Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02955240 2017-01-13
WO 2016/036493 PCT/US2015/045255
LIQUID ASHLESS ANTIOXIDANT ADDITIVE FOR LUBRICATING COMPOSITIONS
FIELD OF THE INVENTION
This application relates to improved antioxidant compositions and lubricating
compositions containing
the same.
BACKGROUND OF THE INVENTION
US patent application 2014/0045736 teaches an antioxidant and antiwear
additive for lubricating
compositions comprising an aromatic amine antioxidant in combination with an
ashless dithiocarbamate.
While alkylated phenyl-a-naphthylamine (APANA) is an aromatic amine
antioxidant, no specific
disclosure of this particular compound is suggested. Rather, only octylated or
nonylated diphenylamine
are specifically discussed.
US 6743759 teaches the combination of ADPA derivative of tolutriazole and
methylenebis(dibutyldithiocarbamate). Optional components include
antioxidants, and APANA is
among the list of literally dozens of possible antioxidant compounds, with no
particular preference
except for nonylated diphenylamine. No suggestion is made that the use of
APANA in particular will
provide a further synergy when used with the primary two-component composition
of the reference.
SUMMARY OF THE INVENTION
Surprisingly, it has been discovered that improved antioxidant protection can
be achieved by providing
in a lubricating composition a three-component liquid antioxidant additive
comprising
(1) solid alkylated-phenyl-a-naphthylamine (APANA), (2) an alkylated
diphenylamine (ADPA)
derivative of triazole, tolutriazole or benzotriazole, and (3) methylenebis(di-
n-butyldithiocarbamate).
The additive may optionally further comprise a mineral oil or synthetic oil.
An antioxidant additive composition wherein the (1) solid alkylated-phenyl-a-
naphthylamine, the (2)
alklylated diphenylamine derivative of triazole, tolutriazole or benzotriazole
and (3) methylenebis(di-n-
butyldithiocarbamate) are each present at the following weight ratios:
(1):(2):(3) being 1-13:1-13:1-13,
preferably, 1-8:1-8:1-8, most preferably 1-2:0.125-1:1-2; optionally wherein
the balance is a mineral oil
or synthetic oil diluent.
A lubricating oil composition comprising a lubricating base at at least 90
wt.%, and an additive
composition comprising, as part of the entire lubricating oil composition (1)
alkylated-phenyl-a -
1
naphthylamine at between 0.01 and 1.0 wt. %, preferably 0.10-0.50 wt. %, more
preferably 0.15-0.30
wt. %; (2) alkylated diphenylamine derivative of triazole, tolutriazole or
benzotriazole at 0.01 to 0.50
wt. %, preferably0.01-0.30 wt. %, more preferably 0.01-0.15 wt. %; and (3)
methylenebis(di-n-
butyldithiocarbamate) at between 0.01 to 1.0wt. %, preferably 0.10-0.50 wt. %,
more preferably 0.15-
0.3 wt%.
The alkylated phenyl-a-naphthylamine (APAN or APANA) may be linear or branched
methylated,
ethylated, propylated, butylated, pentylated, hexylated, heptylated,
octylated, nonylated, decylated,
undecylated, dodecylated, tridecylated, and tetra-decylated, preferably an
octylated phenyl-a-
naphthylamine. Commercial examples of alkylated phenyl-a-naphthylamines are
Irganoxt L-06
manufactured by BASF Corporation, VANLUBE 1202 supplied by Vanderbilt
Chemicals, LLC, and
Naugalubet APAN manufactured by Chemtura Corporation.
The diphenylamine derivative of triazole, tolutriazole or benzotriazole is the
reaction product of
triazole, benzotriazole or tolutriazole with formaldehyde or paraformaldehyde
and diphenylamine or
alkylated diphenylamines. The alkylated diphenylamines may be linear or
branched methylated,
ethylated, propylated, butylated, pentylated, hexylated, heptylated,
octylated, nonylated, decylated,
undecylated, dodecylated, tridecylated, and tetra-decylated, preferably
octylated diphenylamine.
Commercial examples of diphenylamine derivatives of tolutriazole are VANLUBE
887 (50 wt.% of
an alkylated diphenylamine derivative of tolutriazole in mineral oil diluent)
and VANLUBE 887E
(50 wt.% of an alkylated diphenylamine derivative of tolutriazole in synthetic
ester diluent)
manufactured by Vanderbilt Chemicals, LLC. The derivative may be made
according to the teaching
of US 6743759.
Methylenebis(di-n-butyldithiocarbamate) may be Vanlube 7723 manufactured by
Vanderbilt
Chemicals, LLC.
