Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
84007077
COMBINED SORTING AND CONCENTRATING
PARTICLES IN A MICROFLUIDIC DEVICE
TECHNICAL FIELD
The present disclosure relates to combined sorting and concentrating, or vice
versa, of
particles in a microfluidic device.
BACKGROUND
Particle separation and filtration have been used in numerous applications
across
industries and fields. Examples of such applications include chemical process
and
fetmentation filtration, water purification/wastewater treatment, sorting and
filtering
components of blood, concentrating colloid solutions, and purifying and
concentrating
environmental samples. Various macro-scale techniques have been developed for
use in these
applications including methods such as centrifugation and filter-based
techniques. Typically,
such techniques require systems that are large, bulky, and expensive and have
complex moving
components.
In certain cases, micro-scale techniques offer advantages over macro-scale
techniques,
in that scaling down allows the use of unique hydrodynamic effects for
particle sorting and
filtration, and thus eliminates the need for large systems with complex moving
components.
Moreover, micro-scale techniques offer the possibility of portable devices
capable of
performing sorting and filtration at much lower cost than larger macro-scale
systems.
However, typical micro-scale sorting and filtration devices can be limited in
the amount of
fluid they can handle over a specified period of time (i.e., low throughput),
potentially placing
such devices at a disadvantage to their macro-scale counterparts.
SUMMARY
The present disclosure is based, at least in part, on the discovery that if
one carefully
controls the geometries and dimensions of microfluidic devices one can not
only transfer
particles between different fluid samples, but also substantially alter the
concentration of
particles within a particular fluid sample. In particular, microfluidic
devices are disclosed that
employ two separate microfluidic modules, e.g., integrated on a single chip or
substrate, in
which one module uses an array of island structures to process a source fluid
sample (e.g., to
transfer particles from the source fluid to a separate second fluid) based on
a combination of
inertial lift forces and fluid shifting, and in which a second module also
uses fluid shifting in
1
Date Recue/Date Received 2022-03-07
84007077
combination with inertial focusing to enhance or increase the concentration of
the particles,
e.g. particles transferred to the second fluid sample. By placing many of each
type of module
in parallel, an ultra-high throughput microfluidic device can be obtained. The
modules can be
arranged in any order, e.g., various modules can be arranged in any order in
series and/or in
parallel.
According to an aspect of the present disclosure, there is provided a
microfluidic
device comprising: a fluid exchange module in a first substrate, the fluid
exchange module
comprising a corresponding first microfluidic channel and a first array of
island structures
in the first microfluidic channel, the first array of island structures being
arranged in one or
more rows that extend along a longitudinal direction of the first microfluidic
channel, each
island structure in a row being spaced apart from an adjacent island structure
in the row to
form an opening, wherein the first array of island structures in the fluid
exchange module
is configured and arranged to shift portions of fluid through the opening
between adjacent
island structures within a row; and a particle concentration module in a
second substrate,
the particle concentration module comprising a corresponding second
microfluidic channel
and a second array of island structures, each island structure in the second
array of island
structures being spaced apart from an adjacent island structure in the second
array of
island structures to form an opening, wherein the second array of island
structures in each
particle concentration module is configured and arranged to shift portions of
fluid through
the opening between adjacent island structures in the second array of island
structures
toward a first side of the second array of island structures, and to focus
particles contained
within the fluid along one or more streamlines on a second opposite side of
the second
array of island structures.
According to another aspect of the present disclosure, there is provided a
method of
extracting and concentrating particles from a first fluid sample, the method
comprising:
providing the first fluid sample to a fluid exchange module of a microfluidic
device;
providing a second fluid sample to the fluid exchange module of the
microfluidic device,
the fluid exchange module comprising a corresponding first microfluidic
channel and a
first array of island structures in the first microfluidic channel, the first
array of island
structures being arranged in one or more rows that extend along a longitudinal
direction of
the first microfluidic channel, each island structure in a row being spaced
apart from an
adjacent island structure in the row to form an opening, wherein the first
fluid sample and
2
Date Recue/Date Received 2023-01-12
84007077
the second fluid sample are provided to the fluid exchange module under
conditions such
that particle-free portions of the first fluid sample are shifted through the
opening between
adjacent island structures within a row, and an inertial lift force causes the
particles in the
first fluid sample to cross streamlines and transfer into the second fluid
sample; passing,
from the fluid exchange module, the second fluid sample containing the
transferred
particles, to a particle concentration module, the particle concentration
module comprising
a corresponding second microfluidic channel and a second array of island
structures
arranged in a row, each island structure within the second array of island
structures being
spaced apart from an adjacent island structure in the row to form an opening,
wherein the
second fluid sample containing the transferred particles is provided to the
particle
concentration module under conditions such that particle-free portions of the
second fluid
sample are shifted through the opening between adjacent island structures
within the
second microfluidic channel, and such that the particles within the second
fluid sample are
focused to one or more streamlines within an inertial focusing section of the
particle
concentration module.
According to another aspect of the present disclosure, there is provided a
microfluidic
device comprising: a fluid exchange module, the fluid exchange module
comprising a
corresponding first microfluidic channel and a first array of island
structures in the first
microfluidic channel, the first array of island structures being arranged in
one or more rows
that extend along a longitudinal direction of the first microfluidic channel,
each island
structure in a row being spaced apart from an adjacent island structure in the
row to form an
opening, wherein the first array of island structures in the fluid exchange
module is configured
and arranged to shift portions of fluid through the opening between adjacent
island structures
within a row to a first side of the first array, leaving a remaining portion
of the fluid on a
second opposite side of the first array, wherein the fluid exchange module
further comprises a
first fluid exchange output fluidly coupled to the first side of the first
array and a second fluid
exchange output fluidly coupled to the second opposite side of the first
array; and a particle
concentration module, the particle concentration module comprising a
corresponding second
microfluidic channel and a second array of island structures, each island
structure in the
second array of island structures being spaced apart from an adjacent island
structure in the
second array of island structures to form an opening, wherein the second array
of island
structures in each particle concentration module is configured and arranged to
shift portions of
fluid through the openings between adjacent island structures in the second
array of island
structures toward a first side of the second array of island structures, and
wherein a shape of
2a
Date Recue/Date Received 2023-01-12
84007077
the second microfluidic channel is configured to give rise to inertial forces
that focus particles
contained within the fluid along one or more streamlines on a second opposite
side of the
second array of island structures, and wherein the particle concentration
module further
comprises a first concentration module output fluidly coupled to the second
opposite side of
the second array of island structures.
According to another aspect of the present disclosure, there is provided a
method of
extracting and concentrating particles from bone marrow aspirate, the method
comprising:
providing the bone marrow aspirate to a fluid exchange module of a
microfluidic device;
providing an aqueous solution to the fluid exchange module of the microfluidic
device, the
fluid exchange module comprising a corresponding first microfluidic channel
and a first array
of island structures in the first microfluidic channel, the first array of
island structures being
arranged in one or more rows that extend along a longitudinal direction of the
first
microfluidic channel, each island structure in a row being spaced apart from
an adjacent island
structure in the row to form an opening, wherein the bone marrow aspirate and
the aqueous
solution are provided to the fluid exchange module under conditions such that
particle-free
portions of the bone marrow aspirate are shifted through the opening between
adjacent island
structures within a row, and an inertial lift force causes the particles in
the bone marrow
aspirate to cross streamlines and transfer into the aqueous solution; passing,
from the fluid
exchange module, the aqueous solution containing the transferred particles, to
a particle
concentration module, the particle concentration module comprising a
corresponding second
microfluidic channel and a second array of island structures arranged in a
row, each island
structure within the second array of island structures being spaced apart from
an adjacent
island structure in the row to form an opening, wherein the aqueous solution
containing the
transferred particles is provided to the particle concentration module under
conditions such
that particle-free portions of the aqueous solution are shifted through the
opening between
adjacent island structures within the second microfluidic channel, and such
that the particles
within the aqueous solution are focused to one or more streamlines within an
inertial focusing
section of the particle concentration module.
According to another aspect of the present disclosure, there is provided a
method of
isolating and concentrating particles from a biological fluid sample, the
method comprising:
obtaining the biological fluid sample; providing the biological fluid sample
to a fluid exchange
module of a microfluidic device; providing a second fluid sample to the fluid
exchange
module of the microfluidic device, the fluid exchange module comprising a
corresponding first
microfluidic channel and a first array of island structures in the first
microfluidic channel, the
2b
Date Recue/Date Received 2023-01-12
84007077
first array of island structures being arranged in one or more rows that
extend along a
longitudinal direction of the first microfluidic channel, each island
structure in a row being
spaced apart from an adjacent island structure in the row to form an opening,
wherein the
biological fluid sample and the second fluid sample are provided to the fluid
exchange module
under conditions such that a first portion of the biological fluid sample
comprising particles
are shifted through the opening between adjacent island structures within a
row, and an inertial
lift force causes the particles in the biological fluid sample to cross
streamlines and transfer
into the second fluid sample; passing, from the fluid exchange module, the
second fluid
sample containing the transferred particles, to a particle concentration
module, the particle
concentration module comprising a corresponding second microfluidic channel
and a second
array of island structures arranged in a row, each island structure within the
second array of
island structures being spaced apart from an adjacent island structure in the
row to form an
opening, wherein the second fluid sample containing the transferred particles
is provided to the
particle concentration module under conditions such that particle-free
portions of the second
fluid sample are shifted through the opening between adjacent island
structures within the
second microfluidic channel, and such that a concentration of the particles
within the second
fluid sample increases; further processing the transferred particles within
the second fluid
sample to provide a processed second fluid sample; and administering the
second fluid sample
containing the transferred and processed particles to a subject.
According to another aspect of the present disclosure, there is provided a
method of
isolating and concentrating particles from a first fluid sample, the method
comprising:
providing the first fluid sample to a fluid exchange module of a microfluidic
device; providing
a second fluid sample to the fluid exchange module of the microfluidic device,
the fluid
exchange module comprising a corresponding first microfluidic channel and a
first array of
island structures in the first microfluidic channel, the first array of island
structures being
arranged in one or more rows that extend along a longitudinal direction of the
first
microfluidic channel, each island structure in a row being spaced apart from
an adjacent island
structure in the row to form an opening, wherein the first fluid sample and
the second fluid
sample are provided to the fluid exchange module under conditions such that a
first type of
particles within the first fluid sample are shifted through the opening
between adjacent island
structures within a row, and an inertial lift force causes a second type of
particles within the
first fluid sample to cross streamlines and transfer into the second fluid
sample; and passing,
from the fluid exchange module, the second fluid sample containing the second
type of
particles, to a particle concentration module, the particle concentration
module comprising a
2c
Date Recue/Date Received 2023-01-12
84007077
corresponding second microfluidic channel and a second array of island
structures arranged in
a row, each island structure within the second array of island structures
being spaced apart
from an adjacent island structure in the row to form an opening, wherein the
second fluid
sample containing the second type of particles is provided to the particle
concentration module
under conditions such that portions of the second fluid sample are shifted
through the opening
between adjacent island structures within the second microfluidic channel, and
such that a
concentration of the second type of particle within the second fluid sample
increases.
