Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03004320 2018-05-03
TITLE: PROCESS AND SYSTEM FOR MAKING CYCLOPENTADIENE AND/OR
DICYCLOPENTADIENE
INVENTOR(s): Larry L. Iaccino and Kevin C.P. Leung
FIELD OF THE INVENTION
[00011 The
present invention relates to processes and systems for making cyclic C5s
including cyclopendadiene and/or dicyclopentadiene. In particular, the present
invention relates
to processes and systems for making cyclopendadiene and dicyclopentadiene from
acyclic C5
hydrocarbons.
BACKGROUND OF THE INVENTION
[0002]
Cyclopentadiene (CPD) and its dimer dicyclopentadiene (DCPD) are highly
desired
raw materials used throughout the chemical industry in a wide range of
products such as polymeric
materials, polyester resins, synthetic rubbers, solvents, fuels, fuel
additives, etc. Typically,
.. cyclopentadiene is produced as a minor byproduct in liquid fed steam
cracking (e.g., naphtha and
heavier feed) processes. As steam cracking processes shift to using lighter
feed (e.g., ethane and
propane feed), less CPD is produced while demand for CPD continues to rise.
Cyclopentane and
cyclopentene also have high value as solvents while cyclopentene may be used
as a monomer to
produce polymers and as a starting material for other high value chemicals.
[0003] Consequently, there is a need for on-purpose CPD production, i.e.,
CPD produced as
a primary product from a feedstock as opposed to CPD produced as a minor
byproduct. US
5,633,421 generally discloses a process for dehydrogenating C2-05 paraffins to
obtain
corresponding olefins. Similarly, US 2,982,798 generally discloses a process
for dehydrogenating
aliphatic hydrocarbons containing 3 to 6, inclusive, carbon atoms. However,
neither US 5,633,421
nor US 2,982,798 discloses production of CPD from acyclic C5 hydrocarbons,
which are desirable
as feedstock because they are plentiful and low cost. Further, many challenges
exist in designing
an on-purpose CPD production process. For example, the reaction converting C5
hydrocarbons
to CPD is extremely endothermic and is favored by low pressure and high
temperature but
significant cracking of n-pentane and other C5 hydrocarbons can occur at
relatively low
temperature (e.g., 450 C-500 C). Other challenges include loss of catalyst
activity due to coking
during the production process and further processing is needed to remove coke
from the catalyst,
- 1 -
CA 03004320 2018-05-03
and the inability to use oxygen-containing gas to directly provide heat input
to the reactor without
damaging the catalyst.
[0004] From the perspective of storage and shipping, DCPD is easier to
handle than CPD as
a feed material for subsequent chemical syntheses. DCPD and CPD are fungible
in many
.. applications. In certain applications DCPD is preferably used directly in
lieu of CPD. For other
applications where CPD is needed, DCPD can be thermally depolymerized (aka
cracked) via retro-
Diels-Alder reaction to CPD at the point of use.
[0005] Conventional processes for making CPD typically produce C5
hydrocarbon stream(s)
comprising CPD at a modest concentration, acyclic diolefins at significant
concentrations, and
mono olefins. Because many of the C5 species have close boiling points, form
azeotropes, and
are reactive at distillation temperatures, CPD recovery from the product
mixture via conventional
distillation is not industrially feasible. In conventional recovery schemes,
CPD is recovered from
other C5 hydrocarbons utilizing dimerization process(es) which causes CPD to
undergo Diets-
Alder reaction to produce DCPD that can easily be separated from the C5
hydrocarbons by
conventional distillation. Unfortunately, CPD can also react with other
diolefins present in the
stream to produce co-dimers, which contaminate the DCPD. Furthermore,
reactions involving
higher-order oligomers also occur at moderate to high temperatures. These side
reactions produce
undesirable co-dimers and higher-order oligomers, which necessitate more
downstream
processing steps, such as repeated, multi-step cracking and dimerization, to
produce DCPD with
.. sufficient purity required for many applications. Such processes are
expensive, low in yield, and
can be prone to fouling.
[0006] Therefore, there is a need for processes and systems for the
production of CPD and/or
DCPD that address the above described challenges.
SUMMARY OF THE INVENTION
10007] It has been found that by combining a catalytic acyclic C5
hydrocarbon conversion
process where production of CPD is favored over acyclic diolefins, and an
effective separation
process thereafter minimizing the Diels-Alder reactions between CPD and
acyclic diolefins, CPD
can be produced at a high yield, from which high-purity DCPD can be produced.
[0008] A first aspect of the present invention relates to a process for
making cyclic C5s
including CPD and/or DCPD comprising: (I) feeding a C5 feedstock comprising at
least one
acyclic C5 hydrocarbon into a first reactor; (II) contacting the at least one
acyclic C5 hydrocarbon
- 2 -
CA 03004320 2018-05-03
with a catalyst under conversion conditions to obtain a first reactor
hydrocarbon effluent
comprising: C5 components including CPD and acyclic diolefins; light
components including
hydrogen and C I -C4 hydrocarbons; one-ring aromatics; and multiple-ring
aromatics; and (III)
separating the first reactor hydrocarbon effluent to produce (i) a light
components-rich fraction
and (ii) a first CS-rich fraction comprising CPD. High-purity DCPD can be
produced from the
first C5-rich fraction, which can contain CPD, by dimerization.
[0009] A
second aspect of the present invention relates to a system for making cyclic
C5s
including CPD and/or DCPD, comprising: (A) a first reactor configured to
receive a C5 feedstock
comprising at least one acyclic C5 hydrocarbon, an optional hydrogen co-
feedstock and an
optional C I -C4 hydrocarbon co-feedstock; (B) a catalyst loaded inside the
first reactor capable of
catalyzing the conversion of the acyclic C5 hydrocarbon under conversion
conditions to produce
a first reactor hydrocarbon effluent comprising: C5 components including CPD
and acyclic
diolefins; light components including hydrogen and Cl-C4 hydrocarbons; one-
ring aromatics; and
multiple-ring aromatics; and (C) a first separation sub-system in direct or
indirect fluid
communication with the first reactor configured to receive at least a portion
of the first reactor
hydrocarbon effluent and to produce (i) a first C5-rich fraction comprising
CPD and depleted of
hydrogen and Cl -C4 hydrocarbons and (ii) a light components-rich fraction
comprising hydrogen
and C I -C4 hydrocarbons. Additional equipment, such as dimerization reactors
and fractionation
columns, can be added to produce DCPD from the first C5-rich fraction.
BRIEF DESCIPTION OF THE DRAWINGS
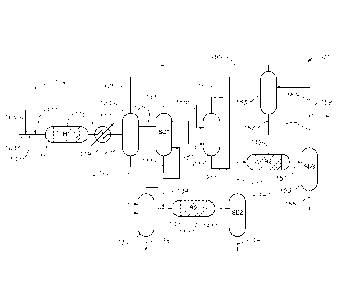
[0010] FIG.
I is a schematic illustration of an exemplary process and system for making
CPD and/or DCPD of the present invention.
[0011] FIG.
2 is a schematic illustration of the details of the first separation sub-
system in
FIG. I.
DETAILED DESCRIPTION OF THE INVENTION
[0012] In
the present disclosure, a process is described as comprising at least one
"step." It
should be understood that each step is an action or operation that may be
carried out once or
multiple times in the process, in a continuous or discontinuous fashion.
Unless specified to the
contrary or the context clearly indicates otherwise, each of the steps in a
process may be conducted
sequentially in the order as they are listed, with or without overlapping with
one or more other
steps, or in any other order, as the case may be. In addition, one or more or
even all steps may be
- 3 -
CA 03004320 2018-05-03
conducted simultaneously with regard to the same or different batch of
material. For example, in
a continuous process, while a first step in a process is being conducted with
respect to a raw
material just fed into the beginning of the process, a second step may be
carried out simultaneously
with respect to an intermediate material resulting from treating the raw
materials fed into the
process at an earlier time in the first step. Preferably, the steps are
conducted in the order
described.
[0013]
Unless otherwise indicated, all numbers indicating quantities in the present
disclosure
are to be understood as being modified by the term "about" in all instances.
It should also be
understood that the precise numerical values used in the specification and
claims constitute
specific embodiments. Efforts have been made to ensure the accuracy of the
data in the examples.
However, it should be understood that any measured data inherently contains a
certain level of
error due to the limitation of the technique and/or equipment used for making
the measurement.
[0014] All
numbers and references to the Periodic Table of Elements are based on the new
notation as set out in Chemical and Engineering News, 63(5), 27 (1985), unless
otherwise
specified.
Definitions
[0015] For
the purpose of this specification and appended claims, the following terms are
defined.
[0016] The
term "cyclic C5" or "cC5" includes, but is not limited to, cyclopentane,
cyclopentene, cyclopentadiene, and mixtures of two or more thereof. The term
"cyclic C5" or
"cCs" also includes alkylated analogs of any of the foregoing, e.g., methyl
cyclopentane, methyl
cyclopentene, and methyl cyclopentadiene. It should be recognized for purposes
of the invention
that cyclopentadiene spontaneously dimerizes over time to form
dicyclopentadicne via Diels-
Alder condensation over a range of conditions, including ambient temperature
and pressure.
[0017] The term "acyclic" includes, but is not limited to, linear and
branched saturates and
non-saturates.
[0018] The
term "aromatic" means a planar cyclic hydrocarbyl with conjugated double
bonds,
such as benzene. As used herein, the term aromatic encompasses compounds
containing one or
more aromatic rings, including, but not limited to, benzene, toluene and
xylene and polynuclear
aromatics (PNAs) which include, but are not limited to, naphthalene,
anthracene, chrysene, and
their alkylated versions. The term "C6+ aromatics" includes compounds based
upon an aromatic
- 4 -
CA 03004320 2018-05-03
ring having six or more ring atoms, including, but not limited to, benzene,
toluene and xylene and
polynuclear aromatics (PNAs) which include, but are not limited to,
naphthalene, anthracene,
chrysene, and their alkylated versions.
[0019] The term "BTX" includes, but is not limited to, a mixture of
benzene, toluene, and
.. xylene (ortho and/or meta and/or para).
[0020] As used herein, the term "rich," when used to describe a component
in a given mixture
or stream produced from a predecessor mixture or stream, means that the
component is present at
a non-negligible concentration in the given mixture or stream that is higher
than its concentration
in the predecessor mixture or stream. Thus, a C5-rich fraction produced from a
predecessor stream
is a fraction comprising C5 hydrocarbons at a non-negligible concentration
that is higher than the
concentration of C5 hydrocarbons in the predecessor stream.
[0021] As used herein, the term "depleted," when used to describe a
component in a given
mixture or stream produced from a predecessor mixture or stream, means that
the component is
present at a concentration (which can be negligible) in the given mixture or
stream that is lower
than its concentration in the predecessor mixture or stream. Thus, a hydrogen-
depleted fraction
produced from a predecessor stream is a fraction comprising hydrogen at a
concentration (which
can be negligible) that is lower than the concentration of hydrogen in the
predecessor stream.
[0022] As used herein, "wt%" means percentage by weight, "vol%" means
percentage by
volume, and "mol%" means percentage by mole. All ranges expressed herein
should include both
end points as two specific embodiments unless specified or indicated to the
contrary.
[0023] An "upper stream" as used herein may be at the very top or the
side of a vessel such
as a fractionation column or a reactor, with or without an additional stream
above it. Preferably,
an upper stream is drawn at a location in the vicinity of the top of the
column. Preferably, an
upper stream is drawn at a location above at least one feed. A "lower stream"
as used herein is at
a location lower than the upper stream, which may be at the very bottom or the
side of a vessel,
and if at the side, with or without an additional stream below it. Preferably,
a lower stream is
drawn at a location in the vicinity of the bottom of the column. Preferably, a
lower stream is
drawn at a location below at least one feed. As used herein, a "middle stream"
is a stream between
an upper stream and a lower stream.
[0024] The term "light hydrocarbons" means hydrocarbons comprising I to 4
carbon atoms
in their molecule structures. The term "light components" means hydrogen and
hydrocarbons
- 5 -
CA 03004320 2018-05-03
comprising 1 to 4 carbon atoms in their molecule structures. The term
"hydrogen" means
molecular Hz.
[0025] The
term "normal boiling point" means boiling point under a pressure of 101
kilopascal. The terms "vapor" and "gas" are both inclusive to mean a phase
that is entirely vapor,
entirely gas and mixtures of gas and vapor.
[0026] As
used herein, the term "essentially free of" means comprising at a
concentration not
higher than 1 wt%, e.g., < 0.8 wt%, < 0.6 wt%, < 0.5 wt%, <0.1 wt%, <0.01 wt%,
or even <
0.001 wt%.
[0027] The
term "mogas" means a mixture of organic compounds suitable as fuel for use in
gasoline internal combustion engine.
[0028] The
term "coke" includes, but is not limited to, a low hydrogen content
hydrocarbon
that is adsorbed on the catalyst composition.
[0029] The
term "Cn" means hydrocarbon(s) having n carbon atom(s) per molecule, wherein
n is a positive integer. Thus, a C5 hydrocarbon feedstock, therefore,
comprises one or more
hydrocarbon, saturated or unsaturated, having 5 carbon atoms per molecule,
such as n-pentane, 2-
methyl-butane, 2,2-dimethylpentane, 1-pentene, 2-
pentene, 2-methyl-2-butene,
3 -methy1-2-butene,1,3-pentadiene, 1,4-
pentadiene, 2-methy1-1,3-butadiene, cyclopentane,
cyclopentene, and the like.
[0030] The
term "Cn+" means hydrocarbon(s) having at least n carbon atom(s) per molecule.
[0031] The term "Cn-" means hydrocarbon(s) having no more than n carbon
atom(s) per
molecule.
[0032] The
term "hydrocarbon" means a class of compounds containing hydrogen bonded to
carbon, and encompasses (i) saturated hydrocarbon compounds, (ii) unsaturated
hydrocarbon
compounds, and (iii) mixtures of hydrocarbon compounds (saturated and/or
unsaturated),
including mixtures of hydrocarbon compounds having different values of n.
[0033] The
term "C5 feedstock" includes a feedstock containing n-pentane, such as a
feedstock which is predominately normal pentane (n-pentane) and/or isopentane
(also referred to
as methylbutane), with smaller fractions of cyclopentane and/or neopentane
(also referred to as
2,2-dimethylpropane).
- 6 -
CA 03004320 2018-05-03
[0034] The term "one-ring aromatics" means aromatic compounds having one
benzene ring
in the molecular structures thereof and includes alkylated versions thereof
such as toluene,
xylenes, and ethylbenzene.
[0035] The term "multiple-ring aromatics" means aromatic compounds having
two or more
aromatic rings in the molecular structures thereof and includes alkylated
versions thereof.
[0036] The term "Group 10 metal" means an element in Group 10 of the
Periodic Table and
includes Ni, Pd, and Pt.
[0037] The term "Group 1 alkali metal" means an element in Group 1 of the
Periodic Table
and includes, but is not limited to, Li, Na, K, Rb, Cs, and a mixture of two
or more thereof, and
excludes hydrogen.
[0038] The term "Group 2 alkaline earth metal" means an element in Group
2 of the Periodic
Table and includes, but is not limited to, Be, Mg, Ca, Sr, Ba, and a mixture
of two or more thereof.
[0039] The term "Group 11 metal" means an element in Group 11 of the
Periodic Table and
includes, but is not limited to, Cu, Ag, Au, and a mixture of two or more
thereof.
[0040] The term "constraint index" is defined in US 3,972,832 and US
4,016,218.
[0041] As used herein, the term "molecular sieve of the MCM-22 family"
(or "material of the
MCM-22 family" or "MCM-22 family material" or "MCM-22 family zeolite")
includes one or
more of:
molecular sieves made from a common first degree crystalline building block
unit cell,
.. which unit cell has the MWW framework topology. (A unit cell is a spatial
arrangement of atoms
which if tiled in three-dimensional space describes the crystal structure.
