Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03026179 2018-11-29
WO 2018/005323 PCT/US2017/039199
APPARATUS AND METHOD FOR FRACTIONAL LIGHT TREATMENT
RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Application No.
62/355,971, filed
June 29, 2016. The entire contents of the foregoing disclosure are herein
incorporated by
reference.
FIELD OF THE INVENTION
The present invention relates to apparatus and methods of performing
fractional light
treatments to human skin and other tissue.
BACKGROUND OF THE INVENTION
Fractional light treatment of the skin has been known for some time and
commercial
products are available in the marketplace that perform fractional skin
treatments. These
treatments may be light-based but also may be RF or ultrasonic (UL)-based and
in addition may
be of a non-ablative, coagulation type or an ablative type, both of which
types are described in
US 8,496,696, assigned to the assignee of the present invention.
Prior to fractional treatment, rather than selected microchannels being
"drilled" into the
skin tissue, large portions of skin tissue were ablated, causing pain to the
patient and sometimes
long recovery times. These are described in the above patent US 8,496,696,
particularly in co1.1,
line 34 to col. 3, line 20. Fractional treatment decreases both the level of
pain experienced and
the recovery time, but these remain concerns in certain circumstances.
Typically, in a fractional treatment, a plurality of spaced apart light beams
impinges on
the skin surface and, depending on whether the energy supplied is for non-
ablative or ablative
treatment, areas of the skin are heated but surrounded by unheated areas (non-
ablative) or
microchannels are formed in the skin and penetrate to a determined depth into
the skin surface,
but again, these microchannels are surrounded by areas of non-treatment. Thus,
the term
"fractional" has the meaning that a "fraction" of the skin surface is treated
with non-ablative,
ablative or a combination of non-ablative light energy.
Generally, and concentrating on the ablative technique while the same
rationale applies to
non-ablative, light beams are delivered to the skin surface as generally
parallel beams, such that
1
CA 03026179 2018-11-29
WO 2018/005323 PCT/US2017/039199
the microchannel formed are generally parallel to one another within the skin
surface. The
delivery system may be a lens system which splits the light beam, generally
from a laser device,
into a number of parallel light beams before it reaches the skin surface. US
Patent Publication
No. 2006/0004347 is one example of such an approach. Another approach is to
use a mirror and
galvometric motor systems distal of the laser light beam to direct the laser
beams onto a number
of rows and columns on the skin surface in seriatim. US Patent Nos. 5,743,902;
5,957,915;
6,328,733 typify such a so-called laser scanning system.
A potential problem with either of the above approaches is that there is one
microchannel
for each laser light beam that penetrates the skin surface and this
potentially exposes the patient
to a longer-term recovery due to the number of microchannels and the potential
possibility of
infection simply due to the number of microchannels drilled into the skin
surface.
Thus, there is a perceived need for a fractional device which forms fewer
microchannels
in the skin surface yet under the skin surface multiplies into a greater
number of microchannels.
It is to this need that the present invention is addressed.
SUMMARY OF THE PRESENT INVENTION
In an aspect, a method of cosmetically treating skin tissue includes the steps
of: providing
a light source to direct one or more light energy beams to the skin tissue;
providing a prism and
interposing the prism between the light source and the skin tissue; activating
the light source; the
one or more light energy beams passing through the prism converge and are
focused to one or
more focal points at or near the skin tissue surface; each of the one or more
light energy beams
each diverge after passing through the one or more focal points to each create
a plurality of
microchannels in the skin tissue. The light source may be one of: coherent or
incoherent light.
In another aspect, the prism is a pyramidal prism having an apex and a base
and wherein
the one or more light energy beams are positioned to pass through the
pyramidal prism in the
direction from the apex and through the base of the prism. The pyramidal prism
may include 3 or
more prism surfaces and may be a flat-top pyramidal prism.
In a further aspect, the number of diverging light beams is correlated with
the number of
prism surfaces. The prism may be two or more prisms, the two or more prisms
being arranged on
their bases arranged adjacent to one another on a plane. The diverging light
beams may produce
one or more of: ablative or coagulative effects in the skin tissue.