BRIEF DESCRIPTION OF THE DRAWINGS
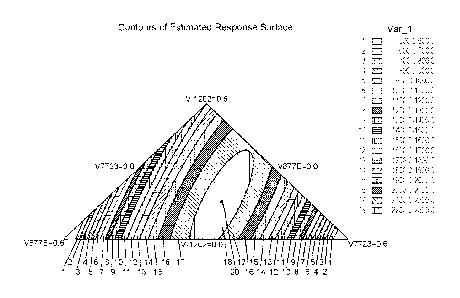
Figure 1 shows a contour plot generated from the data in Table 1.
DESCRIPTION OF THE INVENTION
The improved antioxidant additive composition of the invention may be
incorporated in the lubricating
compositions by known methods in an amount effective to produce the desired
oxidation inhibiting
characteristics. In one embodiment of the invention, the amount may range from
about 0.01 to 5.0
2
CA 2955240 2018-05-30
CA 02955240 2017-01-13
WO 2016/036493 PCT/US2015/045255
percent by weight based on the total weight of the lubricating composition. In
another embodiment of
the invention, the amount range is about 0.1 to 3.0 percent of the additive
based on the total weight of
the lubricating composition. In a preferred embodiment, the additive is
present at about 0.25 to 1.0
percent. The compositions impart metal deactivating as well as oxidation
inhibiting properties to
natural and synthetic lubricants formulated as oils or greases.
The base oils employed as lubricant vehicles are typical oils used in
automotive and industrial
applications such as, among others, turbine oils, hydraulic oils, compressor
oils, heat transfer oils,
transmission oils, automotive and industrial gear oils, greases, shock
absorber fluids, metal working
fluids, aviation oils, two-stroke engine oils, natural gas engine oils, marine
oils, railroad oils, crankcase
oils and diesel oils. Natural base oils include mineral oils, petroleum oils,
and vegetable oils. The base
oil may also be selected from oils derived from petroleum hydrocarbon and
synthetic sources. The
hydrocarbon base oil may be selected from naphthenic, aromatic, and paraffinic
mineral oils. The
synthetic oils may be selected from, among others, ester-type oils (such as
silicate esters, pentaerythritol
esters and carboxylic acid esters), severely hydrogenated mineral oils,
silicones, silanes, polysiloxanes,
alkylene polymers, poly-alpha-olefins and poly-alkylene-glycol ethers.
The lubricating compositions optionally contain the necessary ingredients to
prepare the composition, as
for example dispersing agents, emulsifiers, demulsifiers, and viscosity
improvers. Greases may be
prepared by adding thickeners, as for example salts and complexes of fatty
acids, polyurca compounds,
clays and quarternary ammonium bentonite. Depending on the intended use of the
lubricant, other
functional additives may be added to enhance a particular property of the
lubricant.
The lubricating compositions may also contain one or more of the following
additives:
1. Borated and/or non-borated ashless dispersants
2. Additional antioxidant compounds
3. Seal swell compositions
4. Organic and organo-metallic friction modifiers
5. Extreme pressure/antiwear agents
6. Viscosity modifiers
7. Pour point depressants
3
8. Metallic detergents
9. Phosphates
10. Antifoamants
11. Rust inhibitors
12. Copper corrosion inhibitors
1. Borated and/or Non-Borated Dispersants
Non-borated ashless dispersants may be incorporated within the final fluid
composition in an amount
comprising up to 10 weight percent on an oil-free basis. Many types of ashless
dispersants listed below
are known in the art. Borated ashless dispersants may also be included.
(A) "Carboxylic dispersants" are reaction products of carboxylic acylating
agents (acids, anhydrides,
esters, etc.) containing at least about 34 and preferably at least about 54
carbon atoms reacted with
nitrogen-containing compounds (such as amines), organic hydroxy compounds
(such aliphatic
compounds including monohydric and polyhydric alcohols, or aromatic compounds
including phenols
and naphthols), and/or basic inorganic materials. Examples of these
"carboxylic dispersants'' are
described in British Patent 1,306,529 and in U.S. Pat. Nos. 3,219,666,
3,316,177, 3,340,281,
3,351,552, 3,381,022, 3,433,744, 3,444,170, 3,467,668, 3,501,405, 3,542,680,
3,576,743, 3,632,511,
4,234,435, and Re 26,433, which disclose carboxylic dispersants.
(B) "Amine dispersants" are reaction products of relatively high molecular
weight aliphatic or alicyclic
halides and amines, preferably polyalkylene polyamines. Examples thereof are
described, for example,
in U.S. Pat. Nos. 3,275,554, 3,438,757, 3,454,555, and 3,565,804 which
disclose amine dispersants.
