Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
HYDRAULIC FRACTURING COMPOSITION, METHOD FOR MAKING AND USE OF
SAME
BACKGROUND
[0001-2] Hydraulic fracturing increases the flow of desirable fluids such as
oil and
gas from a subterranean formation and involves placing a fracturing fluid into
a subterranean
formation or zone at a rate and pressure sufficient to impart a stress in the
formation or zone
with attendant production of a fracture in the formation or zone. Some
fracturing fluids
contain a viscosifying or gelling agent such as a polysaccharide that breaks
shortly before or
after placement in the formation.
[0003] Beyond creating the fracture, the fracturing fluid also transports a
proppant
into the fracture. The proppant is supposed to keep the fracture open after
release of the
hydraulic pressure. Further, the proppant establishes conductive channels in
which the
desirable fluids flow to the borehole. Since the proppant provides a higher
conductivity than
the surrounding rock, the fracture has greater potential for production of
hydrocarbons.
However, some fracturing fluids break before the fracture closes, and the
proppant separates
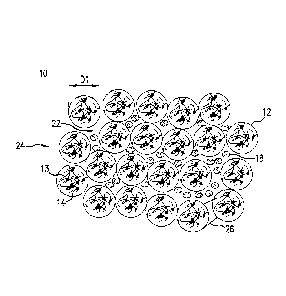
from the fracturing fluid and settles at the bottom of the fracture. In this
situation, the
proppants settle and concentrate at the bottom of the fracture, and thus the
geometry of the
fracture and well productivity is impaired.
[0004] Accordingly, compositions and methods that provide relatively high
permeability and that enhance the production of hydrocarbons from fractured
formations are
highly desired.
BRIEF DESCRIPTION
[0005] The above and other deficiencies are overcome by, in an embodiment, a
hydraulic fracturing composition comprising: a superabsorbent polymer in an
expanded state
and configured to break in response to a breaking condition; a plurality of
proppant particles
disposed in the superabsorbent polymer prior to release of the plurality of
proppant particles
from the superabsorbent polymer in response to breaking the superabsorbent
polymer; a well
treatment agent comprising a pH buffering agent, and a fluid to expand the
superabsorbent
polymer into the expanded state, wherein the superabsorbent polymer comprises
a repeat unit
derived from an acrylate, an acrylamide, a vinylpyrrolidone, a vinyl acetate,
a vinyl alcohol, a
saccharide, a 2-acrylamide-2-methylpropanesulfonic acid, a derivative thereof,
or a
combination thereof; the superabsorbent polymer comprises a plurality of
crosslinks, and the
1
CA 2970488 2018-11-30
pH buffering agent maintains the pH of the hydraulic fracturing composition at
about 7 to
about 8.
[0006] In another embodiment, a process for disposing a plurality of proppant
particles in a fracture comprises disposing a hydraulic fracturing composition
in a fracture in
a downhole environment, the hydraulic fracturing composition comprising: a
superabsorbent
polymer in an expanded state and configured to break in response to a breaking
condition,
such that a decomposed polymer is formed from the superabsorbent polymer, a
plurality of
proppant particles disposed in the superabsorbent polymer prior to release of
the plurality of
proppant particles from the superabsorbent polymer in response to breaking the
superabsorbent polymer, a well treatment agent comprising a pH buffering
agent, and a fluid
to expand the superabsorbent polymer into the expanded state; and breaking the
superabsorbent polymer after disposing in the fracture; and releasing the
plurality of proppant
particles from the superabsorbent polymer to dispose the plurality of proppant
particles in the
fracture, wherein the superabsorbent polymer comprises a repeat unit derived
from an
acrylate, an acrylamide, a vinylpyrrolidone, a vinyl acetate, a vinyl alcohol,
a saccharide, a 2-
acrylamide-2-methylpropanesulfonic acid, a derivative thereof, or a
combination thereof; and
the superabsorbent polymer comprises a plurality of crosslinks, and the pH
buffering agent
maintains the pH of the hydraulic fracturing composition at about 7 to about
8.
BRIEF DESCRIPTION OF THE DRAWINGS
[0007] The following descriptions should not be considered limiting in any
way.
With reference to the accompanying drawings, like elements are numbered alike:
[0008] FIG. 1 shows proppant particles disposed in a superabsorbent polymer in
an
expanded state according to an embodiment;
[0009] FIG. 2 shows proppant particles disposed in a superabsorbent polymer in
an
expanded state according to an embodiment;
[0010] FIG. 3 shows a superabsorbent polymer in an unexpanded state;
[0011] FIG. 4 shows a decomposed polymer and proppant particles;
[0012] FIG. 5 shows a hydraulic fracturing composition disposed in a fracture
before a breaking condition;
[0013] FIG. 6 shows a response of the hydraulic fracturing composition of FIG.
5 to
a breaking condition;
2
CA 2970488 2018-11-30
[0014] FIG. 7 shows a separated fluid and proppant particles disposed in a
fracture
before the fracture closes;
[0015] FIG. 8 shows an effect on fracture size for proppant particles that
settle
before the fracture closes;
2a
CA 2970488 2018-11-30
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
[0016] FIG. 9 shows proppant particles disposed in guar or a superabsorbent
polymer
as a function of time at 180 F;
[0017] FIG. 10 shows addition of a breaker to guar or a superabsorbent polymer
at
180 F;
[0018] FIG. 11 shows a fracture cell during injection of a hydraulic
fracturing
composition;
[0019] FIG. 12 shows a fracture cell after injection of water into a hydraulic
fracturing composition disposed in the fracture cell;
[0020] FIG. 13 shows the viscosity difference of a SPP fluid alone and a SPP
fluid
combined with linear gel systems;
[0021] FIG. 14A and FIG. 14B, collectively referred to as FIG. 14, shows the
effect
of SPP and a linear gel on the foam quality of a foam fracturing fluid;
[0022] FIG 15 shows a fracture cell after alternating injection of a proppant-
containing fluid comprising SPP, proppant particles, a fluid to expand the
SPP, and a
proppant-free fluid comprising water and a lubricant; and
[0023] FIG. 16 shows a fracture cell after alternating injection of a proppant-
free fluid
comprising SPP fluid and a proppant-containing fluid comprising water, a
lubricant, and
proppant particles.
[0024] FIG. 17 shows an effect of fluid pH on viscosity for a superabsorbent
polymer.
DETAILED DESCRIPTION
[0025] A detailed description of one or more embodiments is presented herein
by way
of exemplification and not limitation.
[0026] It has been found that a hydraulic fracturing composition described
herein
creates fractures in a formation and transports proppant particles into the
fractures without
changing the geometry of the fractures so that hydrocarbon transmission
through the fractures
and recovery are optimized. The proppant particles remain suspended in the
hydraulic
fracturing composition without settling to the bottom of the fractures, which
enhances
production from a well.
[0027] As shown in FIG. 1, the hydraulic fracturing composition 10 includes a
superabsorbent polymer 12 (e.g., a plurality of superabsorbent polymer
particles 12), a
plurality of proppant particles 18 disposed in the superabsorbent polymer 12,
and a fluid (not
shown) to expand the superabsorbent polymer 12 into the expanded state. In the
expanded
3
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
state, the superabsorbent polymer 12 is configured to break in response to a
breaking
condition, and a decomposed polymer is formed from breaking the superabsorbent
polymer
12. Upon breaking of the superabsorbent polymer 12, the plurality of proppant
particles 18
are released from the superabsorbent polymer 12.
[0028] The superabsorbent polymer 12 includes a plurality of polymer chains 13
having internal crosslinks 14 between the polymer chains 13 of the
superabsorbent polymer
12. In an embodiment, the proppant particles 18 are included in a space 22
between adjacent
superabsorbent polymer particles 12. In some embodiments, the proppant
particles 18 are
disposed in the space 22 and confined by intra-particle crosslinks 26 of the
superabsorbent
polymer particles 12. It is contemplated that the fluid surrounds an exterior
24 of the
superabsorbent polymer 12, its interior space 22, inside the particles 12, or
a combination
thereof.
[0029] The superabsorbent polymer 12 is a crosslinked, neutralized or
partially
neutralized polymer that is capable of absorbing large amounts of aqueous
liquids, such as
water, brine, acid, or base, with swelling and the formation of a gel or
viscous material, and
retains the absorbed fluid under a certain pressure or temperature. The
superabsorbent
polymer has internal crosslinks, surface crosslinks, or a combination thereof
Superabsorbent
polymer particles are particles of superabsorbent polymers or superabsorbent
polymer
compositions. The acronym SAP may be used in place of superabsorbent polymer,
superabsorbent polymer composition, and particles or fibers (and the like)
herein.
[0030] The SAP has a hydrophilic network that retains large amounts of aqueous
liquid relative to the weight of the SAP. In an embodiment, the SAPs herein
are a variety of
organic polymers that react with or absorb water and swell when contacted with
an aqueous
fluid. Non-limiting examples of such SAPs are a polysaccharide material (that,
e.g., in a dry
state, absorbs and retains a weight amount of water equal to or greater than
its own weight),
poly 2-hydroxyethyl acrylate, polyalkyl acrylate, polyacrylamide, poly
methacrylamide, poly
vinylpyrrolidone, and poly vinyl acetate. In one embodiment, the SAP is a
copolymer of
acrylamide with, for example, maleic anhydride, vinyl acetate, ethylene oxide,
ethylene
glycol, acrylonitrile, or a combination thereof Production of SAPs are, e.g.,
from acrylamide
(AM) or acrylic acid and its salts.
[0031] In an embodiment, the SAP is polymerized from nonionic, anionic,
cationic
monomers, or a combination thereof. Polymerization to form the SAP can be via
free-radical
polymerization, solution polymerization, gel polymerization, emulsion
polymerization,
4
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
dispersion polymerization, or suspension polymerization. Moreover,
polymerization can be
performed in an aqueous phase, in inverse emulsion, or in inverse suspension.
[0032] Examples of nonionic monomers for making the SAP include nonionic
monomers such as acrylamide, methacrylamide, N,N-di(Ci-C8 alkyl)acrylamide
such as N,N-
dimethylacrylamide, vinyl alcohol, vinyl acetate, allyl alcohol, hydroxyethyl
methacrylate,
acrylonitrile, and derivatives thereof. Such derivatives include, for example,
acrylamide
derivatives, specifically alkyl-substituted acrylamides or aminoalkyl-
substituted derivatives
of acrylamide or methacrylamide, and are more specifically acrylamide,
methacrylamide, N-
methylacrylamide, N-methylmethacrylamide, N,N-dimethylacrylamide, N-
ethylacrylamide,
N,N-diethylacrylamide, N-cyclohexylacrylamide, N-benzylacrylamide, N,N-
dimethylaminopropylacrylamide, N,N-dimethylaminoethylacrylamide, N-tert-butyl
acrylamide, N-vinylformamide, N-vinylacetamide, acrylonitrile,
methacrylonitrile, or a
combination thereof.
