Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
METHOD OF OBTAINING OR MAINTAINING OPTICAL TRANSMITTANCE INTO
DEAERATED LIQUID
[0001] This application is a PCT application claiming priority to U.S.
Patent
Application Serial Nos. 14/592,219 and 14/596,691, the disclosures of each of
which are
incorporated herein by reference in their entirety.
BACKGROUND
[0002] Measurement of parameters in liquids using optical sensors is
commonplace.
Reliable measurement of such parameters generally requires light to pass into
the liquid,
which generally requires light to first pass through a reasonably transparent
medium, e.g.,
a light transference medium. Reliability issues can arise in the event of
obstruction of
optical transference through the medium, which may be caused by particulate
matter.
[0003] Generally, boiler liquids are deaerated liquids that have unique
features. Some
unique features of boiler liquids include having very low levels of dissolved
oxygen (e.g.,
less than about 10 ppb dissolved oxygen in conventional boiler feedwater) and
having a
pH of from about 9 to about 11. Particularly in boiler systems utilizing a
form of
treatment control based on light detection and/or measurement (e.g.,
fluorometry), some
amount of corrosion will occur over time and deposit in the form of
particulate matter onto
a light transference medium, thereby causing some amount of optical
obstruction of the
light transference medium. Regarding detection and measurement methods that
utilize
light transference, the unique conditions of deaerated liquids, particularly
boiler liquid,
present a challenge to the user when a light transference medium becomes
optically
obstructed. Ideally, optical obstruction can be altogether prevented, and if
optical
obstruction occurs, it can be removed without disrupting detection,
measurement, and/or
treatment control via the light transference.
SUMMARY
[0004] A method of obtaining, or of maintaining, optical transference
into deaerated
liquid in contact with a light transference medium is provided. The method
comprises
applying ultrasonic energy at a wavelength (2\,) into deaerated liquid in
contact with a light
transference medium. The ultrasonic energy at wavelength (2\,) originates at a
distance (d)
from an optical signal transmitted into the light transference medium such
that optical
1
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
transference into the deaerated liquid via the light transference medium is
obtained or
maintained.
[0005] A clean-in-place method of maintaining optical transference
through a light
transference medium operably connected to a boiler system is provided. The
clean-in-
place method comprises contacting a stream of boiler water with a wetted
surface of a
light transference medium in optical communication with an optical sensor.
Data related
to a parameter of the boiler liquid measured by the optical sensor is input to
a control
scheme of a boiler system. The optical sensor is electronically isolated from
the control
scheme, which maintains control of the boiler system based on the input data
related to the
parameter of the boiler liquid. A liquid chemical agent contacts the wetted
surface of the
light transference medium for a period of time and at a concentration
sufficient to clean
the wetted surface of the light transference medium. The liquid chemical agent
is selected
from an acid, a chelant, a reducing agent, and combinations thereof. The
liquid chemical
agent is removed from the wetted surface of the light transference medium, and
the optical
sensor is electronically de-isolated from the control scheme.
[0006] Additionally, a clean-in-place method of maintaining optical
transference
through a light transference medium operably connected to a boiler system is
provided.
The clean-in-place method comprises flowing a stream of boiler liquid to
contact a wetted
surface of a light transference medium in optical communication with an
optical sensor.
Data related to a parameter of the boiler liquid measured by the optical
sensor is input to a
control scheme of a boiler system. The flow of the stream of boiler liquid to
contact the
wetted surface of the light transference medium is discontinued. The optical
sensor is
electronically isolated from the control scheme, which maintains control of
the boiler
system based on the input data related to the parameter of the boiler liquid.
A liquid
chemical agent contacts the wetted surface of the light transference medium
for a period of
time and at a concentration sufficient to clean the wetted surface of the
light transference
medium. The liquid chemical agent is selected from an acid, a chelant, a
reducing agent,
and combinations thereof. The liquid chemical agent is removed from the wetted
surface
of the light transference medium, the flow of the stream of boiler liquid to
contact the
wetted surface of the light transference medium is resumed, and the optical
sensor is
electronically de-isolated from the control scheme.
2
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWING(S)
[0007] FIG. 1 illustrates an embodiment of a system capable of
performing at least one
inventive method described herein;
[0008] FIG. 2 illustrates a variation of the illustrative embodiment
shown in FIG. 1;
[0009] FIG. 3 illustrates an embodiment of a system capable of performing
at least one
inventive method described herein;
[0010] FIG. 4 illustrates an embodiment of a system capable of
performing at least one
inventive method described herein;
[0011] FIG. 5A illustrates an embodiment of a system incorporating a
sleeve as
described herein;
[0012] FIG. 5B illustrates an embodiment of a system incorporating a
lossy surface;
[0013] FIG. 6 is a plot of experimental data collected during the
execution of Example
1;
[0014] FIG. 7 is a plot of experimental data collected during the
execution of Example
2;
[0015] FIG. 8 is a plot of experimental data collected during the
execution of Example
3;
[0016] FIG. 9 illustrates an embodiment of a system that may be used to
carry out the
methods disclosed herein;
[0017] FIG. 10 is a plot of results of Example 4 related to treatment using
urea
hydrochloride;
[0018] FIG. 11 is a plot of results of Example 5 related to treatment
using oxalic acid;
and
[0019] FIG. 12 is a plot of results of Example 6 related to treatment
using sodium
hydrosulfite.
DETAILED DESCRIPTION
[0020] While embodiments encompassing the general inventive concepts may
take
various forms, there is shown in the drawings and will hereinafter be
described various
illustrative and preferred embodiments with the understanding that the present
disclosure
is to be considered an exemplification and is not intended to be limited to
the specific
embodiments.
3
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
[0021] A method of maintaining optical transference into deaerated
(e.g., degassed)
liquid in contact with a light transference medium. The method includes
applying
ultrasonic energy into the deaerated liquid so as to contact the light
transference medium
at a particular locus of points suitable for obtaining, or for maintaining,
reliability in a
system comprising an optical sensor. In certain embodiments, liquid is
deaerated prior to
being utilized in a heating application (e.g., a boiler). Liquid is generally
deaerated in an
attempt to minimize corrosion of metals that come in contact with the steam
and/or liquid.
Illustrative deaerated liquids include, but are not limited to, deaerated
boiler make-up
feedwater and boiler liquid, which further includes boiler blowdown liquid and
boiler
condensate liquid. The phrase "deaeratedboiler make-up feedwater" is used to
describe
the boiler make-up feedwater that has undergone a deaeration process. The term
is not
used to describe the various boiler liquids, as it is understood by those
skilled in the art
that boiler liquids have already undergone a deaeration process prior to
becoming boiler
liquid.
[0022] As it pertains to this disclosure, unless otherwise indicated,
"controller" refers
to an electronic device having components such as a processor, memory device,
digital
storage medium, cathode ray tube, liquid crystal display, plasma display,
touch screen, or
other monitor, and/or other components. Controllers include, for example, an
interactive
interface that guides a user, provides prompts to the user, or provides
information to the
user regarding any portion of the method of the invention. Such information
may include,
for example, building of calibration models, data collection of one or more
parameters,
measurement location(s), management of resulting data sets, etc.
[0023] The controller is preferably operable for integration and/or
communication
with one or more application-specific integrated circuits, programs, computer-
executable
instructions or algorithms, one or more hard-wired devices, wireless devices,
and/or one or
more mechanical devices such as liquid handlers, hydraulic arms, servos, or
other devices.
Moreover, the controller is operable to integrate feedback, feed-forward, or
predictive
loop(s) resulting from, inter alia, the parameters measured by practicing the
method(s) of
the present disclosure. Some or all of the controller system functions may be
at a central
location, such as a network server, for communication over a local area
network, wide area
network, wireless network, extranet, the Internet, microwave link, infrared
link, and the
like, and any combinations of such links or other suitable links. In addition,
other
4
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
components such as a signal conditioner or system monitor may be included to
facilitate
signal transmission and signal-processing algorithms.
[0024] By way of example, the controller is operable to implement the
method of the
invention in a semi-automated or fully-automated fashion. In another
embodiment, the
controller is operable to implement the method in a manual or semi-manual
fashion.
[0025] Data transmission of any of the measured parameters or signals to
a user,
chemical pumps, alarms, or other system components is accomplished using any
suitable
device, such as a wired or wireless network, cable, digital subscriber line,
internet, etc.
