Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03009463 2018-06-21
[DESCRIPTION]
[Invention Title]
AUSTENITIC STEEL MATERIAL HAVING EXCELLENT HYDROGEN-
EMBRITTLEMENT RESISTANCE
[Technical Field]
The present disclosure relates to an austenitic steel
material having high hydrogen-embrittlement resistance, and
more particularly, to an austenitic steel material having
high hydrogen-embrittlement resistance and suitable for
applications such as high-pressure hydrogen gas tanks,
pipes, and transfer facilities.
[Background Art]
Many efforts have been made to reduce environmental
pollutants and greenhouse gas emissions for the prevention
of global warming and environmental pollution. Thereamong,
a technology using hydrogen as an energy source has made a
great deal of progress in recent years. Unlike fossil fuels
such as coal and petroleum, hydrogen, the most
environmentally friendly energy source, is attracting
attention as a novel energy source for the future with
little emission of pollutants. In particular, there is
great interest in hydrogen as a fuel for hydrogen vehicles
using fuel cells.
Hydrogen vehicles, including high-pressure gas
Page 1
CA 03009463 2018-06-21
containers for storing hydrogen compressed to high pressure,
are the most common type of hydrogen vehicles, and such
containers are required to have high strength for
durability against high pressure, low hydrogen permeability
for minimizing the loss of hydrogen caused by the
penetration of hydrogen, and high hydrogen-embrittlement
resistance for preventing embrittlement caused by hydrogen
permeation.
Basically, hydrogen storage containers and facilities
are aimed at reducing storage loss caused by penetration of
hydrogen, and thus face centered cubic (FCC)-structure
materials having high hydrogen permeability may be suitable
therefor. A representative FCC-structure material used for
these applications is Cr-Ni-based austenitic stainless
steel. Such austenitic stainless steels are used as
materials for high-pressure gas containers or liners and
pipes of high-pressure gas containers owing to their high
hydrogen-embrittlement resistance under high-pressure
hydrogen gas environments.
In recent years, however, hydrogen gas is used at
high pressures on the level of tens or hundreds of
megapascals (MPa) for enabling long-distance driving and
the storage of a large amount of hydrogen through a single
operation of charging hydrogen. Therefore, in the case of
Page 2
CA 03009463 2018-06-21
using austenitic stainless steels having ordinary strength,
a large thickness is required to withstand loads under
high-pressure conditions, and thus, it may be difficult to
avoid an increase in the weight and size of containers or
facilities, thereby limiting commercialization thereof.
As a technique for solving these problems, Japanese
Patent Application Laid-open Publication No. H5-98391 and
International Patent Publication No. 2014-111285 disclose a
technique of increasing the strength of austenitic
stainless steel by cold working. However, when strength is
increased by cold working, ductility and toughness decrease,
and the stability of austenite decreases, thereby causing
the formation of strain-induced martensite. Thus, this
technique is not suitable for hydrogen containers. In
addition, Korean Patent Application Laid-Open Publication
No. 10-2006-0018250 discloses a technique of securing the
stability of austenite by performing a cold working process
twice in different directions. According to the technique,
however, chromium (Cr) and nickel (Ni), expensive alloying
elements, are added in large amounts to increase the
stability of austenite, thereby incurring high costs.
In addition, Korean Patent Application Laid-Open
Publication No. 10-2011-0004491 and Korean Patent
Application Laid-Open Publication No. 10-2013-0045931
Page 3
CA 03009463 2018-06-21
disclose a technique of guaranteeing the formation of
stable austenite and thus improving the hydrogen-
embrittlement resistance of austenitic stainless steel by
replacing nickel (Ni), an expensive alloying element, with
manganese (Mn), an inexpensive alloying element. However,
since this technique still uses a large amount of an
expensive alloying element, commercialization of the
technique is limited in terms of economical aspects.
