Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02980540 2017-09-21
WO 2016/154102
PCT/US2016/023387
BALLISTIC RESISTANT COMPOSITE MATERIAL
CROSS-REFERENCE TO RELATED APPLICATION
[0001] Thisapplication claims the benefit of co-pending United States
Provisional
Application Serial No. 62/138,548, filed on March 26, 2015, the disclosure of
which is
incorporated by reference herein in its entirety.
FIELD OF THE DISCLOSURE
[0002] The present disclosure relates to a composite formed with a
bimodal binder.
In particular, the present disclosure relates to a ballistic resistant
composite formed from a
plurality of fibers and a bimodal binder and a method of forming the same.
DESCRIPTION OF THE RELATED ART
[0003] Ballistic resistant articlesmay contain high-strength fibers which
can be
formed into various articles, such as vests, helmets, vehicle panels,
additional articles of
clothing, and additional items for military or police applications which
resist penetration of
bullets, shrapnel, and shells. Exemplary high-strength fibers are polyethylene
fibers, aramid
fibers, graphite fibers, nylon fibers, glass fibers, and the like. For many
applications, such as
ballistic resistant articles of clothing, the fibers may be used in a woven or
knitted fabric. For
other applications, the fibers may be encapsulated or embedded in a polymeric
matrix
material to form woven or non-woven composites.
[0004] Hard or rigid body armor provides good ballistic resistance but
can be bulky
and stiff Therefore, body armor garments, such as ballistic resistant vests,
are preferably
formed from flexible or soft armor materials. However, while such flexible or
soft armor
materials have good ballistic resistant qualities, these materials may also
exhibit low abrasion
resistance, which affects the durability of the armor. Additionally, it is
necessary for hard and
soft ballistic resistant articles to withstand environmental conditions which
may degrade the
ballistic resistance of the material. For example, due to the nature of
military applications,
ballistic resistant articles may be exposed to a variety of environmental
conditions which may
degrade the material, such as sea water, gasoline, gun lubricant, and
petroleum. As such, the
ballistic resistant articles are formed to resist such degradation when
exposed to
environmental conditions or substances.
1
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
[0005] Referring to FIG. 1, one method for producing soft ballistic
resistant body
armor is with a belt press, such as a steel belt press 2. Illustratively,
steel belt press 2 is an
isobaric steel belt pressor double belt press which includes a first belt 10,
a second belt 12, a
first plurality of rollers 4 supporting first belt 10, a second plurality of
rollers 6 supporting
second belt 12, and a temperature unit 8. As shown in FIG. 1, first plurality
of rollers 4
rotates in a clockwise direction and is positioned above second plurality of
rollers 6.
Conversely, second plurality of rollers 6 rotates in a counter-clockwise
direction, which in
combination with the clockwise rotation of first plurality of rollers 4,
advances a compositel4
through steel belt press 2.
[0006] Compositel4 may be comprised of high-performance fibers and a
binder. The
binder may be at least partially formed of a polymeric material and may be
applied to the
fibers through conventional coating processes (e.g., casting, dispersions).
[0007] As composite 14 enters steel belt press 2, first and second belts
10, 12 are
configured to apply a continuous pressure up to approximately 70 bar or 1,000
psi to
composite 14 as compositel4 advances through steel belt press 2. Additionally,
compositel4passes through temperature unit 8 which includes a heating portion
8a and a
cooling portion 8b. As such, composite14 receives continuous high pressure
from first and
second belts 10, 12 while being both heated and cooled, which results in a
high degree of
compaction and a reduction of air voids in composite 14. It is believed that
the compaction
from steel belt press 2 removes voids and other interstices within composite
14, thereby
providing a smooth surface which is resistant to corrosive and degrading
conditions.
[0008] One disadvantage of using steel belt press 2 to produce ballistic
resistant
composite 14 is that the costs associated with producing composite 14 may be
high, due to
the high expense of steel belt press 2. However, other less expensive
processing techniques
configured to apply pressure, heat, and cooling to composite14 may not be
configured to
apply similar levels of pressure and/or continuous pressure. Additionally,
body armor and
other ballistic resistant materials produced by these less expensive
processing techniques
should be configured to withstand environmental conditions (e.g., fuel, salt
water, humidity,
etc.) thought to degrade the material properties of such composites without
compromising the
ballistic resistant properties thereof. Therefore, a need exists for a low-
cost method of
producing soft ballistic resistant articles which can withstand various
environmental
conditions.
2
CA 02980540 2017-09-21
WO 2016/154102
PCT/US2016/023387
SUMMARY OF THE DISCLOSURE
[0009] The present disclosure provides a ballistic resistant composite
which includes
a plurality of fibers and a bimodal binder applied to the plurality of fibers.
[0010] In one form thereof, the present disclosure providesa ballistic
resistant
composite comprising a plurality of fibers and a bimodal binder applied to the
plurality of
fibers. The binder has a crystalline component with a melting temperature and
an amorphous
component with a softening temperature. The crystalline component and the
amorphous
component have at least one of the following properties relative to one
another: (1) the
melting temperature of the crystalline component is less than the softening
temperature of the
amorphous component; (2) at a temperature above the melting temperature of the
crystalline
component, a viscosity of the crystalline component is less than a viscosity
of the amorphous
component; and (3) at a temperature above the melting temperature of the
crystalline
component, a surface energy of the crystalline component is less than a
surface energy of the
amorphous component.
[0011] In certain embodiments, the crystalline component is a wax
material selected
from the group consisting of carnauba wax, stearamide wax, polyethylene wax,
paraffin wax,
polyolefin wax, and microcrystalline wax, and the amorphous component is a
polymeric
material selected from the group consisting of acrylic, polyurethane, nitrile
rubber,
acrylonitrile butadiene copolymer, and fluorocarbon. Additionally, the
plurality of fibers
may be comprised of polyethylene.
[0012] In certain embodiments, the plurality of fibers defines at least a
first fiber ply
and a second fiber ply oriented 90 degrees from the first fiber ply.
[0013] In certain embodiments, the amorphous component comprises 60-95
wt.% of
the bimodal binder and the crystalline component comprises 5-40 wt.% of the
bimodal
binder.
[0014] In certain embodiments, the melting temperature of the crystalline
component
is about 50-140 C.
[0015] In another form thereof, the present disclosure provides amethod
of forming a
ballistic resistant composite comprising providing a first plurality of fibers
in a unidirectional
orientation, providing a second plurality of fibers in a unidirectional
orientation, and
providing a binder having an amorphous component and a crystalline component.
The
method further comprises coating the first plurality of fibers with the
binder, coating the
second plurality of fibers with the binder, positioning the first plurality of
fibers at a 90
3
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
degree angle to the second plurality of fibers, heating the first and second
pluralities of fibers
to a temperature within a melting temperature range of the crystalline
component, applying a
pressure of less than one bar to the first and second pluralities of fibers
when at a temperature
within the melting point range of the crystalline component, and cooling the
first and second
pluralities of fibers.
[0016] In certain embodiments, said pressure step includes is
conductedwith a flat-
bed laminator.
[0017] In certain embodiments, said applying step includes applying a
first pressure
of less than 0.5 psi to the composite during said heating step and applying a
second pressure
of 10 psi ¨ 300 psi when the composite is at the temperature within the
melting point range of
the crystalline component.