Such crystal structures
are discussed in the "Atlas of Zeolite Framework Types", Fifth edition, 2001);
molecular sieves made from a common second degree building block, being a
2-dimensional tiling of such MWW framework topology unit cells, forming a
monolayer of one
unit cell thickness, preferably one c-unit cell thickness;
molecular sieves made from common second degree building blocks, being layers
of one
or more than one unit cell thickness, wherein the layer of more than one unit
cell thickness is made
from stacking, packing, or binding at least two monolayers of one unit cell
thickness. The stacking
of such second degree building blocks may be in a regular fashion, an
irregular fashion, a random
fashion, or any combination thereof; and
- 7 -
k = CA 03004320 2018-05-03
molecular sieves made by any regular or random 2-dimensional or 3-dimensional
combination of unit cells having the MWW framework topology.
[0042] The MCM-22 family includes those molecular sieves having an X-
ray diffraction
pattern including d-spacing maxima at 12.4 0.25, 6.9 0.15, 3.57 0.07, and 3.42
0.07 Angstrom.
The X-ray diffraction data used to characterize the material are obtained by
standard techniques
using the K-alpha doublet of copper as incident radiation and a diffractometer
equipped with a
scintillation counter and associated computer as the collection system.
[0043] As used herein, the term "molecular sieve" is used
synonymously with the term
"microporous crystalline material."
[0044] As used herein, the term "carbon selectivity" means the moles of
carbon in the
respective cyclic C5, CPD, Cl, and C2-4 formed divided by total moles of
carbon in the C5
feedstock converted. The term "carbon selectivity to cyclic C5 of at least
30%" means that
30 moles of carbon in the cyclic C5 is formed per 100 moles of carbon in the
C5 feedstock (such
as n-pentane) converted.
10045] As used herein, the term "conversion" means the moles of carbon in
the acyclic C5
hydrocarbon(s) that is converted to a product. The term "conversion of at
least 70% of said acyclic
C5 hydrocarbon(s) to a product" means that at least 70% of the moles of said
acyclic C5
hydrocarbon(s) was converted to a product.
[0046] As used herein, the term "ferrosilicate" means an iron-
containing microporous
crystalline structure that contains iron in the framework structure and/or in
the channel system.
[0047] The term "alkylated naphthalene(s)" includes monoalkyl,
dialkyl, trialkyl, and
tetraalkyl naphthalenes.
The CS Feedstock
[0048] A C5 feedstock comprising acyclic C5 hydrocarbon(s) useful
herein is obtainable from
crude oil or natural gas condensate, can include virgin C5, and can include
cracked C5 (in various
degrees of unsaturation: alkenes, dialkenes, alkynes) produced by refining and
chemical
processes, such as fluid catalytic cracking (FCC), reforming, hydrocracking,
hydrotreating,
coking, and steam cracking.
[0049] In one or more embodiments, the C5 feedstock useful in the
process of this invention
comprises pentane, pentene, pentadiene and mixtures of two or more thereof.
Preferably, the C5
feedstock comprises at least about 50 wt%, or 60 wt%, or 75 wt%, or 90 wt%
saturated acyclic
- 8 -
CA 03004320 2018-05-03
C5 hydrocarbon(s), ideally n-pentane, or in the range from about 50 wt% to
about 100 wt%
saturated acyclic C5 hydrocarbon(s), ideally n-pentane. Preferably, 2-
methylbutane is present at
less than 10 wt%.
[0050] The C5 feedstock, optionally, does not comprise C6 aromatic
compounds, such as
benzene. Preferably C6 aromatic compounds are present at less than 5 wt%, or
less than
1 wt%, or less than 0.01 wt%, or even 0 wt%.
[0051] The C5 feedstock, optionally, does not comprise toluene and/or one
or more of the
xylenes (ortho, meta, and para). Preferably, toluene and xylenes (ortho, meta,
and para) are
present in the C5 feedstock at less than 5 wt%, preferably less than 1 wt%,
preferably present at
less than 0.01 wt%, preferably at 0 wt%.
[0052] The C5 feedstock, optionally, does not comprise C6+ aromatic
compounds, preferably
C6+ aromatic compounds are present at less than 5 wt%, preferably less than 1
wt%, preferably
present at less than 0.01 wt%, preferably at 0 wt%.
[0053] The C5 feedstock, optionally, does not comprise C6+ compounds,
preferably C6+
compounds are present at less than 5 wt%, preferably less than 1 wt%,
preferably present at less
than 0.01 wt%, preferably at 0 wt%, preferably any C6+ aromatic compounds are
present at less
than 5 wt%, preferably less than 1 wt%, preferably present at less than 0.01
wt%, preferably at 0
wt%.
[0054] In the present invention, the acyclic C5 hydrocarbon(s) contained
in the C5 feedstock
is fed into a first reactor loaded with a catalyst, where the acyclic C5
hydrocarbons contact the
catalyst under conversion conditions, whereupon at least a portion of the
acyclic C5
hydrocarbon(s) molecules arc converted into CPD molecules, and a reaction
product containing
CPD and, optionally, other cyclic hydrocarbons (e.g., C5 cyclic hydrocarbons
such as
cyclopentane and cyclopentene) exits the first reactor as a first reactor
hydrocarbon effluent.
Preferably, a hydrogen co-feedstock comprising hydrogen and, optionally, light
hydrocarbons,
such as Cl-C4 hydrocarbons, is also fed into the first reactor. Preferably, at
least a portion of the
hydrogen co-feedstock is admixed with at least a portion, preferably the
entirety, of the C5
feedstock prior to being fed into the first reactor. The presence of hydrogen
in the feed mixture
at the inlet location, where the feed first comes into contact with the
catalyst, prevents or reduces
the formation of coke on the catalyst particles. The catalyst composition,
which is described in
greater detail below, may comprise a microporous crystalline metallosilicate,
preferably having a
- 9 -
CA 03004320 2018-05-03
constraint index in the range of less than 12, a Group 10 metal in combination
with a Group 1
alkali metal and/or a Group 2 alkaline earth metal; and, optionally, a Group
11 metal. The catalyst
can be made by using a method described in greater detail below.
[0055] The first reactor can be a plug flow reactor or other reactor
configurations. The
catalyst can be loaded as a fixed bed, a catalyst particle fluid, and the
like. As used herein, the
term "reactor" refers to any vessel(s) in which a chemical reaction occurs.
Reactor includes both
distinct reactors as well as reaction zones within a single reactor apparatus
and, as applicable,
reaction zones across multiple reactors. In other words and as is common, a
single reactor may
have multiple reaction zones. Where the description refers to a first and
second reactor, the person
I() of ordinary skill in the art will readily recognize such reference
includes two reactors, as well as
a single reactor having first and second reaction zones. Likewise, a first
reactor hydrocarbon
effluent and a second reactor effluent will be recognized to include the
effluent from the first
reaction zone and the second reaction zone of a single reactor, respectively.
[0056] As used herein, the term "moving bed" reactor refers to a zone or
vessel with
contacting of solids (e.g., catalyst particles) and gas flows such that the
superficial gas velocity
(U) is below the velocity required for dilute-phase pneumatic conveying of
solid particles in order
to maintain a solids bed with void fraction below 95%. In a moving bed
reactor, the solids (e.g.,
catalyst material) may slowly travel through the reactor and may be removed
from the bottom of
the reactor and added to the top of the reactor. A moving bed reactor may
operate under several
flow regimes including settling or moving packed-bed regime (U<Umf), bubbling
regime
(Umf<U<Umb), slugging regime (Umb<U<Uc), transition to and turbulent
fluidization regime
(U,<U<Utr), and fast-fluidization regime (U>Utr), where Umf is minimum
fluidizing velocity, Umb
is minimum bubbling velocity, Ue is the velocity at which fluctuation in
pressure peaks, and Ut, is
transport velocity. These different fluidization regimes have been described
in, for example,
Kunii, D., Levenspiel, 0., Chapter 3 of Fluidization Engineering, 2" Edition,
Butterworth-Heinemann, Boston, 1991 and Walas, S. M., Chapter 6 of Chemical
Process
Equipment, Revised 2' Edition, Butterworth-Heinemann, Boston, 2010.
[0057] As used herein, the term "settling bed" reactor refers to a zone
or vessel wherein
particulates contact with gas flows such that the superficial gas velocity (U)
is below the minimum
velocity required to fluidize the solid particles (e.g., catalyst particles),
the minimum fluidization
velocity (Um), U<Umf, in at least a portion of the reaction zone, and/or
operating at a velocity
- 10 -
CA 03004320 2018-05-03
higher than the minimum fluidization velocity while maintaining a gradient in
gas and/or solid
property (such as, temperature, gas or solid composition, etc.) axially up the
reactor bed by using
reactor internals to minimize gas-solid, back-mixing. Description of the
minimum fluidization
velocity is given in, for example, Kunii, D., Levenspiel, 0., Chapter 3 of
Fluidization Engineering,
2nd Edition, Butterworth-Heinemann, Boston, 1991 and Walas, S. M., Chapter 6
of Chemical
Process Equipment, Revised 2nd Edition, Butterworth-Heinemann, Boston, 2010. A
settling bed
reactor may be a "circulating settling bed reactor," which refers to a
settling bed with a movement
of solids (e.g., catalyst material) through the reactor and at least a partial
recirculation of the solids
(e.g., catalyst material). For example, the solids (e.g., catalyst material)
may have been removed
from the reactor, regenerated, reheated and/or separated from the product
stream and then returned
back to the reactor.
[0058] As used herein, the term "fluidized bed" reactor refers to a zone
or vessel with
contacting of solids (e.g., catalyst particles) and gas flows such that the
superficial gas velocity
(U) is sufficient to fluidize solid particles (i.e., above the minimum
fluidization velocity Unif) and
is below the velocity required for dilute-phase pneumatic conveying of solid
particles in order to
maintain a solids bed with void fraction below 95%. As used herein, the term
"cascaded fluid-
beds" means a series arrangement of individual fluid-beds such that there can
be a gradient in gas
and/or solid property (such as, temperature, gas or solid composition,
pressure, etc.) as the solid
or gas cascades from one fluid-bed to another. Locus of minimum fluidization
velocity is given
.. in, for example, Kunii, D., Levenspiel, 0., Chapter 3 of Fluidization
Engineering, 2' Edition,
Butterworth-Heinemann, Boston, 1991 and Walas, S. M., Chapter 6 of Chemical
Process
Equipment, Revised 2nd Edition, Butterworth-Heinemann, Boston, 2010. A
fluidized bed reactor
may be a moving fluidized bed reactor, such as a "circulating fluidized bed
reactor," which refers
to a fluidized bed with a movement of solids (e.g., catalyst material) through
the reactor and at
least a partial recirculation of the solids (e.g., catalyst material). For
example, the solids (e.g.,
catalyst material) may have been removed from the reactor, regenerated,
reheated and/or separated
from the product stream and then returned back to the reactor.
[0059] As used herein, the term "riser" reactor (also known as a
transport reactor) refers to a
zone or vessel (such as, vertical cylindrical pipe) used for net upwards
transport of solids (e.g.,
catalyst particles) in fast-fluidization or pneumatic conveying fluidization
regimes. Fast
fluidization and pneumatic conveying fluidization regimes are characterized by
superficial gas
- 11 -
CA 03004320 2018-05-03
velocities (U) greater than the transport velocity (Utr). Fast fluidization
and pneumatic conveying
fluidization regimes are also described in Kunii, D., Levenspiel, 0., Chapter
3 of Fluidization
Engineering, 2lici Edition, Butterworth-Heinemann, Boston, 1991 and Walas, S.
M., Chapter 6 of
Chemical Process Equipment, Revised 2nd Edition, Butterworth-Heinemann,
Boston, 2010. A
fluidized bed reactor, such as a circulating fluidized bed reactor, may be
operated as a riser reactor.
[0060] As used herein, the term "co-current" refers to a flow of two
streams (e.g., stream (a),
stream (b)) in substantially the same direction. For example, if stream (a)
flows from a top portion
to a bottom portion of at least one reaction zone and stream (b) flows from a
top portion to a
bottom portion of at least one reaction zone, the flow of stream (a) would be
considered co-current
to the flow of stream (b). On a smaller scale within the reaction zone, there
may be regions where
flow may not be co-current.
[0061] As used herein, the term "counter-current" refers to a flow of two
streams (e.g., stream
(a), stream (b)) in substantially opposing directions. For example, if stream
(a) flows from a top
portion to a bottom portion of the at least one reaction zone and stream (b)
flows from a bottom
portion to a top portion of the at least one reaction zone, the flow of stream
(a) would be considered
counter-current to the flow of stream (b). On a smaller scale within the
reaction zone, there may
be regions where flow may not be counter-current.
Acyclic C5 Conversion Process
[0062] The process for the conversion of an acyclic C5 hydrocarbon to a
product comprising
cyclic C5 compounds comprises contacting the C5 feedstock and, optionally,
hydrogen under
acyclic C5 conversion conditions in the presence of one or more catalyst
compositions, including
but not limited to the catalyst compositions described herein, to form said
product. The product
of the process for conversion of an acyclic CS feedstock comprises cyclic CS
compounds. The
cyclic CS compounds can comprise one or more of cyclopentane, cyclopentene,
cyclopentadiene,
and includes mixtures thereof.
[0063] In one or more embodiments, the acyclic C5 conversion conditions
include at least a
temperature, a partial pressure, and a weight hourly space velocity (WHSV).
The temperature is
in the range of about 400 C to about 700 C, or in the range from about 450 C
to about 650 C,
preferably, in the range from about 500 C to about 600 C. The partial pressure
is in the range of
about 3 to about 100 psi (21 to 689 kilopascal), or in the range from about 3
to about 50 psi (21 to
345 kilopascal), preferably, in the range from about 3 to about 20 psi (21 to
138 kilopascal). The
- 12 -
CA 03004320 2018-05-03
=
weight hourly space velocity is in the range from about 1 to about 50 hr', or
in the range from
about 1 to about 20 hr-'. Such conditions include a molar ratio of the
optional hydrogen co-feed
to the acyclic C5 hydrocarbon in the range of about 0 to 3, or in the range
from about 0.5 to about
2. Such conditions may also include co-feed Cl¨C4 hydrocarbons with the
acyclic C5 feed.
[0064] In one or more embodiments, this invention relates to a process for
conversion of n-
pentane to cyclopentadiene comprising the steps of contacting n-pentane and,
optionally,
hydrogen (if present, typically H2 is present at a molar ratio of hydrogen to
n-pentane of 0.01 to
3.0) with one or more catalyst compositions including, but not limited to, the
catalyst compositions
described herein, to form cyclopentadiene at a temperature of 400 C to 700 C,
a partial pressure
of 3 to about 100 psia, and a weight hourly space velocity of 1 to about
50 hr-I.
[0065] In the presence of the catalyst, a number of desired and
undesirable side reactions
may take place. The net effect of the reactions is the production of hydrogen
and the increase of
total volume (assuming constant total pressure). One particularly desired
overall reaction (i.e.,
intermediate reaction steps are not shown) is:
n-pentane CPD + 3H2.
[0066] Additional overall reactions include, but are not limited to:
n-pentane 4 1,3-pentadiene + 2H2,
n-pentane 4 1-pentene + H2,
n-pentane 4 2-pentene + H2,
n-pentane 4 2-methyl-2-butene + Hz,
n-pentane 4 cyclopentane + Hz,
cyclopentane 4 cyclopentene + H2, or
cyclopentene --> CPD + H2.
