Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
UPGRADING OF HYDROCARBON MATERIAL
FIELD
[0001] The present disclosure relates to the upgrading of hydrocarbon
materials,
including the upgrading of heavy hydrocarbon materials.
BACKGROUND
[0002] Commonly, heavy oils and/or bitumen are difficult to transport from
their
production areas due to their high viscosities at typical handling
temperatures. For example in
Canada, there are typically three specifications that must be met by any oil
to be acceptable for
pipeline transport. The viscosity should be below the maximum viscosity limit
(e.g. <350 cSt at
7.5 C), the density should be below the maximum density limit (e.g. <940 kg=m-
3 at 15.6 C, or
>19 API), and the olefins (including di-olefins) content should be below the
maximum limit
(e.g. < 1 wt% as 1-decene equivalent).
[0003] As is well known, light oils generally have much lower viscosity
values and
therefore flow easier through pipelines than heavy oils. Regardless of the
recovery method used
for their extraction, including costly thermal enhanced oil recovery (EOR)
methods, heavy oils
and bitumen generally need to be diluted by blending the oil with low density
and low viscosity
diluents, typically gas condensate, naphtha and/or lighter oil to make the
heavy oils and/or
bitumen transportable over long distances. For example in Canada, when adding
diluent to
bitumen to produce transportable oil (also known as "DilBit"), the volume of
diluent is typically
30 to 35% of the total product. There are several disadvantages of adding
diluent to bitumen to
produce transportable oil, including:
[0004] Higher capital cost - Well remoteness makes the construction of
pipelines for
sending or returning of the diluents to the bitumen production zone
considerably more
expensive;
[0005] Higher operating cost - The use of diluents constitutes the single
largest
component of the operating cost of running a bitumen extraction facility;
1
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
[0006] Higher capital and operating costs - The added diluent occupies
valuable pipeline
space that otherwise could be used to transport more partially upgraded
bitumen;
[0007] Lower value ¨ product - bitumen sells at a lower price vs. partially
upgraded
bitumen.
[0008] The upgrading of heavy oil and/or bitumen to a product that meets
the
specifications for pipeline transport is known in the industry. Upgrading has
become an attractive
alternative for converting heavy oil and/or bitumen into transportable oil or
oil that requires less
diluent to be transportable, and in some cases upgrading is the only viable
alternative in order to
transport heavy oil to refineries and market places.
[0009] One upgrading approach involves the chemical processing of the heavy
oil and/or
bitumen by a suitable combination of conversion and separation steps. Most
chemical
processing for converting heavy oil and/or bitumen into transportable oil are
cracking based
systems and usually include at least one form of cracking, e.g. thermal
cracking, and at least one
form of hydro-proccssing. The cracking step is employed to reduce the
viscosity and density of
the heavy oil and/or bitumen. The hydro-processing step is employed to reduce
the olefin and
di-olefin content of the heavy oil and/or bitumen.
[0010] Moderate thermal cracking such as visbreaking or more severe thermal
processes
such as coking systems have been proposed in the prior art to reduce the
viscosity and density of
heavy oils and/or bitumen.
[0011] A disadvantage of these processes is the production of cracked
material
comprising olefins and di-olefins. If left untreated, olefins, and more
particularly the more
reactive conjugated C4 and C5 di-olefins (i.e. butadiene, 1,3-pentadiene, 2-
methyl-1,3-butadiene)
may react with each other, with oxygen (such as oxygen in the air) or other
reactive compounds
(e.g. organic acids, carbonyls, amines, etc.), to form long chain polymers
(polymerization
reaction) commonly referred to as gums. Gums of this nature are known to foul
process
equipment. It has been reported in U.S. Patent 6,210,560 assigned to Exxon
Research and
Engineering Company, that these polymerization reactions occur at a
significant rate for
petroleum oils having a di-olefins value per UOP-326 of 4 grams iodine/110
grams oil or greater
2
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
in the temperature range of 232 C to 324 C; particularly 260 C to 304 C. Below
this
temperature range, the reaction rate is too slow for significant
polymerization formation to occur
and above this temperature range the chemical bonds are broken thermally
faster than they are
formed. It has also been reported in U.S. Patent Application Publication No.
2012/0273394 Al
assigned to UOP LLC, that the fouling tendencies of oils having a di-olefins
value per UOP-326
of less than about 2 grams iodine/110 grams oil are deemed acceptable.
[0012] As excessive olefins content in hydrocarbon streams can lead to
fouling of
refining equipment and pipelines, methods to reduce the amount of olefins in a
hydrocarbon
stream are sought in the industry. A particular challenge is to reduce the
amount of olefins in
facilities where it is impractical to supply or generate sufficient
adscititious hydrogen to make
use of hydro-processing.
SUMMARY
[0013] In one aspect, there is provided a process for upgrading a
hydrocarbon material
comprising: treating a hydrocarbon material-comprising feed, wherein the
treating includes
cracking a hydrocarbon material-comprising feed, such that an upgraded
intermediate is
produced; and in the absence, or the substantial absence, of adscititious
diatomic hydrogen,
reducing the content of olefinic material within at least a fraction of the
upgraded intermediate
such that an olefinic material content-reduced product is produced.
[0014] In another aspect, there is provided a process for upgrading a
hydrocarbon
material comprising: treating a hydrocarbon material-comprising feed, wherein
the treating
includes cracking the hydrocarbon material-comprising feed within a reaction
zone, such that an
upgraded intermediate is produced; separating a feed material into at least an
olefin-comprising
treatment fraction and a treatment bypass fraction, wherein the feed material
includes at least a
fraction of the upgraded intermediate reducing the content of olefinic
material within the olefin-
comprising treatment fraction such that an olefin-depleted intermediate is
produced; separating
the treatment bypass fraction into a heavier hydrocarbon material-comprising
fraction and a
lighter hydrocarbon material-comprising fraction; combining at least the
lighter hydrocarbon
material-comprising fraction and the olefin¨depleted intermediate such that an
olefinic material
content-reduced product is produced; producing an upgraded product including
the olefinic
3
material content-reduced product; and supplying at least a fraction of the
heavier hydrocarbon
material-comprising fraction to the reaction zone, such that the hydrocarbon
material-comprising
feed includes at least a fraction of the heavier hydrocarbon material-
comprising fraction.
[0014 a] In a further aspect, there is provided a process for upgrading a
heavy hydrocarbon
feed material to an upgraded product comprising: thermally cracking the heavy
hydrocarbon feed
material within a reaction zone disposed at a temperature of at least 300
degrees Celsius, such
that an upgraded intermediate is produced, separating the upgraded
intermediate into at least an
olefin-comprising treatment fraction and a treatment bypass fraction, wherein,
at a pressure of
one (1) atmosphere, the boiling point range of the olefin-comprising treatment
fraction is
between 25 degrees Celsius and 365 degrees Celsius, reducing the content of
olefinic material
within the olefin-comprising treatment fraction by alkylating the olefin-
comprising treatment
fraction such that an olefin-depleted intermediate is produced; separating the
treatment bypass
fraction into a heavier hydrocarbon material-comprising fraction and a lighter
hydrocarbon
material-comprising fraction; the boiling point range of the lighter
hydrocarbon material-
comprising fraction is between 80 degrees Celsius and 450 degrees Celsius;
combining at least
the lighter hydrocarbon material-comprising fraction and the olefin¨depleted
intermediate such
that an olefinic material content-reduced product is produced; producing the
upgraded product
including the olefinic material content-reduced product; and supplying at
least a fraction of the
heavier hydrocarbon material-comprising fraction upstream the reaction zone,
such that the
heavy hydrocarbon material-comprising feed includes at least a fraction of the
heavier
hydrocarbon material-comprising fraction.
[0014 b] In still another aspect, there is provided a process for upgrading
a heavy
hydrocarbon feed material to an upgraded product comprising: thermally
cracking the heavy
hydrocarbon feed material within a reaction zone disposed at a temperature of
at least 300
degrees Celsius, such that an upgraded intermediate is produced; separating
the upgraded
intermediate into at least an olefin-comprising treatment fraction, a heavier
hydrocarbon
material-comprising fraction, and a lighter hydrocarbon material-comprising
fraction, wherein
the heavy hydrocarbon feed material includes at least a fraction of the
upgraded intermediate,
reducing the content of olefinic material within the olefin-comprising
treatment fraction by
alkylating the olefin-comprising treatment fraction such that an olefin-
depleted intermediate is
3a
Date Recue/Date Received 2020-08-21
produced; wherein, at a pressure of one (1) atmosphere, the boiling point
range of the olefin-
comprising treatment fraction is between 25 degrees Celsius and 365 degrees
Celsius, combining
at least the lighter hydrocarbon material-comprising fraction and the
olefin¨depleted
intermediate such that an olefinic material content-reduced product is
produced; wherein the
boiling point range of the lighter hydrocarbon material-comprising fraction is
between 80
degrees Celsius and 450 degrees Celsius, producing the upgraded product
including the olefinic
material content-reduced product; and supplying at least a fraction of the
heavier hydrocarbon
material-comprising fraction upstream of the reaction zone, such that the
heavy hydrocarbon
feed material includes at least a fraction of the heavier hydrocarbon material-
comprising fraction.