[Disclosure]
[Technical Problem]
An aspect of the present disclosure may provide an
austenitic steel material having high hydrogen-
embrittlement resistance without expensive alloying
elements.
[Technical Solution]
According to an aspect of the present disclosure, an
austenitic steel material having high hydrogen-
embrittlement resistance may include, by wt%, carbon (C):
0.1% to 0.5%, copper (Cu): 5% or less (excluding 0%),
nitrogen (N): 1% or less (excluding 0%), manganese (Mn):
[Mn]-10.7[C]+24.5, chromium (Cr): 10% or less, nickel
(Ni): 5% or less, molybdenum (Mo): 5% or less, silicon (Si):
4% or less, aluminum (Al): 5% or less, and a balance of
iron (Fe) and inevitable impurities, wherein the austenitic
Page 4
steel material has a T-E12/T-El1 ratio of 0.5 or greater,
where T-E12 is an elongation at break in a tensile test
performed under high-pressure hydrogen conditions of 25 C
and 70 MPa, and T-Ell is an elongation at break in a
tensile test performed under atmospheric conditions of 25 C
and 1 atm, . and
wherein the austenitic steel material has stacking fault
energy (SFE) defined by Formula 1 below within a range of
30 mJ/m2 or greater,
[Formula 1]
SFE(mJ/m2) = 1.6[Ni] - 1.3[Mn] + 0.06[Mn]2 - 1.7[Cr] +
0.01[Cr]2 + 15[Mo] - 5.6[Si] + 1.6[Cu] + 5.5[Al] - 60([C] +
1.2[N])'2 + 26.3([C] + 1.2[N]) ([Cr] + [Mn] + [Mo])
1/2 +
0.6{[Ni]([Cr] + [Mn])I 1/2
where each of [Ni], [Mn], [Cr], [Mo], [Si], [Cu], [Al], [C],
and [N] refers to a content (wt%) of a corresponding
element.
[Advantageous Effects]
One of various effects of the present disclosure is
that the austenitic steel material of the present
disclosure has high hydrogen-embrittlement resistance
without expensive alloying elements.
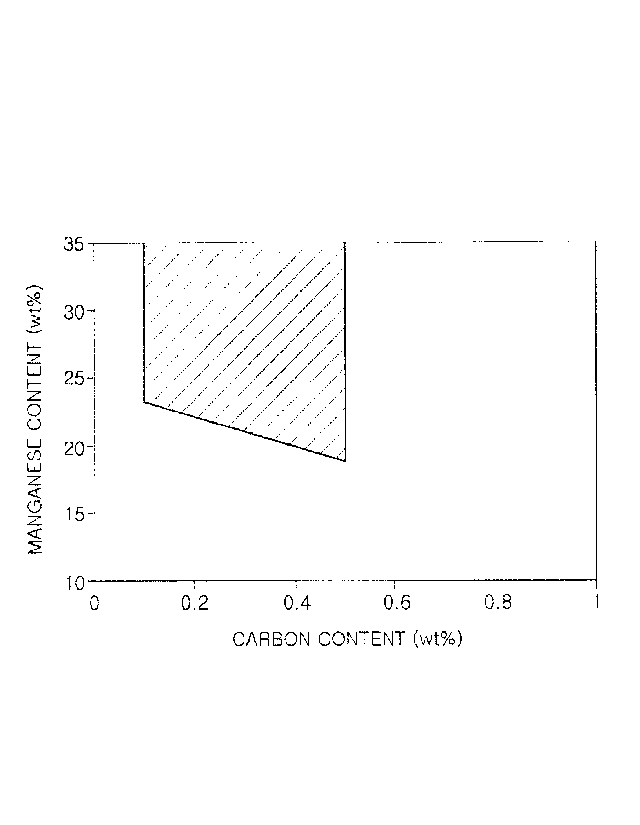
[Description of Drawings]
FIG. 1 is a graph illustrating carbon and manganese
Page 5
CA 3009463 2020-02-13
content ranges according to the present disclosure.