2
CA 03026179 2018-11-29
WO 2018/005323 PCT/US2017/039199
In another aspect, the plurality of prisms may be arranged as an array of
microlenses
mounted in a tube having a distal portion and a proximal portion, the array
being mounted in the
distal portion and the light energy being received from the proximal portion
to travel through the
tube and to the distal portion. The method further may include the steps of
placing the distal
portion of the tube in contact with the skin tissue and activating the light
energy to pass through
the tube, through the array and into the skin tissue.
In another aspect, the one or more diverging light beams produce a fractional
effect in the
skin tissue. Further, the angular orientation of the pyramidal surfaces to the
base determines the
angle of the diverging light energy beams. The angular orientation of the
light energy impinging
on the pyramidal surfaces determines the angle of the diverging light energy
beams. The method
may further include the step of interposing a heat absorbing material between
the base of the
prism and the skin tissue.
In an aspect, a device for cosmetically treating skin tissue includes: a light
source to
direct one or more light energy beams to the skin tissue; a prism, the prism
being interposed
between the light source and the skin tissue; when the light source is
activated, the one or more
light energy beams pass through the prism, become converged and are focused to
one or more
focal points at or near the skin tissue surface; and, wherein each of the one
or more light energy
beams each diverge after passing through the one or more focal points to each
create a plurality
of microchannels in the skin tissue. The light source may be one of: coherent
or incoherent light.
In a further aspect, the prism is a pyramidal prism having an apex and a base
and wherein
the one or more light energy beams are positioned to pass through the
pyramidal prism in the
direction of from the apex and through the base of the prism. Further, the
pyramidal prism may
include 3 or more prism surfaces. Also, the pyramidal prism may be a flat-top
pyramidal prism.
The number of diverging light beams is correlated with the number of prism
surfaces.
In yet a further aspect, the prism may comprise two or more prisms, the two or
more
prisms being arranged on their bases arranged adjacent to one another on a
plane. Further, the
diverging light beams produce one or more of: ablative or coagulative effects
in the skin tissue.
The plurality of prisms may be arranged as an array of microlenses mounted in
a tube having a
distal portion and a proximal portion, the array being mounted in the distal
portion and the light
3
CA 03026179 2018-11-29
WO 2018/005323 PCT/US2017/039199
energy being received from the proximal portion to travel through the tube and
to the distal
portion.
BRIEF DESCRIPTION OF THE DRAWINGS
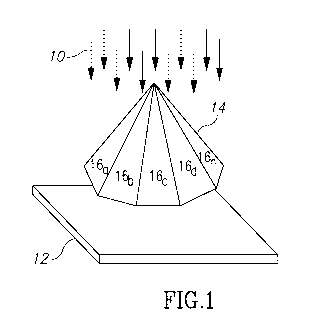
Fig. 1 illustrates a perspective view of a prism structure in accordance with
the present
invention.
Fig. 2 illustrates a side interior view of light beams passing through the
prism of Fig. 1.
Fig. 3 illustrates further side interior and top views of light beams passing
through a
prism flat-top prism in accordance with the present invention.
Figs. 4 and 5 illustrate prism structures in the form of an array of
microlenses.
Fig. 6 illustrates an array of microlenses mounted in a hollow tube or funnel-
like
structure.
Fig. 7 illustrates the interaction of a donut-shaped light source impinging on
a prism
similar to the prism of Fig. 3.
Fig. 8 illustrates a prism similar to those of Figs. 1 to 3 and further
including an isolation
layer of material interposed between the prism bottom surface and a patient's
skin tissue.
Figs. 9A and 9B illustrate an array of Fresnel lenses mounted on a plate.
Fig. 10 illustrates a lens array mounted on a holding plate in accordance with
the present
invention.
DETAILED DESCRIPTION OF THE PRESENT INVENTION
The present invention provides a solution to the potential problems described
above by
directing a number of laser light beams to impinge on the skin surface at a
common point by
being focused at or near (either above or below) the skin surface but then
diverging to create a
plurality of subsurface microchannels in the case of ablative treatment or
areas of heating in the
case of non-ablative heating. This treatment technique described in the
present invention may
become known as "3D Fractional" in that a single or several beam(s) are
divided or scanned into
multiple three-dimensional beam coverage within the patient's skin tissue.