According to another aspect of the present disclosure, there is provided a
method of
isolating and concentrating particles from a first fluid sample, the method
comprising:
providing the first fluid sample to a fluid exchange module of a microfluidic
device; providing
a second fluid sample to the fluid exchange module of the microfluidic device,
the fluid
exchange module comprising a corresponding first microfluidic channel and a
first array of
island structures in the first microfluidic channel, the first array of island
structures being
arranged in one or more rows that extend along a longitudinal direction of the
first
microfluidic channel, each island structure in a row being spaced apart from
an adjacent island
structure in the row to fonn an opening, wherein the first fluid sample and
the second fluid
sample are provided to the fluid exchange module under conditions such that a
first type of
particles within the first fluid sample are shifted through the opening
between adjacent island
structures within a row, and an inertial lift force causes a second type of
particles within the
first fluid sample to cross streamlines and transfer into the second fluid
sample; and passing,
from the fluid exchange module, the first fluid sample containing the first
type of particles, to
a particle concentration module, the particle concentration module comprising
a corresponding
second microfluidic channel and a second array of island structures arranged
in a row, each
island structure within the second array of island structures being spaced
apart from an
adjacent island structure in the row to form an opening, wherein the first
fluid sample
containing the first type of particles is provided to the particle
concentration module under
conditions such that portions of the first fluid sample are shifted through
the opening between
adjacent island structures within the second microfluidic channel, and such
that a
concentration of the first type of particle within the first fluid sample
increases.
In general, in one aspect, the subject matter of the present disclosure can be
embodied
in a microfluidic device that includes: a first fluid sample input port; a
fluid sample input port;
a fluid exchange module in a first substrate, the fluid exchange module
comprising a
corresponding first microfluidic channel and a first array of island
structures in the first
microfluidic channel, the first array of island structures being arranged in
one or more rows
2d
Date Recue/Date Received 2023-01-12
84007077
that extend along a longitudinal direction of the first microfluidic channel,
each island
structure in a row being spaced apart from an adjacent island structure in the
row to form an
opening, in which the first array of island structures in each fluid exchange
module is
configured and arranged to shift portions of fluid through the opening between
adjacent island
structures within a row; and a particle concentration module in a second
substrate, the particle
concentration module that includes a corresponding second microfluidic channel
and a second
array of island structures, each island structure in the second array being
spaced apart from an
adjacent island structure to form an opening, in which the second array island
structures in
each particle concentration module is configured and arranged to shift
portions of the product
fluid through the opening between adjacent island structures in the second
array toward a first
side of the second array of island structures, and to focus particles
contained within the
product fluid along one or more streamlines on a second opposite side of the
second array of
island structures.
Implementations of the device can have one or more of the following features.
For
example, in some implementations, an output of the first microfluidic channel
of the fluid
exchange module is fluidly coupled to an input of the second microfluidic
channel of the
particle concentration module. The fluid exchange module can be arranged to
receive in the
2e
Date Recue/Date Received 2023-01-12
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
first microfluidic channel a first fluid sample from the first fluid sample
input port and a
second fluid sample from the second fluid sample input port.
In some implementations, an output of the second microfluidic channel of the
particle
concentration module is fluidly coupled to an input of the first microfluidic
channel of the
fluid exchange module. The particle concentration module can be arranged to
receive in the
second microfluidic channel a first fluid sample from the first fluid sample
input port, in
which the fluid exchange module is arranged to receive in the first
microfluidic channel a
second fluid sample from the second fluid sample input port.
In some implementations, the first substrate and the second substrate are the
same
substrate.
In some implementations, the first array of island structures in each fluid
exchange
module is configured and arranged to shift portions of fluid through the
opening between
adjacent island structures within a row due to reduced fluidic resistance
beyond the opening,
and the second array island structures in each particle concentration module
is configured and
arranged to shift portions of the product fluid through the opening between
adjacent island
structures in the second array toward a first side of the second array of
island structures due
to reduced fluidic resistance beyond the opening.
In some implementations, for the fluid exchange module, a distance between a
first
wall of the first microfluidic channel and the first array of island
structures progressively
increases along the longitudinal direction of the first microfluidic channel.
For the fluid
exchange module, a distance between a second wall of the first microfluidic
channel and the
first array of island structures can progressively decrease along the
longitudinal direction of
the microfluidic channel.
In some implementations, for the particle concentration module, a distance
between a
first wall of the second microfluidic channel and the second array of island
structures
progressively increases along the longitudinal direction of the second
microfluidic channel.
For the particle concentration module, the second array of island structures
and a second wall
of the second microfluidic channel can be arranged and configured to define an
undulating
fluid pathway between the island structures of the second array and the second
wall along the
longitudinal direction of the second microfluidic channel. A curvature of the
second wall can
alternate between regions of high curvature and regions of low curvature. Each
island
structure within the second array of island structures can include a
triangular prism.
3
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
In some implementations, the device includes: multiple fluid exchange modules
arranged in parallel; and multiple particle concentration modules arranged in
parallel.
In some implementations, the microfluidic device includes a filter, the filter
being
fluidly coupled to the first fluid sample input port and fluidly coupled to
either the fluid
exchange module or the particle concentration module arranged downstream from
the filter,
in which each filter includes an array of post structures.
In some implementations, the microfluidic device includes a filter, the filter
being
fluidly coupled to one of the fluid exchange module or the particle
concentration module
arranged upstream of the filter and to the other of the fluid exchange module
or the particle
concentration module arranged downstream of the filter, in which the filter
includes an array
of post structures.
In some implementations, the microfluidic device includes an inertial
concentrator,
the inertial concentrator being fluidly coupled to either the fluid exchange
module or the
particle concentration module arranged upstream of the inertial concentrator
and fluidly
coupled to the other one of the fluid exchange module or the particle
concentration module
arranged downstream of the inertial concentrator, in which the inertial
concentrator includes a
third microfluidic channel having a cross-section transverse to a longitudinal
direction of the
third microfluidic channel, and in which a size of the cross-section
periodically increases and
decreases along the longitudinal direction of the third microfluidic channel.
In another aspect, the subject matter of the present disclosure can be
embodied in a
method of extracting and concentrating particles from a first fluid sample,
the method
including: providing the first fluid sample to a fluid exchange module of a
microfluidic
device; providing a second fluid sample to the fluid exchange module of the
microfluidic
device, the fluid exchange module including a corresponding first microfluidic
channel and a
first array of island structures in the first microfluidic channel, the first
array of island
structures being arranged in one or more rows that extend along a longitudinal
direction of
the first microfluidic channel, each island structure in a row being spaced
apart from an
adjacent island structure in the row to form an opening, in which the first
fluid sample and the
second fluid sample arc provided to the fluid exchange module under conditions
such that
particle-free portions of the first fluid sample are shifted through the
opening between
adjacent island structures within a row, and an inertial lift force causes the
particles in the
first fluid sample to cross streamlines and transfer into the second fluid
sample; passing, from
the fluid exchange module, the second fluid sample containing the transferred
particles, to a
4
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
particle concentration module, the particle concentration module comprising a
corresponding
second microfluidic channel and a second array of island structures arranged
in a row, each
island structure within the second array being spaced apart from an adjacent
island structure
in the row to form an opening, in which the second fluid sample containing the
transferred
particles is provided to the particle concentration module under conditions
such that particle-
free portions of the second fluid sample are shifted through the opening
between adjacent
island structures within the second microfluidic channel, and such that the
particles within the
second fluid sample are focused to one or more streamlines within an inertial
focusing section
of the particle concentration module.
Implementations of the method can have one or more of the following features.
For
example, in some implementations, the first fluid sample is whole blood and
the second fluid
sample is a buffer solution.
In some implementations, the particles are white blood cells. The white blood
cells
can be neutrophils.
In some implementations, the method further includes filtering the first fluid
sample
prior to providing the first fluid sample to the fluid exchange module.
In some implementations, the method further includes: passing, from the fluid
exchange module, the second fluid sample containing the transferred particles
to a filter; and
filtering the second fluid sample in the filter prior to passing the second
fluid sample to the
particle concentration module.
In some implementations, the method further includes focusing, for the second
fluid
sample output from the fluid exchange module, the particles to one or more
streamlines
within the second fluid sample in a third microfluidic channel prior to
passing the second
fluid sample containing the transferred particles to the particle
concentration module, in
which, for the second fluid sample, the one or more streamlines at an output
of the third
microfluidic channel are aligned to an inertial focusing side of the particle
concentration
module.
In some implementations, the method further includes obtaining at an output of
the
particle concentration module a portion of the second fluid sample containing
a higher
concentration of the particles relative to a concentration of the particles in
the second fluid
sample at an input to the particle concentration module. The particle
concentration within the
second fluid sample at the output of the particle concentration module can be
between 10
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
times and 100 times more than the particle concentration within the second
fluid sample at
the input of the particle concentration module.
Implementations of the subject matter described herein provide several
advantages.
For example, in some implementations, the microfluidic systems and methods
described
herein can be used to isolate particles within a continuously flowing fluid,
increase the
concentration of particles within a continuously flowing fluid without the
need for
centrifugation, and/or obtain purified fluid samples with low particle
concentration. In some
implementations, the microfluidic systems and methods described herein can be
used to shift
particles from one fluid to another fluid, e.g., from whole blood to a buffer
solution. The
continuous flow microfluidic techniques described herein offer high volumetric
capacity and
throughput, substantial and tunable fluid volume reduction, and high particle
yields with
inexpensive and simple instruments that can be implemented into various point-
of-care
devices. In particular, the presently described techniques offer significant
advantages over
existing centrifugation techniques, especially in applications where the size
and expense of
centrifugation is prohibitive. In some implementations, the presently
described techniques
also provide streamlined processing and simple integration with other
microfluidic modules.
For clinical applications, the systems described herein can be configured as
both self-
contained and disposable. In contrast, for bioprocessing/industrial
applications, the devices
canbe configured for continuous flow/processing.
For the purposes of this disclosure, a "sample" (sometimes referred to as
"fluid" or
"fluid sample") is capable of flowing through a microfluidic channel. The
sample can include
one or more of a fluid suspension or any sample that can be put into the form
of a fluid
suspension, and that can flow or be driven through the microfluidic channel.
For the purposes of this disclosure, a fluid can include any type of fluid,
e.g., liquid or
gas. The fluid can include industrial fluids, environmental fluids or fluids
used by other
entities that disperse particles in such fluids for industrial or other types
of processing. For
example, the fluids can include oils or aqueous solutions. The fluid can
include biological
fluids, e.g., whole blood, plasma, buff coat, cerebrospinal fluid, bone marrow
aspirate,
peritoneal, branchioalveolar, ascitcs, urine, or other bodily fluids.
Particles contained in the
fluid can include biological particles, e.g., circulating tumor cells, red
blood cells, white
blood cells, bone marrow cells, bacteria, fungi, virus, algae, any prokaryotic
or eukaryotic
cells, sperm, eggs, organelles, exosomes, or other types of biological
particles that occur
either naturally or arc introduced artificially into the fluid. The particles
can include droplets,
6
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
bubbles, pollutants, precipitates, organic and inorganic particles, beads,
bead labeled analytes,
magnetic beads, and/or magnetically labeled analytes.
For the purposes of this disclosure, the term channel refers to a structure in
which a
fluid can flow.
For the purposes of this disclosure, the term microfluidic system refers to a
fluidic
system, device, channel, or chamber that generally has at least one cross-
sectional dimension
in the range of about 10 nm to about 5 mi.
For the purposes of this disclosure, the terms gap or opening refer to an area
in which
fluids or particles can flow. For example, a gap or opening can be a space
between two
obstacles through which fluids flow.
