Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
3-((Piperazin-1-3TDmethyl)-Phenyl Amide Derivatives and Their Use as Retinoid-
Related
Orphan Receptor Gamma (RORy) Modulators
The present invention relates to novel retinoid-related orphan receptor gamma
(RORy)
modulators and their use in the treatment of diseases mediated by RORy.
Background of the Invention
Retinoid-related orphan receptors (RORs) are transcription factors which
belong to the
steroid hormone nuclear receptor superfamily (Jetten & Joo (2006) Adv. Dev.
Biol. 16:313-355).
The ROR family consists of three members, ROR alpha (RORa), ROR beta (ROM and
ROR
gamma (RORy), each encoded by a separate gene (RORA, RORB and RORC,
respectively). RORs
contain four principal domains shared by the majority of nuclear receptors: an
N-terminal A/B
domain, a DNA-binding domain, a hinge domain, and a ligand binding domain.
Each ROR gene
generates several isoforms which differ only in their N-terminal A/B domain.
Two isoforms of
RORy have been identified: RORyl and RORyt (also known as RORy2). RORy is a
term used to
describe both RORyl and/or RORyt.
While RORyl is expressed in a variety of tissues including thymus, muscle,
kidney and
liver, RORyt is exclusively expressed in the cells of the immune system. RORyt
has been identified
as a key regulator of Th17 cell differentiation. Th17 cells are a subset of T
helper cells which
produce IL-17 and other proinflammatory cytokines. Th17 cells have been shown
to have key
functions in several mouse autoimmune disease models including experimental
autoimmune
encephalomyelitis (EAE) and collagen-induced arthritis (CIA). In addition,
Th17 cells or their
products have been shown to be associated with the pathology of a variety of
human inflammatory
and autoimmune disorders including multiple sclerosis, rheumatoid arthritis,
psoriasis, ankylosing
spondylitis, Crohn's disease and asthma (Jetten (2009) NucLRecept.SignaL 7:
e003; Manel et al.
(2008) Nat. ImmunoL 9:641-649; Miossec & Kolls (2012) Nat
Rev.Drug.Discov.10:763-776). The
pathogenesis of chronic autoimmune diseases including multiple sclerosis and
rheumatoid arthritis
arises from the break in tolerance towards self-antigens and the development
of auto-aggressive
effector T cells infiltrating the target tissues. Studies have shown that Th17
cells are one of the
important drivers of the inflammatory process in tissue-specific autoimmunity
(Steinman (2008) J.
Exp. Med. 205:1517-1522; Leung et al. (2010) Cell. MoL Immunot 7:182-189).
There is evidence
that Th17 cells are activated during the disease process and are responsible
for recruiting other
inflammatory cells types, especially neutrophils, to mediate pathology in the
target tissues (Korn et
al. (2009) Annu. Rev. ImmunoL 27:485-517).
RORyt plays a critical role in the pathogenic responses of Th17 cells (Ivanov
et al. (2006)
Cell 126:1121-1133). RORyt deficient mice show very little Th17 cells. In
addition, RORyt
deficiency resulted in amelioration of EAE. Further support for the role of
RORyt in the
pathogensis of autoimmune or inflammatory diseases can be found in the
following references:
Jetten & Joo (2006) Adv.Dev.BioL 16:313-355; Meier et al. (2007) Immunity
26:643-654; Aloisi &
1
Date Recue/Date Received 2021-10-08
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Pujol-Borrell (2006) Nat. Rev. Immunol. 6:205-217; Jager et al. (2009) J.
Immuno1.183:7169-7177;
Serafini et al. (2004) Brain Pathol.14:164-174; Magliozzi et al. (2007) Brain
130:1089-1104;
Barnes (2008) Nat.Rev.Immuno1.8:183-192; Miossec & Kolls (2012)
Nat.Rev.Drug.Discov.10:763-
776.
In light of the role RORy plays in the pathogenesis of diseases, it is
desirable to prepare
compounds that modulate RORy activity, which can be used in the treatment of
diseases mediated
by RORy.
Summary of the Invention
The invention is directed to novel RORy modulators and their use in the
treatment of
diseases mediated by RORy. Specifically, the invention is directed to
compounds according to
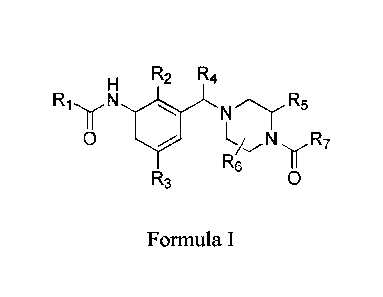
Formula I.
R2 R4
0 R
Re I I
R3 0
Formula I
wherein R1 to R7 are defined below, and to pharmaceutically-acceptable salts
thereof.
The invention is also directed to novel RORy modulators and their use in the
treatment of
diseases mediated by RORy wherein the compounds are according to Formula II:
R2 R4
0
R6 II
R3 0
Formula II
wherein R1 to R7 are defined below, and to pharmaceutically-acceptable salts
thereof.
In another aspect, this invention provides for the use of the compounds of
Formula I and
Formula II for the treatment of diseases mediated by RORy. Examples of such
diseases include
but are not limited to autoimmune or inflammatory diseases such as multiple
sclerosis, rheumatoid
arthritis, psoriasis and ankylo sing spondylitis. In yet another aspect, the
invention is directed to
methods of treating such diseases.
This invention also provides for the use of compounds of Formula I and Formula
II, or a
pharmaceutically acceptable salt thereof of, for the treatment of diseases
mediated by RORy.
Examples of such diseases include but are not limited to autoimmune or
inflammatory diseases such
as multiple sclerosis, rheumatoid arthritis, psoriasis and ankylosing
spondylitis. In yet another
aspect, the invention is directed to methods of treating such diseases.
2
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Detailed Description of the Invention
The present invention provides for, a compound of Formula I or a
pharmaceutically
acceptable salt thereof.
R2 R4
N
RlyJyLNR5
0 N R 7
R6 11
R3 0
Formula I
wherein R1 is:
a) a 6 membered heteroaryl optionally substituted with one substituent
selected from the
group consisting of: C(0)NHRIRf, CN, CHF2 and CH2F, and said RI is
independently
selected from hydrogen or methyl; or
b) a 6 membered heteroaryl substituted with two sub stituents selected from
the group
consisting of: i) F and methyl, ii) CN and methyl, and iii) Cl and methyl; or
c) a methyl substituted with a substituent selected from the group consisting
of:
i) a C4-C6 cycloalkyl substituted with OH or two F;
ii) a 4 to 8 membered heterocycloalkyl optionally substituted one or more
times,
suitably 1 to 2 times, with C(0)Ra, OH, CN, oxo or C1-C3 alkyl, wherein said
heterocycloalkyl containing 1 or 2 heteroatoms independently selected from 0
and N, and said Ra is Cl-C2 alkyl optionally substituted with methyl or ethyl;
iii) a 4 to 6 membered monocyclic sulfone;
iv) oxazolyl or isooxozolyl substituted with methyl;
v) difluoromethoxy or difluoroethoxy; and
vi) NRaSO2CH3, wherein Ra is Cl-C2 alkyl; or
d) a C2-05 alkyl substituted with
i) SO2Rb,
ii) CF3,
iii) CN,
iv) methoxy,
v) OH,
vi) C(0)NRbRb,
vii) NRbRc,
viii) a 5 or 6 membered heterocycloalkyl containing one (1) oxygen (0),
ix) a cyclopropyl substituted with CN, or
x) 0-CHF2,
wherein Rb is independently selected from methyl or ethyl, and Re is C(0)CH3;
or
3
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
e) CH2-0-Rd, wherein Rd is a Cl -C4 alkyl optionally substituted with i)
methoxy, ii) CN, iii)
CHF2 or iv) cyclopropyl substituted with CN; or
f) C3-C6 cycloalkyl substituted with a substituent selected from the group
consisting of
i) NHC(0)CH3,
SO2CH3,
vii) OH, and
viii) Cl-C3 alkyl substituted with OH; or
g) a 4 to 6 membered monocyclic heterocycloalkyl containing one (1) nitrogen
(N)
heteroatom, and optionally a second heteroatom which is oxygen (0), wherein
said
heterocycloalkyl is substituted with S02C113 or C(0)Re, and said Re is C1-C3
alkyl; or
h) - tetrahydro-2H-thiopyran 1,1-dioxide;
R2 is C1-C3 alkyl or halo;
R3 is halo or CN;
R4 is H;
R5 is C I -C3 alkyl;
R6 is H or methyl; and
R7 is selected from the group consisting of:
- C3-C6 cycloalkyl optionally substituted with methyl;
- methyl substituted with C3-05 cycloalkyl; and
- C2 or C3 alkyl optionally substituted with two F.
The present invention provides, in one embodiment, a compound of Faimula I or
a
pharmaceutically acceptable salt thereof, wherein RI is:
a) - 6 membered heteroaryl containing 1 or 2 N, wherein said heteroaryl is
optionally
substituted with C(0)NH2 or CN;
b) 6 membered heteroaryl containing 1 or 2N, wherein said heteroaryl is
substituted with i)
F and methyl, or ii) CN and methyl;
c) C2-C3 alkyl substituted with i) SO2Rb, ii) CF3, or iii) CN, wherein Rb
is methyl or ethyl;
or
d) methyl substituted with a substituent selected from the group consisting
of:
i) C4-C6 cycloalkyl substituted with OH or two F;
ii) 4 or 5 membered heterocycloalkyl optionally substituted one or more times,
suitably
1 or 2 times with C(0)Ra, wherein said heterocycloalkyl contains 1 0 or 1 N,
and said
Ra is CI-C2 alkyl;
4
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
R2 is Cl-C3 alkyl;
R3 is halo or CN;
R4 is H;
R5 is C 1 -C3 alkyl;
R6 is H; and
R7 is a C3-C6 cycloalkyl or a methyl substituted with C3-05 cycloalkyl.
The present invention provides in another embodiument for a compound of
Formula Ia or a
pharmaceutically acceptable salt thereof.
R2 R4
N
I I R1NR5
0 N R7
Re I I
R3
Formula Ia
wherein R1 is:
- 6 membered heteroaryl optionally substituted with one substituent selected
from the group
consisting of: C(0)NH2, CN, CHF2 and CH2F;
- 6 membered heteroaryl substituted with two substituents selected from the
group
consisting
of: i) F and methyl, ii) CN and methyl, and iii) Cl and methyl;
- methyl substituted with a substituent selected from the group consisting of:
i) C4-C6 cycloalkyl substituted with OH or two F;
ii) 4 to 8 membered heterocycloalkyl optionally substituted with C(0)Ra, OH,
CN, oxo
or Cl -C3 alkyl, wherein said heterocycloalkyl containing 1 or 2 heteroatoms
independently selected from 0 and N, and said Ra is Cl-C2 alkyl optionally
substituted
with methyl or ethyl;
iii) 4 to 6 membered monocyclic sulfone;
iv) oxazolyl or isooxozolyl substituted with methyl;
v) difluoromethoxy or difluoroethoxy; and
vi)NRaSO2CH3, wherein Ra is C1-C2 alkyl;
- C2-05 alkyl substituted with i) SO2Rb, ii) CF3, iii) CN, iv) methoxy, v) OH,
vi)
C(0)NRbRb, vii) NRbRe, viii) 5 or 6 membered heterocycloalkyl containing 1 0,
ix) cyclopropyl
substituted with CN, or x) 0-CHF2, wherein Rb is methyl or ethyl, and Re is
C(0)C113;
- CH2-0-Rd, wherein Rd is C1-C4 alkyl optionally substituted with i) methoxy,
ii) CN, iii)
CHF2 or iv) cyclopropyl substituted with CN;
5
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
- C3-C6 cycloalkyl substituted with a substituent selected from the group
consisting of:
i) NHC(0)CH3, ii) SO2CH3, iii) OH, and iv) Cl -C3 alkyl substituted with OH;
- 4 to 6 membered monocyclic heterocycloalkyl containing 1 N heteroatom,
wherein said
heterocycloalkyl is substituted with SO2CH3 or C(0)Re, and said Re is Cl-C3
alkyl; or
R1 is tetrahydro-2H-thiopyran 1,1-dioxide;
R2 is C1-C3 alkyl or halo;
R3 is halo or CN;
R4 is H;
R5 is C1-C3 alkyl;
R6 is H or methyl; and
R7 is selected from the group consisting of:
- C3-C6 cycloalkyl optionally substituted with methyl;
- methyl substituted with C3-05 cycloalkyl; and
- C2 or C3 alkyl optionally substituted with two F.
One embodiment of the invention, is a compound of Formula Ia or a
pharmaceutically
acceptable salt thereof, wherein R1 is:
- 6 membered heteroaryl containing 1 or 2 N, wherein said heteroaryl is
optionally
substituted with C(0)NH2 or CN;
- 6 membered heteroaryl containing 1 or 2 N, wherein said heteroaryl is
substituted with i) F
and methyl, or ii) CN and methyl;
- C2-C3 alkyl substituted with i) SO2Rb, ii) CF3, or iii) CN, wherein Rb is
methyl or ethyl;
or
- methyl substituted with a substituent selected from the group consisting of:
i) C4-C6 cycloalkyl substituted with OH or two F;
ii) 4 or 5 membered heterocycloalkyl optionally substituted with C(0)Ra,
wherein said
heterocycloalkyl containing 1 0 or 1 N, and said Ra is Cl -C2 alkyl;
R2 is C1-C3 alkyl;
R3 is halo or CN;
R4 is H;
R5 is C1-C3 alkyl;
R6 is H; and
R7 is i) C3-C6 cycloalkyl or ii) methyl substituted with C3-05 cycloalkyl.
As will be discussed further herein, compounds of Formulas Ia, and Formulas HI-
IX are all
subsets of Formula I. Unless specifically noted to the contrary all of the
following embodiments
are applicable to all compounds of Formula I, Formula Ia and Formulas In
one embodiment,
6
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
the invention relates to the compounds of Formula I, wherein RI is a pyridinyl
substituted with
C(0)NH2, or C(0)NHCH3, or CN. In one embodiment, this invention also relates
to compounds
of any of the above embodiments, wherein RI is pyridinyl substituted with
C(0)NH2, or
C(0)NHCH3, preferably C(0)NH2. In one embodiment, this invention also relates
to compounds
of any of the above embodiments, wherein R1 is pyridinyl substituted with CN.
In one embodiment, the invention relates to the compounds of Formula I,
wherein RI is
methyl substituted with tetrahydrofuran. In one embodiment, this invention
also relates to
compounds of any of the above embodiments, wherein R1 is methyl substituted
with
hydroxycyclohexyl.
In another embodiment, the invention relates to compounds of Formula I wherein
R1 is a
methyl substituted with difluorocyclobutyl. In another embodiment, this
invention relates to
compounds of Formula I wherein R1 is methyl substituted with
acetylpyrrolidinyl. In another
embodiment, this invention relates to compounds of Formula I wherein R1 is a
methyl substituted
with propionylazetidine.
In another embodiment, the invention relates to the compounds of Formula I,
wherein R1 is
an ethyl substituted with CF3. In another embodiment, this invention also
relates to compounds of
Formula I wherein R1 is propyl substituted with CN. In another embodiment,
this invention also
relates to compounds of Formula I, wherein R1 is ethyl substituted with SO2Rb
wherein Rb is
methyl or ethyl. In one embodiment, the Rb in the SO2Rb is methyl.
In one embodiment, the invention also relates to compounds of Formula I
wherein R2 is
methyl.
In one embodiment, the invention also relates to compounds of any of the above
embodiments, wherein R3 is Cl. In one embodiment, this invention also relates
to compounds of
any of the above embodiments, wherein R3 is F. In one embodiment, the
invention also relates to
compounds of any of the above embodiments, wherein R3 is CN.
In one embodiment, the invention also relates to compounds of any of the above
embodiments, wherein R5 is methyl. In one embodiment, the invention also
relates to compounds
of any of the above, wherein R6 is H.
In one embodiment, this invention also relates to compounds of any of the
above
embodiments, wherein R7 is a C3-C6 cycloalkyl. In one embodiment, R7 is
cyclopentyl. In
another embodiment, R7 is cyclohexyl.
In another embodiment, this invention also relates to compounds of any of the
above
embodiments, wherein R7 is a methyl substituted with C3-05 cycloalkyl. In one
embodiment the
C3-05 cycloalkyl is cyclopropyl. In one embodiment, R7 is a methyl substituted
with cyclopropyl.
In one embodiment, a compound of the present invention, or a pharmaceutically
acceptable
salt thereof, is selected from:
7
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-3-
(methylsulfonyl)propanamide;
(S)-N-(5-chloro-3-((4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-4-
(methylsulfonyl)butanamide;
.. (S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-
2-methylpheny1)-3-
(ethylsulfonyl)propanamide;
(S)-N-(5-chloro-34(4-(cyclohexanecarbony1)-3-methylpiperazin-1-ypmethyl)-2-
methylpheny1)-3-
(methylsulfonyppropanamide;
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-
1 0 4,4,4-trifluorobutanamide;
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylphenyl)-2-
(3,3-difluorocyclobutypacetamide;
(S)-N-(5-chloro-3-((4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-4-
cyanobutanamide;
N-(5-chloro-3-a(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
((S)-tetrahydrofuran-3-yflacetamide;
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylphenyl)-2-
((R)-tetrahydrofuran-3-y1)acctamidc;
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
((1s,4R)-4-hydroxycyclohexyl)acetamide;
N-(5-chloro-3-4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin- 1 -yl)methyl)-2-
methylpheny1)-2-
(( 1 r,45)-4-hydroxycyclohexyl)acetamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
cyanoisonicotinamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
cyanoisonicotinamide;
(S)-N-(5-chloro-344-(2-cyclopropylacety1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-6-
cyanonicotinamide;
(S)-N-(5-chloro-3 -44-(2-cyclopropylacety1)-3-methylpiperazin-1-y1)methyl)-2-
methylphenyl)-6-
cyanonicotinamide;
(S)-N-(5-chloro-3-04-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-6-
cyanonicotinamide;
(S)-N-(5-chloro-3-04-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-6-
cyanonicotinamide;
(S)-N-(5-chloro-3-04-(2-cyclopropylacety1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-5-
cyano-6-methylnicotinamide;
8
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-N-(5-chloro-344-(2-cyclopropylacety1)-3-methylpiperazin-l-yOmethyl)-2-
methylphenyl)-5-
cyano-6-methylnicotinamide;
(S)-N-(5-chloro-34(4-(2-cyclopropylacety1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-5-
fluoro-6-methylnicotinamide;
(S)-N-(5-chloro-34(4-(2-cyclopropylacety1)-3-methylpiperazin-1 -yl)methyl)-2-
methylpheny1)-6-
cyano-5-methylnicotinamide;
2-(1-acetylpyrrolidin-3-y1)-N-(34(S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-1-yOmethyl)-5-
fluoro-2-methylphenypacetamide;
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1 -yOmethyl)-2-
methylpheny1)-2-
(1-propionylazetidin-3-ypacetamide;
(S)-N5-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-ypmethyl)-2-
methylphenyppyridine-2,5-dicarboxamide;
(S)-N-(5-cyano-344-(2-cyclopropylacety1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-5-
fluoro-6-methylnicotinamide; or
(S)-N-(5-cyano-34(4-(2-cyclopropylacety1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-5-
fluoro-6-methylnicotinamide.
One aspect of the invention is a pharmaceutical composition comprising a
compound of
Formula I, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable carrier
or diluent.
The present invention provides, in another aspect, a compound of Formula II or
a
pharmaceutically acceptable salt thereof:
R2 R4
RlJNRs
R6 1
CN 0
Formula II
wherein
R1 is
i) 6 membered heteroaryl containing 1 or 2 N, said heteroaryl is
substituted with
methyl or methoxy; or
ii) phenyl substituted with i) CN or ii) methyl substituted with 1 to 3 F;
R2 is Cl -C3 alkyl;
R4 is H;
R5 is Cl -C3 alkyl;
R6 is H; and
9
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
R7 is i) C3-C6 cycloalkyl; or ii) methyl substituted with C3-05 cycloalkyl.
In one embodiment, compounds of Formula II, or a pharmaceutically acceptable
salt, is
selected from:
(S)-N-(5-cyano-34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylphenyl)-6-
methylnicotinamide;
(S)-N-(5-cyano-34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylpheny1)-2-
methylisonicotinamide;
(S)-N-(5-cyano-34(4-(2-cyclopropylacety1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-2-
methylpyrimidine-5-carboxamide;
(S)-N-(5-cyano-34(4-(2-cyclopropylacety1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-6-
methylnicotinamide;
(S)-N-(5-cyano-2-methy1-3-43-methyl-4-(spiro[2.3Thexane-5-carbonyl)piperazin-l-
y1)methyl)pheny1)-6-methylnicotinamide;
N-(3-(((3S)-4-(bicyclo[3.1.0]hexane-3-carbony1)-3-methylpiperazin-l-y1)methyl)-
5-cyano-2-
methylpheny1)-6-methylnicotinamide;
(S)-N-(5-cyano-34(4-(2-cyclopropylacety1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-6-
methoxynicotinamide;
(S)-N-(5-cyano-34(4-(2-cyclopropylacety1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-6-
methoxynicotinamide;
(S)-N-(5-cyano-344-(cyclobutanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-6-
methylnicotinamide;
(S)-3-cyano-N-(5-cyano-3-44-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylphenyl)benzamide; or
(S)-N-(5-cyano-344-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-2-
methylpyrimidine-5-carboxamide.
One aspect of the invention is a pharmaceutical composition comprising a
compound of
Formula II, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable carrier
or diluent.
In one embodiment, the invention also relates to compounds of Formula II
wherein R2 is
methyl.
In one embodiment, the invention also relates to compounds of Formula II
wherein R5 is
methyl.
In one embodiment, this invention also relates to compounds of Formula II
wherein R7 is a
C3-C6 cycloalkyl. In one embodiment, R7 is cyclopentyl. In another embodiment,
R7 is
cyclohexyl.
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
In another embodiment, this invention also relates to compounds of Formula II
wherein R7
is a methyl substituted with C3-05 cycloalkyl. In one embodiment the C3-05
cycloalkyl is
cyclopropyl. In another embodiment the C3-05 cycloalkyl is cyclopentyl
The present invention provides, in another aspect, a compound of Formula III
or a
pharmaceutically acceptable salt thereof:
R2 R4
Ri N
N R5
0 R 7
R6 I
R3 0
Formula III
wherein
RI is a 6 membered heteroaryl ring optionally substituted with one substituent
selected from
the group consisting of C(0)NRIRf, CN, CHF2 and CH2F;
Rf is independently selected from hydrogen or methyl;
R2 is Cl-C3 alkyl or halo;
R3 is halo or CN;
R4 is H;
R5 is Cl-C3 alkyl;
R6 is H or methyl; and
R7 is selected from the group consisting of:
- a C3-C6 cycloalkyl optionally substituted with methyl;
- a methyl substituted with C3-05 cycloalkyl; and
- a C2 or C3 alkyl optionally substituted with two F.
Suitably, for a compound of Formula III, R1 is a 6 membered heteroaryl ring
optionally
substituted with one substituent selected from the group consisting of
C(0)N1211tf, CN, CHF2 and
CH2F. In one embodiment, Rf is independently selected from hydrogen or methyl.
In one
embodiment both Rf groups are hydrogen. In another embodiment one of Rf is
hydrogen and the
other is methyl. In another embodiment both Rf groups are methyl.
In one embodiment, the 6 membered heteroaryl R1 ring is a pyridinyl or a
pyrimidinyl ring.
In another embodiment, the RI ring is a pyridine. In another embodiument the
R1 ring is a
pyridimine ring.
In one embodiment the pyridine ring is a 2-pyridinyl, a 3-pyridinyl or a 4-
pyridinyl ring
which is substituted. In one embodiment the 3-pyridinyl ring (also called a
nicotinamide when
coupled with the adjacent amide linkage), and is substituted in the 6-position
by cyano or C(0)N112
or C(0)NHCH3. In another embodiment the pyridine ring is an isonicotinamde and
substituted in
the adjacent 2-position by cyano.
11
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Suitably, for a compound of Formula III, R7 is a C3-C6 cycloallcyl optionally
substituted
with methyl. The C3-C6 cycloalkyl which may be optionally substituted is
suitably a cyclopropyl
or cyclopentyl ring. In one embodiment the R7 ring is a cyclopentyl ring. In
another
embodiment, R7 is a cyclopropyl ring.
Suitably, for a compound of Formula III, R7 is methyl substituted with C3-05
cycloallcyl.
Suitably, the C3-05 ring is a cyclopropyl or a cyclopentyl ring. In one
embodiment, R7 is a
cyclopropylmethyl.
Suitably, for a compound of Formula III, R7 is a C2 or C3 alkyl optionally
substituted
with two F, the alkyl chain is a straight of branched C3 carbon chain, such as
propyl or isopropyl.
In one embodiment the first carbon in the chain attached to the carbonyl is
the one optionally
substituted with two fluorines.
Suitably, for compounds of Formula III, R2 is Cl -C3 alkyl or halo. In one
embodiment,
R2 is a Cl-C3. In another embodiment, R2 is methyl.
Suitably, for compounds of Formula III, R3 is halo or CN. In one embodiment,
R3 is halo.
In another embodiment, R3 is fluorine or chlorine. In one embodiment, R3 is
fluorine. In
another embodiment, R3 is chlorine. In another embodiment, R3 is cyano. In one
embodiment,
when R3 is fluorine, chlorine or cyano, R2 is methyl.
Suitably, for compounds of Formula III, R5 is Cl-C3 alkyl. In one embodiment
R5 is
methyl.
Suitably, for compounds of Formula III, R6 is H or methyl. In one embodiment,
R6 is
hydrogen.
In one embodiment, a compound of the Formula III, or a pharmaceutically
acceptable salt
thereof is selected from:
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-2-
cyanoisonicotinamide;
(S)-N-(5-chloro-344-(2-cyclopropylacety1)-3-methylpiperazin-l-yOmethyl)-2-
methylpheny1)-2-
cyanoisonicotinamide;
(S)-N-(5-chloro-344-(2-cyclopropylacety1)-3-methylpiperazin-l-yOmethyl)-2-
methylpheny1)-6-
cyanonicotinamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylphenyl)-6-
cyanonicotinamide;
(S)-N5-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylphenyl)pyridine-2,5-dicarboxamide;
S)-N5-(3-44-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-5-fluoro-2-
methylphenyl)pyridine-2,5-dicarboxamide;
(S)-N5-(5-chloro-344-(2-cyclopropylacety1)-3-methylpiperazin-l-y1)methyl)-2-
methylphenyl)pyridine-2,5-dicarboxamide;
12
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-1\15-(34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-5-fluoro-
2-methylpheny1)-
N2-methylpyridine-2,5-dicarboxamide;
(S)-N5-(344-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-5-fluoro-2-
methylpheny1)-
N2-methylpyridine-2,5-dicarboxamide;
(S)-N-(5-cyano-344-(2-cyclopropylacety1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-6-
(fluoromethypnicotinamide;
(S)-N-(5-cyano-34(4-(2-cyclopropylacety1)-3-methylpiperazin-l-yOmethyl)-2-
methylphenyl)-6-
(fluoromethypnicotinamide;
(S)-N-(5-cyano-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylphenyl)-3-
(difluoromethypbenzamide;
(S)-N-(5-cyano -34(4-(cyclopentanecarbony1)-3-methylpiperazin- 1 -ypmethyl)-2-
methylpheny1)-2-
(difluoromethypisonicotinamide; or
(S)-N-(5-cyano-344-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylphenyl)-2-
(difluoromethyl)isonicotinamide.
One aspect of the invention is a pharmaceutical composition comprising a
compound of
Formula III, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable
carrier or diluent.
The present invention provides, in another aspect, a compound of Formula IV or
a
pharmaceutically acceptable salt thereof
R2 R4
R5
I I
0 R 7
R6 I I
R3 0
Formula IV
wherein R1 is a 6 membered heteroaryl ring substituted with two substituents
selected from
the group consisting of: i) F and methyl, ii) CN and methyl, and iii) Cl and
methyl;
R2 is Cl-C3 alkyl or halo;
R3 is halo or CN;
R4 is H;
R5 is C1-C3 alkyl;
R6 is H or methyl; and
R7 is selected from the group consisting of a C3-C6 cycloalkyl optionally
substituted with
methyl; a methyl substituted with C3-05 cycloalkyl; and a C2 or C3 alkyl
optionally
substituted with two F.
13
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Suitably for a compound of Formula IV, R1 is a 6 membered heteroaryl ring
substituted
with two substituents selected from F and methyl. In another embodiment, R1 is
a 6 membered
heteroaryl ring substituted with two substituents selected from CN and methyl.
In another
embodiment R1 is a 6 membered heteroaryl ring substituted with two
substituents selected from Cl
.. and methyl.
In one embodiment, the 6 membered heteroaryl R1 ring is a pyridinyl or a
pyrimidinyl ring.
In another embodiment, the R1 ring is a pyridine. In another embodiument the
RI ring is a
pyridimine ring.
When R1 is a pyridinyl, it is suitably di-substituted in the 5, 6 position
(the core being
called a nictoinamide derivative). In one embodiment the methyl is in the 6-
position. In another
embodiment, the methyl is in the 5-position.
Suitably, for a compound of Formula TV, R7 is a C3-C6 cycloalkyl optionally
substituted
with methyl. The C3-C6 cycloalkyl which may be optionally substituted is
suitably a cyclopropyl
or cyclopentyl ring. In one embodiment the R7 ring is a cyclopentyl ring. In
another
embodiment, R7 is a cyclopropyl ring.
Suitably, for a compound of Formula IV, R7 is methyl substituted with C3-05
cycloalkyl.
Suitably, the C3-05 ring is a cyclopropyl or a cyclopentyl ring. In one
embodiment, R7 is a
cyclopropylmethyl.
Suitably, for a compound of Formula IV, R7 is a C2 or C3 alkyl optionally
substituted
.. with two F, the alkyl chain is a straight of branched C3 carbon chain, such
as propyl or isopropyl.
In one embodiment the first carbon in the chain attached to the carbonyl is
the one optionally
substituted with two fluorines.
Suitably, for compounds of Formula IV, R2 is Cl-C3 alkyl or halo. In one
embodiment,
R2 is a C1-C3. In another embodiment, R2 is methyl.
Suitably, for compounds of Formula IV, R3 is halo or CN. In one embodiment, R3
is halo.
In another embodiment, R3 is fluorine or chlorine. In one embodiment, R3 is
fluorine. In
another embodiment, R3 is chlorine. In another embodiment, R3 is cyano. In one
embodiment,
when R3 is fluorine, chlorine or cyano, R2 is methyl.
Suitably, for compounds of Formula IV, R5 is Cl-C3 alkyl. In one embodiment R5
is
.. methyl.
Suitably, for compounds of Formula IV, R6 is H or methyl. In one embodiment,
R6 is
hydrogen.
In one embodiment, a compound of the Formula IV, or a pharmaceutically
acceptable salt
thereof is selected from:
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-5-
cyano-6-methylnicotinamide;
14
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-N-(5-chloro-3-44-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-5-
cyano-6-methylnicotinamide;
(S)-5-chloro-N-(5-chloro-344-(2-cyclopropylacety1)-3-methylpiperazin-l-
yOmethyl)-2-
methylpheny1)-6-methylnicotinamide;
(S)-N-(5-chloro-34(4-(2-cyclopropylacety1)-3-methylpiperazin-l-yOmethyl)-2-
methylpheny1)-5-
cyano-6-methylnicotinamide;
(S)-N-(5-chloro-34(4-(2-cyclopropylacety1)-3-methylpiperazin-l-yOmethyl)-2-
methylpheny1)-5-
cyano-6-methylnicotinamide;
(S)-N-(5-chloro-34(4-(2-cyclopropylacety1)-3-methylpiperazin-l-y1)methyl)-2-
methylphenyl)-5-
1 0 fluoro-6-methylnicotinamide;
(S)-5-chloro-N-(5-cyano-3-04-(2-cyclopropylacety1)-3-methylpiperazin-l-
y1)methyl)-2-
methylpheny1)-6-methylnicotinamide;
(S)-5-chloro-N-(34(4-(2,2-difluorobutanoy1)-3-methylpiperazin-l-yl)methyl)-5-
fluoro-2-
methylpheny1)-6-methylnicotinamide;
(S)-N-(5-chloro-3-((4-(2-cyclopropylacety1)-3-methylpiperazin-l-y1)methyl)-2-
methylphenyl)-6-
cyano-5-methylnicotinamide; )
(S)-5-chloro-N-(5-fluoro-3 ((4-isobutyry1-3 -methylpiperazin- 1 -yl)methyl)-2-
methylpheny1)-6-
methylnicotinamide;
(S)-N-(344-butyry1-3-methylpiperazin-1 -yl)methyl)-5 -fluoro-2-methylpheny1)-5-
chloro-6-
methylnicotinamide;
(S)-5 -chloro-N-(5-chloro-344-(cyclopropanecarbony1)-3 -methylpiperazin- 1 -
yl)methyl)-2-
methylpheny1)-6 -methylnicotinamide;
(S)-N-(5-cyano-344-(2-cyclopropylacety1)-3-methylpiperazin-1-yemethyl)-2-
methylpheny1)-5-
fluoro-6-methylnicotinamide;
(S)-N-(5 -cyano -3 #4-(2-cyclopropylacety1)-3-methylpiperazin-1 -yOmethyl)-2-
methylpheny1)-5-
fluoro-6-methylnicotinamide;
(S)-N-(344-butyry1-3-methylpiperazin-1-yOmethyl)-5-chloro-2-methylpheny1)-5-
fluoro-6-
methylnicotinamide;
(S)-5-chloro-N-(344-(2-cyclopropylacety1)-3 -methylpiperazin-1-yOmethyl)-5-
fluoro-2-
methylpheny1)-6-methylnicotinamide; or
(S)-N-(5-chloro-344-(2,2-difluorobutanoy1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-5-
fluoro-6-methylnicotinamide.
One aspect of the invention is a pharmaceutical composition comprising a
compound of
Formula IV, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable
carrier or diluent.
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Suitably, a compound of Formula IV is (S)-N-(5-chloro-3-44-(2-
cyclopropylacety1)-3-
methylpiperazin-l-yOmethyl)-2-methylpheny1)-5-fluoro-6-methylnicotinamide, or
a
pharmaceutically acceoptable salt thereof.
One embodiment of the invention is a pharmaceutical composition comprising (S)-
N-(5-
chloro-344-(2-cyclopropylacety1)-3-methylpiperazin-1-yOmethyl)-2-methylpheny1)-
5-fluoro-6-
methylnicotinamide, or a pharmaceutically acceoptable salt thereof and a
pharmaceutically
acceptable carrier or excipient.
The present invention provides, in another aspect, a compound of Formula V or
a
pharmaceutically acceptable salt thereof
R2 R4
Ri R5
I I
0 K L/NRr
R6 I I
R3
Formula V
wherein
R1 is a methyl substituted with a substituent selected from the group
consisting of:
i) a C4-C6 cycloalkyl substituted with OH or two F;
ii) a 4 to 8 membered heterocycloalkyl ring optionally substituted one or more
times,
suitably 1 or 2 times, with C(0)Ra, OH, CN, oxo or Cl-C3 alkyl, wherein said
heterocycloalkyl contains 1 or 2 heteroatoms each independently selected from
0
and N, and said Ra is a Cl-C2 alkyl optionally substituted with methyl or
ethyl;
iii) a 4 to 6 membered monocyclic sulfone;
iv) oxazolyl or isooxozolyl substituted with methyl;
v) difluoromethoxy or difluoroethoxy; and
NRa'SO2CH3, wherein Ra' is a Cl-C2 alkyl;
R2 is Cl -C3 alkyl or halo;
R3 is halo or CN;
R4 is H;
R5 is C1-C3 alkyl;
R6 is H or methyl; and
R7 is selected from the group consisting of
i) a C3-C6 cycloalkyl optionally substituted with methyl;
ii) a methyl substituted with a C3-05 cycloalkyl; and
iii) a C2 or C3 alkyl optionally substituted with two F.
16
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Suitably, for compounds of Formula V, R1 is a methyl substituted with a
substituent selected
from the group consisting of:
i) a C4-C6 cycloalkyl substituted with OH or two F;
ii) a 4 to 8 membered heterocycloalkyl ring optionally substituted with
C(0)Ra, OH, CN,
oxo or C1-C3 alkyl, wherein said heterocycloalkyl contains 1 or 2 heteroatoms
each
independently selected from 0 and N, and said Ra is a Cl-C2 alkyl optionally
substituted with methyl or ethyl;
iii) a 4 to 6 membered monocyclic sulfone;
iv) oxazolyl or isooxozolyl substituted with methyl;
v) difluoromethoxy or difluoroethoxy; and
vi) NRa' SO2CH3, wherein Ra' is a C1-C2 alkyl.
In one embodiment, R1 is a methyl substituted with a C4-C6 cycloalkyl ring
substituted with
OH or two F. In one embodiment, the C4-C6 ring is a cyclohexyl ring. In
another embodiment,
the C4-C6 ring is a cyclobutyl ring.
In one embodiment, R1 is a methyl substituted with a 4 to 8 membered
heterocycloalkyl
ring optionally substituted one or more times, suitably 1 or 2 times, with
C(0)Ra, OH, CN, oxo or
Cl -C3 alkyl; the heterocycloalkyl ring contains 1 or 2 heteroatoms each
independently selected
from 0 and N, and Ra is a Cl-C2 alkyl optionally substituted with methyl or
ethyl. In another
embodiment, the heterocycloalkyl ring is a 4 or 5 membered heterocycloalkyl
ring. The 4 to 5
membered heterocyclic alkyl ring is optionally substituted with C(0)Ra,
wherein said
heterocycloalkyl contains a 0 or a N, and said Ra is C1-C2 alkyl. It is
recognized that the
substitution on the heterocyclic ring may be on a ring carbon or onthe ring
nitrogen, such as in a 1-
acetylpyrrolidin-3-y1 ring, etc.
In one embodiument the 4 to 8 membered heterocycloalkyl ring is a tetrahydro-
2H-pyranyl
ring, such as a tetrahydro-2H-pyran-2-yl, a tetrahydro-2H-pyran-4-yl, or a
tetrahydro-2H-pyran-3-
yl; a tetrahydrofuranyl ring, such as tetrahydrofuran-2-y1 or tetrahydrofuran-
3-y1; a morpholino; a
pyrrolidinyl, such as a pyrrolidin-l-yl, or a pyrrolidin-3-y1; or an
oxazolidinyl ring, such as a
oxazolidin-3-yl.
In one embodiment, the R1 methyl substituted by a 4 to 8 membered
heterocycloalkyl
ring is substituted by oxo. In another embodiment R1 methyl substituted by a 4
to 8 membered
heterocycloalkyl ring is substituted by C(0)Ra. In another embodiment, the R1
methyl
substituted by a 4 to 8 membered heterocycloalkyl ring is disubstituted with
an oxo and a Cl-C3
alkyl, suitably methyl or ethyl. In one embodiment the R1 methyl substituted
by a 4 to 8
membered heterocycloalkyl ring is a pyrrolidnyl ring disubstituted with oxo
and methyl or ethyl.
It is recognized that the nitrogen in the heterocyclic ring may be substituted
by the optional
17
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
substitutent, such as the C1-3 alkyl, e.g. a 1-methyl-2-oxopyrrolidin-3-yl, or
a 1-ethy1-5-
oxopyrrolidin-3-yl.
Suitably, for a compound of Formula V, R7 is a C3-C6 cycloalkyl optionally
substituted
with methyl. The C3-C6 cycloalkyl which may be optionally substituted is
suitably a cyclopropyl
.. or cyclopentyl ring. In one embodiment the R7 ring is a cyclopentyl ring.
In another
embodiment, R7 is a cyclopropyl ring.
Suitably, for a compound of Formula V, R7 is methyl substituted with C3-05
cycloalkyl.
Suitably, the C3-05 ring is a cyclopropyl or a cyclopentyl ring. In one
embodiment, R7 is a
cyclopropylmethyl group.
Suitably, for a compound of Formula V. R7 is a C2 or C3 alkyl optionally
substituted with
two F, the alkyl chain is a straight of branched C3 carbon chain, such as
propyl or isopropyl. In
one embodiment the first carbon in the chain attached to the carbonyl is the
one optionally
substituted with two fluorines.
Suitably, for compounds of Formula V. R2 is C1-C3 alkyl or halo. In one
embodiment,
.. R2 is a Cl-C3 alkyl. In another embodiment, R2 is methyl.
Suitably, for compounds of Formula V, R3 is halo or CN. In one embodiment, R3
is halo.
In another embodiment, R3 is fluorine or chlorine. In one embodiment, R3 is
fluorine. In
another embodiment, R3 is chlorine. In another embodiment, R3 is cyano. In one
embodiment,
when R3 is fluorine, chlorine or cyano, R2 is methyl.
Suitably, for compounds of Formula V, R5 is C I-C3 alkyl. In one embodiment R5
is
methyl.
Suitably, for compounds of Formula V. R6 is H or methyl. In one embodiment, R6
is
hydrogen..
In one embodiment, a compound of the Formula V, or a pharmaceutically
acceptable salt
thereof is selected from:
(S)-N-(344-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-5-fluoro-2-
methylpheny1)-2-
(oxetan-3-yDacetamide;
-yl)methyl)-5-fluoro-2-methylphenyl)-3-
N-(3-(((S)-4 -(cyclopentanecarbony1)-3-methylpiperazin- 1 -yOmethyl)-5-fluoro-
2-methylpheny1)-3-
(tetrahydrofuran-2-y1)propanamide;
N-(3-0(S)-4-(cyclopentanecarbony1)-3-methylpiperazin- l -yOmethyl)-5-fluoro-2-
methylpheny1)-2-
(tetrahydrofuran-3-ypacetamide;
N-(3-4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-5-fluoro-2-
methylpheny1)-2-
(tetrahydrofuran-2-ypacetamide;
18
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-5-fluoro-2-
methylpheny1)-2-
(tetrahydrofuran-2-y1)acetamide;
(S)-N-(34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-5-fluoro-2-
methylpheny1)-2-
(tetrahydro-2H-pyran-4-y1)acetamide;
N-(3-(((S)-4 -(cyclopentanecarbony1)-3-methylpiperazin- 1 -yl)methyl)-5-fluoro-
2-methylpheny1)-2-
(tetrahydro-2H-pyran-2-y1)acetamide;
N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin- 1 -yl)methyl)-5-fluoro-2-
methylpheny1)-2-
(tetrahydro-2H-pyran-2-y1)acetamide;
cis-N-(3-(((S)-4 -(cyclopentanecarbony1)-3-methylpiperazin-1 -yOmethyl)-5-
fluoro-2-
methylpheny1)-2-(3-hydroxycyclopentypacetamide;
N-(3 4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-5-fluoro-2-
methylpheny1)-2-
(3-hydroxycyclohexyl)acetamide;
N-(3 -R(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-5-fluoro-2-
methylphenyl)-2-
(hexahydrofuro [2,3-1)] furan-3-yOacetamide;
(S)-N-(344-(cyclopentanecarbony1)-3-methylpiperazin-1-ypmethyl)-5-fluoro-2-
methylpheny1)-2-
(2-methyloxazol-4-y1)acetamide;
(S)-N-(344-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-5-fluoro-2-
methylpheny1)-2-
(2-methyloxazol-4-ypacetamide;
(S)-N-(344-(cyclopentanecarbony1)-3-methylpiperazin-1 -yl)methyl)-5-fluoro-2-
methylpheny1)-2-
(3-methylisoxazol-5-ypacetamide;
(S)-N-(344-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-5- chloro-2-
methylpheny1)-2-
(3-methylisoxazol-5-yl)acetamide;
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
(tetrahydrofuran-2-y1)acetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
(tetrahydrofuran-2-yDacetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-ypmethyl)-2-
methylpheny1)-2-
(2-methyltetrahydrofuran-3-ypacetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-2-
(2-methyltetrahydrofuran-3-yl)acetamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin- 1 -yl)methyl)-2-
methylpheny1)-2-
(tetrahydro-2H-pyran-4-yDacetamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin- 1 -yOmethyl)-2-
methylpheny1)-2-
(tetrahydro-2H-pyran-4-yDacetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
(tetrahydro-2H-pyran-2-yDacetamide;
19
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylphcny1)-2-
(tetrahydro-2H-pyran-2-y1)acetamide;
(S)-N-(5-chloro-3-((4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
morpholinoacetamide;
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
morpholinoacetamide;
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-4-
hydroxycyclohexanecarboxamide;
N-(5-chloro-3-a(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
(5-methyltetrahydrofuran-3-yl)acetamide;
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3 -methylpiperazin-l-yOmethyl)-2-
methylpheny1)-2-
(5-methyltetrahydrofuran-3-ypacetamide;
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylphenyl)-2-
(tetrahydro-2H-pyran-3-ypacetamide;
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3 -methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
(tetrahydro-2H-pyran-3-y1)acetamide;
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-2-
(2-methyltetrahydro-2H-pyran-4-ypacetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3 -methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
(4-methyltetrahydrofuran-3-ypacetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
(4-methyltetrahydrofuran-3-y1)acetamide;
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin- 1 -ypmethyl)-2-
methylpheny1)-2-
(3-methyltetrahydro-2H-pyran-4-ypacetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin- 1 -yl)methyl)-2-
methylpheny1)-2-
(3-methyltetrahydro-2H-pyran-4-ypacetamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-ypmethyl)-2-
methylpheny1)-2-
(2-oxopyrrolidin-1-yl)acetamide;
(S)-N-(5-chloro-3((4-(cyclopentanecarbony1)-3 -methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
(2-oxopyrrolidin-1-yl)acetamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-
2-(2-oxooxazolidin-3-ypacetamide;
N-(5-chloro-34(S)-4-(2-cyclopropylacety1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
(tetrahydrofuran-3-ypacetamide;
N-(5 -cyano-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
(tetrahydrofuran-3-ypacetamide;
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
(1,1-dioxidotetrahydro-2H-thiopyran-4-yDacetamide;
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
(3-oxomorpholino)acetamide;
(S)-N-(5-chloro-3-((4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
(1,1-dioxidothietan-3-y1)acetamide;
(S)-N-(5-chloro-3-((4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylphenyl)-2-
(3,3-difluorocyclobutyl)acetamide;
(S)-N-(344-(cyclopentanecarbony1)-3-methylpiperazin-1-ypmethyl)-5-fluoro-2-
methylpheny1)-2-
(3,3-difluorocyclobutyl)acetamide;
(S)-N-(5-chloro-3-((4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-4-
cyanobutanamide;
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
((S)-tetrahydrofuran-3-ypacetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1 -yl)methyl)-2-
methylpheny1)-
2-((R)-tetrahydrofuran-3-y1)acetamide;
N-(2,5-dichloro-3-a(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
yl)methyl)pheny1)-2-
(tetrahydrofuran-3-yl)acetamide;
N-(2,5-dichloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
ypmethyl)pheny1)-24S)-
tetrahydrofuran-3-yl)acetamide;
N-(2,5-dichloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
yOmethyl)pheny1)-2-((R)-
tetrahydrofuran-3-y1)acetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-ypmethyl)-2-
methylpheny1)-2-
((1s,4R)-4-hydroxycyclohexyl)acetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-
2-((1r,4S)-4-hydroxycyclohexyl)acetamide;
N-(3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-5-fluoro-2-
methylphenyl)-2-
(( 1 r,4S)-4-hydroxycyclohexyl)acetamide;
N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-5-fluoro-2-
methylpheny1)-2-
((1s,4R)-4-hydroxycyclohexyl)acetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
((S)-1-methyl-5-oxopyrrolidin-3-yl)acetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
((R)-1-methy1-5-oxopyrrolidin-3-ypacetamide;
N-(5-chloro-3-0(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylphenyl)-2-
((S)-1-ethyl-5-oxopyrrolidin-3-y1)acetamide;
21
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
N-(5-chloro-3-4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-ypmethyl)-2-
methylpheny1)-2-
((R)-1-ethyl-5-oxopyrrolidin-3-yDacetamide;
N-(5-chloro-3-4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
((R)-1-methyl-2-oxopyrrolidin-3-yl)acetamide; E69
N-(5-chloro-3-4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-ypmethyl)-2-
methylpheny1)-2-
((S)-1-methyl-2-oxopyrrolidin-3-y1)acetamide;
(S)-N-(5-chloro-34(4-(2-cyclopropylacety1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
(4-hydroxycyclohexypacetamide;
& (S)-N-(5-chloro-3 -04-(2-cyclopropylacety1)-3-methylpiperazin-1 -yOmethyl)-2-
methylpheny1)-2-
(4-hydroxycyclohexyl)acetamide;
cis N-(3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-5-
fluoro-2-
methylpheny1)-2-(2-hydroxycyclopentypacetamide;
trans N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-5-fluoro-
2-
methylphenyl)-2-(2-hydroxycyclopentypacetamide;
Cis (S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-
2-
methylpheny1)-2-(3-hydroxycyclobutypacetamide;
trans (S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-
yl)methyl)-2-
methylpheny1)-2-(3-hydroxycyclobutyl)acetamide;
N-(3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-5-fluoro-2-
methylpheny1)-2-
(2-hydroxycyclohexyl)acetamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
(3-hydroxyazetidin-1-y1)acetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
(1,1-dioxidotetrahydrothiophen-3-ypacetamide;
N-(5 -chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1 -yl)methyl)-
2-methylpheny1)-2-
(1,1-dioxidotetrahydrothiophen-3-yDacetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-2-
(1,1-dioxidotetrahydrothiophen-2-ypacetamide;
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-
(1,1-dioxidotetrahydro-2H-thiopyran-3-yl)acetamide;
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-2-
(1,1-dioxidotetrahydro-2H-thiopyran-3-y1)acetamide;
2-(1-acetylpyrrolidin-3-y1)-N-(3-(((S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-1-yl)methyl)-5-
fluoro-2-methylphenyl)acetamide;
(S)-2-(1-acetylazetidin-3-y1)-N-(5-chloro-344-(cyclopentanecarbony1)-3-
methylpiperazin-1-
yOmethyl)-2-methylphenypacetamide;
22
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-N-(5-chloro-3-44-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-2-
(N-methylmethylsulfonamido)acetamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin- 1 -ypmethyl)-2-
methylpheny1)-2-
(N-methylmethylsulfonamido)acetamide;
2-(1-acetylpyrrolidin-3-y1)-N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-1-
yOmethyl)-2-methylphenypacetamide;
2-(1-acetylpyrrolidin-3-y1)-N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-l-
y1)methyl)-2-methylphenypacetamide;
2-((R)-1-acetylpyrrolidin-2-y1)-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-1-
yOmethyl)-2-methylphenyl)acetamide;
2-((S)-1-acetylpyrrolidin-2-y1)-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-l-
y1)methyl)-2-methylphenypacetamide;
24(S)-l-acetylpiperidin-3-y1)-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-
methylpiperazin- 1 -
yl)methyl)-2-methylphenypacetamide; or
2-((R)-1 -acetylpiperidin-3 -y1)-N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-
3-methylpiperazin-1-
yl)methyl)-2-methylphenyl)acetamide.
One aspect of the invention is a pharmaceutical composition comprising a
compound of
Formula V, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable carrier
or diluent.
The present invention provides, in another aspect, a compound of Formula VI or
a
pharmaceutically acceptable salt thereof:
R2 R4
0 L) LINR7
R6 II
R3 0
Formula VI
wherein R1 is a C2-05 alkyl substituted with
i) SO2Rb,
ii) CF3,
iii) CN,
iv) methoxy,
v) OH,
vi) C(0)NRbRb,
vii) NRbRc
viii) 5 or 6 membered heterocycloalkyl containing an Oxygen
ix) cyclopropyl substituted with CN, or
23
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
x) 0-CHF2,
Rb is independently selected from methyl or ethyl,
Rc is -C(0)CH3;
R2 is a C1-C3 alkyl or halo;
R3 is halo or CN;
R4 is H;
R5 is C1-C3 alkyl;
R6 is H or methyl; and
R7 is selected from the group consisting of
i) a -C3-C6 cycloalkyl optionally substituted with methyl;
ii) a methyl substituted with a C3-05 cycloalkyl; and
iii) a - C2 or C3 alkyl optionally substituted with two F.
Suitably, for a compound of Formula VI, R1 is a C2-05 alkyl substituted with
i) SO2Rb,
ii) CF3,
iii) CN,
iv) methoxy,
v) OH,
vi) C(0)NRbRb,
vii) NRbRc
viii) 5 or 6 membered heterocycloalkyl containing 1 0,
ix) cyclopropyl substituted with CN, or
x) 0-CHF2.
Suitably, Rb is independently selected from methyl or ethyl.
Suitably, Rc is -C(0)CH3.
The R1 C2-05 alkyl carbon chain may be branched or straight. In one embodiment
the
C3-05 alkyl is a branched chain. Suitably, the C2-05 alkyl carbon is ethyl,
propyl, butyl or an
isobutyl.
Suitably, for a compound of Formula VI, R7 is a C3-C6 cycloalkyl optionally
substituted
with methyl. The C3-C6 cycloalkyl which may be optionally substituted is
suitably a cyclopropyl
or cyclopentyl ring. In one embodiment the R7 ring is a cyclopentyl ring. In
another
embodiment, R7 is a cyclopropyl ring.
Suitably, for a compound of Formula VI, R7 is methyl substituted with C3-05
cycloalkyl.
Suitably, the C3-05 ring is a cyclopropyl or a cyclopentyl ring. In one
embodiment, R7 is a
cyclopropyl methyl.
24
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Suitably, for a compound of Formula VI, R7 is a C2 or C3 alkyl optionally
substituted
with two F, the alkyl chain is a straight of branched C3 carbon chain, such as
propyl or isopropyl.
In one embodiment the first carbon in the chain attached to the carbonyl is
the one optionally
substituted with two fluorines.
Suitably, for compounds of Formula VI, R2 is Cl -C3 alkyl or halo. In one
embodiment,
R2 is a Cl -C3 alkyl. In another embodiment, R2 is methyl.
Suitably, for compounds of Formula VI, R3 is halo or CN. In one embodiment, R3
is halo.
In another embodiment, R3 is fluorine or chlorine. In one embodiment, R3 is
fluorine. In
another embodiment, R3 is chlorine. In another embodiment, R3 is cyano. In one
embodiment,
when R3 is fluorine, chlorine or cyano, R2 is methyl.
Suitably, for compounds of Formula VI, R5 is Cl -C3 alkyl. In one embodiment
R5 is
methyl.
Suitably, for compounds of Formula VI, R6 is H or methyl. In one embodiment,
R6 is
hydrogen.
In one embodiment, a compound of the Formula VI, or a pharmaceutically
acceptable salt
thereof is selected from:
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-2-
(tetrahydrofuran-2-y1)propanamide;
N-(5-ehloro-3-(4S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylphenyl)-2-
(tetrahydrofuran-2-yppropanamide;
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylphenyl)-2-
(tetrahydrofuran-3-yl)propanamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylphenyl)-4-
methoxybutanamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylphenyl)-4-
methoxybutanamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin- 1 -yOmethyl)-2-
methylpheny1)-2-
(2-methoxyethoxy)acetamide;
(S)-N-(5-chloro-3-((4-(2-cyclopropylacety1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-4-
methoxybutanamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylpheny1)-3-
(1-cyanocyclopropyppropanamide;
(S)-N-(5-chloro-3 ((4-(cyclopentanecarbony1)-3 -methylpiperazin-1 -yOmethyl)-2-
methylpheny1)-4-
cyano-4-methylpentanamide;
(S)-N-(5-chloro-3 ((4-(eyclopentanecarbony1)-3-methylpiperazin- 1 -yOmethyl)-2-
methylpheny1)-2-
hydroxy-2-methylpropanamide;
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-N-(5-ehloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-3-
hydroxy-3-methylbutanamide;
(S)-N-(5-chloro-344-(eyelopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-3-
(methylsulfonyl)propanamide;
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-4-
(methylsulfonyl)butanamide;
(S)-N-(5-eyano-344-(eyclopentaneearbony1)-3-methylpiperazin-1-ypmethyl)-2-
methylphenyl)-4-
(methylsulfonyl)butanamide;
(S)-N-(5-ehloro-34(4-(eyelopentaneearbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-3 -
(ethylsulfonyl)propanamide;
(S)-N-(5-cyano-3-(0-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylpheny1)-3-
(ethylsulfonyl)propanamide;
(S)-N-(5-cyano-344-(eyelopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-3-
(ethylsulfonyl)propanamide;
(S)-N-(5-chloro-344-(2-cyclopentylacety1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-3-
(methylsulfonyl)propanamide;
(S)-N-(5-chloro-344-(2-cyclopentylacety1)-3-methylpiperazin-1-ypmethyl)-2-
methylpheny1)-3-
(methylsulfonyppropanamide;
(S)-N-(5-ehloro-344-(cyclohexanecarbony1)-3-methylpiperazin-1 -yl)methyl)-2-
methylpheny1)-3-
(methylsulfonyl)propanamide;
(S)-N-(5-chloro-344-(eyelopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-
4,4,4-trifluorobutanamide;
(S)-N-(5-ehloro-344-(eyelopentaneearbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-
5,5,5-trifluoropentanamide;
(S)-N-(5-ehloro-344-(eyelopentaneearbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-4-
eyanobutanamide,
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-4-
cyanobutanamide;
(S)-N-(5-ehloro-3((4 -(2-eyelopropylacety1)-3-methylpiperazin-1 -yl)methyl)-2-
methylpheny1)-4-
cyano-4-methylpentanamide;
(S)-N-(5-ehloro-2-methy1-3-((3-methyl-4-(spiro [2.3]hexane-5-
earbonyl)piperazin-l-
ypmethyl)pheny1)-3-(methylsulfonyl)propanamide;
N-(3 -(((3 S)-4-(bicyclo [3.1.0Thexane-3-carbony1)-3-methylpiperazin-1-
yOmethyl)-5-ehloro-2-
methylpheny1)-3-(methylsulfonyl)propanamide;
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-3-
(N-methylacetamido) propanamide;
26
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylpheny1)-4-
(Nmethylacetamido) butanamide; or
(S)-N1-(5-chloro-3-44-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylphenyl)-
N4,N4-dimethylsuccinamide.
One aspect of the invention is a pharmaceutical composition comprising a
compound of
Formula VI, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable
carrier or diluent.
The present invention provides, in another aspect, a compound of Formula VII
or a
pharmaceutically acceptable salt thereof:
R2 R4
RILI N
0 N R7
R6
R3 0
Formula VII
wherein
R1 is - CH2-O-Rd,
Rd is a Cl-C4 alkyl optionally substituted with i) methoxy, ii) CN, iii) CHF2
or iv) cyclopropyl
substituted with CN;
R2 is Cl-C3 alkyl or halo;
R3 is halo or CN;
R4 is H;
R5 is C1-C3 alkyl;
R6 is H or methyl; and
R7 is selected from the group consisting of
i) a -C3-C6 cycloalkyl optionally substituted with methyl;
ii) a methyl substituted with a C3-05 cycloalkyl; and
iii) a - C2 or C3 alkyl optionally substituted with two F.
Suitably, for a compound of Formula VII, R1 is - CH2-0-Rd, and Rd is a Cl-C4
alkyl
optionally substituted with i) methoxy, ii) CN, iii) CHF2 or iv) cyclopropyl
substituted with CN.
The Rd C1-4 alkyl carbon chain may be branched or straight. In one embodiment,
the C1-C4
carbon chain is methyl or is a methylene group. When the Cl-C4alkyl group of
Rd is substituted
with a cyclopropyl, it is recognized that the cyano may be substituted on the
1-position of the
cyclopropyl ring (the same position substituted to the C1-4 carbon chain).
27
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Suitably, for a compound of Formula VII, R7 is a C3-C6 cycloalkyl optionally
substituted
with methyl. The C3-C6 cycloalkyl which may be optionally substituted is
suitably a cyclopropyl
or cyclopentyl ring. In one embodiment the R7 ring is a cyclopentyl ring. In
another
embodiment, R7 is a cyclopropyl ring.
Suitably, for a compound of Formula VII, R7 is methyl substituted with C3-05
cycloalkyl.
Suitably, the C3-05 ring is a cyclopropyl or a cyclopentyl ring. In one
embodiment, R7 is a
methyl cyclopropyl.
Suitably, for a compound of Formula VII, R7 is a C2 or C3 alkyl optionally
substituted
with two F, the alkyl chain is a straight of branched C3 carbon chain, such as
propyl or isopropyl.
