Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
HEALTH MONITORING SYSTEMS AND METHODS
BACKGROUND
[0001] Advances in software, electronics, sensor technology and materials
science have
revolutionized patient monitoring technologies. In particular, many devices
and systems are
becoming available for a variety of health monitoring applications. However,
improvements may
yet be desired for health monitoring devices and systems that provide one or
more of effective data
collection and/or manipulation for parameter determination.
[0002] Further alternatives for patients and their physicians may then be
developed to
include robust and convenient monitors that in some instances may collect and
transfer short-term or
long-term data and/or monitor events in real-time, and in some cases may
include multi-variable
parameter determination.
SUMMARY
[0003] Described herein are several alternative medical monitoring devices,
systems and/or
methods for parameter determination, in some instances for long-term sensing
and/or recording of
cardiac and/or respiratory and/or temperature data of one or more individuals,
such as a neonate,
infant, mother/parent, athlete, or patient. A number of alternative
implementations and applications
are summarized and/or exemplified herein below and throughout this
specification.
[0004] In one alternative aspect, the developments hereof may include an
implementation
wherein a health device is configured for monitoring one or a plurality of
physiological parameters
of one or more individuals from time-concordant measurements collected by one
or a plurality of
sensors, including one or a variety of one or more of, but not limited to,
electrodes for measuring
ionic potential changes for electrocardiograms (ECGs), and/or one or more
light sources and one or
more photodetectors, in some cases including LED-photodiode pairs, for
optically based oxygen
saturation measurements, and/or one or more temperature sensors, and/or one or
more xyz
accelerometers for movement and exertion measurements, and the like. In some
implementations,
methods and devices of the developments hereof may be used to generate a
respiration waveform.
Other implementations may include a circuit that mimics a driven right-leg
circuit (sometimes
referred to herein as "a proxy driven right-leg circuit") that may permit
reduction in common mode
1
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
noise in a small-footprint device conveniently adhered or having the capacity
to be adhered to an
individual.
[0005] In another alternative aspect hereof, a blood pressure determination
may in some
cases be made from a determination of pulse transit time. The pulse transit
time is the time for the
cardiac pressure wave to travel from the heart to other locations in the body.
Measurements of pulse
transit time may then be used to estimate blood pressure. Heart beat timing
from ECG or otherwise
and photoplethysmogram (aka PPG) signals can be used to generate pulse transit
time. Note, such
signals may be generated from conventional or other to-be-developed processes
and/or devices or
systems; or, such signals may be taken from one or more wearable health
monitoring devices such as
those also described hereinbelow.
[0006] In another alternative aspect, the developments hereof may include
in some instances
one or more methods and/or devices for measuring and/or determining oxygen
saturation parameters
from time concordant pulse oximetry signals and ECG signals. In some
implementations, ECG
signals may be used to define intervals, or "frames" of pulse oximetry data
that are collected and
averaged for determining the constant and main periodic components (e.g., DC
and AC components)
of the pulse oximetry signals from which, in turn, values for oxygen
saturation may be determined.
Patient-wearable devices of such implementations with pulse oximetry and ECG
sensors may be
particularly useful when placed on a patient's chest for such signal
acquisition.
[0007] These as well as other alternative and/or additional aspects are
exemplified in a
number of illustrated alternative and/or additional implementations and
applications, some of which
are shown in the figures and characterized in the claims section that follows.
However, as will be
understood by the ordinarily skilled artisan, the above summary and the
detailed description below
do not describe the entire scope of the inventions hereof and are indeed not
intended to describe each
illustrated embodiment or every possible implementation of the present
inventions nor provide any
limitation on the claims or scope of protection herein set forth below.
BRIEF DESCRIPTION OF THE DRAWINGS
[0008] The drawings include:
[00091 Fig. 1, which includes and is defined by sub-part Figs. 1A-1R,
illustrates several
alternatives of the present developments, including a variety of isometric,
top and bottom plan and
elevational views of devices and alternative conductive adhesive structures.
2
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
100101 Fig. 2, which includes and is defined by sub-part Figs. 2A-2D,
provides circuit diagrams
of alternatives to, in FIGs. 2A-2C, a driven right leg circuit, and in FIG.
2D, pulse oximetry.
[0011] Fig. 3 is a flow chart including alternative methods of use.
[0012] Fig. 4 illustrates an exemplary computer system or computing
resources with which
implementations hereof may be utilized.
[0013] Fig. 5, which includes and is defined by sub-part Figs. 5A-5D,
provides alternative
screenshots of alternative software implementations according hereto.
[0014] Figs. 6A and 6B illustrate features of one embodiment for measuring
oxygen saturation
using pulse oximetry signals and electrocardiogram signals.
[0015] Fig. 6C is a flow chart showing steps of one embodiment for
determining oxygen
saturation values.
[0016] Figs. 6D and 6E illustrate an embodiment for determining depth of
respiration values.
[0017] Figs. 7A, 7B and 7C set forth flow diagrams for alternative
methodologies hereof.
DETAILED DESCRIPTION
[0018] While the inventions hereof are amenable to various modifications
and alternative
forms, specifics hereof have been shown herein by way of non-limitative
examples in the drawings
and the following description. It should be understood, however, that the
intention is not to limit the
inventions to the particular embodiments described. The intention is to cover
all modifications,
equivalents, and alternatives falling within the spirit and scope of the
inventions whether described
here or otherwise being sufficiently appreciable as included herewithin even
if beyond the literal
words or figures hereof.
[0019] In one aspect, a system hereof may include a device for monitoring
physiological
parameters such as one or more or all of electrocardiogram (aka ECG or EKG),
photoplethysmogram (aka PPG), pulse oximetry, temperature and/or patient
acceleration or
movement signals.
[0020] Moreover, systems hereof may be established to measure and/or
process such signals of a
patient using or including any one or more of the following elements: (a) a
circuit, sometimes
flexible as in or on or forming a flexible or flex circuit board, embedded in
or on a flat elastic
substrate or board having a top surface and a bottom surface, the circuit
having one or more of (i) at
least one sensor mounted in or on or adjacent the bottom surface of the flat
elastic substrate, the at
3
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
least one sensor being capable of electrical or optical communication with the
patient. In some
implementations, a circuit may include (ii) at least one signal processing
module for receiving and/or
accepting signals from the at least one sensor in some implementations also
providing for
transforming such signals for storage as patient data; and/or (iii) at least
one memory module for
receiving and/or accepting and storing patient data, and/or (iv) at least one
data communication
module for transferring patient data, stored or otherwise to an external
device, and/or (v) a control
module for controlling the timing and operation of the at least one sensor,
one or more of the at least
one signal processing module, the at least one memory module, the at least one
data communication
module, and/or the control module capable of receiving commands to implement
transfer of patient
data by the at least one data communication module and to erase and/or wipe
patient data from the at
least one memory module. In some implementations, a system hereof may include
(b) a conductive
adhesive removably attached to the bottom surface of the flat elastic
substrate, the conductive
adhesive capable of adhering to skin of the patient or other user and in some
non-limiting examples a
system hereof may be capable of conducting an electrical signal substantially
only in a direction
perpendicular to the bottom surface of the flat elastic substrate, and/or in
some implementations may
include a conductive portion adjacent the sensor or sensors and a non-
conductive portion. In some
implementations, the conductive adhesive is an anisotropically conductive
adhesive in that it
comprises regions of material that conducts current substantially only in a
direction perpendicular to
the skin (i.e. "z-axis" conduction).
[0021] In some implementations, devices hereof will be for comprehensive
long-term cardiac
monitoring, inter alia. Features of such may but not necessarily include any
one or more of a Lead 1
ECG, PPG, pulse oximeter, accelerometer, temperature sensor and/or a button or
other indicator for
manual patient event marking. Such a device may be adapted to store up to, for
example, about two
weeks of continuous data (though more or less will also be feasible in
alternative implementations),
which may in some implementations be downloaded to a clinic or other computer
in a short time
period, as for one example, in only about 90 seconds (though more or less time
will be viable in
alternative implementations) via computer connection, whether wireless or
wired as in one example
by USB or other acceptable data connection. A companion software data analysis
package may be
adapted to provide automated event capture and/or allow immediate or delayed,
local data
interpretation.
4
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
[0022] Intermittent cardiac anomalies are often difficult for physicians to
detect and/or diagnose,
as they would typically have to occur during a physical examination of the
patient. A device hereof
may address this problem with what in some implementations may be a continuous
or substantially
continuous monitoring of one or a number of vital signs.
[0023] Some alternative features may include but not be limited to one or
more of (i) a driven
"Right Leg" circuit with electrodes located only on the chest, and/or (ii) a
"z-Axis" or anisotropic
conductive adhesive electrode interface that may permit electrical
communication only between an
electrode and a patient's skin immediately beneath the electrode, and/or (iii)
data transmission to and
interpretation by a local computer accessible to CCU/ICU personnel, and/or
(iv) a unique
combination of hardware that may allow correlation of multiple data sources in
time concordance to
aid in diagnosis.
[0024] In some alternative non-limiting implementations, devices and
systems hereof may
provide 1) reusability (in some cases near or greater than about 1000
patients) that may allow
recouping cost of the device in just about 10-15 patient tests; and/or 2) one
or more of ECG
waveform data, inertial exertion sensing, manual event marking, temperature
sensing and/or pulse
oximetry, any one or all of which in time concordance to better detect and
analyze arrhythmic
events; and/or 3) efficient watertightness or waterproofing (for the
patient/wearer to be able to swim
while wearing the device); and/or 4) a comprehensive analysis package for
typically immediate,
local data interpretation. An alternative device may be adapted to take
advantage of flex-circuit
technology, to provide a device that is light-weight, thin, durable, and
flexible to conform to and
move with the patient's skin during patient/wearer movement.
f00251 Figs. 1 and 2 illustrate examples of alternative implementations of
devices that may be so
adapted.
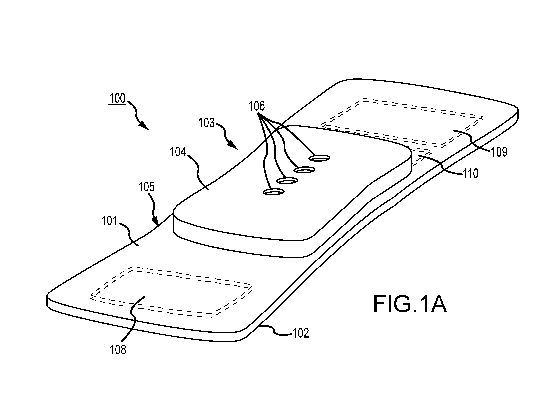
[0026] Fig. 1, which is defined by and includes all of sub-part Figs. 1A-
1P, shows a device 100
that has a component side or top side 101, patient side or circuit side 102,
and one or more inner
electrical layer(s), generally identified by the reference 103 and an
elongated strip layer 105. The
strip layer 105 may have electronics thereon and/or therewithin. FIG. 1 A
shows isometrically these
in what may in some non-limitative implementations be considered a
substantially transparent device
together with some other elements that may he used herewith. FIG. I B is more
specifically directed
to a top side 101 plan view and FIG. 1C to an underside, patient side 102 plan
view and FIG. ID a
first elevational, side view.
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
[00271 Many of the optional electronics hereof may be disposed in the
electronics layer or layers
103, and as generally indicated here, the electronics may be encapsulated in a
material 104 (see
FIGs. 1A, 1B, 1D and 1K for some examples, and see Figs. 1N, 10, 1P and 1Q
described further
below, e.g.), medical grade silicone, plastic or the like, or potting
material, to fix them in operative
position on or in or otherwise functionally disposed relative to the elongated
strip layer 105. The
potting or other material may in many implementations also or alternatively
provide a waterproof or
watertight or water resistant coverage of the electronics to keep them
operative even in water or
sweat usage environments. One or more access points, junctions or other
functional units 106 may
be provided on and/or through any side of the encapsulation material 104 for
exterior access and/or
communication with the electronics disposed therewithin, or thereunder. FIGs.
1A, 1B and 1D show
four such accesses 106 on the top side. These may include high Z data
communication ports and/or
charging contacts, inter alia. This upper or component side 101 of device 100
may be coated in a
silicone compound for protection and/or waterproofing, with only, in some
examples, a HS USB
connector exposed via, e.g., one or more ports 106, for data communication or
transfer and/or for
charging.
[0028] The elongated strip layer 105 may be or may include a circuit or
circuit portions such as
electrical leads or other inner layer conductors, e.g., leads 107 shown in
FIG. 1D, for communication
between the electronics 103 and the electrically conductive pads or contacts
108, 109 and 110
described further below (108 and 109 being in some examples, high
impedance/high Z silver or
copper/silver electrodes for electrocardiograph, ECG, and 110 at times being a
reference electrode).