FIG. 2 is an image of a fracture surface of a
specimen of Inventive Example 1 after a room-temperature
tensile test.
[Best Mode]
Containers for storing and transferring hydrogen are
basically required to have low hydrogen permeability, and
thus it is needed to guarantee the formation of an FCC
structure having low hydrogen permeability in the case of
steel materials for hydrogen containers. In particular, it
is necessary to stably maintain the FCC structure in spite
Page 5a
CA 3009463 2020-02-13
CA 03009463 2018-06-21
of externally-caused deformation such as deformation caused
by plastic working or plastic deformation caused by an
external load applied during use.
Furthermore, in recent years, attempts have been
constantly made to address the above-described economical
demerits of austenitic stainless steels, existing steel
materials having high hydrogen-embrittlement resistance, by
replacing expensive nickel with inexpensive manganese and
adding carbon to stabilize austenite at room temperature.
However, in such high-carbon, high-manganese austenitic
steel materials, planar slips easily occur because partial
dislocations easily develop owing to low stacking fault
energy, and thus dislocations easily accumulate on slip
planes to result in high work hardening. In addition, the
addition of carbon for stabilizing austenite induces
dynamic strain aging and thus markedly improves work
hardening of the steel materials. Therefore, such high-
carbon, high-manganese austenitic steel materials are not
suitable for applications requiring hydrogen-embrittlement
resistance.
Thus, the inventors have tried to improve the
hydrogen-embrittlement resistance of steel materials by
properly adjusting a relationship between carbon and
manganese while relatively decreasing the content of carbon,
Page 6
CA 03009463 2018-06-21
and as a result, the inventors have invented the present
invention.
Hereinafter, an austenitic steel material having high
hydrogen-embrittlement resistance will be described in
detail, according to an aspect of the present disclosure.
First, alloying elements of the austenitic steel
material and the content ranges of the alloying elements
will be described in detail. In the following description,
the content of each element is given in wt% unless
otherwise mentioned.
Carbon (C): 0.1% to 0.5%
Carbon (C) is an element stabilizing austenite and
increasing the strength of the steel material. Particularly,
carbon (C) decreases transformation points Ms and Md at
which austenite transforms into F-martensite or a-
martensite during a cooling or processing process. If the
content of carbon (C) is insufficient, the stability of
austenite is insufficient, and austenite easily undergoes
strain-induced transformation into E-martensite or a-
martensite by external stress. Therefore, an FCC structure
may not be maintained, and thus hydrogen-embrittlement
resistance may markedly decrease. Therefore, according to
the present disclosure, it may be preferable that the
Page 7
CA 03009463 2018-06-21
,
content of carbon (C) be within the range of 0.1% or
greater, more preferably within the range of 0.15% or
greater, and even more preferably within the range of 0.2%
or greater. However, if the content of carbon (C) is
excessively high, dislocations and dynamic strain aging may
occur to result in an increase in the work hardening of the
steel material and a decrease in the hydrogen-embrittlement
resistance of the steel material, and carbides may easily
precipitate to result in a decrease in the ductility or
toughness of the steel material. Therefore, according to
the present disclosure, it may be preferable that the
content of carbon (C) be adjusted to be within the range of
0.5% or less, and more preferably within the range of 0.45%
or less.
Manganese (Mn): [Mn]-10.7[C]+24.5 (where each of [Mn]
and [C] refers to the weight percent (wt%) of a
corresponding element)
In the present disclosure, the content of manganese
(Mn) may be determined by considering a relationship with
carbon (C) and other alloying elements. FIG. 1 illustrates
a manganese content range for improving hydrogen-
embrittlement resistance by stably guaranteeing austenite
or e-martensite having low hydrogen permeability after a
Page 8
CA 03009463 2018-06-21
room-temperature tensile test. The graph of FIG. 1 shows
results that the inventors have obtained through various
experiments.
