Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
VAGINAL INSERTED ESTRADIOL PHARMACEUTICAL
COMPOSITIONS AND METHODS
CROSS-REFERENCES TO RELATED APPLICATIONS
[0001] The present application claims priority to U.S. Provisional Pat. Appl.
No.
62/264,309, filed December 7, 2015; U.S. Provisional Pat. Appl. No.
62/296,552, filed
February 17, 2016; U.S. Provisional Pat. Appl. No. 62/324,838, filed April 19,
2016; U.S.
Provisional Pat. Appl. No. 62/329,940, filed April 29, 2016; and U.S.
Provisional Pat. Appl.
No. 62/348,820, filed June 10, 2016; which applications are incorporated
herein by reference
in their entirety.
FIELD OF THE INVENTION
[0002] This application is directed to pharmaceutical compositions, methods,
and devices
related to hormone replacement therapy.
BACKGROUND OF THE INVENTION
[0003] Postmenopausal women frequently suffer from atrophic vaginitis or
vulvar and
vaginal atrophy (hereinafter "vulvovaginal atrophy" or "VVA") with symptoms
including,
for example, vaginal dryness, vaginal odor, vaginal or vulvar irritation or
itching, dysuria
(pain, burning, or stinging when urinating), dyspareunia (vaginal pain
associated with sexual
activity), or vaginal bleeding associated with sexual activity. Other symptoms
include
soreness; with urinary frequency and urgency; urinary discomfort and
incontinence also
occurring ("estrogen-deficient urinary state(s)"). One symptom of vaginal
atrophy is an
increased vaginal pH, which creates an environment more susceptible to
infections. The
mucosal epithelium of the VVA patients also reported to show signs of severe
atrophy and
upon cytological examination accompanied by an increased number of the
parabasal cells and
a reduced number of superficial cells.
[0004] Each of these VVA-related states manifest symptoms associated with
decreased
estrogenization of the vulvovaginal tissue, and can even occur in women
treated with oral
administration of an estrogen-based pharmaceutical drug product. Although VVA
is most
common with menopausal women, it can occur at any time in a woman's life
cycle. VVA
symptoms also interfere with sexual activity and satisfaction. Women with
female sexual
dysfunction (FSD) are almost 4 times more likely to have VVA than those
without FSD.
1
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0005] Estrogen treatment has proven to be very successful in controlling
menopausal
symptoms, including VVA and FSD. Several studies have shown that the symptoms
connected with vaginal atrophy are often relieved by estrogen treatment given
either
systemically or topically. The existing treatments have numerous problems, for
example
compliance issues with patients not completing or continuing treatment due to
the problems
associated with the form of treatment.
[0006] Accordingly, there remains a need in the art for treatments for VVA and
FSD that
overcome these limitations.
BRIEF SUMMARY OF THE INVENTION
[0007] Disclosed herein is, among other things, a new soft gel vaginal
pharmaceutical
composition and dosage form containing solubilized estradiol for the treatment
of VVA. The
soft gel vaginal pharmaceutical composition has been designed to mitigate
common
limitations found with other vaginal forms of estradiol. The soft gel vaginal
pharmaceutical
composition eases vaginal administration, provides improved safety of
insertion, minimizes
vaginal discharge following administration, and provides a more effective
dosage form
having improved efficacy, safety and patient compliance.
[0008] According to various aspects and embodiments of this disclosure, a soft
gel vaginal
pharmaceutical composition as a treatment for post-menopausal women suffering
with
moderate to severe symptoms of VVA is provided.
[0009] Provided herein is a suppository comprising: a) a therapeutically
effective amount
of estradiol; and b) a solubilizing agent comprising a medium chain oil.
[0010] In some embodiments, the suppository includes about 1 lig to about 25
lig of
estradiol. For example, the suppository can include about 1 lig to about 10
lig of estradiol;
and about 10 lig to about 25 lig of estradiol.
[0011] In some embodiments, the estradiol is solubilized.
[0012] In some embodiments, the medium chain oil includes at least one C6-C12
fatty acid
or a glycol, monoglyceride, diglyceride, or triglyceride ester thereof
[0013] In some embodiments, the solubilizing agent includes at least one ester
selected
from the group consisting of: an ester of caproic fatty acid, an ester of
caprylic fatty acid, an
2
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
ester of capric fatty acid, and combinations thereof For example, the
solubilizing agent can
include a caprylic/capric triglyceride.
[0014] In some embodiments, the suppository further includes a capsule. For
example, the
capsule can be a soft gelatin capsule.
[0015] Also provided herein is a suppository comprising: a) a therapeutically
effective
amount of estradiol; b) a caprylic / capric triglyceride; c) a non-ionic
surfactant comprising
PEG-6 palmitostearate and ethylene glycol palmitostearate; and d) a soft
gelatin capsule.
[0016] In some embodiments, a suppository provided herein includes about 25
lig of
estradiol, wherein administration of the suppository to a patient provides, in
a plasma sample
from the patient: 1) a corrected geometric mean peak plasma concentration
(Cmax) of estradiol
of about 19 pg*hr/mL to about 29 pg*hr/mL; and 2) a corrected geometric mean
area under
the curve (AUC)0_24 of estradiol of about 75 pg*hr/mL to about 112 pg*hr/mL.
[0017] In some embodiments, a suppository provided herein includes about 25 pg
of
estradiol, wherein administration of the suppository to a patient provides, in
a plasma sample
from the patient: 1) a corrected geometric mean peak plasma concentration
(Cmax) of estrone
of about 9 pg*hr/mL to about 14 pg*hr/mL; and 2) a corrected geometric mean
area under the
curve (AUC)0_24 of estrone of about 43 pg*hr/mL to about 65 pg*hr/mL.
[0018] In some embodiments, a suppository provided herein includes about 25 pg
of
estradiol, wherein administration of the suppository to a patient provides, in
a plasma sample
from the patient: 1) a corrected geometric mean peak plasma concentration
(Cmax) of estrone
sulfate of about 416 pg*hr/mL to about 613 pg*hr/mL; and 2) a corrected
geometric mean
area under the curve (AUC)0_24 of estrone sulfate of about 3598 pg*hr/mL to
about 5291
pg*hr/mL.
[0019] In some embodiments, a suppository provided herein includes about 10 pg
of
estradiol, wherein administration of the suppository to a patient provides, in
a plasma sample
from the patient: 1) a corrected geometric mean peak plasma concentration
(Cmax) of estradiol
of about 12 pg*hr/mL to about 18 pg*hr/mL; and 2) a corrected geometric mean
area under
the curve (AUC)0_24 of estradiol of about 42 pg*hr/mL to about 63 pg*hr/mL. In
some
embodiments, the suppository further provides a corrected geometric mean time
to peak
plasma concentration (Tmax) of estradiol of about 1 hrs to about 3 hrs.
3
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0020] In some embodiments, a suppository provided herein includes about 10
lig of
estradiol, wherein administration of the suppository to a patient provides, in
a plasma sample
from the patient: 1) a corrected geometric mean peak plasma concentration
(Cmax) of estrone
of about 4 pg*hr/mL to about 7 pg*hr/mL; and 2) a corrected geometric mean
area under the
curve (AUC)0_24 of estrone of about 20 pg*hr/mL to about 31 pg*hr/mL. In some
embodiments, the suppository further provides a corrected geometric mean time
to peak
plasma concentration (Tmax) of estrone of about 4 hrs to about 8 hrs.
[0021] In some embodiments, a suppository provided herein includes about 10
lig of
estradiol, wherein administration of the suppository to a patient provides, in
a plasma sample
from the patient: 1) a corrected geometric mean peak plasma concentration
(Cmax) of estrone
sulfate of about 10 pg*hr/mL to about 16 pg*hr/mL; and 2) a corrected
geometric mean area
under the curve (AUC)0_24 of estrone sulfate of about 56 pg*hr/mL to about 84
pg*hr/mL. In
some embodiments, the suppository further provides a corrected geometric mean
time to peak
plasma concentration (Tmax) of estrone sulfate of about 4 hrs to about 7 hrs.
[0022] In some embodiments, a suppository provided herein includes about 4 lig
of
estradiol, wherein administration of the suppository to a patient provides, in
a plasma sample
from the patient: 1) a corrected geometric mean peak plasma concentration
(Cmax) of estradiol
of about 4 pg*hr/mL to about 8 pg*hr/mL; and 2) a corrected geometric mean
area under the
curve (AUC)0_24 of estradiol of about 16 pg*hr/mL to about 26 pg*hr/mL. In
some
embodiments, the suppository further provides a corrected geometric mean time
to peak
plasma concentration (Tmax) of estradiol of about 0.25 hrs to about 2 hrs.
[0023] In some embodiments, a suppository provided herein includes about 4 lig
of
estradiol, wherein administration of the suppository to a patient provides, in
a plasma sample
from the patient: 1) a corrected geometric mean peak plasma concentration
(Cmax) of estrone
of about 1 pg*hr/mL to about 3 pg*hr/mL; and 2) a corrected geometric mean
area under the
curve (AUC)0_24 of estrone of about 8 pg*hr/mL to about 13 pg*hr/mL. In some
embodiments, the suppository further provides a corrected geometric mean time
to peak
plasma concentration (Tmax) of estrone of about 1 hrs to about 4 hrs.
[0024] In some embodiments, a suppository provided herein includes about 4 lig
of
estradiol, wherein administration of the suppository to a patient provides, in
a plasma sample
from the patient: 1) a corrected geometric mean peak plasma concentration
(Cmax) of estrone
sulfate of about 4 pg*hr/mL to about 7 pg*hr/mL; and 2) a corrected geometric
mean area
4
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
under the curve (AUC)0_24 of estrone sulfate of about 22 pg*hr/mL to about 34
pg*hr/mL. In
some embodiments, the suppository further provides a corrected geometric mean
time to peak
plasma concentration (Tmax) of estrone sulfate of about 1 hrs to about 3 hrs.
[0025] Also provided herein is a suppository comprising about 1 [ig to about
25 pg of
estradiol, wherein administration of the suppository to a patient provides a
corrected
geometric mean peak plasma concentration (Cmax) of estradiol that is less than
about 30
pg*hr/mL. For example, administration of the suppository to a patient provides
a corrected
geometric mean peak plasma concentration (Cmax) of estradiol that is less than
about 18
pg*hr/mL.
[0026] In some embodiments, a suppository comprising about 1 [ig to about 25
[ig of
estradiol is provided, wherein administration of the suppository to a patient
provides a
corrected geometric mean area under the curve (AUC)0-24 of estradiol that is
less than about
112 pg*hr/mL. For example, administration of the suppository to a patient
provides a
corrected geometric mean area under the curve (AUC)0-24 of estradiol that is
less than about
63 pg*hr/mL.
[0027] In some embodiments, a suppository comprising about 1 [ig to about 25
[ig of
estradiol is provided, wherein administration of the suppository to a patient
provides a
corrected geometric mean peak plasma concentration (Cmax) of estrone that is
less than about
14 pg*hr/mL. For example, administration of the suppository to a patient
provides a corrected
geometric mean peak plasma concentration (Cmax) of estrone that is less than
about 7
pg*hr/mL.
[0028] In some embodiments, a suppository comprising about 1 [ig to about 25
[ig of
estradiol is provided, wherein administration of the suppository to a patient
provides a
corrected geometric mean area under the curve (AUC)0-24 of estrone that is
less than about
65 pg*hr/mL. For example, administration of the suppository to a patient
provides a corrected
geometric mean area under the curve (AUC)0-24 of estrone that is less than
about 31
pg*hr/mL.
[0029] In some embodiments, a suppository comprising about 1 [ig to about 25
[ig of
estradiol is provided, wherein administration of the suppository to a patient
provides a
corrected geometric mean peak plasma concentration (Cmax) of estrone sulfate
that is less than
about 613 pg*hr/mL. For example, administration of the suppository to a
patient provides a
5
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
corrected geometric mean peak plasma concentration (Cmax) of estrone sulfate
that is less than
about 16 pg*hr/mL.
[0030] In some embodiments, a suppository comprising about 1 pg to about 25 pg
of
estradiol is provided, wherein administration of the suppository to a patient
provides a
corrected geometric mean area under the curve (AUC)0-24 of estrone sulfate
that is less than
about 5291 pg*hr/mL. For example, administration of the suppository to a
patient provides a
corrected geometric mean area under the curve (AUC)0-24 of estrone sulfate
that is less than
about 84 pg*hr/mL.
[0031] Further provided herein is a suppository comprising about 1 pg to about
25 pg of
estradiol, wherein administration of the suppository to the proximal region of
the vagina of a
patient provides a therapeutically effective concentration of estradiol over
24 hours in the
proximal region of the vagina.
[0032] This disclosure also provides a method of treating an estrogen-
deficient state, the
method comprising administering to a patient in need thereof, a suppository as
provided
herein. In some embodiments, a method of treating vulvovaginal atrophy is
provided, the
method comprising administering to a patient in need thereof, a suppository as
provided
herein.
[0033] In some embodiments of the methods provided herein, treatment includes
reducing
the severity of one or more symptoms selected from the group consisting of:
vaginal dryness,
dyspareunia, vaginal or vulvar irritation, vaginal or vulvar burning, vaginal
or vulvar itching,
dysuria, and vaginal bleeding associated with sexual activity.
[0034] In some embodiments of the methods provided herein treatment includes
reducing
the vaginal pH of the patient. For example, treatment includes reducing the
vaginal pH of the
patient to a pH of less than about 5Ø
[0035] In some embodiments of the methods provided herein treatment includes a
change
in cell composition of the patient. For example, the change in cell
composition includes
reducing the number of parabasal vaginal cells or increasing the number of
superficial
vaginal cells. In some embodiments, the number of parabasal vaginal cells in
the patient are
reduced by at least about 35% (e.g., at least about 50%). In some embodiments,
the number
of superficial vaginal cells are increased by at least about 5% (e.g., at
least about 35%).
6
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0036] Further provided herein is a method for reducing vaginal discharge
following
administration of a suppository, the method comprising administering to a
patient in need
thereof, a suppository provided herein, wherein the vaginal discharge
following
administration of the suppository is compared to the vaginal discharge
following
administration of a reference drug.
[0037] Also provided herein is a method for treating female sexual dysfunction
in a female
subject in need thereof The method includes administering to the subject a
vaginal
suppository as described herein. In some embodiments, the method includes
administering to
the subject a vaginal suppository comprising: (a) a pharmaceutical composition
comprising:
a therapeutically effective amount of estradiol; a caprylic/capric
triglyceride; a non-ionic
surfactant comprising PEG-6 palmitostearate and ethylene glycol
palmitostearate; and (b) a
soft gelatin capsule; wherein the vaginal suppository includes from about 1
microgram to
about 25 micrograms of estradiol; wherein estradiol is the only active hormone
in the vaginal
suppository. In some embodiments, the vaginal suppository does not include a
hydrophilic
gel-forming bioadhesive agent in the solubilizing agent. In some embodiments,
treating
female sexual dysfunction includes increasing the subject's desire, arousal,
lubrication,
satisfaction, and or/orgasms.
BRIEF DESCRIPTION OF THE DRAWINGS
[0038] The above-mentioned features and objects of the this disclosure will
become more
apparent with reference to the following description taken in conjunction with
the
accompanying drawings wherein like reference numerals denote like elements and
in which:
[0039] Fig. 1 is a flow diagram illustrating a process in accordance with
various
embodiments of the invention;
[0040] Fig. 2 illustrates a suppository in accordance with various embodiments
of the
invention;
[0041] Fig. 3 is a linear plot of mean plasma estradiol - baseline adjusted
concentrations
versus time (N=34);
[0042] Fig. 4 is a semi-logarithmic plot of mean plasma estradiol - baseline
adjusted
concentrations versus time (N=34);
7
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0043] Fig. 5 is a linear plot of mean plasma estrone - baseline adjusted
concentrations
versus time (N=33);
[0044] Fig. 6 is a semi-logarithmic plot of mean plasma estrone - baseline
adjusted
concentrations versus time (N=33);
[0045] Fig. 7 is a linear plot of mean plasma estrone sulfate - baseline
adjusted
concentrations versus time (N=24); and
[0046] Fig. 8 is a semi-logarithmic plot of mean plasma estrone sulfate -
baseline adjusted
concentrations versus time (N=24).
[0047] Fig. 9 is a study schematic diagram.
[0048] Fig. 10 shows the percentage change in superficial cells at 12 weeks
compared to
placebo.
[0049] Fig. 11 shows the percentage change in superficial cells at week 2,
week 6, week 8,
and week 12 compared to placebo.
[0050] Fig. 12 shows percentage change in superficial cells per dose for each
of week 2,
week 6, week 8, and week 12 compared to placebo.
[0051] Fig. 13 shows the percentage change in parabasal cells at 12 weeks
compared to
placebo.
[0052] Fig. 14 shows the percentage change in parabasal cells at week 2, week
6, week 8,
and week 12 compared to placebo.
[0053] Fig. 15 shows the percentage change in parabasal cells per dose for
each of week 2,
week 6, week 8, and week 12 compared to placebo
[0054] Fig. 16 shows the percentage change in pH at 12 weeks compared to
placebo.
[0055] Fig. 17 shows the percentage change in pH at week 2, week 6, week 8,
and week 12
compared to placebo.
[0056] Fig. 18 shows the percentage change in pH per dose for each of week 2,
week 6,
week 8, and week 12 compared to placebo.
[0057] Fig. 19A shows the change in visual assessments from baseline to week
12 in
vaginal color in a modified itent to treat (MITT) population.
8
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0058] Fig. 19B shows the change in visual assessments from baseline to week
12 in
vaginal epithelial integrity in a modified itent to treat (MITT) population.
[0059] Fig. 19C shows the change in visual assessments from baseline to week
12 in
vaginal epithelial thickness a modified itent to treat (MITT) population.
[0060] Fig. 19D shows the change in visual assessments from baseline to week
12 in
vaginal secretions in a modified itent to treat (MITT) population.
[0061] Fig. 20A shows the correlation between the total sum of four visual
assessments and
dyspareunia at week 12 in an intent to treat (ITT) population.
[0062] Fig. 20B shows the correlation between the total sum of four visual
assessments and
vaginal dryness at week 12 in an intent to treat (ITT) population.
[0063] Fig. 21 shows baseline adjusted estradiol serum concentration (pg/mL)
assessed on
Day 1 (squares) and Week 12 (diamonds) for four treatment artms.
[0064] Fig. 22 shows baseline adjusted estradiol serum concentration (pg/mL)
assessed on
Day 14 (squares) and Week 12 (diamonds) for four treatment artms.
[0065] Fig. 23 shows estradiol plasma levels measured in subjects following a
supine
period after administration of the estradiol formulation, compared with plasma
levels
measured in subjects following an ambulatory period after administration of
the estradiol
formulation.
[0066] Fig. 24 shows mean change from baseline in Total FSFI score at Week 12.
[0067] Fig. 25A shows the mean change from baseline to week 12 in the
individual FSFI
lubrication score.
[0068] Fig. 25B shows the mean change from baseline to week 12 in the
individual FSFI
arousal score.
[0069] Fig. 25C shows the mean change from baseline to week 12 in the
individual FSFI
satisfaction score.
[0070] Fig. 25D shows the mean change from baseline to week 12 in the
individual FSFI
desire score.
[0071] Fig. 25E shows the mean change from baseline to week 12 in the
individual FSFI
orgasm score.
9
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
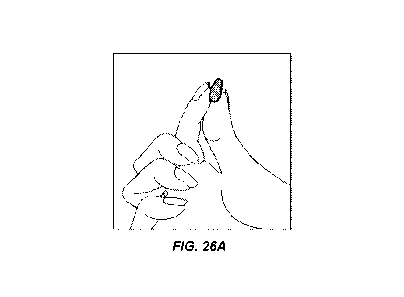
[0072] Fig. 26A shows an estradiol softgel capsule held with the larger end
between the
fingers.
[0073] Fig. 26B shows insertion of an estradiol softgel capsule in a reclining
position. The
softgel is inserted into the lower third of the vagina with the smaller end
up.
[0074] Fig. 26C shows insertion of an estradiol softgel capsule in a standing
position. The
softgel is inserted into the lower third of the vagina with the smaller end
up.
DETAILED DESCRIPTION OF THE INVENTION
[0075] In the following detailed description of embodiments of this
disclosure, reference is
made to the accompanying drawings in which like references indicate similar
elements, and
in which is shown by way of illustration specific embodiments in which this
disclosure may
be practiced. These embodiments are described in sufficient detail to enable
those skilled in
the art to practice this disclosure, and it is to be understood that other
embodiments may be
utilized and that other changes may be made without departing from the scope
of the this
disclosure. The following detailed description is, therefore, not to be taken
in a limiting sense,
and the scope of this disclosure is defined only by the appended claims. As
used in this
disclosure, the term "or" shall be understood to be defined as a logical
disjunction (i.e.,
and/or) and shall not indicate an exclusive disjunction unless expressly
indicated as such with
the terms "either," "unless," "alternatively," and words of similar effect.
I. Definitions
[0076] The term "active pharmaceutical ingredient" ("API") as used herein,
means the
active compound(s) used in formulating a drug product.
[0077] The term "co-administered" as used herein, means that two or more drug
products
are administered simultaneously or sequentially on the same or different days.
[0078] The term "drug product" as used herein means at least one active
pharmaceutical
ingredient in combination with at least one excipient and provided in unit
dosage form.
[0079] The term "area under the curve" ("AUC") refers to the area under the
curve defined
by changes in the blood concentration of an active pharmaceutical ingredient
(e.g., estradiol
or progesterone), or a metabolite of the active pharmaceutical ingredient,
over time following
the administration of a dose of the active pharmaceutical ingredient. "AUCo_."
is the area
under the concentration-time curve extrapolated to infinity following the
administration of a
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
dose. "AUCo_t" is the area under the concentration-time curve from time zero
to time t
following the administration of a dose, wherein t is the last time point with
a measurable
concentration.
[0080] The term "Cmax" refers to the maximum value of blood concentration
shown on the
curve that represents changes in blood concentrations of an active
pharmaceutical ingredient
(e.g., progesterone or estradiol), or a metabolite of the active
pharmaceutical ingredient, over
time.
[0081] The term "Tmax" refers to the time that it takes for the blood
concentration an active
pharmaceutical ingredient (e.g., estradiol or progesterone), or a metabolite
of the active
pharmaceutical ingredient, to reach the maximum value.
[0082] The term "bioavailability," which has the meaning defined in 21 C.F.R.
320.1(a),
refers to the rate and extent to which an API or active ingredient or active
moiety is absorbed
from a drug product and becomes available at the site of action. For example,
bioavailability
can be measured as the amount of API in the blood (serum or plasma) as a
function of time.
Pharmacokinetic (PK) parameters such as AUC, C., or T. may be used to measure
and
assess bioavailability. For drug products that are not intended to be absorbed
into the
bloodstream, bioavailability may be assessed by measurements intended to
reflect the rate
and extent to which the API or active ingredient or active moiety becomes
available at the
site of action.
[0083] The term "bioequivalent," which has the meaning defined in 21 C.F.R.
320.1(e),
refers to the absence of a significant difference in the rate and extent to
which the API or
active ingredient or active moiety in pharmaceutical equivalents or
pharmaceutical
alternatives becomes available at the site of drug action when administered at
the same molar
dose under similar conditions in an appropriately designed study. Where there
is an
intentional difference in rate (e.g., in certain extended release dosage
forms), certain
pharmaceutical equivalents or alternatives may be considered bioequivalent if
there is no
significant difference in the extent to which the active ingredient or moiety
from each product
becomes available at the site of drug action. This applies only if the
difference in the rate at
which the active ingredient or moiety becomes available at the site of drug
action is
intentional and is reflected in the proposed labeling, is not essential to the
attainment of
effective body drug concentrations on chronic use, and is considered medically
insignificant
11
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
for the drug. In practice, two products are considered bioequivalent if the
90% confidence
interval of the AUC, Cmax, or optionally Tmax is within 80.00% to 125.00%.
[0084] The term "bio-identical," "body-identical," or "natural" used in
conjunction with the
hormones disclosed herein, means hormones that match the chemical structure
and effect of
those that occur naturally or endogenously in the human body. An exemplary
natural estrogen
is estradiol.
[0085] The term "bio-identical hormone" or "body-identical hormone" refers to
an active
pharmaceutical ingredient that is structurally identical to a hormone
naturally or
endogenously found in the human body (e.g., estradiol and progesterone).
[0086] The term "estradiol" refers to (170)-estra-1,3,5(10)-triene-3,17-dio1.
Estradiol is
also interchangeably called 170-estradio1, oestradiol, or E2, and is found
endogenously in the
human body. As used herein, estradiol refers to the bio-identical or body-
identical form of
estradiol found in the human body having the structure:
f.--- õ---t\
H
/1
, ... . _
H 1 H
Ho, ......- .......-
[0087] Estradiol is supplied in an anhydrous or hemi-hydrate form. For the
purposes of this
disclosure, the anhydrous form or the hemihydrate form can be substituted for
the other by
accounting for the water or lack of water according to well-known and
understood
techniques.
[0088] The term "solubilized estradiol" means that the estradiol or a portion
thereof is
solubilized or dissolved in the solubilizing agent(s) or the formulations
disclosed herein.
Solubilized estradiol may include estradiol that is about 80% solubilized,
about 85%
solubilized, about 90% solubilized, about 95% solubilized, about 96%
solubilized, about 97%
solubilized, about 98% solubilized, about 99% solubilized or about 100%
solubilized. In
some embodiments, the estradiol is "fully solubilized" with all or
substantially all of the
estradiol being solubilized or dissolved in the solubilizing agent. Fully
solubilized estradiol
may include estradiol that is about 97% solubilized, about 98% solubilized,
about 99%
solubilized or about 100% solubilized. Solubility can be expressed as a mass
fraction (%w/w,
which is also referred to as wt%).
12
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0089] The term "progesterone" refers to pregn-4-ene-3,20-dione. Progesterone
is also
interchangeably called P4 and is found endogenously in the human body. As used
herein,
progesterone refers to the bio-identical or body-identical form of
progesterone found in the
human body having the structure:
Fi A
j
[0090] The term "solubilized progesterone" means that the progesterone or a
portion
thereof is solubilized or dissolved in the solubilizing agent(s) or the
formulations disclosed
herein. In some embodiments, the progesterone is "partially solubilized" with
a portion of the
progesterone being solubilized or dissolved in the solubilizing agent and a
portion of the
progesterone being suspended in the solubilizing agent. Partially solubilized
progesterone
may include progesterone that is about 1% solubilized, about 5% solubilized,
about 10%
solubilized, about 15% solubilized, about 20% solubilized, about 30%
solubilized, about 40%
solubilized, about 50% solubilized, about 60% solubilized, about 70%
solubilized, about 80%
solubilized, about 85% solubilized, about 90% solubilized or about 95%
solubilized. In other
embodiments, the progesterone is "fully solubilized" with all or substantially
all of the
progesterone being solubilized or dissolved in the solubilizing agent. Fully
solubilized
progesterone may include progesterone that is about 97% solubilized, about 98%
solubilized,
about 99% solubilized or about 100% solubilized. Solubility can be expressed
as a mass
fraction (%w/w, which is also referred to as wt%).
[0091] The terms "micronized progesterone" and "micronized estradiol," as used
herein,
include micronized progesterone and micronized estradiol having an X50
particle size value
below about 15 microns or having an X90 particle size value below about 25
microns. The
term "X50" means that one-half of the particles in a sample are smaller in
diameter than a
given number. For example, micronized progesterone having an X50 of 5 microns
means
that, for a given sample of micronized progesterone, one-half of the particles
have a diameter
of less than 5 microns. Similarly, the term "X90" means that ninety percent
(90%) of the
particles in a sample are smaller in diameter than a given number.
13
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0092] The term "glyceride" is an ester of glycerol (1,2,3-propanetriol) with
acyl radicals
of fatty acids and is also known as an acylglycerol. If only one position of
the glycerol
molecule is esterified with a fatty acid, a "monoglyceride" or
"monoacylglycerol" is
produced; if two positions are esterified, a "diglyceride" or "diacylglycerol"
is produced; and
if all three positions of the glycerol are esterified with fatty acids, a
"triglyceride" or
"triacylglycerol" is produced. A glyceride is "simple" if all esterified
positions contain the
same fatty acid; whereas a glyceride is "mixed" if the esterified positions
contained different
fatty acids. The carbons of the glycerol backbone are designated sn-1, sn-2
and sn-3, with sn-
2 being in the middle carbon and sn-1 and sn-3 being the end carbons of the
glycerol
backbone.
[0093] The term "solubilizing agent" refers to an agent or combination of
agents that
solubilize an active pharmaceutical ingredient (e.g., estradiol or
progesterone). For example
and without limitation, suitable solubilizing agents include medium chain oils
and other
solvents and co-solvents that solubilize or dissolve an active pharmaceutical
ingredient to a
desirable extent. Solubilizing agents suitable for use in the formulations
disclosed herein are
pharmaceutical grade solubilizing agents (e.g., pharmaceutical grade medium
chain oils). It
will be understood by those of skill in the art that other excipients or
components can be
added to or mixed with the solubilizing agent to enhance the properties or
performance of the
solubilizing agent or resulting formulation. Examples of such excipients
include, but are not
limited to, surfactants, emulsifiers, thickeners, colorants, flavoring agents,
etc. In some
embodiments, the solubilizing agent is a medium chain oil and, in some other
embodiments,
the medium chain oil is combined with a co-solvent(s) or other excipient(s).
[0094] The term "medium chain" is used to describe the aliphatic chain length
of fatty acid
containing molecules. "Medium chain" specifically refers to fatty acids, fatty
acid esters, or
fatty acid derivatives that contain fatty acid aliphatic tails or carbon
chains that contain 6 (C6)
to 14 (C14) carbon atoms, 8 (C8) to 12 (C12) carbon atoms, or 8 (C8) to 10
(C10) carbon
atoms.
[0095] The terms "medium chain fatty acid" and "medium chain fatty acid
derivative" are
used to describe fatty acids or fatty acid derivatives with aliphatic tails
(i.e., carbon chains)
having 6 to 14 carbon atoms. Fatty acids consist of an unbranched or branched
aliphatic tail
attached to a carboxylic acid functional group. Fatty acid derivatives
include, for example,
fatty acid esters and fatty acid containing molecules, including, without
limitation, mono-, di-
14
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
and triglycerides that include components derived from fatty acids. Fatty acid
derivatives also
include fatty acid esters of ethylene or propylene glycol. The aliphatic tails
can be saturated
or unsaturated (i.e., having one or more double bonds between carbon atoms).
In some
embodiments, the aliphatic tails are saturated (i.e., no double bonds between
carbon atoms).
Medium chain fatty acids or medium chain fatty acid derivatives include those
with aliphatic
tails having 6-14 carbons, including those that are C6-C14, C6-C12, C8-C14, C8-
C12, C6-
C10, C8-C10, or others. Examples of medium chain fatty acids include, without
limitation,
caproic acid, caprylic acid, capric acid, lauric acid, myristic acid, and
derivatives thereof
[0096] The term "oil," as used herein, refers to any pharmaceutically
acceptable oil,
especially medium chain oils, and specifically excluding peanut oil, that can
suspend or
solubilize bioidentical progesterone or estradiol, including starting
materials or precursors
thereof, including micronized progesterone or micronized estradiol as
described herein.
[0097] The term "medium chain oil" refers to an oil wherein the composition of
the fatty
acid fraction of the oil is substantially medium chain (i.e., C6 to C14) fatty
acids, i.e., the
composition profile of fatty acids in the oil is substantially medium chain.
As used herein,
"substantially" means that between 20% and 100% (inclusive of the upper and
lower limits)
of the fatty acid fraction of the oil is made up of medium chain fatty acids,
i.e., fatty acids
with aliphatic tails (i.e., carbon chains) having 6 to 14 carbons. In some
embodiments, about
25%, about 30%, about 35%, about 40%, about 45%, about 50%, about 55%, about
60%,
about 65%, about 70%, about 75%, about 85%, about 90% or about 95% of the
fatty acid
fraction of the oil is made up of medium chain fatty acids. As used herein,
"predominantly"
means that greater than or equal to 50% of the fatty acid fraction of the oil
is made up of
medium-chain fatty acids, i.e., fatty acids with aliphatic carbon chains
having 6 to 14 carbon
atoms. Those of skill in the art that will readily appreciate that the terms
"alkyl content" or
"alkyl distribution" of an oil can be used in place of the term "fatty acid
fraction" of an oil in
characterizing a given oil or solubilizing agent, and these terms are used
interchangeable
herein. As such, medium chain oils suitable for use in the formulations
disclosed herein
include medium chain oils wherein the fatty acid fraction of the oil is
substantially medium
chain fatty acids, or medium chain oils wherein the alkyl content or alkyl
distribution of the
oil is substantially medium chain alkyls (C6-C12 alkyls). It will be
understood by those of
skill in the art that the medium chain oils suitable for use in the
formulations disclosed herein
are pharmaceutical grade (e.g., pharmaceutical grade medium chain oils).
Examples of
medium chain oils include, for example and without limitation, medium chain
fatty acids,
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
medium chain fatty acid esters of glycerol (e.g., for example, mono-, di-, and
triglycerides),
medium chain fatty acid esters of propylene glycol, medium chain fatty acid
derivatives of
polyethylene glycol, and combinations thereof
[0098] The term "ECN" or "equivalent carbon number" means the sum of the
number of
carbon atoms in the fatty acid chains of an oil, and can be used to
characterize an oil as, for
example, a medium chain oil or a long-chain oil. For example, tripalmitin
(tripalmitic
glycerol), which is a simple triglyceride containing three fatty acid chains
of 16 carbon
atoms, has an ECN of 3 xl6=48. Conversely, a triglyceride with an ECN=40 may
have
"mixed" fatty acid chain lengths of 8, 16 and 16; 10, 14 and 16; 8, 14 and 18;
etc. Naturally
occurring oils are frequently "mixed" with respect to specific fatty acids,
but tend not to
contain both long chain fatty acids and medium chain fatty acids in the same
glycerol
backbone. Thus, triglycerides with ECN's of 21-42 typically contain
predominantly medium
chain fatty acids; while triglycerides with ECN's of greater than 43 typically
contain
predominantly long chain fatty acids. For example, the ECN of corn oil
triglyceride in the
USP would be in the range of 51-54. Medium chain diglycerides with ECN's of 12-
28 will
often contain predominanty medium chain fatty chains, while diglycerides with
ECN's of 32
or greater will typically contain predominanty long chain fatty acid tails.
Monoglycerides will
have an ECN that matches the chain length of the sole fatty acid chain. Thus,
monoglyceride
ECN's in the range of 6-14 contain mainly medium chain fatty acids, and
monoglycerides
with ECN's 16 or greater will contain mainly long chain fatty acids.
[0099] The average ECN of a medium chain triglyceride oil is typically 21-42.
For
example, as listed in the US Pharmacopeia (USP), medium chain triglycerides
have the
following composition as the exemplary oil set forth in the table below:
Fatty-acid Tail Length % of oil Exemplary Oil
6 <2.0 2.0
8 50.0-80.0 70.0
10 20.0-50.0 25.0
12 <3.0 2.0
14 <1.0 1.0
and would have an average ECN of 3*[(6*0.02) + (8*0.70) + (10*0.25) +
(12*0.02) +
(14*0.01)1 = 25.8. The ECN of the exemplary medium chain triglycerides oil can
also be
expressed as a range (per the ranges set forth in the USP) of 24.9 ¨ 27Ø For
oils that have
mixed mono-, di-, and triglycerides, or single and double fatty acid glycols,
the ECN of the
16
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
entire oil can be determined by calculating the ECN of each individual
component (e.g., C8
monoglycerides, C8 diglycerides, C10 monoglycerides, and C10 monoglycerides)
and taking
the sum of the relative percentage of the component multiplied by the ECN
normalized to a
monoglyceride for each component. For example, the oil having C8 and C10 mono-
and
diglycerides shown in the table below has an ECN of 8.3, and is thus a medium
chain oil.
Fatty-acid Chain % of oil ECN as % of oil ECN as % of oil
Length (chain length) x (% in normalized to
oil) monoglyceride
C8 monoglyceride 47 8 x 0.47 = 3.76 3.76
Cio monoglyceride 8 10 x 0.08 = 0.8 0.8
C8 diglyceride 38 2 x (8 x 0.38) = 6.08 6.08/2 = 3.04
Cio diglyceride 7 2 X (10 X 0.07) = 1.4 1.4/2 = 0.7
OIL ECN 8.3
(normalized to
monoglycerides)
[0100] Expressed differently, ECN can be calculated as each chain length in
the
composition multiplied by its relative percentage in the oil: (8 * .85) + (10
* .15) = 8.3.
[0101] The term "excipients," as used herein, refers to non-API ingredients
such as
solubilizing agents, anti-oxidants, oils, lubricants, and others used in
formulating
pharmaceutical products.
[0102] The term "patient" or "subject" refers to an individual to whom the
pharmaceutical
composition is administered.
[0103] The term "pharmaceutical composition" refers to a pharmaceutical
composition
comprising at least a solubilizing agent and estradiol. As used herein,
pharmaceutical
compositions are delivered, for example via suppository (i.e., vaginal
suppository), or
absorbed vaginally.
[0104] The term "progestin" means any natural or man-made substance that has
pharmacological properties similar to progesterone.
[0105] The terms "treat," "treating," and "treatment" refer to any indicia of
success in the
treatment or amelioration of an injury, disease, or condition, including any
objective or
subjective parameter such as abatement; remission; diminishing of symptoms or
making the
injury, disease, or condition more tolerable to the patient; slowing in the
rate of degeneration
or decline; or improving a patient's physical or mental well-being. The
treatment or
17
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
amelioration of symptoms can be based on objective or subject parameters,
including the
results of a physical examination, neuropsychiatric examinations, or
psychiatric evaluation.
[0106] The terms "atrophic vaginitis," "vulvovaginal atrophy," "vaginal
atrophy," and
"VVA" are used herein interchangeably. The molecular morphology of VVA is well
known
in the medical field.
[0107] As used herein, "sexual dysfunction" refers to a condition having one
or more
symptoms of difficulty during any one or more stages. The dysfunction can
prevent an
individual from enjoying sexual activity. Non-limiting examples of symptoms of
sexual
dysfunction include: reduced sexual desire, reduced sexual pleasure, reduced
sexual arousal
and excitement, aversion to and avoidance of genital sexual contact, inability
to attain or
maintain arousal, and persistent or recurrent delay of, or absence of orgasm.
Sexual
dysfunction may be lifelong (no effective performance ever) or acquired (after
a period of
normal function); generalized or limited to certain situations or certain
partners; and total or
partial.
[0108] As used herein, "sexual desire" refers to the frequency of wanting to
engage in
sexual activity and/or the frequency of engaging in sexual activity as
perceived by the
individual. Sexual desire can be expressed, for example, in one or more
cognitive activities,
including the frequency of sexual thoughts, the extent of enjoyment of movies,
books, music,
etc. having sexual content and/or the extent of enjoyment or pleasure of
thinking and
fantasizing about sex as perceived by the individual.
[0109] As used herein, "sexual arousal" refers to the frequency of becoming
sexually
aroused, how readily sexual arousal occurs and/or if arousal is maintained, as
perceived by
the individual. Psychologically, arousal can include factors such as increased
desire for
sexual activity and excitement related to sexual activity. Physiologically,
arousal can include
increased blood flow to the genitals, causing clitoral engorgement, as well as
vaginal
lubrication.
[0110] As used herein, "lubrication" refers to wetness in and around the
vagina before,
during, or after sexual activity. Increasing lubrication can include
increasing the frequency of
lubrication; decreasing the difficulty of becoming lubricated; and/or
decreasing the difficulty
in maintaining lubrication.
18
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0111] As used herein, "satisfaction" refers to one or more positive emotions
(e.g.,
contentment, fulfillment, gratification, and the like) related to a sexual
activity or sexual
relationship. Satisfaction can include, for example, satisfaction with
occurrence of sexual
arousal or orgasm, satisfaction with the amount of closeness with a partner,
and satisfaction
with overall sex life.
[0112] As used herein, "orgasm" refers to the highest point of sexual
excitement
characterized by a subjective experience of intense pleasure marked normally
by vaginal
contractions in females. Increasing orgasm can include increasing the
frequency, duration,
and/or intensity of orgasms in a subject. Increasing orgasm can also include
decreasing the
difficulty of reaching orgasm.
11. Introduction
[0113] Provided herein are pharmaceutical compositions comprising solubilized
estradiol
designed to be absorbed vaginally. The pharmaceutical compositions disclosed
herein are
designed to be absorbed and have their therapeutic effect locally, e.g., in
vaginal or
surrounding tissue. Further disclosed herein are data demonstrating efficacy
of the
pharmaceutical compositions disclosed, as well as methods relating to the
pharmaceutical
compositions. Generally, the pharmaceutical compositions disclosed herein are
useful in
VVA, dyspareunia, and other indications caused by decrease or lack of
estrogen.
[0114] Additional aspects and embodiments of this disclosure include:
providing increased
patient ease of use while potentially minimizing certain side effects from
inappropriate
insertion, minimizing incidence of vulvovaginal mycotic infection compared to
incidence of
vulvovaginal mycotic infection due to usage of other vaginally applied
estradiol products;
and, improved side effect profile (e.g., pruritus) compared to, for example,
VAGIFEM
(estradiol vaginal tablets, Novo Nordisk; Princeton, NJ).
III. Pharmaceutical compositions
Functionality
[0115] According to embodiments, the pharmaceutical compositions disclosed
herein are
alcohol-free or substantially alcohol-free. The pharmaceutical compositions
offer provide for
improved patient compliance because of improvements over the prior offering.
According to
embodiments, the pharmaceutical compositions disclosed herein are encapsulated
in soft
gelatin capsules, which improve comfort during use. According to embodiments,
the
19
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
pharmaceutical compositions are substantially liquid, which are more readily
absorbed in the
vaginal tissue, and also are dispersed over a larger surface area of the
vaginal tissue.
Estradiol
[0116] According to embodiments, the pharmaceutical compositions disclosed
herein are
for vaginal insertion in a single or multiple unit dosage form. According to
embodiments, the
estradiol in the pharmaceutical compositions is at least about: 75%, 76%, 77%,
78%, 79%,
80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%,
95%,
96%, 97%, 98%, 99%, or 100% solubilized. According to embodiments and where
the
estradiol is not 100% solubilized, the remaining estradiol is present in a
micronized
(crystalline) form that is absorbable by the body and retains biological
functionality, either in
its micronized form or in another form which the micronized form is converted
to after
administration.
[0117] According to embodiments, all or some of the estradiol is solubilized
in a
solubilizing agent during manufacturing process. According to embodiments, all
or some of
the estradiol is solubilized following administration (e.g., the micronized
portion where the
estradiol is not 100% solubilized is solubilized in a body fluid after
administration).
According to embodiments, because the estradiol is solubilized, the
solubilizing agents taught
herein, with or without additional excipients other than the solubilizing
agents, are liquid or
semi-solid. To the extent the estradiol is not fully solubilized at the time
of
administration/insertion, the estradiol should be substantially solubilized at
a body
temperature (average of 37 C) and, generally, at the pH of the vagina (ranges
from 3.8 to 4.5
in healthy patients; or 4.6 to 6.5 in VVA patients).
[0118] According to embodiments, the estradiol can be added to the
pharmaceutical
compositions disclosed herein as estradiol, estradiol hemihydrate, or other
grade estradiol
forms used in pharmaceutical compositions or formulations.
[0119] According to embodiments, estradiol dosage strengths vary. Estradiol
(or estradiol
hemihydrate, for example, to the extent the water content of the estradiol
hemihydrate is
accounted for) dosage strength of is from at least about 1 microgram (lig or
[tg) to at least
about 50 [tg. Specific dosage embodiments contain at least about: 1 [tg, 2
[tg, 3 [tg, 4 [tg, 5
[tg, 6 [tg, 7 [tg, 8 [tg, 9 [tg, 10 [tg, 11 [tg, 12 [tg, 13 [tg, 14 [tg, 15
[tg, 16 [tg, 17 [tg, 18 [tg, 19
[tg, 20 [tg, 21 [tg, 22 [tg, 23 [tg, 24 [tg, 25 [tg, 26 [tg, 27 [tg, 28 [tg,
29 [tg, 30 [tg, 31 [tg, 32
[tg, 33 [tg, 34 [tg, 35 [tg, 36 [tg, 37 [tg, 38 [tg, 39 [tg, 40 [tg, 41 [tg,
42 [tg, 43 [tg, 44 [tg, 45
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
lig, 46 lig, 47 lig, 48 lig, 49 lig, or 50 lig estradiol. According to
embodiments, the
pharmaceutical compositions contain at least about 2.5 lig; 4 lig 6.25 lig,
7.5 lig, 12.5 lig,
18.75 lig of estradiol. According to embodiments, the pharmaceutical
compositions contain
from about 1 lig to about 10 lig, from 3 lig to 7 lig, from about 7.5 lig to
12.5 lig, from about
10 lig to about 25 lig, about 1 lig, about 2.5 lig, from about 23.5 lig to
27.5 lig, from about
7.5 lig to 22.5 lig, from 10 lig to 25 lig of estradiol. The lowest clinically
effective dose of
estradiol is used for treatment of VVA and other indications set forth herein.
In some
embodiments, the estradiol dosage is about 4 lig. In one embodiment, the
estradiol dosage is
about 10 lig. In another embodiment, the estradiol dosage is about 25 lig.
Solvent System
[0120] According to embodiments, the solvent system that solubilizes the
estradiol are
medium chain fatty acid based solvents, together with other excipients.
According to
embodiments, the solvent system includes non-toxic, pharmaceutically
acceptable solvents,
co-solvents, surfactants, and other excipients suitable for vaginal delivery
or absorption.
[0121] According to embodiments, oils having medium chain fatty acids as a
majority
component are used as solubilizing agents to solubilize estradiol. According
to embodiments,
the solubilizing agents comprise medium chain fatty acid esters (e.g., esters
of glycerol,
ethylene glycol, or propylene glycol) or mixtures thereof According to
embodiments, the
medium chain fatty acids comprise chain lengths from C6 to C14. According to
embodiments
the medium chain fatty acids comprise chain lengths from C6 to C12. According
to
embodiments the medium chain fatty acids substantially comprise chain lengths
from C8-
C10. ECN's for medium chain oils will be in the range of 21-42 for
triglycerides, 12-28 for
diglycerides, and 6-14 for monoglycerides.
[0122] According to embodiments, the medium chain fatty acids are saturated.
According
to embodiments, the medium chain fatty acids are predominantly saturated,
i.e., greater than
about 60% or greater than about 75% saturated.