In many implementations, the strip layer 105 may be or may include flex
circuitry understood to
provide acceptable deformation, twisting, bending and the like, and yet retain
robust electrical
circuitry connections therewithin. Note, though the electronics 103 and
electrodes 108, 109, 110 are
shown attached to layer 105; on top for electronics 103, and to the bottom or
patient side for
electrodes 108, 109, 110; it may be that such elements may be formed in or
otherwise disposed
within the layer 105, or at least be relatively indistinguishably disposed in
relative operational
positions in one or more layers with or on or adjacent layer 105 in practice.
Similarly, the leads or
traces 107 are shown embedded (by dashed line representation in FIG. 1D);
however, these may be
on the top or bottom side, though more likely top side to insulate from other
skin side electrical
communications. If initially top side (or bottom), the traces may be
subsequently covered with an
insulative encapsulant or like protective cover (not separately shown), and/or
in many
6
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
implementations, a flexible material to maintain a flexible alternative for
the entire, or majority of
layer 105.
[0029] On the patient side 102, the ECG electrodes 108, 109 and 110 may be
left exposed for
substantially direct patient skin contact (though likely with at least a
conductive gel applied
therebetween); and/or, in many implementations, the patient side electrodes
108, 109 and/or 110
may be covered by a conductive adhesive material as will be described below.
The electrodes may
be plated with or may be a robust high conductive material, as for example,
silver/silver chloride for
biocompatibility and high signal quality, and in some implementations may be
highly robust and, for
one non-limiting example, be adapted to withstand over about one thousand
(1000) alcohol cleaning
cycles between patients. Windows or other communication channels or openings
111, 112 (Fig. 1C)
may be provided for a pulse oximeter, for example, for LEDs and a sensor. Such
openings 111, 112
(e.g., Fig. 1C) would typically be disposed for optimum light communication to
and from the patient
skin. An alternative disposition of one or more light conduits 111a/112a (and
111b/112b) is shown
in a non-limiting example in FIG. 1D more nearly disposed and/or connected to
the electronics 103.
A variety of alternative placements may be usable herein/herewith, some of
which further described
below.
[0030] In some implementations, sampling of the ambient light (with the
LEDs off) may be
provided, and then subtracting this from each of the pulse-ox signals in order
to cancel out the noise
caused by sunlight or other ambient light sources.
[0031] The LEDs and one or more photodiode sensors may also and/or
alternatively be covered
with a layer of silicone to remove any air gap between the sensor/LEDs and the
patient skin. Some
examples of such are set forth in respective FIGs. 1H and/or 1K and/or 1L
and/or 1M and/or 1N, 10,
1P and 1Q; where a silicone layer or covering 121 and/or 121a and/or 121b
and/or 121c and/or 121d
is shown covering/surrounding the light conduits and/or sensors/LEDs
111c/111d/112c. LED 111c
(FIGs. 1H and/or 1K and/or one or more of IL, 1M, IN, 10, IP and/or 1Q) might
be a Red LED,
LED 111d (FIGs. 1H and/or 1K and/or one or more of 1L--1Q) might be an IR
(infrared) LED and
the device 112c (FIGs. 1H and 1K and/or one or more of 1L--1Q)) might be a
sensor. Alternative
and/or additional LEDs might be provided; for a first example, one or more
additional or alternative
colors of LEDs (not shown) might be provided not unlike those shown in FIGs.
1H and/or 1K and/or
one or more of 1L--1Q, as for example a Green LED (not shown) for additional
and/or alternative
functionality as described further below.
7
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
[0032] Other alternative LED and sensor arrays or arrangements are shown in
FIGs. 1L and 1M
wherein one or more LEDs are more centrally disposed within epoxy/light-pipe
121c on a substrate
105a and one or more sensors or photodiodes are more peripherally disposed. In
FIG. 1L two LEDs
111c and 1 1 Id (not unlike LEDs 111c and 1 1 Id of Figs. 1H and/or 1K, but
for
positioning/geometry) are shown relatively centrally disposed relative to one
or more sensors, here,
two sensors or photodiodes 112c and 112d. As above-described for Figs. 1H
and/or 1K, LED 111c
might be a Red LED, and LED 111d might be an IR (infrared) LED and the devices
112c and/or
112d might be one or more sensors, here two sensors or photodiodes 112c and
112d. In FIG. 1M,
four LEDs 111c, 111d, 111e and 111f (not unlike LEDs 111c and 111d of Figs. 1H
and/or 1K and/or
1L, but for number, positioning and/or geometry) are shown relatively
centrally disposed relative to
one or more sensors, here, four sensors or photodiodes 112c, 112d, 112e and
112f. As above-
described for Figs. 1H and/or 1K and/or IL, LED 111c might be a Red LED, and
LED 111d might
be an IR (infrared) LED, and/or 111e might also be a Red LED, and LED 11 if
might be an IR
(infrared) LED and the devices 112c, 112d, 112e and/or 112f might be one or
more sensors, here
four sensors or photodiodes 112c, 112d, 112e and 112f.
[0033] Placing the LEDs in the more centrally disposed positioning of Figs.
IL and 1M and
surrounding those more centrally disposed LEDs with sensors or photodiodes, as
opposed to the
more or relatively conventional method of a central sensor or photodiode
surrounded by LEDs,
provides a geometry that may be disposed to capture a much greater percentage
of the emitted light,
emitted from the LEDs. In the more or relatively conventional geometry,
substantially all of the
emitted light facing away from the photodiode is wasted. In the geometries of
and/or described for
Figs. 1L and 1M, much more light, perhaps as much as virtually all of the
emitted light may be
captured by the sensors or photodiodes, or in some cases, a significantly
higher efficiency capture
than conventional. Due to the higher efficiency light capture, fewer LEDs
might thus be required
than conventional Multi-LED integrated sensors. This may contribute to
significantly reducing
power consumption and yet achieve similar or better measurement results. In
sum, a geometry of
LEDs, such as the red and IR combinations described above, combined with an
array of photodiodes
(or sensors) is shown and described that may enable a higher concentration of
light into the
subcutaneous region of the subject (patient/infant/neonate/mother/athlete,
e.g.). The combination of
LEDs and photodiodes/sensors might also be referred to in some implementations
as a High-
Efficiency Integrated Sensor. This arrangement may be implemented in
determination of Sp02
(peripheral capillary oxygen saturation). Note, in some practical
implementations, the sensors
8
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
shown in Figs. 1L and 1M, e.g., may be about 5mm2 and the diameter of the
exterior circle
encompassing the sensors and LEDs might be a corresponding about 8 mm. In some
implementations, it may be that about 3.2 mm red may be set for a preferred
distance from the center
of the red LED light source to the center of the corresponding sensor or
sensors, and may be a
preferred distance of about 3.7 mm set from the center of the IR LED light
source to the
corresponding sensor or sensors therefor.
[0034] This silicone layer or covering 121/121a/121b/121c/121d/121e may
reduce the light lost
to reflection off the skin, and thereby greatly increase the signal and reduce
the noise caused by
motion of the skin relative to the sensor. In some implementations this
silicone might be referred to
as a light pipe and in some situations may be clear, colorless, and/or medical
grade silicone. As
described further below, the silicone layer or covering 121 and/or 121a and/or
121b and/or 121c
and/or 121d and/or lens surface 121e (sometimes referred to herein in short by
121/121a/121b/121c/121d/121e but having the same meaning hereof) may
also/alternatively be
referred to as a light pipe or lens 121/121a/121b/121c/121d/121e herein
inasmuch as how it may be
involved in light transmitting or to be transmitted therethreough, whether
upon emission or received
upon reflection or both.
[0035] In one or more implementations, an encapsulant and/or lens
121/121a/121b/121c/121d/121e hereof may be made from a medical grade silicone
that is one or
more of clear, colorless, soft, low durometer. Exemplars of such specialized
silicones that may be
used herewith are known as "tacky gels" (several suppliers), and typically
have very high-tack
adhesives, preferably embedded on both sides. A low durometer silicone
combined with double-
sided adhesive on the tacky gel allows the construction of a lens
121/121a/121b/121c/121d/121e that
may be both conforming to the electronic sensors and skin, as well as, in some
implementations,
exhibiting properties of motion artifact reduction by limiting movement
between the skin-lens-
sensor interface. A lens according hereto may also/alternatively be specially
shaped such that it can
be trapped between layers of the composite adhesive strip (see e.g.,
alternatives of FIGs. 1D, 1G and
II and 1J), and in some implementations, with a raised portion the size of the
opening, often a
rectangular opening, in the adhesive strip that allows the lens to protrude
slightly on the patient side
of the adhesive strip (see further detail relative to FIG. 1K, described
below).
[0036] In FIG. 1K an implementation of a further alternative silicone
covering or encapsulant
121a for the LEDs and sensor 111c/111d/112c, may include a convex lens at or
adjacent the
9
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
covering external surface 121b. In many implementations, the external surface
and lens are one and
the same and/or the lens may be defined by the surface 121b of the encapsulant
material 121a. What
this provides is a structure and method for interfacing pulse oximetry LED
emitters 111c/111d and
one or more photodiode sensors 112c with the skin surface, whether chest or
forehead (e.g., infant or
neonate) or otherwise mounted on the patient or user body.
[0037] More particularly, as otherwise described herein, a system and/or
device 100 hereof may
utilize one or multiple LED emitters 111c/Illd (and/or 111e and/or 1110 of
selected wavelengths
and one or multiple photodiodc sensors. However, In order to maximize coupling
of the LED/sensor
combination to the skin 1001 of a wearer 1000, an encapsulant and/or lens
121/121a/121b/121c/121d/121e comprised of optically clear, medical grade
silicone may be molded
onto or molded such that it may be later attached in covering relationship on
the LED/sensor
combination111c/111d/112c. In many implementations, as for example in Fig. 1K,
the lens 121b
may be partially spherical or perhaps hemispherical in nature, though it need
not be; see e.g., Figs
1N- 1Q, described below. Curvature of other shapes may be useful as well.
Curvature may reduce
loss of skin contact when the device 100 may be moved, whether by wearer
motion or otherwise.
I.e., motion of the wearer 1000 or the device 100 relative to the wearer 1000
in Fig. 1K can result in
a quasi-rolling contact of the lens on and in relation to the skin 1001.
Better maintained skin contact
means better data acquisition without interruption and/or with reduced noise.
In some
implementations, including those above and below (though not directly shown
therein, i.e.,
alternatively included or not included therewith), or as described with
specific reference to Fig. 1Q
below, a thin silicone adhesive 113e may be used on and between the silicone
layer
121/121a/121b/121c/121d/121e to assist with maintenance of the skin contact
relative to the silicone
encapsulant 121/121a/121b/121c/121d/121e. See description of Fig. IQ below,
e.g.
[0038] Moreover, related to the function of maintaining contact is the
light piping effect that
may be achieved when LEDs and sensors, even of different heights are
communicating substantially
with little or substantially without air gap interruption through the light
pipe of the encapsulant
material 121a/121c/121d/121e from the emission to the skin and back from the
skin to the sensors.
With no air gap from emitter to and through the light pipe 121a/121c/121d/121e
and/or sometimes
including a curved surface 121b substantially constant contact with the skin,
there is thus no air gap
or with little or substantially no air gap interruption in transmission into
and through and reflected
back on return from within the skin and back to the sensor via the same light
pipe material
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
121a/121c/121d/121e (transmission and reflection both referring to light
travel). This reduces
inefficiencies caused by light wave scattering at air gap interfaces (air gaps
allow for light to bounce
off the skin or other surface). I.e., encapsulation of the LEDs and the
sensor; provides no air- gap
and a light pipe effect to and the curved surface provides high quality low
scattering transmission
into the skin and reception of reflection from the skin and bone. The light
pipe and curved lens
surface maintain uninterrupted contact skin and lens reduces lost signals due
skin reflection. The
signal to noise ratio goes down and data acquisition goes up in quality.
[0039] Such an encapsulant 121/121a/121c/121d and/or a lens 121b/121e may
thus serve one or
multiple purposes, including in some instances, inter alia: 1) providing a
"light-pipe" effect to assure
equal or otherwise high quality coupling of the different height LEDs and
sensors, as well as
substantially constant coupling to the skin to reduce motion artifact; 2)
focusing of emitted light
through the skin to the bone; and, 3) focusing of reflected light through the
skin to the photodiode
sensors.
[0040] As a further note, for a curved lens 121b option as from Fig. 1K,
the radius of the lens
may be designed to maximize 1) through 3). The height of the lens may be
designed to allow it to
protrude above composite adhesive 113 of the device 100 and into the skin, but
not deep enough to
disturb the capillary bed which would also result in bad data. Moreover, the
radius of curvature and
the angles of LED lightwave emission are not necessarily highly controlled and
need not be because
the LEDs used to penetrate the skin, e.g., the red and infra-red and/or green
LEDs; provide a very
wide array of angles of emission, and thus a large number of reflected array
of lightwaves will be
focused back to the sensor by a large variety of curved surfaces. I.e., the
curved surface is helpful
for maintaining contact through movement (accidental or on purpose), and is
less important to the
angles of transmission through the skin and reflection back to the sensor. In
other words, many
different radii of curvature will be effective with very little difference in
data/wave transmission and
reflection; the wide angle emission of LED takes care of what might be a
variety of radii. Rather,
the curvature may have more limitation in the maintenance of contact due to
movement of the device
100¨ e.g., flatter curvatures won't roll readily, and very small radii of
curvature will not transmit or
receive as much data.
