Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
=
CA 2968441 2017-05-26
TRANSFER OF NATURAL GAS DIRECT FROM A PIPELINE TO LIQUID
STORAGE
DESCRIPTION
Background Information
Natural gas is primarily moved in a gaseous form by pipeline. Beyond certain
distances and by
restrictions imposed by remote, scattered or undersea field locations, the
movement of natural
gas from reserves to market is in many instances not economically viable by
existing pipeline or
LNG technologies. Advances in Pressurized LNG, CNG and Gas Liquid
transportation have
been proposed by industry in recent years, but frustrated by the lack of
accurate Equation of
State methodology for densities in the region adjacent to the Critical Point
of natural gas mixes
region.
Prior work by Morris et al has shown that under certain storage conditions
through the addition of
light-hydrocarbon and other solvents, it is possible to store natural gas as a
dense phase gas (US
Patent 6,217,626) or a liquid matrix phase of sufficient packing density to be
economically
attractive alongside LNG (liquid natural gas), PLNG (pressurized LNG), and CNG
(compressed
natural gas) modes of transportation (US Patent 7,607,310). The light-
hydrocarbons referred to
here as solvents are ethane, propane and butane, or mixtures of all three
components in the form
of NGL (natural gas liquids) or propane and butane in the form of LPG (liquid
petroleum gas).
Again this work too was limited by the available Equation of State methodology
of the day. This
invention now ventures closer to the critical point of the mixtures, into the
realm of better densities
for storage of natural gas below -62C previously attributed to the warmer
reaches of PLNG. Here
we obtain more competitive densities of natural gas containment roughly two
thirds that of LNG
without resorting to the high energy demands of its processing.
The increased densities for the gas mixes are achieved by addressing their
properties and
seeking lower levels of compressibility (Z) factor associated with the gas
behavior equations. The
reduction in the compressibility factor is brought about through the addition
of the light
hydrocarbon solvent which introduces an order of more intense intermolecular
attraction forces
that result in the denser packing of natural gas under the selective
conditions of storage. APRD
methodology by the VMG organization began to filter into use from 2007
onwards. This method
WSLEGAL\076832 \00002\ I 8038266v1
- -
CA 2968441 2017-05-26
improves on the milestone work of the Peng Robinson Equation of State and
methods of CostaId
determining liquid densities.
It is on this basis that more accurate investigations were able to be carried
out in the region
approaching the critical points of rich gas mixes examined here. This work
showed the existence
of a band of temperatures and pressure storage conditions for a liquid phase
of natural gas and
NGLs referred to here as Matrix Gas that offers superior Net Storage Densities
for the natural gas
component hitherto not available to the industry.
It is also important to note that the conditions favorable to enhanced storage
of gas in this manner
are limited as defined by this invention, and only attainable using the stated
light hydrocarbon
solvents. Other heavier hydrocarbons with larger molecules require much higher
pressures to
achieve similar behavior characteristics of high density packing of the
natural gas component in
fuel mixes at/near ambient temperatures - ref. Hibino, (US Patent 6,584,780).
However, the use
of such heavier solvents is not part of the claims of this invention. Their
vehicle fuel service
conditions also run contrary to the objectives of light weight, low pressure
containment
components sought by this invention to achieve economies of scale in storage
and transport of
bulk quantities of natural gas.
The stored density values of this invention are predicated on attaining
storage conditions in the
liquid region at or near the inflection points of graphic representations of
the bubble curves of the
particular phase envelope of the storage mix as described in US Patent
Application 11/750,942.
The present invention further defines its limiting boundaries of Pressure
/Temperature shown in
tabular form in the Figures.
Adjustment of the mol percentage of the solvent at each storage point to a
best performing level
was an additional requirement, to further identify the ideal liquid mix at
each coordinate of
pressure and temperature shown in the Figures. This is a critical requirement,
to achieve the listed
maximum volumetric ratio of the stored natural gas at each of the specified
conditions.
