Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
COMPOSITIONS TO REDUCE PAIN COMPRISING AN OPIOID/TOLL-LIKE
RECEPTOR 4 ANTAGONIST, DEXTRO ENANTIOMERS THEREOF, AND
METHODS OF USE THEREFOR
RELATED APPLICATIONS
[0001] This application claims priority from U.S. Patent Application No.
13/799,287, filed on
March 13, 2013; U.S. Patent Application No. 13/837,099, filed on March 15,
2013; U.S.
Patent Application No. 13/841,100, filed on March 15, 2013; U.S. Patent
Application
No.13/851,773, filed on March 27, 2013 and U.S. Patent Application No.
13/851,267, filed
on March 27, 2013, each of which is incorporated by reference, herein, in
their entireties.
FIELD
[0002] This disclosure relates to compositions comprising an opioid/TLR4
antagonist and
dextro enantiomers thereof, compositions comprising combinations of an
opioid/TLR4
antagonist and dextro enantiomers thereof with an alpha-2 adrenergic receptor
agonist,
acetyl-para-aminophenol (APAP), a cyclooxygenase (COX) inhibitor, and/or an
alpha-2-delta
ligand, particularly those that exhibit a synergistic effect for the
treatment, prevention and
reversal of pain. This disclosure further relates to methods of treatment,
prevention, and
reversal of pain comprising administration of the compositions defined above.
BACKGROUND
[0003] It is well established in medical literature that treatments currently
available for pain
have limitations. Opioid drugs cause tolerance, dependence and side effects
sufficiently
serious to prompt recent action by the FDA to further restrict the drugs.
NSAIDs, taken for
prolonged periods of time, are known to cause gastro-intestinal bleeding as
well as toxicity to
the liver, kidneys, and other organs. Newly approved treatments, like the
calcium channel
alpha-2-delta ligands gabapentin and pregabalin and the serotonin and
norepinephrine
reuptake inhibitors milnacipran and duloxetine, require high doses to show
nominal
effectiveness, have a high dropout rate and carry many side effects.
[0004] This disclosure is a novel approach for the treatment of pain. It is
directed to the
treatment of neuropathic and nociceptive pain with an allodynic component. The
1
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
components of the combination are directed to reducing neuropathic pain and
the allodynic
component associated with nociceptive pain. Specific combinations of drugs and
the dosage
needed to create that effect is the subject of the disclosure.
[0005] In essence the opioid receptor antagonists exert their action in a site
other than the
opioid receptors. That site is the immune system receptor TLR4 located on glia
cells.
SUMMARY
[0006] The disclosure provides a composition comprising a compound that is an
opioid/TLR4 antagonist, dextro enantiomeric mixture thereof, or a
pharmaceutically
acceptable salt thereof and its use for the treatment, prevention, and
reversal of pain. The
disclosure also provides synergistic compositions comprising an opioid/TLR4
antagonist,
dextro enantiomeric mixtures thereof, or pharmaceutically acceptable salts
thereof and an
alpha-2 adrenergic receptor agonist, acetyl-para-aminophenol (APAP), a
cyclooxygenase
(COX) inhibitor, and/or an alpha-2-delta ligand. The disclosure further
provides a method of
use of these synergistic compositions for the treatment, prevention, and
reversal of pain,
particularly neuropathic pain.
[0007] In one embodiment, the disclosure provides a composition for treatment
of pain in a
mammal comprising a synergistic ratio of (a) an opioid/TLR4 antagonist, or
pharmaceutically
acceptable salts or solvates thereof and (b) a direct-acting alpha-2
adrenergic agonist, or
pharmaceutically acceptable salts or solvates thereof. In a further
embodiment, the
opioid/TLR4 antagonist is selected from a group consisting of naltrexone,
norbinaltorphimine, nalmefene, naloxone, nalorphine, methylnaltrexone,
samidorphan,
cyprodime, naltrindole, amentoflavone, naltriben, norbinaltorphimine, 6-B-
naltrexol,
metabolites and pro drugs thereof, including all enantiomeric and epimeric
forms as well as
the appropriate mixtures thereof, or pharmaceutically acceptable salts or
solvates of any
thereof. In another embodiment, the opioid/TLR4 antagonist is naltrexone as
well as pro
drugs thereof or any enantiomeric and epimeric forms thereof, as well as the
appropriate
mixtures thereof, or pharmaceutically acceptable salts or solvates of any
thereof.
[0008] In a further embodiment, the opioid/TLR4 antagonist is naltrexone in a
sustained
release formulation, as well as pro drugs thereof or any enantiomeric and
epimeric forms
thereof, as well as the appropriate mixtures thereof, or pharmaceutically
acceptable salts or
solvates of any thereof. In another embodiment, the opioid/TLR4 antagonist is
(+)¨naltrexone
(dextro-naltrexone), as well as appropriate mixtures thereof, as well as pro
drugs thereof, or
2
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
pharmaceutically acceptable salts or solvates thereof. Dextro -naltrexone ((+)-
naltrexone)
blocks only the TLR-4 while not blocking the morphine receptors. Therefore,
the side effects
arising from blocking of the morphine receptors by the racemic naltrexone (a
mix of (-)-
naltrexone and (+)-naltrexone), which are caused by (-)-naltrexone, are
eliminated by use of
(+)-naltrexone.
[0009] In another embodiment, the direct-acting alpha 2 adrenergic agonist is
selected from a
group consisting of apraclonidine, brimonidine, clonidine, detomidine,
dexmedetomidine,
guanabenz, guanfacine, lofexidine, medetomidine, romifidine, tizanidine,
tolonidine, xylazine
and fadolmidine, or pharmaceutically acceptable salts or solvates of any
thereof.
[0010] In another embodiment, the direct-acting alpha-2 adrenergic agonist is
clonidine, or
pharmaceutically acceptable salts or solvates thereof. In another embodiment,
the direct-
acting alpha-2 adrenergic agonist is clonidine in a sustained release
formulation, or
pharmaceutically acceptable salts or solvates thereof.
[0011] In another embodiment, the opioid/TLR4 antagonist is naltrexone, or
pharmaceutically acceptable salts or solvates thereof, in a therapeutically
effective amount
and the direct-acting alpha-2 adrenergic agonist is clonidine, or
pharmaceutically acceptable
salts or solvates thereof, in therapeutically effective amount. In a further
embodiment,
naltrexone and clonidine, or pharmaceutically acceptable salts or solvates of
any thereof, are
in a weight to weight combination range which corresponds to a synergistic
combination
range of the order of 90:1 to 22.5:1 parts by weight. In another embodiment,
the dose range
of naltrexone, or pharmaceutically acceptable salts or solvates thereof, is
about 0.004 mg/kg-
0.71 mg/kg per day. And wherein, the dose range of clonidine, or
pharmaceutically
acceptable salts or solvates thereof, is about 0.00018 mg/kg - 0.0086mg/kg per
day. In
another embodiment, the human dose range of naltrexone is 0.25 mg - 50 mg per
day and the
dose range of clonidine is 0.0125 mg - 0.6 mg, wherein said composition is
formulated into a
single fixed combination dosage form. In another embodiment, the human dose
range of
naltrexone is 0.25 mg - 15 mg per day and the dose range of clonidine is
0.0125 mg - 0.3 mg,
wherein said composition is formulated into a single fixed combination dosage
form. In a
further embodiment, the composition is administered once, twice, three or four
times through
the day.
[0012] In another embodiment, the therapeutically effective dose of the
pharmaceutical
composition is administered systemically, including but are not limited to
mucosal, nasal,
oral, parenteral, gastrointestinal, topical or sublingual routes.
3
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[0013] In some embodiments, the combination is in a single dosage form, and
said single
dosage form is in the form of tablets, lozenges, troches, hard candies,
liquid, powders, sprays,
creams, salves and suppositories.
[0014] In some embodiments the composition is for treating, preventing and
reversing pain.
In a further embodiment, the pain is back pain. In another embodiment, the
pain is
neuropathic pain. In another embodiment, the pain is migraine. In another
embodiment, the
pain is trigeminal neuralgia. In another embodiment, the pain is vulvodynia.
In another
embodiment, the pain is irritable bowel syndrome. In another embodiment, the
pain is post
herpetic neuralgia. In another embodiment, the pain is diabetic neuropathy. In
another
embodiment, the pain is nociceptive pain with an allodynic component.
[0015] In one embodiment, the disclosure provides a composition for treatment
of pain in a
mammal comprising a synergistic ratio of (a) an opioid/TLR4 antagonist, or
pharmaceutically
acceptable salts or solvates thereof and (b) a acetyl-para-aminophenol, or
pharmaceutically
acceptable salts or solvates thereof. In another embodiment, the opioid/TLR4
antagonist is
selected from a group consisting of naltrexone, norbinaltorphimine, nalmefene,
naloxone,
nalorphine, methylnaltrexone, samidorphan, cyprodime, naltrindole,
amentoflavone,
naltriben, norbinaltorphimine, 6-B-naltrexol, metabolites and pro drugs
thereof, including all
enantiomeric and epimeric forms as well as the appropriate mixtures thereof,
or
pharmaceutically acceptable salts or solvates of any thereof. In another
embodiment, the
opioid/TLR4 antagonist is naltrexone as well as pro drugs thereof or any
enantiomeric and
epimeric forms thereof, as well as the appropriate mixtures thereof, or
pharmaceutically
acceptable salts or solvates of any thereof.
[0016] In a further embodiment, the opioid/TLR4 antagonist is naltrexone in a
sustained
release formulation, as well as pro drugs thereof or any enantiomeric and
epimeric forms
thereof, as well as the appropriate mixtures thereof, or pharmaceutically
acceptable salts or
solvates of any thereof.
[0017] In an embodiment, the opioid/TLR4 antagonist can be (+)¨naltrexone
(dextro-
naltrexone), as well as appropriate mixtures thereof, as well as pro drugs
thereof, or
pharmaceutically acceptable salts or solvates thereof. In another embodiment,
the acetyl-para-
aminophenol or pharmaceutically acceptable salts or solvates thereof, is the
second
compound. In another embodiment, the opioid/TLR4 antagonist is naltrexone, or
pharmaceutically acceptable salts or solvates thereof, in a therapeutically
effective amount
and the acetyl-para-aminophenol is in therapeutically effective amount.
4
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[0018] In another embodiment, the naltrexone and acetyl-para-aminophenol, or
pharmaceutically acceptable salts or solvates of any thereof, are in a weight
to weight
combination range which corresponds to a synergistic combination of 3:200
parts by weight.
In a further embodiment, the dose range of naltrexone, or pharmaceutically
acceptable salts
or solvates thereof, is about 0.004 mg/kg-0.71 mg/kg and the dose range of
acetyl-para-
aminophenol, or pharmaceutically acceptable salts or solvates thereof, is
about 5 mg/kg -
57mg/kg per day. In another embodiment, the human dose range of naltrexone is
0.25 mg -
50 mg per day and the dose range of acetyl-para-aminophenol is 325 mg - 4000
mg, wherein
said composition is formulated into a single fixed combination dosage form. In
another
embodiment, the human dose range of naltrexone is 0.25 mg - 15 mg per day and
the human
the dose range of acetyl-para-aminophenol is 325 mg - 4000 mg, wherein said
composition is
formulated into a single fixed combination dosage form.
[0019] In one embodiment, the composition is administered once, twice, three
or four times
through the day. In another embodiment, the therapeutically effective dose of
the
pharmaceutical composition is administered systemically, including but are not
limited to
mucosal, nasal, oral, parenteral, gastrointestinal, topical or sublingual
routes.
[0020] In another embodiment, the combination is in a single dosage form, and
said single
dosage form is in the form of tablets, lozenges, troches, hard candies,
liquid, powders, sprays,
creams, salves and suppositories.
[0021] In another embodiment, the description provides a composition for
treating,
preventing and reversing pain.
[0022] In another embodiment, the description provides a method of treating
neuropathic
pain, nociceptive pain, nociceptive pain with an allodynic component,
migraine,
inflammation, osteoarthritis, rheumatoid arthritis, psoriatic arthritis,
trigeminal neuralgia,
vulvodynia, irritable bowel syndrome, post herpetic neuralgia, or diabetic
neuropathy in a
mammal in need thereof, comprising administering to the mammal a
therapeutically effective
amount of a composition.
[0023] In one embodiment, the description provides a composition for treatment
of pain in a
mammal comprising a synergistic ratio of an opioid/TLR4 antagonist, or
pharmaceutically
acceptable salts or solvates thereof and a cyclooxygenase (COX) inhibitor, or
pharmaceutically acceptable salts or solvates thereof.
[0024] In another embodiment, the opioid/TLR4 antagonist is selected from a
group
consisting of naltrexone, norbinaltorphimine, nalmefene, naloxone, nalorphine,
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
methylnaltrexone, samidorphan, cyprodime, naltrindole, amentoflavone,
naltriben,
norbinaltorphimine, 6-B-naltrexol, metabolites and pro drugs thereof,
including all
enantiomeric and epimeric forms as well as the appropriate mixtures thereof,
or
pharmaceutically acceptable salts or solvates of any thereof. In another
embodiment, A
composition comprising the formulation of claim 43, wherein, the opioid/TLR4
antagonist is
naltrexone as well as pro drugs thereof or any enantiomeric and epimeric forms
thereof, as
well as the appropriate mixtures thereof, or pharmaceutically acceptable salts
or solvates of
any thereof.
[0025] In one embodiment the opioid/TLR4 antagonist is naltrexone in a
sustained release
formulation, as well as pro drugs thereof or any enantiomeric and epimeric
forms thereof, as
well as the appropriate mixtures thereof, or pharmaceutically acceptable salts
or solvates of
any thereof. In another embodiment, the opioid/TLR4 antagonist is
(+)¨naltrexone (dextro-
naltrexone), as well as appropriate mixtures thereof, as well as pro drugs
thereof, or
pharmaceutically acceptable salts or solvates thereof.
[0026] In one embodiment, the cyclooxygenase (COX) inhibitor is selected from
a group
consisting of aspirin diflunisal, salsalate. ibuprofen, dexibuprofen,
naproxen, fenoprofen,
ketoprofen, dexketoprofen, flurbiprofen, oxaprozin, loxoprofen. indomethacin,
tolmetin,
,sulindac, etodolac, ketorolac, iclofenac, nabumetone. piroxicam, meloxicam,
tenoxicam,
droxicam, lornoxicam, isoxicam. mefenamic acid,meclofenamic acid, flufenamic
acid,
tolfenamic acid. celecoxib, rofecoxib, valdecoxib, parecoxib, lumiracoxib,
etoricwdb,
firocwdb, sulphonanilides, nimesulide, licofelone, lysine, clonixinate,
hyperforin, figwort,
calcitriol or pharmaceutically acceptable salts or solvates of any thereof. In
another
embodiment, the cyclooxygenase (COX) inhibitor is ibuprofen, or
pharmaceutically
acceptable salts or solvates thereof.
[0027] In a particular embodiment, the opioid/TLR4 antagonist is naltrexone,
or
pharmaceutically acceptable salts or solvates thereof, in a therapeutically
effective amount
and the cyclooxygenase (COX) inhibitor is ibuprofen, or pharmaceutically
acceptable salts or
solvates thereof, in a therapeutically effective amount. In a further
embodiment, the
naltrexone and ibuprofen, or pharmaceutically acceptable salts or solvates of
any thereof, are
in a weight to weight combination range which corresponds to a synergistic
combination of
1:90 parts by weight. In one embodiment, the dose range of naltrexone, or
pharmaceutically
acceptable salts or solvates thereof, is about 0.004 mg/kg-0.71 mg/kg and the
dose range of
ibuprofen, or pharmaceutically acceptable salts or solvates thereof, is about
3 mg/kg -
6
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
35mg/kg per day. In another embodiment the human dose range of naltrexone is
0.25 mg - 50
mg per day and the dose range of ibuprofen is 200 mg - 2400 mg, wherein said
composition
is formulated into a single fixed combination dosage form.
[0028] In a particular embodiment, the human dose range of naltrexone is 0.25
mg - 15 mg
per day and the human the dose range of ibuprofen is 200 mg - 2400 mg, wherein
said
composition is formulated into a single fixed combination dosage form. In
another
embodiment, the composition is administered once, twice, three or four times
through the
day. In another embodiment, the therapeutically effective dose of the
pharmaceutical
composition is administered systemically, including but are not limited to
mucosal, nasal,
oral, parenteral, gastrointestinal, topical or sublingual routes.
[0029] In another embodiment, the combination is in a single dosage form, and
wherein, said
single dosage form is in the form of tablets, lozenges, troches, hard candies,
liquid, powders,
sprays, creams, salves and suppositories.
[0030] In one embodiment, the disclosure provides a composition for treating,
preventing and
reversing pain.
[0031] In another embodiment is provided a method of treating neuropathic
pain, nociceptive
pain, nociceptive pain with an allodynic component, migraine, trigeminal
neuralgia,
vulvodynia, irritable bowel syndrome, post herpetic neuralgia, or diabetic
neuropathy in a
mammal in need thereof, comprising administering to the mammal in a
therapeutically
effective amount of a combination.
[0032] In one embodiment, the disclosure provides a composition for treatment
of pain in a
mammal comprising a synergistic ratio of (a) an opioid/TLR4 antagonist, or
pharmaceutically
acceptable salts or solvates thereof and (b) an alpha-2-delta ligand, or
pharmaceutically
acceptable salts or solvates thereof. In a further embodiment, the opioid/TLR4
antagonist is
selected from a group consisting of naltrexone, norbinaltorphimine, nalmefene,
naloxone,
nalorphine, methylnaltrexone, samidorphan, cyprodime, naltrindole,
amentoflavone,
naltriben, norbinaltorphimine, 6-alpha-naltrexol, 6-beta-naltrexol metabolites
and pro drugs
thereof, including all enantiomeric and epimeric forms as well as the
appropriate mixtures
thereof, or pharmaceutically acceptable salts or solvates of any thereof. In
another
embodiment, the opioid/TLR4 antagonist is naltrexone as well as pro drugs
thereof or any
enantiomeric and epimeric forms thereof, as well as the appropriate mixtures
thereof, or
pharmaceutically acceptable salts or solvates of any thereof.
7
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[0033] In one embodiment, the opioid/TLR4 antagonist is naltrexone in a
sustained release
formulation, as well as metabolites and pro drugs thereof or any enantiomeric
and epimeric
forms thereof, as well as the appropriate mixtures thereof, or
pharmaceutically acceptable
salts or solvates of any thereof. In another embodiment, the opioid/TLR4
antagonist is (+)¨
naltrexone (dextro-naltrexone), as well as appropriate mixtures thereof, as
well as metabolites
or pro drugs thereof, or pharmaceutically acceptable salts or solvates
thereof. In an
embodiment, the alpha-2-delta ligand is selected from Gabapentin or Pregabalin
or
pharmaceutically acceptable salts or solvates of any thereof.
[0034] In one embodiment, the alpha-2-delta ligand inhibitor is Gabapentin, or
pharmaceutically acceptable salts or solvates thereof. In another embodiment,
the alpha-2-
delta ligand inhibitor is pregabalin, or pharmaceutically acceptable salts or
solvates thereof.
[0035] In one embodiment, the opioid/TLR4 antagonist is naltrexone, or
pharmaceutically
acceptable salts or solvates thereof, in a therapeutically effective amount
and the alpha-2-
delta inhibitor is Gabapentin or Pregabalin, or pharmaceutically acceptable
salts or solvates
thereof, in a therapeutically effective amount. In another embodiment, the
opioid/TLR4
antagonist is dextro naltrexone, or pharmaceutically acceptable salts or
solvates thereof, in a
therapeutically effective amount and the alpha-2-delta inhibitor is Gabapentin
or Pregabalin,
or pharmaceutically acceptable salts or solvates thereof, in a therapeutically
effective amount.
In an embodiment, the naltrexone and alpha-2-delta ligand, or pharmaceutically
acceptable
salts or solvates of any thereof, are in a weight to weight combination range
which
corresponds to a synergistic combination of 1:30-1:125 parts by weight.
[0036] In one embodiment, the dose range of naltrexone, or pharmaceutically
acceptable salts
or solvates thereof, is about 0.004 mg/kg-0.71 mg/kg. In another embodiment,
the human
dose range of naltrexone is 0.25 mg - 50 mg per day. In another embodiment,
the human dose
range of naltrexone is 0.25 mg - 25 mg per day. In another embodiment, the
human dose
range of naltrexone is 0.25 mg - 15 mg per day.
[0037] In one embodiment, the composition is formulated into a single fixed
combination
dosage form and wherein, the composition is administered once, twice, three or
four times
through the day. In another embodiment, the therapeutically effective dose of
the
pharmaceutical composition is administered systemically, including but are not
limited to
mucosal, nasal, oral, parenteral, gastrointestinal, topical or sublingual
routes. In yet another
embodiment, the combination is in a single dosage form, and wherein, said
single dosage
8
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
form is in the form of tablets, lozenges, troches, hard candies, liquid,
powders, sprays,
creams, salves and suppositories.
[0038] In one embodiment, the disclosure provides a composition for treating,
preventing and
reversing pain. In another embodiment, the disclosure provides a method of
treating
neuropathic pain, nociceptive pain with an allodynic component, migraine,
trigeminal
neuralgia, vulvodynia, irritable bowel syndrome, post herpetic neuralgia, or
diabetic
neuropathy in a mammal in need thereof, comprising administering to the mammal
in a
therapeutically effective amount of a combination.
[0039] In one embodiment, the disclosure provides a method for the treatment
of pain in a
mammal comprising administration to said mammal a therapeutically effective
amount of a
composition comprising an opioid/TLR4 antagonist, or a dextro enantiomer of
the
opioid/TLR4 antagonist or a racemic mixture thereof, or a pharmaceutically
acceptable salt or
solvate thereof. In another embodiment is provided a method for the treatment
of pain in a
mammal comprising administration to said mammal a therapeutically effective
amount of a
composition predominantly comprising a dextro enantiomer of an opioid/TLR4
antagonist or
a pharmaceutically acceptable salt or solvate thereof.
[0040] In another embodiment is provided a method for the treatment of pain in
a mammal
comprising administration of a composition comprising a therapeutically
effective amount of
an opioid/TLR4 antagonist. The opioid/TLR4 antagonist is selected from a group
consisting
of naltrexone, norbinaltorphimine, nalmefene, naloxone, nalorphine,
methylnaltrexone,
samidorphan, cyprodime, naltrindo le, amentoflavone, naltriben,
norbinaltorphimine, 6-beta-
naltrexol, and 6-alpha-naltrexol, including all enantiomeric and epimeric
forms as well as the
appropriate mixtures thereof, as well as pro drugs or metabolites thereof or
pharmaceutically
acceptable salts or solvates of any thereof. In another embodiment the method
of treatment of
pain in a mammal comprises administration to said mammal a pharmaceutical
composition
containing a therapeutically effective amount of naltrexone, naloxone or
nalmefene, or a
predominantly dextro enantiomeric mixture of naltrexone, naloxone or
nalmefene, or a
pharmaceutically acceptable salt or solvate of any thereof. In an embodiment
the method for
treatment of pain in a mammal comprises administration to said mammal a
pharmaceutical
composition containing a therapeutically effective amount of naltrexone or a
pharmaceutically acceptable salt or solvate thereof.
9
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[0041] In another embodiment, the method for treatment of pain in a mammal
comprises
administration to said mammal a pharmaceutical composition containing a
therapeutically
effective amount of naloxone or a pharmaceutically acceptable salt or solvate
thereof.
[0042] In another embodiment the method for treatment of pain in a mammal
comprises
administration to said mammal a pharmaceutical composition containing a
therapeutically
effective amount of nalmefene or a pharmaceutically acceptable salt or solvate
thereof.
[0043] In another embodiment the method comprises administration to said
mammal a
pharmaceutical composition containing a therapeutically effective amount of
predominantly
dextro naltrexone mixture or a pharmaceutically acceptable salt or solvate
thereof. In one
embodiment the method comprises administration to said mammal a pharmaceutical
composition containing a therapeutically effective amount of predominantly
dextro naloxone
mixture or a pharmaceutically acceptable salt or solvate thereof.
[0044] In another embodiment the method comprises administration to said
mammal a
pharmaceutical composition containing a therapeutically effective amount of
predominantly
dextro nalmefene mixture or a pharmaceutically acceptable salt or solvate
thereof. In another
embodiment the amount of the opioid/TLR4 antagonist varies from about
0.004mg/kg to
about 4.3mg/kg, preferably from about 0.004mg/kg to about 0.71mg/kg, and most
preferably
from about 0.004mg/kg to about 0.21 mg/kg.
[0045] In one embodiment the amount of naltrexone varies from about 0.004mg/kg
to about
4.3mg/kg, preferably from about 0.004mg/kg to about 0.71mg/kg, and most
preferably from
about 0.004mg/kg to about 0.21 mg/kg. In another embodiment, the amount of
dextro
naltrexone varies from about 0.004mg/kg to about 4.3mg/kg, preferably from
about
0.004mg/kg to about 0.71mg/kg, and most preferably from about 0.004mg/kg to
about 0.21
mg/kg.
[0046] In one embodiment the disclosure provides a method for treatment of
pain wherein
the human dose range of naltrexone, or a pharmaceutically acceptable salt or
solvate thereof,
varies from about 0.25mg - 50mg per day, preferably from about 0.25mg - 25mg
per day,
most preferably from about 0.25 mg ¨ 15 mg per day wherein said dose is
formulated into a
single dosage form. In another embodiment, the composition comprises greater
than 50% to
60% dextro enantiomer. In yet another embodiment the composition comprises
greater than
60% dextro enantiomer. In one embodiment, the composition comprises greater
than 70%
dextro enantiomer. In another embodiment, the composition comprises greater
than 80%
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
dextro enantiomer. In yet another embodiment, the composition comprises
greater than 90%
dextro enantiomer.
[0047] In one embodiment, the disclosure provides a method for treatment of
pain wherein
the single fixed dosage form is administered once, twice, three or four times
through the day.
In another embodiment, a therapeutically effective dose is administered
systemically, via
routes of mucosal, nasal, oral, parenteral, gastrointestinal, tropical or
sublingual. In yet
another embodiment, the composition is in a single dosage form, and said
single dosage form
is in the form of tablets, lozenges, troches, hard candies, liquid, powders,
sprays, creams,
salves and suppositories.
[0048] In one embodiment, the disclosure provides a method for treatment of
pain wherein
the pharmaceutical composition is used for the treatment, prevention and
reversal of
neuropathic pain, back pain, chronic pain, diabetic neuropathic pain,
trigeminal neuralgia
pain, phantom limb pain, complex regional pain syndrome pain, post herpetic
pain, causalgia
pain, idiopathic pain, inflammatory pain, cancer pain,postoperative pain,
fibromyalgia pain,
headache pain, migraine pain, allodynia pain, vulvodynia pain, interstitial
cystitis pain,
irritable bowel syndrome (IBS), arthritic joint pain and tendinitis. In
another embodiment, the
disclosure provides a method in which the pharmaceutical composition is used
for the
treatment, prevention and reversal of nociceptive pain with an allodynic
component.
Brief Description of the Figures
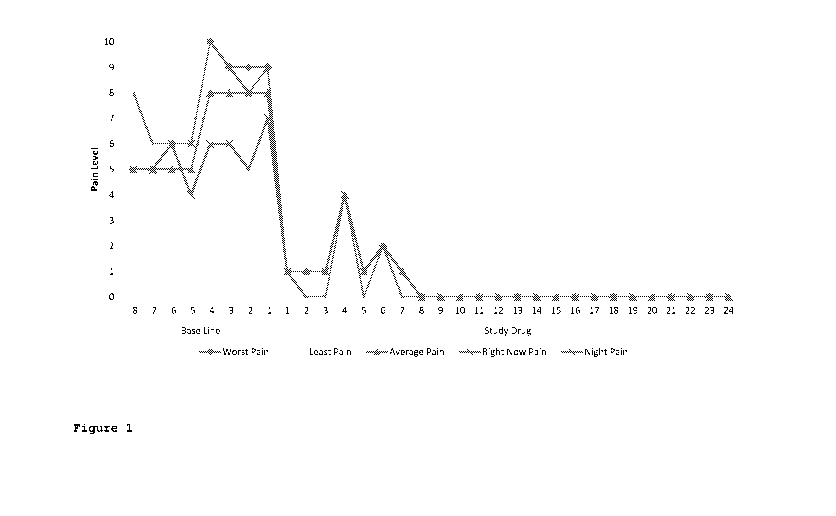
[0049] Figure 1 is a graph of the various pain intensity scores over time in a
patient suffering
from lower back pain.
[0050] Figure 2 is a graph of the percent relief over time reported by the
above patient
suffering from lower back pain. .
[0051] Figure 3 is a graph of the various pain intensity scores over time in a
patient suffering
from pain associated with vulvodynia.
[0052] Figure 4 is a graph of the percent relief over time reported by the
above patient
suffering from pain associated with vulvodynia over time.
[0053] Figure 5 is a graph of the various pain intensity scores over time in a
patient suffering
from pain associated with trigeminal neuralgia.
[0054] Figure 6 is a graph of the percent relief reported by the above patient
suffering from
pain associated with trigeminal neuralgia over time.
11
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[0055] Figure 7 is a graph of various IBS symptom intensity scores over time
in a patient
suffering from IBS.
[0056] Figure 8 is a graph of various headache parameter intensity scores over
time in a
patient suffering from migraine.
[0057] Figure 9 is a graph of the worst pain intensity by day of the ATNC05
group (N=44)
versus the placebo group (N=34) in the Double-Blind phase. Missing data were
imputed by
Baseline Observation Carried Forward (BOCF).
[0058] Figure 10 is a graph of the least pain intensity by day of the ATNC05
group (N=44)
versus the placebo group (N=34) in the Double-Blind phase.. Missing data were
imputed by
BOCF.
[0059] Figure 11 is a graph of the average pain intensity by day of the ATNC05
group
(N=44) versus the placebo group (N=34) in the Double-Blind phase. Missing data
were
imputed by BOCF.
[0060] Figure 12 is a graph of the "right now" pain intensity by day of the
ATNC05 group
(N=44) versus the placebo group (N=34) in the Double-Blind phase. Missing data
were
imputed by BOCF.
[0061] Figure 13 is a graph of the night pain intensity by day of the ATNC05
group (N=44)
versus the placebo group (N=34) in the Double-Blind phase. Missing data were
imputed by
BOCF.
[0062] Figure 14 is a graph depicting the Open Phase Relief by Day compared
with a
baseline measurement.
[0063] Figure 15 is a graph demonstrating in the Oswestry Disability Index
score of cervical
pain over time comparing the ATNC05 group, Placebo Group, and Open-Label Phase
group.
[0064] Figure 16 is a graph demonstrating the Oswestry Disability Index score
of lumbar
pain over time in the ATNC05 group, Placebo group, and Open-Label Phase group.
[0065] Figure 17 is a graph demonstrating the Pittsburgh Insomnia Rating Scale
score over
time comparing ATNC05, Placebo, and Open-Label Phase. Figure 18 is a graph
depicting the
Roland-Morris Low Back Pain Disability Questionnaire (RMQ) scores over time
comparing
ATNC05, Placebo, and Open-Label Phase.
[0066] Figure 19 is a graph depicting change in pulse from baseline over time
comparing
ATNC05, Placebo, and Open-Label Phase.
[0067] Figure 20 is a graph depicting change in the systolic blood pressure
(BP) from
baseline over time comparing ATNC05, Placebo, and Open-Label Phase.
12
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[0068] Figure 21 is a graph depicting change in the diastolic blood pressure
(BP) from
baseline over time comparing ATNC05, Placebo, and Open-Label Phase.
[0069] Figure 22 is a graph depicting the level of cervical pain and
anxiety/irritability over
time in a patient with cervical pain treated with naltrexone 2 mg twice daily.
[0070] Figure 23 is a graph depicting the level of pain and enjoyment of life
over time for a
patient with cervical pain treated with on naltrexone 12.5 mg per day.
[0071] Figure 24 is a graph depicting Right Now Pain Severity score after the
initial dose(on
average two hours) compared to the baseline period mean comparing ATNC05
(N=44) and
Placebo (N=34) in the Double-Blind phase.
[0072] Figure 25 is a graph depicting the response rates as measured by PGI-I
during Week 3
during both the double-blind and open-label phases.
[0073] Figure 26 is a graph depicting the daily mean Average Pain Scores by
day with
standard error bars comparing ATNC05 and placebo groups. The Baseline Period
Mean is
shown on the chart as Day 0. Missing data were imputed by BOCF.
[0074] Figure 27 is a graph depicting the summary of pain severity scores by
week for
placebo in the Double-Blind phase. It shows the five pain severity measures.
Missing data
were imputed by BOCF.
[0075] Figure 28 is a graph depicting the summary of pain severity scores by
week for
ATNC05 in the Double-Blind phase. It shows the five pain severity measures.
Missing data
were imputed by BOCF.
[0076] Figure 29 is a graph depicting the summary of pain interference scores
by week for
placebo in the Double-Blind phase. It shows the nine interference measures.
Missing data
were imputed by BOCF.
[0077] Figure 30 is a graph depicting the summary of pain interference scores
by week for
ATNC05 in the Double-Blind phase. It shows the nine interference measures.
Missing data
were imputed by BOCF.
[0078] Figure 31 is a graph depicting the ODI score by week for each treatment
group. For
each subject, the score was taken for their primary area of back pain
(cervical or lumbar, one
per subject). Missing data in the double-blind period were imputed by BOCF.
[0079] Figure 32 is a graph depicting the average Roland-Morris score by week
for each
treatment group. Missing data in the double-blind period were imputed by BOCF
[0080] Figure 33 is a graph depicting the average PIRS-20 score by week for
each treatment
group. Missing data in the double-blind period were imputed by BOCF.
13
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[0081] Figure 34 is a graph depicting the average length of time subjects were
able to stand
on one leg, in seconds.
[0082] Figure 35 is a graph depicting the weekly mean BPI-Severity for
subjects during the
open-label extension phase.
[0083] Figure 36 is a graph depicting the weekly mean BPI-Interference scores
for subjects
during the open-label extension phase.
[0084] Figure 37 is a graph depicting the mean number of doses of other pain
medications
taken per week per subject.