[0033] Examples of anionic monomers for making the SAP include ethylenically
unsaturated anionic monomers containing acidic groups including a carboxylic
group, a
sulfonic group, a phosphonic group, a salt thereof, a derivative thereof, or a
combination
thereof. In an embodiment, the anionic monomer is acrylic acid, methacrylic
acid, ethacrylic
acid, maleic acid, maleic anhydride, fumaric acid, itaconic acid, a-
chloroacrylic acid, 13-
cyanoacrylic acid, 13-methylacrylic acid (crotonic acid), a-phenylacrylic
acid, (3-
acryloyloxypropionic acid, sorbic acid, a-chlorosorbic acid, 2'-
methylisocrotonic acid,
cinnamic acid, p-chlorocinnamic acid, 13-stearyl acid, citraconic acid,
mesaconic acid,
glutaconic acid, aconitic acid, 2-acrylamido-2-methylpropane sulphonic acid,
allyl sulphonic
acid, vinyl sulphonic acid, allyl phosphonic acid, vinyl phosphonic acid, or a
combination
thereof
[0034] Examples of cationic monomers for making the SAP include an N,N-di-C1-
C8
alkylamino-Ci-C8 alkylacrylate (e.g., N,N-dimethyl amino ethyl acrylate), N,N-
di-C1-C8
alkylamino-Ci-C8 alkylmethacrylate (e.g., N,N-dimethyl amino ethyl
methacrylate),
including a quaternary form (e.g., methyl chloride quaternary forms),
diallyldimethyl
ammonium chloride, N,N-di-C1-C8 alkylamino-Ci-C8 alkylacrylamide, and a
quaternary form
thereof such as acrylamidopropyl trimethyl ammonium chloride.
[0035] In an embodiment, the SAP is an amphoteric SAP, containing both
cationic
substituents and anionic substituents. The cationic substituents and anionic
substituents occur
in various stoichiometric proportions, including one-to-one, or one
substituent is present in a
greater stoichiometric amount than the other substituent. Representative
amphoteric SAPs
include terpolyrners of nonionic monomers, anionic monomers and cationic
monomers.
[0036] In an embodiment, the SAP includes a guar gum and carrageenan. Suitable
materials include those disclosed in Japanese Patent Application No. P2003-
154262A.
[0037] According to an embodiment, the guar gum used in the SAP includes
natural
guar gum as well as enzyme treated guar gum; the latter having been obtained
by treating
natural guar gum with galactosidase, mannosidase, or another enzyme. The guar
gum may
further be a galactomannan derivative prepared by treating natural guar gum
with chemicals
to introduce carboxyl groups, hydroxyl alkyl groups, sulfate groups, phosphate
groups, and
the like. In addition, in an embodiment, a natural polysaccharide, other than
guar and
carrageenan, is included. Exemplary natural polysaccharides include starch,
cellulose,
xanthan gum, agar, pectin, alginic acid, tragacanth gum, pluran, gellan gum,
tamarind seed
gum, cardlan, gum arabic, glucomannan, chitin, chitosan, hyaluronic acid, and
the like.
[0038] Carrageenan is an ionic linear polysaccharide that includes repeating
galactose units that individually may be sulfated or unsulfated. Specific
carrageenan types
include kappa, iota, lambda, and the like. In some embodiments, a mixture of
carrageenan
types is used. In a specific embodiment, a carrageenan or a carrageenan-like
material that
form a gel is used. In addition to natural carrageenan, suitable carrageenans
include enzyme-
treated substances of natural carrageenan or derivatized carrageenan, e.g.,
those prepared by
treating natural carrageenan (e.g., with a chemical) to introduce a functional
group (e.g., a
carboxyl group, hydroxyl alkyl group, sulfate group, phosphate group, and the
like).
[0039] The SAP includes a plurality of crosslinks among the polymer chains of
the
SAP. According to an embodiment, the crosslinks are covalent and result from
crosslinking
the SAP with a crosslinker. In an embodiment, the crosslinker is an
ethylenically unsaturated
monomer that contains, e.g., two sites of ethylenic unsaturation (i.e., two
ethylenically
unsaturated double bonds), an ethylenically unsaturated double bond and a
functional group
that is reactive toward a functional group (e.g., an amide group) of the
polymer chains of the
SAP, or several functional groups that are reactive toward functional groups
of the polymer
chains of the SAP. In an embodiment, the degree of crosslinking in the SAP
herein is
selected to control the amount of swelling (i.e., fluid absorption or volume
expansion) of the
SAP.
[0040] Exemplary crosslinkers include a diacrylamide or methacrylamide of a
diamine such as a diacrylamide of piperazine; an acrylate or methacrylate
ester of a di, tri,
6
CA 2970488 2018-11-30
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
tetrahydroxy compound including ethyleneglycol diacrylate, polyethyleneglycol
diacrylate,
trimethylopropane trimethacrylate, ethoxylated trimethylol triacrylate,
ethoxylated
pentaerythritol tetracrylate, and the like; a divinyl or diallyl compound
separated by an azo
group such as a diallylamide of 2,2'-azobis(isobutyric acid) or a vinyl or
allyl ester of a di or
tri functional acid. Additional crosslinkers include water-soluble diacrylates
such as
poly(ethylene glycol) diacrylate (e.g., PEG 200 diacrylate) or PEG 400
diacrylate and
polyfunctional vinyl derivatives of a polyalcohol such as ethoxylated (9-20)
trimethylol
triacrylate. Further examples of the crosslinker include aliphatic unsaturated
amides, such as
methylenebisacrylamide or ethylenebisacrylamide; aliphatic esters of polyols
or alkoxylated
polyols with ethylenically unsaturated acids, such as di(meth)acrylates or
tri(meth)acrylates
of butanediol, ethylene glycol, polyglycols, trimethylolpropane; di- and
triacrylate esters of
trimethylolpropane (which is oxyalkylated (such as ethoxylated) with an
alkylene oxide such
ethylene oxide); acrylate and methacrylate esters of glycerol or
pentaerythritol; acrylate and
methacrylate esters of glycerol and pentaerythritol oxyethylated with, e.g.,
ethylene oxide;
allyl compounds (such as allyl(meth)acrylate, alkoxylated allyl(meth)acrylate
reacted with,
e.g., ethylene oxide, triallyl cyanurate, triallyl isocyanurate, maleic acid
diallyl ester, poly-
ally' esters, tetraallyloxyethane, triallylamine, tetraallylethylenediamine,
diols, polyols,
hydroxy allyl or acrylate compounds and allyl esters of phosphoric acid or
phosphorous
acid); or monomers that are capable of crosslinking, such as N-methylol
compounds of
unsaturated amides, such as of methacrylamide or acrylamide, and the ethers
derived
therefrom. A combination of the crosslinkers also can be employed.
[0041] In an embodiment, the SAP is a particle (or fiber or other format) that
includes
surface crosslinks, which occur external to the interior of the SAP. The
surface crosslinks,
e.g., result from addition of a surface crosslinker to the SAP particle and
heat-treatment. The
surface crosslinks increase the crosslink density of the SAP near its surface
with respect to
the crosslinking density of the interior of the SAP. Some surface crosslinkers
have a
functional group that is reactive toward a group of the polymer chains of the
SAP, e.g., an
acid or amide group. The surface crosslinker are one of the previously
mentioned
crosslinkers and include a functional group such as an alcohol, amine,
aldehyde, or
carboxylate group. In an embodiment, surface crosslinkers have multiple
different functional
groups such as polyols, polyamines, polyaminoalcohols, and alkylene
carbonates. Ethylene
glycol, diethylene glycol, triethylene glycol, polyethylene glycol, glycerol,
polyglycerol,
propylene glycol, diethanolamine, triethanolamine, polypropylene glycol, block
copolymers
of ethylene oxide and propylene oxide, sorbitan fatty acid esters, ethoxylated
sorbitan fatty
7
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
acid esters, trimethylolpropane, ethoxylated trimethylolpropane,
pentaerythritol, ethoxylated
pentaerythritol, polyvinyl alcohol, sorbitol, ethylene carbonate, and
propylene carbonate can
be used. The surface crosslinkers also provide the SAP with a chemical
property that the
polymer chains of the SAP did not have before surface crosslinking and control
chemical
properties of the SAP, e.g., hydrophobicity, hydrophilicity, or adhesiveness
of the SAP to
other materials such as minerals (e.g., silicates) or other chemicals such as
petroleum
compounds (e.g., hydrocarbons, asphaltene, and the like). Other crosslinkers
include borate,
titanate, zirconate, aluminate, chromate, or a combination thereof Boron
crosslinkers
include, e.g., boric acid, sodium tetraborate, encapsulated borates, and the
like. In some
embodiments, borate crosslinkers are used with buffering agents and pH control
agents such
as sodium hydroxide, magnesium oxide, sodium sesquicarbonate, and sodium
carbonate,
amines (such as hydroxyalkyl amines, anilines, pyridines, pyrimidines,
quinolines,
pyrrolidines, and carboxylates such as acetates and oxalates), delay agents
such as sorbitol,
aldehydes, sodium gluconate, and the like. Zirconium crosslinkers, e.g.,
zirconium lactates
(e.g., sodium zirconium lactate), triethanolamines, 2,2'-iminodiethanol, or a
combination
thereof are used in certain embodiments. Titanates for crosslinking include,
e.g., lactates and
triethanolamines, and the like.
[0042] In an embodiment, the SAP includes a repeat unit that comprises an
acrylate,
an acrylamide, a vinylpyrrolidone, a vinyl ester (e.g., a vinyl acetate), a
vinyl alcohol, a
derivative thereof, or a combination thereof According to an embodiment, the
SAP is a
polyacrylamide having crosslinks that are polyethylene glycol diacrylate. In
some
embodiments, the SAP is polyacrylic acid, wherein the crosslinks are vinyl
ester oligomer. In
an embodiment, the SAP is poly(acrylic acid) partial sodium salt graft
poly(ethylene glycol),
which is commercially available from Sigma Aldrich. Further, the SAP can be in
a number
of formats, including a particle (e.g., a powder), fiber, strand, braid, and
the like, or a
combination thereof The size of the SAP is from 10 [tm to 100,000 p.m,
specifically 50 p.m
to 10,000 tim, and more specifically 50 tim to 1,000 [tm. As used herein,
"size" refers to the
largest linear dimension, e.g., a diameter in a spherical particle Particles
of the SAP are any
shape including spherical, angular, and polyhedral. According to an
embodiment, the SAP is
a particle with pores or spaces between the polymer chains of the SAP that
admits entrance of
a fluid or proppant particle therein. The hydraulic fracturing composition
includes a plurality
of SAP particles (or other format such as fiber or braid) that coalesces
together and form a
single mass of SAP, herein also referred to as the superabsorbent polymer
(SAP). Moreover,
although FIG. 1 shows the SAP as a plurality of superabsorbent polymer
particles 12, the
8
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
SAP is a plurality of superabsorbent polymer fibers 12 as shown in FIG. 2 in
some
embodiments. A combination of the various formats of the SAP is contemplated
for some
embodiments.
[0043] The SAP with crosslinks is useful as a carrier for a fluid or proppant
particles.
In a fracturing operation (e.g., hydraulic fracturing), the proppant particles
disposed in the
SAP remain in the fracture and prop open the fracture when pressure used to
form the
fracture is released as SAPs are broken in response to the breaking condition.
The proppant
particles have a size from 1 i_tm to 2000 m, specifically 10 p.m to 1000 p.m,
and more
specifically 10 [tm to 500 p.m. Further, the proppant particles have any shape
including
spherical, angular, and polyhedral and are monodisperse or polydisperse with
an average
particle size distribution that is unimodal or multimodal, e.g., bimodal.
[0044] In an embodiment, due to the relative size of the SAP and the proppant
particles, the proppant particles are disposed between neighboring SAP
particles (FIG. 1
item) 12 or fibers (FIG. 2 item 12), e.g., in pores or channels formed by
voids or spaces 22
between such adjacent SAP particles or fibers or are disposed within
individual SAP particles
or fibers in the expanded state of the SAP.