[0067] Fluids inside the first reactor are essentially in gas phase. At the
outlet of the first
reactor, a first reactor hydrocarbon effluent, preferably in gas phase, is
obtained. The first reactor
hydrocarbon effluent may comprise a mixture of the following hydrocarbons,
among others:
heavy components comprising more than 8 carbon atoms such as multiple-ring
aromatics; C8, C7,
and C6 hydrocarbons such as one-ring aromatics; CPD (the desired product);
unreacted C5
feedstock material such as n-pentane; C5 by-products such as pentenes
(1-pentene, 2-pentene, e.g.), pentadienes (1,3-pentadiene, 1,4-pentadiene,
e.g.), cyclopentane,
- 13 -
CA 03004320 2018-05-03
cyclopentene, 2-methylbutane, 2-methyl-l-butene, 3-methy1-1-butene, 2-methyl-
1,3-butadiene,
2,2-dimethylpropane, and the like; C4 by-products such as butane, 1-butene,
2-butene, 1,3-butadiene, 2-methylpropane, 2-methyl-1-propene, and the like; C3
by-products such
as propane, propene, and the like; C2 by-products such as ethane and ethene,
methane, and
hydrogen.
[0068] The first reactor hydrocarbon effluent may comprise CPD at a
concentration of
C(CPD)1 wt%, based on the total weight of the C5 hydrocarbons in the first
reactor hydrocarbon
effluent; and al< C(CPD)1 a2, where al and a2 can be, independently, 15, 16,
18, 20, 22, 24,
25, 26, 28, 30, 32, 34, 35, 36, 38, 40, 45, 50, 55, 60, 65, 70, 75, 80, or 85
as long as al< a2.
[0069] The first reactor hydrocarbon effluent may comprise acyclic
diolefins at a total
concentration of C(ADO)1 wt%, based on the total weight of the C5 hydrocarbons
in the first
reactor hydrocarbon effluent; and bl < C(ADO)1 < b2, where bl and b2 can be,
independently,
20, 18, 16, 15, 14, 12, 10, 8, 6, 5,4, 3, 2, 1, or 0.5, as long as b 1 <b2.
Preferably, 0.5 < C(ADO)
< 10. Preferably, the acyclic diolefins comprise 1,3-pentadiene at a
concentration of C(PTD)1
wt%, based on the total weight of C5 components in the first reactor
hydrocarbon effluent; and cl
< C(PTD)1 < c2, where cl and c2 can be, independently, 20, 18, 16, 15, 14, 12,
10, 8, 6, 5, 4, 3,
2, 1, 0.5, or 0.3, as long as cl <c2.
[0070] As a result of the use of the catalyst and the choice of reaction
conditions in the first
reactor, a high CPD to acyclic diolefin molar ratio in the first reactor
hydrocarbon effluent can be
.. achieved such that C(CPD)1/C(ADO)1 > 1.5, preferably 1.6, 1.8, 2.0, 2.2,
2.4, 2.5, 2.6, 2.8, 3.0,
3.2, 3.4, 3.5, 3.6, 3.8, 4.0, 5.0, 6.0, 8.0, 10, 12, 14, 15, 16, 18, or 20.
The high ratio of
C(CPD)1/C(ADO)1 significantly reduces CPD loss as a result of Diels-Alder
reactions between
CPD and acyclic dienes in subsequent processing steps, and therefore, allows
the processes of the
present invention to achieve high DCPD yield and high DCPD purity for the
subsequently
.. produced DCPD fractions.
[0071] Desirably, the total absolute pressure and temperature of the
first reactor hydrocarbon
effluent should be maintained at levels such that the dimerization of CPD to
form DCPD is
substantially avoided, and the Diels-Alder reactions between CPD and acyclic
dienes are
substantially inhibited.
.. Catalyst Composition
- 14 -
CA 03004320 2018-05-03
[0072] Catalyst compositions useful herein include microporous
crystalline metallosilicates,
such as crystalline aluminosilicates, crystalline ferrosilicates, or other
metal containing crystalline
silicates (such as those where the metal or metal containing compound is
dispersed within the
crystalline silicate structure and may or may not be a part of the crystalline
framework).
Microporous crystalline metallosilicate framework types useful as catalyst
compositions herein
include, but are not limited to, MWW, MFI, LTL, MOR, BEA, TON, MTW, MTT, FER,
MRE,
MFS, MEL, DDR, EUO, and FAU.
[0073] Particularly suitable microporous metallosilicates for use herein
include those of
framework type MWW, MFI, LTL, MOR, BEA, TON, MTW, MTT, FER, MRE, MFS, MEL,
DDR, EUO, and FAU where one or more metals from groups 8, 11, and 13 of the
Periodic Table
of the Elements (preferably one or more of Fe, Cu, Ag, Au, B, Al, Ga, and/or
In) are incorporated
in the crystal structure during synthesis or impregnated post crystallization.
It is recognized that
a metallosilicate may have one or more metals present and, for example, a
material may be referred
to as a ferrosilicate, but it will most likely still contain small amounts of
aluminum.
[0074] The microporous crystalline metallosilicates preferably have a
constraint index of less
than 12, alternately from Ito 12, alternately from 3 to 12. Aluminosilicates
useful herein have a
constraint index of less than 12, such as 1 to 12, alternately 3 to 12, and
include, but are not limited
to Zeolite beta, mordenite, faujasite, Zeolite L, ZSM-5, ZSM-11, ZSM-22,
ZSM-23, ZSM-35, ZSM-48, ZSM-50, ZSM-57, ZSM-58, MCM-22 family materials, and
mixtures of two or more thereof. In a preferred embodiment, the microporous
crystalline
aluminosilicate has a constraint index of about 3 to about 12 and is ZSM-5.
[0075] ZSM-5 is described in US 3,702,886. ZSM-11 is described in US
3,709,979.
ZSM-22 is described in US 5,336,478. ZSM-23 is described in US 4,076,842. ZSM-
35 is
described in US 4,016,245. ZSM-48 is described in US 4,375,573. ZSM-50 is
described in US
4,640,829, and ZSM-57 is described in US 4,873,067. ZSM-58 is described in
US 4,698,217. Constraint index and a method for its determination are
described in
US 4,016,218.
[0076] The MCM-22 family material is selected from the group consisting
of MCM-22, PSH-
3, SSZ-25, MCM-36, MCM-49, MCM-56, ERB-1, EMM-10, EMM-10-P, EMM-12, EMM-13,
UZM-8, UZM-8HS, ITQ-1, ITQ-2, ITQ-30, and mixtures of two or more thereof.
- 15 -
CA 03004320 2018-05-03
[0077] Materials of the MCM-22 family include MCM-22 (described in US
4,954,325), PSH-
3 (described in US 4,439,409), SSZ-25 (described in US 4,826,667), ERB-1
(described in EP 293
032), ITQ-1 (described in US 6,077,498), and ITQ-2 (described in
WO 97/17290), MCM-36 (described in US 5,250,277), MCM-49 (described in
US 5,236,575), MCM-56 (described in US 5,362,697), and mixtures of two or more
thereof.
Related zeolites to be included in the MCM-22 family are UZM-8 (described in
US 6,756,030) and UZM-8HS (described in US 7,713,513), both of which are also
suitable for
use as the molecular sieve of the MCM-22 family.
[0078] In one or more embodiments, the microporous crystalline
metallosilicate has an Si/M
molar ratio greater than about 3, or greater than about 25, or greater than
about 50, or greater than
about 100, or greater than 400, or in the range from about 100 to about 2,000,
or from about 100
to about 1,500, or from about 50 to 2,000, or from about 50 to 1,200.
[0079] In one or more embodiments, the microporous crystalline
aluminosilicate has an
SiO2/Al2O3 molar ratio greater than about 3, or greater than about 25, or
greater than about 50,
or greater than about 100, or greater than 400, or in the range from about 100
to about 400, or
from about 100 to about 500, or from about 25 to about 2,000, or from about 50
to about 1,500,
or from about 100 to 1,200, or from about 100 to 1000.
[0080] In another embodiment of the invention, the microporous
crystalline metallosilicate
(such as an aluminosilicate) is combined with a Group 10 metal or metal
compound, and,
optionally, one, two, three, or more Group 1, 2, or 11 metals or metal
compounds.
[0081] In one or more embodiments, the Group 10 metal includes, or is
selected from the
group consisting of, Ni, Pd, and Pt, preferably Pt. The Group 10 metal content
of said catalyst
composition is at least 0.005 wt%, based on the weight of the catalyst
composition. In one or
more embodiments, the Group 10 content is in the range from about 0.005 wt% to
about 10 wt%,
or from about 0.005 wt% up to about 1.5 wt%, based on the weight of the
catalyst composition.
[0082] In one or more embodiments, the Group 1 alkali metal includes, or
is selected from
the group consisting of, Li, Na, K, Rb, Cs, and mixtures of two or more
thereof, preferably Na.
[0083] In one or more embodiments, the Group 2 alkaline earth metal is
selected from the
group consisting of Be, Mg, Ca, Sr, Ba, and mixtures of two or more thereof.
[0084] In one or more embodiments, the Group 1 alkali metal is present as
an oxide and the
metal is selected from the group consisting of Li, Na, K, Rb, Cs, and mixtures
of two or more
- 16 -
CA 03004320 2018-05-03
thereof. In one or more embodiments, the Group 2 alkaline earth metal is
present as an oxide and
the metal is selected from the group consisting of 13e, magnesium, calcium Sr,
Ba, and mixtures
of two or more thereof. In one or more embodiments, the Group 1 alkali metal
is present as an
oxide and the metal is selected from the group consisting of Li, Na, K, Rb,
Cs, and mixtures of
two or more thereof; and the Group 2 alkaline earth metal is present as an
oxide and the metal is
selected from the group consisting of Be, Mg, Ca, Sr, Ba, and mixtures of two
or more thereof.
[0085] In one or more embodiments, the Group 11 metal includes, or is
selected from the
group consisting of, silver, gold, copper, preferably silver or copper. The
Group 11 metal content
of said catalyst composition is at least 0.005 wt%, based on the weight of the
catalyst composition.
In one or more embodiments, the Group 11 content is in the range from about
0.005 wt% to about
10 wt%, or from about 0.005 wt% up to about 1.5 wt%, based on the weight of
the catalyst
composition.
[0086] In one or more embodiments, the catalyst composition has an Alpha
Value (as
measured prior to the addition of the Group 10 metal, preferably platinum) of
less than 25,
alternately less than 15, alternately from 1 to 25, alternately from 1.1 to
15.
[0087] In one or more embodiments of aluminosilicates, the molar ratio of
said Group 1 alkali
metal to Al is at least about 0.5, or from at least about 0.5 up to about 3,
preferably at least about
1, more preferably at least about 2.
[0088] In one or more embodiments of aluminosilicates, the molar ratio of
said Group 2
alkaline earth metal to Al is at least about 0.5, or from at least about 0.5
up to about 3, preferably
at least about 1, more preferably at least about 2.
[0089] In one or more embodiments, the molar ratio of said Group 11 metal
to Group 10 metal
is at least about 0.1, or from at least about 0.1 up to about 10, preferably
at least about 0.5, more
preferably at least about 1. In one or more embodiments, the Group 11 alkaline
earth metal is
present as an oxide and the metal is selected from the group consisting of
gold, silver, and copper,
and mixtures of two or more thereof.
[0090] Preferably, catalyst compositions useful herein are employed at
conversion conditions,
including a temperature in the range of from 400 to 800 C, a pressure in the
range of from 10 to
1,000 kilopascal absolute, and a WHSV in the range of 1 to 100 hr'. In one or
more embodiments,
the use of the catalyst compositions of this invention provides a conversion
of at least about 60%,
or at least about 75%, or at least about 80%, or in the range from about 60%
to about 80%, of said
- 17-
CA 03004320 2018-05-03
acyclic C5 feedstock under acyclic C5 conversion conditions of an n-pentane
containing feedstock
with equimolar H2, a temperature in the range of about 550 C to about 600 C,
an n-pentane partial
pressure between 3 and 10 psia, and an n-pentane weight hourly space velocity
of 10 to 20 hr.*
[0091] In one or more embodiments, the use of any one of the catalyst
compositions of this
invention provides a carbon selectivity to cyclic C5 compounds of at least
about 30%, or at least
about 40%, or at least about 50%, or in the range from about 30% to about 80%,
under acyclic C5
conversion conditions including an n-pentane feedstock with equimolar 112, a
temperature in the
range of about 550 C to about 600 C, an n-pentane partial pressure between 3
and 10 psia, and an
n-pentane weight hourly space velocity between 10 and 20 hr-t.
[0092] In one or more embodiments, the use of any one of the catalyst
compositions of this
invention provides a carbon selectivity to cyclopentadiene of at least about
30%, or at least about
40%, or at least about 50%, or in the range from about 30% to about 80%, under
acyclic C5
conversion conditions including an n-pentane feedstock with equimolar H2, a
temperature in the
range of about 550 C to about 600 C, an n-pentane partial pressure between 3
and 10 psia, and an
n-pentane weight hourly space velocity between 10 and 20 hr-I.
[0093] The catalyst compositions of this invention can be combined with a
matrix or binder
material to render them attrition resistant and more resistant to the severity
of the conditions to
which they will be exposed during use in hydrocarbon conversion applications.
The combined
compositions can contain 1 to 99 wt% of the materials of the invention based
on the combined
weight of the matrix (binder) and material of the invention. The relative
proportions of
microcrystalline material and matrix may vary widely, with the crystal content
ranging from about
1 to about 90 wt% and more usually, particularly when the composite is
prepared in the form of
beads, extrudates, pills, oil drop formed particles, spray dried particles,
etc., in the range of about
2 to about 80 wt% of the composite.
[0094] During the use of the catalyst compositions in the processes of this
invention, coke
may be deposited on the catalyst compositions, whereby such catalyst
compositions lose a portion
of its catalytic activity and become deactivated. The deactivated catalyst
compositions may be
regenerated by conventional techniques including high pressure hydrogen
treatment and
combustion of coke on the catalyst compositions with an oxygen-containing gas.
- 18-
CA 03004320 2018-05-03
[0095] Useful catalyst compositions comprise a crystalline
aluminosilicate or ferrosilicate,
which is, optionally, combined with one, two, or more additional metals or
metal compounds.
Preferred combinations include:
1) a crystalline aluminosilicate (such as ZSM-5 or Zeolite L) combined with
a Group 10 metal
(such as Pt), a Group 1 alkali metal (such as sodium or potassium) and/or a
Group 2 alkaline earth
metal;
2) a crystalline aluminosilicate (such as ZSM-5 or Zeolite L) combined with
a Group 10 metal
(such as Pt) and a Group 1 alkali metal (such as sodium or potassium);
3) a crystalline aluminosilicate (such as a ferrosilicate or an iron
treated ZSM-5) combined with
a Group 10 metal (such as Pt), a Group 1 alkali metal (such as sodium or
potassium);
4) a crystalline aluminosilicate (Zeolite L) combined with a Group 10 metal
(such as Pt) and a
Group 1 alkali metal (such as potassium); and
5) a crystalline aluminosilicate (such as ZSM-5) combined with a Group 10
metal (such as Pt),
a Group 1 alkali metal (such as sodium), and a Group 11 metal (such as silver
or copper).
[0096] Another useful catalyst composition is a group 10 metal (such as Ni,
Pd, and Pt,
preferably Pt) supported on silica (e.g., silicon dioxide) modified by a Group
1 alkali metal silicate
(such as Li, Na, K, Rb, and/or Cs silicates) and/or a Group 2 alkaline earth
metal silicate (such as
Mg, Ca, Sr, and/or Ba silicates), preferably potassium silicate, sodium
silicate, calcium silicate
and/or magnesium silicate, preferably potassium silicate and/or sodium
silicate. The Group 10
metal content of the catalyst composition is at least 0.005 wt%, based on the
weight of the catalyst
composition, preferably, in the range from about 0.005 wt% to about 10 wt%, or
from about 0.005
wt% up to about 1.5 wt%, based on the weight of the catalyst composition. The
silica (SiO2) may
be any silica typically used as catalyst support such as those marketed under
the tradenames of
DAVISIL 646 (Sigma Aldrich), DAVISON 952, DAVISON 948 or DAVISON 955 (Davison
Chemical Division of W.R. Grace and Company).
