Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03014598 2018-08-14
WO 2017/142579 PCT/US2016/036301
TITLE
RAPID EXCHANGE SHEATHLESS PREDILATATION ANGIOPLASTY AND STENT
DEPLOYMENT CATHETER
CROSS-REFERENCE TO RELATED APPLICATION
The present application is a Continuation-in-Part of U.S. Patent Application
Serial No. 15/045,833, filed on February 17, 2016, which is incorporated
herein by
reference in its entirety.
FIELD OF THE INVENTION
The present invention relates to a unitary catheter assembly that is used to
predilate a blockage within a coronary artery and to deploy a stent across the
blockage.
BACKGROUND OF THE INVENTION
Percutaneous coronary intervention ("PCI"), or "angioplasty", is an invasive
procedure that is used to open a blockage in a coronary artery, that is, an
artery
that provides blood to the heart. In a PCI procedure, a cardiologist inserts a
catheter into an artery in the upper arm or thigh of a patient and guides the
catheter through the arteries to the affected coronary artery. With the
catheter in
place, the doctor threads a guide wire across the blockage. After the wire is
across
the blockage and positioned distal to the blockage, the cardiologist then
advances
the catheter with a deflated balloon. The balloon is inflated to dilate the
blockage
to make enough room for the insertion of a second catheter with its own
balloon
and stent. After dilating the blockage, the balloon is deflated and the first
catheter
is removed proximally.
At this point in the procedure, complications can occur. For example,
coronary dissections or ruptures in the wall of the artery can occur and shut
down
blood flow. If the guide wire, which is in position across the blockage, is
lost or
pulled back proximal of the blockage during the first catheter exchange, such
an
occurrence can result in a heart attack if rewiring the artery is not possible
after
breakage of the wall.
Assuming that such complications do not occur, the second catheter with
another balloon and a stent or metal mesh surrounding the balloon is advanced
1
CA 03014598 2018-08-14
WO 2017/142579 PCT/US2016/036301
distally along the guide wire to the area of the blockage. The second balloon
is
inflated, which expands the stent and completely opens the blockage. The
second
balloon is then deflated, leaving the stent in place, and the second catheter
is then
removed.
An improved device for performing the above procedure without requiring
the insertion and removal of two separate catheters is required.
BRIEF SUMMARY OF THE INVENTION
Briefly, the present invention provides a coronary predilatation and stent
deployment catheter assembly that includes a single sheathless unitary
catheter
body having a proximal end and a distal end, a predilatation balloon located
at the
distal end of the body, and a stent inflation balloon located along the body a
distance of between about 5 millimeters and about 15 millimeters proximally of
the
predilatation balloon. An expandable stent is disposed over the stent balloon.
A
predilatation balloon inflation connection is located proximally of the stent
inflation
balloon and in fluid communication with the predilatation balloon through a
predilatation inflation lumen. A stent balloon inflation connection is located
proximally of the stent inflation balloon and in fluid communication with the
stent
inflation balloon through a stent balloon inflation lumen. At least one
radiopaque
marker is disposed on the predilatation balloon and at least one radiopaque
marker
is disposed on the stent inflation balloon. A guidewire lumen extends through
the
body distal of the stent balloon inflation connection about 25-30 centimeters
from
the distal end of the body and extending through the body to the distal end of
the
body.
Further, the present invention provides a sheathless catheter assembly that
includes a single unitary catheter body having a proximal end and a distal
end, a
first balloon located proximally of the distal end, and a second balloon
located a
distance of between about 5 millimeters and about 15 millimeters proximally of
the
first balloon. A balloon-expandable stent is disposed over the second balloon.
A
first balloon inflation connection is located proximally of the second balloon
and in
fluid communication with the first balloon though a first inflation lumen and
a
second balloon inflation connection is located proximally of the second
balloon and
in fluid communication with the second balloon through a second inflation
lumen.
At least one radiopaque marker is disposed on the first balloon and at least
one
radiopaque marker is disposed on the second balloon. A guide wire lumen having
2
CA 03014598 2018-08-14
WO 2017/142579 PCT/US2016/036301
a lumen proximal end extends through the catheter body distal of the first
balloon
inflation connection at about 25-30 centimeters from the distal end of the
body and
a lumen distal end exiting the catheter body distally of the first balloon. A
guide
wire extends through the guide wire lumen.