[0045] The proppant particles include a ceramic, sand, a mineral, a nutshell,
gravel,
glass, resinous particles, polymeric particles, or a combination thereof. In
an embodiment,
the proppant particles are selected depending on the particular application of
the hydraulic
fracturing composition. Examples of the ceramic include an oxide-based
ceramic, nitride-
based ceramic, carbide-based ceramic, boride-based ceramic, silicide-based
ceramic, or a
combination thereof In an embodiment, the oxide-based ceramic is silica
(SiO2), titania
(TiO2), aluminum oxide, boron oxide, potassium oxide, zirconium oxide,
magnesium oxide,
calcium oxide, lithium oxide, phosphorous oxide, and/or titanium oxide, or a
combination
thereof The oxide-based ceramic, nitride-based ceramic, carbide-based ceramic,
boride-
based ceramic, or silicide-based ceramic contain a nonmetal (e.g., oxygen,
nitrogen, boron,
carbon, or silicon, and the like), metal (e.g., aluminum, lead, bismuth, and
the like), transition
metal (e.g., niobium, tungsten, titanium, zirconium, hafnium, yttrium, and the
like), alkali
metal (e.g., lithium, potassium, and the like), alkaline earth metal (e.g.,
calcium, magnesium,
strontium, and the like), rare earth (e.g., lanthanum, cerium, and the like),
or halogen (e.g.,
fluorine, chlorine, and the like). Exemplary ceramics include zirconia,
stabilized zirconia,
mullite, zirconia toughened alumina, spinel, aluminosilicates (e.g., mullite,
cordierite),
perovskite, silicon carbide, silicon nitride, titanium carbide, titanium
nitride, aluminum
carbide, aluminum nitride, zirconium carbide, zirconium nitride, iron carbide,
aluminum
9
oxynitride, silicon aluminum oxynitride, aluminum titanate, tungsten carbide,
tungsten
nitride, steatite, and the like, or a combination thereof.
[0046] Examples of suitable sands for the proppant particles include, but are
not
limited to, Arizona sand, Wisconsin sand, Badger sand, Brady sand, and Ottawa
sand. In an
embodiment, the proppant particles made of a mineral such as bauxite are
sintered to obtain a
hard material. In an embodiment, the bauxite or sintered bauxite has a
relatively high
permeability such as the bauxite material disclosed in US Patent No.
4,713,203.
[0047] Naturally occurring proppant particles include nut shells such as
walnut,
coconut, pecan, almond, ivory nut, brazil nut, and the like; seed shells of
fruits such as plum,
olive, peach, cherry, apricot, and the like; seed shells of other plants such
as maize (e.g., corn
cobs or corn kernels); wood materials such as those derived from oak, hickory,
walnut,
poplar, mahogany, and the like. Such materials are particles formed by
crushing, grinding,
cutting, chipping, and the like.
[0048] In an embodiment, the proppant particles are coated, e.g., with a
resin. That
is, individual proppant particles have a:coating applied thereto. In this
manner, if the
proppant particles are compressed during or subsequent to, e.g., fracturing,
at a pressure great
enough to produce fine particles therefrom, the fine particles remain
consolidated within the
coating so they are not released into the formation. It is contemplated that
fine particles
decrease conduction of hydrocarbons (or other fluid) through fractures or
pores in the
fractures and are avoided by coating the proppant particles. Coating for the
proppant
particles include cured, partially cured, or uncured coatings of, e.g., a
thermoset or
theinioplastic resin. Curing the coating on the proppant particles occurs
before or after
disposal of the proppant particles in the SAP or before or after disposal of
the hydraulic
fracturing composition downhole, for example.
[0049] In an embodiment, the coating is an organic compound that includes
epoxy,
phenolic, polyurethane, polycarbodiimide, polyamide, polyamide imide, furan
resins, or a
combination thereof. The phenolic resin is, e.g., a phenol formaldehyde resin
obtained by the
reaction of phenol, bisphenol, or derivatives thereof with formaldehyde.
Exemplary
thermoplastics include polyethylene, acrylonitrile-butadiene styrene,
polystyrene, polyvinyl
chloride, fluoroplastics, polysulfide, polypropylene, styrene acrylonitrile,
nylon, and
phenylene oxide. Exemplary thermosets include epoxy, phenolic (a true
thermosetting resin
such as resole or a thermoplastic resin that is rendered thermosetting by a
hardening agent),
polyester resin, polyurethanes, epoxy-modified phenolic resin, and derivatives
thereof
CA 2970488 2018-11-30
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
[0050] In an embodiment, the curing agent for the coating is nitrogen-
containing
compounds such as amines and their derivatives; oxygen-containing compounds
such as
carboxylic acid terminated polyesters, anhydrides, phenol-formaldehyde resins,
amino-
formaldehyde resins, phenol, bisphenol A and cresol novolacs, phenolic-
terminated epoxy
resins; sulfur-containing compounds such as polysulfides, polymercaptans; and
catalytic
curing agents such as tertiary amines, Lewis acids, Lewis bases; or a
combination thereof
[0051] In an embodiment, the proppant particles include a crosslinked coating.
The
crosslinked coating typically provides crush strength, or resistance, for the
proppant particles
and prevents agglomeration of the proppant particles even under high pressure
and
temperature conditions. In some embodiments, the proppant particles have a
curable coating,
which cure subsurface, e.g. downhole or in a fracture. The curable coating
cures under the
high pressure and temperature conditions in the subsurface reservoir. Thus,
the proppant
particles having the curable coating are used for high pressure and
temperature conditions.
[0052] According to an embodiment, the coating is disposed on the proppant
particles
by mixing in a vessel, e.g., a reactor. Individual components, e.g., the
proppant particles and
resin materials (e.g., reactive monomers used to form, e.g., an epoxy or
polyamide coating)
are combined in the vessel to form a reaction mixture and are agitated to mix
the components.
Further, the reaction mixture is heated at a temperature or at a pressure
commensurate with
forming the coating. In another embodiment, the coating is disposed on the
particle via
spraying such as by contacting the proppant particles with a spray of the
coating material.
The coated proppant particles are heated to induce crosslinking of the
coating.
[0053] In addition to the proppant particles and the SAP, the hydraulic
fracturing
composition includes a breaker in some embodiments. The breaker contacts the
SAP to
break the SAP. In an embodiment, the breaker contacts the SAP and breaks a
bond in the
backbone of the polymer chains of the SAP, a bond in the crosslinker, a bond
between the
crosslinker and a polymer chain of the SAP, or a combination thereof That is,
breaking the
SAP includes disintegrating, decomposing, or dissociating the SAP such as by
breaking
bonds in the backbone of the SAP, breaking crosslinks among chains of the SAP,
changing a
geometrical conformation of the superabsorbent polymer, or a combination
thereof. In this
way, the viscosity of the hydraulic fracturing composition decreases. In some
embodiments,
the breaker breaks the SAP to form a decomposed polymer such as a plurality of
fragments
that have a lower molecular weight than the SAP. After breaking the SAP, the
plurality of
proppant particles is released from the SAP.
11
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
[0054] According to an embodiment, the breaker includes an oxidizer such as a
peroxide, a persulfate, a perphosphate, a perborate, a percarbonate, a
persilicate, an oxyacid
of a halogen, an oxyanion of halogen, a peracid, a derivative thereof, or a
combination
thereof.
[0055] In one embodiment, the breaker is persulfate, such as sodium
persulfate,
ammonium persulfate, potassium persulfate, potassium peroxymonosulfate (Caro's
acid), or a
combination thereof The breaker is, e.g., an oxyacid or oxyanion of halogen,
for instance,
hypochlorous acid, a hypochlorite, chlorous acid and chlorites, chloric acid
and chlorates,
perchloric acid and perchlorate, a derivative thereof, or a combination
thereof
[0056] In an embodiment, a peroxide breaker has oxygen-oxygen single bonds in
its
molecular structure. The peroxide breaker is hydrogen peroxide or another
material to
provide peroxide or hydrogen peroxide for breaking the SAP Metal peroxides
such as
sodium peroxide, calcium peroxide, zinc peroxide, magnesium peroxide, or other
peroxides
such as superoxides, organic peroxides, and the like can be used.
[0057] Additionally, in an embodiment, the peroxide breaker is a stabilized
peroxide
breaker with the hydrogen peroxide bound, inhibited, or the like by another
compound or
molecule prior to contact with, e.g., an aqueous fluid such as water such that
it forms or
releases hydrogen peroxide when contacted by the aqueous fluid. Exemplary
stabilized
peroxide breakers include an adduct of hydrogen peroxide with another molecule
and include
carbamide peroxide or urea peroxide (C(=0)(NH2)2.H202), a percarbonate (e.g.,
sodium
percarbonate (2Na2CO3.3H202), potassium percarbonate, ammonium percarbonate,
and the
like), and the like. The stabilized peroxide breakers also include compounds
that undergo
hydrolysis in water to release hydrogen peroxide, e.g., sodium perborate. In
an embodiment,
hydrogen peroxide stabilized with appropriate surfactants also is used as the
stabilized
peroxide breaker.
[0058] According to an embodiment, the breaker is the peracid, e.g., peracetic
acid,
perbenzoic acid, a derivative thereof, or a combination thereof Additionally,
a variety of
peroxycarboxylic acids is employed as the peracid breaker. The
peroxycarboxylic acid
includes an ester peroxycarboxylic acid, an alkyl ester peroxycarboxylic acid,
a
sulfoperoxycarboxylic acid, or a combination thereof Peroxycarboxylic acid (or
percarboxylic acid) are acids having a general formula R(CO3H)õ. In an
embodimentõ the R
group is saturated or unsaturated as well as substituted or unsubstituted. As
described herein,
R is an alkyl, alkenyl, arylalkyl, arylalkenyl, cycloalkyl, cycloalkenyl,
aromatic, heterocyclic,
or ester group, or a combination thereof (e.g., an alkyl ester group), with n
being 1, 2, or 3.
12
CA 02970488 2017-06-09
WO 2016/100048
PCT/US2015/064815
Exemplary ester groups include aliphatic ester groups, such as R10C(0)R2,
where Wand R2
independently are a group (e.g., an alkyl group) described above for R such
that It' and R2
are, e.g., independently small carbon chain alkyl groups, such as a Ci-05
alkyl group.
[0059] One skilled in the art will appreciate that peroxycarboxylic acids may
not be
as stable as carboxylic acids, and their stability may increase with
increasing molecular
weight. Thermal decomposition of the peracids proceeds by, e.g., free radical
and nonradical
paths, by photodecomposition or radical-induced decomposition, or by the
action of metal
ions or complexes. In an embodiment, the percarboxylic acid peracids are made
by direct,
acid catalyzed equilibrium action of hydrogen peroxide with a carboxylic acid,
by
autoxidation of aldehydes, or from acid chlorides, and hydrides, or carboxylic
anhydrides
with hydrogen or sodium peroxide.
[0060] Exemplary peroxycarboxylic acids include peroxyformic, peroxyacetic,
peroxypropionic, peroxybutanoic, peroxypentanoic, peroxyhexanoic,
peroxyheptanoic,
peroxyoctanoic, peroxynonanoic, peroxydecanoic, peroxyundecanoic,
peroxydodecanoic,
peroxylactic, peroxycitric, peroxymaleic, peroxyascorbic, peroxyhydroxyacetic
(peroxyglycolic), peroxyoxalic, peroxymalonic, peroxysuccinic, peroxyglutaric,
peroxyadipic, peroxypimelic, peroxysuberic, peroxysebacic acid, and the like.