[0097] For more information on useful catalyst compositions, please see:
1) US
2017/0121253; 2) US 2017/0121245; 3) US 9,856,187; 4) US 9,849,440; and 5) US
2017/0121246.
Cooling of the first reactor hydrocarbon effluent
[0098] To prevent undesirable side reactions such as thermal cracking,
condensation of
PNAs, and premature Diels-Alder reactions of reactive diolefinic species,
especially CPD, it is
- 19-
CA 03004320 2018-05-03
highly desired that the first reactor hydrocarbon effluent is cooled down once
it exits the first
reactor. To that end, the first reactor hydrocarbon effluent may be passed
through at least one heat
exchanger located next to the outlet from the first reactor where its
temperature is lowered to a
range from Tel C to Tc2 C, where Tcl and Tc2 can be, independently, 20, 50,
80, 150, 200, 250,
300, 350, 400, or 450 C, as long as Tel < Tc2. Alternately or additionally,
the first reactor
hydrocarbon effluent may be contacted with a quench liquid so that the
temperature is lowered to
a range from Tc1 C to Tc2 C, where Tel and Tc2 can be, independently, 20, 40,
50, 60, 80, 100,
120, 140, 150, 160, 180, 200, 220, 240, 250, 260, 280, 300, 320, 340, 350,
360, 380, 400, 420,
440, or 450, as long as Tel < Tc2. Upon cooling, a majority of the components
from the first
reactor hydrocarbon effluent are still in gas or vapor phase.
Washing/quenching of the first reactor hydrocarbon effluent
[00991 The first reactor hydrocarbon effluent comprises non-negligible
amounts of heavy
components, including but not limited to: polynuclear aromatic species
(naphthalene and alkylated
naphthalenes, anthracene and alkylated anthracenes, phenanthrene and alkylated
phenanthrenes),
DCPD, products formed as a result of undesired Diels-Alder reactions between
CPD and acyclic
diolefins. It is highly desired that these heavy components, especially C8+
hydrocarbons, are at
least partly removed from the first reactor hydrocarbon effluent such that
contamination of the
C5-rich fraction and subsequent contamination of DCPD fractions by them are
avoided. For
example, naphthalene is very difficult to be removed from DCPD by
distillation; also naphthalene
and heavier PNAs can condense to form solids which can foul equipment.
Therefore, naphthalene
and heavier PNAs are desirably removed from the first reactor hydrocarbon
effluent before it is
further processed.
[00100] Advantageously, such heavy components can be effectively removed in a
vessel by
contacting the stream of the first reactor hydrocarbon effluent, preferably
after it is being partially
cooled down, with a wash oil. To that end, the wash oil, desirably in liquid
phase during operation,
can be sprayed into the washing vessel as liquid droplets when contacting the
substantially vapor
stream of the first reactor hydrocarbon effluent. Additionally or
alternatively, the substantially
vapor stream of the first reactor hydrocarbon effluent can be sent to a
suitable gas-liquid contacting
washing vessel capable of handling fouling services (e.g., a tower with grids
and/or random
packing). Sufficient contact between the first reactor hydrocarbon effluent
and the liquid wash
oil results in the extraction of the heavy components (i.e., C8+ hydrocarbons)
from the
- 20 -
CA 03004320 2018-05-03
substantially vapor stream of the first reactor hydrocarbon effluent into the
wash oil liquid. A
small amount of the wash oil may be entrained in the first reactor hydrocarbon
effluent vapor
stream at a low vapor pressure. The entrained wash oil can be removed
subsequently where
necessary.
1001011 In the washing vessel, the first reactor hydrocarbon effluent vapor
stream can be
quenched down further to a temperature in a range from 10 to 300 C, preferably
from 20 to 100 C.
Thus, from the washing vessel, a vapor stream of the first reactor hydrocarbon
effluent, washed
and cooled, is obtained. In addition, a wash oil liquid stream, comprising
multiple-ring aromatics
mentioned above, may also be obtained.
[00102] Various wash oils can be used. Non-limiting examples of the wash
oil include:
cyclohexane; monoalkyl, dialkyl, and trialkyl cyclohexanes; benzene;
monoalkyl, dialkyl, and
trialkyl benzenes; monoalkyl, dialkyl, trialkyl, and tetraalkyl naphthalenes;
other alkylated
multiple-ring aromatics; and mixtures and combinations thereof. Preferred wash
oils are: alkyl
benzenes and mixtures thereof (herein referred to as light wash oil); and
alkyl naphthalenes and
mixtures thereof (herein referred to as heavy wash oil). More preferably,
toluene, especially
relatively pure toluene with a purity of at least 50 wt%, or
alkylnaphthalene(s), especially those
with purity of at least 50 wt%, is used as the wash oil.
[00103] In the fluid channel from the first reactor to the washing vessel,
including the heat
exchanger in between, if any, and inside the washing vessel, dimerization
between CPD molecules
may occur to form DCPD, and CPD may react with acyclic diolefins to form other
C10+
hydrocarbons. A major portion of these heavy components, if formed, are
partitioned in the wash
oil liquid stream exiting the washing vessel. If the wash oil liquid stream is
sent to a fuel
disposition or other low value disposition directly, a portion of the CPD
produced in the first
reactor would be downgraded to low value. To reduce such undesirable yield
loss, one can treat
the wash oil liquid stream, together with other, down-stream produced streams
also comprising
such heavy components and/or wash oil, in a vessel operated under conditions
favoring reverse
dimerization, to obtain an upper C5-rich stream and a lower wash oil-rich
stream containing
residual C8+ and the wash oil. The upper CS-rich stream may be fed directly or
indirectly to a
second reactor as part of the first CS-rich fraction. The lower wash oil-rich
stream can be further
distilled to recover at least a portion of the wash oil, which can be recycled
to the wash vessel
directly or indirectly. Such conditions favoring reverse dimerization include,
e.g., a temperature
-21 -
CA 03004320 2018-05-03
in the range from 150 to 350 C, preferably, from 170 to 260 C, a pressure in a
range from 21 to
345 kilopascal absolute, preferably from 21 to 138 kilopascal absolute, and a
residence time from
0.01 to 10 hours, preferably from 0.1 to 4 hours.
Separation of the first reactor hydrocarbon effluent
[00104] The first reactor hydrocarbon effluent, which is preferably cooled
at the outlet of the
first reactor as described above, and washed in a washing vessel as described
above is then
supplied via a fluid communication channel to a first separation sub-system
and processed in the
first separation sub-system to obtain a C5-rich fraction that is depleted of
C1-C4 hydrocarbons
and hydrogen, and desirably, depleted of heavy components such as C8+
hydrocarbons. Due to
the nature of the reactions taking place in the first reactor, substantial
volume of hydrogen is
present in the first reactor hydrocarbon effluent. Effective separation of
hydrogen and Cl-C4 light
hydrocarbons from the C5 hydrocarbons (including CPD) needs to take into
consideration that
much of the C5 hydrocarbons can be held as vapor in the hydrogen/light
hydrocarbon stream.
Thus, desirably, a compression train with inter-stage cooling and liquid/vapor
separation can be
advantageously used as the first separation sub-system to minimize the loss of
C5 hydrocarbons
to the hydrogen and light hydrocarbon stream.
[00105] Exemplary compression trains with inter-stage cooling and
liquid/vapor separation are
those comprising at least 3-stages of compression/inter-stage cooling with an
exiting pressure
from the last stage of at least 100 psia (689 kilopascal absolute).
[00106] From the first separation sub-system (a compression train, e.g.),
one or more streams
of C5-rich hydrocarbon (the first C5-rich fraction) may be obtained from the
multiple stages.
Where multiple streams of the first C5-rich fractions are obtained, two or
more of them may be,
optionally, combined into a single first CS-rich rich fraction stream and then
processed together
subsequently. The first C5-rich fraction generally comprises: (i) CPD; (ii)
unreacted C5
hydrocarbon(s) from the C5 feedstock such as n-pentane; and (iii) cyclopentane
and cyclopentene.
[00107] The first CS-rich fraction may further comprise a portion of the
wash oil, especially if
the wash oil contains C6 and C7 hydrocarbons, such as cyclohexane and
alkylcyclohexanes,
benzene and alkylbenzenes (e.g., toluene). Such wash oil may be removed
subsequently where
necessary. Even if high boiling point wash oils such as alkyl naphthalenes are
used, the first C5-
rich fraction may comprise C6 hydrocarbons (as by-products from the first
reactor) such as
benzene at a low concentration.
- 22 -
CA 03004320 2018-05-03
[00108] From the first separation sub-system (the first liquid/vapor
separator in a multi-stage
compression train, e.g.), an optional heavy-containing stream may be produced,
especially at one
of the early stages, comprising wash oil and C8+ hydrocarbons (DCPD, and other
products as a
result of the Diets-Alder reactions between CPD and other dienes, e.g.), and
the like. Such heavy
stream can be in non-negligible quantity if high boiling point wash oil such
as
methylnaphthalene(s) is used. If such heavy stream is produced from the
compression train, it
may be advantageously combined with the wash oil liquid stream produced from
the washing
vessel described above, and then processed together subsequently.
[00109] From the first separation sub-system (a compression train, e.g.),
a light components-
rich fraction comprising hydrogen and C1-C4 hydrocarbons is also obtained.
This light
components-rich fraction is desirably depleted of C5 components, especially
CPD, or at least
minimized, such that C5 molecules are used to the highest degree in the
process of the present
invention.
Separation of the light components-rich fraction and recycling of hydrogen
and/or light
hydrocarbons
[00110] A significant component of the light components-rich fraction
coming from the first
separation sub-system separating the first reactor hydrocarbon effluent is
hydrogen gas.
Cl-C4 hydrocarbons are produced at small quantities in the first reactor from
the C5 feedstock.
Alternatively, in certain exemplary processes of the present invention, a Cl-
C4 light hydrocarbon,
.. such as CH4, may be supplied to the first reactor as a co-feedstock,
resulting in higher
concentrations of the Cl-C4 light hydrocarbons in the light components-rich
fraction obtained
from the first separation sub-system.
[00111] Given the large quantity of hydrogen produced in the process, it
is desirable to separate
the light components-rich fraction to obtain a higher purity hydrogen stream,
which can be used
or sold as a highly valuable industrial gas. To that end, various processes
and equipment may be
used to recover and concentrate hydrogen, such as pressure-swing adsorption
(PSA), rapid cycle
pressure-swing adsorption (RCPSA), thermal-swing adsorption (TSA), cryogenic
processes,
membrane separation, and the like, with PSA or RCPSA being preferred. By using
any of these
processes or any combinations thereof, it is possible to obtain three gas
streams from the light
components-rich fraction: a hydrogen-rich stream comprising hydrogen at a
purity of at least 95
mol%, based on the total moles of the hydrogen-rich stream; a middle stream
comprising hydrogen
- 23 -
CA 03004320 2018-05-03
and C 1 -C4 hydrocarbons that is preferably low in C2+ hydrocarbons; and a Cl-
C4-rich
hydrocarbon stream which may also contain C5+ hydrocarbons which can be
subsequently
recovered by washing or low temperature fractionation (absorber, e.g.). A
portion of the
hydrogen-rich stream and/or a portion of the middle stream (if Cl-C4
hydrocarbon is co-fed into
the first reactor) can be recycled to the first reactor. Additionally or
alternatively, at least a portion
of the middle stream and/or the Cl -C4 hydrocarbon stream can be used as fuel
gas to produce the
thermal energy needed for certain steps (such as the conversion process in the
first reactor) in the
process of the present invention. Alternatively, the Cl-C4-rich hydrocarbon
stream can be utilized
as a feedstock for other process such as light olefins production and/or
further processed to obtain
an LPG fraction.
[00112] As discussed above, the recycle hydrogen may be advantageously admixed
with at
least a portion of the C5 feedstock before being fed into the first reactor to
reduce coke formation
on the catalyst particles, thereby increasing the life of the catalyst used in
the first reactor.
Additionally or alternatively, the recycle hydrogen may be fed separately into
the first reactor.
Additionally or alternatively, the recycle hydrogen may be utilized for
rejuvenation or reduction
of the catalyst.
Dimerization of the first CS-rich fraction
[00113] The first C5-rich fraction advantageously comprises CPD at a high
concentration in a
range from cal wt% to ca2 wt%, based on the total weight of C5 hydrocarbons in
the first C5-rich
fraction, where cal and ca2 can be, independently, 20, 25, 30, 35, 40, 45, 50,
55, 60, 65, 70, 75,
80, 85, or 90, as long as cal < ca2. Such CPD may be used directly as a CPD
feed for the
production of, e.g., norbornene, vinyl norbornene, ethylidene norbornene,
hydrocarbon resin
adhesives or tackifiers, unsaturated polyester resins, cyclopentane, and/or
cyclopentene.
[00114] Additionally or alternatively, at least a portion of the first C5-
rich fraction can be
supplied to a first dimerization reactor (the second reactor in the system)
operating under a first
set of dimerization conditions, where a portion of the CPD is advantageously
converted into
DCPD. This can be highly desirable because DCPD is much more stable than CPD,
and therefore
can be stored and/or transported to a different location where it is used as
DCPD or converted into
CPD and used for the production of value-added products.
[00115] The first dimerization reactor (the second reactor in the system)
can be advantageously
a plug flow reactor, a back mixed reactor, a continuous stirred-tank reactor,
a boiling point reactor,
- 24 -
CA 03004320 2018-05-03
and/or a baffled reactor; additionally the reactor may contain heat transfer
devices such as coils.
The first dimerization reactor may consist of one or more reaction zones
within a single vessel or
in multiple vessels and may include one or more heat exchanging devices within
the reaction zones
or between the reaction zones.
[00116] The first set of dimerization conditions in the first dimerization
reactor can
advantageously include: a temperature in the range from Tb1 C to Tb2 C, where
Tbl and Tb2
can be, independently, 30, 50, 60, 80, 100, 120, 140, 150, 160, 180, 200, 220,
240, or 250, as long
as Tbl < Tb2; an absolute pressure in the range from Pbl kilopascal to Pb2
kilopascal, where Pbl
and Pb2 can be, independently, 345, 350, 400, 450, 500, 550, 600, 700, 800,
900, 1000, 1500,
2000, 2500, 3000, 3500, 4000, 4500, 5000, 6000, 6500, 6894, or 7000, as long
as Pbl < Pb2; and
a residence time in the range from Trl minutes to Tr2 minutes, where Trl and
Tr2 can be,
independently, 1, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140,
150, 160, 170, 180,
190, 200, 210, or 220, as long as Trl < Tr2. Preferably, if two dimerization
reactors in series are
utilized in the system, the first set of dimerization conditions include a
temperature in the range
from 70 to 130 C, a total pressure in the range from 689 to 3447 kilopascal
absolute, and a
residence time in the range from 20 to 200 minutes, such as from 100 to 200
minutes; preferably,
if three dimerization reactors in series are utilized in the system, the first
set of dimerization
conditions include a temperature in the range from 90 to 140 C, a total
pressure in the range from
689 to 3447 kilopascal absolute, and a residence time in the range from 1 to
30 minutes.
[00117] A portion of the CPD contained in the first CS-rich fraction
supplied into the first
dimerization reactor is converted into DCPD. At the outlet of the second
reactor (the first
dimerization reactor), a second reactor effluent is obtained comprising CPD
and DCPD.
