features and characteristics to the cured
preform and ceramic material.
42
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
[00137] The precursor may be a siloxane backbone additive, such as
vinyl substituted and hydrogen terminated polydimethyl siloxane, which formula
is shown below.
CHk C1113. CH3 CH3.
1 1 1 1
H- Si -0-St Si -0¨Si - H
I i1
CH3. C CH3 CH3
C -x-
[00138] This precursor may have a molecular weight (mw) from about
300 mw to about 10,000 mw, and may preferably have a molecular weight from
about 400 mw to 800 mw, and may have a viscosity preferably from about 20 cps
to about 300 cps. The percentage of methylvinylsiloxane units "X" may be from
1% to 100%. The percentage of the dimethylsiloxane units "Y" may be from 0%
to 99%. This precursor may be used to provide branching and decrease the
cure temperature, as well as, other features and characteristics to the cured
preform and ceramic material.
[00139] The precursor may be a siloxane backbone additive, such as
allyl terminated polydimethyl siloxane, which formula is shown below.
_
CH3_ ta1-13 CH3. CH3
1 1 1 C
C- S1- 0-Si - 0- ' Si - 0 ¨ -
i 1
11 1
C:= G CH3 GH3 CH CH3
-X-
[00140] This precursor may have a molecular weight (mw) from about
400 mw to about 10,000 mw, and may have a viscosity preferably from about 40
cps to about 400 cps. The repeating units are the same. This precursor may be
used to provide UV curability and to extend the polymeric chain, as well as,
other
features and characteristics to the cured preform and ceramic material.
[00141] The precursor may be a siloxane backbone additive, such as
vinyl terminated polydimethyl siloxane, which formula is shown below.
43
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
CH la CH3. CH3 CH3,
I 11 I C
C- -Si - ___ Si - Cc¨ Si - C
I I
C Ã113. CH3. CH 3 CHa
X
[00142] This precursor may have a molecular weight (mw) from about
200 mw to about 5,000 mw, and may preferably have a molecular weight from
about 400 mw to 1,500 mw, and may have a viscosity preferably from about 10
cps to about 400 cps. The repeating units are the same. This precursor may be
used to provide a polymeric chain extender, improve toughness and to lower
cure temperature down to for example room temperature curing, as well as,
other
features and characteristics to the cured preform and ceramic material.
[00143] The precursor may be a siloxane backbone additive, such as
silanol (hydroxy) terminated polydimethyl siloxane, which formula is shown
below.
CH3 CH3 CH I& C1113,
I I;
HO- Si - Si 0- I Si - ¨ Si -Oil
l Ii 11
CH3 tCH3 r CIA
[00144] This precursor may have a molecular weight (mw) from about
400 mw to about 10,000 mw, and may preferably have a molecular weight from
about 600 mw to 1,000 mw, and may have a viscosity preferably from about 30
cps to about 400 cps. The repeating units are the same. This precursor may be
used to provide a polymeric chain extender, a toughening mechanism, can
generate nano- and micro- scale porosity, and allows curing at room
temperature, as well as other features and characteristics to the cured
preform
and ceramic material.
[00145] The precursor may be a siloxane backbone additive, such as
silanol (hydroxy) terminated vinyl substituted dimethyl siloxane, which
formula is
shown below.
44
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
cH3 CH CH3,
I
HQ - Si - Si ! Sii Si -OH
I
C143 G CH ! CH
CH3 ; 3,
G
I
X
[00146] This precursor may have a molecular weight (mw) from about
400 mw to about 10,000 mw, and may preferably have a molecular weight from
about 600 mw to 1,000 mw, and may have a viscosity preferably from about 30
cps to about 400 cps. The percentage of methylvinylsiloxane units "X" may be
from 1% to 100%. The percentage of the dimethylsiloxane units "Y" may be from
0% to 99%. This precursor may be used, among other things, in a dual-cure
system; in this manner the dual-cure can allow the use of multiple cure
mechanisms in a single formulation. For example, both condensation type cure
and addition type cure can be utilized. This, in turn, provides the ability to
have
complex cure profiles, which for example may provide for an initial cure via
one
type of curing and a final cure via a separate type of curing.
[00147] The precursor may be a siloxane backbone additive, such as
hydrogen (hydride) terminated polydimethyl siloxane, which formula is shown
below.
_ _
CH3 CH3 CH3 CH&
I
H- - 0- Si CI __ Si 0¨ Si H
1
CH3 CH3 CHa CHa
X
[00148] This precursor may have a molecular weight (mw) from about
200 mw to about 10,000 mw, and may preferably have a molecular weight from
about 500 mw to 1,500 mw, and may have a viscosity preferably from about 20
cps to about 400 cps. The repeating units are the same. This precursor may be
used to provide a polymeric chain extender, as a toughening agent, and it
allows
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
lower temperature curing, e.g., room temperature, as well as, other features
and
characteristics to the cured preform and ceramic material.
[00149] The precursor may be a siloxane backbone additive, such as di-
phenyl terminated siloxane, which formula is shown below.
CH 31 CH3 CHI CH3
1 1 1
C)- Sii- 0 -SE- ____________ Si -0--Si -
1 1 1
CH3. R CH a CH3
X
[00150] Where here R is a reactive group, such as vinyl, hydroxy, or
hydride. This precursor may have a molecular weight (mw) from about 500 mw
to about 2,000 mw, and may have a viscosity preferably from about 80 cps to
about 300 cps. The percentage of methyl - R - siloxane units "X" may be from
1% to 100%. The percentage of the dimethylsiloxane units "Y" may be from 0%
to 99%. This precursor may be used to provide a toughening agent, and to
adjust the refractive index of the polymer to match the refractive index of
various
types of glass, to provide for example transparent fiberglass, as well as,
other
features and characteristics to the cured preform and ceramic material.
[00151] The precursor may be a siloxane backbone additive, such as a
mono-phenyl terminated siloxane, which formulas are shown below.
0113. CH3 CHa CH3,
I I
CH13¨ Sii- -
1 1 1 1
CH3. RCHa CH3
[00152] Where R is a reactive group, such as vinyl, hydroxy, or hydride.
This precursor may have a molecular weight (mw) from about 500 mw to about
2,000 mw, and may have a viscosity preferably from about 80 cps to about 300
cps. The percentage of methyl - R - siloxane units "X" may be from 1 /0 to
100%.
The percentage of the dimethylsiloxane units "Y" may be from 0% to 99%. This
46
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
precursor may be used to provide a toughening agent and to adjust the
refractive
index of the polymer to match the refractive index of various types of glass,
to
provide for example transparent fiberglass, as well as, other features and
characteristics to the cured preform and ceramic material.
[00153] The precursor may be a siloxane backbone additive, such as
diphenyl dimethyl polysiloxane, which formula is shown below.
CH3 CH3 CH&
CH3- Si - OE __ Si -C16¨ Si - CI-13
013 C1136:"11:
X
[00154] This precursor may have a molecular weight (mw) from about
500 mw to about 20,000 mw, and may have a molecular weight from about 800
to about 4,000, and may have a viscosity preferably from about 100 cps to
about
800 cps. The percentage of dimethylsiloxane units "X" may be from 25% to 95%.
The percentage of the diphenyl siloxane units "Y" may be from 5% to 75%. This
precursor may be used to provide similar characteristics to the mono-phenyl
terminated siloxane, as well as, other features and characteristics to the
cured
preform and ceramic material.
[00155] The precursor may be a siloxane backbone additive, such as
vinyl terminated diphenyl dimethyl polysiloxane, which formula is shown below.
CI-6 CH CH3
I ! I l *C:
Si:- -Sii- G _______________ St CI C
I
1/4.. CH3, CF13 I CH3
J
[00156] This precursor may have a molecular weight (mw) from about
400 mw to about 20,000 mw, and may have a molecular weight from about 800
to about 2,000, and may have a viscosity preferably from about 80 cps to about
600 cps. The percentage of dimethylsiloxane units "X" may be from 25% to 95%.
The percentage of the diphenyl siloxane units "Y" may be from 5% to 75%. This
47
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
precursor may be used to provide chain extension, toughening agent, changed or
altered refractive index, and improvements to high temperature thermal
stability
of the cured material, as well as, other features and characteristics to the
cured
preform and ceramic material.
[00157] The precursor may be a siloxane backbone additive, such as
hydroxy terminated diphenyl dimethyl polysiloxane, which formula is shown
below.
CH a CH3 ¨ CH&
I I
HO¨ Si St ____ Si ¨ 0:¨ --- OH
CH a CH3 0
CHia
X
[00158] This precursor may have a molecular weight (mw) from about
400 mw to about 20,000 mw, and may have a molecular weight from about 800
to about 2,000, and may have a viscosity preferably from about 80 cps to about
400 cps. The percentage of dimethylsiloxane units "X" may be from 25% to 95%.
The percentage of the diphenyl siloxane units "Y" may be from 5% to 75%. This
precursor may be used to provide chain extension, toughening agent, changed or
altered refractive index, and improvements to high temperature thermal
stability
of the cured material, can generate nano- and micro- scale porosity, as well
as
other features and characteristics to the cured preform and ceramic material.
[00159] A variety of cyclosiloxanes can be used as reactive molecules in
the formulation. They can be described by the following nomenclature system or
formula: DxD*y, where "D" represents a dimethyl siloxy unit and "D*"
represents a
substituted methyl siloxy unit, where the "*" group could be vinyl, allyl,
hydride,
hydroxy, phenyl, styryl, alkyl, cyclopentadienyl, or other organic group, x is
from
0-8, y is >=1, and x+y is from 3-8.
[00160] The precursor batch may also contain non-silicon based cross-
linking agents, be the reaction product of a non-silicon based cross linking
agent
and a siloxane backbone additive, and combinations and variation of these. The
non-silicon based cross-linking agents are intended to, and provide, the
48
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
capability to cross-link during curing. For example, non-silicon based cross-
linking agents that can be used include: cyclopentadiene (CP),
methylcyclopentadiene (MeCP), dicyclopentadiene ("DCPD"),
methyldicyclopentadiene (MeDCPD), tricyclopentadiene (TCPD), piperylene,
divnylbenzene, isoprene, norbornadiene, vinylnorbornene, propenylnorbornene,
isopropenylnorbornene, methylvinylnorbornene, bicyclononadiene,
methylbicyclononadiene, propadiene, 4-vinylcyclohexene, 1,3-heptadiene,
cycloheptadiene, 1,3-butadiene, cyclooctadiene and isomers thereof. Generally,
any hydrocarbon that contains two (or more) unsaturated, C=C, bonds that can
react with a Si-H, Si-OH, or other Si bond in a precursor, can be used as a
cross-
linking agent. Some organic materials containing oxygen, nitrogen, and sulphur
may also function as cross-linking moieties.
[00161] The precursor may be a reactive monomer. These would
include molecules, such as tetramethyltetravinylcyclotetrasiloxane ("TV"),
which
formula is shown below.
Ç.
t
0( 0 \\
_
[00162] This precursor may be used to provide a branching agent, a
three-dimensional cross-linking agent, as well as, other features and
characteristics to the cured preform and ceramic material. (It is also noted
that in
certain formulations, e.g., above 2%, and certain temperatures, e.g., about
from
about room temperature to about 60 C, this precursor may act as an inhibitor
to
cross-linking, e.g., in may inhibit the cross-linking of hydride and vinyl
groups.)
[00163] The precursor may be a reactive monomer, for example, such
as trivinyl cyclotetrasiloxane,
49
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
0, t,
,si_
0, 0
\\,
[00164] divinyl cyclotetrasiloxane,
r<>0
Sr. Si¨
/
Oi
[00165] trivinyl monohydride cyclotetrasiloxane,
/
,si"si_ H
o
\S\ k
.-. 0
/
\--Si,. Si
[00166]
[00167] divinyl dihydride cyclotetrasiloxane,
ro-
0 0
0, Si
[00168] and a hexamethyl cyclotetrasiloxane, such as,
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
1 0 /
--- Si Si H ' Si'
/ 1
0 0 0
/,
[00169] The precursor may be a silane modifier, such as vinyl phenyl
methyl silane, diphenyl silane, diphenyl methyl silane, and phenyl methyl
silane
(some of which may be used as an end capper or end termination group). These
silane modifiers can provide chain extenders and branching agents. They also
improve toughness, alter refractive index, and improve high temperature cure
stability of the cured material, as well as improving the strength of the
cured
material, among other things. A precursor, such as diphenyl methyl silane, may
function as an end capping agent, that may also improve toughness, alter
refractive index, and improve high temperature cure stability of the cured
material, as well as, improving the strength of the cured material, among
other
things.
[00170] The precursor may be a reaction product of a silane modifier
with a vinyl terminated siloxane backbone additive. The precursor may be a
reaction product of a silane modifier with a hydroxy terminated siloxane
backbone additive. The precursor may be a reaction product of a silane
modifier
with a hydride terminated siloxane backbone additive. The precursor may be a
reaction product of a silane modifier with TV. The precursor may be a reaction
product of a silane. The precursor may be a reaction product of a silane
modifier
with a cyclosiloxane, taking into consideration steric hindrances. The
precursor
may be a partially hydrolyzed tetraethyl orthosilicate, such as TES 40 or
Silbond
40. The precursor may also be a methylsesquisiloxane such as SR-350
available from General Electric Company, Wilton, Conn. The precursor may
also be a phenyl methyl siloxane such as 604 from Wacker Chemie AG. The
precursor may also be a methylphenylvinyisiloxane, such as l-2 C from Wacker
Chemie AG.
51
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00171] The precursors may also be selected from the following:
SiSiB HF2020, TRIMETHYLSILYL TERMINATED METHYL HYDROGEN
SILICONE FLUID 63148-57-2; SiSiB HF2050 TRIMETHYLSILYL
TERMINATED METHYLHYDROSILOXANE DIMETHYLSILOXANE
COPOLYMER 68037-59-2; SiSiB HF2060 HYDRIDE TERMINATED
METHYLHYDROSILOXANE DIMETHYLSILOXANE COPOLYMER 69013-23-6;
SiSiB HF2038 HYDROGEN TERMINATED POLYDIPHENYL SILOXANE;
SiSiB HF2068 HYDRIDE TERMINATED METHYLHYDROSILOXANE
DIMETHYLSILOXANE COPOLYMER 115487-49-5; SiSiB HF2078 HYDRIDE
TERMINATED POLY(PHENYLDIMETHYLSILOXY) SILOXANE PHENYL
SILSESQUIOXANE, HYDROGEN-TERMINATED 68952-30-7; SiSiB VF6060
VINYLDIMETHYL TERMINATED VINYLMETHYL DIMETHYL POLYSILOXANE
COPOLYMERS 68083-18-1; SiSiB VF6862 VINYLDIMETHYL TERMINATED
DIMETHYL DIPHENYL POLYSILOXANE COPOLYMER 68951-96-2; SiSiB
VF6872 VINYLDIMETHYL TERMINATED DIMETHYL-METHYLVINYL-
DIPHENYL POLYSILOXANE COPOLYMER; SiSiB PC9401 1,1,3,3-
TETRAMETHYL-1,3-DIVINYLDISILOXANE 2627-95-4; SiSiB PF1070
SILANOL TERMINATED POLYDIMETHYLSILOXANE (0F1070) 70131-67-8;
SiSiB 0F1070 SILANOL TERMINATED POLYDIMETHYSILOXANE 70131-67-
8; OH-ENDCAPPED POLYDIMETHYLSILOXANE HYDROXY TERMINATED
OLYDIMETHYLSILOXANE 73138-87-1; SiSiB VF6030 VINYL TERMINATED
POLYDIMETHYL SILOXANE 68083-19-2; and, SiSiB HF2030 HYDROGEN
TERMINATED POLYDIMETHYLSILOXANE FLUID 70900-21-9.
[00172] Thus, in additional to the forgoing type of precursors, it is
contemplated that a precursor may be a compound of the following general
formula.
RA 11 RI ___
1
E1- 0:- S - 0 ___________ Si - 0 ¨Ez
I I
R2 R4
52
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00173] Wherein end cappers El and E2 are chosen from groups such
as trimethyl silicon (-Si(CH3)3), dimethyl silicon hydroxy (-Si(CH3)20H),
dimethyl
silicon hydride (-Si(CH3)2H), dimethyl vinyl silicon (-Si(CH3)2(CH=CH2)),
(-Si(CH3)2(C6H5)) and dimethyl alkoxy silicon (-Si(CH3)2(0R). The R groups R1,
R2, R3, and R4 may all be different, or one or more may be the same. Thus, for
example, R2 is the same as R3, R3 is the same as R4, R1 and R2 are different
with
R3 and R4 being the same, etc. The R groups are chosen from groups such as
hydride (-H), methyl (Me)(-C), ethyl (-C-C), vinyl (-C=C), alkyl (-
R)(CnH2n,1), allyl
(-C-C=C), aryl (s R), phenyl (Ph)(-C6H5), methoxy (-0-C), ethoxy (-O-C-C),
siloxy
(-0-Si-R3), alkoxy (-O-R), hydroxy (-0-H), phenylethyl (-C-C-C6H5) and
methyl,phenyl-ethyl (-C-C(-C)(-C6H5).
