Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02990420 2017-12-20
WO 2017/003826 1
PCT/US2016/039090
MEDICATION TRACKING SYSTEM AND METHOD USING HYBRID
ISOLATED MAGNETIC DIPOLE PROBE
The invention generally relates to a system and method for providing energy to
a
container to activate devices in the containers to respond with identification
data, and more
particularly, to a system and method that provides energy having magnetic
field
dominance in the near field of the activation energy to activate wireless
devices, and that
also includes directional energy control.
BACKGROUND
Medications and other medical articles designated for certain patients,
whether
prescription or over-the-counter, are often stored in cabinets that may or may
not be
refrigerated. Accurate inventory tracking of medical articles is imperative to
be sure that
the needed medical articles are where they should be, that there are enough of
them, and
when used, that they are accounted for. Other reasons for tracking medical
articles include
monitoring expiration dates, recalls, and various other factors. Detection of
supply
depletion is also a purpose of tracking medical articles. Such cabinets may
consist of
refrigerators ranging in size from quite small to quite large, to non-
refrigerated cabinets, to
cabinets having a plurality of stacked drawers, to single trays each of which
has a
predeteimined collection of medical articles. Containers may be locked or
unlocked.
Locked containers may include electrically-controlled mechanical locks that
are opened by
matching the identification of a user with authorized users contained in a
database. Other
containers for storing or transporting medical articles are encountered in a
healthcare
environment.
In another system becoming more in demand, medical articles are tracked from
the
manufacturer's facility to delivery at the healthcare facility, and all
through the healthcare
facility until the medical articles are either administered to a patient or
disposed of in some
other way.
An important use of such wireless tracking systems is to be sure that the
correct
patient receives the correct medication. Positively identifying the patient
with an
identification device, positively identifying a medication with a wireless
tracking device,
and using a database that ties the two together can be a highly effective
system in avoiding
medication errors.
CA 02990420 2017-12-20
WO 2017/003826 2
PCT/US2016/039090
Among the current systems being used for the tracking of items, the barcode
tracking system is wireless and has advantages. Wireless barcode tracking
systems
continue to present a useful alternative, especially in retail stores and
other areas where use
of a line of sight reader does not present a problem. However in the
healthcare field where
medical cabinets are used, a line of sight system is less preferable. Some
cabinets store
many medical articles and reading each one by scanning it with a barcode
reader can
involve too much time for busy healthcare personnel. Instead, a wireless
system that does
not require a line of sight tracking system to identify medical articles would
be preferable.
In the healthcare field, a radio frequency identification ("RFID") tracking
system
has been found to excel. The RFID system does not require line of sight to
make the
identification. RFID systems typically include RFID stickers or labels, i.e.,
a sticker or
label that includes an RFID tag, affixed to the inventory item, e.g., bottles,
vials, boxes,
syringes, bandages, etc. In a predominant system available today, each RFID
tag has a
unique identification number.
Each medical article has an RFID tag attached and the identification number of
the
RFID tag is entered into a database and correlated with the name of the
medical article to
which the tag is attached. A processor programmed to read the database matches
that
RFID tag identification number to the medical article to which it was
originally attached so
that the particular medical article can be determined to be present in the
container. The
database often includes an array of the data regarding the medical article to
which the
RFID tag is attached, such as the name, dose, manufacture date, expiration
date,
temperature requirements, and other data.
In another embodiment, the RFID tag itself has a programmable memory that can
be programmed with identifying data about the nature of the very medical
article to which
it is attached thereby immediately identifying the medical article without the
need to refer
to a database. EPC Gen2/ISO 18000-63 standard RFID tags are available in many
different configurations. Some of these tags are delivered preprogrammed with
a 48-bit
read-only write-protected unique ID. These preprogrammed tags with a unique ID
are the
same price as those tags that do not have a preprogrammed unique ID. This
system also
has advantages.
CA 02990420 2017-12-20
WO 2017/003826 3
PCT/US2016/039090
RFID tags may be incorporated into or attached to articles to be tracked. In
some
cases, the tag may be attached to the outside of an article with adhesive,
tape, or other
means and in other cases, the tag may be inserted within the article, such as
being included
in the packaging, located within the container of the article, or sewn into a
garment. Some
RFID tags are manufactured with a unique identification number which is
typically a
simple serial number of a few bytes with a check digit. In some cases, no
check digit is
stored. the error correction codes are generated on the fly by the RFID tag
and reader.
This identification number is incorporated into the tag during manufacture.
The user
cannot alter this serial/identification number and manufacturers guarantee
that each serial
number is used only once. This configuration represents the low cost end of
the
technology in that the RFID tag is read-only and it responds to an
interrogation signal only
with its identification number. Typically, the tag continuously responds with
its
identification number. Data transmission to the tag is not possible. These
tags are very
low cost and are produced in enomious quantities. There are no EPC Gen2/ISO
18000-63
standard based RFID tags currently available in the configuration described
above. The
simplest RFID tags conforming to these standards include a minimum of 96 bits
of
programmable memory.
Such read-only RFID tags typically are permanently attached to an article to
be
tracked and, once attached, the serial number of the tag is associated with
its host article in
.. a computer database. For example, a particular type of medicine may be
contained in
hundreds or thousands of small vials. Upon manufacture, or receipt of the
vials at a health
care institution, an RFID tag is attached to each vial. Each vial with its
permanently
attached RFID tag will be checked into the database of the health care
institution upon
receipt. The RFID identification number may be associated in the database with
the type
of medicine, size of the dose in the vial, and perhaps other infoimation such
as the
expiration date of the medicine. Thereafter, when the RFID tag of a vial is
interrogated
and its identification number read, the database of the health care
institution can match that
identification number with its stored data about the vial. The contents of the
vial can then
be determined as well as any other characteristics that have been stored in
the database.
This system requires that the institution maintain a comprehensive database
regarding the
articles in inventory rather than incorporating such data into an RFID tag.
CA 02990420 2017-12-20
WO 2017/003826 4
PCT/US2016/039090
An object of the tag is to associate it with an article throughout the
article's life in a
particular facility, such as a manufacturing facility, a transport vehicle, a
health care
facility, a storage area, or other, so that the article may be located,
identified, and tracked,
as it is moved. For example, knowing where certain medical articles reside at
all times in a
health care facility can greatly facilitate locating needed medical supplies
when
emergencies arise. Similarly, tracking the articles through the facility can
assist in
generating more efficient dispensing and inventory control systems as well as
improving
work flow in a facility. Additionally, expiration dates can be monitored and
those articles
that are older and about to expire can be moved to the front of the line for
immediate
dispensing. This results in better inventory control and lowered costs.
RFID tags may be applied to containers or articles to be tracked by the
manufacturer, the receiving party, or others. In some cases where a
manufacturer applies
the tags to the product, the manufacturer will also supply a respective
database file that
links the identification number of each of the tags to the contents of each
respective article.
That manufacturer supplied database can be distributed to the customer in the
form of a
file that may easily be imported into the customer's overall database thereby
saving the
customer from the expense of creating the database.
Many RFID tags used today are passive in that they do not have a battery or
other
autonomous power supply and instead, must rely on the interrogating energy
provided by
an RFID reader to provide power to activate the tag. Passive RFID tags require
an
electromagnetic field of energy of a certain frequency range and certain
minimum intensity
in order to achieve activation of the tag and transmission of its stored data.
RFID tags may
be activated by electric field energy and by magnetic field energy. Another
choice is an
active RFID tag; however, such tags require an accompanying battery to provide
power to
activate the tag, thus increasing the expense of the tag and making them
undesirable for
use in a large number of applications.
Depending on the requirements of the RFID tag application, such as the
physical
size of the articles to be identified, their location, and the ability to
reach them easily, tags
may need to be read from a short distance or a long distance by an RFID
reader. Such
distances may vary from a few centimeters to ten or more meters. Additionally,
in the
U.S. and in other countries, the frequency range within which such tags are
peimitted to
operate is limited. As an example, lower frequency bands, such as 125 KHz and
13.56
CA 02990420 2017-12-20
WO 2017/003826 5
PCT/US2016/039090
MHz, may be used for RFID tags in some applications. At this frequency range,
the
electromagnetic energy ("EM") is less affected by liquids and other dielectric
materials,
but suffers from the limitation of a short interrogating distance. At higher
frequency bands
where RFID use is pennitted, such as 915 MHz and 2.4 GHz, the RFID tags can be
interrogated at longer distances, but they de-tune more rapidly as the
material to which the
tag is attached varies. It has also been found that at these higher
frequencies, closely
spaced RFID tags will de-tune each other as the spacing between tags is
decreased.
