Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
METHODS FOR TREATMENT OF OPIOID DEPENDENCY AND
WITHDRAWAL USING NORIBOGAINE
FIELD OF THE INVENTION
[0001] This disclosure is directed to a method of treating dependency on an
opioid drug,
including acute and post-acute withdrawal symptoms, comprising treating an
opioid-
dependent patient with noribogaine, noribogaine derivative, or
pharmaceutically
acceptable salt or solvate thereof
STATE OF THE ART
[0002] Substance abuse is a serious public health problem throughout the
world. Heroin
and other opioids, including prescription painkillers, are widely abused and
account for a
large percentage of illicit drug use. Opioid abuse is also linked to
approximately 50% of
violent crimes in the United States and costs the U.S. economy billions of
dollars per year.
[0003] Opioid-dependent patients exhibit withdrawal symptoms soon after
cessation of
opioids, with the worst symptoms generally occurring 48 to 72 hours after the
last opioid
dose. Due to the severity and duration of withdrawal symptoms, opioid-
dependent patients
have a high rate of relapse.
[0004] Acute withdrawal from drug dependence is characterized by dramatic and
traumatic symptoms, including sweating, racing heart, palpitations, muscle
tension,
tightness in the chest, difficulty breathing, tremor, nausea, vomiting,
diarrhea, grand mal
seizures, heart attacks, strokes, hallucinations and delirium tremens (DTs).
Once acute
withdrawal symptoms have subsided, post-acute withdrawal syndrome can last for
months
or years. Post-acute withdrawal symptoms include fatigue, depression, lack of
motivation,
and increased pain sensitivity.
[0005] Numerous treatments have been developed in attempts to ameliorate acute
and
post-acute withdrawal symptoms. However, in most cases, treatment of
withdrawal
requires use of other addictive substances (e.g., morphine, buprenorphine or
methadone).
Treatment may require that the opioid-dependent patient attend a clinic daily
for an
extended amount of time.
1
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0006] There is a significant need for an effective, non-addictive treatment
for opioid
depdency that addresses acute and post-acute opioid withdrawal symptoms, as
well as the
underlying dependency, including cravings.
SUMMARY OF THE INVENTION
[0007] While the prior art suggests that ibogaine at higher doses is useful as
a treatment
for opioid dependency, use of ibogaine is associated with hallucinations and
other negative
side effects, including death. In the United States, ibogaine is classified as
a Schedule I
controlled substance.
[0008] Noribogaine is a metabolite of ibogaine found in human, dog, rat, and
monkey.
Noribogaine compounds have been suggested to have a greater and longer lasting
activity
in humans than ibogaine for reducing craving for addictive substances and
treating
chemical dependency. U.S. Patent No. 6,348,456, incorporated by reference
herein in its
entirety, discloses highly purified noribogaine and teaches that it should be
provided at
dosages from about 0.01 to about 100 mg per kg body weight per day to treat
dependency.
[0009] Recent studies have shown that noribogaine reduces opioid-associated
withdrawal in dependent patients at a serum level of between 50 nanograms per
milliliter
(ng/mL) and 180 ng/mL. Although an increase in QT interval has been observed
with
noribogaine treatment, the increase is concentration-dependent and mean QT
interval
prolongation of less than about 30 milliseconds (ms) is observed at
therapeutic
noribogaine plasma levels. See, for example, U.S. Patent Publication Nos.
2015/0231145,
2015/0231146, and 2015/0231147, each of which is incorporated herein by
reference in its
entirety.
[0010] This invention is predicated on the surprising discovery that
administration to a
patient of an aggregate amount of up to about 200 mg of noribogaine or
pharmaceutically
acceptable salt or solvate thereof per day results in a dramatic reduction of
withdrawal
symptoms, thereby allowing the patient to abstain from opioid abuse for a
period of time.
This amount, when provided as two or more doses, is sufficient to reduce
withdrawal
symptoms while maintaining the patient's QT interval at less than about 500
ms. This
amount, when provided as two or more doses, is also sufficient to reduce
withdrawal
symptoms without prolonging the patient's QT interval by more than about 60 ms
2
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
(compared to baseline, e.g., prior to administration of noribogaine or
noribogaine
derivative). In one embodiment, the patient is treated with an aggregate
amount of up to
about 200 mg of noribogaine, noribogaine derivative, or pharmaceutically
acceptable salt
or solvate thereof per day for between 2 and 14 days.
[0011] In one embodiment, such methods are achieved by administration of a
loading
(initial) dose of noribogaine, noribogaine derivative, or a pharmaceutically
acceptable salt
or solvate thereof, followed by periodic administration during the day of one
or more
therapeutic maintenance doses. Such therapeutic maintenance doses can be
continued for
several days or longer until the patient is deemed suitable for
discontinuation of treatment
by the attending clinician.
[0012] In one embodiment, the loading dose of noribogaine, noribogaine
derivative, or a
pharmaceutically acceptable salt or solvate thereof is below that dose which
was found to
be most efficacious as a single bolus dose in methadone-dependent patients
(i.e., 120 mg).
Without being bound by theory, it is believed that the loading dose provides
an increase in
serum concentration that is low enough to avoid unacceptable QT interval
prolongation,
while the therapeutic maintenance doses are sufficient to maintain an
efficacious serum
concentration without inducing an unacceptable QT interval prolongation.
[0013] In one embodiment, noribogaine, noribogaine derivative, or a
pharmaceutically
acceptable salt or solvate thereof is administered to the patient at an
aggregate dose that
provides therapeutic results, a maximum QT interval prolongation of less than
about 50
milliseconds (ms), and a maximum QT interval of less than 500 ms. In a
preferred
embodiment, the aggregate dose range is between about 50 mg and about 200 mg
per day.
[0014] Without being bound by theory, it is also contemplated that, for at
least a subset
of opioid-dependent patients, noribogaine serum concentration is less
predictive of
efficacy (e.g., reduced withdrawal symptoms) than opioid metabolism. That is,
patients
with similar noribogaine serum concentrations may nonetheless have very
different scores
on one or more opiate withdrawal scales, depending on each patient's abilty to
metabolize
opioid(s) and eliminate them, e.g. remove the opioid(s) from the brain and/or
circulatory
system.
3
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0015] Opioid metabolism varies by patient and is dependent on factors
including
genetics, age, ethnicity, disease (e.g., hepatic impairment, renal
impairment), and sex. See,
Howard S. Smith, "Opioid Metabolism," Mayo Clin. Proc. 84(7): 613-624, which
is
incorporated herein by reference in its entirety. For example, patients having
allelic
variability, mutations, or the like in one or more enzymes that are involved
in the
metabolic pathway for a given opioid may have altered metabolic processing of
that
opioid.
[0016] In one aspect, this disclosure relates to a method for treating an
opioid-dependent
patient being treated according to a noribogaine, or noribogaine derivative,
treatment
protocol, wherein said patient inefficiently, insufficiently or slowly
metabolizes opioids,
said method comprising selecting and/or identifying a patient that
inefficiently,
insufficiently or slowly metabolizes opioid, and altering the treatment
protocol to include a
revised treatment protocol. In one embodiment, the revised treatment protocol
can be or
include one or more of increasing an amount of noribogaine or noribogaine
derivative
administered, increasing a duration of noribogaine or noribogaine derivative
administration, co-administering an opioid antagonist or partial opioid
antagonist, and
combinations thereof It should be understood that the identifying or selecting
can be done
in response to a clinician (e.g., a nurse, a physician's assistant, a
physician, a medical
provider for dependent patients, and the like) determining that the patient is
a slow or
inefficient metabolizer and/or that the patient continues to have high levels
of opioid in the
brain or body (e.g., high enough to at least partially counteract an effect of
noribogaine or
noribogaine derivative). Those high opioid levels can result in a more
challenging
experience while receiving the noribogaine treatment. Furthermore, the
identifying can
include the use of urine, blood or other assays to determine quantities or
levels of opioid in
the system of the patient.
[0017] In one embodiment, the opioid antagonist is naloxone. In one
embodiment, the
opioid antagonist is naltrexone. In one embodiment, the opioid antagonist is
nalmefene. In
one embodiment, the opioid antagonist is nalorphine. In one embodiment, the
opioid
antagonist is naloxone, levallorphan, cyprodime, naltrindole, or
norbinaltorphimine
[0018] In one aspect is provided a method for treating opioid drug abuse in an
opioid-
dependent human patient, the method comprising administering to the patient a
therapeutic
4
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
dosage of noribogaine, noribogaine derivative, or pharmaceutically acceptable
salt or
solvate thereof, such that the therapeutic dosage is between about 60 mg and
about 200 mg
per day, wherein the therapeutic dosage is administered as at least two
subdoses, thereby
inhibiting or ameliorating said abuse while maintaining a QT interval in the
patient of less
than about 500 ms during said treatment.
[0019] In some embodiments, the method comprises:
a) administering an initial dose of noribogaine, noribogaine derivative, or
pharmaceutically acceptable salt or solvate thereof, wherein the initial dose
is between
about 50 mg and about 120 mg; and
b) administering at least one therapeutic maintenance dose of noribogaine,
noribogaine derivative, or pharmaceutically acceptable salt or solvate
thereof, wherein the
at least one therapeutic maintenance dose is between about 5 mg and about 50
mg.
[0020] In some embodiments, the method further comprises administering an
effective
amount of an opioid antagonist or partial antagonist. In one embodiment, the
opioid
antagonist or partial antagonist is administered concurrently with
noribogaine, noribogaine
derivative, or pharmaceutically acceptable salt or solvate thereof In one
embodiment, the
opioid antagonist or partial antagonist is administered after administration
of noribogaine,
noribogaine derivative, or pharmaceutically acceptable salt or solvate thereof
In one
embodiment, the opioid antagonist or partial antagonist is naloxone,
nalmefene,
naltrexone, nalorphine, levallorphan, cyprodime, naltrindole, or
norbinaltorphimine.
[0021] In some aspects is provided a method for treating an opioid-dependent
patient
being treated according to a noribogaine or noribogaine derivative treatment
protocol,
wherein the patient insufficiently metabolizes opioid, said method comprising
identifying
the patient that insufficiently metabolizes opioid, and altering the treatment
protocol to
include a revised protocol. In some embodiments, the noribogaine or
noribogaine
derivative is in salt and/or solvate form. In some embodiments, the revised
protocol
includes increasing the amount of noribogaine or noribogaine derivative
administered. In
some embodiments, the revised protocol includes increasing the amount of time
of
noribogaine or noribogaine derivative administration. In some embodiments, the
revised
protocol includes co-administering an opioid antagonist or partial opioid
antagonist with
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
the noribogaine or noribogaine derivative. In some embodiments, the revised
protocol
includes a combination of these revisions.
[0022] In some embodiments, insufficient metabolism refers to a decrease in
opioid
level by less than 90% over 1 to 2 days.
[0023] In some embodiments, the opioid antagonist is naloxone, nalmefene,
naltrexone,
nalorphine, levallorphan, cyprodime, naltrindole, or norbinaltorphimine. In a
preferred
embodiment, the opioid antagonist is naloxone.
[0024] In some embodiments, the noribogaine or noribogaine derivative
treatment
protocol comprises administering to the patient a therapeutic dosage of
noribogaine,
noribogaine, noribogaine derivative or pharmaceutically acceptable salt or
solvate thereof,
such that the therapeutic dosage is between about 60 mg and about 200 mg per
day.
[0025] In some embodiments, the patient has an acceptable risk level for QT
interval
prolongation.
[0026] In some embodiments, the average serum concentration of noribogaine,
noribogaine derivative, or pharmaceutically acceptable salt or solvate thereof
is
maintained between about 30 ng/mL and about 180 ng/mL during the treatment
period.
[0027] In preferred embodiments, the patient does not exhibit QT interval
prolongation
of more than 60 ms.
[0028] In some embodiments, at least one withdrawal symptom is attenuated.
[0029] In some aspects is provided a method for treating an opioid-dependent
patient
being treated according to a noribogaine or noribogaine derivative treatment
protocol, the
method comprising:
periodically administering to the patient a unit dose of noribogaine,
noribogaine
derivative, or pharmaceutically acceptable salt or solvate thereof;
monitoring an initial opioid level and one or more subsequent periodic opioid
levels of the patient; and
modifying the treatment protocol. In some embodiments, the protocol is
modified
by increasing the amount of noribogaine or noribogaine derivative, prolonging
the
6
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
noribogaine or noribogaine derivative treatment, adding naloxone, and/or
reducing the
amount of the periodic unit dose when a subsequent opioid level falls below at
least about
10% of the initial opioid level.
[0030] In one embodiment, the modified protocol is performed until the patient
is
substantially free of opioids. In one embodiment, the treatment protocol is
modified by
reducing the amount of the periodic unit dose when the opioid level falls
below at least
about 10% of the initial opioid level or where the patient is substantially
free of opioid in
his or her system.
[0031] In some embodiments, the method further comprises monitoring at least
one
indicator of withdrawal and modifying the treatment protocol based on the at
least one
indicator, such that noribogaine or noribogaine derivative treatment is
modified by
reducing the amount of the periodic unit dose when the indicator indicates
substantially no
withdrawal.
[0032] In one aspect is provided a method for treating an opioid-dependent
patient being
treated according to a noribogaine or noribogaine derivative treatment
protocol, wherein
said patient has insufficient metabolization of opioid, said method comprising
selecting a
patient that has insufficient metabolization of opioid and altering the
treatment protocol to
include co-administration of an opioid antagonist, such as naloxone. In one
embodiment,
insufficient metabolism refers to a decrease in opioid level in the brain by
less than 90%
over 1 to 2 days. In one embodiment, insufficient metabolism refers to the
patient not
having beeing substantially free of opioid or to the patient not having had a
majority of
opioid released from his/her system. In one embodiment, the substantial
freedom from
opioid or the opioid in the system can be determined by assessing the amount
of opioid
released into plasma or urine, or as otherwise determined by a clinician.
[0033] In some embodiments, the opioid antagonist is administered after at
least a first
dose of noribogaine, noribogaine derivative, or pharmaceutically acceptable
salt or solvate
thereof is administered. In some embodiments, the opioid antagonist is
administered
concurrently with at least one dose of noribogaine, noribogaine derivative, or
pharmaceutically acceptable salt or solvate thereof In some embodiments, the
opioid
antagonist is administered at a sub-therapeutic dose.
7
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0034] In some embodiments, at least one indicator of withdrawal symptoms
and/or
mood state is measured prior to and at least one time during treatment with
noribogaine,
noribogaine derivative, or pharmaceutically acceptable salt or solvate thereof
In some
embodiments, at least one indicator of withdrawal symptoms is selected from
the group
consisting of Clinical Opiate Withdrawal Scale (COWS), Subjective Opiate
Withdrawal
Scale (SOWS), Objective Opiate Withdrawal Scale (00WS), and Profile of Mood
States
(POMS). In one embodiment, the patient reports no urge to use the opioid for
at least one
day after cessation of treatment with noribogaine, noribogaine derivative, or
pharmaceutically acceptable salt or solvate thereof
[0035] In some embodiments, the opioid is buprenorphine. In a preferred
embodiment,
the patient has not used buprenorphine for at least 5 days prior to treatment
with
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof
[0036] In especially preferred embodiments, the patient has no history of
methadone use
within the previous 10 days to about 3 weeks. In other preferred embodiments,
the patient
has no history of buprenorphine use within the previous 10 days to about 3
weeks.
[0037] In one aspect is provided a method for pretreating an opioid-dependent
patient
scheduled for treatment with an opioid antagonist, which method comprises
administering
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof
to said patient in an amount sufficient to attenuate withdrawal symptoms and
to allow the
patient to be opioid-free for a period of time prior to initiation of opioid
antagonist
administration. In a preferred embodiment, the patient is opioid-free for at
least 7 days
prior to initiation of opioid antagonist administration.
[0038] In some embodiments, the method comprises:
a) administering an initial dose of noribogaine, noribogaine derivative, or
pharmaceutically acceptable salt or solvate thereof, wherein the initial dose
is between
about 50 mg and about 120 mg; and
b) administering at least one therapeutic maintenance dose of noribogaine,
noribogaine derivative, or pharmaceutically acceptable salt or solvate
thereof, wherein the
at least one therapeutic maintenance dose is between about 5 mg and about 50
mg.
8
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0039] In some embodiments, the at least one therapeutic maintenance dose is
administered from about 6 hours to about 24 hours after the initial dose.
[0040] In some embodiments, the opioid antagonist is selected from the group
consisting
of nalmefene, naltrexone, nalorphine, levallorphan, cyprodime, naltrindole,
and
norbinaltorphimine.
[0041] In preferred embodiments, the amount of noribogaine, noribogaine
derivative, or
pharmaceutically acceptable salt or solvate thereof administered is between
about 60 mg
and about 200 mg per day.
[0042] In especially preferred embodiments, a QT interval of less than about
500 ms is
maintained in the patient during said treatment. In preferred embodiments,
administration
of noribogaine or noribogaine derivative does not result in a maximum QT
interval
prolongation of more than about 60 ms. More preferably, administration of
noribogaine or
noribogaine derivative does not result in a maximum QT interval prolongation
of more
than about 50 ms.
[0043] In some embodiments, the average serum concentration of noribogaine,
noribogaine derivative, or pharmaceutically acceptable salt or solvate thereof
is
maintained between about 20 ng/mL and about 180 ng/mL during the treatment
period.
[0044] In some embodiments, at least two therapeutic maintenance doses are
administered, and further wherein the therapeutic maintenance doses are
administered
from about 6 hours to about 24 hours after the previous dose.
[0045] In some embodiments, at least one withdrawal symptom is attenuated. In
some
embodiments, the at least one withdrawal symptom is a symptom of acute
withdrawal.
[0046] In some aspects is provided a method for treating an opioid-dependent
patient
with an opioid antagonist which method comprises: identifying and/or selecting
an opioid-
dependent patient who has been pretreated with noribogaine, noribogaine
derivative, or
pharmaceutically acceptable salt or solvate thereof so as to be opioid-free
for at least 7
days, and then administering the opioid antagonist in a sufficient amount to
inhibit a
pharmacological effect of opioids in the patient. In some embodiments, the
opioid
antagonist is nalmefene, naltrexone, nalorphine, levallorphan, cyprodime,
naltrindole, or
9
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
norbinaltorphimine. In some embodiments, the opioid antagonist is in a
controlled-release
formulation.
[0047] In some embodiments, the opioid antagonist is orally administered. In
some
embodiments, the opioid antagonist is administered by injection.
[0048] In some embodiments, relapse of opioid use by the patient is inhibited.
[0049] In some embodiments, the method further comprises checking for opioid
detoxification in the patient by naloxone challenge and/or a urine test prior
to
administration of the opioid antagonist.
[0050] In some aspects is provided a method for opioid detoxification of an
opioid-
dependent patient prior to treatment with an opioid antagonist, which method
comprises
administering noribogaine, noribogaine derivative, or pharmaceutically
acceptable salt or
solvate thereof to said patient for a time sufficient for the patient not to
have opioids in
his/her body prior to initiation of opioid antagonist administration.
[0051] In some aspects is provided a kit of parts comprising a plurality of
unit doses of
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof,
and at least one unit dose of an opioid antagonist or partial antagonist.
[0052] In some embodiments, the opioid antagonist is naloxone. In some
embodiments,
the unit dose of naloxone is formulated for intravenous, subcutaneous, and/or
intramuscular injection.
[0053] In some embodiments, the opioid antagonist is nalmefene, naltrexone,
nalorphine, levallorphan, cyprodime, naltrindole, or norbinaltorphimine.