[0018] In certain embodiments, the composite may be heated and/or cooled
for as
little as 0.01 seconds, 0.50 seconds, 1.0 seconds, 1.5 seconds, 2.0 seconds,
2.5 seconds, 3.0
seconds, or as much as 1 minute, 2 minutes, 3 minutes, 4 minutes, or 5
minutes, or any range
delimited by any pair of the foregoing values.
[0019] Also provided is amethod of forming a ballistic resistant
composite
comprising:
providing a first fiber ply comprising a plurality of unidirectionally
oriented first
fibers, wherein said first fibers are coated with a first bimodal binder that
comprises an
amorphous component and a crystalline component;
providing a second fiber ply comprising a plurality of unidirectionally
oriented second
fibers, wherein said second fibers are coated with a second bimodal binder
that comprises an
amorphous component and a crystalline component;
positioning the first fiber ply and second fiber ply in a stacked arrangement,
heating the first fiber ply and the second fiber ply to a temperature within a
melting
temperature range of the crystalline component;
applying a pressure of less than one bar to the first fiber ply and to the
second fiber
ply when said plies are at a temperature within the melting temperature range
of the
crystalline component, whereby the first fiber ply and second fiber ply are
attached to each
other and thereby form a ballistic resistant composite; and
cooling the first fiber ply and the second fiber ply.
4
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
BRIEF DESCRIPTION OF THE DRAWINGS
[0020] The above-mentioned and other features and advantages of this
disclosure, and
the manner of attaining them, will become more apparent and will be better
understood by
reference to the following description of embodiments taken in conjunction
with the
accompanying drawings, wherein:
[0021] FIG. 1 is a schematic cross-sectional view of an isobaric steel
belt press and a
composite material being formed therein;
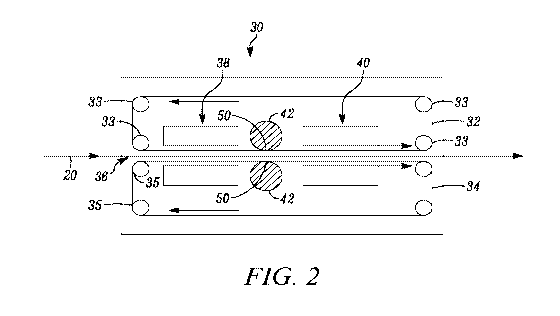
[0022] FIG. 2 is a schematic view of a flat-bed laminator; and
[0023] FIG. 3 is a schematic view of an extended flat-bed laminator with
a plurality
of coating devices.
DETAILED DESCRIPTION
[0024] The present disclosure relates to a ballistic resistant composite
20 including a
bimodal binder, the composite formable in a manner in which it is exposed to
low pressure
for a short duration of time and at a controlled temperature. More
particularly, the bimodal
binder of composite 20 allows the composite to be formed with a flat-bed
laminator, for
example, which may be less expensive than other processing methods, such as a
steel belt
press.
[0025] Composite 20 includes a plurality of fibers 20a embedded in a
bimodal
polymeric matrix or binder material 20b (Fig. 3). Bimodal binder material 20b
includes a
first mode of an amorphous component and a second mode of a crystalline
component, as
detailed further herein. More particularly, the amorphous component comprises
the majority
component of binder material 20b and may be chemically and/or physically
incompatible
with the crystalline component. For example, as detailed further herein, at
least the softening
temperature, or alternatively, the glass-transition temperature,and material
structure of the
amorphous component is different from that of the crystalline component.
A. Fiber Material
[0026] Ballistic resistant composite 20 includes fiber material 20a which
is embedded
with bimodal binder material 20b. Fiber material 20a is formed from a
plurality of fibers,
each of which has an elongate body with a length much greater than the
transverse
dimensions of width and thickness. The cross-sections of the fibers of fiber
material 20a may
be circular, flat, or oblong. Accordingly, the term "fiber" includes
filaments, ribbons, strips,
CA 02980540 2017-09-21
WO 2016/154102
PCT/US2016/023387
and the like having regular or irregular cross-sections. Each fiber of fiber
material 20a also
may be of regular or irregular multi-lobal cross-section projecting from the
linear or
longitudinal axis of the fiber.
[0027] Exemplary fiber material 20a comprises a non-woven, cross-plied,
unidirectional fabric. More particularly, fiber material 20a includes a
plurality of plies of
unidirectional fibers oriented in a cross-ply configuration in which a first
ply of fiber material
20a is oriented 90-degrees to an adjacent second ply of fibers. The fibers
within each ply are
adjacent and parallel to each other and, therefore, are oriented in a
unidirectional
arrangement. In one embodiment, each fiber may be approximately 0.063 inches
or 1.588
mm in diameter.
[0028] Fiber material 20a may be comprised of polyethylene fibers, aramid
fibers,
graphite fibers, nylon fibers, glass fibers, and the like. For example, in one
embodiment,
fiber material 20a is comprised of ultra-high molecular weight polyethylene,
such as
Honeywell 1150-denier SPECTRA Merge 95121 UHMWPE fibers and/or Honeywell 1300-
denier SPECTRA Merge 95159 UHMWPE fibers.Each fiber ply of the fiber material
20a
may have a fiber areal density of from about 15 g/m2 to about 250 g/m2,
typically from about
20 g/m2 to about 100 g/m2, and often from about 25 g/m2 to about 70 g/m2, and
most
preferably about 35 g/m2.The fiber areal density refers to the weight of the
fibers only (i.e.,
not including the binder) per unit area. Additional details of fiber material
20a may be
disclosed in U.S. Patent No. 7,994,075, issued on August 9, 2011, and U.S.
Patent No.
8,017,530, issued on September 13, 2011, the complete disclosures of which are
expressly
incorporated by reference herein.
B. Bimodal Binder Material
[0029] Bimodal binder material 20b is applied to fiber material 20a to
form ballistic
resistant composite 20. Exemplary binder material 20b is a bimodal binder
comprised
oftheamorphous component which has amorphous phases discernible through
magnification
and the crystalline component which has crystalline phases discernible through
magnification, as is known to one of ordinary skill in the art.
1. Amorphous Component
[0030] The amorphous component of binder material 20bis characterized as
amorphous because it does not have long-range order which is characteristic of
a crystalline
6
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
material. The lack of long-range order allows the amorphous component to be
flexible which
allows for flexibility in composite 20 and may be necessary when forming soft
body armor
that is configured to bend and move when being worn. The exemplary amorphous
component
of binder material 20b defines the majority component of binder material 20b.
For example,
the amorphous component may comprise at least 55%, 60%, 65%, 70%, 75%, 80%,
85%,
90%, 95%, 99% or within any range delimited by any pair of the foregoing
values, of the
overall weight of binder material 20b.
[0031] The amorphous component may be comprised of a liquid or powder
resin,
such as a polyurethane resin, acrylic resin, nitrile rubber resin,
acrylonitrile butadiene
copolymer resin, a fluorocarbon resin, polybutadiene resin, polyisoprene
resin, ethylene-
propylene resin, polysulfide resin, polyacrylate resin, polyester resin,
and/or polyether resin.
For example, the amorphous component of binder material 20b may be a
waterborne
dispersion of an acrylonitrile butadiene copolymer, supplied at 40% solids,
such as TYLAC
873 commercially available from Mallard Creek Polymers of Charlotte, NC000000,
and/or a
waterborne dispersion of a fluorocarbon resin, such as NUVA 2040 commercially
available
from Clariant GMBH Corporation of Germany.