Providing an internal RFID system in such a cabinet can pose challenges. Where
internal articles can have random placement within the cabinet, the RFID
system must be
such that there are no "dead zones" that the RFID system is unable to reach.
In general,
dead zones are areas in which the level of coupling between an RFID reader
antenna and
an RFID tag is not adequate for the system to perform a successful read of the
tag. The
existence of such dead zones may be caused by orientations in which the tag
and the reader
antennae are in orthogonal planes. Thus, articles placed in dead zones may not
be detected
thereby resulting in inaccurate tracking of tagged articles. Fresnel zones
(null energy and
high energy regions) occur when reflected RF energy collides with transmitted
energy or
other reflected energy waves. The most common null energy region (dead zone)
occurs
when reflected energy collides with transmitted energy at ninety degrees out
of phase.
Often in the medical field, there is a need to read a large number of tags
attached to
articles in such an enclosure, and as mentioned above, such enclosures have
limited access
due to security reasons. The physical dimension of the enclosure may need to
vary to
accommodate a large number of articles or articles of different sizes and
shapes. In order
to obtain an accurate identification and count of such closely-located medical
articles or
devices, a robust electromagnetic energy field must be provided at the
appropriate
frequency within the enclosure to surround all such stored articles and
devices to be sure
that their tags are all are activated and read. Such medical devices may have
the RFID tags
attached to the outside of their containers and may be stored in various
orientations with
the RFID tag (and associated antenna) pointed upwards, sideways, downward, or
at some
other angle in a random pattern.
Generating such a robust EM energy field is not an easy task. Where the
enclosure
has a size that is resonant at the frequency of operation, it can be easier to
generate a robust
EM field since a resonant standing wave may be generated within the enclosure.
CA 02990420 2017-12-20
WO 2017/003826 6
PCT/US2016/039090
However, in the RFID field the usable frequencies of operation are strictly
controlled and
are limited. The U.S. FCC, and other national authorities around the world,
have
established regulations that define the frequency bands in which wireless
systems (RFID,
WiFiTM, Bluetooth, etc.) can operate license free. The UHF band in the U.S.
extends from
902.5 MHz to 928.0 MHz and was selected for RFID technology because of the low
attenuation of this frequency in free space (i.e., it provides the longest
read distance and
therefore is ideal for supply chain management). It has been found that
enclosures are
desired for the storage of certain articles that do not have a resonant
frequency matching
one of the allowed RFID frequencies. Thus, a robust EM field must be
established in
another way.
Once activated, the RFID tags transmit their respective identifications that
are
received by a receive antenna and conducted to an RFID reader to determine
their presence
in the container. This is commonly referred to as reading the RFID tag. In
order to read
the tags, an injection probe or probes are placed within a storage cabinet
along with a
receive antenna or antennas. In another embodiment, the injection probe and
receive
antenna are the same device and both functions are accomplished by switching
the device
between an energy injection mode and an energy receive mode. The receive
antennas are
interfaced with the RFID reader, which can be permanently mounted at the
cabinet. The
system sends activating energy, also known as interrogation signals, via the
injection probe
which emits that activating energy in the storage container. The activating
energy is strong
enough (also described as having a high enough power level) to activate the
passive RFID
tags. Those activated tags then respond with their stored data. The receive
antennas
receive the responsive data from the RFID tags and this data is forwarded to
the RFID
reader.
In the healthcare environment, cabinets enabled with RFID tracking systems
employ a Faraday cage, which is a conductive chamber completely surrounding
the
container area. The Faraday cage prevents the RFID tracking system located
inside the
container from reading RFID tags outside the container area which would cause
an error.
The Faraday cage also preserves the RF energy within the enclosure for use in
identifying
RFID tags.
Various problems exist with RFID tag activation in an enclosed space. As
discussed above, there are often nulls or dead spaces or dead zones in which
tags will not
CA 02990420 2017-12-20
WO 2017/003826 7
PCT/US2016/039090
receive enough RF activating energy to be activated. Placing many RFID
activation
probes throughout the enclosed container will increase the chances of
activating all RFID
tags, but at the cost of more wires, probes, and antennas. Use of a large
quantity of
antennas also results in larger enclosures and increased read process time.
Increasing the
power level in the container may help but there are limits imposed by FCC on
the power
level. For example, in the U.S., a maximum transmit power of 4 watts (EIRP -
equivalent
isotopically radiated power) is allowed. Additionally, power levels that are
too high may
increase the chances of reading RFID tags located outside the container even
though the
Faraday cage exists. It has been found that Faraday cages used as containers
that must
allow access may leak the activation energy outside the container and the
tracking system
may detect RFID tags on medical articles located outside the container thereby
causing a
tracking error.
Another problem is the effect that liquids have on an RFID reader. Liquids may
actually absorb the activation energy resulting in the failure to activate and
RFLD tag.
Other errors are caused by tags next to each other (tags positioned in close
proximity to or
directly against one another) detuning each other such that they are not
activated by the RF
activation field. Many such conventional designs can suffer from poor results
obtained
due to the static nature (tag positions are fixed) of the interrogations. In
an application
where the field is static, a tag may lie in a RF null created by multipath,
resulting in a
failed interrogation.
Further, many conventional solutions use the traditional combined
transmit/receive
antenna configuration. In this arrangement, a single wireless EM conduction
device
operates as both a transmit antenna and as a receive antenna. This
configuration works
well in traditional applications where the RFID reader antenna radiates into
open space and
objects are in the far-field region of the antenna for minimum RFID reader
antenna
detuning. Far-field is described as a boundary region where the angular field
distribution
of the antenna is essentially independent of distance from the source.
However, in
applications where the RFID tags to be read are in the interior space of a
container that is
within the near-field of the transmitting device, problems can arise.
Reflections of the
transmitted energy can establish the null zones within that container. For
purposes of
discussion herein, the wireless EM conduction device for the interior of a
container is
referred to as an "injection probe" because it is injecting activating RF
energy into a closed
CA 02990420 2017-12-20
WO 2017/003826 8
PCT/US2016/039090
space. In a case such as this, i.e., where the target space is closed, the
traditional combined
transmit/receive antenna approach and combined transmit and receive systems
can
encounter problems in activating an RFID tag that falls within a null zone.
As RFID tagged products enter the RFID reader antenna's near-field region, it
has
an adverse effect on the RFID reader's antenna tuning resulting in reduced
RFID reader
receiver sensitivity. This results in RFID reader antenna detuning and
presents a challenge
for the RFID reader's receiver in terms of energy reflected back into the RFID
reader
receiver competing with energy reflected back by the tagged items. Still
further, RF signal
propagation in contained environments is not well defined, with huge amplitude
variations
in resonant versus null locations within a drawer or chamber. When RFID tags
are placed
in a chamber's null locations, the tags cannot be powered and cannot be
read/interrogated,
ultimately causing errors in tracking medical articles.
Another problem exists when a tag is in its minimum field strength (such as
between two transmitting antennas) with respect to its ability to turn on and
participate in
the interrogation. When this occurs the RFID reader may be unable to detect
the tags' faint
responses resulting in a failed interrogation. This is a common problem in a
high
product/tag density application where high concentration of items exists
within the RF Tx
and Rx paths. A similar problem with conventional solutions occurs when the
items being
tracked include large amounts of liquids. Conventional RFID cabinet systems
typically
use the electric field to communicate to passive RFID tags. Depending on the
frequency
used, some frequencies can be greatly attenuated by liquid items within the
container
resulting in a failed interrogation due to insufficient field strength. To
lessen such effects,
some manufacturers use larger RFID tags so that they will be more immune to
detuning
caused by a large number of tags located near each other. Also, it is thought
that larger
tags somewhat overcome the detuning of liquids. However, larger tags result in
difficulty
of handling the medications. This is discussed further below.
The above cause great difficulties for those RFID systems that are designed
and
developed to track RFID tags on items in the near field (distances less than
approximately
one wavelength from the antenna). The wavelength for electromagnetic energy of
915
MHz is 12.91 inches (32.77 cm), which is typical for RFID-enabled enclosures
employed
for storing medication, such as drawer systems, metal cabinets, refrigerators,
and freezers.
Integrating antenna systems in metallized or shielded enclosures used for
tracking stored
CA 02990420 2017-12-20
WO 2017/003826 9
PCT/US2016/039090
items tagged with RFID tags or smart labels, such as refrigerators, freezers,
drawers,
cabinets, et., presents challenges due to the large amounts of energy that
reflect off the
enclosure walls and any metallized element inside the enclosure. Irrespective
of energy
reflections inside of a metallized enclosure it is difficult to set up
electromagnetic waves in
volume-restricted metallized enclosure, especially those enclosures that are
non-resonant at
the frequency of interest.