[0014 c] In yet another aspect, there is provided a process for upgrading a
heavy
hydrocarbon feed material to an upgraded product comprising: thermally
cracking the heavy
hydrocarbon feed material in a reaction zone disposed at a temperature of at
least 300 degrees
Celsius, such that an upgraded intermediate is produced, wherein the thermal
cracking is
effected; separating the upgraded intermediate into at least a light olefin-
comprising treatment
fraction and a treatment bypass fraction, wherein the heavy hydrocarbon feed
material includes
at least a fraction of the upgraded intermediate; wherein, at a pressure of
one (1) atmosphere, the
boiling point range of the light olefin-comprising treatment fraction is
between 25 degrees
Celsius and 200 degrees Celsius; reducing the content of light olefinic
material within the olefin-
compri sing treatment fraction by alkylating the olefin-comprising treatment
fraction such that a
light olefinic material-depleted intermediate is produced; combining the light
olefinic material-
depleted intermediate and the treatment bypass fraction so as to produce a
light olefinic material
content-reduced product; and producing the upgraded product including the
light olefinic
material content-reduced product
3b
Date Recue/Date Received 2020-08-21
[0015] In another aspect, there is provided a process for upgrading a
hydrocarbon
material comprising: treating a hydrocarbon material-comprising feed, wherein
the treating
includes cracking the hydrocarbon material-comprising feed within a reaction
zone, such that an
upgraded intermediate is produced; separating a feed material into at least an
olefin-comprising
treatment fraction, a heavier hydrocarbon material-comprising fraction, and a
lighter
hydrocarbon material-comprising fraction, wherein the feed material includes
at least a fraction
of the upgraded intermediate reducing the content of olefinic material within
the olefin-
comprising treatment fraction such that an olefin-depleted intermediate is
produced; combining
at least the lighter hydrocarbon material-comprising fraction and the
olefin¨depleted
intermediate such that an olefinic material content-reduced product is
produced; producing an
upgraded product including the olefinic material content-reduced product; and
supplying at least
a fraction of the heavier hydrocarbon material-comprising fraction to the
reaction zone, such that
the hydrocarbon material-comprising feed includes at least a fraction of the
heavier hydrocarbon
material-comprising fraction.
[0016] In another aspect, there is provided a process for upgrading a
hydrocarbon
material comprising: treating a hydrocarbon material-comprising feed, wherein
the treating
includes cracking the hydrocarbon material-comprising feed, such that an
upgraded intermediate
is produced; separating a feed material into at least a light olefin-
comprising treatment fraction
and a treatment bypass fraction, wherein the feed material includes at least a
fraction of the
upgraded intermediate; reducing the content of light olefinic material within
the olefin-
comprising treatment fraction such that a light olefinic material-depleted
intermediate is
produced; combining the light olefinic material-depleted intermediate and the
treatment bypass
fraction so as to produce a light olefinic material content-reduced product;
and producing an
upgraded product including the light olefinic material content-reduced
product.
[0017] In another aspect, there is provided a process for upgrading a
hydrocarbon
material comprising: supplying a hydrogen donor material to a reaction zone;
supplying a
4
Date Recue/Date Received 2020-08-21
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
hydrocarbon material-comprising feed to the reaction zone; treating the
hydrocarbon material-
comprising feed, wherein the treating includes cracking the hydrocarbon
material-comprising
feed in the presence of a hydrogen donor material within the reaction zone,
such that an
upgraded hydrocarbon material is produced.
BRIEF DESCRIPTION OF DRAWINGS
[0018] The preferred embodiments will now be described with the following
accompanying drawings, in which:
[0019] Figure 1 is a process flow diagram of a process according to one
embodiment;
[0020] Figure 1A is a process flow diagram of a process according to
another
embodiment;
[0021] Figure 2 is a process flow diagram of a process according to
another embodiment;
[0022] Figure 3 is a process flow diagram of a process according to
another embodiment;
[0023] Figure 4 is a process flow diagram of a process according to
another embodiment;
[0024] Figure 5 is a process flow diagram of a process according to
another embodiment;
[0025] Figure 5A is a process flow diagram of a process according to
another
embodiment;
[0026] Figure 6 is a process flow diagram of a process according to
another embodiment;
[0027] Figure 7 is a process flow diagram of a process according to
another embodiment;
[0028] Figure 8 is a process flow diagram of a process according to
another
embodiment;
[0029] Figure 9 is a process flow diagram of a process according to
another embodiment;
[0030] Figure 10 is a process flow diagram of a process according to
another
embodiment;
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
[0031] Figure 11 is a process flow diagram of a process according to
another
embodiment; and
[0032] Figure 12 is a process flow diagram of a process according to
another
embodiment.
DETAILED DESCRIPTION
[0033] The present disclosure relates to the upgrading of hydrocarbon
material. In some
embodiments, for example, the upgrading is of heavy hydrocarbon material.
Exemplary
embodiments may relate to heavy hydrocarbon materials, but it is understood,
unless the context
suggests otherwise, that such embodiments are also applicable to hydrocarbon
materials,
generally.
[0034] As used herein, the following terms have the following meanings:
[0035] "Hydrocarbon" is an organic compound consisting primarily of
hydrogen and
carbon, and, in some instances, may also contain heteroatoms such as sulfur,
nitrogen and
oxygen.
[0036] "Hydrocarbon material" is a material consisting of at least one
hydrocarbon.
[0037] "Heavy hydrocarbon material" is, in some embodiments, for example,
hydrocarbon material that includes at least 10 weight percent of hydrocarbon
material that boils
above 500 C. In some of these embodiments, for example, the heavy hydrocarbon
material is a
hydrocarbon material that includes at least 20 weight percent of hydrocarbon
material that boils
above 500 C. In some of these embodiments, for example, the heavy hydrocarbon
material is a
hydrocarbon material includes at least 40 weight percent of hydrocarbon
material that boils
above 500 C. In some of these embodiments, for example, the heavy hydrocarbon
material is a
hydrocarbon material includes at least 60 weight percent of hydrocarbon
material that boils
above 500 C. In some of these embodiments, for example, the heavy hydrocarbon
material is a
hydrocarbon material includes at least 80 weight percent of hydrocarbon
material that boils
above 500 C. In some of these embodiments, for example, the heavy hydrocarbon
material is a
hydrocarbon material that boils above 500 C.
6
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
[0038] In some embodiments, for example, the heavy hydrocarbon material is
a
hydrocarbon material having an API (American Petroleum Institute) gravity of
less than 22 . In
some embodiments, for example, the heavy hydrocarbon material is a hydrocarbon
material
having an API gravity of less than 20 . In some embodiments, for example, the
heavy
hydrocarbon material is a hydrocarbon material having an API gravity of less
than 150. In some
embodiments, for example, the heavy hydrocarbon material is a hydrocarbon
material having an
API gravity of less than 10 . In some embodiments, for example, the heavy
hydrocarbon
material has an API gravity of less than 5 . In some embodiments, for example,
the heavy
hydrocarbon material is a hydrocarbon material having an API gravity of less
than 0 . In some
embodiments, for example, the heavy hydrocarbon material is a hydrocarbon
material having an
API gravity of less than -5 .
[0039] In some embodiments, for example, the heavy hydrocarbon material
includes, or
in some embodiments, consists of, residuum or resid. Exemplary residuum
includes various
heavy crude and refinery fractions. In this respect, in some embodiments, for
example, the
heavy hydrocarbon material includes, or in some embodiments, consists of,
fresh resid
hydrocarbon feeds, a bottoms stream from any refinery process, such as
petroleum atmospheric
tower bottoms, vacuum tower bottoms, or a bottoms stream from a coker or a
thermal cracking
unit, or a bottoms stream from a fluid catalytic cracking ("FCC") unit
operation or a resid fluid
catalytic cracking (RFCC) unit operation, hydrocracked atmospheric tower,
vacuum tower, FCC,
or RFCC bottoms, straight run vacuum gas oil, hydrocracked vacuum gas oil,
fluid catalytically
cracked slurry oils or cycle oils, as well as other similar hydrocarbon
materials, or any
combination thereof, each of which may be straight run, process derived,
hydrocracked, or
otherwise partially treated (for example, desulfurized). The above-described
heavy hydrocarbon
material may also include various impurities, such as sulphur, nitrogen,
oxygen, halides, and
metals.
[0040] In some embodiments, for example, the heavy hydrocarbon material
includes, or
in some embodiments, consists of, a crude, such as an heavy and/or an ultra-
heavy crude. Crude
refers to hydrocarbon material which have been produced and/or retorted from
hydrocarbon-
containing formations and which has not yet been distilled and/or fractionally
distilled in a
treatment facility to produce multiple components with specific boiling range
distributions, such
7
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
as atmospheric distillation methods and/or vacuum distillation methods.
Exemplary crudes
include coals, bitumen, oil sands, heavy oil or crude oil.
[0041] In some embodiments, for example, the heavy hydrocarbon material is
included
within an asphaltene-comprising heavy hydrocarbon-comprising material includes
an asphaltene
content of less than 40 weight %, based on the total weight of the heavy
hydrocarbon material. In
some of these embodiments, for example, the asphaltene-comprising heavy
hydrocarbon-
comprising material includes an asphaltene content of less than 20 weight %,
based on the total
weight of the heavy hydrocarbon material. In some of these embodiments, for
example, the
asphaltene-comprising heavy hydrocarbon-comprising material includes an
asphaltene content of
less than 15 weight %, based on the total weight of the heavy hydrocarbon
material.
[0042] The term "asphaltenes" as used herein refers to the heaviest and
most polar
molecules component of a carbonaceous material such as crude oil, bitumen or
coal and are
defined as a solubility class of materials that are insoluble in an n-alkane
(usually npentane or n-
heptane) but soluble in aromatic solvents such as toluene. In crude oil,
asphaltenes are found,
along with saturated and aromatic hydrocarbons and resins ("SARA").
Asphaltenes consist
primarily of carbon, hydrogen, nitrogen, oxygen, and sulfur, as well as trace
amounts of
vanadium and nickel. The density is approximately 1.2 g/cc and the hydrogen to
carbon atomic
ratio is approximately 1.2, depending on the asphaltenes source and the
solvent used for
extraction. The asphaltenes fraction is also responsible for a large
percentage of the contaminants
contained in the bitumen (for example Athabasca bitumen is typically 72%-76%
w/w of the
metals, 53%-58% w/w of coke precursors, and 26%-31% w/w of the heteroatoms -
sulphur,
nitrogen and oxygen), making bitumen very challenging to process into clean
and valuable
products.