For the purposes of this disclosure, the term rigid island structure refers to
a physical
structure through which a particle generally cannot penetrate.
For the purposes of this disclosure, the term volume reduction means
processing a
suspension of cells/particles such that the product of the process has a
higher concentration
(and therefore smaller volume) of the cells/particles than the input.
For the purposes of this disclosure, the term a particle-free layer is
understood to be
an elongated region of a continuously flowing fluid sample within a
microfluidic device that
is substantially free of one or more different types of particles.
For the purposes of this disclosure, the term absolute particle yield is
understood to
mean the total number of particles in the product divided by the total number
particles in the
input.
For the purposes of this disclosure, the term relative yield is understood to
mean the
total number of particles in the product divided by the total number of
particles in the output
(i.e., product plus waste).
For the purposes of this disclosure, the term length fraction is understood to
mean the
fraction of that stream occupied by particles (as opposed to space between
particles).
For the purposes of this disclosure, the term fluidic resistance refers to the
ratio of
pressure drop across a channel (e.g., a microfluidic channel) to the flow rate
of fluid through
the channel.
Particles within a sample can have any size which allows them to be ordered
and
focused within the microfluidic channel. For example, particles can have an
average
hydrodynamic size that is between 1 pirn and 100 urn. The particle size is
limited only by
channel geometry; accordingly, particles that arc larger and smaller than the
above-described
7
84007077
particles and focused with the microchannel can be used. The size of particles
(e.g., cells, eggs,
bacteria, fungi, virus, algae, any prokaryotic or eukaryotic cells,
organelles, exosomes,
droplets, bubbles, pollutants, precipitates, organic and inorganic particles,
magnetic beads,
and/or magnetically labeled analytes), such as the average hydrodynamic
particle size or
average diameter, can be determined using standard techniques well known in
the field.
In some implementations, multiple particles within a fluid can be focused
along a
streamline of the fluid.
In some implementations, inertial focusing (sometimes referred to as
"localizing") of a
particle to a streamline can be achieved by varying a flow rate of a fluid
carrying suspended
particles flowed through a channel of constant cross-section. In some
implementations,
focusing can be achieved by a reduction in the area of a cross-section of a
channel through
which a flux of particles passes. Particles can be localized within an area
having a width of,
e.g., 1.05, 2, 3, 4, or 5 times the width of the particles. Localization can
occur at any location
within the channel, e.g., at an unobstructed portion of the channel.
Localization can occur in a
portion of the channel having less than 50%, 40%, 30%, 20%, 10%, 5%, 2%, 1%,
or 0.1%
reduction in cross-sectional area.
Unless otherwise defined, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
belongs. Although methods, materials, and devices similar or equivalent to
those described
herein can be used in the practice or testing of the present invention,
suitable methods,
materials and devices are described below. In case of conflict with any
document mentioned
herein, the present specification, including definitions, will control. In
addition, the materials,
methods, and examples are illustrative only and not intended to be limiting.
The details of one or more embodiments are set forth in the accompanying
drawings
and the description below. Other features, objects, and will be apparent from
the description
and drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a top view of the general architecture of a representative
microfluidic device
according to the present disclosure.
8
Date Recue/Date Received 2022-03-07
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
FIG 2 is a schematic that illustrates a top view of a portion of the fluid
sample
receiving sections, the fluid sample filter sections, and the buffer sample
receiving sections of
the device shown in FIG 1.
FIG 3 is a schematic that illustrates a top view of a portion of the fluid
exchange
module of the device shown in FIG 1.
FIG 4 is a schematic that illustrates a top view of both the product
receptacle and the
waste receptacle for the fluid exchange module shown in FIG. 3.
FIG. 5 is a schematic that illustrates a top view of the entrance portion to
the particle
concentration module of the device shown in FIG. 1.
FIG 6 is a schematic that illustrates a top view of the particle concentration
module of
the device shown in FIG 1.
FIG 7 is a schematic that illustrates a top view of the particle concentration
module of
the device shown in FIG 1.
FIG. 8 is a schematic that illustrates a top view of the waste section and
product
output section for the particle concentration module shown in FIG. 7.
FIG. 9 is a schematic that illustrates a generalized cross-section of a
microfluidic
device according to the present disclosure.
FIG. 10 is a schematic that illustrates a top view of a microfluidic chip that
includes
the device according to the present disclosure.
FIG 11 is a schematic that illustrates a top view of an interface layer that
is laser
welded to the substrate containing microfluidic devices according to the
present disclosure.
FIG 12 is a series (FIGS. 12A, 12B) of plots of white blood cell relative
yield
distribution and white blood cell absolute yield distribution for different
experimental runs of
the microfluidic device according to the present disclosure.
FIG 13 is a series (FIGS. 13A, 13B) of plots of white blood cell relative
yield
following the fluid exchanger module (the "fractionator") and white blood cell
relative yield
following the particle concentration module for different experimental runs of
the
microfluidic device according to the present disclosure.
FIG. 14 is a series (FIGS. 14A, 14B) of plots of the relative ncutrophil yield
and the
absolute neutrophil yield from for different experimental runs of the
microfluidic device
according to the present disclosure.
9
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
FIG 15 illustrates a plot of white blood cell (WBC) relative yield versus
sample
neutrophil fraction for the different experimental runs of the microfluidic
device according to
the present disclosure.
FIG 16 illustrates plots of red blood cell (RBC) and platelet depletion data
DETAILED DESCRIPTION
Overview of Combined Microfluidic Particle Sorter and Concentrator
FIG 1 is a schematic that illustrates a top view of the general architecture
of a
representative microfluidic device 100 according to the present disclosure. In
particular, the
schematic illustrates, among other things, the outlines of various
microfluidic channels, ports,
reservoirs, and output receptacles for receiving, transporting, shifting,
adjusting and/or
storing fluid samples. The device 100 is designed to receive a fluid sample,
e.g., blood,
containing a suspension of one or more different types of particles, in a
fluid exchange
module to isolate a subpopulation of particles from the bulk fluid (e.g., by
extracting and
transferring one or more types of particles from the fluid sample to a second
different
solution), and then enrich the concentration of the extracted subpopulation of
particles for
subsequent analysis and processing in a particle concentration module.
Alternatively, the
fluid, e.g., if dilute, can first be passed through the particle concentration
module and then
through the fluid exchange module. The various channels, ports and reservoirs,
among other
structures for manipulating fluids and particles, are fabricated within a
single device layer. A
surface of the device layer is sealed with a lid layer (not shown in FIG. 1)
that serves as a
cover to the channels and reservoirs of the device layer. An optional manifold
layer (not
shown in FIG. 1) can be arranged on a surface of the lid layer to provide
simultaneous fluidic
coupling of the various through-holes to a macroscopic output/input connection
(e.g., tubing).
For example, all modules can be arranged and fixed on and/or fabricated on the
same
substrate, or each module can be arranged and fixed on and/or fabricated on
individual
substrates and then connected via fluid conduits and/or mechanical connections
of the
substrates.
The microfluidic device 100 can be sub-divided into separate sections
referenced as
follows: a fluid sample receiving section 102, a fluid sample filter section
104, a buffer
sample receiving section 106, a fluid exchanger module (also referred to
herein as a fluid
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
force fractionation (FFF) module or an inertial exchanger) 108, a particle
concentration
module (also referred to herein as an inertial concentrator) 110, a fluid
exchanger module
product receptacle section 112, a fluid exchanger module waste receptacle
section 114, a fluid
exchanger module waste reservoir 116, a particle concentration module input
section 118, a
particle concentration module waste section 120, and a particle concentration
module product
output section 122. An overview of how the device 100 operates will be
provided first,
followed by a discussion of the different sections in detail.
In a first step, a fluid sample containing one or more different types of
particles enters
the chip through the fluid sample receiving section 102. The fluid sample
receiving section
102 can include a series of holes into which the fluid sample can be
introduced. For instance,
each hole can be coupled to corresponding tubing through which the fluid
sample is
delivered. Alternatively, or in addition, the fluid sample receiving section
102 can include
valves that can be opened and closed manually or through an automated process
to control
over the delivery of the fluid sample to the device 100. Other mechanisms for
introducing
fluid samples to a microfluidic device as known by those of ordinary skill in
the art can also
be utilized. The fluid sample can be driven into the device 100 using, e.g., a
pump system that
applies pressure to the fluid sample and enables continuous flow of the sample
through the
device 100.
Upon receiving the fluid sample in the device 100, the fluid sample passes to
the fluid
sample filter section 104 that is configured to filter particles contained in
an incoming fluid
according to the particle size (e.g., average diameter), such that only
particles of a pre-defined
size or less are able to pass to the next stage of the system. At the end of
the filter section
104, the device 100 includes a buffer sample receiving section 106 configured
to receive a
second fluid sample (referred to as a buffer sample or buffer stream for the
purpose of the
example device 100). The buffer sample receiving section 106 includes multiple
holes for
receiving the buffer sample, in which the holes are arranged just upstream of
the fluid
exchanger module 108. Similar to the fluid sample receiving section, each hole
can be
coupled to corresponding tubing through which the fluid sample is delivered.
Alternatively, or
in addition, the buffer sample receiving section 106 can include valves that
can be opened
and closed manually or through an automated process to control over the
delivery of the
buffer fluid sample to the device 100.
In some embodiments, both the filtered fluid sample and the buffer fluid
sample then
enter the fluid exchanger module 108. In other embodiments, the filtered fluid
sample and the
11
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
buffer fluid sample first enter the particle concentration module. The buffer
and fluid sample
propagate within the fluid exchanger module 108 under conditions that enable
laminar flow.
That is, the fluids flow under conditions such that there is no turbulent
mixing between the
buffer and fluid sample. Rather, both the buffer and fluid sample propagate
substantially side
by side as parallel streams over the length of the fluid exchanger module 108.
While in the
module 108, at least a first type of particles are transferred from the fluid
sample to the buffer
sample so that by the end of the module 108 most, if not all, of the at least
first type of
particles have been extracted from the fluid sample. As will be explained, the
process of
transferring particles from the sample fluid to the buffer can rely, in part,
on a combination of
extracting the fluid sample at openings between island structures within the
module 108, as
well as inertial lift forces, which force particles away from the extracted
fluid and into the
buffer sample. Because the inertial lift force is size-dependent, it can be
employed to
fractionate (e.g., sort) particles based on size. Fractionation is
accomplished by repeatedly (1)
using the inertial lift force to move large particles away from a channel wall
and then (2)
shifting the fluid that is free of the large particles into an adjacent
channel. After many
iterations, the large particles can be moved from the source fluid (e.g., the
fluid sample)
across streamlines into an adjacent destination fluid (e.g., the buffer fluid
sample).
At the end of the fluid exchanger module 108, the fluid sample enters the
fluid
exchanger module waste station 114. Though referred to as a "waste station,"
the fluid sample
can be disposed of, re-used for other purposes or processed for further
analysis.
On the other hand, the buffer fluid sample, which now contains the transferred
particles, enters the fluid exchanger product receptacle section 112. In the
present example,
the fluid exchanger product receptacle section 112 includes through-holes into
which the
particle-containing buffer sample passes out of the device 100 and into a
manifold layer (not
shown) that directs the buffer sample back into the device 100 at the particle
concentration
module input section 118. In alternative implementations, the buffer sample
containing the
transferred particles can be fluidly coupled directly to the particle
concentration module 110
without having to exit and re-enter the device 100.