(C) ''Mannich dispersants" are the reaction products of alkyl phenols in which
the alkyl group contains
at least about 30 carbon atoms with aldehydes (especially formaldehyde) and
amines (especially
polyalkylenc polyamines). The materials described in U.S. Pat. Nos. 3,036,003,
3,236,770, 3,414,347,
3.448,047, 3,539,633, 3,586,629, 3,591,598, 3,634,515, 3,725,480, and
3,726,882 which disclose
Mannich dispersants.
(D) Post-treated dispersants are obtained by reacting carboxylic, amine or
Mannich dispersants with
reagents such as urea, thiourea, carbon disulfide, aldehydes, ketones,
carboxylic acids, hydrocarbon-
substituted succinic anhydrides, nitriles, epoxides, boron compounds,
phosphorus compounds or the
4
CA 2955240 2018-05-30
like. U.S. Pat. Nos. 3,200,107, 3,282,955, 3,367,943, 3,513,093, 3,639,242,
3,649,659, 3,442,808,
3,455.832, 3,579,450, 3,600,372, 3,702,757, and 3,708,422 which disclose post-
treated dispersants.
(E) Polymeric dispersants are interpolymers of oil-solubilizing monomers such
as decyl methacrylate,
vinyl decyl ether and high molecular weight olefins with monomers containing
polar substituents, e.g.,
aminoalkyl acrylates or acrylamides and poly-(oxyethylene)-substituted
acrylates. Polymeric
dispersants are disclosed in U.S. Pat. Nos. 3,329,658, 3,449,250, 3,519,656,
3,666,730, 3,687,849, and
3,702,300 which disclose polymeric dispersants.
Borated dispersants arc described in U.S. Pat. Nos. 3,087,936 and 3,254,025
which disclose borated
dispersants.
Also included as possible dispersant additives are those disclosed in U.S.
Pat. Nos. 5,198,133 and
4,857,214. The dispersants of these patents compare the reaction products of
an alkenyl succinimide or
succinimide ashless dispersant with a phosphorus ester or with an inorganic
phosphorus-containing
acid or anhydride and a boron compound.
2. Additional antioxidant compounds
Other antioxidants may be used in the compositions of the present invention,
if desired. Typical
antioxidants include hindered phenolic antioxidants, secondary aromatic amine
antioxidants, sulfurized
phenolic antioxidants, oil-soluble copper compounds, organo-molybdenum
compounds, phosphorus-
containing antioxidants, organic sulfides, disulfides and polysulfides and the
like.
Illustrative examples of sterically hindered phenolic antioxidants include
ortho-alkylated phenolic
compounds such as 2,6-di-tert-butylphenol, 4-methyl-2,6-di-tert-butylphenol,
2,4,6-tri-tert-
butylphenol, 4-(N,N-dimethylaminomethyl)-2,6 -di-tert-butylphenol, 4-ethyl-2,6-
di- tertbutylphenol,
2,6-distyry1-4- nonylphenol, 1,6-hexamethylene-bis(3,5-di-tert-buty1-4-
hydroxyhydrocinnamate), 3,5-
di-tert-buty1-4- hydroxyhydrocinnamic acid, Clo-C14 alkyl esters, 3,5-di-tert-
buty1-4-
hydroxyhydrocinnamic acid, C7-C9 alkyl esters, 3,5-di-tert-butyl-4-
hydroxyhydrocinnamic acid, iso-
octyl ester, 3,5-di-tert-buty1-4- hydroxyhydrocinnamic acid, butyl ester, 3,5-
di-tert-butyl-
hydroxyhydrocinnamic acid, methyl ester, tetrakis-(3-(3,5-di-tert-buty1-4-
hydroxypheny1)-
propionyloxymethyl)methane, thiodiethylene bis(3,5-di- tert-buty1-4-
hydroxyhydrocinnamate),
octadecyl 3,5-di-tert-buty1-4- hydroxyhydrocinnamate, N,N'- bis(3,5-di-tert-
buty1-4-
hydroxyphenylpropionyl)hexamethylene diamine, N,N'- bis(3,5-di-tert-buty1-4-
CA 2955240 2018-05-30
hydroxyphenylpropionyl)trimethylene diamine, N,1\11-bis(3,5-di-tert-buty1-4-
hydroxyphenylpropionyl)
hydrazine and their analogs and homologs. Mixtures of two or more such
hindered phenolic
compounds are also suitable.
Other preferred hindered phenol antioxidants for use in the compositions of
this invention are
methylene-bridged alkylphenols, and these can be used singly or in
combinations with each other, or in
combinations with sterically-hindered unbridged phenolic compounds.
Illustrative methylene-bridged
compounds include 4,4'-methylenebis(6-tert-butyl-o-cresol), 4,4-
methylenebis(2-tert-amyl-o-cresol),
2,2'-methylenebis(4-methyl-6-tert-butylphenol), 4,4'-methylenebis(2,6-di-tert-
butylphenol), and
similar compounds. Particularly preferred are mixtures of methylene-bridged
alkylphenols such as are
described in U.S. Pat. No. 3,211,652.