Any suitable interface standard(s), such as an ethernet interface, wireless
interface (e.g.,
IEEE 802.11a/b/g/n, 802.16, Bluetooth, optical, infrared, other
radiofrequency, any other
suitable wireless data transmission method, and any combinations of the
foregoing),
universal serial bus, telephone network, the like, and combinations of such
interfaces/connections may be used. As used herein, the term "network"
encompasses all
of these data transmission methods. Any of the components, devices, sensors,
etc., herein
described may be connected to one another and/or the controller using the
above-described
or other suitable interface or connection. In an embodiment, information
(collectively
referring to all of the inputs or outputs generated by the method of the
invention) is
received from the system and archived. In another embodiment, such information
is
processed according to a timetable or schedule. In a further embodiment, such
information is processed in real-time. Such real-time reception may also
include, for
example, "streaming data" over a computer network.
[0026] As it pertains to this disclosure, unless otherwise indicated,
"control scheme"
refers to providing output based on input from a controller as defined herein.
[0027] A method of obtaining, or of maintaining, optical transference
into deaerated
liquid in contact with a light transference medium. The method comprises
applying
ultrasonic energy at a wavelength (2\,) into deaerated liquid in contact with
a light
transference medium. In certain embodiments, the ultrasonic energy at
wavelength (2\,)
originates at a distance (d) from an optical signal transmitted into the light
transference
medium so as to obtain or maintain optical transference into the deaerated
liquid via the
light transference medium. Preferably, the distance (d) is defined by Formula
1 below:
d = (a + 0.5*n) *)\,
Formula 1
5
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
wherein 2\, is the wavelength of the ultrasonic energy, a is a constant
ranging from
about -0.2 to about 0.2, and n is an integer ranging from 1 to 30. In certain
embodiments,
a is a constant ranging from about -0.15 to about 0.15, or from about -0.1 to
about 0.1.
[0028] Ultrasonic energy follows the laws of acoustics. If the speed (v)
of the
ultrasonic energy is known, or approximately known, wavelength (2\,) can be
defined by
frequency (f) according to Formula 2 below:
v = f *)\,
Formula 2
The speed (v) of the ultrasonic energy will be known or approximately known
based on
the medium of travel of the ultrasonic energy. For example, ultrasonic energy
travels
through deaerated water at a speed (v) of approximately 4800 ft/s at 68 F
(approximately
1480 m/s at 20 C). Assuming a constant medium of travel, and therefore a
constant speed
(v), the frequency (f) and wavelength (2\,) of the ultrasonic energy are
proportionally
related to one another.
[0029] The terms "optical" and "light" are used interchangeably herein.
Utilization of
the phrase "into deaerated liquid" is intended to cover light transmission in
any direction
between the deaerated liquid, the light transference medium, a light source,
and/or a light
detector. For example, the optical signal may originate from within the
deaerated liquid
and be transferred to a sensor via the light transference medium (e.g.,
fluorometric
emission), or from a light source through the light transference medium and
into the
deaerated liquid (e.g., fluorometric excitation). Illustrative embodiments of
optical
sensors that perform optical measurements using optical signals include, but
are not
limited to, devices capable of detecting or sensing absorbance, colorimetric,
refractometric, spectrophotometric, luminometric, and/or fluorometric signals,
or images.
In a preferred embodiment, the optical signal comprises a fluorometric
excitation and/or
emission.
[0030] The method is directed to obtaining or maintaining optical
transference into
deaerated liquid in contact with a light transference medium. The method can
be utilized
to remove obstructions that may be present on the light transference medium.
Removal of
obstruction from the light transference medium sufficient to allow for optical
transference,
thereby allowing for performance of an optical measurement of the deaerated
liquid, is
also achieved by the method of the present invention.
[0031] An advantage of the present invention is that the preferred
method can be
performed without interrupting the process responsible for supplying the
deaerated liquid.
6
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
For example, a boiler and its related treatment processes can continue to
operate during
performance of the preferred method described herein.
[0032] Ultrasonic energy is applied into deaerated liquid in contact
with a light
transference medium. The ultrasonic energy is applied to effectuate removal of
optical
obstruction that may be present on the light transference medium, particularly
at a location
of the light transference medium where an optical signal passes through, or
should pass
through.
[0033] In preferred embodiments, the deaerated liquid in contact with
the light
transference medium is flowing across the light transference medium as defined
herein. In
other embodiments, the deaerated liquid in contact with the light transference
medium is
not flowing across the light transference medium, i.e., is static.
[0034] In embodiments where the deaerated liquid flows across the light
transference
medium, the liquid may do so under conditions described as laminar, turbulent,
and/or
transitional flow, though the deaerated liquid may be static while in contact
with the light
transference medium. The deaerated liquid may have a Reynolds number of from
about 0
to about 4000, including from about 400 to about 3000, and including about 800
to about
2300.
[0035] For embodiments where the deaerated liquid is flowing across the
light
transference medium, the ultrasonic energy may originate upstream or
downstream from a
location of a light transference medium where an optical signal passes
through, or should
pass through. In a preferred embodiment, the ultrasonic energy originates
upstream from a
location of a light transference medium where an optical signal passes
through, or should
pass through.
[0036] The ultrasonic energy may have a frequency of from about 20 kHz
to about
200 kHz. The ultrasonic energy may have a frequency of from about 20 kHz, or
from
about 25 kHz, or from about 30 kHz, or from about 40 kHz, to about 200 kHz, or
to about
150 kHz, or to about 100 kHz, or to about 80 kHz, or to about 70 kHz, or to
about 60 kHz.
In some embodiments, the ultrasonic energy has a frequency of from about 20
kHz to
about 80 kHz. In further embodiments, the ultrasonic energy has a frequency of
from
about 30 kHz to about 60 kHz, which includes about 40 kHz. In even further
embodiments, the ultrasonic energy has a frequency of from about 25 kHz to
about
30 kHz, which includes about 28 kHz.
7
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
[0037] In certain embodiments, the ultrasonic energy is applied at a
rate of from about
1 W/cm2/sec to about 400 W/cm2/sec. The ultrasonic energy may be applied at a
rate of
from about 1 W/cm2/sec, or from about 10 W/cm2/sec, or from about 50
W/cm2/sec, or
from about 100 W/cm2/sec, to about 400 W/cm2/sec, or to about 300 W/cm2/sec,
or to
about 200 W/cm2/sec.
[0038] The wavelength of the ultrasonic energy is dependent upon the
frequency and
the velocity of the ultrasonic energy, which is essentially constant. The
frequency, and
therefore the wavelength, is chosen so as to provide enough energy to prevent
or remove
particulate matter that may become deposited onto a light transference medium
in contact
with deaerated liquid. Ideally, the frequency of the ultrasonic energy will be
sufficient to
remove such particulate matter, or prevent the particulate matter from
depositing onto the
light transference medium, while not damaging the light transference medium.
However,
a user may attempt to minimize or prevent damage to the light transference
medium by
utilizing one or more of several modifications discussed herein.
[0039] As described herein, the ultrasonic energy originates at a distance
(d) from an
optical signal transmitted into the light transference medium, which is
preferably set to
optimize the energy applied into the deaerated liquid at a point relative to
the light
transference medium, to effectively obtain or maintain light transference.
Preferably, the
distance (d) is defined by Formula 1 herein. For example, in embodiments that
apply
ultrasonic energy using an ultrasonic probe, the tip of the ultrasonic probe
is located at a
distance (d) such that particulate matter deposited onto the light
transference medium
becomes dislodged, thereby maintaining optical transference into the deaerated
liquid in
contact with the light transference medium. In certain embodiments, the
distance (d)
within certain ranges defined herein, thereby causing the ultrasonic energy to
"originate"
from the distance (d).
[0040] In certain embodiments, the ultrasonic energy originates at a
distance of from
about 30% to about 70%, or from about 35% to about 65%, or from about 40% to
about
60%, of the wavelength of the ultrasonic energy. In other embodiments, the
ultrasonic
energy originates at a distance from an optical signal transmitted into the
light transference
medium of from about 80% to about 120%, or from about 85% to about 115%, or
from
about 90% to about 110%, of the wavelength of the ultrasonic energy. In
certain
embodiments, the ultrasonic energy originates at a distance of from about 130%
to about
170%, or from about 135% to about 165%, or from about 140% to about 160%, of
the
8
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
wavelength of the ultrasonic energy. In other embodiments, the ultrasonic
energy
originates at a distance from an optical signal transmitted into the light
transference
medium of from about 180% to about 220%, or from about 185% to about 215%, or
from
about 190% to about 210%, of the wavelength of the ultrasonic energy. In
certain
embodiments, the ultrasonic energy originates at a distance of from about 230%
to about
270%, or from about 235% to about 265%, or from about 240% to about 260%, of
the
wavelength of the ultrasonic energy. In other embodiments, the ultrasonic
energy
originates at a distance from an optical signal transmitted into the light
transference
medium of from about 280% to about 320%, or from about 285% to about 315%, or
from
about 290% to about 310%, of the wavelength of the ultrasonic energy. In
certain
embodiments, the ultrasonic energy originates at a distance of from about 330%
to about
370%, or from about 335% to about 365%, or from about 340% to about 360%, of
the
wavelength of the ultrasonic energy. In other embodiments, the ultrasonic
energy
originates at a distance from an optical signal transmitted into the light
transference
medium of from about 380% to about 420%, or from about 385% to about 415%, or
from
about 390% to about 410%, of the wavelength of the ultrasonic energy. In
certain
embodiments, the ultrasonic energy originates at a distance of from about 430%
to about
470%, or from about 435% to about 465%, or from about 440% to about 460%, of
the
wavelength of the ultrasonic energy. In other embodiments, the ultrasonic
energy
originates at a distance from an optical signal transmitted into the light
transference
medium of from about 480% to about 520%, or from about 485% to about 515%, or
from
about 490% to about 510%, of the wavelength of the ultrasonic energy.