Additionally, the present invention provides a coronary predilatation and
stent deployment catheter assembly comprising a single sheathless unitary
catheter body having a proximal end and a distal end, a predilatation balloon
located at the distal end of the body, and a predilatation balloon connection
located
proximally of the predilatation balloon. A predilatation inflation lumen
provides
fluid communication between the predilatation balloon and the predilatation
balloon
connection. At least one radiopaque marker is disposed on the predilatation
balloon. A stent balloon is located along the body proximally of the
predilatation
balloon and a stent balloon inflation connection is located proximally of the
stent
balloon. A stent balloon inflation lumen provides fluid communication between
the
stent balloon and the stent balloon inflation connection and at least one
radiopaque
marker is disposed on the stent balloon. An expandable stent is disposed over
the
balloon. A guide wire lumen extends through the body between a lumen proximal
end, distal of the stent balloon connection at about 25-30 centimeters from
the
distal end of the body, and a lumen distal end, distal of the predilatation
balloon.
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings, which are incorporated herein and constitute
part of this specification, illustrate the presently preferred embodiments of
the
invention, and, together with the general description given above and the
detailed
description given below, serve to explain the features of the invention. In
the
drawings:
FIG. 1 is a side elevational view of a sheathless catheter assembly according
to a first embodiment of the present invention;
FIG. 2 is a sectional view of the sheathless catheter assembly of FIG. 1,
taken along lines 2-2 of FIG. 1;
FIG. 3 is a sectional view of the sheathless catheter assembly of FIG. 1,
taken along lines 3-3 of FIG. 1;
FIG. 4 is a sectional view of the sheathless catheter assembly of FIG. 1,
taken along lines 4-4 of FIG. 1;
3
CA 03014598 2018-08-14
WO 2017/142579 PCT/US2016/036301
FIG. 5 is a sectional view of the sheathless catheter assembly of FIG. 1,
taken along lines 5-5 of FIG. 1;
FIG. 6 is a flow chart illustration an exemplary operation of the sheathless
catheter assembly of FIG. 1;
FIG. 7 is a side elevational view, in section, of a coronary artery showing a
guide wire being passed through a blockage in the artery;
FIG. 8 is a side elevational view, in section, of the coronary artery of FIG.
8,
with a predilatation balloon of the sheathless catheter assembly of FIG. 1
inflated
at the site of the blockage;
FIG. 9 is a side elevational view, in section, of the coronary artery of FIG.
8,
with a stent inflation balloon of the sheathless catheter assembly of FIG. 1
inflated
to expand a stent at the site of the blockage;
FIG. 10 is a side elevational view of a valvuloplasty catheter assembly with a
self-expanding stent valve according to a second embodiment of the present
invention;
FIG. 11 is a sectional view of the valvuloplasty catheter assembly of FIG. 10,
taken along lines 11-11 of FIG. 10;
FIG. 12 is a sectional view of the valvuloplasty catheter assembly of FIG. 10,
taken along lines 12-12 of FIG. 10;
FIG. 13 is a sectional view of the valvuloplasty catheter assembly of FIG. 10,
taken along lines 13-13 of FIG. 10;
FIG. 14 is a front elevational view of a valve used with the catheter
assembly of FIG. 10 after valve deployment;
FIG. 15 is a side elevational view of the valvuloplasty catheter assembly
shown in FIG. 10, with a dilatation balloon expanded across a heart valve;
FIG. 16 is a side elevational view of the valvuloplasty catheter assembly
shown in FIG. 10, with a sheathed valve located across the heart valve;
FIG. 17 is an enlarged elevational view of the valvuloplasty catheter
assembly shown in FIG. 10, with the sheath retracted and the valve expanded
across the heart valve;
FIG. 18 is a side elevational view of a valvuloplasty catheter assembly with a
balloon expandable stent valve according to a third embodiment of the present
invention;
FIG. 19 is is a side elevational view of a sheathless catheter assembly
according to a third embodiment of the present invention;
4
CA 03014598 2018-08-14
WO 2017/142579
PCT/US2016/036301
FIG. 20 is a sectional view of the sheathless catheter assembly of FIG. 19,
taken along lines 20-20 of FIG. 19;
FIG. 21 is a sectional view of the sheathless catheter assembly of FIG. 19,
taken along lines 21-21 of FIG. 19;
FIG. 22 is a sectional view of the sheathless catheter assembly of FIG. 19,
taken along lines 22-22 of FIG. 19;
FIG. 23 is a sectional view of the sheathless catheter assembly of FIG. 19,
taken along lines 23-23 of FIG. 19;
FIG. 24 is a sectional view of the sheathless catheter assembly of FIG. 19,
taken along lines 24-24 of FIG. 19;
FIG. 25 is a sectional view of the sheathless catheter assembly of FIG. 19,
taken along lines 25-25 of FIG. 19;
FIG. 26 is a side elevational view, in section, of the coronary artery of FIG.