[00174] In general, embodiments of formulations for polysilocarb
formulations may for example have from about 0% to 50% MH, about 20% to
about 99% MH, about 0% to about 30% siloxane backbone additives, about 1%
to about 60% reactive monomers, about 30% to about 100% TV, and, about 0%
to about 90% reaction products of a siloxane backbone additives with a silane
modifier or an organic modifier reaction products.
[00175] In mixing the formulations sufficient time should be used to
permit the precursors to become effectively mixed and dispersed. Generally,
mixing of about 15 minutes to an hour is sufficient. Typically, the precursor
formulations are relatively, and essentially, shear insensitive, and thus the
type of
pumps or mixing are not critical. It is further noted that in higher viscosity
formulations additional mixing time may be required. The temperature of the
formulations, during mixing should preferably be kept below about 45 C, and
preferably about 10 C. (It is noted that these mixing conditions are for the
pre-
catalyzed formulations.)
The Reaction Type Process
[00176] In the reaction type process, in general, a chemical reaction is
used to combine one, two or more precursors, typically in the presence of a
solvent, to form a precursor formulation that is essentially made up of a
single
polymer that can then be, catalyzed, cured and pyrolized. This process
provides
53
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
the ability to build custom precursor formulations that when cured can provide
plastics having unique and desirable features such as high temperature, flame
resistance and retardation, strength and other features. The cured materials
can
also be pyrolized to form ceramics having unique features. The reaction type
process allows for the predetermined balancing of different types of
functionality
in the end product by selecting functional groups for incorporation into the
polymer that makes up the precursor formulation, e.g., phenyls which typically
are not used for ceramics but have benefits for providing high temperature
capabilities for plastics, and styrene which typically does not provide high
temperature features for plastics but provides benefits for ceramics.
[00177] In general a custom polymer for use as a precursor formulation
is made by reacting precursors in a condensation reaction to form the polymer
precursor formulation. This precursor formulation is then cured into a preform
through a hydrolysis reaction. The condensation reaction forms a polymer of
the
type shown below.
- -
Rt
Si __ 0 0¨ St - ¨ Si - -
End 1 i End 2:
R2
1112!
_
A2 A
[00178] Where R1 and R2 in the polymeric units can be a hydride (-H), a
methyl (Me)(-C), an ethyl (-C-C), a vinyl (-C=C), an alkyl (-R)(CnH2n,i), an
unsaturated alkyl (-CnH2n-i), a cyclic alkyl (-CnH2n-1), an allyl (-C-C=C), a
butenyl
(-C4H7), a pentenyl (-05H9), a cyclopentenyl (-05H7), a methyl cyclopentenyl (-
05H6(CH3)), a norbornenyl (-CxHy, where X = 7-15 and Y = 9 -18), an aryl (s
R), a
phenyl (Ph)(-C6H5), a cycloheptenyl (-C7H11), a cyclooctenyl (-C81-113), an
ethoxy
(-O-C-C), a siloxy (-0-Si-R3), a methoxy (-0-C), an alkoxy, (-O-R), a hydroxy,
(-
0-H), a phenylethyl (-C-C-C6H5) a methyl,phenyl-ethyl (-C-C(-C)(-C6H5)) and a
vinylphenyl-ethyl (-C-C(C6H4(-C=C))). R1 and R2 may be the same or different.
The custom precursor polymers can have several different polymeric units,
e.g.,
A1, A2, An, and may include as many as 10, 20 or more units, or it may contain
54
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
only a single unit, for example, MHF made by the reaction process may have
only a single unit.
[00179] Embodiments may include precursors, which include among
others, a triethoxy methyl silane, a diethoxy methyl phenyl silane, a diethoxy
methyl hydride silane, a diethoxy methyl vinyl silane, a dimethyl ethoxy vinyl
silane, a diethoxy dimethyl silane. an ethoxy dimethyl phenyl silane, a
diethoxy
dihydride silane, a triethoxy phenyl silane, a diethoxy hydride trimethyl
siloxane,
a diethoxy methyl trimethyl siloxane, a trimethyl ethoxy silane, a diphenyl
diethoxy silane, a dimethyl ethoxy hydride siloxane, and combinations and
variations of these and other precursors, including other precursors set forth
in
this specification.
[00180] The end units, Si End 1 and Si End 2, can come from the
precursors of dimethyl ethoxy vinyl silane, ethoxy dimethyl phenyl silane, and
trimethyl ethoxy silane. Additionally, if the polymerization process is
properly
controlled a hydroxy end cap can be obtained from the precursors used to
provide the repeating units of the polymer.
[00181] In general, the precursors are added to a vessel with ethanol (or
other material to absorb heat, e.g., to provide thermal mass), an excess of
water,
and hydrochloric acid (or other proton source). This mixture is heated until
it
reaches its activation energy, after which the reaction typically is
exothermic.
Generally, in this reaction the water reacts with an ethoxy group of the
silicon of
the precursor monomer, forming a hydroxy (with ethanol as the byproduct).
Once formed this hydroxy becomes subject to reaction with an ethoxy group on
the silicon of another precursor monomer, resulting in a polymerization
reaction.
This polymerization reaction is continued until the desired chain length(s) is
built.
[00182] Control factors for determining chain length, among others, are:
the monomers chosen (generally, the smaller the monomers the more that can
be added before they begin to coil around and bond to themselves); the amount
and point in the reaction where end cappers are introduced; and the amount of
water and the rate of addition, among others. Thus, the chain lengths can be
from about 180 mw (viscosity about 5 cps) to about 65,000 mw (viscosity of
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
about 10,000 cps), greater than about 1000 mw, greater than about 10,000 mw,
greater than about 50,000 mw and greater. Further, the polymerized precursor
formulation may, and typically does, have polymers of different molecular
weights, which can be predetermined to provide formulation, cured, and ceramic
product performance features.
[00183] Upon completion of the polymerization reaction the material is
transferred into a separation apparatus, e.g., a separation funnel, which has
an
amount of deionized water that, for example, is from about 1.2x to about 1.5x
the
mass of the material. This mixture is vigorously stirred for about less than 1
minute and preferably from about 5 to 30 seconds. Once stirred the material is
allowed to settle and separate, which may take from about 1 to 2 hours. The
polymer is the higher density material and is removed from the vessel. This
removed polymer is then dried by either warming in a shallow tray at 90 C for
about two hours; or, preferably, is passed through a wiped film distillation
apparatus, to remove any residual water and ethanol. Alternatively, sodium
bicarbonate sufficient to buffer the aqueous layer to a pH of about 4 to about
7 is
added. It is further understood that other, and commercial, manners of mixing,
reacting and separating the polymer from the material may be employed.
[00184] Preferably a catalyst is used in the curing process of the
polymer precursor formulations from the reaction type process. The same
polymers, as used for curing the precursor formulations from the mixing type
process can be used. It is noted that, generally unlike the mixing type
formulations, a catalyst is not necessarily required to cure a reaction type
polymer. Inhibitors may also be used. However, if a catalyst is not used,
reaction time and rates will be slower. The curing and the pyrolysis of the
cured
material from the reaction process is essentially the same as the curing and
pyrolysis of the cured material from the mixing process and the reaction
blending
process.
[00185] The reaction type process can be conducted under numerous
types of atmospheres and conditions, e.g., air, inert, N2, Argon, flowing gas,
56
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
static gas, reduced pressure, ambient pressure, elevated pressure, and
combinations and variations of these.
The Reaction Blending Type Process
[00186] In the reaction blending type process precursor are reacted to
from a precursor formulation, in the absence of a solvent.
For example, an embodiment of a reaction blending type process has a
precursor formulation that is prepared from MHF and Dicyclopentadiene
("DCPD"). Using the reactive blending process a MHF/DCPD polymer is created
and this polymer is used as a precursor formulation. (It can be used alone to
form
a cured or pyrolized product, or as a precursor in the mixing or reaction
processes.) MHF of known molecular weight and hydride equivalent mass; "P01"
(P01 is a 2% Pt(0) tetravinylcyclotetrasiloxane complex (e.g.,
tetramethyltetravinylcyclotetrasiloxane) in tetravinylcyclotetrasiloxane,
diluted 20x
with tetravinylcyclotetrasiloxane to 0.1% of Pt(0) complex. In this manner 10
ppm Pt is provided for every 1`)/0 loading of bulk cat.) catalyst 0.20 wt% of
MHF
starting material (with known active equivalent weight), from 40 to 90%; and
Dicyclopentadiene with 83% purity, from 10 to 60% are utilized. In an
embodiment of the process, a sealable reaction vessel, with a mixer, can be
used for the reaction. The reaction is conducted in the sealed vessel, in air;
although other types of atmosphere can be utilized. Preferably, the reaction
is
conducted at atmospheric pressure, but higher and lower pressures can be
utilized. Additionally, the reaction blending type process can be conducted
under numerous types of atmospheres and conditions, e.g., air, inert, N2,
Argon,
flowing gas, static gas, reduced pressure, ambient pressure, elevated
pressure,
and combinations and variations of these.
[00187] In an embodiment, 850 grams of MHF (85% of total polymer
mixture) is added to reaction vessel and heated to about 50 C. Once this
temperature is reached the heater is turned off, and 0.20% by weight P01
Platinum catalyst is added to the MHF in the reaction vessel. Typically, upon
addition of the catalyst bubbles will form and temp will initially rise
approximately
2-20 C.
57
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00188] When the temperature begins to fall, about 150 g of DCPD (15
wt% of total polymer mixture) is added to the reaction vessel. The temperature
may drop an additional amount, e.g., around 5-7 C.
[00189] At this point in the reaction process the temperature of the
reaction vessel is controlled to, maintain a predetermined temperature profile
over time, and to manage the temperature increase that may be accompanied by
an exotherm. Preferably, the temperature of the reaction vessel is regulated,
monitored and controlled throughout the process.
[00190] In an embodiment of the MHF/DCPD embodiment of the
reaction process, the temperature profile can be as follows: let temperature
reach
about 80 C (may take ¨15-40 min, depending upon the amount of materials
present); temperature will then increase and peak at ¨104 C, as soon as
temperature begins to drop, the heater set temperature is increased to 100 C
and the temperature of the reaction mixture is monitored to ensure the polymer
temp stays above 80 C for a minimum total of about 2 hours and a maximum
total of about 4 hours. After 2-4 hours above 80 C, the heater is turned off,
and
the polymer is cooled to ambient. It being understood that in larger and
smaller
batches, continuous, semi-continuous, and other type processes the temperature
and time profile may be different.
[00191] In larger scale, and commercial operations, batch, continuous,
and combinations of these, may be used. Industrial factory automation and
control systems can be utilized to control the reaction, temperature profiles
and
other processes during the reaction.
[00192] Table C sets forth various embodiments of reaction blending
processes.
58
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00193] Table C
4 lc
c cn
m m r=i m E t=1 t=1
eD ,7 sm. om om 4= om eD om 4=
IT ; '4EgEg ig ig ':14, Eg 4
4 i
9. r7, 9.,7, 9.,7, , r7, E r7: ,S, CT
A4 0 rD t'D 0 '¨' 0 0 0 M 0
r7
tPD 0' " co co rii ( )e'r ( )rii 0
: ..,
tetramethylcyclotet
rasiloxane (Dzi.) 4 4 4 4 0 4 4 240.51
MHF 33 35 34 33 0 39
39 2145.345
VMF 5 7 6 0 5 11 21 592.959 118.59
TV 4 4 4 0 4 4 12 344.52 86.13
VT 0200 125 127 126 0 2 254 258
9451.206 4725.60
VT 0020 24 26 25 0 2 52 56 1965.187
982.59
VT 0080 79 81 80 0 2 162 166 6041.732
3020.87
Styrene 2 104.15 52.08
Dicyclopentadiene 2 132.2 66.10
1,4-divinylbenzene 2 130.19 65.10
isoprene 2 62.12 31.06
1,3 Butadiene 2 54.09 27.05
Catalyst 10 ppm Pt
Catalyst LP 231
[00194] In the above table, the "degree of polymerization" is the number
of monomer units, or repeat units, that are attached together to form the
polymer.
"Equivalents /mol" refers to the molar equivalents. "Grams/mole of vinyl"
refers to the amount of a given polymer needed to provide 1 molar equivalent
of
vinyl functionality. "VMH" refers to methyl vinyl fluid, a linear vinyl
material from
the ethoxy process, which can be a substitute for TV. The numbers "0200" etc.
for VT are the viscosity in centipoise for that particular VT.
Curing and Pyrolysis
[00195] Precursor formulations, including the polysilocarb precursor
formulations from the above types of processes, as well as others, can be
cured
to form a solid, semi-sold, or plastic like material. Typically, the precursor
formulations are spread, shaped, or otherwise formed into a preform, which
would include any volumetric structure, or shape, including thin and thick
films.
In curing, the polysilocarb precursor formulation may be processed through an
initial cure, to provide a partially cured material, which may also be
referred to,
59
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
for example, as a preform, green material, or green cure (not implying
anything
about the material's color). The green material may then be further cured.
Thus,
one or more curing steps may be used. The material may be "end cured," i.e.,
being cured to that point at which the material has the necessary physical
strength and other properties for its intended purpose. The amount of curing
may be to a final cure (or "hard cure"), i.e., that point at which all, or
essentially
all, of the chemical reaction has stopped (as measured, for example, by the
absence of reactive groups in the material, or the leveling off of the
decrease in
reactive groups over time). Thus, the material may be cured to varying
degrees,
depending upon its intended use and purpose. For example, in some situations
the end cure and the hard cure may be the same. Curing conditions such as
atmosphere and temperature may affect the composition of the cured material.
[00196] In making the precursor formulation into a structure, or preform,
the precursor formulation, e.g., polysilocarb formulation, can be, for
example,
formed using the following techniques: spraying, spray drying, atomization,
nebulization, phase change separation, flowing, thermal spraying, drawing,
dripping, forming droplets in liquid and liquid-surfactant systems, painting,
molding, forming, extruding, spinning, ultrasound, vibrating, solution
polymerization, emulsion polymerization, micro-emulsion polymerization,
injecting, injection molding, or otherwise manipulated into essentially any
volumetric shape. These volumetric shapes may include for example, the
following: spheres, pellets, rings, lenses, disks, panels, cones,
frustoconical
shapes, squares, rectangles, trusses, angles, channels, hollow sealed
chambers,
hollow spheres, blocks, sheets, coatings, films, skins, particulates, beams,
rods,
angles, slabs, columns, fibers, staple fibers, tubes, cups, pipes, and
combinations and various of these and other more complex shapes, both
engineering and architectural.
[00197] The forming step, the curing steps, and the pyrolysis steps may
be conducted in batch processes, serially, continuously, with time delays
(e.g.,
material is stored or held between steps), and combinations and variations of
these and other types of processing sequences. Further, the precursors can be
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
partially cured, or the cure process can be initiated and on going, prior to
the
precursor being formed into a volumetric shape. These steps, and their various
combinations may be, and in some embodiments preferably are, conducted
under controlled and predetermined conditions (e.g., the material is exposed
to a
predetermined atmosphere, and temperature profile during the entirely of its
processing, e.g., reduced oxygen, temperature of cured preform held at about
140 C prior to pyrolysis). It should be further understood that the system,
equipment, or processing steps, for forming, curing and pyrolizing may be the
same equipment, continuous equipment, batch and linked equipment, and
combinations and variations of these and other types of industrial processes.
Thus, for example, a spray drying technique could form cured particles that
are
feed directly into a fluidized bed reactor for pyrolysis.
[00198] The polysilocarb precursor formulations can be made into neat,
non-reinforced, non-filled, composite, reinforced, and filled structures,
intermediates, end products, and combinations and variations of these and
other
compositional types of materials. Further, these structures, intermediates and
end products can be cured (e.g., green cured, end cured, or hard cured),
uncured, pyrolized to a ceramic, and combinations and variations of these
(e.g.,
a cured material may be filled with pyrolized material derived from the same
polysilocarb as the cured material).
[00199] The precursor formulations may be used to form a "neat"
material, (by "neat" material it is meant that all, and essentially all of the
structure
is made from the precursor material or unfilled formulation; and thus, there
are no
fillers or reinforcements).
[00200] The polysilocarb precursor formulations may be used to coat or
impregnate a woven or non-woven fabric, made from for example carbon fiber,
glass fibers or fibers made from a polysilocarb precursor formulation (the
same
or different formulation), to from a prepreg material. Thus, the polysilocarb
precursor formulations may be used to form composite materials, e.g.,
reinforced
products. For example, the formulation may be flowed into, impregnated into,
absorbed by or otherwise combined with a reinforcing material, such as carbon
61
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
fibers, glass fiber, woven fabric, grapheme, carbon nanotubes, thin films,
precipitates, sand, non-woven fabric, copped fibers, fibers, rope, braided
structures, ceramic powders, glass powders, carbon powders, graphite powders,
ceramic fibers, metal powders, carbide pellets or components, staple fibers,
tow,
nanostructures of the above, polymer derived ceramics, any other material that
meets the temperature requirements of the process and end product, and
combinations and variations of these. The reinforcing material may also be
made from, or derived from the same material as the formulation that has been
formed into a fiber and pyrolized into a ceramic, or it may be made from a
different precursor formulation material, which has been formed into a fiber
and
pyrolized into a ceramic.