[0085] Figure 38 is a graph depicting the number of migraine days per week.
The number of
migraine days in the treatment period was converted to weekly frequency by
dividing by 3.
The difference in the changes from baseline in the Treatment Period is
significant with
p=0.049.
[0086] Figure 39 is a graph depicting the subjects' reported change in energy
level during the
treatment period.
[0087] Figure 40 is a graph depicting the subjects' reported change in
activity level during
the treatment period.
[0088] Figure 41 is a graph depicting the mean joint pain (for subjects who
reported
concomitant joint pain) during the back pain study.
[0089] Figure 42 is a graph depicting the long-term pain relief of
participants surveyed after
the back pain study as percentage of subjects with post-treatment follow-up
and as a
percentage of all subjects who received ATNC05.
DETAILED DESCRIPTION
[0090] The composition and methods of use disclosed herein will now be
described more
fully, with reference to the accompanying drawings, in which embodiments are
disclosed.
However, the compositions and methods of use therefor described should not be
construed as
limited to the embodiments set forth herein. Rather, these embodiments are
provided so that
this disclosure will be thorough and complete, and will fully convey the scope
of this work to
those skilled in the art.
14
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Opioid/TLR4 Antagonists
[0091] Various u-opioid receptor ligands have been tested and were found to
also possess
action as agonists or antagonists of Toll-like receptor 4 (TLR4). Toll-like
receptors, found in
the glia, are a class of receptors that play a key role in the innate immune
system. They
recognize pathogen-associated molecular patterns (PAMPs) such as
lipopolysaccharide (LPS)
that are expressed on infectious agents, and mediate the production of
cytokines necessary for
the development of effective immunity. Opioid agonists such as morphine act as
TLR4
agonists, while opioid antagonists such as naloxone and naltrexone were found
to be TLR4
antagonists. The disclosure provides combination therapies including
opioid/TLR4
antagonists and any of the substances described in the sections below. In this
section, dosage
regimens for combinations of opioid/TLR4 antagonists and the substances
described below
are provided.
[0092] Activation of TLR4 by opioid agonists such as morphine leads to
downstream release
of inflammatory modulators including TNF-a and interleukin-1. Constant low-
level release of
these modulators is thought to reduce the efficacy of opioid drug treatment
with time and to
be involved in both the development of tolerance to opioid analgesic drugs and
in the
emergence of side effects such as hyperalgesia and allodynia which can become
problems
following extended use of opioid drugs.
[0093] Accordingly, the disclosure relates to u-opioid receptor ligand as
ligands of TLR4 as
well and contemplates that allodynia is caused by activation of TLR4. Blockage
of TLR4
accordingly will eliminate allodynia.
[0094] Several opioid antagonist drugs were found to act as antagonists for
TLR4, including
naloxone, naltrexone and nalmefene. However it was found that not only the
"normal" (-)
enantiomers, but also the "unnatural" (+) enantiomers of these drugs acted as
TLR4
antagonists. The unnatural enantiomers of the opioid antagonists, (+)-
naltrexone and (+)-
naloxone, dextro-naltrexone and dextro-naloxone, have been discovered to act
as selective
antagonists of TLR4. Since (+)-naloxone and (+)-naltrexone lack affinity for
opioid
receptors, they do not block the effects of opioid analgesic drugs, and so can
be used to
counteract the TLR4-mediated side effects of opioid agonists without affecting
analgesia. (+)-
Naloxone was also found to be neuroprotective, and both (+)-naloxone and (+)-
naltrexone are
effective in their own right at treating symptoms of neuropathic pain in
animal models.
[0095] The best known opioid receptor antagonists are naltrexone, naloxone and
nalmefene.
Naltrexone is an opioid receptor antagonist used primarily in the management
of alcohol
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
dependence and opioid dependence. A dose of 50-300 mg once daily is
recommended for
most patients. Naloxone is an opioid inverse agonist: it is a drug used to
counter the effects of
opiate overdose.
[0096] Low dose naltrexone describes the off label use of naltrexone at doses
less than 15 mg
per day for indications other than chemical dependency or intoxication.
[0097] It has been suggested in the literature that low dose naltrexone exerts
the opposite
effect of naltrexone in full dose. While the full dose naltrexone blocks the
opiate system, the
low dose naltrexone promotes the production of endorphins by the mechanism of
up
regulation caused by partial opiate receptor blockage. The beneficial effect
of naltrexone was
attributed to the increase in endorphins. The beneficial effect of low dose
naltrexone can be
further explained by its antagonism of TLR4.
[0098] Other opioid receptor antagonists used in clinical or scientific
practice which also can
be used for the treatment of pain include but are not limited to the
following: naloxone,
nalmefene, norbinaltorphimine, nalorphine, methylnaltrexone, samidorphan,
cypro dime,
naltrindole, amentoflavone, naltriben, norbinaltorphimine, and the naltrexone
metabolite 6-B-
naltrexol.
[0099] Our understanding of pathological pain has primarily revolved around
neuronal
mechanisms. However, neighboring glia, were TLL4 reside, including astrocytes
and
microglia; have recently been recognized as powerful modulators of pain.
Studies show that
TLRs can be activated not only by well-known "non-self' molecular signals but
also by
endogenous signals (IL-10, TNFa, IL-6 and NO) produced during chronic
neuropathic pain
states. Fibronectin, an endogenous TLR4 ligand that is produced in response to
tissue injury,
leads to an up regulation of the purinoceptor P2X4, which is expressed
exclusively on
microglia.
[00100] The cause of neuropathic pain can be traced to the nervous system
going awry.
Blocking TLR4s with an opioid receptor antagonist solves the perplexing
problem of
neuropathic pain. The disclosure includes findings from studies including a
double-blind
placebo-controlled clinical trial of 78 subjects treated with the opioid
receptor naltrexone
which proved the efficacy of this treatment for pain.
[00101] The package insert for full dose naltrexone reports single-digit
incidence of
nervousness, insomnia, and anxiety. In contrast, a study by Ploesser
evaluating side effects of
low dose naltrexone showed double digit incidence of nervousness, insomnia,
and anxiety.
This disclosure contemplates the use of an alpha-2 adrenergic agonist in order
to abate the
16
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
adverse effects caused by the low dose opioid/TLR4 antagonist. The direct-
acting alpha-2
adrenergic agonist is selected from a group consisting of apraclonidine,
brimonidine,
clonidine, detomidine, dexmedetomidine, guanabenz, guanfacine, lofexidine,
medetomidine,
romifidine, tizanidine, tolonidine, xylazine and fadolmidine, or
pharmaceutically acceptable
salts or solvates of any thereof.
[00102] This disclosure provides an opioid/TLR4 antagonist, or a
pharmaceutically
acceptable salt or solvates thereof. The opioid/TLR4 antagonist may be
selected from a group
consisting of (but not limited to) naltrexone, norbinaltorphimine, nalmefene,
naloxone,
nalorphine, methylnaltrexone, samidorphan, cyprodime, naltrindole,
amentoflavone,
naltriben, norbinaltorphimine, 6-B-naltrexol and metabolites thereof,
including all
enantiomeric and epimeric forms as well as the appropriate mixtures thereof,
as well as pro
drugs or metabolites thereof or pharmaceutically acceptable salts or solvates
of any thereof.
In a particular embodiment of a method and a pharmaceutical composition, the
opioid/ TLR4
antagonist is (+)-naltrexone (dextro-naltrexone), as well as pro drugs thereof
or any
enantiomeric and epimeric forms thereof, as well as the appropriate mixtures
thereof, or
pharmaceutically acceptable salts or solvates of any thereof.
[00103] In a particular embodiment of a method and a pharmaceutical
composition, the
dose range of naltrexone, or a pharmaceutically acceptable salt or solvate
thereof, or of any
opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or solvate
thereof when
combined with any of the substances described below, is about 0.001 to about
1.0 mg/kg, e.g.
is about 0.004 mg/kg- about 0.71 mg/kg (e.g. is about 0.004 mg/kg, 0.005mg/kg,
0.006
mg/kg, 0.007 mg/kg, 0.008 mg/kg, 0.009 mg/kg, 0.010 mg/kg, 0.011 mg/kg, 0.012
mg/kg,
0.013 mg/kg, 0.014 mg/kg, 0.015 mg/kg, 0.016 mg/kg, 0.017 mg/kg, 0.018 mg/kg,
0.019
mg/kg, 0.02 mg/kg, 0.03 mg/kg, 0.04 mg/kg, 0.05 mg/kg, 0.06 mg/kg, 0.07 mg/kg,
0.08
mg/kg, 0.09 mg/kg, 0.10 mg/kg, 0.11 mg/kg, 0.12 mg/kg, 0.13 mg/kg, 0.14 mg/kg,
0.15
mg/kg, 0.16 mg/kg, 0.17 mg/kg, 0.18 mg/kg, 0.19 mg/kg, 0.20 mg/kg, 0.21 mg/kg,
0.22
mg/kg, 0.23 mg/kg, 0.24 mg/kg, 0.25 mg/kg, 0.26 mg/kg, 0.27 mg/kg, 0.28 mg/kg,
0.29
mg/kg, 0.30 mg/kg, 0.31 mg/kg, 0.32 mg/kg, 0.33 mg/kg, 0.34 mg/kg, 0.35 mg/kg,
0.36
mg/kg, 0.37 mg/kg, 0.38 mg/kg, 0.39 mg/kg, 0.40 mg/kg, 0.41 mg/kg, 0.42 mg/kg,
0.43
mg/kg, 0.44 mg/kg, 0.45 mg/kg, 0.46 mg/kg, 0.47 mg/kg, 0.48 mg/kg, 0.49 mg/kg,
0.50
mg/kg, 0.51 mg/kg, 0.52 mg/kg, 0.53 mg/kg, 0.54 mg/kg, 0.55 mg/kg, 0.56 mg/kg,
0.57
mg/kg, 0.58 mg/kg, 0.59 mg/kg, 0.60 mg/kg, 0.61 mg/kg, 0.62 mg/kg, 0.63 mg/kg,
0.64
17
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
mg/kg, 0.65 mg/kg, 0.66 mg/kg, 0.67 mg/kg, 0.68 mg/kg, 0.69 mg/kg, 0.70 mg/kg,
or 0.71
mg/kg.
[00104] In a particular embodiment of a method and a pharmaceutical
composition, the
dose range of naltrexone, or a pharmaceutically acceptable salt or solvate
thereof, or of any
opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or solvate
thereof when
combined with any of the substances described below, is administered in a
range of about
0.001 to about 1.0 mg/kg, e.g. a range of about 0.004 mg/kg to about 0.71
mg/kg (e.g. the
range is about 0.004 to about 0.35 mg/kg, about 0.35 to about 0.71 mg/kg,
about 0.004 to
about 0.24 mg/kg, about 0.24 to about 0.47 mg/kg, about 0.47 to about 0.71
mg/kg, about
0.004 to about 0.18 mg/kg, about 0.18 to about 0.35 mg/kg, about 0.35 to about
0.53 mg/kg,
about 0.53 to about 0.71 mg/kg, about 0.004 to about 0.07 mg/kg, 0.07 to about
0.14 mg/kg,
about 0.14 to about 0.21 mg/kg, about 0.21 to about 0.28 mg/kg, 0.28 to about
0.35 mg/kg,
about 0.35 to about 0.42 mg/kg, about 0.42 to about 0.49 mg/kg, about 0.49 to
about 0.56
mg/kg, about 0.56 to about 0.63 mg/kg, about 0.63 to about 0.71 mg/kg, 0.004
to about 0.047
mg/kg, 0.024 to about 0.71 mg/kg, about 0.18 to about 0.53 mg/kg, or about
0.18 to about
0.71 mg/kg).
[00105] In a particular embodiment of a method and a pharmaceutical
composition, the
human dose range of naltrexone, or a pharmaceutically acceptable salt or
solvate thereof, or
of any opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or
solvate thereof, is
about 0.1 mg to about 100 mg per day, e.g. is about 0.25 mg to about 15 mg per
day, about
0.25 mg to about 25 mg per day, or about 0.25 mg to about 50 mg per day (e.g.
about 0.25
mg, 0.26 mg, 0.27 mg, 0.28 mg, 0.29 mg, 0.30 mg, 0.31 mg, 0.32 mg, 0.33 mg,
0.34 mg, 0.35
mg, 0.36 mg, 0.37 mg, 0.38 mg, 0.39 mg, 0.40 mg, 0.41 mg, 0.42 mg, 0.43 mg,
0.44 mg, 0.45
mg, 0.46 mg, 0.47 mg, 0.48 mg, 0.49 mg, 0.50 mg, 0.51 mg, 0.52 mg, 0.53 mg,
0.54 mg, 0.55
mg, 0.56 mg, 0.57 mg, 0.58 mg, 0.59 mg, 0.60 mg, 0.61 mg, 0.62 mg, 0.63 mg,
0.64 mg, 0.65
mg, 0.66 mg, 0.67 mg, 0.68 mg, 0.69 mg, 0.70 mg, 0.71 mg, 0.72 mg, 0.73 mg,
0.74 mg, 0.75
mg, 0.76 mg, 0.77 mg, 0.78 mg, 0.79 mg, 0.80 mg, 0.81 mg, 0.82 mg, 0.83 mg,
0.84 mg, 0.85
mg, 0.86 mg, 0.87 mg, 0.88 mg, 0.89 mg, 0.90 mg, 0.91 mg, 0.92 mg, 0.93 mg,
0.94 mg, 0.95
mg, 0.96 mg, 0.97 mg, 0.98 mg, 0.99 mg, 1 mg, 2 mg, 3 mg, 3 mg, 4 mg, 5 mg, 6
mg, 7 mg, 8
mg, 9 mg, 10 mg, 11 mg, 12 mg, 13 mg, 14 mg, 15 mg, 16 mg, 17 mg, 18 mg, 19
mg, 20 mg,
21 mg, 22 mg, 23 mg, 24 mg, 25 mg, 26 mg, 27 mg, 28 mg, 29 mg, 30 mg, 31 mg,
32 mg, 33
mg, 34 mg, 35 mg, 36 mg, 37 mg, 38 mg, 39 mg, 40 mg, 41 mg, 42 mg, 43 mg, 43
mg, 44
mg, 45 mg, 46 mg, 47 mg, 48 mg, 49 mg, or 50 mg).
18
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
In a particular embodiment of a method and a pharmaceutical composition, the
dose range of
naltrexone, or a pharmaceutically acceptable salt or solvate thereof, or of
any opioid/TLR4
antagonist, or a pharmaceutically acceptable salt or solvate thereof when
combined with any
of the substances described below, is administered in a range of about 0.001
to about 1.0 mg
per day, e.g. a range of about 0.25 mg to about 15 mg per day (e.g. the range
is about 0.25 to
about 7.6 mg, about 7.6 to about 15 mg, about 0.25 to about 5.1 mg, about 5.1
to about 10.8
mg, about 10.8 to about 15 mg, about 0.25 to about 3.93 mg, about 3.93 to
about 7.6 mg,
about 7.6 to about 11.3 mg, about 11.3 to about 15 mg, about 0.25 to about 10
mg, 5.1 to
about 15 mg, about 3.9 to about 11.3 mg, or about 3.9 to about 15 mg per day),
about 0.25 to
about 25 mg per day (e.g. the range is about 0.25 to about 12.6 mg, about 12.6
to about 25
mg, about 0.25 to about 8.5 mg, about 8.5 to about 16.7 mg, about 16.7 to
about 25 mg, about
0.25 to about 6.43 mg, about 6.43 to about 12.6 mg, about 12.6 to about 18.7
mg, about 18.7
to about 25 mg, about 0.25 to about 16.7 mg, 8.5 to about 25 mg, about 6.4 to
about 18.8 mg,
or about 6.4 to about 25 mg per day), or about 0.25 to about 50 mg per day
(e.g. the range is
about 0.25 to about 25 mg, about 25 to about 50 mg, about 0.25 to about 16.8
mg, about 16.8
to about 33.4 mg, about 33.4 to about 50 mg, about 0.25 to about 12.6 mg,
about 12.6 to
about 25.1 mg, about 25.1 to about 37.5 mg, about 37.5 to about 50 mg, about
0.25 to about
33.4 mg, 16.8 to about 50 mg, about 12.6 to about 37.5 mg, or about 12.6 to
about 50 mg per
day).
Combinations of Opioid/TLR4 Antagonists with Alpha-2-adrenergic agonists
[00106] This disclosure also provides a combination, comprising an
opioid/TLR4
antagonist, and pharmaceutically acceptable salts or solvates of any thereof,
and alpha-2
adrenergic agonist, and pharmaceutically acceptable salts or solvates of any
thereof. The
alpha-2 adrenergic agonist can be selected from a group consisting of
apraclonidine,
brimonidine, clonidine, detomidine, dexmedetomidine, guanabenz, guanfacine,
lofexidine,
medetomidine, romifidine, tizanidine, tolonidine, xylazine and fadolmidine, or
pharmaceutically acceptable salts or solvates of any thereof.
[00107] In certain embodiments, the alpha-2 adrenergic agonist is
clonidine. Clonidine
is a sympatholytic medication, classified as a direct-acting a-2 adrenergic
agonist. It is an
imidazoline derivative. An alternative hypothesis that has been proposed is
that clonidine acts
centrally as an imidazoline-2 receptor agonist. The imidazoline-2 receptor is
an allosteric
binding site of monoamine oxidase and is involved in pain modulation and
neuroprotection.
Clonidine is used to treat medical conditions such as high blood pressure,
ADHD,
19
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
anxiety/panic disorder, and certain pain conditions. The therapeutic doses
most commonly
employed have ranged from about 0.1 to about 1.0 mg, e.g. from about 0.2 mg to
about 0.6
mg (e.g. about 0.20 mg, 0.21 mg, 0.22 mg, 0.23 mg, 0.24 mg, 0.25 mg, 0.26 mg,
0.27 mg,
0.28 mg, 0.29 mg, 0.30 mg, 0.31 mg, 0.32 mg, 0.33 mg, 0.34 mg, 0.35 mg, 0.36
mg, 0.37 mg,
0.38 mg, 0.39 mg, 0.40 mg, 0.41 mg, 0.42 mg, 0.43 mg, 0.44 mg, 0.45 mg, 0.46
mg, 0.47 mg,
0.48 mg, 0.49 mg, 0.50 mg, 0.51 mg, 0.52 mg, 0.53 mg, 0.54 mg, 0.55 mg, 0.56
mg, 0.57 mg,
0.58 mg, 0.59 mg or 0.60 mg) per day given in divided doses.
[00108] Clonidine enhances the pain treatment effect of naltrexone by
agonism of the
imidazoline-2 receptor, while its sympatholytic properties are the cause of
the abatement of
naltrexone's adverse reactions.
[00109] In a particular embodiment of a method and a pharmaceutical
composition, the
alpha-2 adrenergic agonist is a pharmaceutically acceptable salt or solvate
thereof. In a
particular embodiment of a method and a pharmaceutical composition, the direct-
acting
alpha-2 adrenergic agonist is in a sustained release formulation, or a
pharmaceutically
acceptable salt or solvate thereof. In a particular embodiment of a method and
a
pharmaceutical composition, the opioid/TLR4 antagonist is naltrexone or a
pharmaceutically
acceptable salt or solvate thereof, in a therapeutically effective amount and
is combined with
a direct-acting alpha-2 adrenergic agonist, or a pharmaceutically acceptable
salt or solvate
thereof, in a therapeutically effective amount.
[00110] In a particular embodiment of a method and a pharmaceutical
composition, the
opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or solvate
thereof, and the
alpha-2 adrenergic agonist, or a pharmaceutically acceptable salt or solvate
thereof, are in a
weight to weight combination range which corresponds to a synergistic
combination range of
the order of about 100:1 to about 1:1 parts by weight, e.g. about 90:1 to
about 22.5:1 parts by
weight (e.g. about 22.5:1, 23:1, 24:1, 25:1, 26:1,27:1, 28:1, 29:1, 30:1,
31:1, 32:1, 33:1, 34:1,
35:1, 36:1, 37:1, 38:1, 39:1, 40:1, 41:1, 42:1, 43:1, 44:1, 45:1, 46:1, 47:1,
48:1, 49:1, 50:1,
41:1, 42:1, 43:1, 44:1, 45:1, 46:1, 47:1, 48:1, 49:1, 50:1, 51:1, 52:1,53:1,
54:1, 55:1, 56:1,
57:1, 58:1, 59:1, 60:1, 61:1, 62:1, 63:1, 64:1, 65:1, 66:1, 67:1, 68:1, 69:1,
70:1, 71:1, 72:1,
73:1, 74:1, 75:1, 76:1, 77:1, 78:1, 79:1, 80:1, 81:1, 82:1, 83:1, 84:1, 85:1,
86:1, 87:1, 88:1,
89:1, 90:1).
[00111] In certain embodiments, the weight to weight combination range of
naltrexone, or a pharmaceutically acceptable salt or solvate thereof, and the
alpha-2
adrenergic agonist, or a pharmaceutically acceptable salt or solvate thereof,
are of the order of
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
about 100:1 to about 1:1 parts by weight, e.g. is about 90:1 to about 22.5:1
parts by weight
(e.g. may range from about (e.g. may range from about 22.5:1 to about 56:1,
about 56:1 to
about 90:1, about 22.5:1 to about 45:1, about 46:1 to about 67.5:1, about
67.6:1 to about 90:1,
about 22.5:1 to about 39:1, about 40:1 to about 56:1, about 57:1 to about
73:1, about 74:1
about 90:1, about 22.5:1 to about 73:1, 39:1 to about 90:1, about 39:1 to
about 73:1, or about
39:1 to about 90:1 parts by weight).
[00112] In a particular embodiment of a method and a pharmaceutical
composition, the
dose range of the opioid/TLR4 antagonist, or a pharmaceutically acceptable
salt or solvate
thereof, is about 0.001 mg/kg to about 1 mg/kg, e.g. is about 0.004 mg/kg to
about 0.71
mg/kg, as described herein.
[00113] In a particular embodiment of a method and a pharmaceutical
composition, the
dose range of the alpha-2 adrenergic agonist, or a pharmaceutically acceptable
salt or solvate
thereof, is about 0.0001 mg/kg to about 0.01 mg/kg per day, e.g. is about
0.00018 mg/kg -
0.0086mg/kg per day (e.g. is about 0.00018 mg/kg, 0.00019 mg/kg, 0.002 mg/kg,
0.0021
mg/kg, 0.0022 mg/kg, 0.0023 mg/kg, 0.0024 mg/kg, 0.0025 mg/kg, 0.0026 mg/kg,
0.0027
mg/kg, 0.0028 mg/kg, 0.0029 mg/kg, 0.003 mg/kg, 0.0031 mg/kg, 0.0032 mg/kg,
0.0033
mg/kg, 0.0034 mg/kg, 0.0035 mg/kg, 0.0036 mg/kg, 0.0037 mg/kg, 0.0038 mg/kg,
0.0039
mg/kg, 0.004 mg/kg, 0.0041 mg/kg, 0.0042 mg/kg, 0.0043 mg/kg, 0.0044 mg/kg,
0.0045
mg/kg, 0.0046 mg/kg, 0.0047 mg/kg, 0.0048 mg/kg, 0.0049 mg/kg, 0.005 mg/kg,
0.0051
mg/kg, 0.0052 mg/kg, 0.0053 mg/kg, 0.0054 mg/kg, 0.0055 mg/kg, 0.0056 mg/kg,
0.0057
mg/kg, 0.0058 mg/kg, 0.0059 mg/kg, 0.006 mg/kg, 0.0061 mg/kg, 0.0062 mg/kg,
0.0063
mg/kg, 0.0064 mg/kg, 0.0065 mg/kg, 0.0066 mg/kg, 0.0067 mg/kg, 0.0068 mg/kg,
0.0069
mg/kg, 0.007 mg/kg, 0.0071 mg/kg, 0.0072 mg/kg, 0.0073 mg/kg, 0.0074 mg/kg,
0.0075
mg/kg, 0.0076 mg/kg, 0.0077 mg/kg, 0.0078 mg/kg, 0.0079 mg/kg, 0.008 mg/kg,
0.0081
mg/kg, 0.0082 mg/kg, 0.0083 mg/kg, 0.0084 mg/kg, 0.0085 mg/kg, or 0.0086
mg/kg).
[00114] In certain embodiments, the dose range of the alpha-2 adrenergic
agonist, or a
pharmaceutically acceptable salt or solvate thereof, is about 0.0001 mg/kg to
about 0.01
mg/kg per day, e.g. may range from about 0.00018 mg/kg to 0.0086 mg/kg per day
(e.g. may
range from about 0.00018 to about 0.0044 mg/kg, about 0.0044 to about 0.0086
mg/kg,
about 0.00018 to about 0.003 mg/kg, about 0.003 to about 0.006 mg/kg, about
0.006 to about
0.0086 mg/kg, about 0.00018 to about 0.003 mg/kg, about 0.003 to about 0.004
mg/kg, about
0.004 to about 0.006 mg/kg, about 0.006 to about 0.0086 mg/kg, about 0.00018
to about
21
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
0.0006 mg/kg, 0.003 to about 0.0086 mg/kg, about 0.003 to about 0.006 mg/kg,
or about
0.003 to about 0.0086 mg/kg per day).
[00115] In a particular embodiment of a method and a pharmaceutical
composition, the
human dose range of the opioid/TLR4 antagonist, or a pharmaceutically
acceptable salt or
solvate thereof, is about 0.1 mg to about 100 mg per day, e.g. is about 0.25
mg to about 50
mg per day, is about 0.25 mg to about 25 mg per day, or is about 0.25 mg -
about 15 mg per
day, as described above.
[00116] In a particular embodiment of a method and a pharmaceutical
composition, the
human the dose range of the alpha-2 adrenergic agonist, or a pharmaceutically
acceptable salt
or solvate thereof, is about 0.01 to about 1 mg per day, e.g. is about 0.0125
mg - about 0.6
mg (e.g. about 0.0125 mg, 0.0126 mg, 0.0127 mg, 0.0128 mg, 0.0129 mg, 0.0130
mg, 0.0131
mg, 0.0132 mg, 0.0133 mg, 0.0134 mg, 0.0135 mg, 0.0136 mg, 0.0137 mg, 0.0138
mg,
0.0139 mg, 0.0140 mg, 0.0141 mg, 0.0142 mg, 0.0143 mg, 0.0144 mg, 0.0145 mg,
0.0146
mg, 0.0147 mg, 0.0148 mg, 0.0149 mg, 0.0150 mg, 0.0160 mg, 0.00170 mg, 0.018
mg, 0.019
mg, 0.02 mg, 0.03 mg, 0.04 mg, 0.05 mg, 0.06 mg, 0.07 mg, 0.08 mg, 0.09 mg,
0.10 mg, 0.11
mg, 0.12 mg, 0.13 mg, 0.14 mg, 0.15 mg, 0.16 mg, 0.17 mg, 0.18 mg, 0.19 mg,
0.20 mg, 0.21
mg, 0.22 mg, 0.23 mg, 0.24 mg, 0.25 mg, 0.26 mg, 0.27 mg, 0.28 mg, 0.29 mg,
0.30 mg, 0.31
mg, 0.32 mg, 0.33 mg, 0.34 mg, 0.35 mg, 0.36 mg, 0.37 mg, 0.38 mg, 0.39 mg,
0.40 mg, 0.41
mg, 0.42 mg, 0.43 mg, 0.44 mg, 0.45 mg, 0.46 mg, 0.47 mg, 0.48 mg, 0.49 mg,
0.50 mg, 0.51
mg, 0.52 mg, 0.53 mg, 0.54 mg, 0.55 mg, 0.56 mg, 0.57 mg, 0.58 mg, 0.59 mg or
0.60 mg).
[00117] In certain embodiments, the dose range of the alpha-2 adrenergic
agonist, or a
pharmaceutically acceptable salt or solvate thereof, is about 0.0001 mg/kg to
about 0.01
mg/kg per day, e.g. may range from about 0.0125 mg to about 0.6 mg per day
(e.g. may
range from about 0.0125 mg to about 0.31 mg, about 0.31 to about 0.6 mg, about
0.0125 mg
to about 0.21 mg, about 0.21 to about 0.40 mg, about 0.40 to about 0.6 mg,
about 0.0125 to
about 0.16 mg, about 0.16 to about 0.31 mg, about 0.31 to about 0.45 mg, or
about 0.45 to
about 0.6 mg, about 0.0125 to about 0.45 mg, 0.16 to about 0.6 mg, about 0.16
to about 0.45
mg, or about 0.16 to about 0.6 mg per day).
[00118] In a particular embodiment of a method and a pharmaceutical
composition, the
human the dose range of the alpha-2 adrenergic agonist, or a pharmaceutically
acceptable salt
or solvate thereof, is about 0.01 to about 1.0 mg , e.g. is about 0.0125 mg to
about 0.3 mg
(e.g. about 0.0125 mg, 0.0126 mg, 0.0127 mg, 0.0128 mg, 0.0129 mg, 0.0130 mg,
0.0131
mg, 0.0132 mg, 0.0133 mg, 0.0134 mg, 0.0135 mg, 0.0136 mg, 0.0137 mg, 0.0138
mg,
22
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
0.0139 mg, 0.0140 mg, 0.0141 mg, 0.0142 mg, 0.0143 mg, 0.0144 mg, 0.0145 mg,
0.0146
mg, 0.0147 mg, 0.0148 mg, 0.0149 mg, 0.0150 mg, 0.0160 mg, 0.00170 mg, 0.018
mg, 0.019
mg, 0.02 mg, 0.03 mg, 0.04 mg, 0.05 mg, 0.06 mg, 0.07 mg, 0.08 mg, 0.09 mg,
0.10 mg, 0.11
mg, 0.12 mg, 0.13 mg, 0.14 mg, 0.15 mg, 0.16 mg, 0.17 mg, 0.18 mg, 0.19 mg,
0.20 mg, 0.21
mg, 0.22 mg, 0.23 mg, 0.24 mg, 0.25 mg, 0.26 mg, 0.27 mg, 0.28 mg, 0.29 mg, or
0.30 mg).
[00119] In certain embodiments, the dose range of the alpha-2 adrenergic
agonist, or a
pharmaceutically acceptable salt or solvate thereof, is about 0.0001 mg/kg to
about 0.01
mg/kg per day, e.g. may range from about 0.0125 mg to about 0.3 mg per day
(e.g. may
range from about 0.0125 mg to about 0.16 mg, about 0.16 to about 0.3 mg, about
0.0125 mg
to about 0.11 mg, about 0.11 to about 0.20 mg, about 0.20 to about 0.3 mg,
about 0.0125 to
about 0.08 mg, about 0.08 to about 0.16 mg, about 0.16 to about 0.23 mg, or
about 0.23 to
about 0.3 mg, about 0.0125 to about 0.23 mg, 0.08 to about 0.3 mg, about 0.08
to about 0.23
mg, or about 0.08 to about 0.3 mg per day).
[00120] In a particular embodiment of a method and a pharmaceutical
composition, the
human dose range of the opioid/TLR4 antagonist, or a pharmaceutically
acceptable salt or
solvate thereof, is about 0.1 to about 100 mg per day, e.g. is about 0.25 mg -
about 50 mg per
day, as described above, and the human the dose range of the alpha-2
adrenergic agonist, or a
pharmaceutically acceptable salt or solvate thereof, is about 0.01 to about 1
mg, e.g. is about
0.0125 mg to about 0.6 mg or any of the ranges described herein (e.g. about
0.0125 mg,
0.0126 mg, 0.0127 mg, 0.0128 mg, 0.0129 mg, 0.0130 mg, 0.0131 mg, 0.0132 mg,
0.0133
mg, 0.0134 mg, 0.0135 mg, 0.0136 mg, 0.0137 mg, 0.0138 mg, 0.0139 mg, 0.0140
mg,
0.0141 mg, 0.0142 mg, 0.0143 mg, 0.0144 mg, 0.0145 mg, 0.0146 mg, 0.0147 mg,
0.0148
mg, 0.0149 mg, 0.0150 mg, 0.0160 mg, 0.00170 mg, 0.018 mg, 0.019 mg, 0.02 mg,
0.03 mg,
0.04 mg, 0.05 mg, 0.06 mg, 0.07 mg, 0.08 mg, 0.09 mg, 0.10 mg, 0.11 mg, 0.12
mg, 0.13 mg,
0.14 mg, 0.15 mg, 0.16 mg, 0.17 mg, 0.18 mg, 0.19 mg, 0.20 mg, 0.21 mg, 0.22
mg, 0.23 mg,
0.24 mg, 0.25 mg, 0.26 mg, 0.27 mg, 0.28 mg, 0.29 mg, 0.30 mg, 0.31 mg, 0.32
mg, 0.33 mg,
0.34 mg, 0.35 mg, 0.36 mg, 0.37 mg, 0.38 mg, 0.39 mg, 0.40 mg, 0.41 mg, 0.42
mg, 0.43 mg,
0.44 mg, 0.45 mg, 0.46 mg, 0.47 mg, 0.48 mg, 0.49 mg, 0.50 mg, 0.51 mg, 0.52
mg, 0.53 mg,
0.54 mg, 0.55 mg, 0.56 mg, 0.57 mg, 0.58 mg, 0.59 mg or 0.60 mg), wherein said
composition is formulated into a single fixed combination dosage form. In a
particular
embodiment of a method and a pharmaceutical composition, the composition is
administered
once, twice, three or four times through the day.