Preferably, the degree of conversion in the second reactor is limited so that
high purity DCPD
may be produced; i.e., the extent of conversion is limited so that the
quantity of CPD co-dimers
with acyclic dienes and mono olefins is maintained below a level so as to be
able to obtain the
desired purity of DCPD. Advantageously, the first C5-rich fraction comprises
CPD at a
concentration of C(CPD)2 wt%, based on the total weight of C5 components in
the first CS-rich
fraction;the second reactor effluent comprises CPD at a concentration of
C(CPD)3 wt% and
DCPD at a concentration of C(DCPD)1, both based on the total weight of C5
hydrocarbons and
C10 hydrocarbons in the second reactor effluent; and C(CPD)3 < C(CPD)2.
- 25 -
CA 03004320 2018-05-03
Separation of the first DCPD-rich fraction
[00118] At least a portion of the second reactor effluent is then supplied
to a second separation
device, such as a distillation column, where a first DCPD-rich fraction (as a
lower stream such as
a bottom effluent from the column, e.g.) and a second C5-rich fraction (as an
upper stream such
as an overhead effluent from the column, e.g.) are obtained. Advantageously,
the first DCPD-rich
fraction can have a DCPD concentration of C(DCPD)1 wt%; and xl < C(DCPD)1 <
x2, wherein
xl and x2 can be, independently, 80, 82, 84, 85, 86, 88, 90, 91, 92, 93, 94,
95, 96, 97, 98, 99, 99.2,
99.4, 99.5, 99.6, 99.8, or 100, as long as xl <x2. Ultra high purity DCPD
(i.e., UHP DCPD) with
a concentration of at least 98 wt%, 99 wt%, or even 99.5 wt%, can be obtained
as the first
DCPD-rich fraction. At least a portion of the first DCPD-rich fraction can be,
optionally, supplied
to at least another separation device, such as a distillation column, where
the purity of the first
DCPD-rich fraction can be further increased. CPD concentration in the second
C5-rich fraction
tends to be lower than in the first C5-rich fraction. Often, the second C5-
rich fraction comprises
CPD at a concentration in a range of from 95.0 wt% to 99.9 wt% based on the
total weight of the
second C5-rich fraction.
Dimerization of the second C5-rich fraction
[00119] At least a portion of the second CS-rich fraction obtained from
the second separation
device may advantageously comprise CPD at a high concentration in the range
from ca3 wt% to
ca4 wt%, based on the total weight of C5 hydrocarbons in the second CS-rich
fraction, where ca3
and ca4 can be, independently, 1, 5, 10, 20, 25, 30, 35, 40, 45, 50, 55, or
60, as long as ca3 < ca4.
Such CPD in the second CS-rich fraction may be directly used as a CPD feed for
the production
of, e.g., norbornene, vinyl norbornene, ethylidene norbornene, hydrocarbon
resin adhesives or
tackifiers, unsaturated polyester resins, cyclopentane, and/or cyclopentene.
[00120] Additionally or alternatively, the second C5-rich fraction can be
supplied to a second
dimerization reactor (the third reactor in the system) operating under a
second set of dimerization
conditions, where a portion of the CPD is advantageously converted into DCPD,
similar to the
operation in the first dimerization reactor (the second reactor in the
system), but preferably
operating at a higher temperature and/or longer residence time to enable
satisfactory conversion
of the lower concentration CPD.
[00121] Thus, the second set of dimerization conditions in the second
dimerization reactor can
advantageously include: a temperature in the range from Tb3 C to Tb4 C, where
Tb3 and Tb4
- 26 -
CA 03004320 2018-05-03
can be, independently, 30, 50, 60, 80, 100, 120, 140, 150, 160, 180, 200, 220,
240, or 250, as long
as Tb3 < Tb4; an absolute pressure in the range from Pb3 kilopascal to Pb4
kilopascal, where Pb3
and Pb4 can be, independently, 345, 350, 400, 450, 500, 550, 600, 700, 800,
900, 1000, 1500,
2000, 2500, 3000, 3500, 4000, 4500, 5000, 6000, 6500, 6894, or 7000, as long
as Pb3 < Pb4; and
.. a residence time in the range from Tr3 minutes to Tr4 minutes, where Tr3
and Tr4 can be,
independently, 1, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140,
150, 160, 170, 180,
190, 200, 210, 220, 230, 240, 250, 260, 270, 280, 290, or 300 as long as Tr3 <
Tr4. Preferably, if
two dimerization reactors in series are utilized in the system, the second set
of dimerization
conditions include a temperature in the range from 75 to 140 C, such as from
100 to 140 C, a total
pressure in the range from 689 to 3447 kilopascal absolute, and a residence
time in the range from
100 to 300 minutes, such as from 150 to 300 minutes; preferably, if three
dimerization reactors in
series are utilized in the system, the second set of dimerization conditions
include a temperature
in the range from 100 to 140 C, a total pressure in the range from 689 to 3447
kilopascal absolute,
and a residence time in the range from 1 to 30 minutes.
[00122] The second dimerization reactor (the third reactor in the system)
can be a reactor
similar to the first dimerization reactor (the second reactor in the system).
[00123] A portion of the CPD contained in the second C5-rich fraction
supplied into the second
dimerization reactor is converted into DCPD. At the outlet of the second
dimerization reactor), a
third reactor effluent is obtained comprising CPD and DCPD. Preferably, the
degree of
conversion in the third reactor is limited so that high purity DCPD may be
produced; i.e., the
extent of conversion is limited so that the quantity of CPD co-dimers with
acyclic dienes and
mono olefins is maintained below a level so as to be able to obtain the
desired purity of DCPD.
Separation of a second DCPD-rich fraction
[00124] At least a portion of the third reactor effluent can then be
supplied to a third separation
device, such as a distillation column, where a second DCPD-rich fraction (as a
lower stream such
as a bottom effluent from the column, e.g.) and a third C5-rich fraction (as
an upper stream such
as an overhead effluent from the column, e.g.) are obtained. Advantageously,
the second DCPD-
rich fraction can have a DCPD concentration of C(DCPD)2 wt%; and x3 < C(DCPD)2
< x4,
wherein x3 and x4 can be, independently, 40, 50, 60, 65, 70, 75, 80, 82, 84,
85, 86, 88, 90, 91, 92,
93, 94, 95, 96, 97, 98, or 99 as long as x3 <x4. Usually, the purity of the
second DCPD-rich
fraction is lower than the first DCPD-rich fraction because of the lower ratio
of CPD to acyclic
- 27 -
CA 03004320 2018-05-03
diolefins in the second C5-rich fraction than the first C5-rich fraction.
Nonetheless, very high
purity DCPD (HP DCPD) with a concentration of at least 90 wt%, or 92 wt%, or
93 wt%, or even
95 wt% can be obtained as the second DCPD-rich fraction. At least a portion of
the second
DCPD-rich fraction can be, optionally, supplied to at least another separation
device, such as a
distillation column, where the purity of the second DCPD-rich fraction can be
further increased.
Likewise, CPD concentration in the third CS-rich fraction tends to be lower
than in the second
C5-rich fraction. Often, the third CS-rich fraction comprises CPD at a
concentration in a range of
from 90.0 wt% to 99.5 wt% based on the total weight of the third C5-rich
fraction.
Dimerization of the third CS-rich fraction
[00125] At least a portion of the third CS-rich fraction obtained from the
third separation device
may advantageously comprise CPD at a concentration in the range from ca5 wt%
to
ca6 wt%, based on the total weight of the C5 hydrocarbons in the third C5-rich
fraction, where
ca5 and ca6 can be, independently, 1, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50,
55, or 60, as long as
ca5 < ca6. Such CPD in the third CS-rich fraction may be directly used as a
CPD feed for the
production of, e.g., norbornene, vinyl norbornene, ethylidene norbornene,
hydrocarbon resin
adhesives or tackifiers, unsaturated polyester resins, cyclopentane, and/or
cyclopentene.
[00126] Additionally or alternatively, at least a portion of the third CS-
rich fraction can be
supplied to a third dimerization reactor (the fourth reactor in the system)
operating under a third
set of dimerization conditions, where a portion of the CAD is advantageously
converted into
DCPD, similar to the operation in the first dimerization reactor (the second
reactor in the system).
[00127] The third dimerization reactor (the fourth reactor in the system)
can be a reactor similar
to the first dimerization reactor (the second reactor in the system), but
preferably operating at a
higher temperature and/or longer residence time to enable satisfactory
conversion of the lower
concentration CPD.
[00128] Desirably, a majority of the CPD contained in the third CS-rich
fraction supplied into
the third dimerization reactor is converted into DCPD. Additionally or
alternatively, it is desirable
to react acyclic C5 diolefins (e.g., 1,3-pentadiene; 1,4-pentadiene, 1,2-
pentadiene, and/or 2-
methyl-1,3-butadiene) with CPD to produce co-dimers in the third dimerization
reactor.
Additionally or alternatively, additional streams containing acyclic C5
diolefins (e.g., steam
cracked naphtha, light cat naphtha, heavy cat naphtha) and/or C6 diolefins
(e.g., methyl
cyclopentadiene and hexadienes) may be added to the feed to the third
dimerization reactor.
- 28 -
CA 03004320 2018-05-03
Additionally, trimers and tetramers of the C5 and C6 species may also be
advantageously
produced. At the outlet of the third dimerization reactor, a fourth reactor
effluent is obtained
comprising CPD and DCPD, preferably in combination with other C5 co-dimers, -
trimers, and/or
-tetramers.
[00129] Thus, the third set of dimerization conditions in the third
dimerization reactor can
advantageously include: a temperature in the range from Tb5 C to Tb6 C,
where Tb5 and Tb6
can be, independently, 30, 50, 60, 80, 100, 120, 140, 150, 160, 180, 200, 220,
240, or 250, as long
as Tb5 < Tb6; an absolute pressure in the range from Pb5 kilopascal to Pb6
kilopascal, where Pb5
and Pb6 can be, independently, 345, 350, 400, 450, 500, 550, 600, 700, 800,
900, 1000, 1500,
2000, 2500, 3000, 3500, 4000, 4500, 5000, 6000, 6500, 6894, or 7000, as long
as Pb5 < Pb6; and
a residence time in the range from Tr5 minutes to Tr6 minutes, where Tr5 and
Tr6 can be,
independently, 100, 200, 300, 400, 500, 600, 700, 800, 900, or 1000, as long
as Tr5 < Tr6.
Preferably, the third set of dimerization conditions include a temperature in
the range from 80 to
150 C, such as from 100 to 150 C, a total pressure in the range from 689 to
3447 kilopascal
absolute, and a residence time in the range from 150 to 300 minutes.
Separation of a third DCPD-rich fraction
[00130] At least a portion of the fourth reactor effluent can then be
supplied to a fourth
separation device, such as a distillation column, where a third DCPD-rich
fraction (as a bottom
effluent from the column, e.g.) and fourth CS-rich fraction (as an overhead
effluent from the
column, e.g.) are obtained. Advantageously, the third DCPD-rich fraction can
have a DCPD
concentration of C(DCPD)3 wt%; and x5 < C(DCPD)3 < x6, wherein x5 and x6 can
be,
independently, 20, 30, 40, 45, 50, 55, 60, 65, 70, 75, 80, 82, 84, 85, 86, 88,
90, 91, 92, 93, 94, or
95, as long as x5 <x6. Usually, the purity of the third DCPD-rich fraction is
lower than the second
DCPD-rich fraction because of the lower ratio of CPD to acyclic dienes in the
third CS-rich
fraction than the second CS-rich fraction. Nonetheless, moderate purity DCPD
with a
concentration of at least 70 wt%, 75 wt%, 80 wt%, 85 wt%, or 90 wt% can be
obtained as the third
DCPD-rich fraction. At least a portion of the third DCPD-rich fraction can be,
optionally, supplied
to at least another separation device, such as a distillation column, where
the purity of the third
DCPD-rich fraction can be further increased. Likewise, CPD concentration in
the fourth CS-rich
fraction tends to be lower than in the third C5-rich fraction.
- 29 -
CA 03004320 2018-05-03
Recycling of C5-rich fractions to the first reactor
[00131] At least a portion of the first, second, third, and fourth C5-rich
fractions described
above, if produced at all in the process of the present invention, can be
recycled to the first reactor
described above, where the unreacted C5 hydrocarbon(s) and partially converted
C5 hydrocarbons
from the C5 feedstock can be further converted into CPD.
[00132] The first, second, third, and fourth CS-rich fractions, if
produced, may contain C6+
hydrocarbons, such as cyclohexane, benzene, toluene, and the like. To prevent
the accumulation
of such C6+ components in the reaction product in the first reactor, it is
highly desirable that, prior
to being recycled to the first reactor, at least a portion of the C6+
components is separated and
removed from the CS-rich stream in a separation device, such as a distillation
column, to produce
a fifth C5-rich stream and a C6+-rich stream. Thus, a purified fifth C5-rich
fraction is then
recycled to the first reactor.
Forming mogas blending components from the C5+ components
[00133] Mogas is a blended mixture comprising C4 through C12 hydrocarbons
having an
initial normal boiling point of about 35 C and a final boiling point of about
200 C. Mogas is used
primarily as fuel for internal combustion engines in automotive vehicles.
There are many different
mogas specifications that have been mandated by various local, state, or
national governmental
agencies. One example is Reid Vapor Pressure (RVP) of final mogas product. The
vapor pressure
of mogas is a measure of its volatility and high vapor pressures resulting in
high evaporative
emissions of smog-forming hydrocarbons.
[00134] From a performance standpoint, an important attribute of mogas is
its octane rating.
Linear paraffinic hydrocarbons (i.e., straight-chain saturated molecules) tend
to have lower octane
ratings than other hydrocarbons such as aromatics, olefins, and branched
paraffins. To that end,
many of the refining processes used in petroleum refineries are designed to
produce hydrocarbons
with these latter molecular configurations. For example, catalytic reforming
is a widely practiced
industrial process used to convert naphtha feed typically having low-octane
ratings into high-
octane liquid products to make premium blending stocks for mogas. The process
converts
paraffins and naphthenes into high-octane aromatic hydrocarbons. However,
naphtha catalytic
reforming is limited to C6+ feedstocks.
[00135] Converting n-pentane to isopentane (a/k/a i-pentane) can result in
a favorable increase
in octane, but also an unfavorable increase in the RVP. Conversion of n-
pentane to cyclopentyl
- 30 -
CA 03004320 2018-05-03
and internal olefinic species ¨ which occurs in the first reactor in the
present invention ¨ favorably
increases the octane and favorably decreases the RVP. DCPD-rich streams may
also be partially
or fully hydrogenated to produce a low RVP / higher octane blend component.
[00136] Thus, additionally or alternatively, at least a portion of the
first, second, third, fourth,
and fifth CS-rich fractions and the C6+-rich stream described above, if
produced at all in the
process of the present invention, can be, optionally, combined with additional
streams containing
diolefins (e.g., steam cracked naphtha, light cat naphtha, heavy cat naphtha)
and can be selectively
hydrogenated to produce a mogas component. Because the first, second, third,
fourth, and fifth
CS-rich fractions contain high concentrations of unsaturated C5 hydrocarbons,
including CPD and
cyclopentene, once partially hydrogenated they tend to have higher octane-
value and lower Reid
Vapor Pressure (RVP) than the acyclic saturated C5 feedstock supplied to the
first reactor. As
used herein, a "selective hydrogenation" process is a treatment of a mixture
comprising both
diolefins and mono olefins with hydrogen in the presence of a selective
hydrogenation catalyst
under selective hydrogenation conditions favoring the conversion of diolefins
into mono olefins
over the conversion of mono olefins into saturates. Such selective
hydrogenation may be carried
out in a hydrogenation reactor having a hydrogenation catalyst loaded therein.