[0032] The exemplary amorphous component of binder material 20b has a
greater
viscosity, surface energy, and/orsoftening temperature than the crystalline
component when
at a temperature within the melting temperature of the crystalline component.
More
particularly, in one embodiment, the crystalline and amorphous components are
selected such
that the melting temperature of the crystalline component is less than the
softening
temperature of the amorphous component and/or a viscosity of the crystalline
component is
less than a viscosity of the amorphous component when at a temperature above
the melting
temperature of the crystalline component. Additionally, the amorphous and
crystalline
components may be selected so that, at a temperature above the melting
temperature of the
crystalline component, a surface energy of the crystalline component is less
than a surface
energy of the amorphous component. For example, in one embodiment, the
softening
temperature of the amorphous component of binder material 20b isless than a
degradation
temperature of fiber material 20a but substantially greater than the melting
temperature of the
crystalline componentsuch that when the crystalline component melts and begins
to flow, the
amorphous component does not does appreciably melt or undergo a physical
change and may
even exhibit a resistance to flow. More particularly, amorphous materials may
not have a
distinct melting point but will start to soften within a softening temperature
range and will
7
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
continue to soften as the temperature increases. Conversely, crystalline
materials have a true
melting temperature and change drastically from a hard solid to a fluid over a
much shorter
temperature range. In this way, the amorphous component is incompatible with
the
crystalline component because the amorphous component is not physically
modified or
chemically reactive with the crystalline component during a physical
transformation of the
crystalline component. In one example, Honeywell 1150-denier SPECTRA Merge
95121
UHMWPE fibers and/or Honeywell 1300-denier SPECTRA Merge 95159 UHMWPE fibers
may have a degradation temperature of about 140 C and the crystalline
component may have
melting temperature up to 140 C, as detailed further herein. Therefore, the
softening
temperature of the amorphous component may be as little as 90 C, 100 C, 110 C,
120 C,
130 C, 140 C,150 C, 160 C, 170 C, 180 C, 190 C, or as great at 200 C, 210 C,
220 C,
230 C, 240 C, 250 C, 260 C, or more, or may be within any range delimited by
any pair of
the foregoing values.
[0033] Alternatively, in one embodiment, the softening temperature of the
amorphous
component may be less than the melting temperature of the crystalline
component. However,
because the viscosity and/or surface energy of the amorphous component is
greater than that
of the crystalline component when at a temperature within the melting
temperature of the
crystalline component, the amorphous component will remain solid or highly
viscous and,
therefore, the crystalline component will flow around the amorphous component
such that the
amorphous and crystalline components do not mix.Additional details of the
amorphous
component of binder material 20b may be disclosed in U.S. Patent No.
7,994,075, issued on
August 9, 2011, and U.S. Patent No. 8,017,530, issued on September 13, 2011,
the complete
disclosures of which are expressly incorporated by reference herein.
2. Crystalline Component
[0034] The crystalline component of binder material 20b is added to, or
doped into,
the amorphous component. The crystalline component of binder material 20b is
characterized as crystalline because it includes a highly ordered molecular
structure defined
by a crystal lattice. The crystal lattice of the crystalline component may be
discernible
through magnification, as is known to one of ordinary skill in the art. Unlike
the amorphous
component, the crystalline component may have less flexibility but is included
in binder
material 20b because it allows for the compaction and densification of
composite 20 at
processing conditions with decreased pressure, thereby increasing the
ballistic resistant
8
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
properties of composite 20. Also, as discussed further below, during
processing of the
present composite, the crystalline material undergoes a phase change, such as
melting, which
allows the crystalline material to flow relative to the amorphous material,
with subsequent re-
solidification to provide desirable properties such as smoothness and density
enhancement to
provide resistance to corrosive environments.
[0035] The crystalline component of binder material 20b is the minority
component
thereof. For example, the crystalline component may comprise 45%, 40%, 35%,
30%, 25%,
20%, 15%, 10%, 5%, 1% or may be within any range delimited by any pair of the
foregoing
values of the overall weight of binder material 20b.
[0036] The crystalline component may be comprised of any crystalline
polymer
which is incompatible with the amorphous component. More particularly, the
crystalline
component may be incompatible with the amorphous component such that the two
components do not mix or act as a single material. For example, the
crystalline component of
the present disclosure may be a wax material, such as a carnauba wax, a
polyethylene wax,
polyolefin wax, a paraffin wax, stearamide wax, and/or a microcrystalline wax.
Waxes are
generally defined as materials that are solids at room temperature but melt or
soften without
decomposing above about 40 C. Waxes are generally organic and insoluble in
water at room
temperature but may be water wettable and may form pastes and gels in some
solvents, such
as non-polar organic solvents. The molecular weight of a wax may range from
about 400 to
about 25,000 g/mol and may have melting points ranging from about 40 C to
about
150 C.Waxes generally do not form stand-alone films like higher order polymers
and
generally are aliphatic hydrocarbons that contain more carbon atoms than oils
and greases.
[0037] The viscosity of waxes may range from low to high, typically
depending on
the molecular weight of the wax and the crystallinity. The viscosity of waxes
above their
melting point is typically low. As used herein, a "low viscosity wax"
describes a wax having
a melt viscosity of less than or equal to about 500 centipoise (cps) at 140 C.
Preferably, a low
viscosity wax has a viscosity of less than about 250 cps at 140 C, most
preferably less than
about 100 cps at 140 C. However, some linear polyethylene waxes (molecular
weight of
about 2,000 to about 10,000 glmol) and polypropylene waxes may have moderate
to high
viscosity, i.e., as high as 10,000 cps after melting. Viscosity values are
measured using
techniques that are well known in the art and may be measured, for example,
using capillary,
rotational or moving body rheometers. A preferred measurement tool is a
Brookfield
rotational viscometer.
9
CA 02980540 2017-09-21
WO 2016/154102
PCT/US2016/023387
[0038]
Suitable waxes include both natural and synthetic waxes and non-exclusively
include animal waxes, such as beeswax, Chinese wax, shellac wax, spermaceti
and wool wax
(lanolin); vegetable waxes, such as bayberry wax, candelilla wax, carnauba
wax, castor wax,
esparto wax, Japan wax, Jojoba oil wax, ouricury wax, rice bran wax and soy
wax; mineral
waxes, such as ceresin waxes, montan wax, ozocerite wax and peat waxes;
petroleum waxes,
such as paraffin wax and microciystalline waxes; and synthetic waxes,
including polyolefin
waxes, polyethylene, polypropylene waxes, Fischer-Tropsch waxes, stearamide
waxes
(including ethylene his-steara.mi de waxes), polyinerized a-olefin waxes,
substituted amide
waxes (e.g. esterified or saponified substituted amide waxes) and other
chemically modified
waxes. Also suitable are waxes described in U.S. Pat. No. 4,544,694, the
complete disclosure
of which is expressly incorporated by reference herein. Of these, the
preferred waxes include
paraffin waxes, micro-crystalline waxes, Fischer-Tropsch waxes, branched and
linear
polyethylene waxes, polypropylene waxes, large particle size polyethylene
waxes, carnauba
waxes, ethylene bis-stearamide (EBS) waxes, and combinations thereof.