23
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[00121] In a particular embodiment of a method and a pharmaceutical
composition, the
alpha-2 adrenergic agonist is clonidine, or a pharmaceutically acceptable salt
or solvate
thereof. In a particular embodiment of a method and a pharmaceutical
composition, the
direct-acting alpha-2 adrenergic agonist is clonidine in a sustained release
formulation, or a
pharmaceutically acceptable salt or solvate thereof. In a particular
embodiment of a method
and a pharmaceutical composition, the opioid/TLR4 antagonist is naltrexone or
a
pharmaceutically acceptable salt or solvate thereof, in a therapeutically
effective amount and
the direct-acting alpha-2 adrenergic agonist is clonidine, or a
pharmaceutically acceptable salt
or solvate thereof, in a therapeutically effective amount. In a particular
embodiment of a
method and a pharmaceutical composition, naltrexone, or a pharmaceutically
acceptable salt
or solvate thereof, and clonidine, or a pharmaceutically acceptable salt or
solvate thereof, are
in a weight to weight combination range which corresponds to a synergistic
combination
range of the order of about 100:1 to about 1:1 parts by weight, e.g. is about
90:1 to about
22.5:1 parts by weight (e.g. about 22.5:1, 23:1, 24:1, 25:1, 26:1, 27:1, 28:1,
29:1, 30:1, 31:1,
32:1, 33:1, 34:1, 35:1, 36:1, 37:1, 38:1, 39:1, 40:1, 41:1, 42:1, 43:1, 44:1,
45:1, 46:1, 47:1,
48:1, 49:1, 50:1, 41:1, 42:1, 43:1, 44:1, 45:1, 46:1, 47:1, 48:1, 49:1, 50:1,
51:1, 52:1,53:1,
54:1, 55:1, 56:1, 57:1, 58:1, 59:1, 60:1, 61:1, 62:1, 63:1, 64:1, 65:1, 66:1,
67:1, 68:1, 69:1,
70:1, 71:1, 72:1, 73:1, 74:1, 75:1, 76:1, 77:1, 78:1, 79:1, 80:1, 81:1, 82:1,
83:1, 84:1, 85:1,
86:1, 87:1, 88:1, 89:1, 90:1).
[00122] In certain embodiments, the weight to weight combination range of
naltrexone, or a pharmaceutically acceptable salt or solvate thereof, and
clonidine, or a
pharmaceutically acceptable salt or solvate thereof, are of the order of about
100:1 to about
1:1 parts by weight, e.g. is about 90:1 to about 22.5:1 parts by weight (e.g.
may range from
about (e.g. may range from about 22.5:1 to about 56:1, about 56:1 to about
90:1, about 22.5:1
to about 45:1, about 45:1 to about 67.5:1, about 67.5:1 to about 90:1, about
22.5:1 to about
40:1, about 40:1 to about 56:1, about 56:1 to about 73:1, about 73:1 about
90:1, about 22.5:1
to about 73:1, 39:1 to about 90:1, about 39:1 to about 73:1, or about 39:1 to
about 90:1 parts
by weight).
[00123] In a particular embodiment, the naltrexone, or pharmaceutically
acceptable
salt or solvate thereof, can be administered as explained above in the section
entitled
"Opioid/TLR4 Antagonists.
[00124] In a particular embodiment of a method and a pharmaceutical
composition, the
dose range of clonidine, or a pharmaceutically acceptable salt or solvate
thereof, is about
24
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
0.0001 to about 0.01 mg/kg per day, e.g. is about 0.00018 mg/kg - about
0.0086mg/kg per
day (e.g. is about 0.00018 mg/kg, 0.00019 mg/kg, 0.002 mg/kg, 0.0021 mg/kg,
0.0022
mg/kg, 0.0023 mg/kg, 0.0024 mg/kg, 0.0025 mg/kg, 0.0026 mg/kg, 0.0027 mg/kg,
0.0028
mg/kg, 0.0029 mg/kg, 0.003 mg/kg, 0.0031 mg/kg, 0.0032 mg/kg, 0.0033 mg/kg,
0.0034
mg/kg, 0.0035 mg/kg, 0.0036 mg/kg, 0.0037 mg/kg, 0.0038 mg/kg, 0.0039 mg/kg,
0.004
mg/kg, 0.0041 mg/kg, 0.0042 mg/kg, 0.0043 mg/kg, 0.0044 mg/kg, 0.0045 mg/kg,
0.0046
mg/kg, 0.0047 mg/kg, 0.0048 mg/kg, 0.0049 mg/kg, 0.005 mg/kg, 0.0051 mg/kg,
0.0052
mg/kg, 0.0053 mg/kg, 0.0054 mg/kg, 0.0055 mg/kg, 0.0056 mg/kg, 0.0057 mg/kg,
0.0058
mg/kg, 0.0059 mg/kg, 0.006 mg/kg, 0.0061 mg/kg, 0.0062 mg/kg, 0.0063 mg/kg,
0.0064
mg/kg, 0.0065 mg/kg, 0.0066 mg/kg, 0.0067 mg/kg, 0.0068 mg/kg, 0.0069 mg/kg,
0.007
mg/kg, 0.0071 mg/kg, 0.0072 mg/kg, 0.0073 mg/kg, 0.0074 mg/kg, 0.0075 mg/kg,
0.0076
mg/kg, 0.0077 mg/kg, 0.0078 mg/kg, 0.0079 mg/kg, 0.008 mg/kg, 0.0081 mg/kg,
0.0082
mg/kg, 0.0083 mg/kg, 0.0084 mg/kg, 0.0085 mg/kg, or 0.0086 mg/kg).
[00125] In certain embodiments, the dose range of clonidine, or a
pharmaceutically
acceptable salt or solvate thereof, is about 0.0001 mg/kg to about 0.01 mg/kg
per day, e.g.
may range from about 0.00018 mg/kg to 0.0086mg/kg per day (e.g. may range from
about
0.00018 to about 0.004 mg/kg, about 0.004 to about 0.0086 mg/kg, about 0.00018
to about
0.003 mg/kg, about 0.003 to about 0.006 mg/kg, about 0.006 to about 0.0086
mg/kg, about
0.00018 to about 0.003 mg/kg, about 0.003 to about 0.4 mg/kg, about 0.004 to
about 0.006
mg/kg, or about 0.006 to about 0.0086 mg/kg, about 0.00018 to about 0.0006
mg/kg, 0.003 to
about 0.0086 mg/kg, about 0.003 to about 0.006 mg/kg, or about 0.003to about
0.0086 mg/kg
per day).
[00126] In a particular embodiment of a method and a pharmaceutical
composition, the
human dose range of naltrexone, or a pharmaceutically acceptable salt or
solvate thereof, is
about 0.1 to about 100 mg per day, e.g. about 0.25 mg - about 50 mg per day,
about 0.25 mg
- about 25 mg per day, or about 0.25 mg - about 15 mg per day, as described
above.
[00127] In a particular embodiment of a method and a pharmaceutical
composition, the
human the dose range of clonidine, or a pharmaceutically acceptable salt or
solvate thereof, is
about 0.01 to about 1.0 mg, e.g. is about 0.0125 mg to about 0.6 mg per day
(e.g. about
0.0125 mg, 0.0126 mg, 0.0127 mg, 0.0128 mg, 0.0129 mg, 0.0130 mg, 0.0131 mg,
0.0132
mg, 0.0133 mg, 0.0134 mg, 0.0135 mg, 0.0136 mg, 0.0137 mg, 0.0138 mg, 0.0139
mg,
0.0140 mg, 0.0141 mg, 0.0142 mg, 0.0143 mg, 0.0144 mg, 0.0145 mg, 0.0146 mg,
0.0147
mg, 0.0148 mg, 0.0149 mg, 0.0150 mg, 0.0160 mg, 0.00170 mg, 0.018 mg, 0.019
mg, 0.02
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
mg, 0.03 mg, 0.04 mg, 0.05 mg, 0.06 mg, 0.07 mg, 0.08 mg, 0.09 mg, 0.10 mg,
0.11 mg, 0.12
mg, 0.13 mg, 0.14 mg, 0.15 mg, 0.16 mg, 0.17 mg, 0.18 mg, 0.19 mg, 0.20 mg,
0.21 mg, 0.22
mg, 0.23 mg, 0.24 mg, 0.25 mg, 0.26 mg, 0.27 mg, 0.28 mg, 0.29 mg, 0.30 mg,
0.31 mg, 0.32
mg, 0.33 mg, 0.34 mg, 0.35 mg, 0.36 mg, 0.37 mg, 0.38 mg, 0.39 mg, 0.40 mg,
0.41 mg, 0.42
mg, 0.43 mg, 0.44 mg, 0.45 mg, 0.46 mg, 0.47 mg, 0.48 mg, 0.49 mg, 0.50 mg,
0.51 mg, 0.52
mg, 0.53 mg, 0.54 mg, 0.55 mg, 0.56 mg, 0.57 mg, 0.58 mg, 0.59 mg or 0.60 mg
per day).
[00128] In a particular embodiment of a method and a pharmaceutical
composition, the
human the dose range of clonidine, or a pharmaceutically acceptable salt or
solvate thereof, is
about 0.01 to about 1.0 mg, e.g. is about 0.0125 mg to about 0.6 mg (e.g. may
range from
about 0.0125 mg to about 0.3 mg, about 0.3 to about 0.6 mg, about 0.0125 mg to
about 0.2
mg, about 0.2 to about 0.40 mg, about 0.4 to about 0.6 mg, about 0.0125 to
about 0.16 mg,
about 0.16 to about 0.3 mg, about 0.3 to about 0.45 mg, or about 0.45 to about
0.6 mg, about
0.0125 to about 0.45 mg, 0.16 to about 0.6 mg, about 0.16 to about 0.45 mg, or
about 0.16 to
about 0.6 mg per day).
[00129] In a particular embodiment of a method and a pharmaceutical
composition, the
human the dose range of clonidine, or a pharmaceutically acceptable salt or
solvate thereof, is
about 0.01 to about 1.0 mg, e.g. is about 0.0125 mg to about 0.3 mg (e.g.
about 0.0125 mg,
0.0126 mg, 0.0127 mg, 0.0128 mg, 0.0129 mg, 0.0130 mg, 0.0131 mg, 0.0132 mg,
0.0133
mg, 0.0134 mg, 0.0135 mg, 0.0136 mg, 0.0137 mg, 0.0138 mg, 0.0139 mg, 0.0140
mg,
0.0141 mg, 0.0142 mg, 0.0143 mg, 0.0144 mg, 0.0145 mg, 0.0146 mg, 0.0147 mg,
0.0148
mg, 0.0149 mg, 0.0150 mg, 0.0160 mg, 0.00170 mg, 0.018 mg, 0.019 mg, 0.02 mg,
0.03 mg,
0.04 mg, 0.05 mg, 0.06 mg, 0.07 mg, 0.08 mg, 0.09 mg, 0.10 mg, 0.11 mg, 0.12
mg, 0.13 mg,
0.14 mg, 0.15 mg, 0.16 mg, 0.17 mg, 0.18 mg, 0.19 mg, 0.20 mg, 0.21 mg, 0.22
mg, 0.23 mg,
0.24 mg, 0.25 mg, 0.26 mg, 0.27 mg, 0.28 mg, 0.29 mg or 0.30 mg).
[00130] In certain embodiments, the dose range of clonidine, or a
pharmaceutically
acceptable salt or solvate thereof, is about 0.0001 mg/kg to about 0.01 mg per
day, e.g. may
range from about 0.0125 to about 0.3 mg (e.g. may range from about 0.0125 mg
to about 0.16
mg, about 0.16 to about 0.3 mg, about 0.0125 mg to about 0.11 mg, about 0.11
to about 0.20
mg, about 0.20 to about 0.3 mg, about 0.0125 to about 0.08 mg, about 0.08 to
about 0.16 mg,
about 0.16 to about 0.22 mg, or about 0.22 to about 0.3 mg, about 0.0125 to
about 0.22 mg,
0.08 to about 0.3 mg, about 0.08 to about 0.22 mg, or about 0.08 to about 0.3
mg per day).
[00131] In a particular embodiment of a method and a pharmaceutical
composition, the
human dose range of naltrexone, or a pharmaceutically acceptable salt or
solvate thereof, is
26
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
about 0.1 to about 100 mg per day, e.g. is about 0.25 mg - about 50 mg per
day, as described
above, and the human the dose range of clonidine, or a pharmaceutically
acceptable salt or
solvate thereof, is about 0.01 to about 1.0 mg, e.g. is about 0.0125 mg to
about 0.6 mg (e.g.
may be any of the dose ranges listed above, e.g. may be about 0.0125 mg,
0.0126 mg, 0.0127
mg, 0.0128 mg, 0.0129 mg, 0.0130 mg, 0.0131 mg, 0.0132 mg, 0.0133 mg, 0.0134
mg,
0.0135 mg, 0.0136 mg, 0.0137 mg, 0.0138 mg, 0.0139 mg, 0.0140 mg, 0.0141 mg,
0.0142
mg, 0.0143 mg, 0.0144 mg, 0.0145 mg, 0.0146 mg, 0.0147 mg, 0.0148 mg, 0.0149
mg,
0.0150 mg, 0.0160 mg, 0.00170 mg, 0.018 mg, 0.019 mg, 0.02 mg, 0.03 mg, 0.04
mg, 0.05
mg, 0.06 mg, 0.07 mg, 0.08 mg, 0.09 mg, 0.10 mg, 0.11 mg, 0.12 mg, 0.13 mg,
0.14 mg, 0.15
mg, 0.16 mg, 0.17 mg, 0.18 mg, 0.19 mg, 0.20 mg, 0.21 mg, 0.22 mg, 0.23 mg,
0.24 mg, 0.25
mg, 0.26 mg, 0.27 mg, 0.28 mg, 0.29 mg, 0.30 mg, 0.31 mg, 0.32 mg, 0.33 mg,
0.34 mg, 0.35
mg, 0.36 mg, 0.37 mg, 0.38 mg, 0.39 mg, 0.40 mg, 0.41 mg, 0.42 mg, 0.43 mg,
0.44 mg, 0.45
mg, 0.46 mg, 0.47 mg, 0.48 mg, 0.49 mg, 0.50 mg, 0.51 mg, 0.52 mg, 0.53 mg,
0.54 mg, 0.55
mg, 0.56 mg, 0.57 mg, 0.58 mg, 0.59 mg or 0.60 mg), wherein said composition
is
formulated into a single fixed combination dosage form. In a particular
embodiment of a
method and a pharmaceutical composition, the composition is administered once,
twice, three
or four times through the day.
[00132] In one embodiment, this disclosure teaches using the direct-action
alpha-2
adrenergic receptor agonist clonidine to abate the adverse effects caused by
the low dose
opioid/TLR4 antagonist naltrexone.
Combinations of Opioid.TLR4 Antagonists with Acetyl-Para-Aminophenol (APAP)
[00133] Acetyl-para-aminophenol (APAP) enhances the pain relief action of
the
opioid/TLR4 antagonist naltrexone. A specific synergistic dose range of the
combination is
herein presented.
[00134] In a dose finding study the combination of the opioid/TLR4
antagonist,
naltrexone and acetyl-para-aminophenol (APAP), acted synergistically, whether
administered
separately, one right after the other, or administered in combination.
[00135] Acetyl-para-aminophenol (APAP) or acetaminophen (used in the United
States Canada, Japan, South Korea, Hong Kong, and Iran) and paracetamol (used
elsewhere)
both come from a chemical name for the compound: para-acetylaminophenol and
para-
acetylaminophenol. In some contexts, it is simply abbreviated as APAP, for
acetyl-para-
aminophenol. Acetyl-para-aminophenol is a widely used over-the-counter
analgesic and
27
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
antipyretic. Acetyl-para-aminophenol is classified as a mild analgesic. It is
commonly used
for the relief of headaches and other minor aches and pains and is a major
ingredient in
numerous cold and flu remedies. In combination with opioid analgesics, acetyl-
para-
aminophenol can also be used in the management of more severe pain such as
post-surgical
pain and providing palliative care in advanced cancer patients. Though acetyl-
para-
aminophenol is used to treat inflammatory pain, it is not generally classified
as an NSAID
because it exhibits only weak anti-inflammatory activity.
[00136] To date, the mechanism of action of acetyl-para-aminophenol is not
completely understood. The main mechanism proposed is the inhibition of
cyclooxygenase
(COX), and recent findings suggest that it is highly selective for COX-2.
While it has
analgesic and antipyretic properties comparable to those of aspirin or other
NSAIDs, its
peripheral anti-inflammatory activity is usually limited by several factors,
one of which is the
high level of peroxides present in inflammatory lesions. However, in some
circumstances,
even peripheral anti-inflammatory activity comparable to NSAIDs can be
observed.
[00137] Acetyl-para-aminophenol enhances the pain treatment effect of
naltrexone by
affecting nociceptive pain.
[00138] This disclosure provides a combination, comprising an opioid/TLR4
antagonist and acetyl-para-aminophenol, and pharmaceutically acceptable salts
or solvate of
any thereof.
[00139] Another embodiment is a combination, comprising an opioid/TLR4
antagonist
and acetyl-para-aminophenol. Another embodiment is a combination, comprising
an
opioid/TLR4 antagonist and acetyl-para-aminophenol, the opioid /TLR4
antagonist is
naltrexone as well as pro drugs and all enantiomeric and epimeric forms
including, but not
limited to, (+)¨naltrexone (dextro-naltrexone), as well as the appropriate
mixtures thereof, or
pharmaceutically acceptable salts or solvates of any thereof.
[00140] Another embodiment is a combination, comprising an opioid/TLR4
antagonist, or a pharmaceutically acceptable salt or solvate thereof, and
acetyl-para-
aminophenol, or a pharmaceutically acceptable salt or solvate thereof. Another
embodiment
is a combination, comprising an opioid/TLR4 antagonist and acetyl-para-
aminophenol in a
weight to weight combination range which corresponds to a synergistic
combination range of
the order of 3:200 parts by weight.
[00141] The dose range of an opioid/TLR4 antagonist, or a pharmaceutically
acceptable salt or solvate thereof, may be about 0.001 mg/kg to about 1.0
mg/kg per day, e.g.
28
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
about 0.004 mg/kg-0.71 mg/kg per day, as described above, in combination with
acetyl-para-
aminophenol, or a pharmaceutically acceptable salt or solvate thereof, at
about 1 mg/kg ¨
about 100 mg/kg per day, e.g. about 5mg/kg - about 57mg/kg per day (e.g. about
5 mg/kg, 6
mg/kg, 7 mg/kg, 8 mg/kg, 9 mg/kg, 10 mg/kg, 11 mg/kg, 12 mg/kg, 13 mg/kg, 14
mg/kg, 15
mg/kg, 16 mg/kg, 17 mg/kg, 18 mg/kg, 19 mg/kg, 20 mg/kg, 21 mg/kg, 22 mg/kg,
23 mg/kg,
24 mg/kg, 25 mg/kg, 26 mg/kg, 27 mg/kg, 28 mg/kg, 29 mg/kg, 30 mg/kg, 31
mg/kg, 32
mg/kg, 33 mg/kg, 34 mg/kg, 35 mg/kg, 36 mg/kg, 37 mg/kg, 38 mg/kg, 39 mg/kg,
40 mg/kg,
41 mg/kg, 42 mg/kg, 43 mg/kg, 43 mg/kg, 44 mg/kg, 45 mg/kg, 46 mg/kg, 47
mg/kg, 48
mg/kg, 49 mg/kg, 50 mg/kg, 51 mg/kg, 52 mg/kg, 53 mg/kg, 53 mg/kg, 54 mg/kg,
55 mg/kg,
56 mg/kg or 57 mg/kg).
[00142] In certain embodiments, the dose range of acetyl-para-aminophenol,
or a
pharmaceutically acceptable salt or solvate thereof, is about 1 mg/kg to about
100 mg/kg per
day, e.g. may range from about 5 mg/kg to about 57 mg/kg per day (e.g. may
range from
about (e.g. may range from about 5 to about 31 mg/kg, about 31 to about 57
mg/kg, about 5
to about 22 mg/kg, about 22 to about 40 mg/kg, about 40 to about 57 mg/kg,
about 5 to about
18 mg/kg, about 18 to about 31 mg/kg, about 31 to about 44 mg/kg, about 44 to
about 57
mg/kg, about 5 to about 44 mg/kg, 22 to about 57 mg/kg, about 18 to about 44
mg/kg about
31 to about 57 mg/kg, or about 18 to about 57 mg/kg per day).
[00143] Another embodiment is a combination, wherein the human dose range
of an
opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or solvate
thereof, is about 0.1
mg to about 100 mg per day, e.g. is about 0.25 mg ¨ about 50 mg per day, about
0.25 mg ¨
about 25 mg per day, or about 0.25 to about 15 mg per day, as described above,
and the dose
range of acetyl-para-aminophenol, or a pharmaceutically acceptable salt or
solvate thereof, is
about 100 to about 10,000 mg, e.g. is about 324 mg - 4000 mg (e.g. is about
324 mg, 325 mg,
326 mg, 327 mg, 328 mg,. 329 mg, 330 mg, 340 mg, 350 mg, 360 mg, 370 mg, 380
mg, 390
mg, 400 mg, 410 mg, 420 mg, 430 mg, 440 mg, 450 mg, 460 mg, 470 mg, 480 mg,
490 mg,
500 mg, 600 mg, 700 mg, 800 mg, 900 mg, 1100 mg, 1200 mg, 1300 mg, 1400 mg,
1500 mg,
1600 mg, 1700 mg, 1800 mg, 1900 mg, 2000 mg, 2100 mg, 2200 mg, 2300 mg, 2400
mg,
2500 mg, 2600 mg, 2700 mg, 2800 mg, 2900 mg, 3000 mg, 3100 mg, 3200 mg, 3300
mg,
3400 mg, 3500 mg, 3600 mg, 3700 mg, 3800 mg, 3900 mg, 4000 mg). The
combination may
also comprise an opioid/TLR4 antagonist, or a pharmaceutically acceptable salt
or solvate
thereof, at a dosage range of about 0.1 mg to about 100 mg per day, e.g. about
0.25 mg ¨
about 50 mg per day, as described above, and acetyl-para-aminophenol or a
pharmaceutically
29
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
acceptable salt or solvate thereof, at a dosage range of about 100 mg to about
10,000 mg, e.g.
about 324 mg - about 4000mg (e.g. is about 324 mg, 325 mg, 326 mg, 327 mg, 328
mg,. 329
mg, 330 mg, 340 mg, 350 mg, 360 mg, 370 mg, 380 mg, 390 mg, 400 mg, 410 mg,
420 mg,
430 mg, 440 mg, 450 mg, 460 mg, 470 mg, 480 mg, 490 mg, 500 mg, 600 mg, 700
mg, 800
mg, 900 mg, 1100 mg, 1200 mg, 1300 mg, 1400 mg, 1500 mg, 1600 mg, 1700 mg,
1800 mg,
1900 mg, 2000 mg, 2100 mg, 2200 mg, 2300 mg, 2400 mg, 2500 mg, 2600 mg, 2700
mg,
2800 mg, 2900 mg, 3000 mg, 3100 mg, 3200 mg, 3300 mg, 3400 mg, 3500 mg, 3600
mg,
3700 mg, 3800 mg, 3900 mg, 4000 mg), wherein said composition is formulated
into a single
fixed combination dosage form.
[00144] In certain embodiments, the dose range of acetyl-para-aminophenol,
or a
pharmaceutically acceptable salt or solvate thereof, is about 100 mg to about
10,000 mg, e.g.
about 324 mg - about 4000mg (e.g. may range from about 324 to about 2162 mg,
about 2162
to about 4000 mg, about 324 to about 1550 mg, about 1550 to about 2776 mg,
about 2776 to
about 4000 mg, about 324 to about 1240 mg, about 1240 to about 2162 mg, about
2160 to
about 3080 mg, about 3080 to about 4000 mg, about 324 to about 3081 mg, 1550
to about
4000 mg, about 1240 to about 3080 mg about 2160 to about 4000 mg, or about
1240 to about
4000 mg per day).
[00145] Another embodiment is a combination, comprising an opioid
antagonist and
acetyl-para-aminophenol, the opioid /TLR4 antagonist is naltrexone in a
sustained release
formulation, as well as pro drugs thereof or any enantiomeric and epimeric
forms thereof, as
well as the appropriate mixtures thereof, or pharmaceutically acceptable salts
or solvates of
any thereof. Another embodiment is a combination, comprising an opioid
antagonist and
acetyl-para-aminophenol, the opioid /TLR4 antagonist is (+)¨naltrexone (dextro-
naltrexone),
as well as pro drugs thereof or any enantiomeric and epimeric forms thereof,
as well as the
appropriate mixtures thereof, or pharmaceutically acceptable salts or solvates
of any thereof.
[00146] Another embodiment is a combination, comprising naltrexone, or a
pharmaceutically acceptable salt or solvate thereof, and acetyl-para-
aminophenol, or a
pharmaceutically acceptable salt or solvate thereof. Another embodiment is a
combination,
comprising naltrexone and acetyl-para-aminophenol in a weight to weight
combination
range which corresponds to a synergistic combination range of the order of
3:200 parts by
weight.
[00147] The dose range of naltrexone, or a pharmaceutically acceptable salt
or solvate
thereof, may be about 0.001 mg/kg to about 1.0 mg/kg, e.g. about 0.004 mg/kg-
0.71 mg/kg,
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
as described above, per day in combination with acetyl-para-aminophenol, or a
pharmaceutically acceptable salt or solvate thereof, at about 1 mg/kg to about
100 mg/kg per
day, e.g. is about 5mg/kg to about about 57mg/kg per day (e.g. may range from
about (e.g.
may range from about 5 to about 31 mg/kg, about 31 to about 57 mg/kg, about 5
to about 22
mg/kg, about 22 to about 40 mg/kg, about 40 to about 57 mg/kg, about 5 to
about 18 mg/kg,
about 18 to about 31 mg/kg, about 31 to about 44 mg/kg, about 44 to about 57
mg/kg, about 5
to about 44 mg/kg, 22 to about 57 mg/kg, about 18 to about 44 mg/kg about 31
to about 57
mg/kg, or about 18 to about 57 mg/kg per day; e.g. may be about 5 mg/kg, 6
mg/kg, 7 mg/kg,
8 mg/kg, 9 mg/kg, 10 mg/kg, 11 mg/kg, 12 mg/kg, 13 mg/kg, 14 mg/kg, 15 mg/kg,
16 mg/kg,
17 mg/kg, 18 mg/kg, 19 mg/kg, 20 mg/kg, 21 mg/kg, 22 mg/kg, 23 mg/kg, 24
mg/kg, 25
mg/kg, 26 mg/kg, 27 mg/kg, 28 mg/kg, 29 mg/kg, 30 mg/kg, 31 mg/kg, 32 mg/kg,
33 mg/kg,
34 mg/kg, 35 mg/kg, 36 mg/kg, 37 mg/kg, 38 mg/kg, 39 mg/kg, 40 mg/kg, 41
mg/kg, 42
mg/kg, 43 mg/kg, 43 mg/kg, 44 mg/kg, 45 mg/kg, 46 mg/kg, 47 mg/kg, 48 mg/kg,
49 mg/kg,
50 mg/kg, 51 mg/kg, 52 mg/kg, 53 mg/kg, 53 mg/kg, 54 mg/kg, 55 mg/kg, 56 mg/kg
or 57
mg/kg).
[00148] Another embodiment is a combination, wherein the human dose range
of
naltrexone, or a pharmaceutically acceptable salt or solvate thereof, is about
0.1 to about 100
mg per day, e.g. is about 0.25 mg ¨ about 50 mg per day, about 0.25 mg ¨ about
25 mg per
day, or about 0.25 mg ¨ about 15 mg, as described above, per day and the dose
range of
acetyl-para-aminophenol, or a pharmaceutically acceptable salt or solvate
thereof, is about
100 mg to about 10,000 mg, e.g. is about 324 mg to about 4000 mg (e.g. may
range from
about 324 to about 2162 mg, about 2162 to about 4000 mg, about 324 to about
1550 mg,
about 1550 to about 2776 mg, about 2776 to about 4000 mg, about 324 to about
1240 mg,
about 1243 to about 2162 mg, about 2160 to about 3080 mg, about 3081 to about
4000 mg,
about 324 to about 3080 mg, 1550 to about 4000 mg, about 1240 to about 30801
mg about
2160 to about 4000 mg, or about 1240 to about 4000 mg per day, e.g. is about
324 mg, 325
mg, 326 mg, 327 mg, 328 mg,. 329 mg, 330 mg, 340 mg, 350 mg, 360 mg, 370 mg,
380 mg,
390 mg, 400 mg, 410 mg, 420 mg, 430 mg, 440 mg, 450 mg, 460 mg, 470 mg, 480
mg, 490
mg, 500 mg, 600 mg, 700 mg, 800 mg, 900 mg, 1100 mg, 1200 mg, 1300 mg, 1400
mg, 1500
mg, 1600 mg, 1700 mg, 1800 mg, 1900 mg, 2000 mg, 2100 mg, 2200 mg, 2300 mg,
2400
mg, 2500 mg, 2600 mg, 2700 mg, 2800 mg, 2900 mg, 3000 mg, 3100 mg, 3200 mg,
3300
mg, 3400 mg, 3500 mg, 3600 mg, 3700 mg, 3800 mg, 3900 mg, 4000 mg). The
combination
may also comprise naltrexone, or a pharmaceutically acceptable salt or solvate
thereof, at a
31
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
dosage range of about 0.1 mg to about 100 mg per day, e.g. is about 0.25 mg ¨
about 50 mg
per day, as described above, and acetyl-para-aminophenol or a pharmaceutically
acceptable
salt or solvate thereof, at a dosage range of about 100 mg to about 10000 mg,
e.g. is about
324 mg to about 4000mg (e.g. may range from about 324 to about 2162 mg, about
2162 to
about 4000 mg, about 324 to about 1550 mg, about 1550 to about 2776 mg, about
2776 to
about 4000 mg, about 324 to about 1243 mg, about 1243 to about 2162 mg, about
2162 to
about 3081 mg, about 3081 to about 4000 mg, about 324 to about 3081 mg, 1550
to about
4000 mg, about 1243 to about 3081 mg about 2162 to about 4000 mg, or about
1243 to about
4000 mg per day; e.g. is about 324 mg, 325 mg, 326 mg, 327 mg, 328 mg,. 329
mg, 330 mg,
340 mg, 350 mg, 360 mg, 370 mg, 380 mg, 390 mg, 400 mg, 410 mg, 420 mg, 430
mg, 440
mg, 450 mg, 460 mg, 470 mg, 480 mg, 490 mg, 500 mg, 600 mg, 700 mg, 800 mg,
900 mg,
1100 mg, 1200 mg, 1300 mg, 1400 mg, 1500 mg, 1600 mg, 1700 mg, 1800 mg, 1900
mg,
2000 mg, 2100 mg, 2200 mg, 2300 mg, 2400 mg, 2500 mg, 2600 mg, 2700 mg, 2800
mg,
2900 mg, 3000 mg, 3100 mg, 3200 mg, 3300 mg, 3400 mg, 3500 mg, 3600 mg, 3700
mg,
3800 mg, 3900 mg, 4000 mg), wherein said composition is formulated into a
single fixed
combination dosage form.
[00149] Described herein is a method of treating neuropathic, nociceptive
and
migraine pain in a mammal in need thereof, comprising administering to the
mammal a
therapeutically effective amount of a combination comprising an opioid/TLR4
antagonist and
acetyl-para-aminophenol, or a pharmaceutically acceptable salt or solvate
thereof. In another
embodiment isdescribed a method of treating neuropathic, nociceptive and
migraine pain in
a mammal in need thereof, comprising administering to the mammal a
therapeutically
effective amount of a combination comprising naltrexone and acetyl-para-
aminophenol, or a
pharmaceutically acceptable salt or solvate thereof.
Combinations of Onioid/TLR4 Antagonists with COX inhibitors
[00150] Most nonsteroidal anti-inflammatory drugs (NSAIDs) act as
nonselective
inhibitors of the enzyme cyclooxygenase (COX), inhibiting both the
cyclooxygenase-1
(COX-1) and cyclooxygenase-2 (COX-2) isoenzymes. This inhibition is
competitively
reversible (albeit at varying degrees of reversibility). COX catalyzes the
formation of
prostaglandins and thromboxane from arachidonic acid. Prostaglandins act as
messenger
molecules in the process of inflammation. This mechanism of action was
elucidated by John
32
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Vane (1927-2004), who received a Nobel Prize for his work. NSAIDs are usually
indicated
for the treatment of acute or chronic conditions where pain and inflammation
are present.
[00151] Nonsteroidal anti-inflammatory drug can be classified based on
their chemical
structure or mechanism of action. Older NSAIDs were known long before their
mechanism of
action was elucidated and were for this reason classified by chemical
structure or origin.
Newer substances are more often classified by mechanism of action.
[00152] Several forms of NSAIDs can be selected from groups consisting of
Salicylates: Aspirin, Diflunisal, Salsalate; Propionic acid derivatives:
Ibuprofen,
Dexibuprofen, Naproxen, Fenoprofen, Ketoprofen, Dexketoprofen, Flurbiprofen,
Oxaprozin,
Loxoprofen; Acetic acid derivatives: Indomethacin, Tolmetin, Sulindac,
Etodolac, Ketorolac,
iclofenac, Nabumetone; Enolic acid (Oxicam) derivatives: Piroxicam, Meloxicam,
Tenoxicam, Droxicam, Lornoxicam, Isoxicam; Fenamic acid derivatives: Mefenamic
acid,
Meclofenamic acid, Flufenamic acid, Tolfenamic acid; Selective COX-2
inhibitors:
Celecoxib, Rofecoxib, Valdecoxib, Pareco)db, Lumiracoxib, Etoricoxib,
Firocoxib,
Sulphonanilides, Nimesulide, LOX (lipooxygenase); and COX 5-LOX/COX
inhibitors:
Licofelone, Lysine, cloni)dnate. Natural: Hyperforin, Figwort,
Calcitriol(Vitamin D).
[00153] Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) it is
used
primarily for fever, pain, dysmenorrhea and inflammatory diseases such as
rheumatoid
arthritis; it is also used for pericarditis. Ibuprofen is a 'core' medicine in
the World Health
Organization's Model List of Essential Medicines necessary to meet the minimum
medical
needs of a basic healthcare system.
[00154] Ibuprofen enhances the pain treatment effect of naltrexone by
inhibiting the
enzyme cyclooxygenase (COX), which converts arachidonic acid to prostaglandin
112
(PGH2). PGH2, in turn, is converted by other enzymes to several other
prostaglandins, which
are mediators of pain, inflammation, and fever. The disclosure teaches use of
a combination
of an opioid/TLR4 antagonist and a cyclooxygenase inhibitor, particularly
ibuprofen, for its
action on nociception and its anti-inflammatory action. The disclosure teaches
that the
combination is synergy as far as the effect on pain treatment.