[00201] The polysilocarb precursor formulation may be used to form a
filled material. A filled material would be any material having other solid,
or semi-
solid, materials added to the polysilocarb precursor formulation. The filler
material may be selected to provide certain features to the cured product, the
ceramic product and both. These features may relate to, or be, for example,
aesthetic, tactile, thermal, density, radiation, chemical, cost, magnetic,
electric,
and combinations and variations of these and other features. These features
may be in addition to strength. Thus, the filler material may not affect the
strength
of the cured or ceramic material, it may add strength, or could even reduce
strength in some situations. The filler material could impart color, magnetic
capabilities, fire resistances, flame retardance, heat resistance, electrical
conductivity, anti-static, optical properties (e.g., reflectivity,
refractivity and
iridescence), aesthetic properties (such as stone like appearance in building
products), chemical resistivity, corrosion resistance, wear resistance,
reduced
cost, abrasions resistance, thermal insulation, UV stability, UV protective,
and
other features that may be desirable, necessary, and both, in the end product
or
material. Thus, filler materials could include carbon black, copper lead
wires,
thermal conductive fillers, electrically conductive fillers, lead, optical
fibers,
ceramic colorants, pigments, oxides, sand, dyes, powders, ceramic fines,
polymer derived ceramic particles, pore-formers, carbosilanes, silanes,
62
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
silazanes, silicon carbide, carbosilazanes, siloxane, powders, ceramic
powders,
metals, metal complexes, carbon, tow, fibers, staple fibers, boron containing
materials, milled fibers, glass, glass fiber, fiber glass, and nanostructures
(including nanostructures of the forgoing) to name a few.
[00202] The polysilocarb formulation and products derived or made from
that formulation may have metals and metal complexes. Filled materials would
include reinforced materials. In many cases, cured, as well as pyrolized
polysilocarb filled materials can be viewed as composite materials. Generally,
under this view, the polysilocarb would constitute the bulk or matrix phase,
(e.g.,
a continuous, or substantially continuous phase), and the filler would
constitute
the dispersed (e.g., non-continuous), phase. Depending upon the particular
application, product or end use, the filler can be evenly distributed in the
precursor formulation, unevenly distributed, distributed over a predetermined
and
controlled distribution gradient (such as from a predetermined rate of
settling),
and can have different amounts in different formulations, which can then be
formed into a product having a predetermined amounts of filler in
predetermined
areas (e.g., striated layers having different filler concentration). It should
be
noted, however, that by referring to a material as "filled" or "reinforced" it
does not
imply that the majority (either by weight, volume, or both) of that material
is the
polysilcocarb. Thus, generally, the ratio (either weight or volume) of
polysilocarb
to filler material could be from about 0.1:99.9 to 99.9:0.1.
[00203] The polysilocarb precursor formulations may be used to form
non-reinforced materials, which are materials that are made of primarily,
essentially, and preferably only from the precursor materials; but may also
include formulations having fillers or additives that do not impart strength.
[00204] The curing may be done at standard ambient temperature and
pressure ("SATP", 1 atmosphere, 25 C), at temperatures above or below that
temperature, at pressures above or below that pressure, and over varying time
periods. The curing can be conducted over various heatings, rate of heating,
and
temperature profiles (e.g., hold times and temperatures, continuous
temperature
change, cycled temperature change, e.g., heating followed by maintaining,
63
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
cooling, reheating, etc.). The time for the curing can be from a few seconds
(e.g., less than about 1 second, less than 5 seconds), to less than a minute,
to
minutes, to hours, to days (or potentially longer). The curing may also be
conducted in any type of surrounding environment, including for example, gas,
liquid, air, water, surfactant containing liquid, inert atmospheres, N2,
Argon,
flowing gas (e.g., sweep gas), static gas, reduced 02, reduced pressure,
elevated
pressure, ambient pressure, controlled partial pressure and combinations and
variations of these and other processing conditions. For high purity
materials,
the furnace, containers, handling equipment, atmosphere, and other components
of the curing apparatus and process are clean, essentially free from, and do
not
contribute any elements or materials, that would be considered impurities or
contaminants, to the cured material. In an embodiment, the curing environment,
e.g., the furnace, the atmosphere, the container and combinations and
variations
of these can have materials that contribute to or effect, for example, the
composition, catalysis, stoichiometry, features, performance and combinations
and variations of these in the preform, the ceramic and the final applications
or
products.
[00205] Preferably, in embodiments of the curing process, the curing
takes place at temperatures in the range of from about 5 C or more, from about
20 C to about 250 C, from about 20 C to about 150 C, from about 75 C to about
125 C, and from about 80 C to 90 C. Although higher and lower temperatures
and various heating profiles, (e.g., rate of temperature change over time
("ramp
rate", e.g., A degrees/time), hold times, and temperatures) can be utilized.
[00206] The cure conditions, e.g., temperature, time, ramp rate, may be
dependent upon, and in some embodiments can be predetermined, in whole or in
part, by the formulation to match, for example the size of the preform, the
shape
of the preform, or the mold holding the preform to prevent stress cracking,
off
gassing, or other phenomena associated with the curing process. Further, the
curing conditions may be such as to take advantage of, preferably in a
controlled
manner, what may have previously been perceived as problems associated with
the curing process. Thus, for example, off gassing may be used to create a
foam
64
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
material having either open or closed structure. Similarly, curing conditions
can
be used to create or control the microstructure and the nanostructure of the
material. In general, the curing conditions can be used to affect, control or
modify the kinetics and thermodynamics of the process, which can affect
morphology, performance, features and functions, among other things.
[00207] Upon curing the polysilocarb precursor formulation a cross
linking reaction takes place that provides in some embodiments a cross-linked
structure having, among other things, an -Ri-Si-C-C-Si-O-Si-C-C-Si-R2- where
R1
and R2 vary depending upon, and are based upon, the precursors used in the
formulation. In an embodiment of the cured materials they may have a cross-
linked structure having 3-coordinated silicon centers to another silicon atom,
being separated by fewer than 5 atoms between silicons.
[00208] During the curing process some formulations may exhibit an
exotherm, i.e., a self heating reaction, that can produce a small amount of
heat to
assist or drive the curing reaction, or that may produce a large amount of
heat
that may need to be managed and removed in order to avoid problems, such as
stress fractures. During the cure off gassing typically occurs and results in
a loss
of material, which loss is defined generally by the amount of material
remaining,
e.g., cure yield. Embodiments of the formulations, cure conditions, and
polysilocarb precursor formulations of embodiments of the present inventions
can
have cure yields of at least about 90%, about 92%, about 100%. In fact, with
air
cures the materials may have cure yields above 100%, e.g., about 101-105%, as
a result of oxygen being absorbed from the air. Additionally, during curing
the
material typically shrinks, this shrinkage may be, depending upon the
formulation, cure conditions, and the nature of the preform shape, and whether
the preform is reinforced, filled, neat or unreinforced, from about 20%, less
than
20%, less than about 15%, less than about 5%, less than about 1`)/0, less than
about 0.5%, less than about 0.25% and smaller.
[00209] Curing of the preform may be accomplished by any type of
heating apparatus, or mechanisms, techniques, or morphologies that has the
requisite level of temperature and environmental control, for example, heated
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
water baths, electric furnaces, microwaves, gas furnaces, furnaces, forced
heated air, towers, spray drying, falling film reactors, fluidized bed
reactors,
lasers, indirect heating elements, direct heating, infrared heating, UV
irradiation,
RF furnace, in-situ during emulsification via high shear mixing, in-situ
during
emulsification via ultrasonication.
[00210] The cured preforms, either unreinforced, neat, filled or
reinforced, may be used as a stand alone product, an end product, a final
product, or a preliminary product for which later machining or processing may
be
performed on. The preforms may also be subject to pyrolysis, which converts
the
preform material into a ceramic.
[00211] In pyrolizing the preform, or cured structure, or cured material, it
is heated to about 600 C to about 2,300 C; from about 650 C to about 1,200
C, from about 800 C to about 1300 C, from about 900 C to about 1200 C and
from about 950 C to 11500C. At these temperatures typically all organic
structures are either removed or combined with the inorganic constituents to
form
a ceramic. Typically at temperatures in the about 650 C to 1,200 C range the
resulting material is an amorphous glassy ceramic. When heated above about
1,200 C the material typically may from nano crystalline structures, or micro
crystalline structures, such as SiC, Si3N4, SiCN, 0 SiC, and above 1,900 C an
a
SiC structure may form, and at and above 2,200 C a SiC is typically formed.
The pyrolized, e.g., ceramic materials can be single crystal, polycrystalline,
amorphous, and combinations, variations and subgroups of these and other
types of morphologies.
[00212] The pyrolysis may be conducted under many different heating
and environmental conditions, which preferably include thermo control, kinetic
control and combinations and variations of these, among other things. For
example, the pyrolysis may have various heating ramp rates, heating cycles and
environmental conditions. In some embodiments, the temperature may be
raised, and held a predetermined temperature, to assist with known transitions
(e.g., gassing, volatilization, molecular rearrangements, etc.) and then
elevated
to the next hold temperature corresponding to the next known transition. The
66
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
pyrolysis may take place in reducing atmospheres, oxidative atmospheres, low
02, gas rich (e.g., within or directly adjacent to a flame), inert, N2, Argon,
air,
reduced pressure, ambient pressure, elevated pressure, flowing gas (e.g.,
sweep
gas, having a flow rate for example of from about from about 15.0 GHSV to
about
0.1 GHSV, from about 6.3 GHSV to about 3.1 GHSV, and at about 3.9 GHSV),
static gas, and combinations and variations of these.
[00213] The pyrolysis is conducted over a time period that preferably
results in the complete pyrolysis of the preform. For high purity materials,
the
furnace, containers, handling equipment, and other components of the pyrolysis
apparatus are clean, essentially free from, free from and do not contribute
any
elements or materials, that would be considered impurities or contaminants, to
the pyrolized material. A constant flow rate of "sweeping" gas can help purge
the furnace during volatile generation. In an embodiment, the pyrolysis
environment, e.g., the furnace, the atmosphere, the container and combinations
and variations of these, can have materials that contribute to or effect, for
example, the composition, stoichiometry, features, performance and
combinations and variations of these in the ceramic and the final applications
or
products.
[00214] During pyrolysis material may be lost through off gassing. The
amount of material remaining at the end of a pyrolysis step, or cycle, is
referred
to as char yield (or pyrolysis yield). The formulations and polysilocarb
precursor
formulations of embodiments of the present formulations can have char yields
for
SiOC formation of at least about 60%, about 70%, about 80%, and at least about
90%, at least about 91`)/0 and greater. In fact, with air pyrolysis the
materials may
have char yields well above 91`)/0, which can approach 100%. In order to avoid
the degradation of the material in an air pyrolysis (noting that typically
pyrolysis is
conducted in inert atmospheres, reduced oxygen atmosphere, essentially inert
atmosphere, minimal oxygen atmospheres, and combinations and variations of
these) specifically tailored formulations can be used. For example,
formulations
high in phenyl content (at least about 11`)/0, and preferably at least about
20% by
67
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
weight phenyls), formulations high in allyl content (at least about 15% to
about
60%) can be used for air pyrolysis to mitigate the degradation of the
material.
[00215] The initial or first pyrolysis step for SiOC formation, in some
embodiments and for some uses, generally yields a structure that is not very
dense, and for example, may not reached the density required for its intended
use. However, in some examples, such as the use of lightweight spheres,
proppants, pigments, and others, the first pyrolysis may be, and is typically
sufficient. Thus, generally a reinfiltration process may be performed on the
pyrolized material, to add in additional polysilocarb precursor formulation
material, to fill in, or fill, the voids and spaces in the structure. This
reinfiltrated
material may then be cured and repyrolized. (In some embodiments, the
reinfiltrated materials is cured, but not pyrolized.) This process of
pyrolization,
reinfiltration may be repeated, through one, two, three, and up to 10 or more
times to obtain the desired density of the final product.
[00216] In some embodiments, upon pyrolization, graphenic, graphitic,
amorphous carbon structures and combinations and variations of these are
present in the Si-O-C ceramic. A distribution of silicon species, consisting
of
SiOxCy structures, which result in 5iO4, SiO3C, SiO2C2, Si0C3, and SiC4 are
formed in varying ratios, arising from the precursor choice and their
processing
history. Carbon is generally bound between neighboring carbons and/or to a
Silicon atom. In general, in the ceramic state, carbon is largely not
coordinated
to an oxygen atom, thus oxygen is largely coordinated to silicon
[00217] The pyrolysis may be conducted in any heating apparatus
that maintains the request temperature and environmental controls. Thus, for
example pyrolysis may be done with gas fired furnaces, electric furnaces,
direct
heating, indirect heating, fluidized beds, kilns, tunnel kilns, box kilns,
shuttle kilns,
coking type apparatus, lasers, microwaves, and combinations and variations of
these and other heating apparatus and systems that can obtain the request
temperatures for pyrolysis.
[00218] Custom and predetermined control of when chemical reactions,
arrangements and rearrangements, occur in the various stages of the process
68
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
from raw material to final end product can provide for reduced costs,
increased
process control, increased reliability, increased efficiency, enhanced product
features, increased purity, and combinations and variation of these and other
benefits. The sequencing of when these transformations take place can be
based upon the processing or making of precursors, and the processing or
making of precursor formulations; and may also be based upon cure and
pyrolysis conditions. Further, the custom and predetermined selection of these
steps, formulations and conditions, can provide enhanced product and
processing features through the various transformations, e.g., chemical
reactions; molecular arrangements and rearrangements; and microstructure
arrangements and rearrangements.
[00219] At various points during the manufacturing process, the polymer
derived ceramic structures, e.g., polysilocarb structures, intermediates and
end
products, and combinations and variations of these, may be machined, milled,
molded, shaped, drilled, etched, or otherwise mechanically processed and
shaped.
[00220] Starting materials, precursor formulations, polysilocarb
precursor formulations, as well as, methods of formulating, making, forming,
curing and pyrolizing, precursor materials to form polymer derived materials,
structures and ceramics, are set forth in Published US Patent Applications,
Publication Nos. 2014/0343220, 2014/0274658, and 2014/0326453, and US
Patent Applications, Serial Nos. 61/946,598, 62/055,397 and 62/106,094, the
entire disclosures of each of which are incorporated herein by reference.
[00221] In preferred embodiments of the polysilocarb derived ceramics
the amounts of Si, 0, C for the total amount of ceramic are set forth in the
Table
1.
69
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
[00222] Table 1
si o C
Lo Hi Lo Hi Lo Hi
Wt% 35.00% 50.00% 10.00% 35.00% 5.00% 30.00%
Mole Ratio 1.000 1.429 0.502 1.755 0.334 2.004
Mole % 15.358% 63.095% 8.821% 56.819% 6.339% 57.170%
[00223] In general, embodiments of the pyrolized polysilocarb ceramics
can have about 30% to about 60% Si, can have about 5% to about 40% 0, and
can have about 3% to about 35% carbon. Greater and lesser amounts are also
contemplated.
[00224] The type of carbon present in preferred embodiments of the
polysilocarb derived ceramics can be free carbon, (e.g., turbostratic,
amorphous,
graphenic, graphitic forms of carbon) and Carbon that is bound to Silicon.
Embodiments having preferred amounts of free carbon and Silicon-bound-
Carbon (Si-C) are set forth in Table 2.
[00225] Table 2
Embodiment % Free Carbon % Si-C type
1 64.86 35.14
2 63.16 36.85
3 67.02 32.98
4 58.59 41.41
65.70 31.66
6 62.72 30.82
7 61.68 34.44
8 69.25 27.26
9 60.00 27.54
[00226] Generally, embodiments of polysilocarb derived ceramics can
have from about 20% free carbon to about 80% free carbon, and from about 20%
Si-C bonded carbon to about 80% Si-C bonded carbon. Greater and lesser
amounts are also contemplated.
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
[00227] Typically, embodiments of the pyrolized polysilocarb ceramics
can have other elements present, such as Nitrogen and Hydrogen.
Embodiments can have the amounts of these other materials as set out in Table
3. (Note that these are typical for embodiments of net materials. If fillers,
additives, or other materials are combined with or into the precursor
formulation;
then such materials can generally be present to a greater or lesser extent in
the
pyrolized ceramic material)
[00228] Table 3
H N
Lo Hi Lo Hi
Wt% 0.00% 2.20% 0% 2%
Mole Ratio 0.000 1.751 0 0.1
Mole % 0.000% 48.827% 0% 3%
[00229] Turning to FIGS. 1A to 1D there are provided various
embodiment of configurations of structural components that are made from
polysilocarb materials. Depending upon the performance requirements, e.g.,
load, stress, strain, impact, environmental, etc., for these members, they can
be
hard cured polysilocarb materials, pyrolized polysilocarb materials, pyrolized
materials that are reinfiltrated to a very high density, filled and unfilled
polysilocarb materials, ready to press sintered SiC (which is derived from
polysilocarb materials as disclosed and taught in US Patent Application Serial
No. 62/055,397, 62/055,461 and 62/112,025, the entire disclosures of each of
which are incorporated herein by reference) and combinations, variations, and
composites of these and other materials, e.g., metal, steel, aluminum, metal
matrix complexes, plastic, carbon fiber, and wood, among others materials. In
FIG. 1A there is shown an embodiment of a column 100 having a web 101 and
an I-beam 102 that is affixed to the column 100 by connection 103. In FIG. 1B
there is shown an embodiment of an I-beam joint 104, in which two I-beams 105,
106 are affixed by connection or joint 107. I-beam 105 has web 108 and and top
flange 120 and bottom flange 121. I-beam 106 has web 109 and top and bottom
flanges (not numbered). In FIG. 1C there is provided an embodiment of a
71
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
column and I-beam configuration 110, having a central square column or post
111 that has an open central space 112. I-beams 113a, 113b, 113c, 113d are
affixed to the post 111. In FIG. 1D there is provided an embodiment of a
column
and beam configuration 114 having a circular central column or post 115,
having
a central circular opening 116. I-beams 116a, 116b, 116c are attached to the
column 115. The configurations can be attached or affixed by know means such
as metal, composite, or polysilocarb flanges and bolts and other known
techniques for connecting structural members.
