Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
1
LOW CO2 EMISSIONS STEAM AND/OR HYDROGEN GENERATION
SYSTEMS AND PROCESSES FOR HYDROCARBONS RECOVERY OR
UPGRADING
FIELD OF THE INVENTION
The present invention relates to systems and processes for generating steam
and/or
hydrogen for use in thermal recovery and/or upgrading of hydrocarbons from
subterranean formations. More specifically, the present invention relates to
reduced
carbon dioxide emissions steam and/or hydrogen generation systems and methods.
BACKGROUND OF THE INVENTION
An increasing pressure on oil companies to reduce greenhouse gas (GHG)
emissions
and water footprint of bitumen extraction and upgrading has led to seeking
alternative
methods that are both environmentally friendlier and economically competitive
with
the conventional ways of bitumen production from tar sands (Charpentier, A.
D., J. A.
Bergerson, et al. (2009). "Understanding the Canadian oil sands industry's
greenhouse
gas emissions." Environmental Research Letters 4(1): 014005; Levi, M. A.
(2009).
The Canadian oil sands: energy security vs. climate change, Council on Foreign
Relations; Bergerson, J. A. and D. W. Keith (2010). "The truth about dirty
oil: Is CCS
the answer?" Environmental Science & Technology 44(16): 6010-6015).
Theiinal hot-water or steam-based oil recovery processes (sometimes also
referred to
as thermal oil processes or thermal oil recovery) such as Hot Water Flooding,
Steam
Flooding, Steam Assisted Gravity Drainage (SAGD), Cyclic Steam Stimulation
(CSS), or recovery processes that start with hot water and/or steam injection
(e.g. in-
situ combustion) use large volumes of water in the form of hot water and/or
steam, to
deliver energy, in the form of heat, underground to mobilize oil in a
subterranean
formation (also sometimes referred to herein as a "subsurface hydrocarbon
reservoir"). "Oil" used herein refers to one or more of oil, heavy oil, and
bitumen.
Sample schematics of the SAGD, CSS, and Steam Flooding processes are shown in
Figures 2, 3 and 4, respectively.
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
2
The injected water is often produced back to the surface with the mobilized
oil and is
then processed by water treatment and re-heated into hot water or steam and re-
injected into the subterranean formation. The typical injected water-to-oil
ratio is
equal to three or higher volumes of water (expressed as cold water equivalent,
that is,
at standard conditions) to one volume oil, i.e. >3 ni3 water per m3 oil.
Often, in most
operations, between about 80 and 90% of the water injected into the reservoir
is
recovered during water treatment operations. This means that make-up water is
required for these processes to replenish the lost water.
The generation of hot water and/or steam is accomplished by combusting fuel,
which
in most operations, is natural gas or methane. In the present disclosure, the
term "fuel"
includes natural gas and methane but does not exclude other fuels (e.g. other
hydrocarbon fuels such as propane, octane, etc.); the terms "natural gas" and
"methane'' herein are interchangeable, and refer to natural gas, methane, or a
combination thereof. The combustion of methane yields heat which is used to
convert
water to hot water and/or steam. In addition, this combustion of fuel produces
large
amounts of carbon dioxide (CO2) which are typically emitted into the
atmosphere.
For steam-based recovery processes such as SAGD and CSS, at a steam-to-oil
ratio
(SOR) equal to about 3 m3/m3 (steam volume expressed as cold water equivalent
volume), about 0.6 tonnes of carbon dioxide is emitted per m3 oil produced. As
shown in Figure 1, at a SOR equal to about 6 m3/m3, about 1.4 tonnes of carbon
dioxide is emitted per m3 oil produced. Thus, these steam-based recovery
processes
have high carbon intensities and thus, given environmental interests in
reducing
carbon dioxide emissions from recovery processes, there is a need for
processes that
generate steam for thermal oil recovery without significant carbon dioxide
emissions.
Further, there is a need to reduce or eliminate make-up water requirements. In
traditional practice, natural gas is combusted in industrial boilers where
water is
conveyed in tubes through a combustion zone. The water is heated and, for
steam
generation, boils within the tubes. For example, most industrial boilers use
fire tubes
or multiple pass boiler tubes. These forms of boilers keep the water and heat
source,
that is, the combustion zone, physically separate and thus heat transfer is
controlled
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
3
by heat transfer surfaces, for example, the heat transfer area of the fire
tubes. A
significant fraction of the heat generated in these types of boilers is lost
with the
combustion gases which are emitted to the environment.
Other direct contact boilers, for example, the method taught in Canadian
Patent No.
2,751,186, combine the combustion process and feed-water within a single
process
unit so that heat transfer is done within the combustion zone and thus there
are no heat
transfer surfaces. This leads to higher thermal efficiency than a typical drum
boiler or
one-through steam generator since in direct contact steam generation, no heat
is lost
with stack gases. However, the product of a direct contact steam generator, if
used
with oxygen as taught by US Patent Nos. 5,680,764 and 6,170,624, is a mixture
of
steam and carbon dioxide. Even in direct contact steam generators, despite
their
increased thermal efficiency with respect to traditional steam generators,
carbon
dioxide is generated within the process.
Steam-based oil recovery processes use large amounts of water and emit carbon
dioxide into the atmosphere. There is potential that the carbon dioxide can be
used
for enhanced oil or gas recovery, see for example Canadian Patent No.
2,576,896 and
Canadian Patent Application No, 2,619,557. In all those cases, the methods of
capturing carbon dioxide for use in enhanced hydrocarbon recovery are
complicated
and capital intensive. However, after injectant (hot water and/or steam and
carbon
dioxide) breakthrough occurs in the recovery process (breakthrough occurs when
the
injected materials reach the production well), a significant fraction of the
injected
carbon dioxide will be produced with the produced oil and will be potentially
released
into the atmosphere. Thus, the capability for the enhanced oil recovery
process to
sequester carbon dioxide is limited. Carbon dioxide sequestration in deep
saline
aquifers is a potential technology for dealing with carbon dioxide emissions
but it has
not yet been proven to be commercially viable and is prone to many risks
especially
since the carbon dioxide must be sequestered underground for many thousands to
tens
of thousands of years.
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
4
SUMMARY OF THE INVENTION
According to a broad aspect of the present invention, there is provided a
steam and/or
hydrogen generation system for use with a bitumen recovery or upgrading
operation
comprising: a decarbonization unit for receiving a first fuel and decomposing
the first
fuel into carbon black and hydrogen; and a combustor for receiving (i) air;
(ii) the
hydrogen or a second fuel, which is the same or different from the first fuel;
and (iii)
water and/or steam, and combusting same to generate heat for heating water to
generate steam.
According to another broad aspect of the present invention, there is provided
a
method for generating steam and/or hydrogen comprising: decarbonizing a first
fuel
to yield carbon black and hydrogen in a decarbonization process; combusting
air with
the hydrogen or a second fuel to generate heat, the second fuel being the same
as or
different from the first fuel; and heating water with the heat generated from
the
combustion to produce steam.
According to yet another broad aspect of the present invention, there is
provided a
steam and/or hydrogen generation system for use with a bitumen recovery or
upgrading operation comprising: a decarbonization unit for carrying out a
decarbonization process that decomposes natural gas into carbon black and
hydrogen;
and a steam generator having a boiler and at least one tube, wherein
combustion of the
hydrogen occurs in the boiler and water passes through the at least one tube
and is
heated from the hydrogen combustion to generate steam.