[0041] In certain embodiments, a parameter of the deaerated liquid in
contact with the
light transference medium is measured by transmitting the optical signal into
the deaerated
liquid via the light transference medium, and detecting a response. In certain
embodiments, the parameter comprises fluorescence, light absorbance,
temperature,
chemiluminescence, optical scattering (e.g., Rayleigh, Mie, and Raman
scatter), imaging,
transmittance, particle size, particle count, or turbidity, or any combination
thereof.
[0042] In certain embodiments, the method is a clean-in-place method. A
clean-in-
place method does not require disassembly of the system in order to conduct
the method.
In other words, the light transference medium is not removed from the system,
and the
system is not disconnected for the purpose of accessing the light transference
medium.
9
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
[0043] In certain embodiments, treatment of the deaerated liquid is
controlled by
utilizing the measured parameter in a control scheme. Treatment of the
deaerated liquid
may include, but is not limited to, at least one of physical treatment and
chemical
treatment. Non-limiting examples of physical treatment include adjustment of
any of the
following parameters of the deaerated liquid: temperature, pressure, physical
phase, flow
rate (e.g., circulation, blowdown, and/or make-up), flow path, and mixing. Non-
limiting
examples of chemical treatment include adjustment of any of the following
parameters, all
related to a treatment chemical: chemical species selection, chemical species
concentration, chemical species dosage rate, chemical species dosage location,
and
deaeration completeness.
[0044] In certain embodiments, the measured parameter is inputted into a
control
scheme. The control scheme is generally an automated method that inputs a
plurality of
several measured parameters and operates several process devices, e.g., pumps,
valves,
etc. For example, a certain measured parameter may indicate that treatment
chemical
concentration has fallen outside a lower tolerance limit. For the present
example, the
measured parameter may trigger the control scheme to operate a feed pump,
which in turn
adds treatment chemical to the process.
[0045] In certain embodiments, the optical transference through the
light transference
medium is at least partially obstructed by particulate matter or scaling. In
some
embodiments, the particulate matter may comprise a metal oxide. In certain
embodiments,
the light transference medium is obstructed by deposition of a chemical
species
comprising iron, copper, manganese, titanium, chromium, nickel, calcium,
magnesium,
oxide, phosphate, carbonate, or silicate, or any combination thereof. In
certain
embodiments, the light transference medium is obstructed by scale comprising
calcium,
magnesium, phosphate, carbonate, or silicate, or any combination thereof.
[0046] In other embodiments, the particulate deposition may comprise
particulate
matter found in raw water, e.g., mud, sand, silt, etc.
[0047] In certain embodiments, the deaerated liquid may be conditioned
prior to
contacting the light transference medium. For example, particularly when the
deaerated
liquid is boiler blowdown liquid or boiler condensate liquid, the deaerated
liquid may be
"enthalpy-rich." At elevated temperature and pressure (e.g., 300-1500 F and
corresponding pressures for saturated steam/liquid), the deaerated liquid may
be
conditioned such that a portion of the enthalpy (measured in the form of
temperature and
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
pressure) are removed prior to the deaerated liquid contacting the light
transference
medium. In certain embodiments, the deaerated liquid in contact with the light
transference medium has a temperature of from about 20 F to about 200 F,
including from
about 40 F to about 150 F, and including from about 60 F to about 130 F. In
certain
embodiments, the deaerated liquid in contact with the light transference
medium has a
pressure of from about 5 psig to about 100 psig, including from about 10 psig
to about
70 psig, and including from about 15 psig to about 50 psig.
[0048] The timing of the application of the ultrasonic energy to the
deaerated liquid
may take any one or more of several forms. In one embodiment, the ultrasonic
energy is
continuously streamed into the deaerated liquid, which preferably includes
while the
system utilizing the deaerated liquid is operational. In another embodiment,
the ultrasonic
energy is applied intermittently, e.g., for a timed duration at timed
intervals. In yet
another embodiment, the ultrasonic energy is applied on an as-needed basis,
which can be
determined, e.g., by comparing historical data related to the relevant sensor
and light
transference medium. For example, if obstruction of the light transference
medium grows
to an unacceptable value, e.g., a setpoint of from about 1 to about 5%
obstruction,
ultrasonic energy is then applied to the wetted surface of the light
transference medium as
described herein.
[0049] Examples of light transference media include a flow cell, an
optical window, a
reflective surface, a refractive surface, a dispersive element, a filtering
element, and an
optical fiber sensor head. In embodiments where the light transference medium
is
transparent or nearly transparent, the light transference medium is generally
constructed of
a material that is transparent or nearly transparent and having a hardness of
at least about 7
on the Mohs scale. The term "transparent or nearly transparent" refers to the
ability of
light to pass through a substance sufficient to use light for detection and/or
measurement
purposes as discussed herein, which includes transparency as defined by ASTM
D1746.
In certain embodiments, the light transference medium is constructed of
quartz, sapphire,
diamond, or boron nitride.
[0050] In certain embodiments, the light transference medium is
constructed of any
suitable transparent or nearly transparent composition, and is coated with a
transparent or
nearly transparent substance having a hardness of at least about 7 on the Mohs
scale. For
example, the light transference medium may be constructed of a substance
having a Mohs
scale hardness of at least about 7 (e.g., quartz), and then coated with a
substance having an
11
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
even higher Mohs scale rating. In certain embodiments, the coating substance
has a Mohs
scale rating of from about 8 to 10, or from about 9 to 10, or 10. Illustrative
embodiments
of substances suitable for coating a light transference medium include, but
are not limited
to, diamond, titanium diboride, boron nitride, and sapphire.
[0051] In certain embodiments, the light transference medium takes the form
of a
reflective surface. In embodiments utilizing a reflective surface, an optical
window may
be utilized in concert with the reflective surface to provide observation from
outside the
deaerated liquid.
[0052] FIG. 1 illustrates a system capable of performing the inventive
method. A
deaerated liquid 10, which flows in a direction 12, contacts a light
transference medium
20. Light transference medium 20 is a flow cell, and deaerated liquid 10 flows
through the
flow cell via direction 12. A light source 25 and a detector 26 are located so
as to transmit
an optical signal 27 through light transference medium 20 and deaerated liquid
10, and
detect the resulting behavior caused by the transmitted optical signal 27,
which may
include fluorescence, light absorbance, temperature, chemiluminescence,
optical scattering
(e.g., Rayleigh, Mie, and Raman scatter), imaging, transmittance, particle
size, particle
count, turbidity, and combinations thereof. An ultrasonic transducer 30 is
operably
attached to an ultrasonic probe 31 having a tip 32 that emits ultrasonic
energy 33 at a
wavelength (2\,), with tip 32 being located at a distance (d) from the optical
signal 27, with
distance (d) being defined by Formula 1 presented herein. Optionally, the
ultrasonic probe
31 may be positioned such that tip 32 emits ultrasonic energy 33 at an angle a
of from 0 to
about 45 degrees, or to about 35 degrees, or to about 25 degrees, or to about
15 degrees, or
to about 5 degrees, as illustrated. In certain embodiments, the ultrasonic
probe 31 is
positioned such that tip 32 projects ultrasonic energy 33 substantially in the
direction of
flow 12 of deaerated liquid 10 across light transference medium 20. FIGs. 1,
2, 4, 5A, and
5B illustrate embodiments including a mount that seals ultrasonic transducer
30 to light
transference medium 20 utilizing a seal 36, which in certain embodiments is a
washer. In
certain embodiments, seal 36 is constructed of an elastomer. Exemplary
embodiments of
elastomers include, but are not limited to, nitrile-butadiene rubber
("nitrile"),
hydrogenated nitrile-butadiene rubber, ethylene propylene diene monomer
("EPDM"),
silicone, fluoroelastomer, and polychloroprene.
