Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02941988 2016-09-08
=
DESCRIPTION
Title of the Invention:
ALUMINUM ALLOY PLATE HAVING EXCELLENT MOLDABILITY AND BAKE
FINISH HARDENING PROPERTIES
Technical Field
[0001]
The present invention relates to an Al-Mg-Si alloy sheet. The aluminum alloy
sheet referred to in the present invention means an aluminum alloy sheet that
is a rolled
sheet such as a hot rolled sheet or a cold rolled sheet and has been subjected
to refining
such as a solution heat treatment and a quenching treatment, but is not yet
subjected to a
press forming and a bake hardening treatment. Further, aluminum is hereinafter
also
referred to as Al.
Background Art
[0002]
In recent years, because of environmental awareness and the like, the
society's
requirement for weight reduction in a vehicle such as an automobile has been
steadily
increasing. In order to respond to such requirement, as a material for a large
body panel
structure (an outer panel or an inner panel) of an automobile instead of a
steel material
such as a steel sheet, application of an aluminum alloy material excellent in
formability
and bake hardenability and lighter in weight has been increasing.
[0003]
Among the large body panel structure of an automobile, for an outer panel
(outer
sheet) such as a hood, a fender, a door, a roof, or a trunk lid, use of an Al-
Mg-Si-based AA
or JIS 6000-series (hereinafter, also simply referred to as a 6000-series)
aluminum alloy
sheet, as a thin and high strength ahuninum alloy sheet, has been studied.
[0004]
The 6000-series aluminum alloy sheet contains Si and Mg as essential
components. In particular, a 6000-series aluminum alloy with excess Si has a
composition in which the Si/Mg mass ratio is 1 or greater, and has excellent
age
hardenability. Because of this, formability for press forming or bending into
the outer
panels of automobiles is secured by lowering the proof stress. In addition, it
has such
bake hardenability (hereinafter referred to also as BH response) that it
undergoes age
hardening upon heating in an artificial aging (hardening) treatment performed
at a
relatively low temperature, such as the baking treatment of formed panels, and
hence
improves in proof stress, thereby ensuring the strength required as a panel.
[0005]
1
CA 02941988 2016-09-08
On the other hand, as is known well, an outer panel of an automobile is
manufactured by applying combined formings, such as stretch forming or bending
forming
in press forming, to an aluminum alloy sheet. For example, in a large outer
panel such as
a hood or a door, the shape of a formed product is made as an outer panel by
press forming
such as stretching, and then joining with an inner panel is executed by hem
work
(hemming) of a flat hem and the like of the outer panel peripheral section to
be formed into
a panel structural body.
[0006]
Here, the 6000-series aluminum alloy had an advantage of having excellent BR
response, but had a problem of having aging properties at room temperature,
that is, of age
hardening during retention at room temperature after solution heat treatment
and
quenching treatment to increase the strength, thereby deteriorating
formability into a panel,
particularly the bendability. For example, in a case where a 6000-series
aluminum alloy
sheet is to be used for an automobile panel, it is placed at room temperature
(standing at
room temperature) for approximately 1 month after the solution heat treatment
and the
quenching treatment (after manufacturing) at an aluminum manufacturer until
forming into
a panel at an automobile manufacturer, and comes to be significantly age
hardened (room-
temperature aged) during that time. Particularly, in the outer panel subjected
to severe
bending, there was such a problem that, although forming was possible without
any
problem immediately after manufacturing, cracking occurred in hem working
after the
lapse of 1 month. Therefore, in the 6000-series aluminum alloy sheet for an
automobile
panel, particularly for an outer panel, it is necessary to suppress room-
temperature aging
over a comparatively long period of approximately 1 month.
[0007]
Moreover, in the case where such room-temperature aging is great, a problem
also
occurs in that the BR response deteriorate and the proof stress is not
improved to the
strength required as a panel by heating during an artificial aging (hardening)
treatment at a
comparatively low temperature, such as a bake treatment and the like of the
panel after
forming described above.
[0008]
Hereto, from the standpoint of the structure of 6000-series aluminum alloy
sheets,
in particular, the compounds (crystals or precipitates) formed by elements
contained
therein, various proposals have been made on property improvements such as
improvements in formability or BR response and inhibition of room-temperature
aging.
Recently, in particular, it has been proposed to make an attempt to directly
examine and
control clusters (aggregates of atoms) which affect the BH response and room-
temperature
aging properties of 6000-series aluminum alloy sheets (Patent Documents 1 to
3).
2
CA 02941988 2016-09-08
Among these, in Patent Document 1, clusters (aggregates of atoms) which affect
BH response and room-temperature aging properties are analyzed through a
direct
examination of the structure of the sheet as such with a transmission electron
microscope at
a magnification of one million and, among the clusters (aggregates of atoms)
observed, the
average number density of clusters having a circle equivalent diameter of in
the range of 1
to 5 nm is regulated so as to be in a certain range, thereby attaining
excellent BH response
and suppressed room-temperature aging.
[0009]
In contrast, in Patent Documents 2 and 3, in place of directly examining
clusters
(aggregates of atoms) as in Patent Document 1, by using a three-dimensional
atom probe
field ion microscope and from positional information of atoms of the sheet,
temporarily
ionized in a high electric field (electric-field evaporation), aggregates of
atoms are
specified, which are defined in relation to a structure of atoms in the sheet
reconstructed by
the analysis. More specifically, atom aggregates are controlled so that they
include ten or
more pieces of either or both of Mg atom and Si atom in total and satisfies a
requirement
that when any atom of the Mg atom and the Si atom contained therein is used as
a
reference, a distance between the atom as the reference and any atom among
other atoms
adjacent thereto is 0.75 nm or less. Namely, the average number density, size
distribution
or proportion of atom aggregates which satisfy these requirements is
specified.
[0010]
Furthermore, in prior patent documents which are relevant to the addition of
Sn
according to the present invention, many methods have been proposed in which
Sn is
positively added to a 6000-series aluminum alloy sheet to suppress room-
temperature
aging and improve BH response (bake hardenability). For example, Patent
Document 4
proposes a method in which Sn is added in an appropriate amount and a solution
treatment
and subsequently preliminary aging are performed to thereby obtain both of
suppressed
room-temperature aging properties and BH response. Patent Document 5 proposes
a
method in which Sn and Cu, which improves formability, are added to improve
formability, BH response and corrosion resistance.
Prior Art Documents
Patent Documents
[0011]
Patent Document 1: JP-A-2009-242904
Patent Document 2: JP-A-2012-193399
Patent Document 3: JP-A-2013-60627
Patent Document 4: JP-A-09-249950
Patent Document 5: JP-A-10-226894
3
CA 02941988 2016-09-08
Summary of the Invention
Problem that the Invention is to Solve
[0012]
However, even in those conventional Al-Mg-Si alloy sheets, there has still
been
room for obtaining both of satisfactory formability and high BH response after
long-term
room-temperature aging.
[0013]
The various outer panels for automobiles are required, from the standpoint of
design, to attain strain-free, beautiful curved-surface configurations and
character lines.
Such requirements are becoming severer year by year since high-strength
aluminum alloy
sheet materials which are difficult to form are being adopted for the purpose
of weight
reduction. There is hence a growing desire in recent years for an aluminum
alloy sheet
having even better formability. However, with the conventional structure
control
described above, it is impossible to meet such requirements.
[0014]
For example, one cause which renders high-strength aluminum alloy sheets
difficult to apply to such outer panels is the problem concerning the shapes
peculiar to
outer panels. Recessed portions having given depths (protrudent portions,
embossed
portions) for attaching devices or members, such as knob mount bases, lamp
mount bases
and license (number plate) mount bases, or for drawing wheel arches are partly
provided to
outer panels.
[0015]
=
In the cases when such a recessed portion is press-formed together with
consecutive curved surfaces around the recessed portion shape, face strains
are prone to
occur and it is difficult to attain the strain-free, beautiful curved-surface
configuration and
character line. Consequently, it is essential for the outer panels that the
occurrence of
such face strains should be inhibited when the raw sheets are formed.
[0016]
The problem of such face strains is not a problem only for those recessed
portions
(protrudent portions) but a problem common to automotive panels which partly
have a
recessed portion (protrudent portion) that may suffer a face strain, such as a
saddle-shaped
portion of a door outer panel, a vertical wall portion of a front fender, a
window corner
portion of a rear fender, a character-line termination portion of a trunk lid
or hood outer
panel, and a root portion of a rear fender pillar.
[0017]
From the standpoint of attaining improved formability for inhibiting the
occurrence of the face strains to overcome the conventional problem described
above, it is
4
CA 02941988 2016-09-08
desirable that a sheet in press forming (having undergone room-temperature
aging after
production) should have a 0.2% proof stress reduced to less than 110 MPa.
However, in
the cases when the proof stress in forming has been reduced as the above, it
is difficult to
attain a 0.2% proof stress of 200 MPa or greater after bake hardening
(hereinafter also
referred to as "after BH"), that is, to attain an increase in 0.2% proof
stress through bake
hardening of 100 MPa or greater. With the conventional structure control with
a DSC
described above, it is difficult to overcome the problem.
[0018]
A first aspect of the present invention has been achieved in order to overcome
the
conventional problem. An object thereof (hereinafter referred to also as first
object) is to
provide an aluminum alloy sheet which combines formability and bake
hardenability, that
is, which can have, in automotive-panel forming, a 0.2% proof stress reduced
to 110 MPa
or less and can have a 0.2% proof stress after BH of 200 MPa or greater.
[0019]
Meanwhile, from the standpoint of attaining improved formability for
inhibiting
the occurrence of the face strains to overcome the conventional problem, it is
desirable that
a sheet in press forming (having undergone room-temperature aging after
production)
should have, not only a 0.2% proof stress reduced to 110 MPa or less but also
a reduced
value of yield ratio, which is the ratio between tensile strength and yield
strength [(0.2%
proof stress)/(tensile strength)]. However, in the cases when the proof stress
in forming
has been reduced as the above, it is difficult to attain a 0.2% proof stress
of 190 MPa or
greater after bake hardening treatment (hereinafter referred to also as "BH"),
that is, to
attain an increase in 0.2% proof stress through bake hardening of 100 MPa or
greater.
[0020]
A second aspect of the present invention has been achieved in order to
overcome
the conventional problem. An object thereof (hereinafter referred to also as
second
object) is to provide an aluminum alloy sheet which can not only have, in
automotive-
panel forming, a 0.2% proof stress reduced to 110 MPa or less and a yield
ratio reduced to
less than 0.50 but also have a 0.2% proof stress after BH of 190 MPa or
greater to thereby
combines formability and bake hardenability and attains both an increase in BH
response
and a reduction in yield ratio.
Means for Solving the Problem
[0021]
The gist of the aluminum alloy sheet according to the first aspect of the
present
invention, which is for achieving the first object and is excellent in terms
of formability
and bake hardenability, is an Al-Mg-Si alloy sheet containing, in terms of
mass %, Mg: 0.2
to 2.0%, Si: 0.3 to 2.0% and Sn: 0.005 to 0.3%, with the remainder being Al
and
5
CA 02941988 2016-09-08
unavoidable impurities, in which a differential scanning calorimetry curve of
the aluminum
alloy sheet has an endothermic peak in a temperature range of 150 to 230 C,
that is an
endothermic peak corresponding to a dissolution of a Mg-Si cluster and that
has a peak
height of 8 pW/mg or less (including 0 W/mg), and has an exothermic peak in a
temperature range of 240 to 255 C, that is an exothermic peak corresponding to
a
formation of a Mg-Si cluster and that has a peak height of 20 AW/mg or larger.
The differential thermal analysis at each of measurement portions in the
aluminum
alloy sheet is performed under the same conditions including a test apparatus
of DSC220G,
manufactured by Seiko Instruments Inc., a reference substance of aluminum, a
sample
container made of aluminum, temperature increase conditions of 15 C/min, an
atmosphere
of argon (50 mIlmin), and a sample weight of 24.5 to 26.5 mg. The differential
thermal
analysis profile ( W) obtained is divided by the sample weight and thereby
normalized
( W/mg). Thereafter, in the range of 0 to 100 C in the differential thermal
analysis
profile, a region where the differential thermal analysis profile is
horizontal is taken as a
reference level of 0, and the height of exothermic peak from the reference
level is
measured.
[0022]
The gist of the aluminum alloy sheet according to the second aspect of the
present
invention, which is for achieving the second object and is excellent in terms
of formability
and bake hardenability, is an Al-Mg-Si alloy sheet containing, in terms of
mass %, Mg: 0.3
to 1.0%, Si: 0.5 to 1.5% and Sn: 0.005 to 0.3%, with the remainder being Al
and
unavoidable impurities, in which a solid-solution amount of Mg + Si in a
solution,
separated by a residue extraction method with hot phenol is 1.0 mass % or more
and 2.0
mass% or less, and
in which atom aggregates observed with a three-dimensional atom probe field
ion
microscope satisfy conditions that either or both of an Mg atom and an Si atom
are
contained therein by a total of 10 pieces or more and that, when any atom of
the Mg atom
and the Si atom contained therein is used as a reference, a distance between
the atom as the
reference and any atom among other atoms adjacent thereto is 0.75 nm or less,
and
regarding the atom aggregates, an average volume proportion (EVi/Vm)x100 is in
a range
of 0.3 to 1.5%, the average volume proportion (EViNAI) being a proportion of
the total
volume of the atom aggregates, in terms of the total volume EVi obtained by
summing up
volumes of the individual atom aggregates Vi (=4/3/ErG3) calculated from a
Guinier radius
r0 of the individual atom aggregates each regarded as a sphere, to a volume
VAI of the
aluminum alloy sheet measured with the three-dimensional atom probe field ion
microscope, in which
an average volume proportion (EVi1.5 or more/EVO X 100 is 20 to 70%, the
average
volume proportion (Vi1.5 or more/Vi) being a proportion of a total volume E
vi1.5 or more
6
CA 02941988 2016-09-08
obtained by summing up volumes VI 5 or more of atom aggregates each having the
Guinier
radius rG of 1.5 nm or larger to a total volume of the atom aggregates EVi.
Effects of the Invention
[0023]
With regard to the first aspect, Sn exerts such effects in the structure of
the Al-Mg-
Si alloy sheet that, at room temperature, it captures (traps) atomic holes and
thereby
inhibits diffusion of Mg and Si at room temperature, inhibits the strength
from increasing
at room temperature and, during the forming of the sheet into panels, improves
the press
formability including hem workability, drawability and punch stretch
formability
(hereinafter, this press formability is referred to also as hem workability as
a
representative). During an artificial aging treatment of the panels, such as a
baking
treatment, it releases the captured holes and hence in turn enhances the
diffusion of Mg and
Si. Consequently, the BH response can be enhanced.
[0024]
However, the present inventors have found that the addition of such Sn poses a
new problem due to peculiar properties of Sn. Specifically, in the cases when
Sn is added
and a sheet is produced by an ordinary method, the addition of Sn rather
leads, depending
on the production conditions, to a decrease in the amount of Mg-Si clusters
which
contribute to strength. There are hence cases where the addition of Sn results
in an
insufficient amount of precipitates which precipitate after a bake hardening
treatment,
making it impossible to obtain the strength required as automotive panels as
described
above.
[0025]
The reason for this is presumed to be because the Sn's effect of capturing and
releasing atomic holes is related with the fact that the solid-solution amount
of Sn in the
matrix is exceedingly small (in an ordinary means, even when the added amount
of Sn is
controlled to equal to or less than a theoretical solid-solution amount, a
large proportion
thereof crystallizes out or precipitates as compounds without coming into a
solid-solution
state). However, this presumption is uncertain.
[0026]
In any case, the addition of Sn itself may become meaningless unless problems
such as the decrease in the amount of Mg-Si clusters which contribute to
strength and the
insufficient amount of precipitates which precipitate after a bake hardening
treatment are
overcome, such problems being regarded as side effects of the addition of Sn.
[0027]
Because of this, in the present aspect, the inventors have ventured to
reconsider
sheet production processes and contrived production conditions concerning, for
example, a
7
CA 02941988 2016-09-08
preliminary aging treatment (reheating treatment) after a solution quenching
treatment as
will be described later, so that addition of Sn does not result in a decrease
in the amount of
Mg-Si clusters which contribute to strength or in an insufficient amount of
precipitates
which precipitate after a bake hardening treatment.