[0123] According to embodiments, estradiol is soluble in the solubilizing
agent at room
temperature, although it may be desirable to warm certain solubilizing agents
during
manufacture to improve viscosity. According to embodiments, the solubilizing
agent is liquid
at between room temperature and about 50 C, at or below 50 C, at or below 40
C, or at or
below 30 C.
21
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0124] According to embodiments, the solubility of estradiol in the medium
chain oil,
medium chain fatty acid, or solubilizing agent (or oil/surfactant) is at least
about 0.01 wt%,
0.02 wt%, 0.05 wt%, 0.06 wt%, 0.08 wt%, 0.1 wt%, 0.2 wt%, 0.3 wt%, 0.4 wt%,
0.5 wt%,
0.6 wt%, 0.7 wt%, 0.8 wt%, 0.9 wt%, 1.0 wt%, or higher.
[0125] According to embodiments, medium chain solubilizing agents include, for
example
and without limitation saturated medium chain fatty acids: caproic acid (C6),
enanthic acid
(C7), caprylic acid (C8), pelargonic acid (C9), capric acid (C10), undecylic
acid(C11), lauric
acid (C12), tridecylic acid (C13), or myristic acid (C14). According to
embodiments, the
solubilizing agent includes oils made of these free medium chain fatty acids,
oils of medium
chain fatty acid esters of glycerin, propylene glycol, or ethylene glycol, or
combinations
thereof These examples comprise predominantly saturated medium chain fatty
acids (i.e.,
greater than 50% of the fatty acids are medium chain saturated fatty acids).
According to
embodiments, predominantly C6 to C12 saturated fatty acids are contemplated.
According to
embodiments, the solubilizing agent is selected from at least one of a solvent
or co-solvent.
[0126] According to embodiments, glycerin based solubilizing agents include:
mono-, di-,
or triglycerides and combinations and derivatives thereof Exemplary glycerin
based
solubilizing agents include MIGLYOLs , which are caprylic/capric triglycerides
(SASOL
Germany GMBH, Hamburg). MIGLYOLs includes MIGLYOL 810 (caprylic/capric
triglyceride), MIGLYOL 812 (caprylic/capric triglyceride), MIGLYOL 816
(caprylic/capric
triglyceride), and MIGLYOL 829 (capryliecapric/succinic triglyceride). Other
caprylic/capric triglyceride solubilizing agents are likewise contemplated,
including, for
example: caproiecaprylic/capric/lauric triglycerides; caprylic/capric/linoleic
triglycerides;
caprylic/capric/succinic triglycerides. According to embodiments, CAPMUL MCM,
medium
chain mono- and di-glycerides, is the solubilizing agent. Other and
triglycerides of
fractionated vegetable fatty acids, and combinations or derivatives thereof
can be the
solubilizing agent, according to embodiments. For example, the solubilizing
agent can be
1,2,3-propanetriol (glycerol, glycerin, glycerine) esters of saturated coconut
and palm kernel
oil and derivatives thereof
[0127] Ethylene and propylene glycols (which include polyethylene and
polypropylene
glycols) solubilizing agents include: glyceryl mono- and di-caprylates;
propylene glycol
monocaprylate (e.g., CAPMUL PG-8 (the CAPMUL brands are owned by ABITEC,
Columbus, Ohio)); propylene glycol monocaprate (e.g., CAPMUL PG-10); propylene
glycol
22
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
mono- and dicaprylates; propylene glycol mono- and dicaprate; diethylene
glycol mono ester
(e.g., TRANSCUTOL , 2-(2-ethoxyethoxy)ethanol, GATTEFOSSE SAS); and diethylene
glycol monoethyl ether. Other combinations of mono- and di- esters of
propylene glycol or
ethylene glycol are expressly contemplated are the solubilizing agent.
[0128] According to embodiments, the solubilizing agent includes combinations
of mono-
and di- propylene and ethylene glycols and mono-, di-, and triglyceride
combinations.
According to embodiments, polyethylene glycol glyceride (GELUCIREO, GATTEFOSSE
SAS, Saint-Priest, France) can be used herein as the solubilizing agent or as
a surfactant. For
example, GELUCIRE 44/14 (PEG-32 glyceryl laurate EP), a medium chain fatty
acid esters
of polyethylene glycol, is a polyethylene glycol glyceride composed of mono-,
di- and
triglycerides and mono- and diesters of polyethylene glycol.
[0129] According to embodiments, commercially available fatty acid glycerol
and glycol
ester solubilizing agents are often prepared from natural oils and therefore
may comprise
components in addition to the fatty acid esters that predominantly comprise
and characterize
the solubilizing agent. Such other components may be, e.g., other fatty acid
mono-, di-, and
triglycerides; fatty acid mono- and diester ethylene or propylene glycols,
free glycerols or
glycols, or free fatty acids, for example. In some embodiments, when an
oil/solubilizing
agent is described herein as a saturated C8 fatty acid mono- or diester of
glycerol, the
predominant component of the oil, i.e., >50 wt% (e.g., >75 wt%, >85 wt% or >90
wt%) is
caprylic monoglycerides and caprylic diglycerides. For example, the Technical
Data Sheet by
ABITEC for CAPMUL MCM C8 describes CAPMUL MCM C8 as being composed of mono
and diglycerides of medium chain fatty acids (mainly caprylic) and describes
the alkyl
content as < 1% C6,? 95% C8, < 5% C10, and < 1.5% C12 and higher.
[0130] For example, MIGLYOL 812 is a solubilizing agent that is generally
described as a
C8-C10 triglyceride because the fatty acid composition is at least about 80%
triglyceride
esters of caprylic acid (C8) and capric acid (C10). However, it also includes
small amounts of
other fatty acids, e.g., less than about 5% of caproic acid (C6), lauric acid
(C12), and myristic
acid (C14). The product information sheet for various MIGLYOLs illustrate the
various fatty
acid components as follows:
Tests 810 812 818 829 840
Caproic acid (C6:0) max. 2.0 max. 2.0 max. 2 max. 2
max. 2
23
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Capiylic acid (C8:0) 65.0 ¨ 80.0 50.0 ¨ 65.0 45 ¨ 65 45
¨ 55 65 ¨
5
Capric acid (C10:0) 20.0 ¨ 35.0 30.0 ¨ 45.0 30-45 30-40 20-35
10 Laurie acid (C12:0) max. 2 max. 2 max. 3 max. 3
max. 2
Myristic acid max. 1.0 max. 1.0 max. 1 max. 1
15 max. 1
(C14:0)
Linoleic acid 2 ¨ 5
(C18:2)
Succinic acid 15 ¨ 20 -
ECN 25.5-26.4 26.1-27 26.52-28.56 26-27.6 25.5-26.4
[0131] According to embodiments, anionic or non-ionic surfactants may be used
in
pharmaceutical compositions containing solubilized estradiol. Ratios of
solubilizing agent(s)
to surfactant(s) vary depending upon the respective solubilizing agent(s) and
the respective
surfactant(s) and the desired physical characteristics of the resultant
pharmaceutical
composition. For example and without limitation, CAPMUL MCM and a non-ionic
surfactant may be used at ratios including 65:35, 70:30, 75:25, 80:20, 85:15
and 90:10. Other
non-limiting examples include: CAPMUL MCM and GELUCIRE 39/01 used in ratios
including, for example and without limitation, 6:4, 7:3, and 8:2; CAPMUL MCM
and
GELUCIRE 43/01 used in ratios including, for example and without limitation,
7:3, and 8:2;
CAPMUL MCM and GELUCIRE 50/13 used in ratios including, for example and
without
limitation, 7:3, and 8:2, and 9:1.
Other Excipients
[0132] According to embodiments, the pharmaceutical composition further
includes a
surfactant. The surfactant can be a nonionic surfactant, cationic surfactant,
anionic surfactant,
or mixtures thereof Suitable surfactants include, for example, water-insoluble
surfactants
having a hydrophilic-lipophilic balance (HLB) value less than 12 and water-
soluble
surfactants having a HLB value greater than 12. Surfactants that have a high
HLB and
hydrophilicity, aid the formation of oil-water droplets. The surfactants are
amphiphilic in
24
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
nature and are capable of dissolving or solubilizing relatively high amounts
of hydrophobic
drug compounds.
[0133] Non-limiting examples, include, Tween, Dimethylacetamide (DMA),
Dimethyl
sulfoxide (DMSO), Ethanol, Glycerin, N-methyl-2-pyrrolidone (NMP), PEG 300,
PEG 400,
Poloxamer 407, Propylene glycol, Phospholipids, Hydrogenated soy
phosphatidylcholine
(HSPC), Distearoylphosphatidylglycerol (DSPG), L-a-
dimyristoylphosphatidylcholine
(DMPC), L-a-dimyristoylphosphatidylglycerol (DMPG), Polyoxyl 35 castor oil
(CREMOPHOR EL, CREMOPHOR ELP), Polyoxyl 40 hydrogenated castor oil (Cremophor
RH 40), Polyoxyl 60 hydrogenated castor oil (CREMOPHOR RH 60), Polysorbate 20
(TWEEN 20), Polysorbate 80 (TWEEN 80), d-a-tocopheryl polyethylene glycol 1000
succinate (TPGS), Solutol HS-15, Sorbitan monooleate (SPAN 20), PEG 300
capryliecapric
glycerides (SOFTIGEN 767), PEG 400 caprylic/capric glycerides (LABRASOL), PEG
300
oleic glycerides (LABRAFIL M-1944C5), Polyoxyl 35 Castor oil (ETOCAS 35),
Glyceryl
Caprylate (Mono- and Diglycerides) (IMWITOR), PEG 300 linoleic glycerides
(LABRAFIL
M-2125C5), Polyoxyl 8 stearate (PEG 400 monosterate), Polyoxyl 40 stearate
(PEG 1750
monosterate), and combinations thereof Additionally, suitable surfactants
include, for
example, polyoxyethylene derivative of sorbitan monolaurate such as
polysorbate,
caprylcaproyl macrogol glycerides, polyglycolyzed glycerides, and the like.
[0134] According to embodiments, the non-ionic surfactant is selected from one
or more of
glycerol and polyethylene glycol esters of long chain fatty acids, for
example, lauroyl
macrogo1-32 glycerides or lauroyl polyoxy1-32 glycerides, commercially
available as
GELUCIRE, including, for example, GELUCIRE 39/01 (glycerol esters of saturated
C12-
C18 fatty acids), GELUCIRE 43/01 (hard fat NF/JPE) and GELUCIRE 50/13
(stearoyl
macrogo1-32 glycerides EP, stearoyl polyoxy1-32 glycerides NF, stearoyl
polyoxylglycerides
(USA FDA IIG)). These surfactants may be used at concentrations greater than
about 0.01%,
and typically in various amounts of about 0.01%-10.0%, 10.1%-20%, and 20.1%-
30%. In
some embodiments, surfactants may be used at concentrations of about 1% to
about 10%
(e.g., about 1% to about 5%, about 2% to about 4%, about 3% to about 8%).
[0135] According to embodiments, non-ionic surfactants include, for example
and without
limitation: one or more of oleic acid, linoleic acid, palmitic acid, and
stearic acid. According
to embodiments, non-ionic surfactants comprise polyethylene sorbitol esters,
including
polysorbate 80, which is commercially available under the trademark TWEEN 80
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
(polysorbate 80) (Sigma Aldrich, St. Louis, MO). Polysorbate 80 includes
approximately
60%-70% oleic acid with the remainder comprising primarily linoleic acids,
palmitic acids,
and stearic acids. Polysorbate 80 may be used in amounts ranging from about 5
to 50%, and
according to embodiments, about 30% of the pharmaceutical composition total
mass.
[0136] According to embodiments, the non-ionic surfactant includes PEG-6
palmitostearate
and ethylene glycol palmitostearate, which are available commercially as
TEFOSE 63
(GATTEFOSSE SAS, Saint-Priest, France), which can be used with, for example,
CAPMUL
MCM having ratios of MCM to TEFOSE 63 of, for example, 8:2 or 9:1. According
to
embodiments, other solubilizing agents/non-ionic surfactants combinations
include, for
example, MIGLYOL 812:GELUCIRE 50/13 or MIGLYOL 812:TEFOSE 63.
[0137] According to embodiments, the surfactant can be an anionic surfactant,
for example:
ammonium lauryl sulfate, dioctyl sodium sulfosuccinate, perfluoro-octane
sulfonic acid,
potassium lauryl sulfate, or sodium stearate. Cationic surfactants are also
contemplated.
[0138] According to embodiments, non-ionic or anionic surfactants can be used
alone with
at least one solubilizing agent or can be used in combination with other
surfactants.
Accordingly, such surfactants, or any other excipient as set forth herein, may
be used to
solubilize estradiol. The combination of solubilizing agent, surfactant, and
other excipients
should be designed whereby the estradiol is absorbed into the vaginal tissue.
According to
embodiments, the pharmaceutical composition will result in minimal vaginal
discharge.
[0139] According to embodiments, the pharmaceutical composition further
includes at least
one thickening agent. Generally, a thickening agent is added when the
viscosity of the
pharmaceutical composition results less than desirable absorption. According
to
embodiments, the surfactant(s) disclosed herein may also provide thickening of
the
pharmaceutical composition that, upon release, will aid the estradiol in being
absorbed by the
vaginal mucosa while minimizing vaginal discharge. Examples of thickening
agents include:
hard fats; propylene glycol; a mixture of hard fat EP/NF/JPE, glyceryl
ricinoleate,
ethoxylated fatty alcohols (ceteth-20, steareth-20) EP/NF (available as
OVUCIRE 3460,
GATTEFOSSE, Saint-Priest, France); a mixture of hard fat EP/NF/JPE, glycerol
monooleate
(type 40) EP/NF (OVUCIRE WL 3264; a mixture of hard fat EP/NF/JPE, glyceryl
monooleate (type 40) EP/NF (OVUCIRE WL 2944); a non-ionic surfactant
comprising PEG-
6 stearate, ethylene glycol palmitostearate, and PEG-32 stearate; TEFOSE 63 or
a similar
product; and a mixture of various hard fats (WITEPSOL ' Sasol Germany GmbH,
Hamburg,
26
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Germany). Other thickening agents such as the alginates, certain gums such as
xanthan gums,
agar-agar, iota carrageenans, kappa carrageenans, etc. Several other compounds
can act as
thickening agents like gelatim and polymers like HPMC, PVC, and CMC. According
to
embodiments, the viscosity of pharmaceutical compositions in accordance with
various
embodiments may comprise from about 50 cps to about 1000 cps at 25 C. A
person of
ordinary skill in the art will readily understand and select from suitable
thickening agents.
[0140] According to embodiments, the thickening agent is a non-ionic
surfactant. For
example, polyethylene glycol saturated or unsaturated fatty acid ester or
diester is the non-
ionic surfactant thickening agent. In embodiments, the non-ionic surfactant
includes a
polyethylene glycol long chain (C16-C20) fatty acid ester and further includes
an ethylene
glycol long chain fatty acid ester, such as PEG-fatty acid esters or diesters
of saturated or
unsaturated C16-C18 fatty acids, e.g., oleic, lauric, palmitic, and stearic
acids. In
embodiments, the non-ionic surfactant includes a polyethylene glycol long
chain saturated
fatty acid ester and further includes an ethylene glycol long chain saturated
fatty acid ester,
such as PEG- and ethylene glycol-fatty acid esters of saturated C16-C18 fatty
acids, e.g.,
palmitic and stearic acids. Such non-ionic surfactant can comprise PEG-6
stearate, ethylene
glycol palmitostearate, and PEG-32 stearate, such as but not limited to TEFOSE
63.
[0141] According to embodiments, TEFOSE 63 is used to provide additional
viscosity
and/or spreadability in the vagina so as to retard flow of the composition out
of the vagina.
While the pharmaceutical composition remains liquid, the viscosity of such a
pharmaceutical
composition causes the liquid to remain in the API absorption area whereby the
pharmaceutical composition is substantially absorbed by the tissue.
Suprisingly, the addition
of an excipient to increase the viscosity and/or spreadability of the
pharmaceutical
compositions herein allows the administration of a pharmaceutical composition
that is liquid
at body temperature but does not excessively discharge from the vagina when
the patient is
standing, which allows the patients to be ambulatory after administration of
the
pharmaceutical compositions.
[0142] According to embodiments, the non-ionic surfactant used as a thickening
agent is
not hydrophilic and has good emulsion properties. An illustrative example of
such surfactant
is TEFOSE 63, which has a hydrophilic-lipophilic balance (HLB) value of about
9-10.
[0143] According to embodiments, the pharmaceutical composition further
includes one or
more mucoadherent agents to improve vaginal absorption of the estradiol by,
for example,
27
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
increasing the viscosity of of the pharmaceutical composition whereby flow out
of the vagina
is retarded. According to other embodiments, alone or in addition to changes
in viscosity, the
mucoadhesive agent causes the pharmaceutical composition to adhere to the
vaginal tissue
chemically or mechanically. For example, a mucoadherent agent can be present
to aid the
pharmaceutical composition with adherence to the mucosa upon activation with
water.
According to embodiments, polycarbophil is the mucoadherent agent. According
to
embodiments, other mucoadherent agents include, for example and without
limitation: poly
(ethylene oxide) polymers having a molecular weight of from about 100,000 to
about
900,000; chitosans; carbopols including polymers of acrylic acid crosslinked
with ally'
sucrose or ally' pentaerythritol; polymers of acrylic acid and C10-C30 alkyl
acrylate
crosslinked with ally' pentaerythritol; carbomer homopolymer or copolymer that
contains a
block copolymer of polyethylene glycol and a long chain alkyl acid ester; and
the like.
According to embodiments, various hydrophilic polymers and hydrogels may be
used as the
mucoadherent agent. According to certain embodiments, the polymers or
hydrogels can swell
in response to contact with vaginal tissue or secretions, enhancing
moisturizing and
mucoadherent effects. The selection and amount of hydrophilic polymer may be
based on the
selection and amount of solubilizing agent. In some embodiments, the
pharmaceutical
composition includes a hydrophilic polymer but optionally excludes a gelling
agent. In
embodiments having a hydrogel, from about 5% to about 10% of the total mass
may
comprise the hydrophilic polymer. In further embodiments, hydrogels may be
employed. A
hydrogel may comprise chitosan, which swell in response to contact with water.
In various
embodiments, a cream pharmaceutical composition may comprise PEG-90M. In some
embodiments, a mucoadherent agent is present in the pharmaceutical
formulation, in the soft
gel capsule, or both.
[0144] According to embodiments, the pharmaceutical compositions include one
or more
thermoreversible gels, typically of the hydrophilic nature including for
example and without
limitation, hydrophilic sucrose and other saccharide-based monomers (U.S. Pat.
No.
6,018,033, which is incorporated by reference).
[0145] According to embodiments, the pharmaceutical composition further
includes a
lubricant. In some embodiments, a lubricant can be present to aid in
formulation of a dosage
form. For example, a lubricant may be added to ensure that capsules or tablets
do not stick to
one another during processing or upon storage. Any suitable lubricant may be
used. For
example, lecithin, which is a mixture of phospholipids, is the lubricant.
28
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0146] According to embodiments, the pharmaceutical composition further
includes an
antioxidant. Any suitable anti-oxidant may be used. For example, butylated
hydroxytoluene,
butylated hydroxyanisole, and Vitamin E TPGS.
[0147] According to embodiments, the pharmaceutical composition includes about
20% to
about 80% solubilizing agent by weight, about 0.1% to about 5% lubricant by
weight, and
about 0.01% to about 0.1% antioxidant by weight.
[0148] The choice of excipient will depend on factors such as, for example,
the effect of
the excipient on solubility and stability. Additional excipients used in
various embodiments
may include colorants and preservatives. Examples of colorants include FD&C
colors (e.g.,
blue No. 1 and Red No. 40), D&C colors (e.g., Yellow No. 10), and opacifiers
(e.g., Titanium
dioxide). According to embodiments, colorants, comprise about 0.1% to about 2%
of the
pharmaceutical composition by weight. According to embodiments, preservatives
in the
pharmaceutical composition comprise methyl and propyl paraben, in a ratio of
about 10:1,
and at a proportion of about 0.005% and 0.05% by weight.
[0149] Generally, the solubilizing agents, excipients, other additives used in
the
pharmaceutical compositions described herein, are non-toxic, pharmaceutically
acceptable,
compatible with each other, and maintain stability of the pharmaceutical
composition and the
various components with respect to each other. Additionally, the combination
of various
components that comprise the pharmaceutical compositions will maintain will
result in the
desired therapeutic effect when administered to a subject.
Solubility of Estradiol
[0150] According to embodiments, solubilizing agents comprising mixtures of
medium
chain fatty acid glycerides, e.g., C6-C12, C8-C12, or C8-C10 fatty acid mono-
and
diglycerides or mono-, di-, and triglycerides dissolve estradiol. As
illustrated in the
Examples, good results were obtained with solubilizing agents that are
predominantly a
mixture of C8-C10 saturated fatty acid mono- and diglycerides, or medium chain
triglycerides (e.g., MIGLYOL 810 or 812). Longer chain glycerides appear to be
not as well
suited for dissolution of estradiol.
[0151] A solubilizing agent comprising propylene glycol monocaprylate (e.g.,
CAPRYOL)
and 2-(2-Ethoxyethoxy)ethanol (e.g., TRANSCUTOL) solubilized estradiol well.
29
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
IV. MANUFACTURE OF THE PHARMACEUTICAL COMPOSITION
[0152] According to embodiments, the pharmaceutical composition is prepared
via
blending estradiol with a pharmaceutically acceptable solubilizing agent,
including for
example and without limitation, at least one medium chain fatty acid such as
medium chain
fatty acids consisting of at least one mono-, di-, or triglyceride, or
derivatives thereof, or
combinations thereof According to embodiments, the pharmaceutical composition
also
includes at least one glycol or derivatives thereof or combinations thereof or
combinations of
at least one glyceride and glycol. The glycol(s) may be used as solubilizing
agents or to
adjust viscosity and, thus, may be considered thickening agents, as discussed
further herein.
Optionally added are other excipients including, for example and without
limitation, anti-
oxidants, lubricants, and the like. According to embodiments, the
pharmaceutical
composition includes sufficient solubilizing agent to fully solubilize the
estradiol. It is
expressly understood, however, the other volumes of solubilizing agent can be
used
depending on the level of estradiol solubilization desired. Persons of
ordinary skill in the art
will know and understand how to determine the volume of solubilizing agent and
other
excipients depending on the desired percent of estradiol to be solubilized in
the
pharmaceutical composition.
[0153] In illustrative embodiments, GELUCIRE 44/14 (lauroyl macrogo1-32
glycerides EP,
lauroyl polyoxy1-32 glycerides NF, lauroyl polyoxylglycerides (USA FDA IIG))
is heated to
about 65 C and CAPMUL MCM is heated to about 40 C to facilitate mixing of
the oil and
non-ionic surfactant, although such heating is not necessary to dissolve the
estradiol.
[0154] Specific Examples disclosed herein provide additional principles and
embodiments
illustrating the manufactures of the pharmaceutical compositions disclosed
herein.
V. DELIVERY VEHICLE
[0155] Generally, the pharmaceutical compositions described herein delivered
intravaginally inside of a delivery vehicle, for example a capsule. According
to embodiments,
the capsules are soft capsules made of materials well known in the
pharmaceutical arts, for
example, gelatin. However, according to embodiments, the delivery vehicle is
integral with
the pharmaceutical composition (i.e., the pharmaceutical composition is the
delivery vehicle).
In such embodiments the pharmaceutical compositions is a gel, cream, ointment,
tablet, or
other preparation that is directly applied and absorbed vaginally.
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0156] According to embodiments, the capsules do not contain one or more of
the
following: a hydrophilic gel-forming bioadhesive agent, a lipophilic agent, a
gelling agent for
the lipophilic agent, and/or a hydrodispersible agent. According to
embodiments, the capsules
do not contain a hydrophilic gel-forming bioadhesive agent selected from:
carboxyvinylic
acid, hydroxypropylcellulose, carboxymethylcellulose, gelatin, xanthan gum,
guar gum,
aluminum silicate, and mixtures thereof According to embodiments, the capsules
do not
contain a lipophilic agent selected from: a liquid triglyceride, a solid
triglyceride (with a
melting point of about 35 C), carnauba wax, cocoa butter, and mixtures
thereof According
to embodiments, the capsules do not contain a hydrophobic colloidal silica
gelling agent.
According to embodiments, the capsules do not contain a hydrodispersible agent
selected
from: polyoxyethylene glycol, polyoxyethylene glycol 7-glyceryl-cocoate, and
mixtures
thereof In some embodiments, the estradiol is formulated as a liquid
composition consisting
of a therapeutically effective amount of estradiol; a caprylic/capric
triglyceride; and a non-
ionic surfactant comprising PEG-6 palmitostearate and ethylene glycol
palmitostearate. In
such embodiments, a hydrophilic gel-forming bioadhesive agent in the liquid
composition. In
some such embodiments, the liquid composition is contained with a gelatin
capsule as
described herein. In some such embodiments, the capsule comprises gelatin and
optionally
one or more further components selected from the group consisting of gelatin,
hydrolyzed
gelatin, sorbitol-sorbitan solution, water, glycerin, titanium dioxide, FD&C
Red #40, ethanol,
ethyl acetate, propylene glycol, polyvinyl acetate phthalate, isopropyl
alcohol, polyethylene
glycol, and ammonium hydroxide.
[0157] According to embodiments, the delivery vehicle is designed for ease of
insertion.
According to embodiments, the delivery vehicle is sized whereby it can be
comfortably
inserted into the vagina. According to embodiments, the delivery vehicle is
prepared in a
variety of geometries. For example, the delivery vehicle is shaped as a tear
drop, a cone with
frustoconical end, a cylinder, a cylinder with larger "cap" portion, or other
shapes suitable for
and that ease insertion into the vagina. According to embodiments, the
delivery vehicle is
used in connection with an applicator. According to other embodiments, the
delivery vehicle
is inserted digitally.
[0158] According to embodiments, a method for the treatment of VVA, including
dyspareunia, vaginal dryness, and estrogen-deficient urinary states (including
urinary tract
infections), is provided wherein a composition for the treatment of VVA is
digitally insert
approximately two inches into the vagina or in the third of the vagina closest
to the opening
31
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
of the vagina and results in at least one of: improved compliance compared to
other products
for the treatment of VVA; improved user experience compared to other products
for the
treatment of VVA; and statistically significantly improved symptoms of VVA,
compared to
placebo or baseline within one of two, four, six, eight, ten, or twelve or
more weeks after
initiation of administration.. According to embodiments, a method for the
treatment of VVA,
including dyspareunia, vaginal dryness, and estrogen-deficient urinary states
(including
urinary tract infections), is provided wherein a delivery vehicle containing a
composition for
the treatment of VVA and a tear drop shape as disclosed herein is insert
approximately two
inches into the vagina or in the third of the vagina closest to the opening of
the vagina and
results in at least one of: improved compliance compared to other products for
the treatment
of VVA; improved user experience compared to other products for the treatment
of VVA;
and statistically significantly improved symptoms of VVA, compared to placebo
or baseline
within one of two, four, six, eight, ten, or twelve or more weeks after
initiation of
administration.
[0159] With reference to Fig. 2, delivery vehicle 200 includes pharmaceutical
composition
202 and capsule 204. Width 208 represents the thickness of capsule 204, for
example about
0.108 inches. The distance from one end of delivery vehicle 200 to another is
represented by
distance 206, for example about 0.690 inches. The size of delivery vehicle 200
may also be
described by the arc swept by a radius of a given length. For example, arc
210, which is
defined by the exterior of gelatin 204, is an arc swept by a radius of about
0.189 inches. Arc
212, which is defined by the interior of capsule 204, is an arc swept by a
radius of about
0.0938 inches. Arc 214, which is defined by the exterior of gelatin 204
opposite arc 210, is an
arc swept by a radius of about 0.108 inches. Suitable capsules of other
dimensions may be
provided. According to embodiments, capsule 204 has dimensions the same as or
similar to
the ratios as provided above relative to each other. In some embodiment, the
gelatin capsule
further comprises one or more components selected from the group consisting of
hydrolyzed
gelatin, sorbitol-sorbitan solution, water, glycerin, titanium dioxide, FD&C
Red #40, ethanol,
ethyl acetate, propylene glycol, polyvinyl acetate phthalate, isopropyl
alcohol, polyethylene
glycol, and ammonium hydroxide.
[0160] According to embodiments, the delivery vehicle is designed to remaining
in the
vagina until the pharmaceutical compositions are released. According to
embodiments,
delivery vehicle dissolves intravaginally and is absorbed into the vaginal
tissue with the
32
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
pharmaceutical composition, which minimizes vaginal discharge. In such
embodiments,
delivery mechanism is made from constituents that are non-toxic, for example,
gelatin.
Design Factors for Vaginally Inserted Pharmaceutical Compositions
[0161] According to embodiments, the pharmaceutical composition is designed to
maximize favorable characteristics that lead to patient compliance (patients
that discontinue
treatment prior to completion of the prescribed course of therapy), without
sacrificing
efficacy. Favorable characteristics include, for example, lack of or reduction
of irritation
relative to other hormone replacement pessaries, lack of or reduction in
vaginal discharge of
the pharmaceutical composition and delivery vehicle relative to other hormone
replacement
pessaries, lack of or reduction of pharmaceutical composition or delivery
vehicle residue
inside the vagina, ease of administration compared to other hormone
replacement pessaries,
or improved efficacy of drug product relative to otherwise similar
pharmaceutical
compositions.
[0162] According to embodiments, the pharmaceutical composition is non-
irritating or
minimizes irritation. Patient irritation includes pain, pruritus (itching),
soreness, excessive
discharge, swelling, or other similar conditions. Patient irritation results
in poor compliance.
Non-irritating or reduced irritation pharmaceutical compositions are measured
relative to
competing hormone pessaries, including tablets, creams, or other intravaginal
estrogen
delivery forms.
[0163] According to embodiments, the pharmaceutical compositions does not
result in
systemic exposure (e.g., blood circulation of estradiol), which improves
safety. According to
other embodiments, the pharmaceutical compositions disclosed herein result in
significantly
reduced systemic exposure (e.g., blood circulation of estradiol) when compared
to other
vaginally administered drugs on the market for the treatment of VVA.
[0164] In certain embodiments, the administration of the pharmaceutical
composition
provides a mean concentration (C,,) value below 20.6 pg/mL on Day 1 of the
treatment,
and/or a C,, value below 19.4 pg/mL on Day 14 of the treatment, and/or a Cave
value below
11.5 pg/mL on Day 83 of the treatment. In certain embodiments, the
administration of the
pharmaceutical composition provides a mean concentration (Cave) value below 10
pg/mL on
Day 1 of the treatment, and/or a Cave value below 7.3 pg/mL on Day 14 of the
treatment,
and/or a Cave value below 5.5 pg/mL on Day 83 of the treatment.
33
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0165] According to embodiments, the pharmaceutical composition does not leave
residue
inside the vagina. Rather, the pharmaceutical composition and delivery vehicle
are
substantially absorbed or dispersed without resulting in unabsorbed residue or
unpleasant
sensations of non-absorbed or non-dispersed drug product. Measurement of lack
of residue is
relative to other vaginally inserted products or can be measured objectively
with inspection of
the vaginal tissues. For example, certain other vaginally inserted products
contain starch
which can result in greater discharge from the vagina following administration
than. In some
embodiments, the pharmaceutical compositions provided herein provide a lower
amount,
duration, or frequency of discharge following administration compared to other
vaginally
inserted products (e.g., compressed tablets).
[0166] According to embodiments, the pharmaceutical composition improves
vaginal
discharge compared to other pessaries, including pessaries that deliver
hormones. Ideally,
vaginal discharge is eliminated, minimized, or improved compared to competing
products.
[0167] According to embodiments, the pharmaceutical compositions disclosed
herein are
inserted digitally. According to embodiments, the pharmaceutical compositions
are digitally
inserted approximately two inches into the vagina without a need for an
applicator.
According to embodiments, the pharmaceutical compositions are designed to be
also inserted
with an applicator, if desired. According to some embodiments, because the
site of VVA is in
the proximal region of the vagina (towards the vaginal opening), the
pharmaceutical
compositions disclosed herein are designed to be inserted in the proximal
portion of the
vagina.
[0168] Through extensive experimentation, various medium chain fatty acid
esters of
glycerol and propylene glycol demonstrated one or more favorable
characteristics for
development as a human drug product. According to embodiments, the
solubilizing agent was
selected from at least one of a solvent or co-solvent. Suitable solvents and
co-solvents include
any mono-, di- or triglyceride and glycols, and combinations thereof
[0169] According to embodiments, the pharmaceutical composition is delivered
via a
gelatin capsule delivery vehicle. According to these embodiments, the
pharmaceutical
composition is a liquid pharmaceutical composition. According to embodiments,
the delivery
vehicle is a soft capsule, for example a soft gelatin capsule. Thus, the
pharmaceutical
composition of such embodiments is encapsulated in the soft gelatin capsule or
other soft
capsule.
34
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0170] According to embodiments, the pharmaceutical composition includes
estradiol that
is at least about 80% solubilized in a solubilizing agent comprising one or
more C6 to C14
medium chain fatty acid mono-, di-, or triglycerides and, optionally, a
thickening agent.
According to embodiments, the pharmaceutical composition includes estradiol
that is at least
about 80% solubilized one or more C6 to C12 medium chain fatty acid mono-, di-
, or
triglycerides, e.g., one or more C6 to C14 triglycerides, e.g., one or more C6
to C12
triglycerides, such as one or more C8-C10 triglycerides. These embodiments
specifically
contemplate the estradiol being at least 80% solubilized. These embodiments
specifically
contemplate the estradiol being at least 90% solubilized. These embodiments
specifically
contemplate the estradiol being at least 95% solubilized. These embodiments
specifically
contemplate the estradiol being fully solubilized.
[0171] As noted above, liquid pharmaceutical compositions are liquid at room
temperature
or at body temperature. For example, in some embodiments, a pharmaceutical
composition
provided herein is a liquid formulation contained within a soft gel capsule.
Gels, hard fats, or
other solid forms that are not liquid at room or body temperature are less
desirable in
embodiments of the pharmaceutical composition that are liquid.
[0172] The thickening agent serves to increase viscosity, e.g., up to about
10,000 cP
(10,000 mPa-s), typically to no more than about 5000 cP, and more typically to
between
about 50 and 1000 cP. In embodiments, the non-ionic surfactant, e.g., GELUCIRE
or
TEFOSE, may be solid at room temperature and require melting to effectively
mix with the
solubilizing agent. However, in these embodiments, the resultant
pharmaceutical composition
remains liquid, albeit with greater viscosity, not solid.
[0173] According to embodiments, the pharmaceutical composition includes
estradiol, the
medium chain solubilizing agent, and the thickening agent as the ingredients
delivered via a
soft capsule delivery vehicle. Other ingredients, e.g., colorants,
antioxidants, preservatives, or
other ingredients may be included as well. However, the addition of other
ingredients should
be in amounts that do not materially change the solubility of the estradiol,
the
pharmacokinetics of the pharmaceutical composition, or efficacy of the
pharmaceutical
composition. Other factors that should be considered when adjusting the
ingredients of the
pharmaceutical composition include the irritation, vaginal discharge,
intravaginal residue, and
other relevant factors, for example those that would lead to reduced patient
compliance.
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Other contemplated ingredients include: oils or fatty acid esters, lecithin,
mucoadherent
agents, gelling agents, dispersing agents, or the like.
VI. METHODS
[0174] According to embodiments, the pharmaceutical compositions disclosed
herein can
be used for the treatment of VVA, including the treatment of at least one VVA
symptom
including: vaginal dryness, vaginal or vulvar irritation or itching, dysuria,
dyspareunia, and
vaginal bleeding associated with sexual activity, among others. According to
embodiments
the methods of treatment are generally applicable to females.
[0175] According to embodiments, the pharmaceutical compositions disclosed
herein can
be used for the treatment of estrogen-deficient urinary states. According to
embodiments, the
pharmaceutical compositions disclosed herein can be used for the treatment of
dyspareunia,
or vaginal bleeding associated with sexual activity.
[0176] According to embodiments, treatment of the VVA, estrogen-deficient
urinary states,
and dyspareunia and vaginal bleeding associated with sexual activity occurs by
administering
the pharmaceutical compositions intravaginally. According to embodiments where
the
delivery vehicle is a capsule, the patient obtains the capsule and inserts the
capsule into the
vagina, where the capsule dissolves and the pharmaceutical composition is
released into the
vagina where it is absorbed into the vaginal tissue. In some embodiments, the
pharmaceutical
composition is completely absorbed into the vaginal tissue. In some
embodiments, the
pharmaceutical composition is substantially absorbed into the vaginal tissue
(e.g., at least
about 80% by weight, at least about 85% by weight, at least about 90% by
weight, at least
about 95% by weight, at least about 97% by weight, at least about 98% by
weight, or at least
about 99% by weight of the composition is absorbed). According to embodiments,
the
capsule is inserted about two inches into the vagina, however the depth of
insertion is
generally any depth that allows for adsorption of substantially all of the
pharmaceutical
composition. According to embodiments, the capsule can also be applied using
an applicator
that deposits the capsule at an appropriate vaginal depth as disclosed herein.
According to
embodiments, the capsule is insert into the lower third of the vagina (i.e.,
the third closest to
the vaginal opening). According to embodiments, the softgel capsule can be
held with the
larger end between the fingers as shown in Fig. 26A.
The subject will select a position that is most comfortable (e.g., a reclining
position as shown
in Fig. 26B or a standing position as shown in Fig. 26C), and the subject will
insert the
36
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
softgel into the lower third of the vagina with the smaller end up. The
softgel capsule will
dissolve rapidly. The softgel can be inserted at any time of day and normal
activities can be
immediately resumed. According to embodiments, the same time of day for all
insertions of
of the softgel is used.
[0177] According to embodiments where the pharmaceutical composition is a
cream, gel,
ointment, or other similar preparation, the pharmaceutical composition is
applied digitally, as
is well known and understood in the art.
[0178] Upon release of the pharmaceutical composition in the vagina, estradiol
is locally
absorbed. For example, following administration of the suppository to the
proximal region of
the vagina of a patient provides a therapeutically effective concentration of
estradiol over 24
hours in the proximal region of the vagina.
[0179] According to embodiments, the timing of administration of the
pharmaceutical
composition of this disclosure may be conducted by any safe means as
prescribed by an
attending physician. According to embodiments, a patient will administer the
pharmaceutical
composition (e.g., a capsule) intravaginally each day for 14 days, then twice
weekly
thereafter. In some such embodiments, the doses administered during the twice
weekly
dosing period are administered approximately 3-4 days apart. Typically, doses
administered
during the twice weekly dosing period do not exceed more than twice in a seven
day period.
[0180] According to embodiments, the pharmaceutical compositions are vaginally
administered with co-administration of an orally administered estrogen-based
(or progestin-
based or progestin- and estrogen-based) pharmaceutical drug product, or patch,
cream, gel,
spray, transdermal delivery system or other parenterally-administered estrogen-
based
pharmaceutical drug product, each of which can include natural, bio-similar,
or synthetic or
other derived estrogens or progestins. According to embodiments, modulation of
circulating
estrogen levels provided via the administration of the pharmaceutical
compositions disclosed
herein, if any, are not intended to be additive to any co-administered
estrogen product and its
associated circulating blood levels. According to other embodiments, co-
administrated
estrogen products are intended to have an additive effect as would be
determined by the
patient physician.
[0181] According to embodiments, a method for estrogenizing vaginal tissue is
provided.
The method includes administration of a (i.e., a suppository) or dosage as
described herein.
Estrogenized vaginal tissue is typically characterized by one or more of the
following
37
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
properties: the presence clear secretions on vaginal walls; rogation and
elasticity of the
vaginal walls; intact vaginal epithelium; and pink tissue color. In contrast,
de-estrogenized
vaginal is characterized by decreased or absent secretions; smooth tissue with
fewer or no
rugae; bleeding of the vaginal surface; development of petechiae (i.e.,
pinpoint, round spots
on the skin due to bleeding, appearing red, brown, or purple); and pale or
transparent tissues.
Accordingly, estrogenizing vaginal tissue according to the method disclosed
herein can
include, increasing the level of vaginal secretions in a subject; increasing
the number of
vaginal rugae in the subject; and/or decreasing bleeding or petechiae in the
subject.
According to embodiments, a method for estrogenizing vaginal tissue is
provided, the method
including administering a suppository so as to provide an estradiol Cmax or
AUC as described
herein. According to embodiments, a method for estrogenizing vaginal tissue is
provided, the
method including administering a suppository so as to provide an estrone Cmax
or AUC as
described herein.
[0182] According to embodiments, a method for estrogenizing the labia majora
and labia
minora (collectively "labia") is provided as described herein. Generally, the
pharmaceutical
composition is inserted digitally into the vagina approximately two inches or
inserted into the
third of the vagina closest to the vaginal opening as shown in Figs. 26A, 26B,
and 26C. The
gelatin capsule containing the pharmaceutical composition dissolves, ruptures,
or otherwise
releases the pharmaceutical composition into the vagina, whereby the lower
third of the
vagina and labia are both reestrogenized. According to some embodiments, the
pharmaceutical composition is a liquid that partially flows to the labia and
directly
reestrogenizes the labia.
[0183] According to embodiments, a method for estrogenizing the vulva is
provided as
described herein. Generally, the pharmaceutical composition is inserted
digitally into the
vagina approximately two inches or inserted into the third of the vagina
closest to the vaginal
opening as shown in Figs. 26A, 26B, and 26C. The gelatin capsule containing
the
pharmaceutical composition dissolves, ruptures, or otherwise releases the
pharmaceutical
composition into the vagina, whereby the lower third of the vagina and vulva
are both
reestrogenized. According to some embodiments, the pharmaceutical composition
is a liquid
that partially flows to the vulval tissue and directly reestrogenizes the
vulva.
[0184] According to embodiments, a method for treating vaginal dryness is
provided. The
method includes administration of a soft gel vaginal estradiol formulation
(i.e., a suppository)
38
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
or dosage as described herein. Treating vaginal dryness according to the
method disclosed
herein can include, decreasing the severity of vaginal dryness by 1%, 5%, 10%,
15%, 20%,
25%, 30%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, or 99%. The
decrease in severity can be obtained following 2 weeks of treatment, or 6
weeks of treatment,
or 8 weeks of treatment, or 12 weeks of treatment. In some embodiments,
vaginal dryness is
assessed using a severity scale, ranging from 0 to 4 points wherein 0
indicates no dryness, 1
indicates mild dryness, 2 indicates moderate dryness, and 3 indicates severe
dryness.
[0185] In some embodiments, the method for treating vaginal dryness includes
reducing the
dryness severity score from 3, prior to treatment of a subject, to 2, after 2
weeks of treatment
of the subject. In some embodiments, the method for treating vaginal dryness
includes
reducing the dryness severity score from 2, prior to treatment of a subject,
to 1, after 2 weeks
of treatment of the subject. In some embodiments, the method for treating
vaginal dryness
includes reducing the dryness severity score from 1, prior to treatment of
subject, to 0, after 2
weeks of treatment of the subject.
[0186] In some embodiments, the method for treating vaginal dryness includes
reducing the
dryness severity score from 3, prior to treatment of a subject, to 2, after 6
weeks of treatment
of the subject. In some embodiments, the method for treating vaginal dryness
includes
reducing the dryness severity score from 2, prior to treatment of a subject,
to 1, after 6 weeks
of treatment of the subject. In some embodiments, the method for treating
vaginal dryness
includes reducing the dryness severity score from 1, prior to treatment of
subject, to 0, after 6
weeks of treatment of the subject.
[0187] In some embodiments, the method for treating vaginal dryness includes
reducing the
dryness severity score from 3, prior to treatment of a subject, to 2, after 8
weeks of treatment
of the subject. In some embodiments, the method for treating vaginal dryness
includes
reducing the dryness severity score from 2, prior to treatment of a subject,
to 1, after 8 weeks
of treatment of the subject. In some embodiments, the method for treating
vaginal dryness
includes reducing the dryness severity score from 1, prior to treatment of
subject, to 0, after 8
weeks of treatment of the subject.
[0188] In some embodiments, the method for treating vaginal dryness includes
reducing the
dryness severity score from 3, prior to treatment of a subject, to 2, after 12
weeks of treatment
of the subject. In some embodiments, the method for treating vaginal dryness
includes
reducing the dryness severity score from 2, prior to treatment of a subject,
to 1, after 12
39
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
weeks of treatment of the subject. In some embodiments, the method for
treating vaginal
dryness includes reducing the dryness severity score from 1, prior to
treatment of subject, to
0, after 12 weeks of treatment of the subject.
[0189] In some embodiments, the method for treating vaginal dryness includes
decreasing
the severity of dryness after two weeks of treatment, wherein the severity is
assessed on a
scale of 0-3 points, and the average decrease ranges from a 0.5-point decrease
to a 1.25-point
decrease. The average decrease can be determined by observing any suitable
number of
subjects. In some embodiments, the number of subjects is at least 100. In some
embodiments, the number of subjects is at least 500. In some embodiments, the
number of
subjects ranges from 700 to 800. In some embodiments, the number of subjects
ranges from
740 to 750. In some embodiments, the vaginal estradiol formulation contains 4
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 10
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 25
lig of
estradiol.
[0190] In some embodiments, the method for treating vaginal dryness includes
decreasing
the severity of dryness after six weeks of treatment, wherein the severity is
assessed on a
scale of 0-3 points, and the average decrease ranges from a 0.75-point
decrease to a 1.5-point
decrease. The average decrease can be determined by observing any suitable
number of
subjects. In some embodiments, the number of subjects is at least 100. In some
embodiments, the number of subjects is at least 500. In some embodiments, the
number of
subjects ranges from 700 to 800. In some embodiments, the number of subjects
ranges from
740 to 750. In some embodiments, the vaginal estradiol formulation contains 4
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 10
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 25
lig of
estradiol.
[0191] In some embodiments, the method for treating vaginal dryness includes
decreasing
the severity of dryness after eight weeks of treatment, wherein the severity
is assessed on a
scale of 0-3 points, and the average decrease ranges from a 0.9-point decrease
to a 1.5-point
decrease. The average decrease can be determined by observing any suitable
number of
subjects. In some embodiments, the number of subjects is at least 100. In some
embodiments, the number of subjects is at least 500. In some embodiments, the
number of
subjects ranges from 700 to 800. In some embodiments, the number of subjects
ranges from
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
740 to 750. In some embodiments, the vaginal estradiol formulation contains 4
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 10
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 25
lig of
estradiol.