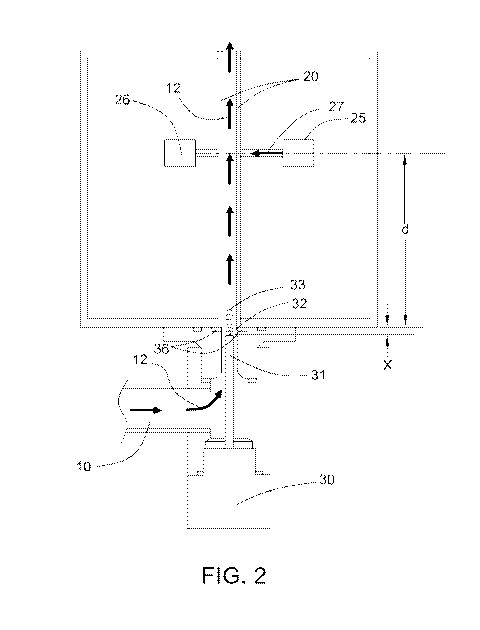
[0053] FIG. 2 illustrates an embodiment, wherein the tip 32 of the
ultrasonic probe 31
is positioned so as to create an offset X from light transference medium 20.
Utilization of
12
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
offset X can be of particular importance to allow sufficient ultrasonic energy
at
wavelength (2\,) into the light transference medium when a relatively soft
material (e.g.,
seal 36) is present in the direction of the ultrasonic energy. The ultrasonic
energy will be
less likely to be dampened by the relatively soft material due to the low
energy level at the
location of the relatively soft material. In embodiments that incorporate a
relatively soft
material between an ultrasonic probe 31 and a light transference medium 20,
the distance
(d) should be measured from a point beyond the relatively soft material to
light
transference medium 20, as illustrated in FIG. 2. In certain embodiments
utilizing
offset X, offset X is defined by Formula 3 below:
X = (b + 0.25 *(2n ¨ 1)) *)\, Formula 3
wherein 2\, is the wavelength of the ultrasonic energy, b is a constant
ranging from
about -0.2 to about 0.2, and n is an integer ranging from 1 to 30. In certain
embodiments,
b is a constant ranging from about -0.15 to about 0.15, or from about -0.1 to
about 0.1.
[0054] FIG. 3 illustrates yet another embodiment of a system capable of
performing
the inventive method. A deaerated liquid 10, which flows in a direction 12,
contacts a
light transference medium 20, which can be, for example, mounted via a tee
pipe
fitting 100. Light transference medium 20 takes the form of an optical window
of a
combination light source/detector 25/26, and deaerated liquid 10 flows across
the optical
window. Combination light source/detector 25/26 is located so as to transmit
an optical
signal 27 through light transference medium 20 (the optical window) and into
the
deaerated liquid 10, and detect the resulting behavior caused by optical
signal 27, which
may include fluorescence, light absorbance, temperature, chemiluminescence,
optical
scattering (e.g., Rayleigh, Mie, and Raman scatter), imaging, transmittance,
particle size,
particle count, turbidity, and combinations thereof. While FIG. 3 shows an
embodiment
utilizing a combination light source/detector 25/26, a person skilled in the
art will readily
recognize that the light source and the detector may be separate units
operably connected
to a control unit (not shown). An ultrasonic transducer 30 is operably
attached to an
ultrasonic probe 31 having a tip 32 that emits ultrasonic energy 33, with tip
32 being
located at a distance (d) from the optical signal 27, with distance (d)
defined by Formula 1
presented herein.
[0055] FIG. 4 illustrates a further embodiment of a system capable of
performing the
inventive method is illustrated. A deaerated liquid 10, which may flow in a
direction 12,
13
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
contacts a light transference medium 20. Light transference medium 20 includes
a
transparent portion 20a and an optional reflective portion 20b, and deaerated
liquid 10
contacts each of transparent portion 20a and reflective portion 20b. A
combination light
source/detector 25/26 is located so as to transmit an optical signal 27
through transparent
portion 20a and into deaerated liquid 10, and detect the resulting behavior
caused by
optical signal 27, which may include fluorescence, light absorbance,
temperature,
chemiluminescence, optical scattering (e.g., Rayleigh, Mie, and Raman
scatter), imaging,
transmittance, particle size, particle count, turbidity, and combinations
thereof. Optical
signal 27 may be transmitted from combination light source/detector 25/26 via
optical
fibers capable of receiving and transmitting fluorescent emission to the
combination light
source/detector 25/26. Alternately, the system may be configured to utilize a
light source
25 and detector 26 in addition to or in place of the combination light
source/detector
25/26, wherein light source 25 and detector 26 are not aligned opposite one
another.
While a combination light source/detector 25/26 is illustrated in this
particular
embodiment, a person of skill in the art will readily recognize that the light
source and the
detector may be separate units connected to a control unit (not shown). An
ultrasonic
transducer 30 is operably attached to an ultrasonic probe 31 having a tip 32
that emits
ultrasonic energy 33, with tip 32 located at a distance (d) from optical
signal 27, with
distance (d) being defined by Formula 1 presented herein.
[0056] In certain embodiments, an enhancer is utilized to assist in
performing the
methods described herein. When utilized, the enhancer allows ultrasonic energy
to be
applied in a manner that provides beneficial removal of obstruction while
protecting the
light transference medium from damage that may be caused by the application of
ultrasonic energy. Particularly when applied at sharp angles (e.g.,
perpendicular) toward
the light transference medium, ultrasonic energy can damage the light
transference
medium. The utilization of one or more enhancers can limit or prevent the
occurrence of
such damage. When utilized, the enhancer may comprise at least one of a sleeve
and a
lossy surface. It is important to note that these particular enhancers may be
used
individually or in combination, or in some embodiments of the methods, not
used at all.
Whether to use an enhancer depends on a number of factors, including, but not
limited to,
the durability of the light transference medium, and the angle and frequency
of the
ultrasonic energy.
14
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
[0057] In embodiments utilizing a sleeve as an enhancer, the sleeve is
generally
positioned so as to protect a portion of the light transference medium located
near the
source of ultrasonic energy. Generally, the sleeve is constructed and
positioned so as to
prevent dampening of the ultrasonic energy in the vicinity of the transmission
of the
optical signal into the light transference medium. More particularly, the
sleeve should
protect the light transference medium from damage that may be caused by
ultrasonic
energy traveling perpendicular or nearly perpendicular from the ultrasonic
energy source
toward the light transference medium. When utilized, the sleeve should be
constructed of
a material suitable for providing protection to the light transference medium.
For
example, the sleeve may be constructed of stainless steel.
[0058] In other embodiments, the sleeve is constructed of a substance
that is not
completely rigid, but is not so soft as to absorb an undesired amount of the
ultrasonic
energy. For example, in embodiments that utilize a sleeve, the sleeve may be
constructed
of a substance compatible with contacting liquid(s). Furthermore, the sleeve
may be
constructed of a substance having a Shore "A" hardness of from about 60 to
about 90. In
certain embodiments, the sleeve is constructed of an elastomer as defined
herein. FIG. 5A
demonstrates an illustrative embodiment of a system that incorporates sleeve
70a into its
design. Example 3 provides further information related to an embodiment of a
sleeve
utilized to prevent over-dampening.
[0059] In embodiments utilizing a lossy surface as an enhancer, the lossy
surface is
generally positioned so as to protect a portion of the light transference
medium located
near the source of ultrasonic energy. Generally, the lossy surface is
positioned so as to
dampen a portion of the ultrasonic energy traveling toward the light
transference medium,
and particularly the ultrasonic energy traveling perpendicular or nearly
perpendicular from
the ultrasonic energy source toward the light transference medium. In certain
embodiments, the lossy surface is a surface that is generally rough, such as,
e.g., a
grooved, threaded, or jagged surface. Generally, a lossy surface is rough such
that at least
a portion of the ultrasonic energy is scattered away when coming in contact
with the lossy
surface. FIG. 5B demonstrates an illustrative embodiment of a system that
incorporates
lossy surface 70b into its design.
[0060] A clean-in-place method of maintaining optical transference
through a light
transference medium operably connected to a boiler system is provided. The
clean-in-
place method comprises contacting a stream of boiler water with a wetted
surface of a
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
light transference medium in optical communication with an optical sensor.