Summary of the Invention
In accordance with a broad aspect of the invention, there is provided a method
of accelerating
the formation of a liquid storage mixture comprising natural gas emerging from
transmission
pipeline or accumulator chamber without the intermediate need for LNG
processing, the mixture
WSLEGAL\076832 \00002\18038266v1
- 2 -
further comprising: methane and one or more light hydrocarbons that are
ethane, propane,
butane or combinations of these light hydrocarbon as "solvents"; the storage
mixture then being
liquefied by Joule Thompson effect as it leaves the pipeline or storage
chamber; with additional
chilling if required, then being done under bulk storage pressure conditions
of 3080 kPa to 7190
kPa and temperature conditions of -63C to -84C of the mixture.
The method of storing natural gas in a liquid matrix may comprise of the
mixture, maintained by
pressure and temperature conditions such that the mol percentage of light
hydrocarbon solvent
ranges from 1 to 9% mol., where the mixture in storage has a net density
greater than that which
would the case for natural gas alone under the same conditions in the form of
CNG or PLNG.
The method of storing natural gas in a liquid matrix may comprise of the
mixture, maintained by
the pressure and temperature conditions, such that the mol percentage of light
hydrocarbon
solvent ranges from 1 to 26% mol. and where the storage yields greater net
density of the stored
mixture, compared to that which would the case for natural gas alone under the
same conditions
in the form of CNG or PLNG.
For mixed service, the method of storing natural gas in a liquid matrix
comprised of the mixture,
maintained by the pressure and temperature conditions, such that the mol
percentage of light
hydrocarbon solvent ranges from 26 to 90% mol.
The method of storing natural gas in a liquid mix comprised of the mixture of
natural gas emerging
from transmission pipeline or accumulator chamber without the intermediate
need for LNG
processing, with methane and one or more light hydrocarbons that are ethane,
propane, butane
or combinations of these light hydrocarbons, as solvent, maintained by the
pressure and
temperature conditions, such that the mol percentage of light hydrocarbons
solvent is preferably
in the range of 5 to 25% mol, yielding net densities of the natural gas
component in the range of
350 to 425 times the density of the natural gas component in the mixture under
standard
conditions of 15C, and 1 atmosphere.
In accordance with another broad aspect of the invention, there is provided an
accumulator
extension apparatus to a natural gas pipeline having an increase in diameter
and/or additional
runs of pipe specifically configured to hold a greater density of the carried
product by virtue of its
operation at higher pressure than the MOP (Maximum Operating Pressure) of the
pipeline, the
operating pressure within the range of 14825 kPa to 22360 kPa.
WSLEGAL\076832\00002\18038266v2 - 3 -
CA 2968441 2018-05-25
In a process chamber and valve apparatus downstream of the accumulator the
pressure in the
accumulator may be relieved to 7190 kPa to 3080 kPa, causing a Joule Thompson
cooling effect
sufficient to bring about a phase change to cause formation of the liquid form
of the carried
product.
The accumulator may include a pre-loading chiller and optional pump
combination to further
increase the density of the carried fluid which is then loaded into a bulk
storage
vessel/transportation system, where transport can be rendered by land, sea, or
air within
composite carbon, Kevlar, aluminum and steel vessels.
The accumulator may include a plurality of process injection equipment
specifically for increasing
the density of the liquid matrix mixture through the introduction of
additional NGLs upstream or
downstream of the process in a process chamber.
The accumulator may include a plurality of process and containment equipment
specifically for
loading and unloading the liquid matrix mixture in combination with the
pressures and natural gas
displacement mixtures available from interconnecting pipelines at each end of
the transport route.
This invention is primarily intended to seek a less capital intensive and
quicker means of
implementing the movement of natural gas from transmission pipeline in the
production field to
one in a distant marketplace. Traditional use of LNG infrastructure is
avoided, and in its place a
less dense fluid carrying the natural gas component is created between high
pressure pipeline
and low pressure carrier. The fluid is simpler to produce from the field,
transport, and convert back
to a gas stream at the market.