[0041] In some implementations, a radii of curvature found useful have been
between about 20
and 40 (both 20.34 mm and 39.94 mm radii of curvature have been found useful)
for a device having
LEDs and sensors in a compartment of about 12.6 mm by 6.6 mm. It may be noted
further that
11
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
LEDs may be on one side or another or on two opposing sides or perhaps at four
or more
substantially equi-distant points around a sensor and may provide desirable
results.
[0042] Note further, pulse oximetry hereof may be with multiple light
sources and/or sensors as
may be one interpretation of the dispositions of FIGs. 1H and 1K, and/or any
one or more of 1L-1Q,
e.g. Typical pulse oximetry circuitry uses one light source (LED) per
wavelength (typically red,
infrared, and sometimes others including green or long time averages of red/TR
for further examples
as described below). However, devices and/or methods hereof may make use of
multiple light
sources for each wavelength. This may allow for interrogation of a wider area
of capillary bed in/on
the patient/wearer in order to reduce the effects of a local motion artifact.
Similarly, multiple
sensors may be used for the same or similar purpose or advantage.
[0043] Furthermore, a combination of driven right leg and/or proxy driven
right leg together with
pulse oximetry can provide additional benefits. The right leg circuit, proxy
right leg and/or driven
right leg, whether for chest or forehead or other electrode placement, can
remove common mode and
power line noise that would/might otherwise be capacitively-coupled into the
pulse oximetry sensor
and reduce effectiveness thereof. A combination of driven right leg and/or
proxy driven right leg
and improved pulse oximetry with a lens as described in and for FIG. 1K and/or
the light pipes of
Figs. 1 H and/or any one or more of Figs. 1L ¨ 1Q may significantly reduce
such noise, and thereby
enhance data acquisition. For driven electrodes see further detail below.
[0044] Thus, measurement of arterial blood oxygen content can be made using
optical signals
(sometimes also referred to as heart beat optical signals), typically from Red
and Infra-Red pulsed
sources, which exhibit different optical absorptions dependent on oxy-
haemoglobin presence or
absence. In sum, a transmissive system is used with light sources and optical
detectors. In many
implementations as described following, a light pipe that encapsulates either
or both the light source
or sources and the one or more sensors may be employed, particularly a light
pipe encapsulating,
meaning having substantially no air gaps, may be used for providing either or
both increased
efficiency in light emission to the skin and/or capturing otherwise lost
photons upon collection.
[0045] Herein, reflective systems are typical, and these often have some
advantages being less
intrusive, and perhaps being more portable. As described herein, such
reflective systems typically
employ a red and an infra-red source and a photo-diode sensor or detector, or
multiple arrangements
of these components. Also as described, one implementation/method employs one
or more central
large area photo-diodes/sensors/detectors, with one or more LED sources, often
one or more of each
12
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
of a red, and an infra-red LED sources adjacent to the photo-diode or in an
array around it. Also as
described, an alternative arrangement uses a central LED set of one or more
light sources, with one
or more of each wavelength type (Red, InfraRed, Green, etc.), and multiple
large area photo-diodes
or light sensors surrounding the central LEDs. Such an arrangement might use
two or three or four
such detectors around the LEDs to collect more light scattering from the LEDs
through the skin and
other tissues; see e.g., FIGs. 1L ancUor 1M.
[0046] A further alternative implementation may employ structural
enhancements to and/or
around the light sources and/or the one or multiple photo-diodes. Described
first are one or more
such enhancements disposed in relation to the central LED arrangement
described above, though the
following could be used with or relative to the prior described central sensor
arrangement as well.
The optical enhancing structures may provide minimal intrusion in the
collection area and may
reduce photo-diode areas or reduce numbers of photodiodes. Cost benefits
and/or increased
efficiency may thus result.
[0047] In Figs. 1N, 10, 1P and 1Q, in the light pipe 121d, the central LED
sources 111c and 111d
arc isolated from the peripheral photo-detectors 112c, 112d, 112e and 112f on
the substrate 105a by
asurrounding barrier wall 122 (here also identified by the alternative
reference B1). A further
optional external barrier 123 surrounding the sensor area is also shown. The
barrier wall 122 (or B1)
and/or wall 123 (or B2) is/or preferably opaque and/or reflective to both red
and IR (or to whatever
other color or wavelength of light is being used, e.g., green etc.) in order
to prevent crosstalk
between the LEDs and the sensors. i.e., preferably want all of the light
leaving the LED area to go
into the skin rather than some of the light rays finding a path to enter the
sensors directly. A
preferable surface for the barrier would be diffuse-reflective (as opposed
generally to relative
absorptive and/or mirror-shiny). An example may be clear anodized aluminum.
Another would be
textured white paint. Operation is shown and described relative to Figs. 1P
and/or 1Q; see below.
[0048] The shape and size of the wall 122 can be chosen appropriate to the
shape and size of the
LED sources, here sources 111c and 111d. For example, the wall 122 could be,
as shown, a circle,
or could be a square or rectangular shape or otherwise (not shown) around the
LEDs 111c, 111d
(noting here also that more or fewer light sources might be included within or
enclosed by the wall
122). The width or thickness of and the material used for the barrier wall 122
can be variable or
varied as needed or desired as well; indeed, the width may depend upon the
material and/or vice
versa in that the relative opacity of any particular material may mean less or
more width necessary to
13
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
provide a particular level of opacity or relative diffuse reflectivity. The
particular wavelengths of
light, i.e., type of light used, and/or the type or types of sensors and/or
the relative and/or overall
geometrical relationships (sensors to light sources, sensors to sensors,
and/or light sources to light
sources) may also figure into the relative dimensions and/or material used for
and/or due to the
relative opacity in relation to the particular wavelengths. There may be
situations where the relative
thicknesses may have more to do with the type of material of the wall, or the
opacity or relative
diffuse reflectivity thereof. In some implementations, the barrier/wall may be
machined, anodized
aluminum, or other similar material, but other implementations are of plastic,
e.g., a molded plastic.
For Red and Infra-Red usage, a driving consideration can be that the material
would perhaps
preferably be opaque or reflective or diffuse reflective to both 660 and 940
nanometers. Thus, in
many situations, very thin aluminum meets this criteria, and thicker plastic
does as well.
[0049] The options for wall 122 will primarily be for the provision of an
optical barrier to
sideways propagation of either the radiation from the LEDs (e.g., as here thus
far, the Red or the
Infra-Red light from, e.g.. LEDs 111c/111d), and the wall 122 is preferably
level or slightly higher
than the optical exit window of the LEDs. It is preferred that the barrier
wall 122 also has a width
sufficient to prevent optical crosstalk of light rays that never enter the
skin or scattering material, but
not so wide that light from the LEDs that is scattered from the skin or other
flesh material, is
prevented from reaching the photo-diode detectors outside the barrier wall.
[0050] An optional external barrier wall 123 might also be employed. This
one assisting with
collection of light reflected from the patient or user. Similar considerations
for size and thickness
and material may be employed with wall 123; the difference being primarily in
collection as opposed
to light generation.
[0051] Where these developments may provide improvement with external
detectors as described
here, Figs. 1N/10, and/or 1P/1Q, is that an optical collecting structure is
added that can collect light
from other regions where detector diodes are not present, and conduct or
reflect some of that
radiation in such a way as to reach one or more of the detectors. Note,
central detectors with
otherwise separately isolated light sources (not shown) may also have similar
improvements.
[0052] Preferred structures of this type may include a transparent optical
medium, here the light
pipe material 121d. This light pipe material may be molded into a shape within
and/or surrounding
the relatively opaque barrier wall 122 (see e.g., Fig. 1Q, described further
below), and contain
sources 111c and 111d (and/or others if/when present) within the wall 122
and/or contain outside the
14
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
wall 122 (between wall 122 and wall 123) the diode detectors 112c, 112d. 112e
and/or 112f (and/or
others if/when present) embedded in that structure 121d with little or
substantially no air gaps
between the detectors and the light pipe material. The detector devices 112c,
112d, 112e and/or
112f may be molded into the optical medium, i.e., light pipe material, itself,
or could be inside
premolded cavities in that optical medium. Optical structures of this type,
could be generally
referred to as "light pipes".
[0053] The shape of the light pipe structure 121d and/or surface 121e can
be chosen in a variety
of ways, depending on the number and size or shape of the detector diodes, and
may be designed in
such a way as to capture scattered light received from the skin or flesh
material not directly in
contact or above any detector diode, and contain it by means of total internal
reflection, and using
scattering reflective surfaces, to redirect rays in a direction towards one or
more of the photo diodes.
In this way, light that would be lost in previous designs, is captured by
devices of these present
implementations. In Figs 1N and 10, the epoxy (light pipe) 121d is relatively
flat, i.e., presenting a
relatively flat surface 121e, not concave or convex, though it may be that
curvature will work with
the barrier wall or walls hereof. In Fig. 1Q, also a relatively or
substantially flat surface 121e is
shown.
[0054] Shown also in Fig. 1Q is an optional thin silicone adhesive 113e on
surface 121e which
may be used to relatively adhere the device to the skin (not shown here) to
reduce movement of the
device relative to the skin and enhance light transmission and reception. If
used, such an adhesive
may preferably be as thin as operably possible so as not to interfere with or
provide refraction of
light waves passing therethrough. A 0.2mm thickness may be so operable. Also,
it may be that a
similar refractive index of the adhesive to the expoxy/encapsulant/light pipe
121/121a/121b/121c/121d/121e might be preferred. This choosing of a similar
refractive index may
be of assistance or may be related to thickness as well as material of
adhesive to be used. E.g., an
appropriate refractive index similarity may result from or lead to an operable
0.2mm thickness.
[00551 Figs. 1P and 1Q show some operative examples and/or alternatives. In
Fig. 1P, where no
light pipe is shown for simplicity, though could be an operable alternative
example, light wave
emissions A, B and C are shown emanating from the exemplar LED 111c. Wave A is
a relative
direct emission meeting no obstacle on its way from the device to the skin
(not shown), whereas
wave B is shown as reflected off the wall 122 (note though waves are sometimes
described, it is
understood that light energy in whatever form is intended herewithin, whether
for example it is or
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
may better be understood as photons which are more particularly as understood
as emitted and/or
collected). Less preferred is a wave C shown not reflected off wall 122; here
shown merely for
highlighting the preference toward most if not all waves leaving the LED
finding a way to be
reflected to exit the LED area and enter the skin of the user (not shown
here). Light collection is
shown relative to the exemplar sensors 112c and 112d, where in Fig. 2P,
relatively direct waves D
are shown as they might enter the sensor area and be captured by the sensors
112c and/or 112d.
Reflected waves E are also shown as they might be reflected off the walls 122
and/or 123. Note, the
floor or top surface of the substrate 105a might also be diffuse reflective to
the waves and assist in
reflecting these ultimately for sensor collection.
[0056] In Fig. 1Q, the light pipe/s 121d are shown as is an optional thin
adhesive 113e. The
relative refractive indices of these materials may or may not affect, or
largely affect the light passing
therethrough. Preference is for similarity of refractive indices to minimize
refraction. Even so,
some refraction may occur as shown for example by emitting light wave B in
Fig. 1Q and in
collected waves E and F, F differing from E by not also being reflected of the
walls 122 and/or 123
as is light wave E. Light wave B is shown both reflected and refracted. Choice
of materials and
sizes and shapes of relative structures can assist in management of relative
reflection and/or
refraction toward increasing efficiency in light emission and/or capture.
[0057] Returning to the adhesive alternatives, FIG. 1D provides a first
example of an adhesive
113 that may be used herewith. The adhesive layer 113 is here a double-sided
adhesive for
application to the bottom side 102 of the device 100, and a second side,
perhaps with a different type
of adhesive for adhering to the skin of the human patient (not shown).
Different types of materials
for adhesion might be used in that the material of choice to which the
adhesive layer is to be attached
are different; typically, circuit or circuit board material for connection to
the device 100, and patient
skin (not separately shown) on the patient side.. A protective backing 114 may
be employed on the
patient side until application to the patient is desired. Note, in many
applications, the adhesive 113
is anisotropic in that it may preferably be only conductive in a single or
substantially a single
direction, e.g., the axis perpendicular to the surface of adhesive contact.
Thus, good electrically
conductive contact for signal communication can be had through such adhesive
to/through the
adhesive to the electrical contacts or electrodes, 108, 109 and 110. Note, a
corresponding one or
more light apertures 111b/112b are shown in the adhesive of 113 of the example
of FIG. 1D to
16
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
communicate light therethrough in cooperation with the light conduit(s)
111a/112a in/through layer
105 for communication of light data typically involved in pulse oximetry.