That is, to obtain a microstructure having high
hydrogen-embrittlement resistance before and after a
tensile test, the content of manganese (Mn) may preferably
be adjusted to be within the range of -10.7[C]+24.5(%) or
greater on the condition that the contents of the other
elements are within ranges proposed in the present
disclosure. If the content of manganese (Mn) is less than -
10.7[C]+24.5(%), the stability of austenite may decrease,
and thus a BCC-based microstructure may be formed by
deformation, thereby decreasing hydrogen-embrittlement
resistance.
Copper (Cu): 5% or less (excluding 0%)
Copper (Cu) stabilizes austenite guaranteeing
hydrogen-embrittlement resistance and facilitates slipping
by increasing stacking fault energy. If the content of
carbon (C) is high, since copper (Cu) has very low solid
solubility in carbides and diffuses slowly in austenite,
copper (Cu) concentrates along boundaries of carbide nuclei
formed in austenite, thereby suppressing the diffusion of
carbon (C) and effectively retarding the growth of carbides.
Page 9
CA 03009463 2018-06-21
As a result, copper (Cu) suppresses the formation of
carbides. Owing to this suppression of carbide formation,
sites to which carbon (C) diffuses are decreased, thereby
improving the hydrogen-embrittlement resistance of the
steel material and the ductility and toughness of the steel
material as well. In the present disclosure, if the content
of copper (Cu) is 0.5% or greater, this effect of
suppressing the formation of carbides may be sufficiently
obtained. However, if the content of copper (Cu) is
excessively high, the hot workability of the steel material
may deteriorate. Therefore, according to the present
disclosure, it may be preferable that the content of copper
(Cu) be adjusted to be within the range of 5% or less, and
more preferably within the range of 3.5% or less.
Nitrogen (N): 1% or less (excluding 0%)
Like carbon (C), nitrogen (N) is an element
stabilizing austenite and thus improving the toughness of
the steel material. Particularly, like carbon (C), nitrogen
(N) is very effective in improving the strength of the
steel material by the effect of solid solution
strengthening. Moreover, as illustrated in Formula 1,
nitrogen (N) is known as an element effectively increasing
stacking fault energy and thus promoting slipping. In the
Page 10
CA 03009463 2018-06-21
present disclosure, however, intended properties may be
obtained without great difficulties even when nitrogen (N)
is not added. Conversely, if the content of nitrogen (N) is
excessively high, coarse nitrides may be formed, and thus
the surface quality and properties of the steel material
may deteriorate. Thus, it may be preferable that the
content of nitrogen (N) be adjusted to be within the range
of 1% or less, and more preferably within the range of 0.5%
or less.
In addition to the above-described elements, the
austenitic steel material of the present disclosure may
further include chromium (Cr), nickel (Ni), molybdenum (Mo),
silicon (Si), and aluminum (Al).
Chromium (Cr): 10% or less
When the content of chromium (Cr) is within a proper
range, chromium (Cr) increases hydrogen-embrittlement
resistance by stabilizing austenite, and increases the
strength of the steel material dissolved in austenite.
Furthermore, chromium (Cr) is an element improving the
corrosion resistance of the steel material. In the present
disclosure, however, intended properties may be obtained
without great difficulties even when chromium (Cr) is not
added. In addition, since chromium (Cr) is a carbide
Page 11
CA 03009463 2018-06-21
forming element, if the content of chromium (Cr) is
excessively high, carbides may be formed along austenite
grain boundaries. Therefore, sites facilitating hydrogen
diffusion may be provided, and the toughness of the steel
material may decrease. Therefore, according to the present
disclosure, it may be preferable that the content of
chromium (Cr) be adjusted to be within the range of 10% or
less, and more preferably within the range of 8% or less.