[0054] In some aspects is provided a pharmaceutical formulation comprising a
dose of
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof
and an effective amount of an opioid antagonist. In some embodiments, the
opioid
antagonist is naloxone, nalmefene, naltrexone, nalorphine, levallorphan,
cyprodime,
naltrindole, or norbinaltorphimine. In some embodiments, the dose of
noribogaine is
between about 5 mg and about 100 mg.
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
BRIEF DESCRIPTION OF THE DRAWINGS
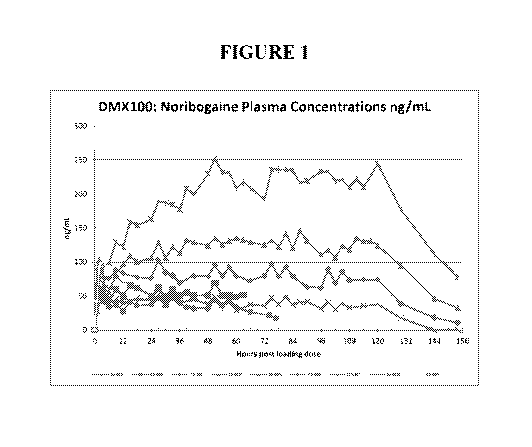
[0055] FIG. 1 shows noribogaine plasma concentrations over time for each
patient in a
clinical study.
[0056] FIG. 2A shows the change in Subjective Opioid Withdrawal Scale (SOWS)
(area
under the curve, AUC) versus noribogaine plasma concentration (AUC) at 3 hours
after
initial noribogaine administration.
[0057] FIG. 2B shows the change in SOWS (AUC) versus noribogaine plasma
concentration (AUC) at 36 hours after initial noribogaine administration.
[0058] FIG. 2C shows the change in SOWS (AUC) versus noribogaine plasma
concentration (AUC) at 60 hours after initial noribogaine administration.
[0059] FIGs. 3A-3I show total SOWS over time compared to urine opioid levels
for
each patient.
[0060] FIG. 4 shows the correlation between urine opioid levels and plasma
opioid
levels.
[0061] FIG. 5 shows mean SOWS score over time. Noribogaine treatment lasted up
to
120 hours, followed by 48 hours of observation after the last dose.
[0062] FIG. 6 shows mean scores for SOWS question #16: "I feel like using
now."
[0063] FIG. 7 shows mean Profile of Mood States (POMS) Total Mood Disturbance
Scores for the first 7 subjects.
DETAILED DESCRIPTION OF THE INVENTION
[0064] It is to be understood that this invention is not limited to particular
embodiments
described, as such may, of course, vary. It is also to be understood that the
terminology
used herein is for the purpose of describing particular embodiments only, and
is not
intended to be limiting, since the scope of this invention will be limited
only by the
appended claims.
11
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0065] The detailed description of the invention is divided into various
sections only for
the reader's convenience and disclosure found in any section may be combined
with that
in another section. Unless defined otherwise, all technical and scientific
terms used herein
have the same meaning as commonly understood by one of ordinary skill in the
art to
which this invention belongs.
[0066] It must be noted that as used herein and in the appended claims, the
singular
forms "a", "an", and "the" include plural referents unless the context clearly
dictates
otherwise. Thus, for example, reference to "a compound" includes a plurality
of
compounds.
I. Definitions
[0067] Unless defined otherwise, all technical and scientific terms used
herein have the
same meaning as commonly understood by one of ordinary skill in the art to
which this
invention belongs. As used herein the following terms have the following
meanings.
[0068] The term "about" when used before a numerical designation, e.g.,
temperature,
time, amount, concentration, and such other, including a range, indicates
approximations
which may vary by ( + ) or ( - ) 10 %, 5 % or 1 %.
[0069] "Administration" refers to introducing an agent into a patient.
Typically, an
effective amount is administered, which amount can be determined by the
treating
physician or the like. Any route of administration, such as oral, topical,
subcutaneous,
peritoneal, intra-arterial, inhalation, vaginal, rectal, nasal, introduction
into the
cerebrospinal fluid, or instillation into body compartments can be used. The
agent may be
administered by direct blood stream delivery, e.g. sublingual, intranasal, or
intrapulmonary
administration.
[0070] The related terms and phrases "administering" and "administration of',
when
used in connection with a compound or pharmaceutical composition (and
grammatical
equivalents) refer both to direct administration, which may be administered to
a patient by
a medical professional or by self-administration by the patient, and/or to
indirect
administration, which may be the act of prescribing a drug. For example, a
physician who
instructs a patient to self-administer a drug and/or provides a patient with a
prescription
12
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
for a drug is administering the drug to the patient. Administration may be via
transdermal
patch, gum, lozenge, sublingual tablet, intranasal, intrapulmonary, oral
administration, or
other administration.
[0071] "Periodic administration" or "periodically administering" refers to
administration
of noribogaine, noribogaine derivative, or salt or solvate thereof one, two,
three, or more
times per day. Periodic administration may also refer to multiple treatments
that occur on a
daily, weekly, or monthly basis.
[0072] "Therapeutic maintenance dose" is the dose of drug that is administered
on a
periodic basis so as to maintain therapeutic serum concentrations of the drug
in the patient.
[0073] "Comprising" or "comprises" is intended to mean that the compositions
and
methods include the recited elements, but not excluding others. "Consisting
essentially
of' when used to define compositions and methods, shall mean excluding other
elements
of any essential significance to the combination for the stated purpose. Thus,
a
composition consisting essentially of the elements as defined herein would not
exclude
other materials or steps that do not materially affect the basic and novel
characteristic(s) of
the claimed invention. "Consisting of' shall mean excluding more than trace
elements of
other ingredients and substantial method steps. Embodiments defined by each of
these
transition terms are within the scope of this invention.
[0074] As used herein, the term "alkyl" refers to monovalent saturated
aliphatic
hydrocarbyl groups having from 1 to 12 carbon atoms, 1 to 10 carbon atoms,
preferably 1
to 6 carbon atoms, and more preferably 1 to 3 carbon atoms. This term
includes, by way
of example, linear and branched hydrocarbyl groups such as methyl (CH3-),
ethyl
(CH3CH2-), n-propyl (CH3CH2CH2-), isopropyl ((CH3)2CH-), n-butyl (CH3CH2CH2CH2-
),
isobutyl ((CH3)2CHCH2-), sec-butyl ((CH3)(CH3CH2)CH-), t-butyl ((CH3)3C-), n-
pentyl
(CH3CH2CH2CH2CH2-), and neopentyl ((CH3)3CCH2-). The term "Cx alkyl" refers to
an
alkyl group having x carbon atoms, wherein x is an integer, for example, C3
refers to an
alkyl group having 3 carbon atoms.
[0075] "Alkenyl" refers to straight or branched hydrocarbyl groups having from
2 to 6
carbon atoms and preferably 2 to 4 carbon atoms and having at least 1 and
preferably from
1 to 2 sites of vinyl (>C=C) unsaturation. Such groups are exemplified, for
example, by
13
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
vinyl, ally!, and but-3-en-1-yl. Included within this term are the cis and
trans isomers or
mixtures of these isomers.
[0076] "Alkynyl" refers to straight or branched monovalent hydrocarbyl groups
having
from 2 to 6 carbon atoms and preferably 2 to 3 carbon atoms and having at
least 1 and
preferably from 1 to 2 sites of acetylenic unsaturation. Examples of such
alkynyl
groups include acetylenyl (-CCH), and propargyl (-CH2CCH).
[0077] "Substituted alkyl" refers to an alkyl group having from 1 to 5,
preferably 1 to 3,
or more preferably 1 to 2 substituents selected from the group consisting of
alkoxy,
substituted alkoxy, acyl, acylamino, acyloxy, amino, substituted amino,
aminocarbonyl,
aminothiocarbonyl, aminocarbonylamino, aminothiocarbonylamino,
aminocarbonyloxy,
aminosulfonyl, aminosulfonyloxy, aminosulfonylamino, amidino, aryl,
substituted aryl,
aryloxy, substituted aryloxy, arylthio, substituted arylthio, carboxyl,
carboxyl ester,
(carboxyl ester)amino, (carboxyl ester)oxy, cyano, cycloalkyl, substituted
cycloalkyl,
cycloalkyloxy, substituted cycloalkyloxy, cycloalkylthio, substituted
cycloalkylthio,
cycloalkenyl, substituted cycloalkenyl, cycloalkenyloxy, substituted
cycloalkenyloxy,
cycloalkenylthio, substituted cycloalkenylthio, guanidino, substituted
guanidino, halo,
hydroxy, heteroaryl, substituted heteroaryl, heteroaryloxy, substituted
heteroaryloxy,
heteroarylthio, substituted heteroarylthio, heterocyclic, substituted
heterocyclic,
heterocyclyloxy, substituted heterocyclyloxy, heterocyclylthio, substituted
heterocyclylthio, nitro, 503H, substituted sulfonyl, sulfonyloxy, thioacyl,
thiol, alkylthio,
and substituted alkylthio, wherein said substituents are defined herein.
[0078] "Substituted alkenyl" refers to alkenyl groups having from 1 to 3
substituents,
and preferably 1 to 2 substituents, selected from the group consisting of
alkoxy,
substituted alkoxy, acyl, acylamino, acyloxy, amino, substituted amino,
aminocarbonyl,
aminothiocarbonyl, aminocarbonylamino, aminothiocarbonylamino,
aminocarbonyloxy,
aminosulfonyl, aminosulfonyloxy, aminosulfonylamino, amidino, aryl,
substituted aryl,
aryloxy, substituted aryloxy, arylthio, substituted arylthio, carboxyl,
carboxyl ester,
(carboxyl ester)amino, (carboxyl ester)oxy, cyano, cycloalkyl, substituted
cycloalkyl,
cycloalkyloxy, substituted cycloalkyloxy, cycloalkylthio, substituted
cycloalkylthio,
cycloalkenyl, substituted cycloalkenyl, cycloalkenyloxy, substituted
cycloalkenyloxy,
cycloalkenylthio, substituted cycloalkenylthio, guanidino, substituted
guanidino, halo,
14
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
hydroxy, heteroaryl, substituted heteroaryl, heteroaryloxy, substituted
heteroaryloxy,
heteroarylthio, substituted heteroarylthio, heterocyclic, substituted
heterocyclic,
heterocyclyloxy, substituted heterocyclyloxy, heterocyclylthio, substituted
heterocyclylthio, nitro, SO3H, substituted sulfonyl, sulfonyloxy, thioacyl,
thiol, alkylthio,
and substituted alkylthio, wherein said substituents are defined herein and
with the proviso
that any hydroxy or thiol substitution is not attached to a vinyl
(unsaturated) carbon atom.
[0079] "Substituted alkynyl" refers to alkynyl groups having from 1 to 3
substituents,
and preferably 1 to 2 substituents, selected from the group consisting of
alkoxy,
substituted alkoxy, acyl, acylamino, acyloxy, amino, substituted amino,
aminocarbonyl,
aminothiocarbonyl, aminocarbonylamino, aminothiocarbonylamino,
aminocarbonyloxy,
aminosulfonyl, aminosulfonyloxy, aminosulfonylamino, amidino, aryl,
substituted aryl,
aryloxy, substituted aryloxy, arylthio, substituted arylthio, carboxyl,
carboxyl ester,
(carboxyl ester)amino, (carboxyl ester)oxy, cyano, cycloalkyl, substituted
cycloalkyl,
cycloalkyloxy, substituted cycloalkyloxy, cycloalkylthio, substituted
cycloalkylthio,
cycloalkenyl, substituted cycloalkenyl, cycloalkenyloxy, substituted
cycloalkenyloxy,
cycloalkenylthio, substituted cycloalkenylthio, guanidino, substituted
guanidino, halo,
hydroxy, heteroaryl, substituted heteroaryl, heteroaryloxy, substituted
heteroaryloxy,
heteroarylthio, substituted heteroarylthio, heterocyclic, substituted
heterocyclic,
heterocyclyloxy, substituted heterocyclyloxy, heterocyclylthio, substituted
heterocyclylthio, nitro, 503H, substituted sulfonyl, sulfonyloxy, thioacyl,
thiol, alkylthio,
and substituted alkylthio, wherein said substituents are defined herein and
with the proviso
that any hydroxy or thiol substitution is not attached to an acetylenic carbon
atom.
[0080] "Alkoxy" refers to the group -0-alkyl wherein alkyl is defined herein.
Alkoxy
includes, by way of example, methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy,
t-butoxy, sec-butoxy, and n-pentoxy.
[0081] "Substituted alkoxy" refers to the group -0-(substituted alkyl) wherein
substituted alkyl is defined herein.
[0082] "Acyl" refers to the groups H-C(0)-, alkyl-C(0)-, substituted alkyl-
C(0)-,
alkenyl-C(0)-, substituted alkenyl-C(0)-, alkynyl-C(0)-, substituted alkynyl-
C(0)-,
cycloalkyl-C(0)-, substituted cycloalkyl-C(0)-, cycloalkenyl-C(0)-,
substituted
cycloalkenyl-C(0)-, aryl-C(0)-, substituted aryl-C(0)-, heteroaryl-C(0)-,
substituted
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
heteroaryl-C(0)-, heterocyclic-C(0)-, and substituted heterocyclic-C(0)-,
wherein alkyl,
substituted alkyl, alkenyl, substituted alkenyl, alkynyl, substituted alkynyl,
cycloalkyl,
substituted cycloalkyl, cycloalkenyl, substituted cycloalkenyl, aryl,
substituted aryl,
heteroaryl, substituted heteroaryl, heterocyclic, and substituted heterocyclic
are as defined
herein. Acyl includes the "acetyl" group CH3C(0)-.
[0083] "Acylamino" refers to the groups -NR38C(0)alkyl, -NR38C(0)substituted
alkyl, -NR38C(0)cycloalkyl, -NR38C(0)substituted
cycloalkyl, -NR38C(0)cycloalkenyl, -NR38C(0)substituted
cycloalkenyl, -NR38C(0)alkenyl, -NR38C(0)substituted
alkenyl, -NR38C(0)alkynyl, -NR38C(0)substituted
alkynyl, -NR38C(0)aryl, -NR38C(0)substituted
aryl, -NR38C(0)heteroaryl, -NR38C(0)substituted heteroaryl, -
NR38C(0)heterocyclic,
and -NR38C(0)substituted heterocyclic wherein R38 is hydrogen or alkyl and
wherein
alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl, substituted
alkynyl,
cycloalkyl, substituted cycloalkyl, cycloalkenyl, substituted cycloalkenyl,
aryl, substituted
aryl, heteroaryl, substituted heteroaryl, heterocyclic, and substituted
heterocyclic are as
defined herein.
[0084] "Acyloxy" refers to the groups alkyl-C(0)O-, substituted alkyl-C(0)O-,
alkenyl-C(0)O-, substituted alkenyl-C(0)O-, alkynyl-C(0)O-, substituted
alkynyl-C(0)O-, aryl-C(0)O-, substituted aryl-C(0)O-, cycloalkyl-C(0)O-,
substituted
cycloalkyl-C(0)O-, cycloalkenyl-C(0)O-, substituted cycloalkenyl-C(0)O-,
heteroaryl-C(0)O-, substituted heteroaryl-C(0)O-, heterocyclic-C(0)O-, and
substituted
heterocyclic-C(0)0- wherein alkyl, substituted alkyl, alkenyl, substituted
alkenyl, alkynyl,
substituted alkynyl, cycloalkyl, substituted cycloalkyl, cycloalkenyl,
substituted
cycloalkenyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl,
heterocyclic, and
substituted heterocyclic are as defined herein.
[0085] "Amino" refers to the group -NH2.
[0086] "Substituted amino" refers to the group -NR39R4 where R39 and R4 are
independently selected from the group consisting of hydrogen, alkyl,
substituted alkyl,
alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, aryl, substituted
aryl, cycloalkyl,
substituted cycloalkyl, cycloalkenyl, substituted cycloalkenyl, heteroaryl,
substituted
16
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
heteroaryl, heterocyclic, substituted heterocyclic, -S02-alkyl, -S02-
substituted
alkyl, -S02-alkenyl, -S02-substituted alkenyl, -S02-cycloalkyl, -S02-
substituted
cylcoalkyl, -S02-cycloalkenyl, -S02-substituted cylcoalkeny1,-S02-aryl, -S02-
substituted
aryl, -S02-heteroaryl, -S02-substituted heteroaryl, -S02-heterocyclic, and -
S02-substituted
heterocyclic and wherein R39 and R4 are optionally joined, together with the
nitrogen
bound thereto to form a heterocyclic or substituted heterocyclic group,
provided that R39
and R4 are both not hydrogen, and wherein alkyl, substituted alkyl, alkenyl,
substituted
alkenyl, alkynyl, substituted alkynyl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl,
substituted cycloalkenyl, aryl, substituted aryl, heteroaryl, substituted
heteroaryl,
heterocyclic, and substituted heterocyclic are as defined herein. When R39 is
hydrogen
and R4 is alkyl, the substituted amino group is sometimes referred to herein
as
alkylamino. When R39 and R4 are alkyl, the substituted amino group is
sometimes
referred to herein as dialkylamino. When referring to a monosubstituted amino,
it is
meant that either R39 or R4 is hydrogen but not both. When referring to a
disubstituted
amino, it is meant that neither R39 nor R4 are hydrogen.
[0087] "Aminocarbonyl" refers to the group -C(0)NR41R42 where R41 and R42 are
independently selected from the group consisting of hydrogen, alkyl,
substituted alkyl,
alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, aryl, substituted
aryl, cycloalkyl,
substituted cycloalkyl, cycloalkenyl, substituted cycloalkenyl, heteroaryl,
substituted
heteroaryl, heterocyclic, and substituted heterocyclic and where R41 and R42
are optionally
joined together with the nitrogen bound thereto to form a heterocyclic or
substituted
heterocyclic group, and wherein alkyl, substituted alkyl, alkenyl, substituted
alkenyl,
alkynyl, substituted alkynyl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl, substituted
cycloalkenyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl,
heterocyclic, and
substituted heterocyclic are as defined herein.
[0088] "Aminothiocarbonyl" refers to the group -C(S)NR41R42 where R41 and R42
are
independently selected from the group consisting of hydrogen, alkyl,
substituted alkyl,
alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, aryl, substituted
aryl, cycloalkyl,
substituted cycloalkyl, cycloalkenyl, substituted cycloalkenyl, heteroaryl,
substituted
heteroaryl, heterocyclic, and substituted heterocyclic and where R41 and R42
are optionally
joined together with the nitrogen bound thereto to form a heterocyclic or
substituted
heterocyclic group, and wherein alkyl, substituted alkyl, alkenyl, substituted
alkenyl,
17
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
alkynyl, substituted alkynyl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl, substituted
cycloalkenyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl,
heterocyclic, and
substituted heterocyclic are as defined herein.
[0089] "Aminocarbonylamino" refers to the group -NR38C(0)NR41R42 where R38 is
hydrogen or alkyl and R41 and R42 are independently selected from the group
consisting of
hydrogen, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl,
substituted
alkynyl, aryl, substituted aryl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl, substituted
cycloalkenyl, heteroaryl, substituted heteroaryl, heterocyclic, and
substituted heterocyclic
and where R41 and R42 are optionally joined together with the nitrogen bound
thereto to
form a heterocyclic or substituted heterocyclic group, and wherein alkyl,
substituted alkyl,
alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, cycloalkyl,
substituted
cycloalkyl, cycloalkenyl, substituted cycloalkenyl, aryl, substituted aryl,
heteroaryl,
substituted heteroaryl, heterocyclic, and substituted heterocyclic are as
defined herein.