[0192] In some embodiments, the method for treating vaginal dryness includes
decreasing
the severity of dryness after twelve weeks of treatment, wherein the severity
is assessed on a
scale of 0-3 points, and the average decrease ranges from a 0.9-point decrease
to a 1.5-point
decrease. The average decrease can be determined by observing any suitable
number of
subjects. In some embodiments, the number of subjects is at least 100. In some
embodiments, the number of subjects is at least 500. In some embodiments, the
number of
subjects ranges from 700 to 800. In some embodiments, the number of subjects
ranges from
740 to 750. In some embodiments, the vaginal estradiol formulation contains 4
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 10
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 25
lig of
estradiol.
[0193] In some embodiments, the method for treating vaginal dryness includes
administering a suppository so as to provide an estradiol Cmax or AUC as
described herein.
According to embodiments, a method for treating vaginal dryness is provided,
the method
including administering a suppository so as to provide an estrone Cmax or AUC
as described
herein.
[0194] According to embodiments, a method for treating vulvar and/or vaginal
itching or
irritation is provided. The method includes administration of a soft gel
vaginal estradiol
formulation (i.e., a suppository) or dosage as described herein. Treating
vulvar and/or
vaginal itching or irritation according to the method disclosed herein can
include, decreasing
the severity of vulvar and/or vaginal itching or irritation by 1%, 5%, 10%,
15%, 20%, 25%,
30%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, or 99%. The
decrease
in severity can be obtained following 2 weeks of treatment, or 6 weeks of
treatment, or 8
weeks of treatment, or 12 weeks of treatment. In some embodiments, vulvar
and/or vaginal
itching or irritation is assessed using a severity scale, ranging from 0 to 4
points wherein 0
indicates no itching or irritation, 1 indicates mild itching or irritation, 2
indicates moderate
itching or irritation, and 3 indicates severe itching or irritation.
41
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0195] In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes reducing the itching/irritation severity score from 3,
prior to treatment of a
subject, to 2, after 2 weeks of treatment of the subject. In some embodiments,
the method for
treating vulvar and/or vaginal itching or irritation includes reducing the
itching/irritation
severity score from 2, prior to treatment of a subject, to 1, after 2 weeks of
treatment of the
subject. In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes reducing the itching/irritation severity score from 1,
prior to treatment of
subject, to 0, after 2 weeks of treatment of the subject.
[0196] In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes reducing the itching/irritation severity score from 3,
prior to treatment of a
subject, to 2, after 6 weeks of treatment of the subject. In some embodiments,
the method for
treating vulvar and/or vaginal itching or irritation includes reducing the
itching/irritation
severity score from 2, prior to treatment of a subject, to 1, after 6 weeks of
treatment of the
subject. In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes reducing the itching/irritation severity score from 1,
prior to treatment of
subject, to 0, after 6 weeks of treatment of the subject.
[0197] In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes reducing the itching/irritation severity score from 3,
prior to treatment of a
subject, to 2, after 8 weeks of treatment of the subject. In some embodiments,
the method for
treating vulvar and/or vaginal itching or irritation includes reducing the
itching/irritation
severity score from 2, prior to treatment of a subject, to 1, after 8 weeks of
treatment of the
subject. In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes reducing the itching/irritation severity score from 1,
prior to treatment of
subject, to 0, after 8 weeks of treatment of the subject.
[0198] In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes reducing the itching/irritation severity score from 3,
prior to treatment of a
subject, to 2, after 12 weeks of treatment of the subject. In some
embodiments, the method
for treating vulvar and/or vaginal itching or irritation includes reducing the
itching/irritation
severity score from 2, prior to treatment of a subject, to 1, after 12 weeks
of treatment of the
subject. In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes reducing the itching/irritation severity score from 1,
prior to treatment of
subject, to 0, after 12 weeks of treatment of the subject.
42
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0199] In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes decreasing the severity of itching/irritation after two
weeks of treatment,
wherein the severity is assessed on a scale of 0-3 points, and the average
decrease ranges
from a 0.3-point decrease to a 0.6-point decrease. The average decrease can be
determined
by observing any suitable number of subjects. In some embodiments, the number
of subjects
is at least 100. In some embodiments, the number of subjects is at least 500.
In some
embodiments, the number of subjects ranges from 700 to 800. In some
embodiments, the
number of subjects ranges from 740 to 750. In some embodiments, the vaginal
estradiol
formulation contains 4 lig of estradiol. In some embodiments, the vaginal
estradiol
formulation contains 10 lig of estradiol. In some embodiments, the vaginal
estradiol
formulation contains 25 lig of estradiol.
[0200] In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes decreasing the severity of itching/irritation after six
weeks of treatment,
wherein the severity is assessed on a scale of 0-3 points, and the average
decrease ranges
from a 0.5-point decrease to a 0.7-point decrease. The average decrease can be
determined
by observing any suitable number of subjects. In some embodiments, the number
of subjects
is at least 100. In some embodiments, the number of subjects is at least 500.
In some
embodiments, the number of subjects ranges from 700 to 800. In some
embodiments, the
number of subjects ranges from 740 to 750. In some embodiments, the vaginal
estradiol
formulation contains 4 lig of estradiol. In some embodiments, the vaginal
estradiol
formulation contains 10 lig of estradiol. In some embodiments, the vaginal
estradiol
formulation contains 25 lig of estradiol.
[0201] In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes decreasing the severity of itching/irritation after eight
weeks of treatment,
wherein the severity is assessed on a scale of 0-3 points, and the average
decrease ranges
from a 0.5-point decrease to a 0.8-point decrease. The average decrease can be
determined
by observing any suitable number of subjects. In some embodiments, the number
of subjects
is at least 100. In some embodiments, the number of subjects is at least 500.
In some
embodiments, the number of subjects ranges from 700 to 800. In some
embodiments, the
number of subjects ranges from 740 to 750. In some embodiments, the vaginal
estradiol
formulation contains 4 lig of estradiol. In some embodiments, the vaginal
estradiol
formulation contains 10 lig of estradiol. In some embodiments, the vaginal
estradiol
formulation contains 25 lig of estradiol.
43
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0202] In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes decreasing the severity of itching/irritation after twelve
weeks of treatment,
wherein the severity is assessed on a scale of 0-3 points, and the average
decrease ranges
from a 0.5-point decrease to a 1.0-point decrease. The average decrease can be
determined
by observing any suitable number of subjects. In some embodiments, the number
of subjects
is at least 100. In some embodiments, the number of subjects is at least 500.
In some
embodiments, the number of subjects ranges from 700 to 800. In some
embodiments, the
number of subjects ranges from 740 to 750. In some embodiments, the vaginal
estradiol
formulation contains 4 ng of estradiol. In some embodiments, the vaginal
estradiol
formulation contains 10 ng of estradiol. In some embodiments, the vaginal
estradiol
formulation contains 25 ng of estradiol.
[0203] In some embodiments, the method for treating vulvar and/or vaginal
itching or
irritation includes administering a suppository so as to provide an estradiol
Cmax or AUC as
described herein. According to embodiments, a method for treating vulvar
and/or vaginal
itching or irritation is provided, the method including administering a
suppository so as to
provide an estrone Cmax or AUC as described herein.
[0204] According to embodiments, a method for treating dyspareunia is
provided. The
method includes administration of a suppository or dosage as described herein.
Treating
dyspareunia according to the method disclosed herein can include, decreasing
the severity of
dyspareunia by 1%, 5%, 10%, 15%, 20%, 25%, 30%, 45%, 50%, 55%, 60%, 65%, 70%,
75%, 80%, 85%, 90%, 95%, or 99%. The decrease in severity can be obtained
following 2
weeks of treatment, or 6 weeks of treatment, or 8 weeks of treatment, or 12
weeks of
treatment. In some embodiments, dyspareunia is assessed using a severity
scale, ranging
from 0 to 4 points wherein 0 indicates no pain associated with sexual activity
(with vaginal
penetration), 1 indicates mild pain associated with sexual activity (with
vaginal penetration),
2 indicates moderate pain associated with sexual activity (with vaginal
penetration), and 3
indicates severe pain associated with sexual activity (with vaginal
penetration).
[0205] In some embodiments, the method for treating dyspareunia includes
reducing the
dyspareunia severity score from 3, prior to treatment of a subject, to 2,
after 2 weeks of
treatment of the subject. In some embodiments, the method for treating
dyspareunia includes
reducing the dyspareunia severity score from 2, prior to treatment of a
subject, to 1, after 2
weeks of treatment of the subject. In some embodiments, the method for
treating dyspareunia
44
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
includes reducing the dyspareunia severity score from 1, prior to treatment of
subject, to 0,
after 2 weeks of treatment of the subject.
[0206] In some embodiments, the method for treating dyspareunia includes
reducing the
dyspareunia severity score from 3, prior to treatment of a subject, to 2,
after 6 weeks of
treatment of the subject. In some embodiments, the method for treating
dyspareunia includes
reducing the dyspareunia severity score from 2, prior to treatment of a
subject, to 1, after 6
weeks of treatment of the subject. In some embodiments, the method for
treating dyspareunia
includes reducing the dyspareunia severity score from 1, prior to treatment of
subject, to 0,
after 6 weeks of treatment of the subject.
[0207] In some embodiments, the method for treating dyspareunia includes
reducing the
dyspareunia severity score from 3, prior to treatment of a subject, to 2,
after 8 weeks of
treatment of the subject. In some embodiments, the method for treating
dyspareunia includes
reducing the dyspareunia severity score from 2, prior to treatment of a
subject, to 1, after 8
weeks of treatment of the subject. In some embodiments, the method for
treating dyspareunia
includes reducing the dyspareunia severity score from 1, prior to treatment of
subject, to 0,
after 8 weeks of treatment of the subject.
[0208] In some embodiments, the method for treating dyspareunia includes
reducing the
dyspareunia severity score from 3, prior to treatment of a subject, to 2,
after 12 weeks of
treatment of the subject. In some embodiments, the method for treating
dyspareunia includes
reducing the dyspareunia severity score from 2, prior to treatment of a
subject, to 1, after 12
weeks of treatment of the subject. In some embodiments, the method for
treating dyspareunia
includes reducing the dyspareunia severity score from 1, prior to treatment of
subject, to 0,
after 12 weeks of treatment of the subject.
[0209] In some embodiments, the method for treating dyspareunia includes
decreasing the
severity of dyspareunia after two weeks of treatment, wherein the severity is
assessed on a
scale of 0-3 points, and the average decrease ranges from a 0.9-point decrease
to a 1.1-point
decrease. The average decrease can be determined by observing any suitable
number of
subjects. In some embodiments, the number of subjects is at least 100. In some
embodiments, the number of subjects is at least 500. In some embodiments, the
number of
subjects ranges from 700 to 800. In some embodiments, the number of subjects
ranges from
740 to 750. In some embodiments, the vaginal estradiol formulation contains 4
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 10
lig of
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
estradiol. In some embodiments, the vaginal estradiol formulation contains 25
lig of
estradiol.
[0210] In some embodiments, the method for treating dyspareunia includes
decreasing the
severity of dyspareunia after six weeks of treatment, wherein the severity is
assessed on a
scale of 0-3 points, and the average decrease ranges from a 1.3-point decrease
to a 1.5-point
decrease. The average decrease can be determined by observing any suitable
number of
subjects. In some embodiments, the number of subjects is at least 100. In some
embodiments, the number of subjects is at least 500. In some embodiments, the
number of
subjects ranges from 700 to 800. In some embodiments, the number of subjects
ranges from
740 to 750. In some embodiments, the vaginal estradiol formulation contains 4
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 10
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 25
lig of
estradiol.
[0211] In some embodiments, the method for treating dyspareunia includes
decreasing the
severity of dyspareunia after eight weeks of treatment, wherein the severity
is assessed on a
scale of 0-3 points, and the average decrease ranges from a 1.5-point decrease
to a 1.8-point
decrease. The average decrease can be determined by observing any suitable
number of
subjects. In some embodiments, the number of subjects is at least 100. In some
embodiments, the number of subjects is at least 500. In some embodiments, the
number of
subjects ranges from 700 to 800. In some embodiments, the number of subjects
ranges from
740 to 750. In some embodiments, the vaginal estradiol formulation contains 4
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 10
lig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 25
lig of
estradiol.
[0212] In some embodiments, the method for treating dyspareunia includes
decreasing the
severity of dyspareunia after twelve weeks of treatment, wherein the severity
is assessed on a
scale of 0-3 points, and the average decrease ranges from a 1.5-point decrease
to a 1.8-point
decrease. The average decrease can be determined by observing any suitable
number of
subjects. In some embodiments, the number of subjects is at least 100. In some
embodiments, the number of subjects is at least 500. In some embodiments, the
number of
subjects ranges from 700 to 800. In some embodiments, the number of subjects
ranges from
740 to 750. In some embodiments, the vaginal estradiol formulation contains 4
lig of
46
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
estradiol. In some embodiments, the vaginal estradiol formulation contains 10
[ig of
estradiol. In some embodiments, the vaginal estradiol formulation contains 25
[ig of
estradiol.
[0213] In some embodiments, the method for treating dyspareunia includes
administering a
suppository so as to provide an estradiol Cmax or AUC as described herein.
According to
embodiments, a method for treating dyspareunia is provided, the method
including
administering a suppository so as to provide an estrone Cmax or AUC as
described herein.
[0214] According to embodiments, a method for treating urinary tract
infections is
provided. As used herein the term "urinary tract infection" refers to an
infection of the
kidneys, ureters, bladder and urethra by a microorganism such as Escherichia
colt,
Staphylococcus saprophyticus, Klebsiella sp., Enterobacter sp., or Proteus sp.
The method
for treating urinary tract infections generally includes administering a soft
gel vaginal
estradiol formulation (i.e., a suppository) as described herein. According to
certain
embodiments, the method further includes decreasing urethral discomfort,
frequency or
urination, hematuria, dysuria, and/or stress incontinence. According to
certain embodiments,
a method for treating urinary tract infections is provided, the method
including administering
a suppository as described herein and decreasing vaginal pH from above 4.5 to
between 3.5
and 4.5 (inclusive). The method can be particularly effective for treating
urinary tract
infections in elderly subjects (e.g., subjects older than 65 years, or older
than 75 years, or
older than 85 years). According to embodiments, a method for treating urinary
tract
infections is provided, the method including administering a suppository so as
to provide an
estradiol Cmax or AUC as described herein. According to embodiments, a method
for treating
urinary tract infections is provided, the method including administering a
suppository so as to
provide an estrone Cmax or AUC as described herein. According to embodiments,
a method
for treating sexual dysfunction is provided. As used herein with respect to
female subjects,
the term "sexual dysfunction" generally refers to pain or discomfort during
sexual
intercourse, diminished vaginal lubrication, delayed vaginal engorgement,
increased time for
arousal, diminished ability to reach orgasm, diminished clitoral sensation,
diminished sexual
desire, and/or diminished arousal. According to embodiments, a method for
treating sexual
dysfunction is provided, the method including administering a suppository so
as to provide an
estradiol Cmax or AUC as described herein. According to embodiments, a method
for treating
sexual dysfunction is provided, the method including administering a
suppository so as to
provide an estrone Cmax or AUC as described herein.
47
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0215] Sexual function and dysfunction can be assessed using the Female Sexual
Function
Index (FSFI) (see, Rosen R, Brown C, Heiman J, et al. "The Female Sexual
Function Index
(FSFI): A Multidimensional Self-Report Instrument for the Assessment of Female
Sexual
Function." Journal of Sex & Marital Therapy 2000. 26: p.191-208). The FSFI is
useful for
assessing various domains of sexual functioning (e.g. sexual desire, arousal,
orgasm,
satisfaction and pain). Accordingly, the method for treating sexual
dysfunction as provided
herein can include administering a vaginal soft gel formulation to a subject
and increasing a
subject's full-scale FSFI score, FSFI-desire score, FSFI-arousal score, FSFI-
lubrication score
and/or FSFI-orgasm score.
Female Sexual Function Index (FSFI)
Question Answer Options
5 = Almost always or always
4 = Most times (more than half the time)
Ql: Over the past 4 weeks, how often did you feel
3 = Sometimes (about half the time)
sexual desire or interest?
2 = A few times (less than half the time)
1 = Almost never or never
5 = Very high
4 = High
Q2: Over the past 4 weeks, how would you rate your
3 = Moderate
level (degree) of sexual desire or interest?
2 = Low
1 = Very low or none at all
0 = No sexual activity
5 = Almost always or always
Q3. Over the past 4 weeks, how often did you feel
4 = Most times (more than half the time)
sexually aroused ("turned on") during sexual activity
3 = Sometimes (about half the time)
or intercourse?
2 = A few times (less than half the time)
1 = Almost never or never
0 = No sexual activity
5 = Very high
Q4. Over the past 4 weeks, how would you rate your
4 = High
level of sexual arousal ("turn on") during sexual
3 = Moderate
activity or intercourse?
2 = Low
1 = Very low or none at all
0 = No sexual activity
5 = Very high confidence
Q5. Over the past 4 weeks, how confident were you
4 = High confidence
about becoming sexually aroused during sexual
3 = Moderate confidence
activity or intercourse?
2 = Low confidence
1 = Very low or no confidence
0 = No sexual activity
5 = Almost always or always
Q6. Over the past 4 weeks, how often have you been
4 = Most times (more than half the time)
satisfied with your arousal (excitement) during sexual
3 = Sometimes (about half the time)
activity or intercourse? Response Options
2 = A few times (less than half the time)
1 = Almost never or never
0 = No sexual activity
Q7: Over the past 4 weeks, how often did you become 5 = Almost always or
always
lubricated ("wet") during sexual activity or 4 = Most times (more than half
the time)
intercourse? 3 = Sometimes (about half the time)
2 = A few times (less than half the time)
48
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
1 = Almost never or never
0 = No sexual activity
1 = Extremely difficult or impossible
Q8. Over the past 4 weeks, how difficult was it to
2 = Very difficult
become lubricated ("wet") during sexual activity or
3 = Difficult
intercourse?
4 = Slightly difficult
= Not difficult
0 = No sexual activity
5 = Almost always or always
Q9: Over the past 4 weeks, how often did you
4 = Most times (more than half the time)
maintain your lubrication ("wetness") until completion
3 = Sometimes (about half the time)
of sexual activity or intercourse?
2 = A few times (less than half the time)
1 = Almost never or never
0 = No sexual activity
1 = Extremely difficult or impossible
Q10: Over the past 4 weeks, how difficult was it to
2 = Very difficult
maintain your lubrication ("wetness") until completion
3 = Difficult
of sexual activity or intercourse?
4 = Slightly difficult
5 = Not difficult
0 = No sexual activity
5 = Almost always or always
Q11. Over the past 4 weeks, when you had sexual
4 = Most times (more than half the time)
stimulation or intercourse, how often did you reach
3 = Sometimes (about half the time)
orgasm (climax)?
2 = A few times (less than half the time)
1 = Almost never or never
0 = No sexual activity
1 = Extremely difficult or impossible
Q12: Over the past 4 weeks, when you had sexual
2 = Very difficult
stimulation or intercourse, how difficult was it for you
3 = Difficult
to reach orgasm (climax)?
4 = Slightly difficult
5 = Not difficult
0 = No sexual activity
5 = Very satisfied 4
Q13: Over the past 4 weeks, how satisfied were you
4 = Moderately satisfied
with your ability to reach orgasm (climax) during
3 = About equally satisfied and dissatisfied
sexual activity or intercourse?
2 = Moderately dissatisfied
1 = Very dissatisfied
0 = No sexual activity
5 = Very satisfied
Q14: Over the past 4 weeks, how satisfied have you
4 = Moderately satisfied
been with the amount of emotional closeness during
3 = About equally satisfied and dissatisfied
sexual activity between you and your partner?
2 = Moderately dissatisfied
1 = Very dissatisfied
5 = Very satisfied
4 = Moderately satisfied
Q15: Over the past 4 weeks, how satisfied have you
3 = About equally satisfied and dissatisfied
been with your sexual relationship with your partner?
2 = Moderately dissatisfied
1 = Very dissatisfied
5 = Very satisfied
4 = Moderately satisfied
Q16: Over the past 4 weeks, how satisfied have you
3 = About equally satisfied and dissatisfied
been with your overall sexual life?
2 = Moderately dissatisfied
1 = Very dissatisfied
0 = Did not attempt intercourse
I = Almost always or always
Q17: Over the past 4 weeks, how often did you
2 = Most times (more than half the time)
experience discomfort or pain during vaginal
3 = Sometimes (about half the time)
penetration?
4 = A few times (less than half the time)
5 = Almost never or never
Q18: Over the past 4 weeks, how often did you 0 = Did not attempt
intercourse
49
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
experience discomfort or pain following vaginal 1 = Almost always or always
penetration? 2 = Most times (more than half the
time)
3 = Sometimes (about half the time)
4 = A few times (less than half the time)
= Almost never or never
0 = Did not attempt intercourse
1 = Very high
Q19. Over the past 4 weeks, how would you rate your
2 = High
level (degree) of discomfort or pain during or
3 = Moderate
following vaginal penetration?
4 = Low
5 = Very low or none at all
FSFI Scorin2 System
Domain Questions Score Range Factor Minimum
Maximum
Desire 1,2 1-5 0.6 1.2 6.0
Arousal 3, 4, 5, 6 0-5 0.3 0 6.0
Lubrication 7, 8, 9, 10 0-5 0.3 0 6.0
Orgasm 11, 12, 13 0-5 0.4 0 6.0
Satisfaction 14, 15, 16 0 (or 1)-5 0.4 0.8 6.0
Pain 17, 18, 19 0-5 0.4 0 6.0
Full Scale Score Range: 2.0 36.0
[0216] In some embodiments, the method for treating sexual dysfunction
includes
5 administering estradiol to the subject and increasing the FSFI-desire
score by at least about
20%, or at least about 25%, or at least about 30% as compared to baseline.
[0217] In some embodiments, the method for treating sexual dysfunction
includes
administering estradiol to the subject and increasing the FSFI-arousal score
by at least about
30%, or at least about 40%, or at least about 50% as compared to baseline.
[0218] In some embodiments, the method for treating sexual dysfunction
includes
administering estradiol to the subject and increasing the FSFI-lubrication
score by at least
about 85%, or at least about 95%, or at least about 115% as compared to
baseline.
[0219] In some embodiments, the method for treating sexual dysfunction
includes
administering estradiol to the subject and increasing the FSFI-orgasm score by
at least about
40%, or at least about 60% as compared to baseline.
[0220] In some embodiments, the method for treating sexual dysfunction
includes
administering estradiol to the subject and increasing the total FSFI score by
at least about
50%, or at least about 55%, or at least about 70% as compared to baseline.
[0221] Examples of other metrics for assessment of sexual function include,
but are not
limited to, Changes in Sexual Function Questionnaire ("CSFQ"; Clayton et al.,
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Psychopharmacol Bull. 33(4):731-45 (1997) and Clayton et al., Psychopharmacol.
Bull.
33(4):747-53 (1997)); the Derogatis Interview for Sexual Functioning¨Self-
Report ("DISF-
SR"; Derogatis, J Sex Marital Ther. 23:291-304 (1997)); the Golombok-Rust
Inventory of
Sexual Satisfaction ("GRISS"; Rust et al., Arch. Sex Behav. 15:157-165
(1986)); the Sexual
Function Questionnaire ("SFQ"; Quirk et al., J Womens Health Gend Based Med.
11:277-289
(2002)); and the Arizona Sexual Experience Scale ("ASEX"; McGahuey et al., J
Sex Marital
Ther. 26:25-40 (2000)), the entire disclosures of which are incorporated
herein by reference.
For assessment using a questionnaire, a measure of sexual dysfunction function
is increased
when the score in the appropriate domain, subscale or subtest is indicative of
sexual
dysfunction, as established for that questionnaire. For instance, a female's
sexual interest is
considered reduced, when assessed using the CSFQ, if the subscale for sexual
interest score is
less than or equal to 9. Conversely, sexual dysfunction is considered improved
when the
score in the appropriate domain, subscale or subtest is indicative of higher
(e.g., normal or
desired) sexual function. For a clinician's assessment, sexual dysfunction may
be assessed in
comparison to a previous point in time for the patient and/or in comparison to
a patient's
peers with respect to age, gender, sexual experience, and health, or may also
be determined
via a validated questionnaire administered by the clinician.
[0222] According to embodiments, the efficacy and safety of the pharmaceutical
compositions described herein in the treatment of the symptoms of VVA may be
determined.
According to embodiments, the size, effect, cytology, histology, and
variability of the VVA
may be determined using various endpoints to determine efficacy and safety of
the
pharmaceutical compositions described herein or as otherwise accepted in the
art, at present
or as further developed. One source of endpoints is with the US Food and Drug
Administration's (FDA) published guidelines for treatment of VVA with
estradiol.
[0223] According to embodiments, a method of treating VVA, including
dyspareunia,
vaginal dryness, and estrogen-deficient urinary states (including urinary
tract infections), is
provided that allows a subject to be ambulatory immediately or within minutes
after a gelatin
capsule containing the pharmaceutical compositions disclosed herein are
administered.
According to embodiments, a gelatin capsule containing a pharmaceutical
composition as
disclosed herein is administered by digitally inserting the gelatin capsule
containing the
pharmaceutical composition into the vagina approximately two inches or
inserting into the
third of the vagina closest to the vaginal opening as shown in Figs. 26A, 26B,
and 26C.
According to embodiments, the gelatin capsule adheres to the vaginal tissue
and dissolves,
51
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
ruptures, or otherwise disintegrates soon after being inserted into the vagina
thereby releasing
the pharmaceutical composition. The pharmaceutical composition spreads onto
the vaginal
tissue and is rapidly absorbed. According to embodiments, the gelatin capsule
is also fully
absorbed by the vaginal tissue. According to some embodiments, a viscosity
enchancer such
as TEFOSE 63 provides increased viscosity to ensure the pharmaceutical
composition stays
within the desired absorption area, thereby estrogenizing the vagina, labia,
and/or vulva. The
combination of high viscosity, bioadhesion, and rapid absorption prevents the
need for
subjects to remain supine after administration to allow the tissue to absorb
the estradiol,
thereby allowing subjects to be ambulatory immediately or almost immediately
after
administration.
[0224] According to embodiments, a method for treating VVA, including
dyspareunia,
vaginal dryness, and estrogen-deficient urinary states (including urinary
tract infections),
without causing non-natural discharge (e.g., discharge of a pharmaceutical
composition or a
component thereof) is provided. According to the method, a soft gelatin
capsule is
administered containing a liquid pharmaceutical composition that is able to be
fully absorbed
by the vaginal tissue. According to embodiments, the pharmaceutical
composition itself is
fully absorbed by the vaginal tissue. According to embodiments, the
pharmaceutical
composition and gelatin capsule are administered in a volume and size,
respectively, that
allows a subject's vaginal tissue to fully absorb the pharmaceutical
composition. According
to embodiments, such absorption will occur contemporaneously with the subject
being
ambulatory. According to the method, the gelatin capsule and liquid
pharmaceutical
composition are fully absorved by the vaginal tissue, wherein the only
discharge that occurs
after estrogenizing the vagina is natural discharge that a woman would have
experienced
prior to menopause. "Natural" vaginal discharge refers to a small amount of
fluid that flows
out of the vagina each day, carrying out old cells that have lined the vagina.
Natural discharge
is usually clear or milky. Non-natural discharge can refer to discharge that
is higher in
volume than natural discharge, different in color than natural discharge, or
different in
consistency than natural discharge. Non-natural discharge can also refer to
the discharge
(e.g., leaking) of a pharmaceutical composition from the vagina.
[0225] According to embodiments, a method of treating VVA, including
dyspareunia,
vaginal dryness, and estrogen-deficient urinary states (including urinary
tract infections),
using a liquid pharmaceutical composition is provided. According to the
method, a soft
gelatin capsule containing a liquid composition for treating VVA is provided
to a subject.
52
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
The subject inserts the soft gelatin capsule containing the liquid composition
for treating
VVA into their vagina either digitally or with an applicator, wherein the soft
gelatin capsule
dissolves, ruptures, or disintegrates and the liquid composition is released
into the vagina.
According to embodiments, the liquid composition for treating VVA is a
pharmaceutical
composition disclosed herein. According to embodiments, the subject inserts
the gelatin
capsule about two inches into the vagina, or in the third of the vagina
closest to the vaginal
opening. According to embodiments, the subject is ambulatory immediately after
or soon
after administration.
[0226] According to embodiments, a method is disclosed herein for treating
VVA,
including dyspareunia, vaginal dryness, and estrogen-deficient urinary states
(including
urinary tract infections), comprising improving the symptoms of VVA, compared
to placebo
or baseline, within two weeks by vaginally administering a composition for the
treatment of
VVA. One of skill in the art will understand that the improvements can be
assessed
statistically as described herein, and that any improvement can be a
statistically significant
improvement. According to embodiments, the composition for the treatment of
VVA is a
liquid pharmaceutical composition as disclosed herein. According to
embodiments, the
composition for the treatment of VVA is a liquid containing from 1 lig to 25
lig of estradiol.
According to embodiments, the method of administration is a method disclosed
herein,
including the insertion method shown in Figs. 26A, 26B, and 26C. According to
embodiments, at the two week point of measurement, the estradiol is not
detected
systemically when measured using standard pharmaceutical pharmacokinetic
parameters,
such as AUC and C.
[0227] According to embodiments, a method is disclosed herein for treating
VVA,
including dyspareunia, vaginal dryness, and estrogen-deficient urinary states
(including
urinary tract infections), comprising improving the symptoms of VVA, compared
to placebo
or baseline, within four weeks by vaginally administering a composition for
the treatment of
VVA. One of skill in the art will understand that the improvements can be
assessed
statistically as described herein, and that any improvement can be a
statistically significant
improvement. According to embodiments, the composition for the treatment of
VVA is a
liquid pharmaceutical composition as disclosed herein. According to
embodiments, the
composition for the treatment of VVA is a liquid containing from 1 lig to 25
lig of estradiol.
According to embodiments, the method of administration is a method disclosed
herein,
including the insertion method shown in Figs. 26A, 26B, and 26C. According to
53
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
embodiments, at the two week point of measurement and/or the four week point
of
measurement, the estradiol is not detected systemically when measured using
standard
pharmaceutical pharmacokinetic parameters, such as AUC and C.
[0228] According to embodiments, a method is disclosed herein for treating
VVA,
including dyspareunia, vaginal dryness, and estrogen-deficient urinary states
(including
urinary tract infections), comprising improving the symptoms of VVA, compared
to placebo
or baseline, within eight weeks by vaginally administering a composition for
the treatment of
VVA. One of skill in the art will understand that the improvements can be
assessed
statistically as described herein, and that any improvement can be a
statistically significant
improvement. According to embodiments, the composition for the treatment of
VVA is a
liquid pharmaceutical composition as disclosed herein. According to
embodiments, the
composition for the treatment of VVA is a liquid containing from 1 pg to 25 pg
of estradiol.
According to embodiments, the method of administration is a method disclosed
herein,
including the insertion method shown in Figs. 26A, 26B, and 26C. According to
embodiments, at the two week point of measurement and/or the eight week point
of
measurement, the estradiol is not detected systemically when measured using
standard
pharmaceutical pharmacokinetic parameters, such as AUC and Cmax.
[0229] According to embodiments, a method is disclosed herein for treating
VVA,
including dyspareunia, vaginal dryness, and estrogen-deficient urinary states
(including
urinary tract infections), comprising improving the symptoms of VVA, compared
to placebo
or baseline, within ten weeks by vaginally administering a composition for the
treatment of
VVA. One of skill in the art will understand that the improvements can be
assessed
statistically as described herein, and that any improvement can be a
statistically significant
improvement. According to embodiments, the composition for the treatment of
VVA is a
liquid pharmaceutical composition as disclosed herein. According to
embodiments, the
composition for the treatment of VVA is a liquid containing from 1 pg to 25 pg
of estradiol.
According to embodiments, the method of administration is a method disclosed
herein,
including the insertion method shown in Figs. 26A, 26B, and 26C. According to
embodiments, at the two week point of measurement and/or the ten week point of
measurement, the estradiol is not detected systemically when measured using
standard
pharmaceutical pharmacokinetic parameters, such as AUC and Cmax.
54
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0230] According to embodiments, a method for treating VVA, including
dyspareunia,
vaginal dryness, and estrogen-deficient urinary states (including urinary
tract infections),
comprising administering a composition containing estradiol for the treatment
of VVA is
provided, wherein the method improves the symptoms of VVA, compared with
baseline or
placebo, in at least one of two weeks, four weeks, six weeks, eight weeks, or
twelve weeks,
wherein the estradiol is not detected systemically using standard
pharmaceutical
pharmacokinetic parameters, such as AUC and C.. One of skill in the art will
understand
that the improvements can be assessed statistically as described herein, and
that any
improvement can be a statistically significant improvement. According to
embodiments, the
composition containing estradiol is a liquid composition as disclosed herein.
According to
embodiments, the copomosition contains 1 [ig to 25 [ig of estradiol.
[0231] According to embodiments, a method for reestrogenizing the vagina,
labia, or vulva
is provided, wherein the method comprises administering a composition
containing estradiol
for the treatment of VVA, wherein the composition is a liquid containing
estradiol or a
synthetic estrogen, and wherein the liquid spreads over a surface area of the
vagina, labia, or
vulva which is larger than the area covered by a solid composition. For
example, the liquid
can spread over a surface area ranging from about 50 cm2 to about 120 cm2
(e.g., from about
50 cm2 to about 60 cm2; or from about 60 cm2 to about 70 cm2; or from about 70
cm2 to about
80 cm2; or from about 80 cm2 to about 90 cm2; or from about 90 cm2 to about
100 cm2; or
from about 100 cm2 to about 110 cm2; or from about 110 cm2 to about 120 cm2;
or from
about 65 cm2 to about 110 cm2). According to embodiments, the subject inserts
a liquid
composition into her vagina in a capsule, such as a hard or soft gelatin
capsule, that then
dissolves, ruptures, disintegrates, or otherwise releases the liquid in the
vagina. According to
embodiments, the liquid contains at least one of a bio-adhesive or viscosity
enhancer to
prevent the liquid from discharging from the vagina before the estradiol or
synthetic estrogen
can be absorbed into the vaginal tissue in a dose sufficient to effect
reestrongenization of the
vagina. According to embodiments, the vagina will be statistically
significantly
reestrogenized within two weeks of administration compared to baseline or
placebo levels.
According to embodiments, the vagina will be statistically significantly
reestrogenized within
four weeks of administration compared to baseline or placebo levels.
[0232] According to embodiments, the vagina will be statistically
significantly
reestrogenized within six weeks of administration compared to baseline or
placebo levels.
According to embodiments, the vagina will be statistically significantly
reestrogenized within
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
eight weeks of administration compared to baseline or placebo levels.
According to
embodiments, the vagina will be statistically significantly reestrogenized
within ten weeks of
administration compared to baseline or placebo levels. According to
embodiments, the vagina
will be statistically significantly reestrogenized within twelve or more weeks
of
administration compared to baseline or placebo levels.
VII. MEASUREMENT OF EFFICACY
[0233] According to embodiments, administration of the pharmaceutical
compositions
described herein resulted in treatment of the VVA, as well as improvement of
one or more of
the associated symptoms. Patients with VVA experience shrinking of the vaginal
canal in
both length and diameter and the vaginal canal has fewer glycogen-rich vaginal
cells to
maintain moisture and suppleness. In addition, the vaginal wall can become
thin, pale, dry, or
sometimes inflamed (atrophic vaginitis). These changes can manifest as a
variety of
symptoms collectively referred to as VVA. Such symptoms include, without
limitations, an
increase in vaginal pH; reduction of vaginal epithelial integrity, vaginal
secretions, or
epithelial surface thickness; pruritus; vaginal dryness; dyspareunia (pain or
bleeding during
sexual intercourse); urinary tract infections; or a change in vaginal color.
According to
embodiments, efficacy is measured as a reduction of vulvar and vaginal atrophy
in a patient
back to premenopausal conditions. According to embodiments, the change is
measured as a
reduction in the severity of one or more atrophic effects measured at baseline
(screening, Day
1) and compared to a measurement taken at Day 15 (end of treatment). Severity
of the
atrophic effect may be measured using a scale of 0 to 3 where, for example,
none = 0, mild =
1, moderate = 2, or severe = 3. Such scoring is implemented to evaluate the
pre-treatment
condition of patients; to determine the appropriate course of a treatment
regime; such as
dosage, dosing frequency, and duration, among others; and post-treatment
outcomes.
[0234] One of the symptoms of VVA is increased vaginal pH. In further aspects
of this
disclosure, treatment with the pharmaceutical compositions described herein
resulted in a
decrease in vaginal pH. A decrease in vaginal pH is measured as a decrease
from the vaginal
pH at baseline (screening) to the vaginal pH at Day 15, according to
embodiments. In some
embodiments, a pH of 5 or greater may be associated with VVA. In some
embodiments, pH
is measured using a pH indicator strip placed against the vaginal wall. In
some embodiments,
a change in vaginal pH is a change in a patient's vaginal pH to a pH of less
than about pH
5Ø In some embodiments, a subject's vaginal pH may be less than about pH
4.9, pH 4.8, pH
56
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
4.7, pH 4.6, pH 4.5, pH 4.4, pH 4.3, pH 4.2, pH 4.1, pH 4.0, pH 3.9, pH 3.8,
pH 3.7, pH 3.6,
or pH 3.5.
[0235] According to embodiments, treatment with the pharmaceutical
compositions
described herein resulted in improvements in the vaginal Maturation Index. The
Maturation
Index is measured as a change in cell composition. According to embodiments
and as related
to VVA, a change in cell composition is measured as the change in percent of
composition or
amount of parabasal vaginal cells, intermediate cells, and superficial vaginal
cells, such as a
change in the composition or amount of parabasal vaginal cells compared with
or, relative to,
a change in superficial vaginal cells. A subject having VVA symptoms often has
an increased
number of parabasal cells and a reduced number of superficial cells (e.g.,
less than about 5%)
compared with women who do not suffer from VVA. Conversely, a subject having
decreasing VVA symptoms, or as otherwise responding to treatment, may
demonstrate an
improvement in the Maturation Index, specifically a decrease in the amount of
parabasal cells
or an increase in the amount of superficial cells compared to baseline
(screening). In
embodiments, a decrease in parabasal cells is measured as a reduction in the
percent of
parabasal cells; the percent reduction may be at least about an 85%, 80%, 75%,
70%, 65%,
60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15% or 10% reduction in the
number of
parabasal cells. In embodiments, a percent reduction may be at least about a
54% reduction in
the number of parabasal cells. In embodiments, an increase in superficial
cells is measured as
an increase in the percent of superficial cells; the percent increase in
superficial cells may be
at least about 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, or 50% increase in
the
number of superficial cells. In further embodiments, a percent increase may be
at least about
a 35% increase in the number of superficial cells.
[0236] In some embodiments, an improvement in the Maturation Index is assessed
as a
change over time. For example, as a change in cell composition measured at a
baseline
(screening) at Day 1 compared to the cell composition measured at Day 15. The
change in
cell composition may also be assessed as a change in the amount of parabasal
cells over time,
optionally in addition to measuring changes in parabasal cells and superficial
cells as
described above. Such cells may be obtained from the vaginal mucosal
epithelium through
routine gynecological examination and examined by means of a vaginal smear.
57
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0237] In various further aspects of this disclosure, treatment with the
pharmaceutical
compositions described herein resulted in any of: an increase in superficial
cells; a decrease
in parabasal cells; and an increase in intermediate cells.
[0238] In further aspects of this disclosure, samples may be collected to
determine
hormone levels, in particular, estradiol levels. In some embodiments, blood
samples may be
taken from a subject and the level of estradiol measured (pg/mL). In some
embodiments,
estradiol levels may be measured at 0 hours (for example, at time of first
treatment), at 1 hour
(for example, post first treatment), at 3 hours, and at 6 hours. In some
embodiments, samples
may be taken at day 8 (for example, post first treatment) and at day 15 (for
example, one day
post the last treatment on day 14). In some embodiments, descriptive
statistics of plasma
estradiol concentrations at each sampling time and observed C. and T. values
may be
measured and the AUC calculated.
[0239] In some embodiments, a suppository can comprise about 25 [ig of
estradiol. In such
cases, administration of the suppository to a patient can provide, in a plasma
sample from the
patient, parameters including one or more parameters selected from: 1) a
corrected geometric
mean peak plasma concentration (Cmax) of estradiol of about 19 pg*hr/mL to
about 29
pg*hr/mL (e.g., 19.55 pg*hr/mL to about 28.75 pg*hr/mL); or 2) a corrected
geometric mean
area under the curve (AUC)0_24 of estradiol of about 75 pg*hr/mL to about 112
pg*hr/mL
(e.g., 75.82 pg*hr/mL to about 111.50). In some embodiments, administration of
the
suppository to a patient provides, in a plasma sample from the patient, one or
more
parameters selected from: 1) a corrected geometric mean peak plasma
concentration (C.) of
estrone of about 9 pg*hr/mL to about 14 pg*hr/mL (e.g., 9.17 pg*hr/mL to about
13.49
pg*hr/mL); and 2) a corrected geometric mean area under the curve (AUC)0_24 of
estrone of
about 43 pg*hr/mL to about 65 pg*hr/mL (e.g., 43.56 pg*hr/mL to about 64.06
pg*hr/mL).
In some embodiments, administration of the suppository to a patient provides,
in a plasma
sample from the patient, provides one or more parameters selected from: 1) a
corrected
geometric mean peak plasma concentration (Cmax) of estrone sulfate of about
416 pg*hr/mL
to about 613 pg*hr/mL (e.g., 416.53 pg*hr/mL to about 612.55 pg*hr/mL); and 2)
a corrected
geometric mean area under the curve (AUC)0_24 of estrone sulfate of about 3598
pg*hr/mL to
about 5291 pg*hr/mL (e.g., 3598.04 pg*hr/mL to about 5291.24 pg*hr/mL).
[0240] In some embodiments, a suppository includes about 25 [ig of estradiol.
In some
such embodiments, administration of the suppository to a patient can provide,
in a plasma
58
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
sample from the patient, parameters including one or more parameters selected
from: 1) a
corrected geometric mean peak plasma concentration (Cmax) of estradiol ranging
from about
20.9 pg/mL to about 32.8 pg/mL (e.g., 20.96 pg/mL to about 32.75 pg/mL); 2) a
corrected
geometric mean area under the curve (AUC)0_24 of estradiol ranging from about
104.3
pg*hr/mL to about 163.1 pg*hr/mL (e.g., 104.32 pg*hr/mL to about 163.0
pg*hr/mL); and 3)
an average concentration (C,g) of estradiol ranging from about 4.3 pg/mL to
about 6.8
pg/mL (e.g., 4.32 pg/mL to about 6.75 pg/mL), as assessed at day 1.
[0241] In some embodiments, administration of a suppository comprising about
25 pg of
estradiol to a patient can provide, in a plasma sample from the patient,
parameters including
one or more parameters selected from: 1) a corrected geometric mean peak
plasma
concentration (C) of estradiol of about 26.2 pg/mL; 2) a corrected geometric
mean area
under the curve (AUC)0_24 of estradiol of about 130 pg*hr/mL; and 3) an
average
concentration (C,g) of estradiol of about 5.4 pg/mL, as assessed at day 1.
[0242] In some embodiments, administration of a suppository comprising about
25 pg of
estradiol to a patient can provide, in a plasma sample from the patient,
parameters including
one or more parameters selected from: 1) a corrected geometric mean peak
plasma
concentration (Cmax) of estradiol ranging from about 9.5 pg/mL to about 15.1
pg/mL (e.g.,
9.60 pg*hr/mL to about 15.00 pg/mL); 2) a corrected geometric mean area under
the curve
(AUC)0_24 of estradiol ranging from about 67.6 pg*hr/mL to about 105.8
pg*hr/mL (e.g.,
67.68 pg*hr/mL to about 105.75 pg*hr/mL); and 3) an average concentration
(C,g) of
estradiol ranging from about 2.7 pg/mL to about 4.4 pg/mL (e.g., 2.80 pg/mL to
about 4.38
pg/mL) of estradiol as assessed at day 14.
[0243] In some embodiments, administration of a suppository comprising about
25 pg of
estradiol to a patient can provide, in a plasma sample from the patient,
parameters including
one or more parameters selected from: 1) a corrected geometric mean peak
plasma
concentration (Cmax) of estradiol of about 12.0 pg/mL; 2) a corrected
geometric mean area
under the curve (AUC)0_24 of estradiol of about 84.6 pg*hr/mL; and 3) an
average
concentration (C,g) of estradiol of about 3.5 pg/mL, as assessed at day 14.
[0244] In some embodiments, administration of a suppository comprising about
25 pg of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) a corrected geometric mean peak plasma concentration (Cmax)
of estrone
conjugates ranging from about 158.8 pg/mL to about 248.3 pg/mL (e.g., 158.88
hr/mL to
59
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
about 248.25 pg*hr/mL); and 2) a corrected geometric mean area under the curve
(AUC)0-24
of estrone conjugates ranging from about 1963.1 pg*hr/mL to about 3067.6
pg*hr/mL (e.g.,
1963.20 pg*hr/mL to about 3067.50 pg*hr/mL) as assessed at day 1.
[0245] In some embodiments, administration of a suppository comprising about
25 pg of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) a corrected geometric mean peak plasma concentration (Cmax)
of estrone
conjugates of about 198.6 pg/mL; and 2) a corrected geometric mean area under
the curve
(AUC)0_24 of estrone conjugates of about 2454 pg*hr/mL as assessed at day 1.
[0246] In some embodiments, administration of a suppository comprising about
25 pg of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) an unadjusted arithmetic mean area under the curve (AUC)0_24
of estradiol
ranging from about 173.5 pg*hr/mL to about 271.3 pg*hr/mL (e.g., from 173.60
pg*hr/mL to
about 271.25 pg*hr/mL; or about 217 pg*hr/mL), as assessed at day 1; 2) a
corrected
arithmetic mean peak plasma concentration (Cavg[0_241) of estradiol ranging
from about 7.2
pg/mL to about 11.4 pg/mL (e.g., from 7.25 pg/mL to about 11.33 pg/mL; or
about 9.06
pg/mL), as assessed at day 1; 3) an unadjusted arithmetic mean area under the
curve (AUC)0_
24 of estradiol ranging from about 137.5 pg*hr/mL to about 215.1 pg*hr/mL
(e.g., from
137.60 pg*hr/mL to about 215.00 pg*hr/mL; or about 172 pg*hr/mL), as assessed
at day 14;
and 4) a corrected arithmetic mean peak plasma concentration (Cavg[0_241) of
estradiol ranging
from about 5.7 pg/mL to about 9.0 pg/mL (e.g., from 5.72 pg/mL to about 8.94
pg/mL; or
about 7.15 pg/mL), as assessed at day 14.