[00155] One embodiment taught by this disclosure is a combination,
comprising an
opioid/TLR4 antagonist and a cyclooxygenase inhibitor wherein, a
cyclooxygenase inhibitor
is selected from a group consisting of aspirin, diclofenac, difluinsal,
etodolac, fenbufen,
fenoprofen, flufenisal, flurbiprofen, ibuprofen, indomethacin, ketoprofen,
ketorolac,
meclofenamic acid, mefenamic acid, nabumetone, naproxen, oxaprozin,
phenylbutazone,
33
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
piroxicam, sulindac, tolmetin, zomepirac, and their pharmaceutically
acceptable salts or
solvates or pharmaceutically acceptable salts or solvates of any thereof.
Another embodiment
is a combination, comprising an opioid antagonist and a cyclooxygenase
inhibitor, the opioid
/TLR4 antagonist is naltrexone as well as pro drugs and all enantiomeric and
epimeric forms,
specifically, (+)¨naltrexone (dextro-naltrexone), as well as the appropriate
mixtures thereof,
or pharmaceutically acceptable salts or solvates of any thereof. Another
embodiment is a
combination, comprising an opioid antagonist and a cyclooxygenase inhibitor,
the opioid
/TLR4 antagonist is naltrexone in a sustained release formulation, as well as
pro drugs
thereof or any enantiomeric and epimeric forms thereof, as well as the
appropriate mixtures
thereof, or pharmaceutically acceptable salts or solvates of any thereof.
Another embodiment
is a combination, comprising an opioid antagonist and a cyclooxygenase
inhibitor, the opioid
/TLR4 antagonist is (+)¨naltrexone (dextro-naltrexone), as well as pro drugs
thereof or any
enantiomeric and epimeric forms thereof, as well as the appropriate mixtures
thereof, or
pharmaceutically acceptable salts or solvates of any thereof.
[00156] Another embodiment is a combination, comprising an opioid/TLR4
antagonist, or a pharmaceutically acceptable salt or solvate thereof, and a
COX inhibitor, or a
pharmaceutically acceptable salt or solvate thereof. Another embodiment is a
combination,
comprising an opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or
solvate
thereof, and a COX inhibitor, or a pharmaceutically acceptable salt or solvate
thereof, in a
weight to weight combination range which corresponds to a synergistic
combination range of
the order of 90:1 parts by weight. Another embodiment is a combination,
comprising the
dose range of an opioid/TLR4 antagonist, or a pharmaceutically acceptable salt
or solvate
thereof, is about 0.001mg/kg to about 1.0 mg/kg per day, e.g. is about 0.004
mg/kg-0.71
mg/kg per day, as described above, and the COX inhibitor, or a
pharmaceutically acceptable
salt or solvate thereof, is about 1 mg/kg to about 50 mg/kg per day, e.g. is
about 3 mg/kg -
35mg/kg per day (e.g. about 3 mg/kg, 4 mg/kg, 5 mg/kg, 6 mg/kg, 7 mg/kg, 8
mg/kg, 9
mg/kg, 10 mg/kg, 11 mg/kg, 12 mg/kg, 13 mg/kg, 14 mg/kg, 15 mg/kg, 16 mg/kg,
17 mg/kg,
18 mg/kg, 19 mg/kg, 20 mg/kg, 21 mg/kg, 22 mg/kg, 23 mg/kg, 24 mg/kg, 25
mg/kg, 26
mg/kg, 27 mg/kg, 28 mg/kg, 29 mg/kg, 30 mg/kg, 31 mg/kg, 32 mg/kg, 33 mg/kg,
34 mg/kg
or 35 mg/kg).
[00157] In an embodiment, the COX inhibitor, or a pharmaceutically
acceptable salt or
solvate thereof, can be administered in a range of a about 1 mg/kg to about 50
mg/kg per day,
e.g. may be administered at a range of about 3 mg/kg to about 35 mg/kg per day
(e.g. may
34
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
range from about 3 to about 19 mg/kg, about 19 to about 35 mg/kg, about 3 to
about 13.7
mg/kg, about 13.7 to about 24.3 mg/kg, about 24.3 to about 35 mg/kg, about 3
to about 11
mg/kg, about 11 to about 19 mg/kg, about 19 to about 27 mg/kg, about 27 to
about 35 mg/kg,
about 3 to about 27 mg/kg, about 13.7 to about 35 mg/kg, about 11 to about 27
mg/kg about
19 to about 35 mg/kg, or about 11 to about 35 mg/kg per day)
[00158] Another embodiment is a combination, comprising the human dose
range of
an opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or solvate
thereof, of about
0.1 mg to about 100 mg, e.g. is about 0.25 mg - 50 mg, about 0.25 mg ¨ about
25 mg, or
about 0.25 mg ¨ about 15 mg, as described above, per day in combination with a
COX
inhibitor, or a pharmaceutically acceptable salt or solvate thereof, at a
dosage range of about
100 mg to about 5000 mg per day, e.g. is about 200 mg - about 2400 mg per day
(e.g. may
range from about 200 to about 1300 mg, about 1300 to about 2400 mg, about 200
to about
933 mg, about 933 to about 1667 mg, about 1667 to about 2400 mg, about 200 to
about 750
mg, about 750 to about 1300 mg, about 1300 to about 1850 mg, about 1850 to
about 2400
mg, about 200 to about 1850 mg, 933 to about 2400 mg, about 750 to about 1850
mg about
1300 to about 2400 mg, or about 750 to about 2400 mg per day; e.g. is about
200 mg, 300
mg, 400 mg, 500 mg, 600 mg, 700 mg, 800 mg, 900 mg, 1100 mg, 1200 mg, 1300 mg,
1400
mg, 1500 mg, 1600 mg, 1700 mg, 1800 mg, 1900 mg, 2000 mg, 2100 mg, 2200 mg,
2300
mg, or 2400 mg).
[00159] Another embodiment is a combination, comprising the human dose
range of
an opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or solvate
thereof, of about
0.1 mg to about 100 mg, e.g. is about 0.25 mg - 50 mg, as described above, per
day and the
human the dose range of a COX 2 inhibitor, or a pharmaceutically acceptable
salt or solvate
thereof, is about 100 to about 5000 mg, e.g. is about 200 mg ¨ about 2400 mg
(e.g. may
range from about 200 to about 1300 mg, about 1300 to about 2400 mg, about 200
to about
930 mg, about 930 to about 1670 mg, about 1670 to about 2400 mg, about 200 to
about 750
mg, about 750 to about 1300 mg, about 1300 to about 1850 mg, about 1850 to
about 2400
mg, about 200 to about 1850 mg, 930 to about 2400 mg, about 750 to about 1850
mg about
1300 to about 2400 mg, or about 750 to about 2400 mg per day; e.g. is about
200 mg, 300
mg, 400 mg, 500 mg, 600 mg, 700 mg, 800 mg, 900 mg, 1100 mg, 1200 mg, 1300 mg,
1400
mg, 1500 mg, 1600 mg, 1700 mg, 1800 mg, 1900 mg, 2000 mg, 2100 mg, 2200 mg,
2300
mg, or 2400 mg), wherein said composition is formulated into a single fixed
combination
dosage form. Another embodiment is a combination, comprising naltrexone, or a
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
pharmaceutically acceptable salt or solvate thereof, and ibuprofen, or a
pharmaceutically
acceptable salt or solvate thereof. Another embodiment is a combination,
comprising
naltrexone and ibuprofen in a weight to weight combination range which
corresponds to a
synergistic combination range of the order of 90:1 parts by weight.
[00160] Another embodiment is a combination, comprising the dose range of
naltrexone, or a pharmaceutically acceptable salt or solvate thereof, at about
0.001 mg/kg to
about 1.0 mg/kg per day, e.g. at about 0.004 mg/kg-0.71 mg/kg per day, as
described above,
with ibuprofen, or a pharmaceutically acceptable salt or solvate thereof, at a
dosage range of
about 1 mg/kg to about 50 mg/kg per day, e.g. at about 3 mg/kg to about
35mg/kg per day
(e.g. in any of the ranges described herein; e.g. about 3 mg/kg, 4 mg/kg, 5
mg/kg, 6 mg/kg, 7
mg/kg, 8 mg/kg, 9 mg/kg, 10 mg/kg, 11 mg/kg, 12 mg/kg, 13 mg/kg, 14 mg/kg, 15
mg/kg, 16
mg/kg, 17 mg/kg, 18 mg/kg, 19 mg/kg, 20 mg/kg, 21 mg/kg, 22 mg/kg, 23 mg/kg,
24 mg/kg,
25 mg/kg, 26 mg/kg, 27 mg/kg, 28 mg/kg, 29 mg/kg, 30 mg/kg, 31 mg/kg, 32
mg/kg, 33
mg/kg, 34 mg/kg or 35 mg/kg). Another embodiment is a combination, comprising
the
human dose range of naltrexone, at about 0.1 to about 100 mg, e.g. at about
0.25 mg ¨ about
50 mg, about 0.25 mg ¨ about 25 mg, or about 0.25 mg - about 15 mg, as
described above, in
combination with ibuprofen, or a pharmaceutically acceptable salt or solvate
thereof, at a
dosage range of about 100 mg to about 5000 mg per day, e.g. about 200 mg to
about 2400 mg
per day (e.g. may range from about 200 to about 1300 mg, about 1300 to about
2400 mg,
about 200 to about 933 mg, about 933 to about 1670 mg, about 1670 to about
2400 mg, about
200 to about 750 mg, about 750 to about 1300 mg, about 1300 to about 1850 mg,
about 1850
to about 2400 mg, about 200 to about 1850 mg, 930 to about 2400 mg, about 750
to about
1850 mg about 1300 to about 2400 mg, or about 750 to about 2400 mg per day;
e.g. is about
200 mg, 300 mg, 400 mg, 500 mg, 600 mg, 700 mg, 800 mg, 900 mg, 1100 mg, 1200
mg,
1300 mg, 1400 mg, 1500 mg, 1600 mg, 1700 mg, 1800 mg, 1900 mg, 2000 mg, 2100
mg,
2200 mg, 2300 mg, or 2400 mg).
[00161] Another embodiment is a combination, comprising the human dose
range of
naltrexone, or a pharmaceutically acceptable salt or solvate thereof, is about
0.1 mg to about
100 mg, e.g. is about 0.25 mg - 50 mg, as described above, and the human the
dose range of
ibuprofen, or a pharmaceutically acceptable salt or solvate thereof, is about
100 mg to about
5000 mg, e.g. is about 200 mg - 2400 mg (e.g. may range from about 200 to
about 1300 mg,
about 1300 to about 2400 mg, about 200 to about 930 mg, about 930 to about
1670 mg, about
1670 to about 2400 mg, about 200 to about 750 mg, about 750 to about 1300 mg,
about 1300
36
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
to about 1850 mg, about 1850 to about 2400 mg, about 200 to about 1850 mg, 930
to about
2400 mg, about 750 to about 1850 mg about 1300 to about 2400 mg, or about 750
to about
2400 mg per day; e.g. is about 200 mg, 300 mg, 400 mg, 500 mg, 600 mg, 700 mg,
800 mg,
900 mg, 1100 mg, 1200 mg, 1300 mg, 1400 mg, 1500 mg, 1600 mg, 1700 mg, 1800
mg,
1900 mg, 2000 mg, 2100 mg, 2200 mg, 2300 mg, or 2400 mg), wherein said
composition is
formulated into a single fixed combination dosage form.
Combinations of Opioid/TLR4 Antagonists with Alpha-2-delta Ligands
[00162] An alpha-2-delta ligand including, but not limited to, Gabapentin
and
pregabalin or a pharmaceutically acceptable salt any thereof, enhances the
pain relief action
of the opioid/TLR4 antagonists including, but not limited to, naltrexone. A
specific
synergistic dose range of the combination is herein presented.
[00163] Voltage-dependent calcium channels alpha-2-delta -1 and alpha-2-
delta -2
subunits are the binding site of the two anticonvulsant drugs, gabapentin
(Neurontin) and
pregabalin (Lyrica), that also find use in treating chronic neuropathic pain.
Gab apentin
(Neurontin) is a pharmaceutical drug, specifically a GABA analog. It was
originally
developed for the treatment of epilepsy, and currently is also used to relieve
neuropathic pain.
Gabapentin provides significant pain relief in about a third of people who
take it for
fibromyalgia or chronic neuropathic pain. Pregabalin is an anticonvulsant drug
used for
neuropathic pain. Recent studies have shown that pregabalin is effective at
treating chronic
pain in disorders such as fibromyalgia. In a dose finding study the
combination of the
opioid/TLR4 antagonist, naltrexone and the calcium channel alpha-2-delta
ligands gabapentin
and pregabalin, acted synergistically, whether administered separately, one
after the other, or
administered in combination. Gabapentin and Pregabalin enhance the pain
treatment effect
of naltrexone by treating pain via another pathway, by binding Voltage-
dependent calcium
channels alpha-2-delta.
[00164] Based upon this, the disclosure first teaches the use of an
opioid/TLR4
antagonist in combination with an alpha-2-delta ligand for their action on
neuropathic pain.
The disclosure also teaches the use of naltrexone, in combination with an
alpha-2-delta ligand
such as Gabapentin or Pregabalin, for their action on neuropathic pain. The
disclosure teaches
that the combination is synergistic as far as the effect on pain.
[00165] Another embodiment is a combination, comprising an opioid/TLR4
antagonist
and an alpha-2-delta ligand. The alpha-2-delta ligand inhibitor may be any
alpha-2-delta
37
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
ligand inhibitor including, but not limited to, Gabapentin or Pregabalin or
pharmaceutically
acceptable salts or solvates of any thereof. Another embodiment is a
combination, comprising
an opioid antagonist and an alpha-2-delta ligand, the opioid /TLR4 antagonist
is naltrexone
as well as pro drugs and all enantiomeric and epimeric forms, specifically,
(+)-naltrexone
(dextro-naltrexone), as well as the appropriate mixtures thereof, or
pharmaceutically
acceptable salts or solvates of any thereof. Another embodiment is a
combination, comprising
an opioid antagonist and an alpha-2-delta ligand, the opioid /TLR4 antagonist
is naltrexone in
a sustained release formulation, as well as pro drugs thereof or any
enantiomeric and
epimeric forms thereof, as well as the appropriate mixtures thereof, or
pharmaceutically
acceptable salts or solvates of any thereof.
[00166] Another embodiment is a combination, comprising an opioid/TLR4
antagonist
and Gabapentin in a weight to weight combination range which corresponds to a
synergistic
combination range of the order of about 1:50 to about 1:125 parts by weight
(e.g. about 1:50,
1:60, 1:70, 1:80, 1:90, 1:100, 1:110, 1:120 or 1:125). Another embodiment is a
combination,
comprising an opioid/TLR4 antagonist and Pregabalin in a weight to weight
combination
range which corresponds to a synergistic combination range of the order of
order of about
1:10 to about 1:100 parts by weight, e.g. about 1:30- about 1:50 parts by
weight (e.g. about
1:30, 1:40, or 1:50). Another embodiment is a combination, comprising the dose
range of an
opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or solvate
thereof, is about
0.001 mg/kg to about 1.0 mg/kg per day, e.g. is about 0.004 mg/kg-0.71 mg/kg
per day, as
described above, with Gabapentin, or a pharmaceutically acceptable salt or
solvate thereof, at
a dosage range of about 0.1 mg/kg to about 100 mg/kg per day, e.g. about 1.3
mg/kg to about
26mg/kg per day (e.g. may range from about 1.3 to about 13.6 mg/kg, about 13.6
to about 26
mg/kg, about 1.3 to about 9.5 mg/kg, about 9.5 to about 17.8 mg/kg, about 17.8
to about 26
mg/kg, about 1.3 to about 7.5 mg/kg, about 7.5 to about 13.6 mg/kg, about 13.6
to about 19.8
mg/kg, about 19.8 to about 26 mg/kg, about 1.3 to about 19.8 mg/kg, 9.5 to
about 26 mg/kg,
about 7.5 to about 19.8 mg/kg about 13.6 to about 26 mg/kg, or about 7.5 to
about 26 mg/kg
per day; e.g. about 1.3 mg/kg, 1.4 mg/kg, 1.5 mg/kg, 1.6 mg/kg, 1.7 mg/kg, 1.8
mg/kg, 1.9
mg/kg, 2 mg/kg, 3 mg/kg, 4 mg/kg, 5 mg/kg, 6 mg/kg, 7 mg/kg, 8 mg/kg, 9 mg/kg,
10 mg/kg,
11 mg/kg, 12 mg/kg, 13 mg/kg, 14 mg/kg, 15 mg/kg, 16 mg/kg, 17 mg/kg, 18
mg/kg, 19
mg/kg, 20 mg/kg, 21 mg/kg, 22 mg/kg, 23 mg/kg, 24 mg/kg, 25 mg/kg or 26
mg/kg).
[00167] Another embodiment is a combination, comprising the dose range of
an
opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or solvate
thereof, is about
38
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
0.001 mg/kg to about 1.0 mg/kg per day, e.g. about 0.004 mg/kg- about 0.71
mg/kg per day,
as described above, with Pregabalin, or a pharmaceutically acceptable salt or
solvate thereof,
at a dosage range of about 0.1 mg/kg to about 10 mg/kg per day, e.g. about 2
mg/kg to about
4 mg/kg per day (e.g. may range from about 2 to about 3 mg/kg, about 3 to
about 4 mg/kg,
about 2 to about 2.6 mg/kg, about 2.6 to about 3.3 mg/kg, about 3.3 to about 4
mg/kg, about 2
to about 2.5 mg/kg, about 2.5 to about 3 mg/kg, about 3 to about 3.5 mg/kg,
about 3.5 to
about 4 mg/kg, about 2 to about 3.5 mg/kg, about 2.6 to about 4 mg/kg, about
2.5 to about 3.5
mg/kg about 3 to about 4 mg/kg, or about 2.5 to about 4 mg/kg per day; e.g.
about 2 mg/kg,
2.1 mg/kg, 2.2 mg/kg, 2.3 mg/kg, 2.4 mg/kg, 2.5 mg/kg, 2.6 mg/kg, 2.7 mg/kg,
2.8 mg/kg,
2.9 mg/kg, 3.0 mg/kg, 3.1 mg/kg, 3.2 mg/kg, 3.3 mg/kg, 3.4 mg/kg, 3.5 mg/kg,
3.6 mg/kg,
3.7 mg/kg, 3.8 mg/kg, 3.9 mg/kg, or 4.0 mg/kg).
[00168] Another embodiment is a combination, comprising an alpha-2-delta
ligand
with an opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or
solvate thereof,
wherein the opioid/TLR4 antagonist is at a dosage range of about 0.1 mg to
about 100 mg,
e.g. about 0.25 mg to about 50 mg , about 0.25 mg - about 25 mg, or a "low"
dose of about
0.25 mg - about 15 mg per day, as described above.
[00169] Another embodiment is a combination, comprising an alpha-2-delta
ligand
with an opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or
solvate thereof,
wherein the opioid/TLR4 antagonist is at a dosage range of about 0.1 mg to
about 100 mg,
e.g. about 0.25 mg - about 50 mg about 0.25 mg - about 25 mg, or a "low" dose
of about
0.25 mg - about 15 mg per day, as described above, and the alpha-2-delta
ligand is
Gabapentin, or a pharmaceutically acceptable salt or solvate thereof, at a
dosage range of
about 1 mg to about 5000 mg per day, e.g. about 100 mg to about 1800 mg per
day (e.g. may
range from about 100 to about 950 mg, about 950 to about 1800 mg, about 100 to
about 670
mg, about 670 to about 1230 mg, about 1230 to about 1800 mg, about 100 to
about 520 mg,
about 520 to about 950 mg, about 950 to about 1375 mg, about 1375 to about
1800 mg, about
100 to about 1375 mg, about 670 to about 1800 mg, about 525 to about 1375 mg
about 950 to
about 1800 mg, or about 525 to about 1800 mg per day; e.g. is about 100 mg,
200 mg, 300
mg, 400 mg, 500 mg, 600 mg, 700 mg, 800 mg, 900 mg, 1100 mg, 1200 mg, 1300 mg,
1400
mg, 1500 mg, 1600 mg, 1700 mg on 800 mg).
[00170] Another embodiment is a combination, comprising an alpha-2-delta
ligand
with an opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or
solvate thereof,
wherein the opioid/TLR4 antagonist is at a dosage range of about 0.1 mg to
about 100 mg,
39
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
e.g. about 0.25 mg ¨ about 50 mg, about 0.25 mg ¨ about 25 mg, or a "low" dose
of about
0.25 mg ¨ about 15 mg per day, as described above, and the alpha-2-delta
ligand is
Pregabalin, or a pharmaceutically acceptable salt or solvate thereof, at a
dosage range of
about 100 mg to about 500 mg per day, e.g. about 150 mg ¨ about 300 mg per day
(e.g. may
range from about 100 to about 220 mg, about 220 to about 300 mg, about 150 to
about 200
mg, about 200 to about 250 mg, about 250 to about 300 mg, about 150 to about
190 mg,
about 190 to about 225 mg, about 225 to about 262 mg, about 262 to about 300
mg, about
150 to about 262 mg, about 200 to about 300 mg, about 187 to about 262 mg
about 225 to
about 300 mg, or about 187 to about 300 mg per day; e.g. is about 150 mg, 160
mg, 170 mg,
180 mg, 190 mg, 200 mg, 210 mg, 220 mg, 230 mg, 240 mg, 250 mg, 260 mg, 270
mg, 280
mg, 290 mg or 300 mg).
[00171] Another embodiment is a combination, comprising the human dose
range of
an opioid/TLR4 antagonist, or a pharmaceutically acceptable salt or solvate
thereof, is about
0.1 mg to about 100 mg, e.g. is about 0.25 mg ¨ about 50 mg, as described
above, and the
human the dose range of Gab apentin, or a pharmaceutically acceptable salt or
solvate thereof,
is about 10 mg to about 5000 mg per day, e.g. is about 100 mg to about 1800 mg
per day (e.g.
may range from about 100 to about 950 mg, about 950 to about 1800 mg, about
100 to about
670 mg, about 670 to about 1230 mg, about 1230 to about 1800 mg, about 100 to
about 520
mg, about 520 to about 950 mg, about 950 to about 1375 mg, about 1375 to about
1800 mg,
about 100 to about 1375 mg, about 670 to about 1800 mg, about 525 to about
1375 mg about
950 to about 1800 mg, or about 525 to about 1800 mg per day; e.g. is about 100
mg, 200 mg,
300 mg, 400 mg, 500 mg, 600 mg, 700 mg, 800 mg, 900 mg, 1100 mg, 1200 mg, 1300
mg,
1400 mg, 1500 mg, 1600 mg, 1700 mg on 800 mg), wherein said composition is
formulated
into a single fixed combination dosage form. Another embodiment is a
combination,
comprising the human dose range of an opioid/TLR4 antagonist, or a
pharmaceutically
acceptable salt or solvate thereof, is about 0.1 mg to about 100 mg, e.g. is
about 0.25 mg ¨
about 50 mg per day, as described above, and the human dose range of
pregabalin, or a
pharmaceutically acceptable salt or solvate thereof, is about 10 to about 1000
mg per day; e.g.
is about 50 mg to about 300 mg per day (e.g. may range from about 50 to about
175 mg,
about 175 to about 300 mg, about 50 to about 133 mg, about 217 to about 300
mg, about 217
to about 300 mg, about 50 to about 112 mg, about 112 to about 175 mg, about
175 to about
237 mg, about 237 to about 300 mg, about 50 to about 237 mg, about 133 to
about 300 mg,
about 112 to about 237 mg about 175 to about 300 mg, or about 112 to about 300
mg per day;
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
e.g. is about 50 mg, 60 mg, 70 mg, 80 mg, 90 mg, 100 mg, 110 mg, 120 mg, 130
mg, 140 mg,
150 mg, 160 mg, 170 mg, 180 mg, 190 mg, 200 mg, 210 mg, 220 mg, 230 mg, 240
mg, 250
mg, 260 mg, 270 mg, 280 mg, 290 mg or 300 mg), wherein said composition is
formulated
into a single fixed combination dosage form.
[00172] Another embodiment is a combination, comprising naltrexone and
Gabapentin in a weight to weight combination range which corresponds to a
synergistic
combination range of the order of about 1:10 to about 1:500 parts by weight,
e.g. about 1:50-
about 1:125 parts by weight (e.g. about 1:50, 1:60, 1:70, 1:80, 1:90, 1:100,
1:110, 1:120 or
1:125). Another embodiment is a combination, comprising naltrexone and
Pregabalin in a
weight to weight combination range which corresponds to a synergistic
combination range of
the order of about 1:1 to about 1:100 parts by weight, e.g. about 1:30- about
1:50 parts by
weight (e.g. about 1:30, 1:40, or 1:50).
[00173] Another embodiment is a combination, comprising the dose range of
naltrexone, or a pharmaceutically acceptable salt or solvate thereof, at a
dosage range of
about 0.001 mg/kg to about 1.0 mg/kg per day, e.g. about 0.004 mg/kg- about
0.71 mg/kg per
day, about 0.25 mg - about 50 mg, about 0.25 mg - about 25 mg, or a "low" dose
of about
0.25 mg - about 15 mg, as described above, per day with Gab apentin, or a
pharmaceutically
acceptable salt or solvate thereof, at a dosage range of about 0.1 mg/kg to
about 100 mg/kg
per day, e.g. about 1.3 mg/kg - about 26mg/kg per day (e.g. may range from
about 1.3 to
about 13.6 mg/kg, about 13.6 to about 26 mg/kg, about 1.3 to about 9.5 mg/kg,
about 9.5 to
about 17.8 mg/kg, about 17.8 to about 26 mg/kg, about 1.3 to about 7.4 mg/kg,
about 7.4 to
about 13.6 mg/kg, about 13.6 to about 19.8 mg/kg, about 19.8 to about 26
mg/kg, about 1.3 to
about 19.8 mg/kg, about 9.5 to about 26 mg/kg, about 7.4 to about 19.8 mg/kg
about 13.6 to
about 26 mg/kg, or about 7.4 to about 26 mg/kg per day; e.g. about 1.3 mg/kg,
1.4 mg/kg, 1.5
mg/kg, 1.6 mg/kg, 1.7 mg/kg, 1.8 mg/kg, 1.9 mg/kg, 2 mg/kg, 3 mg/kg, 4 mg/kg,
5 mg/kg, 6
mg/kg, 7 mg/kg, 8 mg/kg, 9 mg/kg, 10 mg/kg, 11 mg/kg, 12 mg/kg, 13 mg/kg, 14
mg/kg, 15
mg/kg, 16 mg/kg, 17 mg/kg, 18 mg/kg, 19 mg/kg, 20 mg/kg, 21 mg/kg, 22 mg/kg,
23 mg/kg,
24 mg/kg, 25 mg/kg or 26 mg/kg).
[00174] Another embodiment is a combination, comprising the dose range of
naltrexone, or a pharmaceutically acceptable salt or solvate thereof, at a
dosage range of
about 0.001mg/kg to about 1.0 mg/kg per day, e.g. about 0.004 mg/kg- about
0.71 mg/kg per
day, about 0.25 mg - about 50 mg, about 0.25 mg - about 25 mg, or a "low" dose
of about
0.25 mg - about 15 mg, as described above, per day with Pregabalin, or a
pharmaceutically
41
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
acceptable salt or solvate thereof, at a dosage range of about 0.2 mg/kg to
about 10 mg/kg per
day, e.g. about 2 mg/kg - about 4 mg/kg per day (e.g. may range from about 2
to about 3
mg/kg, about 3 to about 4 mg/kg, about 2 to about 2.7 mg/kg, about 2.7 to
about 3 mg/kg,
about 3 to about 4 mg/kg, about 2 to about 2.5 mg/kg, about 2.5 to about 3
mg/kg, about 3 to
about 3.5 mg/kg, about 3.5 to about 4 mg/kg, about 2 to about 3.5 mg/kg, about
2.7 to about 4
mg/kg, about 2.5 to about 3.5 mg/kg about 3 to about 4 mg/kg, or about 2.5 to
about 4 mg/kg
per day; e.g. about 2 mg/kg, 2.1 mg/kg, 2.2 mg/kg, 2.3 mg/kg, 2.4 mg/kg, 2.5
mg/kg, 2.6
mg/kg, 2.7 mg/kg, 2.8 mg/kg, 2.9 mg/kg, 3.0 mg/kg, 3.1 mg/kg, 3.2 mg/kg, 3.3
mg/kg, 3.4
mg/kg, 3.5 mg/kg, 3.6 mg/kg, 3.7 mg/kg, 3.8 mg/kg, 3.9 mg/kg, or 4.0 mg/kg).
[00175] The combination with the opioid/TLR4 antagonist may also include
Gabapentin, or a pharmaceutically acceptable salt or solvate thereof, at a
dosage range of
about 1 mg to about 5000 mg per day, e.g. about 100 mg to about 1800 mg per
day (e.g. may
range from about 100 to about 950 mg, about 950 to about 1800 mg, about 100 to
about 670
mg, about 670 to about 1230 mg, about 1230 to about 1800 mg, about 100 to
about 525 mg,
about 525 to about 950 mg, about 950 to about 1375 mg, about 1375 to about
1800 mg, about
100 to about 1375 mg, about 670 to about 1800 mg, about 525 to about 1375 mg
about 950 to
about 1800 mg, or about 525 to about 1800 mg per day; e.g. is about 100 mg,
200 mg, 300
mg, 400 mg, 500 mg, 600 mg, 700 mg, 800 mg, 900 mg, 1100 mg, 1200 mg, 1300 mg,
1400
mg, 1500 mg, 1600 mg, 1700 mg on 800 mg). The combination with the opioid/TLR4
antagonist may also include Pregabalin, or a pharmaceutically acceptable salt
or solvate
thereof, at a dosage range of about 10 mg to about 1000 mg per day, e.g. about
150 mg to
about 300 mg per day (e.g., is in a range from about e.g. may range from about
100 to about
225 mg, about 225 to about 300 mg, about 150 to about 200 mg, about 200 to
about 250 mg,
about 250 to about 300 mg, about 150 to about 187 mg, about 187 to about 225
mg, about
225 to about 262 mg, about 262 to about 300 mg, about 150 to about 262 mg,
about 200 to
about 300 mg, about 187 to about 262 mg about 225 to about 300 mg, or about
187 to about
300 mg per day; e.g. is about 150 mg, 160 mg, 170 mg, 180 mg, 190 mg, 200 mg,
210 mg,
220 mg, 230 mg, 240 mg, 250 mg, 260 mg, 270 mg, 280 mg, 290 mg or 300 mg). One
embodiment is a combination, comprising the human dose range of naltrexone, or
a
pharmaceutically acceptable salt or solvate thereof, of about 0.1 mg to about
100 mg per day,
e.g. about 0.25 mg - about 50 mg per day, as described above, and the human
the dose range
of Gab apentin, or a pharmaceutically acceptable salt or solvate thereof, of
about 10 mg to
about 5000 mg, e.g. about 100 mg to about 1800 mg (e.g. may range from about
100 to about
42
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
950 mg, about 950 to about 1800 mg, about 100 to about 670 mg, about 670 to
about 1230
mg, about 1230 to about 1800 mg, about 100 to about 525 mg, about 525 to about
950 mg,
about 950 to about 1375 mg, about 1375 to about 1800 mg, about 100 to about
1375 mg,
about 670 to about 1800 mg, about 525 to about 1375 mg about 950 to about 1800
mg, or
about 525 to about 1800 mg per day; e.g. is about 100 mg, 200 mg, 300 mg, 400
mg, 500 mg,
600 mg, 700 mg, 800 mg, 900 mg, 1100 mg, 1200 mg, 1300 mg, 1400 mg, 1500 mg,
1600
mg, 1700 mg or1800 mg), wherein said composition is formulated into a single
fixed
combination dosage form.
[00176] Another embodiment is a combination, comprising the human dose
range of
naltrexone, or a pharmaceutically acceptable salt or solvate thereof, of about
0.1 mg to about
100 mg, e.g. about 0.25 mg ¨ about 50 mg per day, as described above, and the
human the
dose range of pregabalin, or a pharmaceutically acceptable salt or solvate
thereof at about 1
mg to about 500 mg per day, e.g. about 50 mg to about 300 mg per day (e.g. may
range from
about 50 to about 175 mg, about 175 to about 300 mg, about 50 to about 133 mg,
about 216
to about 300 mg, about 216 to about 300 mg, about 50 to about 112 mg, about
112 to about
175 mg, about 175 to about 237 mg, about 237 to about 300 mg, about 50 to
about 237 mg,
about 133to about 300 mg, about 112 to about 237 mg about 175 to about 300 mg,
or about
112 to about 300 mg per day; e.g. is about 50 mg, 60 mg, 70 mg, 80 mg, 90 mg,
100 mg, 110
mg, 120 mg, 130 mg, 140 mg, 150 mg, 160 mg, 170 mg, 180 mg, 190 mg, 200 mg,
210 mg,
220 mg, 230 mg, 240 mg, 250 mg, 260 mg, 270 mg, 280 mg, 290 mg or 300 mg),
wherein
said composition is formulated into a single fixed combination dosage form.
[00177] Another embodiment comprises administering the combination of an
opioid/TLR4 antagonist and an alpha-2-delta ligand once, twice, three or four
times through
the day. Another embodiment comprising the therapeutically effective dose of
the
pharmaceutical composition of an opioid/TLR4 antagonist and an alpha-2-delta
ligand is
administered systemically by such routes including but are not limited to
mucosal, nasal, oral,
parenteral, gastrointestinal, topical or sublingual routes. Another embodiment
comprising,
said combination of an opioid/TLR4 antagonist and an alpha-2-delta ligand is
in a single
dosage form, and said single dosage form is in the form of tablets, lozenges,
troches, hard
candies, liquid, powders, sprays, creams, salves and suppositories.
[00178] The pharmaceutical composition comprising the combination of an
opioid/TLR4 antagonist and an alpha-2-delta ligand may be used for the
treatment,
prevention and reversal of neuropathic pain, back pain, chronic pain, diabetic
neuropathic
43
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
pain, trigeminal neuralgia pain, phantom limb pain, complex regional pain
syndrome pain,
acute herpetic pain, post herpetic pain, causalgia pain, idiopathic pain,
inflammatory pain,
cancer pain, postoperative pain, fibromyalgia pain, headache pain, migraine
pain, allodynia
pain, vulvodynia pain, interstitial cystitis pain, irritable bowel syndrome
(IBS), arthritic joint
pain and tendinitis.
