Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03041417 2019-04-23
CHITOOLIGOSACCHARIDE OF SPECIFIC STRUCTURE,
PREPARATION METHOD THEREFOR AND USE THEREOF
Technical Field
The present invention belongs to the technical field of chitooligosaccharides
application, and particularly relates to a chitooligosaccharide having a
specific
structure and a preparation method and application thereof.
Background Art
Chitooligosaccharides (COS) are oligomers with a degree of polymerization of
less than 20 formed by linking glucosamine and N-acetylglucosamine by 13-1,4
glycosidic bonds, and have anti-inflammatory, anti-tumor and immunoregulation
and other biological activities. Studies show that the structure of
chitooligosaccharide, including the degree of polymerization, degree of
deacetylation and the distribution of acetyl sites in oligosaccharides and
other
structural features, plays a decisive role in the exerting of its biological
activity.
Traditional processes for the preparation of chitosan involve high-temperature
and
strong alkali deacetylation of chitin, and the product chitosan has better
acid
solubility only in the case that the degree of deacetylation is >80%.
Therefore, the
degree of deacetylation of chitosan in the existing industry is generally
>80%, and
chitosan contains a small amount of acetyl groups therein. The degree of
deacetylation of chitooligosaccharide prepared by using the above chitosan as
a
substrate is also >80%, so the acetylated monosaccharide component contained
in
the chitooligosaccharide chain is also reduced correspondingly, and the
corresponding number of different chitooligosaccharide components is limited.
Homogeneous deacetylation of chitin can be achieved by a low-temperature
alkali method (Kurita K, Sannan T, Iwakura Y. Studies on chitin, .4. Evidence
for
formation of block and random copolymers of N-acetyl-D-glucosamine and
CA 03041417 2019-04-23
D-gluco s am ine by heterogeneous and homogeneous
hydrolyses [J]
Makromolekulare Chemie-Macromolecular Chemistry and Physics, 1977, 178(12):
3197-3202. Liu Dasheng, Wei Yuanan, Jiang Linbin, et al. Study on the
preparation
of water-soluble chitosan by deacetylation of ultrafine chitin [J]. Food
Science and
Technology, 2007, 32(9): 108-110.), by which a low degree of deacetylation
chitosan with a degree of deacetylation >50% can be obtained. Due to higher
contents of both N-acetylglucosamine (hereinafter referred to as A) and
glucosamine
(hereinafter referred to as D) in the sugar chain of this type of chitosan,
hydrolase
enzymes such as chitinase and chitosanase capable of recognizing A and D can
hydrolyze this type of chitosan. Studies have found that different chitosan
hydrolases have large difference in glycosidic bond recognition at the
hydrolysis site.
For example, glycoside hydrolase family 18 (GH18) chitinase can only recognize
and hydrolyze A-A and A-D type glycosidic bonds, while GH19 chitinase can only
recognize and hydrolyze A-A and D-A type glycosidic bonds; and as for
chitosanase,
according to the difference in glycosidic bond recognition, it is generally
classified
into three subtypes (I, II and III): type I can recognize and hydrolyze D-D
and A-D,
type II can only recognize D-D, and type III can recognize D-D and D-A
glycosidic
bonds. Even if the hydrolysis substrate is the same low-degree of
deacetylation
chitosan, because of the difference in the recognition of the substrate by
these
different enzymes, chitooligosaccharides having different structure types,
such as
different degrees of polymerization and distribution of acetyl sites, can be
obtained
using different chitosan hydrolases, and these structurally different
chitooligosaccharides may have large difference in specific biological
activities.
Description of the Invention
It is an object of the present invention to provide a novel
chitooligosaccharide
having a specific structure and a preparation method thereof. In addition, the
present
inventors have also found the use of the novel chitooligosaccharide in
medicine,
especially in the field of anti-hepatoma.
2
CA 03041417 2019-04-23
In order to achieve the above object, the technical solution of the present
invention is as follows:
a chitooligosaccharide having a specific structure, the structure of the
chitooligosaccharide being as shown in formula (I),
OH OH OH
HO VOW VOW
HO OH
HO HO
NH2 NHR NH2
II
(1)
wherein n is 0-18; and R is H or COCH3.