[0028]
The inventors have further discovered that a DSC (differential scanning
calorimetry curve) of this sheet can be applied as a standard of the structure
which can,
even when Sn has been added thereto, prevent the Mg-Si clusters that
contribute to
strength from being diminished and increase or ensure the amount of
precipitates that
precipitate after a bake hardening treatment. Specifically, in the present
aspect, based on
the DSC, an endothermic peak corresponding to the dissolution of relatively
small Mg-Si
clusters, which do not contribute to strength, is controlled and meanwhile an
exothermic
peak corresponding to the formation of relatively large Mg-Si clusters, which
contribute to
strength, is enhanced. Thus, Mg-Si clusters that do not contribute to strength
are
suppressed and the Mg-Si clusters that contribute to strength are increased,
thereby
obtaining desired BH response.
[0029]
As a result, according to the present aspect, it is possible to provide an
aluminum
alloy sheet which combines formability and bake hardenability and which
contains Sn and
can have a 0.2% proof stress in automotive-panel forming reduced to 110 MPa or
less and
have a 0.2% proof stress after BH of 200 MPa or greater.
[0030]
In the second aspect, first in order to ensure formability of the Al-Mg-Si
alloy
sheet into the outer panels (hereinafter, this press formability is referred
to also as hem
workability as a representative), the sheet in forming is aimed to have a 0.2%
proof stress
reduced to 110 MPa or less and a yield ratio reduced to less than 0.50.
[0031]
Because of this, the solid-solution amount of Mg and Si is controlled in the
present aspect in addition to the alloy composition including Mg and Si.
Furthermore, by
adding Sn, the BH response is enhanced while ensuring the formability. As will
be
described later, Sn has an important effect of attaining both an increase in
BH response and
a reduction in yield ratio by reducing the volume proportion of atom
aggregates which
inhibit the yield ratio from being reduced, even when the solid-solution
amount of Mg + Si
is increased.
[0032]
Furthermore, in the present aspect, the size distribution of atom aggregates
observed with a three-dimensional atom probe field ion microscope is further
specified in
8
CA 02941988 2016-09-08
1
order to control Mg-Si atom aggregates, in addition to the means described
above, so that
the yield ratio during sheet forming can be reliably reduced to less than
0.50.
[0033]
The term "atom aggregates observed with a three-dimensional atom probe field
ion microscope" here means known atom aggregates including the measurement
methods
described in Patent Documents 2 and 3, and does not mean atom aggregates
(clusters)
observed by directly examining the sizes and state thereof in the structure of
the sheet with
a high-magnification TEM by using the structure of the sheet as such, as in
Patent
Document 1. In other words, as in Patent Documents 2 and 3, those are the atom
aggregates in a three-dimensional structure of atoms (three-dimensional atom
map)
obtained by a reconstruction through analysis from the flight times and
positions of atoms
of the sheet which have temporarily ionized in a high electric field (electric-
field
evaporation) with a three-dimensional atom probe field ion microscope, as the
details of
the measuring method will be described later. Those are the atom aggregates
which are
defined to satisfy the given requirements specified in claim 1 (that is,
deemed to be atom
aggregates) in the three-dimensional structure of atoms.
[0034]
In the present aspect, in order to reduce a yield ratio to less than 0.50, as
a size
distribution of the atom aggregates observed with a three-dimensional atom
probe field ion
microscope, the proportion of atom aggregates that satisfy the requirements
that Mg atom
and/or Si atom is contained and the distance between the atoms are 0.75 nm or
less, is
regulated so as to be in a certain range in terms of volume proportion. In
addition, the
proportion of relatively large atom aggregates which each have a Guinier
radius rG of 1.5
nm or larger, among those atom aggregates, is increased in a certain range in
terms of
volume proportion.
[0035]
As a result, according to the present aspect, it is possible to provide an
aluminum
alloy sheet which combines formability and bake hardenability and which
contains Sn and
can not only have, in automotive-panel forming, a 0.2% proof stress reduced to
110 MPa
or less and a yield ratio reduced to less than 0.50 but also have a 0.2% proof
stress after
BH of 190 MPa or greater.
Brief Description of the Drawing
[0036]
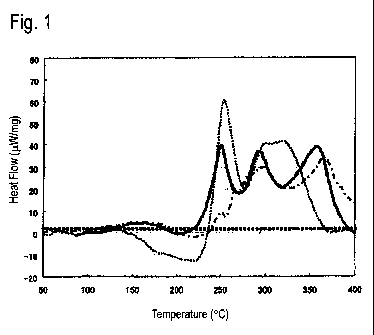
[Fig. 1] Fig. 1 is an explanatory view which shows each DSC of Examples
according to the
first aspect.
Modes for Carrying Out the Invention
9
CA 02941988 2016-09-08
[0037]
(First Aspect)
The first aspect of the present invention will be explained below in detail
with
respect to each requirement.
[0038]
(Chemical Component Composition)
First, the chemical component composition of the Al-Mg-Si (hereinafter
referred
to also as 6000-series) aluminum alloy sheet according to the present aspect
is explained
below. The 6000-series aluminum alloy sheet targeted by the present aspect,
as, for
example, the sheet for the automotive outer panels, is required to have
various properties
such as excellent formability, BH response, strength, weldability, and
corrosion resistance.
Consequently, such requirements are also met by means of the composition. In
addition,
in the present aspect, Sn is incorporated to suppress the room-temperature
aging of the
sheet after production and to reduce a 0.2% proof stress in the panel forming
to 110 MPa
or less. Thus, the formability into automotive panels or the like, which are
particularly
problematic in face strains thereof, in automotive panel structures, can be
improved.
Simultaneously therewith, a 0.2% proof stress after bake hardening of 200 MPa
or greater
is rendered possible by means of the composition.
[0039]
In order to satisfy such requirements, the aluminum alloy sheet according to
the
present aspect has a composition which includes, in terms of mass %, Mg: 0.2
to 2.0%, Si:
0.3 to 2.0% and Sn: 0.005 to 0.3%, with the remainder being Al and unavoidable
impurities. All the content indicated in % of the elements means that in mass
%. In this
description, percentage on mass basis (mass %) is the same as percentage on
weight basis
(wt%). With respect to the content of a chemical component, there are cases
where "X%
or less (exclusive of 0%)" is expressed by "more than 0% and X% or less".
[0040]
In the present aspect, elements other than Mg, Si and Sn are impurities or
elements which may be contained, and may have contents (permissible amounts)
on levels
of the elements in accordance with the AA or JIS standards, etc.
[0041]
Namely, there are cases, in the present aspect also, where not only high-
purity Al
base metal but also 6000-series alloys containing elements other than Mg and
Si as
additive elements (alloying elements) in large amounts, other aluminum alloy
scrap
materials, low-purity Al base metal, and the like are used in large quantities
as melted raw
materials for the alloy, from the standpoint of resource recycling. In such
cases, other
elements such as shown below are inevitably included in substantial amounts.
Since
refining performed for intentionally diminishing these elements itself leads
to an increase
CA 02941988 2016-09-08
=
in cost, it is necessary to accept some degree of inclusion. There are useful
content
ranges which permit inclusion of such elements in substantial amounts but do
not inhibit
the object or effects of the present aspect.
[0042]
Consequently, in the present aspect, inclusion of such elements shown below is
permissible within the range of equal to or less than the upper limits
specified below,
which are in accordance with the AA or JIS standards or the like.
[0043]
Specifically, the aluminum alloy sheet may further contain one kind or two or
more kinds selected from the group consisting of Fe: 1.0% or less (exclusive
of 0%), Mn:
1.0% or less (exclusive of 0%), Cr: 0.3% or less (exclusive of 0%), Zr: 0.3%
or less
(exclusive of 0%), V: 0.3% or less (exclusive of 0%), Ti: 0.1% or less
(exclusive of 0%),
Cu: 1.0% or less (exclusive of 0%), Ag: 0.2% or less (exclusive of 0%), and
Zn: 1.0% or
less (exclusive of 0%), within those ranges, in addition to the basic
composition shown
above.
[0044]
In the cases where these elements are contained, the content of Cu is
preferably
0.7% or less and more preferably 0.3% or less, because Cu is prone to impair
the corrosion
resistance when contained in a large amount. Mn, Fe, Cr, Zr, and V are prone
to yield
relatively coarse compounds when contained in large amounts, and are prone to
impair the
hem workability (hem bendability), which is addressed by the present aspect.
Consequently, the content of Mn is preferably 0.6% or less and more preferably
0.3% or
less, and the content of each of Cr, Zr and V is preferably 0.2% or less and
more preferably
0.1% or less.
[0045]
The content range of each element and the purposes and permissible amount
thereof in the 6000-series aluminum alloy are explained below in order.
[0046]
Si: 0.3 to 2.0%
Si, together with Mg, is an essential element for obtaining the strength
(proof
stress) required as automotive panels by forming aging precipitates which
contribute to an
improvement in strength, during an artificial aging treatment such as a baking
treatment,
and thus exhibiting an age hardenability. In the case where the addition
amount of Si is
too small, the amount of precipitates after artificial aging is too small and
the increase in
strength through baking is too small. Meanwhile, in the case where the content
of Si is
too high, the Si forms coarse crystals with impurity Fe, etc., resulting in a
considerable
decrease in formability such as bendability. In addition, too high Si contents
increases not
only the strength just after sheet production but also the room-temperature
aging amount
11
CA 02941988 2016-09-08
after the production, thereby increases the strength before forming too much,
and reduces
the formability into automotive panels or the like, which are particularly
problematic in
face strains thereof, in automotive panel structures. Consequently, the
content of Si is
regulated so as to be in the range of 0.3 to 2.0%.
[0047]
For attaining an excellent age hardenability in a baking treatment performed
at a
lower temperature for a shorter period after forming into panels, it is
preferable to employ
a 6000-series aluminum alloy composition in which Si/Mg is 1.0 or larger in
terms of mass
ratio so that Si has been incorporated further excessively relative to the Mg
than in the so-
called excess-Si type.
[0048]
Mg: 0.2 to 2.0%
Mg, together with Si, is also an important element for forming the clusters
specified in the present aspect. It is an essential element for obtaining the
proof stress
required as panels by forming, together with the Si, aging precipitates which
contribute to
an improvement in strength, during an artificial aging treatment such as a
baking treatment,
and thus exhibiting an age hardenability. In the case where the content of Mg
is too low,
the amount of precipitates after artificial aging is too small and the
strength after baking is
thus too low. Meanwhile, in the case where the content of Mg is too high, the
Mg forms
coarse crystals with impurity Fe, etc., resulting in a considerable decrease
in formability
such as bendability. In addition, too high Mg contents increases not only the
strength just
after sheet production but also the room-temperature aging amount after the
production,
thereby increases the strength before forming, and reduces the formability
into automotive
panels or the like, which are particularly problematic in face strains
thereof, in automotive
panel structures. Consequently, the content of Mg is regulated so as to be in
the range of
0.2 to 2.0%.
[0049]
Sn: 0.005 to 0.3%
Sn, at room temperature, has the effect of capturing (trapping) atomic holes
to
thereby inhibit room-temperature diffusion of Mg and Si and inhibit the
strength from
increasing at room temperature, and during the forming of the sheet into
panels, improving
the press formability including hem workability, drawability and punch stretch
formability
(hereinafter, this press formability is referred to also as hem workability as
a
representative). During an artificial aging treatment of the panels, such as a
baking
treatment, it releases the captured holes and hence in turn enhances the
diffusion of Mg and
Si, thereby enhancing the BH response. In the case where the content of Sn is
lower than
0.005%, the Sn cannot sufficiently trap holes and is unable to exhibit the
effects thereof.
Meanwhile, in the case where the content of Sn is higher than 0.3%, the Sn
segregates at
12
CA 02941988 2016-09-08
'
grain boundaries and it is prone to cause intergranular cracks. A preferred
lower limit of
the content of Sn is 0.01%. An upper limit of the content of Sn is preferably
0.2%, more
preferably 0.1% and further preferably 0.06%.
[0050]
(Structure)
The composition described above is employed and furthermore, in the present
aspect, the 6000-series aluminum alloy sheet is made to have the following
structure. In
order to ensure high strength as automotive panels or the like, a DSC of this
sheet is used
as a measure for ensuring the amount of precipitates which precipitate after a
bake
hardening treatment, and an endothermic peak and an exothermic peak in
specific
temperature ranges, which affect, in particular, the strength before baking
and an increase
in strength through the baking are controlled. In other words, a DSC of this
sheet is used
to control an endothermic peak and an exothermic peak in specific temperature
ranges,
which affect, in particular, the strength before baking and an increase in
strength through
the baking, so that the addition of Sn does not result in a decrease in the
amount of Mg-Si
clusters which contribute to strength or result in insufficient amount of
precipitates which
precipitate after a bake hardening treatment.
[0051]
More specifically, in the present aspect, based on the DSC, an endothermic
peak
corresponding to the dissolution of relatively small Mg-Si clusters, which do
not contribute
to strength, is controlled and meanwhile an exothermic peak corresponding to
the
formation of relatively large Mg-Si clusters, which contribute to strength, is
enhanced.
Thus, Mg-Si clusters that do not contribute to strength are suppressed and the
Mg-Si
clusters that contribute to strength are increased, thereby obtaining desired
BH response.
[0052]
Here, the differential scanning calorimetry curve (DSC) is a heating curve
from
solid phase, obtained by measuring the thermal changes during melting of
aluminum alloy
sheet after the refining treatment, by differential thermal analysis performed
under the
following conditions.
Specifically, the differential thermal analysis at each of measurement
portions in
the aluminum alloy sheet is performed under the same conditions including a
test apparatus
of DSC220G, manufactured by Seiko Instruments Inc., a reference substance of
aluminum,
a sample container made of aluminum, temperature increase conditions of 15
C/min, an
atmosphere of argon (50 mL/min), and a sample weight of 24.5 to 26.5 mg. The
differential thermal analysis profile OM) obtained is divided by the sample
weight and
thereby normalized (p,W/mg). Thereafter, in the range of 0 to 100 C in the
differential
thermal analysis profile, a region where the differential thermal analysis
profile is
13
CA 02941988 2016-09-08
horizontal is taken as a reference level of 0, and the height of exothermic
peak from the
reference level is measured.
[0053]
In the present aspect, the number (number density) of Mg-Si clusters that have
a
relatively small size and are apt to dissolve during temperature increase in
DSC, which are
regarded as Mg-Si clusters not contributing to strength, is inhibited first.
In the case
where the number of such Mg-Si clusters that are apt to dissolve during
temperature
increase in DSC increases upon BH, the number (number density) of Mg-Si
clusters that
have a relatively large size and are less apt to dissolve during temperature
increase in DSC,
which are regarded as contributive to strength, in turn decreases upon an
artificial age
hardening treatment, making it impossible to increase strength after BH.
Specifically, an
increase in 0.2% proof stress of 100 MPa or greater and a strength (0.2% proof
stress) after
BH of 200 MPa or greater cannot be obtained, although this depends on the BH
conditions.
[0054]
Because of this, in the present aspect, the peak height of an endothermic peak
A in
a temperature range of 150 to 230 C, as an endothermic peak corresponding to
the
dissolution of Mg-Si clusters that are apt to dissolve during temperature
increase in DSC
and do not contribute to strength, is regulated (reduced) to 8 pW/mg or less
(inclusive of 0
JAW/mg). Consequently, that the endothermic peak in the temperature range of
150 to
230 C has a peak height of 8 W/mg indicates a critical number density which
is
permissible with respect to the adverse influence on strength of the Mg-Si
clusters having a
relatively small size and not contributing to strength. Although a sheet in
which such
Mg-Si clusters having a relatively small size and not contributing to strength
are absent
(i.e., the number density thereof is 0) is difficult to produce due to
limitations on its
production, the present aspect includes such the case. Because of this, the
feature in
which the peak height of the endothermic peak A is 8 1.1W/Ing or less involves
the case of 0
p,W/mg, in which such Mg-Si clusters having a relatively small size and not
contributing to
strength are absent.