7, with a predilatation balloon of the sheathless catheter assembly of FIG. 19
inflated at the site of the blockage; and
FIG. 27 is a side elevational view, in section, of the coronary artery of FIG.
7, with a stent inflation balloon of the sheathless catheter assembly of FIG.
19
inflated to expand a stent at the site of the blockage.
DETAILED DESCRIPTION OF THE INVENTION
In the drawings, like numerals indicate like elements throughout. Certain
terminology is used herein for convenience only and is not to be taken as a
limitation on the present invention. The terminology includes the words
specifically
mentioned, derivatives thereof and words of similar import. As used herein,
the
term "fluid" can mean and material that flows, including a liquid or a gas.
The
term "proximal" defines a location closer to the inserting physician and the
term
"distal" defines a location farther from the inserting physician. The term
"about" is
interpreted to mean a range of + 100/0 of the listed value.
The embodiments illustrated below are not intended to be exhaustive or to
limit the invention to the precise form disclosed. These embodiments are
chosen
and described to best explain the principle of the invention and its
application and
practical use and to enable others skilled in the art to best utilize the
invention.
Reference herein to "one embodiment" or "an embodiment" means that a
particular feature, structure, or characteristic described in connection with
the
embodiment can be included in at least one embodiment of the invention. The
5
CA 03014598 2018-08-14
WO 2017/142579 PCT/US2016/036301
appearances of the phrase "in one embodiment" in various places in the
specification are not necessarily all referring to the same embodiment, nor
are
separate or alternative embodiments necessarily mutually exclusive of other
embodiments. The same applies to the term "implementation."
As used in this application, the word "exemplary" is used herein to mean
serving as an example, instance, or illustration. Any aspect or design
described
herein as "exemplary" is not necessarily to be construed as preferred or
advantageous over other aspects or designs. Rather, use of the word exemplary
is
intended to present concepts in a concrete fashion.
Additionally, the term "or" is intended to mean an inclusive "or" rather than
an exclusive "or". That is, unless specified otherwise, or clear from context,
"X
employs A or B" is intended to mean any of the natural inclusive permutations.
That is, if X employs A; X employs B; or X employs both A and B, then "X
employs
A or B" is satisfied under any of the foregoing instances. In addition, the
articles
"a" and "an" as used in this application and the appended claims should
generally
be construed to mean "one or more" unless specified otherwise or clear from
context to be directed to a singular form.
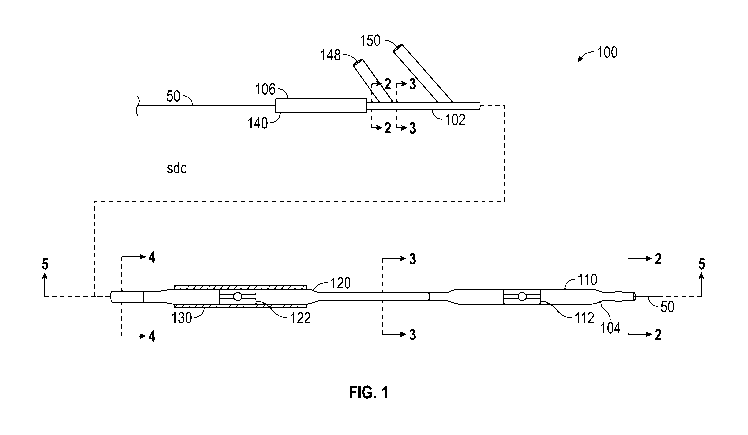
Referring to FIGS. 1-5, a first exemplary embodiment of a catheter assembly
100 according to the present invention is shown. Catheter assembly 100 is used
to
open up blockages within coronary arteries.
