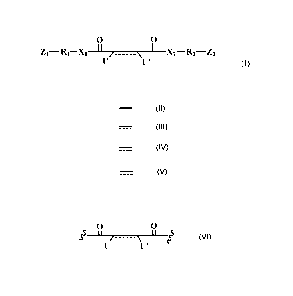Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
NOVEL LINKERS AND THEIR USES IN SPECIFIC
CONJUGATION OF DRUGS TO A BIOLOGICAL MOLECULE
FIELD OF THE INVENTION
The present invention relates to linkers used for the specific conjugation of
com-
pounds, in particular, cytotoxic agents to pairs of sulfur atoms of a
biological molecule at
two drugs per linker. The present invention also relates to methods of making
cell-binding
agent-drug (cytotoxic agent) conjugates in a specific manner comprising either
modifica-
tion of drugs with these linkers first, followed by reaction with prepared
cell-binding
agents; or modification of cell-binding agents with these linkers first,
followed by reaction
with drugs.
BACKGROUND OF THE INVENTION
The big challenge of chemotherapeutic drugs is their narrow therapeutic
windows due
to they normally cannot discriminate between normal and malignant cells, thus
causes side
effects which limit the tolerated doses below the clinically effective ones.
In contrast,
immunotherapy, normally in the form, of monoclonal antibodies (mAb) can
specifically
bind to certain proteins or molecules of malignant cells, leaving normal cells
unharmed,
and thus has less side effects and bigger therapeutic windows than
chemotherapy. Mono-
clonal antibodies (inAb) can target against malignant cells by several
mechanisms, such as,
1). Making the cancer cell more visible to the immune system (Villaruz, L. C.
et al, 2014,
Transl Lung Cancer Res, 3, 2-14; Camacho, L. H. 2015 Cancer Med 4, 661-72);
2). Block-
ing growth signals (Dillman, R. 0. 2011, Cancer Biother Radiopharm, 26, 1-64;
Ferris, R.
L. et al 2010. J Clin Oncol, 28, 4390-9); 3). Stopping new blood vessels from
forming
(Arrillaga-Romany, 1., et al, 2014, Expert Opin Investig Drugs, 23, 199-210);
4). Deliver-
ing radiation to cancer cells (Chapuy, B. et al, 2007, Biotechnol J. 2, 1435-
43); 5). Deliver-
ing chemotherapy drug to cancer cells (Chari R. J. 2008 Acc Chem Res. 41, 98-
107;
Mullard A. 2013, Nature Reviews Drug Discovery 12, 329-332; Zhao, R. J. 2012,
J. Med.
Chem., 55, 766-782); 6). Delivering enzyme to cancer cells (Francis R. J. et
al. 2002, Br. J.
Cancer 87, 600-7). One of these above strategies, delivering chemotherapy to
cancer cells
called antibody¨drug conjugates (ADCs), which enables to target and deliver
drugs to
cancer cells leaving normal cells largely unaffected by the exquisite
targeting ability of
antibodies, has been intensely exploitation in the last two decades. In
particular, since US
FDA approvals of Adcetris (brentuximab vedotin) in 2011 and Kadcyla (ado-
trastuzumab
1
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
emtansine) in 2013, the applications of antibody-drug conjugate (ADC) as a
promise
targeted treatment of cancers have been exploded and almost every major
pharmaceutical
and biotech company has adopted this approach (Chari, R. et al. Angew. Chem.,
Int. Ed.
2014, 53, 3796-3827; Sievers, E. L. et al. Annu Rev Med. 2013, 64, 15-29;
Mehrling, T.
Future Oncol, 2015, 11, 549). Currently there are more than 50 ADC drugs in
the clinic
trials according to www.clinictrails.gov.
The first-generation ADCs, including Kadcyla and Adcetris, are produced
through
nonselective conjugation of native lysine amines or interchain cysteine thiols
on an anti-
body respectively to a cytotoxic drug. Since there are over 50 surface-exposed
lysines and
8 hinge cysteine residues in IgG1 antibodies, this nonselective conjugation
results in
randomly cross-linkage of cytotoxic drugs to practically all areas of the
antibody molecule,
particularly having a diverse population of ADCs with a wide distribution of
drugs per
antibody (DAR) (Wang, L., et al. 2005 Protein Sci. 14, 2436; Hamblett, K. J.,
et al. 2004
Clin. Cancer Res. 10, 7063). Thus some of the undesired ADC subpopulation
could lead
to shorter circulation half-life, lower efficacy, potentially increased off-
target toxicity and
a wide range of in vivo pharmacokinetic (PK) properties (Hamblett, K. J. et
al, Clin.
Cancer Res. 2004, 10, 7063-7070; Adem, Y. T. et al, Bioconjugate Chem. 2014,
25,
656-664; Boylan, N. J. Bioconjugate Chem., 2013, 24, 1008-1016; Strop, P., et
al 2013
Chem. Biol. 20, 161-167). In addition, with this classical conjugation, the
batch-to-batch
consistency in ADC production can be challenging and may require diligent
manufactur-
ing capabilities (Wakankar, A. mAbs, 2011, 3, 161-172).
Therefore, biotechnology companies and academic institutions are highly
focusing on
establishing novel reliable methods for site-specific ADC conjugation. So far,
there are
several approaches developed in recent years for site selective ADC
preparation
(Panowski, S. 2014, mAbs 6, 34). They include incorporation of unpaired
cysteines, e.g.
engineered reactive cysteine residues, called THIOMAB from Genentech
(Junutula, J. R.,
et al 2010 Clin. Cancer Res. 16, 4769; Junutula, J. R., et al 2008 Nat
Biotechnol. 26, 925-
32; US Patents 8,309,300; 7,855,275; 7,521,541; 7,723,485, W02008/141044),
genetical-
ly introduced glutamine tag with Streptoverticillium mobaraense
transglutaminase (mTG)
(Strop, P., Bioconjugate Chem., 2014, 25, 855-862; Strop, P., et al., 2013,
Chem. Biol. 20,
161-167; US Patent 8,871,908 for Rinat-Pfizer) or with Microbial
transglutaminase
(MTGase) (Dennler, P., et al, 2014, Bioconjug. Chem. 25, 569-578. US pat appl
20130189287 for Innate Pharma; US Pat 7,893,019 for Bio-Ker S.r.l. (IT)),
incorporation
of thiolfucose (Okeley, N. M., et al 2013 Bioconjugate Chem. 24, 1650),
incorporation of
2
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
unnatural amino acids through mutagenesis (Axup, J.Y., et al., 2012, Proc.
Natl. Acad.
Sci. 109, 16101-16106; Zimmerman, E.S., et al., 2014, Bioconjug. Chem. 25, 351-
361;
Wu, P., et al, 2009 Proc. Natl. Acad. Sci. 106, 3000-3005; Rabuka, D., et al,
2012 Nat.
Protoc. 7, 1052-67; US Pattent 8,778,631 and US Pat Appl. 20100184135,
W02010/081110 for Sutro Biopharma; W02006/069246. 2007/059312. US Patents
7,332.571, 7,696,312, and 7,638,299 for Ambrx; W02007/130453, US patents
7,632,492
and 7,829,659 for Allozyne), Incorporation of selenocysteine into antibodies
(Hofer, T., et
a12009, Biochemistry 48, 12047-12057; US Patent 8,916,159 for US National
Cancer
Institute), Convertion of cysteines located in the CXPXR consensus sequence to
formylglycine (FGly) with formylglycine generating enzyme (FGE) (Drake, P.M.,
et al.,
2014, Bioconjug. Chem. 25, 1331-1341. Carrico, I. S. et al 7,985,783;
8,097,701;
8,349,910, and US Pat Appl 20140141025, 20100210543 for Redwood Bioscience),
and
through glycoengineeringly introduction of sialic acid with the use of
galactosyl- and
sialytransferases (Zhou, Q., et al 2014, Bioconjug. Chem., 25, 510-520, US Pat
Appl
20140294867 for Sanofi-Genzyme). These above methods have produced nearly
homoge-
neous product profiles, but they are required antibody-engineering processes
and
reoptimization of cell culture conditions. Moreover, expression yields for
genetic encoding
of an unnatural amino acid were typically not promisingly high enough (Tian,
F., et al,
2014, Proc. Natl. Acad. Sci. U. S. A. 111, 1766-71) which has a significant
impact on the
cost of goods of the ADC. In addition, it has been known that ADCs obtained by
conjuga-
tion to cysteine side chains often display limited stability in circulation,
leading to prema-
ture disconnection of the cytotoxic payload before the tumor site is reached
(Junutula, J. R.,
et al 2008, Nat. Biotechnol. 26, 925-32).
The disulfide bond structures of the four subclasses of IgG antibodies were
known in
the 1960s (Milstein C. Biochem J 1966, 101:338 - 351; Pink JR, Milstein C.
Nature 1967,
214:92-94; Frangionc B, Milstein C. Nature 1967, 216:939 - 941; Pink JR,
Milstein C.
Nature 1967, 216:941 -942; Frangione B, et al. Biochem J 1968, 106,15 -21;
Frangione
B, Milstein C. J Mol Biol 1968; 33:893 -906; Edelman GM, et al. Proc Natl Acad
Sci
USA 1969; 63:78 -85; Frangione B, et al. Nature 196, 221:145 -148,
Spiegelberg, H. L. et
al Biochemistry, 1975, 10, 2157-63 ). Disulfide bond structure is critical for
the structure,
stability, and biological functions of IgG molecules. Among the four
subclasses of IgG
antibodies, IgGi, IgG2, IgG3 and 'gat, each IgG contains a total of 12 intra-
chain disulfide
bonds; each disulfide bond is associated with an individual IgG domain. The
two heavy
chains are connected in the hinge region by a variable number of disulfide
bonds: 2 for
3
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
IgGi and IgG4, 4 for IgG2 and 11 for IgG3. The light chain of the IgGi is
connected to the
heavy chain by a disulfide bond between the last cysteine residue of the light
chain and the
fifth cysteine residue of the heavy chain. But, for IgG2, IgG3 and IgG4, the
light chain is
linked to the heavy chain by a disulfide bond between the last cysteine
residue of the light
chain and the third cysteine residue of the heavy chain (Liu, H. and May, K.,
2012, mAbs
4, 17-23). On the ranks of the susceptibility of disulfide bonds in human IgG1
antibodies
by experimental reduction, differential alkylation, and LC-MS analysis (Liu,
H, et al Anal.
Chem., 2010, 82. 5219-5226), inter chain disulfide bonds are more susceptible
to reduc-
tion than intra chain disulfide bonds, and the disulfide bonds between the
light chain and
heavy chain were more susceptible than disulfide bonds between the two heavy
chains.
The upper disulfide bond of the two inter heavy chain disulfide bonds was more
suscepti-
ble than the lower one. Furthermore, disulfide bonds in the CH2 domain were
the most
susceptible to reduction. Disulfide bonds in VL, CL, VH, and CH1 domains had
similar
and moderate susceptibility, while disulfide bonds in the CH3 domain were the
least
susceptible to reduction (Liu, H, et al Anal. Chem.. 2010, 82, 5219-5226).
Based on the more susceptibility of inter chain disulfide bonds in human IgG1
anti-
bodies, several institutions and companies adopted the chemically specific
conjugation
strategy through rebridging reduced interchain disulfide bonds of a native
antibody, such
as, using bromo or dibromo-maleimides, called next generation maleimides
(NGMs)
(Schumacher. F.F., et al 2014, Org. Biomol. Chem. 12, 7261-7269; UCL Cancer
Institute),
applying bis-alkylating reagents via a three-carbon bridge (Badescu, G., et
al., 2014.
Bioconjug. Chem. 25, 1124-1136., W02013/190272, W02014/064424 for PolyTherics
Ltd), with di-substituted heteroaryl bridge (US Pat Appl. 2015/0105539 for
Concortis
Biosystem), or through di-maleimide as a bridge (W02014/114207). We have also
used
bromo maleimide and dibromomaleimide linkers to conjugate both drugs and
antibodies
for a quite while (W02014/009774. PCT/1B2012/053554). However, these above
bridge
linkers were designed in the way to conjugate only one cytotoxic agents to a
pair of disul-
fide bonds, and therefore at most of time they only produced ADCs at DAR less
than 2
(drugs per antibody), due to limited numbers (about two pairs) of reduced
disulfide bonds
are more accessible for conjugation.
As one of the major issues for ADCs is the limited numbers or amount of
cytotoxic
compound that ultimately reaches the tumor, and thus the favorable DAR over 3
is much
important factor for improvement of ADC therapeutical index (Epenetos. A. A.
et al,
Cancer Res., 1986, 46, 3183-3191; Chari, R. V. Ace. Chem. Res., 2008, 41, 98-
107, Zhao,
4
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
R. Y. et al, 2011, J. Med. Chem. 54, 3606-3623), we therefore disclose novel
disulfur
bridge linkers of this invention that not only are able to conjugate two or
more drugs per
linker for achieving higher DARs (>4), but also can selectively rebridge pairs
of reduced
inter chain disulfide bonds on surface of antibody, which are generated by
overloaded
TCEP or DTT reduction agents. And the over reduced pairs of thiol groups that
are inac-
cessibly reached by the bridge linkers can be recoupled (regenerated) by an
oxide, e.g.
dehydroascorbic acid (DHAA) or Cu(II), to form back disulfide bonds at the end
of conju-
gation. In principal, this rebridging back of reduced disulfide bonds results
in more stable
or longer half-life of ADCs in comparison with traditional thiol linked ADCs.
Furthermore,
as the "ring-opened" succinimide ring linker bearing mono-thioether bond has
improved in
vitro stability, improved PK exposure, and improved efficacy as compared to
the mono-
thiol-maleimide-conjugated ADCs (Tumey, L. N, et al, 2014. Bioconjug. Chem.
25, 1871-
80; Lyon, R. P, et al. 2014, Nat. Biotechnol. 32, 1059-62), due to the latter
is prone to
payload loss via a retro-Michael type reaction of the maleimide conjugation
(Shen, B. Q, et
al, 2012, Nat Biotechnol. 30, 184-9; Tumey, L. N, et al, 2014 Bioconjug Chem.
25. 1871-
80), the bridge linkers of this invention containing a 2,3-disubstituted
succinic group, or 2-
monosubstituted, or 2,3-disubstituted fumaric or malcic (trans (E)- or cis (Z)-
butenedioic)
group have less payload loss as compared to their nonhydrolyzed bromo or
dibromo-
maleimide linkers which were tested in our lab. In other words, the methods of
the instant
invention can be used to for the immunoconjugates that carry a combination of
drugs, in
particular different drugs, which can be delivered simultaneously and
specifically to a
particular target site, where the therapeutic molecules in the medicament are
highly homo-
geneous, with lot-to-lot consistency. The major advantages of such
immunoconjugates
include: simultaneous targeted delivery of multiple drugs that act
synergistically in target-
ing malignant cells; combining drugs that act in different phases of the cell
cycle to in-
crease the number of target cells exposed to a particular pharmaceutical drug
or effect;
minimized exposure to non-target cells, tissues or organs; precise control
over drug pay-
loads and drug ratios leading to homogenous final products. In short, the
bridge linkers of
the invention can make homogeneous production of specific ADCs in a simple
manner.
SUMMARY OF THE INVENTION
The present invention provides linkers containing a 2,3-disubstituted succinic
group,
or 2-monosubstituted, or 2,3-disubstituted fumaric or maleic (trans (E)- or
cis (Z)-
butenedioic) group to link two drugs to a cell-binding agent (e.g., an
antibody). The
5
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
preferred formula of the cell-binding molecule-linker-drug conjugates can be
represented
Cb4s --Drug2 n
as: , wherein Cb is a cell-binding agent, L Cb is a
cell-binding
agent, L is a linker containing succinic, fumaric or maleic group; Drugl and
Drug2 are a
drug molecule; n is an integer from 1 to 30; and two S (sulfur) elements from
Cb bridgely
link to L, which covalently connects two or more drugs. The advantages in
applying the
linker in the cell molecule-drug conjugate are: a). Retaining the stability of
the conjugates
by covalently cross-linking (re-bridging) the pairs of reduced disulfur atoms
of the cell-
binding agents, particularly of antibodies; b). Enabling conjugation of the
cytotoxic
agents/drugs to specific sites of a cell-binding molecule, e.g. the inter
chain disulfide bond
sites of IgG antibodies, resulting in homogeneous production of ADC.
In one aspect of the present invention, the linker is represented by Formula
(I)
0 0
Z1¨R1¨X1 ___________________________ X2¨R2¨Z2
U' (I)
Wherein
¨ represents an optional single bond;
= represents either a single bond or a double bond;
U and U' represent the same or different leaving group that can be substituted
by a
thiol. Such leaving groups are, but arc not limited to, a halide (e.g.,
fluoride, chloride,
bromide, and iodide), methanesulfonyl (mesyl). p-toluenesulfonyl (tosyl),
trifluoromethylsulfonyl (trifl ate), trifluoromethylsulfonate, nitrophenol, N-
hydroxysuccinimide (NHS), phenol; dinitrophenol; pentafluorophenol,
tetrafluorophenol,
difluorophenol, monofluorophenol, pentachlorophenol, imidazole,
dichlorophenol,
tetrachlorophenol, 1-hydroxybenzotriazole, 2-ethyl-5-phenylisoxazolium-3'-
sulfonate, or
an intermediate molecule generated with a condensation reagent for Mitsunobu
reactions.
It provided that when = represents a single bond, both U and U' are not H;
when
= represents a double bond, either U or U' can be H, but are not H at the same
time.
Zi and Z2 are the same or different a function group that enables to react
with a cyto-
toxic drug, to form a disulfide, ether, ester, thioether, thioester, peptide,
hydrazone,
carbamate, carbonate, amine (secondary, tertiary, or quarter), imine,
cycloheteroalkyane,
heteroaromatic, alkyloxime or amide bond;
R1 and R2 are the same or different, and are absent, linear alkyl having from
1-6 car-
bon atoms, branched or cyclic alkyl having from 3 to 6 carbon atoms, linear,
branched or
6
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
cyclic alkenyl or alkynyl, or 1-6 carbon atoms of esters, ether, amide, or
polyethyleneoxy
unit of formula (OCH2CH2)p, wherein p is an integer from 0 to about 1000, or
combina-
tion thereof.
Additionally Ri and 121 are respectively a chain of atoms selected from C, N,
0, S, Si.
and P, preferably having 0-500 atoms, which covalently connects to Xi or X/
and Zi or
Z7. The atoms used in forming the Ri and R7 may be combined in all chemically
relevant
ways, such as forming alkylene, alkenylene, and alkynylene, ethers,
polyoxyalkylene,
esters, amines, imines, polyamines, hydrazines, hydrazones, amides, ureas,
semicarbazides, carbazides, alkoxyamines, alkoxylamines, urethanes, amino
acids, pep-
tides, acyloxylamines, hydroxamic acids, or combination thereof.
X1 and X2 are independently selected from NH, N(R3), 0, S or CH2; R3 is H,
linear al-
kyl having from 1-6 carbon atoms, branched or cyclic alkyl having from 3 to 6
carbon
atoms, linear, branched or cyclic alkenyl or alkynyl, or 1-6 carbon atoms of
esters, ether,
amide, or polyethyleneoxy unit of formula (0CH2CH2)p, wherein p is an integer
from 0 to
about 1000, or combination thereof.
In another aspect, this invention provides a cell-binding agent-drug conjugate
of For-
mula (II), in which the cell-binding agent. Cb, and the drug. Drugl and Drug2,
have
reacted at the ends of the bridge linker:
0 0
Drug1¨R1 X1 ____________________________ X2¨R2¨Drug2
_____________________________________________________ In
Cb (11)
Wherein:
Ch represents a cell-binding agent, preferred an antibody;
Inside the bracket (parentheses) are the linker-drug components that are
conjugated to
pairs of sulfur atoms of the cell-binding molecule. The sulfur atoms are
preferred pairs of
thiols reduced from the interchain disulfide bonds of the cell-binding agent
by a reduction
agent, such as DTT and/or TCEP;
Drugi and Drug2 represent the same or different cytotoxic agents, which linked
to the
cell-binding agent via the bridge linker by a disulfide, thioether. thioester,
peptide, hydra-
zone. ether, ester, carbamate. carbonate, cycloheteroalkyane, heteroaromatic,
alkoxime or
amide bond;
7
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
n is 1 ¨ 30; "¨", "=", Ri, R2, Xi and X2 are described the same previously in
Formula (I).
In a further aspect, the present invention provides a modified cell-binding
agent of
Formula (III), in which the cell-binding agent, Cb, through its pair of thiols
generated with
reduction of disulfide bonds, has reacted with the bridge linker, which has Z1
and Z2, the
function groups capable of reacting with a drug:
0 0
Z1-R1-X1/ -------------------- \ X2¨R2¨Z2
/S
In
Cb (III)
Wherein "='', Cb, Z1, Z2, n, RI, R2, X1, and X,) are defined the
same as in
Formula (1) and (M.
In an even further aspect, the present invention provides a modified drug of
Formula
(IV), in which the drug, Drug' and Drug2, have reacted with the linker of
Formula (I),
which still has the 2.3-disubstituted succinic group, or 2-monosubstituted, or
2,3-
disubstituted fumaric or maleic (trans (E)- or cis (Z)-butenedioic) group
capable of react-
ing with a pair of sulfur atoms of the cell-binding agent:
0 0
Drug1-121 ____________________________ X2¨R2¨Drug2
11 U' (IV)
Wherein "¨", "=", Drugi, Drug2, U. U', RI, R2, X1, and X2 are defined the same
as in Formula (I) and (II).
The present invention further relates to a method of making a cell-binding
molecule-
drug conjugate of Formula (II), wherein the drugs, Drugi and Drug2 are linked
to a cell-
binding agent via the bridge linker.
The present invention also relates to a method of making a modified cell-
binding mol-
ecule of Formula (III), wherein the cell-binding molecule is reacted with the
bridge linker
of Formula (I).
The present invention also relates to a method of making a modified drug of
formula
(IV), wherein the drug is reacted with the bridge linker of Formula (I).
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows the synthesis of a bridge linker containing polyethylene
glycols and
the application of this linker in the conjugation of an antibody with drugs
via amide bonds.
8
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Figure 2 shows the synthesis of a bridge linker containing polyethylene
glycols and
the application of this linker in the conjugation of drugs to an antibody via
amide bonds.
Figure 3 shows the synthesis of a bridge linker containing polyethylene
glycols and
the application of this linker in the conjugation of drugs to an antibody via
oxime linkage.
Figure 4 shows the synthesis of a bridge linker containing polyethylene
glycols and
the application of this linker in the conjugation of two drugs to an antibody
via hydrazone
linkage.
Figure 5 shows the synthesis of bridge linkers containing polyethylene glycols
and the
application in the conjugation of two different drugs per linker to an
antibody via amide
linkage.
Figure 6 shows the synthesis of bridge linkers and the application in the
conjugation
of two different drugs per linker to an antibody via hinder amide linkage.
Figure 7 shows the synthesis of bridge linkers containing peptides or
polyethylene
glycols and the application of these linkers in the conjugation of two
(different) drugs to
an antibody via hydrazone linkage..
Figure 8 shows the synthesis of the conjugatable analogs of MMAE, Tubulysin
and
PBD cytotoxic drugs.
Figure 9 shows the synthesis of the conjugatable analogs of PBD, MMAF, and
Tubulysin D cytotoxic drugs.
Figure 10 shows the synthesis of the conjugates of cell-binding molecule-
tubulysin
analogs via the bridge-linker.
Figure 11 shows the synthesis of the conjugates of both PBD dimer analog and
Tubulysin B analog per linker, or both MMAE and Tubulysin D analog per linker,
to an
antibody.
Figure 12 shows the synthesis of the conjugates of both PBD dimer analog and
MMAF analog per linker, or both PBD dimer and Tubulysin B analog per linker,
to an
antibody.
Figure 13 shows the synthesis of the conjugates of both Maytansinoid analog
and
Tubulysin B analog per linker, to an antibody.
Figure 14 shows the synthesis of the conjugates of both Maytansinoid analog
and
PBD dimer analog per linker, or two Tubulysin B analogs per linker, to an
antibody.
Figure 15 shows the synthesis of the conjugates of both two MMAF analogs per
link-
er, or two Tubulysin B analogs per linker, containing polyethylene glycols, to
an antibody
9
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Figure 16 shows the comparison of the anti-tumor effect of conjugate compounds
127.
129 and 142 with T-DM1 using human gastric tumor N87 cell model at dosing, 3
mg/kg,
i.v., one injection. All the four conjugates did not cause the animal body
weight loss (top
figure). The animals at control group were sacrificed at day 37 due to the
tumor volume
larger than 1500 mm3and they were too sick. The three compounds 127, 129 and
142 were
better than T-DM1: All 6/6 animals at the groups of compound 127 and 129 had
complete-
ly no tumor measurable at day 13 till day 60 (the end of experiment). All 6/6
animals at the
group of Compound 142 group had no tumor measurable at day 21 and 2/6 animals
had
tumor growth (measurable) back at days 48, which still inhibited the tumor
growth for over
55 days. In contrast T-DM1 at dose of 3 mg/Kg was not able to eradicate the
tumors com-
pletely although it had inhibited the tumor growth for about 28 days.
DETAILED DESCRIPTION OF THE INVENTION
DEFINITIONS
"Alkyl" refers to an aliphatic hydrocarbon group which may be straight or
branched
having 1 to 8 carbon atoms in the chain. "Branched" means that one or more
lower C
numbers of alkyl groups such as methyl, ethyl or propyl are attached to a
linear alkyl
chain. Exemplary alkyl groups include methyl, ethyl, n-propyl, i-propyl, n-
butyl, t-butyl,
n-pentyl, 3-pentyl, octyl, nonyl, decyl, cyclopentyl, cyclohexyl, 2,2-
dimethylbutyl, 2,3-
dimethylbutyl, 2,2-dimethylpentyl, 2,3-dimethylpentyl, 3,3-dimethylpentyl,
2,3.4-
trimethylpentyl, 3-methyl-hexyl, 2,2-dimethylhexyl, 2,4-dimethylhexyl, 2,5-
dimethylhexyl, 3,5-dimethylhexyl, 2,4-dimethylpentyl, 2-methylheptyl. 3-
methylheptyl, n-
heptyl, isoheptyl, n-octyl, and isooctyl. A Ci-C8 alkyl group can be
unsubstituted or substi-
tuted with one or more groups including, but not limited to, -C1-C8 alkyl,-0-
(Ci-C8 alkyl).
-aryl, -C(0)R', -0C(0)R', -C(0)OR', -C(0)NH2. -C(0)NHR', -C(0)N(W)2, -
NHC(0)R', -
SW, -S(0)2R', -S(0)W, -OH, -halogen, -N3, -NH2, -NH(R'), -N(R) 2 and -CN;
where each
R' is independently selected from -C1-C8 alkyl and aryl.
"Halogen" refers to fluorine, chlorine, bromine or iodine atom; preferably
fluorine
and chlorine atom.
"Heteroalkyl" refers to C7-C8 alkyl in which one to four carbon atoms are inde-
pendently replaced with a heteroatom from the group consisting of 0, S and N.
"Carbocycle" refers to a saturated or unsaturated ring having 3 to 8 carbon
atoms as a
monocycle or 7 to 13 carbon atoms as a bicycle. Monocyclic carbocycles have 3
to 6 ring
atoms, more typically 5 or 6 ring atoms. Bicyclic carbocycles have 7 to 12
ring atoms,
arranged as a bicycle [4,5], [5,5], [5,6] or [6,6] system, or 9 or 10 ring
atoms arranged as a
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
bicycle [5,6] or [6,6] system. Representative C3-C8 carbocycles include, but
are not limited
to, -cyclopropyl, -cyclobutyl, -cyclopentyl, -cyclopentadienyl, -cyclohexyl, -
cyclohexenyl,
-1,3-cyclohexadienyl, -1,4-cyclohexadienyl, -cycloheptyl. -1,3-
cycloheptadienyl, -1,3,5-
cycloheptatrienyl, -cyclooctyl, and -cyclooctadienyl.
A "C3-C8 carbocycle" refers to a 3-, 4-, 5-. 6-. 7- or 8-membered saturated or
unsatu-
rated nonaromatic carbocyclic ring. A C3-C8 carbocycle group can be
unsubstituted or
substituted with one or more groups including, but not limited to, -C1-C8
alkyl,-0-(C1-C8
alkyl), -aryl, -C(0)R', -0C(0)R', -C(0)OR', -C(0)NH2, -C(0)NHR', -C(0)N(R')/, -
NHC(0)R', -SW, -S(0)R',-S(0)2R', -OH, -halogen, -N3, -NW -NH(R'), -N(R') / and
-CN;
where each R' is independently selected from -C1-C8 alkyl and aryl.
"Alkenyr refers to an aliphatic hydrocarbon group containing a carbon-carbon
double
bond which may be straight or branched having 2 to 8 carbon atoms in the
chain. Exem-
plary alkenyl groups include ethenyl, propenyl, n-butenyl, i-butenyl, 3-
methylbut-2-enyl,
n-pentenyl, hexylenyl, heptenyl, octenyl.
"Alkynyl" refers to an aliphatic hydrocarbon group containing a carbon-carbon
triple
bond which may be straight or branched having 2 to 8 carbon atoms in the
chain. Exem-
plary alkynyl groups include ethynyl, propynyl, n-butynyl, 2-butynyl, 3-
methylbutynyl, 5-
pentynyl, n-pentynyl, hexylynyl, heptynyl, and octynyl.
"Alkylene" refers to a saturated, branched or straight chain or cyclic
hydrocarbon rad-
ical of 1-18 carbon atoms, and having two monovalent radical centers derived
by the
removal of two hydrogen atoms from the same or two different carbon atoms of a
parent
alkane. Typical alkylene radicals include, but are not limited to: methylene (-
CH2-), 1,2-
ethyl (-CH2CH2-), 1,3-propyl (-CH2CH2CH2-), 1,4-butyl (-CH2CH2CH2CH2-), and
the
like.
"Alkenylene" refers to an unsaturated, branched or straight chain or cyclic
hydrocar-
bon radical of 2-18 carbon atoms, and having two monovalent radical centers
derived by
the removal of two hydrogen atoms from the same or two different carbon atoms
of a
parent alkene. Typical alkenylene radicals include, but are not limited to:
1,2-ethylene (-
CH=CH-).
"Alkynylene" refers to an unsaturated, branched or straight chain or cyclic
hydrocar-
bon radical of 2-18 carbon atoms, and having two monovalent radical centers
derived by
the removal of two hydrogen atoms from the same or two different carbon atoms
of a
parent alkyne. Typical alkynylene radicals include, but are not limited to:
acetylene,
propargyl and 4-pentynyl.
11
08/13/2019 TUE 16:18 FAX
2004/057
"Aryl" or Ax refers to an aromatic or hetero aromatic group, composed of one
or sev-
eral rings, comprising three to fourteen carbon atoms, preferentially six to
ten carbon
atoms. The term of "hetero aromatic group" refers one or several carbon on
aromatic
group, preferentially one, two, three or four carbon atoms are replaced by 0,
N, Si, Se, P
or S, preferentially by 0, S, and N. The term aryl or Ar also refers to an
aromatic group,
wherein one or several H atoms are replaced independently by -R', -halogen, -
OR', or -
SR', -NR'R", -N=NR', -N=R', -NR'R",-NO2, -S(0)R', -S(0)2R', -S(0)20R', -
OS(0)20R', -PR'R", -P(0)R'R", -P(OR")(OR"), -P(0)(OR')(OR") or - -
0P(0)(OR')(OR") wherein R', R" are independently H, alkyl, alkenyl, alkynyl,
heteroal-
kyl, aryl, arylalkyl, carbonyl, or pharmaceutical salts.
"Heterocycle" refers to a ring system in which one to four of the ring carbon
atoms
are independently replaced with a heteroatom from the group of 0, N, S, Se, B,
Si and P.
Preferable heteroatoms are 0, N and S. Heterocycles are also described in The
Handbook
of Chemistry and Physics, 78th Edition, CRC Press, Inc., 1997-1998, P. 225 to
226. Pre-
ferred nonaromatic heterocyclic include, but are not limited to epoxy,
aziridinyl, thiiranyl,
pyrrolidinyl, pyrazolidinyl, imidazolidinyl, oxiranyl, tetrahydrofuranyl,
dioxolanyl, tetra-
hydropyranyl, dioxanyl, dioxolanyl, piperidyl, piperazinyl, motpholinyl,
pyranyl, imidaz-
olinyl, pyrrolinyl, pyrawlinyl, thiazolidinyl, tetrahydrothiopyranyl,
dithianyl, thiomor-
,=
pholinyl, dihydropyranyl, tetrahydropyranyl, dihydropyranyl,
tetrahydropyridyl, dihydro-
pyridyl, tefrahydropyrimidinyl, dihydrothiopyranyl, azepanyl, as well as the
fused systems
resulting from the condensation with a phenyl group.
The term "heteroaryl" or aromatic heterocycles refers to a 5 to 14, preferably
5 to 10
membered aromatic hetero, mono-, bi- or multicyclic ring. Examples include
pyrrolyl,
pyridyl, pyrazolyl, thienyl, pyrimidinyl, pyrazinyl, tetrazolyl, indolyl,
quinolinyl, purinyl,
imidazolyl, thienyl, thiazolyl, benzothiazolyl, furanyl, benwfuranyl, 1,2,4-
thiadiazolyl,
isothiazolyl, triazoyl, tetrazolyl, isoquinolyl, benzothienyl, isobenzofuryl,
pyrazolyl,
carbazolyl, benzimidazolyl, isoxazolyl, pyridyl-N-oxide, as well as the fused
systems
resulting from the condensation with a phenyl group.
"Alkyl", "cycloalkyl", "alkenyl", "allcynyl", "aryl", "heteroaryl",
"heterocyclic" and
the like refer also to the corresponding "ancylene", "cycloalkylene",
"alkenylene", "al-
kynylene", "arylene", "heteroarylene", "heterocyclene" and the likes which are
formed by
the removal of two hydrogen atoms.
"Arylalkyl" refers to an acyclic alkyl radical in which one of the hydrogen
atoms
12
CA 2991975 2019-08-13
PAGE 4157* RCVD AT 811312019 4:23:43 PM [Eastern Daylight lime]'
SVR:OTT2350FAX01/14* DNIS:3905* CSID: *ANI:6132308821* DURATION (mm-ss):10-02
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
bonded to a carbon atom, typically a terminal or sp3 carbon atom, is replaced
with an aryl
radical. Typical arylalkyl groups include, but are not limited to, benzyl, 2-
phenylethan- 1-
yl, 2-phenylethen-l-yl, naphthylmethyl, 2-naphthylethan-1-yl. 2-naphthylethen-
1-yl,
naphthobenzyl, 2-naphthophenylethan-1-y1 and the like.
"HeteroarylalkyF refers to an acyclic alkyl radical in which one of the
hydrogen at-
oms bonded to a carbon atom, typically a terminal or sp3 carbon atom, is
replaced with a
heteroaryl radical. Typical heteroarylalkyl groups include, but are not
limited to, 2-
benzimidazolylmethyl, 2-furylethyl and the like.
Examples of a "hydroxyl protecting group" include, but are not limited to,
methoxymethyl ether, 2-methoxyethoxymethyl ether, tetrahydropyranyl ether,
benzyl
ether, p-methoxybenzyl ether. trimethylsily1 ether, triethylsilyl ether,
triisopropylsilyl
ether, t-butyldimethylsilyl ether, triphenylmethylsilyl ether, acetate ester,
substituted
acetate esters, pivaloate, benzoate, methanesulfonate and p-toluenesulfonate.
"Leaving group" refers to a functional group that can be substituted by
another func-
tional group. Such leaving groups are well known in the art, and examples
include, but are
not limited to, a halide (e.g.. chloride, bromide, and iodide),
methanesulfonyl (mesyl), p-
toluenesulfonyl (tosyl), trifluoromethylsulfonyl (triflatc), and
trifluoromethylsulfonate.
The following abbreviations may be used herein and have the indicated
definitions:
Boc, tert-butoxy carbonyl; BroP, bromotrispyrrolidinophosphonium
hexafluorophosphate;
CDI, 1,1'-carbonyldiimidazole; DCC, dicyclohexylcarbodiimide; DCE,
dichloroethane;
DCM, dichloromethane; DIAD, diisopropylazodicarboxylate; DIBAL-H, diisobutyl-
aluminium hydride; DIPEA, diisopropylethylamine; DEPC, diethyl
phosphorocyanidate;
DMA, N.N-dimethyl acetamide; DMAP, 4-(N, N-dimethylamino)pyridine; DMF, N,N-
dimethylformamide; DMSO, dimethylsulfoxide; DTT, dithiothreitol; EDC, 1-(3-
dimethylaminopropy1)-3-ethylcarbodiimide hydrochloride; ESI-MS, electrospray
mass
spectrometry; HATU, 0-(7-azabenzotriazol-1-y1)-N, N, N', N' -
tetramethyluronium
hexafluorophosphate; HOBt, 1-hydroxybenzotriazole; HPLC, high pressure liquid
chro-
matography; NHS. N-Hydroxysuccinimidc; MMP, 4-methylmorpholine; PAB, p-
aminobenzyl; PBS, phosphate-buffered saline (pH 7.0-7.5); PEG, polyethylene
glycol;
SEC, size-exclusion chromatography; TCEP, tris(2-carboxyethyl)phosphine; TFA,
trifluoroacetic acid; THF, tetrahydrofuran; Val, valine.
"Pharmaceutically" or "pharmaceutically acceptable" refer to molecular
entities and
compositions that do not produce an adverse, allergic or other untoward
reaction when
administered to an animal, or a human, as appropriate.
13
=
08/13/2019 TUE 16: 19 FAX 0005/057
"Pharmaceutically acceptable solvate" or "solvate" refer to an association of
one or
more solvent molecules and a disclosed compound. Examples of solvents that
form phar-
maceutically acceptable solvates include, but are not limited to, water,
isopropanol, etha-
nol, methanol, DMSO, ethyl acetate, acetic acid and ethanolamine.
"Pharmaceutically acceptable excipient" includes any carriers, diluents,
adjuvants, or
vehicles, such as preserving or antioxidant agents, fillers, disintegrating
agents, wetting
agents, emulsifying agents, suspending agents, solvents, dispersion media,
coatings,
antibacterial and antifimgal agents, isotonic and absorption delaying agents
and the like.
The use of such media and agents for pharmaceutical active substances is well
known in
the art. Except insofar as any conventional media or agent is incompatible
with the active
ingredient, its use in the therapeutic compositions is contemplated.
Supplementary active
ingredients can also be incorporated into the compositions as suitable
therapeutic combi-
nations.
As used herein, "pharmaceutical salts" refer to derivatives of the disclosed
compounds
wherein the parent compound is modified by making acid or base salts thereof.
The phar-
maceutically acceptable salts include the conventional non-toxic salts or the
quaternary
ammonium salts of the parent compound formed, for example, from non-toxic
inorganic
or organic acids. For example, such conventional non-toxic salts include those
derived
from inorganic acids such as hydrochloric, hydrobromic, sulfuric, sulfamic,
phosphoric,
nitric and the like; and the salts prepared from organic acids such as acetic,
propionic,
succinic, tartaric, citric, methanesulfonic, benzenesulfonic, glucuronic,
glutamic, benzoic,
salicylic, toluenesulfonic, oxalic, fumaric, maleic, lactic and the like.