[0039] For
example, exemplary crystalline materials of binder material 20bmay be a
waterborne dispersion of carnauba wax, supplied at 35% solids, such as
HYDROCERTM EC-
35 wax commercially available from Shamrock Technologies Inc. of Newark, NJ; a
waterborne dispersion of large particle size polyethylene wax, supplied at 40%
solids, such as
LL405 commercially available from Michelman, Inc. of Cincinnati, Ohio; a
waterborne
dispersion of high density polyethylene wax, supplied at 35% solids, such as
Michelman, Inc.
LL411; a waterborne dispersion of paraffin wax, supplied at 32% solids, such
as Michelman,
Inc. 454; a waterborne dispersion of microcrystalline wax, supplied at 40%
solids, such as
Michelman, Inc. HL-480; and/or a waterborne dispersion of Fischer Tropsch
polyethylene
wax, supplied at 40% solids, such as Michelman, Inc. ME98040.
[0040] The
exemplary crystalline component of binder material 20b may have a low
melt viscosity and a melting temperature of 50 C, 55 C, 60 C, 65 C, 70 C, 75
C, 80 C,
85 C, 90 C, 95 C, 100 C, 105 C,110 C, 115 C, 120 C, 125 C, 130 C, 135 C, 140
C, or any
range delimited by any pair of the foregoing values. More particularly, a
carnauba wax may
have a melting point of approximately 75-85 C and a low melt viscosity.
Similarly, a micro-
crystalline wax may be have a melting temperature of approximately 60-90 C and
a low melt
viscosity. Additionally, a Fischer-Tropsch wax may have a melting temperature
of 95-100 C
and a low melt viscosity. Also, a paraffin wax may have a melting temperature
of 50-70 C
and a low melt viscosity. Additionally, polyethylene waxes may have a melting
temperature
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
of 90-140 C and, depending on the structure of the polyethylene wax, may have
low,
moderate, or high viscosity. As such, the crystalline component has a sharp
melting
temperature range that may only span about 5-50 C which allows the crystalline
component
to melt and cool rapidly.
[0041] As detailed herein, the melting point and melt viscosity of the
crystalline
component may be different than the softening temperature and viscosity of the
amorphous
component.In this way, when the crystalline component is exposed to a
temperature within its
melting point range, the crystalline component melts rapidly and begins to
flow around the
solid amorphous component which does not appreciably melt at temperatures
within the
melting point range of the crystalline component. The molten crystalline
component is then
able to fill any voids within the blended binder material 20b and also within
composite 20.
Additionally, when the crystalline component is exposed to a temperature that
is less than its
melting point range, the crystalline component cools rapidly to again form a
solid phase.
However, because the crystalline component flowed around the solid amorphous
component
when at its melting temperature, the crystalline component is embedded and
mixed with the
amorphous component once cooled.
[0042] For example, if an amorphous synthetic rubber defines the
amorphous
component of binder 20b and a crystalline polyurethane resin defines the
crystalline
component of binder 20b, a cast film of binder material 20b maintains discreet
regions of the
crystalline polyurethane resin within the larger mass of the amorphous
synthetic rubber.
Upon applying heat to composite 20, for example in a flat-bed laminator, the
amorphous
regions of the synthetic rubber remain solid but the discreet crystalline
regions of the
polyurethane resin melt and flow into voids within fiber material 20a which
improves the
fluid resistance of composite 20 by reducing capillary forces and reducing the
total effective
surface area of composite 20. In this way, composite 20 remains flexible due
to the
amorphous synthetic rubber but has improved ballistic resistance due to the
crystalline
polyurethane resin.
[0043] Alternatively, in one embodiment, the softening temperature of the
amorphous
component may be less than the melting temperature of the crystalline
component. However,
because the viscosity and/or surface energy of the amorphous component is
greater than that
of the crystalline component when at a temperature greater than the melting
temperature of
the crystalline component, the amorphous component will remain solid or highly
viscous and,
therefore, the crystalline component will flow around the amorphous component
and the
11
CA 02980540 2017-09-21
WO 2016/154102
PCT/US2016/023387
amorphous and crystalline components do not mix. More particularly, in one
embodiment,
the crystalline and amorphous components are selected such that the melting
temperature of
the crystalline component is less than the softening temperature of the
amorphous component
and/or the viscosity of the crystalline component is less than the viscosity
of the amorphous
component when at a temperature above the melting temperature of the
crystalline
component. Additionally, the amorphous and crystalline components may be
selected so
that, at a temperature above the melting temperature of the crystalline
component, the surface
energy of the crystalline component is less than the surface energy of the
amorphous
component.Additional details of the crystalline component of binder material
20b may be
disclosed in U.S. Patent No. 7,994,075, issued on August 9, 2011, and U.S.
Patent No.
8,017,530, issued on September 13, 2011, the complete disclosures of which are
expressly
incorporated by reference herein.
C. Ballistic Resistant Composite
[0044] To form bimodal binder material 20b, the crystalline and amorphous
components are mixed together through various processes.
1. Preparing the Bimodal Binder
[0045] In one embodiment, the crystalline and amorphous components may be
mixed
by forming wet blend emulsions and/or wet blend solutions. More particularly,
the wet blend
emulsion and/or wet blend solution includes a solvent in which both the
amorphous
component and the crystalline component are soluble. This wet blend solution
and/or wet
blend emulsionthen may be cast into a dry film in which the crystalline
component and the
amorphous component are maintained in discreet regions in this dry film.
[0046] Binder material 20b may also be mixed by coarsely dispersing a
solid form of
the crystalline component into either a waterborne emulsion of the amorphous
component or
into a solvent-based solution of the amorphous component (FIG. 3).
2. Applying the Bimodal Binder to the Fibers
[0047] Once mixed, binder material 20b is applied to fiber material 20a
to form
composite 20. Binder material 20b is applied to fiber material 20a through
various processes,
such as with a spray gun,fiber pultrusion,fiber impregnation, hot melt
extrusion, gravure
coating, and/or other roll coating methods. For example, a fiber impregnation
method may
12
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
be used to apply binder material 20b to fiber material 20a. Using the fiber
impregnation
method, an excess of a waterborne emulsion or dispersion of binder material
20b is applied to
fiber material 20a. Then a series of stationary bars and pressure rollers
squeeze out the
excess binder material 20b to form composite 20. Composite 20 may then be
temporarily
cast onto and transported by a silicone-coated release paper and, when the
water is dried,
composite 20 is wound onto a roll for further processing.
[0048] Additionally, a combination of the aforementioned methods may be
used to
apply binder material 20b to fiber material 20a. For example, a single
waterborne emulsion
of the amorphous component may be applied to fiber material 20a through the
fiber
impregnation method. Next, the crystalline component may be applied in a dry
form to the
surface of composite 20 by way of an electrostatic sprayer. The dry form of
the crystalline
component can be applied to fiber material 20a either before or after the
water from the
waterborne emulsion of the amorphous component has dried.
[0049] Additionally, in one embodiment, the amorphous component may be
applied
to fiber material 20b as a solvent-cast film using the aforementioned fiber
impregnation
method. The crystalline component is then applied in a dry form to the surface
of the
solvent-cast film of the amorphous component.