FIG. 3 shows what is called an RFID "flag" tag. The tag, which has commonly
been in use for many years, includes a "flag" portion 72 on which is mounted
an RFID
device 76, and a mounting portion 74 that comprises a clear base on which a
layer of clear
adhesive is deposited. The mounting portion is adhered to the vial of
medication for
example. Because the mounting portion is clear, the label 75 placed on the
vial by the
manufacturer can be read through the mounting portion thus not obscuring
expiration
dates, dose size, name of the medication, name of the patient, and any other
data placed on
the vial. The commonly-used flag tags that are relatively large and
consequently
unwieldy. They take up excessive space in a storage container, interfere with
each other
during handling, and are difficult to handle. These more common tags are in
widespread
use because they contain a much larger RFID tag coupling device (antenna).
This is
necessary for many manufacturers of tracking systems because the larger-sized
RFID tag
coupling devices are able to collect more activating RF energy in those
tracking systems
that are inefficient and have dead zones or "weak zones." FIG. 4 on the other
hand shows
the smaller-sized RFID flag tags that are preferable. Medications on which
such smaller-
sized tags are mounted are easier to handle, take less room, and are easier to
store in
containers. Even though the users of RFID tracking systems prefer the smaller
RFID tags,
many manufacturers cannot use them because they will not be activated with
their RFID
tracking systems and tracking errors will result.
Typical antennas used in RFID applications are microstrip antennas, patch
antennas, and wire-based antennas. Although these types of antennas perform
well for far
field applications, they can generate null areas or regions, or low power
(weak) areas or
regions of activating RF energy at localized points in the near field due to
the large
aperture or effective area of the antenna. In addition, these antennas radiate
an energy
wave that is more linear than circular, which can result in loss of RFD tag
interrogation
energy due to the tag being cross polarized when positioned in the near field.
CA 02990420 2017-12-20
WO 2017/003826 10
PCT/US2016/039090
Tracking small form factor medications in small non-resonant enclosures
requires
smaller RFID tag sizes in order for the tagged items to fit easily into a tray
or drawer
pockets without impeding the loading of medications, dispensing of
medications, or the
opening and closing action of the drawer, container, or enclosure in which the
medications
are stored. Certain small form-factor RFID tags that operate in the 915 MHz
industrial,
scientific, and medical ("ISM") radio bands include both a magnetic antenna
loop/feature
and a folded dipole so that both magnetic and electrical field energy can be
harvested to
operate the RFID tag. By definition, the RFID tags attached to small
medication form
factors stored in small non-resonant enclosures will be in close proximity
(near field at 915
MHz) to the activating RF energy injection probes.
Certain antennas do not perform well under the difficult conditions of a
relatively
small container. For example, thin profile microstrip antennas have narrow
bandwidth and
poor radiation efficiency with a lossy substrate and therefore these planar
patch antennas
are not a good choice for a low cost solution. Additionally, a relatively
large size of a
microstrip antenna is required for performance at a frequency of around 900
MHz which
makes it undesirable in most applications where space is at a premium.
Hence, those of skill in the art have identified a need for using a much
smaller
RFID flag tag on medical articles to be stored in a container, for using less
power to
activate all RFID tags in a container, and for having a much higher success
rate of tag
activation and reading. The present invention fulfills these needs and others.
SUMMARY OF THE INVENTION
Briefly and in general terms, the present invention is directed to a system
and
method for activating and reading RFID tags located in non-resonant enclosures
using a
hybrid IMD probe to inject electromagnetic energy ("EM") for powering and
interrogating
the RFID tags. The IMD probe can be employed as a passive injector of EM or
combined
with dynamic impedance matching and/or beam steering capability.
In accordance with an aspect of the invention, a probe is provided having a
capacitively-loaded inductive loop that sets up a magnetic dipole mode and
provides a
magnetic near field that is as strong or stronger than the electric near
field. Another aspect
is that the capacitively-loaded loop effectively provides a self-resonant
structure that is
decoupled from the local environment.
CA 02990420 2017-12-20
WO 2017/003826 11
PCT/US2016/039090
In an additional aspect, a probe is provided with an RFID reading system that
comprises a capacitively coupled inductive loop causing electrical currents to
be strongly
localized in the probe region and thereby not propagating to the ground.
Fringing
electrical currents are minimized resulting in a large magnetic near field
component that is
much more likely to activate RFID tags within the radiation field of the
probe.
In other aspects, there is provided a tracking system for tracking medical
articles
stored in the interior volume of a container, the interior volume of the
container having a
size selected to receive a plurality of medical articles each of which has a
wireless
identification device associated therewith that has individual identification
data, and each
wireless identification device configured to respond with identification data
upon receiving
activation energy, the interior volume of the container having a resonant
frequency that is
different from a frequency of operation of the wireless identification device,
the system
comprising, electromagnetic shielding ("EM") located about the interior volume
of the
container, an electromagnetic energy conducting probe located within the EM
shielding,
the probe having a radiation pattern directed to the interior volume of the
container,
wherein the probe comprises a main conductive element having capacitive
coupling across
at least one slot of the main conductive element thereby forming an isolated
electric field
that fills the interior of the container, and wherein the main conductive
element is spaced
apart from a ground plane by a selected distance thereby fonning a robust
magnetic field
that is orthogonal to the electric field and that also fills the interior of
the container, a
signal source producing activating RF energy having a frequency that is
different from the
resonant frequency of the interior volume of the container, and coupled to the
probe, and a
processor connected with the signal source, the processor being programmed to
control the
signal source to deliver RF energy to the probe for injecting into the
interior of the
.. container to activate identification devices in the interior, the processor
further being
programmed to stop the signal source from delivering RF energy to the probe to
allow the
probe to receive identification signals from activated identification devices
in the interior.
In accordance with further aspects, the probe comprises a hybrid isolated
magnetic
dipole device in which the electric and magnetic fields are circularly
polarized. The probe
includes a parasitic element located at a selected position in relation to the
main
conductive element such that the parasitic element alters the direction of the
radiation
pattern. The probe includes a controllable active tuning element connected
with the
CA 02990420 2017-12-20
WO 2017/003826 12
PCT/US2016/039090
parasitic element to alter the effect of the parasitic element on the main
conductive element
to controllably change the direction of the radiation pattern.
In yet a further aspect, the medical article tracking system further comprises
a dual
probe circuit in which a plurality of probes are co-located and positioned in
relation to
each other to provide multiple radiation patterns into the interior volume.
In an additional aspect, the medical article tracking system further comprises
an
active tuned impedance matching circuit connected with the probe that controls
impedance
of the probe to more closely match the impedance of the interior volume of the
container
whereby increased efficiency in electromagnetic energy transfer into the
interior of the
container results.
In accordance with method aspects, there is provided a method for tracking
medical
articles stored in the interior volume of a container, the interior volume of
the container
having a size selected to receive a plurality of medical articles each of
which has a wireless
identification device associated therewith that has individual identification
data, and each
.. wireless identification device configured to respond with identification
data upon receiving
activation energy, the interior volume of the container having a resonant
frequency that is
different from a frequency of operation of the wireless identification device,
the method
comprising shielding the interior volume of the container from the passage of
electromagnetic ("EM") energy, injecting activating RF energy into the
interior volume in
a radiation pattern with a probe that comprises a main conductive element
having
capacitive coupling across at least one slot of the main conductive element
thereby
forming an isolated electric field that fills the interior of the container,
and wherein the
main conductive element is spaced apart from a ground plane by a selected
distance
thereby forming a robust magnetic field that is orthogonal to the electric
field and that also
fills the interior of the container, delivering activating RF energy to the
probe from a signal
source, the activating energy having a frequency that is different from the
resonant
frequency of the interior volume of the container, and controlling the signal
source to
deliver the activating energy to the probe for injection into the interior
volume, and
controlling the signal source to stop delivering activating energy to the
probe so that the
.. probe may then receive responsive identification signals from activated
identification
devices.