This invention seeks to illustrate the finite limits of temperature and
pressure where the densest
storage of natural gas within a light hydrocarbon solvent can be achieved.
These processes are
based on temperature/pressure/constituent specifications not previously
defined by prior art In
addition, this invention aims to respect the work of others in the field, and
intrude with improved
performance on boundaries established by these technologies. Nevertheless,
limits to the
invention are shown in tabulations in Figures 2, 3 and 4.
These figures provide an overlay of the results of the present methodology on
claim areas of this
invention and of others abutting this invention. The density trends of this
invention and others
clearly establish the superiority of the invention within the sweep of a band
of
pressure/temperature coordinates outlined in heavy lined borders on the
diagrams. Under these
WS LEGAL\ 076832100002 \18038266v2
- 4 -
CA 2968441 2018-05-25
conditions there is a reduction in the energy requirement to produce the
stored liquid and
compress and chill it to containment conditions compared to CLNG and CNG
processing.
Favorable ratios of material intensity and the fiscal capital needs per the
unit of contained gas are
attainable in this region.
The invention seeks to achieve in an energy efficient manner, at pressures
below 5820 kPa, and
through the use of light hydrocarbon solvents to achieve packing densities of
natural gas
components that are an order of magnitude ahead of prior art, raised to two
thirds that of LNG.
(This yields a net storage density gain of the order 400:1 compared to 600:1
for LNG).
Investigation of higher pressures above the critical pressures of most mixes
(about 6850 psig)
revealed declining benefits in volumetric ratio for this technology over those
of simple natural gas
storage (as CNG or PLNG).
No benefits were found at temperatures below -85C, where the performance band
tails off with
higher percentage mol mixes required to sustain better net densities of the
stored natural gas.
Beyond a certain point of concentration of these solvents, it is noted that
their addition becomes
ineffective in improving the yield of natural gas from the gas matrix. The
maximum density of this
matrix mix that yields gains in improved storage packing of the natural gas
component beyond
that of simple natural gas lies in the region of 336 kg/m3.
High density mixes with high concentration of heavier hydrocarbons do not
yield optimal densities
of the natural gas component as is found when using the lighter hydrocarbons
as in this invention.
This invention creates a superior storage region abutting claim areas of
earlier industry practices,
using ethane, propane and butane based solvents or mixtures thereof classified
as NGLs or
LPGs. The base natural gas mixes used are consistent with practical clean
burning levels
promoted by N. American gas specifications. The method is equally applicable
to leaner and
richer base mixes, with adjustments made to the solvent mol percent to achieve
a balanced
storage mix.
Description of the Invention
The invention enables the bulk storage of natural gas to be efficiently
rendered within a liquid light
hydrocarbon matrix, which is then maintained in liquid form under conditions
of pressure and
temperature, yielding a packing net density of the natural gas component that
is greater than
WSLEGAL1076832\ 00002\ 18038266v2
- 5 -
CA 2968441 2018-05-25
CA 2968441 2017-05-26
those previously discovered for Compressed Natural Gas or Compressed LNG mixes
under these
conditions in earlier developments in this field. The solvent components
comprise of ethane,
propane and butane based hydrocarbon mixes or combinations therein as found in
the form of
NGL and LPG blends.
The natural gas or rich natural gas blends delivered from the production field
exit the transmission
pipeline to be compressed into a gas phase accumulator configuration of
pipeline or dedicated
storage chamber operating at a higher pressure than the MOP of the
transmission pipeline.
From gaseous storage the NGL enriched mix is de-pressured into a process
chamber where it
undergoes a phase change to a liquid form suited for bulk storage and
transportation without
having to undergo processing in an LNG Plant. Depending on ultimate storage
density, the liquid
mix may require additional additive, chilling or compression. This product
state is achieved using
less energy and capital expenditure than is required for the production of a
bulk storage and
transport mix of traditional LNG.