In one embodiment the first carbon in the chain attached to the carbonyl is
the one optionally
substituted with two fluorines.
Suitably, for compounds of Formula VII, R2 is Cl -C3 alkyl or halo. In one
embodiment,
R2 is a Cl-C3 alkyl. In another embodiment, R2 is methyl.
Suitably, for compounds of Formula VII, R3 is halo or CN. In one embodiment,
R3 is halo.
In another embodiment, R3 is fluorine or chlorine. In one embodiment, R3 is
fluorine. In
another embodiment, R3 is chlorine. In another embodiment, R3 is cyano. In one
embodiment,
when R3 is fluorine, chlorine or cyano, R2 is methyl.
Suitably, for compounds of Formula VII, R5 is Cl-C3 alkyl. In one embodiment
R5 is
methyl.
Suitably, for compounds of Formula VII, R6 is H or methyl. In one embodiment,
R6 is
hydrogen..
In one embodiment, a compound of the Formula VI is selected from:
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1 -yl)methyl)-2-
methylpheny1)-2-
(difluoromethoxy)acetarnide;
(S)-N-(5-chloro-3((4-(cyclopentanecarbony1)-3-methylpiperazin- I -yl)methyl)-2-
methylpheny1)-3-
(difluoromethoxy)propanamide;
(S)-N-(5-chloro-34(4-(cyc1opentanecarbony1)-3 -methylpiperazin-l-yOmethyl)-2-
methylpheny1)-2-
((1 -cyanocyclopropyl)methoxy)acetamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin- 1 -yl)methyl)-2-
methylpheny1)-2-
(2,2-difluoroethoxy)acetamide; or
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-2-
(2-cyano-2-methylpropoxy)acetamide.
One aspect of the invention is a pharmaceutical composition comprising a
compound of
Formula VI, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable
carrier or diluent.
28
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
The present invention provides, in another aspect, a compound of Formula VIII
or a
pharmaceutically acceptable salt thereof:
R2 R4
1=21-N
R5
0 N
R6 I
R3 0
Formula VIII
wherein
R1 is a C3-C6 cycloalkyl substituted with a substituent selected from the
group consisting of
i)- NHC(0)CH3, ii) SO2CH3, iii) OH, and iv) Cl-C3 alkyl substituted with OH;
R2 is CI-C3 alkyl or halo;
R3 is halo or CN;
R4 is H;
R5 is C1-C3 alkyl;
R6 is H or methyl; and
R7 is selected from the group consisting of
i) a -C3-C6 cycloalkyl optionally substituted with methyl;
ii) a methyl substituted with a C3-05 cycloalkyl; and
a - C2 or C3 alkyl optionally substituted with two F.
Suitably, for a compound of Formula VIII, R1 is a C3-C6 cycloalkyl substituted
with a
substituent selected from the group consisting of i)- NHC(0)CH3, ii) -S02CH3,
iii) -OH, and iv) a -
Cl-C3 alkyl substituted with OR
Suitably, for a compound of Formula VIII, R7 is a C3-C6 cycloalkyl optionally
substituted with methyl. The C3-C6 cycloalkyl which may be optionally
substituted is suitably a
cyclopropyl or cyclopentyl ring. In one embodiment the R7 ring is a
cyclopentyl ring. In
another embodiment, R7 is a cyclopropyl ring.
Suitably, for a compound of Formula VIII, R7 is methyl substituted with C3-05
cycloalkyl. Suitably, the C3-05 ring is a cyclopropyl or a cyclopentyl ring.
In one embodiment,
R7 is a methyl cyclopropyl.
Suitably, for a compound of Formula VIII, R7 is a C2 or C3 alkyl optionally
substituted
with two F, the alkyl chain is a straight of branched C3 carbon chain, such as
propyl or isopropyl.
In one embodiment the first carbon in the chain attached to the carbonyl is
the one optionally
substituted with two fluorines.
Suitably, for compounds of Formula VIII, R2 is Cl-C3 alkyl or halo. In one
embodiment,
R2 is a Cl-C3 alkyl. In another embodiment, R2 is methyl.
29
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Suitably, for compounds of Formula VIII, R3 is halo or CN. In one embodiment,
R3 is halo.
In another embodiment, R3 is fluorine or chlorine. In one embodiment, R3 is
fluorine. In
another embodiment, R3 is chlorine. In another embodiment, R3 is cyano. In one
embodiment,
when R3 is fluorine, chlorine or cyano, R2 is methyl.
Suitably, for compounds of Formula VIII, R5 is Cl-C3 alkyl. In one embodiment
R5 is
methyl.
Suitably, for compounds of Formula VIII, R6 is H or methyl. In one embodiment,
R6 is
hydrogen..
In one embodiment, a compound of the Formula VIII, or a pharmaceutically
acceptable salt
thereof is selected from:
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-2-
(methylsulfonyl)cyclobutanecarboxamide;
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-3-
(methylsulfonyl)cyclobutanecarboxamide;
(1r,3S)-3-acetamido-N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
y1)methyl)-5-
fluoro-2-methylphenypcyclobutanecarboxamide;
(1r,3S)-3-acetamido-N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
y1)methyl)-5-
fluoro-2-methylphenypeyelobutanecarboxamide;
(1 s,3R)-3-acetamido-N-(3 4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1 -
yl)methyl)-5-
fluoro-2-methylphenypcyclobutanecarboxamide;
(1s,3R)-3-acetamido-N-(3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-5-
fluoro-2-methylphenyl)cyclobutanecarboxamide;
(cis) N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylpheny1)-2-(2-hydroxypropan-2-yl)cyclopropanecarboxamide; or
N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-ypmethyl)-2-
methylpheny1)-2-
(2-hydroxypropan-2-y1)cyclopropanecarboxamide.
One aspect of the invention is a pharmaceutical composition comprising a
compound of
Formula VIII, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable
carrier or diluent.
The present invention provides, in another aspect, a compound of Formula IX or
a
pharmaceutically acceptable salt thereof:
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
R2 R4
R1 R5
0 R7
R6
R3 0
Formula IX
wherein
RI is a 4 to 6 membered monocyclic heterocycloalkyl containing one (1)
nitrogen (N) heteroatom,
and optionally an additional heteroatom which is oxygen (0), wherein said
heterocycloalkyl is
substituted with SO2CH3 or C(0)Re, and said Re is a C1-C3 alkyl; or
R1 is a tetrahydro-2H-thiopyran 1,1-dioxide;
R2 is C1-C3 alkyl or halo;
R3 is halo or CN;
R4 is H;
R5 is C1-C3 alkyl;
R6 is H or methyl; and
R7 is selected from the group consisting of
i) a -C3-C6 cycloalkyl optionally substituted with methyl;
ii) a methyl substituted with a C3-05 cycloalkyl; and
iii) a - C2 or C3 alkyl optionally substituted with two F.
In one embodiment of the invention, R1 is a 4 to 6 membered monocyclic
heterocycloalkyl
containing one (1) nitrogen (N) heteroatom, and optionally an additional
heteroatom which is
oxygen (0), and wherein said heterocycloalkyl ring is substituted with SO2CH3
or C(0)Re, and Re
is a Cl-C3 alkylSuitably when R1 is a 4 to 6 membered monocyclic
heterocycloalkyl it is a
piperidinyl ring, a pyrrolidonyl ring or an azetidinyl ring. Suitably, the
ring is a piperidinyl or
pyrrolidonyl ring. In another embodiment the ring is a morpholinyl ring. It is
recognized that
either the ring carbon atom or the ring nitrogen atom of the heterocyclic ring
may be substituted.
In another embodiment R1 is a tetrahydro-2H-thiopyran 1,1-dioxide.
Suitably, for a compound of Formula IX, R7 is a C3-C6 cycloalkyl optionally
substituted
with methyl. The C3-C6 cycloalkyl which may be optionally substituted is
suitably a cyclopropyl
or cyclopentyl ring. In one embodiment the R7 ring is a cyclopentyl ring. In
another
embodiment, R7 is a cyclopropyl ring.
Suitably, for a compound of Formula IX, R7 is methyl substituted with C3-05
cycloalkyl.
Suitably, the C3-05 ring is a cyclopropyl or a cyclopentyl ring. In one
embodiment, R7 is a
cyclopropylmethyl.
Suitably, for a compound of Formula IX, R7 is a C2 or C3 alkyl optionally
substituted
with two F, the alkyl chain is a straight of branched C3 carbon chain, such as
propyl or isopropyl.
31
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
In one embodiment the first carbon in the chain attached to the carbonyl is
the one optionally
substituted with two fluorines.
Suitably, for compounds of Formula IX, R2 is C1-C3 alkyl or halo. In one
embodiment,
R2 is a Cl-C3 alkyl. In another embodiment, R2 is methyl.
Suitably, for compounds of Formula IX, R3 is halo or CN. In one embodiment, R3
is halo.
In another embodiment, R3 is fluorine or chlorine. In one embodiment, R3 is
fluorine. In
another embodiment, R3 is chlorine. In another embodiment, R3 is cyano. In one
embodiment,
when R3 is fluorine, chlorine or cyano, R2 is methyl.
Suitably, for compounds of Formula IX, R5 is C1-C3 alkyl. In one embodiment R5
is
methyl.
Suitably, for compounds of Formula IX, R6 is H or methyl. In one embodiment,
R6 is
hydrogen.
In one embodiment, a compound of the Formula IX, or a pharmaceutically
acceptable salt
thereof is selected from:
(S)-1-acetyl-N-(3-04-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-5-
fluoro-2-
methylphenyl)azetidine-3-carboxamide;
(S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-
(1 -propionylazetidin-3-ypacetamide;
(R)-1-acetyl-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylphenyl)pyrrolidine-3-carboxamide;
(S)-1-acetyl-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1 -
yOmethyl)-2-
methylphenyl)pyrrolidine-3-carboxamide;
(R)-1 -acetyl-N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3 -methylpiperazin-
1 -yl)methyl)-2-
methylphenyl)pyrrolidine-2-carboxamide;
(S)-1 -acetyl-N-(5 -chloro-344-(cyclopentanecarbony1)-3 -methylpiperazin- 1 -
yl)methyl)-2-
methylphenyl)piperidine-4-carboxamide;
(S)-1 -acetyl-N-(5 -chloro-3-4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylphenyl)piperidine-3 -carboxamide;
(R)-1-acetyl-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylphenyl)piperidine-3-carboxamide;
(S)-1-acetyl-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
yHmethyl)-2-
methylphenyl)pyrrolidine-2-carboxamide;
(S)-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-
2-
methylpheny1)-1-propionylpyrrolidine-2-carboxamide;
(S)-N-(5-chloro-3-0(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-ypmethyl)-
2-
methylpheny1)-1-isobutyrylpyrrolidine-2-carboxamide;
32
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-2-(1-acetylpiperidin-4-y1)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-
methylpiperazin-l-
y1)methyl)-2-methylphenypacetamide;
4-Acetyl-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylphenyl)morpholine-2-carboxamide;
4-Acetyl-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
yOmethyl)-2-
methylphenyl)morpholine-2-carboxamide;
(S)-N-(344-(cyclopentanecarbony1)-3-methylpiperazin-l-yemethyl)-5-fluoro-2-
methylpheny1)-1-
(methylsulfonyl)azetidine-3-carboxamide;
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylphenyl)tetrahydro-2H-thiopyran-4-carboxamide 1,1-dioxide; or
N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylphenyptetrahydro-211-thiopyran-3-carboxamide 1,1-dioxide.
One aspect of the invention is a pharmaceutical composition comprising a
compound of
Formula IX, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable
carrier or diluent.
Compounds outside the scope of Formulas I- IX include:
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3 -methylpiperazin-1 -yOmethyl)-2-
methylpheny1)-4-
oxo-4-(pyrrolidin-1 -yl)butanamide, or a pharmaceutically acceptable salt
thereof.
Terms and Definitions
"Alkyl" refers to a monovalent saturated hydrocarbon chain having the
specified number
of member atoms. For example, C1-C6 alkyl refers to an alkyl group having from
1 to 6 member
atoms. Alkyl groups may be optionally substituted with one or more substituent
as defined herein.
The alkyl groups as used herein, may be straight or branched. Representative
branched alkyl
groups have one, two, or three branches. Examples of alkyl include methyl,
ethyl, propyl (n-propyl
and isopropyl), butyl (n-butyl, isobutyl, and t-butyl), pentyl (n-pentyl,
isopentyl, and neopentyl),
and hexyl.
"Cycloalkyl" refers to a saturated hydrocarbon ring having the specified
number of
member atoms. Cycloalkyl groups are monocyclic ring systems or are fused or
bridged bicyclic
ring systems. For example, C3-C7 cycloalkyl refers to a cycloalkyl group
having from 3 to 7
member atoms. Cycloalkyl groups may be optionally substituted with one or more
substituent as
defined herein. Examples of cycloalkyl include cyclopropyl, cyclobutyl,
cyclopentyl, and
cyclohexyl.
"Enantiomeric excess" or "ee" is the excess of one enantiomer over the other
expressed
as a percentage. As a result, since both enantiomers are present in equal
amounts in a racemic
33
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
mixture, the enantiomeric excess is zero (0% ee). However, if one enantiomer
was enriched such
that it constitutes 95% of the product, then the enantiomeric excess would be
90% ee (the amount
of the enriched enantiomer, 95%, minus the amount of the other enantiomer,
5%).
"Enantiomerically pure" refers to products whose enantiomeric excess is 99% ee
or
greater.
"Half-life" refers to the time required for half of a quantity of a substance
to be converted
to another chemically distinct species in vitro or in vivo.
"Halo" refers to the halogen radicals fluoro, chloro, bromo, and iodo.
"Heteroaryl" refers to an aromatic ring containing from 1 to 4 heteroatoms as
member
atoms in the ring. Heteroaryl rings containing more than one heteroatom may
contain different
heteroatoms. Heteroaryl rings may be optionally substituted with one or more
substituent as
defined herein. Heteroaryl rings are monocyclic ring systems, or are fused or
bridged bicyclic
ring systems. Monocyclic heteroaryl rings have from 5 to 7 member atoms.
Bicyclic heteroaryl
rings have from 7 to 11 member atoms. Bicyclic heteroaryl rings include those
rings wherein
phenyl and a monocyclic heterocycloalkyl ring are attached forming a fused,
spiro, or bridged
bicyclic ring system, and those rings wherein a monocyclic heteroaryl ring and
a monocyclic
cycloalkyl, cycloalkenyl, heterocycloalkyl, or heteroaryl ring are attached
forming a fused, spiro, or
bridged bicyclic ring system. Suitable xamples of heteroaryl include but are
not limited to
pyrrolyl, pyrazolyl, irnidazolyl, oxazolyl, isoxazolyl, oxadiazolyl,
thiazolyl, isothiazolyl,
thiadiazolyl, furanyl, furazanyl, thienyl, triazolyl, pyridinyl, pyrimidinyl,
pyridazinyl, pyrazinyl,
triazinyl, tetrazinyl, tetrazolyl, indolyl, isoindolyl, indolizinyl,
indazolyl, purinyl, quinolinyl,
isoquinolinyl, quinoxalinyl, quinazolinyl, pteridinyl, cinnolinyl,
benzimidazolyl, finopyridinyl, and
naphthyridinyl. Suitable examples of 6 membered heteroaryls include but are
not limited to
pyridinyl, pyrimidinyl, pyridazinyl, pyrazinyl, and triazinyl.
Suitable examples of 5 membered heteroaryls include but are not limited to
pyrrolyl,
pyrazolyl, imidazolyl, oxazolyl, isoxazolyl, oxadiazolyl, thiazolyl,
isothiazolyl, thiadiazolyl,
furanyl, furazanyl, thienyl, triazolyl, and tetrazolyl.
"Heteroatom" refers to a nitrogen, sulphur, or oxygen atom, also abbreviated
herein as N,
S or 0 respectively.
"Heterocycloalkyl" refers to a saturated ring containing from 1 to 4
heteroatoms as
member atoms in the ring, unless otherwise indicated. Heterocycloalkyl rings
are not aromatic.
Heterocycloalkyl groups containing more than one heteroatom may contain
different heteroatoms.
Heterocycloalkyl groups may be optionally substituted with one or more
substituents as defined
herein. Heterocycloalkyl groups are monocyclic ring systems or are fused,
spiro, or bridged
bicyclic ring systems. Monocyclic heterocycloalkyl rings have from 4 to 7
member atoms.
Bicyclic heterocycloalkyl rings have from 7 to 11 member atoms. Examples of
heterocycloalkyl
include pyrrolidinyl, tetrahydrofuranyl, dihydrofuranyl, pyranyl,
tetrahydropyranyl, dihydropyranyl,
34
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
tetrahydrothienyl, pyrazolidinyl, oxazolidinyl, thiazolidinyl, piperidinyl,
homopiperidinyl,
piperazinyl, morpholinyl, thiamorpholinyl, azepinyl, 1,3-dioxolanyl, 1,3-
dioxanyl, 1,3-oxathiolanyl,
1,3-dithianyl, azetidinyl, oxetanyl, azabicylo[3.2.1]octyl, and
oxabicylo[2.2.1]heptyl.
"Member atoms" refers to the atom or atoms that form a chain or ring. Where
more
than one member atom is present in a chain and within a ring, each member atom
is covalently
bound to an adjacent member atom in the chain or ring. Atoms that make up a
substituent group
on a chain or ring are not member atoms in the chain or ring.
"Optionally substituted" indicates that a group, such as alkyl, alkenyl,
alkynyl, aryl,
cycloalkyl, cycloalkenyl, heterocycloalkyl, or heteroaryl, may be
unsubstituted, or the group may
be substituted with one or more substituent as defined.
"RORy" refers to all isoforms encoded by the RORC gene which include RORyl and
RORyt.
"RORy modulator" refers to a chemical compound that inhibits, either directly
or
indirectly, the activity of RORy. RORy modulators include antagonists and
inverse agonists of
RORy.
"Pharmaceutically acceptable" refers to those compounds, materials,
compositions, and
dosage forms which are, within the scope of sound medical judgment, suitable
for use in contact
with the tissues of human beings and animals without excessive toxicity,
irritation, or other
problem or complication, commensurate with a reasonable benefit/risk ratio.
"Substituted" in reference to a group indicates that one or more hydrogen atom
attached
to a member atom within the group is replaced with a substituent selected from
the group of
defined substituents. It should be understood that the term "substituted"
includes the implicit
provision that such substitution be in accordance with the permitted valence
of the substituted atom
and the substituent and that the substitution results in a stable compound
(i.e. one that does not
spontaneously undergo transformation such as by rearrangement, cyclization, or
elimination and
that is sufficiently robust to survive isolation from a reaction mixture).
When it is stated that a
group may contain one or more substituent, one or more (as appropriate) member
atom within the
group may be substituted. In addition, a single member atom within the group
may be substituted
with more than one substituent as long as such substitution is in accordance
with the permitted
valence of the atom.
The compounds according to Formula I may contain one or more asymmetric center
(also
referred to as a chiral center) and may, therefore, exist as individual
enantiomers, diastereomers, or
other stereoisomeric forms, or as mixtures thereof. Chiral centers, such as
chiral carbon atoms,
may also be present in a substituent such as an alkyl group. Where the
stereochemistry of a chiral
center present in Formula I, or in any chemical structure illustrated herein,
is not specified the
structure is intended to encompass all individual stereoisomers and all
mixtures thereof. Thus,
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
compounds according to Formula I containing one or more chiral center may be
used as racemic
mixtures, enantiomerically enriched mixtures, or as enantiomerically pure
individual stereoisomers.
Individual stereoisomers of a compound according to Formula I which contain
one or more
asymmetric center may be resolved by methods known to those skilled in the
art. For example,
such resolution may be carried out (1) by formation of diastereoisomeric
salts, complexes or other
derivatives; (2) by selective reaction with a stereoisomer-specific reagent,
for example by
enzamatic oxidation or reduction; or (3) by gas-liquid or liquid
chromatography in a chiral
enviomment, for example, on a chiral support such as silica with a bound
chiral ligand or in the
presence of a chiral solvent. The skilled artisan will appreciate that where
the desired
stereoisomer is converted into another chemical entity by one of the
separation procedures
described above, a further step is required to liberate the desired form.
Alternatively, specific
stereoisomers may be synthesized by asymmetric synthesis using optically
active reagents,
substrates, catalysts or solvents, or by converting one enantiomer to the
other by asymmetric
transformation.
The compounds according to Formula I may also contain double bonds or other
centers of
geometric asymmetry. Where the stereochemistry of a center of geometric
asymmetry present in
Formula I, or in any chemical structure illustrated herein, is not specified,
the structure is intended
to encompass the trans (E) geometric isomer, the cis (Z) geometric isomer, and
all mixtures thereof.
Likewise, all tautomeric forms are also included in Formula I whether such
tautomers exist in
equilibrium or predominately in one form.
In certain embodiments, compounds according to Formula I may be present as a
free base
or free acid.
In certain embodiments, compounds according to Formula I may contain an acidic
functional group. In certain other embodiments, compounds according to Formula
I may contain a
basic functional group. Thus, the skilled artisan will appreciate that
pharmaceutically-acceptable
salts of the compounds according to Formula I or II may be prepared. Indeed,
in certain
embodiments of the invention, pharmaceutically-acceptable salts of the
compounds according to
Formula I or II may be preferred over the respective free base or free acid
because such salts may
impart greater stability or solubility to the molecule thereby facilitating
formulation into a dosage
form. Accordingly, the invention is further directed to the use of
pharmaceutically-acceptable
salts of the compounds according to Formula I or II.
As used herein, the term "pharmaceutically acceptable salts" refers to salts
that retain the
desired biological activity of the subject compound and exhibit minimal
undesired toxicological
effects. These pharmaceutically-acceptable salts may be prepared in situ
during the final isolation
and purification of the compound, or by separately reacting the purified
compound in its free acid
or free base form with a suitable base or acid, respectively. Suitable
pharmaceutically acceptable
salts include those described by Berge, Bighley, and Monkhouse, J. Pharm. Sci.
(1977) 66, pp 1-19.
36
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Salts of the disclosed compounds containing a basic amine or other basic
functional group
may be prepared by any suitable method known in the art, including treatment
of the free base with
an inorganic acid, such as hydrochloric acid, hydrobromic acid, sulfuric acid,
nitric acid,
phosphoric acid, and the like, or with an organic acid, such as acetic acid,
trifluoroacetic acid,
maleic acid, succinic acid, mandelic acid, fumaric acid, malonic acid, pyruvic
acid, oxalic acid,
glycolic acid, salicylic acid, pyranosidyl acid, such as glucuronic acid or
galacturonic acid, alpha-
hydroxy acid, such as citric acid or tartaric acid, amino acid, such as
aspartic acid or glutamic acid,
aromatic acid, such as benzoic acid or cinnamic acid, sulfonic acid, such as p-
toluenesulfonic acid,
methanesulfonic acid, ethanesulfonic acid or the like. Examples of
pharmaceutically acceptable
salts include sulfates, pyrosulfates, bisulfates, sulfites, bisulfites,
phosphates, chlorides, bromides,
iodides, acetates, propionates, decanoates, caprylates, acrylates, formates,
isobutyrates, caproates,
heptanoates, propiolates, oxalates, malonates succinates, suberates,
sebacates, fumarates, maleates,
butyne-1,4-dioates, hexyne-1,6-dioates, benzoates, chlorobenzoates,
methylbenzoates,
dinitrobenzoates, hydroxybenzoates, methoxybenzoates, phthalates,
phenylacetates,
phenylpropionates, phenylbutrates, citrates, lactates, y-hydroxybutyrates,
glycolates, tartrates
mandelates, and sulfonates, such as xylenesulfonates, methanesulfonates,
propanesulfonates,
naphthalene-l-sulfonates and naphthalene-2-sulfonates.
Salts of the disclosed compounds containing an acidic functional group can be
prepared by
reacting with a suitable base. Such a pharmaceutically acceptable salt may be
made with a base
which affords a pharmaceutically acceptable cation, which includes alkali
metal salts (especially
sodium and potassium), alkaline earth metal salts (especially calcium and
magnesium), aluminum
salts and ammonium salts, as well as salts made from physiologically
acceptable organic bases such
as trimethylamine, triethylamine, morpholine, pyridine, piperidine, picoline,
dicyclohexylamine,
NX-dibenzylethylenediamine, 2-hydroxyethylamine, bis-(2-hydroxyethyl)amine,
tri-(2-
.. hydroxyethyl)amine, procaine, dibenzylpiperidine, dehydroabietylamine, N,N'
-
bisdehydroabietylamine, glucamine, N-methylglucamine, collidine, choline,
quinine, quinoline, and
basic amino acid such as lysine and arginine.
Other salts, which are not pharmaceutically acceptable, may be useful in the
preparation of
compounds of this invention and these should be considered to form a further
aspect of the
invention. These salts, such as trifluoroacetate, while not in themselves
pharmaceutically
acceptable, may be useful in the preparation of salts useful as intermediates
in obtaining the
compounds of the invention and their pharmaceutically acceptable salts.
If a compound of the invention containing a basic amine or other basic
functional group is
isolated as a salt, the corresponding free base form of that compound may be
prepared by any
.. suitable method known to the art, including treatment of the salt with an
inorganic or organic base,
suitably an inorganic or organic base having a higher pKa than the free base
form of the compound.
Similarly, if a compound of the invention containing an acidic functional
group is isolated as a salt,
37
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
the corresponding free acid form of that compound may be prepared by any
suitable method known
to the art, including treatment of the salt with an inorganic or organic acid,
suitably an inorganic or
organic acid having a lower pKa than the free acid form of the compound.
As used herein, the term "compounds of the invention" means both the compounds
according to Formula I and II (as a free base or free acid), and the
pharmaceutically-acceptable
salts thereof. The term "a compound of the invention" also appears herein and
refers to both a
compound according to Formula I or II (as a free base or free acid), and its
pharmaceutically-
acceptable salts.
The invention also includes various deuterated forms of the compounds of
Formula I.
Each available hydrogen atom attached to a carbon atom may be independently
replaced with a
deuterium atom. A person of ordinary skill in the art will know how to
synthesize deuterated forms
of the compounds of Formula I. Commercially available deuterated starting
materials may be
employed in the preparation of deuterated forms of the compounds of Formula I,
or they may be
synthesized using conventional techniques employing deuterated reagents (e.g.
lithium aluminum
deuteride).
The compounds of the invention may exist in solid or liquid form. In the solid
state, the
compounds of the invention may exist in crystalline or noncrystalline form, or
as a mixture thereof.
For compounds of the invention that are in crystalline form, the skilled
artisan will appreciate that
pharmaceutically-acceptable solvates may be formed wherein solvent molecules
are incorporated
into the crystalline lattice during crystallization. Solvates may involve
nonaqueous solvents such as
ethanol, isopropanol, DMSO, acetic acid, ethanolamine, and ethyl acetate, or
they may involve
water as the solvent that is incorporated into the crystalline lattice.
Solvates wherein water is the
solvent that is incorporated into the crystalline lattice are typically
referred to as "hydrates."
Hydrates include stoichiometric hydrates as well as compositions containing
vaiable amounts of
water. The invention includes all such solvates.
The skilled artisan will further appreciate that certain compounds of the
invention that
exist in crystalline form, including the various solvates thereof, may exhibit
polymorphism (i.e. the
capacity to occur in different crystalline structures). These different
crystalline forms are typically
known as "polymorphs." The invention includes all such polymorphs. Polymorphs
have the
same chemical composition but differ in packing, geometrical arangement, and
other descriptive
properties of the crystalline solid state. Polymorphs, therefore, may have
different physical
properties such as shape, density, hardness, deformability, stability, and
dissolution properties.
Polymorphs typically exhibit different melting points, IR. spectra, and X-ray
powder diffraction
patterns, which may be used for identification. The skilled artisan will
appreciate that different
polymorphs may be produced, for example, by changing or adjusting the reaction
conditions or
reagents, used in making the compound. For example, changes in temperature,
pressure, or solvent
38
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
may result in polymorphs. In addition, one polymorph may spontaneously convert
to another
polymorph under certain conditions.
The compounds of Formula I and pharmaceutically acceptable salts thereof may
be
employed alone or in combination with other therapeutic agents. Combination
therapies according
to the present invention thus comprise the administration of at least one
compound of Formula I or
a pharmaceutically acceptable salt thereof, and the use of at least one other
therapeutically active
agent. A compound of Formula I or pharmaceutically acceptable salt thereof,
and the other
therapeutically active agent(s) may be administered together in a single
pharmaceutical
composition or separately and, when administered separately this may occur
simultaneously or
sequentially in any order.
In a further aspect, there is provided a combination product comprising a
compound of
FaLmula I or a pharmaceutically acceptable salt thereof, together with one or
more other
therapeutically active agents, and optionally a pharmaceutically acceptable
carrier or excipient.
Suitable other therapeutic agents include, but are not limited to, (1) TNF-
alpha inhibitors;
(2) non-selective COX-1/COX-2 inhibitors; (3) COX-2 inhibitors; (4) other
agents for treatment of
inflammatory and autoimmune diseases including glucocorticoids, methotrexate,
leflunomide,
sulfasalazine, azathioprine, cyclosporin, tacrolimus, penicillamine,
bucillamine, actarit, mizoribine,
lobenzarit, ciclesonide, hydroxychloroquine, d-penicillamine, aurothiomalate,
auranofin or
parenteral or oral gold, cyclophosphamide, Lymphostat-B, BAFF/APRIL
inhibitors, such as
belimumab, and CTLA-4-Ig or mimetics thereof; (5) leukotriene biosynthesis
inhibitor, 5-
lipoxygenase (5-LO) inhibitor or 5-lipoxygenase activating protein (FLAP)
antagonist; (6) LTD4
receptor antagonist; (7) PDE4 inhibitor; (8) antihistamine H1 receptor
antagonists; (9) al- and a2-
adrenoceptor agonist; (10) anticholinergic agents; (11) fl-adrenoceptor
agonists; (12) insulin-like
growth factor type I (IGF-1) mimetic; (13) glucocorticosteroids; (14) kinase
inhibitors such as
.. inhibitors of the Janus Kinases (JAK 1 and/or JAK2 and/or JAK 3 and/or
TYK2), p38 MAPK and
IKK2; (15) B-cell targeting biologies such as rituximab; (16) selective
costimulation modulators
such as abatacept; (17) interleukin inhibitors, such as IL-1 inhibitor
anakinra, IL-6 inhibitors
tocilizumab or sirulcumab, IL-12/1L-23 inhibitor ustelcinumab, IL-23 inhibitor
guselkumab, and
anti-IL17 antibodies; (18) anti-GM-CSF antibodies; (19) checkpoint blockade
and other
immunotherapies, such as anti-PD-1/anti-PD-L1 antibodies, including
pembrolizumab and
nivolumab, and anti-CTLA4 antibodies, including ipilimumab; (20) BET
inhibitors, such as
GSK525762; and (21) other oncology agents, such as fluorouracil, bevacizumab,
irinotecan
hydrochloride, capecitabine, cetuximab, ramucirumab, oxaliplatin, leucovorin
calcium,
panitumumab, regorafenib, ziv-aflibercept, trastuzumab, imatinib mesylate,
sunitinib malate,
sorafenib tosylate, paclitaxel, everolimus, erlotinib hydrochloride,
gemcitabine hydrochloride,
mitomycin C, dabrafenib, trametinib, lapatinib, ofatumumab, topotecan,
doxorubicin hydrochloride,
and ibrutinib.
39
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
Compound Preparation
The compounds according to Formula I may be prepared using conventional
organic
syntheses. Suitable synthetic routes are depicted below in the following
general reaction scheme.
The skilled artisan will appreciate that if a substituent described herein is
not compatible
with the synthetic methods described herein, the substituent may be protected
with a suitable
protecting group that is stable to the reaction conditions. The protecting
group may be removed at
a suitable point in the reaction sequence to provide a desired intermediate or
target compound.
Suitable protecting groups and the methods for protecting and de-protecting
different substituents
using such suitable protecting groups are well known to those skilled in the
art; examples of which
may be found in T. Greene and P. Wuts, Protecting Groups in Chemical Synthesis
(3rd ed.), John
Wiley & Sons, NY (1999). In some instances, a substituent may be specifically
selected to be
reactive under the reaction conditions used. Under these circumstances, the
reaction conditions
convert the selected substituent into another substituent that is either
useful as an intermediate
.. compound or is a desired substituent in a target compound.
Scheme 1
R2 0 R2 R2
02N 02N 0 N
,c 2
OH A OH b
LA,NI3oc
R5 R6
R3 R3 L,NBoc R3
1 2 Re 4
3
H R2
R2 R2
d,e 02N
R5 f NI27 H2N R5 R N R5
o N R
, N N,
I I 7
R6 R6 I I
R3 0 R3 0 R3 0
5 6 Formula I
[Exemplary conditions: a) BI-13=THF, THF, 0 C-RT.; b) PCC, CH2C12; c)
NaBH(OAc)3, CH2C12, 3; d)
Me0H; e) 12.7CO2H, DIPEA, HATU, DMF; 0 Fe, HOAc, 60 C; g) R1CO2H, DIPEA, HATU,
DMF.]
Scheme 1 represents a general reaction scheme for preparing compounds of
Formula I
where R1 to R7 are as defined above. The starting material or reagents
described are either
commercially available or is made from commercially available starting
materials using methods
known to those skilled in the art.
Benzoic acids 1 may be reduced by BH3-THF to provide benzyl alcohol 2. Alcohol
2 may
be oxidized by PCC to the corresponding aldehyde followed by reductive
amination with 3 to
provide nitro compound 4. The Boc protection of 4 may be removed by treatment
with HC1 and
the resulting amine reacted with various acids to provide nitro compound 5.
Nitro compound 5
may be reduced to amine 6 which is then reacted with various acids to give
final compounds of
Formula I.
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Scheme 2
R2 0 R2 R2
02N 40
OH a 02N L 11) OH b,c 02N io . N R5 NB oc
HN'y R5 R6
R3 R3 y NBoc R3
1 2 Rs 4
3
R2
H R2
d, e N
N R5 f, g RiyN
0 upi LIXR7
0 NB oc
R6 R3 0
R3
5 Formula I
[Exemplary conditions: a) BH3-THF, THF, 0 C-RT; b) PCC, C112C12; c)
NaBH(OAc)3, CH2C12, 3; d) Pd/C,
Me0H, H2; e) R1CO2H, DIPEA, HATU, DMF; f) HC1, Me0H; g) It7CO2H, DIPEA, HATU,
DMF].
Scheme 2 represents another reaction scheme for preparing compounds of Formula
I where
R1 to R7 are as defined above. The starting material or reagents described are
either commercially
available or is made from commercially available starting materials using
methods known to those
skilled in the art.
Benzoic acids 1 may be reduced by BH3=THF to provide benzyl alcohol 2. Alcohol
2 may
be oxidized by PCC to corresponding aldehyde followed by reductive amination
with 3 to provide
nitro compound 4. Reduction of nitro compound 4 with Pd/C in the presence of
H2 afforded the
amine which may be reacted with various acids to give amide 5. The Boc
protection of 5 may be
removed by treatment with HC1 and the resulting amine reacted with various
acids to provide final
compounds of Formula I.
Examples
Abbreviations
ACN Acetonitrile
AffiN 2,2' -Azobisisobutyronitrile
Aq. Aqueous
Boc t-Butoxycarbonyl
Bn Benzyl
DAST Diethylaminosulfur trifluoride
DCC Dicyclohexylcarbodiimide
DCM Dichloromethane
DIPEA N,N-diisopropylethylamine
DMAP Dimethylaminopyridine
DMF N,N-dimethylformamide
DMSO Dimethylsulphoxide
Dppf l'-Bis(diphenylphosphino)ferrocene
DPPP Diphenyl- 1 -pyrenylphosphine
41
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
EDC N-(3-Dimethylaminopropy1)-N'-ethylcarbodiimide hydrochloride
ESI Electron Spray Ionization
Et0Ac ethyl acetate
HATU 0-(7-Azabenzotriazol-1-y1)-N,1V,V,NI-tetramethyluronium
hexafluorophosphate
HOBt Hydroxybenzotriazole
LCMS Liquid Chromatography Mass Spectrometry
LDA Lithium diisopropylamide
MDAP Mass Directed Automated Preparative liquid chromatography
MS mass spectrometry
MsC1 Methanesulfonyl chloride
NBS N-bromosuccinamide
PCC Pyridinium chlorochromate
RT room temperature
sat. saturated
SM starting material
TEA Triethylamine
TFA Trifluoroacetic acid
THF Tetrahydrofuran
TsC1 4-Methyl-benzenesulfonyl chloride
Chromatography
Unless stated otherwise, all chromatography was carried out using silica
columns.
LCMS Conditions:
1) Acidic conditions:
Mobile phase: water containing 0.05 TFA / acetonitrile
Column: Agilent SB-C18 4.6 x 30 mm 1.8 um
Detection: MS and photodiode array detector (PDA)
2) Basic conditions:
Mobile phase: 10mM NH4HCO3 aqueous / acetonitrile
Column: Waters XBridge C18 4.6 x 50 min 3.5 lam
Detection: MS and photodiode array detector (PDA)
MDAP Conditions:
1) Acidic conditions:f
Instrument: Waters Mass Directed Auto-purification System
Column: Waters Sunfire Prep C18 column (5 um, 19x50 mm)
Mobile phase: water containing 0.05% TFA / acetonitrile.
2) Basic conditions:
42
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Instrumnet: Mass Directed Auto-purification System
Column: Xbridge Prep C18 column (5 urn, 19x50 mm)
Mobile phase: water containing 0.05% ammonia/ acetonitrile.
In the procedures that follow, after each starting material, reference to an
intermediate is
typically provided. This is provided merely for assistance to the skilled
chemist. The starting
material may not necessarily have been prepared from the batch referred to.
Description 1
cis-2-(3-Hydroxycyclopentyl)acetic acid (D1)
HO
The mixture of 2-oxabicyclo[3.2.1]octan-3-one (300 mg) and potassium hydroxide
(267 mg) in
methanol (30 mL) and water (5 mL) was stirred at 20 C for 12 hours and then
concentrated under
vacuum, 1 M HC1 (aq.) was added to pH = 5, extracted with Et0Ac (3x10 mL),
combined organic
layer was dried and concentrated, the crude product was purified by column
chromatography (silica
gel, petroleum ether/Et0Ac = 1:1) to afford the title compound (150 mg) as
sticky oil. MS (ESI):
C7111203 requires 144; found 143 [M-1-1]-.
Description 2
Methyl 2-(4-hydroxycyclohexyDacetate (D2)
HO
The mixture of Rh/A1 (1 g) and methyl 2-(4-hydroxyphenyl)acetate (6 g) was
dissloved in Me0H
(150 mL). The reaction mixture was hydrogenated at 50 C for 6 hours under 30
bar of H2. The
reaction mixture was filtered and the filtrate was concentrated to afford the
title compound (4 g) as
yellow solid. MS (ESI): C9111603 requires 172; found 195 [M+Nar.
Description 3
2-(4-Hydroxycyclohexyl)acetic acid (D3)
mr0H
HO
The mixture of potassium hydroxide (2.61 g) and methyl 2-(4-
hydroxycyclohexypacetate (D2, 4 g)
was dissloved in Me0H (30 mL) and water (30 mL). The reaction mixture was
stirred for 6 hours
at 80 C. The reaction mixture was concentrated and acidified with 2 M HC1(aq.)
to pH = 1, then
extrated with DCM (2x50 mL). The organic layer was dried with anhydrous sodium
sulfate.
Filtered, the filtrate was concentrated to afford the title compound (3 g) as
yellow solid. MS (ESI):
C8111403 requires 158; found 157 [m-H].
43
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Description 4
2-(3-Hydroxycyclohexyl)acetic acid (D4)
HO OH
0
The mixture of NaOH (0.5 g), Rh/C(1 g) and 2-(3-hydroxyphenyl)acetic acid (1.1
g) in water (60
.. mL) was hydrogenated at 80 C for 12 hours under 10 bar of H2. Filtered, the
filtrate was acidified
withl M HC1 (aq.) to pH = 5 and then extracted with Et0Ac (3x10 mL). Combined
organic layer
was dried and evaporated to afford the title compound (600 mg) as yellow oil.
MS (ESI): C81-11403
requires 158; found 157 [M-HI.
Description 5
.. (E)-ethyl 4-hydroxybut-2-enoate (D5)
0
To a solution of (E)-4-ethoxy-4-oxobut-2-enoic acid (9 g) in THF (50 mL), BH3-
THF (1 M, 45 mL)
solution in THF (100 mL) was added dropwise at -10 C. The reaction mixture was
gradually
warmed to RT and stirred for 12 hours. The reaction was quenched by adding
AcOH/H20 (1:1, v/v,
5 mL) with stirring until no more gas evolution occurred. The mixture was
concentrated under
vacuum, the residue was treated with saturated NaHCO3 solution (50 mL) and
then extracted with
Et0Ac (2x50 mL). The combined organic layer was dried over anhydrous MgSO4 and
concentrated
to afford the title compound (2 g) as yellow oil. MS (ESI): C6111003 requires
130; found 131
[M+H1+ .
.. Description 6
Trans-(E)-ethyl 4((3-bromotetrahydrofuran-2-yl)oxy)but-2-enoate (D6)
Br
To a solution of (E)-ethyl 4-hydroxybut-2-enoate (D5, 1 g) in DCM (50 mL), 2,3-
dihydrofuran
(0.539 g) and NBS (1.368 g) were added at 0 C. The reaction mixture was
gradually warmed to RT
and stirred for 12 hours. To the resulting mixture was added water (100 mL)
and then extracted
with Et0Ac (2x30 mL). The combined organic layer was washed with brine (30
mL), dried over
anhydrous MgSO4 and concentrated. The residue was purified by column
chromatography (silica
gel, petroleum ether/Et0Ac = 10:1) to afford the title compound (500 mg) as
yellow oil. MS (ESI):
C10H15BrO4 requires 278; found 279 [M+H].
44
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Description 7
Ethyl 2-(hexahydrofuro[2,3-b]furan-3-yl)acetate (D7)
0
0 0
To a solution of trans-(E)-ethyl 4-((3-bromotetrahydrofuran-2-yl)oxy)but-2-
enoate (D6, 300 mg) in
benzene (30 mL) was added tributylstannane (313 mg) and AJBN (1 mg) at 0 C.
The reaction
mixture was gradually warm to 80 C and stirred for 4 hours. Water (10 mL) was
added, extracted
with Et0Ac (2x10 mL). The combined organic layer was washed with brine (10
mL), dried over
anhydrous MgSO4 and concentrated. The residue was purified by column
chromatography (silica
gel, petroleum ether/Et0Ac = 5:1) to afford the title compound (200 mg) as
yellow oil. MS (EST):
C10111604 requires 200; found 201 [M+Hr.
Description 8
2-(Hexahydrofuro[2,3-b]furan-3-ypacetic acid (P8)
0 OH
0
The mixture of ethyl 2-(hexahydrofuro[2,3-b]furan-3-yOacetate (D7, 180 mg) and
sodium
hydroxide (36.0 mg) in ethanol (20 mL) and water (10 mL) was stirred at 80 C
for 2 hours and
then concentrated. The residue was acidified with 2 M HC1 (aq.) to pH = 1 and
extracted with
Et0Ac (10 mL). The organic layer was dried and concentrated under vacuum to
afford the title
compound (100 mg) as white solid. MS (ESI): C81-11204 requires 172; found 173
[M+Hr.
Description 9
(5-Methyltetrahydrofuran-3-yl)methanol (D9)
0
The mixture of 4-methylbenzenesulfonic acid (1.925 g) and 2-
(hydroxymethyl)pentane-1,4-diol
(1.5 g) in toluene (30 mL) was stirred at 130 C for 3 hours and then diluted
with Et0Ac (100 mL),
washed with water (100 mL). The organic layer was dried and concentrated under
vacuum. The
residue was purified by column chromatography (silica gel, petroleum
ether/Et0Ac = 4:1) to afford
the title compound (200 mg) as yellow oil. 11-1 NMR (500 MHz, DMSO-d6): 4.61-
4.60 (m, 1H),
3.89-3.83 (m, 2H), 3.60-3.56 (m, 1H), 3.37-3.27 (m, 2H), 2.38-2.33 (m, 1H),
1.73-1.71 (m, 1H),
1.42-1.40 (m, 1H), 1.15-1.10 (m, 3H).
Description 10
(3,3-Difluorocyclobutyl)methanol (D10)
j=r0H
To the mixture of 3,3-difluorocyclobutanecarboxylic acid (1 g) in THF (20 mL)
was added borane
(1 M in THF, 29.4 mL) during 30 mm. The reaction mixture was stirred at 20 C
for 6 hours, then
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
concentrated under vacuum to afford the title compound (1 g) as white solid.
IHNMR (500 MHz,
CDC13): 3.72-3.70 (m, 2H), 2.68-2.62 (m, 2H), 2.40-2.33 (m, 3H).
Description 11
(5-Methyftetrahydrofuran-3-yl)methyl 4-methylbenzenesulfonate (D11)
To the mixture of (5-methyltetrahydrofuran-3-yl)methanol (D9, 200 mg) in
pyridine (5 mL) was
added 4-methylbenzene-1 -sulfonyl chloride (985 mg). The reaction mixture was
stirred at RT
overnight and then diluted with Et0Ac (25 mL) and water (20 mL). The organic
layer was
separated and washed with water (20 mL) and brine (20 mL), dried over Na2SO4
and concentrated.
The residue was purified by column chromatography (silica gel, petroleum
ether/Et0Ac = 8:1) to
afford the title compound (300 mg) as yellow oil. MS (ESI): CBI-118045
requires 270; found 271
[M+H]=
Description 12
(3,3-Difluorocyclobutyl)methyl 4-methylbenzenesulfonate (D12)
7C-.10Ts
D12 was prepared using a similar procedure to that described for D11. MS
(ESI): C121-114F203S
requires 276; found 294 [M+NH4].
Description 13
2-(5-Methyltetrahydrofuran-3-yl)acetonitrile (D13)
The mixture of (5-methyltetrahydrofuran-3-yl)methyl 4-methylbenzenesulfonate
(D11, 300 mg)
and NaCN (65.3 mg) in DMSO (5 mL) was stirred at 80 C overnight. The reaction
mixture was
poured into water (50 mL) and extracted with Et0Ac (2x10 mL). The combined
organic layer was
washed with water (10 mL) and brine (10 mL), dried and concentrated under
vacuum to afford the
title compound (180 mg). 111 NMR (500 MHz, CDC13): 4.24-3.90 (m, 2H), 3.51-
3.44 (m, 1H), 2.62
(brs, 1H), 2.55-2.42 (m, 2H), 1.92-1.75 (m, 2H), 1.28-1.24 (m, 3H).
Description 14
2-(3,3-Difluorocyclobutypacetonitrile (D14)
i(r-CN
D14 was prepared using a similar procedure to that described for D13.111 NMR
(500 MHz,
DMSO-d6): 2.84-2.82 (m, 2H), 2.63-2.49 (m, 3H), 2.43-2.39 (m, 2H).
Description 15
2-(5-Methyltetrahydrofuran-3-yl)acetic acid (D15)
46
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
8
The mixture of 2-(5-methyltetrahydrofuran-3-yl)acetonitrile (D13, 180 mg) and
potassium
hydroxide (161 mg) in ethanol (2 mL) and water (2 mL) was added stirred at 70
C overnight and
then evaporated under vacuum. The residue was acidified to pH = 1 with 2 M HCl
(aq.) and then
extracted with DCM (10 mL). The organic layer was dried over MgSO4 and
concentrated to afford
the title compound (100 mg) as yellow oil (cis/trans mixture). ITINMR (500
MHz, CDC13): 4.15-
3.87 (m, 2H), 3.62-3.32 (m, 1H), 2.79-2.19 (m, 311), 1.81-1.66 (m, 2H), 1.31-
1.18 (m, 3H).
Description 16
2-(3,3-difluorocyclobutyl)acetic acid (D16)
FOH
D16 was prepared using a similar procedure to that described for D15. '1-1NMR
(500 MHz,
DMSO-d5): 12.21 (brs, 1H), 2.72-2.62 (m, 2H), 2.55-2.26 (m, 5H).
Description 17
2-Methyltetrahydro-211-pyran-4-ol (D17)
OH
A mixture of but-3-en-1-ol (7.21 g), 2,4,6-trimethy1-1,3,5-trioxane (4.40 g)
and 20% H2SO4 (12 g)
was heated to 85 C for 2 days. The mixture was cooled to RT and extracted with
ether (4x50 mL).
Combined organic layer was dried and evaporated to afford the title compound
(7.5 g). MS (El):
C61-11202 requires 116; found 116 [M].
Description 18
2-Methyldihydro-211-pyran-4(311)-one (D18)
0
To a solution of' 2-methyltetrahydro-2H-pyran-4-ol (D17, 12.5 g) in DCM (200
mL), PCC (23.20 g)
was added in several portions at 0 C. After addition, the mixture was stirred
at RT overnight. To
the mixture was added petroleum ether (200 mL) and silica gel (100 g). The
mixture was stirred for
mm then filtered through Celite, and the cake was washed with Et0Ac-petroleum
ether (1: 4,
100 mL). Filtrate was dried and evaporated to leave the crude product, which
was purified by
column chromatography (silica gel, petroleum ether/Et0Ac = 20:1 to 10:1) to
afford the title
compound (6.5 g) as pale yellow oil. '11 NMR (400 MHz, CDC13): 4.31-4.26 (m,
1H), 3.78-3.66 (m,
30 2H), 2.63-2.54 (m, 1H), 2.43-2.25 (m, 3H), 1.32 (d, J= 6.4 Hz, 3H).
Description 19
47
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Ethyl 2-(2-methyldihydro-211-pyran-4(311)-ylidene)acetate (D19)
0,, 0
To an ice-cooled solution of ethyl 2-(diethoxyphosphoryl)acetate (14.04 g) in
DMF (100 mL),
sodium hydride (2.505 g) was added in several protions. After addition, the
mixture was stirred at
0 C for 30 mm, and 2-methyldihydro-2H-pyran-4(3H)-one (D18, 6.5 g) was added
dropwise. The
reaction mixture was stirred at 0 C for 1 hour, poured into ice water (200
mL), extracted with
Et0Ac (3 x100 mL). The combined organic layer was washed with brine (200 mL),
dried and
evaporated to leave the crude product, which was purified by column
chromatography (silica gel,
petroleum ether/Et0Ac = 20:1) to afford title compound (12.5 g) as colorless
oil. MS (El):
C101-11603 requires 184; found 184 [1\41+.
Descriptions 20-21
Descriptions 20-21 were prepared using a similar procedure to that described
for D19.
D20 Ethyl 2-(3-methyldihydro-2H-pyran-4(3H)-ylidene)acetate
D21 Ethyl 2-(2-methyldihydrofuran-3(2H)-ylidene)acetate
MS (EST): C10111603 requires 184; found 185 [M+H]+.
D20
0
D21
MS (ESI): C9111403 requires 170; found 171 [M+H].
\cp¨' 0
Description 22
Ethyl 2-(2-methyltetrahydro-2H-pyran-4-yl)acetate (D22)
0õ,.- 0
A mixture of ethyl 2-(2-methyldihydro-2H-pyran-4(3H)-ylidene)acetate (D19,
12.5 g) and Pd/C (1
g) in ethanol (150 mL) was stirred at RT overnight under H2 atmosphere (1
atm). The mixture was
filtered through Celite, and the cake was washed with DCM, The filtrate was
concentrated under
vacuum to afford the title compound (12.2 g) as colorless oil. MS (El):
C10111803 requires 186;
found 186 [M]E .
Descriptions 23-24
Descriptions 23 and 24 were prepared using a similar procedure to that
described for D22.
D23 Ethyl 2-(3-methyltetrahydro-2H-pyran-4-ypacetate
D24 Ethyl 2-(2-methyltetrahydrofuran-3-ypacetate
MS (EST): C10111803 requires 186; found 187 [M+H].
D23
0
48
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
MS (ESI): C9111603 requires 172; found 173 [M+Hr.
1124
0 0
Description 25
2-(2-Methyltetrahydro-2H-pyran-4-yl)acetic acid (D25)
OH
A mixture of ethyl 2-(2-methyltetrahydro-2H-pyran-4-ypacetate (D22, 12.2 g)
and sodium
hydroxide (3.93 g) THF (40 mL) and water (80 mL) was heated to 60 C for 3
hours. The mixture
was concentrated, and the residual aqueous phase was adjusted to pH = 2 with
dilute HC1, extracted
with Et0Ac (3 x50 mL). Combined organic layer was dried and evaporated to
leave the crude
product, which was purified by column chromatograohy (silica gel, petroleum
ether/Et0Ac = 4:1 to
2:1) to afford the title compound (7.15 g) as colorless oil. MS (El): C8141403
requires 158; found
158 [Mr.
Descriptions 26-27
Descriptions 26 and 27 were prepared using a similar procedure to that
described for D25.
D26 2-(3-Mcthylictrahydro-211-pyran-4-ypacctic acid
D27 2-(2-Methyltetrahydrofuran-3-yDacetic acid
D26 MS (EST): C8H1403 requires 158; found 157 [M-
H].
0
OH
D27 MS (ESI): C7H1203 requires 144; found 143 [M-
HI.
0 0
Description 28
3-(Chloromethyl)-4-methyltetrahydrofuran (D28)
<"\rct
0
A mixture of 3-(allyloxy)prop-1-ene (5 g) and iron III chloride (9.92 g) in
THF (100 mL) was
cooled to 0 C and then NaBH4 (2.89 g) was added. The resulting suspension was
stirred at RT
overnight under 02 atmosphere. The mixture was poured into water (100 mL) and
extracted with
ether (3 x50 mL). The combined organic layer was dried and evaporated to
afford the crude title
compound (7.5 g). MS (El): C6Hl1C10 requires 134; found 134 [M]4.
Description 29
2-(4-Methyltetrahydrofuran-3-yl)acetonitrile (D29)
49
CN
0
A mixture of 3-(chloromethyl)-4-methyltetrahydrofuran (D28, 7.5 g) and NaCN (4
g) in DMS0
(100 mL) was heated to 90 C for 20 hours, then poured into ice (200 g),
extracted with Et0Ac
(4x50 mL). The combined organic layer was washed with saturated LiC1 aqueous
solution (2x100
mL), dried and evaporated to afford the crude title compound (4.0 g) as brown
oil. MS (EST):
CJIIINO requires 125; found 124 [M-Hr.
Description 30
2-(4-Methyltetrahydrofuran-3-Aacetic acid (D30)
6ThrOH
0
A mixture of 2-(4-methyltetrahydrofuran-3-yl)acetonitrile (D29, 4.0 g) and
potassium hydroxide
(1.793 g) in ethanol (50 mL) was heated at 85 C for 24 hours. The mixture was
evaporated to
dryness, and the residue was dissolved in water (100 mL) and washed with ether
(2x50 mL). The
aqueous layer was acidified to pH = 3 with KHSO4 solution and then extracted
with ether (3 x50
mL). The combined organic layer was dried and evaporated. The residue was
purified by column
chromatography (silica gel, petroleum ether/Et0Ac = 5:1) to afford title
compound (800 mg) as
yellow oil (cis and trans isomers). NMR (400 MHz, CDC13): 4.14-3.94 (m,
211), 3.57-3.33 (m,
2H), 2.68-2.10 (m, 3H), 2.19-1.80 (m, 1H), 1.05-0.95 (m, 3H).
Description 31
2-(1-Iodoethyl)tetrahydrofuran (D31)
Cr I
The mixture of (Z)-hex-4-en-l-ol (2.2 g), PdC12(dppf)-CH2C12 adduct (0.538 g)
and 1-
iodopyrrolidine-2,5-dione (5.93 g) in toluene (100 mL) was a stirred at RT for
2 hours and then
TM
filtered through Celite. The filtrate was concentrated under vacuum, and the
residue was partitioned
between water (100 mL) and Et0Ac (100 mL). The organic layer was dried over
Na2SO4, filtered
and concentrated. The crude product was purified by column chromatography
(silica gel, petroleum
ether/Et0Ac = 8:1) to afford the title compound (2.78 g). NMR (400 MHz,
CDC13): 4.20-4.14
(m, 1H), 3.98-3.93 (m, 1H), 3.87-3.82 (m, 1H), 3.75-3.70 (m, 1H), 2.10-1.91
(m, 3H), 1.92 (d, J
=7.0 Hz, 3H), 1.69-1.60 (m, 1H). MS (El): C6H1 JO requires 226; found 226 [M].
Description 32
2-(Tetrahydrofuran-2-yl)propanenitrile (D32)
cr
Date Recue/Date Received 2022-05-09
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
The mixture of 2-(1-iodoethyl)tetrahydrofuran (D31, 2.71 g) and NaCN (1.176 g)
in water (12 mT )
and ethanol (20 mL) was refluxed for 18 hours. The mixture was evaporated
under vacuum to
afford the crude title compound (1.5 g). MS (EST): C7IIIIN0 requires 125;
found no mass.
Description 33
.. 2-(Tetrahydrofuran-2-yl)propanoic acid (D33)
conccOH
The mixture of 2-(tetrahydrofuran-2-yl)propanenitrile (D32, 1.5 g) and KOH
(4.5 g) in ethanol (16
mL) were heated at 85 C for 18 hours and then concentrated. The residue was
acidified to pH = 5
with 4 M HC1 (aq.) and extracted with DCM (8 mL). The organic layer was dried
over Na2SO4,
.. filtered and concentrated. The crude product was purified by column
chromatography (silica gel,
petroleum ether/Et0Ac = 3:1) to afford the title compound (410 mg) as orange
oil. MS (ESI):
C7111203 requires 144; found 145 [M+H].
Description 34
Ethyl 2-(dihydrofuran-3(211)-ylidene)propanoate (D34)
To a solution of sodium hydride (1.394 g) in THF (60 mL) at 0 C was added
dropwise ethyl 2-
(diethoxyphosphoryl)propanoate (8.29 g) under N2. The mixture was stirred at 0
C for 30 min until
the mixture became clear, then dihydrofuran-3(2H)-one (1.5 g) was added. The
mixture was stirred
at RT for 2 hours and then quenched by water (100 mL). The mixture was
extracted with Et0Ac
(3 x50 mL). The combined organic layer was washed with brine (100 mL), dried
and evaporated
under vacuum. The crude product was purified by column chromatography (silica
gel, petroleum
ether/Et0Ac = 30:1) to afford the title compound (1.3 g). MS (ESI): C9I11403
requires 170; found
171 [M+11]+.
Description 35
Ethyl 2-(tetrahydrofuran-3-yl)propanoate (D35)
The mixture of ethyl 2-(dihydrofuran-3(2H)-ylidene)propanoate (D34, 2.0 g) and
10% palladium
on carbon (0.250 g) in methanol (15 mL) was stirred at 30 C under H2 (1 atm)
for 2 hours. The
mixture was filtered through Celite, and the filtrate was concentrated under
vacuum to afford the
title compound (2.1 g) as yellow oil. MS (ESI): C9111603 requires 172; found
173 [M+H]t
51
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Description 36
2-(Tetrahydrofuran-3-yl)propanoic acid (D36)
OH
0 0
To a mixture of ethyl 2-(tetrahydrofuran-3-yl)propanoate (D35, 2.1g) in
ethanol (10 mL) and water
(10 mL) was added LiOH aqueous solution (4 M, 15.24 mL). The mixture was
stirred at 30 C for 4
hours and then evaporated to remove most Et0H. The residue was washed with
Et0Ac (2x50 mL),
the aqueous layer was adjusted to pH = 3 with 3 M HC1 (aq.) and then extracted
with Et0Ac (4x50
mL). The combined organic was dried and evaporated under vacuum to afford the
title compound
(1.2 g) as yellow oil. IHNMR (500 MHz, DMSO-d6): 12.18 (s, 1H), 3.79-3.58 (m,
3H), 3.35-3.28
(m, 1H), 2.28-2.23 (m, 2H), 2.00-1.91 (m, 1H), 1.55-1.50 (m, 1H), 1.10-1.00
(m, 3H). MS (El):
C7111203 requires 144; found 143 [M-11]-.
Description 37
4-Methoxybutanoic acid (D37)
To a solution of methyl 4-methoxybutanoate (555.6 mg) in THF (6 mL), sodium
hydroxide (6.31
mL) (2M aqueous solution) was added, the reaction mixture was stirred
overnight. Most solvent
was removed by rotavap, diluted with water (5 mL), washed with DCM (5 mL). The
aqueous layer
was acidified with 3 M HC1 (aq.) to pH = 2, extracted with Et0Ac twice (2x15
mL). The combined
organic layer was dried over Na2SO4 and filtered. The filtrate was
concentrated to afford the title
.. compound (491.3 mg) as colorless oil. 1H NMR (400 MHz, DMSO-d6): 12.02 (s,
1H), 3.30 (t, J=
6.6 Hz, 2H), 3.21 (s, 3H), 2.23 (t, J= 7.3 Hz, 2H), 1.71 (quin, J= 6.9 Hz,
2H).