The chitooligosaccharide of the present invention has a degree of
deacetylation
of 50% to 80%. Preferably, the chitooligosaccharide has a degree of
deacetylation of
56% to 78%, and more preferably, the chitooligosaccharide has a degree of
deacetylation of 60% to 70%, further more preferred degree of deacetylation is
60-65%, and the most preferred degree of deacetylation is 62%. The degree of
deacetylation refers to a fraction of deacetylated chain segments accounting
for the
total chain segments in the chitooligosaccharide structure represented by the
formula
(I). Assuming that the number of the repeating structural units of
chitooligosaccharide is 100, in which there are 22 acetylglucosamine
structural units
and 78 glucosamine structural units, the corresponding degree of deacetylation
is
78%. By controlling the quantitative proportion of R being COCH3 in the
formula
(I), the corresponding degree of deacetylation can be controlled.
The low-degree of deacetylation chitosan described herein refers to chitosan
having a degree of deacetylation of less than 80%.
The present invention also provides a preparation method of the
chitooligosaccharide having a specific structure, which comprises: obtaining
the
chitooligosaccharide by enzymatic hydrolysis of a chitosan substrate, the
enzyme
used can specifically recognize and hydrolyze the glycosidic bond formed by
glucosamine and glucosamine, so that all the components of the
3
CA 03041417 2019-04-23
chitooligosaccharide obtained by hydrolysis have both the reducing ends and
non-reducing ends thereof being glucosamine. The specific steps of the
preparation
method of the chitooligosaccharide are as follows: (1) deacetylating chitin to
obtain
a chitosan substrate, and obtaining chitosans with different degrees of
deacetylation
by controlling the deacetylation reaction time; and (2) using enzymes
specifically
recognizing and hydrolyzing D-D type glycosidic bonds to hydrolyze the
chitosan
substrate to obtain the chitooligosaccharide product.
Preferably, the chitosan has a degree of deacetylation of 50% to 80%, further
preferably 56% to 78%, and even more preferably, the chitooligosaccharide has
a
degree of deacetylation of 60% to 70%, and a still more preferred degree of
deacetylation is 60-65%, and the most preferred degree of deacetylation is
62%. The
degree of deacetylation of chitosan can be controlled by controlling the
reaction time
of deacetylation of chitin.
In the present invention, the deacetylation reaction of chitin is carried out
under
alkaline and low temperature conditions. Bases which can be used for
deacetylation
include sodium hydroxide and potassium hydroxide, and the preferred base is
sodium hydroxide. The temperature for deacetylation ranges from 40 C to 90 C,
and
preferably, the temperature ranges from 50 C to 60 C. The concentration of the
alkaline solution ranges from 40% to 50%, preferably 45%. The time for the
deacetylation reaction ranges from 0.5 h to 24 h, preferably from 2 h to 6 h.
In a specific embodiment of the present invention, chitin is deacetylated in a
45
wt% sodium hydroxide solution at 60 C, and the deacetylation reaction time is
controlled to be from 0.5 to 24 hours, and chitosan having a degree of
deacetylation
of 50%-80% can be obtained.
The present invention uses an enzyme capable of specifically recognizing and
hydrolyzing D-D type glycosidic bonds to hydrolyze the chitosan substrate.
Preferably, the enzyme is a neutral protease. The neutral protease used in the
examples of the present invention is a neutral protease crude protease derived
from
Bacillus subtilis.
4
CA 03041417 2019-04-23
Different enzymes require different hydrolysis conditions for the hydrolysis
of
chitosan substrate. Depending on the nature of the enzyme used, one skilled in
the
art can experiment to determine suitable hydrolysis conditions. In the present
invention, a neutral protease is preferably used, and the specific conditions
for the
neutral protease to hydrolyze chitosan are as follows: the amount of the
neutral
protease is 2 wt% to 25 wt% of the substrate, preferably 5 wt% to 20 wt%, and
more
preferably 5 wt% to 15 wt%; the hydrolysis temperature is 25-55 C, preferably
30-45 C, and more preferably 35-45 C; and the hydrolysis time is 30-60 h, and
preferably 40-50 h.