Nickel (Ni): 5% or less
Nickel (Ni) is an element very effective in
stabilizing austenite. Particularly, nickel (Ni) decreases
transformation points Ms and Md at which austenite
transforms into c-martensite or a-martensite during a
cooling or processing process. Moreover, as illustrated in
Formula 1, nickel (Ni) is known as an element effectively
increasing stacking fault energy and thus promoting
slipping. In the present disclosure, however, intended
properties may he obtained without great difficulties even
when nickel (Ni) is not added. Since nickel (Ni) is an
expensive element, if the content of nickel (Ni) is
excessively high, the economical feasibility of the steel
material decreases. Therefore, according to the present
disclosure, it may be preferable that the content of nickel
Page 12
CA 03009463 2018-06-21
(Ni) be within the range of 5% or less.
Molybdenum (Mo): 5% or less
If molybdenum (Mo) is added to the steel material in
an appropriate amount, molybdenum (Mo) stabilizes austenite
and improves the hydrogen-embrittlement resistance of the
steel material by decreasing transformation points Ms and
Md at which austenite transforms into c-martensite or a-
martensite during a cooling or processing process. In
addition, molybdenum (Mo) dissolves in the steel material
and improves the strength of the steel material. In
addition, molybdenum (Mo) segregates along grain boundaries
of austenite, thereby improving the stability of grain
boundaries and decreasing the energy of grain boundaries.
As a result, molybdenum (Mo) suppresses the precipitation
of carbides along grain boundaries. Moreover, as
illustrated in Formula 1, molybdenum (Mo) is known as an
element effectively increasing stacking fault energy and
thus promoting slipping. In the present disclosure, however,
intended properties may be obtained without great
difficulties even when molybdenum (Mo) is not added. Since
molybdenum (Mo) is an expensive element, if the content of
molybdenum (Mo) is excessively high, the economical
feasibility of the steel material decreases. Therefore,
Page 13
CA 03009463 2018-06-21
according to the present disclosure, it may be preferable
that the content of molybdenum (Mo) be adjusted to be
within the range of 5% or less, and more preferably, within
the range of 4% or less.
Silicon (Si): 4% or less
Silicon (Si) improves the castability of molten steel.
In particular, silicon (Si), added to the austenitic steel
material, dissolves in the austenitic steel material and
effectively increases the strength of the austenitic steel
material. In the present disclosure, however, intended
properties may be obtained without great difficulties even
when silicon (Si) is not added. If the content of silicon
(Si) is excessively high, stacking fault energy decreases,
thereby causing partial dislocations and concentration of
stress and thus decreasing the hydrogen-embrittlement
resistance of the steel material. Therefore, according to
the present disclosure, it may be preferable that the
content of silicon (Si) be within the range of 4% or less.
Aluminum (Al): 5% or less
If aluminum (Al) is added to the steel material in an
appropriate amount, aluminum (Al) stabilizes austenite and
improves the hydrogen-embrittlement resistance of the steel
Page 14
CA 03009463 2018-06-21
material by decreasing transformation points Ms and Md at
which austenite transforms into c-martensite or cx-
martensite during a cooling or processing process. In
addition, aluminum (Al) dissolves in the steel material and
increases the strength of the steel material. In addition,
aluminum (Al) affects the mobility of carbon (C) in the
steel material and effectively suppresses the formation of
carbides, thereby increasing the toughness of the steel
material. In addition, aluminum (Al) induces cross slips by
markedly increasing stacking fault energy, and suppresses
partial dislocations and thus decreases concentration of
stress, thereby increasing hydrogen-
embrittlement
resistance. In the present disclosure, however, intended
properties may be obtained without great difficulties even
when aluminum (Al) is not added. Preferably, aluminum (Al)
may be added in an amount of 0.2% or greater so as to
further improve hydrogen-embrittlement resistance.
Conversely, if the content of aluminum (Al) is excessively
high, the castability and surface quality of steel may
deteriorate because of the formation of oxides and nitrides.
Thus, it may be preferable that the content of aluminum (Al)
be adjusted to be within the range of 5% or less.