Description 38
Ethyl 2-(2-oxocyclohexyl)acetate (D38)
The mixture of 4-(cyclohex-1-en-1 -yl)morpholine (2.0 g) and ethyl 2-
bromoacetate (3.0 g) in
benzene (30 mL) was stirred at 50 C overnight. The reaction mixture was
concentrated. Water (50
mL) and Et0Ac (50 mL) were added to the residue. The resulting mixture was
extracted. The
organic layer was dried over Na2SO4 and concentrated to afford the title
compound (1g) as yellow
oil. MS (ESI): C10111603 requires 184; found 185 [M+H].
Description 39
2-(2-0xocyclohexypacetic acid (D39)
0
anccOH
52
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
The mixture of ethyl 2-(2-oxocyclohexyl)acetate (D38, 1 g) and potassium
hydroxide (0.305 g) in
methanol (20 mL) and water (20 mL) was stirred at 60 C for 2 hours and then
concentrated under
vacuum. The residue was acidified with 2 M HC1 (aq.) to pH = 1 and then
extracted with Et0Ac
(50 mL). The organic layer was dried and concentrated afford the title
compound (300 mg) as white
solid. MS (ESI): C8111203 requires 156; found 155
Description 40
2-(2-0xocyclopentyl)acetic acid (D40)
0
.601,0H
The mixture of ethyl 2-(2-oxocyclopentyl)acetate (600 mg) and potassium
hydroxide (396 mg) in
methanol (20 mL) and water (20 mL) was stirred at 60 C for 3 hours and then
concentrated under
vacuum. The residue was acidified with 2 M HCl (aq.) to pH = 1 and extracted
with Et0Ac (10
mL). The organic layer was dried and concentrated under vacuum to afford the
title compound (300
mg) as oil. MS (EST): C7111003 requires 142; found 141 [M-Hr.
Description 41
Methyl 4-(methylthio)butanoate (D41)
The mixture of methyl 4-bromobutanoate (905 mg) and sodium methanethiolate
(526 mg) in DMF
(16 mL) was heated at 80 C overnight. The mixture was diluted with water (15
mL) and extracted
with Et0Ac (40 mL). The organic layer was dried over Na2SO4, filtered and
concentrated to afford
the title compound (760 mg) as colorless oil. 11-I NMR (400 MHz, CDC13): 3.67
(s, 3H), 2.53 (t, J=
7.2 Hz, 2H), 2.44 (t, J= 7.4 Hz, 2H), 2.09 (s, 311), 1.92 (quin, J= 7.2 Hz,
2H). MS (El): C61-11202S
requires 148; found 148 [M].
Description 42
Methyl 4-(methylsulfonyl)butanoate (D42)
Methyl 4-(methylthio)butanoate (D41, 741 mg) was added into acetic anhydride
(2.363 mL) and
acetic acid (2.4 mL) cooling with ice bath and stirred for 30 mm, then 11202
(30%) (5.11 mL) was
added dropwise during 15 mm. The mixture was stirred at 0 C for 18 hours, a
trace amount of
Mn02 was added to quench excess H202, and the mixture was stirred for another
30 min. The
organic solvents was removed under vacuum. The residue was partitioned between
saturated
Na2CO3 solution (10 mL) and Et0Ac (30 mL). The organic layer was washed with
brine (30 mL),
dried over Na2SO4 and concentrated to afford the title compound (440 mg) as
colorless oil. 1H
NMR (400 MHz, CDC13): 3.69 (s, 3H), 3.13-3.07 (m, 2H), 2.92 (s, 3H), 2.54 (t,
J= 7.0 Hz, 2H),
2.21-2.11 (m, 2H). MS (ESI): C6111204S requires 180; found 181 [M+H].
53
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Description 43
4-(Methylsulfonyl)butanoic acid (D43)
oõo 9
OH
The mixture of methyl 4-(methylsulfonyl)butanoate (D42, 0.44 g) and LiOH
aqueous solution (2 M,
.. 3.66 mL) in THE (10 mL) were stirred at RT overnight. The mixture was
diluted with water (8 mL)
and Et0Ac (16 mL). The organic layer was discarded, and the aqueous phase was
acidified to pH =
5-6 with 1 M HC1 (aq.), extracted with Et0Ac (20 mL). The organic layer was
dried over
Na2SO4, filtered and concentrated to afford the title compound (310 mg) as
white solid. 1H NMR
(400 MHz, DMSO-d6): 12.21 (brs, 111), 3.13 (t, J= 7.7 Hz, 2H), 2.96 (s, 3H),
2.39 (t, J= 7.2 Hz,
.. 2H), 1.89 (quin, J= 7.7 Hz, 2H). MS (EST): C5111004S requires 166; found
167 [M+H].
Description 44
Ethyl 2-(thietan-3-ylidene)acetate (D44)
sJ
To a solution of thietan-3-one (8 g) in DCM (100 mL), ethyl 2-
(triphenylphosphoranylidene)acetate
(31.6 g) in DCM (50 mL) was added dropwise over 30 min at 0 C. The reaction
mixture was
gradually warmed to RT and stirred 6 hours. The mixture was concentrated and
the residue was
partitioned between brine (50 mL) and Et0Ac (50 mL). The aqueous layer was
extracted with
Et0Ac (2x50 mL). Combined organic layer was dried over anhydrous MgSat and
concentrated to
afford the title compound (5 g) as yellow oil. MS (EST): C7I-11002S requires
158; found 159 [M+H].
.. Description 45
D45 Ethyl 2-(dihydro-2H-thiopyran-4(311)-ylidene)acetate
sD-
0 \¨
Description 45 was prepared using a similar procedure to that described for
D44. MS (ESI):
C9111402S requires 186; found 187 [M+H].
Description 46
Ethyl 2-(1,1-dioxidothietan-3-ylidene)acetate (D46)
o#¨/ 11,0
To a solution of ethyl 2-(thietan-3-ylidene)acetate (D44, 500 mg) in DCM (50
mL) was added 3-
chlorobenzoperoxoic acid (1091 mg). The mixture was stirred overnight at RT.
The mixture was
filtered, and the filtrate was concentrated. The residue was partitioned
between saturated NaHCO3
solution (20 mL) and Et0Ac (20 mL). The aqueous layer was extracted with Et0Ac
(2x10 mL).
54
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Combined organic layer was dried over anhydrous MgSO4 and concentrated to
afford the title
compound (300 mg) as yellow solid. MS (ESI): C7111004S requires 190; found 191
{1\4+H}.
Description 47
Ethyl 2-(1,1-dioxidodihydro-2H-thiopyran-4(311)-ylidene)acetate (D47)
/
= )/-0
0 \---
Description 47 was prepared using a similar procedure to that described for
D46. MS (ESI):
C91114045 requires 218; found 219 [M+H].
Description 48
Ethyl 2-(1,1-dioxidothietan-3-ypacetate (D48)
o=s/Dr
The mixture of ethyl 2-(1,1-dioxidothietan-3-ylidene)acetate (D46, 300 mg) and
10% Pd/C (168
mg) in methanol (10 mL) was hydrogenated at 20 C for 12 hours under H2
atmosphere (1 atm).
The mixture was filtered through Celite and the filtrate was concentrated
under vacuum to afford
the title compound (200 mg) as yellow oil. MS (ESI): C7I-112045 requires 192;
found 193 [M+Hr.
Description 49
Ethyl 2-(1,1-dioxidotetrahydro-211-thiopyran-4-yl)acetate (D49)
0õ(\
/ ____________________________________ \
\---
To the mixture of nickel II chloride (594 mg) and NaBH4 (433 mg) in ethanol
(20 mL) was added
ethyl 2-(1,1-dioxidodihydro-2H-thiopyran-4(3H)-ylidene)acetate (D47, 500 mg)
at 0 C. The
reaction mixture was gradually allowed to warm to RT and stirred at RT for 6
hr. The mixture was
concentrated and the residue was treated with saturated NaC1 solution (50 mL)
and extracted with
Et0Ac (2x50 mL). The combined organic layer was washed with brine (2x50 mL),
dried over
anhydrous MgSO4, separated and concentrated to give the title compound (300
mg) as yellow oil.
MS (ESI): C91-11604S requires 220; found 221 [M+H].
Description 50
2-(1,1-Dioxidothietan-3-yl)acetic acid (P50)
0,s/roFf
0
The mixture of potassium hydroxide (88 mg) and ethyl 2-(1,1-dioxidothietan-3-
yl)acetate (D48,
150 mg) in methanol (20 mL) and water (20 mL) was stirred at 80 C for 2 hours.
The reaction
mixture was concentrated under vacuum. The residue was acidified with 2 M HCl
(aq.) to pH = 1
and extracted with Et0Ac (20 mL). The organic layer was dried and concentrated
under vacuum to
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
afford the title compound (120 mg) as white solid. MS (ESI): C5H804S requires
164; found 163
Description 51
2-(1,1-Dioxidotetrahydro-211-thiopyran-4-yl)acetic acid (D51)
0
0
Description 51 was prepared using a similar procedure to that described for
D50. MS (EST):
C71-11204S requires 192; found 215 [M+Nar.
Description 52
Ethyl 3-(tosyloxy)cyclobutanecarboxylate (D52)
Ts0
0
To a solution of ethyl 3-hydroxycyclobutanecarboxylate (800 mg) in DCM (40 mL)
was added
TsC1 (1.14 g) and pyridine (0.898 mL) at RT. The mixture was then stirred at
RT overnight. The
mixture was washed with water (2x20 mL). The combined organic layer was dried
over Na2SO4,
filtered and concentrated under vacuum to leave the crude as yellow oil, which
was purified by
flash chromatography (silica gel, petroleum ether/Et0Ac = 20:1) to afford the
title compound (600
mg) as colorless oil. MS (ESI): C14I-118055 requires 298; found 299 [M+H].
Description 53
Ethyl 3-(methylthio)cyclobutanecarboxylate (D53)
,s
To a solution of ethyl 3-(tosyloxy)cyclobutanecarboxylate (D52, 1.6 g) in DMF
(50 mL) was added
sodium methanethiolate (3.76 g). The reaction mixture was stirred at 90 C
overnight. Solvent was
removed under vacuum, and the residue was partition between Et0Ac (20 mL) and
water (20 mL).
The organic layer was dried and concentrated under vacuum to afford the title
compound (600 mg)
as brown oil. MS (El): C81-11402S requires 174; found 174 [Mr.
Description 54
3-(Methylthio)cyclobutanecarboxylic acid (D54)
OH
0
To a solution of ethyl 3-(methylthio)cyclobutanecarboxylate (D53, 400 mg) in
methanol (10 mL)
was added a solution of NaOH (184 mg) in water (5 mL) at RT, the mixture was
stirred at RT
overnight and then concentrated under vacuum, the residue was acidified to pH
= 5 with 1M HC1.
56
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
The resulting solid was collected by filtration to afford the title compound
(300 mg) as yellow solid.
MS (ESI): C61-11002S requires 146; found no mass.
Description 55
Ethyl 2-(dihydro-211-thiopyran-3(411)-ylidene)acetate (D55)
LO
To a mixture of dihydro-2H-thiopyran-3(4H)-one (410 mg) in DMF (3 mL) were
added ethyl 2-
(diethoxyphosphorypacetate (1187 mg) and K2CO3 (488 mg). The reaction mixture
was stirred at
80 C for 3 hours. The mixture was poured into water (50 mL) and extracted with
Et0Ac (30 mL).
The organic layer was dried over anhydrous sodium sulfate and concentrated.
The residue was
purified by column chromatography (silica gel, petroleum ether) to afford the
title compound (584
mg) as colorless oil. MS (ESI): C91-11402S requires 186; found 187 [M+H]t
Description 56
Ethyl 2-(tetrahydro-211-thiopyran-3-yDacetate (D56)
0
To ethanol (6 mL) was added NaBH4 (471 mg). The mixture was cooled in an ice
bath and nickel II
chloride (404 mg) was added. Ethyl 2-(tetrahydro-211-thiopyran-3-yl)acetate
(D55, 500 mg) in
ethanol (1 mL) was added slowly into above mixture. The reaction mixture was
warmed to RT and
stirred at RT overnight. The mixture was concentrated under vacuo to afford
the title compound
(500 mg) as dark oil. MS (ESI): C91-116025 requires 188; found 189 [M+H].
Description 57
2-(Tetrahydro-211-thiopyran-3-yl)acetic acid (D57)
sr,OH
o
To the solution of ethyl 2-(tetrahydro-2H-thiopyran-3-yl)acetate (D56, 500 mg)
in Me0H (12 mL)
was added a solution of sodium hydroxide (212 mg) in water (12.00 mL). The
mixture was stirred
at 18 C overnight. After the reaction completed, the organic solvent was
removed under vacuo. The
aqueous layer was washed with DCM (10 mL) and acidified with 1 M HC1 (aq.) to
pH = 6 and
extracted with Et0Ac (50 mL). The organic layer was washed with brine (50 mL),
dried and
evaporated to afford the title compound (400 mg) as yellow solid. MS (ESI):
C71-11202S requires
160; found no mass.
Description 58
Ethyl 2-(dihydrothiophen-3(211)-ylidene)acetate (D58)
57
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
To a solution of ethyl 2-(diethoxyphosphoryl)acetate (346 mg) in anhydrous THF
(2 mL) in water-
bath, sodium hydride (37.0 mg) was added portion wise. The mixture was stirred
for 0.5 hour at
18 C. Then the reaction mixture was cooled to -70 C and added dihydrothiophen-
3(2H)-one (105
mg). The reaction mixture was stirred for 1 hour at -70 C, then poured into
water (2 mL), extracted
with Et0Ac (2x10 mL). The organic layer was dried over Na2SO4, filtered and
concentrated. The
residue was purified by column chromatography (silica gel, petroleum ether/
THF =100:1) to afford
the title compound (142 mg) as colorless oil. MS (ESI): C8111202S requires
172; found 173 [M+Hr.
Description 59
Ethyl 2-(tetrahydrothiophen-3-yl)acetate (D59)
NaBH4 (156 mg) was dissolved in ethanol (4 mL), which was added nicke111
chloride (107 mg) in
ice-water bath. Ethyl 2-(dihydrothiophen-3(2H)-ylidene)acetate (D58, 142 mg)
was added into
above mixture slowly, and then the reaction mixture was warmed to RT slowly
and stirred
overnight. The mixture was concentrated under vacuo, and the residue was
purified by column
chromatography (silica gel, petroleum ether/Et0Ac = 100:1) to afford the title
compound (118 mg)
as colorless oil. MS (ESI): C81-11402S requires 174; found 175 [M+H]t
Description 60
2-(Tetrahydrothiophen-3-yl)acetic acid (D60)
OH
0
To a solution of ethyl 2-(tetrahydrothiophen-3-ypacetate (D59, 118 mg) in Me0H
(5 ml) was
added a solution of sodium hydroxide (54.2 mg) in water (5.00 mL). The mixture
was stirred
overnight at RT. After removal of the organic solvent under vacuo, the residue
was acidified with 3
M HC1 (aq.) to pH < 6, extracted with Et0Ac (15 mL). The organic layer was
separated and
condensed to afford the title compound (88 mg) as white solid. MS (ESI):
C6111002S requires 146;
found 145 [M-111-.
Description 61
2-(Tetrahydrothiophen-2-ypacetic acid (D61)
\¨OH
0
To a mixture of 2,2,2-trifluoroacetic acid (8.02 g) and triethylsilane (9.81
g) were added 2-
(thiophen-2-yDacetic acid (2 g) and BF3-Et20 (1.908 g). The reaction mixture
was gradually warm
to RT and stirred at 90 C for 4 days. The mixture was concentrated and brine
(50 mL) was added to
the residue. The mixture was extracted with Et0Ac (2x50 mL). The combined
organic layer was
washed with brine (50 mL), dried over anhydrous MgSO4 and concentrated to
afford the title
compound (1 g) as yellow oil. MS (ESI): C61-11002S requires 146; found 145 [M-
HI.
58
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Description 62
Ethyl 1-bromocyclo butanecarboxylate (D62)
To a solution of ethyl cyclobutanecarboxylate (10 g) in CC14 (100 mL) was
added NBS (20.83 g)
and AIBN (1.281 g). The mixture was stirred at 80 C for 2 hours. Water (50 mL)
was added and
the mixture was extracted with Et0Ac (3 x50 mL). The combined organic was
washed with
saturated NaHCO3 (50 mL), water (50 mL) and brine (50 mL), dried over MgSO4
and evaporated.
The residue was purified by column chromatography (silica gel, petroleum
ether/Et0Ac = 100:1) to
afford the title compound (10 g) as yellow oil. 1H NMR (400 MHz, CDC13): 4.26
(q, J= 14.4, 7.2
Hz, 2H), 2.95-2.88 (m, 2H), 2.67-2.59 (m, 2H), 2.26-2.21 (m, 1H), 1.91-1.86
(m, 1H), 1.32 (t, J=
7.2 Hz, 3H).
Description 63
Cyclobut- 1-enecarboxylic acid (D63)
0
OH
II
To a solution of ethyl 1-bromocyclobutanecarboxylate (D62, 4 g) in toluene (50
mL) was added
potassium hydroxide (5.42 g). The mixture was stirred at 110 C for 2 hours and
evaporated under
vacuum. To the residue was added water (50 mL). The mixture was adjusted to pH
= 6 and
extracted with Et0Ac (3 x50 mL). The combined organic layer was washed with
saturated NaHCO3
(50 mL), water (50 mL) and brine (50 mL), dried over MgSO4 and evaporated to
afford the title
.. compound (1.8 g) as brown solid. 1HNMR (400 MHz, DMSO-d6): 12.25 (brs, 1H),
6.75 (s, 1H),
2.58 (t, J= 3.2 Hz, 2H), 2.38 (t, J= 2.8 Hz, 21).
Description 64
2-(Acetylthio)cyclobutanecarboxylic acid (D64)
0
rE-OH
______________________________________ 0
To a solution of cyclobut-l-enecarboxylic acid (D63, 500 mg) in CC14 (10 mL)
was added
thioacetic acid (776 mg). The mixture was stirred at RT for 48 hours and
evaporated under vacuum.
To the residue was added water (50 mL), and the mixture was extracted with
Et0Ac (3 x50 mL).
The combined organic layer was washed with water (50 mL) and brine (50 mL),
dried over MgSO4
and evaporated to afford the crude title compound (300 mg) as brown oil. MS
(ESI): C7I-11103S
requires 174; found no mass.
Description 65
3-Methylenecyclobutanecarboxylic acid (D65)
59
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
HOyfr
0
To a solution of 3-methylenecyclobutanecarbonitrile (5.0 g) in ethanol (20 mL)
was added KOH
aqueous solution (35%, 34.4 g) and the resulting mixture was heated to reflux
overnight. The
ethanol was removed under reduced pressure. The residue was cooled to below 10
C and acidified
with concentrated HC1 to pH = 5. The mixture was extracted with Et0Ac (2x50
mL). The
combined organic extracts were dried over anhydrous sodium sulfate and
concentrated under
vacuum to afford the title compound (6.01 g) as yellow oil. MS (BSI): C6H802
requires 112; found
111 [M-HI.
Description 66
3-Methylenecyclobutanecarboxylate (D66)
,01(Cr.
0
The mixture of 3-methylenecyclobutanecarboxylic acid (D65, 6.0 g), K2CO3
(14.79 g) and Me2SO4
(7.67 mL) in acetone (100 mL) was heated to reflux for 2 hours. The reaction
mixture was cooled
to RT and filtered. The solvent was removed under reduced pressure, and the
residue was purified
with column chromatography (silica gel, petroleum ether/Et0Ac = 20:1) to
afford the title
compound (2.1 g) as colorless oil. 'H NMR (500 MHz, DMSO-d6): 4.80-4.75 (m,
2H), 3.62 (s, 3H),
3.20-3.12 (m, 1H), 2.89-2.81 (m, 4H).
Description 67
Methyl spiro[2.3]hexane-5-carboxylate (D67)
To an ice-cooled (0 C) solution of diethylzinc (1.0 M in hexane) (39.6 mL) in
DCM (30 mL) was
added dropwise a solution of TFA (3.05 mL) in DCM (10 mL). After one hour of
stirring,
diiodomethane (10.62 g) in DCM (10 mL) was then introduced. After 40 min, the
solution of
methyl 3-methylene cyclobutanecarboxylate (D66, 2.0 g) in DCM (4 mL) was added
dropwise. The
reaction was stirred at RT for 2 hours and then quenched with saturated NH4C1
solution (30 mL).
The organic layer was separated, dried and concentrated to afford the title
compound (1.9 g) as pale
yellow oil. MS (EST): C8111202 requires 140; found 139 [M-H].
Description 68
Spiro[2.3]hexane-5-carboxylic acid (D68)
H0,11,-0A
0
The mixture of methyl spiro[2.3]hexane-5-carboxylate (D67, 0.50 g) and LiOH
aqueous solution (2
M, 5.35 mL) in THF (10 mL) was stirred at RT overnight. The mixture was
diluted with water (8
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
mL) and Et0Ac (16 mL). Extracted, the organic layer was discarded, and the
aqueous layer was
acidified with 1 M HCl (aq.) to pH = 5-6 and then extracted with Et0Ac (20
mL). The organic
layer was dried over anhydrous sodium sulfate, filtered and concentrated to
afford the title
compound (200 mg) as pale yellow oil. MS (ESI): C2111002 requires 126; found
125 [M-11]-.
Description 69
Benzyl 2-(difluoromethoxy)acetate (1)69)
>-F
41)
Benzyl 2-hydroxyacetate (1246 mg), sodium sulfate (213 mg), and acetonitrile
(16 mL) were
placed in a 100 ml two-necked flask fitted with a magnetic stirrer, a dropping
funnel and a
refluxing condenser. 2,2-difluoro-2-(fluorosulfonyl)acetic acid (3.10 mL) was
then added with
stirring at 45 C. After addition, the mixture was further stirred for 2 hours
at this temperature. The
reaction mixture was poured into 10% aqueous sodium carbonate solution (50 mL)
and was
extracted with Et0Ac (2x50 mL). The combined extracts were washed with water
(50 mL) and
brine (50 mL), dried over sodium sulfate, and concentrated. The residue was
purified by column
chromatography (silica gel, petroleum ether/Et0Ac = 4:1) to afford the title
compound (301 mg) as
colorless oil. 11-1NMR (500 MHz, CDC13): 7.41-7.33 (m, 5H), 6.36 (t, J.= 73.2
Hz, 1H), 5.23 (s,
2H), 4.46 (s, 2H). 19F NMR (376 MHz, CDC13): -86.0, -86.2. MS (El): C10H10F203
requires 216;
found 216 [M].
Description 70
2-(Difluoromethoxy) acetic acid (1)70)
F0OH
Benzyl 2-(difluoromethoxy)acetate (D69, 280 mg) was dissolved in Et0Ac (12
mL). DIPEA (0.226
mL) was added. Then palladium on carbon (50 mg, 10 %) was added under nitrogen
atmosphere.
The reaction was hydrogenated for 24 hours (1 bar of H2). Filtered through a
pad of Celite and
concentrated to afford the title compound as ammonium salt (200 mg). MS (ESI):
C3H4F203
requires 126; found 125 [M-HI.
Description 71
Benzyl 3-hydroxypropanoate (1)71)
01rOH
Oxetan-2-one (8.0 g) was added slowly to a stirred solution of sodium
methoxide (0.300 g) in
phenylmethanol (72.0 g) at 0 C. Stirring was continued for a further 12 hours
at 50 C. The reaction
61
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
mixture was washed with water, dried and distilled to get the title compound
as colorless oil. MS
(ESI): C10111203 requires 180; found 181 [M+H].
Description 72
Benzyl 3-(difluoromethoxy)propanoate (D72)
F.y.00
F 0
Benzyl 3-hydroxypropanoate (D71, 1.0 g), sodium sulfate (0.158 g), and
acetonitrile (16 mL) were
placed in a 100 ml two-necked flask fitted with a magnetic stirrer, a dropping
funnel and a
refluxing condenser. 2,2-Difluoro-2-(fluorosulfonyl)acetic acid (2.294 mL) was
then added with
stirring at 45 C. After addition, the mixture was further stirred for 2 hours
at this temperature. The
reaction mixture was poured into 10 % aqueous sodium carbonate solution (30
mL) and was
extracted with Et0Ac (3 x30 mL). The combined extracts were washed with water
(30 mL) and
brine (30 mL), dried over anhydrous sodium sulfate and concentrated.The
residue was purified by
column chromatography (silica gel, petroleum ether/Et0Ac = 5:1) to afford the
title compound
(100 mg) as colorless oil. 1H NMR (400 MHz, CDC13): 7.41-7.38 (m, 5H), 6.22
(t, J= 74.4 Hz, 1H),
5.19 (s, 2H), 4.18 (t, J= 6.4 Hz, 2H), 2.74 (t, J= 6.0 Hz, 2H). 19F (376 MHz,
CDCI3): -84.5, -84.7.
MS (ESI): C11H12F203 requires 230; found no mass.
Description 73
3-(Difluoromethoxy)propanoic acid (D73)
0
F OOH
The mixture of benzyl 3-(difluoromethoxy)propanoate (D72, 100 mg), D1PEA
(0.076 mL) and
palladium on carbon (50 mg, 10 %) in Et0Ac (12 mL) was hydrogenated at RT
under hydrogen
atmosphere (1 atm) for 24 hours. The mixture was filtered through a pad of
Celite and concentrated
to afford the title compound as the ammonium salt (60 mg). MS (ESI): C4H6F203
requires 140;
found 139 [M-HT.
Description 74
1-Methyl-5-oxopyrrolidine-3-carboxylic acid (D74)
\oH
A mixture of 2-methylenesuccinic acid (20 g) and methanamine (17.90 g) (40%
solution in H20)
was heated to 115 C for 2 hours and then cooled to RT. The mixture was
evaporated to remove
most of solvent. The residue was acidified to pH = 3 with concentrated HC1
acid, and the resulting
white solid was collected by suction to leave the crude product as white
solid, which was further
purified by recrystallization from Et0Ac (30 mL) to afford the title compound
(12 g) as white solid.
MS (ESI): C6H9NO3 requires 143; found 142 LM-III.
Description 75
62
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
4-(2-Diazoacety1)-1-methylpyrrolidin-2-one (D75)
0
,N
To a suspension of 1-methyl-5-oxopyrrolidine-3-carboxylic acid (D74, 2.86 g)
in DCM (40 mL)
was added 0.5 mL of DMF and then oxalyl chloride (5 mL) was added dropwise.
After addition the
mixture was stirred at RT for 1 hour and then evaporated to leave the crude
acyl chloride. The
above crude acyl chloride was re-dissolved in a mixture of THF (20
mL)/acetonitrile (20 mL) and
then cooled to 0 C. TMS-diazomethane (20 mL) (2M solution in hexane) was added
dropwise.
After addition the mixture was stirred at RT overnight. The mixture was
evaporated to give the
crude product, which was purified by column chromatography (silica gel,
DCM/Me0H = 10:1) to
afford the title compound (1 g) as brown oil. MS (ESI): C7119N302 requires
167; found 168 [M+Hr.
Description 76
2-(1-Methyl-5-oxopyrrolidin-3-yl)acetic acid (D76)
OH
¨N
0
0
To a solution of 4-(2-diazoacety1)-1-methylpyrrolidin-2-one (D75, 1 g) in THF
(100 mL) and
distillated water (50 mL) was added silver nitrate (1.016 g). The mixture was
stirred at RT
overnight. The mixture was evaporated to remove THF. The aqueous phase was
adjusted to pH = 3
with 1 M HC1(aq.) and then extracted with Et0Ac (6x50 mL). The combined
organic layer was
dried, filtered, and evaporated to afford the title compound (800 mg) as brown
oil. MS (EST):
C7HIIN03 requires 157; found 156 [M-Hr.
Description 77
Tert-butyl 3-(2-ethoxy-2-oxoethyl)-2-oxopyrrolidine -1-carboxylate (D77)
o
Boc,N6 _______________________________ 7¨C)
The solution of tert-butyl 2-oxopyrrolidine-1-carboxylate (5 g) in anhydrous
THF (50 mL) was
cooled to -78 C and then LDA (2.89 g) solution in THF was added dropwise. The
mixture was
stirred at -78 C for 1 hour, and then ethyl 2-bromoacetate (13.50 mL) was
added dropwise. After
addition the mixture was stirred at -78 C for 1 hour and then warm to RT and
stirred for 16 hours.
The reaction mixture was partitioned between water (50 mL) and Et0Ac (50 mL).
The aqueous
layer was extracted twice with Et0Ac (2x50 mL). The combined extracts were
dried, seperated and
concentrated under reduced pressure to leave the crude product, which was
purified by column
chromatography (silica gel, petroleum ether/Et0Ac = 1:1) to afford the title
compound (3 g) as
yellow oil. MS (EST): C13H211\105 requires 271; found 172 [M-F2H-Bocr.
Description 78
63
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Ethyl 2-(2-oxopyrro lidin-3-yl)acetate hydrochloride acid salt (D78)
0 r¨
HNo= Ha
To a solution of tert-butyl 3-(2-ethoxy-2-oxoethyl)-2-oxopyrrolidine -1-carbo
xylate (D77, 3 g) in
Me0H (20 mL) was added the solution of hydrogen chloride (13.82 mL) in
dioxane. The mixture
was stirred at RT for 2 hours and then concentrated to afford the title
compound (2 g) as yellow
solid. MS (ESI): C8H13NO3 requires 171; found 172 [M+11]+.
Description 79
Ethyl 2-(1-methyl-2-oxopyrrolidin-3-yl)acetate (D79)
To a solution of ethyl 2-(2-oxopyrrolidin-3-ypacetate hydrochloride acid salt
(D78, 500 mg) in
THF (10 mL) was added NaH (105 mg). The mixture was stirred at 0 C for 10
minutes and then
Mel (0.274 mL) was added. The mixture was stirred at RT overnight. Water (40
mL) was added
and extracted with Et0Ac (3x50 mL). The combined organic layer was washed with
saturated
NaHCO3 (50 mL), water (50 mL) and brine (50 mL), dried over MgSO4 and
concentrated to afford
the title compound (300 mg) as yellow oil. MS (ESI): C9H15NO3 requires 185;
found 186 [M+H].
Description 80
2-(1-Methyl-2-oxopyrrolidin-3-ypacetic acid (D80)
0
OH
¨N
0
Ethyl 2-(1-methy1-2-oxopyrrolidin-3-yl)acetate (D79, 300 mg) was dissolved in
THF (5 mL) and
water (5 mL), then sodium hydroxide (4 M, 2.025 mL) aqueous solution was
added. The mixture
was stirred at RT for 1 hour. Water (30 mL) was added and the mixture was
washed with Et0Ac
(30 mL). The water phase was adjusted to pH = 6 with 2 M HC1 (aq.) and
lyophilized to afford the
crude title compound (200 mg) as yellow oil. MS (ESI): C7RIN03 requires 157;
found 158
[M+Hr.
Description 81
1-Ethyl-5-oxopyrrolidine-3-carboxylic acid (D81)
0 0H
A mixture of 2-methylenesuccinic acid (10 g) and ethanamine (50 mL) (2M
solution in THF) in
isopropanol (50 mL) was heated to 120 C overnight and then evaporated to
remove most of solvent.
The residue was acidified with concentrated HC1 solution to pH = 2 and then
evaporated under
64
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
vacuum. To the residue was added a mixture of DCM/Me0H (60 mL, 5:1), and then
anhydrous
sodium sulfate (20 g) was added. The mixture was stirred at RT for 2 hours and
then filtered. The
filtrate was evaporated to get the title compound (4 g) as sticky oil. MS
(EST): C7IIIIN03 requires
157; found 156 [M-1-1]-.
Description 82
1-Ethyl-5-oxopyrrolidine-3-carbonyl chloride (D82)
Jict0
To a solution of 1-ethyl-5-oxopyrrolidine-3-carboxylic acid (D81, 1.6 g) in
dry DCM (6 mL) was
added sulfurous dichloride (4.84 g). The mixture was stirred at 40 C overnight
and then evaporated
under vacuum. The residue was re-dissolved in anhydrous DCM (10 mL) and
concentrated under
reduced pressure to get the title compound (1.8 g) as brown oil. MS (ESI):
C8H13NO3 requires 171;
found 172 [M+H] (sample was converted to corresponding methyl ester by
dissolved in Me0H
and sent to LCMS).
Description 83
4-(2-Diazoacety1)-1-ethylpyrrolidin-2-one (D83)
0
1-Ethyl-5-oxopyrrolidine-3-carbonyl chloride (D82, 1.6 g) was dissolved in
acetonitrile (12 mL)
and THF (12 mL). A solution of (diazomethyl)trimethylsilane (9.1 mL) in ether
(2 M) was added.
The reaction mixture was stirred at 25 C for 5 hours. After the reaction
completed, the solvents
were removed under vacuum to leave the crude product, which was purified by
column
chromatography (silica gel, petroleum ether/ THF = 2:1) to afford the title
compound (1.4 g) as
yellow solid. MS (ESI): C8H11N302 requires 181; found 182 [M+H].
Description 84
2-(1-Ethyl-5-oxopyrrolidin-3-yl)acetic acid (D84)
OH
0
To the mixture of 4-(2-diazoacety1)-1-ethylpyrrolidin-2-one (D83. 1.4 g) in
THF (50 mL) and water
(25 mL) was added AgNO3 (1.575 g). The reaction mixture was stirred at 26 C
for 2 days. The
mixture was acidified with 1 M HC1 (aq.) to pH = 3 and concentrated under
vacuum. The residue
was washed with Et0Ac and filtered. The filtrate was dried, seperated and
concentrated under
vacuum to afford the title compound (700 mg) as yellow oil. MS (EST):
C81113NO3 requires 171;
found 172 [M+H].
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Description 85
Ethyl 1-cyanocyclopropanecarboxylate (D85)
0
To the mixture of ethyl 2-cyanoacetate (5 g) and potassium carbonate (18.33 g)
in acetone (20 mL)
was added 1,2-dibromoethane (9.96 g) over a period of 10 min. The reaction
mixture was heated to
reflux overnight. More 1,2-dibromoethane (9.96 g) was added and the reaction
mixture was
refluxed for another 2 hours. The reaction mixture was filtered through a pad
of Celite and the cake
was rinsed with aetone (20 mL). The combined filtrate was concentrated under
reduced pressure to
give the title compound (5 g) as orange oil. MS (EST): C7H9NO2 requires 139;
found 140 [M+H]t
Description 86
1-(HydroxymethyDcyclopropanecarbonitrile (D86)
N
To a mixture of ethyl 1-cyanocyclopropanecarboxylate (D85, 4 g) in
dimethoxyethane (80 mL) and
methanol (8 mL) was added sodium borohydride (115 mmol). The mixture was
stirred at RT for 18
hours. The solution was diluted with saturated NaHCO3 aqueous solution (100
mL) and then
extracted with 10% Me0H / DCM (3 x100 mL). The combined organic layer was
dried over
anhydrous sodium sulfate, seperated and concentrated under vacuum to afford
the title compound
(1.3 g) as pale brown oil. 1H NMR (400 MHz, DMSO-d6): 5.30 (t, J= 6.0 Hz, 1H),
3.39 (d, J= 5.8
Hz, 2H), 1.17-1.12 (m, 2H), 0.94-0.90 (m, 2H).
Description 87
1-Formylcyclopropanecarbonitrile (D87)
To a solution of 1-(hydroxymethyl)cyclopropanecarbonitrile (D86, 1.1 g) in DCM
(10 mL) was
added Dess-Martin reagent (4.80 g). The reaction mixture was stirred at 12 C
overnight and then
poured into saturated NaHCO3 aqueous solution (30 mL) until no gas released.
Saturated Na2S203
aqueous solution (30 mL) was added and then the mixture was extracted with
Et0Ac (30 mL). The
organic layer was washed with brine (30 mL), dried, seperated and evaporated
under vacuo to
afford the title compound (550 mg). MS (ESI): C5H5NO requires 95; found no
mass.
Description 88
Ethyl 3-(1-cyanocyclopropyDacrylate (D88)
N
0
To the mixture of 1-formylcyclopropanecarbonitrile (P87, 550 mg) in anhydrous
DCM (3 mL) was
added ethyl 2-(triphenylphosphoranylidene)acetate (2418 mg). The mixture was
stirred at RT for
66
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
16 hours and then concentrated under reduced pressure to leave the crude
product, which was
purified by column chromatography (silica gel, petroleum ether/Et0Ac = 25:1)
to afford the title
compound (289 mg) as white solid. MS (ESI): C911111\102 requires 165; found
166 [M+H].
Description 89
Ethyl 3-(1-cyanocyclopropyl)propanoate (D89)
0
The mixture of ethyl 3-(1-cyanocyclopropyl)acrylate (D88, 200 mg) and Pd/C (30
mg, 10%) in
ethanol (7 mL) was stirred under hydrogen atmosphere (1 atm) overnight. The
mixture was filtrated
and the filtrate was concentrated under vacuum to afford the title compound
(170 mg) as colorless
oil. MS (ES1): C9H13NO2 requires 167; found 168 [M+Hr.
Description 90
3-(1-Cyanocyclopropy1)- propanoic acid (D90)
0
To a solution of ethyl 3-(1-cyanocyclopropyl)propanoate (D89, 170 mg) in the
mixture solvent of
ethanol (10 ml) and water (10 mL) was added sodium hydroxide (81 mg). The
reaction mixture
was stirred at 18 C overnight and then evaporated under vacuum. The aqueous
layer was acidified
with 1 M HC1 (aq.) to pH = 5-6 and extracted with Et0Ac (20 mL). The organic
layer was dried,
separated and evaporated under vacuum to afford the title compound (80 mg) as
yellow oil. MS
(ESI): C7H9NO2 requires 139; found 138 [M-1-1I.
Description 91
Methyl 4-cyano-4-methylpentanoate (D91)
0
0
A solution of lithium diisopropylamide (11.2 g) in anhydrous TIM (70 mL) was
cooled to -78 C
and then isobutyronitrile (9.6 g) was added dropwise, the reaction mixture was
stirred at -78 C for
additional 2 hours and then the solution of methyl acrylate (6 g) in anhydrous
THE (15 mL) was
added slowly, after addition the reaction mixture was stirred at -78 C until
the reaction was
complete (approximately 60 min). The reaction mixture was then poured into
aqueous saturated
NH4C1 solution (100 mL), the organic layer was separated and washed with water
(100 mL) and
brine (100 ml), dried and concentrated to afford the title compound (9 g) as
yellow oil. MS (ESI):
C81-113NO2 requires 155; found 156 [M+Hr.
Description 92
4-Cyano-4-methylpentanoic acid (D92)
OH
N7. 0
67
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
To a solution of methyl 4-eyano-4-methylpentanoate (D91, 1.2 g) in methanol
(12 mL) and water
(12 mL) was added potassium hydroxide (0.9 g), the reaction mixture was
stirred at 25 C overnight.
The organic solvent was removed under vacuo, the residual aqueous phase was
washed with DCM
(10 mL), the aqueous phase was then acidified with 1 M HC1 (aq.) to pH = 6 and
then extracted
with Et0Ac (20 mL), the organic layer was dried and evaporated under vacuo to
afford the title
compound (0.8 g) as brown solid. 'H NMR (400 MHz, DMSO-d6): 12.30 (s, 1 H),
2.36 (t, J= 8.4
Hz, 2 H), 1.78 (t, J= 8.4 Hz, 2 H), 1.30 (s, 6 H). MS (ESI): C7IIIIN02
requires 141; found 140 WI-
HI.
Description 93
3-Hydroxy-2,2-dimethylpropanenitrile (D93)
N
To a mixture of ethyl 2-cyano-2-methylpropanoate (2.5 g) in THF (20 mL) and
water (50 mL) was
added NaBH4 (3.35 g) portionwise. After addition the mixture was stirred at RT
for 6 hours.
Hydrochloric acid (6 M) was added to quench the reaction mixture, and then
extracted with Et0Ac
(50 mL). The extract was washed with water (50 mL), dried over anhydrous
magnesium sulfate and
concentrated under reduced pressure to afford the title compound (1 g). 'H NMR
(400 MHz,
Me0D-d4): 5.45 (s, 1H), 3.36 (s, 211), 1.21 (s, 611).
Description 94
Tricyclo[2.2.1.02,61heptan-3-y1 acetate (D94)
OAc
To a solution of acetic acid (10.17 g) and bicyclo[2.2.1]hepta-2,5-diene (15.6
g) was added
BF3=Et20 (1.073 mL). The mixture was stirred at 100 C for 6 hours and then
evaporated. To the
residue was added brine (200 mL) and then extracted with ether (2x200 mL). The
combined
organic layer was washed with brine (100 mL), dried over anhydrous MgSO4,
separated and
concentrated to afford the title compound (8 g) as yellow oil. MS (ESI):
C9111202 requires 152;
found 153 [M+Hr.
Description 95
Tricyclo[2.2.1.02,61heptan-3-ol (D95)
N.OH
To the mixture of sodium (1.511 g) in Me0H (60 mL), was added
tricyclo[2.2.1.02,6]heptan-3-y1
acetate (D94, 6 g). The mixture was stirred at 80 C for 6 hours and then
evaporated. To the residue
was added brine (50 mL) and then extracted with ether (2x50 mL). The combined
organic layer
was washed with brine (50 mL), dried over anhydrous MgSO4, separated and
concentrated to afford
the title compound (1.5 g) as yellow oil. MS (ESI): C7I-1100 requires 110;
found 111 [M+H].
68
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Description 96
Tricyclo[2.2.1.02,6]heptan-3-one (D96)
To the mixture of water (10 mL), sulfuric acid (17.81 g) and chromiumVI oxide
(4.54 g) was added
tricyclo[2.2.1.02,6]heptan-3-ol (D95, 1 g). The mixture was stirred at 0 C for
6 hours. Water (50
mL) was added and the resulting mixture was extracted with ether (2x50 mL).
The combined
organic layer was dried over anhydrous MgSO4 and concentrated to afford the
title compound (800
mg) as yellow oil. MS (ESI): C71-180 requires 108; found 109 [M+Hr.
Description 97
Bicyclo[3.1.0]hexane-3-carboxylic acid (D97)
H0,1(07
0
To the solution of KOtBu (2594 mg) in diethyl ether (20 mL) was added
tricyclo[2.2.1.02,6]heptan-
3-one (D96, 500 mg) at 0 C. The mixture was stirred at 0 C for 6 hours and
then poured into water
(50 mL). The mixture was extracted with ether (2x20 mL), dried over anhydrous
MgSO4 and
concentrated to afford the title compound (200 mg) as yellow oil. MS (ESI):
C7111002 requires 126;
found 125 [M-HI.
Description 98
2-(Methoxycarbonyl) cyclopropanecarboxylic acid (D98)
0,0H
0õ 0
KOH (1.14 g) was added to the mixture of dimethyl cyclopropane-1,2-
dicarboxylate (3 g) in
Me0H (50 mL). The reaction mixture was heated to 80 C for 5 hours. The
reaction mixture was
cooled to RT and was partition between water (100 mL) and Et0Ac (50 mL). The
organic layer
was discarded. The water phase was adjusted to pH ¨ 2 with 1 M HC1 (aq.) and
extracted with
Et0Ac (50 mL). The organic layer was dried and concentrated under vacuum to
afford the title
compound (1.5 g) as yellow solid. MS (ESI): C6H804 requires 144; found 143 [M-
HI.
Description 99
3-(2-Diazoacetyl) cyclobutanone (D99)
o=0_4=N7-_N
0
To a solution of 3-oxocyclobutanecarboxylic acid (2 g) in DCM (10 mL) was
added SOC12 (3.84
mL). The reaction mixture was stirred at 20 C for 2 hours. The mixture was
evaporated under
reduced pressure to give acyl chloride. To a solution of the above crude acyl
chloride in TITF (5 mL)
and acetonitrile (5 mL) was added (diazomethyl)trimethylsilane (1 M in hexane,
35 mL). The
reaction mixture was stirred at 20 C overnight and then evaporated under
vacuum to leave the
69
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
crude product, which was purified by column chromatography (silica gel,
petroleum ether/Et0Ac =
2:1) to afford the title compound (400 mg) as yellow oil. MS (EST): C6H6N202
requires 138; found
139 [M+H]t
Description 100
2-(3-0xocyclobutypacetic acid (D100)
0
0
To a solution of 3-(2-diazoacetyl)cyclobutanone (D99, 400 mg) in THE (20 mL)
and water (10 mL)
was added AgNO3 (590 mg). The reaction mixture was stirred at RT overnight and
then evaporated
under vacuum to remove most of THF. The water phase was extracted with Et0Ac
(3 x10 mL). The
combined organic layer was dried over anhydrous Na2SO4 and evaporated under
vacuum to afford
the title compound (360 mg) as yellow oil. MS (ESI): C6H803 requires 128;
found 129 [M+H].
Description 101
(R)-2-((tert-butoxycarbonyl)amino)pentanoic acid (D101)
H0,11), N 5,)
0
To a mixture of (R)-2-aminopentanoic acid (2.343 g) and Na2CO3 (2.120 g) in
water (60.00 mL)
was added dropwise a solution of Boc20 (4.88 mL) in THF (20 mL). The mixture
was stirred at RT
overnight. The mixture was washed with ether (2x20 mL), and the water phase
was adjusted to pH
= 3 with KHSO4 solution and then extracted with Et0Ac (2x30 mL). The combined
organic was
dried and evaporated to afford the title compound (4 g) as sticky oil. MS
(EST): C10H19N04 requires
217; found 216 [M-H].
Description 102
(R)-ethyl 2-(N-benzy1-2-((tert-butoxycarbonyl)amino) pentanamido)acetate
(D102)
o
0
To a mixture of ethyl 2-(benzylamino)acetate (3.38 g), (R)-2-((tert-
butoxycarbonyl)amino)pentanoic acid (D101, 8.9 g) and DIPEA (3.05 mL) in DCM
(50 mL) was
added HATU (6.65 g). The mixture was stirred at RT for 2 days. To the mixture
was added 1 M
KHSO4 solution (50 mL), which resulted in large amount of precipitate. The
mixture was filtered,
and the filtrate was stirred at RT for 5 mm and then the organic layer was
separated. The organic
layer was then washed with saturated NaHCO3 solution (50 mL), KHSO4 solution
(50 mL, 1 M)
and then sat NaHCO3 solution (50 mL), dried over Na2SO4, filtered and
evaporated to afford the
title compound (8.9 g) as yellow oil. MS (ESI): C211-132N205 requires 392;
found 393 [M+H].
Description 103
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(R)-1-benzy1-3-propylpiperazine-2,5-dione (D103)
HN
(3,NBn
The (R)-ethyl2-(N-benzy1-2-((tert-butoxycarbonyl)amino)pentanamido)acetate
(D102, 3.2 g) in
toluene was heated at 85 C overnight and then concentrated to leave the crude
as brown oil, which
was diluted with saturated NaHCO3 solution (50 mL) and Et0Ac (50 mL). The
organic layer was
dried over Na2SO4, filtered and concentrated to leave a brown oil, which was
purified by reverse
phase column chromatography (C18 column, 10-95% CH3CN in H20 with 0.01%
NR00O3) to
afford the title compound (900 mg) as white solid. MS (ES!): C14H18N202
requires 246; found 247
[1\4+11r.
Description 104
(R)-1-benzy1-3-propylpiperazine (D104)
rf
HN
L. NBn
A solution of (R)-1-benzy1-3-propylpiperazine-2,5-dione (D103, 1100 mg) in dry
THF (25 mL)
was flushed with nitrogen for 5 min. The solution was cooled to 0 C in an ice
bath, and LiAlat
(678 mg) was added in portions. The reaction was stirred at RT for 1 hour,
then another batch of
LiA1H4 (275 mg) was added, and the reaction was stirred at RT for 36 hours.
The mixture was
quenched with Na2SO4.10 H20 in an ice bath. The white precipitate was filtered
and washed with a
mixture of DCM (20 mL) and Me0H (20 mL). The filtrate was concentrated to give
the crude as
green oil, which was purified by reverse phase column chromatography (C18
column, 10-95%
CH3CN in H20 with 0.01% NH4HCO3) to afford the title compound (750 mg) as pale
orange solid.
MS (ER): C14H22N2 requires 218; found 219 [M+H].
Description 105
(R)-(4-benzy1-2-propylpiperazin-1-y1) (cyclopentyl) methanone (D105)
o
To the mixture of (R)-1-benzy1-3-propylpiperazine (D104, 180 mg) and TEA
(0.230 mL) in DCM
(10 mL) was added slowly cyclopentanecarbonyl chloride (120 mg) at RT and
stirred for 15 mm.
The mixture was diluted with water (5 inL), and the organic layer was washed
with brine and dried
over Na2SO4, filtered and concentrated to afford the title compound (250 mg)
as white solid. MS
(ES!): C201130N20 requires 314; found 315 [M+H].
Description 106
71
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(R)-cyclopenty1(2-propylpiperazin-1-yOmethanone (D106)
o
CrjL'N
The mixture of (R)-(4-benzy1-2-propylpiperazin-l-y1)(cyclopentyl)methanone
(D105, 240 mg) and
10% palladium-carbon (50 mg) in ethanol (5 mL) was hydrogenated at RT under
hydrogen
atmosphere (1 atm) for 16 hours. The mixture was filtered through Celite and
the filtrate was
concentrated under reduced pressure to afford the title compound (150 mg) as
oil. MS (ESI):
C13H24N20 requires 224; found 225 [M+H].
Description 107
Ethyl 2-(2-methyloxazol-4-yl)acetate (D107)
cr:rf
The mixture of acetamide (200 mg) and ethyl 4-chloro-3-oxobutanoate (1672 mg)
in ethanol (20
mL) was stirred at 50 C for 1 hour and then concentrated. The crude product
was purified by
column chromatography (silica gel, petroleum ether/Et0Ac = 5:1) to afford the
title compound
(150 mg) as yellow solid. MS (ESI): C81111NO3 requires 169; found 170 [M+Hr.
Description 108
2-(2-methyloxazol-4-y1) acetic acid (D108)
OH
NO
0 -7Y-Y
The mixture of ethyl 2-(2-methyloxazol-4-ypacetate (D107,150 mg) and potassium
hydroxide (149
mg) in ethanol (20 mL) was stirred at 80 C for 3 hours. The reaction mixture
was concentrated
under vacuum, 1M HC1 (aq.) was added to pH = 5, extracted with Et0Ac (3 x10
mL). Combined
organic layer was dried and evaporated to afford the title (100 mg) as yellow
solid. MS (ESI):
C6H7NO3 requires 141; found 142 [M+H]t
Description 109
2-(3-Methylisoxazol-5-ypacetic acid (D109)
OH
N-.'Yo0
To a solution of 3,5-dimethylisoxazole (4.5 g) in dry THF (60 mL), n-
butyllithium (23.17 mL, 2.5
M) was added dropwise under nitrogen at -75 C. During the addition,
temperature was kept below -
55 C. After addition, the mixture was stirred for 30 min then poured onto dry
ice (50 g). Water (50
mL) and Et0Ac (30 mL) were added and the mixture was stirred at RT for 30 mm,
the organic
layer was discarded, the aqueous layer was adjust to pH = 2 with HCI,
extracted with Et0Ac (2x20
mL). Combined organic layer was dried over magnesium sulfate, filtered and
concentrated under
72
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
reduced pressure, the crude product was triturated with petroleum ether/Et0Ac
(10:1, 30 mL) to
afford the title compound (2.3 g) as brown solid. MS (ESI): C6117NO3 requires
141; found 142
[M+H].
Description 110
Methyl 2,6-dichloro-5-fluoronicotinate (0110)
CINCI
To a mixture of 2,6-dichloro-5-fluoronicotinic acid (5 g) and one drop of DMF
in DCM (20 mL),
oxalyl chloride (5 mL) was added dropwise at RT. The mixture was stirred at RT
for 1 hour, and
then concentrated. The resulting acyl chloride was re-dissolved in DCM (10
mL), and then added
.. dropwise to a mixture of DCM (20 mL) and Me0H (20 mL). The resulting
mixture was stirred at
RT for another 1 hour, and then concentrated to afford the title compound (6
g) as pale yellow oil.
MS (ESI): C7H4C12FNO2 requires 223; found 224 [M+H].
Description 111
Methyl 2-chloro-5-fluoro-6-methylnicotinate (D111)
0
-'1\1-' CI
A mixture of methyl 2,6-dichloro-5-fluoronicotinate (D110, 6 g), 2,4,6-
trimethy1-1,3,5,2,4,6-
trioxatriborinane (3.36 g), K2CO3 (9.99 g) and Pd(Ph3P)4 (1.548 g) in 1,4-
dioxane (50 mL) was
heated to 110 C for 20 hours. The mixture was filtered, and the filtrate was
concentrated. The
residue was purified by column chromatography (silica gel, petroleum
ether/Et0Ac = 10:1) to
afford the title compound (3.5 g) as yellow oil. MS (ESI): C8117C1FN02
requires 203; found 204
[M+H].
Description 112
Methyl 5-fluoro-6-methylnicotinate (D112)
0
.. A mixture of methyl 2-chloro-5-fluoro-6-methylnicotinate (D111, 4.2 g),
Pd/C (0.5 g) and sodium
acetate (6.77 g) in Et0Ac (50 mL) was stirred at RT overnight under hydrogen
atmosphere (1 atm).
The mixture was filtered, and the filtrate was concentrated. The residue was
purified by column
chromatography (silica gel, petroleum ether/Et0Ac = 10:1) to afford the title
compound (3.5 g) as
white solid. MS (ESI): C8H8FNO2 requires 169; found 170 [M+Hr.
Description 113
5-Fluoro-6-methylnicotinic acid (0113)
73
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
0
To a solution of methyl 5-fluoro-6-methylnicotinate (D112, 2.3 g) in THF (10
mL) and methanol
(10 mL) was added a solution of NaOH (0.707 g) in water (5 mL). The mixture
was stirred at RT
for 1 hour, and then concentrated under vacuum. To the residue was added water
(5 m1). The pH of
the mixture was adjusted to 3. The solid was collected and dried under vacuum
to afford the title
compound (800 mg) as white solid. 1H NMR (400 MHz, DMSO-d6): 8.83 (s, 1H),
8.00 (dd, .1= 9.6,
1.2 Hz, 1H), 2.57 (s, 3H). MS (ESI): C7H6FNO2 requires 155; found 156 [M+H].
Description 114
Ethyl 5-cyano-2-hydroxy-6-methylnicotinate (D114)
NOH
0
A mixture of diethyl 2-(ethoxymethylene)malonate (21.6 g) and 3-aminobut-2-
enenitrile (8.2 g)
was stirred at 150 C for 2 hours and standing overnight. Filtered, the solid
was washed with ice-
cold methanol to give the title compound (5 g) as yellow solid. MS (ESI):
C101110N203 requires 206;
found 207 [M+H].
Description 115
Ethyl 2-chloro-5-cyano-6-methylnicotinate (D115)
NrC)
0
A mixture of ethyl 5-cyano-2-hydroxy-6-methylnicotinate (D114, 3.0 g) in
phosphoryl trichloride
(22.3 g) was stirred at 90 C for 5 hours and standing overnight. The solution
was concentrated
under vacuum. The residue was poured into ice. The resulting mixture was
filtered to afford the
title compound (3 g) as yellow solid. MS (ESI): C10H9C1N202 requires 224;
found 225 [M+H].
Description 116
Ethyl 5-cyano-6-methylnicotinate (D116)
I
N 0
To a mixture of ethyl 2-chloro-5-cyano-6-methylnicotinate (D115, 1.5 g),
methanol (50 mL) and
palladium (10% on carbon, 0.071 g) was added ammonium formate (6.32 g). The
mixture was
stirred at RT for 3 hours, and then filtered. The solution was concentrated
under vacuum. The
residue was purified by column chromatography (silica gel, petroleum
ether/Et0Ac = 4:1) to afford
the title compound (1 g) as white solid. MS (EST): C10H10N202 requires 190;
found 191 [M+Hf'.
Description 117
74
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
5-Cyano-6-methylnicotinic acid (D117)
= =
rOH
N 0
To a mixture of ethyl 5-cyano-6-methylnicotinate (D116, 1 g), methanol (15 mL)
and water (30 mL)
was added sodium hydroxide (2.1 g). The mixture was stirred at RT for 30 min.
The pH of the
.. solution was adjusted to 4 with hydrochloric acid, extracted with Et0Ac (2x
100 mL). Combined
organic layer was concentrated under vacuum to afford the title compound (800
mg) as white solid.
1H NMR (400 MHz, Me0D-d4): 9.20 (s, 1H), 8.62 (s, 1H), 2.83 (s, 3H). MS (ESI):
C8H6N202
requires 162; found 163 [M+H].
Description 118
Methyl 6-(hydroxymethyl)nicotinate (D118)
HO N
A solution of dimethyl pyridine-2,5-dicarboxylate (5 g) in THF (50 mL) was
cooled to 0 C and
then NaBH4 (1.454 g) was added in several portions. The reaction mixture was
stirred at RT
overnight, aq NH4C1 solution (50 mL) was added and the mixture was extracted
with Et0Ac (3x20
mL). The combined organic layer was dried over Na2SO4, Filtered, the filtrate
was concentrated
under vacuum. The residue was purified by column chromatography (silica gel,
petroleum
ether/Et0Ac = 5:1) to afford the title compound (3 g) as white solid. MS
(EST): C8H9NO3 requires
167; found 168 [M+H].
Description 119
Methyl 6-(fluoromethyDnieotinate (D119)
0
A solution of methyl 6-(hydroxymethyptheotinate (D118, 2.0 g) in DCM (40 mL)
was cooled to -
78 C and then DAST (1.9 g) was added dropwise. The reaction mixture was
stirred at -78 C for 4
hours, quenched with saturated NaHCO3 aqueous solution (40 mL). The organic
layer was
.. separated, dried over Na2SO4. Filtered, the filtrate was concentrated under
vacuum, the residue was
purified by column chromatography (silica gel, petroleum ether/Et0Ac = 10:1)
to afford the title
compound (250 mg) as white solid. MS (ESI): C8H8FNO2 requires 169; found 170
{M+H}.
Description 120
6-(Fluoromethypnicotinic acid (D120)
OH
0
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
To a solution of methyl 6-(fluoromethyDnicotinate (D119, 250 mg) in methanol
(20 mL), NaOH
(118 mg) solution in water (10 mL) was added. The reaction mixture was stirred
at 30 C overnight.
Most solvent was evaporated off, the residue was acidified to pH = 3 with 1 M
hydrochloric acid
and then lyophilized to get the crude title compound (200 mg) as white solid.
11-1 NMR (400 MHz,
DMSO-d6): 9.04 (d, J= 2.0 Hz, 1H), 8.35 (dd, J= 8.0, 2.3 Hz, 1H), 7.62 (d, J=
8.0 Hz, 1H), 5.53
(d, J= 46.4 Hz, 2H). MS (ESI): C7H6FNO2 requires 155; found 156 [M+H].
Description 121
Methyl 6-carbamoylnicotinate (D121)
0
N"-
0
To a solution of 5-(methoxycarbonyl)picolinic acid (12 g) in DCM (80 mL),
oxalyl dichloride (8.41
g) was added dropwise over 15 minutes. This mixture stirred at RT for 3 hours
and then solvent
was removed under vacuum. The crude acyl chloride was re-dissolved with DCM
(40 mL), added
dropwise over 15 minutes into concentrated ammonia aqueous solution (20 mL) at
0 C. The
reaction mixture was stirred for 15 minutes, filtered, the filtrate was
concentrated to afford the title
compound (11 g) as white solid. MS (EST): C8H8N203 requires 180; found 181
[M+H].
Description 122
6-Carbamoylnicotinic acid (D122)
0
H2N
OH
0
To a solution of methyl 6-carbamoylnicotinate (D121, 11 g) in THF (20 mL) and
water (20 mL),
NaOH (24.42 g) was added portion-wise over 15 minutes. This mixture stirred at
RT for 3 hours.
The solution was neutralized with 2 M HC1 (aq.) to pH = 5, and extracted with
Et0Ac (2x60 mL).