[00230] Turning to FIG. 2 there is shown an embodiment of building
structural members 200. Depending upon the performance requirements, e.g.,
load, stress, strain, impact, environmental, etc., for these members, they can
be
hard cured polysilocarb materials, pyrolized polysilocarb materials, pyrolized
materials that are reinfiltrated to a very high density, filled and unfilled
polysilocarb materials, ready to press sintered SiC (which is derived from
polysilocarb materials as disclosed and taught in US Patent Application Serial
No. 62/055,397, 62/055,461 and 62/112,025, the entire disclosures of each of
which are incorporated herein by reference) and combinations, variations, and
composites of these and other materials, e.g., metal, steel, aluminum, metal
matrix complexes, plastic, carbon fiber, and wood, among others materials. The
configurations can be attached or affixed by know means such as metal,
composite, or polysilocarb flanges and bolts and other known techniques for
connecting structural members. The building support members 200 have a
column 201, having a web 203 that is on and affixed to a base or pad 202.
Girts
204, 205 (for holding side walls not shown, which can also be made from
polysilocarb materials) are attached to column 201. Roof cross I-beam 206 is
attached to and supported by column 201. Roof purlins 207, 208, 209 are
attached to cross I-beam 206, and support roof panels or members not shown,
which can also be made from polysilocarb materials).
[00231] Turning to FIGS 3A and 3B there is provided an embodiment of
an armored vehicle 300 having a front 301. The vehicle can have body panels or
armor plates incorporated into or on body panels that prevent or mitigate
ballistic
72
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
objects, e.g., bullets, projectiles, and shrapnel, and an explosion's energy
and
force. Depending upon the performance requirements, e.g., load, stress,
strain,
impact, environmental, etc., for these members, they can be hard cured
polysilocarb materials, pyrolized polysilocarb materials, pyrolized materials
that
are reinfiltrated to a very high density, filled and unfilled polysilocarb
materials,
ready to press sintered SiC (which is derived from polysilocarb materials as
disclosed and taught in US Patent Application Serial No. 62/055,397,
62/055,461
and 62/112,025, the entire disclosures of each of which are incorporated
herein
by reference) and combinations, variations, and composites of these and other
materials, e.g., metal, steel, aluminum, metal matrix complexes, plastic,
carbon
fiber, and wood, among others materials. In the embodiment of armored vehicle
300 there is a lower polysilocarb based ballistic plate 305, which protects
the
bottom of the vehicle, there are polysilocarb based door panels 302, 303,
polysilocarb quarter panel 304 and polysilocarb hood assembly 307.
[00232] Turning to FIG. 4, there is provided an embodiment of a
polysilocarb mobile building 400 that can serve as for example a house,
dwelling,
office, business or other purpose. Depending upon the performance
requirements, e.g., load, stress, strain, impact, environmental, etc., for the
building, its panels and structural members, they can be hard cured
polysilocarb
materials, pyrolized polysilocarb materials, pyrolized materials that are
reinfiltrated to a very high density, filled and unfilled polysilocarb
materials, ready
to press sintered SiC (which is derived from polysilocarb materials as
disclosed
and taught in US Patent Application Serial No. 62/055,397, 62/055,461 and
62/112,025, the entire disclosures of each of which are incorporated herein by
reference) and combinations, variations, and composites of these and other
materials, e.g., metal, steel, aluminum, metal matrix complexes, plastic,
carbon
fiber, and wood, among others materials. The mobile building 400 has an upper
side walls 401a, 401b (for an upper story, or second floor) having a window
402,
and lower side walls 401c, 401d (for a lower story or first floor). The
building 400
has a door 403 and awning 404.
73
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00233] These building can be manufacture or readily assembled at a
site or location, e.g., a disaster site. The polysilocarb building can be,
among
other benefits over convention buildings, lighter, and preferably
substantially
lighter than a comparable building made from steel.
[00234] Turning to FIG. 5 there is provided an embodiment of a
polysilocarb shipping container 500. Depending upon the performance
requirements, e.g., load, stress, strain, impact, environmental, etc., for the
shipping container, its panels, lifting lugs, locking devices, and structural
members, they can be hard cured polysilocarb materials, pyrolized polysilocarb
materials, pyrolized materials that are reinfiltrated to a very high density,
filled
and unfilled polysilocarb materials, ready to press sintered SiC (which is
derived
from polysilocarb materials as disclosed and taught in US Patent Application
Serial No. 62/055,397, 62/055,461 and 62/112,025, the entire disclosures of
each of which are incorporated herein by reference) and combinations,
variations, and composites of these and other materials, e.g., metal, steel,
aluminum, metal matrix complexes, plastic, carbon fiber, and wood, among
others materials. The shipping container 500 has a roof plate 501, a sidewall
502, a base 503, and lifting openings or lugs 504, 505 that are formed in the
base 503. The container 500 has doors 506, 507 that have locking mechanism
508. The polysilocarb container can be, among other benefits over conventional
container, lighter, and preferably is substantially lighter than a comparable
container made from steel.
[00235] Turning to FIG. 6 there is provided an embodiment of a
multilayer building panel 600. One, two, three or all of the layers, 602, 603,
604,
605, 606, 607, 608, 609, depending upon the performance requirements, e.g.,
load, stress, strain, fire resistance, impact, environmental, etc., for the
panel, the
layers can be hard cured polysilocarb materials, pyrolized polysilocarb
materials,
pyrolized materials that are reinfiltrated to a very high density, filled and
unfilled
polysilocarb materials, ready to press sintered SiC (which is derived from
polysilocarb materials as disclosed and taught in US Patent Application Serial
No. 62/055,397, 62/055,461 and 62/112,025, the entire disclosures of each of
74
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
which are incorporated herein by reference) and combinations, variations, and
composites of these and other materials, e.g., metal, steel, aluminum, metal
matrix complexes, plastic, carbon fiber, paper, and wood, among others
materials. Further, the surfaces 601 and 610 can be coated with a polysilocarb
material.
[00236] Turning to FIG. 7 there is provided an embodiment of body
armor 700, e.g., a bullet proof vest, which is made from ballistic
polysilocarb
based materials. The body armor 700 has members that prevent or mitigate
ballistic objects, e.g., bullets, projectiles, and shrapnel, and an
explosion's energy
and force. Depending upon the performance requirements, e.g., load, stress,
strain, impact, environmental, etc., for these members, they can be hard cured
polysilocarb materials, pyrolized polysilocarb materials, pyrolized materials
that
are reinfiltrated to a very high density, filled and unfilled polysilocarb
materials,
ready to press sintered SiC (which is derived from polysilocarb materials as
disclosed and taught in US Patent Application Serial No. 62/055,397,
62/055,461
and 62/112,025, the entire disclosures of each of which are incorporated
herein
by reference) and combinations, variations, and composites of these and other
materials, e.g., metal, steel, aluminum, metal matrix complexes, plastic,
carbon
fiber, and wood, among others materials.
[00237] Turning to FIG. 8 there is provided an embodiment of a
polysilocarb rope 800 made from polysilocarb based fibers 801, 802. Depending
upon the performance requirements, e.g., load, stress, strain, impact,
environmental, etc., for the rope, the fibers can be hard cured polysilocarb
materials, pyrolized polysilocarb materials, pyrolized materials that are
reinfiltrated to a very high density, filled and unfilled polysilocarb
materials, ready
to press sintered SiC (which is derived from polysilocarb materials as
disclosed
and taught in US Patent Application Serial No. 62/055,397, 62/055,461 and
62/112,025, the entire disclosures of each of which are incorporated herein by
reference) and combinations, variations, and composites of these and other
materials, e.g., metal, steel, metal matrix complexes, plastic, nylon, carbon
fiber,
and natural fibers, among others materials.
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00238] Turning to FIG. 9 there is provided an embodiment of a trailer
900 having a polysilocarb based frame 901. Depending upon the performance
requirements, e.g., load, stress, strain, impact, environmental, etc., for the
trailer,
the frame can be hard cured polysilocarb materials, pyrolized polysilocarb
materials, pyrolized materials that are reinfiltrated to a very high density,
filled
and unfilled polysilocarb materials, ready to press sintered SiC (which is
derived
from polysilocarb materials as disclosed and taught in US Patent Application
Serial No. 62/055,397, 62/055,461 and 62/112,025, the entire disclosures of
each of which are incorporated herein by reference) and combinations,
variations, and composites of these and other materials, e.g., metal, steel,
aluminum, metal matrix complexes, plastic, carbon fiber, and wood, among
others materials.
[00239] Turning to FIG. 10 there is provided an embodiment of a
abrasion device 1000, e.g., a grinding wheel. The abrasive medium as well as
the support or structural component of the device can be made from, or based
upon polysilocarbs. Depending upon the performance requirements, e.g., load,
stress, strain, impact, environmental, etc., for the abrasive members and the
supporting material can be hard cured polysilocarb materials, pyrolized
polysilocarb materials, pyrolized materials that are reinfiltrated to a very
high
density, filled and unfilled polysilocarb materials, ready to press sintered
SiC
(which is derived from polysilocarb materials as disclosed and taught in US
Patent Application Serial No. 62/055,397, 62/055,461 and 62/112,025, the
entire
disclosures of each of which are incorporated herein by reference) and
combinations, variations, and composites of these and other materials, e.g.,
metal, steel, aluminum, metal matrix complexes, plastic, carbon fiber, natural
fibers, and wood, among others materials.
[00240] Turning to FIG. 11 there is provided an embodiment of a
abrasion device 1100, e.g., a belt grinder, which has a drive wheel 1101 that
drives a grinding belt 1102 having polysilocarb based abrasives. Depending
upon the performance requirements for the abrasives, e.g., load, temperature,
material to be ground, etc., the abrasive materials can be pyrolized
polysilocarb
76
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
materials, pyrolized materials that are reinfiltrated to a very high density,
filled
and unfilled polysilocarb materials, ready to press sintered SiC (which is
derived
from polysilocarb materials as disclosed and taught in US Patent Application
Serial No. 62/055,397, 62/055,461 and 62/112,025, the entire disclosures of
each of which are incorporated herein by reference) and combinations,
variations, and composites of these and other materials, e.g., metal, grit,
diamond, and grinding and abrasive materials.
[00241] Turning to FIG. 11 there is provided an embodiment of a
polysilocarb tubular 1200. The tubular 1200 has an outer surface 1201, inner
surface 1202, a circular wall 1203, and an opening or passage 1204. Depending
upon the performance requirements, e.g., load, stress, strain, impact,
environmental, etc., for the tubular it can be hard cured polysilocarb
materials,
pyrolized polysilocarb materials, pyrolized materials that are reinfiltrated
to a very
high density, filled and unfilled polysilocarb materials, ready to press
sintered SiC
(which is derived from polysilocarb materials as disclosed and taught in US
Patent Application Serial No. 62/055,397, 62/055,461 and 62/112,025, the
entire
disclosures of each of which are incorporated herein by reference) and
combinations, variations, and composites of these and other materials, e.g.,
metal, steel, aluminum, metal matrix complexes, plastic, and carbon fiber,
among
others materials.
[00242] Turning to FIG. 13 there is provided an embodiment of an
automotive break assembly. The break assembly has a piston 1301, a caliper
1302, a first break pad 1303a, a second break pad 1303b, a rotor 1304, a hub
1305 and a break line 1306. In addition to the pads 1303a, 1303b and the rotor
1304 the other components of the break assembly can also be made from
polysilocarb based materials. Depending upon the performance requirements,
e.g., load, stress, strain, impact, environmental, etc., for the components
can be
hard cured polysilocarb materials, pyrolized polysilocarb materials, pyrolized
materials that are reinfiltrated to a very high density, filled and unfilled
polysilocarb materials, ready to press sintered SiC (which is derived from
polysilocarb materials as disclosed and taught in US Patent Application Serial
77
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
No. 62/055,397, 62/055,461 and 62/112,025, the entire disclosures of each of
which are incorporated herein by reference) and combinations, variations, and
composites of these and other materials, e.g., metal, steel, aluminum, metal
matrix complexes, plastic, and carbon fiber, among others materials.
[00243] Examples
[00244] The following examples are provided to illustrate various
embodiments of processes, precursors, polysilocarb formulations, prepregs,
cured preforms, applications, apparatus, equipment, devices and ceramics of
the
present inventions. These examples are for illustrative purposes, and should
not
be viewed as, and do not otherwise limit the scope of the present inventions.
The
percentages used, unless specified otherwise, are weight percents of the total
formulation, preform or structure.
[00245] EXAMPLE 1
[00246] A polysilocarb formulation using the mixing type method is
formulated. The formulation is made by mixing together at room temperature
70% of the MHF precursor and a molecular weight of about 800 and 30% of the
allyl terminated precursor having a molecular weight of about 500 in a vessel
and
placing in storage for later use. The polysilocarb formulation has good shelf
life
and room temperature and the precursors have not, and do not react with each
other. The polysilocarb formulation has a viscosity of about 12 cps.
[00247] EXAMPLE 2
[00248] A polysilocarb formulation using the mixing type method is
formulated. The formulation is made by mixing together at room temperature
60% of the MHF precursor and a molecular weight of about 800 and 40% of the
vinyl terminated precursor having a molecular weight of about 9,400 in a
vessel
and placing in storage for later use. The polysilocarb formulation has good
shelf
life and room temperature and the precursors have not, and do not react with
each other. The polysilocarb formulation has a viscosity of about 200 cps.
[00249] EXAMPLE 3
[00250] A polysilocarb formulation using the mixing type method is
formulated. The formulation is made by mixing together 50% of the MH
78
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
precursor having a molecular weight of about 800 and 50% of the vinyl
terminated precursor having a molecular weight of about 800 in a vessel and
placing in storage for later use. The polysilocarb formulation has good shelf
life
and room temperature and the precursors have not, and do not react with each
other. The polysilocarb formulation has a viscosity of about 55 cps.
[00251] EXAMPLE 4
[00252] A polysilocarb formulation using the mixing type method is
formulated. The formulation is made by mixing together 40% of the MH
precursor having a molecular weight of about 1,000 and 60% of the vinyl
terminated precursor having a molecular weight of about 500 in a vessel and
placing in storage for later use. The polysilocarb formulation has good shelf
life
and room temperature and the precursors have not, and do not react with each
other. The polysilocarb formulation has a viscosity of about 25 cps.
[00253] EXAMPLE 5
[00254] A polysilocarb formulation using the mixing type method is
formulated. The formulation is made by mixing together 30% of the MHF
precursor having a molecular weight of about 800 and 70% of the vinyl
terminated precursor having a molecular weight of about 500 in a vessel and
placing in storage for later use. The polysilocarb formulation has good shelf
life
and room temperature and the precursors have not, and do not react with each
other. The polysilocarb formulation has a viscosity of about 10 cps.
[00255] EXAMPLE 6
[00256] The polysilocarb formulation of Example 1 has 40% of an about
80 micron to about 325 mesh SiC filler added to the formulation to make a
filled
polysilocarb formulation, which can be kept for later use.
[00257] EXAMPLE 7
[00258] The polysilocarb formulation of Example 2 has 30 % of an about
80 micron to about 325 mesh SiC filler added to the formulation to make a
filled
polysilocarb formulation, which can be kept for later use.
79
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00259] EXAMPLE 8
[00260] A polysilocarb formulation using the mixing type method is
formulated. The formulation is made by mixing together 10% of the MHF
precursor haivng a molecular weight of about 800 and 73% of the styrene
(phenylethyl) precursor (having 10% X) and a molecular weight of about 1,000,
and 16% of the TV precursor, and 1`)/0 of the OH terminated precursor, having
a
molecular weight of about 1,000 in a vessel and placing in storage for later
use.
The polysilocarb formulation has good shelf life and room temperature and the
precursors have not, and do not react with each other. The polysilocarb
formulation has a viscosity of about 72 cps.
[00261] EXAMPLE 9
[00262] A polysilocarb formulation using the mixing type method is
formulated. The formulation is made by mixing together 0-90% of the MH
precursor having a molecular weight of about 800, and 0-90% of the styrene
precursor (having 10% X) and a molecular weight of about 1000, and 0-30% of
the TV precursor, and 0-30% of the vinyl terminated precursor having a
molecular weight of about 9400 and 0-20% of the OH terminated precursor,
having a molecular weight of about 800 in a vessel and placing in storage for
later use. The polysilocarb formulation has good shelf life and room
temperature
and the precursors have not, and do not react with each other. The
polysilocarb
formulation has a viscosity of about 100 cps.