[0050] Regardless of the method selected to apply binder material 20b to
fiber
material 20a, fiber material 20a may be scoured with de-ionized water and
dried before
binder material 20b is applied thereto. Fiber material 20a then may be plasma
treated at an
energy flux of 50-80 watts/ft2/min, preferably 67 watts/ft2/min. Binder
material 20b is
subsequently applied to fiber material 20a through one or more of the
aforementioned
processes for adhering the individual fibers of fiber material 20a together
and for adhering the
various plies of fiber material 20a to each other. More particularly, fiber
material 20a is
coated with binder material 20b at a resin content of 5-30%, and preferably at
a resin content
of 17%. Once binder material 20b is applied to fiber material 20a, fiber
material 20a may be
rolled onto spools and stored as rolls until further processing occurs.
3. Flat-Bed Laminator
[0051] Referring to FIG. 2, after binder material 20b is applied to fiber
material 20a,
binder material 20b is applied to at least another layer of fiber material 20a
to define a second
ply of fibers. The various layers of plies of fibers are then positioned in a
stacked
13
CA 02980540 2017-09-21
WO 2016/154102
PCT/US2016/023387
arrangement and each ply is oriented 90 degrees from adjacent plies. Any
number of plies
may be included in composite 20 to accommodate various applications for
composite 20.
[0052] Composite 20 then may be formed witha flat-bed laminator 30 which
includes
a first or upper belt 32rotatable about a plurality of rollers 33 and a second
or lower belt 34
rotatable about a plurality of rollers 35. First and second belts 32, 34 may
be coated with a
non-stick coating, for example a fluoropolymer-based material such asTEFLON ,
commercially available from E. I. DuPont De Nemours and Company of Wilmington,
Delaware. First and second belts 32, 34 are spaced apart from each other by a
passageway 36
for composite 20 to pass through. As shown in FIG. 2, illustrative first belt
32 rotates in a
counter-clockwise direction and second belt 34 rotates in a clockwise
direction which
advances composite 20 through flat-bed laminator 30. In one embodiment, first
and second
belts 32, 34 rotate at a speed of 1-15 meters/second, and preferably 3
meters/second.Illustratively, first and second belts 32, 34 have approximately
the same length
such that composite 20 is in contact with both first and second belt 32, 34
for approximately
the same length of time.
[0053] Flat-bed laminator 30 of FIG. 2further includes a heating portion
or zone 38, a
cooling portion or zone 40, and a plurality of nip or pressure rollers 42
positioned
intermediate heating portion 38 and cooling portion 40. As composite 20
advances within
flat-bed laminator 30, composite 20 is heated in heating portion 38. For
example, heating
portion 38 may be configured for operation at temperatures of as little as 50
C, 60 C, 70 C,
80 C, or as great as 90 C, 100 C, 110 C, 120 C, 130 C, 140 C, 150 C, or any
range
delimited by any pair of the foregoing values. The temperature of heating
portion 38 is
within the melting temperature range of the crystalline component such that
the crystalline
component of binder 20b melts in heating portion 38. In one embodiment,
composite 20 may
be heated for as little as 0.01 seconds, 0.50 seconds, 1.0 seconds, 1.5
seconds, 2.0 seconds,
2.5 seconds, 3.0 seconds, or as much as 1 minute, 2 minutes, 3 minutes, 4
minutes, or 5
minutes, or any range delimited by any pair of the foregoing values.The
heating time and
temperature are based on the nature of the crystalline component and the
specific melting
temperature range thereof
[0054] As composite 20 leaves heating portion 38, pressure is applied to
composite
20 through pressure rollers 42 while the crystalline component is melted.
Pressure rollers
may be comprised of various materials, such as metals (e.g., steel), polymers
(e.g., elastic
rubber), and/or ceramics.Additionally, one of pressure rollers 42 may have a
fixed position
14
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
and the other of pressure rollers 42 may be movable when a force is applied
thereto, such that
when a force is applied to one of pressure rollers 42, a force also is applied
to composite 20.
More particularly, pressure rollers 42 may apply a pressure of less than one
bar to composite
20. For example, pressure rollers 42 may apply a nip pressure to composite 20
of 10 psi, 30
psi, 50 psi, 70 psi, 90 psi, 110 psi, 130 psi, 150 psi, 170 psi, 190 psi, 210
psi, 230 psi, 250 psi,
270 psi, 290 psi, 310 psi, or within any range delimited by any pair of the
foregoing values.
In one embodiment, pressure rollers may apply a pressure of 14 psi to
composite 20.The
greatest pressure applied to composite 20 occurs at a tangent 50 of pressure
rollers 42 which
is parallel to first and second belts 32, 34. However, due to the circular
cross-section of
pressure rollers 42, smaller amounts of pressure are applied to composite 20
as the surfaces of
pressure rollers 42 adjacent tangent 50 are in contact with composite 20. For
example, as a
portion of composite 20 moves through flat-bed laminator 30, an increasing
amount of
pressure is gradiently applied to composite 20 as composite 20 is initially
positioned between
pressure rollers 42. As composite 20 moves toward tangent 50 of pressure
rollers 42, greater
pressure is applied to composite 20, with the greatest pressure applied to
composite 20 when
directly between tangents 50 of pressure rollers 42. Additionally, as
composite 20 moves
past tangent 50, a decreasing amount of pressure is gradiently applied to
composite 20 until
composite 20 is no longer positioned between pressure rollers 42.
[0055] Different designs of flat-bed laminator 30 may apply different
pressures to
composite 20. For example, if pressure rollers 42 have outer surfaces
comprised of steel, the
contact footprint of pressure rollers 42 on composite 20 is relatively small
and the average
point pressure applied to composite 20 is large. However, if pressure rollers
42 have outer
surfaces comprised of elastic rubber, the contact footprint of pressure
rollers 32 on composite
20 is relatively large and the average point pressure applied to composite 20
is small.
[0056] Pressure from pressure rollers 42 is applied to composite 20 for
about 0.02
seconds to about 5 seconds. More particularly, pressure may be applied to
composite 20 for a
duration of time of as little as about 0.01 seconds, 0.50 seconds, 1.0
seconds, 1.5 seconds, 2.0
seconds, 2.5 seconds, or as great as 3.0 second, 3.5 seconds, 4.0 seconds, 4.5
seconds, 5.0
seconds, or within any range delimited by any pair of the foregoing values. In
one
embodiment, pressure may be applied to composite 20 for a timeduration of 0.01-
0.05
seconds. Additionally, because pressure rollers 42 have circular cross-
sections, the
aforementioned times signify the total time duration that composite 20
experiences pressure.
For example, using an order of magnitude calculation, if the length of the
footprint between
CA 02980540 2017-09-21
WO 2016/154102
PCT/US2016/023387
pressure rollers 42 is one cm and the line speed of flat-bed laminator 30 is 5
meters/minute,then the residence time that composite 20 experiences pressure
applied by
pressure rollers 42 is 0.12 seconds. However, because 0.12 seconds represents
the total
amount of time that composite 20 experiences pressure from pressure rollers
42, there is a
gradient of rising pressure for the first 0.06 seconds and a gradient of
decreasing pressure for
the last 0.06 seconds.As such, in the embodiment of Fig. 2, the pressure
applied by pressure
rollers 42 to composite 20 is not continuous because pressure is not applied
to composite 20
when passing through heating portion 38 and cooling portion 40. Alternatively,
flat-bed
laminator 30 may be configured to apply pressure to composite 20 as composite
20 is still
within heating portion 38 and the crystalline component has melted. However,
pressure will
not be applied to composite 20 when passing through cooling portion 40 in
order to minimize
stresses applied to composite 20 during cooling. As such, flat-bed laminator
30 is not
configured to apply continuous pressure to composite 20.