CA 02990420 2017-12-20
WO 2017/003826 13
PCT/US2016/039090
The features and advantages of the invention will be more readily understood
from
the following detailed description that should be read in conjunction with the
accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a view of an automated dispensing cabinet ("ADC") having multiple
drawers in which articles are stored, the ADC having an article tracking
system and a
built-in computer configured for tracking the stored articles by processing
data regarding
articles put into the ADC and removed from the ADC, and communicating over one
or
more networks;
FIG. 2 is a view of a 2.3 ft3 refrigerated cabinet in which medical articles
are
stored, and tracked, the cabinet in this case having a keypad and a display on
the front door
for interfacing with the programming of a processor in the cabinet;
FIG. 3 is a view of a medication vial having a commonly-used RFID "flag tag"
attached thereto, the flag tag in this diagram has a relatively large size
that makes the vial
bulky and can interfere with the handling of articles and storing the articles
in a container;
FIG. 4 is a view of the same medication vial of FIG. 3 but in this figure, a
compact
RFID flag tag is attached, the compact flag tag being much smaller than that
of the
previous figure due to the smaller size of the RF energy coupling device used
in the RFID
tag mounted thereon;
FIG. 5 is a schematic block diagram of an RFID tracking system comprising an
RFID reader positioned for scanning the interior of an article storage
container, and having
two separate RF energy conducting devices acting in one mode as RF probes for
injecting
activating RF energy into the container, and operating in a second mode as RF
probes for
receiving the RF responses of the activated RFID tags attached to articles
stored in the
container, the RF probes connected to conduct the RFID tag responses through a
receiver
to extract data and then to the processor of the RFID reader for further
processing; the
processor of the reader also programmed for frequency control over the signal
generator
for providing frequency hopping and timing control of the activating RF energy
injected
into the storage container by the RF probes;
CA 02990420 2017-12-20
WO 2017/003826 14
PCT/US2016/039090
FIG. 6 is a perspective view of a larger refrigerated cabinet, in this case a
12 ft3
cabinet, with the front door open showing an embodiment of the placement of
multiple RF
probes in the cabinet for tracking medical articles put into, stored, and
taken out of or
removed from the cabinet;
FIG. 7 is a top view of a hybrid isolated magnetic dipole ("IMD") probe used
for
injecting activating RF energy into a container to activate RFID tags stored
therein to
respond with their individual identification data, the hybrid IMD probe having
a single
main element conductor located parallel to and distanced away from a circuit
board with at
least one slot in the single conductor for capacitive coupling that
establishes a robust, but
isolated, electric field in a container, and the spacing of the single main
element above the
circuit board to also establish a robust magnetic field in a container, a
dynamic impedance
matching device is shown located next to the main element and connected
thereto for
matching the impedance of the main element to the impedance of the container,
and two
parasitic elements;
FIG. 8 is a perspective view of the hybrid IMD probe of FIG. 7 depicting the
electric near field and the magnetic near field created by the probe, further
showing the
relative locations of the parasitic elements in relation to the main
conducting element of
the probe, wherein the parasitic element located beside the main element
functions to steer
the radiation pattern of the probe,
FIG. 9 is a perspective view of a hybrid IMD probe having two parasitic
elements
each with an active tuning element and a third active tuning element under the
main
conductive element of the probe, wherein the first and second parasitic
elements and all
three active tuning elements are usable to control the energy pattern, or
beam, of the main
IMD element in the internal storage area of the storage container;
FIG. 10 is a perspective view of a hybrid IMD probe similar to that of FIGS. 7
and
8 but lacking a parasitic element under the main conducting slotted element of
the probe;
FIG. 11 is a top view of a dual hybrid IMD probe circuit board with two hybrid
IMD probes located at ninety degrees from each other, to establish eight
separate and
selectable radiation patterns or beams for providing activating RF energy to a
container to
activate RFID tags locate on articles in the container;
CA 02990420 2017-12-20
WO 2017/003826 15
PCT/US2016/039090
FIG. 12 is another embodiment of a hybrid IMD device having the same main
conductive element as the IMD devices above, but being mounted orthogonally to
the
circuit board;
FIG. 13 is a perspective view of another embodiment of a hybrid IMD probe in
which the main conducting element has two slots for capacitive coupling, and
also
showing a parasitic element with an associated active tuning element for
providing
selectable beams from the probe;
FIG. 14 is a block diagram of the control over a hybrid IMD probe including
its
parasitic elements in injecting RF activating energy into a cavity or
container to activate
RFID tags in the container;
FIG. 15 is a perspective view of a code tray showing a single level of various
medical articles, each of which has an attached RFID tag, and showing a paper
with an
expiration date printed thereon indicating the earliest date of expiration
when one or more
of the stored medical articles in the tray expires, the tray being sealed with
transparent
plastic material; and
FIG. 16 is a system for reading the RFID tags of the medical articles in the
tray of
the FIG. 15 comprising a box which provides electromagnetic shielding around
the tray,
and an RFID reader as well as a hybrid IMD probe for activating and reading
RFID tags
that are within the tray.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Referring now in more detail to the exemplary drawings for purposes of
illustrating
embodiments of the invention, wherein like reference numerals designate
corresponding or
like elements among the several views, there is shown in FIG. 1 a
representative medical
dispensing cabinet 40 also known as an automated dispensing cabinet ("ADC")
40. The
.. ADC comprises a plurality of movable drawers 42. In this embodiment, there
are five
drawers that slide outwardly from the cabinet to provide access to the
contents of the
drawers. Each drawer may be thought of as a container having an interior
volume in
which medical articles may be stored. The cabinet also comprises an integral
computer 44
that may be used to control access to the drawers and to generate data
concerning access to
and contents of the drawers, and to communicate with other systems. In this
embodiment,
CA 02990420 2017-12-20
WO 2017/003826 16
PCT/US2016/039090
the computer generates data concerning the number and type of items in the
drawers, the
names of the patients for whom they have been prescribed, the prescribed
medications and
their prescribed administration dates and times, as well as other information.
In an embodiment, the ADC 40 comprises an RFID tracking system that tracks the
contents of the drawers by activating RFID tags attached to the contents. The
computer 44
may receive unique identification numbers from the RFID tags attached to the
stored items
and pass those identification numbers to an inventory control computer that
has access to a
database for matching the identification numbers to item descriptions, or
perform those
steps itself. The ADC of FIG. 1 also includes a user interface comprising a
display 46, a
typing keyboard 48, and a keypad 50. In another embodiment, the computer 44
contains a
database and is capable of displaying the name of the medical article, the
dose, the patient
name for which it was prepared, and other data/information on the display and
may accept
commands from the user interface.
As used in regard to the embodiments herein, "tag" is meant to refer to an
RFID
transponder. Such tags typically have a coupling element, such as an antenna,
and an
electronic microchip, also referred to as an integrated circuit ("IC"). The IC
includes data
storage, also referred to as memory.
A cabinet exemplified by the ADC 40 of FIG. 1 may be located at a nursing
station
on a particular floor of a health care institution and may contain
prescriptions for the
patients of that floor. As prescriptions are prepared for the patients of that
floor, they are
delivered and placed into the cabinet 40. They are logged into the integral
computer 44,
which may notify the pharmacy of their receipt at the cabinet. A drawer 42 may
also
contain non-prescription medical supplies or items for dispensing to the
patients as
determined by the nursing staff or physicians. At the appropriate time, a
nurse would
access the drawer in which the medical items are stored through the use of the
computer
44, remove a particular patient's prescriptions and any needed non-
prescription items, and
then close the drawer so that it is secured. In order to access the cabinet,
the nurse may
need to provide various information and may need a secure access code. The
drawers 42
may be locked or unlocked as conditions require.
In another embodiment, the drawers may be unlocked and accessible at any time
by
any one as desired. In another embodiment, one or more drawers may contain
controlled
CA 02990420 2017-12-20
WO 2017/003826 17
PCT/US2016/039090
substances, such as narcotics, and must be locked. In a further embodiment,
all drawers,
or no drawers, or only select drawers may be refrigerated.
The computer 44 in some cases may be in communication with other facilities of
the institution. For example, the computer 44 may notify the pharmacy of the
health care
institution that a patient's prescription has been removed from the cabinet 40
for
administration at a particular day and time. The computer may also notify the
finance
department of the health care institution, and/or other entities, of the
removal of
prescriptions and other medical items for administration to a particular
patient. This
medication may then be applied to the patient's account. Further, the computer
44 may
communicate to the institution's administration department for the purpose of
updating a
patient's Medication Administration Record (MAR), or e-MAR. The computer 44 of
the
medication cabinet 40 may be wirelessly connected to other computers of the
health care
institution or may have a wired connection. The cabinet may be mounted on
wheels and
may be moved about as needed or may be stationary.
Although not shown, each of the five drawers of the ADC 40 contains a door, or
drawer, sensor that detects when the respective drawer is opened. A door-open
signal is
generated and received by the integral computer 44 of the ADC. The signal is
stored in a
database along with the time of receipt for possible future reference. The
same sensor or a
different sensor may detect when the drawer is closed and generates a door-
closed signal.