Further addition salts
include ammonium salts such as tromethamine, meglumine, epolamine, etc., metal
salts
such as sodium, potassium, calcium, zinc or magnesium.
The pharmaceutical salts of the present invention can be synthesized from the
parent
compound which contains a basic or acidic moiety by conventional chemical
methods.
Generally, such salts can be prepared via reaction the free acidic or basic
forms of these
compounds with a stoichiometric amount of the appropriate base or acid in
water or in an
organic solvent, or in a mixture of the two. Generally, non-aqueous media like
ether, ethyl
acetate, ethanol, isopropanol, or acetonitrile are preferred. Lists of
suitable salts are found
in Retnington's Pharmaceutical Sciences, 17th ed., Mack Publishing Company,
Easton, PA,
1985, p. 1418.
"Administering" or "administration" refers to any mode of transferring,
delivering, in-
troducing or transporting a pharmaceutical drug or other agent to a subject.
Such modes =
14
CA 2991975 2019-08-13
PAGE 5/57* RCVD AT 8113/2019 4:23:43 PM [Eastern Daylight Time]
SVR:OTT2350FAX01114 * DNIS:3905* CSID: *ANI:6132308821 * DURATION (mm-ss):10-
02
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
include oral administration, topical contact, intravenous, intraperitoneal,
intramuscular,
intralesional. intranasal, subcutaneous or intrathecal administration. Also
contemplated by
the present invention is utilization of a device or instrument in
administering an agent.
Such device may utilize active or passive transport and may be slow-release or
fast-release
delivery device.
The novel conjugates disclosed herein use the bridge linkers. Examples of some
suita-
ble linkers and their synthesis are shown in Figures 1 to 15.
THE BRIDGE LINKERS
The synthetic routes to produce bridge linkers as well as the preparation of
the conju-
gates of drugs to a cell binding molecules of the present invention are shown
in Figures 1-
15. The bridge linkers possess two elements: a) A Substituent that is a 2,3-
disubstituted
succinic group; or 2-monosubstituted, or 2,3-disubstituted fumaric group; or 2-
monosubstituted, or 2,3-disubstituted maleic group; which can react to a pair
of thiols to
form covalent thioether bonds, and b) A group, such as but not limited to, a
disulfide,
maleimide, haloacetyl, aldehyde, ketone, azide, amine, alkoxyamine, hydrazide,
ethenesulfonyl, acyl halide (acid halide), acryl (acryloyl), and/or acid
anhydride group.
capable of reaction with a drug. The bridge substituents of 2,3-disubstituted
succinic group;
or 2-monosubstituted, or 2,3-disubstituted fumaric group; or 2-
monosubstituted. or 2,3-
di substituted maleic group; can be introduced by direct condensation of these
2,3-
di substituted succinic acid, or 2-monosubstituted or 2,3-di substituted
fumaric or maleic
with an amine, an alcohol, or a thiol group to form amide, ester or thioester
bonds. The
synthesis of these bridge linkers is exampled in the Figures 1, 3, 4, 5, 6, 7,
10, 11, 12, 13,
14 and 15.
Preferably, the bridge linkers are compounds of the Formula (I) below:
0 0
Z1¨R1¨X1 ___________________________ X2 R2 ¨ Z2
U U' (I)
Wherein
¨ represents an optional single bond;
= represents either a single bond or a double bond;
It provided that when = represents a single bond, both U and U' are not H;
when
= represents a double bond, either U or U' can be H, but are not H at the same
time.
CA 02991975 2018-01-10
WO 2015/155753 PC T/IB2015/056083
0
ill II csS
Wherein the component: U U' ,
which can be 2,3-disubstituted
succinic group, or 2-monosubstituted or 2,3-disubstituted fumaric group, or 2-
monosubstituted or 2,3-disubstituted maleic group, is capable of reacting with
a pair of
sulfur atoms of the cell-binding agent; The sulfur atoms are preferred pairs
of thiols re-
duced from the interchain disulfide bonds of the cell-binding agent by a
reducing agent,
such as dithiothreitol (DTT), dithiocrythritol (DTE), L-glutathione (GSH) and
tris (2-
carboxyethyl) phosphine (TCEP), or/and beta mercaptoethanol (13-ME. 2-ME).
U and U' represent the same or different leaving group that can be substituted
by a
thiol. Such leaving groups are, but are not limited to, a halide (e.g.,
fluoride, chloride,
bromide, and iodide), methanesulfonyl (mesyl), p-toluenesulfonyl (tosyl),
trifluoromethyl-
sulfonyl (Inflate), trifluoromethylsulfonate, nitrophenol, N-
hydroxysuccinimide (NHS),
phenol; dinitrophenol; pentafluorophenol, tetrafluorophenol, difluorophenol,
monofluorophenol, pentachlorophenol, imidazole, dichlorophenol,
tetrachlorophenol, 1-
hydroxybenzotriazole, 2-ethyl-5-phenylisoxazolium-3'-sulfonate, or an
intermediate
molecule generated with a condensation reagent for Mitsunobu reactions.
Zi and Z2 are the same or different a function group that enables to react
with a cyto-
toxic drug, to form a disulfide, thioether, thioester, peptide, hydrazone,
ether, ester,
carbamate, carbonate, amine (secondary, tertiary, or quarter), imine,
cycloheteroalkyane,
heteroaromatic, alkoxime or amide bond;
R1 and R2 are the same or different, and are absent, linear alkyl having from
1-6 car-
bon atoms, branched or cyclic alkyl having from 3 to 6 carbon atoms, linear,
branched or
cyclic alkenyl or alkynyl, or 1-6 carbon atoms of esters, ether, amide, or
polyethyleneoxy
unit of formula (OCH2CH2)p, or polypropyleneoxy unit of formula
(OCH2(CH3)CH2)p
wherein p is an integer from 0 to about 1000, or combination thereof.
Additionally Ri and R2 are respectively a chain of atoms selected from C, N,
0, S, Si.
and P. preferably having 0-500 atoms, which covalently connects to Xi or X2
and Zi or
The atoms used in forming the R1 and R7 may be combined in all chemically
relevant
ways, such as forming alkylene, alkenylene, and alkynylene, ethers,
polyoxyalkylene,
esters, amines, imines, polyamines, hydrazines, hydrazones, amides, ureas,
semicarbazides, carbazides, alkoxyamines, alkoxylamines, urethanes, amino
acids, pep-
tides, acyloxylamines, hydroxamic acids, or combination thereof.
16
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Xi and X2 are independently selected from NH, N(R3), 0, S or Cfb; Wherein R3
is H.
linear alkyl having from 1-6 carbon atoms, branched or cyclic alkyl having
from 3 to 6
carbon atoms, linear, branched or cyclic alkenyl or alkynyl, or 1-6 carbon
atoms of esters,
ether, amide, or polyethyleneoxy unit of formula (0CH2CH2)p, wherein p is an
integer
from 0 to about 1000, or combination thereof.
In another embodiment, Ri, R), and R3, can be respectively a chain of atoms
selected
from C, N, 0, S, Si, and P which covalently connects the cell-surface binding
molecule
and/or the conjugated drug. The atoms used in forming the bridge linker may be
combined
in all chemically relevant ways, such as forming alkylene, alkenylene, and
alkynylene,
ethers, polyoxyalkylene, esters, amines, imines, polyamines, hydrazines,
hydrazones,
amides, ureas, semicarbazides, carbazides, alkoxyamines, alkoxylamines,
urethanes,
amino acids, acyloxylamines, hydroxamic acids, and many others. In addition,
it is to be
understood that the atoms forming the linker (L) may be either saturated or
unsaturated, or
may be radicals, or may be cyclized upon each other to form divalent cyclic
structures,
including cyclo alkanes, cyclic ethers, cyclic amines, arylenes,
heteroarylenes, and the like
in the linker.
Examples of the functional groups, Zi and Z), which enable linkage of a
cytotoxic
drug, include groups that enable linkage via a disulfide, thioether,
thioester, peptide,
hydrazone, ester, carbamate, carbonate, alkoxime or an amide bond. Such
functional
groups include, but are not limited to, thiol, disulfide, amino, carboxy,
aldehydes, ketone,
maleimido, haloacetyl, hydrazines, alkoxyamino, and/or hydroxy.
Examples of the functional groups, Zi and Z2, that enable reaction with the
terminal
of amine of a drug/ cytotoxic agent can be, but not limited to, N-
hydroxysuccinimide
esters, p-nitrophenyl esters, dinitrophenyl esters, pentafluorophenyl esters,
carboxylic
acid chlorides or carboxylic acid anhydride; With the terminal of thiol can
be, as but not
limited to. pyridyldisulfides, nitropyridyldisulfides, maleimides,
haloacetates,
methylsulfone phenyloxadiazole (ODA), carboxylic acid chlorides and carboxylic
acid
anhydride; With the terminal of ketone or aldehyde can be, as but not limited
to, amines,
alkoxyamines, hydrazines, acyloxylamine, or hydrazide; With the terminal of
azide can
be, as but not limited to, alkyne. Examples of these function groups are
displayed below:
0 0 0
((1µ1-0-1L,
0 N-hydroxysuccinimide ester; 0 maleimide;
17
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
0 0
.,S A .õ..L..,.....c22. x --iLss
R5 S disulfide; '2 haloacetyl; 1 acyl
halide (acid
0
0
¨S--A2¨cSS
II csS
halide), 0 ethenesulfonyl; acryl (acryloyl);
0 0
Ts' "---)Ly mõ...0 A
......õ,-1L,7 ...,..`22õ
¨2.---(2?- 2-(tosyloxy)acetyl; - 2 2-(Mesyloxy)acetyl;
0
0 02\.....LL
02N....14..\ 0 ..........A 4,7 i:k...
X2 ---tk
X2---2- 2-(nitrophenoxy)acetyl; 02N 2-
0
(dinitrophenoxy)acetyl; X2-***1 2-
(fluorophenoxy)-acetyl;
0 0
F X2 õ _...,,,
'12a,
2-(difluorophenoxy)-acetyl;
TfA, it.2) - c..õ 2-
I
I
R3 *
(((trifluoromethyl)-sulfonyl)oxy)acetyl; 1 ketone, or aldehyde,
F F 0
N-N
F = Oik%
X2A. k % = css
Me02S_
F F 2-(pentafluorophenoxy)acetyl; 0 ,
tk
methylsulfone phenyloxadiazole (ODA); 0 X()2 R , 1 Ci X2
acid
...----
r..-----%.......sS
1-12N-soesS = NrS R
anhydride, alkyloxyammo; azido, 3 alkynyl, or
H2NHNilss-S hydrazide. Wherein X1 is F, Cl, Br, I or Lv3; X2 is 0, NH, N(Ri),
or CH2; R5
and R3 are H, Ri. aromatic, heteroaromatic, or aromatic group wherein one or
several H
atoms are replaced independently by -R1, -halogen, -0R1, -SR], -NR1R2, - NO2, -
S(0)R1, -
S(0)2R1, or -COORI; Lv3 is a leaving group selected from nitrophenol; N-
hydroxysuc-
cinimide (NHS); phenol; dinitrophenol; pentafluorophenol; tetrafluorophenol;
difluorophenol; monofluorophenol; pentachlorophenol; triflate; imidazole;
dichlorophenol;
tetrachlorophenol; 1-hydroxybenzotriazole; tosylate; mesylate; 2-ethy1-5-
phenylisoxazolium-3'-sulfonate, anhydrides formed its self, or formed with the
other
18
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
anhydride, e.g. acetyl anhydride, formyl anhydride; or an intermediate
molecule generated
with a condensation reagent for peptide coupling reactions, or for Mitsunobu
reactions.
In preferred embodiments, R1, R7, and R3, are linear alkyl having from 1-6
carbon at-
oms, or polyethyleneoxy unit of formula (OCH2CH2)1, p = 1-100
The key step of synthesis of the bridge linker containing 2,3-disubstituted
succinic
group, or 2-monosubstituted or 2,3-disubstituted fumaric group. or 2-
monosubstituted or
2,3-disubstituted maleic group is the condensation of the 2,3-disubstituted
succinic acid, or
2-monosubstituted or 2,3-di substituted fumaric acid, or 2-monosubstituted or
2,3-
di substituted maleic acid, or its acid derivatives, with the other components
containing an
amine (1 or 20 amines), alcohol, or thiol on their terminal, as shown in the
following
scheme (Ia):
0 0 0 0
----------------------- 11 , + X ¨R¨ss5 cs5 R X
Lvi x.,v2 \fl X R
U' U U' (Ia)
Wherein X is X1 or X2 described in Formula (I) as NH, N(R3), 0. or S; R is R1
and/or
R2 that described in Formula (I); R3 is the same defined in Formula (I).
Lvi and Lv-, arc the same or independently OH; F; Cl; Br; I; nitrophenol; N-
hydroxy-
succinimide (NHS); phenol; dinitrophenol; pentafluorophenol;
tetrafluorophenol;
difluorophenol; monofluorophenol; pentachlorophenol; triflate; imidazole;
dichlorophenol;
tetrachlorophenol; 1-hydroxybenzotriazole; tosylate; mesylate; 2-ethy1-5-
phenylisoxazolium-3'-sulfonate, anhydrides formed its self, or formed with the
other
anhydride, e.g. acetyl anhydride, formyl anhydride; or an intermediate
molecule generated
with a condensation reagent for peptide coupling reactions, or for Mitsunobu
reactions, e.g.
condensation reagents are: EDC (N-(3-Dimethylaminopropy1)-N'-
ethylcarbodiimide),
DCC (Dicyclohexyl-carbodiimide), N,N'-Diisopropylcarbodiimide (DIC). N-
Cyclohexyl-
N'-(2-morpholino-ethyl)carbodiimide metho-p-toluenesulfonate (CMC,or CME-CDI),
1,1'-
Carbonyldiimi-dazole (CDI),TBTU (0-(Benzotriazol-1-y1)-N,N.N',N'-
tetramethyluronium
tetrafluoroborate), N,N,N',N1-Tetramethy1-0-(1H-benzotriazol-1-yl)uronium
hexafluorophosphate (HBTU), (Benzotriazol-1-
yloxy)tris(dimethylamino)phosphonium
hexafluorophosphate (BOP), (Benzotriazol -1-yloxy)tripyrrolidinophosphonium
hexafluorophosphate (PyBOP), Diethyl cyanophosphonate (DEPC), Chloro-N,N,N'.N'-
tetramethylformamidinium hexafluorophosphate, 1-[Bis(dimethylamino)methylene]-
1H-
1.2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (HATU), 1-
[(Dimethylami-
no)(morpholino) methylene]-1H-[1,2,3]triazolo[4.5-b]pyridine-l-ium 3-oxide
19
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
hexafluorophosphate (HDMA), 2-Chloro-1,3-dimethylimidazolidinium
hexafluorophosphate (CIP), Chlorotripyrrolidinophosphonium hexafluorophosphate
(PyCloP), Fluoro-N,N,N',N'-bis(tetramethylene)formamidinium
hexafluorophosphate
(BTFFH), N,N.N',N'-Tetramethyl-S-(1-oxido-2-pyridyl)thiuronium
hexafluorophosphate,
0-(2-0xo-1(2H)pyridy1)-N,N,N',N'-tetramethyluronium tetrafluoroborate (TPTU),
S-(1-
Oxido-2-pyridy1)-N,N,N',NLtetramethylthiuronium tetrafluoroborate, 0-
REthoxycarbonyl)
cyano-methylenamino j-N,N,N',N'-tetramethyluronium hexafluorophosphate (HOTU),
(1-
Cyano-2-ethoxy-2-oxoethylidenaminooxy) dimethylamino-morpholino-carbenium
hexafluorophosphate(COMU), 0-(Benzotriazol-1-y1)-N,N,N',N'-bis(tetramethylene)
uronium hexafluorophosphate (HBPyU), N-Benzyl-N'-cyclohexylcarbodiimide (with,
or
without polymer-bound), Dipyrrolidino(N-succinimidyl-oxy)carbenium hexafluoro-
phosphate (HSPyU), Chlorodipyrrolidinocarbenium hexafluorophosphate (PyClU), 2-
Chloro-1,3-dimethylimidazolidinium tetrafluoroborate(CIB), (Benzotriazol-l-
yloxy)
dipiperidinocarbenium hexafluorophosphate (HBPipU), 0-(6-Chlorobenzotriazol-1-
y1)-
N,N,N',N'-tetramethyluronium tetrafluoroborate (TCTU),
Bromotris(dimethylamino)-
phosphonium hexafluorophosphate (BroP), Propylphosphonic anhydride (PPACA, T3P
),
2-Morpholinoethyl isocyanide (MEI), N,N,N',1\1'-Tetramethy1-0-(N-
succinimidyeuronium
hexafluorophosphate (HSTU), 2-Bromo-1-ethyl-pyridinium tetrafluoroborate
(BEP), 0-
[(Ethoxycarbonyl)cyanomethylenamino]-N,N,N'N'-tetra-methyluronium
tetrafluoroborate
(TOTU), 4-(4,6-Dimethoxy-1,3,5-triazin-2-y1)-4-methylmorpholinium chloride
(MMTM,
DMTMM), N,N,N1,NLTetramethyl-0-(N-succinimidyl)uronium tetrafluoroborate
(TSTU),
0-(3,4-Dihydro-4-oxo-1,2,3-benzotriazin-3-y1)-N,N.N',N'-tetramethyluronium
tetrafluoro-
borate (TDBTU), 1,1'-(Azodicarbonyl)dipiperidine (ADD), Di-(4-chlorobenzyl)
azodicar-
boxylate (DCAD), Di-tert-butyl azodicarboxylate (DBAD), Diisopropyl
azodicarboxylate
(DIAD), Diethyl azodicarboxylate (DEAD).
The detail examples of the synthesis of the bridge linkers are shown in the
figures
1-10. Normally the bridge substituents of 2,3-disubstituted succinic group, or
2-
monosubstituted or 2,3-disubstituted fumaric group, or 2-monosubstituted or
2,3-
disubstituted maleic group can be condensated with linker components
containing function
groups capable to react to drugs of desired conjugation.
CELL-BINDING AGENT-DRUG CONJUGATES
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
The conjugates of the present invention can be represented by the following
formula,
Cb4
rug2
n, wherein Cb is a cell-binding agent, L is linker containing succinic,
fumaric or maleic group, Drugj and Drug2 are a drug molecule. n is an integer
from 1 to
30, and two S (sulfur) elements from Cb bridgely link to L, which covalently
connects two
or more drugs (per bridge linker L).
The bridge linker L may be composed of one or more linker components.
Exemplary
linker components include 6-malcimidocaproyl ("MC"), maleimidopropanoyl
("MP"),
valinc-citrulline ("val-cit" or "vc"), alanine-phenylalanine ("ala-phe" or
"af"), p-
aminobenzyloxycarbonyl ("PAB"), 4-thiopentanoate ("SPP"), 4-(N-
maleimidomethyl)-
cyclohexane-1 carboxylate ("MCC"), (4-acetyl)aminobenzoate (STAB"), 4-thio-
butyrate
(SPDB), 4-thio-2-hydroxysulfonyl-butyrate (2-Sulfo-SPDB), ethyleneoxy ¨CH2CH20-
- as
one or more repeating units ("EO" or "PEO"). Additional linker components are
known in
the art and some are described herein.
Example structures of these components containing linkers are:
0
0/11.?\ s
H H
0 ; (MC, 6-maleimidocaproyl containing)
0
0
0 (MP, malcimidopropanoyl containing)
(22:-HN
N
IT H
0 ,(PAB, p-aminobenzyloxycarbonyl containing)
0
0 0 0
}\/,\V
H S
N
.."21
11R S
0 0 0
0
0
IN/\44A1...rt?\ s
0
071..20 \ s )21 NH 0 H
HN II VNI1' S
0 0
0
21
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
(ME, maleimidoethyl containing).
0 0 H 0
H2N N H H 4SI_T jk'N.L
H HN)r_eN ¨ H2¨ II HN
psS
0"
(valine-citrulline containing)
0 0
0 0 0
c=
111--111A¨N11 41, Nje24 SC)-1(1IN/\'
0 0
(MCC. 4-(N-maleimidomethyl)cyclohexane-1 carboxylate)
0 NH0 0 0 0 c_
S II
t/'N N -1021 N'tN N )1(}4
OH H H
((4-acetyl)aminobenzoate containing)
0 0 0
H H
y v.7.1( Ar.,css
HO3S 11,1 HO3S i/
0
(4-thio-2-hydroxysulfonyl-butyrate, 2-sulfo-SPDB)
Preferably, the conjugates have the following Formula (II):
0 0
I I
Drugi¨R1 X1 ¨2¨ ¨2 ¨Drug2
/S
______________________________________________________ In
Ch (II)
wherein:
Cb represents a cell-binding agent, preferably an antibody, which conjugates
to Drugi
and Drug, via a pair of sulfur atoms (thiols). The conjugatable thiol groups
can generally
be generated from dithiothreitol (DTT), dithiocrythritol (DTE), L-glutathione
(GS H)
and tris (2-carboxyethyl) phosphine (TCEP), or/and beta mercaptoethanol (p-ME,
2-ME)
reduction of pairs of disulfide bonds on the surface of cell-binding molecule.
Drugi and Drug2 represent the same or different cytotoxic agents, linked to
the cell-
binding agent via the bridge linker through an alkyl, alkylene, alkenylene,
alkynylene,
ether, polyoxyalkylene, ester, amine, imine, polyamine, hydrazine, hydrazone,
amide, urea,
semicarbazide, carbazide, alkoxyamine, urethanes, amino acid, peptide,
acyloxylamine,
22
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
hydroxamic acid, disulfide, thioether, thioester, carbamate, carbonate,
heterocyclic ring,
heteroalkyl, heteroaromatic, or alkoxime bond, or combination thereof.
n is 1 ¨ 30; "¨'', "=", R1, R2, Xi and X2 are described the same previously in
Formula (I).
As described in more detail below. Drugi and Drug, can be any of many small
mole-
cule drugs, including, but not limited to, tubulysins, calicheamicins,
auristatins,
maytansinoids, CC-1065 analogs, morpholinos doxorubicins, taxanes,
cryptophycins,
epothilones, and benzodiazepine dimers (e.g., dimmers of pyrrolobenzodiazepine
(PBD) or
tomaymycin), indolinobenzodiazepines, imidazobenzothiadiazepines, or
oxazolidinobenzodiazepines).
To synthesize the conjugate, the cell-binding agent can be first modified with
the
bridge linkers of the present invention through reduction of disulfide bonds
of the cell-
binding molecule. The yielded a pair of free thiols can react to the bridge
linker of Formula
(I) at pH 5-9 aqueous media with or without addition of 0-30% of water mixable
(miscible)
organic solvents, such as DMA, DMF, ethanol, methanol, acetone, acetonitrile,
THF,
isopropanol, dioxane, propylene glycol, or ethylene diol, to introduce the
reactive groups
of Zi and Z2 containing disulfide, maleimido, haloacetyl, azide, 1-yne,
ketone, aldehyde,
alkoxyamino, or hydrazide groups. Then a reactive group of a cytotoxic agent
reacts to the
modified cell-binding molecule accordingly. For example, synthesis of the cell-
binding
agent-drug conjugates linked via disulfide bonds is achieved by a disulfide
exchange
between the disulfide bond in the modified cell-binding agent and a drug
containing a free
thiol group. Synthesis of the cell-binding agent-drug conjugates linked via
thioether is
achieved by reaction of the maleimido or haloacetyl or ethylsulfonyl modified
cell-binding
agent and a drug containing a free thiol group. Synthesis of conjugates
bearing an acid
labile hydrazone can be achieved by reaction of a carbonyl group with the
hydrazide
moiety in the linker, by methods known in the art (see, for example, P. Hamann
et al.,
Cancer Res. 53, 3336-334, 1993; B. Laguzza et al.. J. Med. Chem., 32; 548-555,
1959; P.
Trail et al., Cancer Res., 57; 100-105, 1997). Synthesis of conjugates bearing
triazole
linkage can be achieved by reaction of a 1-yne group of the drug with the
azido moiety in
the linker, through the click chemistry (Huisgen cycloaddition) (Lutz, J-F. et
al, 2008, Adv.
Drit2 Del. Rev. 60, 958-970; Sletten, E. M. et al 2011, Acc Chem. Research 44,
666-676).
Alternatively, the drug can react with the bridge linkers of the present
invention that
have conjugated to a cell-binding molecule to give a modified cell-binding
molecule linker
of Formula (III) bearing functionalities. For example, a thiol-containing drug
can be
23
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
reached with the modified cell-binding molecule bridge linker of Formula (III)
bearing a
maleimdo, or a haloacetyl. or an ethylsulfonyl substituent at pH 5.5-9.0 in
aqueous buffer
to give a cell-binding molecule-drug conjugate via a thioether linkage. A
thiol-containing
drug can undergo disulfide exchange with a modified bridge linker of Formula
(III) bear-
ing a pyridyldithio moiety to give a conjugate a disulfide bond linkage. A
drug bearing a
hydroxyl group or a thiol group can be reacted with a modified bridge linker
of Formula
(III) bearing a halogen, particularly the alpha halide of carboxylates, in the
presence of a
mild base, e.g. pH 8.0-9.5, to give a modified drug bearing an ether or thiol
ether link. A
hydroxyl group containing drug can be condensed with a bridge cross linker of
Formula (I)
bearing a carboxyl group, in the presence of a dehydrating agent, such as EDC
or DCC, to
give ester linkage, then the subject drug modified bridge linker undergoes the
conjugation
with a cell-binding molecule. A drug containing an amino group can condensate
with a
carboxyl ester of NHS, imidazole. nitrophenol; N-hydroxysuccinimide (NHS);
phenol;
dinitrophenol; pentafluorophenol; tetrafluorophenol; difluorophenol;
monofluorophenol;
pentachlorophenol; triflate; imidazole; dichlorophenol; tetrachlorophenol; 1-
hydroxybenzotriazole; tosylate; mesylate; 2-ethyl-5-phenylisoxazolium-3'-
sulfonate on the
cell-binding molecule-bridge linker of Formula (III) to give a conjugate via
amide bond
linkage.
The conjugate may be purified by standard biochemical means, such as gel
filtration
on a Sephadex G25 or Sephacryl S300 column, adsorption chromatography, and ion
ex-
change or by dialysis. In some cases, a small molecule as a cell-binding agent
(e.g. folic
acid, melanocyte stimulating hormone, EGF etc) conjugated with a small
molecular drugs
can be purified by chromatography such as by HPLC, medium pressure column
chroma-
tography or ion exchange chromatography.
MODIFIED CELL-BINDING AGENTS/MOLECULES
The cell-binding agent modified by reaction with linkers of the present
invention are
preferably represented by the Formula (III)
0 0
Z1¨R1¨X1 / -------- X2 R2¨ Z2
/S
In
Cb (III)
Wherein "¨", "=", Cb, Zi, Z2, n, RI, R2, X1, and X2 are defined the same as in
Formula (I) and (II).
24
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
In preferred embodiments, Z1 and Z2 are a disulfide substituent, maleimido,
haloacetyl,
alkoxyamine, azido, ketone, aldehyde, hydrazine, alkyne, an N-
hydroxysuccinimide ester,
or a carboxyl ester formed with phenol; dinitrophenol; pentafluorophenol;
tetrafluoro-
phenol; difluorophenol; monofluorophenol; pentachlorophenol; triflate;
imidazole;
dichlorophenol; tetrachlorophenol; 1-hydroxybenzotriazole; tosylate; mesylate;
2-ethy1-5-
phenylisoxa-zolium-3'-sulfonate. Zi and Z2 can then react with a cytotoxic
agent through
disulfide, thioether, hydrazone, amide, alkoxime, carbamate, ester, ether bond
or hetero-
aromatic ring. The modified cell-binding agent can be prepared via a reaction
of the cell-
binding agent with the bridge linkers of Formula (I) as described in Formula
(II) above.
In order to achieve a higher conjugation yield of the alkyne group on the
bridge link-
ers with a pair of free thiols on the cell-binding molecule, preferably on an
antibody, a
small percentage of organic co-solvent may be required to add to the reaction
mixture, as
well in the solution after the reaction to maintain solubility of the Formula
(III) in aqueous
solution. To modify the cell-binding agents, the cross-linking reagent (bridge
linker) of
Formula (I) can be first dissolved in a polar organic solvent that is miscible
with water, for
example different alcohols, such as methanol, ethanol, and propanol, acetone,
acetonitrile,
tetrahydrofuran (THF), 1,4-dioxane, dimethyl formamide (DMF), dimethyl
acetamide
(DMA), or dimethylsulfoxide (DMSO) at a high concentration, for example 1-500
mM.
Meanwhile, the cell-binding molecule, such as antibody dissolved in an aqueous
buffer pH
5-9.5, preferably pH 6-8.5, at 1-35 mg/ml concentration was treated with 1-20
equivalent
of TCEP or DTT for 20 min to 12 hour. After the reduction, DTT can be removed
by SEC
chromatographic purification. TCEP can be optionally removed by SEC
chromatography
too, or staying in the reaction mixture for the next step reaction without
purification.
Furthermore, the reduction of antibodies or the other cell-binding agents with
TCEP can be
performed with a bridge linker of Formula (I), for which the cross-linking
conjugation for
the cell-binding molecules can be achieved simultaneously along with the TCEP
reduction.
The aqueous solutions for the modification of cell-binding agents are buffered
be-
tween pH 6 and 9, preferably between 6.5 and 7.5 and can contain any non-
nucleophilic
buffer salts useful for these pH ranges. Typical buffers include phosphate,
triethanolamine
HCl, HEPES, and MOPS buffers, which can contain additional components, such as
cyclodextrins, sucrose and salts, for examples, NaCl and KC1. After the
addition of the
bridge linker of Formula (I) into the solution containing the reduced cell-
binding molecules,
the reaction mixture is incubated at a temperature of from 4 C to 45 C,
preferably at
ambient temperature. The progress of the reaction can be monitored by
measuring the
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
decrease in the absorption at 254 nm, or increase in the absorption at 280 nm,
or the other
appropriate wavelength. After the reaction is complete, isolation of the
modified cell-
binding agent can be performed in a routine way, using for example gel
filtration chroma-
tography, or adsorptive chromatography.
The extent of modification can be assessed by measuring the absorbance of the
nitropyridine thione, dinitropyridine dithione, pyridine thionc,
carboxamidopyridine
dithione and dicarboxamidopyridine dithione group released via UV spectra. For
the
conjugation without a chromophore group, the modification or conjugation
reaction can be
monitored by LC-MS, preferably by UPLC-QTOF mass spectrometry, or Capil-
lary electrophoresis¨mass spectrometry (CE-MS). The bridge cross-linkers
described
herein have diverse functional groups that can react with any drugs,
preferably cytotoxic
agents that possess a suitable substituent. For examples, the modified cell-
binding mole-
cules bearing an amino or hydroxyl substituent can react with drugs bearing an
N-hydroxysuccinimide (NHS) ester, the modified cell-binding molecules bearing
a thiol
substituent can react with drugs bearing a maleimido or haloacetyl group.
Additionally,
the modified cell-binding molecules bearing a carbonyl (ketone or aldehyde)
substituent
can react with drugs bearing a hydrazide or an alkoxyamine. One skilled in the
art can
readily determine which linker to use based on the known reactivity of the
available func-
tional group on the linkers.
MODIFIED CYTOTOXIC DRUGS
The cytotoxic drugs modified by reaction with cross-linkers of the present
invention
are preferably represented by the Formula (IV):
0 0
Drugi¨R1 X1 ------------ II _________ X2¨R2¨Drug2
U' (IV)
Wherein "¨", "=", U, U', Drug', Drug2, R1, R2, X1, and X2 are defined the same
as in Formula (I) and (II).
The modified drugs can be prepared via reaction of the drug with the linkers
of the
Formula (I) to give a modified drug of Formula (IV) bearing functionality of
an 2,3-
disubstituted succinic group, or 2-monosubstituted or 2,3-disubstituted
fumaric group, or
2-monosubstituted or 2,3-disubstituted maleic group. But for drugs containing
a thiol, or
the drugs undergoing to link a cell-binding molecule via the bridge linkers
through
thioether, thioester or disulfide bond, it is therefore preferred that the
Drug) or Drug2 may
be synthesized to connect to R1, or R2 in a piece of components via the
linkage of thioether,
26
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
thioester or disulfide bond first. Then the synthesized Ri-Drugi or R2-Drug2
component is
assembled to 2.3-disubstituted succinic acid, or 2-monosubstituted or 2,3-
disubstituted
fumaric acid, or 2-monosubstituted or 2,3-disubstituted maleic acid to form
the bridge
linker modified drugs of Formula (IV).
For examples of the synthesis, a thiol-containing drug can be reacted with the
linker of
components R1 or R2 bearing a malcimdo substituent at neutral pH in aqueous
buffer to
give a Ri-Drugi or R2-Drug2 compartment bearing thioether linkage, and
following by
condensation with either 2,3-disubstituted succinic acid, or 2-monosubstituted
or 2,3-
di substituted fumaric acid, or 2-monosubstituted or 2.3-disubstituted maleic
acid to give a
modified drug of Formula (IV) bearing thioether linkage. A drug bearing a
hydroxyl group
can be reacted with a linker component R1 or R2 bearing a halogen, or a
tosylate, or a
mesylate, in the presence of a mild base, to give a Ri-Drugi or R2-Drug2
compartment
bearing ether linkage, and following by condensation with 2,3-disubstituted
succinic acid,
or 2-monosubstituted or 2.3-disubstituted fumaric acid, or 2-monosubstituted
or 2,3-
disubstituted maleic acid to give a modified drug of Formula (IV) bearing
thioether link-
age. A hydroxyl group containing drug can be condensed with a linker of
Formula (I)
bearing a carboxyl group, in the presence of a dehydrating agent, such as EDC
or
dicyclohexylcarbodiimide (DCC), to give a modified drug of Formula (IV) via
ester link-
age. A drug bearing a thiol group can also react the linker of components R1
or R2 bearing
a maleimido or a vinylsulfonyl, or a haloacetyl group, give a Ri-Drugi or R2-
Drug2 com-
partment bearing thioether linkage, and following by condensation with a
compartment of
2,3-disubstituted succinic acid, or 2-monosubstituted or 2,3-disubstituted
fumaric acid, or
2-monosubstituted or 2,3-disubstituted maleic acid to give a modified drug of
Formula
(IV) bearing thioether linkage. An amino group containing drug can similarly
undergo
condensation with a carboxyl group on the bridge linker of Formula (I) to give
a modified
drug of Formula (IV) bearing amide bonds. The modified drug can be purified by
standard
methods such as column chromatography over silica gel or alumina,
crystallization, pre-
paratory thin layer chromatography, ion exchange chromatography, or HPLC.
CELL-BINDING AGENTS
The cell-binding molecule that comprises the conjugates and the modified cell-
binding
agents of the present invention may be of any kind presently known, or that
become
known, molecule that binds to, complexes with, or reacts with a moiety of a
cell population
sought to be therapeutically or otherwise biologically modified.
27
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
The cell binding agents include, but are not limited to, large molecular
weight proteins
such as, for example, full-length antibodies (polyclonal antibodies,
monoclonal antibodies,
dimers, multimers, multispecific antibodies (e.g., bispecific antibodies);
single chain
antibodies; fragments of antibodies such as Fab, Fab', F(ab'),, Fv [Parham, J.
Immunol.
131, 2895-2902 (1983)], fragments produced by a Fab expression library, anti-
idiotypic
(anti-Id) antibodies. CDR's, diabody, triabody, tetrabody, miniantibody, small
immune
proteins (SIP), and epitope-binding fragments of any of the above which immuno-
specifically bind to cancer cell antigens, viral antigens, microbial antigens
or a protein
generated by the immune system that is capable of recognizing, binding to a
specific
antigen or exhibiting the desired biological activity (Miller et al (2003) J.
of Immunology
170:4854-4861); interferons (such as type I, II, III); peptides; lymphokines
such as IL-2,
IL-3, IL-4, IL-5, IL-6, IL-10, GM-CSF, interferon-gamma (IFN-y); hormones such
as
insulin, TRH (thyrotropin releasing hormones), MSH (melanocyte-stimulating
hormone),
steroid hormones, such as androgens and estrogens, melanocyte-stimulating
hormone
(MSH); growth factors and colony-stimulating factors such as epidermal growth
factors
(EGF). granulocyte-macrophage colony-stimulating factor (GM-CSF), transforming
growth factors (TGF), such as TGFct, TGFI3, insulin and insulin like growth
factors (IGF-I,
IGF-II) G-CSF, M-CSF and GM-CSF [Burgess, Immunology Today, 5, 155-158
(1984)];
vaccinia growth factors (VGF); fibroblast growth factors (FGFs); smaller
molecular weight
proteins, poly-peptide, peptides and peptide hormones, such as bombesin,
gastrin, gastrin-
releasing peptide; platelet-derived growth factors; interleukin and cytokines,
such as
interleukin-2 (IL-2), interleukin-6 (IL-6), leukemia inhibitory factors,
granulocyte-
macrophage colony-stimulating factor (GM-CSF); vitamins, such as folate;
apoproteins
and glycoproteins, such as transferrin [O'Keefe et al, 260 J. Biol. Chem. 932-
937 (1985)];
sugar-binding proteins or lipoproteins, such as lectins; cell nutrient-
transport molecules;
and small molecular inhibitors, such as prostate-specific membrane antigen
(PSMA)
inhibitors and small molecular tyrosine kinase inhibitors (TKI), non-peptides
or any other
cell binding molecule or substance, such as bioactive polymers (Dhar, et al,
Proc. Natl.
Acad. Sci. 2008, 105, 17356-61); bioactive dendrimers (Lee, et al, Nat.
Biotechnol. 2005,
23, 1517-26; Almutairi, et al; Proc. Natl. Acad. Sci. 2009, 106, 685-90);
nanoparticles
(Liong, et al, ACS Nano, 2008, 19, 1309-12; Medarova, et al, Nat. Med. 2007,
13, 372-7;
Javier, et al. Bioconjugate Chem. 2008, 19, 1309-12); liposomes (Medinai, et
al, Curr.
Phar. Des. 2004, 10, 2981-9); viral capsides (Flenniken, et al, Viruses
Nanotechnol. 2009,
327, 71-93).
28
08/13/2019 TUE 16: 19 FAx
2006/057
In general, a monoclonal antibody is preferred as a cell-surface binding agent
if an ap-
propriate one is available. And the antibody may be murine, human, humanized,
chimeric,
or derived from other species.
Production of antibodies used in the present invention involves in vivo or in
vitro pro-
cedures or combinations thereof. Methods for producing polyclonal anti-
receptor peptide
antibodies are well-known in the art, such as in U.S. Pat. No. 4,493,795 (to
Nestor et al). A
monoclonal antibody is typically made by fusing myeloma cells with the spleen
cells from
a mouse that has been immunized with the desired antigen (Kohler, G.;
Milstein, C.