The liquid matrix mix is held in phase and loaded onto the bulk
storage/transport vessel against
a backpressure provided by the storage accumulator or transmission pipeline.
For marine transportation an articulated tug barge configuration of the vessel
is the preferred
means of providing quick turn-round and transitory storage for the bulk liquid
at each end of the
voyage. The Tug section uncouples and latches from one barge to the next at
each terminal
minimizing port charges, and having the voyage fuel preloaded and available on
the newly
coupled barge.
On arrival at its destination, the natural gas matrix mix is offloaded either
in its transport
composition that could be an enhanced form to directly meet particular market
specifications, or
be subject to processing whereby the solvent component can be extracted as a
market specific
feedstock form of ethane, propane, butane, NGL, or LPG or even recycled into
the containment
vessels for reuse on a subsequent delivery trip.
The invention enhances the acquisition of natural gas from so called remote
"stranded" reserves
not able to be economically served by LNG vessels or undersea pipeline
technology, on or
offshore. It enables the delivery of natural gas mixes to market for storage
or onward pipeline
transmission. Notwithstanding, these mixtures can be conveyed to their
destination by land or air
modes considering the light pressure containment requirements.
wsl EGAL\076832 \ 00002 \ I 8038266v I
- 6 -
CA 2968441 2017-05-26
BRIEF DESCRIPTION OF THE FIGURES
In the detailed description of the invention reference is made to the
accompanying illustrations:
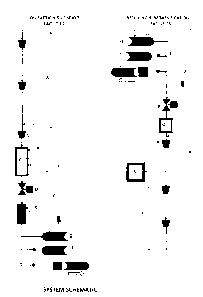
FIG 1. SYSTEM SCHEMATIC
Schematic Representation of Method Showing Direct Pipeline to Ship Transfer of
Liquid Cargo
FIG 2. VOLUMETRIC STORAGE RATIOS OF METHANE MIXES CONSTITUENT
mol% OF ETHANE IN BEST MATRIX MIX
Regions of Optimal Volumetric Ratio of Natural Gas Storage in an Ethane Based
Solvent, Defined by Best mol% Ethane for Each Temperature and Pressure
Point.
FIG 3. VOLUMETRIC STORAGE RATIOS OF METHANE MIXES CONSTITUENT
mol% OF PROPANE IN BEST MATRIX MIX
Regions of Optimal Volumetric Ratio of Natural Gas Storage in a Propane Based
Solvent, Defined by Best mol% Propane for Each Temperature and Pressure
Point.
FIG 4. VOLUMETRIC STORAGE RATIOS OF METHANE MIXES CONSTITUENT
mol% OF BUTANE IN BEST MATRIX MIX
Regions of Optimal Volumetric Ratio of Natural Gas Storage in a Butane Based
Solvent Defined by Best mol% Butane for Each Temperature and Pressure Point.
DETAILED DESCRIPTION OF THE INVENTION AND FIGURES
FIG. 1 shows in schematic form the step by step handling of the gas emerging
from a transmission
pipeline (A). The gas can be either burner tip thermal rating or enriched
mixes boosted with NGLs.
At various points in the process enrichment can be injected at points labeled
(K).
WSLEGAL\076832\00002\18038266v1
- 7 -
The gas flow-stream is compressed from pipeline MOP conditions (typically 6500
to 14725 kPa)
to storage conditions of the order of 20550 kPa using compression facilities
(B). This storage
pressure is limited by the avoidance of fall out of NGL liquids in the
specific gas composition.
The storage space (C) is in the form of a final leg of parallel pipelines or
one of increased diameter,
or cavern type facility to provide several days of production capacity.
From the storage the product flows through a turbo expander or pressure
reducing valve (D) into
a cold temperature chamber (E). Here it experiences a drop to the range of
7190 to 3085 kPa
bulk storage pressures, dependent on the behavior of the specific gas
composition. Here it
undergoes a phase change to the liquid state by virtue of the Joule Thompson
effect. At this point
a touch of additional chilling can be provided if needed to reach the desired
liquid density.