[0058] The adhesive may thus be placed or disposed on the device 100, in
some implementations
substantially permanently, or with some replaceability. In some
implementations, the device as
shown in FIGs. 1A-1D and/or 1G without (or with in some implementations) the
adhesive may be
reusable. In many such cases, the adhesive layer 113 may be removed and
replaced before each
subsequent use, though subsequent re-use of and with a layer 113 is not
foreclosed. In a first or
subsequent use with a replaceable adhesive layer 113, it may be that the user
applying the device to
the patient, e.g., the physician or technician or even the patient,
him/herself, applies the conductive
transfer adhesive 113 to the patient side 102 of the device 100. The
protective backing 114 may then
be removed, and the device adhered to the patient and activated.
[0059] Activation of the device after application to a patient/wearer may
occur in a number of
ways; in some, it may be pre-set that an affirmative activation interaction
may not be necessary from
the doctor or patient or like due to either an inertial and/or a pulse
oximeter activation which may be
substantially automatically activating, e.g., upon receiving sufficient
minimum input (movement in
case of inertial system or light reflection of blood flow for pulse oximetry);
however, a button may
be provided at an access 106 or in some other location adjacent the
electronics to allow the patient to
start or stop the device or otherwise mark an event if desired. In one
exemplar implementation the
device may be worn for a period such as two weeks for collection of data
substantially continuously,
or at intervals as may be preferred and established in or by the systems
hereof.
[0060] After a monitoring period is over, a physician, technician, patient
or other person may
then remove the device from the patient body, in some instances remove the
adhesive, in some
instances with alcohol, and may establish a data communication connection for
data transfer, e.g., by
wireless communication or by insertion/connection of a USB or like data
connector to download the
data. The data may then be processed and/or interpreted and in many instances,
interpreted
immediately if desired. A power source on board may include a battery and this
can then also be re-
charged between uses, in some implementations, fully recharged quickly as
within about 24 hours,
after which the device could then be considered ready for the next patient or
next use.
[0061] Some alternative conductive adhesives may be used herewith. FIGs.
1E, 1F and 1G show
one such alternative conductive adhesive 113a; a bottom plan view in FIG lE
and elevational side
views thereof in FIGs. 1F and 1G (as being connected to a device 100 in FIG.
1G). In some
17
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
implementations, the conductivity may be anisotropic as introduced above; in
some conductive
primarily if not entirely in the direction of the Z-Axis; perpendicular to the
page (into and/or out of
the page) in FIG. 1E, and/or vertically or transversally relative to the long
horizontal shown axis of
device 100 in the implementation view of FIG. 1F.
[0062] The implementation of this particular example includes a composite
adhesive 113a which
itself may include some non-conductive portion(s) 113b and some one or more
conductive portions
113c. The adhesive composite 113a may, as described for adhesive 113 above be
double sided such
that one side adheres to the patient while the other side would adhere to the
underside 102 of the
device 100 (see FIG. 1G) so that one or more conductive portions 113c may be
disposed or placed in
electrically communicative and/or conductive contact with the integrated
electrodes on the electronic
monitoring device 100. Since the electrodes would operate better where they
may be electrically
isolated or insulated from each other, yet each making electrical contact or
communication with the
patient's skin, the adhesive may further be more specifically disposed in some
implementations as
follows.
[0063] As shown in FIGs. lE and 1F, three isolated conductive portions 113c
may be disposed
separated from each other by a body portion 113b which may be non-conductive.
These could then
correspond to the electrodes 108, 109, 110 from the above-described examples,
and as more
particularly shown schematically in FIG. 1G (note the scale is exaggerated for
the adhesive 113a and
thus, exact matching to the electrodes of device 100 is not necessarily
shown). In some examples,
the electrode areas 113c may be a conductive hydrogel that may or may not be
adhesive, and in
some examples, may be made of a conductive an adhesive conductive material
such as 3M
Corporation 9880 Hydrogel adhesive (3M Company, St. Paul, Minnesota). These
areas 113c may
then be isolated from each other by a non-conductive material 113b such as 3M
Corporation 9836
tape or 3M double-sided Transfer Adhesive 9917 (3M. St. Paul, MN) or
equivalent. The additional
layer 113d, if used, might be a 3M 9917 adhesive together with the 113b of a
9836 material. These
constructs may provide the effect of creating a low electrical impedance path
in the Z-axis direction
(perpendicular to page for FIG. 1E and vertically/transversally for FIGs. 1F
and 1G) for the
electrode areas 113c, and high electrical impedance path between the
electrodes in the X/Y
directions. (See Figs. 1E, IF and 1G; coplanar with the page in FIG. lE and
horizontal and
perpendicular to the page in FIGs. IF and 1G). Thus, a composite adhesive
strip can ensure not only
device adhering to the patient, but also that the electrodes whether two or as
shown three electrodes
are conductively connected by conductive portions of the adhesive strip, where
the combination of
18
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
conductive and non-conductive portions can then reduce signal noise and/or
enhance noise free
characteristics. Electrodes that move relative to skin can introduce noise;
that is, electrodes
electrically communicative/connected to the skin via a gel may move relative
to the skin and thus
introduce noise. However, with one or more conductive adhesive portions in a
composite adhesive
connected to respective electrodes and then substantially securely connected
to the skin will keep the
respective electrodes substantially fixed relative to the skin and thereby
reduce or even eliminate
electrode movement relative to the skin. Removal of such movement would then
remove noise
which would thereby provide a clean signal that can allow for monitoring
cardiac P waves which
enhances the possibility to detect arrhythmias that couldn't otherwise be
detected. Further
description is set forth below.
[0064] In some implementations, a further optional connective and/or
insulative structure 113d
may be implemented as shown in FIGs. 1F and/or 1G, to provide further
structural and insulative
separation between electrodes with connected to a device 100 on the underside
102 thereof (see FIG.
1G). Though shown separate in FIGs. 1F and 1G, it may be contiguous with the
insulative adhesive
113b of these views.
[0065] Further alternatives related to the adhesive may be used. In some
implementations, a
composite adhesive strip may be used having properties to reduce one or more
motion artifacts.
Typical ECG attachment systems use a conductive gel located over the
electrode. Here, however, a
hydrogel adhesive may be used which is embedded in a continuous sheet of
laminated adhesives that
cover the selected regions or the entire footprint of the device. The fact
that the hydrogel itself has
strong adhesive properties coupled with the complete coverage of the device
with adhesives may
assure a strong bond between the device and the patient's skin. Contributing
to motion artifact
reduction may be an alternative vertical placement of the device on the
sternum which results in
reduced motion artifacts for one or more of ECG signals, photoplethysmography
waveforms, and
oxygen saturation signals.
[0066] In some implementations, composite adhesive improvements may include
water-proof
encapsulation of the hydrogel adhesive to prevent ohmic impedance reduction
resulting in reduction
of signal amplitude. This may also help prevent hydrocolloid adhesive
degradation. In particular, as
shown the non-limitative alternative exemplar in FIGs. 11 and 1J; several
layers may be used.
Herein, Layer 1 may be a hydrocolloid that is an adhesive designed for long
term skin contact by
absorbing sweat and cells. Layer 2 may then also be a layer designed for long-
term skin contact,
however, this layer 2 isolates Layer 3 from contacting the skin. The smaller
dimensions of Layer 2
19
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
create a gap between Layers 1 and 3. When Layer 1 and 3 bond together, it
forms a water-tight seal
around Layer 2. This layer, Layer 2, also isolates the Hydrocolloid from the
Hydrogel Adhesive,
protecting the adhesive properties of the Hydrocolloid. Layers 3 and 5 would
then generally be
waterproof layers that are electrically isolating, double-sided adhesives.
These two layers
encapsulate the hydrogel adhesive, preventing a "short circuit" described
relative to layer 4 below.
Layer 4 is the hydrogel adhesive that is the conductive element hereof. The
three islands of hydrogel
adhesive of Layer 4 must be kept electrically isolated from each other.
However as the hydrocolloid
in layer 1 absorbs sweat, it too becomes conductive and creates a potential
"short circuit" between
the three islands of hydrogel adhesive in Layer 4, reducing signal amplitude.
Nevertheless, this
"short circuit" may be prevented by layers 3 and 5, described above.
[0067] In some one or more additional alternative implementations,
temperature may be a
parameter determined hereby. This may be by a single sensor or plural sensors
as described herein.
In some temperature implementations, infant or neonate temperature may be
sought data for capture
hereby, or temperature may be used with other users, adult or otherwise.
[0068] Infant and/or neonate temperature sensing can be of significant
assistance in health
monitoring. Forehead or other use may be one such application. Another set of
possible
applications may include methods and apparatuses for sensing the temperature
of both an infant and
a mother engaged in so-called "Kangaroo Care". There is evidence that pre-
mature infants may
benefit more from constant contact with a parent's or the mother's skin than
from being placed in an
incubator. There is also evidence of lower mortality rates.
[0069] An apparatus 100a for dual temperature sensing, the infant wearer
1000 and the mother
1010 or ambient air 1011, is shown in the accompanying figure, FIG. 1R. The
substrate 1105 is
preferably a small, flexible circuit board, in some examples, approximately
twenty (20) mm X thirty
(30) mm. The board 1105 may be disposed to contain circuitry 1103 for, for
example, sensing
relative X-Y-Z position and/or acceleration, and/or Bluetooth or other
wireless data/signal
connectivity, as well as, in many examples, a replaceable and/or rechargeable
battery for extended
use, as for example, seven (7) days of continuous monitoring (circuit element
alternatives not all
separately shown in FIG. 1R). The apparatus 100a may be held to the infant
with an adhesive, such
as the composite adhesive 1113 shown in FIG. 1R, which may further be, for
example, a disposable,
medical grade, double-sided adhesive.
[0070] Each of two temperature sensors 1111a and 1111b may be disposed on
alternative
opposing sides 1101, 1102 of the apparatus 100a, and may be thermally isolated
from each other, as
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
well as often being waterproof, water tight or water resistant. A thermally
insulating or isolation
layer 1103a may provide the thermal isolation of the electronics 1103 and/or
sensors 1111a and
1111b. A further spacer 1103b may be disposed through the insulating/isolating
layer 1103a to
provide a throughway for electronic communication of the sensor 1111b to the
electronics layer
1103. A silicone bead 1104 may be provided for isolating and assisting in
giving a waterproof or
water-resistant seal on the "infant side" 1102, and a silicone cover 1121 may
provide a waterproof or
waterproof barrier on the "mother side" 1101. The sensor 1111b on the "mother
side" or top or
exterior side 1101 may be slightly protruding relative to the cover 1121 with
in many
implementations a thin/thinner layer of covering material and/or silicone
thercover. The sensor
1111a on the child side or patient or circuit side 1102 may be protruding
past, or through the
adhesive and/or disposed exposed or also/alternatively covered with a thin
protectant layer for water
proofness, or tightness or resistance.
[0071] The thermally insulating layer may provide one or two or more
functions. It may provide
for or allow the "infant side" sensor 1111a to reach equilibrium, thus
providing an accurate "core
temperature" of the infant. It may also or alternatively isolate the infant's
temperature reading from
the mother's or ambient. The "mother side" sensor 1111b does not have to
provide an accurate core
temperature for the mother. Typically, the function of sensor 111 lb would be
to differentiate
whether or not the infant is in the correct direct contact with the mother's
skin; i.e., to provide a
relative measurement for determining whether the infant is in relative contact
or not in relative
contact with the mother. If the infant is facing the wrong way, but is still
in the "pouch" the sensor
will read that environment's ambient temperature. If the infant is out of the
pouch, it will read the
room ambient temperature. The relative differences would be interpretable to
provide an indication
of what position the infant is in; whether in contact, or in close association
in a controlled "pouch"
environment (but not in contact), or outside the pouch in a further removed
environment.
[0072] An alarm from a Bluetooth or otherwise wirelessly connected device
may be used to alert
the mother (or health care professional) that the infant is no longer in the
correct desired position, or
no longer in the "pouch".
[00731 Some alternative implementations hereof may include a driven right
leg ECG circuit with
one or more chest only electrodes ("Driven Chest Electrode"). In addition to
the electrodes used to
measure a single or multiple lead electrocardiogram signal, a device 100 may
use an additional
electrode, as for example the reference electrode 110 (see FIGs. 1A, IC, ID
and 1G, e.g.) to reduce
common mode noise. Such an electrode may function in a manner similar to the
commonly-used
21
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
driven right leg electrode, but may here be located on the patient's chest
rather than on the patient's
right leg but nevertheless this third/reference electrode may play the role of
the leg electrode. This
chest electrode may thus mimic a right leg electrode and/or be considered a
proxy driven right leg
electrode. A circuit, or portion of an overall circuit, adapted to operate in
this fashion may include a
number of amplifier stages to provide gain, as well as filtering to ensure
circuit stability and to shape
the overall frequency response. Such a circuit may be biased to control the
common mode bias of the
electrocardiogram signal. This driven chest electrode implementation may be
used in conjunction
with a differential or instrumentation amplifier to reduce common mode noise.
In this case, the sense
electrode may be used as one of the electrocardiogram electrodes.
Alternatively, a single-ended
electrocardiogram amplifier may be used where the differential
electrocardiogram signal is
referenced to ground or to some other known voltage.