[00263] EXAMPLE 10
[00264] A polysilocarb formulation using the mixing type method is
formulated. The formulation is made by mixing together 70% of the MHF
precursor and 30% of the vinyl terminated precursor having a molecular weight
of
about 500 and about 42% of a submicron and a 325 mesh silica in a vessel and
placing in storage for later use. The polysilocarb formulation has good shelf
life
and room temperature and the precursors have not, and do not react with each
other. The polysilocarb formulation has a viscosity of about 300 cps.
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00265] EXAMPLE 11
[00266] A polysilocarb formulation using the mixing type method is
formulated. The formulation is made by mixing together 20-80% of the MH
precursor having a molecular weight of about 800, and 0-10% of the TV
precursor, and 5-80% of the vinyl terminated precursor having a molecular
weight of about and about 500 of submicron, 325 mesh, and 8 micron SiC in a
vessel and placing in storage for later use. The polysilocarb formulation has
good shelf life and room temperature and the precursors have not, and do not
react with each other. The polysilocarb formulation has a viscosity of about
300
cps.
[00267] EXAMPLE 12
[00268] 40 ppm of a platinum catalyst is added to the polysilocarb
formulation of Example 6 and these catalyzed formulations are added drop wise
(e.g., drops of the formulation are dropped into) to a 50-120 C hot water
bath to
cure the formulation. The time in the hot water bath was about 1-2 minutes.
The
cured drop structures were then pyrolized at 950 C for about 60 minutes. The
pyrolized structures were hollow spheres with densities of less than about 1
g/cc,
diameters of about 60 microns to about 2 mm, and crush strengths of about 0.5
¨
2 ksi.
[00269] EXAMPLE 13
[00270] A precursor formulation of having 75% MHF, 15% TV, and 10%
VT is formed using the mixing type process and stored.
[00271] EXAMPLE 14a
[00272] 1 % catalyst (10 ppm platinum and 0.5% LUPEROX 231
peroxide) is added to the precursor formulation of Example 13. The catalyzed
precursor is then impregnated into a reinforcing material and cured to form a
composite.
[00273] EXAMPLE 14b
[00274] The cured material of Example 14a is pyrolized to form a
polysilocarb derived ceramic composite material.
81
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00275] EXAMPLE 14c
[00276] 1`)/0 catalyst (10 ppm platinum and 0.5% LUPEROX 231
peroxide) is added to the precursor formulation of Example 13. Using a tower
forming and cure system, the catalyzed polysilocarb formulation is formed from
a
sonic nozzle having an internal diameter of 0.180 inches into droplets that
fall
from the nozzle into and through an 8 foot curing tower. The temperature at
the
top of the tower is from 495-505 C the temperature at the bottom of the tower
is
650 C. There are no discrete temperature zones in the tower. Airflow up the
tower is by convection. The collection pan is maintained at 110 C. The
forming
and curing are done in air. The preform beads are removed from the pan and
post (hard) cured at 200 C in air for 2 hours. The hard cured preform
proppants
are pyrolized at 1000 C in an argon atmosphere for 2 hours. The cure yield is
from 99% to 101%. The char yield is 86%.
[00277] EXAMPLE 14d
[00278] 1`)/0 catalyst (10 ppm platinum and 0.5% LUPEROX 231
peroxide) is added to the formulation of Example 13, and the polysilocarb
formulation is formed into a prepreg having carbon fiber reinforcement. The
prepreg curing is done in Argon and at 200 C for 2 hours. The hard cured
preform are pyrolized at 1000 C under vacuum for 5 hours.
[00279] EXAMPLE 15
[00280] A polysilocarb precursor formulation having 70% MHF, 20% TV,
and 10% VT is formed using the mixing type process and placed in a container.
[00281] EXAMPLE 16a
[00282] 1`)/0 catalyst (10 ppm platinum and 0.5% LUPEROX 231
peroxide) is added to the precursor formulation of Example 15. The catalyzed
precursor is then impregnated into a reinforcing material and cured to form a
composite.
[00283] EXAMPLE 16b
[00284] The cured material of Example 16a is pyrolized to form a
polysilocarb derived ceramic composite material.
82
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
[00285] EXAMPLE 16c
[00286] 1`)/0 catalyst (10 ppm platinum and 0.5% LUPEROX 231
peroxide) is added to the formulation of Example 15, and the polysilocarb
formulation is formed into a prepreg having carbon fiber reinforcement. The
prepreg curing is done in Argon and at 200 C for 2 hours. The hard cured
preform are pyrolized at 1000 C under vacuum for 5 hours.
[00287] EXAMPLE 17
[00288] Using a tower forming and cure system, a polysilocarb
formulation from the mixing type process and having 70% MHF, 20% TV, 10%
VT and 1`)/0 catalyst (10 ppm platinum and 0.5% LUPEROX 231 peroxide) is
formed from a sonic nozzle having an internal diameter of 0.180 inches into
droplets that fall from the nozzle into and through an 8 foot curing tower.
The
temperature at the top of the tower is from 495-505 C the temperature at the
bottom of the tower is 650 C. There are no discrete temperature zones in the
tower. Airflow up the tower is by convection. The collection pan is maintained
at
110 C. The forming and curing are done in air. The preform proppants are
removed from the pan and post (hard) cured at 200 C in air for 2 hours. The
hard cured preform beads are pyrolized at 1000 C under vacuum for 2 hours.
The cure yield is from 99% to 101%. The char yield is 86%.
[00289] EXAMPLE 18a
[00290] The pyrolized preform of Example 16c, is infused with a
polysiloxane precursor formulation and pyrolized.
[00291] EXAMPLE 18b
[00292] The pyrolized preform of Example 18a, is infused with a
polysiloxane precursor formulation and pyrolized.
[00293] EXAMPLE 19
[00294] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 61 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of
Moles Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
83
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
Methyltriethoxysilane (FIG.
37)
120.00 19.5% 178.30 0.67 47.43% 0.67 2.02
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00% -
-
Dimethyldiethoxysilane (FIG.
42)
70.00 11.4% 148.28 0.47 33.27% 0.47 0.94
Methyldiethoxysilane (FIG. 39) 20.00 3.3% 134.25 0.15
10.50% 0.15 0.30
Vinylmethyldiethoxysilane
(FIG. 40) 20.00 3.3% 160.29 0.12 8.79%
0.12 0.25
Trimethyethoxysilane (FIG. 48) 0.00 0.0% 118.25 - 0.00% -
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 320.00 52.0% 58.08 5.51
Ethanol in hydrolyzer 0.00 0.0% 46.07 -
Water in hydrolyzer 64.00 10.4% 18.00 3.56
HCI 0.36 0.1% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00295] EXAMPLE 20
[00296] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 72 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane
of Si of Et0H
Phenyltriethoxysilane (FIG. 45) 234.00 32.0% 240.37 0.97
54.34% 0.97 2.92
Phenylmethyldiethoxysilane
(FIG. 38) 90.00 12.3% 210.35 0.43 23.88%
0.43 0.86
Dimethyldiethoxysilane (FIG.
42) 0.00 0.0% 148.28 - 0.00% - -
Methyldiethoxysilane (FIG. 39) 28.50 3.9% 134.25 0.21
11.85% 0.21 0.42
Vinylmethyldiethoxysilane (FIG.
40) 28.50 3.9% 160.29 0.18 9.93%
0.18 0.36
Trimethyethoxysilane (FIG. 48) 0.00 0.0% 118.25 - 0.00% -
Acetone in hydrolyzer 0.00 0.0% 58.08 -
Ethanol in hydrolyzer 265.00 36.3% 46.07 5.75
Water in hydrolyzer 83.00 11.4% 18.00 4.61
HCI 0.36 0.0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00297] EXAMPLE 21
[00298] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 61 C for 21 hours.
84
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 142.00 21.1% 240.37 0.59
37.84% 0.59 1.77
Phenylmethyldiethoxysilane
(FIG. 38) 135.00 20.1% 210.35 0.64
41.11% 0.64 1.28
Dimethyldiethoxysilane (FIG.
42) 0.00 0.0% 148.28 - 0.00% -
-
Methyldiethoxysilane (FIG. 39) 24.00 3.6% 134.25 0.18
11.45% 0.18 0.36
Vinylmethyldiethoxysilane
(FIG. 40) 24.00 3.6% 160.29 0.15 9.59%
0.15 0.30
Trimethyethoxysilane (FIG. 48) 0.00 0.0% 118.25 - 0.00% -
-
Acetone in hydrolyzer 278.00 41.3% 58.08 4.79
Ethanol in hydrolyzer 0.00 0.0% 46.07 -
Water in hydrolyzer 69.00 10.2% 18.00 3.83
HCI 0.36 0.1% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00299] EXAMPLE 22
[00300] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 72 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Methyltriethoxysilane (FIG.
37) 0.00 0.0% 178.30 -
0.00% - -
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00% -
-
Dimethyldiethoxysilane (FIG.
42) 56
7.2% 148.28 0.38 17.71% 0.38 0.76
Methyldiethoxysilane (FIG. 39) 182 23.2% 134.25 1.36
63.57% 1.36 2.71
Vinylmethyldiethoxysilane
(FIG. 40) 64 8.2% 160.29 0.40 18.72%
0.40 0.80
Triethoxysilane (FIG. 44) 0.00 0.0% 164.27 - 0.00% -
-
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 0.00 0.0% 58.08 -
Ethanol in hydrolyzer 400.00 51.1% 46.07 8.68
Water in hydrolyzer 80.00 10.2% 18.00 4.44
HCI 0.36 0.0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
[00301] EXAMPLE 23
[00302] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 61 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 198.00 26.6% 240.37 0.82
52.84% 0.82 2.47
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00% - -
Dimethyldiethoxysilane (FIG.
42)
109.00 14.6% 148.28 0.74 47.16% 0.74 1.47
Methyldiethoxysilane (FIG. 39) 0.00 0.0% 134.25 - 0.00% -
-
Vinylmethyldiethoxysilane
(FIG. 40) 0.00 0.0% 160.29 - 0.00% - -
Trimethyethoxysilane (FIG. 48) 0.00 0.0% 118.25 - 0.00% -
-
Acetone in hydrolyzer 365.00 49.0% 58.08 6.28
Ethanol in hydrolyzer 0.00 0.0% 46.07 -
Water in hydrolyzer 72.00 9.7% 18.00 4.00
HCI 0.36 0.0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00303] EXAMPLE 24
[00304] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 72 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 180.00 22.7% 240.37 0.75
44.10% 0.75 2.25
Phenylmethyldiethoxysilane
(FIG. 38) 50.00 6.3% 210.35 0.24 14.00%
0.24 0.48
Dimethyldiethoxysilane (FIG.
42)
40.00 5.0% 148.28 0.27 15.89% 0.27 0.54
Methyldiethoxysilane (FIG. 39) 30.00 3.8% 134.25 0.22
13.16% 0.22 0.45
Vinylmethyldiethoxysilane
(FIG. 40) 35.00 4.4% 160.29 0.22 12.86%
0.22 0.44
Trimethyethoxysilane (FIG. 48) 0.00 0.0% 118.25 - 0.00% -
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 0.00 0.0% 58.08 -
Ethanol in hydrolyzer 380.00 48.0% 46.07 8.25
Water in hydrolyzer 76.00 9.6% 18.00 4.22
86
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
HCI 0.36 0.0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00305] EXAMPLE 25
[00306] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 72 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 190.00 23.8% 240.37 0.79
47.22% 0.79 2.37
Phenylmethyldiethoxysilane
(FIG. 38) 75.00 9.4% 210.35 0.36 21.30%
0.36 0.71
Dinnethyldiethoxysilane
(FIG. 42) 45.00 5.6% 148.28 0.30 18.13%
0.30 0.61
Methyldiethoxysilane (FIG.
39)
30.00 3.8% 134.25 0.22 13.35% 0.22 0.45
Vinylnnethyldiethoxysilane
(FIG. 40) 0.00 0.0% 160.29 - 0.00% -
-
Trinnethyethoxysilane (FIG.
48) 0.00 0.0% 118.25 - 0.00%
-
-
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 0.00 0.0% 58.08 -
Ethanol in hydrolyzer 380.00 47.7% 46.07 8.25
Water in hydrolyzer 76.00 9.5% 18.00 4.22
HCI 0.36 0.0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00307] EXAMPLE 26
[00308] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 61 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 0.00 0.0% 240.37 - 0.00%
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00% - -
Dimethyldiethoxysilane (FIG.
42) 235.00 31.5% 148.28 1.58 83.32%
1.58 3.17
Methyldiethoxysilane (FIG. 39) 0.00 0.0% 134.25 - 0.00% -
Vinylmethyldiethoxysilane
(FIG. 40) 0.00 0.0% 160.29 - 0.00% - -
87
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
TES 40 (FIG. 35) 66.00 8.8% 208.00 0.32 16.68%
0.32 1.27
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 370.00 49.6% 58.08 6.37
Ethanol in hydrolyzer 0.00 0.0% 46.07 -
Water in hydrolyzer 74.00 9.9% 18.00 4.11
HCI 0.36 0.0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00309] EXAMPLE 27
[00310] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 61 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane of
Si of Et0H
Phenyltriethoxysilane (FIG. 45) 0.00 0.0% 240.37 - 0.00%
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00% -
-
Dimethyldiethoxysilane (FIG.
42)
95.00 11.8% 148.28 0.64 34.95% 0.64 1.28
Methyldiethoxysilane (FIG. 39) 60.80 7.6% 134.25 0.45 24.71%
0.45 0.91
Vinylmethyldiethoxysilane
(FIG. 40) 73.15 9.1% 160.29 0.46 24.90%
0.46 0.91
TES 40 (FIG. 35) 58.90 7.3% 208.00 0.28 15.45%
0.28 1.13
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 430.00 53.4% 58.08 7.40
Ethanol in hydrolyzer 0.00 0.0% 46.07 -
Water in hydrolyzer 86.00 10.7% 18.00 4.78
HCI 0.36 0.0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00311] EXAMPLE 28
[00312] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 61 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane of
Si of Et0H
Phenyltriethoxysilane (FIG. 45) 0.00 0.0% 240.37 - 0.00%
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00% -
-
Dimethyldiethoxysilane (FIG.
42)
140.00 17.9% 148.28 0.94 58.38% 0.94 1.89
88
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
Methyldiethoxysilane (FIG. 39) 0.00 0.0% 134.25 - 0.00% -
-
Vinylmethyldiethoxysilane
(FIG. 40) 0.00 0.0% 160.29 - 0.00% - -
TES 40 (F(G. 35) 140.00 17.9% 208.00 0.67 41.62%
0.67 2.69
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 420.00 53.6% 58.08 7.23
Ethanol in hydrolyzer 0.00 0.0% 46.07 -
Water in hydrolyzer 84.00 10.7% 18.00 4.67
[00313] EXAMPLE 29
[00314] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 61 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 0.00 0.0% 240.37 - 0.00%
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00%- -
Dimethyldiethoxysilane (FIG.
42)
20.00 2.6% 148.28 0.13 10.67% 0.13 0.27
Methyldiethoxysilane (FIG. 39) 0.00 0.0% 134.25 - 0.00% -
Vinylmethyldiethoxysilane
(FIG. 40) 0.00 0.0% 160.29 - 0.00% - -
TES 40 (FIG. 35) 235.00 30.0% 208.00 1.13 89.33%
1.13 4.52
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 440.00 56.2% 58.08 7.58
Ethanol in hydrolyzer 0.00 0.0% 46.07 -
Water in hydrolyzer 88.00 11.2% 18.00 4.89
[00315] EXAMPLE 30
[00316] A polysilocarb formulation is combined with a reinforcing
material to form a preform, which is partially or completely cured to form a
reinforced composite material in the shape of a component part. The component
part is cured, and preferably hard cured. The hard cured component part is
pyrolized to form a polysicocarb derived reinforced composite component part,
which is taken through from two to five reinfiltration, cure and pyrolysis
cycles.
The end reinforced component part is ready for further processing, e.g.,
machining to tolerances, assembly steps, integration steps, or use. The
89
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
polysicocarb derived ceramic component part may find applications, or be
designed for use in, or as, among other things: friction members such as
breaks,
break pads, break discs, and clutches; building materials, such as beams,
girders, columns, I-beams, channels, studs (e.g., a 2x4 replacement), wall
panels, roofing, decking, and flooring; aerospace members, such as, high
temperature engine components, air frame members, skin or outer coverings,
cockpit components, seating frames, passenger compartment components,
carpet, and fabric; equipment (including automotive) components, such as,
tubes, rods, custom frame members, hollow rectangular tubes, hollow octagonal
tubes, multisided rods, panels, firewalls, liners, seating components, dash
components, multisided hollow tubes, circular tubes, channels, I-beams, bands,
and joints; and consumer goods, including consumer electronics, appliances,
furniture and home goods. The use of the polysilocarb derived ceramic
composite component parts can provide several features, including among other
things, high temperature resistance, corrosion resistance, UV resistance,
lightweight, low cost (significantly lower, by as much as several orders of
magnitude over silicon carbide and conventional polymer derived ceramics),
increased strength to weight rations (e.g., stronger and lighter, same
strength
and lighter, stronger and same weight), and fire resistance.