The combined organic layer was washed with water (3x 80 mL) and brine (2x40
mL), dried over
Na2SO4 and filtered. The filtrate was concentrated under vacuum to afford the
title compound (2 g)
as white solid. MS (ESI): C7116N203 requires 166; found 167 [M+Hr.
Description 123
Methyl 5,6-dichloronicotinate (D123)
CI N
0
0
The mixture of 5,6-dichloronicotinic acid (5 g) and sulfurous dichloride (3.10
g) in methanol (20
mL) was stirred overnight at 25 C. Cold water (100 mL) was added and the
resulted mixture was
neutralized with saturated NaHCO3 solution. The aqueous layer was extracted
with DCM (2x100
76
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
mL). The combined organic layers were dried over Na2SO4, filtered, and
concentrated under
vacuum to afford the title compound (5 g) as white solid. MS (ESI): C7H5C12NO2
requires 205;
found 206 [M+Hr.
Description 124
Methyl 5-chloro-6-methylnicotinate (D124)
CI
To a solution of methyl 5,6-dichloronicotinate (11123,2 g), methylboronic acid
(0.581 g), K2CO3
(2.68 g) and Pd(PPh3)4 (0.561 g) in 1,4-dioxane (100 mL) was stirred at 75 C
overnight. The
reaction was filtered and the filtrate concentrated under vacuum to give the
residue, which was
purified by column chromatography (silica gel, petroleum ether/Et0Ac = 1:1) to
afford the title
compound (420 mg) as yellow solid. MS (ESI): C8H8C1NO2 requires 185; found 186
[M+H].
Description 125
5-Chloro-6-methylnicotinic acid (11125)
0
.. The mixture of methyl 5-chloro-6-methylnicotinate (D124, 450 mg), sodium
hydroxide (485 mg) in
methanol (20 mL) and water (5 mL) was stirred at RT for 1 hour. 4 M HC1 (aq.)
was used to
adjusted the solution to pH = 4. The solution was concentrated under vacuum
then treated with
Et0Ac (20 inL) and washed with water (2x10 mL). The combined organic layer was
dried over
Na2SO4, concentrated to give the title compound (400 mg) as white solid. MS
(ESI): C7H6C1NO2
requires 171; found 172 [M+H].
Description 126
5-Chloro-6-methylnicotinoyl chloride (D126)
CI CI
0
To a solution of 5-chloro-6-methylnicotinie acid (D125, 80 mg) in DCM (5 mL)
were added oxalyl
chloride (0.122 mL) and a drop of DMF. The mixture was stirred for 30 mm. The
mixture was
concentrated to afford the title compound (100 mg) as yellow solid. MS (EST):
C8H8C1NO2 requires
185; found 186 [M+H] (sample was converted to corresponding methyl ester by
dissolved in
Me0H and sent to LCMS).
Description 127
3-(Difluoromethyl)benzoyl chloride (D127)
77
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
CI
0
To the mixture of 3-(difluoromethypbenzoic acid (202 mg) and two drop of DMF
in dry DCM (3
mL) was added dropwise oxalyl dichloride (298 mg). After addition the mixture
was stirred at RT
for 30 mm. The solvent was removed under vacuum. The residue was re-dissolved
in dry DCM (2
mL) and concentrated to give the title compound (230 mg) as colorless oil. MS
(ESI): C9H8F202
requires 186; found no mass (sample was converted to corresponding methyl
ester by dissolved in
Me0H and sent to LCMS).
Description 128
4-Bromo-2-(difluoromethyflpyridine (D128)
B r
To a solution of 4-bromopicolinaldehyde (1 g) in DCM (20 mL) stirred under
nitrogen atmosphere
at 0 C was added DAST (1.065 mL). The reaction mixture was stirred at RT
overnight. To the
mixture was added water, and then extracted with DCM (3 x50 mL). The organic
phase was washed
with saturated NaHCO3, water, and brine, then dried over MgSO4 and filtered to
give the title
compound (800 mg) as yellow oil. MS (ESI): C6H4BrF2N requires 207; found no
mass.
Description 129
Methyl 2-(difluoromethyflisonicotinate (D129)
N =
F I
0
To a mixture of 4-bromo-2-(difluoromethyl)pyridine (D128, 900 mg), DPPP (357
mg),
triethylamine (0.603 mL) in methanol (20 mL) and DMF (5 mL) was passed CO gas
for 3 min. The
mixture was then heated to 120 C in a sealed vial at 16 atm for 8 hours. After
cooling to RT, the
mixture was concentrated. Water was added, extracted with Et0Ac (3 x50 mL).
The organic layer
was washed with saturated NaHCO3 solution (10 mL), water (10 mL), and brine
(10 mL), dried
over MgSO4, filtered and concentrated. The residue was purified by column
chromatography (silica
gel, petroleum ether/Et0Ac = 5:1) to afford the title compound (500 mg) as
yellow oil. MS (EST):
C8H7F2NO2 requires 187; found 188 [M+H].
Description 130
2-(Difluoromethyflisonicotinic acid (D130)
0
A mixture of methyl 2-(difluoromethyDisonicotinate (D129, 500 mg), NaOH (1069
mg) in water
(10 mL) and Me0H (10 mL) was stirred at RT overnight. The mixture was
concentrated and
78
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
adjusted to pH < 7 with 2 M HCI, then extracted with Et0Ac (3x50 mL). The
organic layer was
washed with saturated NaHCO3 solution (10 mL), water (10 mL), and brine (10
mL), dried over
MgSO4, filtered and concentrated to afford the title compound (400 mg) as
white solid. MS (ESI):
C7H5F2NO2 requires 173; found 174 [M+H]t
Description 131
2-(Difluoromethyl)isonicotinoyl chloride (11131)
Fy1H(C1
0
To a suspension of 2-(difluoromethyl)isonicotinic acid (11130, 160 mg) and two
drop of DMF in
anhydrous DCM (2 mL) was added dropwise oxalyl dichloride (176 mg). After
addition the
mixture was stirred for 1 hour at RT, then evaporated under vacuum. The
residue was re-dissolved
in anhydrous DCM (2 mL) and concentrated again to afford the title compound
(245 mg) as
colorless oil. MS (ESI): C8H7F2NO2 requires 187; found 188 [M+H] (sample was
converted to
corresponding methyl ester by dissolved in Me0H and sent to LCMS).
Description 132
5-Bromo-3-methylpicolinonitrile (11132)
Br
I
N
N
To a solution of 2,5-dibromo-3-methylpyridine (5 g) in DMF (20 mL) was added
cyanocopper
(1.785 g). The mixture was stirred at 120 C overnight and then cooled to RT.
The mixture was
partitioned between Et0Ac (50 mL) and water (50 mL). The organic layer was
washed with brine
(50 mL), dried over Na2SO4 and concentrated under vacuum to leave the crude,
which was purified
by column chromatography (silica gel, petroleum ether/Et0Ac = 4:1) to afford
the title compound
(600 mg, 13.14 % yield) as white solid. MS (ESI): C7H5BrN2 requires 195; found
196 [M+Hr.
Description 133
Methyl 6-cyano-5-methylnicotinate (11133)
0
1\1-
A mixture of 5-bromo-3-methylpicolinonitrile (11132, 700 mg), palladiumil
acetate (160 mg),
DPPP (394 mg) and TEA (1.486 mL) in methanol (12 mL) and DMF (3 mL) was heated
to
120 C for 12 hours under CO atmosphere (10 atm). After cooling to RT, the
mixture was
concentrated under vacuum, the residue was purified by column chromatography
(silica gel,
petroleum ether/Et0Ac = 4:1) to afford the title compound (300 mg) as sticky
oil. MS (ESI):
C9H8N202 requires 176; found 177 [M+H].
79
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Description 134
6-Cyano-5-methylnicotinic acid (D134)
OH
N
N
A mixture of methyl 6-cyano-5-methylnicotinate (D133, 250 mg) and LiOH (68.0
mg) in THE (15
mL) and water (5 mL) was stirred at RT overnight. The mixture was diluted with
water (10 mL)
and EA (16 mL), the organic phase was discarded, the water phase was acidified
to pH = 6 with 1M
HC1 (aq.) and then extracted with Et0Ac (20 mL), the organic phase was dried
over Na2SO4,
filtered and concentrated to afford the title compound (160 mg) as pale solid.
MS (ESI): C8H6N202
requires 162; found 163 [M+H]+.
Description 135
5-Fluoro-2-methy1-3-nitrobenzoic acid (D135)
02N
OH
5-Fluoro-2-methylbenzoic acid (20 g) was added portion-wise to ice-cooled con,
sulfuric acid (98%,
80 mL), the mixture was stirred at 0 C until all solid dissolved, and then the
mixture of nitric acid
(65%, 6 mL) and H2SO4. (98%, 12 mL) was added portion-wise, the mixture was
warmed gradually
to RT and stirred at RT for 6 hours. The mixture was poured into ice (500 g),
the resulting solid
was collected and washed with water (100 mL), the solid was re-dissolved in
Et0Ac (200 mL) and
washed with brine. The organic layer was dried over anhydrous Na2SO4.
Filtered, the filtrate was
concentrated under vacuum to afford the title compound (11 g) as brown solid.
IHNMR (400 MHz,
Me0D-d4): 7.84 (dd, J= 8.7, 2.6 Hz, 1H), 7.78 (dd, J= 7.8, 2.8 Hz, 1H), 2.55
(s, 3H). MS (ESI):
C8H6FNO4 requires 199; found 198 N-Hr.
Description 136
5-Chloro-2-methyl-3-nitrobenzoic acid (D136)
02N
OH
CI
To a solution of 5-chloro-2-methylbenzoic acid (8.5 g) in con. sulfuric acid
(98%, 150 mL), nitric
acid (65%, 17.1 mL) was added dropwise at 0 C and the mixture was warmed
gradually to RT and
stirred at RT for 5 hours. The mixture was poured into ice (-500 g), the
resulting solid was
collected and washed with water three times, dried to afford title compound
(10.7 g), with its regio-
isomer. 1H NMR (400 MHz, DMSO-d6): 8.19 (d, J= 2.3 Hz, 1H), 8.02 (d, J-= 2.3
Hz, 1H), 2.47 (s,
411). MS (ESI): C8H6CINO4 requires 215; found 238 [M+Na]t
Description 137
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
5-Bromo-2-methyl-3-nitrobenzoic acid (11137)
0
02N
OH
Br
2-Methyl-3-nitrobenzoic acid (5.0 g) was dissolved in conc. H2SO4 (20 mL) at 0
C. To this solution,
NBS (6.2 g) was added gradually. The resulting mixture was stirred at 0 C for
2 hours, and then
warmed to 40 C. After stirring at 40 C for 3 hours, the mixture was poured
into ice water. The
white solid precipitate was filtered and dried to give the title compound (7.0
g) as off-white solid.
MS (EST): C8H6BrN0.4 requires 259; found no mass.
Description 138
(5-Fluoro-2-methyl-3-nitrophenyl) methanol (D138)
02N 40
OH
A mixture of 5-fluoro-2-methyl-3-nitrobenzoic acid (11135, 11 g) and BH3-THF
(1 M, 72 mL) was
heated to 80 C for 2 hours. Me0H (20 mL) was added slowly to the mixture to
quench the reaction,
then the mixture was concentrated under vacuum to remove the solvents. The
residue was dissolved
in DCM (50 mL) and washed with saturated NaHCO3 solution (2x50 mL) and brine
(2x50 mL).
The organic phase was dried over Na2SO4, filtered and the filtrate was
concentrated to afford the
title compound (9 g) as yellow solid. 114 NMR (400 MHz, Me0D-d4): 7.54-7.44
(m, 2H), 4.68 (s,
211), 2.31 (s, 3H). MS (ESI): C8H8FNO3 requires 185; found 186 [M+H]+.
Description 139
(5-Chlorol-2-methyl-3-nitrophenyl) methanol (D139)
02N
OH
CI
To a solution of 5-chloro-2-methyl-3-nitrobenzoic acid (D136, 23.8 g) in THF
(400 mL) was added
BH3=THF (166 mL, 1 M in THF) dropwise at 0 C. The mixture was warmed gradually
to RT and
stirred overnight. Me0H was added dropwise until no gas released. The mixture
was poured into
water (100 mL), extracted with Et0Ac (2x100 mL). Combined organic layer was
dried over
Na2SO4. Filtered, the filtrate was concentrated under vacuum. The residue was
purified by column
chromatography (silica gel, petroleum ether/Et0Ac = 4:1) to afford the title
compound (20.5 g).
MS (BSI): C8H8C1NO3 requires 201; found 200 [M-HI.
Description 140
(2,5-Dichloro-3-nitrophenyl)methanol (D140)
81
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
02N 401
OH
CI
2,5-Dichloro-3-nitrobenzoic acid (2.2 g) was dissovled in THE (50 mL) at 0 C.
Then to this
solution, NaBH4 (1.64 g) was added gradually under ice bath. Then BF3=Et20
(5.5 mL) was added
dropwise carefully at 0 C. Subsequently the reaction mixture was stirred at RT
overnight. Methanol
was added slowly to quench the reaction. After remove of the solvent, the
residue was extracted by
Et0Ac (2x20 mL) and water (2x20 mL). The combined organic phases were dried
over sodium
sulfate and concentrated in vacuo to afford the title compound (1.9 g) as
yellow solid. 'H NMR
(400 MHz, CDC13): 7.83 (d, J= 2.2 Hz, 1H), 7.73 (d, J= 2.4 Hz, 1H), 4.86 (s,
2H), 2.20 (brs, 1H).
MS (ESI): C7H5C12NO3 requires 221; found 222 [M+H].
Description 141
5-Chloro-1-(chloromethyl)-2-methyl-3-nitrobenzene (D141)
02N
a
(5-Chloro-2-methyl-3-nitrophenyl) methanol (D139, 7 g) was dissolved in
sulfurous dichloride
(24.78 g). After stirred at 80 C overnight, the mixture was concentrated to
give the title compound
(7 g) as yellow solid. MS (EST): C8H7C12NO2 requires 219; found no mass.
Description 142
5-Fluoro-2-methy1-3-nitrobenzaldehyde (P142)
02N0
To a mixture of (5-fluoro-2-methyl-3-nitrophenyl)methanol (D138, 9 g) in DCM
(100 mL) was
added PCC (14 g) in several portions. The mixture was stirred at RT overnight.
The solvent was
removed under vacuum to give a crude product, which was purified by column
chromatography
(silica gel, petroleum ether/Et0Ac ¨ 20:1) to afford the title compound (5 g)
as pale yellow solid.
111 NMR (400 MHz, CDC13): 10.37 (d, J= 2.2 Hz, 111), 7.80 (dd, J= 7.8, 2.9 Hz,
1H), 7.74 (dd, J=
7.2, 2.8 Hz, 1H), 2.75 (d, J= 0.7 Hz, 3H). MS (ESI): C8H6FNO3 requires 185;
found no mass.
Description 143
5-Chlorol-2-methyl-3-nitrobenzaldehyde (D143)
02No
CI
82
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
To a solution of (5-chloro-2-methyl-3-nitrophenyl)methanol (D139, 1.823 g) in
DCM (20 mL) was
added PCC (2.047 g) portionwise at RT.The reaction mixture was stirred at RT
overnight. The
mixture was extracted by DCM (2x20 mL) and water (2x20 mL). The combined
organic phase was
dried over Na2SO4. Filtered, the filtrate was concentrated under vacuum to
afford the title
compound (1 g). 1H NMR (400 MHz, CDC13): 10.34 (s, 1H), 8.02 (d, J= 2.0 Hz,
1H), 7.97 (d,
2.3 Hz, 1H), 2.75 (s, 3H). MS (EST): C8H6C1NO3 requires 199; found 200 [M+H].
Description 144
2,5-Dichloro-3-nitrobenzaldehyde (D144)
ct
02N
4
D144 was prepared using a similar procedure to that described for D143.111 NMR
(400 MHz,
CDC13): 10.49 (s, 1H), 8.30-7.73 (m, 2H). MS (ESI): C7H3C12NO3 requires 219
found 219 [M]
Description 145
(S)-tert-butyl 4-(5-bromo-2-methy1-3-nitrobenzoy1)-2-methylpiperazine-1-
carboxylate (D145)
0
02N 401
Boc
Br
5-Bromo-2-methyl-3-nitrobenzoic acid (D137, 7 g) and DIPEA (9.40 mL) were
dissolved in DMF
(10 mL). To this solution, HATU (12.28 g) was added gradually. After stirring
at RT for 30 min,
(S)-tert-butyl 2-methylpiperazine-1 -carboxylate (6.47 g) was added. The
mixture was stirred at RT
overnight. As the starting material (5-bromo-2-methyl-3-nitrobenzoic acid)
still remained, more
(S)-tert-butyl 2-methylpiperazine-l-carboxylate (2.0 g) and HATU (4.0 g) were
added. The mixture
was stirred at RT overnight. Water (30 mL) was added, extracted with Et0Ac
(2x30 mL). The
organic phase was dried over Na2SO4. Filtered, the filtrate was concentrated
to dryness. The residue
was triturated with Et0Ac, the resulting solid was filtered through a Buchner
funnel, to afford the
title compound (3.8 g). MS (ESI): C18H24BrN305 requires 441; found 464 [M+Na].
Description 146
(S)-tert-butyl 3-methylpiperazine-1-carboxylate (D146)
NH
Boc" N ""--)
To a solution of (S)-2-methylpiperazine (500 mg) in DCM (5 mL) was added Et3N
(1010 mg) and
(Boc)20 (1198 mg) in DCM (3 mL) dropwise. The mixture was stirred at 0 C for 2
hours. DCM
(10 inL), water (5 mL) and 30% NaHSO4 (10 mL) aqueous solution were added to
the reaction
mixture. The resulted mixture was stirred for 10 min, and to the aqueous layer
was added saturated
Na2CO3 solution until pH = 8, extracted with isopropyl alcohol: chloroform=1:
3 (5x20 mL). The
83
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
combined organic layer was washed with brine (5 mL), dried over Na2SO4,
filtered and
concentrated to afford the title compound (562 mg) as pale yellow oil, MS
(ESI): C10H20N202
requires 200; found 201 [M+H].
Description 147
(S)-tert-butyl 4-(cyclopentanecarbony1)-3-methylpiperazine-1-carboxylate
(D147)
E 0
Boc'N',)
To a solution of (S)-tert-butyl 3-methylpiperazine-l-carboxylate (D146, 15 g)
and triethylamine
(31.3 mL) in DCM (300 mL) stirred at RT under nitrogen was added
cyclopentanecarbonyl
chloride (12.91 g) dropwise. The reaction mixture was stirred at RT overnight.
The mixture was
concentrated to afford the title compound (24 g) as yellow oil, MS (ESI):
C16H281\1203 requires 296;
found 297 [M+H].
Description 148
(S)-cyclopenty1(2-methylpiperazin-1-yflmethanone (D148)
0
HN N¨b
To a solution of (S)-tert-butyl 4-(cyclopentanecarbonyl) -3-methylpiperazine-1-
carboxylate (P147,
24 g) in DCM (300 mL) stirred at RT was added TFA (31.2 mL) slowly. The
mixture was stirred at
RT overnight. The reaction mixture was evaporated. Saturated NaHCO3 solution
(100 mL) was
added and extracted with Et0Ac (3x50 mL). The organic layer was dried over
Na2SO4, filtered, thc
filtrate was evaporated to give the title compound (15 g) as yellow oil, MS
(ESI): Ci IHNN20
requires 196; found 197 [M+H].
Description 149
(S)-tert-butyl 4-(5-fluoro-2-methy1-3-nitrobenzy1)-2-methylpiperazine-1-
carboxylate (D149)
02N
To a solution of 5-fluoro-2-methyl-3-nitrobenzaldehyde (D142, 10 g) and (S)-
tert-butyl 2-
.. methylpiperazine-l-carboxylate (12.03 g) in DCM (120 mL) was added drops of
acetic acid (3.28 g)
and the mixture was stirred at RT for 1 hour. Sodium triacetoxyhydroborate
(23.15 g) was added to
the mixture in ice-bath and the mixture was stirred at RT overnight and
quenched with saturated
NaHCO3 solution. The organic layer was dried with anhydrous Na2SO4, filtered
and the filtrate
evaporated in vacuo to give the title compound (22.17 g) as a syrup. MS (ESI):
C18H26FN304
requires 367; found 368 [M+H].
Descriptions 150
84
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-tert-butyl 4-(5-chloro-2-methy1-3-nitrobenzy1)-2-methylpiperazine-1-
carboxylate (D150)
02N
LN,Boc
CI
To a solution of 5-chloro-2-methyl-3-nitrobenzaldehyde (D143, 20 g) in DCM
(260 mL), (S)-tert-
butyl 2-methylpiperazine-1-carboxylate (24.08 g) was added. The mixture was
stirred for 10 min at
room temperture, sodium triacetoxyhydroborate (25.4 g) was added portionwise.
The reaction
mixtiure was stirred overnight at 20 C. The mixture was washed with brine,
dried with
anhydrous Na2SO4. Filtered, the filtrate was concentrated to give crude
product, which was purified
by column chromatography (silica gel, petroleum ether/Et0Ac = 20:1) to afford
the title compound
(37 g) as white solid. MS (ESI): C18H26C1N304 requires 383; found 384 [M+H]t
Description 151
(S)-tert-butyl 4-(5-bromo-2-methy1-3-nitrobenzy1)-2-methylpiperazine-1-
carboxylate (D151)
02.Nuiiu
N-Boc
Br
(S)-tert-butyl 4-(5-bromo-2-methyl-3-nitrobenzoy1)-2-methylpiperazine-l-
carboxylate (D145, 3.8 g)
was dissolved in THF (20 mL) at 0 C. To this solution, NaBH4 (1.625 g) was
added gradually
under an ice bath, then BF3=Et20 (5.44 mL) was added dropwise carefully. The
mixture was stirred
at 0 C for 2 hours and at RT overnight. Methanol was added to quench the
reaction. After removal
of the solvent, the residue was extracted with Et0Ac (2x20 mL) and water (2x20
mL). The
combined organic phases were dried over Na2SO4 and concentrated in vacuo to
afford the title
compound (4.28 g) as pale yellow oil. MS (ESI): C181126BrN304 requires 427;
found 428 [M+1-1]+.
Description 152
(S)-tert-butyl 4-(5-cyano-2-methy1-3-nitrobenzy1)-2-methylpiperazine-1-
carboxylate (D152)
02N
A mixture of (S)-tert-butyl 4-(5-bromo-2-methy1-3-nitrobenzy1)-2-
methylpiperazine-1-carboxylate
(D151, 1.28 g), dicyanozinc (0.505 g) and
tetrakis(triphenylphosphine)palladium(0) (0.276 g) in a
sealed tube was stirred at 150 C in the microwave for 5 hours. The reaction
mixture was diluted
with Et0Ac (20 mL), poured into water (50 mL), and then filtrated. The
filtrate was extracted with
Et0Ac (20 mL). The organic phase was dried and concentrated. The residue was
purified by
column chromatography (silica gel, petroleum ether/Et0Ac = 9:1 to 2:1) to
afford the title
compound (370 mg). MS (ESI): C19H26N404 requires 374; found 397 [M+Na].
Description 153
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-cyclopenty1(4-(5-fluoro-2-methyl-3-nitrobenzy1)-2-methylpiperazin-1-
yOmethanone (D153)
co 40 N,Th ,:ro
0
A mixture of 5-fluoro-2-methyl-3-nitrobenzaldehyde (D142, 4.4 g) and (S)-
cyclopenty1(2-
methylpiperazin-1-yl)methanone (D148, 4.6 g) in anhydrous DCM (50 mL) was
stirred at RT for
10 mm. NaBH(OAc)3(4.9 g) was added in several portions. The reaction mixture
was stirred at RT
overnight. After the reaction completed, Me0H was added dropwise to quench the
reaction. When
the gaseous evoluation had ceased, the solvents was removed under vacuum to
give the crude
product, which was purified by column chromatography (silica gel, petroleum
ether/Et0Ac = 100:1)
to afford the title compound (7 g) as yellow oil. MS (ESI):
C19H26FN303requires 363; found 364
[M+Hr.
Descriptions 154-155
Descriptions 154-155 were prepared using a similar procedure to that described
for D153.
D154 (S)-(4-(5-chloro-2-methy1-3-nitrobenzy1)-2-methylpiperazin-1-
y1)(cyclopentypmethanone
D155 (S)-cyclopenty1(4-(2,5-dichloro-3-nitrobenzy1)-2-methylpiperazin-l-
yOmethanone
D154 02N MS (ESI): C19H26C1N303 requires 379; found 380 [M+Hr.
io N so'
CI 0
D155 CI MS (ESI): C18H23C12N303 requires 399; found 400
[M+H].
02N, N
CI 0
Description 156
(S)-(4-(3-amino-5-fluoro-2-methylbenzy1)-2-methylpiperazin-1-
y1)(cyclopentyflmethanone
(D156)
H2N
NciN
0
A mixture of (S)-cyclopenty1(4-(5-fluoro-2-methyl-3-nitrobenzy1)-2-
methylpiperazin-1-
yl)methanone (D153, 7.0 g), HCOONH4 (1.8 g) and zinc powder (1.44 g) in
methanol (60 mL) and
water (60 mL) was stirred at 80 C for 4hours. After the reaction completed,
the solvent was
removed in vacuo, the residue was extracted with Et0Ae (4x50 mL). The combined
organic extract
was washed with brine (100 mL), dried over anhydrous sodium sulfate and
concentrated to afford
the title compound (5.1 g) as pale yellow oil. MS (EST): C19H28FN30 requires
333; found 334
[M+H].
Description 157
86
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-1-
y1)(cyclopentypmethanone
(D157)
Fi2N
CI
To a solution of (S)-(4-(5-chloro-2-methy1-3-nitrobenzy1)-2-methylpiperazin-1-
yl)(cyclopentyl)methanone (D154, 2.4 g) in acetic acid (40 mL) was added iron
(3.53 g) portion-
wise under vigorous stirring. After addition, the resulting mixture was
stirred for another 4 hours.
The solid was filtered off and the cake was washed three times with Et0Ac
(3x10 mL). The filtrate
was collected and the solvent was removed in vacuo. The residue was dissolved
in Et0Ac and
washed with aqueous Na2CO3 solution and brine. The organic layer was
separated, dried over
Na2SO4, giltered and solvent removed to afford the title compound (1.8 g). MS
(ESI): C19H28C1N30
requires 349; found 350 [M+H].
Description 158
(S)-(4-(3-amino-2,5-dichlorobenzy1)-2-methylpiperazin-1-
yI)(cyclopentyl)methanone (D158)
.1
H2N
NON;ro
To a solution of compound (S)-cyclopenty1(4-(2,5-dichloro-3-nitrobenzy1)-2-
methylpiperazin-1-
y1)methanone (D155, 122 mg) in DCM (20 mL), Mill chloride dihydrate (492mg)
was added and
the mixture was stirred at RT for 2 days . The pH value of the mixtrue was
adjusted to about 8 by
NaHCO3 solution. Subsequently it was extracted with DCM (2x10 mL), washed by
water (2x10
mL). The organic layer was concentrated in vacuo to give the title compound
(91 mg) as yellow oil.
MS (ESI): C18H25C12N30 requires 369; found 370 [M+H].
Description 159
(S)-tert-butyl 4-(3-amino-5-chloro-2-methylbenzyI)-2-methylpiperazine-1-
carboxylate (D159)
H2. io
CI
Iron (7.54 g) was added in to a solution of (S)-tert-butyl 4-(5-chloro-2-
methyl-3 -nitrobenzy1)-2-
methylpiperazine-l-carboxylate (D150, 3.7 g) in acetic acid (20 mL) at 0 C and
stirred at this
temperature for 5 mm and then at RT for 3hours. After the reaction was
complete, the reaction
mixture was concentrated to remove most of the solvent. The residue was taken
up in DCM (100
mL) and the mixture was filtered through Celite. The aerate was concentrated
and the pH adjusted
to about 8 by saturated NaHCO3 solution. The mixture was extracted with DCM
(3x30 mL), the
organic layer was dried via Na2SO4, filtered and the filtrate was concentrated
to give the title
compound as brown oil. MS (EST): C18H25C1N302 requires 353; found 354 [M+Hr.
87
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Description 160
(S)-tert-butyl 4-(3-amino-5-cyano-2-methylbenzyI)-2-methylpiperazine-1-
carboxylate (D160)
H2N
I
To a solution of (S)-tert-butyl 4-(5-cyano-2-methy1-3-nitrobenzy1)-2-
methylpiperazine-1-
carboxylate (D152, 1010 mg) in ethanol (10 mL) was added tinII chloride
dihydrate (2587 mg).
The mixture was stirred at RT overnight. The pH value of the mixture was
adjusted to about 8 by
NaHCO3 solution. The white precipitate was filtered through Celite and the
filtrate was
concentrated, and then extracted with Et0Ac (2x20 mL). The combined organic
phases were
washed with water (2x10 mL). The resulting organic phase was concentrated in
vacuo to afford the
title compound (630 mg) as yellow oil. MS (ESI): C19H28N402 requires 344;
found 345 [M+H].
Description 161
(S)-1-(5-chloro-2-methyl-3-nitrobenzy1)-3-methylpiperazine (1D161)
.2N
LNH
CI
To a solution of (S)-tert-butyl 4-(5-chloro-2-methy1-3-nitrobenzy1)-2-
methylpiperazine-1-
carboxylate (D150, 10 g) in DCM (20 mL), TFA (16 mL) was added dropwise at
room temperature.
After addition, the reaction mixture was stirred for 3.5 hours at room
temperature. Solvent was
removed under vacuum. The residue was diluted with DCM (40 mL), neutralized
with saturated
Na2CO3 solution to pH = 10, then 2 M NaOH was added until pH = 11. Extracted,
the aqueous
layer was extracted with DCM (20 mL) again. Combined organic layer was dried
over Na2SO4.
Filtered, the filtrate was concentrated to dryness to afford the title
compound (7.96 g) as pale
yellow oil. MS (ESI): C331118C1N302 requires 283; found 284 [M+H].
Description 162
(S)-4-methy1-34(3-methylpiperazin-1-yl)methyl)-5-nitrobenzonitrile (D162)
02N
I
A mixture of (S)-tert-butyl 4-(5-cyano-2-methy1-3-nitrobenzy1)-2-
methylpiperazine-1-carboxylate
(D152, 1.0 g) in methanol (10 mL) was added HCl (4 M in dioxane, 6.68 mL). The
mixture was
stirred at RT for 18 hours, and then concentrated. The residue was diluted
with DCM (40 mL),
neutralized with saturated Na2CO3 solution to pH = 10. Extracted, the aqueous
layer was extracted
with DCM (20 mL) again. Combined organic layer was dried over Na2SO4.
Filtered, the filtrate was
88
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
concentrated to dryness to afford the title compound (724 mg) as pale yellow
oil. MS (ESI):
C14H18N402 requires 274; found 275 [M+H].
Description 163
(S)-1-(4-(5-chloro-2-methy1-3-nitrobenzy1)-2-methylpiperazin-1-y1)-2-
cyclopropylethanone
(D163)
02NLN
so
)(NV
CI
2-Cyclopropylacetic acid (2.84 mL) and DIPEA (10.80 mL) were dissolved in DMF
(10 mL), to
this solution, HATU (12.54 g) was added gradually. The reaction mixture was
stirred at RT for 1
hour. Then (S)-1-(5-chloro-2-methy1-3-nitrobenzy1)-3-methylpiperazine (1)161,
6.5 g) in DMF (2
mL) was added into the mixture, which was stirred at RT overnight. Water (30
mL) was added,
extracted with Et0Ac (2x20 mL), combined organic layer was dried over Na2SO4.
Filtered, the
filtrate was concentrated to dryness to afford the title compound (8 g) as
yellow oil. MS (EST):
C18H24C1N303 requires 365; found 366 [M+H].
Descriptions 164-165
Descriptions 164 and 165 were prepared using a similar procedure to that
described for Dl 63.
1)164 (S)-1-(4-(5-chloro-2-methy1-3-nitrobenzy1)-2-methylpiperazin-1-y1)-2-
cyclopentylethanone
1)165 (S)-(4-(5-chloro-2-methy1-3-nitrobenzy1)-2-methylpiperazin-1-
1)(cyclohexyl)methanone
D164 MS (ES!): C20H28C1N303 requires
02N
393; found 394 [M+H].
1)165 MS (EST): C20H28C1N303 requires
02N
393, found 394 [M+H].
Description 166
(S)-1-(4-(5-chloro-2-methy1-3-nitrobenzy1)-2-methylpiperazin-1-y1) butan-l-one
(1)166)
02N
1\--1111(\/
To a solution of (S)-1-(5-chloro-2-methy1-3-nitrobenzy1)-3-methylpiperazine
(D161, 1 g) and
D1PEA (0.911 g) in DCM (20 mL) was added dropwise butyryl chloride (0.563 g).
The reaction
was stirred at RT overnight. The reaction was quenched with saturated NaHCO3
aqueous solution
(3 mL), extracted with DCM (2x20 mL). The combined organic layer was dried
over Na2SO4,
filtered, and concentrated in vacuo. The residue was purified by column
chromatography (silica gel,
petroleum ether/Et0Ac = 1:1 to 0:1) to afford the title compound (1 g) as
white solid. MS (ES!):
C17H24C1N303 requires 353; found 354 [M+H]t
89
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Description 167
(S)-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-4-methyl-5-
nitrobenzonitrile (D167)
02N
11 0
To the mixture of (S)-4-methy1-34(3-methylpiperazin-l-yOmethyl)-5-
nitrobenzonitrile (D162, 0.5
g) and triethylamine (0.922 g) in DCM (6 mL), cyclopentanecarbonyl chloride
(0.242 g) was added
dropwise. The mixture was stirred at 23 C for 30 min. The mixture was washed
with brine (10 mL),
and the organic layer was dried and evaporated under vacuum to afford the
crude title compound
(0.5 g). MS (ESI): C20H26N403 requires 370; found 371 [M+H].
Description 168
(S)-3-((4- (2-cyclopropylacety1)-3-methylpiperazin-1-yOmethyl)-4-methyl-5-
nitrobenzonitrile
(D168)
02N Nr-)="µµ
11 0
2-Cyclopropylacetic acid (281 mg), DIPEA (0.979 mL) and HATU (1421 mg) were
dissolved in
DMF (2 mL). To this solution, (S)-4-methyl-3((3-methylpiperazin-1 -yl)methyl)-
5-
nitrobenzonitrile (D162, 512 mg) was added. The reaction mixture was stirred
at RT overnight.
Et0Ac (20 mL) and water (10 mL) was added, extracted, the organic layer was
dried over Na2SO4.
Filtered, the filtrate was concentrated to dryness. The residue was purified
by column
chromatography (silicon gel, petroleum ether/Et0Ac = 1:0 to 1:1.5) to afford
the title compound
(320 mg) as yellow oil. MS (ESI): CI9H24N403 requires 356, found 357 [M+Hr.
Description 169
(S)-1-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-1-yI)-2-
cydopropylethanone
(D169)
H2N 40
CI
To a solution of (S)-1-(4-(5-chloro-2-methy1-3-nitrobenzy1)-2-methylpiperazin-
1-y1)-2-
cyclopropylethanone (D163, 600 mg) in methanol (50 mL), Raney nickel (96 mg)
was added under
nitrogen. The mixture was warmed to 50 C, hydrazine hydrate (0.257 mL, 6.56
mmol) was added.
The reaction mixture was stirred at 50 C for 1 hour. Filtered, the filtrate
was concentrated under
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
vacumm to afford the title compound (500 mg) as pale yellow oil. MS (ESI):
C181126C1N30 requires
335, found 336 [M+H].
Description 170
(S)-1-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-1-y1)-2-
cyclopentylethanone
(D170)
H2N
NSs
NO
CI
To a solution of compound (S)-1-(4-(5-chloro-2-methy1-3-nitrobenzy1)-2-
methylpiperazin-1-y1)-2-
cyclopentylethanone (D162, 980 mg) in ethanol (10 mL), tinII chloride
dihydrate (2386 mg) was
added and the mixture was stirred at RT overnight. The pH value of the mixture
was adjusted to
about 8 by NaHCO3 solution. The white precipitate was filtered through Celite
and the filtrate was
concentrated, and then extracted with Et0Ac (2x20 mL). The combined organic
phases were
washed with water (2x10 mL). The resulting organic phase was concentrated in
vacuo to afford the
title compound (730 mg) as yellow oil. MS (ESI): C20H30C1N30 requires 363,
found 364 [M+H]t
Description 171
(S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-1-
y1)(cyclohexyl)methanone
(D171)
H2N
Ltµl
CI 0
D171 was prepared using a similar procedure to that described for D170. MS
(ESI): C20H30C1N30
requires 363, found 364 [M+H].
Description 172
(S)-1-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-1-yl)butan-1-one
(D172)
H2N
CI
To a mixture of (S)-1-(4-(5chloro-2-methy1-3-nitrobe1)-2-methylpiperazin-1-
y1)butan-1-one
(D166, 1 g) and iron (1.578 g) in Me0H (50 mL) was added a solution of N114C1
(1.512 g) in water
(5 mL). The mixture was stirred at 70 C for 1 hour. The mixture was filtered
and the filtrate was
concentrated and dissolved with Et0Ac (200 mL) and water (50 mL). The organic
layer was
washed by brine, dried over Na2SO4, and concentrated to afford the title
compound (800 mg) as
yellow solid. MS (ESI): C17H26C1N30 requires 323; found 324 [M+H].
Description 173
91
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-3-amino-5-04-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-4-
methylbenzonitrile (D173)
H2N
NON'11(0 µ
I I 0
The mixture of (S)-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1) methyl)-
4-methyl-5-
nitrobenzonitrile (D167, 0.4 g) and Pd/C (20 mg, 10%) in Me0H (30 mL) was
stirred at 28 C for 4
hours under hydrogen atmosphere (latm). After filtration, the filtrate was
concentrated under vacuo
to afford the title compound (0.35 g) as colorless oil. MS (EST): C20H28N40
requires 340; found 341
[M+11]+.
Description 174
(S)-3-amino-5-44-(2-cyclopropylacety1)-3-methylpiperazin-1-yOmethyl)-4-
methylbenzonitrile
(D174)
H2N
I I 0
D174 was prepared using a similar procedure to that described for D173. MS
(ESI): C19H26N40
requires 326, found 327 [M+1-1]'.
Description 175
(S)-tert-butyl 4-(3-amino-5-(methoxycarbony1)-2-methylbenzy1)-2-
methylpiperazine-1-
carboxylate (D175)
HN
0 (21"
The mixture of (S)-tert-butyl-4-(5-bromo-2-methyl-3-nitrobenzy1)-2-
methylpiperazine-1-
carboxylate (D151, 16 g), DPPP (1.541 g), diacetoxypalladium (0.419 g) and
triethylamine (10.41
mL) in DMF (10 mL) and methanol (200 mL) was heated to 120 C for 12 hours
under CO
atmosphere (10 atm). The mixture was filtered, the filtrate was concentrated
under vacuum. The
residue was re-dissolved in Et0Ac (20 mL) and then washed with brine (50 mL),
the organic layer
was dried over anhydrous sodium sulfate and evaporated under vacuum to leave
the crude product,
which was purified by column chromatography (silica gel, petroleum ether/Et0Ac
= 5:1) to afford
the title compound (13g) as yellow solid. MS (ESI): C20H3IN304 requires 377,
found 378 [M+Hr.
Description 176
(R)-(4-(5-chloro-2-methy1-3-nitrobenzy1)-2-propylpiperazin-1-
y1)(cyclopentyl)methanone
(D176)
92
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
02N
Nacp
CI 0
The mixture of (R)-cyclopenty1(2-propylpiperazin-1 -yl)methanone (D106, 150
mg) and 5-chloro-1-
(chloromethyl)-2-methy1-3-nitrobenzene (D141, 147 mg) in DMF (6 mL) was heated
at 60 C for 2
hours, and then cooled to RT. The mixture was diluted with water (20 mL) and
extracted with
Et0Ac (20 mL). The organic layer was dried over Na2SO4, filtered and
concentrated to leave the
crude as brown oil, which was purified by column chromatography (silica gel,
petroleum
ether/Et0Ac = 20:1) to afford the title compound (101 mg) as pale yellow
solid. MS (ESI):
C211-130C1N303 requires 407; found 408 [M+H].
Description 177
(R)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-propylpiperazin-1-y1)(cyclopentyl)
methanone
(D177)
H2N so
CI 0
To a solution of (R)-(4-(5-chloro-2-methy1-3-nitrobenzy1)-2-propylpiperazin-1-
y1)
(cyclopentyl)methanone (D176, 100 mg) in Me0H (10 mL) was added Raney Ni (46.0
mg)
.. portionwise. The mixture was heated to 50 C and hydrazine monohydrate
(0.084 mL) was slowly
added during 10 min. After addition the mixture was stirred at 50 C for 5 min.
The mixture was
filtered through a thin pad of Celite and concentrated to afford the title
compound (90 mg) as pale
orange solid. MS (ESI): C211132C1N30 requires 377; found 378 [M+H].
Description 178
(S)-N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
y1)methyl)-2-
methylphenyl)pyrrolidine-3-carboxamide (D178)
C
Nay Boc 0
CI 0
To the solution of (R)-1-(tert-butoxycarbonyl)pyrrolidine-2-carboxylic acid
(176 mg) and (S)-(4-
(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-1-
y1)(cyclopentyl)methanone (D157, 260
mg) in DCM (18 mL) was added HATU (424 mg) at RT. After stirred at RT for 10
min, DIPEA
(0.260 mL) was added to the mixture. Then the mixture was stirred at 45 C for
2 days. The mixture
was washed with saturated NaHCO3 aqueous solution and brine, dried by
anhydrous sodium sulfate,
filtrated and concentrated under vacuum to get crude product which was
purified by column
chromatography (silica gel, petroleum ether/Et0Ac = 10:1) to afford the title
compound (310 mg)
as oil. MS (ESI): C291-143C1N404 requires 546; found 547 [M+11]+.
93
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Descriptions 179-201
Descriptions 179-201 were prepared using a similar procedure to that described
for D178, with the
specified reaction base or solvent listed in the table.
D179 Tert-butyl 2-(24(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-l-y1)methyl)-
2-methylphenyl)amino)-2-oxoethyl)pyrrolidine-1-carboxylate
D180 Tert-butyl 3-(245-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-
1-yOmethyl)-
2-methylphenypamino)-2-oxoethyl)piperidine-1-carboxylate
D181 (S)-tert-butyl 445-chloro-3-04-(cyclopentanecarbony1)-3-methylpiperazin-1-
yOmethyl)-2-
methylphenyl)carbamoyl)piperidine-l-carboxylate
D182 (S)-tert-butyl 345-chloro-34(S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-1-ypmethyl)-
2-methylphenyl)carbamoyDpiperidine-1-carboxylate
D183 (R)-tert-butyl 3-45-chloro-34(S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-1-
yOmethyl)-2-methylphenyl)carbamoyl)piperidine-l-carboxylate
D184 (S)-tert-butyl 2-45-chloro-34(S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-1-y1)methyl)-
2-methylphenyl)carbamoyl)pyrrolidine-1-carboxylate
D185 (S)-tert-butyl 4-(245-chloro-3-44-(cyclopentanecarbony1)-3-
methylpiperazin-1-y1)methyl)-
2-methylphenyl)amino)-2-oxoethyl)piperidine-1-carboxylate
D186 (S)-tert-butyl (345-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-
l-y1)methyl)-2-
methylphenyl)amino)-3-oxopropyl)(methyl)carbamate
D187 (S)-tert-butyl 3-(245-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-
1-y1)methyl)-
2-methylphenypamino)-2-oxoethypazetidine-1-carboxylate,Trifluoroacetic acid
salt
D188 (S)-tert-butyl (445-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylphenyflamino)-4-oxobutyl)(methypcarbamate
D189 Tert-butyl 2-45-chloro-3-4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-
1-ypmethyl)-2-
methylphenyl)carbamoyl)morpholine-4-carboxylate
D190 Tert-butyl 3-(2434(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
yl)methyl)-5-fluoro-
2-methylphenypamino)-2-oxoethyppyrrolidine-1-carboxylate
D191 Tert-butyl ((1S,30-343-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
yl)methyl)-5-
fluoro-2-methylphenyl)carbamoyl)cyclobutyl)carbamate
D192 Tert-butyl ((lR,3s)-34(3-4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-
1 -yl)methyl)-5-
fluoro-2-methylphenyl)carbamoyl)cyclobutypcarbamate
D193 (S)-tert-butyl 34(344-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-5-fluoro-2-
methylphenyl)carbamoyl)azetidine-1-carboxylate
D194 (S)-tert-butyl (245-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-
yOmethyl)-2-
methylphenyl)amino)-2-oxoethyl)(methyl)carbamate
D195 Methyl 245-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
yOmethyl)-2-
methylphenyl)carbamoyl)cyclopropanecarboxylate
94
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
D196 N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-Amethyl)-2-
methylpheny1)-2-(tetrahydrothiophen-2-yDacetamide
D197 N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-
2-
methylpheny1)-2-(tetrahydro-2H-thiopyran-3-yBacetamide
D198 S-(2-((5- chloro-3-(4S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
yOmethy0-2-
methylphenyl)carbamoyl)cyclobutyl) ethanethioate
D199 (S)-N-(5-chloro-3-04-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-
2-
methylpheny1)-3-(methylthio)cyclobutanecarboxamide
D200 N-(3-0(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-5-
fluoro-2-
methylpheny1)-2-(2-oxocyclohexypacetamide
D201 N-(3-(0S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-5-
fluoro-2-
methylphenyl)-2-(2-oxocyclopentyl)acetamide
Structure Base/Solvent Characterization
13(:)
H DIPEA/DMF MS (ESI): C301-145C1N404
ociN N 00 NoN.,y0,,,
D179 requires 560; found 561
ci
[M+H] -
o
H D1PEA/DMF MS (ESD: C31H47C1N404
D180
..,,,,-,....õ..ThrN ao N,-...,,, jr)
requires 574; found 575
'=14-'
13 oc CI o [M+H].
Boc,NarH DIPEA/DMF MS (ESD: C301145C1N404
N
D181 0 101 Nacr0 requires 560; found 561
a o [M+H].
r' H DIPEA/DMF MS (ESD: C30H45C1N404
D182 Boc'N 110 try:flp requires 560; found 561
,,,.N
CI o [M+H].
H DIPEA/DMF MS (ESD: C30H45C1N404
D183 Bo
e N (3rN 40 NLININ';(0 requires 560; found 561
a o [M+H].
cily.H DIPEA/DCM MS (ESD: C29---,3 IIA
C1N40
, ---, 4
N ,õ
D184 Bo C 0 40 NON iiro
requires 546; found 547
a 0 [M+14]+-
H DIPEA/DMF MS (ESI): C31I-147C1N404
N .di N-,-
D185 Boo' NMC IP L0 requires 574; found 575
a o [M+1-1]+.
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Boc
H IDIPEA/DMF MS (EST):
N N
C28H43C1N404 requires
D186 0 L,,,,.N,r0
IlD 534; found 535 [M+1-1]+.
CI
H DIPEA/DMF MS (EST):
D187 soersirn"" so Nocro = TFA
C291143C1N404 requires
CI o
546; found 547 [M+111+.
cilH 0 No,
c DIPEA/DMF MS (EST): C2A5C11\1404
D188 Boc NTO requires 548; found 549
a
0 [M+11]+.
11r N
DTEA/DMF MS (EST): C291-143C1N405
Bac
1 - 0 INI's
D189 0 L,N,ro requires 562; found 563
a
IIC [M+1-1]+.
H DIPEA/DMF MS (ESI): C30H45FN404
N
Boc-NYI 11110 Nl.,:.;(0 requires 544; found 545
D190 F o [M+11] .
H DIPEA/DMF MS (ESI): C29}143FN404
Boel\L"'n N
D191 µ--Ir Si NOco requires 530; found 531
[M+11]+.
F o
Ed
Boe =-ciyi-i DIPEA/DMF MS (ESI): C291-143FN404
N
requires 530; found 531
D192
0 0 N" requires
[M+Hr=
F 0
Boo, DIPEA/DMF MS (ESI): C281-141FN404
D193 NaiH
N
0 la Ocr0 requires 516; found 517
F o [M+II].
H DIPEA/DMF MS (ESI): C271-141CIN404
BocN
I D194 8 NOcC>
requires 520; found 521
CI o [M+1-1]+.
olil
1(0 TEA/DMF MS (ESI): C25H34C1N304
D195
requires 475; found 476
0, 0 N
ci o [M+Ii]+-
96
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
No base/DMF MS (ER): C25H36C1N302S
N = NI requires 477; found 478
D196
CI
[M+H].
TEA/DCM MS (EST): C261138C1N302S
sN N,Thc
D197 ro.
up requires 491; found 492
[M-H].
DIPEA/DMF MS (ESI): C26H36C1N303S
N
PYFI = D198 tõ
requires 505; found 506
\ 0
o
/7
CI
TEA/DMF MS (EST): C25H36C1N302S
D199 \-Thor NIO/ 0\;'0's
requires 477; found 478
[m+fir.
0
0 No base/DMF MS (EST): C27H38FN303
D200 lanOrN = NONcr0 requires 471; found 472
[M+1-1]+.
No base/DMF MS (EST): C26H36FN303
D201 '_201=-
0 NON;y:>'µ requires 457; found 458
0 [M+H] .
Description 202
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-(3-oxocyclobutypacetamide (D202)
N
et1:1Thi) NILCIN.
CI 0
To a solution of 2-(3-oxocyclobutypacetic acid (D100, 360 mg) in DCM (5 mL)
was added (S)-(4-
(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-l-y1)(cyclopentypmethanone
(D157, 1475
mg), EDC (1077 mg), HOBt (861 mg) and DIPEA (1.472 mL). The reaction mixture
was stirred at
40 C overnight. The mixture was evaporated under vacuum to give the title
compound (420 mg) as
yellow oil. MS (EST): C25H34C1N303 requires 459; found 460 [M+H].
Description 203
(2S)-tert-butyl 4-(5-chloro-2-methy1-3-(2-(tetrahydrothiophen-3-
yl)acetamido)benzyl)-2-
methylpiperazine-1-carboxylate (D203)
97
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
N tal
CI
To a suspension of (S)-tert-butyl 4-(3-amino-5-chloro-2-methylbenzy1)-2-
methylpiperazine-l-
carboxylate (D159, 146 mg) in DCM (4 mL) were added HATU (157 mg), 2-
(tetrahydrothiophen-
3-yl)acetic acid (D60, 100 mg) and triethylamine (83 mg). The mixture was
stirred for overnight at
RT. The mixture was washed with brine, separated, dried over sodium sulfate
anhydrous and
concentrated to give the title compound (320 mg) as yellow gum. MS (ESI):
C24H36C1N303S
requires 481; found 482 [M+H].
Description 204
(S)-tert-butyl 4-(5-ehloro-3-(5-fluoro-6-methylnicotinamido)-2-methylbenzyl)-2-
methylpiperazine-1-carboxylate (D204)
I H
0Boc
CI
A solution of (S)-tert-butyl 4-(3-amino-5-chloro-2-methylbenzy1)-2-
methylpiperazine-1-
carboxylate (D159, 913 mg), 5-fluoro-6-methylnicotinic acid (D113, 400 mg),
HATU (980 mg)
and DIPEA (0.450 mL) in DCM (100 mL) was stirred at RT for 18 hours. The
mixture was
concentrated under vacuum to afford the title compound (1.2 g) as red oil
which was used directly
for next step without further purification. MS (ESI): C251132C1FN403 requires
490, found 491
[M+1-1]+.
Descriptions 205-211
Descriptions 205-211 were prepared using a similar procedure to that described
for Description 204,
with the specified reaction base or solvent listed in the table.
D205 (S)-tert-butyl 4-(5-chloro-3-(4-cyano-4-methylpentanamido)-2-
methylbenzy1)-2-
methylpiperazine-1-carboxylate
D206 (S)-tert-butyl 4-(5-chloro-3-(2-(4-hydroxycyclohexypacetamido)-2-
methylbenzy1)-2-
methylpiperazine-l-carboxylate
D207 (S)-tert-butyl 4-(5-chloro-2-methy1-3-(3-
(methylsulfonyl)propanamido)benzy1)-2-
methylpiperazine-l-carboxylate
D208 (S)-tert-butyl 4-(3-(5-chloro-6-methylnicotinamido)-5-fluoro-2-
methylbenzy1)-2-
methylpiperazine-l-carboxylate
D209 (5)-tert-butyl 4-(5-chloro-3-(5-chloro-6-methylnicotinamido)-2-
methylbenzy1)-2-
methylpiperazine-l-carboxylate
D210 (S)-tert-butyl 4-(5-cyano-3-(6-methoxynicotinamido)-2-methylbenzy1)-2-
methylpiperazine-
1-carboxylate
98
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
D211 (S)-tert-butyl 4-(3-(5-chloro-6-methylnicotinamido)-5-(methoxycarbony1)-2-
methylbenzy1)-
2-methylpiperazine-1-carboxylate
Structure Base/Solvent Characterization
TEA/DCM MS (EST): C251{37C1N403
0: D205 o 110 B0 requires 476; found 477
CI U\4+11]+.
No base/DMF MS (EST): C26H40C1N304
D206 HO
IC's' NsBoc requires 493; found 494
CI [M+11]+.
H DIPEA/DMF MS (EST): C22}134C1N3055
a&h
D207 N 8 IP requires 487; found 488
Boo
ci [M+H]F.
H DEPEA/DMF MS (ESI): C251-132C1FN403
D208 N requires 490; found 491
o LNN'Boc ilv1+14] .
DIPEA/DMF MS (ESI): C251-132C12N403
N
D209 CI N-- requires 506; found 507
N. o N,Boc [1\4+11] -
CI
DIPEA/DMF MS (ESI): C26H33N504
,F1
D210 0 11$
L,-N-Boc requires 479; found 480
[Wa]+.
INI
TEA/DMF MS (ESI): C271135C1N405
ci D211 requires 530; found 531
[M+Hr.
o o'
Description 212
(S)-tert-butyl 4-(5-cyano-2-methy1-3-(2-methylpyrimicline-5-
earboxamido)benzy1)-2-
methylpiperazine-1-carboxylate (D212)
0
I I
To a solution of (S)-tert-butyl 4-(3-amino-5-cyano-2-methyl benzy1)-2-
methylpiperazine-1-
carboxylate (D160, 600 mg), 2-methylpyrimidine-5-carboxylic acid (300 mg) and
DMAP (316 mg)
in DCM (15 mL) was added DCC (530 mg). The mixture was stirred at RT
overnight. Filtered, the
99
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
filtrate was concentrated under reduced pressure to leave the crude product,
which was purified by
chromatography (silica gel, petroleum ether/Et0Ac = 1:1) to afford the title
compound (800 mg) as
white solid. MS (ESI): C25H32N603 requires 464; found 465 [M+H].
Description 213
(S)-tert-butyl 4-(5-cyano-3-(3-cyanobenzamido)-2-methylbenzy1)-2-
methylpiperazine-1-
carboxylate (D213)
NI N"'s
0 (N,Boc
I I
To a suspension of 3-cyanobenzoic acid (431 mg) in DCM (10 mL) was added
oxalyl chloride
(0.288 mL) dropwise. The reaction mixture was stirred at 40 C for 2 hours, and
then concentrated.
The residue was re-dissolved in pyridine (1 mL), and then added to a solution
of (S)-tert-butyl 4-(3-
amino-5-cyano-2-methylbenzy1)-2-methylpiperazine-l-carboxylate (D160, 630 mg)
in pyridine (2
mL). The reaction mixture was stirred at RT overnight. The resulting mixture
was diluted with
Et0Ac (20 mL), and then washed with brine (10 mL). The organic phase was
separated, dried over
MgSO4, filtered and concentrated. The residue was purified by column
chromatography (silica gel,
petroleum ether/Et0Ac = 1:0 to 2.5:1) to afford the title compound (915 mg).
MS (ESI):
C27H311\1503 requires 473; found 474 [M+II]+.
Description 214
(S)-tert-butyl 4-(5-cyano-2-methy1-3-(6-methylnicotinamido)benzy1)-2-
methylpiperazine-1-
carboxylate (D214)
I N
Ns
0 t=N'Boc
I I
Description 214 was prepared using a similar procedure to that described for
D213. MS (ESI):
C26H33N503requires 463; found 464 [M+H].
Description 215
(S)-tert-butyl 4-(5-chloro-3-(6-cyano-5-methylnicotinamido)-2-methylbenzy1)-2-
.. methylpiperazine-l-carboxylate (D215)
I H
0
CI
6-Cyano-5-methylnicotinic acid (D134, 160 mg) was dissolved in sulfurous
dichloride (235
mg) .The reaction mixture was stirred for 3 hours at 60 C. The mixture was
then concentrated to
100
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
dryness under reduced pressure, and the residue re-dissolved in DCM (10 inL)
was slowly added to
the mixture of (S)-tert-butyl 4-(3-amino-5-chloro-2-methylbenzy1)-2-
methylpiperazine-l-
carboxylate (D159, 349 mg) and DIPEA (383 mg) at 0 C. The reaction mixture was
allowed to
warm to RT and stirred for 1 hour. The mixture was diluted with water (15 mL),
the organic layer
was dried over anhydrous sodium sulfate, filtered and concentrated to afford
the title compound
(300 mg) as yellow oil. MS (EST): C26H32C1N503 requires 497; found 498 [M+Hr.
Description 216
(S)-3-44-(tert-butoxycarbony1)-3-methylpiperazin-1-y1)methyl)-5-(5-chloro-6-
methylnicotinamido)-4-methylbenzoic acid (D216)
I H
Boc
0 OH
To a solution of (S)-tert-butyl 4-(3-(5-chloro-6-methylnicotinamido)-5-
(methoxycarbony1)-2-
methylbenzy1)-2-methylpiperazine-l-carboxylate (D211, 1.1 g) in the mixture
solvents of ethanol
(10 mL) and water (10 mL), was added lithium hydroxide (0.099 g). The reaction
mixture was
stirred for 10 hours at 40 C. After removal of the organic solvent under
vacuum, the aqueous layer
was acidified to pH-3 with 1 M HCI (aq.) and extracted with Et0Ac (30 mL). The
organic layer
was dried over anhydrous sodium sulfate, filtered and concentrated to afford
the title compound (1
g) as yellow solid. MS (EST): C26H33CIN405 requires 516; found 517 [M+H].
Description 217
(S)-tert-butyl 4-(5-carbamoy1-3-(5-chloro-6-methylnicotinamido)-2-
methylbenzy1)-2-
methylpiperazine-l-carboxylate (D217)
ciN
0 LNB
0 NH2
To a suspension of (S)-344-(tert-butoxycarbony1)-3-methylpiperazin-1-ypmethyl)-
5-(5-chloro-6-
methylnicotinamido)-4-methylbenzoic acid (D216, 320 mg) in DMF (2 mL), was
added HATU
(282 mg) and NH4HCO3 (489 mg). The mixture was stirred overnight at RT. The
mixture was
washed with brine, dried over anhydrous sodium sulfate, filtered, and
concentrated to afford the
title compound (370 mg) as yellow solid. MS (EST): C26H34C1N504 requires 515;
found 516
[M+H].
Description 218
(S)-tert-butyl 4-(3-(5-chloro-6-methylnicotinamido)-5-cyano-2-methylbenzy1)-2-
methylpiperazine-l-carboxylate (D218)
101
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
I H
ti&
0'Bac
I I
To a solution of (S)-tert-butyl 4-(5-carbamoy1-3-(5-chloro-6-
methylnicotinamido)-2-
methylbenzy1)-2-methylpiperazine-l-carboxylate (D217, 270 mg) in DCM (3 mL)
was added
triethylamine (106 mg) and 2,2,2-trifluoroacetic anhydride (330 mg). The
mixture was stirred for
0.5 hour at 13 C. The mixture was washed with brine, dried over anhydrous
sodium sulfate, filtered,
and concentrated to afford the title compound (300 mg) as yellow solid. MS
(EST): C261132C1N503
requires 497; found 498 [M+H].