According to still another aspect of the present invention, there is provided
a method
for generating steam and/or hydrogen comprising: decarbonizing natural gas to
yield
carbon black and hydrogen; injecting the hydrogen and air into a combustor;
igniting
the hydrogen and air; and feeding water through at least one tube passing
through the
ignited hydrogen and air to generate steam.
Further and other aspects of the invention will become apparent to one skilled
in the
art when considering the following detailed description of the preferred
embodiments
provided herein.
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
BRIEF DESCRIPTION OF THE DRAWINGS
Drawings are included for the purpose of illustrating certain aspects of the
invention.
5 Such drawings and the description thereof are intended to facilitate
understanding and
should not be considered limiting of the invention. Drawings are included, in
which:
Figure 1 is a graph illustrating the amount of carbon dioxide produced versus
the
steam-to-oil ratio (100% steam quality generated with efficiency of the steam
generator equal to 0.75) from a prior art steam-based recovery process
operating at
2,100 1cPa.
Figure 2a and 2b show schematic side and front cross-sectional views,
respectively, of
a subterranean formation undergoing a prior art Steam Assisted Gravity
Drainage
("SAGD") thermal recovery process.
Figure 3 is a schematic view of a prior art Cyclic Steam Stimulation thermal
recovery
process.
Figure 4 is a schematic view of a prior art Steam Flood thermal recovery
process.
Figure 5 is a schematic flow diagram of a natural gas ("NG") decarbonization
process.
Figure 6 is a schematic flow diagram showing bitumen recovery coupled to NG
decarbonization technology and oxy-fired NG combustion, according to one
embodiment of the present invention.
Figure 7 is a schematic flow diagram showing a zero emissions bitumen recovery
process, according to another embodiment of the present invention.
Figure 8 is a schematic flow diagram showing a process for production of
hydrogen
by NG decomposition for synthetic oil production from bitumen, according to
yet
another embodiment of the invention.
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
6
Figure 9 is a schematic flow diagram showing a process for zero emissions
synthetic
oil production from bitumen using hydrogen production from NG decarbonization,
according to still another embodiment of the invention.
Figure 10 is a graph showing energy intensity and CO2 intensity (CO2I) of
steam
generation for bitumen recovery for various processes.
Figure 11 is a graph showing energy intensity and CO2 intensity (CO2I) of
steam
generation for bitumen upgrading for various processes.
Figure 12 is a graph showing average values of steam energy requirements and
GHG
emissions from steam generation via a prior art process for some SAGD projects
in
Alberta, Canada.
DETAILED DESCRIPTION
The detailed description set forth below in connection with the appended
drawings is
intended as a description of various embodiments of the present invention and
is not
intended to represent the only embodiments contemplated by the inventor. The
detailed description includes specific details for the purpose of providing a
comprehensive understanding of the present invention. However, it will be
apparent
to those skilled in the art that the present invention may be practiced
without these
specific details.
In this description, the word "zero" means near zero or substantially zero.
The word
"emissions" refers to carbon dioxide emissions. Further, the word "steam"
refers to
steam, hot water, or a combination thereof. The words "air" and "oxygen" are
interchangeable and each includes (i) pure oxygen and/or (ii) a combination of
oxygen
and other gases.
In one broad aspect of the present invention, there is provided a process for
generating
steam by decarbonizing the input fuel to produce carbon black, a solid stable
form of
carbon, and hydrogen. In a preferred embodiment, the input fuel is natural
gas;
however, other hydrocarbon fuels may be used. De-carbonizing the fuel into
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
7
hydrogen, a high energy content fuel, helps reduce downstream carbon dioxide
emissions, thereby obviating the requirement for subsequent carbon dioxide
sequestration. Water can be generated from hydrogen combustion with air.
More specifically, the process uses natural gas decarbonization ("NGD" and
sometimes also referred to as "natural gas decomposition") technology which is
a
process that produces hydrogen via natural gas decarbonization, in the field
of
bitumen extraction and/or upgrading. The present invention provides a process
for
low emissions steam generation for steam assisted gravity drainage (SAGD) and
cyclic steam stimulation (CSS) applications of bitumen recovery. This process
produces hydrogen and carbon black from a known NGD technology (shown for
example in Figure 5). In one embodiment, the process used to produce hydrogen
and
carbon black from natural gas may be either the Thermal Black process or a
variant
thereof.
The produced hydrogen is then used to generate steam for thermal oil recovery.
The
present invention may offer an environmentally friendlier alternative to the
existing
technologies that burn natural gas to generate steam for bitumen recovery.
This
method presents also an alternative to the conventional steam methane
reforming
(SMR) process widely used to produce hydrogen for bitumen upgrading
(Gaudemack,
B. and S. Lynum (1998). "Hydrogen from natural gas without release of CO2 to
the
atmosphere." International journal of hydrogen energy 23(12): 1087-1093).
Another application of the present invention may be in bitumen upgrading.
Hydrogen
produced from the SMR process may be added to a bitumen upgrader to produce
synthetic crude oil (SCO). For example, the SMR process generates about 10
tons
CO2 emitted per ton H2 produced, while the present invention may result in
about 1
ton CO2 emissions per ton H2. Therefore, application of the present invention
may
potentially result in approximately 90% reduction of CO2 emissions from the
bitumen
upgrading ¨ hydrotreating ¨ process. CO2 emissions may be further reduced if a
fraction of the produced hydrogen is used to provide at least part of the
energy
required for the process.
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
8
With reference to Figure 5, the Thermal Black process involves decomposing
natural
gas in the absence of oxygen. A reactor is preheated to greater than 1,000 C,
preferably about 1,300 C, and natural gas is injected into the reactor and is
decomposed into carbon and hydrogen. The carbon/hydrogen mixture is cooled
with
water and the carbon is separated from the hydrogen in large bag filters.
Thermal
decomposition of methane is moderately endothermic with reaction chemistry
given
by:
CH4 + Heat C + 2H2 (AH=75.6 kJ/mol) (1)
The energy requirement per mole of hydrogen produced (37.8 kJ/mol) is
considerably
less than that for the steam reforming process (63.3 kJ/mol H2). An energy
penalty
incurred is typically less than 10% of the heat of methane combustion ¨
equivalent to
burning 14% of the hydrogen product.
The present invention comprises using the hydrogen by-product from the
decarbonization of NG as fuel to preheat another reactor or for other
applications,
such as steam generation for bitumen recovery and hydrogen production for
hydrotreatment of bitumen to produce synthetic crude oil.
The conventional method of producing hydrogen used for upgrading bitumen to
synthetic crude oil (SCO) is through steam methane reforming (SMR). The
process
involves a catalytic conversion of methane and steam to hydrogen and CO2.
About
fifty percent of the hydrogen produced comes from steam and the other (about)
fifty
percent from methane. The reactions of SMR are as follows:
CH4 + H20 ¨+ CO + 3H2 (2)
CO + H20 <¨>. CO2 + H2 (3)
Reaction (2) involves a highly endothermic synthetic gas generation reaction
(760-
925 C). To ensure a minimum concentration of CH4 in the product stream, the
process
generally employs a steam to carbon ratio of 3 to 5 at a process temperature
of 815 C
and pressures up to 35 bar. Conversion levels of this reaction are usually
below 90%.
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
9
Water-gas shift reaction (3), an exothermic reaction (200-400 C), following a
heat
recovery step, reduces the CO content of the product to compositions less than
0.5%.
The synthetic gas generation and the gas-shift reaction have energy
requirements of
83.7 kJ/mole H2 and -41.2 kJ/mole H2, respectively, whereas the overall
theoretical
energy requirement of SMR is 40.75 kJ/mole H2. The SMR process generates
between about 8.7 and 9.8 tons CO2A011 H2 produced. The thermal efficiency of
SMR
can range from 65% to 89%.