[0057] After pressure is applied to composite 20 with rollers 42,
composite 20 moves
through cooling portion 40 and then exits flat-bed laminator 30. In one
embodiment, cooling
portion 40 is configured for temperatures less than the melting temperature of
the crystalline
component of binder material 20b. For example, cooling portion 40 may be
configured for
operation at temperatures of 0 C, 5 C, 10 C, 15 C, 20 C, 25 C, 30 C, 35 C, 40
C, 45 C,
50 C, 60 C, 70 C, 80 C, 90 C or within any range delimited by any pair of the
foregoing
values, depending on the particular crystalline component included within
binder material
20b. Because the length of cooling portion 40 is approximately the same as the
length of
heating portion 38, composite 20 may be cooled for approximately the same
amount of time
it is heated. More particularly, composite 20 may be cooled for as little as
0.01 seconds, 0.50
seconds, 1.0 seconds, 1.5 seconds, 2.0 seconds, 2.5 seconds, 3.0 seconds, or
as much as 1
minute, 2 minutes, 3 minutes, 4 minutes, or 5 minutes, or any range delimited
by any pair of
the foregoing values. As composite 20 passes through cooling portion 40, it is
necessary that
composite 20 remain flat and is not bent so as to minimize stresses applied to
composite 20
during cooling. Alternatively, because heat transfer occurs rapidly in the
crystalline
component, cooling portion 40 may be eliminated from flat-bed laminator 30 if
it is possible
for composite 20 to radiate sufficient heat to its surroundings to decrease
its temperature
below the melting temperature of the crystalline component to allow the
crystalline
component to solidify.
16
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
[0058] Additionally, as detailed herein, it may be desirableto minimize
or eliminate
stresses to composite 20 when cooling below the melting temperature of the
crystalline
component of binder material 20b. Because pressure is not applied to composite
20 when
cooling, stresses are not introduced into the crystal structure of the
crystalline component.
Also, because heat transfer occurs rapidly within the crystalline component,
the discreet
portions of the crystalline component which flowed around the amorphous
component and
into any voids within fiber material 20a when the crystalline component melted
are
maintained without chemically or physically mixing with the amorphous
component. As
such, the amorphous and crystalline components remain discreet within binder
material 20b
and are incompatible with each other.
[0059] Additionally, first and second belts 32, 34 may apply a low
pressure to
composite 20 which is less than the pressure applied by rollers 42. Because
the pressure
applied by first and second belts 32, 34 is low, additional stresses are not
introduced into
composite 20 when passing through cooling portion 40. Alternatively, belts 32,
34 may not
apply any pressure to composite 20 when passing through cooling portion 40. In
one
example, first and second belts 32, 34 may apply a pressure to composite 20 of
as little as
0.01 psi, 0.05 psi, 0.10 psi, 0.15 psi, 0.20 psi, or 0.25 psi, or as great as
1.0 psi, 2.0 psi, 3.0
psi, 4.0 psi, 5.0 psi, 6.0 psi, 7.0 psi, 8.0 psi, 9.0, psi or 10.0 psi, or
within any range delimited
by any pair of the foregoing values, as composite 20 passes through heating
portion 38 and
cooling portion 40. In one embodiment, the pressure applied by first and
second belts 32, 34
is less than 0.5 psi. More particularly, the pressure applied by first and
second belts 32, 34 is
applied for a time duration which is inversely proportional to the belt speed
of flat-bed
laminator 30. In one embodiment, the residence time that pressure is applied
to composite 20
by first and second belts 32, 34 ranges from as little as 1 second, 3 seconds,
5 seconds, 7
seconds, 9 seconds, or 11 seconds, or as much as 1 minute, 2 minutes, 3
minutes, 4 minutes,
or 5 minutes, or any range delimited by any pair of the foregoing values. As
such, composite
20 may experience two distinct pressures ¨ a first low pressure applied by
first and second
belts 32, 34 when passing through heating and/or cooling portions 38, 40, and
a second
higher pressure applied by pressure rollers 42.
[0060] Referring to FIG. 3, composite 20 also may be formed with an
alternative
embodiment of flat-bed laminator 30 which is shown as flat-bed laminator 30'.
Flat-bed
laminator 30' includes first belt 32 which is rotatable in a counter-clockwise
direction about
rollers 33 and a second belt 34' which is rotatable in a clockwise direction
about a plurality of
17
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
rollers 35'. In one embodiment, first and second belts 32, 34' rotate at a
speed of 1-15
meters/second, and preferably at a speed of 3 meters/second. Illustratively,
the length of
second belt 34' is greater than the length of first belt 32 such that
composite 20 is in contact
with second belt 34' for a longer period of time than with first belt 32.
[0061] Referring still to FIG. 3, flat-bed laminator 30' further includes
heating
portion 38, cooling portion 40, and pressure rollers 42 positioned
therebetween. Based on the
location of pressure rollers 42, composite 20 is not under pressure when
moving through
heating portion 38 and cooling portion 40 but does receive a nip pressure when
passing
between rollers 42 of 10 psi, 30 psi, 50 psi, 70 psi, 90 psi, 110 psi, 130
psi, 150 psi, 170 psi,
190 psi, 210 psi, 230 psi, 250 psi, 270 psi, 290 psi, 310 psi, or within any
range delimited by
any pair of the foregoing values.
[0062] Flat-bed laminator 30' of FIG. 3 also includes at least one
coating device 44.
Illustratively, flat-bed laminator 30' includes a first coating device 44a and
a second coating
device 44b, which may be hot melt applicators or a dry coating applicator.
More particularly,
coating devices 44a, 44b are positioned upstream of heating and cooling
portions 38, 40 and
first coating device 44a is configured to apply binder material 20b contained
therein on
second belt 34' while second coating device 44b is configured to apply binder
material 20b
contained therein on top of fiber material 20a before fiber material 20a
enters passageway 36.
[0063] As shown in FIG. 3, fiber material 20a moves along a plurality of
rollers 46
toward first and second belts 32, 34'. First coating device 44a applies a
predetermined
amount of binder material 20b to second belt 34'. As such, when fiber material
20a contacts
second belt 34', the binder material 20b on second belt 34' is applied to the
lower surface of
fiber material 20a. Additionally, binder material 20b from second coating
device 44b is
applied to the top surface of fiber material 20b before fiber material 20b
contacts first belt 32.
In this way, binder material 20b is applied to both the top and bottom
surfaces of fiber
material 20a to define composite 20 before composite 20 enters passageway 36,
heating
portion 38, cooling portion 40, and pressure rollers 42.
[0064] As composite 20 enters heating portion 38, the crystalline
component of
binder material 20b melts and flows into fiber material 20a to integrate with
fiber material
20a. Pressure is applied to composite 20 by pressure rollers 42. More
particularly, and as
detailed herein with respect to flat-bed laminator 30, pressure rollers 42 may
apply a pressure
for a duration of time of as little as about 0.01 seconds, 0.50 seconds, 1.0
seconds, 1.5
seconds, 2.0 seconds, 2.5 seconds, or as great as 3.0 second, 3.5 seconds, 4.0
seconds, 4.5
18
CA 02980540 2017-09-21
WO 2016/154102
PCT/US2016/023387
seconds, 5.0 seconds, or within any range delimited by any pair of the
foregoing values.After
pressure is applied to composite 20 with rollers 42, composite 20 moves
through cooling
portion 40 and exits flat-bed laminator 30'. As such, the pressure applied by
pressure rollers
42 to composite 20 is not continuous because pressure is not applied to
composite 20 as
composite 20 passes through heating portion 38 and cooling portion 40.