Catheter assembly 100 is specifically designed for use within narrow
coronary arteries that have an inside diameter of typically 6 French or less.
The
fact that catheter assembly 100 is sheathless allows catheter assembly 100 to
be
inserted into such narrow arteries. Sheathed catheters are too wide in
diameter to
.. fit into these arteries, given the additional width of the sheath itself.
Catheter assembly 100 has a unitary catheter body 102 that incorporates a
predilatation balloon 110 at a distal end 104 of body 102 and a combination
stent
balloon 120 and stent 130 are located proximally of predilatation balloon 110.
In
an exemplary embodiment, predilatation balloon 110 has a deflated diameter of
about 2.5 millimeters and a length of about 15 millimeters. Also, stent
balloon 110
can include a radiopaque marker 112 disposed on an exterior thereof to allow
for
imaging and locating stent balloon 110 within a blood vessel 52 (shown in FIG.
8)
during an angioplasty procedure.
In an exemplary embodiment, stent balloon 120 and stent 130 are located
between about 10 millimeters and about 15 millimeters proximally from
6
CA 03014598 2018-08-14
WO 2017/142579 PCT/US2016/036301
predilatation balloon 110. In an exemplary embodiment, catheter body 102 can
be
constructed from polytetrafluoroethylene, although those skilled in the art
will
recognize that catheter body 102 can be constructed from other material.
Further,
each of predilatation balloon 110 and stent balloon 120 inflate upon
introduction of
.. an inflation fluid therein, and contract toward their original size upon
release or
withdrawal of the inflation fluid from inside each of predilatation balloon
110 and
stent balloon 120.
Catheter assembly 100 also includes a proximal end 106. As shown in FIG.
2, catheter assembly 100 includes a guide wire lumen 108 that extends from
proximal end 106, through catheter body 102, to distal end 104. Guide wire
lumen
108 is sized to allow a guide wire 50 to extend fully therethrough between
proximal
end 102 and distal end 104.
A predilatation balloon inflation connection 148 is located distally of
proximal
end 106. Predilatation balloon inflation connection 148 is releasably
connectable to
an inflation source (not shown) that provides an inflation fluid such as, for
example, saline, to inflate predilatation balloon 110. As shown in FIGS. 3 and
5, a
predilatation inflation lumen 149 provides fluid communication between
predilatation balloon inflation connection 148 and predilatation balloon 110.
Predilatation inflation lumen 149 extends through stent balloon 120.
A stent balloon inflation connection 150 is located distally of predilatation
balloon inflation connection 148. While stent balloon inflation connection 150
is
shown as being located distally of predilatation balloon inflation connection
148,
those skilled in the art will recognize that stent balloon inflation
connection 150 can
be located proximally of predilatation balloon inflation connection 148
without
departing from the scope of the present invention.
Stent balloon inflation connection 150 is releasably connectable to an
inflation source (not shown) that provides an inflation fluid such as, for
example,
saline, to inflate stent balloon 120. The same fluid source that is used to
inflate
predilatation balloon 110 can be used to inflate stent balloon 120. As shown
in
FIGS. 4 and 5, a stent balloon inflation lumen 152 provides fluid
communication
between stent balloon inflation connection 150 and stent balloon 120.
Referring
back to FIG. 1, stent balloon 120 includes at least one radiopaque marker 122
that
allows the treating physician to locate stent balloon 120 within blood vessel
52.
Stent 130 is an expandable stent as is well known in the art. Stent 130 is
not self-expanding, but is expanded by the inflation of stent balloon 120.