The present invention may be used with any hot water or steam-based thermal
recovery processes including water flooding, steam flooding, Steam Assisted
Gravity
Drainage (SAGD), Cyclic Steam Stimulation (CSS), or combined steam-additive
processes where the additive can be one or more of non-condensable gas,
solvent, or
surfactant. The present invention may also be used in other thermal oil
recovery
applications and for other substances for co-injection, which may further
improve
thermal oil recovery processes.
According to another broad aspect of the present invention, there is provided
a hot
water and/or steam generation system having: a NGD process, which may be
powered
by natural gas or produced hydrogen from the NGD process; and a steam
generator
having an inlet for hydrogen and water, an oxidant in forms of oxygen or air,
a
combustion chamber, and an outlet. In the combustion chamber, heat is added
directly
to the water which converts it to hot water and/or steam.
Further, the resulting steam arising from the water and from the hydrogen
combustion
reactions may be used as an injectant in a thermal oil process. An additive
may be
added to the injectant steam. In one embodiment, the additive is one or more
of
hydrocarbons, propane, butane, pentane, hexane, natural gas condensates,
diluent,
naphtha. The carbon black produced from the process is a by-product that may
be sold
for other industrial applications.
In one embodiment, the produced hydrogen or other fuel is combusted in a
boiler and
water is passed through at least one tube in the boiler and heated therein
until it is hot
water and/or steam. An oxy-fired combustor and air-fired H2 combustor
described
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
hereinbelow may have a boiler for combusting fuel or hydrogen, and at least
one tube
for the passage and heating of water therethrough for steam generation. The
product
water from the combustor and/or combustion chamber is re-cycled as make-up
water
for hot water and/or steam generation.
5
For use in thermal oil recovery processes such as SAGD or CSS, the steam
resulting
from the steam generation process of the present invention is between about
500 and
about 15,000 kPa. In CSS operations, the preferred range is between about
8,000 and
about 13,000 kPa whereas in SAGD, the preferred range is from about 500 to
about
10 7,000 kPa. The processes described herein may optionally add light
hydrocarbons or
other substances to the mixture of steam to act as a further solvent in the
subterranean
formation.
In another embodiment, the steam generation system described herein may be
located
remotely at well sites, in contrast to the standard practice of building a
central plant
for steam generation. .
In the embodiments described herein, carbon dioxide emissions from fuel
combustion
may be reduced or even obviated since carbon is extracted from the fuel in the
form of
carbon black, a marketable product, prior to steam generation. Steam is then
generated by combusting the remaining hydrogen extracted from the fuel and,
when
hydrogen is combusted, the result is steam and heat, without carbon dioxide.
Through
the use of the present invention, water is created as a by-product of hydrogen
combustion. Therefore, the need for make-up water may potentially be reduced
or
eliminated in the process.
A steam and/or hydrogen generation system is described herein, which comprises
a
decarbonization unit for receiving a first fuel and decomposing the first fuel
into
carbon black and hydrogen; and a combustor for receiving (i) air; (ii) the
hydrogen or
a second fuel, which is the same or different from the first fuel; and (iii)
water and/or
steam, and combusting same to generate heat for heating water to generate
steam.
In another embodiment, a steam and/or hydrogen generation system is provided
herein, which comprises a decarbonization unit for carrying out a
decarbonization
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
11
process that decomposes natural gas into carbon black and hydrogen; and a
steam
generator having a boiler and at least one tube, wherein combustion of the
hydrogen
occurs in the boiler and water passes through the at least one tube and is
heated from
the hydrogen combustion to generate steam.
In one embodiment, the steam and/or hydrogen generation system is located
remotely
at a well site.
Methods for generating steam and/or hydrogen are provided herein. In one
embodiment, the method comprises decarbonizing a first fuel to yield carbon
black
and hydrogen in a decarbonization process; combusting oxygen with the hydrogen
or
a second fuel to generate heat, the second fuel being the same as or different
from the
first fuel; and
heating water with the heat geneiated from the combustion to produce steam. In
another embodiment, the method comprises decarbonizing natural gas to yield
carbon
black and hydrogen; injecting the hydrogen and oxygen into a combustor;
igniting the
hydrogen and oxygen; and feeding water through at least one tube passing
through the
ignited hydrogen and oxygen to generate steam.
In one embodiment, the produced hydrogen and air are injected into the
combustor at
a pressure between about 500 and about 15,000 kPa. The steam generated may be
used as an injectant in a thermal oil process. In a further embodiment, the
method is
carried out remotely at a well site.
Business-as-usual case of a bitumen recovery plant
Conventional in situ bitumen recovery processes use steam generated from NG-
fired
steam generators. Steam to oil ratio (SOR) is a key unit of measuring
efficiency of the
bitumen recovery process. SOR measures the volume of steam injected into a
bitumen
reservoir to mobilize and recover a unit volume of bitumen. For example,
considering
a business-as-usual (BAU) case of a bitumen recovery plant having an SOR of 3
m3/m3 (wet basis) and producing bitumen at 147,850 bbl/d (about 23,508 m3/d)
by
burning NG, the BAU bitumen recovery plant uses about 3,605 tons/d NG (15 C
and
15 bar) to generate high quality steam (e.g. the steam has a quality of about
0.96 to
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
12
1.0 produced at 35 bar) for use in SAGD operations. High quality steam refers
to high
pressure steam of quality above about 0.95. The NG used in this example is
composed
of 95 mol.% C1-14, 2.5 mol.% C2H6, 1.6 mol.% N2, 0.7 mol.% CO2 and 0.2 mol.%
C3H8 and has low heating value (LEIV) of 48 kJ/kg.
This BAU process provides 113,347 GJ/d for thermal recovery of bitumen. The
process emits 6,380 tons/d CO2 emissions, equivalent to 43 kg-0O2/bbl bitumen
produced. Assuming 90% of the steam is recovered as water and reused in steam
generation, this plant has a water consumption footprint of 7,045 in3/d (6,430
tons/d).
Bitumen recovery coupled to NGD technology
One embodiment of the present invention involves applying the NG
decarbonization
technology to the BAU bitumen recovery process, which comprises decomposition
(or thermal cracking) of NG to produce hydrogen and pure carbon. After
separating
the hydrogen from the carbon, the hydrogen is combusted in air to produce
steam for
bitumen recovery. Combustion of hydrogen produces pure water, which may be
sufficient to reduce or eliminate the need for make-up water.
NG decarbonization is energy intensive and its energy requirement may be
supplied
by oxy-fired NG combustion (referred to herein as "oxy-fired combustion") or
burning a fraction of the hydrogen produced from the NO decarbonization
(referred to
herein as "hydrogen combustion").
In an oxy-fired combustion method, a mix of steam generated and flue gas
stream,
which is rich in CO2, is injected into a hydrocarbon reservoir to recover
bitumen and
to sequester a portion of the CO2. In hydrogen combustion, a fraction of the
hydrogen
product from the NG decarbonzation process is combusted to provide heat for
the NG
process, whereby only steam is generated in the process. The steam is then
pumped
underground to mobilize bitumen.