[0090] "Aminothiocarbonylamino" refers to the group -NR38C(S)NR41R42 where R38
is
hydrogen or alkyl and R41 and R42 are independently selected from the group
consisting of
hydrogen, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl,
substituted
alkynyl, aryl, substituted aryl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl, substituted
cycloalkenyl, heteroaryl, substituted heteroaryl, heterocyclic, and
substituted heterocyclic
and where R41 and R42 are optionally joined together with the nitrogen bound
thereto to
form a heterocyclic or substituted heterocyclic group, and wherein alkyl,
substituted alkyl,
alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, cycloalkyl,
substituted
cycloalkyl, cycloalkenyl, substituted cycloalkenyl, aryl, substituted aryl,
heteroaryl,
substituted heteroaryl, heterocyclic, and substituted heterocyclic are as
defined herein.
[0091] "Aminocarbonyloxy" refers to the group -0-C(0)NR41R42 where R41 and R42
are
independently selected from the group consisting of hydrogen, alkyl,
substituted alkyl,
alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, aryl, substituted
aryl, cycloalkyl,
substituted cycloalkyl, cycloalkenyl, substituted cycloalkenyl, heteroaryl,
substituted
heteroaryl, heterocyclic, and substituted heterocyclic and where R41 and R42
are optionally
joined together with the nitrogen bound thereto to form a heterocyclic or
substituted
heterocyclic group, and wherein alkyl, substituted alkyl, alkenyl, substituted
alkenyl,
alkynyl, substituted alkynyl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl, substituted
18
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
cycloalkenyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl,
heterocyclic, and
substituted heterocyclic are as defined herein.
[0092] "Aminosulfonyl" refers to the group -S02NR41R42 where R41 and R42 are
independently selected from the group consisting of hydrogen, alkyl,
substituted alkyl,
alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, aryl, substituted
aryl, cycloalkyl,
substituted cycloalkyl, cycloalkenyl, substituted cycloalkenyl, heteroaryl,
substituted
heteroaryl, heterocyclic, and substituted heterocyclic and where R41 and R42
are optionally
joined together with the nitrogen bound thereto to form a heterocyclic or
substituted
heterocyclic group, and wherein alkyl, substituted alkyl, alkenyl, substituted
alkenyl,
alkynyl, substituted alkynyl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl, substituted
cycloalkenyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl,
heterocyclic, and
substituted heterocyclic are as defined herein.
[0093] "Aminosulfonyloxy" refers to the group -0-S02NR41R42 where R41 and R42
are
independently selected from the group consisting of hydrogen, alkyl,
substituted alkyl,
alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, aryl, substituted
aryl, cycloalkyl,
substituted cycloalkyl, cycloalkenyl, substituted cycloalkenyl, heteroaryl,
substituted
heteroaryl, heterocyclic, and substituted heterocyclic and where R41 and R42
are optionally
joined together with the nitrogen bound thereto to form a heterocyclic or
substituted
heterocyclic group, and wherein alkyl, substituted alkyl, alkenyl, substituted
alkenyl,
alkynyl, substituted alkynyl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl, substituted
cycloalkenyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl,
heterocyclic, and
substituted heterocyclic are as defined herein.
[0094] "Aminosulfonylamino" refers to the group -NR38-S02NR41R42 where R38 is
hydrogen or alkyl and R41 and R42 are independently selected from the group
consisting of
hydrogen, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl,
substituted
alkynyl, aryl, substituted aryl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl, substituted
cycloalkenyl, heteroaryl, substituted heteroaryl, heterocyclic, and
substituted heterocyclic
and where R41 and R42 are optionally joined together with the nitrogen bound
thereto to
form a heterocyclic or substituted heterocyclic group, and wherein alkyl,
substituted alkyl,
alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, cycloalkyl,
substituted
19
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
cycloalkyl, cycloalkenyl, substituted cycloalkenyl, aryl, substituted aryl,
heteroaryl,
substituted heteroaryl, heterocyclic, and substituted heterocyclic are as
defined herein.
[0095] "Amidino" refers to the group -C(=NR43)NR41R42 where R41, R42, and R43
are
independently selected from the group consisting of hydrogen, alkyl,
substituted alkyl,
alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, aryl, substituted
aryl, cycloalkyl,
substituted cycloalkyl, cycloalkenyl, substituted cycloalkenyl, heteroaryl,
substituted
heteroaryl, heterocyclic, and substituted heterocyclic and where R41 and R42
are optionally
joined together with the nitrogen bound thereto to form a heterocyclic or
substituted
heterocyclic group, and wherein alkyl, substituted alkyl, alkenyl, substituted
alkenyl,
alkynyl, substituted alkynyl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl, substituted
cycloalkenyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl,
heterocyclic, and
substituted heterocyclic are as defined herein.
[0096] "Aryl" or "Ar" refers to a monovalent aromatic carbocyclic group of
from 6 to 14
carbon atoms having a single ring (e.g., phenyl) or multiple condensed rings
(e.g.,
naphthyl or anthryl) which condensed rings may or may not be aromatic (e.g.,
2-benzoxazolinone, 2H-1,4-benzoxazin-3(4H)-one-7-yl, and the like) provided
that the
point of attachment is at an aromatic carbon atom. Preferred aryl groups
include phenyl
and naphthyl.
[0097] "Substituted aryl" refers to aryl groups which are substituted with 1
to 5,
preferably 1 to 3, or more preferably 1 to 2 substituents selected from the
group consisting
of alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl,
substituted alkynyl,
alkoxy, substituted alkoxy, acyl, acylamino, acyloxy, amino, substituted
amino,
aminocarbonyl, aminothiocarbonyl, aminocarbonylamino, aminothiocarbonylamino,
aminocarbonyloxy, aminosulfonyl, aminosulfonyloxy, aminosulfonylamino,
amidino, aryl,
substituted aryl, aryloxy, substituted aryloxy, arylthio, substituted
arylthio, carboxyl,
carboxyl ester, (carboxyl ester)amino, (carboxyl ester)oxy, cyano, cycloalkyl,
substituted
cycloalkyl, cycloalkyloxy, substituted cycloalkyloxy, cycloalkylthio,
substituted
cycloalkylthio, cycloalkenyl, substituted cycloalkenyl, cycloalkenyloxy,
substituted
cycloalkenyloxy, cycloalkenylthio, substituted cycloalkenylthio, guanidino,
substituted
guanidino, halo, hydroxy, heteroaryl, substituted heteroaryl, heteroaryloxy,
substituted
heteroaryloxy, heteroarylthio, substituted heteroarylthio, heterocyclic,
substituted
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
heterocyclic, heterocyclyloxy, substituted heterocyclyloxy, heterocyclylthio,
substituted
heterocyclylthio, nitro, SO3H, substituted sulfonyl, sulfonyloxy, thioacyl,
thiol, alkylthio,
and substituted alkylthio, wherein said substituents are defined herein.
[0098] "Aryloxy" refers to the group -0-aryl, where aryl is as defined herein,
that
includes, by way of example, phenoxy and naphthoxy.
[0099] "Substituted aryloxy" refers to the group -0-(substituted aryl) where
substituted
aryl is as defined herein.
[0100] "Arylthio" refers to the group -S-aryl, where aryl is as defined
herein.
[0101] "Substituted arylthio" refers to the group -S-(substituted aryl), where
substituted
aryl is as defined herein.
[0102] "Carbonyl" refers to the divalent group -C(0)- which is equivalent to -
C(=0)-.
[0103] "Carboxy" or "carboxyl" refers to -COOH or salts thereof
[0104] "Carboxyl ester" or "carboxy ester" refers to the
groups -C(0)0-alkyl, -C(0)0-substituted alkyl, -C(0)0-alkenyl, -C(0)0-
substituted
alkenyl, -C(0)0-alkynyl, -C(0)0-substituted alkynyl, -C(0)0-aryl, -C(0)0-
substituted
aryl, -C(0)0-cycloalkyl, -C(0)0-substituted
cycloalkyl, -C(0)0-cycloalkenyl, -C(0)0-substituted
cycloalkenyl, -C(0)0-heteroaryl, -C(0)0-substituted heteroaryl, -C(0)0-
heterocyclic,
and -C(0)0-substituted heterocyclic wherein alkyl, substituted alkyl, alkenyl,
substituted
alkenyl, alkynyl, substituted alkynyl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl,
substituted cycloalkenyl, aryl, substituted aryl, heteroaryl, substituted
heteroaryl,
heterocyclic, and substituted heterocyclic are as defined herein.
[0105] "(Carboxyl ester)amino" refers to the
group -NR38-C(0)0-alkyl, -NR38-C(0)0-substituted
alkyl, -NR38-C(0)0-alkenyl, -NR38-C(0)0-substituted
alkenyl, -NR38-C(0)0-alkynyl, -NR38-C(0)0-substituted
alkynyl, -NR38-C(0)0-aryl, -NR38-C(0)0-substituted
aryl, -NR38-C(0)0-cycloalkyl, -NR38-C(0)0-substituted
cycloalkyl, -NR38-C(0)0-cycloalkenyl, -NR38-C(0)0-substituted
21
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
cycloalkenyl, -NR38-C(0)0-heteroaryl, -NR38-C(0)0-substituted
heteroaryl, -NR38-C(0)0-heterocyclic, and -NR38-C(0)0-substituted heterocyclic
wherein
R38 is alkyl or hydrogen, and wherein alkyl, substituted alkyl, alkenyl,
substituted alkenyl,
alkynyl, substituted alkynyl, cycloalkyl, substituted cycloalkyl,
cycloalkenyl, substituted
cycloalkenyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl,
heterocyclic, and
substituted heterocyclic are as defined herein.
[0106] "(Carboxyl ester)oxy" refers to the group -0-C(0)0-alkyl,
substituted -0-C(0)0-alkyl, -0-C(0)0-alkenyl, -0-C(0)0-substituted
alkenyl, -0-C(0)0-alkynyl, -0-C(0)0-substituted
alkynyl, -0-C(0)0-aryl, -0-C(0)0-substituted
aryl, -0-C(0)0-cycloalkyl, -0-C(0)0-substituted
cycloalkyl, -0-C(0)0-cycloalkenyl, -0-C(0)0-substituted
cycloalkenyl, -0-C(0)0-heteroaryl, -0-C(0)0-substituted
heteroaryl, -0-C(0)0-heterocyclic, and -0-C(0)0-substituted heterocyclic
wherein alkyl,
substituted alkyl, alkenyl, substituted alkenyl, alkynyl, substituted alkynyl,
cycloalkyl,
substituted cycloalkyl, cycloalkenyl, substituted cycloalkenyl, aryl,
substituted aryl,
heteroaryl, substituted heteroaryl, heterocyclic, and substituted heterocyclic
are as defined
herein.
[0107] "Cyano" refers to the group -CN.
[0108] "Cycloalkyl" refers to cyclic alkyl groups of from 3 to 10 carbon atoms
having
single or multiple cyclic rings including fused, bridged, and spiro ring
systems. One or
more of the rings can be aryl, heteroaryl, or heterocyclic provided that the
point of
attachment is through the non-aromatic, non-heterocyclic ring carbocyclic
ring. Examples
of suitable cycloalkyl groups include, for instance, adamantyl, cyclopropyl,
cyclobutyl,
cyclopentyl, and cyclooctyl. Other examples of cycloalkyl groups include
bicycle[2,2,2,1octanyl, norbornyl, and spirobicyclo groups such as
spiro[4.51dec-8-yl.
[0109] "Cycloalkenyl" refers to non-aromatic cyclic alkyl groups of from 3 to
10 carbon
atoms having single or multiple cyclic rings and having at least one >C=C<
ring
unsaturation and preferably from 1 to 2 sites of >C=C< ring unsaturation.
22
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0110] "Substituted cycloalkyl" and "substituted cycloalkenyl" refers to a
cycloalkyl or
cycloalkenyl group having from 1 to 5 or preferably 1 to 3 substituents
selected from the
group consisting of oxo, thione, alkyl, substituted alkyl, alkenyl,
substituted alkenyl,
alkynyl, substituted alkynyl, alkoxy, substituted alkoxy, acyl, acylamino,
acyloxy, amino,
substituted amino, aminocarbonyl, aminothiocarbonyl, aminocarbonylamino,
aminothiocarbonylamino, aminocarbonyloxy, aminosulfonyl, aminosulfonyloxy,
aminosulfonylamino, amidino, aryl, substituted aryl, aryloxy, substituted
aryloxy, arylthio,
substituted arylthio, carboxyl, carboxyl ester, (carboxyl ester)amino,
(carboxyl ester)oxy,
cyano, cycloalkyl, substituted cycloalkyl, cycloalkyloxy, substituted
cycloalkyloxy,
cycloalkylthio, substituted cycloalkylthio, cycloalkenyl, substituted
cycloalkenyl,
cycloalkenyloxy, substituted cycloalkenyloxy, cycloalkenylthio, substituted
cycloalkenylthio, guanidino, substituted guanidino, halo, hydroxy, heteroaryl,
substituted
heteroaryl, heteroaryloxy, substituted heteroaryloxy, heteroarylthio,
substituted
heteroarylthio, heterocyclic, substituted heterocyclic, heterocyclyloxy,
substituted
heterocyclyloxy, heterocyclylthio, substituted heterocyclylthio, nitro, 503H,
substituted
sulfonyl, sulfonyloxy, thioacyl, thiol, alkylthio, and substituted alkylthio,
wherein said
substituents are defined herein.
[0111] "Cycloalkyloxy" refers to -0-cycloalkyl.
[0112] "Substituted cycloalkyloxy" refers to -0-(substituted cycloalkyl).
[0113] "Cycloalkylthio" refers to -5-cycloalkyl.
[0114] "Substituted cycloalkylthio" refers to -S-(substituted cycloalkyl).
[0115] "Cycloalkenyloxy" refers to -0-cycloalkenyl.
[0116] "Substituted cycloalkenyloxy" refers to -0-(substituted cycloalkenyl).
[0117] "Cycloalkenylthio" refers to -S-cycloalkenyl.
[0118] "Substituted cycloalkenylthio" refers to -S-(substituted cycloalkenyl).
[0119] "Guanidino" refers to the group -NHC(=NH)Nt12.
[0120] "Substituted guanidino" refers to -NR44c( NR44)Nr 44)2 where each R44
is
independently selected from the group consisting of hydrogen, alkyl,
substituted alkyl,
23
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
aryl, substituted aryl, heteroaryl, substituted heteroaryl, heterocyclic, and
substituted
heterocyclic and two R44 groups attached to a common guanidino nitrogen atom
are
optionally joined together with the nitrogen bound thereto to form a
heterocyclic or
substituted heterocyclic group, provided that at least one R44 is not
hydrogen, and wherein
said substituents are as defined herein.
[0121] "Halo" or "halogen" refers to fluoro, chloro, bromo and iodo and
preferably is
fluoro or chloro.
[0122] "Haloalkyl" refers to alkyl groups substituted with 1 to 5, 1 to 3, or
1 to 2 halo
groups, wherein alkyl and halo are as defined herein.
[0123] "Haloalkoxy" refers to alkoxy groups substituted with 1 to 5, 1 to 3,
or 1 to 2
halo groups, wherein alkoxy and halo are as defined herein.
[0124] "Haloalkylthio" refers to alkylthio groups substituted with 1 to 5, 1
to 3, or 1 to 2
halo groups, wherein alkylthio and halo are as defined herein.
[0125] "Hydroxy" or "hydroxyl" refers to the group -OH.
[0126] "Heteroaryl" refers to an aromatic group of from 1 to 10 carbon atoms
and 1 to 4
heteroatoms selected from the group consisting of oxygen, nitrogen and sulfur
within the
ring. Such heteroaryl groups can have a single ring (e.g., pyridyl, pyridinyl
or furyl) or
multiple condensed rings (e.g., indolizinyl or benzothienyl) wherein the
condensed rings
may or may not be aromatic and/or contain a heteroatom provided that the point
of
attachment is through an atom of the aromatic heteroaryl group. In one
embodiment, the
nitrogen and/or the sulfur ring atom(s) of the heteroaryl group are optionally
oxidized to
provide for the N-oxide (N¨>0), sulfinyl, and/or sulfonyl moieties. Preferred
heteroaryls
include pyridinyl, pyrrolyl, indolyl, thiophenyl, and furanyl.
[0127] "Substituted heteroaryl" refers to heteroaryl groups that are
substituted with from
1 to 5, preferably 1 to 3, or more preferably 1 to 2 substituents selected
from the group
consisting of the same group of substituents defined for substituted aryl.
[0128] "Heteroaryloxy" refers to -0-heteroaryl.
[0129] "Substituted heteroaryloxy" refers to the group -0-(substituted
heteroaryl).
24
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0130] "Heteroarylthio" refers to the group -S-heteroaryl.
[0131] "Substituted heteroarylthio" refers to the group -S-(substituted
heteroaryl).
[0132] "Heterocycle" or "heterocyclic" or "heterocycloalkyl" or "heterocycly1"
refers to
a saturated or partially saturated, but not aromatic, group having from 1 to
10 ring carbon
atoms and from 1 to 4 ring heteroatoms selected from the group consisting of
nitrogen,
sulfur, or oxygen. Heterocycle encompasses single ring or multiple condensed
rings,
including fused bridged and spiro ring systems. In fused ring systems, one or
more the
rings can be cycloalkyl, aryl, or heteroaryl provided that the point of
attachment is through
the non-aromatic heterocyclic ring. In one embodiment, the nitrogen and/or
sulfur atom(s)
of the heterocyclic group are optionally oxidized to provide for the N-oxide,
sulfinyl,
and/or sulfonyl moieties.
[0133] "Substituted heterocyclic" or "substituted heterocycloalkyl" or
"substituted
heterocycly1" refers to heterocyclyl groups that are substituted with from 1
to 5 or
preferably 1 to 3 of the same substituents as defined for substituted
cycloalkyl.
[0134] "Heterocyclyloxy" refers to the group -0-heterocycyl.
[0135] "Substituted heterocyclyloxy" refers to the group -0-(substituted
heterocycyl).
[0136] "Heterocyclylthio" refers to the group -S-heterocycyl.
[0137] "Substituted heterocyclylthio" refers to the group -S-(substituted
heterocycyl).
[0138] Examples of heterocycle and heteroaryls include, but are not limited
to, azetidine,
pyrrole, imidazole, pyrazole, pyridine, pyrazine, pyrimidine, pyridazine,
indolizine,
isoindole, indole, dihydroindole, indazole, purine, quinolizine, isoquinoline,
quinoline,
phthalazine, naphthylpyridine, quinoxaline, quinazoline, cinnoline, pteridine,
carbazole,
carboline, phenanthridine, acridine, phenanthroline, isothiazole, phenazine,
isoxazole,
phenoxazine, phenothiazine, imidazolidine, imidazoline, piperidine,
piperazine, indoline,
phthalimide, 1,2,3,4-tetrahydroisoquinoline, 4,5,6,7-
tetrahydrobenzo[b]thiophene,
thiazole, thiazolidine, thiophene, benzo[b]thiophene, morpholinyl,
thiomorpholinyl (also
referred to as thiamorpholinyl), 1,1-dioxothiomorpholinyl, piperidinyl,
pyrrolidine, and
tetrahydrofuranyl.
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0139] "Nitro" refers to the group -NO2.
[0140] "Oxo" refers to the atom (=0) or (-0).
[0141] "Spiro ring systems" refers to bicyclic ring systems that have a single
ring carbon
atom common to both rings.
[0142] "Sulfonyl" refers to the divalent group -S(0)2-.