[0055]
Meanwhile, in the present aspect, Mg-Si clusters which have a relatively large
size
and are less apt to dissolve during temperature increase in DSC and which
contribute to
strength are yielded in a large amount to improve the BH response. Because of
this, the
peak height of an exothermic peak B in a temperature range of 240 to 255 C,
which
corresponds to the formation of Mg-Si clusters that contribute to strength, is
heightened
(increased) to 20 p,Wimg or more. Consequently, that the exothermic peak in
the
temperature range of 240 to 255 C has a peak height of 20 W/mg indicates a
minimum
value of the number density of Mg-Si clusters having a relatively large size
and
contributing to strength, the minimum value being necessary for obtaining the
14
CA 02941988 2016-09-08
1
improvement in BH response which is addressed by the present aspect (an
increase in 0.2%
proof stress of 100 MPa or greater and a 0.2% proof stress after BH of 200 MPa
or greater)
though it differs by the BH condition. Hence, the higher the number density,
the better,
and the larger (higher) the peak height of the exothermic peak in the
temperature range of
240 to 255 C, the better. However, in view of limitations on sheet production,
an upper
limit thereof is about 80 4W/mg.
[0056]
Fig. 1 shows DSCs of three kinds of aluminum alloy sheet in Examples which
will
be given later, i.e., Invention Example 8 and Comparative Example 9 in Table 2
and
Comparative Example 25 in Table 3. Invention Example 8 is indicated by a thick
continuous line, Comparative Example 9 is indicated by a dotted line and
Comparative
Example 25 is indicated by a dot-and-dash line.
[0057]
In Fig. 1, the DSC of Comparative Example 9 has an endothermic peak A in the
temperature range of 150 to 230 C, which has a peak height exceeding (larger
than) 8
W/mg as shown in Table 2, which will be given later, showing that the number
density of
Mg-Si clusters having a relatively small size and not contributing to strength
is too high.
Meanwhile, the exothermic peak B in the temperature range of 240 to 255 C also
has a
peak height as high (large) as 20 pW/mg or more, showing that the number
density of Mg-
Si clusters having a relatively large size and contributing to strength is
also high.
However, since the number density of the Mg-Si clusters having a relatively
small size and
not contributing to strength is too high, the adverse influences thereof are
too greater.
Therefore, the desired BH response (an increase in 0.2% proof stress of 100
MPa or greater
and a 0.2% proof stress after BH of 200 MPa or greater) cannot be obtained.
[0058]
The DSC of Comparative Example 25 in Fig. 1 has an endothermic peak A in the
temperature range of 150 to 230 C, which has a peak height as low (small) as 8
W/mg or
less as shown in Table 2, which will be given later, showing that the number
density of
Mg-Si clusters having a relatively small size and not contributing to strength
is low.
Meanwhile, the exothermic peak B in the temperature range of 240 to 255 C also
has a
peak height as low (small) as less than 20 JAW/mg, showing that the number
density of Mg-
Si clusters having a relatively large size and contributing to strength is
also too low.
Because of this, the desired BH response (an increase in 0.2% proof stress of
100 MPa or
greater and a 0.2% proof stress after BH of 200 MPa or greater) cannot be
obtained.
[0059]
In contrast, the DSC of Invention Example 8 in Fig. 1 has an endothermic peak
A
in the temperature range of 150 to 230 C, which has a peak height as low
(small) as 8
ilW/mg or less as shown in Table 2, which will be given later, showing that
the number
CA 02941988 2016-09-08
density of Mg-Si clusters having a relatively small size and not contributing
to strength is
low. Meanwhile, the exothermic peak B in the temperature range of 240 to 255 C
has a
peak height as high (large) as 20 JAW/mg or more, showing that the number
density of Mg-
Si clusters having a relatively large size and contributing to strength is
high. Because of
this, the desired BH response (an increase in 0.2% proof stress of 100 MPa or
greater and a
0.2% proof stress after BH of 200 MPa or greater) is obtained.
[0060]
(Production Process)
Next, a process for producing the aluminum alloy sheet according to the
present
aspect is explained. The aluminum alloy sheet according to the present aspect
is
produced through production steps which themselves are common or known, by
subjecting, after casting, an aluminum alloy slab having the 6000-series
component
composition to a homogenizing heat treatment, hot rolling and cold rolling to
obtain a
given sheet thickness, followed by a refining treatment such as a solution
quenching
treatment.
[0061]
However, for obtaining the structure specified with a DSC according to the
present aspect, during those production steps, the average cooling rate in a
quenching
treatment after a solution treatment is controlled and in addition, the
conditions for a
preliminary aging treatment after the quenching treatment are regulated so as
to be in a
preferred range, as will be described later. With respect to other steps,
there are preferred
conditions for obtaining the structure specified with a DSC according to the
present aspect.
Unless such preferred conditions are employed, it is difficult to obtain the
structure
specified with a DSC according to the present aspect.
[0062]
(Melting and casting cooling rate)
First, in melting and casting steps, an aluminum alloy molten metal that has
been
melted and regulated so as to have a component composition within the 6000-
series
composition range is cast by a suitably selected ordinary melting and casting
method, such
as a continuous casting method or a semi-continuous casting method (DC casting
method).
Here, it is preferable that the average cooling rate, during the casting, from
the liquidus
temperature to the solidus temperature is as high (quick) as possible at 30
C/min or greater.
[0063]
In the case where such temperature (cooling rate) control in a high-
temperature
range during casting is not performed, the cooling rate in this high-
temperature range is
inevitably low. When an average cooling rate in the high-temperature range is
low as the
above, the amount of crystals yielded coarsely in the temperature range of
this high-
temperature range is increased and also unevenness in the size and amount of
the crystals
16
CA 02941988 2016-09-08
along the width direction and thickness direction of the slab is increased. As
a result, the
basic mechanical properties, such as strength and elongation, which are a
prerequisite for
the 6000-series aluminum alloy sheet are reduced.
[0064]
(Homogenizing heat treatment)
Next, the aluminum alloy slab obtained by casting is subjected to a
homogenizing
heat treatment prior to hot rolling. The purpose of this homogenizing heat
treatment
(soaking treatment) is to homogenize the structure, that is, to eliminate
segregation within
the grains in the structure of the slab. The conditions are not particularly
limited so long
as the purpose is achieved therewith, and the treatment may be an ordinary one
conducted
once or in one stage.
[0065]
A homogenizing heat treatment temperature is suitably selected from the range
of
500 C or more and lower than the melting point, and a homogenizing time is
suitably
selected from the range of 4 hours and longer. In the case where the
homogenizing
temperature is low, the segregation within grains cannot be sufficiently
eliminated, and
these act as starting points for fracture, resulting in decreases in stretch
flangeability and
bendability. Thereafter, hot rolling may be started immediately.
Alternatively, hot
rolling may be started after holding and cooling to an appropriate
temperature.
[0066]
After the homogenizing heat treatment has been performed, cooling to room
temperature may be performed so that the average cooling rate in the range of
300 C to
500 C is 20 to 100 C/hr, followed by reheating to 350 C to 450 C at an average
heating
rate of 20 to 100 C/hr to start hot rolling in this temperature range.
[0067]
In the cases when the average cooling rate after the homogenizing heat
treatment
and the reheating rate conducted thereafter do not satisfy those conditions,
the possibility
of forming coarse Mg-Si compounds increases, resulting in decreases in the
basic
mechanical properties, such as strength and elongation, that are a
prerequisite for the 6000-
series aluminum alloy sheet before exhibiting the effect of Sn.
[0068]
(Hot rolling)
The hot rolling is constituted of a slab rough rolling step and a finish
rolling step
in accordance with the thickness of the plate to be rolled. In these rough
rolling step and
finish rolling step, rolling mills such as a reverse type and a tandem type
are suitably used.
[0069]
In the cases when the hot-rolling (rough-rolling) start temperature exceeds
the
solidus temperature, burning occurs and, hence, the hot rolling itself is
difficult to carry
17
CA 02941988 2016-09-08
out. Meanwhile, in the cases when the hot-rolling start temperature is lower
than 350 C,
the hot-rolling load is too high, rendering the hot rolling itself difficult.
Consequently, the
hot-rolling start temperature is preferably in the range of 350 C to the
solidus temperature,
more preferably in the range of 400 C to the solidus temperature.
[0070]
(Annealing of the hot-rolled plate)
Annealing (rough annealing) before cold rolling is not always necessary for
the
hot-rolled plate. However, it may be performed in order to further improve
properties
such as formability by making the grains smaller and optimizing the texture.
[0071]
(Cold rolling)
In cold rolling, the hot-rolled sheet is rolled to produce a cold-rolled sheet
(including a coil) having a desired final sheet thickness. However, for making
the grains
even smaller, it is desirable that the total cold rolling ratio should be 60%
or greater
regardless of the number of passes.
[0072]
(Solution treatment and quenching treatment)
After the cold rolling, a solution treatment is performed, followed by a
treatment
for quenching to room temperature. The solution and quenching treatments may
be a
heating and a cooling performed on an ordinary continuous heat treatment line,
and are not
particularly limited. However, from the standpoint of obtaining a sufficient
solid-solution
amount of each element and because it is desirable that the grains should be
finer as stated
above, it is desirable that the treatments should be conducted under such
conditions of
heating at a heating rate of 5 C/sec or greater to a solution treatment
temperature which is
520 C or higher and lower than the melting temperature, and then holding for
0.1 to 10
seconds.
[0073]
From the standpoint of suppressing the formation of coarse intergranular
compounds that reduce the formability and hem workability, it is desirable
that the average
cooling rate from the solution treatment temperature to the quenching stop
temperature,
which is room temperature, should be 3 C/s or greater. In the case where the
rate of
cooling to room temperature after the solution treatment is too low, coarse Mg-
Si and
elemental Si are yielded during the cooling, resulting in impaired
formability. In addition,
the solid-solution amount after the solution treatment is reduced, resulting
in a decrease in
BH response. In order to secure that cooling rate, means such as air cooling
with fans or
water cooling with mist or spray or by immersion, etc. and conditions therefor
are selected
and used for the treatment for quenching to room temperature.
[0074]
18
CA 02941988 2016-09-08
(Preliminary aging treatment: reheating treatment)
After having thus undergone the solution treatment and the subsequent
quenching
treatment to be cooled to room temperature, the cold-rolled sheet is subjected
to a
preliminary aging treatment (reheating treatment) within 1 hour. In the case
where the
room-temperature holding period from termination of the treatment for
quenching to room
temperature to initiation of the preliminary ageing treatment (initiation of
heating) is too
long, the small Mg-Si clusters that do not contribute to strength are yielded
in a large
amount as clusters that are apt to dissolve upon room-temperature aging,
making it difficult
to suppress a peak height of the endothermic peak in the temperature range of
150 to
230 C to 8 pW/mg or less. Consequently, the shorter the room-temperature
holding
period, the better. The solution and quenching treatments and the reheating
treatment
may be consecutively performed so that there is substantially no pause
therebetween, and a
lower limit of the period is not particularly determined.
[0075]
In this preliminary aging treatment, the rate of temperature increase to a
preliminary-aging temperature and the period of holding in a preliminary-aging
temperature range are regulated. It is preferable that the temperature
increase rate, of
these, should be as high (quick) temperature increase rate as possible at 1
C/s or higher and
preferably 5 C/s or higher, for suppressing the formation of the small Mg-Si
clusters not
contributing to strength. In the case where the temperature increase rate is
less than
1 C/s, Mg-Si clusters that are apt to dissolve during temperature increase in
DSC and that
do not contribute to strength are yielded in a large amount, making it
difficult to suppress a
peak height of the endothermic peak in the temperature range of 150 to 230 C
to 8 gW/mg
or less.
[0076]
The temperature and holding period in the preliminary aging treatment is a
holding in a temperature range of 60 to 120 C for 10 hr or more and 40 hr or
less. Here,
the holding in the temperature range of 60 to 120 C may be a heat treatment in
which the
temperature is constant or the temperature is sequentially changed by
temperature increase
or annealing, within that temperature range. In short, the temperature may be
continuously changed by annealing, temperature increase, etc., so long as it
is held in a
temperature range of 60 to 120 C for that period of 10 hr or more and 40 hr or
less.
[0077]
In the case where the preliminary-aging temperature is lower than 60 C or the
holding period is less than 10 hr, the formation of precipitate nuclei is
insufficient and this
is prone to result in a DSC in which an exothermic peak in the range of the
exothermic
peak B in the temperature range of 240 to 255 C has a peak temperature higher
than
255 C. This means that the amount of Mg-Si clusters having a relatively large
size and
19
CA 02941988 2016-09-08
contributing to strength decreases, and it becomes impossible to regulate the
exothermic
peak B in the temperature range of 240 to 255 C so as to have a peak height as
high (large)
as 20 W/mg or more. As a result, the BH response decreases.
[0078]
Meanwhile, in the case where the preliminary-aging temperature exceeds 120 C
= or the holding period exceeds 40 hr, precipitate nuclei are yielded in
too large an amount in
this preliminary aging treatment. Because of this, the Mg-Si clusters having a
relatively
large size and contributing to strength in turn decreases, making it
impossible to make the
exothermic peak B in the temperature range of 240 to 255 C have a peak height
as high
(large) as 20 W/mg or more, in a DSC. Consequently, this also results in a
decrease in
BH response. In addition, strength during forming is too high.
[0079]
Namely, unless the preliminary aging treatment is regulated to fall within
these
preferable conditions, it is difficult to attain a 0.2% proof stress, in
forming of automotive
panels, reduced to 110 MPa or less and a 0.2% proof stress after BH of 200 MPa
or greater.
[0080]
The second aspect of the present invention will be explained below in detail
with
respect to each requirement.
[0081]
(Chemical Component Composition)
First, the chemical component composition of the Al-Mg-Si (hereinafter
referred
to also as 6000-series) aluminum alloy sheet according to the present aspect
is explained
below. The 6000-series aluminum alloy sheet targeted by the present aspect,
as, for
example, the sheet for the automotive outer panels, is required to have
various properties
such as excellent formability, BH response, strength, weldability, and
corrosion resistance.
Consequently, such requirements are also met by means of the composition. In
addition,
in the present aspect, Sn is incorporated to suppress the room-temperature
aging of the
sheet after production, to reduce a 0.2% proof stress in the panel forming to
110 MPa or
less and to reduce a yield ratio to less than 0.50. Thus, the formability into
automotive
panels or the like, which are particularly problematic in face strains
thereof, in automotive
panel structures, is improved. Simultaneously therewith, a 0.2% proof stress
after bake
hardening of 190 MPa or greater is rendered possible by means of the
composition.
[0082]
In order to satisfy such requirements, the aluminum alloy sheet according to
the
present aspect has a composition which includes, in terms of mass %, Mg: 0.3
to 1.0%, Si:
0.5 to 1.5% and Sn: 0.005 to 0.3%, with the remainder being Al and unavoidable
impurities. All the content indicated in % of the elements means that in mass
%. In this
description, percentage on mass basis (mass %) is the same as percentage on
weight basis
CA 02941988 2016-09-08
(wt%). With respect to the content of a chemical component, there are cases
where "X%
or less (exclusive of 0%)" is expressed by "more than 0% and X% or less".
[0083]
In the present aspect, elements other than the Mg, Si and Sn are impurities or
elements which may be contained, and may have contents (permissible amounts)
on levels
of the elements in accordance with the AA or JIS standards, etc.
[0084]
Namely, for the same reasons as in the first aspect, inclusion of such other
elements shown below is permissible in the present aspect within the range of
equal to or
less than the upper limits specified below, which are in accordance with the
AA or JIS
standards or the like.
[0085]
Specifically, the aluminum alloy sheet may further contain one kind or two or
more kinds selected from the group consisting of Fe: 1.0% or less (exclusive
of 0%), Mn:
0.4% or less (exclusive of 0%), Cr: 0.3% or less (exclusive of 0%), Zr: 0.3%
or less
(exclusive of 0%), V: 0.3% or less (exclusive of 0%), Ti: 0.1% or less
(exclusive of 0%),
Cu: 0.4% or less (exclusive of 0%), Ag: 0.2% or less (exclusive of 0%), and
Zn: 1.0% or
less (exclusive of 0%), within those ranges, in addition to the basic
composition shown
above.
[0086]
In the cases where these elements are contained, the content of Cu is
preferably
0.3% or less, because Cu is prone to impair the corrosion resistance when
contained in a
large amount. Mn, Fe, Cr, Zr, and V are prone to yield relatively coarse
compounds when
contained in large amounts, and are prone to impair the hem workability (hem
bendability),
which is addressed by the present aspect. Consequently, the content of Mn is
preferably
0.35% or less, and the content of each of Cr, Zr and V is preferably 0.2% or
less and more
preferably 0.1% or less.