One apparatus for performing this function is shown in Fig. 1. Fig. 1
illustrates a plurality
of light beams 10 traveling more or less in a parallel fashion from a light
source (not shown),
which may be a laser light source of a known type. Prior to reaching the
epidermis 12 of the skin,
4
CA 03026179 2018-11-29
WO 2018/005323 PCT/US2017/039199
a prism 14 is interposed between the light beams 10 and the epidermis/skin
surface 12. The
purpose of the prism, shown in Fig. 1 as a pyramidal prism, is to redirect the
light beams 10
striking the prism surfaces 16a, 16b, 16c, ...16n to a central focal point at
the bottom flat surface
of the prism just before (or even just after) the light beams enter into the
epidermis.
Turning now to Fig. 2, this figure shows a side view of two exemplary beams 20
and 22
as they strike the surfaces 24 and 26 of the prism after traveling through the
air. As illustrated,
the light beams 20 and 22 will be bent upon striking the prism surface
according to the well-
known Snell's law. By adjusting the angular orientation of the prism sides
with respect to the
base of the prism, shown in Fig. 2 as angle a2, the beams hitting prism at
points 28 and 30 will
be refracted/deflected in a way so that they converge just above, at or below
point 33 on the base
of the prism. The skin tissue penetration point depends on the angle the laser
light beam(s) hit
the prism's side surface, the angle of the prism side surfaces with respect to
the base of the prism
and at which point or points along the prism side surface the laser light beam
hits. These are all
variables that can be controlled by a programmed controller in order to
deliver the treatment
desired. The skin surface is shown in Fig. 2 at 34. After impinging on the
epidermis at 34, the
beams will then begin to diverge from one another into the skin tissue 34A as
can be seen in Fig.
2 as well as in Fig. 3 in which beams 31, 37 and 39 will converge at point 35
on the bottom
surface of the prism and then diverge as beams 44, 46 and 48 respectively
within the skin tissue
42. Once within the tissue, the beams, depending on the particular
specifications of the beams
44, 46 and 48, will drill microchannels 36, 38 and 40 and/or areas of
coagulation. Thus, one
single area of the skin produces multiple channels/ areas of coagulation, thus
reducing the
disturbance of the epidermis skin surface.
As mentioned above, in prior art laser fractional devices, a single spot of
light on the skin
generates a single channel or area of coagulation. Each spot of light on the
skin is characterized,
among other things, by a certain spot area based on the beam characteristics
known to those
skilled in the art. In addition, the single channel or area of coagulation
defines a certain volume
in the skin directly affected by the laser beam, having a certain surface.
Laser wavelength and
other laser beam parameters affect this volume and therefore its surface. It
is another aspect of
the invention to increase these overall surfaces with a single spot of light
on the skin. It has been
found that the larger the overall surface of the channels or area of
coagulation in the skin, the
better the clinical effect of skin rejuvenation as a result of a stronger
healing response and
CA 03026179 2018-11-29
WO 2018/005323 PCT/US2017/039199
stronger collagen production. However, the larger the overall area of spots of
light on the skin,
and the density of the affected tissue through which light penetrates into the
skin to create these
channels or areas of coagulation, the higher the risk for complications and
the longer the time for
the tissue to heal.
In prior art systems, in which a single channel or area of coagulation in the
skin is
generated per a single spot of light on the skin, in order to increase the
number of channels or
areas of coagulation in the skin and their surfaces, an increase in the area
of the spot of light on
the skin is required. Therefore, according to this aspect of the invention
there is provided a
system and method to increase the overall surface of the channels or areas of
coagulation in the
skin without the need to increase the areas of spot of lights on the skin.
According to another
aspect of the invention, at least one of the multiple channels or areas of
coagulation has a main
longitudinal axis which is not approximately perpendicular to the skin
surface. According to this
aspect of the invention, at least two channels or areas of coagulation are not
parallel to one
another. They define two separate volumes of tissue directly affected by a
laser beam yet they
share a common spot on the skin through which a laser beam penetrates the
skin. In addition, in
the present invention, the utilization of a combination of ablative and non-
ablative coagulation
treatments may be "custom blended" to achieve the desired results in the 3D
volume of tissue
affected by the impingement of light (or RF energy or UL energy) on the
tissue.