The loading rack (F) provides for loading a vessel (G), holding an empty
vessel (H) or dispatching
a loaded vessel (I). An ocean going Articulated Tug-Barge type vessel is the
preferred means of
conveyance shown here, as it minimizes the turn round time at the terminus
ends of the voyage.
This does not exclude the use of conventional ships. Purging lines and draw-
down compression
(Y) of purge gas from the storage system are illustrated here.
At the delivery end of the voyage the Unloading rack (L) is equipped with the
ability to unload the
vessel (M) using the higher back pressure from the transmission pipeline and
draw down the
resulting heel gas using the interconnects (Z). Provision is made for a
standby loaded vessel (N)
and departing empty vessel (0). The departing vessel can be fuelled by
sufficient heel gas left in
its storage system.
The offloaded product flashes back to a gas after leaving the vessel passing
through a pressure
control station (P) into a heat exchange chamber (Q) where it is warmed to a
gas state suited to
recompression (R) to storage (S) or transmission pipeline entry pressure.
FIG. 2 shows in tabular form the conditions, expressed as coordinates of
temperature and
pressure, where the matrix mix of an ethane C2 solvent and methane Cl (Natural
gas) yield the
best net volumetric values of storage for the methane (natural gas) component.
The lower number in each box shows the storage value VN for methane in the
form of CNG or
PLNG as a ratio relative to methane (natural gas) at standard conditions. The
middle figure shows
the mol % rounded to the nearest whole number where the best performance from
a gas matrix
mix of this methane and ethane can be obtained under these same storage
conditions. The value
WSLEGAL\ 076832 \ 00002 \ 18038266v2
- 8 -
CA 2968441 2018-05-25
of the volumetric ratio for the matrix mix is shown as a net value of the
contained methane on the
upper line of each box.
Where the best performance volumetric ratio numbers of the matrix mixture far
exceed the
CNG/PLNG performance volumetric ratio under the same conditions, the box is
defined by thicker
lines.
It will be noted that the best performing conditions sweep in an arc from a
condition at 3425 kPa
and -84.5C to a condition at 6850 kPa and -51 C. The coordinate boxes on the
higher pressure
side of this arc require a lower % mol of ethane to achieve these higher
volumetric ratios than do
their counterparts positioned on the lower pressure side of the arc.
Of note is the net volumetric ratio of 387 for the matrix mix achieved over a
ratio value of 112 for
PLNG, at conditions of 3425 psig, -84.5C. Mol % solvent here is only 9%.
Within the scope of the invention is the coordinate condition of 6365 kPa and -
67.8C. The matrix
mix yields a net volumetric ratio here of 330 compared to the PLNG figure of
202. A mol% of 14
is required for the ethane.
Dropping down to 4110 kPa for the matrix mix at -67.8C yields a net volumetric
ratio of 299 against
what is now CNG which yields a volumetric ratio of 95. The mol% of solvent
involved here is a
higher figure of 27 percent.
Surrounding the heavy box region are coordinate boxes defined by lighter
lines. These areas are
regions where PLNG and CNG technologies begin to outstrip the matrix mix
volumetric
performance.
Adding solvent to natural gas is ineffective for these outer conditions,
indeed the best numbers
come from adding a minimal 1% mol of solvent to the methane (natural gas).
FIG. 3 shows in tabular form the conditions, expressed as coordinates of
temperature and
pressure, where the matrix mix of a propane C3 solvent and methane Cl (Natural
gas) yield the
best net volumetric values of storage for the methane (natural gas) component.
The lower number in each box shows the storage value VN for methane in the
form of CNG or
PLNG as a ratio relative to methane (natural gas) at standard conditions. The
middle figure shows
the mol % rounded to the nearest whole number where the best performance from
a gas matrix
WSLEGAL \ 076832100002 18038266v2 - 9 -
CA 2968441 2018-05-25
mix of this methane and propane can be obtained under these same storage
conditions. The
value of the volumetric ratio for the matrix mix is shown as a net value of
the contained methane
on the upper line of each box.