The present invention also provides the use of the chitooligosaccharide having
a specific structure as a medicine.
The present invention further provides the use of the chitooligosaccharide
having a specific structure in the preparation of an anti-hepatoma medicine.
The present invention also provides a pharmaceutical composition comprising
the chitooligosaccharide having a specific structure or a pharmaceutically
acceptable
salt of the chitooligosaccharide as an active ingredient.
The pharmaceutical composition of the present invention further comprises, in
addition to the chitooligosaccharide of the present invention, a
pharmaceutically
acceptable carrier. By "pharmaceutically acceptable carrier" herein is meant a
pharmaceutically acceptable material, ingredient or medium, such as liquid or
solid
fillers, diluents, adjuvants, solvents or encapsulating materials, including
carrying or
transporting the main pharmaceutical agents from one organ or part of the body
to
another organ or part of the body. Each carrier must be "acceptable" and
compatible
with other forms of medicines without causing harm to the patient. Some
examples
of pharmaceutically acceptable carriers include: sugars such as lactose,
glucose and
sucrose; starches such as wheat starch and potato starch; cellulose and its
derivatives
such as sodium carboxymethyl cellulose, ethyl cellulose, cellulose acetate,
powdered
tragacanth, malt, gelatin, talcum powder; adjuvants such as cocoa butter and
suppository wax; oils such as peanut oil, cottonseed oil, safflower oil,
sesame oil,
5
CA 03041417 2019-04-23
olive oil, corn oil and soybean oil; glycols such as butanediol; polyols such
as
glycerol, sorbitol, mannitol and polyethylene glycol; esters such as ethyl
oleate and
ethyl laurate; agar; buffers such as magnesium hydroxide and aluminum
hydroxide;
alginic acid; pyrogen-free water; physiological saline; Ringer's solution;
ethanol;
phosphate buffer, and other non-toxic compatible substances applied in
pharmaceutical preparations.
Compared with the prior art, the present invention has the following
advantageous effects.
1. The chitosan having a degree of deacetylation of less than 80% was
hydrolyzed by chitosan hydrolase of different hydrolysis site recognition
types, on
the basis of which the structure of the product chitooligosaccharide was
identified
and evaluated for the difference in the anti-cancer activities thereof The
inventors
have surprisingly found that one chitooligosaccharide having a specific
structural
type is superior to other chitooligosaccharides in terms of anti-hepatoma
activity,
and has great application potential in the field of anti-hepatoma. The
chitooligosaccharide having a specific structure has the structural regularity
that,
both the reducing ends and the non-reducing ends of all the components of the
chitooligosaccharide are glucosamine.
2. The chitooligosaccharide having a specific structure obtained by the
present
invention has better pharmacological activity and can be used in the field of
medicine. Specifically, the chitooligosaccharide having a specific structure
disclosed
in the present invention has an activity of inhibiting the growth of liver
cancer cells,
and can be applied to the preparation of anti-hepatoma medicines or
pharmaceutical
compositions.
Brief Description of the Drawings
Figure 1 is the 'H-NMR spectrum of the chitosan having a degree of
deacetylation of 62% in Example 1 of the present invention.
Figure 2 is the MALDI-TOF mass spectra of different types of
6
CA 03041417 2019-04-23
chitooligosaccharides in Example 2 of the present invention.
Figures 3A-3D are 1H-NMR and DC-NMR spectra of chitooligosaccharides
COS-62-PA and COS-62-NP in Example 2 of the present invention. Therein,
Figures 3A and 3B correspond to the 1H-NMR and 13C-NMR spectra of COS-62-PA;
and Figures 3C and 3D correspond to the '11-NMR and '3C-NMR spectra of
COS-62-NP.