Stent
7
CA 03014598 2018-08-14
WO 2017/142579 PCT/US2016/036301
130 remains expanded after stent balloon 120 is deflated. Further, in an
exemplary embodiment, stent 130 has an expanded size of customarily known,
industry standard, and well-used coronary stents within typical ranges of
between
about 2.5 millimeters and about 4 millimeters in diameter and between about 12
.. millimeters and about 33 millimeters in length. Additionally, in an
exemplary
embodiment, stent 130 does not include a graft, although those skilled in the
art
will recognize that a graft may be utilized with stent 130.
To use catheter assembly 100, and as explained in flowchart 600 of FIG. 6,
in step 602, guide wire 50 is inserted into the patient's blood vessel 52,
such as,
for example, through a femoral artery, and guided into the coronary artery
that
has a blockage 54 to be cleared using known methods, as shown in FIG. 7. After
guide wire 50 is in place such that a portion of guide wire 50 extends
distally of
blockage 54, in step 604, catheter assembly 100 is inserted over guide wire 50
by
inserting a distal end of guide wire 50 into guide wire lumen 108 in proximal
end
104 of catheter body 102.
In step 606, catheter 100 is advanced distally along guide wire 50 until
predilatation balloon 110 is located within blockage 54, as shown in FIG. 8.
The
location of predilatation balloon 110 is determined by observing the location
of
radiopaque marker 112 within blood vessel 52 using known techniques.
Predilatation balloon 110 is then inflated in step 608 by connecting
predilatation
balloon inflation connection 148 to a source of inflation fluid (not shown),
and
pumping the inflation fluid through predilatation balloon inflation connection
148
and predilatation inflation lumen 149 to predilatation balloon 110 to inflate
predilatation balloon 110 and open up blood vessel 52, as shown in FIG. 8.
After predilatation balloon 110 is fully expanded, and blockage 54 is opened
up, in step 610, the fluid is released from predilatation balloon 110,
allowing
predilatation balloon 110 to contract toward its original size. In step 612,
catheter
assembly 100 is advanced distally in blood vessel 52 until stent balloon 120
and
stent 130 are located within blockage 54. Stent balloon 120 is located within
blood
vessel 52 by observing radiopaque markings 122 on stent balloon 120.
In step 614, stent balloon 120 is then inflated by connecting stent balloon
inflation connection 150 to a source of inflation fluid (not shown), and
pumping the
inflation fluid through stent balloon inflation connection 150 and stent
balloon
inflation lumen 152 to stent balloon 120 to inflate stent balloon 120 and
expand
stent 130, as shown in FIG. 9. In step 616, the fluid is released from stent
8
CA 03014598 2018-08-14
WO 2017/142579 PCT/US2016/036301
balloon 120, allowing stent balloon 120 to contract toward its original size,
while
leaving stent 130 in its expanded condition. In step 618, catheter assembly
100 is
withdrawn proximally through blood vessel 52 and removed.
The inventive catheter assembly and method of the present invention
obviates the need for two or more catheters, along with several catheter
exchanges or manipulations to perform the method. This in turn decreases the
chance of losing the position of the guide wire during the catheter balloon
extraction. Further, increased pushability and turgor of the inventive
assembly
may improve the ease of advancing the catheter through calcific and tortuous
arteries, especially when part of the inventive catheter assembly is already
distally
past the blockage.
Additionally, the lower cost of a single catheter, along with less time and
radiation exposure required for catheter laboratory (Cath Lab) personnel may
significantly decrease the cost of an angioplasty procedure. Further, patient
safety
.. and convenience may be enhanced by eliminating exchanges of catheters over
the
guide wire.
An alternative embodiment of a catheter assembly 200 according to the
present invention is shown in FIGS. 10-16. Catheter assembly 200 is used to
install
a stent valve as a replacement for a damaged or calcified coronary valve.
Catheter
assembly 200 incorporates a valvuloplasty balloon and a self-expanding stent
valve
in the same assembly, eliminating the need for separate insertions of a
valvuloplasty balloon and a stent valve by separate catheters or other
insertion
devices. Catheter assembly 200 reduces the amount of catheters required to
perform a stent valve insertion procedure, reducing the risk of injury to the
patient.