[00317] EXAMPLE 31
[00318] The polysilocarb derived ceramic composite component parts of
Example 30 where the polysilocarb formulation includes a vinyl terminated
siloxane.
[00319] EXAMPLE 32
[00320] The polysilocarb derived ceramic composition component parts
of Example 30 where the polysilocarb formulation is 60% MHF, 20% TV, 5% Vt
and 10% MVF (a reacted formulation of all vinylmethyldiethoxysilane, e.g., the
formulations of Examples 66-68)
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00321] EXAMPLE 33
[00322] The polysilocarb derived ceramic composite component parts of
Example 30 where the polysilocarb formulation includes about 10% of the vinyl
terminated siloxane.
[00323] EXAMPLE 34
[00324] A polysilocarb formulation is combined with a reinforcing
material to form a precursor, which is partially or completely cured to form a
reinforced composite material in the shape of a component part. The component
part is cured, and preferably end cured. The end component part is ready for
further processing, e.g., machining to tolerances, assembly steps or use. The
polysicocarb derived plastic component part may find applications, or be
designed for use in, or as, among other things: building materials, such as
beams, girders, columns, I-beams, channels, a 2x4 replacement, wall panels,
decking, and flooring; aerospace members, such as, cockpit components,
seating components, passenger compartment components; equipment
components, such as, tubes, rods, custom frame members, hollow rectangular
tubes, hollow octagonal tubes, multisided rods, multisided hollow tubes,
circular
tubes, channels, I-beams, bands, joints. The use of the polysilocarb derived
reinforced composite component parts can provide several features, including
among other things, higher temperature resistance (including the ability to
retain,
or substantially maintain structural integrity during exposure to higher
temperatures), include increased strength to weight rations (e.g., stronger
and
lighter, same strength and lighter, stronger and same weight), light weight,
low
cost, UV resistance, corrosion resistance, fire resistance, and fire
retardation.
[00325] EXAMPLE 35
[00326] The polysilocarb derived ceramic composite component parts of
Example 30 where the polysilocarb formulation includes a vinyl terminated
siloxane.
[00327] EXAMPLE 36
[00328] The polysilocarb derived ceramic composition component parts
of Example 30 where the polysilocarb formulation is 60% MHF, 20% TV, 5% Vt
91
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
and 10% MVF (a reacted formulation of all vinylmethyldiethoxysilane, e.g., the
formulations of Examples 66-68).
[00329] EXAMPLE 37
[00330] The polysilocarb derived ceramic composite component parts of
Example 30 where the polysilocarb formulation includes about 10% of the vinyl
terminated siloxane.
[00331] EXAMPLE 38
[00332] A polysilocarb formulation is combined with a reinforcing
material to form a precursor to a reinforced composite, which is cured to form
a
reinforced composite material in the shape of a component part. The component
part is cured, and preferably hard cured. The hard cured component part is
pyrolized to form a polysicocarb derived composite component part, which is
then infiltrated with a polysilocarb formulation, which is then cured. This
material
can be referred to as a polysilocarb plastic-ceramic, or plastic-ceramic,
reinforced
structure is ready for further processing, assembly steps or use.
[00333] EXAMPLE 39
[00334] The polysilocarb derived ceramic composition component part,
of Examples 30-38 is a grinding or cutting member having cutting material
distributed throughout its structure. Preferable the cutting member is a
cutting
wheel having a cutting or abrasive material, e.g., polycrystalline diamond
compact (PDC), Aluminum oxide, or diamond, that is evenly distributed through
out the cutting wheel. In this manner as the wheel is worn, the cutting
material
will continue to be exposed on the surface of the well, and preferably be in
an
amount that changes very little, if at all, as the wheel is used and worn.
[00335] EXAMPLE 40
[00336] A polysilocarb formulation is cured to into the volumetric shape
of a bead. The end cured polysilocarb derived beads are, for example, added to
paints, glues, plastics, and building materials, such as dry wall, sheet rock,
gypsum board, MDF board, plywood, plastics and particleboard. The end cured
polysilocarb derived beads, as additives, can provide, among other things,
binding (e.g., serve as a binder), water resistivity, fire resistance, fire
retardation,
92
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
fire protection and strength; as well as, abrasion resistance, wear
resistance,
corrosion resistance and UV resistance, if located at or near the surface of
the
shape.
[00337] In addition to a bead the polysilocarb additives can be in the
form of a fine powder, fines, a power or other dispersible forms. The
dispersible
form can be obtained by grinding or crushing larger cured structures. They
also
may be obtained through the curing process if done under conditions that cause
the structure to fracture, crack or break during curing. These dispersible
forms
may also be obtained by other processing techniques, for example, spray curing
or drying.
[00338] EXAMPLE 41
[00339] A polysilocarb formulation is cured to into the volumetric shape
of a bead. The beads are then pyrolized to for a polysilocarb derived ceramic
bead. The polysilocarb derived ceramic beads are added, for example, to
paints,
glues, plastics, and building materials, such as dry wall, sheet rock, gypsum
board, MDF board, plywood, plastics and particleboard. The ceramic
polysilocarb beads, as additives, can provide, among other things, fire
resistance, fire retardation, fire protection and strength.
[00340] In addition to a bead the polysilocarb additives can be in the
form of a fine power, fines, a power or other dispersible forms. The
dispersible
form can be obtained by grinding or crushing larger cured or pyrolized
structures.
They also may be obtained through the curing or pyrolysis process if done
under
conditions that cause the structure to fracture, crack or break during curing
or
pyrolysis.
[00341] EXAMPLE 42
[00342] A polysilocarb formulation is applied to structural components
(e.g., beams, girders, columns) of a high rise building. The viscosity of the
formulation is such that the liquid formulation stays adhered (e.g., does not
significantly drip or run) to the structural building components until the
curing
process is complete. The formulation is further designed to cure, preferably
to a
hard cure, under ambient conditions in less than a two-day period. (If quicker
93
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
curing times are required, or if higher temperatures are desired to assure a
hard
cure than a heat source can be used, the formulation can be adjusted and
both.)
[00343] The coating provides protection from fire to the structural
building components, such that the polysilocarb coated structural component
meets or exceeds the requirements of ASTM E-119, the entire disclosure of
which is incorporated herein by reference. These coatings are also UV stable,
corrosion resistance and are water repellent.
[00344] Thus these coatings can be applied to, or utilized with, for
example, assemblies of masonry units and composite assemblies of structural
materials for buildings, including bearing and other walls and partitions,
columns,
girders, beams, slabs, and composite slab and beam assemblies for floors and
roofs, as well as other assemblies and structural units that constitute
permanent
integral parts of a finished building. Thus, these coatings can impart or
provide
Standard Fire Test performance exposures of at least about 1/2-h, about 2-h,
about 4-h and about 6-h or more.
[00345] This polysilocarb protective coating is non-halogenated, e.g., it
does not contain any halogens. Thus, the protective coating can be
substantially
free from halogens, i.e., the amount of halogens in any off gassing is so low
as to
render the level acceptable or permissible for human exposure; can be
essentially free from halogens, i.e., the amount of halogens is so low as to
render
their presence in any off gassing undetectable by normal gas sensing devices
used by those of skill in the art to test for the presence of halogens; and,
can be
free from halogens, i.e., the off gases contain no detectable or measurable
presence of halogens. This polysilocarb protective coating is also non-toxic
and
will not produce toxic or dangerous gasses during exposure to fire or high
temperatures. Thus, it is a non-toxic off gassing coating. Additionally, it
will not
produce any harmful or toxic degradation products. Essentially, upon exposure
to flame, the polysilocarb coating goes through a pyrolysis reaction turning
the
coating into a ceramic. The polysilocarb protective coating is further an
active
fire retardant or active fire suppressant. By active fire retardant it is
meant that
94
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
when exposed to flame the coating undergoes reactions that remove oxygen,
heat, and both, from the environment.
[00346] In this manner the polysilocarb coating preferably is capable of
providing flame protection to the substrate through, for example, one, two or
all of
the following flame retardation mechanisms: an endothermic reaction; thermal
shielding; and quenching (e.g., removal of oxygen as a combustion source).
[00347] Additionally, it is preferred that the coating be hard cured to
optimize the active fire retardation mechanisms; and also reduce the creation
of
any hydrogen off gassing. (These general mechanisms, and benefits are
applicable to the embodiments of other Examples in this specification, as well
as,
to other embodiments and other formulations and components in addition to the
embodiments of the present Examples)
[00348] The polysilocarb coating, or additive, can also impart, or
enhance, the thermal barrier properties of a material.
[00349] Such coated steel or concrete structures, for example, may
have the fire resistance properties set out in Table I, or they more
preferably may
exceed those properties
[00350] Table I
Furnace-Flame Temperature Temperature ( F) on unexposed
Time = t ( F) on exposed surface at surface at time t
time t
0.5 hr 1,550 <1,000
1 hr 1,700 <1,000
2 hr 1,850 <1,000
4 hr 2,000 <1,000
8 hr 2,300 <1,000
[00351] EXAMPLE 43
[00352] The building structural component fire resistant coating of
Example 42 is made from a polysilocarb formulation having 41`)/0 MHF and 59%
TV.
[00353] EXAMPLE 44
[00354] The building structural component fire resistant coating of
Example 42 is made from a polysilocarb formulation having 41`)/0 MHF and 59%
MVF.
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00355] EXAMPLE 45
[00356] The building structural component fire resistant coating of
Example 40 is made from a polysilocarb formulation having from about 40% MHF
to about 55% MHF and from about 60% MVF to about 55% MVF.
[00357] EXAMPLE 46
[00358] The building structural component fire resistant coating of
Examples 42 ¨ 45 is applied by flame or thermal spraying the polysilocarb
formulation on the building structural component. In this process, preferably
the
polysilocarb formulation is cured and in the form of a fine powered, which is
then
thermal sprayed onto the building structural member.
[00359] EXAMPLE 47
[00360] The polysilocarb formulations of Examples 42-45 to which
ceramic beads or fines, and preferably polysilocarb ceramic beads or fines,
are
added.
[00361] EXAMPLE 48
[00362] In this example a conventional flame retardant or flame resistant
wrap or outer cover is impregnated with a polysilocarb formulation. The
impregnated material is then cured, and preferably hard cured, and affixed to
the
building structural members, as it would generally be done. Additionally, to
add
additional fire resistivity to an existing structure, existing (e.g.,
presently installed
in a building) fire protective wraps or covers can be impregnated with a
polysilocarb formulation and cured.
[00363] EXAMPLE 49
[00364] A cured polysilocarb formulation is used as a fire retardant
additive for a plastic article. The polysilocarb formulation may be hard
cured,
which is preferable, provided that the hard cure material performs adequately
in
the plastic molding, extruding or forming process to make the plastic article.
[00365] The plastic may be, for example, thermal setting, thermoplastic,
polyolefins, polyamide, engineering plastics, textile adhesives coatings
(TAC),
plastic foams, styrenic alloys, acrylonitrile butadiene styrene (ABS),
polyurethanes, polystyrenes, acrylics, polycarbonates (PC), epoxies,
polyesters,
96
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
nylon, polyethylene, high density polyethylene (HDPE), very low density
polyethylene (VLDPE), low density polyethylene (LDPE), polypropylene (PP),
polyvinyl chloride (PVC), polyethylene terephthalate (PET), polybutylene
terephthalate (PBT), poly ether ethyl ketone (PEEK), polyether sulfone (PES),
bis
maleimide, and viscose (cellulose acetate).
[00366] The polysilocarb additive is preferable in the form of a powder,
beads or pellets that are selected to readily match with the plastic starting
material (e.g., resin), so that the polysilocarb additive can be processed
with the
plastic, preferably with the existing manufacturing equipment, and formed into
the
desired plastic article (e.g., part or component), which could include, for
example,
automotive components, aircraft components, marine components, components
of consumer goods, e.g., lawn mowers, electronics, appliances, furniture, home
goods (e.g., window treatments, and carpeting).
[00367] The plastic articles with the polysilocarb fire retardant additive
most preferably will have the ability to pass VO testing by UL-94 (burning
stops
within 10 seconds on a vertical specimen; drips of particles allowed as long
as they are not inflamed.) The entirety of UL-94 testing procedures and
standards is incorporated herein by reference.
[00368] These articles, depending upon their application and use
requirements may have the ability to pass HB slow burning on a horizontal
specimen; burning rate < 76 mm/min for thickness < 3 mm and burning
stops before 100 mm), V2 (burning stops within 30 seconds on a vertical
specimen; drips of flaming particles are allowed), V1 (burning stops within 30
seconds on a vertical specimen; drips of particles allowed as long as they
are not inflamed), 5VB (burning stops within 60 seconds on a vertical
specimen; no drips allowed; plaque specimens may develop a hole), 5VA
(burning stops within 60 seconds on a vertical specimen; no drips allowed;
plaque specimens may not develop a hole), testing by UL-94, among other
testing regimes.
97
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00369] For foams these polysilocarb added plastic articles may have
the ability to pass HF-1 (burning stops within 2 seconds; afterglow less than
30s; no burning drips allowed), burning stops within 3 seconds; afterglow
less than 30s; burning drips allowed, testing by UL-94, among other testing
regimes.
[00370] The polysilocarb fire retardant additive may be used to replace
existing fire retardants such as ATH (aluminum trihydrate) based, Bromine
based, Phosphorous based, Chlorine based, Antimony based Melamine and
others know to those of skill in the art. These existing fire retardants have
many
disadvantages, include the presence of halogens, the creation of harmful or
hazardous degradation products, hazardous starting materials, creation of
hazardous wastes during manufacturing, high cost, among others. The
polysilocarb fire retardants avoid, e.g., they do not have, any of these
disadvantages.
[00371] In particular, and preferably, the polysilocarb fire retardant
additive is non-halogenated, e.g., it does not contain any halogens. Thus, it
can
be substantially free from halogens, i.e., the amount of halogens in any off
gassing is so low as to render the level acceptable or permissible for human
exposure; can be essentially free from halogens, i.e., the amount of halogens
is
so low as to render their presence in any off gassing undetectable by normal
gas
sensing devices used by those of skill in the art to test for the presence of
halogens; and, can be free from halogens, i.e., the off gases contain no
detectable or measurable presence of halogens. This polysilocarb fire
retardant
additive is also non-toxic and will not produce toxic or dangerous gasses
during
exposure to fire or high temperatures. Thus, it is a non-toxic off gassing
additive.
Additionally, and still more preferably, to the extent any degradation
products are
produced by the polysilocarb additive, they are non-toxic.
[00372] EXAMPLE 50
[00373] The polysilocarb formulation of Example 49 having 70% MHF,
20% TV, and 10% VT. The polysilocarb is hard cured, ground, and sieved to a
particle size of less than 10 pm and a specific gravity of less than about
1.4.
98
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
The plastic is loaded with less than about 20% of the hard cured polysilocarb
fire
retardant. The plastic may be PP, PET-PBT alloys, and PC-ABS alloys.
[00374] EXAMPLE 51
[00375] The polysilocarb formulation of Example 49 having 95% MHF
and 5% TV.
[00376] EXAMPLE 52
[00377] The polysilocarb formulation of Example 49 having 41`)/0 MHF
and 59% TV.
[00378] EXAMPLE 53
[00379] The polysilocarb hard cured additive of Example 49 has a
particle size, less than about 75 microns, greater than about 0.05 microns,
from
about 0.1 to about 50 microns, preferably from about 3 to about 10 microns,
and
a specific gravity of from about 0.5 to about 2.0, preferably about 1 to about
1.5.
The plastic article has from about 0.05%% polysilocarb fire retardant to about
100`)/0 fire retardant, preferably about 30% to about 50
[00380] EXAMPLE 54
[00381] The polysilocarb fire retardant additive of Examples 49-53 also
contain a ceramic, and preferably a ceramic polysilocarb fire retardant
additive.
The ceramic additive may be from about 1`)/0 to about 100`)/0 of the total
additive.
[00382] EXAMPLE 55
[00383] The polysilocarb fire retardant additive of Examples 49-54 is
combined with a conventional fire retardant and used in a plastic article. The
polysilocarb fire retardant additive can be from about 1`)/0 to about 99% of
the
total fire retardant additives used.
[00384] EXAMPLE 56
[00385] A polysilocarb formulation is applied in liquid form to a substrate
to form a coating. The coating is then cured increasing the ability of the
substrate to resisting burning when subject to a fire. The formulation may be
catalyzed or uncatalyzed. The coating may be one, two, three or more coats
(e.g., layers) of the polysilocarb formulation. Preferably the coating cures,
to a
hard cure, in less than 2 days under ambient conditions. However, external
heat
99
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
source can be used to cure the coating. The substrates may be existing
articles
such as for example, the interior walls of a house or building, the wood frame
of a
building prior to installation of the walls, floors, roofs, decks, the
exterior of a
structure (e.g., house, office or barn), the ground or brush (e.g., to form a
fire
break), and they may be building supply materials such as dry wall, plywood
and
2 x 4s that are coated before being used in a building.
[00386] Preferably the coating provides the ability for the wood substrate
to meet or exceed Class A materials under ASTM E84.
[00387] EXAMPLE 57
[00388] The fire resistant coating of Example 56 is made from a
polysilocarb formulation having 41`)/0 MHF and 59% TV.