Comparison of BAU case and NGD-added cases
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
13
Performance of the BAU bitumen recovery process was compared with that of the
NGD-added processes. Since steam energy requirements constitute more than 90%
of
the in situ bitumen recovery processes, the performance of the SAGD process is
evaluated by the thermal efficiency of the steam generation system, which is
based on
a first-law energy balance given by:
*steam " }inseam
Net steam generation efficiency (7,G) = (4)
ThttalavrerFRothor
The first and second numerator terms represent the mass flow (kg/s) and mass
enthalpy (kJ/kg) of steam, respectively whereas the first and second
denominator
terms represent mass flow (kg/s) and the LHV of NG, respectively. The term
Eother is
the sum of heat and work requirements related to auxiliary equipment such as
compressors, pumps, and oxygen production from air separation unit (ASU):
E other = Kompressor I/I/Pump + EAST' (5)
Similarly, boiler efficiencies for fuel boilers were calculated from:
.1-Iste,arn
fuel-boiler = (6)
mfue=Lnviuci
liNG-boiler and iH2-boiler were used to represent efficiencies of NG and
hydrogen boilers,
respectively. Similar to Equation (4), the net boiler efficiencies were
calculated when
applicable (in processes where auxiliaries are included).
The energy intensity of bitumen production was calculated as the sum of all
the
energy input (fuel, heat and power) into the process divided by the volume of
bitumen
produced. The energy intensities from this study are presented as gigajoule
(GJ)
energy per m3 bitumen. The quality of energy used for steam generation is
factored in
by accounting for efficiency losses of generating such energy by applying the
2nd Law
of Thermodynamics:
QAT c thrgcLHVNC =¨ (7)
rea
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
14
For a unit time (s), QNG is the energy content (GJ) of NG used to generate
electric
power, Qe/ (GJ) and lie/ is the power plant efficiency. This implies the
primary energy
content of NG is used in calculations instead of using the electricity value
directly.
Using Equation (7) the quality of energy input from electricity was accounted
for by
considering the energy content of NG used to generate electricity at 0.45
efficiency
(assumed as the efficiency for a NG-fired electricity generation plant).
Emissions from the system were assessed by taking inventory of CO2 emissions
generated from energy use and from chemical process conversion. Carbon dioxide
emissions intensity (CO2I) for bitumen production was evaluated by dividing
the sum
of the CO2 produced from energy consumption and generated by chemical
reactions
by the volume of bitumen produced. The capacity of a process to reduce CO2
emissions was presented in terms of CO2 reduction potential (CRP), a value
computed
by dividing the amount of CO2 reduction a process achieves by the CO2
emissions
from the BAU process expressed as a percentage. Since water is lost in the BAU
case
but produced in the NGD-added cases, process water footprint was calculated as
the
water lost or produced divided by the volume of the produced bitumen.
The integrated NGD processes described herein were modelled using ASPEN
PLUS software. The modelling results were used to compute the overall
material
and energy balances, and CO2 emissions of the integrated processes, which are
provided hereinbelow.
Bitumen recovery coupled to NGD and oxy-fired combustion
High quality steam can be produced from hydrogen combustion steam generators
and
by using the heat from oxy-fired combustion flue gases. Air or oxygen may be
used
for hydrogen combustion. For heat generation, direct contact steam generation
may be
used.
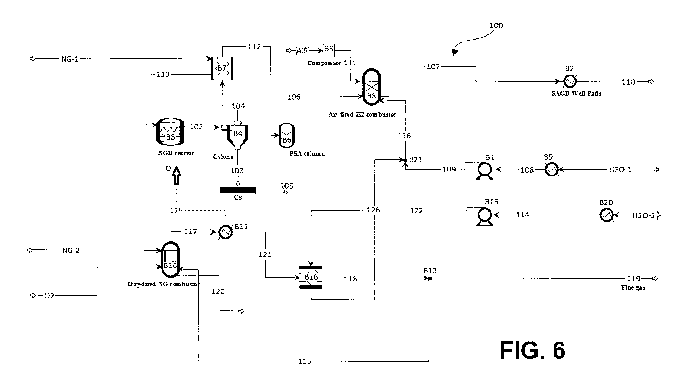
Figure 6 shows a process 100 for bitumen recovery coupled with NGD technology
and oxy-fired combustion. In this sample embodiment, natural gas NG-1 is fed
to an
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
NGD reactor B3, where the natural gas is decomposed thermally to produce
hydrogen
and carbon black. The NGD reactor is a stoichiometric reactor (Rstoic), which
converts methane, ethane and propane in natural gas to elemental carbon and
hydrogen.
5
The product from the NGD reactor is fed to a cyclone B4 for gas-solid
separation
(stream 102). The high temperature hydrogen product from the cyclone is cooled
in a
heat exchanger B7 (stream 104) where its energy supplies the sensible heat for
heating the NG-1 prior to entering the reactor B3 (stream 113). Carbon black
CB is
10 separated out from the cyclone and collected (stream 103). The remaining
hydrogen
product is then sent to a pressure swing adsorption vessel B6 (stream 112),
where the
hydrogen product is separated from impurities (stream 105).
After purification, the hydrogen product is fed to an air-fired hydrogen
combustor B8
15 (stream 106), wherein it is combusted using air as oxidant to produce
steam (stream
107). The combustor B8 may be for example, an equilibrium (REquil) reactor,
requiring that reaction stoichiometry be specified. The air, prior to entering
the
combustor B8 (stream 111), may be compressed by a compressor B5. The reaction
of
hydrogen and oxygen is specified in the air-fired hydrogen combustor B8. It is
also
possible to use oxygen as an oxidant in combustor B8 but air was used in this
sample
embodiment as an illustration.
Water streams H20-1 and H20-2 are preheated via heat exchangers B9 and B20,
respectively. After heating, H20-1 and H20-2 are pumped to reservoir injection
pressures (e.g. 35 bar) by pumps B1 and B15, respectively (streams 108 and
114),
prior to entering combustor B8 (streams 109 and 116) for the production of
steam for
thermal oil recovery. H20-1 and H20-2 may be from the same or different
source.
In this sample embodiment, the heat requirements of the NGD reactor B3 are
supplied
through oxy-fired combustion. Natural gas NG-2 and oxygen 02 are supplied to
an
oxy-fired combustor B10. Combustor B10 may be for example, a REquil reactor.
The
reactions of methane, propane and ethane with oxygen to produce carbon dioxide
and
water are specified in the oxy-fired combustor. NG-2 may be from the same or
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
16
different source as NG-1. Energy Q for the NGD reactor B3 is extracted from
the flue
gas stream of the oxy-fired NG combustor B10 (stream 125).
The flue gas from the combustor B10 goes through a series of heat exchangers
B11
and B16 (streams 117 and 121, respectively) and is then used to produce high
quality
steam for thermal bitumen recovery. A percentage of the flue gas stream is
recycled to
the combustor B10 to regulate the combustion flame temperature therein (stream
115). The flue gas stream is thereafter cooled to a temperature below the dew
point
(stream 119). In one embodiment, H20-2 may pass through heat exchanger B16
(stream 122) before entering combustor B8 (streams 126 and 116).
Optionally, the flue gas from the oxy-fired combustion may be used for thermal
enhanced oil recovery (TEOR). This may further the process CO21 and improve
thermal oil recovery. For example, the flue gas (stream 119) may be compressed
to 35
bar and injected into reservoir.
The steam produced by combustor B8 may then be injected into SAGD well pads B2
for thermal oil recovery (streams 107 and 110).
Example 1 below provides an illustration of this embodiment where a bitumen
recovery plant having an SOR of 3 m3/m3 (wet basis) and producing bitumen at
about
147,850 bbl/d (23,508 m3/d) is considered. This plant generates high quality
steam at
about 240 C and 35 bar.