Figure 4 is a bar data graph showing the effect of chitooligosaccharides
having
different structural types on the growth of HepG2 cells in Example 3 of the
present
invention.
Figures 5A-5B are bar data graphs showing the effects of different
concentrations of chitooligosaccharide COS-62-NP and positive drug 5Fu on the
growth of HepG2 cells in Example 4 of the present invention; wherein Figure 5A
corresponds to a schematic diagram of COS-62-NP inhibiting cell survival rate,
and
Figure 5B corresponds to a schematic diagram of the positive drug 5Fu
inhibiting
cell survival rate.
Figures 6A-6E are the 'H-NMR spectra corresponding to chitosans having
different degrees of deacetylation in Example 5 of the present invention;
wherein the
degree of deacetylation corresponding to Figure 6A is 56%, the degree of
deacetylation corresponding to Figure 6B is 66%, the degree of deacetylation
corresponding to Figure 6C is 70%, the degree of deacetylation corresponding
to
Figure 6D is 74%, and the degree of deacetylation corresponding to Figure 6E
is
78%.
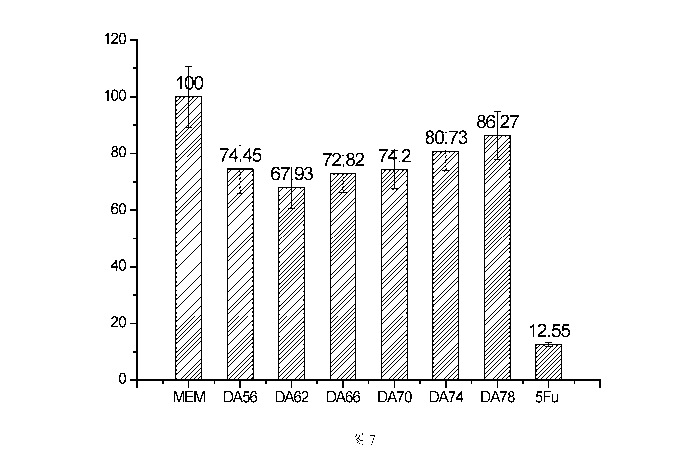
Figure 7 is a bar data graph showing the effects of chitosans having different
degrees of deacetylation on the growth of HepG2 cells in Example 5 of the
present
invention.
Detailed Description
The embodiments of the present invention will be described in detail below
with reference to the following examples. However, one skilled in the art will
7
CA 03041417 2019-04-23
understand that, the following examples are intended to only illustrate the
invention
but not intended to limit the scope of the present invention. Those examples,
in
which the specific conditions are not specified therein, are carried out
according to
the conventional conditions or the conditions recommended by the manufacturer.
The reagents or instruments used, for which the manufacturers are not
specified, are
all conventional products that are commercially available.
Example 1: Preparation of low-degree of deacetylation chitooligosaccharides of
different structural types
Low-degree of deacetylation chitosan was prepared by reference to the method
by Liu Dasheng et al. (Liu Dasheng, Wei Yuanan, Jiang Linbin, et al. Study on
the
preparation of water-soluble chitosan by deacetylation of ultrafine chitin
[J]. Food
Science and Technology, 2007, 32(9): 108-110.), and the specific method is as
follows: weighing 30 g of ultrafinely pulverized chitin powder, adding it to
10 times
of 45% sodium hydroxide solution, stirring evenly, then heating up to 60 C for
deacetylation reaction for 2 h, centrifuging after the reaction completion,
washing
the precipitate with a 60% ethanol-water mixed solution until it was non-
alkaline,
and drying it to obtain a product. The degree of deacetylation was measured by
'1i-NMR (see Figure 1), and the degree of deacetylation was determined to be
62%
based on the 11-1-NMR spectrum in Figure 1. Two portions of the prepared
chitosan
were weighed, each portion of 10 g, were each added to a thermostatic reaction
vessel containing 200 mL of 1.5% aqueous acetic acid solution, and thoroughly
stirred to completely dissolve it. The reaction temperature was adjusted to 40
C, 1 g
of neutral protease dry powder (abbreviated as NP, enzyme activity: 50,000
U/g,
purchased from Shandong Taian Xindeli Bioengineering Co., Ltd.) and 1 g of
papain powder (abbreviated as PA, enzyme activity: 50,000 U/g, purchased from
Nanning Pangbo Biological Engineering Co., Ltd.) were added therein
respectively,
and constant temperature reaction was carried out for 48 hours. After the
reaction
was completed, insoluble substance was removed by centrifugation, and the
supernatant was concentrated to 50-100 mL by a rotary evaporator at 40 C, and
then
8
CA 03041417 2019-04-23
freeze-dried to obtain the finished product chitooligosaccharides, which were
named
as COS-62-NP (referring to the chitooligosaccharide product obtained after
hydrolysis by neutral protease) and COS-62-PA (referring to the
chitooligosaccharide product obtained after hydrolysis by papain).