[0143] "Substituted sulfonyl" refers to the group -S02-alkyl, -S02-substituted
alkyl, -S02-alkenyl, -S02-substituted alkenyl, -S02-cycloalkyl, -S02-
substituted
cylcoalkyl, -502-cycloalkenyl, -502-substituted cylcoalkenyl, -502-aryl, -502-
substituted
aryl, -502-heteroaryl, -502-substituted heteroaryl, -502-heterocyclic, -S02-
substituted
heterocyclic, wherein alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl,
substituted alkynyl, cycloalkyl, substituted cycloalkyl, cycloalkenyl,
substituted
cycloalkenyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl,
heterocyclic, and
substituted heterocyclic are as defined herein. Substituted sulfonyl includes
groups such as
methyl-S02-, phenyl-502-, and 4-methylpheny1-502-. The term "alkylsulfonyl"
refers
to -502-alkyl. The term "haloalkylsulfonyl" refers to -502-haloalkyl where
haloalkyl is
defined herein. The term "(substituted sulfonyl)amino" refers to -
NH(substituted
sulfonyl), and the term "(substituted sulfonyl)aminocarbonyl" refers to -
C(0)NH(substituted sulfonyl), wherein substituted sulfonyl is as defined
herein.
[0144] "Sulfonyloxy" refers to the group -0502-alkyl, -0502-substituted
alkyl, -0502-alkenyl, -0502-substituted alkenyl, -0502-cycloalkyl, -0502-
substituted
cylcoalkyl, -0502-cycloalkenyl, -0502-substituted
cylcoalkeny1,-0502-aryl, -0502-substituted aryl, -0502-heteroaryl, -0502-
substituted
heteroaryl, -0502-heterocyclic, -0S02-substituted heterocyclic, wherein alkyl,
substituted
alkyl, alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, cycloalkyl,
substituted
cycloalkyl, cycloalkenyl, substituted cycloalkenyl, aryl, substituted aryl,
heteroaryl,
substituted heteroaryl, heterocyclic, and substituted heterocyclic are as
defined herein.
[0145] "Thioacyl" refers to the groups H-C(S)-, alkyl-C(S)-, substituted alkyl-
C(S)-,
alkenyl-C(S)-, substituted alkenyl-C(S)-, alkynyl-C(S)-, substituted alkynyl-
C(S)-,
cycloalkyl-C(S)-, substituted cycloalkyl-C(S)-, cycloalkenyl-C(S)-,
substituted
cycloalkenyl-C(S)-, aryl-C(S)-, substituted aryl-C(S)-, heteroaryl-C(S)-,
substituted
26
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
heteroaryl-C(S)-, heterocyclic-C(S)-, and substituted heterocyclic-C(S)-,
wherein alkyl,
substituted alkyl, alkenyl, substituted alkenyl, alkynyl, substituted alkynyl,
cycloalkyl,
substituted cycloalkyl, cycloalkenyl, substituted cycloalkenyl, aryl,
substituted aryl,
heteroaryl, substituted heteroaryl, heterocyclic, and substituted heterocyclic
are as defined
herein.
[0146] "Thiol" refers to the group -SH.
[0147] "Thiocarbonyl" refers to the divalent group -C(S)- which is equivalent
to -C(=S)-.
[0148] "Thione" refers to the atom (=S).
[0149] "Alkylthio" refers to the group -S-alkyl wherein alkyl is as defined
herein.
[0150] "Substituted alkylthio" refers to the group -S-(substituted alkyl)
wherein
substituted alkyl is as defined herein.
[0151] "Compound" or "compounds" as used herein is meant to include the
stereoiosmers and tautomers of the indicated formulas.
[0152] "Stereoisomer" or "stereoisomers" refer to compounds that differ in the
chirality
of one or more stereocenters. Stereoisomers include enantiomers and
diastereomers.
[0153] "Tautomer" refer to alternate forms of a compound that differ in the
position of a
proton, such as enol-keto and imine-enamine tautomers, or the tautomeric forms
of
heteroaryl groups containing a ring atom attached to both a ring -NH- moiety
and a ring
=N- moiety such as pyrazoles, imidazoles, benzimidazoles, triazoles, and
tetrazoles.
[0154] As used herein, the term "phosphate ester" refers to any one of the
mono-, di- or
triphosphate esters of noribogaine, wherein the mono-, di- or triphosphate
ester moiety is
bonded to the 12-hydroxy group and/or the indole nitrogen of noribogaine.
[0155] As used herein, the term "phosphate ester" refers to any one of the
mono-, di- or
triphosphate esters of noribogaine, wherein the mono-, di- or triphosphate
ester moiety is
bonded to the 12-hydroxy group and/or the indole nitrogen of noribogaine.
[0156] As used herein, the term "monophosphate" refers to the group -
P(0)(OH)2.
27
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0157] As used herein, the term "diphosphate" refers to the group ¨P(0)(OH)-
0P(0)(OH)2.
[0158] As used herein, the term "triphosphate" refers to the group ¨P(0)(OH)-
(0P(0)(OH))20H.
[0159] As used herein, the term "ester" as it refers to esters of the mono-,
di- or
triphosphate group means esters of the monophosphate can be represented by the
formula
¨P(0)(0R45)2, where each R45 is independently hydrogen, Ci-C12 alkyl, C3-Cio
cycloalkyl,
C6-C14 aryl, heteroaryl of 1 to 10 carbon atoms and 1 to 4 optionally oxidized
heteroatoms
selected from the group consisting of oxygen, nitrogen, and sulfur and the
like, provided
that at least one R45 is not hydrogen. Likewise, exemplary esters of the di-
or triphosphate
can be represented by the formulas ¨P(0)(0R45)-0P(0)(0R45)2
and -P(0)(0R45)-(0P(0)(0R45))20R45, where R45 is as defined above.
[0160] As used herein, the term "hydrolyzable group" refers to a group that
can be
hydrolyzed to release the free hydroxy group under hydrolysis conditions.
Examples of
hydrolysable group include, but are not limited to those defined for R above.
Preferred
hydrolysable groups include carboxyl esters, phosphates and phosphate esters.
The
hydrolysis may be done by chemical reactions conditions such as base
hydrolysis or acid
hydrolysis or may be done in vivo by biological processes, such as those
catalyzed by a
phosphate hydrolysis enzyme. Nonlimiting examples of hydrolysable group
include
groups linked with an ester-based linker (-C(0)0- or -0C(0)-), an amide-based
linker
(-C(0)NR46- or ¨NR46C(0)-), or a phosphate-linker (-P(0)(0R46)-0-, -0-
P(S)(0R46)-0-, -
0-P(S)(SR46)-0-, -S-P(0)(0R46)-0-, -0-P(0)(0R46)-S-, -S-P(0)(0R46)-S-
, -0-P(S)(0R46)-S-, -S-P(S)(OR 46)-0-, -0-P(0)(R46)-0-, -0-P(S)(R46)-0-
, -S-P(0)(R46)-0-, -S-P(S)(R46)-0-, -S-P(0)(R46)-S-, or -0-P(S)(R46)-S-) where
R46 can be
hydrogen or alkyl.
[0161] Substituted groups of this invention, as set forth above, do not
include polymers
obtained by an infinite chain of substituted groups. At most, any substituted
group can be
substituted up to five times.
[0162] "Noribogaine" refers to the compound:
28
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
HO C2H5
as well as noribogaine derivatives or pharmaceutically acceptable salts and
pharmaceutically acceptable solvates thereof It should be understood that
where
"noribogaine" is mentioned herein, one more polymorphs of noribogaine can be
utilized
and are contemplated. In some embodiments, noribogaine is noribogaine
glucuronide. In
some embodiments, noribogaine does not refer to noribogaine derivatives,
although in
such embodiments noribogaine may still refer to solvates and/or salts of
noribogaine.
[0163] Noribogaine can be prepared by demethylation of naturally occurring
ibogaine:
H3C0 C2H5
which is isolated from Tabernanthe iboga, a shrub of West Africa.
Demethylation may be
accomplished by conventional techniques such as by reaction with boron
tribromide/methylene chloride at room temperature followed by conventional
purification.
See, for example, Huffman, et al., J. Org. Chem. 50:1460 (1985), which
incorporated
herein by reference in its entirety. Noribogaine can be synthesized as
described, for
example in U.S. Patent Pub. Nos. 2013/0165647, 2013/0303756, and 2012/0253037,
and
PCT Patent Publication No. WO 2013/040471 (includes description of making
noribogaine polymorphs), each of which is incorporated herein by reference in
its entirety.
[0164] "Noribogaine derivatives" refer to esters or 0-carbamates of
noribogaine, or
pharmaceutically acceptable salts and/or solvates of each thereof Also
encompassed
within this invention are derivatives of noribogaine that act as prodrug forms
of
noribogaine. A prodrug is a pharmacological substance administered in an
inactive (or
significantly less active) form. Once administered, the prodrug is metabolized
in vivo into
an active metabolite. Noribogaine derivatives include, without limitation,
those
compounds set forth in U.S. Patent Nos. 6,348,456; 8,362,007; and 8,741,891;
as well as
in U.S. Patent Application Publication Nos. US2015/0231146; U52014/0315891;
29
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
US2013/0131046; US2013/0165647; US2013/0165425; and US2013/0165414; each of
which is incorporated herein by reference in its entirety.
[0165] In some embodiments, the methods of the present disclosure entail the
administration of a prodrug of noribogaine. A prodrug of noribogaine refers to
a
compound that metabolizes, in vivo, to noribogaine. In some embodiments, the
prodrug is
selected to be readily cleavable either by a cleavable linking arm or by
cleavage of the
prodrug entity that binds to noribogaine such that noribogaine is generated in
vivo. In one
preferred embodiment, the prodrug moiety is selected to facilitate binding to
the II and/or
K receptors in the brain either by facilitating passage across the blood brain
barrier or by
targeting brain receptors other than the II and/or lc receptors. Examples of
prodrugs of
noribogaine are provided in United States Patent No. 8,741,891, the entire
content of
which is incorporated herein by reference.
[0166] This invention is not limited to any particular chemical form of
noribogaine, and
the drug may be given to patients either as a free base, solvate, or as a
pharmaceutically
acceptable acid addition salt. In the latter case, the hydrochloride salt is
generally
preferred, but other salts derived from organic or inorganic acids may also be
used.
Examples of such acids include, without limitation, those described below as
"pharmaceutically acceptable salts" and the like.
[0167] "Pharmaceutically acceptable composition" refers to a composition that
is
suitable for administration to a human. Such compositions include various
excipients,
diluents, carriers, and such other inactive agents well known to the skilled
artisan.
[0168] "Pharmaceutically acceptable salt" refers to pharmaceutically
acceptable salts,
including pharmaceutically acceptable partial salts, of a compound, which
salts are derived
from a variety of organic and inorganic counter ions well known in the art and
include, by
way of example only, hydrochloric acid, hydrobromic acid, phosphoric acid,
sulfuric acid,
methane sulfonic acid, phosphorous acid, nitric acid, perchloric acid, acetic
acid, tartaric
acid, lactic acid, succinic acid, citric acid, malic acid, maleic acid,
aconitic acid, salicylic
acid, thalic acid, embonic acid, enanthic acid, oxalic acid and the like, and
when the
molecule contains an acidic functionality, include, by way of example only,
sodium,
potassium, calcium, magnesium, ammonium, tetraalkylammonium, and the like.
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0169] "Therapeutically effective amount" or "therapeutic amount" refers to an
amount
of a drug or an agent that, when administered to a patient suffering from a
condition, will
have the intended therapeutic effect, e.g., alleviation, amelioration,
palliation or
elimination of one or more manifestations of the condition in the patient. The
therapeutically effective amount will vary depending upon the patient and the
condition
being treated, the weight and age of the subject, the severity of the
condition, the salt or
solvate of the active drug portion chosen, the particular composition or
excipient chosen,
the dosing regimen to be followed, timing of administration, the manner of
administration
and the like, all of which can be determined readily by one of ordinary skill
in the art. The
full therapeutic effect does not necessarily occur by administration of one
dose, and may
occur only after administration of a series of doses. Thus, a therapeutically
effective
amount may be administered in one or more administrations. For example, and
without
limitation, a therapeutically effective amount of noribogaine or noribogaine
derivative, in
the context of treating opioid drug dependency, refers to an amount of
noribogaine or
noribogaine derivative that attenuates the physical dependency and/or symptoms
of acute
withdrawal for at least about 2 hours beyond control (placebo), at least about
5 hours
beyond control, preferably at least about 10 hours beyond control, and more
preferably at
least about 15 hours beyond control. In an especially preferred embodiment, a
therapeutically effective amount of noribogaine or noribogaine derivative
attenuates the
physical dependency and/or symptoms of acute withdrawal for at least about 24
hours or
at least about 48 hours. In one embodiment, a therapeutically effective amount
of
noribogaine or noribogaine derivative, in the context of treating opioid drug
dependency,
refers to an amount of noribogaine or noribogaine derivative that reduces
withdrawal
symptoms (e.g., as determined by evaluation on an opioid withdrawal scale) for
at least
about 2 hours, 5 hours, 10 hours, 24 hours, or 48 hours. In one embodiment, a
therapeutically effective amount of noribogaine or noribogaine derivative, in
the context
of treating opioid drug dependency, refers to an amount of noribogaine or
noribogaine
derivative that reduces cravings for the opioid of dependency for at least
about 2 hours, 5
hours, 10 hours, 24 hours, or 48 hours. In one embodiment, a therapeutically
effective
amount of noribogaine or noribogaine derivative, in the context of treating
opioid drug
dependency, refers to an amount of noribogaine or noribogaine derivative that
improves
mood of the patient for at least about 2 hours, 5 hours, 10 hours, 24 hours,
or 48 hours.
31
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0170] A "therapeutic level" of a drug is an amount of noribogaine,
noribogaine
derivative, or pharmaceutical salt or solvate thereof that is sufficient to
treat opioid drug
dependency or to treat, prevent, or attenuate acute withdrawal symptoms, but
not high
enough to pose any significant risk to the patient. Therapeutic levels of
drugs can be
determined by tests that measure the actual concentration of the compound in
the blood of
the patient. This concentration is referred to as the "serum concentration."
Where the
serum concentration of noribogaine is mentioned, it is to be understood that
the term
"noribogaine" encompasses any form of noribogaine, including derivatives
thereof
[0171] The term "noribogaine treatment protocol" refers to a treatment
protocol for
administration of noribogaine or derivative that provides a therapeutic
aggregate dose of
noribogaine (or derivative, or salt or solvate thereof) such that the
patient's QT interval is
expected to remain below about 500 ms, and preferably below about 470 ms. An
alternate
protocol is the treatment protocol that is changed based on the factors
described herein
(e.g., inefficient/insufficient opioid metabolism, clearance of opioid from
the body, etc.).
[0172] The term "dose" refers to a range of noribogaine, noribogaine
derivative, or
pharmaceutical salt or solvate thereof that provides a therapeutic serum level
of
noribogaine or noribogaine derivative when given to a patient in need thereof
The dose is
recited in a range, for example from about 20 mg to about 120 mg, and can be
expressed
either as milligrams or as mg/kg body weight. The attending clinician will
select an
appropriate dose from the range based on the patient's weight, age, degree of
dependency,
health, and other relevant factors, all of which are well within the skill of
the art.
[0173] The term "aggregate dosage" refers to a combined dosage, for example
the total
amount of noribogaine, noribogaine derivative, or pharmaceutically acceptable
salt or
solvate thereof administered over a 24-hour period, where smaller amounts are
administered more than once per day.
[0174] The term "unit dose" refers to a dose of drug that is given to the
patient to
provide therapeutic results, independent of the weight of the patient. In such
an instance,
the unit dose is sold in a standard form (e.g., 10 mg or 20 mg tablet). The
unit dose may be
administered as a single dose or a series of subdoses. In some embodiments,
the unit dose
provides a standardized level of drug to the patient, independent of weight of
the patient.
Many medications are sold based on a dose that is therapeutic to all patients
based on a
32
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
therapeutic window. In such cases, it is not necessary to titrate the dosage
amount based
on the weight of the patient.
[0175] "Treatment," "treating," and "treat" are defined as acting upon a
disease,
disorder, or condition with an agent to reduce or ameliorate harmful or any
other undesired
effects of the disease, disorder, or condition and/or its symptoms.
"Treatment," as used
herein, covers the treatment of a human patient, and includes: (a) reducing
the risk of
occurrence of the condition in a patient determined to be predisposed to the
condition but
not yet diagnosed as having the condition, (b) impeding the development of the
condition,
and/or (c) relieving the condition, i.e., causing regression of the condition
and/or relieving
one or more symptoms of the condition. "Treating" or "treatment of' a
condition or
patient refers to taking steps to obtain beneficial or desired results,
including clinical
results such as the reduction of symptoms. For purposes of this invention,
beneficial or
desired clinical results include, but are not limited to: treating opioid drug
dependency;
treating, preventing, eliminating, and/or attenuating acute withdrawal
symptoms; treating,
preventing, and/or attenuating long-term (post-acute) withdrawal symptoms;
treating,
preventing, eliminating, and/or attenuating cravings; and preventing relapse
of opioid drug
use.
[0176] As used herein, the term "patient" refers to humans.
[0177] As used herein, the term "opiate" refers to naturally-occurring
alkaloids found in
the opium poppy. These include codeine, morphine, oripavine, pseudomorphine,
and
thebaine. Also included are opium, opium poppy, poppy straw, and extracts and
concentrates thereof
[0178] As used herein, the term "opioid" refers to naturally-occurring opiates
and
synthetic or semi-synthetic opioids that have psychoactive effects. Non-
limiting examples
include acetyl-alpha-methylphentanyl, acetylmethadol, alfentanil,
allylprodine,
alphacetylmethadol, alphamethadol, alpha-methylfentanyl, alpha-
methylthiofentanyl,
alphaprodine, anileridine, benzylmorphine, benzethidine, betacetylmethadol,
beta-
hy droxyfentanyl, beta-hydroxy-3-methylfentanyl, betameprodine,
betacetylmethadol,
beta-hydroxyfentanyl, beta-hydroxy-3-methylfentanyl, betameprodine,
betamethadol,
betaprodine, bezitramide, buprenorphine, butorphanol, carfentanil,
clonitazene, codeine,
desomorphine, dextromoramide, dextropropoxyphene, dezocine, diampromide,
33
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
diamorphone, diethylthiambutene, dihydrocodeine, dihydroetorphine,
dihydromorphine,
dimenoxadol, dimepheptanol, dimethyl- thiambutene, dioxaphetyl butyrate,
diphenoxylate,
difenoxin, dipipanone, eptazocine, ethoheptazine, ethylmethylthiambutene,
ethylmorphine,
etonitazene, etorphine, etoxeridine, fentanyl, furethidine, heroin,
hydrocodone,
hydromorphone, hydroxypethidine, isomethadone, ketobemidone, levo-
alphacetylmethadol, levomethorphan, levorphanol, levophenacylmorphan,
levomoramide,
lofentanil, loperamide, laudanum, meperidine, meptazinol, metazocine,
methadone, 3-
methylfentanyl, 3-methylthiofentanyl, metopon, morphine, morpheridine, MPPP (1-
methy1-4-pheny1-4-propionoxypiperidine), myrophine, narceine, nicomorphine,
noracymethadol, norlevorphanol, normethadone, nalorphine, nalbuphene,
normorphine,
norpipanone, opium, oxycodone, oxymorphone, papaveretum, para-fluorofentanyl,
paregoric, PEPAP (1-(-2-phenethyl)-4-phenyl-4-acetoxypiperidine), pentazocine,
phenadoxone, phenampromide, phenomorphan, phenazocine, phenoperidine,
piminodine,
piritramide, propheptazine, promedol, properidine, propiram, propoxyphene,
racemoramide, racemethorphan, racemorphan, remifentanil, sufentanil,
tapentadol,
thiofentanyl, tilidine, tramadol, trimeperidine, mixtures of any of the
foregoing, salts of
any of the foregoing, derivatives of any of the foregoing, and the like. The
term opioids
also encompasses opioid intermediates, including 4-cyano-2-dimethylamino-4,4-
diphenyl
butane, 2-methy1-3-morpholino-1,1-diphenylpropane-carboxylic acid, 4-cyano-1-
methy1-
4-phenylpiperidine, ethyl-4-phenylpiperidine-4-carboxylate, and 1-methy1-4-
phenylpiperidine-4-carboxylic acid. Many opioids are Schedule I or Schedule II
drugs in
the US. In some embodiments, one or more of the above-listed opioids can be
specifically
excluded.