[0087]
The content range of each element and the purposes and permissible amount
thereof in the 6000-series aluminum alloy are explained below in order.
[0088]
Si: 0.5 to 1.5%
Si, together with Mg, is an essential element for obtaining the strength
(proof
stress) required as automotive panels by forming aging precipitates which
contribute to an
improvement in strength, during an artificial aging treatment such as a baking
treatment,
and thus exhibiting an age hardenability. Furthermore, solute Si is an element
that
improves the work hardenability, and Si, when present in a solid-solution
state, has the
21
CA 02941988 2016-09-08
effect of lowering the yield ratio, which is a ratio between tensile strength
and yield
strength [(0.2% proof stress)/(tensile strength)], less than 0.50.
[0089]
In the case where the content of Si is too low, the amount of precipitates
after an
artificial age hardening treatment is too small, resulting in a decrease in
the strength
increase due to baking and resulting in a reduced amount of solute Si and
hence in too
large a yield ratio exceeding 0.50. Meanwhile, in the case where the content
of Si is too
high, the Si forms coarse crystals with impurity Fe, etc., resulting in a
considerable
decrease in formability such as bendability. In addition, too high Si contents
increases not
only the strength just after sheet production but also the room-temperature
aging amount
after the production, thereby increases the strength before forming too much,
and reduces
the formability into automotive panels or the like, which are particularly
problematic in
face strains thereof, in automotive panel structures. Consequently, the
content of Si is
regulated so as to be in the range of 0.5 to 1.5%.
[0090]
For attaining an excellent age hardenability in a baking treatment performed
at a
lower temperature for a shorter period after forming into panels, it is
preferable to employ
a 6000-series aluminum alloy composition in which Si/Mg is 1.0 or larger in
terms of mass
ratio so that Si has been incorporated further excessively relative to the Mg
than in the so-
called excess-Si type.
[0091]
Mg: 0.3 to 1.0%
Mg, together with Si, is also an important element for forming the atom
aggregates specified in the present aspect. It is an essential element for
obtaining the
proof stress required as panels by forming, together with the Si, aging
precipitates which
contribute to an improvement in strength, during an artificial aging treatment
such as a
baking treatment, and thus exhibiting an age hardenability. Furthermore,
solute Mg, like
Si, is an element that improves the work hardenability, and Mg, when present
in a solid-
solution state, has the effect of lowering the yield ratio, which is a ratio
between tensile
strength and yield strength [(0.2% proof stress)/(tensile strength)], to less
than 0.50.
[0092]
In the case where the content of Mg is too low, the amount of precipitates
after an
artificial age hardening treatment is too small, resulting in a decrease in
the strength
increase due to baking and resulting in a reduced amount of solute Mg and
hence in too
large a yield ratio exceeding 0.50. Meanwhile, in the case where the content
of Mg is too
high, the Mg forms coarse crystals with impurity Fe, etc., resulting in a
considerable
decrease in formability such as bendability. In addition, too high Mg contents
increases
not only the strength just after sheet production but also the room-
temperature aging
22
CA 02941988 2016-09-08
amount after the production, thereby increases the strength before forming,
and reduces the
formability into automotive panels or the like, which are particularly
problematic in face
strains thereof, in automotive panel structures. Consequently, the content of
Mg is
regulated so as to be in the range of 0.3 to 1.0%.
[0093]
Sn: 0.005 to 0.3%
Sn has the important effect of attaining both an increase in BH response and a
reduction in yield ratio by reducing the volume proportion of atom aggregates
which
enhance the 0.2% proof stress in panel forming, even when the solid-solution
amount of
Mg + Si, which will be described later, is increased. In general, for
increasing the solid-
solution amount of Mg + Si, it is effective to increase the amount of Mg
and/or Si to be
contained in the sheet. However, such increase in Mg and Si contents in the
sheet result
not only in an increase in 0.2% proof stress in panel forming but also in an
increase in the
volume proportion of atom aggregates which inhibit yield ratio reduction. It
has hence
been difficult, with any of conventional compositions or production processes,
to attain all
of an increase in BH response, a reduction in proof stress and a reduction in
yield ratio.
In contrast, according to the present aspect, atom aggregates that inhibit
yield ratio
reduction can be diminished even when the solid-solution amount of Mg + Si is
increased
to enhance the BH response, by incorporating Sn in an amount within the range
shown
2.0 above. Thus, all of an increase in BH response, a reduction in proof
stress, and a .
reduction in yield ratio can be attained.
[0094]
Sn, at room temperature, has the effect of capturing (trapping) atomic holes
to
thereby inhibit room-temperature diffusion of Mg and Si and inhibit the
strength increase
at room temperature (room-temperature age hardening), and during the forming
of the
sheet into panels, improving the press formability including hem workability,
drawability
and punch stretch formability (hereinafter, this press formability is referred
to also as hem
workability as a representative). During an artificial aging treatment of the
panels, such
as a baking treatment, it releases the captured holes and hence in turn
enhances the
diffusion of Mg and Si, thereby enhancing the BH response.
[0095]
In the case where the content of Sn is lower than 0.005%, the effects
described
above, i.e., the effect of lowering the volume proportion of atom aggregates
that inhibit
yield ratio reduction, even when the solid-solution amount of Mg + Si is
increased, and
thereby attaining both an increase in BH response and a reduction in yield
ratio and the
effect of suppressing room-temperature age hardening. Meanwhile, in the case
where the
content of Sn is higher than 0.3%, the Sn segregates at grain boundaries and
it is prone to
cause intergranular cracks. A preferred lower limit of the content of Sn is
0.01%. An
23
CA 02941988 2016-09-08
3
upper limit of the content of Sn is preferably 0.2%, more preferably 0.1% and
further
preferably 0.06%
[0096]
(Solid-solution amount Mg and Si)
The composition described above is employed. Furthermore, in the present
aspect, the total solid-solution amount of Mg and Si contained in the sheet
(solid-solution
amount of Mg + Si) is increased and ensured so as to be in a specified range
of 1.0 mass%
or more and 2.0 mass% or less, in order to enhance the BH response. In the
case where
the solid-solution amount of Mg + Si is less than 1.0 mass%, the BH response
cannot be
ensured even when the composition described above is employed. The larger the
solid-
solution amount of Mg + Si, the more the BH response improves. However, due to
the
composition and production described above, there are limitations on the
contents and
solid-solution amount of Mg and Si. In addition, too high amount of solid-
solution pose a
problem in that the volume proportion of the atom aggregates increases to
result in
increases in proof stress and yield ratio during panel forming. An upper limit
thereof is
hence 2.0 mass %.
[0097]
The solid-solution amount of Mg + Si in a sheet is measured by dissolving a
sample of the sheet to be examined, by a residue extraction method using hot
phenol,
separating the solid/liquid by filtration with a filter having a mesh of 0.1
m, and regarding
the total content of Mg and Si in the separated solution as the solid-solution
amount of Mg
+ Si.
[0098]
The residue extraction method with hot phenol is performed specifically in the
following manner. First, phenol is introduced into a decomposition flask and
heated, and
each sheet sample to be examined is then transferred to this decomposition
flask and
decomposed by heating. Subsequently, benzyl alcohol is added thereto, followed
by
performing a suction filtration with the filter, thereby separating the
solid/liquid by
filtration. The solution separated is quantitatively analyzed to determine the
total content
of Mg and Si therein. For this quantitative analysis, use is suitably made of
atomic
absorption spectrometry (AAS), inductively coupled plasma optical emission
spectroscopy
(ICPOES) or the like. For the suction filtration, a membrane filter having a
diameter of
47 mm and having a mesh (capture particle diameter) of 0.1 pm is used as
stated above.
This examination and a calculation are made with respect to three samples
obtained from
total of three portions, i.e., one central portion in the sheet-width
direction of the test sheet
and two portions located respectively at both ends of the sheet-width-
direction from the
central portion. The solid-solution amount of Mg + Si (mass %) of these
samples are
averaged.
24
CA 02941988 2016-09-08
=
[0099]
(Aggregates of atoms)
The composition and structure described above are employed. In addition, in
the
present aspect, the structure of the 6000-series aluminum alloy sheet is
regulated in the size
distribution of aggregates of Mg and Si atoms observed with a three-
dimensional atom
probe filed ion microscope, in order to reduce a yield ratio to less than 0.50
and to ensure
BH response. Thus, both an increase in BH response and a reduction in yield
ratio are
attained not only by the effects of the Sn but also by regulating the atom
aggregates
(clusters) present in the structure of the sheet.
[0100]
(Definition of aggregate of atoms)
In the present aspect, one which satisfies some conditions (requirements)
specified
through an examination and analysis based on the principle of three-
dimensional atom
probe field ion microscope is defined as an aggregate of atoms, as described
in the section
Effects. Specifically, defined as an aggregate of atoms is one which satisfies
some
conditions (requirements) specified in the present aspect with respect to a
three-
dimensional structure of atoms (three-dimensional atom map) obtained by a
reconstruction
through analysis from the flight times and positions of atoms of the sheet
which have
temporarily ionized in a high electric field (electric-field evaporation) with
a three-
dimensional atom probe field ion microscope.
[0101]
Consequently, the aggregates of atoms specified in the present aspect are not
real
atom aggregates (clusters) exist in 6000-series aluminum alloy sheets, such as
ones
observed by directly examining the structure of a sheet as such with a high-
magnification
TEM (transmission electron microscope) as in Patent Document 1. However, they
correlate deeply with the state in which the real atom aggregates (clusters)
exist in 6000-
seires aluminum alloy sheets, such as ones directly observed with a high-
magnification
TEM. Because of this, even if the examination of atom aggregates in the
present aspect is
indirect or simulative, the atom aggregates satisfactorily correlate with the
state in which
those real atom aggregates (clusters) exist, the state considerably affecting
a reduction in
yield ratio and an increase in BH response. It hence provides a measure for
ensuring a
reduction in yield ratio and an increase in BH response by means of the
structure (atom
,aggregates).
[0102]
The sheet to be examined here is a 6000-series aluminum alloy sheet which has
undergone refining such as a solution treatment and a quenching treatment and
which has
undergone neither press forming nor a bake hardening treatment. The structure
of
CA 02941988 2016-09-08
arbitrary central portion in the sheet-thickness direction of this sheet is
examined with a
three-dimensional atom probe field ion microscope.
[0103]
(Requirements which the atom aggregates should meet)
Requirements (prerequisites) for being defined (regarded) as atom aggregates
in
the present aspect are explained below.
[0104]
The requirements which the atom aggregates in the present aspect should meet
are
the same as in Patent Documents 2 and 3. First, either or both of an Mg atom
and an Si
atom are contained by a total of 10 pieces or more. Although an upper limit on
the
number of the Mg atom and/or Si atom included in the atom aggregate is not
particularly
determined, an upper limit of the number of the Mg atom and/or Si atom
included in the
atom aggregate is about 10,000 in view of limitations on production.
[0105]
Furthermore, the ones are regarded as atom aggregates, in which when any atom
of the Mg atom and the Si atom contained therein is used as a reference, a
distance
between the atom as the reference and any atom among other atoms adjacent
thereto is
0.75 nm or less. The distance therebetween of 0.75 rim is a value which has
been
experimentally fixed in order that atom aggregates in each of which the
distance between
atoms of Mg and/or Si is short and which each have a size that considerably
affects a
reduction in yield ratio and an increase in BH response, and the volume
proportion thereof
are specified with satisfactory reproducibility, although the technical
meaning of the value
has not been fully elucidated.
[0106]
The atom aggregates specified in the present aspect mostly are ones each
including both of Mg atom and Si atom. However, they may include one which
includes
Mg atoms but contains no Si atom and one which includes Si atoms but contains
no Mg
atom. Furthermore, they need not to be composed of Mg atoms and/or Si atoms
only, and
it is highly probable that Al atoms are contained besides these.
[0107]
Moreover, depending on the component composition of the aluminum alloy sheet,
there inevitably are cases where atoms contained as alloying elements or
impurities, such
as Fe, Mn, Cu, Cr, Zr, V. Ti, Zn, Ag, etc., are contained in the atom
aggregates and those
other atoms are counted by the 3DAP analysis. However, even in the cases when
such
other atoms (derived from alloying elements or impurities) are contained in
the atom
aggregates, they are on a low level as compared with the total number of Mg
atoms and Si
atoms. Consequently, even in the cases when such other atoms are contained in
the atom
aggregates, those meet the limitations (requirements) function as the atom
aggregates
26
CA 02941988 2016-09-08
according to the present aspect like the atom aggregates composed of Mg atoms
and/or Si
atoms only. Thus, the atom aggregates specified in the present aspect may
contain any
other atoms so long as they satisfy the limitations.
[0108]
The wording "when any atom of the Mg atom and the Si atom contained therein is
used as a reference, a distance between the atom as the reference and any atom
among
other atoms adjacent thereto is 0.75 nm or less" means that each of all the Mg
atoms and Si
atoms present in each aggregate of atoms has at least one Mg atom or Si atom
therearound
within a distance of 0.75 nm or less.
[0109]
In the limitation on the distance between atoms in the atom aggregates
according
to the present aspect, when any atom of the Mg atom and the Si atom contained
therein is
used as a reference, each of all the distances between the atom as the
reference and all
atoms among other atoms adjacent thereto may not be 0.75 iun or less, and on
the contrary,
each of them all may be 0.75 nm or less. In other words, other Mg atom or Si
atom may
be adjacent at a distance exceeding 0.75 nm, and it is sufficient that in the
periphery of a
specific Mg atom or Si atom (serving as a reference), at least one Mg atom or
Si atom is
present which satisfies the specified distance (spacing).
[0110]
In the case where there is one adjacent other Mg atom or Si atom which
satisfies
the specified distance, the number of Mg atom and/or Si atom which satisfy the
requirement concerning distance and which should be counted is 2, including
the specific
Mg atom or Si atom (serving as a reference). Meanwhile, in the case where
there are two
adjacent other Mg atoms and/or Si atoms which satisfy the specified distance,
the number
of Mg atoms and/or Si atoms which satisfy the requirement concerning distance
and which
should be counted is 3, including the specific Mg atom or Si atom (serving as
a reference).
[0111]
(Regulation of the atom aggregates)
First, in the present aspect, as the total volume of atom aggregates which
satisfy
the given requirements explained above, including the number of Mg atoms
and/or Si
atoms and the distance between atoms, the total volume IVi is determined by
summing up
the volumes of the individual atom aggregates Vi (=4/3nrG3) calculated from
the Guinier
radii rG of the individual atom aggregates each regarded as a sphere. Then,
the average
volume proportion of this total volume Vi to the volume VAI of the aluminum
alloy sheet
measured with the three-dimensional atom probe field ion microscope,
(EViNA0x100, is
regulated so as to be in the range of 0.3 to 1.5%.
[0112]
27
CA 02941988 2016-09-08
Furthermore, in the present aspect, in addition to the regulation of the
volume
proportion of atom aggregates, the average volume proportion of the total
volume Evi1.5 or
more, which is the total volume of atom aggregates each having the Guinier
radius rG of 1.5
nm or larger among the atom aggregates satisfying those requirements, to the
total volume
of the atom aggregates EVi, (EVi1.5 or more/Vi), is regulated so as to be in
the range of 20
to 70%. Namely, the individual atom aggregates which each satisfy the
requirements are
divided at a Guinier radius rG of 1.5 nm, and the average volume proportion of
the total
volume ZVil..5 or more obtained by summing up the volumes V1.5 or more of the
individual atom
aggregates each having a Guinier radius rG of 1.5 Inn or larger to the total
volume of the
atom aggregates V, (Vii.5 or more/EV1)x 100, is regulated so as to be in the
range of 20 to
70%.
[0113]
Guinier radius rG is determined in the following manner. The individual atom
aggregates which each satisfy the requirements are each regarded as a sphere,
and the
largest of values of radius of gyration Ig of each atom aggregate is taken as
the radius of
gyration Ig of the atom aggregate. The Guinier radius rG is a radius obtained
by
converting this radius of gyration Ig by using the equation which will be
described later.
The definition of Guinier radius and the method for calculation thereof which
will be
described later are known by Patent Documents 2 and 3.
[0114]
Due to those structure regulations in combination with the compositional
regulation, it is possible to make the 6000-series aluminum alloy sheet have,
in
automotive-panel forming, a 0.2% proof stress reduced to 110 MPa or less and a
yield ratio
reduced to less than 0.50 and further have a 0.2% proof stress after BH of 190
MPa or
greater.