[0247] In some embodiments, administration of a suppository comprising about
25 pg of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) an unadjusted arithmetic mean area under the curve (AUC)0_24
of estrone
ranging from about 335.1 pg*hr/mL to about 523.8 pg*hr/mL (e.g., from 335.20
pg*hr/mL to
about 523.75 pg*hr/mL; or about 419 pg*hr/mL), as assessed at day 1; 2) a
corrected
arithmetic mean peak plasma concentration (Cavg[0_241) of estrone ranging from
about 13.9
pg/mL to about 21.9 pg/mL (e.g., from 14.00 pg/mL to about 21.88 pg/mL; or
about 17.5
pg/mL), as assessed at day 1; 3) an unadjusted arithmetic mean area under the
curve (AUC)0_
24 of estrone ranging from about 343.1pg*hr/mL to about 536.2 pg*hr/mL (e.g.,
from 343.20
pg*hr/mL to about 536.25 pg*hr/mL; or about 429 pg*hr/mL), as assessed at day
14; and 4) a
corrected arithmetic mean peak plasma concentration (Cavg[0_241) of estrone
ranging from
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
about 14.3 pg/mL to about 22.4 pg/mL (e.g., from 14.32 pg/mL to about 22.38
pg/mL; or
about 17.9 pg/mL), as assessed at day 14.
[0248] In some embodiments, administration of a suppository comprising about
25 ng of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) an unadjusted arithmetic mean area under the curve (AUC)0_24
of estrone
conjugates ranging from about 7,300.7 pg*hr/mL to about 11,407.6 pg*hr/mL
(e.g., from
7,300.80 pg*hr/mL to about 11,407.50 pg*hr/mL; or about 9,126 pg*hr/mL), as
assessed at
day 1; 2) a corrected arithmetic mean peak plasma concentration (Cmg[0_241) of
estrone
conjugates ranging from about 303.9 pg/mL to about 475.1 pg/mL (e.g., from
304.00 pg/mL
to about 475.00 pg/mL; or about 380 pg/mL), as assessed at day 1; 3) an
unadjusted
arithmetic mean area under the curve (AUC)0_24 of estrone conjugates ranging
from about
7,943.9 pg*hr/mL to about 12,412.6 pg*hr/mL (e.g., from 7,944.00 pg*hr/mL to
about
12,412.50 pg*hr/mL; or about 9,930 pg*hr/mL), as assessed at day 14; and 4) a
corrected
arithmetic mean peak plasma concentration (Cavg[0_241) of estrone conjugates
ranging from
about 331.1 pg/mL to about 517.4 pg/mL (e.g., from 331.20 pg/mL to about
517.50 pg/mL;
or about 414 pg/mL), as assessed at day 14.
[0249] In some embodiments, a suppository can comprise about 10 ng of
estradiol. In such
cases, administration of the suppository to a patient can provide, in a plasma
sample from the
patient, one or more parameters selected from: 1) a corrected geometric mean
peak plasma
concentration (Cmax) of estradiol of about 12 pg*hr/mL to about 18 pg*hr/mL
(e.g., 12.22
pg*hr/mL to about 17.98 pg*hr/mL); 2) a corrected geometric mean area under
the curve
(AUC)0_24 of estradiol of about 42 pg*hr/mL to about 63 pg*hr/mL (e.g., 42.18
pg*hr/mL to
about 62.02 pg*hr/mL); and 3) a corrected geometric mean time to peak plasma
concentration (Tmax) of estradiol of about 1 hrs to about 3 hrs (e.g., 1.49
hrs to about 2.19
hrs). In some embodiments, administration of the suppository to a patient
provides, in a
plasma sample from the patient, one or more parameters selected from: 1) a
corrected
geometric mean peak plasma concentration (Cmax) of estrone of about 4 pg*hr/mL
to about 7
pg*hr/mL (e.g., 4.38 pg*hr/mL to about 6.44 pg*hr/mL); 2) a corrected
geometric mean area
under the curve (AUC)0_24 of estrone of about 20 pg*hr/mL to about 31 pg*hr/mL
(e.g., 20.60
pg*hr/mL to about 30.30 pg*hr/mL); and 3) a corrected geometric mean time to
peak plasma
concentration (Tmax) of estrone of about 4 hrs to about 8 hrs (e.g., 4.99 hrs
to about 7.34 hrs).
In some embodiments, administration of the suppository to a patient provides,
in a plasma
sample from the patient, one or more parameters selected from: 1) a corrected
geometric
61
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
mean peak plasma concentration (Cmax) of estrone sulfate of about 10 pg*hr/mL
to about 16
pg*hr/mL (e.g., 10.34 pg*hr/mL to about 15.20 pg*hr/mL); 2) a corrected
geometric mean
area under the curve (AUC)0_24 of estrone sulfate of about 56 pg*hr/mL to
about 84 pg*hr/mL
(e.g., 56.61 pg*hr/mL to about 83.25 pg*hr/mL); and 3) a corrected geometric
mean time to
peak plasma concentration (Tmax) of estrone sulfate of about 4 hrs to about 7
hrs (e.g., 4.67
hrs to about 6.86 hrs).
[0250] In some embodiments, a suppository includes about 10 pg of estradiol.
In some
such embodiments, administration of the suppository to a patient can provide,
in a plasma
sample from the patient, a corrected geometric mean peak plasma concentration
(Cmax) of
estradiol ranging from about 4.7 pg/mL to about 7.6 pg/mL (e.g., 4.80 pg*hr/mL
to about
7.50 pg*hr/mL), as assessed at day 1. In some embodiments, administration of a
suppository
comprising about 10 pg of estradiol to a patient can provide, in a plasma
sample from the
patient, a corrected geometric mean peak plasma concentration (Cmax) of
estradiol ranging
from about 2.3 pg*hr/mL to about 3.8 pg*hr/mL (e.g., 2.40 pg*hr/mL to about
3.75
pg*hr/mL) of estradiol as assessed at day 14.
[0251] In some embodiments, administration of a suppository comprising about
10 pg of
estradiol to a patient provides, in a plasma sample from the patient, a
corrected geometric
mean peak plasma concentration (Cmax) of estradiol of about 6.0 pg/mL, as
assessed at day 1.
In some embodiments, administration of a suppository comprising about 10 pg of
estradiol to
a patient can provide, in a plasma sample from the patient, a corrected
geometric mean peak
plasma concentration (Cmax) of estradiol of about 3.0 pg/mL, as assessed at
day 14.
[0252] In some embodiments, administration of a suppository comprising about
10 pg of
estradiol to a patient provides, in a plasma sample from the patient, a
corrected geometric
mean area under the curve (AUC)0_24 of estradiol ranging from about 17.5 pg/mL
to about
27.4 pg/mL (e.g., 17.52 pg*hr/mL to about 27.37 pg*hr/mL), as assessed at day
1. In some
embodiments, administration of a suppository comprising about 10 pg of
estradiol to a
patient can provide, in a plasma sample from the patient, a corrected
geometric mean area
under the curve (AUC)0_24 of estradiol ranging from about 10.9 pg*hr/mL to
about 17.2
pg*hr/mL (e.g., 10.96 pg*hr/mL to about 17.13 pg*hr/mL) of estradiol as
assessed at day 14.
[0253] In some embodiments, administration of a suppository comprising about
10 pg of
estradiol to a patient provides, in a plasma sample from the patient, a
corrected geometric
mean area under the curve (AUC)0_24 of estradiol of about 21.9 pg*hr/mL, as
assessed at day
62
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
1. In some embodiments, administration of a suppository comprising about 10
[ig of
estradiol to a patient can provide, in a plasma sample from the patient, a
corrected geometric
mean area under the curve (AUC)0_24 of estradiol of about 13.7 pg*hr/mL, as
assessed at day
14.
[0254] In some embodiments, administration of a suppository comprising about
10 [ig of
estradiol to a patient provides, in a plasma sample from the patient, an
average concentration
(Cavg) of estradiol ranging from about 0.6 pg/mL to about 1.1 pg/mL (e.g.,
0.64 pg/mL to
about 1.0 pg/mL), as assessed at day 1. In some embodiments, administration of
a
suppository comprising about 10 [ig of estradiol to a patient can provide, in
a plasma sample
from the patient, an average concentration (Cavg) of estradiol ranging from
about 0.1 pg/mL
to about 0.3 pg/mL (e.g., 0.16 pg/mL to about 0.25 pg/mL) of estradiol as
assessed at day 14.
[0255] In some embodiments, administration of a suppository comprising about
10 [ig of
estradiol to a patient provides, in a plasma sample from the patient, an
average concentration
(Cavg) of estradiol of about 0.8 pg/mL, as assessed at day 1. In some
embodiments,
administration of a suppository comprising about 10 [ig of estradiol to a
patient can provide,
in a plasma sample from the patient, an average concentration (Cavg) of
estradiol of about 0.2
pg/mL, as assessed at day 14.
[0256] In some embodiments, administration of a suppository comprising about
10 [ig of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) a corrected geometric mean peak plasma concentration (Cmax)
of estrone
conjugates ranging from about 72.1 pg/mL to about 112.8 pg/mL (e.g., 72.16
pg/mL to about
112.75 pg/mL); and 2) an average concentration (Cavg) of estrone conjugates
ranging from
about 6.3 pg/mL to about 10.1 pg/mL (e.g., 6.40 pg/mL to about 10.00 pg/mL) as
assessed at
day 1.
[0257] In some embodiments, administration of a suppository comprising about
10 [ig of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) a corrected geometric mean peak plasma concentration (Cmax)
of estrone
conjugates of about 90.2 pg/mL; and 2) an average concentration (Cavg) of
estrone conjugates
of about 8.0 pg/mL, as assessed at day 1.
[0258] In some embodiments, administration of a suppository comprising about
10 [ig of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) an unadjusted arithmetic mean area under the curve (AUC)0_24
of estradiol
63
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
ranging from about 110.3 pg*hr/mL to about 172.6 pg*hr/mL (e.g., from 110.40
pg*hr/mL to
about 172.50 pg*hr/mL; or about 138 pg*hr/mL), as assessed at day 1; 2) a
corrected
arithmetic mean peak plasma concentration (Cõg[0_241) of estradiol ranging
from about 4.6
pg/mL to about 7.8 pg/mL (e.g., from 4.61 pg/mL to about 7.20 pg/mL; or about
5.76
pg/mL), as assessed at day 1; 3) an unadjusted arithmetic mean area under the
curve (AUC)0_
24 of estradiol ranging from about 87.9 pg*hr/mL to about 137.4 pg*hr/mL
(e.g., from 88.00
pg*hr/mL to about 137.50 pg*hr/mL; or about 110 pg*hr/mL), as assessed at day
14; and 4) a
corrected arithmetic mean peak plasma concentration (Cavg[0_241) of estradiol
ranging from
about 3.6 pg/mL to about 5.8 pg/mL (e.g., from 3.67 pg/mL to about 5.74 pg/mL;
or about
4.59 pg/mL), as assessed at day 14.
[0259] In some embodiments, administration of a suppository comprising about
10 ug of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) an unadjusted arithmetic mean area under the curve (AUC)0_24
of estrone
ranging from about 370.3 pg*hr/mL to about 578.8 pg*hr/mL (e.g., from 370.40
pg*hr/mL to
about 578.75 pg*hr/mL; or about 463 pg*hr/mL), as assessed at day 1; 2) a
corrected
arithmetic mean peak plasma concentration (Cavg[0_241) of estrone ranging from
about 15.4
pg/mL to about 24.2 pg/mL (e.g., from 15.44 pg/mL to about 24.13 pg/mL; or
about 19.3
pg/mL), as assessed at day 1; 3) an unadjusted arithmetic mean area under the
curve (AUC)0_
24 of estrone ranging from about 371.1 pg*hr/mL to about 580.1 pg*hr/mL (e.g.,
from 371.20
pg*hr/mL to about 580.00 pg*hr/mL; or about 464 pg*hr/mL), as assessed at day
14; and 4) a
corrected arithmetic mean peak plasma concentration (Cavg[0_241) of estrone
ranging from
about 15.4 pg/mL to about 24.2 pg/mL (e.g., from 15.44 pg/mL to about 24.13
pg/mL; or
about 19.3 pg/mL), as assessed at day 14.
[0260] In some embodiments, administration of a suppository comprising about
10 ug of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) an unadjusted arithmetic mean area under the curve (AUC)0_24
of estrone
conjugates ranging from about 4,745.5 pg*hr/mL to about 7,414.9 pg*hr/mL
(e.g., from
4,745.60 pg*hr/mL to about 7,415.00 pg*hr/mL; or about 5,932 pg*hr/mL), as
assessed at
day 1; 2) a corrected arithmetic mean peak plasma concentration (Cavg[0-24])
of estrone
conjugates ranging from about 197.5 pg/mL to about 308.8 pg/mL (e.g., from
197.60 pg/mL
to about 308.75 pg/mL; or about 247 pg/mL), as assessed at day 1; 3) an
unadjusted
arithmetic mean area under the curve (AUC)0_24 of estrone conjugates ranging
from about
7,182.3 pg*hr/mL to about 11,222.6 pg*hr/mL (e.g., from 7,182.40 pg*hr/mL to
about
64
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
11,222.50 pg*hr/mL; or about 8,978 pg*hr/mL), as assessed at day 14; and 4) a
corrected
arithmetic mean peak plasma concentration (Cõg[0_241) of estrone conjugates
ranging from
about 299.1 pg/mL to about 467.6 pg/mL (e.g., from 299.20 pg/mL to about
467.50 pg/mL;
or about 374 pg/mL), as assessed at day 14.
[0261] In some embodiments, a suppository can comprise about 4 pg of
estradiol. In such
cases, administration of the suppository to a patient can provide, in a plasma
sample from the
patient, one or more parameters selected from: 1) a corrected geometric mean
peak plasma
concentration (Cmax) of estradiol of about 4 pg*hr/mL to about 8 pg*hr/mL; 2)
a corrected
geometric mean area under the curve (AUC)0_24 of estradiol of about 16
pg*hr/mL to about
26 pg*hr/mL; and 3) a corrected geometric mean time to peak plasma
concentration (Tmax) of
estradiol of about 0.25 hrs to about 2 hrs. In some embodiments,
administration of the
suppository to a patient provides, in a plasma sample from the patient, one or
more
parameters selected from: 1) a corrected geometric mean peak plasma
concentration (Cmax) of
estrone of about 1 pg*hr/mL to about 3 pg*hr/mL; 2) a corrected geometric mean
area under
the curve (AUC)0_24 of estrone of about 8 pg*hr/mL to about 13 pg*hr/mL; and
3) a corrected
geometric mean time to peak plasma concentration (Tmax) of estrone of about 1
hrs to about 4
hrs. In some embodiments, administration of the suppository to a patient
provides, in a
plasma sample from the patient, one or more parameters selected from: 1) a
corrected
geometric mean peak plasma concentration (Cmax) of estrone sulfate of about 4
pg*hr/mL to
about 7 pg*hr/mL; 2) a corrected geometric mean area under the curve (AUC)0_24
of estrone
sulfate of about 22 pg*hr/mL to about 34 pg*hr/mL; and 3) a corrected
geometric mean time
to peak plasma concentration (Tmax) of estrone sulfate of about 1 hrs to about
3 hrs.
[0262] In some embodiments, a suppository includes about 4 pg of estradiol. In
some such
embodiments, administration of the suppository to a patient can provide, in a
plasma sample
from the patient, one or more parameters selected from: 1) a corrected
geometric mean peak
plasma concentration (Cmax) of estradiol ranging from about 2.0 pg/mL to about
3.3 pg/mL
(e.g., 2.08 pg*hr/mL to about 3.25 pg*hr/mL); and 2) a corrected geometric
mean area under
the curve (AUC)0_24 of estradiol ranging from about 9.5 pg*hr/mL to about 15.1
pg*hr/mL
(e.g., ; 9.60 pg*hr/mL to about 15.0 pg*hr/mL), as assessed at day 1. In some
embodiments,
administration of a suppository comprising about 4 pg of estradiol to a
patient can provide, in
a plasma sample from the patient, one or more parameters selected from: 1) a
corrected
geometric mean peak plasma concentration (Cmax) of estradiol ranging from
about 1.0
pg*hr/mL to about 1.7 pg*hr/mL (e.g., 1.04 pg*hr/mL to about 1.63 pg*hr/mL) of
estradiol,
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
and 2) a corrected geometric mean area under the curve (AUC)0_24 of estradiol
ranging from
about 5.7 pg*hr/mL to about 9.1 pg*hr/mL (e.g., 5.76 pg*hr/mL to about 9.0
pg*hr/mL).
[0263] In some embodiments, administration of a suppository comprising about 4
pg of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) a corrected geometric mean peak plasma concentration (Cmax)
of estradiol of
about 2.6 pg/mL; and 2) a corrected geometric mean area under the curve
(AUC)0_24 of
estradiol of about 12 pg*hr/mL, as assessed at day 1. In some embodiments,
administration
of a suppository comprising about 10 pg of estradiol to a patient can provide,
in a plasma
sample from the patient, one or more parameters selected from: 1) a corrected
geometric
mean peak plasma concentration (Cmax) of estradiol of about 1.3 pg/mL; 2) a
corrected
geometric mean area under the curve (AUC)0_24 of estradiol of about 7.2
pg*hr/mL, as
assessed at day 14.
[0264] In some embodiments, administration of a suppository comprising about 4
pg of
estradiol to a patient provides, in a plasma sample from the patient, a
corrected geometric
mean peak plasma concentration (Cmax) of estrone conjugates ranging from about
0.3 pg/mL
to about 0.5 pg/mL (e.g., 0.32 pg/mL to about 0.5 pg/mL) as assessed at day 1.
[0265] In some embodiments, administration of a suppository comprising about 4
pg of
estradiol to a patient provides, in a plasma sample from the patient, a
corrected geometric
mean peak plasma concentration (Cmax) of estrone conjugates of about 0.4 pg/mL
as assessed
at day 1.
[0266] In some embodiments, administration of a suppository comprising about 4
pg of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) an unadjusted arithmetic mean area under the curve (AUC)0_24
of estradiol
ranging from about 73.3 pg*hr/mL to about 114.7 pg*hr/mL (e.g., from 73.36
pg*hr/mL to
about 114.63 pg*hr/mL; or about 91.7 pg*hr/mL), as assessed at day 1; 2) a
corrected
arithmetic mean peak plasma concentration (Cavg[0_241) of estradiol ranging
from about 3.1
pg/mL to about 4.8 pg/mL (e.g., from 3.14 pg/mL to about 4.90 pg/mL; or about
3.92
pg/mL), as assessed at day 1; 3) an unadjusted arithmetic mean area under the
curve (AUC)0_
24 of estradiol ranging from about 69.7 pg*hr/mL to about 108.9 pg*hr/mL
(e.g., from 69.76
pg*hr/mL to about 109.00 pg*hr/mL; or about 87.2 pg*hr/mL), as assessed at day
14; and 4)
a corrected arithmetic mean peak plasma concentration (Cavg[0_241) of
estradiol ranging from
66
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
about 2.8 pg/mL to about 4.6 pg/mL (e.g., from 2.90 pg/mL to about 4.54 pg/mL;
or about
3.63 pg/mL), as assessed at day 14.
[0267] In some embodiments, administration of a suppository comprising about 4
[ig of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) an unadjusted arithmetic mean area under the curve (AUC)0_24
of estrone
ranging from about 231.9 pg*hr/mL to about 362.4 pg*hr/mL (e.g., from 232.00
pg*hr/mL to
about 362.50 pg*hr/mL; or about 290 pg*hr/mL), as assessed at day 1; 2) a
corrected
arithmetic mean peak plasma concentration (Cavg[0_241) of estrone ranging from
about 10.3
pg/mL to about 16.3 pg/mL (e.g., from 10.40 pg/mL to about 16.25 pg/mL; or
about 13
pg/mL), as assessed at day 1; 3) an unadjusted arithmetic mean area under the
curve (AUC)0_
24 of estrone ranging from about 261.5 pg*hr/mL to about 408.8 pg*hr/mL (e.g.,
from 261.60
pg*hr/mL to about 408.75 pg*hr/mL; or about 327 pg*hr/mL), as assessed at day
14; and 4) a
corrected arithmetic mean peak plasma concentration (Cavg[0_241) of estrone
ranging from
about 10.8 pg/mL to about 17.1 pg/mL (e.g., from 10.88 pg/mL to about 17.00
pg/mL; or
about 13.6 pg/mL), as assessed at day 14.
[0268] In some embodiments, administration of a suppository comprising about 4
[ig of
estradiol to a patient provides, in a plasma sample from the patient, one or
more parameters
selected from: 1) an unadjusted arithmetic mean area under the curve (AUC)0_24
of estrone
conjugates ranging from about 4,062.3 pg*hr/mL to about 6,347.6 pg*hr/mL
(e.g., from
4,062.40 pg*hr/mL to about 6,347.50 pg*hr/mL; or about 5,078 pg*hr/mL), as
assessed at
day 1; 2) a corrected arithmetic mean peak plasma concentration (C,g[0_241) of
estrone
conjugates ranging from about 172.7 pg/mL to about 270.1 pg/mL (e.g., from
172.80 pg/mL
to about 270.00 pg/mL; or about 216 pg/mL), as assessed at day 1; 3) an
unadjusted
arithmetic mean area under the curve (AUC)0_24 of estrone conjugates ranging
from about
4,138.3 pg*hr/mL to about 6,466.3 pg*hr/mL (e.g., from 4,138.40 pg*hr/mL to
about
6,466.25 pg*hr/mL; or about 5173 pg*hr/mL), as assessed at day 14; and 4) a
corrected
arithmetic mean peak plasma concentration (Cavg[0_241) of estrone conjugates
ranging from
about 172.7 pg/mL to about 270.1 pg/mL (e.g., from 172.80 pg/mL to about
270.00 pg/mL;
or about 216 pg/mL), as assessed at day 14.
[0269] A pharmaceutical composition provided herein can result in
substantially local
delivery of estradiol. For example, plasma concentrations of estradiol,
estrone, and estrone
sulfate measured in the plasma of a patient following administration of a
pharmaceutical
67
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
composition as provided herein be statistically similar to those measured
following
administration of a placebo formulation (i.e., a similar formulation lacking
the estradiol).
Accordingly, in some embodiments, the plasma concentrations of estradiol,
estrone, or
estrone sulfate measured following administration of a pharmaceutical
composition provided
herein may be low compared to RLD formulations.
[0270] In some embodiments, a suppository can include about 1 [ig to about 25
[ig of
estradiol. Upon administration the suppository to a patient, a plasma sample
from the patient
can provide a corrected geometric mean peak plasma concentration (Cmax) of
estradiol that is
less than about 30 pg*hr/mL. For example, administration of the suppository to
a patient
provides a corrected geometric mean peak plasma concentration (Cmax) of
estradiol that is less
than about 18 pg*hr/mL. In some embodiments, administration of the suppository
to a patient
provides a corrected geometric mean area under the curve (AUC)0-24 of
estradiol that is less
than about 112 pg*hr/mL. For example, administration of the suppository to a
patient
provides a corrected geometric mean area under the curve (AUC)0-24 of
estradiol that is less
than about 63 pg*hr/mL.
[0271] In some embodiments, administration of the suppository to a patient
provides a
corrected geometric mean peak plasma concentration (Cmax) of estrone that is
less than about
14 pg*hr/mL. For example, administration of the suppository to a patient
provides a corrected
geometric mean peak plasma concentration (Cmax) of estrone that is less than
about 7
pg*hr/mL. In some embodiments, administration of the suppository to a patient
provides a
corrected geometric mean area under the curve (AUC)0-24 of estrone that is
less than about
65 pg*hr/mL. For example, administration of the suppository to a patient
provides a corrected
geometric mean area under the curve (AUC)0-24 of estrone that is less than
about 31
pg*hr/mL.
[0272] In some embodiments, administration of the suppository to a patient
provides a
corrected geometric mean peak plasma concentration (Cmax) of estrone sulfate
that is less than
about 613 pg*hr/mL. For example, administration of the suppository to a
patient provides a
corrected geometric mean peak plasma concentration (Cmax) of estrone sulfate
that is less than
about 16 pg*hr/mL. In some embodiments, administration of the suppository to a
patient
provides a corrected geometric mean area under the curve (AUC)0-24 of estrone
sulfate that
is less than about 5291 pg*hr/mL. For example, administration of the
suppository to a patient
68
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
provides a corrected geometric mean area under the curve (AUC)0-24 of estrone
sulfate that
is less than about 84 pg*hr/mL.
[0273] In further aspects of this disclosure, capsule disintegration may be
determined. In
some embodiments, delivery vehicle disintegration or absorption (presence or
absence of the
delivery vehicle after administration) at day 1 of treatment (for example, at
6 hours post first
treatment) and at day 15 (for example, one day post the last treatment on day
14).
[0274] The pharmaceutical compositions can be formulated as described herein
to provide
desirable pharmacokinetic parameters in a subject (e.g., a female subject) to
whom the
composition is administered. In some embodiments, a pharmaceutical composition
as
described herein produces desirable pharmacokinetic parameters for estradiol
in the subject.
In some embodiments, a pharmaceutical composition as described herein produces
desirable
pharmacokinetic parameters for one or more metabolites of estradiol in the
subject, for
example, estrone or total estrone.
[0275] Following the administration of a composition comprising estradiol to a
subject, the
concentration and metabolism of estradiol can be measured in a sample (e.g., a
blood, serum,
or plasma sample) from the subject. Estradiol is typically converted
reversibly to estrone,
and both estradiol and estrone can be converted to the metabolite estriol. In
postmenopausal
women, a significant proportion of circulating estrogens exist as sulfate
conjugates,
especially estrone sulfate. Thus, estrone can be measured with respect to
"estrone" amounts
(excluding conjugates such as estrone sulfate) and "total estrone" amounts
(including both
free, or unconjugated, estrone and conjugated estrone such as estrone
sulfate).
[0276] The pharmaceutical compositions of this disclosure can be characterized
for one or
more pharmacokinetic parameters of estradiol or a metabolite thereof following
administration of the composition to a subject or to a population of subjects.
These
pharmacokinetic parameters include AUC, Cmax, Cavg, and Tmax. AUC is a
determination of
the area under the curve (AUC) plotting the blood, serum, or plasma
concentration of drug
along the ordinate (Y-axis) against time along the abscissa (X-axis). AUCs are
well
understood, frequently used tools in the pharmaceutical arts and have been
extensively
described. C. is well understood in the art as an abbreviation for the maximum
drug
concentration in blood, serum, or plasma of a subject. T. is well understood
in the art as an
abbreviation for the time to maximum drug concentration in blood, serum, or
plasma of a
subject.
69
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0277] In some embodiments, one or more pharmacokinetic parameters, e.g., AUC,
Cmax,
Cavg, or Tmax, is measured for estradiol. In some embodiments, one or more
pharmacokinetic
parameters, e.g., AUC, C., Cavg, or Tmax, is measured for estrone. In some
embodiments,
one or more pharmacokinetic parameters, e.g., AUC, Cmax, Cavg, or Tmax, is
measured for total
estrone. Any pharmacokinetic parameter can be a "corrected" parameter, wherein
the
parameter is determined as a change over a baseline level.
[0278] Any of a variety of methods can be used for measuring the levels of
estradiol,
estrone, or total estrone in a sample, including immunoassays, mass
spectrometry (MS), high
performance liquid chromatography (HPLC) with ultraviolet fluorescent
detection, liquid
chromatography in conjunction with mass spectrometry (LC-MS), tandem mass
spectrometry
(MS/MS), and liquid chromatography-tandem mass spectrometry (LC-MS/MS). In
some
embodiments, the levels of estradiol, estrone, or total estrone are measured
using a validated
LC-MS/MS method. Methods of measuring hormone levels are well described in the
literature.
Statistical Measurements
[0279] According to embodiments, pharmacokinetics of the pharmaceutical
composition
disclosed herein are measured using statistical analysis. According to
embodiments, Analysis
of Variance ("ANOVA") or Analysis of CoVariance ("ANCOVA") are used to
evaluate
differences between a patient receiving treatment with a pharmaceutical
composition
comprising an active pharmaceutical composition (for example, a pharmaceutical
composition comprising estradiol) and a patient receiving treatment with a
placebo (for
example, the same pharmaceutical composition but without estradiol) or a
reference drug. A
person of ordinary skill in the art will understand how to perform statistical
analysis of the
data collected.
VIII. EXAMPLES
[0280] The following examples are of pharmaceutical compositions, delivery
vehicles, and
combinations thereof Methods of making are also disclosed. Data generated
using the
pharmaceutical compositions disclosed herein are also disclosed.
EXAMPLE 1: Pharmaceutical Composition
[0281] In embodiments, estradiol is procured and combined with one or more
pharmaceutically acceptable solubilizing agents. The estradiol is purchased as
a
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
pharmaceutical grade ingredient, often as micronized estradiol, although other
forms can also
be used. In embodiments, the pharmaceutical composition includes estradiol in
a dosage
strength of from about 1 lig to about 50 [tg. In embodiments, the
pharmaceutical composition
includes 10 lig of estradiol. In embodiments, the pharmaceutical composition
includes 25 lig
of estradiol.
[0282] In embodiments, the estradiol is combined with pharmaceutically
acceptable
solubilizing agents, and, optionally, other excipients, to form a
pharmaceutical composition.
In embodiments, the solubilizing agent is one or more of CAPMUL MCM, MIGLYOL
812,
GELUCIRE 39/01, GELUCIRE 43/01, GELUCIRE 50/13, and TEFOSE 63.
[0283] GELUCIRE 39/01 and GELUCIRE 43/01 each have an HLB value of 1.
GELUCIRE 50/13 has an HLB value of 13. TEFOSE 63 has an HLB value of between 9
and
10.
[0284] Various combinations of pharmaceutically acceptable solubilizing agents
were
combined with estradiol and examined as shown in Table 1.
Table 1: Capmul MCM ("MCM"), Gelucire 39/01 ("39/01"), Gelucire
43/01("43/01"),
Gelucire 50/13("50/13"), and Tefose ("Tefose 63")
# Vehicle system Ratio Physical Physical state
Viscosity Melting Dispersion
state @ @ 37 C after (cps) Time @ in
water
Room ¨30 minutes 37 C 37 C
Temperat
ure
1 MCM: 39/01 8:2 Solid Clear liquid 50
(a) 37 C Start: 6 min Small oil
Finish: 12
drops on top
min
2 MCM: 39/01 7:3 Solid Clear liquid Start: 9 Mill
Finish: 19
min
3 MCM: 39/01 6:4 Solid Clear liquid Start: 20
min Finish:
32 min
4 MCM: 43/01 8:2 Solid Liqnidwith
solid particles
5 MCM: 43/01 7:3 Solid Liqnidwith
6 MCM:50/13 9:1 Liquid/ Liquid/cloudy 140@
25 C Clear after Uniformly
cloudy 20 min cloudy
dispersion
7 MCM:50/13 8:2 Liquid/ Liquid/cloudy 190@
25 C Uniformly
cloudy cloudy
..................
dispersion
8 MCM:50/13 7:3 Semisolid Semisolid
9 MCM:TEFOSE 9:1 Semisolid
Liquid/cloudy 150@ 25 C Start: 1 min Uniformly
63 Finish: 5 cloudy
min
dispersion
10 MCM:TEFOSE 8:2 Semisolid Semisolid 240@ 25 C
....IIIIIIIIIIIIIIIIIIIII. Uniformly
71
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
63
cloudy
________________________________________________________________________
dispersion
11 MCM:TEFOSE 7:3 Semisolid Semisolid
380@25 c Semisolid Uniformly
63 after 30 min
cloudy
at 37 C,
dispersion
doesn't melt
at 41 C
12 MIGLYOL 812: 9:1 Semisolid Semisolid 140g 25 C
2 phases, oil
50/13 L.. ___ ontop
13 MIGLYOL 812: 9:1 Liquid/ Liquid/cloudy 90g 25 C
Start: 1 min 2 phases, oil
TEFOSE 63 cloudy Finish: 5 on top
min
[0285] Pharmaceutical compositions in Table 1 that were liquid or semisolid at
room
temperature were tested using a Brookfield viscometer (Brookfield Engineering
Laboratories,
Middleboro, MA) at room temperature. Pharmaceutical compositions appearing in
Table 1
that were solid at ambient temperature were tested using a Brookfield
viscometer at 37 C.
[0286] Pharmaceutical compositions appearing in Table 1 that were solid at
room
temperature were assessed at 37 C to determine their melting characteristics.
The viscosity
of the gels can be important during encapsulation of the formulation. For
example, in some
cases, it is necessary to warm the formulation prior to filing of the gelatin
capsules. In
addition, the melting characteristics of the composition can have important
implications
following administration of the formulation into the body. For example, in
some
embodiments, the formulation will melt at temperatures below about 37 C.
Pharmaceutical
Composition 11 (Capmul MCM/Tefose 63), for example, did not melt at 37 C or
41 C.
[0287] A dispersion assessment of the pharmaceutical compositions appearing in
Table 1
was performed. The dispersion assessment was performed by transferring 300 mg
of each
vehicle system in 100 mL of 37 C water, without agitation, and observing for
mixing
characteristics. Results varied from formation of oil drops on the top to
separation of phases
to uniform, but cloudy dispersions. Generally speaking, it is believed that
formulations able
to readily disperse in aqueous solution will have better dispersion
characteristics upon
administration. It was surprisingly found, however, as shown below in Examples
7-9, that
formulations that did not readily disperse in aqueous solution (e.g.,
Formulation 13) and
instead formed two phases upon introduction to the aqueous solution were found
to be the
most effective when administered to the human body.
EXAMPLE 2: Delivery Vehicle
[0288] In embodiments, the pharmaceutical composition is delivered in a
gelatin capsule
delivery vehicle. The gelatin capsule delivery vehicle includes, for example,
gelatin (e.g.,
72
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Gelatin, NF (150 Bloom, Type B)), hydrolyzed collagen (e.g., GELITA , GELITA
AG,
Eberbach, Germany), glycerin, sorbitol special, or other excipients in
proportions that are
well known and understood by persons of ordinary skill in the art. Sorbitol
special may be
obtained commercially and may tend to act as a plasticizer and humectant.
[0289] A variety of delivery vehicles were developed, as show in Table 2, Gels
A through
F. In Table 2, each delivery vehicle A through F differs in the proportion of
one or more
components.
Table 2: Gelatin Capsule Delivery Vehicles
A B C D E F
Ingredient % w/w % w/w % w/w % w/w % w/w % w/w
Gelatin, NF (150 Bloom, Type B) 41.0 41.0 41.0 41.0 43.0
43.0
Glycerin 99.7%, USP 6.0 6.0 6.0 6.0 18.0 18.0
-
Sorbitol Special, USP 15.0 15.0 15.0 15 0
GELITA ( hydrolyzed collagen) 3:::: ::::
.:.:.:.:.:.:.:.:.:.:.:.:.:.:.:.:.:.:.:.:.::::::: :.
,,,.,.,.,.,.,.,.,.,.,.,.,.,.,.,.,.,.,.,., 3.0
Citric acid :: 0.1 0.5 1 :: 0.1
Purified Water 35.0 37.9 37.5 37.0 36.0
38.9
Total 100.0 100.0 100.0 100.0 100.0 100.0
Dissolution gel strips, Avg of 3 48 min 50 nun 75 min 70 min
:.:=:.
(500 mL DH20, 50 rpm @ 37 C) (42,45,58) (50,51,50) (76,75,74) (70,71,70)
..
... .
Dissolution gel strips, Avg of 3
(500 mL pH 4 buffer, 50 rpm A 70 miniii ....
: :
: :
.. .....
:::
: :
... ii 78 min 82 min
37 C)
= = :::::
= =
..............
[0290] Each delivery vehicle A through F was prepared at a temperature range
from about
45 C to about 85 C. Each molten delivery vehicle A through F was cast into a
film, dried,
and cut into strips. The strips were cut into uniform pieces weighing about
0.5 g, with about
0.5 mm thickness. Strips were placed into a USP Type 2 dissolution vessel in
either water or
pH 4 buffer solution and the time for them to completely dissolve was recorded
(see Table 2).
Delivery vehicle A had the fastest dissolution in both water and pH 4 buffer
solution.
EXAMPLE 3: Pharmaceutical Compositions and Delivery Vehicle
[0291] Various combinations of the pharmaceutical compositions from Table 1
and from
Table 2 were prepared. The combinations are shown in Table 3.
Table 3
Trial Pharmaceutical Composition Ratio Batch Size g Delivery
Vehicle
1 MCM:39/01 8:2 750 A
2 MCM:50/13 8:2 750 A
3 MCM:TEFOSE 63 8:2 750 A
73
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
4 MCM:TEFOSE 63 8:2 750
MIGLYOL 812:TEFOSE 63 9:1 750 A
[0292] Each aliquot of the pharmaceutical compositions of Table 3 about 300 mg
to about
310 mg. Batch size was as listed in Table 3. To encapsulate the vehicle
system, each 300 mg
to about 310 mg pharmaceutical composition aliquot was encapsulated in about
200 mg of
5 the gelatin capsule delivery vehicle. Thus, for example, in Trial 1, the
pharmaceutical
composition denoted by MCM:39/01 was encapsulated in gelatin capsule delivery
vehicle A
for a total encapsulated weight of about 500 mg to about 510 mg. The aliquot
size is arbitrary
depending on the concentration of the estradiol and the desired gelatin
capsule delivery
vehicle size. Artisans will readily understand how to adjust the amount of
estradiol in the
pharmaceutical composition to accommodate a given size of delivery vehicle,
when the
delivery vehicle encapsulates the pharmaceutical composition.
EXAMPLE 4: Estradiol Solubility
[0293] In various experiments, solubilizing agents were tested to determine
whether they
were able to solubilize 2 mg of estradiol for a total pharmaceutical
composition weight of 100
mg. The solubilizing agents were considered suitable if estradiol solubility
in the solubilizing
agent was greater than or equal to about 20 mg/g. Initial solubility was
measured by
dissolving micronized estradiol into various solubilizing agents until the
estradiol was
saturated (the estradiol/solubilizing agent equilibrated for three days),
filtering the
undissolved estradiol, and analyzing the resulting pharmaceutical composition
for estradiol
concentration by HPLC.
Table 4: Solubility of Solubilizing Agents (*denotes literature reference)
Ingredient Solubility (mg/g)
PEG 400 105*
Propylene Glycol 75*
Polysorbate 80 36*
TRANSCUTOL HP 141
CAPMUL PG8 31.2
74
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
EXAMPLE 5: Pharmaceutical Compositions
[0294] The following pharmaceutical compositions are contemplated.
Gel mass
Ingredient % w/w Qty/Batch (kg)
Gelatin 150 Bloom Limed Bone, NF 41.00 82.00
Hydrolyzed Gelatin 3.00 6.00
Glycerin 99.7% 6.00 12.00
Sorbitol Special, NF 15.00 30.00
Opatint White G-18006 1.20 2.40
Opatine Red DG-15001 0.06 0.12
Purified Water, USP 33.74 67.48
Total 100.00 200.00 Kg
Pharmaceutical Composition 1: 10 ug estradiol
Ingredients Qty/Capsule (mg) % w/w
Qty/Batch
Estradiol hemihydrate micronized, USP 0.010 0.003 0.10 g
CAPMUL MCM, NF (Glyceryl 240.0 79.997 2.40 kg
Caprylate/Caprate or Medium Chain
Mono- and Diglycerides)
GELUCIRE 50/13 (stearoyl polyoxyl- 60.0 20.0 600.0 g
32 glycerides NF)
Total 300.0 100.0 3.0 kg
Pharmaceutical Composition 2: 10 ug estradiol
Ingredients Qty/Capsule (mg) % w/w
Qty/Batch
Estradiol hemihydrate micronized, USP 0.010 0.003 0.10 g
MIGLOYL 812 (medium chain 270.0 89.997 2.70 kg
triglyceride)
TEFOSEg 63 (mixture of PEG-6 30.0 10.0 300.0 g
stearate or ethylene glycol
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
palmitostearate or PEG-32 stearate;
polyoxyl 6 and polyoxyl 32
palmitostearate / glycol stearate)
Total 300.0 100.0 3.00 kg
Pharmaceutical Composition 3: 25 lig estradiol
Ingredients Qty/Capsule (mg) % w/w
Qty/Batch
Estradiol hemihydrate micronized, USP 0.026* 0.009 0.26 g
MIGLOYL 812 (medium chain 270.0 89.991 2.70 kg
triglyceride)
TEFOSE 63 (mixture of PEG-6 30.02 10.0 300.0g
stearate or ethylene glycol
palmitostearate or PEG-32 stearate;
polyoxyl 6 and polyoxyl 32
palmitostearate / glycol stearate)
Total 300.0 100.0 3.00 kg
* 1.0 mg estradiol is equivalent to 1.03 mg estradiol hemihydrate
Pharmaceutical Composition 4: 4 in estradiol
Ingredients Qty/Capsule (mg) % w/w
Qty/Batch
(alternate
batch size)
Estradiol hemihydrate micronized, USP 0.0041* 0.001 0.041 g
(0.615 g)
MIGLOYL 812 (medium chain 269.99 89.999 2700.0 g
triglyceride)
(40.50 kg)
TEFOSE 63 (mixture of PEG-6 30.0 10.0 300.0 g
stearate or ethylene glycol (4.50
kg)
palmitostearate or PEG-32 stearate;
polyoxyl 6 and polyoxyl 32
palmitostearate / glycol stearate)
Total 300.0 100.0 3000.0 g
45.0 kg
* 1.0 mg estradiol is equivalent to 1.03 mg estradiol hemihydrate
76
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Pharmaceutical Composition 5: 10 lig estradiol
Ingredients Qty/Capsule (mg) % w/w
Qty/Batch
Estradiol hemihydrate micronized, USP 0.0103* 0.003 1.545 g
MIGLOYL 812 (medium chain 269.99 89.997 40.5 kg
triglyceride)
TEFOSE 63 (mixture of PEG-6 30.0 10.0 4.50 kg
stearate or ethylene glycol
palmitostearate or PEG-32 stearate;
polyoxyl 6 and polyoxyl 32
palmitostearate / glycol stearate)
Total 300.0 100.0 45.00 kg
* 1.0 mg estradiol is equivalent to 1.03 mg estradiol hemihydrate
Pharmaceutical Composition 6: 25 lig estradiol
Ingredients Qty/Capsule (mg) % w/w
Qty/Batch
Estradiol hemihydrate micronized, USP 0.026* 0.009 3.90 g
MIGLOYL 812 (medium chain 269.97 89.991 40.50 kg
triglyceride)
TEFOSE 63 (mixture of PEG-6 30.0 10.0 4.50 kg
stearate or ethylene glycol
palmitostearate or PEG-32 stearate;
polyoxyl 6 and polyoxyl 32
palmitostearate / glycol stearate)
Total 300.0 100.0 45.00 kg
* 1.0 mg estradiol is equivalent to 1.03 mg estradiol hemihydrate
Pharmaceutical Composition 7: Placebo
Ingredients Qty/Capsule (mg) % w/w
Qty/Batch
Estradiol hemihydrate micronized, USP 0.00 0.00 0.00 g
MIGLOYL 812 (medium chain 270.0 90.0 40.5 kg
triglyceride)
TEFOSE 63 (mixture of PEG-6 30.0 10.0 4.5 kg
stearate or ethylene glycol
palmitostearate or PEG-32 stearate;
77
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
polyoxyl 6 and polyoxyl 32
palmitostearate / glycol stearate)
Total 300.0 100.0
3000.0 g
[0295] In the Examples below, TX-004HR is Pharmaceutical Compositions 4, 5,
and 6
(TX-004HR 4 ug, TX-004HR 10 ug, and TX-004HR 25 ug) compared to Pharmaceutical
Composition 7.
EXAMPLE 6: Process
[0296] Fig. 1 illustrates an embodiment of a method making pharmaceutical
composition
comprising estradiol solubilized in CapmulMCM/Gelucire solubilizing agent
encapsulated in
a soft gelatin delivery vehicle 100. In operation 102, the CapmulMCM is heated
to 40 C 5
C. Heating may be accomplished through any suitable means. The heating may be
performed in any suitable vessel, such as a stainless steel vessel. Other
pharmaceutical
compositions can be made using the same general method by substituting various
excipients,
including the solubilizing agent.
[0297] In operation 104, GELUCIRE is mixed with the CapmulMCM to form the
finished
solubilizing agent. As used herein, any form of GELUCIRE may be used in
operation 104.
For example, one or more of GELUCIRE 39/01, GELUCIRE 43/01, GELUCIRE 50/13 may
be used in operation 104. Mixing is performed as would be known to persons of
ordinary
skill in the art, for example by impeller, agitator, stirrer, or other like
devices used to mix
pharmaceutical compositions. Operation 104 may be performed under an inert or
relatively
inert gas atmosphere, such as nitrogen gas. Mixing may be performed in any
vessels that are
known to persons of ordinary skill in the art, such as a stainless steel
vessel or a steel tank.
[0298] In operation 106 estradiol is mixed into the solubilizing agent. In
embodiments, the
estradiol in micronized when mixed into the solubilizing agent. In other
embodiments, the
estradiol added is in a non-micronized form. Mixing may be facilitated by an
impeller,
agitator, stirrer, or other like devices used to mix pharmaceutical
compositions. Operation
106 may be performed under an inert or relatively inert gas atmosphere, such
as nitrogen gas.
[0299] In embodiments, however, the addition of estradiol may be performed
prior to
operation 104. In that regard, operations 104 and 106 are interchangeable with
respect to
timing or can be performed contemporaneously with each other.
78
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0300] In operation 110, the gelatin delivery vehicle is prepared. Any of the
gelatin
delivery vehicles described herein may be used in operation 110. In
embodiments, gelatin,
hydrolyzed collagen, glycerin, and other excipients are combined at a
temperature range from
about 45 C to about 85 C and prepared as a film. Mixing may occur in a steel
tank or other
container used for preparing gelatin delivery vehicles. Mixing may be
facilitated by an
impellor, agitator, stirrer, or other devices used to combine the contents of
gelatin delivery
vehicles. Operation 110 may be performed under an inert or relatively inert
gas atmosphere,
such as nitrogen gas. In embodiments, the gelatin delivery vehicle mixture is
degassed prior
to being used to encapsulate the pharmaceutical composition.
[0301] In operation 112, the gelatin delivery vehicle encapsulates the
pharmaceutical
composition, according to protocols well known to persons of ordinary skill in
the art. In
operation 112, a soft gelatin capsule delivery vehicle is prepared by
combining the
pharmaceutical composition made in operation 106 with the gelatin delivery
vehicle made in
operation 110. The gelatin may be wrapped around the material, partially or
fully
encapsulating it or the gelatin can also be injected or otherwise filled with
the pharmaceutical
composition made in operation 106.