[0061] In an embodiment, the peracid includes a combination of several
peroxycarboxylic acids. According to one embodiment, the composition includes
a C2-C4
peroxycarboxylic acid, a C8-C12 peroxycarboxylic acid, an ester
peroxycarboxylic acid, an
alkyl ester peroxycarboxylic acids, or a mono- or di-peroxycarboxylic acid
having up to 12
carbon atoms, and more specifically 2 to 12 carbon atoms. In an embodiment,
the
peroxycarboxylic acid includes peroxyacetic acid (POAA) (i.e., peracetic acid
having the
formula CH3C000H) or peroxyoctanoic acid (POOA) (i.e., peroctanoic acid having
the
formula, e.g., of n-peroxyoctanoic acid: CH3(CH7)6C000H).
[0062] In an embodiment, the peracid is an ester peroxycarboxylic acid. As
used
herein, ester peroxycarboxylic acid refers to a molecule having the formula:
0 0
R2_o_c _cII
_O¨OH
wherein and R2
are independently an organic group (e.g., alkyl, linear or cyclic, aromatic
or saturated) or a substituted organic group (e.g., with a heteroatom or
organic group). In an
embodiment, the ester peroxycarboxylic acid is made by employing methods used
for making
13
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
peroxycarboxylic acid such as combining the corresponding ester carboxylic
acid with an
oxidizing agent, e.g., hydrogen peroxide.
[0063] Exemplary alkyl esterperoxycarboxylic acids include monomethyl
monoperoxyglutaric acid, monomethyl monoperoxyadipic acid, monomethyl
monoperoxyoxalie acid, monomethyl monoperoxymalonic acid, monomethyl
monoperoxysuccinic acid, monomethyl monoperoxypimelic acid, monomethyl
monoperoxysuberic acid, and monomethyl monoperoxysebacic acid; mono ethyl
monoperoxyoxalic acid, monoethyl monoperoxymalonic acid, monoethyl
monoperoxysuccinic acid, monoethyl monoperoxyglutaric acid, monoethyl
monoperoxyadipic acid, monoethyl monoperoxypimelic acid, monoethyl
monoperoxysuberic
acid, and monoethyl monoperoxysebacic acid; monopropyl monoperoxyoxalic acid,
monopropyl monoperoxymalonic acid, monopropyl monoperoxysuccinic acid,
monopropyl
monoperoxyglutaric acid, monopropyl monoperoxyadipic acid, monopropyl
monoperoxypimelic acid, monopropyl monoperoxysuberic acid, monopropyl
monoperoxysebacic acid, in which propyl is n- or isopropyl; monobutyl
monoperoxyoxalic
acid, monobutyl monoperoxymalonic acid, monobutyl monoperoxysuccinic acid,
monobutyl
monoperoxyglutaric acid, monobutyl monoperoxyadipic acid, monobutyl
monoperoxypimelic acid, monobutyl monoperoxysuberic acid, monobutyl
monoperoxysebacic acid, in which butyl is n-, iso-, or t-butyl; and the like.
[0064] In some embodiments, the peracid breaker is a sulfoperoxycarboxylic
acid.
Sulfoperoxycarboxylic acids, which also are referred to as sulfonated
peracids, include the
peroxycarboxylic acid form of a sulfonated carboxylic acid. In some
embodiments, the
sulfonated peracid is a mid-chain sulfonated peracid, i.e., a peracid that
includes a sulfonate
group attached to a carbon that is at least one carbon (e.g., at least the
three position) from the
carbon of the percarboxylic acid group in the carbon backbone of the
percarboxylic acid
chain, wherein the at least one carbon is not in the terminal position. As
used herein, the term
"terminal position" refers to the carbon on the carbon backbone chain of a
percarboxylic acid
that is furthest from the percarboxyl group. Thus, in an embodiment,
sulfoperoxycarboxylic
acid has the following formula:
so3-X+ c),
R3¨c ¨R4¨c ¨0-0H
14
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
wherein R3 is hydrogen or a substituted or unsubstituted alkyl group; R4 is a
substituted or
unsubstituted alkyl group; X is hydrogen, a cationic group, or an ester
forming moiety; or
salts or esters thereof.
[0065] In some embodiments, R3 is a substituted or unsubstituted Cin alkyl
group; X is
hydrogen, a cationic group, or an ester forming moiety; R4 is a substituted or
unsubstituted Cfl
alkyl group; m=1 to 10; n=1 to 10; and m+n is less than 18; or salts, esters,
or a combination
thereof. In some embodiments, R3 is hydrogen. In other embodiments, R3 is a
substituted or
unsubstituted alkyl group. In some embodiments, R3 is a substituted or
unsubstituted alkyl
group that does not include a cycloalkyl group. In some embodiments, R3 is a
substituted
alkyl group. In some embodiments, R3 is an unsubstituted Ci-C9alkyl group. In
some
embodiments, R3 is an unsubstituted C7 or C8 alkyl. In other embodiments, R3
is a substituted
C8-C10 alkyl group. In some embodiments, R3 is a substituted C8-C10 alkyl
group and is
substituted with at least 1, or at least 2 hydroxyl groups. In still yet other
embodiments, R3is
a substituted C1-C9 alkyl group In some embodiments, R31is a substituted C1-C9
substituted
alkyl group and is substituted with an ¨S03H group. In other embodiments, R3
is a C9-C10
substituted alkyl group. In some embodiments, R3 is a substituted C9-C10 alkyl
group wherein
at least two of the carbons on the carbon backbone form a heterocyclic group.
In some
embodiments, the heterocyclic group is an epoxide group.
[0066] In an embodiment, R4 is a substituted C1-C10 alkyl group. In some
embodiments, R4 is a substituted C8-C10 alkyl. In some embodiments, R4 is an
unsubstituted
C6-C9 alkyl. In other embodiments, R4 is a C8-Cin alkyl group substituted with
at least one
hydroxyl group. In some embodiments, R4 is a C10 alkyl group substituted with
at least two
hydroxyl groups. In other embodiments, R4 is a C8 alkyl group substituted with
at least one
¨S03H group. In some embodiments, R4 is a substituted C9 group, wherein at
least two of the
carbons on the carbon backbone form a heterocyclic group. In some embodiments,
the
heterocyclic group is an epoxide group. In some, embodiments, R4 is a C8-C9
substituted or
unsubstituted alkyl, and R4 is a C7-C8 substituted or unsubstituted alkyl.
[0067] According to an embodiment, in the hydraulic fracturing composition,
the
breaker is encapsulated in an encapsulating material to prevent the breaker
from contacting
the SAP. The encapsulating material is configured to release the breaker in
response to the
breaking condition. The breaker is a solid or liquid. As a solid, the breaker
is, e.g., a
crystalline or granular material. In an embodiment, the solid is encapsulated
or provided with
a coating to delay its release or contact with the SAP. Encapsulating
materials are the same
or different as the coating material noted above with regard to the proppant
particles.
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
Methods of disposing the encapsulating material on the breaker are the same or
different as
for disposing the coating on the proppant particles. In an embodiment, a
liquid breaker is
dissolved in an aqueous solution or another suitable solvent.
[0068] In an embodiment, the encapsulation material is a polymer that releases
the
breaker in a controllable way, e.g., at a controlled rate or concentration.
Such material is a
polymer that degrades over a period of time to release the breaker and is
chosen depending on
the release rate desired. Degradation of the polymer of the encapsulation
material polymer
occurs, e.g., by hydrolysis, solvolysis, melting, and the like. In an
embodiment, the polymer
of the encapsulation material is a homopolymer or copolymer of glycolate and
lactate, a
polycarbonate, a polyanhydride, a polyorthoester, a polyphosphazene, or a
combination
thereof
[0069] According to an embodiment, the encapsulated breaker is an encapsulated
hydrogen peroxide, encapsulated metal peroxides (e.g., sodium peroxide,
calcium peroxide,
zinc peroxide, and the like) or any of the peracids or other breaker herein.
[0070] In the hydraulic fracturing composition, the fluid is included to
contact and
expand the SAP into the expanded state. The fluid is an aqueous fluid that
includes water,
brine, an acid such as a mineral acid or an organic acid, or a base. The brine
is, for example,
seawater, produced water, completion brine, or a combination thereof. The
properties of the
brine can depend on the identity and components of the brine. Seawater, as an
example,
contains numerous constituents such as sulfate, bromine, and trace metals,
beyond typical
halide-containing salts. In some embodiments, produced water is water
extracted from a
production reservoir (e.g., hydrocarbon reservoir) or produced from the
ground. Produced
water also is referred to as reservoir brine and contains components such as
barium,
strontium, and heavy metals. In addition to the naturally occurring brines
(seawater and
produced water), completion brine is synthesized from fresh water by addition
of various
salts such as KCl, NaCl, ZnC12, MgCl2, or CaCl2 to increase the density of the
brine, such as
10.6 pounds per gallon of CaCl2 brine. Completion brines typically provide a
hydrostatic
pressure optimized to counter the reservoir pressures downhole. In an
embodiment, the
above brines are modified to include an additional salt. In an embodiment, the
additional salt
included in the brine is NaCl, KC1, NaBr, MgCl2, CaCl2, CaBr/, ZnBr2, NH4C1,
sodium
formate, cesium formate, and the like. The salt is present in the brine in an
amount from
about 0.5 weight percent (wt%) to about 50 wt%, specifically about 1 wt% to
about 40 wt?/o,
and more specifically about 1 wt% to about 25 wt%, based on the weight of the
fluid.
16
CA 02970488 2017-06-09
WO 2016/100048
PCT/US2015/064815
[0071] According to an embodiment, the fluid is a mineral acid that includes
hydrochloric acid, nitric acid, phosphoric acid, sulfuric acid, boric acid,
hydrofluoric acid,
hydrobromic acid, perchloric acid, or a combination thereof. In some
embodiments, the fluid
is an organic acid that includes a carboxylic acid, sulfonic acid, or a
combination thereof
Exemplary carboxylic acids include formic acid, acetic acid, chloroacetic
acid, dichloroacetic
acid, trichloroacetic acid, trifluoroacetic acid, propionic acid, butyric
acid, oxalic acid,
benzoic acid, phthalic acid (including ortho-, meta- and para-isomers), and
the like.
Exemplary sulfonic acids include alkyl sulfonic acid or aryl sulfonic acid.
Alkyl sulfonic
acids include, e.g., methane sulfonic acid. Aryl sulfonic acids include, e.g.,
benzene sulfonic
acid or toluene sulfonic acid. In one embodiment, the alkyl group may be
branched or
unbranched and contains from one to about 20 carbon atoms and is substituted
or
unsubstituted. In an embodiment, the aryl group is alkyl-substituted, i.e., is
an alkylaryl
group, or is attached to the sulfonic acid moiety via an alkylene group (i e ,
an arylalkyl
group). In an embodiment, the aryl group is substituted with a heteroatom. The
aryl group
has from 3 carbon atoms to 20 carbon atoms and includes, e.g., a polycyclic
ring structure.
[0072] In an embodiment, the hydraulic fracturing composition further includes
a
well treatment agent for flow assurance, performance enhancement, and fluid
stability.
Suitable well treatment agents include those that can adress undesired effects
caused by scale
formation, salt formation, paraffin deposition, asphaltene deposition, foaming
agent
deposition, emulsification, gas hydrate formation, corrosion, foaming agents,
oxygen
scavengers, H2S scavengers, biocides, surfactants, or a combination thereof
Specific well
treatment agents include a scale inhibitor, a tracer, a pH-buffering agent, or
a combination
thereof
[0073] The well treatment agents can be used in liquid or solid form, as-is or
in the
form of a salt or other complex. The well treatment agent can be coated,
encapsulated,
incorporated into a binder, adsorbed onto a matrix, or absorbed into a matrix.