FIG. 2 presents a different type of cabinet container 60. In this embodiment,
the
cabinet is a refrigerator having a small size, such as 2.3 cubic ft (ft3). In
this embodiment,
the front door 62 includes a keypad 64 and a display 66, as well as a handle
to control
whether the door is open or closed. The lower section 68 includes a processor,
the RFID
electronics, as well as communication electronics, power control, and any
additional
processors that may be needed. Although this embodiment shows a small
refrigerator
having a display and keypad, they are not necessary to the invention. The
refrigerator 60
may have no user interface and the RFID tracking system of the refrigerator
may
automatically track the contents of the refrigerator and automatically
communicate the
results to a remote computer, or smartphone, or other device.
FIGS. 3 and 4 present views of different RFID "flag-tags" 72 and 73 in use
today.
Both are attached to medical vials 70 that have the same size. The medical
vial in both
CA 02990420 2017-12-20
WO 2017/003826 18
PCT/US2016/039090
figures also have the same label 71 attached to the vial, on which is written
various
information about the contents of the vial, such as the name of the drug in
the vial, the
dose, the quantity, the expiration date, the manufacturer, the prescribing
physician, and
possibly more information or less information. The "flag tags" of FIGS. 3 and
4 are given
this name because they include a length of paper 74 and 75 respectively or a
"flag" portion
upon which an RFID tag 76 and 77 respectively is mounted, and a mounting
portion
comprising a length of a clear attachment strip 78 and 79, having a clear
adhesive, that is
placed over the vial's label 71 to attach the flag tag to the vial 70. The
mounting portion
comprising the attachment strip and adhesive may be a tape material and are
clear so that
any information written on the label 71 of the vial 70 can be read through the
mounting
portion even though the respective flag tag is attached.
In FIG. 3, the RFID flag-tag 72 is a typical size in use today as discussed
previously, which is relatively large. The reason for the large size of the
flag-tag is so that
it can mount an RFID tag 76 that has a large coupling element 81 or antenna.
The
coupling element must be large enough to receive and collect an operational
amount of
activation RF energy to activate the RFID integrated circuit 82 of the RFID
tag 80. Such
large RFID tags are used on medical articles that are to be stored in
containers having
RFID tracking systems that do not provide a robust RF energy activation field.
This field
may also be referred to herein as an interrogation field or a reading field.
Thus the
coupling element 81 for RFID tags used in an environment such as this must be
larger to
collect more RF energy to activate the RFID tag 76.
On the other hand, the RFID flag-tag 73 of FIG. 4 is much smaller than that of
FIG.
3. This is due to the coupling element 83 or antenna of the RFID tag 77 of
FIG. 4 being
much smaller. The integrated circuit 84 of the RFID tag of FIG. 4 is
approximately the
same size as the integrated circuit 82 of the RFID tag 80 of FIG. 3. With an
RFID tag 77
of the type of FIG. 4, a much stronger RF activating energy field must
surround the RFID
tag to activate it. RFID tracking systems that are designed with more
efficient energy
transfer, such as provided by the present invention, can successfully operate
with the
smaller sized RFID tags as shown in FIG. 4 and still produce a one-hundred
percent read
rate (also referred to as "interrogation rate," "activation rate," "detection
rate," and possibly
other names). The advantage of using the smaller tags of FIG. 4 is that they
take less room
CA 02990420 2017-12-20
WO 2017/003826 19
PCT/US2016/039090
in a container, do not visibly obscure the existence of other medical
articles, so not
interfere with each other, and are easier to handle.
FIG. 5 provides an RFID tracking system 130 in accordance with aspects of the
invention in which an RFID reader 132 provides activating RF energy with a
signal
generator 92 to two RF energy conduction devices 134 and 136 that both operate
in one
mode as RF energy injection probes that provide activating RF energy to a
container 96
interior 98. This activating energy activates RFID tags in the interior of the
container
which then respond with RF identification data. hi this embodiment, the same
EM energy
conduction devices 134 and 136 also operate in a second mode as receiving
probes that
receive the responses of the activated RFID tags that are present in the
container and that
have been activated by the activating RF energy. The receiving probes 134 and
136
communicate those responses 102 to the RFID reader's receiver 106. The
receiver is
shown broken in this figure for the purpose of clarity in the figure. In this
embodiment, it
is a single receiver that extracts the identification data from the RF
response signals of the
activated RFID tags in the interior 98 of the container 96 and communicates
that
identification data to the processor 104. In another embodiment, multiple
receivers may be
used. Thus in this embodiment, the RF energy probes 134 and 136 operate
wirelessly as
both an energy injection probe and as a receiving probe.
The system of FIG. 5 also comprises an RF energy conduction device switch 138
.. for selectively switching the RF probes 134 and 136 to either injection
mode or receive
mode as desired. Also, the processor 104 of the RFID reader 132 has been
programmed
for frequency control over the signal generator 92 for providing frequency
hopping of the
activating RF energy injected into the storage container 96.
The temi "probes" has been adopted for the energy transfer devices in this
.. disclosure, as opposed to the word "antenna," because the energy transfer
device or
devices are injecting and receiving EM energy from a cavity, which in this
disclosure has
been termed a "container."
FIG. 6 is a view of a much larger storage container 140, in this case a 12 ft3
refrigerated cabinet. Although not shown in the figure, medical articles may
be stored in
this refrigerator. Shelves have also been removed for clarity of the figure. A
total of four
RF probes 134, 136, 142, and 144 are mounted in the refrigerator interior 146
to scan the
CA 02990420 2017-12-20
WO 2017/003826 20
PCT/US2016/039090
entire interior volume of the cabinet. FIG. 6 shows the particular placement
of multiple
probes in a 12 ft3 refrigerator; however, in another embodiment more or fewer
such
devices may be used depending on the circumstances and they may be placed in
different
locations. In this case, the four devices are mounted with two 134 and 136 on
the back
wall and two 142 and 144 on the left wall. The location of the RF energy
conduction
devices may also vary depending on particular circumstances of the container
shape, size,
and the type of RFID tags used.
The performance of RFID tags will vary from one design to another. "Read
perfoimance" can be defined by a variety of RFID tag characteristics: read
distance of a
single tag in free space, probe polarization (linear or circular), sensitivity
to adjacent tags,
sensitivity to metal in close proximity, sensitivity to liquids in close
proximity, sensitivity
to detuning from packaging materials, location of the RFID tag in the
enclosure, but also
the orientation of the RFID tag, proximity of the tag to the enclosure walls
and the drawer
material (surfaces), among others. All of the above performance
characteristics affect the
statistical probability that a tag can be identified in an RF-enabled
enclosure with multiple
probes. In addition to variations in performance between differing tag
designs,
performance can also vary from one tag to another of the same design.
Variations in the
tag assembly process, the tag antenna material, and possibly the integrated
circuit ("IC")
characteristics can result in performance variation within a group of one tag
type/design.
What has been needed, but not available, is an RF energy injection probe that
can
overcome the above sensitivities and performance-degrading conditions so that
all RFID
tags in the interior of a container are activated. A device satisfying this
need has been
found to be a hybrid isolated magnetic dipole ("IMD") probe. The hybrid IMD
probe has
been found to provide superior efficiency, isolation, and selectivity
characteristics and has
a relatively small size due to the configuration of the elements used. The
hybrid IMD
probe excites a magnetic dipole mode from a metal structure in such a fashion
as to
minimize the fringing fields typically generated between a probe element and
an adjacent
ground plane. A current is induced on the probe structure and a strong
electric field is
generated on the structure in the plane of the IMD element instead of a strong
fringing
field to the ground plane. By minimizing the coupled fields to the ground
plane, with the
circuit board of a wireless device taking the place of the ground plane,
improved efficiency
CA 02990420 2017-12-20
WO 2017/003826 21
PCT/US2016/039090
and isolation can be obtained. Single and multi-resonant elements can be
created to
address a wide range of frequency bands.
The hybrid IMD probe confines current flow on the probe main conductive
element
and thereby optimizes the isolation. Near-field emissions are controlled.
Other probe
designs have strong current flows radiating out onto their ground plane board
and lose
large amounts of energy resulting in lower probe efficiency. The hybrid IMD
probe design
provides a solution for accurately and repeatedly identifying RFID tags
attached to both
large and small medication form factors in small non-resonant RF-enabled
enclosures.
RFID tag interrogation performance in a non-resonant cavity can be improved by
using the
hybrid IMD probes disclosed here instead of electric probes or magnetic loops
or half
loops. The near-field magnetic properties along with high cross polarization
characteristics of the hybrid IMD probe main element provide unique
capabilities when the
hybrid IMD probe is used as an energy injection probe in the cavity. The
improvement
from using hybrid LIVID probes as injection probes in the non-resonant cavity
compared to
typical electric or magnetic probes is due to the ability of the hybrid IMD
probe to act as a
magnetic and electric field probe simultaneously as a result of the high cross
polarization
of the IMD main element.