(1975). Nature 256: 495-497). The detailed procedures are described in
"Antibodies--A
Laboratory Manual", Harlow and Lane, eds., Cold Spring Harbor Laboratory
Press, New
York (1988). Particularly monoclonal antibodies are produced by immunizing
mice, rats,
hamsters or any other mammal with the antigen of interest such as the intact
target cell,
antigens isolated from the target cell, whole virus, attenuated whole virus,
and viral pro-
teins. Splenocytes are typically fused with myeloma cells using polyethylene
glycol (PEG)
6000. Fused hybrids are selected by their sensitivity to HAT (hypoxanthine-
aminopterin-
thymine). Hybridomas producing a monoclonal antibody useful in practicing this
invention
are identified by their ability to immunoreact specified receptors or inhibit
receptor activity
on target cells.
A monoclonal antibody used in the present invention can be produced by
initiating a
monoclonal hybridoma culture comprising a nutrient medium containing a
hybridoma that
secretes antibody molecules of the appropriate antigen specificity. The
culture is main-
tained under conditions and for a time period sufficient for the hybridoma to
secrete the
antibody molecules into the medium. The antibody-containing medium is then
collected.
The antibody molecules can then be further isolated by well-known techniques,
such as
using protein-A affinity chromatography; anion, cation, hydrophobic, or size
exclusive
chromatographies (particularly by affinity for the specific antigen after
protein A, and
sizing column chromatography); centrifugation, differential solubility, or by
any other
standard technique for the purification of proteins.
Media useful for the preparation of these compositions are both well-known in
the art
and commercially available and include synthetic culture media. An exemplary
synthetic
medium is Dulbecco's minimal essential medium (DMEM; Dulbecco et al., Virol.
8, 396
(1959)) supplemented with 4.5 gm/1 glucose, 0-20 mM glutamine, 0-20% fetal
calf serum,
several ppm amount of heavy metals, such as Cu, Mn, Fe, or Zn, etc, or/and the
heavy
29
CA 2991975 2019-08-13
PAGE 6157* RCVD AT 8113/2019 4:23:43 PM [Eastern Daylight Time] *
SVR:OTT2350FAX01114 * DNIS:3905* CSID: *ANI:6132308821 'DURATION (mm-ss):1 0-
02
08/13/2019 TUE 16: 19 FAX
.0007/057
metals added in their salt forms, and with an anti-foaming agent, such as
polyoxyethyIene-
polyoxypropylene block copolymer.
In addition, antibody-producing cell lines can also be created by techniques
other than
fusion, such as direct transformation of B lymphocytes with oncogenic DNA, or
transfec-
tion with an oncovirus, such as Epstein-Barr virus (EBV, also called human
herpesvirus 4
(HBV-4)) or Kaposi's sarcoma-associated herpesvirus (KSHV). See, U.S. Pat.
Nos.
4,341,761; 4,399,121; 4,427,783; 4,444,887; 4,451,570; 4,466,917; 4,472,500;
4,491,632;
4,493,890. A monoclonal antibody may also be produced via an anti-receptor
peptide or
peptides containing the carboxyl terminal as described well-known in the art.
See Niman et
al., Proc. Natl. Acad. Sci. USA, 80: 4949-4953 (1983); Geysen et al., Proc.
Natl. Acad. Sci.
, USA, 82: 178-182 (1985); Lei etal. Biochemistry 34(20): 6675-
6688, (1995). Typically,
the anti-receptor peptide or a peptide analog is used either alone or
conjugated to an im-
munogenic carrier, as the immunogen for producing anti-receptor peptide
monoclonal
antibodies.
There are also a number of other well-known techniques for making monoclonal
anti-
bodies as binding molecules in this invention. Particularly useful are methods
of making
fully human antibodies. One method is phage display technology which can be
used to
select a range of human antibodies binding specifically to the antigen using
methods of
affinity enrichment. Phage display has been thoroughly described in the
literature and the
construction and screening of phage display libraries are well known in the
art, see, e.g.,
Dente et al, Gene. 148(1):7-13 (1994); Little et al, Biotechnol Adv. 12(3):539-
55 (1994);
Clackson et al., Nature 352:264-628 (1991); Huse et al., Science 246:1275-1281
(1989).
Monoclonal antibodies derived by hybridoma technique from another species than
human, such as mouse, can be humanized to avoid human anti-mouse antibodies
when
infused into humans. Among the more common methods of humanization of
antibodies are
complementarity-determining region grafting and resurfacing. These methods
have been
extensively described, see e.g. U.S. Pat. Nos. 5,859,205 and 6,797,492; Liu et
al, Immunol
Rev. 222:9-27 (2008); Almagro et al, Front Biosci. 13: 1619-33 (2008); Lazar
et at, Mol
Immunol. 44(8):1986-98 (2007); Li et al, Proc. Natl. Acad. Sci. U S A.
103(10):3557-62
(2006). Fully human antibodies can also be prepared by immunizing transgenic
mice,
rabbits, monkeys, or other mammals, carrying large portions of the human
immunoglobu-
lin heavy and light chains, with an immunogen. Examples of such mice are: the
Xen-
omouse. (Abgenix/Amgen), the HuMAb-Mouse (Medarex/BMS), the
VelociMouse (Regeneron), see also U.S. Pat. No. 6,596,541,
CA 2991975 2019-08-13
PAGE 7/57* RCVD AT 8/13/2019 4:23:43 PM [Eastern Daylight Time]'
SVR:OTT235QFAX01/14* DNIS:3905* CSID: *ANI:6132308821 * DURATION (mm-ss):10-02
08/13/2019 TUE 16: 19
FAX EI 0 0. 8 / 0 5 7
= =
6,207,418, No. 6,150,584, No. 6,111,166, No. 6,075,181, No. 5,922,545, Nos.
5,661,016,
5,545,806, 5,436,149 and 5,569,825. In human therapy, murine variable regions
and hu-
man constant regions can also be fused to construct called "chimeric
antibodies" that are
considerably less immunogenic in man than murMe mAbs (Kipriyanov et al, Mol
Biotech-
nol. 26:39-60 (2004); Houdebine, Curr Opin Bioteclmol. 13:625-9 (2002)). In
addition,
site-directed mutagenesis in the variable region of an antibody can result in
an antibody
with higher affinity and specificity for its antigen (Bralmigan et al, Nat Rev
Mol Cell Biol.
3:964-70, (2002)); Adams et al, J Immunol Methods. 231:249-60 (1999)) and
exchanging
constant regions of a mAb can improve its ability to mediate effector
functions of binding
and cytotoxicity.
Antibodies inununospecific for a malignant cell antigen can also be obtained
commer-
cially or produced by any method known to one of skill in the art such as,
e.g., chemical
synthesis or recombinant expression techniques. The nucleotide sequence
encoding anti-
bodies immunospecific for a malignant cell antigen can be obtained
commercially, e.g.,
from the GenBank database or a database like it, the literature publications,
or by routine
cloning and sequencing.
Apart from an antibody, a peptide or protein that bind/block/target or in some
other
way interact with the epitopes or corresponding receptors on a targeted cell
can be used as
a binding molecule. These peptides or proteins could be any random peptide or
proteins
that have an affinity for the epitopes or corresponding receptors and they
don't necessarily
have to be of the immunoglobulin family. These peptides can be isolated by
similar tech-
niques as for phage display antibodies (Szardenings, J Recept Signal Transduct
Res. 2003;
23(4):307-49). The use of peptides from such random peptide libraries can be
similar to
antibodies and antibody fragments. The binding molecules of peptides or
proteins may be
conjugated on or linked to a large molecules or materials, such as, but is not
limited, an
albumin, a polymer, a liposome, a nano particle, a denclzimer, as long as such
attachment
permits the peptide or protein to retain its antigen binding specificity.
Examples of antibodies used for conjugation of drugs via the bridge linkers of
this
prevention for treating cancer, autoimmune disease, and/or infectious disease
include, but
are not limited to, 3F8 (anti-GD2), Abagovomab (anti CA-125), Abciximab (anti
CD41
(integrin alpha-llb), Adalimumab (anti-TNF-a), Adecatumumab (anti-EpCAM,
CD326),
Afelimomab (anti-TNF'-a); Afutuzumab (anti-CD20), Alacizumab pegol (anti-
VEGFR2),
ALD518 (anti-IL-6), Alemtuzumab (Campath, MabCampath, anti- CD52), Altumomab
(anti-CEA), Anatumomab (anti-TAG-72), Anrukinzumab (1MA-638, anti-M-13),
31
CA 2991975 2019-08-13
PAGE 8/57* RCVD AT 811312019 4:23:43 PM [Eastern Daylight Time)*
SVR.OTT235QFAX01/14* DNIS:3905* CSID:*ANI:6132308821* DURATION (mm-ss):10-02
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Apolizumab (anti-HLA-DR), Arcitumomab (anti-CEA), Aselizumab (anti-L-selectin
(CD62L), Atlizumab (tocilizumab, Actemra, RoActemra, anti-IL-6 receptor),
Atorolimumab (anti-Rhesus factor). Bapineuzumab (anti-beta amyloid),
Basiliximab
(Simulect, antiCD25 (a chain of IL-2 receptor), Bavituximab (anti-
phosphatidylserine),
Bectumomab (LymphoScan, anti-CD22), Belimumab (Benlysta, LymphoStat-B, anti-
BAFF), Benralizumab (anti-CD125), Bertilimumab (anti-CCL11 (eotaxin-1)),
Besilesomab (Scintimun, anti-CEA-related antigen), Bevacizumab (Avastin, anti-
VEGF-
A), Biciromab (FibriScint. anti-fibrin II beta chain), Bivatuzumab (anti-CD44
v6),
Blinatumomab (BiTE, anti-CD19), Brentuximab (cACl 0, anti-CD30 TNFRSF8),
Briakinumab (anti-IL-12, IL-23) Canakinumab (Ilaris, anti-IL-1), Cantuzumab
(C242, anti-
CanAg), Capromab, Catumaxomab (Removab, anti-EpCAM, anti-CD3), CC49 (anti-TAG-
72), Cedelizumab (anti-CD4), Certolizumab pegol (Cimzia anti-TNF-a), Cetuximab
(Er-
bitux, IMC-C225, anti-EGFR), Citatuzumab bogatox (anti-EpCAM), Cixutumumab
(anti-
IGF-1), Clenoliximab (anti-CD4), Clivatuzumab (anti-MUC1), Conatumumab (anti-
TRAIL-R2), CR6261 (anti-Influenza A hemagglutinin), Dacetuzumab (anti-CD40),
Daclizumab (Zenapax, anti-CD25 (a chain of IL-2 receptor)), Daratumumab (anti-
CD38
(cyclic ADP ribose hydrolase), Denosumab (Prolia, anti-RANKL), Detumomab (anti-
B-
lymphoma cell), Dorlimomab, Dorlixizumab, Ecromeximab (anti-GD3 ganglioside),
Eculizumab (Soliris, anti-CS), Edobacomab (anti-endotoxin). Edrecolomab
(Panorex,
MAb17-1A, anti-EpCAM). Efalizumab (Raptiva, anti-LFA-1 (CD11a), Efungumab
(Mycograb, anti-Hsp90), Elotuzumab (anti-SLAMF7), Elsilimomab (anti-IL-6).
Enlimomab pegol (anti-ICAM-1 (CD54)), Epitumomab (anti-episialin), Epratuzumab
(anti-CD22), Erlizumab (anti-ITGB2 (CD18)), Ertumaxomab (Rexomun, anti-
HER2/neu,
CD3), Etaracizumab (Abegrin, anti-integrin a[33), Exbivirumab ( anti-hepatitis
B surface
antigen), Fanolesomab (NeutroSpec, anti-CD15), Faralimomab (anti-interferon
receptor),
Farletuzumab (anti-folate receptor 1), Felvizumab (anti-respiratory syncytial
virus),
Fezakinumab (anti-IL-22), Figitumumab (anti-IGF-1 receptor), Fontolizumab
(anti-IFN-1),
Foravirumab (anti-rabies virus glycoprotein), Fresolimumab (anti-TGF-13),
Galiximab
(anti-CD80), Gantenerumab (anti- beta amyloid), Gavilimomab (anti-CD147
(basigin)),
Gemtuzumab (anti-CD33), Girentuximab (anti-carbonic anhydrase 9),
Glembatumumab
(CR011, anti-GPNMB), Golimumab (Simponi, anti-TNF-a), Gomiliximab (anti-CD23
(IgE receptor)). lbalizumab (anti-CD4), Ibritumomab (anti-CD20), Igovomab
(Indimacis-
125, anti-CA-125), Imciromab (Myoscint, anti-cardiac myosin), Infliximab
(Remicade,
anti-TNF-a), Intetumumab (anti-CD51), Inolimomab (anti-CD25 (a chain of IL-2
recep-
32
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
tor)), Inotuzumab (anti-CD22), Ipilimumab (anti-CD152), Iratumumab (anti- CD30
(TNFRSF8)), Keliximab (anti-CD4), Labetuzumab (CEA-Cide, anti-CEA),
Lebrikizumab
(anti- IL-13), Lemalesomab (anti-NCA-90 (granulocyte antigen)), Lerdelimumab
(anti-
TGF beta 2), Lexatumumab (anti-TRAIL-R2), Libivirumab (anti-hepatitis B
surface
antigen), Lintuzumab (anti-CD33), Lucatumumab (anti-CD40), Lumiliximab (anti-
CD23
(IgE receptor), Mapatumumab (anti-TRAIL-R1), Maslimomab (anti- T-cell
receptor),
Matuzumab (anti-EGFR), Mepolizumab (Bosatria, anti-IL-5), Metelimumab (anti-
TGF
beta 1), Milatuzumab (anti-CD74), Minretumomab (anti-TAG-72), Mitumomab (BEC-
2,
anti-GD3 ganglioside), Morolimumab (anti-Rhesus factor), Motavizumab (Numax,
anti-
respiratory syncytial virus), Muromonab-CD3 (Orthoclone OKT3. anti-CD3),
Nacolomab
(anti-C242), Naptumomab (anti-5T4), Natalizumab (Tysabri, anti-integrin
a4),Nebacumab
(anti-endotoxin), Necitumumab (anti-EGFR), Nerelimomab (anti-TNF-a),
Nimotuzumab
(Theracim, Theraloc, anti-EGFR). Nofetumomab, Ocrelizumab (anti-CD20),
Odulimomab
(Afolimomab, anti-LFA-1 (CD11 a)), Ofatumumab (Arzen-a, anti-CD20), Olaratumab
(anti-PDGF-R a), Omalizumab (Xolair, anti-IgE Fc region), Oportuzumab (anti-
EpCAM),
Oregovomab (OvaRex, anti-CA-125), Otelixizumab (anti-CD3), Pagibaximab (anti-
lipoteichoic acid), Palivizumab (Synagis, Abbosynagis, anti-respiratory
syncytial virus),
Panitumumab (Vectibix, ABX-EGF, anti-EGFR), Panobacumab (anti- Pseudotnonas
aeruginosa), Pascolizumab (anti-IL-4), Pemtumomab (Theragyn, anti-MUC1),
Pertuzumab
(Omnitarg, 2C4, anti-HER2/neu), Pexelizumab (anti-05), Pintumomab (anti-
adenocarcinoma antigen), Priliximab (anti-CD4), Pritumumab (anti-vimentin),
PRO 140
(anti-CCR5), Racotumomab (1E10, anti-(N-glycolylneuraminic acid (NeuGc, NGNA)-
gangliosides GM3)), Rafivirumab (anti-rabies virus glycoprotein), Ramucirumab
(anti-
VEGFR2), Ranibizumab (Lucentis, anti-VEGF-A), Raxibacumab (anti-anthrax toxin,
protective antigen), Regavirumab (anti-cytomegalovirus glycoprotein B),
Reslizumab
(anti-IL-5). Rilotumumab (anti-HGF), Rituximab (MabThera, Rituxanmab, anti-
CD20),
Robatumumab (anti-IGF-1 receptor), Rontalizumab (anti-IFN-a), Rovelizumab
(LeukArrest, anti-CD11, CD18), Ruplizumab (Antova, anti-CD154 (CD4OL)),
Satumomab
(anti-TAG-72), Sevirumab (anti-cytomegalovirus), Sibrotuzumab (anti-FAP),
Sifalimumab
(anti-IFN-a), Siltuximab (anti-IL-6), Siplizumab (anti-CD2), (Smart) MI95
(anti-CD33),
Solanezumab (anti-beta amyloid), Sonepcizumab (anti-sphingosine-l-phosphate).
Sontuzumab (anti-episialin), Stamulumab (anti-myostatin), Sulesomab
(LeukoScan, (anti-
NCA-90 (granulocyte antigen), Tacatuzumab (anti-alpha-fetoprotein),
Tadocizumab (anti-
integrin a1d33), Talizumab (anti-IgE), Tanezumab (anti-NGF), Taplitumomab
(anti-CD19),
33
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Tefibazumab (Aurexis. (anti-clumping factor A), Telimomab, Tenatumomab (anti-
tenascin
C), Teneliximab (anti-CD40), Teplizumab (anti-CD3), TGN1412 (anti-CD28),
Ticilimumab (Tremelimumab, (anti-CTLA-4), Tigatuzumab (anti-TRAIL-R2), TNX-650
(anti-IL-13), Tocilizumab (Atlizumab, Actemra, RoActemra, (anti-IL-6
receptor),
Toralizumab (anti-CD154 (CD4OL)), Tositumomab (anti-CD20), Trastuzumab
(Herceptin,
(anti-HER2/neu), Tremelimumab (anti-CTLA-4), Tucotuzumab celmoleukin (anti-
EpCAM), Tuvirumab (anti-hepatitis B virus), Urtoxazumab (anti- Escherichia
coli),
Ustekinumab (Stelara. anti-IL-12, IL-23), Vapaliximab (anti-A0C3 (VAP-1)),
Vedolizumab, (anti-integrin 47), Veltuzumab (anti-CD20). Vepalimomab (anti-
A0C3
(YAP-1), Visilizumab (Nuvion, anti-CD3), Vitaxin (anti-vascular integrin
avb3),
Volociximab (anti-integrin a513i), Votumumab (HumaSPECT, anti-tumor antigen
CTAA16.88), Zalutumumab (HuMax-EGFr, (anti-EGFR), Zanolimumab (HuMax-CD4,
anti-CD4), Ziralimumab (anti-CD147 (basigin)), Zolimomab (anti-CD5),
Etanercept
(Enbre10), Alefacept (Amevivel0), Abatacept (Orencia0), Rilonacept (Arcalyst),
14F7
[anti-lRP-2 (Iron Regulatory Protein 2)], 14G2a (anti-GD2 ganglioside, from
Nat. Cancer
Inst. for melanoma and solid tumors), J591 (anti-PSMA, Weill Cornell Medical
School for
prostate cancers), 225.28S [anti-HMW-MAA (High molecular weight-melanoma-
associated antigen), Sorin Radiofarmaci S.R.L. (Milan, Italy) for melanoma],
COL-1 (anti-
CEACAM3, CGM1, from Nat. Cancer Inst. USA for colorectal and gastric cancers),
CYT-
356 (OncoltadO, for prostate cancers), HNK20 (OraVax Inc. for respiratory
syncytial
virus), ImmuRAIT (from Immunomedics for NHL), Lym-1 (anti-HLA-DR10, Peregrine
Pharm. for Cancers), MAK-195F [anti-TNF (tumor necrosis factor; TNFA, TNF-
alpha;
TNFSF2), from Abbott! Knoll for Sepsis toxic shock], MEDI-500 [T10B9, anti-
CD3,
TRa13 (T cell receptor alpha/beta), complex, from MedImmune Inc for Graft-
versus-host
disease]. RING SCAN [ anti-TAG 72 (tumour associated glycoprotein 72), from
Neoprobe
Corp. for Breast, Colon and Rectal cancers], Avicidin (anti-EPCAM (epithelial
cell adhe-
sion molecule), anti-TACSTD1 (Tumor-associated calcium signal transducer 1),
anti-
GA733-2 (gastrointestinal tumor-associated protein 2), anti-EGP-2 (epithelial
glycoprotein
2); anti-KSA; KS1/4 antigen; M4S; tumor antigen 17-1A; CD326, from NeoRx Corp.
for
Colon, Ovarian, Prostate cancers and NHL]; LymphoCide (Immunomedics, NJ),
Smart
1D10 (Protein Design Labs), Oncolym (Techniclone Inc, CA). Allomune
(BioTransplant,
CA), anti-VEGF (Genentech, CA); CEAcide (Immunomedics, NJ), IMC-1C11 (ImClone
Systems, NJ) and Cetuximab (ImClone, NJ) .
34
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Other antibodies as cell binding molecules/ligands include, but are not
limited to, are
antibodies against the following antigens: Aminopeptidase N (CD13), Annexin
Al, B7-H3
(CD276, various cancers), CA125 (ovarian), CA15-3 (carcinomas), CA19-9
(carcinomas),
L6 (carcinomas), Lewis Y (carcinomas), Lewis X (carcinomas), alpha fetoprotein
(carci-
nomas), CA242 (colorectal), placental alkaline phosphatase (carcinomas),
prostate specific
antigen (prostate), prostatic acid phosphatase (prostate), epidermal growth
factor (carcino-
mas), CD2 (Hodgkin's disease, NHL lymphoma, multiple myeloma), CD3 epsilon (T
cell
lymphoma, lung, breast, gastric, ovarian cancers, autoimmune diseases,
malignant ascites),
CD19 (B cell malignancies), CD20 (non-Hodgkin's lymphoma), CD22 (leukemia, lym-
phoma, multiple myeloma, SLE), CD30 (Hodgkin's lymphoma), CD33 (leukemia, auto-
immune diseases), CD38 (multiple myeloma), CD40 (lymphoma, multiple myeloma,
leukemia (CLL)), CD51 (Metastatic melanoma. sarcoma), CD52 (leukemia), CD56
(small
cell lung cancers, ovarian cancer, Merkel cell carcinoma, and the liquid
tumor, multiple
myeloma), CD66e (cancers), CD70 (metastatic renal cell carcinoma and non-
Hodgkin
lymphoma), CD74 (multiple myeloma), CD80 (lymphoma), CD98 (cancers), mucin
(car-
cinomas), CD221 (solid tumors), CD227 (breast, ovarian cancers), CD262 (NSCLC
and
other cancers), CD309 (ovarian cancers), CD326 (solid tumors), CEACAM3
(colorectal,
gastric cancers), CEACAM5 (carcinoembryonic antigen; CEA, CD66e) (breast,
colorectal
and lung cancers), DLL4 (delta-like-4), EGFR (Epidermal Growth Factor
Receptor, van-
ous cancers), CTLA4 (melanoma), CXCR4 (CD184, Heme-oncology, solid tumors),
Endoglin (CD105, solid tumors), EPCAM (epithelial cell adhesion molecule,
bladder,
head, neck, colon, NHL prostate, and ovarian cancers), ERBB2 (Epidermal Growth
Factor
Receptor 2; lung, breast, prostate cancers), FCGR1 (autoimmune diseases), FOLR
(folate
receptor, ovarian cancers), GD2 ganglioside (cancers). G-28 (a cell surface
antigen
glyvolipid, melanoma), GD3 idiotype (cancers), Heat shock proteins (cancers),
HER1
(lung, stomach cancers), HER2 (breast, lung and ovarian cancers), HLA-DR10
(NHL),
HLA-DRB (NHL, B cell leukemia), human chorionic gonadotropin (carcinoma).
IGF1R
(insulin-like growth factor 1 receptor, solid tumors, blood cancers), IL-2
receptor (interleu-
kin 2 receptor, T-cell leukemia and lymphomas), 1L-6R (interleukin 6 receptor,
multiple
myeloma, RA. Castleman's disease, IL6 dependent tumors), Integrins (av(33,
a5131, (16134,
a11133,ct5135, avI35, for various cancers), MAGE-1 (carcinomas). MAGE-2
(carcinomas),
MAGE-3 (carcinomas), MAGE 4 (carcinomas), anti-transferrin receptor
(carcinomas), p97
(melanoma), MS4A1 (membrane-spanning 4-domains subfamily A member 1, Non-
Hodgkin's B cell lymphoma, leukemia), MUC1 or MUC1-KLH (breast, ovarian,
cervix,
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
bronchus and gastrointestinal cancer), MUC16 (CA125) (Ovarian cancers), CEA
(colorec-
tal), gp100 (melanoma), MARTI (melanoma), MPG (melanoma), MS4A1 (membrane-
spanning 4-domains subfamily A, small cell lung cancers. NHL), Nucleolin, Neu
oncogene
product (carcinomas), P21 (carcinomas), Paratope of anti-(N-glycolylneuraminic
acid,
Breast, Melanoma cancers), PLAP-like testicular alkaline phosphatase (ovarian,
testicular
cancers), PSMA (prostate tumors), PSA (prostate), ROB04, TAG 72 (tumour
associated
glycoprotein 72, AML, gastric, colorectal, ovarian cancers), T cell
transmembrane protein
(cancers), Tie (CD202b), TNFRSF1OB (tumor necrosis factor receptor superfamily
mem-
ber l OB, cancers), TNFRSF13B (tumor necrosis factor receptor superfamily
member 13B,
multiple myeloma, NHL, other cancers, RA and SLE). TPBG (trophoblast
glycoprotein,
Renal cell carcinoma), TRAIL-R1 (Tumor necrosis apoprosis Inducing ligand
Receptor
Llymphoma, NHL, colorectal, lung cancers), VCAM-1 (CD106, Melanoma), VEGF,
VEGF-A, VEGF-2 (CD309) (various cancers). Some other tumor associated antigens
recognized by antibodies have been reviewed (Gerber, et al, mAbs 1:3, 247-253
(2009);
Novellino et al, Cancer Immunol Immunother. 54(3), 187-207 (2005). Franke, et
al, Cancer
Biother Radiopharm. 2000, 15, 459-76).
The cell-binding agents, more preferred antibodies, can be any agents that are
able to
against tumor cells, virus infected cells, microorganism infected cells,
parasite infected
cells, autoimmune cells, activated cells, myeloid cells, activated T-cells, B
cells, or mela-
nocytes. More specifically the cell binding agents can be any agent/molecule
that is able to
against any one of the following antigens or receptors: CD3, CD4, CD5, CD6,
CD7, CD8,
CD9, CD10, CD11a, CD11b, CD11c, CD12w, CD14, CD15, CD16, CDw17, CD18,
CD19, CD20, CD21, CD22, CD23, CD24, CD25, CD26, CD27, CD28, CD29, CD30,
CD31, CD32, CD33, CD34, CD35, CD36, CD37, CD38, CD39, CD40, CD41, CD42,
CD43, CD44, CD45, CD46, CD47, CD48, CD49b, CD49c, CD51, CD52, CD53, CD54,
CD55, CD56. CD58, CD59. CD61, CD62E, CD62L, CD62P, CD63, CD66, CD68, CD69,
CD70, CD72, CD74, CD79, CD79a, CD79b, CD80, CD81, CD82, CD83, CD86, CD87,
CD88, CD89, CD90, CD91, CD95, CD96, CD98, CD100, CD103, CD105, CD106,
CD109, CD117, CD120, CD125, CD126, CD127, CD133, CD134, CD135, CD138,
CD141.CD142.CD143, CD144, CD147, CD151, CD147, CD152, CD154, CD156,
CD158, CD163, CD166, .CD168, CD174, CD180, CD184, CDw186, CD194, CD195,
CD200, CD200a, CD200b, CD209, CD221, CD227, CD235a, CD240, CD262, CD271,
CD274, CD276 (B7-H3), CD303, CD304, CD309, CD326, 4-1BB, 5AC, 5T4 (Trophob1ast
glycoprotein, TPBG, 5T4, Wnt-Activated Inhibitory Factor 1 or WAIF1),
Adenocarcinoma
36
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
antigen, AGS-5, AGS-22M6, Activin receptor-like kinase 1, AFP, AKAP-4, ALK,
Alpha
intergrin, Alpha v beta6, Amino-peptidase N, Amyloid beta, Androgen receptor,
Angiopoietin 2, Angiopoietin 3, Annexin Al, Anthrax toxin protective antigen,
Anti-
transferrin receptor, A0C3 (VAP-1), B7-H3, Bacillus anthracis anthrax, BAFF (B-
cell
activating factor), B-lymphoma cell, bcr-abl, Bombesin, BORIS, C5, C242
antigen, CA125
(carbohydrate antigen 125, MUC16), CA-IX (or CAIX, carbonic anhydrase 9),
CALLA,
CanAg, Canis lupus familiaris IL31, Carbonic anhydrase IX, Cardiac myosin,
CCL11(C-C
motif chemokine 11), CCR4 (C-C chemokine receptor type 4, CD194), CCR5, CD3E
(epsilon), CEA (Carcinoembryonic antigen), CEACAM3, CEACAM5 (carcinoembryonic
antigen), CFD (Factor D), Ch4D5, Cholecystokinin 2 (CCK2R), CLDN18 (Claudin-
18),
Clumping factor A, CRIPTO, FCSF1R (Colony stimulating factor 1 receptor,
CD115),
CSF2 (colony stimulating factor 2, Granulocyte-macrophage colony-stimulating
factor
(GM-CSF)), CTLA4 (cytotoxic T-lymphocyte-associated protein 4), CTAA16.88
tumor
antigen, CXCR4 (CD184), C-X-C chemokine receptor type 4, cyclic ADP ribose
hydrolase,
Cyclin Bl, CYP1B1, Cytomegalovirus, Cytomegalovirus glycoprotein B,
Dabigatran,
DLL4 (delta-like-ligand 4). DPP4 (Dipeptidyl-peptidase 4), DRS (Death receptor
5), E.
coli shiga toxin type-1, E. coli shiga toxin type-2. ED-B, EGFL7 (EGF-like
domain-
containing protein 7), EGFR, EGFRH, EGFRvIH, Endoglin (CD105), Endothelin B
receptor, Endotoxin, EpCAM (epithelial cell adhesion molecule), EphA2,
Episialin,
ERBB2 (Epidermal Growth Factor Receptor 2), ERBB3, ERG (TMPRSS2 ETS fusion
gene), Escherichia coli, ETV6-AML, FAP (Fibroblast activation protein alpha),
FCGR1,
alpha-Fetoprotein, Fibrin II, beta chain, Fibronectin extra domain-B, FOLR
(folate recep-
tor), Folate receptor alpha. Folate hydrolase, Fos-related antigen 1.F protein
of respiratory
syncytial virus, Frizzled receptor, Fucosyl GMl. GD2 ganglioside, G-28 (a cell
surface
antigen glyvolipid), GD3 idiotype, GloboH, Glypican 3, N-glycolylneuraminic
acid, GM3,
GMCSF receptor a-chain, Growth differentiation factor 8, GP100, GPNMB
(Transmembrane glycoprotein NMB), GUCY2C (Guanylate cyclase 2C, guanylyl
cyclase
C(GC-C), intestinal Guanylate cyclase, Guanylate cyclase-C receptor, Heat-
stable entero-
toxin receptor (hSTAR)), Heat shock proteins, Hemagglutinin, Hepatitis B
surface antigen,
Hepatitis B virus, HER1 (human epidermal growth factor receptor 1), HER2,
HER2/neu,
HER3 (ERBB-3), IgG4, HGF/SF (Hepatocyte growth factor/scatter factor), HHGFR,
HIV-
1. Histone complex, HLA-DR (human leukocyte antigen), HLA-DR10, HLA-DRB ,
HMWMAA, Human chorionic gonadotropin, HNGF, Human scatter factor receptor
kinase,
HPV E6/E7, Hsp90, hTERT, ICAM-1 (Intercellular Adhesion Molecule 1), Idiotype,
37
CA 02991975 2018-01-10
WO 2015/155753
PCT/IB2015/056083
IGF1R (IGF-1, insulin-like growth factor 1 receptor), IGHE, IFN-y, Influeza
hemag-
glutinin, IgE, IgE Fc region, IGHE, IL-1, IL-2 receptor (interleukin 2
receptor), IL-4. IL-5,
IL-6, IL-6R (interleukin 6 receptor), IL-9, IL-10, IL-12, IL-13, IL-17, IL-
17A, IL-20, IL-
22, IL-23, 1L3 IRA, ILGF2 (Insulin-like growth factor 2), Integrins (a4,
alls133, avf33. a4137,
a5f31, a6f34, a7f37, a11133. a5f35, avf35), Interferon gamma-induced protein,
ITGA2, ITGB2,
LCK, Le, Legumain, Lewis-Y antigen, LFA-1(Lymphocyte function-associated
antigen 1, CD11 a), LHRH, LINGO-1, Lipoteichoic acid, LIVIA, LMP2, LTA, MAD-CT-
1, MAD-CT-2, MAGE-1, MAGE-2, MAGE-3, MAGE Al, MAGE A3, MAGE 4,
MART 1, MCP-1, MW (Macrophage migration inhibitory factor, or glycosylation-
inhibiting factor (GIF)), MS4A1 (membrane-spanning 4-domains subfamily A
member 1),
MSLN (mesothelin), MUC1(Mucin 1, cell surface associated (MUC1) or polymorphic
epithelial mucin (PEM)), MUC1-KLH. MUC16 (CA125), MCP1(monocyte chemotactic
protein 1). MelanA/MART1, ML-IAP, MPG, MS4A1 (membrane-spanning 4-domains
subfamily A). MYCN, Myelin-associated glycoprotcin, Myostatin, NA17, NARP-1,
NCA-
90 (granulocyte antigen), Nectin-4 (ASG-22ME), NGF, Neural apoptosis-regulated
pro-
teinase 1, NOGO-A, Notch receptor. Nucleolin, Neu oncogene product, NY-BR-1,
NY-
ESO-1, OX-40, OxLDL (Oxidized low-density lipoprotein), 0Y-TES1, P21, p53
nonmutant, P97, Page4, PAP, Paratope of anti-(N-glycolylneuraminic acid),
PAX3, PAX5,
PCSK9, PDCD1 (PD-1. Programmed cell death protein 1,CD279), PDGF-Ra (Alpha-
type
platelet-derived growth factor receptor), PDGFR-13, PDL-1, PLAC1, PLAP-like
testicular
alkaline phosphatase, Platelet-derived growth factor receptor beta, Phosphate-
sodium co-
transporter, PMEL 17, Polysialic acid, Proteinase3 (PR1), Prostatic carcinoma,
PS
(Phosphatidylserine), Prostatic carcinoma cells, Pseudomonas aeruginosa, PSMA,
PSA,
PSCA, Rabies virus glycoprotein, RHD (Rh polypeptide 1 (RhPI). CD240), Rhesus
factor,
RANKL, RhoC, Ras mutant, RGS5, ROB04, Respiratory syncytial virus, RON,
Sarcoma
translocation breakpoints, SART3, Sclerostin, SLAMF7 (SLAM family member
7), Selectin P, SDC1 (Syndecan 1), sLe(a), Somatomedin C, SIP (Sphingosine-1 -
phosphate), Somatostatin, Sperm protein 17, SSX2, STEAP1 (six-transmembrane
epitheli-
al antigen of the prostate 1), STEAP2, STn, TAG-72 (tumor associated
glycoprotein 72),
Survivin, T-cell receptor, T cell transmembrane protein, TEM1 (Tumor
endothelial marker
1), TENB2, Tenascin C (TN-C), TGF-
13 (Transforming growth factor beta), TGF-
f31, TGF-f32 (Transforming growth factor-beta 2), Tie (CD202b), Tie2, TIM-1
(CDX-014),
Tn, TNF,
TNFRSF8, TNFRSF1OB (tumor necrosis factor receptor superfamily
member 10B), TNFRSF13B (tumor necrosis factor receptor superfamily member
13B),
38
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
TPBG (trophoblast glycoprotein), TRAIL-R1 (Tumor necrosis apoprosis Inducing
ligand
Receptor 1), TRAILR2 (Death receptor 5 (DR5)), tumor-associated calcium signal
trans-
ducer 2, tumor specific glycosylation of MUCL TWEAK receptor,
TYRP1(glycoprotein
75), TRP-2, Tyrosinase, VCAM-1 (CD106), VEGF, VEGF-A, VEGF-2 (CD309),
VEGFR-1, VEGFR2, or vimentin, WT1, XAGE 1, or cells expressing any insulin
growth
factor receptors, or any epidermal growth factor receptors.
In another specific embodiment, the cell-binding ligand-drug conjugates via
the bridge
linkers of this invention are used for the targeted treatment of cancers. The
targeted cancers
include, but are not limited, Adrenocortical Carcinoma, Anal Cancer, Bladder
Cancer,
Brain Tumor (Adult, Brain Stem Glioma, Childhood, Cerebellar Astrocytoma,
Cerebral
Astrocytoma, Ependymoma, Medulloblastoma, Supratentorial Primitive
Neuroectodermal
and Pineal Tumors, Visual Pathway and Hypothalamic Glioma), Breast Cancer,
Carcinoid
Tumor, Gastrointestinal, Carcinoma of Unknown Primary, Cervical Cancer, Colon
Cancer,
Endometrial Cancer, Esophageal Cancer, Extrahepatic Bile Duct Cancer, Ewings
Family
of Tumors (PNET), Extracranial Germ Cell Tumor, Eye Cancer, Intraocular
Melanoma,
Gallbladder Cancer, Gastric Cancer (Stomach), Germ Cell Tumor, Extragonadal,
Gesta-
tional Trophoblastic Tumor, Head and Neck Cancer, Hypopharyngeal Cancer, Islet
Cell
Carcinoma, Kidney Cancer (renal cell cancer), Laryngeal Cancer, Leukemia
(Acute Lym-
phoblastic, Acute Myeloid, Chronic Lymphocytic, Chronic Myelogenous, Hairy
Cell), Lip
and Oral Cavity Cancer, Liver Cancer, Lung Cancer (Non-Small Cell, Small Cell,
Lym-
phoma (AIDS-Related, Central Nervous System, Cutaneous T-Cell, Hodgkin's
Disease,
Non-Hodgkin's Disease, Malignant Mesothelioma, Melanoma, Merkel Cell
Carcinoma,
Metasatic Squamous Neck Cancer with Occult Primary, Multiple Myeloma, and
Other
Plasma Cell Neoplasms, Mycosis Fungoides, Myelodysplastic Syndrome, Myeloproli-
ferative Disorders, Nasopharyngeal Cancer, Neuroblastoma, Oral Cancer,
Oropharyngeal
Cancer, Osteosarcoma, Ovarian Cancer (Epithelial, Germ Cell Tumor, Low
Malignant
Potential Tumor), Pancreatic Cancer (Exocrine, Islet Cell Carcinoma),
Paranasal Sinus and
Nasal Cavity Cancer, Parathyroid Cancer, Penile Cancer, Pheochromocytoma
Cancer,
Pituitary Cancer, Plasma Cell Neoplasm, Prostate Cancer Rhabdomyosarcoma,
Rectal
Cancer, Renal Cell Cancer (kidney cancer), Renal Pelvis and Ureter
(Transitional Cell),
Salivary Gland Cancer, Sezary Syndrome, Skin Cancer, Skin Cancer (Cutaneous T-
Cell
Lymphoma, Kaposi's Sarcoma, Melanoma), Small Intestine Cancer, Soft Tissue
Sarcoma,
Stomach Cancer, Testicular Cancer, Thymoma (Malignant), Thyroid Cancer,
Urethral
39
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Cancer, Uterine Cancer (Sarcoma), Unusual Cancer of Childhood, Vaginal Cancer,
Vulvar
Cancer, Wilms Tumor.