Data related
to a parameter of the boiler liquid measured by the optical sensor is input to
a control
scheme of a boiler system. The optical sensor is electronically isolated from
the control
scheme, which maintains control of the boiler system based on the input data
related to the
parameter of the boiler liquid. A liquid chemical agent contacts the wetted
surface of the
light transference medium for a period of time and at a concentration
sufficient to clean
the wetted surface of the light transference medium. The liquid chemical agent
is selected
from an acid, a chelant, a reducing agent, and combinations thereof. The
liquid chemical
agent is removed from the wetted surface of the light transference medium, and
the optical
sensor is electronically de-isolated from the control scheme.
[0061] A clean-in-place method of maintaining optical transference
through a light
transference medium operably connected to a boiler system is also provided.
The clean-
in-place method comprises flowing a stream of boiler liquid to contact a
wetted surface of
a light transference medium in optical communication with an optical sensor.
Data related
to a parameter of the boiler liquid measured by the optical sensor is input to
a control
scheme of a boiler system. The flow of the stream of boiler liquid to contact
the wetted
surface of the light transference medium is discontinued. The optical sensor
is
electronically isolated from the control scheme, which maintains control of
the boiler
system based on the input data related to the parameter of the boiler liquid.
A liquid
chemical agent contacts the wetted surface of the light transference medium
for a period of
time and at a concentration sufficient to clean the wetted surface of the
light transference
medium. The liquid chemical agent is selected from an acid, a chelant, a
reducing agent,
and combinations thereof. The liquid chemical agent is removed from the wetted
surface
of the light transference medium, the flow of the stream of boiler liquid to
contact the
wetted surface of the light transference medium is resumed, and the optical
sensor is
electronically de-isolated from the control scheme.
[0062] The terms "optical" and "light" are used interchangeably herein.
Utilization of
the phrase "into boiler liquid" is intended to cover light transmission in any
direction
between the boiler liquid, the light transference medium, a light source,
and/or a light
detector. For example, the optical signal may originate from within the boiler
liquid and
be transferred to a sensor via the light transference medium (e.g.,
fluorometric emission),
or from a light source through the light transference medium and into the
boiler liquid
(e.g., fluorometric excitation). Illustrative embodiments of optical sensors
that perform
16
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
optical measurements using optical signals include, but are not limited to,
devices capable
of detecting or sensing absorbance, colorimetric, refractometric,
spectrophotometric,
luminometric, and/or fluorometric signals, or images. In a preferred
embodiment, the
optical signal comprises a fluorometric excitation and/or emission.
[0063] The method is directed to obtaining or maintaining optical
transference into
boiler liquid in contact with a light transference medium. The method can be
utilized to
remove obstructions that may be present on the light transference medium.
Removal of
obstruction from the light transference medium sufficient to allow for optical
transference,
thereby allowing for performance of an optical measurement of the boiler
liquid, is also
achieved by the methods of the present invention.
[0064] The term "clean-in-place" is utilized herein to describe a method
that is
performed without disassembly of the system. For example, the light
transference
medium is not removed from the system, and the system is not disconnected to
gain
physical access to the light transference medium (e.g., to be manually wiped),
to carry out
a clean-in-place method. Related to the methods described herein, the light
transference
medium remains operably connected to a boiler system, though the stream of
boiler liquid
may be diverted so as to not contact the wetted surface of the light
transference medium
during performance of the disclosed methods.
[0065] When performing the methods described herein, the optical
transference
through the light transference medium may be at least partially obstructed by
particulate
matter. The particulate matter may comprise particulate matter typically found
in raw
water, e.g., mud, sand, silt, etc. The particulate matter may comprise a metal
oxide. The
oxide may be of a metal selected iron, copper, manganese, titanium, chromium,
or nickel,
or any combination thereof. Metal oxide deposition is of particular concern
for boiler
liquid, particularly boiler blowdown liquid. In certain embodiments, the
particulate matter
comprises at least one of silica, a calcium oxide, a calcium salt, a magnesium
oxide, and a
magnesium salt.
[0066] The timing of the contacting of the liquid chemical agent to the
wetted surface
of the light transference medium may take any one or more of several forms. In
certain
embodiments, the liquid chemical agent is added continuously to the boiler
liquid, which
preferably includes during operation of the system utilizing the liquid
chemical agent. In
other embodiments, the liquid chemical agent is added intermittently to the
boiler liquid,
e.g., for a timed duration at timed intervals. In further embodiments, the
liquid chemical
17
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
agent is added on an as-needed basis, which can be determined, e.g., by
comparing
historical data related to the relevant sensor and light transference medium.
For
embodiments where the flow of the stream the boiler liquid is discontinued,
the liquid
chemical agent may be contacted intermittently or on an as-needed basis.
[0067] In embodiments of the present invention, the light transference
medium is in
optical communication with an optical sensor, which allows the optical sensor
to be
utilized to monitor a substance using optical detection methods. For example,
a flow cell
is generally used to allow for fluorometric detection of a component of a
liquid flowing
through a conduit. The flow cell allows for light to pass between a
fluorometer and the
flowing liquid via the wall of the flow cell, thereby allowing the fluorometer
to carry out
its monitoring without physically contacting the flowing liquid. For the given
situation,
the fluorometer is said to be in optical communication with the flow cell.
[0068] Examples of light transference media include, but are not limited
to, a flow
cell, an optical window, a reflective surface, a refractive surface, a
dispersive element, a
filtering element, and an optical fiber sensor head. The light transference
medium may be
constructed of a material that is transparent or nearly transparent. The light
transference
medium may have a hardness of at least about 7 on the Mohs scale. The term
"transparent
or nearly transparent" refers to the ability of light to pass through a
substance sufficient to
use light for detection and/or measurement purposes as discussed herein, which
includes
transparency as defined by ASTM D1746. The hardness of the light transference
medium
becomes increasingly important when ultrasonic energy is utilized to
supplement the
general clean-in-place methods disclosed herein. In certain embodiments, the
light
transference medium is constructed of quartz, sapphire, or diamond.
[0069] In certain embodiments, the light transference medium is
constructed of any
suitable transparent or nearly transparent composition, and is coated with a
transparent or
nearly transparent substance having a hardness of at least about 7 on the Mohs
scale. For
example, the light transference medium may be constructed of a substance
having a Mohs
scale hardness of at least about 7 (e.g., quartz), and then coated with a
substance having an
even higher Mohs scale rating. In certain embodiments, the coating substance
has a Mohs
scale rating of from about 8 to 10, or from about 9 to 10, or 10. Illustrative
embodiments
of substances suitable for coating a light transference medium include, but
are not limited
to, diamond, titanium diboride, boron nitride, and sapphire.
18
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
[0070] In certain embodiments, the light transference medium takes the
form of a
reflective surface. In embodiments utilizing a reflective surface, an optical
window may
be utilized in concert with the reflective surface to provide observation from
outside the
boiler liquid.
[0071] In certain embodiments, treatment of the boiler liquid is controlled
by utilizing
the measured parameter in a control scheme. Treatment of the boiler liquid may
include,
but is not limited to, at least one of physical treatment and chemical
treatment. Non-
limiting examples of physical treatment include adjustment of any of the
following
parameters of the boiler liquid: temperature, pressure, physical phase, flow
rate (e.g.,
circulation, blowdown, and/or make-up), flow path, and mixing. Non-limiting
examples
of chemical treatment include adjustment of any of the following parameters,
all related to
a treatment chemical: chemical species selection, chemical species
concentration,
chemical species dosage rate, chemical species dosage location, and deaeration
completeness.
[0072] In the methods disclosed herein, the measured parameter is inputted
into a
control scheme. The control scheme is generally an automated method that
inputs a
plurality of several measured parameters and operates several process devices,
e.g.,
pumps, valves, etc. For example, a certain measured parameter may indicate
that
treatment chemical concentration has fallen outside a lower tolerance limit.
For the
present example, the measured parameter may trigger the control scheme to
operate a feed
pump, which in turn adds treatment chemical to the process.
[0073] In certain embodiments, the optical sensor is electronically
isolated from the
control scheme. A sensor is said to be electronically isolated if it generates
data that is
intentionally ignored or otherwise intentionally not acted upon by a
controller, or provides
no data because of an action of the controller (e.g., automatically shut down)
or the user
(e.g., unplugged). A sensor that is electronically isolated in the exemplary
manner may
allow for the sensor to be cleaned, e.g., via liquid chemical treatment,
without providing
false or misleading data acquired during said liquid chemical treatment. An
electronically
isolated sensor would not need to be physically isolated from the stream of
boiler liquid,
but isolated only from the control scheme. The term "meaningful data" as used
herein
refers to data that describes a parameter of a substance and may be input into
and reliably
acted upon by a control scheme.