[00389] EXAMPLE 57
[00390] The fire resistant coating of Example 56 is made from a
polysilocarb formulation having 41`)/0 MHF and 59% MVF
[00391] EXAMPLE 58
[00392] The building structural component fire resistant coating of
Example 56 is made from a polysilocarb formulation having from about 40% MHF
to about 55% MHF and from about 60% MVF to about 55% MVF.
[00393] EXAMPLE 59
[00394] Plastic components are made from cured polysilocarb
formulations. These structure components have the ability to maintain their
structural integrity while burning. As the components burn they will to a
greater
or lessor extent pyrolize into a ceramic, and thus maintain their strength and
structural integrity, and more preferably potentially increase those features.
These components may, for example, be used as overhead luggage binds, or
interior components of an aircraft. They may be other structural plastic
members.
[00395] Additionally, the plastic component does not have to be made
up completely of the polysilocarb formulation. Rather, the polysilocarb
formulation can be distributed within in a preselected pattern, on, or around
the
100
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
component, so that the increase in strength can be obtained as the
polysilocarb
is turned to a ceramic upon exposure to a fire.
[00396] EXAMPLE 60
[00397] A polysilocarb formulation is formed into a fiber that is cured and
then pyrolized. If needed or desirable, the ceramic polysilocarb fiber may be
subject to additional reinfiltration, curing and pyrolysis steps (one to five
or more)
to provide a ceramic, or they may be subjected to additional reinfiltration
and
curing steps (one to three or more) to provide a plastic-ceramic. Preferably,
the
polysilocarb formulations are made using phenyltriethoxysilane (FIG. 45),
phenylmethyldiethoxysilane (FIG. 38), methyldiethoxysilane (FIG. 39) and
Vinylmethyldiethoxysilane (FIG. 40), as well as, dimethyldiethoxysilane and
methytriethoxysilane. The mass percentages of the phenyltriethoxysilane
and phenylmethyldiethoxysilane (or dimethyldiethoxysilane and
methytriethoxysilane) would likely range from 10% to 80% with the
preferred range around 40-60% (of either, or total of both).
[00398] Fibers may be made from the polysilocarb formulations of
Examples 61 and 62.
[00399] For the formation of flame retardant fabrics the polysilocarb fiber
may be cured, preferably hard cured, but not necessarily pyrolized.
[00400] EXAMPLE 61
[00401] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 61 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 145.00 18.5% 240.37 0.60
34.58% 0.60 1.81
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00% - -
Dimethyldiethoxysilane (FIG.
42)
0.00 0.0% 148.28 0.57 32.88% 0.57 1.55
Methyldiethoxysilane (FIG. 39) 77.00 9.8% 134.25 - 0.00%
-
Vinylmethyldiethoxysilane
(FIG. 40) 91.00 11.6% 160.29 0.57 32.54%
0.57 1.14
101
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
-
Trimethyethoxysilane (FIG. 48) 0.00 0.0% 118.25 -
0.00% -
Acetone in hydrolyzer 395.00 50.3% 58.08 6.80
Ethanol in hydrolyzer 0.00 0.0% 46.07 -
Water in hydrolyzer 76.00 9.7% 18.00 4.22
HCI 0.36 0.0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00402] EXAMPLE 62
[00403] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 61 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 0.00 0.00% 240.37 - 0.0%
Phenylmethyldiethoxysilane
(FIG. 38) 145.00 18.4% 210.35 0.69 34.47% 0.69
1.38
Dimethyldiethoxysilane (FIG.
-
42) 0.00 0.00% 148.28 - 0.00% -
Methyldiethoxysilane (FIG. 39) 88.00 11.2% 134.25 0.66
32.78% 0.66 1.31
Vinylmethyldiethoxysilane
(FIG. 40) 105.00 13.3% 160.29 0.66 32.76%
0.66 1.31
Trimethyethoxysilane (FIG. 48) 0.00 0.0% 118.25 -
0.00% -
Acetone in hydrolyzer 375.00 47.5% 58.08 6.46
Ethanol in hydrolyzer 0.00 0.0% 46.07 -
Water in hydrolyzer 75.00 9.5% 18.00 4.17
HCI 0.36 0.0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00404] EXAMPLE 63
[00405] The polysilocarb fiber of Examples 60-62 has a colorant added
to the polysilocarb formulation. In this manner a color polysilocarb ceramic
fiber
is obtained. The coloration preferably extends throughout the fiber and is
more
preferably evenly distributed throughout the entirety of the volume of the
fiber,
e.g., the whole fiber, not just the surface is colored. The colors may be
essentially any color, e.g., red, blue, green, orange, yellow, purple, etc.
The
colorant may also be of a non-visible wavelength, but which can be seen by
102
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
machines, and thus could have application for example in machine vision
control
systems or processing.
[00406] EXAMPLE 64
[00407] The colored polysilocarb fiber of Examples 60-63 has a
predetermined and matched color to a composite material. In this manner the
colored ceramic polysilocarb fiber is the reinforcement for a composite
material.
The fiber's color is matched to the composite's color, and in, particular to
the final
color of the matrix material in the composite. Thus, as the composite wears or
ages its color will not change, and the exposure of the reinforcing fibers
will go
essentially undetected, because they are the same color as the matrix
material.
[00408] EXAMPLE 65
[00409] Several different colored polysilocarb fibers of Examples 60-63
each have a predetermined and different color. The first and typically the
outer
most has a color identical to or matched to a composite material. The inner
fibers may have different colors to provide a visual indication of wear or
aging,
e.g., transition to yellow and then red fibers to indicate the end of useful
or
recommended life of a composite part. Alternatively, different color fibers
could
be in the outer area of the composite to indicate a new composite and then
matched color fibers can be below this outer area, to indicate that use has
occurred, e.g., loss of white fibers on outer surface means that the part has
been
used, or that an initial period, e.g., a break in period for the part, has
been
completed. In this manner the colored ceramic polysilocarb fiber can be used
to
both enhance the ascetics of a part, as well as, provide an indication about
use
of the part. Additionally, in some applications it may be desirable or
ascetically
pleasing to have the colored fibers as a different color from the matrix
material.
[00410] EXAMPLE 66
[00411] Using the reaction type process an MVF precursor formulation
was made using the following formulation. The temperature of the reaction was
maintained at 72 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of
Moles Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
103
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
Phenyltriethoxysilane (FIG. 45) 0.00 0.0% 240.37 - 0.00%
- -
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00% - -
Dimethyldiethoxysilane (FIG.
42) 0.00 0.0% 148.28 - 0.00% -
-
Methyldiethoxysilane (FIG. 39) 0.00 0.0% 134.25 - 0.00% -
-
Vinylmethyldiethoxysilane
(FIG. 40) 1584.00 41.1% 160.29 9.88 100.00%
9.88 19.76
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 0.00 0.0% 58.08 -
Ethanol in hydrolyzer 1875.00 49.0% 46.07 40.70
Water in hydrolyzer 370.00 9.7% 18.00 20.56
HCI (pH 2, 36g/100g water) 0.36 36.00 0.01
Sodium bicarbonate 0.84 84.00 0.01
[00412] EXAMPLE 67
[00413] Using the reaction type process an MVF precursor formulation
was made using the following formulation. The temperature of the reaction was
maintained at 61 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of Moles
Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 0.00 0.0% 240.37 - 0.00%
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00% - -
Dimethyldiethoxysilane (FIG.
42) 0.00 0.0% 148.28 - 0.00% -
-
Methyldiethoxysilane (FIG. 39) 0.00 0.0% 134.25 - 0.00% -
-
Vinylmethyldiethoxysilane
(FIG. 40) 1584.00 42.0% 160.29 9.88 100.00%
9.88 19.76
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 1825.00 48.3% 58.08 31.42
Ethanol in hydrolyzer 0.00 0.0% 46.07 -
Water in hydrolyzer 365.00 9.7% 18.00 20.28
HCI (pH 2, 36g/100g water) 0.36 36.00 0.01
Sodium bicarbonate 0.84 84.00 0.01
[00414] EXAMPLE 68
[00415] Using the reaction type process an MVF precursor formulation
was made using the following formulation. The temperature of the reaction was
maintained at 61 C for 21 hours.
104
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
Moles of % of Total
% of Reactant/ Moles of
Moles Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 0.00 0.0% 240.37 - 0.00%
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00% -
-
Dimethyldiethoxysilane (FIG.
42) 0.00 0.0% 148.28 - 0.00% -
-
Methyldiethoxysilane (FIG. 39) 0.00 0.0% 134.25 - 0.00% -
-
Vinylmethyldiethoxysilane
(FIG. 40) 33.00 41.9% 160.29 2.06 100.00%
2.06 4.12
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 380.00 48.3% 58.08 6.54
Ethanol in hydrolyzer 0.00 0.0% 46.07 -
Water in hydrolyzer 76.00 9.7% 18.00 4.22
HCI (pH 2, 36g/100g water) 0.36 36.00 0.01
Sodium bicarbonate 0.84 84.00 0.01
[00416] EXAMPLE 69
[00417] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 72 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of
Moles Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Methyltriethoxysilane (FIG.
37) 0.00 0.0% 178.30 -
0.00% - -
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00% -
-
Dimethyldiethoxysilane (FIG.
42) 56
7.2% 148.28 0.38 17.71% 0.38 0.76
Methyldiethoxysilane (FIG. 39) 182 23.2% 134.25 1.36 63.57%
1.36 2.71
Vinylmethyldiethoxysilane
(FIG. 40) 64 8.2% 160.29 0.40 18.72%
0.40 0.80
Triethoxysilane 0.00 0.0% 164.27 - 0.00% -
-
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 0.00 0.0% 58.08 -
Ethanol in hydrolyzer 400.00 51.1% 46.07 8.68
Water in hydrolyzer 80.00 10.2% 18.00 4.44
HCI 0.36 0.0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
105
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
[00418] EXAMPLE 70
[00419] Using the reaction type process a precursor formulation was
made using the following formulation. The temperature of the reaction was
maintained at 61 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of
Moles Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Methyltriethoxysilane (FIG.
37) 0.00 0.0% 178.30 -
0.00% - -
Phenylmethyldiethoxysilane
(FIG. 38) 0.00 0.0% 210.35 - 0.00% -
-
Dimethyldiethoxysilane (FIG.
42) 56
7.2% 148.28 0.38 17.71% 0.38 0.76
Methyldiethoxysilane (FIG. 39) 182 23.2% 134.25 1.36
63.57% 1.36 2.71
Vinylmethyldiethoxysilane
(FIG. 40) 64 8.2% 160.29 0.40 18.72% 0.40
0.80
Triethoxysilane 0.00 0.0% 164.27 - 0.00%
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 400.00 51.1% 58.08 6.89
Ethanol in hydrolyzer 0.00 0.0% 46.07 -
Water in hydrolyzer 80.00 10.2% 18.00 4.44
HCI 0.36 0.0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00420] EXAMPLE 71
[00421] Proppants for use in hydraulic fracturing are made from the
formulations of Examples 22, 60 and 61.
[00422] EXAMPLE 72
[00423] A polysilocarb formulation having 80% MHF, 15% TV, and 5%
VT is made into proppants for use in hydraulic fracturing.
[00424] EXAMPLE 73
[00425] A polysilocarb formulation having 95% MHF and 5% TV is made
into proppants for use in hydraulic fracturing.
[00426] EXAMPLE 74
[00427] A polysilocarb formulation having 90% MHF, 5% TV, and 5% VT
is made into proppants for use in hydraulic fracturing.
106
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00428] EXAMPLE 75
[00429] A blast and impact shield is formed using one of more layers of
polysilocarb formulations. Layers of polysilocarb formulations are hard cured
on
top of a substrate. The layers are reinforced and can be done so with varied
weave patterns between the layers. The composite layers structure provides
protection against blasts, projectiles and explosions. This shield can weigh
less
than conventional shields and armor, while providing equal or better
protection.
[00430] EXAMPLE 76
[00431] A ballistic composite structure was made using 12 Layers
of 1200 g/sq. meter fiberglass, 20 layers of unidirectional carbon fiber
oriented at 0,45,-45,90 in 5 sections to make 20 layers, and 1 layer of
0.032 steel as face sheet. The fiberglass was bonded together using, a
polysilocarb batch, and a warm press at 150-160 C for 1-2 hours and a
minimum of 500 psi pressure to form a fiberglass plate. The 20 layers of
carbon fiber cloth were bonded together under same conditions to form a
carbon fiber plate. The fiberglass plate, the carbon fiber plate and the
steel sheet were then bonded in one step using a polysilocarb batch as the
bonder between the steel and the carbon fiber plate and between the
carbon fiber plate and the fiberglass plate. The composite had the steel
face sheet bonded to the carbon fiber plate backed up with the thicker
fiberglass sheet, with the bullets hitting the steel plate. The polysilocarb
batch can be the batches of Exhibits 76A, 76B, 760 and 15.
[00432] Monolithic ceramic plates, such as SiC, alumina, boron
nitride may used in addition to or instead of the steel plate. Can also use
ceramic composites made from our polymer and ceramic or carbon fiber.
[00433] EXAMPLE 76A
[00434] Using the reaction type process a polysilocarb precursor
formulation was made using the following formulation. The temperature of the
reaction was maintained at 72 C for 21 hours.
107
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
Moles of % of Total
% of Reactant/ Moles of
Moles Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 180.00 22.7% 240.37 0.75
44.10% 0.75 2.25
Phenylmethyldiethoxysilane 50.00
(FIG. 38) 6.3% 210.35 0.24 14.00%
0.24 0.48
Dimethyldiethoxysilane (FIG.
42) 40.00
5.0% 148.28 0.27 15.89% 0.27 0.54
Methyldiethoxysilane (FIG. 39) 30.00 3.8% 134.25 0.22
13.16% 0.22 0.45
Vinylmethyldiethoxysilane
(FIG. 40) 35.00 4.4% 160.29 0.22 12.86%
0.22 0.44
Trinnethyethoxysilane (FIG.
48) 0.00 0.0% 118.25 0.00% -
-
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 0.00 0.0% 58.08
Ethanol in hydrolyzer 380.00 48.0% 46.07 8.25
Water in hydrolyzer 76.00 10.9% 18.00 4.22
HCI (pH 2, 36g/100g water) 0.36 0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00435] EXAMPLE 76B
[00436] Using the reaction type process a polysilocarb precursor
formulation was made using the following formulation. The temperature of the
reaction was maintained at 61 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of
Moles Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 180.00 22.7% 240.37 0.75
44.10% 0.75 2.25
Phenylmethyldiethoxysilane 50.00
(FIG. 38) 6.3% 210.35 0.24 14.00%
0.24 0.48
Dimethyldiethoxysilane (FIG.
42) 10.00
1.3% 148.28 0.07 3.95% 0.07 0.13
Methyldiethoxysilane (FIG. 39) 45.00 5.7% 134.25 0.34
19.63% 0.34 0.67
Vinylmethyldiethoxysilane
(FIG. 40) 51.00 6.4% 160.29 0.32 18.64%
0.32 0.64
Trinnethyethoxysilane (FIG.
48) 0.00 0.0% 118.25 0.00% -
-
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 380.00 47.9% 58.08 6.54
Ethanol in hydrolyzer 0.00 0.0% 46.07 0.00
Water in hydrolyzer 76.00 9.6% 18.00 4.22
HCI (pH 2, 36g/100g water) 0.36 0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
108
CA 02940678 2016-08-24
WO 2015/131168 PCT/US2015/018211
[00437] EXAMPLE 760
[00438] Using the reaction type process a polysilocarb precursor
formulation was made using the following formulation. The temperature of the
reaction was maintained at 61 C for 21 hours.
Moles of % of Total
% of Reactant/ Moles of
Moles Moles
Reactant or Solvent Mass Total MW solvent Silane of Si
of Et0H
Phenyltriethoxysilane (FIG. 45) 170.00 21.4% 240.37 0.71
40.76% 0.71 2.12
Phenylmethyldiethoxysilane 3200
(FIG. 38) 4.0% 210.35 0.15 8.77%
0.15 0.30
Dimethyldiethoxysilane (FIG.
42) 9.00 1.1% 148.28 0.06 3.50% 0.06 0.12
Methyldiethoxysilane (FIG. 39) 55.00 6.9% 134.25 0.41
23.61% 0.41 0.82
Vinylmethyldiethoxysilane
(FIG. 40) 65.00 8.2% 160.29 0.41 23.37%
0.41 0.81
Trinnethyethoxysilane (FIG.
48) 0.00 0.0% 118.25 0.00% - -
Hexane in hydrolyzer 0.00 0.0% 86.18 -
Acetone in hydrolyzer 385.00 48.5% 58.08 6.63
Ethanol in hydrolyzer 0.00 0.0% 46.07 0.00
Water in hydrolyzer 77.00 9.7% 18.00 4.28
HCI (pH 2, 36g/100g water) 0.36 0% 36.00 0.01
Sodium bicarbonate 0.84 0.1% 84.00 0.01
[00439] EXAMPLE 76D
[00440] The ballistic composite of Example 76 was withstood shots from
a 22 Mag, a 45, a 22 rifle, a 280, a 9mm, a 22 piston, and 223.