In another specific embodiment, the cell-binding-drug conjugates via the
bridge likers
of this invention are used in accordance with the compositions and methods for
the treat-
ment or prevention of an autoimmune disease. The autoimmune diseases include,
but are
not limited, Achlorhydra Autoimmune Active Chronic Hepatitis, Acute
Disseminated
Encephalomyelitis, Acute hemorrhagic leukoencephalitis, Addison's Disease,
Agammaglobulinemia, Alopecia areata, Amyotrophic Lateral Sclerosis, Ankylosing
Spon-
dylitis, Anti-GBM/TBM Nephritis, Antiphospholipid syndrome, Anti synthetase
syndrome,
Arthritis, Atopic allergy, Atopic Dermatitis, Autoimmune Aplastic Anemia,
Autoimmune
cardiomyopathy, Autoimmune hemolytic anemia, Autoimmune hepatitis, Autoimmune
inner ear disease, Autoimmune lymphoproliferative syndrome, Autoimmune
peripheral
neuropathy, Autoimmune pancreatitis, Autoimmune polyendocrine syndrome Types
I, II,
& III, Autoimmune progesterone dermatitis, Autoimmune thrombocytopenic
purpura,
Autoimmune uveitis, Balo disease/Balo concentric sclerosis, Bechets Syndrome,
Berger's
disease. Bickerstaffs encephalitis. Blau syndrome, Bullous Pemphigoid,
Castleman's
disease, Chagas disease, Chronic Fatigue Immune Dysfunction Syndrome, Chronic
in-
flammatory demyelinating polyneuropathy, Chronic recurrent multifocal
ostomyelitis,
Chronic lyme disease, Chronic obstructive pulmonary disease, Churg-Strauss
syndrome,
Cicatricial Pemphigoid, Coeliac Disease, Cogan syndrome, Cold agglutinin
disease, Com-
plement component 2 deficiency, Cranial arteritis, CREST syndrome, Crohns
Disease (a
type of idiopathic inflammatory bowel diseases), Cushing's Syndrome. Cutaneous
leukocytoclastic angiitis. Dego's disease, Dercum's disease, Dermatitis
herpetiformis,
Dermatomyositis, Diabetes mellitus type 1, Diffuse cutaneous systemic
sclerosis, Dress-
ler's syndrome, Discoid lupus erythematosus, Eczema, Endometriosis, Enthesitis-
related
arthritis, Eosinophilic fasciitis, Epidermolysis bullosa acquisita, Erythema
nodosum,
Essential mixed cryoglobulinemia, Evan's syndrome, Fibrodysplasia ossificans
progressiva. Fibromyalgia, Fibromyositis, Fibrosing aveolitis, Gastritis,
Gastrointestinal
pemphigoid, Giant cell arteritis, Glomerulonephritis, Goodpasture's syndrome,
Graves'
disease. Guillain-Barre syndrome, Hashimoto's encephalitis, Hashimoto's
thyroiditis,
Haemolytic anaemia, Henoch-Schonlein purpura. Herpes gestationis, Hidradenitis
suppurativa, Hughes syndrome (See Antiphospholipid syndrome), Hypogamma-
globulinemia, Idiopathic Inflammatory Demyelinating Diseases, Idiopathic
pulmonary
fibrosis, Idiopathic thrombocytopenic purpura (See Autoimmune thrombocytopenic
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
purpura), IgA nephropathy (Also Berger's disease), Inclusion body myositis,
Inflammatory
demyelinating polyneuopathy, Interstitial cystitis, Irritable Bowel Syndrome ,
Juvenile
idiopathic arthritis, Juvenile rheumatoid arthritis, Kawasaki's Disease.
Lambert-Eaton
myasthenic syndrome, Leukocytoclastic vasculitis, Lichen planus, Lichen
sclerosus, Linear
IgA disease (LAD), Lou Gehrig's Disease (Also Amyotrophic lateral sclerosis),
Lupoid
hepatitis, Lupus erythematosus, Majeed syndrome, Moniere's disease,
Microscopic
polyangiitis, Miller-Fisher syndrome, Mixed Connective Tissue Disease,
Morphea, Mucha-
Habermann disease, Muckle¨Wells syndrome, Multiple Myeloma, Multiple
Sclerosis,
Myasthenia gravis, Myositis, Narcolepsy, Neuromyelitis optica (Devic's
Disease),
Neuromyotonia, Occular cicatricial pemphigoid, Opsoclonus myoclonus syndrome,
Ord
thyroiditis, Palindromic rheumatism, PANDAS (Pediatric Autoimmune
Neuropsychiatric
Disorders Associated with Streptococcus), Paraneoplastic cerebellar
degeneration, Parox-
ysmal nocturnal hemoglobinuria, Parry Romberg syndrome, Parsonnage-Turner
syndrome.
Pars planitis. Pemphigus, Pemphigus vulgaris, Pernicious anaemia, Perivenous
encephalo-
myelitis, POEMS syndrome, Polyarteritis nodosa, Polymyalgia rheumatica,
Polymyositis,
Primary biliary cirrhosis, Primary sclerosing cholangitis, Progressive
inflammatory neu-
ropathy, Psoriasis, Psoriatic Arthritis, Pyoderma gangrenosum, Pure red cell
aplasia,
Rasmussen's encephalitis, Raynaud phenomenon, Relapsing polychondritis,
Reiter's syn-
drome, Restless leg syndrome, Retroperitoneal fibrosis, Rheumatoid arthritis,
Rheumatoid
fever, Sarcoidosis, Schizophrenia, Schmidt syndrome, Schnitzler syndrome,
Scleiitis,
Scleroderma, Sjogren's syndrome, Spondyloarthropathy, Sticky blood syndrome,
Still's
Disease, Stiff person syndrome, Subacute bacterial endocarditis, Susac's
syndrome, Sweet
syndrome. Sydenham Chorea, Sympathetic ophthalmia, Takayasu's arteritis,
Temporal
arteritis (giant cell arteritis), Tolosa-Hunt syndrome, Transverse Myelitis,
Ulcerative
Colitis (a type of idiopathic inflammatory bowel diseases), Undifferentiated
connective
tissue disease, Undifferentiated spondyloarthropathy, Vasculitis, Vitiligo,
Wegener's
granulomatosis, Wilson's syndrome, Wiskott-Aldrich syndrome
In another specific embodiment, a binding molecule used for the conjugate via
the
bridge linkers of this invention for the treatment or prevention of an
autoimmune disease
can be. but are not limited to, anti-elastin antibody; Abys against epithelial
cells antibody;
Anti-Basement Membrane Collagen Type IV Protein antibody; Anti-Nuclear
Antibody;
Anti ds DNA; Anti ss DNA, Anti Cardiolipin Antibody IgM, IgG; anti-celiac
antibody;
Anti Phospholipid Antibody IgK, IgG; Anti SM Antibody; Anti Mitochondria'
Antibody;
Thyroid Antibody; Microsomal Antibody, T-cells antibody; Thyroglobulin
Antibody, Anti
41
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
SCL-70; Anti-Jo; Anti-U<sub>1RNP</sub>; Anti-La/SSB; Anti SSA; Anti SSB; Anti
Perital Cells
Antibody; Anti Histones; Anti RNP; C-ANCA; P-ANCA; Anti centromere; Anti-
Fibrillarin, and Anti GBM Antibody, Anti-ganglioside antibody; Anti-Desmogein
3 anti-
body; Anti-p62 antibody; Anti-sp100 antibody; Anti-Mitochondrial(M2) antibody;
Rheu-
matoid factor antibody; Anti-MCV antibody; Anti-topoisomerase antibody; Anti-
neutrophil cytoplasmic(cANCA) antibody.
In certain preferred embodiments, the binding molecule for the conjugate in
the pre-
sent invention, can bind to both a receptor and a receptor complex expressed
on an activat-
ed lymphocyte which is associated with an autoimmune disease. The receptor or
receptor
complex can comprise an immunoglobulin gene superfamily member (e.g. CD2, CD3,
CD4. CD8, CD19, CD20, CD22, CD28, CD30, CD33, CD37, CD38, CD56, CD70, CD79,
CD79b, CD90, CD125, CD147, CD152/CTLA-4, PD-1, or ICOS), a TNF receptor super-
family member (e.g. CD27, CD40, CD95/Fas, CD134/0X40, CD137/4-1BB, INF-R1,
TNFR-2, RANK, TACI, BCMA, osteoprotegerin, Apo2/TRAIL-R1, TRAIL-R2, TRAIL-
R3, TRAIL-R4, and APO-3), an integrin, a cytokine receptor, a chemokine
receptor, a
major histocompatibility protein, a lectin (C-type, S-type, or I-type), or a
complement
control protein.
In another specific embodiment, useful cell binding ligands that are
immunospecific
for a viral or a microbial antigen are humanized or human monoclonal
antibodies. As used
herein, the term "viral antigen" includes, but is not limited to, any viral
peptide, polypep-
tide protein (e.g. HIV gp120, HIV nef, RSV F glycoprotein, influenza virus
neuramimi-
dase, influenza virus hemagglutinin, HTLV tax, herpes simplex virus
glycoprotein (e.g.
gB, gC, gD, and gE) and hepatitis B surface antigen) that is capable of
eliciting an immune
response. As used herein, the term "microbial antigen" includes, but is not
limited to, any
microbial peptide, polypeptide, protein, saccharide, polysaccharide, or lipid
molecule (e.g.,
a bacteria, fungi, pathogenic protozoa, or yeast polypeptides including, e.g.,
LPS and
capsular polysaccharide 5/8) that is capable of eliciting an immune response.
Examples of
antibodies available 1 for the viral or microbial infection include, but are
not limited to,
Palivizumab which is a humanized anti-respiratory syncytial virus monoclonal
antibody for
the treatment of RSV infection; PR0542 which is a CD4 fusion antibody for the
treatment
of HIV infection; Ostavir which is a human antibody for the treatment of
hepatitis B virus;
PROTVIR which is a humanized IgG<sub>1</sub> antibody for the treatment of
cytomegalovirus;
and anti-LPS antibodies.
42
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
The cell binding molecules¨drug conjugates via the bridge linkers of this
invention
can be used in the treatment of infectious diseases. These infectious diseases
include, but
are not limited to, Acinetobacter infections. Actinomycosis, African sleeping
sickness
(African trypanosomiasis), AIDS (Acquired immune deficiency syndrome),
Amebiasis,
Anaplasmosis, Anthrax, Arcanobacterium haemolyticum infection, Argentine
hemorrhagic
fever, Ascariasis. Aspergillosis. Astrovirus infection, Babesiosis, Bacillus
cereus infection,
Bacterial pneumonia, Bacterial vaginosis, Bacteroides infection,
Balantidiasis,
Baylisascaris infection, BK virus infection, Black piedra, Blastocystis
hominis infection,
Blastomycosis, Bolivian hemorrhagic fever, Borrelia infection. Botulism (and
Infant
botulism), Brazilian hemorrhagic fever, Brucellosis, Burkholderia infection,
Buruli ulcer,
Calicivirus infection (Norovirus and Sapovirus), Campylobacteriosis,
Candidiasis
(Moniliasis; Thrush), Cat-scratch disease, Cellulitis, Chagas Disease
(American
trypanosomiasis), Chancroid, Chickenpox, Chlamydia, Chlamydophila pneumoniae
infec-
tion, Cholera, Chromoblastomycosis, Clonorchiasis, Clostridium difficile
infection,
Coccidioidomycosis, Colorado tick fever, Common cold (Acute viral
rhinopharyngitis;
Acute coryza). Creutzfeldt-Jakob disease, Crimean-Congo hemorrhagic fever,
Cryptococ-
cosis, Cryptosporidiosis, Cutaneous larva migrans, Cyclosporiasis,
Cysticercosis, Cyto-
megalovirus infection, Dengue fever, Dientamoebiasis, Diphtheria,
Diphyllobothriasis,
Dracunculiasis, Ebola hemorrhagic fever, Echinococcosis, Ehrlichiosis.
Enterobiasis
(Pinworm infection), Enterococcus infection, Enterovirus infection, Epidemic
typhus,
Erythema infectiosum (Fifth disease), Exanthem subitum, Fasciolopsiasis,
Fasciolosis,
Fatal familial insomnia, Filariasis, Food poisoning by Clostridium
perfringens, Free-living
amebic infection, Fusobacterium infection, Gas gangrene (Clostridial
myonecrosis),
Geotrichosis, Gerstmann-Straussler-Scheinker syndrome, Giardiasis, Glanders,
Gnathosto-
miasis. Gonorrhea. Granuloma inguinale (Donovanosis), Group A streptococcal
infection,
Group B streptococcal infection, Haemophilus influenzae infection, Hand, foot
and mouth
disease (HFMD), Hantavirus Pulmonary Syndrome, Helicobacter pylori infection,
Hemo-
lytic-uremic syndrome, Hemorrhagic fever with renal syndrome, Hepatitis A,
Hepatitis B,
Hepatitis C, Hepatitis D, Hepatitis E, Herpes simplex, Histoplasmosis,
Hookworm infec-
tion, Human bocavirus infection, Human ewingii ehrlichiosis, Human
granulocytic
anaplasmosis, Human metapneumovirus infection, Human monocytic ehrlichiosis,
Human
papillomavirus infection, Human parainfluenza virus infection, Hymenolepiasis,
Epstein-
Barr Virus Infectious Mononucleosis (Mono). Influenza, Isosporiasis, Kawasaki
disease,
Keratitis, Kingella kingae infection, Kuru, Lassa fever, Legionellosis
(Legionnaires'
43
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
disease), Legionellosis (Pontiac fever), Leishmaniasis, Leprosy,
Leptospirosis, Listeriosis,
Lyme disease (Lyme borreliosis), Lymphatic filariasis (Elephantiasis),
Lymphocytic
choriomeningitis, Malaria, Marburg hemorrhagic fever, Measles, Melioidosis
(Whitmore's
disease), Meningitis, Meningococcal disease, Metagonimiasis, Microsporidiosis,
Molluscum contagiosum, Mumps, Murine typhus (Endemic typhus), Mycoplasma pneu-
monia, Mycetoma, Myiasis, Neonatal conjunctivitis (Ophthalmia neonatorum),
(New)
Variant Creutzfeldt-Jakob disease (vCJD, nvCJD), Nocardiosis, Onchocerciasis
(River
blindness), Paracoccidioidomycosis (South American blastomycosis),
Paragonimiasis,
Pasteurellosis, Pediculosis capitis (Head lice), Pediculosis corporis (Body
lice), Pediculosis
pubis (Pubic lice, Crab lice), Pelvic inflammatory disease, Pertussis
(Whooping cough),
Plague, Pneumococcal infection, Pneumocystis pneumonia, Pneumonia,
Poliomyelitis,
Prevotella infection, Primary amoebic meningoencephalitis, Progressive
multifocal
leukoencephalopathy, Psittacosis, Q fever, Rabies, Rat-bite fever, Respiratory
syncytial
virus infection, Rhinosporidiosis. Rhinovirus infection, Rickettsial
infection, Rickettsial-
pox, Rift Valley fever, Rocky mountain spotted fever, Rotavirus infection,
Rubella, Sal-
monellosis, SARS (Severe Acute Respiratory Syndrome), Scabies,
Schistosomiasis, Sepsis,
Shigellosis (Bacillary dysentery), Shingles (Herpes zoster), Smallpox
(Variola),
Sporotrichosis, Staphylococcal food poisoning, Staphylococcal infection,
Strongyloidiasis,
Syphilis, Taeniasis, Tetanus (Lockjaw), Tinea barbae (Barber's itch), Tinea
capitis (Ring-
worm of the Scalp), Tinea corporis (Ringworm of the Body), Tinea cruris (Jock
itch),
Tinea manuum (Ringworm of the Hand), Tinea nigra, Tinea pedis (Athlete's
foot), Tinea
unguium (Onychomycosis), Tinea versicolor (Pityriasis versicolor),
Toxocariasis (Ocular
Larva Migrans), Toxocariasis (Visceral Larva Migrans), Toxoplasmosis,
Trichinellosis,
Trichomoniasis, Trichuriasis (Whipworm infection), Tuberculosis, Tularemia,
Ureaplasma
urealyticum infection, Venezuelan equine encephalitis, Venezuelan hemorrhagic
fever,
Viral pneumonia, West Nile Fever. White piedra (Tinea blanca), Yersinia
pseudotuber-
culosis infection, Yersiniosis, Yellow fever, Zygomycosis.
The cell binding molecule, which is more preferred to be an antibody described
in this
patent that are against pathogenic strains include, but are not limit,
Acinetobacter
baumannii, Actinomyces israelii, Actinomyces gerencseriae and
Propionibacterium
propionicus, Trypanosoma brucei, HIV (Human immunodeficiency virus), Entamoeba
histolytica, Anaplasma genus. Bacillus anthracis, Arcanobacterium
haemolyticum, Junin
virus, Ascaris lumbricoides, Aspergillus genus, Astroviridae family. Babesia
genus, Bacil-
lus cereus, multiple bacteria, Bacteroides genus, Balantidium coli,
Baylisascaris genus, BK
44
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
virus, Piedraia hortae, Blastocystis hominis, Blastomyces dermatitides,
Machupo virus,
Borrelia genus, Clostridium botulinum, Sabia, Brucella genus, usually
Burkholderia
cepacia and other Burkholderia species, Mycobacterium ulcerans, Caliciviridae
family,
Campylobacter genus. usually Candida albicans and other Candida species,
Bartonella
henselae, Group A Streptococcus and Staphylococcus, Trypanosoma cruzi,
Haemophilus
ducreyi, Varicella zoster virus (VZV), Chlamydia trachomatis, Chlamydophila
pneumoniae, Vibrio cholerae, Fonsecaea pedrosoi, Clonorchis sinensis,
Clostridium
difficile, Coccidioides immitis and Coccidioides posadasii, Colorado tick
fever virus,
rhinoviruses, coronaviruses, CID prion, Crimean-Congo hemorrhagic fever virus.
Crypto-
coccus neoformans, Cryptosporidium genus, Ancylostoma braziliense; multiple
parasites,
Cyclospora cayetanensis, Taenia solium, Cytomegalovirus, Dengue viruses (DEN-
1, DEN-
2, DEN-3 and DEN-4) ¨ Flaviviruses, Dientamoeba fragilis, Corynebacterium
diphtheriae,
Diphyllobothrium, Dracunculus medinensis, Ebolavirus, Echinococcus genus,
Ehrlichia
genus, Enterobius vermicularis. Enterococcus genus, Enterovirus genus,
Rickettsia
prowazekii, Parvovirus B19, Human herpesvirus 6 and Human herpesvirus 7.
Fasciolopsis
buski, Fasciola hepatica and Fasciola gigantica, FFI prion, Filarioidea
superfamily, Clos-
tridium perfringens, Fusobacterium genus, Clostridium perfringens; other
Clostridium
species, Geotrichum candidum, GSS prion, Giardia intestinalis, Burkholderia
mallei,
Gnathostoma spinigerum and Gnathostoma hispidum, Nei sseria gonorrhoeae,
Klebsiella
granulomatis, Streptococcus pyogenes, Streptococcus agalactiae, Haemophilus
influenzae,
Enteroviruses, mainly Coxsackie A virus and Enterovirus 71, Sin Nombre virus,
Helico-
bacter pylon, Escherichia coli 0157:H7, Bunyaviridae family, Hepatitis A
Virus, Hepatitis
B Virus, Hepatitis C Virus, Hepatitis D Virus, Hepatitis E Virus, Herpes
simplex virus 1,
Herpes simplex virus 2, Histoplasma capsulatum. Ancylostoma duodenale and
Necator
americanus, Hemophilus influenzae, Human bocavirus, Ehrlichia ewingii,
Anaplasma
phagocytophilum, Human metapneumovirus, Ehrlichia chaffeensis, Human papilloma-
virus, Human parainfluenza viruses, Hymenolepis nana and Hymenolepis diminuta,
Ep-
stein-Barr Virus, Orthomy-xoviridae family, Isospora belli, Kingella kingae,
Klebsiella
pneumoniae, Klebsiella ozaenas, Klebsiella rhinoscleromotis, Kuru prion, Lassa
virus,
Legionella pneumophila, Legionella pneumophila, Leishmania genus,
Mycobacterium
leprae and Mycobacterium lepromatosis, Leptospira genus, Listeria
monocytogenes,
Borrelia burgdorferi and other Borrelia species, Wuchereria bancrofti and
Brugia malayi,
Lymphocytic choriomeningitis virus (LCMV), Plasmodium genus, Marburg virus,
Measles
virus, Burkholderia pseudomallei, Neisseria meningitides, Metagonimus
yokagawai,
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Microsporidia phylum, Molluscum contagiosum virus (MCV), Mumps virus,
Rickettsia
typhi, Mycoplasma pneumoniae, numerous species of bacteria (Actinomycetoma)
and
fungi (Eumycetoma), parasitic dipterous fly larvae. Chlamydia trachomatis and
Neisseria
gonorrhoeae, vCJD prion, Nocardia asteroides and other Nocardia species,
Onchocerca
volvulus, Paracoccidioides brasiliensis. Paragonimus westermani and other
Paragonimus
species, Pasteurella genus, Pediculus humanus capitis, Pediculus humanus
corporis,
Phthirus pubis, Bordetella pertussis, Yersinia pestis, Streptococcus
pneumoniae, Pneumo-
cystis jirovecii, Poliovirus, Prevotella genus, Naegleria fowleri, JC virus,
Chlamydophila
psittaci, Coxiella burnetii, Rabies virus, Streptobacillus moniliformis and
Spirillum minus,
Respiratory syncytial virus, Rhinosporidium seeberi, Rhinovirus, Rickettsia
genus, Rick-
ettsia akari, Rift Valley fever virus, Rickettsia rickettsii, Rotavirus,
Rubella virus, Salmo-
nella genus, SARS coronavirus, Sarcoptes scabiei, Schistosoma genus. Shigella
genus,
Varicella zoster virus, Variola major or Variola minor, Sporothrix schenckii,
Staphylococ-
cus genus. Staphylococcus genus, Staphylococcus aureus, Streptococcus
pyogenes,
Strongyloides stercoralis, Treponema pallidum, Taenia genus, Clostridium
tetani,
Trichophyton genus, Trichophyton tonsurans, Trichophyton genus, Epidermophyton
floccosum, Trichophyton rubrum, and Trichophyton mentagrophytes, Trichophyton
rubrum, Hortaea wemeckii, Trichophyton genus, Malassezia genus, Toxocara canis
or
Toxocara cati, Toxoplasma gondii, Trichinella spiralis, Trichomonas vaginalis,
Trichuris
trichiura, Mycobacterium tuberculosis, Francisella tularensis, Ureaplasma
urealyticum,
Venezuelan equine encephalitis virus, Vibrio colerae, Guanarito virus, West
Nile virus,
Trichosporon beigelii, Yersinia pseudotuberculosis, Yersinia enterocolitica,
Yellow fever
virus, Mucorales order (Mucormycosis) and Entomophthorales order
(Entomophthora-
mycosis), Pseudomonas aeruginosa, Campylobacter (Vibrio) fetus, Aeromonas
hydrophila,
Edwardsiella tarda, Yersinia pestis, Shigella dysenteriae, Shigella flexneri,
Shigella sonnei,
Salmonella typhimurium, Treponema pertenue, Treponema carateneum, Bonelia
vincentii.
Borrelia burgdorferi, Leptospira icterohemorrhagiae. Pneumocystis carinii.
Brucella
abortus, Brucella suis, Brucella melitensis, Mycoplasma spp., Rickettsia
prowazeki, Rick-
ettsia tsutsugumushi, Clamydia spp.; pathogenic fungi (Aspergillus fumigatus,
Candida
albicans, Histoplasma capsulatum); protozoa (Entomoeba histolytica,
Trichomonas tenas,
Trichomonas hominis, Tryoanosoma gambiense, Trypanosoma rhodesiense,
Leishmania
donovani, Leishmania tropica, Leishmania braziliensis, Pneumocystis pneumonia,
Plasmo-
dium vivax, Plasmodium falciparum, Plasmodium malaria); or Helminiths
(Schistosoma
japonicum, Schistosoma mansoni. Schistosoma haematobium, and hookworms).
46
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Other antibodies as cell binding ligands used in this invention for treatment
of viral
disease include, but are not limited to, antibodies against antigens of
pathogenic viruses,
including as examples and not by limitation: Poxyiridae, Herpesviridae,
Adenoviridae,
Papovaviridae, Enteroviridae, Picomaviridae, Parvoviridae, Reoviridae,
Retroviridae,
influenza viruses, parainfluenza viruses, mumps, measles. respiratory
syncytial virus,
rubella, Arboviridae, Rhabdoviridae, Arenaviridae, Non-A/Non-B Hepatitis
virus,
Rhinoviridae, Coronaviridae, Rotoviridae, Oncovirus [such as, HBV
(Hepatocellular
carcinoma), HPV (Cervical cancer, Anal cancer), Kaposi's sarcoma-associated
herpesvirus
(Kaposi's sarcoma), Epstein-Barr virus (Nasopharyngeal carcinoma. Burkitt's
lymphoma,
Primary central nervous system lymphoma). MCPyV (Merkel cell cancer), SV40
(Simian
virus 40). HCV (Hepatocellular carcinoma), HTLV-I (Adult T-cell
leukemia/lymphoma)],
Immune disorders caused virus: [such as Human Immunodeficiency Virus (AIDS)];
Cen-
tral nervous system virus: [such as, JCV (Progressive multifocal
leukoencephalopathy),
MeV (Subacute sclerosing panencephalitis), LCV (Lymphocytic choriomeningitis),
Arbovirus encephalitis, Orthomyxoviridae (probable) (Encephalitis lethargica).
RV (Ra-
bies), Chandipura virus. Herpesviral meningitis, Ramsay Hunt syndrome type II;
Po-
liovirus (Poliomyelitis, Post-polio syndrome), HTLV-I (Tropical spastic
paraparesis)];
Cytomegalovirus (Cytomegalovirus retinitis, HSV (Herpetic keratitis));
Cardiovascular
virus [such as CBV (Pericarditis, Myocarditis)]; Respiratory system/acute
viral
nasopharyngitis/viral pneumonia: [Epstein-Ban vims (EBV infection/Infectious
mononu-
cleosis), Cytomegalovirus; SARS coronavirus (Severe acute respiratory
syndrome)
Orthomyxoviridae: Influenzavirus A/B/C (Influenza/Avian influenza),
Paramyxovirus:
Human parainfluenza viruses (Parainfluenza), RSV (Human respiratory syncytial
virus),
hMPV]; Digestive system virus [MuV (Mumps), Cytomegalovirus (Cytomegalovirus
esophagitis); Adenovirus (Adenovirus infection); Rotavirus, Norovirus,
Astrovirus, Coro-
navirus; HBV (Hepatitis B virus), CBV, HAV (Hepatitis A virus), HCV (Hepatitis
C
virus), HDV (Hepatitis D virus), HEV (Hepatitis E virus). HGV (Hepatitis G
virus)];
Urogenital virus [such as. BK virus, MuV (Mumps)].
According to a further object, the present invention also concerns
pharmaceutical
compositions comprising the conjugate via the bridge linkers of the invention
together with
a pharmaceutically acceptable carrier, diluent. or excipient for treatment of
cancers, infec-
tions or autoimmune disorders. The method for treatment of cancers, infections
and auto-
immune disorders can be practiced in vitro, in vivo, or ex vivo. Examples of
in vitro uses
include treatments of cell cultures in order to kill all cells except for
desired variants that
47
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
do not express the target antigen; or to kill variants that express undesired
antigen. Exam-
ples of ex vivo uses include treatments of hematopoietic stem cells (HSC)
prior to the
performance of the transplantation (HSCT) into the same patient in order to
kill diseased or
malignant cells. For instance, clinical ex vivo treatment to remove tumour
cells or lym-
phoid cells from bone marrow prior to autologous transplantation in cancer
treatment or in
treatment of autoimmune disease, or to remove T cells and other lymphoid cells
from
allogeneic bone marrow or tissue prior to transplant in order to prevent graft-
versus-host
disease, can be carried out as follows. Bone marrow is harvested from the
patient or other
individual and then incubated in medium containing serum to which is added the
conjugate
of the invention, concentrations range from about 1 pM to 0.1 mM, for about 30
minutes to
about 48 hours at about 37 C. The exact conditions of concentration and time
of incuba-
tion (=dose) are readily determined by the skilled clinicians. After
incubation, the bone
marrow cells are washed with medium containing serum and returned to the
patient by i.v.
infusion according to known methods. In circumstances where the patient
receives other
treatment such as a course of ablative chemotherapy or total-body irradiation
between the
time of harvest of the marrow and reinfusion of the treated cells, the treated
marrow cells
are stored frozen in liquid nitrogen using standard medical equipment.
For clinical in vivo use, the conjugate via the linkers of the invention will
be supplied
as solutions or as a lyophilized solid that can be redissolved in sterile
water for injection.
Examples of suitable protocols of conjugate administration are as follows.
Conjugates are
given weekly for 8-20 weeks as an i.v. bolus. Bolus doses are given in 50 to
500 ml of
normal saline to which human serum albumin (e.g. 0.5 to 1 mL of a concentrated
solution
of human serum albumin. 100 mg/mL) can be added. Dosages will be about 50 i.tg
to 20
mg/kg of body weight per week, i.v. (range of 10 pg to 200 mg/kg per
injection). 4-20
weeks after treatment, the patient may receive a second course of treatment.
Specific
clinical protocols with regard to route of administration, excipients,
diluents, dosages,
times, etc., can be determined by the skilled clinicians.
Examples of medical conditions that can be treated according to the in vivo or
ex vivo
methods of killing selected cell populations include malignancy of any types
of cancer,
autoimmune diseases, graft rejections, and infections (viral, bacterial or
parasite).
The amount of a conjugate which is required to achieve the desired biological
effect,
will vary depending upon a number of factors, including the chemical
characteristics, the
potency, and the bioavailability of the conjugates, the type of disease, the
species to which
48
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
the patient belongs, the diseased state of the patient, the route of
administration, all factors
which dictate the required dose amounts, delivery and regimen to be
administered.
In general terms, the conjugates via the linkers of this invention may be
provided in an
aqueous physiological buffer solution containing 0.1 to 10% w/v conjugates for
parenteral
administration. Typical dose ranges are from 1 g/kg to 0.1 g/kg of body
weight per day; a
preferred dose range is from 0.01 mg/kg to 20 mg/kg of body weight per day, or
per week,
or an equivalent dose in a human child. The preferred dosage of drug to be
administered is
likely to depend on such variables as the type and extent of progression of
the disease or
disorder, the overall health status of the particular patient, the relative
biological efficacy
of the compound selected, the formulation of the compound, the route of
administration
(intravenous, intramuscular, or other), the pharmacokinetic properties of the
conjugates by
the chosen delivery route, and the speed (bolus or continuous infusion) and
schedule of
administrations (number of repetitions in a given period of time).
The conjugates via the linkers of the present invention are also capable of
being ad-
ministered in unit dose forms, wherein the term "unit dose" means a single
dose which is
capable of being administered to a patient, and which can be readily handled
and packaged,
remaining as a physically and chemically stable unit dose comprising either
the active
conjugate itself, or as a pharmaceutically acceptable composition, as
described hereinafter.
As such, typical total daily/weekly/biweekly/monthly dose ranges are from 0.01
to 100
mg/kg of body weight. By way of general guidance, unit doses for humans range
from 1
mg to 3000 mg per day, or per week, per two week or per month. Preferably the
unit dose
range is from 1 to 500 mg administered one to four times a week, and even more
prefera-
bly from 1 mg to 100 mg, once a week. Conjugates provided herein can be
formulated into
pharmaceutical compositions by admixture with one or more pharmaceutically
acceptable
excipients. Such unit dose compositions may be prepared for use by oral
administration,
particularly in the form of tablets, simple capsules or soft gel capsules; or
intranasal,
particularly in the form of powders, nasal drops, or aerosols; or dermally.
for example,
topically in ointments, creams, lotions, gels or sprays, or via trans-dermal
patches.
DRUGS/CYTOTOXIC AGENTS
Drugs that can be conjugated to a cell-binding molecule in the present
invention are
small molecule drugs including cytotoxic agents, which can be linked to or
after they are
modified for linkage to the cell-binding agent. A "small molecule drug" is
broadly used
herein to refer to an organic, inorganic, or organometallic compound that may
have a
49
08/13/2019 TUE 16: 20 FAX
2009/0.57
=
molecular weight of for example 100 to 1800, more suitably from 120 to 1400.
Small
molecule drugs are well characterized in the art, such as in W005058367A2, and
in U.S.
Patent No. 4,956,303.
Drugs that are known include, but not limited to,
1). Chemotherapeutic agents: a). Alkylating agents: such as Nitrogen mustards:
chlo-
rambucil, chlornaphazine, cyclophosphamide, dacarbazine, estramustine,
ifosfamide,
mechlorethamine, mechlorethamine oxide hydrochloride, mannomustine,
mitobronitol,
melphalan, mitolactol, pipobroman, novembichin, phenesterine, prednimustine,
thiotepa,
trofosfamide, uracil mustard; CC-1065 (including its adozelesin, carzelesin
and bizelesin
synthetic analogues); Duocarmycin (including the synthetic analogues, KW-2189
and CBI-
TMI); Benzodiazepine dimers (e.g, dimmers of pyrrolobenzodiazepine (PBD) or
tomay-
mycin, indolinobenzodiazepines, imidazobenzothiadiazepines, or oxazolidino-
benzodiazepines); Nitrosoureas: (carmustine, lomustine, chlorozotocin,
fotemustine,
nimustine, ranimustine); Alkylsulphonates: (busulfan, treosulfan, improsulfan
and piposul-
fan); Triazenes: (dacarbazine); Platinum containing compounds: (carboplatin,
cisplatin,
oxaliplatin); aziridines, such as benzodopa, caxboquone, meturedopa, and
uredopa; ethyl-
enimines and methylamelamines including altretamine, triethylenemel-amine,
trie-
tylenephosphoramide, triethylenethiophosphaoramide and trimethylolomel-amine];
b).
Plant Alkaloids: such as Vinca alkaloids: (vincristine, vinblastine,
vindesine, vinorelbine,
navelbin); Taxoids: (paclitaxel, docetaxol) and their analogs, Maytansinoids
(DM I, DM2,
DM3, DM4, maytansine and ansamitocins) and their analogs, cryptophycins
(particularly
cryptophycin 1 and cryptophycin 8); epothilones, eleutherobin, discodermo-
lide, bry-
ostatins, dolostatins, auristatins, tubulysins, cephalostatins;
pancratistatin; a sarcodictyin;
spongistatin; c). DNA Topoisomerase Inhibitors: such as [Epipodophyllins: (9-
aminocamptothecin, camptothecin, crisnatol, daunomycin, etoposide, etoposide
phosphate,
irinotecan, mitoxantrone, novantrone, retinoic acids (retinols), teniposide,
topotecan, 9-
nitrocamptothecin (RFS 2000)); mitomycins: (mitomycin C)]; d). Anti-
metabolites: such as
([Anti-folate: DHFR inhibitors: (methotrexate, trimetrexate, denopterin,
pteropterin,
aminopterin (4-aminopteroic acid) or the other folic acid analogues); IMP
dehydrogenase
Inhibitors: (mycophenolic acid, tiazofirin, ribavirin, EICAR); Ribonucleotide
reductase
Inhibitors: (hydroxyurea, deferoxamine)]; [Pyrimidine analogs: Uracil analogs:
(ancita-
bine, azacitidine, 6-azauridine, capecitabine (Xeloda), carmofur, cytarabine,
dideoxyuridine, doxifluridine, enocitabine, 5-Fluorouracil, floxuricline,
ratitrexed
CA 2991975 2019-08-13
PAGE 9157* RCVD AT 811312019 4:23:43 PM [Eastern Daylight Time]*
SVR:OTT235QFAX01114* DNIS:3905* CSID: *ANI:6132308821* DURATION (mm-ss):10-02
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
(Tomudex)); Cytosine analogs: (cytarabine, cytosine arabinoside, fludarabine);
Purine
analogs: (azathioprine, fludarabine, mercaptopurine, thiamiprine,
thioguanine)]; folic acid
replenisher, such as frolinic acid}; e). Hormonal therapies: such as {Receptor
antagonists:
[Anti-estrogen: (megestro1, raloxifene, tamoxifen); LHRH agonists: (goscrclin,
leuprolide
acetate); Anti-androgens: (bicalutamide, flutamide. calusterone,
dromostanolone propio-
nate, epitiostanol, goserelin, leuprolidc, mepitiostanc, nilutamidc,
testolactonc, trilostane
and other androgens inhibitors)]; Retinoids/Deltoids: [Vitamin D3 analogs: (CB
1093, EB
1089 KH 1060, cholecalciferol, ergocalciferol); Photodynamic therapies:
(verteporfin,
phthalocyanine, photosensitizer Pc4. demethoxy-hypocrellin A); Cytokines:
(Interferon-
alpha, Interferon-gamma, tumor necrosis factor (TNFs), human proteins
containing a TNF
domain)]}; f). Kinase inhibitors, such as BIBW 2992 (anti-EGFR/Erb2),
imatinib,
gefitinib, pegaptanib, sorafenib, dasatinib, sunitinib, erlotinib, nilotinib,
lapatinib, axitinib,
pazopanib. vandetanib, E7080 (anti-VEGFR2), mubritinib, ponatinib (AP24534),
bafetinib
(INNO-406), bosutinib (SKI-606), cabozantinib, vismodegib, iniparib,
ruxolitinib,
CYT387, axitinib, tivozanib, sorafenib, bevacizumab, cetuximab, Trastuzumab,
Ranibizumab, Panitumumab. ispinesib; g). antibiotics, such as the enediyne
antibiotics
(e.g. calicheamicins, especially calichcamicin yl, 61, al and 131, see, e.g.,
J. Med. Chem.,
39 (11), 2103-2117 (1996), Angew Chem Intl. Ed. Engl. 33:183-186 (1994);
dynemicin,
including dynemicin A and deoxydynemicin; esperamicin, kedarcidin, C-1027.
maduropeptin, as well as neocarzinostatin chromophore and related
chromoprotein
enediyne antiobiotic chromomophores), aclacinomysins, actinomycin,
authramycin,
azaserine, bleomycins, cactinomycin, carabicin, carminomycin, carzinophilin;
chromomycins, dactinomycin, daunorubicin, detorubicin, 6-diazo-5-oxo-L-
norleucine,
doxorubicin, morpholino-doxorubicin, cyanomorpholino-doxorubicin, 2-pyrrolino-
doxorubicin and deoxydoxorubicin, epirubicin, esorubicin, idarubicin,
marcellomycin,
nitomycins, mycophenolic acid, nogalamycin, olivomycins, peplomycin,
potfiromycin,
puromycin, quelamycin, rodorubicin, streptonigrin, streptozocin, tubercidin,
ubenimex,
zinostatin, zorubicin; f). Others: such as Polyketides (acetogenins),
especially bullatacin
and bullatacinone; gemcitabine, epoxomicins (e. g. carfilzomib), bortezomib,
thalidomide,
lenalidomide, pomalidomide, tosedostat, zybrestat, PLX4032, STA-9090,
Stimuvax,
allovectin-7, Xegeva, Provenge, Yervoy, Isoprenylation inhibitors (such as
Lovastatin),
Dopaminergic neurotoxins (such as 1-methyl-4-phenylpyridinium ion), Cell cycle
inhibi-
tors (such as staurosporine), Actinomycins (such as Actinomycin D.
dactinomycin),
Bleomycins (such as bleomycin A2, bleomycin B2, peplomycin), Anthracyclines
(such as
51
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
daunorubicin. doxorubicin (adriamycin), idarubicin, epirubicin, pirarubicin,
zorubicin,
mtoxantrone, MDR inhibitors (such as verapamil), Ca2+ATPase inhibitors (such
as
thapsigargin), Histone deacetylase inhibitors (Vorinostat, Romidepsin,
Panobinostat,
Valproic acid, Mocetinostat (MGCD0103), Belinostat, PCI-24781, Entinostat,
SB939,
Resminostat, Givinostat, AR-42, CUDC-101, sulforaphane, Trichostatin A) ;
Thapsigargin,
Celecoxib, glitazones, epigallocatechin gallate, Disulfiram, Salinosporamide
A.; Anti-
adrenals, such as aminoglutethimide, mitotane, trilostane; aceglatone;
aldophosphamide
glycoside; aminolevulinic acid; amsacrine; arabinoside, bestrabucil;
bisantrene; edatraxate;
defofamine; demecolcine; di aziquone; eflornithine (DFMO), elfomithine;
elliptinium
acetate, etoglucid; gallium nitrate; gacytosine, hydroxyurea; ibandronate,
lentinan;
lonidamine; mitoguazone; mitoxantrone; mopidamol; nitracrine; pentostatin;
phenamet;
pirarubicin; podophyllinic acid; 2-ethylhydrazide; procarbazine; PSK ;
razoxane; rhizoxin;
sizofiran; spirogermanium; tenuazonic acid; triaziquone; 2, 2',2"-
trichlorotriethylamine;
trichothecenes (especially T-2 toxin, verrucarin A, roridin A and anguidine);
urethane,
siRNA, antisense drugs, and a nucleolytic enzyme.