[0179] As used herein, the term "QT interval" refers to the measure of the
time between
the start of the Q wave and the end of the T wave in the electrical cycle of
the heart.
Prolongation of the QT interval refers to an increase in the QT interval.
[0180] As used herein, a "patient who has been evaluated for acceptable QT
interval
prolongation", "patient at low risk for QT interval prolongation" and the like
refers to a
patient who has been pre-screened by a clinician and determined to have an
acceptably
low risk for QT interval prolongation. Exemplary criteria for pre-screening
are described
herein, including history of using drugs that are known to prolong QT interval
in at least a
subset of patients (e.g., cocaine, methadone, and a number of prescription
drugs), personal
34
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
or familial history of cardiac conditions (including long QT syndrome), and a
longer-than-
average QT interval. A continuously updated list of medications that are known
to cause a
prolongation of the QT interval is maintained at www.crediblemeds.org. In one
embodiment, the patient is prescreened by treatment with a low dose of
noribogaine,
noribogaine derivative, or salt or solvate thereof and the patient's QT
interval is evaluated
for a period of time to determine whether a therapeutic amount of the drug
would result in
an unacceptable QT interval in that patient. In one embodiment, a patient who
previously
exhibited unacceptable QT interval prolongation upon treatment with ibogaine,
noribogaine, noribogaine derivative, or salt or solvate thereof is deemed to
have an
unacceptable risk of QT interval prolongation.
[0181] As used herein, the terms "addiction" and "dependence" are used
interchangeably
to refer to the patient's inability to stop using the opioid, even when it
would be in his/her
best interest to stop. The DSM-5 criteria for opioid dependency include:
A problematic pattern of opioid use leading to clinically significant
impairment or distress, as manifested by at least two of the
following, occurring within a 12-month period:
= Opioids are often taken in larger amounts or over a longer period than
was
intended.
= There is a persistent desire or unsuccessful efforts to cut down or
control opioid
use.
= A great deal of time is spent in activities necessary to obtain the
opioid, use the
opioid, or recover from its effects.
= Craving, or a strong desire or urge to use opioids.
= Recurrent opioid use resulting in a failure to fulfill major role
obligations at
work, school, or home.
= Continued opioid use despite having persistent or recurrent social or
interpersonal problems caused or exacerbated by the effects of opioids.
= Important social, occupational, or recreational activities are given up
or
reduced because of opioid use.
= Recurrent opioid use in situations in which it is physically hazardous.
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
= Continued opioid use despite knowledge of having a persistent or
recurrent
physical or psychological problem that is likely to have been caused or
exacerbated by the substance.
= Tolerance, as defined by either of the following:
o A need for markedly increased amounts of opioids to achieve intoxication
or desired effect.
o A markedly diminished effect with continued use of the same amount of an
opioid.
= Withdrawal, as manifested by either of the following:
o The characteristic opioid withdrawal syndrome (refer to Criteria A and B
of
the criteria set for opioid withdrawal).
o Opioids (or a closely related substance) are taken to relieve or avoid
withdrawal symptoms.
[0182] The term "solvate" as used herein refers to complexes with solvents in
which
noribogaine, or noribogaine derivative, is reacted or from which noribogaine,
or
noribogaine derivative, is precipitated or crystallized. For example, a
complex with water
is known as a "hydrate". Solvates of noribogaine and noribogaine derivatives
are within
the scope of the invention. It will be appreciated by those skilled in organic
chemistry that
many organic compounds can exist in more than one crystalline form. For
example,
crystalline form may vary based on the solvate used. Thus, all crystalline
forms of
noribogaine, noribogaine derivative, or the pharmaceutically acceptable
solvates thereof
are within the scope of the present invention.
Compositions of the Invention
[0183] As will be apparent to the skilled artisan upon reading this
disclosure, this
invention provides compositions for treating substance abuse in a subject,
comprising
noribogaine, noribogaine derivatives, prodrugs of noribogaine,
pharmaceutically
acceptable salts and/or solvates of each thereof This invention further
provides
compositions for treating, attenuating, or preventing withdrawal symptoms in a
drug-
dependent subject, comprising noribogaine, noribogaine derivatives, prodrugs
of
noribogaine, pharmaceutically acceptable salts and/or solvates of each thereof
36
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0184] In some embodiments, the composition comprises a noribogaine
derivative.
Noribogaine derivatives include, without limitation, those compounds set forth
in U.S.
Patent Nos. 6,348,456; 8,362,007; and 8,741,891; as well as in U.S. Patent
Application
Publication Nos. US2014/0315891; US2013/0131046; US2013/0165647;
US2013/0165425; and US2013/0165414; each of which is incorporated herein by
reference in its entirety.
[0185] In some embodiments, the composition is formulated for oral,
transdermal,
internal, pulmonary, rectal, nasal, vaginal, lingual, intravenous,
intraarterial,
intramuscular, intraperitoneal, intracutaneous or subcutaneous delivery.
[0186] In one embodiment, the noribogaine derivative is represented by Formula
I:
Oil
or a pharmaceutically acceptable salt and/or solvate thereof,
wherein R is hydrogen or a hydrolyzable group such as hydrolyzable esters of
from about
1 to 12 carbons.
[0187] Generally, in the above formula, R is hydrogen or a group of the
formula:
0
¨C¨X
wherein X is a Ci-C12 group, which is unsubstituted or substituted. For
example, X may be
a linear alkyl group such as methyl, ethyl, n-propyl, n-butyl, n-pentyl, n-
hexyl, n-heptyl, n-
octyl, n-nonyl, n -decyl, n-undecyl or n-dodecyl, or a branched alkyl group,
such as i-
propyl or sec-butyl. Also, X may be a phenyl group or benzyl group, either of
which may
be substituted with lower alkyl groups or lower alkoxy groups. Generally, the
lower alkyl
and/or alkoxy groups have from 1 to about 6 carbons. For example, the group R
may be
acetyl, propionyl or benzoyl. However, these groups are only exemplary.
37
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0188] Generally, for all groups X, they may either be unsubstituted or
substituted with
lower alkyl or lower alkoxy groups. For example, substituted X may be o-, m-
or p-methyl
or methoxy benzyl groups.
[0189] C1-C12 groups include Ci-C12 alkyl, C3-C12 cycloalkyl, C6-C12 aryl, C7-
C12
arylalkyl, wherein Cx indicates that the group contains x carbon atoms. Lower
alkyl refers
to Ci-C4 alkyl and lower alkoxy refers to Ci-C4 alkoxy.
[0190] In one embodiment, the noribogaine derivative is represented by Formula
II:
R1
L-R5
.%
R4
R-
or a pharmaceutically acceptable salt and/or solvate thereof,
wherein
- is a single or double bond;
RI- is halo, OR2, or C1-C12 alkyl optionally substituted with 1 to 5 RE);
R2 is hydrogen or a hydrolysable group selected from the group consisting of -
C(0)R', -C(0)OR' and -C(0)N(R)2 where each Rx is selected from the
group consisting of C1-C6 alkyl optionally substituted with 1 to 5 RE), and
each RY is independently selected from the group consisting of hydrogen,
Ci-C6 alkyl optionally substituted with 1 to 5 Rth, C6-C14 aryl optionally
substituted with 1 to 5 Rth, C3-C10 cycloalkyl optionally substituted with 1
to 5 Rth, C1-C10 heteroaryl having 1 to 4 heteroatoms and which is
optionally substituted with 1 to 5 Rth, Ci-Cio heterocyclic having 1 to 4
heteroatoms and which is optionally substituted with 1 to 5 Rth, and where
each RY, together with the nitrogen atom bound thereto form a Ci-C6
heterocyclic having 1 to 4 heteroatoms and which is optionally substituted
with 1 to 5 Rth or a Ci-C6 heteroaryl having 1 to 4 heteroatoms and which is
optionally substituted with 1 to 5 Rth;
38
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
R3 is selected from the group consisting of hydrogen, Ci-C12 alkyl optionally
substituted with 1 to 5 R19, aryl optionally substituted with 1 to 5 R19, -
C(0)R6, -C(0)NR6R6 and -C(0)0R6;
R4 is selected from the group consisting of hydrogen, -(CH2)m0R8, -
CR7(OH)R8, -(CH2)mCN, -(CH2)mCOR8, -(CH2)mCO2R8, -(CH2)mC(0)NR7
R8, -(CH2)mC(0)NR7NR8R8, -(CH2)mC(0)NR7NR8C(0)R9,
and -(CH2)mNR7R8;
m is 0, 1, or 2;
L is a bond or Ci-C12 alkylene;
R5 is selected from the group consisting of hydrogen, Ci-C12 alkyl substituted
with
1 to 5 RI-9, Ci-C12 alkenyl substituted with 1 to 5 RI-9,
R7, -SO2NR7R8, -0-C(0)R9, -C(0)0R8, -C(0)NR7R8, -NR7R8, -
NHC(0)R9, and -NR7C(0)R9;
each R6 is independently selected from the group consisting of hydrogen, C1-
C12
alkyl, C2-C12 alkenyl, C2-C12 alkynyl, C6-Cio aryl, Ci-C6 heteroaryl haying 1
to 4 heteroatoms, and C1-C6 heterocycle haying 1 to 4 heteroatoms, and
wherein the alkyl, alkenyl, alkynyl, aryl, heteroaryl, and heterocycle are
optionally substituted with 1 to 5 RI-9;
X1 is selected from the group consisting of 0 and S;
Y is C1-C4 alkylene or C6-Cio arylene, or a combination thereof;
n is 1, 2, or 3;
R7 and R8 are each independently selected from the group consisting of
hydrogen,
Ci-C12 alkyl optionally substituted with 1 to 5 RI-9, Ci-C6 heterocycle
haying 1 to 4 heteroatoms and which is optionally substituted with 1 to 5
R' ,
C3-C10 cycloalkyl optionally substituted with 1 to 5 RI-9, C6-Cio aryl
optionally substituted with 1 to 5 R19 and Ci-C6 heteroaryl haying 1 to 4
heteroatoms optionally substituted with 1 to 5 R19;
R9 is selected from the group consisting of Ci-C12 alkyl optionally
substituted with
1 to 5 RI-9, C1-C6 heterocycle haying 1 to 4 heteroatoms optionally
substituted with 1 to 5 R19, C3-Cio cycloalkyl optionally substituted with 1
to 5 RI-9, C6-C10 aryl optionally substituted with 1 to 5 RI-9 and C1-C6
heteroaryl haying 1 to 4 heteroatoms optionally substituted with 1 to 5 R19;
39
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
Rth is selected from the group consisting of CI-CI alkyl, phenyl, halo, -OR", -
CN, -COR11, -CO2R11, -C(0)NHR11, -NR11R11, _C(0)NRiiRii, _c(0)NHN
HRH, -C(0)NR11NHR11, -C(0)NR11NR11R11, _C(0)NHNR11C(0)R11, -C(
0)NHNHC(0)R11, -SO2NR11R11, _C(0)NR11NR11C(0)R11,
and -C(0)NRHNHC(0)R11; and
RH is independently hydrogen or Ci-C12 alkyl;
provided that:
when L is a bond, then R5 is not hydrogen;
when - is a double bond, R1 is an ester hydrolyzable group, R3 and R4 are
both hydrogen, then -L-R5 is not ethyl;
when - is a double bond, R1 is -OH, halo or Cl-C12 alkyl optionally
substituted with 1 to 5 R1 , then R4 is hydrogen; and
when - is a double bond, R1 is OR2, R4 is hydrogen, -L-R5 is ethyl, then R2 is
not a hydrolyzable group selected from the group consisting of an ester,
amide, carbonate and carbamate.
[0191] In one embodiment, the noribogaine derivative is represented by Formula
III:
R12 R14
R13
III
or a pharmaceutically acceptable salt and/or solvate thereof,
wherein
- is a single or double bond;
R12 is halo, -OH, -SH, -NH2, -S(0)2N(R17)2, -
Rz-L1-CHR18R19, where Rz is 0, S or NR17;
L1 is alkylene, arylene, -C(0)-alkylene, -C(0)-arylene, -C(0)0-arylene, -C(0)0-
alkylene, -C(0)NR20-alkylene, -C(0)NR20-arylene, -C(NR20)NR20-alkylene
or -C(NR20)NR20-arylene, wherein L1 is configured such that -0-L1-R18 is _
OC(0)-alkylene-R18, -0C(0)0-arylene-R18, -0C(0)0-alkylene-R18, -
OC(0)-arylene-R18, -0C(0)NR20-alkylene-R18, -0C(0)NR20-arylene-R18, -
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
OC(NR29)NR29-alkylene-R18 or -0C(NR29)NR29-arylene-R18, and wherein
the alkylene and arylene are optionally substituted with 1 to 2 RI-6;
RI-3 is hydrogen, -S(0)20R29, -S(0)2R29, -C(0)R15, -C(0)NR15R15, -C(0)0R15, C1-
C12 alkyl optionally substituted with 1 to 5 R16, C1-C12 alkenyl optionally
substituted with 1 to 5 R16, or aryl optionally substituted with 1 to 5 R16;
R14 is hydrogen, halo, -0R17, -CN, CI-Cu alkyl, CI-Cu alkoxy, aryl or aryloxy,
where the alkyl, alkoxy, aryl, and aryloxy are optionally substituted with 1
to 5 R16;
each R15 is independently selected from the group consisting of hydrogen, Cl-
C12
alkyl, C2-C12 alkenyl, C2-C12 alkynyl, aryl, heteroaryl, and heterocycle, and
wherein the alkyl, alkenyl, alkynyl, aryl, heteroaryl, and heterocycle are
optionally substituted with 1 to 5 R16;
R16 is selected from the group consisting of phenyl, halo, -0R17, -
CN, -COR17, -CO2R17, -NR17R17, -NR17C(0)R17, -NR17S02R17, -C(0)NR17
R17, -C(0)NR17NR17R17, -SO2NR17R17 and -C(0)NR17NR17C(0)R17;
each R17 is independently hydrogen or CI-Cu alkyl optionally substituted with
from 1 to 3 halo;
R18 is hydrogen, -C(0)R29, -C(0)0R29, -C(0)N(R29)2 or -N(R29)C(0)R29;
R19 is hydrogen, -
N(R29)2, -C(0)N(R29)2, -C(NR29)N(R29)2, -C(NS02R29)N(R29)2, -NR29C(0)
N(R29)2, -NR29C(S)N(R29)2, -NR29C(NR29)N(R29)2, -NR29C(NS02R29)N(R2
9)2 or tetrazole; and
each R29 is independently selected from the group consisting of hydrogen, Cl-
C12
alkyl and aryl;
provided that:
when - is a double bond and R13 and R14 are hydrogen, then R12 is not
hydroxy;
when - is a double bond, R14 is hydrogen, R12 is
R20, and L is alkylene, then _o_c_R29 are not
methoxy;
when - is a double bond, R14 is hydrogen, Rz is 0, L1 is -C(0)-
alkylene, -C(0)-arylene, -C(0)0-arylene, -C(0)0-alkylene, -C(0)NR29-
alkylene, or -C(0)NR29-arylene, then none of R18, R19 or R29 are hydrogen.
41
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0192] In one embodiment, the noribogaine derivative is represented by Formula
IV:
R210 C2H5
L2,
-R22 IV
or a pharmaceutically acceptable salt and/or solvate thereof,
wherein
R21 is selected from the group consisting of hydrogen, a hydrolysable group
selected from the group consisting of -C(0)R23, -C(0)NR24-25
x and -C(0)0R26, where R23
is selected from the group consisting of hydrogen, alkyl, substituted alkyl,
alkenyl,
substituted alkenyl, alkynyl and substituted alkynyl, R24 and R25 are
independently
selected from the group consisting of hydrogen, alkyl, substituted alkyl,
alkenyl,
substituted alkenyl, alkynyl, substituted alkynyl, aryl, substituted aryl,
heteroaryl,
substituted heteroaryl, heterocyclic and substituted heterocyclic, R26 is
selected from the
group consisting of alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl,
substituted alkynyl, aryl, substituted aryl, heteroaryl, substituted
heteroaryl, heterocyclic
and substituted heterocyclic, provided that R21 is not a saccharide or an
oligosaccharide;
L2 is selected from the group consisting of a covalent bond and a cleavable
linker
group;
R22 is selected from the group consisting of hydrogen, alkyl, substituted
alkyl,
alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, aryl, substituted
aryl, cycloalkyl,
substituted cycloalkyl, heteroaryl, substituted heteroaryl, heterocyclic, and
substituted
heterocyclic, provided that R is not a saccharide or an oligosaccharide;
provided that when L2 is a covalent bond and R22 is hydrogen, then R21 is
selected
-
from the group consisting of -C(0)NR24 x25 and -C(0)0R26; and
further provided that when R21 is hydrogen or -C(0)R23 and L2 is a covalent
bond,
then R22 is not hydrogen.
[0193] In one embodiment, the noribogaine derivative is represented by Formula
V:
42
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
,0
R27
28
V
or a pharmaceutically acceptable salt and/or solvate thereof,
wherein:
refers to a single or a double bond provided that when is a single bond,
Formula V refers to the corresponding dihydro compound;
R27 is hydrogen or S020R29;
R28 is hydrogen or S020R29;
R29 is hydrogen or Ci- C6 alkyl;
provided that at least one of R27 and R28 is not hydrogen.
[0194] In one embodiment, the noribogaine derivative is represented by Formula
VI:
,0
R3 ss,
'R31
VI
or a pharmaceutically acceptable salt and/or solvate thereof,
wherein:
refers to a single or a double bond provided that when is a single bond,
Formula VI refers to the corresponding vicinal dihydro compound;
R3 is hydrogen, a monophosphate, a diphosphate or a triphosphate; and
R31 is hydrogen, a monophosphate, a diphosphate or a triphosphate;
provided that both R3 and R31 are not hydrogen;
wherein one or more of the monophosphate, diphosphate and triphosphate groups
of R3
and R31 are optionally esterified with one or more Ci-C6 alkyl esters.
[0195] In some embodiments, the unit dose of noribogaine, noribogaine
derivative, or
pharmaceutically acceptable salt or solvate thereof is from about 10 mg to
about 120 mg.
In one embodiment, the unit dose is about 10 mg. In one embodiment, the unit
dose is
about 20 mg. In one embodiment, the unit dose is about 30 mg. In one
embodiment, the
43
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
unit dose is about 40 mg. In one embodiment, the unit dose is about 50 mg. In
one
embodiment, the unit dose is about 60 mg. In one embodiment, the unit dose is
about 70
mg. In one embodiment, the unit dose is about 80 mg. In one embodiment, the
unit dose is
about 90 mg. In one embodiment, the unit dose is about 100 mg. In one
embodiment, the
unit dose is about 110 mg. In one embodiment, the unit dose is about 120 mg.
[0196] In some embodiments, the formulation is designed for periodic
administration,
such as once, twice, three time, four times or five time daily with
noribogaine, noribogaine
derivative, or a pharmaceutically acceptable salt or solvate thereof In some
embodiments,
the administration is once daily, or once every second day, once every third
day, three
times a week, twice a week, or once a week. The dosage and frequency of the
administration depends on the route of administration, content of composition,
age and
body weight of the patient, condition of the patient, QT interval (before
and/or during
treatment), without limitation. Determination of dosage and frequency suitable
for the
present technology can be readily made a qualified clinician.