19
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
[0074] In certain embodiments, flow of the stream of boiler liquid in
contact with the
wetted surface of the light transference medium is discontinued in order to
carry out the
contacting the liquid chemical agent step. A light transference medium can be
said to
undergo "system isolation" when the flow of the stream of boiler liquid is
discontinued to
carry out a clean-in-place method such as, e.g., those disclosed herein.
System isolation
allows for the liquid chemical agent to contact the wetted surface of the
light transference
medium for an extended period of time, as opposed to dosing the liquid
chemical
treatment into the flowing stream of boiler liquid.
[0075] After the liquid chemical agent has contacted the wetted surface
for a period of
time and at a concentration sufficient to clean the wetted surface, the liquid
chemical agent
is removed from the wetted surface and flow of the stream of boiler liquid is
resumed. In
certain embodiments, the liquid chemical agent is removed by resuming the flow
of the
stream of boiler liquid to contact the wetted surface of the light
transference medium.
[0076] In embodiments that carry out system isolation, the liquid
chemical agent may
be brought into contact with the wetted surface and remain static for a period
of time. In a
further embodiment, after the liquid chemical agent has been removed from the
wetted
surface, a further liquid chemical agent, whether it be the same species of
liquid chemical
agent or a different species of liquid chemical agent, may be brought into
contact with the
wetted surface and remain static for a period of time, prior to resuming the
flow of the
stream of boiler liquid to contact the wetted surface of the light
transference medium. In
other embodiments that carry out system isolation, the liquid chemical agent
may contact
the wetted surface by being passed across the wetted surface for a period of
time, e.g., in a
liquid chemical treatment loop, or the liquid chemical agent may be pass
across the wetted
surface only once.
[0077] In embodiments of the inventive methods, cleaning via liquid
chemical agent
contact requires that the liquid chemical agent contacts the wetted surface
for a period of
time and at a concentration sufficient to clean the wetted surface of the
light transference
medium. The period of time and the concentration generally depend on each
other, with a
shorter period of contact time generally necessary to achieve cleaning using
higher liquid
chemical agent concentrations, and a longer period of contact time for lower
liquid
chemical agent concentration, assuming that all other factors remain constant
(temperature, species of liquid chemical agent, materials of construction,
etc.). A period
of time sufficient to clean the wetted surface may be nearly instantaneous,
e.g., 1 second
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
or less, for a given liquid chemical agent dosed to a slightly-obstructed
light transference
medium at a reasonably high concentration and otherwise under preferential
conditions.
Cleaning a heavily-obstructed light transference medium may require a
significantly
longer contact time, e.g., 20 minutes or greater, depending on inter alia
liquid chemical
agent selection and concentration. In a preferred embodiment, the period of
time is from
about 30 seconds to about 20 minutes, including from about 1 minute to about
10 minutes.
[0078] The liquid chemical agent should be selected and dosed so as to
provide
cleaning of the wetted surface without corroding or otherwise damaging the
surfaces
contacted by the liquid chemical agent. With few exceptions, a higher
concentration of
liquid chemical agent will generally provide better cleaning activity when
contacting the
wetted surface. One notable exception is sulfuric acid, which may perform
better when
fully-protonated. In embodiments that utilize sulfuric acid, the sulfuric acid
may have a
concentration of from about 5 weight percent to about 98 weight percent in
aqueous
solution. In a preferred embodiment that utilizes sulfuric acid, the sulfuric
acid has a
concentration of from about 5 weight percent to about 15 weight percent,
including about
10 weight percent, in aqueous solution. In embodiments that utilize citric
acid, the citric
acid may have a concentration of from about 5 weight percent to about 30
weight percent
in aqueous solution. In a preferred embodiment that utilizes citric acid, the
citric acid has
a concentration of from about 5 weight percent to about 15 weight percent,
including
about 10 weight percent, in aqueous solution.
[0079] In embodiments that do not carry out system isolation of the
light transference
medium subject to the clean-in-place methods disclosed herein, the liquid
chemical agent
may contact the wetted surface of the light transference medium at a flow rate
of about
1 mL/min to about 40 mL/min at a concentration of about 0.1 weight percent to
about 80
weight percent, depending on, inter alia, the liquid chemical agent utilized.
In a preferred
embodiment, the liquid chemical agent contacts the wetted surface of the light
transference
medium at a flow rate of about 1 mL/min to about 40 mL/min at a concentration
of about
1 weight percent to about 20 weight percent chelant in aqueous solution. In
another
preferred embodiment, the liquid chemical agent contacts the wetted surface of
the light
transference medium at a flow rate of about 1 mL/min to about 40 mL/min at a
concentration of about 0.1 weight percent to about 10 weight percent reducing
agent in
aqueous solution. In yet another preferred embodiment, the liquid chemical
agent contacts
the wetted surface of the light transference medium at a flow rate of about 1
mL/min to
21
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
about 40 mL/min at a concentration of about 30 weight percent to about 60
weight percent
acid in aqueous solution. Each preferred embodiment is further described
herein in the
context of components of the liquid chemical agent.
[0080] In certain embodiments that undergo electronic isolation or
system isolation of
a light transference medium and/or optical sensor, control of the boiler
system may be
maintained based on data input into the control scheme prior to the electronic
or system
isolation. Control of the boiler system may be maintained based on data
gathered from a
period of time previous to the electronic or system isolation. The period of
time previous
to the electronic or system isolation may be, e.g., the last recorded value(s)
prior to the
electronic or system isolation, or e.g., one minute, or five minutes, or one
hour, or five
hours, etc. The gathered data may be manipulated as is known in the art to
implement the
maintenance of boiler system control. Averaging data over a period of time is
an example
of manipulating data.
[0081] As mentioned in the previous paragraph, the boiler system may be
maintained
based on the last recorded value prior to the electronic or system isolation.
By way of
example, the optical sensor may input a data point related to a parameter of
the boiler
liquid, and the optical sensor and its corresponding light transference medium
may be
electronically or systemically isolated immediately following the input of the
data point.
In this preferred embodiment of the invention, the control scheme continues to
maintain
control of the boiler system as if the optical sensor continues to input the
same data that
was input immediately prior to the electronic or system isolation. Instead of
utilizing the
immediate predecessor data point to maintain control, further exemplary
embodiments
may utilize, for example, several prior data points, a mean of several data
points, a median
of several data points, a mode of several data points, or a statistical trend
of several data
points.
[0082] In embodiments of the inventive methods, the liquid chemical
agent comprises
a component selected from an acid, a chelant, a reducing agent, and
combinations thereof.
Single component liquid chemical agents can be used to successfully clean a
light
transference medium according to the inventive methods disclosed herein.
However, in a
particularly preferred embodiment, the liquid chemical agent comprises an acid
of one
chemical species and a chelant of a second chemical species. In another
particularly
preferred embodiment, the liquid chemical agent comprises a reducing agent of
one
chemical species and a chelant of a second chemical species. The phrase "of
one chemical
22
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
species...of a second chemical species" is used to describe the utilization of
distinct
chemicals for each named genus. For example, a liquid chemical agent
comprising a
reducing agent of one chemical species and a chelant of a second chemical
species may be
a liquid chemical agent comprising sodium hyposulfite (a reducing agent of one
chemical
species) and oxalic acid (a chelant of a second chemical species). An
exemplary
embodiment of a liquid chemical agent comprising an acid of one chemical
species and a
chelant of a second chemical species is a liquid chemical species comprising
urea
hydrochloride (an acid of one chemical species) and oxalic acid (a chelant of
a second
chemical species).
[0083] In certain embodiments, the component of the liquid chemical agent
is an acid
selected from urea hydrochloride, hydrochloric acid, sulfuric acid, phosphoric
acid, nitric
acid, acetic acid, citric acid, carbonic acid, bicarbonic acid, sulfamic acid,
or any
combination thereof. In a preferred embodiment, the acid in the liquid
chemical agent is
urea hydrochloride.
[0084] When selected, the acid may be present in the liquid chemical agent
at a
concentration of from about 5 weight percent to about 98 weight percent in
aqueous
solution, including from about 20 weight percent to about 80 weight percent
acid in
aqueous solution, and further including at least about 20 weight percent, or
at least about
30 weight percent, or about 40 weight percent to about 50 weight percent, to
about 60
weight percent, to about 70 weight percent, to about 80 weight percent acid in
aqueous
solution. In a preferred embodiment, the acid is present in the liquid
chemical agent at a
concentration of about 30 weight percent to about 60 weight percent.
[0085] In certain embodiments, the component of the liquid chemical
agent is a
chelant selected from: citric acid, oxalic acid, ethylenediaminetetraacetic
acid, diethylene
triamine pentaacetic acid, an organic phosphonate, salts thereof, or any
combination
thereof. In a preferred embodiment, the chelant in the liquid chemical agent
is oxalic acid.