2). An anti-autoimmune disease agent includes, but is not limited to,
cyclosporine, cy-
closporine A, aminocaproic acid, azathioprine, bromocriptine, chlorambucil,
chloroquine,
cyclophosphamide, corticosteroids (e.g. amcinonide, betamethasone, budesonide,
hydro-
cortisone, flunisolide, fluticasone propionate, fluocortolone danazol,
dexamethasone,
Triamcinolone acetonide, beclometasone dipropionate), DHEA, enanercept,
hydroxychloroquine, infliximab, meloxicam, methotrexate, mofetil,
mycophenylate, pred-
nisone, sirolimus, tacrolimus.
3). An anti-infectious disease agent includes, but is not limited to, a).
Aminoglyco-
sides: amikacin, astromicin, gentamicin (netilmicin, sisomicin, isepamicin),
hygromycin B,
kanamycin (amikacin, arbekacin, bekanamycin, dibekacin, tobramycin), neomycin
(framycetin, paromomycin. ribostamycin), netilmicin, spectinomycin,
streptomycin, to-
bramycin, verdamicin; b). Amphenicols: azidamfenicol, chloramphenicol.
florfenicol,
thiamphenicol; c). Ansamycins: geldanamycin, herbimycin; d). Carbapenems:
biapenem,
doripenem, ertapenem, imipenem/cilastatin, meropenem, panipenem; e). Cephems:
carbacephem (loracarbef), cefacetrile, cefaclor, cefradine, cefadroxil,
cefalonium,
cefalmidine, cefalotin or cefalothin. cefalexin, cefaloglycin, cefamandole,
cefapirin,
cefatrizine, cefazaflur, cefazedone, cefazolin, cefbuperazone, cefcapene,
cefdaloxime,
cefepime, cefminox, cefoxitin, cefprozil, cefroxadine, ceftezole, cefuroxime.
cefixime,
cefdinir, cefditoren. cefepime, cefetamet, cefmenoxime, cefodizime, cefonicid,
52
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
cefoperazone, ceforanide. cefotaxime, cefotiam, cefozopran, cephalexin,
cefpimizole,
cefpiramide, cefpirome, cefpodoxime, cefprozil, cefquinome, cefsulodin,
ceftazidime,
cefteram, ceftibuten, ceftiolene, ceftizoxime, ceftobiprole, ceftriaxone,
cefuroxime,
cefuzonam, cephamycin (cefoxitin, cefotetan, cefmetazole), oxacephem
(flomoxef,
latamoxef); f). Glycopeptides: bleomycin, vancomycin (oritavancin.
telavancin),
teicoplanin (dalbavancin), ramoplanin; g). Glycylcyclincs: c. g. tigecycline;
g). 13-
Lactamase inhibitors: penam (sulbactam, tazobactam), clavam (clavulanic acid);
i).
Lincosamides: clindamycin, lincomycin; j). Lipopeptides: daptomycin, A54145,
calcium-
dependent antibiotics (CDA); k). Macrolides: azithromycin, cethromycin,
clarithromycin,
dirithromycin, erythromycin, flurithromycin, josamycin, ketolide
(telithromycin,
cethromycin), midecamycin, miocamycin, oleandomycin, rifamycins (rifampicin,
rifampin,
rifabutin, rifapentine), rokitamycin, roxithromycin, spectinomycin,
spiramycin. tacrolimus
(FK506), troleandomycin, telithromycin; 1). Monobactams: aztreonam, tigemonam;
m).
Oxazolidinones: linezolid; n). Penicillins: amoxicillin, ampicillin
(pivampicillin, hetacillin.
bacampicillin, metampicillin, talampicillin), azidocillin, azlocillin.
benzylpenicillin,
benzathine benzylpenicillin, benzathine phenoxymethyl-penicillin,
clometocillin, procaine
benzylpenicillin, carbcnicillin (carindacillin), cloxacillin, dicloxacillin,
epicillin,
flucloxacillin, mecillinam (pivmecillinam), mezlocillin, meticillin,
nafcillin, oxacillin,
penamecillin, penicillin, pheneticillin, phenoxymethylpenicillin,
piperacillin, propicillin.
sulbenicillin. temocillin, ticarcillin; o). Polypeptides: bacitracin,
colistin, polymyxin B; p).
Quinolones: alatrofloxacin, balofloxacin, ciprofloxacin, clinafloxacin,
danofloxacin,
difloxacin, enoxacin, enrofloxacin, floxin, garenoxacin, gatifloxacin,
gemifloxacin,
grepafloxacin, kano trovafloxacin, levofloxacin, lomefloxacin, marbofloxacin,
moxifloxacin, nadifloxacin, norfloxacin, orbifloxacin, ofloxacin, pefloxacin,
trovafloxacin,
grepafloxacin, sitafloxacin, sparfloxacin, temafloxacin, tosufloxacin,
trovafloxacin; q).
Streptogramins: pristinamycin, quinupristin/dalfopristin); r). Sulfonamides:
mafenide,
prontosil, sulfacetamide, sulfamethizole, sulfanilimide, sulfasalazine,
sulfisoxazole, trime-
thoprim, trimethoprim-sulfamethoxazole (co-trimoxazole); s). Steroid
antibacterials: e.g.
fusidic acid; t). Tetracyclines: doxycycline, chlortetracycline, clomocycline,
demeclocycline, lymecycline, meclocycline, metacycline, minocycline,
oxytetracycline,
penimepicycline, rolitetracycline, tetracycline, glycylcyclines (e.g.
tigecycline); u). Other
types of antibiotics: annonacin, arsphenamine, bactoprenol inhibitors
(Bacitracin),
DADAL/AR inhibitors (cycloserine), dictyostatin. discodermolide, eleutherobin,
epothilone, ethambutol, etoposide, faropenem, fusidic acid, furazolidone,
isoniazid,
53
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
laulimalide, metronidazole. mupirocin, mycolactone, NAM synthesis inhibitors
(e. g.
fosfomycin), nitrofurantoin, paclitaxel, platensimycin, pyrazinamide,
quinupristin/dalfopristin, rifampicin (rifampin), tazobactam tinidazole,
uvaricin;
4). Anti-viral drugs: a). Entry/fusion inhibitors: aplaviroc, maraviroc,
vicriviroc, gp41
(enfuvirtide), PRO 140, CD4 (ibalizumab); b). Integrase inhibitors:
raltegravir,
elvitegravir, globoidnan A; c). Maturation inhibitors: bevirimat, vivecon; d).
Neuramini-
dase inhibitors: oseltamivir, zanamivir, peramivir; e). Nucleosides
knucleotides: abacavir,
aciclovir, adefovir. amdoxovir, apricitabine, brivudine, cidofovir, clevudine,
dexelvucitabine, didanosine (ddI), elvucitabine, emtricitabine (FTC),
entecavir,
famciclovir, fluorouracil (5-FU), 3'-fluoro-substituted 2', 3' -
dideoxynucleoside analogues
(e.g. 3.-fluoro-2',3'-dideoxythymidine (FLT) and 3' -fluoro-2',3' -dideoxyg
uanosine
(FLG), fomivirsen, ganciclovir, idoxuridine. lamivudine (3TC), 1-nucleosides
(e.g. P-1-
thymidine and P-1-2'-deoxycytidine), penciclovir, racivir, ribavirin,
stampidine, stavudine
(d4T), taribavirin (viramidine), telbivudine, tenofovir, trifluridine
valaciclovir,
valganciclovir, zalcitabine (ddC), zidovudine (AZT); f). Non-nucleosides:
amantadine.
ateviridine, capravirine, diarylpyrimidines (etravirine, rilpivirine),
delavirdine, docosanol,
emivirine, efavirenz, foscarnet (phosphonoformic acid), imiquimod, interferon
alfa,
loviride, lodenosine, methisazone, nevirapine, NOV-205, peginterferon alfa,
podophyllotoxin, rifampicin, rimantadine, resiquimod (R-848), tromantadine;
g). Protease
inhibitors: amprenavir, atazanavir, boceprevir, darunavir, fosamprenavir,
indinavir,
lopinavir, nelfinavir, pleconaril, ritonavir, saquinavir, telaprevir (VX-950),
tipranavir; h).
Other types of anti-virus drugs: abzyme, arbidol, calanolide a, ceragenin,
cyanovirin-n,
diarylpyrimidines, epigallocatechin gallate (EGCG), foscarnet, griffithsin,
taribavirin
(viramidine), hydroxyurea, KP-1461, miltefosine, pleconaril, portmanteau
inhibitors,
ribavirin, seliciclib.
5). The drugs used for conjugates via a bridge linker of the present invention
also in-
clude radioisotopes. Examples of radioisotopes (radionuclides) are 3H, "C,
14C, 18F, 32p,
35S, 'Cu.
68 86 99 111 123 124 125 131 133 177 2U At, S, Cu.
Ga, Y, Tc, In, I, L L Xe, Lu, At, or 213Bi. Radioiso-
tope labeled antibodies are useful in receptor targeted imaging experiments or
can be for
targeted treatment such as with the antibody-drug conjugates of the invention
(Wu et al
(2005) Nature Biotechnology 23(9): 1137-1146). The cell binding molecules,
e.g. an
antibody can be labeled with ligand reagents through the bridge linkers of the
present
patent that bind, chelate or otherwise complex a radioisotope metal, using the
techniques
described in Current Protocols in Immunology, Volumes 1 and 2, Coligen et al,
Ed. Wiley-
54
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Interscience, New York, Pubs. (1991). Chelating ligands which may complex a
metal ion
include DOTA. DOTP, DOTMA, DTPA and TETA (Macrocyclics, Dallas, Tex. USA).
6). The pharmaceutically acceptable salts, acids or derivatives of any of the
above
drugs.
In another embodiment, the drug in the Formula (II) and (IV) can a chromophore
mol-
ecule, for which the conjugate can be used for detection, monitoring, or study
the interac-
tion of the cell binding molecule with a target cell. Chromophore molecules
are a com-
pound that have the ability to absorb a kind of light, such as UV light,
florescent light, IR
light, near IR light, visual light; A chromatophore molecule includes a class
or subclass of
xanthophores, erythrophores, iridophores, leucophores, melanophores, and
cyanophores; a
class or subclass of fluorophore molecules which are fluorescent chemical
compounds re-
emitting light upon light; a class or subclass of visual phototransduction
molecules; a class
or subclass of photophore molecules; a class or subclass of luminescence
molecules; and
a class or subclass of luciferin compounds.
The chromophore molecule can be selected from, but not limited, Non-protein
organic
fluorophores, such as: Xanthene derivatives (fluorescein, rhodamine, Oregon
green, eosin,
and Texas red); Cyanine derivatives: (cyanine, indocarbocyanine,
oxacarbocyanine,
thiacarbocyanine, and merocyanine); Squaraine derivatives and ring-substituted
squaraines, including Seta, SeTau, and Square dyes; Naphthalene derivatives
(dansyl and
prodan derivatives); Coumarin derivatives; Oxadiazole derivatives
(pyrklyloxazole,
nitrobenzoxadiazole and benzoxadiazole); Anthracene derivatives
(anthraquinones, includ-
ing DRAQ5, DRAQ7 and CyTRAK Orange); Pyrene derivatives (cascade blue, etc);
Oxazine derivatives (Nile red, Nile blue, cresyl violet, oxazine 170 etc).
Acridine deriva-
tives (proflavin, acridine orange, acridine yellow etc). Arylmethine
derivatives (auramine,
crystal violet, malachite green). Tetrapyrrole derivatives (porphin,
phthalocyanine, biliru-
bin).
Or a chromophore molecule can be selected from any analogs and derivatives of
the
following fluorophore compounds: CF dye (Biotium), DRAQ and CyTRAK probes
(BioStatus), BODIPY (Invitrogen), Alexa Fluor (Invitrogen), DyLight Fluor
(Thermo
Scientific, Pierce), Atto and Tracy (Sigma Aldrich), FluoProbes (Interchim),
Abberior
Dyes (Abberior), DY and MegaStokes Dyes (Dyomics). Sulfo Cy dyes (Cyandye),
HiLyte
Fluor (AnaSpec), Seta, SeTau and Square Dyes (SETA BioMedicals), Quasar and
Cal
Fluor dyes (Biosearch Technologies), SureLight Dyes (APC, RPEPerCP.
Phycobilisomes)(Columbia Biosciences), APC, APCXL, RPE, BPE (Phyco-Biotech);
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Examples of the widely used fluorophore compounds which are reactive or
conjugatable with the linkers of the invention are: Allophycocyanin (APC),
Aminocou-
marin, APC-Cy7 conjugates, BODIPY-FL, Cascade Blue, Cy2, Cy3, Cy3.5, Cy3B,
Cy5,
Cy5.5, Cy7, Fluorescein, FluorX, Hydroxycoumarin, IR-783, Lissamine Rhodamine
B,
Lucifer yellow, Methoxycoumarin, NBD, Pacific Blue, Pacific Orange, PE-Cy5
conju-
gates, PE-Cy7 conjugates, PerCP, R-Phycoerythrin(PE), Red 613, Seta-555-Azide,
Seta-
555-DBCO, Seta-555-NHS, Seta-580-NHS, Seta-680-NHS, Seta-780-NHS, Seta-APC-
780, Seta-PerCP-680, Seta-R-PE-670, SeTau-380-NHS, SeTau-405-Maleimide, SeTau-
405-NHS, SeTau-425-NHS, SeTau-647-NHS, Texas Red, TRTTC, TruRed, X-Rhodamine.
The fluorophore compounds that can be linked to the linkers of the invention
for study
of nucleic acids or proteins are selected from the following compounds or
their derivatives:
7-AAD (7-aminoactinomycin D, CG-selective), Acridine Orange, Chromomycin A3,
CyTRAK Orange (Biostatus. red excitation dark), DAPI, DRAQ5, DRAQ7, Ethidium
Bromide, Hoechst33258, Hoechst33342, LDS 751, Mithramycin, PropidiumIodide
(PI),
SYTOX Blue, SYTOX Green, SYTOX Orange, Thiazole Orange, TO-PRO: Cyanine
Monomer, TOTO-1, TO-PRO-1, TOTO-3, TO-PRO-3, YOSeta-1, YOY0-1. The
fluorophore compounds that can be linked to the linkers of the invention for
study cells are
selected from the following compounds or their derivatives: DCFH
(27'Dichorodihydro-
fluorescein, oxidized form), DHR (Dihydrorhodamine 123, oxidized form, light
catalyzes
oxidation), Fluo-3 (AM ester. pH > 6), Fluo-4 (AM ester. pH 7.2), Indo-1 (AM
ester,
low/high calcium (Ca2+)), SNARF(pH 6/9). The preferred fluorophore compounds
that
can be linked to the linkers of the invention for study proteins/antibodies
are selected from
the following compounds or their derivatives: Allophycocyanin(APC), AmCyanl
(tetram-
er, Clontech), AsRed2 (tetramer, Clontech), Azami Green (monomer, MBL),
Azurite, B-
phycoerythrin(BPE), Cerulean, CyPet, DsRed monomer (Clontech), DsRed2 ("RFP",
Clontech), EBFP, EBFP2, ECFP. EGFP (weak dimer, Clontech), Emerald (weak
dimer,
Invitrogen), EYFP (weak dimer, Clontech). GFP (S65A mutation), GFP (S65C
mutation),
GFP (S65L mutation), GFP (S65T mutation), GFP (Y66F mutation), GFP (Y66H muta-
tion), GFP (Y66VV mutation), GFF'uv, HcRedl, J-Red, Katusha, Kusabira Orange
(mono-
mer. MBL), mCFP, mCherry, mCitrine, Midoriishi Cyan (dimer, MBL), mKate
(TagFP635, monomer, Evrogen), mKeima-Red (monomer, MBL), mKO, mOrange,
mPlum. mRaspberry, mRFP1 (monomer, Tsien lab), mStrawberry, mTFP1,
mTurquoise2.
P3 (phycobilisome complex), Peridinin Chlorophyll (PerCP), R-
phycoerythrin(RPE), T-
Sapphire, TagCFP (dimer, Evrogen), TagGFP (dimer, Evrogen), TagRFP (dimer,
56
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Evrogen), TagYFP (dimer, Evrogen), tdTomato (tandem dimer), Topaz, TurboFP602
(dimer, Evrogen), TurboFP635 (dimer, Evrogen), TurboGFP (dimer, Evrogen),
TurboRFP
(dimer, Evrogen), TurboYFP (dimer, Evrogen), Venus, Wild Type GFP, YPet,
ZsGreenl
(tetramer, Clontech), ZsYellowl (tetramer, Clontech).
In another embodiment, the drug in the Formula (II) and (IV) can be a
polyalkylene
glycols that are used for extending the half-life of the cell-binding molecule
when admin-
istered to a mammal. Polyalkylene glycols include, but are not limited to,
poly(ethylene
glycols) (PEGs), poly(propylene glycol) and copolymers of ethylene oxide and
propylene
oxide; particularly preferred are PEGs, and more particularly preferred are
monofunctionally activated hydroxyPEGs (e.g., hydroxyPEGs activated at a
single
terminus, including reactive esters of hydroxyPEG-monocarboxylic acids,
hydroxyPEG-
monoaldehydes, hydroxyPEG-monoamines, hydroxyPEG-monohydrazides, hydroxyPEG-
monocarbazates, hydroxyPEG-monoiodoacetamides, hydroxyPEG-monomaleimides,
hydroxyPEG-monoorthopyridyl disulfides, hydroxyPEG-monooximes, hydroxyPEG-
monophenyl carbonates, hydroxyPEG-monophenyl glyoxals, hydroxyPEG-
monothiazolidine-2-thiones, hydroxyPEG-monothioesters, hydroxyPEG-monothiols,
hydroxyPEG-monotriazines and hydroxyPEG-monovinylsulfones).
In certain such embodiments, the polyalkylene glycol has a molecular weight of
from
about 10 Daltons to about 200 kDa, preferably about 88 Da to about 40 kDa; two
branches
each with a molecular weight of about 88 Da to about 40 kDa; and more
preferably two
branches, each of about 88 Da to about 20 kDa. In one particular embodiment,
the
polyalkylene glycol is poly(ethylene) glycol and has a molecular weight of
about 10 kDa;
about 20 kDa, or about 40 kDa. In specific embodiments, the PEG is a PEG 10
kDa (linear
or branched), a PEG 20 kDa (linear or branched), or a PEG 40 kDa (linear or
branched). A
number of US patents have disclosed the preparation of linear or branched "non-
antigenic"
PEG polymers and derivatives or conjugates thereof, e.g., U.S. Pat. Nos.
5,428,128;
5,621,039; 5,622,986; 5,643,575; 5,728,560; 5,730,990; 5,738,846; 5.811,076;
5,824,701;
5.840,900; 5,880,131; 5,900,402; 5.902,588; 5,919,455; 5,951,974; 5,965,119;
5,965,566;
5,969,040; 5,981,709; 6,011,042; 6,042,822; 6,113,906; 6,127,355; 6,132,713;
6,177,087,
and 6.180,095. The structure of the conjugates of the antibody-polyalkylene
glycols via the
bridge linker is as following Pg01:
57
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
R'
[ 0
R3Ri_xi . S
i
iM3
8 niAb
I
R4-(....{o3_¨R2-- X2 I s/
R" m4 0 n
Pg01
Wherein mAb is an antibody; n is 1-30; R' and R" are independently H or CH3;
m3 and
m4 are independently 0-5000; "¨'', ":"--='', Xt, X2, RI, R2, and R3 are the
same defined
in Formula (I) and (II); R4 is OH. H, or R1, or R3 that is defined as in
Formula (I).
In yet another embodiment, the preferred cytotoxic agents that conjugated to a
cell-
binding molecule via a bridge linker of this patent are tubulysins,
maytansinoids, taxanoids
(taxanes), CC-1065 analogs, daunorubicin and doxorubicin compounds,
benzodiazepine
dimers (e.g., dimers of pyrrolobenzodiazepine (PBD), tomaymycin, anthramycin,
indolinobenzodiazepines, imidazobenzothiadiazepines, or oxazolidino-
benzodiazepines),
calicheamicins and the cnediyne antibiotics, actinomycin, azaserincs,
blcomycins,
epirubicin, tamoxifen, idarubicin, dolastatins, auristatins (e.g. monomethyl
auristatin E,
MMAE , MMAF, auristatin PYE, auristatin TP, Auristatins 2-AQ, 6-AQ, EB (AEB),
and
EFP (AEFP)), duocarmycins, thiotepa, vincristines, hemiasterlins, nazumamides,
microginins, radiosumins, alierobactins, microsclerodermins, theonellamides,
esperamicins, PNU-159682, and their analogues and derivatives above thereof.
Tubulysins that are preferred for conjugation in the present invention are
well known
in the art and can be isolated from natural sources according to known methods
or prepared
synthetically according to known methods (e. g. Balasubramanian, R.; et al. J.
Med.
Chem., 2009, 52, 238-240. Wipf, P.; et al. Org. Lett., 2004, 6, 4057-4060.
Pando, O.; et al.
J. Am. Chem. Soc., 2011, 133, 7692-7695. Reddy, J. A.; et al. Mol.
Pharmaceutics, 2009,
6, 1518-1525. Raghavan, B.; et al. J. Med. Chem., 2008, 51, 1530-1533.
Patterson, A. W.;
et al. J. Org. Chem., 2008, 73, 4362-4369. Pando, 0.; et al. Org. Lett., 2009,
11(24), pp
5567-5569. Wipf, P.; et al. Org. Lett., 2007, 9 (8), 1605-1607. Friestad, G.
K.; Org. Lett.,
2004, 6, pp 3249-3252. Hillary M. Peltier. H. M.; et al. J. Am. Chem. Soc.,
2006, 128,
16018-16019. Chandrasekhar, S.; et al. J. Org. Chem., 2009, 74, 9531-9534.
Liu, Y.; et al.
Mol. Pharmaceutics, 2012, 9, 168-175. Friestad, G. K.; et al. Org. Lett.,
2009, 11, 1095-
1098. Kubicek, K.; et al., Angew Chem Int Ed Engl, 2010. 49: p. 4809-12. Chai,
Y.; et al.,
Chem Biol, 2010, 17: 296-309. Ullrich, A.; et al., Angew Chem Int Ed Engl,
2009, 48,
4422-5. Sani, M.; et al. Angew Chem Int Ed Engl, 2007, 46, 3526-9. Domling,
A.; et al..
Angew Chem Int Ed Engl. 2006. 45, 7235-9. Patent applications: Zanda, M. ; et
al, Can.
Pat. Appl. CA 2710693 (2011). Chai, Y.; et al. Eur. Pat. Appl. 2174947 (2010),
PCT WO
58
CA 02991975 2018-01-10
WO 2015/155753 PC T/IB2015/056083
2010034724. Leamon, C.; et al. PCT WO 2010033733, WO 2009002993. Ellman, J.;
et al,
PCT WO 2009134279; PCT WO 2009012958, US appl. 20110263650, 20110021568,
Matschiner, G.; et al, PCT WO 2009095447.Vlahov. I.; et al, PCT WO 2009055562,
WO
2008112873. Low. P.; et al, PCT WO 2009026177. Richter, W., PCT WO 2008138561.
Kjems, J.; et al, PCT WO 2008125116. Davis, M.; et al, PCT WO 2008076333.
Diener, J.;
et al, U.S. Pat. Appl. 20070041901, WO 2006096754. Matschiner, G.; et al, PCT
WO
2006056464. Vaghefi, F.; et al, 5 PCT WO 2006033913. Doemling, A., Ger. Offen.
DE
102004030227; PCT WO 2004005327; WO 2004005326; W02004005269. Stanton, M.; et
al, U.S. Pat. Appl. Publ. 20040249130. Hoefle, G.; et al. Ger. Offen. DE
10254439 ; DE
10241152; DE 10008089. Leung, D.; et al, WO 2002077036. Reichenbach, H.; et
al, Ger.
Offen. DE 19638870; Wolfgang, R.; US 20120129779, Chen, H.,US appl.
20110027274.
The preferred structure of tubulysins for conjugation of cell binding
molecules are de-
scribed in the patent application of PCT/IB2012/053554.
Examples of the structures of the conjugates of the antibody-tubulysin analogs
via
the bridge linker are T01, T02, T03, T04, T05, T06 and T07 as following:
r'l Nit, A wOAc N____))0k --Xis. ji0
1 \mAb
11
N / N 0
I iI I s ' H
0 1/
n...r(g 4)0t., OAc N j j0.(
N¨R2-- X2 0
H
No N / N OH n
_I
01 I S H _
0 TO1
_
0 0,R1,xi......./y s¨
ClIty 0 OAc OH
N4t ''N 1.1)-jki I mAb
i N 0 i /
1 0 µ.. 1 S H .......(....."S
(V 0 OAc N 0
OH
NO ,., N 11)AN
I ty I1 n
. H 0 n
_ TO2
59
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
H _
0 N,_ ,x. 0
it1 I S
CL, 0 OAc
N OH : \mAb
i
/ N 0 s /
iI 0 µ0
0
S H H ''es
H 0 OAc 0 Nõ R2... x2 0
OH
le (N N/ ..LN.)Cli:INHN
¨t 0 µ =` 1 0 n
_
103
4Z3 _
Cls/H,c,o )().(0Ac NJ* jzo 0
Xl... s
NI N i NI! \
--, ,
1 O R,,,, \'µ I S 1 Z 0 1
1
1 mAb
010 Z'3s/
CV 0 OAc 0 X17''\\43
R2
1 0 \
S H 0 ¨ n
_ TO4
__________________________________ 0 4 Z3 ¨
0 NR1 HO
S
mA N
/ 0
/ 1 NX4yNT OH
b 1 I /
\ 0µ 0 s A 0
S
X2 __________________ R2 ------------
0 H 0 0
(.11/, OH
NI ' NX4NJAN
I 0 \ 0 I S H 0 _ n
TO5
¨ cv Ti
OAc 4 Z3 ¨
N 0
OH
0.., xi 4,
N =A
S...1,- =R,,..N 0 1 / N 0
1 µo
mAb : S H 0 Z'3
N : 0414 0 OAc 0
s -\--X2\ 0 / o.L Ny,k OH
Of R2¨N / N
0
_ ¨ n T06
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
H 0 NVOAc
OH
Ri 0
mAb I
N : H 0 OAc
0
OH
0 E.2
01
I S¨ff H 0
¨ ¨ n TO7
Wherein mAb is an antibody; Z3 and Z'3 are independently H,
OP(0)(01\41)(01\42),
OCH2OP(0)(0M1)(0M2), 0S03M1, R1, or 0-glycoside (glucoside, galactoside,
mannoside, glucuronoside, alloside, fructoside, etc), NH-glycoside, S-
glycoside or CH2-
glycoside; M1 and M2 are independently H, Na, K, Ca, Mg, NH4, NR1R2R3; n is 1-
30;
"¨", "=", Xli X2, Ri, R2 and R3 are the same defined in Formula (I) and (II).
Calicheamicins and their related enediyne antibiotics that are preferred for
cell-
binding molecule-drug conjugates of this patent are described in: Nicolaou, K.
C. et al.
Science 1992, 256, 1172-1178; Proc. Natl. Acad. Sci USA. 1993, 90, 5881-5888),
U.S.
Patent Nos. 4,970,198; 5,053,394; 5,108,912; 5,264,586; 5,384,412; 5,606,040;
5,712,374;
5,714,586; 5,739,116; 5,770,701; 5,770,710; 5,773,001; 5,877,296; 6,015,562;
6,124,310;
8,153,768. An Example of the structure of the conjugate of the antibody-
Calicheamicin
analog via the bridge linker is CO1 as the following:
0
¨ ..................õ........õRi-s HO H p ¨
o xi CH3 0 N-1
I a OCH
-......... 3
S'.........V0`.1µ1
1-1....3.---\....c1
S 0 II CH3 OH
Z. 1 H3...C4..c. OCH3
\
mAb
\\\ 1 H3C0
OH 8 H3C0
R2 _____________________________________________ S HO OH 0
1 40
S
¨2 CH3
ClsH3 CH H3C 0 NI)
0 \ -......s. 3
H3C I CH3
OCH30H c2H5H HO 0 HI NN
0 .
HO H3C
¨ H3C0 n
OH r H3C0 C01.
Wherein mAb is an antibody; n is 1-30; --", "=", XI, X2, RI, R? and R3 arc the
same
defined in Formula (I) and (II).
Maytansinoids that are preferred to be used in the present invention including
maytansinol and its analogues are described in U.S. Patent Nos. 4,256,746,
4.361,650,
61
08/13/2019 TUE 16: 20 FAX
0010/057 .
= -
.
4,307,016, 4,294,757, 4,294,757, 4,371,533, 4,424,219, 4,331,598, 4,450,254,
4,364,866,
4,313,946, 4,315,929 4,362,663, 4,322,348, 4,371,533, 4,424,219, 5,208,020,
5,416,064,
5,208,020; 5,416,064; 6,333.410; 6,441,163; 6,716,821, 7,276,497, 7,301,019,
7,303,749,
7,368,565, 7,411,063, 7,851,432, and 8,163,888. An example of the structure of
the
conjugate of the antibody- Maytansinoids via the bridge linker is as the
following M01:
mAb
I x _\/õ.
i 1V- 1 X2-IVICo
0 N
0 0 ....-
Me M = = s
ss.
===
---- ----
1
-- -- X , Hscdmi aX
H3C0 e -- N 0 I
RI H I ", M01.
Wherein niAb is an antibody; n is 1-30; "¨", "=", XI, X2, RI, R2 and R3 are
the same .
'
defined in Formula (I) and (II).
Taxanes, which includes Paclitaxel (Taxon), a cytotoxic natural product, and
docet- l=
axel (Taxotere074), a semi-synthetic derivative, and their analogs which are
preferred for
conjugation via the bridge linkers of the present patent are exampled in:. K
C. Nicolaou et =
;
,
al., J. Am. Chem. Soc. 117, 2409-2420, (1995); Ojima et al, J. Med. Chem.
39:3889-3896
(1996); 40:267-278 (1997); 45, 5620-5623 (2002); Ojima et al., Proc. Natl.
Acad. Sci.,
96:4256-4261 (1999; Kim etal., Bull. Korean Chem. Soc., 20, 1389-1390 (1999);
Miller,
et al. J. Med. Chem., 47, 4802-4805(2004); U.S. Patent No. 5,475,011
5,728,849,
5,811,452; 6,340,701; 6,372,738; 6,391,913, 6.436,931; 6,589,979; 6,596,757;
6,706,708;
7,008,942; 7,186,851; 7,217,819; 7,276,499; 7,598,290; and 7,667,054.
Examples of the structures of the conjugate of the antibody- taxanes via the
bridge
linker are as the following Tx01, Tx02 and Tx03.
mAb
I / .
S S
1
R .A...... X 1W). ,e, ---.71 X2."'""+R.2
/ o
>1
o P- ....yt. e >1...00
itNH 0
. -a
.t.
t
V
i
i
C---. - ,s-/ --... E n
f
OH i OAc OH 6 ft 0Ae CrlAz. OS I A -
0 H5 0 HO
i
0 0
1
I . Me0 41113
WV OMe Me0 tdila
WV OMe I
" Tx01
f
i
62
I
i
i
;
1
CA 2991975 2019-08-13
1
PAGE 10/57 *11µ..vu RI al ij/L019 4 : 2 J :4 3 rm [Eastern Daylight Time] '
SYR:0TT2350FAX01114* DNIS:3905* CSID: *ANI:6132308821 * DURATION (mm-ss):10-02
CA 02991975 2018-01-10
WO 2015/155753
PCT/IB2015/056083
mAb
S/ ------------------------------------- NS
R
0 0 0
0 Q.
C-e-NH 0 Cel-NH0
\ c
I OH " OH 5 -0-Ac OH OH
H õAC
0 0
Me0 Me0
OMe OMe I n
______________________________________________________________________ Tx02
mAb
S/ -- \S
0 0 n
OH o 0 0 OH
CrANH
420 0 Ceir 0 .14)* 0
I A Hi )C08H HO 0 OAc OH HO 0 OAc
0 0
Me0 (111-
OMe
Me0
ta-
w OMe
n Tx03
Wherein mAb is an antibody; n is 1-30; "-", "=", X1, X2, Ri and R2 are the
same
defined in Formula (I) and (II).
CC-1065 analogues and doucarmycin analogs are also preferred to be used for a
con-
jugate with the bridge linkers of the present patent. The examples of the CC-
1065 ana-
logues and doucarmycin analogs as well as their synthesis are described in:
e.g.
Warpehoski, et al, J. Med. Chem. 31:590-603 (1988), D. Boger et al., J. Org.
Chem; 66;
6654-6661, 2001; U. S. Patent Nos: 4169888, 4391904, 4671958, 4816567,
4912227,
4923990, 4952394, 4975278, 4978757, 4994578, 5037993, 5070092, 5084468,
5101038,
5117006, 5137877, 5138059, 5147786, 5187186, 5223409, 5225539, 5288514,
5324483,
5332740, 5332837, 5334528, 5403484, 5427908, 5475092, 5495009, 5530101,
5545806,
5547667, 5569825, 5571698, 5573922, 5580717, 5585089, 5585499, 5587161,
5595499,
5606017, 5622929, 5625126, 5629430, 5633425, 5641780, 5660829, 5661016,
5686237,
5693762, 5703080, 5712374, 5714586, 5739116, 5739350, 5770429, 5773001,
5773435,
5786377 5786486, 5789650, 5814318, 5846545, 5874299, 5877296, 5877397,
5885793,
5939598, 5962216, 5969108, 5985908, 6060608, 6066742, 6075181, 6103236,
6114598,
6130237, 6132722, 6143901, 6150584, 6162963, 6172197, 6180370, 6194612,
6214345.
6262271, 6281354, 6310209, 6329497, 6342480, 6486326, 6512101, 6521404,
6534660,
63
CA 02991975 2018-01-10
WO 2015/155753 PC T/IB2015/056083
6544731, 6548530, 6555313, 6555693, 6566336, 6,586,618, 6593081, 6630579,
6356,397, 6759509, 6762179, 6884869, 6897034, 6946455, 7,049,316, 7087600,
7091186, 7115573, 7129261, 7214663, 7223837, 7304032, 7329507, 7,329,760,
7.388,026, 7,655,660, 7,655,661, 7,906,545, and 8,012,978. Examples of the
structures
of the conjugate of the antibody- CC-1065 analogs via the bridge linker are as
the follow-
ing CC01, CCO2, and CC03.
_ iz .....
N /
Cl/A H / X3 XI
N f (111011 \' VO
Ill N
010 1.1 o ii
1 mAb
1 /
044 ' S
X'3
"
Cl Ni / 0 \ ...XO
N /
Oil 0 N
H -2
I.* 0 lli
n
_
OZ4 _
CCOI
1 fig
C1/44 0 1
140 (10 0 Z 0 --= 010 1101 0 N 0--
H
0. 0 0 \
X3
1 µ112X?-7-----7-1LX1R/iX'3 0
S
N. 's
1 n
mAb CCO2
_
el Cl.,.t. H
N fit X3-R1---Xi -
110
Z40 N /
6....-S
inõ,...0 iiii-I ig * x,3
I /mAb
i Ilito o R2 / µsco
*,(2
Z940 1:6I N
N n
- 0 H - CCO3
Wherein mAb is an antibody; n is 1-30; Z4 and Z'4 are independently H,
P0(0M1)(01\42),
CH2P0(01VII)(0M2), SO3M1, CH3N(CH2CH2)2NC(0)-, 0(CH2CH2)2NC(0)-, R1, or
glycoside; X3 and X'3 are independently 0, NH, NHC(0), 0C(0). -C(0)0, RI, or
absent;
"-", "=", Xi, X2, Ri, R2, Mi, and M2 are the same defined in Formula (I) and
(II).
64
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Daunorubicin/Doxorubicin Analogues are also preferred for conjugation via the
bridge linkers of the present patent. The preferred structures and their
synthesis are exam-
pled in: Hurwitz, E., et al., Cancer Res. 35, 1175-1181 (1975). Yang. H. M.,
and Reisfeld,
R. A., Proc. Natl. Acad. Sci. 85, 1189-1193 (1988); Pietersz, C. A., E., et
al., E., et al.,"
Cancer Res. 48, 926-9311(1988); Trouet, et al., 79, 626-629 (1982); Z. Brich
et al., J.
Controlled Release, 19, 245-258 (1992); Chen et al., Syn. Comm., 33, 2377-
2390, 2003;
King et al., Bioconj. Chem., 10, 279-288, 1999; King et al., J. Med. Chem.,
45, 4336-
4343, 2002; Kratz et al., J Med Chem. 45, 5523-33. 2002; Kratz et al., Biol
Pharm Bull.