The particle concentration module 110 contains three regions: a filter region,
a
focusing region, and a concentrator region. Upon entering the module 110, the
buffer sample
containing the particles propagates through the filter region, where the
filter region is
configured to filter particles contained in an incoming fluid according to the
particle size
(e.g., average diameter), such that only particles of a pre-defined size or
less are able to pass
12
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
to the next stage of the system. The buffer sample then passes to the focusing
region. The
focusing region employs structures that are configured to induce inertial
focusing of the
particles within the buffer sample along one or more streamlines. By focusing
the particles
along defined streamlines, the particles can be positioned at precise
locations prior to entering
the concentrator region, which enables, in certain implementations, the
concentrator to more
effectively enrich the particle concentration within the buffer sample. The
concentrator region
contains an array of structures configured and arranged to increase the
concentration of the
particles within the buffer. In particular, the particles within the buffer
are subject to inertial
lift forces that cause them to migrate across fluid streamlines toward
equilibrium positions
within the channel cross-section. Concentration of the particles is
accomplished by repeatedly
(1) using the inertial forces to move the particles away from channel walls
and then (2)
shifting or siphoning particle-free buffer sample into an adjacent channel.
This results in two
fluid outputs from the particle concentration module 110: an enriched buffer
solution
containing a high concentration of the extracted particles and a particle-free
buffer sample.
At the end of the particle concentration module 110, the enriched buffer fluid
passes
to the particle concentration module product output 122, where it can be
collected for further
analysis and/or processing. The particle-free buffer sample passes to the
waste section 120.
Each of the fluid exchanger module 108 and the particle concentration module
110 employ
multiplexing to establish an ultra-high throughput device capable of
processing large amounts
of sample fluid to obtain highly concentrated subpopulations of particles over
relatively short
time periods.
Sample Receiving and Filter Section
FIG 2 is a schematic that illustrates a top view of a portion of the fluid
sample
receiving sections 102, the fluid sample filter sections 104, and the buffer
sample receiving
sections 106 of the device 100. To increase the microfluidic device
throughput, the foregoing
sections are replicated multiple times on the chip. In the present example,
the sections are
arranged in parallel. As explained above, the fluid sample receiving section
102 includes
multiple through-holes 200 into which the fluid sample can be introduced. Each
through-hole
200 can be coupled, e.g., at one end to a corresponding tubing through which
the fluid sample
is delivered. Alternatively, in some implementations, the device 100 includes
a separate
manifold layer (not shown) that is located above the through-holes 200 and
that is configured
to simultaneously fluidly couple each of the through-holes 200 to a single
macroscopic input
connection (e.g., tubing). Alternatively, or in addition, the fluid sample
receiving section 102
13
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
can include valves that can be opened and closed manually or through an
automated process
to control over the delivery of the fluid sample to the device 100. Other
mechanisms for
introducing fluid samples to a microfluidic device as known by those of
ordinary skill in the
art can also utilized. The fluid sample can be driven into the device 100
using, e.g., a pump
system that applies pressure to the fluid sample and enables continuous flow
of the sample
through the device 100.
From each through-hole 200, the fluid sample passes to a corresponding fluid
sample
filter section 104. In the present example, each fluid sample filter section
104 includes a first
region 202 containing two separate straight channels 203, arranged in
parallel, through which
the fluid sample propagates. At the end of the two channels 203, the fluid
sample merges
again and flows into the second region 204 of the filter 104. Although two
parallel channels
are shown in each filter section 104 in FIG 2, the first region 202 can
include a single
microfluidic channel or more than two microfluidic channels.
Each filter section 104 also includes the second region 204 fluidly coupled to
the first
region 202, in which the second region 204 contains multiple islands or post
structures 205
arranged in one or more staggered arrays that act as filters for the fluid
sample. The array of
post structures 205 in the second region 204 are arranged and configured to
filter particles
contained in the fluid sample according to the particle size (e.g., average
diameter), such that
only particles of a pre-defined size or less are able to pass to the next
stage of the system. For
instance, in the case the fluid sample contains complex matrices, such as bone
marrow
aspirate, the array of posts 205 can be configured to remove bone chips and
fibrin clots to
improve the efficiency of device operations to be performed downstream (e.g.,
enriching
particle concentration within a fluid and/or transferring particles from one
fluid to another
fluid). In the example arrangement shown in FIG 2, the posts 205 within the
second region
204 have a substantially triangular prism shape, in which the pillar size
(e.g., approximate
diameter across the short face of each pillar) and array offset spacing are
designed to deflect
particles above a certain size, thereby separating them from the main
suspension. Typically,
the size limit is determined based on the maximum particle size that is
desired to pass through
later stages of the device 100. For example, the array of posts 205 can be
configured to
filter/block passage of particles that have an average diameter greater than
50%, greater than
60%, greater than 70%, greater than 80% or greater than 90% of the minimum
width of a
microfluidic channel in the subsequent fluid exchanger module 108.
14
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
In the particular example shown in FIG. 2, the fluid sample enters region 204
generally along the direction indicated by arrow 209 until the fluid sample
comes into contact
with a wall/divider 207 that forces the fluid to propagate through the
openings between posts
205, which function to filter the fluid sample. The fluid sample is forced
around the
wall/divider 207 passing through another one or more arrays of posts 205 and
then continuing
on in the direction of arrows 209. Any number or arrangement of such post
arrays can be
included in the filter section 104 to achieve the desired level of fluid
sample filtering.
Furthermore, the order in which the first and second regions of the filter
section 104 are
arranged is not vital to the operation of the device 100. That is, the first
region 202 containing
the straight channels can be arranged upstream or downstream of the second
region 204
containing the post arrays 205 so long as the two regions are fluidly coupled
together. For
instance, as shown in FIG 2, the order in which the first and second regions
202, 204 of the
filter section 104 are arranged alternates for each through-hole 200 in order
to enable tighter
packing of the microfluidic channels on the chip.
Following the fluid sample filter section 104, the filtered fluid sample
passes into
channels 211 to create multiple streams that arc fluidly coupled to fluid
exchanger module
108. At the entrance to the fluid exchanger module 108, the filtered fluid
sample streams
propagate side by side with a second fluid (e.g., a buffer fluid sample). The
buffer fluid
sample enters the device in buffer sample receiving sections 106, which each
include a
through-hole 213 for receiving the buffer fluid sample. Similar to through-
holes 200, the
through-holes 213 can be fluidly coupled at one end to a corresponding tubing
through which
the buffer sample is delivered. Alternatively, in some implementations, a
separate manifold
layer (not shown) can be used to introduce fluid to each of the through-holes
213. The
through-holes 213 are arranged upstream of the entrance to the fluid exchanger
module 108.
Buffer fluid entering from through-holes 213 is split into multiple fluid
streams, one for each
filtered fluid sample stream. In some cases, the buffer fluid passes through a
fluid resistor that
ensures the correct flow ratio between the filtered fluid sample and the
buffer sample. For
instance, in the present example shown in FIG 2, the buffer sample streams
each pass
through a sinusoidal-like channel that functions to increase the fluid
resistance.
A wall or other divider 215 maintains separation between each pair of buffer
sample/fluid sample streams. Both the filtered fluid sample and the buffer
propagate under
conditions that promote laminar flow, such that any mixing between the sample
and buffer is
limited to that due to diffusion. Given the location at which the buffer fluid
stream enters the
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
fluid exchanger module 108, the buffer fluid stream propagates closest to the
wall/divider 215
whereas the filtered fluid sample propagates furthest away from the
wall/divider 215.
Fluid Exchanger Module
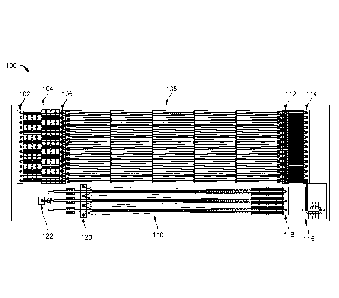
FIG 3 is a schematic that illustrates a top view of a portion of the fluid
exchanger
module 108. The purpose of fluid exchanger module 108 is to deplete the
filtered fluid
sample of large particles. That is, the fluid exchanger module 108 is
configured to sort a
desired sub-population of particles (e.g., relatively large particles) from
the filtered fluid
sample and transfer those particles to the buffer solution. Thus, the fluid
exchanger module
108 "exchanges" the fluid in which the desired particles are suspended. This
process also can
be referred to as "fractionation." To fractionate the filtered fluid sample,
the fluid exchanger
108 includes multiple island structures 300 arranged in one or more arrays, in
which each
island 300 is separated from an adjacent island in the array by a gap through
which fluid can
flow. In the example shown in FIG. 3, the fluid exchanger module 108 actually
includes two
separate arrays, each having three rows of islands 300, in which the arrays
are separated from
one another by the wall/divider 215. The islands 300 are illustrated as
substantially
rectangular structures with their elongated sides extending generally in the
same direction as
fluid flow, though other shapes and orientations can be used instead.
Furthermore, the number
of rows of islands and the number of island arrays can also be varied from one
or more
depending on the desired configuration.
Fractionation using the fluid exchanger module 108 is accomplished by
repeatedly (1)
shifting or extracting portions of the filtered fluid sample that are free
from particles through
the gaps between the islands, while simultaneously relying on (2) inertial
lift forces to move
particles within the fluid sample away from the locations where the fluid is
extracted. After
multiple iterations, the particles in the filtered fluid sample can be moved
across fluid
streamlines and into a second different fluid propagating alongside the fluid
sample (e.g., into
the buffer). The inertial forces within the fluid arise from particles flowing
at relatively high
speeds near microfluidic channel walls. Thus, for example, when the fluid
sample propagates
near the walls of the islands 300, the particles within the fluid sample will
experience inertial
forces pushing the particles away from the islands. Fluid extraction or
shifting, on the other
hand, is controlled by the relative fluidic resistance encountered by the
fluid as it propagates
through the arrays. For a microfluidic channel in which the fluidic resistance
varies over the
length of the channel, fluid will tend to follow in a direction towards
reduced fluidic
16
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
resistance, thus leading to portions of the fluid being shifted away from the
primary direction
of propagation.
In FIG. 3, the fluidic resistance of the channels is controlled by the
geometry of the
outer boundaries of each channel. For instance, with respect to each array,
the distance
between the outer channel wall 305 and the islands progressively increases
along the
direction of fluid flow, leading to lower fluidic resistance. In contrast, the
distance between
the wall 307 of the divider 215 and the islands 300 progressively decreases
along the length
of the array in the direction of fluid flow, leading to increased fluidic
resistance. As a result,
fluid is shifted through the gaps between islands 300 in the directions
indicated by arrows
304. For relatively large particles within the fluids, the particles are also
subject to inertial
lift forces that push the particles away from the gaps, the portions of fluid
extracted through
the gaps are substantially particle-free.