It is highly desired
that the selectively hydrogenated mogas component comprises diolefins at a
total concentration
not higher than 2.0 wt%, preferably not higher than 1.0 wt%, preferably not
higher than 0.5 wt%,
and ideally not higher than 0.1 wt% based on the total weight of the mogas
component. In such
aspects, it is preferably that at least 25 wt% of the third CS-rich fraction
is recycled to the first
reactor and at least 50 wt% of the third C5-rich fraction is subjected to
hydrogenation. This mogas
component can then be blended with additional mogas components to obtain mogas
with the
desired composition and properties.
[00137] Additionally or alternatively, prior to or after hydrogenation
thereof, at least a portion
of the first, second, third, fourth, and fifth CS-rich fractions described
above, if produced at all in
the process of the present invention, and/or a portion of their hydrogenated
products, may be
separated to obtain high-purity cyclopentene, cyclopentane, 2-methyl-1,3-
butadiene, and/or
1,3-pentadiene, each of which can be used or sold as valuable industrial
materials.
[00138] Non-limiting examples of hydrogenation catalyst include: palladium-
based or
nickel-based catalysts. Exemplary hydrogenation conditions include: a
temperature in the range
from 30 ¨ 250 C and a pressure in the range from 1,700 ¨ 5,500 kilopascal
absolute.
-31 -
CA 03004320 2018-05-03
[00139] The present invention can be used to convert low value C5 feedstock
into higher value
CPD, DCPD, mogas components with high octane and/or lower RVP, cyclopentene,
cyclopentane, 1,3-pentadiene, and the like, and hydrogen.
Description according to the drawings
[00140] The drawings schematically illustrate the block flow diagrams of
exemplary system(s)
and sub-system(s) thereof of the present invention operating to implement
exemplary process(es)
or aspects thereof of the present invention. It should be understood that only
major components
are shown in the drawings. Auxiliary equipment such as control valves, pumps,
heat exchangers,
reboilers, recycle loop, and the like, although not all shown in all drawings,
are used liberally
throughout the whole process to manipulate stream and equipment thermodynamic
conditions.
[00141] In the system 101 shown in FIG. 1, a C5 feedstock stream 103
comprising n-pentane
at, e.g., at least 50 wt% is combined with a hydrogen co-feedstock stream 105
to form a combined
stream 107, which is then combined with a recycle third CS-rich stream 109 to
form a combined
feed stream 111, which is fed to a first reactor 113 (also labeled R1). The
molar ratio of hydrogen
to the C5 feedstock in stream 111 can range from 0.1 to 3.0, preferably from
0.3 to 2.0, more
preferably from 0.5 to 1.5. A major purpose of co-feeding hydrogen is to
prevent coke formation
on the catalyst, especially at locations where the in-situ produced hydrogen
is at a relatively low
concentration. The reactor 113 may be a fixed bed reactor with a bed of
catalyst 115 loaded
therein. The catalyst 115 is chosen from the compositions described above.
Reactions of the C5
hydrocarbons in the presence of the catalyst particles are highly endothermic.
Thus, reactor 113
is heated by external heating to maintain an internal temperature in the range
from 450 C to 800 C.
The weight hourly space velocity is in the range from 1 to 100 hour-1. A
substantial portion of the
C5 hydrocarbons in the feed Ill is converted into CPD and byproducts such as
acyclic diolefins,
acyclic mono olefins, cyclopentane, cyclopentene; light components including
hydrogen and
C 1 -C4 hydrocarbons; one-ring aromatics; and multiple-ring aromatics at a
total conversion of
n-pentane in the range from 50% to 99%. At the outlet of the first reactor
113, a first reactor
hydrocarbon effluent 117 is drawn at a temperature in the range from 500 to
800 C and at a total
absolute pressure in the range from 20 to 700 kilopascal absolute.
[00142] The first reactor hydrocarbon effluent 117 can comprise CPD at a total
concentration
in the range from 15 wt% to 80 wt%, on the basis of the total weight of C5
hydrocarbons in the
first reactor hydrocarbon effluent 117. Once it exits the first reactor 113,
the first reactor
- 32 -
CA 03004320 2018-05-03
hydrocarbon effluent stream 117 is promptly cooled down by one or more heat
exchanger 119 to
obtain a stream 121 to avoid undesired side reactions such as thermal
cracking, condensation of
PNAs, and premature Diels-Alder reactions of reactive diolefinic species,
especially CPD. A
quantity of wash oil (not shown) may be added prior to and/or within exchanger
119 to help
prevent fouling.
[00143] The cooled stream 121 and a wash oil steam 125 are then fed into a
washing vessel
123, where the first reactor hydrocarbon effluent is also quenched down to
obtain a washed first
reactor hydrocarbon effluent stream 129. The wash oil used in the example
shown in FIGs. 1 and
2 comprises alkylnaphthalene(s) and/or alkylbenzene at a total concentration
of at least 50 wt%,
although other wash oil as described above may be used. Stream 129 comprises
C5 components
and light components from the first reactor hydrocarbon effluent. Stream 129
may also contain
C6, C7, C8, and the wash oil at non-negligible amounts. A wash oil bottom
stream 127,
comprising the wash oil, one-ring aromatics and multiple-ring aromatics, is
also obtained from
the washing vessel 123.
[00144] The upper stream 129, as clean first reactor hydrocarbon effluent,
is then supplied to
a first separation sub-system 131 (also labeled SD1), where a first C5-rich
stream 133, one or
more additional CS-rich streams 134 (one shown in FIG. 1), and a light
component stream 161
comprising hydrogen and Cl-C4 hydrocarbons are obtained. The CS-rich streams
133 and 134
are advantageously depleted of CI-C4 hydrocarbons. Stream 133 can comprise one
or more of
C6, C7, C8+, and the heavy wash oil at non-negligible amounts. Stream 134
desirably comprises
C6, C7, C8+, and the heavy wash oil at significantly lower concentrations than
stream 133.
Preferably, stream 134 is essentially free of C10+ and the heavy wash oil.
Stream 161 is fairly
large in total volume, given the amount of hydrogen produced in the first
reactor 113. To recover
the non-negligible amount of C5 components present in stream 161, stream 161
is further
contacted with a wash oil stream 165 in vessel 163 (sometimes also called
"debutanizer" or
"debutanizer section") to obtain a stream 167 comprising H2 and Cl -C4
hydrocarbons and
depleted of C5 components. Wash oil stream 125 exiting debutanizer vessel 163
is then recycled
to the washing vessel 123 as described above. Stream 167 can be further
separated by using
various equipment and processes (now shown), such as PSA, RCPSA, TSA,
cryogenic method,
and membrane separation, to obtain one or more of the following: (i) a high-
purity H2 stream; (ii)
a H2/C1-C4 hydrocarbon mixture stream; and (iii) a Cl-C4-rich hydrocarbon
stream.
- 33 -
CA 03004320 2018-05-03
[00145] Stream 133, to the extent it may comprise one or more of C6,
toluene, C8+, and the
heavy wash oil at non-negligible concentration(s), is fed into a heavy wash
oil (e.g.,
alkylnapthalene)-removal column 135 together with stream 127 described above,
where an upper
stream 137 rich in C5 and depleted of C10+, and a lower stream 138 comprising
C7 and C8+ are
obtained. Stream 138 may be purified in a subsequent distillation column (not
shown) to obtain
an alkylnapthalene-rich stream, which can be recycled to vessel 163 and/or
washing vessel 123
described above. Efforts should be taken to reduce reactions between CPD and
acyclic diolefins
in heat exchanger 119, vessels 123, 135, and the front end of the first
separation sub-system 131.
Nonetheless, because such side reactions may take place at various degrees, it
is highly desirable
that column 135 is operated under a condition such that reverse dimerization
reaction is favored
over dimerization, such that heavy components such as DCPD, reaction products
between DCPD
and acyclic diolefins are converted into CPD and other C5 components, and
therefore CPD and
other C5 components that otherwise would be lost to side reactions are at
least partially recovered.
To that end, the conditions in the column 135 comprise advantageously a column
bottom
temperature in the range from 150 to 350 C, preferably from 170 to 260 C, and
a total absolute
pressure in the range from 3 psia to 50 psia (21 to 345 kilopascal absolute),
preferably from 20
psia to 40 psia (138 to 276 kilopascal absolute), and a residence time in the
range from 0.01 to 10
hours, preferably from 0.1 to 4 hours.
[00146] Stream 137 and stream 134, both CS-rich and depleted of C10+,
together as the first
.. C5-rich fraction obtained from the first separation sub-system, is then
delivered to the second
reactor (also labeled R2, and called first dimerization reactor) 139 operating
under a first set of
dimerization conditions to convert a portion of the CPD contained therein into
DCPD. The first
set of dimerization conditions advantageously comprise: a temperature in the
range from 30 to
250 C, preferably from 70 to 140 C, such as from 90 to 130 C, and a total
absolute pressure in
the range from 50 psia to 1000 psia (345 to 6895 kilopascal absolute),
preferably from 100 psia to
500 psia (689 to 3447 kilopascal absolute), and a residence time in the range
from 1 to 220
minutes, preferably from 20 to 200 minutes, such as from 100 to 200 minutes.
Such conditions
are optimized to favor the dimerization reaction between CPD molecules and to
minimize the
reactions between CPD and other diolefins.
[00147] From the reactor 139, a second reactor effluent 141 comprising CPD,
other C5
hydrocarbons, and DCPD is then fed into a second separation device 143 (SD2),
which can be a
- 34 -
CA 03004320 2018-05-03
distillation column. From column 143, an ultra high-purity DCPD lower stream
147 and an upper
stream comprising CPD and other C5 hydrocarbons are obtained. Stream 147 can
comprise DCPD
at a concentration of at least 95 wt%, such as 96 wt%, 98 wt%, 99 wt%, or even
higher, based on
the total weight of the C10 hydrocarbons of the stream 147. Stream 147 may be
purified in a
subsequent distillation column (not shown) to obtain (1) an ultra high-purity
DCPD, which
comprises DCPD at a concentration of at least 95 wt%, such as 96 wt%, 98 wt%,
99 wt%, or even
higher, based on the total weight of the stream; (2) a light wash oil-rich
stream, which can be
recycled to vessel 163 and/or washing vessel 123 described above (not shown).
[00148] Upper stream 145, which is the second C5-rich fraction in the
process of the present
invention, is then fed into a second dimerization reactor (the third reactor
of the present invention,
R3) 149 operated under a second set of dimerization conditions, where the
remaining CPD in
stream 147 is partly converted into DCPD. The second set of dimerization
conditions
advantageously comprise: a temperature in the range from 30 to 250 C,
preferably from 100 to
140 C, and a total absolute pressure in the range from 50 psia to 1000 psia
(345 to 6895 kilopascal
absolute), preferably from 100 psia to 500 psia (689 to 3447 kilopascal
absolute), and a residence
time in the range from 1 to 300 minutes, preferably from 150 to 300 minutes.
Such conditions are
optimized to maximize recovery of the remaining CPD while achieving on-spec
production of a
subsequent DCPD fraction.
[00149] From the reactor 149, a third reactor effluent 151 comprising CPD,
other C5
hydrocarbons, and DCPD is then fed into a third separation device 153 (SD3),
which can be a
distillation column. From column 153, a high-purity DCPD lower stream 155 and
an upper stream
comprising CPD and other C5 hydrocarbons 157 are obtained. Stream 155 can
comprise DCPD
at a concentration of at least 90 wt%, such as 92 wt%, 94 wt%, 95 wt%, or even
higher, based on
the total weight of the C10 hydrocarbons of the stream 155. Stream 155 may be
purified in a
subsequent distillation column (not shown) to obtain (1) a high-purity DCPD,
which comprises
DCPD at a concentration of at least 90 wt%, such as 92 wt%, 94 wt%, 95 wt%, or
even higher,
based on the total weight of the stream; (2) a light wash oil-rich stream,
which can be recycled to
vessel 163 and/or washing vessel 123 described above (not shown).
[00150] DCPD streams 147 and 155 may be sold or delivered as products. The
user may
convert these streams back into CPD or other compounds, depending on the
intended applications.
- 35 -
CA 03004320 2018-05-03
1001511 Upper stream 157, which is the third CS-rich fraction in the
process of the present
invention, can be fed into a third dimerization reactor (not shown), where the
remaining CPD
therein can be converted into an additional amount of DCPD, which can be
separated and
recovered as a third DCPD-rich fraction in a fourth separation device (not
shown), if so desired.
If a third dimerization reactor is utilized, the preferred modes of operation
for the first dimerization
reactor and second dimerization reactor can be advantageously adjusted for the
purpose of
producing DCPD products at optimal quality levels, each with optimal
quantities. Typically, the
third DCPD-rich fraction would have a lower purity than the first and second
DCPD-rich fractions
produced upstream in the process as described above.
[00152] As shown in FIG. 1, the third CS-rich fraction stream 157 from the
third separation
device 153 is divided into two streams 159 and 161. To the extent streams 157,
159, and 161 may
comprise C6+ in addition to C5 hydrocarbons, stream 161 is then separated in
distillation column
163 to obtain a fifth CS-rich stream 165 that is depleted with C6+ and a C6-
rich stream 167.
Stream 165 can then be recycled to the first reactor 113 (R1) as stream 109,
as described above.
Stream 167 may be purged or used in other applications, such as an untreated
mogas component
as described below. It has been found that in this particular embodiment,
without the distillation
column 163, if the weight ratio of stream 161 to stream 159 is higher than
0.4:0.6, accumulation
of C6+ species may occur in the system. It is highly desired that stream 161
is subjected to
purification in column 163 before being recycled to the first reactor to
eliminate such restriction
on the recycle ratio.
[00153] Stream 159 (and, optionally, a portion of the first C5-rich
fraction stream 137, and a
portion of the second C5-rich fraction stream 145, not shown in FIG. 1) can be
used for many
purposes, due to the many useful components contained therein: CPD,
cyclopentane,
cyclopentene, pentene, pentadiene, 2-methylbutadiene, and the like.
[00154] For example, stream 159 (and other CS-rich fraction streams, and C6-
rich stream 167)
can be partly or entirely converted into a mogas component by selective
hydrogenation to convert
at least a portion of the dienes therein to mono olefins and/or saturates. The
high concentrations
of cyclopentane and cyclopentene in stream 159 after hydrogenation makes it
particularly suitable
for mogas blending due to the high octane and lower Reid Vapor Pressure values
of cyclopentane
and cyclopentene relative to the starting feedstock of acyclic C5 hydrocarbon
such as n-pentane.
- 36 -
CA 03004320 2018-05-03
The C6-rich stream 167 may be used directly as a mogas component after
selective hydrogenation
as well.
[00155] For another example, before or after selective hydrogenation,
stream 159 (and other
CS-rich fraction streams) may be separated to obtain at least one pure stream
of the following:
cyclopentane, cyclopentene, pentene, 1,3-pentadiene, I ,4-pentadiene, and 2-
methylbutadiene.
100156] FIG. 2 schematically illustrates an exemplary first separation sub-
system 201 useful
in the process and system of the present invention, particularly in the
exemplary process illustrated
in FIG. 1. The first separation sub-system 201 in HG. 2 comprises a
compression train including
multiple-stage compression, cooling, and liquid/vapor separation. In the
process of this figure,
the upper stream 129 comprising a majority of cleaned first reactor effluent
obtained from column
123 is first fed into a first-stage compressor 203, from which a stream 205 at
a higher pressure is
obtained. Stream 205 is then cooled by a first-stage heat exchanger 207 to
obtain a liquid/vapor
mixture stream 209, which is fed into a first-stage liquid/vapor separation
device (such as a drum)
211 to obtain a first-stage lower liquid stream 215 comprising C5
hydrocarbons, but depleted of
hydrogen and C I -C4 hydrocarbons and a first-stage upper vapor stream 213
comprising C5
hydrocarbons and rich in hydrogen and Cl-C4 hydrocarbons. Stream 213 is then
compressed by
a second-stage compressor 217 to obtain a stream 219 with an even higher
pressure, which is then
cooled by a second-stage heat exchanger 221 to obtain a second-stage lower
temperature
liquid/vapor mixture stream 223, which is separated in a second-stage
liquid/vapor separation
device (such as a drum) 225 to obtain a second-stage lower liquid stream 229
comprising C5
hydrocarbons, but depleted of hydrogen and C1-C4 hydrocarbons and a second-
stage vapor stream
227 comprising C5 hydrocarbons and rich in hydrogen and Cl-C4 hydrocarbons.