Jan. 21, 56-61 , 1998; Lau et al., Bioorg. Med. Chem. 3, 1305-1312. 1995;
Scott et al.,
Bioorg. Med.1 Chem. Lett. 6, 1491-1496; 1996; Watanabe et al., Tokai J.
Experimental
Clin. Med. 15, 327-334, 1990; Zhou et al., J. Am. Chem. Soc. 126, 15656-7,
2004; WO
01/38318; U.S. Patent No.). 5,106,951; 5,122.368; 5,146,064; 5,177,016;
5,208,323;
5.824,805; 6,146,658; 6,214,345; 7569358; 7,803,903; 8,084,586; 8,053,205.
Examples of
the structures of the conjugate of the antibody- CC-1065 analogs via the
bridge linker are
as the following Da01, Da02, Da03 and Da04.
0 H 0 0 OH
OH
OH OH
H3C H3C0 0 OH 0
OH 0 --c4OH
xi.% /NH
/S ________________________________________________________
mAb Da01
mAb
,X2 S`
µ,õ===. 2
x3\ 0 0
I OH
0 011 3
I OH OH
011101411101 'SOH 411010111/10 SOH
OH
H3C0
0 OH r,- HC0
X 0 OH 116"f%X
3 OH
NH2 N112 I
n Da02
CA 02991975 2018-01-10
WO 2015/155753
PCT/IB2015/056083
mAb
R2--x2
3
X3,\
0 OH
'SOH 4 *lb H
k 0 k 0
0 OH 0 OH
H3C0 H3C0
OH OH
n
0("IN
IMe 1--' Me0 Da03
,mAckb
X3/R2-'X2, _______________________________________ Xi ,=X'3
/4 OH OHO DH OHO
HOJ
01.10110 11O' 40(100*
" OH 0 OMe OHO Me
I Me Q) 44 - 411* n Da04
Wherein mAb is an antibody; n is 1-30; X3 and X'3 are independently H, 0, NH,
NHC(0),
NHC(0)NH, C(0), Rt. or OC(0); "-", "=", Xi, X2, RI, and R2 are the same
defined
in Formula (I) and (II).
Auristatins and dolastatins are preferred in conjugation via the bridge
linkers of this
patent. The auristatins (e. g. auristain E (AE) auristatin EB (AEB).
auristatin EFP (AEFP),
monomethyl auristatin E (MMAE), Monomethylauristatin (MMAF), Auristatin F
phenylene diamine (AFP) and a phenylalanine variant of MMAE) which are
synthetic
analogs of dolastatins, are described in Int. J. Oncol. 15:367-72 (1999);
Molecular Cancer
Therapeutics, vol. 3, No. 8, pp. 921-932 (2004); U.S. Application Nos.
11134826,
20060074008, 2006022925. U.S. Patent Nos. 4414205, 4753894, 4764368, 4816444,
4879278, 4943628, 4978744, 5122368, 5165923, 5169774,5286637, 5410024,
5521284,
5530097, 5554725, 5585089, 5599902, 5629197, 5635483, 5654399, 5663149,
5665860,
5708146, 5714586, 5741892, 5767236, 5767237, 5780588, 5821337, 5840699,
5965537,
6004934, 6033876, 6034065, 6048720, 6054297, 6054561, 6124431, 6143721,
6162930,
6214345, 6239104, 6323315, 6342219, 6342221, 6407213, 6569834,
6620911,6639055,
6884869, 6913748, 7090843, 7091186, 7097840, 7098305, 7098308, 7498298,
7375078.
7462352, 7553816, 7659241, 7662387, 7745394, 7754681, 7829531, 7837980,
7837995.
66
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
7902338, 7964566, 7964567, 7851437, 7994135. Examples of the structures of the
conju-
gate of the antibody-auristatins via the bridge linker are as the following
Au01, Au02,
Au03, Au04, and Au05.
_
0 xrRi '',1,(1:0() ry,/,,yQrlitH OH -
N
(110
R2 Xtrll 0 rrempilrfsi OH
XS 1
*I n
- I 0 ", 1 ,..0 0 ,-0 0 -Au01
0 X H si? H
Thi = ;)cThriSjilr
* Z'3
I 0 ____.,== 1,0 0 S i 0 0 OH
mAb\ H 0 H
S ,c,2 R2õNYyNõ..ANry,,,,nr,N
0 * Z3
0 OH - n Au02
H
_
H
I
c CNV *N)cm<I-NH
OH Z3 VN\N
H 1 mAb
H . Z
0 N-112"--X24\S'
NX*1(11\11-se--*?(N)C\r"NeQl"cN 0
I 0 , _co 0 ___
0 0 OH Z13 n
¨ ¨
Au03
¨ H ¨
NillAilTrIlr,1NrN
(110
=,...õ. .....0 0 .....0 0 X4,, 0 s
Rf¨Xi
0 1 /
.=%
1 mAb
\\NXI(LAN.%YlµCV(NH
S
I o - 7-r. I _O o
......00 0 X ' 4 ........ 13 ....." 2
Ix - 2
0 n Au04
\ y ki j)( H
NIr = NrsrierN 110 0 ¨
I 0 A,, I 0 0 0 0 X3 OH Xi S\
1 mAb
N , z
1101
- R S'
N = 1:)CriClirir / 2.'1(2
I ; 0 0 0 OH ,,..., I _... X'3 0 n
....0 - Au05
Wherein mAb is an antibody; n is 1-30; X3 and X'3 are independently CH2, 0,
NH,
NHC(0), NHC(0)NH, C(0), OC(0) R1, or absent; X4 and X'4 are independently CH2,
67
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
C(0), C(0)NH, C(0)N(121), R1, NHRi, NR1, C(0)R1 or C(0)0; Z3 and T3 are inde-
pendently H, R1, OP(0)(0M1)(0M2), NHRi, OCH2OP(0)(0M1)(0M2), 0S03M1, or 0-
glycoside (glucoside, galactoside, mannoside, glucuronoside, alloside,
fructoside), NH-
glycoside, S-glycoside, or CH2-glycoside; M1 and M2 are independently H, Na,
K, Ca,
Mg, NH4, NR1R2R3; "¨", "=", Xi, X2, R1, R2 and R3 are the same defined in
Formula
(I) and (II).
The benzodiazepine dimers (e. g. dimmers of pyrrolobenzodiazepine (PBD) or
(tomaymycin), indolinobenzodiazepines, imidazobenzothiadiazepines, or
oxazolidinobenzodiazepines) which are preferred cytotoxic agents according to
the present
invention are exampled in the art: US Patent Nos . 8,163.736; 8,153,627;
8,034,808;
7.834,005; 7,741,319; 7,704,924; 7,691,848; 7,678,787; 7,612,062; 7,608,615;
7,557,099;
7,528,128; 7,528,126; 7,511,032; 7.429,658; 7,407,951; 7,326,700; 7.312,210;
7,265,105;
7.202,239; 7,189,710; 7,173,026; 7,109,193; 7,067,511; 7,064,120; 7,056,913;
7,049,311;
7.022,699; 7,015,215; 6,979,684; 6,951,853; 6,884,799; 6,800,622; 6,747,144;
6,660,856;
6,608,192; 6,562,806; 6,977,254; 6.951,853; 6,909,006; 6,344,451; 5.880,122;
4,935,362;
4.764,616; 4,761,412; 4,723,007; 4,723,003; 4,683,230; 4,663,453; 4,508,647;
4,464,467;
4,427,587; 4,000,304; US patent appl. 20100203007, 20100316656, 20030195196.
Exam-
ples of the structures of the conjugate of the antibody- benzodiazepine dimers
via the
bridge linker are as the following PB01, PB02, PB03, PB04, PB05, PB06, PB07.
PB08.
PB09. PB10 and PB11.
¨ M1O3S.H HNSO/M,
¨1: ¨
N¨ X3 1.0s
R3 Me Me TO* r,\=
0 0
mAb
M1 3 HN--(S03M2
0=--N'T3
R3 OMe Me 0 n
PB01
0 ¨
ith r&
RF
OMe
(I) Me: .N
11CP SmAb =CNI71\13 g.WP
0 0 /
=7... X2
crisl ar
Rf=3 N g*SP OMe 0 me o R3
0 n
0
PB02
68
CA 02991975 2018-01-10
WO 2015/155753 PC T/IB2015/056083
- M I 03 HHiNT-_-:- : S 3M2
Ri-Xi -
lel Is\W 0 110 1\f-r i
N-X3 0
N
m
R3 ome _e S,
0 0 Mi0S H . -%.
N
,S031V12 i /mAb
1,...3
HN--trS I ./\/\)) ... N - X'3 I S
it"--0 1Lr- 1
N
-3 OMe Me0
- 4 Ir 0 0 PB 03
- MOS g N..x4-Ri-xi 0 _
me Me0 N ¨ R3 1 S%Ab
0 0x'4 Ii /
MOS ki / µ1127 X2 S
-
N ./\/\.0=C/
140: ii01 NO-\o
R3 Me Me0 R3
-
0 0 -nPB 04
- X3 Ri-----,, xi HNe 0 _
M103 ' 14 SO3M2 s
-r.--
4, N
OMe Me0
S
X'3¨ R2¨ X2
0
M103 H ..........S03M2
N HN 1--
_it N 41 I
I Me Me0 1.1 N 41 n
0 0 -
PB()5
_ A4103 ki N-4' 0 _
A N
Me Me0
1 mAb
M203 H
X'4- R2- X2 S
/
N N-----
s: 0
- A N me Me0 N 41, - n
0 0 PB06
69
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
X3 ___________________________________________________ Ri--------X1 0 -
_
miO3 ,k-f
"%mAb _
N me Me
0 ___________________________________________________ Sf
M2 3
R3
X'3-------R2 X2 \co
0
SO3M1
111N-*i
ki
N
R3 N
a_.\
n R3
-
PB07
_
Me Me
0
-
0
Ri------Xi 0 -
X3 ________________________________
X3 SO3M2
-
M1 3' ikii
1 -
S `'s-
mAb 1
Me Me0 1 ,/
N
X2
In 0 S
0 X'3------1%2
iS0
Mi0 M2 0
HN--- 3
3 ' ki
_ n
PB08
X31
Me Me 1
N
0
-
0
- X3---------R f--------X)
_
M10õ...}...35 II
Ns.
mAb
Ri=u3 4 0me Me0 HN¨e. ,S11 1 N R3
0 X'3-------.R2---0 ¨R2--Q¨x2 /s"
- 0
M2 ,....k3S Nil
1-11N 70
iik 0 * al 10=--\R3 _
N n
PB09
fe---CIN 1p 0me Me0 ...-
0
_ _3
_
0
-
HIN..../S 31\12
a .
- 1\1103 g
Ir-N,
e * 1-1-- /
Ri iXJ 0
SN
x4 N illpo o me Me0 4
1 mAb
0 $03M2 1 s
1 /
1-1N-ito
,Th c 0 H
mik,30 N
I .
V4 _ n
PB10
N WI o me Me()
- 0
--e--
0
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
_ _
N.Z....4. vPi...
0....--X'3, ):1 0 N
X4 Ri
N
me Me0 S
I
0 0 doe,mAb
=sr,D. /R2µ s
X'4--CrN
N X3 X2 0
OMe Me0 n
- 0 0 _ PB
11
Wherein mAb is an antibody; n is 1-30; X3 and X'3 are independently CH2, 0,
NH,
NHC(0), NHC(0)NH, C(0), OC(0), 0C(0)(NR3), RI, NHRi, NRi, C(0)R1 or absent; X4
and X'4 are independently CH), C(0), C(0)NH, C(0)N(R1), R1, NHR1, NR1, C(0)R1
or
C(0)0; M1 and Mµ, are independently H, Na, K, Ca, Mg, NH4, NR1R2R3; "-", "=",
X1, X/, R1,12/ and R3 are the same defined in Formula (I) and (II). In
addition, R1 and/or R/
can be absent.
In yet another embodiment, two or more different cytotoxic agents are
preferred con-
jugated to a cell-binding molecule via a bridge linker of this patent. The two
or more
different cytotoxic agents can be selected from any combinations of
tubulysins,
maytansinoids, taxanoids (taxanes), CC-1065 analogs, daunorubicin and
doxorubicin
compounds. benzodiazepine dimers (e.g., dimers of pyrrolobenzodiazepine (PBD),
tomaymycin, anthramycin, indolinobenzodiazepines, imidazobenzothiadiazepines,
or
oxazolidino-benzodiazepines), calicheamicins and the enediyne antibiotics,
actinomycin,
azaserines, bleomycins, epirubicin, tamoxifen, idarubicin, dolastatins,
auristatins (e.g.
monomethyl auristatin E, MMAE , MMAF, auristatin PYE, auristatin TP,
Auristatins 2-
AQ, 6-AQ, EB (AEB), and EFP (AEFP)), duocarmycins, thiotepa, vincristines,
hemiasterlins, nazumamides, microginins, radiosumins, alterobactins,
microsclerodermins,
theonellamides, esperamicins, PNU-159682, and their analogues and derivatives
above
thereof. Examples of the structures of the conjugates containing two or more
different
cytotoxic agents via the bridge linker are as the following Z01, Z02, Z02,
Z04, Z05, Z06,
Z07, Z08, Z09, Z10, Z12, Z13, Z14, Z15, Z16, Z17 and Z18:
0 H 0 ry,,(#,ArrH OH 1
Xi, deX3_ Xvr,N-----11, N N
S Ri 1NT E N
I I O-- I ....,0 *
mi 1 0 ...-0 0
i
X2-R2---X3'
I01 Ac0 \e" ,-. H
S 0 - v 0 %,..0
1L)N- T tr
.µ""...==/N n
HO ITU": Nf
S
0 H I zol
71
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
* N0==\113
= '0
Ev3 3`===.....,.. 0
0 Ri....õ....4 ,,
* OH I I 3 \
I
1
I mAb
ce'N.(NH 0 y re .
o
X3 '"'112 SZ I
OH x2 O
n Z02_
- ,R1---- X3=%,. N.- _:.--4
m S.----
0 ni--AT me Me0 N R3
Ab
tc..3 0
X 0
R \ / 3N Xi(N.,-LLNISlyil
N 110
n R2 NI
_ 0 a 1 .--0 -.0 0o OH ¨ Z03
XA¨R1¨X1, 0 _
_
i
\
vp --/--:Cr 4
¨3
ga X4
0
CV11.tsik0 N OACNeON X3'...,R2 xr ,oe S
22 0 n
¨
I \ 0 ZO4
_
X3 k N ---\, s, _
mAb
N 1 i
Me Me
i
R3 N 0
0 i /
i /
alh X4
H `'v _P
)0.14.i61 401 X3'---R2--X S2---%
N ' /
¨ N4 1 S H 0 ZO5
1 µ
0 s
....-===-"N
0 -
-
C!. \X
Me0 N :' '4 3 \ 0
= Xi .
S
0
----
== a: INreµO
H3C0µ Ho H /
r oil x4 ..,R2 s
µ
H
0 OAcNik x3 X2
OH
- n Z06
72
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
,0 \ -
,-, 0.-...)--k / R1 0 -
Me0 X
a \ V I, 0 Ni....X43 \ A....N
N " #1
A
mAb
0 0,N,-...--- s V
...-'
....-
,.= A N..µ0 X3' /X2
H3C0' HO H / ''''122
NN."'"'
I /\",1:) * ...r.D._..\
Rf=d73 * . Me Me0 N
R3 n
0 - ZO7
- 0
_
03"---Ns / 0
Ri..X1-'.....1\.....s
Me0 N r 1
,0*
0 0 mAb
....'
...--
.,\,.....---- //
.= j, N1 'µ.0
H3C0µ fla H
( 0/ X4 X2
y. ut %)r,c:11,11rH
N
R, _ 0 .--'0 0 X3
Z08
-
- .,,Ri---)(3 N-=-:?f,
=,,,s,..-- r_Cr 4
* NI -=--/¨% 0 --- N
:Me Me0 -3 mAb
R3
0 OH
0
NNSr/
X2 X/3 Xr. 1-1 c?
µ
_ n ZO9
_
-
HN--%0S03M2
- 0 Ri MI93 NH
i \X3--- N 0 . - - - \ R 7S
mAb
Me Me 0 0
H H
NsiNtrX \ .....x,3,,NX(N...f.AN-rteõ....e.QtAno.N (IP
0 R2 I
_ 0
...."...... I ...-0 --'0 0 OH - n Z10
- M103 NH HN-isSO3M2 -
0 RI
Me Me
mAb
.,0 ciµiciir0 1\1 io
H 9,
Il'2"
_ T'tor 1 ;j_cc, 0 -0 0 _ n
0
0 OH Z11
73
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
HN
M1 O3 ' ¨iS03M2 -
NH
14....fl X4-Ri 0
X4
N Me Me Xr4kr s N.,.
0 0 : mAb
_. )criki S? ''citrii\i'Vrki 0 R2....x2yv
N "st".6'4N , /
I 0 ....i...-1. . I --O 0 ¨0 0 0 OH i''3 o
-n Z12
_
- M103, SO
3M2 -
NH HN--
0-X 0
4--Ri X4 j õ(
N
-Xi N-rs'N,,
N
Me Me
0 0 : mAb
I ./
.trH i
N 101 Xfr-s/
r,
11144:1 1P-.Cro 0 --co 0 0 x,32 0 _
n Z13
-
0 -
\ N...r...cryThrRiirg s
,0 0 ,0 0 0 X3-111 ''mAb
/
X'4
Ng 0 x:4Ac Jt 0 4 tp
4 4. N N, X3' --lx2.,x2....CS
_ ;T 0 ,44' I -if -IN
S H 0 0 - n
Z14
SO3M2 0 iO3S n HN-4 11{1 ,,t -
I me Me0
- M /......),--N I Xi S
X3 ''mAb
,i4 *
i 0101 #NO-
0 0
gh OH
H
rTh ,N.4tt xr(c.Dyk 4-w-P X3'¨R2 X2
-n
OH
N 0 1 S H
1 µ 0 Z15
_ M103 S..$ = / HN/S- 31VI 1 X/ S
2 R _
aah 0= ,
X4
N q*IP I imie Me0 * 111.3¨X3 1 \mAb
0 0
X,4
(V, 0 x:.70:j 0 4 R2¨X2-AN
X3, / 0
N4 4411Skl\T )Njjk -n
= S H 0 Z16
74
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
e\ ....3...
_ ......õ....¨X3--N
............Ri P _
a 0 \ e p.
Xi Me0
r....."' 0
s=µ= \ H3C0- HO H
Sy2\ _x,3
NH 0õ .%)ry,%.1risc..lyll
........Ak.... N 61
¨2 NXr a N
0
I ....---, I ,0 0 0
¨ 0 ¨0 0 OH ¨ n Z17
\ ..:0
_ ........õ...X3¨N
......õ...R1 .9 _
a \ 0 o
4. .....
Al Me0 N Cs
./S-.../....40 A
r
mAb ...-
s., --
H3C0 HO H
S'),......X2 ,
N4 41(/`SI&N ,Nfik OH
¨ H 0 ¨ Z18
Wherein mAb is an antibody; n is 1-30; X3 and X'3 are independently CH2. 0,
NH,
NHC(0), NHC(0)NH, C(0), OC(0), OC(0)(NR3), RI, NHRi, NRi, C(0)R1 or absent; X4
and X'4 are independently H, CH2, OH, 0, C(0), C(0)NH, C(0)N(121), R1, NHRi,
NR1,
C(0)R1 or C(0)0; Mi and M/ are independently H, Na, K, Ca, Mg, NH4, NR1R2R.3;
"¨", "=", Xi, X2, Ri, R., and R3 are the same defined in Formula (I) and (II).
In
addition, R1 and/or R2 can be absent.
In yet another embodiment, cell-binding ligands or receptors can be conjugated
to a
cell-binding molecule via a bridge linker of this patent. These conjugated
cell-binding
ligands or receptors, in particular, antibody-receptor conjugates, can be not
only to work as
a targeting conductor/director to deliver the conjugate to malignant cells,
but also be used
to modulate or co-stimulate a desired immune response or altering signaling
pathways. In
the immunotherapy, the cell-binding ligands or receptors are preferred to
conjugate to an
antibody of TCR (T cell receptors) T cell, or of CARs (chimeric antigen
receptors) T cells,
or of B cell receptor (BCR), or the cytotoxic cells. The cell-binding ligands
or receptors are
selected, but not limited, from: Folate derivatives (binding to the folate
receptor, a protein
over-expressed in ovarian cancer and in other malignancies) (Low, P. S. et al
2008, Acc.
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Chem. Res. 41, 120-129); Glutamic acid urea derivatives (binding to the
prostate specific
membrane antigen, a surface marker of prostate cancer cells) (Hillier, S. M.et
al, 2009,
Cancer Res. 69, 6932-6940); Somatostatin (also known as growth homono-
inhibiting
hormone (GHIFI) or somatotropin release-inhibiting factor (SRI:Fr)) or
somatotropin re-
lease-inhibiting hormone) and its analogues such as octreotide (Sandostatin)
and lanreotide
(Somatuline) (particularly for neuroendocrine tumors, GH-producing pituitary
adenoma,
paraganglioma, nonfunctioning pituitary adenoma, pheochromocytomas) (Ginj, M.,
et al,
2006, Proc. Natl. Acad. Sci. U.S.A. 103, 16436-16441); certain Aromatic
sulfonamides,
specific to carbonic anhydrase IX (a marker of hypoxia and of renal cell
carcinoma) (Merl,
D., et al, Nat. Rev. Drug Discov. 2011, 10, 767-777); Pituitary adenylate
cyclase activat-
ing peptide (PACAP) (PAC1) for pheochromocytomas and paragangliomas;
Vasoactive
intestinal peptide (VIP/PACAP) (VPAC1, VPAC2) for cancers of lung, stomach,
colon,
rectum, breast, prostate, pancreatic ducts, liver, and urinary bladder;
Cholecystokinin
(CCK) (CCK1 (formerly CCK-A) and CCK2 for small cell lung cancers, medullary
thy-
roid carcinomas, astrocytomas, and ovarian cancers; Bombesin(Pyr-Gln-Arg-Leu-
Gly-
Asn-Gln-Trp-Ala-Val-Gly-H is-Leu-Met-NW)/gastrin-releasing peptide (GRP) (BB
1,
GRP receptor subtype (BB2), the BB3 and BB4) for renal cell, breast, lung,
gastric and
prostate carcinomas, and neuroblastoma (and neuroblastoma (Ohisson. B., et al,
1999,
Scand. J. Gastroenterology 34 (12): 1224-9; Weber, H. C., 2009, Cur. Opin.
Endocri.
Diab. Obesity 16(1): 66-71, Gonzalez N, et al, 2008, Cur. Opin. Endocri. Diab.
Obesity
15(1), 58-64 ); Neurotensin (NTR1, NTR2, NTR3) for small cell lung cancer,
neuroblastoma, pancreatic and colonic cancer; Substance P (NK1 receptor) for
Glial
tumors; Neuropeptide Y (Y1¨Y6) for breast carcinomas; Homing Peptides include
RGD
(Arg-Gly-Asp), NGR (Asn-Gly-Arg), the dimeric and multimeric cyclic RGD
peptides
(e.g. cRGDfV) that recognize receptors (integrins) on tumor surfaces
(Laakkonen P,
Vuorinen K. 2010, Integr Biol (Camb). 2(7-8): 326-337; Chen K, Chen X. 2011,
Theranostics. 1:189-200; Garanger E, et al, Anti-Cancer Agents Med Chem. 7
(5): 552-
558; Kerr, J. S. et al, Anticancer Research, 19(2A), 959-968; Thumshirn, G, et
al, 2003
Chem. Eur. J. 9,2717-2725), and TAASGVRSMH and LTLRWVGLMS (chondroitin
sulfate proteoglycan NG2 receptor) and F3 peptide (31 amino acid peptide that
binds to
cell surface-expressed nucleolin receptor) (Zitzmann, S., 2002 Cancer Res.,
62,18, pp.
5139-5143, Temminga, K., 2005, Drug Resistance Updates, 8,381-402; P.
Laakkonen
and K. Vuorinen, 2010 Integrative Biol, 2(7-8), 326-337; M. A. Burg, 1999
Cancer Res.,
59(12), 2869-2874; K. Porkka, et al 2002, Proc. Nat. Acad. Sci. USA 99(11),
7444-9);
76
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Cell Penetrating Peptides (CPPs) (Nakase I, et al, 2012, J. Control Release.
159(2),181-
188); Peptide Hormones, such as luteinizing hormone-releasing hormone (LHRH)
ago-
nists and antagonists, and gonadotropin-releasing hormone (GnRH) agonist, acts
by
targeting follicle stimulating hormone (FSH) and luteinising hormone (LH), as
well as
testosterone production, e.g. buserelin (Pyr-His-Trp-Ser-Tyr-D-Ser(OtBu)-Leu-
Arg-Pro-
NHEt), Gonadorclin (Pyr-His-Trp-Ser-Tyr-Gly-Lcu-Arg-Pro-Gly-NH2), Goscrelin
(Pyr-
His-Trp-Ser-Tyr-D-Ser(OtBu)-Leu-Arg-Pro-AzGly-NH?), Histrelin (Pyr-His-Trp-Ser-
Tyr-
D-His(N-benzy1)-Leu-Arg-Pro-NHEO,leuprolide (Pyr-His-Trp-Ser-Tyr-D-Leu-Leu-Arg-
Pro-NHEt), Nafarelin (Pyr-His-Trp-Ser-Tyr-2Na1-Leu-Arg-Pro-G1y-NH2),
Triptorelin
(Pyr-His-Trp-Ser-Tyr-D-Trp-Leu-Arg-Pro-Gly-NH2), Nafarclin, Deslorelin.
Abarelix (Ac-
D-2Nal-D-4-chloroPhe-D-3-(3-pyridyl)Ala-Ser-(N-Me)Tyr-D-Asn-Leu-isopropylLys-
Pro-
DAla-NH2), Cetrorelix (Ae-D-2Nal-D-4-chloroPhe-D-3-(3-ppidyl)Ala-Ser-Tyr-D-Cit-
Leu-Arg-Pro-D-Ala-NH2), Degarelix (Ac-D-2Nal-D-4-chloroPhe-D-3-(3-pyridyl)Ala-
Ser-
4-aminoPhe(L-hydrooroty1)-D-4-aminoPhe(carba-moy1)-Leu-isopropylLys-Pro-D-Ala-
NH2), and Ganirelix (Ac-D-2Nal-D-4-chloroPhe-D-3-(3-pyridyl)Ala-Ser-Tyr-D-(N9,
N10-
diethyl)-homoArg-Leu-(N9. N10-diethyl)-homoArg-Pro-D-Ala-NH2) (Thundimadathil,
J.,
J. Amino Acids, 2012,967347, doi:10.1155/2012/ 967347; Boccon-Gibod, L.; et
al. 2011,
Therapeutic Advances in Urology 3 (3): 127-140; Debruyne, F., 2006, Future
Oncology,
2(6), 677-696,); and Pattern Recognition Receptors (PRRs), such as Toll-like
receptors
(TLRs), C-type lectins and Nodlike Receptors (NLRs) (Fukata, M., et al, 2009,
Setnin.
Immunol. 21, 242-253; Maisonneuve, C., et al, 2014, Proc. Natl. Acad. Sci. U.
S. A. 111,
1-6; Bolos, I., et al, 2011, Structure /9,447-459; Means, T. K., et al, 2000,
Life Sci. 68,
241-258) that range in size from small molecules (imiquimod, guanisine and
adenosine
analogs) to large and complex biomacromolecules such as lipopolysaccharide
(LPS),
nucleic acids (CpG DNA, polyI:C) and lipopeptides (Pam3CSK4) (Kasturi, S. P.,
et al,
2011, Nature 470, 543-547; Lane, T., 2001, J. R. Soc. Med. 94,316; Hotz, C.,
and
Bourquin, C., 2012, Oncoimmunology 1,227-228; Dudek, A. Z., et al, 2007, Clin.
Can-
cer Res. 13,7119-7125).
The cell-binding ligands or receptors can be Ig-based and non-Ig-based protein
scaf-
fold molecules. The Ig-Based scaffolds can be selected, hut not limited, from
Nanobody (a
derivative of VHH (camelid Ig)) (Muyldermans S., 2013 Annu Rev Biochem. 82,775-
797); Domain antibodies (dAb, a derivative of VH or VL domain) (Holt, L. J, et
al, 2003,
Trends Biotechnol. 21,484-490); Bispecific T cell Engager (BiTE, a bispecific
diabody)
(Baeuerle, P. A, et al, 2009, Curr. Opin. Mol. Ther. 11,22-30); Dual Affinity
ReTargeting
77
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
(DART, a bispecific diabody) (Moore P. A. P, et al. 2011, Blood 117(17), 4542-
4551);
Tetravalent tandem antibodies (TandAb, a dimerized bispecific diabody)
(Cochlovius, B,
et al. 2000, Cancer Res. 60(16):4336-4341). The Non-Ig scaffolds can be
selected, but not
limited, from Anticalin (a derivative of Lipocalins) (Skerra A. 2008, FEBS J.,
275(11):
2677-2683; Beste G, et al, 1999 Proc. Nat. Acad. USA. 96(5):1898-1903; Skerra,
A. 2000
Biochim Biophys Acta, 1482(1-2):337-350; Skerra, A. 2007. CUff Opin
Biotechnol.
18(4):295-304; Skerra, A. 2008, FEBS J. 275(11):2677-2683); Adnectins (10th
FN3
(Fibronectin)) (Koide, A, et al, 1998 J. Mol. Biol., 284(4):1141-1151; Baton i
V, 2002,
Protein Eng. 15(12): 1015-1020; Tolcher, A. W, 2011, Clin. Cancer Res.
17(2):363-371;
Hackel, B. J, 2010, Protein Eng. Des. Sel. 23(4):211-219); Designed Ankyrin
Repeat
Proteins (DARPins) (a derivative of ankrin repeat (AR) proteins) (Boersma,
Y.L, et al,
2011 CUff Opin Biotechnol. 22(6): 849-857), e.g. DARPin C9, DARPin Ec4 and
DARPin
E69_LZ3_E01 (Winkler J, et al, 2009 Mol Cancer Ther. 8(9), 2674-2683; Patricia
M-K.
M., et al, Clin Cancer Res. 2011;17(1):100-110; Boersma Y. L, et al, 2011 J.
Biol. Chem.
286(48),41273-41285); Avimers (a domain A/low-density lipoprotein (LDL)
receptor)
(Boersma Y. L. 2011 J. Biol. Chem. 286(48): 41273-41285; Silverman J, et al,
2005 Nat.
Biotechnol., 23(12):1556-1561).
Examples of the structures of the conjugate of the antibody-cell-binding
ligands or re-
ceptors via the bridge linker are as the following: LB01 (PMSA ligand
conjugate), LB02
(Folate receptor conjugate), LB03 (Somatostatin receptor conjugate), LB04
(Octreotide, a
Somatostatin analog receptor conjugate). LB05 (Larireotide, a Somatostatin
analog recep-
tor conjugate), LB06 (CAIX receptor conjugate), LB07(CAIX receptor conjugate),
LB08
(luteinizing hormone-releasing hormone (LH-RH) ligand and GnRH conjugate),
LB09
(luteinizing hormone-releasing hormone (LH-RH) and GnRH ligand conjugate),
LB10
(GnRH antagonist, Abarelix conjugate), LB11 (cobalamin, VB12 analog
conjugate), LB12
(Gastrin releasing peptide receptor (GRPr), MBA conjugate), LB13 (av433
integrin receptor,
cyclic RGD pentapeptide conjugate), LB14 (hetero-bivalent peptide ligand for
VEGF
receptor conjugate), LB15 (Neuromedin B conjugate), LB 16 (a G-protein coupled
recep-
tor, bombesin conjugate) and LB17 (a Toll-like receptor, TLR2 conjugate).
78
CA 02991975 2018-01-10
WO 2015/155753 PC
T/IB2015/056083
HOOC 0
_( 7t1/4 de--,..-X34.A.X.1.0
0 ,1.
_
,..-=S
HOOC N N COOH /m3 1
mAbH H
/ HOOC 0 1 /
X13 X2,r
5, x
0
-Vmoc a l_iT COOH ma ¨n
LB01
0 0 0.1-.0H
¨(
N s X3
HN N 121
III( ---S
0
H 0 ,tO .
7 1
X'3 X2 \CS
HN Al Nra 4* zil,. .R2
0
412N JS'" N Nr
LB02
0113 =
0 40 OH
\x/ 0
_ ¨
H
7 0 \ HN N fsl 0
liii O
/ j1(1) N H HN Xi X4 S\ S ,/ft:
'4. N NH2
_...... S--;
mAb 0 1 HO 43
'....,.. = /10,
, 40 OH
2v2 \NJ 0
0 i(R/2 N
Hyl(N N
H
H H
0
¨ 0 * HA M4 n
LB 03
mAb
1 X'4¨ R2 ....1,? --
_____________ I % X2 S 000
sS x' __________ NH
NH NH
0 NH 10 0 0* *
0 NH
7 H .Fi H
HO HO
0 0
0
Y NH/ NH
H Y\ N JIIih, 0 NH NH
HO
/ \ N Aim LOH0olv L.OH
H 7 0 I).4// 4
0 H 0 H
NH2 NH2 1 n
LB04
79
CA 02991975 2018-01-10
WO 2015/155753
PCT/IB2015/056083
mAb
Sr ..S"' I 1 0
ii...õ, Xir'R1
\x4 I 1
0* NH, NH 101101 NH2 i
HN
O NH 4 OH 0 NH
s: H * OH
Sr.N Tx- H
:Orz0 0 s/ HO 0
HO 0 s/S/slr N
Aft,. I.- o 0 NH NH
i HO
NJIlni,1> 1...=== 0
0 NH NH
i
N 0.1),,,/, 4
H 7 0 01,,Ltv
4
HNyNNY.T.m.NNH H - 0
O H
1 I NH21 m3 I 0 H '11.-/NNH21 m
4 In
L:1305
_
NiNcL=N .31._3
FIN
NN
f
HN S SO,N -
=
mAb 1 0H N¨,N /
\S.. I X, / y,
"..4.,% 4 ,...../.."=%.,..3.. 11-..õ ...õ0/".elk
A ;Is.%
N S SONHm3
- R( 0 \ - FIN." H 2
0 LB06
_ N=N
)ID . 1-3 , ¨
0 xi /X4-"ls=-='"%.,N .õ===
I1 HgliV2H 0 N S SO2N H2
S H
______________________________________ -N 4ek OH
mAb T M3
sl 0 1102C 49> OH
N=N
0 R2 ..i: N S SO2N113
H
HN CO2H H 0
¨ OH
N /1114 n
0
0 CO2H OH
LB07
___ H2N
HN.,,.,.NH2
iTNH_
7 L...N0HOjcr
ilf
NI'L iZy tsLA xT INIf
01111014210 A...,..i10
,c. a 0 -- NH 11N.. .õSlo Xi
* tillkill OH / ''''c õAi Xsep
N
H2N<>
7 N,- ., _
HO
n ' HN ... NH2 H m4
/7-1I s
NH 1 \
H ilf11 - r H 0 \ I /mAb
N.)k
So'S
01 olOr
'' NH OH * HINN......4...) / 72
.x
H 1111111511 --)--- N'
H M5 ¨ n
LB08
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
1_,....- -------R
HN e),- x3 - HN,...N117.2...."'"--
.......T.,
_ ir NH
0 NH Xi _
HO
L. N.s.;2,..1r, Ii? Ry
H
NANly - N 1 H
0 I H 0 44 H 0 A H 0 0 S
.... NH II0 OH HNµ _..0
p --1
0 IN 0 [
NmAb
H
*
- ____, N, 3 ------- .,2 NH2- in3 /
HN.,.NH2 -
- HI> S
iiNH NH
v^0
110
0 i 1101 HO A HO
*
0 IN '''.. NH * 'r- HIX,
_ H OH ...7
NH2_ ma _
_ n
LB 09
¨
N X3-
0 CI ¨
H H22Tir HO * /....iti
H I 1 H 0 HN /
CN,Iris.N N N NreyN 0 zi * k Xi
HN4 0 H 0 H
1
0 AcHN opii\S\mAb
11(t?
N _,_,_ ,õ
..CH 112:110.1 HO\ * 1
0 S
y o H o 1 . 0 H o
ceNy^N N N Ny-N)cN
N
* \-- X2
I
_ OtiHN-S.,. 0 0 H 0 H 0 H E 0 H
\ ...R2
HO * N ta /
t / AcHN X' n
H2N
LB 10
I I, NH2 NH2 I I NH2 NH2 I 1
0 0 0 0
rN--1(2 H , s rNi(2 H S
,4, 1 =
0 0
mat... (a /0 .0014
-O.-i4' A R7 N__
OOH \ i '
Co3+ / NH 0
OH \I 4, NH
\
/\
/ /Co\ /
:) ipT N .66 \ .>.-cmiN 'N N
1IV .66 R1
N Nst s. R2 T 0 lip=,, ,
NH1 OH 1:p NH2
0 H -r
I 04-NH2 H,N=ro lm3 I 0 N 0
1NH2 2 0 I M4
s................. ------------------------------ ......,....,S 0
5 R7 =5'-deoxyadenosyl, Me, OH, or CN, mAb
LB 11
81
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
_
_ 0 Ri-X3 al NH HN'N S'
/ \ 0 _ -
H 0 )(TH 0 iL.N.,er
S NT../A.N N.s.AN N NH2
/ 4 N
_ .'I M3
mAb H2N 0 I* NH HN:
\ S'
H2-X3 0
i \ -
H 0 If 0 N
s,)1,X2 HN- _ NH21 m .--A
4,3 N N
- 0 H 0 -
2 H 0 H 0 It . , _ H 0 4- n
H2N 0 I
LB12,
mAb
1 Q. S.
.....õ,.
.'"-. -s-',S 0
x2
' _______ u"----Xi I
I ,,/
.2 I I µRi I
X3-NH
4 µ
0___<
0 4 0 ,e3--NH
0
HN HN-
0 NH 0 NH
0
HI:i.....X Mj......
HHN HN
NH NH r-INH
. 0 NH H )
N. HNNH .., -A
N
1 I 0 2 1 m3 1 0 0 IIN 1121M4 I n LB13
_
I I H _
Ac-A-G-P-T-W-C-E-D-D-W-Y-Y-C-W-L-F-G-T-G-G-Cr-N (
it \
H..-X, 1 0
/
3
-------N / M 3 \
H mAb
I ______________________________ I
Ac-V-C-W-E-D-S-W-G-G-E-V-C-F-R-Y-D-G-P-G-G-G-NY--NH2 H2 X2 si
¨ ZINI'-
X(3 M4 _n
LB14
OH -
0 R1 ..2,. T-I ' IST-"G-N-L-IV-A-T-
G-H-F-M-NH13
= H
,S Xl x3-N
r
mAb 1 0 H
\c, X2( wisi N
o R2 ' )G-N-L-W-A-T-G-H-F-M-NH2
0 \ H m4
Xv 11
3 ---N _
LB15
- 0 -
Ri jz ( X\ s
H Pyr-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Me1-N -X3 ).-i µ----mAb
1113
_( Pyr-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Met-Lx, -R2 X2/r....1
3 In4 -n
LB16
82
08/13/2019 TUE 16: 20 FAX
2011/057
R'
o
0
¨(C161133-*G%---= N"---"s
AeliN 0 nI3 1 \mAb
OH R.';
0
(cõHeyNs-^1)(31--(TI-N---N/N=47, 0
O AcHN 0 ¨4
LB17
Wherein mAb is an antibody; n is l--30; X3 and X'3 are independently CH2, 0,
NH,
NHC(0), NHC(0)NI-1, C(0), OC(0), OC(0)(NR3), RI, NBRI, NRI, C(0)Ri or absent;
X4
and X'.4 are independently H, CH2, OH, 0, C(0), C(0)NH, C(0)N(R1), RI, NI{Ri,
C(0)R1 or C(0)0; MI and M2 are independently H, Na, K, Ca, Mg, Nth, NRIR2R3;
and m4 is 0-5000, "¨", "=", Xi, X2, Ri, R2 and R3 are the same defined in
Formula (I)
and (II). In addition, RI and/or R2 can be absent.