During operation of fluid exchanger 108, the filtered fluid sample enters both
island
arrays closer to the walls 305 of the channels, whereas the buffer fluid
stream enters the
island arrays closer to the walls 307 of divider 215. On average, both the
filtered fluid sample
and buffer fluid follow a horizontal trajectory through the fluid exchanger
108. Though the
fluid sample has been filtered prior to this stage, it can still contain one
or more different sub-
populations of particles having different sizes. Depending on the size of the
gaps between
islands 300 and the flow speed of the fluid sample, larger particles can
experience a strong
repulsive inertial lift force while flowing alongside the islands 300, which
causes those
particles to follow a trajectory with a component that leads from the filtered
fluid sample
stream (closer to walls 305) across fluid streamlines and into to the buffer
fluid stream (closer
to walls 307). Smaller particles can experience a relatively weaker inertial
lift force while
flowing alongside the islands 300. As a result, the smaller particles can
follow the same
average trajectory as the filtered fluid sample and can not be transferred
into the buffer fluid
stream. At the output of the fluid exchanger 108, the filtered fluid stream
will leave the arrays
without one or more of the sub-populations of particles (e.g., without the
relatively large
particles), whereas the buffer fluid stream will have picked up the one or
more sub-
populations of particles. In some embodiments, one or more of the fluid
samples that enter
fluid exchange module 108 can come from the particle concentration module 110,
described
in further detail below.
As indicated above, the inertial lift force is highly size dependent, such
that large
particles can experience a larger force than small particles. Additionally,
the fraction of fluid
17
84007077
that is extracted through gaps between islands 300 can be adjusted based on
the island design
and configuration. Further discussion on the parameters and design principles
for such fluid
exchangers can be found in U.S. Provisional Application No. 62/074,213, filed
November 3, 2014, and U.S. Provisional Application No. 62/074,315, filed
November 3, 2014.
Fluid Exchanger Module Product Receptacle and Fluid Exchanger Module Waste
Receptacle
After passing through the fluid exchanger module 108, the processed fluid
sample
stream that is depleted of the large particles and the buffer sample stream
pass to the fluid
exchanger module waste receptacle 114 and the fluid exchanger module product
receptacle
112, respectively. FIG. 4 is a schematic that illustrates a top view of both
the product
receptacle 112 and the waste receptacle 114. The direction of fluid flow is
noted at the bottom
of the figure. Upon leaving the fluid exchanger module 108, the buffer fluid
steam continues
to travel close to the walls of divider 215 until it passes into channels 403.
In some
implementations, the channels 403 include a fluid resistor for adjusting the
flow rate of the
buffer sample stream. For instance, the channels 403 can have a sinusoidal-
like shape that to
increase the fluid resistance. Following passage through channels 403, the
buffer streams enter
into through-holes 400 that fluidly couple the buffer streams to the particle
concentration
module 110. For instance, the through-holes 400 can be coupled to a connector,
such as tubing,
that allows the buffer streams to pass into the particle concentration module.
Alternatively, the
through-holes 400 can be coupled to a manifold that redirect the buffer fluid
streams to the
particle concentration module. In some implementations, buffer streams pass
directly from the
fluid exchanger module 108 to the particle concentration module 110 without
first propagating
through channels and/or through-holes 400.
The processed fluid sample streams (depleted of large particles), in contrast,
pass from
the fluid exchanger module 108 through microfluidic channels 401 and channels
405 to the
fluid exchanger waste receptacle 114. The waste receptacle section 114 can
include, for
example, through-holes 406 that collect the processed fluid stream. Again, the
through-holes
can be coupled to tubing or to a manifold that redirects the fluid stream. In
some
implementations, the waste receptacle section 114 does not include through-
holes and instead
contains a reservoir to receive the processed fluid sample streams. The number
of channels
used to couple the fluid exchanger module 108 to the waste receptacle section
114 and to the
18
Date Recue/Date Received 2022-03-07
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
product receptacle section 112 can be modified from that shown in FIG. 4. For
instance, in
some implementations, one channel, three channels, four channels or more can
be used to
couple the processed fluid sample streams from fluid exchanger module 108 to
the waste
receptacle section 114. Similarly, in some implementations, one channel, three
channels, four
channels or more can be sued to couple the buffer fluid streams from the fluid
exchanger
module 108 to the product receptacle section 112.
Particle Concentration Module
As explained above, the buffer fluid stream containing the one or more sub-
populations of particles extracted from the sample fluid stream passes from
the fluid
exchanger product receptacle section 112 to the particle concentration module
110. The
particle concentration module 110 is configured and arranged to further enrich
the
concentration of the sub-population of particles within the buffer stream
through a
combination of inertial focusing techniques and fluid shifting.
FIG. 5 is a schematic that illustrates a top view of the entrance portion to
the particle
concentration module 110 (e.g., corresponding to particle concentration module
input section
118 shown in FIG 1). The general direction of fluid flow is indicated at the
bottom of the
page. The buffer fluid stream containing the one or more sub-populations of
particles enters
the particle concentration module 110 at through-holes 500. The through-holes
500 can be
fluidly coupled to the through-holes 400 from the product receptacle section
112 using, e.g.,
tubing or a manifold. Upon entering the particle concentration module 110, the
buffer fluid
stream passes to one or more filter arrays. The filter arrays can be
constructed similar to the
filter arrays shown in FIG. 2. For example, the filter arrays can include
multiple post
structures 505 arranged and configured to filter particles contained in the
buffer stream
according to the particle size (e.g., average diameter), such that only
particles of a pre-defined
size or less are able to pass to the next stage of the system. As shown in FIG
5, the arrays of
posts 505 are arranged on either side of the buffer stream flow (indicated by
arrows 506). The
filter arrays can also include walls/dividers 507 so that the buffer streams
506 are forced
around the walls 507 and through the posts 505. The posts 505 of the arrays
can be
configured and arranged to filter particles of the same size as those
previously filtered or
particles having a smaller size.
The filter arrays are fluidly coupled to a particle focusing section of the
particle
concentration module 110. The particle focusing section is configured to pre-
focus particles
19
84007077
exiting the filter arrays to a desired fluid streamline position before
enriching the particle
concentration. An advantage of pre-focusing the particles is that, in certain
implementations, it
reduces the distribution of particles across the channel width to a narrow
lateral extent. The
focused line of particles then can be repositioned so that the probability of
the particles
inadvertently entering the wrong channel or being extracted with waste fluid
is reduced.
Pre-focusing can be achieved using inertial focusing, where the structure and
arrangement of the fluid pathways are designed to generate forces that drive
particles within a
fluid sample to desired streamlines. The particle focusing section shown in
FIG. 5 includes a
dividing wall 502 that separates two microfluidic channels 504 fluidly coupled
to the output of
the filter arrays. Each channel 504 has an undulating pathway defined by the
surfaces of the
dividing wall 502 and the outer channel walls, where the contour of the
dividing wall surfaces
match the contour of the outer channel wall it is facing. With the undulating
pathways shown
in FIG. 5, the microfluidic channels alternate between regions having
relatively high curvature
and regions having relatively low curvature.
In general, "focusing" particles refers to re-positioning the particles across
a lateral
extent of the channel and within a width that is less than the channel width.
For example, the
techniques disclosed herein can localize particles suspended in a fluid within
a length of the
channel having a width of 1.05, 2,4, 6, 8, 10, 20, 30, 40, 50, 60, 70, 80, 90,
or 100 times the
average diameter of the particles. In some implementations, the particles are
focused to a
streamline of a fluid. In some implementations, a streamline defines a width
that is
substantially equal to or slightly greater than an average hydraulic diameter
of the particle,
which can be, but is not limited to, between about 1 gm and about 100 gm.
Further discussion on the parameters and design principles for fabricating
inertial
focusing structures can be found, for example, in U.S. Patent No. 8,186,913,
U.S. Provisional
Application No. 62/074,213, filed November 3, 2014, and U.S. Provisional
Application
No. 62/074,315, filed November 3, 2014.
For instance, various channel geometries can require a predetermined particle
to
volume ratio of the particle to be focused in order to achieve a required
interparticle spacing
and thereby maintain ordering and focusing of that particle. In particular,
the particle to
volume ratio of a particle suspended within a fluid can be calculated and
adjusted as needed to
achieve focusing within certain channel geometries. In general, a maximum
particle to
Date Recue/Date Received 2022-03-07
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
volume ratio for a specified particle size and channel geometry can be
determined using the
formula:
MaxVolumeFraction = 2Nrca2/3hw
where N is the number of focusing positions in a channel, a is the focused
particle
diameter, h is the channel height, and w is the channel width. Thus, samples
can be diluted or
concentrated to attain a predetermined ratio before and/or during introduction
of the sample
into
the system. Particle to volume ratios of a sample within the channels
described herein can
have any value sufficient to enable ordering and focusing of particles. In
general, the particle
to volume ratio can be less than about 50%. In other embodiments, particle to
volume ratios
can be less than about 40%, 30%, 20%, 10%, 8%, or 6%. More particularly, in
some
embodiments, particle to volume ratios can be in a range of about 0.001 % to
about 5%, and
can preferably be in a range of about 0.01 % to about 4%.
In general, there arc certain parameters within straight, symmetric, and
asymmetric
microfluidic channels that lead to optimal ordering and focusing conditions
for particles
suspended within a sample. These parameters can include, for example, channel
geometries,
particle size with respect to channel geometries, properties of fluid flow
through micro fluidic
channels, and forces associated with particles flowing within micro fluidic
channels under
laminar flow conditions. It is presently believed that the forces acting on
the particles can be
referred to as inertial forces, however, it is possible that other forces
contribute to the
focusing and ordering behaviors. Exemplary inertial forces can include, but
are not limited to,
inertial lift down shear gradients and away from channel walls, Dean drag
(viscous drag),
pressure drag from Dean flow, and centrifugal forces acting on individual
particles. The
theory discussed below is meant to be solely descriptive and exemplary and,
while the
behavior of systems designed using these principles can be predicted using
this theory, the
theory presented should not be considered as limiting the invention to any of
the parameters
associated with any of the system embodiments disclosed herein or any
particular theory of
operation.
In general, inertial lift forces in laminar microfluidic systems, such as
those described
in the embodiments herein, can act to focus randomly distributed particles
continuously and
at high rates into a single streamline. Particle geometry dependence can be
used to develop
21
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
systems for high-throughput separations. Channel geometry can be changed to
reduce
focusing particles from an annulus to four points, to two points, and then to
a single point
within the channel. Two additional levels of particle ordering can be
observed, in particular,
longitudinally along the channel length and rotationally (for asymmetric
particles). In
general, separation, ordering, and focusing is primarily controlled by a ratio
of particle size to
channel size and the flow characteristics of the system. Advantageously, the
focusing is
independent of particle density.
Lateral migration of particles in shear flow arises from the presence of
inertial lift,
attributed mainly to the shear-gradient-induced inertia (lift in an unbounded
parabolic flow)
that is directed down the shear gradient toward the wall, and the wall induced
inertia which
pushes particles away from the wall. Particles suspended in fluids are
subjected to drag and
lift forces that scale independently with the fluid dynamic parameters of the
system. Two
dimensionless Reynolds numbers can be defined to describe the flow of
particles in closed
channel systems: the channel Reynolds number (Re), which describes the
unperturbed
channel flow, and the particle Reynolds number (Rp), which includes parameters
describing
both the particle and the channel through which it is translating.