[0065] Because the crystalline component of binder material 20b has a
lower melt
viscosity and lower surface energy, and may have a lower melting temperature,
than the
amorphous component, there may be greater wetting, greater displacement of
air, and greater
compaction in the crystalline component when pressure rollers 42 apply
pressure to
composite 20. As such, the presence of voids, air pockets, interstices, or
other internal
openings within the amorphous component is decreased during formation of
composite 20 in
flat-bed laminator 30, 30'. In this way, composite 20 includes a smooth
surface generally
free of voids which reduces capillary forces and the total effective surface
area of composite
20, thereby increasing the ballistic resistance of composite 20 because
environmental
conditions, such as sea water, gasoline, petroleum, solvents, and lubricants
do not penetrate
composite 20. Furthermore, besides decreasing voids at the surface of
composite 20, internal
voids, pockets, and channels within composite 20 are removed or displaced
through the
compaction of composite 20, thereby reducing the tendency for wicking of
fluids or other
infiltration. Composite 20 formed according to the aforementioned disclosure
may be used
for ballistic resistant articles and is resistant to environmental conditions
which may degrade
composite 20.
EXAMPLES
Example A: Salt Water Testing of Bimodal Binder Material
[0066] Various samples of composites were formed with varying levels of a
crystalline component within a binder material. These samples of the
composites were then
exposed to salt water for an extended period of time to determine if the
presence of the
crystalline component affected the corrosive resistance of the composites.
19
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
Formation of the Composites
[0067] To form these samples, various concentrations of a binder material
were
formed according to Table 1 to define a Comparative Example 1, an Example 1 of
composite
20, and an Example 2 of composite 20.
TABLE 1: FORMULATIONS
Cmparatwe
Amorphous Component
87.500% 65.625% 75.000%
¨ TYLAC 873
Amorphous Component
12.500 /0 12.500% 0 /0
¨ Clariant NUVA 2040
Crystalline Component ¨
Shamrock 0% 21.875% 25.00%
HYDROCERTM EC-35
[0068] The binder material was applied tofiber material comprised of
Honeywell
1150-denier SPECTRA Merge 95121 UHMWPE fiber. More particularly, using a
fiber
impregnation coater, the binder material was applied to a first unidirectional
fiber web and
the coated fiber web was dried. The dried fiber web was wound onto a roll. A
second roll of
a second unidirectional fiber web also was coated with the fiber impregnation
coater, dried,
and wound onto a roll. The first coated fiber web on the first roll was cut
into squares. The
second roll of wound fiber was installed at or near the entrance of flat-bed
laminator 30, 30'
and the second fiber web was unrolled and fed through flat-bed laminator 30,
30'. The
temperature of heating portion 38 of flat-bed laminator 30, 30' was set to a
temperature
below the melting temperature of the crystalline component. As the second
coated fiber web
began to travel into flat-bed laminator 30, 30', the squares of the first
coated fiber web were
placed on top of the second coated fiber web prior to the second fiber web
entering flat-bed
laminator 30, 30'. The fiber direction of each square of the first fiber web
was positioned in a
90-degree orientation to the fiber direction of the second fiber web.
Additionally, each
square of the first fiber web was positioned to rearwardlyabut the previous,
adjacent square
on the second fiber web to define a continuous, coated, two-ply fiber
material. This
continuous, two-ply fiber material entered flat-bed laminator 30, 30' and the
pressure applied
by rollers 42 adhered the squares of the first fiber web to the second fiber
web. However, the
pressure from rollers 42 and the heat of heating portion 38 did not melt the
crystalline
component of the binder material, if any crystalline component was present in
the binder
CA 02980540 2017-09-21
WO 2016/154102
PCT/US2016/023387
material. The two-ply fiber web formed of the cut squares of the first fiber
web and the
continuous roll of the second fiber web was then wound onto a roll after
passing through flat-
bed laminator 30, 30' and adhered together. The temperature of heating portion
38 of flat-
bed laminator 30, 30' was then increased to a temperature within the melting
point range of
the crystalline component within the binder material, if any crystalline
component was
present. The roll of the two-ply fiber material was then unrolled and the two-
ply fiber
material passed through flat-bed laminator 30, 30'. Because the temperature of
heating
portion 38 was within the melting point range of the crystalline component
with the binder
material, compaction or densification was imparted to the two-ply fiber
material when
passing through rollers 42. After passing through flat-bed laminator 30, 30',
the two-ply
fiber material was cut into squares and ballistic samples were produced by
stacking 52 layers
of the two-ply fiber material. The total areal density of each sample, or the
total weight per
area of multiple layers of the fabric, was 0.89 pounds/ft2.
Testing of the Composites
[0069] Comparative Example 1, Example 1, and Example 2 were each soaked
in salt
water at a concentration of 3.5% sea salt in tap waterfor 24 hours.
Comparative Example 1,
Example 1 and Example 2 were hung to drip dry for 15 minutes. Next,
Comparative
Example 1, Example 1 and Example 2 were each placed onto a clay block or
platform, as
disclosed further in NIJ STD 0101.06 Level III, and 357 Magnum SJHP Remington
shots
were fired at Comparative Example 1, Example 1 and Example 2 at a velocity of
1430 +/- 30
ft/sec.
TABLE 2: SALT WATER TESTING RESULTS
...............................................................................
...............................................................................
......................................................................
Exa
ipmmwoommilipp!
Comparative Example 1 ¨ Sample 1 51.3 Complete Complete
Comparative Example 1¨ Sample 2 45.1 Complete Complete
Comparative Example 1 Average 48.2 Complete Complete
Example 1¨ Sample 1 41.9 39.3 49.5
Example 1 ¨ Sample 2 44.7 43.2 42.5
Example 1 Average 43.3 41.3 46.0
Example 2 ¨ Sample 1 50.4 46.1 45
Example 2 ¨ Sample 2 53.8 49.5 50.9
Example 2 Average 52.1 47.8 48.0
21
CA 02980540 2017-09-21
WO 2016/154102
PCT/US2016/023387
[0070] Each of the two samples of Comparative Example 1, Example 1 and
Example
2 were each shot three times with 357 Magnum SJHP Remington bullets to
determine the
depth each bullet penetrated into each sample. As shown in Table 2, Shots 2
and 3 on each
sample of Comparative Example 1 fully penetrated the composite, as indicated
by
"Complete" in Table 2. However, none of the samples of Example 1 or Example 2
were fully
penetrated. More particularly, the bullets penetrated the least into the
samples of Example 1,
which contained a fluorocarbon amorphous component and carnauba wax
crystalline
component.
Example B: Bimodal Binder Material with Varying Concentrations of Amorphous
and
Crystalline Components
[0071] Various samples of composites were formed with varying levels of a
crystalline component within a binder material. These samples of the
composites were then
shot with 9mm bullets to determine if the presence of the crystalline
component affected the
ballistic resistance of the composites.