[0197] In some embodiments, the formulation designed for administration in
accordance
with the methods provide herein can be suitable for a variety of delivery
modes including,
without limitation, oral and transdermal delivery. Formulations suitable for
internal,
pulmonary, rectal, nasal, vaginal, lingual, intravenous, intra-arterial,
intramuscular,
intraperitoneal, intracutaneous and subcutaneous routes may also be used.
Possible
formulations include tablets, capsules, pills, powders, aerosols,
suppositories, parenterals,
and oral liquids, including suspensions, solutions and emulsions. Sustained
release dosage
forms may also be used. All formulations may be prepared using methods that
are
standard in the art (see e.g., Remington's Pharmaceutical Sciences, 16th ed.,
A. Oslo
editor, Easton Pa. 1980).
[0198] In a preferred embodiment, the formulation is designed for oral
administration,
which may conveniently be provided in tablet, caplet, sublingual, liquid or
capsule form.
In certain embodiments, the noribogaine is provided as noribogaine HC1, with
dosages
reported as the amount of free base noribogaine. In some embodiments, the
noribogaine
HC1 is provided in hard gelatin capsules containing only noribogaine HC1 with
no
excipients.
44
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0199] The opioid antagonist (or partial antagonist) may be any agent that
blocks opioid
interaction with a receptor, e.g. an opioid receptor, so as to block the
effects of the opioid
in a mammal. In some embodiments, the opioid antagonist (or partial
antagonist) blocks
subjective effects. "Subjective effects" include the feeling of being "high"
that is normally
associated with opioid drug use. In one embodiment, the amount of opioid
antagonist (or
partial antagonist) administered is sufficient to reduce or eliminate opioid
cravings in the
patient. Where the term "opioid antagonist" is used herein, it is to be
understood that a
partial opioid antagonist may be substituted.
[0200] Any formulation of the opioid antagonist may be used in the methods
described
herein. In some embodiments, the opioid antagonist is formulated for oral,
transdermal,
internal, pulmonary, rectal, nasal, vaginal, lingual, intravenous,
intraarterial,
intramuscular, intraperitoneal, intracutaneous or subcutaneous delivery. In
one
embodiment, the opioid antagonist is formulated for oral administration. In a
preferred
embodiment, the opioid antagonist is formulated for injection. In a
particularly preferred
embodiment, the opioid antagonist is in an extended-release formulation.
III. Methods of the Invention
[0201] As will be apparent to the skilled artisan upon reading this
disclosure,
embodiments of the present invention provide a method for treating opioid
abuse,
including acute and post-acute withdrawal symptoms, in an opioid-dependent
patient. In
some embodiments, the patient is evaluated for acceptable QT interval
prolongation prior
to administration of noribogaine, noribogaine derivative, or pharmaceutically
acceptable
salt or solvate thereof
[0202] In some aspects, this invention further relates to treatment of the
patient with an
opioid antagonist or partial antagonist.
[0203] In some aspects, the patient insufficiently metabolizes the opioid(s)
and the
method comprises administering to the patient noribogaine, noribogaine
derivative, or
pharmaceutically acceptable salt or solvate thereof according to an altered
treatment
protocol. That is, the patient having insufficient opioid metabolism is
administered
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
for a longer duration, at a higher dose, and/or in combination with a drug
that facilitates
metabolism of the opioid.
[0204] Embodiments of the present invention further provide a method for
treating an
opioid-dependent patient comprising periodically administering noribogaine,
noribogaine
derivative, or pharmaceutically acceptable salt or solvate thereof to the
patient, monitoring
the patient's opioid level over time, and reducing the amount of the periodic
dose when
the opioid level falls below a desired threshold. Without being bound by
theory, it is
believed that a low serum concentration of noribogaine, noribogaine
derivative, or
pharmaceutically acceptable salt or solvate thereof (e.g., as low as 20 ng/mL
to 100
ng/mL) will be effective at reducing withdrawal symptoms in a patient having
little or no
opioid remaining in the brain and/or body. In one embodiment, the amount of
noribogaine,
noribogaine derivative, or pharmaceutically acceptable salt or solvate thereof
administered
to the patient is reduced about 24 to about 72 hours after administration of
the first dose of
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof
Patient Pre-screening and Monitoring
[0205] Without being bound by theory, it is believed that patients who are
predisposed
to QT interval prolongation are more likely to experience dangerous or life-
threatening QT
interval prolongation upon treatment with noribogaine, noribogaine derivative,
or
pharmaceutically acceptable salt or solvate thereof Accordingly, this
invention is directed
to minimizing the risk of an adverse QT interval prolongation in a patient
during
noribogaine treatment by excluding from treatment any patient having an
increased risk
factor for an unacceptable QT interval prolongation during the treatment. In
one aspect,
this invention is further directed to pre-screening a patient for a risk
factor for an
unacceptable QT interval prolongation.
[0206] An "acceptable risk level" in this context indicates that the patient
is at low risk
of developing QT interval prolongation of greater than 60 milliseconds upon
administration of noribogaine, noribogaine derivative, or pharmaceutically
acceptable salt
or solvate thereof That is, the patient does not have one or more risk factors
associated
with increased QT interval prolongation. Risk factors associated with
increased QT
interval prolongation include, without limitation: genetic factors (e.g., pre-
disposition to
prolonged QT interval); pre-existing condition (e.g., cardiac condition or
other condition
46
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
that makes the patient susceptible to QT interval prolongation and/or causes
QT interval
prolongation); current or previous use of drugs (prescription or illicit) that
are known to
have a risk of QT interval prolongation; and previous unacceptable QT response
(i.e.,
greater than 60 milliseconds (ms) prolongation or QT interval over 500 ms) to
ibogaine,
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof
[0207] In one embodiment, patients deemed suitable for such treatment have
been
evaluated for one or more of the following: genetic/familial risk factors,
preexisting
conditions, history of using drugs (e.g., prescription or illicit) that are
known to have a risk
of QT interval prolongation, and/or previous unacceptable QT response to
ibogaine,
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof
Preferably, all of such risk factors are included in the evaluation, as well
as others deemed
appropriate by the attending or pre-screening clinician.
[0208] Pre-screening of patients before treatment with noribogaine,
noribogaine
derivative, or a pharmaceutically acceptable salt or solvate thereof and/or
monitoring of
patients during noribogaine treatment may be performed to ensure that QT
interval is not
prolonged beyond a certain value. For example, QT interval greater than about
500 ms can
be considered dangerous for individual patients. For example, pre-screening
and/or
monitoring may be necessary at high levels of noribogaine treatment. Pre-
screening may
be done by the clinician administering noribogaine or by any other qualified
professional.
[0209] In one embodiment, a patient receiving noribogaine, noribogaine
derivative, or a
pharmaceutically acceptable salt or solvate thereof is monitored in a clinical
setting.
Monitoring may be necessary to ensure the QT interval is not prolonged to an
unacceptable degree. A "clinical setting" refers to an inpatient setting
(e.g., inpatient
clinic, hospital, rehabilitation facility) or an outpatient setting with
frequent, regular
monitoring (e.g., outpatient clinic that is visited daily to receive dose and
monitoring).
Monitoring includes monitoring of QT interval. Methods for monitoring of QT
interval are
well-known in the art, for example by ECG. In one embodiment, the patient is
monitored
after administration of the initial dose. In one embodiment, the patient is
further monitored
after administration of at least one therapeutic maintenance dose. In one
embodiment, the
patient is monitored for a subset of the treatment, e.g., one day, two days,
three days, four
days, one week. In one embodiment, a patient receiving noribogaine,
noribogaine
47
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
derivative, or a pharmaceutically acceptable salt or solvate thereof is not
monitored in a
clinical setting.
[0210] In one aspect, this invention is directed to a method for pre-screening
an opioid-
dependent patient prior to administration of noribogaine, noribogaine
derivative, or
pharmaceutically acceptable salt or solvate thereof in order to determine
whether the
patient has an acceptable risk of QT interval prolongation. In one embodiment,
one or
more of the following risk factors is evaluated: genetic/familial risk
factors, preexisting
conditions, history of using drugs (e.g., prescription or illicit) that are
known to have a risk
of QT interval prolongation, and/or previous unacceptable QT response to
ibogaine,
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof
In one embodiment, the risk associated with a history of using a drug that is
known to
have a risk of QT interval prolongation is based on the half-life of the drug,
the amount of
drug consumed by the patient, and the last time the patient used the drug. In
a preferred
embodiment, the patient is deemed to have an acceptable risk of QT interval
prolongation
if the patient has none of the risk factors. In one embodiment, the patient is
deemed to
have an acceptable risk of QT interval prolongation if the patient has one or
more of the
risk factors and is determined to nonetheless be acceptable for noribogaine
administration
by a clinician or pre-screening individual. For example, a clinician may
determine that a
familial risk factor is not sufficient to disqualify the patient from
noribogaine treatment. In
one embodiment, a patient who is deemed to have an acceptable risk of QT
interval
prolongation is administered noribogaine, noribogaine derivative, or
pharmaceutically
acceptable salt or solvate thereof
[0211] In one aspect, this invention relates to a method for prescreening an
opioid-
dependent patient for tolerance of noribogaine, noribogaine derivative, or a
pharmaceutically acceptable salt or solvate thereof In one embodiment,
prescreening of
the patient comprises ascertaining that noribogaine treatment will not result
in a maximum
QT interval over about 500 ms. In one embodiment, prescreening of the patient
comprises
ascertaining that noribogaine treatment will not result in a maximum QT
interval over
about 470 ms. In one embodiment, prescreening comprises ascertaining that
noribogaine
treatment will not result in a maximum QT interval over about 450 ms. In one
embodiment, prescreening comprises ascertaining that noribogaine treatment
will not
result in a maximum QT interval over about 420 ms.
48
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0212] As it relates to pre-screening or pre-selection of patients, patients
may be selected
based on any criteria as determined by the skilled clinician. Such criteria
may include, by
way of non-limiting example, pre-treatment QT interval, pre-existing cardiac
conditions,
risk of cardiac conditions, familial history of cardiac conditions, current
drug (prescription
and/or illicit) use, age, sex, general health, and the like. The following are
examples of
selection criteria for disallowing noribogaine treatment or restricting dose
of noribogaine
administered to the patient: high QT interval before treatment (e.g., such
that there is a risk
of the patient's QT interval exceeding about 500 ms during treatment);
congenital long QT
syndrome; bradycardia; hypokalemia or hypomagnesemia; recent acute myocardial
infarction; uncompensated heart failure; and taking other drugs that increase
QT interval.
In some embodiments, the methods can include selecting and/or
administering/providing
noribogaine to a patient that lacks one more of such criteria. In a preferred
embodiment,
the patient is not currently taking any medications or illicit drugs that are
known to have a
risk of QT interval prolongation. In an especially preferred embodiment, the
patient has
not taken a medication or illicit drug that is known to have a risk of QT
interval
prolongation for a period of time sufficient for the residual serum
concentration of the
drug in the patient is so low as to have no significant effect on QT interval
prolongation
upon administration with noribogaine, noribogaine derivative, or a
pharmaceutically
acceptable salt or solvate thereof One of skill in the art would understand
that the required
period of time between the last time the medication or illicit drug was taken
and the
administration of noribogaine treatment may vary depending, for example, on
the half-life
of the medication or illicit drug.
[0213] In one embodiment, this invention relates to pre-screening a patient to
determine
if the patient is at risk for prolongation of the QT interval beyond a safe
level. In one
embodiment, a patient at risk for prolongation of the QT interval beyond a
safe level is not
administered noribogaine. In one embodiment, a patient at risk for
prolongation of the QT
interval beyond a safe level is administered noribogaine at a limited dosage.
In one
embodiment, a patient with an acceptable risk of QT interval prolongation is
administered
noribogaine, noribogaine derivative, or salt or solvate thereof
[0214] In one embodiment, this invention relates to monitoring a patient who
is
administered a therapeutic dose of noribogaine, noribogaine derivative, or
salt or solvate
thereof In one embodiment, the dose of noribogaine is reduced if the patient
has serious
49
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
adverse side effects. In one embodiment, the noribogaine treatment is
discontinued if the
patient has serious adverse side effects. In one embodiment, the adverse side
effect is a QT
interval that is prolonged beyond a safe level. The determination of a safe
level of
prolongation is within the skill of a qualified clinician.
Noribogaine Administration
[0215] In one aspect, this invention relates to treatment and/or attenuation
of opioid drug
use in a dependent patient comprising administering a therapeutically
effective amount of
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof
under an altered treatment protocol. In one embodiment, the patient has
insufficient
metabolism of the opioid. In one embodiment, the patient is pre-screened for
acceptable
QT interval (or acceptable expected prolongation of the QT interval) prior to
treatment.
[0216] In one embodiment, the method further comprises administering an opioid
antagonist or partial antagonist to the patient, as described herein.
[0217] In one embodiment, the amount of noribogaine or noribogaine derivative
administered at a given time is dependent on the patient's current QT
interval. In one
embodiment, the amount of noribogaine or noribogaine derivative administered
at a given
time is dependent on the patient's expected QT interval. That is, the QT
interval (or
prolongation thereof) that is expected to result in the patient upon
administration of a
given amount of noribogaine is determined (e.g., based n the patient's current
QT interval
and the desired dose). If the expected QT interval is greater than 500 ms, and
preferably if
greater than 480 ms, then the dose of noribogaine is not administered or a
lower dose is
administered. Expected QT interval (or prolongation) can be determined, for
example,
based on the average QT prolongation for a given dose (see, e.g., U.S. Pub.
No.
2015/0231147, which is incorporated herein by reference in its entirety).
[0218] In one aspect, this invention relates to treatment and/or attenuation
of opioid drug
abuse in a dependent patient comprising administering a therapeutically
effective amount
of noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate
thereof, wherein the amount of noribogaine, noribogaine derivative, or salt or
solvate
thereof administered to the patient is reduced once the patient's opioid
levels fall below a
desired threshold.
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0219] In one embodiment, this invention further relates to a method for
attenuating
(reducing) withdrawal symptoms in a human patient susceptible to such symptoms
due to
opioid drug dependency. In one embodiment, the withdrawal symptoms are
symptoms of
acute withdrawal. In one embodiment, the withdrawal symptoms are symptoms of
post-
acute withdrawal.
[0220] In a preferred embodiment, the patient is administered an initial dose
of
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof,
followed by one or more therapeutic maintenance doses. In one embodiment, the
one or
more therapeutic maintenance doses are administered periodically.
[0221] In one aspect, a QT interval of less than about 500 ms is maintained
during
noribogaine or noribogaine derivative treatment. In one embodiment, a QT
interval of less
than about 470 ms is maintained during treatment. Preferably, a QT interval of
less than
about 450 ms is maintained during treatment. In one embodiment, a QT interval
of less
than about 420 ms is maintained during treatment. Treatment includes the
initial dose and
subsequent therapeutic maintenance dose(s).
[0222] In one embodiment, the QT interval is not prolonged more than about 60
ms
during noribogaine or noribogaine derivative treatment. In a preferred
embodiment, the
QT interval is not prolonged more than about 50 ms during noribogaine or
noribogaine
derivative treatment. In one embodiment, the QT interval is not prolonged more
than about
40 ms. In a preferred embodiment, the QT interval is not prolonged more than
about 30
ms. In one embodiment, the QT interval is not prolonged more than about 20 ms.
[0223] In one embodiment, the method of treatment with noribogaine or
noribogaine
derivative maintains an average serum concentration of noribogaine or
noribogaine
derivative between about 20 ng/mL and about 200 ng/mL. In one embodiment, the
method
of treatment with noribogaine or noribogaine derivative maintains an average
serum
concentration of noribogaine or noribogaine derivative between about 20 ng/mL
and about
180 ng/mL, or about 40 ng/mL and about 180 ng/mL. In one embodiment, the
average
serum concentration of noribogaine or noribogaine derivative is maintained
between about
20 ng/mL and about 150 ng/mL, or about 40 ng/mL and about 150 ng/mL. In one
embodiment, the average serum concentration of noribogaine or noribogaine
derivative is
maintained between about 20 ng/mL and about 100 ng/mL, or about 40 ng/mL and
about
51
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
100 ng/mL. In one embodiment, the average serum concentration of noribogaine
or
noribogaine derivative is maintained between about 50 ng/mL and about 180
ng/mL. In
one embodiment, the average serum concentration of noribogaine or noribogaine
derivative is maintained between about 50 ng/mL and about 150 ng/mL. In one
embodiment, the average serum concentration of noribogaine or noribogaine
derivative is
maintained between about 50 ng/mL and about 100 ng/mL. The ranges include both
extremes as well as any subranges between.
[0224] In one embodiment, the dosage of noribogaine, noribogaine derivative,
or
pharmaceutically acceptable salt or solvate thereof provides a maximum serum
concentration (Cmax) of less than about 250 ng/mL. In one embodiment, the
dosage of
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof
provides a Cmax between about 20 ng/mL and about 200 ng/mL. In a preferred
embodiment, the dosage of noribogaine, noribogaine derivative, or
pharmaceutically
acceptable salt or solvate thereof provides a C. between about 40 ng/mL and
about 180
ng/mL. In one embodiment, the dosage of noribogaine, noribogaine derivative,
or
pharmaceutically acceptable salt or solvate thereof provides a C. between
about 60
ng/mL and about 180 ng/mL. In one embodiment, the dosage of noribogaine,
noribogaine
derivative, or pharmaceutically acceptable salt or solvate thereof provides a
maximum
serum concentration (Cmax) of less than about 200 ng/mL. In one embodiment,
the dosage
of noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate
thereof provides a maximum serum concentration (Cmax) of less than about 120
ng/mL.
[0225] In one embodiment, the aggregate dosage of noribogaine, noribogaine
derivative,
or salt or solvate thereof is between about 40 mg and about 250 mg per day,
and
preferably about 60 mg to about 200 mg per day. In some embodiments, the
initial dose of
noribogaine, noribogaine derivative, or salt or solvate thereof is from about
20 mg to about
120 mg, and preferably between about 60 mg and about 100 mg. In one
embodiment, the
one or more therapeutic maintenance doses are from about 5 mg to about 50 mg,
and
preferably between about 10 mg and about 30 mg. In one embodiment, each of the
one or
more therapeutic maintenance doses may or may not comprise the same amount of
noribogaine, noribogaine derivative, or salt or solvate thereof For example,
the clinician
may adjust the dose up or down depending on factors including the patient's
expected or
actual QT interval (or prolongation thereof), expected or actual noribogaine
or noribogaine
52
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
derivative plasma concentration, and/or how well the dose is working (e.g. to
attenuate
withdrawal symptoms).
[0226] In one embodiment, the altered treatment protocol comprises increasing
the
amount of noribogaine, noribogaine derivative, salt, or solvate thereof that
is administered
to the patient. In one embodiment, the increase is an increase in one or more
unit doses of
noribogaine or noribogaine derivative and/or an increase in the aggregate
daily amount
administered to the patient. When the amount of noribogaine or noribogaine
derivative
administered is increased, the patient may be evaluated for QT interval
prolongation.
[0227] In one embodiment, the one or more therapeutic maintenance doses are
administered periodically. In one embodiment, the one or more therapeutic
maintenance
doses are administered about every 4 hours to about every 48 hours, and
preferably about
every 6 hours to about every 12 hours.