Re = (UmDk)/ v and Rp = Rc (a2/ Dh2)= (Um a2)/( vi),)
Both dimensionless groups depend on the maximum channel velocity, Um, the
kinematic viscosity of the fluid, and v = p/p (# and p being the dynamic
viscosity and density
of the fluid, respectively), and Db, the hydraulic diameter, defined as
2wh/(w+h) (w and h
being the width and height of the channel). The particle Reynolds number has
an additional
dependence on the particle diameter, a. The definition of Reynolds number
based on the
mean channel velocity can be related to Itc by Re=2/312Ø
Inertial lift forces dominate particle behavior when the particle Reynolds
number is of
order 1. Typically, particle flow in microscale channels is dominated by
viscous interactions
with Rp<<l. In these systems, particles are accelerated to the local fluid
velocity because of
viscous drag of the fluid over the particle surface. Dilute suspensions of
neutrally buoyant
particles are not observed to migrate across streamlines, resulting in the
same distribution
seen at the inlet, along the length, and at the outlet of a channel. As Rp
increases, migration
22
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
across streamlines occurs in macroscale systems. In a cylindrical tube,
particles were
observed to migrate away from the tube center and walls to form a focused
annulus. The
theoretical basis for this "tubular pinch" effect is a combination of inertial
lift forccs acting on
particles at high particle Reynolds numbers. The dominant forces on rigid
particles are the
"wall effect," where an asymmetric wake of a particle near the wall leads to a
lift force away
from the wall, and the shear-gradient-induced lift force that is directed down
the shear
gradient and toward the wall.
Channels with curvature create additional drag forces on particles. When
introducing
curvature into rectangular channels, secondary flows develop perpendicular to
the streamwise
direction due to the nonuniform inertia of the fluid. In a parabolic velocity
profile, faster
moving fluid elements within the center of a curving channel can develop a
larger inertia than
elements near the channel edges. These elements can move toward the channel
outer edge,
and in order to conserve mass at all points where the fluid is recirculated
along the top and
bottom of the channel. Two dimensionless numbers can be written to
characterize this flow,
the Dean number (De) based on the maximum velocity in the channel, and the
curvature ratio
(6). The Dean number, De = Rc (Dh/2r)[/2 and the curvature ratio, 6 = Dh/2r,
where r is the
average radius of curvature of the channel. For moderate De <75 observed in
the microfluidic
systems described herein, the secondary rotational flow, or Dean flow,
consists of only two
vortices. The velocity magnitude of the Dean flow scales as UD pDe2/(l.0h) and
therefore,
Stokes drag on suspended particles due to this secondary flow becomes
significant for large
De. In general, the drag due to to Dean flow, or Dean drag (FD) scales as
FD (pUm2aDh2)/r.
in short, three flow regimes can be considered: (1) At low fluid velocities,
the ratio of lift to
drag forces, Rf, may be larger than 1 over the majority of the channel cross
section; however,
the magnitudes of Fz and FD are too low to create focused streams within the
length of
channel. (2) At intermediate fluid velocities, Re may be greater or equal to 1
over a limited
region of the channel cross section, and the magnitude of forces is large
enough to create
focusing to one or more streams. (3) For high fluid velocities, Rf is less
than 1 over the entire
channel cross section, and Dean drag is dominant, leading to particle mixing.
23
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
Referring again to device 100, the buffer fluid stream passes from the
inertial focusing
section of the particle concentration module 110 to microfluidic channels
configured to
increase the concentration of one or more sub-populations of particles within
the buffer
stream. FIG 6 is a schematic that illustrates a top view of the portion of the
particle
concentration module 110 for enriching particle concentration. The direction
of fluid flow
through the module 110 is shown at the bottom of the page. In particular, the
buffer stream
exits the microfluidic channels 504 of the pre-focusing section and passes
into microfluidic
channels 512 or 514. A first pair of channels 512, 514 remains separated from
a second pair
of channels 512, 514 by wall 502. Each of channels 512 and 514 are themselves
separated
from one another by an array of post structures 506. The post structures are
spaced apart from
one another by gaps through which fluid can be extracted (along, e.g., a
direction indicated
by arrows 510).
Generally, the pre-focusing positioned the particles within the buffer stream
along one
or more streamlines that are closer to the surfaces of wall 502 so that, as
the buffer stream
exits the pre-focuser, the particles are aligned to the channels 512. The
design and
configuration of the channels 512 are similar to channels 504 in that the
pathway is
undulating and alternates between areas of relatively high curvature and areas
of relatively
low curvature. This channel shape again gives rise to inertial forces that
focus one or more
sub-populations of particles to streamlines within channels 512. At the same
time, however,
channels 514 arc configured to have a decreasing fluidic resistance such that
portions of the
buffer fluid are repeatedly extracted into channels 514 as indicated by arrows
510, similar to
the fluid extraction that takes place in the fluid exchanger module 108. That
is, the distance
between the outer walls 516 and the island structures 506 progressively
increases along the
length of the channels 514. For example, FIG. 7 is a schematic that
illustrates the walls 516
are oriented at an angle with respect to islands 506, such that the width of
channels 514
continues to increase downstream (the distance to the wall from the island
structures is much
greater further down the channel, as shown in FIG. 7, than near the input, as
shown in FIG
6). Because the inertial focusing drives the particles to streamlines away
from the gaps where
fluid extraction takes place, the extracted portions of fluid arc
substantially particle-free. By
repetitively removing/siphoning particle-free buffer fluid from the channels
512, the
concentration of particles relative to the buffer fluid in channels 512
substantially increases
relative to their concentration upon exiting channels 504.
24
84007077
Design parameters that are relevant for establishing the amount of particle-
free fluid
extracted at each gap between islands 506 and for positioning the focused
particles along one
or more streamlines include, among others, the lengths of the channels 512,
514, the widths of
the channels 512, 514, the spacing between islands 506, and the flow speed of
fluid through
the particle concentration module 110. For example, the maximum flow rate in
which a
particle-free layer can form and be extracted through the gaps between islands
506 follows a
generally linear relationship with the width of the particle concentration
channels (as measured
in the x-direction of FIG. 7 between a channel wall 512 and the opposing wall
of the divider
502). In another example, the yield of the device 100 can be adversely
affected by flow rates
through the particle concentration module 110 that are too low (i.e., low
Reynolds numbers)
such that inertial forces are not large enough to focus particles to
streamlines.
Further discussion on the parameters and design principles for fabricating
structures
that combine inertial focusing and fluid extraction to enhance particle
concentration can be
found, for example, in U.S. Provisional Application No. 62/074,213, filed
November 3, 2014,
and U.S. Provisional Application No. 62/074,315, filed November 3, 2014,
Particle Concentration Module Waste and Particle Concentration Module Product
Output
Upon exiting the particle concentration module, the buffer stream containing
the
enriched population of particles is passed to a product output section 122
where the particles
can be collected for further processing and/or analysis. The portions of the
particle-free buffer
fluid that have been extracted are passed to a waste section 120, where the
buffer fluid can be
disposed of or further analyzed and/or processed. FIG. 8 is a schematic that
illustrates a top
view of the waste section 120 and product output section 122. As shown in FIG.
8, channels
514, which include the particle-free buffer solution, are fluidly coupled to
the waste section
120. The waste section 120 can include one or more through-holes 800 into
which the particle-
free buffer solution passes. The channels containing the particle enriched
buffer solution, in
contrast, are fluidly coupled to the product output section 122. In the
implementation depicted
in FIG. 8, the channels coupled to product output 122 can include one or more
fluid resistor
sections, such as fluid resistors 802 and 804. The fluid resistors 802 and 804
can be configured
and arranged to obtain the correct flow rates for the buffer fluid as it
passes to the product
output 122. For instance, in the present example shown in FIG. 8, the fluid
resistors 802 and
804 include sinusoidal-shaped channels that increase the fluid
Date Recue/Date Received 2022-03-07
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
resistance. The center channel that is coupled to through-hole 806 includes
the additional
fluid resistor 804 to modify the buffer fluid flow rate in that channel so
that it matches the
flow rate of buffer solution coming from the longer upper and lower channels
that are also
coupled to the through-hole 806.
By using the particle concentration module 110, it can be possible, in some
implementations, to enhance the concentration of particles that have been
transferred to a
second fluid sample from a first fluid sample by substantial amounts. For
example, the
concentration can be enhanced between about 5 times and about 100 times the
original
concentration of the particles prior to entering the particle concentration
module including,
e.g., about 10 times, about 20 times, about 30 times, about 40 times, about 50
times, about 60
times, about 70 times, about 80 times, or about 90 times the original particle
concentration.
In addition, by combining the fluid exchanger module 108 and the particle
concentration module 110 in a single microfluidic device, it is possible to
extract
substantially high yields of desired sub-populations of particles from a fluid
sample. For
example, in some implementations, the device 100 can be used to obtain
particle yields from
an initial fluid sample greater than about 70%, greater than about 80%,
greater than about
85%, greater than about 90%, greater than about 95%, greater than about 99%,
greater than
about 99.9%, or greater than 99.99% yield, where yield can be understood to
mean the
percentage of number of desired particles extracted from the fluid sample
relative to the
number of desired particles originally contained within the fluid sample when
the fluid
sample was introduced into the device.
In the examples disclosed above, the fluid exchange module can be used to
deplete
relatively large particles from a fluid sample and transfer those particles to
a second fluid
such as a buffer fluid. As a result, the fluid sample that is depleted becomes
the waste,
whereas the buffer solution becomes the product. It is also possible, in some
implementations, to keep the fluid sample that is depleted of the relatively
large particles as
the product and remove the second fluid to which the relatively large
particles have been
transferred as the waste. In such cases, the device can be configured to alter
(e.g., enrich) the
concentration of the fluid sample that is kept as product in the particle
concentration module
instead of the second fluid sample that contains the transferred particles.
For example, the
fluid exchange module fractionator can be designed to have a cutoff size for
removing white
blood cells and red blood cells from a blood sample and then enrich the
concentration of the
platelets remaining in the blood sample stream.
26
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
Fabrication of Microfluidic Devices
A process for fabricating a microfluidic device according to the present
disclosure is
set forth as follows. A substrate layer is first provided. The substrate layer
can include, e.g.,
glass, plastic or silicon wafer. An optional thin film layer (e.g., SiO2) can
be formed on a
surface of the substrate layer using, for example, thermal or electron beam
deposition. The
substrate and optional thin film layer provide a base in which the
microfluidic channels
depicted throughout FIGS. 2-8 can be formed. The thickness of the substrate
can fall within
the range of approximately 500 pm to approximately 10 mm. For example, the
thickness of
the substrate 210 can be 600 pm, 750 pm, 900 pm, 1 mm, 2 mm, 3 mm, 4 mm, 5 mm,
6 mm,
7 mm, 8 mm, or 9 mm. Other thicknesses are possible as well.
The microfluidic channels formed within the substrate include the different
fluid flow
pathways for the fluid sample and the buffer, such as the straight channels,
the filter arrays,
the fluidic resistors, the channels within the fluid exchanger module, and the
channels within
the particle concentration module. The microfluidic channels can be formed, in
some
implementations, by depositing a polymer (e.g., polydimethylsiloxane (PDMS),
polymethylmethacrylate (PMMA), polycarbonate (PC), or cyclo olefin polymer
(COP)) in a
mold that defines the fluidic channel regions. The polymer, once cured, then
can be
transferred and bonded to a surface of a support layer. For example, PDMS can
be first
poured into a mold (e.g., an SU-8 mold fabricated with two step
photolithography
(MicroChem)) that defines the microfluidic network of channels. The PDMS then
is cured
(e.g., heating at 65 C for about 3 hours). Prior to transferring the solid
PDMS structure to
the support layer, the surface of the substrate layer is treated with 02
plasma to enhance
bonding. Alternatively, if the microfluidic channels are fabricated in other
substrate
materials, such as a glass or silicon wafer, the channels can be formed using
standard
semiconductor photolithography processing to define the channel regions in
combination
with wet and/or dry etching techniques to fabricate the channels.