The other element of the austenitic steel material is
iron (Fe). However, impurities of raw materials or
Page 15
CA 03009463 2018-06-21
manufacturing environments may be inevitably included in
the austenitic steel material, and such impurities may not
be removed from the austenitic steel material. Such
impurities are well-known to those of ordinary skill in the
art, and thus descriptions thereof will not be given in the
present disclosure. In addition, addition of effective
elements other than the above-described elements is not
excluded.
For example, the austenitic steel material of the
present disclosure may have stacking fault energy (SEF)
expressed by Formula 1 below within the range of 30 mJ/m2
or greater.
[Formula 1]
SFE (mJ/m2) = 1.6[Ni] - 1.3[Mn] + 0.06[Mn]2 - 1.7[Cr] +
0.01[Cr]2 + 15[Mo] - 5.6[Si] + 1.6[Cu] + 5.5[Al] - 60([C] +
1.2 [N])1/2 + 26.3([C] + 1.2[N]) ([Cr] + [Mn] + [Mo]) 1/2 +
0.6{ [Ni] + [Mn])I 1/2
(where each of [Ni], [Mn], [Cr], [No], [Si], [Cu],
[Al], [C], and [N] refers to the content (wt%) of a
corresponding element).
In general, high-manganese steels having a high
manganese content like the austenitic steel material of the
present disclosure have relatively low stacking fault
energy compared to general carbon steels and thus easily
Page 16
CA 03009463 2018-06-21
have partial dislocations, and since slipping of such
partial dislocations is limited to particular slip planes,
dislocation accumulation and stress concentration are
easily caused. Such concentration of stress facilitates
diffusion of hydrogen, and thus a phenomenon in which the
fracture strength of a material decreases because of
diffusion of hydrogen, that is, embrittlement caused by
hydrogen, is likely to occur in high-manganese steels like
the austenitic steel material of the present disclosure.
Therefore, according to the present disclosure, the
deformation behavior of the austenitic steel material is
particularly controlled by adjusting stacking fault energy
through control of alloying elements and contents thereof.
Based on results of research conducted by the inventors,
the inventors have found that if stacking fault energy
defined by Formula 1 above is adjusted to be 30 mJ/m2 or
greater, the possibility of hydrogen embrittlement is
markedly reduced.
The degree of work hardening of a steel material
caused by concentration of stress may be measured by
measuring a strain hardening rate in a tensile test. For
example, the austenitic steel material of the present
disclosure may have a strain hardening rate of 14000 N/mm2
or less in a tensile test performed under atmospheric
Page 17
CA 03009463 2018-06-21
conditions of 25 C and 1 atm. The strain hardening rate may
be calculated from true strain and true stress. If the
strain hardening rate in a tensile test is greater than
14000 N/mm2, concentration of stress caused by dislocations
is excessively high, and thus hydrogen easily diffuses and
accumulates. Thus, hydrogen embrittlement may occur.
For example, the austenitic steel material of the
present disclosure may have a tensile strength of 800 MPa
or less in a tensile test performed under atmospheric
conditions of 25 C and 1 atm. If the tensile strength of
the austenitic steel material is greater than 800 MPa,
hydrogen-embrittlement resistance may deteriorate because
of high work hardening caused by concentration of stress.
For example, the austenitic steel material of the
present disclosure may have a microstructure including
austenite in an area fraction of 95% or greater. If the
area fraction of austenite is less than 95%, intended
hydrogen-embrittlement resistance may not be obtained.
For example, the microstructure of the austenitic
steel material of the present disclosure may be austenite,
or E-martensite and austenite after a tensile test
performed under atmospheric conditions of 25 C and 1 atm.
If the microstructure of the austenitic steel material has
ferrite, intended hydrogen-embrittlement resistance may not
Page 18
CA 03009463 2018-06-21
be obtained.