[0302] In embodiments, operation 112 is completed in a suitable die to provide
a desired
shape. Vaginal soft gel capsules may be prepared in a variety of geometries.
For example,
vaginal soft gel capsules may be shaped as a tear drop, a cone with
frustoconical end, a
cylinder, a cylinder with larger "cap" portion as illustrated in Fig. 2, or
other shapes suitable
for insertion into the vagina. The resulting pharmaceutical composition
encapsulated in the
soft gelatin delivery vehicle may be inserted digitally or with an applicator.
EXAMPLE 7: Study of Estradiol Pharmaceutical composition on the Improvement of
Vulvovaginal Atrophy (VVA)
[0303] The objective of this study was designed to evaluate the efficacy and
safety of a
pharmaceutical composition comprising 10 pg estradiol (i.e., Pharmaceutical
Composition 2)
in treating moderate to severe symptoms of VVA associated with menopause after
14 days of
treatment, and to estimate the effect size and variability of vulvovaginal
atrophy endpoints. In
addition, the systemic exposure to estradiol from single and multiple doses of
the
pharmaceutical composition was investigated.
[0304] This study was a phase 1, randomized, double-blind, placebo-controlled
trial to
evaluate safety and efficacy of the pharmaceutical composition in reducing
moderate to
79
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
severe symptoms of vaginal atrophy associated with menopause and to
investigate the
systemic exposure to estradiol following once daily intravaginal
administrations of a
pharmaceutical composition for 14 days.
[0305] Postmenopausal subjects who met the study entry criteria were
randomized to one
of two treatment groups (pharmaceutical composition or placebo). During the
screening
period subjects were asked to self-assess the symptoms of VVA, including
vaginal dryness,
vaginal or vulvar irritation or itching, dysuria, vaginal pain associated with
sexual activity,
and vaginal bleeding associated with sexual activity. Subjects with at least
one self-assessed
moderate to severe symptom of VVA identified by the subject as being most
bothersome to
her were eligible to participate in the study.
[0306] Clinical evaluations were performed at the following time points:
Screening Period (up to 28 days);
Visit 1¨Randomization/Baseline (day 1);
Visit 2¨Interim (day 8); and
Visit 3¨End of the treatment (day 15).
[0307] Eligible subjects were randomized in a 1:1 ratio to receive either
pharmaceutical
composition comprising estradiol 10 pg or a matching placebo vaginal softgel
capsule, and
self-administered their first dose of study medication at the clinical
facility under the
supervision of the study personnel. Serial blood samples for monitoring of
estradiol level
were collected at 0.0, 1.0, 3.0, and 6.0 hours relative to first dose
administration on day 1.
Subjects remained at the clinical site until completion of the 6-hour blood
draw and returned
to clinical facility for additional single blood draws for measurement of
estradiol
concentration on day 8 (before the morning dose) and day 15. Subjects were
provided with
enough study medication until the next scheduled visit and were instructed to
self-administer
their assigned study treatment once a day intravaginally at approximately the
same time ( 1
hour) every morning. Each subject was provided with a diary in which she was
required to
daily record investigational drug dosing dates and times. Subjects returned to
clinical facility
on day 8 for interim visit and on day 15 for end of treatment assessments and
post study
examinations. Capsule disintegration state was assessed by the investigator at
day 1 (6 hours
post-dose) and day 15.
[0308] The study involved a screening period of up to 28 days before
randomization and
treatment period of 14 days. Selection of dosage strength (estradiol 10 pg)
and treatment
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
regimen (once daily for two weeks) was based on the FDA findings on safety and
efficacy of
the RLD.
Number of Subjects (Planned and Analyzed)
[0309] Up to 50 (25 per treatment group) postmenopausal female subjects 40 to
75 years
old with symptoms of moderate to severe VVA were randomized. 50 subjects were
enrolled,
48 subjects completed the study, and 48 subjects were analyzed.
Diagnosis and Main Criteria for Inclusion
[0310] Fifty female subjects were enrolled in the study. Post-menopausal
female subjects
40 to 75 years of age, with a mean age was 62.3 years were enrolled. Subjects'
mean weight
(kg) was 71.2 kg with a range of 44.5-100 kg. Subjects' mean height (cm) was
162.6 cm with
a range of 149.9-175.2 cm, and the mean BMI (kg/m2) was 26.8 kg/m2 with a
range of 19-33
kg/m2. Criteria of inclusion in the study included: self-identification of at
least one moderate
to severe symptom of VVA, for example, vaginal dryness, dyspareunia, vaginal
or vulvar
irritation, burning, or itching, dysuria, vaginal bleeding associated with
sexual activity, that
was identified by the subject as being most bothersome to her; <5% superficial
cells on
vaginal smear cytology; vaginal pH>5.0; and estradiol level < 50 pg/mL.
Subject who were
judged as being in otherwise generally good health on the basis of a pre-study
physical
examination, clinical laboratory tests, pelvic examination, and mammography
were enrolled.
Estradiol 10 ug or Placebo, Dose, and Mode of Administration
[0311] Subjects were randomly assigned (in 1:1 allocation) to self-administer
one of the
following treatments intravaginally once daily for 14 days:
Treatment A: The pharmaceutical composition of Example 5 (Pharmaceutical
Composition 2: 10 lig estradiol); or
Treatment B: Placebo vaginal softgel capsule, containing the same formulation
as
Treatment A, except for the 10 jig of estradiol.
[0312] The estradiol formulation was a tear drop shaped light pink soft gel
capsule.
Treatment B had the same composition, appearance, and route of administration
as the
Treatment A, but contained no estradiol.
81
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Duration of Treatment
[0313] The study involved a screening period of up to 28 days before
randomization and a
treatment period of 14 days.
Criteria for Evaluation
[0314] Efficacy Endpoints:
Change from baseline (screening) to day 15 in the Maturation Index (percent of
parabasal vaginal cells, superficial vaginal cells, and intermediate vaginal
cells) of the
vaginal smear. Data for this endpoint are shown in Tables 6-8.
Change from baseline (screening) to day 15 in vaginal pH. Data for this
endpoint are
shown in Table 9.
Change from baseline (randomization) to day 15 in severity of the most
bothersome
symptoms: (1) vaginal dryness; (2) vaginal or vulvar irritation, burning, or
itching; (3)
dysuria; (4) dyspareunia; (5) vaginal bleeding associated with sexual
activity. Data for
this endpoint are shown in Tables 13 and 15.
Change from baseline (randomization) to day 15 in investigator's assessment of
the
vaginal mucosa. Data for this endpoint are shown in Tables 18-21.
[0315] Unless otherwise noted, the efficacy endpoints were measured as a
change-from
Visit 1¨Randomization/Baseline (day 1) to Visit 3¨End of the treatment (day
15), except
for vaginal bleeding which was expressed as either treatment success or
failure.
[0316] Other endpoints include:
Vital signs, weight, changes in physical exam, pelvic and breast exam, and
adverse
events were evaluated as part of the safety endpoints.
Concentration of estradiol at each sampling time.
Peak concentration of estradiol on day 1 and sampling time at which peak
occurred.
Delivery vehicle disintegration to measure the amount of residual delivery
vehicle
remains in the vagina post treatment.
[0317] Results from the assessment of plasma concentrations of estradiol are
presented in
Table 5.
Table 5
Safety Results: The descriptive statistics for Day i. plasma estradiol C. and
Tmax are
provided below.
82
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Estradiol 10 ittg Placebo
Cmax Tmax Cmax Tmax
24 24 26 26
Mean + SD 30.7 + 7.47 2.12 1.73 27.5 17.26 4.00
+ 2.68
Geometric Mean 29.9 24.7
Median 29.8 1.00 22.1 6.00
Min, Max 19.7, 52.3 1.00, 6.00 15.1, 90.0 0.00,
6.00
CV% 24.3% 81.3% 62.9% 67.1%
Maturation Index Results
[0318] Vaginal cytology data was collected as vaginal smears from the lateral
vaginal walls
according to standard procedures to evaluate vaginal cytology at screening and
Visit 3-End
of treatment (day 15). The change in the Maturation Index was assessed as a
change in cell
composition measured at Visit 1-Baseline (day 1) compared to the cell
composition
measured at Visit 3-End of treatment (day 15). The change in percentage of
superficial,
parabasal, and intermediate cells obtained from the vaginal mucosal epithelium
from a
vaginal smear was recorded. Results from these assessments are presented in
Tables 6, 7, and
8.
Table 6: Primary Efficacy Analysis Results of Change from Baseline (Screening)
to Day 15
in the Maturation Index of the Vaginal Smear (Percent Parabasal Cells)
Difference Estradiol
10
Between ttg
vs.
Estradiol Treatment 90% CI for
Placebo P-
Population Statistics 10 ittg Placebo Means
Difference value
Treat Intent-to-
24 24
Least-
Squares -54.4 -4.80 -49.6 (-60.4, -38.8)
<0.0001
Mean
-53.8
Mean SD -5.4 22.3
39.7
Median -6o.o -5.0
Min, Max -100.0, 0.0 -60.0, 60.0
1 Confidence interval for the estradiol 10 tg-Placebo from ANCOVA with
treatment as a fixed effect and baseline as a
covariate.
2 P-value for treatment comparison from ANCOVA with treatment as a fixed
effect and baseline as a covariate.
83
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Table 7: Primary Efficacy Analysis Results of Change from Baseline (Screening)
to Day 15
in the Maturation Index of the Vaginal Smear (Superficial Cells)
Difference
Estradiol 10
Between ttg
vs.
Estradiol Treatment 90% CI for
Placebo P-
Population Statistics 10 ittg Placebo Means Difference
value
Treat Intent-to-
24 24
Least-
Squares 35.2 8.75 26.5 (15.4, 37.6)
0.0002
Mean
Mean SD 35.2 26.4 8.8 18.7
Median 40.0 0.0
Min, Max 0.0, 80.0 0.0, 90.0
1 Confidence interval for the estradiol 10 tg-Placebo from ANOVA with
treatment as a fixed effect.
2 P-value for treatment comparison from ANOVA with treatment as a fixed
effect.
Table 8: Primary Efficacy Analysis Results of Change from Baseline (Screening)
to Day 15
in the Maturation Index of the Vaginal Smear (Intermediate Cells)
Difference
Estradiol 10
Between ttg
vs.
Estradiol Treatment 90% CI for
Placebo P-
Population Statistics 10 ittg Placebo Means Difference
value2
Treat Intent-to-
24 24
Least-
Squares 18.7 -3,54 22.3 (11.1, 33.5)
0.0017
Mean
Mean SD 18.5 42.7 -3.3 21.6
Median 22.5 -5.0
-6o.o,
Min, Max -6o.o, 20.0
100.0
1 Confidence interval for the estradiol 10 tg-Placebo from ANCOVA with
treatment as a fixed effect and baseline as a
covariate.
2 P-value for treatment comparison from ANCOVA with treatment as a fixed
effect and baseline as a covariate.
Change in pH Results
[0319] Vaginal pH was measured at Screening and Visit 3¨End of treatment (day
15). The
pH measurement was obtained by pressing a pH indicator strip against the
vaginal wall. The
subjects entering the study were required to have a vaginal pH value greater
than 5.0 at
screening. pH values were recorded on the subject's case report form. The
subjects were
advised not to have sexual activity and to refrain from using vaginal douching
within 24
hours prior to the measurement. Results from these assessments are presented
in Table 9.
84
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Table 9: Primary Efficacy Analysis Results of Change from Baseline (Screening)
to Day 15
in Vaginal pH
Estradiol 10
Difference lag
vs.
Estradiol Between 90% CI for
Placebo P-
Population Statistics 10 lug Placebo
Treatment Means Difference' value2
Treat Intent-to-
24 24
Least-
(-0.9oo, -
Squares -0.974 -0.339 -0.635
0.0002
Mean 0.368)
M -0.917 -0.396
ean SD
0.686 0.659
Median -1.00 -0.500
-1.5o,
Min, Max -2.00, 0.500
o.5oo
Confidence interval for the estradiol 10 ig-Placebo from ANCOVA with treatment
as a fixed effect and baseline as a
covariate.
2 P-value for treatment comparison from ANCOVA with treatment as a fixed
effect and baseline as a covariate.
Most Bothersome Symptoms Data
[0320] Subjects were asked to specify the symptom that she identified as the
"most
bothersome symptom." During the screening period all of the subjects were
provided with a
questionnaire to self-assess the symptoms of VVA: (1) vaginal dryness; (2)
vaginal or vulvar
irritation, burning, or itching; (3) dysuria; (4) dyspareunia; (5) vaginal
bleeding associated
with sexual activity. Each symptom, with the exception of vaginal bleeding
associated with
sexual activity, was measured on a scale of 0 to 3, where 0 = none, 1 = mild,
2 = moderate,
and 3 = severe. Vaginal bleeding associated with sexual activity was measured
in a binary
scale: N = no bleeding; Y = bleeding. The subject's responses were recorded.
All randomized
subjects were also provided a questionnaire to self-assess the symptoms of VVA
at Visit 1¨
Randomization/Baseline (day 1) and at Visit 3¨End of the treatment (day 15).
Subjects
recorded their self-assessments daily in a diary and answers were collected on
days 8 and 15
(end of treatment). Pre-dose evaluation results obtained at Visit 1 were
considered as baseline
data for the statistical analyses. Data from these assessments are presented
in Tables 10 and
11.
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Table 10: Baseline Characteristics for Vaginal Atrophy Symptoms (ITT
Population)
VVA Symptom
Estradiol 10 lig vs.
Placebo
Statistics Estradiol 10 lig Placebo P-value'
Vaginal dryness N of Subjects 24 24
Mean 2.292 2.375 0.68231
Vaginal or vulvar irr- N of Subjects 24 24
itation/burning/itching Mean 0.875 1.333 0.08721
Pain, burning or stinging N of Subjects 24 24
when urinating Mean 0.583 0.625 0.87681
Vaginal pain associated N of Subjects2 12 12
with sexual activity Mean 2.083 2.333 0.54281
Vaginal bleeding N of Subjects2 12 12
associated with sexual
Percent3 25.00 33.33 0.31463
activity
1P-value for treatment comparison from ANOVA/ANCOVA with treatment as a fixed
effect and Baseline as a
covariate when appropriate.
2 N = number of subjects sexually active at baseline.
3 Percent of subjects with bleeding, evaluated using Fisher's Exact Test.
Table 11: Additional Efficacy Analysis Results of Change from Baseline
(Randomization) to
Day 15 in Severity of Vaginal Atrophy Symptoms
Least-Squares Mean Difference
Between
Estradiol 10 lug
Statistical Estradiol Treatment 90% CI for
vs. Placebo P-
Symptom Method' 10 Placebo Means Difference2 value
Vaginal dryness
ANCOVA 0.980 0.729 0.251 -0.706, 0.204)
0.3597
Vaginal or vulvar
Irritation/burning
ANCOVA 0.694 0.514 0.180 -0.549, 0.189)
0.4159
/itching
Pain/Burning/
Stinging
(Urination) ANCOVA 0.391 0.359 0.032 -0.263,0.200)
0.8185
Vaginal pain
associatedwith
ANOVA 0.800 0.500 0.300 -1.033, 0.433)
0.4872
sexual activity
'ANOVA model contained a fixed effect for treatment. ANCOVA added baseline as
a covariate to the model.
2 Confidence interval for the difference between estradiol to ng and Placebo
treatment least-squares means.
[0321] Changes to the most bothersome symptom from the baseline was scored
according
to the evaluation of VVA symptoms generally set forth above. Tables 13 and 14
show a
comparison between the pharmaceutical composition 1 and placebo generally for
most
bothersome symptom and vaginal atrophy symptom. It is noteworthy to point out
that these
measurement demonstrated a trend of improvement, though not statistically
significant, at
day 15.
86
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Table 13: Primary Efficacy Analysis Results of Change from Baseline
(Randomization) to
Day 15 in Severity of the Most Bothersome VVA
Difference
Estradiol 10
Between ittg
vs.
Estradiol Treatment 90% CI for
Placebo P-
Population Statistics 10 lag Placebo Means
Difference' value2
Treat Intent-to-
24 24
Least-
(-0.497,
Squares -1.043 -1.042 -0.002 0.9951
0.493)
Mean
Mean SD -1.043 -1.042
0.928 1.08
Median -1.00 -1.00
-3.00,
Min, Max -3.00, 0.00
o.00
1Confidence interval for the estradiol 10 tg-Placebo from ANOVA with
treatment as a fixed effect.
2 P-value for treatment comparison from ANOVA with treatment as a fixed
effect.
Table 14 - Additional Efficacy Analysis Results of Change from Baseline
(Randomization) to
Day 15 in Severity of Vaginal Atrophy Symptoms
Least-Squares Mean TX-12-
004-
HR vs.
Statistical TX-12-004- Difference Between 90% CI for Placebo P-
Symptom Method' HR Placebo Treatment Means
Difference2 value
Dryness ANCOVA -0.980 -0.729 -0.251 (-
0.706, 0.204) 0.3597
Irritation ANCOVA -0.694 -0.514 -0.180 (-
0.549, 0.189) 0.4159
Pain (Sex) ANOVA -0.800 -0.500 -0.300 (-
1.033, 0.433) 0.4872
Pain/Burning/
ANCOVA -0.391 -0.359 -0.032 (-0.263, 0.200)
0.8185
Stinging (Urination)
lANOVA model contained a fixed effect for treatment. ANCOVA added baseline as
a covariate to the model.
2 Confidence interval for the difference between TX-12-004-HR and Placebo
treatment least-squares means.
[0322] With respect to the most bothersome symptoms data presented in Tables
13 and 14,
the period over which the data was measured is generally considered
insufficient to make
meaningful conclusions. However, the trends observed as part of this study
suggest that the
data will show improvement of the most bothersome symptoms when data for a
longer time
period is collected.
[0323] The absence or presence of any vaginal bleeding associated with sexual
activity was
also measured as one of the most bothersome symptoms. The data for vaginal
bleeding
associated with sexual activity is reported in Table 15.
87
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Table 15: Primary Efficacy Analysis Results of Change from Baseline
(Randomization) to
Day 15 in Vaginal Bleeding Associated with Sexual Activity
Baseline (Randomization) and Day 15 Summary of Vaginal Bleeding
Bleeding/ No
Bleeding Bleeding/ No Bleeding/ No Bleeding/ No
Treatment N* (Success)2
Bleeding (Failure) Bleeding (Failure) Bleeding (NC)
Estradiol 10 ttg 10 2 (100%) 0 0 8
Placebo 10 1 (20%) 3 1 5
P-Value for
Estradiol 10 ittg vs. 0.1429
Placebo'
*N = Total number of patients within each treatment group who were sexually
active at both Baseline and Day 15 and
provided a response at both visits.
NC = No Change ¨ not considered in the statistical comparison.
1P-value for treatment comparison from Fisher's Exact Test.
2 Percent is based on the number of subjects classified as either a Success or
a Failure (N=2 for estradiol 10 jig; N=5 for
Placebo
Estradiol Level/Pharmacokinetics Data
[0324] In this study, the systemic exposure to estradiol following once daily
intravaginal
administration of estradiol 10 jig for 14 days was investigated. Descriptive
statistics of the
plasma estradiol concentrations taken at each sampling time and the observed
Cmax and Tmax
values were recorded in Tables 16 and 17. No statistically significant
difference in the
systemic concentration of estradiol 10 jig versus the placebo group was
observed, which
suggests the estradiol is not carried into the blood stream where it will have
a systemic effect.
Rather, it remains in localized tissues; the effect of estradiol is therefore
believed be local to
the location of administration (i.e., the vagina). The lower limits of
detection of the assays
used to measure the pharmacokinetic data may have affected the measured the
accuracy of
the PK values presented. Additional PK studies were performed with more
accurate assays in
Examples 8 and 9.
[0325] For the purpose of monitoring the estradiol level during the study
blood samples
were collected at 0.0, 1.0, 3.0, and 6.0 hours relative to dosing on day 1;
prior to dosing on
day 8; and prior to dosing on day 15. Efforts were made to collect blood
samples at their
scheduled times. Sample collection and handling procedures for measurement of
estradiol
blood level was performed according to procedure approved by the sponsor and
principal
investigator. All baseline and post-treatment plasma estradiol concentrations
were determined
88
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
using a validated bioanalytical (UPLC-MS/MS) methods. These data are shown in
Tables 16
and 17.
Table 16: Descriptive Statistics of Estradiol Concentrations (pg/mL) at Each
Sampling Time
Treatment Sampling Time
Pre-dose Day Pre-dose Day
Estradiol 10 ittg 0 Hour 1 Hour 3 Hours 6 Hours 8 15
N 24 24 24 24 24
22
Mean + SD 20.1 + 5.74 28.7 + 5.89 25.7 + 5.71 23.4
+ 7.91 21.4 + 9.28 23.4 + 8.72
Median 20.2 28.9 24.7 22.3 20.7 20.7
Min, Max 2.63, 38.3 18.8, 43.9 19.3, 47.5
3.31, 52.3 2.09, 52.2 17.9, 54.7
Placebo
N 26 26 26 26 25
24
Mean + SD 20.5 + 4.29 21.0
+ 6.14 19.0 + 5.92 26.9 17.36 29.9 + 22.51 28.1 16.80
Median 20.8 20.8 20.9 21.7 21.6 21.1
Min, Max 4.03, 29.1 3.19, 41.2 3.15, 26.9
15.1, 90.0 15.0, 116.2 14.7, 81.3
Table 17: Descriptive Statistics of Estradiol Cma and Tma on Day 1
Estradiol 10 ittg Placebo
Cmax Tmax Cmax Tmax
N 24 24 26 26
Mean + SD 30.7 + 7.47 2.12 1.73 27.5 17.26 4.00 +
2.68
Geometric Mean 29.9 24.7 -
Median 29.8 1.00 22.1 6.00
Min, Max 19.7, 52.3 1.00, 6.00 15.1, 90.0 0.00,
6.00
CV% 24.3% 81.3% 62.9% 67.1%
Assessment of Vaginal Mucosa Data
[0326] The investigators rated the vaginal mucosal appearance at day 1 (pre-
dose) and day
15. Vaginal color, vaginal epithelial integrity, vaginal epithelial surface
thickness, and
vaginal secretions were evaluated according to the following degrees of
severity: none, mild,
moderate, or severe using scales 0 to 3, where 0 = none, 1 = mild, 2 =
moderate, and 3 =
severe. Results from these investigators rated assessments are presented in
Tables 18, 19, 20,
and 21.
89
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Table 18: Primary Efficacy Analysis Results of Change from Baseline
(Randomization) to
Day 15 in Investigator's Assessment of the Vaginal Mucosa (Assessment of
Vaginal Color)
Difference
Estradiol 10
Between lug
vs.
Estradiol Treatment 90% CI for
Placebo P-
Population Statistics 10 lug Placebo Means Difference'
value2
Treat Intent-to-
24 24
Least-
(o.434,
-
squares -0.199 -0.009 -0.191
0.1945
0.052)
Mean
Mean SD -0.333 0.125
0.565 0.741
Median 0.00 0.00
Min, Max -2.00, 0.00
2.00
1 Confidence interval for the estradiol 10 tg-Placebo from ANCOVA with
treatment as a fixed effect and baseline as a
covariate.
2 P-value for treatment comparison from ANCOVA with treatment as a fixed
effect and baseline as a covariate.
Table 19: Primary Efficacy Analysis Results of Change from Baseline
(Randomization) to
Day 15 in Investigator's Assessment of the Vaginal Mucosa (Assessment of
Vaginal
Epithelial Integrity)
Difference
Estradiol 10
Between lug
vs.
Estradiol Treatment 90% CI for
Placebo P-
Population Statistics 10 lug Placebo Means Difference'
value2
Treat Intent-to-
24 24
Least-
(-0.726, -
squares -0.342 0.176 -0.518 o.00m
o.311)
Mean
Mean SD -0.417 0.250
0.584 0.442
Median 0.00 0.00
Min, Max -1.00, 1.00 0.00, 1.00
1 Confidence interval for the estradiol 10 tg-Placebo from ANCOVA with
treatment as a fixed effect and baseline as a
covariate.
2 P-value for treatment comparison from ANCOVA with treatment as a fixed
effect and baseline as a covariate.
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Table 20: Primary Efficacy Analysis Results of Change from Baseline
(Randomization) to
Day 15 in Investigator's Assessment of the Vaginal Mucosa (Assessment of
Vaginal
Epithelial Surface Thickness)
Difference
Estradiol 10
Between lug
vs.
Estradiol Treatment 90% CI for
Placebo P-
Population Statistics 10 lug Placebo Means Difference'
value2
Treat Intent-to-
24 24
Least-
(-0.024,
squares -0.034 -0.133 0.099
o.182o
o )
Mean .221
Mean SD -0.125 -0.042
0.338 0.550
Median 0.00 0.00
Min, Max -1.00, 0.00 -1.00, 1.00
1 Confidence interval for the estradiol 10 tg-Placebo from ANCOVA with
treatment as a fixed effect and baseline as a
covariate.
2 P-value for treatment comparison from ANCOVA with treatment as a fixed
effect and baseline as a covariate.
Table 21: Primary Efficacy Analysis Results of Change from Baseline
(Randomization) to
Day 15 in Investigator's Assessment of the Vaginal Mucosa (Assessment of
Vaginal
Secretions)
Difference
Estradiol 10
Between lug
vs.
Estradiol Treatment 90% CI for
Placebo P-
Population Statistics 10 lug Placebo Means Difference'
value2
Treat Intent-to-
24 24
Least-
(-0.661, -
squares -0.643 -0.274 -0.369
0.0401
0.076)
Mean
Mean SD -0.792 -0.125
0.779 0.741
Median -1.00 0.00
-2.00,
Min, Max -2.00, 1.00
2.00
1 Confidence interval for the estradiol 10 tg-Placebo from ANCOVA with
treatment as a fixed effect and baseline as a
covariate.
2 P-value for treatment comparison from ANCOVA with treatment as a fixed
effect and baseline as a covariate.
Delivery Vehicle Disintegration Data
[0327] Assessment of capsule disintegration in the vagina (presence or
absence) at Day 1
(6 hours after dosing) and Day 15. Results of this assessment is presented in
Table 22.
91
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Table 22: Capsule Disintegration State in the Vagina on Day 1 and Day 15
Estradiol 10 pg Placebo
Dayl Day 15 Day 1 Day 15
No evidence of
capsule present 23 (95.8%) 24 (100.0%) 26 (100.0%) 24 (92.3%)
Evidence of capsule 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%)
present
Assessment not 1 (4.2%) 0 (0.0%) 0 (0.0%) 2 (7.7%)
done
[0328] Serum hormone level data was collected to measure the serum
concentrations of
estradiol. These data were used for screening inclusion and were determined
using standard
clinical chemistry methods.
Appropriateness of Measurements
[0329] The selection of the efficacy measurements used in this study was based
on FDA's
recommendations for studies of estrogen and estrogen/progestin drug products
for the
treatment of moderate to severe vasomotor symptoms associated with the
menopause and
moderate to severe symptoms of vulvar and vaginal atrophy associated with the
menopause
(Food and Drug Administration, Guidance for Industry, Estrogen and
Estrogen/Progestin
Drug Products to Treat Vasomotor Symptoms and Vulvar and Vaginal Atrophy
Symptoms ¨
Recommendations for Clinical Evaluation. January 2003, hereby incorporated by
reference).
[0330] Standard clinical, laboratory, and statistical procedures were utilized
in the trial. All
clinical laboratory procedures were generally accepted and met quality
standards.
Statistical Methods:
Efficacy:
[0331] Analysis of variance (ANOVA) was used to evaluate the change from
baseline
differences between the subjects receiving estradiol 10 jig and placebo
capsules for all
efficacy endpoints, except for vaginal bleeding, to estimate the effect size
and variability of
the effect. In some cases, for example, for some vaginal atrophy symptoms, the
change from
baseline (post dose response) was correlated with the baseline value (p<0.05),
so baseline
was included as a covariate to adjust for this correlation (Analysis of
Covariance,
ANCOVA). The 90% confidence intervals on the differences between estradiol 10
jig and
92
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
placebo endpoint means were determined to evaluate the effect size. The change
from
baseline in vaginal bleeding associated with sexual activity was evaluated in
terms of the
proportion of subjects who had treatment success or failure. Any subject
reporting bleeding at
baseline who did not report bleeding at Day 15 was considered to have been
successfully
treated. Any subject reporting bleeding at day 15 was considered a treatment
failure,
regardless of whether they reported baseline bleeding or not. Subjects
reporting no bleeding
at both baseline and day 15 were classified as no-change and were excluded
from the
statistical evaluation. The difference in the proportion of subjects with
success between the
two treatment groups was statistically evaluated using Fisher's Exact Test.
Results of this
difference in proportion are presented in Table 10.
Measurements of Treatment Compliance
[0332] Subjects were required to complete a diary in order to record treatment
compliance.
Diaries were reviewed for treatment compliance at day 8 and day 15 visits. A
total of 45
subjects (21 subjects in the estradiol 10 [ig group and 24 subjects in the
placebo group) were
100% compliant with the treatment regimen.
[0333] Due to the investigative nature of the study, no adjustments were made
for
multiplicity of endpoints.
Safety:
[0334] The frequency and severity of all adverse events were summarized
descriptively by
treatment group.
[0335] Results: All forty eight (48) subjects who completed the study were
included in the
primary efficacy analyses. The results of efficacy analyses are presented
throughout Tables 5,
6, and 7.
Conclusions
Efficacy
[0336] The two-week treatment with pharmaceutical composition 10 [ig led to a
statistically significant greater mean decrease in percent of parabasal cells
than did placebo
treatment (54% vs. 5%, p<0.0001), as illustrated in Table 6. At the same time,
a significantly
greater mean increase in the percent of superficial cells was observed with
the pharmaceutical
composition (35%) than with the placebo capsules (9%), with the difference
being highly
93
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
statistically significant (p=0.0002), as illustrated in Table 7. The
difference in pH reduction
between the pharmaceutical composition (0.97 units) compared to that for the
placebo (0.34
units) was only slightly greater than 0.5 units, but the difference was
detected as statistically
significant (p=0.0002), as illustrated in Table 9.
[0337] While the decrease in severity of the most bothersome symptom was
essentially the
same (-1 unit) for both pharmaceutical composition and placebo, the reductions
in the
severity of the individual symptoms of vaginal dryness, irritation and pain
during sexual
activity were all marginally better for the active treatment than for the
placebo treatment.
None of the differences between the two treatments, all of which were < 0.3
units, were
detected as statistically significant. There was no difference between the two
treatments in
regard to reduction of pain/burning/stinging during urination (-0.4 unit
reduction). The
length of the study was not long enough to show a separation between the most
bothersome
symptoms in the pharmaceutical composition and placebo. However, the trends of
most
bothersome symptoms suggest that with a suitable period of time, significantly
significant
differences between the two treatments would be observed.
[0338] The two-week treatment with estradiol 10 pg capsules showed no
statistically
detectable difference in regard to reduction of severity from baseline
according to the
investigator's assessment of vaginal color or vaginal epithelial surface
thickness.
Pharmaceutical composition capsules did demonstrate a statistically
significant greater
reduction than did placebo in severity of atrophic effects on vaginal
epithelial integrity (-0.34
vs. 0.18, p=0.0001) and vaginal secretions (-0.64 vs. -0.27, p=0.0401).
[0339] Descriptive statistical analyses (mean, median, geometric mean,
standard deviation,
CV, minimum and maximum, Cmax, and Tmax) were conducted on the estradiol
concentrations
at each sampling time, the peak concentration on day 1 and the time of peak
concentration.
Results from this assessment are presented in Tables 16 and 17.
[0340] A pharmaceutical composition comprising estradiol 10 pg outperformed
placebo
treatment in regard to improvement in the Maturation Index, reduction in
vaginal pH,
reduction in the atrophic effects on epithelial integrity and vaginal
secretions. The lack of
statistical significance between the two treatments in regard to reduction of
severity for the
most bothersome symptom, and the individual vaginal atrophy symptoms of
dryness,
irritation, pain associated with sexual activity, and pain/burning/stinging
during urination, is
not unexpected given the small number of subjects in the study and the short
duration of
94
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
therapy. Too few subjects in the study had vaginal bleeding associated with
sexual activity to
permit any meaningful evaluation of this vaginal atrophy symptom.
[0341] Of the 48 subjects enrolled in the study, 45 subjects were 100%
compliant with the
treatment regimen. Of the remaining three subjects, one removed herself from
the study due
to personal reasons and the other two subjects each missed one dose due to an
adverse event.
Safety
[0342] Although the Day 1 mean plasma estradiol peak concentration for the
pharmaceutical composition was somewhat higher than that for the Placebo
(ratio of
geometric means = 1.21: Test Product (estradiol 10 pg) 21% > Placebo), no
statistically
significant difference was determined. However, the assay methods were
questionable,
resulting in questionable PK data. Additional PK studies were performed in
Examples 8 and
9.
[0343] There were no serious adverse events in the study.
[0344] Overall, the pharmaceutical composition comprising estradiol 10 pg was
well
tolerated when administered intravaginally in once daily regimen for 14 days.
EXAMPLE 8: PK Study (25 i.tg formulation)
[0345] A PK study was undertaken to compare the 25 pg formulation disclosed
herein
(Pharmaceutical Composition 3) to the RLD. The results of the PK study for
estradiol are
summarized in Table 23. The p values for these data demonstrate statistical
significance, as
shown in Table 24.
Table 23: Statistical Summary of the Comparative Bioavailability Data for
Unscaled Average
BE studies of Estradiol, Least Square Geometric Means of Estradiol, Ratio of
Means and
90% Confidence Intervals, Fasting/Fed Bioequivalence Study (Study No.: ESTR-1K-
500-
12), Dose 25 pg estradiol
Parameter Test N RLD N Ratio (%) 90% C.I.
Cmax 44.18 -
(pg/mL)
23.0839 36 42.7024 36 54.06
66.14
AUC0_24 23.72 -
89.2093 36 292.0606 36 30.54
(pg.hr/mL) 39.34
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Table 24: P-values for Table 23
P-Va1ne
Effect
ATIC:
Trezhneut
Sequence 0.44711
Period 04104 ,0.7:7, I
[0346] As illustrated in Table 23, baseline adjusted PK data illustrates that
the formulations
disclosed herein unexpectedly show a 54% decrease in C. and a 31% decrease in
the AUC
relative to the RLD. This result is desirable because the estradiol is
intended only for local
absorption. These data suggest a decrease in the circulating levels of
estradiol relative to the
RLD. Moreover, it is noteworthy to point out that the Cmax and AUC levels of
estradiol
relative to placebo are not statistically differentiable, which suggests that
the formulations
disclosed herein have a negligible systemic effect. As shown in Table 24,
there was no
significant difference between the test and reference products due to sequence
and period
effects. However, there was a significant difference due to treatment effect
for both Cmax and
AUC.
[0347] Pharmacokinetics for circulating total estrone, a metabolite of
estradiol, is show in
Table 25. These data show that the total circulating estrone for the
formulations disclosed
herein resulted in a 55% decrease in the Cmax for circulating estrone, and a
70% decrease in
the AUC for circulating estrone.
Table 25: Statistical Summary of the Comparative Bioavailability Data for
Unscaled Average
BE studies of Estrone, Least Square Geometric Means, Ratio of Means and 90%
Confidence
Intervals, Fasting/Fed Bioequivalence Study (Study No.: ESTR-1K-500-12), Dose
25 lig
estradiol
Parameter Test N RLD N Ratio (%) 90% C.I.
Cmax
10.7928 36 23.5794 36 45.77
32.95 to
(pg/mL) 63.59
AUC0_24 19.8 -
51.2491 36 165.4664 36 30.97
(pg.hr/mL) 48.45
96
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Table 26: P-values for Table 25
EftWA.
Tr tntIZEUt 4".V.Z1:µ,2 (x>31
asap:Rem-a, <',1524 ,N64
,t)I10
[0348] There was a significant difference between test and reference products
due to
treatment effect whereas there was no significant difference due to sequence
and period
effects for C.. For AUC, there was a significant difference between test and
reference
products due to treatment, sequence, and period effects.
[0349] PK for circulating total estrone sulfate is shown in Table 27. These
data show that
the total circulating estrone sulfate for the pharmaceutical compositions
disclosed herein
resulted in a 33% decrease in the C. and a 42% decrease in the AUC for
circulating estrone
sulfate.
Table 27: Statistical Summary of the Comparative Bioavailability Data for
Unscaled Average
BE studies of Estrone Sulfate, Least Square Geometric Means of Estrone
Sulfate, Ratio of
Means and 90% Confidence Intervals, Fasting/Fed Bioequivalence Study (Study
No.: ESTR-
1K-500-12), Dose 25 lig estradiol
Parameter Test N RLD N Ratio (%) 90% C.I.
Cmax 53.84 -
490.0449 36 730.5605 36 67.08
(pg/mL) 83.57
AUC0_24 43.23 -
4232.9914 36 7323.0827 36 57.80
(pg.hr/mL) 77.29
Table 28: P-values for Table 27
F-Value
Effeit
Trutzstat f¶s::!:i
Stgg2liert 5.235
Periati ;::! 12.7g
[0350] There was a significant difference between test and reference products
due to
treatment effect whereas there was no significant difference due sequence and
period effects
for both C. and AUC.
97
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
EXAMPLE 9: PK Study (10 ug formulation)
103511 A PK study was undertaken to compare the 10 ug formulation disclosed
herein
(Pharmaceutical Composition 2) to the RLD. The results of the PK study for
estradiol are
summarized in Table 29-40, and Figs. 9-14.
103521 A PK study was undertaken to compare pharmaceutical compositions
disclosed
herein having 10 ug of estradiol to the RLD. The results of the PK study for
estradiol are
summarized in Tables 29-34, which demonstrate that the pharmaceutical
compositions
disclosed herein more effectively prevented systemic absorption of the
estradiol. Table 35
shows that the pharmaceutical compositions disclosed herein had a 28%
improvement over
the RLD for systemic blood concentration C. and 72% AUC improvement over the
RLD.
Table 29: Summary of Pharmacokinetic Parameters of Test product (T) of
Estradiol -
Baseline adjusted (N=34)
Arithmetic
Pharmacokinetic Mean Coefficient
Median Minimum Maximum
Parameter Standard of Variation
Deviation
15.7176
Cmax (pg/mL) 50.3761 13.9000 6.5000 49.6000
7.9179
AUC 0 53.0100 _24 (pg.hr/mL) 36.9041 49.9750
24.3000 95.1500
19.5629
tmax (hr) 1.98 1.29 65.34 2.00 1.00 8.05
Table 30: Summary of Pharmacokinetic Parameters of Reference product (R) of
Estradiol -
Baseline adjusted (N=34)
Arithmetic
Pharmacokinetic Mean Coefficient
Median Minimum Maximum
Parameter Standard of Variation
Deviation
1882
Cmax (pg/mL) 24. 49.2877 24.1500 1.0000 55.3000
11.9218
163.
AUC0_24 (pg.hr/mL) 43.9960 158.0375 2.0000 304.8500
72.08586
913
tmax (hr) 10.53 5.58 52.94 8.06 2.00 24.00
Table 31: Geometric Mean of Test Product (T) and Reference product (R) of
Estradiol -
Baseline adjusted (N=34)
Geometric Mean
Pharmacokinetic Parameter
Test Product (T) Reference Product (R)
Cmax (pg/mL) 14.3774 20.3837
98
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
AUC0_24 (pg.hr/mL) 49.6231 132.9218
tmax (hr) 1.75 9.28
Table 32: Statistical Results of Test product (T) versus Reference product (R)
for Estradiol -
Baseline adjusted (N=34)
Geometric Least
Square Mean Intra T/R 90%
Pharmacokinetic
Test Reference Subject Ratio Confidence
Parameter
Product Product CV % % Interval
(T) (R)
Cmax (pg/mL) 14.4490 20.1980 60.68 71.54* 56.82-
90.08
AUC0-24 49.7310 131.0400 70.64 37.95* 29.21-49.31
(pg.hr/mL)
* Comparison was detected as statistically significant by ANOVA (a=o.05).
[0353] The PK data for total estrone likewise demonstrated reduced systemic
exposure
when compared to the RLD. Table 33 shows the pharmaceutical compositions
disclosed
herein reduced systemic exposure by 25% for C. and 49% for AUC.
Table 33: Summary of Pharmacokinetic Parameters of Test product (T) of Estrone
- Baseline
adjusted (N=33)
Arithmetic
Pharmacokinetic Mean Coefficient
. Median Minimum Maximum
Parameter Standard of Variation
Deviation
Cmax (pg/mL) 6.84854 96.1149
5.4000 1.3000 36.3000
6.582
34.7051
AUC 0_24 (pg.hr/mL) 80.5476 30.8500 3.3500 116.7500
27.9541
tmax (hr) 9.12 8.83 96.80 4.00 1.00 24.00
Table 34: Summary of Pharmacokinetic Parameters of Reference product (R) of
Estrone -
Baseline adjusted (N=33)
Arithmetic
Pharmacokinetic Mean Coefficient
. Median Minimum Maximum
Parameter Standard of Variation
Deviation
8333
Cmax (pg/mL) 8. 80.9086 6.7000
2.7000 30.3000
7.1469
AUC 0-24 (pg.hr/mL) 63.0042 73.8814
51.2800 8.8000 214.0000
46.5484
tmax (hr) 11.16 7.24 64.95 10.00 4.00 24.00
99
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Table 35: Geometric Mean of Test Product (T) and Reference product (R) of
Estrone ¨
Baseline adjusted (N=33)
Geometric Mean
Pharmacokinetic Parameter
Test Product (T) Reference Product (R)
Cmax (pg/mL) 5.1507 6.9773
AUC0_24 (pg.hr/mL) 24.2426 48.2377
tmax (hr) 5.87 9.07
Table 36: Statistical Results of Test product (T) versus Reference product (R)
for Estrone ¨
Baseline adjusted (N=33)
Geometric Least
Square Mean Intra T/R 90%
Pharmacokinetic
Test Reference Subject Ratio Confidence
Parameter
Product Product CV % % Interval
(T) (R)
Cmax (pg/mL) 5.1620 6.9280 47.59 74.50* 61.69-
89.97
AUC0-24 24.1960 47.9020 73.66
50.51* 38.37-66.50
(pg.hr/mL)
* Comparison was detected as statistically significant by ANOVA (a=o.05).
[0354] The PK data for estrone sulfate likewise demonstrated reduced systemic
exposure
when compared to the RLD. Table 37 shows the pharmaceutical compositions
disclosed
herein reduced systemic exposure by 25% for C. and 42% for AUC.
Table 37: Summary of Pharmacokinetic Parameters of Test product (T) of Estrone
Sulfate ¨
Baseline adjusted (N=24)
Arithmetic
Pharmacokinetic Mean Coefficient
. Median Minimum Maximum
Parameter Standard of Variation
Deviation
13.9042
Cmax (ng/mL) 50.6339 11.1500 1.3000
39.0000
7.0402
97.9953
AUC0-24 (ng=hr/mL) 82.5408 76.2750 5.1025 338.0000
80.8861
tmax (hr) 6.33 4.56 71.93 4.00 4.00 24.00
Table 38: Summary of Pharmacokinetic Parameters of Reference product (R) of
Estrone
Sulfate ¨ Baseline adjusted (N=24)
Pharmacokinetic Arithmetic Coefficient
Median Minimum Maximum
Parameter Mean of Variation
100
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Standard
Deviation
Cmax (ng/mL) 19.254233 59.0173 15.2000
7.0000 53.7000
11.36
177.6208
AUC 0_24 (ng.hr/mL) 93.5931 124.0000 20.0000 683.0500
166.2408
tmax (hr) 10.33 5.58 54.05 10.00 2.00 24.00
Table 39: Geometric Mean of Test Product (T) and Reference product (R) of
Estrone Sulfate
¨ Baseline adjusted (N=24)
Geometric Mean
Pharmacokinetic Parameter
Test Product (T) Reference Product (R)
Cmax (ng/mL) 12.1579 16.8587
AUC0_24 (ng.hr/mL) 66.5996 121.5597
tmax (hr) 5.49 8.83
Table 40: Statistical Results of Test product (T) versus Reference product (R)
for Estrone
Sulfate ¨ Baseline adjusted (N=24)
Geometric Least
Square Mean Intra T/R 90%
Pharmacokinetic
Test Reference Subject Ratio Confidence
Parameter
Product Product CV % % Interval
(T) (R)
Cmax (ng/mL) 12.3350 16.5470 48.02 74.55* 59.43-
93.51
AUC0-24 68.5260 118.4170 73.87 57.87* 41.68-80.35
(ng.hr/mL)
* Comparison was detected as statistically significant by ANOVA (a=o.05).
EXAMPLE 10: Randomized, double-blind, placebo-controlled multicenter study of
Estradiol Vaginal Softgel Capsules for Treatment of VVA.
Investigational Plan
[0355] The study was a randomized, double-blind, placebo-controlled
multicenter study
design. Postmenopausal subjects who meet the study entry criteria will be
randomized in a
1:1:1:1 ratio to receive Estradiol Vaginal Softgel Capsule 4 pg, Estradiol
Vaginal Softgel
Capsule 10 pg, Estradiol Vaginal Softgel Capsule 25 pg, or matching placebo.
Subjects will
be asked to self-assess the symptoms of vulvar or vaginal atrophy including
vaginal pain
associated with sexual activity, vaginal dryness, vulvar or vaginal itching or
irritation by
completing the VVA symptom self-assessment questionnaire and identification of
her MBS
101
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
at screening visit 1A to determine eligibility for the study. The VVA symptom
Self-
Assessment Questionnaire, vaginal cytology, vaginal pH, and vaginal mucosa
will be
assessed at screening visit 1B. These assessments will determine continued
eligibility and
will be used as the baseline assessments for the study. Randomized subjects
will then
complete the Questionnaire during visits 3, 4, 5, and 6.
[0356] The primary efficacy endpoints for the study included: (A) change from
baseline to
week 12 in the percentage of vaginal superficial cells (by vaginal cytologic
smear) compared
to placebo; (B) change from baseline to week 12 in the percentage of vaginal
parabasal cells
(by vaginal cytologic smear) compared to placebo; (C) change from baseline at
week 12 in
vaginal pH as compared to placebo; and (D) change from baseline to week 12 on
the severity
of the MBS of dyspareunia (vaginal pain associated with sexual activity)
associated with
VVA as compared to placebo.