[00179] Another embodiment is a method of treating neuropathic and
nociceptive pain
with an allodynic component and migraine in a mammal in need thereof,
comprising
administering to the mammal a therapeutically effective amount of a
combination comprising
an opioid/TLR4 antagonist and an alpha-2-delta ligand, or pharmaceutically
acceptable salts
or solvates of any thereof. Another embodiment is a method of treating
neuropathic and
nociceptive pain with an allodynic component and migraine in a mammal in need
thereof,
comprising administering to the mammal a therapeutically effective amount of a
combination
comprising naltrexone and Gabapentin or Pregabalin, or pharmaceutically
acceptable salts or
solvates of any thereof.
Disease states
[00180] Pain can be classified as either acute or chronic. Acute pain can
be caused by
damage to tissue and generally has a sudden onset and a limited duration.
Chronic pain tends
to last longer than acute pain and is usually associated with a long-term
illness. It is usually
more resistant to treatment, and can be the defining characteristic of a
disease (such as
fibromyalgia). It can be the result of damaged tissue, but more often is
attributed to nerve
damage. Pain can also be classified by the kind of damage that causes it.
Nociceptive pain is
pain caused by tissue damage, while neuropathic pain is pain caused by nerve
damage.
Nociceptive pain may be further divided into three different sub-categories:
visceral, deep
somatic, and superficial somatic pain.
[00181] Examples of pain include but are not limited to: acute pain,
chronic pain,
muscle pain, joint pain, chest pain, neck pain, shoulder pain, hip pain,
abdominal pain, carpal
tunnel syndrome, knee pain, back pain, myofascial pain syndrome, fibromyalgia,
arthritic
pain, headache, migraine headache, Piriformis syndrome, whiplash, chronic
muscle pain,
nociceptive pain, visceral pain, deep somatic pain, superficial somatic pain,
neuropathic pain,
central pain syndrome, complex regional pain syndrome, diabetic peripheral
neuropathy, pain
associated with shingles, postherpetic neuralgia, neuralgia, trigeminal
neuralgia, sciatica pain,
arachnoiditis (spinal pain), central pain syndrome, phantom limb pain, phantom
body pain,
44
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
neuropathy, compartment syndrome, acute herpetic pain, post herpetic pain,
causalgia pain,
idiopathic pain, inflammatory pain, cancer pain, postoperative pain,
vulvodynia pain,
interstitial cystitis pain, irritable bowel syndrome (IBS), tendinitis,
breakthrough pain, and
incident pain.
[00182] Allodynia is a clinical feature of many painful conditions,
including but not
limited to: back pain, chronic pain, neuropathic pain, diabetic neuropathic
pain, trigeminal
neuralgia pain, phantom limb pain, complex regional pain syndrome pain, acute
herpetic
pain, post herpetic pain, causalgia pain, idiopathic pain, inflammatory pain,
cancer pain,
postoperative pain, fibromyalgia pain, headache pain, migraine pain,
vulvodynia pain,
interstitial cystitis pain, irritable bowel syndrome (IBS), arthritic joint
pain and tendinitis. It
becomes apparent that allodynia plays a role in every kind of pain.
[00183] The disclosure offers a new explanation for the occurrence of
allodynia, or
"memory pain", connecting the dots of existing knowledge from animal model
studies along
with the vast information gleaned from the clinical trials disclosed herein,
it is now evident
that allodynia is caused by abnormal endogenous activation of TLR4 that in
turn trigger a
pro-inflammatory cascade. The clinical trial for back pain disclosed herein
verified that the
pain is interrupted by the opioid/TLR4 antagonist naltrexone. Additionally,
TLR4 antagonism
can play a role in improving nociceptive pain by affecting the allodynic
component of
nociceptive pain.
[00184] The compositions and methods of use therefore described in this
application
may be used to prevent, reduce, or eliminate any type of pain, including those
types of pain
that have an allodynic component. The prevention, reduction, or elimination of
pain may be
measured in many ways, including (but not limited to): percent relief of pain,
decrease in
relative severity of the pain, decrease in frequency of breakout pain,
reduction in the duration
of pain, reduction in the level of pain, reduction in the frequency of night
pain, reduction in
the patient's Oswestry disability index level, reduction in the patient's
Pittsburgh Insomnia
scale rating, a decrease in insomnia, a decrease in disability, a decrease in
the patient's score
on the Roland-Morris low back pain and disability questionnaire, an increase
in amount of
time patient can stand, an increase in amount of time patient can stand on one
leg, an
increase in the patient's quality of life, an increase in the patient's energy
levels, or an
increase in the patient's activity levels.
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Pharmaceutical Compositions
[00185] As used herein the term "pharmaceutical composition" refers to a
preparation
of one or more of the components described herein, or physiologically
acceptable salts or
prodrugs thereof, with other chemical components such as physiologically
suitable carriers
and excipients. The purpose of a pharmaceutical composition is to facilitate
administration of
a compound to an organism. The term "prodrug" refers a precursor compound that
can
hydrolyze, oxidize, or otherwise react under biological conditions (in vitro
or in vivo) to
provide the active compound.
[00186] The term "excipient" refers to an inert or inactive substance added
to a
pharmaceutical composition to further facilitate administration of a compound.
Non-limiting
examples of excipients include calcium carbonate, calcium phosphate, various
sugars and
types of starch, cellulose derivatives, gelatin, vegetable oils and
polyethylene glycols.
[00187] The pharmaceutical compositions of the present disclosure comprise
an
opioid/TLR4 antagonist, dextro enantiomer thereof, pro-drugs, salts, or
solvates thereof and
may also include one or more additive drugs (e.g., additional active
ingredients), such as, but
not limited to those listed above that may be suitable for combination
therapy.
[00188] One pharmaceutical compositions of the present disclosure comprises
an
opioid/TLR4 antagonist or pharmaceutically acceptable salts thereof. Another
pharmaceutical
composition of the present disclosure comprises a dextro-enantiomer of an
opioid/TLR4
antagonist or the pharmaceutically acceptable salt thereof.
[00189] The disclosure also provides synergistic compositions comprising an
opioid/TLR4 antagonist, dextro enantiomeric mixtures thereof, or
pharmaceutically
acceptable salts thereof and an alpha-2 adrenergic receptor agonist, acetyl-
para-aminophenol
(APAP), a cyclooxygenase (COX) inhibitor, and/or an alpha-2-delta ligand.
Therefore, the
pharmaceutical compositions of the present disclosure also comprise an
opioid/TLR4
antagonist, dextro enantiomeric mixture thereof, or pharmaceutically
acceptable salts thereof
in combination with an alpha-2 adrenergic receptor agonist. The pharmaceutical
compositions of the present disclosure may also comprise an opioid/TLR4
antagonist, dextro
enantiomeric mixture thereof, or pharmaceutically acceptable salts thereof in
combination
with an acetyl-para-aminophenol (APAP). The pharmaceutical compositions of the
present
disclosure also comprise an opioid/TLR4 antagonist, dextro enantiomeric
mixture thereof, or
pharmaceutically acceptable salts thereof in combination with a cyclooxygenase
(COX)
inhibitor. The pharmaceutical compositions of the present disclosure also
comprise an
46
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
opioid/TLR4 antagonist, dextro enantiomeric mixture thereof, or
pharmaceutically acceptable
salts thereof in combination with an alpha-2-delta ligand.
[00190] Synergy, as described, for example, by Chou and Talalay, Adv.
Enzyme Regul.
vol. 22, pp. 27-55 (1984), occurs when the effect of the compounds when
administered in
combination is greater than the additive effect of the compounds when
administered alone as
a single agent. In general, a synergistic effect is most clearly demonstrated
at sub-optimal
concentrations of the compounds when administered alone. Synergy can be in
terms of lower
cytotoxicity, increased decrease in pain, or some other beneficial effect of
the combination
compared with the individual components. For example, synergy can encompass
the
reduction of side effects associated with one or more of the substances in the
combination
because one or more of the substances can be used at a lower dose while still
maintaining or
enhancing pharmaceutical effectiveness.
[00191] Furthermore, the pharmaceutical compositions of the present
disclosure may
comprise an opioid/TLR4 antagonist, dextro enantiomeric mixture thereof, or
pharmaceutically acceptable salts thereof in combination with an alpha-2
adrenergic receptor
agonist and acetyl-para-aminophenol (APAP); an opioid/TLR4 antagonist, dextro
enantiomeric mixture thereof, or pharmaceutically acceptable salts thereof in
combination
with an alpha-2 adrenergic receptor agonist and a cyclooxygenase (COX)
inhibitor; or an
opioid/TLR4 antagonist, dextro enantiomeric mixture thereof, or
pharmaceutically acceptable
salts thereof in combination with an alpha-2 adrenergic receptor agonist and
an alpha-2-delta
ligand.
[00192] The pharmaceutical compositions of the present disclosure may also
comprise
an opioid/TLR4 antagonist, dextro enantiomeric mixture thereof, or
pharmaceutically
acceptable salts thereof in combination with acetyl-para-aminophenol (APAP)
and a
cyclooxygenase (COX) inhibitor, or it may comprise an opioid/TLR4 antagonist,
dextro
enantiomeric mixture thereof, or pharmaceutically acceptable salts thereof in
combination
with acetyl-para-aminophenol (APAP) and an alpha-2-delta ligand. The
pharmaceutical
compositions of the present disclosure may also comprise an opioid/TLR4
antagonist, dextro
enantiomeric mixture thereof, or pharmaceutically acceptable salts thereof in
combination
with a cyclooxygenase (COX) inhibitor and an alpha-2-delta ligand.
[00193] The pharmaceutical compositions of the present disclosure may also
comprise
an opioid/TLR4 antagonist, dextro enantiomeric mixture thereof, or
pharmaceutically
47
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
acceptable salts thereof in combination with a cyclooxygenase (COX) inhibitor
in
combination with acetyl-para-aminophenol (APAP) and an alpha-2-delta ligand.
[00194] The
pharmaceutical compositions of the present disclosure may comprise an
opioid/TLR4 antagonist, dextro enantiomeric mixture thereof, or
pharmaceutically
acceptable salts thereof in combination with an alpha-2 adrenergic receptor
agonist, acetyl-
para-aminophenol (APAP), and a cyclooxygenase (COX) inhibitor. It may also
comprise an
opioid/TLR4 antagonist, dextro enantiomeric mixture thereof, or
pharmaceutically acceptable
salts thereof in combination with an alpha-2 adrenergic receptor agonist, a
cyclooxygenase
(COX) inhibitor, and an alpha-2-delta ligand. The pharmaceutical composition
may comprise
an opioid/TLR4 antagonist, dextro enantiomeric mixture thereof, or
pharmaceutically
acceptable salts thereof in combination with an alpha-2 adrenergic receptor
agonist, acetyl-
para-aminophenol (APAP), and an alpha-2-delta ligand.
[00195] The
pharmaceutical composition may also comprise an opioid/TLR4
antagonist, dextro enantiomeric mixture thereof, or pharmaceutically
acceptable salts thereof
in combination with an alpha-2 adrenergic receptor agonist, acetyl-para-
aminophenol
(APAP), an alpha-2 adrenergic receptor agonist, and a cyclooxygenase (COX)
inhibitor.
[00196] The term
"combination" refers to two or more therapeutic agents to treat a
therapeutic condition or disorder described in the present disclosure. Such
combination of
therapeutic agents may be in the form of a single pill, capsule, or
intravenous solution.
However, the term "combination" also encompasses the situation when the two or
more
therapeutic agents are in separate pills, capsules, or intravenous solutions.
Likewise, the term
"combination therapy" refers to the administration of two or more therapeutic
agents to treat
a therapeutic condition or disorder described in the present disclosure. Such
administration
encompasses co-administration of these therapeutic agents in a substantially
simultaneous
manner, such as in a single capsule having a fixed ratio of active ingredients
or in multiple, or
in separate containers (e.g., capsules) for each active ingredient. In
addition, such
administration also encompasses use of each type of therapeutic agent in a
sequential manner,
either at approximately the same time or at different times. In either case,
the treatment
regimen will provide beneficial effects of the drug combination in treating
the conditions or
disorders described herein. The dosage form can be prepared by various
conventional mixing,
comminution and fabrication techniques readily apparent to those skilled in
the chemistry of
drug formulations.
48
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[00197] For example, the method of treating a disease according to the
invention can
comprise (i) administration of the opioid/TLR4 antagonist, dextro enantiomeric
mixture
thereof, or pharmaceutically acceptable salts thereof and (ii) administration
of one or more
of: an alpha-2 adrenergic receptor agonist, acetyl-para-aminophenol (APAP),
and an alpha-2-
delta ligand, or any pharmaceutically acceptable salts thereof simultaneously
or sequentially
in any order, in jointly therapeutically effective amounts, preferably in
synergistically
effective amounts, e.g. in daily or intermittently dosages corresponding to
the amounts
described herein. The individual combination partners of the combination of
the invention
can be administered separately at different times during the course of therapy
or concurrently
in divided or single combination forms. Furthermore, the term administering
also
encompasses the use of a pro-drug of a combination partner that convert in
vivo to the
combination partner as such. The instant invention is therefore to be
understood as
embracing all such regimens of simultaneous or alternating treatment and the
term
"administering" is to be interpreted accordingly.
[00198] Frequency of dosage can vary depending on the compound used and the
particular condition to be treated or prevented. In general, the use of the
minimum dosage
that is sufficient to provide effective therapy is preferred. Patients can
generally be
monitored for therapeutic effectiveness using assays suitable for the
condition being treated
or prevented, which will be familiar to those of ordinary skill in the art.
Different dosage
regimens may be used to treat any of the disease states referenced herein. In
some
embodiments, a daily dosage, such as any of the exemplary dosages described
herein, is
administered once, twice, three times, or four times a day for three, four,
five, six, seven,
eight, nine, or ten days. In some embodiments, the compounds described herein
may be used
on the order of about 10 times per day to about once per six months (e.g.,
about 10, 9, 8, 7, 6,
5, 4, 3, 2, 1 times per day to about 31, 30, 29, 28, 27, 26, 25, 24, 23, 22,
21, 20, 19, 18, 17,
16, 15, 14, 13, 12, 11, 10, 9, 8, 7, 6, 5, 4, 3, 2, or 1 times per month). In
some embodiments,
dosing frequencies are once per day, once per week, once per two weeks, once
per three
weeks, and once per month. Depending on the type of disorder treated, a
shorter treatment
time (e.g., up to five days) may be employed along with a high dosage, or a
longer treatment
time (e.g., ten or more days, or weeks, or a month, or longer) may be employed
along with a
low dosage. In some embodiments, the dosing regimen may change over time
depending on
the condition being treated and the patient's response to the treatment.
49
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[00199] The disclosure provides a composition comprising a compound that is
an
opioid/TLR4 antagonist, dextro enantiomeric mixture thereof, or a
pharmaceutically
acceptable salt thereof and its use for the treatment, prevention, and
reversal of pain. The
disclosure also provides synergistic compositions comprising an opioid/TLR4
antagonist,
dextro enantiomeric mixtures thereof, or pharmaceutically acceptable salts
thereof and an
alpha-2 adrenergic receptor agonist, acetyl-para-aminophenol (APAP), a
cyclooxygenase
(COX) inhibitor, and/or an alpha-2-delta ligand. The disclosure further
provides a method of
use of these synergistic compositions for the treatment, prevention, and
reversal of pain,
particularly neuropathic pain.
[00200] The pharmaceutical compositions of the present disclosure may be
manufactured by processes well known in the art, e.g., by means of
conventional mixing,
dissolving, granulating, grinding, pulverizing, dragee-making, levigating,
emulsifying,
encapsulating, entrapping or by lyophilizing processes.
[00201] The compositions for use in accordance with the present disclosure
thus may
be formulated in conventional manner using one or more pharmaceutically
acceptable
carriers comprising excipients and auxiliaries, which facilitate processing of
the active
compounds into preparations which can be used pharmaceutically. Proper
formulation is
dependent upon the route of administration chosen.
[00202] The term "administration" or any variation thereof as used herein
is meant any
way of administration. The one or more opioid/TLR4 antagonists, dextro
enantiomers
thereof, pro-drugs, salts, or solvates thereof may be administered alone. The
one or more
opioid/TLR4 antagonists, dextro enantiomers thereof, pro-drugs, salts, or
solvates thereof and
at least one additional drug may be administered in one therapeutic dosage
form or in two or
more separate therapeutic dosages such as in separate capsules, tablets or
injections. In the
case of the two or more separate therapeutic dosages, the administration may
be such that the
periods between the administrations vary or are determined by the
practitioner. The second
drug and any other additional drugs may be administered within the therapeutic
response time
of the first drug. The second drug and any other additional drugs may be also
administered
after the therapeutic response time of the first drug. The one or more of
opioid/TLR4
antagonists, dextro enantiomers thereof, pro-drugs, salts, or solvates thereof
and at least one
additional drug which may be administered either at the same time, or
separately, or
sequentially, according to the disclosure, do not represent a mere aggregate
of known agents,
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
but a new combination with the valuable property that the effectiveness of the
treatment is
achieved at a much lower dosage of said at least one additional drug.
[00203] The pharmaceutical compositions of the present disclosure may be
administered by any convenient route, for example, by infusion or bolus
injection, by
absorption through epithelial or mucocutaneous linings (e.g., oral mucosa,
rectal and
intestinal mucosa, etc.) and may be administered together with any other
therapeutic agent.
Administration can be systemic or local.
[00204] Various delivery systems are known, e.g., encapsulation in
liposomes,
microparticles, microcapsules, or capsules that may be used to administer the
compositions of
the disclosure. Methods of administration include but are not limited to
intradermal,
intramuscular, intraperitoneal, intravenous, subcutaneous, epidural, oral,
sublingual,
intranasal, intracerebral, intravaginal, transdermal, rectally (including by
suppository or
enema), by inhalation, or topically to the cars, nose, eyes, or skin. The mode
of
administration is left to the discretion of the practitioner, and will depend
in part upon the site
of the medical condition and the severity of thereof.
[00205] For example, for injection the composition of the disclosure may be
formulated in aqueous solutions, preferably in physiologically compatible
buffers such as
Hank's solution, Ringer's solution, or physiological saline buffer. For
transmucosal
administration, penetrants appropriate to the barrier to be permeated are used
in the
formulation. Such penetrants for example DMSO, or polyethylene glycol are
generally
known in the art.
[00206] For oral administration, the composition can be formulated readily
by
combining the active components with any pharmaceutically acceptable carriers
known in the
art. Such "carriers" may facilitate the manufacture of such as tablets, pills,
dragees, capsules,
liquids, gels, syrups, slurries, suspensions, and the like, for oral ingestion
by a patient.
Pharmacological preparations for oral use can be made using a solid excipient,
optionally
grinding the resulting mixture, and processing the mixture of granules, after
adding suitable
auxiliaries if desired, to obtain tablets or dragee cores. Suitable excipients
are, in particular,
fillers such as sugars, including lactose, sucrose, mannitol, or sorbitol;
cellulose preparations
such as, for example, maize starch, wheat starch, rice starch, potato starch,
gelatin, gum,
methyl cellulose, hydroxypropylmethyl-cellulose, sodium carbomethylcellulose,
and/or
physiologically acceptable polymers such as polyvinylpyrrolidone (PVP). If
desired,
51
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
disintegrating agents may be added, such as cross-linked polyvinyl
pyrrolidone, agar, or
alginic acid or a salt thereof such as sodium alginate.
[00207] Dragee cores are provided with suitable coatings. For this purpose,
concentrated sugar solutions may be used which may optionally contain gum
arabic, talc,
polyvinyl pyrrolidone, carbopol gel, polyethylene glycol, titanium dioxide,
lacquer solutions
and suitable organic solvents or solvent mixtures.
[00208] Pharmaceutical compositions, which can be used orally, include push-
fit
capsules made of gelatin as well as soft, sealed capsules made of gelatin and
a plasticizer,
such as glycerol or sorbitol. The push-fit capsules may contain the active
ingredients in
admixture with filler such as lactose, binders such as starches, lubricants
such as talc or
magnesium stearate and, optionally, stabilizers. In soft capsules, the active
components may
be dissolved or suspended in suitable liquids, such as fatty oils, liquid
paraffin, or liquid
polyethylene glycols.
[00209] Dyestuffs or pigments may be added to the tablets or dragee
coatings for
identification or to characterize different combinations of active NSAID
doses. In addition,
stabilizers may be added.
[00210] Pharmaceutical compositions for parenteral administration include
aqueous
solutions of the active preparation in a water-soluble form. Additionally,
suspensions of the
active preparation may be prepared as oily injection suspensions. Suitable
lipophilic solvents
or vehicles include fatty oils such as sesame oil, or synthetic fatty acids
esters such as ethyl
oleate, triglycerides or liposomes. Aqueous injection suspensions may contain
substances,
which increase the viscosity of the suspension, such as sodium carboxymethyl,
cellulose,
sorbitol or dextran. Optionally, the suspension may also contain suitable
stabilizers or agents,
which increase the solubility of the compounds, to allow for the preparation
of highly
concentrated solutions.
[00211] Alternatively, the composition may be in a powder form for
constitution
before use with a suitable vehicle, e.g., sterile, pyrogen-free water. The
exact formulation,
route of administration and dosage may be chosen by the physician familiar
with the patient's
condition. (See for example Fingl, et al., 1975, in "The Pharmacological Basis
of
Therapeutics", Chapter I, p. 1). Depending on the severity and responsiveness
of the
condition treated, dosing can also be a single administration of a slow
release composition,
with course of treatment lasting from several days to several weeks or until
cure is effected or
diminution of the disease state is achieved.
52
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[00212] In an embodiment for non-human animal administration the term
"pharmaceutical" as used herein may be replaced by "veterinary".
EXAMPLES
[00213] The examples disclosed in the instant application are case studies
from clinical
trials. These examples are non-limiting and are merely representative.
Example 1: A Pharmaceutical Composition Comprising Naltrexone and Clonidine
and
Clinical Trials Thereof
[00214] Naltrexone and clonidine were evaluated alone and in combination on
a
human subject with the purpose of finding whether or not a combination of the
two
compounds offers synergistic advantage. Two aspects were evaluated for
synergy: one aspect
was pain treatment effect comparing the amounts used weight to weight, and the
other aspect
was an assessment of synergy of side effects.
[00215] The components of the combination were administered to a subject in
the
following manner: naltrexone 4.5mg was given in the morning. The clonidine
dose was
divided into two doses, a dose of 0.1mg in the morning and a dose of 0.2mg at
bedtime. The
morning/daytime combination of naltrexone/clonidine 2.25mg/ 0.025mg
respectively was
given in the morning, and the night/bedtime combination of
naltrexone/clonidine of 2.25mg/
0.05mg and 2.25mg/0.1mg respectively was given at night. The pain treatment
effect of
naltrexone and clonidine was evaluated after one hour post-dose. Side effects
were assessed
over the next 24 hours.
[00216] To determine synergy, the amounts of naltrexone and clonidine
administered
alone were compared to the combination combined amounts. For proper weight to
weight
(W/W) comparison between naltrexone and clonidine an adjustment for the higher
potency of
clonidine needed to be made based on the dose of each compound given by
itself, where the
naltrexone dose was 4.5 mg and the clonidine dose was 0.3 mg. Clonidine is 15
times more
potent than naltrexone (4.5/0.3=15). Naltrexone and clonidine were
administered at fixed
dose ratios of 90:1, 45:1 and 22.5:1 to a human subject afflicted with
neuropathic back pain.
[00217] Table 1 illustrates the naltrexone/clonidine ratios that exhibit
weight to weight
(W/W) synergy in a human subject. The 90:1 combination represents a 2-fold
lower dose of
naltrexone and 4-fold lower dose of clonidine when administered alone. The
45:1
combination represents a 2-fold lower dose of naltrexone and 2-fold lower dose
of clonidine
when administered alone.
53
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Table 1: Naltrexone/Clonidine Ratios And Weight to Weight (W/VV) Synergy
Ratio Naltrexone Clonidine Clonidine Total dose
Interaction
mg mg Potency reversal Naltrexone +
Adjustment of pain Adjusted
(x15) clonidine mg
4.5:0 4.50 100 4.5
0:0.3 0.300 4.500 50 4.5
90:1 2.25 0.025 0.375 100 2.625 synergy
45:1 2.25 0.050 0.750 100 2.25+0.75=3.0 synergy
22.5:1 2.25 0.1 1.500 100 2.25+1.5=3.75 synergy
[00218] The 22.5:1 ratio represents a 2-fold lower dose of naltrexone and
the same
dose of clonidine when administered alone. The fixed dose ratio of 90:1, 45:1
and 22.5:1
demonstrated weight to weight synergy with neuropathic back pain completely
blocked by
the doses of 2.25mg/ 0.025mg, 2.25mg/0.05mg and 2.25mg/0.1mg of
naltrexone/clonidine
respectively.
[00219] Table 2 demonstrates the side effect synergy of the
naltrexone/clonidine
combination: alertness and anxiety from naltrexone are counteracted by the
somnolence and
calmness caused by clonidine. Naltrexone administered by itself at a 4.5mg
dosage carries a
high incidence of alertness, insomnia and anxiety which deters compliance.
Clonidine
administered by itself at a .3mg dosage at bedtime causes excessive somnolence
that carries
over through the next day.
Table 2: Synergy of Side Effects for Naltrexone/Clonidine
Ratio Naltrexone mg Clonidine mg Interaction Side effects
4.5:0 4.5 Alertness,
Insomnia, anxiety
0:0.3 0.3 Excessive
Somnolence,
calmness
90:1 2.25 0.025 synergy No side effects
45:1 2.25 0.05 synergy Somnolence
Rarely
22.5:1 2.25 0.1 synergy Somnolence
Rarely
54
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[00220] The 90:1 ratio is a suitable synergistic ratio for morning/daytime
contemplated
administration. It embodies a lower adjusted total weight to weight dose of
2.625mg
compared to 4.5mg of naltrexone and clonidine alone. Furthermore, it offers
balance between
the side effects: alertness and anxiety from naltrexone are counteracted by
calmness from
clonidine.
[00221] The 45:1 ratio is a suitable synergistic ratio for night/bedtime
contemplated
use. It embodies a lower adjusted total weight to weight dose of 2.35mg
compare to 4.5mg of
naltrexone and clonidine alone. This ratio provides additional sedation for
more restorative
night sleep.
[00222] The 22.5:1 ratio is a synergistic ratio for night/bedtime use as it
provides
additional sedative effect.
[00223] To summarize the naltrexone/clonidine synergistic effect, the
disclosure
teaches that the optimal contemplated naltrexone, or a pharmaceutically
acceptable salt or
solvate thereof, to clonidine, or a pharmaceutically acceptable salt or
solvate thereof,
combination dosage ratio range is between 90:1 and 22.5:1. This dosage ratio
range exhibits
synergy of weight to weight proportion and of side effect profile.
[00224] The clinical trial described below demonstrated statistically
significant
improvement of sleep compared to baseline as measured by the Pittsburgh
Insomnia Rating
Scale (PIRS 20). This suggests that the naltrexone side effects of alertness
and insomnia were
not only counteracted, but sleep was improved with the co-administration of
the clonidine.
(See Figure 17, below).
Use of Combination of Opioid/TLR4 Antagonist and direct-acting Alpha-2
Adrenergic
Antagonist to treat Lower Back Pain
[00225] A 59 year old female presented with a 15 year history of lower back
pain
(LBP), leg weakness and pain. Additionally, she had a lifelong history of
migraine headaches
averaging two headache-days a week with an average intensity of 5 on a scale
of 10, where
is intolerable pain. Prior to entering the double blind clinical trial, she
had used duloxetine,
topiramate, and non-steroidal anti-inflammatory drugs (NSAIDs) on a regular
basis, all of
which were discontinued upon entering the trial.
[00226] Prior to dosing, the subject could not stand on either leg for any
length of time.
Her baseline average back pain score the week before receiving study drug was
6.5 on a scale
of 10, and her headache pain level was rated as 10. Eight minutes after the
first dose
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
combination of naltrexone/clonidine 2.25mg/0.025mg she reported that her
headache had
begun to resolve. After one hour her migraine pain had resolved to 0; her LBP
had become 5
for a 60% improvement by her own report. After six hours, her LBP had fully
resolved to a
score of 0 out of 10.
[00227] The subject continued the combination of naltrexone/clonidine
2.25mg/0.025mg twice daily for 27 days during which time she reported no lower
back pain
or migraine pain. At the end of the 27-day treatment period she was able to
stand separately
on each leg for longer than 20 seconds.
[00228] During an 18-month post-study follow up period with no study
medication, the
subject was seen on six different occasions and maintained a self-reported
long-term 80%
improvement of her LBP and migraines compared to the period before receiving
the study
drug.
[00229] This case study shows that the combination of naltrexone/clonidine
at the dose
of 2.25mg/0.025mg twice daily is successful in treating acute migraine
headache attack and
chronic LBP. It also appears to have had a long-term prophylactic impact on
migraine
headaches. Table 3 tabulates the various pain intensity scores over time and
percent relief for
the example of lower back pain. The drawing in Figure 1 is a graph of the
various pain
intensity scores over time in the case of lower back pain. The drawing in
Figure 2 is a graph
of the percent relief reported by the case of lower back pain over time.
56
0
Table 3: An Example of Lower Back Pain
t..)
o
1¨
.6.
Baseline Study Drug
o
o
o
--.1
--.1
Days in
Trial -8 -7 -6 -5 -4 -3 -2 -1 1 2 3 4
5 6 7 8 9 10 11 12 13 14 15 16 17 18
19 20 21 22 23 24
Worst
5 5 6 6 10 9 9 9 1 1 1 4 1 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Pain
Least
4 5 4 6 6 6 8 8 1 1 1 4 1 1
1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0
Pain
Average
5 5 5 8 8 8 8 1 1 1 4 1 2 1 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Pain
Right
Now 5 5 6 4 6 6 5 7 1 1 1 4 1 2
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0
Pain
Night
8 6 6 6 10 9 8 9 1 0 0 4 0 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Pain
Relief % 70 70 70 50 70 60 60 70
70 70 70 80 80 80 80 90
90 90 90 90 90 90 90 90 P
"
.,
..
"
cn
u,
..
"
,
.,
,
,
w
Iv
n
cp
w
.6.
-a-,
w
u,
--.1
--.1
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Use of Combination of Opioid/TLR4 Antagonist and direct-acting Alpha-2
Adrenergic
Antagonist to treat Vulvodynia
[00230]
Vulvodynia is a chronic pain syndrome that affects the vulvar area and often
occurs without an identifiable cause or visible pathology. A 55 year old
female with a six
year history of vulvodynia presented with constant pain and sensitivity in the
vulva which
interfered with her daily living and prevented her from having sexual
intercourse. The subject
entered open label study for three weeks. She improved dramatically after the
first dose. After
three days her pain level went from 9 to 0 (on an 11 point pain scale). After
taking two
capsules the first day, she noticed that she felt aggression, and so she
reduced the dose to one
capsule a day. One capsule still offered her full relief but without adverse
events. Table 4
tabulates the various pain intensity scores over time and percent relief for
the example of
Vulvodynia. The drawing in Figure 3 is a graph of the various pain intensity
scores over time
in the case of Vulvodynia pain. The drawing in Figure 4 is a graph of the
percent relief
reported by the case of Vulvodynia pain over time. This case study
demonstrates complete
reversal of vulvodynia pain starting after the first dose of ATNC05. The
effect was
maintained throughout the 30-day treatment period. The patient reported
increased energy
level, improvement of sleep quality, and resolution of headaches.
Table 4: An Example of Vulvodynia
atmfdmmiitfamcomimmiNimmaimimimaimmaimaiiii
11.04.0**1111=11111111111117111111111111111111111111111111111111111111111111111
1111111111111111111111111111111111111111111111111111111111111111111111111111111
1111111111111111111111111111111111111111111111111111111111111111111111111111111
111111111111119
iip411111111i "'III pill EN pie", Ili
ENNEMg 1 1 1............ ....... .........
........
8 0 0 8 8 8 8 8 6 0 6 0 0 0 0 0 0 0 0
iiti:04,00111ili ___________________________________________________ 3 7 8 6 6
6 6 6 0 0 0 0 0 0 0 0 0 0 0
ii.M04gOilp41 ______________________________________________________ 7 7 8 6 6
6 6 6 0 0 0 0 0 0 0 0 0 0 0
Ewg4p.!,:11Ø*E
8 7 8 6 6 6 6 6 0 0 0 0 0 0 0 0 0 0 0
1 ____________ 1
iiNg4.1M#111 6 0 0 8 8 8 8 8 6 0 6 0 0 0 0 0 0 0
0
ipplip1111111111111111 ________________________________________________
11111(rØ5.04.11111iii 10 10 10 10 10 10 10
10 10
60 0 90 0 0 0 0 0 0 0 0
Ttntnt
aNi
o 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
58
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
o o o 00000 o o o o o o o o o o o
AvagPth U o o 000 o o o o o o o o o o o o o
EX(0,101
Pain o o o o 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
img4.1mi 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
7,11.11111111111111111 1 1 1 1 1 1
madatiiiimm 0 0 0 0 0 0 10 10 10 10 10 10 10
10 10 10 10 10
i*omommuii 0 0 100 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 0
Use of Combination of Opioid/TLR4 Antagonist and direct-acting Alpha-2
Adrenergic
Antagonist to treat Trigeminal Neuralgia
[00231]
Trigeminal neuralgia, also known as suicide disease, is a neuropathic disorder
characterized by episodes of intense pain in the face, originating from the
trigeminal nerve. It
has been described as among the most painful conditions known to mankind. This
case study
is of a 40 year old male with a three month history of trigeminal neuralgia.