The hybrid IMD probe is formed by coupling one element to another in a manner
that forms a capacitively-loaded inductive loop, setting up a magnetic dipole
mode. This
magnetic dipole mode provides a single resonance and forms a probe that is
efficient and
well isolated from the surrounding structure. This is, in effect, a self-
resonant structure
that is de-coupled from the local environment.
The hybrid IMD probe involves placing a conductor in close proximity to a slot
or
conductive regions of an IMD probe to create a reactive section capable of
increasing the
bandwidth of the IMD probe. The conductor can be capacitively coupled to the
IMD
probe or can be connected to a portion of the IMD probe. Lumped reactance in
the foul' of
capacitors and/or inductors can be incorporated into the probe structure, to
both the driven
element and/or the coupled element, to provide additional adjustment to the
frequency
response. Increases in both efficiency and bandwidth have been documented from
this
technique which more efficiently utilizes the volume that the probe occupies.
WO 2017/003826 22
PCT/US2016/039090
A first type of hybrid IMD probe (Type 1) 160 as shown in the top view of FIG.
7
comprises a pair of conductors 162 and 164 placed in close proximity to each
other with
portions of each conductor positioned in parallel with each other. One
conductor 162 is
connected to a signal source 166 and a second conductor 164 is grounded 168 on
one end.
The overall structure of the main element 170 can be considered as a
capacitively-loaded
inductive loop. The capacitance is formed by the coupling between two parallel
conductors 162 and 164 with the inductive loop formed by connecting the second
element
164 to ground 168. The length of the overlap region between the two conductors
along
with the separation 172 between conductors is used to adjust the resonant
frequency of the
probe 160. A wider bandwidth can be obtained by increasing the separation
between the
conductors, with an increase in overlap region used to compensate for the
frequency shift
that results from the increased separation. This type of hybrid IMD probe
requires a
ground plane 174 for operation. With a ground plane 174 coupled to the IMD
probe 170,
this hybrid IMD probe can be considered a half-loop radiator, providing a
strong magnetic
field component in the near-field of the probe as well as a strong electric
field.
Also shown in FIG. 7 is an active impedance matching circuit 188 in block
form.
The main element 170 of the IMD probe 160 is connected with the matching
circuit to
vary the impedance of the IMD probe to a value as close to the impedance of
the container
with which it is associated as possible so that energy is efficiently
transferred between the
two. Such impedance matching circuits are known in the art. U.S. Patent No.
8,384,545 to
Hussain et al. provides a non-limiting description of such a circuit usable
here.
FIG. 8 shows a perspective view of the IMD probe 160 of FIG. 7 and further
shows the magnetic field "H" 180 and the electric field "E" 182 created by the
IMD probe.
It will be noted that the electric field "E" is in the X plane while the
magnetic field "H" is
in the orthogonal Y plane. Both fields are robust and fill the entire interior
of a container
60 such as that shown in FIG. 2.
An advantage of this hybrid IMD type of probe structure is the method in which
the probe is fed or excited. This leaves great flexibility for reduced-space
integration. The
probe size reduction is obtained by the capacitive loading that is equivalent
to using a low
loss, high dielectric constant material. At resonance a cylindrical current
going back and
forth around the loop is formed. This generates a magnetic field "H" 180 along
the axis of
Date Recue/Date Received 2023-01-06
CA 02990420 2017-12-20
WO 2017/003826 23
PCT/US2016/039090
the loop which is the main mechanism of radiation. The electrical field "E"
182 remains
highly confined between the two elements 162 and 164. This reduces the
interaction with
surrounding metallic objects and obtains high isolation.
In accordance with one aspect of the invention, the hybrid IMD probe 160 of
FIGS.
7 and 8 provides high energy efficiency. The hybrid IMD probe comprises a
capacitively-
coupled inductive loop 170 where multiple components of a part of the loop are
capacitively coupled together to create a robust electric field and the
inductive coupling of
the components to the ground plane create an equally robust magnetic field.
FIG. 8
provides a perspective view of an IMD main element 170 situated above a ground
plane
.. 174. The ground plane 174 may include an impedance matching circuit 188
incorporated
therein. The main element of the probe 170 consists of a slot region 172 and
prong type
feed and ground legs 184. A current is induced around the U-shaped probe
structure 170
through a feed port and ground of the wireless device. The current is induced
in order to
generate a strong electric field in the slot region, in the plane of the 'MD
element 170
instead of a strong fringing field to the ground plane 174 below it. This
minimizes the
coupled fields to the ground plane 174. With a circuit board of a wireless
device acting as
the ground plane, an improved efficiency and isolation may be obtained.
Different
configurations of these resonant elements may be made in order to address a
wide range of
frequency bands.
The length of the IMD element 170 may be modified to be longer or shorter
dependent on the frequency desired. For instance, longer IMD elements 170 show
improved lower frequency ranges. In addition the center slot capacitive region
172 may be
wide or narrow. In addition multiple slot regions may be formed, as is
provided in FIG.
13. The height of the IMD element 170 above the ground plane 174 also affects
the
frequency range functionality of the probe. By displacing the portions of the
structure in
three dimensions, the IMD element can be optimized at various frequency
regions. Lower
frequencies will be more efficient when implemented with increased height,
such as 6 mm,
while higher frequencies will be more efficient with lower heights, such as 4
mm. As well,
the height above the ground plane for optimal efficiency varies as probe
operation varies
from 1800 MHz to 2200 MHz. Discrete steps in height are applicable, as well as
variable
and continuous increases or decreases in element height as a function of
element length.
W02017/003826 24
PCT/US2016/039090
U.S. Patent No. 7,777,686 provides further non-limiting details on modifying
an IMD
probe.
The embodiment of a hybrid IMD probe shown in FIGS. 7 and 8 comprises an
isolated main probe element 170, a first parasitic element 210, and a first
active tuning
element 212. The first parasitic element 210 and its associated first active
tuning element
212 are positioned to one side of the main probe element. In one embodiment,
the first
active tuning element is adapted to provide a split resonant frequency
characteristic
associated with the probe 170. The first active tuning element may be adapted
to rotate the
radiation pattern associated with the IMD probe 160. This rotation may be
effected by
controlling the current flow through the parasitic element 210. In one
embodiment, the
first parasitic element 210 is positioned on a substrate 174. This
configuration may
become particularly important in applications where space is the critical
constraint. In one
embodiment, the parasitic element is positioned at a pre-determined angle with
respect to
the main probe element 170. For example, the first parasitic element 210 may
be
positioned parallel to the main probe element 170, or it may be positioned
perpendicular to
the main probe element. The parasitic element may further comprise multiple
parasitic
sections.
In one embodiment of the present invention, the first active tuning element
212
comprises at least one of the following: voltage controlled tunable
capacitors, voltage
controlled tunable phase shifters, FETs, and switches.
In another embodiment of the present invention, the probe 160 further
comprises a
plurality of parasitic elements, and a plurality of active tuning elements, as
is shown in
FIGS. 7 and 8. In this embodiment, the probe 160 includes a first parasitic
element 210
and a first active tuning element 212 associated with the first parasitic
element, wherein
the first parasitic element and the first active element 212 are positioned to
one side of the
main probe element 170. The embodiment also includes a second parasitic
element 232
and a second active tuning element 234 associated with the second parasitic
element. The
second parasitic element and the second active tuning element are positioned
below the
main probe element 170. In this case, the second parasitic and active tuning
elements are
used to tune the frequency characteristic of the probe 230, and in another
embodiment, the
first parasitic and active tuning elements are used to provide beam steering
capability for
the probe.
Date Recue/Date Received 2023-01-06
CA 02990420 2017-12-20
WO 2017/003826 25
PCT/US2016/039090
In one embodiment of the present invention, the radiation pattern associated
with
the probe is rotated in accordance with the first parasitic and active tuning
elements. In
some embodiments, this rotation may be ninety degrees.
In another embodiment of the present invention shown in FIG. 9, the probe 230
further includes a third active tuning element 240 associated with the main
probe element
192. This third active tuning element is adapted to tune the frequency
characteristics
associated with the probe.
Referring now to FIG. 10, a different embodiment of a hybrid IMD probe 244 is
shown. In this embodiment, the hybrid IMD probe 244 includes a first parasitic
element
210 and associated first active tuning element 212 but does not include the
second parasitic
element located under the main element 170 as shown in FIGS. 7, 8, and 9. The
embodiment therefore has fewer parts, less programming in that a second active
tuning
element does not need to be controlled, nor is there a third active tuning
element that needs
to be controlled (see FIG. 9 for the first, second, and third active tuning
elements 212, 232,
and 240).