[0357] The secondary efficacy endpoints for the study included: (E) change
from baseline
to weeks 2, 6, and 8 in the percentage of vaginal superficial cells (by
vaginal cytologic smear)
compared to placebo; (F) change from baseline to weeks 2, 6, and 8 in the
percentage of
vaginal parabasal cells (by vaginal cytologic smear) compared to placebo; (G)
change from
baseline to weeks 2, 6, and 8 in vaginal pH as compared to placebo; (H) change
from
baseline to weeks 2, 6, and 8 on the severity of the MBS of dyspareunia
(vaginal pain
associated with sexual activity) associated with VVA as compared to placebo;
(I) change
from baseline to weeks 2, 6, 8, and 12 on the severity of vaginal dryness and
vulvar or
vaginal itching or irritation associated with VVA as compared to placebo; (J)
change in
visual evaluation of the vaginal mucosa from baseline to weeks 2, 6, 8, and 12
compared to
placebo; (K) assessment of standard PK parameters as defined in the SAP for
serum estradiol,
estrone, and estrone conjugates at Screening Visit 1A, days 1, 14, and 84 of
treatment in a subset of
subjects (PK substudy) utilizing baseline corrected and uncorrected values [as
outlined in the
Statistical Analysis Plan (SAP)]; and (L) change from baseline in the Female
Sexual
Function Index (FSFI) at week 12 compared to placebo.
[0358] The safety endpoints for the study included: (1) Adverse events; (2)
Vital signs; (3)
Physical examination findings; (4) Gynecological examination findings; (5)
Clinical
laboratory tests; (6) Pap smears; and (7) Endometrial biopsy.
[0359] Approximately 100 sites in the United States and Canada screened
approximately
1500 subjects to randomize 747 subjects in this study (modified intent to
treatment
102
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
population, or all subjects who have taken at least one dose of the
pharmaceutical
compositions disclosed herein), with a target of 175 subjects randomized to
each treatment
group (175 in each active treatment group and 175 in the placebo group to
complete 560
subjects). Actual subjects enrolled are 186 subjects in the 4 pg formulation
group, 188
subjects in the 10 pg formulation group, 186 subjects in the 25 pg formulation
group, and 187
subjects in the placebo group, for a total of 747 subjects in the study.
Within each treatment
group, 15 subjects also participated in a PK substudy. Subjects were assigned
to one of four
treatment groups: (1) 4 pg formulation; (2) 10 pg formulation; (3) 25 pg
formulation; and (4)
placebo.
[0360] Most subjects participated in the study for 20-22 weeks. This included
a 6 to 8 week
screening period (6 weeks for subjects without an intact uterus and 8 weeks
for subjects with
an intact uterus), 12 weeks on the investigational product, and a follow-up
period of
approximately 15 days after the last dose of investigational product. Some
subjects'
involvement lasted up to 30 weeks when an 8-week wash-out period was
necessary. Subjects
who withdrew from the study were not replaced regardless of the reason for
withdrawal.
[0361] The study schematic diagram shown in Fig. 9. There were two treatment
periods;
once daily intravaginal administration of one of the listed investigational
products for 2
weeks, followed by a twice weekly intravaginal administration for 10 weeks.
[0362] The subject inclusion criteria included: (1) postmenopausal female
subjects between
the ages of 40 and 75 years (at the time of randomization) with at least: 12
months of
spontaneous amenorrhea (women <55 years of age with history of hysterectomy
without
bilateral oophorectomy prior to natural menopause must have follicle
stimulating hormone
(FSH) levels > 40 mIU/mL); or 6 months of spontaneous amenorrhea with follicle
stimulating hormone (FSH) levels > 40 m1U/mL; or At least 6 weeks postsurgical
bilateral
oophorectomy.
[0363] The subject inclusion criteria also included: (2) <5% superficial cells
on vaginal
cytological smear; (3) Vaginal pH > 5.0; (4) Moderate to severe symptom of
vaginal pain
associated with sexual activity considered the most bothersome vaginal symptom
by the
subject at screening visit 1A; (5) Moderate to severe symptom of vaginal pain
associated with
sexual activity at screening visit 1B; (6) Onset of moderate to severe
dyspareunia in the
postmenopausal years; (7) Subjects were sexually active (i.e., had sexual
activity with vaginal
103
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
penetration within approximately 1 month of screening visit 1A); and (8)
Subjects anticipated
having sexual activity (with vaginal penetration) during the conduct of the
trial
[0364] For subjects with an intact uterus, the subject inclusion criteria also
included: (9)
subjects had an acceptable result from an evaluable screening endometrial
biopsy. The
endometrial biopsy reports by the two central pathologists at screening
specified one of the
following: proliferative endometrium; weakly proliferative endometrium;
disordered
proliferative pattern; secretory endometrium; endometrial tissue other (i.e.,
benign, inactive,
or atrophic fragments of endometrial epithelium, glands, stroma, etc.);
endometrial tissue
insufficient for diagnosis; no endometrium identified; no tissue identified;
endometrial
hyperplasia; endometrial malignancy; or other findings (endometrial polyp not
present,
benign endometrial polyp, or other endometrial polyp). Identification of
sufficient tissue to
evaluate the biopsy by at least one pathologist was required.
[0365] For subjects with a Body Mass Index (BMI) less than or equal to 38
kg/m2, the
subject inclusion criteria also included: (10) BMI values were rounded to the
nearest integer
(ex. 32.4 rounds down to 32, while 26.5 rounds up to 27).
[0366] In general, the inclusion criteria also included: (11) in the opinion
of the
investigator, the subject was believed likely to comply with the protocol and
complete the
study.
[0367] The exclusion criteria included: (1) use of oral estrogen-, progestin-,
androgen-, or
SERM-containing drug products within 8 weeks before screening visit 1A (entry
of washout
was permitted); use of transdermal hormone products within 4 weeks before
screening visit
1A (entry of washout was permitted); use of vaginal hormone products (rings,
creams, gels)
within 4 weeks before screening visit 1A (entry of washout was permitted); use
of
intrauterine progestins within 8 weeks before screening visit 1A (entry of
washout was
permitted); use of progestin implants/injectables or estrogen
pellets/injectables within 6
months before screening visit 1A (entry of washout was not permitted); or use
of vaginal
lubricants or moisturizers within 7 days before the screening visit 1B vaginal
pH assessment.
[0368] The exclusion criteria also included: (2) a history or active presence
of clinically
important medical disease that might confound the study or be detrimental to
the subject,
including, for example: hypersensitivity to estrogens; endometrial
hyperplasia; undiagnosed
vaginal bleeding; a history of a chronic liver or kidney dysfunction/disorder
(e.g., Hepatitis C
or chronic renal failure); thrombophlebitis, thrombosis, or thromboembolic
disorders;
104
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
cerebrovascular accident, stroke, or transient ischemic attack; myocardial
infarction or
ischemic heart disease; malignancy or treatment for malignancy, within the
previous 5 years,
with the exception of basal cell carcinoma of the skin or squamous cell
carcinoma of the skin
(a history of estrogen dependent neoplasia, breast cancer, melanoma, or any
gynecologic
cancer, at any time, excluded the subject); and endocrine disease (except for
controlled
hypothyroidism or controlled non-insulin dependent diabetes mellitus).
[0369] The exclusion criteria also included: (3) recent history of known
alcohol or drug
abuse; (4) history of sexual abuse or spousal abuse that was likely to
interfere with the
subject's assessment of vaginal pain with sexual activity; (5) current history
of heavy
smoking (more than 15 cigarettes per day) or use of e-cigarettes; (6) use of
an intrauterine
device within 12 weeks before screening visit 1A; (7) use of an
investigational drug within 60
days before screening visit 1A; (8) any clinically important abnormalities on
screening
physical exam, assessments, electrocardiogram (ECG), or laboratory tests; (9)
known
pregnancy or a positive urine pregnancy test; and (10) current use of
marijuana.
[0370] In this study, if a subject discontinued or was withdrawn, the subject
was not
replaced. At the time of consent, each subject was given a unique subject
number that
identified their clinical site and sequential number. In addition to the
assigned subject
number, subject initials were used for identification. The clinical trial was
performed in
compliance with standard operating procedures as well as regulations set forth
by FDA,
ICH E6 (R1) guidelines, and other relevant regulatory authorities. Compliance
was achieved
through clinical trial-specific audits of clinical sites and database review.
Statistical Methods
[0371] Efficacy. The primary objective of the trial was to assess the efficacy
of estradiol
vaginal softgel capsules (4 [ig, 10 [ig, and 25 g) when compared to placebo
on vaginal
superficial cells, vaginal parabasal cells, vaginal pH, and on the symptom of
moderate to
severe dyspareunia (vaginal pain associated with sexual activity) as the MBS
at week 12. To
account for the multiple comparisons of testing placebo to each of the three
doses of estradiol
(4 [ig, 10 [ig, and 25 g) and the multiple testing of the four co-primary
endpoints, a closed
procedure was performed (see, Edwards D, Madsen J. "Constructing multiple
procedures for
partially ordered hypothesis sets." Stat Med 2007:26-5116-24, incorporated by
reference
herein).
105
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0372] Determination of Sample Size. The sample size needed per dose vs.
placebo for
each test of hypothesis in the modified intent-to-treat (MITT) population to
achieve a given
power was calculated using reference data from other studies (see, Bachman,
G., et al.
"Efficacy and safety of low-dose regimens of conjugated estrogens cream
administered
vaginally."Menopause, 2009. 16(4): p.719-27; Simon, J., et al. "Effective
Treatment of
Vaginal Atrophy With an Ultra¨Low-Dose Estradiol Vaginal Tablet." Obstetrics &
Gynecology, 2008. 112(5):p. 1053-60; FDA Medical Officer's Review of Vagifem
[NDA 20-
908, March 25, 1999, Table 6, p 121, each incorporated by reference herein).
Table 41 below
provides the effect sizes, power, and sample size determinations for each of
the primary
endpoints. In general, subjects in the study met all inclusion/exclusion
criteria and had
moderate to severe dyspareunia as their most bothersome symptom of VVA. Based
on the
power analysis and the design considerations, approximately 175 subjects per
treatment arm
were enrolled.
Table 41: Power Analysis and Sample Size Determinations
Four Primary Endpoints in a Closed Procedure
Mean Change from Baseline to Week 12 Compared to Placebo (MMRM)
Power (One-way ANOVA, Alpha=0.005, one-tailed)
Primary Endpoint Effect Size (%)* Power Based Upon
N=140 per group per MITT
% Parabasal Cells 150.3% >0.999
% Superficial Cells 115.3% >0.999
Vaginal pH 77.4% >0.999
Severity of Dyspareunia** 30.0%, 41.2%, 70.5% 0.50, 0.80, > 0.999
*Range from 30% (Vagifem 10 tig; see, Simon 2008, supra), 41.2% (Vagifem 25
tig; see, FDA 1999, supra),
70.5% (Premarin cream 2/week; see, Bachman 2009, supra)
** Effect Size is calculated for all primary endpoints as 100% times
difference (treated minus placebo) in mean
changes at week 12 from baseline.
[0373] All subjects who were randomly assigned and had at least 1 dose of
investigational
product formed the intent-to-treat (ITT) population. The Modified intent-to-
treat (MITT)
population was defined as all ITT subjects with a baseline and at least one
follow-up value
for each of the primary endpoints, each subject having taken at least one dose
of
investigational product, and was the primary efficacy population. The efficacy-
evaluable
(EE) population was defined as all MITT subjects who completed the clinical
trial, were at
least 80% compliant with investigational product, had measurements for all
primary efficacy
106
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
endpoints, and were deemed to be protocol compliant, with no significant
protocol violations.
The safety population included all ITT subjects.
[0374] The primary efficacy analyses were conducted on the MITT subjects with
supportive efficacy analyses conducted on the EE population. For analysis
purposes, subjects
were required to complete all visits, up to and including Visit 6 (week 12),
to be considered
as having completed the study.
[0375] Analysis of Efficacy Endpoints. For all numerically continuous efficacy
endpoints,
which included the four primary endpoints (mean change from baseline to week
12), active
treatment group means were compared to placebo using an ANCOVA adjusting for
the
baseline level.
[0376] Primary and secondary efficacy endpoints were measured at baseline and
at 2, 6, 8,
and 12 weeks. The analysis examined change from baseline. Therefore, ANCOVAs
were
based on a repeated measures mixed effects model (MMRM) where the random
effect was
subject and the two fixed effects were treatment group and visit (2, 6, 8, and
12 weeks).
Baseline measures and age were used as covariates. ANCOVAs were therefore not
calculated
independently for each study collection period. The analyses started with the
full model but,
interaction terms for visit (week 2, 6, 8, and 12) with treatment only
remained where
statistically significant (p<0.05).
[0377] The following three pair-wise comparisons were performed using the
appropriate
ANCOVA contrast for week 12 (primary) and weeks 2, 6, and 8 (secondary)
changes from
baseline: (1) active treatment, high dose group vs placebo; (2) active
treatment, middle dose
group vs placebo; and (3) active treatment, low dose group vs placebo.
[0378] Safety outcome measures. Adverse events, vital signs, physical
examination
findings, gynecological examination findings, clinical laboratory tests, pap
smears, and
endometrial biopsy were the safety parameters. Adverse events and SAEs were
summarized
for each treatment group and overall for all active treatment groups with the
proportion of
subjects reporting each event. Actual values and change from baseline in vital
signs, and all
laboratory test parameters were summarized for each treatment group and
overall for all
active treatment groups with descriptive statistics at each assessment
obtained.
[0379] Endometrial Biopsy Assessment. Three independent pathologists with
expertise in
gynecologic pathology, blinded to treatment and to each other's readings,
determined the
107
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
diagnosis for endometrial biopsy slides during the conduct of the study. All
visit 6, early
termination, and on-treatment unscheduled endometrial biopsies were centrally
read by three
of the pathologists. Each pathologist's report was classified into one of the
following three
categories: category 1: not hyperplasia/not malignancy - includes
proliferative endometrium,
weakly proliferative endometrium, disordered proliferative pattern, secretory
endometrium,
endometrial tissue other (i.e., benign, inactive or atrophic fragments of
endometrial
epithelium, glands, stroma, etc.), endometrial tissue insufficient for
diagnosis, no
endometrium identified, no tissue identified, other; category 2: hyperplasia-
includes simple
hyperplasia with or without atypia and complex hyperplasia with or without
atypia; category
3: malignancy ¨ endometrial malignancy.
[0380] The final diagnosis was based on agreement of two of the three reads.
Consensus
was reached when two of the three pathologist readers agreed on any of the
above categories.
For example, any 2 subcategories of "not hyperplasia/not malignancy" were
classified as
"Category 1: not hyperplasia/not malignancy." If all three readings were
disparate (i.e., each
fell into a different category ¨ category 1, 2, or 3), the final diagnosis was
based on the most
severe of the three readings.
[0381] The analysis population for endometrium hyperplasia was the endometrial
hyperplasia (EH) population. An EH subject at week 12 was one who was randomly
assigned
and took at least 1 dose of investigational product, with no exclusionary
protocol violation (as
detailed at the Statistical Analysis Plan), and had a pretreatment endometrial
biopsy and a
biopsy on therapy.
Treatment of Subjects
[0382] The study used a double-blind design. Investigational product was
supplied as 3
doses of Estradiol Vaginal Softgel Capsules (4 pg, 10 pg, and 25 pg) and
matching placebo
capsules. All subjects manually inserted one capsule into the vaginal cavity
daily for 14 days
(2 weeks) followed by twice weekly for 10 weeks according to one of the
following treatment
arms:
Table 42. Treatment Arms and Administration
Regimen Capsules Capsules
Treatment 1 capsule daily of 4 pg vaginal 1 capsule twice weekly of 4
pg vaginal
1 softgel for 2 weeks softgel for 10 weeks
Treatment 1 capsule daily of 10 pg vaginal 1 capsule twice weekly of
10 pg vaginal
108
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Regimen Capsules Capsules
2 softgel for 2 weeks softgel for 10 weeks
Treatment 1 capsule daily of 25 [ig vaginal 1 capsule twice weekly of
25 [ig vaginal
3 softgel for 2 weeks softgel for 10 weeks
Treatment 1 capsule daily of placebo vaginal 1 capsule twice weekly of
placebo
4 softgel for 2 weeks vaginal softgel for 10 weeks
[0383] Investigational product was dispensed to all eligible subjects at visit
2. Each subject
was provided a total of 30 soft gel capsules of investigational product in a
labeled bottle,
allowing for extra capsules for accidental loss or damage. A second bottle was
dispensed at
Visit 5. Each subject was trained by the clinical site to self-administer
intravaginally one
capsule daily at approximately the same hour for 2 weeks (14 days). The drug
administration
instructions included: "Remove vaginal capsule from the bottle; find a
position most
comfortable for you; insert the capsule with the smaller end up into vaginal
canal for about 2
inches." Starting on Day 15, each subject administered 1 capsule twice weekly
for the
remaining 10 weeks. Twice weekly dosing was approximately 3-4 days apart, and
generally
did not exceed more than twice in a seven day period. For example, if the Day
15 dose was
inserted on Sunday, the next dose was inserted on Wednesday or Thursday. At
randomization
visit 2 (day 1), subjects received their first dose of investigational product
at the clinical
facility under the supervision of the study personnel.
[0384] The investigational estradiol vaginal softgel drug products used in the
study are
pear-shaped, opaque, light pink softgel capsules. The capsules contain the
solubilized
estradiol pharmaceutical compositions disclosed herein as Pharmaceutical
Compositions 4-7.
When the softgel capsules come in contact with the vaginal mucosa, the soft
gelatin capsule
releases the pharmaceutical composition, into the vagina. In embodiments, the
soft gelatin
capsule completely dissolves.
[0385] The placebo used in the study contained the excipients in the
investigational
estradiol vaginal softgel capsule without the estradiol (see, e.g.,
Pharmaceutical Composition
7). The packaging of the investigational products and placebo were identical
to maintain
adequate blinding of investigators. Neither the subject nor the investigator
was able to
identify the treatment from the packaging or label of the investigational
products.
[0386] A subject was required to use at least 80% of the investigational
product to be
considered compliant with investigational medication administration. Capsule
count and
109
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
diary cards were be used to determine subject compliance at each study visit.
Subjects were
randomly assigned in a 1:1:1:1 ratio to receive Estradiol Vaginal Softgel
Capsule 4 pg
(Pharmaceutical Composition 4), Estradiol Vaginal Softgel Capsule 10 pg
(Pharmaceutical
Composition 5), Estradiol Vaginal Softgel Capsule 25 pg (Pharmaceutical
Composition 6), or
placebo (Pharmaceutical Composition 7).
[0387] Concomitant medications/treatments were used to treat chronic or
intercurrent
medical conditions at the discretion of the investigator. The following
medications were
prohibited for the duration of the study: investigational drugs other than the
investigational
Estradiol Vaginal Softgel Capsule; estrogen-, progestin-, androgen (i.e.,
DHEA) or SERM-
containing medications other than the investigational product; medications,
remedies, and
supplements known to treat vulvar/vaginal atrophy; vaginal lubricants and
moisturizers (e.g.,
Replens) be discontinued 7 days prior to Visit 1B vaginal pH assessment; and
all medications
excluded before the study.
Efficacy Assessments
[0388] Vaginal cytological smears were collected from the lateral vaginal
walls according
to standard procedures and sent to a central laboratory to evaluate vaginal
cytology. The
percentage of superficial, parabasal, and intermediate cells was determined.
All on-
therapy/early termination vaginal cytology results were blinded to the
Sponsor, Investigators,
and subjects.
[0389] Vaginal pH was determined at screening Visit 1B and visits 3, 4, 5, and
6/end of
treatment. Subjects were not allowed to use vaginal lubricants or moisturizers
within 7 days
of the screening vaginal pH assessment or at any time afterwards during the
study. The
subjects were advised not to have sexual intercourse and to refrain from using
vaginal
douching within 24 hours prior to the measurement for all scheduled vaginal pH
assessments.
After insertion of an unlubricated speculum, a pH indicator strip was applied
to the lateral
vaginal wall until it became wet, taking care to avoid cervical mucus, blood
or semen that are
known to affect vaginal pH. The color of the strip was compared immediately
with a
colorimetric scale and the measurement was recorded.
[0390] During the gynecological examinations, the investigator performed a
visual
evaluation of vaginal mucosa using a four-point scale (0 = none, 1 = mild, 2 =
moderate, and
3 = severe) to assess parameters of vaginal secretions, vaginal epithelial
integrity, vaginal
epithelial surface thickness, and vaginal color according to the table below.
110
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Assessment Severity
Criteria
No atrophy Mild Moderate Severe
Vaginal normal clear superficial coating of scant not covering
none, inflamed,
secretions secretions noted on secretions, difficulty the
entire vaginal ulceration noted,
vaginal walls with speculum vault, may need need
lubrication with
insertion lubrication with speculum
insertion to
speculum insertion prevent pain
to prevent pain
Vaginal normal vaginal surface vaginal surface
vaginal surface has
epithelial bleeds with scraping bleeds with light petechiae before
integrity contact contact and bleeds
with light contact
Vaginal rogation and poor rogation with smooth, some
smooth, no elasticity,
epithelial elasticity of vault some elasticity noted
elasticity of constriction of the
surface of vaginal vault vaginal vault upper one third of
thickness vagina or loss of
vaginal tone
(cystocele and
rectocele)
Vaginal color pink lighter in color pale in color
transparent, either no
color or inflamed
[0391] The VVA symptom self-assessment questionnaire, shown below, is an
instrument
for subjects to self-assess their symptoms of vulvar or vaginal atrophy,
including vaginal pain
associated with sexual activity, vaginal dryness, vulvar or vaginal itching,
or irritation. At
screening visit 1A subjects were asked to complete the questionnaire and
identify their most
bothersome symptoms, and the results of the survey were used to determine
initial eligibility
for the study. At visit 1A, subjects were also asked to indicate which
moderate or severe
symptoms bothered them most. The questionnaire was administered again at
screening visit
1B and used to determine continued eligibility for the study.
VVA SYMPTOMS SELF-ASSESSMENT
Severity Score
Please Rate your Vulvar
(Please select only ONE)
and/or Vaginal
Symptoms 0 = None 1 = Mild 2 = Moderate 3 = Severe
1 Pain associated
with sexual
activity (with
vaginal
penetration).
2 Vaginal dryness.
3 Vulvar and/or
vaginal itching
or irritation.
111
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0392] Randomized subjects were asked to complete the VVA Symptom Self-
Assessment
Questionnaire at visits 3, 4, 5, and 6. Subjects were asked to indicate if no
sexual activity
with vaginal penetration was experience since the previous visit. Screening
visit 1B
evaluation results were considered as Baseline data for the statistical
analyses.
[0393] The Female Sexual Function Index (FSFI) is a brief, multidimensional
scale for
assessing sexual function in women (see, Rosen, 2000, supra 26: p.191-208,
incorporated by
reference herein). The scale consists of 19 items that assess sexual function
over the past 4
weeks and yield domain scores in six areas: sexual desire, arousal,
lubrication, orgasm,
satisfaction, and pain. Further validation of the instrument was conducted to
extend the
validation to include dyspareunia/vaginismus (pain), and multiple sexual
dysfunctions (see,
Weigel, M., et al. "The Female Sexual Function Index (FSFI): Cross-Validation
and
Development of Clinical Cutoff Scores." Journal of Sex & Marital Therapy,
2005. 31: p. 1-
20, incorporated by reference herein). The FSFI was conducted at Visits 2 and
6. Subjects
participating in the PK substudy were not assessed using FSFI.
Safety Assessments
[0394] A complete medical history, including demographic data (age and
race/ethnicity)
gynecological, surgical, and psychiatric history and use of tobacco and
alcohol was recorded
at the washout/screening visit 1A prior to any washout period; this history
included a review
of all past and current diseases and their respective durations as well as any
history of
amenorrhea.
[0395] A complete physical examination was conducted at screening visit 1A and
visit
6/end of treatment. The physical examination included, at a minimum,
examination of the
subject's general appearance, HEENT (head, eyes, ears, nose, and throat),
heart, lungs,
musculoskeletal system, gastrointestinal (GI) system, neurological system,
lymph nodes,
abdomen, and extremities. The subject's height was measured at
washout/screening visit 1A
only and body weight (while the subject is lightly clothed) was be measured at
washout/screening visit 1A and end of treatment. BMI was calculated at
washout/screening
visit 1A. Vital signs (body temperature, heart rate [HR], respiration rate
[RR], and sitting
blood pressure [BP]) were measured at all visits after the subject had been
sitting for >10
minutes. If the initial BP reading was above 140 mmHg systolic or 90 mmHg
diastolic, the
112
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
option for a single repeat assessment performed 15 minutes later was provided.
A standard
12-lead ECG was obtained at screening visit 1A and visit 6 or early
termination.
[0396] Subjects were required to have a pelvic examination and Pap smear
performed
during the screening visit 1B and visit 6 or early termination. The Pap smear
was required for
all subjects with or without an intact uterus and cervix. For subjects without
an intact cervix
the Pap smear was obtained by sampling the apex of the vaginal cuff All
subjects were
required to have a Pap smear done during screening, regardless of any recent
prior
assessment. Subjects who discontinued the study after 2 weeks of
investigational product
were required to have an end of treatment Pap smear. Subjects had a breast
examination
performed during screening visit 1A and at visit 6 or early termination.
[0397] Endometrial biopsies were performed by a board-certified gynecologist
at screening
and at visit 6/end of treatment. Unscheduled endometrial biopsies were
performed during the
study, when indicated for medical reasons. The screening biopsy was performed
at screening
visit 1B, after the subject's initial screening visit assessments indicated
that the subject was
otherwise an eligible candidate for the study.
[0398] At screening, endometrial biopsies were read centrally by two
pathologists. A
candidate subject was excluded from the study if at least one pathologist
assessed the
endometrial biopsy as endometrial hyperplasia, endometrial cancer,
proliferative
endometrium, weakly proliferative endometrium, or disordered proliferative
pattern, or if at
least one pathologist identified an endometrial polyp with hyperplasia,
glandular atypia of
any degree (e.g., atypical nuclei), or cancer. Additionally, identification of
sufficient tissue to
evaluate the biopsy by at least one pathologist was required for study
eligibility. The option
for one repetition of the screening endometrial biopsy was made available when
an initial
endometrial biopsy was performed and both of the primary pathologists reported
endometrial
tissue insufficient for diagnosis, no endometrium was identified, or no tissue
was identified,
and if the subject had met all other protocol-specified eligibility criteria
to date. The visit 6
(or early termination) endometrial biopsies and on treatment unscheduled
biopsies were
assessed by three pathologists.
[0399] During the study, at early termination, and at the end of the study,
any subject with
a diagnosis of endometrial hyperplasia was withdrawn and treated with 10 mg of
Medroxyprogesterone acetate (MPA) for 6 months unless deemed otherwise by the
PI. For
113
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
unscheduled biopsies, the histological diagnosis of endometrial polyp did not
force
withdrawal unless atypical nuclei were present.
[0400] A urine pregnancy test was conducted at screening visit 1A unless the
subject had a
history of tubal ligation, bilateral oophorectomy, or was >55 years of age and
had
experienced cessation of menses for at least 1 year.
[0401] Blood samples for blood chemistry, hematology, coagulation tests, and
hormone
levels and urine samples for urine analysis were collected and sent to a
central laboratory.
Blood Chemistry (sodium, potassium, chloride, total cholesterol, blood urea
nitrogen (BUN),
iron, albumin, total protein, aspartate aminotransferase (AST), alanine
aminotransferase
(ALT), alkaline phosphatase, creatinine, calcium, phosphorous, uric acid,
total bilirubin,
glucose and triglycerides (must be fasting minimum of 8 hours). A fasting
glucose of > 125
mg/dL will require a HgAl C) was monitored. Hematology (complete blood count
(CBC)
including white blood cell count and differential, red blood cell count,
hemoglobin,
hematocrit, and platelet count) was monitored. Hormone Levels (follicle-
stimulating
hormone (FSH) (not required for subjects with >12 months of spontaneous
amenorrhea or
bilateral oophorectomy), estradiol, estrone, and estrone conjugates and SHBG
for subjects in
the PK substudy) were monitored. Urine Analysis (appearance, specific gravity,
protein, and
pH) was conducted.
Pharmacokinetic Assessment
[0402] Seventy-two subjects were also enrolled in a pharmacokinetic (PK)
substudy. In
those subjects participating in the PK substudy, time Oh serum blood samples
were obtained
at screening visit 1A, day 1, and day 14 prior to dosing for baseline. The
baseline was
characterized by the average of the two pre-treatment samples. Serum blood
samples were
then obtained on day 1 and day 14 at five post dose time points (2h, 4h, 6h,
10h, and 24h). On
study days 1 (visit 2) and 14 (visit 3) a baseline pretreatment blood sample
(Time Oh) was
collected from each subject prior to insertion of the investigational product.
After insertion of
the product, blood samples were drawn at 2, 4, 6, 10, and 24 hours following
insertion. The
last PK sample (approximately day 84) was obtained 4 days following the last
insertion of
investigational product.
[0403] Blood samples were analyzed to characterize area under the curve (AUC),
time of
maximum concentration (tmax), minimum concentration (Cmm), and maximum
concentration
(Cmax). Blood samples were also analyzed to measure the levels of estradiol,
estrone, and
114
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
estrone conjugates. No fasting requirements were applied. Sex hormone binding
globulin
(SHBG) levels were obtained at pre-treatment baseline (day 1, visit 2), and
day 14 at the Oh
and on the day 84 final hormone blood draw.
[0404] A symptoms/complaints and medications diary was dispensed at all visits
and
subjects were instructed on completion. The subjects used the diary to record
symptoms/complaints (including stop and start dates and treatment received)
and prior
medications/treatments (including indication, stop, and start dates). A copy
of the diary was
made at each visit and re-dispensed to the subject. A dosing diary was
dispensed at visit 2 and
at visit 3 and subjects were be instructed on completion. Subjects recorded
investigational
product usage and sexual activity. The dosing diary dispensed at visit 3 was
re-dispensed at
visits 4 and 5. A copy of the diary was made at each visit prior to re-
dispensing to the subject.
Study Visits
[0405] Study visits were typically conducted so as to include the activities
outlined in
Table 43.
Table 43. Schedule of Assessments ¨ Main Study
Washout Visit 1A Visit 1B Visit 2: Visit
3:
Screening Screening Randomization
Interim
/ Baseline
Activity
Week -14 to Week -6 to Week -4 to Week 0 Week 2
-6 O O Day 1 Day 14
( 3d)
Informed consent X X
Demographics/Medical and X X
Gynecological history and prior
medications
Weight X X
Height and BMI calculation X X
Vital signs X X X X X
MBS X
Subject VVA Self-Assessment X X X
Questionnaire
Physical examination including breast X
exam
Laboratory safety tests (Hematology, X
Serum Chemistry, FSHP, Urinalysis)
12-Lead ECG X
Pelvic exam X
Vaginal pH X X
Papanicolaou (Pap) smear X
Investigator assessment of vaginal X X
mucosa
Vaginal cytological smear X X
Mammogram X
115
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Washout Visit 1A Visit 1B Visit 2:
Visit 3:
Screening Screening Randomization
Interim
/ Baseline
Activity
Week -14 to Week -6 to Week -4 to Week 0 Week 2
-6 O O Day 1 Day 14
( 3d)
Endometrial biopsy X
Diary Dispense X X X X X
Diary Collection X X X X
FSFI X
Satisfaction Survey
Urine pregnancy test X
Randomization X
Dispense Investigational Product bottle X
Re-dispense Investigational Product X
bottle
Treatment administration instruction X X
Collect unused investigational product X
and used bottles ; assess compliance
Adverse event monitoring X X X X
Concomitant medications X X X X
Table 43¨continued.
Visit visit visit 6: Telephon
4: 5: End of
Interi Interi Treatmen Interview
Activity m m t or Early
Term
Week Week Week 12 Week 14
6 8 Day 84 approxim
Day Day ( 3d) ately 15
42 56 days after
( 3d) ( 3d) last dose
of IP
Informed consent
Demographics/Medical
and Gynecological
history and prior
medications
Weight X
Height and BMI
calculation
Vital signs X X X
MBS
Subject VVA Self- X X X
Assessment
Questionnaire
Physical examination X
including breast exam
Laboratory safety tests X
(Hematology, Serum
Chemistry, FSHP,
Urinalysis)
12-Lead ECG X
Pelvic exam X
116
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Visit Visit Visit 6: Telephon
4: 5: End of
Interi Interi Treatmen Interview
Activity m m t or Early
Term
Week Week Week 12 Week 14
6 8 Day 84 approxim
Day Day ( 3d) ately 15
42 56 days after
( 3d) ( 3d) last dose
of IP
Vaginal pH X X X
Papanicolaou (Pap) X
smear
Investigator assessment X X X
of vaginal mucosa
Vaginal cytological X X X
smear
Mammogram
Endometrial biopsy X
Diary Dispense X X
Diary Collection X X X
FSFI X
Satisfaction Survey X
Urine pregnancy test
Randomization
Dispense Investigational X
Product bottle
Re-dispense X
Investigational Product
bottle
Treatment administration X X
instruction
Collect unused X X X
investigational product
and used bottles ; assess
compliance
Adverse event X X X X
monitoring
Concomitant medications X X X X
[0406] Washout Period Visit (if applicable; Weeks -14 to -6). The purpose of
this visit
was to discuss the study with a potential subject and obtain informed consent
that is signed
and dated before any procedures, including washout are performed. Subjects who
agreed to
discontinue current treatment began washout after the consent form was signed.
A
symptoms/complaints and medication diary was dispensed at this visit and the
subject was
instructed in how to complete the diary. Once the washout period was
completed, the subject
will return to the site for visit 1A.
[0407] The activities and assessments conducted during the visit included:
informed
consent; demographics; medical/gynecological history; collection of prior and
concomitant
117
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
medication information; height, body weight measurement and BMI calculation;
collection of
vital signs (body temperature, HR, RR, and BP); dispensation of
symptoms/complaints diary
and instruction in how to complete the diary
[0408] Screening Period Visits (Visits 1A and 1B). Subjects not requiring
washout begin
screening procedures at visit 1A as described above for the washout period.
With the
exception of vital signs, procedures performed at washout will not be repeated
at screening
visit 1A. In general, screening visits 1A and 1B were completed within 6 weeks
(42 days) for
subjects without a uterus or within 8 weeks (56 days) for subjects with a
uterus. All screening
assessments were completed prior to randomization. The investigators reviewe d
the results
from all screening procedures and determined if the subjects were eligible for
enrollment into
the study.
[0409] Visit 1A (approximately Week -6 to 0). Visit 1A was conducted after the
wash-out
period (if applicable) or after the subject provided informed consent. The
subject was advised
to fast for 8 hours prior to the visit for blood draws.
[0410] Procedures and evaluations conducted at the visit included: informed
consent;
demographics; medical/gynecological history; collection of the
symptoms/complaints and
medications diary from washout (if applicable) and review with the subject;
recording of
prior medication information; recording and assessment of adverse events (AEs)
starting
from the signing of informed consent; height, body weight measurement and BMI
calculation; collection of vital signs (body temperature, HR, RR, and BP);
physical
examination; breast examination (including a mammogram conducted up to nine
months
prior to Visit 2); urine pregnancy test as required; blood and urine sample
collection for blood
chemistry (minimum fast of 8 hrs), hematology, and urinalysis; serum FSH as
required; 12-
Lead ECG.
[0411] At visit 1A, the VVA symptom self-assessment questionnaire was
conducted and
most bothersome symptoms were identified, with the subject self-identifying
moderate or
severe pain with sexual activity as her MBS to continue screening. The
symptoms/complaints
and medications diary was dispensed, and subjects were instructed in how to
complete the
diary. Subjects were instructed to refrain from use of vaginal lubricants for
7 days and sexual
intercourse/vaginal douching for 24 hours prior to the vaginal pH assessment
to be done at
visit 1B.
118
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0412] Visit 1B (approximately Week -4 to Week 0). Visit 1B was conducted
after the
subject's initial screening visit and after the other screening results
indicated that the subject
was otherwise an eligible candidate for the study (preferably around the
middle of the
screening period).
[0413] Procedures and evaluations conducted at the visit included: VVA symptom
self-
assessment questionnaire, the subject having indicated moderate to severe pain
with sexual
activity with vaginal penetration in order to continue screening; collection
of vital signs
(body temperature, HR, RR, and BP); pelvic examination; investigator
assessment of vaginal
mucosa as described above; assessment of vaginal pH (sexual intercourse or
vaginal
douching within 24 hrs prior to the assessment being prohibited, and a
subject's vaginal pH
being >5.0 to continue screening); Pap smear; vaginal cytological smear (one
repetition being
permitted during screening if no results were obtained from the first smear);
endometrial
biopsy performed as described above; review of the symptoms/complaints and
medications
diary with the subject.
[0414] Visit 2 (Week 0; Randomization/Baseline). Subjects who met entry
criteria were
randomized to investigational product at this visit. Procedures and
evaluations conducted at
the visit included: self-administration of FSFI by subjects not participating
in the PK
substudy; review of the symptoms/complaints and medications diary with the
subject; review
of evaluations performed at screening visits and verification of present of
all inclusion criteria
and the absence of all exclusion criteria; collection of vital signs (body
temperature, HR, RR,
and BP); randomization, with subjects meeting all entry criteria being
randomized and
allocated a bottle number; dispensation of investigational product and
instruction in how to
insert the capsule vaginally, with subjects receiving their first dose of
investigational product
under supervision; dispensation of dosing diary and instruction on completion
of the
treatment diary, including recording investigational product usage and sexual
activity.
[0415] Visit 3 (Week 2, Day 14 3 days). Procedures and evaluations conducted
at the
visit included: completion of the VVA symptom self-assessment questionnaire;
review of the
symptoms/complaints and medications diaries with the subject; collection of
vital signs (body
temperature, HR, RR, and BP); Assessment of vaginal mucosa; assessment of
vaginal pH
(with sexual intercourse or vaginal douching within 24 hrs prior to the
assessment being
prohibited); vaginal cytological smear; collection of unused investigational
product and bottle
for assessment of compliance/accountability; re-dispensation of
investigational product and
119
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
re-instruction in how to insert the capsule vaginally if necessary; review of
the completed
dosing diary with the subject.
[0416] Visit 4 (Week 6, Day 42 3 days). Procedures and evaluations conducted
at the
visit included: completion of the VVA symptom self-assessment questionnaire;
review of the
symptoms/complaints and medications diary with the subject; collection of
vital signs (body
temperature, HR, RR, and BP); assessment of vaginal mucosa as described above;
vaginal
cytological smear; assessment of vaginal pH (with sexual intercourse or
vaginal douching
within 24 hrs prior to the assessment being prohibited); collection of unused
investigational
product for assessment of compliance/accountability; re-dispensation of
investigational
product and re-instruction in how to insert the capsule vaginally if
necessary; review of the
completed dosing diary with the subject.
[0417] Visit 5 (Week 8, Day 56 3 days). Procedures and evaluations conducted
at the
visit included: completion of the VVA symptom self-assessment questionnaire;
review of the
symptoms/complaints and medications diary with the subject; collection of
vital signs (body
temperature, HR, RR, and BP); assessment of vaginal mucosa as described above;
vaginal
cytological smear; assessment of vaginal pH (with sexual intercourse or
vaginal douching
within 24 hrs prior to the assessment being prohibited); collection of unused
investigational
product for assessment of compliance/accountability; re-dispensation of
investigational
product and re-instruction in how to insert the capsule vaginally if
necessary; review of the
completed dosing diary with the subject.
[0418] Visit 6 (Week 12, Day 84 3 days or early termination). This visit was
performed if a subject withdraws from the study before visit 6. Procedures
performed at this
visit included: completion of the VVA symptom self-assessment questionnaire;
review of the
subject the dosing diary, symptoms/complaints, and medications diaries with
the subject;
collection of blood and urine sample collection for blood chemistry (minimum
fast of 8 hrs),
hematology, and urinalysis; collection of vital signs (body temperature, HR,
RR, and BP) and
weight; performance of 12-lead-ECG; collection of unused investigational
product and
container for assessment of compliance/accountability; physical examination;
breast exam;
assessment of vaginal mucosa as described above; assessment of vaginal pH
(with sexual
intercourse or vaginal douching within 24 hrs prior to the assessment being
prohibited);
vaginal cytological smear; Pap smear; endometrial biopsy; self-administration
of FSFI by
120
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
subjects not participating in the PK substudy; self-administration of survey
titled
"Acceptability of product administration Survey" by subjects.
[0419] Follow-up Interview (approximately 15 days after the last dose of
investigational product). Each subject who received investigational product
received a
follow-up phone call, regardless of the duration of therapy, approximately 15
days following
the last dose of investigational product. The follow-up generally took place
after receipt of all
safety assessments (e.g., endometrial biopsy and mammography results). The
follow-up
included: review of ongoing adverse events and any new adverse events that
occurred during
the 15 days following the last dose of investigational product; review of
ongoing concomitant
medications and any new concomitant medications that occurred during the 15
days
following the last dose of investigational product; and discussion of all end
of study safety
assessments and determination if further follow up or clinic visit is
required.
PK Substudy Visit Procedures and Schedule
[0420] Screening Visit 1A. In addition to the procedures listed described
above, activities
in the PK substudy also included: provision of informed consent by subject and
agreement to
participate in the PK substudy; collection of a serum blood sample during the
visit for
baseline assessment of estradiol, estrone, and estrone conjugates.
[0421] Visit 2 (Week 0, Day 1). In addition to the procedures listed described
above,
activities in the PK substudy also included collection of serum blood sample
obtained prior to
the administration of investigational product (timepoint Oh) for baseline
assessment of
estradiol, estrone, estrone conjugates, and SHBG. The investigational product
was self-
administered by the subject after the pre-treatment blood sample has been
taken. After
investigational product administration, serum blood samples were obtained at
2h, 4h, 6h, 10h,
and 24h timepoints for estradiol, estrone, and estrone conjugates (serum
samples were
generally taken within +/- 5 minutes at 2h and 4h, within +/- 15 minutes at
6h, and within +/-
lh at 10h and 24h). The subject was released from the site after the 10 hour
sample and
instructed to return to the site the next morning for the 24 hour blood draw.
The subject was
instructed not to self-administer the day 2 dose until instructed by the site
personnel to dose at
the clinical site. The subject was released from the clinical site following
the 24 hour blood
sample and administration of the day 2 dose.
[0422] Visit 3 (Week 2, Day 14). The visit must occurred on day 14 with no
visit window
allowed. In addition to the procedures listed above, the PK substudy included
collection of a
121
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
serum blood sample prior to the administration of day 14 dose (timepoint Oh)
for SHBG and
PK assessments. The subject self-administered the day 14 dose at the clinical
site, and serum
blood samples were obtained at 2h, 4h, 6h, 10h, and 24h timepoints for
estradiol, estrone, and
estrone conjugates. The subject was released from the site after the 10 hour
sample and
instructed to return to the site the next morning for the 24 hour blood draw.
The subject was
instructed not to self-administer the day 15 dose until instructed by the site
personnel to dose
at the clinical site. The subject was released from the clinical site
following the 24 hour blood
sample and administration of the day 15 dose. The subject was be instructed to
administer the
next dose of study drug on day 18 or day 19 and continue dosing on a bi-weekly
basis at the
same time of day for each dose.
[0423] Visit 6 (Week 12, Day 84 3 days, or at early termination). The visit
took place
4 days after last IP dose or early termination. A serum sample for estradiol,
estrone, and
estrone conjugates and SHBG was drawn in addition to the procedures described
above.
[0424] PK sub-study visits were typically conducted so as to include the
activities outlined
in Table 44.
Table 44. Schedule of Assessments for PK Sub-study
visit 2:
Visit 1A Visit 1B
Washout Randomization / Visit 3:
Interim
Screening Screening
Baseline
Activity
Week 2
Week -14 Week -4 to Week 0
Week -6 to 0 Day 14
to - 6 0 Day 1
(no window)
PK sub-study Informed X X
consent
Demographics/Medical
and Gynecological history X X
and prior medications
Weight X X
Height and BMI X X
calculation
Vital signs X X X X X
MBS X
Subject VVA Self-
Assessment X X X
Questionnaire
Physical examination X
including breast exam
Laboratory safety tests
(Hematology, Serum X
Chemistry, FSHP,
Urinalysis)
122
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Visit 2:
Visit 1A Visit 1B
WashoutRandomization / Visit 3: Interim
Screening Screening
Baseline
Activity
Week 2
Week -14 Week -4 to Week 0
Week -6 to 0 Day 14
to - 6 0 Day 1
(no window)
PK Serum Blood Samples
(Estradiol, Estrone, X X X
Estrone Conjugates)
Serum blood samples for X X
SHBG
12-Lead ECG X
Pelvic exam X
Vaginal pH X X
Papanicolaou (Pap) smear X
Investigator assessment of X X
vaginal mucosa
Vaginal cytological smear X X
Mammogram X
Endometrial biopsy X
Diary Dispense X X X X X
Diary Collection X X X X
Satisfaction Survey
Urine pregnancy test X
Randomization X
Dispense new
Investigational Product X
(IP) bottle
Re-dispense
Investigational Product X
(IP) bottle
IP administration X X
instruction
Collect unused IP and
used bottles; assess X
compliance
Adverse event monitoring X X X X
Concomitant medications X X X X
Table 44-continued
Visit 6: End
Visit 4: Visit 5: of treatment Telephone
Interim Interim or Early Interview
Term
Activity
Week 12 Week 14
Week 6 Week 8
Day 84 ( 3d) approximately
Day 42 Day 56
(4 days after 15 days after
( 3d) ( 3d)
last IP dose) last dose of IP
PK sub-study Informed
consent
123
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Visit 6: End
Visit 4: Visit 5: of treatment Telephone
Interim Interim or Early Interview
Term
Activity
Week 12 Week 14
Week 6 Week 8
Day 84 ( 3d) approximately
Day 42 Day 56
(4 days after 15 days after
( 3d) ( 3d)
last IP dose) last dose of IP
Demographics/Medical
and Gynecological
history and prior
medications
Weight X
Height and BMI
calculation
Vital signs X X X
MBS
Subject VVA Self-
Assessment X X X
Questionnaire
Physical examination X
including breast exam
Laboratory safety tests
(Hematology, Serum X
Chemistry, FSHP,
Urinalysis)
PK Serum Blood
Samples (Estradiol, X
Estrone, Estrone
Conjugates)
Serum blood samples X
for SHBG
12-Lead ECG X
Pelvic exam X
Vaginal pH X X X
Papanicolaou (Pap) X
smear
Investigator assessment X X X
of vaginal mucosa
Vaginal cytological X X X
smear
Mammogram
Endometrial biopsy X
Diary Dispense X X
Diary Collection X X X
Satisfaction Survey X
Urine pregnancy test
Randomization
Dispense new
Investigational Product X
(IP) bottle
Re-dispense
Investigational Product X
(IP) bottle
124
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Visit 6: End
Visit 4: Visit 5: of treatment
Telephone
Interim Interim or Early
Interview
Term
Activity
Week 12 Week 14
Week 6 Week 8
Day 42 Day 56 Day 84 ( 3d)
approximately
( 3d) 3d)
(4 days after 15 days after
(
last IP dose) last dose of IP
IP administration X X
instruction
Collect unused IP and
used bottles; assess X X X
compliance
Adverse event X X X X
monitoring
Concomitant X X X X
medications
[0425] An Adverse Event (AE) in the study was defined as the development of an
undesirable medical condition or the deterioration of a pre-existing medical
condition
following or during exposure to a pharmaceutical product, whether or not
considered casually
related to the product. An AE could occur from overdose of investigational
product. In this
study, an AE can include an undesirable medical condition occurring at any
time, including
baseline or washout periods, even if no study treatment has been administered.