Description 219
(S)-tert-butyl 3-45-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-
1-y1)methyl)-
2-methylphenyl)carbamoyppyrrolidine-1-carboxylate (D219)
Boc¨NO
õ N
."r
0
CI 0
(S)-1-(Tert-butoxycarbonyl)pyrrolidine-3-carboxylic acid (50 mg) was dissolved
in DCM (20 mL)
and cooled to 0 C. Oxalyl chloride (0.024 mL) was added dropwise under a
nitrogen atmosphere,
followed by a few drops of DMF. The reaction mixture was allowed to warm to 25
C and stirred
for 1 h. The mixture was then concentrated to dryness under reduced pressure,
and the residue
redissolved in DCM (20 mL) was slowly added to the mixture of (S)-(4-(3-amino-
5-chloro-2-
methylbenzy1)-2-methylpiperazin-l-y1)(cyclopentypmethanone (D157, 81 mg) and
DIPEA (0.081
mL) at 0 C. The reaction mixture was allowed to warm to RT and stirred for 1
hour. The mixture
was dulited with water (15 mL), the organic phase was dried over anhydrous
sodium sulfate,
filtered and concentrated to afford the title compound (80 mg) as yellow oil.
MS (ESI):
C29H43C1N404 requires 546; found 547 [M+Hr.
Descriptions 220-222
Descriptions 220-222 were prepared using a similar procedure to that described
for Description 219,
with the specified reaction base or solvent listed in the table.
D220 Tert-butyl 3-(24(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-l-yOmethyl)-
2-methylphenypamino)-2-oxoethyppyrrolidine-l-carboxylate
D221 (R)-tert-butyl 345-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-l-
ypmethyl)-2-methylphenyl)carbamoyepyrrolidine-l-carboxylate
D222 (S)-6-cyano-N-(3 44-(cyclopentanecarbony1)-3-methylpiperazin-l-yl)methyl)-
5-fluoro-2-
methylphenyl)nicotinamide
102
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
Structure Base/Solvent Characterization
DIIIEA/DCM MS (ESI): C301145C1N404
D220 110
N 0 (õN ,r0 requires 560; found 561
Boc CI [M+H]+.
Boc-41fl 1 TEA/DCM MS (ESD: C29H43CIN404
rEr
D221 o NON11)0 requires 546; found 547
iM+Hr=
NC N I K2CO3/CH3CN MS (ESD: C26H30FN502
=-=
11
D222 requires 463; found 464
iM+141-=
Description 223
(S)-N-(5-chloro-3-4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-
2-
methylphenyl)pyrrolidine-3-carboxamide, hydrochloride acid salt (D223)
HNO
=401 = HCI
0
CI 0
The mixture of (S)-tert-butyl 34(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-1-
yDmethyl)-2-methylphenyl)carbamoyDpyrrolidine-1-carboxylate (D219, 200 mg) in
Me0H (20 mL)
was added HCl (0.022 mL) and the mixture was stirred at 60 C for 1 hour. Then
the reaction
mixture was concentrated to afford the title compound (300 mg) as white solid.
MS (ESD:
C24H35C1N402 requires 446; found 447 [M+H].
Descriptions 224-242
Descriptions 224-242 were prepared using a similar procedure to that described
for Description 223,
with the specified reaction base or solvent listed in the table.
D224 (R)-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
yDmethyl)-2-
methylphenyl)pyrrolidine-3-carboxamide, 2 hydrochloride acid salt
D225 N-(5 -chloro-3 -(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1 -
yl)methyl)-2-
methylpheny1)-2-(pyrrolidin-3-yDacetamide, 2 hydrochloride acid salt
D226 (R)-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
yDmethy0-2-
methylphenyl)pyrrolidine-2-carboxamide, 2 hydrochloride acid salt
D227 (S)-N-(5-chloro-344-(cyclopentanecarbony0-3-methylpiperazin-l-yl)methyl)-
2-
methylphenyl)piperidine-4-carboxamide, 2 hydrochloride acid salt
103
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
D228 (S)-N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3 -m etbylpi perazin -1 -
yl)methyl)-2-
methylphenyl)piperidine-3 -carboxamide, 2 hydrochloride acid salt
D229 (R)-N-(5 -chloro-3 A(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1 -
yl)methyl)-2-
methylphenyl)piperidine-3-carboxamide, 2 hydrochloride acid salt
D230 (S)-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
yOmethyl)-2-
methylphenyl)pyrrolidine-2-carboxamide, 2 hydrochloride acid salt
D231 (S)-N-(5 -chloro-344-(cyclopentanecarbony1)-3 -methylpiperazin- 1 -
yl)methyl)-2-
methylpheny1)-2-(piperidin-4-ypacetamide, 2 hydrochloride acid salt
D232 N-(5 -chloro-3 -a(S)-4-(cyclopentanecarbony1)-3 -methylpiperazin-1 -
yl)methyl)-2-
methylpheny1)-2-(pyrrolidin-2-yl)acetamide, 2 hydrochloride acid salt
D233 N-(5 -chloro-3 -a(S)-4-(cyclopentanecarbony1)-3 -methylpiperazin- 1 -
yl)methyl)-2-
methylpheny1)-2-(piperidin-3-yl)acetamide, 2 hydrochloride acid salt
D234 (S)-N-(5 -chloro-3 #4-(cyclopentanecarbony1)-3-methylpiperazin- 1 -
yl)methyl)-2-
methylpheny1)-3 -(methylamino)propanamide
D235 (S)-2-(azetidin-3-y1)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-
methylpiperazin-l-
yOmethyl)-2-methylphenypacetamide,Trifluoroacetic acid salt
D236 (S)-N-(5 -chloro-3 44-(cyclopentanecarbony1)-3 -methylpiperazin- 1 -
yl)methyl)-2 -
methylpheny1)-4-(methylamino)butanamide, 2 hydrochloride acid salt
D237 N-(5 -chloro-3 4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1 -
yl)methyl)-2-
methylphenyl)morpholine-2-carboxamide, 2 hydrochloride acid salt
D238 N-(3 -(((S)-4-(cyclopentanecarbony1)-3 -methylpiperazin- 1 -yl)methyl)-5 -
fluoro-2-
methylpheny1)-2-(pyrrolidin-3 -yl)acetamide, 2 hydrochloride acid salt
D239 (1 r,3 S)-3 -amino-N-(3 -(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-
1 -yl)methyl)-5 -
fluoro-2-methylphenyl)cyclobutanecarboxamide, 2 hydrochloride acid salt
D240 (1 s,3R)-3-amino-N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin- 1 -
yl)methyl)-5 -
fluoro-2-methylphenyl)cyclobutanecarboxamide, 2 hydrochloride acid salt
D241 (S)-N-(3 #4-(cyclopentanecarbony1)-3-methylpiperazin-1 -yl)methyl)-5 -
fluoro-2-
methylphenyl)azetidine-3-carboxamide
D242 (S)-N-(5 -chloro-3 4(4-(cyclopentanecarbony1)-3 -methylpiperazin-1 -
yl)methyl)-2-
methylpheny1)-2-(methylamino)acetamide
Structure Acid/Solvent Characterization
May M HC1/Me0H MS (ESI): C24H35C1N402
0 = 2 HCI
requires 446; found 447
D224 CI 0
[M+H].
104
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
I-1(nH HC1/1v1e01-1 MS (ESI): C25H37C1N402
N IPAu
N;0 = 2 HCI requires 460; found 461
D225 a
a [M+Hr.
IICl/IVIe0H MS (ESI): C241135C1N402
H c, Ccf: =2HCI
requires 446; found 447
D226 a o
[M+1-1]+.
HNarH HC1/THF MS (ESD: C251-137C1N402
N
D227 , .0 NoN;r0 = 2 HCI requires 460; found 461
CI 0 {M H]+.
rTh H FICYTHF MS (ESI): C251137C1N402
HN,..-= ,õg_N so õ,...õ
.L_rco = D228 2 HCI requires 460; found 461
CI 0 [M+1-11+.
HaH HC1/11-1F MS (ESI): C251137C1N402
rN
D229 0 410 Nocz, = 2 HCI
requires 460; found 461
CI 0
[M+1-11-'.
cil,rrH HC1/Me0H MS (ESI): C241135C1N402
N 4,6. Nj,-,
D230 " o le- 1,,,,c0 = 2 HCI requires 446; found 447
CI 0
[M+1-1]+.
" Alb HC1/Et0H MS (ESI): C26H39C1N402
D231 2 HO
HraThci VP NOIN;r0 = requires 474; found 473
CI 0
[M-1-1]-.
H H HC1/TUF MS (EST): C251137C1N402
N 2NdI C.norN 110 Nacr0 =
D232 requires 460; found 461
CI 0
[1\4 }1].
N iii... ,...., 1-1CUMe0H MS (EST): C261-139C1N402
Mor uji NL,,,,c,C) = 2 HCI
D233 N requires 474; found 475
H
CI 0
[M+I-1]+.
H H HC1/1VIe0H MS (ESI): C23H35C1N402
D234 requires 434; found 435
a
0 [M+H].
H
TFA/DCM MS (ESI): C241135C1N402
D235 I-IN'i-/ " .--,,,
0 0 NON;r0 - TFA
requires 446; found 447
CI 0
105
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
[M+H]
2 HCI
HCl/Me0H MS (EST): C24H37C1N402 0 =
D236
requires 448; found 449
[M+H].
0,1r14 HC1fEt0H MS (ESI):
= 2 HCI
D237 H 0 10 C241135C1N403 requires
Cl
462; found 463 [M+HTF.
HC1/iPrOH MS (EST): C25/137FN402
D238 HNc(0 * requires 444; found 445
[M+H]+.
2 HCI
H2N,õ H HC1/Et0H MS (ESI): C241135FN402
D239 o= NO:y0' requires 430; found 431
[M+1-1]+.
2HCI
H2NrFrl HCl/Et0H MS (EST): C241135FN402
D240
requires 430; found 431
0
[M+H]+.
= 2HCI
Hr.\ Id , HC1/iPrOH MS (ESI): C23H33FN402
D241 no( LLi1II requires 416; found 417
0 [M+11]+.
HC1/Et0H MS (EST): C22H33C1N402
D242 Nacr-0 requires 420; found 421
[M+111+.
Description 243
(S)-N-(5-cyano-2-methyl-34(3-methylpiperazin-l-yl)methyl)pheny1)-2-
methylpyrimidine-5-
carboxamide, 2 hydrochloride acid salt (D243)
H
8 LJ c.,NH = 2 HCI
I
5 To a solution of (S)-tert-butyl 4-(5-cyano-2-methyl-3-(2-methyl
pyrimidine-5-
carboxamido)benzy1)-2-methylpiperazine-1 -earboxylate (D212, 800 mg) in
methanol (4 nip and
DCM (10 mL) was added hydrogen chloride in dioxane (4 M, 4.31 mL). The mixture
was stirred at
50 C for 30 mm then concentrated under reduced pressure to afford the title
compound (900 mg) as
yellow solid. MS (ESI): C20H24N60 requires 364; found 365 [M+H] .
106
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Description 244
(S)-3-cyano-N-(5-cyano-2-methy1-3-43-methylpiperazin-1-
yl)methypphenyl)benzamide,
dihydrochloride, 2 hydrochloride acid salt (D244)
NJZ2N
0 III 1L.õNH = 2 MCI
11
(S)-tert-butyl 4-(5-cyano-3-(3-cyanobenzamido)-2-methylbenzy1)-2-
methylpiperazine-l-
carboxylate (D213, 915 mg) was added into aq. HC1 solution (5 M, 3.48 mL) in
isopropanol (20
mL). The reaction mixture was stirred at 50 C overnight. The solvent was
removed in vacuo to
give the title compound (750 mg) as white solid. MS (EST): C221-123N50
requires 373; found 374
[M+H].
Description 245
(S)-N-(5-cyano-2-methyl-3-((3-methylpiperazin-1-yl)methyl)pheny1)-6-
methylnicotinamide, 2
hydrochloride acid salt (1)245)
H
- 2 HCI
0 L.NH
To a solution of (S)-tert-butyl 4-(5-cyano-2-methy1-3-(6-
methylnicotinamido)benzy1)-2-
methylpiperazine-l-carboxylate (D214, 150 mg) in THF (2 mL) was added HC1 in
dioxane (0.809
mL). The mixture was stirred at RT for 18 hours. The mixture was concentrated
to obtain the title
compound (130 mg) as pale yellow solid. MS (ESI): C211-125N50 requires 363;
found 364 [M+H].
Descriptions 246-253
Descriptions 246-253 were prepared using a similar procedure to that described
for Description 245,
with the specified reaction base or solvent listed in the table.
D246 (S)-N-(5-ehloro-2-methy1-34(3-methylpiperazin-l-yl)methyl)pheny1)-4-cyano-
4-
methylpentanamide, 2 hydrochloride acid salt
D247 (S)-N-(5-chloro-2-methy1-34(3-methylpiperazin-l-yOmethyl)pheny1)-2-(4-
hydroxycyclohexypacetamide, 2 hydrochloride acid salt
D248 (S)-N-(5-chloro-2-methy1-34(3-methylpiperazin-l-y1)methyl)pheny1)-3-
(methylsulfonyl)propanamide, 2 hydrochloride acid salt
D249 (S)-5-chloro-N-(5-fluoro-2-methy1-34(3-methylpiperazin-l-ypmethyl)pheny1)-
6-
methylnicotinamide, 2 hydrochloride acid salt
D250 (S)-5-chloro-N-(5-chloro-2-methy1-34(3-methylpiperazin-l-
y1)methyl)pheny1)-6-
methylnicotinamide, 2 hydrochloride acid salt
107
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
D251 (S)-N-(5-cyano-2-rnethy1-343-methylpiperazin-l-yDmethyl)pheny1)-6-
methoxynicotinamide,Trifluoroacetic acid salt
D252(S)-N-(5-chloro-2-methy1-343-methylpiperazin-l-yl)methyl)pheny1)-6-cyano-5-
methylnicotinamide, 2 hydrochloride acid salt
D253 (S)-5-chloro-N-(5-cyano-2-methy1-343-methylpiperazin-l-AmethyDpheny1)-6-
methylnicotinamide, 2 hydrochloride acid salt
Structure Base/Solvent Characterization
,fi__ H HC1/Me011 MS (ESI):
C20H29C1N40
N re-1
D246 " 0 I. L.14i.'s = 2 HCI
requires 376; found 377
CI [M+H].
H
HC1/1,4-Dioxane MS (ESI): C211-132C1N302
N
D247 H Mr 0 N-0,,;.: = 2 HCI
o =requires 393; found 394
CI
[M+H].
(i) y
F 1 2 HCI HCFMe0H MS (ESI):
.rõ,...ir I'll ,----,- =
D248 o io C17H26C1N303S requires
o LNH
CI 387; found 388 [M+H].
HHC1/Me0H MS (ESI): C20H24C1FN40
..õ.Ar
et . 2 HCI
D249 10 requires 390; found 391
0
F [M+141+.
N H HC1/Me0H MS (ESI: C201124C12N40
D250 ci
IT, ',,I N 2 HCI requires 406; found 407
ci
7.o.,,c:lir N TFA/DCM MS (ESI): C211125N502
i
D251 0 ao i,,NH = TFA requires 379; found 380
[M+H].
tII
N
hk, HC1/Me0H MS (ESI):
C211124C1N50
=.,
,
I H
D252 -, N Ark requires 397; found 398 tr).=''
o IWP LNH = 2 HCI
[M+Hr.
CI
A
i HCl/Me0H MS (ESD:
C211124C1N50 l
ci requires 397; found 398
D253 =
0 011 L,,,, NH 2 HCI
[M+H].
I I
N
108
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Description 254
(S)-N-(5-chloro-2-methyl-3-03-methylpiperazin-l-Amethypphenyl)-5-fluoro-6-
methylnicotinamide, 3 Trifluoroacetic acid salt (D254)
I H
= 3 TFA
0
CI
(S)-tert-butyl 4-(5-chloro-3-(5-fluoro-6-methylnicotinamido)-2-methylbenzy1)-2-
methylpiperazine-
l-carboxylate (D204, 6.5 g) was added into HC1 solution (30 mL, 4 M in 1,4-
Dioxane). The
mixture was stirred at RT for 1 hour. The solyent was removed under vacuum and
the crude
product was washed with DCM/Me0H = 10:1 (2x2 mL) to afford the title compound
(7 g) as
yellow solid. MS (ESI) C201124C1FN40 requires: 390, found 391 [M+H].
Description 255
N-(5-chloro-2-methy1-3-4(S)-3-methylpiperazin-l-Amethyflphenyl)-2-
(tetrahydrothiophen-
3-yl)acetamide, 2 hydrochloride acid salt (D255)
N..m.õsk
= 2 HCI
\s ¨1 0
CI
To a solution of (2S)-tert-butyl 4-(5-chloro-2-methy1-3-(2-(tetrahydrothiophen-
3-
yOacetamido)benzy1)-2-methylpiperazine-1-carboxylate (D203, 48 mg) in Me0H (1
mL), was
added a solution of hydrogen chloride in dioxane (4 M, 1 mL). The mixture was
stirred for 2 hours
at 50 C. The solvent was removed under vacuum to give the title compound (210
mg) as yellow
gum. MS (ESI): C19H28C1N30S requires 381; found 382 [M+Hr.
Description 256
N-(5-chloro-3-(4S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylphenyl)-2-(tetrahydrothiophen-3-yflacetamide (D256)
cn N. y-0
CI 0
To a solution of N-(5-chloro-2-methy1-3-(((S)-3-methylpiperazin-1-
yl)methyl)pheny1)-2-
(tetrahydrothiophen-3-yl)acetamide (D255, 39 mg) in anhydrous DCM (2 mL) was
added
triethylamine (20.66 mg) and cyclopentanecarbonyl chloride (20.31 mg). The
reaction mixture was
stirred for 1 hour at 18 C. The mixture was washed with brine, and the organic
phase was dried
109
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
over anhydrous sodium sulfate, filtered and concentrated to give the title
compound (220 mg) as
yellow gum. MS (EST): C25H36C1N302S requires 477; found 478 [M+H].
Description 257
(S)-2-chloro-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylphenypacetamide (D257)
Nasi'yo'sµ
CI 0
2-Chloroacetyl chloride (97 mg) solution in DCM (10 mL) was slowly added to
the mixture of (S)-
(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-l-
y1)(cyclopentyl)methanone (D157, 240
mg) and TEA (0.159 mL) in DCM (20 mL) at 0 C. The reaction mixture was allowed
to warm to
RT and stirred for 1 hour. The mixture was dulited with water (15 mL. The
organic layer was dried
over anhydrous sodium sulfate, filtered and concentrated. The residue was
purified by column
chromatography (silica gel, petroleum ether/Et0Ae = 5:1) to afford the title
compound (240 mg) as
white solid. MS (ESI): C211-129C12N302 requires 425; found 426 [M+H].
Description 258
(S)-54344-(cyclopentanecarbony1)-3-methylpiperazin-l-yl)methyl)-5-fluoro-2-
methylphenyl)carbamoyl)picolinic acid, Trifluoroacetic acid salt (D258)
0
HO
I H
0
= TFA
To a suspension of (S)-6-eyano-N-(344-(cyclopentanecarbonyl)-3-methylpiperazin-
1-y1)methyl)-
5-fluoro-2-methylphenyl)nicotinamide (D222, 120 mg) in ethanol (5 mL), 3 M
NaOH solution (2.2
20 mL) was added dropwise. The reaction mixture was stirred at 100 C for 3
hours. Solvent was
removed by rotavap, 3 M HCl (aq.) was added dropwise to pH = 5. The residue
was diluted with
Et0Ae (10 mL) then washed with brine (10 mL). Organic layer was separated,
dried over MgSO4,
filtered and concentrated. The residue was purified by reverse phase column
chromatography (C18
column, 0-35% CH3CN in H20 with 0.05 % TFA) to afford the title compound (80
mg) as white
25 solid. MS (ESI): C26H31FN404 requires 482; found 483 [M+H]t
Example 1
(S)-N-(344-(cyclopentanecarbony1)-3-methylpiperazin-l-yl)methyl)-5-fluoro-2-
methylpheny1)-2-(oxetan-3-ybacetamide (El)
110
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
N
NlõNTO
2-(Oxetan-3-y1) acetic acid (0.035 mL) and DIPEA (0.157 mL) were dissolved in
DMF (3 mL).To
this solution, HATU (182 mg) was added gradually. The reaction mixture was
stirred at RT for 1
hour. Then (S)-(4-(3-amino-5-fluoro-2-methylbenzy1)-2-methylpiperazin-1-
yl)(cyclopentyl)methanone (D156, 100 mg) in DMF (2 mL) was added into the
mixture, which was
stirred at RT overnight. Water (10 mL) was added, extracted with Et0Ac (2x10
mL). The
combined organic layer was dried over Na2SO4 and filtered. The filtrate was
concentrated to
dryness, and the residue was purified by MADP to get the title (40 mg) as
white solid. 'H NMR
(400 MHz, DMSO-d6): 9.45 (s, 1H), 7.20 (dd, J= 10.5, 2.0 Hz, 1H), 6.95 (d, J=
9.5 Hz, 1H), 4.69
(dd, J= 7.5, 6.2 Hz, 2H), 4.54 (brs, 0.5H), 4.35 (t, J= 6.1 Hz, 2H), 4.19 (d,
J= 10.0 Hz, 1H), 3.74
(d, J= 13.0 Hz, 0.5H), 3.45-3.38 (m, 2H), 3.32-3.25 (m, 1H), 3.18 (t, J= 12.3
Hz, 0.5H), 2.96-2.86
(m, 1H), 2.77 (d, J= 7.8 Hz, 2.5H), 2.72 (d, J= 12.2 Hz, 1H), 2.66-2.58 (m,
1H), 2.14 (s, 3H),
2.11-1.44 (m, 10H), 1.28-1.04 (m, 3H). 19F NMR (376 MHz, DMSO-d6): -117.9. MS
(ESI):
C24H34FN303requires 431; found 432 [M+H].
Examples 2-42
Examples 2-42 were prepared using a similar procedure to that described for
Example 1, with the
specified reaction base or solvent listed in the table.
E2 N-(3-a(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-5-fluoro-2-
methylpheny1)-3-(tetrahydrofuran-2-y1)propanamide, Trifluoroacetic acid salt
E3 N-(3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-5-fluoro-
2-
methylpheny1)-2-(tetrahydrofuran-3-yDacetamide
E4 N-(3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-5-fluoro-
2-
methylphenyl)-2-(tetrahydrofuran-2-ypacetamide, Trifluoroacetic acid salt
E5 (S)-N-(34(4-(cyclopentanecarbony1)-3-methylpiperazin-1 -yl)methyl)-5-fluoro-
2-
methylpheny1)-2-(tetrahydro-2H-pyran-4-ypacetamide
E6 N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-5-fluoro-2-
methylpheny1)-2-(tetrahydro-2H-pyran-2-y1)acetamide, Trifluoroacetic acid salt
E7 eis-N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-ypmethyl)-5-
fluoro-2-
methylpheny1)-2-(3-hydroxycyclopentyl)acetamide
E8 N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-5-fluoro-2-
methylpheny1)-2-(3-hydroxycyclohexypacetamide
E9 N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-5-fluoro-2-
methylpheny1)-2-(hexahydrofuro[2,3-b]furan-3-ypacetamide
111
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
MO (S)-N-(3 -(cyclopentanecarbony1)-3-methylpiperazin-1 -yl)methyl)-5 -
fluoro-2-
methylpheny1)-2-(2-methyloxazol-4 -yl)acetamide, Trifluoroacetic acid salt
Ell (S)-N-(3 -((4 -(cyclopentanecarbony1)-3 -methylpiperazin- 1 -yl)methyl)-5-
fluoro-2-
methylpheny1)-2-(3-methylisoxazol-5-ypacetamide
E12 (S)-N-(34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-ypmethyl)-5- chloro-
2-
methylpheny1)-2-(3-methylisoxazol-5-ypacetamide
E13 N-(5 -chloro-3 -(((S)-4 -(cyclopentanecarbony1)-3 -methylpiperazin- 1 -
yl)methyl)-2 -
methylpheny1)-2-(tetrahydrofuran-2 -yl)acetamide, Trifluoroacetic acid salt
E14 N-(5 -chloro-3 -(((S)-4 -(cyclopentanecarbony1)-3 -methylpiperazin-1 -
yl)methyl)-2 -
methylpheny1)-2-(2-methyltetrahydrofuran-3-yl)acetamide, Trifluoroacetic acid
salt
E15 (S)-N-(5-chloro-3((4-(cyclopentanecarbony1)-3-methylpiperazin-1 -
yl)methyl)-2
methylpheny1)-2-(tetrahydro-2H-pyran-4-ypacetamide, Trifluoroacetic acid salt
E16 N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylphenyl)-2-(tetrahydro-2H-pyran-2-y1)acetamide, Trifluoroacetic acid salt
.. E17 (S)-N-(5 -chloro-344-(cyclopentanecarbony1)-3-methylpiperazin- 1 -
yl)methyl)-2 -
methylpheny1)-2-morpholinoacetamide, Trifluoroacetic acid salt
E18 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-
2-
methylpheny1)-4-hydroxycyclohexanecarboxamide
E19 N-(5 -chloro-3 -((( S)-4 -(cyclopentanecarbony1)-3-methylpiperazin-1 -
yl)methyl)-2 -
.. methylpheny1)-2-(5-methyltetrahydrofuran-3-ypacetamide, Trifluoroacetic
acid salt
E20 N-(5-chloro-3-4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-
2-
methylpheny1)-2-(tetrahydro-2H-pyran-3-ypacetamide, Trifluoroacetic acid salt
E21 N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
yl)methyl)-2-
methylpheny1)-2-(2-methyltetrahydro-2H-pyran-4-yl)acetamide
E22 N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-
2-
methylpheny1)-2-(4-methyltetrahydrofuran-3-ypacetamide, Trifluoroacetic acid
salt
E23 N-(5 -chloro-34(S)-4-(cyclopentanecarbony1)-3 -methylpiperazin-1 -
yl)methyl)-2-
methylpheny1)-2-(3-methyltetrahydro-2H-pyran-4-yl)acetamide, Trifluoroacetic
acid salt
E24 N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1 -
yl)methyl)-2-
methylpheny1)-2-(tetrahydrofuran-2-yppropanamide, Trifluoroacetic acid salt
E25 N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1 -
yl)methyl)-2-
methylpheny1)-2-(tetrahydrofuran-3-yl)propanamide
E26 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-
2-
methylpheny1)-4-methoxybutanamide, Trifluoroacetic acid salt
E27 (S)-N-(5-ehloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylphenyl)-2-(2-methoxyethoxy)acetamide
112
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
E28 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-2-(2-oxopyrro1idin-1-y1)acetamide, Trifluoroacetic acid salt
E29 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-
2-
methylpheny1)-2-(2-oxooxazolidin-3-y1)acetamide
E30 N-(5-chloro-34(S)-4-(2-cyclopropylacety1)-3-methylpiperazin-l-yOmethyl)-2-
methylpheny1)-
2-(tetrahydrofuran-3 -yl)acetamide
E31 (S)-N-(5-chloro-344-(2-cyclopropylacety1)-3-methylpiperazin-l-ypmethyl)-2-
methylpheny1)-
4-methoxybutanamide
E32 N-(5-cyano -3-(((S)-4 -(cyclopentanecarbony1)-3-methylpiperazin-1 -
yOmethyl)-2-
methylpheny1)-2-(tetrahydrofuran-3-yl)acetamide
E33 (S)-N-(5-chloro-3((4-(cyclopentanecarbony1)-3 -methylpiperazin-1 -
yl)methyl)-2-
methylpheny1)-3-(1-cyanocyclopropyl)propanamide, Trifluoroacetic acid salt
E34 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylpheny1)-2-(difluoromethoxy)acetamide
E35 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-
2-
methylpheny1)-4-cyano-4-methylpentanamide
E36 (S)-N-(5-chloro-3((4-(cyclopentanecarbony1)-3 -methylpiperazin-l-
yl)methyl)-2-
methylphenyl)tetrahydro-2H-thiopyran-4-carboxamide 1,1-dioxide
E37 N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3 -methylpiperazin-1 -
yl)methyl)-2-
methylphenyl)tetrahydro-211-thiopyran-3-carboxamide 1,1-dioxide
E38 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-
2-
methylpheny1)-2-(1,1-dioxidotetrahydro-2H-thiopyran-4-y1)acetamide
E39 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-3-(difluoromethoxy)propanamide, Trifluoroacetic acid salt
E40 (S)-N-(5-chloro-3((4-(cyclopentanecarbony1)-3 -methylpiperazin- 1 -
yptnethyl)-2-
methylpheny1)-2-(3-oxomorpholino)acetamide
E41 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin- 1 -
yl)methyl)-2-
methylpheny1)-2-hydroxy-2-methylpropanamide
E42 (S)-N-(5-chloro-3 -44-(cyclopentanecarbony1)-3 -methylpiperazin-1 -
yl)methyl)-2 -
methylpheny1)-3-hydroxy-3-methylbutanamide
Structure Base/Solvent Characterization
1H NMR (400 MHz, DMSO-
H
d6): 9.34 (s, 111), 7.21 (d, J=
E2 0 N0 DIPEA/DMF 10.1 Hz, 1H), 6.95 (d, J= 8.7
Hz, 1H), 4.55 (brs, 0.5H), 4.12
= TFA
(brs, 1H), 3.84-3.65 (m, 2.5H),
113
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
3.65-3.52 (m, 1H), 3.41 (brs,
2H), 3.30 (brs, 2.5H), 2.99-2.55
(m, 3.5H), 2.47-2.35 (m, 2H),
2.15 (s, 3H), 2.05-1.37 (m,
14H), 1.33-0.96 (m, 3H). 19F
NMR (376 MHz, DMSO-d6): -
74.4, -117.6. MS (ESI):
C261138FN303requires 459;
found 460 [M+H].
NMR (400 MHz, DMSO-
d6): 9.37 (s, 1H), 7.20 (dd, J=
10.4, 2.6 Hz, 111), 6.96 (dd, J=
9.4, 2.4 Hz, 1H), 4.55 (brs,
0.5H), 4.19 (brs, 1H), 3.87-3.58
(m, 3.5H), 3.45-3.31 (m, 3H),
3.25-3.12 (m, 0.5H), 2.98-2.85
E3 0 0 11,A NT DIPEAJDMF
(m, 1H), 2.85-2.52 (m, 3.5H),
2.47-2.38 (m, 2H), 2.16 (s, 3H),
2.10-1.40 (m, MI), 1.30-1.04
(m, 3H). 19F NMR (376 MHz,
DMSO-d6): -73.4, -118Ø MS
(ESI): C25H36FN303requires
445; found 446 [M+H].
'H NMR (400 MHz, DMSO-
d6): 9.39 (s, 1H), 7.24 (dd, J=
10.4, 2.1 Hz, 111), 6.95 (d, J=
9.3 Hz, 1H), 4.54 (brs, 0.5H),
4.26-4.09 (m, 2H), 3.86-3.70
401 (m, 1.5H),
3.68-3.57 (m, 1H),
E4 N 0 DIPEA/DMF
3.46-3.38 (m, 2H), 3.25-3.10
(m, 0.5H), 2.98-2.53 (m, 4.5H),
= TFA
2.48-2.42 (m, 1H), 2.15 (s, 3H),
2.10-1.43 (m, 1411), 1.32-1.03
(m, 311).19F NMR (376 MHz,
DMSO-d6): -74.0, -117.9. MS
(ESI): C25H36FN303requires
114
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
445; found 446 [M+H].
1H NMR (400 MHz, Me0D-
d4): 7.08 (dd, J= 9.8, 2.8 Hz,
111), 6.99 (dd, J= 9.3, 2.8 Hz,
1H), 4.66 (brs, 0.511), 4.36-4.22
(m, 1H), 3.95 (dd, J= 11.1,3.4
Hz, 2H), 3.83 (d, J= 13.3 Hz,
N rath 0.511), 3.54-3.41 (m, 4H), 3.40-
E5 c:1 tipLNo D1PEA/DMF 3.33 (m, 0.5H), 3.08-2.91 (m,
1.511), 2.83 (d, J= 11.6 Hz,
1H), 2.77-2.67 (m, 111), 2.37 (d,
J= 7.2 Hz, 2H), 2.28-2.21 (m,
3H), 2.19-1.56 (m, 1311), 1.50-
1.14 (m, 5H). MS (ESI):
C26H38FN303requires 459;
found 460 [M+H].
1H NMR (400 MHz, DMSO-
d6): 9.35 (s, 1H), 7.26 (d, J=
10.4 Hz, 111), 6.94 (d, J= 8.1
Hz, 111), 4.54 (brs, 0.5H), 4.26-
4.11 (m, 1H), 3.87 (d, J= 11.4
Hz, 1H), 3.79-3.64 (m, 1.5H),
3.46-3.38 (m, 2H), 3.24-3.13
so
(m, 0.5H), 2.99-2.85 (m, 1H),
E6 0 NO D1PEA/DMF
2.84-2.68 (m, 1.5H), 2.68-2.52
= TEA (m, 2H), 2.45-2.35 (m, 1H),
2.15 (s, 311), 2.10-1.38 (m,
15H), 1.32-1.02 (m, 4H). 19F
NMR (376 MHz, DMSO-d6): -
74.4, -116.3. MS (EST):
C26H38FN303requires 459;
found 460 [M+Hr.
1H NMR (500 MHz, DMSO-
E7
H
No base d6): 9.34 (s, 1H), 7.17 (dd, J=
HO
/DMF 10.6, 2.7 Hz, 111), 6.96 (d, J=
9.1 Hz, 1H), 4.54 (brs, 0.5H),
115
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
4.50 (d, J= 3.8 Hz, 1H), 4.23-
4.16 (m, 1H), 4.12-4.06 (m,
1H), 3.75 (d, J= 14.8 Hz,
0.5H), 3.45-3.35 (m, 2H), 3.24-
3.15 (m, 0.5H), 2.95-2.87 (m,
1H), 2.81-2.70 (m, 1.5H), 2.66-
2.59 (m, 111), 2.42-2.37 (m,
2H), 2.24-2.18 (m, 1H), 2.15 (s,
3H), 2.12-1.37 (m, 15H), 1.26-
1.05 (m, 4H). MS (ESI):
C26H38FN303requires 459;
found 460 [M+H]t
1H NMR (500 MHz, DMSO-
d6): 9.33 (s, 1H), 7.22-7.17 (dd,
J= 10.0, 2.0 Hz, 111), 6.97-6.95
(d, J= 9.8 Hz, 1H), 4.55 (brs,
0.5H), 4.36-4.19 (m, 2H), 3.85
(brs, 1H), 3.77-3.71 (m, 0.5H),
3.44-3.38 (m, 2H), 3.23-3.17
HO,corN No base
T
E8 o 1.1 140:1(0
/DMF (m, 0.5H), 2.95-2.86 (m, 1H),
0 2.81-2.70 (m, 1.5H), 2.66-
2.59 (m, 1H), 2.27-2.18 (m,
2H), 2.15 (brs, 3H), 2.08-1.34
(m, 16H), 1.28-0.98 (m, 6H),
MS (ESI): C27atoFN303
requires 473; found 474
[M+H].
1HNMR (500 MHz, DMSO-
d6): 9.44 (s, 1H), 7.21 (dd, J=
10.1, 2.2Hz, 1H), 6.97 (d, J=
N 8.5 Hz, 1H), 5.61 (d, J= 5.0 Hz,
N No base
E9 0 o
1H), 4.54 (brs, 0.5H), 4.25-4.14
/DMF
(m, 1H), 3.88 (t, J= 7.7 Hz,
1H), 3.79-3.69 (m, 2.5H), 3.46-
3.35 (m, 3H), 3.19 (t, J= 12.6
Hz, 0.511), 2.96-2.69 (m, 3.5H),
116
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
2.68-2.52 (m, 311), 2.16 (s, 311),
2.14-1.45 (m, 1311), 1.30-1.05
(m, 3H). MS (ESD:
C271138FN304requires 487;
found 488 [M+H].
IHNMR (400 MHz, DMSO-
d6): 9.67 (s, 111), 7.83 (s, 1H),
7.50 (d, J= 9.5 Hz, 111), 7.26
(d, J= 6.3 Hz, 1H), 4.80 (brs,
No base 0.511), 4.57-4.20 (m, 3H), 4.05
MO 0 /DMF (d, J= 12.3 Hz, 0.5H), 3.62 (s,
2H), 3.44-2.85 (m, 6H), 2.38 (s,
= TFA
311), 2.23 (s, 3H), 1.86-1.43 (m,
8H), 1.37-1.10 (m, 3H). MS
(ESD: C25H33FN403 requires
456; found 457 [M+H].
IHNMR (400 MHz, DMSO-
d6): 9.75 (s, 1H), 7.22 (dd, J=
10.4, 2.6 Hz, 114), 6.99 (dd, J=
9.3, 2.0 Hz, 1H), 6.28 (s, 1H),
4.54 (brs, 0.5H), 4.25-4.15 (m,
1H), 3.94 (s, 2H), 3.75 (d, J=
11.8 Hz, 0.5H), 3.46-3.37 (m,
N
Ell 7-ir TEA/DMF 2H),
3.19 (t, J= 12.2 Hz, 0.5H),
\ 0
0 2.96-2.86 (m, 1H), 2.84-2.69
(m, 1.511), 2.66-2.59 (m, 1H),
2.22 (s, 311), 2.18 (s, 311), 2.15-
1.45 (m, 10H), 1.29-1.05 (m,
3H). MS (ESD: C25H33FN403
requires 456; found 457
[M+Hr.
'H NMR (400 MHz, DMSO-
H d6): 9.78 (s, 111), 7.41 (d, J=
N
E12 Nf) I 0 TEA/DMF 2.3
Hz, 1H), 7.19 (s, 1H), 6.28
(s, 114), 4.55 (brs, 0.511), 4.25-
4.15 (m, 1H), 3.93 (s, 2H), 3.75
117
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(d, J= 12.8 Hz, 0.5H), 3.42
(brs, 2H), 3.23-3.13 (m, 0.5H),
2.96-2.86 (m, 1H), 2.83-2.67
(m, 1.5H), 2.65-2.56 (m, 111),
2.21 (s, 3H), 2.19 (s, 3H), 2.15-
1.45 (m, 10H), 1.29-1.03 (m,
310. MS (ESD: C25H33C1N403
requires 472; found 473
[M+H].
111 NMR (400 MHz, Me0D-
d4): 7.59 (s, 11), 7.48 (s, 111),
4.60 (brs, 1H), 4.41-4.07 (m,
3.5H), 3.94 (q, J= 7.2 Hz, 1H),
a-1N
3.88-3.70 (m, 1H), 3.64-3.34
E13 N y,0 DIPEA/DMF
(m, 2.5H), 3.25-2.91 (m, 3.5H),
CI
2.69-2.56 (m, 21), 2.30 (s, 31),
= TEA
2.21-1.51 (m, 1211), 1.48-1.15
(m, 3H). MS (ESI):
C25H36C1N303requires 461;
found 462 [M+H].
1H NMR (400 MHz, DMSO-
d6): 9.51 (d, J= 4.9 Hz, 1H),
7.38 (d, J= 1.7 Hz, 1H), 7.16
(brs, 1H), 4.54 (brs, 0.5H), 4.19
(d, J= 10.6 Hz, 1H), 4.03-3.91
(m, 0.511), 3.86-3.46 (m, 3H),
E14 DWEA/DMF 3.41
(s, 2H), 3.17 (t, J= 11.9
CI
= TFA 100 Hz,
0.5H), 3.01-2.52 (m, 4H),
2.47-2.21 (m, 2H), 2.17 (s, 3H),
2.13-1.40 (m, 12.5H), 1.30-0.98
(m, 6H). MS (EST):
C261138C1N303requires 475;
found 476 [M+H].
118
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
NMR (400 MHz, DMSO-
d6): 9.42 (s, 1H), 7.38 (d, J=
1.8 Hz, 1H), 7.16 (s, 111), 4.54
(brs, 0.5H), 4.19 (d, J= 9.4 Hz,
111), 3.91-3.65 (m, 2.511), 3.47-
H
3.37 (m, 2H), 3.31-3.07 (m,
EIS 0 DIPEA/DMF 2.5H), 2.99-2.82 (m, 1H), 2.81-
CI
2.55 (m, 2.511), 2.29 (d, J=7.1
= TFA
Hz, 2H), 2.16 (s, 3H), 2.12-1.41
(m, 1311), 1.36-0.97 (m, 5H).
MS (ER): C261138C1N303
requires 475; found 476
[M+H].
'H NMR (400 MHz, DMSO-
d6): 9.52-9.06 (m, 1 H), 7.78-
6.95 (m, 2 H), 4.89-4.02 (m, 3
H), 3.86 (d, J= 11.0 Hz, 2 H),
El6 0 DIPEA/DMF 3.51-3.11 (m, 5 H), 3.05-2.58
CI
(m, 3 H), 2.46-2.07 (m, 411),
= TFA
1.90-0.99 (m, 18 H). MS (ESI):
C26H38C1N303requires 475;
found 476 [M+H].
IFINMR (400 MHz, Me0D-
d4): 7.67 (d, J= 2.3 Hz, 1H),
7.50 (d, J= 2.4 Hz, 1H), 4.58
(brs, 0.5H), 4.38-3.86 (m,
r-
8.5H), 3.64-3.35 (m, 511), 3.35-
E17 o,) 0
NI N DIPEA/DMF
3.31 (m, 2H), 3.30-3.27 (m, 0.5
CI
= TFA H), 3.18-2.91 (m, 2.5H), 2.33
(s, 3H), 2.03-1.17 (m, 12H). MS
(ESI): C25H37C1N403requires
476; found 477 [M+H].
NMR (400 MHz, Me0D-
H
µ,0
El8 0 ILN0 D1PEA/DMF
d4): 7.27 (d, J= 1.8 Hz, 1H),
7.21 (d, J= 1.6 Hz, 1H), 4.74-
CI
4.52 (m, 1H), 4.38-4.20 (m,
119
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
11-I), 4.01-3.73 (m, 1H), 3.64-
3.33 (m, 2.5H), 3.12-2.60 (m,
3.5H), 2.55-2.31 (m, 1H), 2.28-
2.20 (m, 3H), 2.20-1.53 (m,
17H), 1.47-1.08 (m, 4H). MS
(ESI): C26H38C1N303requires
475; found 476 [M+H].
NMR (400 MHz, Me0D-
d4): 7.36 (s, 2H), 4.47 (brs, 1H),
4.24-3.75 (m, 5H), 3.56-3.30
(m, 2H), 3.20-3.12 (m, 0.5H),
N 3.05-2.81
(m, 2.5H), 2.75-2.60
E19 0 8 LN 0 DIPEA/DMF (m,
1.5H), 2.53-2.36 (m, 211),
CI 2.28-2.12
(m, 3.5H), 1.91-1.42
- TFA
(11, 10H), 1.40-1.03 (m, 7H).
MS (ESI): C261138C1N303
requires 475; found 476
[M+1-1]+.
NMR (400 MHz, Me0D-
d4): 7.50 (s, 1H), 7.48 (s, 1H),
4.62 (brs, 0.5H), 4.36 (brs, 2H),
4.17 (d, J= 11.9 Hz, 0.5H),
3.98-3.78 (m, 2H), 3.65-3.34
E20 DIPEA/DMF
(m, 3.511), 3.29-2.86 (m, 4.5H),
CI
2.41-2.31 (m, 2H), 2.29 (s, 3H),
= TFA
2.22-1.56 (m, 13H), 1.49-1.16
(m, 411). MS (EST):
C26H38C1N301 requires 475;
found 476 [M+H].
IIINMR (400 MHz, Me0D-
d4): 7.32 (d, J= 2.0 Hz, 1H),
N 1"µ's 7.21 (d, J=
1.7 Hz, 1H),4.65
E21 0 DIPEA/DMF
(brs, 0.5H), 4.40-4.18 (m, 1H),
CI 3.97 (dd, J=
11.5, 3.7 Hz, 1H),
3.89-3.66 (m, 1H), 3.55-3.32
(m, 411), 3.07-2.41 (m, 4H),
120
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
2.34 (d, .1= 7.2 Hz, 1.511), 2.25
(s, 311), 2.22-1.50 (m, 1311),
1.50-0.94 (m, 8H). MS (ESI):
C271140C1N303 requires 489;
found 488 [M-HI.
IFI NMR (400 MHz, Me0D-
d4): 7.46-7.35 (m, 2H), 4.54
(brs, 1H), 4.38-4.25 (m, 211),
4.17-3.80 (m, 2.5H), 3.58-3.23
(m, 5H), 3.18-2.83 (m, 3.5H),
E22 N 0
DIPEA/DMF 2.70-2.49 (m, 2H), 2.44-2.25
CI
(m, 2H), 2.19 (s, 3H), 1.88-1.06
= TFA
(m, 11H), 1.05-0.88 (m, 3H).
MS (ESI): C26H38C1N303
requires 475; found 476
[M+II]+.
1HNMR (400 MHz, Me0D-
d4): 7.51 (d, J= 1.7 Hz, 111),
7.48 (d, J= 1.7 Hz, 1H),5.04-
4.93 (m, 0.5H), 4.63 (brs, 1H),
4.49-4.28 (m, 211), 4.18 (d, J=
giath
13.2 Hz, 0.511), 3.98-3.77 (m,
E23 0 III L,.,.N,r0 DIPEA/DMF
1H), 3.74-3.53 (m, 2I1), 3.52-
CI
3.35 (m, 3H), 3.26-2.67 (m,
= TFA
4H), 2.51-2.16 (m, 611), 2.01-
1.17 (m, 15H), 1.12-0.86 (m,
3}1). MS (ESI): C27a40CIN303
requires 489; found 490
[M+II] .
11INMR (400 MHz, CDC13):
8.81 (s, 1H), 8.15 (s, 111), 7.16
c
(d, J= 1.6 Hz, 1H),5.02-4.98
E24 rl ocro
DIPEA/DMF (m, 2H), 4.66-4.62 (m, 1.5H),
CI 0
4.28-4.17 (m, 2.51I), 4.12-4.01
= TFA
(m, 2H), 3.93-3.84 (m, 0.5H),
3.60-3.54 (m, 1.511), 2.26-2.20
121
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(m, 111), 2.82-2.71 (m, 211),
2.51-2.38 (m, 2H), 2.27 (s, 3H),
2.05-1.96 (m, 4H), 1.83-1.60
(m, 7H), 1.38-1.34 (m, 311),
1.02-0.97 (m, 311). MS (ESI):
C26H38C1N303 requires 475;
found 476 [M+H].
111 NMR (400 MHz, DMSO-
d6): 9.46 (d, J= 4.8 Hz, 1H),
7.33 (dd, J= 6.8, 2.0 Hz, 1H),
7.19 (s, 1H), 4.52-4.50 (m,
0.511), 4.28-4.20 (m, 111), 3.84-
3.75 (m, 2.511), 3.65-3.62 (m,
1H), 3.50-3.42 (m, 3H), 3.25-
E25 0 N -110( RP N,N.1.1(0 DIPEA/DMF 3.20 (m, 0.5H), 2.94-2.90 (m,
1H), 2.80-2.61(m, 2.5H), 2.50-
2.30 (m, 2H), 2.19-2.16 (m,
3H), 2.15-1.80 (m, 3H), 1.80-
1.45 (m, 9H), 1.30-1.00 (m,
6H). MS (ESI): C261138C1N303
requires 475; found 476
[M+Hr=
11-INMR (400 MHz, Me0D-
d4): 7.51 (s, 2H), 4.65 (brs, 1H),
4.49-4.36 (m, 2H), 4.19 (d, J=
11.0 Hz, 0.5H), 3.65-3.38 (m,
INYM".(0 511), 3.35
(s, 3H), 3.29-2.97 (m,
E26 o 41,-P DIPEA/DMF
3.511), 2.52 (t, J= 7.3 Hz, 211),
TEA 2.29 (s,
3H), 1.97 (quin, J= 6.7
=
Hz, 2H), 1.92-1.56 (m, 8H),
1.49-1.19 (m, 3H). MS (EST):
C241136C1N303 requires 449;
found 450 [M+Hr.
1H NMR (400 MHz, DMSO-
E27 N
DIPEA/DCM d6): 9.22 (s, 1H), 7.58 (d, J=
2.0 Hz, 1H), 7.18 (s, 1H), 4.54
122
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(brs, 0.5H), 4.20 (d, J= 10.5
Hz, 1H), 4.11 (s, 2H), 3.75 (d, J
= 13.6 Hz, 0.5H), 3.70 (dd, J=
5.5, 3.8 Hz, 2H), 3.53 (dd, J=
5.5, 3.8 Hz, 2H), 3.48-3.37 (m,
2H), 3.27 (s, 3H), 3.18 (t, J=
12.4 Hz, 0.511), 2.95-2.85 (m,
1H), 2.83-2.68 (m, 1.511), 2.61
(d, J= 11.0 Hz, 1H), 2.17 (s,
3H), 2.15-1.45 (m, 10H), 1.27-
1.03 (m, 3H). MS (ESI):
C24H36C1N304requires 465;
found 466 [M+H].
111NMR (400 MHz, Me0D-
d4): 7.57 (d, J= 2.2 Hz, 1H),
7.53 (d, J= 2.2 Hz, 1H), 4.65
(brs, 1H), 4.50-4.35 (m, 211),
4.20 (brs, 2.511), 3.60 (t, J= 7.1
Hz, 2H), 3.51-3.37 (m, 2.5H),
aThor_ 401
E28 DIPEA/DMF
3.29-2.96 (m, 3.5H), 2.45 (t, J=
CI 0
8.1 Hz, 2H), 2.30 (s, 3H), 2.13
= TFA
(quin, J= 7.6 Hz, 211), 1.97-
1.57 (m, 8H), 1.49-1.20 (m,
3H). MS (ES1): C25H35C1N403
requires 474; found 475
[M+Hr=
IHNMR (400 MHz, Me0D-
d4): 7.37 (d, J= 2.2 Hz, 111),
7.23 (d, J= 1.7 Hz, 1H), 4.72-
4.50 (m, 0.5H), 4.43 (t, J= 8.1
HZ, 211), 4.36-4.23 (m, 1H),
E29 j 8 NaicrO. DIPEA/DMF
4.16 (s, 2H), 3.83 (d, J= 13.7
Hz, 0.5H), 3.77 (t, J= 8.1 Hz,
2H), 3.60-3.40 (m, 2H), 3.40-
3.33 (m, 0.5H), 3.07-2.89 (m,
1.5H), 2.82 (d, J= 10.8 Hz,
123
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/070753
1H), 2.71 (t, J= 10.4 Hz, 1H),
2.26 (s, 3H), 2.23-2.12 (m, 1H),
2.09-1.54 (m, 9H), 1.40-1.16
(m, 3H). MS (ESD:
C24H33C1N404 requires 476;
found 477 [M+H].
NMR (400 MHz, Me0D-
d4): 7.21 (d, J= 2.1 Hz, 1H),
7.11 (d, J= 1.8 Hz, 1H),4.57
(brs, 0.5H), 4.22 (d, J= 13.8
Hz, 0.511), 4.03 (brs, 0.5H),
3.89-3.76 (m, 2H), 3.68 (q, J=
7.7 Hz, 1H), 3.60 (d,J= 13.2
Hz, 0.5H), 3.45-3.22 (m, 3.5H),
E30 = try
2.91-2.80 (m, 0.5H), 2.72 (t, J=
N
No-' 0 DIPEA/DMF
8.9 Hz, 111), 2.66-2.56 (m, 2H),
ci
2.48-2.27 (m, 2.5H), 2.19 (d, J
= 6.6 Hz, 1H), 2.15 (s, 3H),
2.13-1.84 (m, 3.5H), 1.67-1.53
(m, 111), 1.28-1.08 (m, 3H),
0.98-0.78 (m, 1H), 0.50-0.35
(m, 211), 0.08 (brs, 2H). MS
(ESD: C24H34C1N303 requires
447; found 448 [M+Hr.
1H NMR (400 MHz, Me0D-
d4): 7.32 (d, J= 2.2 Hz, 1H),
7.21 (d, J= 2.0 Hz, 1H), 4.67
(brs, 0.5H), 4.32 (d, J= 13.4
Hz, 0.5H), 4.14 (brs, 0.5H),
E31 NON''s
D1PEA/DMF 3.70 (d, J= 12.7 Hz, 0.5H),
3.54-3.44 (m, 4H), 3.43-3.36
(m, 0.5H), 3.35 (s, 3H), 2.95 (t,
J= 12.8 Hz, 0.5H), 2.82 (t, J=
9.8 Hz, 1H),2.71 (d, J= 11.5
Hz, 1H), 2.50 (t, J= 7.3 Hz,
2H), 2.43 (dd, J= 14.9, 6.4 Hz,
124
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
0.5H), 2.30 (d, õI= 6.4 Hz, 111),
2.25 (s, 3H), 2.23-2.13 (m,
1.511), 2.11-1.90 (m, 311), 1.36-
1.18 (m, 3H), 1.05-0.93 (m,
1H), 0.59-0.48 (m, 211), 0.18
(brs, 2H). MS (ESI):
C23H34C1N303requires 435;
found 436 [M+H].
111 NMR (400 MHz, Me0D-
d4): 7.68 (s, 1H), 7.54 (s, 1H),
4.67 (brs, 0.5H), 4.41-4.19 (m,
1H), 4.04-3.73 (m, 3.5H), 3.63-
E32
H
NO\;''s)pµ
O-1 8 DIPEA/DMF
I I 2.86-2.65 (m, 3H), 2.63-2.48
(m, 2H), 2.37 (s, 3H), 2.29-1.51
(m, 1211), 1.43-1.13 (m, 3H).
MS (ESI): C26H36N403 requires
452; found 453 [M+H].
NerH
TEA DIPEA/DMF 111NMR (400 MHz, DMS0-
d6): 9.64 (s, 1H), 9.23 (brs, 111),
ci
7.64 (s, 111), 7.52 (s, 1H), 4.78
(brs, 0.511), 4.55-4.30 (m,
2.5H), 4.20-4.00 (m, 0.5H),
3.40-3.10 (m, 2H), 3.05-2.85
(m, 211), 2.66-2.61 (m, 2H),
E33
2.25 (s, 311), 1.85-1.50 (m,
1211), 1.34-1.32 (m, 111), 1.25-
1.10 (m, 3H), 1.00-0.85(m,
2.5H). 19F (376 MHz, DMSO-
d6): -69.2, -71.1. MS (EST):
C26H35C1N402requires 470;
found 471 [M+Hr.
125
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
7 H DIPEA/DMF NMR (400 MHz, CDC13):
N-Th's
7.97 (brs, 1H), 7.88 (s, 1H),
0 1,õNTo
ci
7.13 (s, 1H), 6.43 (t, J= 72.8
Hz, 1H), 4.78 (brs, 0.5H), 4.54
(s, 2H), 4.44-4.40 (m, 0.5H),
4.15 (brs, 0.5H), 3.70-3.65 (m,
0.5H), 3.49-3.30 (m,2.5H),
E34 2.87-2.63 (m, 3.511), 2.25 (s,
3H), 2.20-2.17(m, 1H), 2.05-
1.95 (m, 1H), 1.85-1.64 (m,
511), 1.67-1.51 (m, 3H), 1.31-
1.22 (m, 3H). 19F (376 MHz,
CDC13): -85.0, -85.2. MS (EST):
C221-110C1F2N303 requires 457;
found 458 [M+H].
)7H DIPEA/DMF IHNMR (500 MHz, DMSO-
N
N 0= Nac,c> d6): 9.54 (s, 1H), 7.42 (brs, 1H),
ci 7.16 (brs, 1H), 4.55 (brs, 0.5H),
4.23-4.20 (m, 111), 3.77-3.73
(m, 0.5H), 3.42 (s, 2H), 3.22-
3.15 (m, 1H), 2.93-2.90 (m,
1H), 2.78-2.71 (m, 2H), 2.56-
E35
2.53 (m, 2H), 2.20 (s, 3H),
2.12-2.03 (m, 2H), 1.90-1.81
(m, 3H), 1.72-1.51 (m, 8H),
1.34 (s, 6H), 1.23-1.10 (m, 3H),
MS (EST): C26H37C1N402
requires 472; found 473
[M+H].
o D1PEAJDMF 1HNMR (400 MHz, Me0D-
H
,õ c/4.): 7.42-7.14 (m, 2H), 4.67
(brs, 1H), 4.31 (brs, 1H), 3.86
E36 CI 0 (brs, 1H), 3.49 (brs, 3H), 3.22
(brs, 3H), 3.10-2.63 (m, 511),
2.50-1.55 (m, 16H), 1.49-1.12
(m, 311). MS (ESI):
126
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
C25H36C1N304S requires 509,
found 510 [m+n].
H DIPEAMMF IHNMR (400 MHz, Me0D-
0=s
OnrN= raµcro d4): 7.32 (s, 111), 7.22 (brs,
1H),
4.66 (brs, 0.5H), 4.37-4.19 (m,
1H), 3.83 (d, J= 12.7 Hz,
0.511), 3.46 (d, J= 7.0 Hz, 211),
3.36-3.32 (m, 1H), 3.29-2.87
E37
(m, 6H), 2.87-2.55 (m, 2H),
2.25 (s, 3H), 2.20-1.46 (m,
14H), 1.41-1.14 (m, 311).
MS (ESI): C25H36C1N304S
requires 509; found 510
[M+II]+.
DIPEA/DMF IHNMR (500 MHz,
advb
gpi d6): 9.46 (s, 1H), 7.42 (d, J=
a'
CI 0 2.0 Hz, 111), 7.17 (s, 111), 4.54
(brs, 0.5H), 4.23-4.20 (m, 1H),
3.77-3.74 (m, 0.5H), 3.46-3.39
(m, 211), 3.21-3.15 (m, 2.5H),
3.14-3.01 (m, 211), 2.93-2.90
E38 (m, 1H), 2.77-2.70 (m, 1.5H),
2.61-2.59 (m, 111), 2.37 (d, J=
7.5 Hz, 2H), 2.18 (s, 3H), 2.16-
2.04 (m, 511), 2.00-1.80 (m,
1H), 1.75-1.48 (m, 9H), 1.23-
1.10 (m, 311). MS (ESI):
C261138C1N304S requires 523;
found 524 [M+H].
DIPEA/DMF NMR (400 MHz, Me0D-
Fr..õ..-IN io m,
.T"
0 c/4): 7.49 (d, J= 2.8 Hz, 2H),
CI
6.41 (t, J= 75.2 Hz, 1H),4.61
E39 (brs, 0.511), 4.29-4.20 (m,
4.5H), 4.23 (t, J= 5.6 Hz, 211),
3.57-3.50 (m, 0.511), 3.39-3.36
(m, 1.5H), 3.14-3.01 (m, 3H),
127
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
2.78 (t, J= 5.6 Hz, 2H), 2.29 (s,
3H), 1.84-1.64 (m, 811), 1.41-
1.27 (m, 3H). 19F (376 MHz,
Me0D-d4): -85.6, -85.4. MS
(ESI): C23H32C1F2N303requires
471; found 472 [M+H].
D1PEA/DMF 1H NMR (400 MHz, CDCI3):
rjl'Iµl-rN = Ni"
0,õJ 0 Ny-LD 8.48 (brs, 1H), 7.95 (s, 1H), 7.06
(s, 1H), 4.77 (brs, 1H), 4.40 (d,
= 12.2 Hz, 0.5H), 4.27 (s, 2H),
4.23-4.07 (m, 2.5H), 3.95 (t, J =
4.8 Hz, 2H), 3.72-3.54 (m, 2H),
E40 3.51-3.23 (m, 3H), 2.98-2.68
(m, 2.5H), 2.64 (d, J = 11.5 Hz,
1H), 2.28-2.10 (m, 4H), 1.98 (t, J
= 11.1 Hz, 1H), 1.92-1.46 (m,
8H), 1.38-1.15 (m, 3H). MS
(ESI): C25H35C1N404 requires
490; found 491 [M+Hr.
HO H DIPEA/DMF 1H NMR (400 MHz, Me0D-
,>.?
N
d4): 7.65 (d, J= 2.0 Hz, 1H),
CI 7.18 (d, J= 1.5 Hz, 1H), 4.66
(brs, 0.5H), 4.59 (brs, 0.5H),
4.36-4.22 (m, 111), 3.83 (d,
J=12.7 Hz, 0.5H), 3.56-3.40 (m,
2H), 3.40-3.34 (m, 0.5H), 3.08-
E41
2.89 (m, 1.5H), 2.82 (d, J=10.5
Hz, 1H), 2.76-2.67 (m, 1H),
2.28 (s, 3H), 2.23-2.12 (m, 1H),
2.09-1.54 (m, 9H), 1.47 (s, 6H),
1.39-1.17 (m, 3H). MS (ESI):
C231134C1N303 requires 435,
found 436 [M+H].