Catheter assembly 200 has a unitary catheter body 202 that incorporates a
valvuloplasty balloon 210 at a distal end 204 of body 202 and a self-expanding
stent valve 230 located proximally of valvuloplasty balloon 210. In an
exemplary
embodiment, valvuloplasty balloon 210 has a deflated diameter of between about
18 millimeters and about 25 millimeters and a length of about 5 centimeters.
Also,
valvuloplasty balloon 210 can include a radiopaque marker 212 disposed on an
exterior thereof to allow for imaging and locating valvuloplasty balloon 210
within a
chamber of a heart 62, such as a left ventricle, during a radiographic or
fluoroscopic procedure.
In an exemplary embodiment, stent valve 230 is located a length "L" of
about 5 millimeters proximally from valvuloplasty balloon 210. In an exemplary
9
CA 03014598 2018-08-14
WO 2017/142579
PCT/US2016/036301
embodiment, the distance between distal end 204 and stent valve 230 is
minimized
because catheter assembly 200 is at least partially inserted into a patient's
heart to
deploy stent valve 230 across a heart valve 64, leaving little room for distal
end
204 in the heart 62.
In an exemplary embodiment, catheter body 202 can be constructed from
polytetrafluoroethylene, although those skilled in the art will recognize that
catheter body 202 can be constructed from other material. Further,
valvuloplasty
balloon 210 inflates upon introduction of an inflation fluid therein, and
contract
toward its original size upon release or withdrawal of the inflation fluid
from inside
of valvuloplasty balloon 210.
Catheter assembly 200 also includes a proximal end 206. As shown in FIG.
11, catheter assembly 200 includes a guide wire lumen 208 that extends from
proximal end 206, through catheter body 202, to distal end 204. Guide wire
lumen
208 is sized to allow a guide wire 50 to extend fully therethrough between
proximal
.. end 202 and distal end 204.
A valvuloplasty balloon inflation connection 248 is located distally of
proximal end 206. Valvuloplasty balloon inflation connection 248 is releasably
connectable to an inflation source (not shown) that provides an inflation
fluid such
as, for example, saline, to inflate valvuloplasty balloon 210. As shown in
FIGS. 11-
13, a valvuloplasty inflation lumen 249 extends partially through catheter
body 202
and provides fluid communication between valvuloplasty balloon inflation
connection 248 and valvuloplasty balloon 210.
In an exemplary embodiment, as shown in FIG. 14, stent valve 230 is a
tricuspid one-way valve having valve flaps 232a, 232b, 232c that are operable
.. between a closed position in which blood flow is restricted from passing
through
stent valve 230, and an open position in which blood flow is allowed to pass
through stent valve 230.
Referring back to FIGS. 10, 12, and 13, stent sheath 250 is disposed at least
partially over catheter body 202, including over stent valve 230. Stent sheath
250
extends proximally toward predilatation balloon inflation connection 248, and
can
optionally include a handle 252 that allow stent sheath 250 to be slid
proximally
along catheter body 202 to release stent valve 230. In the event that stent
valve
230 needs to be repositioned while stent valve 230 is still disposed over
catheter
body 202, sheath 250 can be slid distally with respect to catheter body 202 to
compress stent valve 230 within sheath 250.
CA 03014598 2018-08-14
WO 2017/142579 PCT/US2016/036301
To use catheter assembly 200, as shown in FIG. 10, using known methods,
guide wire 50 is inserted into the patient's blood vessel, such as, for
example,
through a femoral artery, and guided toward heart 62 to the heart valve 64
that
has a blockage to be cleared. After guide wire 50 is in place such that a
portion of
guide wire 50 extends distally of heart valve 64, catheter assembly 200 is
inserted
over guide wire 50 by inserting a distal end of guide wire 50 into guide wire
lumen
208 in proximal end 204 of catheter body 202.
Catheter assembly 200 is then advanced distally along guide wire 50 until
valvuloplasty balloon 210 is located across heart valve 64, as shown in FIG.
10.
The location of valvuloplasty balloon 210 is determined by observing the
location of
radiopaque marker 212 within heart 62 using known techniques. Valvuloplasty
balloon 210 is then inflated connecting valvuloplasty balloon inflation
connection
248 to a source of inflation fluid (not shown), and pumping the inflation
fluid
through valvuloplasty balloon inflation connection 248 and valvuloplasty
inflation
.. lumen 249 to valvuloplasty balloon 210 to inflate valvuloplasty balloon 210
and
open up heart valve 64, as shown in FIG. 15.