[0228] In one embodiment, the one or more therapeutic maintenance doses are
provided
as sustained release formulations of noribogaine, noribogaine derivative, or
salt or solvate
thereof As would be apparent to one of skill in the art, the total dosage in
the sustained
release formulation may be higher than the desired dose at the time
administered, because
the noribogaine, noribogaine derivative, or salt or solvate thereof is
released slowly over
time. As will also be apparent to one of skill in the art, sustained
formulations may be
administered less frequently than non-sustained release formulations to
achieve the same
results (e.g., maintenance of a desired serum drug level while maintaining a
maximum QT
interval of less than 500 ms and a QT interval prolongation of less than 60
ms).
[0229] In some embodiments, the patient is administered periodically, such as
once,
twice, three times, four times, five times, or six times daily with
noribogaine, noribogaine
derivative, or a pharmaceutically acceptable salt or solvate thereof In some
embodiments,
the administration is once daily, or once every second day, once every third
day, three
times a week, twice a week, or once a week. The dosage and frequency of the
administration depends on the route of administration, dosage, age and body
weight of the
patient, condition of the patient, and risk of QT prolongation, without
limitation.
Determination of dosage and frequency suitable for the present technology can
be readily
made a qualified clinician.
53
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0230] In some embodiments, the altered treatment protocol comprises
increasing the
frequency of administration of noribogaine or noribogaine derivative. For
example, where
the noribogaine treatment protocol includes administering a unit dose of
noribogaine or
noribogaine derivative every 6 hours, the altered protocol may include
administering a unit
dose every 1, 2, 3, 4, or 5 hours, or an increment thereof In one embodiment,
the
increased frequency of administration is coupled with an increase in the
aggregate amount
of noribogaine or noribogaine derivative administered to the patient. In one
embodiment,
the frequency of noribogaine or noribogaine derivative administration is
increased for a
portion of the protocol (e.g., for 0.5, 1, 2, 3, 4 or more days).
[0231] In one embodiment, the altered treatment protocol comprises increasing
the
duration of time that noribogaine, noribogaine derivative, or salt, or solvate
thereof is
administered to the patient. For example, a standard noribogaine treatment
protocol may
provide periodic administration of noribogaine or noribogaine derivative for
between two
and seven days, and a patient having insufficient metabolism of the opioid may
be
administered noribogaine or noribogaine derivative for three to ten days. Such
determination is within the ability of a skilled clinician, based on factors
including the
patient's age, gender, health, and the speed at which the opioid is
metabolized (e.g., as
determined by serum and/or urine analysis).
[0232] The patient may suffer from dependency upon any opioid or opiate. In a
preferred embodiment, the opioid is selected from the group consisting of
heroin, opiate,
morphine, codeine, oxycodone, and hydrocodone. In one embodiment, the opioid
is
heroin. In one embodiment, the opioid is morphine. In a preferred embodiment,
the patient
is not currently using methadone. In one embodiment, the patient has not used
methadone
for at least 5 days prior to noribogaine or noribogaine derivative treatment.
In one
embodiment, the patient has not used methadone for at least one week, two
weeks, three
weeks, four weeks, or more prior to noribogaine or noribogaine derivative
treatment. In a
preferred embodiment, the patient is not currently using buprenorphine. In one
embodiment, the patient has not used buprenorphine for at least 5 days prior
to
noribogaine or noribogaine derivative treatment. In one embodiment, the
patient has not
used buprenorphine for at least one week, two weeks, three weeks, four weeks,
or more
prior to noribogaine or noribogaine derivative treatment.
54
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
Administration of Opioid Antagonist
Co-administration of Opioid Antagonist
[0233] In one aspect, a patient having insufficient metabolism of opioid is
administered
an opioid antagonist or partial opioid antaonist in combination with
noribogaine or
noribogaine derivative. Without being bound by theory, it is believed that the
antagonist
can bypass or reduce the need for the patient to metabolize the opioid in
order for
noribogaine or noribogaine derivative to have its full effect on reduction of
withdrawal
symptoms and/or treatment of opioid dependency.
[0234] In one embodiment, the opioid antagonist can be one or more of
naloxone,
naltrexone, nalorphine, nalmefene, levallorphan, cyprodime, naltrindole, and
norbinaltorphimine. In one embodiment, the opioid antagonist is administered
at a dose
that is sub-therapeutic, i.e., below the dose generally used for other
indications. In some
embodiments, the opioid antagonist is administered intravenously. In one
embodiment, the
dose of the opioid antagonist is titrated. In some embodiments, one or more of
the listed
opioid antagonists can be specifically excluded from the methods and kits and
of the
technology.
[0235] In one embodiment, the opioid antagonist is administered after at least
one dose
of noribogaine or noribogaine derivative. In one embodiment, the opioid
antagonist is
administered at the same time as at least one dose of noribogaine or
noribogaine
derivative. In one embodiment, the opioid antagonist is administered
periodically or
continuously for a period of time. Determination of the amount of time for
administration
of the opioid antagonist is within the ability of a skilled clinician, for
example for a period
of time sufficient for the patient's withdrawal symptoms to be alleviated by
noribogaine or
noribogaine derivative.
[0236] In one embodiment, the opioid antagonist is administered without regard
for the
opioid metabolism of the patient.
[0237] In one embodiment, the opioid antagonist is naloxone. Naloxone is used
to
counteract side effects of opioid analgesia, to reverse opioid overdose, and
in opioid
formulations to deter abuse of the opioid (e.g., Suboxone). In one embodiment,
about 0.1
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
to about 4 mg of naloxone is administered. In one embodiment, about 0.1 to
about 2 mg of
naloxone is administered. In one embodiment, about 0.1 to about 1 mg of
naloxone is
administered. In one embodiment, about 0.1 to about 0.5 mg of naloxone is
administered.
In one embodiment, the naloxone is administered periodically (e.g., every 2-30
minutes or
every 1-5 hours) or continuously until the desired response is achieved. In
one
embodiment, the naloxone is administered by intravenous, subcutaneous, and/or
intramuscular injection.
[0238] In one embodiment, the opioid antagonist is naloxone, nalmefene,
naltrexone,
nalorphine, levallorphan, cyprodime, naltrindole, or norbinaltorphimine.
Opioid Antagonist Following Noribogaine Treatment
[0239] Opioid antagonist (or partial antagonist) therapy may be used to
prevent relapse
to opioid abuse. Opioid antagonists attenuate or block the physiological
effects (e.g., the
subjective pleasurable effects) of opioids. For example, naltrexone is an
opioid antagonist
that is approved for use in treating opioid dependence and alcohol dependence.
A
drawback of opioid antagonists such as naltrexone for opioid dependence is
that,
heretofor, a period of opioid abstinence to reduce or completely eliminate
opioid in the
body is required before the opioid antagonist can be administered. This
detoxification
period is generally 7-14 days or longer, depending on the opioid being used.
Few opioid-
dependent patients can meet this requirement.
[0240] In one aspect, this invention is predicated on the surprising discovery
that
administration of noribogaine to an opioid-dependent patient, for example on a
periodic
basis, results in a reduction in opioid withdrawal symptoms during the
treatment period as
well as for a time afterward, such that the patient is opioid-free for at
least 7 days. After
the noribogaine treatment and opioid abstinence for the required period of
time, the patient
optionally can be administered an opioid antagonist. In a preferred
embodiment, the opioid
antagonist is naltrexone. In an especially preferred embodiment, the opioid
antagonist such
as naltrexone is administered in a controlled-release formulation. Thus, some
embodiments relate to methods of using noribogaine treatment as a pre-
treatment or as a
detoxification treatment prior to treatment with an opioid antagonist such as
naltrexone.
Other embodiments relate to methods of treating opioid dependency using an
opioid
antagonist such as naltrexone by selecting a patient that been pre-treated
with noribogaine
56
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
or that has gone through detoxification to eliminate opioid from the body
prior to
receiving the naltrexone treatment, and administering the opioid antagonist to
the patient.
[0241] This invention is further predicated on the surprising discovery that
administration to a patient of an aggregate amount of up to about 200 mg of
noribogaine,
noribogaine derivative, or pharmaceutically acceptable salt or solvate thereof
per day
results in a dramatic reduction of withdrawal symptoms, thereby allowing the
patient to
abstain from opioid abuse for a period of time. In one embodiment, the patient
is treated
with an aggregate amount of up to about 200 mg of noribogaine, noribogaine
derivative,
or pharmaceutically acceptable salt or solvate thereof per day for between 2
and 14 days,
as described herein.
[0242] As will be apparent to the skilled artisan upon reading this
disclosure, the present
invention provides a method for treating opioid abuse, including acute and
post-acute
withdrawal symptoms, in an opioid-dependent patient, comprising administering
to a
patient scheduled to receive opioid antagonist therapy a therapeutic dose of
noribogaine,
noribogaine derivative, or pharmaceutically acceptable salt or solvate thereof
such that the
patient is opioid-free for a period of time (e.g., for at least 5 days) prior
to initiation of
opioid antagonist therapy.
[0243] The present invention further provides a method for treating an opioid-
dependent
patient who has been pretreated with noribogaine or noribogaine derivative so
as to be
opioid-free (e.g., for at least 5 days), comprising administering to the
patient an opioid
antagonist in a sufficient amount to block at least one physiological effect
of an opioid.
[0244] In one aspect, this invention relates to treatment and/or attenuation
of opioid drug
use in a dependent patient comprising administering a therapeutically
effective amount of
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof,
such that the patient is opioid free for at least about 5 days. In one
embodiment, a patient
who has been pre-treated with noribogaine, noribogaine derivative, or
pharmaceutically
acceptable salt or solvate thereof is administered an opioid antagonist. In
one embodiment,
this invention further relates to a method for attenuating withdrawal symptoms
in a human
patient susceptible to such symptoms due to opioid drug dependency. In one
embodiment,
the withdrawal symptoms are symptoms of acute withdrawal. In one embodiment,
the
withdrawal symptoms are symptoms of post-acute withdrawal.
57
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0245] In a preferred embodiment, the patient is administered an initial dose
of
noribogaine, noribogaine derivative, or pharmaceutically acceptable salt or
solvate thereof,
followed by one or more therapeutic maintenance doses. In one embodiment, the
one or
more therapeutic maintenance doses are administered periodically.
[0246] In one embodiment, this invention relates to a method for pretreating
an opioid-
dependent patient scheduled for treatment with an opioid antagonist, which
method
comprises administering noribogaine, noribogaine derivative, or
pharmaceutically
acceptable salt or solvate thereof to said patient in an amount sufficient to
attenuate
withdrawal symptoms and to allow the patient to be opioid-free for at least 7
days prior to
initiation of opioid antagonist administration.
[0247] In one embodiment, this invention relates to a method for treating an
opioid-
dependent patient with an opioid antagonist which method comprises:
identifying an
opioid-dependent patient who has been pretreated with noribogaine or
noribogaine
derivative so as to be opioid-free for at least 7 days, and then administering
the opioid
antagonist in a sufficient amount to inhibit a pharmacological effect of
opioids in the
patient.
[0248] The opioid antagonist is preferably administered to a patient having
residual
noribogaine or noribogaine derivative in the patient's serum so as to reduce
the risk of
relapse during the time between cessation of noribogaine treatment and
commencement of
opioid antagonist treatment.
[0249] The dosage and administration of opioid antagonist (e.g. naltrexone) to
be used
can be determined by a skilled clinician. For example, the number of days of
opioid
abstinence required before the patient can be administered opioid antagonist
depends upon
factors including the opioid of dependency, the amount of opioid used by the
patient, the
opioid antagonist being administered, and the availability of medical support
(e.g. in-
patient facility) in the case of sudden onset of withdrawal. Generally, a
patient will abstain
from opioid use for between at least about 5 days and about 14 days prior to
opioid
antagonist administration, and preferably at least about 7 days to about 14
days.
[0250] In one embodiment, the opioid antagonist is naloxone, nalmefene,
naltrexone,
nalorphine, levallorphan, cyprodime, naltrindole, or norbinaltorphimine.
58
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
Evaluation of Patient Opioid Metabolism
[0251] In one aspect, this disclosure relates to methods for screening a
patient population
for rate (or expected rate) of opioid metabolism. In one embodiment, a patient
is screened
for rate (or expected rate) of opioid metabolism. The patient may be screened
prior to
intial noribogaine or noribogaine derivative administration, during treatment
with
noribogaine or noribogaine derivative, or both.
[0252] In one aspect, this disclosure relates to methods for screening a
patient population
or a patient for opioid levels (e.g., serum and/or urine). In one embodiment,
administration
of noribogaine or noribogaine derivative (amount, duration, and/or co-
administration of
opioid antagonist or partial antagonist) is determined based on the opioid
levels of the
patient as described herein.
[0253] In one embodiment, this disclosure relates to selection of a patient
having a
desired rate of opioid metabolism and/or a desired level of opioid and
determining the
noribogaine or noribogaine derivative treatment protocol or altering a
protocol based on
the rate or level.
[0254] In one embodiment is provided a method for screening a patient for rate
(or
expected rate) of opioid metabolism, the method comprising evaluating the
amount of time
required for the patient's opioid levels (e.g., serum and/or urine) to fall
below a desired
level. In one embodiment, the desired level is an amount of opioid(s) below
which
noribogaine or noribogaine derivative is capable of reducing withdrawal
symptoms. In one
embodiment, a patient who does not exhibit an opioid level below the desired
level within
a predetermined period of time (e.g., 1 to 2 days) has insufficient opioid
metabolism.
[0255] In one embodiment, the desired level is less than about 3,000 ng/mL
total
detectable opioid in urine, or equivalent (e.g., serum equivalent). In one
embodiment, the
desired level is less than about 2,000 ng/mL in urine, or equivalent. In a
preferred
embodiment, the desired level is less than about 1,500 ng/mL in urine, or
equivalent. In
one embodiment, the desired level is less than about 1,000 ng/mL in urine, or
equivalent.
In one embodiment, the desired level is less than about 900 ng/mL in urine, or
equivalent.
In one embodiment, the desired level is less than about 800 ng/mL in urine, or
equivalent.
In one embodiment, the desired level is less than about 700 ng/mL in urine, or
equivalent.
59
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
In one embodiment, the desired level is less than about 600 ng/mL in urine, or
equivalent.
In one embodiment, the desired level is less than about 500 ng/mL in urine, or
equivalent.
In one embodiment, the desired level is less than about 400 ng/mL in urine, or
equivalent.
In one embodiment, the desired level is less than about 300 ng/mL in urine, or
equivalent.
[0256] In one embodiment, the predetermined period of time is between 0 and 4
days
after administration of noribogaine, noribogaine derivative, or salt and/or
solvate thereof
In one embodiment, the predetermined period of time is 0 days after
administration of
noribogaine or noribogaine derivative. In one embodiment, the predetermined
period of
time is 1 days after administration of noribogaine or noribogaine derivative.
In one
embodiment, the predetermined period of time is 2 days after administration of
noribogaine or noribogaine derivative. In one embodiment, the predetermined
period of
time is 3 days after administration of noribogaine or noribogaine derivative.
In one
embodiment, the predetermined period of time is 4 days after administration of
noribogaine or noribogaine derivative.
[0257] In one embodiment, at least one indicator of withdrawal symptoms is
measured
prior to and at least one time during treatment with noribogaine or
noribogaine derivative
and/or the opioid antagonist. In one embodiment, at least one indicator of
withdrawal
symptoms is assessed as may be described in a group of assessments such as
Clinical
Opiate Withdrawal Scale (COWS), Subjective Opiate Withdrawal Scale (SOWS),
Objective Opiate Withdrawal Scale (00WS), and Profile of Mood States (POMS).
In one
embodiment, the dose of noribogaine or noribogaine derivative and/or opioid
antagonist is
adjusted based on the indicator. For example, a patient exhibiting higher
levels of
withdrawal/withdrawal symptoms may be administered more noribogaine or
noribogaine
derivative and/or altered dose (more or less) of opioid antagonist.
Alternatively, a patient
exhibiting lower levels of withdrawal/withdrawal symptoms may be administered
less
noribogaine or noribogaine derivative and/or altered dose (more or less) of
opioid
antagonist. In some embodiments, a patient exhibiting high levels of
withdrawal/withdrawal symptoms is administered an additional therapeutic agent
to
address one or more symptoms. Determination of whether, what, and how much
additional
therapeutic agent to administer is within the ability of a skilled clinician.
In one
embodiment, the patient reports no urge to use the opioid for at least one day
after
cessation of noribogaine or noribogaine derivative treatment.
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0258] In one embodiment is provided a method for screening a patient for
expected rate
of opioid metabolism, the method comprising determining the ability of the
patient to
metabolize an opioid of interest based on the allelic variant(s) or genetic
mutation(s) of at
least one enzyme that is involved in metabolism of the opioid of interest. In
one
embodiment, the enzyme is a cytochrome P450 enzyme. In one embodiment, the
enzyme
is a glucuronidation enzyme (UGT). In one embodiment, the enzyme is selected
from the
group consisting of CYP3A4, CYP2D6, CYP2B6, CYP2C8, CYP2C19, CYP2C9, and
UGT2B7. Allelic variations of the enzymes that affect metabolism of opioids
are known in
the art. Methods for determining allelic variant or genetic mutation of a
given gene in a
patient are well-known in the art.
[0259] In one embodiment is provided a method for screening a patient for
expected rate
of opioid metabolism, the method comprising evaluating the patient's age,
ethnicity, sex,
and diseases (e.g., hepatic impairment, renal impairment) to determine whether
the patient
is expected to have a fast, intermediate (normal) or slow rate of opioid
metabolism.
[0260] In one embodiment, the noribogaine or noribogaine derivative treatment
protocol
for a patient having insufficient opioid metabolism is adjusted as described
herein.
[0261] In one embodiment, the amount and/or duration of noribogaine or
noribogaine
derivative treatment is reduced for a patient having a fast rate of opioid
metabolism.
[0262] In one embodiment, the amount and/or duration of noribogaine treatment
or
noribogaine derivative is reduced once the patient's opioid levels fall below
a desired
threshold.
IV. Kit of Parts
[0263] In one aspect, this disclosure relates to a kit of parts comprising a
composition
comprising an opioid antagonist or partial opioid antagonist and a second
composition
comprising noribogaine, noribogaine derivative, or salt or solvate thereof,
and a means for
administering the compositions to a patient in need thereof The means for
administration
to a patient can include, for example, any one or combination of a
pharmaceutically
acceptable formulation comprising noribogaine, noribogaine derivative, or a
pharmaceutically acceptable salt or solvate thereof and/or the antagonist
(e.g., a pill,
61
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
transdermal patch, injectable, and the like, without limitation) and
optionally a means for
dispensing and/or administering the formulation (e.g., a syringe, a needle, an
IV bag
comprising the composition, a vial comprising the composition, an inhaler
comprising the
composition, etc, without limitation). In one embodiment, the kit of parts
further
comprises instructions for dosing and/or administration of the compositions.
[0264] In some embodiments, the kit of parts further comprises a dosing
treatment
schedule in a readable medium. In some embodiments, the dosing treatment
schedule
includes the amount of noribogaine or noribogaine derivative required to
achieve a desired
average serum level is provided. In some embodiments, the kit of parts
includes a dosing
treatment schedule that provides an attending clinician the ability to select
a dosing
regimen of noribogaine or noribogaine derivative and/or antagonist, for
example based on
the sex of the patient, mass of the patient, QT interval (and/or expected
prolongation
thereof) and the serum level that the clinician desires to achieve. In an
embodiment, the
storage medium can include an accompanying pamphlet or similar written
information that
accompanies the unit dose form in the kit. In an embodiment, the storage
medium can
include electronic, optical, or other data storage, such as a non-volatile
memory, for
example, to store a digitally-encoded machine-readable representation of such
information.