Suitable
coatings include the thermoplastic, thermosetting, and crosslinked coatings
described above
for use with proppants. Suitable encapsulants include those described above
for use with
breakers. The same thermoplastic, thermosetting, and crosslinked materials
that can be used
as a coating or an encapsulant are also suitable for use as a binder, or a
matrix for adsorption
of the well treatment agents. Matrices for absorption of the well treatment
materials are
porous, preferably microporous, and can be organic (e.g., an open-celled
polymer foam such
as a polyurethane foam) or inorganic (e.g., zeolites, metal silicates, and
aluminophosphates).
17
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
[0074] Scale inhibitors can be used to control or prevent scale formation in
the well,
among other functions. Scale inhibitors can be a carboxylic, sulfonic, or
phosphonic acid-
containing compound, a carboxylic, sulfonic, or phosphonic group-containing
polymer, or a
combination thereof, for example amino trimethylene phosphonic acid, 1-hydroxy
ethylidene-1,1-diphosphonic acid, 2-phosphonobutane-1,2,4-tricarboxylic acid,
2-
hydroxyethyl-amino-bis(methylenephosphonic acid), ethylenediamine
tetrakis(methylene
phosphonic acid), tetramethylenediamine tetrakis(methylene phosphonic acid),
hexamethylenediamine tetrakis(methylene phosphonic acid), 2-hydroxy
phosphonoacetic
acid, diethylene triamine penta(methylene phosphonic acid),
bis(hexamethylenetriamine
penta(methylene phosphonic acid)), polyaminopolyether methylenephosphonate or
a salt
thereof, phosphino-polycarboxylate, polyacrylic acid, polymaleic acid, acrylic
acid
copolymers, sulfonated polyacrylate copolymers, polyvinyl sulfonate,
carboxymethyl inulin,
polyaspartate, or a combination thereof.
[0075] The scale inhibitor is present in the hydraulic fracturing composition
in an
amount effective to inhibit scale to the desired degree, which can be, for
example, about
0.001 wt% to about 10 wt%, or about 0.01 wt% to about 10 wt%, or about 0.01
wt% to about
wt?/o, preferably about 0.1 wt% to about 2 wt%, each based on total weight of
the
composition.
[0076] A tracer can be used to later detect or infer information about the
well,
borehole or the drilled formations. Tracers used during drilling can be mud
tracers and
filtrate tracers. Tracers can be oil- or water-soluble. Examples of tracers
include a
fluorinated benzoic acid, perfluorinated hydrocarbon, alcohol, ketone, organic
acid,
halogenated compound, or a combination thereof
[0077] Exemplary perfluorinated hydrocarbons are perfluorinated CI-CB
hydrocarbons, for example, tetrafluoromethane, tetrafluoroethane,
tetrafluoropropane, and the
like.
[0078] Examples of alcohols include C1-C24 monofunctional and polyfunctional
alcohols such as methanol, ethanol, glycol, propanol, propanediol, butanol,
pentanol,
pentaerythritol, hexanol, octanol, decanol, dodecanol, and the like. Preferred
alcohols are
C10-C24 monofunctional alcohols.
[0079] Exemplary ketones are C1-C18 ketones and diketones such as acetone,
cyclopropanone, methyl ethyl ketone, cyclohexanone, acetylacetone,
benzophenone, and the
like.
18
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
[0080] Exemplary organic acids include Ci-C18 mono-, di and tricarboxylic
acids.
Examples of organic acids are acetic acid, propanoic acid, butanoic acid,
oxalic acid, malonic
acid, succinic acid, glutaric acid, adipic acid, sebacic acid, citric acid,
and the like.
[0081] The halogenated compounds can be mono, di, tri and tetrachlorinated C1-
C12
hydrocarbons. Examples include methylene chloride, chloroform, carbon
tetrachloride,
trichloroethylene, tetrachloroethylene, hexachlorocyclohexane, benzyl
chloride, benzal
chloride, benzotrichloride, and the like.
[0082] The tracer is present in the hydraulic fracturing composition in an
amount
effective to trace the desired fluid or composition, which can be, for
example, about 0.001
wt% to about 10 wt%, or about 0.001 wt% to about 5 wt%, or about 0.01 wt% to
about 5
wt%, preferably about 0.01 wt% to about 1 wt%, each based on total weight of
the
composition.
[0083] A buffering agent can be a weak acid or base used to maintain the pH of
a
solution near a chosen value after the addition of another acid or base The
function of a
buffering agent is to prevent a rapid change in pH when acids or bases are
added to the
solution. For buffers in acid regions, the pH is adjusted to a desired value
by adding a strong
acid such as HC1 to the buffering agent. For alkaline buffers, a strong base
such as NaOH is
added. Alternatively, a buffer combination can be made from a combination,
e.g., a mixture,
of an acid and its conjugate base. For example, an acetate buffer can be made
from a mixture
of acetic acid and sodium acetate. Similarly, an alkaline buffer can be made
from a mixture
of the base and its conjugate acid. pH-Buffering agents differ from pH-
adjusting agents in
that a buffering agent maintains the pH of the fluid in a desired range, for
example a pH from
about 6 to about 9, preferably a pH from about 6.5 to about 8.5, most
preferably a pH of
about 7 to about 8 at a downhole temperature of a subterranean well. Examples
of buffering
agents include alkali and alkaline earth salts of carbonates, bicarbonate,
acetate, citrate,
gluconate, phosphate, borate, or tartrate, for example sodium carbonate and
potassium
carbonate, CaCO3, sodium sesquicarbonate, and potassium sesquicarbonate,
oxides of
alkaline earth metals such as MgO and CaO, an organic polyelectrolytes, or a
combination
thereof.
[0084] The buffering agent is present in the hydraulic fracturing composition
in an
amount effective to buffer the composition, which can be, for example, about
0.005 wt% to
about 10 wt%, or about 0.01 wt% to about 10 wt%, or about 0.01 wt% to about 5
wt%, or
about 0.1 wt% to about 2 wt%, each based on total weight of the composition.
19
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
[0085] The buffering agent can optionally be used in combination with a slow-
release
breaking agent, for example, a slow release acid. The acid can be glyoxal, a
solid acid, an
encapsulated acid, a coated acid, or a combination thereof. Glyoxal is a
dialdehyde that can
slowly release acids. Slow release of acids can overcome the buffering agent,
and result in
gradual reduction of the fluid pH until a selected pH value is attained that
is suitable for
breaking the SAP.
[0086] When used, the foregoing well treatment agents can be continuously
injected
through a downhole injection point in the completion, or periodic squeeze
treatments can be
undertaken to place the additive in the reservoir matrix for subsequent
commingling with
produced fluids.
[0087] Besides the SAP, the hydraulic fracturing composition includes a
viscous
polymer in in some embodiments The viscous polymer includes guar gums, high-
molecular
weight polysaccharides composed of mannose and galactose sugars, xanthan gum,
guar, or
starch or guar derivatives such as hydropropyl guar (HPG), carboxymethyl guar
(CMG), and
carboxymethylhydroxypropyl guar (CMHPG), galactomannan gums, glucomannan gums,
guars, derived guars, cellulose derivatives, or a combination thereof
Cellulose derivatives
such as hydroxyethylcellulose (HEC), carboxymethylcellulose (CMC),
hydroxypropylcellulose (HPC), and carboxymethylhydroxyethylcellulose (CMHEC);
hydropropyl starch; or lignosulfonate also is used.
[0088] According to an embodiment, the viscous polymer is includes a repeat
unit
that comprises an acrylate, an acrylamide, a vinylpyrrolidone, a vinyl ester
(e.g., a vinyl
acetate), a vinyl alcohol, a 2-acrylamide-2-methylpropanesulfonic acid, a
derivative thereof,
or a combination thereof In some embodiments, the viscous polymer is
polyacrylic acid.
[0089] In an exemplary embodiment, the viscous polymer comprises a linear
polymer
such as a polyacrylamide, a guar, a guar derivative, glycerol, a
polysaccharide such as
cellulose and starch, or a combination thereof Without wishing to be bound by
theory, it is
believed that the presence of a viscous polymer in the hydraulic fracturing
composition
increases the viscosity, thus the proppant-suspension ability of the
composition. The
presence of the viscous polymer also helps to reduce the friction pressure.
When the
hydraulic fracturing composition is a foam fluid, the viscous polymer further
stabilizes the
foam fluid by improving the foam quality and foam half-life.
[0090] The viscous polymer forms a viscous gel due to contact with the fluid
of the
hydraulic fracturing composition (or another fluid such as water, brine, or
other downhole
fluid). When the viscous polymer comprises glycerol, a linear polymer such as
linear
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
polyacrylamide, a guar, a guar derivative, a polysaccharide such as cellulose
and starch, or a
combination thereof, the formed viscous gel can be referred to as a linear
gel. In some
embodiments, a combination of fluids is used, a first fluid to expand the SAP
and a second
fluid to gel the viscose polymer. Without wishing to be bound by theory, it is
believed that
the viscous polymer has increased viscosity due to long polymer chains that
becomes
entangled. Entangled polymer chains of the viscous polymer creates networks,
giving
complex viscosity behavior. In an embodiment, the viscous polymer is a
copolymer that
contains two or more different monomers that are arranged randomly or in
blocks. Moreover,
the viscosity of the viscous polymer is increased by crosslinking the polymer
chains of the
viscose polymer. Crosslinkers for the viscous polymer include borate,
titanate, zirconate,
aluminate, chromate, or a combination thereof Boron crosslinked viscose
polymers include,
e.g., guar and substituted guars crosslinked with boric acid, sodium
tetraborate, or
encapsulated borates; borate crosslinkers may be used with buffering agents
and pH control
agents such as sodium hydroxide, magnesium oxide, sodium sesquicarbonate, and
sodium
carbonate, amines (such as hydroxyalkyl amines, anilines, pyridines,
pyrimidines, quinolines,
and pyrrolidines, and carboxylates such as acetates and oxalates) and with
delay agents such
as sorbitol, aldehydes, and sodium gluconate. Zirconium crosslinked viscose
polymers
include, e.g., those crosslinked by zirconium lactates (e.g., sodium zirconium
lactate),
triethanolamines, 2,2'-iminodiethanol, or a combination thereof. Titanates for
crosslinking
include, e.g., lactates and triethanolamines, and the like.
[0091] In an embodiment, the hydraulic fracturing composition includes an SAP,
for
example an SAP having crosslinked polymer particles such as a polyacrylic
acid,
polyacrylamide, a polysaccharide, or a combination thereof; a plurality of
proppant particles;
a fluid to expand the SAP, and a viscose polymer. Once the SAP is combined
with the fluid,
it expands while maintaining its shape. The viscous polymer is a linear
polymer that hydrates
in the fluid and has a viscosity determined by entanglement of the hydrated
linear polymer. It
is contemplated that the entangled linear polymers can be crosslinked in-situ
to form a
crosslinked gel. Thus, the hydraulic fracturing composition has beneficial
rheological
properties including tunable viscosity and breaking properties.
[0092] The hydraulic fracturing composition can also comprise a surfactant.
Useful
surfactants include fatty acids of up to 22 carbon atoms such as stearic acids
and esters and
polyesters thereof, poly(alkylene glycols) such as poly(ethylene oxide),
poly(propylene
oxide), and block and random poly(ethylene oxide-propylene oxide) copolymers
such as
those marketed under the trademark PLURONIC by BASF. Other surfactants include
21
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
polysiloxanes, such as homopolymers or copolymers of poly(dimethylsiloxane),
including
those having functionalized end groups, and the like. Other useful surfactants
include those
having a polymeric dispersant having poly(alkylene glycol) side chains, fatty
acids, or
fluorinated groups such as perfluorinated C14 sulfonic acids grafted to the
polymer backbone.