Example 2: Composition and structure identification of different types of
chitooligosaccharides
The chitooligosaccharide composition of the above three chitooligosaccharides
were identified using MALDI-TOF mass spectrometry. The specific method is as
follows: weighing three prepared or purchased chitooligosaccharide samples
COS-62-NP, COS-62-PA and COS-MP-162, preparing each of them into an
aqueous solution having a concentration of 2 mg/mL with ultrapure water,
sucking
out 1 pL for each sample, spotting onto the sample plate, after natural
drying, each
adding 1 L of matrix 2,5-dihydroxybenzoic acid (DHB) solution, and after
drying,
using an autoflex III smartbeam type MALDI-TOF mass spectrometer (Bruker
corporation) for detection (positive ion reflection mode). The results of mass
spectrometry were shown in Figure 2: respectively correspond to the mass
spectra of
COS-62-NP, COS-62-PA and COS-MP-162; and for easy distinction, in Figure 2, A
represents N-acetylglucosamine, D represents glucosamine, the subsequent
numbers
represent the numbers of such monosaccharides contained, and the sum of the
two is
the degree of polymerization of the oligosaccharide. From the results of mass
spectrometry in Figure 2, it can be seen that the commercial product
chitooligosaccharide COS-MP-162 consists mainly of fully deacetylated
chitooligosaccharide component, while the low-degree of deacetylation
chitooligosaccharides (COS-62-NP and COS-62-PA) consist of oligosaccharide
components containing more N-acetylglucosamine. Further, it can be known from
the mass spectra in Figure 2 that the degrees of polymerization of the low-
degree of
deacetylation chitooligosaccharides (COS-62-NP and COS-62-PA) were between 2
and 20. Moreover, even with the same substrate, when using different enzymes
for
hydrolysis, oligosaccharides had their structures significantly different. The
degree
9
CA 03041417 2019-04-23
of deacetylation of the chitooligosaccharide obtained by neutral protease
hydrolysis
of low-degree of deacetylation chitosan in the detection range is higher, only
1-3
acetyl groups existing in the acetyl group-containing oligosaccharide
component,
while the degree of deacetylation of the chitooligosaccharide obtained by
papain
hydrolysis of low-degree of deacetylation chitosan in the detection range is
lower,
containing 3- 6 acetyl groups.
Because MALDI-TOF mass spectrometry can only better detect the
components with a molecular weight of below 2000, for further confirmation of
the
structural characteristics of different types of chitooligosaccharides
prepared, the
structural characteristics of the reducing ends and non-reducing ends of COS-
62-PA
and COS-62-NP were analyzed by using '1-I-NMR and '3C-NMR, as shown in
Figures 3A-3D, wherein Figures 3A and 3B correspond to the '1-I-NMR and
'3C-NMR spectra of COS-62-PA; and Figures 3C and 3D correspond to the
'H-NMR and '3C-NMR spectra of COS-62-NP. The results of the spectra of Figures
3A-3D showed that, the reducing end of COS-62-PA contained both
monosaccharide units A and D (as shown in Figure 3A), while the non-reducing
end
also contained both A and D (as shown in Figure 3B). The results indicated
that
papain may contain multiple hydrolysis types of chitosan hydrolase enzymes at
the
same time, thereby producing chitooligosaccharide products with complex
reducing
end and non-reducing end structures. Relatively speaking, the structure of
COS-62-NP is more regular: both the reducing end and the non-reducing end were
composed of D sugar units (as shown in Figures 3C and 3D), and had easily
distinguished structural characteristics. This may be due to the fact that,
that plays
the role of chitosan hydrolysis in the neutral protease is a relatively single
enzyme,
and according to the reducing end and non-reducing end properties of the
product, it
should be a type II chitosanase that can only recognize and hydrolyze D-D
glycosidic bonds. This is in good agreement with the MALDI-TOF mass
spectrometry results of COS-62-NP (as shown in Figure 2) in which the
oligosaccharide component contained at least two D monosaccharides.