[00441] EXAMPLE 77
[00442] A polysilocarb formulation is pyrolized in the form of a
volumetric structure. The ceramic polysilocarb derived volumetric structure
exhibits reflective and refractive optical properties, such as opalescence,
shine,
twinkle, and sparkle. These optical properties are present when the structure
is
black in color, (e.g., no colorant has been added to the formulation); or if
the
structure is colored (e.g., any color other than black, e.g., white, yellow,
red, etc.).
[00443] EXAMPLE 78
[00444] The volumetric structure of Example 77 is a work surface, such
as a table top, a bench top, an insert, or a kitchen counter top, to name a
few.
109
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00445] EXAMPLE 79
[00446] The volumetric structure of Example 78 has other colorings or
additive to provide simulated granite like appearance.
[00447] EXAMPLE 80
[00448] The volumetric structures of Example 77 are small beads that
are black and exhibit a twinkle, opalescence or shin. These beads are
incorporated into a paint formulation. The patent formulation is for example
applied to automobiles or appliances. It provides a flat or mat finish, which
is for
example popular on newer BMWs and Mercedes, but adds to that mat finish an
inner sparkle or luster. Thus, the polysiloxane based paint formulation
provides a
sparkle mat finish to an automobile, appliance or other article.
[00449] EXAMPLE 81
[00450] Pyrolized polysilocarb beads having a size of from about 100 to
about 1,000 microns are added to a paint formulation at a loading of from
about
1% to about 40%.
[00451] EXAMPLE 82
[00452] The paint of Example 81 in which the paint formulation, is an
automotive paint, and is colored blue and the beads are the same blue color as
the paint, and have size of 350 microns (+/- 5%) and a loading of about 25%.
[00453] EXAMPLE 83
[00454] The paint of Example 81 in which the beads are not colored,
i.e., they are black, and have a size ranging from about 300-500 microns, and
the
paint is a black, although not necessarily the same black as the beads.
[00455] EXAMPLE 84
[00456] A latex paint formulation having pyrolized polysilocarb power
added into the formulation, the power has a size range of about 0.5 ¨ 100
microns, and the powder has a loading of about 15%.
[00457] EXAMPLE 85
[00458] The paint formulation of Example 83 is an enamel.
[00459] EXAMPLE 86
[00460] The polysilocarb ceramic pigments can be made from the
1o
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
pyrolysis of any polysilocarb batches that are capable of being pyrolized. The
polysilocarb pigment material can be provided, for example, as beads, powder,
flakes, fines, or other forms that are capable of being dispersed or suspended
in
the paint formulation (e.g., platelets, spheres, crescents, angular, blocky,
irregular or amorphous shapes). Beads can have a size of from about 100 to
about 1,000 microns in diameter. Powders can have a particle size range of
from
about 0.5 to about 100 microns in diameter. Any subset range within these
ranges can create the desired effect or color. Larger and smaller sizes may
also
provide the desired effects in other formulations. For example: 300 ¨ 500
micron range beads; 350 (+/- 5%) micron beads; 5 ¨ 15 micron range powder.
Particle size ranges for a particular polysilocarb ceramic pigment preferably
range as tight as +/- 10% and more preferably +/- 5%. The range may also be
broader in certain applications, e.g., 100 ¨ 1000 for beads, and e.g., 0.5 ¨
100
for powders. The density and hardness of the polysilocarb ceramic pigment can
be varied, controlled and predetermined by the precursor formulations used, as
well as the curing and pyrolzing conditions. The polysilocarb ceramic pigments
can provided enhanced corrosion resistance, scratch resistance and color (UV)
stability to paint formulations. Optical properties or effects of the
polysilocarb
ceramic pigment can, among other ways, be controlled by the use of different
gases and gas mixtures, as well as other curing and pyrolysis conditions. The
polysilocarb ceramic pigment loading can be used anywhere from a 1`)/0 to a
40%
in order to achieve the desired effect. Further, the use of the polysilocarb
ceramic pigments can provide enhanced flame retardant benefits. The
polysilocarb ceramic pigments have a further advantage of being low dusting,
and easily mixed into any type of paint formulations, e.g., latex, enamel,
polyurethanes, automotive OEM and refinish, alkyd, waterborne, acrylic and
polyol coatings formulations. The polysilocarb ceramic pigments can also be
used as a fine colorant in inks and graphic arts formulations.
[00461] EXAMPLE 87
[00462] A polysilocarb formulation is formed into the shape of a larger
internal diameter (from about 5 inches to about 36 inches, or larger) tube
111
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
structure. This tube structure is reinforced with reinforcing material
arranged to
provide both hoop strength (e.g., burst and crush) and axial strength (e.g.,
bending, elongation, and compression). The thickness of the wall of the tube
is
dependent upon the end use requirements, the reinforcement material, and
whether the material is a plastic, ceramic or plastic-ceramic. By way of
example,
the thickness could range from about less than a 1/4 inch to 5 inches or more.
These polysilocarb tube structures are then cured, to preferable to an end
cure.
They may also be pyrolized into a ceramic, which may be subject to additional
reinfiltration, curing and pyrolysis steps (one to five or more) to provide a
ceramic, or they may be subjected to additional reinfiltration and curing
steps
(one to three or more) to provide a plastic-ceramic. Once so formed, the tube
structures can be subject to: further processing, e.g., machining to
specification;
the incorporation of end assemblies, e.g., flanges, couplings, joints,
connectors;
the incorporation of secondary lines (external, internal and both), e.g., data
lines,
choke lines, kill lines, hydraulic lines, electric lines, etc.; they may have
a second
tube formed around them, or may be inserted into or over a second tube forming
a tube-in-tube structure (e.g., a double walled pipeline); and the addition of
sensors and monitoring equipment (although it should be noted that break
detection devices, in for example the form of wires, or optical fibers, could
be
incorporated into the wall of the tube structure during formation). These tube
structures provide many features include increased strength to weight rations
(e.g., stronger and lighter, same strength and lighter, stronger and same
weight).
[00463] EXAMPLE 88
[00464] The polysilocarb tube structure of EXAMPLE 87 is a marine
riser for off shore hydrocarbon exploration and production activities. Each
riser
section is about 75 feet long and the riser sections are capable of being
assembled into a deep sea rise that can extend down from the surface to the
sea
floor for depth of more than 5,000 ft, 10,000 ft, 15,000 ft, 20,000 ft or
more. The
riser has is substantially less dense than traditional steel risers. Thus, the
riser
wall can have a density of less than about 5 g/cm3, and less than about 3
g/cm3,
which is substantially less than the density of steel, which is about 7.8
g/cm3.
112
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
The reduced density of the polysilocarb riser reduces the amount of flotation
needed, and thus reduces the over all outer diameter of the as deployed riser.
The smaller outer diameter provides the advantage of reducing the amount of
surface area that is subject to currents and thus reduces the stresses place
upon
the riser.
[00465] EXAMPLE 89
[00466] A polysilocarb formulation is coated onto the surface of a pipe
and soft cured. The polysilocarb formulation can be net or it can be filled,
for
example, with pyrolized polysilocarb ceramic fines (along the lines of Example
.
This process is repeated until the desired thickness of the coating is
obtained.
Once the desired thickness of the coating has been obtained the soft cured
layers are end cured. A coating along the exterior of the pipe can provide
thermal insulation to the pipe, as well as, corrosion resistance. An internal
coating to the pipe may also provide thermal insulation for the materials in
the
pipe, and provides corrosion resistance. The corrosion resistance provided by
the cured polysilocarb internal coating can enable the replacement of more
expensive stainless steel and other high priced alloys with lower priced
metals.
[00467] EXAMPLE 90
[00468] A polysilocarb formulation is added as a layer, component, or
the entire out covering, e.g., insulation, for electrical wiring. The
polysilocarb
formulation is hard cured and provides both mechanical strength, and fire
resistance to the wiring covering.
[00469] EXAMPLE 91
[00470] High purity alpha, beta and both, SiC is made by curing a
polysilocarb formulation, grinding the formulation to about 0.1 pm to about 10
mm. The cured particles are then pyrolized, and controlling the shrinkage
during
pyrolysis the end size of the pyrolized particles can be predetermined and
controlled. Preferably, the SiC particles are less than 1 pm. Shrinkage rates
can be controlled by the polysilocarb formulation and by the pyrolzing
conditions.
The pyrolysis is conducted in excess of 1,400 C, and more preferable at about
1,600 C, under vacuum, in an inert atmosphere or under a reduced pressure
113
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
inert atmosphere. At about 1,650 C primarily beta SiC will be produced. At
temperatures above 2,200 C to about 2,400 C primarily alpha SiC will be
produced.
[00471] EXAMPLE 92
[00472] A polysilocarb formulation is hard cured to form polysilocarb
cured aggregate. The aggregate is be added to concrete.
[00473] EXAMPLE 93
[00474] A polysilocarb formulation is formed into a porous ceramic
structure to serve as a support for a catalyst. The porous polysilocarb
ceramic
support can be in the shape of beads, pellets, honeycombs, and any other shape
or configuration that is used as a catalyst support or system. The porous
polysilocarb ceramic support can be a catalytic converter for a vehicle. In
addition to the support for the catalyst, the outer structure, housing,
components
and assembly of the catalytic device can also be made from a polysilocarb
plastic, ceramic or plastic-ceramic.
[00475] EXAMPLE 94
[00476] A polysilocarb formulation is formed into a ceramic exhaust for a
vehicle, such as an automobile, truck, and motorcycle.
[00477] EXAMPLE 95
[00478] A polysilocarb formulation is coated on a wall material and
cured. The coating provides a vapor barrier, reducing and preferably
preventing,
for example, odors in the wall from fire damage, from being smelled in the
room.
[00479] EXAMPLE 96
[00480] The treatment of pyrolized polysiloxane materials, such as for
example, proppants and other volumetric shapes, with silanes, anti-static
agents
and combinations of these has the ability to increase, and significantly
increase
the strength of the pyrolized materials.
[00481] Thus, treating composition may optionally contain conventional
additives such as rheology modifiers, fillers, coalescents such as glycols and
glycol ethers to aid in proppant storage stability, antifoaming agents such as
Drew L-139 (commercially available from Drew Industries, a division of Ashland
114
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
Chemical), antistatic agents such as Emerstat 6660A (commercially available
from Cognis) or Katex 6760 (from Pulcra Chemicals), dust suppression agents,
and/or other conventional additives. Additives may be present in the coatings
composition from trace amounts (such as <about 0.1`)/0 by weight the total
composition) up to about 5.0% by weight of the total composition.
[00482] The preferable treating solution contains a silane, Silquest
A1100 from Momentive and has the following chemical formula,
H2NCH2CH2CH2Si(OCH2C1-13)3.
[00483] To treat proppant the following procedure may be utilized.
Wash the Proppant in water (current procedure) to remove fines, Wash the
Proppant in Silane/Antistat aqueous solution for 5min (at 25C). Remove
Proppant and save all the excess Silane/Antistat solution for multiple use.
Dry the
Proppant at 105-110C for 30mins-1hr (preferably it should be completely dry).
[00484] By way of example, 40 mesh proppant having a crush strength
of 13,200 psi was treated using the above procedure and exhibited crush
strengths that exceeded 17,600 psi, and more. The fine percentage for these
silane treated proppants was less than 1.7%, and lower.
[00485] EXAMPLE 97
[00486] A polymer derived ceramic having 41`)/0 Si, 31 % 0, and 27 % C
(with 27.5% of the carbon being the Si-C bonded type, and the remaining carbon
being the free carbon type), is used in structural components, members,
abrasives, devices and applications for the ceramics that are set forth in the
above Examples and embodiments of the figures.
[00487] EXAMPLE 98
[00488] A polymer derived ceramic having 45 % Si, 22 % 0, and 33% C
(with 34.4% of the carbon being the Si-C bonded type, and the remaining carbon
being the free carbon type), is used in structural components, members,
abrasives, devices and applications for the ceramics that are set forth in the
above Examples and embodiments of the figures.
115
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
[00489] EXAMPLE 99
[00490] A polymer derived ceramic black pigment having 44 % Si, 31 %
0, and 25% C (with 27.3% of the carbon being the Si-C bonded type, and the
remaining carbon being the free carbon type), is used in structural
components,
members, abrasives, devices and applications for the ceramics that are set
forth
in the above Examples and embodiments of the figures.
[00491] EXAMPLE 100
[00492] A polymer derived ceramic black pigment having 50 % Si, 20 %
0, and 30% C (with 25% of the carbon being the Si-C bonded type, and the
remaining carbon being the free carbon type), is used in structural
components,
members, abrasives, devices and applications for the ceramics that are set
forth
in the above Examples and embodiments of the figures.
[00493] EXAMPLE 101
[00494] A polysilocarb batch having 75% MH, 15% TV, 10% VT and 1%
catalyst (10 ppm platinum and 0.5% Luperox 231 peroxide) is cured and
pyrolized to form a ceramic suitable for several applications including as a
black
ceramic pigment.
[00495] EXAMPLE 102
[00496] A polysilocarb batch having 70% MH, 20% TV, 10% VT and 1`)/0
catalyst (10 ppm platinum and 0.5% Luperox 231 peroxide) is cured and
pyrolized to form a ceramic suitable for several applications including as a
black
ceramic pigment.
[00497] EXAMPLE 103
[00498] A polysilocarb batch having 50% by volume carbon black is
added to a polysilocarb batch having 70% MH, 20% TV, 10% VT and 1% catalyst
(10 ppm platinum and 0.5% Luperox 231 peroxide) is cured and pyrolized to form
a ceramic suitable for several applications including as a black ceramic
filled
pigment.
[00499] EXAMPLE 104
[00500] A ceramic polysilocarb proppant is placed down hole in a deep
hydrocarbon production well, the proppant functions as a "cracking" medium for
116
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
the hydrocarbons in the formation by placing the proppant into the reservoir
and
using the heat of the formation in combination with to crack to lighter
hydrocarbons in situ. The proppant may further have a catalysis incorporated
into it to enhance the ability to crack the hydrocarbons.
[00501] EXAMPLE 105
[00502] The polysicocarb formulation is formed into a fiberglass like
material, blown insulation like material, fiber mats, and similar types of
insulating,
and insulation structures and configurations.
[00503] EXAMPLE 106
[00504] Sand, or any material with porosity, including micro-porosity has
polysilocarb formulations vacuum infiltrated into the material. The vacuum
infiltrated material can be cured and pyrolized. This step can be repeated.
This
vacuum infiltration process with he polysilocarb formulations gives
predetermined
enhanced materials.
[00505] EXAMPLE 107
[00506] The polysilocarb formulations are pyrolized to form a black
pigment capable of being use in sintering process.
[00507] EXAMPLE 108
[00508] Polysilocarb ceramics are formed into beads. The beads are
used to fill voids in vehicle panels, doors, vests, other structures, etc. The
beads
are lightweight and impart ballistic resistant capabilities to the panels. The
beads
can further provide fire resistance to the panels, vehicles and structures.
[00509] EXAMPLE 109
[00510] Polysilocarb ceramics can be formed into fire place boxes, grills,
ovens, fire fighting tools, gun lockers, safes, waste containers and ball
bearings.
[00511] EXAMPLE 110
[00512] The polysilocarb ceramics can be forming into powers for
addition to and use in cosmetics.
[00513] EXAMPLE 111
[00514] The polysilocarb formulations as cured, pyrolized and both
beads can be added to cement, concrete or other flowable-curing building
117
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
materials, in conjunction with or as a replace for aggregate. The ceramic has
considerably greater strength than glass beads, and is considerably lighter
than
most aggregate. It can be added to the building material while it is flowable.
[00515] It is noted that there is no requirement to provide or address the
theory underlying the novel and groundbreaking processes, materials,
performance or other beneficial features and properties that are the subject
of, or
associated with, embodiments of the present inventions. Nevertheless, various
theories are provided in this specification to further advance the art in this
area.
These theories put forth in this specification, and unless expressly stated
otherwise, in no way limit, restrict or narrow the scope of protection to be
afforded the claimed inventions. These theories many not be required or
practiced to utilize the present inventions. It is further understood that the
present inventions may lead to new, and heretofore unknown theories to explain
the function-features of embodiments of the methods, articles, materials,
devices
and system of the present inventions; and such later developed theories shall
not
limit the scope of protection afforded the present inventions.
[00516] The various embodiments of formulations, plastics, articles,
components, parts, uses, applications, methods, activities and operations set
forth in this specification may be used for various other fields and for
various
other activities, uses and embodiments. Additionally, these embodiments, for
example, may be used with: existing systems, articles, components, operations
or activities; may be used with systems, articles, components, operations or
activities that may be developed in the future; and with such systems,
articles,
components, operations or activities that may be modified, in-part, based on
the
teachings of this specification. Further, the various embodiments and examples
set forth in this specification may be used with each other, in whole or in
part,
and in different and various combinations. Thus, for example, the
configurations
provided in the various embodiments and examples of this specification may be
used with each other; and the scope of protection afforded the present
inventions
should not be limited to a particular embodiment, example, configuration or
118
CA 02940678 2016-08-24
WO 2015/131168
PCT/US2015/018211
arrangement that is set forth in a particular embodiment, example, or in an
embodiment in a particular Figure.
[00517] The invention may be embodied in other forms than those
specifically disclosed herein without departing from its spirit or essential
characteristics. The described embodiments are to be considered in all
respects
only as illustrative and not restrictive.
119