Polymer backbones include those based on a polyester, a poly(meth)acrylate, a
polystyrene, a
poly(styrene-(meth)acrylate), a polycarbonate, a polyamide, a polyimide, a
polyurethane, a
polyvinyl alcohol, or a copolymer comprising at least one of these polymeric
backbones.
Additionally, the surfactant is anionic, cationic, zwitterionic, or non-ionic.
[0093] Exemplary cationic surfactants include but are not limited to alkyl
primary,
secondary, and tertiary amines, alkanolamides, quaternary ammonium salts,
alkylated
imidazolium, and pyridinium salts. Additional examples of the cationic
surfactant include
primary to tertiary alkylamine salts such as, e.g., monostearylammonium
chloride,
distearylammonium chloride, tristearylammonium chloride; quaternary
alkylammonium salts
such as, e.g., monostearyltrimethylammonium chloride,
distearyldimethylammonium
chloride, stearyldimethylbenzylammonium chloride, monostearyl-
bis(polyethoxy)methylammonium chloride, alkylpyridinium salts such as, e.g., N-
cetylpyridinium chloride, N-stearylpyridinium chloride; N,N-
dialkylmorpholinium salts; fatty
acid amide salts such as, e.g., polyethylene polyamine; and the like.
[0094] Exemplary anionic surfactants include alkyl sulfates, alkyl sulfonates,
fatty
acids, sulfosuccinates, and phosphates. Examples of an anionic surfactant
include anionic
surfactants having a carboxyl group such as sodium salt of alkylcarboxylic
acid, potassium
salt of alkylcarboxylic acid, ammonium salt of alkylcarboxylic acid, sodium
salt of
alkylbenzenecarboxylic acid, potassium salt of alkylbenzenecarboxylic acid,
ammonium salt
of alkylbenzenecarboxylic acid, sodium salt of polyoxyalkylene alkyl ether
carboxylic acid,
potassium salt of polyoxyalkylene alkyl ether carboxylic acid, ammonium salt
of
polyoxyalkylene alkyl ether carboxylic acid, sodium salt of N-acylsarcosine
acid, potassium
salt of N-acylsarcosine acid, ammonium salt of N-acylsarcosine acid, sodium
salt of N-
acylglutamic acid, potassium salt of N-acylglutamic acid, ammonium salt of N-
acylglutamic
acid; anionic surfactants having a sulfonic acid group; anionic surfactants
having a
phosphonic acid; and the like.
[0095] In an embodiment, the nonionic surfactant is, e.g., an ethoxylated
fatty
alcohols, alkyl phenol polyethoxylates, fatty acid esters, glycerol esters,
glycol esters,
polyethers, alkyl polyglycosides, amineoxides, or a combination thereof
Exemplary
nonionic surfactants include fatty alcohols (e.g., cetyl alcohol, stearyl
alcohol, cetostearyl
22
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
alcohol, oleyl alcohol, and the like); polyoxyethylene glycol alkyl ethers
(e.g., octaethylene
glycol monododecyl ether, pentaethylene glycol monododecyl ether, and the
like);
polyoxypropylene glycol alkyl ethers (e.g., butapropylene glycol monononyl
ethers);
glucoside alkyl ethers (e.g., decyl glucoside, lauryl glucoside, octyl
glucoside);
polyoxyethylene glycol octylphenol ethers (e.g., Triton X-100 (octyl phenol
ethoxylate));
polyoxyethylene glycol alkylphenol ethers (e.g., nonoxyno1-9); glycerol alkyl
esters (e.g.,
glyceryl laurate); polyoxyethylene glycol sorbitan alkyl esters (e.g.,
polysorbates such as
sorbitan monolaurate, sorbitan monopalmitate, sorbitan monostearate, sorbitan
tristearate,
sorbitan monooleate, and the like); sorbitan alkyl esters (e.g.,
polyoxyethylene sorbitan
monolaurate, polyoxyethylene sorbitan monopalmitate, polyoxyethylene sorbitan
monostearate, polyoxyethylene sorbitan monooleate, and the like); cocamide
ethanolamines
(e.g., cocamide monoethanolamine, cocamide diethanolamine, and the like);
amine oxides
(e.g., dodecyldimethylamine oxide, tetradecyldimethyl amine oxide, hexadecyl
dimethylamine oxide, octadecylamine oxide, and the like); block copolymers of
polyethylene
glycol and polypropylene glycol (e.g., poloxamers available under the trade
name Pluronics,
available from BASF);polyethoxylated amines (e.g., polyethoxylated tallow
amine);
polyoxyethylene alkyl ethers such as polyoxyethylene stearyl ether;
polyoxyethylene
alkylene ethers such as polyoxyethylene oleyl ether; polyoxyalkylene
alkylphenyl ethers such
as polyoxyethylene nonylphenyl ether; polyoxyalkylene glycols such as
polyoxypropylene
polyoxyethylene glycol; polyoxyethylene monoalkylates such as polyoxyethylene
monostearate; bispolyoxyethylene alkylamines such as bispolyoxyethylene
stearylamine;
bispolyoxyethylene alkylamides such as bispolyoxyethylene stearylamide;
alkylamine oxides
such as N,N-dimethylalkylamine oxide; and the like.
[0096] Zwitterionic surfactants (which include a cationic and anionic
functional
group on the same molecule) include, e.g., betaines, such as alkyl ammonium
carboxylates
(e.g., RCH3)3N+-CH(R)C001 or sulfonates (sulfo-betaines) such as [RN4-
(CH3)2(CH2)3S034
where R is an alkyl group). Examples include n-dodecyl-N-benzyl-N-
methylglycine
[C12H25N+(CH2C6H5)(CH3)CH2C001, N-allyl N-benzyl N-methyltaurines
[C.1-1211--1N+(CH2C6H5)(CH3)CH2CH2S03].
[0097] In an embodiment, the surfactant is a viscoelastic surfactant. The
surfactant is
viscoelastic because, unlike numerous surfactants, which form Newtonian
solutions with a
viscosity slightly higher than water even at high concentrations, it is
capable of forming
viscoelastic fluids at a lower concentration. This specific rheological
behavior is mainly due
to the types of surfactant aggregates that are present in the fluids. In low
viscosity fluids, the
23
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
surfactant molecules aggregate in spherical micelles whereas, in viscoelastic
fluids, long
micelles, which can be described as worm-like, thread-like or rod-like
micelles, are present
and entangle.
[0098] The viscoelastic surfactant of the invention is usually ionic. It may
be cationic,
anionic or zwitterionic depending on the charge of its head group. When the
surfactant is
cationic, it is associated with a negative counterion, which can be an
inorganic anion such as
a sulfate, a nitrate, a perchlorate or a halide such as Cl, Br or with an
aromatic organic anion
such as salicylate, naphthalene sulfonate, p and m chlorobenzoates, 3,5 and
3,4 and 2,4-
dichlorobenzoates, t-butyl and ethyl phenate, 2,6 and 2,5-dichlorophenates,
2,4,5-
trichlorophenate, 2,3,5,6-tetrachlorophenate, p-methyl phenate, m-
chlorophenate, 3,5,6-
trichloropicolinate, 4-amino-3,5,6-trichlorpicolinate, 2,4-
dichlorophenoxyacetate. When the
surfactant is anionic, it is associated with a positive counterion, for
example, Na+ or K+.
When it is zwitternionic, it is associated with both negative and positive
counterions, for
example, Cl and Na+ or K+. Other viscoelastic surfactant has been described in
US Patent
Numbers 7,081,439 and 7,279,446.
[0099] The hydraulic fracturing composition can be a liquid or a foam. A
liquid
includes a surfactant based fluid, a linear gel fluid, or a crosslinked gel
fluid. A surfactant-
based fluid can refer to the hydraulic fracturing composition comprising a
viscoelastic
surfactant. A linear gel fluid can refer to the hydraulic fracturing
composition comprising a
linear gel. Similarly, a crosslinked gel fluid refers to the hydraulic
fracturing composition
comprising a crosslinked gel.
[0100] A foam fluid is a stable mixture of gas and liquid. It is generally
described by
its foam quality, i.e. the ratio of gas volume to the foam volume. The foam
half-life is
another important parameter to evaluate the stability of foam fluids. The half-
life of a foam
fluid is the time necessary for one-half of the liquid used to generate the
foam to break out of
the foam under atmospheric conditions. A foam system is mainly used in
fracturing low
pressure or water sensitive formations.
[0101] Water-soluble polymers, such as guar gums, high-molecular weight
polysaccharides composed of mannose and galactose sugars, or guar derivatives
such as
hydropropyl guar (HPG), carboxymethylhydropropyl guar (CMHPG), can be used to
prepare
the liquid phase of the foam fluids. Crosslinking agents based on boron,
titanium, zirconium
or aluminum complexes can be used to increase the effective molecular weight
of the
polymer and make them better suited for use in high-temperature wells.
24
CA 02970488 2017-06-09
WO 2016/100048
PCT/US2015/064815
[0102] Polymer-free, liquid phase of foam fluids can be obtained using
viscoelastic
surfactants. These fluids are noimally prepared by mixing in appropriate
amounts suitable
surfactants such as anionic, cationic, nonionic and zwitterionic surfactants
in aqueous
solutions. The viscosity of viscoelastic surfactant fluids is attributed to
the three dimensional
structure formed by the components in the fluids. When the concentration of
surfactants in a
viscoelastic fluid significantly exceeds a critical concentration, and in most
cases in the
presence of an electrolyte, surfactant molecules aggregate into species such
as micelles,
which can interact to foim a network exhibiting viscosity and elastic behavior
to further
stabilize the foamed systems. Meanwhile, the surfactant also acts as foaming
agent to create
the stable dispersion of gas in viscous liquid.
[0103] In an embodiment, various additional additives are included in the
hydraulic
fracturing composition. Exemplary additional additives include a lubricant, a
non-emulsifier,
a clay stabilizer, a biocide, an acid, a corrosion inhibitor, a pH-adjusting
agent, or a
combination thereof.
[0104] In an embodiment, the non-emulsifier of the additional additive is a
combination of the above surfactants or a combination of surfactant with a
short chain
alcohol or polyol such as lauryl sulfate with isopropanol or ethylene glycol.
The non-
emulsifier prevents formation of emulsions in the hydraulic fracturing
composition.
[0105] In an embodiment, the additional additive is the lubricant such as a
polyacrylamide, petroleum distillate, hydrotreated light petroleum distillate,
a short chain
alcohol (e.g., methanol), or polyol (e.g., ethylene glycol or glycerol). Such
lubricants
minimize friction and include, e.g., a polymer such as polyacrylamide,
polyisobutyl
methacrylate, polymethyl methacrylate, or polyisobutylene as well as water-
soluble
lubricants such as guar, guar derivatives, polyacrylamide, and polyethylene
oxide. In an
embodiment, the lubricant comprises a guar, a guar derivative, glycerol,
polyacrylamide, a
polysaccharide such as cellulose and starch, or a combination thereof
[0106] The clay stabilizer of the additional additive prevents the clay
downhole from
swelling under contact with the hydraulic fracturing composition or applied
fracturing
pressure. In an embodiment, the clay stabilizer includes a quaternary amine, a
brine (e.g.,
KC1 brine), choline chloride, tetramethyl ammonium chloride, and the like.