[0074] A circuit or sub-circuit 200 using a transistor 201 as shown in Fig.
2 may be such a circuit
(aka module) and may thus include as further shown in FIG. 2A, a sense
electrode 202, a drive
electrode 203, and an amplifier 204. Both the sense and drive electrodes 202,
203 are placed on the
patient's chest such that they provide an electrical connection to the
patient. The amplifier 204 may
include gain and filtering. The amplifier output is connected to the drive
electrode, the inverting
input to the sense electrode, and the non-inverting input to a bias voltage
205. The amplifier
maintains the voltage of the sense electrode at a level close to the bias
voltage. An electrocardiogram
signal may then be measured using additional electrodes. Indeed, as was the
case for the improved
conductivity through use of anisotropic adhesive portions above, here also or
alternatively, the use of
this third electrode as a proxy for a right leg electrode (i.e., proxy driven
right leg electrode) can
provide signal reception otherwise unavailable. Clean signals may thus allow
for receiving cardiac P
waves which enhances the possibility to detect arithythmias that couldn't
otherwise be detected.
[0075] Further alternative descriptions of circuitry include that which is
shown in FIGs. 2B and
2C; in which are shown non-limiting alternatives in which three adjacent
electrodes El, E2, and E3
may be used to pick up the ECG signal, one of which electrodes playing the
role of the distant limb
electrode of traditional ECG monitors. Because the electrode-patient interface
has an associated
impedance (Rel and Re2), current flowing through this interface will cause a
difference in voltage
between the patient and the electrode. The circuit may use a sense electrode
(El) to detect the patient
voltage. Because this exemplar circuit node has a high impedance to circuit
ground (GND), very
little current flows through the electrode interface, so that the voltage drop
between the patient and
22
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
this node is minimized. The first of these alternative, non-limiting circuits
(FIG. 2B) also contains
an amplifier (U1) whose low-impedance output is connected to a separate drive
electrode (E2). The
amplifier uses negative feedback to control the drive electrode such that the
patient voltage (as
measured by the sense electrode El) is equal to the bias voltage (V1). This
may effectively maintain
the patient voltage equal to the bias voltage despite any voltage difference
between the driven
electrode (E2) and the patient. This can include voltage differences caused by
power line-induced
current flowing between the drive electrode and the patient (through Re2).
This arrangement differs
from a traditional 'driven-right-leg' circuit in at least two ways: the driven
electrode is placed on the
patient's chest (rather than the right leg), and the ECG signal is a single-
ended (not differential)
measurement taken from a third electrode (E3). Because all electrodes are
located on the patient's
chest in a chest-mounted example, a small device placed there may contain all
the necessary
electrodes for ECG measurement. One possible benefit of the single-ended
measurement is that gain
and filtering circuitry (U2 and associated components (Fig. 2C)) necessary to
condition the ECG
signal prior to recording (ECG Output) requires fewer components and may be
less sensitive to
component tolerance matching. The examples of FIGs. 2A, 2B and 2C are non-
limiting examples
and not intended to limit the scope of the claims hereto as other circuits
with other circuit elements
can be formed by skilled artisans in view hereof and yet remain within the
spirit and scope of claims
hereof.
[0076] In many implementations, a system hereof may include other circuitry
operative together
with the ECG electrodes, which may thus be accompanied by other sensors to
provide time
concordant traces of: i) ECG p-, qrs-, and t- waves; ii) 02 Saturation, as
measured by Pulse
Oxymetry; and/or iii) xyz acceleration, to provide an index of physical
activity. Such circuitry may
be implemented to one or more of the following electrical specifications. The
overall system might
in some implementations include as much as two weeks (or more) of continuous
run time; gathering
data during such time. Some implementations may be adapted to provide as many
or even greater
than 1000 uses. Alternatives may include operability even after or during
exposure to fluids or
wetness; in some such examples being water resistant, or waterproof, or
watertight, in some cases
continuing to be fully operable when fully submerged (in low saline water).
Other implementations
may include fast data transfer, as for an example where using an HS USB for
full data transfer in less
than about 90 seconds. A rechargeable battery may typically be used.
23
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
[0077] A further alternative implementation may include an electronic
"ground": In a device
hereof, mounted entirely on a flexible circuit board, the ground plane
function may be provided by
coaxial ground leads adjacent to the signal leads. The main contribution of
this type of grounding
system may be that it may allow the device the flexibility required to conform
and adhere to the skin.
100781 For electrocardiograph; EKG or ECG, some implementations may include
greater than
about 10 Meg Ohms input impedance; some implementations may operate with a 0.1
¨ 48 Hz
bandwidth; and some with an approximate 256 Hz Sampling Rate; and may be
implementing 12 Bit
Resolution. For PPG and Pulse Oximeter, operation may be with 660 and 940 nm
Wavelength;
about 80 ¨ 100 Sp02 Range; a 0.05 ¨ 4.8 Hz Bandwidth; a 16 Hz Sampling Rate;
and 12 bit
resolution. For an accelerometer: a 3-Axis Measurement may be employed, and in
some
implementations using a 2 G Range; with a 16 Hz Sampling Rate; and a 12 Bit
Resolution.
[0079] For pulse oximetry, an option for PPG ambient light subtraction may
be included. A
method and circuitry for reducing errors in pulse oximetry caused by ambient
light is described and a
circuitry option shown in FIG. 2D. Here a correlated double sampling technique
is shown for use to
remove the effect of ambient light, photo- detector dark current, and flicker
noise.
[0080] The schematic shown in FIG. 2D may be used where, first, the noise
signal may be
measured. The light sources are turned off, switch Si is closed, and switch S2
is open. This allows
charge proportional to the noise signal to accumulate on Cl. Then switch Si is
opened. At this point
the voltage on Cl is equal to the noise signal voltage. Next, the light signal
may be measured. The
light source is turned on, switch S2 is closed, and charge is allowed to flow
through Cl and C2 in
series. Then, S2 is opened, and the voltage is held on C2 until the next
measurement cycle when the
whole process is repeated.
[0081] If Cl is much larger than C2, nearly all the voltage will appear on
C2, and the voltage on
C2 will be equal to the noise-free signal (s). Otherwise, the voltage on C2
will be a linear
combination of the previous C2 voltage (p) and the noise-free signal: (C2 * s
+ Cl * p) / (Cl + C2).
This has the effect of applying a first-order, low-pass, IIR discrete-time
filter to the signal. If this
filtering effect is not desired, the voltage on C2 may be discharged to zero
before the signal is
measured each cycle, so that the signal held on C2 is simply: (C2 * s) / (Cl +
C2).
[0082] This circuit may be used with a trans-impedance amplifier in place
of resistor R, a
phototransistor in place of the photodiode, and FETs in place of the switches.
The output may be
followed by additional buffering, amplification, filtering and processing
stages.
24
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
[0083] Some summary methodologies may now be understood with relation to FIG.
3, though
others may be understood through and as parts of the remainder of the
disclosure hereof. A flow
chart 300 as in Fig. 3 may demonstrate some of the alternatives; where an
initial maneuver 301
might be the application of the device 100 to the patient. Indeed, this might
include some one or
more of the alternatives for adhesive application as described here above,
whether by/through use of
an adhesive such as that 113 of Fig.1D, or that of FIGs. 1E, 1F ancUor 1G.
Then, as shown, in
moving by flow line 311, a data collection operation 302 may be implemented.
Note, this might
include a continuous or substantially continuous collection or an interval or
periodic collection or
perhaps even a one-time event collection. This may depend upon the type of
data to be collected
and/or be dependent upon other features or alternatives, as for example
whether a long term quantity
of data is desired, for ECG for example, or whether for example a relative
single data point might be
useful, as in some cases of pulse oximetry (sometimes a single saturation
point might be of interest,
as for example, if clearly too low, though comparison data showing trending
over time, may indeed
be more typical).
[0084] Several alternatives then present in FIG. 3, flow chart 300; a first
such might be the
following of flowline 312 to the transmission of data operation 303, which
could then involve either
wireless or wired (e.g., USB or other) data communication from the device 100
to data analysis
and/or storage devices and/or systems (not separately shown in FIG. 3; could
include computing
devices, see e.g., FIG. 4 described below, or the like). Options from this
point also appear; however,
a first such might include following flow line 313 to the data analysis
operation 304 for analyzing
the data for determination of the relative health and/or for condition
diagnosis of a patient.
Computing systems, e.g., a computer (could be of many types, whether hand-
held, personal or
mainframe or other; see FIG. 4 and description below) could be used for this
analysis; however, it
could be that sufficient intelligence might be incorporated within the
electronics 103 of device 100
such that some analysis might be operable on or within device 100 itself. A
non-limiting example,
might be a threshold comparison, as for example relative to pulse oximetry
where when a low (or in
some examples, perhaps a high) threshold level is reached an indicator or
alarm might be activated
all on/by the electronics 103 of the device 100.
[0085] A similar such example, might be considered by the optional
alternative flow path 312a
which itself branches into parts 312b and 312c. Following flow path 312 a, and
then, in a first
example path 312b, a skip of the transmit data operation 303 can be understood
whereby analysis
304 might be achieved without substantial data transfer. This could explain on
board analysis,
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
whether as for example according to the threshold example above, or might in
some instances
include more detailed analysis depending upon how much intelligence is
incorporated on/in the
electronics 103. Another view is relative to how much transmission may be
involved even if the
transmission operation 303 is used; inasmuch as this could include at one
level the transmission of
data from the patient skin through the conductors 108, 109 and/or 110 through
the traces 107 to the
electronics 103 for analysis there. In other examples, of course, the
transmission may include off-
board downloading to other computing resources (e.g., FIG. 4). In some cases,
such off-loading of
the data may allow or provide for more sophisticated analysis using higher
computing power
resources.
[0086] Further alternatives primarily may involve data storage, both when
and where, if used. As
with intelligence, it may be that either some or no storage or memory may be
made available in/by
the electronics 103 on-board device 100. If some storage, whether a little or
a lot, is made available
on device 100, then, flow path 312a to and through path 312c may be used to
achieve some storing
of data 305. This may in many cases then, though not necessarily be before
transmission or analysis
(note, for some types of data multiple paths may be taken simultaneously, in
parallel though perhaps
not at the same time or serially (e.g., paths 312b and 312c need not be taken
totally to the exclusion
of the other), so that storage and transmission or storage and analysis may
occur without necessarily
requiring a completion of any particular operation before beginning or
otherwise implementing
another). Thus, after (or during) storage 305, flow path 315a may be followed
for stored data which
may then be transmitted, by path 315b to operation 303, and/or analyzed, by
path 315c to operation
304. In such a storage example, which in many cases may also be an on-board
storage example, data
can be collected then stored in local memory and later off-loaded/transmitted
to one or more robust
computing resources (e.g., FIG. 4) for analysis. Frequently, this can include
long term data
collection, e.g., in the manner of days or weeks or even longer, and may thus
include remote
collection when a patient is away from a doctor's office or other medical
facilities. Thus, data can
be collected from the patient in the patient's real world circumstances. Then,
after collection, the
data can be transmitted from its storage on device 100 back to the desired
computing resource (FIG.
4, e.g.), and such transmission might be wireless or wired or come combination
of both, as for
example a blue tooth or Wi-Fi connection to a personal computer (FIG. 4 for
one example) which
might then communicate the data over the internet to the designated computer
for final analysis.
Another example might include a USB connection to a computer, either to a PC
or a mainframe
(FIG. 4), and may be to the patient computer or to the doctor computer for
analysis.
26
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
[0087] If little or no storage or memory is resident on device 100 (or in
some examples even
where there may be a large amount of resident memory available), then,
relatively soon after
collection, the data would need to or otherwise might desirably either or both
be transmitted and then
stored, see path 313a after operation 303, and/or transmitted and analyzed,
paths 312 and 313. If
path 313a is used, then, more typically, the data storage may be in/on
computing resources (not
shown in FIG. 3, but see FIG. 4 described below) off-board (though on-board
memory could be used
as well), and then, any of paths 315a, 315b and 315c may be used.
[0088] A feature hereof may include an overall system including one or more
devices 100 and
computing resources (see Fig. 4, for example) whether on-board device(s) 100,
or separate, as for
example in personal or mobile or hand-held computing devices (generally by
FIG. 4), the overall
system then providing the ability for the physician or doctor to have
immediate, in-office analysis
and presentation of collected test data. This would in some implementations
allow for on-site data
analysis from the device without utilization of a third party for data
extraction and analysis.
[0089] Alternative implementations hereof may thus include one or more
hardware and software
combinations for multiple alternative data source interpretations. As noted
above, a device 100
hereof includes hardware that monitors one or more of various physiologic
parameters, then
generates and stores the associated data representative of the monitored
parameters. Then, a system
which includes hardware such as device 100 and/or the parts thereof, and
software and computing
resources (FIG. 4, generally) for the processing thereof. The system then
includes not only the
collection of data but also interpretation and correlation of the data.