128
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
DEPEA/DMF 1H NMR (400 MHz, Me0D-
H(;>'rN NI ==õ. NI TO c/4): 7.59 (d, J= 1.7 Hz,
1H),
CI 7.17 (d, J= 1.0 Hz, 1H), 4.66
(brs, 0.5H), 4.37-4.22 (m, 1H),
3.83 (d, J= 13.9 Hz, 0.5H),
3.54-3.39 (m, 2H), 3.39-3.33
(m, 0.5H), 3.08-2.89 (m, 1.5H),
E42
2.81 (d, J= 11.2 Hz, 1H),2.76-
2.66 (m, 1H), 2.56 (s, 2H), 2.28
(s, 3H), 2.23-2.12 (m, 1H),
2.08-1.56 (m, 9H), 1.42-1.16
(m, 9H). MS (ESI):
C24H36C1N303 requires 449,
found 450 [M+111+.
Example 43
(S)-N-(5-ehloro-34(4-(cyclopentaneearbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-3-(methylsulfonyl)propanamide (E43)
0 ,0 H
io0
CI
3-(Methylsulfonyl)propanoic acid (65.2 mg) and D1PEA (0.15 mL) were dissolved
in DMF (3 mL).
To this solution, HATU (217 mg) was added gradually. The reaction mixture was
stirred at RT for
1 hour, then (S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-1-
y1)(cyclopentyl)methanone (D157, 100 mg) in DMF (2 mL) was added into the
mixture, which was
stirred at RT for 2 days. Water (10 mL) was added, extracted with Et0Ac (2x 10
mL). The organic
layer was dried over Na2SO4and filtered.The filtrate was concentrated under
vacuum. The residue
was purified by MADP to afford the title compound (55 mg) as white solid. 1H
NMR (400 MHz,
Me0D-d4): 7.27 (d, J= 2.0 Hz, 1H), 7.11 (d, J= 1.8 Hz, 1H), 4.56 (brs, 0.5H),
4.26-4.11 (m, 1H),
3.73 (d, J= 12.8 Hz, 0.5H), 3.42 (t, J= 7.3 Hz, 2H), 3.36 (d, J= 7.7 Hz, 2H),
3.33-3.24 (m, 0.5H),
2.93 (s, 3H), 2.91-2.79 (m, 3.5H), 2.72 (d, J= 11.2 Hz, 1H), 2.65-2.57 (m,
1H), 2.17 (s, 3H), 2.13-
1.44 (m, 10H), 1.28-1.07 (m, 311). MS (ESI): C23H34C1N304S requires 483; found
484 [M+H].
Example 44
(S)-N-(5-ehloro-34(4-(cyclopentaneearbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-4-(methylsulfonyDbutanamide (E44)
129
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
,S-rN &hi INI'-')=sy0s"
0 0 w
CI 0
To a solution of 4-(methylsulfonyl)butanoic acid (D43, 73.1 mg) and (S)-(4-(3-
amino-5-chloro-2-
methylbenzy1)-2-methylpiperazin-1 -y1)(cyclopentyl)methanone (D157, 140 mg) in
DCM (100 mL)
was added HATU (228 mg) at 0 C, then stirred at RT for 10 mm. 13113EA (0.105
mL) was added,
and the reaction mixture was stirred at RT overnight. The mixture was washed
with saturated
NaHCO3 solution and brine, dried over Na2SO4 and filtered. The filtrate was
concentrated to
dryness under vacuum to get crude product, which was purified by MDAP to
afford the title
compound (150 mg) as white solid. 'FINMR (400 MHz, CDC13): 7.76 (s, 1H), 7.33
(brs, 1H), 7.10
(s, 1H), 4.75 (brs, 0.511), 4.39 (d, J=13.3 Hz, 0.5H), 4.12 (brs, 0.5H), 3.66
(d, J= 13.1 Hz, 0.5H),
3.48-3.25 (m, 2.511), 3.18 (t, J= 7.0 Hz, 2H), 2.95 (s, 3H), 2.91-2.79 (m,
1.5H), 2.78-2.66 (m, 3H),
2.62 (d, J= 11.0 Hz, 1H), 2.32 (quin, J= 6.9 Hz, 2H), 2.22 (s, 3H), 2.20-2.13
(m, 1H), 2.04-1.66
(m, 7H), 1.60-1.50 (m, 2H), 1.35-1.15 (m, 3H). MS (ESI): C241136C1N304S
requires 497; found 498
[M+Hr=
Example 45
(S)-N-(5-cyano-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-Amethyl)-2-
methylpheny1)-4-(methylsulfonyl)butanamide (E45)
Fri
?=-=,;----T, 10..;(0
N I
Example 45 was prepared using a similar procedure to that described for E44,
with DIPEA/DMF as
the base/solvent. NMR
(400 MHz, DMSO-d6): 9.61 (s, 1H), 7.79 (d, J= 1.0 Hz, 111), 7.52 (s,
111), 4.54 (brs, 0.5H), 4.24-4.14 (m, 1H), 3.74 (d, J= 13.1 Hz, 0.5H), 3.52-
3.40 (m, 211), 3.22-3.15
(m, 2.5H), 2.99 (s, 3H), 2.96-2.86 (m, 1H), 2.82-2.66 (m, 1.5H), 2.64-2.52 (m,
3H), 2.29 (s, 311),
2.16-1.44 (m, 12H), 1.27-1.03 (m, 311). MS (ESI): C25H36N4.04S requires 488;
found 489 [M+H].
Example 46
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-3-(ethylsulfonyl)propanamide (E46)
,0 H
Nacx)0
CI 0
3-(Ethylsulfonyl)propanoic acid (71.2 mg) and DIPEA (0.150 mL) were dissolved
in DMF (3 mL).
To this solution, HATU (217 mg) was added gradually. The reaction mixture was
stirred at RT for
min, then (S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-1-
30 yl)(cyclopentyl)methanone (D157, 100 mg) was added into the mixture,
which was stirred at RT
130
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
overnight. Water (10 mL) was added, extracted with Et0Ac (2x10 mL). The
organic layer was
dried over Na2SO4 and filtered. The filtrate was concentrated under vacuum.
The residue was
purified by MADP to afford the title compound (20 mg) as white solid. 1H NMR
(400 MHz,
Me0D-d4): 7.39 (d, J= 2.1 Hz, 111), 7.23 (d, J = 1.7 Hz, 1H), 4.68 (brs,
0.5H), 4.38-4.24 (m, 111),
3.85 (d, J= 13.3 Hz, 0.5H), 3.57-3.36 (m, 4.511), 3.17 (q, J= 7.5 Hz, 2H),
3.09-2.89 (m, 3.5H),
2.84 (d, J= 11.2 Hz, 1H), 2.77-2.68 (m, 1H), 2.28 (s, 3H), 2.25-1.54 (m, 10H),
1.40 (t, J= 7.5 Hz,
3H), 1.37-1.21 (m, 3H). MS (ESI): C24H36C1N304S requires 497; found 498 [M+H].
Example 47
(S)-N-(5-cyano-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yflmethyl)-2-
methylpheny1)-3-(ethylsulfonyl)propanamide, Trifluoroacetic acid salt (E47)
'"'"'SnrN so Norco = TFA
Example 47 was prepared using a similar procedure to that described for E46,
II-1 NMR (400 MHz,
Me0D-d4): 7.90 (d, J= 1.1 Hz, 1H), 7.79 (s, 1H), 4.62 (brs, 1H), 4.35 (brs,
211), 4.24-4.13 (m,
0.5H), 3.52 (t, J= 7.0 Hz, 2H), 3.42-3.37 (m, 2H), 3.24-2.80 (m, 8.5H), 2.44
(s, 3H), 1.96-1.56 (m,
811), 1.48-1.22 (m, 6H). MS (EST): C25H36N404S requires 488; found 489 [M+H]t
Example 48
(S)-N-(5-chloro-3-04-(cydopentanecarbony1)-3-methylpiperazin-l-Amethyl)-2-
methylpheny1)-2-(1,1-dioxidothietan-3-yflacetamide (E48)
0 =0'SrN NI":slp
0
0
2-(1,1-Dioxidothietan-3-yl)acetic acid (D50, 40 mg) and HATU (93 mg) were
added into the
solution of (S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-l-
y1)(cyclopentypmethanone (D157, 85 mg) in DMF (10 mL). The reaction mixture
was stirred for 6
hours at RT. Water (20 mL) was added, extracted with DCM (50 mL). The organic
layer was dried
over Na2SO4 and filtered. The filtrate was concentrated, and the crude product
was purified by
MDAP to afford the title compound (8 mg) as white solid. 111 NMR (500 MHz,
DMSO-d6): 9.57 (s,
111), 7.43 (d, J= 2.0 Hz, 1H), 7.16 (s, 1H), 4.54 (brs, 0.5H), 4.35-4.27 (m,
211), 4.19 (d, J= 11.3
Hz, 1H), 3.97-3.91 (m, 2H), 3.77-3.72 (m, 0.5H), 3.45-3.36 (m, 211), 3.21-3.13
(m, 0.5H), 2.95-
2.68 (m, 5.5H), 2.65-2.57 (m, 1H), 2.17 (s, 311), 2.11-1.45 (m, 10H), 1.26-
1.05 (m, 3H). MS (ESI):
C24H34C1N3045 requires 495; found 496 [M+H].
Example 49
(S)-N-(5-chloro-34(4-(2-cyclopentylacety1)-3-methylpiperazin-1-Amethyl)-2-
methylpheny1)-
3-(methylsulfonyflpropanamide, Trifluoroacetic acid salt (E49)
131
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
0õ'0 H
µS N
N"s = TEA
0 1N,r0
CI
3-(Methylsulfonyl)propanoic acid (62.7 mg), DIPEA (0.144 mL) and HATU (209 mg)
were
dissolved in DMF (2 mL). To this solution, (S)-1-(4-(3-amino-5-chloro-2-
methylbenzy1)-2-
methylpiperazin-l-y1)-2-cyclopentylethanone (D170, 100 mg) was added. The
reaction mixture
was stirred at RT overnight. Et0Ac (20 mL) and water (10 mL) were added into
the reaction
mixture. After 10 min stirring, the Et0Ac layer was seperated and condensed.
The residue was
purified by MDAP (acidic elution) to get the title compound (45 mg) as white
solid.1H NMR (400
MHz, Me0D-d4): 7.57 (d, J= 2.1 Hz, 1H), 7.52 (d, J= 1.8 Hz, 1H), 4.62 (brs, 1
H), 4.52-4.35 (m,
2H), 4.10 (d, J= 7.1 Hz, 1H), 3.67-3.35 (m, 511), 3.23-3.08 (m, 1H), 3.04 (s,
3H), 2.99 (t, J=7.1
Hz, 2H), 2.57-2.35 (m, 2H), 2.31 (s, 3H), 2.25-2.08 (m, 1H), 1.84 (brs, 2H),
1.74-1.07 (m, 10H).
19F NMR (376 MHz, Me0D-d4): -78.9. MS (ESI): C24H36C1N304 S requires 497,
found 498
[M+Hi+-
Example 50
(S)-N-(5-ehloro-34(4-(cyclohexaneearbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-3-(methylsulfonyl)propanamide (E50)
oõo H
0 NO
CI
0
To a solution of 3-(methylsulfonyl)propanoic acid (62.7 mg), HATU (209 mg) and
D1PEA (0.144
mL) in DMF (10 mL), (S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-
1-
y1)(cyclohexypmethanone (D171, 100 mg) was added into the mixture, which was
stirred at RT for
2 days. After extraction with Et0Ac/1120, dry, and condense, the residue was
purified by MDAP
(basic elution) to get the required product (S)-N-(5-chloro-34(4-
(cyclohexanecarbony1)-3-
methylpiperazin-l-yOmethyl)-2-methylpheny1)-3-(methylsulfonyl)propanamide (7
mg) as white
solid. ill NMR (400 MHz, Me0D-d4): 7.39 (s, 1H), 7.23 (brs, 1H), 4.67 (brs,
0.5H), 4.38-4.16 (m,
1H), 3.80 (d, J= 12.7 Hz, 1H), 3.63-3.35 (m, 4.5H), 3.05 (s, 3H), 3.03-2.89
(m, 2.5H), 2.87-2.51
(m, 311), 2.28 (s, 3H), 2.24-1.94 (m, 211), 1.87-1.12 (m, 1311). MS (ESD:
C241136C1N304S requires
497, found 498 [M+H].
Example 51
(S)-N-(5-chloro-3-04-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-4,4,4-trifluorobutanamide (E51)
132
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
0 10 Nair-0 µ
0 0
To a solution of (S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-l-
y1)(cyclopentyl)methanone(D157, 138.2 mg) and 4,4,4-trifluorobutanoic acid
(74.5 mg) in
anhydrous DMF (5 mL), HATU (302.4 mg) and D1PEA (0.207 mL) were added. The
resulting
reaction mixture was stirred overnight at RT. Diluted with DCM (10 mL), washed
with water twice
(2x 20 mL), the organic layer was separated and concentrated to dryness under
vacuum. The
residue was purified with MDAP to afford the title compound (90.5 mg) as white
solid. II-I NMR
(400 MHz, Me0D-d4): 7.33 (d, J= 2.2 Hz, 1H), 7.22 (d, .1= 2.0 Hz, 1H), 4.72-
4.53 (m, 1.5H),
4.36-4.22 (m, 1H), 3.83 (d, J= 13.4 Hz, 0.5H), 3.52-3.40 (m, 2H), 3.40-3.33
(m, 0.5H), 3.07-2.90
(m, 1.5H), 2.82 (d, J= 11.0 Hz, 111), 2.75-2.66 (m, 3H), 2.66-2.52 (m, 211),
2.25 (s, 3H), 2.23-2.12
(m, 1H), 2.10-1.53 (m, 9H), 1.39-1.16 (m, 3H). I9F NMR (376 MHz, Me0D-d4): -
68.2. MS (EST):
C23H31C1F3N302 requires: 473, found 474 [M+H].
Example 52
(S)-N-(5-chloro-34(4-(eyelopentaneearbony1)-3-methylpiperazin-1-yDmethyl)-2-
methylpheny1)-5,5,5-trifluoropentanamide (E52)
= N
FFrYi .(õ r!,s;r0
CI
E52 was prepared using a similar procedure to that described for E51. IHNMR
(400 MHz, Me0D-
d4): 7.33 (d, J= 2.0 Hz, 111), 7.21 (d, J= 1.5 Hz, 1H), 4.66 (brs, 0.5H), 4.37-
4.21 (m, 1H), 3.83 (d,
J= 13.2 Hz, 0.5H), 3.53-3.40 (m, 211), 3.40-3.33 (m, 0.5H), 2.90-3.07 (m,
1.5H), 2.82 (d, J= 11.0
Hz, 1H), 2.75-2.66 (m, 1H), 2.54 (t, J= 7.3 Hz, 211), 2.36-2.11 (m, 6H), 2.09-
1.53 (m, 1111), 1.39-
1.15 (m, 3H). '9F NMR (376 MHz, Me0D-d4): -67.9. MS (EST): C24H33C1F3N302
requires 487;
found 488 [M+H].
Example 53
(S)-N-(5-chloro-344-(cyclopentaneearbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-2-(3,3-difluorocyclobutypacetamide (E53)
FN l'asµ
Cl
To a solution of (S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-1 -
y1)(cyclopentypmethanone (D157, 117 mg) and 2-(3,3-difluorocyclobutyl)acetic
acid (D16, 50 mg)
in DMF (10 mL), HATU (127 mg) was added at RT. The reaction mixture was
stirred for 6 hours.
Water (20 mL) was added, extracted with DCM (50 mL). The organic layer was
dried over Na2SO4
133
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
and filtered. The filtrate was concentrated and the residue was purified with
MDAP to afford the
title compound (20 mg) as white solid. 111 NMR (500 MHz, DMSO-d6): 9.45 (s,
1H), 7.40 (s, 1H),
7.16 (s, 1H), 4.54 (brs, 0.5H), 4.20 (d, J= 8.8 Hz, 1H), 3.75 (d, J= 12.6 Hz,
0.5H), 3.45-3.36 (m,
2H), 3.21-3.13 (m, 0.5H), 2.95-2.86 (m, 1H), 2.80-2.68 (m, 3.5H), 2.65-2.55
(m, 3H), 2.44-2.31 (m,
2H), 2.17 (s, 3H), 2.13-1.45 (m, 11H), 1.27-1.04 (m, 3H). MS (ESI)
C25H34C1F2N302requires 481;
found 482 [M+H].
Example 54
(S)-N-(34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-Amethyl)-5-fluoro-2-
methylpheny1)-2-(3,3-difluorocyclobutyl)acetamide (E54)
110 10
E54 was prepared using a similar procedure to that described for E53. 1H NMR
(500 MHz, DMSO-
d6): 9.42 (s, 1H), 7.20 (dd, J= 10.1, 2.2 Hz, 1H), 6.96 (d, J= 8.5 Hz, 1H),
4.54 (brs, 0.5H), 4.19 (d,
J= 11.7 Hz, 1H), 3.74 (d, J= 13.6 Hz, 0.5H), 3.45-3.36 (m, 2H), 3.19 (t, J=
11.7 Hz, 0.5H), 2.95-
2.87 (m, 1H), 2.82-2.67 (m, 3.5H), 2.66-2.56 (m, 3H), 2.44-2.30 (m, 2H), 2.15
(s, 3H), 2.10-1.45
(m, 11H), 1.30-1.05 (m, 3H). MS (EST): C25H34P3N302requires 465; found 466
[M+H].
Example 55
(S)-N-(5-chloro-3-04-(cyclopentanecarbony1)-3-methylpiperazin-1-Amethyl)-2-
methylpheny1)-4-cyanobutanamide, Trifluoroacetic acid salt (E55)
Nt:c Norco,
= TFA
.. To a solution of (S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-
1-
yl)(cyclopentyl)methanone (D157, 99.1 mg) and 4-cyanobutanoic acid (37.1 mg)
in anhydrous
DMF (6 mL), HATU (162 mg) and DIPEA (0.15 inL) was added, the resulting
reaction mixture
was stirred overnight at rt. The mixture was sent to MDAP for purification to
afford the title
compound (73.5 mg) as white solid. 1H NMR (400 MHz, Me0D-d4): 7.52 (s, 1H),
7.48 (s, 1H),
4.61 (brs, 1H), 4.30 (brs, 2H), 4.15 (d, J= 12.5 Hz, 0.5H), 3.60-3.45 (m,
0.5H), 3.45-3.34 (m, 2H),
3.26-2.83 (m, 3.5H), 2.69-2.53 (m, 4H), 2.29 (s, 3H), 2.04 (quin, J= 7.2 Hz,
2H), 1.98-1.56 (m,
8H), 1.50-1.20 (m, 3H). MS (ESI): C24H33C1N402 requires 444; found 445 [M+H].
Examples 56&57
N-(5-chloro-3-(4S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-Amethyl)-2-
.. methylpheny1)-2((S)-tetrahydrofuran-3-ypacetamide & N-(5-chloro-3-(((S)-4-
134
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-methylpheny1)-2-((R)-
tetrahydrofuran-3-Aacetamide
(E56 & E57)
cllirO'sµ
., ci
To a solution of 2-(tetrahydrofuran-3-yl)acetic acid (130 mg) and (S)-(4-(3-
amino-5-chloro-2-
methylbenzy1)-2-methylpiperazin-l-y1)(cyclopentyl)methanone (D157, 350 mg) in
DMF (20 mL),
HATU (456 mg) and D1PEA (0.349 mL) were added. The reaction mixture was
stirred at 50 C for
1 hour. Water (20 mL) was added, extracted with Et0Ac (50 mL). The organic
layer was dried over
Na2SO4 and filtered. The filtrate was concentrated, and the crude product was
purified by MDAP to
afford N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylpheny1)-2-(tetrahydrofuran-3-y1)acetamide (240 mg), which was separated
by preparative
chiral SFC to afford the title compounds (70 mg and 78 mg) as white solid.
Isomer 1: 11-1NMR
(400 MHz, DMSO-d6): 9.45 (s, 1H), 7.39 (d, J= 2.3 Hz, 111), 7.16 (s, 1H), 4.54
(brs, 0.5H), 4.19 (d,
J= 11.0 Hz, 1H), 3.81 (dd, J= 8.0, 7.0 Hz, 1H), 3.78-3.71 (m, 1.5H), 3.65 (q,
J= 7.4 Hz, 111),
3.45-3.37 (m, 2H), 3.37-3.34 (m, 1H), 3.17 (t, J= 13.1 Hz, 0.5H), 2.96-2.86
(m, 1H), 2.82-2.68 (m,
1.511), 2.65-2.53 (m, 2H), 2.47-2.40 (m, 2H), 2.17 (s, 3H), 2.13-1.44 (m,
1211), 1.27-1.05 (m, 311).
MS (ESI): C25H36C1N303requires 461; found 462 [M+11]+. Isomer 2: 1H NMR (400
MHz, DMSO-
d6): 9.46 (s, 111), 7.39 (d, J= 2.0 Hz, 111), 7.16 (s, 1H), 4.54 (brs, 0.5H),
4.19 (d, J=11 8 Hz, 1H),
3.81 (dd, J= 8.3, 7.0 Hz, 1H), 3.78-3.71 (m, 1.511), 3.65 (q, J= 7.7 Hz, 1H),
3.45-3.38 (m, 2H),
3.17 (t, J= 11.9 Hz, 0.5H), 2.96-2.86 (m, 1H), 2.81-2.68 (m, 1.5H), 2.65-2.53
(m, 211), 2.47-2.40
(m, 211), 2.17 (s, 3H), 2.13-1.45 (m, 1211), 1.27-1.05 (m, 3H). MS (ESI):
C25H36C1N303requires
461; found 462 [M+Hr.
Example 58
N-(2,5-diehloro-3-(((S)-4-(eyclopentanecarbony1)-3-methylpiperazin-1-
y1)methyDpheny1)-2-
(tetrahydrofuran-3-yDacetamide (E58)
CI
N
141ffl 1\(,,,N1r0
CI 0
To a suspension of 2-(tetrahydrofuran-3-yl)acetic acid (39.4 mg) in DCM (10
), oxalyl chloride
(0.030 mL) was added dropwise. The reaction mixture was stirred at 40 C for 30
min. Solvent was
removed by rotavap, then re-dissolved with DCM (1 mL), added to a solution of
(S)-(4-(3-amino-
2,5-dichlorobenzy1)-2-methylpiperazin-1-y1)(cyclopentypmethanone (D158, 70 mg)
in pyridine (2
mL). The reaction mixture was stirred at RT overnight. The reaction mixture
was diluted with
DCM (10 mL) then washed with brine (10 mL). DCM layer was separated and
concentrated. The
135
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
residue was purified by MADP to afford the title compound (7 mg) as white
solid. 111 NMR (400
MHz, Me0D-d4): 7.71 (d, J= 1.5 Hz, 1H), 7.35 (d, J= 2.0 Hz, 1H), 4.61 (brs,
0.5H), 4.33-4.17 (m,
1H), 3.90-3.51 (m, 5.511), 3.45-3.27 (m, 1.5H), 3.01-2.67 (m, 3.511), 2.67-
2.41 (m, 3.511), 2.27 (brs,
1H), 2.14-1.99 (m, 1.5H), 1.84-1.43 (m, 1011), 1.35-1.13 (m, 3H). MS (ESI):
C241133C12N303
requires 481; found 482 [M+11]+.
Examples 59&60
N-(2,5-dichloro-3-R(S)-4-(cyclopentaneearbony1)-3-methylpiperazin-1-
yOmethyDphenyl)-2-
((S)-tetrahydrofuran-3-ypacetamide & N-(2,5-dichloro-34(S)-4-
(cyclopentanecarbony1)-3-
methylpiperazin-1-y1)methyl)phenyl)-2-((R)-tetrahydrofuran-3-y1)acetamide (E59
& E60)
ci
N
11P) N'y-C) (0-3 ON yi:1)
ci 0 CI
N-(2,5-dichloro-34(S)-4-(cyclopentaneearbony1)-3-methylpiperazin-l-
yOmethyl)pheny1)-2-
(tetrahydrofuran-3-ypacetamide (E58) (20 mg) was separated by chiral SFC to
afford the title two
compounds (6 mg and 7 mg). Isomer 1: 111 NMR (400 MHz, CDC13): 8.39 (d, J= 1.0
Hz, 111), 7.69
(s, 1H), 4.78 (brs, 0.5H), 4.44 (d, J= 13.6 Hz, 0.5H), 4.14 (brs, 0.511), 3.98
(dd, J= 8.7, 6.9 Hz,
1H), 3.95-3.87 (m, 1H), 3.79 (q, J= 7.5 Hz, 111), 3.69 (d, J= 13.3 Hz, 0.5H),
3.58-3.46 (m, 3H),
3.39 (t, J= 12.2 Hz, 0.5H), 3.07-2.92 (m, 0.5H), 2.90-2.73 (m, 311), 2.67 (d,
J= 11.0 Hz, 111), 2.55
(d, J= 8.0 Hz, 2H), 2.30-2.15 (m, 2H), 2.14-2.05 (m, 1H), 1.97-1.61 (m, 9H),
1.41-1.22 (m,
3H).MS (ESI): C24H33C12N303 requires 481; found 482 [M+Hr. Isomer 2: 111 NMR
(400 MHz,
CDC13): 8.39 (d, J= 1.0 Hz, 111), 7.69 (s, 1H), 4.78 (brs, 0.511), 4.44 (d, J=
13.6 Hz, 0.51I), 4.14
(brs, 0.5H), 3.98 (dd, J= 8.7, 6.9 Hz, 1H), 3.95-3.86 (m, 1H), 3.79 (q, J= 7.5
Hz, 111), 3.69 (d, J=
13.3 Hz, 0.5H), 3.58-3.45 (m, 311), 3.39 (t, J= 13.1 Hz, 0.511), 3.05-2.92 (m,
0.5H), 2.90-2.73 (m,
3H), 2.67 (d, J¨ 11.0 Hz, 1H), 2.55 (d, J= 7.3 Hz, 2H), 2.28-2.15 (m, 211),
2.10 (t, J= 11.9 Hz,
111), 1.97-1.63 (m, 9H), 1.41-1.26 (m, 3H). MS (ESI): C24H33C12N303 requires
481; found 482
[M+Hr=
Examples 61&62
N-(5-chloro-3-(((S)-4-(eyelopentanecarbony1)-3-methylpiperazin-l-yl)methyl)-2-
methylpheny1)-2-((ls,4R)-4-hydroxycyclohexyl)acetamide & N-(5-ehloro-3-(((S)-4-
(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-methylpheny1)-2-
((lr,4S)-4-
hydroxycyclohexyl)acetamide (E61 & E62)
MHO, Nasl's(C)
Kr.
CI 0 CI 0
136
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
2-(4-Hydroxycyclohexyl)acetic acid (D3, 150 mg) and HATU (361 mg) were added
to the (5)-(4-
(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-l-
y1)(cyclopentyl)methanone (D157, 332
mg) solution in DMF (20 mL), the reaction mixture was continued to stir for 6
hours at RT. Water
(20 mL) was added and extracted with Et0Ac (50 mL), the organic layer was
dried over Na2SO4.
.. Filtered, the filtrated was concentrated and the residue was purified by
preparative HPLC to afford
the two compounds (48 mg and 18 mg) as white solid. Isomer 1: 111 NMR (500
MHz, DMSO-d6):
9.35 (s, 1H), 7.38 (s, 111), 7.15 (s, 1H), 4.54 (brs, 0.5H), 4.47 (d, J= 4.4
Hz, 1H), 4.19 (d, J= 10.1
Hz, 111), 3.75 (d, J= 12.9 Hz, 0.5H), 3.45-3.36 (m, 2H), 3.36-3.33 (m, 1H),
3.17 (t, J= 12.1 Hz,
0.5H), 2.97-2.86 (m, 1H), 2.81-2.68 (m, 1.5H), 2.60 (d, J= 9.5 Hz, 1H), 2.21
(d, J= 6.9 Hz, 2H),
2.16 (s, 3H), 2.14-1.45 (m, 15H), 1.29-0.95 (m, 7H). MS (ESI): C271140C1N303
requires 489; found
490 [M+H]t . Isomer 2: 1H NMR (500 MHz, DMSO-d6): 9.37 (s, 1H), 7.38 (d, J=
1.6 Hz, 1H),
7.15 (s, 1H), 4.54 (brs, 0.5H), 4.30 (d, J= 3.2 Hz, 1H), 4.20 (d, J= 8.5 Hz,
111), 3.79-3.70 (m,
1.5H), 3.46-3.36 (m, 2H), 3.18 (t, J= 11.7 Hz, 0.511), 2.91 (quin, J= 7.4 Hz,
111), 2.81-2.68 (m,
1.5H), 2.65-2.57 (m, 1H), 2.26 (d, J= 7.3 Hz, 2H), 2.17 (s, 311), 2.13-1.38
(m, 19H), 1.27-1.05 (m,
3H). MS (ESI): C271140C1N303 requires 489; found 490 [M+H].
Examples 63-70
Examples 63-70 were prepared using a similar procedure to that described for
E61 and E62, with
the specified reaction base or solvent listed in the table.
E63: N-(3-(4S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-5-
fluoro-2-
methylpheny1)-2-((lr,45)-4-hydroxycyclohexypacetamide
E64: N-(3 -(45)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-ypmethyl)-5-
fluoro-2-
methylpheny1)-2-((1s,4R)-4-hydroxycyclohexyl)acetamide
E65: N-(5-chloro-3-0(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
y1)methyl)-2-
methylpheny1)-2-((S)-1-methyl-5-oxopyrrolidin-3-y1)acetamide
E66: N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-
2-
methylpheny1)-2-((R)-1-methyl-5-oxopyrrolidin-3-ypacetamide
E67: N-(5-chloro-3-4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylpheny1)-2-45)-1-ethyl-5-oxopyrrolidin-3-ypacetamide
E68: N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-
2-
.. methylpheny1)-24(R)-1-ethyl-5-oxopyrrolidin-3-ypacetamide
E69: N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-
2-
methylpheny1)-2-((R)-1-methyl-2-oxopyrrolidin-3-ypacetamide
E70: N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylpheny1)-2-((S)-1-methyl-2-oxopyrrolidin-3-ypacetamide
Base/Solv
Structure Characterization
ent
137
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Isomer 1: 1H NMR (500 MHz,
DMSO-d6): 9.32 (s, 111), 7.19
(dd, J= 10.2, 2.4 Hz, 1H), 6.95
(d, J= 9.5 Hz, 1H), 4.54 (brs,
0.5H), 4.47 (d, J= 4.4 Hz, 111),
4.19 (d, J= 11.7 Hz, 111), 3.75
(d, J= 12.3 Hz, 0.5H), 3.45-
3.36 (m, al), 3.36-3.33 (m,
1H), 3.19 (t, J= 11.7 Hz, 0.5H),
2.96-2.87 (m, 1H), 2.82-2.69
(m, 1.511), 2.66-2.59 (m, 111),
2.21 (d, J= 6.9 Hz, 211), 2.14
(s, 311), 2.08 - 1.46 (m, 15H),
1.31 -0.97 (m, 711). MS (EST):
HoeCricC' 1):1 NON.;r0 C271140FN303 requires 473;
No base
E63&E64 found 474 [M+H].
/DMF
MIsomer 2: 1H NMR (500 MHz, r tac o
H. DMSO-d6): 9.34 (s, 1H), 7.20
(dd, J= 10.2, 2.7 Hz, 111), 6.95
(d, J= 8.8 Hz, 1H), 4.54 (brs,
0.511), 4.30 (d, 2.5 Hz, 1H),
4.19 (d, J= 10.7 Hz, 1H), 3.79-
3.69 (m, 1.511), 3.46-3.36 (m,
2H), 3.19 (t, J= 12.8 Hz, 0.5H),
2.97-2.86 (m, 1H), 2.83-2.68
(m, 1.5H), 2.67-2.58 (m, 1H),
2.27 (d, J= 7.6 Hz, 2H), 2.15
(s, 3H), 2.08-1.37 (m, 19H),
1.27-1.05 (m, 3H). MS (EST):
C27H40FN303 requires 473;
found 474 [M+H].
Isomer 1: 1H NMR (400 MHz,
1161 rac,C> CDC13): 7.96-7.86 (m, 1H),
ci 0 DWEA 7.59 (s, 111), 7.10 (d, J= 11.2
E65&E66
/DIVIF Hz, 1H), 4.75-4.65 (m, 0.5H),
=Noco
4.36-4.25 (m, 0.511), 4.13-4.08
138
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(m, 0.5H), 3.71-3.61 (m, 1.5H),
3.44-3.26 (m, 2.511), 3.22-3.15
(m, 1H), 2.99-2.50 (m, 10H),
2.26-2.09 (m, 4.5H), 2.04-1.65
(m, 8 H), 1.63-1.50 (m, 2H),
1.37-1.18 (m, 3H). MS (ESI):
C26H37C1N403 requires 488;
found 489 [M+H].
Isomer 2: 11-1NMR (400 MHz,
CDC13): 7.71-7.63 (m, 2H),
7.10 (s, 111), 4.76-4.67 (m,
0.511), 4.37-4.28 (m, 0.5H),
4.16-4.08 (m, 0.5H), 3.71-3.60
(m, 1.511), 3.45-3.25 (m, 2.5H),
3.23-3.15 (m, 1H), 2.98-2.52
(m, 1011), 2.26-2.05 (m, 4.511),
2.04-1.65 (m, 8H), 1.63-1.50
(m, 2H), 1.34-1.18 (m, 311). MS
(ESD: C261137C1N403requires
488; found 489 [M+H].
Isomer 1: `11NMR (400 MHz,
sõ
N0y0
DMSO-d6): 9.52 (s, 1H), 7.41
CI 0 (d, J= 1.6 Hz, 111), 7.17 (brs,
1H), 4.56 (brs, 0.511), 4.30-4.22
,-N2-/ N3;0
(m, 1H), 3.77-3.71 (m, 1H),
CI 0 3.55-3.50 (m, 1H), 3.41 (s, 2H),
3.25-3.05 (m, 5H), 2.92-2.84
DIPEA (m, 1H), 2.75-2.55 (m, 4H),
E67&E68
/DMF 2.46-2.42 (m, 1H), 2.17 (s, 3H),
2.06-1.75 (m, 2.5H), 1.75-1.45
(m, 8H), 1.23-1.10 (m, 6H). MS
(ESD: C271139C1N403requires
502; found 503 [M+H].
Isomer 2: 1HNMR (400 MHz,
DMSO-d6): 9.53 (s, 1H), 7.41
(d, J= 1.6 Hz, 1H), 7.17 (brs,
139
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
1H), 4.56 (brs, 0.5H), 4.29-4.22
(m, 1H), 3.77-3.71 (m, 1H),
3.55-3.50 (m, 1H), 3.41 (s, 2H),
3.25-3.07 (m, 511), 2.93-2.84
(m, 1H), 2.80-2.57 (m, 411),
2.48-2.45 (m, 1H), 2.17 (s, 3H),
2.10-1.75 (m, 2.5H), 1.77-1.50
(m, 8H), 1.23-1.10 (m, 311),
1.02-0.96 (m, 3H). MS (ESI):
C271439CIN403requires 502;
found 503 LM+1-1]+.
Isomer 1: 111NMR (400 MHz,
CDC13): 9.44-9.39 (brs, 1H),
7.81 (s, 1H), 7.07 d, J= 2.0 Hz,
1H), 4.80 (brs, 0.5H), 4.45-4.40
(m, 0.511), 4.15 (brs, 0.5H),
3.68-3.61 (m, 0.5H), 3.45-3.37
(m, 4.511), 2.93-2.88 (m, 4.5H),
2.88-2.82 (m, 2H), 2.80-2.70
(m, 1H), 2.70-2.60 (m, 2H),
2.42-2.38 (m, 111), 2.28 (s, 311),
Na;r0 2.20-2.18 (m, 1H), 2.00-1.55
(m, 10H), 1.33-1.23 (m, 311).
DIPEA
E69&E70 H MS (EST): C261437C1N403
/DMF
Nocro requires 488; found 489
Isomer 2: IIINMR (400 MHz,
CDC13): 9.41-9.40 (brs, 111),
7.82 (s, 1H), 7.07 (s, 1H), 4.82
(brs, 0.511), 4.45-4.40 (m,
0.5H), 4.13 (brs, 0.5H), 3.68-
3.61 (m, 0.5H), 3.45-3.37 (m,
4.511), 3.00-2.88 (m, 4.5H),
2.85-2.82 (m, 2H), 2.80-2.70
(m, 1H), 2.70-2.60 (m, 2H),
2.42-2.37 (m, 111), 2.28 (s, 3H),
140
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
2.19-2.18 (m, 1H), 2.00-1.55
(m, 10H), 1.33 (d, J= 6.4 Hz,
1.5H), 1.23 (d, J= 6.8
Hz,1.511). MS (ESI):
C26H37C1N403requires 488;
found 489 [M+H].
Example 71
(S)-N-(5-chloro-34(4-(cyclopentanecarbonyl)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-cyanoisonicotinamide, Trifluoroacetic acid salt (E71)
N11=;p0 Ir
CI 0
= TFA
To a solution of (S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-1-
yl)(cyclopentyl)methanone (D157, 115.5 mg) and 2-cyanoisonicotinic acid (56.6
mg) in anhydrous
DMF (5 mL), HATU (194.0 mg) and DIPEA (0.173 mL were added at RT. The
resulting reaction
mixture was stirred overnight. Diluted with DCM (15 mL), washed with water (10
mL), the organic
layer was separated and solvent was removed under vacuum. The residue was
purified with MDAP
to afford the title compound (73.9 mg) as white solid. 1H NMR (400 MHz, Me0D-
d4): 8.93 (d, J=
4.9 Hz, 1H), 8.36 (s, 1H), 8.15 (dd, J= 5.1, 1.7 Hz, 111), 7.58 (s, 2H), 4.63
(brs, 111), 4.35 (brs, 2H),
4.18 (d,.. T = 14.9 Hz, 0.511), 3.65-3.48 (m, 0.5H), 3.47-3.33 (m, 211), 3.25-
2.84 (in, 3.511), 2.35 (s,
3H), 1.99-1.55 (m, 8H), 1.52-1.21 (m, 3H). MS (ES1): C26H30C1N502 requires
479; found 480
[MI-H]t
Examples 72-76
Examples 72 to 76 were prepared using a similar procedure to that described
for E71, with the
specified reaction base or solvent listed in the table.
E72 (S)-N-(5-chloro-344-(2-cyclopropylacety1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-
2-cyanoisonicotinamide, Trifluoroacetic acid salt
E73 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylpheny1)-5-cyano-6-methylnicotinamide, Trifluoroacetic acid salt
E74 (S)-N-(5-cyano-3-44-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-
2-
methylphenyl)-6-methylnicotinamide
E75 (S)-N-(5-cyano-344-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-2-methylisonicotinamide
E76 (S)-5-chloro-N-(5-chloro-344-(2-cyclopropylacety1)-3-methylpiperazin-l-
y1)methyl)-2-
methylpheny1)-6-methylnicotinamide
141
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Structure Base/Solvent Characterization
111 NMR (400 MHz, Me0D-
d4): 8.93 (dd, J= 5.1, 0.7 Hz,
1H), 8.36 (s, 1H), 8.15 (dd, J=
5.0, 1.6 Hz, 1H), 7.58 (brs, 2H),
4.62 (brs, 0.5H), 4.55-4.43 (m,
0.5H), 4.35 (brs, 2H), 4.03 (d, J
Ni1 N s = 9.5 Hz 0.5H) 3.66-3.50 (m,
E72 o 40 DipEA/DmF
1H), 3.46-3.37 (m, 2H), 3.26-
= TFA 2.85 (m, 2.5H), 2.56-2.24
(m,
5H), 1.49-1.22 (m, 3H), 1.08-
0.93 (m, 1H), 0.63-0.48 (m,
2H), 0.25-0.14 (m, 2H). MS
(EST): C25H28C1N502requires
465; found 466 [M+Hr.
'H NMR (400 MHz, Me0D-
d4): 9.21 (d, J= 2.2 Hz, 1H),
8.64 (d, J= 2.2 Hz, 1H), 7.55
(s, 2H), 4.61 (brs, 0.5H), 4.30
" (brs, 2H), 4.21-4.08 (m, 1H),
E73 =O (0 D1PEA/DMF
3.53 (t, J= 12.6 Hz, 1H), 3.38
NN;'
(d, J= 14.4 Hz, 2H), 3.21-2.88
ci = TFA (m, 3.5H), 2.84 (s, 3H), 2.35 (s,
3H), 1.99-1.55 (m, 8H), 1.50-
1.20 (m, 3H). MS (ESI):
C271132C1N502requires 493;
found 494 [M+H].
1H NMR (400 MHz, DMSO-
d6): 10.23 (s, 1H), 9.03 (s, 1H),
8.21 (dd, J= 8.2, 1.9 Hz, 1H),
I
7.78 (s, 1H), 7.63 (s, 1H), 7.44
N E74 NO\;;r JD'
TEA/DMF (d, J= 8.3 Hz, 1H), 4.56 (brs,
I I 0.5H), 4.21 (d, J= 9.5 Hz, 1H),
3.76 (d, J= 12.8 Hz, 0.5H),
3.59-3.43 (m, 2H), 3.19 (t, J=
12.4 Hz, 0.5H), 2.92 (quin, J=
142
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
7.8 Hz, 1H), 2.83-2.70 (m,
1.5H), 2.68-2.60 (m, 1H), 2.56
(s, 3H), 2.33 (s, 3H), 2.23-1.45
(m, 10H), 1.30-1.05 (m, 311).
MS (ESI): C27H33N502 requires
459; found 460 [M+Hr.
NMR (400 MHz, Me0D-
d4): 8.52 (d, J= 5.1 Hz, 114),
7.71 (s, 1H), 7.68-7.58 (m, 211),
7.54 (s, 1H), 4.58 (brs, 0.5H),
4.29-4.14 (m, 1H), 3.75 (d, J=
13.2 Hz, 0.5H), 3.54-3.39 (m,
E75 o Nay-0' D1PEA/DMF
2H), 3.33-3.26 (m, 0.5H), 3.00-
I Io 2.83 (m, 1.5H), 2.77-2.58 (m,
NI
2H), 2.56 (s, 311), 2.32 (s, 3H),
2.21-1.43 (m, 10H), 1.30-1.06
(m, 3H). MS (ESI): C27H33N502
requires 459; found 460
[M+Hr=
DWEA/DMF '11NMR (400 MHz, Me0D-
Ar H
CI ig& c/4): 8.95 (d, J= 0.8 Hz, 1H),
0 õCV
8.37 (d, J= 0.6 Hz, 1H), 7.38-
7.32 (d, J= 22 Hz, 2H), 4.73-
4.67 (m, 0.5H), 4.36-4.33 (d,
0.511), 4.16-4.15 (m, 0.5H),
3.75-3.72 (d, 0.511), 3.57-3.48
(m, 2H), 3.46-3.40 (m, 0.5H),
E76 3.01-2.95 (m, 0.5H), 2.88-2.86
(m, 111), 2.76-2.72 (m, 4H),
2.48-2.40 (m, 1H), 2.32 (s, 4H),
2.27-2.20 (m, 3H), 1.37-1.25
(m, 3H), 1.02-0.99 (m, 1H),
0.56-0.54 (m, 2H), 0.21-0.20
(m, 2H). MS (EST):
C251130C12N402 requires 488;
found 489 [M+H].
143
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Example 77
(S)-N-(5-chloro-34(4-(2-cyclopropylacety1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-
6-cyanonicotinamide, Trifluoroacetic acid salt (E77)
I I-1
0" = TFA
0
ci 8 v
To a solution of (S)-1-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-
l-y1)-2-
cyclopropylethanone (D169, 122.8 mg) in DCM (5 mL), 6-cyanonicotinic acid
(61.9 mg), DMAP
(4.9 mg) and EDC (165.5 mg) were added at RT. The resulting reaction mixture
was stirred
overnight. Diluted with DCM (15 mL), washed with water (10 mL). The organic
layer was
separated and solvent was removed under vacuum. The residue was purified with
MDAP to afford
the title compound (100.9 mg) as white solid. 1H NMR (400 MHz, Me0D-d4): 9.23
(d, J=1.5 Hz,
1H), 8.52 (dd, J= 8.1, 2.2 Hz, 1H), 8.06 (d, J= 8.1 Hz, 1H), 7.62 (d, J= 2.0
Hz, 1H), 7.59 (d, J=
2.2 Hz, 1H), 4.66 (brs, 0.5H), 4.57-4.41 (m, 2.5H), 4.07 (brs, 0.5H), 3.76-
3.41 (m, 3H), 3.27-2.98
(m, 2H), 2.51-2.26 (m, 5H), 1.35 (brs, 3H), 1.07-0.93 (m, 1H), 0.63-0.48 (m,
2H), 0.25-0.11 (m,
2H). MS (ESI): C25H28C1N502requires 465; found 466 [M+H]t
Example 78
(S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-Amethyl)-2-
methylpheny1)-6-cyanonicotinamide, Trifluoroacetic acid salt (E78)
N
I H = TFA
0
CI 0
To a solution of (S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-l-
y1)(cyclopentyl)methanone (D157, 115.5 mg) and 6-cyanonicotinic acid (57.2 mg)
in anhydrous
DMF (5 mL), HATU (255.3 mg) and D1PEA (0.173 mL) were added at RT. The
resulting reaction
mixture was stirred overnight. Diluted with DCM (15 mL), washed with water (10
mL). The
organic layer was separated and solvent was removed under vacuum. The residue
was purified with
MDAP to afford the title compound (90.4 mg) as white solid. 1H NMR (400 MHz,
Me0D-d4): 9.23
(d, J= 1.5 Hz, 1H), 8.52 (dd, J= 8.1, 2.2 Hz, 1H), 8.06 (dd, J= 8.2, 0.6 Hz,
1H), 7.65-7.57 (m, 2H),
4.67 (brs, 1H), 4.54-4.38 (m, 211), 4.21 (d, J= 14.4 Hz, 0.511), 3.65-3.40 (m,
2.5H), 3.29-2.99 (m,
3.5H), 2.36 (s, 3H), 2.00-1.56 (m, 8H), 1.52-1.21 (m, 3H). MS (ESI):
C26H30C1N502requires 479;
found 480 [M+Hr.
Example 79
144
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-N-(5-chloro-34(4-(2-cyclopropylacety1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-
5-cyano-6-methylnicotinamide, Trifluoroacetic acid salt (E79)
kl
NTh-r''' 40 INI'M = TFA
0
CI
To a solution of (S)-1-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-
1-y1)-2-
cyclopropylethanone (D169, 120.9 mg) in anhydrous DMF (5 it-IL), 5-cyano-6-
methylnicotinic acid
(D117, 62.8 mg), HATU (274 mg) and D1PEA (0.126 mL) were added. The reaction
mixture was
stirred overnight. After the reaction, the mixture was sent to MDAP for
purification (acidic
condition), to give the title compound (62.5 mg) as white solid. 1H NMR (400
MHz, Me0D-d4):
9.21 (s, 1H), 8.64 (s, 111), 7.55 (s, 211), 4.63 (brs, 111), 4.46 (brs, 1H),
4.29 (brs, 2H), 4.11-3.92 (m,
1H), 3.65-3.46 (m, 1H), 3.20-2.90 (m, 311), 2.84 (s, 3H), 2.54-2.21 (m, 511),
1.50-1.22 (m, 3H),
1.01 (brs, 111), 0.56 (d, J= 7.6 Hz, 2H), 0.19 (d, J= 4.2 Hz, 2H). 19F NMR
(376 MHz, Me0D-d4): -
77.2. MS (ESI): C26H30C1N502 requires 479, found 480 [M+H].
Example 80
(S)-N-(5-chloro-3-04-(2-cyclopropylacety1)-3-methylpiperazin-1-yOmethyl)-2-
methylpheny1)-
5-fluoro-6-methylnicotinamide (E80)
I H
F1-1µ1
0
CI 0 V
The mixture of (S)-N-(5-chloro-2-methy1-343-methylpiperazin-1-yOmethypphenyl)-
5-fluoro-6-
methylnicotinamide, 2 hydrochloric acid salt (D254, 3000 mg), 2-
cyclopropylacetic acid (777 mg),
HATU (3689 mg) and D1PEA (3344 mg) in. DCM (20 mL) was stirred overnight.
After reaction
completed, water (100 ml) was added, extracted with DCM twice (2 x 100 mL).
Combined
organic layer was dried over Na2SO4. Filtered, the filtrate was concentrated
to dryness. The residue
was purified by MDAP to get the title compound (1802 mg). 1H NMR (400 MHz,
Me0D-d4): 8.87
(s, 1H), 8.08 (dd, J= 10.0, 1.2 Hz, 1H), 7.36 (d, J= 2.0 Hz, 111), 7.31 (d, J=
2.0 Hz, 1H), 4.67-
4.70 (m, 0.511), 4.33 (d, J= 12.8 Hz, 0.5H), 4.15 (m, 0.5H), 3.74 (d, J¨ 13.2
Hz, 0.5H), 3.55-3.47
(m, 2H), 3.42-3.36 (m, 0.5H), 3.00-2.93 (m, 0.5H), 2.83-2.86 (m, 1H), 2.75,
(d, J= 11.6 Hz, 1H),
2.61 (d, J= 2.8 Hz, 311), 2.47-2.42 (m, 0.5H), 2.31 (s, 311), 2.26-2.15 (m,
2.511), 2.09-2.00 (m, 111),
1.35-1.24 (m, 311), 1.04-0.96 (m, 1H), 0.53-0.55 (m, 2H), 0.17-0.20 (m, 2H).
MS (EST):
C25H30C1FN402requires 472; found 473 [M+Hr.
Examples 81-90
Examples 81-90 were prepared using a similar procedure to that described for
E80, with the
specified reaction base or solvent listed in the table.
145
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
E81 (S)-N-(5-cyano-34(4-(2-cyclopropylaccty1)-3-mcthylpiperazin-l-yOmethyl)-2-
mcthylpheny1)-
2-methylpyrimidine-5-carboxamide
E82 (S)-N-(5-cyano-34(4-(2-cyclopropylacety1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-
6-methylnicotinamide
E83 (S)-N-(5-cyano-2-methy1-3-43-methyl-4-(spiro[2.3]hexane-5-
carbonyl)piperazin-1-
y1)methyl)pheny1)-6-methylnicotinamide
E84 N-(3-(((3S)-4-(bicyclo[3.1.01hexane-3-carbony1)-3-methylpiperazin-1-
yl)methyl)-5-cyano-2-
methylpheny1)-6-methylnicotinamide
E85 (S)-N-(5-cyano-34(4-(2-cyclopropylacety1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-
6-methoxynicotinamide, Trifluoroacetic acid salt
E86 (S)-5-chloro-N-(5-cyano-344-(2-cyclopropylacety1)-3-methylpiperazin-l-
y1)methyl)-2-
methylpheny1)-6-methylnicotinamide
E87 (S)-5-chloro-N-(344-(2,2-difluorobutanoy1)-3-methylpiperazin-l-yOmethyl)-5-
fluoro-2-
methylpheny1)-6-methylnicotinamide
E88 (S)-N-(5-chloro-344-(2-cyclopropylacety1)-3-methylpiperazin-l-ypmethyl)-2-
methylpheny1)-
4-cyano-4-methylpentanamide
E89 (S)-N-(5-chloro-2-methy1-343-methyl-4-(spiro[2.31hexane-5-
carbonyl)piperazin-l-
yOmethyl)pheny1)-3-(methylsulfonyl)propanamide
E90 N-(3-(((3S)-4-(bicyclo[3.1.0]hexane-3-carbony1)-3-methylpiperazin-l-
yOmethyl)-5-chloro-2-
methylpheny1)-3-(methylsulfonyl)propanamide
Structure Base/Solvent Characterization
1H NMR (400 MHz, DMS0-
do): 10.40 (s, 1H), 9.20 (s, 2H),
7.81 (d, J= 2.0 Hz, 1H),7.65
(d, J= 1.2 Hz, 1H), 4.52-4.50
(m, 0.5H), 4.25-4.00 (m, 1H),
3.60-3.55 (m, 0.5H), 3.52-3.48
r-N1 H (m, 2H), 3.30-3.10 (m,
0.5H),
E81 ao
0 TEA/DCM 3.00-2.95 (m, 0.5H), 2.73 (s,
NI 8 v 3H),
2.64-2.61 (m, 1H), 2.35 (s,
3H), 2.23-1.91 (m, 4H), 1.23-
1.05 (m, 3H), 0.95 (brs, 1H),
0.50-0.40 (m, 2H), 0.15-0.07
(m, 2H). MS (ESI): C25H30N602
requires 446; found 447
N+Hr.
146
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
H TEA/DMF 111
NMR (400 MHz, CDC13):
10.23 (s, 1H), 9.04 (d, J= 1.6
0 LJ
Hz, 1H), 8.22 (dd, J= 8.4, 1.6
I I 0
Hz, 111), 7.78 (s, 1H), 7.64 (s,
1H), 7.44 (d, J= 8.4 Hz, 1H),
4.55-4.49 (m, 0.5H), 4.25-4.00
(m, 1H), 3.56-3.52 (m, 0.511),
E82 3.52-3.47 (m, 2H), 2.80-2.64
(m, 3H), 2.57 (s, 311), 2.34 (s,
3H), 2.25-1.92 (m, 4H), 1.23-
1.18 (m, 3H), 0.95 (brs, 111),
0.50-0.40 (m, 2H), 0.17-0.05
(m, 211). MS (ESI): C26113iN502
requires 445; found 446
EM Hit
DIPEA/DMF NMR (400 MHz,
CDC13):
H
9.02 (s, 1H), 8.17-8.14 (m, 211),
7.97 (brs, 1H), 7.46 (brs, 1H),
I
7.34 (d, J= 7.6 Hz, 1H), 4.76
(brs, 0.5H), 4.41-4.38 (m,
0.511), 3.92-3.86 (m, 0.511),
3.51-3.37 (m, 411), 2.92-2.90
(m, 0.511), 2.71-2.67 (m, 0.5H),
E83
2.67 (s, 3H), 2.58-2.53 (m,
2.5H), 2.41 (s, 3H), 2.21-2.12
(m, 311), 2.03-1.97 (m, 1H),
1.29 (d, J= 6.4 Hz, 1.5H), 1.22
(d, J= 6.4 Hz, 1.5H), 0.47-
0.38(m, 411). MS (EST):
C281133N502 requires 471; found
472 [M+H].
Li
DIPEA/DMF 'H NMR (500 MHz, DMS0-
I
0 do: 10.32
(brs, 1H), 9.05 (s,
E84
111), 8.26 (d, J= 8.0 Hz, 1H),
I I
7.48 (d, J= 7.5 Hz, 1H), 5.70-
5.65 (m, 1H), 3.88-3.82 (m,
147
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
5H), 2.58 (s, 311), 2.35 (brs,
411), 2.27-1.76 (m, 8H), 1.64-
1.04 (m, 6H), 0.45-0.15 (m,
211). MS (ESI): C271133C114402
requires 480; found 481
[M+H].
0 N DIPEA/DMF 1H NMR (400 MHz, Me0D-
y0
0 = TFA d4): 8.82 (d, J= 2.0 Hz, 1H),
I I
8.24 (dd, J= 8.7, 2.1 Hz, 1H),
7.89-7.71 (m, 2H), 6.93 (d, J=
8.6 Hz, 1H), 4.56 (brs, 1H),
4.41 (brs, 0.5H), 4.23 (brs, 2H),
4.01 (s, 3.5H), 3.63-3.41 (m,
E85 1H), 3.25-2.63 (m, 411), 2.43 (s,
3H), 2.34 (brs, 2H), 1.50-1.21
(m, 311), 1.10-0.93 (m, 111),
0.56 (d, J= 7.6 Hz, 2H), 0.19
(d, J= 4.6 Hz, 2H). '9F NMR
(376 MHz, Me0D-d4): -77.4.
MS (EST): C26H31N503 requires
461; found 462 [M+H].
D1PEA/DCM 1H NMR (DMSO-d6, 400
ci N MHz): 10.35 (brs, 1H), 8.96 (d,
1,õ Ny0
J= 1.6 Hz, 1H), 8.38 (d, J= 2.0
Hz, 1I1), 7.78 (d, J= 1.6 Hz,
1H), 7.65 (s, 1H), 4.56 (brs,
0.5H), 4.27-4.22 (m, 0.5H),
4.10-4.01 (m, 0.5H), 3.61-3.55
E86 (m, 0.5H), 3.55-3.49 (m, 2H),
3.30-3.22 (m, 0.5H), 2.80-2.70
(m, 1.5H), 2.67-2.60 (m, 411),
2.33 (s, 3H), 2.25-1.82 (m, 411),
1.21-1.11 (m, 311), 0.93 (brs,
1H), 0.43 (d, J= 7.6 Hz, 2H),
0.11 (brs, 211). MS (EST):
C26H30C1N602requires 479;
148
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
found 480 [M+H].
D1PEA/DMF 1H NMR (DMSO-d6, 400
N
F MHz): 8.90 (s, 1H), 8.37 (d, J=
0 2.0 Hz, 1H), 7.15-7.08 (m, 2H),
4.65 (brs, 0.5H), 4.49 (brs,
0.5H), 4.31-4.27 (m, 0.5H),
4.08-4.05 (m, 0.5H), 3.53 (s,
3H), 3.49-3.42 (m, 0.5H), 3.17-
E87 3.13 (m, 0.5H), 2.93-2.86 (m,
1H), 2.79-2.76 (m, 1H), 2.62-
2.61 (m, 3H), 2.32 (s, 3H),
2.31-2.05 (m, 4H), 1.43-1.30
(m, 3H), 1.09-1.05 (m, 3H). MS
(BSI): C241128C1F3N402 requires
496; found 497 [M+Hr.
DIPEA/DMF 1H NMR (400 MHz, DMS0-
;1-N 40
0 d6): 9.62 (s, 1H), 9.23 (brs, 1H),
7.64 (s, 1H), 7.51 (s, 1H), 4.78
(brs, 1H), 4.42 (brs, 3H), 3.98-
3.91 (m, 1H), 3.27-2.95 (m,
2H), 2.60-2.55 (m, 2H), 2.30-
2.22 (m, 4H), 1.88 (t, J= 7.6
E88 Hz, 2H), 1.35-1.30 (m, 8H),
1.20-1.19 (m, 2H), 0.95 (brs,
1H), 0.45 (d, J= 7.6 Hz, 2H),
0.11-0.08 (m, 2H). 19F (376
MHz, Me0D-d4): -73.6, -75.5.
MS (EST): C25H35C1N402
requires 458; found 459
[M+H]t
9 H DIPEA/DMF 1H NMR (400 MHz, CDC13):
N
N71'õ0
7.66 (brs, 1H), 7.57-7.53 (m,
0 NTO
E89
1H), 7.11 (brs, 1H), 4.75 (brs,
0.5H), 4.40-4.35 (m, 0.5H),
CI
3.87 (m, 0.511), 3.51 (t, J= 75.2
149
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Hz, 2H), 3.44-3.35 (m, 4H),
3.25-3.30 (m, 5H), 2.91-2.83
(m, 0.511), 2.72-2.54 (m, 4H),
2.23 (s, 311), 2.19-2.12 (m, 311),
1.98-1.94 (m, 1H), 1.28-1.21
(m, 3H), 0.49-0.38 (m, 4H). MS
(EST): C241134C1N304S requires
495; found 496 [M+Hr.
0 H D1PEA/DMF
1H NMR (500 MHz, Me0D-
õo
IN"Th' d4): 7.38
(d, J= 2.5 Hz, 1H),
0
7.23 (d, J= 1.5 Hz, 1H), 4.60
(brs, 0.5H), 4.23-4.15 (m, 1H),
3.72-3.68 (m, 1H), 3.55-3.44
(m, 4.511), 3.05 (s, 3H), 2.99 (t,
J= 7.0 Hz, 211), 2.81-2.78 (m,
E90
1H), 2.71-2.69 (m, 111), 2.28 (s,
3H), 2.25-1.82 (m, 6.5H), 1.34-
1.30 (m, 3.5H), 1.21 (d, J= 6.5
Hz, 2H), 0.45-0.40 (m, 1H),
0.40-0.24 (m, 1H). MS (ESI):
C.24H34C1N304S requires 495;
found 496 [M+Hr.