The patient had a
gradual onset of pain in the right side of his face that started six weeks
after a root canal. The
pain involved the right check, upper jaw and the upper front teeth. The pain
was constant and
described as burning, stinging and aching. It was aggravated by eating,
brushing teeth,
flossing, chewing and talking. The subject's pain level on average was 5, but
it would peak to
9. Ibuprofen brought the pain level from 9 to 7 for two hours, vicoden brought
his pain level
from 9 to 5, but relief lasted only three to four hours. The subject entered
open label study for
3 weeks. After he was on the study drug at a dose of two capsules a day for
one week his pain
level fell to 2 and he reported being 90% better than before participating in
the study. He
stated that one dose had a lasting effect of up to 24 hours, unlike previous
medications he had
tried. Table 5 tabulates the various pain intensity scores over time and
percent relief for the
example of Trigeminal Neuralgia. The drawing in Figure 5 is a graph of the
various pain
intensity scores over time in the case of Trigeminal Neuralgia pain. The
drawing in Figure 6
is a graph of the percent relief reported by the case of Trigeminal Neuralgia
pain over time.
Table 5: An Example of Trigeminal Neuralgia
tdy Drug
...............................................................................
...............................................................................
...............................................................................
.................
...............................................................................
...............................................................................
...............................................................................
.................
...............................................................................
...............................................................................
...............................................................................
..................
11=11130$011,12111:9
*iWbittiPAitli:i*: 6 6 6 6 6 6 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 1 1 1 1 1 1 1
sPIi 4 4 4 4 4 4 2 2 2 2 2 2 2 2
2 2 2 2 2 2 2 2 0 0 0 0 0 0 0
5 5 5 5 5 2 2 2 2 2 2 2 2 2 2 2
2 2 2 2 2 1 1 1 1 1 1 1
59
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
4 4 4 4 4 4 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 0 0 0 0 0 0 0
imotou 6 6 6 6 6 5 4 2 2 4 4 4 4 3 3 3 4 4 3 3 3 1 1 1 1 1 1 1 1
V0011tiiMi
90 90 90 90 90 90 90 90 90 90 90 90 90 90 90 90 96 96 96 96 96 96 96
Use of Combination of Opioid/TLR4 Antagonist and direct-acting Alpha-2
Adrenergic
Antagonist to treat Irritable Bowel Syndrome (IBS)
[00232] A 42 year old male had an eight year history of IBS averaging
two attacks per
year. His present flare-up had persisted for seven days with symptoms include
left lower
quadrant abdominal pain, cramping and bloating. Physical examination showed
tenderness of
the left lower quadrant and hyperactive bowel sounds. The subject reported a
pain rating of 8.
He enrolled in the open label phase of the trial with a baseline pain level of
8, lower back
pain of 7. Seventy-five minutes after his initial dose, his pain, cramping,
and lower back pain
had become zero. Four hours later his pain recurred but was resolved after a
second dose was
taken. Table 6 tabulates the various IBS symptom intensity scores over time
for the example
of IBS. The drawing in Figure 7 is a graph of the various IBS symptom
intensity scores over
time in the case of IBS.
Table 6: An Example of Irritable Bowel Syndrome
An Example of Irritable Bowel Syndrome
Hours Post Dose
0.00 0.25 0.50 0.75 1 3 4 5 6 7 8 9 12 21 22
23 24 25 28
IBS 8 4 2 1
0 3 3 4 5 4 2 0 0 0 0 0 0 0 0
Cramping 8 5 2 0 0 2 3 3 2 0 0 0
3 2 2 2 2 2
Back Pain 7 3 2 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0
Bloating 7 5 2 0 0 2 3 3 2 1 0 0 3 2 2 1 1
Use of Combination of Opioid/TLR4 Antagonist and direct-acting Alpha-2
Adrenergic
Antagonist to treat Acute Migraine
[00233] A 45 year old man presented with an acute migraine. His
symptoms included
pain level of 8 on a 0 to 10 point scale, with 10 being intolerable pain;
nausea level of 5; and
noise and light disturbance of 6. Seventy-five minutes after administration
his nausea was at
3 while all his other symptoms went to zero, and he was able to look directly
into the light.
He was symptom-free for four hours, after which his headache and other
symptoms recurred.
He took two additional capsules of study drug and the symptoms resolved
completely within
one hour. Symptoms did not recur. Table 7 tabulates the various headache
parameters
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
intensity scores over time for the example of migraine. The drawing in Figure
8 is a graph of
the various headache parameters intensity scores over time in the case of
migraine headache.
Table 7: An Example of Migraine
Migraine Example
Hours Post Dose
0 0.3 0.5 0.8 1 2 3 4 5 6 7 8 9
10 11 12 13 14 15
Headache 8 8 3 3 2 0 0 0 0 8 0 0 0 0 0 0 0 0 0
Light 6 6 3 3 2 0 0 0 0 6 0 0 0 0 0 0 0 0 0
Noise 6 6 3 3 2 0 0 0 0 6 0 0 0 0 0 0 0 0 0
Use of Combination of Opioid/TLR4 Antagonist and direct-acting Alpha-2
Adrenergic
Antagonist to treat Leg Pain
[00234] A 61 year old woman with a ten year history of leg pain and
weakness caused
by L-5 radiculopathy was incapable of standing for longer than 10 minutes. She
was not able
to stand on one leg for any length of time on either side without losing
balance immediately.
The subject of this case had seen eleven spine surgeons, repeatedly told that
surgery would
not be beneficial for her pain. She tried oral medication such as NSAIDs,
anticonvulsants,
muscle relaxants, and anti-depressants (bupropion, duloxetine). Aside from
some relief from
NSAIDs, none of the other medications had any meaningful effect. She received
spinal
decompression on a DRX 9000 and obtained about 10% relief. An epidural steroid
injection
provided 5% relief for two weeks, three perispinal injections with botulinum
toxin offered
10% relief for two months, and three perispinal Etanercept injections offered
10% relief for
two months.
[00235] The subject in this case study has been on naltrexone/clonidine at
the dose of
2.25mg/0.025mg twice daily for three years with improvement of up to 80% of
her
symptoms. Recently she increased the dose to 6 units which she prefers to take
at night for
the control of night pain and insomnia. With the current regiment her pain
level stabilizes at a
self-reported score of between 0-3 on a 10-point scale, and she has been able
to stand on
either leg for longer than 60 seconds. Without medication pain recurs within
one to three
days.
61
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Clinical Trial Excerpts
[00236] A double-blind, placebo-controlled, randomized, proof-of-concept
clinical
trial was conducted in order to determine the efficacy and safety of a
combination of low
dose naltrexone and low dose clonidine (code-named ATNC05) for the treatment
of
symptoms of back pain. The studies were conducted under the regulatory
oversight of the
Food and Drug Administration (FDA) and the Independent Institutional Review
Board
(presently known as Shulman IRB). The subjects were screened to verify the
diagnosis of
chronic back pain (longer than three months). After a one-week baseline
period, during
which subjects completed daily pain questionnaires, 78 subjects were enrolled
in the study
and treated for a three-week Double-Blind treatment period with either placebo
or ATNC05
(the first 24 subjects were randomized in a 1:4 ratio, and the remaining were
randomized in a
1:1 ratio). Subjects who were non-responders in the Double-Blind were offered
the option to
continue into the Open-Label extension phase, during which subjects were
treated with
ATNC05 for three weeks. There were 44 subjects in the ATNC05 group, and 34
subjects in
the placebo group. There were 27 subjects who began the Open-Label phase.
Table 8: Demographic Characteristics of Clinical Trial Subjects
Placebo ATNC05 Total
34 44 78
Age (years)
Mean(SD) 45.6 (11.4) 48.0 (8.5) 46.9 (9.9)
Median 46 49 47
Range 19-70 21-63 19-70
Gender, n (%)
Female 24 (70.6) 28 (63.6) 52 (66.7)
Male 10 (29.4) 16 (36.4) 26 (33.3)
Race, n (%)
White 29 (85.3) 32(72.7) 61(78.2)
Black 1(2.9) 5(11.4) 6(7.7)
More than One Race 4(11.8) 7(15.9) 11(14.1)
Ethnicity, n (%)
Hispanic 24 (70.6) 23(52.3) 47(60.3)
Non-Hispanic 10 (29.4) 21(47.7) 31(39.7)
Weight (Lb)
Mean(SD) 177.5 (44.5) 177.3 (49.0) 177.4 (46.8)
Median 165 176.5 170.5
Range 106-338 115-352 106-352
Height (inches)
Mean(SD) 66.0 (3.1) 66.1 (3.3) 66.1 (3.2)
Median 65.5 66 66
62
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Placebo ATNC05 Total
Range 60-74 61-77 61-77
Baseline Period Brief Pan Inventory (BPI) Severity Scores - Mean (SD)
Worst Pain 6.60 (1.14) 6.64 (1.14) 6.62 (1.11)
Least Pain 4.90 (1.40) 4.64 (1.40) 4.75 (1.28)
Average Pain 5.49 (1.02) 5.56 (1.02) 5.53 (1.02)
Right Now Pain 5.42 (1.23) 5.48 (1.23) 5.45 (1.22)
Night Pain 5.74 (1.35) 6.01 (1.35) 5.89 (1.42)
Baseline Period Brief Pan Inventory (BPI) Interference Scores - Mean (SD)
General Activity 5.46 (1.48) 5.57 (1.48) 5.52 (1.46)
Mood 4.73 (1.62) 4.99 (1.62) 4.88 (1.80)
Walking 5.02 (1.76) 4.97 (1.76) 4.99 (1.91)
Normal Work 5.47 (1.23) 5.62 (1.23) 5.55 (1.42)
Relationships 4.42 (1.72) 4.09 (1.72) 4.24 (2.05)
Sleep 5.77 (1.16) 5.83 (1.16) 5.80 (1.52)
Enjoyment of Life 5.15 (1.53) 5.30 (1.53) 5.23 (1.73)
Standing Ability 5.33 (1.56) 5.22 (1.56) 5.27 (1.81)
Sitting 5.03 (1.70) 5.02 (1.70) 5.03 (1.79)
Office Encounters Measures Scores - Mean (SD)
CGI-S 5.28 (0.83) 5.32 (0.80) 5.30 (0.82)
Oswestry Disability Index 41.69 (8.56) 42.12 (7.37) 41.36 (9.45)
Roland-Morris Disability 14.83 (4.43) 15.78 (4.50) 14.13 (4.30)
Pittsburgh Insomnia Rating (PIRS-20) 35.42 (7.83) 36.06 (7.28) 34.93 (8.29)
Duration of Subject Back Pain - Mean (SD)
Duration (Years)* 7.88 (6.16) 9.07 (5.90) 8.67 (5.96)
[00237] The demographic characteristics of the subjects are summarized in
Table 8.
[00238] Table 9 shows 23 subjects out of 78 who entered the study for
back pain had
concomitant migraine or tension headache. This finding represents a higher
incidence of
headaches than found in the general population. Similarly, table 10 shows 30
out of
78subjects had joint pain, most of which was associated with tendinitis rather
than arthritic
pain.
Table 9: Baseline Migraine Subject Characteristics
Placebo ATNC05 All
Migraine Migraine Migraine
Subjects Subjects Subjects
13 23
Age (years)
41.5 46.2 44.2
Mean(SD) (12.62) (8.32) (10.42)
Median 39.5 45 43
Range 19 - 70 33 - 59 19 - 70
Gender, n
63
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Female I 10 (100) I 10 (76.9) I 20 (86.9)
Male I 0(0) I 3(23.1) I 3(13.0)
Race, n (%)
White 9 (90) 7 (53.8) 15 (65.2)
Black 0 (0) 2 (15.4) 2 (8.6)
More than One Race 1(10) 4 (30.7) 5(21.7)
Ethnicity, n (%)
Hispanic 7 (70) 9 (69.2) 16 (69.5)
Non-Hispanic 3 (30) 4 (30.7) 7 (30.4)
Duration in Years¨ Mean (SD) Average Severity on 11-point scale ¨ Mean (SD) N
Placebo Migraine Subjects 17.9(11.23) 6.4 (1.17)
10
ATN035 Migraine Subjects 23.1 (13.62) 5.8 (1.48)
13
All Migraine Subjects 20.8 (12.64) 6.0 (1.36)
23
Open-Label Migraine Subjects 17.1 (9.87) 6.4 (1.33)
9
Table 10: Baseline Joint Pain (Tendinitis) Characteristics
Placebo ATNC05 Total
Joint Pain (Tendinitis)
6 10 16
Average Severity 5.10 5.80 5.53
Average Duration (Years) 3.36 4.58 4.12
[00239] The subjects took 1 capsule containing naltrexone/clonidine
2.25mg/0.025mg
respectively or placebo twice daily and were instructed to increase the dose
if the pain
reduction effect was not sufficient. Subjects who did not respond had the
option of continuing
in an open label phase, receiving study drug for 3 weeks.
[00240] While in the study the subjects completed a daily pain
questionnaire regarding
back pain. Subjects who had concomitant headaches and/or joint pain recorded
progression of
those symptoms as well. The study subjects were evaluated in the office, in
person four times
during the course of the study and for subjects in open phase six times.
Disability and sleep
quality questionnaires along with safety data were collected during the office
encounters.
Results
[00241] Table 11 presents the daily improvement of pain scores of study
drug vs.
placebo measured on a scale of 0-10, with 10 being most severe during the
blinded study
drug period.
Table 11: Daily Improvement of Pain Scores of Study Drug vs. Placebo by Day
N of Subjects N of Mean (SE) Mean (SE) Placebo ATNC05
Study Receiving Subjects Average Pain Average Pain Treatment Treatment
Day Placebo (n of Receiving Score for Score for
Effect Effect p-value
64
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
subjects ATNC05 (n Placebo ATNC05
Imputed by of subjects Subjects Subjects
BOCF) Imputed by
BOCF)
2 34 (1) 44 (0) 4.77 (0.33) 3.09 (0.29) -0.72 -
2.49 0.00029
3 34 (1) 44 (2) 4.63 (0.33) 3.07 (0.29) -0.87 -
2.51 0.00072
4 34 (1) 44 (2) 4.74 (0.34) 2.77 (0.29) -0.75 -
2.81 0.00003
34 (2) 44 (2) 4.59 (0.33) 2.84 (0.31) -0.90 -2.74
0.00020
6 34 (3) 44 (1) 4.51 (0.35) 2.31 (0.28) -0.98 -
3.27 0.00001
7 34 (3) 44 (2) 4.60 (0.37) 2.48 (0.29) -0.90 -
3.10 0.00003
8 34 (3) 44 (2) 4.83 (0.38) 2.59 (0.32) -0.66 -
2.99 0.00003
9 34 (4) 44 (5) 4.69 (0.37) 2.24 (0.32) -0.81 -
3.33 0.00000
34 (4) 44 (3) 4.66 (0.36) 2.14 (0.31) -0.84 -3.44
0.00000
11 34 (4) 44 (3) 4.66 (0.34) 2.14 (0.32) -0.84 -
3.44 0.00000
12 34 (4) 44 (2) 4.66 (0.36) 1.89 (0.33) -0.84 -
3.69 0.00000
13 34 (4) 44 (2) 4.78 (0.39) 1.86 (0.32) -0.72 -
3.72 0.00000
14 34 (4) 44 (3) 4.86 (0.42) 1.84 (0.33) -0.63 -
3.74 0.00000
34 (4) 44 (4) 4.69 (0.41) 1.89 (0.32) -0.80 -3.69
0.00000
16 34 (4) 44 (5) 4.61 (0.41) 1.82 (0.34) -0.89 -
3.76 0.00000
17 34 (4) 44 (6) 4.69 (0.41) 1.86 (0.35) -0.80 -
3.72 0.00000
18 34 (4) 44 (6) 4.55 (0.39) 1.93 (0.34) -0.95 -
3.65 0.00000
19 34 (4) 44 (7) 4.58 (0.40) 1.84 (0.36) -0.92 -
3.74 0.00000
34 (4) 44 (7) 4.43 (0.35) 1.93 (0.37) -1.07 -3.65
0.00001
21 34 (5) 44 (7) 4.59 (0.39) 1.93 (0.36) -0.90 -
3.65 0.00000
22 26* 36* 4.42 (0.45) 1.14 (0.27) -1.07 -4.44
0.00000
23 18* 28* 3.28 (0.56) 1.61 (0.39) -2.22 -3.97
0.02064
24 13* 24* 3.46 (0.69) 1.21 (0.31) -2.03 -4.37
0.00820
[00242] Table 11 shows that back pain scores were significantly lower in
the study
drug subjects compared with placebo (P = 0.001) from Day 2 onwards. The drug
impact
increases as time progressed showing a 5 point improvement.
[00243] Pain intensity scores of study drug vs. placebo, as reported daily
by the
subjects for Worst Pain, Least Pain, Average Pain, Right-Now Pain, and Night
Pain during
the previous 24-hours are summarized in the following 5 tables and graphs. The
data and
graphs show consistent treatment impact on all pain intensity measures.
Improvement begins
starting the first day. The study drug impact increases as time progresses
showing
approximately a 5 point (on a scale of 0-10 with 10 being most severe)
improvement
compared to baseline by the end of the study. The Study Drug group exhibited
resolution of
pain. Table 12 tabulates the worst pain intensity scores over time for the
study drug group
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
versus placebo group in the clinical trial subjects. The drawing in Figure 9
is a graph of the
treatment impact on worst pain by day of study drug group versus placebo group
in the
clinical trial subjects. Table 13 tabulates the least pain intensity scores
over time for the study
drug group versus placebo group in the clinical trial subjects. The drawing in
Figure 10 is a
graph of the treatment impact on least pain by day of study drug group versus
placebo group
in the clinical trial subjects. Table 14 tabulates the average pain intensity
scores over time for
the study drug group versus placebo group in the clinical trial subjects. The
drawing in Figure
11 is a graph of the treatment impact on average pain by day of study drug
group versus
placebo group in the clinical trial subjects. Table 15 tabulates the right now
pain intensity
scores over time for the study drug group versus placebo group in the clinical
trial subjects.
The drawing in Figure 12 is a graph of the treatment impact on right now pain
by day of
study drug group versus placebo group in the clinical trial subjects. Table 16
tabulates the
night pain intensity scores over time for the study drug group versus placebo
group in the
clinical trial subjects. The drawing in Figure 13 is a graph of the treatment
impact on night
pain by day of study drug group versus placebo group in the clinical trial
subjects.
Table 12: Worst Pain Scores (Scale: 0-10) By Treatment Group
Double-Blind Mean Worst Pain Scores by Group By Day (BOCF)
Placebo ATNC05
Treatment Day N (n BOCF) Ave. N (n BOCF) Ave.
Baseline Period
Mean 34 6.60 44 6.62
1*
2 34(1) 5.49 44(0) 4.09
3 34(1) 5.07 44(2) 4.01
4 34(1) 5.27 44(2) 3.78
34 (2) 5.33 44 (2) 3.62
6 34(3) 5.18 44(1) 3.02
7 34(3) 5.29 44(2) 3.18
8 34(3) 5.44 44(2) 3.20
9 34(4) 5.42 44(5) 2.78
10 34(4) 5.24 44(3) 2.80
11 34 (4) 5.33 44 (3) 2.75
12 34(4) 5.27 44(2) 2.45
13 34 (4) 5.51 44 (2) 2.38
14 34(4) 5.30 44(3) 2.30
15 34(4) 5.28 44(4) 2.48
16 34(4) 5.16 44(5) 2.17
17 34 (4) 5.22 44 (6) 2.35
18 34 (4) 5.31 44 (6) 2.30
19 34(4) 5.28 44(7) 2.24
20 34(4) 5.16 44(7) 2.27
21 34(5) 5.41 44(7) 2.46
22 26* 5.15 28* 1.46
23 18* 4.11 24* 2.11
24 13* 4.23 28* 1.75
*Day 1 answers were not tabulated for this question because subjects completed
the BPI on Day 1 within hours of their first
dose of study drug. Data were not imputed by BOCF during the taper-off
periods, days 22-24.
66
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
Table 13: Least Pain Scores (Scale: 0-10) By Treatment Group
Double-Blind Mean Least Pain Scores by Group by Day (BOCF)
Placebo ATNC05
Treatment Day N (n BOCF) Ave. N (n BOCF) Ave.
Baseline Period
Mean 34 4.93 44 4.70
1*
2 34(1) 4.29 44(0) 2.52
3 34(1) 4.11 44(2) 2.56
4 34 (1) 4.23 44 (2) 2.33
5 34(2) 4.15 44(2) 2.44
6 34(3) 4.14 44(1) 2.04
7 34 (3) 4.31 44 (2) 2.03
8 34 (3) 4.31 44 (2) 2.03
9 34(4) 4.39 44(5) 1.81
10 34(4) 4.25 44(3) 1.73
11 34(4) 4.07 44(3) 1.75
12 34(4) 4.04 44(2) 1.49
13 34 (4) 4.31 44 (2) 1.40
14 34(4) 4.34 44(3) 1.50
15 34(4) 4.26 44(4) 1.46
16 34(4) 4.34 44(5) 1.34
17 34 (4) 4.26 44 (6) 1.35
18 34 (4) 4.26 44 (6) 1.39
19 34(4) 4.23 44(7) 1.42
20 34(4) 4.08 44(7) 1.51
21 34 (5) 4.21 44 (7) 1.51
22 26* 4.23 36* 0.97
23 18* 3.17 28* 1.43
24 13* 3.38 24* 1.00
*Day 1 answers were not tabulated for this question because subjects completed
the BPI on Day 1 within hours of their first
dose of study drug. Data were not imputed by BOCF during the taper-off
periods, days 22-24.
Table 14: Average Pain Scores (Scale: 0-10) By Treatment Group
Double-Blind Mean Average Pain Scores by Group by Day (BOCF)
Placebo AT NC05
Treatment Day N (n BOCF) Ave. N (n BOCF) Ave.
Baseline Period Mean 34 5.50 44 5.55
1*
2 34(1) 4.77 44(0) 3.09
3 34 (1) 4.63 44 (2) 3.07
4 34(1) 4.74 44(2) 2.77
5 34(2) 4.59 44(2) 2.84
6 34(3) 4.51 44(1) 2.31
7 34(3) 4.60 44(2) 2.48
8 34(3) 4.83 44(2) 2.59
9 34(4) 4.69 44(5) 2.24
10 34(4) 4.66 44(3) 2.14
11 34(4) 4.66 44(3) 2.14
12 34 (4) 4.66 44 (2) 1.89
13 34(4) 4.78 44(2) 1.86
14 34(4) 4.86 44(3) 1.84
15 34(4) 4.69 44(4) 1.89
16 34 (4) 4.61 44 (5) 1.82
17 34(4) 4.69 44(6) 1.86
18 34(4) 4.55 44(6) 1.93
19 34(4) 4.58 44(7) 1.84
67
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
20 34(4)
4.43 44(7)
4.59 44(7)
4.42 36*
3.28 28*
1.93
21 34 (5)
1.93
22 26*
1.14
23 18*
1.61
24 13* 3.46 24*
1.21
*Day 1 answers were not tabulated for this question because subjects completed
the BPI on Day 1 within hours of their first
dose of study drug. Data were not imputed by BOCF during the taper-off
periods, days 22-24.
Table 15 Right Now Pain Scores (Scale: 0-10) By Treatment Group
Double-Blind Mean Right Now Pain Scores by Group by Day (BOCF)
Placebo ATNC05
Treatment Day N (n BOCF) Ave. N (n BOCF) Ave.
Baseline Period
Mean 34 5.42 44 5.53
1 34 4.65 44 2.77
2 34(1) 4.58 44(0) 2.82
3 34(1) 4.50 44(2) 2.94
4 34 (1) 4.32 44 (2) 2.76
5 34(2) 4.38 44(2) 2.76
6 34 (3) 4.21 44 (1) 2.22
7 34 (3) 4.33 44 (2) 2.35
8 34(3) 4.64 44(2) 2.49
9 34(4) 4.56 44(5) 2.18
10 34(4) 4.38 44(3) 1.94
11 34(4) 4.50 44(3) 2.06
12 34 (4) 4.38 44 (2) 1.76
13 34(4) 4.70 44(2) 1.69
14 34(4) 4.59 44(3) 1.74
15 34(4) 4.50 44(4) 1.68
16 34(4) 4.53 44(5) 1.72
17 34(4) 4.35 44(6) 1.79
18 34(4) 4.44 44(6) 1.70
19 34(4) 4.47 44(7) 1.75
20 34(4) 4.41 44(7) 1.83
21 34(5) 4.45 44(7) 1.90
22 26* 4.42 36* 1.03
23 18* 3.22 28* 1.26
24 13* 3.38 24* 1.08
*Day 1 answers were not tabulated for this question because subjects completed
the BPI on Day 1 within hours of their first
dose of study drug. Data were not imputed by BOCF during the taper-off
periods, days 22-24.
Table 16 Night Pain Scores (Scale: 0-10) By Treatment Group
Double-Blind Mean Night Pain Scores by Group by Day (BOCF)
Placebo ATNC05
Treatment Day N (n BOCF) Ave. N (n BOCF) Ave.
Baseline Period
Mean 34 5.71 44 6.04
1*
2 34(1) 4.84 44(0) 3.43
3 34(1) 4.78 44(2) 3.47
4 34(1) 4.84 44(2) 3.26
34 (2) 4.71 44 (2) 3.26
6 34(3) 4.44 44(1) 2.70
7 34(3) 4.70 44(2) 2.91
8 34(3) 4.84 44(2) 2.81
9 34(4) 4.60 44(5) 2.38
10 34(4) 4.54 44(3) 2.19
11 34 (4) 4.66 44 (3) 2.49
12 34(4) 4.60 44(2) 2.10
13 34(4) 4.80 44(2) 2.08
14 34(4) 4.77 44(3) 2.03
68
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
15 34(4) 4.66 44(4) 2.05
16 34(4) 4.69 44(5) 1.91
17 34(4) 4.63 44(6) 2.05
18 34(4) 4.72 44(6) 2.01
19 34(4) 4.81 44(7) 2.09
20 34(4) 4.54 44(7) 2.11
21 34(5) 4.63 44(7) 2.16
22 26* 4.27 36* 1.28
23 18* 3.50 28* 1.64
24 13* 3.54 24* 1.25
*Day 1 answers were not tabulated for this question because subjects completed
the BPI on Day 1 within hours of their first
dose of study drug. Data were not imputed by BOCF during the taper-off
periods, days 22-24.
[00244] The Open Phase enrolled 27 subjects who had not responded to the
initial
treatment during the Study Drug Phase. Subjects received study drug twice
daily for three to
four weeks.
[00245] The Open Phase results are summarized in Table 17 and in Figure 14.
Table
17 tabulates the daily improvement of pain scores in the open phase and the
drawing in
Figure 14 graphs the Open Phase Relief by Day. It shows that back pain
symptoms were
significantly lower in the open phase period compare with baseline
measurement. Subjects
who did not respond in the blinded study drug period, presumably the subjects
who might
have received the placebo in the blinded phase of the trial, were given the
study drug
containing low dose naltrexone and clonidine 2.25mg/0.025mg respectively for
three weeks.
Improvement begins starting the second day. The drug impact increases as time
progress
showing approximately a 5 point (on a scale of 0-10 with 10 being most severe)
improvement
compared to baseline by the end of the study.
Table 17 Daily Improvement of Average Pain Scores Open Phase
Days in Open Phase Count Treatment with study Standard
P(Treatment>Placebo
drug compare to base Deviation
line
Day 2 Of Open Phase 22 -3.94 0.18 <.001
Day 3 Of Open Phase 22 -4.05 0.15 <.001
Day 4 Of Open Phase 21 -4.16 0.15 <.001
Day 5 Of Open Phase 21 -4.23 0.15 <.001
Day 6 Of Open Phase 22 -4.27 0.15 <.001
Day 7 Of Open Phase 21 -4.29 0.15 <.001
Day 8 Of Open Phase 21 -4.32 0.15 <.001
Day 9 Of Open Phase 20 -4.35 0.15 <.001
Day10 Of Open Phase 20 -4.42 0.15 <.001
69
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Dayl 1 Of Open Phase 19 -4.50 0.15 <001
Day12 Of Open Phase 19 -4.57 0.15 <001
Day13 Of Open Phase 17 -4.62 0.16 <001
Day14 Of Open Phase 16 -4.65 0.15 <001
Day15 Of Open Phase 15 -4.71 0.16 <001
Day16 Of Open Phase 14 -4.73 0.17 <001
Day17 Of Open Phase 14 -4.73 0.17 <001
Day18 Of Open Phase 10 -4.72 0.17 <001
Day19 Of Open Phase 18 -4.71 0.17 <001
Day20 Of Open Phase 18 -4.69 0.18 <001
Day21 Of Open Phase 9 -4.68 0.19 <001
Day22 Of Open Phase 8 -4.69 0.19 <001
Day23 Of Open Phase 3 -4.68 0.21 <001
Day24 Of Open Phase 3 -4.69 0.22 <001
Day25 Of Open Phase 3 -4.71 0.23 <001
Day26 Of Open Phase 2 -4.74 0.25 <001
Day27 Of Open Phase 1 -4.78 0.27 <001
Day28 Of Open Phase 1 -4.80 0.29 <001
Day29 Of Open Phase 1 -4.82 0.30 <001
[00246] Figure 14 shows an average 4.5 point drop in pain score during the
Open
Phase.
[00247] The clinical trial found that the opioid/TLR4 antagonist
naltrexone, in
combination with the alpha two adrenergic receptor agonist clonidine, treated
and reversed
chronic pain conditions, including chronic back pain, chronic headaches,
including migraines
and chronic joint pain. Additionally, the study drug reversed long standing
trigeminal
neuralgia, tactile allodynia, vulvodynia and IBS.
[00248] The Oswestry Disability Index is considered the gold standard for
assessing
the disability level of back pain for those, who suffer back pain, to assess
their disability
level. The drawing in Figure 15 shows the change in the Oswestry Disability
Index of the
cervical pain over time in the study drug group versus the placebo group. The
drawing in
Figure 16 shows the change in the Oswestry Disability Index of the lumbar pain
over time in
the study drug group versus the placebo group.
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[00249] The Pittsburgh Insomnia Rating Scale (PIRS) was developed by the
University
of Pittsburgh's Western Psychiatric Institute and Clinic to assess insomnia.
The Total Score is
a sum of all nineteen responses to questions on the questionnaire with
possible values of zero
to sixty. Higher scores indicate greater degree of insomnia. The drawing in
Figure 17 shows
the change in the Pittsburgh Insomnia Rating Scale over time in the study drug
group versus
the placebo group. The Study Drug treatment group showed statistically
significant
improvement over the placebo group in PIRS 20 Total Score at Week 1 and at
Week 3.
[00250] The drawing in Figure 18 graphs average the Roland-Morris Low Back
Pain
and Disability Questionnaire (RMQ) scores for the Study Drug Group and Placebo
at
Baseline, Week 1, and Week 3. The Roland-Morris Questionnaire is a self-
administered
disability measure in which greater levels of disability are reflected by
higher numbers on a
24-point scale. The RMQ has been shown to yield reliable measurements, which
are valid for
inferring the level of disability, and to be sensitive to change over time for
groups of patients
with lower back pain.
[00251] From the clinical trial it is concluded that the opioid/TLR4
antagonist
naltrexone, in combination with the alpha-2 adrenergic receptor agonist
clonidine, treated and
reversed the chronic pain conditions chronic back pain, chronic headaches
including
migraines and chronic joint pain. Additionally, the study drug reversed long
standing
trigeminal neuralgia, vulvodynia and irritable bowel syndrome.
Example 2: A Pharmaceutical Composition Comprising Opioid/TLR4 Antagonists and
Acetyl-Para-Aminophenol (APAP) for use in treatment of Pain.
[00252] Naltrexone and acetyl-para-aminophenol were evaluated alone and in
combination on a human subject with the purpose of finding whether or not a
combination of
the two compounds offers a synergistic advantage for the pain treatment effect
comparing the
amounts used weight to weight.
[00253] The components of the combination were administered to a subject as
follows,
the naltrexone dose administered by itself was 4.5mg and the acetyl-para-
aminophenol dose
administered by itself was 1000mg, The naltrexone/acetyl-para-aminophenol
combination
dose was 2.25mg/325 respectively. The pain treatment effect of naltrexone and
acetyl-para-
aminophenol was evaluated two hours post-dose.
[00254] To determine synergy, the amounts of naltrexone and acetyl-para-
aminophenol administered alone were compared to the combination combined
amounts. For
71
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
proper weight to weight (W/W) comparison between naltrexone and acetyl-para-
aminophenol
an adjustment for the higher potency of naltrexone was made based on the dose
of each
compound given by itself. Naltrexone is 222 times more potent than acetyl-para-
aminophenol
(1000/4.5=222). Naltrexone and acetyl-para-aminophenol were administered at
fixed dose
ratios of 3:200 to a human subject afflicted with neuropathic back pain.
[00255] Table 18 illustrates the naltrexone/acetyl-para-aminophenol ratio
that exhibit
weight to weight (W/W) synergy in a human subject. The 3:200 combinations
represent a 2-
fold lower dose of naltrexone and 3-fold lower dose of acetyl-para-aminophenol
when
administered together.
Table 18: Naltrexone/acetyl-para-aminophenol Ratio and Weight to Weight (W/VV)
Synergy
Ratio Naltrexone acetyl-para- naltrexone Total dose
Naltrexone + Interaction
mg aminophenol e Potency reversal Adjusted acetyl-para-
mg Adjustment of pain aminophenol mg
(x222)
4.5:0 4.50 1000 100 1000
0:800 - 1000 50 1000
1:90 2.25 325 500 100 500+325=825 Synergy
[00256] To summarize the naltrexone/acetyl-para-aminophenol synergistic
effect, the
disclosure teaches that the optimal contemplated naltrexone, or a
pharmaceutically acceptable
salt or solvate thereof, to acetyl-para-aminophenol, combination dosage ratio
range is 3:200,
and this dosage ratio exhibits synergy of weight to weight proportion.
Example 3: A Pharmaceutical Composition Comprising Opioid/TLR4 Antagonists and
a
cyclooxygenase (COX) inhibitor for use in treatment of Pain.
[00257] Naltrexone and ibuprofen were evaluated alone and in combination on
a
human subject with the purpose of finding whether or not a combination of the
two
compounds offers a synergistic advantage for the pain treatment effect
comparing the
amounts used weight to weight.
[00258] The components of the combination were administered to a subject as
follows:
the naltrexone dose administered alone was 4.5mg, and the ibuprofen dose
administered
alone was 800mg. The dose of the naltrexone/ibuprofen combination was
2.25mg/200,
respectively. The pain treatment effect was evaluated one hour post-dose.