[0086] When selected, the chelant may be present in the liquid chemical
agent at a
concentration of from about 0.1 weight percent to about 20 weight percent
chelant in
aqueous solution, including at least about 0.1 weight percent, or at least
about 0.5 weight
percent or at least about 1 weight percent, to about 3 weight percent, or
about 5 weight
percent, or about 10 weight percent, or about 20 weight percent chelant in
aqueous
solution. In a preferred embodiment, the chelant is present in the liquid
chemical agent at
23
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
a concentration of from about 1 weight percent to about 3 weight percent
chelant in
aqueous solution.
[0087] In certain embodiments, the component of the liquid chemical
agent is a
reducing agent selected from an acid sulfite, an acid bisulfite, an acid
hydrosulfite, an acid
phosphite, phosphoric acid, oxalic acid, formic acid, ascorbic acid, or
erythorbic acid, salts
thereof, or any combination thereof. In a preferred embodiment, the reducing
agent in the
liquid chemical agent is sodium hydrosulfite.
[0088] When selected, the reducing agent may be present in the liquid
chemical agent
at a concentration of from about 0.1 weight percent to about 10 weight percent
reducing
agent in aqueous solution, including from about 0.1 weight percent, or about
0.3 weight
percent, or about 0.5 weight percent, to about 3 weight percent, or to about 7
weight
percent, or to about 10 weight percent reducing agent in aqueous solution. In
a preferred
embodiment, the reducing agent is present in the liquid chemical agent at a
concentration
of from about 0.5 weight percent to about 3 weight percent reducing agent in
aqueous
solution.
[0089] Of note, a particular chemical species may overlap into any two,
and in some
instances, all three, of the three chemical genuses of the present invention.
For example,
citric acid and oxalic acid can be considered both an acid and a chelant.
Furthermore,
several acids, including oxalic acid and phosphoric acid, can be considered to
be reducing
agents in addition to acids and/or chelants.
[0090] Though different reference numerals may be utilized in FIG. 9
compared to
other figures presented herewith, like-named elements are to be construed as
being the
same or similar to like-named elements present in other figures (e.g., FIGs. 1-
5B). For
example, light transference medium 20 of FIG. 1 should be construed as being
the same or
similar to light transference medium 1020 of FIG. 9, and so forth. Referring
to FIG. 9,
operation of a boiler treatment system generally involves boiler liquid 1010
flows through
solenoid valve 1012a and continues through light transference medium (e.g.,
flow cell)
1020, contacting wetted surface 1021, and usually out to auxiliary operations
via valve
1012b or to treatment or a drain via valve 1012c. A parameter of boiler liquid
1010 is
measured using optical sensor 1022 (e.g., fluorometer), which is in operable
communication with light transference medium 1020, and data related to the
parameter is
input into a control scheme (e.g., relayed to controller 1100). Optical sensor
1022 is
electronically isolated from the control scheme, which maintains control of
the boiler
24
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
system based upon the previously input data. A liquid chemical agent, e.g.,
present in
container 1050, is brought into contact with wetted surface 1021 via pump
1054, flowing
through valve 1052 and check valve 1056 and on to wetted surface 1021.
Optionally,
boiler liquid 1010 can be diverted to bypass line 1070 by closing valve 1012a
and opening
valve 1012d. As is readily recognized by one skilled in the art, valves 1012a
and 1012d
can be replaced by a single three-way valve (not shown), which could be
operably
configured to divert boiler liquid 1010 from wetted surface 1021 of light
transference
medium 1020 and to bypass line 1070. Optionally, valves 1012a, 1012b, 1012c,
and
1012d can be operably actuated to provide system isolation of light
transference medium
1020. The liquid chemical agent may continuously or intermittently contact
wetted
surface 1021, or may be periodically contacted and removed from wetted surface
via
system isolation as described herein.
[0091] To supplement the more general clean-in-place methods disclosed
herein,
ultrasonic energy may be applied as described herein into the liquid chemical
agent during
at least a portion of the contacting of the liquid chemical agent to the
wetted surface of the
light transference medium. When utilized, the ultrasonic energy further
effectuates
cleaning of the wetted surface of the light transference medium. When
utilized, the
ultrasonic energy may be applied prior to, simultaneously, and/or subsequent
to contacting
the liquid chemical agent to the wetted surface of the light transference
medium. The
ultrasonic energy may be applied via an ultrasonic probe and ultrasonic
transducer in a
manner disclosed in U.S. Patent Application Publication No. 2013/0186188,
filed January
19, 2012, to Bradley et al., or in a manner disclosed in U.S. Patent
Application Serial No.
14/592,219, filed January 8, 2015, to Hicks et al., each disclosure of which
is incorporated
herein by reference in its entirety. The embodiment illustrated in FIG. 9
includes optional
ultrasonic probe 1201 operably attached to optional ultrasonic transducer
1202, which may
be configured as described herein, and more particularly as in FIGs. 1-5B
presented
herewith.
[0092] The following examples further illustrate the invention but, of
course, should
not be construed as in any way limiting its scope.
EXAMPLE 1
[0093] FIG. 6 is a plot of obstruction of light transference media in
the form of
particulate deposition onto flow cells. Two light transference media (flow
cells for this
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
Example) were used to perform the experiment: ultrasonic energy was applied to
the test
flow cell, and no cleaning method was implemented for the control flow cell.
In the
example, the flow cells were initially clean and of the same type. The flow
cells were
exposed to the same blowdown stream of a 1500 psi recovery boiler of a paper
mill. The
flow cells were quartz glass tubes, each having an outer diameter of 0.312"
(7.9 mm), an
inner diameter of 0.236" (6 mm), and a length of 4.69" (11.9 cm). The
ultrasonic energy
was applied via a probe positioned at a distance (d) of 58 mm according to
FIG. 1 and
Formula 1 (i.e., n = 3), with the tip of the ultrasonic probe positioned flush
with the end of
the light transference medium. The blowdown of the recovery boiler flowed
through the
flow cells after being conditioned from saturated, e.g., 1515 psig at 597 F,
to less than
40 psig and less than 120 F. The conditioned blowdown is expected to flow
through the
flow cells under laminar flow, as the flow rate is approximately 500 mL/min
and having a
Reynolds Number of approximately 1800.
[0094] Ultrasonic energy was applied to the test flow cell at 40 kHz,
which was
intermittently applied at 2.2% duty. In other words, the ultrasonic energy was
applied to
the test flow cell for 1 minute per 45 minutes (i.e., 1 minute / 45 minutes =
0.022).
[0095] The experiment was carried out over 20 days for the control flow
cell, which
continued to accumulate particulate deposition up to about 75% obstruction.
The
experiment was carried out over 14 days for the test flow cell, which
accumulated virtually
no obstruction over the 14-day trial. Obstruction of the light transference
medium was
virtually eliminated by the application of ultrasonic energy at a frequency of
40 kHz.
EXAMPLE 2
[0096] FIG. 7 is a plot of particulate deposition being removed from a
light
transference medium, in this instance a flow cell, over time by the
application of ultrasonic
energy to the flow cell. In the example, the flow cell was exposed to a
blowdown stream
of a powerhouse boiler. The flow cell was the same as the test flow cell of
Example 1
herein, except that the distance (d) was 56 mm, and the tip of the ultrasonic
probe was
10 mm beyond the end of the flow cell (offset (X) = 10 mm) as shown in FIG. 2.
Offset
(X) fits Formula 3, with n = 1. The flow cell was approximately 100%
obstructed at the
beginning of the experiment. Blowdown of the powerhouse boiler flowed through
the
flow cell at 300 mL/min after being conditioned as in Example 1 herein.
26
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
[0097] Ultrasonic energy was applied to the flow cell at 28 kHz, which
was
intermittently applied at 50% duty (i.e., one minute "on" for every minute
"off'), as
opposed to the 2.2% duty to the test flow cell of Example 1. The experiment
was carried
out over approximately 250 minutes. By approximately the 170th minute,
substantially all
of the particulate deposition had been removed, and the flow cell was
substantially
unobstructed.
EXAMPLE 3
[0098] FIG. 8 is a plot of the effect of the utilization of an enhancer
in combination
with an EPDM washer used to seal the ultrasonic energy source to a light
transference
medium, for this Example flow cells as described in Examples 1 and 2 herein.