[0090] For example, an electrocardiogram trace that reveals a ventricular
arrhythmia during
intense exercise may be interpreted differently than the same arrhythmia
during a period of rest.
Blood oxygen saturation levels that vary greatly with movement can indicate
conditions that may be
more serious than when at rest, inter alia. Many more combinations of the four
physiologic
parameters are possible, and the ability of software hereof to display and
highlight possible problems
will greatly aid the physician in diagnosis. Thus, a system as described
hereof can provide beneficial
data interpretation.
[0091] Some of the features which can assist toward this end may be
subsumed within one or
more of operations 303 and 304 of FIG. 3, wherein data collected on a device
100 can rather simply
be communicated/transmitted to computing resources (again, whether on-board
device 100 or
discrete therefrom as e.g., FIG. 4). For an example, when a patient having had
a device applied
27
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
(operation 301) may return to a physician's office after a test period wherein
data was collected
(operation 302) the device is connected via one or more data transmission
alternatives, as for
example, USB to a computer (Windows or Mac) (generally with reference to FIG.
4 and description
thereof) in the office, allowing immediate analysis by the physician while the
patient waits (note, the
device 100 may first have been removed from the patient or might remain
thereon pending
transmission and analysis for determination of whether more data may be
desired). In some
implementations, data analysis time may be relatively quick, at approximately
15 minutes in some
implementations, and might be achieved with a user-friendly GUI (Graphic User
Interface) to guide
the physician through the analysis software.
[0092] The analysis/software package may be disposed to present the
physician with results in a
variety of formats. In some implementations, an overview of the test results
may be presented,
either together with or in lieu of more detailed results. In either case, a
summary of detected
anomalies and/or patient-triggered events may be provided, either as part of
an overview and/or as
part of the more detailed presentation. Selecting individual anomalies or
patient-triggered events
may provide desirable flexibility to allow a physician to view additional
detail, including raw data
from the ECG and/or from other sensors. The package may also allow data to be
printed and saved
with annotations in industry-standard EHR formats.
[0093] In one implementation, patient data may be analyzed with software
having the one or
more of the following specifications. Some alternative capabilities may
include: 1.Data Acquisition;
i.e., loading of data files from device; 2. Data Formatting; i.e., formatting
raw data to industry
standard file formats (whether, e.g., aECG (xml); DICOM; or SCP-ECG) (note,
such data formatting
may be a part of Acquisition, Storage or Analysis, or may have translation
from one to another (e.g.,
data might be better stored in a compact format that may need translation or
other un-packing to
analyze)); 3. Data Storage (whether local, at a clinic/medical facility level
or e.g., in the Cloud
(optional and allows offline portable browser based presentation/analysis); 4.
Analysis which inter
alia, may include, e.g., noise filtering (High pass/Low pass digital
filtering); and/or QRS (Beat)
detection (in some cases, may include Continuous Wave Transform (CWT) for
speed and accuracy);
and/or 5. Data/Results Presentation, whether including one or more graphical
user interface(s)
(GUIs) perhaps more particularly with an overall Summary and/or General
Statistics and/or
Anomaly Summary of Patient triggered event(s); presentation of additional
levels of detail whether
of Strip view(s) of anomaly data by incident (previous, next) Blood Oxygen
saturation, stress
28
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
correlation or the like; and/or allowing care provider
bookmarking/annotations/notes by incident
and/or Print capability.
[0094] Further, on alternative combinations of hardware with proprietary
software packages: I)
One on-device software package may be adapted to store the measurements from
the data signals
acquired from one or more of EKG/ECG (whether right leg and/or p-, qrs- and/or
t- waves), or 02
saturation, or xyz acceleration, in a time concordant manner, so that a
physician may access a
temporal history of the measurements (say, in some examples, over a 1-2 week
interval), which
would provide useful information on what the patient's activity level was
prior to, during, and after
the occurrence of a cardiac event. ii) an alternative to alternately manage
the real-time transmission
of the real-time measured parameters to a nearby station or relay. And/or;
iii) an off-device ECG
analysis software aimed at recognizing arrhythmias.
[0095] The software mentioned above may be industry understood software
provided by a 3rd
party, or specially adapted for the data developed and transmitted by and /or
received from a
wearable device 100 hereof. Thorough testing using standard (MIT-BIH/AHA/NST)
arrhythmia
databases, FDA 510(k) approvals preferred. Such software may be adapted to
allow one or more of
automated ECG analysis and interpretation by providing callable functions for
ECG signal
processing, QRS detection and measurement, QRS feature extraction,
classification of normal and
ventricular ectopic beats, heart rate measurement, measurement of PR and QT
intervals, and rhythm
interpretation.
[0096] In many implementations, the software may be adapted to provide and/or
may be made
capable of supplying one or more of the following measurements:
Table 1:
I. Heart Rate Min, Max and Average
2. QRS duration average
3. PR interval average
4. QT interval average
5. ST deviation average
and, may be adapted to recognize a broad range of arrhythmias such as those
set forth here:
Table 2A:
I. SINUS RHYTHM
2. SINUS RHYTHM + IVCD
3. SINUS BRADYCARDIA
4. SINUS BRADYCARDIA + IVCD
5. SINUS TACHYCARDIA
29
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
6. PAUSE
7. UNCLASSIFIED RHYTHM
8. ARTIFACT
[0097] This first group of 8 given above are arrhythmia types that may be
recognizable even if
there is no discernible P wave. They are the ones typically recognized by
existing products in the
outpatient monitoring market that we propose to address.
[0098] A second set or group of arrhythmias; below, may require a
discernible and measurable P
wave. Some implementations hereof may be adapted to be able to detect and
recognize them, as
device 100 may be able as described above to detect P waves, depending of
course, and for example,
on whether the strength of the P wave which may be affected by device 100
placement or patient
physiology.
Table 2B:
9. ATRIAL FIBRILLATION/FLUTTER SVR (slow)
10. ATRIAL FIBRILLATION/FLUTTER CVR (normal rate)
11. ATRIAL FIBRILLATION/FLUTTER RVR (rapid
12. FIRST DEGREE AV BLOCK + SINUS RHYTHM
13. FIRST DEGREE AV BLOCK + SINUS TACHYCARDIA
14. FIRST DEGREE AV BLOCK + SINUS BRADYCARDIA
15. SECOND DEGREE AV BLOCK
16. THIRD DEGREE AV BLOCK
17. PREMATURE ATRIAL CONTRACTION
18. SUPRAVENTRICULAR TACHYCARDIA
19. PREMATURE VENTRICULAR CONTRACTION
20. VENTRICULAR COUPLET
21. VENTRICULAR BIGEMINY
22. VENTRICULAR TRIGEMINY
23. IDIOVENTRICULAR RHYTHM
24. VENTRICULAR TACHYCARDIA
25. SLOW VENTRICULAR TACHYCARDIA
[0099] Further in alternative software implementations; some sample
screenshots are shown in
FIG. 5. A first such alternative is shown in FIG. 5A, which is an example
screenshot showing ECG
and Oxygen Saturation data taken by using a patch device such as a device 100
hereof. An
extremely clean signal is shown (no filtering or smoothing has been done on
this data). Distinct p-
waves are also shown (3 of which are shown as an example with arrows). P wave
detection can be
extremely important for ECG anomaly detection. Oxygen Saturation, as measured
by Pulse
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
Oxymetry, is shown on the bottom plot. This is data taken by a device on the
chest, and is taken in
time concordance with the ECG data.
[0100] Another alternative is shown in Fig. 5B, which is an example
screenshot of Analysis
Software. This is a sample of ECG data taken from the MIT-BIH Arrhythmia
Database, Record
205. As analyzed by the Analysis system hereof, we see in the Event
Occurrences Summary list
(top, left) five (5) anomaly types (plus normal sinus rhythm). This list also
shows the number of
occurrences of each anomaly, total duration of the anomaly in the complete
ECG, and the percent
time this anomaly occurs in the complete ECG. To view specific instances of
each anomaly, the
user double clicks the specific row in the Event Occurrences Summary list, as
shown in Figure 5C.
[0101] As introduced, Fig. 5C is an example screenshot showing specific
instance of Ventricular
Tachycardia. The ECG plot automatically navigates to the specific time in the
ECG waveform, and
marks the beginning and end of the event. More detailed data about this
specific event is now shown
in the Occurrence Details: HR Average, HR Max, etc. for the duration of this
event. To show the
instances of another anomaly in this ECT, the user can click on the Premature
Ventricular
Contraction (PVC) row of the Event Occurrences Summary, as shown Figure 5D.
[0102] As introduced, Fig. 5D is an example screenshot showing specific
instance of Premature
Ventricular Contraction. This shows occurrences of the PVC. The Start Times
list (middle top)
shows all instances of PVC occurrences in this ECG, and lists the start time
for each occurrence. In
this case, the user can click on the PVC that starts at 00:15:27 (the 11th
occurrence). The ECG plot
is automatically taken to this point in time to show and indicate the PVC
instances in the waveform.
Since there are 3 instances of a PVC in this timeslot, all 3 occurrences are
marked.
[0103] As mentioned above, in one aspect of the developments hereof, ECG
signals collected in
time concordance with pulse oximetry signals may be used to reduce the noise
in the pulse oximetry
signals and to permit the calculation of values for oxygen saturation,
particularly in circumstances
where sensors pulse oximetry data are placed on noise-prone locations of a
patient, such as the chest.
In some embodiments, this aspect may be implemented by the following steps:
(a) measuring an
electrocardiogram signal over multiple heart beats; (b) measuring one or more
pulse oximetry
signals over multiple heart beats such that the electrocardiogram signal and
the one or more pulse
oximetry signals are in time concordance over one or more heart beats; (c)
comparing a portion of
the electrocardiogram signal and the one or more pulse oximetry signals in
time concordance over
one or more heart beats to determine a constant component and a primary
periodic component of
31
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
each of the one or more pulse oximetry signals; and (d) determining oxygen
saturation from the
constant components and primary periodic components of the one or more pulse
oximetry signals.
Measurement of the ECG signals and pulse oximetry signals may be implemented
by embodiments
of devices hereof. In particular, pulse oximetry signals may be a reflective
infrared signal and a
reflective red light signal collected by a photodetector in a device hereof.
Alternatives may include
other colors, as for example green in addition to or in lieu of one or both of
red and infrared. Such
alternatives are described further below.
[0104]
Intervals of pulse oximetry signals corresponding to heart beats may be
determined by
comparing such signals to the time concordant ECG signals. For example (not
intended to be
limiting), successive R-wave peaks of a time concordant ECG signal may be used
to identify such
intervals, although other features of the ECG signal may be used as well. Once
such intervals are
identified, values at corresponding times within the intervals may be averaged
to reduce signal noise
and to obtain more reliable values for the constant components (sometimes
referred to as the "DC
components") and the main periodic components (sometimes referred to as the
"AC components")
of the pulse oximetry signals, e.g. Warner et al, Anesthesiology, 108: 950-958
(2008). The number
of signal values recorded in an interval depends on the signal sampling rate
of the detectors and
processing electronics employed. Also, as the intervals may vary in duration,
the averaging may be
applied to a subset of values in the intervals. As described below, oxygen
saturation values may be
computed from such DC and AC components using conventional algorithms. The
number of heart
beats or intervals over which such averages may be computed may vary widely,
as noted below. In
some embodiments, signals from one or more heart beats or intervals may be
analyzed; in other
embodiments, signals from a plurality of heart beats or intervals may be
analyzed; and in some
embodiments, such plurality may be in the range of from 2 to 25, or in the
range of from 5 to 20, or
in the range of from 10 to 20.
[0105] As
described, a method of pulse oximetry measures photoplethysmogram signals at
red
and infrared wavelengths. The DC or mean value is estimated and subtracted,
and the ratio of AC or
pulsatile signal is estimated and/or averaged. Linear regression between the
two signals can be used
as described below. However, performance is limited because similar noise
exists in both the red
and infrared signals. Photoplethysmography taken using green light (-550nm) is
more resilient to
motion noise because the light is absorbed much more by blood than by water or
other tissue.
However, the difference between oxygenated and deoxygenated blood in the green
region of the
32
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
spectrum is much less than red. In an alternative, a green PPG signal (or long
time average of red/IR
(see below)) may be used to determine the shape of the pulsatile signal. A
weighted average of any
number of different wavelengths (such as green, red and infrared) may be used
to estimate the shape
of the pulsatile waveform.
[0106] In further alternative implementations, a linear regression
algorithm for Oxygen
Saturation may be used. As such, either or both the patient's ECG signal
and/or a green (or other
color) LED PPG signal may be used. For a first example, an ECG signal may be
used to determine
when heart beats occur. The beat locations allow correlated time averaging of
each of the two
photoplethysmogram signals. A linear regression of the ensemble averages may
then be used to
determine the linear gain factor between the two signals. This gain factor can
be used to determine
the patient oxygen saturation.