Stream 227 is
then compressed by a third-stage compressor 231 to obtain a stream 233 with an
even higher
pressure, which is then cooled by a third-stage heat exchanger 235 to obtain a
lower temperature
third-stage liquid/vapor mixture stream 237, which is separated in a third-
stage liquid/vapor
separation device (such as a drum) 239 to obtain a third-stage lower liquid
stream 241 comprising
C5 hydrocarbons, but depleted of hydrogen and C I -C4 hydrocarbons and a third-
stage upper
vapor stream 161 comprising rich in hydrogen and C I -C4 hydrocarbons and,
optionally,
comprising C5 hydrocarbons at a lower concentration. Stream 161 is then fed to
a vessel 163 as
illustrated in FIG. 1 and described above.
- 37 -
CA 03004320 2018-05-03
[00157] As shown in FIG. 2, stream 215, to the extent it may comprise non-
negligible
concentrations of at least one of the wash oil, C7, and C8+ (such as DCPD),
can be fed into the
heavy wash oil-removal column 135 together with stream 127, where it is
processed to obtain a
CS-rich stream 137 depleted with heavy wash oil as described above in
connection with FIG. 1.
Downstream streams 229 and 241, to the extent they tend to comprise lower
concentrations of the
heavy wash oil, C7, and C8+, may be combined to form a single stream 134,
which is then
combined with stream 137 as the first C5-rich fraction directly fed into the
first dimerization
reactor 139 (R2) as illustrated in FIG. 1.
[00158] It is contemplated, though not shown, that streams 215, 229, and
241, to the extent
they may all contain non-negligible concentrations of at least one of the
heavy wash oil, C7, and
C8+ (such as DCPD), may be all delivered to the heavy wash oil-removal column
135 along with
stream 127, where the C5-rich stream 137 is obtained and delivered to the
first dimerization
reactor 139.
[00159] It is also contemplated, though not shown, that streams 215, 229,
and 241, to the extent
they may all contain the heavy wash oil, C7, and C8+ at sufficiently low
concentrations, if any at
all, may be combined with stream 137 and then delivered directly to the first
dimerization reactor
139.
Industrial Applicability
[00160] The first hydrocarbon reactor effluent obtained during the the
acyclic C5 conversion
process containing cyclic, branched and linear C5 hydrocarbons and,
optionally, containing any
combination of hydrogen, C4 and lighter byproducts, or Co and heavier
byproducts is a valuable
product in and of itself. Preferably, CPD and/or DCPD may be separated from
the reactor effluent
to obtain purified product streams, which are useful in the production of a
variety of high value
products.
[00161] For example, a purified product stream containing 50 wt% or greater,
or preferably 60
wt% or greater of DCPD is useful for producing hydrocarbon resins, unsaturated
polyester resins,
and epoxy materials. A purified product stream containing 80 wt% or greater,
or preferably 90
wt% or greater of CPD is useful for producing Diets-Alder reaction products
formed in accordance
with the following reaction Scheme (I):
- 38 -
CA 03004320 2018-05-03
Scheme
+ % 4+2 cycloaddition
R Diels-Alder reaction
product.
where R is a heteroatom or substituted heteroatom, substituted or
unsubstituted C 1 -050
hydrocarbyl radical (often a hydrocarbyl radical containing double bonds), an
aromatic radical, or
any combination thereof. Preferably, substituted radicals or groups contain
one or more elements
from Groups 13-17, preferably from Groups 15 or 16, more preferably nitrogen,
oxygen, or sulfur.
In addition to the monoolefin DieIs-Alder reaction product depicted in Scheme
(I), a purified
product stream containing 80 wt% or greater, or preferably 90 wt% or greater
of CPD can be used
to form Diels-Alder reaction products of CPD with one or more of the
following: another CPD
molecule, conjugated dienes, acetylenes, allenes, disubstituted olefins,
trisubstituted olefins,
cyclic olefins and substituted versions of the foregoing. Preferred Diels-
Alder reaction products
include norbornene, ethylidene norbornene, substituted norbornenes (including
oxygen-containing norbomenes), norbornadienes, and tetracyclododecene, as
illustrated in the
following structures:
0
norbornene ethylidene norbornene tetracyclododecene
norbornadiene oxygen substituted
norbornene.
[00162] The foregoing Diels-Alder reaction products are useful for producing
polymers and
copolymers of cyclic olefins copolymerized with olefins such as ethylene. The
resulting cyclic
olefin copolymer and cyclic olefin polymer products are useful in a variety of
applications, e.g.,
packaging film.
[00163] A purified product stream containing 99 wt% or greater of DCPD is
useful for
producing DCPD polymers using, for example, ring opening metathesis
polymerization (ROMP)
catalysts. The DCPD polymer products are useful in forming articles,
particularly molded parts,
e.g., wind turbine blades and automobile parts.
[00164] Additional components may also be separated from the reactor effluent
and used in
the formation of high value products. For example, separated cyclopentene is
useful for producing
polycyclopentene, also known as polypentenamer, as depicted in Scheme (II).
- 39 -
CA 03004320 2018-05-03
Scheme II
010' ROMP
catalyst
[00165] Separated cyclopentane is useful as a blowing agent and as a
solvent. Linear and
branched C5 products are useful for conversion to higher olefins and alcohols.
Cyclic and
non-cyclic C5 products, optionally, after hydrogenation, are useful as octane
enhancers and
transportation fuel blend components.
[00166] Separated cyclopentane is useful as a blowing agent and as a
solvent. Linear and
branched C5 products are useful for conversion to higher olefins and alcohols.
Cyclic and
non-cyclic CS products, optionally, after hydrogenation, are useful as octane
enhancers and
transportation fuel blend components.
Examples
[00167] The following non-limiting examples 1-7 illustrate the invention.
Examples 1-5 are
obtained by using simulation. In these examples, the respective first reactor
effluents are fed
forward to the quench/wash section (123), a compression train section (SDI,
131), and a
debutanization section (163) in similar manners as discussed above. All the
recovered C5-rich
fractions produced from the quench/wash section (135), the compression train
section (SDI, 131),
and debutanization section (163) are routed to the heavy wash oil removal
column (135), and
subsequently to a first dimerization reactor (R2, 139), an ultra high-purity
DCPD recovery column
(SD2, 143), a second dimerization reactor (R3, 149), and then a high-purity
DCPD recovery
column (SD3, 153).
Example 1
[00168] In this example, a first reactor effluent produced from pure n-
pentane feedstock, a pure
hydrogen co-feedstock with 1:2 hydrogen/n-pentane molar ratio, without co-
feeding a light
hydrocarbon or recycling of any down-stream C5-rich fractions to the first
reactor. The process
temperature, pressure, weight hourly space velocity, and molecular weight at
the reactor inlet are
475 C, 62 psia (401.9 kilopascal absolute), 15 hr-', and 49.01 g/mol,
respectively. Temperature
and pressure at the reactor outlet are 575 C and 10 psia (68.9 kilopascal
absolute), respectively.
The reactions generate an additional 1.87 moles of molecules in the first
reactor effluent exiting
the outlet per mole of molecules in the total feed material at the inlet. This
1.87-fold molar
- 40 -
CA 03004320 2018-05-03
expansion has the effects of lowering the molecular weight and density of the
stream mixture from
49.01 g/mol at the inlet to 27.05 g/mol at the outlet and from 3.08 kg/m3 at
the inlet to 0.26 kg/m3
at the outlet, respectively. The pressure drop from the inlet to the outlet of
the first reactor is
calculated to be about 52 psi (359 kilopascal). Composition of the first
reactor effluent at the
.. outlet is given in Table I below.
[00169] The entire third CS-rich fraction is used as a mogas blend for making
mogas, the
composition of the mogas blend is provided in Table I below as well.
[00170] In this example, to produce 100 tons of CPD in stream 117, a total
weight of 403 tons
of n-pentane feed is fed to the system (representing a total CPD yield of 24.8
wt%, based on all
weight of the n-pentane feed), a total weight of 13.1 tons of hydrogen is
produced, a total weight
of 82 tons of UHP DCPD with purity level exceeding 99.0 wt% (stream 147) is
produced, a total
weight of 11 tons of DCPD with purity level exceeding 90.0 wt% (stream 155) is
produced, and
a total weight of 238 tons of mogas blend is produced.
Example 2
[00171] The reactor inlet and outlet temperature and pressure remain the same
as in Example
1 above. However, in this example, a C5-rich stream, produced as 35% of the
third CS-rich
fraction obtained by separating the third reactor effluent produced from a
second dimerization
reactor described above, is recycled to the first reactor, where it is admixed
with n-pentane before
being fed into the first reactor. Hydrogen is co-fed at H2/(all C5
hydrocarbons except (iso-05
hydrocarbons and CPD)) molar ratio of 1:2. It has been experimentally found
that the reaction
pathway from iso-05 hydrocarbons to CPD is kinetically inhibited under the
reaction conditions.
The composition of the total feed to the first reactor is given in Table I
below.
[00172] The remaining 65% of the third CS-rich fraction is used as a mogas
blend for making
mogas. The composition of the mogas blend is provided in Table I below as
well.
[00173] In this example, to produce 100 tons of CPD in stream 117, a total
weight of 308 tons
of n-pentane feed is fed into the system (representing a CPD yield of 32.5
wt%, based on the total
weight of the n-pentane feed), a total weight of 11.7 tons of hydrogen is
produced, a total weight
of 85 tons of UHP DCPD (stream 147) with purity level exceeding 99.0 wt% is
produced, a total
weight of 8 tons of DCPD (stream 155) with purity level exceeding 90.0 wt% is
produced, and a
total weight of 146 tons of mogas blend is produced.
- 41 -
CA 03004320 2018-05-03
[00174] To produce the same amount of CPD, Example 2 (with 35% recycle of the
third
C5-rich fraction to the first reactor) requires 23.4% less of fresh n-pentane
feed than Example 1
(without recycle of any of the C5-rich fraction to the first reactor).
[00175] To produce the same amount of CPD, Example 2 produces 10.8% less of
hydrogen
than Example 1, due to using partially unsaturated feed vs. a fully saturated
feed. This has the
benefit of reduced volumetric flow rates in the reactor(s) and downstream
equipment. For
example, the first reactor in Example 2 shows a 7.8% reduction in volumetric
flow than in
Example 1. This may have significant impacts on the equipment sizing of the
downstream quench
tower(s), gas compressor(s), and debutanizer(s).
ti [00176] The enthalpy changes of the stream across the first reactor
also shows significant
reduction in Example 2 compared to Example 1. This translates into a 10.9 %
reduction in furnace
firing in Example 2, which can have a significant impact of the equipment
sizing of the reactor(s)
and fuel costs. The amount of heat required for sustaining the endothermic
reactions in the first
reactor is comparatively lower when using a partially converted C5 feedstock.
[00177] To produce 100 tons of CPD, Example 2 shows a 38.6 wt% of reduction of
materials
diverted to mogas production. Furthermore, it can be seen from Table I that
the mogas stream in
Example 2 has a slightly higher octane value of CS+ byproduct than in Example
1 since more
kinetically limited isomerization and aromatization products will be
concentrated into a smaller
byproduct stream.
[00178] Thus, clearly, it may be advantageous to recycle at least part of
the C5-containing
streams to the CPD reactor(s). This is especially beneficial if demand for the
partially converted
C5 hydrocarbons is reduced, e.g., during certain seasons when the RVP spec on
mogas limits the
amount of C5 hydrocarbons that can be blended. This allows the plant to
continue to operate at
desired DCPD product rates with lower quantities of co-products.
Table I
Composition of First Composition of
Total Feed Composition Reactor Hydrocarbon Mogas Blend
(wt%) Effluent at Exit (wt%) (wt%)
Example Example Example
Component 1 2 1 2 1 2
Hydrogen 1.30 1.30 4.51 4.28
Methane 0.00 0.00 1.50 1.34
- 42 -
CA 03004320 2018-05-03
, .
Composition of First Composition of
Total Feed Composition Reactor Hydrocarbon Mogas Blend
(wt%) Effluent at Exit (wt%) (wt%)
Example Example
Example
Component 1 2 1 2 1 2
Ethylene 0.00 0.00 0.11 0.10 - -
_
Ethane 0.00 0.01 0.97 0.87 - -
Propylene 0.00 0.04 0.73 0.70 0.04
0.07
Propane 0.00 0.06 0.75 0.72 0.05
0.08
Isobutane 0.00 0.00 0.00 0.00 0.00
0.00
Isobutylene 0.00 0.03 0.14 0.16 0.03
0.05
_ 1-butene 0.00 0.18 0.73 0.82 0.14
0.27
1,3-butadiene 0.00 , 0.05 0.19 0.22 0.04 0.07
n-butane 0.00 0.22 0.79 0.93 0.17
0.33
_
t-2-butene 0.00 0.23 0.80 0.94 0.18
0.35
c-2-butene 0.00 0.18 0.58 0.70 0.13
0.27
3-methyl-1-butene 0.00 0.02 0.05 0.06 0.01
0.03
1,4-pentadiene 0.00 0.02 0.05 0.05 0.01
0.02
._
Isopentane 0.00 0.10 0.22 0.30 0.06
0.14
-
1-pentene 0.00 1.28 3.99 3.98 1.19
1.95
r
2-methy14-butene 0.00 0.09 0.19 0.26 0.06
0.13
Isoprene 0.00 0.02 0.06 0.08 0.01
0.03
_
n-pentane 98.70 88.73 32.64 30.87
9.81 15.27
t-2-pentene 0.00 2.51 7.72 7.67 2.34
3.81
_
c-2-pentene 0.00 1.41 4.35 4.31 1.32
2.14
_
2-methyl-2-butene 0.00 0.15 0.33 0.46 0.10
0.23
. CPD 0.00 0.17 24.51 25.57 0.08
0.25
t-1,3-pentadiene 0.00 0.91 2.78 2.81 0.82
1.38
-
c-1,3-pentadiene 0.00 0.74 2.28 2.32 0.67
1.12
Cyclopentene 0.00 1.14 3.43 3.51 1.03
1.73
Cyclopentane 0.00 0.18 0.56 0.56 0.17
0.27
Benzene 0.00 0.25 0.72 0.89 0.19
0.37
Toluene 0.00 0.00 0.00 0.00 - -
Meta-xylene 0.00 0.00 0.00 0.00 - ..
DCPD 0.00 0.00 0.00 0.00 - -
_ .