Examples 91&92
(S)-N-(5-chloro-3-04-(2-cyclopropylacety1)-3-methylpiperazin-1-yl)methyl)-2-
methylphenyly
2-(4-hydroxycyclohexyl)acetamide & (S)-N-(5-chloro-34(4-(2-cyclopropylacety1)-
3-
methylpiperazin-1-yl)methyl)-2-methylpheny1)-2-(4-hydroxycyclohexyl)acetamide
(E91 &
E92)
HO
= No..., v HVO
.Ø1rN 40 N.,Th
0 .sso
ci 8 8 v
The 2-cyclopropylacetic acid (50.8 mg) and HATU (92 mg) was added into the
solution of (S)-N-
(5-ehloro-2-methy1-343-methylpiperazin-l-yOmethyl)pheny1)-2-(4-
hydroxycyclohexypacetamide
(D247, 100 mg) in DMF (10 mL). The reaction mixture was stirred for 6 hours at
40 C. The
reaction mixture was added water (20 mL) and extracted with DCM (100 mL). The
organic layer
was separated, dried and filtered. The filtrate was concentrated and the
residue was purified by
column chromatography (silica gel, petroleum ether/Et0Ac =1:1) then MDAP to
give the title
150
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
compounds (10 mg and 15 mg) as yellow solid. Isomer 1:1H NMR (500 MHz, Me0D-
d4): 7.31
(brs, 1H), 7.23 (s, 1H), 4.69 (brs, 0.5H), 4.39-4.32 (m, 0.5H), 4.18 (brs,
0.5H), 3.94 (brs, 1H), 3.73-
3.71 (m, 0.5H), 3.52-3.26 (m, 3H), 2.97-2.90 (m, 0.5H), 2.86-2.81 (m, 1H),
2.72 (d, J= 11.5 Hz,
1H), 2.45-2.43 (m, 111), 2.38-2.18 (m, 8H), 2.10-1.95 (m, 211), 1.80-1.76 (m,
2H), 1.62-1.55 (m,
.. 5.511), 1.35(d, J= 6.5 Hz, 1.5H), 1.25 (d, J= 6.5 Hz, 1.5H), 1.01(brs, 1H),
0.58-0.54 (m, 2H), 0.20
(brs, 2H). MS (ESI): C26H38C1N303requires 475; found [M+H]t Isomer 2: 1H NMR
(400 MHz,
Me0D-d4): 7.31 (d, J= 2.0 Hz, 111), 7.23 (d, J= 1.5 Hz, 1H), 4.69 (brs, 1H),
4.35-4.32 (m, 0.5H),
4.15 (brs, 0.5H), 3.73-3.70 (brs, 0.5H), 3.55-3.35 (m, 4H), 3.00-2.95 (m,
0.511), 2.88-2.80 (m, 111),
2.72 (d, J= 11.0 Hz, 1H), 2.47-2.43 (m, 0.5H), 2.32-2.17 (m, 8H), 2.09-1.97
(m, 3H), 1.89-1.80 (m,
3H), 1.35-1.13 (m, 7H), 1.00 (brs, 1H), 0.57-0.53 (m, 1.511), 0.20 (brs,
1.511). MS (ESI):
C26H38C1N303requires 475; found [M+1-1r.
Example 93
(S)-N-(5-chloro-34(4-(2-cyclopropylacety1)-3-methylpiperazin-l-yl)methyl)-2-
methylpheny1)-
6- cyano-5-methylnicotinamide (E93)
NN
NTsµ"
0 IP
CI
To a solution of (S)-N-(5-chloro-2-methy1-343-methylpiperazin-l-
ypmethyl)pheny1)-6-cyano-5-
methylnicotinamide (D252, 50 mg) in DCM (10 mL) was added 2-cyclopropylacetic
acid (12.58
mg), HATU (57.3 mg) and DIPEA (0.066 mL). After stirred for 8 hours, the
mixture was
concentrated, the residue was purified by MDAP to afford the title compound
(17 mg) as white
solid. 1H NMR (400 MHz, CDC13): 9.09-9.06 (brs, 111), 8.61 (brs, 0.511), 8.45
(m, 0.511), 8.27 (s,
1H), 7.61 (s, 1H), 7.18 (brs, 1H), 4.63 (brs, 0.5H), 4.23-4.14 (m, 0.5H), 3.98
(brs, 0.5H), 3.58-3.26
(m, 311), 2.83-2.71 (m, 111), 2.66 (s, 311), 2.60-2.50 (m, 1H), 2.40-2.31 (m,
0.5H), 2.27 (s, 3H),
2.25-2.00 (m, 3H), 1.90-1.80 (m, 0.5H), 1.34-1.08 (m, 3.5H), 1.00 (brs, 111),
0.55 (brs, 2H), 0.19-
0.11 (d, J= 4.0 Hz, 2H). MS (ESI): C26H30C1N802requires 479; found 480 [M+H].
Examples 94&95
cis N-(3-0(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1.-Amethyl)-5-fluoro-
2-
methylpheny1)-2-(2-hydroxycyclopentyl)acetamide & trans N-(34(S)-4-
(cyclopentanecarbony1)-3-methylpiperazin-1-Amethyl)-5-fluoro-2-methylpheny1)-2-
(2-
hydroxycyclopentyDacetamide (E94 & E95)
cQsN N 114,1 N'Th=;70
0 0
151
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
To a solution of N-(3-(((S)-4-(cyclopentanecarbony1)-3-methyl piperazin-1 -
ypmethyl)-5-fluoro-2-
methylpheny1)-2-(2-oxocyclopentypacetamide (D201, 150 mg) in ethanol (30 mL)
was added
NaBH4 (196 mg) at 0 C. The reaction mixture was stirred at 20 C for 6 hours.
The mixture was
concentrated and to the residue was added water (20 mL), extracted with Et0Ac
(20 mL). The
organic layer was dried and concentrated to leave the crude product, which was
purified by
preparative HPLC and then chiral HPLC to get two isomers (10 mg and 40 mg) as
yellow solid.
Isomer 1: 111 NMR (500 MHz, DMSO-d6): 9.34 (s, 1H), 7.23 (d, J= 10.0 Hz, 1H),
6.95 (d, J= 9.5
Hz, 1H), 4.54 (brs, 0.5H), 4.47-4.45 (m, 111), 4.23-4.16 (m, 1H), 4.02 (brs,
1H), 3.78-3.72 (m,
0.5H), 3.45-3.39 (m, 2H), 3.23-3.15 (m, 0.5H), 2.95-2.88 (m, 1H), 2.77-2.70
(m, 1.5H), 2.66-2.61
(m, 111), 2.36-2.31 (m, 1H), 2.20-1.92 (m, 6H), 1.90-1.37 (m, 13H), 1.35-1.09
(m, 511). MS (ESI):
C26H38FN303 requires 459; found 460 [M+Ht. Isomer 2: 111 NMR (500 MHz, DMSO-
d6): 9.37 (s,
1H), 7.19 (d, J= 10.0 Hz, 1H), 6.96 (d, J= 9.0 Hz, 111), 4.65 (d, J = 5.0 Hz,
1H), 4.55 (brs, 0.5H),
4.23-4.16 (m, 111), 3.78-3.65 (m, 1.511), 3.44-3.36 (m, 2H), 3.22-3.15 (m,
0.5H), 2.95-2.88 (m, 1H),
2.80-2.70 (m, 1.511), 2.66-2.60 (m, 111), 2.22-1.39 (m, 20H), 1.30-1.07 (m,
511). MS (ESI):
C26}138FN303 requires 459; found 460 [M+H]t
Examples 96&97
Cis (S)-N-(5-chloro-3-44-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-
2-
methylpheny1)-2-(3-hydroxycyclobutyl)acetamide & trans (S)-N-(5-chloro-34(4-
(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-methylpheny1)-2-(3-
hydroxycyclobutyl)acetamide (E96&E97)
HO -cnor lel "3 µ.(0 µ HO OS
N )(C>
CI C I
To an ice-cooled solution of (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-
methylpiperazin-l-
y1)methyl)-2-methylpheny1)-2-(3-oxocyclobutyl)acetamide (D202, 320 mg) in Me0H
(2 mL) was
added NaBH4 (26.3 mg). The reaction mixture was stirred at 0 C overnight. The
mixture was
evaporated under vacuum to leave the crude product, which was purified by
preparative HPLC and
chiral HPLC to afford the title compounds (10 mg and 10 mg) as white solids.
Isomer 1: 111 NMR
(400 MHz, CDC13): 7.70 (s, 1H), 7.01 (s, 1H), 6.92 (s, 1H), 4.69 (brs, 1H),
4.33 (d, J= 12.8 Hz,
0.5H), 4.19-4.11 (m, 1H), 4.08-4.03 (m, 0.511), 3.59 (d, J'12.8 Hz, 1H), 3.39-
3.20 (m, 3H), 2.86-
2.64 (m, 2H), 2.56 (d, J= 10.8 Hz, 2H), 2.47 (d, J=7.2 Hz, 2H), 2.14 (s, 3H),
2.12-2.07 (m, 2H),
1.93-1.67 (m, 8H), 1.50 (brs, 2H), 1.25-1.05 (m, 5H). MS (ESI):
C25H36C1N303requires 461; found
462 [M+H]t Isomer 2: 1H NMR (400 MHz, DMSO-d6): 9.38 (brs, 1H), 7.37 (d, J=
2.0 Hz, 1H),
7.15 (s, 111), 4.95 (d, J= 6.0 Hz, 111), 4.54 (brs, 0.5H), 4.28-4.18 (m,
1.51), 3.76-3.73 (m, 0.511),
3.30 (brs, 3H), 3.21-3.15 (m, 111), 2.95-2.87 (m, 1H), 2.80-2.67 (m, 1.5H),
2.65-2.57 (m, 1H), 2.48-
2.43 (m, 21I), 2.16 (s, 3H), 2.13-1.94 (m, 5H), 1.90-1.45 (m, 711), 1.24-1.09
(m, 5H). MS (ESI):
C251136C1N303requires 461; found 462 [M+H].
152
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Example 98
N-(3-0(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-5-fluoro-2-
methylpheny1)-2-(2-hydroxycyclohexyl)acetamide (E98)
OH
aryi
0 tip NL,,_,NyID
0
To a solution of N-(3-(((S)-4-(cyclopentanecarbony1)-3-methyl piperazin-1-
yl)methyl)-5-fluoro-2-
methylpheny1)-2-(2-oxocyclohexyl)acetamide (D200, 100 mg) in ethanol (30 mL)
was added
NaBH4 (127 mg). The reaction mixture was stirred at 20 C for 6 hours. The
mixture was
concentrated and to the residue was added water (20 mL), then extracted with
Et0Ac (10 mL). The
organic layer was dried and concentrated to leave the crude product, which was
purified by MDAP
to afford the title compound (8 mg) as white solid. 'H NMR (400 MHz, DMSO-d6):
9.33 (s, 1H),
7.21 (d, J= 11.0 Hz, 1H), 6.95 (d, J= 8.0 Hz, 1H), 4.56 (brs, 0.5H), 4.42-4.19
(m, 1.5I1), 3.90-3.80
(m, 111), 3.76-3.72 (m, 0.511), 3.45-3.37 (m, 211), 3.19-3.15 (m, 0.511), 3.00-
2.90 (m, 1H), 2.89-2.71
(m, 1.5H), 2.70-2.55 (m, 111), 2.35-2.08 (m, 6H), 2.06-1.75 (m, 2.511), 1.75-
1.30 (m, 1411), 1.28-
1.00 (m, 5H). MS (ESI): C271140FN303 requires 473; found 474 [M+Hr.
Example 99
(S)-N-(5-chloro-3-04-(cyclopentanecarbony1)-3-methylpiperazin-l-Amethyl)-2-
methylpheny1)-2-((1-cyanocyclopropyl)methoxy)acetamide, Trifluoroacetic acid
salt (E99)
= TFA
0
CI 0
To a solution of 1-(hydroxymethyl)cyclopropanecarbonitrile (D86, 47.8 mg) in
anhydrous THF (3
mL) was added sodium iodide (59.1 mg). The mixture was stirred for 0.5 hour at
0 C, followed by
adding (S)-2-chloro-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-
yOmethyl)-2-
methylphenypacetamide (D257, 140 mg) and sodium iodide (59.1 mg). The reaction
mixture was
stirred for 2 hours at 18 C. The mixture was concentrated, and the residue was
purified by column
chromatography (silica gel, petroleum ether/Et0Ac = 3/1) and MDAP to afford
the title compound
(20 mg) as white solid. 1H NMR (400 MHz, Me0D-d4): 7.68 (s, 1 H), 7.35 (s,
1H), 4.45 (brs, 1 H),
4.18 (s, 3H), 4.14-4.10 (m, 1H), 3.58 (s, 211), 3.45-3.36 (m, 1H), 3.21 (brs,
2H), 3.00-2.92 (m, 2H),
2.24 (s, 3H), 1.80-1.53 (m, 9.5H), 1.26 (brs, 1.5 H), 1.24 (m, 2H), 1.16 (d,
.1= 4.8 Hz, 2H), 1.05-
1.02 (m, 2H); 19F NMR (376 MHz, DMSO-d6): -74Ø MS (ESI):
C26H35C1N403requires 486; found
487 [M+14]+.
Examples 100-101
Examples 100-101 were prepared using a similar procedure to that described for
E99.
153
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
E100 (S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-
2-
methylpheny1)-2-(2,2-difluoroethoxy)acetamide, trifluoroacetic acid salt
E101 (S)-N-(5-ehloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylpheny1)-2-(2-cyano-2-methylpropoxy)acetamide
Structure Characterization
1H NMR (400 MHz, CDC13): 8.34 (s, 1H),
F 0 lir I\ILõ1
1
.0 8.14 (brs, 1H), 7.21 (d, J= 2.4 Hz,
1H),
01 0 5.99 (dt, J= 54.8, 3.2 Hz, 1H),
5.01(brs,
0.5H), 4.67 (brs, 0.511), 4.40-4.20 (m,
411), 3.90 (dt, J= 14, 3.6 Hz, 2.5H), 3.80-
3.60 (m, 1.511), 3.31(brs, 111), 2.82-2.70
E100
(m, 2H), 2.60-2.54 (m, 1H), 2.27 (s, 3H),
1.86-1.72(m, 611), 1.61-1.58 (m, 2H),
1.25-1.15 (m, 3H). 19F (376 MHz,
CDC13): -125.9, -126.1. MS (ES1):
C23H32C1F2N303 requires 471; found 472
[M4+1]+.
1H NMR (500 MHz, DMSO-d5): 9.25 (s,
0--liN 1\l's:sifrO
0 1H), 7.57 (s, 1H), 7.20 (s, 1H),
4.55 (brs,
ci 0 0.5H), 4.225-4.20 (m, 3H), 3.76-
3.74 (m,
0.5H), 3.59 (s, 2H), 3.45-3.42 (m, 2H),
E101 3.25-3.15 (m, 0.511), 2.92-2.90 (m,
111),
2.76-2.60 (m, 2H), 2.20 (s, 3H), 2.10-1.50
(m, 10.511), 1.34 (s, 6H), 1.22-1.09 (in,
3H), MS (ESI): C26H37C1N403requires
488; found 489 [M+H].
Example 102
(S)-N-(5-chloro-34(4-(eyelopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-2-(3-hydroxyazetidin-l-y1)acetamide (E102)
Ho'CiN 1) 40
c, 0
The solution of (S)-2-chloro-N-(5-chloro-344-(cyclopentanecarbony1)-3-
methylpiperazin-1-
ypmethyl)-2-methylphenypacetamide (D257, 50 mg), K2CO3 (32.4 mg) and azetidin-
3-ol (17.14
mg) in DMF (5 mL) was stirred under nitrogen at RT overnight. Water (10 mL)
was added,
154
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
extracted with Et0Ac (20 mL). The organic layer was concentrated and the
residue was purified by
MDAP to afford the title compound (28.0 mg) as white solid. 111 NMR (400 MHz,
CDC13): 9.07 (s,
111), 8.00 (d, J= 2.0 Hz, 1H), 7.05 (s, 111), 4.80 (brs, 0.5H), 4.60-4.52 (m,
111), 4.44-4.41(brs,
0.511), 4.13 (brs, 0.5H), 3.85 (dd, J= 8.0, 6.0 Hz, 2H), 3.68-3.62 (m, 0.5H),
3.50-3.40 (m, 1H),
3.36-3.16 (m, 3.5H), 3.24-3.20 (m, 2H), 2.92-2.60 (m, 3.511), 2.26 (s, 3H),
2.22-2.18 (m, 2H), 2.01-
1.90 (m, 1H), 1.85-1.70 (m, 8H), 1.32 (d, J= 6.4 Hz, 1.5H), 1.23 (d, J= 6.8
Hz, 1.5H). MS (ESI):
C24H35C1N403requires 460; found 461 [M+H].
Example 103
(R)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-propylpiperazin-1-yl)methyl)-2-
methylpheny1)-3-(methylsulfonyl)propanamide (E103)
TS NacP
ci
The mixture of 3-(methylsulfonyl)propanoic acid (72.5 mg) in SOC12 (2 mL) was
refluxed for 2
hours. The mixture was then concentrated to dryness under reduced pressure to
afford the crude
acyl chloride. The above acyl chloride was dissolved in DCM (4mL) and added
slowly to the
mixture of (R)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-propylpiperazin-1-
yl)(cyclopentyl)methanone (D177, 90 mg) and TEA (0.033 mL) in DCM (12 mL) at 0
C. The
mixture was stirred at RT for 1 hour, diluted with water (10 mL) and extracted
with Et0Ac (10
mL). The organic phase was dried over Na2SO4, filtered and concentrated to
leave the crude as
brown oil, which was purified by MDAP to afford the title compound (90 mg) as
white solid. 11-1
NMR (400 MHz, CDC13): 7.66-7.59 (m, 2H), 7.15 (brs, 1H), 4.63 (brs, 0.5H),
4.46-4.42 (m, 0.511),
3.88 (brs, 0.511), 3.71-3.67 (m, 0.5H), 3.52 (t, J= 6.8 Hz, 2H), 3.42-3.31 (m,
3H), 3.04-3.00 (m,
511), 2.88-2.73 (m, 4H), 2.23 (s, 311), 2.16-1.98 (m, 3H), 1.86-1.58 (m, 8H),
1.23-1.21 (m, 211),
0.96-0.90 (m, 3H). MS (ESI): C25H38C1N304S requires 511; found 512 [M+H].
Example 104
N-(5-chloro-3-a(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-Amethyl)-2-
methylpheny1)-2-(1,1-dioxidotetrahydrothiophen-3-y1)acetamide, Trifluoroacetic
acid salt
(E104)
N
(s-jThOr L=,-N = TFA
CI
To a solution of N-(5-chloro-3-0(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-
l-ypmethyl)-2-
methylpheny1)-2-(tetrahydrothiophen-3-ypacetamide (D256, 32 mg) in TFA (1 mL)
was added 30%
hydrogen peroxide (9.11 mg). The mixture was stirred for 10 mm, which was
diluted with methanol
(2 mL) and purified by MDAP to afford the title compound (34 mg) as white
solid.IHNMR (400
155
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
MHz, Me0D-d4): 7.50 (s, 1H), 7.46 (s, 1H), 4.62 (brs, 1H), 4.30-4.10 (m, 3H),
3.67-3.65 (in, 0.5H),
3.55-3.45 (m, 1H), 3.20-3.15 (m,1H), 3.15-3.00 (m, 4H), 3.00-2.80 (m, 3H),
2.75-2.60 (m, 2.5H),
2.50-2.40 (m, 1H), 2.28 (s, 3H), 1.97-1.55 (m, 1011), 1.38-1.26 (m, 3H). 19F
(376 MHz, Me0D-d4):
-77.2. MS (EST): C251136C1N304S requires 509; found 510 [M+Hr.
Examples 105-108
Examples 105-108 were prepared using a similar procedure to that described for
E104.
E105 N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
yOmethyl)-2-
methylpheny1)-2-(1,1-dioxidotetrahydrothiophen-2-y1)acetamide
E106 N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-
2-
methylpheny1)-2-(1,1-dioxidotetrahydro-2H-thiopyran-3-yl)acetamide,
trifluoroacetic acid salt
E107 N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin- 1 -
yl)methyl)-2-
methylpheny1)-2-(methylsulfonyl)cyclobutanecarboxamide
E108 (S)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-ypmethyl)-
2-
methylpheny1)-3-(methylsulfonyl)cyclobutanecarboxamide
Structure Characterization
0, p H 1H NMR (500 MHz, DMSO-d6): 9.63 (s,
s_nor-i Nac0
111), 7.41 (d, J= 2.0 Hz, 1H), 7.18 (s, 111),
4.55 (brs, 0.5H), 4.25-4.20 (m, 1H), 3.76-
3.74 (m, 0.5H), 3.44-3.40 (m, 311), 3.19-
3.14 (m, 1.5H), 3.05-2.96 (m, 1H), 2.93-
E105 2.84 (m, 2H), 2.73-2.71 (m, 111),
2.64-2.60
(m, 2H), 2.42-2.36 (m, 111), 2.19 (s, 3H),
2.10-1.96 (m, 3.5H), 1.69-1.49 (m, 1011),
1.24-1.10 (m, 311). MS (ESI):
C251136C1N304S requires 509; found 510
[M+Hr.
11-INMR (400 MHz, Me0D-d4): 7.47 (m,
N,Th ,;(0
= TFA
0 µ1111 211), 4.62 (brs, 0.511), 4.32 (brs,
211), 4.18-
,s,
d 0 Cl 4.14 (m, 0.5H), 3.2-3.46 (m, 0.5H),
3.38-
3.32 (m, 2H), 3.14-3.10 (m, 2.5H), 3.02-
2.93 (m, 511), 2.60-2.45 (m, 3H), 2.25 (s,
E106
3H), 2.15-2.11 (m, 1H), 2.00-1.55 (m, 11H),
1.43-1.30 (m, 2H), 1.24-1.21 (m, 2H). 19F
(376 MHz, Me0D-d4): -77.2. MS (ESI):
C26H38C1N304S requires 523; found 524
156
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
2 ,1(H IH NMR (400 MHz, CDC13): 7.91-7.87
(m,
Os . facci)
dr, 0 211), 7.08 (s, 1H), 4.79 (brs,
0.511), 4.45-
a 0 4.41(brs, 0.5H), 4.11-4.03 (m, 1.5H),
3.82-
3.78 (q, J= 8.4 Hz, 1H), 3.65 (brs, 0.511),
3.43-3.27 (m, 2.511), 2.92 (s, 311), 2.90-2.68
E107 (m, 2H), 2.65-2.42 (m, 311), 2.30-
2.22 (t, J
= 8.8 Hz, 2H), 2.23 (s, 3H), 2.20-2.18 (m,
1.511), 2.00-1.85 (m, IH), 1.80-1.62 (m,
811), 1.31-1.21 (m, 3H). MS (EST):
C23H36CIN304S requires 509; found 510
[M+H]+.
0 11-1 NMR (400 MHz, CDC13): 7.80 (s, 1H),
7.11 (s, 111), 7.00 (s, 111), 4.78 (brs, 1H),
iLj1=1-"y;õ0
0 L..N 4.74-4.50 (m, 111), 4.48-4.35 (m,
0.5H),
ci 0 4.20-4.09 (m, 0.5H), 3.94-3.82 (m,
1H),
3.73-3.62 (m, 0.5H), 3.54-3.28 (m, 3.5H),
E108 3.02-2.91 (m, 1H), 2.86 (s, 3H), 2.84-
2.73
(m, 5H), 2.65 (d, J= 8.8 Hz, 1H), 2.31-2.15
(m, 4H), 2.10-1.96 (m, 111), 1.95-1.52 (m,
711), 1.37-1.15 (m, 3H). MS (ESI):
C23H36C1N304S requires 509; found 510
[M+Hr=
Example 109
2-(1-acetylpyrrolidin-3-y1)-N-(34(S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-1-
yl)methyl)-5-fluoro-2-methylphenypacetamide (E109)
0
101
0
N-(3-4(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-5-fluoro-2-
methylpheny1)-2-
(pyrrolidin-3-y1)acetamide, 2 hydrochloride acid salt (D238, 100 mg) and TEA
(0.054 mL) were
dissolved in DCM (10 mL). To this solution, acetyl chloride (0.017 mL) was
added gradually. The
reaction mixture was stirred at RT for 2 hours. Water (10 ) was added, the
DCM layer was
seperated and the aqueous layer was extracted with DCM (10 mL) again. Combined
DCM layers
were dried over Na2SO4. Filtered, the filtrate was concentrated under vacuum.
The residue was
purified by MADP to afford the title compound (27 mg) as white solid. NMR (400
MHz,
DMSO-d6): 9.52 (d, J= 7.9 Hz, 1H), 7.28 (td, J= 10.6, 2.3 Hz, 1H), 7.03 (d, J=
8.4 Hz, 1H), 4.61
157
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(brs, 0.5H), 4.26 (d, .1= 9.5 Hz, 1H), 3.81 (d, J= 13.0 Hz, 0.511), 3.70 (dd,
.1= 10.0, 7.2 Hz, 0.5H),
3.64-3.55 (m, 1H), 3.54-3.43 (m, 3H), 3.32-3.13 (m, 1.5H), 3.05-2.93 (m,
1.511), 2.89-2.76 (m,
1.5H), 2.74-2.62 (m, 1.5H), 2.55-2.48 (m, 2H), 2.22 (d, J= 2.8 Hz, 3H), 2.19-
2.02 (m, 2H), 1.99 (s,
3H), 1.96-1.50 (m, 1011), 1.37-1.13 (m, 31I). 19F NMR (376 MHz, DMSO-d6): -
117.9. MS (ESI):
C271139FN403 requires 486; found 487 [M+Hr.
Examples 110-126
Examples 110-126 were prepared using a similar procedure to that described for
E109, with the
specified reaction base or solvent listed in the table.
E110 (1r,3S)-3-acetamido-N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
yl)methyl)-5-
fluoro-2-methylphenyl)cyclobutanecarboxamide, Trifluoroacetic acid salt
E111 (1s,3R)-3-acetamido-N-(34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
y1)methy1)-5-
fluoro-2-methylphenyl)cyclobutanecarboxamide, Trifluoroacetic acid salt
E112 (S)-1-acetyl-N-(3-44-(cyclopentanecarbony1)-3-methylpiperazin-1-
yl)methyl)-5-fluoro-2-
methylphenyflazetidine-3-carboxamide
E113 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-
2-
methylphenyl)-2-(1-propionylazetidin-3-y1)acetamide
E114 (R)-1-acetyl-N-(5-ch1oro-34(S)-4-(cyclopentanecarbony1)-3-methy1piperazin-
1-y1)methyl)-
2-methylphenyl)pyrrolidine-3-carboxamide
E115 (S)-1-acetyl-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-
1-yOmethyl)-
2-methylphenyl)pyrrolidine-3-carboxamide
E116 (R)-1-acetyl-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-
l-y1)methyl)-
2-methylphenyppyrrolidine-2-carboxamide
E117 (S)-1-acetyl-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-
yOmethyl)-2-
methylphenyppiperidine-4-carboxamide
E118 (S)-1-acetyl-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-
1-yOmethyl)-
2-methylphenyl)piperidine-3-carboxamide
E119 (R)-1-acetyl-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-
1-yOmethyl)-
2-methylphenyl)piperidine-3-carboxamide
E120 (S)-1-acety1-N-(5-chloro-34(S)-4-(cyc1opentanecarbony1)-3-methylpiperazin-
1-y1)methyl)-
2-methylphenyl)pyrrolidine-2-carboxamide
E121 (S)-N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
yOmethyl)-2-
methylpheny1)-1-propionylpyrrolidine-2-carboxamide
E122 (S)-N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-
y1)methyl)-2-
methylpheny1)-1-isobutyrylpyffolidine-2-carboxamide
E123 (S)-2-(1-acetylpiperidin-4-y1)-N-(5-chloro-344-(cyclopentanecarbony1)-3-
methylpiperazin-
1-ypmethyl)-2-methylphenyl)acetamide
158
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
E124 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-Amethyl)-2-
methylpheny1)-3-(N-methylacetamido) Propanamide
E125 (S)-N-(5-chloro-344-(cyclopentanecarbony1)-3-methylpiperazin-l-yDmethyl)-
2-
methylpheny1)-4-(N-methylacetamido) butanamide
E126 4-Acetyl-N-(5-chloro-3-(((S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
yDmethyl)-2-
methylphenyl)morpholine-2-carboxamide, Trifluoroacetic acid salt
Structure Base/Solvent
Characterization
1HNMR (400 MHz, Me0D-d4):
7.29 (dd, J= 9.6, 2.5 Hz, 1H),
7.24 (dd, J= 8.9, 2.5 Hz, 1H),
5.10-4.91 (m, 1.5H), 4.63 (brs,
1H), 4.50 (quin, J= 15.7 Hz,
ii 1H), 4.39 (brs, 2H), 4.19(d
J=
13.0 Hz 0.5H), 3.64-3.34 (m,
E110 0Nacr0 Pyridine/TI-IF
0 3.5H), 3.29-2.94 (m, 4.5H),
= WA 2.71-2.57 (m, 2H), 2.44-
2.30 (m,
2H), 2.30-2.25 (m, 3H), 1.93 (s,
3H), 1.90-1.56 (m, 8H), 1.46-
1.20 (m, 3H). MS (ESD:
C26H37FN403 requires 472;
found 473 [M+H].
1HNMR (400 MHz, Me0D-d4):
7.31 (dd, J= 9.3, 2.4 Hz, 1H),
7.25 (dd, J= 9.0, 2.4 Hz, 1H),
5.08-4.90 (m, 1.5H), 4.64 (brs,
1H), 4.40 (brs, 2H), 4.34-4.10
(m, 1.5H), 3.63-3.34 (m, 2.5H),
El11 0 10 Nac,C) Pyridine/THF
3.27-2.95 (m, 4.5H), 2.58 (qd, J
0
= TFA = 8.1, 2.7 Hz, 2H),
2.31-2.16 (m,
5H), 1.92 (s, 3H), 1.90-1.55 (m,
8H), 1.51-1.19 (m, 311). MS
(ESD: C26H37FN403 requires
472; found 473 [M+Hr.
159
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
NMR (400 MHz, DMSO-d6):
9.50 (s, 111), 7.19 (dd, J= 10.3,
2.4 Hz, 1H), 6.92 (d, J= 8.1 Hz,
1H), 4.47 (brs, 0.5H), 4.29-4.04
(m, 311), 4.00-3.79 (m, 2H), 3.68
0 (d, J= 13.2 Hz, 0.5H), 3.59-3.46
)t,N
E112 TEA/DCM (m, 1H), 3.41-3.30 (m, 2H),
1$ r0,,;.crO 3.19-3.05 (m, 0.511), 2.91-2.79
0 111), 2.76-2.62 (m, 1.5H),
2.60-2.49 (m, 1H), 2.08 (s, 3H),
2.04-1.72 (m, 2H), 1.69 (s, 311),
1.67-1.37 (m, 8H), 1.23-0.98 (m,
3H). MS (ESI): C25H35FN403
requires 458; found 459 [M+Hr.
11-1NMR (400 MHz, Me0D-d4):
7.35 (d, J= 2.0 Hz, 1H), 7.23 (d,
J= 1.8 Hz, 1H), 4.67 (brs,
0.5H), 4.46-4.37 (m, 1H), 4.37-
4.25 (m, 1H), 4.18 (t, J= 9.2 Hz,
1H), 4.02 (dd, J= 8.9, 5.7 Hz,
111), 3.87 (d, J= 13.1 Hz, 0.5H),
N do.h
E113 INin tip TEA/DCM 3.77 (dd, J= 10.0, 5.7 Hz, 1H),
3.54-3.42 (m, 211), 3.42-3.31 (m,
0.5H), 3.14-2.91 (m, 2.5H),
2.88-2.66 (m, 4H), 2.27 (s, 3H),
2.23-1.91 (m, 2H), 1.88 (s, 3H),
1.84-1.53 (m, 8H), 1.40-1.16 (m,
3H). MS (ESI): C26H37C1N4.03
requires 488; found 489 [M+H].
TEA/DCM 1H NMR (400 MHz, CDC13):
0 0 11$ LNJII 7.73 (brs, 1H), 7.24-7.18 (m,
1H), 7.14-7.08 (m, 111), 4.76
E114 (brs, 0.5H), 4.43-4.37 (m, 0.5H),
4.12 (brs, 0.5H), 3.94-3.61 (m,
3.5H), 3.60-3.26 (m, 4H), 3.22-
3.05 (m, 111), 2.90-2.80
160
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/070753
(m,1.5H), 2.78-2.70 (brs, 1H),
2.65-2.60 (d, J= 9.8 Hz, 111),
2.47-2.40 (m, 0.5H), 2.31-2.25
(m, 1H), 2.22 (d, J= 6.0 Hz,
3H), 2.20-2.14 (m, 111), 2.09 (d,
J= 6.0 Hz, 3H), 2.03-1.80 (m,
2.511), 1.80-1.68 (m, 511), 1.31-
1.17 (m, 311). MS (ESI):
C26H37C1N403requires 488,
found 489 [M+H].
TEA/DCM NMR (400 MHz, CDC13):
- NCINI;(0
7.71 (brs, 1H), 7.30-7.26 (m,
0 1H), 7.12 (brs, 1H), 4.75 (brs,
0.5H), 4.40-4.37 (m, 0.5H), 4.12
(brs, 0.5H), 3.96-3.60 (m, 4H),
3.58-3.28 (m, 411), 3.22-3.18 (m,
0.5H), 3.12-3.07 (m, 0.5H),
2.90-2.80 (m, 1.5H), 2.75-2.71
E115 (m, 1H), 2.64-2.60 (m, 1H),
2.48-2.41 (m, 0.5H), 2.35-2.25
(m, 114), 2.22 (d, J= 6.0 Hz,
311), 2.21-2.17 (m, 111), 2.08 (d,
J= 6.0 Hz, 3H), 2.02-1.96 (m,
1H), 1.96-1.65 (m, 5.5H), 1.57-
1.50 (m, 3H), 1.32-1.20 (m, 3H).
MS (ESI): C26H37C1N403
requires 488; found 489 [M+H].
CD. 11 TEA/DCM 1H NMR (400 MHz, CDC13):
N
0 Nallp 9.46 (s, 1H), 8.02 (d, J= 2.0 Hz,
0
111), 7.03 (s, 1H), 4.84 (d, J=
ci 0
8.0 Hz, 1H), 4.77 (brs, 0.511),
E116 4.42-4.38 (m, 0.5H), 4.12 (brs,
0.5H), 3.62-3.50 (m, 1.5H),
3.49-3.45 (m, 1.511), 3.45-3.30
(m, 2H), 2.91-2.63 (m, 4.5H),
2.25 (s, 3H), 2.19-2.17 (m, 51I),
161
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
2.10-1.81 (m, 6H), 1.79-1.76 (m,
5H), 1.31-1.22 (m, 311). MS
(ESI): C26H37C1N403 requires
488; found 489 [M+Hr.
No base/THF 1H NMR (400 MHz, CDC13):
7.75 (brs, 111), 7.18-7.10(m
o 10 Na.l,ro's 211), 4.80-4.64 (m, 1.511), 4.45-
CI 0 4.38 (m, 0.511), 4.13 (brs, 0.5H),
3.98-3.88 (m, 1H), 3.70-3.63 (m,
0.5H), 3.43-3.30 (m, 2.5H),
3.20-3.15 (m, 1H), 2.95-2.80 (m,
E117 1.5H), 2.80-2.70 (m, 211), 2.65-
2.56 (m, 2H), 2.30 (s, 311), 2.20-
12.17 (m, 1H), 2.13 (s, 3H),
2.07-1.97 (m, 311), 1.90-1.61 (m,
811), 1.60-1.56 (m, 2H), 1.31-
1.20 (m, 311). MS (ESI):
C271139C1N403 requires 502;
found 503 [M+Hr.
H No base/THF 111 NMR (400 MHz, CDC13):
1N IN = Nac0 7.98-7.96(m, 1H), 7.77 (brs,
0.211), 7.61 (brs, 0.811), 7.13 (s,
111), 4.83 (brs, 0.5H), 4.46-4.41
(m, 0.5H), 4.20-4.08 (m, 1.5H),
3.68-3.60 (m, 211), 3.50-3.25 (m,
E118 3.5H), 2.96-2.77 (m, 3H), 2.70-
2.58 (m, 2H), 2.24 (s, 3H), 2.22-
2.15(m, 4H), 2.01-1.95 (m, 2H),
1.87-1.70 (m, 8H), 1.60-1.58 (m,
3H), 1.30-1.20 (m, 3H). MS
(ESI): C271139C1N403 requires
502; found 503 [M+H]t
H No base/THF 111 NMR (400 MHz, CDC13):
E119
7.96 (brs, 0.611), 7.77 (brs,
ci 0.311), 7.61
(s, 111), 7.13 (brs,
1.5H), 4.78 (brs, 0.511), 4.50-
162
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
4.42 (m, 0.5H), 4.20-4.10 (m,
1H), 3.72-3.58 (m, 2H), 3.50-
3.30 (m, 3.5H), 2.90-2.58 (m,
4.511), 2.25 (brs, 311), 2.20-2.16
(m, 4H), 2.02-1.96 (m, 2H),
1.87-1.70 (m, 8H), 1.30-1.20 (m,
311). MS (ESI): C271139C1N403
requires 502; found 503 [M+Hr.
H TEA/DCM 111 NMR (400
MHz, CDC13):
NCy;r0 9.46 (s,
111), 8.02(brs, 1H), 7.02
(d, J= 6.0 Hz 111), 4.82 (d, J=
ci 0
7.6 Hz, 111), 4.76 (brs, 0.511),
4.38-4.12 (m, 0.5H), 4.11 (brs,
0.511), 3.62-3.57 (m, 1.5H),
3.51-3.46 (m, 1.511), 3.37-3.30
E120
(m, 2H), 2.90-2.64 (m, 4.511),
2.25(s, 3H), 2.15-2.13 (m, 5H),
2.09-2.00 (m, 1H), 1.97-1.74 (m,
8.5H), 1.66-1.50 (m, 2.5H),
1.31-1.22 (m, 3H). MS (ESI):
C261137C1N403requires 488;
found 489 [M+Hr.
TEA/DCM NMR (400 MHz,
CDC13):
\ N
9.48 (d, J= 3.2 Hz, 1H), 8.02 (d,
0
J= 4.8 Hz, 111), 7.03 (d, J= 7.6
0i 0
Hz, 111), 4.87 (d, J= 8.0 Hz,
111), 4.76 (brs, 0.511), 4.42-4.38
(m, 0.5H), 4.11 (brs, 0.5H),
3.66-3.32 (m, 5H), 2.92-2.75 (m,
E121
2.5H), 2.65-2.63 (m, 2H), 2.42-
2.39 (m, 2H), 2.24 (s, 3H), 2.19-
1.96 (m, 4H), 1.85-1.75 (m, 7}1),
1.57-1.50 (m, 2H), 1.30 (d, J=
6.4 Hz, 1.5H), 1.22-1.18 (m,
4.5H). MS (ESI). C271139C1N403
requires 502; found 503
163
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
[M+H] .
H TEA/DCM IHNMR (400 MHz, CDC13):
= N-Th';(0
9.48 (d, J= 3.2 Hz, 1H), 7.99 (d,
J= 6.4 Hz, 1H), 7.03 (d, J= 6.4
ci
Hz, 1H), 4.87 (d, J= 7.2 Hz,
111), 4.76 (brs, 0.511), 4.42-4.38
(m, 0.5H), 4.10 (brs, 0.5H),
3.63-3.60 (m, 1.511), 3.57-3.53
E122 (m, 111), 3.43-3.30 (m, 2.5H),
2.91-2.72 (m, 3.511), 2.65-2.60
(m, 2H), 2.23 (s, 3H), 2.16-2.09
(m, 3H), 1.98-1.62 (m, 8H),
1.56-1.50 (m, 2H), 1.28 (d, J=
6.4 Hz, 1.5H), 1.21-1.15 (m,
7.5H). MS (ESI): C281141C1N403
requires 516; found 517 [M+H].
TEA/DCM IHNMR (400 MHz, Me0D-
oiNrnr.,.,- N= r11 LõNs d4): 7.35 (brs, 1H), 7.25 (brs, H),
CI 4.69 (brs, 0.5H), 4.55 (d, J=
13.0 Hz, 1H), 4.41-4.24 (m, 1H),
4.03-3.76 (m, 1.511), 3.50 (brs,
2H), 3.39 (d, J= 12.3 Hz, 0.511),
E123 3.16 (t, J= 12.1 Hz, 1H), 3.10-
2.58 (m, 4.511), 2.48-2.35 (m,
2H), 2.31-2.24 (m, 311), 2.23-
2.00 (m, 6H), 1.98-1.50 (m,
10H), 1.43-1.12 (m, 5H). MS
(ER): C281441C1N403 requires
516; found 517 [M+H] .
TEA/DCM 111 NMR (400
MHz, Me0D-d4):
N 7.32 (d, J= 12.0 Hz, 1H), 7.21
(brs, 1H), 4.66 (brs, 111), 4.31 (d,
E124 CI
140 J= 14.3 Hz, 1H), 3.89-3.63 (m,
2.511), 3.56-3.36 (m, 2H), 3.12
(s, 211), 3.05-2.88 (m, 2.5H),
2.86-2.54 (m, 4H), 2.36-1.98 (m,
164
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
8H), 1.96-1.47 (m, 811), 1.43-
1.12 (m, 3H).
MS (ESI): C25H37C1N403
requires 476; found 477
[MA-1] .
TEA/DCM 1H NMR (400 MHz, Me0D-d4):
0
0L 7.36 (d, J= 3.4 Hz, 1H),7.21
ci
1410 (brs, 1H), 4.66 (brs, 0.5H),
4.41-
4.18 (m, 1H), 3.83 (d, J= 12.5
Hz, 0.5H), 3.52-3.34 (m, 4.5H),
3.15-2.85 (m, 4.5H), 2.82 (d, J=
E125
11.0 Hz, 1H),2.71 (t, J= 9.4 Hz,
1H), 2.53-2.35 (m, 2H), 2.26 (s,
3H), 2.22-1.50 (m, 15H), 1.43-
1.08 (m, 3H).
MS (ESI): C26H39C1N403
requires 490; found 491 [M+H].
H TEA/THF 1H NMR (400 MHz, Me0D-d4):
7.66 (brs, 1H), 7.57 (brs, 1H),
0 1,I\lsxC; 4.81-4.59 (m, 1.5H), 4.52-4.42
ci
(m, 21.1), 4.38-3.99 (m, 3.5H),
3.92-3.36 (m, 5H), 3.30-2.95 (m,
E126 4H), 2.93-2.78 (m, 111), 2.31
(brs, 3H), 2.17 (s, 311), 1.99-1.54
(m, 8H), 1.53-1.08 (m, 3H). 19F
NMR (376 MHz, Me0D-d4): -
77.3. MS (ESI): C261137C1N404
requires 504; found 505 [M+Hr.
Example 127
(S)-2-(1-acetylazetidin-3-yl)-N-(5-chloro-34(4-(cyclopentanecarbony1)-3-
methylpiperazin-1-
ypmethyl)-2-methylphenyl)acetamide (E127)
NIYThrN
0 NO
0 CI
To a solution of (S)-2-(azetidin-3-y1)-N-(5-chloro-344-(cyclopentanecarbony1)-
3-methylpiperazin-
l-y1)methyl)-2-methylphenypacetamide, Trifluoroacetic acid salt (D235, 54.9
mg) in acetonitrile (2
165
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
mL), Et3N (41 pL) was added by pipette, followed by propionyl chloride (15
L). The reaction
mixture was stirred for 1 hour at RT. The mixture was sent to MDAP for
purification (basic eluent),
giving the title compound (21.6 mg) as white solid. 1IINMR (400 MHz, Me0D-d4):
7.32 (s, 1H),
7.21 (s, 1H), 4.66 (brs, 0.5H), 4.38 (t, J= 8.6 Hz, 1H), 4.34-4.24 (m, 111),
4.16 (t, J= 9.2 Hz, 111),
3.99 (dd, J= 8.6, 5.9 Hz, 111), 3.83 (d, Jr 13.9 Hz, 0.5H), 3.75 (dd, J= 9.8,
5.9 Hz, 1H), 3.40-3.53
(m, 211), 3.40-3.34 (m, 0.5H), 3.14-2.90 (m, 2.5H), 2.88-2.75 (m, 3H), 2.75-
2.66 (m, 111), 2.25 (s,
3H), 2.20-1.50 (m, 12H), 1.40-1.15 (m, 3H), 1.09 (t, J= 7.5 Hz, 311). MS
(ESI): C271139C1N403
requires 502; found 503 [M+Hr.
Example 128
(S)-N-(34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-5-fluoro-2-
methylpheny1)-1-(methylsulfonyDazetidine-3-carboxamide (E128)
0, 0
N\..3r1-4
N
0
0
(S)-N-(344-(cyclopentanecarbony1)-3-methylpiperazin-1-yOmethyl)-5-fluoro-2-
methylphenyl)azetidine-3-carboxamide (D241, 100 mg) and TEA (0.05 mL) were
dissolved in
DCM (10 inL). To this solution, MsC1 (0.022 mL) was added gradually. The
reaction mixture was
stirred at RT for 1 hour. Water (10 mL) was added into the reaction mixture to
quench the reaction.
The DCM layer was seperated and the aqueous layer was extracted with DCM (10
mL) again.
Combined DCM layer was dried over Na2SO4 and filtered. The filtrate was
concentrate under
vacuum. The residue was purified by MADP to afford the title compound (53 mg)
as white solid.
NMR (400 MHz, DMSO-d6): 9.51 (s, 1H), 7.21 (dd, J= 10.3, 2.4 Hz, 1H), 6.92 (d,
J= 8.4 Hz,
1H), 4.47 (brs, 0.5H), 4.20-4.07 (m, 1H), 4.03-3.91 (m, 4H), 3.68 (d, J= 12.5
Hz, 0.5H), 3.56 (quin,
J= 7.6 Hz, 1H), 3.40-3.30 (m, 2H), 3.12 (t, J= 12.0 Hz, 0.5H), 2.96 (s, 3H),
2.85 (quin, J= 7.2 Hz,
1H), 2.77-2.49 (m, 2.5H), 2.09 (s, 3H), 2.05-1.37 (m, 10H), 1.23-0.99 (m,
311). 19F NMR (376 MHz,
DMSO-d6): -117.8. MS (ESI): C241135FN404S requires 494; found 495 [M+H].
Example 129
(S)-N-(5-chloro-3-44-(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-
methylpheny1)-2-(N-methylmethylsulfonamido)acetamide, Trifluoroacetic acid
salt
p H
S", N
Nnor 110 Co
CI 0
= TEA
E129 was prepared using a similar procedure to that described for E128. 1H NMR
(400 MHz,
Me0D-d4): 7.53 (d, J= 1.8 Hz, 1H), 7.41 (s, 1H), 4.88 (brs, 0.5H), 4.52 (brs,
1H), 4.26 (brs, 2H),
166
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
4.13-3.98 (m, 2.511), 3.51-3.23 (m, 311), 3.18-2.78 (m, 1011), 2.21 (s, 311),
1.90-1.46 (m, 8H), 1.40-
1.08 (m, 3H). MS (ESI): C23H35C1N404S requires 498; found 499 [M+H].
Examples 130&131
2-(1-acetylpyrrolidin-3-yl)-N-(5-chloro-3-(aS)-4-(cyclopentaneearbonyl)-3-
methylpiperazin-1-
.. yl)methyl)-2-methylphenyl)acetamide & 2-(1-acetylpyrrolidin-3-y1)-N-(5-
chloro-3-4(S)-4-
(cyclopentanecarbony1)-3-methylpiperazin-l-yOmethyl)-2-methylphenyl)acetamide
(E130 &
E131)
N 401
'NJ 8 \x(?) ca'ssNIN Nlõ--11,;:ro
0CI 0
To a solution of N-(5-chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-
l-yOmethyl)-2-
methylpheny1)-2-(pyrrolidin-3-yl)acetamide (D225, 120 mg) in DCM (10 mL) were
added acetyl
chloride (20.43 mg) and triethylamine (79 mg). After stirred for 2 hours, the
mixture was
concentrated to give a white solid, which was purified by preparative chiral
HPLC (OZ-H column
(5 lam, 4.6x250 mm) at a column temperature of 40 C with 10 mM DEA buffer /n-
hexane and 10
mM DEA buffer /Et0H as mobile phase with a flow rate of 60 g /min) to give the
title compounds
.. (10 mg and 12 mg). Isomer 1: 11-1NMR (400 MHz, CDC13): 7.77-7.73 (m, 111),
7.13-7.07 (m, 2H),
4.76 (brs, 0.5H), 4.45-4.36 (m, 0.5H),4.12 (brs, 0.5H), 3.83-3.75 (m, 111),
3.70-3.54 (m, 1.511),
3.50-3.26 (m, 411), 3.22-3.10 (m, 1H), 2.93-2.70 (m, 3.5H), 2.64-2.40 (m, 3H),
2.35-2.26 (m, 0.511),
2.22 (s, 3H), 2.20-2.13 (m, 2H), 2.06 (s, 3H), 1.96-2.02 (m, 2H), 1.90-1.68
(m, 7H), 1.31-1.20 (m,
311). MS (ESI): C271139C1N403 requires 502, found 503 [M+H]t Isomer 2: 'H NMR
(400 MHz,
CDC13): 7.77-7.71 (m, 1H), 7.23-7.14 (brs, 1H), 7.10 (brs, 1H), 4.76 (brs,
0.5H), 4.12 (brs, 0.513),
4.06-3.97 (m, 0.5H), 3.83-3.76 (m, 1H), 3.70-3.54 (m, 1.5H), 3.50-3.26 (m,
4H), 3.22-3.11(m, 1H),
2.94-2.70 (m, 3.511), 2.64-2.42 (m, 3H), 2.35-2.27 (m, 0.5H), 2.23 (s, 3H),
2.20-2.13 (m, 2H), 2.05
(s, 311), 2.02-1.94 (m, 211), 1.90-1.66 (m, 7H), 1.33-1.19 (m, 31). MS (ES1):
C271139C1N403
requires 502, found 503 [M+H].
Examples 132-135
Examples 132-135 were prepared using a similar procedure to that described for
E130 and E131,
with the specified reaction base or solvent listed in the table.
E132&E133: 24(R)-1-acetylpyrrolidin-2-y1)-N-(5-chloro-34(S)-4-
(cyclopentanecarbony1)-3-
methylpiperazin-l-yOmethyl)-2-methylphenyl)acetamide & 2-((S)-1-
acetylpyrrolidin-2-y1)-N-(5-
.. chloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-ypmethyl)-2-
methylphenyflacetamide
E134&E135 2-((S)-1-acetylpiperidin-3-y1)-N-(5-chloro-3-(((S)-4-
(cyclopentanecarbony1)-3-
methylpiperazin-1-yl)methyl)-2-methylphenyl)acetamide & 2-((R)-1-
acetylpiperidin-3-y1)-N-(5-
167
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
chloro-3-(05)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-2-
methylphenyl)acetamide
Structure Base/ Solvent Characterization
H No base Isomer 1: 111NMR (400 MHz,
(Inor.N io õco
/THF CDC13): 8.86-8.82 (m, 1H), 7.79 (s,
111), 7.07 (s, 111), 4.80 (brs, 0.511),
H 4.45-4.34 (m, 1.511), 4.13 (brs,
0.5H),
lo3.68-3.55 (m, 2H), 3.50-3.33 (m, 411),
2.93-2.70 (m, 411), 2.64-2.60 (m, 1H),
a
2.51-2.47 (m, 1H), 2.29 (s, 3H), 2.19-
2.16 (m, 1.5H), 2.11-2.08 (m, 5H),
2.00-1.83 (m, 4H), 1.80-1.72 (m, 5H),
1.32-1.22 (m, 3H). MS (ESI):
E132 C271139C1N403requires 502; found
503 [M+H]. Isomer 2: 1H NMR (400
E133 MHz, CDC13): 8.80 (brs, 1H), 7.81
(brs, 111), 7.07 (brs, 1H), 4.87 (brs,
0.5H), 4.45-4.34 (m, 1.511), 4.17 (brs,
0.511), 3.68-3.60 (m, 2H), 3.50-3.33
(m, 4H), 2.99-2.75 (m, 4H), 2.65-2.60
(brs, 1H), 2.51-2.45 (m, 1H), 2.30 (s,
3H), 2.30-2.25 (m, 1.5H), 2.15-2.05
(m, 5H), 2.00-1.83 (m, 411), 1.80-1.58
(m, 5H), 1.32-1.22 (m, 311). MS
(EST): C271139C1N403requires 502;
found 503 [M+11]+.
TEA/DCM Isomer 1: 114 NMR (400 MHz,
Na0CDC13): 8.46 (brs, 111), 7.73-7.64 (m,
:10
CI 0 111), 7.12
(brs, 1H), 4.77 (brs, 0.5H),
4.43-4.38 (m, 0.5H), 4.26-4.13 (m,
E134 Nacci>
0.514), 3.90-3.85 (m, 1H), 3.64-3.58
CI 0 (m, 1.5H), 3.46-3.32 (m, 4H), 2.91-
E135
2.75 (m, 3.511), 2.68-2.65 (m, 1H),
2.51-2.37 (m, 1H), 2.28 (s, 3H), 2.18
(s, 3H), 2.21-2.17 (m, 3.5H), 1.99-
1.58 (m, 12H), 1.34-1.21 (m, 3H). MS
168
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(ESI): C281-141C1N403requires 516;
found 517 [M+Hr. Isomer 2: 1H
NMR (400 MHz, CDC13): 8.46 (brs,
1H), 7.72-7.65 (m, 1H), 7.11 (brs,
1H), 4.78 (brs, 0.5H), 4.43-4.38 (m,
0.5H), 4.25-4.14 (m, 0.5H), 3.90-3.83
(m, 1H), 3.69-3.60 (m, 1.511), 3.47-
3.30 (m, 4H), 2.98-2.85 (in, 3.511),
2.67-2.60 (m, 1H), 2.47-2.37 (m, 1H),
2.28 (s, 311), 2.18 (s, 3H),2.21-2.18
(m, 3.5H), 2.00-1.48 (m, 12H), 1.34-
1.23 (m, 3H). MS (EST):
C281{0C1N403requires 516; found
517 [M+11]+.
Example 136
(S)-N5-(5-chloro-34(4-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylphenyl)pyridine-2,5-dicarboxamide (E136)
0
H2N
I H
N
0
CI 0
6-Carbamoylnicotinic acid (D122, 100 mg) and DIPEA (0.150 mL) were dissolved
in DMF (3 mL).
To this solution, HATU (217 mg) was added gradually. The reaction mixture was
stirred at RT for
1 hour, then (S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-l-
y1)(cyclopentypmethanone (D157, 100 mg) was added into the mixture, which was
stirred at RT
for 2 days. Water (10 mL) was added, extracted with Et0Ac (2x10 mL), the
organic layer was
dried over Na2SO4. Filtered, the filtrate was concentrated under vacuum. The
residue was purified
by MADP to afford the title compound (30 mg) as white solid. 1H NMR (400 MHz,
DMSO-d6):
10.32 (s, 1H), 9.14 (d, J= 0.9 Hz, 1H), 8.48 (dd, J¨ 8.1, 1.8 Hz, 1H), 8.27
(brs, 1H), 8.18 (d, J-
8.1 Hz, 1H), 7.81 (brs, 1H), 7.43 (d, J= 2.0 Hz, 1H), 7.29 (s, 1H), 4.56 (brs,
0.5H), 4.21 (d, J= 8.6
Hz, 1H), 3.77 (d, J= 12.5 Hz, 0.5H), 3.53-3.41 (m, 2H), 3.19 (t, J= 11.6 Hz,
0.511), 2.92 (quin, J-
7.4 Hz, 1H), 2.84-2.70 (m, 1.5H), 2.64 (d, J= 9.0 Hz, 111), 2.24 (s, 311),
2.18-1.42 (m, 10H), 1.30-
1.04 (m, 3H). MS (ESI): C26H32C1N503 requires 497; found 496 EM-11I.
Examples 137-138
Examples 137-138 were prepared using a similar procedure to that described for
E136.
E137 (S)-N5-(344-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-5-
fluoro-2-
methylphenyl)pyridine-2,5-dicarboxamide
169
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
E138 (S)-N5-(5-ehloro-34(4-(2-cyclopropylacety1)-3-methylpiperazin-1-
yl)methyl)-2-
methylphenyl)pyridine-2,5-dicarboxamide
Structure Base/Solvent Characterization
1HNMR (400 MHz, DMSO-d6):
10.58 (s, 1H), 9.20 (s, 11I), 8.54
(d, J= 6.4 Hz, 1H), 8.27 (s, 1H),
8.16 (d, J= 8.4 Hz, 1H), 7.82 (s,
1H), 7.19 (dd, J= 10.0 Hz, 3.2
o Hz, 1H), 7.09 (d, J= 8.8 Hz,
H2N N
111), 4.56 (brs, 0.5H), 4.23-4.19
E137 o Ni"),;;(0 DIPEA/DMF
(m, 1H), 3.78-3.74 (m, 0.5H),
3.50-3.45 (m, 2H), 2.95-2.90 (m,
1H), 2.77-2.74 (m, 1H), 2.69-
2.63 (m, 1H), 2.21 (s, 3H), 2.17-
1.49 (m, 11H), 1.26-1.04 (m,
3H). MS (ESI): C26H32FN503
requires 481; found 482 [M+11]+.
111 NMR (400 MHz, DMSO-d6):
10.33 (s, 1H), 9.14 (s, 111), 8.48
(dd, J= 8.0, 1.8 Hz, 1H), 8.29
(brs, 1H), 8.18 (d, J= 8.3 Hz,
1H), 7.82 (brs, 1H), 7.43 (d, J=
1.8 Hz, 1H), 7.29 (s, 111), 4.56
(brs, 0.511), 4.20 (d, .1= 12.5 Hz,
0
H2N 0.5H), 4.06 (brs, 0.5H), 3.63 (d,
E138 I
N
N DIPEA/DMF J=13.6 Hz, 0.511), 3.53 - 3.41
a *
(m, 2H), 3.19 (t, J= 11.8 Hz,
0.511), 2.83-2.69 (m, 1.5H), 2.63
(d, J= 11.0 Hz, 111), 2.41-1.83
(m, 7H), 1.29-1.06 (m, 311),
1.01-0.87 (m, 1H), 0.49-0.37 (m,
2H), 0.16-0.03 (m, 2H). MS
(ESI): C25H30C1N503 requires
483; found 484 [M+H]+.
Example 139
170
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-N5-(34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-yflmethyl)-5-fluoro-2-
methylpheny1)-N2-methylpyridine-2,5-dicarboxamide, Trifluoroacetic acid salt
(E139)
0
I
n Nacx).`sµ
0
= TFA
(S)-54(34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-5-fluoro-2-
methylphenyl)carbamoyl)picolinic acid, Trifluoroacetic acid salt (11258, 80
mg) and DIPEA (0.070
mL) were dissolved in DMF (3 mL). To this solution, HATU (82 mg) was added
gradually. The
reaction mixture was stirred at RT for 10 min. Then methanamine, hydrochloride
acid salt (14.49
mg) was added into the mixture, which was stirred at RT overnight. Water (10
mL) was added,
extracted with Et0Ac (2x10 mL), the organic layer was dried over Na2SO4.
Filtered, the filtrate
.. was concentrated under vacuum. The residue was purified by MADP to afford
the title compound
(27 mg) as white solid. 1H NMR (400 MHz, Me0D-d4): 9.17 (d, J= 1.6 Hz, 1H),
8.49 (dd, J= 8.1,
2.2 Hz, 111), 8.28-8.17 (m, 1H), 7.41 ¨7.33 (m, 2H), 4.98 (brs, 0.5H), 4.67
(brs, 1H), 4.45 (brs, 2H),
4.20 (d, J= 13.1 Hz, 0.5H), 3.66-3.37 (m, 2.5H), 3.29-3.01 (m, 3.5H), 3.00 (s,
3H), 2.35 (s, 311),
2.03-1.55 (m, 8H), 1.50-1.20 (m, 3H). '9F NMR (376 MHz, Me0D-d4): -77.2, -
116.9. MS (ESI):
.. C271134FN503 requires 495; found 496 [M+Hr.