[0115]
In the case where the average volume proportion of atom aggregates which
satisfy
those requirements, (IVi/Vm)x100, is less than 0.3%, the absolute number of
relatively
large atom aggregates having a Guinier radius rG of 1.5 urn or larger and
effective for an
increase in BH response and a reduction in yield ratio is insufficient.
Because of this,
even when the composition is satisfied, it is impossible to attain the
increase in BH
response and reduction in yield ratio. Meanwhile, in the case where the
average volume
proportion EVi/VAx100 exceeds 1.5%, the number of atom aggregates which
satisfy the
requirements, including the distance between atoms being 0.75 nm or less, is
too large,
making it impossible to attain reduction in 0.2% proof stress and reduction in
yield ratio in
panel forming.
[0116]
28
CA 02941988 2016-09-08
= A
Furthermore, also in the case where the average volume proportion (ViI.5 or
more/EVi)x 100 of relatively large atom aggregates having a Guinier radius rG
of 1.5 nm or
larger and effective for an increase in BH response and a reduction in yield
ratio is less
than 20%, the absolute number of these atom aggregates is insufficient and a
reduction in
yield ratio cannot be attained even when the composition is satisfied or even
when the
average volume proportion of atom aggregates which satisfy those requirements
satisfies
the limitation. Meanwhile, the larger the number or proportion of relatively
large atom
aggregates having a Guinier radius rG of 1.5 nm or larger, the easier the
attainment of a
reduction in yield ratio. However, it is difficult, from the standpoint of
production, to
E
increase the average volume proportion (EVi 5 or more/VO100 beyond 70%, and
this 70%
is determined as an upper limit in view of limitations on production.
[0117]
(Principle of measurement with 3DAP and method of the measurement therewith)
The principle of a measurement with a 3DAP and a method of the measurement
therewith are also known from Patent Documents 1 to 3. The 3DAP (three-
dimensional
atom probe) is configured of a field ion microscope (FIM) and a time-of-flight
mass
spectrometer attached thereto. Due to such configuration, this is a local
analyzer in which
individual atoms in a metal surface can be observed with the field ion
microscope and
these atoms can be identified by time-of-flight mass spectrometry.
Furthermore, since the
3DAP can simultaneously analyze the kinds and positions of atoms emitted from
a sample,
it can be an exceedingly effective means for analyzing the structure of atom
aggregates.
Because of this, it is used as a known technique for, for example, structural
analysis of
magnetic recording films, electronic devices, steel materials or the like, as
stated above.
Recently, it is used for determination or the like of atom aggregates in the
structure of an
aluminum alloy sheet, as described above.
[0118]
The 3DAP utilizes the phenomenon called electric-field evaporation, in which
atoms of a sample themselves are ionized in a high electrical field. When a
high voltage
necessary for causing atoms of a sample to undergo electric-field evaporation
is applied to
the sample, atoms are ionized from the sample surface, and they pass through
the probe
hole and reach a detector.
[0119]
This detector is a position sensitive detector, which performs mass
spectrometry
for individual ions (identification of elements that are kinds of atom) and
measures the
time of flight of each ion to the detector and which can thereby
simultaneously determine
the positions detected (atom structure positions). Consequently, the 3DAP can
simultaneously measure the positions and atom kinds of atoms present at the
tip of the
sample, and hence has the feature of being able to three-dimensionally
reconstitute and
29
CA 02941988 2016-09-08
observe the structure of atoms present in the tip of the sample. In addition,
because
electric-field evaporation takes place in order from the surface of the sample
tip, the depth-
direction distribution of atoms from the sample tip can be examined with
atomic-level
resolution.
[0120]
Since the 3DAP utilizes a high electric field, the sample to be analyzed is
required
to have high electroconductivity, like metals, etc., and the shape of the
sample is generally
required to be an ultrafine needle shape having a tip diameter of about 100 nm
or less.
Because of this, a sample is taken from, for example, a central portion in a
sheet-thickness
direction of an aluminum alloy sheet to be examined, and this sample is cut
with a precise
cutting device and electropolished to produce a sample for analysis which has
an ultrafine
needle-shaped tip portion. A measuring method is as follows. "LEAP 3000",
manufactured by Imago Scientific Instruments Corp., is, for example, used, and
a high-
pulse voltage on the order of 1 kV is applied to the aluminum alloy sheet
sample having a
tip formed in a needle-shape, thereby continuously ionize millions of atoms
from the
sample tip. The ions are detected by the position sensitive detector. Mass
spectrometry
of each ion (identification of the element that is the kind of atom) is
conducted on the basis
of the time of flight from the emission of the individual ion from the sample
tip, which is
caused by the pulse-voltage application, to the arrival at the detector.
[0121]
Furthermore, the feature in which the electric-field evaporation takes place
regularly in order form the surface of the sample tip is utilized to suitably
give a depth-
direction coordinate to a two-dimensional map which shows ion arrival sites,
and analysis
software "WAS" is used to conduct a three-dimensional mapping (construction of
three-
dimensional structure of atoms: atom map). Thus, a three-dimensional atom map
for the
sample tip can be obtained.
[0122]
This three-dimensional atom map is farther processed by a maximum separation
method, which is a method for defining atoms belonging to a precipitate or to
an atom
aggregate, to analyze aggregates of atoms (atom aggregates). In this analysis,
the number
of either or both of Mg atom and Si atom (ten pieces or more in total), the
distance
(spacing) between adjacent Mg atoms and/or Si atoms, and the number of Mg
atoms and/or
Si atoms which have the specific narrow spacing (0.75 nm or less) are given as
parameters.
[0123]
Then, atom aggregates which satisfy conditions in which either or both of an
Mg
atom and an Si atom are contained by a total of 10 pieces or more and, when
any atom of
the Mg atom and the Si atom contained therein is used as a reference, a
distance between
the atom as the reference and any atom among other atoms adjacent thereto is
0.75 nm or
CA 02941988 2016-09-08
less, are defined as atom aggregates according to the present aspect. In
addition, the
dispersion state in which atom aggregates according to the definition is
evaluated, and the
number density of atom aggregates is quantified by examining three or more
samples and
averaging the measured values in terms of average density per 1 m3
(pieces/m3).
[0124]
That is, a maximum radius of gyration Ig when each of the atom aggregates
being
examined is regarded as a sphere by using the analysis software originally
specific to the
3DAP is acquired by using the following formula of Math. 1.
[Math. 1]
In
¨ E[(x1 x)2 (y -y)2 +(z1 -z)2]
= _____________________________________________
[0125]
In the formula of Math. 1, Ig represents a radius of gyration automatically
calculated by the software specific to the three-dimensional atom probe field
ion
microscope. x, y and z respectively represent an x axis, a y axis and a z axis
which are
invariable in the measuring layout of the three-dimensional atom probe field
ion
microscope. xi, yi and z, respectively represent the lengths of the x axis, y
axis and z axis,
and are spacial coordinates for the Mg atoms and/or Si atoms which constitute
the atom
aggregate. "x bar" and the like in which "-" is placed on the top of each of
"x", "y" and
"z" also represent the lengths of the x, y and z axes, but are barycentric
coordinates for the
atom aggregate. n represents the number of Mg atoms and/or Si atoms which
constitute
the atom aggregate.
[0126]
Next, a maximum of the radius of gyration Ig of each of the individual atom
aggregates is taken as the radius of gyration 1g of the atom aggregate, and
converted to a
Guinier radius rG by using the relationship rG = 'I(5/3) 1g of the following
formula of Math.
2. This Guinier radius rG obtained by the conversion is regarded as the
radius of the atom
aggregate.
[Math. 2]
r = = 1
3 g
[0127]
On the basis of this, the volumes Vi (=4/3nrG3) of the individual atom
aggregates
which satisfy those requirements are summed up to determine the total volume
EVi.
31
CA 02941988 2016-09-08
Meanwhile, the volume of the needle-shaped sample which has undergone the
electric-
field evaporation (i.e., which has disappeared due to electric-field
evaporation) is taken as
the volume VA1 of the aluminum alloy sheet measured with the three-dimensional
atom
probe field ion microscope, and the average volume proportion of the total
volume of the
atom aggregates thereto, (EVi/Viu)x100, is determined. Furthermore, the
average volume
proportion of the total volume EVi1.5 or more Of atom aggregates each having a
Guinier
radius r0 of 1.5 nm or larger to the total volume V of the atom aggregates,
(EVi1.5 or
more/Vi) x 100, is also determined. The measurement of each average volume
proportion
of atom aggregates with the 3DAP is made on arbitrary ten regions of central
portions in
the sheet-thickness direction in the 6000-series aluminum alloy sheet which
has undergone
the refining, and the measured values (calculated values) thereof are
averaged.
[0128]
The calculation formula for calculating the radius of an atom aggregate and
the
methods for measuring and converting the radius of gyration Ig for Guinier
radius rG are
based on quotations from M. K. Miller: Atom Probe Tomography, (Kluwer
Academic/Plenum Publishers, New York, 2000), p.184. Calculation formulae for
the
radius of an atom aggregate are described in many documents other than this.
For
example, "(2) Three-dimensional Atom Probe Analysis" on page 140 of
"Microstructural
Evolution in Low Alloy Steels under High Dose Ion Irradiation" (Katsuhiko
Fujii, Koji
Fukuya, Tadakatsu Ohkubo, Kazuhiro Hono, et al.) describes including the
formula of
Math. 1 and the formula for conversion to Guinier radius rG (in this document,
however,
the symbol of the radius of gyration Ig is described as ro).
[0129]
(Efficiency of atom detection by 3DAP)
Currently, the efficiency of the detection of atoms by the 3DAP is about 50%
at
the most with respect to the ionized atoms, and the remaining atoms cannot be
detected.
In the cases when the efficiency of the detection of atoms by the 3DAP changes
considerably due to, for example, an improvement in the future, there is a
possibility that
the results of measurements, with a 3DAP, of the average number density
(pieces/ m3) of
each of atom aggregates of the sizes specified in the present aspect may vary.
Consequently, from the standpoint of conducting the measurements with
reproducibility, it
is preferable that the efficiency of the detection of atoms with a 3DAP should
be kept
approximately constant at about 50%.
[0130]
(Production Process)
Next, a process for producing the aluminum alloy sheet according to the
present
aspect is explained. The aluminum alloy sheet according to the present aspect
is
produced through production steps which themselves are common or known, by
32
CA 02941988 2016-09-08
subjecting, after casting, an aluminum alloy slab having the 6000-series
component
composition to a homogenizing heat treatment, hot rolling and cold rolling to
obtain a
given sheet thickness, followed by a refining treatment such as a solution
quenching
treatment.
[0131]
However, for obtaining the structure including the atom aggregates specified
with
a 3DAP according to the present aspect, during those production steps, the
average cooling
rate in a quenching treatment after a solution treatment is controlled and in
addition, the
conditions for a preliminary aging treatment after the quenching treatment are
regulated so
as to be in a preferred range, as will be described later. With respect to
other steps, there
are preferred conditions for obtaining the structure specified in the present
aspect. Unless
such preferred conditions are employed, it is difficult to obtain the
structure according to
the present aspect.
[0132]
(Melting and casting cooling rate)
First, in melting and casting steps, an aluminum alloy molten metal that has
been
melted and regulated so as to have a component composition within the 6000-
series
composition range is cast by a suitably selected ordinary melting and casting
method, such
as a continuous casting method or a semi-continuous casting method (DC casting
method).
Here, it is preferable that the average cooling rate, during the casting, from
the liquidus
temperature to the solidus temperature is as high (quick) as possible at 30
C/min or greater.
[0133]
In the case where such temperature (cooling rate) control in a high-
temperature
range during casting is not performed, the cooling rate in this high-
temperature range is
inevitably low. When an average cooling rate in the high-temperature range is
low as the
above, the amount of crystals yielded coarsely in the temperature range of
this high-
temperature range is increased and also unevenness in the size and amount of
the crystals
along the width direction and thickness direction of the slab is increased. As
a result, the
basic mechanical properties, such as strength and elongation, which are a
prerequisite for
the 6000-series aluminum alloy sheet are reduced.
[0134]
(Homogenizing heat treatment, hot rolling, annealing of hot-rolled plate, cold
rolling, and
solution and quenching treatments)
Subsequently, the aluminum alloy slab obtained by casting is subjected to the
treatments of a homogenizing heat treatment, hot rolling, annealing of the hot-
rolled plate
(according to need), cold rolling, and solution and quenching treatments in
the same
manners as in the first aspect. The conditions for these treatments are the
same as in the
first aspect, and explanations thereon are omitted here.
33
CA 02941988 2016-09-08
[0135]
(Preliminary aging treatment: reheating treatment)
After having thus undergone the solution treatment and the subsequent
quenching
treatment to be cooled to room temperature, the cold-rolled sheet is subjected
to a
preliminary aging treatment (reheating treatment) within a period which is as
short as
possible and is up to 1 hour (60 minutes).
[0136]
In the case where the room-temperature holding period from termination of the
quenching treatment to room temperature to initiation of the preliminary aging
treatment
(initiation of heating) is too long and exceeds 1 hour, it becomes impossible
to regulate the
total volume of atom aggregates to 1.5% or less in terms of average volume
proportion, the
atom aggregates satisfying the requirements concerning the number of Mg atoms
and/or Si
atoms and the distance between atoms. In addition, relatively large clusters
are less apt to
be yielded, making it impossible to increase the average volume proportion of
atom
aggregates each having a Guinier radius rG of 1.5 nm or larger to the atom
aggregates
which satisfy the requirements to 20% or higher. As a result, the BH response
decreases,
and a reduction in yield ratio is also difficult. Consequently, the shorter
the room-
temperature holding period, the better. The solution and quenching treatments
and the
reheating treatment may be consecutively performed so that there is
substantially no pause
therebetween , and a lower limit of the period is not particularly determined.
[0137]
In this preliminary aging treatment, the rate of temperature increase to a
preliminary-aging temperature and the period of holding in a preliminary-aging
temperature range are regulated. It is preferable that the temperature
increase rate, of
these, should be as high (quick) temperature increase rate as possible at 1
C/s or higher,
preferably 5 C/s or higher, for suppressing the formation of small atom
aggregates not
contributing to strength. In the case where the temperature increase rate is
less than
1 C/s, small atom aggregates not contributing to strength are yielded in a
large amount,
making it impossible to increase the average volume proportion of atom
aggregates each
having a Guinier radius rG of 1.5 nm or larger to the atom aggregates which
satisfy the
requirements to 20% or higher. As a result, the BH response decreases, and a
reduction in
yield ratio is also difficult.
[0138]
The temperature and holding period in the preliminary aging treatment is a
holding in a temperature range of 60 to 120 C for 10 hr or more and 40 hr or
less. Here,
the holding in the temperature range of 60 to 120 C may be a heat treatment in
which the
temperature is constant or the temperature is sequentially changed by
temperature increase
or annealing, within that temperature range. In short, the temperature may be
34
CA 02941988 2016-09-08
continuously changed by annealing, temperature increase, etc., so long as it
is held in a
temperature range of 60 to 120 C for that period of 10 hr or more and 40 hr or
less.
[0139]
In the case where the preliminary-aging temperature is lower than 60 C or the
holding period is less than 10 hr, the formation of precipitate nuclei is
insufficient, making
it impossible to increase the average volume proportion of atom aggregates
each having a
Guinier radius rG of 1.5 nm or larger to the atom aggregates which satisfy the
requirements
to 20% or higher. As a result, the BH response decreases.
[0140]
Meanwhile, in the case where the preliminary-aging temperature exceeds 120 C
or the holding period exceeds 40 hr, precipitate nuclei are yielded in too
large an amount in
this preliminary aging treatment. Because of this, the amount of atom
aggregates having
a relatively large size and contributing to strength decreases. As a result,
the average
volume proportion of atom aggregates which satisfy the requirements increases
beyond
1.5%, making it impossible to enable the sheet in forming to have a yield
ratio reduced to
less than 0.50.
[0141]
Namely, unless the preliminary aging treatment is regulated to fall within
these
preferable conditions, it is difficult to produce a sheet which has, in
automotive-panel
forming, a 0.2% proof stress reduced to 110 MPa or less and a yield ratio
reduced to less
than 0.50 and further has a 0.2% proof stress after BH of 190 MPa or greater.