The drugs/ cytotoxic agents used for conjugation via a bridge linker of the
present
patent can be any analogues and/or derivatives of drugs/molecules described in
the
present patent. One skilled in the art of drugs/cytotoxic agents will readily
understand
that each of the drugs/cytotoxic agents described herein can be modified in
such a manner
that the resulting compound still retains the specificity and/or activity of
the starting
compound. The skilled artisan will also understand that many of these
compounds can be
used in place of the drugs/cytotoxic agents described herein. Thus, the
drugs/cytotoxic
agents of the present invention include analogues and derivatives of the
compounds
described herein.
EXAMPLES
The invention is further described in the following examples, which are not
intended
to limit the scope of the invention. Cell lines described in the following
examples were
maintained in culture according to the conditions specified by the American
Type Culture
Collection (ATCC) or Deutsche Sammlung von Milcroorganismen und Zellkulturen
GmbH, Braunschweig, Germany (DMSZ), or The Shanghai Cell Culture Institute of
Chinese Acadmy of Science, unless otherwise specified. Cell culture reagents
were ob-
tamed from Invitrogen Corp., unless otherwise specified. All anhydrous
solvents were
commercially obtained and stored in Sure-seal bottles under nitrogen. All
other reagents
83
CA 2991975 2019-08-13
PAGE 11/57' RCVD AT 8/1312019 4:23:43 PM [Eastern Daylight Time]
SVR:OTT2350FAX01114 * DNIS:3905* CSID: *ANI:6132308821* DURATION (mm-ss):10-02
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
and solvents were purchased as the highest grade available and used without
further
purification. The preparative HPLC separations were performed with Varain
PreStar
HPLC. NMR spectra were recorded on Varian Mercury 400 MHz Instrument. Chemical
shifts (.delta.) are reported in parts per million (ppm) referenced to
tetramethylsilane at
0.00 and coupling constants (J) are reported in Hz. The mass spectral data
were acquired
on a Waters Xevo QTOF mass spect equipped with Waters Acquity UPLC separations
module and Acquity TUV detector.
Example 1: tert-Butyl 3-(2-(2-hydroxyethoxy)ethoxy)propanoate (84)
0
HO0)--H =Aok, 2
2 ______________________________________________ 2
Na/THF 0
83 84
To 350 mL of anhydrous THF was added 80 mg (0.0025 mol) of sodium metal and
diethylene glycol 83 (150.1 g, 1.41 mol) with stirring. After the sodium had
completely
dissolved, tert-butyl acrylate (24 mL, 0.33 mol) was added. The solution was
stirred for 20
h at room temperature and neutralized with 8 mL of 1.0 M HC1. The solvent was
removed
in vacuo and the residue was suspended in brine (250 mL) and extracted with
ethyl acetate
(3 x 125 mL). The combined organic layers were washed with brine (100 mL) then
water
(100 mL), dried over sodium sulfate, and the solvent was removed. The
resulting colorless
oil was dried under vacuum to give 60.27 g (78% yields) of product 84. 1H NMR:
1.41 (s,
9H). 2.49 (t, 2H, J=6.4 Hz), 3.59-3.72 (m, 10H). ESI MS m/z- C11I-12105 (M-H),
cacld.
233.15, found 233.40.
Example 2. tert-Butyl 3-(2-(2-(tosyloxy)ethoxy)ethoxy)propanoate (85)
H00. "0,1 MsCl/Pyr
MsO 0 'f=--o
/2 DCM
0 0
84 85
A solution of 84 (10.0 g, 42.70 mmol) in dichloromethane (50.0 mL) was treated
with
pyridine (20.0 mL). A solution of methanesulfonyl chloride (7.50 g, 65.81
mmol) in 50
mL dichloromethane was added dropwise via an addition funnel over 30 minutes.
After 5
h TLC analysis revealed that the reaction was complete. The pyridine
hydrochloride that
had formed was filtered off and the solvent was removed. The residue was
purified on
silica gel by eluting from with 20% ethyl acetate in hexane to with neat ethyl
acetate to
give 10.39 g (76% yield) of compound 85. 1H NMR: 1.40 (s, 9H), 3.23 (s, 3H),
2.45 (t,
2H, J=6.4 Hz), 3.54-3.70 (in, 10H); ESI MS m/z+ C12F11507S (M+H), cacld.
313.10, found
313.30.
84
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Example 3. tert-Butyl 3-(2-(2-azidoethoxy)ethoxy)propanoate (86)
Ts0(''%==' 1-3-/Y 4- N34.====1-3--7.1( \f--
0 85 0 86
To 50 mL of DMA was added tert-butyl 3-(2-(2-(Mesyloxy)ethoxy)ethoxy)ethoxy)-
propanoate 85 (4.0 g, 12.81 mmol) and sodium azide (0.90 g. 13.84 mmol) with
stirring.
The reaction was heated to 80 C. After 4 h TLC analysis revealed that the
reaction was
complete. The reaction was cooled to room temperature and quenched with water
(25
mL). The aqueous layer was separated and extracted into ethyl acetate (3 x 35
mL). The
combined organic layers were dried over anhydrous magnesium sulfate, filtered,
concen-
trated in vacuo and purified on silica gel by eluting from with 15% ethyl
acetate in hexane
to with neat ethyl acetate to give 2.88 g (87% yield) of compound 86. 1H NMR
(CDC13):
1.40 (s, 9H), 2.45 (t, 2H, J=6.4 Hz), 3.33 (t, 2H, J=5.2 Hz), 3.53-3.66 (m.
8H). ESI MS
m/z+ C11H22N307 (M+H), cacld. 260.13, found 260.20.
Example 4. 3-(2-(2-azidoethoxy)ethoxy)propanoic acid (87).
/oHC1
N3+00.1
Diox
12 " ane /2 " 87
0 0
15 The azide compound 86 (2.51 g, 9.68 mmol) dissolved in 1,4-dioxane (30
mL) and
was added 10 ml of HC1 (conc.). The mixture was stirred for 35 min, diluted
with Et0H
(30 ml) and toluene (30 ml) and evacuated under vacuum. The crude mixture was
purified
on silica gel using a mixture of methanol (from 5% to 10%) and 1% formic acid
in meth-
ylene chloride as the eluant to give title compound 87 (1.63 g, 83% yield),
ESI MS m/z-
20 C7H12N304 (M-H), cacld. 202.06, found 202.30.
Example 5. 2,5-dioxopyrrolidin-l-y1 3-(2-(2-azidoethoxy)ethoxy)propanoate
(88).
0
To compound 87 (1.60 g, 7.87 mmol) in 30 mL of dichloromethane was NHS (1.08
g,
9.39 mmol) and EDC (3.60 g, 18.75 mmol) with stirring. After 8 h TLC analysis
revealed
that the reaction was complete, the reaction mixture was evaporated and
purified on silica
gel using a mixture of ethyl acetate (from 5% to 10%) in methylene chloride as
the eluant
to give title compound 88 (1.93 g, 82% yield). ESI MS m/z+ C11H17N406 (M+H).
cacld.
301.11, found 301.20.
Example 6. (4R)-4-(2-((lR,3R)-1-acetoxy-34(2S,3S )-N,3-dimethy1-2-((R)-1-
methylpiperidine-2-carboxamido)pentanamido)-4-methylpentyl)thiazole-4-
carboxamido)-
08/13/291.9 TUE 16: 20 FAX
2012/057
5-(3-(3-(2-(2-azidoethoxy)ethoxy)propanamido)-4-hydroxypheny1)-2-
methylpentanoic
acid (94)
oit
qv. 0õ);.,N3
N N OH 0
N S H
I 0
To a solution of (4R)-4-(24(1R,3R)-1-acetoxy-3-((2S,3 S)-N,3-dimethy1-24(R)-1-
methylpiperidine-2-carboxamido)pentanamido)-4-methylpentyl)thiazole-4-
carboxamido)-
5-(3-amino-4-hydroxypheny1)-2-methylpentanoic acid, 93 (Huang Y. et al, Med
Chem.
#4,I, 249th ACS National Meeting, Denver, CO, Mar. 22-26, 2015; W02014009774)
(100
mg, 0.131 mmol) in the mixture of DMA (10 ml) and NaH2PO4 buffer (5 ml, 1.0 M,
pH
7.5) was added compound 88 (80.0 mg, 0.266 mmol) in four portions in 2 h. The
mixture
was stirred overnight, concentrated and purified on C-18 preparative HPLC (3.0
x 25 cm,
25 ml/min) eluted with from 80% water/20% methanol to 10% water/90% methanol
in 45
min to afford the title compound (101.5 mg, 82% yield). LC-MS (EST) m/z calcd.
for
C45H70N9011S [M+H]: 944.48, found: 944.70.
Example 7. (4R)-4-(241R,3R)-1-acetoxy-342S,3S)-N,3-dimethyl-2-((R)-1-methyl-
=
piperidine-2-carboxamido)pentanamido)-4-methylpentyl)thiazole-4-carboxamido)-5-
(3-(3-
(2-(2-aminoethoxy)ethoxy)propanamido)-4-hydroxypheny1)-2-methylpentanoic acid
(95).
d,.sh OHH
iN44. ift 119) N
NH2
OH
N 0
0 S H
Compound 94 (100.0 mg, 0.106 mmol) in methanol (25 ml) containing 0.1% HCI in
a
hydrogenation vessel was added Pd/C (25 mg, 10% Pd, 50% wet). After air was
vacuumed
20 out in the vessel, 35 psi H2 was conducted in. The mixture was shaken
for 4 h, filtered
through Celitea, concentrated, and purified on C-18 preparative HPLC (3.0 x 25
cm, 25
ml/min) eluted with from 85% water/15% methanol to 15% water/85% methanol in
45
min to afford the title compound (77.5 mg, 79% yield). LC-MS (ESI) m/z calcd.
for
C45H72N7011S [M+Hr: 918.49, found: 918.60.
25 Example 8. 4-(benzyloxy)-3-methoxybenzoic acid
Bn I to
H3C0 COOH
5
86
= CA 2991975 2019-08-13
PAGE 12/57' RCVD AT 8113/2019 4:23:43 PM [Eastern Daylight Time]'
SVR:OTT2350FAX01/14 DNIS:3905* CSID: *ANI:6132308821* DURATION (mm-ss):10-02
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
4-Hydroxy-3-methoxybenzoic acid (50.0g. 297.5 mmol) in the mixture of ethanol
(350 ml) and NaOH solution (2.0 M. 350 ml) was added RnBr (140.0 g, 823.5
mmol). The
mixture was stirred at 65 C for 8 h, concentrated, co-evaporated with water
(2 x 400 ml)
to -400 ml, acidified with 6 M HC1 to pH 3.0, filtered the solid, crystallized
with Et0H,
dried over the oven at 45 C with vacuum to afford the title compound (63.6 g,
83% yield).
ESI MS m/z+ 281.2 (M + Na).
Example 9. 4-(benzyloxy)-5-methoxy-2-nitrobenzoic acid
Bn0 to NO2
H3C0 COOH
4-(Benzyloxy)-3-methoxybenzoic acid (63.5 g, 246.0 mmol) in the mixture of
CH2C12
(400 ml) and HOAc (100 ml) was added FIN03 (fuming, 25.0 ml, 528.5 mmol). The
mix-
ture was stirred for 6 h, concentrated, crystallized with Et0H, dried over the
oven at 40 C
with vacuum to afford the title compound (63.3 g, 85% yield). ES1 MS nth+
326.1 (M +
Na).
Example 10. (2S,4R)-methyl 4-hydroxypyiTolicline-2-carboxylate,, hydrochloric
salt.
HO
COOMe
Trans-4-hydroxy-L-proline (15.0 g, 114.3 mmol) in dry methanol (250 mL) at 0 -
4
was added dropwise thionyl chloride (17 mL, 231 mmol). The resulting mixture
was
stirred for at RT overnight, concentrated, crystallized with Et0H/hexa.ne to
provide the
title compound (18.0 g, 87% yield), ESI MS m/z+ 168.2 + Na).
Example 11. (2S,4R)-1-tert-butyl 2-methyl 4-hydroxypyrrolidine-1,2-
dicarboxylate
HQ,
COOMe
Roc
To a solution of trans-4-hydroxy-L-proline methyl ester (18.0 g, 107.0 mmol)
in the
mixture of Me0H (150 ml) and sodium bicarbonate solution (2.0 M, 350 ml) was
added
(BOC)10 (30.0 g, 137.6 mmol) in three portions in 4 h. After stirring for an
additional 4 h,
the reaction was concentrated to -350 ml and extracted with Et0Ac (4 x 80 mL).
The
combined organic layers were washed with brine (100 mL), dried (MgSO4),
filtered,
concentrated and purified by SiO2 chromatography (1:1 hexanes/Et0Ac) to give
the title
compound (22.54 g, 86% yield). ESI MS rrilz+ 268.2 (M + Na).
87
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Example 12. (S)-1-tert-butyl 2-methyl 4-oxopyrrolidine-1,2-dicarboxylate
0
n"COOMe
Boe
The title compound prepared through Dess-Martin oxidation was described in:
Franco
Manfre et al. J. Org. Chem. 1992, 57, 2060-2065. Alternatively Swern oxidation
procedure
is as following: A solution of (COC)-, (13.0 ml, 74.38 mmol) in CH1C12 (350
ml) cooled
to -78 C was added dry DMS0 (26.0 mL). The solution was stirred at -78 C for
15 min
and then (2S,4R)-1-tert-butyl 2-methyl 4-hydroxypyrrolidine-1,2-dicarboxylate
(8.0 g,
32.63 mmol) in CH2C12 (100 ml). After stirred at -78 C for 2 h, triethylamine
(50 ml,
180.3 nunol) was added clropwise, and the solution was warmed to room
temperature. The
mixture was diluted with NaH11304 (400 ml, 1.0 M) solution and separated. The
aqueous
layer was extracted with CH2C12 (2 x 60 nil). The organic layers were
combined, dried
over MgSO4, filtered, concentrated and purified by SiO2 chromatography (7:3
hexanes/
Et0Ac) to give the title compound (6.73 g, 85% yield). ESI MS m/z+ 266.2 (M +
Na).
Example 13. (S)-1-tert-butyl 2-methyl 4-methylenepyrrolidine-1,2-
clicarboxylate
COOMe -1111' COOMe
Boc Boc
A solution of methyl triphenylphosphonium bromide (19.62 g, 55.11 mrnol) in TI-
IF
(150 mL) at 0 C was potassium-t-butoxide (6.20 g, 55.30 mmol) in anhydrous THF
(80
mL). After stirred at 0 C for 2 h, the resulting yellow ylide suspension was
added the
solution of (S)-1-tert-butyl 2-methyl 4-oxopyrrolidine-1,2-dicarboxylate (6.70
g, 27.55
mmol) in THE (40 mL). After stirring at RT for 1 h, the reaction mixture was
concen-
trated, diluted with Et0Ac (200 mL), washed with H2O (150 mL), brine (150 mL),
dried
over MgSO4, concentrated purified on SiO2 flash chromatography (9:1
hexanes/Et0Ac) to
yield the title compound (5.77 g, 87% yield). ELMS ni/z+ 264 (M + Na).
Example 14. (S)-methyl 4-methylenepyrrolidine-2-carboxylate
COOMe
Boc
(S)-1-tert-butyl 2-methyl 4-methylenepyrrolidine-1,2-dicarboxylate (5.70 g,
23.63
mmol) in Et0Ac (40 ml) at 4 C. was added HC1 (10 ml, 12 M). The mixture was
stirred
88
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
for 1 h, diluted with toluene (50 ml), concentrated, and crystallized with
Et0II/hexane to
yield the title compound as HC1 salt (3.85 g, 92% yield). EIMS in/z+ 142.2 (M
+H).
Example 15. (S)-methyl 1-(4-(benzyloxy)-5-methoxy-2-nitrobenzoy1)-4-
methylenepyrrolidine-2-carboxylate
Bn0 NO2 Bn0
NO2 COOMe
H3C0 COOH H3C0
0
A catalytic amount of DMF (30 pl) was added to a solution of 4-(benzyloxy)-5-
methoxy-2-nitrobenzoic acid (2.70 g, 8.91 mtnol) and oxalyl chloride (2.0 InL,
22.50
mmol) in anhydrous CH-)C12 (70 mL) and the resulting mixture was stirred at
room tem-
perature (RT) for 2 h. Excess CH2C12 and oxalyl chloride was removed with
rotavap. The
actyl chloride was resuspended in fresh CH)C12 (70 mL) and was added dropwise
to a
solution of 4-methylene-L-proline methyl ester HC1 salt (1.58 g, 8.91 mmol),
Et3N (6 mL)
at 0 C under argon atmosphere. The reaction mixture was allowed to warm to RT
and
stirring was continued for 8 h. After removal of CH-CI, and El3N, the residue
was parti-
tioned between FLO and Et0Ac (70/70 mL). The aqueous layer was further
extracted with
Et0Ac (2 x 60 mL). The combined organic layers were washed with brine (40 mL),
dried
(MgSO4) and concentrated. Purification of the residue with flash
chromatography (silica
gel, 2:8 hexanes/Et0Ac) yielded (S)-methyl 1-(4-(benzyloxy)-5-methoxy-2-
nitrobenzoy1)-
4-methylenepyrrolidine-2-carboxylate (2.88 g, 76.1% yield); EIMS rn/z 449.1
(IMr+Na).
Example 16. (S)-1-(4-(benzyloxy)-5-rnethoxy-2-nitrobenzoy1)-4-methylenepyrro-
lidine-2-carbaldehyde
Bn0 * NO2 COOCH3
Bn0
¨1111PP. NO2 CHO
H3C0 H3C0
0 0
To a vigorously stirred solution of (S)-methyl 1-(4-(benzyloxy)-5-methoxy-2-
nitrobenzoy1)-4-methylenepyrrolidine-2-carboxylate (2.80 g, 6.57 mmol) in
anhydrous
CH4211(60 mL) was added dropwise solution of DIBAL-H (10 mL of a 1.M solution
in
CH2C12) at -78 C under argon atmosphere. After the mixture was stirred for an
additional
90 min, excess reagent was decomposed by addition of 2 ml of methanol followed
by 5%
HC1 (10 mL). The resulting mixture was allowed to warm to 0 C. Layers were
separated
and the aqueous layer was further extracted with CH2C12 (3 x 50 mL). Combined
organic
layers were washed with brine, dried (MgSO4) and concentrated. Purification of
the resi-
due with flash chromatography (silica gel, 95:5 CHC13/Me0H) yielded (S)-1-(4-
89
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
(benzyloxy)-5-methoxy-2-nitrobenzoy1)-4-methylenepyrrolidine-2-carbaldehyde
(2.19 g,
84% yield). EIMS na/z 419.1 ([M]+Na).
Example 17. (S)-8-(benzyloxy)-7-methoxy-2-methylene-2,3-dihydro-1H-benzoIel-
pyrroloI1,2-aJazepin-5(11 aH)-one
Bn0 oil NO2 CHO Bn0
¨1110.
H3C0 H3C0
0 0
(S)-1-(4-(benzylox.y)-5-methoxy-2-ni trobenzoy1)-4-meth ylenepyrrolidine-2-
carbaldehycle (2.18 g, 5.50 in-mol) and Na.2S204 (8.0g. 45.97 mmol) in the
mixture of TI-IF
(60 nil) and F110 (40 ml) were stirred at RT for 20 h. Solvents were removed
under high
vacuum. The residue was re-suspended in Me0H (60 mI_,), and HC1 (6M) was added
dropwise until pH - 2. The resulting mixture was stirred at RT for 1 h. The
reaction was
work-up by removing most of Me011, then diluted with Et0Ac (100 mi..). The
Et0Ac
solution was washed with sat. aq. NaHCO3, brine, dried (MgSO4), and
concentrated.
Purification of the residue with flash chromatography (silica gel, 97:3
CHC13/Me0H)
yielded (S)-8-(benzyloxy)-7-methoxy-2-methylene-2,3-dihydro-1H-
benzoIeilpyrrolo[1,2-
a]azepin-5(11aH)-one (1.52 g, 80%). EIMS m/z 372.1 ([M]++Na).
Example 18. (S)-8-hydroxy-7-methoxy-2-methylene-2,3-dihydro-111-benzo[ei-
pp-m-10[1,2-a] a.zepin-5(11a.H)-one
Bn0 oil N=7/
HO oil N=o;
H3C0 H3C0
0 0
(S)-8-(benzyloxy)-7-methoxy-2-methylene-2,3-dihydro-1H-benzo[e]pyrrolo[1,2-
a]azepin-5(11_all.)-one (1.50 g, 4.32 mmol) in 70 ml of C11.2C11 at 0 C was
added 25 ml of
CH2S03H. 'The mixture was stirred at 0 C for 10 min then RT for 2 h, diluted
with CH2C12
, neutralized with cold 1.0 M NaHCO3 to pH 4, filtered. The aqueous layer was
extracted
with CH2C12(3x 60 ml). The organic layers were combined, dried over Na2SO4,
filtered,
evaporated and purified on SiO2 chromatography eluted with C1-13011/CH.2C1.2
(1:15) to
afford 811 mg (73% yield) of the title product. EIMS miz 281.1 (1[M]+-1-Na).
Example 19. (1 1 aS,1 1 a'S )-8,8'-(pentane-1,5-diylbis(oxy))bis(7-methoxy-2-
methylene-2,3-dihydro-1H-benzo[e]pyrrolo[1,2-a] [1,4]diazepin-5(11aH)-one)
(97)
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
HO N=7* 0/\/\.0
H3C0 Cs2CO3
Butanone OCH3 H3C0
0 0 0
To a stirred suspended solution of Cs2CO3 (0.761 g, 2.33 mmol) in butanone (8
ml)
were added (S)-8-hydroxy-7-methox y-2-methylene-2,3-dihydro-1H-
benzo[e]pyrrolol1,2-
al1,4_1diazepin-5(1 laH)-one (401 mg, 1.55 mmol) and 1,5-diiodopentane (240
mg, 0.740
mmol). The mixture was stirred at RT overnight, concentrated, and purified on
SiO2
chromatography eluted with Et0Ac/CH2C12 (1:10) to afford 337 mg (78% yield) of
the
title product. ElMS m/z 607.2 ([M] +Na).
Example 20. (S)-7-tnethoxy-84(5-4(S)-7-methoxy-2-methylene-5-oxo-
2,3,5,10.11,11a-hexahydro-1H-benzo[e]pyrrolo[1,2-a][1.4]diazepin-8-
yl)oxy)pentyl)oxy)-
2-methylene-2,3-dihydro-1Erbenzo[e]pyrrolo[l ,2-a][1,41diazepin-5(1 1af1)-one
(98)
0...."/\...0
NaB1-14
11.'F OCH3 H3C0
_______________________________________________ 10.
0 0
N gal *h N-4
-N 11P OCH3 H3C0
gj OCH3 H3C0
0 0 0 0
(1 1 aS,11 a'S)-8,8'-(pentane-1,5-diylbis(oxy))bis(7-methoxy-2-methylene-2,3-
dihydro-1H-
benzo[e]pyrrolo[1,2-a][1,4]diazepin-5(11aH)-one) (150 mg, 0.256 mmol) in
anhydrous
dichloromethane (1 mL) and absolute ethanol (1.5 mL) was added sodium
borohydride in
methoxyethyl ether (85 1, 0.5 M, 0.042mmol) at 0 C. The ice bath was removed
after 5
minutes and the mixture was stirred at room temperature for 3 hours, then
cooled to 0 C,
quenched with saturated ammonium chloride, diluted with dichloromethane, and
separat-
ed. The organic layer was washed with brine, dried over anhydrous Na2SO4 and
filtered
through Celite and concentrated. The residue was purified by reverse phase
HPLC (C18
column, acetonitrile/ water). The corresponding fractions were extracted with
dichloro-
methane and concentrated to afford the title compound (98), (S)-7-methoxy-84(5-
(((S)-7-
melhoxy-2-methylene-5-oxo-2,3,5,10,11,11a-hexahydro-1H-benzo[elpyrrolo[1,2-
a][1,4]diazepin-8-yl)oxy)pentypoxy)-2-methylene-2,3-dihydro-1H-
benzo[e]pyrrolo[1,2-
a][1,4]diazepin-5(11aH)-one, (64.7 mg, 43%), MS m/z+ 609.2 (M + Na), 625.3 (M
+ K),
627.2 (M + Na+ H20); the full reduced compound, (11aS,11a'S)-8,8'-(pentanc-1,5-
diylbis(oxy))bis(7-methoxy-2-methylene-2,3,11,11a-tetrahydro-1H-
benzo[e]pyrrolo[1,2-
a][1,4]diazepin-5(10H)-one), (16.5 mg, 11.1%), MS in/z+ 611.2 (M + Na), 627.2
(M + K),
629.2 (M + Na+ H/0); and the unreacted starting material (10.2 mg, 6.8%), MS
m/z+
607.2 (M + Na), 625.2 (M + Na+ 1+0).
91
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Example 21. (S)-8-((5-(((S)-10-(3-(2-(2-azidoethoxy)ethoxy)propanoyl )-7-
methoxy-
2-methylene-5-oxo-2,3,5 ,10,11,11a-hexahydro-1H -benzo [ e]pyrrolo [1,2-a] [
1,4]di azepin-8-
yl)oxy)pentyl)oxy)-7-methoxy-2-methylene-2,3-dihydro-1H-benzo[e]pyrrolo[1,2-
a] [1,4] diazepin-5( llaH)- one, (99)
2
OMe Me
0 0 99
To the mixture of compound 98 (60.0 mg, 0.102 mmol) and compound 88 (40.5 mg,
0.134 mmol) in dichloromethane (5 ml) was added EDC (100.5 mg, 0.520 mmol).
The
mixture was stirred at RT overnight, concentrated, and purified on SiO2
chromatography
eluted with Et0Ac/CH2C12 (1:6) to afford 63.1 nig (81% yield) of the title
product 99. ESI
MS m/z+ C40H50N709 (M+H), cacld. 772.36, found 772.30.
Example 22. (S)-8-((5-(((S)-10-(3-(2-(2-aminoethoxy)ethoxy)propanoy1)-7-
methoxy-
2-methylene-5-oxo-2,3,5,10,11,11a-hexahydro-1H-benzo[e]pyrrolo[1,2-
a][1,4]diazepin-8-
yl)oxy)pentypoxy)-7-methoxy-2-methylene-2,3-dihydro-1H-benzo[e]pyrrolo[1,2-
a][1,4]diazepin-5(11aH)-one, (100).
2
N isrr
Me Me
0 :x?
o=2
the compound 99 (60 mg, 0.078 mmol) in the mixture of THF (5 ml) and
NaH2PO4 buffer (50 mM, pH 5.0, 1 ml) was added PPh3 (70 mg, 0.267 mmol). The
mix-
ture was stirred at RT overnight, concentrated, and purified on C-18
chromatography
eluted with water/CH3CN (from 90% water to 35% water in 35 min) to afford 45.1
mg
(79% yield) of the title product 100 after dried with high vacuum pump. ESI MS
m/z+
C40H52N509 (M+H), cacld. 746.37, found 746.50.
Example 23. (S)-tert-butyl 2-(hydroxymethyppyrrolidine-1-carboxylate
g.y0H
TIIF, 0 'V
13oe 0 13oc
Boc-L-proline (10.0 g, 46.4 mmol) dissolved in 50 mL THF was cooled to 0 C,
to
which BH3 in THF (1.0 M, 46.4 mL) was added carefully. The mixture was stirred
at 0 C
for 1.5 h then poured onto ice water and extracted with ethyl acetate. The
organic layer
92
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
was washed with brine (50 mL), dried over anhydrous Na2SO4, and concentrated
under
reduced pressure to give the title compound (8.50 g, 91% yield) as a white
solid. IFI NMR
(500 MHz, CDC13) 6 3.94 (dd, J = 4.9, 2.7 Hz, 2H), 3.60 (ddd, J = 18.7, 11.9,
9.3 Hz. 2H),
3.49 - 3.37 (m, 1H), 3.34 - 3.23 (m, 1H), 2.06 - 1.91 (m, 1H), 1.89 - 1.69 (m,
2H), 1.65 -
1.51 (m, 1H), 1.49 - 1.40 (m, 9H).
Example 24. (S)-tcrt-butyl 2-formylpyrrolidinc-1-carboxylate
CL.OH S03-pyr. v.-
Et31, DMSO CNIM
Boc Boc
To a solution of (S)-tert-butyl 2-(hydroxymethyl)pyrrolidinc-1-carboxylatc
(13.0 g,
64.6 mmol) in dimethyl sulfoxide (90 mL) was added triethylamine (40 mL) and
the
stirring was continued for 15 min. The mixture was cooled over ice bath and
sulfur triox-
ide-pyridine complex (35.98 g, 226 mmol) was added in portions over a 40 mm
period.
The reaction was warmed to r.t. and stirred for 2.5 h. After addition of ice
(250 g), the
mixture was extracted with dichloromethane (150 mL x 3). The organic phase was
washed
with 50% citric acid solution (150 mL), water (150 mL), saturated sodium
bicarbonate
solution (150 mL), and brine (150 mL), dried over anhydrous Na2SO4. Removal of
solvent
in vacuo yielded the title compound (10.4 g, 81% yield) as dense oil which was
used
without further purification. 11-1 NMR (500 MHz, CDC13) 6 9.45 (s, 1H), 4.04
(s, 1H), 3.53
(dd, J = 14.4, 8.0 Hz, 2H), 2.00- 1.82 (m, 4H), 1.44 (d, J = 22.6 Hz, 9H).
Example 25. (4R,5S)-4-methy1-5-pheny1-3-propionyloxazolidin-2-one
0 0
HN C1 ).L1.1-k0
n-BuLi, THF )1,h
Ph -78 to -50 C
n-Butyllithium in hexane (21.6 mL, 2.2 M, 47.43 mmol) was added dropwise at -
78
C to a stirred solution of 4-methyl-5-phenyloxazolidin-2-one (8.0 g, 45.17
mmol) in THF
(100 mL) under N2. The solution was maintained at -78 C for 1 h then
propionyl chloride
(4.4 mL, 50.59 mmol) was added slowly. The reaction mixture was warmed to -50
C,
stirred for 2 h then quenched by addition of a saturated solution of ammonium
chloride
(100 mL). The organic solvent was removed in vacuo and the resultant solution
was
extracted with ethyl acetate (3 x 100 mL). The organic layer was washed with
saturated
sodium bicarbonate solution (100 mL) and brine (100 mL), dried over Na2SO4,
filtered
and concentrated in vacuo. The residue was purified by column chromatography
(20%
ethyl acetate/hexanes) to afford the title compound as dense oil (10.5 g, 98%
yield). 1H
93
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
NMR (500 MHz, CDC13) 6 7.45 -7.34 (m, 3H), 7.30 (d, J= 7.0 Hz, 2H), 5.67 (d,
J= 7.3
Hz, 1H). 4.82 -4.70 (m, 1H), 2.97 (dd, J = 19.0, 7.4 Hz, 2H), 1.19 (t, J = 7.4
Hz, 3H),
0.90 (d, J = 6.6 Hz, 3H).
Example 26. (S)-tert-butyl 2-((1R,2R)-1-hydroxy-2-methy1-3-((4R,5S)-4-methyl-2-
oxo-5-phenyloxazolidin-3-y1)-3-oxopropyl)pyrrolidine-l-carboxylate.
0 0 0
LN't( "Bu2BTf, Et3N)...
0 C then
11
Boc
Ph -78 C, CH2C12 B0c OH 0
To a solution of (4R,5S)-4-methyl-5-phenyl-3-propionyloxazolidin-2-one (9.40
g,
40.4 mmol) in dichloromethane (60 mL) was added Et3N (6.45 mL, 46.64 mmol) at
0 C,
followed by 1M dibutylboron triflate in dichloromethane (42 mL, 42 mmol). The
mixture
was stirred at 0 C for 45 min, cooled to -70 C, (S)-tert-butyl 2-
formylpyrrolidine-1-
carboxylate (4.58 g, 22.97 mmol) in dichloromethane (40 mL) was then added
slowly over
a 30 min period. The reaction was stirred at -70 C for 2 h, 0 C 1 h, and
r.t. 15 mm, and
then quenched with phosphate buffer solution (pH 7, 38 mL). After the addition
of Me0H-
30% H202 (2:1, 100 mL) at below 10 C and stiffing for 20 min, water (100 mL)
was
added and the mixture was concentrated in vacuo. More water (200 mL) was added
to the
residue and the mixture was extracted with ethyl acetate (3 x 100 mL). The
organic layer
was washed with 1N KHSO4 (100 mL), sodium bicarbonate solution (100 mL) and
brine
(100 mL), dried over anhydrous Na2SO4 and concentrated in vacuo. The residue
was
purified by flash column chromatography (10% - 50% ethyl acetate/hexanes) to
afford the
title compound as a white solid (7.10g, 71% yield). 1H NMR (500 MHz, CDC13) 6
7.39
(dt, J= 23.4, 7.1 Hz, 3H), 7.30(d. J= 7.5 Hz, 2H), 5.67 (d, J= 7.1 Hz, 1H),
4.84 - 4.67
(m, 1H), 4.08 - 3.93 (m, 3H), 3.92 - 3.84 (m, 1H), 3.50 (d, J = 9.0 Hz, 1H),
3.24 (d, J =
6.7 Hz, 1H), 2.15 (s, 1H), 1.89 (dd, J = 22.4, 14.8 Hz, 3H), 1.48 (d, J = 21.5
Hz, 9H), 1.33
(d, J = 6.9 Hz, 3H), 0.88 (d, J = 6.4 Hz, 3H).
Example 27. (S)-tert-butyl 2-((1R,2R)-1-methoxy-2-methy1-3-((4R,5S)-4-methyl-2-
oxo-5-phenyloxazolidin-3-y1)-3-oxopropyl)pyrrolidine-1-carboxylate
Me3OBF4
C3
yLif.T1 proton sponge
MS, C1CH2CH2C1
Boc OH 0 0 C to r.t. Hoe 0,, 0
To a mixture of (S)-tert-butyl 2-((lR,2R)-1-hydroxy-2-methy1-3-((4R.5S)-4-
methyl-
2-oxo-5-phenyloxazolidin-3-y1)-3-oxopropyl)pyn-olidine-1-carboxylate. (5.1 g
11.9 mmol)
94
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
and molecular sieves (4 A, 5 g) was added anhydrous dichloroethane (30 mi..)
under
The mixture was stirred at room temperature for 20 min and cooled to 0 C.
Proton sponge
(6.62 g, 30.9 mato was added, followed by trimethyloxonium tetrafluoroborate
(4.40 g,
29.7 mmol). Stirring was continued for 2 h at 0 C and 48 h at r.t. The
reaction mixture
was filtrated and the filtrate was concentrated and purified by column
chromatography
(20-70% ethyl acetate/hexanes) to afford the title compound as a colorless
solid (1.80 g,
35% yield). 1H NMR (500 MHz, CDC13) 6 7.46 - 7.27 (m, 5H), 5.65 (s, 1H), 4.69
(s, 1H),
3.92 (s, H), 3.83 (s, I H), 3.48 (s, 3H), 3.17 (s, 2H), 2.02 - 1.68 (m, 5H),
1.48 (d, J= 22.3
Hz, 9H), 1.32 (t, J = 6.0 Hz, 3H), 0.91 -0.84 (m, 3H).
Example 28. (2R,3R)-3-((S)-1-(tert-butoxycarbonyl)pyrrolidin-2-y1)-3-methoxy-2-
methylpropanoic acid
rjN1 Li0H, H2021., cy,lf.OH
r THF, H20
Boc 0 0 0 C Boe 0 0
To a solution of (S)-tert-butyl 2-((lR,2R)-1-methoxy-2-methy1-3-((4R,5S)-4-
methyl-
2-oxo-5-phenyloxazolidin-3-y1)-3-oxopropyl)pynolidine-1-carboxylate (1.80 g,
4.03
mmol) in TFIF (30 mL) and H20 (7.5 mL), 30(.) 11201 (1.44 mL, 1.4.4 mmol) was
added
over a 5 min period at 0 C , followed by a solution of LiOH (0.27 g, 6.45
mmol) in water
(5 mi.). After stirring at 0 C for 3 It I N sodium sulfite (15,7 mi..) was
added and the
mixture was allowed to warm to r.t. and stirred overnight. 'MP was removed in
vacuo and
the aqueous phase wa.s wash with dichloromethane (3 x 50 mL) to remove the
oxazolidinone auxiliary. The aqueous phase was acidified to pii 3 with IN HCi
and ex-
tracted with ethyl acetate (3 x 50 mL). The organic layer was washed with
brine (50 mL),
dried over Na2SO4, filtered and concentrated in vacuo to afford the title
compound as
colorless oil (1.15 g, 98% yield). 1H NMR (500 MHz, CDC13) 6 3.99 - 3.74 (m,
2H), 3.44
(d, J = 2.6 Hz, 3H), 3.23 (s, 1H), 2.60- 2.45 (m, 1H), 1.92 (tt, J = 56.0,
31.5 Hz, 3H), 1.79
- 1.69 (m, 1H), 1.58- 1.39 (m, 9H), 1.30- 1.24 (m, 3H).
Example 29. (4S,5S)-ethyl 4-((tert-butoxycarbonyl)amino)-5-methy1-3-
oxoheptanoate
CDI, THF, then _
Boc
O \,,0y7y,0
H1:1f4 H
0 õO
Boc Mg 0 0
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
To an ice-cooled solution of (2S,3S)-2-((tert-butoxycarbonyl)amino)-3-methyl-
pentanoic acid (4.55 g, 19.67 mmol) in THF (20 mL) was added 1,1'-
carbonyldiimidazole
(3.51 g, 21.63 mmol). After evolution of gas ceased, the resultant mixture was
stirred at
r.t. for 3.5 h. A solution of freshly prepared isopropylmagnesium bromide in
THF (123
mmol, 30mL) was added dropwise to a pre-cooled (0 C) solution of ethyl
hydrogen
malonate (6.50 g, 49.2 mmol) at such a rate to keep the internal temperature
below 5 C.