Where the best performance volumetric ratio numbers of the matrix mixture far
outstrip the
CNG/PLNG performance volumetric ratio under the same conditions, the box is
defined by thicker
lines.
It will be noted that the best performing conditions sweep in an arc from a
condition at 3425 kPa
and -84.5C to a condition at 6850 kPa and -51C. The coordinate boxes on the
higher pressure
side of this arc require a lower % mol of ethane to achieve these higher
volumetric ratios than do
their counterparts positioned on the lower pressure side of the arc.
Of note is the net volumetric ratio of 388 for the matrix mix achieved over a
ratio value of 112 for
PLNG, at conditions of 3425 kPa, -84.5 C. Mol % solvent here is 11%.
Within the scope of this invention is the coordinate condition of 5480 kPa and
-67.8C. The matrix
mix yields a net volumetric ratio here of 349 compared to the PLNG figure of
202. A mol% of 11
is required for the propane.
Dropping down to 4110 kPa for the matrix mix at -73.0 yields a net volumetric
ratio of 327 against
what is now CNG which yields a volumetric ratio of 109. The mol% of solvent
involved here is a
higher figure of 22 percent.
Surrounding the heavy box region are coordinate boxes in defined by lighter
lines. These areas
are regions where PLNG and CNG technologies begin to exceed the matrix mix
volumetric
performance. Adding solvent to natural gas is ineffective for these outer
conditions.
FIG. 4. shows the behavior of butane C4 solvent matrix mixes. Equal parts of i-
butane and of
n-butane were used in calculations for butane mix and the tabulated values
represent an average
of the differing values of the critical temperatures and critical pressures of
these two forms of this
light hydrocarbon. Overall the critical properties of butane exhibit a higher
critical temperature and
a lower critical pressure than the aforementioned ethane and propane
components of a matrix
mix.
WSLEGAL\076832\00002\18038266v2 - 10 -
CA 2968441 2018-05-25
CA 2968441 2017-05-26
Once again the best values for superior volumetric performance begin at the
coordinate condition
of 3425 kPa psig pressure and -84.5C and assume an arc rotating counter
clockwise towards the
higher pressure region of the table.
The best performing condition here is at 4110 kPa and -81.7C where the
volumetric ratio of the
matrix mix is 395 for 5% mol solvent content. This compares to a volumetric
ratio of 265 for CNG
under the same conditions.
At a condition of 900 psig pressure and -65C temperature a matrix mix with
butane based solvent
requires 9% mol of solvent to achieve a volumetric ratio of 351 that compares
to a volumetric ratio
of 241 for CNG under the same conditions.
At a condition of lower pressure of 3425 kPa psig and the same -65C
temperature the matrix mix
requires 21% mol of solvent to achieve a volumetric ratio of 312. The same
conditions yield a
volumetric ratio of 170 for CNG.
The use of NGL compositions that have constituent parts of ethane, propane and
butane and
LPG compositions that also include propane and butane in their make-up is also
considered for
suitable solvent mixes. The use of these solvents will straddle the same
general
pressure/temperature arcs presented in FIGS. 2, 3 and 4, the exact positioning
being dependent
on the composition of the solvent.
All Matrix storage mixes are suited to transportation and fixed location
storage. The mixes having
lighter % mol in the form of solvent are particularly suited for transport
modes for natural gas
mixes where the solvent does not take up an undue portion of the tonnage
allocated for cargo in
the design of the vessel.
The Matrix mixes having a heavier % mol in the form of solvent are more suited
to fixed storage
such as bulk reserves and smaller peak shaving plants where utilities
supplement transmission
pipeline deliveries during times of high demand. More costly LNG plants are
presently employed
by over 50 utilities in the US and Canada for peak shaving purposes.