Examples
[0142]
The present invention will be explained below in more detail by reference to
Examples. However, the present invention should not, of course, be construed
as being
limited by the following Examples, and can be suitably modified unless the
modifications
depart from the gist of the present invention described hereinabove and
hereinafter. All
such modifications are included in the technical range of the present
invention.
[0143]
(Examples according to the first aspect)
Next, Examples according to the first aspect of the present invention are
explained. 6000-series aluminum alloy sheets were individually produced so as
to differ
in the structure specified with a DSC in the present aspect, by changing the
conditions for a
preliminary aging treatment performed after solution and quenching treatments.
After a
holding at room temperature for 30 days after the production of the sheets, BH
response
(bake hardenability), As proof stress as an index of press formability and hem
workability
as bendability are examined and evaluated.
CA 02941988 2016-09-08
[0144]
For individually producing the structure specified with a DSC, the 6000-series
aluminum alloy sheets having the compositions shown in Table 1 was produced by
variously changing conditions such as the average cooling rate in the
quenching treatment
after a solution treatment and the temperature and holding period in the
subsequent
preliminary aging treatment as shown in Tables 2 and 3. With respect to the
indications
of the contents of elements within Table 1, a value of the element expressed
by a blank
indicates that the content is below a detection limit.
[0145]
Specific conditions for aluminum alloy sheet production were as follows. Slabs
of aluminum alloys respectively having the compositions shown in Table 1 were
commonly produced through casting by the DC casting method. In this casting,
the
average rate of cooling from the liquidus temperature to the solidus
temperature was set at
50 C/min in common with all the Examples. Subsequently, the slabs were
subjected to a
soaking treatment of 540 C x 6 hours performed in one stage only, followed by
initiation
of hot rough rolling at that temperature, in common with all the Examples.
Thereafter,
they were hot-rolled, in the succeeding finish rolling, to a thickness of 3.5
mm to obtain
hot-rolled sheets, in common with all the Examples. The hot-rolled aluminum
alloy
sheets were subjected to rough annealing of 500 C x 1 minute and then to cold
rolling at a
processing rate of 70% without performing intermediate annealing during the
cold-rolling
passes, to obtain cold-rolled sheets having a thickness of 1.0 mm, in common
with all the
Examples.
[0146]
Furthermore, the cold-rolled sheets were each continuously subjected to a
refining
treatment (T4) with continuous type heat treatment facilities while unwinding
and winding
each sheet, in common with all the Examples. Specifically, a solution
treatment was
performed by heating at an average rate of heating to 500 C of 10 C/sec and
holding for
10 seconds after the temperature reached a target temperature of 560 C,
followed by
cooling to room temperature by water cooling or air cooling so as to result in
the average
cooling rates shown in Tables 2 and 3. After this cooling and after the
subsequent
required periods shown in Table 2 at room temperature, a preliminary aging
treatment was
performed by using an atmospheric furnace and an oil bath and using the
temperature
increase rates, reached temperatures, average cooling rates, and holding
periods shown in
Tables 2 and 3. As for the cooling after this preliminary aging treatment,
water cooling or
gradual cooling (natural cooling) was conducted in order to change the average
rate of
cooling.
[0147]
36
CA 02941988 2016-09-08
From the final product sheets which each had been allowed to stand at room
temperature for 30 days after the refining treatment, test sheets (blanks)
were cut out and
the DSC and properties of the test sheets were examined and evaluated. The
results
thereof are shown in Table 3.
[0148]
(DSC)
The structure in each of ten portions of the central portion in the sheet-
thickness
direction in each test sheet was examined for the DSC. In the DSC
(differential scanning
calorimetry curves) of this sheet, as for the average value for these ten
portions, the peak
height (W/mg) of an endothermic peak in the temperature range of 150 to 230 C
as an
endothermic peak corresponding to the dissolution of Mg-Si clusters not
contributing to
strength and the peak height (pW/mg) of an exothermic peak in the temperature
range of
240 to 255 C as an exothermic peak corresponding to the formation of Mg-Si
clusters
contributing to strength were determined.
[0149]
The differential thermal analysis of each of the measurement portions in each
test
sheet was performed under the same conditions including a test apparatus of
DSC220G,
manufactured by Seiko Instruments Inc., a reference substance of aluminum, a
sample
container made of aluminum, temperature increase conditions of 15 C/min, an
atmosphere
of argon (50 mL/min), and a sample weight of 24.5 to 26.5 mg. The differential
thermal
analysis profile (p,W) obtained was divided by the sample weight and thereby
normalized
(p,W/mg). Thereafter, in the range of 0 to 100 C in the differential thermal
analysis
profile, a region where the differential thermal analysis profile was
horizontal was taken as
a reference level of 0, and the height of exothermic peak from the reference
level was
measured. The results thereof are shown in Tables 2 and 3.
[0150]
(Bake hardenability)
The test sheets which had been allowed to stand at room temperature for 30
days
after the refilling treatment were each examined for 0.2% proof stress (As
proof stress) as a
mechanical property through a tensile test. Furthermore, these test sheets
were aged at
room temperature for 30 days, subsequently subjected to an artificial age
hardening
treatment of 170 C x 20 minutes (after BID, and then examined for 0.2% proof
stress
(proof stress after B1-1) through a tensile test, in common with the test
sheets. The BH
response of each test sheet was evaluated on the basis of the difference
between these 0.2%
proof stresses (increase in proof stress).
[0151]
With respect to the tensile test, No. 5 specimens (25 mm x 50 mmGL x sheet
thickness) according to JIS Z2201 were cut out of each sample sheet to perform
the tensile
37
CA 02941988 2016-09-08
=
test at room temperature. Here, the tensile direction of each specimen was set
so as to be
perpendicular to the rolling direction. The tensile rate was set at 5 mm/min
until the 0.2%
proof stress and at 20 mm/min after the proof stress. The number N of
examinations for
mechanical property was 5, and an average value therefor was calculated. With
respect to
the specimens to be examined for the proof stress after BH, a 2% pre-strain as
a simulation
of sheet press forming was given to the specimens by the tensile tester,
followed by
performing the BH treatment.
[0152]
(Hem workability)
Hem workability was evaluated only with respect to the test sheets which had
been allowed to stand at room temperature for 30 days after the refining
treatment. In the
test, strip-shaped specimens having a width of 30 mm were used and subjected
to 90
bending at an inward bending radius of 1.0 mm with a down flange. Thereafter,
an inner
plate having a thickness of 1.0 mm was nipped, and the specimen was subjected,
in order,
to pre-hem working in which the bent part was further bent inward to
approximately 130
and flat-hem working in which the bent part was further bent inward to 180
and the end
portion was brought into close contact with the inner plate.
[0153]
The surface state, such as the occurrence of rough surface, a minute crack or
a
large crack, of the bent part (edge bent part) of the flat hem was visually
examined and
visually evaluated on the basis of the following criteria. In the following
criteria, ratings
of 0 to 2 are on an acceptable level, and ratings of 3 and larger are
unacceptable.
0, no crack and no rough surface; 1, slight rough surface; 2, deep rough
surface; 3,
minute surface crack; 4, linearly continued surface crack.
[0154]
Invention Examples Nos. 0, 1, 8, and 13 in Table 2 and Nos. 16 to 24 in Table
3,
which employ alloys Nos. 0 to 12 shown in Table 1, each is not only within the
component
composition range according to the present aspect and has been produced under
conditions
within preferred ranges but also has undergone the refining treatment,
including the
solution quenching treatment and the preliminary aging treatment, under
preferred
conditions. Because of this, these Invention Examples satisfy the DSC
requirements
specified in the present aspect, as shown in Tables 2 and 3. That is, in the
DSCs of these
sheets, the endothermic peak in the temperature range of 150 to 230 C as an
endothermic
peak corresponding to the dissolution of Mg-Si clusters not contributing to
strength had a
peak height of 8 pW/mg or less, while the exothermic peak in the temperature
range of 240
to 255 C as an exothermic peak corresponding to the formation of Mg-Si
clusters
contributing to strength had a peak height of 20 AW/mg or larger.
[0155]
38
CA 02941988 2016-09-08
As a result, the Invention Examples each show excellent BH response although
the bake hardening is performed after the refining treatment and subsequent
room-
temperature aging and is a treatment conducted at a low temperature for a
short period of
time. Furthermore, as shown in Table 3, even after the refining treatment and
subsequent
room-temperature aging, they each have a relatively low As proof stress and
hence show
excellent press formability into automotive panels or the like and excellent
hem
workability. That is, the Invention Examples, even when having undergone an
automotive-baking treatment after room-temperature aging, were able to exhibit
not only
high BH response with a 0.2% proof stress difference of 100 MPa or greater and
a 0.2%
proof stress after BH of 170 MPa or greater but also press formability with an
As 0.2%
proof stress of 110 MPa or less and satisfactory bendability.
[0156]
In contrast, Comparative Examples 2 to 7, 9 to 13, 14, and 15 in Table 2,
which
employed alloy example 1, 2 or 3 in Table 1 like Invention Examples, each have
the
preliminary aging treatment conditions outside the preferred ranges, as shown
in Table 2.
As a result, they each gave a DSC which was outside the range specified in the
present
aspect, and show enhanced room-temperature aging and, in particular, a
relatively high As
proof stress after 30-day room-temperature holding, as compared with the
Invention
Examples having the same alloy composition. Because of this, they are poor in
press
formability into automotive panels or the like and in hem workability and are
poor also in
BH response.
[0157]
In Comparative Example 2, the average cooling rate in the quenching treatment
to
room temperature performed after the solution treatment is too low. Because of
this, the
exothermic peak B in the temperature range of 240 to 255 C has a peak height
as low
(small) as less than 20 ,W/mg, although the endothermic peak A in the
temperature range
of 150 to 230 C has a peak height of 8 W/mg or less, showing that the number
density of
Mg-Si clusters having a relatively large size and contributing to strength is
low. This is
because due to the low cooling rate in the quenching treatment to room
temperature, coarse
Mg2Si and elemental Si were yielded during the cooling. Neither the desired
press
formability with an As 0.2% proof stress of 110 MPa or less nor satisfactory
bendability is
obtained. In addition, the BH response is low.
[0158]
In Comparative Examples 3 and 9, the period from the quenching treatment to
room temperature after the solution treatment to the preliminary aging
treatment (initiation
of heating) is too long. Because of this, Mg-Si clusters that are apt to
dissolve during
temperature increase in DSC and do not contribute to strength have been
yielded in a large
amount, and the endothermic peak A in the temperature range of 150 to 230 C
has a peak
39
CA 02941988 2016-09-08
=
height higher (larger) than 8 OV/mg, as shown in Fig. 1. Meanwhile, the
exothermic
peak B in the temperature range of 240 to 255 C has a peak height which also
is as high
(large) as 20 t.W/mg or more, showing that the number density of Mg-Si
clusters having a
relatively large size and contributing to strength is high. However, since the
number
density of Mg-Si clusters having a relatively small size and not contributing
to strength is
too high, the adverse influences thereof are too greater. Therefore, the
desired press
formability with an As 0.2% proof stress of 110 MPa or less and satisfactory
bendability
cannot be obtained. In addition, the BH response is low.
[0159]
In Comparative Examples 4 and 10, the temperature increase rate in the
preliminary aging treatment is too low. Because of this, Mg-Si clusters that
are apt to
dissolve during temperature increase in DSC and do not contribute to strength
have
undesirably been yielded in a large amount, and the endothermic peak A in the
temperature
range of 150 to 230 C has a peak height higher (larger) than 8 p.W/mg, as
shown in Fig. 1.
Meanwhile, the exothermic peak B in the temperature range of 240-255 C has a
peak
height which also is as high (large) as 20 l.LW/mg or more, showing that the
number density
of Mg-Si clusters having a relatively large size and contributing to strength
is high.
However, since the number density of Mg-Si clusters having a relatively small
size and not
contributing to strength is too high, the adverse influences thereof are too
greater.
Therefore, the desired press formability with an As 0.2% proof stress of 110
MPa or less
and satisfactory bendability cannot be obtained. In addition, the BH response
is low.
[0160]
In Comparative Examples 5, 11 and 14, the period of holding in the range of 60
to
120 C in the preliminary aging treatment is 1 hour, which is too short.
Because of this,
Mg-Si clusters that are apt to dissolve during temperature increase in DSC and
do not
contribute to strength have been yielded in a large amount, and the
endothermic peak A in
the temperature range of 150 to 230 C has a peak height higher (larger) than 8
p,W/mg, as
shown in Fig. 1. Meanwhile, the exothermic peak B in the temperature range of
240 to
255 C has a peak height which also is as high (large) as 20 W/mg or more,
showing that
the number density of Mg-Si clusters having a relatively large size and
contributing to
strength is high. However, since the number density of Mg-Si clusters having a
relatively
small size and not contributing to strength is too high, the adverse
influences thereof are
too greater. Therefore, the desired press formability with an As 0.2% proof
stress of 110
MPa or less and satisfactory bendability cannot be obtained. In addition, the
BH response
is low.
[0161]
In Comparative Examples 6, 12 and 15, the period of holding in the range of 60
to
120 C in the preliminary aging treatment is 48 hours, which is too long.
Because of this,
CA 02941988 2016-09-08
the exothermic peak B in the temperature range of 240 to 255 C has a peak
height as low
(small) as less than 20 W/mg, showing that the number density of Mg-Si
clusters having
a relatively large size and contributing to strength is low. As a result, the
desired press
formability with an As 0.2% proof stress of 110 MPa or less and satisfactory
bendability
cannot be obtained. In addition, the BH response is low.
[0162]
In Comparative Example 7, the reached temperature in the preliminary aging
treatment is 130 C, which exceeds the upper limit of 120 C and is too high.
Because of
this, the amount of Mg-Si clusters having a relatively large size and
contributing to
strength has decreased, and the exothermic peak B in the temperature range of
240 to
255 C thus has a peak height as low (small) as less than 20 11W/mg, showing
that the
number density of the Mg-Si clusters having a relatively large size and
contributing to
strength is low. As a result, the BH response is low and the As 0.2% proof
stress exceeds
110 MPa and is too high, and press formability and satisfactory bendability
cannot be
obtained, too.
[0163]
Comparative Examples 25 to 34 in Table 3 have been produced within preferred
ranges, including the conditions for the preliminary aging treatment. However,
since they
employed alloys Nos. 13 to 22 shown in Table 1, the contents of Mg and Si,
which are
essential elements, therein are outside the ranges according to the present
aspect or the
content of impurity elements therein is too high. Because of this, these
Comparative
Examples 24 to 33 each show, in particular, a relatively high As proof stress
after 30-day
room-temperature holding as compared with the Invention Examples, as shown in
Table 3,
and hence are poor in press formability into automotive panels or the like and
in hem
workability or are poor in BH response.
[0164]
Comparative Example 25 is alloy 13 shown in Table 1, in which the Si content
is
too low.
Comparative Example 26 is alloy 14 shown in Table 1, in which the Si content
is
too high.
Comparative Example 27 is alloy 15 shown in Table 1, in which the Sn content
is
too low.
Comparative Example 28 is alloy 16 shown in Table 1, in which the Sn content
is
too high and cracking occurred during the hot rolling, making the sheet
production
impossible.
Comparative Example 29 is alloy 17 shown in Table 1, in which the Fe content
is
too high.
41
CA 02941988 2016-09-08
Comparative Example 30 is alloy 18 shown in Table 1, in which the Mn content
is
too high.
Comparative Example 31 is alloy 19 shown in Table 1, in which the Cr content
and Ti content are too high.
Comparative Example 32 is alloy 20 shown in Table 1, in which the Cu content
is
too high.
Comparative Example 33 is alloy 21 shown in Table 1, in which the Zn content
is
too high.
Comparative Example 34 is alloy 22 shown in Table 1, in which the Zr content
and V content are too high.
[0165]
Those results of the Examples establish that, for improving formability and BH
response after room-temperature aging, it is necessary that all the
requirements concerning
composition and DSC specified in the present aspect should be satisfied.