Amine antioxidants, especially oil-soluble aromatic secondary amines may also
be used in the
compositions of this invention. Although aromatic secondary monoamines are
preferred, aromatic
secondary polyamines are also suitable. Illustrative aromatic secondary
monoamines include
diphenylamine, alkyl diphenylamines containing 1 or 2 alkyl substituents each
having up to about 16
carbon atoms, phenyl-f3-naphthylamine, and phenyl-a -napthylaminc.
A preferred type of aromatic amine antioxidant is an alkylated diphenylamine
of the general formula:
RI-C6H4-NH-C61-14-R2
where R1 is an alkyl group (preferably a branched alkyl group) having 4 to 12
carbon atoms, (more
preferably 8 or 9 carbon atoms) and R2 is a hydrogen atom or an alkyl group
(preferably a branched
alkyl group) having 4 to 12 carbon atoms, (more preferably 8 or 9 carbon
atoms). Most preferably, RI
and R2 are the same. One such preferred compound is available commercially as
Naugalube 438L, a
material which is understood to be predominately a 4,4'-dinonyldiphenylamine
(i.e., bis(4-
nonylphenyl)(amine)) in which the nonyl groups are branched. Another such
preferred compound is
available commercially as VANLUBEC0 961 or IRGANOX L57, a material which is
understood to
be a mixture of butylated and octylated alkylated diphenylamines.
Another useful type of antioxidant are 2,2,4-trimethy1-1,2-dihydroquinoline
(TMDQ) polymers and
homologs containing aromatized terminal units such as those described in U.S.
Patent 6,235,686.
6
CA 2955240 2018-05-30
Mixtures of different antioxidants may also be used.
3. Seal Swell Compositions
Compositions which are designed to keep seals pliable are also well known in
the art. A preferred seal
swell composition is isodecyl sulfolanc. The seal swell agent is preferably
incorporated into the
composition at about 0.1-3 weight percent. Substituted 3-alkoxysulfolanes are
disclosed in U.S. Pat.
No. 4,029,587.
4. Friction Modifiers
Friction modifiers are also well known to those skilled in the art. A useful
list of friction modifiers are
included in U.S. Pat. No. 4,792,410. U.S. Pat. No. 5,110,488 discloses metal
salts of fatty acids and
especially zinc salts. Useful friction modifiers include fatty phosphites,
fatty acid amides, fatty
epoxides, borated fatty epoxides, fatty amines, glycerol esters, borated
glycerol esters alkoxylated fatty
amines, borated alkoxylated fatty amines, metal salts of fatty acids,
sulfurized olefin, fatty
imidazolines, molybdenum dithiocarbamates (e.g., U.S. Pat. No. 4,259,254),
molybdate esters (e.g.,
U.S. Pat. No. 5,137,647 and U.S. Pat. No. 4,889,647), molybdate amine with
sulfur donors (e.g., U.S.
Pat. No. 4,164,473), and mixtures thereof
The preferred friction modifier is a borated fatty epoxide as previously
mentioned as being included
for its boron content. Friction modifiers are preferably included in the
compositions in the amounts of
0.1- weight percent and may be a single friction modifier or mixtures of two
or more.
5. Extreme Pressure/Antiwear Agents
Dialkyl dithiophosphate succinates may be added to provide antiwear
protection. Zinc salts are
preferably added as zinc salts of phosphorodithioic acids or dithiocarbamic
acid. Among the preferred
compounds for use are zinc, diisooctyl dithiophosphate and zinc dibenzyl
dithiophosphate and amyl
dithiocarbamic acid. Also included in lubricating compositions in the same
weight percent range as the
zinc salts to give antiwear/extreme pressure performance are dibutyl hydrogen
phosphite (DBPH) and
triphenyl monothiophosphate, and the thiocarbamate ester formed by reacting
dibutyl amine-carbon
disulfide- and the methyl ester of acrylic acid. The thiocarbamate is
described in U.S. Pat. No.
7
CA 2955240 2018-05-30
4,758,362 and the phosphorus-containing metal salts are described in U.S. Pat.
No. 4,466,894.
Antimony or lead salts may also be used for extreme pressure. The preferred
salts are of
dithiocarbamic acid such as antimony diamyldithiocarbamate. Examples of
commercial anti-wear and
Extreme Pressure agents that may be used include VANLUBE 727, VANLUBE 7611M,
VANLUBE 9123, VANLUBE 871 and VANLUBE 981 all manufactured by Vanderbilt
Chemicals, LLC. Triaryl phosphates may also be used as antiwear additives and
include triphenyl
phosphate, tricresol phosphate and tri-butylatedphenyl phosphate.