After forming the microfluidic channels within the substrate, the substrate,
also
referred to as the "fluidic layer," can be covered with a lid layer. The lid
layer seals the fluidic
layer microchannels (i.e., forms the "ceiling" of each channel) and is aligned
and bonded to
the fluidic layer. Bonding can be achieved using, e.g., an adhesive. The lid
layer can include
through-holes that are aligned with the through-holes formed in the fluidic
layer, so as to
27
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
allow fluids to be introduced and withdrawn from the device. Alternatively, or
in addition, the
through-holes can be formed in the fluid layer such that, in some
implementations, the lid
layer is not necessary. In some implementations, a third interface layer
couples to the surface
of the lid layer. The interface layer can include a manifold that enables
macro-scale
connections to the device.
FIG 9 is a schematic that illustrates a cross-section of a device, such as
device 100,
according to the present disclosure. The view shown in FIG. 9 is a generalized
cross-section
and does not correspond to any one particular location in the device 100. As
shown in the
cross-section, the device 100 includes a fluidic layer 900, in which the
microfluidic channels
are formed. A top surface of fluidic layer 900 is bonded to the lid layer 902
(not shown in
FIG 1). The through-holes of the fluidic layer 900 can be aligned with the
through-holes in
the lid layer. The interface layer 904 (not shown in FIG. 1) includes one or
more manifold
sections 906 for establishing a common macro-connection (e.g., to tubing) to
multiple
through-holes of the device 100. For instance, the interface layer 904 can
include a first
manifold section for providing a single coupling connection to the multiple
through-holes 200
(see FIG. 2) and another manifold section for providing a single coupling
connection from the
multiple through-holes 800 (see FIG. 8). The interface layer 904 can be laser
welded to the lid
layer 902. In some implementations, a manifold of the interface layer 904
includes a port 908
for enabling the macro-connection.
Additional information about microfluidic channel networks and their
fabrication can
be found, for example, in U.S. Patent App. Publication No. 2011/0091987, U.S.
Patent No.
8,021,614, and U.S. Patent No. 8,186,913, each of which is disclosed herein by
reference in
its entirety.
28
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
Microfluidic Device Dimensions
For generally spherical particles being transported through a microfluidic
device, the
depth (e.g., as measured into/out of the page for FIGS. 2-8) and width (e.g.,
as measured
along the x-direction in FIGS. 2-8) of the microfluidic channels can be, for
example, in the
range of about 2 times to about 50 times the diameter of the type of particle
for which the
device 100 is designed to enrich. With respect to the island structures 300
that form the gaps
through which fluid is extracted in the fluid exchanger module 108 or the
island structures
506 that form the gaps through which fluid is extracted in the particle
concentration module
110, the width of the structures can be, e.g., up to about 10 times the width
of an adjacent
microfluidic channel, whereas the length of those structures can be between
about 0.25 times
the adjacent channel width up to about 50 times the adjacent channel width.
In some implementations, the length of the island structures 300 (as measured
generally along the z-direction in FIG. 3) can be between, e.g., about 10 gm
to about 5 mm
long, including about 100 gm long, about 250 gm long, about 500 gm long, about
750 gm
long, or about 1 mm long. In some implementations, the width of the structures
300 (as
measured generally along the x-direction in FIG. 3) can be between, e.g.,
about 1 gm wide to
about 1 mm wide, including about 10 gm wide, about 50 m wide, about 100 gm
wide, about
250 gm wide, about 500 gm wide, or about 750 gm wide. In some implementations,
the
distance between adjacent islands 300 (as measured generally along the z-
direction in FIG. 3)
can be between, e.g., about 1 gm to about 1 mm, including about 10 gm, about
50 gm, about
100 gm, about 250 gm, about 500 gm, or about 750 m. In some implementations,
the
distance between the outermost islands 300 of the arrays and the microfluidic
channel walls
(e.g., walls 305 or 307) can vary between about 1 um to about 500 gm,
including, for
example, about 10 gm, about 20 gm, about 30 gm, about 40 gm, about 50 gm,
about 60 gm,
about 70 gm, about 80 gm, about 90 gm, about 100 gm, about 150 gm, about 200
gm, about
250 gm, about 300 gm, about 350 gm, about 400 gm, or about 450 gm. In some
implementations, the length of the fluidic exchanger module 108 (as measured
generally
along the z-direction from one end of the array of islands 300 to the other
end of the array of
islands 300) can be between, e.g., about 10 mm to about 100 mm, including
about 20 mm,
about 30 mm, about 40 mm, about 50 mm, about 60 mm, about 70 mm, about 80 mm,
or
about 90 mm.
29
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
In contrast to the island structures 300, the island structures of the
particle
concentration module 110 have a generally triangular prism shape with a
maximum width
generally corresponding to one base of the triangular face. In some
implementations, the
maximum length of the island structures 506 can be between, e.g., about 10 pm
to about 5
mm long, including about 100 pm long, about 250 p.m long, about 500 p.m long,
about 750
pm long, or about 1 mm long. In some implementations, the distance between
adjacent
islands 506 (as measured generally along the z-direction in FIG. 7) can be
between, e.g.,
about 1 um to about 1 mm, including about 10 pm, about 50 gm, about 100 pm,
about 250
pm, about 500 um, or about 750 p.m. In some implementations, the distance
between the
outermost islands 506 of the arrays and the microfluidic channel walls (e.g.,
walls 516 or
walls of divider 502) can vary between about 1 p.m to about 500 gm, including,
for example,
about 10 gm, about 20 gm, about 30 pm, about 40 gm, about 50 gm, about 60 p.m,
about 70
pm, about 80 gm, about 90 gm, about 100 gm, about 150 pm, about 200 pm, about
250 gm,
about 300 pm, about 350 um, about 400 um, or about 450 p.m. In some
implementations, the
length of the particle concentration module 110 (as measured generally along
the z-direction
from one end of the array of islands 506 to the other end of the array of
islands 506) can be
between, e.g., about 10 mm to about 100 mm, including about 20 mm, about 30
mm, about
40 mm, about 50 mm, about 60 mm, about 70 mm, about 80 mm, or about 90 mm.
As an example, for a generally spherical particle having a diameter of about 8
microns, a microfluidic device having two microfluidic channels separated by
an array of
rigid structures similar to the configuration shown in FIG. 1 can have the
following
parameters: each microfluidic channel can have a depth about 52 pm, each
microfluidic
channel can have a range of widths between about 10 pm to about 5000 p.m, each
island
structure can have a width of about 50 pm, each island structure can have a
length of about
200 pm.
Ap plications
The new microfluidic techniques and devices described herein can be used in
various
different applications. For example, the techniques and devices disclosed
herein can be used
to isolate and enrich the concentration of cells or other particles from a
fluid sample. Such
cells or particles can include, e.g., blood cells in general as well as fetal
blood cells in
maternal blood, bone marrow cells, and circulating tumor cells (CTCs), sperm,
eggs, bacteria,
fungi, virus, algae, any prokaryotic or cukaryotic cells, organelles,
exosomes, droplets,
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
bubbles, pollutants, precipitates, organic and inorganic particles, magnetic
beads, and/or
magnetically labeled analytes). Alternatively, or in addition, the techniques
and devices
disclosed herein can be used to extract purified fluid samples, from which
particles and/or
cells have been extracted. Such fluids can include, e.g., blood, aqueous
solutions, oils, or
gases. Examples of specific applications are set forth below.
Centrifugation Replacement
The combined fluid exchanger and particle concentration device described
herein can
be used as a replacement for centrifugation. In general, centrifugation is
understood to
include the concentrating of sub-components within a fluid through the
application of
centrifugal forces to the fluid. Typically, this process requires devices that
have moving
parts, which are prone to wear and breakage. Moreover, the moving parts
require complex
and costly fabrication processes. Another problem with centrifugation is that
it is a process
typically applied in a closed system, i.e., centrifugation requires manually
transferring
samples to and from a centrifuge.
In contrast, the presently disclosed device is capable of substantially
increasing the
concentration of fluid components using relatively simple micro-structures
without the need
for moving parts. The techniques can be implemented as part of a single open
microfluidic
system, such that fluid samples can be transfen-ed between the fluid exchanger
module and
the particle concentration module, among other sections of the device without
manual
interference. Additionally, the device described herein can be used for
applications requiring
large throughput (i.e., volume rate of fluid that can be processed). For
example, the devices
disclosed herein can be configured to enable up to 10, 25, 50, 75, 100, 250,
500, 1000, 5000,
or 10000 ttlimin of fluid flow. Other flow rates are also possible. Varying
the channel sizes
can alter the maximum volumetric flow rate of which the device is capable.
Furthermore,
because the channels in the fluid exchanger module and the particle
concentration module are
multiplexed (i.e., multiple copies of the fluid exchanger and particle
concentration structures
are used in parallel), even higher rates of flow can be achieved. Thus, in
certain
implementations, the device and techniques disclosed herein can provide
substantial cost and
time saving advantages over traditional centrifugation processes. Examples of
applications
where a microfluidic replacement for a centrifuge device can be useful include
bone marrow
and urine analysis.
31
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
Detecting Infectious Agents
in addition, the device and techniques disclosed herein can be used as part of
a
research platform to study analytcs of interest (e.g., proteins, cells,
bacteria, pathogens, and
DNA) or as part of a diagnostic assay for diagnosing potential disease states
or infectious
agents in a patient. By extracting, focusing and enriching particle
concentration, the
microfluidic device described herein can be used to measure many different
biological
targets, including small molecules, proteins, nucleic acids, pathogens, and
cancer cells.
Further examples are described below.
Rare Cell Detection
The microfluidic device and methods described herein can be used to detect
rare cells,
such as circulating tumor cells (CTC) in a blood sample or fetal cells in
blood samples of
pregnant females. For example, the concentration of primary tumor cells or
CTCs can be
enhanced in a blood sample for rapid and comprehensive profiling of cancers.
Thus, the
microfluidic device can be used as a powerful diagnostic and prognostic tool.
The targeted
and detected cells could be cancer cells, stem cells, immune cells, white
blood cells or other
cells including, for example, circulating endothelial cells (using an antibody
to an epithelial
cell surface marker, e.g., the Epithelial Cell Adhesion Molecule (EpCAM)), or
circulating
tumor cells (using an antibody to a cancer cell surface marker, e.g., the
Melanoma Cell
Adhesion molecule (CD146)). The systems and methods also can be used to detect
small
molecules, proteins, nucleic acids, or pathogens.
Isolation and Concentration of Nucleated Cells
The microfluidic device and techniques disclosed herein can be used to isolate
and
concentrate nucleated cells (e.g., white blood cells) from complex input
fluids, such as blood
and bone marrow. For instance, the device can be used to isolate neutrophils
from blood for
radiolabeling and subsequent injection and nuclear imaging ("leuko-imaging")
and/or for
enriching progenitor cells from bone marrow aspirate for subsequent injection
into orthopedic
injury sites.
Fluid Exchange
The microfluidic device and methods described herein can be used to shift
cells from
one carrier fluid to another carrier fluid. For example, the particle shifting
techniques
32
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
disclosed could be used to shift cells into or out of a fluid stream
containing reagents, such as
drugs, antibodies, cellular stains, magnetic beads, cryoprotectants, lysing
reagents, and/or
other other analytcs.