72
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[00259] To determine synergy, the amounts of naltrexone and ibuprofen
administered
alone were compared to the combination combined amounts. For proper weight to
weight
(W/W) comparison between naltrexone and ibuprofen an adjustment for the higher
potency
of naltrexone was made based on the dose of each compound given by itself.
Naltrexone is
178 times more potent than ibuprofen (800/4.5=178). Naltrexone and ibuprofen
were
administered at fixed dose ratios of 1:90 to a human subject afflicted with
neuropathic back
pain.
[00260] Table 19 illustrates the naltrexone/ibuprofen ratio that exhibit
weight to weight
(W/W) synergy in a human subject. The 1:90 combinations represent a 2-fold
lower dose of
naltrexone and 4-fold lower dose of ibuprofen when administered together.
Table 19: Naltrexone/ibuprofen Ratios and Weight to Weight (W/VV) Synergy
Ratio Naltrexone Ibuprofen Naltrexone % reversal Total dose
Naltrexone Interaction
mg e mg Potency of + Adjusted
Adjustment pain ibuprofen mg
(x178)
4.5:0 4.50 800 100 800
0:800 - 800 50 800
1:90 2.25 200 400.00 100 200+400=600 Synergy
[00261] To summarize the naltrexone/ibuprofen synergistic effect, the
disclosure
teaches that the optimal contemplated naltrexone to ibuprofen combination
dosage ratio is
1:90. This dosage ratio exhibits synergy of weight to weight proportion.
Example 4: A Pharmaceutical Composition Comprising Opioid/TLR4 Antagonists and
an Alpha 2 Delta Ligand for use in treatment of Pain.
[00262] Naltrexone and Gabapentin or Pregabalin were evaluated alone and in
combination on a human subject with the purpose of finding whether or not a
combination of
the two compounds offers a synergistic advantage for the pain treatment effect
comparing the
amounts used weight to weight.
[00263] The components of the combination were administered to a subject as
follows:
the naltrexone dose administered alone was 4.5mg, and the Gabapentin and
pregabalin dose
administered alone was 1800mg and 300mg respectively. The dose of the
naltrexone/
Gabapentin combination was 2.25mg/300 respectively and the
naltrexone/pregabalin
73
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
combination was 2.25mg/150 respectively, the pain treatment effect was
evaluated one hour
post-dose.
[00264] To determine synergy, the amounts of naltrexone and Gabapentin or
pregabalin administered alone were compared to the combination combined
amounts. For
proper weight to weight (W/W) comparison between naltrexone and Gabapentin or
pregabalin an adjustment for the higher potency of naltrexone was made based
on the dose of
each compound given by itself. Naltrexone is 200 times more potent than
gabapentin
(200/4.5=200). Naltrexone and Gabapentin were administered at fixed dose
ratios of 1:50-
1:125 to a human subject afflicted with neuropathic back pain. The 1:125
combinations
represent a 2-fold lower dose of naltrexone and a 6-fold lower dose of
Gabapentin
[00265] Table 20 illustrates the naltrexone/Gabapentin ratio that exhibit
weight to
weight (W/W) synergy in a human subject. The 1:50 combinations represent a 2-
fold lower
dose of naltrexone and 18 fold lower dose of Gabapentin. The 1:125
combinations represent a
2-fold lower dose of naltrexone and 6-fold lower dose of Gabapentin.
Table 20: Naltrexone/ Gabapentin Ratios and Weight to Weight (W/VV) Synergy
Ratio Naltrexone Gabapentin Naltrexone % Total dose Naltrexone
Interaction
mg mg Potency revers + Adjusted
Adjustment al of Gabapentin mg
(x200) pain
4.5:0 4.50 900.00 100 900
0:900 - 1800 50 1800
1:50 2.25 100 450.00 100 100+450=550 Synergy
1:125 2.25 300 450.00 100 300+450=850 Synergy
[00266] Table 21 illustrates the naltrexone/pregabalin ratio that exhibit
weight to
weight (W/W) synergy in a human subject. The 1:30 combinations represent a 2-
fold lower
dose of naltrexone and 4 fold lower dose of pregabalin when administered
together. The 1:50
combinations represent a 2-fold lower dose of naltrexone and 2.4 fold lower
dose of
Pregalbin when administered together.
Table 21: Naltrexone/pregabalin Ratios and Weight to Weight (W/W) Synergy
Ratio Naltrexone pregabalin Naltrexone % Total dose Naltrexone +
Interaction
mg mg Potency reversal Adjusted pregabalin mg
Adjustment of pain
(x75)
4.5:0 4.50 300.00 100 300
74
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
0:900 - 300- 50 300 -
1:30 2.25 75 150.00 100 150+75=225 Synergy
1:40 2.25 100 150.00 100 150+100=250 Synergy
1:50 2.25 125 150.00 100 150+125=275 Synergy
Example 5: A Pharmaceutical Composition Comprising Opioid/TLR4 Antagonists and
Dextro Enantiomers Thereof for use in treatment of Pain.
[00267] The clinical trials demonstrated that adverse effects were minimal
and
transient with no indication of addiction. There were no reports of tolerance
or withdrawal
symptoms.
[00268] The following tables and graphs demonstrate safety of naltrexone
for the
treatment of pain. The data was obtained from a 78 subject clinical trial
where naltrexone
2.25 mg/clonidine 0.025 mg were administered twice daily. Table 22 tabulates
the change of
the pulse from baseline by visit of the study drug group and the placebo
group. The drawing
in Figure 19 graphs the change in the pulse from baseline of the study drug
group versus the
placebo group. Table 23 tabulates the change of the systolic blood pressure
(BP) from
baseline by visit of the study drug group and the placebo group. The drawing
in Figure 20
graphs the change in the systolic blood pressure (BP) from baseline of the
study drug group
versus the placebo group. Table 24 tabulates the change of the diastolic blood
pressure (BP)
from baseline by visit of the study drug group and the placebo group. The
drawing in Figure
21 graphs the change in the diastolic blood pressure (BP) from baseline of the
study drug
group versus the placebo group The data shows that there were no clinically
significant
changes in pulse, systolic blood pressure and diastolic blood pressure over a
three to four
week treatment, either during treatment period or Open Phase (OP).
Table 22 Pulse Change from Baseline by Visit
Mean Change in Pulse From From Pre-Initial Dose In Double-Blind and Open-Label
Phase
Placebo ATNC05 Open-Label Phase
N Mean (SE) N Mean (SE) N Mean (SE)
Pre-Initial Dose 34 0.00 (0.00) 44 0.00 (0.00) 27 0.00
(0.00)
Post Initial Dose 34 -0.32 (0.71) 44 0.80 (0.80) 28 0.07 (0.83)
Week 1 32 0.81 (0.62) 43 0.98 (1.06) 25 -0.61 (0.93)
Week 3 30 0.07 (1.20) 41 -0.44 (0.86) 23
0.35 (1.07)
End of Taper-Off Phase 31 0.00 (0.00) 42 0.00 (1.10) 24 0.00
(0.00)
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
Table 23 Systolic BP Change from Baseline by Visit
Mean Change in Systolic Blood Pressure From Pre-Initial Dose In Double-Blind
and Open-Label
Phase
Placebo ATNC05 Open-Label Phase
N Mean (SE) N Mean (SE) N Mean (SE)
Pre-Initial Dose 34 0.00 (0.00) 44 0.00 (0.00) 27 0.00
(0.00)
Post Initial Dose 34 -0.26 (1.18) 44 0.00 (1.63) 28 -1.86
(1.53)
Week 1 32 0.94 (1.56) 43 -1.58 (1.19) 25 -0.87
(2.06)
Week 3 30 1.59 (2.30) 41 -0.39 (1.58) 23 -2.43
(1.94)
End of Taper-Off Phase 4 -9.25 (11.69) 12 -2.83 (3.97) 2 1.00
(15.00)
Table 24 Diastolic Change from Baseline by Visit
Mean Change in Diastolic Blood Pressure From Pre-Initial Dose In Double-Blind
and Open-Label
Phase
Placebo ATNC05 Open-Label Phase
N Mean (SE) N Mean (SE) N Mean (SE)
Pre-Initial Dose 34 0.00 (0.00) 44 0.00 (0.00) 27 0.00
(0.00)
Post Initial Dose 34 0.91 (0.61) 44 0.68 (0.90) 28 0.11
(1.17)
Week 1 32 2.94 (0.90) 43 -0.56 (0.93) 25 0.17
(1.29)
Week 3 30 2.34 (1.66) 41 0.83 (1.09) 23 0.61
(1.16)
End of Taper-Off Phase 4 -3.50 (6.24) 12 -1.83 (2.65) 2 5.00
(5.00)
Example 6: Validation of Use of Dextro Enantiomer of Naltrexone
[00269] The
following demonstrates adverse effects in two human subjects who were
dosed with naltrexone alone: their experience validates the need to use the
dextro enantiomer
of naltrexone because of the side effects they experienced.
The Case of a Human Subject using a low dose of Naltrexone (2 mg/12 hours) for
treatment of cervical pain.
[00270] A human
subject used naltrexone in a low dose of 2 mg twice daily for
cervical pain. The subject experienced a significant relief of his pain
however he discontinued
using the drug after 2 weeks because he was unable to tolerate the side
effects that included
anxiety and irritability.
[00271] Table 25
tabulates the change of pain and side effects for this patient (on
Naltrexone 2mg twice daily) by day. The drawing in Figure 22 graphs the change
of pain and
side effects over the same period. Table 25 and Figure 22 show naltrexone's
effect on pain
76
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
and side effects. The pain treatment effect of naltrexone continued for a few
days after
discontinuation. The side effects resolved within one day of treatment
discontinuation.
Table 25 Pain and Side Effects By Day (Naltrexone 2mg/day twice daily)
Day 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24
Cervical Pain 8 3 3 3 3 2 2 2 2 2 2 2 2 2
2 2 2 2 2 2 2 4 4 8
Anxiety &
Irritability 0 6 6 6 6 6 6 6 6 6 6 6 6 6 6 0 0 0 0 0 0 0 0 0
Naltrexone
Daily Dose 0 4 4 4 4 4 4 4 4 4 4 4 4 4 4
0 0 0 0 0 0 0 0 0
The Case of a Human Subject using Naltrexone (12.5 mg/day) for treatment of
cervical
pain.
[00272] A second subject used 12.5 mg per day of naltrexone for cervical
pain. Table
26 tabulates the change of pain and enjoyment of life for this patient (on
Naltrexone
12.5mg/day) by day. The drawing in Figure 23 graphs the change of pain and
enjoyment of
life for this patient by day. As with the subject above (on low-dose
Naltrexone), he too
experienced relief of his pain, but this patient (on 12.5 mg Naltrexone/day)
elected to
discontinue the treatment after 10 days because of adverse effects. At this
medium dose,
naltrexone caused interference with his ability to experience pleasure,
particularly from food
and alcohol. In addition, his libido was reduced, and he stated that he felt
"blah".
Table 26 Example 2 Pain and Side Effects by Day (Naltrexone 12.5mg/day)
Day 1 2 3 4 5 6 7 8 9 10 11 12 13 14
Cervical Pain 6 3 3 3 3 3 3 3 3 3 3 3 3
3
Enjoyment
of Life 10 1 1 1 1 1 1 1 1 1 1 1 1
10
Naltrexone
Daily Dose 12.5 12.5 12.5 12.5 12.5
12.5 12.5 12.5 12.5 12.5 12.5 12.5 0 0
Example 7: Validation of Use of Dextro Enantiomer of Naltrexone
The Investigational product: ATNC05
[00273] ATNC05 is the code name for the investigational product discussed
in this
application. It consists of capsules containing naltrexone 2.25 mg and
clonidine 0.025 mg to
be administered orally for neuropathic lower back pain as 1 to 2 capsules
taken twice daily.
[00274] Naltrexone was approved for opioid addiction in 1984 and for
alcohol
dependence in 1995. A dose of 50 mg once daily is recommended for most
patients.
Clonidine, a direct acting alpha 2 adrenergic agonist and an imidazoline
receptor agonist, was
77
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
approved for hypertension in 1974 and in an extended release form for the
treatment of
attention deficit hyperactivity disorder in 2009. The therapeutic doses most
commonly
employed for clonidine have ranged from 0.2 mg to 0.6 mg per day given in
divided doses.
[00275] ATNC05 represents a low dose of naltrexone and clonidine. At the
anticipated
therapeutic dose of ATNC05, two capsules per day, the dose of naltrexone is 9%
of the
recommended dose for the approved indication, and the dose of clonidine is 8%
(0.05 mg/0.6
mg) of the upper range of the common therapeutic dose for the approved
indications. In some
cases, as demonstrated by the preliminary data, a higher dose of ATNC05 was
needed. At a
dose of four capsules per day, the doses of naltrexone and clonidine are 18%
and 16% of the
approved doses, respectively.
[00276] Dextro-naltrexone ((+)-naltrexone) blocks only the TLR-4 while not
blocking
the morphine receptors. Therefore, the side effects arising from blocking of
the morphine
receptors by the racemic naltrexone (a mix of (-)-naltrexone and (+)-
naltrexone), which are
caused by (-)-naltrexone, are eliminated by use of (+)-naltrexone.
Mechanism of Action and Scientific Rationale of the Investigational Product
Preliminary Clinical Results with ATNC05 for Neuropathic Lower Back Pain
[00277] A Phase II study was conducted using ATNC05 for neuropathic back
pain
under IND 110491. The Clinical Study Report for this trial was submitted to
the Division of
Anesthesia, Analgesia, and Addiction Products on July 7, 2013. The study was a
78-subject
double-blind placebo-controlled randomized proof-of-concept clinical trial.
Following a one-
week Baseline period, subjects were administered ATNC05 or placebo for three
weeks. The
study had an open-label extension phase for non-responders in the double-blind
phase, during
which all subjects received ATNC05 with no comparator. Missing data were
imputed by the
Baseline Observation Carried Forward (BOCF) methodology.
Primary Endpoint
[00278] The primary end point for the study was the change from baseline to
Week 3
in Average Pain. For the placebo group, pain was reduced by 0.91 points. For
the ATNC05
arm, pain was reduced by 3.69 points. The treatment effect size is 2.78
1.351 (99%
confidence interval), which is significant, with p<0.00001. The analysis is
summarized in
Table 27.
Table 27: Primary End Point - Change in Average Pain in Week 3
Imputation Treatment N Mean Average Pain Intensity ¨ Week 3
78
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Change (Standard
Baseline WK3 Error) p-value
BOCF Placebo 34 5.50 4.59 -0.91 (0.36) 0.00000
BOCF ATNC05 44 5.55 1.86 -3.69 (0.34)
Groups All Groups in Double Blind Phase
Method t-Test, 2-sided
P Value <0.00001
Mean Difference -2.78
Standard Error 0.35
99% Confidence Interval (-4.131 to -1.429)
Immediate Effect of ATNC05 on Neuropathic Back Pain
[00279] As part of the post-initial dose office assessment, subjects
reported their Right
Now Pain Severity score one to four hours after the initial dose (on average,
two hours). The
change from the baseline period mean was compared. For the placebo group, pain
was
reduced by 1.96. For the ATNC05 arm, pain was reduced by 3.88. The treatment
effect size is
1.92 1.488 (99% confidence interval), which is significant, with p<0.00001
(Figure 24,
Table 28).
Table 28: Mean change from base line to Post-Initial Dose in Right Now Pain
Intensity
Mean Right Now Pain Intensity ¨ Post-Initial Dose
Change (Standard
Imputation Treatment N Baseline WK1 Error) p-value
BOCF Placebo 34 6.61 4.37 -1.96 (0.39) 0.00000
BOCF ATNC05 44 6.60 2.44 -3.88 (0.38)
Groups All Groups in Double Blind Phase
Method t-Test, 2-sided
P Value <0.00001
Mean Difference -1.92
Standard Error 0.39
99% Confidence Interval (-3.408 to -0.432)
79
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[00280] This indicates that ATNC05 causes immediate significant relief of
neuropathic
back pain. By contrast, other treatments for neuropathic lower back pain take
weeks to
provide relief.
Subject Global Impression of Improvement (PGI-I)
[00281] The Patient Global Impression ¨ Improvement (PGI-I) asks subjects
to report
"what percent [pain] relief has the study medication provided," compared to
the subject's
pain before treatment. The item is scored on a percentage scale, where 0%
corresponds to no
relief, and 100% corresponds to total relief. Subjects with missing data were
not imputed
because there was no baseline measure of PGI-I.
[00282] Figure 25 shows the response rates as measured by PGI-I during
Week 3
during both the double-blind and open-label phases. The sections on the x-axis
indicate
ranges of improvement. The columns in the charts are a histogram of the number
of subjects
with a mean PGI-I in Week 3 in each range. The lines indicate the cumulative
distribution of
the subjects; it shows the percentage of subjects whose score is in that range
or one greater.
[00283] Table 29 shows the response rates across study phases. The
percent indicates
the percentage of subjects reporting improvement of the threshold or greater.
Table 29: Summary of Response Rates based on Week 3 PGI-I
:777
Response A'ENC05 Placebo Open-Label
'lives hold ol Response Respons& Responsa:
Improvement Rate (n=40) Rate (n=30) Rate (n=23)
35/40 6/30 22/23
=
.=
(87.50%) (20.00%) (95.70%)
33/40 6/30 20/23
.==
(82.50%) (20.00%) (87.00%)
28/40 6/30 12/23
(70.00%) (20.00%) (52.20%)
[00284] ATNC05 subjects had a high improvement in PGI-I. 7 out of 10 of
ATNC05
subjects reported 90% or greater improvement, 8 out of 10 subjects reported
70% or greater
improvement, and nearly 9 out of 10 subjects reported of 50% or greater
improvement during
Week 3 of the double-blind phase. Subjects showed even stronger improvement in
the Open-
Label phase, where nearly 9 out of 10 subjects reported 70% or greater
improvement, and
95.7% of subjects reported improvement of 50% or greater. By comparison, 2 out
of 10
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
placebo subjects reported 70% or greater improvement, consistent with the
placebo effect
size observed in other studies.
[00285] Of the 40 ATNC05 subjects who completed the double-blind phase, 4
subjects
were non-responders. Three of these subjects responded to ATNC05 in the open-
label phase.
It is likely that these subjects would have had a faster response to ATNC05
with a higher
dose.
[00286] All 18 placebo subjects (non-responders in the double-blind phase)
who
completed the open-label phase responded to ATNC05. This indicates a response
rate of
98.2% (57/58) among the subjects who completed a three-week treatment period
with
ATNC05 in either double-blind or open-label phase.
Brief Pain Inventory Measures
[00287] The following tables and charts are excerpted from the Clinical
Study Report
in order to demonstrate that ATNC05 has a significant treatment effect and
represents a
substantial improvement when compared to published data on available
therapies.
BPI-Severity Measures
[00288] The groups had nearly identical Baseline Period Mean Average Pain
Scores;
5.50 for placebo subjects and 5.55 for ATNC05 subjects. On the second day of
the treatment
period, subjects receiving ATNC05 already showed a large and statistically
significant
improvement in Average Pain scores. On every day of the treatment period,
subjects on
ATNC05 reported a statistically significant treatment effect compared to the
placebo group.
On each day during the main treatment period of Study Days 2-21, the p-value
was less than
0.0001.
[00289] Table 30 shows the daily self-reported mean Average Pain Scores for
subjects
starting on Study Day 2 during the Double-Blind phase (Average Pain is
measured over the
previous 24 hours, and taking this measure on Day 1 would have included time
in the
Baseline Period; Day 1 data were discussed earlier). The p-value column shows
the result of a
Student's t-test comparing the Placebo Treatment Effect and the ATNC05
Treatment Effect.
Please note that data were not imputed by BOCF during the taper-off periods
(days 22-24).
[00290] Figure 26 shows the daily mean Average Pain Scores by day with
standard
error bars. The Baseline Period Mean is shown on the chart as Day 0.
81
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
[00291] Table 31 shows the mean weekly pain scores for each of the five
back pain
severity measures. For each pain measurement and period, the p-value is based
on a Student's
t-test comparing the groups' mean change in the score from the Baseline Period
Mean for that
score. For all groups in all time-frames, ATNC05 subjects had significantly
greater
improvement than the placebo subjects. In the main treatment period (Week 1,
Week 2, and
Week 3), the improvement has a p-value of <0.00001.
[00292] Figure 27 shows the summary of weekly pain severity scores in
graphical form
for subjects in the placebo treatment group. It shows the five severity
measures for each week
of the treatment period.
[00293] Figure 28 shows the same summary graph for subjects receiving
ATNC05. As
illustrated, placebo group subjects do not show a strong trend of improvement,
while the
ATNC05 subjects show a strong downward trend in all the pain severity
measures.
Table 30: Mean Average Pain Scores of ATNC05 and Placebo by Day (BOCF)
N of Subjects
N of Subjects Receiving Mean (SE)
Receiving ATNC05 (n of Mean (SE) Average Pain
Placebo (n of subjects Average Pain Score for Placebo
ATNC05
Study subjects Imputed Imputed by Score for Placebo ATNC05
Treatment Treatment
Day by BOCF) BOCF) Subjects Subjects Effect Effect p-
value
2 34 (1) 44 (0) 4.77 (0.33) 3.09(0.29) -0.72 -
2.49 0.00029
3 34 (1) 44 (2) 4.63 (0.33) 3.07(0.29) -0.87 -
2.51 0.00072
4 34 (1) 44 (2) 4.74(0.34) 2.77(0.29) -0.75 -
2.81 0.00003
34 (2) 44 (2) 4.59 (0.33) 2.84 (0.31) -0.90 -2.74
0.00020
6 34 (3) 44 (1) 4.51 (0.35) 2.31 (0.28) -0.98 -
3.27 0.00001
7 34 (3) 44 (2) 4.60 (0.37) 2.48(0.29) -0.90 -
3.10 0.00003
8 34 (3) 44 (2) 4.83 (0.38) 2.59(0.32) -0.66 -
2.99 0.00003
9 34 (4) 44 (5) 4.69 (0.37) 2.24(0.32) -0.81 -
3.33 0.00000
34 (4) 44 (3) 4.66 (0.36) 2.14 (0.31) -0.84 -3.44
0.00000
11 34 (4) 44 (3) 4.66(0.34) 2.14(0.32) -0.84 -
3.44 0.00000
12 34 (4) 44 (2) 4.66 (0.36) 1.89 (0.33) -0.84 -
3.69 0.00000
13 34 (4) 44 (2) 4.78 (0.39) 1.86(0.32) -0.72 -
3.72 0.00000
14 34 (4) 44 (3) 4.86(0.42) 1.84 (0.33) -0.63 -
3.74 0.00000
34 (4) 44 (4) 4.69(0.41) 1.89(0.32) -0.80 -3.69
0.00000
16 34(4) 44(5) 4.61 (0.41) 1.82 (0.34) -0.89 -
3.76 0.00000
17 34 (4) 44 (6) 4.69(0.41) 1.86 (0.35) -0.80 -
3.72 0.00000
18 34 (4) 44 (6) 4.55 (0.39) 1.93 (0.34) -0.95 -
3.65 0.00000
19 34 (4) 44 (7) 4.58(0.40) 1.84 (0.36) -0.92 -
3.74 0.00000
34 (4) 44 (7) 4.43 (0.35) 1.93 (0.37) -1.07 -3.65
0.00001
21 34 (5) 44 (7) 4.59 (0.39) 1.93 (0.36) -0.90 -
3.65 0.00000
82
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
1 22 26* 36* 4.42(0.45) 1.14(0.27) -1.07 -
4.44 0.00000
23 18* 28* 3.28(0.56) 1.61 (0.39) -2.22 -
3.97 0.02064
24 13* 24* 3.46(0.69) 1.21 (0.31) -2.03 -
4.37 0.00820
Table 31: Summary of Back Pain Severity Measures by Week (BOCF)
= luseline Week 1 Week 2 Week 3
'Faller-OW.1
F.:.....::U:U:U:um:=:=:=:== placebo 6.61 T 5.19 ..........1-5.36
' 5.25 4.89
= A'11\1(l'05 6.65 3.33 2.65 2.28
1.84
.:
:
.:
'Worst Pain . p-value .i 0.00000 0.00000 0.00000 0.00000
. Placebo 4.91 4.19 4.25 4.23 4.08
..
:
:
:.:
...
= l'I'N(l'05 : 4.64 2.16 1.66 1.41
0.93
.==
:
,east Pain p-value .. 0.00000 0.00000 0.00000 0.00001
Placebo 5.50 4.62 4.72 4.59 4.19
.==
.== .==
..
:
:
...
...
= MN:05 : 5.55 2.57 2.08 1.86 1.30
:
..
:
ifµverage Pain P-value 0.00000 0.00000 0.00000 0.00003
!,---
Placebo 6.61 4.37 4.52 4.44 4.41
..
Night lif4w iit ATN( '05 6.60 2.44 1.98 1.72 1.08
g---
Paw p-value :: 0.00000 0.00000 0.00000 0.00066
iõ 1111111111
==================== ,...--
====================== Placebo 6.61 4.68 4.68 4.66 4.33
:.:
= ATINC05.: 6.65 2.94 2.28 2.02
1.32
:
:
p=light Pain .:... p-value i 0.00000 0.00000 0.00000 0.00000
BPI-Interference Measures
[00294] Table 32 shows the mean weekly pain scores for each of the nine
back pain
interference measures. For each pain measurement and period, the p-value is
based on a
Student's t-test comparing the groups' mean change in the score from the
Baseline Period
Mean for that score.
[00295] Figure 29 shows the summary of weekly pain interference scores in
graphical
form for subjects in the placebo treatment group. Figure 30 shows the same
summary graph
for subjects receiving ATNC05. As illustrated, placebo group subjects do not
show a strong
trend of improvement, while the ATNC05 subjects show a strong downward trend
in all the
pain interference measures.
83
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
Table 32: Summary of Back Pain Interference Measures by Week (BOCF)
Baseline Week I Week 2 Week
3========:::F%-l'aper-:(:)ft
.i:.:=:.:
.:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=
:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:=:. ============ ======
============== .:=:::
6.61 4.56 4.63 4.71 4.64
= NENIC05 :: 6.61 2.52 1.91
1.70 0.90
:
:
( ;client! Activity p-\=3l110 . 0.00000 0.00000 0.00000
0.00018
Placebo . 6.61 3.81 3.83 3.92 4.37
:.
=
:...
=
.. ATN("05 :: 6.67 2.10 1.58 1.41 0.77
:: ..
=
Mood p-value 0.00000 0.00005 0.00002
0.00021
=
=
. Placebo ' 5.02 4.17 4.18 4.28 4.49
:
4.97 2.44 1.84 1.69 0.75
.:
ii Walking. Abilit p-value 0.00000 0.00001 0.00000
0.00000
....
===============================================================================
=======
.Placdo 5.47 4.41 4.46 4.47 4.58
:
.:
:
..=
..
AINC05 5.62 2.58 1.95 1.68 0.89
.:
. .::
:
ii Normal Work. Ind 101119, !louse Work p-value 0.00000 0.00000
0.00000 0.00001
'l
Placebo 6.61 3.35 3.63 3.61 4.16
:: AINC05 6.52 1.78 1.35 1.23 0.98
=
.:
:
.: Relationships with Other People p-value .i: 0.00591 0.00008
0.00003 0.00074
'll ================
14444.1
Placebo . 5.77 4.46 4.41 4.38 4.54
.== = NENIC05 ..i 5.85 2.71 2.07 1.80 1.18
:
:
Sleep Quality p-vall/C . 0.00000 0.00000 0.00000
0.00044
Placebo ' 5.15 4.00 4.18 4.33 4.65
:.
..
:
..=
= ATN("05 5.30 2.13 1.68 1.58
1.35
:
..
:
I ii.loyment oilife p-value 0.00000 0.00001 0.00000
0.00104
Placebo 6.61 4.31 4.33 4.38 4.38
...
=:
:...
=... NINO/5 :: 6.63 2.30 1.71 1.60
1.11
=
.:
....
Standing Ability p-value 0.00000 0.00000 0.00000
0.00081
....
Placebo 5.03
===============================================================================
=====
4.25 4.24 4.33 4.36
:.
..
:
: ,===---
..
= A.11\1(:05 ===.: 5.03 2.37 1.69
1.53 1.11
. .
:
:
::$ittilig Ability: p-value 0.00000 0.00000 0.00000
0.00000
Oswestry Disability Index Results
[00296] The
Oswestry Disability Index (ODI) is a 10 item questionnaire which
measures permanent functional disability caused by back pain. It was measured
during each
office visit, and is measured on a scale of 0 to 100.
[00297] Figure 31
shows the average ODI score for each treatment group during the
Baseline Period, and at the Week 1 and Week 3 visits. The Open-Label results
are shown for
Week 1 and Week 3. Double-Blind period were imputed by BOCF when needed.
During the
baseline period, both Placebo and ATNC05 subjects had mean scores around 40
points. By
84
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Week 3, placebo subjects improved by 4.9 points, while ATNC05 subjects dropped
by 27.5
points. In both Week 1 and Week 3, the difference between the placebo and
ATNC05 groups
was statistically significant, with p-values of 0.00005 and <0.00001,
respectively.
Roland-Morris Low Back Pain and Disability Questionnaire Results
[00298] The Roland-Morris Low Back Pain and Disability Questionnaire is a
24 item
questionnaire which asks the subjects to choose statements about back pain
which apply to
them. The statements cover a variety of impairments caused by back pain. It
was measured
during each office visit, and is measured on a scale of 0 to 24 (where each
statement the
subject agrees with increases the score by one).
[00299] Figure 32 shows the average Roland-Morris score for each treatment
group
during the Baseline Period, and at the Week 1 and Week 3 visits. The Open-
Label results are
shown for Week 1 and Week 3. Double-Blind period were imputed by BOCF when
needed.
In the baseline period, placebo subjects scored a mean of 15.75, while ATNC05
subjects
scored a mean of 14.18. In Week 3, the Placebo subjects scored a mean of
14.98, a change of
0.77. The ATNC05 subjects had a Week 3 mean of 3.85, a change of 10.33 points
from their
baseline period. In the third week of the Open-Label phase, subjects scored a
mean of 1.89
points. In both Week 1 and Week 3, the difference between the placebo and
ATNC05 groups
was statistically significant, with p-values of <0.00001.
Pittsburgh Insomnia Scale Results
[00300] The Pittsburgh Insomnia Scale (PIRS-20) is a 20 item questionnaire
which
measures the difficulty subjects have with sleeping due to pain. It was
measured during each
office visit and is measured on a sale of 0 to 60.
[00301] Figure 33 shows the average PIRS-20 score for each treatment group
during
the Baseline Period, and at the Week 1 and Week 3 visits. The Open-Label
results are shown
for Week 1 and Week 3. Double-Blind period were imputed by BOCF when needed.
In the
baseline period, placebo subjects scored a mean of 36.05, while ATNC05
subjects scored a
mean of 34.81. In Week 3, the Placebo subjects scored a mean of 30.57, a
change of 5.48.
The ATNC05 subjects had a Week 3 mean of 12.88, a change of 21.93 points from
their
baseline period. In the third week of the Open-Label phase, subjects scored a
mean of 6.96
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
points. In both Week 1 and Week 3, the difference between the placebo and
ATNC05 groups
was statistically significant, with p-values of 0.00010 and <0.00001,
respectively.
Ability to Stand on One Leg Results
[00302] Part of the evaluation of back pain included the length of time
subjects are
able stand on one leg. This was measured during office visits. The
investigator postulates that
involvement of the Lumbar 5 dermatome results in difficulty with standing on
one leg; the
Lumbar 5 dermatome innervates the superior gluteal nerve supplying the
musculature
involved in standing. The particular muscles involved with standing are the
gluteus minimus,
gluteus medius, and the tensor fasciae latae. The investigator hypothesizes
that ATNC05 will
improve the duration that subjects are able to stand on one leg. This measure
was taken only
for subjects who had difficulty standing on one leg during the Baseline Period
office visits
(N=20).
[00303] Figure 34 shows the average length of time these subjects were able
to stand
on one leg, in seconds. During the baseline period, both groups have a similar
ability to stand
on one leg. While the placebo group never exceeds 5 seconds of standing on one
leg, by the
end of the first week, ATNC05 subjects are able to stand on one leg for over
24 seconds on
average. By the end of the open-label period, subjects are able to stand on
one leg for over 59
seconds on average.
Open-Label Phase Results
[00304] The study had an Open-Label Extension phase, during which subjects
who did
not respond to the medication (either true non-responders who received ATNC05
or placebo
subjects) were given the opportunity to receive ATNC05 for three weeks. 27
subjects entered
the Open-Label phase, and 21 completed.
[00305] Figure 35 and Figure 36 show the weekly mean BPI-Severity and BPI-
Interference scores, respectively, for subjects during the Open-Label
extension phase. Since
most of the subjects in the Open-Label extension were originally randomized
placebo, the
Baseline Period Means are shown for the placebo group for the various BPI
measures.
Use of Other Pain Medications
[00306] Subjects on ATNC05 took fewer doses of other pain medications than
placebo
subjects throughout the treatment period. In Week 3, ATNC05 subjects took a
mean of 0.5
doses, a reduction from baseline of 4.16 doses, while placebo subjects took
2.87 doses, a
86
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
reduction from baseline of 0.95 doses. Table 33 shows both the total number of
doses taken
and the number per subject as a table. Figure 37 shows the mean number of
doses taken per
week. This indicates that ATNC05 relieved the subjects' symptoms, so they did
not need to
take other pain medications.
Table 33: Doses of Concomitant Analgesics and/or Muscle Relaxants by Week
Baseline Week I Week 2 Week 3
Total Placebo 130 60 68 86
1)oses ATNI('05 205 81 35 20
1)oses per Placebo 3.82 1.88 2.13 2.87
Subject ATNIC05 4.66 1.88 0.88 0.5
Post-Treatment Sustained Effect of ATNC05
[00307] Table 34 shows the results of post-study questionnaires, collected
by in-person
and telephone interviews with subjects after the treatment period. 60 or more
days after
treatment ended. Of 58 patients who completed a treatment with ATNC05, 32 were
available
for this follow up. These data were collected outside of the original
protocol. Responders
analysis was done as a percentage of patients who had the post-study follow-up
and as a
percentage of all ATNC05 treated patients. Reversal is defined as 70% or more
improvement
in the Patient Global Impression of Improvement (PGI-I).