The austenitic steel material of the present
disclosure may be manufactured by a general steel material
manufacturing method using a steel slab having the above-
described composition. For example, the austenitic steel
material of the present disclosure may be manufactured by
reheating, rough rolling, finish rolling, and cooling a
steel slab having the above-described composition.
In this case, the temperature of the finish rolling
process may be adjusted to be greater than a non-
crystallization temperature. If the finish rolling process
is performed at a temperature equal to or lower than the
non-crystallization temperature, the strength of the steel
material may be excessively high due to excessive formation
and accumulation of dislocations, thereby promoting
concentration of stress and fracture caused by hydrogen. In
addition, ferrite inducing hydrogen embrittlement during
tensile deformation may be early formed, and thus it may be
difficult to obtain intended hydrogen-embrittlement
resistance.
In addition, the steel material may be cooled through
an accelerated cooling process after a rolling process, so
as to suppress the formation of carbides. The reason for
this is that if carbides are formed, the elongation of the
Page 19
CA 03009463 2018-06-21
steel material decreases, and in particular, hydrogen
accumulates along boundaries between carbides and austenite,
thereby decreasing hydrogen-embrittlement resistance. Since
elements such as carbon (C), chromium (Cr), and molybdenum
(Mo) are main carbide forming elements, whether or not to
perform accelerated cooling and the rate of accelerated
cooling are determined according to the contents of such
elements as expressed by the following formula.
[Formula 2]
Cooling rate (00/3) 15[C] + [Cr] + [Mo]
(where each of [C], [Cr], and [Mo] refers to the
content (wt%) of a corresponding element).
[Mode for Invention]
Hereinafter, the present disclosure will be described
more specifically through examples. However, the following
examples are for illustrative purposes only and are not
intended to limit the scope of the present invention. The
scope of the present invention is defined by the appended
claims, and modifications and variations reasonably made
therefrom.
Slabs having compositions shown in Table 1 below were
prepared, and then rolled materials were manufactured by
hot rolling and cooling the slabs. At that time, the same
process conditions were applied to all the examples except
Page 20
CA 03009463 2018-06-21
finish rolling temperatures and cooling rates as shown in
Table 2 below. Referring to Table 2, a cooling rate in
Comparative Example 5 is not stated, and this means that
simple air cooling was performed.
Thereafter, microstructures of the rolled materials
were observed, and the fraction of austenite in each of the
rolled materials was measured. Then, a tensile test was
performed on the rolled materials under atmospheric
conditions of 25 C and 1 atm, and then the tensile strength,
strain hardening rate, elongation at break and
ferrite fraction of each of the rolled materials were
measured. Independently of this, a tensile test was
performed on the rolled materials under high-pressure
hydrogen conditions of 25 C and 70 MPa, and elongation at
break T-E12 was measured. Results thereof are shown in
Table 3 below.