6. Viscosity Modifiers
Viscosity modifiers (VM) and dispersant viscosity modifiers (DVM) are well
known. Examples of
VMs and DVMs are polymethacrylates, polyacrylates, polyolefins, styrene-maleic
ester copolymers,
and similar polymeric substances including homopolymers, copolymers and graft
copolymers.
Summaries of viscosity modifiers can be found in U.S. Pat. Nos. 5,157,088,
5,256,752 and 5,395,539.
The VMs and/or DVMs preferably are incorporated into the fully- formulated
compositions at a level
of up to 10% by weight.
7. Pour Point Depressants (PPD)
These components are particularly useful to improve low temperature qualities
of lubricating oil. A
preferred pour point depressant is an alkylnaphthalene. Pour point depressants
are disclosed in U.S.
Pat. Nos. 4,880,553 and 4,753,745. PPDs are commonly applied to lubricating
compositions to reduce
viscosity measured at low temperatures and low rates of shear. The pour point
depressants are
preferably used in the range of 0.1-5 weight percent.
8. Detergents
Lubricating compositions in many cases also preferably include detergents.
Detergents as used herein
are preferably metal salts of organic acids. The organic acid portion of the
detergent is preferably a
sulphonate, carboxylate, phenate, or salicylate. The metal portion of the
detergent is preferably an
alkali or alkaline earth metal. Preferred metals are sodium, calcium,
potassium and magnesium.
8
CA 2955240 2018-05-30
Preferably, the detergents are overbased, meaning that there is a
stoichiometric excess of metal over
that needed to form the neutral metal salt.
Preferred overbased organic salts are the sulfonate salts having a
substantially oleophilic character and
which are formed from organic materials. Organic sulfonates are well known
materials in the lubricant
and detergent arts. The sulfonate compound should preferably contain on
average from about 10 to
about 40 carbon atoms, more preferably from about 12 to about 36 carbon atoms
and most preferably
from about 14 to about 32 carton atoms on average. Similarly, the phenates,
oxy-lates and carboxylates
preferably have a substantially oleophilic character.
Examples of detergents can be found in U. S. Patent Nos. 2228654, 2250188,
2252663, 2865956,
3150089, 3256186 and 3410798. The amount of the overbased salt utilized in the
composition is
preferably from about 0.1 to about 10 weight percent on an oil free basis. The
overbased salt is usually
made up in about 50% oil with a TBN range of 10-600 on an oil free basis.
Borated and non-borated
overbased detergents are described in U.S. Pat. Nos. 5,403,501 and 4,792,410.
9. Phosphates
The lubricating compositions can also preferably include at least one
phosphorus acid, phosphorus acid
salt, phosphorus acid ester or derivative thereof including sulfur-containing
analogs preferably in the
amount of 0.002-1.0 weight percent. The phosphorus acids, salts, esters or
derivatives thereof include
compounds selected from phosphorus acid esters or salts thereof, phosphites,
phosphorus-containing
9
CA 2955240 2018-05-30
CA 02955240 2017-01-13
WO 2016/036493 PCT/US2015/045255
amides, phosphorus-containing carboxylic acids or esters, phosphorus
containing ethers and mixtures
thereof
In one embodiment, the phosphorus acid, ester or derivative can be a
phosphorus acid, phosphorus acid
ester, phosphorus acid salt, or derivative thereof. The phosphorus acids
include the phosphoric,
phosphonic, phosphinic, and thiophosphoric acids including dithiophosphoric
acid as well as the
monothiophosphoric, thiophosphinic and thiophosphonic acids.
One class of compounds arc adducts of 0,0-dialkyl-phosphorodithioates and
esters of malcic or fumaric
acid. The compounds can be prepared by known methods as described in U.S. Pat.
No. 3,359,203, as for
example 0,0-di(2-ethylhexyl) S-(1,2-dicarbobutoxyethyl) phosphorodithioate.
Another class of compounds useful to the invention are dithiophosphoric acid
esters of carboxylic acid
esters. Preferred are alkyl esters having 2 to 8 carbon atoms, as for example
3-[[bis(1-
methylethoxy)phosphinothioyl]thio] propionic acid ethyl ester.