Relationship
to Investigational Product
[0426] The investigators determined the relationship to the investigational
product for each
AE (Not Related, Possibly Related, or Probably Related). The degree of
"relatedness" of the
adverse event to the investigational product was described as follows: not
related¨no
temporal association and other etiologies are likely the cause;
possible¨temporal
association, but other etiologies are likely the cause. However, involvement
of the
investigational product cannot be excluded; probable¨temporal association,
other etiologies
are possible but unlikely. The event may respond if the investigational
product is
discontinued.
EXAMPLE 11: Efficacy results of randomized, double-blind, placebo-controlled
multicenter study.
[0427] Each of the three doses showed statistical significance compared with
placebo for
the primary endpoints. Each of the three doses showed statistical significance
compared with
placebo for the secondary endpoints. Table 45 shows the statistical
significance of the
experimental data for each of the four co-primary endpoints. Each of the
dosages met each of
125
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
the four co-primary endpoints at a statistically significant level. The 25 mcg
dose of TX-
004HR demonstrated highly statistically significant results at the p < 0.0001
level compared
to placebo across all four co-primary endpoints. The 10 mcg dose of TX-004HR
demonstrated highly statistically significant results at the p < 0.0001 level
compared to
placebo across all four co-primary endpoints. The 4 mcg dose of TX-004HR also
demonstrated highly statistically significant results at the p < 0.0001 level
compared to
placebo for the endpoints of superficial vaginal cells, parabasal vaginal
cells, and vaginal pH;
the change from baseline compared to placebo in the severity of dyspareunia
was at the p =
0.0255 level.
Table 45: Statistical Significance of Results for Co-Primary Endpoints (Based
on Mean
Change from Baseline to Week 12 Compared to Placebo)
25 mcg 10 mcg 4 mcg
Superficial Cells P < 0.0001 P < 0.0001 P < 0.0001
Parabasal Cells P < 0.0001 P < 0.0001 P < 0.0001
Vaginal pH P < 0.0001 P < 0.0001 P < 0.0001
Severity of
P = 0.0001 P = 0.0001 P = 0.0255
Dyspareunia
[0428] Statistical improvement over placebo was also observed for all three
doses at the
first assessment at week two and sustained through week 12. The
pharmacokinetic data for all
three doses demonstrated low systemic absorption, supporting the previous
Phase 1 trial data.
TX-004HR was well tolerated, and there were no clinically significant
differences compared
to placebo-treated women with respect to adverse events. There were no drug-
related serious
adverse events reported.
[0429] As shown in the data below, in the MITT population (n=747) at week 12,
all TX-
004HR doses compared with placebo significantly decreased the percentage of
parabasal
cells and vaginal pH, significantly increased the percentage of superficial
cells, and
significantly reduced the severity of dyspareunia (all 1310.00001 except
dyspareunia at 4 [ig
p=0.0149).
[0430] At weeks 2, 6, and 8, the percentage of parabasal cells and vaginal pH
significantly
decreased p<0.00001); the percentage of superficial cells significantly
increased (p<0.00001);
and the severity of dyspareunia significantly improved from baseline with all
TX-004HR
doses vs placebo (4 [ig p<0.03; 10 [ig and 25 [ig p(0.02).
126
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
[0431] Moderate-to-severe vaginal dryness was reported by 93% at baseline and
significantly improved (p<0.02) for all doses at weeks 2, 6, 8, and 12 (except
4 [ig at week 2).
Vulvar and/or vaginal itching or irritation significantly improved (p<0.05)
for 10 [ig at weeks
8 and 12, and for 25 [ig at week 12.
[0432] TX-004HR was well tolerated, had high acceptability, and no treatment-
related
serious AEs were reported in the safety population (n=764). There were no
clinically
significant differences in any AEs or treatment-related SAEs between TX-004HR
and
placebo. Very low to negligible systemic levels of estradiol were observed.
[0433] All TX-004HR doses were safe and effective and resulted in very low to
negligible
systemic absorption of E2 in women with VVA and moderate-to-severe
dyspareunia. Onset
of effect was seen as early as 2 weeks and was maintained throughout the study
and
acceptability was very high. This novel product provides a promising new
treatment option
for women experiencing menopausal VVA.
Cytology
[0434] Vaginal cytology data was collected as vaginal smears from the lateral
vaginal walls
according to procedures presented above to evaluate vaginal cytology at
screening and Visit
6¨End of treatment (day 84). The change in the Maturation Index was assessed
as a change
in cell composition measured at Visit 1¨Baseline (day 1) compared to the cell
composition
measured at Visit 3¨End of treatment (day 84). The change in percentage of
superficial,
parabasal, and intermediate cells obtained from the vaginal mucosal epithelium
from a
vaginal smear was recorded. Results from these assessments for superficial
cells are
presented in Table 46 and Table 47, as well as Fig. 10, Fig. 11, and Fig. 12.
Results from
these assessments for parabasal cells are presented in Table 48 and Table 49,
as well as Fig.
13, Fig. 14, and Fig. 15.
Superficial cells
Table 46: Superficial Cells P-values by Treatment Week
4 [ig 10 [ig 25 [ig
Week 2 (O.0001 (O.0001 (O.0001
Week 6 (O.0001 (O.0001 (O.0001
Week 8 (O.0001 (O.0001 (O.0001
127
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Week 12 (0.0001 (0.0001 (0.0001
Table 47: Superficial Cells Change in Severity from Baseline by Treatment Week
(change in percent of total vaginal cells)
4 lig 10 lig 25 lig Placebo
Week 2 31.35(1.496) 31.93(1.488) 38.85(1.5)
6.05(1.498)
Week 6 18.41(1.536) 16.88(1.543) 22.65(1.532)
5.43(1.525)
Week 8 19.04(1.561) 17.41(1.558) 23.88(1.554)
5.98(1.551)
Week 12 17.5(1.542) 16.72(1.54) 23.2(1.529)
5.63(1.537)
[0435] The study showed the formulations disclosed herein across all doses
increased the
percentage of superficial cells across all dosages in a statistically
significant way.
Parabasal cells
Table 48: Parabasal Cells P-values by Treatment Week
4 lig 10 lig 25 lig
Week 2 (0.0001 (0.0001 (0.0001
Week 6 (0.0001 (0.0001 (0.0001
Week 8 (0.0001 (0.0001 (0.0001
Week 12 (0.0001 (0.0001 (0.0001
Table 49: Parabasal Cells Change in Severity from Baseline by Treatment Week
(change in
percent of total vaginal cells)
4 lig 10 lig 25 lig Placebo
Week 2 -40.23(1.719) -44.42(1.708) -45.6(1.723) -7(1.72)
Week 6 -39.36(1.75) -43.55(1.752) -45.61(1.746) -
9.23(1.741)
Week 8 -41.87(1.768) -43.78(1.764) -45.08(1.762) -
7.86(1.76)
Week 12 -40.63(1.755) -44.07(1.751) -45.55(1.745) -
6.73(1.75)
[0436] The increase of superficial cells and decrease of parabasal cells
showed statistical
significance over placebo at week 2 and for every week thereafter, including
at week 12.
128
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Administration of the pharmaceutical formulation resulted in rapid onset of
action, as early as
two weeks after the initial administration. Rapid onset of action may be
coupled with the
rapid absorption demonstrated in the pharmacokinetic data presented below.
pH
[0437] Vaginal pH was measured at Screening and Visit 6¨End of treatment (day
84). The
pH measurement was obtained as disclosed herein. Results from these
assessments are
presented in Table 50 and Table 51, and Fig. 16, Fig. 17, and Fig. 18.
Table 50: pH P-values by Treatment Week
4 lig 10 lig 25 lig
Week 2 (0.0001 (0.0001 (0.0001
Week 6 (0.0001 (0.0001 (0.0001
Week 8 (0.0001 (0.0001 (0.0001
Week 12 (0.0001 (0.0001 (0.0001
Table 51: pH Change in Severity from Baseline by Treatment Week (change in pH)
4 lig 10 lig 25 lig Placebo
Week 2 -1.23(0.064) -1.37(0.064) -1.3(0.065) -
0.28(0.064)
Week 6 -1.32(0.066) -1.4(0.066) -1.48(0.066) -
0.3(0.065)
Week 8 -1.35(0.067) -1.46(0.067) -1.45(0.066) -
0.38(0.066)
Week 12 -1.32(0.066) -1.42(0.066) -1.34(0.066) -
0.28(0.066)
[0438] The decrease in vaginal pH was observed at statistically significant
levels at week 2
and through the end of the study. Surprisingly, the pH decreased in all three
pharmaceutical
formulations tested and at all three dosages of over a full pH unit for all
three doses.
Most bothersome symptoms
Dyspareunia
[0439] Subjects were asked to specify the symptom that she identified as the
"most
bothersome symptom." During the screening period all of the subjects were
provided with a
questionnaire to self-assess the symptoms of VVA: (1) dyspareunia; (2) vaginal
dryness; and
129
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
(3) vaginal or vulvar irritation, burning, or itching. Each symptom was
measured on a scale of
0 to 3, where 0 = none, 1 = mild, 2 = moderate, and 3 = severe. Each subject
was given a
questionnaire at each visit and the responses were recorded. All randomized
subjects were
also provided a questionnaire to self-assess the symptoms of VVA at Visit 1
and on each
subsequent visit through Visit 6¨End of the treatment (day 84). Subjects
recorded their self-
assessments daily in a diary and answers were collected on visits 8 and 15
(end of treatment).
Pre-dose evaluation results obtained at Visit 1 were considered as baseline
data for the
statistical analyses. Data from these assessments for dyspareunia are
presented in Table 52
and Table 53. Data from these assessments for dryness are presented in Table
54 and Table
55.
Table 52: Dyspareunia P-values by Treatment Week
4 ug 10 ug 25 ug
Week 2 0.026 0.0019 0.0105
Week 6 0.0069 0.0009 < 0.0001
Week 8 0.0003 < 0.0001 < 0.0001
Week 12 0.0149 < 0.0001 < 0.0001
Table 53: Dyspareunia Change in Severity from Baseline by Treatment Week
(0 to 3 severity scale)
4 ug 10 ug 25 ug Placebo
Week 2 -0.99(0.072) -1.08(0.072) -1.02(0.073) -
0.76(0.072)
Week 6 -1.3(0.072) -1.37(0.072) -1.48(0.072) -
1.03(0.07)
Week 8 -1.52(0.073) -1.64(0.074) -1.62(0.075) -
1.15(0.072)
Week 12 -1.52(0.071) -1.69(0.071) -1.69(0.071) -
1.28(0.07)
[0440] Each of the 4 ug, 10 ug, and 25 ug formulations tests demonstrated an
early onset
of action at week 2 for the most bothersome symptom of dyspareunia, evidenced
by the
statistically significant results (measured by p-value) in Table 52. After two
weeks, each dose
demonstrated separation from placebo in improvement in the most bothersome
symptom of
dyspareunia.
130
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0441] Coupled with the PK data presented below, these results show that the
formulations
disclosed herein provide a bolus of estradiol within two hours of
administration, which
resulted in a decrease in the severity of dyspareunia as early as two weeks
later. Estradiol is
rapidly absorbed at around two hours, which is significantly faster than the
formulations of
the prior art that sought an extended release profile. The rapid absorption of
estradiol is
believed to be a result of administration with a liquid formulation.
[0442] Surprisingly, the 4 lig formulation showed clinical effectiveness at
two weeks along
with the 25 lig and 10 lig dosage levels. These data demonstrate that 4 lig is
an effective
dose, and can be effective as early as two weeks after administration for the
most bothersome
symptom of dyspareunia.
Dryness
Table 54: Dryness P-values by Treatment Week
4 lig 10 lig 25 lig
Week 2 0.1269 0.0019 0.0082
Week 6 0.0094 0.0001 0.0005
Week 8 0.0128 < 0.0001 0.0008
Week 12 0.0014 < 0.0001 < 0.0001
Table 55: Dryness Change in Severity from Baseline by Treatment Week (0 to 3
severity
scale)
4 lig 10 lig 25 lig Placebo
Week 2 -0.86(0.066) -1.01(0.065) -0.96(0.066) -
0.72(0.066)
Week 6 -1.14(0.067) -1.27(0.068) -1.23(0.067) -0.9
(0.067)
Week 8 -1.25(0.069) -1.44(0.068) -1.34(0.068) -
1.01(0.068)
Week 12 -1.27(0.068) -1.47(0.067) -1.47(0.067) -
0.97(0.067)
[0443] Each of the 4 lig, 10 lig, and 25 lig formulations tests demonstrated
an early onset
of action at week 2 for the most bothersome symptom of dryness, evidenced by
the
statistically significant results (measured by p-value) in Table 54. After two
weeks, each dose
demonstrated separation from placebo in improvement in the most bothersome
symptom of
dryness.
131
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Irritation/Itching
Table 56: Irritation/Itching P-values by Treatment Week
4 ng 10 ng 25 ng
Week 2 0.9616 0.2439 0.6518
Week 6 0.7829 0.2328 0.4118
Week 8 0.0639 0.0356 0.0914
Week 12 0.0503 0.0055 0.0263
Table 57: Irritation/Itching Change in Severity from Baseline by Treatment
Week
(0 to 3 severity scale)
4 ng 10 ng 25 ng Placebo
Week 2 -0.47(0.054) -0.56(0.053) -0.51(0.054) -
0.47(0.054)
Week 6 -0.57(0.055) -0.64(0.055) -0.61(0.055) -
0.55(0.055)
Week 8 -0.74(0.056) -0.76(0.056) -0.73(0.056) -
0.59(0.056)
Week 12 -0.75(0.055) -0.81(0.055) -0.77(0.055) -
0.6(0.055)
[0444] Vulvar and/or vaginal itching or irritation significantly improved
(p<0.05) for 10 ng
at weeks 8 and 12, and for 25 ng at week 12. Moreover, the trend for 4 ng was
an
improvement in itching week over week to nearly being statistically
significant at week 12.
[0445] Coupled with the PK data presented below, these results show that the
formulations
disclosed herein provide a bolus of estradiol within two hours of
administration, which
resulted in a decrease in the severity of dryness as early as two weeks later.
Estradiol is
rapidly absorbed at around two hours, which is significantly faster than the
formulations of
the prior art that sought an extended release profile. The rapid absorption of
estradiol is
believed to be a result of administration with a liquid formulation.
[0446] Surprisingly, the 4 ng formulation showed clinical effectiveness at two
weeks along
with the 25 ng and 10 ng dosage levels. These data demonstrate that 4 ng is an
effective
dose, and can be effective as early as two weeks after administration for the
most bothersome
symptom of dryness.
132
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
[0447] As described above, each dose was compared with placebo for change from
baseline to week 12 in the percentages of vaginal superficial cells and
parabasal cells, vaginal
pH, and severity of dyspareunia (co-primary endpoints). The proportion of
responders
(defined as women with >2 of the following at week 12: vaginal superficial
cells >5%,
vaginal pH (5.0, >1 category improvement from baseline dyspareunia score) was
compared
in TX-004HR groups vs placebo. Pre-specified subgroup analyses of co-primary
endpoints
were analyzed by age (<56 years, 57-61 years, and >62 years), BMI (<24 kg/m2,
25-28
kg/m2, and >29 kg/m2), uterine status, parity, and vaginal births.
Pharmacokinetic (PK)
parameters were compared with placebo in a sub-analysis of the main study.
[0448] The proportion of responders was significantly higher for all TX-004HR
dose
groups vs placebo (p<0.0001 for all). All TX-004HR doses vs placebo
significantly improved
percentage of superficial and parabasal cells, vaginal pH, and severity of
dyspareunia at 12
weeks. Subgroup analyses showed generally similar results for percentage of
superficial and
parabasal cells and vaginal pH irrespective of age, BMI, uterine status,
parity, and vaginal
births. Severity of dyspareunia was significantly reduced at 12 weeks with all
TX-004HR
doses vs placebo in most subgroups (Table 57A).
[0449] The PK sub-analysis (n=72), described in more detail below, found AUC
and Ca,,g
parameters for E2 and estrone (El) with 4 pg and 10 pg TX-004HR to be similar
to placebo.
Increases occurred in E2 AUC and Cavg with 25 pg vs placebo but remained
within the
normal postmenopausal range. E2 levels at day 84 were similar between the TX-
004HR
groups and placebo, indicating no systemic drug accumulation.
[0450] All doses of TX-004HR were associated with robust efficacy and
demonstrated a
statistically significant difference vs placebo for increasing superficial
cells, decreasing
parabasal cells and vaginal pH, and reducing the severity of dyspareunia. Age,
BMI, uterine
status, parity and vaginal births generally did not affect TX-004HR efficacy.
These results
occurred with negligible systemic absorption of TX-004HR estradiol doses of 4
pg, 10 pg,
and 25 pg.
Table 57A. Change from baseline to week 12 in the severity of dyspareunia
(LS mean change SE).
Key clinical factors TX-004HR TX-004HR TX-004HR
Placebo
(n=187) 4 ttg 10 ttg 25
ttg
(n=186) (n=188)
(n=186)
Age, years 56 n=52 -1.25 0.119 n=50 -1.58 0.122
n=61 -1.77 0.112T n=65 -1.86 0.1081
57-61 n=53 -1.39 0.118 n=50 -1.42 0.121
n=49 -1.63 0.121 n=47 -1.79 0.125*
>62 n=58 -1.19 0.122 n=51 -1.52 0.126 n=44 -1.66
0.138T n=47 -1.38 0.135
133
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
BMI, < 24 n=56 -1.14 0.115 n=58 -1.48
0.113* n=56 -1.6 0.117T n=51 -1.72 0.1231
kg/m2 25 to 28 n=57 -1.48 0.118 n=45 -1.51 0.131
n=52 -1.78 0.124 n=58 -1.77 0.117
> 29 n=50 -1.21 0.125 11=48 -1.56 0.125
11=46 -1.71 0.129T n=50 -1.57 0.124*
Uterine Intact 11=101 -1.35 0.086 11=82 -1.66
0.095* 11=84 -1.74 0.095T n=85 -1.81 0.0941
status Non-intact n=62 -1.15 0.115 n=69 -1.35 0.108
n=70 -1.63 0.108T n=74 -1.55 0.107*
Pregnancy Pregnancy = 0 n=16 -1.18 0.220 n=17 -1.28 0.217
n=19 -1.26 0.209 n=13 -1.64 0.257
status Pregnancy? 1 n=147 -1.28 0.073 n=134 -1.55
0.075* n=135 -1.74 0.076 n=146 -1.70 0.0731
Vaginal Vaginal birth = 0 n=26 -1.19 0.171 n=22 -1.74
0.189* n=29 -1.68 0.161* n=31 -1.76 0.160*
births Vaginal birth > 1 n=121 -1.30 0.080 n=112 -
1.51 0.082 n=106 -1.77 0.0851 n=115 -1.69 0.0821
*p<0.05; tp<0.01; Ip<0.001; _p.<0.0001 vs placebo.
[0451] Visual evaluation of the vaginal epithelium, a secondary endpoint of
the trial, was
performed during gynecological examinations at baseline and weeks 2, 6, 8, and
12. A four-
point score (0 = none, 1 = mild, 2 = moderate, 3 = severe) was used to assess
changes in
vaginal color, vaginal epithelial integrity, vaginal epithelial surface
thickness, and vaginal
secretions. Change from baseline to each time point was compared with placebo
using the
mixed effect model repeat measurement (MMRM) analysis.
[0452] At baseline, women had mean scores of 1.8 for vaginal color, 1.5 for
epithelial
integrity, 1.9 for epithelial surface thickness, and 1.7 for secretions. These
scores were
consistent with VVA reflecting pallor, diminished vaginal wall integrity and
thickness, and
secretions. Significant improvements from baseline at weeks 2, 6, 8 and 12
(Table 57B; Fig.
19A-Fig. 19D) were observed for all 3 doses of TX-004HR compared with placebo
in vaginal
color (white to pink), epithelial integrity, epithelial surface thickness and
secretions (p<0.001
for all). After 12 weeks, women in the active TX-004HR treatment groups had
mean scores
less than 1 in all four characterized categories. Vaginal visual examination
of women in the 3
TX-004HR groups had greater reported improvements from baseline in all vaginal
parameters examined than placebo subjects and at all time points. These
improved vaginal
visual scores reflect other observed measures of efficacy of TX-004HR (4 pg,
10 pg, and 25
pg) at treating moderate-to-severe VVA in postmenopausal women, with
negligible to very
low systemic E2 absorption.
Table 57B: Change from baseline at week 12 in vaginal parameters
Vaginal Parameters, mean (SD) TX-004HR TX-004HR TX-004HR
Placebo
4 iug 10 iug 25 iug
(n=171) (n=173) (n=175) (n=175)
Vaginal epithelial Baseline 1.8 (0.61) 1.7 (0.59) 1.8 (0.60)
1.7 (0.64)
color 12 weeks 0.8 (0.67) 0.7 (0.64)
0.8 (0.68) 1.2 (0.80)
Change -1.0 (0.82) -1.1 (0.80) -1.0 (0.88)
-0.6 (0.83)
LS Mean (SE) -0.97 (0.05)* -1.06 (0.05)*
-0.96 (0.05)* -0.60 (0.05)
Vaginal epithelial Baseline 1.6 (0.84) 1.4 (0.83) 1.5 (0.77)
1.5 (0.84)
integrity 12 weeks 0.5 (0.69) 0.4 (0.57)
0.5 (0.66) 0.9 (0.91)
Change -1.0 (0.93) -1.0 (0.89) -1.0 (0.91)
-0.6 (0.98)
134
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
LS Mean (SE) -0.97 (0.05)* -1.07 (0.05)* -
1.01 (0.05)* -0.60 (0.05)
Vaginal epithelial Baseline 1.9 (0.67) 1.8 (0.63) 1.9 (0.59)
1.9 (0.65)
surface thickness 12 weeks 0.9 (0.66) 0.8 (0.63) 0.9 (0.69)
1.3 (0.85)
Change -1.0(0.76) -1.0(0.79) -0.9(0.80)
-0.6(0.82)
LS Mean (SE) -0.98 (0.05)* -1.03 (0.05)* -
0.94 (0.05)* -0.61 (0.05)
Vaginal Baseline 1.8 (0.68) 1.7 (0.66) 1.7 (0.63)
1.8 (0.63)
secretions 12 weeks 0.8 (0.69) 0.6 (0.67) 0.7 (0.71)
1.1 (0.84)
Change -1.0(0.82) -1.0(0.86) -1.0(0.85)
-0.7(0.79)
LS Mean (SE) -1.01 (0.05)* -1.06 (0.05)* -
1.04 (0.05)* -0.64 (0.05)
Data is mean (SD) unless otherwise noted; *MMRMp<0.0001 vs placebo.
[0453] A direct correlation was observed between the total sum of the
individual visual
examination score and severity of dyspareunia (r=0.31; P<0.0001) as well as
the severity of
vaginal dryness (r=0.38; P<0.0001) at 12 weeks when all subjects were analyzed
independent
of treatment. See, Fig. 20A and Fig. 20B. Interestingly, women treated with
placebo also
showed some improvements in their scores at week 2, but while women treated
with TX-
004HR showed continued improvements through 12 weeks of treatment, such
continued
improvements were not observed to the same extent with the placebo. Three
possible
explanations for the improvements observed with the placebo include the
potential
lubricating effect of the excipient Miglyol, a fractionated coconut oil
contained in all softgel
capsules, improved appearance based on vaginal lubrication caused by increased
sexual
activity and/or bias on the part of the physicians performing the examinations
as they may
anticipate improvement. Nevertheless, TX-004HR still significantly improved
evaluated
signs and symptoms of VVA better than placebo.
[0454] Since visual inspection of the vagina with the 4-point assessment tool
positively
correlated with dyspareunia and vaginal dryness in this study, this tool may
help healthcare
professionals diagnose VVA and assess its treatment, and provide a vehicle for
health care
professionals to initiation discussion with their patients about a sensitive
topic. Several large-
scale studies have shown that it is difficult for patients to discuss
vulvovaginal health openly
with their health care professionals because they are either embarrassed,
uninformed about
VVA and its treatments, or believe that the topic is not appropriate for
discussion. Therefore,
of the 50% of postmenopausal women who have symptoms of VVA, far fewer seek
treatment. Visual examination of the vagina may help practitioners identify
women at risk of
dyspareunia and vaginal dryness, and allow them to proactively engage women in
conversations about VVA symptoms such as dyspareunia and dryness and discuss
available
treatment options.
135
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
EXAMPLE 12: Pharmacokinetics results in randomized, double-blind, placebo-
controlled multicenter study.
[0455] While some approved local estrogens effectively treat VVA, systemic
estradiol may
increase with local administration. TX-004HR is a new low-dose vaginal softgel
capsule
containing solubilized natural estradiol designed to provide excellent
efficacy with negligible
systemic absorption. Up to three times lower systemic estrogen levels were
previously
reported with TX-004HR vs an approved low-dose vaginal estradiol tablet. The
present
studies show that VVA efficacy can be achieved with negligible systemic
absorption as
measured by PK in postmenopausal women with moderate-to-severe dyspareunia.
[0456] The terms "minimal systemic effect," "low systemic absorption," and
"negligible
systemic absorption," as used herein, mean that the disclosed formulations and
methods result
in low to minimal absorption of estradiol in women, especially women with VVA
and/or
dyspareunia. In fact, it has surprisingly been found that the disclosed
formulations and
methods result in negligible to very low systemic absorption of estradiol,
which remains in
the postmenopausal range. The finding is borne out by the examples provided
herein that
demonstrate that the C. and AUC levels of estradiol relative to placebo were
not
statistically differentiable, which indicates that the formulations disclosed
herein have a
negligible systemic effect. As such, the disclosed formulations and methods
advantageously
provide local benefits in patients with VVA and/or dyspareunia (i.e., the
disclosed
formulations are extremely effective in increasing the superficial cells,
decreasing parabasal
cells, and decreasing pH) without increasing systemic levels.
[0457] A PK substudy was part of a large, multicenter, double-blind,
randomized, placebo-
controlled phase 3 trial evaluating the efficacy and safety of TX-004HR (4 ug,
10 ug, and 25
ug) compared with placebo for treating postmenopausal moderate-to-severe
dyspareunia.
Women received TX-004HR or placebo once daily for 2 weeks then twice weekly
for 10
wks.
[0458] In this study, the systemic exposure to estradiol following once daily
intravaginal
administration of estradiol 25 ug, 10 ug, 4 ug, and placebo were investigated
on days 1, 14,
and 84 as described herein. Descriptive statistics of the plasma estradiol
concentrations taken
at each sampling time and the observed C. values were recorded, as shown in
the tables
below and Fig. 21 and Fig. 22, for estradiol, estrone, and estrone conjugates
for all three
136
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
doses. Serum estradiol, estrone, estrone conjugates, and sex hormone binding
globulin were
measured.
[0459] For PK, serum was sampled pre-dose and at 2, 4, 6, 10, and 24 h post-
dose on days
1 and 14 for estradiol, estrone (El), and estrone conjugates (ElCs). Baseline-
adjusted results
are shown here; unadjusted data will be presented. Efficacy endpoints were
change from
baseline to week 12 for vaginal superficial cells (%), vaginal parabasal cells
(%), vaginal pH,
and severity of dyspareunia. Secondary endpoints were severity of dryness and
itching/irritation. Blood chemistry was tested at week 12.
[0460] The substudy randomized 72 women (mean age 59 y) at 11 centers. Mean
area
under the concentration-time curve (AUC) and average concentration (Cavg) for
estradiol were
not significantly different vs placebo with 4 ug and 10 ug TX-004HR, but were
significantly
higher with 25 ug at day 1 (AUC 130 vs 13.8 h*pg/mL and Cavg 5.4 vs 0.4 pg/mL)
and day
14 (AUC 84.6 vs 7.1 h*pg/mL and Cavg 3.5 vs -0.2 pg/mL).
[0461] Mean estradiol peak concentration (Cmax) was not significantly
different with 4 ug
(day 1: 2.6 pg/mL; day 14: 1.3 pg/mL) vs placebo (day 1: 2.1 pg/mL; day 14:
1.0 pg/mL),
and although significant, was negligible with 10 ug (day 1: 6.0 pg/mL; day 14:
3.0 pg/mL)
and very low for 25 ug (day 1: 26.2 pg/mL; day 14: 12.0 pg/mL).
[0462] El and ElCs AUC, Cavg, Cmax, Cmin did not differ vs placebo, except for
El Cs on
day 1 when AUC was significantly higher with 25 ug (2454 vs 83.0 h*pg/mL),
Cmax with 10
ug and 25 ug (90.2 and 198.6 pg/mL, respectively vs 27.1 pg/mL), and Cavg with
10 [ig (8.0
vs -33.7 pg/mL).
[0463] In the overall study TX004-HR showed robust efficacy for symptoms of
dyspareunia, vaginal dryness and irritation at 12 weeks with all 3 doses
compared with
placebo.
[0464] Vaginal TX-004HR resulted in negligible to very low systemic absorption
of
estradiol, which remained in the postmenopausal range. TX-004HR improved the
signs and
symptoms of VVA. This study supports local benefits of estradiol without
increasing
systemic exposure.
[0465] The pharmacokinetic data for estradiol demonstrates the rapid
absorption of the
formulations disclosed herein for all three doses. Surprisingly, while the
pharmacokinetic
137
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
data was extremely low for all three doses, each dose was extremely effective
in increasing
the superficial cells, decreasing parabasal cells, and decreasing pH.
104661 The pharmaceutical compositions disclosed herein provide an improved
safety
profile over other options for treating VVA. The combination of low systemic
estradiol,
while retaining efficacy was a surprising result for all three doses.
Estradiol Concentration
Table 58: Pharmacokinetics Estradiol Baseline (pg/mL)
4 lig 10 lig 25 lig
Placebo
Baseline 4.7(4.41) 5(3.52) 3.6(1.86)
4.6(2.56)
Table 59: Pharmacokinetics Estradiol Day 1 (pg/mL)
4 lig 10 lig 25 lig
Placebo
Predose 3.1(1.56) 4.9(3.47) 3.6(1.81)
4.1(2.45)
2 hour 6.1(2.3) 10.4(4.89) 28.7(17.91)
4.8(3.33)
4 hour 4.3(1.68) 6.7(3.59) 16.1(14.75)
5(3.59)
6 hour 3.7(1.96) 5.7(3.16) 9.7(6.86)
4.8(3.53)
hour 3.7(1.47) 5.5(2.92) 6.2(2.37) 5.2(3.61)
24 hour 4.2(2.02) 5.4(4.44) 6.2(8.43)
5.1(4.42)
Table 60: Pharmacokinetics Estradiol Day 14 (pg/mL)
4 lig 10 lig 25 lig
Placebo
Predose 3.5(1.63) 3.8(2.56) 5.2(2.89)
4.2(3.07)
2 hour 4.3(2.01) 6.3(2.29) 15.3(7.72)
4.2(2.44)
4 hour 4(1.7) 5.9(2.55) 11(4.86)
4.7(3.2)
6 hour 3.9(1.92) 5.1(2.32) 7.9(3.35)
4.7(2.97)
10 hour 3.8(2.12) 5(3) 6.8(3.76)
5.1(3.53)
24 hour 3.6(1.89) 3.7(2.05) 4.9(4.35)
3.9(2.43)
138
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Table 61: Pharmacokinetics Estradiol End of Study (pg/mL)
4 lig 10 lig 25 lig
Placebo
Post Dosing 4.3(2.69) 4.8(2.57) 6.7(11.51)
4.4(2.6)
Estradiol Area Under the Curve (0-24 hours)
Table 62: Estradiol Area Under the Curve (0-24 hours) (h*pg/mL)
4 lig 10 lig 25 lig
Placebo
Day 1 91.7(37.86) 138.2(75.22) 217.4(99.02)
116.6(77.3)
Day 14 87.2(42.77) 110.1(54.57) 171.6(80.13)
104.2(66.39)
Table 63: Estradiol Area Under the Curve (0-24 hours) (Baseline Adjusted)
(h*pg/mL)
4 lig 10 lig 25 lig
Placebo
Day 1 12(13.89) 21.9(19.16) 130.4(111.95)
13.8(28.86)
Day 14 7.2(12.08) 13.7(18.77) 84.6(62.7)
7.1(20.28)
Table 64: Estradiol Area Under the Curve (0-24 hours) P-values Pairwise Test
vs. 4 lig
lig 25 lig
Day 1 0.0242 < 0.0001
Day 14 0.1777 0.0005
10 Table 65: Estradiol Area Under the Curve (0-24 hours) P-values Pairwise
Test vs. Placebo
4 lig 10 lig 25 lig
Day 1 0.2292 0.4028 0.0021
Day 14 0.3829 0.7724 0.0108
Table 66: Estradiol Area Under the Curve (0-24 hours) P-values Pairwise Test
vs. 4 lig
(Baseline Adjusted)
10 lig 25 lig
Day 1 0.082 0.0001
139
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Day 14 0.2373 < 0.0001
Table 67: Estradiol Area Under the Curve (0-24 hours) P-values Pairwise Test
vs. Placebo
(Baseline Adjusted)
4 pg 10 pg 25 pg
Day 1 0.8134 0.3238 0.0002
Day 14 0.979 0.3235 < 0.0001
Table 68: Estradiol Area Under the Curve (0-24 hours) Ratio (Day 14) of Day 14
to Day 1
4 pg 10 pg 25 pg Placebo
AUC Ratio of Day 14 0.971(0.2358) 0.876(0.1937) 0.955(0.6633)
0.949(0.225)
to Day 1
Pairwise test vs 4 ug 0.2022 0.9246
Pairwise test vs 0.7859 0.3101 0.9748
Placebo
Estradiol Cmax
Table 69: Cma,jp
4 pg 10 pg 25 pg Placebo
Day 1 6.5(2.13) 10.9(5) 29.8(17.51)
6.6(4.85)
Day 14 4.8(2.31) 7.3(2.36) 15.7(7.61) 5.5(3.43)
Table 70: Cmax (Baseline Adjusted) (pg/mL)
4 lig 10 pg 25 pg Placebo
Day 1 2.6(2.17) 6(4.44) 26.2(18.19)
2.1(3.48)
Day 14 1.3(1.08) 3(1.73) 12(7.32) 1(1.81)
Table 71: Cmax P-values Pairwise Test vs. 4 fig
10 pg 25 pg
Day 1 0.0013 < 0.0001
140
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Day 14 0.0033 < 0.0001
Table 72: Cmax P-values Pairwise Test vs. Placebo
4 p.g 10 p.g 25 p.g
Day 1 0.9586 0.0116 (0.0001
Day 14 0.5174 0.0702 < 0.0001
Table 73: C. P-values Pairwise Test vs. 4 p.g (Baseline Adjusted)
10 p.g 25 p.g
Day 1 0.0055 < 0.0001
Day 14 0.002 < 0.0001
Table 74: C. P-values Pairwise Test vs. Placebo (Baseline Adjusted)
4 p.g 10 p.g 25 p.g
Day 1 0.6074 0.0059 < 0.0001
Day 14 0.5088 0.0022 < 0.0001
Table 75: Cma Ratio Da 14 ofDay 14 to Day 1
4 p.g 10 p.g 25 p.g Placebo
Cma, Ratio of Day 14 to 0.77(0.2633) 0.804(0.3245)
0.929(1.5011) 0.933(0.2406)
Day 1
Pairwise test vs 0.7399 0.6702
Pairwise test vs 0.0702 0.1946 0.9931
Placebo
Estradiol
Table 76: Caig (pg/mL)
4 p.g 10 p.g 25 p.g
Placebo
Day 1 3.9(1.46) 5.8(3.13) 9.1(4.13)
4.9(3.22)
141
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Day 14 3.6(1.78) 4.6(2.27) 7.1(3.34)
4.3(2.77)
Table 77: Cavg (Baseline Adjusted) (pg/mL)
4 ng 10 ng 25 ng
Placebo
Day 1 0(1.93) 0.8(0.95) 5.4(4.66)
0.4(1.35)
Day 14 0.1(0.68) 0.2(1.22) 3.5(2.61) -
0.2(1.28)
Table 78: CavE P-values Pairwise Test vs. 4 Lig
10 ng 25 ng
Day 1 0.0294 < 0.0001
Day 14 0.1777 0.0005
Table 79: C,g P-values Pairwise Test vs. Placebo
4 ng 10 ng 25 ng
Day 1 0.267 0.4028 0.0021
Day 14 0.3829 0.7724 0.0108
Table 80: Cav. P-values Pairwise Test vs. 4 = Baseline Ad.usted
10 ng 25 ng
Day 1 0.1076 0.0001
Day 14 0.7759 < 0.0001
Table 81: C,g P-values Pairwise Test vs. Placebo (Baseline Adjusted)
4 ng 10 ng 25 ng
Day 1 0.5126 0.2564 0.0001
Day 14 0.4098 0.3629 < 0.0001
142
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Table 82: Cõg Ratio (Day 14) of Day 14 to Day 1
4 ng 10 ng 25 ng Placebo
C,g Ratio of Day 14 to 0.77(0.2633) 0.804(0.3245)
0.929(1.5011) 0.933(0.2406)
Day 1
Pairwise test vs 0.7399 0.6702
Pairwise test vs 0.0702 0.1946 0.9931
Placebo
Estradiol Tinõ
Table 83: T._(1-11
4 ng 10 ng 25 ng
Placebo
Day 1 7(9.36) 6.1(8.04) 4.6(7.09)
8.6(6.74)
Day 14 9.3(8.86) 4(2.57) 2.7(1.94) 7.2(3)
Table 84: T. P-values Pairwise Test vs. 4 fig
10 ng 25 ng
Day 1 0.7566 0.3834
Day 14 0.0206 0.004
Table 85: T. P-values Pairwise Test vs. Placebo
4 ng 10 ng 25 ng
Day 1 0.5705 0.3255 0.0943
Day 14 0.3576 0.0019 < 0.0001
Estrone Concentration
Table 86: Pharmacokinetics Estrone Baseline (pg/mL)
4 ng 10 ng 25 ng
Placebo
Baseline 15.9(6.02) 19.7(9.18) 16.3(7.71)
20.4(9.67)
143
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Table 87: Pharmacokinetics Estrone Day 1 (pg/mL)
4 ug 10 ug 25 ug Placebo
Predose 14.7(4.44) 21(8.51) 17.2(8.5)
18.3(8.54)
2 hour 13.3(4.52) 20(8.53) 18.9(6.7)
18.9(11.25)
4 hour 13(4.68) 19.3(7.4) 19.4(7.06)
19.9(13.87)
6 hour 13.9(6.04) 19.6(8.89) 19.1(8.1)
19(11.69)
hour 13.4(4.94) 19.7(8.53) 18.8(7.18)
19.3(11.65)
24 hour 14.3(5.92) 21.2(9.89)
16.6(6.06) 22.9(17.18)
Table 88: Pharmacokinetics Estrone Day 14 (pg/mL)
4 ug 10 ug 25 ug Placebo
Predose 15.8(5.15) 21.7(14.25)
18.6(8.49) 18.7(9.38)
2 hour 13.6(5.3) 19.7(10.2)
19.8(9.08) 17.3(7.99)
4 hour 14(5.25) 21(13.46) 19.9(7.26)
20.4(11.41)
6 hour 14(5.11) 20.7(10.4)
19.3(6.47) 16.1(7.54)
10 hour 14.2(5.51) 20.1(11.93)
19.3(8.24) 19(8.17)
24 hour 14.5(4.69) 20.1(9.34)
16.7(6.09) 18.9(8.24)
5 Table 89: Pharmacokinetics Estrone End of Study (pg/mL)
4 ug 10 ug 25 ug Placebo
Post Dosing 4.328(2.7619) 4.643(2.5807) 6.652(11.508)
4.363(2.5982)
Estrone Area Under the Curve (0-24 hours)
Table 90: Estrone Area Under the Curve (0-24 hours) (h*pg/mL)
4 ug 10 ug 25 ug Placebo
Day 1 290.2(123.67) 462.7(195.64) 419.1(147.85)
467.9(278.78)
Day 14 326.6(114.09) 464.1(243.92) 428.7(161.75)
426.8(180.67)
144
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Table 91: Estrone Area Under the Curve (0-24 hours) (Baseline Adjusted)
(h*pg/mL)
4 p.g 10 p.g 25 p.g Placebo
Day 1 7.2(20.91) 10.9(24.55) 44.3(54.27)
43.5(97.41)
Day 14 15(41.53) 43.2(84.87) 55.6(78.06)
17.4(45.27)
Table 92: Estrone Area Under the Curve (0-24 hours) P-values Pairwise Test vs.
4 p.g
10 p.g 25 p.g
Day 1 0.003 0.0076
Day 14 0.042 0.0393
Table 93: Estrone Area Under the Curve (0-24 hours) P-values Pairwise Test vs.
Placebo
4 p.g 10 p.g 25 p.g
Day 1 0.0193 0.9487 0.519
Day 14 0.0621 0.6117 0.9738
Table 94: Estrone Area Under the Curve (0-24 hours) P-values Pairwise Test vs.
4 p.g
(Baseline Adjusted)
10 p.g 25 p.g
Day 1 0.6195 0.0104
Day 14 0.2251 0.0658
Table 95: Estrone Area Under the Curve (0-24 hours) P-values Pairwise Test vs.