[Table 1]
No. Alloying composition (wt%) DO
Mn Cu N Cr Ni Mo Si Al
(wt%)
*CE1 0.62 18.2 0.06 0.012 0.13 17.866
CE2 0.46 16 0.13 0.021 0.2 19.578
CE3 0.42 23.2 5.32 0.016 1.52 20.006
CE4 0.83 15.2 0.32 0.017 5.3 0.32 15.619
CE5 0.13 19.5 0.021 0.2 23.109
"1E1 0.41 31.8 0.015 1.72 20.113
1E2 0.29 29.8 0.35 0.022 0.86 21.397
1E3 0.42 27.3 0.51 0.022 2.08 1.51 20.006
1E4 0.28 31.2 0.022 1.08 1.75 0.35 21.504
Page 21
CA 03009463 2018-06-21
1E5 0.38 28.5 1.1 0.018 0.16 0.31 1.74
20.434
where CIO refers to -10.7C (wt%) + 24.5
*CE: Comparative Example, **IE: Inventive Example
[Table 2]
Finish rolling
No. Cooling rate (
C/sec)
temperature ( C)
Comparative Example 1 910 11.5
Comparative Example 2 870 5.6
Comparative Example 3 865 7.2
Comparative Example 4 892 15.2
Comparative Example 5 856 -
Inventive Example 1 912 15.4
Inventive Example 2 905 12.7
Inventive Example 3 922 13.6
Inventive Example 4 915 20.4
Inventive Example 5 932 15.6
[Table 3]
No. Stacking Before After tensile test elongation
fault tensile at break
energy test ratio
(mJ/m2) Austenite Ferrite Tensile Strain
fraction fraction strength hardening
(area%) (area%) (MPa) rate
(N/mm2)
*CE1 19.7 100 0 1015 18653 0.1
CE2 5.5 96 12 948 19320 0.13
CE3 34.9 100 Not measured (cracks)
0E4 30.0 92.5 0 1135 21396 0.07
CE5 -5.1 62 28 832 17504 0.08
**IE1 53.0 100 0 760 9854 0.75
1E2 31.5 100 0 658 4850 0.97
1E3 42.9 100 0 715 6512 0.91
1E4 33.3 100 0 672 4385 0.96
1E5 42.2 100 0 675 5214 0.92
Page 22
CA 03009463 2018-06-21
*CE: Comparative Example, **IE: Inventive Example
Referring to Table 3, after tensile deformation at
room temperature, each of Inventive Examples 1 to 5
satisfying the composition ranges proposed in the present
disclosure had stable austenite without ferrite, a low
strain hardening rate, and low tensile strength. In
particular, since Inventive Examples 1 to 5 were rolled at
a finish rolling temperature higher than a non-
crystallization temperature, the formation and accumulation
of dislocations were suppressed, and since Inventive
Examples 1 to 5 were cooled at a cooling rate satisfying
the range proposed in the present disclosure, the formation
of carbides was effectively suppressed. As a result,
austenitic steel materials having high hydrogen-
embrittlement resistance, that is, having a high elongation
at break ratio, could be obtained.
However, Comparative Example 1 had carbon and
manganese contents outside the ranges proposed in the
present disclosure and particularly, a high strain
hardening rate because of an excessively high carbon
content, and thus, the elongation at break ratio of
Comparative Example 1 was low. That is, Comparative Example
1 had poor hydrogen-embrittlement resistance.
Page 23
=
CA 03009463 2018-06-21
Particularly, in Comparative Example 2 having a
manganese content outside of the range proposed in the
present disclosure, austenite was unstable, and thus
ferrite susceptible to hydrogen embrittlement was formed
after tensile deformation. That is, Comparative Example 2
had poor hydrogen-embrittlement resistance.
In Comparative Example 3 having carbon and manganese
contents and stacking fault energy within the ranges
proposed in the present disclosure but a copper content
greater than the range proposed in the present disclosure,
cracks were formed in the rolled material, and thus a
normal specimen could not obtained.
Since Comparative Example 4 had a carbon content
greater than the range proposed in the present disclosure,
Comparative Example 4 had a high strain hardening rate and
carbides excessively precipitated along austenite grain
boundaries, and thus the hydrogen-embrittlement resistance
of Comparative Example 4 was poor.
In addition, since Comparative Example 5 had a
manganese content outside the range proposed in the present
disclosure, an intended microstructure could not be
obtained, and thus the hydrogen-embrittlement resistance of
Comparative Example 5 was poor.
FIG. 2 is an image of a fracture surface of a
Page 24
CA 03009463 2018-06-21
specimen of Inventive Example 1 after the room-temperature
tensile test. Referring to FIG. 2, fracture occurred in a
dimple type which is typical of ductile fracture.
While exemplary embodiments have been shown and
described above, it will be apparent to those skilled in
the art that modifications and other embodiments could be
made therefrom. That is, such modifications and other
embodiments could be made without departing from the scope
of the present invention as defined by the appended claims.
Page 25