Each
EPDM washer used to seal the flow cells had a Shore "A" hardness of about 55
to about
75. For this Example, the enhancer was a sleeve covering the EPDM washer and
part of
the light transference medium of the test flow cell. The control flow cell
incorporated the
EPDM washer seal but did not incorporate an enhancer. In the example, each of
the two
flow cells were initially 100% obstructed by particulate deposition and were
exposed to
the same conditioned blowdown stream as in Examples 1 and 2, except that the
pre-
conditioned blowdown stream was initially saturated at 700 psig and 503 F. The
conditioned blowdown stream (less than 40 psig and less than 120 F) flowed
through the
flow cells at approximately 300 mL/min. Ultrasonic energy was applied to both
the
control flow cell and the test flow cell. The distance (d) for the test flow
cell was 62 mm,
and the tip of the ultrasonic probe was 6 mm beyond each flow cell (offset (X)
= 6 mm,
but only for the control flow cell). Because the EPDM washer was covered by an
enhancer for the test flow cell, the distance (d) followed FIG. 1 and Formula
1, with n = 2.
For the control flow cell, the distance (d) was 56 mm and offset (X) was 6 mm,
as shown
in FIG. 2, which falls within the parameters of Formula 3. However,
incorporation of the
enhancer of the test flow cell was clearly beneficial in minimizing the
dampening effect of
the EPDM washer.
[0099] The sleeve was constructed of 316-stainless steel "thin wall"
tubing, having an
outer diameter slightly less than the 0.236" inner diameter of the test flow
cell. The
ultrasonic energy was applied to each flow cell at 20 kHz, which was
intermittently
applied at 50% duty. As shown in FIG. 8, the control sample showed little
removal of
obstruction at 20 kHz at 50% duty for the 2000-minute test. However,
utilization of a
27
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
sleeve covering the EPDM washer and a portion of the test flow cell allowed
for nearly
complete removal of the obstruction after about 1000 minutes of 20 kHz
ultrasonic energy
at 50% duty.
EXAMPLE 4
[0100] Three cleaning chemicals have been tested to be able to remove iron
oxide
particles from boiler water stream.
[0101] Urea hydrochloride, available as DC-14 from Nalco Company, 1601
West
Diehl Road, Naperville, IL 60563, was tested as a liquid cleaning agent to
clean a wetted
surface of a flow cell used to monitor a boiler blowdown stream of a power
house boiler.
A control flow cell was continuously exposed to the same boiler blowdown
stream and
was not cleaned. The duration of the test was approximately 28 days. The urea
hydrochloride had a concentration of 30-60% by weight in aqueous solution,
having a pH
of 1.5. Aqueous urea hydrochloride at this concentration generally vaporizes
at normal
operational conditions. The urea hydrochloride was dosed once per day at full
concentration, i.e., not further diluted, and allowed to contact the wetted
surface of the test
flow cell for 3 minutes under system isolation.
[0102] FIG. 10 illustrates the results of testing. The spikes in cell
obstruction
represent the periods of time during which the urea hydrochloride was dosed to
the wetted
surface of the flow cell. Notice the measured increase in cell obstruction of
the control
flow cell versus the significantly lower cell obstruction of the test flow
cell during non-
treatment time periods. For example, at Day 15, the control flow cell is
nearly 40%
obstructed while the test flow cell is less than 10% obstructed, which is
believed to be
unobstructed. While not wishing to be bound by theory, any amount of measured
obstruction that is less than 10% is believed to be caused by light absorbance
of water or
LED decay of the optical sensor.
EXAMPLE 5
[0103] Oxalic acid was tested as a liquid cleaning agent to clean a
wetted surface of a
flow cell used to monitor a boiler blowdown stream of a power house boiler. A
control
flow cell was continuously exposed to the same boiler blowdown stream and was
not
cleaned. The duration of the test was approximately 25 days. The oxalic acid
had a
concentration of 12,000 ppm by weight in aqueous solution, having a pH of 2,
which was
28
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
stable at 50 C. A higher pH would be expected at a lower concentration. The
oxalic acid
was dosed at full concentration, i.e., not further diluted, and allowed to
contact the wetted
surface of the test flow cell for 10 minutes under system isolation. FIG. 11
illustrates the
results of testing, with the spikes in cell obstruction represent the periods
of time during
which the oxalic acid was dosed to the wetted surface of the flow cell. Notice
the
measured increase in cell obstruction of the control flow cell versus the
significantly lower
cell obstruction of the test flow cell during non-treatment time periods. For
example, at
Day 11, the control flow cell is approximately 10% obstructed while the test
flow cell is
almost completely unobstructed. Furthermore, when oxalic acid is not dosed to
the test
flow cell (e.g., Days 16-20), obstruction of the test flow cell generally
tracks the
obstruction of the control flow cell. However, obstruction of the test flow
cell decreases
dramatically after oxalic acid contacts the test flow cell's wetted surface.
EXAMPLE 6
[0104] Sodium hydrosulfite was tested as a liquid cleaning agent to
clean a wetted
surface of a flow cell used to monitor a boiler blowdown stream of a power
house boiler.
A control flow cell was continuously exposed to the same boiler blowdown
stream and
was not cleaned. The duration of the test was approximately 38 days. The
sodium
hydrosulfite had a concentration of 0.8-2.4 weight percent in aqueous
solution, which
decomposes to sulfur dioxide at above 50 C. The sodium hydrosulfite was dosed
at full
concentration, i.e., not further diluted, and allowed to contact the wetted
surface of the test
flow cell for 10 minutes under system isolation.
[0105] FIG. 12 illustrates the results of testing. The spikes in cell
obstruction
represent the periods of time during which the sodium hydrosulfite was dosed
to the
wetted surface of the test flow cell under system isolation. Notice the
measured increase
in cell obstruction of the control flow cell versus the significantly lower
cell obstruction of
the test flow cell, particularly beginning at approximately Day 28.
EXAMPLE 7
[0106] Experiments were performed to test certain combinations of
components of the
liquid cleaning agents of Examples 1-3. The following aqueous agents were
obtained or
prepared: sodium hydrosulfite at 0.8-2.4% by weight; oxalic acid at 1.2% by
weight (as
dihydrate); and urea hydrochloride as 30-60% by weight. The following
combinations
were created from the aqueous agents, each blended at 1:1 volume ratios:
29
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
Combination No. Agents
1 Sodium hydrosulfite + oxalic acid
2 Urea hydrochloride + oxalic acid
3 Urea hydrochloride + sodium hydrosulfite
[0107] Flow cells of various obstructions (60-100%) were placed in each
of the
combined liquid cleaning agents for 10 minutes at a time, removed, and
observed to
determine each combination's performance. Combinations 2 and 3 removed some of
the
obstructing deposition, and Combination 1 removed substantially all of the
obstructing
deposition.
[0108] All references, including publications, patent applications, and
patents, cited
herein are hereby incorporated by reference to the same extent as if each
reference were
individually and specifically indicated to be incorporated by reference and
were set forth
in its entirety herein.
[0109] The use of the terms "a" and "an" and "the" and "at least one"
and similar
referents in the context of describing the invention (especially in the
context of the
following claims) are to be construed to cover both the singular and the
plural, unless
otherwise indicated herein or clearly contradicted by context. The use of the
term "at least
one" followed by a list of one or more items (for example, "at least one of A
and B") is to
be construed to mean one item selected from the listed items (A or B) or any
combination
of two or more of the listed items (A and B), unless otherwise indicated
herein or clearly
contradicted by context. The terms "comprising," "having," "including," and
"containing"
are to be construed as open-ended terms (i.e., meaning "including, but not
limited to,")
unless otherwise noted. Recitation of ranges of values herein are merely
intended to serve
as a shorthand method of referring individually to each separate value falling
within the
range, unless otherwise indicated herein, and each separate value is
incorporated into the
specification as if it were individually recited herein. All methods described
herein can be
performed in any suitable order unless otherwise indicated herein or otherwise
clearly
contradicted by context. The use of any and all examples, or exemplary
language (e.g.,
"such as," "illustrative") provided herein, is intended merely to better
illuminate the
invention and does not pose a limitation on the scope of the invention unless
otherwise
claimed. No language in the specification should be construed as indicating
any non-
claimed element as essential to the practice of the invention.
CA 02973098 2017-07-05
WO 2016/112291
PCT/US2016/012648
[0110] Preferred embodiments of this invention are described herein,
including the
best mode known to the inventors for carrying out the invention. Variations of
those
preferred embodiments may become apparent to those of ordinary skill in the
art upon
reading the foregoing description. The inventors expect skilled artisans to
employ such
variations as appropriate, and the inventors intend for the invention to be
practiced
otherwise than as specifically described herein. Accordingly, this invention
includes all
modifications and equivalents of the subject matter recited in the claims
appended hereto
as permitted by applicable law. Moreover, any combination of the above-
described
elements in all possible variations thereof is encompassed by the invention
unless
otherwise indicated herein or otherwise clearly contradicted by context.
31