A third class of ashless dithiophosphates for use with the present invention
include:
(i) those of the formula
CH2¨COORi
(R-0)2-P-S-CH-000R1
wherein R and R1 arc independently selected from alkyl groups having 3 to 8
carbon atoms
(commercially available as VANLUBE 7611M, from R. T. Vanderbilt Co., Inc.);
(ii) dithiophosphoric acid esters of carboxylic acid such as those
commercially available as
IRGALUBE 63 from BASF Corp.;
(iii) triphenylphosphorothionates such as those commercially available as
IRGALUBE TPPT from
BASF Corp.; and
10. Antifoamants
Antifoaming agents are well-known in the art as silicone or fluorosilicone
compositions. Such
antifoam agents are available from Dow Corning Corporation and Union Carbide
Corporation. A
preferred fluorosilicone antifoam product is Dow FS-1265. Preferred silicone
antifoam products are
Dow Coming DC-200 and Union Carbide UC-L45. Also, a siloxane polyether
copolymer antifoamer
available from OSI Specialties, Inc. of Farmington Hills, Michigan may also be
included. One such
material is sold as SILWET'LL-7220. The antifoam products are preferably
included in the
compositions of this invention at a level of 5 to 80 parts per million with
the active ingredient being on
an oil-free basis.
11. Rust inhibitors
Embodiments of rust inhibitors include metal salts of alkylnaphthalenesulfonic
acids.
12. Copper corrosion inhibitors
Embodiments of copper corrosion inhibitors which may optionally be added
include thiazoles,
triazoles and thiadiazoles. Example embodiments of such compounds include
benzotriazole,
tolyltriazole, octyltriazole, decyltriazole, dodecyltriazole, 2-mercapto
benzothiazole, 2,5- dimercapto-
1,3,4- thiadiazole, 2-mercapto-5-hydrocarbylthio-1,3,4-thiadiazoles, 2-
mercapto-5- hydrocarbyldithio-
1,3,4- thiadiazoles, 2,5-bis(hydrocarbylthio)-1,3,4-thiadiazoles, and 2,5-
bis(hydrocarbyldithio)-1,3,4-
thiadiazoles.
Working Examples
The following examples are given for the purpose of illustrating the invention
and are not intended to
limit the invention. All percentages and parts are based on weight unless
otherwise indicated.
RPVOT Testing
The Rotating Pressure Vessel Oxidation Test (RPVOT, ASTM D 2272) is a turbine
oil oxidation test
used as a quality control tool for new and used turbine oils of known
composition, as well as a research
tool for estimating the oxidative stability of experimental oils. The test
evaluates the oxidative stability
of a turbine oil at elevated temperatures and oxygen pressures and in the
presence of a copper coil
oxidation catalyst and water. A rotating glass pressure vessel provides
maximum oil-oxygen contact.
11
CA 2955240 2018-05-30
Results are reported as the time to a 25 psi drop in oxygen pressure. The test
oil, copper coil and water
are placed in the glass oxidation pressure vessel. The vessel is sealed and
pressurized to 90 psi of
oxygen. The pressurized vessel is placed in a high temperature bath maintained
at 150 C and
continuously rotated throughout the test period. The test is monitored for
consumption of oxygen. The
time from the start of the test to the point when the pressure of the vessel
has dropped 25 psi is defined
as the oxidation life or oxidation induction time.
Example 1
In this series of experiments, test fluids were blended as defined in the
table below. The APANA was
an octylated phenyl-ct-naphthylamine available commercially as Vanlube 1202
from Vanderbilt
Chemicals, LLC. ODPA derivative of tolutriazole is the octylated diphenylamine
derivative of
tolutriazole. 50% diluted in a polyol ester diluent, available commercially as
Vanlubeg 887E from
Vanderbilt Chemicals, LLC. The methylenebis(dibutyldithiocarbamate) (MBDTC) is
available
commercially as Vanlubee 7723. The three additives were blended into an ISO 32
Group II base oil
and tested in the RPVOT. Experiments were run in duplicate and the results
averaged. The weight
percentages given throughout are with respect to the entire lubricating
composition including the base
oil. The test results are presented in Table 1 below.
TABLE 1
Oil ID APANA ODPA MBDTC Number of RPVOT Life Synergistic
(wt%) derivative of (wt%) RPVOT (average in Effect
(minutes
tolutriazole* tests minutes) above expected
(wt%) value)
A 0.6 2 1719 NR
0.6 2 78 NR
=
0.3 2 179 NR
0.3 0.3 2 2011 1113
0.3 0.15 2 1130 181
0.15 0.3 2 2180 2052
0.2 0.1 0.2 2 2299 0
(invention)
*ODPA derivative of tolutriazole is present as a 50 wt. % diluted in Polyol
ester HATCOLTm 2965.
The data shown here and throughout the other tables is given as weight % of
the ODPA derivative of
tolutriazole alone.
12
CA 2955240 2018-05-30
Note from the test results in Table 1 that the best performing oil was that
which contained all three
additives, the APANA, the ODPA derivative of tolutriazole and the MBDTC. The
results in the table
below show antioxidant synergies that exist: (1) synergy between APANA and
ODPA derivative of
tolutriazole, (2) synergy between APANA and MBDTC, and (3) synergy between
ODPA derivative of
tolutriazole and MBDTC.