[0107] According to an embodiment, the additional additive is the pH-adjusting
agent, which adjusts pH of the hydraulic fracturing composition. The pH-
adjusting agent is
an organic or inorganic base, organic or inorganic acid, or a buffering agent,
which is any
appropriate combination of acid and conjugate base. Exemplary inorganic bases
include
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
those represented by MOH, where M is a metal from group 1 or 2 of the periodic
table, a
transition metal, or a metal or metalloid from group 13, 14, or 15; carbonate
salt; bicarbonate
salt; or a combination thereof. Exemplary inorganic acids include HC1, HBr,
fluoroboric
acid, sulfuric acid, nitric acid, acetic acid, formic acid, methanesulfonic
acid, propionic acid,
chloroacetic or dichloroacetic acid, citric acid, glycolic acid, lactic acid,
or a combination
thereof. In an embodiment, the pH-adjusting agent is selected to avoid
imparting favorable
characteristics to the hydraulic fracturing composition. In an embodiment, the
pH-adjusting
agent is selected to avoid damage to the surface equipment containing the
hydraulic
fracturing composition or to avoid damaging the wellbore or subterranean
formation.
[0108] In an embodiment, the additional additive to the hydraulic fracturing
composition is the biocide that prevents injection of a microbe (e.g.,
bacteria) downhole. The
biocide kills, eliminates, or reduces bacteria in the hydraulic fracturing
composition such as
water (e.g., when using river water as the fluid). In this way, introduction
of live bacteria into
the formation is prevented, thus reducing production of, e.g., sour gas.
[0109] According to an embodiment, the biocide does not interfere with the
other
components of the hydraulic fracturing composition and is not a health risk.
In an
embodiment, the biocide is an aldehyde such as glutaraldehyde. Examples of the
biocide
include non-oxidizing and oxidizing biocides. Exemplary oxidizing biocides
include
hypochlorite bleach (e.g., calcium hypochlorite and lithium hypochlorite),
peracetic acid,
potassium monopersulfate, potassium peroxymonosulfate,
bromochlorodimethylhydantoin,
dichloroethylmethylhydantoin, chloroisocyanurate, trichloroisocyanuric acids,
dichloroisocyanuric acids, chlorinated hydantoins, and the like. Additional
oxidizing
biocides include, e.g., bromine products such as stabilized sodium
hypobromite, activated
sodium bromide, or brominated hydantoins. Other oxidizing biocides include
chlorine
dioxide, ozone, inorganic persulfates such as ammonium persulfate, or
peroxides, such as
hydrogen peroxide and organic peroxides.
[0110] Exemplary non-oxidizing biocides include dibromonitfilopropionamide,
thiocyanomethylthiobenzothlazole, methyldithiocarbamate,
tetrahydrodimethylthladiazonethione, tributyltin oxide, bromonitropropanediol,
bromonitro styrene, methylene bisthiocyanate, chloromethylisothlazolone,
methylisothiazolone, benzisothlazolone, dodecylguanidine hydrochloride,
polyhexamethylene biguanide, tetrakis(hydroxymethyl) phosphonium sulfate,
glutaraldehyde,
alkyldimethylbenzyl ammonium chloride, didecyldimethylammonium chloride,
poly[oxyethylene-(dimethyliminio) ethylene (dimethyliminio) ethylene
dichloride],
26
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
decylthioethanamine, terbutylazine, and the like. Additional non-oxidizing
biocides are
quaternary ammonium salts, aldehydes and quaternary phosphonium salts. In an
embodiment, quaternary biocides have a fatty alkyl group and three methyl
groups, but in the
phosphonium salts, the methyl groups, e.g., are substituted by hydroxymethyl
groups without
substantially affecting the biocidal activity. In an embodiment, they also are
substituted with
an aryl group. Examples include formaldehyde, glyoxal, furfural, acrolein,
methacrolein,
propionaldehyde, acetaldehyde, crotonaldehyde, pyridinium biocides,
benzalkonium chloride,
ceramide, cetyl trimethyl ammonium chloride, benzethonium chloride,
cetylpyridinium
chloride, chlorphenoctium amsonate, dequalinium acetate, dequalinium chloride,
domiphen
bromide, laurolinium acetate, methylbenzethonium chloride, myristyl-gamma-
picolinium
chloride, ortaphonium chloride, triclobisonium chloride, alkyl dimethyl benzyl
ammonium
chloride, cocodiamine, dazomet, 1-(3-chloroally1)-chloride.3,5,7-triaza-1 -
azoniaadamantane,
or a combination thereof.
[0111] In an embodiment, the biocide is encapsulated or coated as discussed
above
with regard to the proppant particles or breaker. In an embodiment, the
biocide is
encapsulated or coated by any suitable encapsulation method using any suitable
encapsulation material. The encapsulation material is any material that does
not adversely
interact or chemically react with the biocide to destroy its utility. In an
embodiment, the
biocide is released from the coating at a selected time.
[0112] In the hydraulic fracturing composition, the proppant particles are
present in
an amount effective to prop open the fracture without the geometry of the
fracture being
altered during settling of the formation when the proppant is released from
the SAP. In a
particular embodiment, the proppant particles are present in a mass
concentration from 0.1
pounds per gallon (lb/gal) to 20 lb/gal, specifically 0.25 lb/gal to 16
lb/gal, and more
specifically 0.25 lb/gal to 12 lb/gal, based on the total volume of the
composition. In an
embodiment, the SAP is present in a mass concentration from 1 pound of SAP per
one
thousand gallons of fluid (ppt) to 200 ppt, specifically 5 ppt to 100 ppt, and
more specifically
15 ppt to 50 ppt, based on the total volume of the composition. In the
hydraulic fracturing
composition, any ratio of the amount of the proppant particles to the amount
of the SAP is
applicable as long as the proppant particles are suspended in the gel formed
by the SAP.
[0113] In an embodiment, the breaker is present in the hydraulic fracturing
composition in a mass concentration from 0 ppt to 20 ppt, specifically 0 ppt
to 15 ppt, and
more specifically, 0 ppt to 10 ppt, based on the total volume of the
composition. In some
embodiments, the biocide is present in an amount from 10 parts per million
(ppm) to 2000
27
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
ppm, specifically 50 ppm to 1500 ppm, and more specifically 50 ppm to 1000
ppm. An
amount of the viscose polymer, if present, is from 0.25 gallons of viscose
polymer per 1000
gallons of fluid (gpt) to 10 gpt, specifically 0.5 gpt to 8 gpt, and more
specifically 0.5 gpt to 4
gpt, based on the total hydraulic fracturing composition volume.
[0114] The hydraulic fracturing composition can be made in a variety of ways.
According to an embodiment, a process for making the hydraulic fracturing
composition
includes contacting a superabsorbent polymer with a fluid to expand the
superabsorbent
polymer into an expanded state and disposing a plurality of proppant particles
in the
superabsorbent polymer to make the hydraulic fracturing composition. As shown
in FIG. 3,
the SAP (e.g., particle 12 or fiber 50) is in an unexpanded state 20 with
internal crosslinks 14
and has a diameter of D2 prior to contact with the fluid (not shown). As
indicated in FIGs. 1
and 2, once contacted with the fluid, the SAP (12 or 50) expands to diameter
Dl (where Dl is
greater than D2) as the fluid is absorbed into the SAP (12 or 50).
Additionally, in the case of
an SAP fiber 50 or an SAP that has a major axis, the length of the SAP 50 can
lengthen upon
expansion caused by absorption of the fluid. It should be noted that the
crosslinks 14 limit
the volumetric expansion and the ultimate size of the SAP 12. In the expanded
state (FIG. 1
or FIG. 2), the proppant particles 18 are disposed in the SAP (12 or 50). The
SAPs (12 or 50)
shown in FIG. 1, FIG. 2, and FIG. 3 represent a single particle, fiber, etc.
of the SAP 12 or a
plurality of such items as well as agglomerates of polymer chains that make
the SAP (12 or
50).
[0115] The additive including the scale inhibitor, tracer, buffering agent, or
a
combination thereof, can be added to the fluid before or after disposing the
SAP (12 or 50)
and the proppant particles 18. Optionally, the additive is added to the SAP
and proppant
particles. According to an embodiment, the breaker is added to the fluid
before or after
disposing the SAP (12 or 50) and the proppant particles 18. Optionally, the
breaker is added
to the SAP and proppant particles.
[0116] In an embodiment, combining the components of the hydraulic fracturing
composition is accomplished in a vessel such as a mixer, blender, and the
like. In some
embodiments, the hydraulic fracturing composition is injected without mixing,
e.g. it is
injected "on the fly". The components are mixed, agitated, stirred, and the
like. In an
embodiment, the components are combined as the hydraulic fracturing
composition is being
disposed downhole.
[0117] The hydraulic fracturing composition herein has advantageous properties
that
include suspending the proppant particles in the SAP for an extended period of
time or at an
28
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
elevated temperature or pressure. The length of time, temperature, or pressure
under which
the proppant particles remain suspended in the SAP is deteimined by the
polymer chains that
make up the SAP as well as the crosslinker compound, degree of crosslinking,
amount of
proppant particles present, concentration of the SAP, and identity of the
fluid.
[0118] Accordingly, the hydraulic fracturing composition includes a highly
crosslinked SAP, lightly crosslinked SAP, or a combination thereof. In the
hydraulic
fracturing composition, the SAP is configured to be broken and to release the
proppant
particles in response to the breaking condition. The breaking condition
includes a
temperature, pH, contact between the breaker and the SAP, a time lapse between
the SAP
being in the expanded state and breaking the superabsorbent polymer. In an
embodiment, the
time the proppant particles are disposed in the SAP prior to release from the
SAP is greater
than or equal to 48 hours at a temperature greater than or equal to 150 F,
specifically greater
than or equal to 36 hours, more specifically greater than or equal to 24
hours, even more
specifically greater than or equal to 18 hours, and yet more specifically
greater than or equal
to 20 minutes, preferably from 10 minutes to 18 hours.
[0119] In an embodiment, the pH for breaking the SAP is a pH effective to
break
bonds in the SAP, crosslinker, between the SAP and crosslinker, or a
combination thereof
Likewise, in an embodiment, the pH causes dissociation between particles of
SAP so that the
proppant particles are released therefrom. In an embodiment, the pH is acidic
or basic so that
ionic groups of the polymer chains in the SAP are neutralized, which affects
the amount of
fluid present in the SAP and causes contraction of the SAP and expulsion of
the proppant
particles. According to an embodiment, the pH is from 1 to 12, specifically 3
to 12, and more
specifically 5 to 11.5.
[0120] In an embodiment, the SAP breaks due to the breaking condition even in
the
absence of the breaker. Thus, in an embodiment, the SAP is broken at a
temperature, pH,
time lapse, and the like without contact with the breaker.
[0121] In an embodiment, the viscosity of the SAP in the expanded state is
from 1
centipoise (cP) to 1000 cP, and specifically 1 cP to 300 cP, as measured by
Ofite M900
rheometer for less than 100 cP viscosity or Grace M5500 rheometer for more
than 100 cP
viscosity at a temperature of 180 F.