[0043] In some embodiments, for example, the heavy hydrocarbon material is
included
within deasphalted heavy hydrocarbon-comprising material. In this respect, to
produce
deasphalted heavy hydrocarbon-comprising material, an asphaltene-comprising
heavy
hydrocarbon-comprising material is deasphalted.
[0044] Deasphalting effects production of the deasphalted heavy
hydrocarbon-
comprising material, such that the asphaltene content of the deasphalted heavy
hydrocarbon-
8
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
comprising material is less than the asphaltene content of the asphaltene-
comprising heavy
hydrocarbon-comprising material. In some embodiments, for example, the
deasphalting is
effected by at least a solvent extraction process. In some embodiments, for
example, the
deasphalting is effected by at least a reactive process.
[0045] As alluded to above, in some embodiments, for example, the
deasphalting is
effected by solvent extraction, as is well known in the art, and is described
in, and amongst other
sources, the article by Billon and others published in 1994 in Volume 49, No.
5 of the journal of
the French Petroleum Institute, pages 495 to 507, in the book "Raffinage et
conversion des
produits lourds du petrole [Refining and Conversion of Heavy Petroleum
Products]" by J. F. Le
Page, S. G. Chatila, and M. Davidson, Edition Technip, pages 17-32. In some
embodiments, for
example, the solvent material is a supercritical fluid at the operating
conditions of the zone
within which the solvent material is separated (for recycling and re-use) from
the heavy
hydrocarbon material which it has previously extracted.
[0046] "Olefin" means an unsaturated hydrocarbon containing one or more
carbon-
carbon double bonds that are not part of an aromatic ring, and, for greater
certainty, includes di-
olefins and cyclo-olefins.
[0047] "Olefinic material" means a material consisting of one or more
olefins.
[0048] "Light olefinic material" means any one or both of the following:
(a) olefinic material that has a weight average molecular weight of less
than 150
g/mol; or
(b) olefinic material that has a normal boiling point temperature (i.e.,
boiling point at
one (1) atmosphere) of less than 200 degrees Celsius.
[0049] "Pipeline specification" refers to a characteristic of an oil that
effects whether it
can be transported by pipeline, and varies from jurisdiction to jurisdiction.
In Canada, for
example, there are three critical specifications that must be met by an oil to
be acceptable for
pipeline transport:
9
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
= The viscosity should be below the maximum viscosity limit (e.g. <350 cSt
at the
pipeline reference temperature, which could be as low as 7.5 C during the
winter
months);
= The density should be below the maximum density limit (e.g. <940 kg.m3 at
15.6 C, or >19 API);
= The olefins content should be below the maximum limit (e.g. < 1 wt% as 1-
decene equivalent) per CAPP olefins in crude oil by proton NMR test.
[0050] The
term "fraction of', with reference to a material, means, depending on the
context, one of (a) a portion of the material with the same, or substantially
the same, composition
as the material as a whole, or (b) a portion of the material that is
compositionally different than
the material as a whole, or either one of (a) or (b).
[0051] The
upgrading of the heavy hydrocarbon material includes cracking of the heavy
hydrocarbon material. Cracking refers to any process for breaking down heavier
hydrocarbon
molecules into lighter hydrocarbon molecules. Exemplary methods of cracking
include thermal
cracking, steam cracking, catalytic cracking, and coking. "Thermal cracking"
refers to an
example of a cracking process that uses heat to perform such breaking of
heavier molecules into
smaller ones. Exemplary thermal cracking processes include visbreaking.
In some
embodiments, for example, the cracking is effected within a cracking unit
operation. In some of
these embodiments, for example, the cracking unit operation effects
visbreaking, and includes a
heater and a soaker.
[0052]
Conversion technologies that accomplish cracking may generate olefins. The
presence of excessive quantities of olefins is undesirable. Olefins
(including, in particular, the
more reactive conjugated diolefins) are considered to be deleterious materials
due to their impact
on refineries:
fouling of heaters, heat exchangers and catalyst beds;
negatively impacting the oxidation stability of refined products such as
gasoline,
jet fuel and diesel; and
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
increasing the refinery hydrogen demand.
[0053]
Accordingly, it is desirable to reduce the olefins content of hydrocarbon
material
to achieve appropriate operational and pipeline specifications.
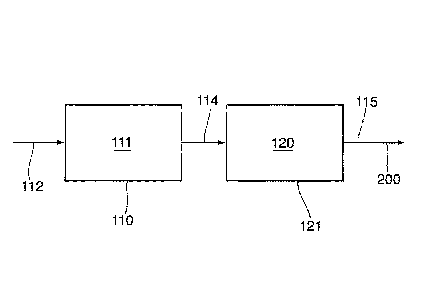
[0054] In
this respect, and referring to Figure 1, the process includes treating a heavy
hydrocarbon material-comprising feed 112. The treating is such that an
upgraded product 200 is
produced. The treating includes cracking the heavy hydrocarbon material-
comprising feed 112
within a reaction zone 111. In some embodiments, for example, the reaction
zone 111 is
disposed at a temperature of at least 300 degrees Celsius, such as, for
example, at least 350
degrees Celsius.
[0055]
Referring to Figure 1A, the heavy hydrocarbon material-comprising feed may be
derived from a heavy hydrocarbon material-comprising supply stream 1012 from
which a portion
has bypassed the process as bypass stream 1014 and then combined with the
upgraded product
20D.
[0056]
Generally, cracking of the heavy hydrocarbon material-comprising feed produces
olefinic material within an upgraded intermediate product 114. In one aspect,
the process further
includes, within a reaction zone 120, effecting a reduction in content of
olefinic material within
the upgraded intermediate 114. In this respect, after the upgraded
intermediate 114 is produced,
a reduction of the content of olefinic material within the upgraded
intermediate 114 is then
effected to produce an olefinic material content-reduced product 115. An
upgraded product 200
is produced including the olefinic material content-reduced product 115.
[0057] In
another aspect, the process further includes creating conditions which
suppress
the formation of olefinic material during the cracking within the reaction
zone 111.
[0058] In
some embodiments, for example, it may be useful to pre-treat the upgraded
intermediate 114, prior to supplying the upgraded intermediate to the reaction
zone 120, such as
by effecting a reduction in the content of di-olefins within the upgraded
intermediate. In some
embodiments, for example, di-olefins (and, particularly, the conjugated
diolefins) are particularly
reactive and may effect fouling of any catalyst material that is disposed
within the reaction zone
120. The pre-treatment may include, for example, catalytic conversion,
adsorption, precipitation,
11
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
or extraction. Where the pre-treatment includes catalytic conversion, the
catalyst material being
used for the pre-treatment may be different than the catalyst material that is
used for effecting, in
the absence, or the substantial absence, of adscititious diatomic hydrogen,
the reducing of the
content of olefinic material within at least a fraction of the upgraded
intermediate. If the pre-
treatment is effected, in the absence, or the substantial absence, of
adscititious diatomic
hydrogen, and results in the reducing of the content of olefinic material
within at least a fraction
of the upgraded intermediate, then the pre-treatment is considered to be
included within the step
of reducing the content of olefinic material within at least a fraction of the
upgraded intermediate
in the absence, or the substantial absence, of adscititious diatomic hydrogen.
(a) Reducing olefinic material content without adscititious diatomic
hydrogen
[0059] In one aspect, the reducing of the content of olefinic material
within at least a
fraction of the upgraded intermediate 114 is effected in the absence, or the
substantial absence,
of adscititious diatomic hydrogen (H2).
[0060] In a related aspect, the reducing of the content of olefinic
material within at least a
fraction of the upgraded intermediate 114 is effected within a reaction zone
120, and the ratio of
the weight of adscititious diatomic hydrogen within the reaction zone 120 to
the weight of
olefinic material within at least a fraction of the upgraded intermediate
within the reaction zone
is less than 0.25, such as, for example, less than 0.1, and includes zero or
substantially zero. In
some embodiments, for example adscititious diatomic hydrogen is absent, or
substantially absent
within the reaction zone 120.
[0061] In some embodiments, for example, the reduction of the content of
olefinic
material within at least a fraction of the upgraded intermediate 114 is
effected by alkylating one
or more aromatic compounds, that are present within the at least a fraction of
the upgraded
intermediate, with the olefinic material. Advantageously, aromatic compounds
are typically
present within heavy hydrocarbon material, and are, therefore, available to
effect conversion of
the olefinic material such that the content of olefinic material within the at
least a fraction of the
upgraded intermediate is reduced. In this respect, the one or more aromatic
compounds with
which the olefinic material participates in the alkylation reaction is present
within the heavy
hydrocarbon material being upgraded. In some embodiments, for example, the one
or more
12
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
aromatic compounds are present within the bitumen or heavy oil from which the
heavy
hydrocarbon material, being upgraded, is derived.
[0062] The alkylation reaction is a reaction comprising the addition of an
olefinic group
to an aromatic group (e.g. the aromatic group is essentially the alkyl
acceptor and would be
available within the hydrocarbon feed). The olefins-aromatics alkylation
reaction produces an
alkylated aromatic compound and effects a reduction in olefinic material
content of the upgraded
intermediate. The reaction can occur without the use of an external source of
olefins, di-olefins,
aromatics and/or hydrogen and, without substantial loss of the volume of
material as compared
to the hydrocarbon feed. The difference between the volume of the reaction
product and the
volume of the upgraded intermediate, whose olefinic material content is being
reduced, is about
0.1% to 10% v/v. Thus, the volume of the product is substantially similar to
the original feed.
The exact volume decrease or increase is dependent on the olefinic material
content of the
upgraded intemediate and other reaction conditions. As would be appreciated,
the olefinic group
that takes part in the reaction may be a sole olefin, or it may be attached to
and/or form part of a
molecule containing at least one other functional group. Similarly, the
aromatic group may he a
sole aromatic hydrocarbon or may be attached to, and/or form part of a
molecule that contains at
least one other functional group.