Figure 1 shows a contour plot generated from the data in Table 1 using a
statistical analysis program
called Statgraphics Centurion' m XVI Version 16.2.04 (64-bit). The program
takes data from designed
experiments like that in Table 1 and provides response surface analyses in the
form of contour plots
where each series of lines represents an increase in response or performance.
The cross near the center
of the contour plot represents the maximum response possible in the series of
experiments. Note the
maximum response comes very close to the mid-point of the plot which is the
area where all three
components are present.
Example 2
In this series of experiments shown in Table 2, different tolutriazoles were
tested and compared to the
ODPA derivative of tolutriazole. Test fluids were blended as defined in the
table below. The APANA
is octylated phenyl-ct-naphthylamine available commercially as Vanlube0 1202
from Vanderbilt
Chemicals, LLC. Note that the concentrations of the tolutriazole and
derivatives of tolutriazoles varied
in the formulations. These additives were blended at equal nitrogen content in
order to maintain
equivalent activity in these experiments. In these blends the 2EHA (2-ethyl
hexamine) derivative of
tolutriazole is a bis(2-ethylhexylamine) derivative of tolutriazole available
commercially as Cuvan
303 from Vanderbilt Chemicals, LLC. The additives were blended into an ISO 32
Group II base oil in
thc presence of 0.2 wt.% of a rust inhibitor VanlubeC) RI-A commercially
available from Vanderbilt
Chemicals, LLC and tested in the RPVOT. Experiments were run in duplicate and
the results averaged.
The test results are presented below.
13
CA 2955240 2018-05-30
CA 02955240 2017-01-13
WO 2016/036493 PCT/US2015/045255
TABLE 2
Oil ID Tolutriazole Tolutriazole Tolutriazole APANA, MBDTC, Number RPVOT
or Or component Wt% Wt% of Life
Tolutriazole Tolutriazole wt% (by RPVOT (average
derivative derivative, wt% Tests in
Type Wt% nitrogen) minutes)
ODPA 0.15 0.0162 0.15 0.15 2 1580
(invention) derivative
of
tolutriazole
2EHA 0.112 0.0162 0.15 0.15 2 1430
derivative
of
tolutriazole
tolutriazole 0.0513 0.0162 0.15 0.15 2 1630
ODPA 0.075 0.0081 0.15 0.3 2 2218
(invention) derivative
of
tolutriazole
2EHA 0.056 0.0081 0.15 0.3 2 1375
derivative
of
tolutriazole
tolutriazole 0.0256 0.0081 0.15 0.3 2 1671
The results show that the ODPA derivative of tolutriazole performs better than
the 2EHA derivative of
tolutriazole in both blends (H versus I and K versus L). The ODPA derivative
of tolutriazole performs
better than tolutriazole itself in one blend (K versus M) and equivalent to
tolutriazole in the other blend
(H versus J). However, it should be pointed out that tolutriazole itself is
not a practical additive to use in
turbine oils and lubricants in general due to its very limited solubility.
Example 3
In this series of experiments shown in Table 3, different antioxidants were
tested and compared to the
APANA (same compound as in Example 2). Test fluids were blended as defined in
the table below. In
these blends NDPA is nonylated diphenylamine available commercially as
Naugalubet 438L from
Chemtura Corporation, MBDTBP is 4,4'-methylenebis(2,6-di-tert-butylphenol)
available commercially
as ETHANOX 4702 from SI Group, 2,6-DTBP is 2,6-di-tert-butylphenol, and TMQ
is 2,2,4-
Trimethy1-1,2-Dihydroquinoline polymer available commercially as Vanlube RD
from Vanderbilt
Chemicals, LLC. The additives were blended into an ISO 32 Group II base oil in
the presence of 0.2 wt.
% of a rust inhibitor Vanlube R1-A commercially available from Vanderbilt
Chemicals, LLC and
tested in the RPVOT as defined below. The test results are presented below.
14
CA 02955240 2017-01-13
WO 2016/036493 PCT/US2015/045255
Table 3
Oil ID Antioxidant Antioxidant ODPA MBDTC Number of RPVOT
Type Wt% derivative (Wt%) RPVOT Life
of tests (average in
tolutriazole minutes)
(Wt%)
N APANA 0.15 0.15 0.15 2 1580
(invention)
0 NDPA 0.15 0.15 0.15 3 1325
P MBDTBP 0.15 0.15 0.15 3 883
Q 2,6-DTBP 0.15 0.15 0.15 2 1081
R TMQ 0.15 0.15 0.15 2 837
S APANA 0.15 0.075 0.3 2 2218
(invention)
T NDPA 0.15 0.075 0.3 2 1797
U MBDTBP 0.15 0.075 0.3 2
1054
/ 2,6-DTBP 0.15 0.075 0.3 2
1385
W TMQ 0.15 0.075 0.3 2 1173
The results show that the inventive blend containing APANA performs better
than the non-inventive
blends containing other commonly used lubricant antioxidants (N versus 0, P, Q
and R, and S versus T,
U, V and W) . It should be pointed out that TMQ itself is not a practical
additive to use in turbine oils
due to its limited solubility.