42
[0166]
[Table 1]
Table 1
Alloy Chemical components of Al-Mg-Si alloy sheet (mass%;
remainder, Al)
No. Mg Si Sn Fe Mn , Cr Zr V Ti Cu
Zn Ag
0 0.64 0.99 0.040
1 0.58 0.90 0.050 0.2
2 0.40 0.82 0.039 0.2 0.05
0.12
3 0.39 1.18 0.058 0.2 0.2 0.01
4 0.34 1.50 0.097 0.2 ,
0.64
0.54 1.31 0.053 0.2 0.22
6 0.55 0.79 0.197 0.2 0.12
7 0.45 0.89 0.042 0.2 0.65 0.05
8 0.64 1.15 0.027 0.2 0.05 0.05
9 1.47 0.53 0.110 0.2 0.3
0.01
0.71 1.00 0.055 0.2 0.05
11 0.47 1.23 0.002 0.7
0.6 09
12 0.55 0.87 0.050 0.2 0.2
0.1 0.1
13 1.53 0.21 0.046 0.2
14 0.40 2.10 0.042 0.2
0.58 1.02 0.002 0.2
16 0.60 1.09 0.455 0.2
17 0.38 0.80 0.051 1.3
18 0.65 1.04 0.046 0.2
1.21 0.01
19 0.51 0.80 0.057 0.2 0.44 0.08
0.36 0.79 0.044 0.2 1.28
21 0.48 1.01 0.052 0.2
1.23
22 0.49 0.94 0.055 0.2 0.4 0.4
* Field in which the value for the element is blank indicates below detection
limit.
43
,
[0167]
[Table 2]
_
Table 2
,
Solution quenching
Preliminary aging
treatment
Alloy No. Solution Required period
Period of
Classification No. Average Temperature
Reached Average
in Table 1 treatment to preliminary
holding
cooling rate increase rate
temperature cooling rate
temperature aging
at 60 to 120 C
_ .
.
C ocis min C/s C
hr C/s .
Inv. Ex. . 0 0 540 100 5 20
100 12 100 _
Inv. Ex. 1 1 540 , 100 5 20
100 12 100 ,
Corn. Ex. 2 1 540 1 5 20
100 , 12 100 P
Corn. Ex. 3 1 540 100 120 20
100 12 100
_
2'
Corn. Ex. 4 1 540 100 5 0.1_
100 12 100 .
,
,
Corn. Ex. 5 1 540 100 5 20
100 1 100 03 '
Corn. Ex. 6 1 540 100 5 20 _
100 48 , 100 rõ
-
,
Corn. Ex. 7 1 540 100 5 20
130 12 100 ,I,
,
.
,
Inv. Ex. 8 2 540 100 5 20 90
12 100
0
,
Corn. Ex. 9 2 540 100 80 20 90
12 100
¨
Corn. Ex. 10 2 540 100 5 0.1 90
12 100
Corn. Ex. 11 _ 2 540 100 5 20 90
3 100
Corn. Ex. 12 2 540 100 5 20 90
48 100
Inv. Ex. 133 540 100 5 20
100 16 0.05
e,
_
.
Corn. Ex. 14 3 540 100 5 5
100 3 100 _
_ _
Com. Ex. 15 3 540 100 5 5
100 45 0.02
¨
44
(Table 2 Continued)
_
Structure of aluminum alloy sheet after Properties of aluminum alloy after
30-day room-temperature holding 30-day room-
temperature holding
0.2% proof
As 0.2%
Proof stress Hem
Differential scanning calorimetry curve stress after
Alloy No proof stress increase workability
.
Classification No. BH
in Table 1
Height of Height of
endothermic exothermic Exothermic peak B
peak A peak B temperature MPa
MPa MPa
C
liW/mg [LW/mg
Inv. Ex. 0 0 1.5 48.2 251 103 220
117 1
Inv. Ex. 1 1 0.9 45.3 251 94 226
132 1 p
Corn. Ex. , 2 1 2.8 18.5 254 127 209
82 3 2
Corn. Ex. 3 1 13.0 60.3 257 133 201
68 3 .."
,
03'
Com. Ex. , 4 1 8.2 54.1 256 114 206
92 2 m
_
''
Corn. Ex. 5 1 8.6 53.3 256 93 183
90 1
,
Corn. Ex. 6 1 1.3 16.2 250 147 262
115 4
1'
Corn. Ex. 7 1 1.0 5.3 251 156 224
68 , 4 .
Inv. Ex. 8 2 0.6 35.4 251 88 206
118 1
Corn. Ex. 9 2 12.3 60.3 254 135 200
65 3
_
Corn. Ex. 10 2 8.3 63.2 256 112 205
93 2
Corn. Ex. 11 2 8.8 58.9 256 90 179
89 , 1
Corn. Ex. 12 2 0.8 15.5 250 144 261
117 4
Inv. Ex. 13 3 2.1 40.6 250 95 216
121 1
_
Corn. Ex. 14 _ 3 9.1 55.7 256 97 192
_ 95 1
Corn. Ex. 15 3 1.7 14.2 250 150 269
119 _ 4
[0168]
.
[Table 3]
_
Table 3
Solution quenching
Preliminary aging
treatment
Alloy No. Solution Required period
Period of
Classification No. Average Temperature Reached
Average
in Table 1 treatment to preliminary
holding
cooling rate increase rate
temperature cooling rate
temperature aging
at 60 to 120 C
C C/s min C/s C
hr C/s .
Inv. Ex. 16 4 540 50 5 20
100 12 100
_
-
Inv. Ex. 17 5 540 20 5 20
100 12 100
_
Inv. Ex. 18 6 540 100 15 20
100 12 100
-
Inv. Ex. 19 7 540 100 5 _ 5
100 12 100 P
Inv. Ex. 20 8 540 100 _ 5 3
100 12 100 2
.."
Inv. Ex. 21 9 540 100 5 20 80
.3' 12 100 ,
_ .3
Inv. Ex. 22 10 540 100 5 20
100 8 100 rõ
Inv. Ex. 23 11 540 100 5 20 80
27 0.1 ,
,
Inv. Ex. 24 12 540 100 5 20 70
32 0.1 '
,
, .
Corn. Ex. 25 13 540 100 5 20
100 12 100 .3
Corn. Ex. 26 14 540 100 5 20
100 12 100
Corn. Ex. 27 15 540 100 5 20
100 12 100
Corn. Ex. 28 16 cracking occurred during
hot rolling _
Corn. Ex. 29 17 540_ 100 5 20
100 12 100
Corn. Ex. 30 _ 18 540 100 5 20
100 12 100
_
Corn. Ex. 31 19 540 100 5 20
100 s 12 100
Corn. Ex. 32 20 540 100 5 20
100 _ 12 100
Corn. Ex. 33 21 540 100 5 20
100 12 100
Com. Ex. 34 22 540 100 5 20
100 12 100
46
(Table 3 Continued)
.
Structure of aluminum alloy sheet after Properties
of aluminum alloy after
30-day room-temperature holding 30-day room-
temperature holding
As 0.2% 0.2% proof
Proof stress
Hem
Differential scanning calorimetry curve proof stress
after .
Alloy No increase workability
.
Classification No. stress BH
in Table 1
Height of Height of
Exothermic peak B
endothermic exothermic
peak A peak B temperature MPa MPa
MPa .
C
1.1W/mg JAW/mg
,
Inv. Ex. 16 4 2.3 50.2 251 101 213
112 2
Inv. Ex. 17 5 2.2 54.2 251 104 222
118 2 p
Inv. Ex. 18 6 5.6 42.9 253 103 207
104 1 2
..'
Inv. Ex. 19 7 2.4 45.0 251 92 208
116 2 ,
.3'
Inv. Ex. 20 8 3.0 68.4 252 107 221
114 1 .3
Inv. Ex. 21 9 3.4 47.1 250 98 201
103 1 ,
,
Inv. Ex. 22 10 4.3 64.9 249 105 215
110 2 0
1'
Inv. Ex. 23 11 2.5 68.1 248 104 219
115 2 2
Inv. Ex. 24 12 3.6 57.3 251 84 195
111 1
Corn. Ex. 25 13 3.6 6.8 258 78 126
48 1
Corn. Ex. 26 14 10.2 53.6 251 125 207
82 4
Corn. Ex. 27 15 1.7 19.5 268 133 255
122 3
Corn. Ex. 28 16 cracking occurred during hot rolling
Corn. Ex. 29 17 1.3 38.2 251 106 209
103 4
Corn. Ex. 30 18 2.2 37.2 251 114 212
98 4
Corn. Ex. 31 19 4.0 36.0 251 103 209
106 4
Corn. Ex. 32 20 4.3 36.6 250 _ 135 246
111 4
Corn. Ex. 33 21 2.3 34.5 251 112 205
93 4
Corn. Ex. 34 22 1.6 39.0 251 108 212
104 4
47
CA 02941988 2016-09-08
[0169]
Next, Examples according to the second aspect of the present invention are
explained. 6000-series aluminum alloy sheets were individually produced so as
to differ
in the structure specified in the present aspect, by changing the conditions
for a preliminary
aging treatment performed after solution and quenching treatments. After a
holding at
room temperature for 30 days after the production of the sheets, BH response
(bake
hardenability), As proof stress as an index of press formability and hem
workability as
bendability are examined and evaluated.
[0170]
For individually producing the structure, the 6000-series aluminum alloy
sheets
having the compositions shown in Table 4 was produced by variously changing
conditions
such as the average cooling rate in the quenching treatment after a solution
treatment and
the temperature and holding period in the subsequent preliminary aging
treatment as shown
in Tables 5 and 6. With respect to the indications of the contents of elements
within Table
4, a value of the element expressed by a blank indicates that the content is
below a
detection limit.
[0171]
Specific conditions for aluminum alloy sheet production were as follows. Slabs
of aluminum alloys respectively having the compositions shown in Table 4 were
commonly produced through casting by the DC casting method. In this casting,
the
average rate of cooling from the liquidus temperature to the solidus
temperature was set at
50 C/min in common with all the Examples. Subsequently, the slabs were
subjected to a
soaking treatment of 540 C x 6 hours performed in one stage only, and were
then reheated
to 500 C to initiate hot rough rolling, in common with all the Examples.
Thereafter, they
were hot-rolled, in the succeeding finish rolling, to a thickness of 3.5 mm to
obtain hot-
rolled sheets, in common with all the Examples. The hot-rolled aluminum alloy
sheets
were subjected to rough annealing of 500 C x 1 minute and then to cold rolling
at a
processing rate of 70% without performing intermediate annealing during the
cold-rolling
passes, to obtain cold-rolled sheets having a thickness of 1.0 mm, in common
with all the
Examples.
[0172]
Furthermore, the cold-rolled sheets were each continuously subjected to a
refining
treatment (T4) with continuous type heat treatment facilities while unwinding
and winding
each sheet, in common with all the Examples. Specifically, a solution
treatment was
performed by heating at an average rate of heating to 500 C of 10 C/sec and
holding for
10 seconds after the temperature reached a target temperature of 560 C,
followed by
cooling to room temperature by water cooling or air cooling so as to result in
the average
cooling rates shown in Tables 5 and 6. After this cooling and after the
subsequent
48
CA 02941988 2016-09-08
required periods shown in Table 2 at room temperature, a preliminary aging
treatment was
performed by using an atmospheric furnace and an oil bath and using the
temperature
increase rates, reached temperatures, average cooling rates, and holding
periods shown in
Tables 5 and 6. As for the cooling after this preliminary aging treatment,
water cooling or
gradual cooling (natural cooling) was conducted in order to change the average
rate of
cooling.
[0173]
From the final product sheets which each had been allowed to stand at room
temperature for 30 days after the refining treatment, test sheets (blanks)
were cut out and
the structure and properties of the test sheets were examined and evaluated.
The results
thereof are shown in Tables 5 and 6.
[0174]
(Structure)
The solid-solution amount of Mg + Si in the sheet, volume proportions of atom
aggregates determined with a three-dimensional atom probe field ion
microscope, etc. were
determined through measurements and analysis by the measuring methods
described
above. In Tables 5 and 6, the average volume proportions (%) of atom
aggregates
determined with a three-dimensional atom probe field ion microscope are
abbreviated to
"average volume proportions of atom aggregates determined with 3DAP (%)".
[0175]
In the "average volume proportions of atom aggregates" in Tables 5 and 6, the
average volume proportion (EViNm)x100 of the total volume EVi of atom
aggregates
which satisfied the requirements specified in the present aspect to the volume
VA! of the
needle-shaped sample which had undergone electric-field evaporation was
determined (in
Tables 2 and 3, it is referred to as EviNmx100). Furthermore, the average
volume
proportion (V11.5 or more/Vi) X 1 00 of the total volume
V-1,5 or more of atom aggregates
having a Guinier radius r0 of 1.5 nm or larger to the total volume EVi of the
atom
aggregates was also determined (in Tables 5 and 6, it is referred to as EViI.5
or
more/V'< 100).
[0176]
(Bake hardenability)
The test sheets which had been allowed to stand at room temperature for 30
days
after the refining treatment were each examined for 0.2% proof stress (As
proof stress) as a
mechanical property through a tensile test. Furthermore, these test sheets
were aged at
room temperature for 30 days, subsequently subjected to an artificial age
hardening
treatment of 170 C x 20 minutes (after BH), and then examined for 0.2% proof
stress
(proof stress after BH) through a tensile test, in common with the test
sheets. The BH
49
CA 02941988 2016-09-08
response of each test sheet was evaluated on the basis of the difference
between these 0.2%
proof stresses (increase in proof stress).
[0177]
With respect to the tensile test, No. 5 specimens (25 mm x 50 mmGL x sheet
thickness) according to JIS Z2201 were cut out of each sample sheet to perform
the tensile
test at room temperature. Here, the tensile direction of each specimen was set
so as to be
perpendicular to the rolling direction. The tensile rate was set at 5 mm/min
until the 0.2%
proof stress and at 20 mm/min after the proof stress. The number N of
examination for
mechanical property was 5, and an average value therefor was calculated. With
respect to
the specimens to be examined for the proof stress after BH, a 2% pre-strain as
a simulation
of sheet press forming was given to the specimens by the tensile tester,
followed by
performing the BH treatment.
[0178]
(Hem workability)
Hem workability was evaluated only with respect to the test sheets which had
been allowed to stand at room temperature for 7 days or 100 days after the
refining
treatment. In the test, strip-shaped specimens having a width of 30 mm were
used and
subjected to 90 bending at an inward bending radius of 1.0 mm with a down
flange.
Thereafter, an inner plate having a thickness of 1.0 mm was nipped, and the
specimen was
subjected, in order, to pre-hem working in which the bent part was further
bent inward to
approximately 130 and flat-hem working in which the bent part was further
bent inward to
180 and the end portion was brought into close contact with the inner plate.
[0179]
The surface state, such as the occurrence of rough surface, a minute crack, or
a
large crack, of the bent part (edge bent part) of the flat hem was visually
examined and
visually evaluated on the basis of the following criteria. In the following
criteria, ratings
of 0 to 2 are on an acceptable level, and ratings of 3 and larger are
unacceptable.
0, no crack and no rough surface; 1, slight rough surface; 2, deep rough
surface; 3,
minute surface crack; 4, linearly continued surface crack.
[0180]
Invention Examples Nos. 35, 36, 43, and 48 in Table 5 and Nos. 51 to 58 in
Table
6, which employ alloys Nos. 23 to 34 shown in Table 4, each is not only within
the
component composition range according to the present aspect and has been
produced under
conditions within preferred ranges but also has undergone the refining
treatment, including
the solution quenching treatment and the preliminary aging treatment, under
preferred
conditions. Because of this, these Invention Examples satisfy the structure
requirements
specified in the present aspect, as shown in Tables 5 and 6. That is, the
solid-solution
amount of Mg + Si is 1.0 mass % or more and 2.0 mass % or less, the average
volume
CA 02941988 2016-09-08
proportion EViN,Ox100 of the total volume Vi of atom aggregates satisfying the
requirements specified in the present aspect to the volume VA1 of the needle-
shaped sample
which has undergone electric-field evaporation is in the range of 0.3 to 1.5%,
and the
average volume proportion (IViI.5 or more' Vi) X100 of the total volumevi V
..1.5 or more of
atom aggregates having a Guinier radius rG of 1.5 nm or larger to the total
volume EVi of
the atom aggregates is 20 to 70%.
[0181]
As a result, the Invention Examples each show excellent BH response although
the bake hardening is performed after the refining treatment and subsequent
room-
temperature aging and is a treatment conducted at a low temperature for a
short period of
time. Furthermore, as shown in Table 6, even after the refining treatment and
subsequent
room-temperature aging, they each have a relatively low As proof stress and a
low yield
ratio and hence show excellent press formability into automotive panels or the
like and
excellent hem workability.