CA 03041417 2019-04-23
Example 3: Comparison of anti-hepatoma activity of chitooligosaccharides of
different structural types
Human hepatoma cell line HepG2 cultured to log phase was digested and then
added to MEM medium (10% FBS, SIP, 1%NAEE), and diluted to 1 x104 cells/ml,
three 96-well cell culture plates were inoculated at 100 l/well, and
culturing was
carried out at 37 C in 5% CO2 incubator overnight until the cells were
completely
attached. The aqueous solutions (10 mg/ml) of three chitooligosaccharides
(COS-62-NP, COS-62-PA and COS-MP-162) were prepared and sterilized by
filtration through a 0.22 pm filter in an intercellular super clean bench. The
three
aqueous solutions of chitooligosaccharides prepared above were diluted to 200
with MEM medium (60 1 of 10 mg/ml oligosaccharide solution was added to
MEM medium 2940 Ill to 3 ml), and three 96-well cell culture plates were
inoculated at 100 p1/well (the final concentration of chitooligosaccharide was
100
[tg/m1), and culturing was continued at 37 C in 5% CO2 incubator. At the same
time,
5-fluorouracil (5Fu) of the same concentration was used as a positive control,
and a
blank medium MEM was used as a negative control. MTT was added at 20 111/well
into the 96-well cell culture plates 72 h after administration, and the
culturing was
continued at 37 C in 5% CO2 incubator for 4 h. The liquid in each well of the
culture plate was sucked out by a lance and discarded, and DMSO was added at
100
The 0D490 values in each well were determined and plotted with OriginPro
8.5 software. The cell survival rates of the three different
chitooligosaccharides and
the positive control drug groups were calculated by using MEM medium group as
100% cell survival rate, and the specific results were shown in Figure 4. The
results
showed that the positive drug 5Fu had the most obvious inhibitory effect on
the
growth of HepG2 cells, and the cell survival rate was only 11.64% of the
control
group; COS-62-NP also had obvious inhibitory effect on the growth of HepG2
cells,
and the cell survival rate was only 69.87% of the control group; while the
inhibitory
effects of the chitooligosaccharides COS-62-PA and COS-MP-162 on HepG2 cells
were not obvious.