Example 4
In this series of experiments shown in Table 4, the various additives were
blended into a 650 solvent
neutral Group I base oil. No other additives were present. The blends were
prepared as defined below. In
these blends APANA * is octylated phenyl-a-naphthylamine available
commercially as Irganox(R) L 06
from BASF Corporation, and APANA ** is octylated phenyl-a-napthylamine
available commercially as
Vanlube 1202 from Vanderbilt Chemicals, LLC. Results of the RPVOT testing are
shown below:
CA 02955240 2017-01-13
WO 2016/036493 PCT/US2015/045255
Table 4
Oil ID APANA (wt%) ODPA MBDTC Number of RPVOT Life
derivative of (wt%) RPVOT tests (average
in
tolutriazole minutes)
(wt%)
X (baseoil 1 20
only)
0.5* 2 148
0.5** 2 138
AA 0.0375 0.425 1 268
AB 0.5 1 94
AC (invention) 0.25* 0.01875 0.2125 1 323
AD 0.25* 0.25 1 178
AE (invention) 0.25** 0.01875 0.2125 1 330
AF 0.25** 0.25 1 179
The results clearly show that the three way combination of APANA, ODPA
derivative of tolutriazole,
and MBDTC is a significantly better antioxidant compared to the non-inventive
examples.
PDSC Testing
Pressurized Differential Scanning Calorimetry (PDSC) is a commonly used
technique for evaluating a
wide variety of engine and industrial lubricating oils. The simplicity of the
test combined with its
excellent repeatability and the ability to easily modify test conditions,
makes it a valuable tool for
quality control and lubricant research. The test evaluates the oxidative
stability of a thin-film of lubricant
under high temperature and high oxygen pressures. Results are generally
reported as the induction time
to an exothermic release of heat caused by oxidation of the thin-film of oil.
A thin-film of oil is placed in
a sample holder and then added to the DSC pressure cell. The cell is
pressurized with the specified gas
and subjected to a specified heating sequence that is accurately controlled by
the DSC computer control
system. The most commonly used heating sequence is the isothermal method. The
experiment is run
until an exothermic release of heat is observed. The time from the start of
the experiment to the
exothermic release of heat represents the onset to oil oxidation and is
reported as the oxidation induction
time. The standardized test procedure ASTM D 6186, Standard Test Method for
Oxidation Induction
Time of Lubricating Oils by Pressure Differential Scanning Calorimetry (PDSC)
was the test procedure
utilized in the following examples.
16
CA 02955240 2017-01-13
WO 2016/036493 PCT/US2015/045255
Example 5
In this series of experiments shown in Table 5, the various additives were
blended into a PAO 6 cSt.
Group IV base oil. No other additives were present. The blends were prepared
as defined below. PDSC
testing was performed in the isothermal mode at 180 C.
Table 5
Oil ID APANA ODPA MBDTC Number of PDSC OIT
(wt%) derivative of (wt%) PDSC tests (average in
tolutriazole minutes)
(wt%)
AG (baseoil 1 0
only)
AH 0.5* 2 94
Al 0.5** 2 94
AJ 0.0375 0.425 1 36
AK 0.5 1 6
AL 0.25 1 14
AM (invention) 0.25* 0.01875 0.2125 2 96
AN 0.25* 0.25 2 70
AO (invention) 0.25** 0.01875 0.2125 1 99
AP 0.25** 0.25 2 92
The results show that the three way combination of APANA, ODPA derivative of
tolutriazole, and
MBDTC performs better than most other combinations (AM and AO versus AJ, AK,
AL, AN and AP)
and about the same as the APANA's alone (AM and AO versus AH and Al). It is
pointed out that the
APANA's have two key negative attributes when used alone that make the three
way combination much
more desirable. These are (1) the APANA's are solids that are difficult to
handle and blend into
lubricants , and (2) the APANA's used by themselves provide a finished
lubricant formulation that is
considerably cost prohibitive. While a blend of APANA/ODPA derivative of
tolutriazole/MBDTC as
described in this invention and examples AM and AO is a liquid product that
blends readily in
lubricating oils. It is additionally noted that the two component AP example,
while providing acceptable
results, is also in the form of a solid.
17