Di-isoprene 0.00 0.00 0.00 0.00 - -
- 43 -
CA 03004320 2018-05-03
Composition of First Composition of
Total Feed Composition Reactor Hydrocarbon Mogas Blend
(wt%) Effluent at Exit (wt%) (wt%)
Example Example Example
Component 1 2 1 2 1 2
Naphthalene 0.00 0.00 2.45 2.55 - -
Methylnaphthalene 0.00 0.000 0.00 0.00 - -
Anthracene 0.00 0.00 1.70 1.77 - -
Pyrene 0.00 0.00 0.19 0.20 - -
TOTAL 100.00 100.00 100.00 100.00 100.00 100.00
Example 3
[00179] In this prophetic example, obtained by simulation, a model third
C5-rich fraction used
as the mogas blend and a corresponding partially hydrogenated mogas component
having the
following compositions in Table II can be obtained:
Table II
Concentration in (wt%)
Components Third CS-rich Fraction Post-Selective Hydrogenation
Propylene 0.22 0.22
Propane 0.27 0.27
Isobutane 0.00 0.00
Isobutylene 0.14 0.14
1-butene 0.75 0.81
1,3-butadiene 0.21 0.00
n-butane 0.91 0.91
t-2-butene 0.95 1.01
c-2-butene 0.72 0.79
3-methyl-1-butene 0.07 0.07
1,4-pentadiene 0.07 0.00
Isopentane 0.34 0.34
1-pentene 6.36 9.11
2-methyl-1-butene 0.30 0.32
Isoprene 0.05 0.00
n-pentane 52.59 52.59
t-2-pentene 12.54 15.21
- 44 -
CA 03004320 2018-05-03
Concentration in (wt%)
Components Third C5-rich Fraction Post-Selective Hydrogenation
c-2-pentene 7.08 9.75
2-methyl-2-butene 0.54 0.56
Cyclopentadiene 0.44 0.00
t-1,3-pentadiene 4.41 0.00
c-1,3-pentadiene 3.61 0.00
Cyclopentene 5.54 5.98
Cyclopentane 0.90 0.90
Benzene 1.01 1.01
C10 (dimers) 0.00 0.00
Total 100.00 100.00
Example 4
[00180] In this example, a first reactor effluent produced from pure n-
pentane feedstock, a pure
hydrogen co-feedstock with 1:1 hydrogen/n-pentane molar ratio, without co-
feeding a light
hydrocarbon or recycling of any down-stream C5-rich fractions to the first
reactor. The process
temperature, pressure, weight hourly space velocity, and molecular weight at
the reactor inlet are
475 C, 62 psia (401.9 kilopascal absolute), a lower weight hourly space
velocity than Example 1
to attain a closer approach to thermodynamic equilibrium, and 49.01 g/mol,
respectively.
Temperature and pressure at the reactor outlet are 575 C and 10 psia (68.9
kilopascal absolute),
respectively. In this example, the reaction system also employs a different
catalyst system from
Example 1. The reactions generate an additional 2.02 moles of molecules in the
first reactor
effluent exiting the outlet per mole of molecules in the total feed material
at the inlet. This
2.02-fold molar expansion has the effects of lowering the molecular weight and
density of the
stream mixture from 36.72 g/mol at the inlet to 18.16 g/mol at the outlet and
from 2.44 kg/m3 at
the inlet to 0.18 kg/m3 at the outlet, respectively. The pressure drop from
the inlet to the outlet of
the first reactor is calculated to be about 52 psi (359 kilopascal).
Composition of the first reactor
effluent at the outlet is given in Table III below.
[00181] The entire third C5-rich fraction is used as a mogas blend for making
mogas, the
composition of the mogas blend is provided in Table III below as well.
- 45 -
CA 03004320 2018-05-03
1001821 In this example, to produce 100 tons of CPD in stream 117, a total
weight of
222 tons of n-pentane feed is fed to the system (representing a total CPD
yield of 45.0 wt%, based
on all weight of the n-pentane feed), a total weight of 18.3 tons of hydrogen
is produced, a total
weight of 54 tons of UHP DCPD with purity level exceeding 99.0 wt% (stream
147) is produced,
a total weight of 44 tons of DCPD with purity level exceeding 90.0 wt% (stream
155) is produced,
and a total weight of 77 tons of mogas blend is produced.
Table III
Composition of First
Total Feed Reactor
Hydrocarbon Composition
Composition Effluent at Exit of Mogas
Component (wt%) (wt%) Blend (wt%)
Hydrogen 2.78 8.01
Methane 0.00 2.02 0.01
Ethylene 0.00 0.11
Ethane 0.00 2.42 0.05
Propylene 0.00 0.98 0.13
Propane 0.00 2.29 0.38
Isobutane 0.00 0.04 0.03
Isobutylene 0.00 0.23 0.17
1-butene 0.00 0.71 0.53
1,3-butadiene 0.00 0.04 0.03
n-butane 0.00 1.63 1.72
t-2-butene 0.00 0.81 0.90
c-2-butene 0.00 0.62 0.79
3-methyl-1- 0.00 0.24 0.60
butene
1,4-pentadiene 0.00 0.14 0.38
Isopentane 0.00 0.54 1.46
1-pentene 0.00 1.69 4.62
2-methyl-1- 0.00 0.93 2.55
butene
Isoprene 0.00 0.43 1.10
n-pentane 97.23 5.35 14.33
t-2-pentene 0.00 4.14 11.37
c-2-pentene 0.00 2.10 5.78
- 46 -
CA 03004320 2018-05-03
Composition of First
Total Feed Reactor
Hydrocarbon Composition
Composition Effluent at Exit of Mogas
Component (wt%) (wt%) Blend (wt%)
2-methyl-2- 0.00 1.49 4.09
butene
CPD 0.00 43.76 1.97
t-1,3-pentadiene 0.00 1.13 2.96
c-1,3-pentadiene 0.00 0.93 2.43
Cyclopentene 0.00 12.21 32.99
Cyclopentane 0.00 3.13 7.97
Benzene 0.00 0.48 0.01
Toluene 0.00 0.38
Meta-xylene 0.00 0.04
DCPD 0.00 0.00
Di-isoprene 0.00 0.00
Naphthalene 0.00 0.87
Methylnaphthalen 0.00 0.07
Anthracene 0.00 0.01
Pyrene 0.00 0.00
TOTAL 100.00 100.00 100.00
Example 5
[00183] In this prophetic example, obtained by simulation, a model third
C5-rich fraction used
as the mogas blend and a corresponding partially hydrogenated mogas component
having the
following compositions in Table IV can be obtained:
- 47 -
CA 03004320 2018-05-03
Table IV
Concentration in (wt%)
Third C5-rich Post-Selective
Components Fraction Hydrogenation
Propylene 0.13 0.08
Propane 0.38 0.26
Isobutane 0.03 0.02
Isobutylene 0.17 0.15
1 -butene 0.53 0.49
1,3-butadiene 0.03 0.00
n-butane 1.72 1.53
t-2-butene 0.90 0.81
c-2-butene 0.79 0.71
3-methyl-1-butene 0.60 0.57
1,4-pentadiene 0.38 0.00
Isopentane 1.46 1.43
1 -pentene 4.62 4.93
2-methyl-1-butene 2.55 3.07
Isoprene 1.10 0.00
n-pentane 14.33 14.30
t-2-pentene 11.37 14.41
c-2-pentene 5.78 8.29
2-methyl-2-butene 4.09 4.64
Cyclopentadiene 1.97 0.08
t-1,3 -pentadi ene 2.96 0.00
c-1,3-pentadiene 2.43 0.00
Cyc 1 opentene 32.99 35.52
Cyclopentane 7.97 8.14
Benzene 0.01 0.01
C I 0 (dimers) 0.00 0.00
Total 100.00 100.00
- 48 -
CA 03004320 2018-05-03
Example 6 ¨ ZSM-5 Catalyst Composition Synthesis
[00184] A synthesis mixture with ¨ 20.3% solids was prepared from 10,000 g of
deionized
(DI) water, 600 g of 50% NaOH solution, 25g of 45% Sodium Aluminate solution,
730 g of
n-propyl amine 100% solution, 80 g of ZSM-5 seed crystals, and 3,190 g of
Ultrasil PMTm.
Modified silica were mixed in a 5-gal pail container and then charged into a 5-
gal autoclave after
mixing. The synthesis mixture had the following molar composition:
SiO2/A1203 ¨ 470
H20/SiO2 ¨ 12.1
OH/SiO2 ¨ 0.16
Na/SiO2 ¨ 0.16
n-PA/Si ¨ 0.25.
[00185] The synthesis mixture was mixed and reacted at 230 F (110 C) at 250
rpm for
72 hours. The resulting product was filtered and washed with DI water and then
dried in the oven
at ¨ 250 F (121 C) overnight. A portion of the as-synthesized crystals were
converted (for
characterization) into the hydrogen form by three ion exchanges with ammonium
nitrate solution
at room temperature, followed by drying at 250 F (121 C) and calcination at
1000 F (540 C) for
6 hours. The resulting ZSM-5 crystals had a 5i02/A1203 molar ratio of 414,
total surface area
(SA)/(micropore SA + mesopore SA) of 490 (440 + 51) m2/g, Hexane sorption of
117 mg/g and
an Alpha value (as measured on the proton form) of 31. A second portion of the
material was
used as synthesized for Pt impregnation.
[00186] ZSM-5 having a SiO2/A1203 molar ratio of 414 and a sodium content of
0.38 wt% was
calcined for 6 hours in nitrogen at 900 F (482 C). After cooling, the sample
was re-heated to
900 F (482 C) in nitrogen and held for three hours. The atmosphere was then
gradually changed
to 1.1, 2.1, 4.2, and 8.4% oxygen in four stepwise increments. Each step was
held for 30 minutes.
The temperature was increased to 1000 F (540 C), the oxygen content was
increased to 16.8%,
and the material was held at 1000 F (540 C) for 6 hours. After cooling, 0.5
wt% Pt was added
via incipient wetness impregnation using an aqueous solution of tetraamine
platinum hydroxide.
The catalyst composition was dried in air at room temperature for 2 hours,
then at 250 F (121 C)
for 4 hours, and lastly calcined in air at 660 F (349 C) for 3 hours. The
catalyst composition
powder was pressed (15 ton), crushed, and sieved to obtain 20-40 mesh particle
size.
- 49 -
CA 03004320 2018-05-03
Example 7 - Catalyst Composition Performance Evaluation
[001871 The above material of Example 6 was evaluated for performance. The
catalyst
composition (0.5 g) was physically mixed with quartz (1.5 g, 60-80 mesh) and
loaded into a
reactor. The catalyst composition was dried for 1 hour under He (100 mL/min,
30 psig (207 kPa),
250 C) then reduced for 1 hour under 1-12 (200 mL/min, 30 psig (207 kPa), 500
C). The catalyst
composition was then tested for performance with feed of n-pentane, H2, and
balance He, typically
at 550 C - 600 C, 5.0 psia (35 kPa-a) C5H12, 1.0 molar H2:C5F112, 14.7 WI
WHSV, and 30 psig
(207 kPa) total. Catalyst composition stability and regenerability was tested
post initial tests at
550 C to 600 C by treatment with H2 (200 mL/min, 30 psig (207 kPa), 650 C) for
5 hours, then
retesting performance at 600 C.
1001881 Cyclopentadiene and three equivalents of hydrogen are produced by
dehydrogenation
and cyclization of n-pentane (Equation 1). This is achieved by flowing n-
pentane over a solid-
state, Pt containing catalyst composition at elevated temperature. The
performance of ZSM-
5(414:1)/0.5%Pt of Example 6 was evaluated based on n-pentane conversion,
cyclic C5 production
(cC5), cracking yields, and stability. These results are summarized in Table
V, Table VI, Table
VII, and Table VIII.
A
C51/12 C5H6 3H2 Equation (1)
Table V
Conversion Selectivity (mol %) Yield (mol
%)
(%)
Temperature C5H12 cC5 CPD Cl C2-4 iC5 cC5 CPD Cl C2-4 iC5 cC5:C1-4
( C)
545 71 33 20 11 21 4.4 24 14 8.1 15 3.1 1.0
570 80 37 26
13 22 3.7 30 21 10 17 3.0 1.1
595 84 40
32 13 22 3.1 34 26 11 18 2.6 1.1
595, Post 76 38 30 16 22 2.4 29 23 12 17
1.8 1.0
H2
- 50 -
CA 03004320 2018-05-03
Table VI
Conversion Selectivity (mol %) Yield (mol %)
(%)
Temperature C5H12 iC5 iC5o iC5= iC5- iC5 iC5o iC5= iC5==
( C)
545 71 4.4 1.1 3.2 0.04 3.1 0.8 2.3
0.03
570 80 3.7 0.8 2.8 0.05 3.0 0.7 2.3
0.04
595 84 3.1 0.7 2.4 0.05 2.6 0.6 2.0
0.05
595, Post 76 2.4 0.6 1.8 0.04 1.8 0.5 1.4
0.03
H2
Table VII
Conversio Selectivity (C %) Yield (C %)
n (%)
Temperature C5H12 cC5 CPD Cl C2- iC cC CF Cl C2- iC cC5:C1-4
( C) 4 5 5 D 4 5
545 71 40 24
2.8 15 5.3 28 17 2. 11 3.7 2.2
0
570 80 45 32
3.1 16 4.5 36 26 2. 13 3.6 2.3
595 84 50 39
3.3 16 3.8 42 33 2. 14 3.2 2.5
8
595, Post H2 76 48 38 4.1 17 3.0 37 29 3. 13 2.3
2.3
1
5 Table VIII
Conversion Selectivity (C %) Yield (C %)
(A)
Temperature C5H12 iC5 iC5o iC5= iC5 == iC5 iC5o iC5= iC5==
( C)
545 71 5.3 1.4 3.8 0.05 3.7 1.0 2.7 0.04
570 80 4.5 1.0 3.5 0.06 3.6 0.8 2.8 0.04
595 84 3.8 0.8 2.9 0.07 3.2 0.7 2.5 0.06
595, Post H2 76 3.0 0.8 2.2 0.05 2.3 0.6 1.7
0.03
[00189] Table V and Table VI show the conversion of n-pentane and selectivity
and yield of
cyclic CS, CPD, iso-Cs, C1, and C2-4 cracking products at varying temperatures
(average values
over 8 hours at each temperature) for a catalyst composition of 0.5 g ZSM-
5(Si:Al2 molar ratio
414:1)/0.5wt%Pt at conditions of 5.0 psia (35 kPa-a) C5K2, 1:1 molar H2:C5,
14.7 WHSV, 45 psia
(310 kPa-a) total. In Table V, the selectivities and yields are expressed on a
molar percentage
-51 -
CA 03004320 2018-05-03
basis for the respective cyclic C5, CPD, iso-05, Cl, and C2-4 of hydrocarbons
formed; i.e., the
molar selectivity is the moles of the respective cyclic C5, CPD, Ci, and C2-4
formed divided by
total moles of pentane converted. In Table VI, the selectivities and yields
are expressed on a
carbon percentage basis for the respective cyclic C5, CPD, iso-05, Cl, and C2-
4 of hydrocarbons
formed; i.e., the carbon selectivity is the moles carbon in the respective
cyclic C5, CPD, iso-05,
Cl, and C2-4 formed divided by total moles of carbon in the pentane converted.
As can be seen,
Table V and Table VI show greater than 80% conversion of pentane, at high
WHSV, and 40%
selectivity to cyclic C5 species at 595 C. While not the specific end product,
cyclopentane and
cyclopentene can be recycled to produce CPD.
t) [00190] Tables VI and VIII further specify the individual iC5 components
which are shown as
totals in Tables V and VII. iC5o is iso pentane; including 2-methyl butane and
3-methyl butane.
iC5= is isopentenes including 2-methyl butene and 3-methyl butene. iC5= is iso-
pentadienes;
including 2-methyl butadiene and 3-methyl butadiene. These results show the
low levels of iso-
pentadienes that are possible with the example catalyst.
[00191] As is apparent from the foregoing general description and the specific
embodiments,
while forms of the invention have been illustrated and described, various
modifications can be
made without departing from the spirit and scope of the invention.
Accordingly, it is not intended
that the invention be limited thereby. Likewise, the term "comprising" is
considered synonymous
with the term "including." Likewise, whenever a composition, an element or a
group of elements
is preceded with the transitional phrase "comprising," it is understood that
we also contemplate
the same composition or group of elements with transitional phrases
"consisting essentially of,"
"consisting of," "selected from the group of consisting of," or "is" preceding
the recitation of the
composition element, or elements and vice versa.
- 52 -