[0265] The term "readable medium" as used herein refers to a representation of
data that
can be read, for example, by a human or by a machine. Non-limiting examples of
human-
readable formats include pamphlets, inserts, or other written forms. Non-
limiting examples
of machine-readable formats include any mechanism that provides (i.e., stores
and/or
transmits) information in a form readable by a machine (e.g., a computer,
tablet, and/or
smartphone). For example, a machine-readable medium includes read-only memory
(ROM); random access memory (RAM); magnetic disk storage media; optical
storage
media; and flash memory devices. In one embodiment, the machine-readable
medium is a
CD-ROM. In one embodiment, the machine-readable medium is a USB drive. In one
embodiment, the machine-readable medium is a Quick Response Code (QR Code) or
other
matrix barcode.
[0266] In some aspects, the machine-readable medium comprises software that
contains
information regarding dosing schedules for noribogaine or noribogaine
derivative and/or
62
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
antagonist, and optionally other drug information. In some embodiments, the
software
may be interactive, such that the attending clinician or other medical
professional can
enter patient information. In a non-limiting example, the medical professional
may enter
the weight and sex of the patient to be treated, and the software program
provides a
recommended dosing regimen based on the information entered. The amount and
timing
of noribogaine or noribogaine derivative recommended to be delivered will be
within the
dosages that result in the serum concentrations as provided herein.
V. Formulations
[0267] In one aspect, this disclosure relates to formulations of noribogaine,
noribogaine
derivative, or pharmaceutically acceptable salt or solvate thereof and an
effective amount
of an opioid antagonist. In some embodiments, the opioid antagonist is
naloxone,
nalmefene, naltrexone, nalorphine, levallorphan, cyprodime, naltrindole, or
norbinaltorphimine. In some embodiments, the dose of noribogaine or
noribogaine
derivative is between about 5 mg and about 200 mg. Preferably, the dose of
noribogaine or
noribogaine derivative is between about 10 mg and about 120 mg.
EXAMPLES
[0268] The following Examples are intended to further illustrate certain
embodiments of
the disclosure and are not intended to limit its scope.
Example 1. Efficacy of Multi-Dose Noribogaine Treatment in Humans
[0269] A phase 1B, open-label, titration study was conducted in a secure in-
patient
clinical unit. A loading dose of noribogaine 80mg was followed by titrated
maintenance
dosing (10, 20, 30, or 40mg, q6h) for up to 5 days. Maintenance dosing
considered
subjective and objective clinical assessments, and change-from-baseline QTc
(AQTcF),
using a predefined algorithm to avoid pronounced QT prolongation (exclusion
with QTcF
>500msec or AQTcF >60msec). Standard safety evaluations, PK and PD assessments
were
conducted. Continuous cardiac monitoring was performed via Holter monitor and
telemetry.
[0270] Nine (9) patients (6 males/3 females; mean age, 31.2 years) were
enrolled.
Documented opioid use included heroin and prescription opioids. Cumulative
noribogaine
63
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
doses ranged from 80mg to 670mg, with 4 patients administered the maximum 20
doses
(mean 653mg, range 640 ¨ 670mg).
[0271] No serious adverse events (AEs) were reported. For treatment-emergent
AEs,
97% were mild in severity, 56% were opioid withdrawal related and 10% were
headaches.
The largest AQTcF by subject ranged between 22 ¨ 51msec (mean 33msec). A
noribogaine concentration-dependent increase in AQTcF was observed,
corresponding to a
predicted AQTcF of 17msec at the geometric mean of peak concentrations of
89.1ng/mL.
No QTc changes resulted in discontinuation.
[0272] Noribogaine plasma concentrations showed high inter-subject
variability, even
between subjects with similar unit/daily/total doses, with Cmax values
following multiple
dosing ranging from 52 to 252 (mean 102) ng/mL. Steady-state plasma
concentrations
were reached between 12 and 24 hours after the 1st dose in 5 subjects and
prior to 48
hours for 2 other subjects. Although plasma concentrations vary among
subjects, they are
consistent within a subject once steady-state has been reached even when the
variation of
doses with a subject is taken into account. Scores on withdrawal, including
cravings, and
mood scales decreased rapidly and substantially following administration of
noribogaine
with very positive subjective experiences in the majority of patients. Follow-
up results
reflect the fragility of this population: 5 subjects relapsed, 3 were lost to
follow-up and 1
remained abstinent (15 wks).
[0273] Noribogaine treatment for up to 5 days was well-tolerated. The
previously noted
QT effect of noribogaine was confirmed and a dosing algorithm with QT criteria
was
successful in avoiding pronounced QT prolongation: no patient experienced QTcF
>500msec or AQTcF >60msec. Clinically significant reductions in withdrawal
symptoms
and mood assessments were seen following noribogaine administration.
[0274] The patients' scores on various Opiate Withdrawal Scales (Clinical,
COWS;
Subjective, SOWS; Objective, 0OWS) and Profile of Mood States (POMS) were
evaluated during treatment. QT interval, pupil diameter, noribogaine serum
concentration,
opioid levels (urine), and adverse events were also monitored and recorded.
Demographic
information for each patient is provided in Table 1.
64
el
m
cr
,-1
m
o
,-1
o
el
ci)
E=1
c.)
a
Table 1: Demographic Data by Patient
02-001 02-002 02-003 02-004 02-005 02-006 02-007 02-008
02-009
, Demographics
,
,
, Gender Female Male Male
Male Male Male Female Female Male
,
.3
,
. Age 24 27 43
41 21 34 24 36 31
,
Black or Lo
.3
.:, Race White White White White
African White Other White White
. American
0
Non- Non- Non- Non- Non- Non- Non- Non- Non-
Latino/non- Latino/non- Latino/non- Latino/non- Latino/non- Latino/non-
Latino/non- Latino/non- Latino/non
Hispanic Hispanic Hispanic Hispanic Hispanic Hispanic Hispanic Hispanic
Hispanic
White and
aboriginal
Body Mass Index at
30 28 22 30 29 28 19 22 21
screening
7r
7r Daily morphine
el
473 to
99)
oe equivalents (McMaster 70mg 600mg 70mg 120mg
140mg 360mg
,-1
720mg
cony table)
,-1
o
el Opioid use >10 yrs >8 yrs >25 yrs >13
yrs >6 yrs >6 yrs >3 yrs >3 yrs >4 yrs
0 Tobacco use Yes Yes Yes Yes
Yes Yes Yes Yes Yes
CA 03024081 2018-11-13
WO 2016/183244 PCT/US2016/031932
[0275] Noribogaine plasma concentrations for each patient are provided in FIG.
1.
Surprisingly, although patients 02-004 and 02-005 had among the lowest and the
highest
noribogaine plasma concentrations, respectively, both patients exhibited few
withdrawal
symptoms at 24 to 48 hours after administration of the initial noribogaine
dose. Other
patients with varying noribogaine plasma concentrations exhibited more/more
intense
withdrawal symptoms. As can be seen in FIGs. 2A-2C, there is little
correlation between
noribogaine plasma concentration and change in SOWS score.
[0276] Also surprisingly, the patients exhibited an improvement in withdrawal
symptoms after their opioid levels (urine) dropped below about 1500 ng/mL,
regardless of
noribogaine plasma concentration. FIGS. 3A through 31 show the correlation
between
SOWS and urine opiate levels for each patient. The data used to generate the
figures is
provided in Tables 2 through 4. Two patients exhibited opioid levels greater
than 2000
ng/mL at day 3 (post noribogaine administration), and both of the patients
left the study
around that time. Table 4 provides SOWS scores by patient over time. Urine
opioid levels
show strong correlation to plasma levels at the same time points (FIG. 4).
Measurement of
plasma opioid levels included morphine, hydromorphone, oxycodone, and morphine-
6-
beta-glucuronide levels.
Table 2: Morphine* levels in urine (ng/mL)
DS Drug Morphine ..
Sum of DS Drug Test Result Numeric Subject ID
Visit L.. 02-001
02-002 02-003 02-004 02-005 02-006 02-007 02-008 02-009
A - Screening 38867 42000 42000 5618 903
84000 2576 42000 27245
B- Day -2 4684
42000 42000 42000 1871 42000 5118 42000 42000
C - Day -1-1 42000 42000 10056 39337 662
42000 3691 17785 7478
D - Day 1 10230 28580 4496 378
816 42000 2829 9346 3763
E - Day 2 3354 32406 531 92 59 26444 1817
F - Day 3 1026 6000 219 37 44 843 477
G - Day 4 134 23 0 0 334
H - Day 5 33 17 1 0
I - First Day of Observation 23 14 2 0
J - Second Day of Observation 20 0 31 0
*Test reported as "morphine" in the urine drug screen represents results of
the
MULTIGENT Opiates Assay, which may detect the following substances with
varying
sensitivity: 6-Monoacetyl morphine, Codeine, Dihydrocodeine, Heroin,
Hydrocodone,
66
CA 03024081 2018-11-13
WO 2016/183244 PCT/US2016/031932
Hydromorphone, Levorphanol, Morphine, Morphine-3-glucuronide, Morphine-6-
glucuronide, Oxycodone, Oxymorphone, or Ranitidine.
Table 3: Oxycodone levels in urine (ng/mL)
DS Drug Oxycodone
Sum of DS Drug Test Result Nun Subject ID
Visit 02-
001 02-002 02-003 02-004 02-005 02-006 02-007 02-008 02-009
A - Screening 12 149 17 102 6108 71 10000 1
123
B- Day -2 900 86 19 9 10000 10000
10000 12 11
C - Day -1-1 4496 24 0 56 2536 2643
10000 0 0
D - Day 1 732 39 0 12 494 2798 10000
34 17
E - Day 2 174 35 0 4 112 802 9983
F - Day 3 44 15 0 11 94 53 1605
G - Day 4 15 0 10 55 21
H - Day 5 12 0 0 25
I - First Day of Observation 5 0 0 26
J - Second Day of Observation 6 0 1 8
67
el
m
cA
,--i
m
o
,.z Table 4: SOWS Scores
,¨,
=
el
ci) Sum of SOWS Score Subject ID
E=1 Visit S Protcol Tim(
02-001 02-002 02-003 02-004 02-005 02-006 02-007 02-008 02-
009 Timepoint Mean SD 90% CI N
c.) C - Day -1-1 26 24 20 9 31 8
30 31 41 24 11 6 9
D - Day 1 0 33 35 24 21 49 16
32 41 22 0 30 i 11" 8r 9
, *
re 1 13 12 11 14 33 15 10 22 21 1 17 7
4 * 9
, .0o,
20 11 7 16 31 4 7 11 36 4 .5. 28 24 2.
. . 16', 10 6' 9
3 13 17 .: : i .. !" 16
13". 7 6
, 36 22 !. Br 4 9
8 6 14 24 5 1 22 18 4 . 12 4" 9
,
r
9:oe
6 22 11 10 20 22 4 1 25 6
14 5* * 8
9 .. '=
o
9 18 18 11 24 19 5 2 18 1e'
Br 4r 8
6 17 13 11 20: 13 5 3 12 .. 1Z' 4r 7
, 12
1 *
16 6r
7-
, 15 21 23 12 8
15 4
r
, 18 28 26 19 22 9 3
18 18' 10 7 * 6
,
1
-1'
0,
, E - Day 2 , 13* 24 27i 11 i 16 6 21 i
7i 3 24 e 5" 7
. ,
...............................................................................
......... :*= ...
cs, 27 25 17 20 4 27 : 11
5 7 1e' 8:5 7 co
le
6r 7 11r .)0 L.
0, 36 29 7 13 6 0 6 18
36
&:* o,
cs, 39 7 15 1 10
39 6i 6i'' 4
jr
...............................................................................
.............
11 7
0-,
. F - Day 3 48 31 15 12 3 0
0 .. 13 .... 48 ......... i 11" 7
,
6 .! 51 27 14 13 Z 2 0 15
51 le 10 6$' 7
, * 10 7 6 * 60
............... 15 23 13 1 1 0 60 9 6
63
o'=
63 10 1 8r 6"
7r 2
:y
G - Day 4 , 72 13 11 3 0
72 7r BY Br 4
, .%,
6r 5r 75 11 12 2 0 75 6$'
4
5re 5* 4re
= 84 9 9 1
0 84 4
,
o, ,o,
87 12 6 3 ir 5i
4! 3
,
87 --ir==
H - Day 5 96 11 9 1 0
96 5 .6'. 5 4
,
6 6r ,,.
99 13 9 1 1 99 15
5' 4
7
r 0.
2 !"
108 7 7 1: 3
108 3i 4
.re
.re 111 7 2: =
111 .. , 5 4r. 4 2
el
1 4 0 1 i
r
,n I_ First Day of Observatil Morning
0 120 : 4 5 $' 4.r 4
oo
,--i
r
-C.6- Evening 6 4 0 4
130 4* 3 2* 4
,--i J - Second Day of Obser Morning 5 2 0 1
144 f 2' f 4
el Evening 4 2 0 1
154 2 2 .'Y 14' 4
0 L - 24 hr Phone call 2 3 0 1 0 5
16 17 6 7 4 8
el
m
cr Table 4: SOWS Scores (cont.)
,-,
m
=
,-,
=
el Sum of SOWS Qu 16 Feel like using
Subject ID
ci)
Visit S Protcol Tim i 02-001 02-002 02-003 02-004 02-005
02-006 02-007 02-008 02-009 Timepoint Mean SD 90%Cl N
E=i C - Day -1-1 4 2 3: 2 2 4: 3
4 4 3.1 1 1 9
C.) D - Day 1 ,
0 4 2 2 4 4 4 3 4 3 0 , 3.3 1 ,
0,
9
a., ,
...............................................................................
........ v 2.2 1r 1 v
1 1 1 1 3 3 3 1 4 3
1' 9
/ 1:Tx
2 1 1 0 3 2 1 1 4 3 2 y 1.8 v
1' 9
%
3
...............................................................................
... 3
3 1 3 t 3 2 1 0 4 2.0% .1 Y
1 ! 9
,
4 1 1 1 3 2 4 : 1 1 0 4 1.6
1r 1Y 9
,
v v
6 2 1 1 4 1 1 0 4 6 1.8 1'
1' 8
, v
9 1 2 1 4 1 1 1 4 9 1.9Y 1v=
1'
8
y
v
12 1 1 1 4 1 1 1 12 1.4' 1r
1 - 7
y
:v
15 3 2 1 2 15 2.0Y 1.Y
1 4
=lv==
,
...
i , 18 2: 4: 1: 1 3: 4: 1
: 18 v 2.3 1: 1 7
,
,
24
...............................................................................
.................. o,
1 E - Day 2 24 2 1 1 1 3 1 1
1.4 Y. 1 OY.
7
0.
,
v v
O 27 3 3 1 0: 1 0
2 27 1.4 Y 1r 1$- 7
36
...............................................................................
...... i,
:
cn
, 36 4 0 1 0: 0 2
3 1.4 2. 1 7 Lo
. ,
:.,
' .4, 39 0 1 0 2
39 0.8 w 1 r' 1r 4
N ,
____
v.
o, o,
' ,. F - Day 48 3 48 2 2 1 0 0 .. 0
2 1.0Y 1 1 7
0, v
v
6 , 51 3 2 1 0: 0 0 2
51
:
1.1 r 1 - 1 - 7
0 60 0 3 1 0: 0 0 60 0.7 Y 1.Y f 6
63
,
=lv= ....
63 : 1: 0: : . :
. 0.5, 1: 1 2
. .
v
o,
G- Day 4 , 72 72 1 1 0
0 0.5 Y 1 OY.
4
y
v
75 0 1 0: 0 75 0.3Y 14v
Dr 4
y
84
:v .0
84 0 0 0 0 0.0 0
4
87
,
=lv= ' ....
87 . 0: 1 0 : : . : 0.3 ir.
1=Y 3
. .
o,
H- Day 5 , 96 v. 96
0 0 0 0 0.0 Y 0 4
y
% 4,-
99 0 0 0: 0 99 0.0V 0
4
y
.0
108 0 0 0: 0
108 0.0, 0 4
7r
-re ,
111 Ø 'Y ' ....
111 . 0: 0 : : . . 0.0, 0
2
el . .
...............................................................................
........ V
v
o,
I - First Day of Obser 120 vati Morning 0 0 0
0 0.0 0 4
oo
%
4,-
v-i E 130 vening 0 ,
0 0 0 0.0V 0 4
144
i,
v-i : J - Second Day of Obser Morning Oi .
Oi 0 0 i = =
0.0
0 4
Ø
, r ...
el Evening 0 0: 0 0
154 0.0 0 .......... 4
1 !0
0 L- 24 hr Phone call 0 0 0 0 0 0
2. 2 196 Y. 0.5 7 1 r'. 8
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
[0277] Average SOWS scores over time are provided in FIG. 5. SOWS scores are
interpreted as follows: Mild withdrawal: score of 1-10; Moderate withdrawal:
score of 11-
20; Severe withdrawal: score of >21. Mean scores for SOWS question 16 ("I feel
like
using now") are provided in FIG. 6. Scores dropped almost immediately after
first
noribogaine administration and generally continued to fall during treatment.
Surprisingly,
all four of the patients who remained in the study by 96 hours post first
noribogaine dose
replied "Not at all" in answer to this question. Scoring for SOWS assessment:
Patient is
asked to score the items "According to how you feel now" on a scale of 0-4 (0:
Not at all;
1: a little; 2: moderately; 3: quite a bit; 4: extremely).
[0278] Mean Profile of Mood States (POMS) Total Mood Disturbance Scores for
first
seven subjects (001 ¨ 007) is provided in FIG. 7. POMS Total Mood Disturbance
Scores
also dropped within the first 24 hours after first noribogaine dose. POMS is a
self-reported
measure to assess changes in positive and negative mood over time. There are
six
subscales: tension/anxiety, depression/dejection, anger/hostility, vigor,
fatigue,
confusion/bewilderment. A comprehensive global impairment score, Total Mood
Disturbance or TMD, is the sum of all negative mood subscales less the
positive mood
assessment of vigor. Negative TMD scores indicate that vigor exceeds the sum
of
negative mood subscales.
[0279] Patients reported various feelings and during the treatment period.
Patient 1
overcame extreme sadness, depression experienced on Day 2, and had a change in
perspective on Day 3/4 of treatment. Patient 1 completed the full 15 week
follow-up
period opioid free. Patient 4 also experienced change in perspective on Day 2
coincident
with significant reduction in withdrawal symptoms, and called noribogaine "a
re-set
button pushed in my brain," with no cravings and substantially shortened
withdrawal
symptoms (2-3 days, versus 2-3 weeks for "cold-turkey").
[0280] Patient 3 also experienced a change in perspective on Day 3/4,
including first
positive thoughts (in sobriety) since teenage years; "I wanted to be happy and
sober at the
same time, which I am now, which is something that never happens to me."
Patient 3 was
opioid-free for Patients 4 and 5 experienced a change in perspective on Day 2,
coincident
with significant reduction in withdrawal symptoms. Patient 6, despite request
of rescue at
60 hours (hydromorphone) to help with sleep (which did not work) had SOWS
score of 0
CA 03024081 2018-11-13
WO 2016/183244
PCT/US2016/031932
at 24 hours post-discharge and had not resumed opiate consumption. Patient 7,
despite
early discharge, reported no cravings 24 hours after discharge.
[0281] The results of this multi-dose study indicate that safe multiple dosing
is possible
with noribogaine, and that exposure response modeling reliably predicts QT
prolongation
in individual patients. The dosing regimen described herein is a safe way of
titrating
dosages. Finally, noribogaine is effective at treating both opioid withdrawal
and
dependency.
[0282] Further examples of the tolerance and efficacy of noribogaine in humans
are
found, by way of non-limiting example, in U.S. Patent App. Pub. Nos.
2015/0231147 and
2015/0231146, each of which is incorporated herein by reference in its
entirety.
71