[0122] The hydraulic fracturing composition is useful e.g., to transport and
dispose
proppant particles in a fracture without the SAP being broken until after
disposal of the
proppant particles to prevent proppant particles from settling and therefore
increase overall
fractured surface area. According to an embodiment, the hydraulic fracturing
composition is
29
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
used to form the fracture. In an embodiment, a process for disposing a
plurality of proppant
particles in a fracture includes disposing a hydraulic fracturing composition
in a downhole
environment. The hydraulic fracturing composition includes an SAP in an
expanded state
and configured to break in response to a breaking condition, such that a
decomposed polymer
is formed from the superabsorbent polymer. The hydraulic fracturing
composition also
includes a plurality of proppant particles disposed in the SAP prior to
release of the plurality
of proppant particles from the SAP in response to breaking the SAP, an
additive comprising a
scale inhibitor, tracer, buffer, or combination thereof, and a fluid to expand
the SAP into the
expanded state. In this method, forming a fracture in the downhole environment
is
accomplished by applying hydraulic force on the downhole environment from the
hydraulic
fracturing composition, disposing the hydraulic fracturing composition in the
fracture,
breaking the superabsorbent polymer after forming the fracture, and releasing
the plurality of
proppant particles from superabsorbent polymer to dispose the plurality of
proppant particles
in the fracture. In this manner, the proppant particles do not settle to the
bottom of the
fracture. The downhole environment is, e.g., a reservoir temperature,
formation water,
formation rock, sand, and the like, which contains, e.g., pores or veins of
various sizes in
such rock, sand, and the like.
[0123] As shown in FIG. 4, after the breaking condition occurs, the SAP is in
a
broken state 30 such that the SAP forms, e.g., a decomposed polymer 32 with
the proppant
particles 18 released from the SAP. Although the decomposed polymer 32 is
shown as being
separated fragments (e.g., polymers, oligomers, monomers, molecules, atoms,
and the like,
which are charged or charge neutral), in an embodiment, the decomposed polymer
is formed
from the SAP by breaking all or some of the crosslinks so that the polymer
chains of the SAP
remain intact. It is contemplated that conformational changes in the SAP
release the
proppant particles from the SAP and ensure good conductivity.
[0124] In an embodiment, the crosslinks or the SAP are degraded by certain
conditions such as heat or pH. Degradation reduces the degree of crosslinking
in the SAP by
breaking a bond in the crosslinker or a bond between the crosslinker and
polymer chains of
the SAP. Generally, decreasing the degree of crosslinking of the SAP increases
the amount
of fluid that is absorbed by the SAP or increases the volumetric increase of
the SAP due to
fluid absorption. In an embodiment, the aforementioned conditions cleave bonds
in the
crosslinks without substantially degrading the polymer backbone of the SAP. In
some
embodiments, these conditions also degrade the polymer backbone of the SAP.
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
[0125] In addition to disposing the hydraulic fracturing composition in the
downhole
environment for hydraulically fracturing the formation, the method also
includes disposal of
other elements such as water, a downhole fluid (e.g., brine or other above-
mentioned fluids),
a viscose polymer, or a combination thereof. Thus, in an embodiment, the
method further
includes disposing water, a viscose polymer, or a combination thereof in the
downhole
environment and forming the fracture with the hydraulic fracturing
composition, water, the
viscose polymer, or a combination thereof The order of addition can be varied
and the time
of injecting each is the same or different. According to an embodiment, for
hydraulically
fracturing a formation, a proppant-free fluid and a proppant-containing fluid
are injected in an
alternating order into a subterranean formation. The proppant-free fluid can
be injected first
followed by the proppant-containing fluid. Alternatively, the proppant-
containing fluid is
injected first followed by the proppant-free fluid.
[0126] In an exemplary embodiment, the proppant-free fluid comprises an
aqueous
carrier comprising water, brine, an acid, or a base and a lubricant. The
lubricant can
comprise a polyacrylamide, a guar, a guar derivative, glycerol, a
polysaccharide such as
cellulose and starch, or a combination thereof. When the lubricant comprises a
polyacrylamide, e.g. MaxPerm 20A, MaxPerm 20A is present in an amount of 0.25
to 15
gallons per one thousand gallons of the proppant-free fluid. When the
lubricant comprises a
guar, the lubricant is present in an amount of 1 to 50 pounds per one thousand
gallons of the
proppant-free fluid. The proppant-containing fluid comprises SPP, a plurality
of proppant
particles disposed in SPP, a fluid to expand the SPP into the expanded state,
and optionally a
linear gel or a viscous polymer comprising a guar, a guar derivative, a
polyacrylamide,
glycerol, a polysaccharide, or a combination thereof The proppant-containing
fluid can be
the hydraulic fracturing composition disclosed herein. By using the method,
high
conductivity channels are created within the proppant pack. The effects are
illustrated in
FIG. 15.
[0127] In another exemplified embodiment, the proppant-free fluid comprises
SPP, a
fluid to expand the SPP, and optionally a viscous polymer comprising a guar, a
guar
derivative, a polyacrylamide, glycerol, a polysaccharide, or a combination
thereof For
proppant free fluid, 20-60 pounds SPPs are nounally prepared in one thousand
gallons of
proppant-free fluids. Proppant containing fluids comprises an aqueous carrier
comprising
water, brine, an acid, or a base, proppants, and a lubricant. The lubricant
can comprise a
polyacrylamide, a guar, a guar derivative, glycerol, a polysaccharide, or a
combination
thereof. When the lubricant comprises a polyacrylamide, e.g. MaxPerm 20A,
MaxPerm 20A
31
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
is present in an amount of 0.25 to 15 gallons per one thousand gallons of the
proppant-free
fluid. When the lubricant comprises a guar, the polymer is present in an
amount of 1 to 50
pounds per one thousand gallons of the proppant-containing fluid.
Heterogeneous proppant
distribution can be achieved by this method. The beneficial effects are
illustrated in FIG. 16.
[0128] In an embodiment, the initial injection of water (or brine) and the
viscous
polymer is, e.g., 15 minutes each although the length of injection times is
different in some
embodiments. The injection time for the hydraulic fracturing composition is
the same or
different as the water or viscose polymer, e.g., having a duration of two
hours. It is
contemplated that the injection time varies and is selected based on
conditions of the
formation and the properties of the hydraulic fracturing composition, other
fluids (e.g., brine),
viscose polymer, and the like.
[0129] A benefit of the hydraulic fracturing composition is that the proppant
particles
remain disposed in the SAP until the breaking condition causes the SAP to
break. As shown
in FIG. 5, a formation 100 is traversed by a tubular 104 disposed in casing
102 although only
the casing 102 or only the tubular 104 is present in some embodiments. The
hydraulic
fracturing composition 120 is transferred from an interior of the tubular 104
to contact the
formation 100 through an aperture (not shown) in the tubular 104. The
hydraulic fracturing
composition 120 (which is similar to or identical to that of FIG. 1 or FIG. 2)
fractures the
formation 100 to create a fracture 106. The proppant particles 18 are disposed
in the SAP 12
until the breaking condition occurs at which point the SAP 12 breaks to form a
decomposed
polymer 122 and releases the proppant particles 18 as shown in FIG. 6. Here,
the SAP 12 is
not broken nor are the proppant particles 18 released from the SAP 12 before
closing of the
fracture 106. Therefore, the proppant particles 18 do not settle to the bottom
of the fracture
106 before the fracture 106 closes so that the geometry of the fracture 106 is
not affected
negatively by breaking the SAP 12. That is, before the fracture 106 closes, it
has a height Hl.
After closing, the fracture has a height H2. After closing of the fracture
commences, the SAP
12 is broken, and the decomposed polymer 122 is formed. Due to the high degree
of
suspension of the proppant particles 18 in the SAP 12, the height H2 of the
fracture 106 does
not vary significantly from the original, pre-closing height H1, such that the
height H2 (post-
closing) is nearly the same size as the original height H1 (pre-closing).
[0130] During the breaking of SAP 12, the formation pressure squeezes the
proppant
particles in-situ from settling to the bottom of the fracture by the broken
fluids leaking off In
this manner, the hydraulic fracturing composition accomplishes enhanced
proppant particles
transport and vertical distribution in the fracture. Consequently, the
conduction of
32
CA 02970488 2017-06-09
WO 2016/100048 PCT/US2015/064815
hydrocarbons or other fluids from the formation 100, through the fracture 106,
into the
tubular 104 (or a space between the tubular 104 and casing 102) is increased
relative to
incomplete or imperfect disposal of the proppant particles 18, which is shown
in FIGs. 7 and
8. Therefore, the hydraulic fracturing composition 120 transports and disposes
the proppant
particles 18 to ensure that the proppant particles 18 prop open the fracture
106 in the same or
substantially the same geometry as the fracture 106 is initially formed and
thus provides more
fractured surface area than if the proppant particles settle to the bottom of
the fracture as
shown in the FIGs. 7 and 8. In this manner, a high conduction pathway for
transmission of
hydrocarbons and other fluids between the formation and the borehole occurs
when using the
hydraulic fracturing composition herein.
[0131] With regard to FIG. 7 and FIG. 8, when using certain fracturing systems
that
do not contain the hydraulic fracturing composition herein, such as proppant
particles 130
suspended in a fluid 132 without the benefit of the SAP to suspend the
proppant particles
130, the proppant particles 130 settle from the fluid 132 and collect on the
bottom of the
fracture 106 before the fracture 106 closes (FIG. 7). Even though the fracture
106 has an
original height H2 before closing (FIG. 7), the height H2 is reduced to a
diminished height
H4 after closing because the proppant particles 130 settle to the bottom of
the fracture 106
before the fracture 106 closed.
[0132] The hydraulic fracturing composition and processes herein are
illustrated
further by the following non-limiting example.
[0133] Superabsorbent polymer (SAP) (QX-A1051; Nippon Shokubai) was mixed
with tap water at a concentration of 40 parts per thousand (ppt) (w/v). The pH
of the mixture
was determined to be about 7. The pH was adjusted to greater than 7 using the
buffer BF-
10L (from Baker Hughes) and a pH of less than 7 using the buffer BF-9L from
Baker
Hughes. The viscosity of the mixtures at different pH's was recorded using a
Chandler
M5550 instrument according to the API RP 39 standard at 20 C and under
atmospheric
pressure. FIG. 17 is a plot showing the effect of pH on viscosity for this
example. As shown
in FIG. 17, a maximum viscosity was recorded at a fluid pH about 7 to about 8
[0134] All ranges disclosed herein are inclusive of the endpoints, and the
endpoints
are independently combinable with each other. The ranges are continuous and
thus contain
every value and subset thereof in the range. Unless otherwise stated or
contextually
inapplicable, all percentages, when expressing a quantity, are weight
percentages. As used
herein, "combination" is inclusive of blends, mixtures, alloys, reaction
products, and the like.
Further As used herein, "a combination thereof' refers to a combination
comprising at least
33
one of the named constituents, components, compounds, or elements, optionally
together
with one or more like constituents, components, compounds, or elements not
named. The use
of the terms "a" and "an" and "the" and similar referents in the context of
describing the
invention (especially in the context of the following claims) are to be
construed to cover both
the singular and the plural, unless otherwise indicated herein or clearly
contradicted by
context. "Or" means "and/or." The conjunction "or" is used to link objects of
a list or
alternatives and is not disjunctive; rather the elements can be used
separately or can be
combined together under appropriate circumstances.
[0001] It should further be noted that the terms "first," "second," "primary,"
"secondary," and the like herein do not denote any order, quantity, or
importance, but rather
are used to distinguish one element from another. The modifier "about" used in
connection
with a quantity is inclusive of the stated value and has the meaning dictated
by the context
(e.g., it includes the degree of error associated with measurement of the
particular quantity).
[0002] While one or more embodiments have been shown and described,
modifications and substitutions may be made thereto without departing from the
spirit and
scope of the invention. Accordingly, it is to be understood that the present
invention has been
described by way of illustrations and not limitation. Embodiments herein can
be used
independently or can be combined.
34
CA 2970488 2018-11-30