[0063] In some embodiments, for example, the alkylation is conducted within
a reaction
zone 120 (such as, for example, within a reactor 121) at a pressure and
temperature which
facilitates olefin alkylation with aromatics (olefins-aromatics alkylation).
In this respect, a
fraction of the upgraded intermediate 114, produced by the cracking of the
heavy hydrocarbon
material-comprising feed 112 within the cracking unit operation 110, is
supplied to the reaction
zone 120 so as to effect olefin alkylation with aromatics. In some
embodiments, for example,
the temperature within the reaction zone is below about 380 C. In some
embodiments, for
example, the temperature within the reaction zone ranges from about 50 C to
about 380 C, such
as, for example, from about 150 C to about 350 C. The pressure within the
reaction zone 200 is
such that the reactants and resultant reaction product are disposed in a
liquid, or substantially
liquid, state. While the transition phase from liquid to vapour is pressure
and temperature
dependent, the methods disclosed herein can be carried out within a reaction
zone at a pressure
from about 0 to about 8 MPa, such as for example, from about 2 MPa to about 5
MPa.
13
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
[0064] In some embodiments, for example, the alkylation has a weight
hourly space
velocity of from about 0.01 to about 201-1, such as, for example, from about
0.02 to about 20h-1.
[0065] In some embodiments, for example, the alkylation is catalyzed with
a catalyst
material disposed within the reaction zone 120. The catalyst material includes
at least one acid
catalyst, and the reaction zone 120 is disposed at a temperature below around
380 C and at a
pressure sufficient for the reactants and resultant product to be disposed in
a liquid, or
substantially liquid, state.
[0066] The catalyst material, operating conditions, and reactor are
selected to allow this
process to achieve desirable olefins content reduction. It is possible to
select different
combinations of the reactor, catalyst material and operating conditions that
will convert the
olefins in the feed to a sufficient degree to meet the desired operating
and/or pipeline
specifications objective(s). The catalyst material is selected such that the
catalyst material can
catalyze the reaction without being poisoned or otherwise inhibited to an
extent that the reaction
cannot occur. In practice, this means that if the catalyst material includes
an acid catalyst, the
reaction conditions are chosen such that the acid catalyst would not become
irreversibly
poisoned with basic compounds present in the feed. The temperature can be
chosen to prevent
the acid catalyst from reacting with basic compounds, or at least, from
becoming irreversibly
bound to the basic compounds.
[0067] Because the upgraded intermediate 114 would generally be produced
as a result of
an upgrading process, such as an upgrading process for the processing of heavy
oils, the
upgraded intermediate 114 may have species that include heteroatoms such as
sulfur, nitrogen
and oxygen. These types of heteroatoms can sometimes be problematic for acid
catalysis because
strong bonds or strong adsorption can be formed between the compounds in the
feed and the acid
sites on the catalyst material, thereby rendering the catalyst material
neutralized or inactive. In
some embodiments, for example, the acid strength of the catalyst material is
selected in such a
way that the compounds in the feed adsorb to form a bond with the acid sites
that do not persist
at the operating temperature of the catalyst material and hence the catalyst
material is not
rendered inactive. In some embodiments, for example, the acid strength of the
catalyst material
14
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
is within the range of strength characterized by temperature programmed
ammonia desorption
within the temperature range of 150 degrees Celsius to 350 degrees Celsius.
[0068] The catalyst material may be a heterogeneous catalyst material
selected from the
group consisting of supported liquid phase catalyst material, solid catalyst
materials, and
supported homogeneous catalyst materials.
[0069] In some embodiments, for example, the supported liquid phase
catalyst material
includes Bronsted acids (e.g. H2SO4, HF) and Lewis acids (e.g. BF3).
[0070] In some embodiments, for example, the heterogeneous catalyst
material has a
particle size and particle morphology suitable for use in a packed bed may be
used. Catalyst
materials that are suitable for use in packed bed reactors are known in the
art. Such catalyst
materials have a smaller chance of contaminating the hydrocarbon product as
the catalyst
material-product separation tends to be easier, allowing for simpler reactor
and operating
configurations. This may be advantageous when used in a field upgrading
application (e.g. when
the upgrading occurs on site), as such field upgrading applications are most
economical when
there is less complex equipment set-up.
[0071] In some embodiments, for example, the heterogeneous catalyst
material includes
large pore catalyst materials that can accommodate bulky olefin and the
potentially bulky
aromatics. The desirable pore size mainly depends on the size of the molecules
being treated and
the size of the materials being produced. In some embodiments, for example,
the catalyst
material includes a pore network with a pore diameter of greater than 0.5
nanometres. In some
embodiments, for example, the pore diameter is within the range of 0.5 to ten
(10) nanometres.
If the pore diameter is too small, larger molecules will not be able to travel
through the pore
network. On the other hand, large pore diameter, as a necessary incident,
reduces the available
surface area for catalyst activity, and also compromises mechanical integrity
of the catalyst
structure.
[0072] The catalyst material has acidic properties and, therefore,
includes at least one
acid catalyst. The acid catalyst may be promoted with metals, even though
metal promoters are
not specifically required by the processes disclosed herein. In some
embodiments, for example,
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
the acid catalyst has sufficient acid strength to catalyze the olefins-
aromatics alkylation reaction,
as well as an acid strength distribution to retain sufficient activity in
contact with the basic
compounds that are present in the upgraded intermediate. The temperature and
acid catalyst are
selected such that an optimal combination of olefins-aromatics alkylation
activity and smallest
amount of catalyst inhibition by compounds that are strongly adsorbing, or are
basic in nature in
the reaction product (with reduced olefins levels) is achieved.
[0073] It is known that among others, the following heterogeneous
catalysts are
catalytically active materials for liquid phase aromatic alkylation:
[0074] (b.1) Zeolites of the framework type FAU, like Y-zeolite (e.g. J.
Mol. Catal. A
2007, 277:1-14 and Appl. Catal. A 1999, 182:407-411);
[0075] (b.2) Zeolites of the framework type BEA, like Beta-zeolite (e.g.
Appl. Catal. A
1997, 153:233-241);
[0076] (b.3) Zeolites of the framework type MOR, like mordenite (e.g. J.
Mol. Catal. A
2004, 223:305-311);
[0077] (b.4) Zcolites of the framework type MFI, like ZSM-5 (e.g. Energy
Fuels 2008,
22:1449-1455);
[0078] (b.5) Zeolites of the framework type MWW, like MCM-22 (e.g. App!.
Catal. A
2005, 292:68-75 and J. Catal. 2005, 236:45-54);
[0079] (b.6) Zeolites of the framework type MTW, like ZSM-12 (e.g. Catal.
Rev.-Sci.
Eng. 2002, 44:375-421);
[0080] (b.7) Amorphous silica-alumina based catalysts (e.g. Ind. Eng.
Chem. Res. 2005,
44:5535-5541);
[0081] (b.8) Natural clays, such as montmorillonite (e.g. Hely. Chim. Acta
1987, 70:577-
586);
16
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
[0082] (b.9)
Solid phosphoric acid (e.g. Ind. Eng. Chem. Res. 2006, 45:7399-7408 and J.
Am. Chem. Soc. 1945, 67:1060-1062);
[0083]
(b.10) Acidic resins, such as sulfonated styrene-divinylbenzene copolymers
(e.g.
React. Func. F'olym. 2000, 44:1-7).
[0084] The
aforementioned list is by no means exhaustive. However, this process can be
performed using appropriate catalysts despite the presence of contaminants
typically found in
industrial feed materials and which may be deleterious to acid catalysts in
general. Thus, in
contrast to some known methods of alkylation, the process of the present
invention can be used
at temperatures below about 380 degrees Celsius with a hydrocarbon feed that
contains potential
catalyst poisons in the feed.
[0085] In
some embodiments, for example, an amorphous silica-alumina catalyst,
material or a crystalline silica-alumina catalyst material, may be used in
this process. The silica-
alumina catalyst material may have a SiO2 to Al2O3 ratio of 0-99 wt% for
example, but in some
circumstances, it may be appropriate to use a catalyst having a SiO2 to Al2O3
ratio ratio of 5-75
wt%. The silica-alumina catalyst material is generally activated by
calcination at a temperature in
the range 500 to 600 degrees Celsius.
[0086] The
choice of catalyst type is based on accessibility and performance in the
presence of basic compounds, such as pyridine, which may be acid catalyst
poisons. Basic
nitrogen compounds are typically present in most hydrocarbon feed materials
that have not been
hydro-processed.
[0087] The
type of catalyst material affects the selection of the reactor and operating
conditions.
[0088] The
operating conditions are selected to match the catalyst employed. There are a
number of guiding principles in selecting appropriate operating conditions.
These are as follows:
[0089] (a)
The temperature range depends on the catalyst selected and the contaminants
present in the hydrocarbon feed.
Olefin-aromatic alkylation reactions are favored
thermodynamically by low temperature. However, the contaminants present in the
hydrocarbon
17
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
feed, in particular basic nitrogen species, tend to deactivate the acid sites
in the catalyst and
render it ineffective after a short exposure time. It has been found however,
that operating the
catalyst at higher temperatures the catalyst can perform the reaction without
being poisoned or
otherwise inhibited to an extent that the reaction cannot occur. If too high a
temperature is used
however the rate of olefins polymerization reactions that promote fouling and
catalyst
deactivation will prevail. Furthermore, the operating temperature needs to
consider the rate of
catalytic or thermal cracking such that the amount of olefins produced during
the cracking
process does not exceed the target olefins concentration at the outlet of the
reactor. The
maximum operating temperature is in the range of about 320 to 380 degrees
Celsius for silica-
alumina catalysts. The lower operating temperature limit is determined by the
activity of the
catalyst in the presence of basic heteroatom feed contaminants, where below
this point olefin-
aromatic alkylation reactions are too slow for significant olefins conversion
to occur. In order to
perform acid catalysis at an industrially meaningful rate, the minimum
operating temperature
must be sufficiently high to avoid excessive catalyst poisoning by
irreversible adsorption of
compounds. In the presence of nitrogen bases in the feed, the minimum
operating temperature is
in the range of from about 200 to 300 degrees Celsius for silica-alumina
catalysts. The
temperature conditions appropriate for acidic resin and SPA catalysts are from
about 50 to 380
degrees Celsius, and more particularly, from about 150 to 350 degrees Celsius.