After valvuloplasty balloon 210 is fully expanded, and valve 64 is opened up,
the fluid is released from valvuloplasty balloon 210, allowing valvuloplasty
balloon
210 to contract toward its original size. Catheter assembly 200 is next
advanced
distally into heart 62 until stent valve 230 is located within across valve
64, as
shown in FIG. 16. Stent valve 230 is advanced across valve 64 by
radioscopically
observing stent valve 230, which is radiopaque.
As shown in FIG. 17, valve 230 is expanded by withdrawing stent sheath
250 proximally in the direction of arrows "F", allowing stent valve 230 to
self-
expand across valve 64. Next, catheter assembly 200 is withdrawn proximally
through the blood vessel and removed.
Alternatively, instead of a balloon expanding stent 130, a catheter assembly
300 incorporates a balloon expandable stent valve 330. In catheter assembly
300,
stent valve 330 is advanced to heart valve 64 in the same manner as described
with respect to stent valve 230 above. However, instead of sliding sheath 250
proximally, stent valve balloon 120 is inflated to expand stent valve 330
across
heart valve 64. In this embodiment, stent valve balloon 120 is located about 5
millimeters proximal of valvuloplasty balloon 310.
An alternative embodiment of a catheter assembly 400 according to the
present invention is shown in FIGS. 19-27. Catheter assembly 400 is used to
open
11
CA 03014598 2018-08-14
WO 2017/142579
PCT/US2016/036301
up blockages within coronary arteries and is similar to catheter assembly 100,
but,
instead of having a guide wire lumen 408 extending wholly through a catheter
body 402 between a distal end 404 and a proximal end 406, catheter assembly
400 can be a "rapid exchange" catheter in which guide wire lumen 408 extends
through catheter body 402 distally of a stent balloon inflation connection 450
and
extends through catheter body 402 to distal end 404 of catheter body 402.
An advantage of catheter assembly 400 is that catheter assembly 400 is
wholly self-contained, meaning that no other instruments such as sheaths or
other
catheters are required to be used with catheter 400. As such, catheter
assembly
400 is a "standalone" device.
Similar to catheter assembly 100, catheter assembly 400 is specifically
designed for use within narrow coronary arteries that have an inside diameter
of
typically 6 French or less. The fact that catheter assembly 400 is sheathless
allows
catheter assembly 400 to be inserted into such narrow arteries. Sheathed
.. catheters are too wide in diameter to fit into these arteries, given the
additional
width of the sheath itself.
Catheter assembly 400 has a unitary catheter body 402 that incorporates a
predilatation balloon 410 at a distal end 404 of body 402 and a combination
stent
balloon 420 and stent 430 are located proximally of predilatation balloon 410.
In
an exemplary embodiment, predilatation balloon 410 has a deflated diameter of
about 2.5 millimeters and a length of about 15 millimeters. Also, stent
balloon 410
can include a radiopaque marker 412 disposed on an exterior thereof to allow
for
imaging and locating stent balloon 410 within a blood vessel 52 (shown in FIG.
26)
during an angioplasty procedure.
In an exemplary embodiment, stent balloon 420 and stent 430 are located
between about 5 millimeters and about 15 millimeters proximally from
predilatation balloon 410. More specifically, stent balloon 420 and stent 430
are
located about 5 millimeters proximally from predilatation balloon 410. The
small
distance of about 5 millimeters can be important when advancing catheter
assembly 400 through a blood vessel 52, particularly a small coronary vessel
that
may not have much distance between a blockage that is being treated and a
patient's heart.
In an exemplary embodiment, catheter body 402 can be constructed from
polytetrafluoroethylene, although those skilled in the art will recognize that
catheter body 402 can be constructed from other material. Further, each of
12
CA 03014598 2018-08-14
WO 2017/142579 PCT/US2016/036301
predilatation balloon 410 and stent balloon 420 inflate upon introduction of
an
inflation fluid therein, and contract toward their original size upon release
or
withdrawal of the inflation fluid from inside each of predilatation balloon
410 and
stent balloon 420.