11
CA 03041417 2019-04-23
Example 4: Effect of concentration on anti-hepatoma activity of
chitooligosaccharide COS-62-NP
The HepG2 cells cultured to log phase were digested and then added to MEM
medium (10% FBS, SIP, 1% NAEE), and diluted to 1x104 cells/ml, three 96-well
cell culture plates were inoculated at 100 td/well, and culturing was carried
out at
37 C in 5% CO2 incubator overnight until the cells were completely attached.
An
aqueous solution of the chitooligosaccharide COS-62-NP (10 mg/ml) was prepared
and sterilized by filtration through a 0.22 lam filter in an intercellular
super clean
bench. It was diluted to 200 pg/ml, 100 pg/ml, 50 tig/ml, 20 jig/ml and 2
ig/m1 in
sequence with MEM medium, three 96-well cell culture plates were inoculated at
100 1.11/well, and culturing was continued at 37 C in 5% CO2 incubator. At the
same
time, 5-fluorouracil (5Fu) of the same concentration was used as a positive
control,
and a blank medium MEM was used as a negative control. MTT was added at 20
111/well into the 96-well cell culture plates 72 h after administration, and
the
culturing was continued at 37 C in 5% CO2 incubator for 4 h. The liquid in
each
well of the culture plate was sucked out by a lance and discarded, and DMSO
was
added at 100 [d/well. The 0D490 values in each well were determined and
plotted
with OriginPro 8.5 software. The cell survival rates of different-
concentration
chitooligosaccharide COS-62-NP and the positive control drug groups were
calculated by using MEM medium group as 100% cell survival rate. The specific
results were shown in Figure 5A and Figure 5B, wherein Figure 5A corresponded
to
a schematic diagram of COS-62-NP inhibiting cell survival rate, and Figure 5B
corresponded to a schematic diagram of the positive drug 5Fu inhibiting cell
survival rate. The results showed that the inhibitory activity of
chitooligosaccharide
COS-62-NP on HepG2 cells increased with the increase of concentration, and
similar results were also obtained for the positive drug 5Fu, but the
inhibitory effect
was more obvious.
Example 5: Effect of degree of deacetylation on the anti-hepatoma activity of
chitooligosaccharide
12
CA 03041417 2019-04-23
In order to determine the effect of degree of deacetylation on the anti-
hepatoma
activity of the product obtained by neutral protease hydrolysis of low-degree
of
deacetylation chitosan, further on the basis of chitosan having a degree of
deacetylation of 62%, by reference to the method by Liu Dasheng et al (Liu
Dasheng, Wei Yuanan, Jiang Linbin, et al. Study on the preparation of water-
soluble
chitosan by deacetylation of ultrafine chitin [J]. Food Science and
Technology, 2007,
32(9): 108-110.), by adjusting the deacetylation reaction time at 60 C
(reaction time
of lh, 3h, 6h, 9h, 12h respectively), chitosans having different degrees of
deacetylation were re-prepared, and the degrees of deacetylation were
determined by
'1-I-NMR, as shown in Figure 6A to Figure 6E, wherein the degree of
deacetylation
corresponding to Figure 6A was 56%, the degree of deacetylation corresponding
to
Figure 6B was 66%, the degree of deacetylation corresponding to Figure 6C was
70%, the degree of deacetylation corresponding to Figure 6D was 74%, and the
degree of deacetylation corresponding to Figure 6E was 78%. According to the
'I-I-NMR spectrum data of Figure 6A to Figure 6E, the degrees of deacetylation
were determined to be 56% (deacetylation reaction time was 1 h), 66%
(deacetylation reaction time was 3 h), 70% (deacetylation reaction time was 6
h),
74% (deacetylation reaction time was 9 h) and 78% (deacetylation reaction time
was
12 h) respectively. The above chitosan substrates were hydrolyzed by reference
to
the method in Example 1 using a neutral protease, and the obtained products
were
sequentially recorded as DA56, DA66, DA70, DA74 and DA78, and the original
COS-62-NP was also recorded as DA62, for the evaluation of anti-hepatoma
activity.
Example 4 was referred to for the specific method for evaluating the
anti-hepatoma activity of the above chitooligosaccharides of different degrees
of
deacetylation, and the final concentration of chitooligosaccharide used was
100
1.1g/mL. At the same time, 5-fluorouracil (5Fu) of the same concentration was
used
as a positive control, and a blank medium MEM was used as a negative control,
and
the results were shown in Figure 7. It can be seen from the data results of
Figure 7
13
CA 03041417 2019-04-23
that chitooligosaccharides having different degrees of deacetylation all had
certain
anti-hepatoma activity, and the anti-hepatoma activity of chitooligosaccharide
was
best when the degree of deacetylation was 62%.
The above is only some of the preferred embodiments of the present invention,
and the present invention is not limited to the contents of the embodiments.
It will be
apparent to those skilled in the art that various changes and modifications
may be
made within the scope of the inventive concept of the technical solution of
the
present invention, and any changes and modifications made are within the scope
of
the present invention.
14