Formation of the Composites
[0072] To form these samples, various concentrations of a binder material
were
formed according to Table 3 to define a Comparative Example 1, an Example 1 of
composite
20, an Example 2 of composite 20, and an Example 3 of composite 20. The
coating and
composite processing conditions for forming these samples are identical to
those of Example
1 above.
TABLE 3: FORMULATIONS
Coni!1!1!1!1!1!1!1!1!pm[c.4r1f9#1.potIggiExample 1
Mpl!le 1!1!1!1!1!Im.4.0p!I2., !1!1!1!1!1!KINmqp!pq15
Amorphous Component
87.500% 65.625% 52.000% 74.374%
¨ Tylac 873
Amorphous Component
12.500% 12.500% 12.500% 12.500%
¨ Clariant Nuva 2040
Crystalline Component¨
0% 21.875% 35.00%
13.125%
Shamrock EC-35
22
CA 02980540 2017-09-21
WO 2016/154102
PCT/US2016/023387
Testing of the Composites
[0073] The samples of Comparative Example 1 and Examples 1-3 were each
placed
onto a clay block or platform, as disclosed further in NIJ STD 0101.06 Level
III, and 9 mm
shots were fired at each sample of Comparative Example 1 and Examples 1-3 at
varying
velocities shown in Table 4. More particularly, Table 4 provides a theoretical
velocity, V50,
at which 50% of the bullets stopped within Comparative Example 1 and Examples
1-3 and
50% of the bullets completely penetrated Comparative Example 1 and Examples 1-
3. For
example, to determine the V50 velocity, a plurality of shots were fired at
each sample of
Comparative Example 1 and Examples 1-3 at varying velocities to determine the
velocity
range at which a bullet completely penetrated the sample and a velocity range
at which a
bullet partially penetrated a sample. These shot groupings on each sample
underwent
statistical analysis to determine the V50 velocity for each sample of
Comparative Example 1
and Examples 1-3 tested.
TABLE 4: 9mm FMJ V50 TESTING RESULTS
Comparative Example 1 ¨ Sample 1 1539
Comparative Example 1 ¨ Sample 2 1632
Comparative Example 1 Average 1586
Example 1 ¨ Sample 1 1722
Example 1 ¨ Sample 2 1798
Example 1 Average 1760
Example 2 ¨ Sample 1 1665
Example 2 ¨ Sample 2 1716
Example 2 Average 1691
Example 3 ¨ Sample 1 1755
Example 3 ¨ Sample 2 1744
Example 3 Average 1750
[0074] As shown in Table 4, Comparative Example 1, which was not a
bimodal
binder, had the lowest V50 velocity compared to Examples 1, 2, and 3. As such,
the samples
of Examples 1-3 were able to withstand bullets shot at higher velocities
without the bullet
fully penetrating the sample. Additionally, Example 1, which contained 15% of
the
crystalline component, and Example 3, which contained 40% of the crystalline
component,
each had similar V50 velocities.
23
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
Example C: Bimodal Binder Material with Varying Crystalline Components
[0075] Various samples of composites were formed with varying levels of
a
crystalline component within a binder material. These samples of the
composites were then
shot with 9mm bullets to determine if the presence of the crystalline
component affected the
ballistic resistance of the composites.
Formation of the Composites
[0076] To form these samples, various concentrations of a binder
material were
formed according to Table 3 to define a Comparative Example 1, an Example 1 of
composite
20, an Example 2 of composite 20, an Example 3 of composite 20, an Example 4
of
composite 20, an Example 5 of composite 20, and an Example 6 of composite 20.
The
coating and composite processing conditions for forming these samples are
identical to those
of Example 1 above.
TABLE 5: FORMULATIONS
Comp
Emm;ion
eimeingile
Amorphous
Component¨ 87.500% 65.625% 65.625% 65.625% 65.625% 65.625% 65.625%
Tylac 873
Amorphous
Component¨
12.500% 12.500% 12.500% 12.500% 12.500% 12.500% 12.500%
Clariant
Nuva 2040
Crystalline
Component¨
0% 21.875% 0% 0% 0% 0% 0%
Shamrock
EC-35
Crystalline
Component¨ 0%
0% 21.875% 0% 0% 0% 0%
Michelman
LL405
Crystalline
Component¨ 0%
0% 0% 21.875% 0% 0% 0%
Michelman
LL411
Crystalline
Component¨ 0%
0% 0% 0% 21.875% 0% 0%
Michelman
454
24
CA 02980540 2017-09-21
WO 2016/154102
PCT/US2016/023387
Crystalline
Component¨
0% 0% 0% 0% 0% 21.875% 0%
Michelman
HL-480
Crystalline
Component¨
0% 0% 0% 0% 0% 0% 21.875%
Michelman
ME98040
Testing of the Composites
[0077] Each
sample of Comparative Example 1 and Examples 1-6 were each placed
onto a clay block or platform, as disclosed further in NIJ STD 0101.06 Level
III, and 9 mm
shots were fired at each sample of Comparative Example 1 and Examples 1-6 at
varying
velocities shown in Table 6. More particularly, Table 6 provides a theoretical
velocity, V50,
at which 50% of the bullets stopped within Comparative Example 1 and Examples1-
6 and
50% of the bullets completely penetratedComparative Example 1 and Examples 1-
6. For
example, to determine the V50 velocity, a plurality of shots were fired at
each sample of
Comparative Example 1 and Examples 1-6 at varying velocities to determine the
velocity
range at which a bullet completely penetrated the sample and a velocity range
at which a
bullet partially penetrated a sample. These shot groupings on each sample
underwent
statistical analysis to determine the V50 velocity for each sample of
Comparative Example 1
and Examples 1-6 tested.
TABLE 6: 9mm FMJ V50 TESTING RESULTS
Example V11
Comparative Example 1 ¨ Sample 1 1539
Comparative Example 1 ¨ Sample 2 1632
Comparative Example 1 Average 1586
Example 1 ¨ Sample 1 1665
Example 1 ¨ Sample 2 1716
Example 1 Average 1691
Example 2 ¨ Sample 1 1765
Example 2 ¨ Sample 2 1739
Example 2 Average 1752
Example 3 ¨ Sample 1 1774
Example 3 ¨ Sample 2 1768
Example 3 Average 1771
Example 4 ¨ Sample 1 1688
Example 4 ¨ Sample 2 1586
CA 02980540 2017-09-21
WO 2016/154102 PCT/US2016/023387
Example 4 Average 1637
Example 5 ¨ Sample 1 1757
Example 5 ¨ Sample 2 1734
Example 5 Average 1746
Example 6 ¨ Sample 1 1733
Example 6 ¨ Sample 2 1728
Example 6 Average 1731
[0078] As shown in Table 5, Comparative Example 1, which was not a
bimodal
binder, had the lowest V50 velocity compared to Examples 1-6. As such, the
samples of
Examples 1-6 were able to withstand bullets shot at higher velocities without
the bullet fully
penetrating the sample. Additionally, Example 3, which contained
microcrystalline wax, had
the greatest V50.
[0079] While the present disclosure has been particularly shown and
described with
reference to preferred embodiments, it will be readily appreciated by those of
ordinary skill in
the art that various changes and modifications may be made without departing
from the spirit
and scope of the invention. It is intended that the claims be interpreted to
cover the disclosed
embodiment, those alternatives which have been discussed above and all
equivalents thereto.
26