The mixture was stirred at r.t. for 1.5 h. This solution of the magnesium
enolate was then
cooled over an ice-water bath, followed by the gradual addition of the
imidazolide solution
over a l h period via a double-ended needle at 0 C. The resultant mixture was
stirred at 0
C for 30 mm then r.t. 64 h. The reaction mixture was quenched by addition of
10%
aqueous citric acid (5 mL), and acidified to pH 3 with an additional 10%
aqueous citric
acid (110 mL). The mixture was extracted with ethyl acetate (150 mL x 3). The
organic
extracts were washed with water (50 mL), saturated aqueous sodium hydrogen
carbonate
(50 mL), and saturated aqueous sodium chloride (50 mL), dried over Na2SO4, and
concen-
trated in vacuo. The residue was purified by column chromatography on silica
gel using
ethyl acetate/hexane (1:4) as an eluent to give title compound (5.50 g, 93%
yield). 'H
NMR (500 MHz, CDC13) 6 5.04 (d, J = 7.8 Hz, 1H), 4.20 (p, J = 7.0 Hz, 3H).
3.52 (t, J =
10.7 Hz, 2H), 1.96 (d, J= 3.7 Hz, 1H), 1.69 (s, 2H), 1.44 (s, 9H), 1.28 (dd,
J= 7.1, 2.9 Hz,
3H), 0.98 (t, J= 6.9 Hz, 3H), 0.92- 0.86 (m, 3H).
Example 30. (3R,4S,5S)-ethyl 4-((tert-butoxycarbonyl)amino)-3-hydroxy-5-methyl-
heptanoate
NaBH4
,Boe.N
Et0Hip
0 0 OH OH
To a solution of (4S,5S)-ethyl 4-((tert-butoxycarbonyeamino)-5-methy1-3-
oxoheptanoate (5.90 g, 19.83 mmol) in ethanol (6 mL) at -60 C was added
sodium
borohydride (3.77 g, 99.2 mmol) in one portion. The reaction mixture was
stirred for 5.5 h
below -55 C then quenched with 10% aqueous citric acid (100 mL). The
resultant solu-
tion was acidified to pH 2 with an additional 10% aqueous citric acid,
followed by extrac-
tion with ethyl acetate (100 mL x 3). The organic extracts were washed with
saturated
aqueous sodium chloride (100 mL), dried over Na2SO4, and concentrated in
vacuo. The
residue was purified by column chromatography (10-50% ethyl acetate/hexane) to
give
pure diastereomer (3R,4S,5S)-ethyl 4-((tert-butoxycarbonyl)amino)-3-hydroxy-5-
methyl-
heptanoate (2.20 g, 37% yield) and a mixture of (3R,4S,5S)-ethyl 4-((tert-
butoxycarbonyl)
96
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
amino)-3-hydroxy-5-methyl-heptanoate and (3S,4S,5S)-ethyl 4-((tert-
butoxycarbony1)-
amino)-3-hydroxy-5-methylheptanoate (2.0g, 34% yield, about 9:1 ratio).1HNMR
(500
MHz, CDC13) 6 4.41 (d, J = 9.3 Hz, 1H), 4.17 (tt, J = 7.1, 3.6 Hz, 2H), 4.00
(t, J = 6.9 Hz,
1H). 3.55 (dd, J= 11.7, 9.3 Hz, 1H), 2.56 - 2.51 (m, 2H), 2.44 (dd, J= 16.4,
9.0 Hz, 1H),
1.79 (d, J= 3.8 Hz, 1H), 1.60 - 1.53 (m, 1H), 1.43 (s, 9H), 1.27 (dd, J= 9.3,
5.0 Hz, 3H),
1.03 -0.91 (m, 7H).
Example 31. (3R,4S,5S)-4-((tert-butoxycarbonyl)amino)-3-hydroxy-5-methyl-
heptanoic acid
1 N NaOH
Et0H 0. Boe..N OH
= OHO 0110
To a solution of compound (3R,4S,5S)-ethyl 4-((tert-butoxycarbonyl)amino)-3-
hydroxy-5-methyl-heptanoate (2.20 g, 7.20 mmol) in ethanol (22 mL) was added 1
N
aqueous sodium hydroxide (7.57 mL, 7.57 mmol). The mixture was stirred at 0 C
for 30
min then r.t. 2 h. The resultant solution was acidified to pH 4 by addition of
1 N aqueous
hydrochloric acid, which was then extracted with ethyl acetate (50 mL x 3).
The organic
extracts were washed with 1 N aqueous potassium hydrogen sulfate (50 mL), and
saturat-
ed aqueous sodium chloride (50 mL), dried over Na2SO4, and concentrated in
vacuo to
give the title compound (1.90 g, 95% yield). 'H NMR (500 MHz, CDC13) 6 4.50
(d, J = 8.7
Hz, 1H), 4.07 (d, J = 5.5 Hz, 1H), 3.59 (d, J = 8.3 Hz, 1H), 2.56 -2.45 (m,
2H), 1.76 -
1.65 (m, 1H), 1.56 (d, J= 7.1 Hz, 1H), 1.45 (s, 9H), 1.26 (t, J= 7.1 Hz, 3H),
0.93 (dd, J=
14.4, 7.1 Hz, 6H).
Example 32. (3R,4S,5S)-4-((tert-butoxycarbonyl)(methyl)amino)-3-methoxy-5-
methylheptanoic acid
Me!, NaH
Boc. 16:C= r.T.OH THF Boc%NvLy.yon
0 0
= 011 0
To a solution of (3R,4S,5S)-4-((tert-butoxycarbonyl)amino)-3-hydroxy-5-methyl-
heptanoic acid (1.90 g, 6.9 mmol) in THF (40 mL) was added sodium hydride (60%
oil
suspension, 1.93 g, 48.3 mmol) at 0 C. After stirring for lh, methyl iodide
(6.6 mL, 103.5
mmol) was added. The stirring was continued at 0 C for 40 h before saturated
aqueous
sodium hydrogen carbonate (50 mL) was added, followed by water (100 mL). The
mixture
was washed with diethyl ether (50 mL x 2) and the aqueous layer was acidified
to pH 3 by
1 N aqueous potassium hydrogen sulfate, then extracted with ethyl acetate (50
mL x 3).
97
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
The combined organic extracts were washed with 5% aqueous sodium thiosulfate
(50 mL)
and saturated aqueous sodium chloride (50 mL), dried over Na2SO4, and
concentrated in
vacuo to give the title compound (1.00g. 48% yield). 'H NMR (500 MHz, CDC13) 6
3.95
(d, J= 75.4 Hz, 2H), 3.42 (d. J= 4.4 Hz, 3H), 2.71 (s, 3H), 2.62 (s, 1H), 2.56
¨ 2.47 (m,
2H), 1.79 (s, 1H), 1.47 (s, 1H), 1.45 (d. J= 3.3 Hz, 9H), 1.13¨ 1.05 (m, 1H),
0.96 (d, J=
6.7 Hz, 3H), 0.89 (td, J = 7.2, 2.5 Hz, 3H).
Example 33. General procedure for the removal of the Boc function with
trifluoroacetic acid.
To a solution of the N-Boc amino acid (1.0 mmol) in methylene chloride (2.5
mL)
was added trifluoroacetic acid (1.0 mL). After being stirred at room
temperature for 1-3 h,
the reaction mixture was concentrated in vacuo. Co-evaporation with toluene
gave the
deprotected product, which was used without any further purification.
Example 34. (S)-tert-butyl 2-((1R,2R)-1-methoxy-3-(((S)-1-methoxy-1-oxo-3-
phenylpropan-2-y1)amino)-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate
L-Phe-OMe
cLirly0H EtiN, DECPPh
Boc 0 0 0 C to r.t. Boc 0 CO2Me
To a solution of (2R,3R)-3-((S)-1-(tert-butoxycarbonyl)pyrrolidin-2-y1)-3-
methoxy-2-
methylpropanoic acid (100 mg, 0.347 mmol) and L-Phenylalanine methyl ester
hydrochlo-
ride (107.8 mg, 0.500 mmol) in DMF (5 mL) at 0 C was added diethyl
cyanophosphonate
(75.6 [tL, 0.451 mmol), followed by Et3N (131 ILL, 0.94 mmol). The reaction
mixture was
stirred at 0 C for 2 h, then warmed to r.t. and stirred overnight. The
reaction mixture was
then diluted with ethyl acetate (80 mL), washed with 1 N aqueous potassium
hydrogen
sulfate (40 mL), water (40 mL), saturated aqueous sodium hydrogen carbonate
(40 mL),
and saturated aqueous sodium chloride (40 mL), dried over Na2SO4, and
concentrated in
vacuo. The residue was purified by column chromatography (15-75% ethyl ace-
tate/hexanes) to afford the title compound (130 mg, 83% yield) as a white
solid. 1H NMR
(500 MHz, CDC13) 6 7.28 (dd, J = 7.9, 6.5 Hz, 2H), 7.23 (t, J = 7.3 Hz, 1H),
7.16 (s. 2H).
4.81 (s, 1H), 3.98 ¨ 3.56 (m, 5H), 3.50 (s, 1H), 3.37 (d, J= 2.9 Hz, 311),
3.17 (dd, J= 13.9,
5.4 Hz, 2H), 3.04 (dd, J = 14.0, 7.7 Hz, 1H), 2.34 (s, 1H), 1.81 ¨ 1.69 (m,
2H), 1.65 (s,
3H), 1.51 ¨ 1.40 (in, 9H), 1.16 (d, J = 7.0 Hz, 3H).
Example 35. (S)-methyl 24(2R,3R)-34(S)-1-((3R,4S,5S)-4-((tert-butoxycarbony1)-
(methyl)amino)-3-methoxy-5-methylheptanoyl)pyrrolidin-2-y1)-3-methoxy-2-
methylpropanamido)-3-phenylpropanoate
98
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Ph
Ph BocOH
Bos
r:c..1r(N)ylyNyol
110yLrrN,T0.1 I 0 0
______________________________________ ID' I 0 0 0 0
O 0 CO2Me Et3N, DECP, DMF CO2Me
To a solution of the deprotected product from (S)-tert-butyl 24(1R,2R)-1-
methoxy-3-
(((S)-1-methoxy-1-oxo-3-phenylpropan-2-yl)amino)-2-methyl-3-
oxopropyl)pyrrolidine-1-
carboxylate (0.29 mmol) and (3R,4S,5S)-4-((tert-butoxycarbonyl)(methyl)amino)-
3-
methoxy-5-methylheptanoic acid (96.6 mg, 0.318 mmol) in DMF (5 mL) at 0 C was
added diethyl cyanophosphonate (58 pi, 0.347 mmol), followed by Et3N (109 Id_
0.78
mmol). The reaction mixture was stirred at 0 C for 2 h, then warmed to r.t.
and stirred
overnight. The reaction mixture was diluted with ethyl acetate (80 mL), washed
with l N
aqueous potassium hydrogen sulfate (40 mL), water (40 mL), saturated aqueous
sodium
hydrogen carbonate (40 mL), and saturated aqueous sodium chloride (40 mL),
dried over
Na2SO4 and concentrated in vacuo. The residue was purified by column
chromatography
(15-75% ethyl acetate/hexanes) to afford the title compound (150 mg, 81%
yield) as a
white solid. LC-MS (ESI) m/z calcd. for C34H55N308 [1\4+Hr: 634.40, found:
634.40.
Example 36. (S)-methyl 2-((2R,3R)-3-((S)-1-((3R,4S,5S)-4-((S)-2-((tert-
butoxycarbonyl)amino)-N,3-dimethylbutanamido)-3-methoxy-5-methylheptanoy1)-
pyrrolidin-2-y1)-3-methoxy-2-methylpropanamido)-3-phenylpropanoate
Ph Boc-Val-OH BocHN\ Ph
BP, DIPEA1), AN N
HI:V.)1)1'1)1Nro
CH2C12 = I
I O. 0 .õ0 0 CO2Me O. 0 O 0 CO2Me
To a solution of the deprotected product from (S)-methyl 2-((2R,3R)-3-((S)-1-
((3R,4S,55)-4-((tert-butoxycarbony1)-(methyl)amino)-3-methoxy-5-
methylheptanoy1)-
pyrrolidin-2-y1)-3-methoxy-2-methylpropanamido)-3-phenylpropanoate (0.118
mmol) and
Boc-Val-OH (51.8 mg. 0.236 mmol) in DCM (5 mL) at 0 C was added
bromotris(dimethylamino)- phosphonium hexafluorophosphate (BroP, 70.1 mg,
0.184
mmol), followed by diisopropylethylamine (70 pL. 0.425 mmol). The mixture was
shield-
ed from light and stirred at 0 C for 30 min then at r.t. for 2 days. The
reaction mixture was
diluted with ethyl acetate (80 mL), washed with 1 N aqueous potassium hydrogen
sulfate
(40 mL), water (40 mL), saturated aqueous sodium hydrogen carbonate (40 mL),
and
saturated aqueous sodium chloride (40 mL), dried over Na/SO4 and concentrated
in vacuo.
The residue was purified by column chromatography (20-100% ethyl
acetate/hexanes) to
afford the title compound (67 mg, 77% yield) as a white solid. LC-MS (ESI) m/z
calcd. for
C39H64N409 [M+I-1]+: 733.47, found: 733.46.
99
CA 02991975 2018-01-10
WO 2015/155753
PCT/IB2015/056083
Example 37. Preparation of compound Boe-N-Me-Val-OH
OH c,i,. Mel, NaH
Boe-N
THF v. Boe-N OH
H I 0
0
To a solution of Boc-L-Val-OH (2.00 g, 9.2 mmol) and methyl iodide (5.74 mL,
92
nunol) in anhydrous THE (40 mL) was added sodium hydride (3.68 g, 92 mmol) at
0 C.
The reaction mixture was stirred at 0 C for 1.5 h, then warmed to r.t. and
stirred for 24 h.
The reaction was quenched by ice water (50 mL). After addition of water (100
inL), the
reaction mixture was washed with ethyl acetate (50 mL x 3) and the aqueous
solution was
acidified to pH 3 then extracted with ethyl acetate (50 mL x 3). The combined
organic
phase was dried over Na2SO4 and concentrated to afford Boc-N-Me-Val-OH (2.00
g, 94%
yield) as a white solid. 1H NMR (500 MHz, CDC13) 6 4.10 (d, J = 10.0 Hz, 1H),
2.87 (s,
3H), 2.37 ¨2.13 (m, 1H), 1.44 (d, J = 26.7 Hz, 9H), 1.02 (d, J = 6.5 Hz, 3H),
0.90 (t, J =
8.6 Hz, 3H).
Example 38. (S)-methyl 24(2R,3R)-3-((S)-14(6S,9S,12S,13R)-12-((S)-sec-buty1)-
6,9-diisopropy1-13-methoxy-2,2,5,11-tetramethy1-4,7,10-trioxo-3-oxa-5,8,11-
triazapenta-
decan-15-oyl)pyrrolidin-2-y1)-3-methoxy-2-methylpropanamido)-3-
phenylpropanoate
ph BocsNYrrOH H 0õ H Ph
H2Nµ,/ 4Iri,õiralrkirLI I 0 Boc;;,N,õõ).k 114;c1c1sQylv.-N-yol
A N i DECP/MMP/DMF 1 A N
./=== I 4:\, 0 0 0 0
.,,,,. I 0,. 0 Al 0 CO2Me
CO2 Me 0 C to r.t.
To a solution of the deprotected product from (S)-methyl 2-((2R,3R)-3-((S)-1-
((3R,4S,5S)-44(S)-2-((tert-butoxycarbonyl)amino)-N,3-dimethylbutanamido)-3-
methoxy-
5-methylheptanoye-pyrrolidin-2-y1)-3-methoxy-2-methylpropanamido)-3-
phenylpropanoate (0.091 mmol) and Boc-N-Me-Val-OH (127 mg, 0.548 mmol) in DMF
(5 mL) at 0 C was added diethyl cyanophosphonate (18.2 iaL, 0.114 mmol),
followed by
4-methylmorpholine (59 pL, 0,548 mmol). The reaction mixture was stirred at 0
C for 2
h, then warmed to r.t. and stirred overnight. The reaction mixture was diluted
with ethyl
acetate (80 mL), washed with 1 N aqueous potassium hydrogen sulfate (40 mL),
water (40
mL), saturated aqueous sodium hydrogen carbonate (40 mL), and saturated
aqueous
sodium chloride (40 mL), dried over sodium sulfate, and concentrated in vacuo.
The
residue was purified by column chromatography (20-100% ethyl acetate/hexanes)
to
afford the title compound (30 mg, 39% yield) as a white solid. LC-MS (ESI) m/z
calcd. for
C45H75N50101M+1-11+: 846.55, found: 846.56.
100
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Example 39. (S)-2-((2R,3R)-3-((S)-1-((6S,9S,12S,13R)-12-((S)-sec-buty1)-6,9-
diisopropy1-13-methoxy-2,2,5,11-tetramethy1-4,7,10-trioxo-3-oxa-5,8,11-
triazapenta-
decan-15-oyl)pyrrolidin-2-y1)-3-methoxy-2-methylpropanamido)-3-phenylpropanoic
acid
y H 0 H
O
N N
BocNil\LAN
I CO2H
(S)-methyl 2-((2R,3R)-3-((S)-1-((6S.9S,12S,13R)-12-((S)-sec-buty1)-6,9-
diisopropyl-
13-methoxy-2,2,5,11-tetramethy1-4,7,10-trioxo-3-oxa-5,8,11-triazapenta-decan-
15-
oyl)pyrrolidin-2-y1)-3-methoxy-2-methylpropanamido)-3-phenylpropanoate (30 mg,
0.035
mmol) in THF (1.0 ml) was added LiOH in water (1.0M, 0.8 ml). The mixture was
stirred
at RT for 35 min, neutralized with 0.5M H3PO4 to pH 6, concentrated and
purified on SiO2
column eluted with CH3OH/CH2C12/HOAc (1:10:0.01) to afford the title compound
(25.0
mg, 85% yield). LC-MS (ESI) m/z calcd. for C44H741\150101M+Hr: 832.54, found:
832.60.
Example 40. (S)-2-((2R,3R)-3-((S)-1-((3R,4S,5S)-4-((S)-N,3-dimethy1-2-((S)-3-
methy1-2-(methylamino)butanamido)butanamido)-3-methoxy-5-methylheptanoy1)-
pyrrolidin-2-y1)-3-methoxy-2-methylpropanamido)-3-phenylpropanoic acid (101)
y H
HN^riN"."'µ
I Pft
0 7 0, 0 ,120 0 CO2H
(S)-2-((2R,3R)-3-((S)-1-((6S.9S,12S,13R)-12-((S)-sec-buty1)-6,9-diisopropy1-13-
methoxy-2,2,5,11-tetramethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapenta-decan-15-
oyl)pyrrolidin-2-y1)-3-methoxy-2-methylpropanamido)-3-phenylpropanoic acid (25
mg,
0.030 mmol) in the mixture of HC1 (conc. 0.3 ml) and 1,4-dioxane (0.9 ml) was
stirred at
RT for 35 min. The mixture was diluted with Et0H (1.0 ml) and toluene (1.0
ml), concen-
trated and re-evaporated with Et0H/toluene (2:1) to afford the title compound
as a white
solid (22 mg, ¨100% yield) for the next step without further purification. LC-
MS (ESI)
m/z calcd. for C39H66N508 [M+H]: 732.48, found: 732.60.
Example 41. (2S)-2-((2R,3R)-3-((2S)-1-((11S,14S,17S)-1-azido-17-((R)-sec-
buty1)-
11,14-diisopropyl-18-methoxy-10,16-dimethyl-9,12,15-trioxo-3.6-dioxa-10,13,16-
triazai-
cosan-20-oyl)pyrrolidin-2-y1)-3-methoxy-2-methylpropanamido)-3-phenylpropanoic
acid
0
H
N3 +.01%.ANYyN,r...1(Nroislr.N
2 0 a ,0 ¨0 0
0 OH 102
101
CA 02991975 2018-01-10
WO 2015/155753
PCT/IB2015/056083
To the crude compound 101 (22 mg, 0.030 mmol) in the mixture of DMA (0.8 ml)
and NaH2PO4 buffer (0.7 ml, 1.0 M, pH 7.5) was added compound 88 (18.0 mg,
0.060
mmol) in four portions in 2 h. The mixture was stirred overnight, concentrated
and puri-
fied on SiO2 column eluted with CH3OH/CH2C12/HOAc (1:8:0.01) to afford the
title
compound (22.5 mg, 82% yield). LC-MS (ESI) m/z calcd. for C46H77N8011 [M+Hr:
917.56, found: 917.60.
Example 42. (2S )-2-((2R,3R)-3-((2S)-1-((11S ,14S ,175 )- 1-amino- 174(R)-sec-
buty1)-
11,14-dii sopropy1-18-methoxy-10,16-dimethy1-9,12,15-trioxo-3,6-dioxa-10,13,16-
triazaicosan-20-oyl)pyrrolidin-2-y1)-3-methoxy-2-methylpropanamido)-3-
phenylpropanoic
acid (103).
0 0
1121NN,õ(".03A ;µ)CroN,,rr1Q,(111,N
2 I I --0 ¨0 0 0 0H 103
Compound 102 (22.0 mg, 0.024 mmol) in methanol (5 ml) in a hydrogenation
vessel
was added Pd/C (5 mg. 10% Pd, 50% wet). After air was vacuumed out in the
vessel, 25
psi H2 was conducted in. The mixture was shaken for 4 h, filtered through
celite, concen-
trated to afford the crude title product (-20 mg, ¨92% yield).for the next
step without
further purification. ESI MS m/z+ C46H79N6011 (M+H), cacld. 891.57, found
891.60.
Example 43. 2,3-dibromosuccinic anhydride (70)
0 Br 0
Hoy.y.OH Br 0
CH2C12 Br
Br 0 0
2,3-dibromosuccinic acid (10.00g. 36.51 mmol) in dry CH2C12 (100 ml) at 0 C
was
added phosphorus pentoxide (12.21 g, 85.84 mmol). The mixture was stirred at 0
C for 2 h
and then RT for 5 h, filtered through short SiO2 column. and rinsed the column
with
Et0Ac/CH2C12 (1:6). The filtered solutions were combined, evaporated and
solidified with
Et0Ac/Hexane to afford the title compound (6.63 g, 71% yield). ESI MS m/z+
C4H2Br203
(M+H), cacld. 256.85. found 256.70.
Example 44. 2,3-dibromo-4-((2-(2-(3-((S)-7-methoxy-8-((5-(((S)-7-methoxy-2-
methylene-5-oxo-2.3,5,11a-tetrahydro-1H-benzo [e]pyrrolo [1,2-a] [1,4]diazepin-
8-
yl)oxy)pentyl)oxy)-2-methylene-5-oxo-2,3,11,11a-tetrahydro-1H-
benzo[e]pyrrolo[1,2-
a][1,4]diazepin-10(5H)-y1)-3-oxopropoxy)ethoxy)ethypamino)-4-oxobutanoic acid
(124)
102
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
0
N
Br4õ 100
Br DMA iNr* H Br
0 Me Me0
70 0 124
0 0
To the compound 100 (40.0 mg. 0.068 mmol) in the mixture of DCM (4 ml) and
DlPEA (12 uL, 0.069 mmol) was added 2,3-dibromosuccinic anhydride (38.0 mg,
0.148
mmol) at 0 C. The mixture was stirred at 0 C for 2 h and then RT 5h. The
mixture was
concentrated and purified on SiO2 column eluted with CH3OH/CH2C12/HOAc
(1:6:0.01) to
afford the title compound (56.5 mg, 83% yield). LC-MS (EST) m/z calcd. for
C44H53Br2N5012 [M+H]: 1002.21, found: 1002.40, 1004.40 (M+2+H).
Example 45. 2,5-dioxopyrrolidin-1-y1 2,3-dibromo-442-(2-(34(S)-7-methoxy-845-
(((S)-7-methoxy-2-methylene-5-oxo-2,3,5,11a-tetrahydro-1H-benzo[e[pyrrolo[1,2-
a][1,4[-
diazepin-8-yl)oxy)pentyl)oxy)-2-methylene-5-oxo-2,3,11,11a-tetrahydro-1H-
benzo[e[pyr-
rolo[1,2-a][1,4]diazepin-10(5H)-y1)-3-oxopropoxy)ethoxy)ethyl)amino)-4-
oxobutanoate
(125)
ki 0
) 0
Br o--N
Me Me0 Br
0 0 0
Compound 125 (55.0 mg, 0.054 mmol) in CH2C12 (3 ml) was added NHS (10.0 mg,
0.086 mmol) and EDC (30.5 mg, 0.158 mmol). The mixture was stirred at RT
overnight,
concentrated and purified on SiO2 column eluted with Et0Ac/CH2C12 (1:5) to
afford the
title compound (50.5 mg, 85% yield). LC-MS (ESI) m/z calcd. for C48I-
156Br2N6014
[M+H]: 1099.22, found: 1099.40, 1101.40 (M+2+H), 1119.50 (M+2+H+H20).
Example 46. (25)-2-((2R,3R)-342S)-14(13S,265,295,32S)-12,13-dibromo-324(R)-
sec-buty1)-26,29-diisopropy1-33-methoxy-14(S)-7-methoxy-8-((5-(((S)-7-methoxy-
2-
methylene-5-oxo-2.3,5,11a-tetrahydro-1H-benzo[e[pyrrolo[1,2-a][1,41diazepin-8-
yl)oxy)pentyl)oxy)-2-methylene-5-oxo-2,3,11,11a-tetrahydro-1H-
benzo[e[pyrrolo[1,2-
a][1,41diazepin-10(5H)-y1)-25,31-dimethy1-1,11,14,24,27,30-hexaoxo-4,7,18,21-
tetraoxa-
10,15,25,28,31-pentaazapentatriacontan-35-oyl)pyrrolidin-2-y1)-3-methoxy-2-
methylpropanamido)-3-phenylpropanoic acid (126)
103
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
n H 0
Br ¨ 1SCrorY"TIT
Br
<;17-
Me Me
0
0 H 0
4101
Compound 1103 (-20 mg, 0.022 mmol) in the mixture of DMA (1 ml) and NaH2PO4
buffer (0.6 ml, 0.15 M, pH 7.5) was added compound 125 (30.0 mg, 0.027 mmol).
The
mixture was stirred for 7 h, concentrated, and purified on C-18 chromatography
(ao 2.0 cm
x 25 cm) eluted with water/CH3CN (from 90% water to l 5% water in 50 min at 10
ml/min) to afford the title product 126 (26.1 mg, 63% yield) after dried with
high vacuum
pump. ESI MS m/z+ C90H130Br2N11022 (M+H), cacld. 1874.77, found 1874.50.
Example 47. Conjugated compound 126 to an antibody for 127.
0
\/('\ Orh--1/
0
mAb
Me Me N-1
HN+0.,,,OLNYyNs.r.AN -- N
12 0 I 0 _o 0 0 n 127
OH
To a mixture of 2.0 mL of 10 mg/ml Herceptin in pH 6.0-8.0, were added of 0.70
-
2.0 mL PBS buffer of 100 mM NaH2PO4. pH 6.5-7.5 buffers, TCEP (28 L, 20 mM in
water) and the compound 126 (14 L, 20 mM in DMA). The mixture was incubated at
RT
for 4-16 h, then DHAA (135 IaL, 50 mM) was added in. After continuous
incubation at
RT overnight, the mixture was purified on G-25 column eluted with 100 mM
NaH2PO4,
50 mM NaCl pH 6.0-7.5 buffer to afford 16.5-17.7 mg of the conjugate compound
127
(82%-88% yield) in 13.1-15.0 ml buffer. The drug/antibody ratio (DAR) (the
combina-
tion of PBD dimer and MMAF per antibody) was 3.85, which was determined via
UPLC-
Qtof mass spectrum. It was 96-99% monomer analyzed by SEC HPLC (Tosoh
Bioscience,
Tskgel G3000SW, 7.8 mm ID x 30 cm, 0.5 ml/min, 100 min) and a single band
measured
by SDS-PAGE gel.
Example 48. (4R)-4-(2-((1R,3R)-1-acetoxy-34(25,35)-N,3-dimethyl-2-((R)-1-
methylpiperidine-2-carboxamido)pentanamido)-4-methylpentyl)thiazole-4-
carboxamido)-
5-(3-(12,13-dibromo-24-((S)-7-methoxy-8-((5-(((S)-7-methoxy-2-methylene-5-oxo-
2.3,5,11a-tetrahydro-1H-benzolelpyrrolol1,2-al [1,41diazepin-8-
yBoxy)pentyl)oxy)-2-
104
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
methylene-5-oxo-2.3,11,11a-tetrahydro-1H-benzo[e]pyrrolo[1,2-a][1,4]diazepin-
10(5H)-
y1)-11,14,24-trioxo-4,7,18,21-tetraoxa-10,15-diazatetracosanamido)-4-
hydroxypheny1)-2-
methylpentanoic acid (128)
ome Me = *
1%01"x
N 0
0 2 '=-=
\,.Br
0
OH 0
N4 0 OAc 0 ftir+Ø+N..,CBr
''SINUNIAN OH 0 2 H 0
N =fi, 0 I S H 128
I 0
Compound 95 (20 mg, 0.021 mmol) in the mixture of DMA (1 ml) and NaH2PO4
buffer (0.6 ml, 0.15 M, pH 7.5) was added compound 125 (30.0 mg, 0.027 mmol).
The
mixture was stirred for 8 h, concentrated, and purified on C-18 chromatography
((I) 2.0 cm
x 25 cm) eluted with water/CH3CN (from 90% water to 20% water in 50 min at 10
ml/min) to afford the title product 128 (26.6 mg, 64% yield) after dried with
high vacuum
pump. ESI MS m/z+ C89H123Br2N12022S (M+H), cacld. 1901.69, found 1901.90.
Example 49. Conjugated compound 128 to an antibody for 129.
N 0
mAb
I Me Me
0
-
X.:4.ij Nyc /
Niyt
N OH 0 2 H
129
N 0 I S H
I 0
To a mixture of 2.0 mL of 10 mg/ml Herceptin in pH 6.0-8.0, were added of 0.70
-
2.0 mL PBS buffer of 100 mM NaH2PO4, pH 6.5-7.5 buffers, TCEP (28 L, 20 mM in
water) and the compound 128 (14 L, 20 mM in DMA). The mixture was incubated
at RT
for 4-16 h, then DHAA (135 viL, 50 mM) was added in. After continuous
incubation at
RT overnight, the mixture was purified on G-25 column eluted with 100 mM
NaH2PO4,
50 mM NaC1 pH 6.0-7.5 buffer to afford 16.4-17.6 mg of the conjugate compound
129
(82%-88% yield) in 13.2-15.1 ml buffer. The drug/antibody ratio (DAR) (the
combina-
tion of PBD dimer and Tubulysin analog per antibody) was 3.9, which was
determined via
UPLC-Qtof mass spectrum. It was 96-99% monomer analyzed by SEC HPLC (Tosoh
Bioscience, Tskgel G3000SW. 7.8 mm ID x 30 cm, 0.5 ml/min, 100 min) and a
single
band measured by SDS-PAGE gel.
105
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
Example 50. Bis(2,5-dioxopyrrolidin-l-y1) 2,3-dibromosuccinate (9)
BrJ 0 0
OH MIS 00¨N)
Br EDC/DCMo_ 0 0
0
0 0
The solution of 2,3-dibromosuccinic acid (5.0 g, 18.25 mmol), N-
hydroxysuccinimide
(NHS) (5.01 g, 43.56 mmol) and EDC (12.02 g, 62.60 mmol) in dichloromethane
(100 ml)
was stirred at RT overnight, concentrated and purified on SiO2 column eluted
with
Et0Ac/CH2C12 (1:6) to afford the title compound (6.74 g, 79% yield). LC-MS
(ESI) m/z
calcd. for C12H1 iBr2N208 [M+Hr: 468.88, [M+H+21+:470.88, found: 468.70,
470.70.
Example 51. (R,R,S,S,R,4R,412)-5,5'-(((12,13-dibromo-11,14-dioxo-4,7,18,21-
tetraoxa-10,15-diazatetracosane-1,24-dioyl)bis(azanediyMbis(4-hydroxy-3,1-
phenylene))-
bis(4-(2-((1R,3R)-1-acetoxy-3-((2S,3S)-N,3-dimethy1-2-((R)-1-methylpiperidine-
2-
carboxamido)pentanamido)-4-methylpentyl)thiazole-4-carboxamido)-2-
methylpentanoic
acid) (141)
,N4,s1,004.cN} Noti
ellrt N OH Tif Br
N ,fle S H
I 0
I H
Br
11\144s?(NUe5A0 INly+O N
4011-1 N OH 0 2 H 0
N *0 I 141
S H
I 0
Compound 95 (40 mg, 0.042 mmol) in the mixture of DMA (1 ml) and NaH2PO4
buffer (0.6 ml, 0.15 M, pH 7.5) was added Bis(2,5-dioxopyrrolidin-1-y1) 2,3-
dibromosuccinate (9) (18.0 mg, 0.038 mmol). The mixture was stirred for 8 h,
concentrat-
ed, and purified on C-18 chromatography (41) 2.0 cm x 25 cm) eluted with
water/CH3CN
(from 90% water/10% CH3CN to 20% water/90% CH3CN in 50 min at 10 ml/min) to
afford the title product 141 (38.5 mg, 49% yield) after dried with high vacuum
pump. ESI
MS m/z+ C94H143Br2N14024S2 (M+H), cacld. 2073.81, found 2073.60.
Example 52. Conjugated compound 141 to an antibody for 142.
106
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
0
al OH
0.4N 0 Xy.,(Ac 0 0-
4fit=LN ..N?Am
OH 0
N I kW mAb
I 0 OH
,N4k 0 XV:cieN 0 +N
Nillr+
1PAN OH 0 2 H 0
142
To a mixture of 2.0 mL of 10 mg/ml Herceptin in pH 6.0-8.0, were added of 0.70
¨
2.0 mL PBS buffer of 100 mM NaH21304, pH 6.5-7.5 buffers, TCEP (28 L, 20 mM
in
water) and the compound 141 (14 L, 20 mM in DMA). The mixture was incubated at
RT
for 4-16 h, then DHAA (135 L. 50 mM) was added in. After continuous
incubation at
RT overnight, the mixture was purified on G-25 column eluted with 100 mM
NaH2PO4,
50 mM NaC1 pH 6.0-7.5 buffer to afford 16.4-17.6 mg of the conjugate compound
92
(82%-88% yield) in 13.1-15.2 ml buffer. The drug/antibody ratio (DAR) was 3.9,
which
was determined via UPLC-Qtof mass spectrum. It was 96-99% monomer analyzed by
SEC HPLC (Tosoh Bioscience, Tskgel G3000SW, 7.8 mm ID x 30 cm, 0.5 ml/min, 100
min) and a single band measured by SDS-PAGE gel.
Example 53. In vitro cytotoxicity evaluation of conjugates 127, 129 and 142 in
com-
parison with T-DM1:
The cell lines used in the cytotoxicity assays were HL-60, a human
promyclocytic
leukemia cell line; NCI-N87, a human gastric carcinoma cell line; BT-474, a
human
invasive ductal carcinoma cell line; and SKOV3, a human ovarian carcinoma cell
line. For
HL-60, NCI-N87, and BT-474 cells, the cells were grown in RPMI-1640 with 10%
FBS.
For SKOV3 cells, the cells were grown in McCoy's 5A Medium with 10% FBS. To
run
the assay, the cells (180 1, 6000 cells) were added to each well in a 96-well
plate and
incubated for 24 hours at 37 C with 5% CO?. Next, the cells were treated with
test com-
pounds (20 1) at various concentrations in appropriate cell culture medium
(total volume,
0.2 mL). The control wells contain cells and the medium but lack the test
compounds. The
plates were incubated for 120 hours at 37 C with 5% CO?. MTT (5 mg/ml) was
then
added to the wells (20 1) and the plates were incubated for 1.5hr at 37 C.
The medium
was carefully removed and DMSO (180 I) was added afterward. After it was
shaken for
15min, the absorbance was measured at 490 nm and 570 nm with a reference
filter of
620nm. The inhibition% was calculated according to the following equation:
inhibition%
[1-(assay-blank)/(control-blank)] x 100.
The cytotoxicity results:
107
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
IC50 (11M) N87 cell (Ag+) SK-OV-3 cell (Ag+ HL60 cell (Ag-
Conjugate 127 0.009 nM 0.010 nM >8 nM
Conjugate 129 0.012 nM 0.015 nM >8 nM
Conjugate 142 0.097 nM 0.083 nM >15 nM
T-DM1 0.263 nM 0.187 nM >15 nM
Specificity of conjugate 127 for N87 cell was over 889 (IC50> 8/ IC50= 0.009),
and for
SK-OV-3 cell was over 800; Specificity of conjugate 129 for N87 cell was over
666 (IC5o>
8/ IC50= 0.012), and for SK-OV-3 cell was over 533; Specificity of conjugate
142 for N87
cell was over 155 (IC50> 15/ IC50= 0.097), and for SK-OV-3 cell was over 180;
Specificity
of conjugate T-DM1 for N87 cell was over 57 (IC5o> 15/ IC50= 0.263), and for
SK-OV-3
cell was over 80.
The three new conjugates 127, 129 and 142 were extremely more potent than the
commercial conjugate T-DM1.
Example 54. Antitumor Activity In vivo.
The in vivo efficacy of conjugates 127, 129 and 142 along with T-DM1 were
evaluat-
ed in a human gastric carcinoma N-87 cell line tumor xenograft models. Five-
week-old
female BALB/c Nude mice (30 animals) were inoculated subcutaneously in the
area under
the right shoulder with N-87 carcinoma cells (5 x 106 cells/mouse) in 0.1 mL
of serum-
free medium. The tumors were grown for 8 days to an average size of 133 mm3.
The
animals were then randomly divided into 5 groups (6 animals per group). The
first group
of mice served as the control group and was treated with the phosphate-
buffered saline
vehicle. The remaining three groups were treated with conjugates 127, 129, 142
and T-
DM1 respectively at dose of 3 mg/Kg administered intravenously. Three
dimensions of the
tumor were measured every 4 days and the tumor volumes were calculated using
the
formula tumor volume =1/2 (length x width x height). The weight of the animals
was also
measured at the same time. A mouse was sacrificed when any one of the
following criteria
was met: (1) loss of body weight of more than 20% from pretreatment weight,
(2) tumor
volume larger than 1500 mm3, (3) too sick to reach food and water, or (4) skin
necrosis. A
mouse was considered to be tumor-free if no tumor was palpable.
108
CA 02991975 2018-01-10
WO 2015/155753 PCT/IB2015/056083
The results were plotted in Figure 16. All the four conjugates did not cause
the animal
body weight loss. And the animals at control group were sacrificed at day 37
due to the
tumor volume larger than 1500 mm3 and they were too sick. All 6/6 animals at
the groups
of compound 127 and 129 had completely no tumor measurable at day 13 till day
60 (the
end of experiment). All 6/6 animals at the group of Compound 142 group had no
tumor
measurable at day 21 and 2/6 animals had tumor growth (measurable) back at
days 48. In
contrast T-DM1 at dose of 3 mg/Kg was not able to eradicate the tumors and it
only inhib-
ited the tumor growth for 28 days.
109