[0090] (b) The pressure should be sufficient to keep most of the
hydrocarbon feed
material in the liquid phase at the operating temperature. This limits the
amount of light olefins
that may be present in the vapour phase and that may pass through the reactor
unconverted. A
typical operating pressure is in the range 0-8 MPa. For operating temperatures
in the range of
about 300 to 380 degrees Celsius, which is typical for silica-alumina
catalysts, the pressure range
is about 2-5 MPa.
[0091] (c) The flow rate is determined by the olefins conversion
requirements for the
selected combination of catalyst and operating conditions. The range of weight
hourly space
velocity (WHSV) is 0.01 to 20 111. The WHSV range is generally in the range of
0.02 to 2 111.
Optimum conditions for olefins conversion are adjusted to meet final product
specifications and
may be determined empirically, depending on changes in feed composition,
selected catalyst
types (i.e. one or more catalyst types used in conjunction) and aging, number
of reactor beds (i.e.
18
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
single vs. multiple with interstage cooling) and other unit constraints.
Process operating
conditions can be optimized using strategies known in the art.
[0092] (d) Depending on the catalyst, it may be beneficial to add water, or
compounds
that may produce water, such as alcohols, to the feed.
[0093] The process may be conducted in a conventional packed bed reactor.
The catalyst
is contained and retained in a process vessel that is designed according to
principles known in the
art. In one embodiment, a single adiabatic packed bed (fixed bed) reactor is
employed. The use
of multiple catalyst beds within the reactor, the use of inter-bed quench feed
stream, the use of
more than one reactor and product recycling may all be considered.
[0094] The adiabatic temperature increase should be controlled. Aromatic
alkylation
with olefins and olefins dimerization (a possible side-reaction) are both
exothermic.
Implementation of heat management strategies in the reactor design is known in
the art.
[0095] The reactor may be operated either in down flow or up flow
configuration. The
operation of the reactor in an up flow configuration with a liquid filled
catalyst bed improves
heat transfer and catalyst wetting, and maximizes liquid holdup. This
configuration also
facilitates removal of heavy products (gums) typical of di-olefins reactions
from the catalyst by
dissolving it in the liquid product, as described in patents related to the
buildup of fouling agents
(e.g. U.S. Patent No. 4,137,274). The operation of the reactor in a down flow
configuration
facilitates maintenance and catalyst replacement, since the contaminated
portion of the catalyst
will be concentrated at the top of the reactor where it is easier to access
and replace.
[0096] From an operational point of view, the reactor may further be
designed for easy
maintenance and catalyst replacement in the field.
[0097] In some embodiments, for example, the olefinic material may include
one or more
cyclo-olefins, and the reducing of the content of olefinic material within the
at least a fraction of
the upgraded intermediate is effected by dehydrogenation of the one or more
cyclo-olefins. The
dehydrogenation of a cyclo-olefin produces an aromatic. In some embodiments,
for example, the
cyclo-olefin includes one or more heteroatoms In some embodiments, for
example, the
dehydrogenation is effected within a reaction zone 120 disposed at a
temperature of from about
19
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
100 degrees Celsius to about 300 degrees Celsius, such as, for example, from
about 125 degrees
Celsius to about 275 degrees Celsius. In some embodiments, for example, the
cyclo-olefin is a
five-membered ring or a six-membered ring, and may include one or more
heteroatoms.
[0098] In
some embodiments, for example, the reaction zone 120 includes a catalyst
material (including a supported catalyst material) that is active for
hydrogenation-
dehydrogenation, and includes catalysts that are active for hydrogenation-
dehydrogenation in the
presence of heteroatom-containing cyclo-olefins (e.g. Ni/A1203, Ni/SiO2,
NiMo/A1203,
CoMo/A1203, FeS, or MoS2).
[0099]
Advantageously, as a result of the dehydrogenation, some diatomic hydrogen is
produced, which can be exploited to effect hydrogenation of di-olefins to
produce mono-olefins.
This has downstream benefits related to the removal of the di-olefins. There
are also immediate
benefits in that the consumption of the produced diatomic hydrogen favours the
equilibrium
towards dehydrogenation of the cyclo-olefins to aromatics.
[00100] In
some embodiments, for example, the catalyst material for the dehydrogenation
includes large pore catalyst materials that can accommodate bulky olefin and
the potentially
bulky aromatics. The desirable pore size mainly depends on the size of the
molecules being
treated and the size of the materials being produced. In some embodiments, for
example, the
catalyst material includes a pore network with a pore diameter of greater than
0.5 nanometres. In
some embodiments, for example, the pore diameter is within the range of 0.5 to
ten (10)
nanometres. If the pore diameter is too small, larger molecules will not be
able to travel through
the pore network. On the other hand, large pore diameter, as a necessary
incident, reduces the
available surface area for catalyst activity, and also compromises mechanical
integrity of the
catalyst structure.
[00101] In
some embodiments, for example, the olefinic material includes one or more
cyclo-olefins, and the reducing of the content of the olefinic material within
the at least a fraction
of the upgraded intermediate is effected by hydrogen disproportionation of the
one or more
cyclo-olefins. The hydrogen disproportionation converts the one or more cyclo-
olefins to cyclo-
paraffin structures or aromatic structures. In some embodiments, for example,
the hydrogen
disproportionation is effected within the reaction zone 111. In some
embodiments, for example,
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
to effect the hydrogen disproportionation, the temperature within the reaction
zone 111 is at least
300 degrees Celsius.
[00102] Referring to Figure 2, in some embodiments, for example, the heavy
hydrocarbon
material-comprising feed, that is the subject of the treating that includes
cracking of the heavy
hydrocarbon material-comprising feed, includes at least a fraction of a
heavier hydrocarbon
material-comprising fraction 134A that has been, along with a lighter
hydrocarbon material-
comprising fraction 134B, separated from a feed material 150. In this respect,
a feed material
150 is provided, and the feed material 150 is separated (for example, by a
separator 130, such as
a fractionator) into at least the heavier hydrocarbon material-comprising
fraction 134A and the
lighter hydrocarbon material-comprising fraction 134B. The heavier hydrocarbon
material-
comprising fraction 134A has a weight average molecular weight that is greater
than that of the
lighter hydrocarbon material-comprising fraction 134B. In some embodiments,
for example, the
separation is effected based on differences in volatilities between the
heavier hydrocarbon
material-comprising fraction 134A and the lighter hydrocarbon material-
comprising fraction
134B. Suitable separation processes that are based on differences in
volatilities between the
heavier hydrocarbon material-comprising fraction 134A and the lighter
hydrocarbon material-
comprising fraction 134B include stripping and distillation. In some
embodiments, for example,
at a predetermined pressure and temperature at which the separation is
effected, the heavier
hydrocarbon material-comprising fraction 134A has a higher boiling point than
the lighter
hydrocarbon material-comprising fraction 134B. In some embodiments, for
example, at a
predetermined temperature at which the separation is effected, the heavier
hydrocarbon material-
comprising fraction 134A has a lower vapour pressure than the lighter
hydrocarbon material-
comprising fraction 134B. In some embodiments, for example, at a predetermined
temperature
at which the separation is effected, the vapour pressure of the heavier
hydrocarbon material-
comprising fraction 134A is less than 90% (such as, for example, less than
80%, such as, for
example, less than 70%, such as, for example, less than 60%, such as, for
example, less than
50%) of the vapour pressure of the lighter hydrocarbon material-comprising
fraction 134B. In
this respect, it is recognized that the additional incremental cost in
configuring a cracking unit
operation 110 for effecting the cracking of lighter hydrocarbon material may
not be justified,
having regard to the fact that, relative to heavier hydrocarbon material,
cracking of lighter
hydrocarbon material is not pronounced and does not significantly contribute
to the production
21
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
of a hydrocarbon material having a pipeline specification Instead of being
processed through the
cracking unit operation 110, the lighter hydrocarbon material-comprising
fraction 134B is
combined with at least a fraction of the olefinic material content-reduced
product 115, such that
the upgraded product 200 is produced.
[00103] Referring to Figure 3, the reducing of the content of olefinic
material within the
upgraded intermediate 114 is effected by separating at least a fraction of the
upgraded
intermediate 114 into at least an olefin-comprising treatment fraction 132 and
a treatment by-
pass fraction 134. In some embodiments, for example, the separating is
effected within a
separator 130, such as a fractionator. In some embodiments, for example, the
separation is
effected based on differences in volatilities between the olefin-comprising
treatment fraction 132
and the treatment by-pass fraction 134. Suitable separation processes that are
based on
differences in volatilities between the olefin-comprising treatment fraction
132 and the treatment
by-pass fraction 134 include stripping and distillation. In some embodiments,
for example, at a
predetermined temperature at which the separation is effected, the olefin-
comprising treatment
fraction 132 has a higher vapour pressure than the treatment by-pass fraction
134. In some
embodiments, for example, at a predetermined temperature at which the
separation is effected,
the vapour pressure of the treatment by-pass fraction 134 is less than 90%
(such as, for example,
less than 80%, such as, for example, less than 70%, such as, for example, less
than 60%, such as,
for example, less than 50%) of the vapour pressure of the olefin-comprising
treatment fraction
132. The content of olefinic material within the olefin-comprising treatment
fraction 132 is
reduced such that an olefin depleted intermediate 136 is produced. In this
respect, in some
embodiments, for example, the reducing of the content of olefinic material
within the olefin-
comprising treatment fraction 132 includes effecting conversion of the
olefinic material within
the olefin-comprising treatment fraction 132, such as by the processes
effected within the
reaction zone 120, as explained above, including, in particular, those which
are carried out in the
absence, or the substantial absence, of adscititious diatomic hydrogen. The
treatment by-pass
fraction 134 may then be combined with at least the olefin-depleted
intermediate 136 to produce
the olefinic material content-reduced product 115, which then may be produced
as at least a part
of the upgraded product 200.