Referring now to FIG. 11, a further embodiment is shown having dual hybrid
11MD
probes . In particular, two hybrid IMD probes 246 and 248 are located on the
same circuit
board 249. Each probe 246 and 248 includes a main conducting element 280 and
282
respectively, and a first parasitic element 284 and 286 with an active tuning
element
associated with both 288 and 290. It will be noted that these two co-located
dual hybrid
IMD probes are oriented so that they are ninety degrees from each other in
this
embodiment. The first parasitic element of each permits four separate
radiation patterns or
"beams" for each probe resulting in a total of eight radiation patterns 249
for the entire
circuit board 249 of dual hybrid IMD probes. Because the two probes are
oriented at a
particular angle to each other, the eight radiation patterns do not overlap in
this
embodiment. However, in other embodiments, overlap may be desired and
different
orientations of the probes in relation to each other may be implemented. This
embodiment
is particularly applicable for use in larger containers, but may also be used
in smaller
containers as well.
CA 02990420 2017-12-20
WO 2017/003826 26
PCT/US2016/039090
FIG. 12 presents a perspective view of a hybrid IMD probe 300 in which the
main
conducting element 302 is mounted orthogonally on the circuit board 304.
Similar
performance can be obtained with this configuration as described above.
A second type of hybrid IMD probe 190 is shown in FIG. 13 and provides two
resonances for use in dual frequency band or multi-band applications. This
second type of
hybrid IMD structure is composed of a planar main element 192 positioned above
a
ground plane 194. Two slots 196 and 198 are formed in one section of the
planar
conductor. The probe is excited in such a way that there are strong electric
fields in the
slot regions, with the slots being dimensioned to resonate at two different
frequencies. The
strong electric fields in the slot regions is a result of opposing currents
flowing on two
portions of a planar conductor that are parallel to one another. The two
opposing currents
on the conductor provide a magnetic field distribution similar to the fields
formed by a half
loop element above a ground plane, as is shown in FIG. 8. The result is a
probe that has
reduced fringing electric fields between the probe conductor and the ground
plane, and a
magnetic field distribution that is similar to a loop. A good mix of electric
and magnetic
fields are present in the near-field. The planar conductor 200 forming the
probe is
typically positioned above and in parallel to a ground plane 194. A conductor
forming a
feed leg 202 and a conductor forming a ground leg 204 are positioned
orthogonal to the
plane of the planar conductor. In this configuration the MID probe forms a
volume
encompassed by the planar conductor and the ground plane, which determines the
frequency bandwidth.
The parasitic element 206 and its associated active tuning element 208 result
in
multiple selectable radiation patterns or beams from the probe.
The planar slot configuration shown in the conductor shown in FIG. 13 provides
equivalent radiated field performance as a pair of capacitive loops, one large
loop and one
small loop. The fields are equivalent due to the orientation of the slot
configuration and
the direction of current flow on individual portions or conductive sides of
the slot.
Unlike other probes, such as the Planar Inverted F-Style Antenna (PIFA), the
hybrid IMD probe 190 has a underlying advantage in that its properties depend
mainly on
the probe structure itself and not the surrounding area. In the hybrid IMD
probe 190, the
electrical currents are strongly localized to the probe region and do not
propagate on the
WO 2017/003826 27
PCT/US2016/039090
ground 194. This is an important feature as any probe employed for identifying
RFID tags
in metallized or shielded enclosures will by definition be in close proximity
to large metal
areas.
U.S. Patent No. 7,911,402 provides further non-limiting details on selecting
or
"steering" probe radiation patterns or beams.
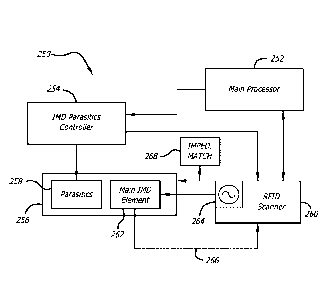
Referring now to FIG. 14, In regard to the hybrid probes shown and described
above having a parasitic element that provides for beam steering or radiation
pattern
selection, a main processor 252 signals the IMD parasitic controller 254 to
select a
particular beam of the probe 256 to activate. The parasitic controller then
controls the
various parasitic elements 258 and active tuning elements associated therewith
to set the
particular beam with which the probe will operate. The main processor 252 then
controls
the RFID reader 260 to provide activating RF energy to the main IMD element
262
through a signal generator 264. The probe 256 operates to inject activating RF
energy to
activate all RFID tags in the beam selected. The probe 256 is then controlled,
in one
embodiment, to receive the responsive signals from activated RFID tags in the
container
of interest, and forward the received responsive signals 266 to the RFID
reader 260. Also
shown is an active impedance matching control to increase energy transfer
between the
probe 256 and a container.
In one case, the beam steering may be dynamic, in that the processor has the
hybrid
IMD probe change beams periodically. In another case, the beam selected for
use by the
probe is selected based on the location of the probe in the container, and
that beam is fixed
in that the probe only operates on that beam, or mode, for the entire life of
the container.
In one embodiment, the probe had four "beams" or "modes" at which the probe
could be
set.
Referring now to FIG. 15, another type of container or storage system is
commonly
known as a tray or code tray, and may have other names. The code is typically
used to
identify the medical purpose of the tray, such as a "code blue" tray to
resuscitate a person
undergoing cardiac arrest. Such a tray may be formed of non-metallic material
such as
composites or plastics. The tray holds all of the medications, tools, and
equipment that are
expected to be required to complete a medical procedure or to handle a
particular medical
event.
Date Recue/Date Received 2023-01-06
CA 02990420 2017-12-20
WO 2017/003826 28
PCT/US2016/039090
A tray is typically laid out and displayed in an easily recognizable fashion.
Color
may be used also to assist in managing the inventory of the tray. This allows
an assistant
to retrieve the correct medication or instrument without delay. In the event
that a surgeon
is looking for the optimum tool or medication, a quick glance at the surgical
tray will allow
the identification of all available tools at his or her disposal. Labels are
often placed on the
tray also that specify what is in the pockets of the tray.
An example of such a medical "tray" is shown in FIG. 15. The tray 320 is a
single
layer and includes various pharmaceuticals 322 and other medical articles,
such as pre-
loaded syringes 324 (epinephrine syringe, lidocaine syringe, and an atropine
syringe). The
entire tray is sealed with clear plastic wrap 326 and an inventory list 328 is
contained just
under the plastic seal so that it is visible and readable without breaking the
seal. The
Required Inventory list in this case identifies the name of the tray, such as
"Childbirth
Tray," lists the contents of the tray, and includes other infoiniation such as
the first
expiration date of any of the articles contained in the tray. The Required
Inventory list
may also contain a plan layout of the tray showing which articles should be
stored where.
It may have multiple pages or only a single page.
The tray 320 has been prepared by a pharmacist at the pharmacy because it has
prescription medications in it (oxycontin for example). The Required Inventory
list may
also include brand names as well as generic names, and National Drug Codes
("NDCs") or
Universal Product Codes ("UPCs") as part of the inventory. State regulations
typically
allow a hospital or other facility to define the contents of its trays, and
therefore they can
be selected based on particular "community" standards and requirements. State
regulations, typically require that the hospital have specific procedures to
ensure accuracy
of tray contents. Such procedures include inventory and restocking procedures,
as well as
detection of expired and recalled medical articles. In the example of FIG. 14,
the tray is
relatively small. However for other purposes, a tray can be much larger with
many more
medical articles. Some trays may include additional layers that may or may not
include
additional items not contained in the top layer.
If the seal is broken, regardless of whether any of the contents were removed,
an
inventory will likely be required. Existing processes require that this be
done manually.
Each of the articles in the tray is examined to determine if it is expired or
recalled, and is
compared against the Required Inventory list to determine if it should be in
the tray. The
CA 02990420 2017-12-20
WO 2017/003826 29
PCT/US2016/039090
Required Inventory list is also referenced for checking that all required
articles are in the
tray and that extra articles are not in the tray. Once it has been restocked,
the tray 320 is
resealed 326 and may be placed on the floor again for medical use. Such
examination and
restocking can take significant amounts of time and if a pharmacist is
required to perform
some of the inventory process, that pharmacist will be unavailable to perform
other duties.
In such a manual procedure, mistakes can be made. Thus, a need has been
identified to
provide a more efficient and accurate system and method to restock such carts
and trays.
Crash carts and trays must be resupplied periodically to replace expired or
recalled
items, and if a cart or a tray was actually used, to replace consumed
articles. As
mentioned, such processes are typically performed manually at a significant
cost in time.
Missing key medical articles in a tray could be devastating in an emergency
situation.