[0107] If/when in the alternative and/or in addition, photoplethysmography
(PPG) using green
light (-550nm) is implemented, the PPG signal may be used determine the shape
of the pulsatile
signal. This lower-noise signal may then be used as the independent variable
for linear regression
with both the red and infrared signals. The ratio of these two regression
results is an estimate of the
correlation between the red and infrared signals. Noise can be reduced by
ensemble averaging over
multiple heart beats as disclosed herein (see e.g., description of frames
below). In addition to or
instead of using an ECG signal to determine beat timing, the green wavelength
PPG signal may be
used. Alternatively, a weighted average of any number of different wavelengths
(such as green, red
and infrared or long time average of red/IR (see below)) may be used. The
ensemble averaging may
be improved by detecting and removing outlier beats, possibly by discarding
beats that have less
correlation to the estimated ensemble average than others, or by estimating
noise and weighting
beats from areas of high noise less. Noise can also be improved through longer
averaging periods.
[0108] As such, included may be a method for health monitoring comprising:
determining from
either or both a user's ECG and/or a first photoplethysmogram PPG signal
and/or a weighted
combination of wavelengths when heart beats occur; time averaging the first
photoplethymogram
PPG signal to generate a first pulse shape template or dataset; time averaging
each of two additional
photoplethysmogram signals correlated to the beat locations; one of the
additional signals being red,
the other additional signal being IR; generating ensemble averages for each of
the red and IR signals;
comparing each of the red and IR ensemble averages to the first pulse shape
template or dataset;
using a linear regression of each of the red and IR ensemble average-
comparisons to the first pulse
33
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
template or dataset to determine the linear gain factor between the two
signals; determining from the
gain factor the patient oxygen saturation.
[0109] In a similar view; included may be a method for determining pulse
oxygenation;
comprising: a) detecting heart beats; using ECG, using green, or using a
weighted combination of
wavelengths; b) generating one or more of a first pulse shape template or a
dataset representing a
first pulse shape, including using green wavelengths, an ensemble average of
green over
approximately the same amount of time as for either red or IR, an ensemble
average of multiple
wavelengths over approximately the same amount of time as for either red or
IR, or an
ensemble average of multiple wavelengths over significantly longer than the
amount of time as for
either red or IR; can use ensemble average gives beat shape; or a long time
average of a single
wavelength of any color; c) obtaining a red pulse shape template or dataset
representing same and
an IR pulse shape template or dataset representing same, and compare each of
these to the first pulse
shape above; and, d) correlating via linear regression between red ensemble
average with the first
pulse shape template or dataset to the IR ensemble average with the first
pulse shape or dataset,
where the ratio of these correlations is then used as the AC ratio for oxygen
saturation.
[0110] The pulse shape template or dataset is in some implementations
similar to the reference
frame template described herein as well in that the pulse shape template
represents a long-term
ensemble average of the PPG signal. However, a difference is that the
reference frame template
described herein elsewhere was there designated for pulse transit time, while
in the present
description related to a first pulse shape or dataset or the like, is for
oxygen saturation.
[0111] While a first method may be one where green light is used for the
beat detection, other
methods will be viable as well, as where ECG is used for beat detection.
Further, the alternatives
include green or a long red and IR average used for the first pulse shape, and
a shorter red and IR is
used for the oxygen saturation comparisons to the first pulse shape. It may be
helpful to understand
that a long red and IR average used for the first pulse waveform shape (or
dataset) is in relation to
the relatively shorter red/jr signals used for the oxygen saturation
measurement. Because the shape
is expected to change slower than the oxygen saturation, a long average can be
used for the shape,
while still using a shorter average (and thus getting faster response times)
for the oxygen saturation
part.
34
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
101121 Note, green has been found desirable because it has a high signal to
noise ratio; the pulse
signal is strong relative to other possible motion noise. However, other
wavelengths could be used
instead of green, i.e. green could be replaced by other colors in the spectrum
of light, keeping in
mind, some colors will behave better or other colors worse in the relationship
of signal to noise.
Note, other colors, even without a desirable signal to noise ratio can be used
herein or herewith.
Similarly, the preference for red and/or IR wavelengths has been that it has
been found that red
and/or IR have provided good relative reflectivity to the particular
oxygenation of hemoglobin blood
in a test subject. Each of oxygenated blood reflects an effective amount
comparatively of red light
and de-doxygenated blood reflects an effective amount comparatively of
infrared, IR, light. Other
colors can be used instead of red and IR throughout, though the other colors
may have less (or more)
effectiveness in particular applications. It should also be noted that as
understood in the art,
whenever any particular color of light is described, a number of discrete
wavelengths may be
understood as falling within such definition, and that utility may fall within
or outside the definition,
though preferences may be identified by general color. Thus colors other than
green or red or IR are
understood to be used and/or such color selection may be limited only by
minimal effectiveness in
either signal to noise ratio and/or reflectiveness related to oxygenation or
other utility.
[0113] ECG or green PPG (or like) or long time average of red/IR (see
below) data may be
recorded in time-concordance with two or more photoplethysmographs of
different light
wavelengths. The heart beats are detected in the ECG or green PPG signal.
These heart beats allow
for definition of a 'frame' of photoplethysmogram data for the time between
two adjacent heart
beats. Two or more of these frames can then be averaged together at each point
in time to create an
average frame for the time interval. Because the photoplethysmogram is
correlated with the
heartbeat, the photoplethysmograph signal is reinforced by this averaging.
However, any motion
artifact or other noise source that is uncorrelated in time with the heartbeat
is diminished. Thus, the
signal-to-noise ratio of the average frame is typically higher than that of
the individual frames.
[0114] Having constructed an average frame for at least two
photoplethysmographs of
different light wavelengths, linear regression can then be used to estimate
the gain between the two
average frame signals. This gain value may be used to estimate blood oxygen
saturation information
or other components present in the blood such as hemoglobin, carbon dioxide or
others. The process
may be repeated for additional and/or alternative light wavelengths in order
to do so.
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
[0115] Exemplar/alternative methods hereof may include determining the gain
between particular
signals, as between the red and IR and/or green frame signals, if/when such
may be used. These
may be found by averaging the two frames together first. This may result in a
signal with reduced
noise. The gain is found by performing linear regression of the red versus
combined and IR versus
combined and then finding the ratio of these two results; or linear regression
of the red versus
combined with green and IR versus combined with green and then finding the
ratio of these two
results; or linear regression of red versus green and IR versus green and then
finding the ratio of
these two results; or by linear regression of combining green with each of red
and IR and using the
ratio of these results.
[0116] Another method involves selecting a possible gain value, multiplying
the average
frame signal by it, and determining the residual error with respect to an
average frame of a different
wavelength. This process may be repeated for a number of potential gain
values. While simple linear
regression finds the global minimum gain value, this method allows for finding
local minima. Thus,
if it is likely that the global minimum represents correlation caused by
motion artifact, venous blood
movement or another noise source, it may be ignored, and a local minimum may
be selected instead.
[0117] Yet another method uses an ensemble average of the red and/or IR
signals over a much
longer time to determine the pulse waveform shape, then fitting shorter time
averaged signals to that
waveform shape. Basically, we replace the green light signal or ECG signal
described above with a
long time average of red/IR.
[0118] As mentioned above, patient wearable devices hereof for implementing
the above aspects
may be particularly useful for monitoring oxygen saturation in noisy regions
for such measurements,
for example, where there is significant local skin movement, such as the chest
location.
[0119] One embodiment of the above aspect hereof is illustrated in Figs. 6A-
6C. In Fig. 6A,
curve A (600) illustrates time varying output of the photodiode of a device
hereof for infrared (IR)
reflection and curve B (602) illustrates time varying output of the photodiode
of the device for red
light reflection. In some embodiments, the skin is alternatively illuminated
by the red and IR LEDs
to generate the signals collected by the same photodiode. In Fig. 6B, time
synchronized (i.e. time
concordant) ECG data (or alternatively/additionally green PPG data or long
time average of red/IR
as introduced above), illustrated by curve C (604), is added to the plot of
Fig. 6A. Peak values in the
ECG data (e.g. peaks 606 and 608) (or green PPG or long time average of red/IR
data, see above)
may be used to define frames or intervals of pulse oximetry data. Additional
consecutive frames or
36
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
intervals are indicated by 612 and 614, and further frames may be similarly
determined. In
accordance with this aspect, pulse oximetry data from a plurality of frames is
collected. The
magnitude of the plurality may vary widely depending on particular
applications. In some
embodiments, the plurality of frames collected is from 5 to 25; in one
embodiment, a plurality is
between 8 and 10 frames. Typically, frames or intervals of pulse oximetry data
contain different
numbers of signal samples. That is, output from the sensors may be sampled at
a predetermined rate,
such a 32 samples per second. If the time between ECG (or green PPG or long
time average of
red/IR) peaks varies, then the number of samples per frame will vary. In one
embodiment, features
in the ECG (or green PPG or long time average of red/IR) data serving as the
starting points of a
frame are selected so that an associated peak in the pulse oximetry data is
approximately in the mid-
point, or center, of the frame, after which a predetermined number of signal
samples are recorded for
each frame. Preferably in this embodiment, the predetermined number is
selected to be large enough
to ensure that the pulse oximetry signal peak is roughly mid-frame. Sample
values corresponding to
time points above the predetermined value are not used. After a plurality of
frames of data is
collected, averages of the values at corresponding time points of the frames
are computed. The
values from such averages AC and DC components of the pulse oximetry data are
determined and
are then used to compute relative oxygen saturation by conventional methods,
such as the ratio-of-
ratios algorithm, e.g. Cypress Semiconductor document No. 001-26779 Rev A
(January 18, 2010).
This basic procedure is summarized in the flow chart of Fig. 6C. Frame size
(in terms of number of
samples) is determined (620). Values of samples at corresponding time points
within each frame are
summed (622), after which average values for each time point are computed
which, in turn, give the
AC and DC components of IR and red and/or green light reflection with reduced
noise. In some
embodiments, values for these components can be used to compute oxygen
saturation using
conventional algorithms (626). Relative values for oxygen saturation may be
converted into
absolute values by calibrating the measurements for particular embodiments.
Calibration may be
carried out in controlled environments where individuals are exposed to
varying atmospheric
concentrations of oxygen and measured oxygen saturation values are related to
corresponding
oxygen levels.
[0120] In addition to the above embodiment for comparing ECG and/or green PPG
or long time
average of red/IR signals with pulse oximetry signals, a range of other
embodiments for such
comparing is within the comprehension of those of ordinary skill in the art.
For example, in order to
find peaks of the AC component of pulse oximetry signals in the presence of
noise, features of the
37
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
time concordant ECG signal that are located at characteristic times preceding
and succeeding the
pulse oximetry maximum and/or minimum values may be used to reliably determine
the pulse
oximetry peak and minimum values when averaged over a plurality of heart beats
(without the need
to average all values of the pulse oximetry signal over the heart beats). For
example, if, within an
interval, the R wave peak of an ECG signal characteristically preceded a pulse
oximetry signal
maximum by x milliseconds and trailed a pulse oximetry signal minimum by y
milliseconds, then
the essential information about the AC component of the pulse oximetry signal
may be obtained by
repeated measurements of just two values of pulse oximetry signals.
[0121] In some embodiments, values for IR or red reflection measured by the
photodiode may be
used to estimate depth and/or rate of respiration. In Fig. 6D, a curve (630)
of Red or IR or green
values over time is illustrated. In Fig. 6E, maximum values and minimum values
of curve (630) are
shown by dashed curves (632) and (634), respectively. The difference between
the maximum and
minimum values at a time point is monotonically related to the depth of breath
in an individual being
monitored. Thus, as illustrated, breaths at time (636) are shallower than
those at time (638). In
some embodiments, depth of breath versus time may be computed and monitored in
an individual.
Over time, the rate of respiration can be evaluated from the curve of maximum
and minimum values
over time.
[0122] Moreover, moving from an appreciation of a derivation of a respiration
waveform from
ECG R-S amplitude and/or R-R intervals, it has been found that a PPG and/or
pulse oximeter as
described herein can be used to relatively directly estimate a respiration
waveform. As the chest
expands and contracts during breathing, the motion hereof shows up as a
wandering baseline artifact
on the PPG signals. The respiration signal may be isolated by filtering out
the PPG data to focus on
the breathing/respiration signal. This may be particularly so with a chest-
mounted PPG.
[0123] In addition, a chest mounted accelerometer may also or alternatively
be used to measure
the respiration waveform, especially when the user is lying on his/her back.
As the chest expands
and contracts, the chest accelerates up and down (or transversely, or
otherwise depending upon
orientation), which can be measured by the accelerometer.
[0124] Either of these, PPG and/or accelerometer, devices and/or methods
may be used discretely
or in combination with each other and/or with the above-described ECG-based
respiration estimation
technique. Using multiple methods may improve accuracy when compared to
estimates based on a
38
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
single method. Respiration rate and depth may then be estimated from the
respiration signal using
time-domain and/or frequency domain methods.