Catheter assembly 400 also includes a proximal end 406. A predilatation
balloon inflation connection 448 is located at proximal end 406. Predilatation
balloon inflation connection 448 is releasably connectable to an inflation
source
(not shown) that provides an inflation fluid such as, for example, saline, to
inflate
predilatation balloon 410. As shown in FIGS. 20-23 and 25, a predilatation
inflation lumen 449 provides fluid communication between predilatation balloon
inflation connection 448 and predilatation balloon 410. Predilatation
inflation
lumen 449 extends through stent balloon 420.
A stent balloon inflation connection 450 is located distally of predilatation
balloon inflation connection 448. While stent balloon inflation connection 450
is
shown as being located distally of predilatation balloon inflation connection
448,
those skilled in the art will recognize that stent balloon inflation
connection 450 can
be located proximally of predilatation balloon inflation connection 448
without
departing from the scope of the present invention.
Stent balloon inflation connection 450 is releasably connectable to an
inflation source (not shown) that provides an inflation fluid such as, for
example,
saline, to inflate stent balloon 420. The same fluid source that is used to
inflate
predilatation balloon 410 can be used to inflate stent balloon 420. As shown
in
FIGS. 21, 22, and 25, a stent balloon inflation lumen 452 provides fluid
communication between stent balloon inflation connection 450 and stent balloon
420. Referring back to FIG. 19, stent balloon 420 includes at least one
radiopaque
marker 422 that allows the treating physician to locate stent balloon 420
within
blood vessel 52 (shown in FIG. 27).
Stent 430 is an expandable stent as is well known in the art. Stent 430 is
not self-expanding, but is expanded by the inflation of stent balloon 420.
Stent
430 remains expanded after stent balloon 420 is deflated. Further, in an
exemplary embodiment, stent 430 has an expanded size of customarily known,
industry standard, and well-used coronary stents within typical ranges of
between
about 2.5 millimeters and about 4 millimeters in diameter and between about 12
millimeters and about 33 millimeters in length. Additionally, in an exemplary
13
CA 03014598 2018-08-14
WO 2017/142579 PCT/US2016/036301
embodiment, stent 430 does not include a graft, although those skilled in the
art
will recognize that a graft may be utilized with stent 430.
As shown in FIGS. 22-25, catheter assembly 400 includes a guide wire
lumen 408 that extends through body 402 from an opening 409 distal of stent
balloon inflation connection 450 and extending through the body to distal end
404
of body 402. In an exemplary embodiment, opening 409 is about 24.5 cm from
distal end 404 of catheter body 402.
Guide wire lumen 408 is sized to allow a guide wire 50 to extend partially
through body 402, between opening 409 and distal end 404. The location of
opening 409 distally of stent balloon inflation connection 450 allows for the
rapid
exchange of catheter 400 over guide wire 50. A benefit of the rapid exchange
of
catheter 400 is that such exchange can be performed faster, and importantly,
safer, since a significant portioh of catheter assembly 400 can be pulled back
by
merely holding the guide wire in place. Once the catheter shaft is reached,
the
exchange is performed under fluoroscopy in a known way (pushing guide wire 50
and pulling catheter assembly 400) until distal tip 406 of catheter assembly
400 is
removed from blood vessel 52.
Guide wire 50 is inserted into blood vessel 52 in a known manner such that
guide wire extends on either side of a blockage 54, as shown in FIG. 7. The
insertion and operation of catheter assembly 400 is the same as the insertion
and
operation of catheter assembly 100 as described above, as well as in FIG. 6,
substituting element numbers beginning with "1" with element numbers beginning
with "4". However, instead of guide wire 50 exiting catheter body 402 at
proximal
end 406, guide wire 50 exits body 402 through opening 409 distal of stent
balloon
inflation connection 450, as is shown in FIG. 19.It will be appreciated by
those
skilled in the art that changes could be made to the embodiments described
above
without departing from the broad inventive concept thereof. It is understood,
therefore, that this invention is not limited to the particular embodiments
disclosed, but it is intended to cover modifications within the spirit and
scope of the
present invention as defined by the appended claims.
14