Example 1 =
In this example, NG-1 is about 2,844.7 tons/d (about 236,906 m3/d at 15 C, 15
bar) of
natural gas and is fed to the NGD reactor B3 where it is decomposed thermally
at
about 1,010 C to produce about 671.3 tons/d hydrogen and about 2,028.6 tons/d
carbon. The hot hydrogen product from the NGD reactor is fed to cyclone B4 to
separate out the carbon. The remaining hydrogen is then cooled in heat
exchanger B7
where its energy supplies the sensible heat for heating the NG-1 feed to the
reactor D3
from 15 C to 1,000 C. The cooled hydrogen goes to pressure swing adsorption
vessel
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
17
B6 for purification. The hydrogen product exiting vessel B6 is combusted with
air in
combustor B8 to produce steam.
The oxy-fired NG combustor B10 supplies heat to the NGD reactor. In this
example,
the oxy-fired NG combustor B10 is fed about 2,005.9 tons/d (167,072 m3/d at 15
C,
bar) NG fuel (NG-2) and about 10,547 tons/d oxygen (02), to produce 11,185
GJ/d of heat for the NGD reactor. The temperature of the flue gas exiting the
oxy-
fired NG combustor B10 is reduced from 1,196 C to 1,035 C after supplying the
heat
requirements of the NGD reactor B3. The flue gas stream generates an
additional
10 82,485 GJ/d of steam using heat exchangers B11 and B16. This steam is
combined
with the steam generated by the hydrogen combustor B8 and used for bitumen
recovery. After steam is generated therefrom, the flue gas stream is cooled to
about
140 C, a temperature below the dew point. About eighty percent of the cooled
flue
gas stream is thereafter recycled back to the combustor B10 to regulate the
15 combustion flame temperature.
= CO2 emi'ssions of this process come mostly from oxy-NG combustion and
from
oxygen production. Commercial vendor data suggest that oxygen production
supplied
at 95% purity has energy requirement below 220 kWh/ton 02. 200 kWh/ton 02 is
the
valued used in the calculations herein but the actual value may be within the
upper
range.
By assuming an emission factor of a NG-fired power plant to be 450 g CO2/kWh,
the
production of oxygen and other auxiliaries contribute ¨759 kg CO2/d and 503 kg
CO2/d, respectively, (equivalent to ¨55 kg CO2/m3 bitumen). The emissions from
oxy-NG combustion is 4,818 kg CO2/d, and when combined with emissions of the
auxiliaries results in a CO21 of'-'260 kg CO2/ ni3 bitumen. This is a CO2
reduction
potential (CRP) equal to 42%-points if flue gases of the oxy-NG combustor are
flared.
The CO21 of this process is relatively high considering that a significant
amount of
carbon emissions were avoided through NG decarbonization. However, it is noted
that
the composition of the oxy-NG combustion flue gas presents some potential
usages. A
CO2-rich flue gas (>70 vol.%) is typical of an oxy-NG combustion. The CO2-rich
flue gas may be injected into underground reservoirs for enhanced bitumen
recovery
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
18
(e.g. above 200 C) or with additional energy input, the flue gas may be
processed in a
CO2 removal unit to produce a stream of >95 vol.% CO2 that is usable for
enhanced
oil recovery.
In this example, the option of using the flue gas for thermal oil recovery was
assessed.
Before the flue gas was injected into the reservoir, the flue gas stream was
compressed from 5 to 35 bar. The steam content of the flue gas stream alone is
able to
produce about 1,330 m3/d bitumen. Assuming that up to 50% of the CO2
eventually
escapes the reservoir formation into the atmosphere, the resulting CO21 of the
process
becomes 174 CO2/ m3 bitumen (a CRP of 61%). In a worst case scenario where
100%
of the CO2 eventually escapes the reservoir formation into the atmosphere, the
result
is a CRP of 39%, a performance worse than the case where the flue gases are
emitted
directly into the atmosphere. This is because the compressor work added to
pressurize
the gas stream to injection pressures yielded negative CPR benefits.
A benefit of this process is that a fraction of the CO2 that would have been
emitted to
the atmosphere is fixed in solid form as carbon black. Besides the lower CO21
and
environmental benefits of this process, production of large amounts of
marketable
carbon black may render this process commercially attractive. In addition to
the large
amount of carbon black produced, combustion of the hydrogen product generates
about 2,720 tons/d of pure water (264 kg/m3 bitumen), which may be sufficient
to
generate steam to produce about 2,267 m3 bitumen and more than half of the
make-up
water needed in the BAU case.
Zero emissions NGD for bitumen recovery
Figure 7 illustrates a zero emissions bitumen recovery process 200 in which a
fraction
of the NGD-produced hydrogen is used to generate heat for NG decomposition.
High
quality steam is produced and used for thermal oil recovery. For heat
generation,
direct contact steam generation may be used.
The process is operated autothermally with its heat requirements supplied
using a
fraction of the hydrogen product from the NGD process while the other fraction
is
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
19
combusted in air to provide energy for high quality steam generation. With
reference
to Figure 7, natural gas NG is fed to an NGD reactor C3 to produce hydrogen
and
carbon black CB. The NGD reactor is for example an RStoic reactor, which
converts
NG to elemental carbon and hydrogen. The hot product from the NGD reactor is
sent
to a cyclone C4 (stream 202) where carbon is separated out from hydrogen
(stream
203). The remaining hydrogen product is then cooled in a heat exchanger C7
(stream
204), where its energy supplies sensible heat for heating the NO before the NO
enters
reactor C3 (stream 213).
The cooled hydrogen is sent to a pressure swing adsorption vessel C6 (stream
212)
where it is separated from impurities. The impurities are collected from
vessel C6
(stream 205). The purified hydrogen is fed to an air-fired 112 combustor C5
(stream
206). It is also possible to use oxygen as an oxidant in combustor C5 but air
was used
in this sample embodiment as an illustration. Combustor C5 is for example a
REquil
reactor.
A supply of air is compressed by a compressor C12 and fed into combustor C5
(stream 217) for combustion with the purified hydrogen to produce heat and
steam.
The reaction of hydrogen and oxygen is specified in the air-fired H2
combustor. The
heat requirements of the NGD reactor are supplied by a fraction of the heat
produced
from combusting the hydrogen product in combustor C5. Combustion products,
which
consist mainly of steam, exit the combustor C5 (stream 207) and pass through a
heat
exchanger, where a portion of the heat extracted Q from the combustion
products is
supplied to reactor C3 (stream 231). The remaining heat portion is fed to heat
exchanger C14 (stream 230) where it is used to heat up a water stream (stream
219)
that has been pumped to reservoir injection pressure (e.g. 35 bar) by pump
C13, to
produce more steam. The steam generated may then be injected into SAGD well
pads
C2 for thermal oil recovery (streams 220 and 209).
In one embodiment, a portion of the combustion products, after cooling, is
recycled
back to combustor C5 to regulate the hydrogen combustion temperature (stream
222).
For example, 50% of the combustion products are recycled back to combustor C5.
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
Stream 211 is a pressure safety vent stream which prevents pressure build up
in
combustor C5. Streams 216 and 221 contain the products of the hydrogen
combustion
process (which are mostly steam) from combustor C5.
5 In this embodiment, NG is used only as a feed for hydrogen production
while the
produced hydrogen is the fuel for the NG decarbonization reaction.
Example 2 below provides an illustration of this embodiment where a bitumen
recovery plant having an SOR of 3 m3/m3 (wet basis) and producing bitumen at
about
10 147,695 bbl/d (23,483 m3/d) is considered. This plant generates high
quality steam at
about 240 C and 35 bar.