[00308] 40 ATNC05 subjects completed a three-week treatment period in the
double-
blind phase (5 of those subjects also completed the open-label phase). In the
open-label
phase, 18 subjects who had previously received placebo completed the open-
label with
ATNC05. In total, 58 (40+18) subjects completed a three-week treatment period
with
ATNC05, with 5 subjects completing two three-week treatment periods.
[00309] Post-study data gathering was not part of the original protocol and
was
collected on an ad hoc basis. A conservative estimate, taking post-study
responders as a
percentage of all subjects who ever completed a course ATNC05 (N=58), gives a
response
rate of 43% who were 70% or more improved 60 days or more after they finished
their three-
week treatment with ATNC05.
[00310] Taking response rate as the percentage of subjects who answered
post-study
questionnaires (N=32) who were responders gives a response rate of 78%.
[00311] Eleven subjects reported a 100% improvement in back pain more than
two
months after they stopped taking ATNC05. This is a response rate of 19%
(11/58). While
87
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
further study would be required to establish this definitively, this hints
toward ATNC05 being
a potential cure for some neuropathic pain syndromes.
Table 34: Responders Analysis of Mean Subject Improvement (PGI-I) 61 or more
day
post-treatment
Long-term Reversal
Long-term Reversal Patients as a
Patients as a Percent Percentage of
of Patients who Patients who
Reported Post-Study completed a three-
Number of Long-term Follow-up Scores week treatment
with
Reversal Patients (N=32) ATNC05 (N=58)
70% or more
25 78.13% 43.10%
improvement
100% improvement 11 34.38% 18.97%
Safety Evaluation
[00312] In the clinical trial for neuropathic back pain, ATNC05 did not
cause serious
adverse events or death. Mild and transient nausea, dizziness, and dry mouth
were the most
commonly observed side effects in the study. A full safety analysis is
available in Section 12
(pp. 136-153) of the Clinical Study Report dated July 7, 2013.
[00313] Four subjects in the double-blind phase had adverse events that
were
distressing enough to cause subjects to withdraw. Two of these subjects were
placebo
subjects and two were ATNC05 subjects. This corresponds to a dropout rate of
4.5% (2/44)
for the ATNC05 group and 5.8% (2/34) in the placebo group. Additionally, two
subjects
withdrew for adverse events during the open-label phase, corresponding to a
dropout rate of
7.4% (2/27) for all subjects entering the open-label phase. At the worst case,
the dropout rate
is 7.4%, based on the study. The adverse symptoms resolved within two days of
ceasing the
medication, and did not require medical attention. The impact on other vital
signs and
laboratory parameters is minimal to nonexistent.
[00314] The components of ATNC05 have separately been demonstrated to be
safe in
decades of clinical use, and it may be concluded from this study that they are
safe in
combination. Naltrexone and clonidine are present in ATNC05 in a low dose of
less than
88
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
10% of their approved doses for other indications. The mean dose of ATNC05 was
2.18
capsules per day in the double-blind phase, and 2.13 capsules per day when the
taper-off
period is included. Information on doses taken in the study is found on pages
128-131 of the
Clinical Study Report. Dose-dependent adverse effects are therefore less
pronounced in
ATNC05.
[00315] ATNC05 is a product which consists of a low dose of two approved
medications. It has a low adverse events profile and the preliminary data show
it to be well-
tolerated.
Comparison of ATNC05 and other Neuropathic Pain Treatments
Efficacy Comparison
[00316] The data from the Phase II trial showed treatment effect more than
four times
larger than drugs recently investigated for neuropathic pain. If data from the
open-label
extension phase is examined, the effect may be as large as six times greater
than that of the
comparable medications for neuropathic pain.
[00317] Table 35 shows the effect size for ATNC05 compared to two other
recently
studied medications approved for neuropathic pain: Cymbalta (duloxetine) and
Lyrica
(pregabalin). The data are taken from reviewer's analyses from statistical
reviews published
by the Center for Drug Evaluation and Research.
[00318] In all four studies cited in the table, missing data were imputed
by the very
conservative method, Baseline Observation Carried Forward (BOCF). Cymbalta
showed an
effect size of 0.5 points on a pain scale. Lyrica showed effect sizes of 0.6
and 0.9 points.
ATNC05 showed an effect size of 2.8, more than 4.5 times larger than the mean
effect size of
the other treatments.
[00319] In the open-label extension phase of the ATNC05 Back Pain Study,
during
which all subjects received ATNC05, the subjects showed a mean improvement of
4.6 points
at the primary endpoint (Week 3). The open-label extension phase consisted of
subjects who
were non-responders in the Double-Blind phase (most subjects in the extension
phase
received placebo during the Double-Blind phase); 27 subjects began the open-
label extension
phase and 22 subjects were evaluated for the primary end point. Of the
subjects who left the
open-label extension phase, two left due to adverse effects, while three were
lost to follow up.
When compared to the improvement experienced by the placebo group, the effect
size of
ATNC05 becomes 3.7 points, over six times more than the mean for Lyrica and
Cymbalta.
89
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[00320] It is the position of the applicant that the open-label results
more accurately
reflect the true net effect of ATNC05. Seven subjects receiving ATNC05 left
the study during
the double-blind phase. However, most of these subjects did not leave due to
adverse events;
they were lost to follow up. Prior to their departing the study, these
subjects showed similar
improvement to the subjects who completed the treatment period, and had they
remained in
the study, the resulting effect size would have been larger. While the open-
label phase did not
have a direct placebo comparator, most of the subjects in the open-label
extension phase were
randomized to the placebo group in the Double-Blind phase, so their results
provide a good
proxy for any placebo effects they may have experienced.
Table 35: Efficacy of ATNC05 compared to existing treatments for neuropathic
pain
Placebo Subjects Active Drug Placebo Active Drug
Effect Size p-value
Subjects Improvement Improvement
Lyrica (pregabalin)2 ¨ 67 70 -0.3 -1.2 0.9
<0.001
Neuropathic Pain
Associated with Spinal
Cord Injury
ATNC05.4 ¨ Neuropathic 34 44 -0.9 -3.7 2.8
0.00000
Back Pain
Reviewer's Primary Efficacy Analysis, BOCF, HMGC Study in Summary Review for
Regulatory Action, page 11
2 Reviewer's Primary Efficacy Analysis, BOCF, Study 125 in Statistical Review
and Evaluation, page 9
3 Reviewer's Primary Efficacy Analysis, BOCF, Study 1107 in Statistical Review
and Evaluation, page 15
4 Double-blind phase primary endpoint, from Clinical Study Report dated July
7, 2013, "ATNC05, a composition to reduce back pain, A Proof-
of-Concept, Randomized, Double-Blind, and Placebo controlled study with an
Open Label Phase extension". Table 11-17, page 40.
9 During open-label extension phase, missing data were not imputed. Open-label
extension phase is compared to the placebo group from
the Double-Blind phase.
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Safety Comparison
[00321] It is well established that treatments currently available for
neuropathic pain
have limitations. Opioid drugs cause tolerance, dependence and side effects
sufficiently
serious to prompt recent action by the FDA to further restrict the drugs.
NSAIDs, taken for a
prolonged periods of time, are known to cause gastro-intestinal bleeding as
well as toxicity to
the liver, kidneys and other organs. Newly approved treatments, like the
calcium channel
alpha-2-delta ligands gabapentin and pregabalin and the serotonin and
norepinephrine
reuptake inhibitors milnacipran and duloxetine, require high doses to show
nominal
effectiveness, have a high dropout rate and carry many side effects.
[00322] In the Phase II trial, ATNC05 was tested for subjects with moderate
chronic
pain (4 points to 8 points on an 11-point scale). Currently available
treatments for moderate
neuropathic pain are NSAIDs, APAP, alpha-2-delta ligands, and serotonin and
norepinephrine reuptake inhibitors. ATNC05 is more effective than NSAIDs and
has fewer
adverse effects. The same is true for APAP, alpha-2-delta ligands, and
serotonin and
norepinephrine reuptake inhibitors. ATNC05 has fewer negative impacts compared
to opioids
used for moderate pain.
Opioid Use and Chronic Neuropathic Back Pain
[00323] The FDA has cited overuse of opioid medication as a serious health
issue.
According to Sharon Hertz, MD, deputy director of FDA's Division of
Anesthesia, Analgesia
and Addiction Products, "There are a limited number of options available for
the treatment of
pain. Opioids are one option, but they carry a significant risk of misuse,
abuse, overdose and
death (FDA Works to Reduce Risk of Opioid Pain Relievers, 2012)."
[00324] Martell et al. (2007) conducted a meta-analysis of opioid use in
chronic back
pain and found that the current prevalence of opioid use disorder associated
with chronic
back pain ranges between 3% and 43%, with a mean of 21%. The differences in
these rates
depend on the criteria used for assessing opioid misuse. Based on these rates
and the
estimated prevalence of chronic neuropathic back pain (estimated as 13.5
million people in an
earlier section), it is estimated that between 405,000 and 5.8 million people
in the United
States misuse opioids due to chronic neuropathic back pain, with a mean
estimate of 2.8
million people.
[00325] Approximately 21% of people with chronic back pain misuse opioids.
Opioids
carry a significant risk of misuse, abuse, overdose and death. By offering a
non-narcotic
alternative for moderate chronic neuropathic lower back pain, ATNC05 has the
potential to
91
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
greatly reduce the risks linked to narcotic medication used in chronic
neuropathic lower back
pain.
Conclusion: ATNC05 as a Breakthrough Therapy
[00326] Chronic neuropathic back pain is a serious condition, and is one of
the leading
causes of disability in the world, as measured by the WHO. The treatment
effect size of
ATNC05 is 4.5 times larger than recently approved treatments for neuropathic
pain
(duloxetine and pregabalin). Most importantly, by offering an effective non-
narcotic
treatment for chronic neuropathic lower back pain of moderate severity, ATNC05
has the
potential to greatly reduce the risks linked to narcotic medication used in
chronic neuropathic
lower back pain because of the lack of effective non-narcotic treatment
options.
[00327] Preliminary data for ATNC05 show reversal of pain after a three-
week course
of treatment, and at least 43% of ATNC05 subjects still had 70% or more relief
of their back
pain two months after ending the three-week treatment course.
[00328] There is a large public health benefit to be realized from meeting
the current
unmet need for an effective non-narcotic treatment for pain.
[00329] Unlike other available treatments for neuropathic back pain, ATNC05
shows
reversal and a "cure" in at least 43% of the subjects in the Phase II trial,
showing 70% or
more improvement of their back pain more than two months after the end of
their three-week
treatment course with ATNC05. This may indicate that the novel mechanism of
action of
ATNC05, TLR-4 antagonism, has a relationship to the pathophysiology of chronic
neuropathic back pain and has the ability to "switch off' the cycle that
perpetuates the
chronicity of neuropathic pain.
[00330] ATNC05 has the potential to revolutionize the treatment of
neuropathic lower
back pain, and alleviate the suffering and economic impact. ATNC05 has the
potential to be a
game-changer for the treatment of back pain and may eliminate the need for
lengthy and
costly treatments with marginal benefit, such as physical therapy and
chiropractic treatment.
Additionally, ATNC05 will most likely reduce the prevalence of spinal surgical
procedures.
[00331] ATNC05 represents an inexpensive treatment option for three primary
reasons. First, it is administered at home and does not have to be
administered by a medical
professional. Second, ATNC05 provides substantial relief after a short course
of treatment,
and this relief endures even after ceasing treatment. Third, even if needed to
be taken for a
92
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
long term, the components of ATNC05 are available generically, so when ATNC05
is
commercialized, the prices will need to be competitive with compounding
variations.
[00332] The applicant states: a) chronic neuropathic back pain is a serious
condition,
b) ATNC05 is superior in efficacy and safety profile to current treatments
(e.g., APAP,
NSAIDs, alpha-2-delta ligands, and antidepressants) and begins to work within
hours of the
initial dose, rather than taking several weeks to reach an effect, c) ATNC05
has a novel
mechanism of action, TLR-4 antagonism, which "switches off' the underlying
cause of
neuropathic pain, d) ATNC05 will be an inexpensive treatment option, since its
components
are generically available, significantly reducing the direct and indirect
costs of chronic
neuropathic back pain, and e) will greatly reduce the need for opioids for
chronic neuropathic
back pain of moderate severity.
Example 8: Preliminary Clinical Results with ATNC05 for Migraine Prevention
[00333] A study was conducted using ATNC05 for neuropathic back pain under
IND
110491. The study was a 78-subject double-blind placebo-controlled randomized
proof-of-
concept clinical trial. Following a one-week Baseline period, subjects were
administered
ATNC05 or placebo for three weeks. The study had an open-label extension phase
for back
pain non-responders in the double-blind phase, during which all subjects
received ATNC05
with no comparator. The Clinical Study Report for this trial was submitted to
FDA on July 7,
2013 and is available upon request. Because this was a proof-of-concept study,
data on
concomitant migraine were collected.
[00334] 23 subjects met the criteria set forth by the International
Headache Society for
migraine (moderate to severe intensity [4 points or greater on an 11-point
scale], occurring
three or more days per month). Ten of these subjects were randomized to
receive placebo,
and 13 were randomized to ATNC05. Nine migraine subjects continued into the
open-label
phase (8 of them were originally randomized placebo, and one was ATNC05). They
were
classified by the investigator's assessment of the IHS criteria during the
initial screening
process.
[00335] These subjects had a mean duration of migraine diagnosis of 20.8
years and a
mean severity of 6 points on an 11-point scale, with no significant difference
between the
treatment groups. Table 36 shows the demographic summary of the migraine
subjects. Table
37 shows the duration and severity of these subjects' migraine diagnosis.
93
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
[00336] During the study, subjects completed daily questionnaires on which
they
recorded headache incidence and intensity. The analysis only considers
migraine days, which
are days where headache intensity was four points or more on an 11-point pain
scale.
Table 36: Demographics of Migraine Subjects
Placebo ATNC05 All
Migraine Migraine Migraine
Subjects Subjects Subjects
13 23
Age (years)
41.5 46.2 44.2
Mean(SD) (12.62) (8.32) (10.42)
Median 39.5 45 43
Range 19 -70 33 -59 19 -70
Gender, n (%)
Female 10 (100) 10 (76.9) 20 (86.9)
Male 0 (0) 3 (23.1) 3 (13.0)
Race, n (%)
White 9 (90) 7 (53.8) 15 (65.2)
Black 0 (0) 2 (15.4) 2 (8.6)
More than One
Race 1(10) 4 (30.7) 5 (21.7)
Ethnicity, n (%)
Hispanic 7 (70) 9 (69.2) 16 (69.5)
Non-Hispanic 3 (30) 4 (30.7) 7 (30.4)
Table 37: Migraine History at Screening ¨ Duration of Condition and Average
Severity
Duraiion in Years Mean (SD) Average Severity tin 11-point scale Mean (SD) NI
Placebo Migraine Subjects 17.9 (11.23) 6.4 (1.17)
10
ATNC05 Migraine Subjects 23.1 (13.62) 5.8 (1.48)
13
All Migraine Sub,jects 20.8 (12.64) 6.0 (1.36)
23
::Open-l.tibel Migraine Subjects 17.1 (9.87) 6.4 (1.33)
9
Migraine Frequency Data from Daily Questionnaire
[00337] Based on the daily questionnaire data, a subject was classified as
having a
Migraine Day on all days upon which the subject reported a headache intensity
of 4 points or
more on an 11-point pain scale (the frequency is filtered by severity). During
the baseline
94
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
period, subjects in the placebo group reported 2.6 migraine days, and ATNC05
subjects
reported 3.85 migraine days.
[00338] In the first week of the treatment period, placebo subjects
reported 1.3
migraine days, a decrease of 1.3 from baseline, and ATNC05 subjects reported
0.15, a
decrease of 3.44 from baseline (Table 38). The difference in the changes from
baseline was
not significant, with p=0.091. This is not because the ATNC05 group did not
improve
(indeed, ATNC05 subjects went from 3.85 to 0.15, a 96% decrease), but because
the placebo
group included two subjects who had a large placebo effect. This distortion is
less likely to
occur in a larger study. ATNC05 started working in the first week.
[00339] In the second week of the treatment period, placebo subjects
reported 1.7
migraine days, an increase from baseline of 0.40, and ATNC05 subjects reported
0.15
migraine days, a decrease from baseline of 3.43 (or 96%). The difference in
the changes from
baseline was highly statistically significant, with p=0.00038 (Table 39).
[00340] In the third week of the treatment period, placebo subjects
reported 1.5
migraine days, a decrease from baseline of 1.10, while ATNC05 subjects
reported 0.0
migraine days, a 100% improvement. The difference in the changes from baseline
in Week 3
is significant with p=0.043 (Table 40).
[00341] Over entire three-week treatment period, placebo subjects reported
2.0
migraine days per week, a decrease of 1.10 migraine days per week from
baseline, and
ATNC05 subjects reported 0.1 migraine days per week, a decrease from baseline
of 3.74
migraine days per week. The number of migraine days in the treatment period
was converted
to weekly frequency by dividing by 3. The difference in the changes from
baseline in the
Treatment Period is significant with p=0.049 (Table 41, Figure 38).
Table 38: Analysis of Change from Baseline in Weekly Frequency To Week 1 In
Migraine Subjects
Weekly Frequency of Migraine Days
Treatment N Baseline Wk 1 Change (Standard Error) p-value
Placebo 10 2.60 1.30 1.30 (1.09) 0.09127
ATNC05 13 3.85 0.15 3.44 (0.82)
Groups All Subjects Migraine Subjects
Method t-Test, 2-sided
P Value 0.09127
Mean Difference 0.091272445
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Standard Error 0.46
95% Confidence Interval (-1.957 to 1.775)
Table 39: Analysis of Change from Baseline in Weekly Frequency To Week 2 In
Migraine Subjects
Weekly Frequency of Migraine Days
Treatment N Baseline Wk 2 Change (Standard Error) p-value
Placebo 10 2.60 1.70 -0.40 (0.34) 0.00038
ATNC05 13 3.85 0.15 3.43 (0.82)
Groups All Subjects Migraine Subjects
Method t-Test, 2-sided
P Value 0.00038
Mean Difference 0.091272445
Standard Error 0.56
95% Confidence Interval (-2.066 to 1.884)
Table 40: Analysis of Change from Baseline in Weekly Frequency To Week 3 In
Migraine Subjects
Weekly Frequency of Migraine Days
Treatment N Baseline WK3 Change (Standard Error) p-value
Placebo 10 2.60 1.50 1.10 (0.85) 0.04284
ATNC05 13 3.85 0.00 3.85 (0.90)
Groups All Migraine Subjects
Method t-Test, 2-sided
P Value 0.04284
Mean Difference -2.75
Standard Error 0.88
95% Confidence Interval (-0.050 to 5.550)
96
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
Table 41: Analysis of Change from Baseline in Weekly Frequency in Entire
Treatment
Period in Migraine Subjects
Weekly Frequency of Migraine Days
Treatment N Baseline Treatment Period Mean Change (Standard Error) p-value
Placebo 10 2.60 2.00 1.10 (0.92) 0.04989
ATNC05 13 3.85 0.10 3.74 (0.86)
Groups All Migraine Subjects
Method t-Test, 2-sided
P Value 0.04989
Mean Difference -2.64
Standard Error 0.89
95% Confidence Interval (-0.209 to 5.489)
Table 42: Migraine Day Incidence Data from Daily Questionnaire, Expressed As
Monthly (Four-Week) Frequency
Baseline Week Week 2 Week
Placebo (N=10) 10.40 ....T 5.20- 6.80 6.00
ATNC05 (N=13) 15.38 0.62 0.62 0.00
)1en-1,abel ( N=9 ) 2.22 0.00 0.00
..::)
[00342] Converting the weekly frequencies to a monthly basis, the evidence
shows that
the ATNC05 migraine subjects went from a mean 15.38 migraine days per month to
nearly
zero throughout three weeks of treatment (Table 42). The reduction started
immediately in
the first week and persisted through the entire treatment period. The placebo
subjects showed
some reduction, attributable mainly to two subjects with a strong placebo
effect. These
subjects showed a strong placebo effect in the back pain parameters in the
study as well.
[00343] Even with the relatively small sample in this study, this
represents a
statistically significant improvement, and reflects the potential of ATNC05 to
be an effective
treatment for chronic migraine prevention. The applicant believes that the
data represents true
and compelling findings.
[00344] As referenced earlier in this section, current treatments for
migraine
prevention tend to only reduce migraine frequency by about 40% in half of
patients. The
preliminary evidence shows that ATNC05 has a larger effect than these
therapies, providing
complete remission of migraine. As part of this project, a meta-analysis will
be undertaken to
97
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
compare the efficacy data from the proposed study and data from studies of
other current
treatments for chronic migraine prevention.
MIDAS
[00345] The Migraine Disability Assessment Test (MIDAS) was used to measure
the
number of migraine days in the three months before the study period and the
three-week
treatment period. It was administered to subjects reporting frequent and
severe migraines
(n=12 ATNC05 subjects). This is a less reliable methodology than daily
questionnaires, since
it depends on subjects' memory, which may be biased.
[00346] Six subjects with >15 headache days per month before the study went
from a
mean of 25.7 headache days per month to a mean of 3.6. Nine subjects with >10
headache
days per month went from a mean of 21.1 headache days per month to mean of
2.4. Twelve
subjects with >6 headache days per month went from a mean of 15.2 headache
days per
month to a mean of 1.8. Subjects with migraine assessed by MIDAS showed
significant
reduction in headache days during the treatment period. This data is in Table
43.
Potential Limitations of the MIDAS Data
[00347] The MIDAS was used on the most severe migraine subjects. The number
of
subjects assessed with MIDAS was too small for statistical analysis (placebo
n=4 and
ATNC05 n=12). However, the reliability of the MIDAS is supported by the fact
that, for the
baseline period, the frequency of migraine reported on the daily questionnaire
(15.38
migraine days per month) and the frequency reported by MIDAS (15.28 migraine
days per
month) differ by only 0.1 days for ATNC05 subjects. The memory bias of the
subjects is
practically non-existent. The applicant believes that the data represent true
and compelling
findings.
Table 43: Summary of Midas-Assessed Subjects with Concomitant Migraine
mums-Assessed Suhjects receiving . \TN( '05
Suhjects with ( or I () ()r more 15 or more
more hezicktchq headtche days::Ne hcadziche
daysWi-
clays L1 lliOfllh. month 111.40,0?,:,,, month
NoFSuhjects 12
9 6
Mean Monthly Frequency in 'three Months Prior:
to Stud, 15.28 21.11 25.67
: Me=an Monthly Frequency during Study Period"' 1.78 2.37
3.56
98
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Percent Improvement in Stud:!, Pciioi 88.3% 88.8% 86.1%
Number of subjects repor1in_2 tern headache
while receiving A IN( '05 10 6 4
* The number of headache days reported during the three-week study period was
multiplied
by 1.33 to get the monthly frequency.
Conclusion of Preliminary Clinical Experience with ATNC05 in Migraine
Prevention
[00348] The preliminary study enrolled 23 migraine subjects in the double-
blind phase
(placebo n = 10, ATNC05 n = 13), with nine of these subjects continuing to the
open-label
phase. Despite the small size of the migraine group (who were a subset of
subjects in a larger
study on back pain), the number of subjects was large enough to determine
statistical
significance of the effect size. ATNC05 provides a statistically significant
reduction in
migraine frequency, compared to placebo.
[00349] Based on the daily questionnaire data, subjects had a reduction of
96% in
migraine frequency in their first and second weeks on ATNC05, and a 100%
reduction in
their third week on ATNC05. The MIDAS assessment shows that migraine subjects
had
reduction of nearly 90% in migraine frequency during the treatment period,
compared to the
three months before the trial.
[00350] In addition to the preventive effect of ATNC05, the investigator
observed
complete resolution of acute migraine upon administration of the first dose of
ATNC05.
Comparison of ATNC05 and other Chronic Migraine Treatments
[00351] A review in Rev. Neurol. (Spain) states that there are only two
treatments
which show efficacy in the prevention of chronic migraine: topiramate
(Topamax) and
pericranial infiltrations of Onabotulinumtcocin A (Botulinum Toxin Type A,
Botox)
(Pascual).Please see Table 44.
[00352] Studies by Silberstein, Lipton, Dodick et al. and Diener et al. for
the treatment
of chronic migraine with topiramate show an effect size of 1.7 and 3.7 fewer
migraine days
per month, respectively. The completion rates in the active treatment group
were 55.8% and
75%, respectively.
[00353] Silberstein, Lipton, Dodick et al. reported that 28.7% of subjects
who
discontinued use of topiramate did so due to lack of efficacy, and 25% of
topiramate
99
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
discontinuations were due to adverse events. A breakdown of reasons for
discontinuation was
not available from Diener et al.
[00354]
Silberstein, Blumenfeld, Cady, et al. studied Onabotulinumtoxin A in chronic
migraine subjects and found an effect size of 2.0 fewer migraine days per
month. The
treatment was well tolerated.
[00355] ATNC05
showed an effect size of 10.56 fewer migraine days per month.
ATNC05 migraine subjects had a 92% completion rate, with only one migraine
subject
discontinuing the study early (on day 15 of the 21-day treatment period) due
to lack of sexual
interest.
[00356] The
preliminary study with ATNC05 showed an effect size of 10.56 fewer
migraine days per month for subjects with migraine and chronic migraine. This
is compared
with effect sizes of 1.7 and 3.7 fewer migraine days in topiramate, and 2.0
with
Onabotulinumtoxin A, the current Standard of Care for chronic migraine
prevention.
[00357] ATNC05
produced fewer side effects than topiramate. In the topiramate
studies, the completion rate was between 55% and 75%. ATNC05 had a 92%
completion rate
for migraine subjects, which indicates that ATNC05 is well-tolerated and
effective compared
to topiramate. Onabotulinumtoxin A has to be administered by a trained
professional. By
contrast, ATNC05 is administered orally at home. The components of ATNC05 are
available
generically, so ATNC05 represents an inexpensive treatment option.
Table 44: Review of Efficacy Studies for Chronic Migraine
Treatment N N Active Placebo Treatme Improveme Improveme Net p- AE
Activ Placeb Completi Completi nt length nt from nt from
Effe value Dropo
e o on Rate on Rate Baseline in Baseline
in ct ut
Migraine Migraine Size
Rate ¨
Days (per Days per Active
month) - Month -
Active Placebo
l'W*.4*.MOMM.Mg AACVE
Ltkt
iiiiiSi.fØ4.rfonigOnngggnnEngnMOnngngEgngn Egngn Ong EgnOnAi:
iiii.:4001111111111.agg.011MMNOOngnOONMgOMMEMMEgNM Egngn Ong Egnngniiiii
Diener et Topiramate 32 27 75% 52% 16 week 3.5 6.3
-0.21-4.1 3.7 <0.05
al. (with and without
Medication
Overuse)
100
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
Toledano6 ATNC05 13 10 92% 100% 3-week 14.96 3.44 4.4 3.68 10.5
0.049 0%
6
Migraine
[00358] The
efficacy of ATNC05 on neuropathic back pain is shown in Table 45. In
the 78-subject study, ATNC05 showed an effect size of 2.78 points (on an 11-
point scale) in
Week 3 of the treatment period.
Table 45: Summary of Results from Phase II Study For Neuropathic Back Pain
Study Treatme N N Active Placebo Treatme Improveme Improveme Net p-value
AE
nt Activ Placeb Completio Completio nt length nt
from nt from Effec Dropou
o n Rate n Rate Baseline in Baseline in t Size
t Rate
Average Average
Back Pain Back Pain Active
Severity at Severity at
Week3- Week3 -
Active Placebo
[00359] The
studies above show that there is an unmet medical need that the current
available treatments for chronic migraine prevention do not adequately
address. ATNC05
provides a large effect size of 10.56 fewer migraine days per month, compared
to 1.7-3.7 for
topiramate, and 2.0 for Onabotulinumtoxin A. ATNC05 is tolerated much better
than
topiramate, as evidenced by the low dropout rate. ATNC05 has a quick onset of
action,
starting immediately (1-2 hours) after the first dose and persisting
throughout the treatment
period. The components of ATNC05 are available generically, so ATNC05
represents an
inexpensive treatment option.
Opioid Use and Chronic Migraine
[00360] The FDA
has cited overuse of opioid medication as a serious health issue.
According to Sharon Hertz, MD, deputy director of FDA's Division of
Anesthesia, Analgesia
and Addiction Products, "There are a limited number of options available for
the treatment of
6 This analysis is based on the subset of migraine subjects in the back pain
study.
7 This summary is of all subjects in the neuropathic back pain study. Missing
data were imputed by Baseline
Observation Carried Forward (BOCF)
101
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
pain. Opioids are one option, but they carry a significant risk of misuse,
abuse, overdose and
death (FDA Works to Reduce Risk of Opioid Pain Relievers, 2012)."
[00361] Buse et al. (2012) studied opioid use and dependence among persons
with
migraine and found 15.9% of migraine sufferers use opioids for acute migraine
symptoms. Of
these, 16.6% meet the standard for opioid dependence. Opioid users reported
greater
disability due to migraine and greater frequency of migraine. Based on the
prevalence figures
cited earlier, it is estimated that between 683,000 and one million people
(15.9% of people
with chronic migraine) in the United States use opioids for chronic migraine,
and between
113,494 and 180,000 of these people (16.6% of chronic migraine patients who
use opioids)
are dependent on opioids. 15.9% of people with migraine use opioids. Opioids
carry a
significant risk of misuse, abuse, overdose and death. By offering a non-
narcotic alternative
for chronic migraine prevention, ATNC05 has the potential to greatly reduce
the risks linked
to narcotic medication used in chronic migraine.
Example 9: Concomitant Joint Pain in Back Pain Clinical Trials with ATMC05
[00362] Figure 41 shows the mean joint pain (for subjects who reported
concomitant
joint pain) during the back pain study. Subjects on ATNC05 reported
significant reductions in
joint pain; subjects reported no joint pain at all during the third week of
the Open-Label
phase.
Example 10: Long-term Pain Relief
[00363] Table 46 shows the results of post-study questionnaires, collected
from the
daily questionnaire forms and telephone interviews. For this analysis, a
subject is classified as
having responded to the treatment if they report 70% or more improvement in
the Patient
Global Impression of Improvement (PGI-I).
[00364] 40 ATNC05 subjects completed a three-week treatment period in the
double-
blind phase (5 of those subjects also completed the open-label phase). In the
open-label
phase, 18 subjects who had previously received placebo completed the open-
label with
ATNC05. In total, 58 (40+18) subjects completed a three-week treatment period
with
ATNC05, with 5 subjects completing two three-week treatment periods.
[00365] Post-study data gathering was not part of the original protocol and
was
collected on a voluntary basis (Table 47, Figure 42). 25 subjects reported 70%
or more
improved 60 days or more after they finished their three-week treatment with
ATNC05. This
102
CA 02942641 2016-09-13
WO 2014/160077 PCT/US2014/025771
number as a percentage of all subjects who completed a course of ATNC05 (N=58)
gives a
response rate of 43%. The response rate as the percentage of subjects who
answered post-
study questionnaires (N=32) is 78%. Accordingly, the post-study reversal of
symptoms
greater than 70% is between 43% and 78%.
[00366] At
least 43% of subjects reported 70% or greater improvement of their back
pain 60 days or more after finishing their three-week treatment period with
ATNC05.
[00367] Eleven
subjects reported a 100% reversal of back pain more than two months
after they stopped taking ATNC05. This is a post-treatment response rate of
19% (11/58).
While further study would be required to establish this definitively, this
hints toward
ATNC05 being a potential cure for some neuropathic pain syndromes.
Table 46: Responders Analysis of Mean Subject Improvement (PGI-I) 61 or more
day
post-treatment
Number Responders as a Percent of Responders as a Percentage
of Subjects who Reported Post- of Subjects who
completed a
Respond Study Follow-up Scores three-week treatment with
ers (N=32) ATNC05 (N=58)
70% or more
25 78.13% 43.10%
improvement
100% improvement 11 34.38% 18.97%
Table 47: Responders Analysis of Mean Subject Improvement (PGI-I) during Post-
Study
Period
30-60 Days Post 61 or More Days
end of Study Post end of Study
Drug Drug
Total Subjects answering Post-Study Questionnaire 27 32
Number of Subjects classified as Responders* 23 27
Responders as a Percent of Subjects who Reported Post-
Study Follow-up Scores 85.19% 84.38%
Responders as a Percent of Initially Randomized
Subjects (N=78) 29.49% 34.62%
Responders as a Percentage of Subjects who Received
ATNC during Double-Blind or Open-Label Phases
(n=71) 37.10% 43.55%
103
CA 02942641 2016-09-13
WO 2014/160077
PCT/US2014/025771
Example 11: Energy and Activity Levels Results
[00368] In the daily pain questionnaire, subjects were asked to respond if
their energy
levels had changed from before the treatment period (options were Increased,
Decreased, and
Stayed the Same). They were also asked to rate if they had been more or less
active in the 24
hours prior to the questionnaire, as compared to before starting the study
drug (subjects
provided a percentage increase or decrease, which was converted to trinary
increase/decrease/stayed the same results).
[00369] Figure 39 shows the subjects' reported change in energy level
during the
treatment period. The percentage is the percentage of questionnaires with the
response in
each group (i.e., the percentage of subject-days reporting the given energy
level). Subjects on
ATNC05 reported increased levels of energy on 70% of subject days, compared to
10% for
subjects in the placebo treatment group.
[00370] Figure 40 shows the subjects' reported change in activity level
during the
treatment period. The percentage is the percentage of questionnaires with the
response in
each group (i.e., the percentage of subject-days reporting the given energy
level). Subjects on
ATNC05 reported an increase in activity of 68% of subject-days during the
Double-Blind
phase and of 75% during the Open-Label phase, compared to 35% for subjects in
the placebo
treatment group. ATNC05 subjects reported a mean percentage increase in
activity level of
more than 50%, while subjects on the placebo reported an increase of 16.78%.
104