A single particle shifting region could contain many parallel fluid streams
(from many
inlets) through which a shifted cell would pass. For example, white blood
cells could be
shifted from a blood stream into a stream containing staining reagents and
then into a buffer
stream.
In bioprocessing and related fields, the devices and techniques described can
be used
to enable sterile, continuous transfer of cells from old media (containing
waste products) into
fresh growth media. Similarly, extracellular fluids and cellular products
(e.g., antibodies,
proteins, sugars, lipids, biopharmaceuticals, alcohols, and various chemicals)
can be extracted
from a bioreactor in a sterile, continuous manner while cells are retained
within the
bioreactor.
Separating and Analyzing Cells
The microfluidic device and methods described herein can be used to
fractionate cells
based on biophysical properties, such as size. For example, the device and
methods can be
used to fractionate blood into separate platelet, red blood cell, and
leulcocyte streams.
Similarly, the device and methods can be used to fractionate leukocytes into
its separate
lymphocyte, monocytc, and granulocyte streams.
The streams of fractionated cells can be isolated by routing them into
separate fluid
outlets. Alternatively, the streams of cells can be detected and analyzed in
real-time (e.g.,
using optical techniques) to determine the number of cells in each stream or
properties, such
as size or granularity, of the cells in each stream.
Techniques can be used to alter cells or their carrier fluid before or during
sorting to
facilitate their fractionation and/or analysis. For example, large beads can
be bound to a
specific cell type increase the effective size of that cell type. Controlled
cell aggregation can
also be used to increase the effective size of cells. The temperature,
density, viscosity,
elasticity, pH, osmotic, and other properties of the fluid can be changed to
either directly
affect the sorting process (e.g., inertial effects are viscosity dependent) or
indirectly affect the
sorting process by altering the properties of cells (e.g., osmotic swelling or
shrinking).
33
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
Fluid Sterilization and Cleansing
The microfluidic device microfluidic device and methods described herein can
be
used to remove pathogens, pollutants, and other particular contaminants from
fluids. By
shifting contaminants across fluid streamlines, contaminants can be removed
from a fluid
sample and collected as a separate waste stream.
Harvesting Algae fOr Biofitels
Harvesting algae from growth media is a major expense in the production of
biofuels
because algae grow in very dilute suspensions at near neutral buoyancy, making
efficient
extraction and concentration of algal biomass difficult. The microfluidic
device and methods
described herein can provide an efficient means of harvesting algae that does
not depend on
either density or filtration. The devices and techniques described enable the
algae in a growth
tank to be extracted from the growth media and concentrated to a high volume
density. This
could be done either as a single step or as part of a continuous process.
Additionally, because
the devices described herein can sort cells in a size-dependent manner, they
can be designed
to sort and concentrate only the larger algae that have reached maturity,
returning smaller,
immature algae to the tank.
EXAMPLES
The invention is further described in the following examples, which do not
limit
the scope of the invention described in the claims.
Device Fabrication
Various experiments were performed to analyze the behavior of a microfluidic
device
that combines a fluid exchanger module with a particle concentration module,
in which the
architecture of the device was designed to follow the structure and
arrangement of the
components illustrated in FIGS. 1-8. The device used in those experiments were
designed and
fabricated as follows.
FIG. 10 is a schematic that illustrates a top view of a microfluidic chip that
includes
the device according to the present disclosure. As shown in FIG. 10, a
substrate 1000, which
is a plastic disc (similar in shape to a DVD or CD), was fabricated to include
two copies of
the microfluidic device 1002. The designs of the devices 1002 are similar to
the architecture
illustrated in FIGS. 1-8. The channels of the devices 1002 were formed using
injection
34
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
molding into the plastic disc substrate. The disc is therefore an example of
the fluidic layer
900 shown in FIG. 9. Each device 1002 is shown in FIG. 10 surrounded by a
rectangular box
outline. This outline is not formed in the actual device and is instead
included in HG. 10 to
illustrate that the general footprint of each device 1002 fits within a
microscope slide area
(e.g., about 75 mm x 25 mm).
A lid layer was thermally bonded to the surface of the disc, followed by laser
welding
of the interface layer that serves as the macro-micro interface to the through-
holes of the
devices 1002. FIG. 11 is a schematic that illustrates a top view of an
interface layer that is
laser welded to the substrate containing the microfluidic devices 1002. The
interface layer
included a series of openings 1100 that are configured to couple to tubing
and, when the
interface layer was bonded to the substrate, were situated over corresponding
through-holes
for introducing fluid samples or withdrawing fluid samples from the devices
1002.
The depths of the microfluidic channels fabricated in the devices 1002 were
all
approximately 52 ptm. The fluid exchanger module of each device 1002 included
30 arrays,
arranged as 15 duplexes (for example, the structure shown in FIG. 3
corresponds to two
separate arrays or 1 duplex, in which each array includes three rows of
islands 300). For the
manufactured device, each array included3 rows of island structures (e.g.,
island structures
300). The particle concentration module of each device 1002 includes 6 arrays,
arranged as 3
duplexes (for example, the structure shown in FIG. 7 corresponds to two arrays
or a single
duplex).
Cell Extraction and Enrichment
Following fabrication, the devices 1002 as manufactured were used to carry out
an
experiment in which whole blood was loaded into the device to extract and
enrich a
concentration of neutrophils.
The blood volume loaded in all experimental runs was 50 mL. It was diluted 1:1
with
50 mL of buffer (1X PBS with 1% Pluronic F-127), bringing the total sample
volume to 100
mL. The median volume processed, calculated from a hematocrit of the input
sample and the
Fluid Exchanger waste, was about 87 mL (87%). The ¨13 mL (13%) loss can have
been due
to dead volume losses in the sample container, syringes, tubing, and fittings.
The diluted
blood sample thus corresponds to the fluid sample as described herein. A
second fluid sample
into which cells from the diluted blood sample would be transferred and
enriched included a
buffer fluid sample (1X PBS with 1% Pluronic F-127). The flow rate into the
devices 1002
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
for the diluted blood sample was about 1.79 mL/min. the flow rate into the
devices for the
buffer sample was about 8.17 mL/min. The flow rate of the product (the buffer
containing the
enriched cells from the blood sample) out of the devices 1002 was about 90
1.,/min. The
waste flow rate out of the devices 1002 was about 9.87 mL/min. The volume
reduction factor
(input volume/product volume) of the fluid exchanger module of each device was
determined
to be about 0.62X. The volume reduction factor of the particle concentration
module of each
device was determined to be about 32X.
For the purposes of evaluating the experiments, we defined the following
parameters:
EP corresponds to the number of white blood cells (WBCs) output from the Fluid
Exchanger
as product, ETV corresponds to the number of WBCs output from the Fluid
Exchanger as
waste, EO corresponds to the total number of WBCs output from the Fluid
Exchanger, CP
corresponds to the number of WBCs output from the Particle Concentration
module as
product, CW corresponds to the number of WBCs output from the Particle
Concentration
module as waste, and CO corresponds to the total number of WBCs output from
the Particle
Concentration module. Using the foregoing parameters, the following
relationships hold true:
EP = CO = CF + CW; EO = EP + EW =CP + CW + EW. Accordingly, for the fluid
exchanger module, the relative yield can be expressed as EP/EO =
(CP+CW)/(CP+CW+EW), and for the particle concentration module, the relative
yield can
be expressed as CF/CO = CP/(CP+CW).
The median relative yield of white blood cells (WBCs) after the fluid
exchanger
module was about 78%, and the median absolute yield (calculated based on the
processed
volume) was a very similar 81%. FIG. 12 illustrates plots of white blood cell
relative yield
distribution (FIG. 12A) and white blood cell absolute yield distribution (FIG.
12B) for the
different experimental runs. The median relative yield after the particle
concentration module
stage was about 100%. That is, essentially all loss of cells occurred in the
Fluid Exchanger
module stage. FIG. 13 illustrates plots of white blood cell relative yield
(FIG. 13A) following
the fluid exchanger module (the "fractionator") and white blood cell relative
yield (FIG. 13B)
following the particle concentration module for the different experimental
runs.
Several approaches can be used to assess the yield of WBC subpopulations.
Taking
neutrophils as an example, as they can be understood to be an important
subpopulation for the
leuko-imaging application, the neutrophil relative yield can be calculated as
36
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
Neutrophil relative yield = WBC relative yield x (Product neutrophil fraction
/
Sample neutrophil fraction)
where product neutrophil fraction is the fraction of all WBCs in the product
that are
neutrophils and sample neutrophil fraction is the fraction of all WBCs in the
blood sample
that are neutrophils. Thus, the neutrophil relative yield can be expressed as
essentially the
overall WBC relative yield adjusted based on the relative enrichment of the
neutrophil
subpopulation. The median neutrophil relative yield is 84%, significantly
higher than the
median WBC relative yield. The second approach is the neutrophil absolute
yield. This is
calculated as the total number of neutrophils in the product divided by the
total number of
neutrophils processed in the sample. The median neutrophil absolute yield is
78%. FIG. 14
illustrates plots of the relative neutrophil yield (FIG. 14A) and the absolute
neutrophil yield
(FIG. 14B) from the devices 1002 for the different experimental runs.
It should be noted that, with neutrophil relative and absolute yield
calculations, these
values depend on hematology analyzer analysis of the product obtained from the
output of the
device, a cell solution whose composition is very different from the
composition the analyzer
is designed to work with (that of whole blood). As such, the hematology
analyzer reports of
the concentration and frequency of WBC subpopulations can not be particularly
accurate.
A third method of assessing the neutrophil yield does not suffer from this
limitation,
but it does depend on collecting a significant body of data and only provides
an estimate of
neutrophil yield across many samples, not on a sample-by-sample basis. In this
method, the
sample neutrophil fraction was plotted against the relative yield for each
fraction, and a best-
fit line is found to relate the quantities. The best-fit line can be extended
to the hypothetical
case of a sample with 100% neutrophil fraction. In this case, the WBC relative
yield and the
neutrophil relative are equivalent. For this dataset, the slope of the best-
fit line is positive:
the higher the neutrophil content in the sample, the higher the overall WBC
yield. This
strongly suggests that the devices 1002 enrich for neutrophils, consistent
with what one
would expect given the larger size of neutrophils. Moreover, using the
extrapolation method
described suggests that the neutrophil yield is in the range of 100%. FIG. 15
illustrates a plot
of WBC relative yield versus sample neutrophil fraction for the different
experimental runs
and includes the best-fit line described above.
The WBC purity was calculated as the total number of WBCs in the product
divided
by the total number of cells in the product (WBCs, RBCs, and platelets). The
overall median
37
CA 02966603 2017-05-02
WO 2016/073448
PCT/US2015/058785
purity was about 97%. Even in the cases where buffer inlets might have been
blocked
(causing RBC carryover), the purity was typically between about 70-90%.
However, runs
that were not subject to either blocked buffer ports or non-uniform injection
had a WBC
purity of about 99%. Thus, with process improvements one would expect the
median WBC
to trend toward about 99%.
The purity data is closely related to the RBC and platelet depletion data as
shown in
FIG. 16. The median RBC depletion was 4.8 log and the median platelet
depletion was 3.7
log. The depletion of both cell types was generally quite good, the exception
being in cases
of a blocked buffer port or non-uniform injection.
OTHER EMBODIMENTS
It is to be understood that while the invention has been described in
conjunction with
the detailed description thereof, the foregoing description is intended to
illustrate and not
limit the scope of the invention, which is defined by the scope of the
appended claims.
38