Any number of beams may be utilized depending on the number of sides that the
prism
possesses. As seen in Fig. 3, the prism may be a flat-topped pyramidal prism
so that a center
beam goes through the prism un-refracted or un-bent, as with beam 37. The
prism may be placed
directly on the epidermis or may be mounted or fixed a predetermined distance
from the skin
surface, as desired, with the angles of the prism sides with respect to the
base being adjusted so
the beams become focused at the epidermis surface. In addition, it may be
desirable to provide
that the beams 31, 37 and 39 are focused not at the surface but below or even
above the skin
surface. The prism may be placed or mounted within a housing open at the top
and bottom with
active or passive cooling of the housing that will cool the prism and thus the
patient's skin.
Figs. 4 and 5 illustrate the application of the principles of the prism
structure of Figs. 1 to
3 as applied to microlens arrays, either in the form of triangular/trapezoid
[not shown]
microlenses or, as in Fig. 5, hexagonal shaped microlenses.
6
CA 03026179 2018-11-29
WO 2018/005323 PCT/US2017/039199
Fig. 6 illustrates an array of microlenses 100 which may be like the arrays
depicted in
Figs. 4 or 5 mounted on a hollow tube or funnel. It is to be understood that
the element 102 may
be solid or may be an open framework. While the distal end 104 may be in
contact or in the
vicinity of the patient's skin tissue to be treated, the proximal end 106 may
be in contact with or
at least aligned with the laser source to be applied through the tube or
funnel, through the array
of prisms and into the patient's skin tissue. While shown as a conical shape,
the element 102 may
be any suitable shape. A device to evacuate smoke from tissue disruption by
the laser, presently
used in some CO2 laser systems, may also be incorporated into the device 110.
The device 110
may be either a disposable or not disposable depending on cost, ease of
sterilization and other
factors.
Fig. 7 illustrates another embodiment of the present invention in which a
donut-shaped
laser light beam 200 is impinged on a prism 202 which may be like the prisms
in Figs. 1, 2 or 3.
Once the light beams have impinged on prism 202, they are bent as shown and
form in the skin a
generally cone shaped ablation/coagulation volume of dimensions much greater
than the initial
contact or entry into the patient's skin surface 204.
Fig. 8 illustrates another embodiment of a device in which an isolation layer
of suitable
material 300 is interposed between the prism 302 and the patient's skin
surface 304.The material
300 may be selected for transmissivity but also may have heat absorptive
characteristics. The
material 300 may be, for example, actively cooled with suitable means to keep
the patient's skin
tissue cool and may include a drug or other substance to enhance the clinical
response or
improve results of the treatment with the interposition of the material 300,
as this arrangement
will affect the focal point 306 due to the greater distance from the skin
compared to when there is
no material 300 present. Of course, the focal point can be manipulated by
changing the angle (a
2 as in Fig.2) to cause deflection of the laser light beam and the focal point
as desired.
Figs. 9A and 9B illustrate yet another embodiment in which an array of Fresnel
lenses
(like that shown as element 400 in Fig. 9A) are mounted on a plate or other
holder 402 as micro
Fresnel lenses 403a to 403n shown in Fig. 9B. These lenses are oriented as
shown in Fig. 9A so
that the parallel laser light beams are focused as desired on a patient's skin
tissue 401 at one or
multiple points. As with the other embodiments described in this application,
the number of
entries of laser light beams into the skin surface will be reduced to a lesser
number than the
treated areas, much like as shown in Figs. 1 and 2.
7
CA 03026179 2018-11-29
WO 2018/005323 PCT/US2017/039199
Fig. 10 illustrates another embodiment of a lens array 500 which includes a
plate or
holder 502 which mounts or otherwise holds a number of microlenses 504a ...
504n which are
oriented to converge light beams 506a to 506n onto a skin surface 508 at focal
point 510.
Returning to Fig. 3, it is seen that a light beam 37 impinges the upper flat
surface 43 of
the flat-topped pyramid. As such, that beam 37 will travel through the prism
undeflected. While
all three beams 31, 37 and 39 may be of the same" type", that is, one of
ablative or non-ablative,
they may be "mixed and matched" so that, for example, the central beam 37 may
be ablative
while any side beams non-ablative or vice-versa, all dependent on the
characteristics of the
planned skin tissue treatment.
Also, while Figs. 1 to 3 illustrate the use of multiple parallel laser beams,
a laser scanner
of the type described above may be used to selectively impinge on desired
sides of the prisms
shown in a pattern to be selected dependent on the desired effects on the skin
tissue.
8