Example 140
(S)-N-(5-cyano-34(4-(cyclobutanecarbony1)-3-methylpiperazin-1-Amethyl)-2-
methylphenyly
6-methylnicotinamide (E140)
H
0 lip INyCi
NI I 0
To a solution of (S)-4-(5-cyano-2-methy1-3-(6-methylnicotinamido)benzy1)-2-
methylpiperazin-1-
ium, 2 hydrochloride acid salt (I)245, 50 mg) and triethylamine (55.5 mg) in
DCM (4 mL),
cyclobutanecarbonyl chloride (32.5 mg) was added at RT. The reaction mixture
was stirred for 1
hour at RT. Solvent was removed under vacuum, the crude product was purified
by MDAP to
afford the title compound (26.3 mg). 1H NMR (400 MHz, DMSO-d6): 10.22 (s, 1H),
9.03 (s, 1H),
.. 8.21 (d, J= 8.3 Hz, 1H), 7.77 (s, 1H), 7.63 (s, 1H), 7.44 (d, J= 7.8 Hz,
1H), 4.51 (brs, 0.511), 4.16
(d, J= 12.3 Hz, 0.5H), 3.91 (brs, 0.511), 3.56-3.40 (m, 2.5H), 3.11 (t, J=
12.8 Hz, 0.511), 2.84-2.58
(m, 3.5H), 2.56 (s, 3H), 2.32 (s, 3H), 2.25-1.65 (m, 8H), 1.24-1.07 (m, 3H).
MS (ESI): C26H31N502
requires 445; found 446 [M+H].
Examples 141-145
171
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Examples 141-145 were prepared using a similar procedure to that described for
E140, with the
specified reaction base or solvent listed in the table.
E141 (S)-N-(5-cyano-344-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-
2-
methylpheny1)-2-methylpyrimidine-5-carboxamide
E142 (S)-3-cyano-N-(5-cyano-34(4-(cyclopentanecarbony1)-3-methylpiperazin-l-
ypmethyl)-2-
methylphenyObenzamide
E143 (S)-5-chloro-N-(5-fluoro-344-isobutyry1-3-methylpiperazin-l-yl)methyl)-2-
methylpheny1)-
6-methylnicotinamide
E144 (S)-N-(3-((4-butyry1-3-methylpiperazin-1 -ypmethyl)-5-fluoro-2-
methylpheny1)-5-chloro-6-
methylnicotinamide
E145 (S)-5-chloro-N-(5-chloro-34(4-(cyclopropanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylpheny1)-6-methylnicotinamide
Structure Base/Solvent Characterization
1H NMR (400 MHz, DMSO-d6):
10.39 (s, 1H), 9.20 (s, 2H), 7.81
(d, J = 1.6 Hz, 1H), 7.66 (s, 1H),
4.56 (brs, 0.5H), 4.23-4.20 (m,
1H), 3.80-3.70 (m, 0.5H), 3.56-
Ti H
E141 ioL 1)0 TEA/DCM 3.48 (m, 2H), 3.25-3.15 (m,
0.5H), 2.95-2.91 (m, 1H), 2.85-
II
2.62 (m, 5.5H), 2.35 (s, 3H),
2.25-1.45 (m, 10H), 1.25-1.15
(m, 3H). MS (ESI): C261-I32N602
requires 460; found 461
[M+Hr.
11-1 NMR (400 MHz, Me0D-d4):
8.25 (s, 1H), 8.18 (d, J = 7.9 Hz,
1H), 7.88 (d, J = 7.8 Hz, 1H),
7.71-7.57 (m, 2H), 7.54 (s, 1H),
N --
E142 N'.=cro
TEA/DCM 4.64-4.52 (m, 0.5H), 4.30-4.09
(m, 1H), 3.76 (d, J = 13.2 Hz,
0.5H), 3.59-3.38 (m, 2H), 3.33-
3.24 (brs, 0.5H), 3.02-2.50 (m,
3.5H), 2.32 (s, 3H), 2.21-1.41
172
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(m, 10H), 1.31-1.09 (m, 3H).
MS (ES!): C28H31N502 requires
469; found 470 [M+H].
111 NMR (400 MHz, Me0D-
d4): 8.94 (d, J= 2 Hz, 1H),
8.37 (d, J= 1.6 Hz, 1H),7.15-
7.09 (m, 2H), 4.69 (s, 0.5H),
4.36-4.26 (m, 111), 3.85-3.81
ciNlN11 (m, 0.5H), 3.56-3.50 (m, 2H),
o r,
E143 D1PEA/DCM 3.44-3.39 (m, 0.5H), 3.01-2.86
(m, 2.5H), 2.80-2.71 (m, 4H),
2.30 (s, 3H), 2.28-1.99 (m,
2H), 1.39-1.24 (m, 3H), 1.19-
0.91 (m, 611). MS (EST):
C241130C1FN402 requires 460;
found 461 [M+H].
-i.)uyN DTPEA/DCM 11-INMR (400 MHz, Me0D-
c Ie'issµ 4): 8.94 (d, J= 1.2 Hz, 1H),
F
8.37 (d, J= 1.6 Hz, 1H), 7.15-
0
7.09 (m, 2H), 4.69(brs, 0.5H),
4.36-4.20 (m, 1H), 3.76-3.73
(m, 0.5H), 3.56-3.50 (m, 2H),
3.44-3.39 (m, 0.5H), 3.01-2.94
E144 (m, 0.5H), 2.88-2.85 (m, 111),
2.78-2.71 (m, 4H), 2.50-2.32
(m, 2H), 2.30 (s, 3H), 2.28-
1.99 (m, 3H), 1.68-1.60 (m,
2H), 1.39-1.24 (m, 3H), 1.01-
0.91 (m, 3H). MS (ESI):
C241130C1FN402 requires 460;
found 461 [M+H]+.
H
DIPEA/DCM 1H NMR (400 MHz, Me0D-
--
N
CI
0 1./ 4): 8.95 (d, J= 1.6 Hz, 1H),
E145 8.37 (d, J= 1.6 Hz, 1H), 7.38-
a
7.32 (dd, J= 19.2, 2.0 Hz, 2H),
4.66 (brs, 0.5H), 4.54 (brs,
173
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
0.5H), 4.29-4.10 (m, 1H),
3.56-3.50 (m, 2.511), 3.04-2.97
(m, 0.5H), 2.87 (br, 1H), 2.80-
2.71 (m, 4H), 2.30 (s, 3H),
2.21-1.95 (m, 3H), 1.39-1.24
(m, 3H), 0.89-0.81 (m, 4H).
MS (EST): C241128C12N402
requires 474; found 475
[M+1-1]+.
Example 146
(S)-N-(5-cyano-3-44-(2-cyclopropylacety1)-3-methylpiperazin-1-yflmethyl)-2-
methylpheny1)-
5-fluoro-6-methylnicotinamide, Trifluoroacetic acid salt (E146)
I NH
= TFA
0
I I 0
To a suspension of 5-fluoro-6-methylnicotinic acid (D113, 57.0 mg) in DCM (10
mL), oxalyl
dichloride (0.048 mL) was added dropwise. The reaction mixture was stirred at
RT under nitrogen
for 2 hours. Solvent was removed by rotavap, then re-dissolved with DCM (1
mL), added to a
solution of (S)-3-amino-5-04-(2-cyclopropylacety1)-3-methylpiperazin-l-
ypmethyl)-4-
methylbenzonitrile (D174, 80 mg) and DIPEA (0.128 mL) in DCM (10 mL). The
reaction mixture
was stirred at RT overnight. Diluted with DCM (20 mL) then washed with brine
(20 mL). DCM
layer was separated, dried over MgSO4 and filtered. The filtrate was
concentrated and purified by
MADP to afford the title compound (51 mg) as white solid. 111NMR (400 MHz,
Me0D-d4): 8.88
(s, 111), 8.09 (dd, J= 9.7, 1.7 Hz, 1H), 7.86 (s, 111), 7.85 (s, 111), 4.59
(brs, 111), 4.31 (brs, 2H),
3.99 (brs, 1H), 3.71-3.32 (m, 2H), 3.23-2.68 (m, 311), 2.61 (d, J= 2.8 Hz,
3H), 2.53-2.15 (m, 511),
1.48-1.22 (m, 3H), 1.07-0.93 (m, 111), 0.60-0.49 (m, 211), 0.23-0.13 (m, 2H).
19F NMR (376 MHz,
Me0D-d4): -77.4, -125.2. MS (ESI): C26H30FN502 requires 463; found 464 [M+H].
Examples 147-148
Examples 147-148 were prepared using a similar procedure to that described for
E146.
E147 (S)-N-(5-cyano-3-44-(2-cyclopropylacety1)-3-methylpiperazin-1-y1)methyl)-
2-
methylpheny1)-6-(fluoromethypnicotinamide, Trifluoroacetic acid salt
E148 (S)-N-(344-butyry1-3-methylpiperazin-l-yl)methyl)-5-chloro-2-
methylpheny1)-5-fluoro-6-
methylnicotinamide
174
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Structure Base/Solvent Characterization
'H NMR (400 MHz, Me0D-d4):
9.13 (d, J= 1.6 Hz, 1H), 8.46
(dd, J= 8.2, 2.1 Hz, 1H), 7.88 (s,
1H), 7.85 (s, 1H), 7.72 (d, J-
8.2 Hz, 1H), 5.57 (d, J= 46.8
Hz, 211), 5.10-4.91 (in, 0.5 H),
4.75-4.41 (m, 1H), 4.32 (brs,
E147
F Cll., 11
N D1PEA/DCM
= TFA 2H),
3.99 (brs, 0.5H), 3.55 (brs,
117 o
0.5H), 3.36 (brs, 1H), 3.23-2.65
I I
(m, 311), 2.46 (s, 3H), 2.34 (brs,
2H), 1.54-1.21 (m, 311), 1.11-
0.87 (m, 111), 0.64-0.41 (m, 2H),
0.28-0.11 (m, 2H). '9F NMR
(376 MHz, Me0D-d4): -78.9.
MS (ESI): C261130FN502 requires
463; found 464 [M+11]+.
NMR (400 MHz, Me0D-d4):
8.88 (s,1H), 8.10-8.07 (dd, J=
9.6, 1.6 Hz 1H), 7.38-7.32 (dd, J
= 20, 2.4 Hz, 2H), 4.69 (m,
0.5H), 4.35-4.19 (m, 1H), 3.76-
3.73 (m, 0.5H), 3.56-3.48 (m,
2H), 3.42-3.36 (m, 0.5H), 2.99-
2.93 (m, 0.511), 2.87-2.85 (m,
E148 DIPEA/DCM 111), 2.77-2.72 (m, 1H), 2.62-
2.61 (m, 3H), 2.49-2.34 (m, 2H),
2.32 (s, 3H), 2.29-1.99 (m, 211),
1.70-1.64 (m, 2H), 1.36-1.24 (in,
3H), 1.00-0.96 (m, 3H). I9F
NMR (376 MHz, Me0D-d4): -
127.2. MS (ESI):
C241130CIFN402 requires 460;
found 461 [M+11]+ .
Example 149
175
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
(S)-N-(5-cyano-3-04-(cyclopentanecarbony1)-3-methylpiperazin-1-yl)methyl)-2-
methylpheny1)-3-(difluoromethyDbenzamide (E149)
0
I 0
To a solution of (S)-3-amino-5-44-(cyclopentanecarbony1)-3-methylpiperazin-1-
ypmethyl)-4-
.. methylbenzonitrile (D173, 330 mg) and triethylamine (98 mg) in anhydrous
DCM (3 mL) was
added the solution of 3-(difluoromethypbenzoyl chloride (D127, 185 mg) in
anhydrous DCM (3
mL) dropwise. The reaction mixture was stirred for 1 hour at RT. After the
reaction completed, the
mixture was washed with brine, and the organic layer was concentrated to give
the crude product
which was purified by MDAP to afford the title compound (260 mg) as white
solid.1H NMR (400
MHz, DMSO-d6): 10.30 (s, 1 H), 8.19-8.16 (m, 2 H), 7.82 (d, J= 7.6 Hz, 1 H),
7.77 (d, J= 1.6 Hz,
1 H), 7.71 (t, J= 7.6 Hz, 1H), 7.64 (s, 1H), 7.16 (t, J= 55.6 Hz, 1H), 4.56
(brs, 0.5H), 4.20 (brs,
1H), 3.80-3.70 (m, 0.5H), 3.55-3.47 (m, 2H), 3.20-3.15 (m, 0.5H), 2.95-2.90
(m, 1 H), 2.82-2.72 (m,
1H), 2.66-2.60 (m, 1.5 H), 2.33 (s, 3 H), 2.27-1.80 (m, 2H), 1.75-1.48 (m,
8H), 1.26-1.15 (m, 3H);
19F NMR (376 MHz, DMSO-d6): -109.9, -110.1. MS (ESI): C28H32P2N402requires
494; found 495
[M+H] .
Examples 150-151
Examples 150-151 were prepared using a similar procedure to that described for
E149, with the
specified reaction base or solvent listed in the table.
E150 (S)-N-(5-cyano-344-(cyclopentanecarbony1)-3-methylpiperazin-l-y1)methyl)-
2-
methylpheny1)-2-(difluoromethyl)isonicotinamide, trifluoroacetic acid salt
E151 (S)-5-chloro-N-(344-(2-cyclopropylacety1)-3-methylpiperazin-l-y1)methyl)-
5-fluoro-2-
methylpheny1)-6-methylnicotinamide
Structure Base/Solvent Characterization
N H TEA/DCM 1H NMR (400 MHz, DMSO-d6):
F Ns N ;,,,c) = TFA
F 0 10.64 (brs, 1H), 8.94 (d, J=
4.8
Hz, 1H), 8.20 (s, 1H), 8.09 (d, J=
3.2 Hz, 1H), 8.00-7.80 (m, 2H),
7.11 (t, J= 54.8 Hz, 1H), 4.80-
E150 4.15 (brs, 1.5H), 3.60-3.10
(brs,
3H), 3.00-2.51 (m, 3.5H), 2.37 (s,
3H), 2.30-2.02 (m, 1.5H), 1.82-
1.50 (m, 8.5H), 1.30-1.16 (m, 3H);
19F NMR (376 MHz, DMSO-d6): -
176
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
74.3, -115.8, -115.9. MS (ESI):
C27H31F2N502 requires 495; found
496 [M+H].
DMAP/DCM IH NMR (400 MHz, Me0D-d4):
H
ri& 8.94 (d, J= 1.2 Hz, 1H), 8.36
(s,
o L.õ.11
1H), 7.14-7.07 (m, 2H), 4.69 (m,
8 v
0.511), 4.33 (d, J= 14 Hz, 0.5H),
4.15 (brs, 0.511), 3.74 (brs, 0.511),
3.65-3.48 (m, 2H), 3.44-3.33 (m,
0.5H), 2.96-2.98 (m, 0.5H), 2.88-
E151 2.90 (m, 0.511), 2.75 (d, J= 11.2
Hz, 1H), 2.73-2.70 (m, 3.5H),
2.47-2.42 (m, 0.5H), 2.32-2.18 (m,
5.5H), 2.13-2.02 (m, 1H), 1.36-
1.25 (m, 311), 1.02-0.99 (m, 111),
0.53-0.55 (m, 2H), 0.17-0.20 (m,
2H). MS (ESI): C251130C1FN402
requires 472; found 473 [M+H].
Examples 152&153
(cis) N-(5-ehloro-34(S)-4-(cyclopentanecarbony1)-3-methylpiperazin-l-
y1)methyl)-2-
methylpheny1)-2-(2-hydroxypropan-2-y1)cyclopropanecarboxamide & (trans) N-(5-
chloro-3-
WS)-4-(cyclopentanecarbony1)-3-methylpiperazin-1-Amethyl)-2-methylpheny1)-2-(2-
hydroxypropan-2-y1)cyclopropaneearboxamide (E152&E153)
th-
0 ir CI NoNyo
OH 0 OH 0
0
CI 0
To the mixture of methyl 245-chloro-34(S)-4-(cyclopentanecarbony1)-3-
methylpiperazin-l-
yOmethyl)-2-methylphenypcarbamoyl)cyclopropaneearboxylate (D195, 100 mg) in
THF (20 mL)
was added methylmagnesium chloride (3 M in ether, 0.210 mL) during 20 minutes
at -50 C. The
reaction mixture was warmed to 0 C and stirred for 3 hours at 0 C. The
reaction mixture was
quenched with water (1 mL) and then filtered. The filtrate was concentrated to
give the crude
product, which was purified by chromatography on silica gel (200-300 mesh,
eluting with
petroleum ether/Et0Ac 1:1) to afford a yellow solid, which was purified by
preparative chiral to
give the title compounds (10 mg and 7 mg). Isomer 1: iHNMR (500 MHz, Me0D-d4):
7.34 (brs,
111), 7.21 (s, 111), 4.68 (brs, 0.5H), 4.32-4.24 (m, 111), 3.86-3.83 (m,
0.511), 3.52-3.44 (m, 3H),
177
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
3.05-2.95 (m, 2H), 2.85-2.81 (m, 1H), 2.73-2.71 (m, 1H), 2.28 (s, 311), 2.25-
2.15 (m, 1H), 2.05-
1.99 (m, 111), 1.91-1.52 (m, 1111), 1.36-1.18 (m, 10H), 1.10-1.02 (m, 1H). MS
(ESI): C26H38C1N303
requires 475; found 476 LM+11r. Isomer 2: 'H NMR (500 MHz, Me0D-d4): 7.34 (d,
J= 1.6 Hz,
1H), 7.22 (s, 1H), 4.68 (brs, 0.5H), 4.34-4.31 (m, 111), 3.86-3.83 (m, 0.511),
3.52-3.44 (m, 2H),
3.04-3.00 (m, 1H), 2.85-2.80 (m, 1H), 2.75-2.70 (m, 1H), 2.28 (s, 3H), 2.21-
2.15 (m, 1H), 2.05-
2.00 (m, 1H), 1.92-1.61 (m, 9H), 1.55-1.53 (m, 1H), 1.28-1.18 (m, 11H), 1.10-
1.02 (m, 211). MS
(ESI): C26H38C1N303, equires 475; found 476 [M+H]t
FAam 11.54
(S)-N1-(5-ehloro-344-(cyclopentanecarbony1)-3-methylpiperazin-1-y1)methyl)-2-
methylpheny1)-N4,N4-dimethylsuccinamide (E154)
0 N01;
To a solution of (S)-(4-(3-amino-5-chloro-2-methylbenzy1)-2-methylpiperazin-l-
y1)(cyclopentyl)methanone (D157, 400 mg) in DCM (10 mL), succinic anhydride
(129.1 mg) and
N,N-dimethylpyridin-4-amine (13.97 mg) were added. The reaction mixture was
stirred for 6 days.
The mixture was washed with water (5 mL), and the organic layer was
concentrated to dryness,
giving the crude (S)-4-45-chloro-3-44-(cyclopentanecarbonyl)-3-methylpiperazin-
l-yOmethyl)-2-
methylphenypamino)-4-oxobutanoic acid (490.3 mg). To a solution of (S)-445-
chloro-3-44-
(cyclopentanecarbony1)-3-methylpiperazin-l-ypmethyl)-2-methylphenypamino)-4-
oxobutanoic
acid (153.9 mg) in anhydrous DMF (5 mL), dimethylamine, hydrochloride acid
salt (84.3 mg) and
HATU (227.9 mg) was added, followed by DIPEA (0.25 mL). The resulting reaction
mixture was
stirred overnight at RT. The mixture was sent to MDAP for purification (basic
elution), giving the
title compound (100.7 mg) as white solid. 'H NMR (500 MHz, Me0D-d4): 7.38 (s,
1H), 7.19 (s,
111), 4.66 (brs, 0.511), 4.36-4.21 (m, 1 H), 3.83 (d, J= 13.2 Hz, 0.511), 3.52-
3.40 (m, 211), 3.39-3.33
(m, 0.511), 3.09 (s, 311), 3.05-2.97 (m, 1.5H), 2.95 (s, 311), 2.82 (d, J=
11.0 Hz, 1H), 2.78-2.67 (m,
511), 2.26 (s, 3H), 2.22-2.12 (m, 1H), 2.07-1.95 (m, 111), 1.92-1.54 (m, 8H),
1.39-1.15 (m, 3H). MS
(EST): C25H37C1N403 requires 476; found 477 [M H].
Example 155
(S)-N-(5-chloro-34(4-(cyclopentaneearbony1)-3-methylpiperazin-1-ypmethyl)-2-
methylpheny1)-4-oxo-4-(pyrrolidin-l-y1)butanamide (E155)
CiN'TINH NCrcro
N
E155 was prepared using a similar procedure to that described for E154. 'H NMR
(500 MHz,
Me0D-d4): 7.38 (s, 1H), 7.19 (s, 111), 4.66 (brs, 0.5H), 4.37-4.20 (m, 111),
3.83 (d, J= 13.2 Hz,
0.5H), 3.54 (t, J= 6.7 Hz, 2H), 3.50-3.36 (m, 4.5H), 3.07-2.90 (m, 1.5 H),
2.82 (d, J= 10.5 Hz, 1H),
178
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
2.78-2.64 (m, 5H), 2.26 (s, 3H), 2.22-2.12 (m, 1H), 2.08-1.55 (m, 13H), 1.39-
1.15 (m, 3H). MS
(ESI): C271139C1N403 requires 501; found 503 [M+H].
Example 156
(S)-N-(5-chloro-34(4-(2,2-difluorobutanoyD-3-methylpiperazin-l-yOmethyl)-2-
methylphenyl)-5-fluoro-6-methylnicotinamide (E156)
I H
F F
0 Lip
CI 0
To a mixture of 2,2-difluorobutanoic acid (50 mg), HATU (229 mg), and DIPEA
(104 mg) in DMF
(5 mL) was added (S)-N-(5-chloro-2-methy1-34(3-methylpiperazin-l-
y1)methyl)pheny1)-5-fluoro-
6-methylnicotinamide (D254, 173 mg). The mixture was stirred at 45 C for 15
hours. The reaction
was quenched with water, extracted with Et0Ac (2x50 mL). The combined organic
layers were
dired over anhydrous sodium sulfate, filtered and concentrated. The residue
was purified by MDAP
to afford the title compound (14 mg). IHNMR (400 MHz, Me0D-d4): 8.89 (s, 1H),
8.09 (dd, J=
9.9, 1.6 Hz, 1H), 7.44-7.27 (m, 2H), 4.65 (brs, 0.5H), 4.49 (brs, 0.511), 4.29
(d, J= 13.6 Hz, 0.5H),
4.07 (d, J= 13.8 Hz, 0.5H), 3.53 (s, 2H), 3.51-3.41 (m, 0.5H), 3.14 (t, J=
12.3 Hz, 0.5H), 2.94-
2.83 (m, 111), 2.78 (d, J= 11.3 Hz, 111), 2.59 (brs, 311), 2.37-2.30 (m, 3H),
2.29-2.02 (m, 411), 1.50-
1.21 (m, 3H), 1.07 (td, J= 7.4, 2.8 Hz, 3H). MS (EST): C24H28C1F3N402requires
496; found 497
[M+H].
Biological Data
As stated above, the compounds according to Formula I are RORy modulators, and
are useful in
the treatment of diseases mediated by RORy. The biological activities of the
compounds according to
Formula I can be determined using any suitable assay for determining the
activity of a candidate
compound as a RORy modulator, as well as tissue and in vivo models.
Fluorescence Energy Transfer (FRET) Assay
The assays were performed in an assay buffer consisting of 50 mM NaF, 50 mM 3-
(N-
morpholino)propanesulfonic acid, pH 7.5, 50 uM 34(3-
cholamidopropyl)dimethylammoniol-
propanesulfonate, 0.1 mg/mL bovine serum albumin, and 10 mM dithiothreitol in
384-well plates
(Greiner 784076, Longwood, FL). The total volume was 10 uL/well. The europium-
labeled SRC1
solution was prepared by adding an appropriate amount of biotinylated SRC and
europium labeled
streptavidin (PerkinElmer Life and Analytical Sciences, Waltham, MA) into
assay buffer, with
final concentrations of 27 and 3.3 riM, respectively. The allophycocyanin
(APC)-labeled-LBD
solution was prepared by adding an appropriate amount of biotinylated RORy-LBD
and APC-
labeled streptavidin (CR130-100; PerkinElmer Life and Analytical Sciences) at
a final
179
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
concentration of 33 nM each. After 15 min of incubation at room temperature, a
20-fold excess of
biotin was added to block theremaining free streptavidin. Equal volumes of
europium-labeled SRC-
and APC-labeled RORy-LBD were then mixed with 0.2 M surrogate agonist N-(2-
chloro-6-
fluorobenzy1)-N42'-methoxy-[1,1'-biphenyl]-4-y1)methyl)benzenesulfonamide
(Zhang, W., et al.,
Mol. Pharmacol. 2012, 82, 583-590) and dispensed into 384-well assay plates at
10 uL
volume/well. The 384-well assay plates had 100 nL of test compound in DMSO
predispensed into
each well. The plates were incubated for 1 h at room temperature and then read
on ViewLux
(PerkinElmer Life and Analytical Sciences) in LANCE mode configured for
europeum-APC labels.
Data were collected and analyzed by Activitybase.
Dual Fluorescence Eneru Transfer (FRET) Assay
This assay is based on the knowledge that nuclear receptors interact with
cofactors
(transcription factors) in a ligand dependent manner. RORy is a typical
nuclear receptor in that it
has an AF2 domain in the ligand binding domain (LBD) which interacts with co-
activators. The
sites of interaction have been mapped to the LXXLL motifs in the co-activator
SRC1(2) sequences.
Short peptide sequences containing the LXXLL motif mimic the behavior of full-
length co-
activator.
The assay measures ligand-mediated interaction of the co-activator peptide
with the purified
bacterial-expressed RORy ligand binding domain (RORy-LBD) to indirectly assess
ligand binding.
RORy has a basal level of interaction with the co-activator SRC1(2) in the
absence of ligand, thus it
is possible to find ligands that inhibit or enhance the RORy/SRC1(2)
interaction.
Materials
Generation of RORy-LBD bacterial expression plasmid
Human RORy Ligand Binding Domain (RORy-LBD) was expressed in E.coli strain
BL21(DE3)
as an amino-terminal polyhistidine tagged fusion protein. DNA encoding this
recombinant protein
was sub-cloned into a modified pET2la expression vector (Novagen). A modified
polyhistidine tag
(MKKHHHHEIILVPRGS) was fused in frame to residues 263-518 of the human RORy
sequence.
Protein Purification
Approximately 50 g E.coli cell pellet was resuspended in 300 mL of lysis
buffer (30 mM
imidazole pH 7.0 and 150 mM NaCl). Cells were lysed by sonication and cell
debris was removed
by centrifugation for 30 minutes at 20,000g at 4 C. The cleared supernatant
was filtered through a
0.45 uM cellulose acetate membrane filter. The clarified lysate was loaded
onto a column (XK-26)
packed with ProBond Nickel Chelating resin (Invitrogen), pre-equilibrated with
30 mM imidazole
pH 7.0 and 150 mM NaCl. After washing to baseline absorbance with the
equilibration buffer, the
column was developed with a gradient from 30 to 500 mM imidazole pH 7Ø
Column fractions
containing the RORy-LBD protein were pooled and concentrated to a volume of 5
mls. The
concentrated protein was loaded onto a Superdex 200 column pre-equilibrated
with 20 mM Tris-Cl
180
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
pH 7.2 and 200 mM NaCl. The fractions containing the desired RORy-LBD protein
were pooled
together.
Protein Biotinylation
Purified RORy-LBD was buffer exchanged by exhaustive dialysis [3 changes of at
least 20
volumes (>8000x)] against PBS [100mM NaPhosphate, pH 8 and 150mM NaCl]. The
concentration of RORy-LBD was approximately 30uM in PBS. Five-fold molar
excess of NHS-
LC-Biotin (Pierce) was added in a minimal volume of PBS. This solution was
incubated with
occasional gentle mixing for 60 minutes at ambient RT. The modified RORy-LBD
was dialyzed
against 2 buffer changes - TBS pH 8.0 containing 5mM DTI', 2mM EDTA and 2%
sucrose - each
at least 20 times of the volume. The modified protein was distributed into
aliquots, frozen on dry
ice and stored at -80 C. The biotinylated RORy-LBD was subjected to mass
spectrometric analysis
to reveal the extent of modification by the biotinylation reagent. In general,
approximately 95% of
the protein had at least a single site of biotinylation and the overall extent
of biotinylation followed
a normal distribution of multiple sites ranged from one to five. A
biotinylated peptide
corresponding to amino acid 676 to 700 (CPSSHSSLTERHKILHRLLQEGSPS) of the co-
activator
steroid receptor coactivator SRC1(2) was generated using similar method.
Assay
Preparation of Europium labeled SRC I (2) peptide: biotinylated SRC1(2)
solution was prepared
by adding an appropriate amount of biotinylated SRC1(2) from the 100uM stock
solution to a
buffer containing 10 mM of freshly added DTT from solid to give a final
concentration of 40 nM.
An appropriate amount of Europium labeled Streptavidin was then added to the
biotinylated
SRC1(2) solution in a tube to give a final concentration of 10 nM. The tube
was inverted gently and
incubated for 15 minutes at room temperature. Twenty-fold excess biotin from
the 10 mM stock
solution was added and the tube was inverted gently and incubated for 10
minutes at room
temperature.
Preparation of APC labeled RORy-LBD: biotinylated RORy-LBD solution was
prepared by
adding an appropriate amount of biotinylated RORy-LBD from the stock solution
to a buffer
containing 10 mM of freshly added DTT from solid to give a final concentration
of 40 nM. An
appropriate amount of APC labeled Streptavidin was then added to the
biotinylated RORy-LBD
solution in a tube to give a final concentration of 20 nM. The tube was
inverted gently and
incubated for 15 minutes at room temperature. Twenty-fold excess biotin from
the 10 mM stock
solution was then added and the tube was inverted gently and incubated for 10
minutes at room
temperature.
Equal volumes of the above-described Europium labeled SRC1(2) peptide and the
APC labeled
RORy-LBD were gently mixed together to give 20nM RORy-LBD, lOnM APC-
Strepavidin, 20nM
SRC1(2) and 5nM Europium-Streptavidin. The reaction mixtures were incubated
for 5 minutes.
Using a Thermo Combi Multidrop 384 stacker unit, 25 ul of the reaction
mixtures per well was
181
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
added to the 384-well assay plates containing lul of test compound per well in
100% DMSO. The
plates were incubated for lhr and then read on ViewLux in Lance mode for
EU/APC.
Jurkat Cell Luciferase Assay
RORy is known to bind to a CNS (conserved non-coding sequences) enhancer
element in the
IL17 promoter. In this assay, RORy activity is indirectly assessed using a
luciferase reporter
construct which contains the human 11,17 promoter having the RORy-specific CNS
enhancer
element. Inhibition of RORy activity by a compound will result in a decrease
in luciferase activity
of Jurkat cells transfected with the reporter construct.
Materials
Jurkat cell line
For the luciferase reporter plasmid, the 3 Kb human IL17 promoter containing
the RORy-
specific CNS enhancer element was PCR amplified from human genomic DNA and
cloned into a
pGL4-Luc2/hygro reporter plasmid sequencially as XhoI-HindIll (1.1 Kb) and
Kpnl-XhoI (1.9 Kb)
fragments. For the 1.1 Kb fragment, PCR was used to amplify human 1L17
proximal promoter
region from genomic DNA of 293T cells using primers as follows: forward
primer, 5'-
CTCGAGTAGAGCAGGACAGGGAGGAA-3' (XhoI site is underlined) and reverse primer, 5'-
AAGCTTGGATGGATGAGrri __________________________________________________ GTGCCT-
3' (HindlII site is underlined). The 1.1 kb DNA bands
were excised, purified, and inserted into pMD19-T Simple Vector (Takara).
After DNA sequencing
confirmation, the 1.1 kb DNA was digested with XhoI and Hindlil and inserted
into Xhol/HindIll
sites of pGL4.31[1uc2P/GAL4UAS/Hygro] (Promega) to generate the pIL17-1kb-lue
reporter
construct. For the 1.9 Kb fragment, PCR was used to amplify human IL17
promoter region from
genomic DNA using primers as follows: forward primer, 5'-
GGTACCTGCCCTGCTCTATCCTGAGT-3' (Kpnl site is underlined) and reverse primer, 5'-
CTCGAGTGGTGAGTGCTGAGAGATGG-3' (Xhol site is underlined). The resulting 1.9 kb
DNA
bands were excised, gel purified, and cloned into a pMD19-T Simple Vector
(Takara). DNA
sequencing analysis revealed that there were three point mutations but none of
which affected
RORy binding. The 1.9 kb DNA fragment was released by double digestion with
Kpnl and XhoI
and inserted into plL17-1kb-luc to generate the luciferase reporter plasmid
"p1L17-3kb-CNS-luc."
To overexpress RORyt, the full-length cDNA of human RORyt identical to the
published sequence
NM 001001523 was cloned into pcDNA3.1 at the Kpnl-NotI cloning sites to
generate the RORyt
overexpression plasmid "CDNA3.1DhRORy49-8".
The luciferase reporter plasmid and the RORyt overexpression plasmid were
transfected into
Jurkat cell line and a stable clone was identified. The stable clone was grown
in 10% dialyzed FBS
in RPMI (1640) with 800ug/m1 geneticin and 400ug/m1 hygromecin.
Assay
Compounds were dissolved in DMSO at three concentrations, 10mM, 400uM and
16uM, and
were dispensed into 384-wells assay plate at 40n1, 12.5n1, 5n1 respectively.
The volume was
182
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
adjusted with pure DMSO to a give a final uniform volume of 40 nl Jurkat cells
described above
were counted and centrifuged. The growth medium was discarded and the cells
were resuspended
with assay medium (phenol red free RPMI) at 1E-6/ml. Cells were added to each
of the compounds
in the assay plates. Cells were either untreated or treated with CD3
microbeads (Miltenyi Biotec) at
1 ul beads per 500,000 cells. Cells were culture overnight and luciferase
assay (Promega) was
performed. Data were collected by ViewLux (using luciferase greiner 384
setting).
Th17 Cell Differentiation Assay
ELISA
Mouse CD4+ cells were purified using the CD4+ T Cell Isolation II Kit
according to
manufacturer's instructions (Miltenyi Biotec). 96 well plates were pre-coated
with anti-mCD3
antibody. Un-coated wells were used as controls. CD4+ Cells were resuspended
in RPMI 1640
complete medium and were added to the 96-well plates. Cytokine cocktail and
the compound were
then added to the wells. Antibodies and cytokines (all from R&D Systems) used
in the assay were
selected from the following: anti-mCD3; anti-mCD28; anti-mIFNy; anti-mIL4; mIL-
6; mIL-23;
mIL-113; hTGF-131. The culture was incubated at 37 C for 3 days and
supernatants were collected
for ELISA. The IL-17 ELISAs were performed according to manufacturer's
instructions (R&D
Systems). The results were analyzed using Prism software with non-linear
regression to determine
pIC50.
Intracellular staining
The Th17 differentiation culture described above was maintained for 5 days and
cells were
analyzed by IL-17 and IFN-y intracellular staining according to manufacturer's
instructions (BD
Biosciences).
Assay Data
The data described below represents a mean pIC50 value of multiple test
results if the test was
performed more than once. It is understood that the data illustrated below may
have reasonable
variation depending on the specific conditions and procedures used by the
person conducting the
testing.
All exemplified compounds were tested in the FRET assay described above. All
tested
compounds were found to have a pIC50 between 5 and 8. Example 80 was found to
have a pIC50 of
about 7.3.
All exemplified compounds except Examples 4, 25, 37, 39, 47, 48, 50, 54, 58-
60, 62, 64, 75, 84,
90, 94, 97-99, 104, 105, 107, 108, 130, 142, 152, and 153 were tested in the
dual FRET assay
described above. All tested compounds were found to have a pIC50 between 6 and
S. Example 80
was found to have a pIC50 of about 7Ø
183
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
All exemplified compounds except Examples 1-7, 9-22, 27-31, 33-39, 41, 45, 47-
49, 51-54, 59,
60, 63-68, 71-73, 75, 78, 79, 81-85, 87, 88, 90, 91, 96-98, 100-103, 107, 108,
113, 115-121, 123,
126, 132, 133, 135-145 and 147-156 were tested in the Jurkat cell luciferase
assay described above.
All tested compounds were found to have a pIC50 between 5 and 8. Example 80
was found to have
a pIC50 of about 8.2.
All exemplified compounds except Examples 3, 7, 9, 10, 18, 22-24, 30, 31, 36,
38, 39, 41, 47,
66-68, 81, 82, 84, 88, 90, 91, 97, 103, 112, 116, 126, 128, 135, 140, 147,149
and 153 were tested
in the Th17 cell differentiation assay described above. All tested compounds
were found to have a
pIC50 between 5 and 9. Example 80 was found to have a pIC50 of about 8.3.
EAE Studies
Experimental Autoimmune Encephalomyelitis (EAE) is an animal model of multiple
sclerosis.
The ability of a test compound to ameliorate EAE can be measured in the EAE
studies. Wild-type
mice of the C57BL/6 (B6) strain are maintained under pathogen-free conditions.
EAE is induced by
intravenous injections of 100 ng of pertussis toxin (List Biological
Laboratories) and subcutaneous
immunization with an emulsion composed of M0G35_55 peptide (300 jig/mouse) in
PBS and an
equal volume of complete Freund's adjuvant containing 5 mg/ml heat-killed
Mycobacterium
tuberculosis H37Ra (Difco Laboratories) on day 0, followed by another
intravenous injections of
100 ng of pertussis toxin on day 2 as described previously (Wang et al.
(2006)J. Clin. Invest. 116:
2434-2441). For treatment of EAE, each compound or vehicle PBS is given orally
from day 0 at
various doses selected from 3, 10, 30 and 100 mg/kg twice a day. Mice are
scored for disease
severity daily using a EAE scoring system (Wang et al. (2006) J. Clin. Invest.
116: 2434-2441): 0,
no overt signs of disease; 1, limp tail or hind limb weakness but not both; 2,
limptail and
paraparesis (weakness, incomplete paralysis of one or two hind limbs); 3,
paraplegia (complete
paralysis of two hind limbs); 4, paraplegia with forelimb weakness or
paralysis; and 5, moribund
state or death. Clinical score data can be expressed as means S.E.M.
Human peripheral blood CD4+ T cell cultures and cytokine analysis
Human biological samples were cryopreserved human CD4+ T cells purchased
from AllCells, LLC and Stemcell Technologies, Inc. The CD4+ T cells were
differentiated
to the Th17 subtype by culturing for 5 days in tissue culture plates coated
with anti-CD3
antibody (2 n/mL) in Iscove's modified Dulbecco's medium (IMDM) containing 10%
HI-
FBS, 55 ttM 2-mercaptoethanol and soluble anti-CD28 (3 ug/mL) in the presence
of a
Th17 skewing cocktail, including IL-113 (10 ng/mL), IL-6 (30 ng/mL), TGFI3
(0.5 ng/mL),
IL-21 (10 ng/mL), IL-23 (10 ng/mL), anti-IFI\TT (10 ug/mL) and anti-IL-4 (10
ttg/mL). To
examine compound effects on Th17 polarization, freshly thawed CD4+ cells in
IMDM
184
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
supplemented with all Th17 polarization cocktail constituents (above) were
seeded at low
cell density (20,000 cells/well) directly into anti-CD3 coated round bottom 96-
well plates
already containing serially diluted compounds. Cells were incubated
undisturbed for 5
days at 37 C. Immediately following culture, supernatant was analyzed for
secreted IL-
17A and IL-22 protein by MSD electrochemiluminescent cytokine assays
(Mesoscale
Discovery) and ELISA (Quantikine assay, R&D Systems), respectively. Compound
treatment(s) were performed in triplicate. Results are presented as the mean
SD or as
mean percent inhibition ( SD) relative to the response to stimulus alone.
Example 80 as tested in this assay had an ILI 7A pIC50 = 7.54 and Inhibition
Activity (ASSYM
MAX) = 94.
In Vitro Percutaneous Studies
The in vitro percutaneous study is aimed to predict the level of percutaneous
penetration
obtained for a compound in a topical formulation for psoriasis. This assay
coupled with the
intrinsic potency of the compound are used to predict the likelihood of
success of a compound to
engage the target. The higher the ratio of the percutaneous penetration to the
intrinsic potency, the
higher the ratio of local skin concentration to the intrinsic potency and
therefore the higher the
chance of a compound to engage the target in a topical formulation.
The compounds can be manufactured in a modified aqueous cream at pH=6.
Aqueous cream composition
Ingredients % w/w
Cetostearyl alcohol 7.2
Cetomacrogol 1000 1.8
White soft paraffm 15.0
Liquid paraffin 6.0
Water 57.0
Na2HPO4 0.6
Citric Acid 0.2
Propylene Glycol 10.0
Methyl paraben 0.1
Caffeine 0.1
API#1 1.0
API#2 1.0
API#3 1.0
185
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
The study can be conducted with dermatomed abdominal human skin sourced from
three skin
donors using 2cm2 Franz diffusion cells. The receiving fluid consisted of
Bovine serum albumin (4%
w/v) in 0.1% w/v sodium azide in Phospate Buffer Saline and can be heated at
37 C in order to
obtain 32 C at the skin surface. The cream formulation can be applied on the
donor side at a 10 mg
dose, i.e. 5 mg/cm2. The samples can be taken at the following time points:
t=0, 3, 6, 9 and 24 h.
The receiver samples can then be assayed using a method based upon protein
precipitation with
acetonitrile followed by LC/MS/MS analysis. The percutaneous flux (in
ng/cm2/hr) can be
determined using the individual API (in a multiple composition) that has
permeated into the
receiver compartment over 24hrs per cm2.
Iminuimod-induced skin inflammation
Imiquimod is an immune modifying agent that potently activates specific Toll-
like receptors
(e.g., TLR7) and induces irritation/ inflammation of the skin that requires
the IL23R/RORy/IL17
.. axis of the immune system (van der Fits et al, (2009) J Immunol; 182:5836-
5845; Gray eta!, (2013)
Nature Immunol; Jun;14(6):584-92). The imiquimod-induced skin inflammation
model can be used
to assess the ability of an RORy inhibitor to reduce Th17-driven inflammation
in mice. For the ear-
only skin inflammation model in which ear thickness was measured with digital
engineer's calipers
(Mitutoyo PK-0505), female wild type C57BL/6NTac mice obtained from Taconic
(Hudson, NY)
at 8 to 12 wk of age received a daily topical dose of 10 mg of commercially
available imiquimod
cream (5%) (Aldara; Mcdicis) distributed over both ears at approximately
11:00h for up to 4
consecutive days. Alternatively, 72 mg of Aldara was distributed over both
ears and the
shaved/depiliated back skin of mice at approximately 11:00h for 3 consecutive
days to examine
RORy-dependent gene expression (RNA isolated from both ears using Qiazol
followed by clean-up
.. on with the RIVeasy protocol (Qiagen, Germantown, MD); Taqman probe/primer
sets for B2M
(Mm00437762_m1), IL-17A (Mm00439619_m1), IL-17F (Mm00521423_m1), or IL-22
(Mm00444241_ml) (Thermo Fisher Scientific, Inc., Waltham, MA) and ex vivo
stimulated (anti-
CD3 (2 ug/ml, clone eBio500A2, eBioscience, San Diego, CA), anti-CD28 (1
ug/ml, clone 37.51,
BD Bioscience, San Jose, CA), recombinant mouse M-lp (20 ng/ml, R&D Systems,
Minneapolis,
MN), and recombinant mouse IL-23 (20 ng/ml, R&D Systems, Minneapolis, MN) IL-
17A protein
expression from whole blood (Meso Scale Discovery, Rockville, MD). For
treatment of the skin
inflammation in these models, each compound or vehicle (methylcellulose in
water, 1% w/v, Sigma
Aldrich, St. Louis, MO) was administered via oral gavage at approximately
08:00h and 16:00h
daily at various doses selected from 1, 3, 10, and 30 mg/kg.
As shown in the table below, E43 at the 10 and 30mg/kg doses resulted in a
statistically
significant reduction of imiquimod-induced ear thickening (Studies 1-4). In
study B, E43 at 10
186
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
mg/kg significantly reduced IL17F and IL22 mRNA levels in the ear skin, while
in study C, only
the reduction in IL17A mRNA levels reached statistical significance. The ex
vivo whole blood
assay performed as part of study A revealed that E43 at 10mg/kg significantly
reduced the amount
of IL17A protein that is expressed following ex vivo stimulation despite no
significant reduction in
ear mRNA levels of IL17A, IL17F, or IL22.
Cytokine
expression in
ex vivo
Ear thickness Ear Th17 Gene Expression
whole blood
BID following 4 daily applications (number of mRNA copies per
assay from
Dose of 10 mg Aldara to the ears 100 ng of whole ear RNA, mean
Study A
(mg/kg (microns S.E.M., n=8 mice
copy number S.E.M., n=8-10
(mean pg/ml
by oral per group) mice per group)
SE .M.,
gavage)
n=8-10 mice
per group)
Study Study Study Study Study IL- IL-
IL-22 IL-17A
1 2 3 4 17A 17F
2101 59488
6737
\ 5 4189 + 321
9
1561 i 5078
$44.3 301.0 352.5 370.7 1324 43310
9224.
Vehicle () H- + + B 0 not in
study
. 933
27.7 17.7 19,5 14.3. 2182 461.2..
8997 9016
2123
+
2060 13$.3
1681 52579 6547
* 1678
A 1+
314
3028 8614 1239
-== = t .
7129 = ".. t...
244.7 296.6 297.8 . 25610
E43 10 233.3 B 5.612
.. not in study
6.3 1285 840
10.6 16.1 16.5 ." . 3623
4426 978
C 1883 not in
study
+850 202
273
187
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
264 '7 not II riot in to In not in t 11
6 11 0_111
111
111,1% 111, 11,1' ,111,11 ,11111
9.0
14.o
* Designates significant difference (ANOVA with Bonferroni post-test analysis
P<0.05)
Designates significant difference (student's t-test P<0.05)
Methods of Use
The compounds of Formula I, Ia, Formula II, and Formulas ifi ¨IX are all
modulators of
RORy and can be useful in the treatment of diseases mediated by RORy,
particularly autoimmune
or inflammatory diseases. Examples of the inflammatory or autoimmune diseases
of the invention
include multiple sclerosis, rheumatoid arthritis, psoriasis, ankylosing
spondylitis, Crohn's disease,
inflammatory bowel disease, Sjorgen's syndrome, optic neuritis, chronic
obstructive pulmonary
disease, asthma, type I diabetes, neuromyelitis optica, Myasthenia Gavis,
uveitis, Guillain-Barre
syndrome, psoriatic arthritis, Gaves' disease and allergy. Accordingly, in
another aspect the
invention is directed to a method of treating autoimmune and inflammatory
diseases mediated by
RORy with an effective amount of a compound of Formula I, Ia, Formula II, and
Formulas III ¨ IX,
or a pharmaceutically acceptable salt thereof. As used herein in this section
unless spefically
indicated to the contrary, a "compound of Formula I" also refers to compounds
of Formulas Ia,
and Formulas III-DC.
In a further aspect, the present invention provides a compound of Formula I,
or a
pharmaceutically acceptable salt thereof, for use in therapy.
In a further aspect, the present invention provides a compound of Formula I,
or a
pharmaceutically acceptable salt thereof, for use in the treatment of
inflammatory and autoimmune
diseases mediated by RORy.
In a further aspect, the present invention provides a compound of Formula I,
or a
pharmaceutically acceptable salt thereof, for use in the treatment of multiple
sclerosis.
In a further aspect, the present invention provides a compound of Formula I,
or a
.. pharmaceutically acceptable salt thereof, for use in the treatment of of
ankylosing spondylitis.
In a further aspect, the present invention provides a compound of Formula I,
or a
pharmaceutically acceptable salt thereof, for use in the treatment of
psoriasis.
In a further aspect, the present invention is directed to a method of
treatment of an
inflammatory or autoimmune disease mediated by RORy, which comprises
administering to a
mammal, particularly a human, in need thereof, an effective amount of a
compound of Formula I,
or a pharmaceutically acceptable salt thereof.
188
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
In yet a further aspect, the present invention is directed to a method of
treating multiple
sclerosis, which comprises administering to a human in need thereof,
aneffective amount of a
compound of Formula I, or a pharmaceutically acceptable salt thereof.
In yet a further aspect, the present invention is directed to a method of
treating of
ankylosing spondylitis, which comprises administering to a human in need
thereof, an teffective
amount of a compound of Formula I, or a pharmaceutically acceptable salt
thereof.
In yet a further aspect, the present invention is directed to a method of
treating psoriasis,
which comprises administering to a human in need thereof, an effective amount
of a compound of
Formula I, or a pharmaceutically acceptable salt thereof.
In a further aspect, the present invention is directed to the use of a
compound of Formula I,
or a pharmaceutically acceptable salt thereof, in the manufacture of a
medicament for use in the
treatment of an inflammatory or autoimmune disease mediated by RORy.
In a yet further aspect, the present invention is directed to the use of a
compound of
Formula I, or a pharmaceutically acceptable salt thereof, in the manufacture
of a medicament for
use in the treatment of multiple sclerosis.
In a yet further aspect, the present invention is directed to the use of a
compound of
Formula I, or a pharmaceutically acceptable salt thereof, in the manufacture
of a medicament for
use in the treatment of of ankylosing spondylitis.
In a yet further aspect, the present invention is directed to the use of a
compound of
Formula I, or a pharmaceutically acceptable salt thereof, in the manufacture
of a medicament for
use in the treatment of of psoriasis.
In a yet further aspect, the present invention is directed to the use of (S)-N-
(5-chloro-3-44-
(2-cyclopropylacety1)-3-methylpiperazin-1-yOmethyl)-2-methylphenyl)-5-fluoro-6-
methylnicotinamide, or a pharmaceutically acceptable salt thereof, in the
manufacture of a
medicament for use in the treatment of of psoriasis.
In yet a further aspect, the present invention is directed to a method of
treating psoriasis in
a human in need thereof, which comprises administering to said human an
effective amount of (S)-
N-(5-chloro-344-(2-cyclopropylacety1)-3-methylpiperazin-l-y1)methyl)-2-
methylpheny1)-5-
fluoro-6-methylnicotinamide, or a pharmaceutically acceptable salt thereof.
In yet a further aspect, the present invention is directed to a method of
treatment of an
inflammatory or autoimmune disease mediated by RORy, which comprises
administering to a
human in need thereof, an effective amount of (S)-N-(5-chloro-344-(2-
cyclopropylacety1)-3-
methylpiperazin-1-yOmethyl)-2-methylpheny1)-5-fluoro-6-methylnicotinamide, or
a
pharmaceutically acceptable salt thereof.
Another aspect is the use of of (S)-N-(5-chloro-3-04-(2-cyclopropylacety1)-3-
methylpiperazin-l-yOmethyl)-2-methylpheny1)-5-fluoro-6-methylnicotinamide, or
a
pharmaceutically acceptable salt thereof for the treatment of psoriasis.
189
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
Another aspect is the use of of (S)-N-(5-chloro-34(4-(2-cyclopropylacety1)-3-
methylpiperazin-l-yOmethyl)-2-methylphenyl)-5-fluoro-6-methylnicotinamide, or
a
pharmaceutically acceptable salt thereof for the treatment of an inflammatory
or autoimmune
disease mediated by RORy.
As used herein, "treat" in reference to a condition means: (1) to ameliorate
or prevent the
condition or one or more of the biological manifestations of the condition,
(2) to interfere with (a)
one or more points in the biological cascade that leads to or is responsible
for the condition or (b)
one or more of the biological manifestations of the condition, (3) to
alleviate one or more of the
symptoms or effects associated with the condition, or (4) to slow the
progression of the condition or
one or more of the biological manifestations of the condition.
As indicated above, "treatment" of a condition includes prevention of the
condition. The
skilled artisan will appreciate that "prevention" is not an absolute term. In
medicine, "prevention"
is understood to refer to the prophylactic administration of a drug to
substantially diminish the
likelihood or severity of a condition or biological manifestation thereof, or
to delay the onset of
such condition or biological manifestation thereof.
The compounds of the invention may be administered by any suitable route of
administration, including both systemic administration and topical
administration. Systemic
administration includes oral administration, parenteral administration,
transdermal administration,
rectal administration, and administration by inhalation. Parenteral
administration refers to routes
of administration other than enteral, transdermal, or by inhalation, and is
typically by injection or
infusion. Parenteral administration includes intravenous, intramuscular, and
subcutaneous
injection or infusion. Inhalation refers to administration into the human
lungs whether inhaled
through the mouth or through the nasal passages. Topical administration
includes application to
the skin as well as intraocular, otic, intravaginal, and intranasal
administration.
The compounds of the invention may be administered once or according to a
dosing
regimen wherein a number of doses are administered at varying intervals of
time for a given period
of time. For example, doses may be administered one, two, three, or four times
per day. Doses
may be administered until the desired therapeutic effect is achieved or
indefinitely to maintain the
desired therapeutic effect. Suitable dosing regimens for a compound of the
invention depend on the
pharmacokinetic properties of that compound, such as absorption, distribution,
and half-life, which
can be determined by the skilled artisan. In addition, suitable dosing
regimens, including the
duration such regimens are administered, for a compound of the invention
depend on the condition
being treated, the severity of the condition being treated, the age and
physical condition of the
individual being treated, the medical history of the individual to be treated,
the nature of concurrent
therapy, the desired therapeutic effect, and like factors within the knowledge
and expertise of the
skilled artisan. It will be further understood by such skilled artisans that
suitable dosing regimens
190
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
may require adjustment given an individual's response to the dosing regimen or
over time as
individual needs change.
Typical daily dosages may vary depending upon the particular route of
administration
chosen. Typical daily dosages for oral administration range from 0.1 mg to
1000 mg. Typical
daily dosages for topical administration range from about 0.001% to about 10%
w/w (weight
percent) and preferably from about 0.01% to about 1% w/w.
Additionally, the compounds of the invention may be administered as prodrugs.
As used
herein, a "prodrug" of a compound of the invention is a functional derivative
of the compound
which, upon administration to an individual, eventually liberates the compound
of the invention in
vivo. Administration of a compound of the invention as a prodrug may enable
the skilled artisan to
do one or more of the following: (a) modify the onset of the compound in vivo;
(b) modify the
duration of action of the compound in vivo; (c) modify the transportation or
distribution of the
compound in vivo; (d) modify the solubility of the compound in vivo; and (e)
overcome or
overcome a side effect or other difficulty encountered with the compound.
Typical functional
derivatives used to prepare prodrugs include modifications of the compound
that are chemically or
enzymatically cleaved in vivo. Such modifications, which include the
preparation of phosphates,
amides, esters, thioesters, carbonates, and carbamates, are well known to
those skilled in the art.
Compositions
The compounds of the invention will normally, but not necessarily, be
formulated into
pharmaceutical compositions prior to administration to an individual.
Accordingly, in another
aspect the invention is directed to pharmaceutical compositions comprising a
compound of the
invention and one or more pharmaceutically-acceptable excipient.
The pharmaceutical compositions of the invention may be prepared and packaged
in bulk
form wherein a safe and effective amount of a compound of the invention can be
extracted and then
given to the individual such as with powders or syrups. Alternatively, the
pharmaceutical
compositions of the invention may be prepared and packaged in unit dosage form
wherein each
physically discrete unit contains a safe and effective amount of a compound of
the invention.
When prepared in unit dosage form, the pharmaceutical compositions of the
invention typically
contain from 0.1 mg to 1000 mg.
The pharmaceutical compositions of the invention typically contain one
compound of the
invention. However, in certain embodiments, the pharmaceutical compositions of
the invention
contain more than one compound of the invention. For example, in certain
embodiments the
pharmaceutical compositions of the invention contain two compounds of the
invention. In
addition, the pharmaceutical compositions of the invention may optionally
further comprise one or
more additional pharmaceutically active compounds.
191
CA 02950211 2016-11-24
WO 2015/180612
PCT/CN2015/079753
As used herein, "pharmaceutically-acceptable excipient" means a
pharmaceutically
acceptable material, composition or vehicle involved in giving form or
consistency to the
pharmaceutical composition. Each excipient must be compatible with the other
ingredients of the
pharmaceutical composition when commingled such that interactions which would
substantially
reduce the efficacy of the compound of the invention when administered to an
individual and
interactions which would result in pharmaceutical compositions that are not
pharmaceutically
acceptable are avoided. In addition, each excipient must of course be of
sufficiently high purity to
render it pharmaceutically-acceptable.
The compound of the invention and the pharmaceutically-acceptable excipient or
excipients
.. will typically be formulated into a dosage form adapted for administration
to the individual by the
desired route of administration. For example, dosage forms include those
adapted for (1) oral
administration such as tablets, capsules, caplets, pills, troches, powders,
syrups, elixers,
suspensions, solutions, emulsions, sachets, and cachets; (2) parenteral
administration such as sterile
solutions, suspensions, and powders for reconstitution; (3) transdermal
administration such as
transdermal patches; (4) rectal administration such as suppositories; (5)
inhalation such as dry
powders, aerosols, suspensions, and solutions; and (6) topical administration
such as creams,
ointments, lotions, solutions, pastes, sprays, foams, and gels.
Suitable pharmaceutically-acceptable excipients will vary depending upon the
particular
dosage form chosen. In addition, suitable pharmaceutically-acceptable
excipients may be chosen
.. for a particular function that they may serve in the composition. For
example, certain
pharmaceutically-acceptable excipients may be chosen for their ability to
facilitate the production
of uniform dosage forms. Certain pharmaceutically-acceptable excipients may be
chosen for their
ability to facilitate the production of stable dosage forms. Certain
pharmaceutically-acceptable
excipients may be chosen for their ability to facilitate the carrying or
transporting of the compound
or compounds of the invention once administered to the individual from one
organ, or portion of
the body, to another organ, or portion of the body. Certain pharmaceutically-
acceptable excipients
may be chosen for their ability to enhance compliance.
Suitable pharmaceutically-acceptable excipients include the following types of
excipients:
Diluents, fillers, binders, disintegrants, lubricants, glidants, granulating
agents, coating agents,
wetting agents, solvents, co-solvents, suspending agents, emulsifiers,
sweetners, flavoring agents,
flavor masking agents, coloring agents, anticalcing agents, hemectants,
chelating agents, plasticizers,
viscosity increasing agents, antioxidants, preservatives, stabilizers,
surfactants, and buffering agents.
The skilled artisan will appreciate that certain pharmaceutically-acceptable
excipients may serve
more than one function and may serve alternative functions depending on how
much of the
excipient is present in the formulation and what other ingredients are present
in the formulation.
Skilled artisans possess the knowledge and skill in the art to enable them to
select suitable
pharmaceutically-acceptable excipients in appropriate amounts for use in the
invention. In addition,
192
CA 02950211 2016-11-24
WO 2015/180612 PCT/CN2015/079753
there are a number of resources that are available to the skilled artisan
which describe
pharmaceutically-acceptable excipients and may be useful in selecting suitable
pharmaceutically-
acceptable excipients. Examples include Remington's Pharmaceutical Sciences
(Mack Publishing
Company), The Handbook of Pharmaceutical Additives (Gower Publishing Limited),
and The
Handbook of Pharmaceutical Excipients (the American Pharmaceutical Association
and the
Pharmaceutical Press).
The pharmaceutical compositions of the invention are prepared using techniques
and
methods known to those skilled in the art. Some of the methods commonly used
in the art are
described in Remington's Pharmaceutical Sciences (Mack Publishing Company).
In one aspect, the invention is directed to a solid oral dosage form such as a
tablet or
capsule comprising a safe and effective amount of a compound of the invention
and a diluent or
filler. Suitable diluents and fillers include lactose, sucrose, dextrose,
mannitol, sorbitol, starch (e.g.
corn starch, potato starch, and pre-gelatinized starch), cellulose and its
derivatives (e.g.
microcrystalline cellulose), calcium sulfate, and dibasic calcium phosphate.
The oral solid dosage
form may further comprise a binder. Suitable binders include starch (e.g. corn
starch, potato
starch, and pre-gelatinized starch), gelatin, acacia, sodium alginate, alginic
acid, tragacanth, guar
gum, povidone, and cellulose and its derivatives (e.g. microcrystalline
cellulose). The oral solid
dosage form may further comprise a disintegrant. Suitable disintegrants
include crospovidone,
sodium starch glycolate, croscarmelose, alginic acid, and sodium carboxymethyl
cellulose. The
oral solid dosage form may further comprise a lubricant. Suitable lubricants
include stearic acid,
magnesuim stearate, calcium stearate, and talc.
The above description fully discloses the invention including preferred
embodiments thereof.
Modifications and improvements of the embodiments specifically disclosed
herein are within the scope
of the following claims. Without further elaboration, it is believed that one
skilled in the are can, using
the preceding description, utilize the present invention to its fullest
extent. Therefore the Examples
herein are to be construed as merely illustrative and not a limitation of the
scope of the present invention
in any way. The embodiments of the invention in which an exclusive property or
privilege is claimed
are defined as follows.
193