The concept of enhancing volume ratio storage through the addition of a
solvent is also suited to
recycling of the solvent on release of the natural gas. Commercial demands
might dictate the sale
of all or part of the solvent - this is possible by simple diversion of the
return flow, downstream of
the offloading process train that splits the natural gas from the solvent.
WSLEGAL \076832 \ 00002 \18038266v1
CA 2968441 2017-05-26
The recent development in low temperature steels, fiber composite technology,
and aluminum
alloys for use at temperatures of -73.5C, coupled with advances in low
temperature fluids
example, makes this invention feasible in a number of process areas. Should
costs of stainless
steel or high nickel steels become excessive for the fabrication of
containment vessels on the
scale envisaged for this technology, the lower pressures suggested here will
minimize the impact.
The invention stems from the dense phase technology of natural gas achieved by
an increase in
light hydrocarbon constituents. This natural gas rich liquid state is achieved
either by the addition
of the light hydrocarbons or the reduction of natural gas methane
concentration employing
commonly used industry technologies. These process systems are not as complex
or costly as
LNG trains. By selective positioning of storage conditions of temperature
/pressure it is possible
to contain the storage mix in a state where its compression factor "Z" is
decreased to the levels
of 0.2 or lower.
Beyond this optimal point there is little benefit in increasing the relatively
incompressible density
with greater expenditure of work, either by further decreasing temperature or
increasing pressure.
IN CONCLUSION
This invention establishes a niche technology wedged between CNG and PLNG.
It requires a simple means of field preparation producing the stored fluid
comprising minimal gas
conditioning in respect of removal of water, nitrogen, carbon dioxide and acid
gas such as H2S.
Liquid formation, direct from a pipeline adjunct storage system, and without
the use of extensive
equipment trains or LNG processing comes about through simple static mixing of
the chilled flow-
streams of solvent and solvent followed by final cooling and compression of
the formed liquid to
storage conditions.
Placing the liquid into containment is done at storage pressure against the
back pressure of a
reusable slug of natural gas mix or suitable non miscible displacement medium
in a similar manner
to the salt water method commonly applied in storage caverns. In this manner
the liquid is
prevented from flashing to the vapor stage during loading.
Containment in vessels designed specifically for the storage conditions is
provided with a
backflow of pipeline gas or alternate displacement medium acting against
controlled pressure
outlet valves to empty the fluid. In this way maximum evacuation of the
containment system is
WSLEGAL\076832\00002\18038266v1
- 12 -
CA 2968441 2017-05-26
possible, any "heel" gas as is common to LNG and CNG systems that is left
behind is measured
and used for fuel gas on the return voyage of delivery vessels.
Gas mixes can be customized at the loading side of the voyage to suit the GJ
heat rating at the
market end of a voyage. Leaner N. American spec transmission gas can be
enhanced to a higher
GJ heat rating by enhancement of the NGL content to balance both the liquid
storage needs and
the export market specifications. On arrival it requires only to be warmed and
unloaded directly
into market transmission pipelines following compression of the re-established
gas phase.
Alternatively, straddle plant process systems can separate portions of the NGL
solvent from the
offloaded flow to yield the local specification natural gas and feedstock
according to local market
conditions for both products.
The overall process of this invention requires common gas field equipment, and
little in the way
of exotic materials and process technology. It requires less energy from field
to market delivery
compared to the complexity of LNG systems or compression energy and attendant
cooling and
evacuation of CNG systems. This enables a greater portion of field reserves to
be available for
the end market.
Coupled with less costly capital and operating costs the invention offers the
market a broad range
of NGL solvents as well as natural gas mixes. Additionally, improved
volumetric ratios are offered
beyond the prior art for transport of natural gas mixes with compressed liquid
hydrocarbons,
exceeding or comparable to those sought for the lower reaches of PLNG systems
at lesser cost.
WSLEGAL\076832\00002\18038266v1
- 13 -