[0125] In some implementations, heart beat timing (e.g., from ECG) and PPG
signals can be used
to determine pulse transit time; i.e., the time for the pressure wave to
travel from the heart to other
locations in the body. Measurements of pulse transit time may then be used to
determine or estimate
blood pressure. Note, the heartbeat timing, ECG and/or PPG signals may be
generated by
conventional or other to-be-developed methods, systems or devices, or may be
developed by
wearable devices such as those otherwise described herein. I.e., the
algorithms hereof may be
separately usable, as well as being usable in the wearable cardiac device.
[0126] As disclosed herein elsewhere, the PPG signals of several heart
beats may be averaged by
correlating each with a respective heartbeat. The result is a PPG frame where
the heart rate-
correlated PPG signal is reinforced while uncorrelated noise is diminished.
Moreover, because the
PPG frame is already correlated to the timing of the heartbeat, pulse transit
time may be estimated by
determining the location of either the peak or minimum with respect to either
the beginning or end of
the frame itself. This may be done either by finding the minimum and/or
maximum sample(s), or by
interpolating the signal to find points between measured samples. For example,
interpolation may be
done with a quadratic fit, a cubic spline, digital filtering, or many other
methods.
[0127] The pulse transit time may also be estimated by correlating the PPG
frame with a sample
signal. By shifting the two signals with respect to each other, the time shift
resulting in the maximum
correlation may be determined. If the sample signal is an approximation of the
expected PPG frame,
then the time shift with maximum correlation may be used to determine the
pulse transit time.
[0128] An exemplar methodology or algorithm herefor is described here and
shown in the
drawing FIGs. 7A, 7B and 7C. Initially, such a method 710 (which includes
and/or is defined by
parts 710a, 710b and/or 710c) takes at least one heartbeat (typical ECG)
signal 712 and at least one
PPG signal 711 as input as shown in FIG. 7A, e.g. The heartbeat timing
information/signal 712 is
used to generate heartbeat timing information by detecting the R-wave or other
ECG feature from
each beat; multiple ECG signals (i.e. different leads from locations on the
body) may be used to
obtain a better estimate of the heartbeat timing information. The PPG 711 may
use a single light
wavelength or signals from multiple light wavelengths. Using the corresponding
heartbeat timing
information related to each PPG signal 711, each PPG signal 711 is segmented
into "frames," see
39
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
PPG Frame 1, PPG Frame 2 and PPG Frame N in FIG. 7A, where each frame contains
the PPG
signal of a single wavelength for the duration of one corresponding beat of
the heart.
[0129] Optionally, but, typically, a PPG signal quality estimate may also
be performed. An
example of this is shown as method part 710b in FIG. 7B. This estimate may
consider the variance
of the PPG signal, the estimated signal-to-noise ratio of the PPG signal, PPG
signal saturation.
patient motion information from an accelerometer or gyroscope, an ECG or
impedance measurement
noise estimate, or other information about the PPG signal quality. Shown in
FIG. 7B is an exemplar
using accelerometer signal 713 in conjunction with PPG signal 711 to generate
a PPG Signal Quality
Value/Estimate 714. This signal quality estimate 714 may then be used in
conjunction with the
heartbeat timing information 712 to generate the gain for each frame, see PPG
Frame 1 Gain, PPG
Frame 2 Gain and PPG Frame N Gain in FIG. 7B, where lower signal quality
results in a lower gain.
To reduce computation time, the signal quality estimate 714 may be omitted and
a constant may be
used for the gain information.
[0130] As shown in FIG. 7C, the gain information (PPG Frame 1 Gain, PPG Frame
2 Gain and
PPG Frame N Gain from FIG. 7B) may be used (here shown as
combined/manipulated) with the
frame information (PPG Frame 1, PPG Frame 2 and PPG Frame N from FIG. 7A) to
create a
weighted, n-sample moving-average frame 715, where the PPG signal that is
correlated with the
heartbeat timing is reinforced while the uncorrelated noise is reduced. The
number of samples
included in the frame (n) 715 may be adapted to reduce noise or decrease
response time. The frames
may be additionally weighted by time in order to increase the contribution of
recent or near-future
frames with respect to frames that are further away and potentially less-
relevant. This additional
weighting by time may be implemented using an IIR or FIR filter.
[0131] Once the average frame 715 has been produced for a given instant in
time, the pulse
transit time 716 may be determined by finding the shift in the frame signal
with respect to the
heartbeat. This may be done simply by finding the sample index 717 where the
signal is at a
minimum or maximum and comparing it with the frame boundary (heartbeat timing)
to determine
the pulse transit time. For a more precise result, the signal may be
interpolated 718 using a spline or
polynomial fit around the minimum or maximum values, allowing the minimum or
maximum to be
determined with greater precision than the sample rate. Finally, the frame may
be compared 719 to a
reference frame template, where the average frame is shifted with respect to
the template. The shift
with the highest correlation between the average frame and the template
indicates the transit time
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
716. This reference template may be a predetermined signal, or it may be
allowed to adapt by using a
long-term frame average with a known transit time.
[0132] Note, such methodologies may be used with PPG and heartbeat timing
information
obtained from a variety of sources, including but not limited to conventional
and/or to-be-developed
technologies; or, may be obtained one or the other alone or together and/or
together with quality
signal (PPG variance, estimated PPG signal-to-noise ratio, PPG signal
saturation, patient motion
accelerometer or gyroscope data, an ECG or impedance measurement noise
estimate, or other
information about the PPG signal quality) obtained from a wearable device
and/or system as
described further hereinbelow.
[0133] Some further alternatives may include data transmission and/or
interpretation by local
medical facilities, whether physician or doctor offices or e.g., ICU/CCU
(Intensive Care/Coronary
Care Units). Accordingly, a device 100 hereof that will measure one or more of
a variety of
physiologic signals, possibly including electrocardiogram, photoplethysmogram,
pulse oximetry
and/or patient acceleration signals will be placed on the patient's chest and
held with an adhesive as
described herein. The device transmits the physiologic signals wirelessly or
by wire (e.g.. USB) to a
nearby base station for interpretation and further transmission, if desired.
The wireless transmission
may use Bluetooth, Wi-Fi, Infrared, RFID (Radio Frequency IDentification) or
another wireless
protocol. The device may be powered by wireless induction, battery, or a
combination of the two.
The device 100 monitors physiological signals and/or collects data
representative thereof. The
collected data may then be transmitted wirelessly or by wire connection, in
real time, to the nearby
base station. The device may be wirelessly powered by the base station or by
battery, removing the
need for wires between the patient and the station.
[0134] Relatedly and/or alternatively, patients or wearers may be monitored
wirelessly in a
hospital, including an ICU (Intensive Care Unit) or other facility. As such,
an ECG signal may be
measured on a patient using a small, wireless patch device hereof. The signal
is then digitized and
transmitted wirelessly to a receiver. The receiver converts the signal back to
analog, such that it
approximates the original ECG signal in amplitude. This output is then
presented to an existing
hospital ECG monitor through the standard electrode leads. This allows the
patient to be monitored
using existing hospital infrastructure without any lead wires necessarily
connecting the patient to the
monitor. Patient chest impedance may be measured as well, allowing the
reconstructed signal to
approximate the ECG signal not only in amplitude, but in output impedance as
well. This can be
41
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
used to detect a disconnected patch. The output impedance may be continuously
variable, or it may
have discrete values that may be selected (e.g. one low value for a connected
device and one high
value to signify the patch has come loose). The impedance may also be used to
signify problems
with the wireless transmission.
[0135] Other alternative implementations may include coupling one or
multiple sensors mounted
to the forehead of an infant. Initially, a method of obtaining oxygen
saturation data by mounting a
device in the forehead of an infant might be used as introduced. However, an
expansion or
alternative may include coupling oxygen saturation sensors with relative
position and temperature
sensors on the same forehead-mounted device. The combined data can be utilized
to ascertain if an
infant is in any danger of suffocation due to a face-down position.
[0136] Thus, some of the alternative combinations hereof may include one or
more of: 1) medical
grade adhesives (from many possible sources) selected for their ability to
maintain in intimate
contact with the skin without damaging it, for several days (up to, say 10
days or two weeks in some
examples), as well as operability with different types of sensors; 2)
conductive electrodes or photo-
sensitive detectors able to supply electrical signals from the skin or from
the photo-response of
cutaneous or subcutaneous tissues to photo-excitation; 3) amplifiers,
microprocessors and memories,
capable of treating these signals and storing them; 4) power supply for the
electronics hereof with
stored or with wirelessly accessible re-chargeability; 5) flex circuits
capable of tying the above
elements together within a flexible strip capable of conforming to a cutaneous
region of interest.
[0137] Examples of physiological parameters that may be subject to
monitoring,
recordation/collection and/or analyzing may include one or more of:
electrocardiograms, photo
responses of photo-excited tissues for e.g., oxygen saturation of blood; pulse
rates and associated
fluctuations; indications of physical activity/acceleration. One or more of
these may be used in
monitoring ambulatory cardiac outpatients over several days and nights, which
could thereby
provide for recording, for post-test analysis, several days' worth of
continuous ECG signals together
with simultaneous recording of 02 saturation and an index of physical
exertion. Similarly, one or
more of these may be used in monitoring ambulatory pulmonary outpatients over
several days and
nights for recording, for post-test analysis, 02 saturation together with
simultaneous recording of an
index of physical activity. Alternatively and/or additionally, one or more of
these could be used for
monitoring in-patients or other patients of interest, as for example neonates,
wirelessly (or in some
cases wired), whether in clinics, emergency rooms, or ICUs, in some instances
detecting the
42
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
parameters of EKG, 02 and/or physical exertion, but instead of storing them
would transmit them
wirelessly to either a bedside monitor or a central station monitor, thus
freeing the patient from
attachment to physical wires. In particular, devices hereof may be adhered to
the forehead of a
neonate for monitoring respiration and oxygen saturation. In further
alternatives, devices hereof
may be used to monitor respiration and ECG of patients suffering from sleep
apnea.
[0138] An exemplary computer system or computing resources which may be used
herewith will
now be described, though it should be noted that many alternatives in
computing systems and
resources may be available and operable within the reasonably foreseeable
scope hereof so that the
following is intended in no way to be limiting of the myriad possible
computational alternatives
properly intended within both the spirit and scope hereof.
[0139] Some of the implementations of the present developments include
various steps. A variety
of these steps may be performed by hardware components or may be embodied in
machine-
executable instructions, which may be used to cause a general-purpose or
special-purpose processor
programmed with the instructions to perform the steps. Alternatively, the
steps may be performed by
a combination of hardware, software, and/or firmware. As such, FIG. 4 is an
example of computing
resources or a computer system 400 with which implementations hereof may be
utilized. According
to the present example, a sample such computer system 400 may include a bus
401, at least one
processor 402, at least one communication port 403, a main memory 404, a
removable storage media
405, a read only memory 406, and a mass storage 407. More or fewer of these
elements may be
used in a particular implementation hereof.
[0140] Processor(s) 402 can be any known processor, such as, but not
limited to, an Intel
Itanium or Itanium 2 processor(s), or AMD Opteron or Athlon MP
processor(s), or
Motorola lines of processors. Communication port(s) 403 can be any of an RS-
232 port for use
with a modem based dialup connection, a 10/100 Ethernet port, a Universal
Serial Bus (USB) port,
or a Gigabit port using copper or fiber. Communication port(s) 403 may be
chosen depending on a
network such a Local Area Network (LAN), Wide Area Network (WAN), or any
network to which
the computer system 400 connects or may be adapted to connect.
[0141] Main memory 404 can be Random Access Memory (RAM), or any other dynamic
storage
device(s) commonly known in the art. Read only memory 406 can be any static
storage device(s)
such as Programmable Read Only Memory (PROM) chips for storing static
information such as
instructions for processor 402.
43
CA 03045025 2019-05-24
WO 2018/112401 PCT/US2017/066805
[0142] Mass storage 407 can be used to store information and instructions.
For example, hard
disks such as the Adaptec family of SCSI drives, an optical disc, an array of
disks such as RAID,
such as the Adaptec family of RAID drives, or any other mass storage devices
may be used.
[0143] Bus 401 communicatively couples processor(s) 402 with the other
memory, storage and
communication blocks. Bus 401 can be a PCl/PCI-X or SCSI based system bus
depending on the
storage devices used.
[0144] Removable storage media 405 can be any kind of external hard-drives,
floppy drives,
IOMEGA Zip Drives, Compact Disc--Read Only Memory (CD-ROM), Compact Disc--Re-
Writable (CD-RW), Digital Video Dis--Read Only Memory (DVD-ROM).
[0145] The components described above are meant to exemplify some types of
possibilities. In no
way should the aforementioned examples limit the scope of the inventions
hereof, as they are only
exemplary embodiments.
[0146] Embodiments of the present inventions relate to devices, systems,
methods, media, and
arrangements for monitoring and processing cardiac parameters and data, inter
alia. While detailed
descriptions of one or more embodiments of the inventions have been given
above, various
alternatives, modifications, and equivalents will be apparent to those skilled
in the art without
varying from the spirit of the inventions hereof. Therefore, the above
description should not be taken
as limiting the scope of the inventions, which is defined by the appended
claims.
44