Example 2
In order to produce an equivalent amount and quality of steam as in Example 1,
a
15 larger amount of NG feed is required since a part of the hydrogen is
used as fuel for
the NG decarbonization process.
Air and hydrogen are fed into combustor C5 to produce high temperature steam
and to
meet the heat requirements of the NG decomposition process. Modeling results
show
20 that about 822.7 tons/d hydrogen is produced from -4,223 tons/d (i.e.
352,725 m3/d at
15 C and 15 bar) NG fed to the NG decomposition reactor C3 at 1,010 C. The hot
hydrogen product from the NG decarbonization reactor C3 is cooled in heat
exchanger C7 where its energy supplies the sensible heat for heating the NG
feed
from about 15 C to about 1,000 C.
About 40,000 tons/d air (19 % excess) is compressed to 5 bar by compressor C12
and
fed into the combustor C5. NG decomposition reaction heat of about 25,000 GJ/d
at
about 1,010 C is supplied by using part of the energy generated from hydrogen
combustion in combustor C5.
In this example, there is zero CO2 emissions when the CO2 content of air used
for
combustion is discounted. The CO2 that would have been emitted into the
atmosphere
is sequestered in solid form as carbon black. In addition, large amounts (e.g.
-4,531
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
21
tons/d) of valuable carbon black are produced as a by-product. The process
produces
about 6,082 tons/d pure water with a water footprint of 259 kg/m3 bitumen,
which
should be enough water to generate steam to produce ¨2,221 in3 bitumen and
more
than half of the make-up water needed in the BAU case.
Table 1 and Figure 10 show how some of the process performance parameters such
as
process energy intensity, process efficiency, CRP and water footprints compare
with
other process configurations. In Table 1 and Figure 10, "OxyNG + NGD +
flaring"
means oxy-NG combustion applied to NGD with flue gases flared. "OxyNG + NGD
(TEOR -50% CO2)' means oxy-NG combustion applied to NGD with 50% escape of
the flue gases used for TEOR. "OxyNG + NGD (TEOR -100% CO2)' means oxy-NG
combustion applied to NGD with 100% escape of the flue gases used for TEOR.
"Zero emissions NGD" means zero emissions NGD process, as described above.
Table 1. Process performance results for steam generation via NGD for bitumen
recovery.
CO2
11 net 1NG n net- reduction Water
Concept short name n net-SG 11 loss
H2Boiler boiler NGboiler potential, footprint
CRP
%- %-%-p oints kg/m3
points points points points points bitumen
BAU case N/A 80 72 72 0 0 414
OxyNG + NGD + flaring 94.6 80 73.7 48.7 13.3 42 -116
OxyNG + NGD (TEOR -
78.8 92 85 50 12 61 -264
50% CO2)
OxyNG + NGD (TEOR -
78.8 92 85 50 12 39 -264
100% CO2)
Zero emissions NGD 79.7 N/A N/A 36.9 25.1 94 -259
Hydrogen production via NG decarbonization
Figures 8 and 9 each show a process wherein the NGD process is integrated with
hydrotreating to produce SCO from bitumen: i) oxy-NG combustion applied to NGD
for bitumen upgrading (Figure 8); and ii) zero emissions hydrogen production
for
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
22
bitumen upgrading (Figure 9). Hydrogen produced from NGD is used as an
alternative to the hydrogen produced from SMR. This approach may result in
significant reductions in CO2 when compared with the conventional SMR
process.,
Further emissions reductions may be realized when a fraction of the hydrogen
product
is used to fuel the NGD reaction. Hydrogen and marketable carbon black are the
main
products of this process. Clean water is also a product from hydrogen
combustion,
which is useful for bitumen recovery. Two approaches for generating heat for
NGD
for these processes are described herein: i) oxy-NG combustion and ii)
hydrogen
combustion.
Process performance of the SMR method, which is taken to be a BAU hydrogen
process, was compared with those of abovementioned processes. The performance
of
a hydrogen process is computed by the thermal efficiency of the system, based
on a
first-law energy balance:
.th,Hz .LHvHz
nnet-H2prod (8)
The first and second numerator terms represent the mass flow (kg/s) and the
LHV of
hydrogen, respectively whereas the first and second denominator terms
represent
mass flow (kg/s) and LHV of NG, respectively.
Similar to Equation (4), the net hydrogen production process efficiency was
calculated by:
fit ph 4.Hvm
__________________________________________________________ Net hydrogen
process efficiency Oprocess) = (9)
771 NG 4.41V NG+E other
CO2 emissions of hydrogen production processes described therein were assessed
by
using the same methods applied in bitumen recovery. CO21 for hydrogen
production
was evaluated by dividing the sum of CO2 produced from energy consumption and
generated by chemical reactions by the mass of produced hydrogen. The capacity
of
the proposed hydrogen processes to reduce CO2 emissions was also presented in
terms
of CRP, computed as the amount of CO2 reduction a process achieves divided by
the
CO2 emissions from the BAU hydrogen process. Likewise, process water footprint
for
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
23
hydrogen production was calculated as the water lost or produced divided by
the mass
of the produced hydrogen.
Oxy-NG combustion applied to NGD for bitumen upgrading
Figure 8 is a schematic flow diagram showing a process 300 wherein NGD
technology is integrated with synthetic oil production from bitumen. A bitumen
upgrader (not shown) is coupled to the NG decarbonization technology.
=
In this embodiment, oxy-fired NG combustion is used to generate heat for NG
decarbonization. High quality steam is produced using the heat from the oxy-NG
combustion flue gases and the produced steam is used for thermal oil recovery.
With reference to Figure 8, natural gas NG-1 is fed to an NGD reactor D3 to
produce
hydrogen and carbon. The NGD reactor D3 is an RStoic reactor, which converts
methane, ethane and propane in NG to elemental carbon and hydrogen. The hot
hydrogen product from reactor D3 passes through a cyclone D4 (stream 302)
where
carbon black CB is separated from the product (steam 303). The remaining
hydrogen
product is sent to a heat exchanger D7 for cooling (stream 304). The energy
extracted
by heat exchanger D7 supplies the sensible heat for heating NG-1 before NG-1
enters
reactor D3 (stream 313). After cooling, the hydrogen product is fed to a
pressure
swing adsorption vessel D6 (stream 312), where the hydrogen is separated from
impurities. The purified hydrogen product may then be used for bitumen
upgrading.
Natural gas NG-2 and oxygen 02 are supplied to an oxy-fired NG combustor D10,
which may be for example a REquil reactor. The reactions of methane, propane
and
ethane with oxygen to produce CO2 and water are specified in the oxy-fired NG
combustor D10. The combustion of NG in combustor D10 is primarily for
providing
the reaction heat requirement for the NGD reactor D3. More specifically, the
flue gas
from combustor D10 are directed to a heat exchanger D14 (stream 317), whereby
heat
Q is extracted from the flue gas and supplied to NGD reactor D3 (stream 322).
Stream 320 is a pressure safety vent stream which prevents pressure build up
in
combustor D10.
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
24
Water H20-2 is preheated via a heat exchanger D5 (stream 314) and is then
pumped
by a pump D15 to a heat exchanger D16 (stream 323). The cooled flue gas
(stream
321) is fed to heat exchanger D16, and the heat extracted from the flue gas is
used to
generate steam from the water H20-2 in the heat exchanger D16. The generated
steam (stream 326) may then be used for theunal recovery of bitumen (e.g. SAGD
well pads D2).