[0182]
That is, the Invention Examples, even when having undergone an automotive-
baking treatment after room-temperature aging, were able to exhibit not only
high BH
response with a 0.2% proof stress difference of 100 MPa or greater and a 0.2%
proof stress
after BH of 190 MPa or greater but also press formability with an As 0.2%
proof stress of
110 MPa or less and a low yield ratio of less than 0.50 and satisfactory
bendability. Thus,
they have succeeded in combining formability and bake hardenability and in
attaining both
an increase in BH response and a reduction in yield ratio.
[0183]
In contrast, Comparative Examples 37 to 42, 44 to 47, 49, and 50 in Table 5,
which employed alloy examples 24, 25 and 26 in Table 4 like Invention
Examples, each
have the preliminary aging treatment condition outside the preferred ranges,
as shown in
Table 5. As a result, either the solid-solution amount of Mg + Si or the
average volume
proportion (YViNm)x100 or the average volume proportion (EVi1.5 or more/EVi) X
100 is
outside the range specified in the present aspect. As a result, they show
enhanced room-
temperature aging and, in particular, a relatively high As proof stress or an
increased yield
ratio after 30-day room-temperature holding, as compared with the Invention
Examples
having the same alloy composition. Because of this, they are poor in press
formability
into automotive panels or the like and in hem workability or are poor in BH
response.
Thus, they have failed to combine formability and bake hardenability and to
attain both an
increase in BH response and a reduction in yield ratio.
[0184]
In Comparative Example 37, the average cooling rate in the quenching treatment
to room temperature performed after the solution treatment is too low. Because
of this,
51
CA 02941988 2016-09-08
coarse Mg-Si and elemental Si were yielded during the cooling, resulting in
low
formability. In addition, the solid-solution amount of after the solution
treatment is low,
and the average volume proportion (EVil 5 or more/EVi) X 1 00 also is less
than 20%.
Furthermore, the BH response is also low.
[0185]
In Comparative Examples 38 and 44, the period from the quenching treatment to
room temperature after the solution treatment to the preliminary aging
treatment (initiation
of heating) is too long. Because of this, the average volume proportion
(EVi1.5 or
more/Vi) X 1 00 is less than 20% and the BH response is low. A reduction in
yield ratio
was also unable to be attained.
[0186]
In Comparative Examples 39 and 45, the temperature increase rate in the
preliminary aging treatment is too low. Because of this, the average volume
proportion
(EVi 1.5 or more/V) x 100 was unable to be increased to 20% or higher,
resulting in low BH
response.
[0187]
In Comparative Examples 40, 46 and 49, the period of holding in the range of
60
to 120 C in the preliminary aging treatment is 1 hour, which is too short.
Because of this,
the formation of precipitate nuclei was insufficient, and the average volume
proportion
(V115 0r more/V1) < 100 was unable to be increased to 20% or higher, resulting
in low BH
response.
[0188]
In Comparative Examples 41, 47 and 50, the period of holding in the range of
60
to 120 C in the preliminary aging treatment is 48 to 45 hours, which is too
long. Because
of this, precipitate nuclei were yielded in too large an amount in the
preliminary aging
treatment. As a result, the amount of atom aggregates having a relatively
large size and
contributing to strength has decreased and the average volume proportion
(EVi/Vm)x100
has increased beyond 1.5%, resulting in a failure in reducing the yield ratio
of the sheet
during forming to less than 0.50.
[0189]
In Comparative Example 42, the reached temperature in the preliminary aging
treatment is 130 C, which exceeds the upper limit of 120 C and is too high.
Because of
this, precipitate nuclei were yielded in too large an amount in the
preliminary aging
treatment. As a result, the amount of atom aggregates having a relatively
large size and
contributing to strength has decreased and the average volume proportion
(EVi/Vm)x100
has increased beyond 1.5%, resulting in too high an As proof stress and a
failure in
reducing the yield ratio of the sheet during forming to less than 0.50.
[0190]
52
CA 02941988 2016-09-08
Comparative Examples 59 to 67 in Table 6 have been produced within preferred
ranges, including the conditions for the preliminary aging treatment. However,
since they
employed alloys Nos. 35 to 43 shown in Table 4, the contents of Mg and Si,
which are
essential elements, therein are outside the ranges according to the present
aspect or the
content of impurity elements therein is too high. Because of this, Comparative
Examples
59 to 67 each show, in particular, too high an As proof stress and too high a
yield ratio after
30-day room-temperature holding as compared with the Invention Examples, as
shown in
Table 6, and hence are poor in press formability into automotive panels or the
like and in
hem workability or are poor in BIT response.
[0191]
Comparative Example 59 is alloy 35 shown in Table 4, in which the Si content
is
too low.
Comparative Example 60 is alloy 36 shown in Table 4, in which the Si content
is
too high.
Comparative Example 61 is alloy 37 shown in Table 4, in which the Sn content
is
too low.
Comparative Example 62 is alloy 38 shown in Table 4, in which the Sn content
is
too high and cracking occurred during the hot rolling, making the sheet
production
impossible.
Comparative Example 63 is alloy 39 shown in Table 4, in which the Fe content
is
too high.
Comparative Example 64 is alloy 40 shown in Table 4, in which the Mn content
is
too high.
Comparative Example 65 is alloy 41 shown in Table 4, in which the Cr content
and Ti content are too high.
Comparative Example 66 is alloy 42 shown in Table 4, in which the Zn content
is
too high.
Comparative Example 67 is alloy 43 shown in Table 4, in which the Zr content
and V content are too high.
[0192]
Those results of the Examples establish that, for improving formability and BH
response after room-temperature aging, it is necessary that all the
requirements concerning
composition and structure specified in the present aspect should be satisfied.
53
[0193]
[Table 4]
Table 4
Alloy Chemical components of Al-Mg-Si alloy sheet
(mass%; remainder, Al)
Classification
No. Mg Si Sn Fe Mn Cr Zr V Ti Cu Zn Ag
23 0.64 0.99 0.040
24 0.58 0.90 0.050 0.2
25 0.40 0.82 0.039 0.2 0.05
0.12
26 0.39 1.18 0.058 0.2 -
0.16 0.01
27 0.36 1.23 0.084 0.2 _
0.33
28 0.54 1.31 0.053 0.2 0.22
Inv. Ex.
29 0.55 0.79 0.197 0.2 0.12
30 0.45 0.93 0.040 0.2 0.35 0.05
31 0.64 1.15 0.027 0.2 0.05
0.05 _
32 0.71 0.72 0.055 0.2
_ 0.03
00'
33 0.47 1.23 0.005 0.7
0.6
34 0.55 0.87 0.050 0.2 0.2
0.1 0.1
35 0.77 0.45 0.046 0.2
36 0.40 2.10 0.042 0.2
37 0.58 1.02 0.002 0.2
38 0.60 1.09 0.455 0.2
Corn. Ex. 39 0.38 0.80 0.051 1.3
40 0.53 0.98 0.046 0.2 0.78
0.01
41 0.51 0.80 0.057 0.2 0.44
0.08
42 0.48 1.01 0.052 0.2
1.23
43 0.49 0.94 0.055 0.2 0.4 0.4
* Field in which the value for the element is blank indicates below detection
limit.
54
[0194]
_
[Table 5]
Table 5
..
Solution quenching treatment
Preliminary aging
Solution Required period
Period Average
Alloy No.Average Temperature
Reached
Classification No.treatment to preliminary
of holding at cooling
in Table 4 cooling rate increase rate
temperature
temperature aging
60 to 120 C rate
C C/s min C/s C
hr C/s ..
Inv. Ex. 35 23 540 100 5 20
100 12 100
Inv. Ex. 36 24 540 100 5 20
100 12 100
Corn. Ex. 37 24 540 1 5 20 100
12 100 p
Corn. Ex. 38 24 540 100 120 20 100
12 100 0
rõ
Corn. Ex. 39 24 540 100 5 0.1 100
12 100 .
,
Corn. Ex. 40 24 540 100 5 20 100
1 100
.3
Corn. Ex. 41 24 540 100 5 20 100
48 100 ''
,
0,
Corn. Ex. 42 24 540 100 5 20 130
12 100 ,
0
.
'
Inv. Ex. 43 _ 25 540 100 5 20 90
12 100
.3
Corn. Ex. 44 25 540 100 80 20 90
12 100
Corn. Ex. 45 25 540 100 5 0.1 90
12 100
,
Corn. Ex. 46 25 540 100 5 20 90
3 100
Corn. Ex. 47 25 540 100 5 20 90
48 100
Inv. Ex. 48 26 540 100 5 20
100 16 0.05
_
Corn. Ex. 49 26 540 100 5 5 100
3 100
_
Com. Ex. 50 26 540 100 5 5 100
45 0.02
-
(Table 5 Continued)
Structure of aluminum alloy sheet after Properties of aluminum alloy sheet
after
30-day room-temperature holding 30-day
room-temperature holding
,
Average volume
Alloy Solid-solution amount of proportions of atom As As 0.2%
0.2% proof Proof ..
Classifi- No. No. in Mg d Si
aggregates determined tensile proof Yield ratio stress stress Hem
an
cation with 3DAP
strength stress after BH increase
Table 4
[(proof stress)/ work-
%)
EVi 1 .5 or more (tensile
strength)] ability
Mg Si Mg+Si EviNAI
-
mass% mass% mass% x100 /Vi MPa MPa
MPa MPa
x100
Inv. Ex. 35 23 0.61 0.89 1.50 0.74 31 221
103 0.466 220 117 1
_
Inv. Ex. 36 24 0.55 0.80 1.35 0.55 26 207 94
_ 0.455 226 132 1 P
_ _
Corn. Ex. 37 24 0.35 0.48 0.83 0.58 8 251
127 0.506 209 82 3 2'
Corn. Ex. -38 24 ' 0.55 0.80 - 1.35 - 1.54 9
253 133 0.527 201 68 3 .
,
_
_ .
Corn. Ex. 39 24 0.55 0.80 1.35 1.15 15 234
114 0.488 206 92 2 .3
0
_ _
rõ
Corn. Ex. 40 24 0.55 0.80 1.35 0.48 10 211 93
0.441 183 90 1
_
Corn. Ex. 41 24 0.55 0.80 1.35 2.45 45 274
147 0.536 262 115 4 ,
0
.
_ _ . .
,
Corn. Ex. 42 24 0.55 0.80 1.35 2.73 42 274
156 0.568 224 68 4
0
Inv. Ex. 43 25 0.38 0.71 1.09 0.42 20 204 88
0.431 206 118 1
_
Corn. Ex. 44 25 0.38 0.71 1.09 1.42 6 264
135 0.512 200 65 3
Corn. Ex. 45 25 0.38 0.71 1.09 1.01 9 227
112 0.492 205 93 2
Corn. Ex. 46 25 0.38 0.71 1.09 0.44 16 202 90
0.444 179 89 1
_
_
Corn. Ex. 47 25 0.38 0.71 1.09 _ 2.27 35 271
144 0.532 261 117 4
Inv. Ex. 48 26 0.37 1.02 1.39 0.55 32 207 _ 95
0.458 216 121 1
_
Corn. Ex. 49 26 0.37 1.02 1.39 0.65 - 17 215 97
0.452 192 95 1
. _
Corn. Ex. 50 26 0.37 1.02 - 1.39 - 2.59 51
282 150 0.531 269 119 4
56
[0195]
[Table 6]
_
Table 6
_Solution quenching treatment
Preliminary aginp .
Solution Required period
Period Average
Alloy No Temperature Reached
. Average
Classification No. treatment to preliminary
of holding at cooling
in Table 4 cooling rate increase rate temperature
-
.
temperature aging
60 to 120 C rate
C C/s min C/s
C hr . 'Cis
-
Inv. Ex. 51 27 540 50 5 20
100 12 100 _
Inv. Ex. 52 28 540 20 5 20
100 12 100 P
.
Inv. Ex. 53 29 540 100 15 20
100 12 _ 100 -
,
Inv. Ex. 54 30 540 100 5 5
100 12 100
_
.3
Inv. Ex. 55 31 540 100 5 3
100 12 100
.
,
Inv. Ex. 56 32 540 100 5 20
100 8 100 .
,
.
Inv. Ex. 57 33 540 100 5 20
80 27 0.1 .
,I,
.3
Inv. Ex. 58 34 540 100 5 20 _
70 32 0.1 .
Corn. Ex. 59 35 540 100 5 20
100 12 100
-
Corn. Ex. 60 36 540 100 5 20
100 12 100
Corn. Ex. 61 37 540 100 5 20
100 12 100
Corn. Ex. 62 38 cracking occurred during
hot rolling
Corn. Ex. 63 39 540 100 5 20
100 12 100
-
Corn. Ex. 64 40 540 100 5 20
100 12 100
. .
Corn. Ex. 65 41 540 100 5 20
100 12 100
Com. Ex. 66 42 540 100 5 20 ,
100 12 100
Corn. Ex. 67 43 540 100 5 20
100 12 100
57
-
(Table 6 Continued)
Structure of aluminum alloy sheet after Properties of aluminum alloy sheet
after
30-day room-temperature holding 30-day
room-temperature holding
Average volume
Alloy Solid-solution amount of proportions of atom As As 0.2%
0.2% proof Proof -
Classifi-
No. No. in Mg d Si Yield
ratio aggregates determined tensile proof stress stress Hem
an
cation with 3DAP strength
stress after BH increase
Table 4
[(proof stress)/ work-
(tensile strength)]
ability
yrevi i .5 or mo
Mg Si Mg+Si EviNAI MPa MPa
MPa MPa =
mass% mass% mass% x100
x100
,
. _ . - .
Inv. Ex. 51 27 0.33 1.08 1.41 0.63 33 202
91 0.450 207 116 2
_
_
Inv. Ex. 52 28 0.39 0.88 1.27 0.74 26 223
104 0.467 222 118 2
Inv. Ex. 53 29 0.52 0.70 1.22 0.78 21 223
103 0.462 207 104 1 P
.
Inv. Ex. 54 30 0.43 0.71 1.14 0.54 20 204
86 0.422 200 114 2 ."
Inv. Ex. 55 31 0.61 1.02 1.64 0.88 37 224
107 0.477 221 114 1 i
_
Inv. Ex. 56 32 0.68 0.62 1.30 0.88 21 216
106 0.491 208 102 2 "
_
_
Inv. Ex. 57 33 0.44 1.05 1.49 0.78 34 224
104 0.464 219 115 , 2
_
Inv. Ex. 58 34 0.52 0.78 1.30 0.32 24 _ 201
84 0.417 195 111 1
2
Com. Ex. 59 35 0.75 0.40 1.15 0.24 13 178
74 0.416 121 47 1
Corn. Ex. 60 36 0.38 1.79 2.17 1.52 66 252
125 0.496 207 82 4
_
_
Corn. Ex. 61 37 0.56 0.88 1.44 1.77 30 259
133 0.514 255 122 3 _
Corn. Ex. 62 38 crackin I occurred durin:. hot
rollinl
Corn. Ex. 63 39 0.35 1 0.69 ' 1.04 0.91
17 219 106 0.483 209 103 4
_
_ _
Corn. Ex. 64 40 0.51 0.87 1.38 1.15 28 237
117 0.494 211 94 4
_ _
Corn. Ex. 65 41 0.49 0.69 1.17 0.79 19 225
103 0.458 209 106 4
Corn. Ex. 66 42 0.46 0.90 1.37 1.05 28 232
112 0.483 205 93 4
_
_
Corn. Ex. 67 43 0.46 0.80 1.26 0.93 24 230
108 0.469 212 104 4
58
CA 02941988 2016-09-08
[0196]
While the present invention has been described in detail with reference to
specific
embodiments thereof, it will be apparent to one skilled in the art that
various changes and
modifications can be made therein without departing from the spirit and scope
of the
present invention.
This application is based on a Japanese patent application filed on March 31,
2014
(Application No. 2014-074045) and a Japanese patent application filed on March
31, 2014
(Application No. 2014-074046), the entire contents thereof being incorporated
herein by
reference.
Industrial Applicability
[0197]
According to the present invention, it is possible to provide 6000-series
aluminum
alloy sheets which combine BH response and formability after room-temperature
aging.
As a result, the -6000-series aluminum alloy sheets are usable in applications
extended to
automotive panels, in particular, outer panels in which problems may arise
concerning the
design of beautiful curved-surface configurations, character lines, etc.
59