22
[00104] Referring
to Figure 4, in some of these embodiments, for example, the upgraded
intermediate 114 may not have been sufficiently cracked by a single pass
through the cracking
unit operation 110, such that excessive amounts of heavy hydrocarbon material
remain present
within the upgraded intermediate 114. It may be desirable, therefore, to
recycle a fraction of the
upgraded intermediate 114 through the cracking unit operation 110 so as to
further reduce the
content of heavy hydrocarbon material within the upgraded intermediate 114. In
this respect,
instead of combining the entirety of the treatment by-pass fraction 134 with
the olefin-depleted
intermediate 136 to effect production of the upgraded product 200, the
treatment by-pass fraction
134 is separated into at least fractions 138A, 138B, with the fraction 138B
being combined with
the olefin-depleted intermediate, and the fraction 138A being supplied to the
cracking unit
operation. In this respect, the heavy hydrocarbon material-comprising feed
includes a
combination of the heavy hydrocarbon material-comprising supply stream 150 and
the fraction
138A.
[00105] By
reducing the content of olefinic material, without requiring any amount, or
any
substantial amount, of diatomic hydrogen supplied from an external source,
cost and
complexities of producing and supplying such diatomic hydrogen are mitigated.
In particular,
most methods to reduce olefinic material content currently involve expensive
hydro-processing
infrastructures such as hydrotreaters and associated equipment, which require
an extraneous
source of hydrogen. Also, many methods to reduce the olefinic material content
of a hydrocarbon
stream require the addition of extraneous components such as diatomic hydrogen
and/or another
hydrocarbon. For example, when upgrading is employed to treat a feed material,
separate
equipment is needed for diatomic hydrogen generation to enable hydro-
processing. In addition to
the required hydro-processing unit, units needed to produce diatomic hydrogen
(e.g. steam
methane reforming) increase the complexity and cost of the oil upgrading
facility and increase
the carbon footprint of the overall process.
(b) Separating
olefinic material from the upgraded intermediate prior to converting the
olefinic material, and then separating lighter hydrocarbon material from the
residual
upgraded intermediate prior to cracking
23
CA 2953853 2019-01-23
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
[00106] In another aspect, because the upgraded intermediate 114 may not
have been
sufficiently cracked by a single pass through the cracking unit operation 110,
such that excessive
amounts of heavy hydrocarbon material remain present within the upgraded
intermediate 114, it
may be desirable to recycle at least a fraction of the upgraded intermediate
114 through the
cracking unit operation 110. This may be desirable if the upgraded
intermediate 114 contains
cyclo-olefins. The recycling of at least a fraction of the upgraded
intermediate 114 may be
effected by separating the treatment by-pass fraction 134 from the upgraded
intermediate 114,
and then supplying a fraction 138A of the treatment by-pass fraction 134 to
the reaction zone 111
of the cracking unit operation 110 (see, for example, Figure 4). However, the
fraction 134 may
include not an insubstantial content of lighter hydrocarbon material, whose
recycling through the
reaction zone 111 may not be justifiable, as it is recognized that additional
incremental cost in
configuring the cracking unit operation 110 for effecting the cracking of
recycled lighter
hydrocarbon material may not be justified, having regard to the fact that,
relative to heavier
hydrocarbon material, cracking of lighter hydrocarbon material is not
pronounced and does not
significantly contribute to the production of a hydrocarbon material having a
pipeline
specification. It is, therefore, desirable to separate, from the treatment by-
pass fraction 134, at
least a heavier hydrocarbon material-comprising fraction 134A and a lighter
hydrocarbon
material-comprising fraction 134B, and recycle only at least some material
(i.e. fraction 138A) of
the heavier hydrocarbon material-comprising fraction 134A through the cracking
unit operation
110, as opposed to simply recycling the entirety of the treatment by-pass
fraction 134 (including
the lighter hydrocarbon material-comprising fraction) through the reaction
zone 111.
Advantageously, where the upgraded intermediate 114 includes cyclo-olefins,
cyclo-olefins
being recycled within the fraction 138A may undergo hydrogen
disproportionation within the
reaction zone 111, thereby further reducing the olefinic content of the
upgraded product 200
being produced.
[00107] In this respect, and referring to Figure 5, the process includes
supplying a heavy
hydrocarbon material-comprising supply stream 150 to the cracking unit
operation such that the
intermediate upgraded product 114 is produced. At least a fraction of the
intermediate upgraded
product 114 is separated into a olefin-comprising treatment fraction 132 and a
treatment by-pass
fraction 134. In some embodiments, for example, the separating is effected
within a separator
130, such as a fractionator. In some embodiments, for example, the separation
is effected based
24
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
on differences in volatilities between the olefin-comprising treatment
fraction 132 and the
treatment by-pass fraction 134. Suitable separation processes that are based
on differences in
volatilities between the olefin-comprising treatment fraction 132 and the
treatment by-pass
fraction 134 include stripping and distillation. In some embodiments, for
example, at a
predetermined temperature at which the separation is effected, the olefin-
comprising treatment
fraction 132 has a higher vapour pressure than the treatment by-pass fraction
134. In some
embodiments, for example, at a predetermined temperature at which the
separation is effected,
the vapour pressure of the treatment by-pass fraction 134 is less than 90%
(such as, for example,
less than 80%, such as, for example, less than 70%, such as, for example, less
than 60%, such as,
for example, less than 50%) of the vapour pressure of the olefin-comprising
treatment fraction
132. In some embodiments, for example, at a pressure of one (1) atmosphere,
the boiling point
range of the olefin-comprising treatment fraction is between 25 degrees
Celsius and 365 degrees
Celsius, such as, for example, between 25 degrees Celsius and 200 degrees
Celsius, such as, for
example, 25 degrees Celsius and 100 degrees Celsius. The content of olefinic
material within
the olefin-comprising treatment fraction is reduced such that an olefin-
depleted intermediate 136
is produced. In this respect, in some embodiments, for example, the reducing
of the content of
olefinic material within the olefin-comprising treatment fraction 132 includes
effecting
conversion of the olefinic material within the olefin-comprising treatment
fraction 132, such as
by the processes effected within the reaction zone 120, as explained above,
including, in
particular, those which are carried out in the absence, or the substantial
absence, of adscititious
diatomic hydrogen.
[00108] The treatment by-pass fraction 134 is then separated (such as by
the separator
130) into at least a heavier hydrocarbon material-comprising fraction 134A and
a lighter
hydrocarbon material-comprising fraction 134B. In some embodiments, for
example, the
separation is effected based on differences in volatilities between the
heavier hydrocarbon
material-comprising fraction 134A and the lighter hydrocarbon material-
comprising fraction
134B. Suitable separation processes that are based on differences in
volatilities between the
heavier hydrocarbon material-comprising fraction 134A and the lighter
hydrocarbon material-
comprising fraction 134B include stripping and distillation. In some
embodiments, for example,
at a predetermined temperature at which the separation is effected, the
heavier hydrocarbon
material-comprising fraction 134A has a lower vapour pressure than that of the
lighter
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
hydrocarbon material-comprising fraction 134B. In some embodiments, for
example, at a
predetermined temperature at which the separation is effected, the vapour
pressure of the heavier
hydrocarbon material-comprising fraction 134A is less than 90% (such as, for
example, less than
80%, such as, for example, less than 70%, such as, for example, less than 60%,
such as, for
example, less than 50%) of the vapour pressure of the lighter hydrocarbon
material-comprising
fraction 134B.
[00109] In some embodiments, for example, where the above-described
separations are
effected based on differences in volatilities, as a necessary incident, the
lighter hydrocarbon
material-comprising fraction 134B has a lower vapour pressure than that of the
olefin-comprising
treatment fraction 132. In some embodiments, for example, at a predetermined
temperature at
which the separation is effected, the vapour pressure of the lighter
hydrocarbon material-
comprising fraction 134B is less than 90% (such as, for example, less than
80%, such as, for
example, less than 70%, such as, for example, less than 60%, such as, for
example, less than
50%) of the vapour pressure of the olefin-comprising treatment fraction 132.
In some
embodiments, for example, at a pressure of one (1) atmosphere, the boiling
point range of the
olefin-comprising treatment fraction 132 is between 25 degrees Celsius and 365
degrees Celsius
(such as, for example, between 25 degrees Celsius and 200 degrees Celsius,
such as, for
example, 25 degrees Celsius and 100 degrees Celsius), and the boiling point
range of the lighter
hydrocarbon material-comprising fraction 134B is between 80 degrees Celsius
and 450 degrees
Celsius (such as, for example, 200 degrees Celsius and 450 degrees Celsius,
such as, for
example, between 360 degrees Celsius and 450 degrees Celsius). In some of
these embodiments,
for example, the lighter hydrocarbon material-comprising fraction 134B
includes hydrocarbon
material that is equivalent to light vacuum gas oil.