In one embodiment, part of the flue gas exiting from exchanger D16 (stream
318) is
recycled back to maintain the combustion temperatures in combustor D10 (stream
315). Optionally, the remaining portion of the flue gas (stream 119) may be
used for
TEOR, which may further reduce the process CO21 and improve the thermal oil
recovery. The flue gas stream of the oxy-NG combustion process (stream 319)
may be
compressed (e.g. from 5 to 35 bar) and injected into reservoir.
Example 3 below illustrates the above-described oxy-NG combustion applied to
NGD
for bitumen upgrading process.
Example 3
In this example, 4,080 ton/d (339,778 m3/d at 15 C, 15 bar) of natural gas NG-
1 is fed
to the NGD reactor D3 at 1,010 C to produce about 878.8 tons/d hydrogen. The
hot
hydrogen product from the NGD reactor is fed to cyclone D4 to separate out the
carbon. The remaining hydrogen is cooled in heat exchanger D7 where its energy
supplies the sensible heat for heating NG-1 from 15 C to 1,000 C. The cooled
hydrogen is sent to vessel D6, where the hydrogen is separated from
impurities.
The oxy-fired NO combustor D10 was fed about 3,000 ton/d (249,837 m3/d at 15
C,
15 bar) NO fuel (NG-2) and ¨11,464 tons/d oxygen (02) delivered at 15 bar.
Energy
from the flue gas generated by the oxy-fired NG combustor D10 is sent to the
NGD
reactor D3, as described above. The flue gas temperature was thereafter
reduced from
about 1,295 C to about 1,116 C, and the flue gas was used to generate steam in
heat
exchanger D16 with water from stream H20-2.
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
Results show that for the production of 878.8 tons/d hydrogen, the heat
requirement
for NGD supplied by the oxy-NG combustion process is about 22,079 GJ/d. CO2
emissions of this process come mostly from oxygen production and oxy-NG
5 combustion, resulting in 1,135 tons/d CO2 and 7,206 tons/d CO2,
respectively.
Alongside the production of hydrogen, 43,700 tons of high quality steam was
produced. Accounting for the energy for hydrogen production and steam
generation
results in emissions of 1.7 kg-0O2/kg-H2 (or 6.6 kg-0O2/GJ steam) produced, if
the
10 flue gas stream is -flared.
The performance results changes when the flue gas stream is used for TEOR. The
steam content of the flue gas stream alone can produce 2,107 m3/d bitumen.
Assuming that up to 50% of the injected CO2 eventually escapes the reservoir
15 formation into the atmosphere, the CRP increases from 83% to 89%.
However an
energy penalty of 2.5%-points is incurred. In a situation where 100% of the
injected
CO2 escapes into the atmosphere, this scenario is unfavorable to almost all
the process
performance parameters, except for water footprint. This is because the
additional
energy input for flue gas compression brings resultant negative energy and CO2
20 sequestration outcomes.
Zero emissions hydrogen production for bitumen upgrading
Figure 9 is a schematic flow diagram for a process 400 for zero emissions
hydrogen
25 production for bitumen upgrading. Bitumen upgrader (not shown) is
coupled to the
NG decarbonization technology of the process. In this embodiment, a fraction
of the
produced hydrogen from NGD is used to generate heat for NG decomposition.
Besides the carbon byproduct from the NG decarbonization, a potential benefit
of this
embodiment may be that little or no carbon dioxide is emitted.
With reference to Figure 9, natural gas NG is fed to an NGD reactor E3, which
is for
example an RStoic reactor, which converts NG to elemental carbon and hydrogen.
The heated product from the NGD reactor E3 is sent to a cyclone E4 for gas-
solid
separation (stream 402), where carbon CB is separated out from the hydrogen
product
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
26
(stream 403). The remaining hydrogen product is cooled in a heat exchanger E7
(stream 404), where the energy extracted from the product supplies the
sensible heat
for heating the NG stream prior to it entering the NOD reactor (stream 413).
The cooled hydrogen product is fed to a pressure swing adsorption vessel E6
where
hydrogen is separated from impurities (stream 412). Impurities exit vessel E6
(stream
405) and purified hydrogen is supplied from vessel E6 (stream 406). A portion
of the
purified hydrogen is sent to an air-fired H2 combustor E5 (stream 418). The
remaining
purified hydrogen may be used for the bitumen upgrading (stream H2).
A supply of air is compressed by compressor E 12 and then fed into combustor
E5
(stream 417). Combustor E5 may be for example a REquil reactor, wherein the
reaction of hydrogen and oxygen is specified. The combustion product from
combustor E5 is sent to a heat exchanger E20 where heat is extracted therefrom
(stream 407).
Stream 411 is a pressure safety vent stream which prevents pressure build up
in
combustor E5.
A portion of the heat Q extracted is used to for the heat requirements of the
NOD
reactor (stream 431) while the other portion (in stream 430) is sent to a heat
exchanger E14 for the production of steam.
Water is pumped by a pump El3 into heat exchanger El4 (stream 419) and is
heated
by the heat supplied by the combustor E5 to produce steam. The generated steam
(streams 420 and 409) may then be used for thermal recovery of bitumen (e.g.
SAGD
well pads E2). After passing through heat exchanger E14, part of the
combustion
product, which is mostly water, is recycled back to combustor E5 (streams 416
and
422) to regulate the combustion temperature and to produce additional high
quality
steam usable for thermal oil recovery. The remaining part (streams 416 and
421) is
combined with stream 420 and used for thermal bitumen recovery in well pads
E2.
Example 4 below illustrates this embodiment.
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
27
Example 4
In this example, 12,648 ton/d (1,053,312 m3/d at 15 C, 15 bar) of NG was fed
to the
NGD reactor E3 at 1,010 C to produce about 3,003 tons/d hydrogen at 1,010 C.
The
hot product from the NGD reactor is sent to cyclone E4 wherein carbon is
separated
out from the hydrogen product. The remaining hydrogen product is cooled in
heat
exchanger E7 where energy is extracted from the hot product to supply the
sensible
heat for heating NG stream from 15 C to 1,000 C before the NG enters reactor
E3.
The cooled hydrogen product was purified in vessel E6.
A portion of the purified hydrogen was sent to combustor E5. Air compressed to
5 bar
by compressor E12 is also sent to combustor E5, where the air is combusted
with the
purified hydrogen. Part of the heat generated from the combustion product is
used to
supply heat to the NGD reactor E3 and the remainder is used to generate steam
in heat
exchanger E14 (stream 430). Water is pumped into heat exchanger E14 at
reservoir
injection pressure of 35 bar and is heated by the heat provided by the
combustion
product. After cooling, about 5% of the products from the combustor was
recycled to
combustor E5.
To meet the heat requirements, of an autothermal process producing 3,003
tons/d 112,
about 50,040 GJ/d heat is required. About 43,000 tons/d air (28% excess air),
compressed to 5 bar was fed into the combustor E5. The emission from this
process is
about 1,042 tons-0O2/d, which comes from auxiliary equipment (e.g. compressor
and
pump). Instead of generating CO2 emissions, the carbon in the NG feed is
sequestered
as 9,070 tons/d of commercially viable carbon black product. The steam
generated in
this example should sufficient to thermally recover about 45,629 bbl/d (7,255
m3/d)
bitumen.
Details of the process performance parameters are presented in Table 2 and
Fig. 10. In
Table 2 and Fig. 10, "OxyNG + NGD + flaring" means oxy-NG combustion applied
to NGD with flue gases flared. "OxyNG + NGD (TEOR -50% CO2)" means oxy-NG
combustion applied to NGD with 50% escape of the flue gases used for TEOR.