Placebo
(Baseline Adjusted)
4 p.g 10 p.g 25 p.g
Day 1 0.1311 0.167 0.9761
Day 14 0.8721 0.2746 0.0886
Table 96: Estrone Area Under the Curve (0-24 hours) Ratio (Day 14) of Day 14
to Day 1
4 p.g 10 p.g 25 p.g
Placebo
145
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
AUC Ratio of Day 14 1.234(0.5824) 1.023(0.2675)
1.039(0.1941) 1.006(0.2316)
to Day 1
Pairwise test vs 0.1722 0.1866
Pairwise test vs 0.1432 0.848 0.6544
Placebo
Estrone Cmax
Table 97: Cma,jp
4 ng 10 ng 25 ng
Placebo
Day 1 15.7(6.07) 23.5(9.87) 21.9(7.73)
25.7(18.43)
Day 14 16(5.5) 23.9(13.45) 22.4(8.95)
22.8(10.89)
Table 98: Cmax (Baseline Adjusted) (pg/mL)
4 ng 10 ng 25 ng
Placebo
Day 1 0.4(3.05) 3.2(2.99) 5.1(4.78) 6.3(12.81)
Day 14 0.6(3.49) 3.7(8.79) 5.6(4.81)
3.4(5.69)
Table 99: Cma P-values Pairwise Test vs. 4 =
ng 25 ng
Day 1 0.007 0.0126
Day 14 0.0301 0.0163
Table 100: Cmax P-values Pairwise Test vs. Placebo
4 ng 10 ng 25 ng
Day 1 0.0373 0.6567 0.4223
Day 14 0.0275 0.7878 0.8979
Table 101: Cmax P-values Pairwise Test vs. 4 ng (Baseline Adjusted)
10 ng 25 ng
146
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Day 1 0.0087 0.0013
Day 14 0.1975 0.0014
Table 102: Cm, P-values Pairwise Test vs. Placebo Baseline Ad.usted
4 [tg 10 [tg 25 [tg
Day 1 0.0659 0.3046 0.71
Day 14 0.0938 0.933 0.2249
Table 103: Cmax Ratio (Day 14) of Day 14 to Da
4 [tg 10 [tg 25 [tg
Placebo
Cmax Ratio of Day 14 to 1.029(0.2346) 1.042(0.3436)
1.041(0.2179) 1.039(0.2916)
Day 1
Pairwise test vs 0.9035 0.8835
Pairwise test vs 0.9188 0.9788 0.982
Placebo
Estrone
Table 104: Call (pg/mL)
4 [tg 10 [tg 25 [tg
Placebo
Day 1 13(4.72) 19.3(8.15) 17.5(6.16)
19.5(11.62)
Day 14 13.6(4.75) 19.3(10.16) 17.9(6.74)
17.8(7.53)
Table 105: Cavg (Baseline Adjusted) (pg/mL)
4 [tg 10 [tg 25 [tg
Placebo
Day 1 -2.3(2.26) -1.1(2.66) 0.7(3.73)
0.1(5.03)
Day 14 -1.7(3.25) -0.9(5.91) 1.1(4.81) -
1.6(3.8)
Table 106: CavE P-values Pairwise Test vs. 4 ug
10 [tg 25 [tg
Day 1 0.0075 0.0207
147
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Day 14 0.042 0.0393
Table 107: Ca,,g P-values Pairwise Test vs. Placebo
4 lig 10 lig 25 lig
Day 1 0.0363 0.9487 0.519
Day 14 0.0621 0.6117 0.9738
Table 108: Ca,,g P-values Pairwise Test vs. 4 lig (Baseline Adjusted)
lig 25 lig
Day 1 0.1345 0.0057
Day 14 0.6351 0.0495
5
Table 109: Cavg P-values Pairwise Test vs. Placebo (Baseline Adjusted)
4 lig 10 lig 25 lig
Day 1 0.0712 0.3751 0.691
Day 14 0.912 0.7058 0.0742
Table 110: Cav Ratio Da 14)ofDay l4toDay 1
4 lig 10 lig 25 lig
Placebo
Ca,,g Ratio of Day 14 to 1.029(0.2346) 1.042(0.3436)
1.041(0.2179) 1.039(0.2916)
Day 1
Pairwise test vs 0.9035 0.8835
Pairwise test vs 0.9188 0.9788 0.982
Placebo
10 Estrone Tmax
Table 111: TmaxADI
4 lig 10 lig 25 lig Placebo
Day 1 14.1(9.37) 11.9(9.76) 9.1(7.43)
12.1(9.39)
148
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Day 14 10.9(9.03) 10.4(8.93) 6.3(6.9)
12.2(9.24)
Table 112: T. P-values Pairwise Test vs. 4 lig
lig 25 lig
Day 1 0.4862 0.0849
Day 14 0.8711 0.0982
Table 113: T. P-values Pairwise Test vs. Placebo
4 lig 10 lig 25 lig
Day 1 0.5341 0.9449 0.2997
Day 14 0.6824 0.5639 0.0391
5
Estrone Conjugates
Table 114: Pharmacokinetics Estrone Conjugates Baseline (pg/mL)
4 lig 10 lig 25 lig
Placebo
Baseline 250.3(162.91) 259.7(208.51) 374.4(586.45)
280.7(171.26)
Table 115: Pharmacokinetics Estrone Conjugates Day 1 (pg/mL)
4 lig 10 lig 25 lig
Placebo
Predose 225.1(215.01) 218.6(147.84) 312.4(410.38)
271.2(153.33)
2 hour 206.8(163.2) 273.1(176.59) 396.6(408.16)
223.4(162.11)
4 hour 241.7(176.87) 267.2(161.79) 413.3(343.25)
241.8(139.77)
6 hour 240.6(181.14) 266(184.92) 477.8(472.66)
265(154.01)
10 hour 223(150.42) 243.5(173.71)
436.4(461) 258(133.21)
24 hour 229.4(186.79) 268.4(221.29) 306.4(322.91)
268.8(153.22)
Table 116: Pharmacokinetics Estrone Conjugates Day 14 (pg/mL)
4 lig 10 lig 25 lig
Placebo
149
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Predose 212.7(140.19) 319.1(326.71)
411.1(624.14) 256.1(133.07)
2 hour 212.4(145.02) 420.4(560.53)
434.3(491.31) 285.6(158.61)
4 hour 240.2(155.7) 429.3(506.01)
505.1(618.47) 273.1(148.76)
6 hour 225.8(164.76) 359.2(346.26)
483.8(515.95) 267.7(181.53)
hour 238.3(152.45) 417.6(517.51)
492.5(598.16) 306.9(178.68)
24 hour 206.4(154.26) 349(345.91)
309.6(380.88) 240.1(115.84)
Table 117: Pharmacokinetics Estrone Conjugates End of Study (pg/mL)
4 ug 10 ug 25 ug
Placebo
Post 237.4(151.19) 221.7(188.05)
499.7(1089.67) 250(148.72)
Dosing
Estrone Conjugates Area Under the Curve (0-24 hours)
5 Table 118: Estrone Conjugates Area Under the Curve (0-24 hours)
(h*pg/mL)
4 ug 10 ug 25 ug Placebo
Day 1 5077.5(3798.39) 5931.9(4209.95)
9126(9186.37) 5637.9(3151.49)
Day 14 5172.9(3382.89)
8978(9811.23) 9930.2(11711.99) 6275.2(3397.54)
Table 119: Estrone Conjugates Area Under the Curve (0-24 hours) (Baseline
Adjusted)
(h*pg/mL)
4 ug 10 ug 25 ug Placebo
Day 1 375.5(843.98) 422.4(473.83) 2454.3(2600.25)
83(229.06)
Day 14 660.5(1230.69) 3767.2(7671.38)
3059(4792.46) 665.4(1552.19)
10 Table 120: Estrone Conjugates Area Under the Curve (0-24 hours) P-values
Pairwise Test vs. 4 ug
10 ug 25 ug
Day 1 0.5219 0.0931
Day 14 0.1392 0.1166
150
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Table 121: Estrone Conjugates Area Under the Curve (0-24 hours) P-values
Pairwise Test vs. Placebo
4 [ig 10 [ig 25 [ig
Day 1 0.639 0.8157 0.1472
Day 14 0.3503 0.2898 0.2246
Table 122: Estrone Conjugates Area Under the Curve (0-24 hours) P-values
Pairwise Test vs. 4 [ig (Baseline Adjusted)
[ig 25 [ig
Day 1 0.8349 0.0028
Day 14 0.1087 0.0537
Table 123: Estrone Conjugates Area Under the Curve (0-24 hours) P-values
Pairwise Test vs. Placebo (Baseline Adjusted)
4 [ig 10 [ig 25 [ig
Day 1 0.1894 0.0134 0.001
Day 14 0.992 0.1225 0.0654
Table 124: Estrone Conjugates Area Under the Curve (0-24 hours) Ratio (Day 14)
of Day 14
to Day 1
4 [ig 10 [ig 25 [ig Placebo
AUC Ratio of Day 14 1.115(0.4539) 1.444(1.0121)
1.107(0.3545) 1.125(0.4522)
to Day 1
Pairwise test vs 0.2279 0.9587
Pairwise test vs 0.9459 0.2427 0.8975
Placebo
Estrone Conjugates Cmax
Table 125: Cma,jpini,
4 [ig 10 [ig 25 [ig Placebo
Day 1 273.1(196.36) 329.4(226.58) 542.1(475.49)
309.8(146.07)
151
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Day 14 289(183.79) 511.7(568.75) 579.5(610.1)
343.6(182.2)
Table 126: C. (Baseline Adjusted) (pg/mL)
4 lig 10 lig 25 lig Placebo
Day 1 35.4(89.09) 90.2(65.2) 198.6(301.53)
27.1(49.69)
Day 14 48.2(132.61) 277.8(493.64) 236.1(372.42)
67(121.81)
Table 127: C. P-values Pairwise Test vs. 4 lig
10 lig 25 lig
Day 1 0.4261 0.0333
Day 14 0.1332 0.0685
Table 128: C. P-values Pairwise Test vs. Placebo
4 lig 10 lig 25 lig
Day 1 0.5369 0.7629 0.0625
Day 14 0.3902 0.2533 0.1356
Table 129: C. P-values Pairwise vs. 4 Ú.tg (Baseline Adjusted)
10 lig 25 lig
Day 1 0.039 0.0345
Day 14 0.0726 0.0579
Table 130: C. P-values Pairwise Test vs. Placebo (Baseline Adjusted)
4 lig 10 lig 25 lig
Day 1 0.7444 0.0033 0.0318
Day 14 0.6735 0.1065 0.0928
152
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Table 131: Cmax Ratio (Day 14) of Day 14 to Da
4 lig 10 lig 25 lig Placebo
Cma, Ratio of Day 14 to 1.13(0.4068) 1.524(1.1682)
1.144(0.4569) 1.11(0.5404)
Day 1
Pairwise test vs 0.1969 0.9226
Pairwise test vs 0.9043 0.1919 0.8406
Placebo
Estrone Conjugates C,,g
Table 132: Call (pg/mL)
4 lig 10 lig 25 lig Placebo
Day 1 215.9(154.77) 247.2(175.41)
380.3(382.77) 244.6(128.1)
Day 14 215.5(140.95) 374.1(408.8) 413.8(488)
261.5(141.56)
Table 133: Cavg (Baseline Adjusted) (pg/mL)
4 lig 10 lig 25 lig Placebo
Day 1 -21.8(88.41) 8(34.21) 36.8(291.72) -
33.7(46.95)
Day 14 -25.3(120.69) 140.2(330.6)
70.3(300.36) -7.9(89.89)
Table 134: CavE P-values Pairwise Test vs. 4 itg
10 lig 25 lig
Day 1 0.5701 0.1004
Day 14 0.1392 0.1166
P-values Pairwise Test vs. Placebo
4 lig 10 lig 25 lig
Day 1 0.5562 0.9602 0.1741
Day 14 0.3503 0.2898 0.2246
153
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Table 136: C,g P-values Pairwise Test vs. 4 lig (Baseline Adjusted)
lig 25 lig
Day 1 0.1804 0.4201
Day 14 0.0606 0.2305
Table 137: Cavg P-values Pairwise Test vs. Placebo (Baseline Adjusted)
4 lig 10 lig 25 lig
Day 1 0.6353 0.0047 0.3473
Day 14 0.6439 0.0928 0.3244
5 Table 138: C,g Ratio (Day 14) of Day 14 to Day 1
4 lig 10 lig 25 lig Placebo
C,g Ratio of Day 14 to 1.13(0.4068) 1.524(1.1682)
1.144(0.4569) 1.11(0.5404)
Day 1
Pairwise test vs 0.1969 0.9226
Pairwise test vs 0.9043 0.1919 0.8406
Placebo
Estrone Conjuax
Table 139: TmaxADI
4 lig 10 lig 25 lig Placebo
Day 1 10.9(8.66) 9.2(9.25) 5.4(2.64) 13.1(9.7)
Day 14 8.4(7.79) 9(8.6) 5.9(2.87) 8.1(6.76)
10 Table 140: Tina P-values Pairwise Test vs. 4 =
10 lig 25 lig
Day 1 0.5609 0.0154
Day 14 0.8173 0.2178
154
CA 03007636 2018-06-06
WO 2017/100378 PCT/US2016/065466
Table 141: Tmax P-values Pairwise Test vs. Placebo
4 p.g 10 p.g 25 p.g
Day 1 0.4893 0.2253 0.003
Day 14 0.9256 0.739 0.2087
[0467] In the phase 3 trial, all doses of TX-004HR compared with placebo (MITT
n=747)
significantly improved the 4 co-primary endpoints at week 2 through week 12,
as well as the
secondary endpoints of vaginal dryness by week 6 and vulvar and/or vaginal
itching or
irritation by week 12 (except 4 pg, p=0.0503), and was well-tolerated with no
treatment-
related serious AEs reported. The phase 3 PK study (n=72) showed no difference
in systemic
E2 levels for 4 pg and 10 p.g TX-004HR vs placebo, as measured by AUC and
Cavg. E2 AUC
and Cavg with 25 p.g TX-004HR was higher than placebo, but average
concentrations
remained within the normal postmenopausal range (Table 142). E2 levels at day
84 were
similar to placebo indicating no systemic drug accumulation. SHBG
concentrations did not
change with treatment. The two phase 2 studies (n=36 for each) of TX-004HR 10
pg and 25
pg resulted in statistically significantly lower E2 absorption than an
approved E2 tablet at
identical doses, with 25 pg TX-004HR demonstrating AUC less than 1/3 that of
the approved
product (Table 143).
Table 142. Phase 3 study PK parameters for E2 (unadjusted mean SD).
AUC0_24 (pg*hr/mL) Cavg (pg/mL)
Day Dose (jig) TX-004HR Placebo p-value TX-004HR Placebo p-value
1 4 91.7 37.9 116.6 77.3 NS 3.92 1.46 4.86 3.22 NS
10 138.2 75.2 116.6 77.3 NS 5.76 3.13 4.86 3.22 NS
217.4 99.0 116.6 77.3 0.0021 9.06 4.13 4.86 3.22 0.0021
14 4 87.2 42.8 104.2 66.4 NS 3.63 1.78 4.34 2.77 NS
10 110.1 54.6 104.2 66.4 NS 4.59 2.27 4.34 2.77 NS
25 171.6 80.1 104.2 66.4 0.0108 7.15 3.34 4.34 2.77 0.0108
Table 143. Phase 2 studies PK parameters for E2 (baseline adjusted geometric
mean).
AUC0_24 (pg*hr/mL) Cmax (pg/mL)
Dose (jig) TX-004HR Vaginal Tablet p-value TX-004HR Vaginal Tablet p-value
10 49.62 132.92 <0.0001 14.38 20.38
0.0194
25 89.21 292.06 <0.0001 23.08 42.70
<0.0001
[0468] With robust efficacy demonstrated as early as 2 weeks and up to 12
weeks at all 3
20 doses, TX-004HR 4 pg and 10 pg showed negligible systemic E2 absorption,
while 25 pg
resulted in very low systemic absorption of E2 in the phase 3 trial. TX-004HR
10 pg and 25
155
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
lig showed lower systemic E2 exposure than equivalent doses of an approved E2
tablet. The
absence of clinically meaningful increases in E2 concentrations paired with
data consistent
with a lack of systemic effects (e.g., no increase in SHBG) shows that TX-004
HR delivers
excellent efficacy with negligible to very low systemic exposure.
[0469] The impact of normal daily activities for 4 hours post dose was
evaluated, in
comparison with the impact of remaining in the supine position for 4 hours
post dose on the
pharmacokinetic (PK) profile of TX-004HR 25 mcg. In two studies, at the same
site, the
same sixteen healthy postmenopausal female subjects were fasted for at least
10 hours prior
to dosing through 4 hours following dosing. Subjects received a 25 mcg dose of
TX-004HR
administered intravaginally by trained female study personnel. Following their
first dose, the
subjects were required to remain in a supine position for 4 hours following
dosing.
Following the second dose, after 5 minutes resting time, the subjects were
instructed to be
ambulatory in the clinic and refrain from reclining for the 4 hours following
dosing. Blood
samples were collected at pre-defined intervals up to 24 hours after dosing.
Plasma samples
were analyzed for estradiol using LC-MS/MS. See, e.g., Fig. 23. PK parameters
were
calculated on an individual and group mean basis with baseline correction.
[0470] The mean C. and AUC0_24 of estradiol was not significantly different
with
ambulation than with supination. On an individual subject basis, the majority
showed similar
Cmax and AUC0_24 levels with ambulation as with supination. There were no
signs of posture
having an effect on absorption rate as evidenced by the similarity in group
average and
individual subject T.. In addition, there was no difference between the group
mean profiles
when compared on an individual time point basis, further demonstrating that
posture had no
effect on absorption. The systemic exposure of estradiol in TX-004HR 25 mcg
was generally
low and occurred regardless of whether the subjects were ambulatory or supine
for 4 hours
after dosing. An important advantage of the formulation is that a woman can be
ambulatory
almost immediately after the formulation is administered, as opposed to other
known
formulations that require a subject to remain in a supine position after
administration.
Generally, other known formulations direct administration before bed at night
because of the
requirement to be supine, which requirement is unnecessary in the
pharmaceutical
compositions disclosed herein because the pharmaceutical compositions
disclosed herein
adhere to the vaginal tissue, the capsule dissolves rapidly, and the
formulation is released into
the vagina and rapidly absorbed by the vaginal tissue. Because activity level
does not
156
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
adversely affect the systemic absorption of estradiol, the formulation of the
invention gives
the patient more flexibility with her dosing regimen.
EXAMPLE 13: Safety results in randomized, double-blind, placebo-controlled
multicenter study.
[0471] Safety endpoints in the study included vital signs, clinical laboratory
tests (blood
chemistry, hematology, hormone levels, urine analysis), ECG, physical and
gynecological
examination findings, pap smears, endometrial biopsies, and adverse events
(AEs). AEs
included undesirable medical conditions occurring at any time during all study
phases
including the washout period, whether or not a study treatment had been
administered. An
AE was considered treatment emergent if it occurred after study drug
administration, or if it
was pre-existing and worsened during 120 days post-dose follow up.
Participants were given
a diary with instructions to record product use, sexual activity,
symptoms/complaints, and
other medications. AEs, concomitant medications, and vital signs were recorded
and assessed
at each study visit from screening to week 12.
[0472] TX-004HR had a favorable safety profile and was well tolerated. No
clinically
significant differences in AEs were observed between treatment and placebo
groups (Table
144). Headache was the most commonly reported TEAE, followed by vaginal
discharge,
nasopharyngitis, and vulvovaginal pruritus (Table 144). Headache was the only
treatment-
related TEAE that was numerically more frequent in women receiving TX-004HR
than those
receiving placebo (3.7% for 4-[tg dose vs 3.1% for placebo). Vaginal discharge
was reported
by numerically fewer women in any of the TX-004HR groups than by women in the
placebo
group. Most TEAEs were mild to moderate in severity. Few participants (1.8%)
discontinued
the study due to AEs.
157
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Table 144. Number (%) of treatment emergent adverse events (TEAE) reported for
>3% in
any treatment arm of the safety population.
TX- TX-
TX-004HR 004HR 004HR Placebo
4 lag 10 lag 25 lag
Preferred Term (n = 191) (n = 191) (n =
190) (n = 192)
Any subject with reported TEAE 97 (50.8) 94 (49.2) 93
(48.9) 111 (57.8)
Headache 12 (6.3) 14 (7.3) 6 (3.2) 15 (7.8)
Vaginal discharge 5 (2.6) 6 (3.1) 4 (2.1) 13 (6.8)
Nasopharyngitis 5 (2.6) 6 (3.1) 7 (3.7) 10 (5.2)
Vulvovaginal pruritus 4 (2.1) 3 (1.6) 7 (3.7) 10 (5.2)
Back pain 9 (4.7) 1 (0.5) 4 (2.1) 8 (4.2)
Urinary tract infection 5 (2.6) 5 (2.6) 8 (4.2) 4 (2.1)
Upper respiratory tract
infection 5 (2.6) 6 (3.1) 3 (1.6) 5 (2.6)
Oropharyngeal pain 1 (0.5) 0 (0) 6 (3.2) 1 (0.5)
[0473] Nine serious TEAEs were reported in 8 subjects; however, none were
considered
related to treatment. Complete heart block, appendicitis, endophthalmitis, and
chronic
obstructive pulmonary disease were each reported by a different participant in
the 25 [ig
group. Sinus node dysfunction and ankle fracture were both reported for one
women, and
arthralgia and malignant melanoma were each reported for one women in the 10
[ig group.
None of the women in the 4 [ig group had reports of serious TEAEs. One woman
in the
placebo group was reported to have a cervical myelopathy. No deaths occurred
during the
study.
[0474] No diagnoses of endometrial hyperplasia or malignancy from endometrial
biopsies
were observed at week 12. Total cholesterol numerically decreased from
baseline to week 12
by a mean of 0.024 mmol/L to 0.07 mmol/L in the treatment groups, and by 0.008
mmol/L in
the placebo group. No clinically meaningful increases in triglycerides were
observed in any
active treatment groups compared with placebo. Sex hormone binding globulin
(SHBG)
concentrations (measured in a subset of 72 women) did not increase with
treatment relative to
placebo or baseline at week 12. No clinically significant changes in any
laboratory
parameters were found.
[0475] The phase 3 clinical trial demonstrated that TX-004HR at 4 [ig, 10 [ig,
and 25 [ig
doses is safe and effective for treating vaginal changes and self-reported
symptoms of VVA
in postmenopausal women. Statistically significant and clinically meaningful
improvements
158
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
in all of the 4 pre-specified co-primary endpoints (increase in the percentage
of vaginal
superficial cells, decrease in the percentage of vaginal parabasal cells and
vaginal pH, and
decrease in severity of the MBS of dyspareunia) occurred as early as 2 weeks
with all 3 doses
of TX-004HR as compared with placebo, and were sustained throughout the 12-
week trial.
Additionally, improvements were found for the secondary endpoints of vaginal
dryness and
vulvar or vaginal irritation and itching. These improvements were achieved
without
increasing systemic estrogen concentrations (4 lig and 10 lig) or with
negligible (25 lig)
systemic estrogen exposure, as found in pharmacokinetic studies. TX-004HR was
also well-
tolerated with no clinically significant differences found between treatment
and placebo
groups in any AEs or treatment-related AEs, and no treatment-related serious
AEs.
[0476] The results demonstrate early onset of action in the clinical signs of
VVA with
statistically significantly improved changes compared with placebo. The
efficacy results here
were somewhat numerically higher than data from a 12-week, randomized,
controlled trial
that compared a 10-pg vaginal estradiol tablet with placebo, which showed
significant
improvements in the percentages of superficial and parabasal cells, and in pH
compared with
placebo (see, Simon et al. Obstet Gynecol. 2008;112:1053-1060). At 12 weeks,
improvements were smaller with the 10- g estradiol tablet (change of 13% in
superficial
cells, -37% in parabasal cells, and -1.3 in vaginal pH) than what was observed
in this study
with the 10-p,g TX-004HR dose (change of 17% in superficial cells, -44% in
parabasal cells,
and -1.4 in vaginal pH). While improvements in some objective (cell and pH)
endpoints were
seen with the estradiol tablet within 2 weeks of treatment, the patient-
reported improvements
in a composite score of subjective symptoms were not observed until 8 weeks of
therapy,
which can be perceived as a disadvantage for many users. That clinical trial
did not assess
individual symptoms. A second randomized, controlled trial of 10- g and 25- g
estradiol
tablets similarly did not find significant improvements over placebo in the
composite score of
vaginal symptoms with either dose until 7 weeks of treatment (week 2, NS).
Likewise, the
SERM, ospemifene, was evaluated in a clinical trial for the treatment of
dyspareunia, and
statistically significant improvements were not observed until week 12. See,
Bachmann et al.
Obstet Gynecol. 2008;111:67-76; Portman et al. Menopause. 2013;20:623-630.
[0477] Importantly, the results reported here showed significant improvement
in
dyspareunia within 2 weeks with all 3 doses of TX-004HR, with reductions in
severity scores
from 1.5 to 1.7 points at week 12, which were comparable or superior to
reductions of 1.2 to
1.6 points reported for other currently approved dyspareunia treatments. See,
VAGIFEMO
159
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
(estradiol vaginal tablets) Prescribing Information. Bagsvaerd, Denmark: Novo
Nordisk
Pharmaceuticals Inc.; 2012; PREMARINO (conjugated estrogens tablets, USP)
Prescribing
Information. Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2010; OSPHENAO
(ospemifene) tablets, for oral use. Prescribing Information. Shionogi, Inc.
2013.
[0478] Additionally, vaginal dryness improved from week 2 with 10 ug and 25 ug
TX-
004HR. None of the currently available products reported as early an onset of
action for the
symptom of vaginal dryness associated with VVA as did TX-004HR. Furthermore,
TX-
004HR 10 ug and 25 ug showed significant improvement in vaginal irritation
and/or itching
at week 12, while none of the currently available products on the market are
reported to
improve these symptoms. See, Portman et al. Maturitas. 2014;78:91-98; Eriksen
et al. Eur J
Obstet Gynecol Reprod Biol. 1992;44:137-144.
[0479] Based on a large survey of postmenopausal women in the United States,
only a
small proportion (7%) of women are thought to receive prescription vaginal
estrogen therapy
alone for their VVA, probably due to lack of information about available
treatments,
avoidance of discussion of the topic with health care practitioners, or
dissatisfaction with
currently available products (see, e.g., Kingsberg et al. J Sex Med.
2013;10:1790-1799).
Eliminating the need for an applicator or individually measuring doses is
intended to give
women a more positive user experience and thus potentially better compliance,
resulting in
overall better efficacy of treatment.
[0480] The results with TX-004HR in this study exemplify one of the advantages
of local
vaginal estrogen therapies: rapid symptom resolution without increasing
systemic estrogen
concentrations. The mean area under the concentration-time curve (AUC) and
average
concentration (C,g) for estradiol were not significantly different from
placebo with 4 ug and
10 ug TX-004HR. Although statistically higher AUC for estradiol was observed
with the 25
ug dose, estradiol levels remained within the postmenopausal range with no
evidence of
accumulation by day 84. Although there was negligible systemic absorption,
rapid efficacy
was observed within 2 weeks of dosing with all doses of TX-004HR.
[0481] TX-004HR was well-tolerated. The 4 most commonly reported TEAEs,
including
vaginal discharge and vulvovaginal pruritus, were experienced by fewer women
in any TX-
004HR group than in the placebo group, and were mostly mild to moderate in
severity. By
comparison, in a 12-week study of the efficacy of ospemifene, vaginal
discharge was
reported more than 6-times more frequently in the ospemifene group than in the
placebo
160
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
group (see, Portman et al. Menopause. 2013;20:623-630). Genital pruritus was
also reported
4-times more frequently in women treated with Vagifem 10-ug tablets than with
placebo in a
12-month randomized study (see, Vagifem0 (estradiol vaginal tablets)
Prescribing
Information. Bagsvaerd, Denmark: Novo Nordisk Pharmaceuticals Inc.; 2012).
Importantly,
endometrial findings after TX-004HR were benign as no hyperplasia or
malignancies were
reported in biopsies at 12 weeks. Onset of effect was seen as early as 2 weeks
and was
maintained throughout the study. TX-004HR was well tolerated as reported here
and
systemic estrogen exposure was negligible to very low as demonstrated by the
pharmacokinetic study.
EXAMPLE 14: Results of Female Sexual Function Index in randomized, double-
blind, placebo-controlled multicenter study.
[0482] The trial was a randomized, double-blind, placebo controlled,
multicenter, phase 3
study. Treatments were self-administered vaginally, once daily, for 2 weeks
and then twice
weekly, for 10 weeks. Female sexual dysfunction (FSD) was evaluated using the
multidimensional Female Sexual Function Index (FSFI) at baseline and at week
12. The FSFI
is a brief, validated, self-reporting questionnaire consisting of 19 questions
designed to assess
the areas of arousal, desire, orgasm, lubrication, and pain. The Index defines
sexual
dysfunction by a total FSFI score (the sum of the individual domain scores) of
<26.55 out of
a possible maximum score of 36.
[0483] Postmenopausal women (40-75 years ; BMI <38 kg/m2) were included if
they had
<5% superficial cells on vaginal cytological smear; vaginal pH >5.0; self-
identified most
bothersome symptom (MBS) of moderate-to severe dyspareunia; and anticipated
sexual
activity (with vaginal penetration) during the trial period. Vulvar and
vaginal atrophy (VVA)
treatments, including vaginal lubricants and moisturizers, were discontinued
within 7 days
prior to screening. Use of oral estrogen-, progestin-, androgen-, or SERM-
containing drug
products were prohibited within 8 weeks of study start. Changes from baseline
in total and
individual domain FSFI scores for each dose were compared with placebo using
ANCOVA
with baseline as a covariate.
[0484] 764 postmenopausal women were randomized to 4 pg (n=191), 10 pg
(n=191), or
25 pg (n=190) vaginal estradiol softgel capsules or placebo (n=192). The
majority of the
women were white (87%) with a mean age of 59 years and a mean BMI of 26.7
kg/m2 (Table
145). The FSFI questionnaire was completed by those who were not in the PK sub-
study
161
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
(n=692; 90.6%). The average baseline total FSFI score of 14.8 for all women
indicated FSD
in the subjects.
Table 145. Summary of subjects enrolled in study
Composition Composition Composition Composition
4 5 6 7
4 pg 10 lug 25 pg
(11=186) (11=188) (11=186) (11=187)
Age, years
Mean+SD 59.8+6.0 58.6+6.3 58.8+6.2
59.4+6.0
Race, n (%)
White 162 (87.1) 165 (87.8) 161
(86.6) 160 (85.6)
Black or African American 20 (10.8) 21 (11.2) 24
(12.9) 21 (11.2)
Asian 3 (1.6) 2 (1.1) 1 (0.5) 1 (0.5)
BMI, kg/m2
Mean+SD 26.6+4.9 26.8+4.7 26.9+4.8
26.6+4.6
Baseline total FSFI Score
Mean+SD 14.8+6.13 15.8+6.24 14.2+6.21
14.4+6.61
Baseline FSFI Pain Score
Mean+SD 1.6+1.11 1.8+1.22 1.7+1.17
1.7+1.20
[0485] The Female Sexual Function Index (FSFI) total summary score is a
numerically
continuous measure that was descriptively summarized at Visits 2 and 6 and the
change in the
total summary score (Visit 6 minus Visit 2) was also descriptively summarized.
The domain
sub-scores and the changes in the domain sub-scores were also descriptively
summarized.
Summaries were by treatment arm, and all active treatment arms combined.
[0486] In addition, the change in mean from baseline of each active treatment
group from
the placebo group for each numerically continuous endpoint was evaluated. The
least square
(LS) mean changes and the 95% CI for the difference in LS Mean changes between
treated
and placebo are provided. The FSFI Questionnaire consists of 19 questions
divided among 6
domains, and has a minimum total score of 2.0 and a maximum score of 36.0
points. The
FSFI questionnaire was administered to the randomized population except for
those subjects
in the PK sub-study. At Baseline, the overall mean Total Score was 14.8 (14.8
for the 4 pg
group; 15.8 for the 10 pg group; 14.2 for the 25 pg group; and 14.4 for the
placebo group).
The LS mean change in the FSFI Total Score and domain scores from Baseline to
Week 12
are summarized in Table 146.
[0487] Change from Baseline to Week 12 in FSFI total score and domains
compared to
placebo was assessed.
162
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
[0488] After 12 weeks, total FSFI scores numerically improved from baseline in
all groups,
including placebo. Total FSFI score significantly increased with the 10 pg
group (P<0.05)
and the 25 pg group (P=0.0019) versus placebo (Fig. 24).
[0489] FSFI lubrication and pain domain scores improved numerically in all
groups
including placebo from baseline to 12 weeks; improvements for the 10 pg group
and the 25
pg group were statistically significantly greater than with placebo (Fig.
25A). The 25 pg
composition significantly improved FSFI arousal (P=0.0085) and satisfaction
(P=0.0073)
domain scores at 12 weeks (Fig. 25B, Fig. 25C). All three doses were
comparable to placebo
in their effect on the FSFI domains of desire and orgasm (Fig. 25D, Fig. 25E).
The 4 pg
composition and placebo provided similar levels of improvement. The
compositions
improved FSFI in a dose-dependent manner, with the 25 ug dose having the
greatest
improvement. All three doses were efficacious, and the numeric improvement in
subjective
symptoms was highest for subjects in the 10 and 25 pg groups. The observed
placebo
response could be attributed to the coconut oil (Miglyol) in the formulation
for the placebo
and the estradiol compositions, which may also contribute to the observed
benefits.
Table 146. Female Sexual Function Index Total and Domain Scores:
4 ittg 10 lug 25 pg Placebo
Category Score
Mean SD Mean SD Mean SD Mean SD
Baseline 14.8 6.13 15.8 6.24 14.2 6.21 14.4
6.61
Week 12 22.6 8.4 24.8 7.59 24.8 7.59 22
8.54
Total
Change 7.98 7.551 8.85 7.361 10.49 8.176
7.74 8.41
LS Mean 7.909 0.9075 9.431 0.0492 10.283
0.0019 7.458 -
Baseline 2.8 1.44 2.9 1.43 2.7 1.5 2.7 1.41
Week 12 3.6 1.61 4.1 1.47 4.1 1.39 3.6
1.52
Arousal
Change 0.88 1.615 1.16 1.632 1.43 1.646
1.02 1.607
LS Mean 0.876 0.9777 1.288 0.0581 1.393
0.008 0.927 -
Baseline 2.6 1.01 2.7 1.13 2.6 1.09 2.7
1.07
Week 12 3.3 1.11 3.5 1.13 3.5 1.06 3.3
1.21
Desire
Change 0.64 1.065 0.78 1.113 0.87 1.105
0.62 1.102
LS Mean 0.626 1 0.801 0.2753 0.849 0.1139
0.628 -
Baseline 2.1 1.25 2.3 1.25 2 1.19 2 1.29
Lubricatio Week 12 3.9 1.84 4.4 1.56 4.3 1.65 3.6
1.77
n
Change 1.84 1.782 2.12 1.612 2.36 1.744
1.64 1.871
LS Mean 1.835 0.4023 2.243 0.0012 2.3
0.0003 1.591 -
Orgasm Baseline 2.7 1.74 2.9 1.74 2.4 1.68 2.4
1.73
163
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
Week 12 3.8 1.89 4.1 1.75 4.1 1.66 3.7
1.97
Change 1.12 1.93 1.09 1.821 1.68 1.857 1.31
1.86
LS Mean 1.162 0.9978 1.273 0.9424 1.59 0.0763
1.189
Baseline 2.9 1.37 3.2 1.43 2.9 1.37 2.9
1.49
Satisfactio Week 12 4.2 1.54 4.4 1.37 4.6 1.35 4.1
1.55
Change 1.31 1.512 1.24 1.534 1.64 1.613
1.23 1.661
LS Mean 1.256 0.8798 1.382 0.3484 1.628 0.0063
1.165
IX. EXEMPLARY EMBODIMENTS
[0490] Exemplary embodiments provided in accordance with the presently
disclosed
subject matter include, but are not limited to, the claims and the following
embodiments:
1. A method for
treating the symptoms of vulvo-vaginal atrophy (VVA)
comprising:
administering a vaginal suppository comprising 4 lig to 25 lig of estradiol to
a
subject having VVA,
wherein the treatment is effective within two weeks of the first
administration.
2. The method of
embodiment 1, wherein adverse events associated with
administering the estradiol, other than headaches, do not differ significantly
from adverse
events associated with administering a placebo.
3. The method of embodiment 1 or embodiment 2, wherein the symptoms
of VVA comprise one or more symptoms selected from vaginal dryness,
dyspareunia, vaginal
or vulvar irritation, burning, or itching, dysuria, and vaginal bleeding
associated with sexual
activity.
4. The method of any one of embodiments 1-3, comprising increasing the
level of vaginal secretions in a subject, as assessed by visual examination.
5. The method of any one of embodiments 1-4, comprising increasing the
number of vaginal rugae in the subject, as assessed by visual examination.
6. The method of any one of embodiments 1-5, comprising decreasing
vaginal bleeding or petechiae in the subject, as assessed by visual
examination.
164
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
7. The method of any one of embodiments 1-6, comprising changing the
color of the vaginal mucosa in the subject from transparent to pink, or from
pale pink to pink,
as assessed by visual examination.
8. The method of any one of embodiments 1-7, wherein the treatment
decreases the severity of vaginal dryness within two weeks.
9. The method of any one of embodiments 1-8, wherein the treatment
decreases the severity of vulvar or vaginal itching within two weeks.
10. The method of any one of embodiments 1-9, wherein the treatment
decreases the severity of dyspareunia within two weeks.
11. The method of any one of embodiments 1-10, wherein the vaginal
suppository further includes a solubilizing agent, wherein the solubilizing
agent includes at
least one C6-C12 fatty acid or a glycol, monoglyceride, diglyceride, or
triglyceride ester
thereof
12. The method of any one of embodiments 1-11, wherein the vaginal
suppository includes 4 pg estradiol.
13. The method of embodiment 12, wherein administering the vaginal
suppository provides, in a plasma sample from the patient, one or more
parameters selected
from:
1) an unadjusted arithmetic mean area under the curve (AUC)0_24 of estradiol
ranging from about 73.3 pg*hr/mL to about 114.7 pg*hr/mL, as assessed at day
1;
2) a corrected arithmetic mean peak plasma concentration (Cavg[0_241) of
estradiol ranging from about 3.1 pg/mL to about 4.8 pg/mL, as assessed at day
1;
3) an unadjusted arithmetic mean area under the curve (AUC)0_24 of estradiol
ranging from about 69.7 pg*hr/mL to about 108.9 pg*hr/mL, as assessed at day
14; and
4) a corrected arithmetic mean peak plasma concentration (Cavg[0_241) of
estradiol ranging from about 2.8 pg/mL to about 4.6 pg/mL, as assessed at day
14.
13. The method of embodiment 12, wherein administering the
vaginal
suppository provides, in a plasma sample from the patient, a corrected
geometric mean peak
165
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
plasma concentration (Cmax) of estradiol ranging from about 2.0 pg/mL to about
3.3 pg/mL,
as assessed at day 1.
14. The method of embodiment 12 or embodiment 13, wherein
administering the vaginal suppository provides, in a plasma sample from the
patient, a
corrected geometric mean peak plasma concentration (Cmax) of estradiol ranging
from about
1.0 pg*hr/mL to about 1.7 pg*hr/mL, as assessed at day 14.
15. The method of any one of embodiments 12-14, wherein administering
the vaginal suppository provides, in a plasma sample from the patient, a
corrected geometric
mean area under the curve (AUC)0_24 of estradiol ranging from about 9.5
pg*hr/mL to about
15.1 pg*hr/mL, as assessed at day 1.
16. The method of any one of embodiments 12-15, wherein administering
the vaginal suppository provides, in a plasma sample from the patient, a
corrected geometric
mean area under the curve (AUC)0_24 of estradiol ranging from about 5.7
pg*hr/mL to about
9.1 pg*hr/mL, as assessed at day 14.
17. The method of any one of embodiments 1-11, wherein the vaginal
suppository includes 10 lig estradiol.
19. The method of embodiment 17, wherein administering the
vaginal
suppository provides, in a plasma sample from the patient, one or more
parameters selected
from:
1) an unadjusted arithmetic mean area under the curve (AUC)0_24 of estradiol
ranging from about 110.3 pg*hr/mL to about 172.6 pg*hr/mL, as assessed at day
1;
2) a corrected arithmetic mean peak plasma concentration (Cavg[0_241) of
estradiol ranging from about 4.6 pg/mL to about 7.8 pg/mL, as assessed at day
1;
3) an unadjusted arithmetic mean area under the curve (AUC)0_24 of estradiol
ranging from about 87.9 pg*hr/mL to about 137.4 pg*hr/mL, as assessed at day
14; and
4) a corrected arithmetic mean peak plasma concentration (Cavg[0_241) of
estradiol ranging from about 3.6 pg/mL to about 5.8 pg/mL, as assessed at day
14.
18. The method of embodiment 17, wherein administering the
vaginal
suppository provides, in a plasma sample from the patient, a corrected
geometric mean peak
166
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
plasma concentration (Cmax) of estradiol ranging from about 4.7 pg/mL to about
7.6 pg/mL,
as assessed at day 1.
19. The method of embodiment 17 or embodiment 18, wherein
administering the vaginal suppository provides, in a plasma sample from the
patient, a
corrected geometric mean peak plasma concentration (Cmax) of estradiol ranging
from about
2.3 pg/mL to about 3.8 pg/mL, as assessed at day 14.
20. The method of any one of embodiments 17-19, wherein administering
the vaginal suppository provides, in a plasma sample from the patient, a
corrected geometric
mean area under the curve (AUC)0_24 of estradiol ranging from about 17.5
pg*hr/mL to about
27.4 pg*hr/mL, as assessed at day 1.
21. The method of any one of embodiments 17-20, wherein administering
the vaginal suppository provides, in a plasma sample from the patient, a
corrected geometric
mean area under the curve (AUC)0_24 of estradiol ranging from about 10.9
pg*hr/mL to about
17.2 pg*hr/mL, as assessed at day 14.
22. The method of any one
of embodiments 17-21, wherein administering
the vaginal suppository provides, in a plasma sample from the patient, a
corrected geometric
mean peak plasma concentration (Cavg) of estradiol ranging from about 0.6
pg/mL to about
1.1 pg/mL, as assessed at day 1.
23. The method of any one of embodiments 17-22, wherein administering
the vaginal suppository provides, in a plasma sample from the patient, a
corrected geometric
mean peak plasma concentration (Cavg) of estradiol ranging from about 0.1
pg/mL to about
0.3 pg/mL, as assessed at day 14.
24. The method of any one of embodiments 1-11, wherein the vaginal
suppository includes 25 [ig estradiol.
27. The method of embodiment 24, wherein administering the vaginal
suppository provides, in a plasma sample from the patient, one or more
parameters selected
from:
1) an unadjusted arithmetic mean area under the curve (AUC)0_24 of estradiol
ranging from about 173.5 pg*hr/mL to about 271.3 pg*hr/mL, as assessed at day
1;
167
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
2) a corrected arithmetic mean peak plasma concentration (Cavg[0_241) of
estradiol ranging from about 7.2 pg/mL to about 11.4 pg/mL, as assessed at day
1;
3) an unadjusted arithmetic mean area under the curve (AUC)0_24 of estradiol
ranging from about 137.5 pg*hr/mL to about 215.1 pg*hr/mL, as assessed at day
14; and
4) a corrected arithmetic mean peak plasma concentration (Cavg[0_241) of
estradiol ranging from about 5.7 pg/mL to about 9.0 pg/mL, as assessed at day
14.
25. The method of embodiment 24, wherein administering the vaginal
suppository provides, in a plasma sample from the patient, a corrected
geometric mean peak
plasma concentration (Cmax) of estradiol ranging from about 20.9 pg/mL to
about 32.8 pg/mL,
as assessed at day 1.
26. The method of embodiment 24 or embodiment 25, wherein
administering the vaginal suppository provides, in a plasma sample from the
patient, a
corrected geometric mean peak plasma concentration (Cmax) of estradiol ranging
from about
9.5 pg/mL to about 15.1 pg/mL, as assessed at day 14.
27. The method of any
one of embodiments 24-26, wherein administering
the vaginal suppository provides, in a plasma sample from the patient, a
corrected geometric
mean area under the curve (AUC)0_24 of estradiol ranging from about 104.3
pg*hr/mL to
about 163.1 pg*hr/mL, as assessed at day 1.
28. The method of any one of embodiments 24-27, wherein administering
the vaginal suppository provides, in a plasma sample from the patient, a
corrected geometric
mean area under the curve (AUC)0_24 of estradiol ranging from about 67.6
pg*hr/mL to about
105.8 pg*hr/mL, as assessed at day 14.
29. The method of any one of embodiments 24-28, wherein administering
the vaginal suppository provides, in a plasma sample from the patient, a
corrected geometric
mean peak plasma concentration (Cavg) of estradiol ranging from about 4.3
pg/mL to about
6.8 pg/mL, as assessed at day 1.
30. The method of any one of embodiments 24-29, wherein administering
the vaginal suppository provides, in a plasma sample from the patient, a
corrected geometric
mean peak plasma concentration (Cavg) of estradiol ranging from about 2.7
pg/mL to about
4.4 pg/mL, as assessed at day 14.
168
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
31. The method of any one of embodiments 12-30, wherein administering
the vaginal suppository provides, in a plasma sample from the patient, a
corrected geometric
mean time to peak plasma concentration (Tmax) of estradiol of about 0.25 hrs
to about 2 hrs.
32. The method of any one of embodiments 1-31, wherein the vaginal
suppository does not include a hydrophilic gel-forming bioadhesive agent in
the solubilizing
agent.
33. The method of any one of embodiments 1-32, wherein estradiol is the
only active hormone in the vaginal suppository.
34. The method of any one of embodiments 1-33, wherein the
administration is conducted daily for two weeks, and twice weekly thereafter.
35. The method of any one of embodiments 1-33, wherein the subject
remains ambulatory for a period of time beginning about 5 minutes after
administering the
vaginal suppository and ending about 4 hours after administering the vaginal
suppository.
36. A method for treating female sexual dysfunction, the method
comprising administering to a female subject in need thereof, a vaginal
suppository
comprising: (a) a liquid composition comprising: a therapeutically effective
amount of
estradiol; a caprylic/capric triglyceride; a non-ionic surfactant comprising
PEG-6
palmitostearate and ethylene glycol palmitostearate; and (b) a soft gelatin
capsule; wherein
the vaginal suppository includes from about 1 microgram to about 25 micrograms
of
estradiol; wherein estradiol is the only active hormone in the vaginal
suppository.
37. The method of embodiment 36, wherein the vaginal suppository does
not include a hydrophilic gel-forming bioadhesive agent in the liquid
composition.
38. The method of embodiment 36, wherein treating female sexual
dysfunction includes increasing the subject's desire, arousal, lubrication,
satisfaction, and
or/orgasms.
39. The method of embodiment 38, wherein the treatment is assessed using
the Female Sexual Function Index.
169
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
40. The method of embodiment 36, wherein the suppository is
administered to the subject daily for two weeks, and twice weekly thereafter.
41. The method of embodiment 36, wherein the suppository includes about
4 lig of estradiol.
42. The method of embodiment 36, wherein the suppository includes about
lig of estradiol.
43. The method of embodiment 36, wherein the suppository includes about
25 lig of estradiol.
44. A method for treating vaginal dryness, the method comprising
10 administering to a female subject in need thereof, a vaginal suppository
comprising: (a) a
liquid composition comprising: a therapeutically effective amount of
estradiol; a
caprylic/capric triglyceride; a non-ionic surfactant comprising PEG-6
palmitostearate and
ethylene glycol palmitostearate; and (b) a soft gelatin capsule; wherein the
vaginal
suppository includes from about 1 microgram to about 25 micrograms of
estradiol; wherein
estradiol is the only active hormone in the vaginal suppository.
45. The method of embodiment 44, wherein the vaginal suppository does
not include a hydrophilic gel-forming bioadhesive agent in the liquid
composition.
46. The method of embodiment 44, wherein the suppository is
administered to the subject daily for two weeks, and twice weekly thereafter.
47. The method of embodiment 44, wherein the suppository includes about
4 lig of estradiol.
48. The method of embodiment 44, wherein the suppository includes about
10 lig of estradiol.
49. The method of embodiment 44, wherein the suppository includes about
25 lig of estradiol.
50. The method of embodiment 44, wherein the treatment reduces vaginal
dryness within two weeks.
170
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
51. A method for treating dyspareunia, the method comprising
administering to a female subject in need thereof, a vaginal suppository
comprising: (a) a
liquid composition comprising: a therapeutically effective amount of
estradiol; a
caprylic/capric triglyceride; a non-ionic surfactant comprising PEG-6
palmitostearate and
ethylene glycol palmitostearate; and (b) a soft gelatin capsule; wherein the
vaginal
suppository includes from about 1 microgram to about 25 micrograms of
estradiol; wherein
estradiol is the only active hormone in the vaginal suppository.
52. The method of embodiment 51, wherein the vaginal suppository does
not include a hydrophilic gel-forming bioadhesive agent in the liquid
composition.
53. The method of embodiment 51, wherein the suppository is
administered to the subject daily for two weeks, and twice weekly thereafter.
54. The method of embodiment 51, wherein the suppository includes about
4 lig of estradiol.
55. The method of embodiment 51, wherein the suppository includes about
10 lig of estradiol.
56. The method of embodiment 51, wherein the suppository includes about
lig of estradiol.
57. The method of embodiment 51, wherein the treatment reduces
dyspareunia within two weeks.
20 58. A method for reestrogenizing the vagina, labia, or vulva,
the method
comprising administering to a female subject in need thereof, a vaginal
suppository
comprising: (a) a liquid composition comprising: a therapeutically effective
amount of
estradiol; a caprylic/capric triglyceride; a non-ionic surfactant comprising
PEG-6
palmitostearate and ethylene glycol palmitostearate; and (b) a soft gelatin
capsule; wherein
25 the vaginal suppository includes from about 1 microgram to about 25
micrograms of
estradiol; wherein estradiol is the only active hormone in the vaginal
suppository.
59. The method of embodiment 58, wherein the suppository is
administered to the subject daily for two weeks, and twice weekly thereafter.
171
CA 03007636 2018-06-06
WO 2017/100378
PCT/US2016/065466
60. The method of embodiment 58, wherein the suppository includes about
4 lig of estradiol.
61. The method of embodiment 58, wherein the suppository includes about
lig of estradiol.
5 62. The method of embodiment 58, wherein the suppository
includes about
25 lig of estradiol.
63. The method of embodiment 58, wherein the vaginal suppository does
not include a hydrophilic gel-forming bioadhesive agent in the liquid
composition.
10 [0491] While the pharmaceutical compositions and methods have been
described in terms
of what are presently considered to be practical and preferred embodiments, it
is to be
understood that the disclosure need not be limited to the disclosed
embodiments. It is
intended to cover various modifications and similar arrangements included
within the spirit
and scope of the claims, the scope of which should be accorded the broadest
interpretation so
as to encompass all such modifications and similar embodiments. This
disclosure includes
any and all embodiments of the following claims.
172