Therefore accuracy in the resupply is mandatory. Often, trays that have
articles that are
just nearing expiration must be returned to the pharmacy for resupply in
advance of
expiration due to the time it takes to process the tray. Any recalled articles
must also be
removed and substitutions made. It is also possible that items foreign to the
crash cart or
tray have been added while they were in the field, and these foreign articles
must be found
and removed.
Unfortunately, the above procedures tend to suffer from significant
shortcomings.
For instance, manual inspections can result in errors as can resupply.
Creating records of
what was done is also generally time consuming and error prone, all of which
drive up the
cost of creating and resupplying the carts and trays. There has therefore been
recognized a
need for improvement in managing such crash carts and trays.
Furthetmore, under the current system, the pharmacy is unable to create
individualized carts for patients. For example, certain patients may be
provided a patient-
specific cocktail of drugs (this may be a mixed vial or a combination of
drugs). Because
these are non-standard drugs or drug combinations, a pharmacist has to double
check a
drug list or a prescription list when creating a cocktail drug or filling a
personalized cart
with medical items.
FIG. 16 shows an embodiment of an inventory management system 340 according
to aspects of the invention. An enclosure 342 is shown, which in this case
creates an EM
energy shielded cage in that all the walls and top and bottom are electrically
shielded to
CA 02990420 2017-12-20
WO 2017/003826 30
PCT/US2016/039090
isolate the enclosure by preventing (or significantly attenuating) EM from
entering or
escaping the enclosure. The enclosure is fitted with a reader 344 configured
to interrogate
RFID tags located within the enclosure. One or more hybrid IMD probes are
locate within
the enclosure 342 and are connected to the reader 344.
The reader 344 is connected to a computer 346 through a connection 348. The
connection 348 may be a wired connection, wireless connection, or any other
suitable
connection for data transfer. In one embodiment, the physical body of the
computing
system may be attached to the enclosure 342. The computing system 346 has a
non-
volatile memory 354 in which is stored at least one database ("db") which may
be a local
database, or other. The non-volatile memory 354 comprises one or more computer
readable media within the computer system 346 and may be located within the
computer
itself or external to the computer. The memory is shown here as being outside
the
computer only for clarity of illustration in the discussion and is not meant
to limit the
invention in any way. In another embodiment, part or all of the local database
may be held
on a server 360. The computing system 346 is also connected to the remote
database 360
at which is located a first remote database 362 and a second remote database
364. As in
the local computer, these remote databases may be stored on a memory that is
internal to
the server or that is external to the server. Further, the server 360 may be
located nearby
the local computer 346 or may be remote therefrom. By remote, it is meant that
it may be
in the same room, or in the same wing, or in the same facility, or may be in
the cloud.
Connection 366 to the server 360 may likewise be a wired connection, wireless
connection, or any other suitable connection for data transfer.
In one embodiment, the data held on the local database 352 may depend on the
location/specialty/facility using computer system 346. For example, if the
computer
system 346 were stationed in an emergency room ("ER"), the local database 352
may hold
only information or data regarding medical articles, medical containers, and
other
inventory most used in an ER. In one embodiment, the remote database 362 at
the server
360 may serve as a main database and contain data for all medical articles,
medical
containers, and other inventory for all medical
locations/facilities/specialties. The local
database 352 may maintain a copy of the portion of data held on the remote
database 362
that is most relevant to the computer system 346, but can access the remote
database 362
CA 02990420 2017-12-20
WO 2017/003826 31
PCT/US2016/039090
when encountering medical items, medical containers, or other inventory for
different
facilities/specialties/locations.
The enclosure 342 has an opening 370 through which a tray 372 may be slid into
the enclosure. The tray is placed completely within the enclosure so that the
front door
374 can be closed over the opening 370 to complete the Faraday cage of the
enclosure 342.
The tray includes a number of medical items 376 with each one having an RFID
tag 378
attached. As discussed previously, each RFID tag has a stored different
identification
number comprising a few bytes with a check digit. The error codes are not
stored in the
tag memory. They are generated on the fly. Manufacturers guarantee that each
serial
-- number is used only once. Some RFID tags have more complex codes for
identifying the
RFID tag. In this case, the tray 372 also has an RFID tag 280 attached to its
outer surface
382. The reader 344 will read those identification numbers from the tags,
communicate
them to the computer which will compare them against one or more databases
either
locally 352 or remotely through a server 362 and/or 364. The process of using
the
-- identification numbers of the tags is discussed below.
Medical item information may include information such as name, lot code, date
of
manufacture, expiration date, dosage, weight, color, and an image of the
medical article.
In one embodiment, the identification ("ID") data may be partially made of
drug codes that
identify the drugs. As an example and not by way of limitation, the
identification data
may use the National Drug Code ("NDC") as part of its data allowing for easy
identification of the attached medical item. Identification data may also have
other
identifying codes that establish the manufacturer, lot code, dosage, drug
type, expiration
date, etc.
Shown in FIG. 16 is an enclosure 342 foinied in accordance with aspects of the
invention by which it is much smaller than an enclosure sized to be resonant
at the
operating frequency of RFID yet the EM field within the enclosure 342 is
highly robust
and effective at exciting and reading all RFID tags located therein due to the
use of a
hybrid IMD probe or probes. Because inventive aspects are incorporated, the
enclosure is
much smaller than other enclosures and is therefore highly desirable in areas
where space
is limited, such as a pharmacy in a healthcare facility. Although not shown,
the front door
374 includes latching hardware to retain it in a closed when it is rotated
upwards and put in
use. A handle 384 assists in managing the configuration of the front door. The
enclosure
W02017/003826 32
PCT/US2016/039090
is formed of a metallic mesh or other EM shielding material to provide an EM
shielded
cage about trays that are slid within it for scanning and inventorying. The
front door in
this embodiment is also formed of an RF shielding material. An RFID reader 344
is
shown in dashed lines which may also contain a hybrid IMD probe or probes, the
electronics, and a battery 388 for the enclosure. The electronics include a
processor,
communications, wired and wireless connections, and a local power source. In
another
embodiment, an AC adapter may be included for using wall power. Communications
ability over networks is provided.
The approximate volume for a resonant enclosure at an RFID operating frequency
of 900 MHz is 3 ft. x 3 ft. x 3 ft. for a total of 27 cubic feet. In one
embodiment, the
enclosure 342 had the dimensions of 2.25 ft. wide by 1.6 ft. long by 0.88 ft.
high for an
approximate volume of 3.15 cubic feet, and with the use of a hybrid IMD probe
or probes,
achieved equally effective electric and magnetic fields within the enclosure
at exciting and
reading all RFID tags located therein. The difference in sizes of the two
enclosures makes
one formed in accordance with the invention more attractive in many situations
where
space is limited.
The above may also be combined with a frequency hopping arrangement and a
Return Signal Sensitivity Indicator arrangement for increasing the likelihood
of activating
all RFID tags in a particular container. U.S. Patent Application Publication
No.
2014/0184391 provides further non-limiting details on such arrangements.
The invention is intended to provide a read process that ensures the highest
statistical probability of identifying all RFID tags contained in the RF-
enabled enclosure.
Although shown and described in the embodiment of a medical article tracking
system and method, the invention can have application to other fields of
tracking outside
the medical field.
A Faraday cage is mentioned; however, this device is also known as a Faraday
shield and Faraday screen. In addition, other EM shielding is usable.
Different EM
shielding can produce the desired isolation of keeping activating RF energy
within the
container so that RFID tags located outside the container are not activated
and read.
Mistakenly reading RFID tags that are located outside the container can cause
errors since
Date Recue/Date Received 2023-01-06
CA 02990420 2017-12-20
WO 2017/003826
33
PCT/US2016/039090
the tracking system of the container will not be able to determine that the
RFID tag is
outside the container and will return a result showing that it is in the
container.
Although RFID tags are used herein as an embodiment, other data carriers that
communicate through electromagnetic energy may also be usable.
Unless the context requires otherwise, throughout the specification and claims
that
follow, the word "comprise" and variations thereof, such as, "comprises" and
"comprising"
are to be construed in an open, inclusive sense, which is as "including, but
not limited to."
Although RFID tags are used herein as an embodiment, other data carriers that
communicate through electromagnetic energy may also be usable.
Unless the context requires otherwise, throughout the specification and claims
that
follow, the word "comprise" and variations thereof, such as, "comprises" and
"comprising" are to be construed in an open, inclusive sense, which is as
"including, but
not limited to."
While the invention has been described in connection with what is presently
considered to be the most practical and preferred embodiments, it is to be
understood that
the invention is not to be limited to the disclosed embodiments and elements,
but, to the
contrary, is intended to cover various modifications, combinations of
features, equivalent
arrangements, and equivalent elements included within the spirit and scope of
the
appended claims.