"OxyNG + NGD (TEOR -100% CO2)" means oxy-NG combustion applied to NGD
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
28
with 100% escape of the flue gases used for TEOR. "Zero emissions NGD" means
zero emissions NGD process, as described above.
Table 2. Process performance results for hydrogen production via NGD for
bitumen upgrading.
CO2
n net flNG fleet- reduction Water
Concept short name H2Boiler boiler NGboiler ilprocess n loss
potential, footprint
CRP
%-%- %-
0/0-points CA-points C/o-points kg/kg-H2
points points points
BAU case N/A 80 75-64 65-89 0 0 17.9
OxyNG + NGD +
flaring N/A 80 76 55 9.5-33.2 82.7 0
OxyNG + NGD
(TEOR -50% 002) N/A 78 72 52 13.3-37 88.8 -6.6
OxyNG + NGD
(TEOR -100% 002) N/A 78 72 52 13.3-37 84.7 -6.6
Zero emissions NGD 83.3 N/A N/A 44 21.3-45 95.7 -3.2
Figure 12 shows the average range of process energy intensity and the CO21
values of
the BAU steam generation process from SAGD project data obtained from publicly
available online database of the Alberta Energy Regulator (AER) (AER. In situ
process presentations. Alberta Energy Regulator, 2013; available at
http://www.aer.ca/data-and-publications/activity-and-data/in-situ-performance-
presentations). The error bars in Figure 12 are associated with the GHG
emissions of
steam generation. Figure 12 shows that steam-based recovery processes have
high
recovery energy requirements, and consequently high GHG emissions intensities.
The GHG emissions presented in Fig. 12 are those associated with the processes
of
steam generation using once-through steam generators having efficiencies of
about
0.85. This efficiency is 5% more than that of the BAU case, however, some of
the
projects operate at steam qualities higher than 0.96. The project data
analysis
accounted only for the energy of NG used to generate steam for SAGD bitumen
recovery and the associated life cycle emissions of natural gas production and
combustion. The life cycle emissions of NG production and combustion were
estimated using an emission factor of 60.2 kg-0O2e/GJ (GHGenius Model 4.03.
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
29
Model background and structure. Natural Resources Canada, 2013). It is noted
that, in
general, the results obtained from computer modelling of the processes of the
present
invention fall mostly within the average range of values shown in Figure 12.
With reference to Table 1 and Figure 10, the results show that the least
performing
concept may achieve a CRP of 42%-points whereas the best performing concept
may
achieve a CRP of 94%-points. The results indicate that the choice of a
particular
process over another may be a function of at least three major factors: energy
penalty,
economic costs and CO21.
Comparing the conventional SMR (BAU case) and the processes of the present
invention, the processes of the present invention appear to be more
competitive and
may potentially offer huge GHG emissions benefits. For example, the SMR
process
generates 9.8 tons CO2/ ton H2 whereas the processes described herein may
potentially emit less than 2 tons-0O2/ton-H2, which may result in a CRP of 85-
96%-
points.
Although the above-mentioned processes are described with respect to natural
gas, the
processes may be operable with other hydrocarbon fuels, such as propane,
octane, etc.
The present invention may provide competitive advantages over existing
teclmology.
These advantages may include:
a) Reduction in carbon dioxide emissions
The processes of the present invention may offer potentially huge emission
reductions. Its application in SAGD or CSS in situ bitumen recovery may reduce
the
CO2 emissions from about 43 kg-0O2/bbl bitumen to about 0 - 17 kg-0O2/bbl
bitumen. This potential reduction in CO2 emissions may help make achievable a
zero
emissions bitumen recovery process. Similarly, integration of the present
invention
with the bitumen upgrading process may reduce CO2 emissions by about 87%.
The avoided CO2 emissions are permanently sequestered in a solid form as
carbon
black, which is also a valuable product. Fixation of carbon emissions in
solids is a
thermodynamically stable, environmentally benign and permanent form of
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
sequestration, with substantially no risk of leaking and no need for post-
sequestration
monitoring. The present invention substantially avoids the processes of post-
combustion capture of CO2 from flue gases, CO2 compression to pipeline
pressures
and the uncertainties associated with storage of CO2 in geological formations.
5
b) Hydrogen production
Hydrogen is a product of the processes of the present invention. The
conventional
SMR process can generate 7 to 11 tons CO2 emissions per ton H2 produced while
the
process described herein may result in about 1 ton CO2 emissions per ton H2.
10 Therefore, application of the present invention may result in about an
80-90%
reduction of the CO2 emissions of the bitumen upgrading ¨ hydrotreating ¨
process.
However, the CO2 emissions may potentially be avoided completely if a fraction
of
the produced hydrogen is used to provide for the process energy requirements.
15 Apart from the potential environmental benefits of the present
invention, capital cost
reduction prospects are envisaged due to the simplicity of the process within
the
context of a thermal recovery process. The process described herein involves
two unit
operations while the SMR has three unit operations.
20 c) Production of water and reduction of process water footprint
The present invention produces about 40-70 kg water for every barrel of
bitumen
produced. Since water consumption footprint and recycling are major
sustainability
challenges associated with bitumen recovery, the generation of water may
present a
promising prospect for the heavy oil industry.
. d) Economic benefits
Economic benefits from byproducts may offset the costs of additional energy
requirements of the processes of the present invention. Carbon black produced
from
the process described herein is a valuable product with many existing and
emerging
markets. Great market potential exists in the rubber, plastics, ink and
metallurgical
industries (Gaudemack and Lynum 1998). The carbon black product may be sold as
is
or reprocessed to a high-tech commercially viable nano-carbon product.
Depending
on the carbon quality, carbon black price may vary from hundreds to thousands
of
dollars. For example, the price of good quality carbon black can be in the
range of
CA 02955749 2017-01-19
WO 2015/010201
PCT/CA2014/050688
31
about $1,000/ton and about $4,000/ton. The total world production of carbon
black is
over 6 million tons annually (Muradov, N. (2000). Thermocatalytic CO2-free
production of hydrogen from hydrocarbon fuels. Proceedings of the 2000
Hydrogen
Program Review, NREL/CP-570-28890). Thus, economic benefits from the carbon
black byproduct may significantly reduce the cost of the producing hydrogen.
e) Simple equipment setup
The present invention involves two characteristic unit operations: (i) NG
decomposition and (ii) gas/solid separation. Conventional NG-fired steam
generation
plants and SMR plants cannot achieve a permanent CO2 sequestration of its CO2
emissions without adding more unit operations, which consequently leads to
additional capital and operating costs.
The previous description of the disclosed embodiments is provided to enable
any
person skilled in the art to make or use the present invention. Various
modifications
to those embodiments will be readily apparent to those skilled in the art, and
the
generic principles defined herein may be applied to other embodiments without
departing from the spirit or scope of the invention. Thus, the present
invention is not
intended to be limited to the embodiments shown herein, but is to be accorded
the full
scope consistent with the claims, wherein reference to an element in the
singular, such
as by use of the article "a" or "an" is not intended to mean "one and only
one" unless
specifically so stated, but rather "one or more". All structural and
functional
equivalents to the elements of the various embodiments described throughout
the
disclosure that are known or later come to be known to those of ordinary skill
in the
art are intended to be encompassed by the elements of the claims. Moreover,
nothing
disclosed herein is intended to be dedicated to the public regardless of
whether such
disclosure is explicitly recited in the claims. For US patent properties, it
is noted that
no claim element is to be construed under the provisions of 35 USC 112, sixth
paragraph, unless the element is expressly recited using the phrase "means
for" or
"step for".