[00110] In a related aspect, the treating includes, separating at least a
fraction of the
upgraded intermediate into at least an olefin-comprising treatment fraction
132, a light
hydrocarbon material-comprising fraction 134B, and a heavy hydrocarbon
material-comprising
fraction 134A. In some embodiments, for example, the separating is effected
within a separator
130, such as a fractionator. In some embodiments, for example, the separation
is effected based
on differences in volatilities between the olefin-comprising treatment
fraction 132, the light
hydrocarbon material-comprising fraction 134B, and the heavy hydrocarbon
material-comprising
26
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
fraction 134A. Suitable separation processes that are based on differences in
volatilities between
these components include stripping and distillation. In some embodiments, for
example, at a
predetermined temperature at which the separation of the olefin-comprising
treatment fraction
132 is effected, the heavier hydrocarbon material-comprising fraction 134A has
a lower vapour
pressure than that of the lighter hydrocarbon material-comprising fraction
134B, and the lighter
hydrocarbon material-comprising fraction 134B has a lower vapour pressure than
that of the
olefin-comprising treatment fraction 132. In
some embodiments, for example, at a
predetermined temperature at which the separation is effected, the vapour
pressure of the heavier
hydrocarbon material-comprising fraction 134A is less than 90% (such as, for
example, less than
80%, such as, for example, less than 70%, such as, for example, less than 60%,
such as, for
example, less than 50%) of the vapour pressure of the lighter hydrocarbon
material-comprising
fraction 134B, and the vapour pressure of the lighter hydrocarbon material-
comprising fraction
134B is less than 90% (such as, for example, less than 80%, such as, for
example, less than 70%,
such as, for example, less than 60%, such as, for example, less than 50%) of
the vapour pressure
of the olefin-comprising treatment fraction 132.
[00111] A
fraction 138A of the produced heavier hydrocarbon material-comprising
fraction 134A may be recycled through the cracking unit operation 110. With
respect to the
produced lighter hydrocarbon material-comprising fraction 134B, instead of
being processed
through the cracking unit operation 110, the lighter hydrocarbon material-
comprising fraction
134B may be combined with at least the produced olefin-depleted intermediate
136 (and, in
some embodiments, for example, a fraction 138B of the heavier hydrocarbon
material-
comprising fraction 134A) to create an olefinic material content-reduced
product 115, which
may then be produced as the upgraded product 200.
[00112]
Referring to Figure 5A, instead of supplying the heavy hydrocarbon material-
comprising supply stream 150 directly to the cracking unit operation 110, the
heavy hydrocarbon
material-comprising supply stream 150 may be combined with the upgraded
intermediate 114 to
form the feed to the separator 130.
(c) Removing light olefinic material from the upgraded intermediate
27
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
[00113] Referring to Figure 6, in another aspect, the reducing of the
content of olefinic
material within the upgraded intermediate 114 is defined by a reduction in the
content of light
olefinic material within at least a fraction of the upgraded intermediate 114
such that the content
of light olefinic material within the olefinic material content-reduced
product is less than the
content of light olefinic material within the at least a fraction of the
upgraded intermediate 114.
In this respect, the ratio of the weight of light olefinic material within the
olefinic material
content-reduced product to the total weight of the olefinic material content-
reduced product is
less than the ratio of the weight of light olefinic material within the
upgraded intermediate 114 to
the total weight of the upgraded intermediate 114.
[00114] It is recognized that, in some embodiments, it is not necessary to
reduce the
content of all of the olefinic material, as the presence of some of the
olefinic material may not
necessarily be as detrimental to the further processing of the upgraded
product 200, including the
olefinic material content-reduced product, as other fractions of the olefinic
material. In some
embodiments, for example, light olefinic material may be a more significant
contributor to the
problems associated with olefinic material, generally (and as above-
described), relative to other
kinds of olefinic material. In this respect, in some of these embodiments, for
example, it may be
sufficient to effect a reduction of the content of at least the light olefinic
material.
[00115] By not making it a requirement to effect a reduction in the content
of all of the
olefinic material, and just a portion of the olefinic material (and,
specifically, the light olefinic
material portion), costs and complexities relating to the processing of the
upgraded intermediate
114 are reduced.
[00116] In some of these embodiments, for example, the reducing of the
content of light
olefinic material within the at least a fraction of the upgraded intermediate
114 includes effecting
conversion of the light olefinic material within at least a fraction of the
upgraded intermediate
114, such as by the processes effected within the reaction zone 120, as
explained above with
respect to olefinic material generally, including, in particular, those
processes which are carried
out in the absence, or the substantial absence, of adscititious diatomic
hydrogen.
[00117] Referring to Figure 7, in some of these embodiments, for example,
the reducing of
the content of olefinic material within the upgraded intermediate 114 is
effected by separating at
28
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
least a fraction of the upgraded intermediate 114 into at least a lighter
olefin-comprising fraction
142 and a heavier fraction 144, and then effecting a reduction in the content
of light olefinic
material within the light olefin-comprising fraction 142 (such as by the
processes effected within
the reaction zone 120, as explained above with respect to olefinic material
generally) such that a
light olefin-depleted intermediate 146 is produced, and then combining the
heavier fraction 144
with at least the light olefin-depleted intermediate 146 to produce a light
olefinic material
content-reduced product. In this respect, the ratio of the weight of light
olefinic material within
the lighter olefin-comprising fraction 142 to the total weight of the lighter
olefin-comprising
fraction 142 is greater than the ratio of the weight of light olefinic
material within the heavier
fraction 144 to the total weight of the heavier fraction 144. An upgraded
product 200 may be
produced including the light olefin content material-reduced product In some
embodiments, for
example, the separation is effected within a separator 130, such as a
fractionator. In some
embodiments, for example, the separation is effected based on differences in
volatilities between
the lighter olefin-comprising fraction 142 and the heavier fraction 144.
Suitable separation
processes that are based on differences in volatilities of the lighter olefin-
comprising fraction 142
and the heavier fraction 144 include stripping and distillation. In some
embodiments, for
example, at a predetermined temperature at which the separation is effected,
the lighter olefin-
comprising fraction 142 has a higher vapour pressure than the heavier fraction
144. In some
embodiments, for example, at a predetermined temperature at which the
separation is effected,
the vapour pressure of the heavier fraction 144 is less than 90% (such as, for
example, less than
80%, such as, for example, less than 70%, such as, for example, less than 60%,
such as, for
example, less than 50%) of the vapour pressure of the lighter olefin-
comprising fraction 142.
[00118] Referring to Figure 8, in a related aspect, the reducing of the
content of olefinic
material within the upgraded intermediate 114 is effected by separating at
least a fraction of the
upgraded intermediate 114 into a more volatile fraction 162 and a less
volatile fraction 164,
wherein the more volatile fraction 164 has a boiling point range, at a
pressure of one (1)
atmosphere, of between 25 degrees Celsius and 200 degrees Celsius, such
boiling point range
being characteristic of a lighter olefinic material. A reduction in the
content of olefinic material
within the more volatile fraction 164 (such as by the processes effected
within the reaction zone
120, as explained above with respect to olefinic material generally) such that
an olefin-depleted
intermediate 166 is produced, and then combining at least a fraction of the
less volatile fraction
29
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
164 with at least the olefin-depleted intermediate 166 to produce an olefinic
material content-
reduced product 115. An upgraded product 200 may be produced including the
olefin content
material-reduced product 115. In some embodiments, for example, the separation
is effected
within a separator 130, such as a fractionator. In some embodiments, for
example, the separation
is effected based on differences in volatilities between the more volatile
fraction and the less
volatile fraction. Suitable separation processes that are based on differences
in volatilities of the
more volatile fraction and the less volatile fraction include stripping and
distillation.
(d) Cracking in the presence of a hydrogen donor material
[00119]
Referring to Figure 9, in some embodiments, for example, the treating of the
heavy hydrocarbon material-comprising feed 112 includes cracking the heavy
hydrocarbon
material-comprising feed within the reaction zone 111, of the cracking unit
operation 110, in the
presence of a hydrogen donor material 108. In some embodiments, for example,
the hydrogen
donor material 108 is another hydrocarbon material-comprising feed that has
hydrogen transfer
or hydrogen donor properties. The treating is such that an upgraded product
200 is produced.
The presence of the hydrogen donor material 108 discourages the production of
olefinic material,
and thereby renders the upgraded product 200 of a quality that meets at least
one pipeline
specification or come closer to meeting at least one pipeline specification.
In some
embodiments, for example, the hydrogen donor material 108 is supplied to the
reaction zone 111.
[00120] In
some embodiments, for example, the ratio of weight of hydrogen donor
material supplied to the reaction zone 111 to weight of heavy hydrocarbon
material is at least
1:20. In some of these embodiments, for example, the ratio is at least 1:5,
such as, for example,
at least 1:4. In some embodiments, for example, the ratio is between 1:20 and
4:1, such as, for
example, between 1:6 and 1:3.
[00121] In
some embodiments, for example, the hydrogen donor material includes
synthetic crude oil.
[00122] In
some embodiments, for example, the hydrogen donor material includes (or, in
some embodiments, for example, is defined by) one or more cycloalkanes, one or
more
naptheno-aromatics, or one or more cycloalkanes and one or more naptheno-
aromatics. The
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
cycloalkane may be substituted or unsubstituted. The naphtheno-aromatic may be
substituted or
unsubstituted. In some embodiments, for example, the hydrogen donor material
includes a
hydrocarbon including a six-membered ring structure that is attached to an
aromatic. In some
embodiments, for example, the hydrogen donor material is substantially free of
five-membered
ring structures that cannot form aromatic rings as a consequence of their
hydrogen donor
activity.
[00123] In some embodiments, for example, the hydrogen donor material
includes tetralin
(i.e. 1,2,3 ,4-tetrahydrohaphthalene).
[00124] The cracking of the heavy hydrocarbon material-comprising feed in
the presence
of a hydrogen donor material, that includes a cycloalkane (such as a five-
membered cyclo-
alkane, and also, in some embodiments, and to some extent, a six-membered
cyclo-alkane) or a
naphtheno-aromatic, produces an intermediate product 114 including a cyclo-
olefin material that
includes one or more cyclo-olefins. In such embodiments, for example, the
treating further
includes effective a reactive process, such that the intermediate product 114
participates within
the reactive process as a reactant and is consumed within the reactive
process. The reactive
process includes dehydogenation of an olefinic material within a reaction zone
170 (such as
within a reactor 172) to produce an olefinic material content-reduced product
115. In some
embodiments, for example the treating includes dehydrogenating at least a
fraction of the cyclo-
olefin material of the intermediate product 114. In this respect, the reactive
process is effected
within the reaction zone 170 disposed at a temperature that is
thermodynamically more
favourable to the dehydrogenation of cyclo-olefins than to the dehydrogenation
of cyclo-alkanes.
Where the cyclo-olefin material is cyclohexene, the temperature of the
reaction zone is from
about 125 degrees Celsius and 275 degrees Celsius. Within this temperature
range, it is believed
that it is thermodynamically favourable to dehydrogenate cyclohexene and
thermodynamically
unfavourable to dehydrogenate cyclohexane. In some embodiments, for example, a
suitable
catalyst material, that is active for dehydrogenation and hydrogenation, is
disposed within the
reaction zone 170. In some embodiments, for example, the catalyst material may
include a
supported metal catalyst that is active for dehydrogenation and hydrogenation
in the presence of
heteroatom-comprising compounds (e.g. Ni/A1203, Ni/SiO2, NiMo/A1203,
CoMo/A1203). In
some embodiments, for example, the catalyst material includes a dispersed
catalyst.
31
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
(e) Upgraded product
[00125] In some embodiments, for example, an upgraded product 200 is
produced and
includes the olefinic material content-reduced product 115. The upgraded
product 200 meets at
least one pipelines specification. In this respect, in some embodiments, for
example, the
upgraded product 200 is supplied to a pipeline for transporting to a refinery.
In some of these
embodiments, for example, the process also includes transporting the upgraded
product 200, via
the pipeleine, to the refinery.
(fi Other exemplary embodiments
[00126] It is understood that any two or more of the above-described
aspects, and any one
of their respective exemplary embodiments, may be combined to create other
embodiments of
the present disclosure, such as the embodiment illustrated in Figures 10, 11,
and 12.
[00127] Each one of illustrate feed material 150 that is derived, in part,
from a
deasphalting process. In this respect, the feed material 150 includes a
deasphalted heavy
hydrocarbon-comprising material.
[00128] Referring to Figures 10, 11, and 12, an asphaltene-comprising heavy
hydrocarbon-comprising material 300 is supplied to a separator 302 to effect
phase separation of
gaseous material 304 and aqueous material 306 from the raw asphaltene-
comprising heavy
hydrocarbon-comprising material such that a dewatered/degassed asphaltene-
comprising heavy
hydrocarbon-comprising material 308 is produced. The material 308 is admixed
with solvent
material 310, 312 in mixers 314, 316 to produce a mixture 318.
[00129] The mixture 318 is separated, within a separator 320, into at least
an asphaltene-
depleted heavy hydrocarbon-comprising material fraction 322 and an asphaltene-
enriched
material fraction 324. The asphaltene content of the asphaltene-depleted heavy
hydrocarbon-
comprising fraction 322 is less than the asphaltene content of the mixture
318. The asphaltene-
enriched material fraction 324, being denser than the asphaltene-depleted
heavy hydrocarbon-
comprising material fraction 322, is recovered as an underflow product, and
the asphaltene-
depleted heavy hydrocarbon-comprising material fraction is recovered as an
overhead product in
32
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
the form of the deasphalted heavy hydrocarbon-comprising material which
defines the feed
material 150.
[00130] In
some embodiments, for example, the asphaltene-enriched material fraction 324
is admixed with solvent material 326 within a mixer 328 and then separated
within a separator
330 into an overflow material mixture 332, including deasphalted heavy
hydrocarbon-
comprising material and solvent material, and an underflow asphaltene-enriched
material
fraction 334.
[00131] The
material 332 is recycled to upstream of the separator 320 to increase recovery
of the deasphalted heavy hydrocarbon-comprising material fraction.
[00132] The
underflow asphaltene-enriched material fraction 334 is supplied to a separator
336, such as a fractionator, to effect separation of the asphaltene-enriched
material fraction 334
into a gaseous solvent-enriched material fraction 338 and a further-enriched
asphaltene material
fraction 340. The gaseous solvent-enriched material fraction 338 may be re-
used within the
process, while the further-enriched asphaltcnc material fraction 340 may be
further treated to
recover water.
[00133] In
each one of the embodiments illustrated in Figures 10, 11, and 12, the feed
material 150, which includes at least a fraction of an upgraded intermediate
114 produced by the
cracking unit operation 110, is supplied to a separator 130 (such as a
fractionator), for effecting
separation of at least the lighter hydrocarbon material-comprising fraction
134B (for example, in
the form of a distillate).
[00134] In
the embodiment, illustrated in Figure 10, the presence of olefinic material
within the upgraded product 200 is minimized by effecting a reduction in
content of the olefinic
material within the upgraded intermediate 114. In this respect, the separator
130 additionally
effects separation of the olefm-comprising treatment fraction 132 (such as,
for example, the
lighter olefinic material-comprising fraction) as a distillate. The olefin-
comprising treatment
fraction 132 is converted into an olefin-depleted intermediate 136 (such as a
light olefin material-
depleted intermediate), such as by the processes effected within the reaction
zone 120, as
explained above with respect to olefinic material generally, namely, any one
of, or any
33
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
combination of alkylation and dehydrogenation (with incidental hydrogenation,
as explained
above). The heavier bottoms product 134A is also recovered. The fraction 138B
of the bottoms
product 134A is combined with both of the olefin-depleted intermediate 136 and
the lighter
hydrocarbon material-comprising fraction 134B to produce the upgraded product
200. A
fraction 138A of the bottoms product 134A is supplied to the cracking unit
operation 110. The
cracking unit operation may comprise, in series, a heater 110a and a soaker
110b, which effects
cracking of the bottoms product fraction 138A to produce the upgraded
intermediate 114 which
is then combined into the feed material 150. In this respect, at least a
fraction of the upgraded
intermediate 114 is recycled through the cracking unit operation 110 in the
form of the fraction
138A of the bottoms product 134A. Because the feed material 150 includes a
deasphalted heavy
hydrocarbon-comprising material, the feed material 150 also typically includes
residual solvent
material deriving from the deasphalting process, and most of the residual
solvent material may
be recovered as a top distillate product 308 from the separator 130.
[00135] In some embodiments, for example, steam stream 1311 supplies steam
to the
separator to reduce partial pressure of hydrocarbon material, and thereby
improve the separation
between distillate cuts to optimize the yields of desired product from the
separator 130.
[00136] In some embodiments, for example, solvent stream 133 is merged with
the
treatment material 132 for purging the solvent loop from olefins and also for
adding diluent to
the upgraded product 200.
[00137] In the embodiment illustrated in Figure 11, the presence of
olefinic material
within the upgraded product 200 is minimized by mitigating formation of the
olefinic material
within the cracking unit operation 110. In this respect, a bottoms product
134A is recovered and
a fraction 138B of the bottoms product 134A is combined with the lighter
hydrocarbon material-
comprising fraction 134B and the stream 312 to produce the upgraded product
200, while a
fraction 138A of the bottoms product 134A is combined with a hydrogen donor
material 310
(such as synthetic crude oil that includes cycloalkanes) to produce a cracking
unit operation feed
3102, and is then supplied to the cracking unit operation 110. The presence of
the hydogen
donor material 310 mitigates formation of olefinic material within the
cracking unit operation
110. The cracking unit operation 110 may comprise, in series, a heater 110a
and a soaker 110b,
34
CA 02953853 2016-12-29
WO 2016/000060 PCT/CA2014/000915
which effects cracking of the feed 3102 to produce the upgraded intermediate
114 which is then
combined into the feed material 150. In this respect, at least a fraction of
the upgraded
intermediate 114 is recycled through the cracking unit operation in the form
of the second
fraction 306 of the bottoms product 134A. Because the feed material 150
includes a deasphalted
heavy hydrocarbon-comprising material, the feed material 150 also typically
includes residual
solvent material deriving from the deasphalting process, and most of the
residual solvent material
may be recovered as a top distillate product 308 from the separator 130.
[00138] In
the embodiment illustrated in Figure 12, the process embodiment illustrated in
Figure 11 is modified such that the presence of olefinic material within the
upgraded product 200
is further minimized by one or both of aromatic alkylation and dehydrogenation
(with incidental
hydrogenation), as described above. The dehydrogenation is also helpful for
converting cyclo-
olefins that may be derived from the supplied hydrogen donor material (such as
from five-
membered cyclo-alkanes). In those embodiments where both of aromatic
alkylation and
dehydrogenation (with incidental hydrogenation) is effected within the
reaction zone 170 of an
olefin treating unit 172, in some of these embodiments, for example, the
reaction zone 170 is
configured such that, as the received treatment material 132 is conducted
through the reaction
zone 170, the treatment material 132 is contacted, sequentially, with a
dehydrogenation/hydrogenation catalyst, and then with an olefin-aromatic
alkylation catalyst. In
this respect, in some embodiments, for example, the olefin treating unit 172
includes an inlet 174
for receiving the treatment material 132 and an outlet 176 for discharging the
olefin-depleted
intermediate 136, and the dehydrogenation/hydrogenation catalyst is disposed
closer to the inlet
174 than the olefin-aromatic alkylation catalyst, and the olefin-aromatic
alkylation catalyst is
disposed closer to the outlet 136 than the dehydrogenation/hydrogenation
catalyst. A fraction
138A of the bottoms product 134A is combined with the hydrogen donor material
310 (such as
synthetic crude oil that contains cycloalkanes) to produce a cracking unit
operation feed 3102
which is then supplied to the cracking unit operation.
[00139] In
the above description, for purposes of explanation, numerous details are set
forth in order to provide a thorough understanding of the present disclosure.
However, it will be
apparent to one skilled in the art that these specific details are not
required in order to practice
the present disclosure.
Although certain dimensions and materials are described for
implementing the disclosed example embodiments, other suitable dimensions
and/or materials
may be used within the scope of this disclosure. All such modifications and
variations, including
all suitable current and future changes in technology, are believed to be
within the sphere and
scope of the present disclosure.
36
Date Recue/Date Received 2020-08-21